User login
Can a Stroke Be Caused by Cervical Manipulation?
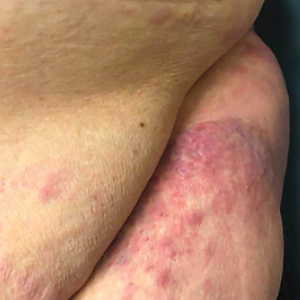
Cervical manipulations have been associated with vascular complications. While the incidence of carotid dissections does not seem to have increased, the question remains open for vertebral artery injuries. We must remain vigilant!
Resorting to joint manipulation for neck pain is not unusual. Currently, cervical manipulation remains a popular first-line treatment for cervicodynia or headaches. Although evidence exists showing that specific joint mobilization can improve this type of symptomatology, there is a possibility that it may risk damaging the cervical arteries and causing ischemic stroke through arterial dissection.
Epidemiologically, internal carotid artery dissection is a relatively rare event with an estimated annual incidence of 1.72 per 100,000 individuals (those most likely to be diagnosed being obviously those leading to hospitalization for stroke) but represents one of the most common causes of stroke in young and middle-aged adults. Faced with case reports that may raise concerns and hypotheses about an associated risk, two studies have sought to delve into the issue.
No Increased Carotid Risk Identified
The first study, of a case-cross design, identified all incident cases of ischemic stroke in the territory of the internal carotid artery admitted to the hospital over a 9-year period using administrative healthcare data, the cases being used as their own control by sampling control periods before the date of the index stroke. Thus, 15,523 cases were compared with 62,092 control periods using exposure windows of 1, 3, 7, and 14 days before the stroke. The study also compared post-medical consultation and post-chiropractic consultation outcomes, knowing that as a first-line for complaints of neck pain or headache, patients often turn to one of these two types of primary care clinicians.
However, data analysis shows, among subjects aged under 45 years, positive associations for both different consultations in cases of subsequent carotid stroke (but no association for those aged over 45 years). These associations tended to increase when analyses were limited to visits for diagnoses of neck pain and headaches. Nevertheless, there was no significant difference between risk estimates after chiropractic or general medical consultation.
A notable limitation of this work is that it did not focus on strokes due to vertebral artery dissections that run through the transverse foramina of the cervical vertebrae.
A Screening Test Lacking Precision
More recently, the International Federation of Orthopedic Manual Physical Therapists has looked into the subject to refine the assessment of the risk for vascular complications in patients seeking physiotherapy/osteopathy care for neck pain and/or headaches. Through a cross-sectional study involving 150 patients, it tested a vascular complication risk index (from high to low grade, based on history taking and clinical examination), developed to estimate the risk for the presence of vascular rather than musculoskeletal pathology, to determine whether or not there is a contraindication to cervical manipulation.
However, the developed index had only low sensitivity (0.50; 95% CI, 0.39-0.61) and moderate specificity (0.63; 95% CI, 0.51-0.75), knowing that the reference test was a consensus medical decision made by a vascular neurologist, an interventional neurologist, and a neuroradiologist (based on clinical data and cervical MRI). Similarly, positive and negative likelihood ratios were low at 1.36 (95% CI, 0.93-1.99) and 0.79 (95% CI, 0.60-1.05), respectively.
In conclusion, the data from the case-cross study did not seem to demonstrate an excess risk for stroke in the territory of the internal carotid artery after cervical joint manipulations. Associations between cervical manipulation sessions or medical consultations and carotid strokes appear similar and could have been due to the fact that patients with early symptoms related to arterial dissection seek care before developing their stroke.
However, it is regrettable that the study did not focus on vertebral artery dissections, which are anatomically more exposed to cervical chiropractic sessions. Nevertheless, because indices defined from joint tests and medical history are insufficient to identify patients “at risk or in the process of arterial dissection,” and because stroke can result in severe disability, practitioners managing patients with neck pain cannot take this type of complication lightly.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cervical manipulations have been associated with vascular complications. While the incidence of carotid dissections does not seem to have increased, the question remains open for vertebral artery injuries. We must remain vigilant!
Resorting to joint manipulation for neck pain is not unusual. Currently, cervical manipulation remains a popular first-line treatment for cervicodynia or headaches. Although evidence exists showing that specific joint mobilization can improve this type of symptomatology, there is a possibility that it may risk damaging the cervical arteries and causing ischemic stroke through arterial dissection.
Epidemiologically, internal carotid artery dissection is a relatively rare event with an estimated annual incidence of 1.72 per 100,000 individuals (those most likely to be diagnosed being obviously those leading to hospitalization for stroke) but represents one of the most common causes of stroke in young and middle-aged adults. Faced with case reports that may raise concerns and hypotheses about an associated risk, two studies have sought to delve into the issue.
No Increased Carotid Risk Identified
The first study, of a case-cross design, identified all incident cases of ischemic stroke in the territory of the internal carotid artery admitted to the hospital over a 9-year period using administrative healthcare data, the cases being used as their own control by sampling control periods before the date of the index stroke. Thus, 15,523 cases were compared with 62,092 control periods using exposure windows of 1, 3, 7, and 14 days before the stroke. The study also compared post-medical consultation and post-chiropractic consultation outcomes, knowing that as a first-line for complaints of neck pain or headache, patients often turn to one of these two types of primary care clinicians.
However, data analysis shows, among subjects aged under 45 years, positive associations for both different consultations in cases of subsequent carotid stroke (but no association for those aged over 45 years). These associations tended to increase when analyses were limited to visits for diagnoses of neck pain and headaches. Nevertheless, there was no significant difference between risk estimates after chiropractic or general medical consultation.
A notable limitation of this work is that it did not focus on strokes due to vertebral artery dissections that run through the transverse foramina of the cervical vertebrae.
A Screening Test Lacking Precision
More recently, the International Federation of Orthopedic Manual Physical Therapists has looked into the subject to refine the assessment of the risk for vascular complications in patients seeking physiotherapy/osteopathy care for neck pain and/or headaches. Through a cross-sectional study involving 150 patients, it tested a vascular complication risk index (from high to low grade, based on history taking and clinical examination), developed to estimate the risk for the presence of vascular rather than musculoskeletal pathology, to determine whether or not there is a contraindication to cervical manipulation.
However, the developed index had only low sensitivity (0.50; 95% CI, 0.39-0.61) and moderate specificity (0.63; 95% CI, 0.51-0.75), knowing that the reference test was a consensus medical decision made by a vascular neurologist, an interventional neurologist, and a neuroradiologist (based on clinical data and cervical MRI). Similarly, positive and negative likelihood ratios were low at 1.36 (95% CI, 0.93-1.99) and 0.79 (95% CI, 0.60-1.05), respectively.
In conclusion, the data from the case-cross study did not seem to demonstrate an excess risk for stroke in the territory of the internal carotid artery after cervical joint manipulations. Associations between cervical manipulation sessions or medical consultations and carotid strokes appear similar and could have been due to the fact that patients with early symptoms related to arterial dissection seek care before developing their stroke.
However, it is regrettable that the study did not focus on vertebral artery dissections, which are anatomically more exposed to cervical chiropractic sessions. Nevertheless, because indices defined from joint tests and medical history are insufficient to identify patients “at risk or in the process of arterial dissection,” and because stroke can result in severe disability, practitioners managing patients with neck pain cannot take this type of complication lightly.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cervical manipulations have been associated with vascular complications. While the incidence of carotid dissections does not seem to have increased, the question remains open for vertebral artery injuries. We must remain vigilant!
Resorting to joint manipulation for neck pain is not unusual. Currently, cervical manipulation remains a popular first-line treatment for cervicodynia or headaches. Although evidence exists showing that specific joint mobilization can improve this type of symptomatology, there is a possibility that it may risk damaging the cervical arteries and causing ischemic stroke through arterial dissection.
Epidemiologically, internal carotid artery dissection is a relatively rare event with an estimated annual incidence of 1.72 per 100,000 individuals (those most likely to be diagnosed being obviously those leading to hospitalization for stroke) but represents one of the most common causes of stroke in young and middle-aged adults. Faced with case reports that may raise concerns and hypotheses about an associated risk, two studies have sought to delve into the issue.
No Increased Carotid Risk Identified
The first study, of a case-cross design, identified all incident cases of ischemic stroke in the territory of the internal carotid artery admitted to the hospital over a 9-year period using administrative healthcare data, the cases being used as their own control by sampling control periods before the date of the index stroke. Thus, 15,523 cases were compared with 62,092 control periods using exposure windows of 1, 3, 7, and 14 days before the stroke. The study also compared post-medical consultation and post-chiropractic consultation outcomes, knowing that as a first-line for complaints of neck pain or headache, patients often turn to one of these two types of primary care clinicians.
However, data analysis shows, among subjects aged under 45 years, positive associations for both different consultations in cases of subsequent carotid stroke (but no association for those aged over 45 years). These associations tended to increase when analyses were limited to visits for diagnoses of neck pain and headaches. Nevertheless, there was no significant difference between risk estimates after chiropractic or general medical consultation.
A notable limitation of this work is that it did not focus on strokes due to vertebral artery dissections that run through the transverse foramina of the cervical vertebrae.
A Screening Test Lacking Precision
More recently, the International Federation of Orthopedic Manual Physical Therapists has looked into the subject to refine the assessment of the risk for vascular complications in patients seeking physiotherapy/osteopathy care for neck pain and/or headaches. Through a cross-sectional study involving 150 patients, it tested a vascular complication risk index (from high to low grade, based on history taking and clinical examination), developed to estimate the risk for the presence of vascular rather than musculoskeletal pathology, to determine whether or not there is a contraindication to cervical manipulation.
However, the developed index had only low sensitivity (0.50; 95% CI, 0.39-0.61) and moderate specificity (0.63; 95% CI, 0.51-0.75), knowing that the reference test was a consensus medical decision made by a vascular neurologist, an interventional neurologist, and a neuroradiologist (based on clinical data and cervical MRI). Similarly, positive and negative likelihood ratios were low at 1.36 (95% CI, 0.93-1.99) and 0.79 (95% CI, 0.60-1.05), respectively.
In conclusion, the data from the case-cross study did not seem to demonstrate an excess risk for stroke in the territory of the internal carotid artery after cervical joint manipulations. Associations between cervical manipulation sessions or medical consultations and carotid strokes appear similar and could have been due to the fact that patients with early symptoms related to arterial dissection seek care before developing their stroke.
However, it is regrettable that the study did not focus on vertebral artery dissections, which are anatomically more exposed to cervical chiropractic sessions. Nevertheless, because indices defined from joint tests and medical history are insufficient to identify patients “at risk or in the process of arterial dissection,” and because stroke can result in severe disability, practitioners managing patients with neck pain cannot take this type of complication lightly.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
QOL Not Harmed With Add-On Pembrolizumab in Cervical Cancer
according to patient-reported outcome analyses from the KEYNOTE-A18 trial.
Progression-free survival data from the trial, reported at the 2023 meeting of the European Society for Medical Oncology, showed a 30% reduction in the risk for progression (P =.002) with the addition of pembrolizumab vs placebo, as well as favorable overall survival trends, reported Leslie Randall, MD, with VCU Health in Richmond, Virginia. Now the new analyses bring the “voices of the patients from this large phase 3 study.”
“I think the data can put us at ease and give patients a measure of comfort because there was no detriment in the quality of life,” said Dr. Randall, who presented the data on March 17 at the Society of Gynecologic Oncology (SGO) 2024 conference.
The phase 3 KEYNOTE-A18 trial enrolled 1060 patients new to treatment who had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease. They were randomly allocated to receive pembrolizumab or placebo plus concurrent chemoradiotherapy.
Patient-reported outcome instruments used in the study were the European Organization for Research and Treatment of Cancer (EORTC) 30-item quality-of-life (QOL) core questionnaire (QLQ-C30), the EORTC cervical cancer–specific module (QLQ-CX24), and the EuroQol EQ-5D-5L visual analog scale (VAS).
Prespecified secondary study endpoints were changes from baseline to week 36 in QLQ-C30 global health status/QOL (GHS/QOL) and physical functioning, and the QLQ-CX24 symptom-experience scale.
At baseline, patient-reported outcome scores were similar between treatment groups. Patients in both treatment groups had an initial decrease in QLQ-C30 GHS/QOL and physical functioning subscale and EQ-5D-5L VAS scores at weeks 3 and 6, but these improved relative to baseline at week 12 and subsequent weeks.
A similar number of patients in the pembrolizumab and placebo groups had improved or stable scores for QLQ-C30 GHS/QOL (76% vs 75%), QLQ-C30 physical functioning (77% vs 77%), QLQ-CX24 symptom experience (85% vs 85%) and EQ-5D-5L VAS (73% vs 76%).
Across all QOL instruments evaluated, there were no meaningful differences in patient-reported outcomes among patients who received pembrolizumab compared with those who received placebo, said Dr. Randall.
The improvement in progression-free survival, coupled with the patient-reported outcomes showing no additional harms to quality of life, support pembrolizumab plus concurrent chemoradiotherapy as a “new standard of care” for patients with high-risk locally advanced cervical cancer, she told attendees.
“We have had stunning success in cervical cancer treatments over the past 10-15 years, and now we see that the addition of pembrolizumab to standard chemotherapy not only improves outcome but very importantly does not significantly impact the quality of life in our patients,” said study discussant R. Wendel Naumann, MD, Atrium Health Levine Cancer Institute, Charlotte, North Carolina.
Funding for KEYNOTE-A18 was provided by Merck. Dr. Randall has disclosed relationships with Seagen/Genmab, Merck, GSK, ImmunoGen, AstraZeneca, and On Target Laboratories. Dr. Naumann has no relevant disclosures.
A version of this article appeared on Medscape.com.
according to patient-reported outcome analyses from the KEYNOTE-A18 trial.
Progression-free survival data from the trial, reported at the 2023 meeting of the European Society for Medical Oncology, showed a 30% reduction in the risk for progression (P =.002) with the addition of pembrolizumab vs placebo, as well as favorable overall survival trends, reported Leslie Randall, MD, with VCU Health in Richmond, Virginia. Now the new analyses bring the “voices of the patients from this large phase 3 study.”
“I think the data can put us at ease and give patients a measure of comfort because there was no detriment in the quality of life,” said Dr. Randall, who presented the data on March 17 at the Society of Gynecologic Oncology (SGO) 2024 conference.
The phase 3 KEYNOTE-A18 trial enrolled 1060 patients new to treatment who had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease. They were randomly allocated to receive pembrolizumab or placebo plus concurrent chemoradiotherapy.
Patient-reported outcome instruments used in the study were the European Organization for Research and Treatment of Cancer (EORTC) 30-item quality-of-life (QOL) core questionnaire (QLQ-C30), the EORTC cervical cancer–specific module (QLQ-CX24), and the EuroQol EQ-5D-5L visual analog scale (VAS).
Prespecified secondary study endpoints were changes from baseline to week 36 in QLQ-C30 global health status/QOL (GHS/QOL) and physical functioning, and the QLQ-CX24 symptom-experience scale.
At baseline, patient-reported outcome scores were similar between treatment groups. Patients in both treatment groups had an initial decrease in QLQ-C30 GHS/QOL and physical functioning subscale and EQ-5D-5L VAS scores at weeks 3 and 6, but these improved relative to baseline at week 12 and subsequent weeks.
A similar number of patients in the pembrolizumab and placebo groups had improved or stable scores for QLQ-C30 GHS/QOL (76% vs 75%), QLQ-C30 physical functioning (77% vs 77%), QLQ-CX24 symptom experience (85% vs 85%) and EQ-5D-5L VAS (73% vs 76%).
