User login
Triple-drug therapy proves effective in CF patients with most common mutation
Reinforcing previous findings, a new study has determined that the next-generation corrector elexacaftor, in combination with tezacaftor and ivacaftor, can effectively treat patients with Phe508del-minimal function genotypes who did not respond to previous cystic fibrosis transmembrane conductance regulator (CFTR) modulator regimens.
“These results provide evidence that , thus addressing the underlying cause of disease in the large majority of patients,” wrote Peter G. Middleton, PhD, of the University of Sydney (Australia) and his coauthors. The study was published in the New England Journal of Medicine.
To further determine if the elexacaftor-tezacaftor-ivacaftor regimen was effective and safe, the researchers launched a randomized, placebo-controlled phase 3 trial of 403 cystic fibrosis patients age 12 or older who had a single Phe508del allele. Patients in the combination group (n = 200) received 200 mg of elexacaftor once daily, 100 mg of tezacaftor once daily, and 150 mg of ivacaftor every 12 hours for 24 weeks. Patients in the other group (n = 203) received matched placebos.
At 14 weeks, patients in the combination group had a change in percentage of predicted forced expiratory volume in 1 second (FEV1) that was 13.8 points higher than the placebo group (95% confidence interval, 12.1-15.4, P less than .001). At 24 weeks, the combination group had a predicted FEV1 difference that was 14.3 percentage points higher (95% confidence interval, 12.7-15.8, P less than .001). The rate of pulmonary exacerbations was 63% lower (rate ratio 0.37; 95% CI, 0.25-0.55, P less than .001) and sweat chloride concentration was 41.8 mmol/L lower (95% CI, –44.4 to –39.3, P less than .001) in the combination group through 24 weeks.
At least one adverse event occurred in 93.1% of patients in the combination group and 96% of patients in the placebo group. Serious adverse events occurred in 28 patients (13.9%) in the combination group and 42 patients (20.9%) in the placebo group. There were no deaths in either group.
The study was funded by Vertex Pharmaceuticals. The authors had disclosures, including receiving personal fees and grants from various pharmaceutical companies and being on the advisory board, owning stock, or being an employee of Vertex Pharmaceuticals.
SOURCE: Middleton PG et al. 2019 Oct 31. N Engl J Med. doi: 10.1056/NEJMoa1908639.
After 30 years, new research from Middleton et al. and others appears to be the breakthrough we’ve been waiting for in treating cystic fibrosis, wrote Francis S. Collins, MD, PhD, of the National Institutes of Health in an accompanying editorial (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMe1911602).
As one of the researchers who discovered the cystic fibrosis gene, he acknowledged the 3 decades of work that followed their discovery and the excitement that comes from being able to counter the common Phe508del CFTR mutation that afflicts so many cystic fibrosis patients. “These findings indicate that it may soon be possible to offer safe and effective molecularly targeted therapies to 90% of persons with cystic fibrosis,” he wrote.
“Yet we must not abandon the patients with cystic fibrosis who have null mutations and will not have a response to these drugs,” he added, noting that those challenges remain “substantial” and potentially will involve in vivo somatic-cell gene editing of airway epithelial cells. That said, what once was a dream 30 years ago now appears to be a reality.
Dr. Collins reported being a coinventor of the original patents on the CFTR gene, for which he donated all royalties to the Cystic Fibrosis Foundation.
After 30 years, new research from Middleton et al. and others appears to be the breakthrough we’ve been waiting for in treating cystic fibrosis, wrote Francis S. Collins, MD, PhD, of the National Institutes of Health in an accompanying editorial (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMe1911602).
As one of the researchers who discovered the cystic fibrosis gene, he acknowledged the 3 decades of work that followed their discovery and the excitement that comes from being able to counter the common Phe508del CFTR mutation that afflicts so many cystic fibrosis patients. “These findings indicate that it may soon be possible to offer safe and effective molecularly targeted therapies to 90% of persons with cystic fibrosis,” he wrote.
“Yet we must not abandon the patients with cystic fibrosis who have null mutations and will not have a response to these drugs,” he added, noting that those challenges remain “substantial” and potentially will involve in vivo somatic-cell gene editing of airway epithelial cells. That said, what once was a dream 30 years ago now appears to be a reality.
