User login
Expanding Medicaid did not reduce preventable hospitalizations for lupus patients
A new study has found that, although the Affordable Care Act (ACA) increased health insurance coverage in states that expanded their Medicaid program, the expansion did not translate into improved access to care or decreased preventable hospitalizations of patients with systemic lupus erythematosus (SLE).
“Our findings emphasize the importance of addressing systemic problems with American healthcare delivery at multiple levels,” wrote Elizabeth A. Brown, PhD, of the Medical University of South Carolina, Charleston, and her coauthors. The study was published in Arthritis Care & Research.
To examine the effects of the ACA’s Medicaid expansion on lupus patients when it was implemented Jan. 1, 2014, the researchers launched a retrospective, quasi‐experimental study using administrative data from eight states to compare eight quarterly time points in the 2 years prior to implementation against seven quarterly time points in the 2 years after implementation. Four states expanded Medicaid under the ACA – Arizona, Kentucky, New Jersey, and New York – and four of them did not – Florida, Georgia, South Carolina, and Wisconsin.
During the study period, there were 204,150 lupus hospitalizations across all eight states during the 15 quarters, 53% of which occurred in the four states that did not expand Medicaid. Although the investigators’ base model found that the probability of a preventable hospitalization did not increase over time (odds ratio, 1.004; 95% confidence interval, 0.996-1.013), the four expansion states had significantly higher odds of having preventable hospitalizations in the final adjusted model (OR, 1.302; 95% CI, 1.119-1.515).
Variables that contributed to higher odds of a preventable hospitalization included age: Lupus patients who were 55-64 years old had considerably higher odds for preventable lupus hospitalizations than did patients who were 20-34 years old (OR, 1.488; 95% CI, 1.415-1.564). In addition, those with a median household income under $42,000 had higher odds of hospitalization when compared with those making over $68,000 (OR, 1.138; 95% CI, 1.415-1.564), as did those on Medicaid compared with those on private insurance (OR, 1.298; 95% CI, 1.238-1.361).
The authors acknowledged their study’s limitations, including relying on potential coding errors within administrative data. In addition, they were unable to factor in Medicaid marketing, enrollment strategies, or other related actions undertaken in each of the eight states.
The study was partially supported by the South Carolina Clinical & Translational Research Institute via a National Center for Advancing Translational Sciences grant. The authors reported no conflicts of interest.
SOURCE: Brown EA et al. Arthritis Care Res. 2019 Sept 28. doi: 10.1002/acr.24080
A new study has found that, although the Affordable Care Act (ACA) increased health insurance coverage in states that expanded their Medicaid program, the expansion did not translate into improved access to care or decreased preventable hospitalizations of patients with systemic lupus erythematosus (SLE).
“Our findings emphasize the importance of addressing systemic problems with American healthcare delivery at multiple levels,” wrote Elizabeth A. Brown, PhD, of the Medical University of South Carolina, Charleston, and her coauthors. The study was published in Arthritis Care & Research.
To examine the effects of the ACA’s Medicaid expansion on lupus patients when it was implemented Jan. 1, 2014, the researchers launched a retrospective, quasi‐experimental study using administrative data from eight states to compare eight quarterly time points in the 2 years prior to implementation against seven quarterly time points in the 2 years after implementation. Four states expanded Medicaid under the ACA – Arizona, Kentucky, New Jersey, and New York – and four of them did not – Florida, Georgia, South Carolina, and Wisconsin.
During the study period, there were 204,150 lupus hospitalizations across all eight states during the 15 quarters, 53% of which occurred in the four states that did not expand Medicaid. Although the investigators’ base model found that the probability of a preventable hospitalization did not increase over time (odds ratio, 1.004; 95% confidence interval, 0.996-1.013), the four expansion states had significantly higher odds of having preventable hospitalizations in the final adjusted model (OR, 1.302; 95% CI, 1.119-1.515).
Variables that contributed to higher odds of a preventable hospitalization included age: Lupus patients who were 55-64 years old had considerably higher odds for preventable lupus hospitalizations than did patients who were 20-34 years old (OR, 1.488; 95% CI, 1.415-1.564). In addition, those with a median household income under $42,000 had higher odds of hospitalization when compared with those making over $68,000 (OR, 1.138; 95% CI, 1.415-1.564), as did those on Medicaid compared with those on private insurance (OR, 1.298; 95% CI, 1.238-1.361).
The authors acknowledged their study’s limitations, including relying on potential coding errors within administrative data. In addition, they were unable to factor in Medicaid marketing, enrollment strategies, or other related actions undertaken in each of the eight states.
The study was partially supported by the South Carolina Clinical & Translational Research Institute via a National Center for Advancing Translational Sciences grant. The authors reported no conflicts of interest.
SOURCE: Brown EA et al. Arthritis Care Res. 2019 Sept 28. doi: 10.1002/acr.24080
A new study has found that, although the Affordable Care Act (ACA) increased health insurance coverage in states that expanded their Medicaid program, the expansion did not translate into improved access to care or decreased preventable hospitalizations of patients with systemic lupus erythematosus (SLE).
“Our findings emphasize the importance of addressing systemic problems with American healthcare delivery at multiple levels,” wrote Elizabeth A. Brown, PhD, of the Medical University of South Carolina, Charleston, and her coauthors. The study was published in Arthritis Care & Research.
To examine the effects of the ACA’s Medicaid expansion on lupus patients when it was implemented Jan. 1, 2014, the researchers launched a retrospective, quasi‐experimental study using administrative data from eight states to compare eight quarterly time points in the 2 years prior to implementation against seven quarterly time points in the 2 years after implementation. Four states expanded Medicaid under the ACA – Arizona, Kentucky, New Jersey, and New York – and four of them did not – Florida, Georgia, South Carolina, and Wisconsin.
During the study period, there were 204,150 lupus hospitalizations across all eight states during the 15 quarters, 53% of which occurred in the four states that did not expand Medicaid. Although the investigators’ base model found that the probability of a preventable hospitalization did not increase over time (odds ratio, 1.004; 95% confidence interval, 0.996-1.013), the four expansion states had significantly higher odds of having preventable hospitalizations in the final adjusted model (OR, 1.302; 95% CI, 1.119-1.515).
Variables that contributed to higher odds of a preventable hospitalization included age: Lupus patients who were 55-64 years old had considerably higher odds for preventable lupus hospitalizations than did patients who were 20-34 years old (OR, 1.488; 95% CI, 1.415-1.564). In addition, those with a median household income under $42,000 had higher odds of hospitalization when compared with those making over $68,000 (OR, 1.138; 95% CI, 1.415-1.564), as did those on Medicaid compared with those on private insurance (OR, 1.298; 95% CI, 1.238-1.361).
The authors acknowledged their study’s limitations, including relying on potential coding errors within administrative data. In addition, they were unable to factor in Medicaid marketing, enrollment strategies, or other related actions undertaken in each of the eight states.
The study was partially supported by the South Carolina Clinical & Translational Research Institute via a National Center for Advancing Translational Sciences grant. The authors reported no conflicts of interest.
SOURCE: Brown EA et al. Arthritis Care Res. 2019 Sept 28. doi: 10.1002/acr.24080
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Expanded Medicaid coverage under the Affordable Care Act did not lead to decreased odds of preventable hospitalizations for lupus patients.
Major finding: After adjusted analysis, the four states with expanded Medicaid had significantly higher odds of having preventable hospitalizations than did the four that did not expand Medicaid (odds ratio, 1.302; 95% confidence interval, 1.119-1.515).
Study details: A retrospective, quasi‐experimental study using data from eight states, four that expanded Medicaid under the ACA and four that did not.
Disclosures: The study was partially supported by the South Carolina Clinical & Translational Research Institute via a National Center for Advancing Translational Sciences grant. The authors reported no conflicts of interest.
Source: Brown EA et al. Arthritis Care Res. 2019 Sept 28. doi: 10.1002/acr.24080.
State medical boards under fire in physician suicides
SAN DIEGO – Physician suicide is “a public health crisis because of the sheer volume of people who are dying,” and many medical authorities are contributing to stigma through “invasive” questionnaires, a prevention advocate said at the annual Psych Congress.
“Physicians fear sharing their mental health struggles with the state medical health board,” said Pamela Wible, MD, a family physician who practices in Eugene, Ore., at the meeting. They “pretend, deny, and lie,” she said, and sometimes they seek care and medication hours away in order to avoid detection.
including the “very worst,” which is that of Alaska.
Dr. Wible, who speaks of suffering from suicidal feelings herself as physician in 2004, is a leading advocate for suicide prevention in the medical profession.
She told colleagues at the Psych Congress that anesthesiologists face the highest risk of suicide, followed by surgeons, ob.gyns., and psychiatrists.
“They end their lives not because they want to die but because they want to stop the pain and they can’t find any other way,” she said. “They have a great work ethic until the end: They’re smiling, doing complex surgeries, and cracking jokes to the surgical team, then they shoot themselves in the closet.”
Colleagues are often shocked, she said: “ ‘Wait a minute. He was just joking with me yesterday. What do you mean he hung himself in his office?’ ‘She just had a newborn baby and she was so happy!’ If you see the smile, you don’t see the pain.”
In 2018, she wrote a Washington Post commentary titled “What I’ve learned from my tally of 757 doctor suicides” that was based on her registry of physician suicides. In the United States, she wrote, 1 million patients lose a physician to suicide each year. Factors contributing to suicides include patient deaths, malpractice suits, “academic distress,” and overwork. “Doctors who need help don’t seek it because they fear mental health care won’t remain confidential,” she wrote. “So they drive out of town, pay cash, and use fake names to hide from state medical boards, hospitals, and insurance plans out of fear that they will lose state licensure, hospital privileges, and health plan participation.”
Dr. Wible oversaw a 2019 research project that analyzed state medical board applications. The goal was to grade the state boards by how intrusively their application questions grill applicants about their mental health history. “Physicians fear sharing their mental health struggles with the state medical health board and with each other,” she said. Some lie, and others – “the really honest physicians” – are so dedicated to telling the truth that “they’ll withhold getting care because they want to correctly check the ‘no’ box.”
Seven states – Alabama, Alaska, Delaware, Florida, Mississippi, Rhode Island, and Washington –received “F” grades for “highly invasive mental health questions unlinked to current impairment that contain confusing, punitive, or adversarial language.”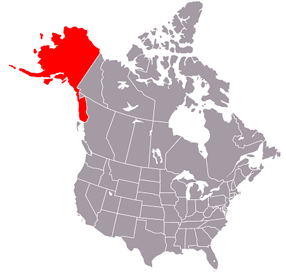
Alaska, Dr. Wible said, asks multiple 25 yes-or-no questions about mental health. One question lists 14 conditions, almost all related to mental health – including depression, “any organic mental disorder,” and “any condition requiring chronic medical or behavioral treatment” – and asks, “Have you ever been diagnosed with, treated for, or do you currently have” any of them. This is “the most invasive mental health question we found on any application,” Dr. Wible wrote on her website.
States also hurt applicants by asking peers of applicants about their mental health, she said. “I’m not against getting peer references, but can we stop getting into everyone’s business with their psych history? What we really want to know is: ‘Are you are safe with patients today?’ ”
Dr. Wible also criticizes state medical boards for asking about mental health impairment over the last 5 years: They don’t get higher than a “C.”
The 13 states with “A” grades either don’t ask about mental health or simply ask about general impairment: Connecticut, Hawaii, Indiana, Kentucky, New Jersey, Maine, Maryland, Massachusetts, Michigan, Nevada, New York, Pennsylvania, and Wyoming.
Massachusetts, for example, asks, “Do you have a medical or physical condition that currently impairs your ability to practice medicine?”
“That is a question anyone can understand,” Dr. Wible said. “I think that’s good wording.”
Going forward, she said, “we’ve got to remove these mental health questions. If we could do this, our profession would be so much better, and we’d lose so many fewer people.”
And, she added, “what we really need to do is share our stories. It’s therapeutic for you and your colleagues, it creates collegial trust and bonding, and it destigmatizes physician mental health.”
Dr. Wible reported no relevant disclosures.
SAN DIEGO – Physician suicide is “a public health crisis because of the sheer volume of people who are dying,” and many medical authorities are contributing to stigma through “invasive” questionnaires, a prevention advocate said at the annual Psych Congress.
“Physicians fear sharing their mental health struggles with the state medical health board,” said Pamela Wible, MD, a family physician who practices in Eugene, Ore., at the meeting. They “pretend, deny, and lie,” she said, and sometimes they seek care and medication hours away in order to avoid detection.
including the “very worst,” which is that of Alaska.
Dr. Wible, who speaks of suffering from suicidal feelings herself as physician in 2004, is a leading advocate for suicide prevention in the medical profession.
She told colleagues at the Psych Congress that anesthesiologists face the highest risk of suicide, followed by surgeons, ob.gyns., and psychiatrists.
“They end their lives not because they want to die but because they want to stop the pain and they can’t find any other way,” she said. “They have a great work ethic until the end: They’re smiling, doing complex surgeries, and cracking jokes to the surgical team, then they shoot themselves in the closet.”
Colleagues are often shocked, she said: “ ‘Wait a minute. He was just joking with me yesterday. What do you mean he hung himself in his office?’ ‘She just had a newborn baby and she was so happy!’ If you see the smile, you don’t see the pain.”
In 2018, she wrote a Washington Post commentary titled “What I’ve learned from my tally of 757 doctor suicides” that was based on her registry of physician suicides. In the United States, she wrote, 1 million patients lose a physician to suicide each year. Factors contributing to suicides include patient deaths, malpractice suits, “academic distress,” and overwork. “Doctors who need help don’t seek it because they fear mental health care won’t remain confidential,” she wrote. “So they drive out of town, pay cash, and use fake names to hide from state medical boards, hospitals, and insurance plans out of fear that they will lose state licensure, hospital privileges, and health plan participation.”
Dr. Wible oversaw a 2019 research project that analyzed state medical board applications. The goal was to grade the state boards by how intrusively their application questions grill applicants about their mental health history. “Physicians fear sharing their mental health struggles with the state medical health board and with each other,” she said. Some lie, and others – “the really honest physicians” – are so dedicated to telling the truth that “they’ll withhold getting care because they want to correctly check the ‘no’ box.”
Seven states – Alabama, Alaska, Delaware, Florida, Mississippi, Rhode Island, and Washington –received “F” grades for “highly invasive mental health questions unlinked to current impairment that contain confusing, punitive, or adversarial language.”
Alaska, Dr. Wible said, asks multiple 25 yes-or-no questions about mental health. One question lists 14 conditions, almost all related to mental health – including depression, “any organic mental disorder,” and “any condition requiring chronic medical or behavioral treatment” – and asks, “Have you ever been diagnosed with, treated for, or do you currently have” any of them. This is “the most invasive mental health question we found on any application,” Dr. Wible wrote on her website.
States also hurt applicants by asking peers of applicants about their mental health, she said. “I’m not against getting peer references, but can we stop getting into everyone’s business with their psych history? What we really want to know is: ‘Are you are safe with patients today?’ ”
Dr. Wible also criticizes state medical boards for asking about mental health impairment over the last 5 years: They don’t get higher than a “C.”
The 13 states with “A” grades either don’t ask about mental health or simply ask about general impairment: Connecticut, Hawaii, Indiana, Kentucky, New Jersey, Maine, Maryland, Massachusetts, Michigan, Nevada, New York, Pennsylvania, and Wyoming.
Massachusetts, for example, asks, “Do you have a medical or physical condition that currently impairs your ability to practice medicine?”
“That is a question anyone can understand,” Dr. Wible said. “I think that’s good wording.”
Going forward, she said, “we’ve got to remove these mental health questions. If we could do this, our profession would be so much better, and we’d lose so many fewer people.”
And, she added, “what we really need to do is share our stories. It’s therapeutic for you and your colleagues, it creates collegial trust and bonding, and it destigmatizes physician mental health.”
Dr. Wible reported no relevant disclosures.
SAN DIEGO – Physician suicide is “a public health crisis because of the sheer volume of people who are dying,” and many medical authorities are contributing to stigma through “invasive” questionnaires, a prevention advocate said at the annual Psych Congress.
“Physicians fear sharing their mental health struggles with the state medical health board,” said Pamela Wible, MD, a family physician who practices in Eugene, Ore., at the meeting. They “pretend, deny, and lie,” she said, and sometimes they seek care and medication hours away in order to avoid detection.
including the “very worst,” which is that of Alaska.