Across all QOL instruments evaluated, there were no meaningful differences in patient-reported outcomes among patients who received pembrolizumab compared with those who received placebo, said Dr. Randall.
The improvement in progression-free survival, coupled with the patient-reported outcomes showing no additional harms to quality of life, support pembrolizumab plus concurrent chemoradiotherapy as a “new standard of care” for patients with high-risk locally advanced cervical cancer, she told attendees.
“We have had stunning success in cervical cancer treatments over the past 10-15 years, and now we see that the addition of pembrolizumab to standard chemotherapy not only improves outcome but very importantly does not significantly impact the quality of life in our patients,” said study discussant R. Wendel Naumann, MD, Atrium Health Levine Cancer Institute, Charlotte, North Carolina.
Funding for KEYNOTE-A18 was provided by Merck. Dr. Randall has disclosed relationships with Seagen/Genmab, Merck, GSK, ImmunoGen, AstraZeneca, and On Target Laboratories. Dr. Naumann has no relevant disclosures.
A version of this article appeared on Medscape.com.
according to patient-reported outcome analyses from the KEYNOTE-A18 trial.
Progression-free survival data from the trial, reported at the 2023 meeting of the European Society for Medical Oncology, showed a 30% reduction in the risk for progression (P =.002) with the addition of pembrolizumab vs placebo, as well as favorable overall survival trends, reported Leslie Randall, MD, with VCU Health in Richmond, Virginia. Now the new analyses bring the “voices of the patients from this large phase 3 study.”
“I think the data can put us at ease and give patients a measure of comfort because there was no detriment in the quality of life,” said Dr. Randall, who presented the data on March 17 at the Society of Gynecologic Oncology (SGO) 2024 conference.
The phase 3 KEYNOTE-A18 trial enrolled 1060 patients new to treatment who had either stage 1B2-2B disease with lymph node involvement or stage 3-4A disease. They were randomly allocated to receive pembrolizumab or placebo plus concurrent chemoradiotherapy.
Patient-reported outcome instruments used in the study were the European Organization for Research and Treatment of Cancer (EORTC) 30-item quality-of-life (QOL) core questionnaire (QLQ-C30), the EORTC cervical cancer–specific module (QLQ-CX24), and the EuroQol EQ-5D-5L visual analog scale (VAS).
Prespecified secondary study endpoints were changes from baseline to week 36 in QLQ-C30 global health status/QOL (GHS/QOL) and physical functioning, and the QLQ-CX24 symptom-experience scale.
At baseline, patient-reported outcome scores were similar between treatment groups. Patients in both treatment groups had an initial decrease in QLQ-C30 GHS/QOL and physical functioning subscale and EQ-5D-5L VAS scores at weeks 3 and 6, but these improved relative to baseline at week 12 and subsequent weeks.
A similar number of patients in the pembrolizumab and placebo groups had improved or stable scores for QLQ-C30 GHS/QOL (76% vs 75%), QLQ-C30 physical functioning (77% vs 77%), QLQ-CX24 symptom experience (85% vs 85%) and EQ-5D-5L VAS (73% vs 76%).
Across all QOL instruments evaluated, there were no meaningful differences in patient-reported outcomes among patients who received pembrolizumab compared with those who received placebo, said Dr. Randall.
The improvement in progression-free survival, coupled with the patient-reported outcomes showing no additional harms to quality of life, support pembrolizumab plus concurrent chemoradiotherapy as a “new standard of care” for patients with high-risk locally advanced cervical cancer, she told attendees.
“We have had stunning success in cervical cancer treatments over the past 10-15 years, and now we see that the addition of pembrolizumab to standard chemotherapy not only improves outcome but very importantly does not significantly impact the quality of life in our patients,” said study discussant R. Wendel Naumann, MD, Atrium Health Levine Cancer Institute, Charlotte, North Carolina.
Funding for KEYNOTE-A18 was provided by Merck. Dr. Randall has disclosed relationships with Seagen/Genmab, Merck, GSK, ImmunoGen, AstraZeneca, and On Target Laboratories. Dr. Naumann has no relevant disclosures.
A version of this article appeared on Medscape.com.
Primary Biliary Cholangitis: Managing a Progressive Liver Disease
Primary biliary cholangitis is a rare progressive autoimmune liver disease that specifically targets biliary epithelial cells. In this article, Dr. Kowdley describes prognostic markers of disease, current and investigational medical therapies, liver transplant, and focusing on maximizing favorable long-term outcomes.
Primary biliary cholangitis is a rare progressive autoimmune liver disease that specifically targets biliary epithelial cells. In this article, Dr. Kowdley describes prognostic markers of disease, current and investigational medical therapies, liver transplant, and focusing on maximizing favorable long-term outcomes.
Primary biliary cholangitis is a rare progressive autoimmune liver disease that specifically targets biliary epithelial cells. In this article, Dr. Kowdley describes prognostic markers of disease, current and investigational medical therapies, liver transplant, and focusing on maximizing favorable long-term outcomes.
Rheumatology Workforce May Have Grown, But It’s Still Not Enough
The number of clinically active rheumatology providers in the United States grew more than 20% from 2009 to 2019, according to a new analysis.
The number of advanced practice providers (APPs) in rheumatology more than doubled during that same time. However, these numerical increases are not enough to meet the projected demand in care, the authors reported.
“Even though we found growth, and if that same growth continued, we’d still be well below the projected needs for the overall aging population of the United States,” lead author Melissa Mannion, MD, MSPH, a pediatric rheumatologist at the University of Alabama at Birmingham, said in an interview.
In a 2015 workforce study by the American College of Rheumatology (ACR), researchers projected that the supply of rheumatologists would decrease by 25% from 2015 to 2030, with an estimated shortage of over 4000 clinical full-time equivalent (cFTE) rheumatology providers.
While the previous study’s findings seem to contrast with this newest analysis, Dr. Dr. Mannion said the findings are complementary, with both studies using different data sources.
The newest findings were published online on February 24 in Arthritis & Rheumatology.
Leveraging Medicare Data
The ACR study estimated the adult rheumatology workforce and cFTEs using both primary and secondary sources, including published data from the American Medical Association and other professional societies, as well as electronic surveys of ACR members and rheumatology fellows in training.
By contrast, this newest study identified rheumatology providers using Medicare administrative data from 2006 to 2020, supplemented by national provider identifier taxonomy codes. Rheumatologists were considered “clinically active” if they provided care for at least five patients in 1 year. To account for rheumatology fellows, who can be categorized as internal medicine physicians, researchers also included internists who prescribed biologic or targeted synthetic disease-modifying antirheumatic drugs (DMARDs) to more than 10 patients a year.
The count also included APPs, both nurse practitioners (NPs) and physician assistants (PAs), who were attached to practices with only rheumatologists, internists, or one other specialty. Like physicians, APPs were included in the count if they prescribed more than 10 patients biologic or targeted synthetic DMARDs in a calendar year and billed at least five patients with a rheumatic condition per year.
Researchers also estimated the number of cFTEs for 2019, using a variety of assumptions to generate an upper and lower range.
Different Numbers, Complementary Conclusions
For the 2019 calendar year, researchers counted 5667 clinically active rheumatologists and 379 NPs/PAs.
This represented a 23% increase in rheumatologists and a 141% increase in the number of APPs in the rheumatology workforce from 2009. The active rheumatology workforce increased by about 100 providers per year over the study period, although growth flattened off in more recent years.
The estimated cFTEs for 2019 ranged from 3457 to 5396, which are “comparable” to the projected 2020 cFTE estimates made in the ACR 2015 workforce study: 3888 to 5777 cFTEs.
Daniel Battafarano, DO, chair of the ACR Workforce Solutions Committee, agreed with Dr. Mannion that these findings complement each other, telling a similar story “but with different numbers,” he told this news organization. Dr. Battafarano, a professor of medicine at the Uniformed Services University of the Health Sciences, Bethesda, Maryland, led the ACR workforce study.
“Both studies project estimates of rheumatology providers and workforce trends, but neither study can claim accurate numbers of rheumatology providers due to the true limitation of either workforce study model,” he said.
The ACR study began with a higher count of rheumatologists, estimating 6013 practicing providers in 2015, while Dr. Mannion’s team calculated 5474 providers for the same year. The ACR study projected that provider numbers would fall to 5886 by 2020, which was higher than the 2019 count in this most recent study.
Dr. Battafarano noted that the studies also dealt with different time frames: While the ACR study used 2015 data to project trends in 5-year increments, this most recent study looked trends in the previous decade.
“We found an increase over time, although toward the end of our study — from 2019 to 2020 — we did have a drop off in number of providers, so maybe we are finding the beginning of what [the ACR researchers] anticipated, or maybe that will be corrected in the future,” Dr. Dr. Mannion said. “I think that our estimates really coordinate with what they found just using a different data source.”
Geographic Disparities and Solutions
The new study also highlighted the scarcity of rheumatology providers in rural counties, which was also an important take away from the 2015 ACR study. In the new analysis, researchers found that 95% of rheumatology practices were in urban settings, which did not change over the study period. Most counties in the United States had fewer than 30 rheumatologists per 1 million adults, with 93% of rural counties having zero adult rheumatologists. In comparison, 48% of urban counties had no adult rheumatologists.
“There’s not enough people, and they’re in very specific locations,” Dr. Dr. Mannion said. “Both things make it harder for people to get care.”
Dr. Battafarano noted that leveraging APPs would be key to increasing patient access to care moving forward — a resource that has more than doubled in the past decade, according to Dr. Dr. Mannion’s analysis.
“Recruiting a significant number of APPs into rheumatology practice should be a primary goal to increase the rheumatology workforce,” he said. Other potential solutions, he added, include employing telemedicine and educating primary care providers on rheumatic disease to help them comanage these conditions with rheumatologists.
This study was partially funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Mannion was supported by the Rheumatology Research Foundation Norman B. Gaylis, MD Clinical Research Award, paid to her institution. Dr. Battafarano had no relevant disclosures.
A version of this article appeared on Medscape.com.
The number of clinically active rheumatology providers in the United States grew more than 20% from 2009 to 2019, according to a new analysis.
The number of advanced practice providers (APPs) in rheumatology more than doubled during that same time. However, these numerical increases are not enough to meet the projected demand in care, the authors reported.
“Even though we found growth, and if that same growth continued, we’d still be well below the projected needs for the overall aging population of the United States,” lead author Melissa Mannion, MD, MSPH, a pediatric rheumatologist at the University of Alabama at Birmingham, said in an interview.
In a 2015 workforce study by the American College of Rheumatology (ACR), researchers projected that the supply of rheumatologists would decrease by 25% from 2015 to 2030, with an estimated shortage of over 4000 clinical full-time equivalent (cFTE) rheumatology providers.
While the previous study’s findings seem to contrast with this newest analysis, Dr. Dr. Mannion said the findings are complementary, with both studies using different data sources.
The newest findings were published online on February 24 in Arthritis & Rheumatology.
Leveraging Medicare Data
The ACR study estimated the adult rheumatology workforce and cFTEs using both primary and secondary sources, including published data from the American Medical Association and other professional societies, as well as electronic surveys of ACR members and rheumatology fellows in training.
By contrast, this newest study identified rheumatology providers using Medicare administrative data from 2006 to 2020, supplemented by national provider identifier taxonomy codes. Rheumatologists were considered “clinically active” if they provided care for at least five patients in 1 year. To account for rheumatology fellows, who can be categorized as internal medicine physicians, researchers also included internists who prescribed biologic or targeted synthetic disease-modifying antirheumatic drugs (DMARDs) to more than 10 patients a year.
The count also included APPs, both nurse practitioners (NPs) and physician assistants (PAs), who were attached to practices with only rheumatologists, internists, or one other specialty. Like physicians, APPs were included in the count if they prescribed more than 10 patients biologic or targeted synthetic DMARDs in a calendar year and billed at least five patients with a rheumatic condition per year.
Researchers also estimated the number of cFTEs for 2019, using a variety of assumptions to generate an upper and lower range.
Different Numbers, Complementary Conclusions
For the 2019 calendar year, researchers counted 5667 clinically active rheumatologists and 379 NPs/PAs.
This represented a 23% increase in rheumatologists and a 141% increase in the number of APPs in the rheumatology workforce from 2009. The active rheumatology workforce increased by about 100 providers per year over the study period, although growth flattened off in more recent years.
The estimated cFTEs for 2019 ranged from 3457 to 5396, which are “comparable” to the projected 2020 cFTE estimates made in the ACR 2015 workforce study: 3888 to 5777 cFTEs.
Daniel Battafarano, DO, chair of the ACR Workforce Solutions Committee, agreed with Dr. Mannion that these findings complement each other, telling a similar story “but with different numbers,” he told this news organization. Dr. Battafarano, a professor of medicine at the Uniformed Services University of the Health Sciences, Bethesda, Maryland, led the ACR workforce study.
“Both studies project estimates of rheumatology providers and workforce trends, but neither study can claim accurate numbers of rheumatology providers due to the true limitation of either workforce study model,” he said.
The ACR study began with a higher count of rheumatologists, estimating 6013 practicing providers in 2015, while Dr. Mannion’s team calculated 5474 providers for the same year. The ACR study projected that provider numbers would fall to 5886 by 2020, which was higher than the 2019 count in this most recent study.
Dr. Battafarano noted that the studies also dealt with different time frames: While the ACR study used 2015 data to project trends in 5-year increments, this most recent study looked trends in the previous decade.
“We found an increase over time, although toward the end of our study — from 2019 to 2020 — we did have a drop off in number of providers, so maybe we are finding the beginning of what [the ACR researchers] anticipated, or maybe that will be corrected in the future,” Dr. Dr. Mannion said. “I think that our estimates really coordinate with what they found just using a different data source.”
Geographic Disparities and Solutions
The new study also highlighted the scarcity of rheumatology providers in rural counties, which was also an important take away from the 2015 ACR study. In the new analysis, researchers found that 95% of rheumatology practices were in urban settings, which did not change over the study period. Most counties in the United States had fewer than 30 rheumatologists per 1 million adults, with 93% of rural counties having zero adult rheumatologists. In comparison, 48% of urban counties had no adult rheumatologists.
“There’s not enough people, and they’re in very specific locations,” Dr. Dr. Mannion said. “Both things make it harder for people to get care.”
Dr. Battafarano noted that leveraging APPs would be key to increasing patient access to care moving forward — a resource that has more than doubled in the past decade, according to Dr. Dr. Mannion’s analysis.
“Recruiting a significant number of APPs into rheumatology practice should be a primary goal to increase the rheumatology workforce,” he said. Other potential solutions, he added, include employing telemedicine and educating primary care providers on rheumatic disease to help them comanage these conditions with rheumatologists.
This study was partially funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Mannion was supported by the Rheumatology Research Foundation Norman B. Gaylis, MD Clinical Research Award, paid to her institution. Dr. Battafarano had no relevant disclosures.
A version of this article appeared on Medscape.com.
The number of clinically active rheumatology providers in the United States grew more than 20% from 2009 to 2019, according to a new analysis.
The number of advanced practice providers (APPs) in rheumatology more than doubled during that same time. However, these numerical increases are not enough to meet the projected demand in care, the authors reported.
“Even though we found growth, and if that same growth continued, we’d still be well below the projected needs for the overall aging population of the United States,” lead author Melissa Mannion, MD, MSPH, a pediatric rheumatologist at the University of Alabama at Birmingham, said in an interview.
