User login
Treating AKs with PDT, other options
SEATTLE – While David Pariser, MD, said during a presentation at the annual Coastal Dermatology Symposium.
“My personal view is that, no matter how good other treatments are eventually going to be, we’re never going to give that up,” Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
During the presentation, he emphasized that it isn’t always clear which actinic keratosis (AK) should be treated and which can be left alone, since most AKs don’t progress to squamous cell carcinoma (SCC). “We know that most squamous cell carcinomas arise near AKs, and many of them have histologic evidence” of AK/SCC continuum at the periphery, he said. Sun protection reduces the incidence of AKs and the incidence of nonmelanoma skin cancer, “so it’s a logical conclusion that treating AKs reduces the development of SCCs, but there are no data to show that.”
Generally, treatment decisions are made based on the presence of symptoms, location, or appearance; if the area is irritated; or there is a progressive or unusual appearance, especially if hyperkeratotic. Physician or patient concerns about cancer can prompt treatment, as should a history of multiple skin cancers or the presence of immunosuppression, he said.
Treatment options include cryosurgery, surgery, topical agents, and photodynamic therapy (PDT); Dr. Pariser focused on the latter because it is a special interest of his.
Field cancerization is based on the idea that a broad area of cells may be at risk for developing into SCC, rather than just individual AKs. Treatment with methyl 5-aminolevulinate (MAL) can reveal the extent of a problem. In some patients, “you can see a lot of fluorescence in areas that look reasonably clinically normal. So this is a piece of evidence of this field cancerization, that maybe we shouldn’t be treating individual AKs, but larger areas,” Dr. Pariser said.
With PDT, there has been some debate about how long to leave the photosensitizer on the skin before applying the light. The longer it remains, the more it spreads to nerves, which can lead to pain during the procedure. A clinical trial comparing 1-, 2-, and 3-hour wait times showed no difference in efficacy. “So 1 hour is what I do for AKs, that’s it,” Dr. Pariser said.
There are two Food and Drug Administration–approved PDT systems, a blue-light system combined with aminolevulinic acid (ALA) and a newer red-light system combined with a nanoemulsion of ALA 7.8% and a proprietary 635-nm red LED light. The nanoemulsion has the theoretical advantage in that it can penetrate more deeply into the epidermis, though this isn’t really an issue when treating AKs, according to Dr. Pariser.
A study comparing nanoemulsion of ALA, compared with a MAL cream, found the nanoemulsion to be superior in achieving complete clearance of all lesions at 12 weeks (78.2% vs. 64.2%; P less than .05). Both treatments achieved best efficacy with LED lamps, and the proprietary red light may reduce pain by allowing use of lower light intensity (Br J Dermatol. 2012 Jan; 166[1]:137-46).
Another study, Dr. Pariser said, looked at whether occlusion during drug incubation improves outcomes of blue light ALA-PDT (J Drugs Dermatol. 2012;11[12]:1483-9). Patients underwent split occlusion on the upper extremities before undergoing blue-light treatment. The median clearance rate of AKs at 8 weeks was higher with occlusion, compared with the nonoccluded areas (75% vs. 47%; P = .006), and at 12 weeks, after a second treatment (89% vs. 70%; P = .00029). There was a higher efficacy with a 3-hour incubation period, compared with studies using a 2-hour incubation period.
Application of heat can also boost success rates by increasing the synthesis of the photoactive agent, Dr. Pariser said. One study found that a simple heating pad applied to the area treated with ALA-PDT and blue light led to an 88% reduction in lesions at 8 and 24 weeks, compared with a reduction of 71% at 8 weeks and 68% at 24 weeks without heat (P less than .0001). “So if you want to give PDT a little extra oomph, add occlusion and heat,” he commented.
He also pointed out the availability of a new 4% 5-fluorouracil cream that contains peanut oil, which has similar efficacy to 5% 5-fluorouracil cream but has been associated with less pruritus, stinging/burning, edema, crusting, scaling/dryness, erosion, and erythema (J Drugs Dermatol. 2016 Oct 1;15[10]: 1218-24).
Dr. Pariser is an investigator and consultant for DUSA/Sun Pharma, Photocure, LEO Pharma, and Biofrontera. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – While David Pariser, MD, said during a presentation at the annual Coastal Dermatology Symposium.
“My personal view is that, no matter how good other treatments are eventually going to be, we’re never going to give that up,” Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
During the presentation, he emphasized that it isn’t always clear which actinic keratosis (AK) should be treated and which can be left alone, since most AKs don’t progress to squamous cell carcinoma (SCC). “We know that most squamous cell carcinomas arise near AKs, and many of them have histologic evidence” of AK/SCC continuum at the periphery, he said. Sun protection reduces the incidence of AKs and the incidence of nonmelanoma skin cancer, “so it’s a logical conclusion that treating AKs reduces the development of SCCs, but there are no data to show that.”
Generally, treatment decisions are made based on the presence of symptoms, location, or appearance; if the area is irritated; or there is a progressive or unusual appearance, especially if hyperkeratotic. Physician or patient concerns about cancer can prompt treatment, as should a history of multiple skin cancers or the presence of immunosuppression, he said.
Treatment options include cryosurgery, surgery, topical agents, and photodynamic therapy (PDT); Dr. Pariser focused on the latter because it is a special interest of his.
Field cancerization is based on the idea that a broad area of cells may be at risk for developing into SCC, rather than just individual AKs. Treatment with methyl 5-aminolevulinate (MAL) can reveal the extent of a problem. In some patients, “you can see a lot of fluorescence in areas that look reasonably clinically normal. So this is a piece of evidence of this field cancerization, that maybe we shouldn’t be treating individual AKs, but larger areas,” Dr. Pariser said.
With PDT, there has been some debate about how long to leave the photosensitizer on the skin before applying the light. The longer it remains, the more it spreads to nerves, which can lead to pain during the procedure. A clinical trial comparing 1-, 2-, and 3-hour wait times showed no difference in efficacy. “So 1 hour is what I do for AKs, that’s it,” Dr. Pariser said.
There are two Food and Drug Administration–approved PDT systems, a blue-light system combined with aminolevulinic acid (ALA) and a newer red-light system combined with a nanoemulsion of ALA 7.8% and a proprietary 635-nm red LED light. The nanoemulsion has the theoretical advantage in that it can penetrate more deeply into the epidermis, though this isn’t really an issue when treating AKs, according to Dr. Pariser.
A study comparing nanoemulsion of ALA, compared with a MAL cream, found the nanoemulsion to be superior in achieving complete clearance of all lesions at 12 weeks (78.2% vs. 64.2%; P less than .05). Both treatments achieved best efficacy with LED lamps, and the proprietary red light may reduce pain by allowing use of lower light intensity (Br J Dermatol. 2012 Jan; 166[1]:137-46).
Another study, Dr. Pariser said, looked at whether occlusion during drug incubation improves outcomes of blue light ALA-PDT (J Drugs Dermatol. 2012;11[12]:1483-9). Patients underwent split occlusion on the upper extremities before undergoing blue-light treatment. The median clearance rate of AKs at 8 weeks was higher with occlusion, compared with the nonoccluded areas (75% vs. 47%; P = .006), and at 12 weeks, after a second treatment (89% vs. 70%; P = .00029). There was a higher efficacy with a 3-hour incubation period, compared with studies using a 2-hour incubation period.
Application of heat can also boost success rates by increasing the synthesis of the photoactive agent, Dr. Pariser said. One study found that a simple heating pad applied to the area treated with ALA-PDT and blue light led to an 88% reduction in lesions at 8 and 24 weeks, compared with a reduction of 71% at 8 weeks and 68% at 24 weeks without heat (P less than .0001). “So if you want to give PDT a little extra oomph, add occlusion and heat,” he commented.
He also pointed out the availability of a new 4% 5-fluorouracil cream that contains peanut oil, which has similar efficacy to 5% 5-fluorouracil cream but has been associated with less pruritus, stinging/burning, edema, crusting, scaling/dryness, erosion, and erythema (J Drugs Dermatol. 2016 Oct 1;15[10]: 1218-24).
Dr. Pariser is an investigator and consultant for DUSA/Sun Pharma, Photocure, LEO Pharma, and Biofrontera. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – While David Pariser, MD, said during a presentation at the annual Coastal Dermatology Symposium.
“My personal view is that, no matter how good other treatments are eventually going to be, we’re never going to give that up,” Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
During the presentation, he emphasized that it isn’t always clear which actinic keratosis (AK) should be treated and which can be left alone, since most AKs don’t progress to squamous cell carcinoma (SCC). “We know that most squamous cell carcinomas arise near AKs, and many of them have histologic evidence” of AK/SCC continuum at the periphery, he said. Sun protection reduces the incidence of AKs and the incidence of nonmelanoma skin cancer, “so it’s a logical conclusion that treating AKs reduces the development of SCCs, but there are no data to show that.”
Generally, treatment decisions are made based on the presence of symptoms, location, or appearance; if the area is irritated; or there is a progressive or unusual appearance, especially if hyperkeratotic. Physician or patient concerns about cancer can prompt treatment, as should a history of multiple skin cancers or the presence of immunosuppression, he said.
Treatment options include cryosurgery, surgery, topical agents, and photodynamic therapy (PDT); Dr. Pariser focused on the latter because it is a special interest of his.
Field cancerization is based on the idea that a broad area of cells may be at risk for developing into SCC, rather than just individual AKs. Treatment with methyl 5-aminolevulinate (MAL) can reveal the extent of a problem. In some patients, “you can see a lot of fluorescence in areas that look reasonably clinically normal. So this is a piece of evidence of this field cancerization, that maybe we shouldn’t be treating individual AKs, but larger areas,” Dr. Pariser said.
With PDT, there has been some debate about how long to leave the photosensitizer on the skin before applying the light. The longer it remains, the more it spreads to nerves, which can lead to pain during the procedure. A clinical trial comparing 1-, 2-, and 3-hour wait times showed no difference in efficacy. “So 1 hour is what I do for AKs, that’s it,” Dr. Pariser said.
There are two Food and Drug Administration–approved PDT systems, a blue-light system combined with aminolevulinic acid (ALA) and a newer red-light system combined with a nanoemulsion of ALA 7.8% and a proprietary 635-nm red LED light. The nanoemulsion has the theoretical advantage in that it can penetrate more deeply into the epidermis, though this isn’t really an issue when treating AKs, according to Dr. Pariser.
A study comparing nanoemulsion of ALA, compared with a MAL cream, found the nanoemulsion to be superior in achieving complete clearance of all lesions at 12 weeks (78.2% vs. 64.2%; P less than .05). Both treatments achieved best efficacy with LED lamps, and the proprietary red light may reduce pain by allowing use of lower light intensity (Br J Dermatol. 2012 Jan; 166[1]:137-46).
Another study, Dr. Pariser said, looked at whether occlusion during drug incubation improves outcomes of blue light ALA-PDT (J Drugs Dermatol. 2012;11[12]:1483-9). Patients underwent split occlusion on the upper extremities before undergoing blue-light treatment. The median clearance rate of AKs at 8 weeks was higher with occlusion, compared with the nonoccluded areas (75% vs. 47%; P = .006), and at 12 weeks, after a second treatment (89% vs. 70%; P = .00029). There was a higher efficacy with a 3-hour incubation period, compared with studies using a 2-hour incubation period.
Application of heat can also boost success rates by increasing the synthesis of the photoactive agent, Dr. Pariser said. One study found that a simple heating pad applied to the area treated with ALA-PDT and blue light led to an 88% reduction in lesions at 8 and 24 weeks, compared with a reduction of 71% at 8 weeks and 68% at 24 weeks without heat (P less than .0001). “So if you want to give PDT a little extra oomph, add occlusion and heat,” he commented.
He also pointed out the availability of a new 4% 5-fluorouracil cream that contains peanut oil, which has similar efficacy to 5% 5-fluorouracil cream but has been associated with less pruritus, stinging/burning, edema, crusting, scaling/dryness, erosion, and erythema (J Drugs Dermatol. 2016 Oct 1;15[10]: 1218-24).
Dr. Pariser is an investigator and consultant for DUSA/Sun Pharma, Photocure, LEO Pharma, and Biofrontera. This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
‘Forward-Oriented’ Vector Holds Potential for Sickle Cell Cure
About 100,000 people in America have sickle cell disease. Of those, an estimated 27 people have undergone experimental gene therapy using conventional vectors—virus-based vehicles for delivering “therapeutic genes.” Now National Institutes of Health researchers have taken the vector idea and revved it up, bringing the possibility of curing sickle cell disease a bit closer.
With gene therapy, doctors add a normal copy of the β-globin gene to the patient’s hematopoietic stem cells, then reinfuse the modified stem cells into the patient to produce normal disc-shaped red blood cells. In animal studies, the new vector was up to 10 times more efficient at incorporating corrective genes into bone marrow stem cells with a carrying capacity of up to 6 times greater viral load than current vectors. The new vectors also can be produced in much higher amounts, saving time and lowering costs.
The researchers call it a “forward-oriented” vector because it changes the usual direction of how gene sequences in globin-containing vectors are read: from right to left. That backward orientation—globin-containing vectors are the only therapeutic vectors in clinical development that use it—the researchers say, “has remained unchallenged for decades despite its negative impacts on efficiency.”
The right-to-left orientation was dictated by the need to prevent the loss of a key molecular component, intron 2, by RNA splicing during the vector preparation. The redesigned forward-reading method crucially leaves intron 2 intact and makes the gene-translation approach less complicated, says John Tisdale, MD, chief of the Cellular and Molecular Therapeutic Branch at the National Heart, Lung, and Blood Institute, who, with Naoya Uchida, MD, PhD, came up with the idea.
In testing, the new vectors also proved longer lasting, remaining in place 4 years after transplantation.
National Institutes of Health is working to accelerate research and development through the Cure Sickle Cell Initiative, launched by NHLBI in 2018 to identify and support the most promising genetic therapies for the more than 20 million people worldwide who have sickle cell disease. NIH holds the patent for the new vector, which still will need clinical testing in humans. Clinical trials are actively enrolling.
About 100,000 people in America have sickle cell disease. Of those, an estimated 27 people have undergone experimental gene therapy using conventional vectors—virus-based vehicles for delivering “therapeutic genes.” Now National Institutes of Health researchers have taken the vector idea and revved it up, bringing the possibility of curing sickle cell disease a bit closer.
With gene therapy, doctors add a normal copy of the β-globin gene to the patient’s hematopoietic stem cells, then reinfuse the modified stem cells into the patient to produce normal disc-shaped red blood cells. In animal studies, the new vector was up to 10 times more efficient at incorporating corrective genes into bone marrow stem cells with a carrying capacity of up to 6 times greater viral load than current vectors. The new vectors also can be produced in much higher amounts, saving time and lowering costs.
The researchers call it a “forward-oriented” vector because it changes the usual direction of how gene sequences in globin-containing vectors are read: from right to left. That backward orientation—globin-containing vectors are the only therapeutic vectors in clinical development that use it—the researchers say, “has remained unchallenged for decades despite its negative impacts on efficiency.”
The right-to-left orientation was dictated by the need to prevent the loss of a key molecular component, intron 2, by RNA splicing during the vector preparation. The redesigned forward-reading method crucially leaves intron 2 intact and makes the gene-translation approach less complicated, says John Tisdale, MD, chief of the Cellular and Molecular Therapeutic Branch at the National Heart, Lung, and Blood Institute, who, with Naoya Uchida, MD, PhD, came up with the idea.
In testing, the new vectors also proved longer lasting, remaining in place 4 years after transplantation.
National Institutes of Health is working to accelerate research and development through the Cure Sickle Cell Initiative, launched by NHLBI in 2018 to identify and support the most promising genetic therapies for the more than 20 million people worldwide who have sickle cell disease. NIH holds the patent for the new vector, which still will need clinical testing in humans. Clinical trials are actively enrolling.
About 100,000 people in America have sickle cell disease. Of those, an estimated 27 people have undergone experimental gene therapy using conventional vectors—virus-based vehicles for delivering “therapeutic genes.” Now National Institutes of Health researchers have taken the vector idea and revved it up, bringing the possibility of curing sickle cell disease a bit closer.
With gene therapy, doctors add a normal copy of the β-globin gene to the patient’s hematopoietic stem cells, then reinfuse the modified stem cells into the patient to produce normal disc-shaped red blood cells. In animal studies, the new vector was up to 10 times more efficient at incorporating corrective genes into bone marrow stem cells with a carrying capacity of up to 6 times greater viral load than current vectors. The new vectors also can be produced in much higher amounts, saving time and lowering costs.
The researchers call it a “forward-oriented” vector because it changes the usual direction of how gene sequences in globin-containing vectors are read: from right to left. That backward orientation—globin-containing vectors are the only therapeutic vectors in clinical development that use it—the researchers say, “has remained unchallenged for decades despite its negative impacts on efficiency.”
The right-to-left orientation was dictated by the need to prevent the loss of a key molecular component, intron 2, by RNA splicing during the vector preparation. The redesigned forward-reading method crucially leaves intron 2 intact and makes the gene-translation approach less complicated, says John Tisdale, MD, chief of the Cellular and Molecular Therapeutic Branch at the National Heart, Lung, and Blood Institute, who, with Naoya Uchida, MD, PhD, came up with the idea.
In testing, the new vectors also proved longer lasting, remaining in place 4 years after transplantation.
National Institutes of Health is working to accelerate research and development through the Cure Sickle Cell Initiative, launched by NHLBI in 2018 to identify and support the most promising genetic therapies for the more than 20 million people worldwide who have sickle cell disease. NIH holds the patent for the new vector, which still will need clinical testing in humans. Clinical trials are actively enrolling.
Autoimmune Hemolytic Anemia: Treatment of Common Types
Autoimmune hemolytic anemia (AIHA) is mediated by antibodies, and in most cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C. AIHA caused by a drug reaction is rare, with an estimated annual incidence of 1:1,000,000 for severe drug-related AIHA.1 This article reviews the management of the more common types of AIHA, with a focus on warm, cold, and drug-induced AIHA; the evaluation and diagnosis of AIHA is reviewed in a separate article.
Warm Autoimmune Hemolytic Anemia
In AIHA, hemolysis is mediated by antibodies that bind to the surface of red blood cells. AIHA in which IgG antibodies are the offending antibodies is referred to as warm AIHA. “Warm” refers to the fact that the antibody binds best at body temperature (37°C). In warm AIHA, testing will show IgG molecules attached to the surface of the red cells, with 50% of patients also showing C3. Between 50% and 90% of AIHA cases are due to warm antibodies.2,3 The incidence of warm AIHA varies by series but is approximately 1 case per 100,000 patients per year; this form of hemolysis affects women more frequently than men.4,5
Therapeutic Options
First Line
Steroids. The goal of therapy in warm AIHA can be hard to define. However, most would agree that a hematocrit above 30% (or higher to prevent symptoms) with a minimal increase in the reticulocyte count—reflective of a significantly slowed hemolytic process—is a reasonable goal. Initial management of warm AIHA is prednisone at a standard dose of 1 mg/kg daily (Table 1).6,7 Patients should be also started on proton-pump inhibitors to prevent ulcers. It can take up to 3 weeks for patients to respond to prednisone therapy. Once the patient’s hematocrit is above 30%, the prednisone is slowly tapered. Although approximately 80% of patients will respond to steroids, only 30% can be fully tapered off steroids. For patients who can be maintained on a daily steroid dose of 10 mg or less, steroids may be the most reasonable long-term therapy. In addition, because active hemolysis leads to an increased demand for folic acid, patients with warm AIHA are often prescribed folic acid 1 mg daily to prevent megaloblastic anemia due to folic acid deficiency.
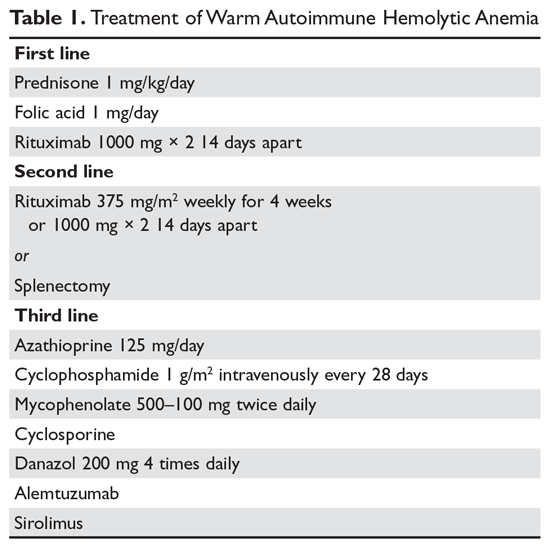
Rituximab. Increasingly, rituximab (anti-CD20) therapy is added to the initial steroids. Two clinical trials showed both increased long-term and short-term responses with the use of rituximab.8,9 An important consideration is that most patients respond gradually to rituximab over weeks, so a rapid response should not be expected. Most studies have used the traditional dosing of 375 mg/m2 weekly for 4 weeks. These responses appear to be durable, but as in immune thrombocytopenia (ITP), repeat treatment with rituximab is effective.
The major side effects of rituximab are infusion reactions, which are often worse with the first dose. These reactions can be controlled with antihistamines, steroids, and, for severe rigors, meperidine. Rarely, patients can develop neutropenia (approximately 1:500) that appears to be autoimmune in nature. Infections appear to be only minimally increased with the use of rituximab.10 One group at risk is chronic carriers of hepatitis B virus, who may experience a reactivation of the virus that can be fatal. Thus, patients being considered for rituximab need to be screened for hepatitis B virus carrier state.11 Patients receiving rituximab are at very slight risk for progressive multifocal leukoencephalopathy, which is more common in patients with cancer and in heavily immunosuppressed patients. The overall risk is unknown but is less than 1:50,000.
Second Line
Splenectomy. For patients who cannot be weaned from steroids or in whom steroid therapy fails, there is no standard therapy. Currently, the 2 main choices are splenectomy or rituximab (anti-CD20) therapy if the patient did not receive it first line. Splenectomy is the classic therapy for warm AIHA. Reported response rates in the literature range from 50% to 80%, with 50% to 60% remaining in remission.12-16 Timing of the procedure is a balance between allowing time for the steroids to work and the risk of toxicity of steroids. In a patient who has low presurgical risk and has either refractory disease or cannot be weaned from high doses of steroids, splenectomy should be done sooner. Splenectomy can be delayed or other therapy tried first in patients who require lower doses of steroids or have medical risk factors for surgery. Most splenectomies are performed via laparoscopy. The small incisions allow for quicker healing, and the laparoscopic approach provides better visualization of the abdomen to find and remove accessory spleens. When splenectomy is performed by experienced surgeons, the mortality rate is low (< 0.5%).17
The most concerning complication of splenectomy is overwhelming post-splenectomy infection (OPSI).18 In adults, the spleen appears to play a minimal role in immunity except for protecting against certain encap-sulated organisms. Splenectomized patients infected with an encapsulated organism (eg, Pneumococcus) will develop overwhelming sepsis within hours. These patients will often have disseminated intravascular coagulation and will rapidly progress to purpura fulminans. Approximately 40% to 50% of patients will die of sepsis even when the infection is detected early. The overall lifetime risk of sepsis may be as high as 1:500. The organism that most commonly causes sepsis in splenectomized patients is Streptococcus pneumoniae, reported in over 50% of cases. Neisseria meningitidis and Haemophilus influenzae have also been implicated in many cases.19 Overwhelming sepsis after dog bites has been reported due to Capnocytophaga canimorsus infections. Patients are also at increased risk of developing severe malaria and severe babesiosis.18
Patients who have undergone splenectomy need to be warned about the risk of OPSI and instructed to report to the emergency department readily if they develop a fever greater than 101°F (38.3°C) or shaking chills. Once in the emergency department, blood cultures should be obtained rapidly and the patient started on antibiotic coverage with vancomycin and ceftriaxone (or levofloxacin if allergic to beta-lactams).20 For patients in remote areas, some physicians will prescribe prophylactic antibiotics to take while they are traveling to a health care provider or even recommend a “standby” antibiotic dose to take while traveling to health care.5 This usually consists of amoxicillin or a macrolide for penicillin-allergic patients.