Dr. Wible, who speaks of suffering from suicidal feelings herself as physician in 2004, is a leading advocate for suicide prevention in the medical profession.
She told colleagues at the Psych Congress that anesthesiologists face the highest risk of suicide, followed by surgeons, ob.gyns., and psychiatrists.
“They end their lives not because they want to die but because they want to stop the pain and they can’t find any other way,” she said. “They have a great work ethic until the end: They’re smiling, doing complex surgeries, and cracking jokes to the surgical team, then they shoot themselves in the closet.”
Colleagues are often shocked, she said: “ ‘Wait a minute. He was just joking with me yesterday. What do you mean he hung himself in his office?’ ‘She just had a newborn baby and she was so happy!’ If you see the smile, you don’t see the pain.”
In 2018, she wrote a Washington Post commentary titled “What I’ve learned from my tally of 757 doctor suicides” that was based on her registry of physician suicides. In the United States, she wrote, 1 million patients lose a physician to suicide each year. Factors contributing to suicides include patient deaths, malpractice suits, “academic distress,” and overwork. “Doctors who need help don’t seek it because they fear mental health care won’t remain confidential,” she wrote. “So they drive out of town, pay cash, and use fake names to hide from state medical boards, hospitals, and insurance plans out of fear that they will lose state licensure, hospital privileges, and health plan participation.”
Dr. Wible oversaw a 2019 research project that analyzed state medical board applications. The goal was to grade the state boards by how intrusively their application questions grill applicants about their mental health history. “Physicians fear sharing their mental health struggles with the state medical health board and with each other,” she said. Some lie, and others – “the really honest physicians” – are so dedicated to telling the truth that “they’ll withhold getting care because they want to correctly check the ‘no’ box.”
Seven states – Alabama, Alaska, Delaware, Florida, Mississippi, Rhode Island, and Washington –received “F” grades for “highly invasive mental health questions unlinked to current impairment that contain confusing, punitive, or adversarial language.”
Alaska, Dr. Wible said, asks multiple 25 yes-or-no questions about mental health. One question lists 14 conditions, almost all related to mental health – including depression, “any organic mental disorder,” and “any condition requiring chronic medical or behavioral treatment” – and asks, “Have you ever been diagnosed with, treated for, or do you currently have” any of them. This is “the most invasive mental health question we found on any application,” Dr. Wible wrote on her website.
States also hurt applicants by asking peers of applicants about their mental health, she said. “I’m not against getting peer references, but can we stop getting into everyone’s business with their psych history? What we really want to know is: ‘Are you are safe with patients today?’ ”
Dr. Wible also criticizes state medical boards for asking about mental health impairment over the last 5 years: They don’t get higher than a “C.”
The 13 states with “A” grades either don’t ask about mental health or simply ask about general impairment: Connecticut, Hawaii, Indiana, Kentucky, New Jersey, Maine, Maryland, Massachusetts, Michigan, Nevada, New York, Pennsylvania, and Wyoming.
Massachusetts, for example, asks, “Do you have a medical or physical condition that currently impairs your ability to practice medicine?”
“That is a question anyone can understand,” Dr. Wible said. “I think that’s good wording.”
Going forward, she said, “we’ve got to remove these mental health questions. If we could do this, our profession would be so much better, and we’d lose so many fewer people.”
And, she added, “what we really need to do is share our stories. It’s therapeutic for you and your colleagues, it creates collegial trust and bonding, and it destigmatizes physician mental health.”
Dr. Wible reported no relevant disclosures.
EXPERT ANALYSIS FROM PSYCH CONGRESS 2019
Help wanted
In a Pediatrics article, Hsuan-hsiu Annie Chen, MD, offers a very personal and candid narrative of her struggle with depression during medical school and residency (Pediatrics. 2019 Sep 1. doi: 10.1542/peds.2019-1210). Dr. Chen knows from personal experience that she was not alone in her cohort as she faced the challenges of sleep deprivation and emotional trauma that continue to be a part of a physician’s education and training. In her discussion of how future medical trainees might be spared some of the long hours she endured, Dr. Chen suggests that this country consider expanding its physician workforce by “increasing the number of medical schools and recruiting foreign medical graduates” as some European countries have done. Dr. Chen now works in the pediatric residency office at Children’s Hospital, Los Angeles.
Ironically, or maybe it was intentionally, the editors of Pediatrics chose to open the same issue in which Dr. Chen’s personal story appears with a Pediatrics Perspective commentary that looks into the murky waters of physician workforce research (Pediatrics. 2019 Sep 1. doi: 10.1542/peds.2019-0469). Gary L. Freed, MD, MPH, at the Child Health Evaluation and Research Center at the University of Michigan, Ann Arbor, claims that, in general, the data currently being generated by workforce research must be interpreted with caution because many of the studies are flawed by one or more biases.
You may have survived the gauntlet of medical school and residency relatively unscathed. But Is part of the problem that your clinic is seeing too many patients with too few physicians? Do your colleagues share your opinion? Is the administration actively recruiting more physicians, but failing to find interested and qualified doctors? Is this a strictly local phenomenon limited to your community, or is it a regional shortage? Do you think your situation reflects a national trend that deserves attention?
Like Dr. Chen, do you think that more medical schools and active recruitment of foreign medical students would allow you to work less hours? Obviously, even if you were a teenager when you entered your residency, opening more medical schools is not going to allow you to shorten your workday. But are more medical schools the best solution for this country’s overworked physicians even in the long term? Dr. Freed’s observations should make you hesitant to even venture a guess.
You, I, and Dr. Chen only can report on how we perceive our own work environment. Your local physician shortage may be in part because the school system in your community has a poor reputation and young physicians don’t want to move there. It may be that the hospital that owns your practice is struggling and can’t afford to offer a competitive salary. Producing more physicians may not be the answer to the physician shortage in communities like yours, even in the long run.
This is a very large country with relatively porous boundaries between the states for physicians. Physician supply and demand seldom dictates where physicians choose to practice. In fact, a medically needy community is probably the least likely place a physician just finishing her training will choose to settle.
Although adding another physician to your practice may decrease your workload, can your personal finances handle the hit that might occur as you see less patients? Particularly, if the new hire turns out to be a rock star who siphons off more of your patients than you anticipated. On the other hand, there is always the chance that, despite careful vetting, your group hires a lemon who ends up creating more trouble than he is worth.
As Dr. Freed suggests, trying to determine just how many and what kind of physicians we need is complicated. It may be just a roll of the dice at best.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In a Pediatrics article, Hsuan-hsiu Annie Chen, MD, offers a very personal and candid narrative of her struggle with depression during medical school and residency (Pediatrics. 2019 Sep 1. doi: 10.1542/peds.2019-1210). Dr. Chen knows from personal experience that she was not alone in her cohort as she faced the challenges of sleep deprivation and emotional trauma that continue to be a part of a physician’s education and training. In her discussion of how future medical trainees might be spared some of the long hours she endured, Dr. Chen suggests that this country consider expanding its physician workforce by “increasing the number of medical schools and recruiting foreign medical graduates” as some European countries have done. Dr. Chen now works in the pediatric residency office at Children’s Hospital, Los Angeles.
Ironically, or maybe it was intentionally, the editors of Pediatrics chose to open the same issue in which Dr. Chen’s personal story appears with a Pediatrics Perspective commentary that looks into the murky waters of physician workforce research (Pediatrics. 2019 Sep 1. doi: 10.1542/peds.2019-0469). Gary L. Freed, MD, MPH, at the Child Health Evaluation and Research Center at the University of Michigan, Ann Arbor, claims that, in general, the data currently being generated by workforce research must be interpreted with caution because many of the studies are flawed by one or more biases.
You may have survived the gauntlet of medical school and residency relatively unscathed. But Is part of the problem that your clinic is seeing too many patients with too few physicians? Do your colleagues share your opinion? Is the administration actively recruiting more physicians, but failing to find interested and qualified doctors? Is this a strictly local phenomenon limited to your community, or is it a regional shortage? Do you think your situation reflects a national trend that deserves attention?
Like Dr. Chen, do you think that more medical schools and active recruitment of foreign medical students would allow you to work less hours? Obviously, even if you were a teenager when you entered your residency, opening more medical schools is not going to allow you to shorten your workday. But are more medical schools the best solution for this country’s overworked physicians even in the long term? Dr. Freed’s observations should make you hesitant to even venture a guess.
You, I, and Dr. Chen only can report on how we perceive our own work environment. Your local physician shortage may be in part because the school system in your community has a poor reputation and young physicians don’t want to move there. It may be that the hospital that owns your practice is struggling and can’t afford to offer a competitive salary. Producing more physicians may not be the answer to the physician shortage in communities like yours, even in the long run.
This is a very large country with relatively porous boundaries between the states for physicians. Physician supply and demand seldom dictates where physicians choose to practice. In fact, a medically needy community is probably the least likely place a physician just finishing her training will choose to settle.
Although adding another physician to your practice may decrease your workload, can your personal finances handle the hit that might occur as you see less patients? Particularly, if the new hire turns out to be a rock star who siphons off more of your patients than you anticipated. On the other hand, there is always the chance that, despite careful vetting, your group hires a lemon who ends up creating more trouble than he is worth.
As Dr. Freed suggests, trying to determine just how many and what kind of physicians we need is complicated. It may be just a roll of the dice at best.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In a Pediatrics article, Hsuan-hsiu Annie Chen, MD, offers a very personal and candid narrative of her struggle with depression during medical school and residency (Pediatrics. 2019 Sep 1. doi: 10.1542/peds.2019-1210). Dr. Chen knows from personal experience that she was not alone in her cohort as she faced the challenges of sleep deprivation and emotional trauma that continue to be a part of a physician’s education and training. In her discussion of how future medical trainees might be spared some of the long hours she endured, Dr. Chen suggests that this country consider expanding its physician workforce by “increasing the number of medical schools and recruiting foreign medical graduates” as some European countries have done. Dr. Chen now works in the pediatric residency office at Children’s Hospital, Los Angeles.
Ironically, or maybe it was intentionally, the editors of Pediatrics chose to open the same issue in which Dr. Chen’s personal story appears with a Pediatrics Perspective commentary that looks into the murky waters of physician workforce research (Pediatrics. 2019 Sep 1. doi: 10.1542/peds.2019-0469). Gary L. Freed, MD, MPH, at the Child Health Evaluation and Research Center at the University of Michigan, Ann Arbor, claims that, in general, the data currently being generated by workforce research must be interpreted with caution because many of the studies are flawed by one or more biases.
You may have survived the gauntlet of medical school and residency relatively unscathed. But Is part of the problem that your clinic is seeing too many patients with too few physicians? Do your colleagues share your opinion? Is the administration actively recruiting more physicians, but failing to find interested and qualified doctors? Is this a strictly local phenomenon limited to your community, or is it a regional shortage? Do you think your situation reflects a national trend that deserves attention?
Like Dr. Chen, do you think that more medical schools and active recruitment of foreign medical students would allow you to work less hours? Obviously, even if you were a teenager when you entered your residency, opening more medical schools is not going to allow you to shorten your workday. But are more medical schools the best solution for this country’s overworked physicians even in the long term? Dr. Freed’s observations should make you hesitant to even venture a guess.
You, I, and Dr. Chen only can report on how we perceive our own work environment. Your local physician shortage may be in part because the school system in your community has a poor reputation and young physicians don’t want to move there. It may be that the hospital that owns your practice is struggling and can’t afford to offer a competitive salary. Producing more physicians may not be the answer to the physician shortage in communities like yours, even in the long run.
This is a very large country with relatively porous boundaries between the states for physicians. Physician supply and demand seldom dictates where physicians choose to practice. In fact, a medically needy community is probably the least likely place a physician just finishing her training will choose to settle.
Although adding another physician to your practice may decrease your workload, can your personal finances handle the hit that might occur as you see less patients? Particularly, if the new hire turns out to be a rock star who siphons off more of your patients than you anticipated. On the other hand, there is always the chance that, despite careful vetting, your group hires a lemon who ends up creating more trouble than he is worth.
As Dr. Freed suggests, trying to determine just how many and what kind of physicians we need is complicated. It may be just a roll of the dice at best.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
In utero Zika exposure can have delayed consequences
WASHINGTON – Evidence continues to mount that infants born to moms infected with Zika virus during pregnancy can have neurodevelopmental abnormalities as they age even if they showed no defects at birth, based on follow-up of 890 Colombian children tracked by epidemiologists from the U.S. Centers for Disease Control and Prevention.
Among the 890 neonates born to mothers apparently infected with Zika during pregnancy and followed for up to 2 years, 40 of the 852 (5%) without a detectable birth defect at delivery went on to show some type of neurodevelopmental sequelae during up to 24 months of age, Margaret Honein, PhD, said at an annual scientific meeting on infectious diseases.
In addition, among the children without birth defects at delivery who received follow-up examinations out to about 2 years, the incidence of “alerts” for possible neurodevelopmental issues was 15%-20% for each of the four domains studied (gross motor, fine motor, hearing and language, and personal and social functions), said Dr. Honein, an epidemiologist and chief of the birth defects branch of the CDC. In contrast, 17 of the 38 children (45%) followed who had identifiable birth defects at delivery also showed neurodevelopmental abnormalities when reexamined as long as 2 years after birth. These possible neurodevelopmental abnormalities, designated as alerts, were identified in comparison with a contemporaneous cohort of children born to uninfected mothers in the same regions of Colombia and assessed by the CDC researchers.
This cohort of children born to mothers who became infected with Zika virus during the 2016 Colombian epidemic will not undergo any planned, additional follow-up beyond the initial 2 years, Dr. Honein noted.
The findings she reported were consistent with observations from a much smaller cohort of 70 infants born to Colombian mothers infected with Zika virus while pregnant who had a normal head circumference and a normal clinical examination at delivery. When assessed once or twice 4-18 months after birth, these 70 infants showed an overall greater than one standard deviation (z-score) drop in their scores on the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) metric by 12 months after birth and continuing out to 18 months, said Sarah B. Mulkey, MD, a fetal-neonatal neurologist at Children’s National Health System in Washington. These deficits were especially pronounced in the mobility and social cognition domains of the four-domain WIDEA metric. The social cognition domain is an important predictor of later problems with executive function and other neurologic disorders, Dr. Mulkey said while reporting her findings in a separate talk at the meeting. She acknowledged that the analysis was flawed by comparing the WIDEA outcomes of the Zika virus–exposed children to healthy children from either inner-city Chicago or Canada. Dr. Mulkey said that she and her associates plan to characterize a population of Zika virus–unexposed children in Colombia to use for future comparisons.
The study reported by Dr. Honein involved an enhanced surveillance program launched by the CDC in 2016 in three regions of Colombia and included 1,190 pregnancies accompanied by Zika symptoms in the mother and with a reported pregnancy outcome, including 1,185 live births. Nearly half of the Zika infections occurred during the first trimester, and 34% occurred during the second trimester. However, fewer than a third of the pregnant women underwent some type of laboratory testing to confirm their infection, either by serology or by a DNA-based assay, and of these 28% had a positive finding. Dr. Honein cautioned that many of the specimens that tested negative for Zika virus may have been false negatives.
The birth defects identified among the infants born from an apparently affected pregnancy included brain abnormalities, eye anomalies, and microcephaly, with 5% of the 1,185 live births showing one or more of these outcomes. The neurodevelopmental deficits identified during follow-up of 890 of the children out to 2 years included seizures; abnormalities of tone, movement, or swallowing; and impairments of vision or hearing.
WASHINGTON – Evidence continues to mount that infants born to moms infected with Zika virus during pregnancy can have neurodevelopmental abnormalities as they age even if they showed no defects at birth, based on follow-up of 890 Colombian children tracked by epidemiologists from the U.S. Centers for Disease Control and Prevention.
Among the 890 neonates born to mothers apparently infected with Zika during pregnancy and followed for up to 2 years, 40 of the 852 (5%) without a detectable birth defect at delivery went on to show some type of neurodevelopmental sequelae during up to 24 months of age, Margaret Honein, PhD, said at an annual scientific meeting on infectious diseases.