In a 2015 workforce study by the American College of Rheumatology (ACR), researchers projected that the supply of rheumatologists would decrease by 25% from 2015 to 2030, with an estimated shortage of over 4000 clinical full-time equivalent (cFTE) rheumatology providers.
While the previous study’s findings seem to contrast with this newest analysis, Dr. Dr. Mannion said the findings are complementary, with both studies using different data sources.
The newest findings were published online on February 24 in Arthritis & Rheumatology.
Leveraging Medicare Data
The ACR study estimated the adult rheumatology workforce and cFTEs using both primary and secondary sources, including published data from the American Medical Association and other professional societies, as well as electronic surveys of ACR members and rheumatology fellows in training.
By contrast, this newest study identified rheumatology providers using Medicare administrative data from 2006 to 2020, supplemented by national provider identifier taxonomy codes. Rheumatologists were considered “clinically active” if they provided care for at least five patients in 1 year. To account for rheumatology fellows, who can be categorized as internal medicine physicians, researchers also included internists who prescribed biologic or targeted synthetic disease-modifying antirheumatic drugs (DMARDs) to more than 10 patients a year.
The count also included APPs, both nurse practitioners (NPs) and physician assistants (PAs), who were attached to practices with only rheumatologists, internists, or one other specialty. Like physicians, APPs were included in the count if they prescribed more than 10 patients biologic or targeted synthetic DMARDs in a calendar year and billed at least five patients with a rheumatic condition per year.
Researchers also estimated the number of cFTEs for 2019, using a variety of assumptions to generate an upper and lower range.
Different Numbers, Complementary Conclusions
For the 2019 calendar year, researchers counted 5667 clinically active rheumatologists and 379 NPs/PAs.
This represented a 23% increase in rheumatologists and a 141% increase in the number of APPs in the rheumatology workforce from 2009. The active rheumatology workforce increased by about 100 providers per year over the study period, although growth flattened off in more recent years.
The estimated cFTEs for 2019 ranged from 3457 to 5396, which are “comparable” to the projected 2020 cFTE estimates made in the ACR 2015 workforce study: 3888 to 5777 cFTEs.
Daniel Battafarano, DO, chair of the ACR Workforce Solutions Committee, agreed with Dr. Mannion that these findings complement each other, telling a similar story “but with different numbers,” he told this news organization. Dr. Battafarano, a professor of medicine at the Uniformed Services University of the Health Sciences, Bethesda, Maryland, led the ACR workforce study.
“Both studies project estimates of rheumatology providers and workforce trends, but neither study can claim accurate numbers of rheumatology providers due to the true limitation of either workforce study model,” he said.
The ACR study began with a higher count of rheumatologists, estimating 6013 practicing providers in 2015, while Dr. Mannion’s team calculated 5474 providers for the same year. The ACR study projected that provider numbers would fall to 5886 by 2020, which was higher than the 2019 count in this most recent study.
Dr. Battafarano noted that the studies also dealt with different time frames: While the ACR study used 2015 data to project trends in 5-year increments, this most recent study looked trends in the previous decade.
“We found an increase over time, although toward the end of our study — from 2019 to 2020 — we did have a drop off in number of providers, so maybe we are finding the beginning of what [the ACR researchers] anticipated, or maybe that will be corrected in the future,” Dr. Dr. Mannion said. “I think that our estimates really coordinate with what they found just using a different data source.”
Geographic Disparities and Solutions
The new study also highlighted the scarcity of rheumatology providers in rural counties, which was also an important take away from the 2015 ACR study. In the new analysis, researchers found that 95% of rheumatology practices were in urban settings, which did not change over the study period. Most counties in the United States had fewer than 30 rheumatologists per 1 million adults, with 93% of rural counties having zero adult rheumatologists. In comparison, 48% of urban counties had no adult rheumatologists.
“There’s not enough people, and they’re in very specific locations,” Dr. Dr. Mannion said. “Both things make it harder for people to get care.”
Dr. Battafarano noted that leveraging APPs would be key to increasing patient access to care moving forward — a resource that has more than doubled in the past decade, according to Dr. Dr. Mannion’s analysis.
“Recruiting a significant number of APPs into rheumatology practice should be a primary goal to increase the rheumatology workforce,” he said. Other potential solutions, he added, include employing telemedicine and educating primary care providers on rheumatic disease to help them comanage these conditions with rheumatologists.
This study was partially funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Mannion was supported by the Rheumatology Research Foundation Norman B. Gaylis, MD Clinical Research Award, paid to her institution. Dr. Battafarano had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Myeloma: FDA Advisers Greenlight Early CAR-T
The FDA asked its Oncologic Drugs Advisory Committee (ODAC) to vote on two separate but similar questions at the March 15 meeting. Much of their discussion centered on higher rates of deaths for patients on the CAR-T therapies during early stages of key studies.
ODAC voted 11-0 to say the risk-benefit assessment appeared favorable for a requested broadening of the patient pool for ciltacabtagene autoleucel (cilta-cel, Carvykti, Johnson & Johnson’s Janssen). J&J is seeking approval for use of the drug for adults with relapsed or refractory multiple myeloma (RRMM) who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and are refractory to lenalidomide.
ODAC voted 8-3 to say the risk-benefit assessment appeared favorable for a requested broadening of the patient pool for idecabtagene vicleucel (ide-cel, Abecma, Bristol Myers Squibb). The company is seeking approval of the drug for people with relapsed or refractory multiple myeloma (RRMM) who have received an IMiD, a PI, and an anti-CD38 antibody.
The FDA staff will consider ODAC’s votes and recommendations, but is not bound by them. Janssen’s parent company, J&J, said the FDA’s deadline for deciding on the request to change the cilta-cel label is April 5. Bristol Myers Squibb (BMS) said there is not a PDUFA deadline at this time for its application.
Both CAR-T treatments currently are approved for RRMM after 4 or more prior lines of therapy, including an IMiD, PI and an anti-CD38 monoclonal antibody. Last year BMS and Janssen filed their separate applications, both seeking to have their drugs used earlier in the course of RRMM.
Data provided in support of both requests for expanded use raised alarms at the FDA, with more deaths seen in the early stage of testing among patients given the CAR-T drugs compared to those given standard-of-care regimens, the agency staff said.
The application for cilta-cel rests heavily on the data from the CARTITUDE-4 trial. As reported in The New England Journal of Medicine last year, progression-free survival (PFS) at 12 months was 75.9% (95% CI, 69.4 to 81.1) in the cilta-cel group and 48.6% (95% CI, 41.5 to 55.3) in the standard-care group.
But the FDA staff review focused on worrying signs in the early months of this study. For example, the rate of death in the first 10 months post randomization was higher in the cilta-cel arm (29 of 208; 14%) than in the standard therapy arm (25 of 211; 12%) based on an analysis of the intent-to-treat (ITT) population, the FDA said.
In its review of the ide-cel application, the FDA staff said the median PFS was 13.3 months in the ide-cel arm (95% CI: 11.8, 16.1), and 4.4 months (95% CI: 3.4, 5.9) in the standard of care (SOC) arm.
However, the rate of deaths in the first 9 months post randomization was higher in the ide-cel arm (45/254; 18%) than in the comparator standard-of-care group (15/132; 11%) in the ITT population, the FDA staff said. In the safety analysis population, the rate of deaths from adverse events that occurred within 90 days from starting treatment was 2.7% in the ide-cel arm and 1.6 % in the standard-regimen group.
ODAC ultimately appeared more impressed by data indicating the potential benefit, measured as progression-free survival (PFS), of the two drugs under review, than they were concerned about the issues about early deaths raised by FDA staff.
Panelist Jorge J. Nieva, MD, of the University of Southern California said the CAR-T drugs may present another case of “front-loaded risk” as has been noted for other treatments for serious medical procedures, such as allogeneic transplantations and thoracic surgeries.
In response, Robert Sokolic, MD, the branch chief for malignant hematology at FDA, replied that the data raised concerns that did in fact remind him of these procedures.
“I’m a bone marrow transplant physician. And that’s exactly what I said when I saw these curves. This looks like an allogeneic transplant curve,” Dr. Sokolic said.
But there’s a major difference between that procedure and CAR-T in the context being considered at the ODAC meeting, he said.
With allogeneic transplant, physicians “counsel patients. We ask them to accept an upfront burden of increased mortality, because we know that down the line, overall, there’s a benefit in survival,” Dr. Sokolic said.
In contrast, the primary endpoint in the key studies for expansion of CAR-T drugs was progression-free survival (PFS), with overall survival as a second endpoint. The FDA staff in briefing documents noted how overall survival, the gold standard in research, delivers far more reliable answers for patients and doctors in assessing treatments.
In the exchange with Dr. Nieva, Dr. Sokolic noted that there’s far less certainty of benefit at this time when asking patients to consider CAR-T earlier in the progression of MM, especially given the safety concerns.
“We know there’s benefit in PFS. We know there’s a safety concern,” Dr. Sokolic said.“That’s not balanced by an overall survival balance on the tail end. It may be when the data are more mature, but it’s not there yet.”
Describing Risks to Patients
ODAC panelists also stressed a need to help patients understand what’s known — and not yet known — about these CAR-T therapies. It will be very challenging for patients to understand and interpret the data from key studies on these medicines, said ODAC panelist Susan Lattimore, RN, of Oregon Health & Science University. She suggested the FDA seek labeling that would be “overtly transparent” and use lay terms to describe the potential risks and benefits.
In its presentations to the FDA and ODAC, J&J noted that the COVID pandemic has affected testing and that the rate of deaths flips in time to be higher in the comparator group.
In its briefing document for the meeting, BMS emphasized that most of the patients in the ide-cel arm who died in the first 6 months of its trial did not get the study drug. There were 9 deaths in the standard-regimen arm, or 6.8% of the group, compared with 30, or 11.8% in the ide-cel group.
In the ide-cel arm, the majority of early deaths (17/30; 56.7%) occurred in patients who never received ide-cel treatment, with 13 of those 17 dying from disease progression, the company said in its briefing document. The early death rate among patients who received the allocated study treatment was similar between arms (5.1% in the ide-cel arm vs 6.8% in the standard regimen arm),the company said.
In the staff briefing, the FDA said the median PFS was 13.3 months in the ide-cel arm, compared with 4.4 months in the standard of care (SOC) arm. But there was a “clear and persistent increased mortality” for the ide-cel group, compared with the standard regimen arm, with increased rates of death up to 9 months. In addition, the overall survival disadvantage persisted to 15 months after randomization, when the survival curves finally crossed, the FDA staff said in its March 15 presentation.
ODAC Chairman Ravi A. Madan, MD, of the National Cancer Institute, was among the panelists who voted “no” in the ide-cel question. He said the risk-benefit profile of the drug does not appear favorable at this time for expanded use.
“There’s a lot of optimism about moving these therapies earlier in the disease states of multiple myeloma,” Dr. Madan said, calling the PFS data “quite remarkable.
“But for me this data at this level of maturity really didn’t provide convincing evidence that ide-cel earlier had a favorable risk benefit assessment in a proposed indication.”
ODAC panelist Christopher H. Lieu, MD, of the University of Colorado, said he struggled to decide how to vote on the ide-cel question and in the end voted yes.
He said the response to the treatment doesn’t appear to be as durable as hoped, considering the significant burden that CAR-T therapy imposes on patients. However, the PFS data suggest that ide-cel could offer patients with RRMM a chance for significant times off therapy with associated quality of life improvement.
“I do believe that the risk-benefit profile is favorable for this population as a whole,” he said. “But it’s a closer margin than I think we would like and patients will need to have in-depth discussions about the risks and benefits and balance that with the possible benefits with their provider.”
The FDA asked its Oncologic Drugs Advisory Committee (ODAC) to vote on two separate but similar questions at the March 15 meeting. Much of their discussion centered on higher rates of deaths for patients on the CAR-T therapies during early stages of key studies.
ODAC voted 11-0 to say the risk-benefit assessment appeared favorable for a requested broadening of the patient pool for ciltacabtagene autoleucel (cilta-cel, Carvykti, Johnson & Johnson’s Janssen). J&J is seeking approval for use of the drug for adults with relapsed or refractory multiple myeloma (RRMM) who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and are refractory to lenalidomide.
ODAC voted 8-3 to say the risk-benefit assessment appeared favorable for a requested broadening of the patient pool for idecabtagene vicleucel (ide-cel, Abecma, Bristol Myers Squibb). The company is seeking approval of the drug for people with relapsed or refractory multiple myeloma (RRMM) who have received an IMiD, a PI, and an anti-CD38 antibody.
The FDA staff will consider ODAC’s votes and recommendations, but is not bound by them. Janssen’s parent company, J&J, said the FDA’s deadline for deciding on the request to change the cilta-cel label is April 5. Bristol Myers Squibb (BMS) said there is not a PDUFA deadline at this time for its application.
Both CAR-T treatments currently are approved for RRMM after 4 or more prior lines of therapy, including an IMiD, PI and an anti-CD38 monoclonal antibody. Last year BMS and Janssen filed their separate applications, both seeking to have their drugs used earlier in the course of RRMM.
Data provided in support of both requests for expanded use raised alarms at the FDA, with more deaths seen in the early stage of testing among patients given the CAR-T drugs compared to those given standard-of-care regimens, the agency staff said.
The application for cilta-cel rests heavily on the data from the CARTITUDE-4 trial. As reported in The New England Journal of Medicine last year, progression-free survival (PFS) at 12 months was 75.9% (95% CI, 69.4 to 81.1) in the cilta-cel group and 48.6% (95% CI, 41.5 to 55.3) in the standard-care group.
But the FDA staff review focused on worrying signs in the early months of this study. For example, the rate of death in the first 10 months post randomization was higher in the cilta-cel arm (29 of 208; 14%) than in the standard therapy arm (25 of 211; 12%) based on an analysis of the intent-to-treat (ITT) population, the FDA said.
In its review of the ide-cel application, the FDA staff said the median PFS was 13.3 months in the ide-cel arm (95% CI: 11.8, 16.1), and 4.4 months (95% CI: 3.4, 5.9) in the standard of care (SOC) arm.
However, the rate of deaths in the first 9 months post randomization was higher in the ide-cel arm (45/254; 18%) than in the comparator standard-of-care group (15/132; 11%) in the ITT population, the FDA staff said. In the safety analysis population, the rate of deaths from adverse events that occurred within 90 days from starting treatment was 2.7% in the ide-cel arm and 1.6 % in the standard-regimen group.
ODAC ultimately appeared more impressed by data indicating the potential benefit, measured as progression-free survival (PFS), of the two drugs under review, than they were concerned about the issues about early deaths raised by FDA staff.
Panelist Jorge J. Nieva, MD, of the University of Southern California said the CAR-T drugs may present another case of “front-loaded risk” as has been noted for other treatments for serious medical procedures, such as allogeneic transplantations and thoracic surgeries.
In response, Robert Sokolic, MD, the branch chief for malignant hematology at FDA, replied that the data raised concerns that did in fact remind him of these procedures.
“I’m a bone marrow transplant physician. And that’s exactly what I said when I saw these curves. This looks like an allogeneic transplant curve,” Dr. Sokolic said.
But there’s a major difference between that procedure and CAR-T in the context being considered at the ODAC meeting, he said.
With allogeneic transplant, physicians “counsel patients. We ask them to accept an upfront burden of increased mortality, because we know that down the line, overall, there’s a benefit in survival,” Dr. Sokolic said.
