User login
Next-generation sequencing test detects pathogens with high sensitivity
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
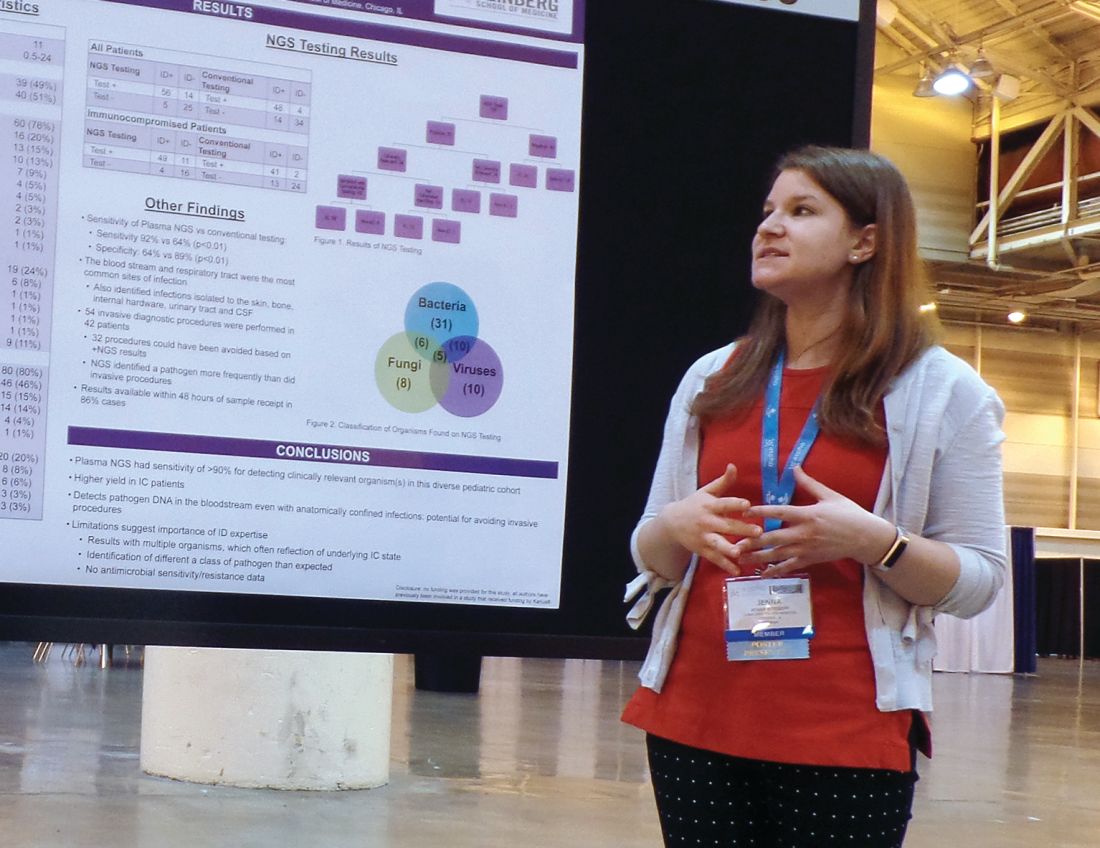
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
NEW ORLEANS – A next-generation sequencing (NGS) test for pathogen detection demonstrated higher sensitivity than conventional testing methods in a cohort of diverse pediatric patients, according to researchers.
The NGS test, which detects sequences of circulating cell-free DNA in plasma, detected pathogens with 92% sensitivity, compared with 64% sensitivity for all conventional testing methods combined (P less than .01).
“While I think we can all recognize that specificity is important, I think sensitivity is more important to be able to get at sources of infection,” said Jenna Rossoff, MD, of Ann & Robert H. Lurie Children’s Hospital of Chicago.
Dr. Rossoff and her colleagues conducted this study and presented the results in a poster at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Lurie Children’s Hospital began using a commercially available NGS pathogen test, the Karius test, in 2016. Dr. Rossoff and her colleagues set out to evaluate how the test affected patient care by conducting a retrospective analysis of tests performed from December 2016 through August 2018.
The researchers studied 100 NGS tests performed for 79 pediatric patients. The patients had a median age of 11 years (range, 0.5-24 years).
Most patients (n = 60) were immunocompromised, largely due to a hematologic malignancy (n = 16), primary immune deficiency (n = 13), hematopoietic cell transplant (n = 10), or solid organ transplant (n = 7).
The remaining 19 patients were immunocompetent, and 9 of them had no underlying diagnosis. The most common diagnosis for this group was neurologic disorder (n = 6).
Results
Of the 100 NGS tests evaluated, 70 were positive for any organism, and 56 of these were deemed clinically relevant.
“What I think is quite remarkable is that, of those clinically relevant organisms, tests on 14, which is 25% of those, were able to identify clinically relevant or pathogenic organisms when no other conventional testing modality was able to identify them,” Dr. Rossoff said. “And these were often in patients who underwent invasive procedures to try to get at the source of their infectious disease.”
In fact, the study included 42 patients who underwent 54 invasive diagnostic procedures, and 32 of those procedures could have been avoided based on positive NGS results, according to Dr. Rossoff and her colleagues.
Dr. Rossoff noted that the most common sites of infection were the bloodstream and respiratory tract, but the NGS test was able to identify pathogens in the bone, skin, cerebrospinal fluid, and urinary tract. She also pointed out that NGS results were available “in a fairly timely manner,” as 86% of test results were available within 48 hours of sample receipt.
Dr. Rossoff and her colleagues did not receive any funding for this study, but they were previously involved in a study funded by Karius, the company that commercialized the NGS test.
SOURCE: Rossoff J et al. ASPHO 2019. Abstract 439.
REPORTING FROM 2019 ASPHO CONFERENCE
Mystery hypoglycemia case highlights troublesome diagnosis
LOS ANGELES – The 69-year-old woman with a history of type 2 diabetes had persistent hypoglycemia despite treatment with hydrocortisone, dextrose, and glucagon. Doctors in South Carolina worried about insulinoma and planned to launch an intra-arterial calcium stimulation test. But the medical team wasn’t quite certain it had the correct diagnosis.
Then along came a suspicious nurse who uncovered the truth: The patient had used syringes and vials of insulin socked away in a cosmetics bag. The diagnosis? An unusual, but not entirely rare, case of factitious hypoglycemia. That doesn’t mean her condition was fictional. Instead, it means she created it herself.

In this case, he said, “the challenge with the patient was that she was denying insulin use very firmly,” and her demeanor didn’t suggest she was lying or had a mental illness. “If you saw the lady, you’d believe her.”
The patient presented with glucose levels that were repeatedly less than 40 mg/dL even though medical personnel fed her and gave her glucose. Her insulin level was high.
“The suspicion was that something in her body was producing insulin or she [was] giving herself or someone from her family was injecting her with insulin,” Dr. Aljehani said. “She denied that she was using insulin and said the last time she had used it was about 3 months earlier. Her husband and multiple family members confirmed the story.”
The results of a C-peptide test, however, suggested she was taking insulin herself. But it wasn’t conclusive.
Nurses monitored the patient during her stay of about 2 weeks. “They were keeping a good eye on her all the time, but nobody noticed anything suspicious. Then, probably 2 or 3 days before the discharge, one of the nurses had noted the patient gave her husband a bag. The nurse was able to take a look inside the bag, and she found empty insulin vials and syringes.”
The patient and her husband still denied that she was taking insulin. A psychiatric examination suggested the patient had a dissociative identity disorder and wasn’t aware she was giving herself insulin, he said.
If the patient’s insulin use hadn’t been discovered, Dr. Aljehani said, the next steps could have included more invasive testing and, potentially, removal of the pancreas.
Factitious hypoglycemia has a long history. The first case appeared in 1927, not long after the discovery of insulin, endocrinologist F.J. Service, MD, PhD, an emeritus professor of medicine at the Mayo Clinic, said in an interview.
Dr. Service, who has written about factitious hypoglycemia, offered these tips about diagnosis and treatment:
- In every patient, he said, do a drug screen for sulfonylureas. “Now that we have multiple classes of diabetes drugs, most of which have a risk for hypoglycemia, one has to have a lab capable of measuring all of them. And that is not easy. It’s not just ‘draw the blood and send to your corner lab.’ ”
- Patients with factitious hypoglycemia don’t tend to have predictable dips in blood sugar during fasting or after meals. Instead, their symptoms are chaotic. “It all depends on when they’re taking [insulin],” he said.
- Patients with factitious hypoglycemia don’t seem ill, but those with insulinomas do. “Patients with insulinomas are totally incapable of living normal lives. They’re incapacitated. Their lives are so disrupted that some of them need ‘babysitters’,” Dr. Service said. If they “get the tumor removed, they are cured. Then they are back to the normal life.”
- Beware that patients may not realize they’re taking a medication that causes factitious hypoglycemia. It’s common, Dr. Service said, for a patient to accidentally take his or her spouse’s medication because of a mix-up.
Ultimately, the goal is to catch factitious hypoglycemia in time. Some physicians haven’t been so fortunate. “They only get to the right answer,” he said, “after the patient has recovered from surgery.”
LOS ANGELES – The 69-year-old woman with a history of type 2 diabetes had persistent hypoglycemia despite treatment with hydrocortisone, dextrose, and glucagon. Doctors in South Carolina worried about insulinoma and planned to launch an intra-arterial calcium stimulation test. But the medical team wasn’t quite certain it had the correct diagnosis.
Then along came a suspicious nurse who uncovered the truth: The patient had used syringes and vials of insulin socked away in a cosmetics bag. The diagnosis? An unusual, but not entirely rare, case of factitious hypoglycemia. That doesn’t mean her condition was fictional. Instead, it means she created it herself.

In this case, he said, “the challenge with the patient was that she was denying insulin use very firmly,” and her demeanor didn’t suggest she was lying or had a mental illness. “If you saw the lady, you’d believe her.”
The patient presented with glucose levels that were repeatedly less than 40 mg/dL even though medical personnel fed her and gave her glucose. Her insulin level was high.
“The suspicion was that something in her body was producing insulin or she [was] giving herself or someone from her family was injecting her with insulin,” Dr. Aljehani said. “She denied that she was using insulin and said the last time she had used it was about 3 months earlier. Her husband and multiple family members confirmed the story.”
The results of a C-peptide test, however, suggested she was taking insulin herself. But it wasn’t conclusive.
Nurses monitored the patient during her stay of about 2 weeks. “They were keeping a good eye on her all the time, but nobody noticed anything suspicious. Then, probably 2 or 3 days before the discharge, one of the nurses had noted the patient gave her husband a bag. The nurse was able to take a look inside the bag, and she found empty insulin vials and syringes.”