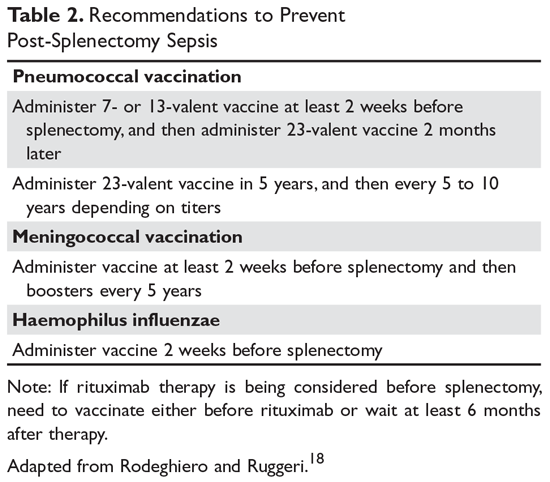
Patients in whom splenectomy is being planned or considered should be vaccinated for pneumococcal, meningococcal, and influenza infections (Table 2).18 If there is a plan to treat with rituximab, patients should first be vaccinated since they will not be able to mount an immune response after being treated with rituximab.
Third Line
The therapeutic options for patients who do not respond to either splenectomy or rituximab are much less certain.5,6 Although intravenous immune globulin is a standard therapy for ITP, response rates are low in warm AIHA.17 Numerous therapies have been reported in small series, but no clear approach has emerged. Options include azathioprine, cyclophosphamide, mycophenolate, cyclosporine, danazol, and alemtuzumab. Our approach has been to use mycophenolate for patients requiring high doses of steroids or transfusions. Patients who respond to lower doses of steroids may be good candidates for danazol to help wean them off steroids.
Treatment of Warm AIHA with Associated Diseases
Warm AIHA can complicate several diseases. Patients with systemic lupus erythematosus (SLE) can develop warm AIHA as part of their disease complex. The initial treatment approach is the same, but data suggest that splenectomy may not be as effective.13,17 Also, many SLE patients have complex medical conditions, making surgeries riskier. For SLE patients who are refractory or cannot be weaned from steroids, rituximab may be the better choice. Babesiosis, particularly in asplenic patients, has been associated with the development of AIHA.21,22
Of the malignances associated with AIHA, chronic lymphocytic leukemia (CLL) has the strongest association.4,23 Series show that 5% to 10% of patients with CLL will have warm AIHA. AIHA can appear concurrent with CLL or develop during the course of the disease. The introduction of purine analogs such as fludarabine led to a dramatic increase in the incidence of warm AIHA in treated patients.24 It is speculated that these powerful agents reduce the number and effectiveness of T cells that hold in check the autoantibody response, leading to warm AIHA.25 However, when these purine analogs are used in com-bination with agents such as cyclophosphamide or rituximab (with their immunosuppressive effects), the rates of warm AIHA have been lower.23
The approach to patients with CLL and warm AIHA depends on the state of their CLL.23 For patients who have low-stage CLL that does not need treatment, the standard approach to warm AIHA should be steroids, splenectomy, and rituximab.24 For patients with higher-stage CLL, the treatment for the leukemia will often provide therapy for the warm AIHA. The combination of rituximab-cyclophosphamide-dexamethasone has been reported to be effective for both the AIHA and CLL components.26 The use of ibrutinib has also been reported to be effective.27
A rare but important variant of warm AIHA is Evans syndrome.28,29 This is the combination of AIHA and ITP. Approximately 1% to 3% of AIHA cases are the Evans variant. The ITP can precede, be concurrent with, or develop after the AIHA. The diagnosis of Evans syndrome should raise concern for underlying disorders. In young adults, immunodeficiency disorders such as autoimmune lymphoproliferative disease (ALPS) need to be considered. In older patients, Evans syndrome is often associated with T cell lymphomas. The sparse literature on Evans syndrome suggests that it can be more refractory to standard therapy, with response rates to splenectomy in the 50% range.28,30 In patients with lymphoma, antineoplastic therapy is crucial. There is increasing data showing that mycophenolate or sirolimus may be effective for patients with ALPS in whom splenectomy or rituximab therapy is unsuccessful.31
Warm AIHA with IgA or IgM Antibodies
In rare patients with warm AIHA, IgA or IgM is the implicated antibody. The literature suggests that patients with IgA AIHA may have more severe hemolysis.32 Patients with IgM AIHA often have a severe course with a fatal outcome.33 In such cases, the patient’s plasma may show spontaneous hemolysis and agglutination. The DAT may not be strongly positive or may show C3 reactivity only. The clinical clues are C3 reactivity with no cold agglutinins and severe hemolysis, sometimes with an intravascular component. Treatment is the same as for warm AIHA, including the use of rituximab.34
Cold Autoimmune Hemolytic Anemia
In cold AIHA, the hemolysis is mediated by IgM antibodies directed against red cells.35 As discussed earlier, the term “cold” refers to the fact that the antibody binds maximally at temperatures below 37°C. The most efficient temperature for binding is called the “thermal amplitude,” and, in theory, the higher the thermal amplitude, the more virulent the antibody. An antibody titer can be calculated at each reaction temperature from 4°C to 37°C by serial dilutions of the patient’s serum prior to incubating with reagent red cells. Rarely, the IgM can fix complement rapidly, leading to intravascular hemolysis. In most patients, complement is fixed through C3, and the C3-coated red cells are taken up by macrophages in the mononuclear phagocyte system, primarily in the liver.3
The DAT in patients with cold AIHA will show cells coated with C3. The blood smear will often show ag-glutination of the blood, and if the blood cools before being analyzed, the agglutination will interfere with the analysis. Titers of cold agglutinin can range from 1:1000 to over 1 million. The IgM autoantibodies are most often directed against the I/i antigens on the red blood cell membrane, with 90% against I antigen.35 The I antigen specificity is typical with primary cold agglutinin disease and after Mycoplasma infection. The i antigen specificity is most typical of Epstein-Barr virus and cytomegalovirus infections in children. In young patients, cold AIHA often occurs following an infection, including viral and Mycoplasma infections, and the course is self-limited.35,36 The hemolysis usually starts 2 to 3 weeks after the illness and will last for 4 to 6 weeks. In older patients, the etiology in over 90% of cases is a B-cell lymphoproliferative disorder, usually with monoclonal kappa B-cells.37 The most common disorders are marginal zone lymphoma, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma.3
Therapeutic Options
It is important to diagnose cold AIHA because the standard therapy for warm AIHA (steroids) is ineffective in cold AIHA. Because C3-coated red cells are taken up primarily in the liver, removing the spleen is also an ineffective therapy. Simple measures to help with cold AIHA should be employed.37 Patients should be kept in a warm environment and should try to avoid the cold. If transfusions are needed, they should be infused via blood warmers to prevent hemolysis. In rare patients with severe hemolysis, therapeutic plasma exchange (TPE) can be considered.38 Given that the culprit antibody is IgM—mostly intravascular—use of TPE may slow the hemolysis to give time for other therapies to take effect.
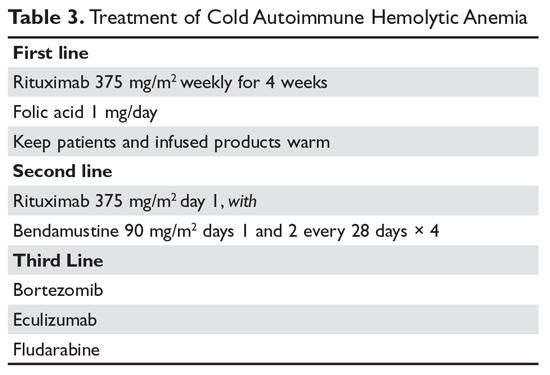
Treatment of cold AIHA remains difficult (Table 3). Because most patients with primary AIHA have underlying lymphoproliferative diseases, chlorambucil has been used in the past to treat cold AIHA. However, responses were rare and the drug could worsen the anemia.38 Currently, the drug of choice is rituximab. Response is seen in 45% to 75% of patients, but is almost always a partial response and retreatment is often necessary.17,37,39 As with other autoimmune hematologic diseases, there can be a delay in response that ranges from 2 weeks to 4 months (median time, 1.5 months).37 Given the lack of robust response (complete and durable) with rituximab, the Berentsen group has explored adding bendamustine to rituximab.40 In a prospective trial, 71% of patients responded with a 40% complete response rate. Therapy was well tolerated, with only 29% of patients needing dose reduction Although more toxic, this combination can be considered in patients with aggressive disease. A small study of the use of bortezomib showed a good response in one-third of patients.41 There is increasing use of the C5 complement inhibitor eculizumab to halt the hemolysis, but further study of this agent is also required.36,42,43 Blockers of C1s complement, which block hemolysis by preventing complement activation, are currently being studied in clinical trials.44
Since most patients with cold AIHA are older, a frequent issue that must be considered is cardiac surgery. The concern is that the hypothermia involved with most heart bypass procedures will lead to agglutination and hemolysis. The development of normothermic bypass has expanded treatment options. A recommended approach in patients who have known cold agglutinins is to measure the thermal amplitude of the antibody preoperatively. If the thermal amplitude is above 18°C, normothermic bypass should be done, if feasible.45 If not feasible, preoperative TPE should be considered.
Paroxysmal Cold Hemoglobinuria
A unique cold AIHA is paroxysmal cold hemoglobinuria (PCH).3,46 This cold hemolytic syndrome most often occurs in children following a viral infection, but in the past it complicated any stage of syphilis.47 The mediating antibody in PCH is an IgG antibody directed against the P antigen on the red blood cell. This antibody binds best at temperatures below 37°C, fixing complement at cold temperatures, but then activates the complement cascade at body temperature.48 Because this antibody can fix complement, hemolysis can be rapid and severe, leading to extreme anemia. The DAT is often weakly positive but can be negative. The diagnostic test for PCH is the Donath–Landsteiner test. This complex test is performed by incubating 3 blood samples, 1 at 0° to 4°C, another at 37°C, and a third at 0° to 4°C and then at 37°C. The diagnosis of PCH is made if only the third tube shows hemolysis.35 PCH can persist for 1 to 3 months but is almost always self-limiting. In severe case, steroids may be of benefit.
Drug-Induced Hemolytic Anemia
AIHA caused by a drug reaction is rare, with a lower incidence than drug-related ITP. The rate of severe drug-related AIHA is estimated at 1:1,000,000, but less severe cases may be missed.1 Most patients will have a positive DAT without signs of hemolysis, but in rare cases patients will have relentless hemolysis resulting in death.
Mechanisms
Multiple mechanisms for drug-induced immune hemolysis have been proposed, including drug-absorption (hapten-induced) and immune complex mechanisms.1,49 The hapten mechanism is most often associated with the use of high-dose penicillin.50 High doses of penicillin or similar drugs such as piperacillin lead to incorporation of the drug into the red cell membrane by binding to proteins. Patients will manifest a positive DAT with IgG antibody but not complement.51 The patient’s plasma will be reactive only with penicillin-coated red cells and not with normal cells. As mentioned, very few patients will have hemolysis, and if they have hemolysis, it will resolve in a few days after discontinuation of the offending drug.52
Binding of a drug-antibody complex to the red cell membrane may cause hemolysis via the immune com-plex mechanism.53 Again, most often the patient will have just a positive DAT, but rarely patients will have life-threatening hemolysis upon exposure or reexposure to the drug. The onset of hemolysis is rapid, with signs of acute illness and intravascular hemolysis. The paradigm drug is quinine, but many other drugs have been implicated. Testing shows a positive Coombs test with anti-complement but not anti-IgG.50 This pattern is due to the effectiveness of the tertiary complex at fixing complement. The patient’s plasma reacts with red cells only when the drug is added.
A form of immune complex hemolysis associated with both disseminated intravascular coagulation (DIC) and brisk hemolysis has been recognized. Patients who receive certain second- and third-generation cephalosporins (especially cefotetan and ceftriaxone.53,54) have developed this syndrome.50,55-59 The clinical symptoms start 7 to 10 days after the drug is administered; often the patient has only received the antibiotic for surgical prophylaxis. Immune hemolysis with acute hematocrit drop, hypotension, and DIC ensues. The patients are often believed to have sepsis and are often reexposed to the cephalosporin, resulting in worsening of the clinical status. The outcome is often fatal due to massive hemolysis and thrombosis.56,60,62
Finally, 8% to 36% of patients taking methyldopa will develop a positive DAT after 6 months of therapy, with less than 1% showing hemolysis.52,63 The hemolysis in these patients is indistinguishable from warm AIHA, consistent with the notion that methyldopa induces an AIHA. The hemolysis often resolves rapidly after the methyldopa is stopped, but the Coombs test may remain positive for months.63 This type of drug-induced hemolytic anemia has been reported with levodopa, procainamide, and chlorpromazine, but fludarabine is the most common cause currently. This form of AIHA is now being seen with increased use of checkpoint inhibitors.64
Diagnosis
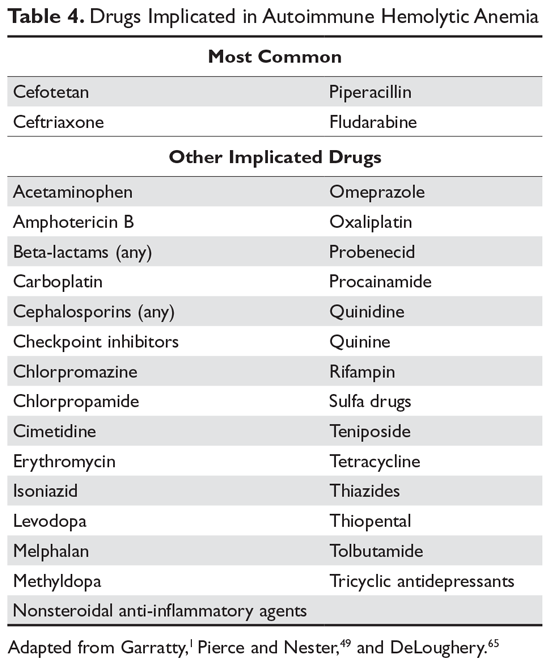
In many patients, the first clue to the presence of drug AIHA is the finding of a positive DAT. Rarely, patients will have severe hemolysis, but in many patients the hemolytic process is mild and may be wrongly assumed to be part of the underlying illness. Finding the offending drug can be a challenge, unless a patient has recently started a new drug; in a hospitalized patient on multiple agents, identifying the problem drug is difficult. Patients recently started on “suspect drugs,” especially the most common agents cefotetan, ceftriaxone, and piperacillin, should have these agents stopped (Table 4).1,49,65 Specialty laboratories such as the Blood Center of Wisconsin or the Los Angeles Red Cross can perform in vitro studies of drug interactions that can confirm the clinical diagnosis of drug-induced AIHA.
Treatment
Therapy for patients with positive DAT without signs of hemolysis is uncertain. If the drug is essential, then the patient can be observed. If the patient has hemolysis, the drug needs to be stopped and the patient observed for signs of end-organ damage. It is doubtful that steroids or other autoimmune-directed therapy is effective. For patients with the DIC-hemolysis syndrome, there are anecdotal reports that TPE may be helpful.1
Summary
AIHA can range from an abnormal laboratory test (positive DAT and signs of hemolysis) to an acute, life-threatening illness. Treatment is guided by the laboratory work-up and evaluation of the patient’s clinical status. While rituximab is promising for many patients, the lack of robust clinical trials hinders the treatment of patients who fail standard therapies.
1. Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143-150.
2. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
3. Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016:226-231.
4. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
5. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
6. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for re¬current venous thrombosis. Lancet. 2010;376:2032-2039.
7. Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood. 2017;129:2971-2979.
8. Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163:393-399
9. Michel M, Terriou L, Roudot-Thoraval F, et al. A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study). Am J Hematol. 2017;92:23-27.
10. Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47:187-198.
11. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297-1309.
12. Coon WW. Splenectomy in the treatment of hemolytic anemia. Arch Surg. 1985;120:625-628.
13. Akpek G, McAneny D, Weintraub L. Comparative response to splenectomy in coombs-positive autoimmune hemolytic anemia with or without associated disease. Am J Hematol. 1999;61:98-102.
14. Patel NY, Chilsen AM, Mathiason MA, et al. Outcomes and complications after splenectomy for hematologic disorders. Am J Surg. 2012;204:1014-1020.
15. Crowther M, Chan YL, Garbett IK, et al. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118:4036-4040.
16. Giudice V, Rosamilio R, Ferrara I, et al. Efficacy and safety of splenectomy in adult autoimmune hemolytic anemia. Open Med (Wars). 2016;11:374-380.
17. Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831-1838.
18. Rodeghiero F, Ruggeri M. Short- and long-term risks of splenectomy for benign haematological disorders: should we revisit the indications? Br J Haematol. 2012;158:16-29.
19. Ahmed N, Bialowas C, Kuo YH, Zawodniak L. Impact of preinjury anticoagulation in patients with traumatic brain injury. South Med J. 2009;102:476-480.
20. Morgan TL, Tomich EB. Overwhelming post-splenectomy infection (OPSI): a case report and review of the literature. J Emerg Med. 2012;43:758-763.
21. Woolley AE, Montgomery MW, Savage WJ, et al. Post-babesiosis warm autoimmune hemolytic anemia. N Engl J Med. 2017;376:939-946.
22. Shatzel JJ, Donohoe K, Chu NQ, et al. Profound autoimmune hemolysis and Evans syndrome in two asplenic patients with babesiosis. Transfusion. 2015;55:661-665.
23. Hodgson K, Ferrer G, Montserrat E, Moreno C. Chronic lymphocytic leukemia and autoimmunity: a systematic review. Haematologica. 2011;96:752-761.
24. Hamblin TJ. Autoimmune complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:230-239.
25. Tertian G, Cartron J, Bayle C, et al. Fatal intravascular au¬toimmune hemolytic anemia after fludarabine treatment for chronic lymphocytic leukemia. Hematol Cell Ther. 1996;38:359-360.
26. Rossignol J, Michallet AS, Oberic L, et al. Rituximab-cyclophosphamide-dexamethasone combination in the management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia. 2011;25:473-478.
27. Hampel PJ, Larson MC, Kabat B, et al. Autoimmune cytopenias in patients with chronic lymphocytic leukaemia treated with ibrutinib in routine clinical practice at an academic medical centre. Br J Haematol. 2018;183:421-427.
28. Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114:3167-3172.
29. Jaime-Pérez JC, Aguilar-Calderón PE, Salazar-Cavazos L, Gómez-Almaguer D. Evans syndrome: clinical perspectives, biological insights and treatment modalities. J Blood Med. 2018;9:171-184.
30. Dhingra KK, Jain D, Mandal S, et al. Evans syndrome: a study of six cases with review of literature. Hematology. 2008;13:356-360.
31. Bride KL, Vincent T, Smith-Whitley K, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood. 2016;127:17-28.
32. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell au¬toantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
33. Garratty G, Arndt P, Domen R, et al. Severe autoimmune hemolytic anemia associated with IgM warm autoantibodies directed against determinants on or associated with glycophorin A. Vox Sang. 1997;72:124-130.
34. Wakim M, Shah A, Arndt PA, et al. Successful anti-CD20 monoclonal antibody treatment of severe autoimmune hemolytic anemia due to warm reactive IgM autoantibody in a child with common variable immunodeficiency. Am J Hematol. 2004;76:152-155.
35. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
36. Berentsen S, Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107-115.
37. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
38. King KE, Ness PM. Treatment of autoimmune hemolytic anemia. Semin Hematol. 2005;42:131136.
39. Barcellini W, Zaja F, Zaninoni A, et al. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biologic studies. Blood. 2012;119:3691-3697.
40. Berentsen S, Randen U, Oksman M, et al. Bendamustine plus rituximab for chronic cold agglutinin disease: results of a Nordic prospective multicenter trial. Blood. 2017;130:537-541.
41. Rossi G, Gramegna D, Paoloni F, et al. Short course of bortezomib in anemic patients with relapsed cold agglutinin disease: a phase 2 prospective GIMEMA study. Blood. 2018;132:547-550.
42. Makishima K, Obara N, Ishitsuka K, et al. High efficacy of eculizumab treatment for fulminant hemolytic anemia in primary cold agglutinin disease. Ann Hematol. 2019;98:1031-1032.
43. Roth A, Huttmann A, Rother RP, et al. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2009;113:38853886.
44. Jäger U, D'Sa S, Schörgenhofer C, et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial. Blood. 2019;133:893-901.
45. Agarwal SK, Ghosh PK, Gupta D. Cardiac surgery and cold-reactive proteins. Ann Thorac Surg. 1995;60:1143-1150.
46. Shanbhag S, Spivak J. Paroxysmal cold hemoglobinuria. Hematol Oncol Clin North Am. 2015;29:473-478.
47. Kumar ND, Sethi S, Pandhi RK. Paroxysmal cold haemoglobinuria in syphilis patients. Genitourin Med. 1993;69:76.
48. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
49. Pierce A, Nester T. Pathology consultation on drug-induced hemolytic anemia. Am J Clin Pathol. 2011;136:7-12.
50. Garratty G. Immune cytopenia associated with antibiotics. Transfusion Med Rev. 1993;7:255-267.
51. Petz LD, Mueller-Eckhardt C. Drug-induced immune hemolytic anemia. Transfusion. 1992;32:202-204.
52. Packman CH, Leddy JP. Drug-related immune hemolytic anemia. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, eds. William’s Hematology. 5th ed. New York: McGraw-Hill; 1995:691-704.
53. Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2009;73-79.
54. Leicht HB, Weinig E, Mayer B, et al. Ceftriaxone-induced hemolytic anemia with severe renal failure: a case report and review of literature. BMC Pharmacol Toxicol. 2018;19:67.
55. Chenoweth CE, Judd WJ, Steiner EA, Kauffman CA. Cefotetan-induced immune hemolytic anemia. Clin Infect Dis. 1992;15:863-865.
56. Garratty G, Nance S, Lloyd M, Domen R. Fatal im¬mune hemolytic anemia due to cefotetan. Transfusion. 1992;32:269-271.
57. Endoh T, Yagihashi A, Sasaki M, Watanabe N. Ceftizoxime-induced hemolysis due to immune complexes: case report and determination of the epitope responsible for immune complex-mediated hemolysis. Transfusion. 1999;39:306-309.
58. Arndt PA, Leger RM, Garratty G. Serology of antibodies to second- and third-generation cephalosporins associated with immune hemolytic anemia and/or positive direct antiglobulin tests. Transfusion. 1999;39:1239-1246.
59. Martin ME, Laber DA. Cefotetan-induced hemolytic anemia after perioperative prophylaxis. Am J Hematol. 2006;81:186-188.
