User login
SABR offers surgery alternative for localized RCC
For patients with localized renal cell carcinoma (RCC), stereotactic ablative body radiotherapy (SABR) may be an effective alternative to surgery, according to findings from a retrospective study.
Patients with smaller tumors and nonmetastatic disease achieved the best outcomes with SABR, reported lead author Rodney E. Wegner, MD, of Allegheny Health Network Cancer Institute, Pittsburgh, and colleagues.
“Radiation therapy is often overlooked in [RCC] as historic preclinical data reported RCC as being relatively radioresistant to external beam radiation at conventional doses,” the investigators wrote in Advances in Radiation Oncology. However, SABR may be able to overcome this resistance by delivering “highly conformal dose escalated radiation,” the investigators noted, citing two recent reports from the International Radiosurgery Oncology Consortium for Kidney (IROCK) that showed promising results (J Urol. 2019 Jun;201[6]:1097-104 and Cancer. 2018 Mar 1;124[5]:934-42).
The present study included 347 patients with RCC from the National Cancer Database who were treated with SABR and not surgery. Most patients (94%) did not have systemic therapy. Similar proportions lacked lymph node involvement (97%) or distant metastasis (93%). About three-quarters of patients (76%) had T1 disease. The median SABR dose was 45 Gy, ranging from 35 to 54 Gy, most frequently given in three fractions.
After a median follow-up of 36 months, ranging from 1 to 156 months, median overall survival across all patients was 58 months. SABR was most effective for patients with nonmetastatic disease who had smaller tumors.
An inverse correlation between tumor size and overall survival was apparent given that patients with tumors 2.5 cm or smaller had the longest median overall survival, at 92 months, with decrements in survival as tumors got larger. Survival dropped to 88 months for tumors 2.6-3.5 cm, 44 months for tumors 3.5-5.0 cm, and finally to 26 months for tumors larger than 5.0 cm. In addition to tumor size and metastatic disease, age was a risk factor for shorter survival.
“The results presented demonstrate excellent post-SABR outcomes, with median overall survival in the range of 7-8 years for smaller lesions,” the investigators wrote. “This is particularly impressive considering that many of these patients were likely medically inoperable.”
The researchers noted that most of kidney SABR is done at academic centers, which highlights the importance of appropriate technology and training for delivering this treatment.
“Further prospective research is needed to verify its safety and efficacy,” the investigators concluded.
No external funding was provided for the project and the investigators reported no conflicts of interest.
SOURCE: Wegner RE et al. Adv Rad Onc. 2019 Aug 8. doi: 10.1016/j.adro.2019.07.018.
For patients with localized renal cell carcinoma (RCC), stereotactic ablative body radiotherapy (SABR) may be an effective alternative to surgery, according to findings from a retrospective study.
Patients with smaller tumors and nonmetastatic disease achieved the best outcomes with SABR, reported lead author Rodney E. Wegner, MD, of Allegheny Health Network Cancer Institute, Pittsburgh, and colleagues.
“Radiation therapy is often overlooked in [RCC] as historic preclinical data reported RCC as being relatively radioresistant to external beam radiation at conventional doses,” the investigators wrote in Advances in Radiation Oncology. However, SABR may be able to overcome this resistance by delivering “highly conformal dose escalated radiation,” the investigators noted, citing two recent reports from the International Radiosurgery Oncology Consortium for Kidney (IROCK) that showed promising results (J Urol. 2019 Jun;201[6]:1097-104 and Cancer. 2018 Mar 1;124[5]:934-42).
The present study included 347 patients with RCC from the National Cancer Database who were treated with SABR and not surgery. Most patients (94%) did not have systemic therapy. Similar proportions lacked lymph node involvement (97%) or distant metastasis (93%). About three-quarters of patients (76%) had T1 disease. The median SABR dose was 45 Gy, ranging from 35 to 54 Gy, most frequently given in three fractions.
After a median follow-up of 36 months, ranging from 1 to 156 months, median overall survival across all patients was 58 months. SABR was most effective for patients with nonmetastatic disease who had smaller tumors.
An inverse correlation between tumor size and overall survival was apparent given that patients with tumors 2.5 cm or smaller had the longest median overall survival, at 92 months, with decrements in survival as tumors got larger. Survival dropped to 88 months for tumors 2.6-3.5 cm, 44 months for tumors 3.5-5.0 cm, and finally to 26 months for tumors larger than 5.0 cm. In addition to tumor size and metastatic disease, age was a risk factor for shorter survival.
“The results presented demonstrate excellent post-SABR outcomes, with median overall survival in the range of 7-8 years for smaller lesions,” the investigators wrote. “This is particularly impressive considering that many of these patients were likely medically inoperable.”
The researchers noted that most of kidney SABR is done at academic centers, which highlights the importance of appropriate technology and training for delivering this treatment.
“Further prospective research is needed to verify its safety and efficacy,” the investigators concluded.
No external funding was provided for the project and the investigators reported no conflicts of interest.
SOURCE: Wegner RE et al. Adv Rad Onc. 2019 Aug 8. doi: 10.1016/j.adro.2019.07.018.
For patients with localized renal cell carcinoma (RCC), stereotactic ablative body radiotherapy (SABR) may be an effective alternative to surgery, according to findings from a retrospective study.
Patients with smaller tumors and nonmetastatic disease achieved the best outcomes with SABR, reported lead author Rodney E. Wegner, MD, of Allegheny Health Network Cancer Institute, Pittsburgh, and colleagues.
“Radiation therapy is often overlooked in [RCC] as historic preclinical data reported RCC as being relatively radioresistant to external beam radiation at conventional doses,” the investigators wrote in Advances in Radiation Oncology. However, SABR may be able to overcome this resistance by delivering “highly conformal dose escalated radiation,” the investigators noted, citing two recent reports from the International Radiosurgery Oncology Consortium for Kidney (IROCK) that showed promising results (J Urol. 2019 Jun;201[6]:1097-104 and Cancer. 2018 Mar 1;124[5]:934-42).
The present study included 347 patients with RCC from the National Cancer Database who were treated with SABR and not surgery. Most patients (94%) did not have systemic therapy. Similar proportions lacked lymph node involvement (97%) or distant metastasis (93%). About three-quarters of patients (76%) had T1 disease. The median SABR dose was 45 Gy, ranging from 35 to 54 Gy, most frequently given in three fractions.
After a median follow-up of 36 months, ranging from 1 to 156 months, median overall survival across all patients was 58 months. SABR was most effective for patients with nonmetastatic disease who had smaller tumors.
An inverse correlation between tumor size and overall survival was apparent given that patients with tumors 2.5 cm or smaller had the longest median overall survival, at 92 months, with decrements in survival as tumors got larger. Survival dropped to 88 months for tumors 2.6-3.5 cm, 44 months for tumors 3.5-5.0 cm, and finally to 26 months for tumors larger than 5.0 cm. In addition to tumor size and metastatic disease, age was a risk factor for shorter survival.
“The results presented demonstrate excellent post-SABR outcomes, with median overall survival in the range of 7-8 years for smaller lesions,” the investigators wrote. “This is particularly impressive considering that many of these patients were likely medically inoperable.”
The researchers noted that most of kidney SABR is done at academic centers, which highlights the importance of appropriate technology and training for delivering this treatment.
“Further prospective research is needed to verify its safety and efficacy,” the investigators concluded.
No external funding was provided for the project and the investigators reported no conflicts of interest.
SOURCE: Wegner RE et al. Adv Rad Onc. 2019 Aug 8. doi: 10.1016/j.adro.2019.07.018.
FROM ADVANCES IN RADIATION ONCOLOGY
Key clinical point:
Major finding: Median overall survival was 92 months among patients with renal tumors no larger than 2.5 cm.
Study details: A retrospective study involving 347 patients with localized renal cell carcinoma (RCC) who were treated with stereotactic ablative body radiotherapy.
Disclosures: No external funding was provided for the study and the investigators reported having no conflicts of interest.
Source: Wegner RE et al. Adv Rad Onc. 2019 Aug 8. doi: 10.1016/j.adro.2019.07.018.
Wrong cuff size throws off pediatric BP by 5 mm Hg
NEW ORLEANS – according to investigators from Columbia University in New York.
There are five cuff sizes in pediatrics, depending on a child’s arm circumference. Ideally, it’s measured beforehand so the right cuff size is used, but that doesn’t always happen in everyday practice.
Sometimes, clinicians just estimate arm size before choosing a cuff or opt for the medium-sized cuff in most kids; other times, the correct size has gone missing, said lead investigator Ruchi Gupta Mahajan, MD, a pediatric nephrology fellow at Columbia.
For those situations, she and her colleagues wanted to quantify how much the wrong cuff size throws off blood pressure readings in children, something that’s been done before in adult medicine, but not in pediatrics.
The idea was to give clinicians a decent estimate of true blood pressure even when the cuff isn’t quite right, something that’s particularly important with an increasing emphasis on catching hypertension as early as possible in children, she said.
After her subjects sat quietly for 10 minutes, Dr. Mahajan took automated blood pressure readings on 137 children; once with the right cuff size, once with a cuff one size too small, and once with a cuff one size too big, with a minute apart between readings.
The children were aged 4-12 years old and were in the office for wellness visits. None of them had heart or kidney disease, and none were on steroids or any other medications that affect blood pressure. There were a few more boys than girls, and almost all the children were Hispanic.
Overall, systolic blood pressure was an average of 5 mm Hg less with the larger cuff and 5 mm Hg more with the smaller cuff. The finding was the same in both girls and boys, and it held across age groups and in under, over, and normal weight children.
“I was really surprised there was no difference between ages, 4-12 years of age, its just a single number: 5. [Even] if [you] don’t have the appropriate cuff size,” the finding means that it’s still possible to have a good estimate of blood pressure, Dr. Mahajan said at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Meanwhile, cuff size didn’t have any statistically significant effect on diastolic pressure.