In addition, among the children without birth defects at delivery who received follow-up examinations out to about 2 years, the incidence of “alerts” for possible neurodevelopmental issues was 15%-20% for each of the four domains studied (gross motor, fine motor, hearing and language, and personal and social functions), said Dr. Honein, an epidemiologist and chief of the birth defects branch of the CDC. In contrast, 17 of the 38 children (45%) followed who had identifiable birth defects at delivery also showed neurodevelopmental abnormalities when reexamined as long as 2 years after birth. These possible neurodevelopmental abnormalities, designated as alerts, were identified in comparison with a contemporaneous cohort of children born to uninfected mothers in the same regions of Colombia and assessed by the CDC researchers.
This cohort of children born to mothers who became infected with Zika virus during the 2016 Colombian epidemic will not undergo any planned, additional follow-up beyond the initial 2 years, Dr. Honein noted.
The findings she reported were consistent with observations from a much smaller cohort of 70 infants born to Colombian mothers infected with Zika virus while pregnant who had a normal head circumference and a normal clinical examination at delivery. When assessed once or twice 4-18 months after birth, these 70 infants showed an overall greater than one standard deviation (z-score) drop in their scores on the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) metric by 12 months after birth and continuing out to 18 months, said Sarah B. Mulkey, MD, a fetal-neonatal neurologist at Children’s National Health System in Washington. These deficits were especially pronounced in the mobility and social cognition domains of the four-domain WIDEA metric. The social cognition domain is an important predictor of later problems with executive function and other neurologic disorders, Dr. Mulkey said while reporting her findings in a separate talk at the meeting. She acknowledged that the analysis was flawed by comparing the WIDEA outcomes of the Zika virus–exposed children to healthy children from either inner-city Chicago or Canada. Dr. Mulkey said that she and her associates plan to characterize a population of Zika virus–unexposed children in Colombia to use for future comparisons.
The study reported by Dr. Honein involved an enhanced surveillance program launched by the CDC in 2016 in three regions of Colombia and included 1,190 pregnancies accompanied by Zika symptoms in the mother and with a reported pregnancy outcome, including 1,185 live births. Nearly half of the Zika infections occurred during the first trimester, and 34% occurred during the second trimester. However, fewer than a third of the pregnant women underwent some type of laboratory testing to confirm their infection, either by serology or by a DNA-based assay, and of these 28% had a positive finding. Dr. Honein cautioned that many of the specimens that tested negative for Zika virus may have been false negatives.
The birth defects identified among the infants born from an apparently affected pregnancy included brain abnormalities, eye anomalies, and microcephaly, with 5% of the 1,185 live births showing one or more of these outcomes. The neurodevelopmental deficits identified during follow-up of 890 of the children out to 2 years included seizures; abnormalities of tone, movement, or swallowing; and impairments of vision or hearing.
WASHINGTON – Evidence continues to mount that infants born to moms infected with Zika virus during pregnancy can have neurodevelopmental abnormalities as they age even if they showed no defects at birth, based on follow-up of 890 Colombian children tracked by epidemiologists from the U.S. Centers for Disease Control and Prevention.
Among the 890 neonates born to mothers apparently infected with Zika during pregnancy and followed for up to 2 years, 40 of the 852 (5%) without a detectable birth defect at delivery went on to show some type of neurodevelopmental sequelae during up to 24 months of age, Margaret Honein, PhD, said at an annual scientific meeting on infectious diseases.
In addition, among the children without birth defects at delivery who received follow-up examinations out to about 2 years, the incidence of “alerts” for possible neurodevelopmental issues was 15%-20% for each of the four domains studied (gross motor, fine motor, hearing and language, and personal and social functions), said Dr. Honein, an epidemiologist and chief of the birth defects branch of the CDC. In contrast, 17 of the 38 children (45%) followed who had identifiable birth defects at delivery also showed neurodevelopmental abnormalities when reexamined as long as 2 years after birth. These possible neurodevelopmental abnormalities, designated as alerts, were identified in comparison with a contemporaneous cohort of children born to uninfected mothers in the same regions of Colombia and assessed by the CDC researchers.
This cohort of children born to mothers who became infected with Zika virus during the 2016 Colombian epidemic will not undergo any planned, additional follow-up beyond the initial 2 years, Dr. Honein noted.
The findings she reported were consistent with observations from a much smaller cohort of 70 infants born to Colombian mothers infected with Zika virus while pregnant who had a normal head circumference and a normal clinical examination at delivery. When assessed once or twice 4-18 months after birth, these 70 infants showed an overall greater than one standard deviation (z-score) drop in their scores on the Warner Initial Developmental Evaluation of Adaptive and Functional Skills (WIDEA) metric by 12 months after birth and continuing out to 18 months, said Sarah B. Mulkey, MD, a fetal-neonatal neurologist at Children’s National Health System in Washington. These deficits were especially pronounced in the mobility and social cognition domains of the four-domain WIDEA metric. The social cognition domain is an important predictor of later problems with executive function and other neurologic disorders, Dr. Mulkey said while reporting her findings in a separate talk at the meeting. She acknowledged that the analysis was flawed by comparing the WIDEA outcomes of the Zika virus–exposed children to healthy children from either inner-city Chicago or Canada. Dr. Mulkey said that she and her associates plan to characterize a population of Zika virus–unexposed children in Colombia to use for future comparisons.
The study reported by Dr. Honein involved an enhanced surveillance program launched by the CDC in 2016 in three regions of Colombia and included 1,190 pregnancies accompanied by Zika symptoms in the mother and with a reported pregnancy outcome, including 1,185 live births. Nearly half of the Zika infections occurred during the first trimester, and 34% occurred during the second trimester. However, fewer than a third of the pregnant women underwent some type of laboratory testing to confirm their infection, either by serology or by a DNA-based assay, and of these 28% had a positive finding. Dr. Honein cautioned that many of the specimens that tested negative for Zika virus may have been false negatives.
The birth defects identified among the infants born from an apparently affected pregnancy included brain abnormalities, eye anomalies, and microcephaly, with 5% of the 1,185 live births showing one or more of these outcomes. The neurodevelopmental deficits identified during follow-up of 890 of the children out to 2 years included seizures; abnormalities of tone, movement, or swallowing; and impairments of vision or hearing.
REPORTING FROM ID WEEK 2019
It’s all in the timing
It is often fun and sometimes exhausting watching the speed with which children run around or switch from one game to another. A lot of us were attracted to pediatrics to share the quick joy of children and also the speed of their physical recovery. We get to see premature infants gain an ounce a day, and see wounds heal in less than a week. We give advice on sleep and see success in a month. We and the families get used to quick fixes.
Parents and children are forewarned and reassured by our knowledge about how long things typically take: Respiratory syncytial virus (RSV) peaks in 5 days, colic lessens in 3 months, changing sleep patterns takes 3 weeks, habit formation 6 weeks, menses come 2 years after breast development, and so on. But the timing of daily parenting is rarely as predictable. Sometimes a child’s clock is running fast, making waiting even seconds for a snack or a bathroom difficult; other times are slow, as when walking down the sidewalk noticing every leaf. The child’s clock is independent of the adult’s – and complicated by clocks of siblings.
Parent pace also is determined by many factors unrelated to the child: work demands, deadlines, train schedules, something in the oven, needs of siblings, and so on. To those can be added intrinsic factors affecting parent’s tolerance to shifting pace to the child’s such as temperament, fatigue, illness, pain, or even adult ADHD. And don’t forget caffeine (or other drugs) affecting the internal metronome. When impatience with the child is a complaint, it is useful to ask, “What makes waiting for your child difficult for you?”
When discussing time, I find it important to discuss the poison “s-word” of parenting – “should.” This trickster often comes from time illusions in childrearing. After seeing so many behaviors change quickly, parents expect all change to be equally fast. She should be able to sleep through the night by now! He should be able to dress and get to the table in 5 minutes. And sometimes it is the parent’s s-word that creates pain – I should love pushing for as long as she wants to swing, if I am a good parent. The problem with thinking “should” is that it implies willful or moral behavior, and it may prompt a judgmental or punitive parental response.
Otherwise well intentioned, cooperative children who take longer to shift their attention from homework to shower can be seen as oppositional. Worse yet, if the example used is from playing video games (something fun) to getting to the bus stop (an undesirable shift), you may hear parents critically say, “He only wants to do what he wants to do.” When examining examples (always key to helping with behavior), pointing out that all kinds of transitions are difficult for this child may be educational and allow for a more reasoned response. And specifically being on electronics puts adults as well as children in a time warp which is hard to escape.
There are many kinds of thwarted expectations, but expectations about how long things take are pretty universal. Frustration generates anger and even can lead to violence, such as road rage. Children – who all step to the beat of a different drummer, especially those with different “clocks” such as in ADHD – may experience frustration most of the day. This can manifest as irritability for them and sometimes as an irritable response back from the parent.
The first step in adapting to differences in parent and child pace is to realize that time is the problem. Naming it, saying “we are on toddler time,” can be a “signal to self” to slow down. Generations of children loved Mr. Rogers because he always conveyed having all the time in the world for the person he was with. It actually does not take as long as it feels at first to do this. Listening while keeping eye contact, breathing deeply, and waiting until two breaths after the child goes silent before speaking or moving conveys your interest and respect. For some behaviors, such as tantrums, such quiet attention may be all that is needed to resolve the issue. We adults can practice this, but even infants can be helped to develop patience by reinforcement with brief attention from their caregivers for tiny increments of waiting.
I sometimes suggest that parents time behaviors to develop perspective, reset expectations, practice waiting, and perhaps even distract themselves from intervening and making things worse by lending attention to negative behaviors. Timing as observation can be helpful for tantrums, breath holding spells, whining, and sibling squabbles; maximum times for baths and video games; minimum times for meals, sitting to poop, and special time. Timers are not just for Time Out! “Visual timers” that show green then yellow then red and sometimes flashing lights as warnings of an upcoming stopping point are helpful for children preschool and older. These timers help them to develop a better sense of time and begin managing their own transitions. A game of guessing how long things take can build timing skills and patience. I think every child past preschool benefits from a wristwatch, first to build time sense, and second to avoid looking at a smartphone to see the hour, then being distracted by content! Diaries of behaviors over time are a staple of behavior change plans, with the added benefit of lending perspective on actually how often and how long a troublesome behavior occurs. Practicing mindfulness – nonjudgmental watching of our thoughts and feelings, often with deep breathing and relaxation – also can help both children and adults build time tolerance.
Children have little control over their daily schedule. Surrendering when you can for them to do things at their own pace can reduce their frustration, build the parent-child relationship, and promote positive behaviors. Plus family life is more enjoyable lived slower. You even can remind parents that “the days are long but the years are short” before their children will be grown and gone.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
It is often fun and sometimes exhausting watching the speed with which children run around or switch from one game to another. A lot of us were attracted to pediatrics to share the quick joy of children and also the speed of their physical recovery. We get to see premature infants gain an ounce a day, and see wounds heal in less than a week. We give advice on sleep and see success in a month. We and the families get used to quick fixes.
Parents and children are forewarned and reassured by our knowledge about how long things typically take: Respiratory syncytial virus (RSV) peaks in 5 days, colic lessens in 3 months, changing sleep patterns takes 3 weeks, habit formation 6 weeks, menses come 2 years after breast development, and so on. But the timing of daily parenting is rarely as predictable. Sometimes a child’s clock is running fast, making waiting even seconds for a snack or a bathroom difficult; other times are slow, as when walking down the sidewalk noticing every leaf. The child’s clock is independent of the adult’s – and complicated by clocks of siblings.
Parent pace also is determined by many factors unrelated to the child: work demands, deadlines, train schedules, something in the oven, needs of siblings, and so on. To those can be added intrinsic factors affecting parent’s tolerance to shifting pace to the child’s such as temperament, fatigue, illness, pain, or even adult ADHD. And don’t forget caffeine (or other drugs) affecting the internal metronome. When impatience with the child is a complaint, it is useful to ask, “What makes waiting for your child difficult for you?”
When discussing time, I find it important to discuss the poison “s-word” of parenting – “should.” This trickster often comes from time illusions in childrearing. After seeing so many behaviors change quickly, parents expect all change to be equally fast. She should be able to sleep through the night by now! He should be able to dress and get to the table in 5 minutes. And sometimes it is the parent’s s-word that creates pain – I should love pushing for as long as she wants to swing, if I am a good parent. The problem with thinking “should” is that it implies willful or moral behavior, and it may prompt a judgmental or punitive parental response.
Otherwise well intentioned, cooperative children who take longer to shift their attention from homework to shower can be seen as oppositional. Worse yet, if the example used is from playing video games (something fun) to getting to the bus stop (an undesirable shift), you may hear parents critically say, “He only wants to do what he wants to do.” When examining examples (always key to helping with behavior), pointing out that all kinds of transitions are difficult for this child may be educational and allow for a more reasoned response. And specifically being on electronics puts adults as well as children in a time warp which is hard to escape.
There are many kinds of thwarted expectations, but expectations about how long things take are pretty universal. Frustration generates anger and even can lead to violence, such as road rage. Children – who all step to the beat of a different drummer, especially those with different “clocks” such as in ADHD – may experience frustration most of the day. This can manifest as irritability for them and sometimes as an irritable response back from the parent.
The first step in adapting to differences in parent and child pace is to realize that time is the problem. Naming it, saying “we are on toddler time,” can be a “signal to self” to slow down. Generations of children loved Mr. Rogers because he always conveyed having all the time in the world for the person he was with. It actually does not take as long as it feels at first to do this. Listening while keeping eye contact, breathing deeply, and waiting until two breaths after the child goes silent before speaking or moving conveys your interest and respect. For some behaviors, such as tantrums, such quiet attention may be all that is needed to resolve the issue. We adults can practice this, but even infants can be helped to develop patience by reinforcement with brief attention from their caregivers for tiny increments of waiting.
I sometimes suggest that parents time behaviors to develop perspective, reset expectations, practice waiting, and perhaps even distract themselves from intervening and making things worse by lending attention to negative behaviors. Timing as observation can be helpful for tantrums, breath holding spells, whining, and sibling squabbles; maximum times for baths and video games; minimum times for meals, sitting to poop, and special time. Timers are not just for Time Out! “Visual timers” that show green then yellow then red and sometimes flashing lights as warnings of an upcoming stopping point are helpful for children preschool and older. These timers help them to develop a better sense of time and begin managing their own transitions. A game of guessing how long things take can build timing skills and patience. I think every child past preschool benefits from a wristwatch, first to build time sense, and second to avoid looking at a smartphone to see the hour, then being distracted by content! Diaries of behaviors over time are a staple of behavior change plans, with the added benefit of lending perspective on actually how often and how long a troublesome behavior occurs. Practicing mindfulness – nonjudgmental watching of our thoughts and feelings, often with deep breathing and relaxation – also can help both children and adults build time tolerance.
Children have little control over their daily schedule. Surrendering when you can for them to do things at their own pace can reduce their frustration, build the parent-child relationship, and promote positive behaviors. Plus family life is more enjoyable lived slower. You even can remind parents that “the days are long but the years are short” before their children will be grown and gone.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
It is often fun and sometimes exhausting watching the speed with which children run around or switch from one game to another. A lot of us were attracted to pediatrics to share the quick joy of children and also the speed of their physical recovery. We get to see premature infants gain an ounce a day, and see wounds heal in less than a week. We give advice on sleep and see success in a month. We and the families get used to quick fixes.
Parents and children are forewarned and reassured by our knowledge about how long things typically take: Respiratory syncytial virus (RSV) peaks in 5 days, colic lessens in 3 months, changing sleep patterns takes 3 weeks, habit formation 6 weeks, menses come 2 years after breast development, and so on. But the timing of daily parenting is rarely as predictable. Sometimes a child’s clock is running fast, making waiting even seconds for a snack or a bathroom difficult; other times are slow, as when walking down the sidewalk noticing every leaf. The child’s clock is independent of the adult’s – and complicated by clocks of siblings.
Parent pace also is determined by many factors unrelated to the child: work demands, deadlines, train schedules, something in the oven, needs of siblings, and so on. To those can be added intrinsic factors affecting parent’s tolerance to shifting pace to the child’s such as temperament, fatigue, illness, pain, or even adult ADHD. And don’t forget caffeine (or other drugs) affecting the internal metronome. When impatience with the child is a complaint, it is useful to ask, “What makes waiting for your child difficult for you?”