60. Bernini JC, Mustafa MM, Sutor LJ, Buchanan GR. Fatal hemolysis induced by ceftriaxone in a child with sickle cell anemia. J Pediatr. 1995;126(5 Pt 1):813-815.
61. Borgna-Pignatti C, Bezzi TM, Reverberi R. Fatal ceftriaxone-induced hemolysis in a child with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1995;14:1116-1117.
62. Lascari AD, Amyot K. Fatal hemolysis caused by ceftriaxone [see comments]. J Pediatr. 1995;126(5 Pt 1):816-817.
63. Petz LD. Drug-induced autoimmune hemolytic anemia. Transfusion Med Rev. 1993;7:242-254.
64. Leaf RK, Ferreri C, Rangachari D, et al. Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors. Am J Hematol. 2019;94:563-574.
65. DeLoughery T. Drug induced immune hematological disease. Allerg Immunol Clin. 1998;18:829-841.
Autoimmune hemolytic anemia (AIHA) is mediated by antibodies, and in most cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C. AIHA caused by a drug reaction is rare, with an estimated annual incidence of 1:1,000,000 for severe drug-related AIHA.1 This article reviews the management of the more common types of AIHA, with a focus on warm, cold, and drug-induced AIHA; the evaluation and diagnosis of AIHA is reviewed in a separate article.
Warm Autoimmune Hemolytic Anemia
In AIHA, hemolysis is mediated by antibodies that bind to the surface of red blood cells. AIHA in which IgG antibodies are the offending antibodies is referred to as warm AIHA. “Warm” refers to the fact that the antibody binds best at body temperature (37°C). In warm AIHA, testing will show IgG molecules attached to the surface of the red cells, with 50% of patients also showing C3. Between 50% and 90% of AIHA cases are due to warm antibodies.2,3 The incidence of warm AIHA varies by series but is approximately 1 case per 100,000 patients per year; this form of hemolysis affects women more frequently than men.4,5
Therapeutic Options
First Line
Steroids. The goal of therapy in warm AIHA can be hard to define. However, most would agree that a hematocrit above 30% (or higher to prevent symptoms) with a minimal increase in the reticulocyte count—reflective of a significantly slowed hemolytic process—is a reasonable goal. Initial management of warm AIHA is prednisone at a standard dose of 1 mg/kg daily (Table 1).6,7 Patients should be also started on proton-pump inhibitors to prevent ulcers. It can take up to 3 weeks for patients to respond to prednisone therapy. Once the patient’s hematocrit is above 30%, the prednisone is slowly tapered. Although approximately 80% of patients will respond to steroids, only 30% can be fully tapered off steroids. For patients who can be maintained on a daily steroid dose of 10 mg or less, steroids may be the most reasonable long-term therapy. In addition, because active hemolysis leads to an increased demand for folic acid, patients with warm AIHA are often prescribed folic acid 1 mg daily to prevent megaloblastic anemia due to folic acid deficiency.

Rituximab. Increasingly, rituximab (anti-CD20) therapy is added to the initial steroids. Two clinical trials showed both increased long-term and short-term responses with the use of rituximab.8,9 An important consideration is that most patients respond gradually to rituximab over weeks, so a rapid response should not be expected. Most studies have used the traditional dosing of 375 mg/m2 weekly for 4 weeks. These responses appear to be durable, but as in immune thrombocytopenia (ITP), repeat treatment with rituximab is effective.
The major side effects of rituximab are infusion reactions, which are often worse with the first dose. These reactions can be controlled with antihistamines, steroids, and, for severe rigors, meperidine. Rarely, patients can develop neutropenia (approximately 1:500) that appears to be autoimmune in nature. Infections appear to be only minimally increased with the use of rituximab.10 One group at risk is chronic carriers of hepatitis B virus, who may experience a reactivation of the virus that can be fatal. Thus, patients being considered for rituximab need to be screened for hepatitis B virus carrier state.11 Patients receiving rituximab are at very slight risk for progressive multifocal leukoencephalopathy, which is more common in patients with cancer and in heavily immunosuppressed patients. The overall risk is unknown but is less than 1:50,000.
Second Line
Splenectomy. For patients who cannot be weaned from steroids or in whom steroid therapy fails, there is no standard therapy. Currently, the 2 main choices are splenectomy or rituximab (anti-CD20) therapy if the patient did not receive it first line. Splenectomy is the classic therapy for warm AIHA. Reported response rates in the literature range from 50% to 80%, with 50% to 60% remaining in remission.12-16 Timing of the procedure is a balance between allowing time for the steroids to work and the risk of toxicity of steroids. In a patient who has low presurgical risk and has either refractory disease or cannot be weaned from high doses of steroids, splenectomy should be done sooner. Splenectomy can be delayed or other therapy tried first in patients who require lower doses of steroids or have medical risk factors for surgery. Most splenectomies are performed via laparoscopy. The small incisions allow for quicker healing, and the laparoscopic approach provides better visualization of the abdomen to find and remove accessory spleens. When splenectomy is performed by experienced surgeons, the mortality rate is low (< 0.5%).17
The most concerning complication of splenectomy is overwhelming post-splenectomy infection (OPSI).18 In adults, the spleen appears to play a minimal role in immunity except for protecting against certain encap-sulated organisms. Splenectomized patients infected with an encapsulated organism (eg, Pneumococcus) will develop overwhelming sepsis within hours. These patients will often have disseminated intravascular coagulation and will rapidly progress to purpura fulminans. Approximately 40% to 50% of patients will die of sepsis even when the infection is detected early. The overall lifetime risk of sepsis may be as high as 1:500. The organism that most commonly causes sepsis in splenectomized patients is Streptococcus pneumoniae, reported in over 50% of cases. Neisseria meningitidis and Haemophilus influenzae have also been implicated in many cases.19 Overwhelming sepsis after dog bites has been reported due to Capnocytophaga canimorsus infections. Patients are also at increased risk of developing severe malaria and severe babesiosis.18
Patients who have undergone splenectomy need to be warned about the risk of OPSI and instructed to report to the emergency department readily if they develop a fever greater than 101°F (38.3°C) or shaking chills. Once in the emergency department, blood cultures should be obtained rapidly and the patient started on antibiotic coverage with vancomycin and ceftriaxone (or levofloxacin if allergic to beta-lactams).20 For patients in remote areas, some physicians will prescribe prophylactic antibiotics to take while they are traveling to a health care provider or even recommend a “standby” antibiotic dose to take while traveling to health care.5 This usually consists of amoxicillin or a macrolide for penicillin-allergic patients.
Patients in whom splenectomy is being planned or considered should be vaccinated for pneumococcal, meningococcal, and influenza infections (Table 2).18 If there is a plan to treat with rituximab, patients should first be vaccinated since they will not be able to mount an immune response after being treated with rituximab.

Third Line
The therapeutic options for patients who do not respond to either splenectomy or rituximab are much less certain.5,6 Although intravenous immune globulin is a standard therapy for ITP, response rates are low in warm AIHA.17 Numerous therapies have been reported in small series, but no clear approach has emerged. Options include azathioprine, cyclophosphamide, mycophenolate, cyclosporine, danazol, and alemtuzumab. Our approach has been to use mycophenolate for patients requiring high doses of steroids or transfusions. Patients who respond to lower doses of steroids may be good candidates for danazol to help wean them off steroids.
Treatment of Warm AIHA with Associated Diseases
Warm AIHA can complicate several diseases. Patients with systemic lupus erythematosus (SLE) can develop warm AIHA as part of their disease complex. The initial treatment approach is the same, but data suggest that splenectomy may not be as effective.13,17 Also, many SLE patients have complex medical conditions, making surgeries riskier. For SLE patients who are refractory or cannot be weaned from steroids, rituximab may be the better choice. Babesiosis, particularly in asplenic patients, has been associated with the development of AIHA.21,22
Of the malignances associated with AIHA, chronic lymphocytic leukemia (CLL) has the strongest association.4,23 Series show that 5% to 10% of patients with CLL will have warm AIHA. AIHA can appear concurrent with CLL or develop during the course of the disease. The introduction of purine analogs such as fludarabine led to a dramatic increase in the incidence of warm AIHA in treated patients.24 It is speculated that these powerful agents reduce the number and effectiveness of T cells that hold in check the autoantibody response, leading to warm AIHA.25 However, when these purine analogs are used in com-bination with agents such as cyclophosphamide or rituximab (with their immunosuppressive effects), the rates of warm AIHA have been lower.23
The approach to patients with CLL and warm AIHA depends on the state of their CLL.23 For patients who have low-stage CLL that does not need treatment, the standard approach to warm AIHA should be steroids, splenectomy, and rituximab.24 For patients with higher-stage CLL, the treatment for the leukemia will often provide therapy for the warm AIHA. The combination of rituximab-cyclophosphamide-dexamethasone has been reported to be effective for both the AIHA and CLL components.26 The use of ibrutinib has also been reported to be effective.27
A rare but important variant of warm AIHA is Evans syndrome.28,29 This is the combination of AIHA and ITP. Approximately 1% to 3% of AIHA cases are the Evans variant. The ITP can precede, be concurrent with, or develop after the AIHA. The diagnosis of Evans syndrome should raise concern for underlying disorders. In young adults, immunodeficiency disorders such as autoimmune lymphoproliferative disease (ALPS) need to be considered. In older patients, Evans syndrome is often associated with T cell lymphomas. The sparse literature on Evans syndrome suggests that it can be more refractory to standard therapy, with response rates to splenectomy in the 50% range.28,30 In patients with lymphoma, antineoplastic therapy is crucial. There is increasing data showing that mycophenolate or sirolimus may be effective for patients with ALPS in whom splenectomy or rituximab therapy is unsuccessful.31
Warm AIHA with IgA or IgM Antibodies
In rare patients with warm AIHA, IgA or IgM is the implicated antibody. The literature suggests that patients with IgA AIHA may have more severe hemolysis.32 Patients with IgM AIHA often have a severe course with a fatal outcome.33 In such cases, the patient’s plasma may show spontaneous hemolysis and agglutination. The DAT may not be strongly positive or may show C3 reactivity only. The clinical clues are C3 reactivity with no cold agglutinins and severe hemolysis, sometimes with an intravascular component. Treatment is the same as for warm AIHA, including the use of rituximab.34
Cold Autoimmune Hemolytic Anemia
In cold AIHA, the hemolysis is mediated by IgM antibodies directed against red cells.35 As discussed earlier, the term “cold” refers to the fact that the antibody binds maximally at temperatures below 37°C. The most efficient temperature for binding is called the “thermal amplitude,” and, in theory, the higher the thermal amplitude, the more virulent the antibody. An antibody titer can be calculated at each reaction temperature from 4°C to 37°C by serial dilutions of the patient’s serum prior to incubating with reagent red cells. Rarely, the IgM can fix complement rapidly, leading to intravascular hemolysis. In most patients, complement is fixed through C3, and the C3-coated red cells are taken up by macrophages in the mononuclear phagocyte system, primarily in the liver.3
The DAT in patients with cold AIHA will show cells coated with C3. The blood smear will often show ag-glutination of the blood, and if the blood cools before being analyzed, the agglutination will interfere with the analysis. Titers of cold agglutinin can range from 1:1000 to over 1 million. The IgM autoantibodies are most often directed against the I/i antigens on the red blood cell membrane, with 90% against I antigen.35 The I antigen specificity is typical with primary cold agglutinin disease and after Mycoplasma infection. The i antigen specificity is most typical of Epstein-Barr virus and cytomegalovirus infections in children. In young patients, cold AIHA often occurs following an infection, including viral and Mycoplasma infections, and the course is self-limited.35,36 The hemolysis usually starts 2 to 3 weeks after the illness and will last for 4 to 6 weeks. In older patients, the etiology in over 90% of cases is a B-cell lymphoproliferative disorder, usually with monoclonal kappa B-cells.37 The most common disorders are marginal zone lymphoma, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma.3
Therapeutic Options
It is important to diagnose cold AIHA because the standard therapy for warm AIHA (steroids) is ineffective in cold AIHA. Because C3-coated red cells are taken up primarily in the liver, removing the spleen is also an ineffective therapy. Simple measures to help with cold AIHA should be employed.37 Patients should be kept in a warm environment and should try to avoid the cold. If transfusions are needed, they should be infused via blood warmers to prevent hemolysis. In rare patients with severe hemolysis, therapeutic plasma exchange (TPE) can be considered.38 Given that the culprit antibody is IgM—mostly intravascular—use of TPE may slow the hemolysis to give time for other therapies to take effect.
Treatment of cold AIHA remains difficult (Table 3). Because most patients with primary AIHA have underlying lymphoproliferative diseases, chlorambucil has been used in the past to treat cold AIHA. However, responses were rare and the drug could worsen the anemia.38 Currently, the drug of choice is rituximab. Response is seen in 45% to 75% of patients, but is almost always a partial response and retreatment is often necessary.17,37,39 As with other autoimmune hematologic diseases, there can be a delay in response that ranges from 2 weeks to 4 months (median time, 1.5 months).37 Given the lack of robust response (complete and durable) with rituximab, the Berentsen group has explored adding bendamustine to rituximab.40 In a prospective trial, 71% of patients responded with a 40% complete response rate. Therapy was well tolerated, with only 29% of patients needing dose reduction Although more toxic, this combination can be considered in patients with aggressive disease. A small study of the use of bortezomib showed a good response in one-third of patients.41 There is increasing use of the C5 complement inhibitor eculizumab to halt the hemolysis, but further study of this agent is also required.36,42,43 Blockers of C1s complement, which block hemolysis by preventing complement activation, are currently being studied in clinical trials.44

Since most patients with cold AIHA are older, a frequent issue that must be considered is cardiac surgery. The concern is that the hypothermia involved with most heart bypass procedures will lead to agglutination and hemolysis. The development of normothermic bypass has expanded treatment options. A recommended approach in patients who have known cold agglutinins is to measure the thermal amplitude of the antibody preoperatively. If the thermal amplitude is above 18°C, normothermic bypass should be done, if feasible.45 If not feasible, preoperative TPE should be considered.
Paroxysmal Cold Hemoglobinuria
A unique cold AIHA is paroxysmal cold hemoglobinuria (PCH).3,46 This cold hemolytic syndrome most often occurs in children following a viral infection, but in the past it complicated any stage of syphilis.47 The mediating antibody in PCH is an IgG antibody directed against the P antigen on the red blood cell. This antibody binds best at temperatures below 37°C, fixing complement at cold temperatures, but then activates the complement cascade at body temperature.48 Because this antibody can fix complement, hemolysis can be rapid and severe, leading to extreme anemia. The DAT is often weakly positive but can be negative. The diagnostic test for PCH is the Donath–Landsteiner test. This complex test is performed by incubating 3 blood samples, 1 at 0° to 4°C, another at 37°C, and a third at 0° to 4°C and then at 37°C. The diagnosis of PCH is made if only the third tube shows hemolysis.35 PCH can persist for 1 to 3 months but is almost always self-limiting. In severe case, steroids may be of benefit.
Drug-Induced Hemolytic Anemia
AIHA caused by a drug reaction is rare, with a lower incidence than drug-related ITP. The rate of severe drug-related AIHA is estimated at 1:1,000,000, but less severe cases may be missed.1 Most patients will have a positive DAT without signs of hemolysis, but in rare cases patients will have relentless hemolysis resulting in death.
Mechanisms
Multiple mechanisms for drug-induced immune hemolysis have been proposed, including drug-absorption (hapten-induced) and immune complex mechanisms.1,49 The hapten mechanism is most often associated with the use of high-dose penicillin.50 High doses of penicillin or similar drugs such as piperacillin lead to incorporation of the drug into the red cell membrane by binding to proteins. Patients will manifest a positive DAT with IgG antibody but not complement.51 The patient’s plasma will be reactive only with penicillin-coated red cells and not with normal cells. As mentioned, very few patients will have hemolysis, and if they have hemolysis, it will resolve in a few days after discontinuation of the offending drug.52
Binding of a drug-antibody complex to the red cell membrane may cause hemolysis via the immune com-plex mechanism.53 Again, most often the patient will have just a positive DAT, but rarely patients will have life-threatening hemolysis upon exposure or reexposure to the drug. The onset of hemolysis is rapid, with signs of acute illness and intravascular hemolysis. The paradigm drug is quinine, but many other drugs have been implicated. Testing shows a positive Coombs test with anti-complement but not anti-IgG.50 This pattern is due to the effectiveness of the tertiary complex at fixing complement. The patient’s plasma reacts with red cells only when the drug is added.
A form of immune complex hemolysis associated with both disseminated intravascular coagulation (DIC) and brisk hemolysis has been recognized. Patients who receive certain second- and third-generation cephalosporins (especially cefotetan and ceftriaxone.53,54) have developed this syndrome.50,55-59 The clinical symptoms start 7 to 10 days after the drug is administered; often the patient has only received the antibiotic for surgical prophylaxis. Immune hemolysis with acute hematocrit drop, hypotension, and DIC ensues. The patients are often believed to have sepsis and are often reexposed to the cephalosporin, resulting in worsening of the clinical status. The outcome is often fatal due to massive hemolysis and thrombosis.56,60,62
Finally, 8% to 36% of patients taking methyldopa will develop a positive DAT after 6 months of therapy, with less than 1% showing hemolysis.52,63 The hemolysis in these patients is indistinguishable from warm AIHA, consistent with the notion that methyldopa induces an AIHA. The hemolysis often resolves rapidly after the methyldopa is stopped, but the Coombs test may remain positive for months.63 This type of drug-induced hemolytic anemia has been reported with levodopa, procainamide, and chlorpromazine, but fludarabine is the most common cause currently. This form of AIHA is now being seen with increased use of checkpoint inhibitors.64
Diagnosis
In many patients, the first clue to the presence of drug AIHA is the finding of a positive DAT. Rarely, patients will have severe hemolysis, but in many patients the hemolytic process is mild and may be wrongly assumed to be part of the underlying illness. Finding the offending drug can be a challenge, unless a patient has recently started a new drug; in a hospitalized patient on multiple agents, identifying the problem drug is difficult. Patients recently started on “suspect drugs,” especially the most common agents cefotetan, ceftriaxone, and piperacillin, should have these agents stopped (Table 4).1,49,65 Specialty laboratories such as the Blood Center of Wisconsin or the Los Angeles Red Cross can perform in vitro studies of drug interactions that can confirm the clinical diagnosis of drug-induced AIHA.

Treatment
Therapy for patients with positive DAT without signs of hemolysis is uncertain. If the drug is essential, then the patient can be observed. If the patient has hemolysis, the drug needs to be stopped and the patient observed for signs of end-organ damage. It is doubtful that steroids or other autoimmune-directed therapy is effective. For patients with the DIC-hemolysis syndrome, there are anecdotal reports that TPE may be helpful.1
Summary
AIHA can range from an abnormal laboratory test (positive DAT and signs of hemolysis) to an acute, life-threatening illness. Treatment is guided by the laboratory work-up and evaluation of the patient’s clinical status. While rituximab is promising for many patients, the lack of robust clinical trials hinders the treatment of patients who fail standard therapies.
Autoimmune hemolytic anemia (AIHA) is mediated by antibodies, and in most cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C. AIHA caused by a drug reaction is rare, with an estimated annual incidence of 1:1,000,000 for severe drug-related AIHA.1 This article reviews the management of the more common types of AIHA, with a focus on warm, cold, and drug-induced AIHA; the evaluation and diagnosis of AIHA is reviewed in a separate article.
Warm Autoimmune Hemolytic Anemia
In AIHA, hemolysis is mediated by antibodies that bind to the surface of red blood cells. AIHA in which IgG antibodies are the offending antibodies is referred to as warm AIHA. “Warm” refers to the fact that the antibody binds best at body temperature (37°C). In warm AIHA, testing will show IgG molecules attached to the surface of the red cells, with 50% of patients also showing C3. Between 50% and 90% of AIHA cases are due to warm antibodies.2,3 The incidence of warm AIHA varies by series but is approximately 1 case per 100,000 patients per year; this form of hemolysis affects women more frequently than men.4,5
Therapeutic Options
First Line
Steroids. The goal of therapy in warm AIHA can be hard to define. However, most would agree that a hematocrit above 30% (or higher to prevent symptoms) with a minimal increase in the reticulocyte count—reflective of a significantly slowed hemolytic process—is a reasonable goal. Initial management of warm AIHA is prednisone at a standard dose of 1 mg/kg daily (Table 1).6,7 Patients should be also started on proton-pump inhibitors to prevent ulcers. It can take up to 3 weeks for patients to respond to prednisone therapy. Once the patient’s hematocrit is above 30%, the prednisone is slowly tapered. Although approximately 80% of patients will respond to steroids, only 30% can be fully tapered off steroids. For patients who can be maintained on a daily steroid dose of 10 mg or less, steroids may be the most reasonable long-term therapy. In addition, because active hemolysis leads to an increased demand for folic acid, patients with warm AIHA are often prescribed folic acid 1 mg daily to prevent megaloblastic anemia due to folic acid deficiency.

Rituximab. Increasingly, rituximab (anti-CD20) therapy is added to the initial steroids. Two clinical trials showed both increased long-term and short-term responses with the use of rituximab.8,9 An important consideration is that most patients respond gradually to rituximab over weeks, so a rapid response should not be expected. Most studies have used the traditional dosing of 375 mg/m2 weekly for 4 weeks. These responses appear to be durable, but as in immune thrombocytopenia (ITP), repeat treatment with rituximab is effective.
The major side effects of rituximab are infusion reactions, which are often worse with the first dose. These reactions can be controlled with antihistamines, steroids, and, for severe rigors, meperidine. Rarely, patients can develop neutropenia (approximately 1:500) that appears to be autoimmune in nature. Infections appear to be only minimally increased with the use of rituximab.10 One group at risk is chronic carriers of hepatitis B virus, who may experience a reactivation of the virus that can be fatal. Thus, patients being considered for rituximab need to be screened for hepatitis B virus carrier state.11 Patients receiving rituximab are at very slight risk for progressive multifocal leukoencephalopathy, which is more common in patients with cancer and in heavily immunosuppressed patients. The overall risk is unknown but is less than 1:50,000.
Second Line
Splenectomy. For patients who cannot be weaned from steroids or in whom steroid therapy fails, there is no standard therapy. Currently, the 2 main choices are splenectomy or rituximab (anti-CD20) therapy if the patient did not receive it first line. Splenectomy is the classic therapy for warm AIHA. Reported response rates in the literature range from 50% to 80%, with 50% to 60% remaining in remission.12-16 Timing of the procedure is a balance between allowing time for the steroids to work and the risk of toxicity of steroids. In a patient who has low presurgical risk and has either refractory disease or cannot be weaned from high doses of steroids, splenectomy should be done sooner. Splenectomy can be delayed or other therapy tried first in patients who require lower doses of steroids or have medical risk factors for surgery. Most splenectomies are performed via laparoscopy. The small incisions allow for quicker healing, and the laparoscopic approach provides better visualization of the abdomen to find and remove accessory spleens. When splenectomy is performed by experienced surgeons, the mortality rate is low (< 0.5%).17
The most concerning complication of splenectomy is overwhelming post-splenectomy infection (OPSI).18 In adults, the spleen appears to play a minimal role in immunity except for protecting against certain encap-sulated organisms. Splenectomized patients infected with an encapsulated organism (eg, Pneumococcus) will develop overwhelming sepsis within hours. These patients will often have disseminated intravascular coagulation and will rapidly progress to purpura fulminans. Approximately 40% to 50% of patients will die of sepsis even when the infection is detected early. The overall lifetime risk of sepsis may be as high as 1:500. The organism that most commonly causes sepsis in splenectomized patients is Streptococcus pneumoniae, reported in over 50% of cases. Neisseria meningitidis and Haemophilus influenzae have also been implicated in many cases.19 Overwhelming sepsis after dog bites has been reported due to Capnocytophaga canimorsus infections. Patients are also at increased risk of developing severe malaria and severe babesiosis.18
Patients who have undergone splenectomy need to be warned about the risk of OPSI and instructed to report to the emergency department readily if they develop a fever greater than 101°F (38.3°C) or shaking chills. Once in the emergency department, blood cultures should be obtained rapidly and the patient started on antibiotic coverage with vancomycin and ceftriaxone (or levofloxacin if allergic to beta-lactams).20 For patients in remote areas, some physicians will prescribe prophylactic antibiotics to take while they are traveling to a health care provider or even recommend a “standby” antibiotic dose to take while traveling to health care.5 This usually consists of amoxicillin or a macrolide for penicillin-allergic patients.
