User login
Benlysta approved for children with SLE
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
The B-lymphocyte stimulator–inhibitor called Benlysta already is approved for use in adults alongside standard therapy for SLE, and this approval marks the first such treatment available for children. Although there are regulatory submissions for use of this drug among children elsewhere, the United States is the first to approve its use among this age group, according to a press release from GSK. According to an FDA press announcement, the agency expedited the review and approval because belimumab could fulfill an unmet need.
The approval is based on a 1-year postapproval commitment study, which assessed efficacy, safety, and pharmacokinetics of 10 mg/kg belimumab plus standard therapy versus placebo plus standard therapy among children with SLE aged 5-11 years (n = 13) and those aged 12-17 years (n = 80). Although the study was not fully powered because of the rarity of the disease in this age group, it did find numerically higher SLE responder index response rates over 1 year among children treated with belimumab plus standard therapy (53%) than in those treated with placebo and standard therapy (44%).
Adverse reactions seen among this age group were consistent with those seen in adults, including nausea, diarrhea, pyrexia, nasopharyngitis, and bronchitis. The most common serious adverse reactions were serious infections. Belimumab has not been studied in combination with certain other drugs, such as other biologics or cyclophosphamide; therefore, combining it with such treatments is not recommended. Acute hypersensitivity reactions – including anaphylaxis and death – have been observed, even among patients who had previously tolerated belimumab.
Infusion reactions were common, so pretreat patients with an antihistamine. Also, do not administer the drug with live vaccines, the FDA noted.
More information can be found in the press announcement on the FDA website.
Statute of limitations in malpractice actions
Question: Regarding the statute of limitations, which of the following is incorrect?
A. The statute stipulates the time period from knowledge of injury to when a lawsuit must be filed, beyond which it will be forever barred.
B. This time period is usually 2 years but varies somewhat from jurisdiction to jurisdiction.
C. It starts to “run” when the cause of action accrues, i.e., when the claimant knew or should have known of the injury, not when the negligent act took place.
D. In the case of minors, disability, and concealment, the statute may be tolled, thereby giving the plaintiff more time to file his/her claim.
E. In some U.S. jurisdictions, the judge may exercise discretion and waive the statutory time period.
Answer: E. At common law, there was no time limit that barred a plaintiff from bringing a claim, although there was a so-called “doctrine of laches” that foreclosed an action that had long lapsed. However, statutory changes in the law now require that complaints be brought in a timely manner so that the evidence remains fresh, accurate, and reliable. Another reason is to provide repose to the wrongdoer, i.e., relief from worrying for an indefinite period of time whether a lawsuit will be brought. It is 2 years for the tort of negligence in most jurisdictions, although states like California and Tennessee place a 1-year limit on medical malpractice claims under some circumstances. In England, actions for negligence-based personal injury have a limitation period of 3 years. Additionally, section 33 allows the court to use its discretion to extend this time period, something that is not available in other common law jurisdictions such as Singapore and the United States.
The statute of limitations does not start to run from the date of the negligent act or omission. For example, if there is a failure to timely diagnose and treat a cancerous condition and the patient suffers harm several years later, time starts to accrue from the date of discovering the injury, not the date of misdiagnosis. The term “discovery rule” defines the accrual period, which begins from the date the injury is discovered or should have been discovered if the party had exercised reasonable diligence. In cases of fraudulent concealment of a right of action, the statute may be tolled (halted) during the period of concealment. Tolling also may apply during legal disability.
In malpractice actions involving minors, the running of the time period may be tolled until the minor reaches a certain age, such as the age of majority, or by the minor’s 10th birthday (Hawaii law). Chaffin v. Nicosia1 dealt with such a situation. As the result of negligent forceps delivery, which injured the optic nerve, the plaintiff became blind in the right eye in early infancy. He brought suit when he was 22 years old. Indiana had two statutes on the issue, one requiring a malpractice suit to be brought within 2 years of the incident, and the other allowing a minor to sue no later than 2 years after reaching the age of 21. The Indiana Supreme Court allowed the case to go forward, reversing the lower court’s decision barring the action.
Courts are apt to closely scrutinize attempts to use the statute of limitations to prevent recovery as taking such actions could deprive the injured plaintiff of an otherwise legitimate claim. In one example, the defendants sought to dismiss the case (so-called motion for summary judgment) by arguing that the plaintiff filed suit some 32 months after she had developed Sheehan syndrome from postpartum hemorrhagic shock, and this exceeded the 2-year statute of limitations. The court ruled: “Since reasonable minds could differ as to when the injury and its operative cause should have been discovered by a reasonably diligent patient, the timeliness of the plaintiff’s claims should be decided by a jury and the motions for summary judgment will therefore be denied.”2
Two very recent cases are illustrative of litigation over statutes of limitations. In the first case, the District of Columbia’s highest court held that BKW, a patient-plaintiff, did not qualify for an extension and rejected his untimely suit against the hospital.3 The patient’s injuries stemmed from alleged unsuccessful venipunctures, and his complaint contained six causes of action, including negligent and intentional infliction of emotional distress and unnecessary pain, suffering, and bodily injury. In the District of Columbia, a plaintiff must serve the defendant with notice of intention to file suit (pre-suit notice) not less than 90 days prior to filing the action. The plaintiff must then file the complaint itself within the 3-year limitations period, with an extension allowed to take into account the 90-day pre-suit notice requirement. The case centered on the “within 90 days” requirement to trigger the statute of limitations extension. BKW, acting on one’s own behalf, conceded that the 3-year period applicable to his claims had lapsed, but because his complaint was filed “within 90 days” after the limitation period expired, it was eligible for an extension. The Court disagreed and dismissed the case, holding that to be eligible for the 90-day extension, a plaintiff must serve the pre-suit notice within 90 days before the limitation period expired.
The second case4 alleged malpractice in the care of a patient who died of anaphylaxis after a nurse infused him with iron dextran. The nurse had allegedly left the patient’s room too soon and did not adequately monitor his reaction to the drug. The patient was admitted to the hospital for removal of a colonic tumor and was to receive treatment for iron deficiency anemia. The nurse, identified in the chart as Agency Nurse RN 104, administered the prescribed intravenous 25-mg test-dose of iron dextran over a 5-minute period, but when the patient began having an anaphylactic-type allergic reaction, the nurse was allegedly not in the patient’s room. The plaintiff and her attorney attempted, on several occasions and without success, to discover the actual identity of the nurse from the hospital’s representatives. Consequently, the complaint designated the nurse as “Agency Nurse RN 104,” and the plaintiff did not provide the name of the nurse, even though doing so was legally required; the exclusion of the nurse’s name would have resulted in case dismissal since the statute of limitations had lapsed. However, the court ruled, “we are satisfied that plaintiff and her attorney acted with reasonable diligence in attempting – with no avail – to ascertain the true identity of “Agency Nurse RN 104” before filing suit and before the 2-year limitations statute ran ...”
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Portions of this article had been previously published in a 2010 issue of Internal Medicine News. For additional information, readers may contact the author at [email protected]
References
1. Chaffin v. Nicosia, 310 N.E.2d 867 (Ind. 1974).
2. Lomeo v. Davis, 53 Pa. D. & C. 4th 49 (Pa. Com. Pl. Jul 24, 2001).
3. Waugh v. Medstar Georgetown University Hospital, District of Columbia Court of Appeals No. 18-CV-329. Decided March 14, 2019.
4. Rosenberg v. Watts, Superior Court N.J. Appellate Div., Docket No. A-4525-16T4. Decided March 21, 2019.
Question: Regarding the statute of limitations, which of the following is incorrect?
A. The statute stipulates the time period from knowledge of injury to when a lawsuit must be filed, beyond which it will be forever barred.
B. This time period is usually 2 years but varies somewhat from jurisdiction to jurisdiction.
C. It starts to “run” when the cause of action accrues, i.e., when the claimant knew or should have known of the injury, not when the negligent act took place.
D. In the case of minors, disability, and concealment, the statute may be tolled, thereby giving the plaintiff more time to file his/her claim.
E. In some U.S. jurisdictions, the judge may exercise discretion and waive the statutory time period.
Answer: E. At common law, there was no time limit that barred a plaintiff from bringing a claim, although there was a so-called “doctrine of laches” that foreclosed an action that had long lapsed. However, statutory changes in the law now require that complaints be brought in a timely manner so that the evidence remains fresh, accurate, and reliable. Another reason is to provide repose to the wrongdoer, i.e., relief from worrying for an indefinite period of time whether a lawsuit will be brought. It is 2 years for the tort of negligence in most jurisdictions, although states like California and Tennessee place a 1-year limit on medical malpractice claims under some circumstances. In England, actions for negligence-based personal injury have a limitation period of 3 years. Additionally, section 33 allows the court to use its discretion to extend this time period, something that is not available in other common law jurisdictions such as Singapore and the United States.
The statute of limitations does not start to run from the date of the negligent act or omission. For example, if there is a failure to timely diagnose and treat a cancerous condition and the patient suffers harm several years later, time starts to accrue from the date of discovering the injury, not the date of misdiagnosis. The term “discovery rule” defines the accrual period, which begins from the date the injury is discovered or should have been discovered if the party had exercised reasonable diligence. In cases of fraudulent concealment of a right of action, the statute may be tolled (halted) during the period of concealment. Tolling also may apply during legal disability.