When discussing time, I find it important to discuss the poison “s-word” of parenting – “should.” This trickster often comes from time illusions in childrearing. After seeing so many behaviors change quickly, parents expect all change to be equally fast. She should be able to sleep through the night by now! He should be able to dress and get to the table in 5 minutes. And sometimes it is the parent’s s-word that creates pain – I should love pushing for as long as she wants to swing, if I am a good parent. The problem with thinking “should” is that it implies willful or moral behavior, and it may prompt a judgmental or punitive parental response.
Otherwise well intentioned, cooperative children who take longer to shift their attention from homework to shower can be seen as oppositional. Worse yet, if the example used is from playing video games (something fun) to getting to the bus stop (an undesirable shift), you may hear parents critically say, “He only wants to do what he wants to do.” When examining examples (always key to helping with behavior), pointing out that all kinds of transitions are difficult for this child may be educational and allow for a more reasoned response. And specifically being on electronics puts adults as well as children in a time warp which is hard to escape.
There are many kinds of thwarted expectations, but expectations about how long things take are pretty universal. Frustration generates anger and even can lead to violence, such as road rage. Children – who all step to the beat of a different drummer, especially those with different “clocks” such as in ADHD – may experience frustration most of the day. This can manifest as irritability for them and sometimes as an irritable response back from the parent.
The first step in adapting to differences in parent and child pace is to realize that time is the problem. Naming it, saying “we are on toddler time,” can be a “signal to self” to slow down. Generations of children loved Mr. Rogers because he always conveyed having all the time in the world for the person he was with. It actually does not take as long as it feels at first to do this. Listening while keeping eye contact, breathing deeply, and waiting until two breaths after the child goes silent before speaking or moving conveys your interest and respect. For some behaviors, such as tantrums, such quiet attention may be all that is needed to resolve the issue. We adults can practice this, but even infants can be helped to develop patience by reinforcement with brief attention from their caregivers for tiny increments of waiting.
I sometimes suggest that parents time behaviors to develop perspective, reset expectations, practice waiting, and perhaps even distract themselves from intervening and making things worse by lending attention to negative behaviors. Timing as observation can be helpful for tantrums, breath holding spells, whining, and sibling squabbles; maximum times for baths and video games; minimum times for meals, sitting to poop, and special time. Timers are not just for Time Out! “Visual timers” that show green then yellow then red and sometimes flashing lights as warnings of an upcoming stopping point are helpful for children preschool and older. These timers help them to develop a better sense of time and begin managing their own transitions. A game of guessing how long things take can build timing skills and patience. I think every child past preschool benefits from a wristwatch, first to build time sense, and second to avoid looking at a smartphone to see the hour, then being distracted by content! Diaries of behaviors over time are a staple of behavior change plans, with the added benefit of lending perspective on actually how often and how long a troublesome behavior occurs. Practicing mindfulness – nonjudgmental watching of our thoughts and feelings, often with deep breathing and relaxation – also can help both children and adults build time tolerance.
Children have little control over their daily schedule. Surrendering when you can for them to do things at their own pace can reduce their frustration, build the parent-child relationship, and promote positive behaviors. Plus family life is more enjoyable lived slower. You even can remind parents that “the days are long but the years are short” before their children will be grown and gone.
Dr. Howard is an assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. Email her at [email protected].
Portico system safe, effective for high-risk TAVR patients
SAN FRANCISCO – An investigational device exemption trial of the Portico valve with FlexNav delivery system showed 1-year clinical results on par with commercially available valves, Gregory P. Fontana, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
In a prospective, open-label study conducted at 52 sites known as PORTICO, Dr. Fontana and colleagues conducted a noninferiority intention-to-treat evaluation of the safety and effectiveness of the self-expanding Portico transcatheter aortic valve replacement system, compared with Food and Drug Administration–approved and commercially available TAVR systems for patients with severe aortic stenosis at high or extreme risk for surgery. Between May 2014 and June 2019, 750 patients from 69 sites were randomized 1:1 to each group. The prespecified primary safety composite endpoint was all-cause mortality, disabling stroke, life-threatening bleeding requiring blood transfusion, acute kidney injury requiring dialysis, or major vascular complications at 30 days, while the primary effectiveness composite endpoint was all-cause mortality or disabling stroke at 1 year.
The mean baseline age of patients was 83 years, 53% were female, and their mean Society of Thoracic Surgeons score was 6.5%. Dr. Fontana, director and chairman of cardiothoracic surgery at the CardioVascular Institute of Los Robles Regional Medical Center, Thousand Oaks, Calif., reported at the meeting sponsored by the Cardiovascular Research Foundation that procedural success was comparable between groups (96.5% for Portico vs, 98.3% for commercially available TAVR, respectively). In addition, patients in both groups met the prespecified primary safety composite endpoint (13.8% vs. 9.6%; P for noninferiority = .03) and the primary effectiveness composite endpoint (14.9% vs. 13.4%, P for noninferiority = .006).
However, the rate of moderate to severe paravalvular leak at 30 days was 6.3% among patients in the Portico valve group, compared with 2.1% of their counterparts in the commercially available TAVR group, a difference that did not reach noninferiority. Dr. Fontana said that a next-generation valve with design modifications to reduce paravalvular leak is being tested in clinical trials.
PORTICO included a separate cohort of 100 patients who underwent Portico valve implantation using the FlexNav Delivery System, which became available after the trial had launched. The primary safety endpoint for the FlexNav cohort was major vascular complication rate at 30 days. This cohort demonstrated no deaths or strokes, low rates of major vascular complications (7.0%) and new permanent pacemaker implants (14.6%), as well as a safety profile comparable with the commercially available valve group in the randomized study (8.0% vs. 9.6%).
“My sense of this device is that presumably it will be another valve we have available to us in the United States,” Pinak B. Shah, MD, a cardiologist at Brigham and Women’s Hospital, Boston, said during a media briefing. “The challenge to all of us is to figure out where it fits in our armamentarium. Is it going to be worth individuals to learn a whole new device when at this point it’s hard to say if there’s a major difference compared to the other self-expanding devices we have now?”
With the new FlexNav delivery system, the Portico valve is characterized by “a very calm, slow delivery,” said Dr. Fontana, who was the study’s coprincipal investigator, along with Raj R. Makkar, MD, director of interventional cardiology at Cedars-Sinai Medical Center, Los Angeles. “The operator can land the valve exactly where they want it. If they’re not happy, they can make some adjustments. I haven’t yet a system in my hands that is as stable as this. The option of having excellent hemodynamics and large cells to engage the coronary system is a unique combination for us in the United States.”
The Portico valve is not currently FDA approved. The PORTICO study was funded by Abbott. Dr. Fontana disclosed grant/research support from Abbott and Medtronic and consulting fees/honoraria from Abbott, Medtronic, and LivaNova.
SAN FRANCISCO – An investigational device exemption trial of the Portico valve with FlexNav delivery system showed 1-year clinical results on par with commercially available valves, Gregory P. Fontana, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
In a prospective, open-label study conducted at 52 sites known as PORTICO, Dr. Fontana and colleagues conducted a noninferiority intention-to-treat evaluation of the safety and effectiveness of the self-expanding Portico transcatheter aortic valve replacement system, compared with Food and Drug Administration–approved and commercially available TAVR systems for patients with severe aortic stenosis at high or extreme risk for surgery. Between May 2014 and June 2019, 750 patients from 69 sites were randomized 1:1 to each group. The prespecified primary safety composite endpoint was all-cause mortality, disabling stroke, life-threatening bleeding requiring blood transfusion, acute kidney injury requiring dialysis, or major vascular complications at 30 days, while the primary effectiveness composite endpoint was all-cause mortality or disabling stroke at 1 year.
The mean baseline age of patients was 83 years, 53% were female, and their mean Society of Thoracic Surgeons score was 6.5%. Dr. Fontana, director and chairman of cardiothoracic surgery at the CardioVascular Institute of Los Robles Regional Medical Center, Thousand Oaks, Calif., reported at the meeting sponsored by the Cardiovascular Research Foundation that procedural success was comparable between groups (96.5% for Portico vs, 98.3% for commercially available TAVR, respectively). In addition, patients in both groups met the prespecified primary safety composite endpoint (13.8% vs. 9.6%; P for noninferiority = .03) and the primary effectiveness composite endpoint (14.9% vs. 13.4%, P for noninferiority = .006).
However, the rate of moderate to severe paravalvular leak at 30 days was 6.3% among patients in the Portico valve group, compared with 2.1% of their counterparts in the commercially available TAVR group, a difference that did not reach noninferiority. Dr. Fontana said that a next-generation valve with design modifications to reduce paravalvular leak is being tested in clinical trials.
PORTICO included a separate cohort of 100 patients who underwent Portico valve implantation using the FlexNav Delivery System, which became available after the trial had launched. The primary safety endpoint for the FlexNav cohort was major vascular complication rate at 30 days. This cohort demonstrated no deaths or strokes, low rates of major vascular complications (7.0%) and new permanent pacemaker implants (14.6%), as well as a safety profile comparable with the commercially available valve group in the randomized study (8.0% vs. 9.6%).
“My sense of this device is that presumably it will be another valve we have available to us in the United States,” Pinak B. Shah, MD, a cardiologist at Brigham and Women’s Hospital, Boston, said during a media briefing. “The challenge to all of us is to figure out where it fits in our armamentarium. Is it going to be worth individuals to learn a whole new device when at this point it’s hard to say if there’s a major difference compared to the other self-expanding devices we have now?”
With the new FlexNav delivery system, the Portico valve is characterized by “a very calm, slow delivery,” said Dr. Fontana, who was the study’s coprincipal investigator, along with Raj R. Makkar, MD, director of interventional cardiology at Cedars-Sinai Medical Center, Los Angeles. “The operator can land the valve exactly where they want it. If they’re not happy, they can make some adjustments. I haven’t yet a system in my hands that is as stable as this. The option of having excellent hemodynamics and large cells to engage the coronary system is a unique combination for us in the United States.”
The Portico valve is not currently FDA approved. The PORTICO study was funded by Abbott. Dr. Fontana disclosed grant/research support from Abbott and Medtronic and consulting fees/honoraria from Abbott, Medtronic, and LivaNova.
SAN FRANCISCO – An investigational device exemption trial of the Portico valve with FlexNav delivery system showed 1-year clinical results on par with commercially available valves, Gregory P. Fontana, MD, reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
In a prospective, open-label study conducted at 52 sites known as PORTICO, Dr. Fontana and colleagues conducted a noninferiority intention-to-treat evaluation of the safety and effectiveness of the self-expanding Portico transcatheter aortic valve replacement system, compared with Food and Drug Administration–approved and commercially available TAVR systems for patients with severe aortic stenosis at high or extreme risk for surgery. Between May 2014 and June 2019, 750 patients from 69 sites were randomized 1:1 to each group. The prespecified primary safety composite endpoint was all-cause mortality, disabling stroke, life-threatening bleeding requiring blood transfusion, acute kidney injury requiring dialysis, or major vascular complications at 30 days, while the primary effectiveness composite endpoint was all-cause mortality or disabling stroke at 1 year.
The mean baseline age of patients was 83 years, 53% were female, and their mean Society of Thoracic Surgeons score was 6.5%. Dr. Fontana, director and chairman of cardiothoracic surgery at the CardioVascular Institute of Los Robles Regional Medical Center, Thousand Oaks, Calif., reported at the meeting sponsored by the Cardiovascular Research Foundation that procedural success was comparable between groups (96.5% for Portico vs, 98.3% for commercially available TAVR, respectively). In addition, patients in both groups met the prespecified primary safety composite endpoint (13.8% vs. 9.6%; P for noninferiority = .03) and the primary effectiveness composite endpoint (14.9% vs. 13.4%, P for noninferiority = .006).
However, the rate of moderate to severe paravalvular leak at 30 days was 6.3% among patients in the Portico valve group, compared with 2.1% of their counterparts in the commercially available TAVR group, a difference that did not reach noninferiority. Dr. Fontana said that a next-generation valve with design modifications to reduce paravalvular leak is being tested in clinical trials.
PORTICO included a separate cohort of 100 patients who underwent Portico valve implantation using the FlexNav Delivery System, which became available after the trial had launched. The primary safety endpoint for the FlexNav cohort was major vascular complication rate at 30 days. This cohort demonstrated no deaths or strokes, low rates of major vascular complications (7.0%) and new permanent pacemaker implants (14.6%), as well as a safety profile comparable with the commercially available valve group in the randomized study (8.0% vs. 9.6%).
“My sense of this device is that presumably it will be another valve we have available to us in the United States,” Pinak B. Shah, MD, a cardiologist at Brigham and Women’s Hospital, Boston, said during a media briefing. “The challenge to all of us is to figure out where it fits in our armamentarium. Is it going to be worth individuals to learn a whole new device when at this point it’s hard to say if there’s a major difference compared to the other self-expanding devices we have now?”
With the new FlexNav delivery system, the Portico valve is characterized by “a very calm, slow delivery,” said Dr. Fontana, who was the study’s coprincipal investigator, along with Raj R. Makkar, MD, director of interventional cardiology at Cedars-Sinai Medical Center, Los Angeles. “The operator can land the valve exactly where they want it. If they’re not happy, they can make some adjustments. I haven’t yet a system in my hands that is as stable as this. The option of having excellent hemodynamics and large cells to engage the coronary system is a unique combination for us in the United States.”
The Portico valve is not currently FDA approved. The PORTICO study was funded by Abbott. Dr. Fontana disclosed grant/research support from Abbott and Medtronic and consulting fees/honoraria from Abbott, Medtronic, and LivaNova.
REPORTING FROM TCT 2019
Universal coverage may be possible without increases in national spending
The Commonwealth Fund and The Urban Institute looked at eight reform scenarios, including ones that build on the Affordable Care Act and expand to universal coverage or single payer. Two scenarios that continue to utilize private insurance show that, conceptually, broad coverage can be achieved without increasing spending.
“This study is important because it shows that there are several health reform approaches that have the potential to increase the number of people with health insurance, make health care more affordable, and slow cost growth,” David Blumenthal, MD, president of The Commonwealth Fund, said during an Oct. 15 conference call introducing the report.
He called the details that separate the varying models “central to the national debate on health care and health insurance coverage as the 2020 campaign season progresses.”
All the scenarios presented in the report have a foundation in various current Democratic health care reform proposals, although no one specific proposal or legislation is profiled within the eight scenarios presented.
“Our hope is that this extensive analysis will clarify for voters and policy makers the implications of the policy choices before us,” said Sara R. Collins, PhD, the vice president of health care coverage and access at The Commonwealth Fund, during the call.
The first of these scenarios, dubbed “Universal Coverage I: Private and Public Options,” includes continued use of private insurance but also involves a public option and is the first of four options presented in the report to achieve universal coverage by actively enrolling people who are not enrolled in a private plan for one year in the public option with income-scaled premiums. This option would not utilize the ACA employer mandate and would remove the “firewall” that prevents individuals with access to employer-sponsored coverage from accessing financial assistance and seeking individual coverage from the insurance marketplace.
This scenario, as with all but one of the scenarios analyzed in the report, covers all essential benefits as defined in the Affordable Care Act. The only single-payer option that does not cover all of these essential benefits still provides coverage for medically necessary care, including dental, vision, hearing, and long-term services.
The Universal Coverage I scenario does not have any penalties for not carrying insurance, but all legal residents that forgo voluntary coverage from an employer or the marketplace will be automatically enrolled in coverage for which they are responsible for a premium payment.
There would be no expanded access to short-term, limited duration plans as the automatic enrollment to those not voluntarily covered by an employer or in the marketplace would make coverage universal. Federal government spending under this plan increases government health care spending in 2020 by $122.1 billion and $1.5 trillion over 10 years. However, total national spending in this scenario would decrease by $22.6 billion or 0.6% in 2020, compared with current law.
“Universal Coverage II: Enhanced Subsidies” is similar to Universal Coverage I in all other respects other than that it includes more generous premium and cost-sharing subsidies. These additional offerings would push federal government spending up $161.8 billion more in 2020, compared with current law, and to $2 trillion more over the next 10 years, while showing a minimal decrease in total national spending of less than 1% compared with current law.
The other two options that would move toward providing everyone with health insurance include a single payer system that covers all ACA essential health benefits, features no premiums, has income-related cost sharing, and covers all legal residents. Private insurance in this scenario is prohibited, and provider payments would be similar to those received in Medicare. Federal government spending would increase in 2020 by $1.5 trillion, compared with current law, and $17.6 trillion over the next 10 years. However, total national spending would decrease by $209.5 billion, or 6%, in 2020 compared with current law. These savings would come from lower provider payments and administrative costs that outweigh increased costs associated with near universal coverage and lower cost-sharing requirements.