Patients in whom splenectomy is being planned or considered should be vaccinated for pneumococcal, meningococcal, and influenza infections (Table 2).18 If there is a plan to treat with rituximab, patients should first be vaccinated since they will not be able to mount an immune response after being treated with rituximab.

Third Line
The therapeutic options for patients who do not respond to either splenectomy or rituximab are much less certain.5,6 Although intravenous immune globulin is a standard therapy for ITP, response rates are low in warm AIHA.17 Numerous therapies have been reported in small series, but no clear approach has emerged. Options include azathioprine, cyclophosphamide, mycophenolate, cyclosporine, danazol, and alemtuzumab. Our approach has been to use mycophenolate for patients requiring high doses of steroids or transfusions. Patients who respond to lower doses of steroids may be good candidates for danazol to help wean them off steroids.
Treatment of Warm AIHA with Associated Diseases
Warm AIHA can complicate several diseases. Patients with systemic lupus erythematosus (SLE) can develop warm AIHA as part of their disease complex. The initial treatment approach is the same, but data suggest that splenectomy may not be as effective.13,17 Also, many SLE patients have complex medical conditions, making surgeries riskier. For SLE patients who are refractory or cannot be weaned from steroids, rituximab may be the better choice. Babesiosis, particularly in asplenic patients, has been associated with the development of AIHA.21,22
Of the malignances associated with AIHA, chronic lymphocytic leukemia (CLL) has the strongest association.4,23 Series show that 5% to 10% of patients with CLL will have warm AIHA. AIHA can appear concurrent with CLL or develop during the course of the disease. The introduction of purine analogs such as fludarabine led to a dramatic increase in the incidence of warm AIHA in treated patients.24 It is speculated that these powerful agents reduce the number and effectiveness of T cells that hold in check the autoantibody response, leading to warm AIHA.25 However, when these purine analogs are used in com-bination with agents such as cyclophosphamide or rituximab (with their immunosuppressive effects), the rates of warm AIHA have been lower.23
The approach to patients with CLL and warm AIHA depends on the state of their CLL.23 For patients who have low-stage CLL that does not need treatment, the standard approach to warm AIHA should be steroids, splenectomy, and rituximab.24 For patients with higher-stage CLL, the treatment for the leukemia will often provide therapy for the warm AIHA. The combination of rituximab-cyclophosphamide-dexamethasone has been reported to be effective for both the AIHA and CLL components.26 The use of ibrutinib has also been reported to be effective.27
A rare but important variant of warm AIHA is Evans syndrome.28,29 This is the combination of AIHA and ITP. Approximately 1% to 3% of AIHA cases are the Evans variant. The ITP can precede, be concurrent with, or develop after the AIHA. The diagnosis of Evans syndrome should raise concern for underlying disorders. In young adults, immunodeficiency disorders such as autoimmune lymphoproliferative disease (ALPS) need to be considered. In older patients, Evans syndrome is often associated with T cell lymphomas. The sparse literature on Evans syndrome suggests that it can be more refractory to standard therapy, with response rates to splenectomy in the 50% range.28,30 In patients with lymphoma, antineoplastic therapy is crucial. There is increasing data showing that mycophenolate or sirolimus may be effective for patients with ALPS in whom splenectomy or rituximab therapy is unsuccessful.31
Warm AIHA with IgA or IgM Antibodies
In rare patients with warm AIHA, IgA or IgM is the implicated antibody. The literature suggests that patients with IgA AIHA may have more severe hemolysis.32 Patients with IgM AIHA often have a severe course with a fatal outcome.33 In such cases, the patient’s plasma may show spontaneous hemolysis and agglutination. The DAT may not be strongly positive or may show C3 reactivity only. The clinical clues are C3 reactivity with no cold agglutinins and severe hemolysis, sometimes with an intravascular component. Treatment is the same as for warm AIHA, including the use of rituximab.34
Cold Autoimmune Hemolytic Anemia
In cold AIHA, the hemolysis is mediated by IgM antibodies directed against red cells.35 As discussed earlier, the term “cold” refers to the fact that the antibody binds maximally at temperatures below 37°C. The most efficient temperature for binding is called the “thermal amplitude,” and, in theory, the higher the thermal amplitude, the more virulent the antibody. An antibody titer can be calculated at each reaction temperature from 4°C to 37°C by serial dilutions of the patient’s serum prior to incubating with reagent red cells. Rarely, the IgM can fix complement rapidly, leading to intravascular hemolysis. In most patients, complement is fixed through C3, and the C3-coated red cells are taken up by macrophages in the mononuclear phagocyte system, primarily in the liver.3
The DAT in patients with cold AIHA will show cells coated with C3. The blood smear will often show ag-glutination of the blood, and if the blood cools before being analyzed, the agglutination will interfere with the analysis. Titers of cold agglutinin can range from 1:1000 to over 1 million. The IgM autoantibodies are most often directed against the I/i antigens on the red blood cell membrane, with 90% against I antigen.35 The I antigen specificity is typical with primary cold agglutinin disease and after Mycoplasma infection. The i antigen specificity is most typical of Epstein-Barr virus and cytomegalovirus infections in children. In young patients, cold AIHA often occurs following an infection, including viral and Mycoplasma infections, and the course is self-limited.35,36 The hemolysis usually starts 2 to 3 weeks after the illness and will last for 4 to 6 weeks. In older patients, the etiology in over 90% of cases is a B-cell lymphoproliferative disorder, usually with monoclonal kappa B-cells.37 The most common disorders are marginal zone lymphoma, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma.3
Therapeutic Options
It is important to diagnose cold AIHA because the standard therapy for warm AIHA (steroids) is ineffective in cold AIHA. Because C3-coated red cells are taken up primarily in the liver, removing the spleen is also an ineffective therapy. Simple measures to help with cold AIHA should be employed.37 Patients should be kept in a warm environment and should try to avoid the cold. If transfusions are needed, they should be infused via blood warmers to prevent hemolysis. In rare patients with severe hemolysis, therapeutic plasma exchange (TPE) can be considered.38 Given that the culprit antibody is IgM—mostly intravascular—use of TPE may slow the hemolysis to give time for other therapies to take effect.
Treatment of cold AIHA remains difficult (Table 3). Because most patients with primary AIHA have underlying lymphoproliferative diseases, chlorambucil has been used in the past to treat cold AIHA. However, responses were rare and the drug could worsen the anemia.38 Currently, the drug of choice is rituximab. Response is seen in 45% to 75% of patients, but is almost always a partial response and retreatment is often necessary.17,37,39 As with other autoimmune hematologic diseases, there can be a delay in response that ranges from 2 weeks to 4 months (median time, 1.5 months).37 Given the lack of robust response (complete and durable) with rituximab, the Berentsen group has explored adding bendamustine to rituximab.40 In a prospective trial, 71% of patients responded with a 40% complete response rate. Therapy was well tolerated, with only 29% of patients needing dose reduction Although more toxic, this combination can be considered in patients with aggressive disease. A small study of the use of bortezomib showed a good response in one-third of patients.41 There is increasing use of the C5 complement inhibitor eculizumab to halt the hemolysis, but further study of this agent is also required.36,42,43 Blockers of C1s complement, which block hemolysis by preventing complement activation, are currently being studied in clinical trials.44

Since most patients with cold AIHA are older, a frequent issue that must be considered is cardiac surgery. The concern is that the hypothermia involved with most heart bypass procedures will lead to agglutination and hemolysis. The development of normothermic bypass has expanded treatment options. A recommended approach in patients who have known cold agglutinins is to measure the thermal amplitude of the antibody preoperatively. If the thermal amplitude is above 18°C, normothermic bypass should be done, if feasible.45 If not feasible, preoperative TPE should be considered.
Paroxysmal Cold Hemoglobinuria
A unique cold AIHA is paroxysmal cold hemoglobinuria (PCH).3,46 This cold hemolytic syndrome most often occurs in children following a viral infection, but in the past it complicated any stage of syphilis.47 The mediating antibody in PCH is an IgG antibody directed against the P antigen on the red blood cell. This antibody binds best at temperatures below 37°C, fixing complement at cold temperatures, but then activates the complement cascade at body temperature.48 Because this antibody can fix complement, hemolysis can be rapid and severe, leading to extreme anemia. The DAT is often weakly positive but can be negative. The diagnostic test for PCH is the Donath–Landsteiner test. This complex test is performed by incubating 3 blood samples, 1 at 0° to 4°C, another at 37°C, and a third at 0° to 4°C and then at 37°C. The diagnosis of PCH is made if only the third tube shows hemolysis.35 PCH can persist for 1 to 3 months but is almost always self-limiting. In severe case, steroids may be of benefit.
Drug-Induced Hemolytic Anemia
AIHA caused by a drug reaction is rare, with a lower incidence than drug-related ITP. The rate of severe drug-related AIHA is estimated at 1:1,000,000, but less severe cases may be missed.1 Most patients will have a positive DAT without signs of hemolysis, but in rare cases patients will have relentless hemolysis resulting in death.
Mechanisms
Multiple mechanisms for drug-induced immune hemolysis have been proposed, including drug-absorption (hapten-induced) and immune complex mechanisms.1,49 The hapten mechanism is most often associated with the use of high-dose penicillin.50 High doses of penicillin or similar drugs such as piperacillin lead to incorporation of the drug into the red cell membrane by binding to proteins. Patients will manifest a positive DAT with IgG antibody but not complement.51 The patient’s plasma will be reactive only with penicillin-coated red cells and not with normal cells. As mentioned, very few patients will have hemolysis, and if they have hemolysis, it will resolve in a few days after discontinuation of the offending drug.52
Binding of a drug-antibody complex to the red cell membrane may cause hemolysis via the immune com-plex mechanism.53 Again, most often the patient will have just a positive DAT, but rarely patients will have life-threatening hemolysis upon exposure or reexposure to the drug. The onset of hemolysis is rapid, with signs of acute illness and intravascular hemolysis. The paradigm drug is quinine, but many other drugs have been implicated. Testing shows a positive Coombs test with anti-complement but not anti-IgG.50 This pattern is due to the effectiveness of the tertiary complex at fixing complement. The patient’s plasma reacts with red cells only when the drug is added.
A form of immune complex hemolysis associated with both disseminated intravascular coagulation (DIC) and brisk hemolysis has been recognized. Patients who receive certain second- and third-generation cephalosporins (especially cefotetan and ceftriaxone.53,54) have developed this syndrome.50,55-59 The clinical symptoms start 7 to 10 days after the drug is administered; often the patient has only received the antibiotic for surgical prophylaxis. Immune hemolysis with acute hematocrit drop, hypotension, and DIC ensues. The patients are often believed to have sepsis and are often reexposed to the cephalosporin, resulting in worsening of the clinical status. The outcome is often fatal due to massive hemolysis and thrombosis.56,60,62
Finally, 8% to 36% of patients taking methyldopa will develop a positive DAT after 6 months of therapy, with less than 1% showing hemolysis.52,63 The hemolysis in these patients is indistinguishable from warm AIHA, consistent with the notion that methyldopa induces an AIHA. The hemolysis often resolves rapidly after the methyldopa is stopped, but the Coombs test may remain positive for months.63 This type of drug-induced hemolytic anemia has been reported with levodopa, procainamide, and chlorpromazine, but fludarabine is the most common cause currently. This form of AIHA is now being seen with increased use of checkpoint inhibitors.64
Diagnosis
In many patients, the first clue to the presence of drug AIHA is the finding of a positive DAT. Rarely, patients will have severe hemolysis, but in many patients the hemolytic process is mild and may be wrongly assumed to be part of the underlying illness. Finding the offending drug can be a challenge, unless a patient has recently started a new drug; in a hospitalized patient on multiple agents, identifying the problem drug is difficult. Patients recently started on “suspect drugs,” especially the most common agents cefotetan, ceftriaxone, and piperacillin, should have these agents stopped (Table 4).1,49,65 Specialty laboratories such as the Blood Center of Wisconsin or the Los Angeles Red Cross can perform in vitro studies of drug interactions that can confirm the clinical diagnosis of drug-induced AIHA.

Treatment
Therapy for patients with positive DAT without signs of hemolysis is uncertain. If the drug is essential, then the patient can be observed. If the patient has hemolysis, the drug needs to be stopped and the patient observed for signs of end-organ damage. It is doubtful that steroids or other autoimmune-directed therapy is effective. For patients with the DIC-hemolysis syndrome, there are anecdotal reports that TPE may be helpful.1
Summary
AIHA can range from an abnormal laboratory test (positive DAT and signs of hemolysis) to an acute, life-threatening illness. Treatment is guided by the laboratory work-up and evaluation of the patient’s clinical status. While rituximab is promising for many patients, the lack of robust clinical trials hinders the treatment of patients who fail standard therapies.
1. Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143-150.
2. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
3. Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016:226-231.
4. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
5. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
6. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for re¬current venous thrombosis. Lancet. 2010;376:2032-2039.
7. Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood. 2017;129:2971-2979.
8. Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163:393-399
9. Michel M, Terriou L, Roudot-Thoraval F, et al. A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study). Am J Hematol. 2017;92:23-27.
10. Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47:187-198.
11. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297-1309.
12. Coon WW. Splenectomy in the treatment of hemolytic anemia. Arch Surg. 1985;120:625-628.
13. Akpek G, McAneny D, Weintraub L. Comparative response to splenectomy in coombs-positive autoimmune hemolytic anemia with or without associated disease. Am J Hematol. 1999;61:98-102.
14. Patel NY, Chilsen AM, Mathiason MA, et al. Outcomes and complications after splenectomy for hematologic disorders. Am J Surg. 2012;204:1014-1020.
15. Crowther M, Chan YL, Garbett IK, et al. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118:4036-4040.
16. Giudice V, Rosamilio R, Ferrara I, et al. Efficacy and safety of splenectomy in adult autoimmune hemolytic anemia. Open Med (Wars). 2016;11:374-380.
17. Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831-1838.
18. Rodeghiero F, Ruggeri M. Short- and long-term risks of splenectomy for benign haematological disorders: should we revisit the indications? Br J Haematol. 2012;158:16-29.
19. Ahmed N, Bialowas C, Kuo YH, Zawodniak L. Impact of preinjury anticoagulation in patients with traumatic brain injury. South Med J. 2009;102:476-480.
20. Morgan TL, Tomich EB. Overwhelming post-splenectomy infection (OPSI): a case report and review of the literature. J Emerg Med. 2012;43:758-763.
21. Woolley AE, Montgomery MW, Savage WJ, et al. Post-babesiosis warm autoimmune hemolytic anemia. N Engl J Med. 2017;376:939-946.
22. Shatzel JJ, Donohoe K, Chu NQ, et al. Profound autoimmune hemolysis and Evans syndrome in two asplenic patients with babesiosis. Transfusion. 2015;55:661-665.
23. Hodgson K, Ferrer G, Montserrat E, Moreno C. Chronic lymphocytic leukemia and autoimmunity: a systematic review. Haematologica. 2011;96:752-761.
24. Hamblin TJ. Autoimmune complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:230-239.
25. Tertian G, Cartron J, Bayle C, et al. Fatal intravascular au¬toimmune hemolytic anemia after fludarabine treatment for chronic lymphocytic leukemia. Hematol Cell Ther. 1996;38:359-360.
26. Rossignol J, Michallet AS, Oberic L, et al. Rituximab-cyclophosphamide-dexamethasone combination in the management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia. 2011;25:473-478.
27. Hampel PJ, Larson MC, Kabat B, et al. Autoimmune cytopenias in patients with chronic lymphocytic leukaemia treated with ibrutinib in routine clinical practice at an academic medical centre. Br J Haematol. 2018;183:421-427.
28. Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114:3167-3172.
29. Jaime-Pérez JC, Aguilar-Calderón PE, Salazar-Cavazos L, Gómez-Almaguer D. Evans syndrome: clinical perspectives, biological insights and treatment modalities. J Blood Med. 2018;9:171-184.
30. Dhingra KK, Jain D, Mandal S, et al. Evans syndrome: a study of six cases with review of literature. Hematology. 2008;13:356-360.
31. Bride KL, Vincent T, Smith-Whitley K, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood. 2016;127:17-28.
32. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell au¬toantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
33. Garratty G, Arndt P, Domen R, et al. Severe autoimmune hemolytic anemia associated with IgM warm autoantibodies directed against determinants on or associated with glycophorin A. Vox Sang. 1997;72:124-130.
34. Wakim M, Shah A, Arndt PA, et al. Successful anti-CD20 monoclonal antibody treatment of severe autoimmune hemolytic anemia due to warm reactive IgM autoantibody in a child with common variable immunodeficiency. Am J Hematol. 2004;76:152-155.
35. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
36. Berentsen S, Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107-115.
37. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
38. King KE, Ness PM. Treatment of autoimmune hemolytic anemia. Semin Hematol. 2005;42:131136.
39. Barcellini W, Zaja F, Zaninoni A, et al. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biologic studies. Blood. 2012;119:3691-3697.
40. Berentsen S, Randen U, Oksman M, et al. Bendamustine plus rituximab for chronic cold agglutinin disease: results of a Nordic prospective multicenter trial. Blood. 2017;130:537-541.
41. Rossi G, Gramegna D, Paoloni F, et al. Short course of bortezomib in anemic patients with relapsed cold agglutinin disease: a phase 2 prospective GIMEMA study. Blood. 2018;132:547-550.
42. Makishima K, Obara N, Ishitsuka K, et al. High efficacy of eculizumab treatment for fulminant hemolytic anemia in primary cold agglutinin disease. Ann Hematol. 2019;98:1031-1032.
43. Roth A, Huttmann A, Rother RP, et al. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2009;113:38853886.
44. Jäger U, D'Sa S, Schörgenhofer C, et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial. Blood. 2019;133:893-901.
45. Agarwal SK, Ghosh PK, Gupta D. Cardiac surgery and cold-reactive proteins. Ann Thorac Surg. 1995;60:1143-1150.
46. Shanbhag S, Spivak J. Paroxysmal cold hemoglobinuria. Hematol Oncol Clin North Am. 2015;29:473-478.
47. Kumar ND, Sethi S, Pandhi RK. Paroxysmal cold haemoglobinuria in syphilis patients. Genitourin Med. 1993;69:76.
48. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
49. Pierce A, Nester T. Pathology consultation on drug-induced hemolytic anemia. Am J Clin Pathol. 2011;136:7-12.
50. Garratty G. Immune cytopenia associated with antibiotics. Transfusion Med Rev. 1993;7:255-267.
51. Petz LD, Mueller-Eckhardt C. Drug-induced immune hemolytic anemia. Transfusion. 1992;32:202-204.
52. Packman CH, Leddy JP. Drug-related immune hemolytic anemia. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, eds. William’s Hematology. 5th ed. New York: McGraw-Hill; 1995:691-704.
53. Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2009;73-79.
54. Leicht HB, Weinig E, Mayer B, et al. Ceftriaxone-induced hemolytic anemia with severe renal failure: a case report and review of literature. BMC Pharmacol Toxicol. 2018;19:67.
55. Chenoweth CE, Judd WJ, Steiner EA, Kauffman CA. Cefotetan-induced immune hemolytic anemia. Clin Infect Dis. 1992;15:863-865.
56. Garratty G, Nance S, Lloyd M, Domen R. Fatal im¬mune hemolytic anemia due to cefotetan. Transfusion. 1992;32:269-271.
57. Endoh T, Yagihashi A, Sasaki M, Watanabe N. Ceftizoxime-induced hemolysis due to immune complexes: case report and determination of the epitope responsible for immune complex-mediated hemolysis. Transfusion. 1999;39:306-309.
58. Arndt PA, Leger RM, Garratty G. Serology of antibodies to second- and third-generation cephalosporins associated with immune hemolytic anemia and/or positive direct antiglobulin tests. Transfusion. 1999;39:1239-1246.
59. Martin ME, Laber DA. Cefotetan-induced hemolytic anemia after perioperative prophylaxis. Am J Hematol. 2006;81:186-188.
60. Bernini JC, Mustafa MM, Sutor LJ, Buchanan GR. Fatal hemolysis induced by ceftriaxone in a child with sickle cell anemia. J Pediatr. 1995;126(5 Pt 1):813-815.
61. Borgna-Pignatti C, Bezzi TM, Reverberi R. Fatal ceftriaxone-induced hemolysis in a child with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1995;14:1116-1117.
62. Lascari AD, Amyot K. Fatal hemolysis caused by ceftriaxone [see comments]. J Pediatr. 1995;126(5 Pt 1):816-817.
63. Petz LD. Drug-induced autoimmune hemolytic anemia. Transfusion Med Rev. 1993;7:242-254.
64. Leaf RK, Ferreri C, Rangachari D, et al. Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors. Am J Hematol. 2019;94:563-574.
65. DeLoughery T. Drug induced immune hematological disease. Allerg Immunol Clin. 1998;18:829-841.
1. Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143-150.
2. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
3. Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016:226-231.
4. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
5. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
6. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for re¬current venous thrombosis. Lancet. 2010;376:2032-2039.
7. Go RS, Winters JL, Kay NE. How I treat autoimmune hemolytic anemia. Blood. 2017;129:2971-2979.
8. Birgens H, Frederiksen H, Hasselbalch HC, et al. A phase III randomized trial comparing glucocorticoid monotherapy versus glucocorticoid and rituximab in patients with autoimmune haemolytic anaemia. Br J Haematol. 2013;163:393-399
9. Michel M, Terriou L, Roudot-Thoraval F, et al. A randomized and double-blind controlled trial evaluating the safety and efficacy of rituximab for warm auto-immune hemolytic anemia in adults (the RAIHA study). Am J Hematol. 2017;92:23-27.
10. Gea-Banacloche JC. Rituximab-associated infections. Semin Hematol. 2010;47:187-198.
11. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297-1309.
12. Coon WW. Splenectomy in the treatment of hemolytic anemia. Arch Surg. 1985;120:625-628.
13. Akpek G, McAneny D, Weintraub L. Comparative response to splenectomy in coombs-positive autoimmune hemolytic anemia with or without associated disease. Am J Hematol. 1999;61:98-102.
14. Patel NY, Chilsen AM, Mathiason MA, et al. Outcomes and complications after splenectomy for hematologic disorders. Am J Surg. 2012;204:1014-1020.