The patient and her husband still denied that she was taking insulin. A psychiatric examination suggested the patient had a dissociative identity disorder and wasn’t aware she was giving herself insulin, he said.
If the patient’s insulin use hadn’t been discovered, Dr. Aljehani said, the next steps could have included more invasive testing and, potentially, removal of the pancreas.
Factitious hypoglycemia has a long history. The first case appeared in 1927, not long after the discovery of insulin, endocrinologist F.J. Service, MD, PhD, an emeritus professor of medicine at the Mayo Clinic, said in an interview.
Dr. Service, who has written about factitious hypoglycemia, offered these tips about diagnosis and treatment:
- In every patient, he said, do a drug screen for sulfonylureas. “Now that we have multiple classes of diabetes drugs, most of which have a risk for hypoglycemia, one has to have a lab capable of measuring all of them. And that is not easy. It’s not just ‘draw the blood and send to your corner lab.’ ”
- Patients with factitious hypoglycemia don’t tend to have predictable dips in blood sugar during fasting or after meals. Instead, their symptoms are chaotic. “It all depends on when they’re taking [insulin],” he said.
- Patients with factitious hypoglycemia don’t seem ill, but those with insulinomas do. “Patients with insulinomas are totally incapable of living normal lives. They’re incapacitated. Their lives are so disrupted that some of them need ‘babysitters’,” Dr. Service said. If they “get the tumor removed, they are cured. Then they are back to the normal life.”
- Beware that patients may not realize they’re taking a medication that causes factitious hypoglycemia. It’s common, Dr. Service said, for a patient to accidentally take his or her spouse’s medication because of a mix-up.
Ultimately, the goal is to catch factitious hypoglycemia in time. Some physicians haven’t been so fortunate. “They only get to the right answer,” he said, “after the patient has recovered from surgery.”
LOS ANGELES – The 69-year-old woman with a history of type 2 diabetes had persistent hypoglycemia despite treatment with hydrocortisone, dextrose, and glucagon. Doctors in South Carolina worried about insulinoma and planned to launch an intra-arterial calcium stimulation test. But the medical team wasn’t quite certain it had the correct diagnosis.
Then along came a suspicious nurse who uncovered the truth: The patient had used syringes and vials of insulin socked away in a cosmetics bag. The diagnosis? An unusual, but not entirely rare, case of factitious hypoglycemia. That doesn’t mean her condition was fictional. Instead, it means she created it herself.

In this case, he said, “the challenge with the patient was that she was denying insulin use very firmly,” and her demeanor didn’t suggest she was lying or had a mental illness. “If you saw the lady, you’d believe her.”
The patient presented with glucose levels that were repeatedly less than 40 mg/dL even though medical personnel fed her and gave her glucose. Her insulin level was high.
“The suspicion was that something in her body was producing insulin or she [was] giving herself or someone from her family was injecting her with insulin,” Dr. Aljehani said. “She denied that she was using insulin and said the last time she had used it was about 3 months earlier. Her husband and multiple family members confirmed the story.”
The results of a C-peptide test, however, suggested she was taking insulin herself. But it wasn’t conclusive.
Nurses monitored the patient during her stay of about 2 weeks. “They were keeping a good eye on her all the time, but nobody noticed anything suspicious. Then, probably 2 or 3 days before the discharge, one of the nurses had noted the patient gave her husband a bag. The nurse was able to take a look inside the bag, and she found empty insulin vials and syringes.”
The patient and her husband still denied that she was taking insulin. A psychiatric examination suggested the patient had a dissociative identity disorder and wasn’t aware she was giving herself insulin, he said.
If the patient’s insulin use hadn’t been discovered, Dr. Aljehani said, the next steps could have included more invasive testing and, potentially, removal of the pancreas.
Factitious hypoglycemia has a long history. The first case appeared in 1927, not long after the discovery of insulin, endocrinologist F.J. Service, MD, PhD, an emeritus professor of medicine at the Mayo Clinic, said in an interview.
Dr. Service, who has written about factitious hypoglycemia, offered these tips about diagnosis and treatment:
- In every patient, he said, do a drug screen for sulfonylureas. “Now that we have multiple classes of diabetes drugs, most of which have a risk for hypoglycemia, one has to have a lab capable of measuring all of them. And that is not easy. It’s not just ‘draw the blood and send to your corner lab.’ ”
- Patients with factitious hypoglycemia don’t tend to have predictable dips in blood sugar during fasting or after meals. Instead, their symptoms are chaotic. “It all depends on when they’re taking [insulin],” he said.
- Patients with factitious hypoglycemia don’t seem ill, but those with insulinomas do. “Patients with insulinomas are totally incapable of living normal lives. They’re incapacitated. Their lives are so disrupted that some of them need ‘babysitters’,” Dr. Service said. If they “get the tumor removed, they are cured. Then they are back to the normal life.”
- Beware that patients may not realize they’re taking a medication that causes factitious hypoglycemia. It’s common, Dr. Service said, for a patient to accidentally take his or her spouse’s medication because of a mix-up.
Ultimately, the goal is to catch factitious hypoglycemia in time. Some physicians haven’t been so fortunate. “They only get to the right answer,” he said, “after the patient has recovered from surgery.”
EXPERT ANALYSIS FROM AACE 2019
Sugary drink intake may be associated with MS severity
PHILADELPHIA – presented at the annual meeting of the American Academy of Neurology. Overall diet quality, however, is not associated with disability, the study showed.
The results do not establish that sugary drinks cause disability, and the potential association needs to be confirmed in larger, longitudinal studies, the researchers said. Nevertheless, “we do know that sodas have no nutritional value, and people with MS may want to consider reducing or eliminating them from their diet,” study lead author Elisa Meier-Gerdingh, MD, of St. Josef Hospital in Bochum, Germany, said in a written statement.
Diet may influence metabolic comorbidities, immune function, oxidative stress, and gut microbiota in patients with MS, but data about diet’s effects on MS progression are limited, the researchers said.
To examine dietary intake and disability in patients with MS, Dr. Meier-Gerdingh and her colleagues conducted a cross-sectional study of 135 participants with MS who were treated at a large referral center in Germany. Participants had a mean age of 44.6 years and mean body mass index of 24.5. Mean disease duration was 13.8 years, and 73% were women. Participants completed a 102-item food frequency questionnaire, which the investigators used to calculate each participant’s Dietary Approaches to Stop Hypertension (DASH) score. The score is a composite measure of dietary quality that favorably scores intake of fruits, vegetables, nuts and legumes, whole grains, and dairy and unfavorably scores intake of sodium, sugar-sweetened beverages, and red and processed meats.
“We chose to study the DASH diet because adherence to the DASH diet is associated with lower risk of other chronic diseases like high blood pressure, diabetes, and cardiovascular diseases,” Dr. Meier-Gerdingh said.
The researchers considered severe disability to be an Expanded Disability Status Scale (EDSS) score of 6 or greater. They assessed the association between overall DASH scores and disability status using logistic regression models that adjusted for age, sex, body mass index, smoking, and symptom duration. They also assessed the association between disability and each nutritional component of the DASH score.
In all, 30 participants had severe disability. “Overall DASH scores were not associated with disability status,” Dr. Meier-Gerdingh and her colleagues said. When they looked at individual DASH score components, patients with higher intakes of sugar-sweetened beverages had a higher risk of severe disability (P for trend = .01). Participants in the highest quartile consumed about 290 calories of sugar-sweetened beverages per day on average, compared with 7 calories per day for patients in the lowest quartile. The top quartile had an average EDSS of 4.1, and the bottom quartile had an average EDSS of 3.4. Other DASH score components were not associated with disability status.
Dr. Meier-Gerdingh had no disclosures. Her coauthors reported research support and personal compensation from pharmaceutical companies.
SOURCE: Meier-Gerdingh E et al. AAN 2019, Abstract P4.2-063.
PHILADELPHIA – presented at the annual meeting of the American Academy of Neurology. Overall diet quality, however, is not associated with disability, the study showed.
The results do not establish that sugary drinks cause disability, and the potential association needs to be confirmed in larger, longitudinal studies, the researchers said. Nevertheless, “we do know that sodas have no nutritional value, and people with MS may want to consider reducing or eliminating them from their diet,” study lead author Elisa Meier-Gerdingh, MD, of St. Josef Hospital in Bochum, Germany, said in a written statement.
Diet may influence metabolic comorbidities, immune function, oxidative stress, and gut microbiota in patients with MS, but data about diet’s effects on MS progression are limited, the researchers said.
To examine dietary intake and disability in patients with MS, Dr. Meier-Gerdingh and her colleagues conducted a cross-sectional study of 135 participants with MS who were treated at a large referral center in Germany. Participants had a mean age of 44.6 years and mean body mass index of 24.5. Mean disease duration was 13.8 years, and 73% were women. Participants completed a 102-item food frequency questionnaire, which the investigators used to calculate each participant’s Dietary Approaches to Stop Hypertension (DASH) score. The score is a composite measure of dietary quality that favorably scores intake of fruits, vegetables, nuts and legumes, whole grains, and dairy and unfavorably scores intake of sodium, sugar-sweetened beverages, and red and processed meats.
“We chose to study the DASH diet because adherence to the DASH diet is associated with lower risk of other chronic diseases like high blood pressure, diabetes, and cardiovascular diseases,” Dr. Meier-Gerdingh said.
The researchers considered severe disability to be an Expanded Disability Status Scale (EDSS) score of 6 or greater. They assessed the association between overall DASH scores and disability status using logistic regression models that adjusted for age, sex, body mass index, smoking, and symptom duration. They also assessed the association between disability and each nutritional component of the DASH score.
In all, 30 participants had severe disability. “Overall DASH scores were not associated with disability status,” Dr. Meier-Gerdingh and her colleagues said. When they looked at individual DASH score components, patients with higher intakes of sugar-sweetened beverages had a higher risk of severe disability (P for trend = .01). Participants in the highest quartile consumed about 290 calories of sugar-sweetened beverages per day on average, compared with 7 calories per day for patients in the lowest quartile. The top quartile had an average EDSS of 4.1, and the bottom quartile had an average EDSS of 3.4. Other DASH score components were not associated with disability status.
Dr. Meier-Gerdingh had no disclosures. Her coauthors reported research support and personal compensation from pharmaceutical companies.
SOURCE: Meier-Gerdingh E et al. AAN 2019, Abstract P4.2-063.
PHILADELPHIA – presented at the annual meeting of the American Academy of Neurology. Overall diet quality, however, is not associated with disability, the study showed.
The results do not establish that sugary drinks cause disability, and the potential association needs to be confirmed in larger, longitudinal studies, the researchers said. Nevertheless, “we do know that sodas have no nutritional value, and people with MS may want to consider reducing or eliminating them from their diet,” study lead author Elisa Meier-Gerdingh, MD, of St. Josef Hospital in Bochum, Germany, said in a written statement.