A second single payer scenario broadens the benefits and would cover all residents in the United States, including undocumented residents. It would have no cost-sharing requirements.
The “optimal levels at which the payments for hospitals and doctors and other providers should be paid are really unknown at this time,” said Linda Blumberg, PhD, fellow at The Urban Institute’s Health Policy Center and one of the report authors, during the call.
Providing total coverage for all people in the United States is estimated to increase federal spending by $2.8 trillion in 2020 compared with current law, and $34 trillion over 10 years, with much of this increase accounted for by the shift in existing state and private spending to the federal government. At the same time, total national spending would increase by approximately $720 billion in 2020 compared with current law. Even though employer, household, and state spending would decrease, these savings would not be enough to offset increases in federal spending as well as the increased consumption of health care that comes with more generous benefits. The offsets from lower administrative costs and lower provider payments also would not offset higher spending.
The report only looks at health care spending and does not present any suggestions on revenue to offset the spending.
SOURCE: Blumberg LJ et al. “From Incremental to Comprehensive Health Insurance Reform: How Various Reform Options Compare On Coverage and Costs.” The Commonwealth Fund and The Urban Institute. 2019 Oct 16.
The Commonwealth Fund and The Urban Institute looked at eight reform scenarios, including ones that build on the Affordable Care Act and expand to universal coverage or single payer. Two scenarios that continue to utilize private insurance show that, conceptually, broad coverage can be achieved without increasing spending.
“This study is important because it shows that there are several health reform approaches that have the potential to increase the number of people with health insurance, make health care more affordable, and slow cost growth,” David Blumenthal, MD, president of The Commonwealth Fund, said during an Oct. 15 conference call introducing the report.
He called the details that separate the varying models “central to the national debate on health care and health insurance coverage as the 2020 campaign season progresses.”
All the scenarios presented in the report have a foundation in various current Democratic health care reform proposals, although no one specific proposal or legislation is profiled within the eight scenarios presented.
“Our hope is that this extensive analysis will clarify for voters and policy makers the implications of the policy choices before us,” said Sara R. Collins, PhD, the vice president of health care coverage and access at The Commonwealth Fund, during the call.
The first of these scenarios, dubbed “Universal Coverage I: Private and Public Options,” includes continued use of private insurance but also involves a public option and is the first of four options presented in the report to achieve universal coverage by actively enrolling people who are not enrolled in a private plan for one year in the public option with income-scaled premiums. This option would not utilize the ACA employer mandate and would remove the “firewall” that prevents individuals with access to employer-sponsored coverage from accessing financial assistance and seeking individual coverage from the insurance marketplace.
This scenario, as with all but one of the scenarios analyzed in the report, covers all essential benefits as defined in the Affordable Care Act. The only single-payer option that does not cover all of these essential benefits still provides coverage for medically necessary care, including dental, vision, hearing, and long-term services.
The Universal Coverage I scenario does not have any penalties for not carrying insurance, but all legal residents that forgo voluntary coverage from an employer or the marketplace will be automatically enrolled in coverage for which they are responsible for a premium payment.
There would be no expanded access to short-term, limited duration plans as the automatic enrollment to those not voluntarily covered by an employer or in the marketplace would make coverage universal. Federal government spending under this plan increases government health care spending in 2020 by $122.1 billion and $1.5 trillion over 10 years. However, total national spending in this scenario would decrease by $22.6 billion or 0.6% in 2020, compared with current law.
“Universal Coverage II: Enhanced Subsidies” is similar to Universal Coverage I in all other respects other than that it includes more generous premium and cost-sharing subsidies. These additional offerings would push federal government spending up $161.8 billion more in 2020, compared with current law, and to $2 trillion more over the next 10 years, while showing a minimal decrease in total national spending of less than 1% compared with current law.
The other two options that would move toward providing everyone with health insurance include a single payer system that covers all ACA essential health benefits, features no premiums, has income-related cost sharing, and covers all legal residents. Private insurance in this scenario is prohibited, and provider payments would be similar to those received in Medicare. Federal government spending would increase in 2020 by $1.5 trillion, compared with current law, and $17.6 trillion over the next 10 years. However, total national spending would decrease by $209.5 billion, or 6%, in 2020 compared with current law. These savings would come from lower provider payments and administrative costs that outweigh increased costs associated with near universal coverage and lower cost-sharing requirements.
A second single payer scenario broadens the benefits and would cover all residents in the United States, including undocumented residents. It would have no cost-sharing requirements.
The “optimal levels at which the payments for hospitals and doctors and other providers should be paid are really unknown at this time,” said Linda Blumberg, PhD, fellow at The Urban Institute’s Health Policy Center and one of the report authors, during the call.
Providing total coverage for all people in the United States is estimated to increase federal spending by $2.8 trillion in 2020 compared with current law, and $34 trillion over 10 years, with much of this increase accounted for by the shift in existing state and private spending to the federal government. At the same time, total national spending would increase by approximately $720 billion in 2020 compared with current law. Even though employer, household, and state spending would decrease, these savings would not be enough to offset increases in federal spending as well as the increased consumption of health care that comes with more generous benefits. The offsets from lower administrative costs and lower provider payments also would not offset higher spending.
The report only looks at health care spending and does not present any suggestions on revenue to offset the spending.
SOURCE: Blumberg LJ et al. “From Incremental to Comprehensive Health Insurance Reform: How Various Reform Options Compare On Coverage and Costs.” The Commonwealth Fund and The Urban Institute. 2019 Oct 16.
The Commonwealth Fund and The Urban Institute looked at eight reform scenarios, including ones that build on the Affordable Care Act and expand to universal coverage or single payer. Two scenarios that continue to utilize private insurance show that, conceptually, broad coverage can be achieved without increasing spending.
“This study is important because it shows that there are several health reform approaches that have the potential to increase the number of people with health insurance, make health care more affordable, and slow cost growth,” David Blumenthal, MD, president of The Commonwealth Fund, said during an Oct. 15 conference call introducing the report.
He called the details that separate the varying models “central to the national debate on health care and health insurance coverage as the 2020 campaign season progresses.”
All the scenarios presented in the report have a foundation in various current Democratic health care reform proposals, although no one specific proposal or legislation is profiled within the eight scenarios presented.
“Our hope is that this extensive analysis will clarify for voters and policy makers the implications of the policy choices before us,” said Sara R. Collins, PhD, the vice president of health care coverage and access at The Commonwealth Fund, during the call.
The first of these scenarios, dubbed “Universal Coverage I: Private and Public Options,” includes continued use of private insurance but also involves a public option and is the first of four options presented in the report to achieve universal coverage by actively enrolling people who are not enrolled in a private plan for one year in the public option with income-scaled premiums. This option would not utilize the ACA employer mandate and would remove the “firewall” that prevents individuals with access to employer-sponsored coverage from accessing financial assistance and seeking individual coverage from the insurance marketplace.
This scenario, as with all but one of the scenarios analyzed in the report, covers all essential benefits as defined in the Affordable Care Act. The only single-payer option that does not cover all of these essential benefits still provides coverage for medically necessary care, including dental, vision, hearing, and long-term services.
The Universal Coverage I scenario does not have any penalties for not carrying insurance, but all legal residents that forgo voluntary coverage from an employer or the marketplace will be automatically enrolled in coverage for which they are responsible for a premium payment.
There would be no expanded access to short-term, limited duration plans as the automatic enrollment to those not voluntarily covered by an employer or in the marketplace would make coverage universal. Federal government spending under this plan increases government health care spending in 2020 by $122.1 billion and $1.5 trillion over 10 years. However, total national spending in this scenario would decrease by $22.6 billion or 0.6% in 2020, compared with current law.
“Universal Coverage II: Enhanced Subsidies” is similar to Universal Coverage I in all other respects other than that it includes more generous premium and cost-sharing subsidies. These additional offerings would push federal government spending up $161.8 billion more in 2020, compared with current law, and to $2 trillion more over the next 10 years, while showing a minimal decrease in total national spending of less than 1% compared with current law.
The other two options that would move toward providing everyone with health insurance include a single payer system that covers all ACA essential health benefits, features no premiums, has income-related cost sharing, and covers all legal residents. Private insurance in this scenario is prohibited, and provider payments would be similar to those received in Medicare. Federal government spending would increase in 2020 by $1.5 trillion, compared with current law, and $17.6 trillion over the next 10 years. However, total national spending would decrease by $209.5 billion, or 6%, in 2020 compared with current law. These savings would come from lower provider payments and administrative costs that outweigh increased costs associated with near universal coverage and lower cost-sharing requirements.
A second single payer scenario broadens the benefits and would cover all residents in the United States, including undocumented residents. It would have no cost-sharing requirements.
The “optimal levels at which the payments for hospitals and doctors and other providers should be paid are really unknown at this time,” said Linda Blumberg, PhD, fellow at The Urban Institute’s Health Policy Center and one of the report authors, during the call.
Providing total coverage for all people in the United States is estimated to increase federal spending by $2.8 trillion in 2020 compared with current law, and $34 trillion over 10 years, with much of this increase accounted for by the shift in existing state and private spending to the federal government. At the same time, total national spending would increase by approximately $720 billion in 2020 compared with current law. Even though employer, household, and state spending would decrease, these savings would not be enough to offset increases in federal spending as well as the increased consumption of health care that comes with more generous benefits. The offsets from lower administrative costs and lower provider payments also would not offset higher spending.
The report only looks at health care spending and does not present any suggestions on revenue to offset the spending.
SOURCE: Blumberg LJ et al. “From Incremental to Comprehensive Health Insurance Reform: How Various Reform Options Compare On Coverage and Costs.” The Commonwealth Fund and The Urban Institute. 2019 Oct 16.
Health care stayed front and center at Democratic debate
This time, it wasn’t just about Medicare-for-all.
Voters got a better look at Democrats’ health care priorities on Tuesday, as
While the debate began on the topic of impeaching President Trump, Sen. Bernie Sanders of Vermont soon steered the discussion back to kitchen-table issues.
“I think what would be a disaster, if the American people believe that all we were doing is taking on Trump,” he said. “We’re forgetting that 87 million Americans are uninsured or underinsured.”
That was only the beginning of a series of health care conversations that lasted through much of the three-hour debate.
With Sen. Elizabeth Warren of Massachusetts polling in second place before the night began, she was pressed to offer more details about what Medicare-for-all would look like under her leadership – in particular, whether she would raise taxes to pay for it.
“I have made clear what my principles are here,” she said. “That is, costs will go up for the wealthy and for big corporations, and for hardworking, middle-class families, costs will go down.”
But Mayor Pete Buttigieg of South Bend, Ind., pushed back, pointing out that, unlike Sen. Sanders – who has said taxes would increase to pay for his universal health care plan – she had not actually said whether she would raise taxes.
“Your signature is to have a plan for everything, except this,” Mr. Buttigieg said. “No plan has been laid out to explain how a multitrillion-dollar hole in this plan that Sen. Warren is putting forward is supposed to get filled in.”
Sen. Amy Klobuchar of Minnesota challenged the practicality of focusing on such a sweeping overhaul as Medicare-for-all. She pushed her support for a public option and noted the importance of issues that get less attention, like long-term care.
“The difference between a plan and a pipe dream is something that you can actually get done,” Sen. Klobuchar said.
But Sen. Warren stood her ground. When she was studying bankruptcy as a professor at Harvard Law School, she said, she noticed that two out of three families that went bankrupt after a medical problem had health insurance. The problem is cost, she said: “That is why hardworking people go broke.”
The candidates also staked their claim on two issues that are critically important to Democratic voters: strengthening gun control measures and guaranteeing access to reproductive health care.
Former Vice President Joe Biden trumpeted his role in securing the now-lapsed assault weapons ban in 1994. Among others, Sen. Kamala Harris of California called for a “comprehensive” background check requirement and a ban on the importation of assault weapons.
And one by one, the candidates vowed to codify abortion access, especially in light of recent conservative attacks in a number of states on the premise of the Supreme Court’s Roe v. Wade decision.
“It’s not an exaggeration to say women will die because these Republican legislatures in these various states who are out of touch with America are telling women what to do with their bodies,” Sen. Harris said, a reference to crackdowns on abortion access in many Republican-controlled states.
After pointing out earlier in the evening that two Planned Parenthood clinics in Ohio recently closed because of a Trump administration policy change, Sen. Cory Booker of New Jersey said he would create an office of reproductive freedom and reproductive rights in his White House.
“It’s an assault on the most fundamental ideal that human beings should control their own body,” Sen. Booker said.
And addressing the opioid crisis, blamed for lowering life expectancy in the United States, many of the candidates called outright for jailing the executives of opioid manufacturers, whom Sen. Harris called “nothing more than some high-level dope dealers.”
“The people who should pay for the treatment are the very people that got people hooked and killed them in the first place,” she said.
The evening was also Sen. Sanders’ first appearance on the debate stage since he had a heart attack and underwent heart surgery just weeks ago. Asked about his health, he seemed impatient: “I’m healthy. I’m feeling great,” Sen. Sanders said as he brought the conversation back to policy.
The debate took place in Westerville, Ohio, a traditionally conservative suburb of Columbus that had turned blue in recent years – a nod to Democrats’ hopes of winning with the support of suburban voters in 2020.
And with those 12 Democrats standing elbow-to-elbow, the debate hosted by CNN and the New York Times had an unusual distinction: the most candidates to ever appear onstage at a presidential debate.
The fifth Democratic debate is scheduled for Nov. 20. The Democratic National Committee plans to hold 12 primary debates in total.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
This time, it wasn’t just about Medicare-for-all.
Voters got a better look at Democrats’ health care priorities on Tuesday, as
While the debate began on the topic of impeaching President Trump, Sen. Bernie Sanders of Vermont soon steered the discussion back to kitchen-table issues.
“I think what would be a disaster, if the American people believe that all we were doing is taking on Trump,” he said. “We’re forgetting that 87 million Americans are uninsured or underinsured.”
That was only the beginning of a series of health care conversations that lasted through much of the three-hour debate.
With Sen. Elizabeth Warren of Massachusetts polling in second place before the night began, she was pressed to offer more details about what Medicare-for-all would look like under her leadership – in particular, whether she would raise taxes to pay for it.
“I have made clear what my principles are here,” she said. “That is, costs will go up for the wealthy and for big corporations, and for hardworking, middle-class families, costs will go down.”
But Mayor Pete Buttigieg of South Bend, Ind., pushed back, pointing out that, unlike Sen. Sanders – who has said taxes would increase to pay for his universal health care plan – she had not actually said whether she would raise taxes.
“Your signature is to have a plan for everything, except this,” Mr. Buttigieg said. “No plan has been laid out to explain how a multitrillion-dollar hole in this plan that Sen. Warren is putting forward is supposed to get filled in.”
Sen. Amy Klobuchar of Minnesota challenged the practicality of focusing on such a sweeping overhaul as Medicare-for-all. She pushed her support for a public option and noted the importance of issues that get less attention, like long-term care.
“The difference between a plan and a pipe dream is something that you can actually get done,” Sen. Klobuchar said.
But Sen. Warren stood her ground. When she was studying bankruptcy as a professor at Harvard Law School, she said, she noticed that two out of three families that went bankrupt after a medical problem had health insurance. The problem is cost, she said: “That is why hardworking people go broke.”
The candidates also staked their claim on two issues that are critically important to Democratic voters: strengthening gun control measures and guaranteeing access to reproductive health care.
Former Vice President Joe Biden trumpeted his role in securing the now-lapsed assault weapons ban in 1994. Among others, Sen. Kamala Harris of California called for a “comprehensive” background check requirement and a ban on the importation of assault weapons.
And one by one, the candidates vowed to codify abortion access, especially in light of recent conservative attacks in a number of states on the premise of the Supreme Court’s Roe v. Wade decision.
“It’s not an exaggeration to say women will die because these Republican legislatures in these various states who are out of touch with America are telling women what to do with their bodies,” Sen. Harris said, a reference to crackdowns on abortion access in many Republican-controlled states.