In malpractice actions involving minors, the running of the time period may be tolled until the minor reaches a certain age, such as the age of majority, or by the minor’s 10th birthday (Hawaii law). Chaffin v. Nicosia1 dealt with such a situation. As the result of negligent forceps delivery, which injured the optic nerve, the plaintiff became blind in the right eye in early infancy. He brought suit when he was 22 years old. Indiana had two statutes on the issue, one requiring a malpractice suit to be brought within 2 years of the incident, and the other allowing a minor to sue no later than 2 years after reaching the age of 21. The Indiana Supreme Court allowed the case to go forward, reversing the lower court’s decision barring the action.
Courts are apt to closely scrutinize attempts to use the statute of limitations to prevent recovery as taking such actions could deprive the injured plaintiff of an otherwise legitimate claim. In one example, the defendants sought to dismiss the case (so-called motion for summary judgment) by arguing that the plaintiff filed suit some 32 months after she had developed Sheehan syndrome from postpartum hemorrhagic shock, and this exceeded the 2-year statute of limitations. The court ruled: “Since reasonable minds could differ as to when the injury and its operative cause should have been discovered by a reasonably diligent patient, the timeliness of the plaintiff’s claims should be decided by a jury and the motions for summary judgment will therefore be denied.”2
Two very recent cases are illustrative of litigation over statutes of limitations. In the first case, the District of Columbia’s highest court held that BKW, a patient-plaintiff, did not qualify for an extension and rejected his untimely suit against the hospital.3 The patient’s injuries stemmed from alleged unsuccessful venipunctures, and his complaint contained six causes of action, including negligent and intentional infliction of emotional distress and unnecessary pain, suffering, and bodily injury. In the District of Columbia, a plaintiff must serve the defendant with notice of intention to file suit (pre-suit notice) not less than 90 days prior to filing the action. The plaintiff must then file the complaint itself within the 3-year limitations period, with an extension allowed to take into account the 90-day pre-suit notice requirement. The case centered on the “within 90 days” requirement to trigger the statute of limitations extension. BKW, acting on one’s own behalf, conceded that the 3-year period applicable to his claims had lapsed, but because his complaint was filed “within 90 days” after the limitation period expired, it was eligible for an extension. The Court disagreed and dismissed the case, holding that to be eligible for the 90-day extension, a plaintiff must serve the pre-suit notice within 90 days before the limitation period expired.
The second case4 alleged malpractice in the care of a patient who died of anaphylaxis after a nurse infused him with iron dextran. The nurse had allegedly left the patient’s room too soon and did not adequately monitor his reaction to the drug. The patient was admitted to the hospital for removal of a colonic tumor and was to receive treatment for iron deficiency anemia. The nurse, identified in the chart as Agency Nurse RN 104, administered the prescribed intravenous 25-mg test-dose of iron dextran over a 5-minute period, but when the patient began having an anaphylactic-type allergic reaction, the nurse was allegedly not in the patient’s room. The plaintiff and her attorney attempted, on several occasions and without success, to discover the actual identity of the nurse from the hospital’s representatives. Consequently, the complaint designated the nurse as “Agency Nurse RN 104,” and the plaintiff did not provide the name of the nurse, even though doing so was legally required; the exclusion of the nurse’s name would have resulted in case dismissal since the statute of limitations had lapsed. However, the court ruled, “we are satisfied that plaintiff and her attorney acted with reasonable diligence in attempting – with no avail – to ascertain the true identity of “Agency Nurse RN 104” before filing suit and before the 2-year limitations statute ran ...”
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Portions of this article had been previously published in a 2010 issue of Internal Medicine News. For additional information, readers may contact the author at [email protected]
References
1. Chaffin v. Nicosia, 310 N.E.2d 867 (Ind. 1974).
2. Lomeo v. Davis, 53 Pa. D. & C. 4th 49 (Pa. Com. Pl. Jul 24, 2001).
3. Waugh v. Medstar Georgetown University Hospital, District of Columbia Court of Appeals No. 18-CV-329. Decided March 14, 2019.
4. Rosenberg v. Watts, Superior Court N.J. Appellate Div., Docket No. A-4525-16T4. Decided March 21, 2019.
Question: Regarding the statute of limitations, which of the following is incorrect?
A. The statute stipulates the time period from knowledge of injury to when a lawsuit must be filed, beyond which it will be forever barred.
B. This time period is usually 2 years but varies somewhat from jurisdiction to jurisdiction.
C. It starts to “run” when the cause of action accrues, i.e., when the claimant knew or should have known of the injury, not when the negligent act took place.
D. In the case of minors, disability, and concealment, the statute may be tolled, thereby giving the plaintiff more time to file his/her claim.
E. In some U.S. jurisdictions, the judge may exercise discretion and waive the statutory time period.
Answer: E. At common law, there was no time limit that barred a plaintiff from bringing a claim, although there was a so-called “doctrine of laches” that foreclosed an action that had long lapsed. However, statutory changes in the law now require that complaints be brought in a timely manner so that the evidence remains fresh, accurate, and reliable. Another reason is to provide repose to the wrongdoer, i.e., relief from worrying for an indefinite period of time whether a lawsuit will be brought. It is 2 years for the tort of negligence in most jurisdictions, although states like California and Tennessee place a 1-year limit on medical malpractice claims under some circumstances. In England, actions for negligence-based personal injury have a limitation period of 3 years. Additionally, section 33 allows the court to use its discretion to extend this time period, something that is not available in other common law jurisdictions such as Singapore and the United States.
The statute of limitations does not start to run from the date of the negligent act or omission. For example, if there is a failure to timely diagnose and treat a cancerous condition and the patient suffers harm several years later, time starts to accrue from the date of discovering the injury, not the date of misdiagnosis. The term “discovery rule” defines the accrual period, which begins from the date the injury is discovered or should have been discovered if the party had exercised reasonable diligence. In cases of fraudulent concealment of a right of action, the statute may be tolled (halted) during the period of concealment. Tolling also may apply during legal disability.
In malpractice actions involving minors, the running of the time period may be tolled until the minor reaches a certain age, such as the age of majority, or by the minor’s 10th birthday (Hawaii law). Chaffin v. Nicosia1 dealt with such a situation. As the result of negligent forceps delivery, which injured the optic nerve, the plaintiff became blind in the right eye in early infancy. He brought suit when he was 22 years old. Indiana had two statutes on the issue, one requiring a malpractice suit to be brought within 2 years of the incident, and the other allowing a minor to sue no later than 2 years after reaching the age of 21. The Indiana Supreme Court allowed the case to go forward, reversing the lower court’s decision barring the action.
Courts are apt to closely scrutinize attempts to use the statute of limitations to prevent recovery as taking such actions could deprive the injured plaintiff of an otherwise legitimate claim. In one example, the defendants sought to dismiss the case (so-called motion for summary judgment) by arguing that the plaintiff filed suit some 32 months after she had developed Sheehan syndrome from postpartum hemorrhagic shock, and this exceeded the 2-year statute of limitations. The court ruled: “Since reasonable minds could differ as to when the injury and its operative cause should have been discovered by a reasonably diligent patient, the timeliness of the plaintiff’s claims should be decided by a jury and the motions for summary judgment will therefore be denied.”2
Two very recent cases are illustrative of litigation over statutes of limitations. In the first case, the District of Columbia’s highest court held that BKW, a patient-plaintiff, did not qualify for an extension and rejected his untimely suit against the hospital.3 The patient’s injuries stemmed from alleged unsuccessful venipunctures, and his complaint contained six causes of action, including negligent and intentional infliction of emotional distress and unnecessary pain, suffering, and bodily injury. In the District of Columbia, a plaintiff must serve the defendant with notice of intention to file suit (pre-suit notice) not less than 90 days prior to filing the action. The plaintiff must then file the complaint itself within the 3-year limitations period, with an extension allowed to take into account the 90-day pre-suit notice requirement. The case centered on the “within 90 days” requirement to trigger the statute of limitations extension. BKW, acting on one’s own behalf, conceded that the 3-year period applicable to his claims had lapsed, but because his complaint was filed “within 90 days” after the limitation period expired, it was eligible for an extension. The Court disagreed and dismissed the case, holding that to be eligible for the 90-day extension, a plaintiff must serve the pre-suit notice within 90 days before the limitation period expired.
The second case4 alleged malpractice in the care of a patient who died of anaphylaxis after a nurse infused him with iron dextran. The nurse had allegedly left the patient’s room too soon and did not adequately monitor his reaction to the drug. The patient was admitted to the hospital for removal of a colonic tumor and was to receive treatment for iron deficiency anemia. The nurse, identified in the chart as Agency Nurse RN 104, administered the prescribed intravenous 25-mg test-dose of iron dextran over a 5-minute period, but when the patient began having an anaphylactic-type allergic reaction, the nurse was allegedly not in the patient’s room. The plaintiff and her attorney attempted, on several occasions and without success, to discover the actual identity of the nurse from the hospital’s representatives. Consequently, the complaint designated the nurse as “Agency Nurse RN 104,” and the plaintiff did not provide the name of the nurse, even though doing so was legally required; the exclusion of the nurse’s name would have resulted in case dismissal since the statute of limitations had lapsed. However, the court ruled, “we are satisfied that plaintiff and her attorney acted with reasonable diligence in attempting – with no avail – to ascertain the true identity of “Agency Nurse RN 104” before filing suit and before the 2-year limitations statute ran ...”