In contrast, the primary endpoint in the key studies for expansion of CAR-T drugs was progression-free survival (PFS), with overall survival as a second endpoint. The FDA staff in briefing documents noted how overall survival, the gold standard in research, delivers far more reliable answers for patients and doctors in assessing treatments.
In the exchange with Dr. Nieva, Dr. Sokolic noted that there’s far less certainty of benefit at this time when asking patients to consider CAR-T earlier in the progression of MM, especially given the safety concerns.
“We know there’s benefit in PFS. We know there’s a safety concern,” Dr. Sokolic said.“That’s not balanced by an overall survival balance on the tail end. It may be when the data are more mature, but it’s not there yet.”
Describing Risks to Patients
ODAC panelists also stressed a need to help patients understand what’s known — and not yet known — about these CAR-T therapies. It will be very challenging for patients to understand and interpret the data from key studies on these medicines, said ODAC panelist Susan Lattimore, RN, of Oregon Health & Science University. She suggested the FDA seek labeling that would be “overtly transparent” and use lay terms to describe the potential risks and benefits.
In its presentations to the FDA and ODAC, J&J noted that the COVID pandemic has affected testing and that the rate of deaths flips in time to be higher in the comparator group.
In its briefing document for the meeting, BMS emphasized that most of the patients in the ide-cel arm who died in the first 6 months of its trial did not get the study drug. There were 9 deaths in the standard-regimen arm, or 6.8% of the group, compared with 30, or 11.8% in the ide-cel group.
In the ide-cel arm, the majority of early deaths (17/30; 56.7%) occurred in patients who never received ide-cel treatment, with 13 of those 17 dying from disease progression, the company said in its briefing document. The early death rate among patients who received the allocated study treatment was similar between arms (5.1% in the ide-cel arm vs 6.8% in the standard regimen arm),the company said.
In the staff briefing, the FDA said the median PFS was 13.3 months in the ide-cel arm, compared with 4.4 months in the standard of care (SOC) arm. But there was a “clear and persistent increased mortality” for the ide-cel group, compared with the standard regimen arm, with increased rates of death up to 9 months. In addition, the overall survival disadvantage persisted to 15 months after randomization, when the survival curves finally crossed, the FDA staff said in its March 15 presentation.
ODAC Chairman Ravi A. Madan, MD, of the National Cancer Institute, was among the panelists who voted “no” in the ide-cel question. He said the risk-benefit profile of the drug does not appear favorable at this time for expanded use.
“There’s a lot of optimism about moving these therapies earlier in the disease states of multiple myeloma,” Dr. Madan said, calling the PFS data “quite remarkable.
“But for me this data at this level of maturity really didn’t provide convincing evidence that ide-cel earlier had a favorable risk benefit assessment in a proposed indication.”
ODAC panelist Christopher H. Lieu, MD, of the University of Colorado, said he struggled to decide how to vote on the ide-cel question and in the end voted yes.
He said the response to the treatment doesn’t appear to be as durable as hoped, considering the significant burden that CAR-T therapy imposes on patients. However, the PFS data suggest that ide-cel could offer patients with RRMM a chance for significant times off therapy with associated quality of life improvement.
“I do believe that the risk-benefit profile is favorable for this population as a whole,” he said. “But it’s a closer margin than I think we would like and patients will need to have in-depth discussions about the risks and benefits and balance that with the possible benefits with their provider.”
The FDA asked its Oncologic Drugs Advisory Committee (ODAC) to vote on two separate but similar questions at the March 15 meeting. Much of their discussion centered on higher rates of deaths for patients on the CAR-T therapies during early stages of key studies.
ODAC voted 11-0 to say the risk-benefit assessment appeared favorable for a requested broadening of the patient pool for ciltacabtagene autoleucel (cilta-cel, Carvykti, Johnson & Johnson’s Janssen). J&J is seeking approval for use of the drug for adults with relapsed or refractory multiple myeloma (RRMM) who have received at least one prior line of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD), and are refractory to lenalidomide.
ODAC voted 8-3 to say the risk-benefit assessment appeared favorable for a requested broadening of the patient pool for idecabtagene vicleucel (ide-cel, Abecma, Bristol Myers Squibb). The company is seeking approval of the drug for people with relapsed or refractory multiple myeloma (RRMM) who have received an IMiD, a PI, and an anti-CD38 antibody.
The FDA staff will consider ODAC’s votes and recommendations, but is not bound by them. Janssen’s parent company, J&J, said the FDA’s deadline for deciding on the request to change the cilta-cel label is April 5. Bristol Myers Squibb (BMS) said there is not a PDUFA deadline at this time for its application.
Both CAR-T treatments currently are approved for RRMM after 4 or more prior lines of therapy, including an IMiD, PI and an anti-CD38 monoclonal antibody. Last year BMS and Janssen filed their separate applications, both seeking to have their drugs used earlier in the course of RRMM.
Data provided in support of both requests for expanded use raised alarms at the FDA, with more deaths seen in the early stage of testing among patients given the CAR-T drugs compared to those given standard-of-care regimens, the agency staff said.
The application for cilta-cel rests heavily on the data from the CARTITUDE-4 trial. As reported in The New England Journal of Medicine last year, progression-free survival (PFS) at 12 months was 75.9% (95% CI, 69.4 to 81.1) in the cilta-cel group and 48.6% (95% CI, 41.5 to 55.3) in the standard-care group.
But the FDA staff review focused on worrying signs in the early months of this study. For example, the rate of death in the first 10 months post randomization was higher in the cilta-cel arm (29 of 208; 14%) than in the standard therapy arm (25 of 211; 12%) based on an analysis of the intent-to-treat (ITT) population, the FDA said.
In its review of the ide-cel application, the FDA staff said the median PFS was 13.3 months in the ide-cel arm (95% CI: 11.8, 16.1), and 4.4 months (95% CI: 3.4, 5.9) in the standard of care (SOC) arm.
However, the rate of deaths in the first 9 months post randomization was higher in the ide-cel arm (45/254; 18%) than in the comparator standard-of-care group (15/132; 11%) in the ITT population, the FDA staff said. In the safety analysis population, the rate of deaths from adverse events that occurred within 90 days from starting treatment was 2.7% in the ide-cel arm and 1.6 % in the standard-regimen group.
ODAC ultimately appeared more impressed by data indicating the potential benefit, measured as progression-free survival (PFS), of the two drugs under review, than they were concerned about the issues about early deaths raised by FDA staff.
Panelist Jorge J. Nieva, MD, of the University of Southern California said the CAR-T drugs may present another case of “front-loaded risk” as has been noted for other treatments for serious medical procedures, such as allogeneic transplantations and thoracic surgeries.
In response, Robert Sokolic, MD, the branch chief for malignant hematology at FDA, replied that the data raised concerns that did in fact remind him of these procedures.
“I’m a bone marrow transplant physician. And that’s exactly what I said when I saw these curves. This looks like an allogeneic transplant curve,” Dr. Sokolic said.
But there’s a major difference between that procedure and CAR-T in the context being considered at the ODAC meeting, he said.
With allogeneic transplant, physicians “counsel patients. We ask them to accept an upfront burden of increased mortality, because we know that down the line, overall, there’s a benefit in survival,” Dr. Sokolic said.
In contrast, the primary endpoint in the key studies for expansion of CAR-T drugs was progression-free survival (PFS), with overall survival as a second endpoint. The FDA staff in briefing documents noted how overall survival, the gold standard in research, delivers far more reliable answers for patients and doctors in assessing treatments.
In the exchange with Dr. Nieva, Dr. Sokolic noted that there’s far less certainty of benefit at this time when asking patients to consider CAR-T earlier in the progression of MM, especially given the safety concerns.
“We know there’s benefit in PFS. We know there’s a safety concern,” Dr. Sokolic said.“That’s not balanced by an overall survival balance on the tail end. It may be when the data are more mature, but it’s not there yet.”
Describing Risks to Patients
ODAC panelists also stressed a need to help patients understand what’s known — and not yet known — about these CAR-T therapies. It will be very challenging for patients to understand and interpret the data from key studies on these medicines, said ODAC panelist Susan Lattimore, RN, of Oregon Health & Science University. She suggested the FDA seek labeling that would be “overtly transparent” and use lay terms to describe the potential risks and benefits.
In its presentations to the FDA and ODAC, J&J noted that the COVID pandemic has affected testing and that the rate of deaths flips in time to be higher in the comparator group.
In its briefing document for the meeting, BMS emphasized that most of the patients in the ide-cel arm who died in the first 6 months of its trial did not get the study drug. There were 9 deaths in the standard-regimen arm, or 6.8% of the group, compared with 30, or 11.8% in the ide-cel group.
In the ide-cel arm, the majority of early deaths (17/30; 56.7%) occurred in patients who never received ide-cel treatment, with 13 of those 17 dying from disease progression, the company said in its briefing document. The early death rate among patients who received the allocated study treatment was similar between arms (5.1% in the ide-cel arm vs 6.8% in the standard regimen arm),the company said.
In the staff briefing, the FDA said the median PFS was 13.3 months in the ide-cel arm, compared with 4.4 months in the standard of care (SOC) arm. But there was a “clear and persistent increased mortality” for the ide-cel group, compared with the standard regimen arm, with increased rates of death up to 9 months. In addition, the overall survival disadvantage persisted to 15 months after randomization, when the survival curves finally crossed, the FDA staff said in its March 15 presentation.
ODAC Chairman Ravi A. Madan, MD, of the National Cancer Institute, was among the panelists who voted “no” in the ide-cel question. He said the risk-benefit profile of the drug does not appear favorable at this time for expanded use.
“There’s a lot of optimism about moving these therapies earlier in the disease states of multiple myeloma,” Dr. Madan said, calling the PFS data “quite remarkable.
“But for me this data at this level of maturity really didn’t provide convincing evidence that ide-cel earlier had a favorable risk benefit assessment in a proposed indication.”
ODAC panelist Christopher H. Lieu, MD, of the University of Colorado, said he struggled to decide how to vote on the ide-cel question and in the end voted yes.
He said the response to the treatment doesn’t appear to be as durable as hoped, considering the significant burden that CAR-T therapy imposes on patients. However, the PFS data suggest that ide-cel could offer patients with RRMM a chance for significant times off therapy with associated quality of life improvement.
“I do believe that the risk-benefit profile is favorable for this population as a whole,” he said. “But it’s a closer margin than I think we would like and patients will need to have in-depth discussions about the risks and benefits and balance that with the possible benefits with their provider.”
Practicing Medicine in Canada’s Far North
In 2019, we interviewed Andrea Prince, MD, who was completing her internship in the Inuit village of Puvirnituq, a town of 2000 inhabitants located in Nunavik, in the Canadian Far North. Five years later, still in her position, what perspective does she have on her practice? Have the challenges of practicing medicine in a remote region within the Inuit community affected her vocation? Would she recommend this experience to young doctors?
Question: What position do you currently hold?
Dr. Prince: I am a full-time general practitioner at Puvirnituq Hospital. My responsibilities range from following up on hospitalized patients to those seen in outpatient clinics for chronic illnesses. Within our medical team, I receive patients in the emergency department (day and night shifts), and I travel to smaller dispensaries nearby, especially to the village of Akulivik. So, it’s quite a varied practice.
More recently, I have been involved in remote continuing medical education projects in collaboration with specialists based in Montreal. In this context, we are increasingly trying to collaborate with doctors from other indigenous communities, such as the Grand Council of the Cree, because our practices are quite similar.
Q: What is the patient volume you see?
Dr. Prince: We see approximately 20-30 patients per day in the clinic, plus about 10 by appointment, and dozens of calls from dispensaries, in addition to patients transferred from other villages. There are four daytime doctors (one at night) and about 15 nurses stationed full-time at Puvirnituq Hospital.
Our practice relies heavily on collaboration with the nursing team, which has an expanded role — they can manage certain patients according to the treatment plan established by the doctor and prescribe treatments (eg, antibiotics for uncomplicated otitis).
Q: Access to care in these isolated regions is considered difficult. Have you observed any improvement in the situation over the past 5 years? What about new material and human resources?
Dr. Prince: For the past year, we have had a Starlink internet connection at the hospital, which facilitates telemedicine exchanges with specialists; we can now send data and medical images to Montreal to obtain expertise much more easily. Previously, everything was done by phone or with significant delays. We do not yet have a cellular network, and all records are currently in paper format.
But the challenges remain numerous. Progress is very slow. Like everywhere in the country, we are experiencing a shortage of staff, particularly an insufficient number of nurses. But the impact is even more dramatic in these isolated territories. We have had to close dispensaries on the coast due to a lack of personnel and only offer emergency services. However, patients have no other options; they cannot drive to another hospital. In Nunavik, the road network is practically nonexistent, and travel to other regions is by plane (about a 2.5-hour medical evacuation trip).
So, sometimes, patients do not seek care in time, and when we finally see them, unfortunately, the issue can be quite advanced.
Q: What are the most pressing logistical needs?
Dr. Prince: We still do not have a scanner in the Far North. This has a significant impact on mortality, especially in the case of accidents and trauma, which are very common in these regions. “Residents of Nunavik are four times more likely to suffer trauma than the rest of Quebec’s population and 40 times more likely to die from it,” as recently reported in La Presse.
There has also been much discussion about cancer mortality, with a risk for death about 70% higher following a lung cancer diagnosis (reported by Medscape Medical News). We do not have a mammogram machine to diagnose breast cancer. Before the COVID-19 pandemic, equipped diagnostic teams sometimes traveled to the region, but this is no longer the case. Today, a patient needing a mammogram will have to travel to Montreal. The same goes for colonoscopies, but visits are becoming less frequent. Therefore, campaigns to screen for certain common types of cancer are practically nonexistent.
As for urgent surgeries (appendicitis, cesarean sections, trauma, etc.), patients must be transferred to Montreal by medical evacuation. We have a visiting surgeon twice a year.
Q: What improvement strategies do you foresee despite the lack of resources?
Dr. Prince: The saying “prevention is better than cure” makes perfect sense in such remote regions under extreme conditions (it is impossible to fly a medevac when it is too windy or during a snowstorm!). That’s why my colleagues and I believe that prevention should be the top priority in terms of healthcare intervention. It may seem obvious, but nothing is simple in the Far North.
Q: In which areas should prevention campaigns be prioritized in your opinion?
Dr. Prince: An example is wearing helmets. Practically no one wears this type of protection in the Far North. They use all-terrain vehicles that are dangerous and for which helmet use is crucial. But they are simply not available in stores. So, communication is difficult: We tell people, “you need a helmet for the ATV, another for the bike, for the snowmobile, for playing hockey, etc.” when it is difficult to obtain one. With traumatologists in Montreal, we had a project to create multifunctional helmets for children — to protect them but also to develop a culture of helmet use, which is not common practice in the community — but these are projects that take a lot of time and are more complex than they seem.
Villages still do not have running water. Therefore, it is difficult to give recommendations to patients as they live in sanitary conditions that are unseen elsewhere in Canada. Without clean water, we cannot ensure that wound care is done properly. Not to mention the occurrence of hepatitis A epidemics, like the one we had to face.
Residents also grapple with significant alcohol and smoking problems, but there is no detox center or dedicated psychological help on site. To follow a detox program, patients would have to leave, move away from their families, and that can be psychologically very destabilizing. I try, in my practice, to talk to my patients about this, especially pregnant women — because many continue to smoke or drink during their pregnancy — but we need more resources.
Q: What about women’s health in this region?
