User login
Lentiviral gene therapy appears effective in X-CGD
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
HOUSTON – , said Donald B. Kohn, MD, of the University of California, Los Angeles.
Seven of nine patients treated were “alive and well” at 12 months’ follow-up after receiving lentiviral vector transduced CD34+ cells, Dr. Kohn reported in a late-breaking clinical trial session at the Transplantation & Cellular Therapy Meetings.
Most patients were able to discontinue antibiotic prophylaxis for this disease, which is associated with severe, recurrent, and prolonged life-threatening infections, he said.
Results of the small study provide “proof of concept” for use of the gene therapy in the disease, though additional studies are needed to formally assess the clinical safety and efficacy of the approach, he said.
The estimated incidence of chronic granulomatous disease is 1 in 200,000 births in the United States, and the X-linked form is most common, occurring in about 60% of patients, Dr. Kohn told attendees of the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Most of these patients are treated with antibacterial or antifungal prophylaxis. While allogeneic hematopoietic stem cell transplantation is also an option, according to Dr. Kohn, the approach is limited by a lack of matched donors and graft-versus-host disease.
Dr. Kohn reported results for nine patients in the United States and the United Kingdom who were treated with the same G1XCGD lentiviral vector. The patients, who ranged in age from 2 to 27 years, underwent CD34+ cell mobilization or bone marrow isolation, transduction with the lentiviral vector, busulfan conditioning, and autologous transplantation.
All patients had confirmed X-linked chronic granulomatous disease, and had had at least one severe infection or inflammatory complication requiring hospitalization.
There were no infusion-related adverse events, and one serious adverse event, which was an inflammatory syndrome that resolved with steroids. Two patients died from complications unrelated to gene therapy, Dr. Kohn reported.
“The other patients are basically doing quite well,” he said.
Of the seven patients alive at the 12-month follow up, six were reported as “clinically well” and off antibiotic prophylaxis, according to Dr. Kohn, while the seventh patient was clinically well and receiving antimicrobial support.
Dr. Kohn is a scientific advisory board member for Orchard Therapeutics, which licensed the lentiviral gene therapy for X-CGD discussed in his presentation. He is also an inventor of intellectual property related to the therapy that UCLA has licensed to Orchard.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
SOURCE: Kohn DB et al. TCT 2019, Abstract LBA1.
REPORTING FROM TCT 2019
Concurrent Keratoacanthomas and Nonsarcoidal Granulomatous Reactions in New and Preexisting Tattoos
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
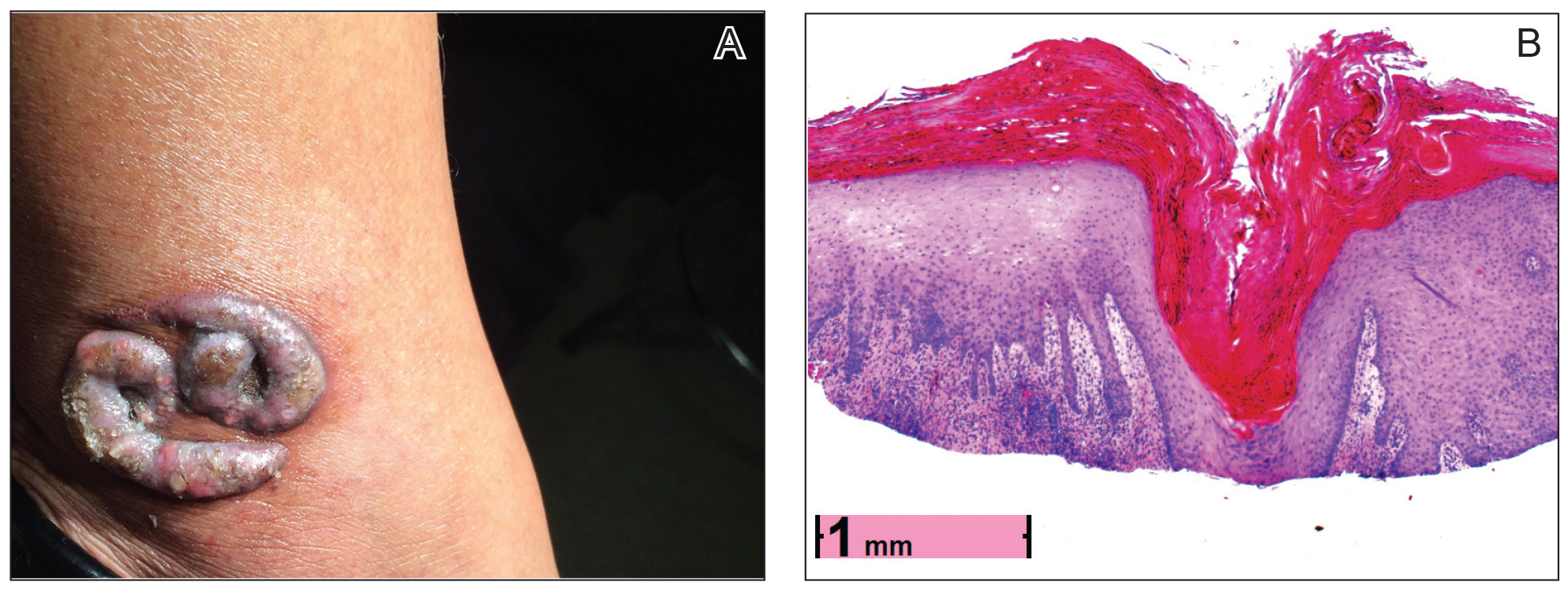
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.

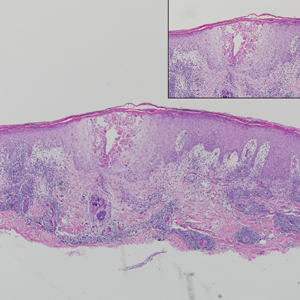
contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).
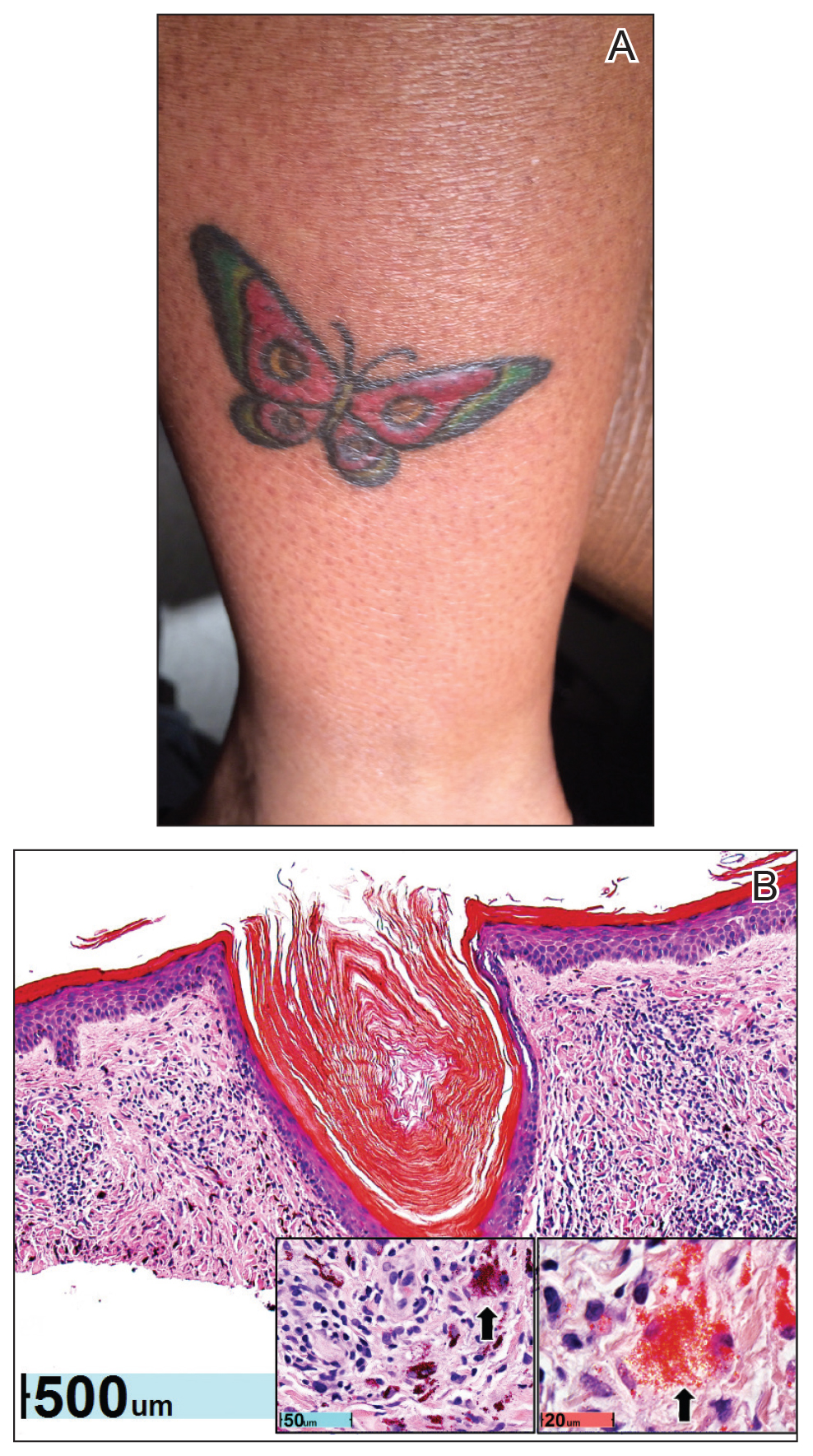
The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.


contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).

The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
To the Editor:
Cutaneous reactions to tattoos are common and histologically diverse. As outlined by Jacob,1 these reactions can be categorized into 4 main groups: inoculative/infective, hypersensitive, neoplastic, and coincidental. A thorough history and physical examination can aid in distinguishing the type of cutaneous reaction, but diagnosis often requires histopathologic clarification. We report the case of a patient who presented with painful indurated nodules within red ink areas of new and preexisting tattoos.
A 48-year-old woman with no prior medical conditions presented with tender pruritic nodules at the site of a new tattoo and within recently retouched tattoos of 5 months’ duration. The tattoos were done at an “organic” tattoo parlor 8 months prior to presentation. Simultaneously, the patient also developed induration and pain in 2 older tattoos that had been done 10 years prior and had not been retouched.
Physical examination revealed 2 smooth and serpiginous nodules nested perfectly within the new red tattoo on the left medial ankle (Figure 1A). Examination of the retouched tattoos on the dorsum of the right foot revealed 4 discrete nodules within the red, heart-shaped areas of the tattoos (Figure 2A). Additionally, the red-inked portions of an older tattoo on the left lateral calf that were outlined in red ink also were raised and indurated (Figure 3A), and a tattoo on the right volar wrist, also in red ink, was indurated and tender to palpation. The remainder of the physical examination was normal.


contiguous dilated follicular infundibula with atypical keratinocytes that had hyperchromatic nuclei, consistent with a keratoacanthoma, as well as a lymphocytic infiltrate in the dermis above a dense infiltrate of lymphocytes and histiocytes (H&E, original magnification ×2.5 [original magnification ×6.2]).