There was no outside funding for the study and Dr. Mahajan reported having no disclosures.
SOURCE: Mahajan RG et al. Joint Hypertension 2019, Abstract P182.
NEW ORLEANS – according to investigators from Columbia University in New York.
There are five cuff sizes in pediatrics, depending on a child’s arm circumference. Ideally, it’s measured beforehand so the right cuff size is used, but that doesn’t always happen in everyday practice.
Sometimes, clinicians just estimate arm size before choosing a cuff or opt for the medium-sized cuff in most kids; other times, the correct size has gone missing, said lead investigator Ruchi Gupta Mahajan, MD, a pediatric nephrology fellow at Columbia.
For those situations, she and her colleagues wanted to quantify how much the wrong cuff size throws off blood pressure readings in children, something that’s been done before in adult medicine, but not in pediatrics.
The idea was to give clinicians a decent estimate of true blood pressure even when the cuff isn’t quite right, something that’s particularly important with an increasing emphasis on catching hypertension as early as possible in children, she said.
After her subjects sat quietly for 10 minutes, Dr. Mahajan took automated blood pressure readings on 137 children; once with the right cuff size, once with a cuff one size too small, and once with a cuff one size too big, with a minute apart between readings.
The children were aged 4-12 years old and were in the office for wellness visits. None of them had heart or kidney disease, and none were on steroids or any other medications that affect blood pressure. There were a few more boys than girls, and almost all the children were Hispanic.
Overall, systolic blood pressure was an average of 5 mm Hg less with the larger cuff and 5 mm Hg more with the smaller cuff. The finding was the same in both girls and boys, and it held across age groups and in under, over, and normal weight children.
“I was really surprised there was no difference between ages, 4-12 years of age, its just a single number: 5. [Even] if [you] don’t have the appropriate cuff size,” the finding means that it’s still possible to have a good estimate of blood pressure, Dr. Mahajan said at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Meanwhile, cuff size didn’t have any statistically significant effect on diastolic pressure.
There was no outside funding for the study and Dr. Mahajan reported having no disclosures.
SOURCE: Mahajan RG et al. Joint Hypertension 2019, Abstract P182.
NEW ORLEANS – according to investigators from Columbia University in New York.
There are five cuff sizes in pediatrics, depending on a child’s arm circumference. Ideally, it’s measured beforehand so the right cuff size is used, but that doesn’t always happen in everyday practice.
Sometimes, clinicians just estimate arm size before choosing a cuff or opt for the medium-sized cuff in most kids; other times, the correct size has gone missing, said lead investigator Ruchi Gupta Mahajan, MD, a pediatric nephrology fellow at Columbia.
For those situations, she and her colleagues wanted to quantify how much the wrong cuff size throws off blood pressure readings in children, something that’s been done before in adult medicine, but not in pediatrics.
The idea was to give clinicians a decent estimate of true blood pressure even when the cuff isn’t quite right, something that’s particularly important with an increasing emphasis on catching hypertension as early as possible in children, she said.
After her subjects sat quietly for 10 minutes, Dr. Mahajan took automated blood pressure readings on 137 children; once with the right cuff size, once with a cuff one size too small, and once with a cuff one size too big, with a minute apart between readings.
The children were aged 4-12 years old and were in the office for wellness visits. None of them had heart or kidney disease, and none were on steroids or any other medications that affect blood pressure. There were a few more boys than girls, and almost all the children were Hispanic.
Overall, systolic blood pressure was an average of 5 mm Hg less with the larger cuff and 5 mm Hg more with the smaller cuff. The finding was the same in both girls and boys, and it held across age groups and in under, over, and normal weight children.
“I was really surprised there was no difference between ages, 4-12 years of age, its just a single number: 5. [Even] if [you] don’t have the appropriate cuff size,” the finding means that it’s still possible to have a good estimate of blood pressure, Dr. Mahajan said at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Meanwhile, cuff size didn’t have any statistically significant effect on diastolic pressure.
There was no outside funding for the study and Dr. Mahajan reported having no disclosures.
SOURCE: Mahajan RG et al. Joint Hypertension 2019, Abstract P182.
REPORTING FROM JOINT HYPERTENSION 2019
Visceral adiposity linked to sixfold increase in masked hypertension
NEW ORLEANS – Visceral adiposity, but not body mass index or total body fat, significantly correlated with elevated 24-hour ambulatory systolic blood pressure, greater systolic variability, and masked hypertension in a study from the University of Pennsylvania, Philadelphia.
Subjects in the highest quartile of visceral fat had a 6.3-fold greater odds of masked hypertension – normal in the office, but high at home – compared with those in the lowest quartile (95% confidence interval, 1.2-33.1).
The study findings suggest that central obesity, in particular, should trigger 24-hour ambulatory blood pressure monitoring (ABPM). “Every obese person should get a 24-hour” ABPM, but “we really need to be pushing [it] in people who have central adiposity. These are the patients ... we really need to focus on” because of the risk of masked hypertension, a “ticking time bomb” that greatly increases the risk of cardiovascular events, said lead investigator Jordana B. Cohen, MD, an assistant professor of medicine at the university.
The study also helps explain why body mass index (BMI) hasn’t been consistently linked to masked hypertension in previous studies; some studies likely included subjects with high BMIs but not central obesity.
Waist circumference, a marker of visceral adiposity, also correlated with elevated 24-hour systolic pressure and greater variability, but a trend for masked hypertension was not statistically significant, Dr. Cohen reported at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
It’s long been known that visceral fat – fat around the abdominal organs – is metabolically active and associated with greater cardiovascular risk, but its relationship to blood pressure hadn’t been well described, so Dr. Cohen and her team decided to take a look.
They ran whole body dual x-ray absorptiometry scans on 96 hypertensive adults on a stable dose of one antihypertensive drug for at least 2 months and correlated the findings with ABPM. Subjects were an average of 58 years old, almost 60% were women, almost half were black, and 54% were obese, with BMIs of at least 30 kg/m2.
After adjustment for age, sex, race, and antihypertensive class, the team found a significant, linear correlation between visceral fat and mean 24-hour systolic blood pressure. Patients with a visceral adiposity of about 0.1 kg/m2, for instance, had a mean pressure of around 130 mm Hg, compared with patients with more than 0.6 kg/m2, who had a mean of almost 150 mm Hg. Findings were similar for waist circumference over a range of 70-150 cm.
The correlations were weak (r = 0.3), but Dr. Cohen said they might improve with ongoing enrollment. Both measures also correlated with systolic variability.
Overall, the highest quartiles of waist circumference and visceral adiposity correlated with the highest mean systolic pressures and greatest variability, compared with the lowest quartiles. Visceral adiposity was the only measure significantly linked with masked hypertension. Trends in those directions for increasing BMI and total body fat mass were not statistically significant.
Mean BMI in the study was 31.7 kg/m2, and mean waist circumference was 104 cm. Mean 24-hour systolic blood pressure was 135 mm Hg and mean 24-hour systolic variability was 13 mm Hg. Almost 30% of the subjects had masked hypertension. Drug classes included beta-blockers, calcium channel blockers, diuretics, ACE-inhibitors, and angiotensin receptor blockers.
Dr. Cohen plans to investigate drug response versus visceral adiposity once the recruitment goal of 150 subjects is reached.
There was no external funding, and the investigators reported that they didn’t have any relevant disclosures.
SOURCE: Cohen JB et al. Joint Hypertension 2019, Abstract P2052.
NEW ORLEANS – Visceral adiposity, but not body mass index or total body fat, significantly correlated with elevated 24-hour ambulatory systolic blood pressure, greater systolic variability, and masked hypertension in a study from the University of Pennsylvania, Philadelphia.
Subjects in the highest quartile of visceral fat had a 6.3-fold greater odds of masked hypertension – normal in the office, but high at home – compared with those in the lowest quartile (95% confidence interval, 1.2-33.1).
The study findings suggest that central obesity, in particular, should trigger 24-hour ambulatory blood pressure monitoring (ABPM). “Every obese person should get a 24-hour” ABPM, but “we really need to be pushing [it] in people who have central adiposity. These are the patients ... we really need to focus on” because of the risk of masked hypertension, a “ticking time bomb” that greatly increases the risk of cardiovascular events, said lead investigator Jordana B. Cohen, MD, an assistant professor of medicine at the university.
The study also helps explain why body mass index (BMI) hasn’t been consistently linked to masked hypertension in previous studies; some studies likely included subjects with high BMIs but not central obesity.
Waist circumference, a marker of visceral adiposity, also correlated with elevated 24-hour systolic pressure and greater variability, but a trend for masked hypertension was not statistically significant, Dr. Cohen reported at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
It’s long been known that visceral fat – fat around the abdominal organs – is metabolically active and associated with greater cardiovascular risk, but its relationship to blood pressure hadn’t been well described, so Dr. Cohen and her team decided to take a look.
They ran whole body dual x-ray absorptiometry scans on 96 hypertensive adults on a stable dose of one antihypertensive drug for at least 2 months and correlated the findings with ABPM. Subjects were an average of 58 years old, almost 60% were women, almost half were black, and 54% were obese, with BMIs of at least 30 kg/m2.
After adjustment for age, sex, race, and antihypertensive class, the team found a significant, linear correlation between visceral fat and mean 24-hour systolic blood pressure. Patients with a visceral adiposity of about 0.1 kg/m2, for instance, had a mean pressure of around 130 mm Hg, compared with patients with more than 0.6 kg/m2, who had a mean of almost 150 mm Hg. Findings were similar for waist circumference over a range of 70-150 cm.
The correlations were weak (r = 0.3), but Dr. Cohen said they might improve with ongoing enrollment. Both measures also correlated with systolic variability.
Overall, the highest quartiles of waist circumference and visceral adiposity correlated with the highest mean systolic pressures and greatest variability, compared with the lowest quartiles. Visceral adiposity was the only measure significantly linked with masked hypertension. Trends in those directions for increasing BMI and total body fat mass were not statistically significant.