After pointing out earlier in the evening that two Planned Parenthood clinics in Ohio recently closed because of a Trump administration policy change, Sen. Cory Booker of New Jersey said he would create an office of reproductive freedom and reproductive rights in his White House.
“It’s an assault on the most fundamental ideal that human beings should control their own body,” Sen. Booker said.
And addressing the opioid crisis, blamed for lowering life expectancy in the United States, many of the candidates called outright for jailing the executives of opioid manufacturers, whom Sen. Harris called “nothing more than some high-level dope dealers.”
“The people who should pay for the treatment are the very people that got people hooked and killed them in the first place,” she said.
The evening was also Sen. Sanders’ first appearance on the debate stage since he had a heart attack and underwent heart surgery just weeks ago. Asked about his health, he seemed impatient: “I’m healthy. I’m feeling great,” Sen. Sanders said as he brought the conversation back to policy.
The debate took place in Westerville, Ohio, a traditionally conservative suburb of Columbus that had turned blue in recent years – a nod to Democrats’ hopes of winning with the support of suburban voters in 2020.
And with those 12 Democrats standing elbow-to-elbow, the debate hosted by CNN and the New York Times had an unusual distinction: the most candidates to ever appear onstage at a presidential debate.
The fifth Democratic debate is scheduled for Nov. 20. The Democratic National Committee plans to hold 12 primary debates in total.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
This time, it wasn’t just about Medicare-for-all.
Voters got a better look at Democrats’ health care priorities on Tuesday, as
While the debate began on the topic of impeaching President Trump, Sen. Bernie Sanders of Vermont soon steered the discussion back to kitchen-table issues.
“I think what would be a disaster, if the American people believe that all we were doing is taking on Trump,” he said. “We’re forgetting that 87 million Americans are uninsured or underinsured.”
That was only the beginning of a series of health care conversations that lasted through much of the three-hour debate.
With Sen. Elizabeth Warren of Massachusetts polling in second place before the night began, she was pressed to offer more details about what Medicare-for-all would look like under her leadership – in particular, whether she would raise taxes to pay for it.
“I have made clear what my principles are here,” she said. “That is, costs will go up for the wealthy and for big corporations, and for hardworking, middle-class families, costs will go down.”
But Mayor Pete Buttigieg of South Bend, Ind., pushed back, pointing out that, unlike Sen. Sanders – who has said taxes would increase to pay for his universal health care plan – she had not actually said whether she would raise taxes.
“Your signature is to have a plan for everything, except this,” Mr. Buttigieg said. “No plan has been laid out to explain how a multitrillion-dollar hole in this plan that Sen. Warren is putting forward is supposed to get filled in.”
Sen. Amy Klobuchar of Minnesota challenged the practicality of focusing on such a sweeping overhaul as Medicare-for-all. She pushed her support for a public option and noted the importance of issues that get less attention, like long-term care.
“The difference between a plan and a pipe dream is something that you can actually get done,” Sen. Klobuchar said.
But Sen. Warren stood her ground. When she was studying bankruptcy as a professor at Harvard Law School, she said, she noticed that two out of three families that went bankrupt after a medical problem had health insurance. The problem is cost, she said: “That is why hardworking people go broke.”
The candidates also staked their claim on two issues that are critically important to Democratic voters: strengthening gun control measures and guaranteeing access to reproductive health care.
Former Vice President Joe Biden trumpeted his role in securing the now-lapsed assault weapons ban in 1994. Among others, Sen. Kamala Harris of California called for a “comprehensive” background check requirement and a ban on the importation of assault weapons.
And one by one, the candidates vowed to codify abortion access, especially in light of recent conservative attacks in a number of states on the premise of the Supreme Court’s Roe v. Wade decision.
“It’s not an exaggeration to say women will die because these Republican legislatures in these various states who are out of touch with America are telling women what to do with their bodies,” Sen. Harris said, a reference to crackdowns on abortion access in many Republican-controlled states.
After pointing out earlier in the evening that two Planned Parenthood clinics in Ohio recently closed because of a Trump administration policy change, Sen. Cory Booker of New Jersey said he would create an office of reproductive freedom and reproductive rights in his White House.
“It’s an assault on the most fundamental ideal that human beings should control their own body,” Sen. Booker said.
And addressing the opioid crisis, blamed for lowering life expectancy in the United States, many of the candidates called outright for jailing the executives of opioid manufacturers, whom Sen. Harris called “nothing more than some high-level dope dealers.”
“The people who should pay for the treatment are the very people that got people hooked and killed them in the first place,” she said.
The evening was also Sen. Sanders’ first appearance on the debate stage since he had a heart attack and underwent heart surgery just weeks ago. Asked about his health, he seemed impatient: “I’m healthy. I’m feeling great,” Sen. Sanders said as he brought the conversation back to policy.
The debate took place in Westerville, Ohio, a traditionally conservative suburb of Columbus that had turned blue in recent years – a nod to Democrats’ hopes of winning with the support of suburban voters in 2020.
And with those 12 Democrats standing elbow-to-elbow, the debate hosted by CNN and the New York Times had an unusual distinction: the most candidates to ever appear onstage at a presidential debate.
The fifth Democratic debate is scheduled for Nov. 20. The Democratic National Committee plans to hold 12 primary debates in total.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
AGA News
Ten tips to help you get a research grant
It’s almost time to submit your application for the American Gastroenterological Association Research Scholar Award, the application deadline is Nov. 13, 2019. Review these tips for writing and preparing your application.
1. Start early. Allow plenty of time to complete your application, give it multiple reviews, and get feedback from others. Most applicants start working on the Specific Aims for the project 6 months in advance of the deadline.
2. Look at examples. Ask your division if there are any templates/prior grant submissions that you can review. There’s no recipe for a successful grant, so the only way to compose one is to have a sense of what has worked in the past. In general, prior awardees are happy to share their applications if you contact them.
3. Request feedback. Ask mentors and colleagues for early feedback on your Specific Aims page. If it makes sense and is interesting to them, reviewers will likely feel the same way.
4. Ask your collaborators for letters of support. In addition to your preceptor, consider including letters of support from prior researchers that you have worked with or any collaborators for the current project, especially if they will help you with a new technique or reagents.
5. Contact the grants staff with questions and concerns early on. If you don’t understand part of the application, aren’t sure if you’re eligible or are having problems with submission, contact the grant staff right away. Don’t wait until the week or day the grant is due when staff may be flooded with calls. They can assist you much better with advance notice, which will allow you to avoid last-minute stress.
6. Each application should be different. Keep in mind the scope of the grant and amount of funding. Don’t just recycle an R01-level application for a 1-year AGA pilot award.
7. More is better than less when it comes to preliminary data. If your expertise in a technique you are proposing is established, you will not need to demonstrate the capability to do the work but will likely need to show preliminary data. If you are looking to build expertise (as a part of your career development), you may need to show that the infrastructure that enables you to do the work is accessible.
8. Don’t take constructive feedback personally. As you share your draft with mentors and colleagues for feedback, you may receive some unanticipated criticism. Try not to take this personally. If you can detach yourself emotionally, you’ll be in a better position to answer critiques and make adjustments.
9. Remember your end goal: To help patients! Even the most basic science proposals are rooted in a clear potential to benefit patients.
10. Stay positive. If you do not succeed on your first application, believe in your work, make it better, and apply again.
Thanks to the following AGA Research Foundation grant recipients for sharing their advice, which resulted in the above 10 tips:
- Arthur Beyder, MD, PhD, 2015 AGA Research Scholar Award
- Barbara Jung, MD, AGAF, 2016 AGA-Elsevier Pilot Research Award
- Benjamin Lebwohl, MD, 2014 AGA Research Scholar Award
- Josephine Ni, MD, 2017 AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
- Sahar Nissim, MD, PhD, 2017 AGA-Caroline Craig Augustyn and Damian Augustyn Award in Digestive Cancer
- Jatin Roper, MD, 2011 AGA Fellowship-to-Faculty Transition Award
- Christina Twyman-Saint Victor, MD, 2015 AGA Research Scholar Award
Visit www.gastro.org/research-funding to review the AGA Research Foundation research grants now open for applications. If you have questions about the AGA awards program, please contact [email protected].
The importance of getting involved for gastroenterology
On Sept. 20, 2019, I had the opportunity to participate in AGA’s Advocacy Day for the second time, joining 40 of our gastroenterology colleagues from across the United States on Capitol Hill to advocate for our profession and our patients.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.), both of whom invited our questions. Both congressmen are friends of AGA, with Rep. McGovern serving as chair of the House Rules Committee, and Sen. Blunt serving as chair of the Senate Labor–Health & Human Services Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
- Removing Barriers to Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies — regardless of whether we remove polyps during these colonoscopies.
- Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
- Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
- National Institutes of Health research funding facilitates innovative research and supports young investigators in our field.

Full of enthusiasm, our six-strong North Carolina contingent (pictured L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina senators, Richard Burr (R) and Thom Tillis (R), on Capitol Hill to convey our “asks.”
At Rep. Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy because of delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown (D-Ohio) has circulated asking the Centers for Medicare & Medicaid Services to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Durham, N.C.; member, AGA Clinical Guidelines Committee.
GI of the week: Arthur Beyder, MD, PhD
Congrats to Arthur Beyder, MD, PhD, who was selected for an NIH Director’s New Innovator Award, part of the NIH director’s high-risk, high-reward research award program. The NIH Director’s New Innovator Award will provide Dr. Beyder with more than $2 million in funding over a 5-year period to continue his project: Does the gut have a sense of touch?
Dr. Beyder’s lab at the Mayo Clinic, Rochester, Minn., recently discovered a novel population of mechanosensitive epithelial sensory cells that are similar to skin’s touch sensors, which prompted a potentially transformative question: “Does the gut have a sense of touch?” We look forward to seeing the results of future research on this topic.
Dr. Beyder – a physician-scientist at the Mayo Clinic – is a 2015 AGA Research Scholar Award recipient and graduate of the 2018 AGA Future Leaders Program. Dr. Beyder currently serves on the AGA Nominating Committee.
Please join us in congratulating Dr. Beyder on Twitter (@BeyderLab) or in the AGA Community.
The NIH director’s high-risk, high-reward research program funds highly innovative, high-impact biomedical research proposed by extraordinarily creative scientists – these awards have one of the lowest funding rates for NIH. Congrats to two additional AGA members who also received a 2019 NIH Director’s New Innovator Award: Maayan Levy, PhD, and Christoph A. Thaiss, PhD, both from the University of Pennsylvania, Philadelphia.
Eight new insights about diet and gut health
During your 4 years of medical school, you likely received only 4 hours of nutrition training. Yet we know diet is so integral to the care of GI patients. That’s why AGA focused the 2019 James W. Freston Conference on the topic: Food at the Intersection of Gut Health and Disease.
Our course directors William Chey, MD, AGAF, Sheila E. Crowe, MD, AGAF, and Gerard E. Mullin, MD, AGAF, share eight points from the meeting that stuck with them and can help all practicing GIs as they consider dietary treatments for their patients.
1. Personalized nutrition is important. Genetic differences lead to differences in health outcomes. One size or recommendation does not fit all. This is why certain diets only work on certain people. There is no one diet for all and for all disease states. Genetic tests can be helpful, but they rely on reporting that isn’t readily available yet.
2. Dietary therapy is key to managing eosinophilic esophagitis (EoE). EoE is becoming more and more prevalent. Genes can’t change that fast, but epigenetic factors can, and the evidence seems to be in food. EoE is not an IgE-mediated disease and therefore most allergy tests will not prove useful; however, food is often the trigger – most common, dairy. Dietary therapy is likely the best way to manage. You want to reduce the number of eliminated foods by way of a reintroduction protocol. The six-food elimination diet is standard, though some are moving to a four-food elimination diet (dairy, wheat, egg, and soy).
3. There has been a reported increase in those with food allergies, sensitivities, celiac disease, and other adverse reactions to food. Many of the food allergy tests available are not helpful. In addition, many afflicted patients are using self-imposed diets rather than working with a GI, allergist, or dietitian. This needs to change.
4. There is currently insufficient evidence to support a gluten-free diet for irritable bowel syndrome (IBS). It is possible that fructans, more than gluten, are causing the GI issues. Typically, the low-FODMAP diet is beneficial to IBS patients if done correctly with the guidance of a dietitian; however, not everyone with IBS improves on it. All the steps are important though, including reintroduction and maintenance.
5. When working with patients on the low-FODMAP or other restrictive diets, it is important to know their food and eating history. Avoidance/restrictive food intake disorder is something we need to be aware of when it comes to patients with a history or likelihood to develop disordered eating/eating disorders. The patient team may need to include an eating disorder therapist.
6. The general population in the United States has increased the adoption of a gluten-free diet although the number of cases of celiac disease has not increased. Many have self-reported gluten sensitivities. Those that have removed gluten, following trends, are more at risk of bowel irregularity (low fiber), weight gain, and disordered eating. Celiac disease is not a do-it-yourself disease, patients will be best served working with a dietitian and GI.
7. Food can induce symptoms in patients with IBD. It can also trigger gut inflammation resulting in incident or relapse. There is experimental plausibility for some factors of the relationship to be causal and we may be able to modify the diet to prevent and manage IBD.
8. The focus on nutrition education must continue! Nutrition should be a required part of continuing medical education for physicians. And physicians should work with dietitians to improve the care of GI patients.
17 fellows advancing GI and patient care
Each year during Digestive Disease Week®, AGA hosts a session titled “Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects.” During the 2019 session, 17 quality improvement initiatives were presented — you can review these abstracts in the July issue of Gastroenterology in the “AGA Section” or review a presenter’s abstract by clicking their name or image. Kudos to the promising fellows featured below, who all served as lead authors for their quality improvement projects.

Manasi Agrawal, MD
Lenox Hill Hospital, New York
@ManasiAgrawalMD
Jessica Breton, MD
Children’s Hospital of Philadelphia
Adam Faye, MD
Columbia University Medical Center, New York
@AdamFaye4
Shelly Gurwara, MD
Wake Forest Baptist Health Medical Center, Winston-Salem, N.C.
Afrin Kamal, MD
Stanford (Calif.) University
Ani Kardashian, MD
University of California, Los Angeles
@AniKardashianMD
Sonali Palchaudhuri, MD
University of Pennsylvania, Philadelphia
@sopalchaudhuri
Nasim Parsa, MD
University of Missouri Health System, Columbia
Sahil Patel, MD
Drexel University, Philadelphia
@sahilr
Vikram Raghu, MD
Children’s Hospital of Pittsburgh
Amit Shah, MD
Children’s Hospital of Philadelphia
Lin Shen, MD
Brigham and Women’s Hospital, Boston
@LinShenMD
Charles Snyder, MD
Icahn School of Medicine at Mount Sinai, New York
Brian Sullivan, MD
Duke University, Durham, N.C.
Ashley Vachon, MD
University of Colorado at Denver, Aurora
Ted Walker, MD
Washington University/Barnes Jewish Hospital, St. Louis
Xiao Jing Wang, MD
Mayo Clinic, Rochester, Minn.
@IrisWangMD
Ten tips to help you get a research grant
It’s almost time to submit your application for the American Gastroenterological Association Research Scholar Award, the application deadline is Nov. 13, 2019. Review these tips for writing and preparing your application.
1. Start early. Allow plenty of time to complete your application, give it multiple reviews, and get feedback from others. Most applicants start working on the Specific Aims for the project 6 months in advance of the deadline.
2. Look at examples. Ask your division if there are any templates/prior grant submissions that you can review. There’s no recipe for a successful grant, so the only way to compose one is to have a sense of what has worked in the past. In general, prior awardees are happy to share their applications if you contact them.
3. Request feedback. Ask mentors and colleagues for early feedback on your Specific Aims page. If it makes sense and is interesting to them, reviewers will likely feel the same way.
4. Ask your collaborators for letters of support. In addition to your preceptor, consider including letters of support from prior researchers that you have worked with or any collaborators for the current project, especially if they will help you with a new technique or reagents.
5. Contact the grants staff with questions and concerns early on. If you don’t understand part of the application, aren’t sure if you’re eligible or are having problems with submission, contact the grant staff right away. Don’t wait until the week or day the grant is due when staff may be flooded with calls. They can assist you much better with advance notice, which will allow you to avoid last-minute stress.
6. Each application should be different. Keep in mind the scope of the grant and amount of funding. Don’t just recycle an R01-level application for a 1-year AGA pilot award.