The lesions continued to enlarge and become increasingly painful despite trials of fluticasone propionate cream 0.05%, clobetasol propionate gel 0.05%, a 7-day course of oral levofloxacin, and a 10-day course of oral amoxicillin-clavulanate. Ultimately, a shave biopsy from the new tattoo on the left medial ankle revealed an early keratoacanthoma (KA)(Figure 1B). Subsequent shave biopsies of the retouched tattoos on the dorsal foot and the preexisting tattoo on the calf revealed KAs and a granulomatous reaction, respectively (Figures 2B and 3B). The left ankle KA was treated with 2 injections of 5-fluorouracil without improvement. The patient ultimately underwent Mohs micrographic surgery of the left ankle KA and underwent total excision with skin graft.
The development of KAs within tattoos is a known but poorly understood phenomenon.2 Keratoacanthomas are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution. They typically present as solitary isolated nodules arising in sun-exposed areas of patients of either sex, with a predilection for individuals of Fitzpatrick skin types I and II and in areas of prior trauma or sun damage.3
Histologically, the proliferative phase is defined by keratin-filled invagination of the epidermis into the dermis, with areas of hyperkeratosis, acanthosis, and mitotic activity within the strands and nodules. A high degree of nuclear atypia underlines the diagnostic difficulty in distinguishing KAs from squamous cell carcinomas (SCCs). A fully developed KA has less prominent cellular atypia and a characteristic buttressing lip of epithelium extending over the edges of an irregular, keratin-filled crater. In the final involution stage of KAs, granulation tissue and fibrosis predominate and apoptotic cells may be noted.4
The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, treatment with BRAF inhibitors, and trauma. Keratoacanthoma incidence also has been associated with chronic scarring diseases such as discoid lupus erythematous5 and lichen planus.6 Although solitary lesions are more typical, multiple generalized KAs can arise at once, as observed in generalized eruptive KA of Grzybowski, a rare condition, as well as in the multiple self-healing epitheliomas seen in Ferguson-Smith disease.
Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Some contest that KAs are benign and self-limited reactive proliferations, whereas others propose they are malignant variants of SCC.3,4,7,8 This debate is compounded by the difficulty in distinguishing KAs from SCC when specimen sampling is inadequate and given documentation that SCCs can develop within KAs over time.7 There also is some concern regarding the remote possibility of aggressive infiltration and even metastasis. One systematic review by Savage and Maize8 attempted to clarify the biologic behavior and malignant potential of KAs. Their review of 445 cases of KA with reported follow-up led to the conclusion that KAs exhibit a benign natural course with no reliable reports of death or metastasis. This finding was in stark contrast to 429 cases of SCC, of which 61 cases (14.2%) resulted in metastasis despite treatment.8
Our patient’s presentation was unique compared to others already reported in the literature because of the simultaneous development of nonsarcoidal granulomatous dermatitis within the older and nonretouched tattoos. Nonsarcoidal granulomatous dermatitis, which encompasses inflammatory skin diseases with histiocytes, is a reactive cutaneous proliferation that also has been reported to occur within tattoos.9,10 Granulomatous tattoo reactions can be further subdivided as foreign body type or sarcoidal type. Foreign body reactions are distinguished by the presence of pigment-containing multinucleated giant cells (as seen in our patient), whereas the sarcoidal type contains compact nodules of epithelioid histiocytes with few lymphocytes.4
The concurrent development of 2 clinically and histologically distinct entities suggests that a similar overlapping pathogenesis underlies each. One hypothesis is that the introduction of exogenous dyes may have instigated an inflammatory foreign body reaction, with the red ink acting as the unifying offender. The formation of granulomas in the preexisting tattoos is likely explained by an exaggerated immune response in the form of a type IV delayed hypersensitivity reaction triggered by reintroduction of the antigen—the red ink—in a presensitized host. Secondly, the parallel development of KAs within the new and retouched tattoos could be a result of the traumatic direct inoculation of the foreign material to which the body was presensitized and subsequent attempt by the skin to degrade and remove it.11
This case provides an example of the development of multiple KAs via a reactive process. Many other similar cases have been described in the literature, including case reports of KAs arising in areas of trauma such as thermal burns, vaccination sites, scars, skin grafts, arthropod bites, and tattoos.2-4,8 Together, the trauma and immune response may lead to localized inflammation and/or cellular hyperplasia, ultimately predisposing the individual to the development of dermoepidermal proliferation. Moreover, the exaggerated keratinocyte proliferation in KAs in response to trauma is reminiscent of the Köbner phenomenon. Other lesions that demonstrate köbnerization also have been reported to occur within new tattoos, including psoriasis, lichen planus, molluscum contagiosum, and verruca vulgaris.1,3
Although KAs are not always a consequence of trauma among humans, trauma-induced KA has been proven as a reliable phenomenon among animal models; an older study showed consistent KA development after animal skin was traumatized from the application of chemical carcinogens.12 Keratoacanthomas within areas of trauma seem to develop rapidly—within a week to a year after trauma—while the development of trauma-related nonmelanoma skin cancers appears to take longer, approximately 1 to 50 years later.13
More research is needed to clarify the pathophysiology of KAs and its precise relationship to trauma and immunology, but our case adds additional weight to the idea that some KAs are primarily reactive phenomena, sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
- Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28:962-965.
- Fraga GR, Prossick TA. Tattoo-associated keratoacanthomas: a series of 8 patients with 11 keratoacanthomas. J Cutan Pathol. 2010;37:85-90.
- Goldsmith LA, Katz SL, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw-Hill; 2012.
- Elder DE, Elenitsas R, Johnson BL Jr, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott, 2005.
- Minicucci EM, Weber SA, Stolf HO, et al. Keratoacanthoma of the lower lip complicating discoid lupus erythematosus in a 14-year-old boy. Pediatr Dermatol. 2007;24:329-330.
- Giesecke LM, Reid CM, James CL, et al. Giant keratoacanthoma arising in hypertrophic lichen planus. Australas J Dermatol. 2003;44:267-269.
- Weedon DD, Malo J, Brooks D, et al. Squamous cell carcinoma arising in keratoacanthoma: a neglected phenomenon in the elderly. Am J Dermatopathol. 2010;32:423-426.
- Savage JA, Maize JC. Keratoacanthoma clinical behavior: a systematic review. Am J Dermatopathol. 2014;36:422-429.
- Schwartz RA, Mathias CG, Miller CH, et al. Granulomatous reaction to purple tattoo pigment. Contact Derm. 1987;16:198-202.
- Bagley MP, Schwartz RA, Lambert WC. Hyperplastic reaction developing within a tattoo. granulomatous tattoo reaction, probably to mercuric sulfide (cinnabar). Arch Dermatol. 1987;123:1557, 1560-1561.
- Kluger N, Plantier F, Moguelet P, et al. Tattoos: natural history and histopathology of cutaneous reactions. Ann Dermatol Venereol. 2011;138:146-154.
- Ghadially FN, Barton BW, Kerridge DF. The etiology of keratoacanthoma. Cancer. 1963;16:603-611.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:e161-168.
Practice Points
- Keratoacanthomas (KAs) are common keratinizing, squamous cell lesions of follicular origin distinguished by their eruptive onset, rapid growth, and spontaneous involution.
- The etiology of KAs remains controversial, but several factors have been correlated with their development, including UV light exposure, chemical carcinogenesis, genetic predisposition, viruses (namely human papillomavirus infection), immunosuppression, scarring disorders, and trauma (including tattoos).
- Because of the unusual histology of KAs and their tendency to spontaneously regress, it is not totally understood where they fall on the benign vs malignant spectrum. Our case adds additional weight to the idea that some KAs are primarily reactive phenomena sharing features of other reactive cutaneous proliferations such as foreign body granulomas.
Infective endocarditis isn’t what it used to be
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
SNOWMASS, COLO. – Infective endocarditis in 2019 is very different from the disease most physicians encountered in training, both in terms of epidemiology and clinical presentation, Patrick T. O’Gara, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The classic description of infective endocarditis provided by Sir William Osler, MD, was of a subacute bacterial infection characterized by a long latent phase of low-grade fever, back pain, weight loss, and night sweats. It was mainly a right-heart disease of younger individuals with an infected native valve, and the predominant pathogens were streptococci, Dr. O’Gara said.
“I think in the current era endocarditis is more often characterized by an acute illness with toxic features in the context of adults with a high burden of degenerative diseases – for example, patients with rheumatoid arthritis or psoriatic arthritis on immunosuppressive therapy, or diabetes, end-stage renal disease, and risk factors for hospital-acquired infection. Injectable drug use is through the roof, there’s a wider prevalence of cardiac implanted electronic devices, which are a wonderful place for bacteria to hide, and Staphylococcus aureus has certainly become the leading pathogen with regard to endocarditis in the United States, especially MRSA, often multidrug resistant,” said Dr. O’Gara, professor of medicine at Harvard Medical School, Boston.
“Also, no talk about endocarditis is sufficient without paying some attention to the opioid crisis in which we find ourselves. It’s one of the top three causes of death among young men in the United States, along with accidents and gun violence. No region of the country is spared. This has completely inundated our ER and hospitalist services and our inpatient cardiology services with folks who are often repeat offenders when it comes to the difficulty in being able to give up an injectable drug use habit. They have multiple infections and hospitalizations, tricuspid valve involvement, and depending upon the aggressiveness of the Staphylococcus organism, typically they have left-sided disease with multiple complications, including aortic regurgitation and heart failure,” the cardiologist continued.
This description underscored one of Dr. O’Gara’s major points about the challenges posed by infective endocarditis in contemporary practice: “Expect the unexpected,” he advised. “When you’ve seen one case of infective endocarditis, you’ve seen one case of infective endocarditis.”
Outcomes are ‘sobering’
In the current era, outcomes are “sobering,” the cardiologist noted. Infective endocarditis carries a 6-month mortality rate of 20%-25% despite early surgery being performed during the index hospitalization in up to 60% of patients, with a relatively high perioperative mortality rate of about 10%. However, the risk of reinfection occurring in a newly implanted cardiac valve is impressively low at about 2%.
Refer early for multimodality imaging and surgical consultation
Transesophageal echocardiography is valuable in assessment of the infected valve. However, when extravalvular extension of the infection is suspected and the echo assessment is nondiagnostic or indeterminate, it’s time to quickly move on to advanced imaging, such as PET-CT.
The ACC/American Heart Association class I recommendations for early surgery in infected native valves haven’t changed substantially in over a decade. Based largely on observational data, there is an association between early surgery and lower in-hospital mortality (Lancet. 2012 Mar 10;379[9819]:965-975).
Class IIa recommendations for native valve surgery include recurrent emboli and a persistent vegetation despite appropriate antibiotic therapy. A “very controversial” class IIb recommendation for surgery because of weak supporting data is the identification of a mobile vegetation larger than 10 mm, particularly if it’s located on an anterior mitral valve leaflet, he said.
If the decision is made to forgo early surgery, be sure to repeat transesophageal echocardiography on day 7-10 to reassess the size of the patient’s vegetation.
“There is an association between size of vegetation and 1-year mortality, with a cut point of greater than 15 mm. Some would argue this constitutes a reasonable indication for early surgery,” Dr. O’Gara noted.
The embolization rate in patients with infective endocarditis is highest during the day before presentation, the day of presentation, and through the first 2 days afterward. The rate drops precipitously within 2 weeks after initiation of appropriate antibiotic therapy. Thus, to utilize early surgery to maximum effect in order to decrease the risk of embolization, it makes sense to operate within the first several days following presentation, before antibiotics have had sufficient time to catch up with the evolving disease process.
Don’t use half measures when it comes to removal of cardiac implanted electronic devices
The guidelines are clear regarding infected pacemakers, implanted cardioverter-defibrillators, and cardiac resynchronization devices: “It all needs to come out,” Dr. O’Gara emphasized. That includes all leads and the generator in patients with documented infection of only one portion of the device system, as a class I, level of evidence B recommendation. Moreover, complete removal of a pacemaker or defibrillator system is deemed “reasonable” as a class IIa recommendation in all patients with valvular infection caused by S. aureus or fungi even in the absence of evidence of device infection.
