User login
‘Put your own oxygen mask on first’
Takeaways from the leadership conference stress the importance of self-care, emotional intelligence, and remaining optimistic.
“Leadership 101: put your own oxygen mask on first @DarwinConwell #AGAleads #AGAForward @AmerGastroAssn”— Dr Michelle T. Long (@DrMTLong)
The inaugural Leadership Development Conference combined participants from three AGA programs for a weekend of networking, mentorship, and mapping out goals and initiatives.
Attendees included the 2020 class of AGA Future Leaders and mentors, Women’s Leadership Conference participants, and mentors and scholars of the new AGA FORWARD Program, an NIH-funded initiative that supports underrepresented minority physicians and scientists.
“Got to meet one of my tweeps heroes today! She’s even more awesome in real life!! #AGALeads #WomenInMedicine #WomenInGI @drfolamay @AmerGastroAssn” — Dr Aline Charabaty (@DCharabaty)
“Dr. Boland (Lynch syndrome) discussing career success in an ever changing scientific environment #AGALeads #AGAForward” — Eric J. Vargas M.D. (@EricJVargasMD)
“7 AGA Presidents, moderated by Dr. Anandasabapathy on Pathways to Leadership and Overcoming Challenges of the Era Presidential Panel @AmerGastroAssn Leadership conference program @SeragHashem @BCMDeptMedicine @KanwalFasiha @Aketwaroo @richashukla84” — Ruben Hernaez (@ruben_hernaez)
The event coincided with International Women’s Day, giving Women’s Leadership Conference attendees the chance to celebrate their journeys and grow into leadership roles with other #WomenInGI.
“#AGALeads #womenleadershipconference #womeninGI #InternationWomensDay with some amazing ladies in GI!! @AmerGastroAssn @AlisonGoldinMD @ibddocmaria @joanwchen” — ReezwanaCMD (@reezwanc)
“#AGAleads #WomeninGI women negotiating in a group are perceived favorably – Ellen Zimmerman, MD”
— Fazia Mir-Shaffi,MD (@Faiziya) March 9, 2019
“What I learned at @AmerGastroAssn #womeninGI Leadership course (after waiting a bit to see what stuck w me)
1. If you say yes to a request, you’re saying yes to doing it well.
2. Knowing your limitations will serve you better than being great at everything” — Laura Targownik (@UofM_GI_Head)
Aline Charabaty Pishvaian, MD, shared some takeaways in the AGA Community forum (community.gastro.org) about challenges women in GI face — a breakout discussion from the Women’s Leadership Conference.
View more insight and takeaways from participants on Twitter using #AGALeads.
Takeaways from the leadership conference stress the importance of self-care, emotional intelligence, and remaining optimistic.
Takeaways from the leadership conference stress the importance of self-care, emotional intelligence, and remaining optimistic.
“Leadership 101: put your own oxygen mask on first @DarwinConwell #AGAleads #AGAForward @AmerGastroAssn”— Dr Michelle T. Long (@DrMTLong)
The inaugural Leadership Development Conference combined participants from three AGA programs for a weekend of networking, mentorship, and mapping out goals and initiatives.
Attendees included the 2020 class of AGA Future Leaders and mentors, Women’s Leadership Conference participants, and mentors and scholars of the new AGA FORWARD Program, an NIH-funded initiative that supports underrepresented minority physicians and scientists.
“Got to meet one of my tweeps heroes today! She’s even more awesome in real life!! #AGALeads #WomenInMedicine #WomenInGI @drfolamay @AmerGastroAssn” — Dr Aline Charabaty (@DCharabaty)
“Dr. Boland (Lynch syndrome) discussing career success in an ever changing scientific environment #AGALeads #AGAForward” — Eric J. Vargas M.D. (@EricJVargasMD)
“7 AGA Presidents, moderated by Dr. Anandasabapathy on Pathways to Leadership and Overcoming Challenges of the Era Presidential Panel @AmerGastroAssn Leadership conference program @SeragHashem @BCMDeptMedicine @KanwalFasiha @Aketwaroo @richashukla84” — Ruben Hernaez (@ruben_hernaez)
The event coincided with International Women’s Day, giving Women’s Leadership Conference attendees the chance to celebrate their journeys and grow into leadership roles with other #WomenInGI.
“#AGALeads #womenleadershipconference #womeninGI #InternationWomensDay with some amazing ladies in GI!! @AmerGastroAssn @AlisonGoldinMD @ibddocmaria @joanwchen” — ReezwanaCMD (@reezwanc)
“#AGAleads #WomeninGI women negotiating in a group are perceived favorably – Ellen Zimmerman, MD”
— Fazia Mir-Shaffi,MD (@Faiziya) March 9, 2019
“What I learned at @AmerGastroAssn #womeninGI Leadership course (after waiting a bit to see what stuck w me)
1. If you say yes to a request, you’re saying yes to doing it well.
2. Knowing your limitations will serve you better than being great at everything” — Laura Targownik (@UofM_GI_Head)
Aline Charabaty Pishvaian, MD, shared some takeaways in the AGA Community forum (community.gastro.org) about challenges women in GI face — a breakout discussion from the Women’s Leadership Conference.
View more insight and takeaways from participants on Twitter using #AGALeads.
“Leadership 101: put your own oxygen mask on first @DarwinConwell #AGAleads #AGAForward @AmerGastroAssn”— Dr Michelle T. Long (@DrMTLong)
The inaugural Leadership Development Conference combined participants from three AGA programs for a weekend of networking, mentorship, and mapping out goals and initiatives.
Attendees included the 2020 class of AGA Future Leaders and mentors, Women’s Leadership Conference participants, and mentors and scholars of the new AGA FORWARD Program, an NIH-funded initiative that supports underrepresented minority physicians and scientists.
“Got to meet one of my tweeps heroes today! She’s even more awesome in real life!! #AGALeads #WomenInMedicine #WomenInGI @drfolamay @AmerGastroAssn” — Dr Aline Charabaty (@DCharabaty)
“Dr. Boland (Lynch syndrome) discussing career success in an ever changing scientific environment #AGALeads #AGAForward” — Eric J. Vargas M.D. (@EricJVargasMD)
“7 AGA Presidents, moderated by Dr. Anandasabapathy on Pathways to Leadership and Overcoming Challenges of the Era Presidential Panel @AmerGastroAssn Leadership conference program @SeragHashem @BCMDeptMedicine @KanwalFasiha @Aketwaroo @richashukla84” — Ruben Hernaez (@ruben_hernaez)
The event coincided with International Women’s Day, giving Women’s Leadership Conference attendees the chance to celebrate their journeys and grow into leadership roles with other #WomenInGI.
“#AGALeads #womenleadershipconference #womeninGI #InternationWomensDay with some amazing ladies in GI!! @AmerGastroAssn @AlisonGoldinMD @ibddocmaria @joanwchen” — ReezwanaCMD (@reezwanc)
“#AGAleads #WomeninGI women negotiating in a group are perceived favorably – Ellen Zimmerman, MD”
— Fazia Mir-Shaffi,MD (@Faiziya) March 9, 2019
“What I learned at @AmerGastroAssn #womeninGI Leadership course (after waiting a bit to see what stuck w me)
1. If you say yes to a request, you’re saying yes to doing it well.
2. Knowing your limitations will serve you better than being great at everything” — Laura Targownik (@UofM_GI_Head)
Aline Charabaty Pishvaian, MD, shared some takeaways in the AGA Community forum (community.gastro.org) about challenges women in GI face — a breakout discussion from the Women’s Leadership Conference.
View more insight and takeaways from participants on Twitter using #AGALeads.
Does BMI affect outcomes after ischemic stroke?
according to research that will be presented at the annual meeting of the American Academy of Neurology.