Diet may influence metabolic comorbidities, immune function, oxidative stress, and gut microbiota in patients with MS, but data about diet’s effects on MS progression are limited, the researchers said.
To examine dietary intake and disability in patients with MS, Dr. Meier-Gerdingh and her colleagues conducted a cross-sectional study of 135 participants with MS who were treated at a large referral center in Germany. Participants had a mean age of 44.6 years and mean body mass index of 24.5. Mean disease duration was 13.8 years, and 73% were women. Participants completed a 102-item food frequency questionnaire, which the investigators used to calculate each participant’s Dietary Approaches to Stop Hypertension (DASH) score. The score is a composite measure of dietary quality that favorably scores intake of fruits, vegetables, nuts and legumes, whole grains, and dairy and unfavorably scores intake of sodium, sugar-sweetened beverages, and red and processed meats.
“We chose to study the DASH diet because adherence to the DASH diet is associated with lower risk of other chronic diseases like high blood pressure, diabetes, and cardiovascular diseases,” Dr. Meier-Gerdingh said.
The researchers considered severe disability to be an Expanded Disability Status Scale (EDSS) score of 6 or greater. They assessed the association between overall DASH scores and disability status using logistic regression models that adjusted for age, sex, body mass index, smoking, and symptom duration. They also assessed the association between disability and each nutritional component of the DASH score.
In all, 30 participants had severe disability. “Overall DASH scores were not associated with disability status,” Dr. Meier-Gerdingh and her colleagues said. When they looked at individual DASH score components, patients with higher intakes of sugar-sweetened beverages had a higher risk of severe disability (P for trend = .01). Participants in the highest quartile consumed about 290 calories of sugar-sweetened beverages per day on average, compared with 7 calories per day for patients in the lowest quartile. The top quartile had an average EDSS of 4.1, and the bottom quartile had an average EDSS of 3.4. Other DASH score components were not associated with disability status.
Dr. Meier-Gerdingh had no disclosures. Her coauthors reported research support and personal compensation from pharmaceutical companies.
SOURCE: Meier-Gerdingh E et al. AAN 2019, Abstract P4.2-063.
REPORTING FROM AAN 2019
Emergency Protocol Improves Survival After Severe Head Injury
Preventing low oxygen, low blood pressure, and hyperventilation in people with head injury has been shown to improve survival, according to observational studies. The guidelines for prehospital management of traumatic brain injury (TBI), developed in 2000, were updated in 2007 to reflect those findings. But are they being followed? And if followed, do they help?
The Excellence in Prehospital Injury Care (EPIC) study, the first time the guidelines were assessed in real-world conditions, trained EMS responders in Arizona and compared patient outcomes before and after the guideline implementation.
The study researchers found “a therapeutic sweet spot” in that the guidelines had an “enormous impact” on people with severe TBI. Implementing the guidelines did not affect overall survival of the entire group, which included > 21,000 patients with moderate, severe, and critical injuries. But further analysis showed that they helped double the survival rate of people with severe TBI and tripled the survival rate in severe TBI patients who had to have a breathing tube inserted by EMS personnel.
Daniel Spaite, MD, who led the study, said the patients with moderate injuries would most likely have survived anyway, and those in critical condition may have had injuries too serious to overcome.
The guidelines also were associated with an overall increase in survival to hospital admission.
According to Bentley Bobrow, MD, co-principal investigator, “It was exciting to see such dramatic outcomes resulting from a simple 2-hour training session with EMS personnel.”
The study “demonstrates the significance of conducting studies in real-world settings and brings a strong evidence base to the guidelines,” said Patrick Bellgowan, PhD, program director at the National Institute of Neurological Disorders and Stroke, which supported the study. “It suggests we can systematically increase the chances of saving lives of thousands of people who suffer severe traumatic brain injuries.”
Preventing low oxygen, low blood pressure, and hyperventilation in people with head injury has been shown to improve survival, according to observational studies. The guidelines for prehospital management of traumatic brain injury (TBI), developed in 2000, were updated in 2007 to reflect those findings. But are they being followed? And if followed, do they help?
The Excellence in Prehospital Injury Care (EPIC) study, the first time the guidelines were assessed in real-world conditions, trained EMS responders in Arizona and compared patient outcomes before and after the guideline implementation.
The study researchers found “a therapeutic sweet spot” in that the guidelines had an “enormous impact” on people with severe TBI. Implementing the guidelines did not affect overall survival of the entire group, which included > 21,000 patients with moderate, severe, and critical injuries. But further analysis showed that they helped double the survival rate of people with severe TBI and tripled the survival rate in severe TBI patients who had to have a breathing tube inserted by EMS personnel.
Daniel Spaite, MD, who led the study, said the patients with moderate injuries would most likely have survived anyway, and those in critical condition may have had injuries too serious to overcome.
The guidelines also were associated with an overall increase in survival to hospital admission.
According to Bentley Bobrow, MD, co-principal investigator, “It was exciting to see such dramatic outcomes resulting from a simple 2-hour training session with EMS personnel.”
The study “demonstrates the significance of conducting studies in real-world settings and brings a strong evidence base to the guidelines,” said Patrick Bellgowan, PhD, program director at the National Institute of Neurological Disorders and Stroke, which supported the study. “It suggests we can systematically increase the chances of saving lives of thousands of people who suffer severe traumatic brain injuries.”
Preventing low oxygen, low blood pressure, and hyperventilation in people with head injury has been shown to improve survival, according to observational studies. The guidelines for prehospital management of traumatic brain injury (TBI), developed in 2000, were updated in 2007 to reflect those findings. But are they being followed? And if followed, do they help?
The Excellence in Prehospital Injury Care (EPIC) study, the first time the guidelines were assessed in real-world conditions, trained EMS responders in Arizona and compared patient outcomes before and after the guideline implementation.
The study researchers found “a therapeutic sweet spot” in that the guidelines had an “enormous impact” on people with severe TBI. Implementing the guidelines did not affect overall survival of the entire group, which included > 21,000 patients with moderate, severe, and critical injuries. But further analysis showed that they helped double the survival rate of people with severe TBI and tripled the survival rate in severe TBI patients who had to have a breathing tube inserted by EMS personnel.
Daniel Spaite, MD, who led the study, said the patients with moderate injuries would most likely have survived anyway, and those in critical condition may have had injuries too serious to overcome.
The guidelines also were associated with an overall increase in survival to hospital admission.
According to Bentley Bobrow, MD, co-principal investigator, “It was exciting to see such dramatic outcomes resulting from a simple 2-hour training session with EMS personnel.”
The study “demonstrates the significance of conducting studies in real-world settings and brings a strong evidence base to the guidelines,” said Patrick Bellgowan, PhD, program director at the National Institute of Neurological Disorders and Stroke, which supported the study. “It suggests we can systematically increase the chances of saving lives of thousands of people who suffer severe traumatic brain injuries.”
New JIA, JIA-associated uveitis guidelines address knowledge gaps
while attempting to address gaps in the clinical screening, monitoring, and treatment of these diseases.
The first guideline – which was published simultaneously in Arthritis Care & Research and Arthritis & Rheumatology – offered 39 recommendations for treating children and adolescents with JIA and nonsystemic polyarthritis, sacroiliitis, and enthesitis. Due to the low quality of the supporting evidence, 31 of the recommendations were labeled as conditional.
“While these recommendations are intended to address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences,” wrote first author Sarah Ringold, MD, of Seattle Children’s Hospital and her coauthors.
These recommendations serve as updates to an initial set on JIA treatment in 2011 and a 2013 addition on the treatment of systemic arthritis. In addition, the ACR has since adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to generate its recommendations, which was described in an interview with Dr. Ringold as providing “greater transparency around the decisions made during the recommendation development process.”
“An important difference from the 2011 guidelines is that is that initial NSAID monotherapy is no longer recommended for children with polyarthritis, given the established benefits of early initiation of DMARD [disease-modifying antirheumatic drug] therapy for these patients,” she added. “In addition, these guidelines also support inactive disease as a treatment goal for children with polyarthritis, with treatment escalation recommended for patients with low disease activity.”
As general recommendations for patients with JIA and polyarthritis, the coauthors strongly recommend using triamcinolone hexacetonide over triamcinolone acetonide for intraarticular glucocorticoid injections, along with using infliximab in combination with a DMARD. Despite the quality of the supporting evidence being very low, they also strongly recommend against adding chronic low-dose glucocorticoids to treatment because of well-known adverse effects like growth suppression, weight gain, osteopenia, and cataract.
Other strong recommendations include choosing treatment with an NSAID in children and adolescents with JIA and active sacroiliitis; adding a tumor necrosis factor inhibitor (TNFi) rather than continued NSAID monotherapy and avoiding methotrexate monotherapy in children and adolescents with active sacroiliitis despite NSAIDs; and choosing NSAID treatment in children and adolescents with JIA and active enthesitis.
Recommendations on JIA with associated uveitis
The second guideline – also published in Arthritis Care & Research and Arthritis & Rheumatology – focused on the screening, monitoring, and treatment of JIA with associated uveitis. Their 19 recommendations serve as updates to 2006 recommendations from the American Academy of Pediatrics on routine ophthalmic screening in children with arthritis and 2012 recommendations from the German Uveitis in Childhood Study Group on proposed ophthalmic screening schedules, neither of which addressed the monitoring of children with an established diagnosis of uveitis or the treatment of uveitis.
“Although the quality of evidence was very low, and most recommendations were therefore conditional, this guideline fills an important clinical gap in the care of children with JIA-associated uveitis and may be updated as better evidence becomes available,” wrote first author Sheila T. Angeles-Han, MD, of the Cincinnati Children’s Hospital Medical Center and her coauthors.
Their strong recommendations include ophthalmic monitoring at least every 3 months in children and adolescents with JIA and controlled uveitis on stable therapy; monitoring within 1 month after each change of topical glucocorticoids in patients who are tapering or discontinuing that treatment; and monitoring within 2 months of changing systemic therapy for patients who are tapering or discontinuing that treatment.
They also strongly recommend education on the warning signs of acute anterior uveitis for children and adolescents with spondyloarthritis, along with tapering topical glucocorticoids before systemic therapy in children and adolescents with JIA and chronic anterior uveitis who are still on 1-2 drops a day of glucocorticoids.
“Our biggest message is that it is critical that uveitis is controlled, since persistent active uveitis can lead to sight-threatening complications and permanent vision loss,” Dr. Angeles-Han said in an interview. “It is important that ocular inflammation is controlled early, exposure to long-term topical glucocorticoids is limited, and that systemic treatment is started promptly.
“We also emphasize that close communication and collaboration between pediatric rheumatologists and ophthalmologists is important to ensure optimal vision outcomes,” she added.
These guidelines also factored in feedback from a patient and parent panel, particularly in regard to recommendations with a low level of supporting evidence.