Dr. Collins reported being a coinventor of the original patents on the CFTR gene, for which he donated all royalties to the Cystic Fibrosis Foundation.
After 30 years, new research from Middleton et al. and others appears to be the breakthrough we’ve been waiting for in treating cystic fibrosis, wrote Francis S. Collins, MD, PhD, of the National Institutes of Health in an accompanying editorial (N Engl J Med. 2019 Oct 31. doi: 10.1056/NEJMe1911602).
As one of the researchers who discovered the cystic fibrosis gene, he acknowledged the 3 decades of work that followed their discovery and the excitement that comes from being able to counter the common Phe508del CFTR mutation that afflicts so many cystic fibrosis patients. “These findings indicate that it may soon be possible to offer safe and effective molecularly targeted therapies to 90% of persons with cystic fibrosis,” he wrote.
“Yet we must not abandon the patients with cystic fibrosis who have null mutations and will not have a response to these drugs,” he added, noting that those challenges remain “substantial” and potentially will involve in vivo somatic-cell gene editing of airway epithelial cells. That said, what once was a dream 30 years ago now appears to be a reality.
Dr. Collins reported being a coinventor of the original patents on the CFTR gene, for which he donated all royalties to the Cystic Fibrosis Foundation.
Reinforcing previous findings, a new study has determined that the next-generation corrector elexacaftor, in combination with tezacaftor and ivacaftor, can effectively treat patients with Phe508del-minimal function genotypes who did not respond to previous cystic fibrosis transmembrane conductance regulator (CFTR) modulator regimens.
“These results provide evidence that , thus addressing the underlying cause of disease in the large majority of patients,” wrote Peter G. Middleton, PhD, of the University of Sydney (Australia) and his coauthors. The study was published in the New England Journal of Medicine.
To further determine if the elexacaftor-tezacaftor-ivacaftor regimen was effective and safe, the researchers launched a randomized, placebo-controlled phase 3 trial of 403 cystic fibrosis patients age 12 or older who had a single Phe508del allele. Patients in the combination group (n = 200) received 200 mg of elexacaftor once daily, 100 mg of tezacaftor once daily, and 150 mg of ivacaftor every 12 hours for 24 weeks. Patients in the other group (n = 203) received matched placebos.
At 14 weeks, patients in the combination group had a change in percentage of predicted forced expiratory volume in 1 second (FEV1) that was 13.8 points higher than the placebo group (95% confidence interval, 12.1-15.4, P less than .001). At 24 weeks, the combination group had a predicted FEV1 difference that was 14.3 percentage points higher (95% confidence interval, 12.7-15.8, P less than .001). The rate of pulmonary exacerbations was 63% lower (rate ratio 0.37; 95% CI, 0.25-0.55, P less than .001) and sweat chloride concentration was 41.8 mmol/L lower (95% CI, –44.4 to –39.3, P less than .001) in the combination group through 24 weeks.
At least one adverse event occurred in 93.1% of patients in the combination group and 96% of patients in the placebo group. Serious adverse events occurred in 28 patients (13.9%) in the combination group and 42 patients (20.9%) in the placebo group. There were no deaths in either group.
The study was funded by Vertex Pharmaceuticals. The authors had disclosures, including receiving personal fees and grants from various pharmaceutical companies and being on the advisory board, owning stock, or being an employee of Vertex Pharmaceuticals.
SOURCE: Middleton PG et al. 2019 Oct 31. N Engl J Med. doi: 10.1056/NEJMoa1908639.
Reinforcing previous findings, a new study has determined that the next-generation corrector elexacaftor, in combination with tezacaftor and ivacaftor, can effectively treat patients with Phe508del-minimal function genotypes who did not respond to previous cystic fibrosis transmembrane conductance regulator (CFTR) modulator regimens.
“These results provide evidence that , thus addressing the underlying cause of disease in the large majority of patients,” wrote Peter G. Middleton, PhD, of the University of Sydney (Australia) and his coauthors. The study was published in the New England Journal of Medicine.