15. Crowther M, Chan YL, Garbett IK, et al. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118:4036-4040.
16. Giudice V, Rosamilio R, Ferrara I, et al. Efficacy and safety of splenectomy in adult autoimmune hemolytic anemia. Open Med (Wars). 2016;11:374-380.
17. Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831-1838.
18. Rodeghiero F, Ruggeri M. Short- and long-term risks of splenectomy for benign haematological disorders: should we revisit the indications? Br J Haematol. 2012;158:16-29.
19. Ahmed N, Bialowas C, Kuo YH, Zawodniak L. Impact of preinjury anticoagulation in patients with traumatic brain injury. South Med J. 2009;102:476-480.
20. Morgan TL, Tomich EB. Overwhelming post-splenectomy infection (OPSI): a case report and review of the literature. J Emerg Med. 2012;43:758-763.
21. Woolley AE, Montgomery MW, Savage WJ, et al. Post-babesiosis warm autoimmune hemolytic anemia. N Engl J Med. 2017;376:939-946.
22. Shatzel JJ, Donohoe K, Chu NQ, et al. Profound autoimmune hemolysis and Evans syndrome in two asplenic patients with babesiosis. Transfusion. 2015;55:661-665.
23. Hodgson K, Ferrer G, Montserrat E, Moreno C. Chronic lymphocytic leukemia and autoimmunity: a systematic review. Haematologica. 2011;96:752-761.
24. Hamblin TJ. Autoimmune complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:230-239.
25. Tertian G, Cartron J, Bayle C, et al. Fatal intravascular au¬toimmune hemolytic anemia after fludarabine treatment for chronic lymphocytic leukemia. Hematol Cell Ther. 1996;38:359-360.
26. Rossignol J, Michallet AS, Oberic L, et al. Rituximab-cyclophosphamide-dexamethasone combination in the management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia. 2011;25:473-478.
27. Hampel PJ, Larson MC, Kabat B, et al. Autoimmune cytopenias in patients with chronic lymphocytic leukaemia treated with ibrutinib in routine clinical practice at an academic medical centre. Br J Haematol. 2018;183:421-427.
28. Michel M, Chanet V, Dechartres A, et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood. 2009;114:3167-3172.
29. Jaime-Pérez JC, Aguilar-Calderón PE, Salazar-Cavazos L, Gómez-Almaguer D. Evans syndrome: clinical perspectives, biological insights and treatment modalities. J Blood Med. 2018;9:171-184.
30. Dhingra KK, Jain D, Mandal S, et al. Evans syndrome: a study of six cases with review of literature. Hematology. 2008;13:356-360.
31. Bride KL, Vincent T, Smith-Whitley K, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood. 2016;127:17-28.
32. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell au¬toantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
33. Garratty G, Arndt P, Domen R, et al. Severe autoimmune hemolytic anemia associated with IgM warm autoantibodies directed against determinants on or associated with glycophorin A. Vox Sang. 1997;72:124-130.
34. Wakim M, Shah A, Arndt PA, et al. Successful anti-CD20 monoclonal antibody treatment of severe autoimmune hemolytic anemia due to warm reactive IgM autoantibody in a child with common variable immunodeficiency. Am J Hematol. 2004;76:152-155.
35. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
36. Berentsen S, Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107-115.
37. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
38. King KE, Ness PM. Treatment of autoimmune hemolytic anemia. Semin Hematol. 2005;42:131136.
39. Barcellini W, Zaja F, Zaninoni A, et al. Low-dose rituximab in adult patients with idiopathic autoimmune hemolytic anemia: clinical efficacy and biologic studies. Blood. 2012;119:3691-3697.
40. Berentsen S, Randen U, Oksman M, et al. Bendamustine plus rituximab for chronic cold agglutinin disease: results of a Nordic prospective multicenter trial. Blood. 2017;130:537-541.
41. Rossi G, Gramegna D, Paoloni F, et al. Short course of bortezomib in anemic patients with relapsed cold agglutinin disease: a phase 2 prospective GIMEMA study. Blood. 2018;132:547-550.
42. Makishima K, Obara N, Ishitsuka K, et al. High efficacy of eculizumab treatment for fulminant hemolytic anemia in primary cold agglutinin disease. Ann Hematol. 2019;98:1031-1032.
43. Roth A, Huttmann A, Rother RP, et al. Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease. Blood. 2009;113:38853886.
44. Jäger U, D'Sa S, Schörgenhofer C, et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial. Blood. 2019;133:893-901.
45. Agarwal SK, Ghosh PK, Gupta D. Cardiac surgery and cold-reactive proteins. Ann Thorac Surg. 1995;60:1143-1150.
46. Shanbhag S, Spivak J. Paroxysmal cold hemoglobinuria. Hematol Oncol Clin North Am. 2015;29:473-478.
47. Kumar ND, Sethi S, Pandhi RK. Paroxysmal cold haemoglobinuria in syphilis patients. Genitourin Med. 1993;69:76.
48. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
49. Pierce A, Nester T. Pathology consultation on drug-induced hemolytic anemia. Am J Clin Pathol. 2011;136:7-12.
50. Garratty G. Immune cytopenia associated with antibiotics. Transfusion Med Rev. 1993;7:255-267.
51. Petz LD, Mueller-Eckhardt C. Drug-induced immune hemolytic anemia. Transfusion. 1992;32:202-204.
52. Packman CH, Leddy JP. Drug-related immune hemolytic anemia. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, eds. William’s Hematology. 5th ed. New York: McGraw-Hill; 1995:691-704.
53. Garratty G. Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program. 2009;73-79.
54. Leicht HB, Weinig E, Mayer B, et al. Ceftriaxone-induced hemolytic anemia with severe renal failure: a case report and review of literature. BMC Pharmacol Toxicol. 2018;19:67.
55. Chenoweth CE, Judd WJ, Steiner EA, Kauffman CA. Cefotetan-induced immune hemolytic anemia. Clin Infect Dis. 1992;15:863-865.
56. Garratty G, Nance S, Lloyd M, Domen R. Fatal im¬mune hemolytic anemia due to cefotetan. Transfusion. 1992;32:269-271.
57. Endoh T, Yagihashi A, Sasaki M, Watanabe N. Ceftizoxime-induced hemolysis due to immune complexes: case report and determination of the epitope responsible for immune complex-mediated hemolysis. Transfusion. 1999;39:306-309.
58. Arndt PA, Leger RM, Garratty G. Serology of antibodies to second- and third-generation cephalosporins associated with immune hemolytic anemia and/or positive direct antiglobulin tests. Transfusion. 1999;39:1239-1246.
59. Martin ME, Laber DA. Cefotetan-induced hemolytic anemia after perioperative prophylaxis. Am J Hematol. 2006;81:186-188.
60. Bernini JC, Mustafa MM, Sutor LJ, Buchanan GR. Fatal hemolysis induced by ceftriaxone in a child with sickle cell anemia. J Pediatr. 1995;126(5 Pt 1):813-815.
61. Borgna-Pignatti C, Bezzi TM, Reverberi R. Fatal ceftriaxone-induced hemolysis in a child with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1995;14:1116-1117.
62. Lascari AD, Amyot K. Fatal hemolysis caused by ceftriaxone [see comments]. J Pediatr. 1995;126(5 Pt 1):816-817.
63. Petz LD. Drug-induced autoimmune hemolytic anemia. Transfusion Med Rev. 1993;7:242-254.
64. Leaf RK, Ferreri C, Rangachari D, et al. Clinical and laboratory features of autoimmune hemolytic anemia associated with immune checkpoint inhibitors. Am J Hematol. 2019;94:563-574.
65. DeLoughery T. Drug induced immune hematological disease. Allerg Immunol Clin. 1998;18:829-841.
Autoimmune Hemolytic Anemia: Evaluation and Diagnosis
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons.1,2 AIHA is mediated by antibodies, and in the majority of cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C (Table 1). This article series reviews the most common types of AIHA, with an overview of evaluation and diagnosis presented in this article and management of warm, cold, and drug-induced AIHA reviewed in a separate article.
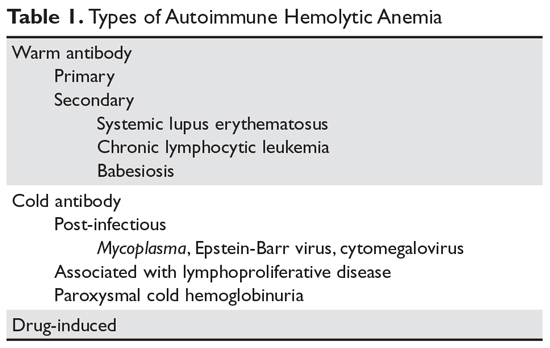
Pathogenesis
In most cases, the ultimate etiology of AIHA is unknown. In warm AIHA, the target epitopes in most cases are Rh proteins.2 What leads the immune system to target these proteins is unidentified, but one theory is that an initial immune response to a foreign antigen starts to cross-react with the Rh proteins and the immune system fails to suppress this autoreactive response, leading to hemolysis. In IgG-mediated (warm) hemolysis, the red cells become coated with IgG molecules, which mark the cells for uptake and destruction by splenic macrophages.3 In "cold" AIHA, IgM molecules fix complement to the surface of red blood cells. Rarely, this can lead to activation of the full complement cascade, resulting in red cell lysis, but more often it is stopped at the C3 stage, leading to C3-coated red cells which are then taken up by hepatic macrophages.4
Suspecting the Diagnosis
In many patients, it is the symptoms and signs of anemia that lead to suspicion of hemolysis. Older patients often present earlier in the course of the disease due to lack of tolerance of anemia, especially if there is a sudden drop in the red blood cell count. Dark, cola-colored urine resulting from the presence of free hemoglobin may be noted by some patients. Patients with rapid-onset hemolysis may note lumbar back pain, and those with cold agglutinins often note symptoms related to agglutination of red cells in the peripheral circulation, such as the development of acrocyanosis in cold weather.5 In rare cases, patients will have abdominal pain when eating cold food due to ischemia related to agglutination of red cells in the viscera. Some patients with cold agglutinins can have an exacerbation of their hemolysis with cold exposure.
Unlike patients with immune thrombocytopenia, those with AIHA may have mild splenomegaly on exam. The presence of enlarged lymph nodes or massive splenomegaly should raise concern about concomitant lymphoma or chronic lymphocytic leukemia.
Making the Diagnosis
The 2 key steps in diagnosis are (1) demonstrating hemolysis and (2) demonstrating the autoimmune component.
Laboratory Evaluation for Hemolysis
Hemolysis is proven by finding evidence of both red cell breakdown and the compensatory increase in red cell production this stimulates (Table 2). The following sections discuss the laboratory tests that are performed to investigate hemolysis.
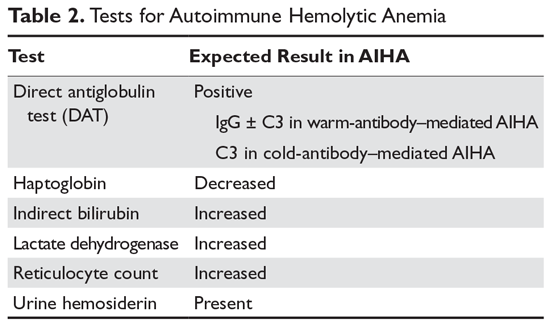
Lactate Dehydrogenase
When red cells undergo hemolysis, they release their contents, which are mostly comprised of hemoglobin but also include lactate dehydrogenase (LDH), an enzyme found in high concentration in red cells. Most patients with hemolysis will have an elevated LDH level, making this a sensitive test. However, because many other processes, including liver disease and pneumonia, also raise the serum LDH level, this finding is not specific for hemolysis.
Serum Bilirubin
Hemoglobin is salvaged by haptoglobin, and the heme moiety is broken down first to bilirubin and then to urobilinogen, which is excreted in the urine.2 Bilirubin produced from the breakdown of heme is not conjugated, but rather is delivered to the liver, where it is conjugated and excreted into the bile. In hemolysis, the concentration of unconjugated bilirubin (indirect bilirubin) is increased, while in liver disease the level of conjugated bilirubin (direct bilirubin) is increased. However, if the patient has concomitant liver disease with an increased direct bilirubin level, the serum bilirubin test is not reliable.
Serum Haptoglobin
Haptoglobin binds free serum hemoglobin and is taken up by the liver. Haptoglobin usually falls to very low levels in hemolysis. A confounder is that haptoglobin is an acute phase reactant and can rise with systemic disease or inflammation. However, patients with advanced liver disease will have low haptoglobin levels due to lack of synthesis, and up to 2% of the population may congenitally lack haptoglobin.1
Serum Hemoglobin
If the hemolysis is very rapid, the amount of free hemoglobin released will overwhelm the binding capacity of haptoglobin and lead to free hemoglobin in the plasma. This can be crudely quantified by examining the plasma color. Even minute amounts of free hemoglobin will turn the plasma pink. In fulminant hemolysis, the plasma will be cola-colored.
Reticulocyte Count
In most patients with hemolysis, the destruction of red cells is accompanied by an increase in the reticulocyte count. Reticulocytes are red cells that still contain RNA and are a marker of red cells that are approximately 24 hours old or less. Traditionally, reticulocytes were measured manually by staining the blood smear with vital blue and counting the percentage of cells that absorb the stain; this percentage needs to be adjusted for the hematocrit. Usually a percentage above 1.5% is considered indicative of an elevated reticulocyte count. Recently, automated complete blood count machines have taken advantage of the fact that reticulocytes will absorb certain stains; these machines can directly measure the reticulocyte count via flow cytometry, which results in an “absolute” reticulocyte count. The reticulocyte count obtained using this method does not have to be corrected for hematocrit, and levels of approximately 90,000/μL are considered raised. However, the reticulocyte count can also be raised in blood loss or in patients who have other causes of anemia (eg, iron deficiency) under treatment. In addition, as many as 25% of patients with AIHA will never have raised counts for various reasons, such as nutritional deficiency, autoimmune destruction of red cell precursors, or lack of erythropoietin.
Blood Smear
The blood smear provides vital information. The hallmark laboratory parameter of AIHA is spherocytes seen on the blood smear. In AIHA, antibodies and/or complement attach to the red cells, and when the antibodies or complement are taken up by macrophages in the spleen some of the red blood cell mem-brane is removed as well, decreasing the surface area of the cell. As the surface area of the red cell decreases with each pass through the spleen, the cell's shape changes from a biconcave disk to a sphere before the cell is destroyed, reflecting the fact that a sphere has the smallest surface area for a given volume. The vast majority of patients with AIHA will have spherocytes on the blood smear. However, spherocytes are not specific to AIHA, as they can be seen in hereditary spherocytosis, Wilson’s disease, clostridial sepsis, and severe burns.
Patients with cold agglutinins will often have red cell agglutination on the blood smear. In addition, patients with AIHA will often have a raised mean corpuscular volume (MCV) for 2 reasons. In patients with brisk reticulocytosis, the MCV will be raised due to the large size of the reticulocyte. In patients with cold agglutinin disease, the MCV may be falsely raised due to clumping of the red blood cells.
Urinary Hemosiderin
When hemoglobin is excreted by the kidney, the iron is deposited in the tubules. When the tubule cells are sloughed off, they appear in the urine. The urine can be stained for iron, and a positive result is another sign of hemolysis. Hemosiderinuria is a later sign of hemolysis, as it takes 1 week for iron-laden tubule cells to be excreted in sufficient quantities to be detected in the urine.
Urinary Hemoglobin
One other sign of hemolysis is the presence of hemoglobin in the urine. A quick way to demonstrate hemoglobinuria is to check the urine with a dipstick followed by a microscopic exam. In hemolysis, the dipstick will detect “blood,” while the microscopic exam will be negative for red cells.
Laboratory Evaluation for Autoimmune Component
The autoimmune component is shown by demonstrating the presence of either IgG molecules or complement on the surface of red blood cells.4,6 This can be done by performing the direct antiglobulin test (DAT) or Coombs test. IgG bound to red cells will not agglutinate them, but if IgM that is directed against IgG or C3 is added, the red cells will agglutinate, proving that there is IgG and/or C3 on the red cell membrane. The finding of a positive DAT in the setting of a hemolytic anemia is diagnostic of AIHA. Beware of individuals with concomitant weak positive DAT and other causes of hemolysis. The strength of the DAT result and the degree of hemolysis must match in order to conclude that the hemolysis is immune-mediated.
There are several pitfalls to the DAT. One is that a positive DAT is found in 1:1000 patients in the normal population and in up to several percent of ill patients, especially those with elevated gamma globulin, such as patients with liver disease or HIV infection.6 Administration of intravenous immunoglobulin (IVIG) can also create a positive DAT. Conversely, patients can have AIHA with a negative DAT.7-9 For some patients, the number of IgG molecules bound to the red cell is below the detection limit of the DAT reagents. Other patients can have IgA or “warm” IgM as the cause of the AIHA.10 Specialty laboratories can test for these possibilities. The diagnosis of DAT-negative AIHA should be made with caution, and other causes of hemolysis, such as hereditary spherocytosis or paroxysmal hematuria, should be excluded.
Transfusion Therapy
Performing transfusions can be very difficult in patients with AIHA.2 The presence of the autoantibody can interfere with typing of the blood and almost always interferes with the crossmatch, since this final step consists of mixing the patient’s serum or plasma with donor red cells. In most patients with AIHA, the autoantibodies will react with any donor cells, rendering a negative crossmatch impossible. Without the crossmatch, the concern is that underlying alloantibodies can be missed. Studies indicate that 15% to 32% of patients will have underlying alloantibodies, which can lead to transfusion reactions.2 However, there are 2 considerations that may mitigate these concerns.11,12 First, patients who have never been transfused or pregnant will rarely have alloantibodies. Second, a patient who has been transfused in the remote past may have an anamnestic antibody response but not an immediate hemolytic reaction.
The transfusion service can take several steps to identify alloantibodies. Occasionally, if the autoantibody is weakly reacting when the patient’s serum is tested against a panel of reagent red cells, the alloantibodies can be identified by their stronger reactions as compared with the weakly reactive autoantibody. The most common technique for identifying alloantibodies is the autoadsorption technique.4,13 This involves incu-bating the patient’s red cells with the patient’s serum to adsorb the autoantibody. After a period of incubation, the cells are pelleted and the serum is collected as the supernatant. The adsorbed serum may be incubated with another sample of the patient’s cells for a second adsorption if the initial agglutination reactions of the patient’s serum with the reagent cells were strong. After 1 to 3 adsorptions, the adsorbed serum is tested with a red cell panel in order to check for “leftover” alloantibodies.
When a patient is first suspected of having AIHA, a generous sample of blood should be given to the transfusion service to allow for adequate testing. Many centers will test the blood not only for blood groups ABO and D but also perform full Rh typing plus check for Kidd, Duffy and Kell status.14 Increasingly, this is performed by direct genetic sequencing for the appropriate genotypes. This can allow transfusion of phenotypically matched red blood cells to lessen the risk of alloantibody formation.
One difficult issue is timing of transfusion. Clinicians are often hesitant to transfuse patients with AIHA due to fear of reactions, but in patients with severe anemia, especially elderly patients or those with heart disease, transfusion can be lifesaving. Since in some cases it may take hours to screen for alloantibodies, it is often preferable to transfuse patients with severe anemia and observe carefully for reaction.
1. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
2. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
3. Seve P, Philippe P, Dufour JF, et al. Autoimmune hemolytic anemia: classification and therapeutic approaches. Expert Rev Hematol. 2008;1:189-204.
4. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
5. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
6. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
7. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607-618.
8. Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156-164.
9. Sachs UJ, Roder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655-656.
10. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell autoantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
11. Petz LD. “Least incompatible” units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 2003;43:1503-1507.
12. Blackall DP. How do I approach patients with warm-reactive autoantibodies? Transfusion. 2011;51:14-17.
13. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662-2668.
14. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons.1,2 AIHA is mediated by antibodies, and in the majority of cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C (Table 1). This article series reviews the most common types of AIHA, with an overview of evaluation and diagnosis presented in this article and management of warm, cold, and drug-induced AIHA reviewed in a separate article.

Pathogenesis
In most cases, the ultimate etiology of AIHA is unknown. In warm AIHA, the target epitopes in most cases are Rh proteins.2 What leads the immune system to target these proteins is unidentified, but one theory is that an initial immune response to a foreign antigen starts to cross-react with the Rh proteins and the immune system fails to suppress this autoreactive response, leading to hemolysis. In IgG-mediated (warm) hemolysis, the red cells become coated with IgG molecules, which mark the cells for uptake and destruction by splenic macrophages.3 In "cold" AIHA, IgM molecules fix complement to the surface of red blood cells. Rarely, this can lead to activation of the full complement cascade, resulting in red cell lysis, but more often it is stopped at the C3 stage, leading to C3-coated red cells which are then taken up by hepatic macrophages.4
Suspecting the Diagnosis
In many patients, it is the symptoms and signs of anemia that lead to suspicion of hemolysis. Older patients often present earlier in the course of the disease due to lack of tolerance of anemia, especially if there is a sudden drop in the red blood cell count. Dark, cola-colored urine resulting from the presence of free hemoglobin may be noted by some patients. Patients with rapid-onset hemolysis may note lumbar back pain, and those with cold agglutinins often note symptoms related to agglutination of red cells in the peripheral circulation, such as the development of acrocyanosis in cold weather.5 In rare cases, patients will have abdominal pain when eating cold food due to ischemia related to agglutination of red cells in the viscera. Some patients with cold agglutinins can have an exacerbation of their hemolysis with cold exposure.
Unlike patients with immune thrombocytopenia, those with AIHA may have mild splenomegaly on exam. The presence of enlarged lymph nodes or massive splenomegaly should raise concern about concomitant lymphoma or chronic lymphocytic leukemia.
Making the Diagnosis
The 2 key steps in diagnosis are (1) demonstrating hemolysis and (2) demonstrating the autoimmune component.
Laboratory Evaluation for Hemolysis
Hemolysis is proven by finding evidence of both red cell breakdown and the compensatory increase in red cell production this stimulates (Table 2). The following sections discuss the laboratory tests that are performed to investigate hemolysis.

Lactate Dehydrogenase
When red cells undergo hemolysis, they release their contents, which are mostly comprised of hemoglobin but also include lactate dehydrogenase (LDH), an enzyme found in high concentration in red cells. Most patients with hemolysis will have an elevated LDH level, making this a sensitive test. However, because many other processes, including liver disease and pneumonia, also raise the serum LDH level, this finding is not specific for hemolysis.
Serum Bilirubin
Hemoglobin is salvaged by haptoglobin, and the heme moiety is broken down first to bilirubin and then to urobilinogen, which is excreted in the urine.2 Bilirubin produced from the breakdown of heme is not conjugated, but rather is delivered to the liver, where it is conjugated and excreted into the bile. In hemolysis, the concentration of unconjugated bilirubin (indirect bilirubin) is increased, while in liver disease the level of conjugated bilirubin (direct bilirubin) is increased. However, if the patient has concomitant liver disease with an increased direct bilirubin level, the serum bilirubin test is not reliable.
Serum Haptoglobin
Haptoglobin binds free serum hemoglobin and is taken up by the liver. Haptoglobin usually falls to very low levels in hemolysis. A confounder is that haptoglobin is an acute phase reactant and can rise with systemic disease or inflammation. However, patients with advanced liver disease will have low haptoglobin levels due to lack of synthesis, and up to 2% of the population may congenitally lack haptoglobin.1
Serum Hemoglobin
If the hemolysis is very rapid, the amount of free hemoglobin released will overwhelm the binding capacity of haptoglobin and lead to free hemoglobin in the plasma. This can be crudely quantified by examining the plasma color. Even minute amounts of free hemoglobin will turn the plasma pink. In fulminant hemolysis, the plasma will be cola-colored.