“This partnership highlighted the importance of incorporating parent and patient preferences into treatment decisions and the need for shared decision-making approaches,” Dr. Ringold said.
Both guidelines were supported by the American College of Rheumatology and the Arthritis Foundation. Several authors reported support from the National Institutes of Health, the Rheumatology Research Foundation, and the Fundación Bechara. Several authors also reported receiving consulting and speaking fees, along with research grants, from numerous pharmaceutical companies.
SOURCES: Ringold S et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23870 ; Angeles-Han ST et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23871 .
while attempting to address gaps in the clinical screening, monitoring, and treatment of these diseases.
The first guideline – which was published simultaneously in Arthritis Care & Research and Arthritis & Rheumatology – offered 39 recommendations for treating children and adolescents with JIA and nonsystemic polyarthritis, sacroiliitis, and enthesitis. Due to the low quality of the supporting evidence, 31 of the recommendations were labeled as conditional.
“While these recommendations are intended to address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences,” wrote first author Sarah Ringold, MD, of Seattle Children’s Hospital and her coauthors.
These recommendations serve as updates to an initial set on JIA treatment in 2011 and a 2013 addition on the treatment of systemic arthritis. In addition, the ACR has since adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to generate its recommendations, which was described in an interview with Dr. Ringold as providing “greater transparency around the decisions made during the recommendation development process.”
“An important difference from the 2011 guidelines is that is that initial NSAID monotherapy is no longer recommended for children with polyarthritis, given the established benefits of early initiation of DMARD [disease-modifying antirheumatic drug] therapy for these patients,” she added. “In addition, these guidelines also support inactive disease as a treatment goal for children with polyarthritis, with treatment escalation recommended for patients with low disease activity.”
As general recommendations for patients with JIA and polyarthritis, the coauthors strongly recommend using triamcinolone hexacetonide over triamcinolone acetonide for intraarticular glucocorticoid injections, along with using infliximab in combination with a DMARD. Despite the quality of the supporting evidence being very low, they also strongly recommend against adding chronic low-dose glucocorticoids to treatment because of well-known adverse effects like growth suppression, weight gain, osteopenia, and cataract.
Other strong recommendations include choosing treatment with an NSAID in children and adolescents with JIA and active sacroiliitis; adding a tumor necrosis factor inhibitor (TNFi) rather than continued NSAID monotherapy and avoiding methotrexate monotherapy in children and adolescents with active sacroiliitis despite NSAIDs; and choosing NSAID treatment in children and adolescents with JIA and active enthesitis.
Recommendations on JIA with associated uveitis
The second guideline – also published in Arthritis Care & Research and Arthritis & Rheumatology – focused on the screening, monitoring, and treatment of JIA with associated uveitis. Their 19 recommendations serve as updates to 2006 recommendations from the American Academy of Pediatrics on routine ophthalmic screening in children with arthritis and 2012 recommendations from the German Uveitis in Childhood Study Group on proposed ophthalmic screening schedules, neither of which addressed the monitoring of children with an established diagnosis of uveitis or the treatment of uveitis.
“Although the quality of evidence was very low, and most recommendations were therefore conditional, this guideline fills an important clinical gap in the care of children with JIA-associated uveitis and may be updated as better evidence becomes available,” wrote first author Sheila T. Angeles-Han, MD, of the Cincinnati Children’s Hospital Medical Center and her coauthors.
Their strong recommendations include ophthalmic monitoring at least every 3 months in children and adolescents with JIA and controlled uveitis on stable therapy; monitoring within 1 month after each change of topical glucocorticoids in patients who are tapering or discontinuing that treatment; and monitoring within 2 months of changing systemic therapy for patients who are tapering or discontinuing that treatment.
They also strongly recommend education on the warning signs of acute anterior uveitis for children and adolescents with spondyloarthritis, along with tapering topical glucocorticoids before systemic therapy in children and adolescents with JIA and chronic anterior uveitis who are still on 1-2 drops a day of glucocorticoids.
“Our biggest message is that it is critical that uveitis is controlled, since persistent active uveitis can lead to sight-threatening complications and permanent vision loss,” Dr. Angeles-Han said in an interview. “It is important that ocular inflammation is controlled early, exposure to long-term topical glucocorticoids is limited, and that systemic treatment is started promptly.
“We also emphasize that close communication and collaboration between pediatric rheumatologists and ophthalmologists is important to ensure optimal vision outcomes,” she added.
These guidelines also factored in feedback from a patient and parent panel, particularly in regard to recommendations with a low level of supporting evidence.
“This partnership highlighted the importance of incorporating parent and patient preferences into treatment decisions and the need for shared decision-making approaches,” Dr. Ringold said.
Both guidelines were supported by the American College of Rheumatology and the Arthritis Foundation. Several authors reported support from the National Institutes of Health, the Rheumatology Research Foundation, and the Fundación Bechara. Several authors also reported receiving consulting and speaking fees, along with research grants, from numerous pharmaceutical companies.
SOURCES: Ringold S et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23870 ; Angeles-Han ST et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23871 .
while attempting to address gaps in the clinical screening, monitoring, and treatment of these diseases.
The first guideline – which was published simultaneously in Arthritis Care & Research and Arthritis & Rheumatology – offered 39 recommendations for treating children and adolescents with JIA and nonsystemic polyarthritis, sacroiliitis, and enthesitis. Due to the low quality of the supporting evidence, 31 of the recommendations were labeled as conditional.
“While these recommendations are intended to address common clinical situations, all treatment decisions must be individualized, with consideration of the unique aspects of each patient’s presentation, medical history, and preferences,” wrote first author Sarah Ringold, MD, of Seattle Children’s Hospital and her coauthors.
These recommendations serve as updates to an initial set on JIA treatment in 2011 and a 2013 addition on the treatment of systemic arthritis. In addition, the ACR has since adopted the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to generate its recommendations, which was described in an interview with Dr. Ringold as providing “greater transparency around the decisions made during the recommendation development process.”
“An important difference from the 2011 guidelines is that is that initial NSAID monotherapy is no longer recommended for children with polyarthritis, given the established benefits of early initiation of DMARD [disease-modifying antirheumatic drug] therapy for these patients,” she added. “In addition, these guidelines also support inactive disease as a treatment goal for children with polyarthritis, with treatment escalation recommended for patients with low disease activity.”
As general recommendations for patients with JIA and polyarthritis, the coauthors strongly recommend using triamcinolone hexacetonide over triamcinolone acetonide for intraarticular glucocorticoid injections, along with using infliximab in combination with a DMARD. Despite the quality of the supporting evidence being very low, they also strongly recommend against adding chronic low-dose glucocorticoids to treatment because of well-known adverse effects like growth suppression, weight gain, osteopenia, and cataract.
Other strong recommendations include choosing treatment with an NSAID in children and adolescents with JIA and active sacroiliitis; adding a tumor necrosis factor inhibitor (TNFi) rather than continued NSAID monotherapy and avoiding methotrexate monotherapy in children and adolescents with active sacroiliitis despite NSAIDs; and choosing NSAID treatment in children and adolescents with JIA and active enthesitis.
Recommendations on JIA with associated uveitis
The second guideline – also published in Arthritis Care & Research and Arthritis & Rheumatology – focused on the screening, monitoring, and treatment of JIA with associated uveitis. Their 19 recommendations serve as updates to 2006 recommendations from the American Academy of Pediatrics on routine ophthalmic screening in children with arthritis and 2012 recommendations from the German Uveitis in Childhood Study Group on proposed ophthalmic screening schedules, neither of which addressed the monitoring of children with an established diagnosis of uveitis or the treatment of uveitis.
“Although the quality of evidence was very low, and most recommendations were therefore conditional, this guideline fills an important clinical gap in the care of children with JIA-associated uveitis and may be updated as better evidence becomes available,” wrote first author Sheila T. Angeles-Han, MD, of the Cincinnati Children’s Hospital Medical Center and her coauthors.
Their strong recommendations include ophthalmic monitoring at least every 3 months in children and adolescents with JIA and controlled uveitis on stable therapy; monitoring within 1 month after each change of topical glucocorticoids in patients who are tapering or discontinuing that treatment; and monitoring within 2 months of changing systemic therapy for patients who are tapering or discontinuing that treatment.
They also strongly recommend education on the warning signs of acute anterior uveitis for children and adolescents with spondyloarthritis, along with tapering topical glucocorticoids before systemic therapy in children and adolescents with JIA and chronic anterior uveitis who are still on 1-2 drops a day of glucocorticoids.
“Our biggest message is that it is critical that uveitis is controlled, since persistent active uveitis can lead to sight-threatening complications and permanent vision loss,” Dr. Angeles-Han said in an interview. “It is important that ocular inflammation is controlled early, exposure to long-term topical glucocorticoids is limited, and that systemic treatment is started promptly.
“We also emphasize that close communication and collaboration between pediatric rheumatologists and ophthalmologists is important to ensure optimal vision outcomes,” she added.
These guidelines also factored in feedback from a patient and parent panel, particularly in regard to recommendations with a low level of supporting evidence.
“This partnership highlighted the importance of incorporating parent and patient preferences into treatment decisions and the need for shared decision-making approaches,” Dr. Ringold said.
Both guidelines were supported by the American College of Rheumatology and the Arthritis Foundation. Several authors reported support from the National Institutes of Health, the Rheumatology Research Foundation, and the Fundación Bechara. Several authors also reported receiving consulting and speaking fees, along with research grants, from numerous pharmaceutical companies.
SOURCES: Ringold S et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23870 ; Angeles-Han ST et al. Arthritis Care Res. 2019 Apr 25. doi: 10.1002/acr.23871 .
FROM ARTHRITIS CARE & RESEARCH
Biomarker-based score predicts poor outcomes after acute ischemic stroke
PHILADELPHIA – A prognostic score for acute ischemic stroke that incorporates copeptin levels, age, recanalization, and National Institutes of Health Stroke Scale score has been externally validated and accurately predicts unfavorable outcome, according to research presented at the annual meeting of the American Academy of Neurology.
Although the four-item score could not be validated for mortality prediction, it had reasonable accuracy for predicting unfavorable functional outcome, defined as disability or mortality 3 months after ischemic stroke, Gian Marco De Marchis, MD, of the department of neurology and the stroke center at University Hospital Basel (Switzerland), said in a presentation.
“The use of a biomarker increases prognostic accuracy, allowing us to personalize prognosis in the frame of individualized, precision medicine,” Dr. De Marchis said.
Copeptin has been linked to disability and mortality at 3 months in two independent, large cohort studies of patients with ischemic stroke, he said.
The four-item prognostic score devised by Dr. De Marchis and his coinvestigators, which they call the CoRisk score, was developed based on a derivation cohort of 319 acute ischemic stroke patients and a validation cohort including another 783 patients in the Copeptin for Risk Stratification in Acute Stroke Patients (CoRisk) Study.