“One possible explanation is that people who are overweight or obese may have a nutritional reserve that may help them survive during prolonged illness,” said Zuolu Liu, MD, of the University of California, Los Angeles, in a press release. “More research is needed to investigate the relationship between BMI and stroke.”
The obesity paradox was first noted when studies suggested that being overweight improved survival in patients with kidney disease or heart disease. Investigators previously examined whether the obesity paradox is observed in stroke, but their studies were underpowered and produced ambiguous results.
Dr. Liu and colleagues sought to evaluate the relationship between BMI and 90-day outcomes of acute ischemic stroke. They examined data for all participants in the FAST-MAG trial, which studied whether prehospital treatment with magnesium improved disability outcomes of acute ischemic stroke. Dr. Liu and colleagues focused on the outcomes of death, disability or death (that is, modified Rankin Scale score of 2-6), and low stroke-related quality of life (that is, Stroke Impact Scale score less than 70). They analyzed potential relationships with BMI univariately and in multivariate models that adjusted for 12 prognostic variables, such as high blood pressure, high cholesterol, and smoking.
Dr. Liu’s group included 1,033 participants in its study. The population’s mean age was 71 years, and 45.1% of the population was female. Mean National Institutes of Health Stroke Scale (NIHSS) score was 10.6, and mean BMI was 27.5 kg/m2.
The investigators found an inverse association between the risk of death and BMI. Adjusted odds ratios for mortality were 1.67 for underweight participants, 0.85 for overweight participants, 0.54 for obese participants, and 0.38 for severely obese participants, compared with participants of normal weight. Similarly, the risk of disability had a U-shaped relationship with BMI. Odds ratios for disability or death were 1.19 for underweight participants, 0.78 for overweight participants, 0.72 for obese participants, and 0.96 for severely obese participants, compared with participants of normal weight. This relationship was attenuated after adjustment for other prognostic factors, however. Dr. Liu’s group did not find a significant association between BMI and low stroke-related quality of life.
The study was limited by the fact that all participants were from Southern California, which potentially reduced the generalizability of the results. The racial and ethnic composition of the study population, however, is similar to that of the national population, said the researchers.
No study sponsor was reported.
SOURCE: Liu Z et al. AAN 2019, Abstract P3.3-01.
according to research that will be presented at the annual meeting of the American Academy of Neurology.
“One possible explanation is that people who are overweight or obese may have a nutritional reserve that may help them survive during prolonged illness,” said Zuolu Liu, MD, of the University of California, Los Angeles, in a press release. “More research is needed to investigate the relationship between BMI and stroke.”
The obesity paradox was first noted when studies suggested that being overweight improved survival in patients with kidney disease or heart disease. Investigators previously examined whether the obesity paradox is observed in stroke, but their studies were underpowered and produced ambiguous results.
Dr. Liu and colleagues sought to evaluate the relationship between BMI and 90-day outcomes of acute ischemic stroke. They examined data for all participants in the FAST-MAG trial, which studied whether prehospital treatment with magnesium improved disability outcomes of acute ischemic stroke. Dr. Liu and colleagues focused on the outcomes of death, disability or death (that is, modified Rankin Scale score of 2-6), and low stroke-related quality of life (that is, Stroke Impact Scale score less than 70). They analyzed potential relationships with BMI univariately and in multivariate models that adjusted for 12 prognostic variables, such as high blood pressure, high cholesterol, and smoking.
Dr. Liu’s group included 1,033 participants in its study. The population’s mean age was 71 years, and 45.1% of the population was female. Mean National Institutes of Health Stroke Scale (NIHSS) score was 10.6, and mean BMI was 27.5 kg/m2.
The investigators found an inverse association between the risk of death and BMI. Adjusted odds ratios for mortality were 1.67 for underweight participants, 0.85 for overweight participants, 0.54 for obese participants, and 0.38 for severely obese participants, compared with participants of normal weight. Similarly, the risk of disability had a U-shaped relationship with BMI. Odds ratios for disability or death were 1.19 for underweight participants, 0.78 for overweight participants, 0.72 for obese participants, and 0.96 for severely obese participants, compared with participants of normal weight. This relationship was attenuated after adjustment for other prognostic factors, however. Dr. Liu’s group did not find a significant association between BMI and low stroke-related quality of life.
The study was limited by the fact that all participants were from Southern California, which potentially reduced the generalizability of the results. The racial and ethnic composition of the study population, however, is similar to that of the national population, said the researchers.
No study sponsor was reported.
SOURCE: Liu Z et al. AAN 2019, Abstract P3.3-01.
according to research that will be presented at the annual meeting of the American Academy of Neurology.
“One possible explanation is that people who are overweight or obese may have a nutritional reserve that may help them survive during prolonged illness,” said Zuolu Liu, MD, of the University of California, Los Angeles, in a press release. “More research is needed to investigate the relationship between BMI and stroke.”
The obesity paradox was first noted when studies suggested that being overweight improved survival in patients with kidney disease or heart disease. Investigators previously examined whether the obesity paradox is observed in stroke, but their studies were underpowered and produced ambiguous results.
Dr. Liu and colleagues sought to evaluate the relationship between BMI and 90-day outcomes of acute ischemic stroke. They examined data for all participants in the FAST-MAG trial, which studied whether prehospital treatment with magnesium improved disability outcomes of acute ischemic stroke. Dr. Liu and colleagues focused on the outcomes of death, disability or death (that is, modified Rankin Scale score of 2-6), and low stroke-related quality of life (that is, Stroke Impact Scale score less than 70). They analyzed potential relationships with BMI univariately and in multivariate models that adjusted for 12 prognostic variables, such as high blood pressure, high cholesterol, and smoking.
Dr. Liu’s group included 1,033 participants in its study. The population’s mean age was 71 years, and 45.1% of the population was female. Mean National Institutes of Health Stroke Scale (NIHSS) score was 10.6, and mean BMI was 27.5 kg/m2.
The investigators found an inverse association between the risk of death and BMI. Adjusted odds ratios for mortality were 1.67 for underweight participants, 0.85 for overweight participants, 0.54 for obese participants, and 0.38 for severely obese participants, compared with participants of normal weight. Similarly, the risk of disability had a U-shaped relationship with BMI. Odds ratios for disability or death were 1.19 for underweight participants, 0.78 for overweight participants, 0.72 for obese participants, and 0.96 for severely obese participants, compared with participants of normal weight. This relationship was attenuated after adjustment for other prognostic factors, however. Dr. Liu’s group did not find a significant association between BMI and low stroke-related quality of life.
The study was limited by the fact that all participants were from Southern California, which potentially reduced the generalizability of the results. The racial and ethnic composition of the study population, however, is similar to that of the national population, said the researchers.
No study sponsor was reported.
SOURCE: Liu Z et al. AAN 2019, Abstract P3.3-01.
FROM AAN 2019
AGA president advocates for increased access to care for digestive disease patients
AGA President David Lieberman, MD, AGAF, was on Capitol Hill advocating for legislation to ensure that digestive disease patients have timely access to lifesaving treatments and touted the importance of increasing access to colorectal cancer screenings. Specifically, Dr. Lieberman sought support for H.R. 1570/S. 668, the Removing Barriers to Colorectal Cancer Screening Act, legislation that would fix the current Medicare screening colonoscopy coinsurance problem. Currently, when a Medicare beneficiary has a screening colonoscopy that turns therapeutic, the procedure is no longer considered a screening and the patient is on the hook for the “surprise” bill. This bipartisan, bicameral legislation would fix this problem for beneficiaries.
Dr. Lieberman also participated in a congressional briefing sponsored by AGA, ACG, and ASGE on the importance of colorectal cancer (CRC) screening and spoke of the geographic, ethnic, and socioeconomic barriers to CRC screening and how it impacts the rates of screening. Rep. James P. McGovern, D-MA, chair of the House Rules Committee, also spoke about the importance of CRC screenings and the number of lives that can be saved with screening. He also stressed that we have made strides in screening because of the research that is funded through the NIH which Congress needs to continue to support.