7. More is better than less when it comes to preliminary data. If your expertise in a technique you are proposing is established, you will not need to demonstrate the capability to do the work but will likely need to show preliminary data. If you are looking to build expertise (as a part of your career development), you may need to show that the infrastructure that enables you to do the work is accessible.
8. Don’t take constructive feedback personally. As you share your draft with mentors and colleagues for feedback, you may receive some unanticipated criticism. Try not to take this personally. If you can detach yourself emotionally, you’ll be in a better position to answer critiques and make adjustments.
9. Remember your end goal: To help patients! Even the most basic science proposals are rooted in a clear potential to benefit patients.
10. Stay positive. If you do not succeed on your first application, believe in your work, make it better, and apply again.
Thanks to the following AGA Research Foundation grant recipients for sharing their advice, which resulted in the above 10 tips:
- Arthur Beyder, MD, PhD, 2015 AGA Research Scholar Award
- Barbara Jung, MD, AGAF, 2016 AGA-Elsevier Pilot Research Award
- Benjamin Lebwohl, MD, 2014 AGA Research Scholar Award
- Josephine Ni, MD, 2017 AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
- Sahar Nissim, MD, PhD, 2017 AGA-Caroline Craig Augustyn and Damian Augustyn Award in Digestive Cancer
- Jatin Roper, MD, 2011 AGA Fellowship-to-Faculty Transition Award
- Christina Twyman-Saint Victor, MD, 2015 AGA Research Scholar Award
Visit www.gastro.org/research-funding to review the AGA Research Foundation research grants now open for applications. If you have questions about the AGA awards program, please contact [email protected].
The importance of getting involved for gastroenterology
On Sept. 20, 2019, I had the opportunity to participate in AGA’s Advocacy Day for the second time, joining 40 of our gastroenterology colleagues from across the United States on Capitol Hill to advocate for our profession and our patients.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.), both of whom invited our questions. Both congressmen are friends of AGA, with Rep. McGovern serving as chair of the House Rules Committee, and Sen. Blunt serving as chair of the Senate Labor–Health & Human Services Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
- Removing Barriers to Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies — regardless of whether we remove polyps during these colonoscopies.
- Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
- Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
- National Institutes of Health research funding facilitates innovative research and supports young investigators in our field.

Full of enthusiasm, our six-strong North Carolina contingent (pictured L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina senators, Richard Burr (R) and Thom Tillis (R), on Capitol Hill to convey our “asks.”
At Rep. Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy because of delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown (D-Ohio) has circulated asking the Centers for Medicare & Medicaid Services to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Durham, N.C.; member, AGA Clinical Guidelines Committee.
GI of the week: Arthur Beyder, MD, PhD
Congrats to Arthur Beyder, MD, PhD, who was selected for an NIH Director’s New Innovator Award, part of the NIH director’s high-risk, high-reward research award program. The NIH Director’s New Innovator Award will provide Dr. Beyder with more than $2 million in funding over a 5-year period to continue his project: Does the gut have a sense of touch?
Dr. Beyder’s lab at the Mayo Clinic, Rochester, Minn., recently discovered a novel population of mechanosensitive epithelial sensory cells that are similar to skin’s touch sensors, which prompted a potentially transformative question: “Does the gut have a sense of touch?” We look forward to seeing the results of future research on this topic.
Dr. Beyder – a physician-scientist at the Mayo Clinic – is a 2015 AGA Research Scholar Award recipient and graduate of the 2018 AGA Future Leaders Program. Dr. Beyder currently serves on the AGA Nominating Committee.
Please join us in congratulating Dr. Beyder on Twitter (@BeyderLab) or in the AGA Community.
The NIH director’s high-risk, high-reward research program funds highly innovative, high-impact biomedical research proposed by extraordinarily creative scientists – these awards have one of the lowest funding rates for NIH. Congrats to two additional AGA members who also received a 2019 NIH Director’s New Innovator Award: Maayan Levy, PhD, and Christoph A. Thaiss, PhD, both from the University of Pennsylvania, Philadelphia.
Eight new insights about diet and gut health
During your 4 years of medical school, you likely received only 4 hours of nutrition training. Yet we know diet is so integral to the care of GI patients. That’s why AGA focused the 2019 James W. Freston Conference on the topic: Food at the Intersection of Gut Health and Disease.
Our course directors William Chey, MD, AGAF, Sheila E. Crowe, MD, AGAF, and Gerard E. Mullin, MD, AGAF, share eight points from the meeting that stuck with them and can help all practicing GIs as they consider dietary treatments for their patients.
1. Personalized nutrition is important. Genetic differences lead to differences in health outcomes. One size or recommendation does not fit all. This is why certain diets only work on certain people. There is no one diet for all and for all disease states. Genetic tests can be helpful, but they rely on reporting that isn’t readily available yet.
2. Dietary therapy is key to managing eosinophilic esophagitis (EoE). EoE is becoming more and more prevalent. Genes can’t change that fast, but epigenetic factors can, and the evidence seems to be in food. EoE is not an IgE-mediated disease and therefore most allergy tests will not prove useful; however, food is often the trigger – most common, dairy. Dietary therapy is likely the best way to manage. You want to reduce the number of eliminated foods by way of a reintroduction protocol. The six-food elimination diet is standard, though some are moving to a four-food elimination diet (dairy, wheat, egg, and soy).
3. There has been a reported increase in those with food allergies, sensitivities, celiac disease, and other adverse reactions to food. Many of the food allergy tests available are not helpful. In addition, many afflicted patients are using self-imposed diets rather than working with a GI, allergist, or dietitian. This needs to change.
4. There is currently insufficient evidence to support a gluten-free diet for irritable bowel syndrome (IBS). It is possible that fructans, more than gluten, are causing the GI issues. Typically, the low-FODMAP diet is beneficial to IBS patients if done correctly with the guidance of a dietitian; however, not everyone with IBS improves on it. All the steps are important though, including reintroduction and maintenance.
5. When working with patients on the low-FODMAP or other restrictive diets, it is important to know their food and eating history. Avoidance/restrictive food intake disorder is something we need to be aware of when it comes to patients with a history or likelihood to develop disordered eating/eating disorders. The patient team may need to include an eating disorder therapist.
6. The general population in the United States has increased the adoption of a gluten-free diet although the number of cases of celiac disease has not increased. Many have self-reported gluten sensitivities. Those that have removed gluten, following trends, are more at risk of bowel irregularity (low fiber), weight gain, and disordered eating. Celiac disease is not a do-it-yourself disease, patients will be best served working with a dietitian and GI.
7. Food can induce symptoms in patients with IBD. It can also trigger gut inflammation resulting in incident or relapse. There is experimental plausibility for some factors of the relationship to be causal and we may be able to modify the diet to prevent and manage IBD.
8. The focus on nutrition education must continue! Nutrition should be a required part of continuing medical education for physicians. And physicians should work with dietitians to improve the care of GI patients.
17 fellows advancing GI and patient care
Each year during Digestive Disease Week®, AGA hosts a session titled “Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects.” During the 2019 session, 17 quality improvement initiatives were presented — you can review these abstracts in the July issue of Gastroenterology in the “AGA Section” or review a presenter’s abstract by clicking their name or image. Kudos to the promising fellows featured below, who all served as lead authors for their quality improvement projects.

Manasi Agrawal, MD
Lenox Hill Hospital, New York
@ManasiAgrawalMD
Jessica Breton, MD
Children’s Hospital of Philadelphia
Adam Faye, MD
Columbia University Medical Center, New York
@AdamFaye4
Shelly Gurwara, MD
Wake Forest Baptist Health Medical Center, Winston-Salem, N.C.
Afrin Kamal, MD
Stanford (Calif.) University
Ani Kardashian, MD
University of California, Los Angeles
@AniKardashianMD
Sonali Palchaudhuri, MD
University of Pennsylvania, Philadelphia
@sopalchaudhuri
Nasim Parsa, MD
University of Missouri Health System, Columbia
Sahil Patel, MD
Drexel University, Philadelphia
@sahilr
Vikram Raghu, MD
Children’s Hospital of Pittsburgh
Amit Shah, MD
Children’s Hospital of Philadelphia
Lin Shen, MD
Brigham and Women’s Hospital, Boston
@LinShenMD
Charles Snyder, MD
Icahn School of Medicine at Mount Sinai, New York
Brian Sullivan, MD
Duke University, Durham, N.C.
Ashley Vachon, MD
University of Colorado at Denver, Aurora
Ted Walker, MD
Washington University/Barnes Jewish Hospital, St. Louis
Xiao Jing Wang, MD
Mayo Clinic, Rochester, Minn.
@IrisWangMD
Ten tips to help you get a research grant
It’s almost time to submit your application for the American Gastroenterological Association Research Scholar Award, the application deadline is Nov. 13, 2019. Review these tips for writing and preparing your application.
1. Start early. Allow plenty of time to complete your application, give it multiple reviews, and get feedback from others. Most applicants start working on the Specific Aims for the project 6 months in advance of the deadline.
2. Look at examples. Ask your division if there are any templates/prior grant submissions that you can review. There’s no recipe for a successful grant, so the only way to compose one is to have a sense of what has worked in the past. In general, prior awardees are happy to share their applications if you contact them.
3. Request feedback. Ask mentors and colleagues for early feedback on your Specific Aims page. If it makes sense and is interesting to them, reviewers will likely feel the same way.
4. Ask your collaborators for letters of support. In addition to your preceptor, consider including letters of support from prior researchers that you have worked with or any collaborators for the current project, especially if they will help you with a new technique or reagents.
5. Contact the grants staff with questions and concerns early on. If you don’t understand part of the application, aren’t sure if you’re eligible or are having problems with submission, contact the grant staff right away. Don’t wait until the week or day the grant is due when staff may be flooded with calls. They can assist you much better with advance notice, which will allow you to avoid last-minute stress.
6. Each application should be different. Keep in mind the scope of the grant and amount of funding. Don’t just recycle an R01-level application for a 1-year AGA pilot award.
7. More is better than less when it comes to preliminary data. If your expertise in a technique you are proposing is established, you will not need to demonstrate the capability to do the work but will likely need to show preliminary data. If you are looking to build expertise (as a part of your career development), you may need to show that the infrastructure that enables you to do the work is accessible.
8. Don’t take constructive feedback personally. As you share your draft with mentors and colleagues for feedback, you may receive some unanticipated criticism. Try not to take this personally. If you can detach yourself emotionally, you’ll be in a better position to answer critiques and make adjustments.
9. Remember your end goal: To help patients! Even the most basic science proposals are rooted in a clear potential to benefit patients.
10. Stay positive. If you do not succeed on your first application, believe in your work, make it better, and apply again.
Thanks to the following AGA Research Foundation grant recipients for sharing their advice, which resulted in the above 10 tips:
- Arthur Beyder, MD, PhD, 2015 AGA Research Scholar Award
- Barbara Jung, MD, AGAF, 2016 AGA-Elsevier Pilot Research Award
- Benjamin Lebwohl, MD, 2014 AGA Research Scholar Award
- Josephine Ni, MD, 2017 AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
- Sahar Nissim, MD, PhD, 2017 AGA-Caroline Craig Augustyn and Damian Augustyn Award in Digestive Cancer
- Jatin Roper, MD, 2011 AGA Fellowship-to-Faculty Transition Award
- Christina Twyman-Saint Victor, MD, 2015 AGA Research Scholar Award
Visit www.gastro.org/research-funding to review the AGA Research Foundation research grants now open for applications. If you have questions about the AGA awards program, please contact [email protected].
The importance of getting involved for gastroenterology
On Sept. 20, 2019, I had the opportunity to participate in AGA’s Advocacy Day for the second time, joining 40 of our gastroenterology colleagues from across the United States on Capitol Hill to advocate for our profession and our patients.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.), both of whom invited our questions. Both congressmen are friends of AGA, with Rep. McGovern serving as chair of the House Rules Committee, and Sen. Blunt serving as chair of the Senate Labor–Health & Human Services Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
- Removing Barriers to Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies — regardless of whether we remove polyps during these colonoscopies.
- Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
- Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
- National Institutes of Health research funding facilitates innovative research and supports young investigators in our field.

Full of enthusiasm, our six-strong North Carolina contingent (pictured L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina senators, Richard Burr (R) and Thom Tillis (R), on Capitol Hill to convey our “asks.”
At Rep. Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy because of delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown (D-Ohio) has circulated asking the Centers for Medicare & Medicaid Services to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Durham, N.C.; member, AGA Clinical Guidelines Committee.
GI of the week: Arthur Beyder, MD, PhD
Congrats to Arthur Beyder, MD, PhD, who was selected for an NIH Director’s New Innovator Award, part of the NIH director’s high-risk, high-reward research award program. The NIH Director’s New Innovator Award will provide Dr. Beyder with more than $2 million in funding over a 5-year period to continue his project: Does the gut have a sense of touch?
Dr. Beyder’s lab at the Mayo Clinic, Rochester, Minn., recently discovered a novel population of mechanosensitive epithelial sensory cells that are similar to skin’s touch sensors, which prompted a potentially transformative question: “Does the gut have a sense of touch?” We look forward to seeing the results of future research on this topic.
Dr. Beyder – a physician-scientist at the Mayo Clinic – is a 2015 AGA Research Scholar Award recipient and graduate of the 2018 AGA Future Leaders Program. Dr. Beyder currently serves on the AGA Nominating Committee.
Please join us in congratulating Dr. Beyder on Twitter (@BeyderLab) or in the AGA Community.
The NIH director’s high-risk, high-reward research program funds highly innovative, high-impact biomedical research proposed by extraordinarily creative scientists – these awards have one of the lowest funding rates for NIH. Congrats to two additional AGA members who also received a 2019 NIH Director’s New Innovator Award: Maayan Levy, PhD, and Christoph A. Thaiss, PhD, both from the University of Pennsylvania, Philadelphia.
Eight new insights about diet and gut health
During your 4 years of medical school, you likely received only 4 hours of nutrition training. Yet we know diet is so integral to the care of GI patients. That’s why AGA focused the 2019 James W. Freston Conference on the topic: Food at the Intersection of Gut Health and Disease.
Our course directors William Chey, MD, AGAF, Sheila E. Crowe, MD, AGAF, and Gerard E. Mullin, MD, AGAF, share eight points from the meeting that stuck with them and can help all practicing GIs as they consider dietary treatments for their patients.
1. Personalized nutrition is important. Genetic differences lead to differences in health outcomes. One size or recommendation does not fit all. This is why certain diets only work on certain people. There is no one diet for all and for all disease states. Genetic tests can be helpful, but they rely on reporting that isn’t readily available yet.
2. Dietary therapy is key to managing eosinophilic esophagitis (EoE). EoE is becoming more and more prevalent. Genes can’t change that fast, but epigenetic factors can, and the evidence seems to be in food. EoE is not an IgE-mediated disease and therefore most allergy tests will not prove useful; however, food is often the trigger – most common, dairy. Dietary therapy is likely the best way to manage. You want to reduce the number of eliminated foods by way of a reintroduction protocol. The six-food elimination diet is standard, though some are moving to a four-food elimination diet (dairy, wheat, egg, and soy).
3. There has been a reported increase in those with food allergies, sensitivities, celiac disease, and other adverse reactions to food. Many of the food allergy tests available are not helpful. In addition, many afflicted patients are using self-imposed diets rather than working with a GI, allergist, or dietitian. This needs to change.
4. There is currently insufficient evidence to support a gluten-free diet for irritable bowel syndrome (IBS). It is possible that fructans, more than gluten, are causing the GI issues. Typically, the low-FODMAP diet is beneficial to IBS patients if done correctly with the guidance of a dietitian; however, not everyone with IBS improves on it. All the steps are important though, including reintroduction and maintenance.
5. When working with patients on the low-FODMAP or other restrictive diets, it is important to know their food and eating history. Avoidance/restrictive food intake disorder is something we need to be aware of when it comes to patients with a history or likelihood to develop disordered eating/eating disorders. The patient team may need to include an eating disorder therapist.
6. The general population in the United States has increased the adoption of a gluten-free diet although the number of cases of celiac disease has not increased. Many have self-reported gluten sensitivities. Those that have removed gluten, following trends, are more at risk of bowel irregularity (low fiber), weight gain, and disordered eating. Celiac disease is not a do-it-yourself disease, patients will be best served working with a dietitian and GI.