Diagnostic accuracy was 82% for the endpoint of unfavorable functional outcome at 3 months, according to Dr. De Marchis.
“The observed outcomes matched well with the expected outcomes,” he said in his presentation.
Further analyses demonstrated that the addition of copeptin indeed contributed to the diagnostic accuracy of the score, improving the classification for 46%; in other words, about half of the patients were reclassified based on addition of the biomarker data.
By contrast, the score is not well suited to predict mortality alone at 3 months, the results of the analyses showed.
The algorithm used to calculate the score based on its four variables is somewhat complex, but available as a free app and online calculator, Dr. De Marchis said.
Dr. De Marchis and his coauthors had nothing to disclose related to their study. A full report on the study was published ahead of print on March 1 in Neurology.
SOURCE: De Marchis GM et al. AAN 2019, Abstract S47.001.
PHILADELPHIA – A prognostic score for acute ischemic stroke that incorporates copeptin levels, age, recanalization, and National Institutes of Health Stroke Scale score has been externally validated and accurately predicts unfavorable outcome, according to research presented at the annual meeting of the American Academy of Neurology.
Although the four-item score could not be validated for mortality prediction, it had reasonable accuracy for predicting unfavorable functional outcome, defined as disability or mortality 3 months after ischemic stroke, Gian Marco De Marchis, MD, of the department of neurology and the stroke center at University Hospital Basel (Switzerland), said in a presentation.
“The use of a biomarker increases prognostic accuracy, allowing us to personalize prognosis in the frame of individualized, precision medicine,” Dr. De Marchis said.
Copeptin has been linked to disability and mortality at 3 months in two independent, large cohort studies of patients with ischemic stroke, he said.
The four-item prognostic score devised by Dr. De Marchis and his coinvestigators, which they call the CoRisk score, was developed based on a derivation cohort of 319 acute ischemic stroke patients and a validation cohort including another 783 patients in the Copeptin for Risk Stratification in Acute Stroke Patients (CoRisk) Study.
Diagnostic accuracy was 82% for the endpoint of unfavorable functional outcome at 3 months, according to Dr. De Marchis.
“The observed outcomes matched well with the expected outcomes,” he said in his presentation.
Further analyses demonstrated that the addition of copeptin indeed contributed to the diagnostic accuracy of the score, improving the classification for 46%; in other words, about half of the patients were reclassified based on addition of the biomarker data.
By contrast, the score is not well suited to predict mortality alone at 3 months, the results of the analyses showed.
The algorithm used to calculate the score based on its four variables is somewhat complex, but available as a free app and online calculator, Dr. De Marchis said.
Dr. De Marchis and his coauthors had nothing to disclose related to their study. A full report on the study was published ahead of print on March 1 in Neurology.
SOURCE: De Marchis GM et al. AAN 2019, Abstract S47.001.
PHILADELPHIA – A prognostic score for acute ischemic stroke that incorporates copeptin levels, age, recanalization, and National Institutes of Health Stroke Scale score has been externally validated and accurately predicts unfavorable outcome, according to research presented at the annual meeting of the American Academy of Neurology.
Although the four-item score could not be validated for mortality prediction, it had reasonable accuracy for predicting unfavorable functional outcome, defined as disability or mortality 3 months after ischemic stroke, Gian Marco De Marchis, MD, of the department of neurology and the stroke center at University Hospital Basel (Switzerland), said in a presentation.
“The use of a biomarker increases prognostic accuracy, allowing us to personalize prognosis in the frame of individualized, precision medicine,” Dr. De Marchis said.
Copeptin has been linked to disability and mortality at 3 months in two independent, large cohort studies of patients with ischemic stroke, he said.
The four-item prognostic score devised by Dr. De Marchis and his coinvestigators, which they call the CoRisk score, was developed based on a derivation cohort of 319 acute ischemic stroke patients and a validation cohort including another 783 patients in the Copeptin for Risk Stratification in Acute Stroke Patients (CoRisk) Study.
Diagnostic accuracy was 82% for the endpoint of unfavorable functional outcome at 3 months, according to Dr. De Marchis.
“The observed outcomes matched well with the expected outcomes,” he said in his presentation.
Further analyses demonstrated that the addition of copeptin indeed contributed to the diagnostic accuracy of the score, improving the classification for 46%; in other words, about half of the patients were reclassified based on addition of the biomarker data.
By contrast, the score is not well suited to predict mortality alone at 3 months, the results of the analyses showed.
The algorithm used to calculate the score based on its four variables is somewhat complex, but available as a free app and online calculator, Dr. De Marchis said.
Dr. De Marchis and his coauthors had nothing to disclose related to their study. A full report on the study was published ahead of print on March 1 in Neurology.
SOURCE: De Marchis GM et al. AAN 2019, Abstract S47.001.
REPORTING FROM AAN 2019
Medicaid coverage decreased in Arkansas following work requirements
WASHINGTON – Early analysis of the implementation of Medicaid work requirements suggests administrative burden on beneficiaries is leading to decreased coverage.
according to state reports.
A study of the early months after Arkansas put its work requirement into effect in June 2018 found those most affected were people already working but unaware of or unable to meet the administrative requirements to demonstrate they were working. These people lost coverage.
Researchers identified “lots of confusion,” Anna Goldman, MD, of Harvard University, Boston, told attendees at the annual meeting of the Society of General Internal Medicine 2019 conference. “People didn’t know if they should report, or if they thought they should, they didn’t know how.”
Additionally, there is no evidence that the implementation of the work rules caused an increase of employment within the Medicaid-eligible population in Arkansas within the first 6 months of the law going into effect.
Arkansas’s rules required individuals aged 30-49 years (applied during the first 6 months of the rollout) to work at least 80 hours a month in a job or doing something equivalent to a job, such as job skills training. Exemptions were available to those who were disabled, pregnant, or caregiving; had student status; or were receiving treatment for substance abuse. Individuals were required to report their work hours each month. Failure to do so for 3 months in a year would cause Medicaid benefits to be suspended for the balance of the year.
Dr. Goldman noted that phone surveys were done in four states (Arkansas, Kentucky, Louisiana, and Texas) of low-income adults earning up to 138% of the federal poverty limit. About 6,000 people were surveyed across the four states, with half coming from 2016 and the other from a survey in 2018. In both years, the surveys were conducted in November and December of survey years. She acknowledged a low response rate of 14%, but she said that weighting was used to minimize nonresponse bias.
With the introduction of the work requirements, researchers found a 13.2% decrease in Medicaid or marketplace insurance and a 7.1% increase in the uninsured rate. No change in employment rates were found.
She noted that the findings that employment didn’t change were not surprising “given that 95% of people were either exempt or working, according to our survey results.”
Dr. Goldman said the survey completed for this study was consistent with studies done in Arkansas that showed most people that would be covered by the work rule were either working or would be exempt from the work requirements laid forth by state regulation.
“Those studies had raised an initial concern that most of the coverage losses would be related to nonreporting and administrative issues as opposed to just removing people who were healthy and didn’t want to get a job,” she explained.
Dr. Goldman noted that this newer study found that of the people required to report their work activities to the state, half didn’t because they either had no Internet access or were confused and could not figure out how to use the reporting system, even though they met the work requirements necessary to keep their Medicaid coverage.
In general, about 52% of the Arkansas population surveyed had heard something about the work requirements, but of those in the age range targeted by the rules, only 22% thought they were subject to the work requirements. Of those informed by the state that they needed to report, only half said they were doing so, Dr. Goldman added.
The survey results were consistent with state reports on the number of people experiencing coverage loss after the rollout, she said.
“One important thing to take away is that information barriers and administrative hassles are key reasons why people are losing coverage. ... Therefore, anything that states can do to automatically exempt people using state data that they already have can possibly reduce unnecessary coverage losses,” Dr. Goldman concluded.
Arkansas’s Medicaid work requirements were blocked by a federal judge in March 2019. Appeals are ongoing.
The authors reported no disclosures.
WASHINGTON – Early analysis of the implementation of Medicaid work requirements suggests administrative burden on beneficiaries is leading to decreased coverage.
according to state reports.
A study of the early months after Arkansas put its work requirement into effect in June 2018 found those most affected were people already working but unaware of or unable to meet the administrative requirements to demonstrate they were working. These people lost coverage.
Researchers identified “lots of confusion,” Anna Goldman, MD, of Harvard University, Boston, told attendees at the annual meeting of the Society of General Internal Medicine 2019 conference. “People didn’t know if they should report, or if they thought they should, they didn’t know how.”
Additionally, there is no evidence that the implementation of the work rules caused an increase of employment within the Medicaid-eligible population in Arkansas within the first 6 months of the law going into effect.
Arkansas’s rules required individuals aged 30-49 years (applied during the first 6 months of the rollout) to work at least 80 hours a month in a job or doing something equivalent to a job, such as job skills training. Exemptions were available to those who were disabled, pregnant, or caregiving; had student status; or were receiving treatment for substance abuse. Individuals were required to report their work hours each month. Failure to do so for 3 months in a year would cause Medicaid benefits to be suspended for the balance of the year.
Dr. Goldman noted that phone surveys were done in four states (Arkansas, Kentucky, Louisiana, and Texas) of low-income adults earning up to 138% of the federal poverty limit. About 6,000 people were surveyed across the four states, with half coming from 2016 and the other from a survey in 2018. In both years, the surveys were conducted in November and December of survey years. She acknowledged a low response rate of 14%, but she said that weighting was used to minimize nonresponse bias.
With the introduction of the work requirements, researchers found a 13.2% decrease in Medicaid or marketplace insurance and a 7.1% increase in the uninsured rate. No change in employment rates were found.
She noted that the findings that employment didn’t change were not surprising “given that 95% of people were either exempt or working, according to our survey results.”
Dr. Goldman said the survey completed for this study was consistent with studies done in Arkansas that showed most people that would be covered by the work rule were either working or would be exempt from the work requirements laid forth by state regulation.
“Those studies had raised an initial concern that most of the coverage losses would be related to nonreporting and administrative issues as opposed to just removing people who were healthy and didn’t want to get a job,” she explained.
Dr. Goldman noted that this newer study found that of the people required to report their work activities to the state, half didn’t because they either had no Internet access or were confused and could not figure out how to use the reporting system, even though they met the work requirements necessary to keep their Medicaid coverage.
In general, about 52% of the Arkansas population surveyed had heard something about the work requirements, but of those in the age range targeted by the rules, only 22% thought they were subject to the work requirements. Of those informed by the state that they needed to report, only half said they were doing so, Dr. Goldman added.
The survey results were consistent with state reports on the number of people experiencing coverage loss after the rollout, she said.