Mean BMI in the study was 31.7 kg/m2, and mean waist circumference was 104 cm. Mean 24-hour systolic blood pressure was 135 mm Hg and mean 24-hour systolic variability was 13 mm Hg. Almost 30% of the subjects had masked hypertension. Drug classes included beta-blockers, calcium channel blockers, diuretics, ACE-inhibitors, and angiotensin receptor blockers.
Dr. Cohen plans to investigate drug response versus visceral adiposity once the recruitment goal of 150 subjects is reached.
There was no external funding, and the investigators reported that they didn’t have any relevant disclosures.
SOURCE: Cohen JB et al. Joint Hypertension 2019, Abstract P2052.
NEW ORLEANS – Visceral adiposity, but not body mass index or total body fat, significantly correlated with elevated 24-hour ambulatory systolic blood pressure, greater systolic variability, and masked hypertension in a study from the University of Pennsylvania, Philadelphia.
Subjects in the highest quartile of visceral fat had a 6.3-fold greater odds of masked hypertension – normal in the office, but high at home – compared with those in the lowest quartile (95% confidence interval, 1.2-33.1).
The study findings suggest that central obesity, in particular, should trigger 24-hour ambulatory blood pressure monitoring (ABPM). “Every obese person should get a 24-hour” ABPM, but “we really need to be pushing [it] in people who have central adiposity. These are the patients ... we really need to focus on” because of the risk of masked hypertension, a “ticking time bomb” that greatly increases the risk of cardiovascular events, said lead investigator Jordana B. Cohen, MD, an assistant professor of medicine at the university.
The study also helps explain why body mass index (BMI) hasn’t been consistently linked to masked hypertension in previous studies; some studies likely included subjects with high BMIs but not central obesity.
Waist circumference, a marker of visceral adiposity, also correlated with elevated 24-hour systolic pressure and greater variability, but a trend for masked hypertension was not statistically significant, Dr. Cohen reported at the joint scientific sessions of the American Heart Association (AHA) Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
It’s long been known that visceral fat – fat around the abdominal organs – is metabolically active and associated with greater cardiovascular risk, but its relationship to blood pressure hadn’t been well described, so Dr. Cohen and her team decided to take a look.
They ran whole body dual x-ray absorptiometry scans on 96 hypertensive adults on a stable dose of one antihypertensive drug for at least 2 months and correlated the findings with ABPM. Subjects were an average of 58 years old, almost 60% were women, almost half were black, and 54% were obese, with BMIs of at least 30 kg/m2.
After adjustment for age, sex, race, and antihypertensive class, the team found a significant, linear correlation between visceral fat and mean 24-hour systolic blood pressure. Patients with a visceral adiposity of about 0.1 kg/m2, for instance, had a mean pressure of around 130 mm Hg, compared with patients with more than 0.6 kg/m2, who had a mean of almost 150 mm Hg. Findings were similar for waist circumference over a range of 70-150 cm.
The correlations were weak (r = 0.3), but Dr. Cohen said they might improve with ongoing enrollment. Both measures also correlated with systolic variability.
Overall, the highest quartiles of waist circumference and visceral adiposity correlated with the highest mean systolic pressures and greatest variability, compared with the lowest quartiles. Visceral adiposity was the only measure significantly linked with masked hypertension. Trends in those directions for increasing BMI and total body fat mass were not statistically significant.
Mean BMI in the study was 31.7 kg/m2, and mean waist circumference was 104 cm. Mean 24-hour systolic blood pressure was 135 mm Hg and mean 24-hour systolic variability was 13 mm Hg. Almost 30% of the subjects had masked hypertension. Drug classes included beta-blockers, calcium channel blockers, diuretics, ACE-inhibitors, and angiotensin receptor blockers.
Dr. Cohen plans to investigate drug response versus visceral adiposity once the recruitment goal of 150 subjects is reached.
There was no external funding, and the investigators reported that they didn’t have any relevant disclosures.
SOURCE: Cohen JB et al. Joint Hypertension 2019, Abstract P2052.
REPORTING FROM JOINT HYPERTENSION 2019
No ‘one size fits all’ approach to managing severe pediatric psoriasis
AUSTIN, TEX. – The way Kelly M. Cordoro sees it, the most for which patient.
“You can look up the dosing and frequency of these drugs; all of that’s available,” she said at the annual meeting of the Society for Pediatric Dermatology. “But how do we think about which drug for which patient? What are the considerations?”
Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, described psoriasis as an autoamplifying inflammatory cascade involving innate and adaptive immunity and noted that various components of that cascade represent treatment targets. “We don’t have a true comprehension of the pathophysiology of psoriasis, but as we learn the pathways, we’re targeting them,” she said. “You can target keratinocyte proliferation with drugs like retinoids and phototherapy. You can broadly target T cells, neutrophils, and dendritic cells with methotrexate, cyclosporine, and phototherapy. The newer drugs target the cytokine milieu, including TNF [tumor necrosis factor]–alpha, IL [interleukin]–17A, IL-12, and IL-23.”
There is no one right answer for which drug to prescribe, she continued, except in the cases of certain comorbidities, contraindications, and genetic variants. “For example, if a patient has psoriatic arthritis, then you have methotrexate and all of the biologics that might be disease modifying,” she said. “If a patient has inflammatory bowel disease, it’s critical to know that IL-17 inhibitors will flare that disease, but anti-TNF and IL-12 and IL-23 inhibitors are okay. If a patient has liver and kidney disease, you want to avoid methotrexate and cyclosporine. If there’s a female of childbearing potential you want to be very cautious with using retinoids. I think the harder question for us is, How about the rest of the patients?”
In addition to a drug’s mechanism of action, patient- and family-related factors play a role in deciding which agent to use. For example, does the patient prefer an oral or an injectable agent? Is the patient able to travel to a phototherapy center? Is it feasible for the family to manage visits for lab work and direct clinical monitoring? Does the family have a high level of health literacy and are you communicating with them in ways that facilitate shared decision making?
“The best way to choose a systemic therapy is to develop an individualized assessment of overall disease burden,” said Dr. Cordoro, who is also division chief of pediatric dermatology at UCSF. “Include psychological burden and subjective data in addition to objective measures like body surface area. Look for triggers. Infants are more commonly affected by viral infections and, in a subset, monogenic forms of psoriasis such as deficiency of interleukin 1 receptor antagonist [DIRA]. In general, we try to take a conservative approach in the developing child. As children hit early adolescence and become post pubertal, you have to start thinking about the psychosocial impact [of psoriasis], and we have to start treating patients with the consideration that chronic uncontrolled inflammation can potentially lead to comorbidities down the road. We see this in adults with severe psoriasis and early onset cardiovascular disease, the so-called psoriatic march from chronic inflammation to cardiovascular disease.”
Dr. Cordoro advises clinicians to rethink the conventional “therapeutic ladder” concept and embrace the idea of “finding the right tool for the job right now.” If a patient presents with a flare from a known trigger such as a strep infection, “maybe you want to treat with something more conservative,” she said. “Once you treat, and if the trigger has been managed, they might be better. But some patients will need the most aggressive treatment right out of the gate.”
Tried and true systemic therapies for psoriasis include methotrexate, cyclosporine, acitretin, and phototherapy, but none is approved by the Food and Drug Administration for use in children. “These drugs have decades of experience behind them,” Dr. Cordoro said. “Methotrexate is slow to start but has a sustained profile, so if you can get the patient to respond, that response tends to persist. Methotrexate also prevents the formation of antidrug antibodies, which is important if you are considering use of a biologic agent later on.”
Cyclosporine is best if you need a rapid rescue drug to get the disease under control before moving on to other options. “One in four patients relapse once cyclosporine is discontinued, so the benefit may not be as sustained as with methotrexate,” she said. “Acitretin is a really nice choice when you can’t or don’t want to immunosuppress the patient, and phototherapy is good if you can get it. The advantages of systemic therapies are that they’re easy on, easy off, and you can combine medications in severe situations. Almost all of these drugs can be combined with another, with few exceptions. I would caution that over immunosuppression is the biggest risk ... so this must be done carefully and only when necessary.”
Biologic agents such as TNF inhibitors and IL-12/23 inhibitors are playing an increasing role in pediatric psoriasis. They can be expensive and difficult for some insurance plans to cover, but offer the convenience of better efficacy and less frequent lab monitoring than conventional systemics. In the United States, etanercept and ustekinumab are approved for moderate to severe pediatric plaque psoriasis in patients as young as age 4 and 12 years, respectively. TNF inhibitors have accumulated the most data in children, while data are accumulating in trials of IL-17 inhibitors, IL-23 inhibitors, and PDE4 inhibitors.
“These drugs have reassuring safety profiles; low rates of infection and adverse reactions,” Dr. Cordoro said of biologic agents. “They’ve changed the landscape completely because now the expectation is complete or near-complete clearance. In contrast to the systemic agents, which may be started and stopped repeatedly, you need to think about continuous therapy, because these drugs are immunogenic,” she noted. “Whether antibodies against them become neutralizing or not is a different case. If a patient does have antibodies, it does not mean you have to stop the drug. Dose escalation can help. Increasing frequency of use of the drug can help, but patients will develop antibodies and it may result in loss of efficacy or reactions to the drug,” she added.
“When you’re thinking about using a biologic agent, think about patients who are chronic, moderate to severe, and who will need more long-term therapy. Most importantly, treatment should be individualized, as there is no ‘one size fits all’ approach.”
Dr. Cordoro disclosed that she is a member of the Celgene Corporation Scientific Steering Committee.
AUSTIN, TEX. – The way Kelly M. Cordoro sees it, the most for which patient.
“You can look up the dosing and frequency of these drugs; all of that’s available,” she said at the annual meeting of the Society for Pediatric Dermatology. “But how do we think about which drug for which patient? What are the considerations?”
Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, described psoriasis as an autoamplifying inflammatory cascade involving innate and adaptive immunity and noted that various components of that cascade represent treatment targets. “We don’t have a true comprehension of the pathophysiology of psoriasis, but as we learn the pathways, we’re targeting them,” she said. “You can target keratinocyte proliferation with drugs like retinoids and phototherapy. You can broadly target T cells, neutrophils, and dendritic cells with methotrexate, cyclosporine, and phototherapy. The newer drugs target the cytokine milieu, including TNF [tumor necrosis factor]–alpha, IL [interleukin]–17A, IL-12, and IL-23.”
There is no one right answer for which drug to prescribe, she continued, except in the cases of certain comorbidities, contraindications, and genetic variants. “For example, if a patient has psoriatic arthritis, then you have methotrexate and all of the biologics that might be disease modifying,” she said. “If a patient has inflammatory bowel disease, it’s critical to know that IL-17 inhibitors will flare that disease, but anti-TNF and IL-12 and IL-23 inhibitors are okay. If a patient has liver and kidney disease, you want to avoid methotrexate and cyclosporine. If there’s a female of childbearing potential you want to be very cautious with using retinoids. I think the harder question for us is, How about the rest of the patients?”
In addition to a drug’s mechanism of action, patient- and family-related factors play a role in deciding which agent to use. For example, does the patient prefer an oral or an injectable agent? Is the patient able to travel to a phototherapy center? Is it feasible for the family to manage visits for lab work and direct clinical monitoring? Does the family have a high level of health literacy and are you communicating with them in ways that facilitate shared decision making?
“The best way to choose a systemic therapy is to develop an individualized assessment of overall disease burden,” said Dr. Cordoro, who is also division chief of pediatric dermatology at UCSF. “Include psychological burden and subjective data in addition to objective measures like body surface area. Look for triggers. Infants are more commonly affected by viral infections and, in a subset, monogenic forms of psoriasis such as deficiency of interleukin 1 receptor antagonist [DIRA]. In general, we try to take a conservative approach in the developing child. As children hit early adolescence and become post pubertal, you have to start thinking about the psychosocial impact [of psoriasis], and we have to start treating patients with the consideration that chronic uncontrolled inflammation can potentially lead to comorbidities down the road. We see this in adults with severe psoriasis and early onset cardiovascular disease, the so-called psoriatic march from chronic inflammation to cardiovascular disease.”
Dr. Cordoro advises clinicians to rethink the conventional “therapeutic ladder” concept and embrace the idea of “finding the right tool for the job right now.” If a patient presents with a flare from a known trigger such as a strep infection, “maybe you want to treat with something more conservative,” she said. “Once you treat, and if the trigger has been managed, they might be better. But some patients will need the most aggressive treatment right out of the gate.”
Tried and true systemic therapies for psoriasis include methotrexate, cyclosporine, acitretin, and phototherapy, but none is approved by the Food and Drug Administration for use in children. “These drugs have decades of experience behind them,” Dr. Cordoro said. “Methotrexate is slow to start but has a sustained profile, so if you can get the patient to respond, that response tends to persist. Methotrexate also prevents the formation of antidrug antibodies, which is important if you are considering use of a biologic agent later on.”
Cyclosporine is best if you need a rapid rescue drug to get the disease under control before moving on to other options. “One in four patients relapse once cyclosporine is discontinued, so the benefit may not be as sustained as with methotrexate,” she said. “Acitretin is a really nice choice when you can’t or don’t want to immunosuppress the patient, and phototherapy is good if you can get it. The advantages of systemic therapies are that they’re easy on, easy off, and you can combine medications in severe situations. Almost all of these drugs can be combined with another, with few exceptions. I would caution that over immunosuppression is the biggest risk ... so this must be done carefully and only when necessary.”
Biologic agents such as TNF inhibitors and IL-12/23 inhibitors are playing an increasing role in pediatric psoriasis. They can be expensive and difficult for some insurance plans to cover, but offer the convenience of better efficacy and less frequent lab monitoring than conventional systemics. In the United States, etanercept and ustekinumab are approved for moderate to severe pediatric plaque psoriasis in patients as young as age 4 and 12 years, respectively. TNF inhibitors have accumulated the most data in children, while data are accumulating in trials of IL-17 inhibitors, IL-23 inhibitors, and PDE4 inhibitors.
“These drugs have reassuring safety profiles; low rates of infection and adverse reactions,” Dr. Cordoro said of biologic agents. “They’ve changed the landscape completely because now the expectation is complete or near-complete clearance. In contrast to the systemic agents, which may be started and stopped repeatedly, you need to think about continuous therapy, because these drugs are immunogenic,” she noted. “Whether antibodies against them become neutralizing or not is a different case. If a patient does have antibodies, it does not mean you have to stop the drug. Dose escalation can help. Increasing frequency of use of the drug can help, but patients will develop antibodies and it may result in loss of efficacy or reactions to the drug,” she added.
“When you’re thinking about using a biologic agent, think about patients who are chronic, moderate to severe, and who will need more long-term therapy. Most importantly, treatment should be individualized, as there is no ‘one size fits all’ approach.”
Dr. Cordoro disclosed that she is a member of the Celgene Corporation Scientific Steering Committee.
AUSTIN, TEX. – The way Kelly M. Cordoro sees it, the most for which patient.
“You can look up the dosing and frequency of these drugs; all of that’s available,” she said at the annual meeting of the Society for Pediatric Dermatology. “But how do we think about which drug for which patient? What are the considerations?”
Dr. Cordoro, professor of dermatology and pediatrics at the University of California, San Francisco, described psoriasis as an autoamplifying inflammatory cascade involving innate and adaptive immunity and noted that various components of that cascade represent treatment targets. “We don’t have a true comprehension of the pathophysiology of psoriasis, but as we learn the pathways, we’re targeting them,” she said. “You can target keratinocyte proliferation with drugs like retinoids and phototherapy. You can broadly target T cells, neutrophils, and dendritic cells with methotrexate, cyclosporine, and phototherapy. The newer drugs target the cytokine milieu, including TNF [tumor necrosis factor]–alpha, IL [interleukin]–17A, IL-12, and IL-23.”
There is no one right answer for which drug to prescribe, she continued, except in the cases of certain comorbidities, contraindications, and genetic variants. “For example, if a patient has psoriatic arthritis, then you have methotrexate and all of the biologics that might be disease modifying,” she said. “If a patient has inflammatory bowel disease, it’s critical to know that IL-17 inhibitors will flare that disease, but anti-TNF and IL-12 and IL-23 inhibitors are okay. If a patient has liver and kidney disease, you want to avoid methotrexate and cyclosporine. If there’s a female of childbearing potential you want to be very cautious with using retinoids. I think the harder question for us is, How about the rest of the patients?”
In addition to a drug’s mechanism of action, patient- and family-related factors play a role in deciding which agent to use. For example, does the patient prefer an oral or an injectable agent? Is the patient able to travel to a phototherapy center? Is it feasible for the family to manage visits for lab work and direct clinical monitoring? Does the family have a high level of health literacy and are you communicating with them in ways that facilitate shared decision making?
“The best way to choose a systemic therapy is to develop an individualized assessment of overall disease burden,” said Dr. Cordoro, who is also division chief of pediatric dermatology at UCSF. “Include psychological burden and subjective data in addition to objective measures like body surface area. Look for triggers. Infants are more commonly affected by viral infections and, in a subset, monogenic forms of psoriasis such as deficiency of interleukin 1 receptor antagonist [DIRA]. In general, we try to take a conservative approach in the developing child. As children hit early adolescence and become post pubertal, you have to start thinking about the psychosocial impact [of psoriasis], and we have to start treating patients with the consideration that chronic uncontrolled inflammation can potentially lead to comorbidities down the road. We see this in adults with severe psoriasis and early onset cardiovascular disease, the so-called psoriatic march from chronic inflammation to cardiovascular disease.”
Dr. Cordoro advises clinicians to rethink the conventional “therapeutic ladder” concept and embrace the idea of “finding the right tool for the job right now.” If a patient presents with a flare from a known trigger such as a strep infection, “maybe you want to treat with something more conservative,” she said. “Once you treat, and if the trigger has been managed, they might be better. But some patients will need the most aggressive treatment right out of the gate.”
Tried and true systemic therapies for psoriasis include methotrexate, cyclosporine, acitretin, and phototherapy, but none is approved by the Food and Drug Administration for use in children. “These drugs have decades of experience behind them,” Dr. Cordoro said. “Methotrexate is slow to start but has a sustained profile, so if you can get the patient to respond, that response tends to persist. Methotrexate also prevents the formation of antidrug antibodies, which is important if you are considering use of a biologic agent later on.”
Cyclosporine is best if you need a rapid rescue drug to get the disease under control before moving on to other options. “One in four patients relapse once cyclosporine is discontinued, so the benefit may not be as sustained as with methotrexate,” she said. “Acitretin is a really nice choice when you can’t or don’t want to immunosuppress the patient, and phototherapy is good if you can get it. The advantages of systemic therapies are that they’re easy on, easy off, and you can combine medications in severe situations. Almost all of these drugs can be combined with another, with few exceptions. I would caution that over immunosuppression is the biggest risk ... so this must be done carefully and only when necessary.”
Biologic agents such as TNF inhibitors and IL-12/23 inhibitors are playing an increasing role in pediatric psoriasis. They can be expensive and difficult for some insurance plans to cover, but offer the convenience of better efficacy and less frequent lab monitoring than conventional systemics. In the United States, etanercept and ustekinumab are approved for moderate to severe pediatric plaque psoriasis in patients as young as age 4 and 12 years, respectively. TNF inhibitors have accumulated the most data in children, while data are accumulating in trials of IL-17 inhibitors, IL-23 inhibitors, and PDE4 inhibitors.