“I think we as general cardiologists have become increasingly impressed about how sick and festering these kinds of patients can become, even when we’re not able to prove that the lead is infected. The lead looks okay on transesophageal echo or PET-CT, blood cultures are negative, the valvular heart disease is really not that advanced, but several days go by and the patient is just not responding. We should have a high index of suspicion that there’s an infection we cannot appreciate. But obviously, you make these difficult decisions in consultation with your electrophysiology colleagues,” he added.
Know when the cardiologist should say ‘no’ to early aggressive surgery
While an aggressive early surgical approach often pays off in terms of prevention of embolic sequelae and a reduction in heart failure, the timing of surgery in the 20%-40% of patients with infective endocarditis who present with stroke or other neurologic complications remains controversial. An international group of Canadian and French cardiac surgeons and neurologists developed a useful algorithm regarding the types of neurologic complications for which early cardiac surgery is a poor idea because of the high risk of neurologic exacerbation. For example, a mycotic neuroaneurysm is grounds for postponement of cardiac surgery for at least 4 weeks (Circulation. 2016 Oct 25;134[17]:1280-92).
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
REPORTING FROM ACC SNOWMASS 2019
Which biologic is best in Crohn’s? Jury’s still out
LAS VEGAS – Biologics have dramatically improved the treatment of Crohn’s disease (CD), an influential gastroenterologist told colleagues, but there is still no clear evidence suggesting which ones are best as first-line treatments.
When it comes to making a choice, insurer policies may play a role, said Gary R. Lichtenstein, MD, lead author of the American College of Gastroenterology’s 2018 treatment guidelines for CD. Otherwise, “you’re left with clinical factors, your own judgment, and some indirect data,” said Dr. Lichtenstein, professor of medicine at the University of Pennsylvania, Philadelphia, who spoke about the guidelines in a presentation at the Crohn’s & Colitis Congress – a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Lichtenstein spoke about recommendations regarding treatment of CD in several areas. The following is a summary of some points he made:
Diagnosis: Some tests aren’t recommended
Classic signs and symptoms of CD include abdominal pain, diarrhea, fatigue, weight loss, failure to grow, anemia, and manifestations outside the intestines. Fecal calprotectin testing is recommended to differentiate inflammatory bowel disease from irritable bowel syndrome; genetic testing and serologic markers are not recommended for diagnosis.
Ileocolonoscopy is recommended for diagnosis and provides details about severity. Several factors suggest higher risk of progressive disease: Young age at diagnosis, initial extensive bowel involvement, perianal/severe rectal disease, and penetrating or stenosing phenotype at diagnosis.
Research is hinting that visceral adiposity may be a risk factor too, Dr. Lichtenstein said, adding that “the greater the number of poor prognostic factors, the worse the likelihood of needing a colectomy.”
Focus of treatment: Don’t just consider symptoms
For patients with moderate to severe disease, guidelines now suggest that physicians not just focus on symptoms but also consider endoscopic signs of response and healing. Guidelines also recommend paying attention to quality of life and levels of stress, anxiety, and depression.
Drug therapy: Biologics stand apart
Budesonide is appropriate for induction therapy in mild to moderate CD. There are many possible treatments for moderate to severe disease, including steroids for induction and thiopurines or methotrexate for maintenance
But biologics stand apart, Dr. Lichtenstein said, noting that “they have really been the mainstay of the treatment of our moderate to severe patients.”
Still, he cautioned that tumor necrosis factor (TNF) inhibitors are linked to a variety of adverse effects, including demyelination, heart failure, auto-immunity, infusion reactions, immunogenicity, infection, bone marrow suppression, and cancer. Specifically, the risk of lymphoma and melanoma may go up, although the absolute risk is low.
He added that a third of patients will not respond to TNF inhibitors, and about half of those who do respond may stop responding after a few years.
Among newer drugs, vedolizumab (Entyvio) is useful as an induction and maintenance drug in CD. “We recognize it has a slow onset of action,” Dr. Lichtenstein said. “Waiting is part of what one needs to do. Those who had prior anti-TNF failures are less likely to respond than those who have been anti-TNF naive.”
The drug has a favorable safety profile, he said.
Ustekinumab (Stelara) also has a favorable safety profile with very low infection risk, although Dr. Lichtenstein said he expects that the drug will be linked to a small increased risk of cancer. “Perhaps I’ll be wrong,” he said, “but time will tell.”
So which biologic is best? Direct head-to-head trials are lacking, Dr. Lichtenstein said. However, a 2018 systematic review and network meta-analysis analyzed clinical trial data and came to these conclusions: Infliximab (Remicade) and adalimumab (Humira) are best for induction of remission in biologic-naive patients; adalimumab and ustekinumab are best for induction of remission in TNF inhibitor–exposed patients; adalimumab and infliximab are best for maintenance of remission; and ustekinumab and infliximab are best in terms of lowest risk of adverse events or infection (Aliment Pharmacol Ther. 2018 Aug;48[4]:394-409).
Dr. Lichtenstein reports many disclosures with multiple drugmakers, including both grants/research support and consulting relationships with Celgene, Janssen Biotech, Salix, Shire, UCB and Warner Chilcott.
LAS VEGAS – Biologics have dramatically improved the treatment of Crohn’s disease (CD), an influential gastroenterologist told colleagues, but there is still no clear evidence suggesting which ones are best as first-line treatments.
When it comes to making a choice, insurer policies may play a role, said Gary R. Lichtenstein, MD, lead author of the American College of Gastroenterology’s 2018 treatment guidelines for CD. Otherwise, “you’re left with clinical factors, your own judgment, and some indirect data,” said Dr. Lichtenstein, professor of medicine at the University of Pennsylvania, Philadelphia, who spoke about the guidelines in a presentation at the Crohn’s & Colitis Congress – a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Lichtenstein spoke about recommendations regarding treatment of CD in several areas. The following is a summary of some points he made:
Diagnosis: Some tests aren’t recommended
Classic signs and symptoms of CD include abdominal pain, diarrhea, fatigue, weight loss, failure to grow, anemia, and manifestations outside the intestines. Fecal calprotectin testing is recommended to differentiate inflammatory bowel disease from irritable bowel syndrome; genetic testing and serologic markers are not recommended for diagnosis.
Ileocolonoscopy is recommended for diagnosis and provides details about severity. Several factors suggest higher risk of progressive disease: Young age at diagnosis, initial extensive bowel involvement, perianal/severe rectal disease, and penetrating or stenosing phenotype at diagnosis.
Research is hinting that visceral adiposity may be a risk factor too, Dr. Lichtenstein said, adding that “the greater the number of poor prognostic factors, the worse the likelihood of needing a colectomy.”
Focus of treatment: Don’t just consider symptoms
For patients with moderate to severe disease, guidelines now suggest that physicians not just focus on symptoms but also consider endoscopic signs of response and healing. Guidelines also recommend paying attention to quality of life and levels of stress, anxiety, and depression.
Drug therapy: Biologics stand apart
Budesonide is appropriate for induction therapy in mild to moderate CD. There are many possible treatments for moderate to severe disease, including steroids for induction and thiopurines or methotrexate for maintenance
But biologics stand apart, Dr. Lichtenstein said, noting that “they have really been the mainstay of the treatment of our moderate to severe patients.”
Still, he cautioned that tumor necrosis factor (TNF) inhibitors are linked to a variety of adverse effects, including demyelination, heart failure, auto-immunity, infusion reactions, immunogenicity, infection, bone marrow suppression, and cancer. Specifically, the risk of lymphoma and melanoma may go up, although the absolute risk is low.
He added that a third of patients will not respond to TNF inhibitors, and about half of those who do respond may stop responding after a few years.
Among newer drugs, vedolizumab (Entyvio) is useful as an induction and maintenance drug in CD. “We recognize it has a slow onset of action,” Dr. Lichtenstein said. “Waiting is part of what one needs to do. Those who had prior anti-TNF failures are less likely to respond than those who have been anti-TNF naive.”
The drug has a favorable safety profile, he said.
Ustekinumab (Stelara) also has a favorable safety profile with very low infection risk, although Dr. Lichtenstein said he expects that the drug will be linked to a small increased risk of cancer. “Perhaps I’ll be wrong,” he said, “but time will tell.”
So which biologic is best? Direct head-to-head trials are lacking, Dr. Lichtenstein said. However, a 2018 systematic review and network meta-analysis analyzed clinical trial data and came to these conclusions: Infliximab (Remicade) and adalimumab (Humira) are best for induction of remission in biologic-naive patients; adalimumab and ustekinumab are best for induction of remission in TNF inhibitor–exposed patients; adalimumab and infliximab are best for maintenance of remission; and ustekinumab and infliximab are best in terms of lowest risk of adverse events or infection (Aliment Pharmacol Ther. 2018 Aug;48[4]:394-409).
Dr. Lichtenstein reports many disclosures with multiple drugmakers, including both grants/research support and consulting relationships with Celgene, Janssen Biotech, Salix, Shire, UCB and Warner Chilcott.
LAS VEGAS – Biologics have dramatically improved the treatment of Crohn’s disease (CD), an influential gastroenterologist told colleagues, but there is still no clear evidence suggesting which ones are best as first-line treatments.
When it comes to making a choice, insurer policies may play a role, said Gary R. Lichtenstein, MD, lead author of the American College of Gastroenterology’s 2018 treatment guidelines for CD. Otherwise, “you’re left with clinical factors, your own judgment, and some indirect data,” said Dr. Lichtenstein, professor of medicine at the University of Pennsylvania, Philadelphia, who spoke about the guidelines in a presentation at the Crohn’s & Colitis Congress – a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Dr. Lichtenstein spoke about recommendations regarding treatment of CD in several areas. The following is a summary of some points he made:
Diagnosis: Some tests aren’t recommended
Classic signs and symptoms of CD include abdominal pain, diarrhea, fatigue, weight loss, failure to grow, anemia, and manifestations outside the intestines. Fecal calprotectin testing is recommended to differentiate inflammatory bowel disease from irritable bowel syndrome; genetic testing and serologic markers are not recommended for diagnosis.
Ileocolonoscopy is recommended for diagnosis and provides details about severity. Several factors suggest higher risk of progressive disease: Young age at diagnosis, initial extensive bowel involvement, perianal/severe rectal disease, and penetrating or stenosing phenotype at diagnosis.
Research is hinting that visceral adiposity may be a risk factor too, Dr. Lichtenstein said, adding that “the greater the number of poor prognostic factors, the worse the likelihood of needing a colectomy.”
Focus of treatment: Don’t just consider symptoms
For patients with moderate to severe disease, guidelines now suggest that physicians not just focus on symptoms but also consider endoscopic signs of response and healing. Guidelines also recommend paying attention to quality of life and levels of stress, anxiety, and depression.
Drug therapy: Biologics stand apart
Budesonide is appropriate for induction therapy in mild to moderate CD. There are many possible treatments for moderate to severe disease, including steroids for induction and thiopurines or methotrexate for maintenance
But biologics stand apart, Dr. Lichtenstein said, noting that “they have really been the mainstay of the treatment of our moderate to severe patients.”
Still, he cautioned that tumor necrosis factor (TNF) inhibitors are linked to a variety of adverse effects, including demyelination, heart failure, auto-immunity, infusion reactions, immunogenicity, infection, bone marrow suppression, and cancer. Specifically, the risk of lymphoma and melanoma may go up, although the absolute risk is low.
He added that a third of patients will not respond to TNF inhibitors, and about half of those who do respond may stop responding after a few years.
Among newer drugs, vedolizumab (Entyvio) is useful as an induction and maintenance drug in CD. “We recognize it has a slow onset of action,” Dr. Lichtenstein said. “Waiting is part of what one needs to do. Those who had prior anti-TNF failures are less likely to respond than those who have been anti-TNF naive.”
The drug has a favorable safety profile, he said.
Ustekinumab (Stelara) also has a favorable safety profile with very low infection risk, although Dr. Lichtenstein said he expects that the drug will be linked to a small increased risk of cancer. “Perhaps I’ll be wrong,” he said, “but time will tell.”