“One important thing to take away is that information barriers and administrative hassles are key reasons why people are losing coverage. ... Therefore, anything that states can do to automatically exempt people using state data that they already have can possibly reduce unnecessary coverage losses,” Dr. Goldman concluded.
Arkansas’s Medicaid work requirements were blocked by a federal judge in March 2019. Appeals are ongoing.
The authors reported no disclosures.
WASHINGTON – Early analysis of the implementation of Medicaid work requirements suggests administrative burden on beneficiaries is leading to decreased coverage.
according to state reports.
A study of the early months after Arkansas put its work requirement into effect in June 2018 found those most affected were people already working but unaware of or unable to meet the administrative requirements to demonstrate they were working. These people lost coverage.
Researchers identified “lots of confusion,” Anna Goldman, MD, of Harvard University, Boston, told attendees at the annual meeting of the Society of General Internal Medicine 2019 conference. “People didn’t know if they should report, or if they thought they should, they didn’t know how.”
Additionally, there is no evidence that the implementation of the work rules caused an increase of employment within the Medicaid-eligible population in Arkansas within the first 6 months of the law going into effect.
Arkansas’s rules required individuals aged 30-49 years (applied during the first 6 months of the rollout) to work at least 80 hours a month in a job or doing something equivalent to a job, such as job skills training. Exemptions were available to those who were disabled, pregnant, or caregiving; had student status; or were receiving treatment for substance abuse. Individuals were required to report their work hours each month. Failure to do so for 3 months in a year would cause Medicaid benefits to be suspended for the balance of the year.
Dr. Goldman noted that phone surveys were done in four states (Arkansas, Kentucky, Louisiana, and Texas) of low-income adults earning up to 138% of the federal poverty limit. About 6,000 people were surveyed across the four states, with half coming from 2016 and the other from a survey in 2018. In both years, the surveys were conducted in November and December of survey years. She acknowledged a low response rate of 14%, but she said that weighting was used to minimize nonresponse bias.
With the introduction of the work requirements, researchers found a 13.2% decrease in Medicaid or marketplace insurance and a 7.1% increase in the uninsured rate. No change in employment rates were found.
She noted that the findings that employment didn’t change were not surprising “given that 95% of people were either exempt or working, according to our survey results.”
Dr. Goldman said the survey completed for this study was consistent with studies done in Arkansas that showed most people that would be covered by the work rule were either working or would be exempt from the work requirements laid forth by state regulation.
“Those studies had raised an initial concern that most of the coverage losses would be related to nonreporting and administrative issues as opposed to just removing people who were healthy and didn’t want to get a job,” she explained.
Dr. Goldman noted that this newer study found that of the people required to report their work activities to the state, half didn’t because they either had no Internet access or were confused and could not figure out how to use the reporting system, even though they met the work requirements necessary to keep their Medicaid coverage.
In general, about 52% of the Arkansas population surveyed had heard something about the work requirements, but of those in the age range targeted by the rules, only 22% thought they were subject to the work requirements. Of those informed by the state that they needed to report, only half said they were doing so, Dr. Goldman added.
The survey results were consistent with state reports on the number of people experiencing coverage loss after the rollout, she said.
“One important thing to take away is that information barriers and administrative hassles are key reasons why people are losing coverage. ... Therefore, anything that states can do to automatically exempt people using state data that they already have can possibly reduce unnecessary coverage losses,” Dr. Goldman concluded.
Arkansas’s Medicaid work requirements were blocked by a federal judge in March 2019. Appeals are ongoing.
The authors reported no disclosures.
REPORTING FROM SGIM 2019
Atypical case of cutaneous MCL mimics SPTCL
An atypical case of cutaneous mantle cell lymphoma (MCL) with histomorphological features mimicking subcutaneous panniculitis-like T-cell lymphoma (SPTCL) highlights a “potential pitfall,” according to investigators.
This unusual case stresses the importance of molecular cytogenetics and/or immunohistochemistry for panniculitis-type lymphomas, reported lead author Caroline Laggis, MD of the University of Utah, Salt Lake City, and colleagues.
“While morphologic features of SPTCL, specifically rimming of adipocytes by neoplastic lymphoid cells, have been documented in other types of lymphomas, this case is exceptional in that the morphologic features of SPTCL are showed in secondary cutaneous involvement by MCL,” the investigators wrote. Their report is in Journal of Cutaneous Pathology.
The patient was a 69-year-old man who presented with 2-year history of night sweats and fever of unknown origin, and, closer to presentation, weight loss and tender bumps under the skin of his pelvic region.
Subsequent computed tomography and excisional lymph node biopsy led to a diagnosis of MCL, with a Mantle Cell Lymphoma International Prognostic Index of 5, suggesting aggressive, intermediate-risk disease. Further imaging showed involvement of the nasopharynx, and cervical and mediastinal lymph nodes.
Bendamustine and rituximab chemotherapy was given unremarkably until the final cycle, at which point the patient presented with tender subcutaneous nodules on his lower legs. Histopathology from punch biopsies revealed “a dense infiltrate of monomorphic, mitotically active lymphoid cells with infiltration between the deep dermal collagen and the adipocytes in subcutaneous fat,” the investigators wrote, noting that the infiltrative cells were blastoid and 70% expressed cyclin D1, supporting cutaneous involvement of his systemic MCL.
Treatment was switched to ibrutinib and selinexor via a clinical trial, which led to temporary improvement of leg lesions; when the lesions returned, biopsy was performed with the same histopathological result. Lenalidomide and rituximab were started, but without success, and disease spread to the central nervous system.
Another biopsy of the skin lesions again supported cutaneous MCL, with tumor cells rimming individual adipocytes.
Because of this atypical morphology, fluorescence in situ hybridization (FISH) was conducted, revealing t(11;14)(q13:32) positivity, thereby “confirming the diagnosis of cutaneous involvement by systemic MCL,” the investigators wrote.
Genomic sequencing revealed abnormalities of “ataxia-telangiectasia mutated, mechanistic target of rapamycin kinase (mTOR), BCL6 corepressor, and FAS-associated factor 1, as well as the expected mutation in IGH-CCND1, leading to cyclin D1 upregulation.”
Subsequent treatment was unsuccessful, and the patient died from his disease.
“The complex and central role that mTOR plays in adipose homeostasis may link our tumor to its preference to the adipose tissue, although further investigation is warranted regarding specific genomic alterations in lymphomas and the implications these mutations have in the involvement of tumor cells with cutaneous and adipose environments,” the investigators wrote.
The investigators did not report conflicts of interest.
SOURCE: Laggis C et al. 2019 Apr 8. doi:10.1111/cup.13471.
An atypical case of cutaneous mantle cell lymphoma (MCL) with histomorphological features mimicking subcutaneous panniculitis-like T-cell lymphoma (SPTCL) highlights a “potential pitfall,” according to investigators.
This unusual case stresses the importance of molecular cytogenetics and/or immunohistochemistry for panniculitis-type lymphomas, reported lead author Caroline Laggis, MD of the University of Utah, Salt Lake City, and colleagues.
“While morphologic features of SPTCL, specifically rimming of adipocytes by neoplastic lymphoid cells, have been documented in other types of lymphomas, this case is exceptional in that the morphologic features of SPTCL are showed in secondary cutaneous involvement by MCL,” the investigators wrote. Their report is in Journal of Cutaneous Pathology.
The patient was a 69-year-old man who presented with 2-year history of night sweats and fever of unknown origin, and, closer to presentation, weight loss and tender bumps under the skin of his pelvic region.
Subsequent computed tomography and excisional lymph node biopsy led to a diagnosis of MCL, with a Mantle Cell Lymphoma International Prognostic Index of 5, suggesting aggressive, intermediate-risk disease. Further imaging showed involvement of the nasopharynx, and cervical and mediastinal lymph nodes.
Bendamustine and rituximab chemotherapy was given unremarkably until the final cycle, at which point the patient presented with tender subcutaneous nodules on his lower legs. Histopathology from punch biopsies revealed “a dense infiltrate of monomorphic, mitotically active lymphoid cells with infiltration between the deep dermal collagen and the adipocytes in subcutaneous fat,” the investigators wrote, noting that the infiltrative cells were blastoid and 70% expressed cyclin D1, supporting cutaneous involvement of his systemic MCL.
Treatment was switched to ibrutinib and selinexor via a clinical trial, which led to temporary improvement of leg lesions; when the lesions returned, biopsy was performed with the same histopathological result. Lenalidomide and rituximab were started, but without success, and disease spread to the central nervous system.
Another biopsy of the skin lesions again supported cutaneous MCL, with tumor cells rimming individual adipocytes.
Because of this atypical morphology, fluorescence in situ hybridization (FISH) was conducted, revealing t(11;14)(q13:32) positivity, thereby “confirming the diagnosis of cutaneous involvement by systemic MCL,” the investigators wrote.
Genomic sequencing revealed abnormalities of “ataxia-telangiectasia mutated, mechanistic target of rapamycin kinase (mTOR), BCL6 corepressor, and FAS-associated factor 1, as well as the expected mutation in IGH-CCND1, leading to cyclin D1 upregulation.”
Subsequent treatment was unsuccessful, and the patient died from his disease.
“The complex and central role that mTOR plays in adipose homeostasis may link our tumor to its preference to the adipose tissue, although further investigation is warranted regarding specific genomic alterations in lymphomas and the implications these mutations have in the involvement of tumor cells with cutaneous and adipose environments,” the investigators wrote.
The investigators did not report conflicts of interest.
SOURCE: Laggis C et al. 2019 Apr 8. doi:10.1111/cup.13471.
An atypical case of cutaneous mantle cell lymphoma (MCL) with histomorphological features mimicking subcutaneous panniculitis-like T-cell lymphoma (SPTCL) highlights a “potential pitfall,” according to investigators.
This unusual case stresses the importance of molecular cytogenetics and/or immunohistochemistry for panniculitis-type lymphomas, reported lead author Caroline Laggis, MD of the University of Utah, Salt Lake City, and colleagues.
“While morphologic features of SPTCL, specifically rimming of adipocytes by neoplastic lymphoid cells, have been documented in other types of lymphomas, this case is exceptional in that the morphologic features of SPTCL are showed in secondary cutaneous involvement by MCL,” the investigators wrote. Their report is in Journal of Cutaneous Pathology.
The patient was a 69-year-old man who presented with 2-year history of night sweats and fever of unknown origin, and, closer to presentation, weight loss and tender bumps under the skin of his pelvic region.
Subsequent computed tomography and excisional lymph node biopsy led to a diagnosis of MCL, with a Mantle Cell Lymphoma International Prognostic Index of 5, suggesting aggressive, intermediate-risk disease. Further imaging showed involvement of the nasopharynx, and cervical and mediastinal lymph nodes.