Dr. Tan is emeritus professor of medicine and former adjunct professor of law at the University of Hawaii, Honolulu. This article is meant to be educational and does not constitute medical, ethical, or legal advice. Portions of this article had been previously published in a 2010 issue of Internal Medicine News. For additional information, readers may contact the author at [email protected]
References
1. Chaffin v. Nicosia, 310 N.E.2d 867 (Ind. 1974).
2. Lomeo v. Davis, 53 Pa. D. & C. 4th 49 (Pa. Com. Pl. Jul 24, 2001).
3. Waugh v. Medstar Georgetown University Hospital, District of Columbia Court of Appeals No. 18-CV-329. Decided March 14, 2019.
4. Rosenberg v. Watts, Superior Court N.J. Appellate Div., Docket No. A-4525-16T4. Decided March 21, 2019.
Psoriatic Arthritis Journal Scan: April 2019
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Systematic review of depression and anxiety in psoriatic arthritis.
Kamalaraj N, El-Haddad C, Hay P, Pile K. Int J Rheum Dis. 2019 Apr 26.
This is the first systematic review of point prevalence of depression and anxiety in patients with psoriatic arthritis. There is a moderate point prevalence of both depression and anxiety in patients with psoriatic arthritis, which is similar or slightly higher than the general population and comparable to that seen in other rheumatic diseases. The effects of treatment for psoriatic arthritis on comorbid depression and anxiety remain unclear.
Amplifying the concept of psoriatic arthritis: The role of autoimmunity in systemic psoriatic disease.
Chimenti MS, Caso F, Alivernini S, et al. Autoimmun Rev. 2019 Apr 5.
Recently, an autoimmune footprint of PsA pathogenesis has been demonstrated with the presence of autoantigens and related autoantibodies in PsA patients' sera. The purpose of this review is to describe the new pathogenetic mechanisms and the different clinical pictures of systemic psoriatic disease, with the ultimate goal of improving the knowledge of this heterogeneous chronic inflammatory condition.
Cardiac and cardiovascular morbidities in patients with psoriatic arthritis: a population-based case control study.
Kibari A, Cohen AD, Gazitt T, et al. Clin Rheumatol. 2019 Apr 1.
A retrospective case control study assessed the prevalence of risk factors associated with cardiovascular disease (CVD) and CVD-related morbidity in a large Middle-Eastern psoriatic arthritis (PsA) cohort. The results emphasize the importance of clinician awareness of the increased risk for CVD-related complications in PsA patients.
Exploring Dimensions of Stiffness in Rheumatoid and Psoriatic Arthritis: The ARAD and OMERACT Stiffness Special Interest Group collaboration.
Sinnathura P, Bartlett SJ, Halls S, et al. J Rheumatol. 2019 Apr 1.
The aims of this study were to: 1) compare stiffness in psoriatic arthritis (PsA) and rheumatoid arthritis (RA) using patient-reported outcomes, 2) explore how dimensions of stiffness are associated with each other and reflect the patient experience, 3) explore how different dimensions of stiffness are associated with physical function.
Acrodermatitis Continua of Hallopeau with Psoriatic Arthritis.
Khosravi-Hafshejani T, Zhou Y, Dutz JP. J Rheumatol. 2019 Apr;46(4):437-438.
Acrodermatitis continua of Hallopeau (ACH) is a form of localized pustular psoriasis that can be associated with psoriatic arthritis. This case report examines a 53-year-old male presented with a 1-year history of fingernail and toenail dystrophy and pustules on the distal toes.
Head and neck cancer cost analysis yields simple bundled payment model
While many factors can influence cost in head and neck cancer, a simple bundled payments model for the disease can be developed based solely on treatment types, researchers reported.
The number of treatment modalities was the biggest driver of cost in an analysis of 150 head and neck cancer patients. Whether those patients needed single-modality, bimodality, or trimodality treatment was in turn driven by the stage of the disease; by contrast, patient factors had no significant cost impacts, the investigators found.
Based on those findings, they developed a three-tiered cost model in which surgery or radiation was the least costly, chemoradiation or surgery plus radiation was next, and surgery plus chemoradiation was associated with the highest cost.
Basing bundled payments on treatment modality is a “simple but clinically robust model” for payment selection in head and neck cancer patients, wrote senior author Matthew C. Ward, MD, of the Levine Cancer Institute at Atrium Health in Charlotte, N.C., and coauthors.
“A tiered system driven by treatment complexity will aid providers who seek to stratify financial risk in a simple and meaningful manner,” Dr. Ward and colleagues wrote in the Journal of Oncology Practice.
As cancer costs rise, bundled payment models seek to “incentivize value and reduce administrative waste” with a single payment per episode of care, shared by all providers contributing to that episode, they wrote. However, there have been few cancer-specific bundled payment programs described to date.
The tiered approach was based on an analysis of 150 patients with stage 0 to IVB head and neck cancer, excluding those with recurrent or metastatic disease and those treated with palliative intent. Most (58%) had stage IVA disease and the oropharynx was the tumor subsite in 48%.
Direct costs could not be published because of institutional policy, according to Dr. Ward and coauthors, who instead reported overall costs of treatment as relative median costs, or the ratio of the cost of a specific treatment versus the cost of surgery alone.
Specifically, surgery plus chemoradiation was the most costly versus surgery alone, with a relative median cost of 3.13 (P less than .001), followed by chemoradiation at 2.18 (P less than .001) surgery and radiation at 1.98 (P less than .001), and radiation alone at 1.66 (P = .013).
The treatment modalities used were driven by groups of stages, the investigators wrote. Compared with stages 0 to I, stages II to IVA were 33% more expensive, while stage IVB was 60% more expensive. Patient factors such as age, smoking, or comorbidities were not associated with cost.
Previous reported studies of cancer-specific bundled payment models have shown decreased costs and favorable outcomes, according to the researchers. Among those is an University of Texas MD Anderson Center report showing that a four-tiered model, based on treatments received and stratified by comorbidities, was feasible in a 1-year pilot study.
The current report validates those previous findings, with some differences, Dr. Ward and coauthors wrote. In particular, the three-tiered model was not stratified by Charlson comorbidity index, which did not correlate with cost in the present analysis, and it included less common disease sites than in the MD Anderson model.
“We felt it important to be inclusive of all patients seen by our multidisciplinary head and neck cancer team to keep the proposed bundled payments model as practical and simple as possible,” they wrote.
Dr. Ward reported a consulting or advisory role with AstraZeneca. Study coauthors provided disclosures related to Blue Earth Diagnostics, Merck, Varian Medical Systems, UpToDate, Gerson Lehrman Group, Osler, AlignRT, Chrysalis Biotherapeutics, and others.
SOURCE: Tom MC et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00665.
While many factors can influence cost in head and neck cancer, a simple bundled payments model for the disease can be developed based solely on treatment types, researchers reported.
The number of treatment modalities was the biggest driver of cost in an analysis of 150 head and neck cancer patients. Whether those patients needed single-modality, bimodality, or trimodality treatment was in turn driven by the stage of the disease; by contrast, patient factors had no significant cost impacts, the investigators found.
Based on those findings, they developed a three-tiered cost model in which surgery or radiation was the least costly, chemoradiation or surgery plus radiation was next, and surgery plus chemoradiation was associated with the highest cost.
Basing bundled payments on treatment modality is a “simple but clinically robust model” for payment selection in head and neck cancer patients, wrote senior author Matthew C. Ward, MD, of the Levine Cancer Institute at Atrium Health in Charlotte, N.C., and coauthors.
“A tiered system driven by treatment complexity will aid providers who seek to stratify financial risk in a simple and meaningful manner,” Dr. Ward and colleagues wrote in the Journal of Oncology Practice.
As cancer costs rise, bundled payment models seek to “incentivize value and reduce administrative waste” with a single payment per episode of care, shared by all providers contributing to that episode, they wrote. However, there have been few cancer-specific bundled payment programs described to date.
The tiered approach was based on an analysis of 150 patients with stage 0 to IVB head and neck cancer, excluding those with recurrent or metastatic disease and those treated with palliative intent. Most (58%) had stage IVA disease and the oropharynx was the tumor subsite in 48%.
Direct costs could not be published because of institutional policy, according to Dr. Ward and coauthors, who instead reported overall costs of treatment as relative median costs, or the ratio of the cost of a specific treatment versus the cost of surgery alone.
Specifically, surgery plus chemoradiation was the most costly versus surgery alone, with a relative median cost of 3.13 (P less than .001), followed by chemoradiation at 2.18 (P less than .001) surgery and radiation at 1.98 (P less than .001), and radiation alone at 1.66 (P = .013).