So which biologic is best? Direct head-to-head trials are lacking, Dr. Lichtenstein said. However, a 2018 systematic review and network meta-analysis analyzed clinical trial data and came to these conclusions: Infliximab (Remicade) and adalimumab (Humira) are best for induction of remission in biologic-naive patients; adalimumab and ustekinumab are best for induction of remission in TNF inhibitor–exposed patients; adalimumab and infliximab are best for maintenance of remission; and ustekinumab and infliximab are best in terms of lowest risk of adverse events or infection (Aliment Pharmacol Ther. 2018 Aug;48[4]:394-409).
Dr. Lichtenstein reports many disclosures with multiple drugmakers, including both grants/research support and consulting relationships with Celgene, Janssen Biotech, Salix, Shire, UCB and Warner Chilcott.
EXPERT ANALYSIS FROM CROHN’S & COLITIS CONGRESS
Comorbid skin conditions common in children with lichen nitidus
as is generalized disease, according to Selcen Kundak, MD, and Yasemin Çakır, MD, of Dr. Behcet Uz Children’s Research and Training Hospital in Izmir, Turkey.
In a retrospective study 10-year study of 17 children with biopsy-confirmed lichen nitidus (LN) who were diagnosed with the disease at a single tertiary care health center between January 2007 and March 2017, the mean age of onset was 9 years and 15 of 17 (88%) were male. The mean skin lesion duration period was 13 months (range 1-48 months).
The generalized form of LN was common in the study population, occurring in 7 of 17 (41%) patients, 2 of whom had severe pruritus. Comorbid skin conditions also occurred in seven (41%) patients; these conditions included lichen planus in one patient, lichen striatus in one patient, nail psoriasis in one patients, and cutaneous features of atopic skin in four patients. In addition, 11 of 17 (65%) patients had multinucleated giant cells.
“Seven of 17 had comorbid skin conditions. Lichen planus, lichen striatus, psoriasis, and atopy are also chronic inflammatory skin conditions, and possibly, there are common triggers for these and LN; however, this is speculative, not proven,” the investigators concluded in Pediatric Dermatology.
Mild to moderate corticosteroids were give to 16 patients; all showed some clinical improvement within 3 weeks. One patient was treated with systemic corticosteroids.
The study authors reported no relevant financial disclosures.
SOURCE: Kundak S et al. Pediatr Dermatol. 2019 Feb 11. doi: 10.1111/pde.13749.
as is generalized disease, according to Selcen Kundak, MD, and Yasemin Çakır, MD, of Dr. Behcet Uz Children’s Research and Training Hospital in Izmir, Turkey.
In a retrospective study 10-year study of 17 children with biopsy-confirmed lichen nitidus (LN) who were diagnosed with the disease at a single tertiary care health center between January 2007 and March 2017, the mean age of onset was 9 years and 15 of 17 (88%) were male. The mean skin lesion duration period was 13 months (range 1-48 months).
The generalized form of LN was common in the study population, occurring in 7 of 17 (41%) patients, 2 of whom had severe pruritus. Comorbid skin conditions also occurred in seven (41%) patients; these conditions included lichen planus in one patient, lichen striatus in one patient, nail psoriasis in one patients, and cutaneous features of atopic skin in four patients. In addition, 11 of 17 (65%) patients had multinucleated giant cells.
“Seven of 17 had comorbid skin conditions. Lichen planus, lichen striatus, psoriasis, and atopy are also chronic inflammatory skin conditions, and possibly, there are common triggers for these and LN; however, this is speculative, not proven,” the investigators concluded in Pediatric Dermatology.
Mild to moderate corticosteroids were give to 16 patients; all showed some clinical improvement within 3 weeks. One patient was treated with systemic corticosteroids.
The study authors reported no relevant financial disclosures.
SOURCE: Kundak S et al. Pediatr Dermatol. 2019 Feb 11. doi: 10.1111/pde.13749.
as is generalized disease, according to Selcen Kundak, MD, and Yasemin Çakır, MD, of Dr. Behcet Uz Children’s Research and Training Hospital in Izmir, Turkey.
In a retrospective study 10-year study of 17 children with biopsy-confirmed lichen nitidus (LN) who were diagnosed with the disease at a single tertiary care health center between January 2007 and March 2017, the mean age of onset was 9 years and 15 of 17 (88%) were male. The mean skin lesion duration period was 13 months (range 1-48 months).
The generalized form of LN was common in the study population, occurring in 7 of 17 (41%) patients, 2 of whom had severe pruritus. Comorbid skin conditions also occurred in seven (41%) patients; these conditions included lichen planus in one patient, lichen striatus in one patient, nail psoriasis in one patients, and cutaneous features of atopic skin in four patients. In addition, 11 of 17 (65%) patients had multinucleated giant cells.
“Seven of 17 had comorbid skin conditions. Lichen planus, lichen striatus, psoriasis, and atopy are also chronic inflammatory skin conditions, and possibly, there are common triggers for these and LN; however, this is speculative, not proven,” the investigators concluded in Pediatric Dermatology.
Mild to moderate corticosteroids were give to 16 patients; all showed some clinical improvement within 3 weeks. One patient was treated with systemic corticosteroids.
The study authors reported no relevant financial disclosures.
SOURCE: Kundak S et al. Pediatr Dermatol. 2019 Feb 11. doi: 10.1111/pde.13749.
FROM PEDIATRIC DERMATOLOGY
Indurated Plaque on the Shoulder
Herpes zoster (HZ) is a painful skin condition caused by reactivation of latent varicella-zoster virus (VZV) in dorsal root ganglion cells.1 Upon reactivation, VZV replicates in the dorsal root ganglion, which ultimately results in inflammation and necrosis of the neuron and intense neuralgia. Reactivation of latent VZV may occur spontaneously or may be induced by various factors including immunosuppression, stress, illness, and trauma. Prior to the development of skin lesions, many patients experience a prodrome of tingling, pain, or pruritus. Herpes zoster classically presents with grouped vesicles on an erythematous base in a unilateral dermatomal distribution; however, more than one adjacent dermatome may be involved, and the lesions can cross the midline. Furthermore, the development of vesicles may be preceded by the development of edematous papules or plaques.1
On histology, VZV closely resembles herpes simplex virus type 1 and herpes simplex virus type 2 infections.2 Classic histologic findings include ballooning degeneration of keratinocytes, acantholysis, nuclear molding, ground-glass nuclear inclusions, marginated chromatin, and multinucleated keratinocytes, as well as necrosis of follicles and sebaceous glands.2 Varicella-zoster virus polymerase chain reaction or immunostaining can be used to confirm the diagnosis.2
Classic mycosis fungoides (MF) presents with well-circumscribed erythematous patches in non–sun-exposed areas and eventually may progress to plaques and tumors.3 Patients with cutaneous T-cell lymphomas, such as MF, are at a higher risk for skin infections including HZ4,5; however, immunocompromised patients, such as those with cutaneous lymphomas, can have atypical clinical presentations of HZ that may be concerning for cutaneous lymphoma.6 Furthermore, cutaneous malignancies can occur in dermatomal distributions that may mimic HZ.7 Therefore, the threshold for biopsy should be lowered in those patients with dermatomal lesions and history concerning for possible malignancy.
Classically, histologic examination of MF demonstrates an infiltrate of haloed cells at the dermoepidermal junction, which are atypical T cells with hyperchromatic cerebriform nuclei that are larger, darker, and more angulated than the benign recruited lymphocytes in the perivascular infiltrate seen in VZV infection (Figure 1).3 Papillary dermal fibrosis typically is present, and the perivascular infiltrate is denser above the postcapillary venule rather than being symmetrical around the vessel (bare underbelly sign). Clusters of these cells may form within the epidermis, which are called Pautrier microabscesses.3 Mycosis fungoides also can exhibit large cell transformation in which small lymphocytes transform into larger cells, thereby associated with a poorer prognosis.8
Lymphomatoid papulosis is a CD30+-predominant form of cutaneous T-cell lymphoma characterized by papules and nodules that spontaneously involute.9 This condition is most commonly associated with MF but can be associated with other lymphomas. This condition may be mistaken for HZ clinically, but histology classically demonstrates large atypical lymphocytes resembling Reed-Sternberg cells in small clusters rather than follicular necrosis (Figure 2).9
Patients with lymphoma may sequentially develop a secondary lymphoma. There have been reports of secondary B-cell lymphomas associated with MF, but this phenomenon is rare.10 The histology depends on the type of B-cell lymphoma present, but follicular necrosis would not be expected (Figure 3).
Unusual hypersensitivity reactions to arthropod attacks have been described in patients with lymphoproliferative disorders and could be mistaken for HZ. Histology may demonstrate a wedge-shaped perivascular and/or interstitial infiltrate containing eosinophils with endothelial swelling (Figure 4), but these findings may vary depending on the type of arthropod involved.11
Our case provided a unique example of HZ in a patient with a known history of MF. Clinically, there was concern for progression of the patient’s underlying disease; however, histology demonstrated ballooning keratinocytes and follicular necrosis, which are classically seen in HZ infection.
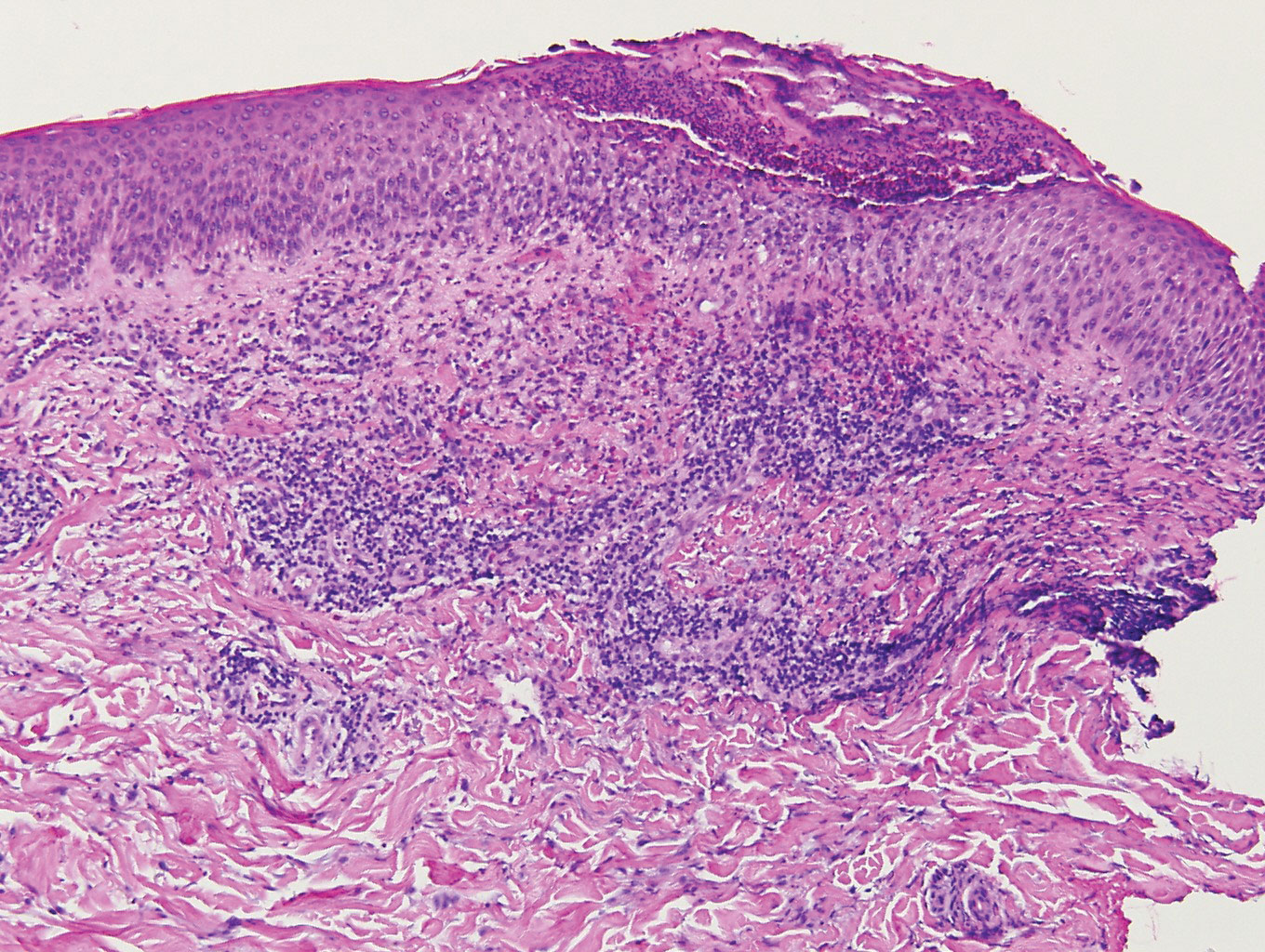
- Downing C, Medoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- Chisholm C, Lopez L. Cutaneous infections caused by Herpesviridae: a review. Arch Pathol Lab Med. 2011;135:1357-1362.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70: 205.e1-205.e16.
- Vonderheid EC, van Voorst Vader PC. Herpes zoster-varicella in cutaneous T-cell lymphomas. Arch Dermatol. 1980;116:408-412.
- Lebas E, Arrese JE, Nikkels AF. Risk factors for skin infections in mycosis fungoides. Dermatology. 2016;232:731-737.
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Niiyama S, Satoh K, Kaneko S, et al. Zosteriform skin involvement of nodal T-cell lymphoma: a review of the published work of cutaneous malignancies mimicking herpes zoster. J Dermatol. 2007;34:68-73.
- Pulitzer M, Myskowski PL, Horwitz SM, et al. Mycosis fungoides with large cell transformation:clinicopathological features and prognostic factors. Pathology. 2014;46:610-616.
- Zackheim HS, Jones C, Leboit PE, et al. Lymphomatoid papulosis associated with mycosis fungoides: a study of 21 patients including analyses for clonality. J Am Acad Dermatol. 2003;49:620-623.
- Barzilai A, Trau H, David M, et al. Mycosis fungoides associated with B-cell malignancies. Br J Dermatol. 2006;155:379-386.
- Vassallo C, Passamonti F, Cananzi R, et al. Exaggerated insect bite-like reaction in patients affected by oncohaematological diseases. Acta Derm Venereol. 2005;85:76-77.
Herpes zoster (HZ) is a painful skin condition caused by reactivation of latent varicella-zoster virus (VZV) in dorsal root ganglion cells.1 Upon reactivation, VZV replicates in the dorsal root ganglion, which ultimately results in inflammation and necrosis of the neuron and intense neuralgia. Reactivation of latent VZV may occur spontaneously or may be induced by various factors including immunosuppression, stress, illness, and trauma. Prior to the development of skin lesions, many patients experience a prodrome of tingling, pain, or pruritus. Herpes zoster classically presents with grouped vesicles on an erythematous base in a unilateral dermatomal distribution; however, more than one adjacent dermatome may be involved, and the lesions can cross the midline. Furthermore, the development of vesicles may be preceded by the development of edematous papules or plaques.1
On histology, VZV closely resembles herpes simplex virus type 1 and herpes simplex virus type 2 infections.2 Classic histologic findings include ballooning degeneration of keratinocytes, acantholysis, nuclear molding, ground-glass nuclear inclusions, marginated chromatin, and multinucleated keratinocytes, as well as necrosis of follicles and sebaceous glands.2 Varicella-zoster virus polymerase chain reaction or immunostaining can be used to confirm the diagnosis.2
Classic mycosis fungoides (MF) presents with well-circumscribed erythematous patches in non–sun-exposed areas and eventually may progress to plaques and tumors.3 Patients with cutaneous T-cell lymphomas, such as MF, are at a higher risk for skin infections including HZ4,5; however, immunocompromised patients, such as those with cutaneous lymphomas, can have atypical clinical presentations of HZ that may be concerning for cutaneous lymphoma.6 Furthermore, cutaneous malignancies can occur in dermatomal distributions that may mimic HZ.7 Therefore, the threshold for biopsy should be lowered in those patients with dermatomal lesions and history concerning for possible malignancy.
Classically, histologic examination of MF demonstrates an infiltrate of haloed cells at the dermoepidermal junction, which are atypical T cells with hyperchromatic cerebriform nuclei that are larger, darker, and more angulated than the benign recruited lymphocytes in the perivascular infiltrate seen in VZV infection (Figure 1).3 Papillary dermal fibrosis typically is present, and the perivascular infiltrate is denser above the postcapillary venule rather than being symmetrical around the vessel (bare underbelly sign). Clusters of these cells may form within the epidermis, which are called Pautrier microabscesses.3 Mycosis fungoides also can exhibit large cell transformation in which small lymphocytes transform into larger cells, thereby associated with a poorer prognosis.8