To further determine if the elexacaftor-tezacaftor-ivacaftor regimen was effective and safe, the researchers launched a randomized, placebo-controlled phase 3 trial of 403 cystic fibrosis patients age 12 or older who had a single Phe508del allele. Patients in the combination group (n = 200) received 200 mg of elexacaftor once daily, 100 mg of tezacaftor once daily, and 150 mg of ivacaftor every 12 hours for 24 weeks. Patients in the other group (n = 203) received matched placebos.
At 14 weeks, patients in the combination group had a change in percentage of predicted forced expiratory volume in 1 second (FEV1) that was 13.8 points higher than the placebo group (95% confidence interval, 12.1-15.4, P less than .001). At 24 weeks, the combination group had a predicted FEV1 difference that was 14.3 percentage points higher (95% confidence interval, 12.7-15.8, P less than .001). The rate of pulmonary exacerbations was 63% lower (rate ratio 0.37; 95% CI, 0.25-0.55, P less than .001) and sweat chloride concentration was 41.8 mmol/L lower (95% CI, –44.4 to –39.3, P less than .001) in the combination group through 24 weeks.
At least one adverse event occurred in 93.1% of patients in the combination group and 96% of patients in the placebo group. Serious adverse events occurred in 28 patients (13.9%) in the combination group and 42 patients (20.9%) in the placebo group. There were no deaths in either group.
The study was funded by Vertex Pharmaceuticals. The authors had disclosures, including receiving personal fees and grants from various pharmaceutical companies and being on the advisory board, owning stock, or being an employee of Vertex Pharmaceuticals.
SOURCE: Middleton PG et al. 2019 Oct 31. N Engl J Med. doi: 10.1056/NEJMoa1908639.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
The challenges of contracting for value, not volume in prescription drugs
NATIONAL HARBOR, MD. – Paying for value over volume is being seen as a key part of driving down the cost of prescription drugs. But setting up value-based contracts can be a challenge.
“In Utah, we thought we would be an appropriate laboratory to try and figure out, ‘Is there a way that we can do this different?’ ” Diana Brixner, PhD, of the University of Utah, Salt Lake City, said at the annual meeting of the Academy of Managed Care Pharmacy. “How can we be creative and have alternatives to high-deductible plans in Utah through value-based–type programs?”
The state considered three different options, she noted. The first option was value-based drug coverage, which pays for only those drugs that are deemed valuable by an independent source. Uptake on these types of contracts has been slow, Dr. Brixner noted, particularly as patient advocates have argued that some drugs may not be cost effective but are still the best choice for certain patients. In those cases, value-based drug coverage has the potential to hinder access.
“There are certainly still different areas and issues that need to be worked out, but in concept, this could potentially help the solution of getting more affordable care to patients,” Dr. Brixner said.
The second option is outcomes-based contracting, which involves working with manufacturers to determine appropriate disease states with vetted outcomes measures and building pharmacy contracts around them.
“We are very optimistic about the potential for outcomes-based contracting as well,” Dr. Brixner said.
CVS has looked into zero copays for preventive medicines, Dr. Brixner said. She added that studies have shown the potential for millions in savings from these kinds of arrangements.
But there are concerns with all of these designs. Drug manufacturers, for instance, have concerns about getting accurate data to determine the payment parameters. Another concern from the manufacturer side is the inability to discuss information about off-label drug use that could be important to negotiating a value-based contract.
For payers, a key concern is making sure there are measurable outcomes, as well as appropriate risk sharing.
In the end, different conditions lend themselves to different types of value-based contracting, Dr. Brixner said. For example, multiple sclerosis is better suited to a value-based drug coverage contract, while rheumatoid arthritis fits better in an outcomes-based contracting design.
Kenneth Schaecher, MD, associate chief medical officer of the University of Utah Health Plan, highlighted some of the challenges of value-based care from a payer perspective, including determining outcomes to use in contracts.
“One of the challenges that we get is trying to decide what is a measure that is important to both the health plans and the patients and the providers,” he said. “If the measure is not reflective of an outcome relative to those, it is going to be very hard to impact it” through a value-based contract. He noted that patient-reported outcomes do not work well in value-based contracts.