The treatment modalities used were driven by groups of stages, the investigators wrote. Compared with stages 0 to I, stages II to IVA were 33% more expensive, while stage IVB was 60% more expensive. Patient factors such as age, smoking, or comorbidities were not associated with cost.
Previous reported studies of cancer-specific bundled payment models have shown decreased costs and favorable outcomes, according to the researchers. Among those is an University of Texas MD Anderson Center report showing that a four-tiered model, based on treatments received and stratified by comorbidities, was feasible in a 1-year pilot study.
The current report validates those previous findings, with some differences, Dr. Ward and coauthors wrote. In particular, the three-tiered model was not stratified by Charlson comorbidity index, which did not correlate with cost in the present analysis, and it included less common disease sites than in the MD Anderson model.
“We felt it important to be inclusive of all patients seen by our multidisciplinary head and neck cancer team to keep the proposed bundled payments model as practical and simple as possible,” they wrote.
Dr. Ward reported a consulting or advisory role with AstraZeneca. Study coauthors provided disclosures related to Blue Earth Diagnostics, Merck, Varian Medical Systems, UpToDate, Gerson Lehrman Group, Osler, AlignRT, Chrysalis Biotherapeutics, and others.
SOURCE: Tom MC et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00665.
While many factors can influence cost in head and neck cancer, a simple bundled payments model for the disease can be developed based solely on treatment types, researchers reported.
The number of treatment modalities was the biggest driver of cost in an analysis of 150 head and neck cancer patients. Whether those patients needed single-modality, bimodality, or trimodality treatment was in turn driven by the stage of the disease; by contrast, patient factors had no significant cost impacts, the investigators found.
Based on those findings, they developed a three-tiered cost model in which surgery or radiation was the least costly, chemoradiation or surgery plus radiation was next, and surgery plus chemoradiation was associated with the highest cost.
Basing bundled payments on treatment modality is a “simple but clinically robust model” for payment selection in head and neck cancer patients, wrote senior author Matthew C. Ward, MD, of the Levine Cancer Institute at Atrium Health in Charlotte, N.C., and coauthors.
“A tiered system driven by treatment complexity will aid providers who seek to stratify financial risk in a simple and meaningful manner,” Dr. Ward and colleagues wrote in the Journal of Oncology Practice.
As cancer costs rise, bundled payment models seek to “incentivize value and reduce administrative waste” with a single payment per episode of care, shared by all providers contributing to that episode, they wrote. However, there have been few cancer-specific bundled payment programs described to date.
The tiered approach was based on an analysis of 150 patients with stage 0 to IVB head and neck cancer, excluding those with recurrent or metastatic disease and those treated with palliative intent. Most (58%) had stage IVA disease and the oropharynx was the tumor subsite in 48%.
Direct costs could not be published because of institutional policy, according to Dr. Ward and coauthors, who instead reported overall costs of treatment as relative median costs, or the ratio of the cost of a specific treatment versus the cost of surgery alone.
Specifically, surgery plus chemoradiation was the most costly versus surgery alone, with a relative median cost of 3.13 (P less than .001), followed by chemoradiation at 2.18 (P less than .001) surgery and radiation at 1.98 (P less than .001), and radiation alone at 1.66 (P = .013).
The treatment modalities used were driven by groups of stages, the investigators wrote. Compared with stages 0 to I, stages II to IVA were 33% more expensive, while stage IVB was 60% more expensive. Patient factors such as age, smoking, or comorbidities were not associated with cost.
Previous reported studies of cancer-specific bundled payment models have shown decreased costs and favorable outcomes, according to the researchers. Among those is an University of Texas MD Anderson Center report showing that a four-tiered model, based on treatments received and stratified by comorbidities, was feasible in a 1-year pilot study.
The current report validates those previous findings, with some differences, Dr. Ward and coauthors wrote. In particular, the three-tiered model was not stratified by Charlson comorbidity index, which did not correlate with cost in the present analysis, and it included less common disease sites than in the MD Anderson model.
“We felt it important to be inclusive of all patients seen by our multidisciplinary head and neck cancer team to keep the proposed bundled payments model as practical and simple as possible,” they wrote.
Dr. Ward reported a consulting or advisory role with AstraZeneca. Study coauthors provided disclosures related to Blue Earth Diagnostics, Merck, Varian Medical Systems, UpToDate, Gerson Lehrman Group, Osler, AlignRT, Chrysalis Biotherapeutics, and others.
SOURCE: Tom MC et al. J Oncol Pract. 2019 Apr 22. doi: 10.1200/JOP.18.00665.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
Good Notes Can Deter Litigation
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
Pretrial screening panels: Do they reduce frivolous claims?
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.
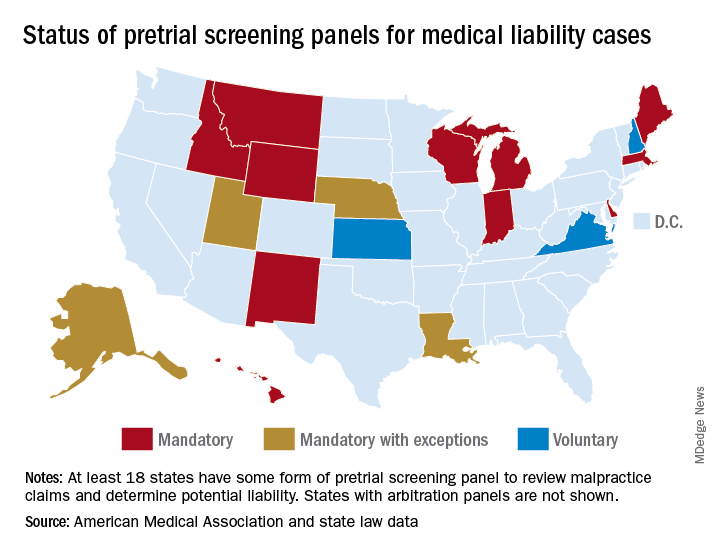
So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”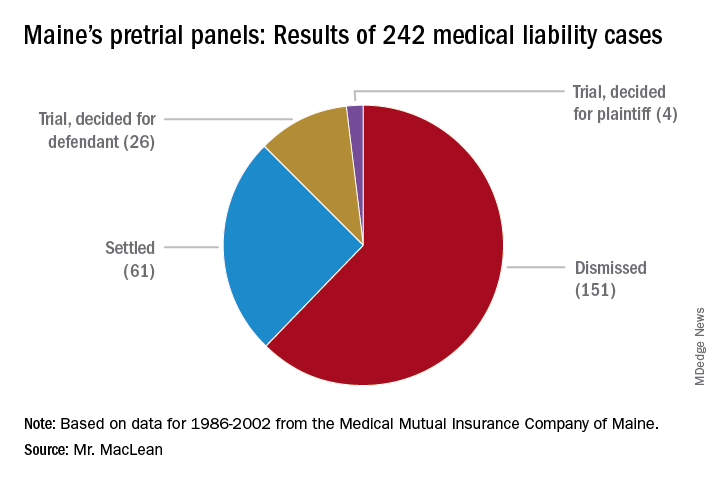
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.

So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
The liability climate for Kentucky physicians has long been bleak, according to Bruce A. Scott, MD, president of the Kentucky Medical Association. Insurance premiums are high, few doctors want to relocate to the Bluegrass State, and an overriding fear of lawsuits weighs heavily on the minds of physicians practicing there.

So the physician community was encouraged when in 2017, Kentucky enacted a law requiring all new malpractice claims to go before a medical review panel. The panel, comprised of an attorney and three health care professionals, would review evidence and opine on whether defendants had breached the standard of care. Plaintiffs could then decide whether to drop or resolve the case, or whether to continue to court.
“We saw it as a modest step forward,” Dr. Scott said in an interview. “[The panel] was hopefully going to speed up justice. Those cases that had merit would be settled, and those cases that didn’t have merit would be eliminated to allow the trial court to move on to the cases that needed to be tried.”
The Kentucky Supreme Court disagreed. In November 2018, state justices struck down the panel law as unconstitutional. Requiring plaintiffs to go before a medical review panel delays access to the courts and impedes their right to a speedy trial, the court ruled.
The end to Kentucky’s short-lived medical review panel raises questions about whether such advisory committees are beneficial in medical liability cases. Do review panels help reduce frivolous claims? What effects do the panels have on case duration and court costs?
At least 17 states have some form of pretrial screening panel that evaluates claims against health care professionals. Most panels include legal experts and medical professionals who review evidence and make a determination about potential negligence. In some states, such as Indiana, a panel review is mandatory, whereas in others, like Kansas, the process is voluntary.* Most panel decisions are nonbinding, and parties can proceed to court if they prefer.
Maine: A success story
Maine has experienced marked success with its medical review panel, which has been active since 1986, said Andrew B. MacLean, an attorney and interim CEO for the Maine Medical Association (MMA). The three-person panel, which includes a judicial expert, an attorney, and a physician, addresses whether the defendant’s actions constitute a deviation from the standard of care, whether acts or omissions caused the alleged injury, and the degree to which potential negligence exists on the part of the health care professional and/or the patient.