Dr. Prince: We are fortunate to have a team of midwives, several of whom are Inuit, who are of great help in accessing contraception, performing cervical cancer screening tests, etc. But some patients with high-risk pregnancies who should be transferred to Montreal refuse to give birth away from their families. Again, if we had the means to allow high-risk women — or those for whom continuous monitoring or a cesarean section may be necessary — to give birth here safely, it would be a big step. As for abortion, it is feasible but remains a very taboo subject in the community.
Regarding violence against women, I have not observed any particularly encouraging developments in the past 5 years, but recently, we met with the mayor about this, hoping that concrete actions will be taken to help victims of violence.
Q: What is the predominant feeling in your daily life in a situation that is slow to evolve?
Dr. Prince: I remain hopeful for my patients. We must continue to fight! Initiatives must also come from the communities themselves; they must be involved in developing solutions. Because patients, too, need to have hope. They have the right to be cared for like other inhabitants of Canada.
On my part, I try to find a balance between feeling good about my caregiving profession and not burning out professionally. But burnout is a subject that concerns many doctors around the world and is increasingly being discussed. We should all have psychological support when entering medicine!
Q: Would you recommend colleagues to come and work in the Far North? What would you tell them?
Dr. Prince: I would tell them they will have no regrets! Yes, it’s difficult, but it’s a unique type of practice and very rewarding on a human level.
Professionally, it is a general practice that is no longer seen in the city today. The spectrum is very broad, ranging from neonatology to geriatrics, from the simplest to the most complex. It’s very stimulating. Diagnostically, practice is also very different from what is done in the metropolis. Without a scanner, you really have to question and investigate to evaluate whether a patient should be evacuated by plane to Montreal or not. It’s not trivial. Decisions must be made judiciously and quickly.
The human experience is also unique. Inuit communities are little known, and the aspects relayed in the media are often negative due to their increased risk for addiction. However, they are cheerful and very warm people, with an extraordinary culture. I have learned a lot from them, including reconsidering the notion of time, reviewing my priorities, and approaching life one day at a time.
I am very grateful to them for accepting me. I am sometimes even greeted with a “Welcome home!” when I return from vacation...Being told that in Nunavik, I am also “at home,” touches me immensely. I have seen children grow up, adolescents become adults. A bond of trust has developed.
Of course, all of this comes with sacrifices like being away from family and loved ones. We miss birthdays, weddings, etc. But without hesitation, it’s worth it!
Q: What are the next steps in your career in Nunavik? Will you stay for a long time?
Dr. Prince: I take it one day at a time, especially since I am about to take maternity leave very soon. But if a full-time return to Nunavik is difficult with a newborn, I know that the Nunavummiuts [inhabitants of Nunavik] will always be part of my life and my practice.
I want to remain involved with these communities, whether on-site (by practicing there a few months a year) or in Montreal where many patients are transferred. Coming to be treated in a big city (Montreal, 1.7M inhabitants), in very large hospitals, can be very stressful for them. They express themselves much less verbally than Westerners, so we must know how to listen to them, dedicate the necessary time to them, consider their culture and beliefs. I would like to be the familiar face they will encounter when they are cared for away from home. It’s a bond I want to preserve.
This story was translated from Medscape France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In 2019, we interviewed Andrea Prince, MD, who was completing her internship in the Inuit village of Puvirnituq, a town of 2000 inhabitants located in Nunavik, in the Canadian Far North. Five years later, still in her position, what perspective does she have on her practice? Have the challenges of practicing medicine in a remote region within the Inuit community affected her vocation? Would she recommend this experience to young doctors?
Question: What position do you currently hold?
Dr. Prince: I am a full-time general practitioner at Puvirnituq Hospital. My responsibilities range from following up on hospitalized patients to those seen in outpatient clinics for chronic illnesses. Within our medical team, I receive patients in the emergency department (day and night shifts), and I travel to smaller dispensaries nearby, especially to the village of Akulivik. So, it’s quite a varied practice.
More recently, I have been involved in remote continuing medical education projects in collaboration with specialists based in Montreal. In this context, we are increasingly trying to collaborate with doctors from other indigenous communities, such as the Grand Council of the Cree, because our practices are quite similar.
Q: What is the patient volume you see?
Dr. Prince: We see approximately 20-30 patients per day in the clinic, plus about 10 by appointment, and dozens of calls from dispensaries, in addition to patients transferred from other villages. There are four daytime doctors (one at night) and about 15 nurses stationed full-time at Puvirnituq Hospital.
Our practice relies heavily on collaboration with the nursing team, which has an expanded role — they can manage certain patients according to the treatment plan established by the doctor and prescribe treatments (eg, antibiotics for uncomplicated otitis).
Q: Access to care in these isolated regions is considered difficult. Have you observed any improvement in the situation over the past 5 years? What about new material and human resources?
Dr. Prince: For the past year, we have had a Starlink internet connection at the hospital, which facilitates telemedicine exchanges with specialists; we can now send data and medical images to Montreal to obtain expertise much more easily. Previously, everything was done by phone or with significant delays. We do not yet have a cellular network, and all records are currently in paper format.
But the challenges remain numerous. Progress is very slow. Like everywhere in the country, we are experiencing a shortage of staff, particularly an insufficient number of nurses. But the impact is even more dramatic in these isolated territories. We have had to close dispensaries on the coast due to a lack of personnel and only offer emergency services. However, patients have no other options; they cannot drive to another hospital. In Nunavik, the road network is practically nonexistent, and travel to other regions is by plane (about a 2.5-hour medical evacuation trip).
So, sometimes, patients do not seek care in time, and when we finally see them, unfortunately, the issue can be quite advanced.
Q: What are the most pressing logistical needs?
Dr. Prince: We still do not have a scanner in the Far North. This has a significant impact on mortality, especially in the case of accidents and trauma, which are very common in these regions. “Residents of Nunavik are four times more likely to suffer trauma than the rest of Quebec’s population and 40 times more likely to die from it,” as recently reported in La Presse.
There has also been much discussion about cancer mortality, with a risk for death about 70% higher following a lung cancer diagnosis (reported by Medscape Medical News). We do not have a mammogram machine to diagnose breast cancer. Before the COVID-19 pandemic, equipped diagnostic teams sometimes traveled to the region, but this is no longer the case. Today, a patient needing a mammogram will have to travel to Montreal. The same goes for colonoscopies, but visits are becoming less frequent. Therefore, campaigns to screen for certain common types of cancer are practically nonexistent.
As for urgent surgeries (appendicitis, cesarean sections, trauma, etc.), patients must be transferred to Montreal by medical evacuation. We have a visiting surgeon twice a year.
Q: What improvement strategies do you foresee despite the lack of resources?
Dr. Prince: The saying “prevention is better than cure” makes perfect sense in such remote regions under extreme conditions (it is impossible to fly a medevac when it is too windy or during a snowstorm!). That’s why my colleagues and I believe that prevention should be the top priority in terms of healthcare intervention. It may seem obvious, but nothing is simple in the Far North.
Q: In which areas should prevention campaigns be prioritized in your opinion?
Dr. Prince: An example is wearing helmets. Practically no one wears this type of protection in the Far North. They use all-terrain vehicles that are dangerous and for which helmet use is crucial. But they are simply not available in stores. So, communication is difficult: We tell people, “you need a helmet for the ATV, another for the bike, for the snowmobile, for playing hockey, etc.” when it is difficult to obtain one. With traumatologists in Montreal, we had a project to create multifunctional helmets for children — to protect them but also to develop a culture of helmet use, which is not common practice in the community — but these are projects that take a lot of time and are more complex than they seem.
Villages still do not have running water. Therefore, it is difficult to give recommendations to patients as they live in sanitary conditions that are unseen elsewhere in Canada. Without clean water, we cannot ensure that wound care is done properly. Not to mention the occurrence of hepatitis A epidemics, like the one we had to face.
Residents also grapple with significant alcohol and smoking problems, but there is no detox center or dedicated psychological help on site. To follow a detox program, patients would have to leave, move away from their families, and that can be psychologically very destabilizing. I try, in my practice, to talk to my patients about this, especially pregnant women — because many continue to smoke or drink during their pregnancy — but we need more resources.
Q: What about women’s health in this region?
Dr. Prince: We are fortunate to have a team of midwives, several of whom are Inuit, who are of great help in accessing contraception, performing cervical cancer screening tests, etc. But some patients with high-risk pregnancies who should be transferred to Montreal refuse to give birth away from their families. Again, if we had the means to allow high-risk women — or those for whom continuous monitoring or a cesarean section may be necessary — to give birth here safely, it would be a big step. As for abortion, it is feasible but remains a very taboo subject in the community.
Regarding violence against women, I have not observed any particularly encouraging developments in the past 5 years, but recently, we met with the mayor about this, hoping that concrete actions will be taken to help victims of violence.
Q: What is the predominant feeling in your daily life in a situation that is slow to evolve?
Dr. Prince: I remain hopeful for my patients. We must continue to fight! Initiatives must also come from the communities themselves; they must be involved in developing solutions. Because patients, too, need to have hope. They have the right to be cared for like other inhabitants of Canada.
On my part, I try to find a balance between feeling good about my caregiving profession and not burning out professionally. But burnout is a subject that concerns many doctors around the world and is increasingly being discussed. We should all have psychological support when entering medicine!
Q: Would you recommend colleagues to come and work in the Far North? What would you tell them?
Dr. Prince: I would tell them they will have no regrets! Yes, it’s difficult, but it’s a unique type of practice and very rewarding on a human level.
Professionally, it is a general practice that is no longer seen in the city today. The spectrum is very broad, ranging from neonatology to geriatrics, from the simplest to the most complex. It’s very stimulating. Diagnostically, practice is also very different from what is done in the metropolis. Without a scanner, you really have to question and investigate to evaluate whether a patient should be evacuated by plane to Montreal or not. It’s not trivial. Decisions must be made judiciously and quickly.
The human experience is also unique. Inuit communities are little known, and the aspects relayed in the media are often negative due to their increased risk for addiction. However, they are cheerful and very warm people, with an extraordinary culture. I have learned a lot from them, including reconsidering the notion of time, reviewing my priorities, and approaching life one day at a time.
I am very grateful to them for accepting me. I am sometimes even greeted with a “Welcome home!” when I return from vacation...Being told that in Nunavik, I am also “at home,” touches me immensely. I have seen children grow up, adolescents become adults. A bond of trust has developed.
Of course, all of this comes with sacrifices like being away from family and loved ones. We miss birthdays, weddings, etc. But without hesitation, it’s worth it!
Q: What are the next steps in your career in Nunavik? Will you stay for a long time?
Dr. Prince: I take it one day at a time, especially since I am about to take maternity leave very soon. But if a full-time return to Nunavik is difficult with a newborn, I know that the Nunavummiuts [inhabitants of Nunavik] will always be part of my life and my practice.
I want to remain involved with these communities, whether on-site (by practicing there a few months a year) or in Montreal where many patients are transferred. Coming to be treated in a big city (Montreal, 1.7M inhabitants), in very large hospitals, can be very stressful for them. They express themselves much less verbally than Westerners, so we must know how to listen to them, dedicate the necessary time to them, consider their culture and beliefs. I would like to be the familiar face they will encounter when they are cared for away from home. It’s a bond I want to preserve.
This story was translated from Medscape France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In 2019, we interviewed Andrea Prince, MD, who was completing her internship in the Inuit village of Puvirnituq, a town of 2000 inhabitants located in Nunavik, in the Canadian Far North. Five years later, still in her position, what perspective does she have on her practice? Have the challenges of practicing medicine in a remote region within the Inuit community affected her vocation? Would she recommend this experience to young doctors?
Question: What position do you currently hold?
Dr. Prince: I am a full-time general practitioner at Puvirnituq Hospital. My responsibilities range from following up on hospitalized patients to those seen in outpatient clinics for chronic illnesses. Within our medical team, I receive patients in the emergency department (day and night shifts), and I travel to smaller dispensaries nearby, especially to the village of Akulivik. So, it’s quite a varied practice.
More recently, I have been involved in remote continuing medical education projects in collaboration with specialists based in Montreal. In this context, we are increasingly trying to collaborate with doctors from other indigenous communities, such as the Grand Council of the Cree, because our practices are quite similar.
Q: What is the patient volume you see?
Dr. Prince: We see approximately 20-30 patients per day in the clinic, plus about 10 by appointment, and dozens of calls from dispensaries, in addition to patients transferred from other villages. There are four daytime doctors (one at night) and about 15 nurses stationed full-time at Puvirnituq Hospital.
Our practice relies heavily on collaboration with the nursing team, which has an expanded role — they can manage certain patients according to the treatment plan established by the doctor and prescribe treatments (eg, antibiotics for uncomplicated otitis).
Q: Access to care in these isolated regions is considered difficult. Have you observed any improvement in the situation over the past 5 years? What about new material and human resources?
Dr. Prince: For the past year, we have had a Starlink internet connection at the hospital, which facilitates telemedicine exchanges with specialists; we can now send data and medical images to Montreal to obtain expertise much more easily. Previously, everything was done by phone or with significant delays. We do not yet have a cellular network, and all records are currently in paper format.
But the challenges remain numerous. Progress is very slow. Like everywhere in the country, we are experiencing a shortage of staff, particularly an insufficient number of nurses. But the impact is even more dramatic in these isolated territories. We have had to close dispensaries on the coast due to a lack of personnel and only offer emergency services. However, patients have no other options; they cannot drive to another hospital. In Nunavik, the road network is practically nonexistent, and travel to other regions is by plane (about a 2.5-hour medical evacuation trip).
So, sometimes, patients do not seek care in time, and when we finally see them, unfortunately, the issue can be quite advanced.
Q: What are the most pressing logistical needs?
Dr. Prince: We still do not have a scanner in the Far North. This has a significant impact on mortality, especially in the case of accidents and trauma, which are very common in these regions. “Residents of Nunavik are four times more likely to suffer trauma than the rest of Quebec’s population and 40 times more likely to die from it,” as recently reported in La Presse.
There has also been much discussion about cancer mortality, with a risk for death about 70% higher following a lung cancer diagnosis (reported by Medscape Medical News). We do not have a mammogram machine to diagnose breast cancer. Before the COVID-19 pandemic, equipped diagnostic teams sometimes traveled to the region, but this is no longer the case. Today, a patient needing a mammogram will have to travel to Montreal. The same goes for colonoscopies, but visits are becoming less frequent. Therefore, campaigns to screen for certain common types of cancer are practically nonexistent.
As for urgent surgeries (appendicitis, cesarean sections, trauma, etc.), patients must be transferred to Montreal by medical evacuation. We have a visiting surgeon twice a year.
Q: What improvement strategies do you foresee despite the lack of resources?
Dr. Prince: The saying “prevention is better than cure” makes perfect sense in such remote regions under extreme conditions (it is impossible to fly a medevac when it is too windy or during a snowstorm!). That’s why my colleagues and I believe that prevention should be the top priority in terms of healthcare intervention. It may seem obvious, but nothing is simple in the Far North.
Q: In which areas should prevention campaigns be prioritized in your opinion?
Dr. Prince: An example is wearing helmets. Practically no one wears this type of protection in the Far North. They use all-terrain vehicles that are dangerous and for which helmet use is crucial. But they are simply not available in stores. So, communication is difficult: We tell people, “you need a helmet for the ATV, another for the bike, for the snowmobile, for playing hockey, etc.” when it is difficult to obtain one. With traumatologists in Montreal, we had a project to create multifunctional helmets for children — to protect them but also to develop a culture of helmet use, which is not common practice in the community — but these are projects that take a lot of time and are more complex than they seem.