The timeliness of the data can also present a challenge, especially when factoring in member turnover from health plans.
But there are examples of success, he noted. Dr. Schaecher highlighted a few examples, including an outcomes-based contract between Cigna and Merck for Januvia and Janumet, which included higher discounts for improvements in hemoglobin A1c across the insured population. Additional discounts were offered if adherence improved. And if both outcomes and adherence improved, Cigna would move the drugs to formulary tiers with lower copays.
NATIONAL HARBOR, MD. – Paying for value over volume is being seen as a key part of driving down the cost of prescription drugs. But setting up value-based contracts can be a challenge.
“In Utah, we thought we would be an appropriate laboratory to try and figure out, ‘Is there a way that we can do this different?’ ” Diana Brixner, PhD, of the University of Utah, Salt Lake City, said at the annual meeting of the Academy of Managed Care Pharmacy. “How can we be creative and have alternatives to high-deductible plans in Utah through value-based–type programs?”
The state considered three different options, she noted. The first option was value-based drug coverage, which pays for only those drugs that are deemed valuable by an independent source. Uptake on these types of contracts has been slow, Dr. Brixner noted, particularly as patient advocates have argued that some drugs may not be cost effective but are still the best choice for certain patients. In those cases, value-based drug coverage has the potential to hinder access.
“There are certainly still different areas and issues that need to be worked out, but in concept, this could potentially help the solution of getting more affordable care to patients,” Dr. Brixner said.
The second option is outcomes-based contracting, which involves working with manufacturers to determine appropriate disease states with vetted outcomes measures and building pharmacy contracts around them.
“We are very optimistic about the potential for outcomes-based contracting as well,” Dr. Brixner said.
CVS has looked into zero copays for preventive medicines, Dr. Brixner said. She added that studies have shown the potential for millions in savings from these kinds of arrangements.
But there are concerns with all of these designs. Drug manufacturers, for instance, have concerns about getting accurate data to determine the payment parameters. Another concern from the manufacturer side is the inability to discuss information about off-label drug use that could be important to negotiating a value-based contract.
For payers, a key concern is making sure there are measurable outcomes, as well as appropriate risk sharing.
In the end, different conditions lend themselves to different types of value-based contracting, Dr. Brixner said. For example, multiple sclerosis is better suited to a value-based drug coverage contract, while rheumatoid arthritis fits better in an outcomes-based contracting design.
Kenneth Schaecher, MD, associate chief medical officer of the University of Utah Health Plan, highlighted some of the challenges of value-based care from a payer perspective, including determining outcomes to use in contracts.
“One of the challenges that we get is trying to decide what is a measure that is important to both the health plans and the patients and the providers,” he said. “If the measure is not reflective of an outcome relative to those, it is going to be very hard to impact it” through a value-based contract. He noted that patient-reported outcomes do not work well in value-based contracts.
The timeliness of the data can also present a challenge, especially when factoring in member turnover from health plans.
But there are examples of success, he noted. Dr. Schaecher highlighted a few examples, including an outcomes-based contract between Cigna and Merck for Januvia and Janumet, which included higher discounts for improvements in hemoglobin A1c across the insured population. Additional discounts were offered if adherence improved. And if both outcomes and adherence improved, Cigna would move the drugs to formulary tiers with lower copays.
NATIONAL HARBOR, MD. – Paying for value over volume is being seen as a key part of driving down the cost of prescription drugs. But setting up value-based contracts can be a challenge.
“In Utah, we thought we would be an appropriate laboratory to try and figure out, ‘Is there a way that we can do this different?’ ” Diana Brixner, PhD, of the University of Utah, Salt Lake City, said at the annual meeting of the Academy of Managed Care Pharmacy. “How can we be creative and have alternatives to high-deductible plans in Utah through value-based–type programs?”
The state considered three different options, she noted. The first option was value-based drug coverage, which pays for only those drugs that are deemed valuable by an independent source. Uptake on these types of contracts has been slow, Dr. Brixner noted, particularly as patient advocates have argued that some drugs may not be cost effective but are still the best choice for certain patients. In those cases, value-based drug coverage has the potential to hinder access.