“These drugs have reassuring safety profiles; low rates of infection and adverse reactions,” Dr. Cordoro said of biologic agents. “They’ve changed the landscape completely because now the expectation is complete or near-complete clearance. In contrast to the systemic agents, which may be started and stopped repeatedly, you need to think about continuous therapy, because these drugs are immunogenic,” she noted. “Whether antibodies against them become neutralizing or not is a different case. If a patient does have antibodies, it does not mean you have to stop the drug. Dose escalation can help. Increasing frequency of use of the drug can help, but patients will develop antibodies and it may result in loss of efficacy or reactions to the drug,” she added.
“When you’re thinking about using a biologic agent, think about patients who are chronic, moderate to severe, and who will need more long-term therapy. Most importantly, treatment should be individualized, as there is no ‘one size fits all’ approach.”
Dr. Cordoro disclosed that she is a member of the Celgene Corporation Scientific Steering Committee.
EXPERT ANALYSIS FROM SPD 2019
Rituximab, bendamustine look better than chemo alone in MCL
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
In older patients with newly diagnosed mantle cell lymphoma (MCL), first-line therapy with rituximab- and bendamustine-based regimens significantly reduced 1-year mortality rates versus chemotherapy alone, according to a retrospective analysis.
“This study evaluated the comparative effectiveness of [rituximab, bortezomib, or bendamustine] in elderly patients newly diagnosed with MCL,” wrote Shuangshuang Fu, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. The findings were reported in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied population-based data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. They identified all patients over age 65 years who received a new diagnosis of MCL between Jan. 1, 1999, and Dec. 31, 2013.
The study cohort included a total of 1,215 patients. Participants were classified into four different groups according to treatment regimen: chemotherapy alone, rituximab plus or minus chemotherapy, bendamustine plus or minus chemotherapy, and bortezomib plus or minus chemotherapy.
At 1-year follow-up, the team analyzed various mortality outcomes, including MCL-specific, all-cause, and noncancer mortality. The bortezomib results were not included in the primary analysis because of small sample size, according to the researchers.
After multivariable analysis, Dr. Fu and colleagues found that 1-year all-cause mortality rate was significantly lower for patients receiving rituximab-based regimens, compared with chemotherapy alone (hazard ratio, 0.38; 95% confidence interval, 0.25-0.59). There was a similar decline for MCL-specific mortality (HR, 0.38; 95% CI, 0.24-0.60).
The 1-year MCL-specific mortality was also significantly reduced in the bendamustine group, compared with chemotherapy alone (HR, 0.49; 95% CI, 0.24-0.99).
“Our findings comparing rituximab with chemotherapy alone further confirmed the benefit of adding rituximab to chemotherapy in newly diagnosed older MCL patients,” they wrote.
The researchers acknowledged that a key limitation of the study was the observational design. As a result, selection bias and unmeasured confounding could have influenced the results.
“Future studies evaluating the comparative effectiveness of those newly approved novel agents for MCL patients were warranted as more data are available,” they concluded.
The study was funded by the Duncan Family Institute, the Cancer Prevention Research Institute of Texas, and the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Fu S et al. Clin Lymphoma Myeloma Leuk. 2019 Aug 30. doi: 10.1016/j.clml.2019.08.014.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
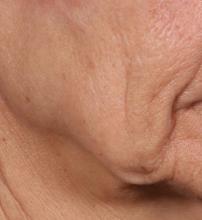
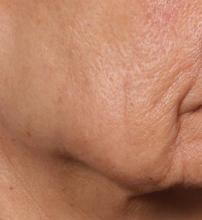
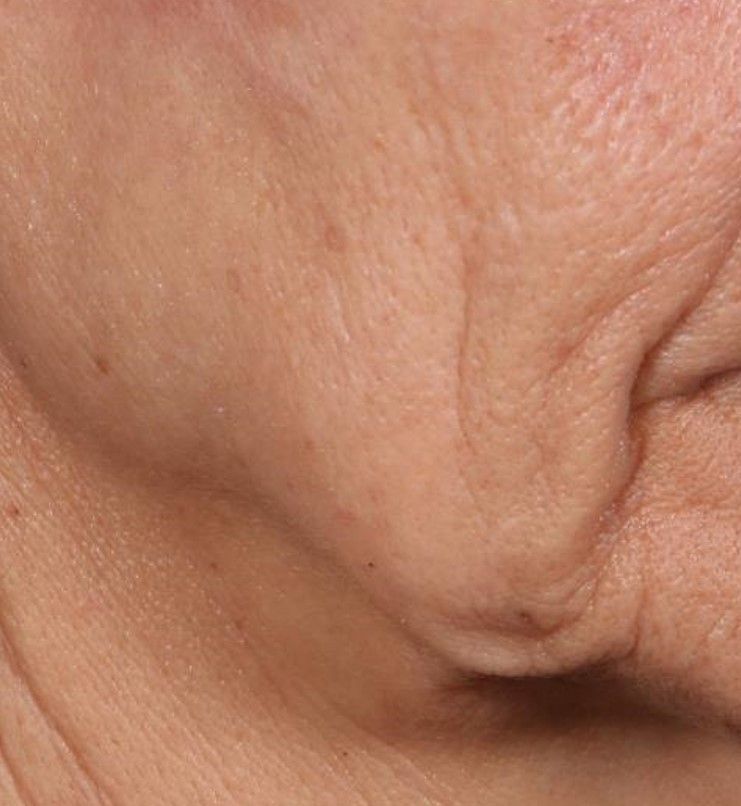
Novel dermal microcoring device holds promise for moderate to severe wrinkles
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
SAN DIEGO – is poised to become a game-changer in the minimally invasive aesthetics field.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the device features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“The idea is to get more significant improvement of tissue laxity by fractionally removing the skin,” Mathew M. Avram, MD, JD, explained at the annual Masters of Aesthetics Symposium. “You can do a facelift by cutting the skin on the side and pulling it back. This is skin tightening and improvement of wrinkles with a thousand micro punches. It’s called fractional tissue extraction.”
Instead of relying on laser, heat, light, or radiofrequency, the device uses needle mechanics to extract microsized cores of full-thickness skin below the size threshold that causes scarring. “Then, you have biomechanical remodeling; you close the channels right away,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “If you remove skin that is smaller than 500 micrometers in size, no scar is left behind.”
In trials of the device being carried out by Cytrellis Biosystems, more than 100 patients have been treated one to two times. During a separate presentation, one of the device investigators, Jill S. Waibel, MD, said that areas of treatment have included the upper and lower cheeks, perioral areas, and the submentum. On average, the amount of skin removed during each treatment session ranges from 5% to 8.5% and the mean down time is 3.8 days. According to combined data from two studies of 30 patients who had 60 areas treated and were followed at 90 or 180 days, subjects experienced an average 1.1 grade improvement on the Lemperle Rating Scale and showed an 80% improvement in moderate or severe wrinkles. In addition, 91% of investigators rated treatment areas as “improved” or “very much improved” on the Global Aesthetic Improvement Scale, and 88% of subjects were “satisfied” or “extremely satisfied” with the results.
“The safety profile of this device is amazing,” said Dr. Waibel, a dermatologist and owner of the Miami Dermatology and Laser Institute. “It provides an entirely new mode of treatment for skin laxity through highly approachable tissue removal with minimal to no pain or downtime. You can visually see the cores close through Optical Coherence Tomography before patients even leave the office. There have also been virtually no side effects except in one patient at another site who had minor postinflammatory hyperpigmentation.”
The device allows for local and scarless treatment of wrinkles in the areas in which they form, she continued, so results are natural and true to the underlying anatomy. “While its target testing has been in more severely lax patients, I think it has a great future for younger patients who want to stave off a future face lift,” Dr. Waibel said. “Initial treatments required preoperative lidocaine injections. However, recent trials using more tolerable analgesic methods have shown that this may not even be necessary. This is very exciting new technology and I have high hopes for the future of this device.”
Histological analysis from baseline to 60-90 days post treatment showed homogenization of elastosis, which signals reorganization of the papillary dermis, Dr. Avram said. It also showed a decrease in the grenz zone, rete ridge flattening, a slight increase in the collagen-to-elastin ratio, and no scarring. Pending clearance, he said, the device could be commercially available in 2020.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, Soliton, and Zalea. He also reported having ownership and/or shareholder interest in Cytrellis, Invasix, and Zalea and intellectual property rights with Cytrellis.
Dr. Waibel disclosed that she has conducted clinical research for AbbVie, Aquavit, Cytrellis, Lumenis, Lutronic, Michelson Diagnostics, RegenX, Sciton, Sebacia, and Syneron/Candela. She is also a consultant for RegenX, Strata, and Syneron/Candela and is a member of the advisory board for Dominion Technologies, Sciton, and Sebacia.
EXPERT ANALYSIS FROM MOA 2019
FDA approves nintedanib for scleroderma interstitial lung disease
The Food and Drug Administration has approved nintedanib (Ofev) for the rare but sometimes deadly form of interstitial lung disease that’s caused by systemic sclerosis, or scleroderma.
Although scleroderma itself is rare, half of those patients present with scleroderma-related interstitial lung disease (SSc-ILD), and it remains the leading cause of death in scleroderma patients because it can lead to loss of pulmonary function. Nintedanib appears to slow the progress of SSc-ILD and is the first treatment approved for it, according to a news release from the FDA.
The approval is based on a randomized, double-blind, placebo-controlled trial of 576 patients aged 20-79 years with SSc-ILD. The primary efficacy endpoint was forced vital capacity, and patients on nintedanib showed less decline than did those on placebo.
The most frequent serious adverse event reported in this trial was pneumonia (2.8% with nintedanib vs. 0.3% with placebo). Adverse reactions that led to permanent dose reductions occurred in 34% of nintedanib patients and 4% of placebo-treated patients; the most common of these was diarrhea.
The full prescribing information, which is available on the FDA website, includes warnings for patients with moderate to severe hepatic impairment, elevated liver enzymes, and drug-induced liver injury, as well as those with gastrointestinal disorders. Nintedanib may cause embryo-fetal toxicity, so women of childbearing age should be counseled to avoid pregnancy while taking this drug.