Bendamustine and rituximab chemotherapy was given unremarkably until the final cycle, at which point the patient presented with tender subcutaneous nodules on his lower legs. Histopathology from punch biopsies revealed “a dense infiltrate of monomorphic, mitotically active lymphoid cells with infiltration between the deep dermal collagen and the adipocytes in subcutaneous fat,” the investigators wrote, noting that the infiltrative cells were blastoid and 70% expressed cyclin D1, supporting cutaneous involvement of his systemic MCL.
Treatment was switched to ibrutinib and selinexor via a clinical trial, which led to temporary improvement of leg lesions; when the lesions returned, biopsy was performed with the same histopathological result. Lenalidomide and rituximab were started, but without success, and disease spread to the central nervous system.
Another biopsy of the skin lesions again supported cutaneous MCL, with tumor cells rimming individual adipocytes.
Because of this atypical morphology, fluorescence in situ hybridization (FISH) was conducted, revealing t(11;14)(q13:32) positivity, thereby “confirming the diagnosis of cutaneous involvement by systemic MCL,” the investigators wrote.
Genomic sequencing revealed abnormalities of “ataxia-telangiectasia mutated, mechanistic target of rapamycin kinase (mTOR), BCL6 corepressor, and FAS-associated factor 1, as well as the expected mutation in IGH-CCND1, leading to cyclin D1 upregulation.”
Subsequent treatment was unsuccessful, and the patient died from his disease.
“The complex and central role that mTOR plays in adipose homeostasis may link our tumor to its preference to the adipose tissue, although further investigation is warranted regarding specific genomic alterations in lymphomas and the implications these mutations have in the involvement of tumor cells with cutaneous and adipose environments,” the investigators wrote.
The investigators did not report conflicts of interest.
SOURCE: Laggis C et al. 2019 Apr 8. doi:10.1111/cup.13471.
FROM JOURNAL OF CUTANEOUS PATHOLOGY
Insomnia symptoms correlate with seizure frequency
PHILADELPHIA – . Insomnia symptoms are not associated with epilepsy type, number of antiepileptic drugs (AEDs), or AED standardized dose, however.
“Given the potential benefits of sleep therapies on epilepsy outcomes, routine screening of insomnia symptoms is warranted,” said lead study author Thapanee Somboon, MD, a researcher at the sleep disorders center at Cleveland Clinic Neurological Institute in Ohio and at Prasat Neurological Institute in Bangkok.
Insomnia is common and associated with depression in patients with epilepsy, but prior studies that looked at the relationship between insomnia and epilepsy-related characteristics yielded limited and conflicting results, according to Dr. Somboon.
To evaluate potential associations between insomnia and epilepsy, Dr. Somboon and colleagues conducted a prospective analysis of data from 270 patients with epilepsy who presented to the Cleveland Clinic Epilepsy Center for an initial evaluation between January and August 2018. The patients completed the Insomnia Severity Index (ISI). An ISI score of 8 or greater indicated clinical insomnia symptoms, and an ISI score of 15 or greater indicated moderate or severe insomnia symptoms.
The researchers used Spearman’s correlation and the Kruskal-Wallis test to evaluate associations among insomnia symptoms and AED standardized dose, monthly seizure frequency, Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Questionnaire (GAD-7), and Quality of Life in Epilepsy-10 (QOLIE10).
Among the 270 patients, the average age was 43.5 years, 58% were female, 74% had focal epilepsy, and 26% had one or more seizures per month. The population’s median ISI score was 7. Nearly half had an ISI score of 8 or greater, and 23% had an ISI score of 15 or greater.
“A positive correlation was found between ISI and PHQ-9 (r = 0.64, P less than .001), GAD-7 (r = 0.68, P less than .001), QOLIE (r = 0.55, P less than .001), and monthly seizure frequency (r = 0.31, P less than .001),” the researchers reported. Insomnia symptoms had a significantly stronger correlation with PHQ-9 and GAD-7 than with seizure frequency.
Dr. Somboon had no disclosures. A coinvestigator has received research support from Jazz Pharmaceuticals.
SOURCE: Somboon T et al. AAN 2019, Abstract P3.6-026.
PHILADELPHIA – . Insomnia symptoms are not associated with epilepsy type, number of antiepileptic drugs (AEDs), or AED standardized dose, however.
“Given the potential benefits of sleep therapies on epilepsy outcomes, routine screening of insomnia symptoms is warranted,” said lead study author Thapanee Somboon, MD, a researcher at the sleep disorders center at Cleveland Clinic Neurological Institute in Ohio and at Prasat Neurological Institute in Bangkok.
Insomnia is common and associated with depression in patients with epilepsy, but prior studies that looked at the relationship between insomnia and epilepsy-related characteristics yielded limited and conflicting results, according to Dr. Somboon.
To evaluate potential associations between insomnia and epilepsy, Dr. Somboon and colleagues conducted a prospective analysis of data from 270 patients with epilepsy who presented to the Cleveland Clinic Epilepsy Center for an initial evaluation between January and August 2018. The patients completed the Insomnia Severity Index (ISI). An ISI score of 8 or greater indicated clinical insomnia symptoms, and an ISI score of 15 or greater indicated moderate or severe insomnia symptoms.
The researchers used Spearman’s correlation and the Kruskal-Wallis test to evaluate associations among insomnia symptoms and AED standardized dose, monthly seizure frequency, Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Questionnaire (GAD-7), and Quality of Life in Epilepsy-10 (QOLIE10).
Among the 270 patients, the average age was 43.5 years, 58% were female, 74% had focal epilepsy, and 26% had one or more seizures per month. The population’s median ISI score was 7. Nearly half had an ISI score of 8 or greater, and 23% had an ISI score of 15 or greater.
“A positive correlation was found between ISI and PHQ-9 (r = 0.64, P less than .001), GAD-7 (r = 0.68, P less than .001), QOLIE (r = 0.55, P less than .001), and monthly seizure frequency (r = 0.31, P less than .001),” the researchers reported. Insomnia symptoms had a significantly stronger correlation with PHQ-9 and GAD-7 than with seizure frequency.
Dr. Somboon had no disclosures. A coinvestigator has received research support from Jazz Pharmaceuticals.
SOURCE: Somboon T et al. AAN 2019, Abstract P3.6-026.
PHILADELPHIA – . Insomnia symptoms are not associated with epilepsy type, number of antiepileptic drugs (AEDs), or AED standardized dose, however.
“Given the potential benefits of sleep therapies on epilepsy outcomes, routine screening of insomnia symptoms is warranted,” said lead study author Thapanee Somboon, MD, a researcher at the sleep disorders center at Cleveland Clinic Neurological Institute in Ohio and at Prasat Neurological Institute in Bangkok.
Insomnia is common and associated with depression in patients with epilepsy, but prior studies that looked at the relationship between insomnia and epilepsy-related characteristics yielded limited and conflicting results, according to Dr. Somboon.
To evaluate potential associations between insomnia and epilepsy, Dr. Somboon and colleagues conducted a prospective analysis of data from 270 patients with epilepsy who presented to the Cleveland Clinic Epilepsy Center for an initial evaluation between January and August 2018. The patients completed the Insomnia Severity Index (ISI). An ISI score of 8 or greater indicated clinical insomnia symptoms, and an ISI score of 15 or greater indicated moderate or severe insomnia symptoms.
The researchers used Spearman’s correlation and the Kruskal-Wallis test to evaluate associations among insomnia symptoms and AED standardized dose, monthly seizure frequency, Patient Health Questionnaire (PHQ-9), Generalized Anxiety Disorder Questionnaire (GAD-7), and Quality of Life in Epilepsy-10 (QOLIE10).
Among the 270 patients, the average age was 43.5 years, 58% were female, 74% had focal epilepsy, and 26% had one or more seizures per month. The population’s median ISI score was 7. Nearly half had an ISI score of 8 or greater, and 23% had an ISI score of 15 or greater.
“A positive correlation was found between ISI and PHQ-9 (r = 0.64, P less than .001), GAD-7 (r = 0.68, P less than .001), QOLIE (r = 0.55, P less than .001), and monthly seizure frequency (r = 0.31, P less than .001),” the researchers reported. Insomnia symptoms had a significantly stronger correlation with PHQ-9 and GAD-7 than with seizure frequency.
Dr. Somboon had no disclosures. A coinvestigator has received research support from Jazz Pharmaceuticals.
SOURCE: Somboon T et al. AAN 2019, Abstract P3.6-026.
REPORTING FROM AAN 2019
Inebilizumab reduces the risk of NMOSD attacks
PHILADELPHIA – , according to results presented at the annual meeting of the American Academy of Neurology. The antibody also reduces the risks of disability worsening, MRI lesion activity, and hospitalization.
No approved therapies are available for NMOSD, which is thought to be a B-cell-mediated disorder. Research suggests that CD19 is a potential therapeutic target in NMOSD. Inebilizumab has a high affinity for CD19 and reliably depleted B cells in preclinical and clinical studies.
Participants did not receive concomitant immunosuppressants
Bruce Cree, MD, PhD, clinical research director at the University of California, San Francisco, Multiple Sclerosis Center, and colleagues conducted a phase 3 study to evaluate the safety and efficacy of inebilizumab in NMOSD. Eligible participants were older than 18 years, had NMOSD, and were seropositive for aquaporin-4 (AQP4) antibodies. Seronegative patients were allowed to participate, provided that they met the 2006 Wingerchuk criteria for neuromyelitis optica. Participants were required to have had one attack in the previous year or two attacks in the 2 previous years. Participants had a wide range of disability. No background use of immune suppressants was permitted.
The trial was conducted at 99 centers in 24 countries. After a 4-week screening period, investigators randomized patients in a 3:1 ratio to inebilizumab or placebo. Treatment was administered on days 1 and 15 by IV infusion. The randomized, controlled period lasted for 197 days. If a participant did not have a relapse by the end of this period, he or she was given the choice of participating in a yearlong open-label study, in which he or she would receive 300 mg of inebilizumab every 6 months. Participants who had an attack were treated for the attack.
An independent eligibility committee reviewed and confirmed the diagnostic criteria for AQP4 seronegative participants. A masked attack-adjudication committee provided real-time assessment of NMOSD attacks. An independent data monitoring committee reviewed patient safety and ensured the ethical conduct of the study.
The trial’s primary endpoint was the time from randomization to first attack. Secondary endpoints included a measure of disability, a measure of visual acuity, cumulative lesions on MRI, and NMOSD-related inpatient hospitalizations.
Treatment reduced the risk of hospitalization
Dr. Cree and colleagues screened 467 subjects and randomized 231. Among those patients, 175 received inebilizumab, and 56 received placebo. The population’s mean age was 43 years. About 90% were women, and 72% were white or Asian. The median baseline disability score was 3.5, indicating moderate disability. More than 90% of patients were AQP4 seropositive. The mean number of attacks at entry was 4.3. About 60% of participants had had prior exposure to immune suppressants.