“There are certainly still different areas and issues that need to be worked out, but in concept, this could potentially help the solution of getting more affordable care to patients,” Dr. Brixner said.
The second option is outcomes-based contracting, which involves working with manufacturers to determine appropriate disease states with vetted outcomes measures and building pharmacy contracts around them.
“We are very optimistic about the potential for outcomes-based contracting as well,” Dr. Brixner said.
CVS has looked into zero copays for preventive medicines, Dr. Brixner said. She added that studies have shown the potential for millions in savings from these kinds of arrangements.
But there are concerns with all of these designs. Drug manufacturers, for instance, have concerns about getting accurate data to determine the payment parameters. Another concern from the manufacturer side is the inability to discuss information about off-label drug use that could be important to negotiating a value-based contract.
For payers, a key concern is making sure there are measurable outcomes, as well as appropriate risk sharing.
In the end, different conditions lend themselves to different types of value-based contracting, Dr. Brixner said. For example, multiple sclerosis is better suited to a value-based drug coverage contract, while rheumatoid arthritis fits better in an outcomes-based contracting design.
Kenneth Schaecher, MD, associate chief medical officer of the University of Utah Health Plan, highlighted some of the challenges of value-based care from a payer perspective, including determining outcomes to use in contracts.
“One of the challenges that we get is trying to decide what is a measure that is important to both the health plans and the patients and the providers,” he said. “If the measure is not reflective of an outcome relative to those, it is going to be very hard to impact it” through a value-based contract. He noted that patient-reported outcomes do not work well in value-based contracts.
The timeliness of the data can also present a challenge, especially when factoring in member turnover from health plans.
But there are examples of success, he noted. Dr. Schaecher highlighted a few examples, including an outcomes-based contract between Cigna and Merck for Januvia and Janumet, which included higher discounts for improvements in hemoglobin A1c across the insured population. Additional discounts were offered if adherence improved. And if both outcomes and adherence improved, Cigna would move the drugs to formulary tiers with lower copays.
REPORTING FROM AMCP NEXUS 2019
Technology softens prior authorization pain points
NATIONAL HARBOR, MD. – Nebulous pricing associated with prior authorization continues to be a major pain point for health care professionals, but this may become a thing of the past – thanks to a technology called real-time pharmacy benefit.
Real-time pharmacy benefits (RTPB) is software or a software component that allows practicing clinicians to look up a patient’s out-of-pocket costs for a specific drug, regardless of the patient’s health insurance coverage. Users can see the costs, copayment, and deductible for branded and generic, as well as compare insurance costs versus cash pricing.
Lindsey Colbert, RN, program manager for care team efficiency at HealthPartners, and Leann McDowell, PharmD, supervisor, pharmacy utilization management at HealthPartners, investigated how integrating RTPB software into their existing platforms and operations could help address pricing nuances and their associated burden on patients and health care professionals. They presented the results of their pilot test and post–pilot test expansion at the annual meeting of the Academy of Managed Care Pharmacy.
“Historically, clinicians were told not to quote prices, because having numerous insurance plans made it difficult to know what was going to be covered,” Ms. Colbert said. “Now, with real-time benefits, clinicians have pricing information readily available to them.”
HealthPartners pilot-tested RTPB at two locations before expanding to additional sites. They found that integrating real-time pharmacy benefits information improved the user experience and added cost savings for patients while improving workflow efficiency.
Health care professionals were more like to use RTPB for inquiries when the perceived patient cost was $50 or more – a price many clinicians perceive to be too expensive for many patients.
Before RTPB implementation, participating health care professionals reported waiting at least 45 minutes to get pricing on drugs requiring prior authorization. Integrating the RTPB software shaved the wait time down to 4 minutes – allowing them to quote drug prices to patients at the point of service.
Despite the benefits, everyone is not on board with RTPB.
Health care professionals already feel burdened by the information requirements of their electronic health records systems. They “count the number of computer clicks they have to make, so getting them to make an additional click to use RTPB requires another buy-in,” Ms. Colbert said.
While participating health care professionals were asked to run every prescription through RTPB, they reported using the software only when they knew a patient would either perceive cost as a potential barrier, or if they knew a drug would be expensive.