Reticulocyte Count
In most patients with hemolysis, the destruction of red cells is accompanied by an increase in the reticulocyte count. Reticulocytes are red cells that still contain RNA and are a marker of red cells that are approximately 24 hours old or less. Traditionally, reticulocytes were measured manually by staining the blood smear with vital blue and counting the percentage of cells that absorb the stain; this percentage needs to be adjusted for the hematocrit. Usually a percentage above 1.5% is considered indicative of an elevated reticulocyte count. Recently, automated complete blood count machines have taken advantage of the fact that reticulocytes will absorb certain stains; these machines can directly measure the reticulocyte count via flow cytometry, which results in an “absolute” reticulocyte count. The reticulocyte count obtained using this method does not have to be corrected for hematocrit, and levels of approximately 90,000/μL are considered raised. However, the reticulocyte count can also be raised in blood loss or in patients who have other causes of anemia (eg, iron deficiency) under treatment. In addition, as many as 25% of patients with AIHA will never have raised counts for various reasons, such as nutritional deficiency, autoimmune destruction of red cell precursors, or lack of erythropoietin.
Blood Smear
The blood smear provides vital information. The hallmark laboratory parameter of AIHA is spherocytes seen on the blood smear. In AIHA, antibodies and/or complement attach to the red cells, and when the antibodies or complement are taken up by macrophages in the spleen some of the red blood cell mem-brane is removed as well, decreasing the surface area of the cell. As the surface area of the red cell decreases with each pass through the spleen, the cell's shape changes from a biconcave disk to a sphere before the cell is destroyed, reflecting the fact that a sphere has the smallest surface area for a given volume. The vast majority of patients with AIHA will have spherocytes on the blood smear. However, spherocytes are not specific to AIHA, as they can be seen in hereditary spherocytosis, Wilson’s disease, clostridial sepsis, and severe burns.
Patients with cold agglutinins will often have red cell agglutination on the blood smear. In addition, patients with AIHA will often have a raised mean corpuscular volume (MCV) for 2 reasons. In patients with brisk reticulocytosis, the MCV will be raised due to the large size of the reticulocyte. In patients with cold agglutinin disease, the MCV may be falsely raised due to clumping of the red blood cells.
Urinary Hemosiderin
When hemoglobin is excreted by the kidney, the iron is deposited in the tubules. When the tubule cells are sloughed off, they appear in the urine. The urine can be stained for iron, and a positive result is another sign of hemolysis. Hemosiderinuria is a later sign of hemolysis, as it takes 1 week for iron-laden tubule cells to be excreted in sufficient quantities to be detected in the urine.
Urinary Hemoglobin
One other sign of hemolysis is the presence of hemoglobin in the urine. A quick way to demonstrate hemoglobinuria is to check the urine with a dipstick followed by a microscopic exam. In hemolysis, the dipstick will detect “blood,” while the microscopic exam will be negative for red cells.
Laboratory Evaluation for Autoimmune Component
The autoimmune component is shown by demonstrating the presence of either IgG molecules or complement on the surface of red blood cells.4,6 This can be done by performing the direct antiglobulin test (DAT) or Coombs test. IgG bound to red cells will not agglutinate them, but if IgM that is directed against IgG or C3 is added, the red cells will agglutinate, proving that there is IgG and/or C3 on the red cell membrane. The finding of a positive DAT in the setting of a hemolytic anemia is diagnostic of AIHA. Beware of individuals with concomitant weak positive DAT and other causes of hemolysis. The strength of the DAT result and the degree of hemolysis must match in order to conclude that the hemolysis is immune-mediated.
There are several pitfalls to the DAT. One is that a positive DAT is found in 1:1000 patients in the normal population and in up to several percent of ill patients, especially those with elevated gamma globulin, such as patients with liver disease or HIV infection.6 Administration of intravenous immunoglobulin (IVIG) can also create a positive DAT. Conversely, patients can have AIHA with a negative DAT.7-9 For some patients, the number of IgG molecules bound to the red cell is below the detection limit of the DAT reagents. Other patients can have IgA or “warm” IgM as the cause of the AIHA.10 Specialty laboratories can test for these possibilities. The diagnosis of DAT-negative AIHA should be made with caution, and other causes of hemolysis, such as hereditary spherocytosis or paroxysmal hematuria, should be excluded.
Transfusion Therapy
Performing transfusions can be very difficult in patients with AIHA.2 The presence of the autoantibody can interfere with typing of the blood and almost always interferes with the crossmatch, since this final step consists of mixing the patient’s serum or plasma with donor red cells. In most patients with AIHA, the autoantibodies will react with any donor cells, rendering a negative crossmatch impossible. Without the crossmatch, the concern is that underlying alloantibodies can be missed. Studies indicate that 15% to 32% of patients will have underlying alloantibodies, which can lead to transfusion reactions.2 However, there are 2 considerations that may mitigate these concerns.11,12 First, patients who have never been transfused or pregnant will rarely have alloantibodies. Second, a patient who has been transfused in the remote past may have an anamnestic antibody response but not an immediate hemolytic reaction.
The transfusion service can take several steps to identify alloantibodies. Occasionally, if the autoantibody is weakly reacting when the patient’s serum is tested against a panel of reagent red cells, the alloantibodies can be identified by their stronger reactions as compared with the weakly reactive autoantibody. The most common technique for identifying alloantibodies is the autoadsorption technique.4,13 This involves incu-bating the patient’s red cells with the patient’s serum to adsorb the autoantibody. After a period of incubation, the cells are pelleted and the serum is collected as the supernatant. The adsorbed serum may be incubated with another sample of the patient’s cells for a second adsorption if the initial agglutination reactions of the patient’s serum with the reagent cells were strong. After 1 to 3 adsorptions, the adsorbed serum is tested with a red cell panel in order to check for “leftover” alloantibodies.
When a patient is first suspected of having AIHA, a generous sample of blood should be given to the transfusion service to allow for adequate testing. Many centers will test the blood not only for blood groups ABO and D but also perform full Rh typing plus check for Kidd, Duffy and Kell status.14 Increasingly, this is performed by direct genetic sequencing for the appropriate genotypes. This can allow transfusion of phenotypically matched red blood cells to lessen the risk of alloantibody formation.
One difficult issue is timing of transfusion. Clinicians are often hesitant to transfuse patients with AIHA due to fear of reactions, but in patients with severe anemia, especially elderly patients or those with heart disease, transfusion can be lifesaving. Since in some cases it may take hours to screen for alloantibodies, it is often preferable to transfuse patients with severe anemia and observe carefully for reaction.
The autoimmune hemolytic anemias (AIHA) are rare but important hematologic diseases. They can range in severity from mildly symptomatic illness to a rapidly fatal syndrome. The incidence of AIHA is estimated to be between 0.6 and 3 cases per 100,000 persons.1,2 AIHA is mediated by antibodies, and in the majority of cases immunoglobulin (Ig) G is the mediating antibody. This type of AIHA is referred to as "warm" AIHA because IgG antibodies bind best at body temperature. "Cold" AIHA is mediated by IgM antibodies, which bind maximally at temperatures below 37°C (Table 1). This article series reviews the most common types of AIHA, with an overview of evaluation and diagnosis presented in this article and management of warm, cold, and drug-induced AIHA reviewed in a separate article.

Pathogenesis
In most cases, the ultimate etiology of AIHA is unknown. In warm AIHA, the target epitopes in most cases are Rh proteins.2 What leads the immune system to target these proteins is unidentified, but one theory is that an initial immune response to a foreign antigen starts to cross-react with the Rh proteins and the immune system fails to suppress this autoreactive response, leading to hemolysis. In IgG-mediated (warm) hemolysis, the red cells become coated with IgG molecules, which mark the cells for uptake and destruction by splenic macrophages.3 In "cold" AIHA, IgM molecules fix complement to the surface of red blood cells. Rarely, this can lead to activation of the full complement cascade, resulting in red cell lysis, but more often it is stopped at the C3 stage, leading to C3-coated red cells which are then taken up by hepatic macrophages.4
Suspecting the Diagnosis
In many patients, it is the symptoms and signs of anemia that lead to suspicion of hemolysis. Older patients often present earlier in the course of the disease due to lack of tolerance of anemia, especially if there is a sudden drop in the red blood cell count. Dark, cola-colored urine resulting from the presence of free hemoglobin may be noted by some patients. Patients with rapid-onset hemolysis may note lumbar back pain, and those with cold agglutinins often note symptoms related to agglutination of red cells in the peripheral circulation, such as the development of acrocyanosis in cold weather.5 In rare cases, patients will have abdominal pain when eating cold food due to ischemia related to agglutination of red cells in the viscera. Some patients with cold agglutinins can have an exacerbation of their hemolysis with cold exposure.
Unlike patients with immune thrombocytopenia, those with AIHA may have mild splenomegaly on exam. The presence of enlarged lymph nodes or massive splenomegaly should raise concern about concomitant lymphoma or chronic lymphocytic leukemia.
Making the Diagnosis
The 2 key steps in diagnosis are (1) demonstrating hemolysis and (2) demonstrating the autoimmune component.
Laboratory Evaluation for Hemolysis
Hemolysis is proven by finding evidence of both red cell breakdown and the compensatory increase in red cell production this stimulates (Table 2). The following sections discuss the laboratory tests that are performed to investigate hemolysis.

Lactate Dehydrogenase
When red cells undergo hemolysis, they release their contents, which are mostly comprised of hemoglobin but also include lactate dehydrogenase (LDH), an enzyme found in high concentration in red cells. Most patients with hemolysis will have an elevated LDH level, making this a sensitive test. However, because many other processes, including liver disease and pneumonia, also raise the serum LDH level, this finding is not specific for hemolysis.
Serum Bilirubin
Hemoglobin is salvaged by haptoglobin, and the heme moiety is broken down first to bilirubin and then to urobilinogen, which is excreted in the urine.2 Bilirubin produced from the breakdown of heme is not conjugated, but rather is delivered to the liver, where it is conjugated and excreted into the bile. In hemolysis, the concentration of unconjugated bilirubin (indirect bilirubin) is increased, while in liver disease the level of conjugated bilirubin (direct bilirubin) is increased. However, if the patient has concomitant liver disease with an increased direct bilirubin level, the serum bilirubin test is not reliable.
Serum Haptoglobin
Haptoglobin binds free serum hemoglobin and is taken up by the liver. Haptoglobin usually falls to very low levels in hemolysis. A confounder is that haptoglobin is an acute phase reactant and can rise with systemic disease or inflammation. However, patients with advanced liver disease will have low haptoglobin levels due to lack of synthesis, and up to 2% of the population may congenitally lack haptoglobin.1
Serum Hemoglobin
If the hemolysis is very rapid, the amount of free hemoglobin released will overwhelm the binding capacity of haptoglobin and lead to free hemoglobin in the plasma. This can be crudely quantified by examining the plasma color. Even minute amounts of free hemoglobin will turn the plasma pink. In fulminant hemolysis, the plasma will be cola-colored.
Reticulocyte Count
In most patients with hemolysis, the destruction of red cells is accompanied by an increase in the reticulocyte count. Reticulocytes are red cells that still contain RNA and are a marker of red cells that are approximately 24 hours old or less. Traditionally, reticulocytes were measured manually by staining the blood smear with vital blue and counting the percentage of cells that absorb the stain; this percentage needs to be adjusted for the hematocrit. Usually a percentage above 1.5% is considered indicative of an elevated reticulocyte count. Recently, automated complete blood count machines have taken advantage of the fact that reticulocytes will absorb certain stains; these machines can directly measure the reticulocyte count via flow cytometry, which results in an “absolute” reticulocyte count. The reticulocyte count obtained using this method does not have to be corrected for hematocrit, and levels of approximately 90,000/μL are considered raised. However, the reticulocyte count can also be raised in blood loss or in patients who have other causes of anemia (eg, iron deficiency) under treatment. In addition, as many as 25% of patients with AIHA will never have raised counts for various reasons, such as nutritional deficiency, autoimmune destruction of red cell precursors, or lack of erythropoietin.
Blood Smear
The blood smear provides vital information. The hallmark laboratory parameter of AIHA is spherocytes seen on the blood smear. In AIHA, antibodies and/or complement attach to the red cells, and when the antibodies or complement are taken up by macrophages in the spleen some of the red blood cell mem-brane is removed as well, decreasing the surface area of the cell. As the surface area of the red cell decreases with each pass through the spleen, the cell's shape changes from a biconcave disk to a sphere before the cell is destroyed, reflecting the fact that a sphere has the smallest surface area for a given volume. The vast majority of patients with AIHA will have spherocytes on the blood smear. However, spherocytes are not specific to AIHA, as they can be seen in hereditary spherocytosis, Wilson’s disease, clostridial sepsis, and severe burns.
Patients with cold agglutinins will often have red cell agglutination on the blood smear. In addition, patients with AIHA will often have a raised mean corpuscular volume (MCV) for 2 reasons. In patients with brisk reticulocytosis, the MCV will be raised due to the large size of the reticulocyte. In patients with cold agglutinin disease, the MCV may be falsely raised due to clumping of the red blood cells.
Urinary Hemosiderin
When hemoglobin is excreted by the kidney, the iron is deposited in the tubules. When the tubule cells are sloughed off, they appear in the urine. The urine can be stained for iron, and a positive result is another sign of hemolysis. Hemosiderinuria is a later sign of hemolysis, as it takes 1 week for iron-laden tubule cells to be excreted in sufficient quantities to be detected in the urine.
Urinary Hemoglobin
One other sign of hemolysis is the presence of hemoglobin in the urine. A quick way to demonstrate hemoglobinuria is to check the urine with a dipstick followed by a microscopic exam. In hemolysis, the dipstick will detect “blood,” while the microscopic exam will be negative for red cells.
Laboratory Evaluation for Autoimmune Component
The autoimmune component is shown by demonstrating the presence of either IgG molecules or complement on the surface of red blood cells.4,6 This can be done by performing the direct antiglobulin test (DAT) or Coombs test. IgG bound to red cells will not agglutinate them, but if IgM that is directed against IgG or C3 is added, the red cells will agglutinate, proving that there is IgG and/or C3 on the red cell membrane. The finding of a positive DAT in the setting of a hemolytic anemia is diagnostic of AIHA. Beware of individuals with concomitant weak positive DAT and other causes of hemolysis. The strength of the DAT result and the degree of hemolysis must match in order to conclude that the hemolysis is immune-mediated.
There are several pitfalls to the DAT. One is that a positive DAT is found in 1:1000 patients in the normal population and in up to several percent of ill patients, especially those with elevated gamma globulin, such as patients with liver disease or HIV infection.6 Administration of intravenous immunoglobulin (IVIG) can also create a positive DAT. Conversely, patients can have AIHA with a negative DAT.7-9 For some patients, the number of IgG molecules bound to the red cell is below the detection limit of the DAT reagents. Other patients can have IgA or “warm” IgM as the cause of the AIHA.10 Specialty laboratories can test for these possibilities. The diagnosis of DAT-negative AIHA should be made with caution, and other causes of hemolysis, such as hereditary spherocytosis or paroxysmal hematuria, should be excluded.
Transfusion Therapy
Performing transfusions can be very difficult in patients with AIHA.2 The presence of the autoantibody can interfere with typing of the blood and almost always interferes with the crossmatch, since this final step consists of mixing the patient’s serum or plasma with donor red cells. In most patients with AIHA, the autoantibodies will react with any donor cells, rendering a negative crossmatch impossible. Without the crossmatch, the concern is that underlying alloantibodies can be missed. Studies indicate that 15% to 32% of patients will have underlying alloantibodies, which can lead to transfusion reactions.2 However, there are 2 considerations that may mitigate these concerns.11,12 First, patients who have never been transfused or pregnant will rarely have alloantibodies. Second, a patient who has been transfused in the remote past may have an anamnestic antibody response but not an immediate hemolytic reaction.
The transfusion service can take several steps to identify alloantibodies. Occasionally, if the autoantibody is weakly reacting when the patient’s serum is tested against a panel of reagent red cells, the alloantibodies can be identified by their stronger reactions as compared with the weakly reactive autoantibody. The most common technique for identifying alloantibodies is the autoadsorption technique.4,13 This involves incu-bating the patient’s red cells with the patient’s serum to adsorb the autoantibody. After a period of incubation, the cells are pelleted and the serum is collected as the supernatant. The adsorbed serum may be incubated with another sample of the patient’s cells for a second adsorption if the initial agglutination reactions of the patient’s serum with the reagent cells were strong. After 1 to 3 adsorptions, the adsorbed serum is tested with a red cell panel in order to check for “leftover” alloantibodies.
When a patient is first suspected of having AIHA, a generous sample of blood should be given to the transfusion service to allow for adequate testing. Many centers will test the blood not only for blood groups ABO and D but also perform full Rh typing plus check for Kidd, Duffy and Kell status.14 Increasingly, this is performed by direct genetic sequencing for the appropriate genotypes. This can allow transfusion of phenotypically matched red blood cells to lessen the risk of alloantibody formation.
One difficult issue is timing of transfusion. Clinicians are often hesitant to transfuse patients with AIHA due to fear of reactions, but in patients with severe anemia, especially elderly patients or those with heart disease, transfusion can be lifesaving. Since in some cases it may take hours to screen for alloantibodies, it is often preferable to transfuse patients with severe anemia and observe carefully for reaction.
1. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
2. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
3. Seve P, Philippe P, Dufour JF, et al. Autoimmune hemolytic anemia: classification and therapeutic approaches. Expert Rev Hematol. 2008;1:189-204.
4. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
5. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
6. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
7. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607-618.
8. Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156-164.
9. Sachs UJ, Roder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655-656.
10. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell autoantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
11. Petz LD. “Least incompatible” units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 2003;43:1503-1507.
12. Blackall DP. How do I approach patients with warm-reactive autoantibodies? Transfusion. 2011;51:14-17.
13. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662-2668.
14. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
1. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359.
2. Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210.
3. Seve P, Philippe P, Dufour JF, et al. Autoimmune hemolytic anemia: classification and therapeutic approaches. Expert Rev Hematol. 2008;1:189-204.
4. Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69:258-271.
5. Berentsen S. How I manage cold agglutinin disease. Br J Haematol. 2011;153:309-317.
6. Zantek ND, Koepsell SA, Tharp DR Jr, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87:707-709.
7. Michel M. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607-618.
8. Garratty G. Immune hemolytic anemia associated with negative routine serology. Semin Hematol. 2005;42:156-164.
9. Sachs UJ, Roder L, Santoso S, Bein G. Does a negative direct antiglobulin test exclude warm autoimmune haemolytic anaemia? A prospective study of 504 cases. Br J Haematol. 2006;132:655-656.
10. Sokol RJ, Booker DJ, Stamps R, et al. IgA red cell autoantibodies and autoimmune hemolysis. Transfusion. 1997;37:175-181.
11. Petz LD. “Least incompatible” units for transfusion in autoimmune hemolytic anemia: should we eliminate this meaningless term? A commentary for clinicians and transfusion medicine professionals. Transfusion. 2003;43:1503-1507.
12. Blackall DP. How do I approach patients with warm-reactive autoantibodies? Transfusion. 2011;51:14-17.
13. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662-2668.
14. Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859-1862.
Global blood supply runs low
Nearly two-thirds of countries worldwide have an insufficient supply of blood for transfusion, according to findings from a recent modeling study including 195 countries and territories.
Low- and middle-income countries are most often affected, reported lead author Nicholas Roberts of the University of Washington in Seattle and colleagues. Among all countries in Oceania, south Asia, and eastern, central, and western sub-Saharan Africa, not one had enough blood to meet estimated needs, they noted.
Shortages are attributable to a variety of challenges, including resource constraints, insufficient component production, and a high prevalence of infectious diseases, according to investigators. And existing guidelines, which call for 10-20 donations per 1,000 people in the population, may need to be revised, investigators suggested.
“These estimates are important, as they can be used to guide further investments in blood transfusion services, for analysis of current transfusion practices, and to highlight need for alternatives to transfusions such as antifibrinolytics, blood saving devices, and implementation of blood management systems,” the investigators wrote in Lancet Haematology.
Blood availability was calculated using the 2016 WHO Global Status Report on Blood Safety and Availability, in which 92% of countries participated. Blood needs were calculated using multiple databases, including the (U.S.) National Inpatient Sample datasets from 2000-2014, the State Inpatient Databases from 2003-2007, and the Global Burden of Disease 2017 study.
The global blood need was almost 305 million blood product units, while the supply totaled approximately 272 million units. These shortages, however, were not distributed equally across the globe. Out of 195 countries, 119 (61%) had an insufficient supply of blood to meet anticipated demand. Within this group, the shortage equated to about 102 million blood product units.
Denmark had the greatest supply of blood products (red blood cell products, platelets, and plasma), at 14,704 units per 100,000 population, compared with South Sudan, the least prepared to meet transfusion needs, with just 46 blood product units available per 100,000 population. This pattern was echoed across the globe; high-income countries were usually better stocked than those with low or middle income.
To meet demands, some of the most affected countries would need to raise their collection goals from single-digit figures to as high as 40 donations per 1,000 population, the investigators advised.
“Many countries face critical undersupply of transfusions, which will become more pronounced as access to care improves,” the investigators wrote.
The study was funded by the National Institutes of Health. The investigators reported having no other financial disclosures.
SOURCE: Roberts N et al. Lancet Haematol. 2019 Oct 17. doi: 10.1016/ S2352-3026(19)30200-5.
Nearly two-thirds of countries worldwide have an insufficient supply of blood for transfusion, according to findings from a recent modeling study including 195 countries and territories.
Low- and middle-income countries are most often affected, reported lead author Nicholas Roberts of the University of Washington in Seattle and colleagues. Among all countries in Oceania, south Asia, and eastern, central, and western sub-Saharan Africa, not one had enough blood to meet estimated needs, they noted.
Shortages are attributable to a variety of challenges, including resource constraints, insufficient component production, and a high prevalence of infectious diseases, according to investigators. And existing guidelines, which call for 10-20 donations per 1,000 people in the population, may need to be revised, investigators suggested.
“These estimates are important, as they can be used to guide further investments in blood transfusion services, for analysis of current transfusion practices, and to highlight need for alternatives to transfusions such as antifibrinolytics, blood saving devices, and implementation of blood management systems,” the investigators wrote in Lancet Haematology.
Blood availability was calculated using the 2016 WHO Global Status Report on Blood Safety and Availability, in which 92% of countries participated. Blood needs were calculated using multiple databases, including the (U.S.) National Inpatient Sample datasets from 2000-2014, the State Inpatient Databases from 2003-2007, and the Global Burden of Disease 2017 study.
The global blood need was almost 305 million blood product units, while the supply totaled approximately 272 million units. These shortages, however, were not distributed equally across the globe. Out of 195 countries, 119 (61%) had an insufficient supply of blood to meet anticipated demand. Within this group, the shortage equated to about 102 million blood product units.
Denmark had the greatest supply of blood products (red blood cell products, platelets, and plasma), at 14,704 units per 100,000 population, compared with South Sudan, the least prepared to meet transfusion needs, with just 46 blood product units available per 100,000 population. This pattern was echoed across the globe; high-income countries were usually better stocked than those with low or middle income.