Protection for patients who are subject to step-therapy protocols was another area that Dr. Lieberman emphasized during his meetings with congressional staff. Step therapy is a utilization management tool where insurers force patients to fail one or more therapies before they will cover the initial therapy recommended by their physician. This policy is more and more common especially for patients with inflammatory bowel disease (IBD) who rely on biologics for treatment. Dr. Lieberman stressed that forcing a patient to fail a medication that they know will be ineffective is in violation of the Hippocratic oath. Restoring the Patient’s Voice Act, legislation soon to be reintroduced by Reps. Raul Ruiz, D-CA, and Brad Wenstrup, R-OH, would provide an expeditated appeals process and provide some common sense exceptions for patients when subjected to step therapy.
Dr. Lieberman stressed the importance of funding the NIH and requested Congress increase their budget by $2 billion in fiscal year 2020. Dr. Lieberman described the NIH as our country’s crown jewel since it invests in biomedical research that will ultimately find cures for countless conditions, increase our country’s economic competitiveness, and spur industries and invests in our country’s best and brightest scientists. We are hopeful that Congress will reject the Trump Administration’s recommendation of a 12% cut for NIH and instead continue to provide the necessary increases the agency needs to remain competitive.
AGA President David Lieberman, MD, AGAF, was on Capitol Hill advocating for legislation to ensure that digestive disease patients have timely access to lifesaving treatments and touted the importance of increasing access to colorectal cancer screenings. Specifically, Dr. Lieberman sought support for H.R. 1570/S. 668, the Removing Barriers to Colorectal Cancer Screening Act, legislation that would fix the current Medicare screening colonoscopy coinsurance problem. Currently, when a Medicare beneficiary has a screening colonoscopy that turns therapeutic, the procedure is no longer considered a screening and the patient is on the hook for the “surprise” bill. This bipartisan, bicameral legislation would fix this problem for beneficiaries.
Dr. Lieberman also participated in a congressional briefing sponsored by AGA, ACG, and ASGE on the importance of colorectal cancer (CRC) screening and spoke of the geographic, ethnic, and socioeconomic barriers to CRC screening and how it impacts the rates of screening. Rep. James P. McGovern, D-MA, chair of the House Rules Committee, also spoke about the importance of CRC screenings and the number of lives that can be saved with screening. He also stressed that we have made strides in screening because of the research that is funded through the NIH which Congress needs to continue to support.
Protection for patients who are subject to step-therapy protocols was another area that Dr. Lieberman emphasized during his meetings with congressional staff. Step therapy is a utilization management tool where insurers force patients to fail one or more therapies before they will cover the initial therapy recommended by their physician. This policy is more and more common especially for patients with inflammatory bowel disease (IBD) who rely on biologics for treatment. Dr. Lieberman stressed that forcing a patient to fail a medication that they know will be ineffective is in violation of the Hippocratic oath. Restoring the Patient’s Voice Act, legislation soon to be reintroduced by Reps. Raul Ruiz, D-CA, and Brad Wenstrup, R-OH, would provide an expeditated appeals process and provide some common sense exceptions for patients when subjected to step therapy.
Dr. Lieberman stressed the importance of funding the NIH and requested Congress increase their budget by $2 billion in fiscal year 2020. Dr. Lieberman described the NIH as our country’s crown jewel since it invests in biomedical research that will ultimately find cures for countless conditions, increase our country’s economic competitiveness, and spur industries and invests in our country’s best and brightest scientists. We are hopeful that Congress will reject the Trump Administration’s recommendation of a 12% cut for NIH and instead continue to provide the necessary increases the agency needs to remain competitive.
AGA President David Lieberman, MD, AGAF, was on Capitol Hill advocating for legislation to ensure that digestive disease patients have timely access to lifesaving treatments and touted the importance of increasing access to colorectal cancer screenings. Specifically, Dr. Lieberman sought support for H.R. 1570/S. 668, the Removing Barriers to Colorectal Cancer Screening Act, legislation that would fix the current Medicare screening colonoscopy coinsurance problem. Currently, when a Medicare beneficiary has a screening colonoscopy that turns therapeutic, the procedure is no longer considered a screening and the patient is on the hook for the “surprise” bill. This bipartisan, bicameral legislation would fix this problem for beneficiaries.
Dr. Lieberman also participated in a congressional briefing sponsored by AGA, ACG, and ASGE on the importance of colorectal cancer (CRC) screening and spoke of the geographic, ethnic, and socioeconomic barriers to CRC screening and how it impacts the rates of screening. Rep. James P. McGovern, D-MA, chair of the House Rules Committee, also spoke about the importance of CRC screenings and the number of lives that can be saved with screening. He also stressed that we have made strides in screening because of the research that is funded through the NIH which Congress needs to continue to support.
Protection for patients who are subject to step-therapy protocols was another area that Dr. Lieberman emphasized during his meetings with congressional staff. Step therapy is a utilization management tool where insurers force patients to fail one or more therapies before they will cover the initial therapy recommended by their physician. This policy is more and more common especially for patients with inflammatory bowel disease (IBD) who rely on biologics for treatment. Dr. Lieberman stressed that forcing a patient to fail a medication that they know will be ineffective is in violation of the Hippocratic oath. Restoring the Patient’s Voice Act, legislation soon to be reintroduced by Reps. Raul Ruiz, D-CA, and Brad Wenstrup, R-OH, would provide an expeditated appeals process and provide some common sense exceptions for patients when subjected to step therapy.
Dr. Lieberman stressed the importance of funding the NIH and requested Congress increase their budget by $2 billion in fiscal year 2020. Dr. Lieberman described the NIH as our country’s crown jewel since it invests in biomedical research that will ultimately find cures for countless conditions, increase our country’s economic competitiveness, and spur industries and invests in our country’s best and brightest scientists. We are hopeful that Congress will reject the Trump Administration’s recommendation of a 12% cut for NIH and instead continue to provide the necessary increases the agency needs to remain competitive.
ICYMI: NIH renames, streamlines gene therapy committee
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
The National Institutes of Health has released an amended guideline on research involving gene therapy.
As part of the streamlining process, the Recombinant DNA Advisory Committee has been renamed as the Novel and Exceptional Technology and Research Advisory Committee to better align with the committee’s original intention – following and providing advice on safety and ethical issues associated with emerging biotechnologies, according to a statement from Francis S. Collins, MD, PhD, director of the NIH.
We previously covered this story; find our coverage at the link below.
Are you ready to celebrate 50 years of DDW®?
With 2019 being the 50th anniversary of Digestive Disease Week® (DDW), this year’s meeting is one you won’t want to miss. AGA looks forward to seeing members May 18 to 21, 2019, in San Diego, California. Register and view additional information on the DDW website. You can learn more about AGA programming and events at DDW by visiting www.gastro.org/DDW.
With 2019 being the 50th anniversary of Digestive Disease Week® (DDW), this year’s meeting is one you won’t want to miss. AGA looks forward to seeing members May 18 to 21, 2019, in San Diego, California. Register and view additional information on the DDW website. You can learn more about AGA programming and events at DDW by visiting www.gastro.org/DDW.
With 2019 being the 50th anniversary of Digestive Disease Week® (DDW), this year’s meeting is one you won’t want to miss. AGA looks forward to seeing members May 18 to 21, 2019, in San Diego, California. Register and view additional information on the DDW website. You can learn more about AGA programming and events at DDW by visiting www.gastro.org/DDW.
Biomarker testing may transform treatment of acute GVHD
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Researchers say they have identified biomarkers that may help guide early treatment decisions in patients with acute graft-versus-host disease (GVHD).