“The vast majority of medical malpractice claims in Maine are resolved at or before the screening panel stage and our state’s relatively small medical malpractice bar has come to accept this and to work cooperatively within the panel process,” Mr. MacLean said in an interview. “This has not been easy, but we’ve achieved such a result through many years of negotiation among representatives of the judiciary, plaintiffs’ and defense bar, professional liability insurers, and the professional organizations of trial lawyers and physicians.”
From 1986 to 2002, pretrial panels in Maine analyzed 242 medical liability cases, according to MMA data. Panelists found unanimously for the defendant in 157 cases and unanimously for the plaintiff in 42. In 43 cases, panelists were split. Of the total 242 cases, 151 were ultimately dismissed, 61 cases were settled, and 30 cases went to trial. Of the 30 cases that went to trial, jurors found for the health care professional 26 times.
A medical panel review is a quicker way to determine liability, and the process generally benefits both parties, said Peter Michaud, MMA associate general counsel. Panel hearings last 1-2 days, whereas court trial can take weeks, said Mr. Michaud, who chaired Maine’s panel for 10 years. At the same time, the patient gets their “day in court” and a chance to share their side of the story, he added.
“If you have a panel that votes 3-0 for no liability, or 3-0 for liability, that’s pretty persuasive to the attorneys,” Mr. Michaud said in an interview. “And it’s something they can use in their discussion with their own clients about what to do next.”
The fact that professionals make up the panel enables the case to unfold more smoothly, Mr. Michaud noted.
“It’s very important because if there’s any game playing going on by counsel, having a person with judicial experience, plus another attorney, cuts through that,” he said. “Also having a medical professional on the panel helps the nonmedical panelists understand and evaluate the expert evidence submitted by both parties.”
Reduced claims, higher costs
In Indiana, physician defendants have experienced similar benefits from the state’s medical review panel. Medical malpractice claims for more than $15,000 must be presented to the panel, comprised of an attorney and three health care professionals. After reviewing evidence, the panel provides its opinions, which are admissible at trial but not conclusive, according to state law.
When sued, health care professionals generally feel more comfortable that their conduct will initially be judged by a panel of peers before being presented to a jury, said J. Richard Moore, an Indianapolis-based medical liability defense attorney.
“In my experience, the medical review panel process does reduce the number of truly frivolous claims,” Mr. Moore said in an interview. “The panel adds another layer of process that requires knowledge and experience. ”
However, while the panel helps eliminate invalid claims, the process often can increase legal expenses, Mr. Moore said. The discovery process – subpoenaing records, taking sworn witnesses testimony, and obtaining paid expert witness opinions – is a major cost of litigation, he explained, and also happens before a case goes before the panel.
“In panel cases, there is really no cost savings with respect to discovery, and the two-phase process tends to increase, rather than reduce, attorney fees and costs,” Mr. Moore said. ”This is particularly true on the defense side because we are typically compensated via hourly billing.”
Such costs are counterbalanced if the panel finds in favor of the medical provider and the case is dropped without any plaintiff payment or settlement, he added.
The value of a case review depends greatly on the panelists, according to Karen E. Beach, an appellate attorney in Bloomfield Hills, Mich. In Michigan, the majority of claims go before a mediation panel that includes three attorneys and two health care professionals, one chosen by the plaintiff and one chosen by the defendant. Within 14 days of the panel hearing, the group submits an evaluation of the case regarding the applicable standard of care.
Panels that have more experience with medical malpractice law are more useful than those with less, said Ms. Beach. Overall, however, the case review process in Michigan is widely regarded as unhelpful in getting medical malpractice cases settled, she said.
“The sense, especially from defendants, is that the panel does not spend enough time on each case, and the assessment of the value is not realistic in the eyes of the attorney/client,” Ms. Beach said in an interview. “In fact, the Michigan Supreme Court is presently examining whether to do away with or modify the case-evaluation process.”
Screening panels have been repealed in at least seven states and overturned by courts on constitutional grounds in another six states, including Kentucky.
Broader studies needed
Little national data exists on the overall impact of medical review panels.
Pretrial screening panels had no significant effect on claims frequency or compensation amounts, according to a 2016 report from the Medicare Payment Advisory Commission (MedPAC).
That report looked at seven state tort reform strategies and concluded that data on pretrial screening panels was older and more limited, compared with that of other reforms. Because few early studies identified any notable effects of screening panels, researchers in later studies typically excluded screening panels from the models being tested, according to the MedPAC report.
Michelle M. Mello, PhD, a law professor at Stanford (Calif.) University and coauthor of the MedPAC report, said she was uncertain why there has not been closer study of pretrial screening panels in recent years. Pretrial screening panels probably have little effect because they apply a low standard to complaints, and thus, few claims get weeded out, she said in an interview. “The statutes don’t require them to do much more than say the plaintiff has a plausible case.”
The last comprehensive study on the effects of pretrial screening panels was published almost 10 years ago.
Researchers at Virginia Military University in Lexington evaluated panel data collected during 1991-2004 and data on malpractice awards from the National Practitioner Data Bank for the analysis. The study found review panels had no significant effect on the number of malpractice awards. However, results showed that states with noneconomic damages caps had markedly fewer malpractice awards (Virginia Economic Journal. 2010;15:35-45).
“The fact that damage caps are binding, while [medical malpractice review panel] recommendations are not, could explain the significance of the former, and the insignificance of the latter,” the authors wrote. “It seems reasonable that reforms must be binding, unavoidable, and obligatory to have real effects.”
*Clarification, 6/5/2019: An earlier version of this story indicated that Utah has a voluntary pretrial screening panel. In Utah, a panel review is mandatory, unless both parties agree to waive the hearing process. The accompanying graphic of the United States has been updated accordingly.
Just a series of fortunate events?
Building a career in hospital medicine
Residents and junior faculty have frequently asked me how they can attain a position similar to mine, focused on quality and leadership in a health care system. When I was first asked to offer advice on this topic, my response was generally something like, “Heck if I know! I just had a series of lucky accidents to get here!”
Back then, I would recount my career history. I established myself as a clinician educator and associate program director soon after Chief Residency. After that, I would explain, a series of fortunate events and health care trends shaped my career. Evidence-based medicine (EBM), the patient safety movement, a shift to incorporate value (as well as volume) into reimbursement models, and the hospital medicine movement all emerged in interesting and often synergistic ways.
A young SHM organization (then known as NAIP) grew rapidly even while the hospitalist programs I led in Phoenix, then at University of California, San Diego, grew in size and influence. Inevitably, it seemed, I was increasingly involved in quality improvement (QI) efforts, and began to publish and speak about them. Collaborative work with SHM and a number of hospital systems broadened my visibility regionally and nationally. Finally, in 2015, I was recruited away from UC San Diego into a new position, as chief quality officer at UC Davis.
On hearing this history, those seeking my sage advice would look a little confused, and then say something like, “So your advice is that I should get lucky??? Gee, thanks a lot! Really helpful!” (Insert sarcasm here).
The honor of being asked to contribute to the “Legacies” series in The Hospitalist gave me an opportunity to think about this a little differently. No one really wanted to know about how past changes in the health care environment led to my career success. They wanted advice on tools and strategies that will allow them to thrive in an environment of ongoing, disruptive change that is likely only going to accelerate. I now present my upgraded points of advice, intertwined with examples of how SHM positively influenced my career (and could assist yours):
Learn how your hospital works. Hospitalists obviously have an inside track on many aspects of hospital operations, but sometimes remain oblivious to the organizational and committee structure, priorities of hospital leadership, and the mechanism for implementing standardized care. Knowing where to go with new ideas, and the process of implementing protocols, will keep you from hitting political land mines and unintentionally encroaching on someone else’s turf, while aligning your efforts with institutional priorities improves the buy-in and resources available to do the work.
Start small, but think big. Don’t bite off more than you can chew, and make sure your ideas for change work on a small scale before trying to sell the world on them. On the other hand, think big! The care you and others provide is dependent on systems that go far beyond your immediate control. Policies, protocols, standardized order sets, checklists, and an array of other tools can be leveraged to influence care across an entire health system, and in the SHM Mentored Implementation programs, can impact hundreds of hospitals.
Broaden your skills. Commit to learning new skills that can increase your impact and career diversity. Procedural skills; information technology; and EMR, EBM, research, public health, QI, business, leadership, public speaking, advocacy, and telehealth, can all open up a whole world of possibilities when combined with a medical degree. These skills can move you into areas that keep you engaged and excited to go to work.
Engage in mentor/mentee relationships. As an associate program director and clinician-educator, I had a lot of opportunity to mentor residents and fellows. It is so rewarding to watch the mentee grow in experience and skills, and to eventually see many of them assume leadership and mentoring roles themselves. You don’t have to be in a teaching position to act as a mentor (my experience mentoring hospitalists and others in leadership and quality improvement now far surpasses my experience with house staff).
The mentor often benefits as much as the mentee from this relationship. I have been inspired by their passion and dedication, educated by their ideas and innovation, and frequently find I am learning more from them, than they are from me. I have had great experiences in the SHM Mentored Implementation program in the role of mentee and mentor.