Nintedanib received both Priority Review and Orphan Drug designation. The former meant the FDA intends to take action on the application within 6 months because the agency has determined that, if approved, it would have important effects on treatment of a serious condition. The latter provides incentives to assist and encourage development of drugs for rare diseases. The drug was approved in 2014 for adult patients with idiopathic pulmonary fibrosis, another interstitial lung disease.
The full release is available on the FDA website.
The Food and Drug Administration has approved nintedanib (Ofev) for the rare but sometimes deadly form of interstitial lung disease that’s caused by systemic sclerosis, or scleroderma.
Although scleroderma itself is rare, half of those patients present with scleroderma-related interstitial lung disease (SSc-ILD), and it remains the leading cause of death in scleroderma patients because it can lead to loss of pulmonary function. Nintedanib appears to slow the progress of SSc-ILD and is the first treatment approved for it, according to a news release from the FDA.
The approval is based on a randomized, double-blind, placebo-controlled trial of 576 patients aged 20-79 years with SSc-ILD. The primary efficacy endpoint was forced vital capacity, and patients on nintedanib showed less decline than did those on placebo.
The most frequent serious adverse event reported in this trial was pneumonia (2.8% with nintedanib vs. 0.3% with placebo). Adverse reactions that led to permanent dose reductions occurred in 34% of nintedanib patients and 4% of placebo-treated patients; the most common of these was diarrhea.
The full prescribing information, which is available on the FDA website, includes warnings for patients with moderate to severe hepatic impairment, elevated liver enzymes, and drug-induced liver injury, as well as those with gastrointestinal disorders. Nintedanib may cause embryo-fetal toxicity, so women of childbearing age should be counseled to avoid pregnancy while taking this drug.
Nintedanib received both Priority Review and Orphan Drug designation. The former meant the FDA intends to take action on the application within 6 months because the agency has determined that, if approved, it would have important effects on treatment of a serious condition. The latter provides incentives to assist and encourage development of drugs for rare diseases. The drug was approved in 2014 for adult patients with idiopathic pulmonary fibrosis, another interstitial lung disease.
The full release is available on the FDA website.
The Food and Drug Administration has approved nintedanib (Ofev) for the rare but sometimes deadly form of interstitial lung disease that’s caused by systemic sclerosis, or scleroderma.
Although scleroderma itself is rare, half of those patients present with scleroderma-related interstitial lung disease (SSc-ILD), and it remains the leading cause of death in scleroderma patients because it can lead to loss of pulmonary function. Nintedanib appears to slow the progress of SSc-ILD and is the first treatment approved for it, according to a news release from the FDA.
The approval is based on a randomized, double-blind, placebo-controlled trial of 576 patients aged 20-79 years with SSc-ILD. The primary efficacy endpoint was forced vital capacity, and patients on nintedanib showed less decline than did those on placebo.
The most frequent serious adverse event reported in this trial was pneumonia (2.8% with nintedanib vs. 0.3% with placebo). Adverse reactions that led to permanent dose reductions occurred in 34% of nintedanib patients and 4% of placebo-treated patients; the most common of these was diarrhea.
The full prescribing information, which is available on the FDA website, includes warnings for patients with moderate to severe hepatic impairment, elevated liver enzymes, and drug-induced liver injury, as well as those with gastrointestinal disorders. Nintedanib may cause embryo-fetal toxicity, so women of childbearing age should be counseled to avoid pregnancy while taking this drug.
Nintedanib received both Priority Review and Orphan Drug designation. The former meant the FDA intends to take action on the application within 6 months because the agency has determined that, if approved, it would have important effects on treatment of a serious condition. The latter provides incentives to assist and encourage development of drugs for rare diseases. The drug was approved in 2014 for adult patients with idiopathic pulmonary fibrosis, another interstitial lung disease.
The full release is available on the FDA website.
CVS-Aetna merger approval gets poor review from physicians
Physician groups are criticizing a judge’s decision to approve the merger of pharmacy chain CVS Health and health insurer Aetna, saying the deal will raise prices and lower quality.
Judge Richard J. Leon of the U.S. District Court for the District of Columbia allowed the merger to move forward on Sept. 4, ruling that the acquisition was legal under antitrust law. The merger was approved by the Department of Justice in October 2018 on the condition that Aetna sell its Medicare prescription drug plan (PDP) business to independently owned competitor, WellCare Health Plans. Judge Leon has been examining the government’s plan since late 2018.
In his decision, Judge Leon acknowledged that the merger will have widespread effects for millions of patients and noted the many industry stakeholders, consumer groups, and state regulatory bodies have raised concerns about the merger.
“Although [the opposition] raised substantial concerns that warranted serious consideration, CVS’s and the government’s witnesses, when combined with the existing record, persuasively support why the markets at issue are not only very competitive today, but are likely to remain so post merger,” Judge Leon wrote. “Consequently, the harms to the public interest the [opposition] raised were not sufficiently established to undermine the government’s conclusion to the contrary. As such, for all of the above reasons, I have concluded that the proposed settlement is well ‘within the reaches’ of the public interest and the government’s motion ... should therefore be granted.”
Patrice A. Harris, MD, president for the American Medical Association, said the judge’s decision fails patients and will likely raise prices, lower quality, reduce choice, and stifle innovation.
“The American people and our health system will not be served well by allowing a merger that combines health insurance giant Aetna Inc. with CVS Health Corporation,” Dr. Harris said in a statement. “For patients and employers struggling with recurrent increases to health insurance premiums, out-of-pocket costs, and prescription drug prices, it’s hard to find any upside to a merger that leaves them with fewer choices. Nothing in the deal guarantees reductions on insurance premiums or prescription drug costs. As for promised efficiency savings, that money will likely go straight to CVS’s bottom line.”
Angus Worthing, MD, government affairs committee chair for the American College of Rheumatology, said his group is concerned that the merger will hinder progress that has been made toward creating cost transparency and will make it easier for costs savings to remain secret.
“We hope that regulators will now actively watch the conduct of the merged company to ensure patients are protected,” Dr. Worthing said in a statement.
In a statement, CVS noted that CVS Health and Aetna have been one company since November 2018, stating that the court’s action “makes that 100 percent clear.”
“We remain focused on transforming the consumer health care experience in America,” they said.
CVS Health announced it would buy Aetna for $69 billion in 2017 and finalized the acquisition in 2018. CVS Health President and CEO Larry J. Merlo said the combined company would connect consumers with the powerful health resources of CVS Health in communities across the country and Aetna’s network of providers to help remove barriers to high quality care and build lasting relationships with patients.
Assistant Attorney General Makan Delrahim of the Justice Department’s antitrust division said the agency was pleased with the court’s decision to approve the government’s plan and finalize the merger.
“The divestiture of Aetna’s individual PDP business provides a comprehensive remedy to the harms the Justice Department identified,” Mr. Delrahim said in a statement. “The entry of the final judgment protects seniors and other vulnerable customers of individual PDPs from the anticompetitive effects that would have occurred if CVS and Aetna had merged their individual PDP businesses.”
Physician groups are criticizing a judge’s decision to approve the merger of pharmacy chain CVS Health and health insurer Aetna, saying the deal will raise prices and lower quality.
Judge Richard J. Leon of the U.S. District Court for the District of Columbia allowed the merger to move forward on Sept. 4, ruling that the acquisition was legal under antitrust law. The merger was approved by the Department of Justice in October 2018 on the condition that Aetna sell its Medicare prescription drug plan (PDP) business to independently owned competitor, WellCare Health Plans. Judge Leon has been examining the government’s plan since late 2018.
In his decision, Judge Leon acknowledged that the merger will have widespread effects for millions of patients and noted the many industry stakeholders, consumer groups, and state regulatory bodies have raised concerns about the merger.
“Although [the opposition] raised substantial concerns that warranted serious consideration, CVS’s and the government’s witnesses, when combined with the existing record, persuasively support why the markets at issue are not only very competitive today, but are likely to remain so post merger,” Judge Leon wrote. “Consequently, the harms to the public interest the [opposition] raised were not sufficiently established to undermine the government’s conclusion to the contrary. As such, for all of the above reasons, I have concluded that the proposed settlement is well ‘within the reaches’ of the public interest and the government’s motion ... should therefore be granted.”
Patrice A. Harris, MD, president for the American Medical Association, said the judge’s decision fails patients and will likely raise prices, lower quality, reduce choice, and stifle innovation.
“The American people and our health system will not be served well by allowing a merger that combines health insurance giant Aetna Inc. with CVS Health Corporation,” Dr. Harris said in a statement. “For patients and employers struggling with recurrent increases to health insurance premiums, out-of-pocket costs, and prescription drug prices, it’s hard to find any upside to a merger that leaves them with fewer choices. Nothing in the deal guarantees reductions on insurance premiums or prescription drug costs. As for promised efficiency savings, that money will likely go straight to CVS’s bottom line.”
Angus Worthing, MD, government affairs committee chair for the American College of Rheumatology, said his group is concerned that the merger will hinder progress that has been made toward creating cost transparency and will make it easier for costs savings to remain secret.
“We hope that regulators will now actively watch the conduct of the merged company to ensure patients are protected,” Dr. Worthing said in a statement.
In a statement, CVS noted that CVS Health and Aetna have been one company since November 2018, stating that the court’s action “makes that 100 percent clear.”
“We remain focused on transforming the consumer health care experience in America,” they said.
CVS Health announced it would buy Aetna for $69 billion in 2017 and finalized the acquisition in 2018. CVS Health President and CEO Larry J. Merlo said the combined company would connect consumers with the powerful health resources of CVS Health in communities across the country and Aetna’s network of providers to help remove barriers to high quality care and build lasting relationships with patients.
Assistant Attorney General Makan Delrahim of the Justice Department’s antitrust division said the agency was pleased with the court’s decision to approve the government’s plan and finalize the merger.