The biomarkers, ST2 and REG3-alpha, were measured during the first month of GVHD treatment and proved more accurate than clinical response for predicting 6-month nonrelapse mortality (NRM). In fact, biomarker assessment revealed patients who responded to treatment but had a high risk of NRM and nonresponders who had a low risk of NRM.
The researchers also found that biomarkers changed over the first month of treatment but remained significant predictors of NRM. This suggests that modifying treatment according to biomarker findings at various time points could result in better outcomes for patients.
“We think this is going to transform the way we treat graft-versus-host disease,” said James L.M. Ferrara, MD, DSc, of the Icahn School of Medicine at Mount Sinai, New York.
Dr. Ferrara and Hrishikesh Srinagesh, along with their colleagues at Mount Sinai, have conducted extensive research with these biomarkers and presented some of their findings at the Acute Leukemia Forum of Hemedicus.
Comparing biomarkers and response
In one study, the researchers evaluated 355 patients who had undergone allogeneic hematopoietic stem cell transplant at 1 of 20 Mount Sinai Acute GVHD International Consortium (MAGIC) centers between January 2016 and February 2018. All patients developed acute GVHD and received systemic steroids as treatment.
Patients provided blood samples weekly for the first month of treatment, and concentrations of ST2 and REG3-alpha were measured in each sample. Both biomarker concentrations were used to calculate the biomarker probability of NRM.
“The concentration of those two biomarkers are put into a computer, and we get … a single number, and that gives us the probability of mortality,” Dr. Ferrara said. “[W]e call this the MAGIC algorithm probability, or MAP. And when a MAP is low, the patient has a very low chance of dying from graft-versus-host disease, when it’s intermediate, they have an intermediate risk, and when it’s high, they have a high risk.”
The researchers then compared the MAP and clinical response for their ability to predict 6-month NRM throughout the first month of therapy for acute GVHD.
MAP bests response
After 1 month of therapy, the MAP was more accurate than clinical response for predicting 6-month NRM. The area under the curve was 0.84 and 0.65, respectively (P less than .001).
Likewise, the MAP after 1 week of therapy was more accurate than clinical response at 1 month for predicting 6-month NRM. The area under the curve was 0.80 and 0.65, respectively (P less than .001).
“[T]he clinical responses were good, but not great, at predicting long-term outcome, where the biomarker, the MAP, was significantly better,” Dr. Ferrara said. “[A]t every time point we tested, the biomarkers were better than the clinical responses.”
The researchers also identified subgroups of clinical responders and nonresponders for whom MAP more accurately predicted 6-month NRM.
The team found that 61% of clinical nonresponders were actually low risk according to MAP. And the incidence of 6-month NRM was significantly lower in the MAP-designated low-risk patients than in MAP-designated high-risk patients – 22% and 56%, respectively (P less than .001).
On the other hand, 10% of clinical responders were high risk according to MAP. The incidence of 6-month NRM was significantly higher in the high-risk patients than in the low-risk patients – 40% and 13%, respectively (P less than .001).
Assessing changes over time
The researchers found that patients who were initially high risk by MAP but had not experienced NRM by 6 months had significant decreases in their MAP after 4 weeks of treatment (P = .003). Patients who did experience NRM had a significant increase in their MAP whether their initial MAP was low (P = .007) or high (P = .024).
“What we found was that patients who lived tended to either have low biomarkers at the start of treatment and stay low or start out with high biomarkers and have reductions over the first month of therapy,” Mr. Srinagesh said. “Conversely, patients who tended to do worse were those who had either increases in their biomarkers or stayed high at all time points.”
The researchers identified a threshold – 0.290 – for separating patients by mortality risk.
“Patients who started out above the threshold and then went below it had a 5-fold reduction in mortality, whereas patients who started out below the threshold and rose above it had a 5-fold increase in mortality,” Mr. Srinagesh said.
MAP in clinical trials and practice
Based on these findings and results from related studies, the researchers theorize that MAP would be a better endpoint for clinical trials than clinical response.
At present, there are three trials in which researchers are using MAP as an endpoint to assess the efficacy of treatment for GVHD (NCT02133924, NCT03459040, and NCT03846479). Dr. Ferrara said a fourth trial is set to begin this summer.
Additionally, MAP is being used in clinical practice. A company called Viracor Eurofins Clinical Diagnostics licensed the MAGIC algorithm and provides three related tests for consumer use.
Viracor’s aGVHD Pre-Symptomatic Algorithm assigns patients to high- and low-risk groups based on results from samples collected 7 days after transplant. The aGVHD Symptomatic Onset Algorithm assigns patients to high-, intermediate-, and low-risk groups. The aGVHD Post-Treatment Algorithm, which can be used 7 days or more after GVHD treatment initiation, stratifies steroid-resistant patients into high- or low-risk groups for both NRM and overall survival.
“We are still in early days of figuring out how to use [the biomarker tests], but … what I’ve heard is that people are finding them to be useful in their clinical practice,” Dr. Ferrara said.
Dr. Ferrara has an ownership interest in and receives royalties from Viracor. Mr. Srinagesh reported having no relevant conflicts of interest. The research was supported by grants from the National Cancer Institute and the American Cancer Society.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
REPORTING FROM ALF 2019
Electronic health records linked to lower patient safety
Higher reliance on electronic health records (EHRs) in ambulatory oncology practice was significantly associated with reduced safety actions among oncology nurses and prescribers, according to results of a statewide survey.
“The purpose of this study was to investigate the degree to which EHRs, satisfaction with technology, and clinician communication enable a safety culture in ambulatory oncology treatment settings,” wrote Minal R. Patel, PhD, MPH, of the University of Michigan, Ann Arbor, and colleagues. The report is published in the Journal of Oncology Practice.
The researchers conducted a statewide survey of 297 oncology nurses and prescribers in 29 ambulatory oncology practices in Michigan. They obtained quantitative data for May to October 2017 from clinician surveys and practice logs at these clinical sites.
The study methodology was built by use of the sociotechnical framework, which examined how EHR technologies influenced the safe administration of chemotherapy.
Eligible survey participants included physicians, nurses, physician assistants, and nurse practitioners who cared for adult patients receiving infusion treatments for cancer.
A total of 438 clinicians were recruited and confirmed to be eligible, and 297 (68%) completed a survey.
After analysis, the researchers found that higher reliance on electronic health records in practice was associated with reduced safety scores (P less than .001). The mean safety score was reported to be 5.3 (standard deviation, 1.1; practice-level range, 4.9-5.4).
In an opposite manner, increased satisfaction with technology and better-quality communication were associated with higher safety actions.
The researchers acknowledged a key limitation of the study was cross-sectional design. As a result, confounding factors could influence the findings.
“Careful attention to technology adoption and updates coupled with high-quality communication skills across clinicians are promising strategies to administer high-risk treatments safely in ambulatory oncology settings,” they concluded.
The study was supported by grant funding from the Agency for Healthcare Research and Quality and the National Cancer Institute. No conflicts of interest were reported.
SOURCE: Patel MR et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00507.
Higher reliance on electronic health records (EHRs) in ambulatory oncology practice was significantly associated with reduced safety actions among oncology nurses and prescribers, according to results of a statewide survey.
“The purpose of this study was to investigate the degree to which EHRs, satisfaction with technology, and clinician communication enable a safety culture in ambulatory oncology treatment settings,” wrote Minal R. Patel, PhD, MPH, of the University of Michigan, Ann Arbor, and colleagues. The report is published in the Journal of Oncology Practice.
The researchers conducted a statewide survey of 297 oncology nurses and prescribers in 29 ambulatory oncology practices in Michigan. They obtained quantitative data for May to October 2017 from clinician surveys and practice logs at these clinical sites.
The study methodology was built by use of the sociotechnical framework, which examined how EHR technologies influenced the safe administration of chemotherapy.
Eligible survey participants included physicians, nurses, physician assistants, and nurse practitioners who cared for adult patients receiving infusion treatments for cancer.