Villages still do not have running water. Therefore, it is difficult to give recommendations to patients as they live in sanitary conditions that are unseen elsewhere in Canada. Without clean water, we cannot ensure that wound care is done properly. Not to mention the occurrence of hepatitis A epidemics, like the one we had to face.
Residents also grapple with significant alcohol and smoking problems, but there is no detox center or dedicated psychological help on site. To follow a detox program, patients would have to leave, move away from their families, and that can be psychologically very destabilizing. I try, in my practice, to talk to my patients about this, especially pregnant women — because many continue to smoke or drink during their pregnancy — but we need more resources.
Q: What about women’s health in this region?
Dr. Prince: We are fortunate to have a team of midwives, several of whom are Inuit, who are of great help in accessing contraception, performing cervical cancer screening tests, etc. But some patients with high-risk pregnancies who should be transferred to Montreal refuse to give birth away from their families. Again, if we had the means to allow high-risk women — or those for whom continuous monitoring or a cesarean section may be necessary — to give birth here safely, it would be a big step. As for abortion, it is feasible but remains a very taboo subject in the community.
Regarding violence against women, I have not observed any particularly encouraging developments in the past 5 years, but recently, we met with the mayor about this, hoping that concrete actions will be taken to help victims of violence.
Q: What is the predominant feeling in your daily life in a situation that is slow to evolve?
Dr. Prince: I remain hopeful for my patients. We must continue to fight! Initiatives must also come from the communities themselves; they must be involved in developing solutions. Because patients, too, need to have hope. They have the right to be cared for like other inhabitants of Canada.
On my part, I try to find a balance between feeling good about my caregiving profession and not burning out professionally. But burnout is a subject that concerns many doctors around the world and is increasingly being discussed. We should all have psychological support when entering medicine!
Q: Would you recommend colleagues to come and work in the Far North? What would you tell them?
Dr. Prince: I would tell them they will have no regrets! Yes, it’s difficult, but it’s a unique type of practice and very rewarding on a human level.
Professionally, it is a general practice that is no longer seen in the city today. The spectrum is very broad, ranging from neonatology to geriatrics, from the simplest to the most complex. It’s very stimulating. Diagnostically, practice is also very different from what is done in the metropolis. Without a scanner, you really have to question and investigate to evaluate whether a patient should be evacuated by plane to Montreal or not. It’s not trivial. Decisions must be made judiciously and quickly.
The human experience is also unique. Inuit communities are little known, and the aspects relayed in the media are often negative due to their increased risk for addiction. However, they are cheerful and very warm people, with an extraordinary culture. I have learned a lot from them, including reconsidering the notion of time, reviewing my priorities, and approaching life one day at a time.
I am very grateful to them for accepting me. I am sometimes even greeted with a “Welcome home!” when I return from vacation...Being told that in Nunavik, I am also “at home,” touches me immensely. I have seen children grow up, adolescents become adults. A bond of trust has developed.
Of course, all of this comes with sacrifices like being away from family and loved ones. We miss birthdays, weddings, etc. But without hesitation, it’s worth it!
Q: What are the next steps in your career in Nunavik? Will you stay for a long time?
Dr. Prince: I take it one day at a time, especially since I am about to take maternity leave very soon. But if a full-time return to Nunavik is difficult with a newborn, I know that the Nunavummiuts [inhabitants of Nunavik] will always be part of my life and my practice.
I want to remain involved with these communities, whether on-site (by practicing there a few months a year) or in Montreal where many patients are transferred. Coming to be treated in a big city (Montreal, 1.7M inhabitants), in very large hospitals, can be very stressful for them. They express themselves much less verbally than Westerners, so we must know how to listen to them, dedicate the necessary time to them, consider their culture and beliefs. I would like to be the familiar face they will encounter when they are cared for away from home. It’s a bond I want to preserve.
This story was translated from Medscape France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Rare Cutaneous Presentation of Burkitt Lymphoma
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.
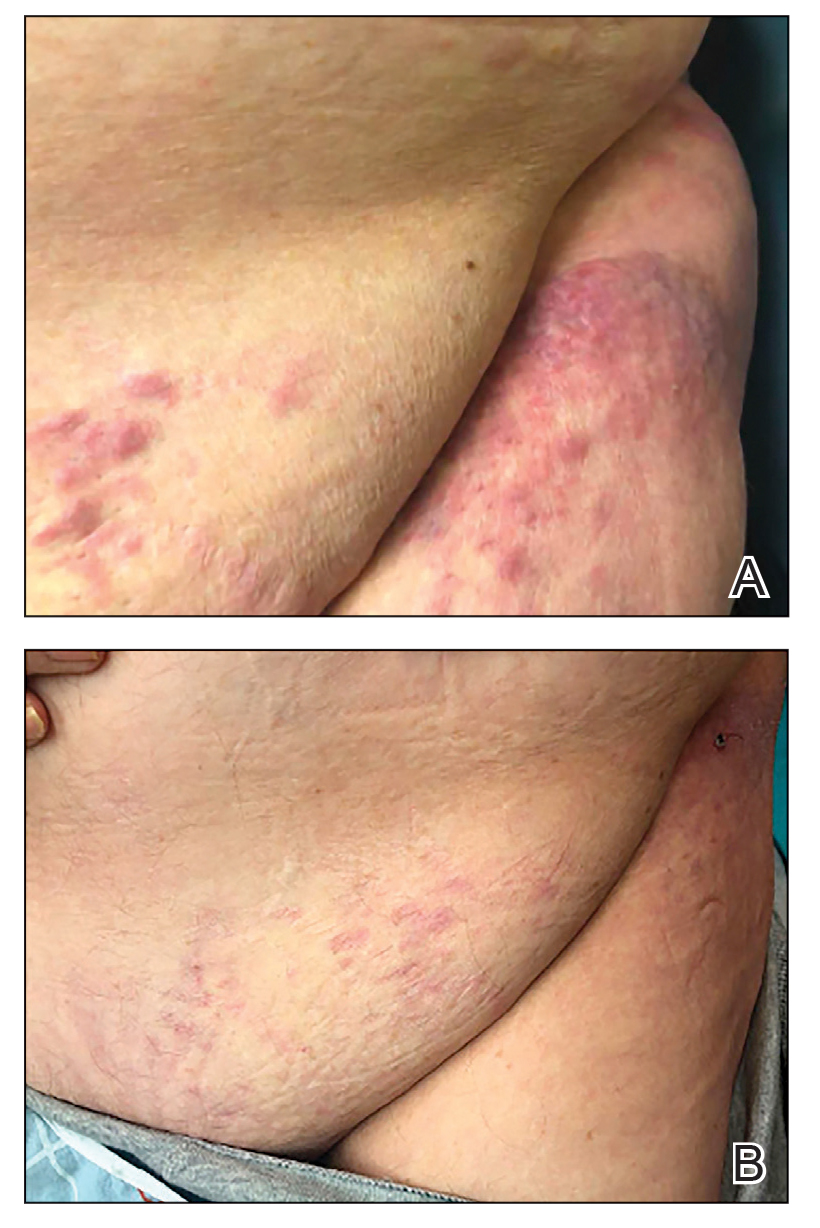
A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.
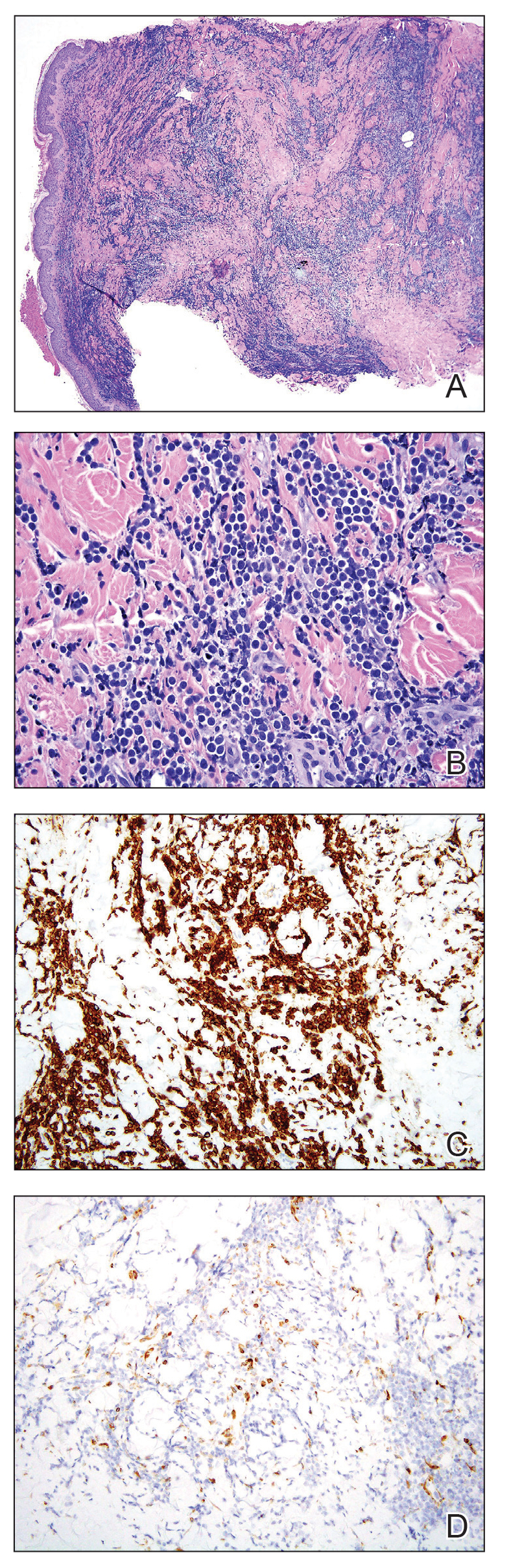
Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.

A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.

Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
To the Editor:
A 73-year-old man was admitted to the hospital with progressive abdominal and hip pain of several weeks’ duration that was accompanied by unilateral swelling of the left leg. He had a medical history of hypertension, hyperlipidemia, and prediabetes. Computed tomography (CT) showed extensive intra-abdominal, retroperitoneal, and pelvic lymphadenopathy in addition to poorly defined hepatic lesions.
A CT-guided core biopsy of a left inguinal lymph node showed Burkitt lymphoma. Fluorescence in situ hybridization was positive for oncogene c-MYC rearrangement on chromosome 8q24 and negative for B-cell lymphoma 2 (BCL2) and B-cell lymphoma 6 (BCL6) gene rearrangements. Flow cytometry demonstrated an aberrant population of κ light chain-restricted CD5−CD10+ B lymphocytes.
The patient’s overall disease burden was consistent with stage IV Burkitt lymphoma. R-miniCHOP chemotherapy—rituximab plus a reduced dose of cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone—was initiated. Approximately 2 weeks after chemotherapy was initiated, the patient developed a firm erythematous eruption on the left hip (Figure 1A). His regimen was then switched to R-EPOCH—rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, and doxorubicin—at the time of discharge, and he was referred to dermatology due to an initial concern of an adverse reaction to R-EPOCH chemotherapy. The patient denied any pain, pruritus, or irritation. Physical examination showed multifocal, subcutaneous, indurated, erythematous and violaceous nodules without epidermal changes. Some nodules on the lateral aspect of the hip coalesced to form firm plaques.

A punch biopsy specimen showed markedly atypical lymphocytes with enlarged nuclei and scant cytoplasm present throughout the dermis (Figures 2A and 2B). Numerous apoptotic cells and cellular debris were seen. Immunohistochemical staining demonstrated that the lymphocytic infiltrate comprised CD79a+ B cells that were positive for Bcl-6 and CD10 and negative for Bcl-2 (Figures 2C and 2D). There also was diminished focal expression of CD20. Ki-67 protein staining was intensely positive and demonstrated a very high proliferative index.

Taken together, these findings were consistent with a diagnosis of cutaneous metastasis of Burkitt lymphoma. The patient’s cutaneous lesions improved after continued aggressive chemotherapy. At follow-up 2 weeks after biopsy, he was receiving his second round of R-EPOCH chemotherapy with appreciable regression of skin lesions (Figure 1B). However, he then developed right-side double vision, ptosis, and right-side facial paresthesia. Although magnetic resonance imaging of the brain and lumbar puncture did not show evidence of central nervous system involvement, the chemotherapy regimen was switched to dose-adjusted CVAD-R—hypercyclophosphamide, vincristine, doxorubicin hydrochloride, and dexamethasone plus rituximab—for empiric treatment of central nervous system disease. Although treatment was complicated by sepsis with extended-spectrum β-lactamase-producing Enterobacter cloacae, Burkitt lymphoma was found to be in remission after 3 cycles of CVAD-R and 5 months of chemotherapy.
Burkitt lymphoma is a B-cell non-Hodgkin malignancy caused by translocation of chromosome 8 and chromosome 14, leading to overexpression of c-MYC and subsequent hyperproliferation of B lymphocytes.1,2 The disease is divided into 3 major categories: sporadic, endemic, and immunodeficiency related.3 The endemic variant is the most prevalent subtype in Africa and is associated with Plasmodium falciparum malaria; the sporadic variant is the most common subtype in the rest of the world.4
Burkitt lymphoma is highly aggressive and is characterized by unusually high rates of mitosis and apoptosis that result in abundant cellular debris and a distinctive starry-sky pattern on histopathology.5,6 Extranodal metastasis is common,7 but cutaneous involvement is exceedingly rare, with only a few cases having been reported.8-14 Cutaneous metastasis of Burkitt lymphoma often is associated with a high overall disease burden and poor prognosis.8,11
Immunodeficiency-related Burkitt lymphoma is particularly aggressive. Notably, 3 of 7 (42.9%) reported cases of cutaneous Burkitt lymphoma occurred in HIV-positive patients.11,13 In one case, cutaneous involvement was the first sign of relapsed disease that had been in remission.12
Although c-MYC rearrangement is required to make a diagnosis of Burkitt lymphoma, the disease also is present in a minority of cases of diffuse large B-cell lymphoma (DLBCL)(6%).15 Although DLBCL typically can be differentiated from Burkitt lymphoma by the large nuclear size and characteristic vesicular nuclei of B cells, few cases of DLBCL with c-MYC rearrangement histologically mimic Burkitt lymphoma. However, key features such as immunohistochemical staining for Bcl-2 and CD10 can be used to distinguish these 2 entities.16 Bcl-2 negativity and CD10 positivity, as seen in our patient, is considered more characteristic of Burkitt lymphoma. This staining pattern in combination with a high Ki-67 fraction (>95%) and the presence of monomorphic medium-sized cells is more consistent with a diagnosis of Burkitt lymphoma than of DLBCL.17
Earlier case reports have documented that cutaneous lesions of Burkitt lymphoma can occur in a variety of ways. Hematogenous spread is the likely route of metastasis for lesions distant to the primary site or those that have widespread distribution.18 Alternatively, other reports have suggested that cutaneous metastases can occur from local invasion and subcutaneous extension of malignant cells after a surgical procedure.10,19 For example, cutaneous Burkitt lymphoma has been reported in the setting of celioscopy, occurring directly at the surgical site.19 In our patient, we believe that the route of metastatic spread likely was through subcutaneous invasion secondary to CT-guided core biopsy, which was supported by the observation that the onset of cutaneous manifestations was temporally related to the procedure and that the lesions occurred on the skin directly overlying the biopsy site.