Participate in a community. When I first joined NAIP, I was amazed that the giants (Wachter, Nelson, Whitcomb, Holman, Williams, Greeno, Howell, Huddleston, Wellikson, and on and on) were not only approachable, they were warm, friendly, interesting, and extraordinarily welcoming. The ever-expanding and evolving community at SHM continues that tradition and offers a forum to share innovative work, discuss common problems and solutions, contact world experts, or just find an empathetic ear. Working on toolkits and collaborative efforts with this community remains a real highlight of my career, and the source of several lasting friendships. So don’t be shy; step right up; and introduce yourself!
Avoid my past mistakes (this might be a long list). Random things you should try to avoid.
- Tribalism – It is natural to be protective of your hospitalist group, and to focus on the injustices heaped upon you from (insert favorite punching bag here, e.g., ED, orthopedists, cardiologists, nursing staff, evil administration penny pinchers, etc). While some of those injustices might be real, tribalism, defensiveness, and circling the wagons generally only makes things worse. Sit down face to face, learn a little bit about the opposing tribe (both about their work, and about them as people), and see how much more fun and productive work can be.
- Storming out of a meeting with the CMO and CEO, slamming the door, etc. – not productive. Administrative leaders are doing their own juggling act and are generally well intentioned and doing the best they can. Respect that, argue your case, but if things don’t pan out, shake their hand, and live to fight another day.
- Using e-mail (evil-mail) to resolve conflict – And if you’re a young whippersnapper, don’t use Twitter, Facebook, Snapchat, or other social media to address conflict either!
- Forgetting to put patients first – Frame decisions for your group around what best serves your patients, not your doctors. Long term, this gives your group credibility and will serve the hospitalists better as well. SHM does this on a large scale with their advocacy efforts, resulting in more credibility and influence on Capitol Hill.
Make time for friends, family, fitness, fun, and reflection. A sense of humor and an occasional laugh when dealing with ill patients, hospital medicine politics, and the EMR all day provides resilience, as does taking the time to foster self-awareness and insight into your own weaknesses, strengths, and how you react to different stressors. A little bit of exercise and time with family and friends can go a long way towards improving your outlook, work, and life in general, while reducing burnout. Oh yeah, it’s also a good idea to choose a great life partner as well. Thanks Michelle!
Dr. Maynard is chief quality officer, University of California Davis Medical Center, Sacramento, Calif.
Building a career in hospital medicine
Building a career in hospital medicine
Residents and junior faculty have frequently asked me how they can attain a position similar to mine, focused on quality and leadership in a health care system. When I was first asked to offer advice on this topic, my response was generally something like, “Heck if I know! I just had a series of lucky accidents to get here!”
Back then, I would recount my career history. I established myself as a clinician educator and associate program director soon after Chief Residency. After that, I would explain, a series of fortunate events and health care trends shaped my career. Evidence-based medicine (EBM), the patient safety movement, a shift to incorporate value (as well as volume) into reimbursement models, and the hospital medicine movement all emerged in interesting and often synergistic ways.
A young SHM organization (then known as NAIP) grew rapidly even while the hospitalist programs I led in Phoenix, then at University of California, San Diego, grew in size and influence. Inevitably, it seemed, I was increasingly involved in quality improvement (QI) efforts, and began to publish and speak about them. Collaborative work with SHM and a number of hospital systems broadened my visibility regionally and nationally. Finally, in 2015, I was recruited away from UC San Diego into a new position, as chief quality officer at UC Davis.
On hearing this history, those seeking my sage advice would look a little confused, and then say something like, “So your advice is that I should get lucky??? Gee, thanks a lot! Really helpful!” (Insert sarcasm here).
The honor of being asked to contribute to the “Legacies” series in The Hospitalist gave me an opportunity to think about this a little differently. No one really wanted to know about how past changes in the health care environment led to my career success. They wanted advice on tools and strategies that will allow them to thrive in an environment of ongoing, disruptive change that is likely only going to accelerate. I now present my upgraded points of advice, intertwined with examples of how SHM positively influenced my career (and could assist yours):
Learn how your hospital works. Hospitalists obviously have an inside track on many aspects of hospital operations, but sometimes remain oblivious to the organizational and committee structure, priorities of hospital leadership, and the mechanism for implementing standardized care. Knowing where to go with new ideas, and the process of implementing protocols, will keep you from hitting political land mines and unintentionally encroaching on someone else’s turf, while aligning your efforts with institutional priorities improves the buy-in and resources available to do the work.
Start small, but think big. Don’t bite off more than you can chew, and make sure your ideas for change work on a small scale before trying to sell the world on them. On the other hand, think big! The care you and others provide is dependent on systems that go far beyond your immediate control. Policies, protocols, standardized order sets, checklists, and an array of other tools can be leveraged to influence care across an entire health system, and in the SHM Mentored Implementation programs, can impact hundreds of hospitals.
Broaden your skills. Commit to learning new skills that can increase your impact and career diversity. Procedural skills; information technology; and EMR, EBM, research, public health, QI, business, leadership, public speaking, advocacy, and telehealth, can all open up a whole world of possibilities when combined with a medical degree. These skills can move you into areas that keep you engaged and excited to go to work.
Engage in mentor/mentee relationships. As an associate program director and clinician-educator, I had a lot of opportunity to mentor residents and fellows. It is so rewarding to watch the mentee grow in experience and skills, and to eventually see many of them assume leadership and mentoring roles themselves. You don’t have to be in a teaching position to act as a mentor (my experience mentoring hospitalists and others in leadership and quality improvement now far surpasses my experience with house staff).
The mentor often benefits as much as the mentee from this relationship. I have been inspired by their passion and dedication, educated by their ideas and innovation, and frequently find I am learning more from them, than they are from me. I have had great experiences in the SHM Mentored Implementation program in the role of mentee and mentor.
Participate in a community. When I first joined NAIP, I was amazed that the giants (Wachter, Nelson, Whitcomb, Holman, Williams, Greeno, Howell, Huddleston, Wellikson, and on and on) were not only approachable, they were warm, friendly, interesting, and extraordinarily welcoming. The ever-expanding and evolving community at SHM continues that tradition and offers a forum to share innovative work, discuss common problems and solutions, contact world experts, or just find an empathetic ear. Working on toolkits and collaborative efforts with this community remains a real highlight of my career, and the source of several lasting friendships. So don’t be shy; step right up; and introduce yourself!
Avoid my past mistakes (this might be a long list). Random things you should try to avoid.
- Tribalism – It is natural to be protective of your hospitalist group, and to focus on the injustices heaped upon you from (insert favorite punching bag here, e.g., ED, orthopedists, cardiologists, nursing staff, evil administration penny pinchers, etc). While some of those injustices might be real, tribalism, defensiveness, and circling the wagons generally only makes things worse. Sit down face to face, learn a little bit about the opposing tribe (both about their work, and about them as people), and see how much more fun and productive work can be.
- Storming out of a meeting with the CMO and CEO, slamming the door, etc. – not productive. Administrative leaders are doing their own juggling act and are generally well intentioned and doing the best they can. Respect that, argue your case, but if things don’t pan out, shake their hand, and live to fight another day.
- Using e-mail (evil-mail) to resolve conflict – And if you’re a young whippersnapper, don’t use Twitter, Facebook, Snapchat, or other social media to address conflict either!
- Forgetting to put patients first – Frame decisions for your group around what best serves your patients, not your doctors. Long term, this gives your group credibility and will serve the hospitalists better as well. SHM does this on a large scale with their advocacy efforts, resulting in more credibility and influence on Capitol Hill.
Make time for friends, family, fitness, fun, and reflection. A sense of humor and an occasional laugh when dealing with ill patients, hospital medicine politics, and the EMR all day provides resilience, as does taking the time to foster self-awareness and insight into your own weaknesses, strengths, and how you react to different stressors. A little bit of exercise and time with family and friends can go a long way towards improving your outlook, work, and life in general, while reducing burnout. Oh yeah, it’s also a good idea to choose a great life partner as well. Thanks Michelle!
Dr. Maynard is chief quality officer, University of California Davis Medical Center, Sacramento, Calif.
Residents and junior faculty have frequently asked me how they can attain a position similar to mine, focused on quality and leadership in a health care system. When I was first asked to offer advice on this topic, my response was generally something like, “Heck if I know! I just had a series of lucky accidents to get here!”
Back then, I would recount my career history. I established myself as a clinician educator and associate program director soon after Chief Residency. After that, I would explain, a series of fortunate events and health care trends shaped my career. Evidence-based medicine (EBM), the patient safety movement, a shift to incorporate value (as well as volume) into reimbursement models, and the hospital medicine movement all emerged in interesting and often synergistic ways.
A young SHM organization (then known as NAIP) grew rapidly even while the hospitalist programs I led in Phoenix, then at University of California, San Diego, grew in size and influence. Inevitably, it seemed, I was increasingly involved in quality improvement (QI) efforts, and began to publish and speak about them. Collaborative work with SHM and a number of hospital systems broadened my visibility regionally and nationally. Finally, in 2015, I was recruited away from UC San Diego into a new position, as chief quality officer at UC Davis.