A total of 438 clinicians were recruited and confirmed to be eligible, and 297 (68%) completed a survey.
After analysis, the researchers found that higher reliance on electronic health records in practice was associated with reduced safety scores (P less than .001). The mean safety score was reported to be 5.3 (standard deviation, 1.1; practice-level range, 4.9-5.4).
In an opposite manner, increased satisfaction with technology and better-quality communication were associated with higher safety actions.
The researchers acknowledged a key limitation of the study was cross-sectional design. As a result, confounding factors could influence the findings.
“Careful attention to technology adoption and updates coupled with high-quality communication skills across clinicians are promising strategies to administer high-risk treatments safely in ambulatory oncology settings,” they concluded.
The study was supported by grant funding from the Agency for Healthcare Research and Quality and the National Cancer Institute. No conflicts of interest were reported.
SOURCE: Patel MR et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00507.
Higher reliance on electronic health records (EHRs) in ambulatory oncology practice was significantly associated with reduced safety actions among oncology nurses and prescribers, according to results of a statewide survey.
“The purpose of this study was to investigate the degree to which EHRs, satisfaction with technology, and clinician communication enable a safety culture in ambulatory oncology treatment settings,” wrote Minal R. Patel, PhD, MPH, of the University of Michigan, Ann Arbor, and colleagues. The report is published in the Journal of Oncology Practice.
The researchers conducted a statewide survey of 297 oncology nurses and prescribers in 29 ambulatory oncology practices in Michigan. They obtained quantitative data for May to October 2017 from clinician surveys and practice logs at these clinical sites.
The study methodology was built by use of the sociotechnical framework, which examined how EHR technologies influenced the safe administration of chemotherapy.
Eligible survey participants included physicians, nurses, physician assistants, and nurse practitioners who cared for adult patients receiving infusion treatments for cancer.
A total of 438 clinicians were recruited and confirmed to be eligible, and 297 (68%) completed a survey.
After analysis, the researchers found that higher reliance on electronic health records in practice was associated with reduced safety scores (P less than .001). The mean safety score was reported to be 5.3 (standard deviation, 1.1; practice-level range, 4.9-5.4).
In an opposite manner, increased satisfaction with technology and better-quality communication were associated with higher safety actions.
The researchers acknowledged a key limitation of the study was cross-sectional design. As a result, confounding factors could influence the findings.
“Careful attention to technology adoption and updates coupled with high-quality communication skills across clinicians are promising strategies to administer high-risk treatments safely in ambulatory oncology settings,” they concluded.
The study was supported by grant funding from the Agency for Healthcare Research and Quality and the National Cancer Institute. No conflicts of interest were reported.
SOURCE: Patel MR et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00507.
FROM JOURNAL OF ONCOLOGY PRACTICE
Courts temporarily block Title X changes
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
[email protected]
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
[email protected]
U.S. District Judge Stanley Bastian for the District of Eastern Washington on April 25 approved a temporary nationwide ban against the program changes in response to legal a challenge by Washington state. The same day, U.S. District Judge for the District of Oregon Michael J. McShane also preliminarily barred the restrictions from taking effect in response to a legal challenge by the American Medical Association and the Planned Parenthood Federation of America.
Judge McShane called the program restrictions “arbitrary and capricious,” and wrote that the rules ignore comprehensive, ethical, and evidence-based health care, and impermissibly interfere with the patient-doctor relationship. Judge Bastian agreed, writing in his order that the plaintiffs have demonstrated that the restrictions violate the central purpose of Title X, which is to equalize access to comprehensive, evidence-based, and voluntary family planning.
“Plaintiffs have demonstrated they are likely to suffer irreparable harm in the absence of a preliminary injunction by presenting facts and argument that the final rule may or likely will: seriously disrupt or destroy the existing network of Title X providers in both the State of Washington and throughout the entire nation,” Judge Bastian wrote in his order.
Changes to the Title X program – scheduled to take effect May 3 – would have made health clinics ineligible for Title X funding if they offer, promote, or support abortion as a method of family planning. Title X grants generally go to health centers that provide reproductive health care – such as STD-testing, cancer screenings, and contraception – to low-income families. Under the rule, the government would withdraw financial assistance to clinics if they allow counseling or referrals associated with abortion, regardless of whether the money is used for other health care services.
HHS officials said that the final rule will provide for clear financial and physical separation between Title X and non–Title X activities, reduce confusion on the part of Title X clinics and the public about permissible Title X activities, and improve program transparency by requiring more complete reporting by grantees about their partnerships with referral agencies.
Washington state and the National Family Planning & Reproductive Health Association sued the U.S. Department of Health & Human Services in early March to block the agency from enforcing the modifications. A separate lawsuit was filed by the American Medical Association and the Planned Parenthood Federation of America to stop the funding changes, and 22 states issued a third legal challenge. The Title X changes impose a “government gag rule” on what information physicians can provide to their patients, according to the plaintiffs.
The American College of Physicians (ACP) and other groups, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics have voiced their opposition to the Title X restrictions. In a joint court brief, the medical societies wrote that the Trump administration’s limitations to the Title X program will create cultural, geographic, and financial barriers to care; erode the physician-patient relationship; and cause extreme, immediate, and irreparable harm to millions of patients.
Washington Attorney General Bob Ferguson said the nationwide ban ensures that clinics across the nation can remain open and continue to provide quality, unbiased health care to women
“Trump’s ‘gag rule’ would have jeopardized health care access to women across the country,” he said in a statement. “Title X clinics, such as Planned Parenthood, provide essential services – now they can keep serving women while we continue to fight to keep the federal government out of the exam room.”
AMA President Barbara L. McAneny, MD, praised Judge McShane’s order. “The new rule would have placed obstacles to health care for low-income patients,” Dr. McAneny said in a statement. “We are pleased the judge shared the AMA’s concern about the physician-patient relationship that the rule would have jeopardized.”
The Trump administration had not said at press time whether it would appeal the order.
Antiabortion organizations, such as the Susan B. Anthony List, have expressed strong support of the Title X funding restrictions.
“The rule advances President Trump’s promise to stop taxpayer funding of abortion businesses like Planned Parenthood,” SBA List President Marjorie Dannenfelser said in a statement. “The Protect Life Rule does not cut family planning funding by a single dime, and instead directs tax dollars to entities that provide health care to women but do not perform abortions.”
[email protected]
Navigating the Oncology Care Model
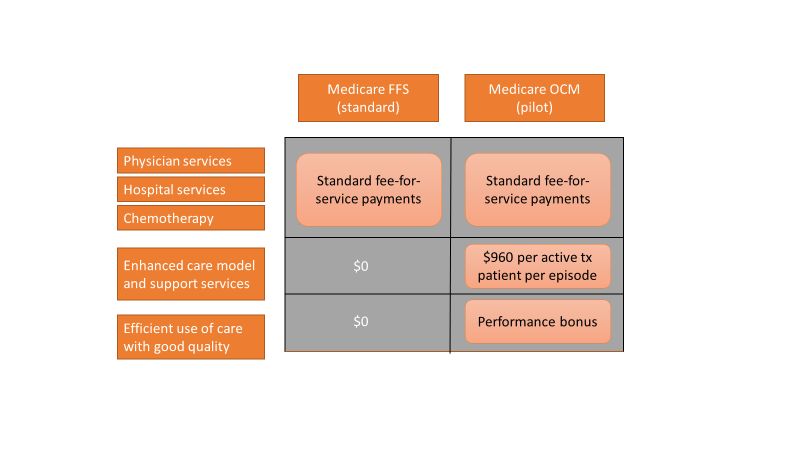
Care of the cancer patient is complex and expensive. During 2001-2011, medical spending to treat cancer increased from $56.8 billion to $88.3 billion in the United States. During this time, ambulatory expenditures for care and treatment increased while inpatient hospital expenditures decreased.1,2 Treatments for cancer have advanced, but costs do not correlate with outcomes. Advanced payment models aimed at ensuring high quality while lowering costs may be the vehicle to help mitigate the financial burden of cancer treatment on patients and society at large.