To meet demands, some of the most affected countries would need to raise their collection goals from single-digit figures to as high as 40 donations per 1,000 population, the investigators advised.
“Many countries face critical undersupply of transfusions, which will become more pronounced as access to care improves,” the investigators wrote.
The study was funded by the National Institutes of Health. The investigators reported having no other financial disclosures.
SOURCE: Roberts N et al. Lancet Haematol. 2019 Oct 17. doi: 10.1016/ S2352-3026(19)30200-5.
Nearly two-thirds of countries worldwide have an insufficient supply of blood for transfusion, according to findings from a recent modeling study including 195 countries and territories.
Low- and middle-income countries are most often affected, reported lead author Nicholas Roberts of the University of Washington in Seattle and colleagues. Among all countries in Oceania, south Asia, and eastern, central, and western sub-Saharan Africa, not one had enough blood to meet estimated needs, they noted.
Shortages are attributable to a variety of challenges, including resource constraints, insufficient component production, and a high prevalence of infectious diseases, according to investigators. And existing guidelines, which call for 10-20 donations per 1,000 people in the population, may need to be revised, investigators suggested.
“These estimates are important, as they can be used to guide further investments in blood transfusion services, for analysis of current transfusion practices, and to highlight need for alternatives to transfusions such as antifibrinolytics, blood saving devices, and implementation of blood management systems,” the investigators wrote in Lancet Haematology.
Blood availability was calculated using the 2016 WHO Global Status Report on Blood Safety and Availability, in which 92% of countries participated. Blood needs were calculated using multiple databases, including the (U.S.) National Inpatient Sample datasets from 2000-2014, the State Inpatient Databases from 2003-2007, and the Global Burden of Disease 2017 study.
The global blood need was almost 305 million blood product units, while the supply totaled approximately 272 million units. These shortages, however, were not distributed equally across the globe. Out of 195 countries, 119 (61%) had an insufficient supply of blood to meet anticipated demand. Within this group, the shortage equated to about 102 million blood product units.
Denmark had the greatest supply of blood products (red blood cell products, platelets, and plasma), at 14,704 units per 100,000 population, compared with South Sudan, the least prepared to meet transfusion needs, with just 46 blood product units available per 100,000 population. This pattern was echoed across the globe; high-income countries were usually better stocked than those with low or middle income.
To meet demands, some of the most affected countries would need to raise their collection goals from single-digit figures to as high as 40 donations per 1,000 population, the investigators advised.
“Many countries face critical undersupply of transfusions, which will become more pronounced as access to care improves,” the investigators wrote.
The study was funded by the National Institutes of Health. The investigators reported having no other financial disclosures.
SOURCE: Roberts N et al. Lancet Haematol. 2019 Oct 17. doi: 10.1016/ S2352-3026(19)30200-5.
FROM LANCET HAEMATOLOGY
Dupilumab shrinks nasal polyps in severe chronic rhinosinusitus
MADRID – In adults with severe chronic rhinosinusitus with nasal polyps (CRSwNP), the monoclonal antibody dupilumab is effective for shrinking the polyps, improving symptoms, and reducing the need for systemic corticosteroids and surgery, according to results of two phase 3 studies reported together at the annual congress of the European Respiratory Society.
“Dupilumab improved all of the disease components, and the improvement was observed in most of them at the first assessment,” reported Jorge F. Máspero, MD, research director, Fundacion Cidea, Buenos Aires.
The data were drawn from multicenter phase 3 trials called LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52. Both included stratifications for asthma and for NSAID-exacerbated respiratory disease (ERD), which are common comorbidities. Findings of the two studies were published together just prior to Dr. Máspero’s presentation at the ERS (Lancet. 2019 Sep 26. doi: 10.1016/S0140-6736(19)31881-1).
For the coprimary end point of endoscopic nasal polyp score (NSP), the reductions were 2.06 and 1.8 at 24 weeks from baseline (both P less than .0001) in SINUS-24 and SINUS-52, respectively. For the nasal congestion or obstruction score, another primary end point, the reductions were 0.89 and 0.87, respectively (both P less than .0001).
There were also major improvements at week 24 on secondary end points, including the Lund-McKay CT score for staging of CRSwNP (P less than .0001), total symptom score (P less than .0001), the UPSIT test for smell (P less than .0001), and SNOT-22 (P less than .0001), a quality of life instrument specific for nasal and sinus diseases.
When these outcomes were graphed, curves for the dupilumab and placebo arms had already separated by 4 weeks, “and then we see the dupilumab patients keep getting better over the course of follow-up, and the effect was seen regardless of comorbidities,” said Dr. Máspero, referring to concomitant asthma or ERD.
The SINUS-24 trial randomly assigned 276 CRSwNP patients to 300 mg dupilumab or placebo, each given subcutaneously every 2 weeks. The SINUS-52 trial, which randomized 448 patients, included the same two arms plus a third arm in which patients also received 300 mg dupilumab every 2 weeks for 24 weeks and then 300 mg every month for an additional 26 weeks.
In a pooled analysis of these trials, patients randomized to dupilumab had a 78% reduction in likelihood of receiving systemic corticosteroids and a 79% reduction in being referred for surgery relative to placebo, Dr. Máspero reported.
Dupilumab, a monoclonal antibody that inhibits the activity of interleukin-4, IL-5, and IL-13, was well tolerated. Among the most common adverse events, there were lower rates of headache (9% vs. 7%), epistaxis (7% vs. 6%), and injection-site erythema (8% vs. 6%) in the dupilumab and placebo arms, respectively, but the rate of serious adverse events (6% vs. 3%) and adverse events leading to treatment discontinuation (5% vs. 3%) were only slightly higher in the active treatment group.
Both trials, which required a bilateral baseline NPS score of 5.0 for entry, recruited a population with relatively severe CRSwNP, according to Dr. Máspero. Of the 724 patients, 204 had ERD.
A restored sense of smell was one of the contributors to an improvement in quality of life.
“The sense of smell improves very quickly after starting dupilumab. Patients reported results within 2 weeks, and there was an almost complete lack of improvement in the placebo group,” Dr. Máspero reported.
Dupilumab is already indicated for the treatment of CRSwNP, but this study confirms a major effect on polyp size, sinus congestion, and symptoms irrespective of the presence of common comorbidities affecting the airways, Dr. Máspero said.
Dr. Maspero reports no potential conflicts of interest.
SOURCE: (Bachert C et al. Lancet. 2019 Sep 26. doi: 10.1016/S0140-6736(19)31881-1.
MADRID – In adults with severe chronic rhinosinusitus with nasal polyps (CRSwNP), the monoclonal antibody dupilumab is effective for shrinking the polyps, improving symptoms, and reducing the need for systemic corticosteroids and surgery, according to results of two phase 3 studies reported together at the annual congress of the European Respiratory Society.
“Dupilumab improved all of the disease components, and the improvement was observed in most of them at the first assessment,” reported Jorge F. Máspero, MD, research director, Fundacion Cidea, Buenos Aires.
The data were drawn from multicenter phase 3 trials called LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52. Both included stratifications for asthma and for NSAID-exacerbated respiratory disease (ERD), which are common comorbidities. Findings of the two studies were published together just prior to Dr. Máspero’s presentation at the ERS (Lancet. 2019 Sep 26. doi: 10.1016/S0140-6736(19)31881-1).
For the coprimary end point of endoscopic nasal polyp score (NSP), the reductions were 2.06 and 1.8 at 24 weeks from baseline (both P less than .0001) in SINUS-24 and SINUS-52, respectively. For the nasal congestion or obstruction score, another primary end point, the reductions were 0.89 and 0.87, respectively (both P less than .0001).
There were also major improvements at week 24 on secondary end points, including the Lund-McKay CT score for staging of CRSwNP (P less than .0001), total symptom score (P less than .0001), the UPSIT test for smell (P less than .0001), and SNOT-22 (P less than .0001), a quality of life instrument specific for nasal and sinus diseases.
When these outcomes were graphed, curves for the dupilumab and placebo arms had already separated by 4 weeks, “and then we see the dupilumab patients keep getting better over the course of follow-up, and the effect was seen regardless of comorbidities,” said Dr. Máspero, referring to concomitant asthma or ERD.
The SINUS-24 trial randomly assigned 276 CRSwNP patients to 300 mg dupilumab or placebo, each given subcutaneously every 2 weeks. The SINUS-52 trial, which randomized 448 patients, included the same two arms plus a third arm in which patients also received 300 mg dupilumab every 2 weeks for 24 weeks and then 300 mg every month for an additional 26 weeks.
In a pooled analysis of these trials, patients randomized to dupilumab had a 78% reduction in likelihood of receiving systemic corticosteroids and a 79% reduction in being referred for surgery relative to placebo, Dr. Máspero reported.
Dupilumab, a monoclonal antibody that inhibits the activity of interleukin-4, IL-5, and IL-13, was well tolerated. Among the most common adverse events, there were lower rates of headache (9% vs. 7%), epistaxis (7% vs. 6%), and injection-site erythema (8% vs. 6%) in the dupilumab and placebo arms, respectively, but the rate of serious adverse events (6% vs. 3%) and adverse events leading to treatment discontinuation (5% vs. 3%) were only slightly higher in the active treatment group.
Both trials, which required a bilateral baseline NPS score of 5.0 for entry, recruited a population with relatively severe CRSwNP, according to Dr. Máspero. Of the 724 patients, 204 had ERD.
A restored sense of smell was one of the contributors to an improvement in quality of life.
“The sense of smell improves very quickly after starting dupilumab. Patients reported results within 2 weeks, and there was an almost complete lack of improvement in the placebo group,” Dr. Máspero reported.
Dupilumab is already indicated for the treatment of CRSwNP, but this study confirms a major effect on polyp size, sinus congestion, and symptoms irrespective of the presence of common comorbidities affecting the airways, Dr. Máspero said.
Dr. Maspero reports no potential conflicts of interest.
SOURCE: (Bachert C et al. Lancet. 2019 Sep 26. doi: 10.1016/S0140-6736(19)31881-1.
MADRID – In adults with severe chronic rhinosinusitus with nasal polyps (CRSwNP), the monoclonal antibody dupilumab is effective for shrinking the polyps, improving symptoms, and reducing the need for systemic corticosteroids and surgery, according to results of two phase 3 studies reported together at the annual congress of the European Respiratory Society.
“Dupilumab improved all of the disease components, and the improvement was observed in most of them at the first assessment,” reported Jorge F. Máspero, MD, research director, Fundacion Cidea, Buenos Aires.
The data were drawn from multicenter phase 3 trials called LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52. Both included stratifications for asthma and for NSAID-exacerbated respiratory disease (ERD), which are common comorbidities. Findings of the two studies were published together just prior to Dr. Máspero’s presentation at the ERS (Lancet. 2019 Sep 26. doi: 10.1016/S0140-6736(19)31881-1).
For the coprimary end point of endoscopic nasal polyp score (NSP), the reductions were 2.06 and 1.8 at 24 weeks from baseline (both P less than .0001) in SINUS-24 and SINUS-52, respectively. For the nasal congestion or obstruction score, another primary end point, the reductions were 0.89 and 0.87, respectively (both P less than .0001).
There were also major improvements at week 24 on secondary end points, including the Lund-McKay CT score for staging of CRSwNP (P less than .0001), total symptom score (P less than .0001), the UPSIT test for smell (P less than .0001), and SNOT-22 (P less than .0001), a quality of life instrument specific for nasal and sinus diseases.
When these outcomes were graphed, curves for the dupilumab and placebo arms had already separated by 4 weeks, “and then we see the dupilumab patients keep getting better over the course of follow-up, and the effect was seen regardless of comorbidities,” said Dr. Máspero, referring to concomitant asthma or ERD.
The SINUS-24 trial randomly assigned 276 CRSwNP patients to 300 mg dupilumab or placebo, each given subcutaneously every 2 weeks. The SINUS-52 trial, which randomized 448 patients, included the same two arms plus a third arm in which patients also received 300 mg dupilumab every 2 weeks for 24 weeks and then 300 mg every month for an additional 26 weeks.
In a pooled analysis of these trials, patients randomized to dupilumab had a 78% reduction in likelihood of receiving systemic corticosteroids and a 79% reduction in being referred for surgery relative to placebo, Dr. Máspero reported.
Dupilumab, a monoclonal antibody that inhibits the activity of interleukin-4, IL-5, and IL-13, was well tolerated. Among the most common adverse events, there were lower rates of headache (9% vs. 7%), epistaxis (7% vs. 6%), and injection-site erythema (8% vs. 6%) in the dupilumab and placebo arms, respectively, but the rate of serious adverse events (6% vs. 3%) and adverse events leading to treatment discontinuation (5% vs. 3%) were only slightly higher in the active treatment group.
Both trials, which required a bilateral baseline NPS score of 5.0 for entry, recruited a population with relatively severe CRSwNP, according to Dr. Máspero. Of the 724 patients, 204 had ERD.
A restored sense of smell was one of the contributors to an improvement in quality of life.
“The sense of smell improves very quickly after starting dupilumab. Patients reported results within 2 weeks, and there was an almost complete lack of improvement in the placebo group,” Dr. Máspero reported.
Dupilumab is already indicated for the treatment of CRSwNP, but this study confirms a major effect on polyp size, sinus congestion, and symptoms irrespective of the presence of common comorbidities affecting the airways, Dr. Máspero said.
Dr. Maspero reports no potential conflicts of interest.
SOURCE: (Bachert C et al. Lancet. 2019 Sep 26. doi: 10.1016/S0140-6736(19)31881-1.
REPORTING FROM ERS 2019
Neurologists consider flu shot safe in most patients with autoimmune neuromuscular disorders
AUSTIN, TEX. – (CIDP), according to a survey presented at the annual meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine. They are more conservative in recommending immunization for patients with a history of Guillain-Barré syndrome, however. Temporally associated disease relapses may be a risk factor for relapse with subsequent immunization, according to the investigators.
Influenza vaccination of patients with autoimmune neuromuscular disorders such as myasthenia gravis, CIDP, or Guillain-Barré syndrome is controversial, and no clear guideline helps clinicians to decide whether vaccination for such patients is appropriate. Tess Litchman, a medical student at Yale University, New Haven, Conn., and colleagues conducted a web-based survey of neurologists throughout the United States to examine current practices for recommending influenza vaccination for patients with myasthenia gravis, CIDP, and Guillain-Barré syndrome.
The researchers received 184 survey responses, with the highest proportions of responses coming from California (8.8%), Connecticut (8.8%), and Texas (8.3%). On average, respondents had been in practice for 15.5 years. Their reported practice specialties were neuromuscular medicine in 50%, general neurology in 20%, mixed specialties in 20%, and other in 10%.
Across practice settings, neurologists followed 6,448 patients with myasthenia gravis, 2,310 patients with CIDP, and 1,907 patients with Guillain-Barré syndrome. Approximately 83% of respondents reported recommending influenza vaccination for all of their patients with myasthenia gravis, 59% reported recommending vaccination for all of their patients with CIDP, and 43% of respondents reported recommending vaccination for all of their patients with Guillain-Barré syndrome. About 2%, 8%, and 15% of respondents reported that they do not recommend influenza vaccination for any of their patients with myasthenia gravis, CIDP, and Guillain-Barré syndrome, respectively.
A temporal association between disease relapse and influenza vaccination was reported in 1.5% of patients with myasthenia gravis, 3.7% of patients with CIDP, and 8.7% of patients with Guillain-Barré syndrome. Recurrent relapses occurred in 87% (26 of 30) of patients with myasthenia gravis, 92% (23 of 25) of patients with CIDP, and 74% (26 of 35) of patients with Guillain-Barré syndrome who received another influenza vaccination.
“According to existing guidelines per the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, all patients with myasthenia gravis and CIDP should be vaccinated, and patients with Guillain-Barré syndrome who did not develop the syndrome due to a flu shot should be vaccinated,” said Richard J. Nowak, MD, director of the program in clinical and translational neuromuscular research at Yale and one of the senior investigators on the study. “This survey demonstrates that clearer guidelines and education from a professional academic neurology society is an unmet need and would be helpful to better inform the neurology community about the possible risks and benefits of immunization in myasthenia gravis, CIDP, and Guillain-Barré syndrome patients. We hope to utilize these initial results to stimulate a larger scale study, and identify whether this topic represents a knowledge gap in the community or an area in which we can improve on the best-practice standard.”
Dr. Nowak had no relevant disclosures. The study was supported by the department of neurology at Yale University; there was no external funding.
SOURCE: Litchman T et al. AANEM 2019, Abstract 16.
AUSTIN, TEX. – (CIDP), according to a survey presented at the annual meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine. They are more conservative in recommending immunization for patients with a history of Guillain-Barré syndrome, however. Temporally associated disease relapses may be a risk factor for relapse with subsequent immunization, according to the investigators.
Influenza vaccination of patients with autoimmune neuromuscular disorders such as myasthenia gravis, CIDP, or Guillain-Barré syndrome is controversial, and no clear guideline helps clinicians to decide whether vaccination for such patients is appropriate. Tess Litchman, a medical student at Yale University, New Haven, Conn., and colleagues conducted a web-based survey of neurologists throughout the United States to examine current practices for recommending influenza vaccination for patients with myasthenia gravis, CIDP, and Guillain-Barré syndrome.
The researchers received 184 survey responses, with the highest proportions of responses coming from California (8.8%), Connecticut (8.8%), and Texas (8.3%). On average, respondents had been in practice for 15.5 years. Their reported practice specialties were neuromuscular medicine in 50%, general neurology in 20%, mixed specialties in 20%, and other in 10%.
Across practice settings, neurologists followed 6,448 patients with myasthenia gravis, 2,310 patients with CIDP, and 1,907 patients with Guillain-Barré syndrome. Approximately 83% of respondents reported recommending influenza vaccination for all of their patients with myasthenia gravis, 59% reported recommending vaccination for all of their patients with CIDP, and 43% of respondents reported recommending vaccination for all of their patients with Guillain-Barré syndrome. About 2%, 8%, and 15% of respondents reported that they do not recommend influenza vaccination for any of their patients with myasthenia gravis, CIDP, and Guillain-Barré syndrome, respectively.
A temporal association between disease relapse and influenza vaccination was reported in 1.5% of patients with myasthenia gravis, 3.7% of patients with CIDP, and 8.7% of patients with Guillain-Barré syndrome. Recurrent relapses occurred in 87% (26 of 30) of patients with myasthenia gravis, 92% (23 of 25) of patients with CIDP, and 74% (26 of 35) of patients with Guillain-Barré syndrome who received another influenza vaccination.
“According to existing guidelines per the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, all patients with myasthenia gravis and CIDP should be vaccinated, and patients with Guillain-Barré syndrome who did not develop the syndrome due to a flu shot should be vaccinated,” said Richard J. Nowak, MD, director of the program in clinical and translational neuromuscular research at Yale and one of the senior investigators on the study. “This survey demonstrates that clearer guidelines and education from a professional academic neurology society is an unmet need and would be helpful to better inform the neurology community about the possible risks and benefits of immunization in myasthenia gravis, CIDP, and Guillain-Barré syndrome patients. We hope to utilize these initial results to stimulate a larger scale study, and identify whether this topic represents a knowledge gap in the community or an area in which we can improve on the best-practice standard.”
Dr. Nowak had no relevant disclosures. The study was supported by the department of neurology at Yale University; there was no external funding.
SOURCE: Litchman T et al. AANEM 2019, Abstract 16.
AUSTIN, TEX. – (CIDP), according to a survey presented at the annual meeting of the American Association of Neuromuscular and Electrodiagnostic Medicine. They are more conservative in recommending immunization for patients with a history of Guillain-Barré syndrome, however. Temporally associated disease relapses may be a risk factor for relapse with subsequent immunization, according to the investigators.
Influenza vaccination of patients with autoimmune neuromuscular disorders such as myasthenia gravis, CIDP, or Guillain-Barré syndrome is controversial, and no clear guideline helps clinicians to decide whether vaccination for such patients is appropriate. Tess Litchman, a medical student at Yale University, New Haven, Conn., and colleagues conducted a web-based survey of neurologists throughout the United States to examine current practices for recommending influenza vaccination for patients with myasthenia gravis, CIDP, and Guillain-Barré syndrome.
The researchers received 184 survey responses, with the highest proportions of responses coming from California (8.8%), Connecticut (8.8%), and Texas (8.3%). On average, respondents had been in practice for 15.5 years. Their reported practice specialties were neuromuscular medicine in 50%, general neurology in 20%, mixed specialties in 20%, and other in 10%.
Across practice settings, neurologists followed 6,448 patients with myasthenia gravis, 2,310 patients with CIDP, and 1,907 patients with Guillain-Barré syndrome. Approximately 83% of respondents reported recommending influenza vaccination for all of their patients with myasthenia gravis, 59% reported recommending vaccination for all of their patients with CIDP, and 43% of respondents reported recommending vaccination for all of their patients with Guillain-Barré syndrome. About 2%, 8%, and 15% of respondents reported that they do not recommend influenza vaccination for any of their patients with myasthenia gravis, CIDP, and Guillain-Barré syndrome, respectively.
A temporal association between disease relapse and influenza vaccination was reported in 1.5% of patients with myasthenia gravis, 3.7% of patients with CIDP, and 8.7% of patients with Guillain-Barré syndrome. Recurrent relapses occurred in 87% (26 of 30) of patients with myasthenia gravis, 92% (23 of 25) of patients with CIDP, and 74% (26 of 35) of patients with Guillain-Barré syndrome who received another influenza vaccination.
“According to existing guidelines per the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices, all patients with myasthenia gravis and CIDP should be vaccinated, and patients with Guillain-Barré syndrome who did not develop the syndrome due to a flu shot should be vaccinated,” said Richard J. Nowak, MD, director of the program in clinical and translational neuromuscular research at Yale and one of the senior investigators on the study. “This survey demonstrates that clearer guidelines and education from a professional academic neurology society is an unmet need and would be helpful to better inform the neurology community about the possible risks and benefits of immunization in myasthenia gravis, CIDP, and Guillain-Barré syndrome patients. We hope to utilize these initial results to stimulate a larger scale study, and identify whether this topic represents a knowledge gap in the community or an area in which we can improve on the best-practice standard.”
Dr. Nowak had no relevant disclosures. The study was supported by the department of neurology at Yale University; there was no external funding.
SOURCE: Litchman T et al. AANEM 2019, Abstract 16.
REPORTING FROM AANEM 2019
Survey: Most physicians who treat STIs in their offices lack key injectable drugs
The majority of physicians surveyed who treat sexually transmitted infections (STIs) in their offices reported that they did not have on-site availability of the two primary injectable drugs for syphilis and gonorrhea, according to researchers from the Centers for Disease Control and Prevention.
This lack of drug availability for immediate treatment is significant because STIs are on the rise in the United States. The numbers of reported cases of Neisseria gonorrhoeae and Treponema pallidum infections dramatically increased between 2013 and 2017, at 75% higher for gonorrhea and 153% higher for syphilis (primary and secondary), according to a research letter in the November issue of Emerging Infectious Diseases.
Optimal, same-day treatment of bacterial STIs with a highly effective regimen is critical for national STI control efforts and can help mitigate the development of drug resistance, the researchers stated. The recommended first-line treatment for uncomplicated gonorrhea is intramuscular ceftriaxone (250 mg), and for primary and secondary syphilis, it’s intramuscular penicillin G benzathine (2.5 million units), instead of using oral antimicrobial drug alternatives, which have been known to facilitate the development of drug resistance.