Investigators said they plan to continue working with clinicians to make RTPB integration more user-friendly by eventually eliminating the additional computer click required to run the program. They also plan to monitor the progress of the National Council for Prescription Drug Programs – developer of RTPB – regarding its adaptation of its new standard.
NATIONAL HARBOR, MD. – Nebulous pricing associated with prior authorization continues to be a major pain point for health care professionals, but this may become a thing of the past – thanks to a technology called real-time pharmacy benefit.
Real-time pharmacy benefits (RTPB) is software or a software component that allows practicing clinicians to look up a patient’s out-of-pocket costs for a specific drug, regardless of the patient’s health insurance coverage. Users can see the costs, copayment, and deductible for branded and generic, as well as compare insurance costs versus cash pricing.
Lindsey Colbert, RN, program manager for care team efficiency at HealthPartners, and Leann McDowell, PharmD, supervisor, pharmacy utilization management at HealthPartners, investigated how integrating RTPB software into their existing platforms and operations could help address pricing nuances and their associated burden on patients and health care professionals. They presented the results of their pilot test and post–pilot test expansion at the annual meeting of the Academy of Managed Care Pharmacy.
“Historically, clinicians were told not to quote prices, because having numerous insurance plans made it difficult to know what was going to be covered,” Ms. Colbert said. “Now, with real-time benefits, clinicians have pricing information readily available to them.”
HealthPartners pilot-tested RTPB at two locations before expanding to additional sites. They found that integrating real-time pharmacy benefits information improved the user experience and added cost savings for patients while improving workflow efficiency.
Health care professionals were more like to use RTPB for inquiries when the perceived patient cost was $50 or more – a price many clinicians perceive to be too expensive for many patients.
Before RTPB implementation, participating health care professionals reported waiting at least 45 minutes to get pricing on drugs requiring prior authorization. Integrating the RTPB software shaved the wait time down to 4 minutes – allowing them to quote drug prices to patients at the point of service.
Despite the benefits, everyone is not on board with RTPB.
Health care professionals already feel burdened by the information requirements of their electronic health records systems. They “count the number of computer clicks they have to make, so getting them to make an additional click to use RTPB requires another buy-in,” Ms. Colbert said.
While participating health care professionals were asked to run every prescription through RTPB, they reported using the software only when they knew a patient would either perceive cost as a potential barrier, or if they knew a drug would be expensive.
Investigators said they plan to continue working with clinicians to make RTPB integration more user-friendly by eventually eliminating the additional computer click required to run the program. They also plan to monitor the progress of the National Council for Prescription Drug Programs – developer of RTPB – regarding its adaptation of its new standard.
NATIONAL HARBOR, MD. – Nebulous pricing associated with prior authorization continues to be a major pain point for health care professionals, but this may become a thing of the past – thanks to a technology called real-time pharmacy benefit.
Real-time pharmacy benefits (RTPB) is software or a software component that allows practicing clinicians to look up a patient’s out-of-pocket costs for a specific drug, regardless of the patient’s health insurance coverage. Users can see the costs, copayment, and deductible for branded and generic, as well as compare insurance costs versus cash pricing.
Lindsey Colbert, RN, program manager for care team efficiency at HealthPartners, and Leann McDowell, PharmD, supervisor, pharmacy utilization management at HealthPartners, investigated how integrating RTPB software into their existing platforms and operations could help address pricing nuances and their associated burden on patients and health care professionals. They presented the results of their pilot test and post–pilot test expansion at the annual meeting of the Academy of Managed Care Pharmacy.
“Historically, clinicians were told not to quote prices, because having numerous insurance plans made it difficult to know what was going to be covered,” Ms. Colbert said. “Now, with real-time benefits, clinicians have pricing information readily available to them.”
HealthPartners pilot-tested RTPB at two locations before expanding to additional sites. They found that integrating real-time pharmacy benefits information improved the user experience and added cost savings for patients while improving workflow efficiency.
Health care professionals were more like to use RTPB for inquiries when the perceived patient cost was $50 or more – a price many clinicians perceive to be too expensive for many patients.