In conclusion, we describe an exceedingly rare presentation of cutaneous Burkitt lymphoma in which a surgical procedure likely served as an inciting event that triggered seeding of malignant cells to the skin. Cutaneous spread of Burkitt lymphoma is infrequently reported; all such reports that provide long-term follow-up data have described it in association with high disease burden and often a lethal outcome.8,11,12 Our patient had complete resolution of cutaneous lesions with chemotherapy. It is unclear if the presence of cutaneous lesions can serve as a prognostic indicator and requires further investigation. However, our case provides preliminary evidence to suggest that cutaneous metastases do not always represent aggressive disease and that cutaneous lesions may respond well to chemotherapy.
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
- Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10:56. doi:10.1186/s13244-019-0733-7
- Dunleavy K, Gross TG. Management of aggressive B-cell NHLs in the AYA population: an adult vs pediatric perspective. Blood. 2018;132:369-375. doi:10.1182/blood-2018-02-778480
- Noy A. Burkitt lymphoma—subtypes, pathogenesis, and treatment strategies. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S37-S38. doi:10.1016/S2152-2650(20)30455-9
- Lenze D, Leoncini L, Hummel M, et al. The different epidemiologic subtypes of Burkitt lymphoma share a homogenous micro RNA profile distinct from diffuse large B-cell lymphoma. Leukemia. 2011;25:1869-1876. doi:10.1038/leu.2011.156
- Bellan C, Lazzi S, De Falco G, et al. Burkitt’s lymphoma: new insights into molecular pathogenesis. J Clin Pathol. 2003;56:188-192. doi:10.1136/jcp.56.3.188
- Chuang S-S, Ye H, Du M-Q, et al. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007;128:558-564. doi:10.1309/EQJR3D3V0CCQGP04
- Baker PS, Gold KG, Lane KA, et al. Orbital burkitt lymphoma in immunocompetent patients: a report of 3 cases and a review of the literature. Ophthalmic Plast Reconstr Surg. 2009;25:464-468. doi:10.1097/IOP.0b013e3181b80fde
- Fuhrmann TL, Ignatovich YV, Pentland A. Cutaneous metastatic disease: Burkitt lymphoma. J Am Acad Dermatol. 2011;64:1196-1197. doi:10.1016/j.jaad.2009.08.033
- Burns CA, Scott GA, Miller CC. Leukemia cutis at the site of trauma in a patient with Burkitt leukemia. Cutis. 2005;75:54-56.
- Jacobson MA, Hutcheson ACS, Hurray DH, et al. Cutaneous involvement by Burkitt lymphoma. J Am Acad Dermatol. 2006;54:1111-1113. doi:10.1016/j.jaad.2006.02.030
- Berk DR, Cheng A, Lind AC, et al. Burkitt lymphoma with cutaneous involvement. Dermatol Online J. 2008;14:14.
- Bachmeyer C, Bazarbachi A, Rio B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54:176. doi:10.1002/(sici)1096-8652(199702)54:2<176::aid-ajh20>3.0.co;2-c
- Rogers A, Graves M, Toscano M, et al. A unique cutaneous presentation of Burkitt lymphoma. Am J Dermatopathol. 2014;36:997-1001. doi:10.1097/DAD.0000000000000004
- Thakkar D, Lipi L, Misra R, et al. Skin involvement in Burkitt’s lymphoma. Hematol Oncol Stem Cell Ther. 2018;11:251-252. doi:10.1016/j.hemonc.2018.01.002
- Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18:510-518. doi:10.1200/JCO.2000.18.3.510
- Nakamura N, Nakamine H, Tamaru J-I, et al. The distinction between Burkitt lymphoma and diffuse large B-cell lymphoma with c-myc rearrangement. Mod Pathol. 2002;15:771-776. doi:10.1097/01.MP.0000019577.73786.64
- Bellan C, Stefano L, Giulia de F, et al. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010;28:53-56. doi:10.1002/hon.916
- Amonchaisakda N, Aiempanakit K, Apinantriyo B. Burkitt lymphoma initially mimicking varicella zoster infection. IDCases. 2020;21:E00818. doi:10.1016/j.idcr.2020.e00818
- Aractingi S, Marolleau JP, Daniel MT, et al. Subcutaneous localizations of Burkitt lymphoma after celioscopy. Am J Hematol. 1993;42:408. doi:10.1002/ajh.2830420421
Practice Points
- Cutaneous metastasis is exceedingly rare in Burkitt lymphoma. When cutaneous involvement does occur, it can represent an uncommon consequence of a surgical procedure, serving as the inciting event for hematogenous spread and local tumor extension into the skin.
- Although cutaneous metasis of Burkitt lymphoma typically is associated with high disease burden and mortality, our case demonstrated that cutaneous spread can be present even in a patient who has a positive outcome. Our patient was able to achieve disease remission and complete resolution of cutaneous lesions with continued chemotherapy, suggesting that cutaneous metastasis does not always portend a poor prognosis.
Hormones and Viruses Influence Each Other: Consider These Connections in Your Patients
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Stefan Bornstein, MD, PhD, professor, made it clear during a press conference at the 67th Congress of the German Society of Endocrinology (DGE) that there is more than one interaction between them. Nowadays, one can almost speak of an “endocrine virology and even of the virome as an additional, hormonally metabolically active gland,” said Dr. Bornstein, who will receive the Berthold Medal from the DGE in 2024.
Many questions remain unanswered: “We need a better understanding of the interaction of hormone systems with infectious agents — from basics to therapeutic applications,” emphasized the director of the Medical Clinic and Polyclinic III and the Center for Internal Medicine at the Carl Gustav Carus University Hospital, Dresden, Germany.
If infectious diseases could trigger diabetes and other metabolic diseases, this means that “through vaccination programs, we may be able to prevent the occurrence of common metabolic diseases such as diabetes,” said Dr. Bornstein. He highlighted that many people who experienced severe COVID-19 during the pandemic, or died from it, exhibited diabetes or a pre-metabolic syndrome.
“SARS-CoV-2 has utilized an endocrine signaling pathway to invade our cells and cause damage in the organ systems and inflammation,” said Dr. Bornstein. Conversely, it is now known that infections with coronaviruses or other infectious agents like influenza can significantly worsen metabolic status, diabetes, and other endocrine diseases.
SARS-CoV-2 Infects the Beta Cells
Data from the COVID-19 pandemic showed that the likelihood of developing type 1 diabetes significantly increases with a SARS-CoV-2 infection. Researchers led by Dr. Bornstein demonstrated in 2021 that SARS-CoV-2 can infect the insulin-producing cells of the organ. They examined pancreatic tissue from 20 patients who died from COVID-19 using immunofluorescence, immunohistochemistry, RNA in situ hybridization, and electron microscopy.
They found viral SARS-CoV-2 infiltration of the beta cells in all patients. In 11 patients with COVID-19, the expression of ACE2, TMPRSS, and other receptors and factors like DPP4, HMBG1, and NRP1 that can facilitate virus entry was examined. They found that even in the absence of manifest newly onset diabetes, necroptotic cell death, immune cell infiltration, and SARS-CoV-2 infection of the pancreas beta cells can contribute to varying degrees of metabolic disturbance in patients with COVID-19.
In a report published in October 2020, Tim Hollstein, MD, from the Institute for Diabetology and Clinical Metabolic Research at UKSH in Kiel, Germany, and colleagues described the case of a 19-year-old man who developed symptoms of insulin-dependent diabetes after a SARS-CoV-2 infection, without the presence of autoantibodies typical for type 1 diabetes.
The man presented to the emergency department with diabetic ketoacidosis, a C-peptide level of 0.62 µg/L, a blood glucose concentration of 30.6 mmol/L (552 mg/dL), and an A1c level of 16.8%. The patient’s history revealed a probable SARS-CoV-2 infection 5-7 weeks before admission, based on a positive antibody test against SARS-CoV-2.
Some Viruses Produce Insulin-Like Proteins
Recent studies have shown that some viruses can produce insulin-like proteins or hormones that interfere with the metabolism of the affected organism, reported Dr. Bornstein. In addition to metabolic regulation, these “viral hormones” also seem to influence cell turnover and cell death.
Dr. Bornstein pointed out that antiviral medications can delay the onset of type 1 diabetes by preserving the function of insulin-producing beta cells. It has also been shown that conventional medications used to treat hormonal disorders can reduce the susceptibility of the organism to infections — such as antidiabetic preparations like DPP-4 inhibitors or metformin.
In a review published in 2023, Nikolaos Perakakis, MD, professor, research group leader at the Paul Langerhans Institute Dresden, Dresden, Germany, Dr. Bornstein, and colleagues discussed scientific evidence for a close mutual dependence between various virus infections and metabolic diseases. They discussed how viruses can lead to the development or progression of metabolic diseases and vice versa and how metabolic diseases can increase the severity of a virus infection.
Viruses Favor Metabolic Diseases...
Viruses can favor metabolic diseases by, for example, influencing the regulation of cell survival and specific signaling pathways relevant for cell death, proliferation, or dedifferentiation in important endocrine and metabolic organs. Viruses are also capable of controlling cellular glucose metabolism by modulating glucose transporters, altering glucose uptake, regulating signaling pathways, and stimulating glycolysis in infected cells.
Due to the destruction of beta cells, enteroviruses, but also the mumps virus, parainfluenza virus, or human herpes virus 6, are associated with the development of diabetes. The timing of infection often precedes or coincides with the peak of development of islet autoantibodies. The fact that only a small proportion of patients actually develop type 1 diabetes suggests that genetic background, and likely the timing of infection, play an important role.
...And Metabolic Diseases Influence the Course of Infection
Infection with hepatitis C virus (HCV), on the other hand, is associated with an increased risk for type 2 diabetes, with the risk being higher for older individuals with a family history of diabetes. The negative effects of HCV on glucose balance are mainly attributed to increased insulin resistance in the liver. HCV reduces hepatic glucose uptake by downregulating the expression of glucose transporters and additionally impairs insulin signal transduction by inhibiting the PI3K/Akt signaling pathway.
People with obesity, diabetes, or insulin resistance show significant changes in the innate and adaptive functions of the immune system. Regarding the innate immune system, impaired chemotaxis and phagocytosis of neutrophils have been observed in patients with type 2 diabetes.
In the case of obesity, the number of natural killer T cells in adipose tissue decreases, whereas B cells accumulate in adipose tissue and secrete more proinflammatory cytokines. Longitudinal multiomics analyses of various biopsies from individuals with insulin resistance showed a delayed immune response to respiratory virus infections compared with individuals with normal insulin sensitivity.
This story was translated from Medscape Germany using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Each Minute of Screen Time May Affect Toddlers’ Development
TOPLINE:
New research shows increased screen time in children aged 12-36 months is associated with reduced verbal interactions between toddlers and their parents, which in turn could affect language development.
METHODOLOGY:
- The study included data from 220 families in Australia.
- Researchers used advanced speech recognition technology to capture children’s screen time and language environment at home during a 16-hour window every 6 months.
- They adjusted for variables such as the sex of the child, the education level of the mother, and psychological distress in the primary caregiver.
TAKEAWAY:
- Increases in screen time were associated with decreases in words spoken near children by adults, vocalizations by children, and back-and-forth interactions between adults and children. This correlation was especially notable at age 36 months.
- At age 36 months, each additional minute of screen time was linked to children hearing 6.6 fewer adult words, making 4.9 fewer vocalizations, and participating in 1.1 fewer conversational interactions.
- Based on the average daily screen time at that age seen in the study — 172 minutes (2.87 hours) — “children could be missing out on 1139 adult words, 843 vocalizations, and 194 conversational turns per day,” the researchers estimated.
IN PRACTICE:
“Identifying different ways that screen time could facilitate parent-child interactions, such as through interactive co-viewing, may be important strategies to support families given the current ubiquitous nature of screen time in families’ lives,” the authors of the study wrote.
What children watch and listen to may be an important consideration, according to a developmental scientist who was not involved with the study.
“It could be that less communicative contact with the caregiver is not as detrimental if the screen time is of high quality and developmentally appropriate, educational content,” Marina Bazhydai, PhD, with Lancaster University in Lancaster, United Kingdom, said in her comments on the research.
SOURCE:
Mary E. Brushe, PhD, with Telethon Kids Institute and the University of Western Australia in Adelaide, was the corresponding author of the study. The research was published online in JAMA Pediatrics.
LIMITATIONS:
The study’s reliance on speech recognition technology did not capture all nuances of screen exposure.
DISCLOSURES:
This study was supported by grants from the Australian National Health and Medical Research Council.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
New research shows increased screen time in children aged 12-36 months is associated with reduced verbal interactions between toddlers and their parents, which in turn could affect language development.
METHODOLOGY:
- The study included data from 220 families in Australia.
- Researchers used advanced speech recognition technology to capture children’s screen time and language environment at home during a 16-hour window every 6 months.
- They adjusted for variables such as the sex of the child, the education level of the mother, and psychological distress in the primary caregiver.
TAKEAWAY:
- Increases in screen time were associated with decreases in words spoken near children by adults, vocalizations by children, and back-and-forth interactions between adults and children. This correlation was especially notable at age 36 months.
- At age 36 months, each additional minute of screen time was linked to children hearing 6.6 fewer adult words, making 4.9 fewer vocalizations, and participating in 1.1 fewer conversational interactions.
- Based on the average daily screen time at that age seen in the study — 172 minutes (2.87 hours) — “children could be missing out on 1139 adult words, 843 vocalizations, and 194 conversational turns per day,” the researchers estimated.
IN PRACTICE:
“Identifying different ways that screen time could facilitate parent-child interactions, such as through interactive co-viewing, may be important strategies to support families given the current ubiquitous nature of screen time in families’ lives,” the authors of the study wrote.
What children watch and listen to may be an important consideration, according to a developmental scientist who was not involved with the study.
“It could be that less communicative contact with the caregiver is not as detrimental if the screen time is of high quality and developmentally appropriate, educational content,” Marina Bazhydai, PhD, with Lancaster University in Lancaster, United Kingdom, said in her comments on the research.
SOURCE:
Mary E. Brushe, PhD, with Telethon Kids Institute and the University of Western Australia in Adelaide, was the corresponding author of the study. The research was published online in JAMA Pediatrics.
LIMITATIONS:
The study’s reliance on speech recognition technology did not capture all nuances of screen exposure.
DISCLOSURES:
This study was supported by grants from the Australian National Health and Medical Research Council.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
New research shows increased screen time in children aged 12-36 months is associated with reduced verbal interactions between toddlers and their parents, which in turn could affect language development.
METHODOLOGY:
- The study included data from 220 families in Australia.
- Researchers used advanced speech recognition technology to capture children’s screen time and language environment at home during a 16-hour window every 6 months.
- They adjusted for variables such as the sex of the child, the education level of the mother, and psychological distress in the primary caregiver.
TAKEAWAY:
- Increases in screen time were associated with decreases in words spoken near children by adults, vocalizations by children, and back-and-forth interactions between adults and children. This correlation was especially notable at age 36 months.
- At age 36 months, each additional minute of screen time was linked to children hearing 6.6 fewer adult words, making 4.9 fewer vocalizations, and participating in 1.1 fewer conversational interactions.
- Based on the average daily screen time at that age seen in the study — 172 minutes (2.87 hours) — “children could be missing out on 1139 adult words, 843 vocalizations, and 194 conversational turns per day,” the researchers estimated.
IN PRACTICE:
“Identifying different ways that screen time could facilitate parent-child interactions, such as through interactive co-viewing, may be important strategies to support families given the current ubiquitous nature of screen time in families’ lives,” the authors of the study wrote.
What children watch and listen to may be an important consideration, according to a developmental scientist who was not involved with the study.