7. Food can induce symptoms in patients with IBD. It can also trigger gut inflammation resulting in incident or relapse. There is experimental plausibility for some factors of the relationship to be causal and we may be able to modify the diet to prevent and manage IBD.
8. The focus on nutrition education must continue! Nutrition should be a required part of continuing medical education for physicians. And physicians should work with dietitians to improve the care of GI patients.
17 fellows advancing GI and patient care
Each year during Digestive Disease Week®, AGA hosts a session titled “Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects.” During the 2019 session, 17 quality improvement initiatives were presented — you can review these abstracts in the July issue of Gastroenterology in the “AGA Section” or review a presenter’s abstract by clicking their name or image. Kudos to the promising fellows featured below, who all served as lead authors for their quality improvement projects.

Manasi Agrawal, MD
Lenox Hill Hospital, New York
@ManasiAgrawalMD
Jessica Breton, MD
Children’s Hospital of Philadelphia
Adam Faye, MD
Columbia University Medical Center, New York
@AdamFaye4
Shelly Gurwara, MD
Wake Forest Baptist Health Medical Center, Winston-Salem, N.C.
Afrin Kamal, MD
Stanford (Calif.) University
Ani Kardashian, MD
University of California, Los Angeles
@AniKardashianMD
Sonali Palchaudhuri, MD
University of Pennsylvania, Philadelphia
@sopalchaudhuri
Nasim Parsa, MD
University of Missouri Health System, Columbia
Sahil Patel, MD
Drexel University, Philadelphia
@sahilr
Vikram Raghu, MD
Children’s Hospital of Pittsburgh
Amit Shah, MD
Children’s Hospital of Philadelphia
Lin Shen, MD
Brigham and Women’s Hospital, Boston
@LinShenMD
Charles Snyder, MD
Icahn School of Medicine at Mount Sinai, New York
Brian Sullivan, MD
Duke University, Durham, N.C.
Ashley Vachon, MD
University of Colorado at Denver, Aurora
Ted Walker, MD
Washington University/Barnes Jewish Hospital, St. Louis
Xiao Jing Wang, MD
Mayo Clinic, Rochester, Minn.
@IrisWangMD
What does the REPLENISH trial reveal about E2/P4’s ability to affect VMS and sleep and appropriate dosing for smokers?
The REPLENISH trial evaluated the oral 17β-estradiol/progesterone (E2/P4) softgel capsule (TX-001HR; 1 mg E2/100 mg P4) approved by the US Food and Drug Administration in October 2018 as Bijuva (TherapeuticsMD) for the treatment of moderate to severe vasomotor symptoms (VMS) due to menopause. In separate subanalyses presented at the annual Scientific Meeting of the North American Menopause Society in Chicago, Illinois (September 25-28, 2019), researchers examined E2/P4’s ability to address VMS according to age and body mass index (BMI), ability to address sleep, and appropriate dosing in smokers versus nonsmokers.
REPLENISH
The REPLENISH trial was a phase 3, randomized, double-blind, placebo-controlled, multicenter trial evaluating the safety and efficacy of E2/P4 for the treatment of VMS in 1,835 postmenopausal women aged 40 to 65 years with a uterus. Women with moderate to severe VMS (≥7/day or ≥50/week) were randomly assigned to E2/P4 (mg/mg) 1/100, 0.5/100, 0.5/50, 0.25/50, or placebo.1
E2/P4 and VMS according to age and BMI
Percent changes in the weekly frequency and severity of moderate to severe VMS from baseline to weeks 4 and 12 versus placebo were analyzed by age (<55 and ≥55 years) and BMI in the study participants.1 The BMI subgroups had similar baseline VMS, but women in the younger age group had higher baseline frequency of moderate to severe VMS than women in the older age group.
Age. The percent changes in VMS frequency from baseline for women treated with E2/P4 were similar at weeks 4 and 12 between age groups. While subgroup analyses were not powered for statistical significance, significant differences were observed between E2/P4 dosages and placebo at week 12. For VMS severity, the percent changes from baseline for women treated with E2/P4 ranged from 16% to 22% at week 4 and 24% to 51% for either age group at week 12.
BMI. When analyzed by BMI, larger percent reductions from baseline in VMS frequency and severity were observed with E2/P4 dosaging versus placebo, with some groups meeting statistical significance at both weeks 4 and 12.
The authors concluded that their subgroup analyses show a consistency of efficacy for VMS frequency and severity among the different age group and BMI populations of women treated with E2/P4.
E2/P4 and sleep outcomes
Participants in the REPLENISH trial took 2 surveys related to sleep—the Medical Outcomes Study (MOS)-Sleep, a 12-item questionnaire measuring 6 sleep dimensions, and the Menopause-specific Quality of Life (MENQOL), which included a “difficulty sleeping” item.2 Except for women treated with E2/P4 0.25/50 at week 12, women receiving E2/P4 reported significantly better change in the MOS-Sleep total, as well as better ratings on sleep problems and disturbance subscales, than women treated with placebo at week 12 and months 6 and 12. The incidence of somnolence was low with E2/P4 treatment. In addition, sleep mediation models showed that E2/P4 improved MOS-sleep disturbances indirectly through improvements in VMS. The study authors concluded that women taking E2/P4 for moderate to severe VMS may experience improved sleep.
Smoking and E2/P4 dosage
Among postmenopausal women, smoking has been shown to reduce the efficacy of hormone therapy.3 Researchers found that nonsmokers (never or past smokers) may benefit more from a lower E2/P4 dosage than current smokers (<15 cigarettes per day).4 (Women smoking ≥15 cigarettes per day or any e-cigarettes were excluded from REPLENISH). Compared with nonsmokers taking placebo, nonsmokers taking any dosage of E2/P4 had a significant and clinically meaningful reduction in VMS frequency and severity beginning at week 4 and maintained through week 12 (except for the E2/P4 dosage of 0.5/50 at week 4 for severity). By contrast, current smokers in any E2/P4 group had no significant VMS improvements from baseline to weeks 4 and 12 compared with placebo, and proportions of smokers who did measure some response to treatment (at both ≥50% and ≥75% levels) were not different from placebo at weeks 4 and 12. In addition, current smokers had significantly lower median levels of systemic estradiol and estrone concentrations with all E2/P4 treatment groups than did nonsmokers, despite both groups having similar estradiol and estrone concentrations at baseline.
- Bitner D, Brightman R, Graham S, et al. E2/P4 capsules effectively treat vasomotor symptoms irrespective of age and BMI. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Kaunitz AM, Kagan R, Graham S, et al. Oral 17β-estradiol/progesterone (E2/P4) improved sleep outcomes in the REPLENISH trial. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Jensen J, Christiansen C, Rodbro P. Cigarette smoking, serum estrogens, and bone loss during hormone-replacement therapy after menopause. N Engl J Med. 1985;313:973-975.
- Constantine GD, Santoro N, Graham S, et al. Nonsmokers may benefit from lower doses of an oral 17β-estradiol/progesterone capsule—data from the REPLENISH trial. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
The REPLENISH trial evaluated the oral 17β-estradiol/progesterone (E2/P4) softgel capsule (TX-001HR; 1 mg E2/100 mg P4) approved by the US Food and Drug Administration in October 2018 as Bijuva (TherapeuticsMD) for the treatment of moderate to severe vasomotor symptoms (VMS) due to menopause. In separate subanalyses presented at the annual Scientific Meeting of the North American Menopause Society in Chicago, Illinois (September 25-28, 2019), researchers examined E2/P4’s ability to address VMS according to age and body mass index (BMI), ability to address sleep, and appropriate dosing in smokers versus nonsmokers.
REPLENISH
The REPLENISH trial was a phase 3, randomized, double-blind, placebo-controlled, multicenter trial evaluating the safety and efficacy of E2/P4 for the treatment of VMS in 1,835 postmenopausal women aged 40 to 65 years with a uterus. Women with moderate to severe VMS (≥7/day or ≥50/week) were randomly assigned to E2/P4 (mg/mg) 1/100, 0.5/100, 0.5/50, 0.25/50, or placebo.1
E2/P4 and VMS according to age and BMI
Percent changes in the weekly frequency and severity of moderate to severe VMS from baseline to weeks 4 and 12 versus placebo were analyzed by age (<55 and ≥55 years) and BMI in the study participants.1 The BMI subgroups had similar baseline VMS, but women in the younger age group had higher baseline frequency of moderate to severe VMS than women in the older age group.
Age. The percent changes in VMS frequency from baseline for women treated with E2/P4 were similar at weeks 4 and 12 between age groups. While subgroup analyses were not powered for statistical significance, significant differences were observed between E2/P4 dosages and placebo at week 12. For VMS severity, the percent changes from baseline for women treated with E2/P4 ranged from 16% to 22% at week 4 and 24% to 51% for either age group at week 12.
BMI. When analyzed by BMI, larger percent reductions from baseline in VMS frequency and severity were observed with E2/P4 dosaging versus placebo, with some groups meeting statistical significance at both weeks 4 and 12.
The authors concluded that their subgroup analyses show a consistency of efficacy for VMS frequency and severity among the different age group and BMI populations of women treated with E2/P4.
E2/P4 and sleep outcomes
Participants in the REPLENISH trial took 2 surveys related to sleep—the Medical Outcomes Study (MOS)-Sleep, a 12-item questionnaire measuring 6 sleep dimensions, and the Menopause-specific Quality of Life (MENQOL), which included a “difficulty sleeping” item.2 Except for women treated with E2/P4 0.25/50 at week 12, women receiving E2/P4 reported significantly better change in the MOS-Sleep total, as well as better ratings on sleep problems and disturbance subscales, than women treated with placebo at week 12 and months 6 and 12. The incidence of somnolence was low with E2/P4 treatment. In addition, sleep mediation models showed that E2/P4 improved MOS-sleep disturbances indirectly through improvements in VMS. The study authors concluded that women taking E2/P4 for moderate to severe VMS may experience improved sleep.
Smoking and E2/P4 dosage
Among postmenopausal women, smoking has been shown to reduce the efficacy of hormone therapy.3 Researchers found that nonsmokers (never or past smokers) may benefit more from a lower E2/P4 dosage than current smokers (<15 cigarettes per day).4 (Women smoking ≥15 cigarettes per day or any e-cigarettes were excluded from REPLENISH). Compared with nonsmokers taking placebo, nonsmokers taking any dosage of E2/P4 had a significant and clinically meaningful reduction in VMS frequency and severity beginning at week 4 and maintained through week 12 (except for the E2/P4 dosage of 0.5/50 at week 4 for severity). By contrast, current smokers in any E2/P4 group had no significant VMS improvements from baseline to weeks 4 and 12 compared with placebo, and proportions of smokers who did measure some response to treatment (at both ≥50% and ≥75% levels) were not different from placebo at weeks 4 and 12. In addition, current smokers had significantly lower median levels of systemic estradiol and estrone concentrations with all E2/P4 treatment groups than did nonsmokers, despite both groups having similar estradiol and estrone concentrations at baseline.
The REPLENISH trial evaluated the oral 17β-estradiol/progesterone (E2/P4) softgel capsule (TX-001HR; 1 mg E2/100 mg P4) approved by the US Food and Drug Administration in October 2018 as Bijuva (TherapeuticsMD) for the treatment of moderate to severe vasomotor symptoms (VMS) due to menopause. In separate subanalyses presented at the annual Scientific Meeting of the North American Menopause Society in Chicago, Illinois (September 25-28, 2019), researchers examined E2/P4’s ability to address VMS according to age and body mass index (BMI), ability to address sleep, and appropriate dosing in smokers versus nonsmokers.
REPLENISH
The REPLENISH trial was a phase 3, randomized, double-blind, placebo-controlled, multicenter trial evaluating the safety and efficacy of E2/P4 for the treatment of VMS in 1,835 postmenopausal women aged 40 to 65 years with a uterus. Women with moderate to severe VMS (≥7/day or ≥50/week) were randomly assigned to E2/P4 (mg/mg) 1/100, 0.5/100, 0.5/50, 0.25/50, or placebo.1
E2/P4 and VMS according to age and BMI
Percent changes in the weekly frequency and severity of moderate to severe VMS from baseline to weeks 4 and 12 versus placebo were analyzed by age (<55 and ≥55 years) and BMI in the study participants.1 The BMI subgroups had similar baseline VMS, but women in the younger age group had higher baseline frequency of moderate to severe VMS than women in the older age group.
Age. The percent changes in VMS frequency from baseline for women treated with E2/P4 were similar at weeks 4 and 12 between age groups. While subgroup analyses were not powered for statistical significance, significant differences were observed between E2/P4 dosages and placebo at week 12. For VMS severity, the percent changes from baseline for women treated with E2/P4 ranged from 16% to 22% at week 4 and 24% to 51% for either age group at week 12.
BMI. When analyzed by BMI, larger percent reductions from baseline in VMS frequency and severity were observed with E2/P4 dosaging versus placebo, with some groups meeting statistical significance at both weeks 4 and 12.
The authors concluded that their subgroup analyses show a consistency of efficacy for VMS frequency and severity among the different age group and BMI populations of women treated with E2/P4.
E2/P4 and sleep outcomes
Participants in the REPLENISH trial took 2 surveys related to sleep—the Medical Outcomes Study (MOS)-Sleep, a 12-item questionnaire measuring 6 sleep dimensions, and the Menopause-specific Quality of Life (MENQOL), which included a “difficulty sleeping” item.2 Except for women treated with E2/P4 0.25/50 at week 12, women receiving E2/P4 reported significantly better change in the MOS-Sleep total, as well as better ratings on sleep problems and disturbance subscales, than women treated with placebo at week 12 and months 6 and 12. The incidence of somnolence was low with E2/P4 treatment. In addition, sleep mediation models showed that E2/P4 improved MOS-sleep disturbances indirectly through improvements in VMS. The study authors concluded that women taking E2/P4 for moderate to severe VMS may experience improved sleep.
Smoking and E2/P4 dosage
Among postmenopausal women, smoking has been shown to reduce the efficacy of hormone therapy.3 Researchers found that nonsmokers (never or past smokers) may benefit more from a lower E2/P4 dosage than current smokers (<15 cigarettes per day).4 (Women smoking ≥15 cigarettes per day or any e-cigarettes were excluded from REPLENISH). Compared with nonsmokers taking placebo, nonsmokers taking any dosage of E2/P4 had a significant and clinically meaningful reduction in VMS frequency and severity beginning at week 4 and maintained through week 12 (except for the E2/P4 dosage of 0.5/50 at week 4 for severity). By contrast, current smokers in any E2/P4 group had no significant VMS improvements from baseline to weeks 4 and 12 compared with placebo, and proportions of smokers who did measure some response to treatment (at both ≥50% and ≥75% levels) were not different from placebo at weeks 4 and 12. In addition, current smokers had significantly lower median levels of systemic estradiol and estrone concentrations with all E2/P4 treatment groups than did nonsmokers, despite both groups having similar estradiol and estrone concentrations at baseline.
- Bitner D, Brightman R, Graham S, et al. E2/P4 capsules effectively treat vasomotor symptoms irrespective of age and BMI. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Kaunitz AM, Kagan R, Graham S, et al. Oral 17β-estradiol/progesterone (E2/P4) improved sleep outcomes in the REPLENISH trial. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Jensen J, Christiansen C, Rodbro P. Cigarette smoking, serum estrogens, and bone loss during hormone-replacement therapy after menopause. N Engl J Med. 1985;313:973-975.
- Constantine GD, Santoro N, Graham S, et al. Nonsmokers may benefit from lower doses of an oral 17β-estradiol/progesterone capsule—data from the REPLENISH trial. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Bitner D, Brightman R, Graham S, et al. E2/P4 capsules effectively treat vasomotor symptoms irrespective of age and BMI. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Kaunitz AM, Kagan R, Graham S, et al. Oral 17β-estradiol/progesterone (E2/P4) improved sleep outcomes in the REPLENISH trial. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.
- Jensen J, Christiansen C, Rodbro P. Cigarette smoking, serum estrogens, and bone loss during hormone-replacement therapy after menopause. N Engl J Med. 1985;313:973-975.
- Constantine GD, Santoro N, Graham S, et al. Nonsmokers may benefit from lower doses of an oral 17β-estradiol/progesterone capsule—data from the REPLENISH trial. Poster presented at: North American Menopause Society Annual Meeting; September 25-28, 2019; Chicago, IL.