Before RTPB implementation, participating health care professionals reported waiting at least 45 minutes to get pricing on drugs requiring prior authorization. Integrating the RTPB software shaved the wait time down to 4 minutes – allowing them to quote drug prices to patients at the point of service.
Despite the benefits, everyone is not on board with RTPB.
Health care professionals already feel burdened by the information requirements of their electronic health records systems. They “count the number of computer clicks they have to make, so getting them to make an additional click to use RTPB requires another buy-in,” Ms. Colbert said.
While participating health care professionals were asked to run every prescription through RTPB, they reported using the software only when they knew a patient would either perceive cost as a potential barrier, or if they knew a drug would be expensive.
Investigators said they plan to continue working with clinicians to make RTPB integration more user-friendly by eventually eliminating the additional computer click required to run the program. They also plan to monitor the progress of the National Council for Prescription Drug Programs – developer of RTPB – regarding its adaptation of its new standard.
REPORTING FROM AMCP NEXUS 2019
New data further define role of PD-L1 status, immunotherapy in metastatic breast cancer
BARCELONA – Programmed death-ligand 1 (PD-L1) status in patients with advanced triple negative or HER2-positive breast cancer appears to identify distinct disease entities with varying likelihood of benefit from immune checkpoint inhibition, according to Giampaolo Bianchini, MD.
This observation, which contrasts with findings in other solid tumors and expands the road map to improved outcomes with immunotherapy for metastatic breast cancer, is based in part on new findings presented at the European Society for Medical Oncology Congress.
Among additional lessons from those findings: PD-L1 assays are not easily interchangeable, and studies with a “one size fits all” approach should be avoided, Dr. Bianchini, head of the Breast Cancer Group – Medical Oncology and clinical translational and immunotherapy research at Ospedale San Raffaele, Milan, said at the congress.
IMPassion130 and PD-L1 assays
In the phase 3 IMpassion130 trial assessing nanoparticle, albumin-bound (nab)-paclitaxel chemotherapy + either the anti-PD-L1 monoclonal antibody atezolizumab or placebo for the first-line treatment of metastatic triple-negative breast cancer (mTNBC), investigators used, and validated, the VENTANA PD-L1 SP142 assay to evaluate PD-L1 expression in immune cells (IC). PD-L1 positivity was defined using a 1% cutoff, meaning that PD-L1-stained IC encompassed at least 1% of the tumor area.
The trial demonstrated significantly improved progression-free survival (PFS) in the atezolizumab arm, both in the intention-to-treat (ITT) analysis (7.2 vs. 5.5 months in the placebo arm; hazard ratio, 0.80), and the PD-L1-positive subgroup (7.5 vs. 5.0 months; HR, 0.62), and the results were published in November 2018 (N Engl J Med. 2018; 379:2108-21).
“IMpassion130 is the first phase 3 trial demonstrating clinical benefit of cancer immunotherapy in patients with PD-L1-positive, metastatic triple-negative breast cancer,” Hope S. Rugo, MD, said at the congress. “The combination of atezolizumab and nab-paclitaxel is now approved in the United States and Europe for this indication.”
In addition, the SP142 antibody (which binds to PD-L1), at the 1% cutoff, predicted PFS and overall survival (OS) with atezolizumab + nab-paclitaxel, compared with nab-paclitaxel + placebo; the absolute improvement in OS in the PD-L1-positive population was 7 months (HR, 0.71), whereas no impact was seen in PFS or OS in patients who were PD-L1-negative using the SP142 assay, said Dr. Rugo, professor of hematology/oncology, and director of breast oncology and clinical trials education at the University of California, San Francisco.
Based on the IMPassion130 findings, the Food and Drug Administration approved the SP142 assay, using the 1% cutoff, as a “companion diagnostic device for selecting TNBC patients for atezolizumab.”
However, questions remain about how to best identify patients who could benefit from the atezolizumab + nab-paclitaxel combination, Dr. Rugo said.
Therefore, she and her colleagues performed a retrospective post hoc subgroup analysis of data from the trial to assess the performance and analytical concordance of the SP142 assay and two other commonly used PD-L1 immunohistochemistry (IHC) assays: the VENTANA SP263 IHC assay typically used as a companion diagnostic with durvalumab, and the Dako PD-L1 IHC 22C3 assay typically used with pembrolizumab.