On hearing this history, those seeking my sage advice would look a little confused, and then say something like, “So your advice is that I should get lucky??? Gee, thanks a lot! Really helpful!” (Insert sarcasm here).
The honor of being asked to contribute to the “Legacies” series in The Hospitalist gave me an opportunity to think about this a little differently. No one really wanted to know about how past changes in the health care environment led to my career success. They wanted advice on tools and strategies that will allow them to thrive in an environment of ongoing, disruptive change that is likely only going to accelerate. I now present my upgraded points of advice, intertwined with examples of how SHM positively influenced my career (and could assist yours):
Learn how your hospital works. Hospitalists obviously have an inside track on many aspects of hospital operations, but sometimes remain oblivious to the organizational and committee structure, priorities of hospital leadership, and the mechanism for implementing standardized care. Knowing where to go with new ideas, and the process of implementing protocols, will keep you from hitting political land mines and unintentionally encroaching on someone else’s turf, while aligning your efforts with institutional priorities improves the buy-in and resources available to do the work.
Start small, but think big. Don’t bite off more than you can chew, and make sure your ideas for change work on a small scale before trying to sell the world on them. On the other hand, think big! The care you and others provide is dependent on systems that go far beyond your immediate control. Policies, protocols, standardized order sets, checklists, and an array of other tools can be leveraged to influence care across an entire health system, and in the SHM Mentored Implementation programs, can impact hundreds of hospitals.
Broaden your skills. Commit to learning new skills that can increase your impact and career diversity. Procedural skills; information technology; and EMR, EBM, research, public health, QI, business, leadership, public speaking, advocacy, and telehealth, can all open up a whole world of possibilities when combined with a medical degree. These skills can move you into areas that keep you engaged and excited to go to work.
Engage in mentor/mentee relationships. As an associate program director and clinician-educator, I had a lot of opportunity to mentor residents and fellows. It is so rewarding to watch the mentee grow in experience and skills, and to eventually see many of them assume leadership and mentoring roles themselves. You don’t have to be in a teaching position to act as a mentor (my experience mentoring hospitalists and others in leadership and quality improvement now far surpasses my experience with house staff).
The mentor often benefits as much as the mentee from this relationship. I have been inspired by their passion and dedication, educated by their ideas and innovation, and frequently find I am learning more from them, than they are from me. I have had great experiences in the SHM Mentored Implementation program in the role of mentee and mentor.
Participate in a community. When I first joined NAIP, I was amazed that the giants (Wachter, Nelson, Whitcomb, Holman, Williams, Greeno, Howell, Huddleston, Wellikson, and on and on) were not only approachable, they were warm, friendly, interesting, and extraordinarily welcoming. The ever-expanding and evolving community at SHM continues that tradition and offers a forum to share innovative work, discuss common problems and solutions, contact world experts, or just find an empathetic ear. Working on toolkits and collaborative efforts with this community remains a real highlight of my career, and the source of several lasting friendships. So don’t be shy; step right up; and introduce yourself!
Avoid my past mistakes (this might be a long list). Random things you should try to avoid.
- Tribalism – It is natural to be protective of your hospitalist group, and to focus on the injustices heaped upon you from (insert favorite punching bag here, e.g., ED, orthopedists, cardiologists, nursing staff, evil administration penny pinchers, etc). While some of those injustices might be real, tribalism, defensiveness, and circling the wagons generally only makes things worse. Sit down face to face, learn a little bit about the opposing tribe (both about their work, and about them as people), and see how much more fun and productive work can be.
- Storming out of a meeting with the CMO and CEO, slamming the door, etc. – not productive. Administrative leaders are doing their own juggling act and are generally well intentioned and doing the best they can. Respect that, argue your case, but if things don’t pan out, shake their hand, and live to fight another day.
- Using e-mail (evil-mail) to resolve conflict – And if you’re a young whippersnapper, don’t use Twitter, Facebook, Snapchat, or other social media to address conflict either!
- Forgetting to put patients first – Frame decisions for your group around what best serves your patients, not your doctors. Long term, this gives your group credibility and will serve the hospitalists better as well. SHM does this on a large scale with their advocacy efforts, resulting in more credibility and influence on Capitol Hill.
Make time for friends, family, fitness, fun, and reflection. A sense of humor and an occasional laugh when dealing with ill patients, hospital medicine politics, and the EMR all day provides resilience, as does taking the time to foster self-awareness and insight into your own weaknesses, strengths, and how you react to different stressors. A little bit of exercise and time with family and friends can go a long way towards improving your outlook, work, and life in general, while reducing burnout. Oh yeah, it’s also a good idea to choose a great life partner as well. Thanks Michelle!
Dr. Maynard is chief quality officer, University of California Davis Medical Center, Sacramento, Calif.
Most measles cases in 25 years prompts government pleas to vaccinate
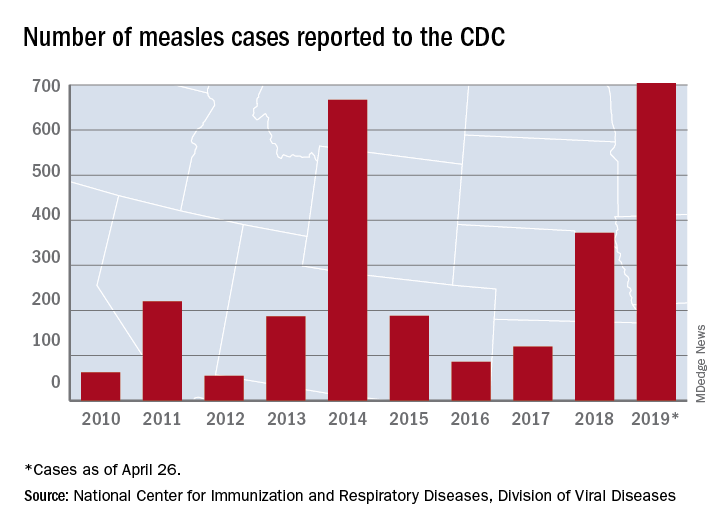
The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”

The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”

The updated figure adds 9 cases to the previous tally of 695 cases as of April 24, when the CDC announced that the number of cases in 2019 had surpassed the total for any year since the disease was considered effectively eliminated from the country in 2000.
Cases have been reported in 22 states, with the largest outbreaks in Washington and New York. The outbreak in Washington, which included 72 cases, was declared over last week. Two outbreaks in New York, however, are the largest and longest-lasting measles outbreaks since the disease was considered eliminated, said Nancy Messonnier, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases. The longer they continue, the “greater the chance that measles will again gain a foothold in the United States,” she said at CDC telebriefing on measles.
The outbreaks are linked to travelers who are exposed to measles abroad and bring it to the United States. The disease then may spread, especially in communities with high rates of unvaccinated people. “A significant factor contributing to the outbreaks in New York is misinformation in the communities about the safety of the measles/mumps/rubella vaccine,” according to the CDC.
National Infant Immunization Week
Until last week, 2014 – with 667 measles cases – had been the year with the most cases since the disease was effectively eliminated. The last time the United States had more measles cases was in 1994, when there were 963 cases for the year.
Health and Human Services Secretary Alex Azar, also at the telebriefing, pointed out that 1994 also was the year that the United States first observed National Infant Immunization Week, which is April 27–May 4 this year. The CDC is marking the 25th anniversary of the annual observance, which highlights “the importance of protecting infants from vaccine-preventable diseases” and celebrates “the achievements of immunization programs in promoting healthy communities,” Secretary Azar said.
Message to health care providers
CDC director Robert Redfield Jr., MD, noted that measles has “no treatment, no cure, and no way to predict how bad a case will be.”
Some patients may have mild symptoms, whereas others may have serious complications such as pneumonia or encephalitis. In 2019, 3% of the patients with measles have developed pneumonia, he said. No patients have died.
Dr. Redfield, a virologist, noted that the CDC is recommending that children aged 6-12 months receive 1 dose of the measles vaccine if traveling abroad.
“As CDC director and as a physician, I have and continue to wholeheartedly advocate for infant immunization,” he said in a statement. “More importantly, as a father and grandfather I have ensured all of my children and grandchildren are vaccinated on the recommended schedule. Vaccines are safe. Vaccines do not cause autism. Vaccine-preventable diseases are dangerous.”
More than 94% of parents vaccinate their children, Dr. Redfield added. “CDC is working to reach the small percentage of vaccine-hesitant individuals so they too understand the importance of vaccines. It is imperative that we correct misinformation and reassure fearful parents so they protect their children from illnesses with long-lasting health impacts.”
About 1.3%, or 100,000 children, in the United States under 2 years old have not been vaccinated, he said.
“I call upon health care providers to encourage parents and expectant parents to vaccinate their children for their own protection and to avoid the spread of vaccine-preventable diseases within their families and communities,” he said. “We must join together as a nation to once again eliminate measles and prevent future disease outbreaks.”
The CDC has a complete list of clinical recommendations for health care providers on its website.
The president weighs in
President Donald Trump said that children should receive vaccinations – his first public comment about vaccines since his inauguration. Previously, he had questioned the safety of vaccines.