Oncology Care Model
The Center for Medicare and Medicaid Innovation designed the Oncology Care Model (OCM), which allows practices and payers in the United States to partner with the Centers for Medicare & Medicaid Services. The goal of the OCM is to provide high quality, highly coordinated cancer care at the same or lower cost. Practice partnerships with the CMS involve payment arrangements that include financial and performance accountability for episodes of cancer care surrounding chemotherapy delivery to patients.3
Practices that have been selected by the CMS have attested to providing a number of enhanced services from 24/7 patient access to an appropriate clinician who can access medical records to having a documented care plan for every patient.4
Payment methodology
An episode of care is defined as a 6-month period that starts at the time of chemotherapy administration. In addition to the standard fee-for-service payment, practices have the ability to earn two other types of payments during an oncology episode.
The per-beneficiary Monthly Enhanced Oncology Services payment is $960 for the entire episode but is paid to practices at $160 per month.
Practices have the potential to earn additional performance-based payments (PBP) based on the difference in cost between the projected and actual cost of the episode. The PBP also incorporates performance on quality metrics, based on Medicare claims and other information submitted by the practice. For example, claims-based measures include hospital, emergency department (ED), and hospice utilization.
To participate in the OCM, practices must choose either a one-sided or two-sided risk model. In the one-sided risk model, practices take on no downside risk but need to achieve a greater reduction in expenditures (4% below the benchmark price). In the two-side risk model, practices need only to reduce expenditures by 2.75% below the benchmark price. But if they fail to meet their savings goals, they must pay the difference to the CMS. The recoupment is capped at 20% of the benchmark amount.
Feedback reports
The CMS sends quarterly feedback reports that contain information on practice demographics, outcomes, expenditures, chemotherapy use, and patient satisfaction. The outcomes include the mortality rate for Medicare beneficiaries treated at the practice, compared with other practices nationally. In addition, the reports include end-of-life metrics and patient satisfaction, as well as details of expenditures on drugs, hospital use, imaging and laboratory services, and a description of chemotherapy usage.
These reports can be a helpful tool for measuring your own use of services, as well as benchmarking it against national figures.
Practice modifications
According to CMS feedback reports, the cost of care per beneficiary per month has increased across all practices since the inception of the OCM. However, there are practices that have been successful in reducing cost of care without negatively affecting mortality.
Drugs, hospital, and ED visits, along with imaging and laboratory evaluation, account for 75% of the cost. Some strategies to reduce expenditure involve targeting those areas.
Consider prescribing drugs conservatively without affecting outcomes. For instance, bisphosphonates for bone metastasis can be given every 12 weeks instead of 4 weeks.5 Similarly, adjuvant chemotherapy can be given for 3 months, instead of 6 months in appropriate stage 3 colon cancer patients.6
Another potential opportunity for savings is the judicious use of pertuzumab in early-stage breast cancer patients.7 These are all evidence-based recommendations with potential for cost savings. Clinical pathways can aid in this process, but physician buy-in is imperative.
In terms of imaging, avoid PET scans when they will not affect your clinical decision making, avoid staging scans in early-stage breast and prostate cancer patients, and avoid surveillance scans among early-stage breast cancer and lymphoma patients. The Choosing Wisely campaign can help guide some of these decisions.8
Another area where good care meets cost effective care is in the early engagement of palliative care. Several studies have shown that early involvement of palliative care improves survival and quality of life.9,10 Palliative care involvement also decreases the emotional burden for patients and oncologists. Appropriate symptom control, particularly of pain, decreases hospitalizations during treatment.
Investing in a robust supportive care team – financial advocates, social work, nutrition, behavioral health, as well as various community services – can help reduce the financial, physical, and emotional distress levels for patients. All of these services ultimately lead to reduced hospitalizations.11 The Monthly Enhanced Oncology Services payment can be put toward these expenses.
Care teams working at the highest level of competence and license can also save time and money. Consider using registered nurses to implement triage pathways to assess side effects and symptom management, or using nurse practitioners, registered nurses, and physician assistants for same-day appointments and to assess symptoms rather than referring patients to the emergency department.
Avoid the ED and hospitalizations by using the infusion center to provide hydration and blood transfusions in a timely fashion.
Telemedicine can be used for symptom management as well as leveraging supportive care services.
Cost for cancer care is very difficult to sustain. The OCM provides early insights into expenditures, challenges, and opportunities. Practices should use this information to build infrastructure and provide high quality, cost-effective care. Value-based cancer care should be the overarching goal for oncology practices and health care organizations.
Dr. Mahesh is the director of hematology-oncology and program director of the Oncology Care Model at Summa Health in Akron, Ohio.
References
1. Siegel RL et al. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Medical Expenditure Panel Survey, Statistical Brief #443. 2014 Jun.
3. CMS: Oncology Care Model.
4. CMS: OCM Frequently Asked Questions.
5. Himelstein AL et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases. JAMA. 2017 Jan 3;317(1):48-58.
6. Grothey A et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-88.
7. Von Minckwitz G et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-31.
8. American Society of Clinical Oncology: Ten Things Physician and Patients Should Question.
9. Temel JS et al. Early palliative care for patients with metastatic non–small cell lung cancer. N Engl J Med. 2010;363(8):733-42.
10. Blayney DW et al. Critical lessons from high-value oncology practices. JAMA Oncol. 2018 Feb 1;4(2):164-71.
11. Sherman DE. Transforming practices through the oncology care model: financial toxicity and counseling. J Oncol Pract. 2017 Aug;13(8):519-22.
Care of the cancer patient is complex and expensive. During 2001-2011, medical spending to treat cancer increased from $56.8 billion to $88.3 billion in the United States. During this time, ambulatory expenditures for care and treatment increased while inpatient hospital expenditures decreased.1,2 Treatments for cancer have advanced, but costs do not correlate with outcomes. Advanced payment models aimed at ensuring high quality while lowering costs may be the vehicle to help mitigate the financial burden of cancer treatment on patients and society at large.

Oncology Care Model
The Center for Medicare and Medicaid Innovation designed the Oncology Care Model (OCM), which allows practices and payers in the United States to partner with the Centers for Medicare & Medicaid Services. The goal of the OCM is to provide high quality, highly coordinated cancer care at the same or lower cost. Practice partnerships with the CMS involve payment arrangements that include financial and performance accountability for episodes of cancer care surrounding chemotherapy delivery to patients.3
Practices that have been selected by the CMS have attested to providing a number of enhanced services from 24/7 patient access to an appropriate clinician who can access medical records to having a documented care plan for every patient.4
Payment methodology
An episode of care is defined as a 6-month period that starts at the time of chemotherapy administration. In addition to the standard fee-for-service payment, practices have the ability to earn two other types of payments during an oncology episode.
The per-beneficiary Monthly Enhanced Oncology Services payment is $960 for the entire episode but is paid to practices at $160 per month.
Practices have the potential to earn additional performance-based payments (PBP) based on the difference in cost between the projected and actual cost of the episode. The PBP also incorporates performance on quality metrics, based on Medicare claims and other information submitted by the practice. For example, claims-based measures include hospital, emergency department (ED), and hospice utilization.
To participate in the OCM, practices must choose either a one-sided or two-sided risk model. In the one-sided risk model, practices take on no downside risk but need to achieve a greater reduction in expenditures (4% below the benchmark price). In the two-side risk model, practices need only to reduce expenditures by 2.75% below the benchmark price. But if they fail to meet their savings goals, they must pay the difference to the CMS. The recoupment is capped at 20% of the benchmark amount.