Enrollment into the randomized, controlled period was stopped at 231 patients after 43 attacks had occurred, based on the recommendation of the independent data monitoring committee, which concluded that further recruitment into the placebo arm would not be ethical. The committee also recommended that all participants be shifted into the open-label period.
Among AQP4 seropositive patients, 88% of the inebilizumab group did not have an attack, compared with 57% of the control group. This result represents a 77% reduction in the risk of a relapse following inebilizumab treatment. The number needed to treat was 3.23. In the population overall, 87% of patients treated with inebilizumab did not have an attack, compared with 60% of controls, which corresponded to a 73% reduction in risk of an attack and a number needed to treat of 3.7.
In addition, Dr. Cree and colleagues found that inebilizumab was associated with a 63% reduction in risk of disability, a 43% reduction in risk of developing new lesions, and a 71% reduction in NMOSD-related hospitalizations. These results were statistically significant and clinically meaningful, he said. The only secondary endpoint that was not met was the difference in binocular low-contrast visual acuity scores. “This [result] may be due to the low frequency of optic neuritis events, a floor effect within the placebo arm (many of those patients had profound visual loss at baseline), or selection of a binocular acuity test that diluted the impact of monocular events,” said Dr. Cree.
Adverse events and serious adverse events were well balanced between the two treatment arms. The rate of infusion-related reactions was low in both arms and was “perhaps slightly lower in the inebilizumab treatment arm,” said Dr. Cree. No deaths occurred during the randomized, controlled period, and the researchers found no pattern of serious adverse events. Two deaths occurred in the open-label period: one related to a severe attack, and the other related to a brain event of unclear etiology without definite diagnosis.
B cells were depleted within approximately 4 weeks of treatment, and this depletion was sustained throughout the randomized, controlled period.
This study was funded by Viela Bio and MedImmune. Dr. Cree has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Abbvie, Akili, Biogen, EMD Serono, GeNeuro and Novartis.
SOURCE: Cree B et al. AAN 2019. Abstract Plen02.001.
PHILADELPHIA – , according to results presented at the annual meeting of the American Academy of Neurology. The antibody also reduces the risks of disability worsening, MRI lesion activity, and hospitalization.
No approved therapies are available for NMOSD, which is thought to be a B-cell-mediated disorder. Research suggests that CD19 is a potential therapeutic target in NMOSD. Inebilizumab has a high affinity for CD19 and reliably depleted B cells in preclinical and clinical studies.
Participants did not receive concomitant immunosuppressants
Bruce Cree, MD, PhD, clinical research director at the University of California, San Francisco, Multiple Sclerosis Center, and colleagues conducted a phase 3 study to evaluate the safety and efficacy of inebilizumab in NMOSD. Eligible participants were older than 18 years, had NMOSD, and were seropositive for aquaporin-4 (AQP4) antibodies. Seronegative patients were allowed to participate, provided that they met the 2006 Wingerchuk criteria for neuromyelitis optica. Participants were required to have had one attack in the previous year or two attacks in the 2 previous years. Participants had a wide range of disability. No background use of immune suppressants was permitted.
The trial was conducted at 99 centers in 24 countries. After a 4-week screening period, investigators randomized patients in a 3:1 ratio to inebilizumab or placebo. Treatment was administered on days 1 and 15 by IV infusion. The randomized, controlled period lasted for 197 days. If a participant did not have a relapse by the end of this period, he or she was given the choice of participating in a yearlong open-label study, in which he or she would receive 300 mg of inebilizumab every 6 months. Participants who had an attack were treated for the attack.
An independent eligibility committee reviewed and confirmed the diagnostic criteria for AQP4 seronegative participants. A masked attack-adjudication committee provided real-time assessment of NMOSD attacks. An independent data monitoring committee reviewed patient safety and ensured the ethical conduct of the study.
The trial’s primary endpoint was the time from randomization to first attack. Secondary endpoints included a measure of disability, a measure of visual acuity, cumulative lesions on MRI, and NMOSD-related inpatient hospitalizations.
Treatment reduced the risk of hospitalization
Dr. Cree and colleagues screened 467 subjects and randomized 231. Among those patients, 175 received inebilizumab, and 56 received placebo. The population’s mean age was 43 years. About 90% were women, and 72% were white or Asian. The median baseline disability score was 3.5, indicating moderate disability. More than 90% of patients were AQP4 seropositive. The mean number of attacks at entry was 4.3. About 60% of participants had had prior exposure to immune suppressants.
Enrollment into the randomized, controlled period was stopped at 231 patients after 43 attacks had occurred, based on the recommendation of the independent data monitoring committee, which concluded that further recruitment into the placebo arm would not be ethical. The committee also recommended that all participants be shifted into the open-label period.
Among AQP4 seropositive patients, 88% of the inebilizumab group did not have an attack, compared with 57% of the control group. This result represents a 77% reduction in the risk of a relapse following inebilizumab treatment. The number needed to treat was 3.23. In the population overall, 87% of patients treated with inebilizumab did not have an attack, compared with 60% of controls, which corresponded to a 73% reduction in risk of an attack and a number needed to treat of 3.7.
In addition, Dr. Cree and colleagues found that inebilizumab was associated with a 63% reduction in risk of disability, a 43% reduction in risk of developing new lesions, and a 71% reduction in NMOSD-related hospitalizations. These results were statistically significant and clinically meaningful, he said. The only secondary endpoint that was not met was the difference in binocular low-contrast visual acuity scores. “This [result] may be due to the low frequency of optic neuritis events, a floor effect within the placebo arm (many of those patients had profound visual loss at baseline), or selection of a binocular acuity test that diluted the impact of monocular events,” said Dr. Cree.
Adverse events and serious adverse events were well balanced between the two treatment arms. The rate of infusion-related reactions was low in both arms and was “perhaps slightly lower in the inebilizumab treatment arm,” said Dr. Cree. No deaths occurred during the randomized, controlled period, and the researchers found no pattern of serious adverse events. Two deaths occurred in the open-label period: one related to a severe attack, and the other related to a brain event of unclear etiology without definite diagnosis.
B cells were depleted within approximately 4 weeks of treatment, and this depletion was sustained throughout the randomized, controlled period.
This study was funded by Viela Bio and MedImmune. Dr. Cree has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Abbvie, Akili, Biogen, EMD Serono, GeNeuro and Novartis.
SOURCE: Cree B et al. AAN 2019. Abstract Plen02.001.
PHILADELPHIA – , according to results presented at the annual meeting of the American Academy of Neurology. The antibody also reduces the risks of disability worsening, MRI lesion activity, and hospitalization.
No approved therapies are available for NMOSD, which is thought to be a B-cell-mediated disorder. Research suggests that CD19 is a potential therapeutic target in NMOSD. Inebilizumab has a high affinity for CD19 and reliably depleted B cells in preclinical and clinical studies.
Participants did not receive concomitant immunosuppressants
Bruce Cree, MD, PhD, clinical research director at the University of California, San Francisco, Multiple Sclerosis Center, and colleagues conducted a phase 3 study to evaluate the safety and efficacy of inebilizumab in NMOSD. Eligible participants were older than 18 years, had NMOSD, and were seropositive for aquaporin-4 (AQP4) antibodies. Seronegative patients were allowed to participate, provided that they met the 2006 Wingerchuk criteria for neuromyelitis optica. Participants were required to have had one attack in the previous year or two attacks in the 2 previous years. Participants had a wide range of disability. No background use of immune suppressants was permitted.
The trial was conducted at 99 centers in 24 countries. After a 4-week screening period, investigators randomized patients in a 3:1 ratio to inebilizumab or placebo. Treatment was administered on days 1 and 15 by IV infusion. The randomized, controlled period lasted for 197 days. If a participant did not have a relapse by the end of this period, he or she was given the choice of participating in a yearlong open-label study, in which he or she would receive 300 mg of inebilizumab every 6 months. Participants who had an attack were treated for the attack.
An independent eligibility committee reviewed and confirmed the diagnostic criteria for AQP4 seronegative participants. A masked attack-adjudication committee provided real-time assessment of NMOSD attacks. An independent data monitoring committee reviewed patient safety and ensured the ethical conduct of the study.
The trial’s primary endpoint was the time from randomization to first attack. Secondary endpoints included a measure of disability, a measure of visual acuity, cumulative lesions on MRI, and NMOSD-related inpatient hospitalizations.
Treatment reduced the risk of hospitalization
Dr. Cree and colleagues screened 467 subjects and randomized 231. Among those patients, 175 received inebilizumab, and 56 received placebo. The population’s mean age was 43 years. About 90% were women, and 72% were white or Asian. The median baseline disability score was 3.5, indicating moderate disability. More than 90% of patients were AQP4 seropositive. The mean number of attacks at entry was 4.3. About 60% of participants had had prior exposure to immune suppressants.
Enrollment into the randomized, controlled period was stopped at 231 patients after 43 attacks had occurred, based on the recommendation of the independent data monitoring committee, which concluded that further recruitment into the placebo arm would not be ethical. The committee also recommended that all participants be shifted into the open-label period.
Among AQP4 seropositive patients, 88% of the inebilizumab group did not have an attack, compared with 57% of the control group. This result represents a 77% reduction in the risk of a relapse following inebilizumab treatment. The number needed to treat was 3.23. In the population overall, 87% of patients treated with inebilizumab did not have an attack, compared with 60% of controls, which corresponded to a 73% reduction in risk of an attack and a number needed to treat of 3.7.
In addition, Dr. Cree and colleagues found that inebilizumab was associated with a 63% reduction in risk of disability, a 43% reduction in risk of developing new lesions, and a 71% reduction in NMOSD-related hospitalizations. These results were statistically significant and clinically meaningful, he said. The only secondary endpoint that was not met was the difference in binocular low-contrast visual acuity scores. “This [result] may be due to the low frequency of optic neuritis events, a floor effect within the placebo arm (many of those patients had profound visual loss at baseline), or selection of a binocular acuity test that diluted the impact of monocular events,” said Dr. Cree.
Adverse events and serious adverse events were well balanced between the two treatment arms. The rate of infusion-related reactions was low in both arms and was “perhaps slightly lower in the inebilizumab treatment arm,” said Dr. Cree. No deaths occurred during the randomized, controlled period, and the researchers found no pattern of serious adverse events. Two deaths occurred in the open-label period: one related to a severe attack, and the other related to a brain event of unclear etiology without definite diagnosis.
B cells were depleted within approximately 4 weeks of treatment, and this depletion was sustained throughout the randomized, controlled period.
This study was funded by Viela Bio and MedImmune. Dr. Cree has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities from Abbvie, Akili, Biogen, EMD Serono, GeNeuro and Novartis.
SOURCE: Cree B et al. AAN 2019. Abstract Plen02.001.
REPORTING FROM AAN 2019