Asked by reporters about the measles outbreaks and his message for parents about having their kids vaccinated, he said: “They have to get the shot. The vaccinations are so important. This is really going around now. They have to get their shots.”
FROM A CDC TELEBRIEFING
Combo respiratory pathogen tests miss pertussis
BALTIMORE – Ann Arbor.
Respiratory pathogen panels are popular because they test for many things at once, but providers have to know their limits, said lead investigator Colleen Mayhew, MD, a pediatric emergency medicine fellow at the University of Michigan.
“Should RPAN be used to diagnosis pertussis? No,” she said at the Pediatric Academic Societies annual meeting. RPAN was negative for confirmed pertussis 44% of the time in the study.
“In our cohort, [it] was no better than a coin flip for detecting pertussis,” she said. Also, even when it missed pertussis, it still detected other pathogens, which raises the risk that symptoms might be attributed to a different infection. “This has serious public health implications.”
“The bottom line is, if you are concerned about pertussis, it’s important to use a dedicated pertussis PCR [polymerase chain reaction] assay, and to use comprehensive respiratory pathogen testing only if there are other, specific targets that will change your clinical management,” such as mycoplasma or the flu, Dr. Mayhew said.
In the study, 102 nasopharyngeal swabs positive for pertussis on standalone PCR testing – the university uses an assay from Focus Diagnostics – were thawed and tested with RPAN.
RPAN was negative for pertussis on 45 swabs (44%). “These are the potential missed pertussis cases if RPAN is used alone,” Dr. Mayhew said. RPAN detected other pathogens, such as coronavirus, about half the time, whether or not it tested positive for pertussis. “Those additional pathogens might represent coinfection, but might also represent asymptomatic carriage.” It’s impossible to differentiate between the two, she noted.
In short, “neither positive testing for other respiratory pathogens, nor negative testing for pertussis by RPAN, is reliable for excluding the diagnosis of pertussis. Dedicated pertussis PCR testing should be used for diagnosis,” she and her team concluded.
RPAN also is a PCR test, but with a different, perhaps less robust, genetic target.
The 102 positive swabs were from patients aged 1 month to 73 years, so “it’s important for all of us to keep pertussis on our differential diagnose” no matter how old patients are, Dr. Mayhew said.
Freezing and thawing the swabs shouldn’t have degraded the genetic material, but it might have; that was one of the limits of the study.
The team hopes to run a quality improvement project to encourage the use of standalone pertussis PCR in Ann Arbor.
There was no industry funding. Dr. Mayhew didn’t report any disclosures.
BALTIMORE – Ann Arbor.
Respiratory pathogen panels are popular because they test for many things at once, but providers have to know their limits, said lead investigator Colleen Mayhew, MD, a pediatric emergency medicine fellow at the University of Michigan.
“Should RPAN be used to diagnosis pertussis? No,” she said at the Pediatric Academic Societies annual meeting. RPAN was negative for confirmed pertussis 44% of the time in the study.
“In our cohort, [it] was no better than a coin flip for detecting pertussis,” she said. Also, even when it missed pertussis, it still detected other pathogens, which raises the risk that symptoms might be attributed to a different infection. “This has serious public health implications.”
“The bottom line is, if you are concerned about pertussis, it’s important to use a dedicated pertussis PCR [polymerase chain reaction] assay, and to use comprehensive respiratory pathogen testing only if there are other, specific targets that will change your clinical management,” such as mycoplasma or the flu, Dr. Mayhew said.
In the study, 102 nasopharyngeal swabs positive for pertussis on standalone PCR testing – the university uses an assay from Focus Diagnostics – were thawed and tested with RPAN.
RPAN was negative for pertussis on 45 swabs (44%). “These are the potential missed pertussis cases if RPAN is used alone,” Dr. Mayhew said. RPAN detected other pathogens, such as coronavirus, about half the time, whether or not it tested positive for pertussis. “Those additional pathogens might represent coinfection, but might also represent asymptomatic carriage.” It’s impossible to differentiate between the two, she noted.
In short, “neither positive testing for other respiratory pathogens, nor negative testing for pertussis by RPAN, is reliable for excluding the diagnosis of pertussis. Dedicated pertussis PCR testing should be used for diagnosis,” she and her team concluded.
RPAN also is a PCR test, but with a different, perhaps less robust, genetic target.
The 102 positive swabs were from patients aged 1 month to 73 years, so “it’s important for all of us to keep pertussis on our differential diagnose” no matter how old patients are, Dr. Mayhew said.
Freezing and thawing the swabs shouldn’t have degraded the genetic material, but it might have; that was one of the limits of the study.
The team hopes to run a quality improvement project to encourage the use of standalone pertussis PCR in Ann Arbor.
There was no industry funding. Dr. Mayhew didn’t report any disclosures.
BALTIMORE – Ann Arbor.
Respiratory pathogen panels are popular because they test for many things at once, but providers have to know their limits, said lead investigator Colleen Mayhew, MD, a pediatric emergency medicine fellow at the University of Michigan.
“Should RPAN be used to diagnosis pertussis? No,” she said at the Pediatric Academic Societies annual meeting. RPAN was negative for confirmed pertussis 44% of the time in the study.
“In our cohort, [it] was no better than a coin flip for detecting pertussis,” she said. Also, even when it missed pertussis, it still detected other pathogens, which raises the risk that symptoms might be attributed to a different infection. “This has serious public health implications.”
“The bottom line is, if you are concerned about pertussis, it’s important to use a dedicated pertussis PCR [polymerase chain reaction] assay, and to use comprehensive respiratory pathogen testing only if there are other, specific targets that will change your clinical management,” such as mycoplasma or the flu, Dr. Mayhew said.
In the study, 102 nasopharyngeal swabs positive for pertussis on standalone PCR testing – the university uses an assay from Focus Diagnostics – were thawed and tested with RPAN.
RPAN was negative for pertussis on 45 swabs (44%). “These are the potential missed pertussis cases if RPAN is used alone,” Dr. Mayhew said. RPAN detected other pathogens, such as coronavirus, about half the time, whether or not it tested positive for pertussis. “Those additional pathogens might represent coinfection, but might also represent asymptomatic carriage.” It’s impossible to differentiate between the two, she noted.
In short, “neither positive testing for other respiratory pathogens, nor negative testing for pertussis by RPAN, is reliable for excluding the diagnosis of pertussis. Dedicated pertussis PCR testing should be used for diagnosis,” she and her team concluded.
RPAN also is a PCR test, but with a different, perhaps less robust, genetic target.
The 102 positive swabs were from patients aged 1 month to 73 years, so “it’s important for all of us to keep pertussis on our differential diagnose” no matter how old patients are, Dr. Mayhew said.
Freezing and thawing the swabs shouldn’t have degraded the genetic material, but it might have; that was one of the limits of the study.
The team hopes to run a quality improvement project to encourage the use of standalone pertussis PCR in Ann Arbor.
There was no industry funding. Dr. Mayhew didn’t report any disclosures.
REPORTING FROM PAS 2019
Poststroke hemorrhage risk higher with clopidogrel/aspirin than aspirin
A secondary analysis of the POINT randomized, clinical trial found that the risk of major hemorrhage associated with clopidogrel plus aspirin was higher than that with placebo plus aspirin (0.9% vs. 0.2%; hazard ratio, 3.57; 95% confidence interval, 1.44-8.85; P = .003). The study, which was published in JAMA Neurology, randomized nearly 4,900 patients at centers worldwide and concluded that overall the risk for hemorrhage was low with either treatment, although it did also find higher risk of minor hemorrhage with the combination than aspirin alone.
We previously covered results from POINT when they were presented at the World Stroke Congress, which can be found below.
SOURCE: Tillman H et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0932.
A secondary analysis of the POINT randomized, clinical trial found that the risk of major hemorrhage associated with clopidogrel plus aspirin was higher than that with placebo plus aspirin (0.9% vs. 0.2%; hazard ratio, 3.57; 95% confidence interval, 1.44-8.85; P = .003). The study, which was published in JAMA Neurology, randomized nearly 4,900 patients at centers worldwide and concluded that overall the risk for hemorrhage was low with either treatment, although it did also find higher risk of minor hemorrhage with the combination than aspirin alone.
We previously covered results from POINT when they were presented at the World Stroke Congress, which can be found below.
SOURCE: Tillman H et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0932.
A secondary analysis of the POINT randomized, clinical trial found that the risk of major hemorrhage associated with clopidogrel plus aspirin was higher than that with placebo plus aspirin (0.9% vs. 0.2%; hazard ratio, 3.57; 95% confidence interval, 1.44-8.85; P = .003). The study, which was published in JAMA Neurology, randomized nearly 4,900 patients at centers worldwide and concluded that overall the risk for hemorrhage was low with either treatment, although it did also find higher risk of minor hemorrhage with the combination than aspirin alone.
We previously covered results from POINT when they were presented at the World Stroke Congress, which can be found below.
SOURCE: Tillman H et al. JAMA Neurol. 2019 Apr 29. doi: 10.1001/jamaneurol.2019.0932.
FROM JAMA NEUROLOGY