“The divestiture of Aetna’s individual PDP business provides a comprehensive remedy to the harms the Justice Department identified,” Mr. Delrahim said in a statement. “The entry of the final judgment protects seniors and other vulnerable customers of individual PDPs from the anticompetitive effects that would have occurred if CVS and Aetna had merged their individual PDP businesses.”
Physician groups are criticizing a judge’s decision to approve the merger of pharmacy chain CVS Health and health insurer Aetna, saying the deal will raise prices and lower quality.
Judge Richard J. Leon of the U.S. District Court for the District of Columbia allowed the merger to move forward on Sept. 4, ruling that the acquisition was legal under antitrust law. The merger was approved by the Department of Justice in October 2018 on the condition that Aetna sell its Medicare prescription drug plan (PDP) business to independently owned competitor, WellCare Health Plans. Judge Leon has been examining the government’s plan since late 2018.
In his decision, Judge Leon acknowledged that the merger will have widespread effects for millions of patients and noted the many industry stakeholders, consumer groups, and state regulatory bodies have raised concerns about the merger.
“Although [the opposition] raised substantial concerns that warranted serious consideration, CVS’s and the government’s witnesses, when combined with the existing record, persuasively support why the markets at issue are not only very competitive today, but are likely to remain so post merger,” Judge Leon wrote. “Consequently, the harms to the public interest the [opposition] raised were not sufficiently established to undermine the government’s conclusion to the contrary. As such, for all of the above reasons, I have concluded that the proposed settlement is well ‘within the reaches’ of the public interest and the government’s motion ... should therefore be granted.”
Patrice A. Harris, MD, president for the American Medical Association, said the judge’s decision fails patients and will likely raise prices, lower quality, reduce choice, and stifle innovation.
“The American people and our health system will not be served well by allowing a merger that combines health insurance giant Aetna Inc. with CVS Health Corporation,” Dr. Harris said in a statement. “For patients and employers struggling with recurrent increases to health insurance premiums, out-of-pocket costs, and prescription drug prices, it’s hard to find any upside to a merger that leaves them with fewer choices. Nothing in the deal guarantees reductions on insurance premiums or prescription drug costs. As for promised efficiency savings, that money will likely go straight to CVS’s bottom line.”
Angus Worthing, MD, government affairs committee chair for the American College of Rheumatology, said his group is concerned that the merger will hinder progress that has been made toward creating cost transparency and will make it easier for costs savings to remain secret.
“We hope that regulators will now actively watch the conduct of the merged company to ensure patients are protected,” Dr. Worthing said in a statement.
In a statement, CVS noted that CVS Health and Aetna have been one company since November 2018, stating that the court’s action “makes that 100 percent clear.”
“We remain focused on transforming the consumer health care experience in America,” they said.
CVS Health announced it would buy Aetna for $69 billion in 2017 and finalized the acquisition in 2018. CVS Health President and CEO Larry J. Merlo said the combined company would connect consumers with the powerful health resources of CVS Health in communities across the country and Aetna’s network of providers to help remove barriers to high quality care and build lasting relationships with patients.
Assistant Attorney General Makan Delrahim of the Justice Department’s antitrust division said the agency was pleased with the court’s decision to approve the government’s plan and finalize the merger.
“The divestiture of Aetna’s individual PDP business provides a comprehensive remedy to the harms the Justice Department identified,” Mr. Delrahim said in a statement. “The entry of the final judgment protects seniors and other vulnerable customers of individual PDPs from the anticompetitive effects that would have occurred if CVS and Aetna had merged their individual PDP businesses.”
Less CPAP time linked to exacerbation in COPD/OSA overlap syndrome
and all‐cause mortality, according to a retrospective cohort study.
“These factors should be taken into account when considering the management and prognosis of these patients,” the researchers said in the Clinical Respiratory Journal.
Prior studies have found that patients with COPD and OSA – that is, with overlap syndrome – “have a substantially greater risk of morbidity and mortality, compared to those with either COPD or OSA alone,” said Philippe E. Jaoude, MD, and Ali A. El Solh, MD, both of the Veterans Affairs Western New York Healthcare System in Buffalo and the University at Buffalo.
To identify factors associated with COPD exacerbation and all‐cause mortality in patients with overlap syndrome, Dr. Jaoude and Dr. El Solh reviewed the electronic health records of patients with simultaneous COPD and OSA. They compared patients with overlap syndrome who had an acute exacerbation of COPD during a 42-month period with a control group of patients with overlap syndrome who did not have exacerbations during that time. Patients with exacerbations and controls were matched 1:1 by age and body mass index.
Eligible patients were aged 42-90 years, had objectively confirmed COPD, and had documented OSA by in-laboratory polysomnography (that is, at least five obstructive apneas and hypopneas per hour). The investigators defined a COPD exacerbation as a sustained worsening of a patient’s respiratory condition that warranted additional treatment.
Of 225 eligible patients, 92 had at least one COPD exacerbation between March 2014 and September 2017. Patients with COPD exacerbation and controls had a mean age of about 68 years. The group of patients with exacerbation had a higher percentage of active smokers (21% vs. 9%) and had poorer lung function (mean forced expiratory volume in 1 second percent predicted: 55.2% vs. 64.5%).
“Although the rate of CPAP adherence between the two groups was not significantly different, the average time of CPAP use was significantly higher in patients with no recorded exacerbation,” the researchers reported – 285.4 min/night versus 238.2 min/night.
In all, 146 patients (79.4%) survived, and 38 patients (20.6%) died during the study period. The crude mortality rate was significantly higher in the group with COPD exacerbations (14% vs. 7%).
“Multivariate logistic regression analysis identified the independent risk factors associated with COPD exacerbations as active smoking, worse airflow limitation, and lower CPAP utilization,” they said. “As for all-cause mortality, a higher burden of comorbidities, worse airflow limitation, and lower time of CPAP use were independently associated with poor outcome.”
The researchers noted that they cannot rule out the possibility that patients who were adherent to CPAP were systematically different from those who were not.
The authors had no conflicts of interest.
SOURCE: Jaoude P et al. Clin Respir J. 2019 Aug 22. doi: 10.1111/crj.13079.
and all‐cause mortality, according to a retrospective cohort study.
“These factors should be taken into account when considering the management and prognosis of these patients,” the researchers said in the Clinical Respiratory Journal.
Prior studies have found that patients with COPD and OSA – that is, with overlap syndrome – “have a substantially greater risk of morbidity and mortality, compared to those with either COPD or OSA alone,” said Philippe E. Jaoude, MD, and Ali A. El Solh, MD, both of the Veterans Affairs Western New York Healthcare System in Buffalo and the University at Buffalo.
To identify factors associated with COPD exacerbation and all‐cause mortality in patients with overlap syndrome, Dr. Jaoude and Dr. El Solh reviewed the electronic health records of patients with simultaneous COPD and OSA. They compared patients with overlap syndrome who had an acute exacerbation of COPD during a 42-month period with a control group of patients with overlap syndrome who did not have exacerbations during that time. Patients with exacerbations and controls were matched 1:1 by age and body mass index.
Eligible patients were aged 42-90 years, had objectively confirmed COPD, and had documented OSA by in-laboratory polysomnography (that is, at least five obstructive apneas and hypopneas per hour). The investigators defined a COPD exacerbation as a sustained worsening of a patient’s respiratory condition that warranted additional treatment.
Of 225 eligible patients, 92 had at least one COPD exacerbation between March 2014 and September 2017. Patients with COPD exacerbation and controls had a mean age of about 68 years. The group of patients with exacerbation had a higher percentage of active smokers (21% vs. 9%) and had poorer lung function (mean forced expiratory volume in 1 second percent predicted: 55.2% vs. 64.5%).
“Although the rate of CPAP adherence between the two groups was not significantly different, the average time of CPAP use was significantly higher in patients with no recorded exacerbation,” the researchers reported – 285.4 min/night versus 238.2 min/night.
In all, 146 patients (79.4%) survived, and 38 patients (20.6%) died during the study period. The crude mortality rate was significantly higher in the group with COPD exacerbations (14% vs. 7%).
“Multivariate logistic regression analysis identified the independent risk factors associated with COPD exacerbations as active smoking, worse airflow limitation, and lower CPAP utilization,” they said. “As for all-cause mortality, a higher burden of comorbidities, worse airflow limitation, and lower time of CPAP use were independently associated with poor outcome.”
The researchers noted that they cannot rule out the possibility that patients who were adherent to CPAP were systematically different from those who were not.
The authors had no conflicts of interest.
SOURCE: Jaoude P et al. Clin Respir J. 2019 Aug 22. doi: 10.1111/crj.13079.
and all‐cause mortality, according to a retrospective cohort study.
“These factors should be taken into account when considering the management and prognosis of these patients,” the researchers said in the Clinical Respiratory Journal.
Prior studies have found that patients with COPD and OSA – that is, with overlap syndrome – “have a substantially greater risk of morbidity and mortality, compared to those with either COPD or OSA alone,” said Philippe E. Jaoude, MD, and Ali A. El Solh, MD, both of the Veterans Affairs Western New York Healthcare System in Buffalo and the University at Buffalo.
To identify factors associated with COPD exacerbation and all‐cause mortality in patients with overlap syndrome, Dr. Jaoude and Dr. El Solh reviewed the electronic health records of patients with simultaneous COPD and OSA. They compared patients with overlap syndrome who had an acute exacerbation of COPD during a 42-month period with a control group of patients with overlap syndrome who did not have exacerbations during that time. Patients with exacerbations and controls were matched 1:1 by age and body mass index.
Eligible patients were aged 42-90 years, had objectively confirmed COPD, and had documented OSA by in-laboratory polysomnography (that is, at least five obstructive apneas and hypopneas per hour). The investigators defined a COPD exacerbation as a sustained worsening of a patient’s respiratory condition that warranted additional treatment.