Lymphomatoid papulosis is a CD30+-predominant form of cutaneous T-cell lymphoma characterized by papules and nodules that spontaneously involute.9 This condition is most commonly associated with MF but can be associated with other lymphomas. This condition may be mistaken for HZ clinically, but histology classically demonstrates large atypical lymphocytes resembling Reed-Sternberg cells in small clusters rather than follicular necrosis (Figure 2).9

Patients with lymphoma may sequentially develop a secondary lymphoma. There have been reports of secondary B-cell lymphomas associated with MF, but this phenomenon is rare.10 The histology depends on the type of B-cell lymphoma present, but follicular necrosis would not be expected (Figure 3).

Unusual hypersensitivity reactions to arthropod attacks have been described in patients with lymphoproliferative disorders and could be mistaken for HZ. Histology may demonstrate a wedge-shaped perivascular and/or interstitial infiltrate containing eosinophils with endothelial swelling (Figure 4), but these findings may vary depending on the type of arthropod involved.11
Our case provided a unique example of HZ in a patient with a known history of MF. Clinically, there was concern for progression of the patient’s underlying disease; however, histology demonstrated ballooning keratinocytes and follicular necrosis, which are classically seen in HZ infection.

Herpes zoster (HZ) is a painful skin condition caused by reactivation of latent varicella-zoster virus (VZV) in dorsal root ganglion cells.1 Upon reactivation, VZV replicates in the dorsal root ganglion, which ultimately results in inflammation and necrosis of the neuron and intense neuralgia. Reactivation of latent VZV may occur spontaneously or may be induced by various factors including immunosuppression, stress, illness, and trauma. Prior to the development of skin lesions, many patients experience a prodrome of tingling, pain, or pruritus. Herpes zoster classically presents with grouped vesicles on an erythematous base in a unilateral dermatomal distribution; however, more than one adjacent dermatome may be involved, and the lesions can cross the midline. Furthermore, the development of vesicles may be preceded by the development of edematous papules or plaques.1
On histology, VZV closely resembles herpes simplex virus type 1 and herpes simplex virus type 2 infections.2 Classic histologic findings include ballooning degeneration of keratinocytes, acantholysis, nuclear molding, ground-glass nuclear inclusions, marginated chromatin, and multinucleated keratinocytes, as well as necrosis of follicles and sebaceous glands.2 Varicella-zoster virus polymerase chain reaction or immunostaining can be used to confirm the diagnosis.2
Classic mycosis fungoides (MF) presents with well-circumscribed erythematous patches in non–sun-exposed areas and eventually may progress to plaques and tumors.3 Patients with cutaneous T-cell lymphomas, such as MF, are at a higher risk for skin infections including HZ4,5; however, immunocompromised patients, such as those with cutaneous lymphomas, can have atypical clinical presentations of HZ that may be concerning for cutaneous lymphoma.6 Furthermore, cutaneous malignancies can occur in dermatomal distributions that may mimic HZ.7 Therefore, the threshold for biopsy should be lowered in those patients with dermatomal lesions and history concerning for possible malignancy.
Classically, histologic examination of MF demonstrates an infiltrate of haloed cells at the dermoepidermal junction, which are atypical T cells with hyperchromatic cerebriform nuclei that are larger, darker, and more angulated than the benign recruited lymphocytes in the perivascular infiltrate seen in VZV infection (Figure 1).3 Papillary dermal fibrosis typically is present, and the perivascular infiltrate is denser above the postcapillary venule rather than being symmetrical around the vessel (bare underbelly sign). Clusters of these cells may form within the epidermis, which are called Pautrier microabscesses.3 Mycosis fungoides also can exhibit large cell transformation in which small lymphocytes transform into larger cells, thereby associated with a poorer prognosis.8

Lymphomatoid papulosis is a CD30+-predominant form of cutaneous T-cell lymphoma characterized by papules and nodules that spontaneously involute.9 This condition is most commonly associated with MF but can be associated with other lymphomas. This condition may be mistaken for HZ clinically, but histology classically demonstrates large atypical lymphocytes resembling Reed-Sternberg cells in small clusters rather than follicular necrosis (Figure 2).9

Patients with lymphoma may sequentially develop a secondary lymphoma. There have been reports of secondary B-cell lymphomas associated with MF, but this phenomenon is rare.10 The histology depends on the type of B-cell lymphoma present, but follicular necrosis would not be expected (Figure 3).

Unusual hypersensitivity reactions to arthropod attacks have been described in patients with lymphoproliferative disorders and could be mistaken for HZ. Histology may demonstrate a wedge-shaped perivascular and/or interstitial infiltrate containing eosinophils with endothelial swelling (Figure 4), but these findings may vary depending on the type of arthropod involved.11
Our case provided a unique example of HZ in a patient with a known history of MF. Clinically, there was concern for progression of the patient’s underlying disease; however, histology demonstrated ballooning keratinocytes and follicular necrosis, which are classically seen in HZ infection.