“It could be that less communicative contact with the caregiver is not as detrimental if the screen time is of high quality and developmentally appropriate, educational content,” Marina Bazhydai, PhD, with Lancaster University in Lancaster, United Kingdom, said in her comments on the research.
SOURCE:
Mary E. Brushe, PhD, with Telethon Kids Institute and the University of Western Australia in Adelaide, was the corresponding author of the study. The research was published online in JAMA Pediatrics.
LIMITATIONS:
The study’s reliance on speech recognition technology did not capture all nuances of screen exposure.
DISCLOSURES:
This study was supported by grants from the Australian National Health and Medical Research Council.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
OTC Birth Control Pill Headed to US Pharmacies: What Your Patients Should Know
Primary care clinicians have largely welcomed the arrival of Opill, the first over-the-counter (OTC) birth control pill from Perrigo, which will reach US pharmacy shelves this month. Although the medicine has a long-track record of safe use, physicians and nurse practitioners may want to ready themselves to answer questions from patients about shifting to the option.
The switch to OTC status for the norgestrel-only contraceptive has the support of many physician groups, including the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists (ACOG).
The end of the prescription-requirement removes a barrier to access for many women, especially those who lack insurance. But it also will take away a chief reason many women in their childbearing years make appointments with doctors, as they will no longer need prescriptions for birth control pills.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology at University of Washington School of Medicine, in Seattle, Washington, said she is also worried that the availability of an OTC pill will lead to missed opportunities to help patients avoid sexually transmitted diseases. For example, patients can get counseling about the need for testing for sexually transmitted diseases at the start of new relationships during a visit made to obtain a prescription for the pill.
“My hope is that they still follow our recommendations, which is to get tested with every partner,” said Dr. Oelschlager, who cares for many patients in their teens. “Adolescents are at a particularly high risk of infection compared to older ones.”
When clinicians do see patients, they may want to raise the issue of the OTC option and proper use. Patients will need to closely read materials provided for Opill, a step they might skip due to the ready access, according to Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, which scrutinizes the safety of medical products.
“When something is sold over the counter, it’s perceived by individuals as being safe,” Dr. Zuckerman told this news organization. “There’s less concern and a little less interest in reading the instructions and reading the warnings.”
Considerations for Safety
The US Food and Drug Administration (FDA) in July approved the sales of a daily 0.075 mg norgestrel tablet without prescription. Perrigo told this news organization that it spent the intervening months ensuring retailers and consumers will receive education on the drug.
One of the biggest challenges for people using Opill may be sticking with the dosing schedule, according to Dr. Oelschlager.
“There are going to be people that have a harder time remembering to take a pill every day,” at the same time, said Dr. Oelschlager, who is chair of ACOG’s Clinical Consensus Gynecology Committee. “We need to watch and see what happens as it becomes more widely available, and people start using it.”
Unexpected vaginal bleeding is the most common adverse event linked to this form of birth control, with over one fifth of participants from one study of the OTC drug reporting this side effect, according to an FDA memo.
“It is more likely to be a tolerability issue rather than a safety issue,” the FDA wrote.
Many prescription of birth control options contain estrogen, which is associated with venous thromboembolism (VTE). But Opill contains only norgestrel, a form of progestin, which is not associated with thrombosis. Patients may be more likely to overestimate their potential risks for VTE than to underestimate them, according to Kwuan Paruchabutr, DNP, president of National Association of Nurse Practitioners in Women’s Health and an assistant professor at Georgetown University in Washington, DC.
“This is a progesterone-only pill: The risk is relatively low” of VTE, Dr. Paruchabutr said.
Clinicians should also take special care with patients who are prescribed drugs for seizures, tuberculosis, HIV/AIDS, and pulmonary hypertension or who are taking supplements containing St John’s wort.
Patients in their childbearing years who take isotretinoin are already expected to use some form of birth control.
“All patients on isotretinoin must be registered in the iPLEDGE program, which mandates monthly contraception counseling and monthly pregnancy tests for persons of childbearing potential,” Terrence A. Cronin, Jr, MD, president of the American Academy of Dermatology, told news organization through email.
Dr. Oelschlager noted that many patients who take isotretinoin may benefit from taking a birth control pill containing estrogen, for which they will need a prescription. At least three pills have an FDA-approved indication for treating moderate acne, including Ortho Tri-Cyclen, Estrostep, and Yaz.
The FDA has posted consumer-friendly information about the OTC pill that clinicians can refer their patients to. For clinicians who want more information, ACOG released a practice advisory about the switch in status for this progestin-only pill.
The Cost
While federal laws mandate employer-based and Medicaid plans cover prescription birth control pills for free, the OTC version will carry a cost, according to A. Mark Fendrick, MD, director of the University of Michigan Center for Value-Based Insurance Design in Ann Arbor, Michigan.
Seven states, including New Mexico and New York, already have laws in effect that require health plans to cover certain OTC contraceptives without a prescription, according to a tally kept by the nonprofit research organization KFF.
Dr. Fendrick said it would be helpful for health plans to offer coverage for the OTC pill without copays even if they are not required to do so.
Priced at about $20 a month, Opill “is likely out of reach for many of the individuals who would most benefit from an OTC option,” Dr. Fendrick told this news organization in an email.
The new pill may be utilized most by those who do not have health insurance or have low incomes and cannot afford to see a doctor for a prescription, according to Sally Rafie, PharmD, a pharmacist specialist at University of California San Diego Health and founder of the Birth Control Pharmacist.
The manufacturer’s suggested retail prices will be $19.99 for a 1-month supply and $49.99 for a 3-month supply. Dublin-based Perrigo said it plans to offer a cost-assistance program for the drug in the coming weeks for people who have low incomes and lack insurance.
A version of this article appeared on Medscape.com.
Primary care clinicians have largely welcomed the arrival of Opill, the first over-the-counter (OTC) birth control pill from Perrigo, which will reach US pharmacy shelves this month. Although the medicine has a long-track record of safe use, physicians and nurse practitioners may want to ready themselves to answer questions from patients about shifting to the option.
The switch to OTC status for the norgestrel-only contraceptive has the support of many physician groups, including the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists (ACOG).
The end of the prescription-requirement removes a barrier to access for many women, especially those who lack insurance. But it also will take away a chief reason many women in their childbearing years make appointments with doctors, as they will no longer need prescriptions for birth control pills.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology at University of Washington School of Medicine, in Seattle, Washington, said she is also worried that the availability of an OTC pill will lead to missed opportunities to help patients avoid sexually transmitted diseases. For example, patients can get counseling about the need for testing for sexually transmitted diseases at the start of new relationships during a visit made to obtain a prescription for the pill.
“My hope is that they still follow our recommendations, which is to get tested with every partner,” said Dr. Oelschlager, who cares for many patients in their teens. “Adolescents are at a particularly high risk of infection compared to older ones.”
When clinicians do see patients, they may want to raise the issue of the OTC option and proper use. Patients will need to closely read materials provided for Opill, a step they might skip due to the ready access, according to Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, which scrutinizes the safety of medical products.
“When something is sold over the counter, it’s perceived by individuals as being safe,” Dr. Zuckerman told this news organization. “There’s less concern and a little less interest in reading the instructions and reading the warnings.”
Considerations for Safety
The US Food and Drug Administration (FDA) in July approved the sales of a daily 0.075 mg norgestrel tablet without prescription. Perrigo told this news organization that it spent the intervening months ensuring retailers and consumers will receive education on the drug.
One of the biggest challenges for people using Opill may be sticking with the dosing schedule, according to Dr. Oelschlager.
“There are going to be people that have a harder time remembering to take a pill every day,” at the same time, said Dr. Oelschlager, who is chair of ACOG’s Clinical Consensus Gynecology Committee. “We need to watch and see what happens as it becomes more widely available, and people start using it.”
Unexpected vaginal bleeding is the most common adverse event linked to this form of birth control, with over one fifth of participants from one study of the OTC drug reporting this side effect, according to an FDA memo.
“It is more likely to be a tolerability issue rather than a safety issue,” the FDA wrote.
Many prescription of birth control options contain estrogen, which is associated with venous thromboembolism (VTE). But Opill contains only norgestrel, a form of progestin, which is not associated with thrombosis. Patients may be more likely to overestimate their potential risks for VTE than to underestimate them, according to Kwuan Paruchabutr, DNP, president of National Association of Nurse Practitioners in Women’s Health and an assistant professor at Georgetown University in Washington, DC.
“This is a progesterone-only pill: The risk is relatively low” of VTE, Dr. Paruchabutr said.
Clinicians should also take special care with patients who are prescribed drugs for seizures, tuberculosis, HIV/AIDS, and pulmonary hypertension or who are taking supplements containing St John’s wort.
Patients in their childbearing years who take isotretinoin are already expected to use some form of birth control.
“All patients on isotretinoin must be registered in the iPLEDGE program, which mandates monthly contraception counseling and monthly pregnancy tests for persons of childbearing potential,” Terrence A. Cronin, Jr, MD, president of the American Academy of Dermatology, told news organization through email.
Dr. Oelschlager noted that many patients who take isotretinoin may benefit from taking a birth control pill containing estrogen, for which they will need a prescription. At least three pills have an FDA-approved indication for treating moderate acne, including Ortho Tri-Cyclen, Estrostep, and Yaz.
The FDA has posted consumer-friendly information about the OTC pill that clinicians can refer their patients to. For clinicians who want more information, ACOG released a practice advisory about the switch in status for this progestin-only pill.
The Cost
While federal laws mandate employer-based and Medicaid plans cover prescription birth control pills for free, the OTC version will carry a cost, according to A. Mark Fendrick, MD, director of the University of Michigan Center for Value-Based Insurance Design in Ann Arbor, Michigan.
Seven states, including New Mexico and New York, already have laws in effect that require health plans to cover certain OTC contraceptives without a prescription, according to a tally kept by the nonprofit research organization KFF.
Dr. Fendrick said it would be helpful for health plans to offer coverage for the OTC pill without copays even if they are not required to do so.
Priced at about $20 a month, Opill “is likely out of reach for many of the individuals who would most benefit from an OTC option,” Dr. Fendrick told this news organization in an email.
The new pill may be utilized most by those who do not have health insurance or have low incomes and cannot afford to see a doctor for a prescription, according to Sally Rafie, PharmD, a pharmacist specialist at University of California San Diego Health and founder of the Birth Control Pharmacist.
The manufacturer’s suggested retail prices will be $19.99 for a 1-month supply and $49.99 for a 3-month supply. Dublin-based Perrigo said it plans to offer a cost-assistance program for the drug in the coming weeks for people who have low incomes and lack insurance.
A version of this article appeared on Medscape.com.
Primary care clinicians have largely welcomed the arrival of Opill, the first over-the-counter (OTC) birth control pill from Perrigo, which will reach US pharmacy shelves this month. Although the medicine has a long-track record of safe use, physicians and nurse practitioners may want to ready themselves to answer questions from patients about shifting to the option.
The switch to OTC status for the norgestrel-only contraceptive has the support of many physician groups, including the American Medical Association, the American Academy of Family Physicians, and the American College of Obstetricians and Gynecologists (ACOG).
The end of the prescription-requirement removes a barrier to access for many women, especially those who lack insurance. But it also will take away a chief reason many women in their childbearing years make appointments with doctors, as they will no longer need prescriptions for birth control pills.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology at University of Washington School of Medicine, in Seattle, Washington, said she is also worried that the availability of an OTC pill will lead to missed opportunities to help patients avoid sexually transmitted diseases. For example, patients can get counseling about the need for testing for sexually transmitted diseases at the start of new relationships during a visit made to obtain a prescription for the pill.
“My hope is that they still follow our recommendations, which is to get tested with every partner,” said Dr. Oelschlager, who cares for many patients in their teens. “Adolescents are at a particularly high risk of infection compared to older ones.”
When clinicians do see patients, they may want to raise the issue of the OTC option and proper use. Patients will need to closely read materials provided for Opill, a step they might skip due to the ready access, according to Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, which scrutinizes the safety of medical products.
“When something is sold over the counter, it’s perceived by individuals as being safe,” Dr. Zuckerman told this news organization. “There’s less concern and a little less interest in reading the instructions and reading the warnings.”
Considerations for Safety
The US Food and Drug Administration (FDA) in July approved the sales of a daily 0.075 mg norgestrel tablet without prescription. Perrigo told this news organization that it spent the intervening months ensuring retailers and consumers will receive education on the drug.
One of the biggest challenges for people using Opill may be sticking with the dosing schedule, according to Dr. Oelschlager.
“There are going to be people that have a harder time remembering to take a pill every day,” at the same time, said Dr. Oelschlager, who is chair of ACOG’s Clinical Consensus Gynecology Committee. “We need to watch and see what happens as it becomes more widely available, and people start using it.”
Unexpected vaginal bleeding is the most common adverse event linked to this form of birth control, with over one fifth of participants from one study of the OTC drug reporting this side effect, according to an FDA memo.
“It is more likely to be a tolerability issue rather than a safety issue,” the FDA wrote.
Many prescription of birth control options contain estrogen, which is associated with venous thromboembolism (VTE). But Opill contains only norgestrel, a form of progestin, which is not associated with thrombosis. Patients may be more likely to overestimate their potential risks for VTE than to underestimate them, according to Kwuan Paruchabutr, DNP, president of National Association of Nurse Practitioners in Women’s Health and an assistant professor at Georgetown University in Washington, DC.
“This is a progesterone-only pill: The risk is relatively low” of VTE, Dr. Paruchabutr said.
Clinicians should also take special care with patients who are prescribed drugs for seizures, tuberculosis, HIV/AIDS, and pulmonary hypertension or who are taking supplements containing St John’s wort.
Patients in their childbearing years who take isotretinoin are already expected to use some form of birth control.
“All patients on isotretinoin must be registered in the iPLEDGE program, which mandates monthly contraception counseling and monthly pregnancy tests for persons of childbearing potential,” Terrence A. Cronin, Jr, MD, president of the American Academy of Dermatology, told news organization through email.
Dr. Oelschlager noted that many patients who take isotretinoin may benefit from taking a birth control pill containing estrogen, for which they will need a prescription. At least three pills have an FDA-approved indication for treating moderate acne, including Ortho Tri-Cyclen, Estrostep, and Yaz.
The FDA has posted consumer-friendly information about the OTC pill that clinicians can refer their patients to. For clinicians who want more information, ACOG released a practice advisory about the switch in status for this progestin-only pill.
The Cost
While federal laws mandate employer-based and Medicaid plans cover prescription birth control pills for free, the OTC version will carry a cost, according to A. Mark Fendrick, MD, director of the University of Michigan Center for Value-Based Insurance Design in Ann Arbor, Michigan.
Seven states, including New Mexico and New York, already have laws in effect that require health plans to cover certain OTC contraceptives without a prescription, according to a tally kept by the nonprofit research organization KFF.
Dr. Fendrick said it would be helpful for health plans to offer coverage for the OTC pill without copays even if they are not required to do so.
Priced at about $20 a month, Opill “is likely out of reach for many of the individuals who would most benefit from an OTC option,” Dr. Fendrick told this news organization in an email.
The new pill may be utilized most by those who do not have health insurance or have low incomes and cannot afford to see a doctor for a prescription, according to Sally Rafie, PharmD, a pharmacist specialist at University of California San Diego Health and founder of the Birth Control Pharmacist.
The manufacturer’s suggested retail prices will be $19.99 for a 1-month supply and $49.99 for a 3-month supply. Dublin-based Perrigo said it plans to offer a cost-assistance program for the drug in the coming weeks for people who have low incomes and lack insurance.
A version of this article appeared on Medscape.com.