Of 225 eligible patients, 92 had at least one COPD exacerbation between March 2014 and September 2017. Patients with COPD exacerbation and controls had a mean age of about 68 years. The group of patients with exacerbation had a higher percentage of active smokers (21% vs. 9%) and had poorer lung function (mean forced expiratory volume in 1 second percent predicted: 55.2% vs. 64.5%).
“Although the rate of CPAP adherence between the two groups was not significantly different, the average time of CPAP use was significantly higher in patients with no recorded exacerbation,” the researchers reported – 285.4 min/night versus 238.2 min/night.
In all, 146 patients (79.4%) survived, and 38 patients (20.6%) died during the study period. The crude mortality rate was significantly higher in the group with COPD exacerbations (14% vs. 7%).
“Multivariate logistic regression analysis identified the independent risk factors associated with COPD exacerbations as active smoking, worse airflow limitation, and lower CPAP utilization,” they said. “As for all-cause mortality, a higher burden of comorbidities, worse airflow limitation, and lower time of CPAP use were independently associated with poor outcome.”
The researchers noted that they cannot rule out the possibility that patients who were adherent to CPAP were systematically different from those who were not.
The authors had no conflicts of interest.
SOURCE: Jaoude P et al. Clin Respir J. 2019 Aug 22. doi: 10.1111/crj.13079.
FROM CLINICAL RESPIRATORY JOURNAL
New study confirms rise in U.S. suicide rates, particularly in rural areas
County-by-county analysis cites links to higher density of gun shops, other factors
Suicide rates in the United States climbed from 1999 to 2016, a new cross-sectional study found, and the increases were highest in rural areas.
“These findings are consistent with previous studies demonstrating higher and more rapidly increasing suicide rates in rural areas and are of considerable interest in light of the work by [Anne] Case and [Angus] Deaton,” wrote Danielle L. Steelesmith, PhD, and associates. “While increasing rates of suicide are well documented, little is known about contextual factors associated with county-level suicide rates.” The findings appear in JAMA Network Open.
To examine those contextual factors, Dr. Steelesmith, of the department of psychiatry and behavioral health at the Ohio State University, Columbus, and associates analyzed county-by-county suicide statistics from 1999 to 2016 for adults aged 25-64 years, noting that they “focused on this age range because most studies on mortality trends have focused on this age range.”
The researchers developed 3-year suicide averages for counties for rate “stabilization” purposes. They placed the counties into four categories (large metropolitan, small metropolitan, micropolitan, and rural), and used various data sources to gather various types of statistics about the communities.
Most of those who died by suicide were men (77%), and most (51%) were aged 45-64 years. The median suicide rate per county rose from 15 per 100,000 (1999-2001) to 21 per 100,000 (2014-2016), reported Dr. Steelesmith and associates.
Rural counties only made up 2% of the suicides, compared with 81% in large and small metropolitan counties, but suicide rates were “increasing most rapidly in rural areas, although all county types saw increases during the period studied,” Dr. Steelesmith and associates wrote.
They added that “counties with the highest excess risk of suicide tended to be in Western states (e.g., Colorado, New Mexico, Utah, and Wyoming), Appalachia (e.g., Kentucky, Virginia, and West Virginia), and the Ozarks (e.g., Arkansas and Missouri).”
In addition to the connections between increasing suicide rates, living in a rural area, and a higher density of gun shops, the researchers cited other contextual factors. Among those factors were higher median age and higher percentages of non-Hispanic whites, numbers of residents without health insurance, and veterans. They also found links between higher suicide rates and worse numbers on indexes designed to measure social capital; social fragmentation; and deprivation, a measure encompassing lower education, employment levels, and income.
“Long-term and persistent poverty appears to be more entrenched and economic opportunities more constrained in rural areas,” Dr. Steelesmith and associates wrote. “Greater social isolation, challenges related to transportation and interpersonal communication, and associated difficulties accessing health and mental health services likely contribute to the disproportionate association of deprivation with suicide in rural counties.”
Dr. Steelesmith and associates cited several limitations. One key limitation is that, because the study looked only at adults aged 25-64 years, the results might not be generalizable to youth or elderly adults.
No study funding was reported. One study author reported serving on the scientific advisory board of Clarigent Health and receiving grant support from the National Institute of Mental Health outside of the submitted work. No other disclosures were reported.
SOURCE: Steelesmith DL et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10936.
County-by-county analysis cites links to higher density of gun shops, other factors
County-by-county analysis cites links to higher density of gun shops, other factors
Suicide rates in the United States climbed from 1999 to 2016, a new cross-sectional study found, and the increases were highest in rural areas.
“These findings are consistent with previous studies demonstrating higher and more rapidly increasing suicide rates in rural areas and are of considerable interest in light of the work by [Anne] Case and [Angus] Deaton,” wrote Danielle L. Steelesmith, PhD, and associates. “While increasing rates of suicide are well documented, little is known about contextual factors associated with county-level suicide rates.” The findings appear in JAMA Network Open.
To examine those contextual factors, Dr. Steelesmith, of the department of psychiatry and behavioral health at the Ohio State University, Columbus, and associates analyzed county-by-county suicide statistics from 1999 to 2016 for adults aged 25-64 years, noting that they “focused on this age range because most studies on mortality trends have focused on this age range.”
The researchers developed 3-year suicide averages for counties for rate “stabilization” purposes. They placed the counties into four categories (large metropolitan, small metropolitan, micropolitan, and rural), and used various data sources to gather various types of statistics about the communities.
Most of those who died by suicide were men (77%), and most (51%) were aged 45-64 years. The median suicide rate per county rose from 15 per 100,000 (1999-2001) to 21 per 100,000 (2014-2016), reported Dr. Steelesmith and associates.
Rural counties only made up 2% of the suicides, compared with 81% in large and small metropolitan counties, but suicide rates were “increasing most rapidly in rural areas, although all county types saw increases during the period studied,” Dr. Steelesmith and associates wrote.
They added that “counties with the highest excess risk of suicide tended to be in Western states (e.g., Colorado, New Mexico, Utah, and Wyoming), Appalachia (e.g., Kentucky, Virginia, and West Virginia), and the Ozarks (e.g., Arkansas and Missouri).”
In addition to the connections between increasing suicide rates, living in a rural area, and a higher density of gun shops, the researchers cited other contextual factors. Among those factors were higher median age and higher percentages of non-Hispanic whites, numbers of residents without health insurance, and veterans. They also found links between higher suicide rates and worse numbers on indexes designed to measure social capital; social fragmentation; and deprivation, a measure encompassing lower education, employment levels, and income.
“Long-term and persistent poverty appears to be more entrenched and economic opportunities more constrained in rural areas,” Dr. Steelesmith and associates wrote. “Greater social isolation, challenges related to transportation and interpersonal communication, and associated difficulties accessing health and mental health services likely contribute to the disproportionate association of deprivation with suicide in rural counties.”
Dr. Steelesmith and associates cited several limitations. One key limitation is that, because the study looked only at adults aged 25-64 years, the results might not be generalizable to youth or elderly adults.
No study funding was reported. One study author reported serving on the scientific advisory board of Clarigent Health and receiving grant support from the National Institute of Mental Health outside of the submitted work. No other disclosures were reported.
SOURCE: Steelesmith DL et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10936.
Suicide rates in the United States climbed from 1999 to 2016, a new cross-sectional study found, and the increases were highest in rural areas.
“These findings are consistent with previous studies demonstrating higher and more rapidly increasing suicide rates in rural areas and are of considerable interest in light of the work by [Anne] Case and [Angus] Deaton,” wrote Danielle L. Steelesmith, PhD, and associates. “While increasing rates of suicide are well documented, little is known about contextual factors associated with county-level suicide rates.” The findings appear in JAMA Network Open.
To examine those contextual factors, Dr. Steelesmith, of the department of psychiatry and behavioral health at the Ohio State University, Columbus, and associates analyzed county-by-county suicide statistics from 1999 to 2016 for adults aged 25-64 years, noting that they “focused on this age range because most studies on mortality trends have focused on this age range.”
The researchers developed 3-year suicide averages for counties for rate “stabilization” purposes. They placed the counties into four categories (large metropolitan, small metropolitan, micropolitan, and rural), and used various data sources to gather various types of statistics about the communities.
Most of those who died by suicide were men (77%), and most (51%) were aged 45-64 years. The median suicide rate per county rose from 15 per 100,000 (1999-2001) to 21 per 100,000 (2014-2016), reported Dr. Steelesmith and associates.
Rural counties only made up 2% of the suicides, compared with 81% in large and small metropolitan counties, but suicide rates were “increasing most rapidly in rural areas, although all county types saw increases during the period studied,” Dr. Steelesmith and associates wrote.
They added that “counties with the highest excess risk of suicide tended to be in Western states (e.g., Colorado, New Mexico, Utah, and Wyoming), Appalachia (e.g., Kentucky, Virginia, and West Virginia), and the Ozarks (e.g., Arkansas and Missouri).”
In addition to the connections between increasing suicide rates, living in a rural area, and a higher density of gun shops, the researchers cited other contextual factors. Among those factors were higher median age and higher percentages of non-Hispanic whites, numbers of residents without health insurance, and veterans. They also found links between higher suicide rates and worse numbers on indexes designed to measure social capital; social fragmentation; and deprivation, a measure encompassing lower education, employment levels, and income.
“Long-term and persistent poverty appears to be more entrenched and economic opportunities more constrained in rural areas,” Dr. Steelesmith and associates wrote. “Greater social isolation, challenges related to transportation and interpersonal communication, and associated difficulties accessing health and mental health services likely contribute to the disproportionate association of deprivation with suicide in rural counties.”
Dr. Steelesmith and associates cited several limitations. One key limitation is that, because the study looked only at adults aged 25-64 years, the results might not be generalizable to youth or elderly adults.
No study funding was reported. One study author reported serving on the scientific advisory board of Clarigent Health and receiving grant support from the National Institute of Mental Health outside of the submitted work. No other disclosures were reported.
SOURCE: Steelesmith DL et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10936.
FROM JAMA NETWORK OPEN