- Downing C, Medoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- Chisholm C, Lopez L. Cutaneous infections caused by Herpesviridae: a review. Arch Pathol Lab Med. 2011;135:1357-1362.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70: 205.e1-205.e16.
- Vonderheid EC, van Voorst Vader PC. Herpes zoster-varicella in cutaneous T-cell lymphomas. Arch Dermatol. 1980;116:408-412.
- Lebas E, Arrese JE, Nikkels AF. Risk factors for skin infections in mycosis fungoides. Dermatology. 2016;232:731-737.
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Niiyama S, Satoh K, Kaneko S, et al. Zosteriform skin involvement of nodal T-cell lymphoma: a review of the published work of cutaneous malignancies mimicking herpes zoster. J Dermatol. 2007;34:68-73.
- Pulitzer M, Myskowski PL, Horwitz SM, et al. Mycosis fungoides with large cell transformation:clinicopathological features and prognostic factors. Pathology. 2014;46:610-616.
- Zackheim HS, Jones C, Leboit PE, et al. Lymphomatoid papulosis associated with mycosis fungoides: a study of 21 patients including analyses for clonality. J Am Acad Dermatol. 2003;49:620-623.
- Barzilai A, Trau H, David M, et al. Mycosis fungoides associated with B-cell malignancies. Br J Dermatol. 2006;155:379-386.
- Vassallo C, Passamonti F, Cananzi R, et al. Exaggerated insect bite-like reaction in patients affected by oncohaematological diseases. Acta Derm Venereol. 2005;85:76-77.
- Downing C, Medoza N, Sra K, et al. Human herpesviruses. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:1400-1424.
- Chisholm C, Lopez L. Cutaneous infections caused by Herpesviridae: a review. Arch Pathol Lab Med. 2011;135:1357-1362.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part I. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70: 205.e1-205.e16.
- Vonderheid EC, van Voorst Vader PC. Herpes zoster-varicella in cutaneous T-cell lymphomas. Arch Dermatol. 1980;116:408-412.
- Lebas E, Arrese JE, Nikkels AF. Risk factors for skin infections in mycosis fungoides. Dermatology. 2016;232:731-737.
- Leinweber B, Kerl H, Cerroni L. Histopathologic features of cutaneous herpes virus infections (herpes simplex, herpes varicella/zoster): a broad spectrum of presentations with common pseudolymphomatous aspects. Am J Surg Pathol. 2006;30:50-58.
- Niiyama S, Satoh K, Kaneko S, et al. Zosteriform skin involvement of nodal T-cell lymphoma: a review of the published work of cutaneous malignancies mimicking herpes zoster. J Dermatol. 2007;34:68-73.
- Pulitzer M, Myskowski PL, Horwitz SM, et al. Mycosis fungoides with large cell transformation:clinicopathological features and prognostic factors. Pathology. 2014;46:610-616.
- Zackheim HS, Jones C, Leboit PE, et al. Lymphomatoid papulosis associated with mycosis fungoides: a study of 21 patients including analyses for clonality. J Am Acad Dermatol. 2003;49:620-623.
- Barzilai A, Trau H, David M, et al. Mycosis fungoides associated with B-cell malignancies. Br J Dermatol. 2006;155:379-386.
- Vassallo C, Passamonti F, Cananzi R, et al. Exaggerated insect bite-like reaction in patients affected by oncohaematological diseases. Acta Derm Venereol. 2005;85:76-77.
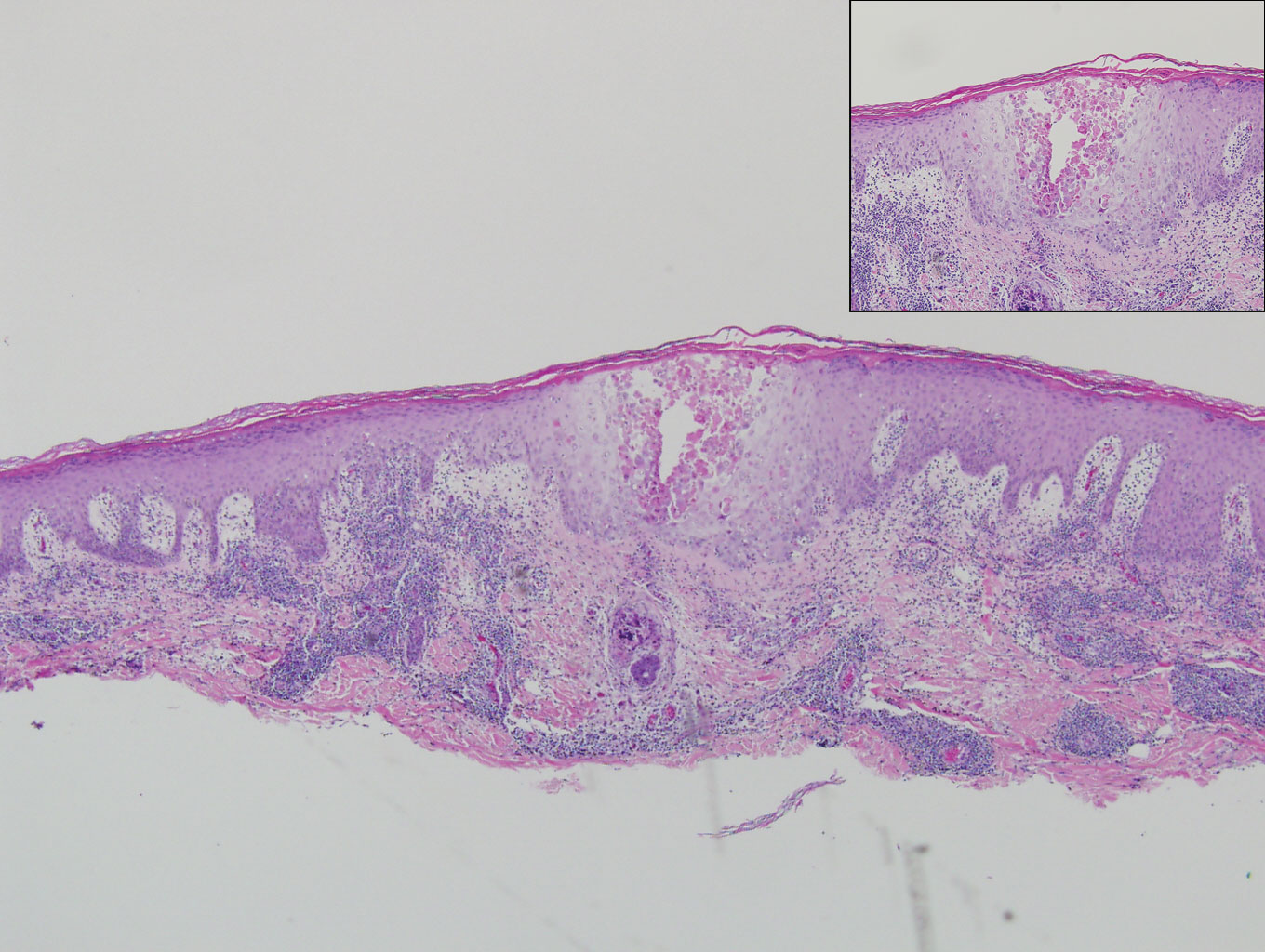
A 66-year-old man with mycosis fungoides presented with a new indurated plaque on the left shoulder. Biopsies of the left shoulder and back lesions were obtained.
Novel transplant regimen improves survival in primary immunodeficiency
HOUSTON – Allogeneic hematopoietic stem cell transplantation (allo-HCT) following a novel reduced-intensity conditioning regimen was largely successful in a heterogeneous cohort of 29 adults and children with primary immunodeficiency in a prospective clinical trial.
At 1 year after transplant, overall survival was 98% and the estimated graft failure–free and graft-versus-host disease (GVHD)–free survival was 82% among the participants, who had various underlying primary immunodeficiencies (PIDs), Dimana Dimitrova, MD, reported at the Transplantation and Cellular Therapy Meetings.
GVHD-free survival was defined in this National Institutes of Health study as the absence of steroid-refractory grade 3-4 acute GVHD and chronic GVHD, noted Dr. Dimitrova of the NIH.
All patients, including 19 adults and 10 children (median age, 25 years), received a serotherapy-free, radiation-free, reduced-intensity conditioning regimen designed to optimize immune reconstitution, minimize toxicity and GVHD, reduce the risk of infectious complications, and enable successful use of alternative donors.
The conditioning platform included pentostatin on day –11 and day –7 at 4 mg/m2 along with 8 days of low-dose cyclophosphamide and 2 days of pharmacokinetically dosed busulfan at 4,600 mmol/min. GVHD prophylaxis included posttransplantation cyclophosphamide, mycophenolate mofetil (MMF), and sirolimus.
All patients received T cell–replete bone marrow or peripheral blood stem cell allografts; 72% received alternative donor grafts, Dr. Dimitrova said.
Two patients died, including one with bacterial sepsis and invasive aspergillosis who died on day +44 and one with presumed viral encephalitis who died on day +110. The patients were high risk overall (median HCT–comorbidity index score of 3, with a range of 0-11), and the two who died had HCT-CI scores of 6 and 8, respectively.
An additional accidental death occurred at 18 months after transplant “in the setting of continued remission, good graft function, and no transplant-related complications,” she said.
Neutrophil recovery occurred at a median of 17 days after transplant; three patients experienced graft failure, including one primary failure with autologous recovery on day +14 and two secondary graft failures.
“Two patients with known underlying difficult-to-engraft diseases required second transplants using different nonmyeloabalative platforms, and nevertheless required donor lymphocyte infusions to avoid threatened secondary graft failure,” she said. “The third patient actually had sufficiently improved infectious disease control and has not needed a second transplant to date.”
Overall GVHD incidence using the novel platform has been extremely low, she said, noting that 14% of patients had grade 2-4 GVHD and 3% had grade 3-4 acute GVHD. There was no steroid-refractory GVHD or chronic GVHD.
Among the infectious complications, other than those that led to the two deaths, were cytomegalovirus reactivation in 7 of 16 patients at risk, BK virus–associated hemorrhagic cystitis in 19 of 22 patients at risk, and a suspected case of viral cardiomyopathy that ultimately resolved.
“Importantly, although many patients had Epstein-Barr virus [EBV] control issues prior to transplant, no patients received preemptive EBV-directed therapy, and no patients had EBV-PTLD [posttransplant lymphoproliferative disorder],” she said.
Additionally, blood stream infections were detected in five patients, there were two cases of confirmed aspergillosis, and one child developed cutaneous candidiasis. Other complications and toxicities appeared to relate to underlying pretransplant issues in the affected organ or exuberant immune responses to existing infection.
“Phenotype reversal was evident to some degree in all evaluable patients, even in those with mixed chimerism or unknown underlying genetic defect,” Dr. Dimitrova said.
All 10 patients with malignancy or lymphoproliferative disease as an additional indication for allo-HCT remain in remission, and most patients who required immunoglobulin replacement therapy prior to transplant have been able to discontinue it, she noted.
The findings of this study are of note, because while it has been known for decades that allo-HCT is a potentially curative therapy for patients with PIDs that arise from defects in cells of hematopoietic origin, it frequently fails because of complicating factors or is not an option, Dr. Dimitrova said.
“These patients will often enter transplant with multiple comorbidities and disease sequelae, particularly as diagnosis of PIDs increases in older children and adults following years of illness,” she explained, adding that related donor options may be limited if family members are also affected.
For this reason, and with the goal of improving access to allo-HCT to all who require it, the novel conditioning platform used in this study was developed.
The platform was well tolerated overall, Dr. Dimitrova said, emphasizing the “notably low” GVHD rates.
“Currently we are investigating reduced MMF with the goal of promoting earlier immune reconstitution, and a separate protocol has opened that includes several modifications to this platform aimed at patients with increased risk of graft failure who may not tolerate mixed chimerism early on,” she said, noting that both protocols are currently enrolling.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Dimitrova reported having no financial disclosures.
SOURCE: Dimitrova D et al. TCT 2019, Abstract 54.
HOUSTON – Allogeneic hematopoietic stem cell transplantation (allo-HCT) following a novel reduced-intensity conditioning regimen was largely successful in a heterogeneous cohort of 29 adults and children with primary immunodeficiency in a prospective clinical trial.
At 1 year after transplant, overall survival was 98% and the estimated graft failure–free and graft-versus-host disease (GVHD)–free survival was 82% among the participants, who had various underlying primary immunodeficiencies (PIDs), Dimana Dimitrova, MD, reported at the Transplantation and Cellular Therapy Meetings.
GVHD-free survival was defined in this National Institutes of Health study as the absence of steroid-refractory grade 3-4 acute GVHD and chronic GVHD, noted Dr. Dimitrova of the NIH.
All patients, including 19 adults and 10 children (median age, 25 years), received a serotherapy-free, radiation-free, reduced-intensity conditioning regimen designed to optimize immune reconstitution, minimize toxicity and GVHD, reduce the risk of infectious complications, and enable successful use of alternative donors.
The conditioning platform included pentostatin on day –11 and day –7 at 4 mg/m2 along with 8 days of low-dose cyclophosphamide and 2 days of pharmacokinetically dosed busulfan at 4,600 mmol/min. GVHD prophylaxis included posttransplantation cyclophosphamide, mycophenolate mofetil (MMF), and sirolimus.
All patients received T cell–replete bone marrow or peripheral blood stem cell allografts; 72% received alternative donor grafts, Dr. Dimitrova said.
Two patients died, including one with bacterial sepsis and invasive aspergillosis who died on day +44 and one with presumed viral encephalitis who died on day +110. The patients were high risk overall (median HCT–comorbidity index score of 3, with a range of 0-11), and the two who died had HCT-CI scores of 6 and 8, respectively.
An additional accidental death occurred at 18 months after transplant “in the setting of continued remission, good graft function, and no transplant-related complications,” she said.
Neutrophil recovery occurred at a median of 17 days after transplant; three patients experienced graft failure, including one primary failure with autologous recovery on day +14 and two secondary graft failures.
“Two patients with known underlying difficult-to-engraft diseases required second transplants using different nonmyeloabalative platforms, and nevertheless required donor lymphocyte infusions to avoid threatened secondary graft failure,” she said. “The third patient actually had sufficiently improved infectious disease control and has not needed a second transplant to date.”
Overall GVHD incidence using the novel platform has been extremely low, she said, noting that 14% of patients had grade 2-4 GVHD and 3% had grade 3-4 acute GVHD. There was no steroid-refractory GVHD or chronic GVHD.
Among the infectious complications, other than those that led to the two deaths, were cytomegalovirus reactivation in 7 of 16 patients at risk, BK virus–associated hemorrhagic cystitis in 19 of 22 patients at risk, and a suspected case of viral cardiomyopathy that ultimately resolved.
“Importantly, although many patients had Epstein-Barr virus [EBV] control issues prior to transplant, no patients received preemptive EBV-directed therapy, and no patients had EBV-PTLD [posttransplant lymphoproliferative disorder],” she said.