William S. Pearson, PhD, of the CDC and colleagues examined the on-site availability of the two injectable therapeutic agents among physicians who treated STIs in their office. They used the 2016 Physician Induction File of the National Ambulatory Medical Care Survey to assess the number of physicians who treat patients with STIs and had injectable antimicrobial drugs available on site. A total of 1,030 physicians (46.2% unweighted response rate), which represents an estimated 330,581 physicians in the United States, completed the Physician Induction File in 2016.
In this survey, physicians who reported evaluating or treating patients for STIs were asked which antimicrobial drugs they had available on site for same-day management of gonorrhea and syphilis, including intramuscular ceftriaxone and penicillin G benzathine at the recommended doses.
The researchers used this information to determine national estimates of reported on-site, same-day availability for these antimicrobial drugs and stratified results by patient-centered medical homes (PCMH) designation and U.S. region. They used multiple logistic regression models to determine if PCMH designation and region were predictive of on-site availability of the two medications.
An estimated 45.2% (149,483) of office-based physicians indicated that they evaluate patients for STIs in their offices. Of these, 77.9% reported not having penicillin G benzathine available on site, and 56.1% reported not having ceftriaxone.
Geographic differences in drug availability were not statistically significant. In addition, physicians in offices not designated PCMHs were more likely than those in offices designated as PCMHs to report lacking on-site availability of ceftriaxone (odds ratio, 2.03) and penicillin G benzathine (OR, 3.20).
“The costs of obtaining and carrying these medications, as well as issues of storage and shelf-life, should be explored to determine if these factors are barriers. In addition, the implications of prescribing alternative treatments or delaying care in situations when medications are not readily available on site should be further explored. Mitigating the lack of medication availability to treat these infections will help public health officials stop the rise in STI disease,” the researchers concluded.
The authors are all employees of the CDC and did not provide other disclosures.
SOURCE: Pearson WS et al. Emerg Infect Dis. 2019. doi: 10.3201/eid2511.190764.
The majority of physicians surveyed who treat sexually transmitted infections (STIs) in their offices reported that they did not have on-site availability of the two primary injectable drugs for syphilis and gonorrhea, according to researchers from the Centers for Disease Control and Prevention.
This lack of drug availability for immediate treatment is significant because STIs are on the rise in the United States. The numbers of reported cases of Neisseria gonorrhoeae and Treponema pallidum infections dramatically increased between 2013 and 2017, at 75% higher for gonorrhea and 153% higher for syphilis (primary and secondary), according to a research letter in the November issue of Emerging Infectious Diseases.
Optimal, same-day treatment of bacterial STIs with a highly effective regimen is critical for national STI control efforts and can help mitigate the development of drug resistance, the researchers stated. The recommended first-line treatment for uncomplicated gonorrhea is intramuscular ceftriaxone (250 mg), and for primary and secondary syphilis, it’s intramuscular penicillin G benzathine (2.5 million units), instead of using oral antimicrobial drug alternatives, which have been known to facilitate the development of drug resistance.
William S. Pearson, PhD, of the CDC and colleagues examined the on-site availability of the two injectable therapeutic agents among physicians who treated STIs in their office. They used the 2016 Physician Induction File of the National Ambulatory Medical Care Survey to assess the number of physicians who treat patients with STIs and had injectable antimicrobial drugs available on site. A total of 1,030 physicians (46.2% unweighted response rate), which represents an estimated 330,581 physicians in the United States, completed the Physician Induction File in 2016.
In this survey, physicians who reported evaluating or treating patients for STIs were asked which antimicrobial drugs they had available on site for same-day management of gonorrhea and syphilis, including intramuscular ceftriaxone and penicillin G benzathine at the recommended doses.
The researchers used this information to determine national estimates of reported on-site, same-day availability for these antimicrobial drugs and stratified results by patient-centered medical homes (PCMH) designation and U.S. region. They used multiple logistic regression models to determine if PCMH designation and region were predictive of on-site availability of the two medications.
An estimated 45.2% (149,483) of office-based physicians indicated that they evaluate patients for STIs in their offices. Of these, 77.9% reported not having penicillin G benzathine available on site, and 56.1% reported not having ceftriaxone.
Geographic differences in drug availability were not statistically significant. In addition, physicians in offices not designated PCMHs were more likely than those in offices designated as PCMHs to report lacking on-site availability of ceftriaxone (odds ratio, 2.03) and penicillin G benzathine (OR, 3.20).
“The costs of obtaining and carrying these medications, as well as issues of storage and shelf-life, should be explored to determine if these factors are barriers. In addition, the implications of prescribing alternative treatments or delaying care in situations when medications are not readily available on site should be further explored. Mitigating the lack of medication availability to treat these infections will help public health officials stop the rise in STI disease,” the researchers concluded.
The authors are all employees of the CDC and did not provide other disclosures.
SOURCE: Pearson WS et al. Emerg Infect Dis. 2019. doi: 10.3201/eid2511.190764.
The majority of physicians surveyed who treat sexually transmitted infections (STIs) in their offices reported that they did not have on-site availability of the two primary injectable drugs for syphilis and gonorrhea, according to researchers from the Centers for Disease Control and Prevention.
This lack of drug availability for immediate treatment is significant because STIs are on the rise in the United States. The numbers of reported cases of Neisseria gonorrhoeae and Treponema pallidum infections dramatically increased between 2013 and 2017, at 75% higher for gonorrhea and 153% higher for syphilis (primary and secondary), according to a research letter in the November issue of Emerging Infectious Diseases.
Optimal, same-day treatment of bacterial STIs with a highly effective regimen is critical for national STI control efforts and can help mitigate the development of drug resistance, the researchers stated. The recommended first-line treatment for uncomplicated gonorrhea is intramuscular ceftriaxone (250 mg), and for primary and secondary syphilis, it’s intramuscular penicillin G benzathine (2.5 million units), instead of using oral antimicrobial drug alternatives, which have been known to facilitate the development of drug resistance.
William S. Pearson, PhD, of the CDC and colleagues examined the on-site availability of the two injectable therapeutic agents among physicians who treated STIs in their office. They used the 2016 Physician Induction File of the National Ambulatory Medical Care Survey to assess the number of physicians who treat patients with STIs and had injectable antimicrobial drugs available on site. A total of 1,030 physicians (46.2% unweighted response rate), which represents an estimated 330,581 physicians in the United States, completed the Physician Induction File in 2016.
In this survey, physicians who reported evaluating or treating patients for STIs were asked which antimicrobial drugs they had available on site for same-day management of gonorrhea and syphilis, including intramuscular ceftriaxone and penicillin G benzathine at the recommended doses.
The researchers used this information to determine national estimates of reported on-site, same-day availability for these antimicrobial drugs and stratified results by patient-centered medical homes (PCMH) designation and U.S. region. They used multiple logistic regression models to determine if PCMH designation and region were predictive of on-site availability of the two medications.
An estimated 45.2% (149,483) of office-based physicians indicated that they evaluate patients for STIs in their offices. Of these, 77.9% reported not having penicillin G benzathine available on site, and 56.1% reported not having ceftriaxone.
Geographic differences in drug availability were not statistically significant. In addition, physicians in offices not designated PCMHs were more likely than those in offices designated as PCMHs to report lacking on-site availability of ceftriaxone (odds ratio, 2.03) and penicillin G benzathine (OR, 3.20).
“The costs of obtaining and carrying these medications, as well as issues of storage and shelf-life, should be explored to determine if these factors are barriers. In addition, the implications of prescribing alternative treatments or delaying care in situations when medications are not readily available on site should be further explored. Mitigating the lack of medication availability to treat these infections will help public health officials stop the rise in STI disease,” the researchers concluded.
The authors are all employees of the CDC and did not provide other disclosures.
SOURCE: Pearson WS et al. Emerg Infect Dis. 2019. doi: 10.3201/eid2511.190764.
FROM EMERGING INFECTIOUS DISEASES
Physician: The nicotine, not the flavors, needs regulation
While most of the recent legislative action on e-cigarettes and other electronic nicotine delivery systems are focusing on the flavors that are appealing, especially to children,
Michael B. Siegel, MD, MPH, a physician and professor at Boston University School of Public Health, said the turning point in the spike in youth e-cigarettes occurred when products like Juul, Sourin, Smok, and Phix were introduced into the market.
Prior to that, he noted that, in 2014, citing that year’s National Youth Tobacco Survey from the Centers for Disease Control and Prevention, 74% of nonsmoking youth e-cigarette users reported using e-cigarettes no more than once per week, while only 4% reported daily use. By 2018, 12% of nonsmoking youth e-cigarette users were using e-cigarettes daily, while 42% of nonsmoking e-cigarette use was no more than once a week, according to that year’s CDC survey.
“All of these brands use a different nicotine formulation from virtually all other e-cigarettes,” Dr. Siegel testified Oct. 16 at a House Energy and Commerce Health Subcommittee hearing. “They use a nicotine salt at very high concentrations.”
His written testimony notes that Juul and similar products use nicotine salt at concentrations of 50 mg/mL, whereas most other e-cigarette products have nicotine concentrations that are less than 25 mg/mL.
“The use of nicotine salts allows nicotine to be absorbed into the bloodstream much more quickly, simulating the pattern you get with a real cigarette,” he continued. “That is why so many youth are now addicted to vaping. It is not the flavors. It’s the nicotine.”
Susanne Tanski, MD, testifying on behalf of the American Academy of Pediatrics agreed with Dr. Siegel that the Food and Drug Administration needs to be doing more to regulate the amount of nicotine that these products are releasing and that the introduction of nicotine salt was a significant cause of addiction.
However, she also targeted flavors as a key issue.
“There is reasonable concern that flavors may also modify the addictiveness of e-cigarettes, but with the thousands of flavor combinations on the market, there has not been specific research yet to test this hypothesis,” Dr. Tanksi, a pediatrician at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., noted in her written testimony. “We know that flavors unto themselves are pleasurable. If you link a pleasurable flavor with a buzz of nicotine from a powerful nicotine delivery system such as the newer e-cigarettes, perhaps this is even more behaviorally and biologically reinforcing to drive the addictiveness of this new generation of products.”
Other panelists also expressed concern about the flavoring of e-cigarettes.
Subcommittee Chair Anna Eshoo (D-Calif.) noted that “flavor is very attractive. It really drives our eating habits and other habits.” She then asked whether “e-cigarettes’ sweet flavors have contributed to youth tobacco use.”
Matthew L. Myers, president of the Campaign for Tobacco-Free Kids, responded by saying that all “of the evidence is that they are the driving force of that and that it has gotten worse over the last 4 years.”
He also noted that while youth use of flavored products has spiked in the last 4 years, there really has not been any growth in adult usage of flavored tobacco.
“What it shows is the introduction of all these flavors has fueled a youth epidemic, but it has had no impact whatsoever” on adults. “Indeed, before Juul was introduced, the most popular e-cigarette flavor was tobacco. So, for smokers who want to quit, that was a viable option until Juul changed the market.”
The subcommittee hearing was held to solicit opinions on H.R. 2339, the “Reversing the Youth Tobacco Epidemic Act of 2019.” Among the provisions in the bill are raising the minimum age of purchasing all tobacco and nicotine products to age 21 years, requiring health warning labels on e-cigarette products, placing restrictions on advertising that are similar to those on smoking products, and prohibiting Internet sales of e-cigarettes.
Dr. Siegel voiced his approval for the bill, providing the provision that bans all flavored tobacco products was removed.
He noted that the current epidemic of vaping illness and deaths recently has not been caused primarily from legit e-cigarette and other vaping products, but rather from black market, THC-laced vaping cartridges.
“A ban on flavored e-cigarettes would create a public health disaster because it would create a new black market for flavored e-liquids,” he testified. “It is nearly certain that we would see more outbreaks similar to what we are seeing now with these tainted THC vape cartridges.”
He continued: “Banning flavored e-liquids is not going to do anything to curtail this respiratory disease outbreak, but it may make the outbreak worse. Why? Because the supply of e-liquids that youth are vaping is going to transition from one dominated by nicotine products to one dominated by THC products, exactly the products that are causing this outbreak.”
Dr. Siegel also spoke to the potential effect it might have on adult smokers who have abandoned combustible tobacco products in favor of e-cigarettes.
“More than 2 million adult smokers in the U.S. have quit smoking completely by switching to flavored electronic cigarettes,” he said. “If these products are banned, many of these ex-smokers will return to cigarette smoking. Most of those who don’t will turn to a new potentially dangerous black market that will be created by this legislation.”
Dr. Tanski, on the other hand, called the provision banning all flavored tobacco products, including menthol, “the single most important policy that Congress can pass to address the youth tobacco epidemic, and a step that Congress took years ago for other flavored cigarettes.”
While most of the recent legislative action on e-cigarettes and other electronic nicotine delivery systems are focusing on the flavors that are appealing, especially to children,
Michael B. Siegel, MD, MPH, a physician and professor at Boston University School of Public Health, said the turning point in the spike in youth e-cigarettes occurred when products like Juul, Sourin, Smok, and Phix were introduced into the market.
Prior to that, he noted that, in 2014, citing that year’s National Youth Tobacco Survey from the Centers for Disease Control and Prevention, 74% of nonsmoking youth e-cigarette users reported using e-cigarettes no more than once per week, while only 4% reported daily use. By 2018, 12% of nonsmoking youth e-cigarette users were using e-cigarettes daily, while 42% of nonsmoking e-cigarette use was no more than once a week, according to that year’s CDC survey.
“All of these brands use a different nicotine formulation from virtually all other e-cigarettes,” Dr. Siegel testified Oct. 16 at a House Energy and Commerce Health Subcommittee hearing. “They use a nicotine salt at very high concentrations.”
His written testimony notes that Juul and similar products use nicotine salt at concentrations of 50 mg/mL, whereas most other e-cigarette products have nicotine concentrations that are less than 25 mg/mL.
“The use of nicotine salts allows nicotine to be absorbed into the bloodstream much more quickly, simulating the pattern you get with a real cigarette,” he continued. “That is why so many youth are now addicted to vaping. It is not the flavors. It’s the nicotine.”
Susanne Tanski, MD, testifying on behalf of the American Academy of Pediatrics agreed with Dr. Siegel that the Food and Drug Administration needs to be doing more to regulate the amount of nicotine that these products are releasing and that the introduction of nicotine salt was a significant cause of addiction.
However, she also targeted flavors as a key issue.
“There is reasonable concern that flavors may also modify the addictiveness of e-cigarettes, but with the thousands of flavor combinations on the market, there has not been specific research yet to test this hypothesis,” Dr. Tanksi, a pediatrician at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., noted in her written testimony. “We know that flavors unto themselves are pleasurable. If you link a pleasurable flavor with a buzz of nicotine from a powerful nicotine delivery system such as the newer e-cigarettes, perhaps this is even more behaviorally and biologically reinforcing to drive the addictiveness of this new generation of products.”
Other panelists also expressed concern about the flavoring of e-cigarettes.
Subcommittee Chair Anna Eshoo (D-Calif.) noted that “flavor is very attractive. It really drives our eating habits and other habits.” She then asked whether “e-cigarettes’ sweet flavors have contributed to youth tobacco use.”
Matthew L. Myers, president of the Campaign for Tobacco-Free Kids, responded by saying that all “of the evidence is that they are the driving force of that and that it has gotten worse over the last 4 years.”
He also noted that while youth use of flavored products has spiked in the last 4 years, there really has not been any growth in adult usage of flavored tobacco.
“What it shows is the introduction of all these flavors has fueled a youth epidemic, but it has had no impact whatsoever” on adults. “Indeed, before Juul was introduced, the most popular e-cigarette flavor was tobacco. So, for smokers who want to quit, that was a viable option until Juul changed the market.”
The subcommittee hearing was held to solicit opinions on H.R. 2339, the “Reversing the Youth Tobacco Epidemic Act of 2019.” Among the provisions in the bill are raising the minimum age of purchasing all tobacco and nicotine products to age 21 years, requiring health warning labels on e-cigarette products, placing restrictions on advertising that are similar to those on smoking products, and prohibiting Internet sales of e-cigarettes.
Dr. Siegel voiced his approval for the bill, providing the provision that bans all flavored tobacco products was removed.
He noted that the current epidemic of vaping illness and deaths recently has not been caused primarily from legit e-cigarette and other vaping products, but rather from black market, THC-laced vaping cartridges.
“A ban on flavored e-cigarettes would create a public health disaster because it would create a new black market for flavored e-liquids,” he testified. “It is nearly certain that we would see more outbreaks similar to what we are seeing now with these tainted THC vape cartridges.”
He continued: “Banning flavored e-liquids is not going to do anything to curtail this respiratory disease outbreak, but it may make the outbreak worse. Why? Because the supply of e-liquids that youth are vaping is going to transition from one dominated by nicotine products to one dominated by THC products, exactly the products that are causing this outbreak.”
Dr. Siegel also spoke to the potential effect it might have on adult smokers who have abandoned combustible tobacco products in favor of e-cigarettes.
“More than 2 million adult smokers in the U.S. have quit smoking completely by switching to flavored electronic cigarettes,” he said. “If these products are banned, many of these ex-smokers will return to cigarette smoking. Most of those who don’t will turn to a new potentially dangerous black market that will be created by this legislation.”
Dr. Tanski, on the other hand, called the provision banning all flavored tobacco products, including menthol, “the single most important policy that Congress can pass to address the youth tobacco epidemic, and a step that Congress took years ago for other flavored cigarettes.”
While most of the recent legislative action on e-cigarettes and other electronic nicotine delivery systems are focusing on the flavors that are appealing, especially to children,
Michael B. Siegel, MD, MPH, a physician and professor at Boston University School of Public Health, said the turning point in the spike in youth e-cigarettes occurred when products like Juul, Sourin, Smok, and Phix were introduced into the market.
Prior to that, he noted that, in 2014, citing that year’s National Youth Tobacco Survey from the Centers for Disease Control and Prevention, 74% of nonsmoking youth e-cigarette users reported using e-cigarettes no more than once per week, while only 4% reported daily use. By 2018, 12% of nonsmoking youth e-cigarette users were using e-cigarettes daily, while 42% of nonsmoking e-cigarette use was no more than once a week, according to that year’s CDC survey.
“All of these brands use a different nicotine formulation from virtually all other e-cigarettes,” Dr. Siegel testified Oct. 16 at a House Energy and Commerce Health Subcommittee hearing. “They use a nicotine salt at very high concentrations.”
His written testimony notes that Juul and similar products use nicotine salt at concentrations of 50 mg/mL, whereas most other e-cigarette products have nicotine concentrations that are less than 25 mg/mL.
“The use of nicotine salts allows nicotine to be absorbed into the bloodstream much more quickly, simulating the pattern you get with a real cigarette,” he continued. “That is why so many youth are now addicted to vaping. It is not the flavors. It’s the nicotine.”
Susanne Tanski, MD, testifying on behalf of the American Academy of Pediatrics agreed with Dr. Siegel that the Food and Drug Administration needs to be doing more to regulate the amount of nicotine that these products are releasing and that the introduction of nicotine salt was a significant cause of addiction.
However, she also targeted flavors as a key issue.
“There is reasonable concern that flavors may also modify the addictiveness of e-cigarettes, but with the thousands of flavor combinations on the market, there has not been specific research yet to test this hypothesis,” Dr. Tanksi, a pediatrician at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., noted in her written testimony. “We know that flavors unto themselves are pleasurable. If you link a pleasurable flavor with a buzz of nicotine from a powerful nicotine delivery system such as the newer e-cigarettes, perhaps this is even more behaviorally and biologically reinforcing to drive the addictiveness of this new generation of products.”
Other panelists also expressed concern about the flavoring of e-cigarettes.
Subcommittee Chair Anna Eshoo (D-Calif.) noted that “flavor is very attractive. It really drives our eating habits and other habits.” She then asked whether “e-cigarettes’ sweet flavors have contributed to youth tobacco use.”
Matthew L. Myers, president of the Campaign for Tobacco-Free Kids, responded by saying that all “of the evidence is that they are the driving force of that and that it has gotten worse over the last 4 years.”
He also noted that while youth use of flavored products has spiked in the last 4 years, there really has not been any growth in adult usage of flavored tobacco.
“What it shows is the introduction of all these flavors has fueled a youth epidemic, but it has had no impact whatsoever” on adults. “Indeed, before Juul was introduced, the most popular e-cigarette flavor was tobacco. So, for smokers who want to quit, that was a viable option until Juul changed the market.”
The subcommittee hearing was held to solicit opinions on H.R. 2339, the “Reversing the Youth Tobacco Epidemic Act of 2019.” Among the provisions in the bill are raising the minimum age of purchasing all tobacco and nicotine products to age 21 years, requiring health warning labels on e-cigarette products, placing restrictions on advertising that are similar to those on smoking products, and prohibiting Internet sales of e-cigarettes.
Dr. Siegel voiced his approval for the bill, providing the provision that bans all flavored tobacco products was removed.
He noted that the current epidemic of vaping illness and deaths recently has not been caused primarily from legit e-cigarette and other vaping products, but rather from black market, THC-laced vaping cartridges.
“A ban on flavored e-cigarettes would create a public health disaster because it would create a new black market for flavored e-liquids,” he testified. “It is nearly certain that we would see more outbreaks similar to what we are seeing now with these tainted THC vape cartridges.”
He continued: “Banning flavored e-liquids is not going to do anything to curtail this respiratory disease outbreak, but it may make the outbreak worse. Why? Because the supply of e-liquids that youth are vaping is going to transition from one dominated by nicotine products to one dominated by THC products, exactly the products that are causing this outbreak.”
Dr. Siegel also spoke to the potential effect it might have on adult smokers who have abandoned combustible tobacco products in favor of e-cigarettes.
“More than 2 million adult smokers in the U.S. have quit smoking completely by switching to flavored electronic cigarettes,” he said. “If these products are banned, many of these ex-smokers will return to cigarette smoking. Most of those who don’t will turn to a new potentially dangerous black market that will be created by this legislation.”
Dr. Tanski, on the other hand, called the provision banning all flavored tobacco products, including menthol, “the single most important policy that Congress can pass to address the youth tobacco epidemic, and a step that Congress took years ago for other flavored cigarettes.”
REPORTING FROM A HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
Vaping-linked lung injuries near 1,500
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-three deaths have been confirmed.
E-cigarette–linked lung injuries, now called EVALI, occurred in all U.S. states (except Alaska), the District of Columbia, and the U.S. Virgin Islands. Seventy percent of patients are male, and 79% are under age 35 years.
Information on the substances used over the previous 3 months before symptom onset was available for 849 patients and included the following:
- 78% reported using THC-containing products, with or without nicotine-containing products;
- 31% reported exclusive use of THC-containing products;
- 58% reported using nicotine-containing products, with or without THC-containing products; and
- 10% reported exclusive use of nicotine-containing products.
CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. CDC is also validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
In a related development, JUUL, maker of e-cigarette products, has announced that it will suspend the sale of nontobacco, nonmenthol flavors (mango, creme, fruit, and cucumber) in the United States, pending review by the Food and Drug Administration. The JUUL announcement comes in advance of an expected FDA ban on flavored e-cigarettes.
The CDC continues its investigation into EVALI but stated, “Since the specific cause or causes of lung injury are not yet known, the only way to assure that you are not at risk while the investigation continues is to consider refraining from use of all e-cigarette, or vaping, products.”
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.