Feedback reports
The CMS sends quarterly feedback reports that contain information on practice demographics, outcomes, expenditures, chemotherapy use, and patient satisfaction. The outcomes include the mortality rate for Medicare beneficiaries treated at the practice, compared with other practices nationally. In addition, the reports include end-of-life metrics and patient satisfaction, as well as details of expenditures on drugs, hospital use, imaging and laboratory services, and a description of chemotherapy usage.
These reports can be a helpful tool for measuring your own use of services, as well as benchmarking it against national figures.
Practice modifications
According to CMS feedback reports, the cost of care per beneficiary per month has increased across all practices since the inception of the OCM. However, there are practices that have been successful in reducing cost of care without negatively affecting mortality.
Drugs, hospital, and ED visits, along with imaging and laboratory evaluation, account for 75% of the cost. Some strategies to reduce expenditure involve targeting those areas.
Consider prescribing drugs conservatively without affecting outcomes. For instance, bisphosphonates for bone metastasis can be given every 12 weeks instead of 4 weeks.5 Similarly, adjuvant chemotherapy can be given for 3 months, instead of 6 months in appropriate stage 3 colon cancer patients.6
Another potential opportunity for savings is the judicious use of pertuzumab in early-stage breast cancer patients.7 These are all evidence-based recommendations with potential for cost savings. Clinical pathways can aid in this process, but physician buy-in is imperative.
In terms of imaging, avoid PET scans when they will not affect your clinical decision making, avoid staging scans in early-stage breast and prostate cancer patients, and avoid surveillance scans among early-stage breast cancer and lymphoma patients. The Choosing Wisely campaign can help guide some of these decisions.8
Another area where good care meets cost effective care is in the early engagement of palliative care. Several studies have shown that early involvement of palliative care improves survival and quality of life.9,10 Palliative care involvement also decreases the emotional burden for patients and oncologists. Appropriate symptom control, particularly of pain, decreases hospitalizations during treatment.
Investing in a robust supportive care team – financial advocates, social work, nutrition, behavioral health, as well as various community services – can help reduce the financial, physical, and emotional distress levels for patients. All of these services ultimately lead to reduced hospitalizations.11 The Monthly Enhanced Oncology Services payment can be put toward these expenses.
Care teams working at the highest level of competence and license can also save time and money. Consider using registered nurses to implement triage pathways to assess side effects and symptom management, or using nurse practitioners, registered nurses, and physician assistants for same-day appointments and to assess symptoms rather than referring patients to the emergency department.
Avoid the ED and hospitalizations by using the infusion center to provide hydration and blood transfusions in a timely fashion.
Telemedicine can be used for symptom management as well as leveraging supportive care services.
Cost for cancer care is very difficult to sustain. The OCM provides early insights into expenditures, challenges, and opportunities. Practices should use this information to build infrastructure and provide high quality, cost-effective care. Value-based cancer care should be the overarching goal for oncology practices and health care organizations.
Dr. Mahesh is the director of hematology-oncology and program director of the Oncology Care Model at Summa Health in Akron, Ohio.
References
1. Siegel RL et al. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Medical Expenditure Panel Survey, Statistical Brief #443. 2014 Jun.
3. CMS: Oncology Care Model.
4. CMS: OCM Frequently Asked Questions.
5. Himelstein AL et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases. JAMA. 2017 Jan 3;317(1):48-58.
6. Grothey A et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-88.
7. Von Minckwitz G et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-31.
8. American Society of Clinical Oncology: Ten Things Physician and Patients Should Question.
9. Temel JS et al. Early palliative care for patients with metastatic non–small cell lung cancer. N Engl J Med. 2010;363(8):733-42.
10. Blayney DW et al. Critical lessons from high-value oncology practices. JAMA Oncol. 2018 Feb 1;4(2):164-71.
11. Sherman DE. Transforming practices through the oncology care model: financial toxicity and counseling. J Oncol Pract. 2017 Aug;13(8):519-22.
Care of the cancer patient is complex and expensive. During 2001-2011, medical spending to treat cancer increased from $56.8 billion to $88.3 billion in the United States. During this time, ambulatory expenditures for care and treatment increased while inpatient hospital expenditures decreased.1,2 Treatments for cancer have advanced, but costs do not correlate with outcomes. Advanced payment models aimed at ensuring high quality while lowering costs may be the vehicle to help mitigate the financial burden of cancer treatment on patients and society at large.

Oncology Care Model
The Center for Medicare and Medicaid Innovation designed the Oncology Care Model (OCM), which allows practices and payers in the United States to partner with the Centers for Medicare & Medicaid Services. The goal of the OCM is to provide high quality, highly coordinated cancer care at the same or lower cost. Practice partnerships with the CMS involve payment arrangements that include financial and performance accountability for episodes of cancer care surrounding chemotherapy delivery to patients.3
Practices that have been selected by the CMS have attested to providing a number of enhanced services from 24/7 patient access to an appropriate clinician who can access medical records to having a documented care plan for every patient.4
Payment methodology
An episode of care is defined as a 6-month period that starts at the time of chemotherapy administration. In addition to the standard fee-for-service payment, practices have the ability to earn two other types of payments during an oncology episode.
The per-beneficiary Monthly Enhanced Oncology Services payment is $960 for the entire episode but is paid to practices at $160 per month.
Practices have the potential to earn additional performance-based payments (PBP) based on the difference in cost between the projected and actual cost of the episode. The PBP also incorporates performance on quality metrics, based on Medicare claims and other information submitted by the practice. For example, claims-based measures include hospital, emergency department (ED), and hospice utilization.
To participate in the OCM, practices must choose either a one-sided or two-sided risk model. In the one-sided risk model, practices take on no downside risk but need to achieve a greater reduction in expenditures (4% below the benchmark price). In the two-side risk model, practices need only to reduce expenditures by 2.75% below the benchmark price. But if they fail to meet their savings goals, they must pay the difference to the CMS. The recoupment is capped at 20% of the benchmark amount.
Feedback reports
The CMS sends quarterly feedback reports that contain information on practice demographics, outcomes, expenditures, chemotherapy use, and patient satisfaction. The outcomes include the mortality rate for Medicare beneficiaries treated at the practice, compared with other practices nationally. In addition, the reports include end-of-life metrics and patient satisfaction, as well as details of expenditures on drugs, hospital use, imaging and laboratory services, and a description of chemotherapy usage.
These reports can be a helpful tool for measuring your own use of services, as well as benchmarking it against national figures.
Practice modifications
According to CMS feedback reports, the cost of care per beneficiary per month has increased across all practices since the inception of the OCM. However, there are practices that have been successful in reducing cost of care without negatively affecting mortality.
Drugs, hospital, and ED visits, along with imaging and laboratory evaluation, account for 75% of the cost. Some strategies to reduce expenditure involve targeting those areas.
Consider prescribing drugs conservatively without affecting outcomes. For instance, bisphosphonates for bone metastasis can be given every 12 weeks instead of 4 weeks.5 Similarly, adjuvant chemotherapy can be given for 3 months, instead of 6 months in appropriate stage 3 colon cancer patients.6
Another potential opportunity for savings is the judicious use of pertuzumab in early-stage breast cancer patients.7 These are all evidence-based recommendations with potential for cost savings. Clinical pathways can aid in this process, but physician buy-in is imperative.
In terms of imaging, avoid PET scans when they will not affect your clinical decision making, avoid staging scans in early-stage breast and prostate cancer patients, and avoid surveillance scans among early-stage breast cancer and lymphoma patients. The Choosing Wisely campaign can help guide some of these decisions.8
Another area where good care meets cost effective care is in the early engagement of palliative care. Several studies have shown that early involvement of palliative care improves survival and quality of life.9,10 Palliative care involvement also decreases the emotional burden for patients and oncologists. Appropriate symptom control, particularly of pain, decreases hospitalizations during treatment.