Additionally, blood stream infections were detected in five patients, there were two cases of confirmed aspergillosis, and one child developed cutaneous candidiasis. Other complications and toxicities appeared to relate to underlying pretransplant issues in the affected organ or exuberant immune responses to existing infection.
“Phenotype reversal was evident to some degree in all evaluable patients, even in those with mixed chimerism or unknown underlying genetic defect,” Dr. Dimitrova said.
All 10 patients with malignancy or lymphoproliferative disease as an additional indication for allo-HCT remain in remission, and most patients who required immunoglobulin replacement therapy prior to transplant have been able to discontinue it, she noted.
The findings of this study are of note, because while it has been known for decades that allo-HCT is a potentially curative therapy for patients with PIDs that arise from defects in cells of hematopoietic origin, it frequently fails because of complicating factors or is not an option, Dr. Dimitrova said.
“These patients will often enter transplant with multiple comorbidities and disease sequelae, particularly as diagnosis of PIDs increases in older children and adults following years of illness,” she explained, adding that related donor options may be limited if family members are also affected.
For this reason, and with the goal of improving access to allo-HCT to all who require it, the novel conditioning platform used in this study was developed.
The platform was well tolerated overall, Dr. Dimitrova said, emphasizing the “notably low” GVHD rates.
“Currently we are investigating reduced MMF with the goal of promoting earlier immune reconstitution, and a separate protocol has opened that includes several modifications to this platform aimed at patients with increased risk of graft failure who may not tolerate mixed chimerism early on,” she said, noting that both protocols are currently enrolling.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Dimitrova reported having no financial disclosures.
SOURCE: Dimitrova D et al. TCT 2019, Abstract 54.
HOUSTON – Allogeneic hematopoietic stem cell transplantation (allo-HCT) following a novel reduced-intensity conditioning regimen was largely successful in a heterogeneous cohort of 29 adults and children with primary immunodeficiency in a prospective clinical trial.
At 1 year after transplant, overall survival was 98% and the estimated graft failure–free and graft-versus-host disease (GVHD)–free survival was 82% among the participants, who had various underlying primary immunodeficiencies (PIDs), Dimana Dimitrova, MD, reported at the Transplantation and Cellular Therapy Meetings.
GVHD-free survival was defined in this National Institutes of Health study as the absence of steroid-refractory grade 3-4 acute GVHD and chronic GVHD, noted Dr. Dimitrova of the NIH.
All patients, including 19 adults and 10 children (median age, 25 years), received a serotherapy-free, radiation-free, reduced-intensity conditioning regimen designed to optimize immune reconstitution, minimize toxicity and GVHD, reduce the risk of infectious complications, and enable successful use of alternative donors.
The conditioning platform included pentostatin on day –11 and day –7 at 4 mg/m2 along with 8 days of low-dose cyclophosphamide and 2 days of pharmacokinetically dosed busulfan at 4,600 mmol/min. GVHD prophylaxis included posttransplantation cyclophosphamide, mycophenolate mofetil (MMF), and sirolimus.
All patients received T cell–replete bone marrow or peripheral blood stem cell allografts; 72% received alternative donor grafts, Dr. Dimitrova said.
Two patients died, including one with bacterial sepsis and invasive aspergillosis who died on day +44 and one with presumed viral encephalitis who died on day +110. The patients were high risk overall (median HCT–comorbidity index score of 3, with a range of 0-11), and the two who died had HCT-CI scores of 6 and 8, respectively.
An additional accidental death occurred at 18 months after transplant “in the setting of continued remission, good graft function, and no transplant-related complications,” she said.
Neutrophil recovery occurred at a median of 17 days after transplant; three patients experienced graft failure, including one primary failure with autologous recovery on day +14 and two secondary graft failures.
“Two patients with known underlying difficult-to-engraft diseases required second transplants using different nonmyeloabalative platforms, and nevertheless required donor lymphocyte infusions to avoid threatened secondary graft failure,” she said. “The third patient actually had sufficiently improved infectious disease control and has not needed a second transplant to date.”
Overall GVHD incidence using the novel platform has been extremely low, she said, noting that 14% of patients had grade 2-4 GVHD and 3% had grade 3-4 acute GVHD. There was no steroid-refractory GVHD or chronic GVHD.
Among the infectious complications, other than those that led to the two deaths, were cytomegalovirus reactivation in 7 of 16 patients at risk, BK virus–associated hemorrhagic cystitis in 19 of 22 patients at risk, and a suspected case of viral cardiomyopathy that ultimately resolved.
“Importantly, although many patients had Epstein-Barr virus [EBV] control issues prior to transplant, no patients received preemptive EBV-directed therapy, and no patients had EBV-PTLD [posttransplant lymphoproliferative disorder],” she said.
Additionally, blood stream infections were detected in five patients, there were two cases of confirmed aspergillosis, and one child developed cutaneous candidiasis. Other complications and toxicities appeared to relate to underlying pretransplant issues in the affected organ or exuberant immune responses to existing infection.
“Phenotype reversal was evident to some degree in all evaluable patients, even in those with mixed chimerism or unknown underlying genetic defect,” Dr. Dimitrova said.
All 10 patients with malignancy or lymphoproliferative disease as an additional indication for allo-HCT remain in remission, and most patients who required immunoglobulin replacement therapy prior to transplant have been able to discontinue it, she noted.
The findings of this study are of note, because while it has been known for decades that allo-HCT is a potentially curative therapy for patients with PIDs that arise from defects in cells of hematopoietic origin, it frequently fails because of complicating factors or is not an option, Dr. Dimitrova said.
“These patients will often enter transplant with multiple comorbidities and disease sequelae, particularly as diagnosis of PIDs increases in older children and adults following years of illness,” she explained, adding that related donor options may be limited if family members are also affected.
For this reason, and with the goal of improving access to allo-HCT to all who require it, the novel conditioning platform used in this study was developed.
The platform was well tolerated overall, Dr. Dimitrova said, emphasizing the “notably low” GVHD rates.
“Currently we are investigating reduced MMF with the goal of promoting earlier immune reconstitution, and a separate protocol has opened that includes several modifications to this platform aimed at patients with increased risk of graft failure who may not tolerate mixed chimerism early on,” she said, noting that both protocols are currently enrolling.
The meeting was held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Dimitrova reported having no financial disclosures.
SOURCE: Dimitrova D et al. TCT 2019, Abstract 54.
REPORTING FROM TCT 2019
New IPF diagnosis test now covered by Medicare
The Envisia Genomic Classifier, produced by Veracyte, has received final Medicare local coverage determination for the diagnosis of idiopathic pulmonary fibrosis (IPF).
Envisia is a complement to high-resolution CT that can help differentiate IPF from other interstitial lung diseases, as more than half of patients with IPF/interstitial lung disease report being misdiagnosed at least once. The test analyzes samples obtained through transbronchial biopsy, a nonsurgical procedure commonly used in lung evaluation. Envisia has been shown to detect usual interstitial pneumonia, a signature of IPF, with high accuracy.
The new policy was issued through the Palmetto GBA MolDx program and will go into effect on April 1, 2019, making Envisia the first commercially available test of its kind, available to the 55 million people who are currently enrolled in Medicare.
“We are pleased that the evidence supporting the Envisia classifier met the MolDx program’s high standards for coverage. This important milestone will enable us to begin making the Envisia Classifier more widely available to patients with suspected IPF so that they can obtain an accurate, timely diagnosis and, in turn, appropriate treatment,” Bonnie Anderson, chairman and chief executive officer of Veracyte, said in a press release.
Find the full press release on the Veracyte website.
The Envisia Genomic Classifier, produced by Veracyte, has received final Medicare local coverage determination for the diagnosis of idiopathic pulmonary fibrosis (IPF).
Envisia is a complement to high-resolution CT that can help differentiate IPF from other interstitial lung diseases, as more than half of patients with IPF/interstitial lung disease report being misdiagnosed at least once. The test analyzes samples obtained through transbronchial biopsy, a nonsurgical procedure commonly used in lung evaluation. Envisia has been shown to detect usual interstitial pneumonia, a signature of IPF, with high accuracy.
The new policy was issued through the Palmetto GBA MolDx program and will go into effect on April 1, 2019, making Envisia the first commercially available test of its kind, available to the 55 million people who are currently enrolled in Medicare.
“We are pleased that the evidence supporting the Envisia classifier met the MolDx program’s high standards for coverage. This important milestone will enable us to begin making the Envisia Classifier more widely available to patients with suspected IPF so that they can obtain an accurate, timely diagnosis and, in turn, appropriate treatment,” Bonnie Anderson, chairman and chief executive officer of Veracyte, said in a press release.
Find the full press release on the Veracyte website.
The Envisia Genomic Classifier, produced by Veracyte, has received final Medicare local coverage determination for the diagnosis of idiopathic pulmonary fibrosis (IPF).
Envisia is a complement to high-resolution CT that can help differentiate IPF from other interstitial lung diseases, as more than half of patients with IPF/interstitial lung disease report being misdiagnosed at least once. The test analyzes samples obtained through transbronchial biopsy, a nonsurgical procedure commonly used in lung evaluation. Envisia has been shown to detect usual interstitial pneumonia, a signature of IPF, with high accuracy.
The new policy was issued through the Palmetto GBA MolDx program and will go into effect on April 1, 2019, making Envisia the first commercially available test of its kind, available to the 55 million people who are currently enrolled in Medicare.
“We are pleased that the evidence supporting the Envisia classifier met the MolDx program’s high standards for coverage. This important milestone will enable us to begin making the Envisia Classifier more widely available to patients with suspected IPF so that they can obtain an accurate, timely diagnosis and, in turn, appropriate treatment,” Bonnie Anderson, chairman and chief executive officer of Veracyte, said in a press release.
Find the full press release on the Veracyte website.
Infographic: Laser Hair Removal
Dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Unfortunately, many patients often undergo laser hair removal treatments at spas by practitioners with limited training. Dermatologists must encourage patients to seek treatment from a board-certified dermatologist.
Full survey results and commentary from Dr. Shari Lipner are available at bit.ly/2tzNbSg.
Dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Unfortunately, many patients often undergo laser hair removal treatments at spas by practitioners with limited training. Dermatologists must encourage patients to seek treatment from a board-certified dermatologist.
Full survey results and commentary from Dr. Shari Lipner are available at bit.ly/2tzNbSg.

Dermatologists are best equipped to treat patients who are interested in removing unwanted hair safely and effectively. Unfortunately, many patients often undergo laser hair removal treatments at spas by practitioners with limited training. Dermatologists must encourage patients to seek treatment from a board-certified dermatologist.
Full survey results and commentary from Dr. Shari Lipner are available at bit.ly/2tzNbSg.

Women survive more often than men do when hospitalized with cirrhosis
Women hospitalized with cirrhosis are less likely to die in the hospital than are men, according to a retrospective analysis of more than half a million patients.
Although women more often had infections and comorbidities, men more often had liver decompensation, which contributed most significantly to their higher mortality rate, reported lead author Jessica Rubin, MD, of the University of California, San Francisco, and her colleagues.
Their findings add to an existing body of knowledge about sex-related differences in chronic liver disease. Women are less likely to develop chronic liver disease; however, when women do develop disease, it often follows a unique clinical course, with milder early disease followed by more severe end-stage disease, meaning many women are too sick for a transplant, or die on the waiting list.
“The reasons behind this ‘reversal’ in [sex] disparities is unknown,” the investigators wrote in Journal of Clinical Gastroenterology.
Considering recent findings that showed a correlation between hospitalization and mortality rates in chronic liver disease, the investigators believed that a comparison of hospital-related outcomes in men and women could explain why women apparently fare worse when dealing with end-stage disease.
The retrospective, cross-sectional study involved 553,017 patients (median age, 57 years) who were hospitalized for cirrhosis between 2009 and 2013. Data were drawn from the National Inpatient Sample (NIS). Inpatient mortality was the primary outcome.
In agreement with previous findings, the minority of patients were women (39%). Against expectations, however, women had a significantly lower mortality rate than that of men (5.7% vs. 6.4%; multivariable analysis odds ratio, 0.86). Better survival was associated with lower rates of decompensation (Baveno IV criteria; 34% vs. 38.8%) and other cirrhosis complications, such as hepatorenal syndrome, variceal bleeding, ascites, and spontaneous bacterial peritonitis. The only cirrhosis complication more common in women than men was hepatic encephalopathy (17.8% vs. 16.8%). Owing to fewer complications, fewer women required liver-related interventions, including transjugular intrahepatic portosystemic shunt (0.8% vs. 1.0%), upper endoscopy (12.8% vs. 13.0%), or paracentesis (17.6% vs. 20.6%).
While less frequent complications and a lower mortality rate might suggest that women were admitted with better overall clinical pictures, not all data supported this conclusion. For instance, women were more likely to have noncirrhosis comorbidities, including diabetes, hypertension, heart failure, stroke, and cancer. Furthermore, women had a higher rate of acute bacterial infection than that of men (34.9% vs. 28.2%), although this disparity should be considered in light of urinary tract infections (UTIs), which were significantly more common among women (18.8% vs. 8.0%).
“Interestingly, infections were a stronger predictor of inpatient mortality in women than men,” the investigators wrote. “Despite this, women in our cohort were less likely to die in the hospital than men.”
Additional analysis revealed etiological differences that may have contributed to differences in mortality rates. For instance, women less often had liver disease due to viral hepatitis (27.6% vs. 35.2%) or alcohol (24.1% vs. 38.7%). In contrast, women more often had autoimmune hepatitis (2.5% vs. 0.4%) or cirrhosis due to unspecified or miscellaneous reasons (45.7% vs. 25.7%).
“Our data suggest that differential rates of ongoing liver injury – including by cofactors such as active alcohol use – explain some but not all of the [sex] difference we observed in hepatic decompensation,” the investigators wrote, before redirecting focus to a clearer clinical finding. “The poor prognosis of decompensated cirrhosis ... provides a reasonable explanation for the higher rates of in-hospital mortality seen among men versus women,” they concluded.
Considering the surprising findings and previously known sex disparities, Dr. Rubin and her colleagues suggested that more research in this area is needed, along with efforts to deliver sex-appropriate care.
“The development of [sex]-specific cirrhosis management programs – focused on interventions to manage the interaction between cirrhosis and other common comorbidities, improving physical function both before and during hospitalization, and postacute discharge programs to facilitate resumption of independent living – would target differential needs of women and men living with cirrhosis, with the ultimate goal of improving long-term outcomes in these patients,” the investigators wrote.
The study was funded by a National Institute on Aging Paul B. Beeson Career Development Award in Aging and a National Institute of Diabetes and Digestive and Kidney Diseases National Research Service Award hepatology training grant. The investigators declared no conflicts of interest.
SOURCE: Rubin et al. J Clin Gastroenterol. 2019 Feb 22. doi: 10.1097/MCG.0000000000001192.
Women hospitalized with cirrhosis are less likely to die in the hospital than are men, according to a retrospective analysis of more than half a million patients.
Although women more often had infections and comorbidities, men more often had liver decompensation, which contributed most significantly to their higher mortality rate, reported lead author Jessica Rubin, MD, of the University of California, San Francisco, and her colleagues.
Their findings add to an existing body of knowledge about sex-related differences in chronic liver disease. Women are less likely to develop chronic liver disease; however, when women do develop disease, it often follows a unique clinical course, with milder early disease followed by more severe end-stage disease, meaning many women are too sick for a transplant, or die on the waiting list.
“The reasons behind this ‘reversal’ in [sex] disparities is unknown,” the investigators wrote in Journal of Clinical Gastroenterology.
Considering recent findings that showed a correlation between hospitalization and mortality rates in chronic liver disease, the investigators believed that a comparison of hospital-related outcomes in men and women could explain why women apparently fare worse when dealing with end-stage disease.
The retrospective, cross-sectional study involved 553,017 patients (median age, 57 years) who were hospitalized for cirrhosis between 2009 and 2013. Data were drawn from the National Inpatient Sample (NIS). Inpatient mortality was the primary outcome.
In agreement with previous findings, the minority of patients were women (39%). Against expectations, however, women had a significantly lower mortality rate than that of men (5.7% vs. 6.4%; multivariable analysis odds ratio, 0.86). Better survival was associated with lower rates of decompensation (Baveno IV criteria; 34% vs. 38.8%) and other cirrhosis complications, such as hepatorenal syndrome, variceal bleeding, ascites, and spontaneous bacterial peritonitis. The only cirrhosis complication more common in women than men was hepatic encephalopathy (17.8% vs. 16.8%). Owing to fewer complications, fewer women required liver-related interventions, including transjugular intrahepatic portosystemic shunt (0.8% vs. 1.0%), upper endoscopy (12.8% vs. 13.0%), or paracentesis (17.6% vs. 20.6%).
While less frequent complications and a lower mortality rate might suggest that women were admitted with better overall clinical pictures, not all data supported this conclusion. For instance, women were more likely to have noncirrhosis comorbidities, including diabetes, hypertension, heart failure, stroke, and cancer. Furthermore, women had a higher rate of acute bacterial infection than that of men (34.9% vs. 28.2%), although this disparity should be considered in light of urinary tract infections (UTIs), which were significantly more common among women (18.8% vs. 8.0%).
“Interestingly, infections were a stronger predictor of inpatient mortality in women than men,” the investigators wrote. “Despite this, women in our cohort were less likely to die in the hospital than men.”
Additional analysis revealed etiological differences that may have contributed to differences in mortality rates. For instance, women less often had liver disease due to viral hepatitis (27.6% vs. 35.2%) or alcohol (24.1% vs. 38.7%). In contrast, women more often had autoimmune hepatitis (2.5% vs. 0.4%) or cirrhosis due to unspecified or miscellaneous reasons (45.7% vs. 25.7%).
“Our data suggest that differential rates of ongoing liver injury – including by cofactors such as active alcohol use – explain some but not all of the [sex] difference we observed in hepatic decompensation,” the investigators wrote, before redirecting focus to a clearer clinical finding. “The poor prognosis of decompensated cirrhosis ... provides a reasonable explanation for the higher rates of in-hospital mortality seen among men versus women,” they concluded.
Considering the surprising findings and previously known sex disparities, Dr. Rubin and her colleagues suggested that more research in this area is needed, along with efforts to deliver sex-appropriate care.
“The development of [sex]-specific cirrhosis management programs – focused on interventions to manage the interaction between cirrhosis and other common comorbidities, improving physical function both before and during hospitalization, and postacute discharge programs to facilitate resumption of independent living – would target differential needs of women and men living with cirrhosis, with the ultimate goal of improving long-term outcomes in these patients,” the investigators wrote.
The study was funded by a National Institute on Aging Paul B. Beeson Career Development Award in Aging and a National Institute of Diabetes and Digestive and Kidney Diseases National Research Service Award hepatology training grant. The investigators declared no conflicts of interest.
SOURCE: Rubin et al. J Clin Gastroenterol. 2019 Feb 22. doi: 10.1097/MCG.0000000000001192.
Women hospitalized with cirrhosis are less likely to die in the hospital than are men, according to a retrospective analysis of more than half a million patients.
Although women more often had infections and comorbidities, men more often had liver decompensation, which contributed most significantly to their higher mortality rate, reported lead author Jessica Rubin, MD, of the University of California, San Francisco, and her colleagues.
Their findings add to an existing body of knowledge about sex-related differences in chronic liver disease. Women are less likely to develop chronic liver disease; however, when women do develop disease, it often follows a unique clinical course, with milder early disease followed by more severe end-stage disease, meaning many women are too sick for a transplant, or die on the waiting list.
“The reasons behind this ‘reversal’ in [sex] disparities is unknown,” the investigators wrote in Journal of Clinical Gastroenterology.
Considering recent findings that showed a correlation between hospitalization and mortality rates in chronic liver disease, the investigators believed that a comparison of hospital-related outcomes in men and women could explain why women apparently fare worse when dealing with end-stage disease.
The retrospective, cross-sectional study involved 553,017 patients (median age, 57 years) who were hospitalized for cirrhosis between 2009 and 2013. Data were drawn from the National Inpatient Sample (NIS). Inpatient mortality was the primary outcome.
In agreement with previous findings, the minority of patients were women (39%). Against expectations, however, women had a significantly lower mortality rate than that of men (5.7% vs. 6.4%; multivariable analysis odds ratio, 0.86). Better survival was associated with lower rates of decompensation (Baveno IV criteria; 34% vs. 38.8%) and other cirrhosis complications, such as hepatorenal syndrome, variceal bleeding, ascites, and spontaneous bacterial peritonitis. The only cirrhosis complication more common in women than men was hepatic encephalopathy (17.8% vs. 16.8%). Owing to fewer complications, fewer women required liver-related interventions, including transjugular intrahepatic portosystemic shunt (0.8% vs. 1.0%), upper endoscopy (12.8% vs. 13.0%), or paracentesis (17.6% vs. 20.6%).
While less frequent complications and a lower mortality rate might suggest that women were admitted with better overall clinical pictures, not all data supported this conclusion. For instance, women were more likely to have noncirrhosis comorbidities, including diabetes, hypertension, heart failure, stroke, and cancer. Furthermore, women had a higher rate of acute bacterial infection than that of men (34.9% vs. 28.2%), although this disparity should be considered in light of urinary tract infections (UTIs), which were significantly more common among women (18.8% vs. 8.0%).
“Interestingly, infections were a stronger predictor of inpatient mortality in women than men,” the investigators wrote. “Despite this, women in our cohort were less likely to die in the hospital than men.”
Additional analysis revealed etiological differences that may have contributed to differences in mortality rates. For instance, women less often had liver disease due to viral hepatitis (27.6% vs. 35.2%) or alcohol (24.1% vs. 38.7%). In contrast, women more often had autoimmune hepatitis (2.5% vs. 0.4%) or cirrhosis due to unspecified or miscellaneous reasons (45.7% vs. 25.7%).
“Our data suggest that differential rates of ongoing liver injury – including by cofactors such as active alcohol use – explain some but not all of the [sex] difference we observed in hepatic decompensation,” the investigators wrote, before redirecting focus to a clearer clinical finding. “The poor prognosis of decompensated cirrhosis ... provides a reasonable explanation for the higher rates of in-hospital mortality seen among men versus women,” they concluded.
Considering the surprising findings and previously known sex disparities, Dr. Rubin and her colleagues suggested that more research in this area is needed, along with efforts to deliver sex-appropriate care.
“The development of [sex]-specific cirrhosis management programs – focused on interventions to manage the interaction between cirrhosis and other common comorbidities, improving physical function both before and during hospitalization, and postacute discharge programs to facilitate resumption of independent living – would target differential needs of women and men living with cirrhosis, with the ultimate goal of improving long-term outcomes in these patients,” the investigators wrote.
The study was funded by a National Institute on Aging Paul B. Beeson Career Development Award in Aging and a National Institute of Diabetes and Digestive and Kidney Diseases National Research Service Award hepatology training grant. The investigators declared no conflicts of interest.
SOURCE: Rubin et al. J Clin Gastroenterol. 2019 Feb 22. doi: 10.1097/MCG.0000000000001192.
FROM JOURNAL OF CLINICAL GASTROENTEROLOGY