Investing in a robust supportive care team – financial advocates, social work, nutrition, behavioral health, as well as various community services – can help reduce the financial, physical, and emotional distress levels for patients. All of these services ultimately lead to reduced hospitalizations.11 The Monthly Enhanced Oncology Services payment can be put toward these expenses.
Care teams working at the highest level of competence and license can also save time and money. Consider using registered nurses to implement triage pathways to assess side effects and symptom management, or using nurse practitioners, registered nurses, and physician assistants for same-day appointments and to assess symptoms rather than referring patients to the emergency department.
Avoid the ED and hospitalizations by using the infusion center to provide hydration and blood transfusions in a timely fashion.
Telemedicine can be used for symptom management as well as leveraging supportive care services.
Cost for cancer care is very difficult to sustain. The OCM provides early insights into expenditures, challenges, and opportunities. Practices should use this information to build infrastructure and provide high quality, cost-effective care. Value-based cancer care should be the overarching goal for oncology practices and health care organizations.
Dr. Mahesh is the director of hematology-oncology and program director of the Oncology Care Model at Summa Health in Akron, Ohio.
References
1. Siegel RL et al. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Medical Expenditure Panel Survey, Statistical Brief #443. 2014 Jun.
3. CMS: Oncology Care Model.
4. CMS: OCM Frequently Asked Questions.
5. Himelstein AL et al. Effect of longer-interval vs. standard dosing of zoledronic acid on skeletal events in patients with bone metastases. JAMA. 2017 Jan 3;317(1):48-58.
6. Grothey A et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177-88.
7. Von Minckwitz G et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-31.
8. American Society of Clinical Oncology: Ten Things Physician and Patients Should Question.
9. Temel JS et al. Early palliative care for patients with metastatic non–small cell lung cancer. N Engl J Med. 2010;363(8):733-42.
10. Blayney DW et al. Critical lessons from high-value oncology practices. JAMA Oncol. 2018 Feb 1;4(2):164-71.
11. Sherman DE. Transforming practices through the oncology care model: financial toxicity and counseling. J Oncol Pract. 2017 Aug;13(8):519-22.
Your recap of the 2019 Gut Microbiota for Health World Summit
On March 23 and 24, AGA and the European Society of Neurogastroenterology and Motility (ESNM) gathered 350+ international clinicians and researchers to network and discuss the latest evidence on the interaction between diet, nutrition and the gut microbiome at the 2019 Gut Microbiota for Health World Summit.
Twenty-three novel abstracts were presented as posters at the meeting. The abstracts covered topics ranging from probiotics to diet to potential microbiome-driven treatments for GI disorders.
Below are some key takeaways (as shared on Twitter) from the meeting. Stay tuned for more news and resources from the 2019 Gut Microbiota for Health World Summit, including an official meeting report in Gastroenterology, on-demand presentation recordings, video clips, and more.
“Excess zinc supplementation can change the gut #microbiota and increase risk AND severity of #cdiff infection, says @joeyzacks #GMFH2019 @cdiffFoundation” — Dr. Caterina Oneto (@caterina_oneto)
“You need a #dietitian for low #FODMAP diet education to ensure the patient consumes a nutritionally adequate diet. @MagnusSimren #GMFH2019” — Kate Scarlata, RDN (@KateScarlata_RD)
“Patients with cirrhosis have increased bacteremia, blood LPS levels and intestinal permeability. This background has led to study the role of gut microbiota in liver disease #GMFH2019” — GutMicrobiota Health (@GMFHx)
“Much anticipated talk on #probiotics happening now at #GMFH2019 led by AGA’s probiotics experts @KashyapPurna & Geoffrey Preidis. This work will culminate in a new AGA guideline on using probiotics in clinical practice. Additional data will be presented at #DDW19” — AGA (@AmerGastroAssn)
“Eric Martens: while a low fibre diet may not drive inflammation in the short term, it may increase disease risk in the long term, due to changes in microbiota & mucus degrading bacteria! #GMFH2019” — Andrea Hardy RD (@AndreaHardyRD)
View additional Twitter coverage of the meeting: #GMFH2019.
On March 23 and 24, AGA and the European Society of Neurogastroenterology and Motility (ESNM) gathered 350+ international clinicians and researchers to network and discuss the latest evidence on the interaction between diet, nutrition and the gut microbiome at the 2019 Gut Microbiota for Health World Summit.
Twenty-three novel abstracts were presented as posters at the meeting. The abstracts covered topics ranging from probiotics to diet to potential microbiome-driven treatments for GI disorders.
Below are some key takeaways (as shared on Twitter) from the meeting. Stay tuned for more news and resources from the 2019 Gut Microbiota for Health World Summit, including an official meeting report in Gastroenterology, on-demand presentation recordings, video clips, and more.
“Excess zinc supplementation can change the gut #microbiota and increase risk AND severity of #cdiff infection, says @joeyzacks #GMFH2019 @cdiffFoundation” — Dr. Caterina Oneto (@caterina_oneto)
“You need a #dietitian for low #FODMAP diet education to ensure the patient consumes a nutritionally adequate diet. @MagnusSimren #GMFH2019” — Kate Scarlata, RDN (@KateScarlata_RD)
“Patients with cirrhosis have increased bacteremia, blood LPS levels and intestinal permeability. This background has led to study the role of gut microbiota in liver disease #GMFH2019” — GutMicrobiota Health (@GMFHx)
“Much anticipated talk on #probiotics happening now at #GMFH2019 led by AGA’s probiotics experts @KashyapPurna & Geoffrey Preidis. This work will culminate in a new AGA guideline on using probiotics in clinical practice. Additional data will be presented at #DDW19” — AGA (@AmerGastroAssn)
“Eric Martens: while a low fibre diet may not drive inflammation in the short term, it may increase disease risk in the long term, due to changes in microbiota & mucus degrading bacteria! #GMFH2019” — Andrea Hardy RD (@AndreaHardyRD)
View additional Twitter coverage of the meeting: #GMFH2019.
On March 23 and 24, AGA and the European Society of Neurogastroenterology and Motility (ESNM) gathered 350+ international clinicians and researchers to network and discuss the latest evidence on the interaction between diet, nutrition and the gut microbiome at the 2019 Gut Microbiota for Health World Summit.
Twenty-three novel abstracts were presented as posters at the meeting. The abstracts covered topics ranging from probiotics to diet to potential microbiome-driven treatments for GI disorders.
Below are some key takeaways (as shared on Twitter) from the meeting. Stay tuned for more news and resources from the 2019 Gut Microbiota for Health World Summit, including an official meeting report in Gastroenterology, on-demand presentation recordings, video clips, and more.
“Excess zinc supplementation can change the gut #microbiota and increase risk AND severity of #cdiff infection, says @joeyzacks #GMFH2019 @cdiffFoundation” — Dr. Caterina Oneto (@caterina_oneto)
“You need a #dietitian for low #FODMAP diet education to ensure the patient consumes a nutritionally adequate diet. @MagnusSimren #GMFH2019” — Kate Scarlata, RDN (@KateScarlata_RD)
“Patients with cirrhosis have increased bacteremia, blood LPS levels and intestinal permeability. This background has led to study the role of gut microbiota in liver disease #GMFH2019” — GutMicrobiota Health (@GMFHx)
“Much anticipated talk on #probiotics happening now at #GMFH2019 led by AGA’s probiotics experts @KashyapPurna & Geoffrey Preidis. This work will culminate in a new AGA guideline on using probiotics in clinical practice. Additional data will be presented at #DDW19” — AGA (@AmerGastroAssn)
“Eric Martens: while a low fibre diet may not drive inflammation in the short term, it may increase disease risk in the long term, due to changes in microbiota & mucus degrading bacteria! #GMFH2019” — Andrea Hardy RD (@AndreaHardyRD)
View additional Twitter coverage of the meeting: #GMFH2019.