In addition, the investigators assessed PD-L1 prevalence and clinical activity.
“We also included an evaluation of important factors related to PD-L1 testing and ... relationship to clinical outcome,” Dr. Rugo said.
In 614 biomarker-evaluable patients, representing 68% of the IMPassion130 ITT population, PD-L1-positive prevalence was 46% with the SP142 assay, 75% with the SP263 assay (also based on a 1% IC cutoff), and 81% with the 22C3 assay (with positivity defined as a combined positive score [CPS] of 1 or more based on an algorithm including both tumor and IC counts).
“Almost all SP142-positive cases are captured by either 22C3 or SP263. However, about a third of patients’ tumors were positive for PD-L1 using only one of the other two assays,” she noted, explaining that “this leads to suboptimal analytical concordance.”
The overall percentage agreement between SP142 and the other assays was only 64%-68%, she said.
Positive percentage agreement rates of 98% for both SP263 and 22C3 suggest that the patients identified as PD-L1 positive using the SP142 assay are captured by the other two assays. However, negative percentage agreement rates were less than 45%.
The HRs for PFS were 0.60 in SP142-positive patients, 0.64 in SP263-positive patients, and 0.68 in 22C3-positive patients, and the HRs for OS were 0.74, 0.75, and 0.78, respectively.
Subgroup analyses indicated that PFS and OS benefit with atezolizumab + nab-paclitaxel vs. nab-paclitaxel alone was greater in double-positive patients (those with SP142 positivity and either SP263 or 22C3 positivity) than in patients who were SP263-positive/SP142-negative or 22C3-positive/SP142-negative.
Dr. Rugo and her colleagues also found that the benefits with atezolizumab + nab-paclitaxel in PD-L1-positive patients were apparent regardless of the source of tissue for testing (breast or distant metastases).
They concluded that the findings of the assays are not equivalent; 22C3 and SP263 identified more patients as PD-L1 positive, and SP142-positivity was encompassed in positive tests for both.
“The clinical benefit in the 22C3-positive and the SP263-positive subgroups appear to be driven by the SP142-positive subgroup, and [SP142] identifies patients with the longest median progression-free and overall survival from the addition of atezolizumab to nab-paclitaxel,” she said “The SP142 assay with an IC cutoff of 1% or greater is the approved diagnostic test used to identify patients with metastatic triple-negative breast cancer who are most likely to benefit from the addition of the checkpoint inhibitor atezolizumab to nab-paclitaxel.”
As for whether the SP142 should be the assay of choice in other settings in which it hasn’t been validated, Dr. Rugo said it is advisable to use the assay that has been validated in a positive trial.
“That’s what we would generally do ... however, recognizing that some countries are not using SP142, and some sites may not have access, certainly you encompass that population in the patients whose tumors are positive by both other assays,” she said. “The risk is that you might overtreat, and the cost of treatment is greater.”
Excess toxicity is also a concern in that situation, she said, adding that “hopefully in the future we’ll be able to figure out ways to have even more patients benefit from the addition of immunotherapy so that won’t be an issue.”
“What this data shows is that you can feel secure that you are encompassing the patient population identified by the parent trial to benefit from the addition of atezolizumab by using either of the other two assays; you’re only missing 1% – so that’s very reasonable,” she said. “The risk is that you’re overtreating; it’s quite likely that there’s a population there that isn’t benefiting as much, but that’s a balance.”
The findings from IMPassion130 with regard to OS in the unselected population that included PD-L1-negative patients (18.7 vs. 21.0 months with vs. without atezolizumab; HR, 0.86) underscore the fact that “one size does not fit all” when it comes to immunotherapy benefit, Dr. Bianchini said.
This is further demonstrated by the post hoc analysis comparing IHC assays, he said, explaining that 63% of IMPassion130 patients who were considered PD-L1-negative based on the SP142 “actually scored as PD-L1-positive by the other tests.
“So the very clinically important question is if there is any evidence from the data that [the PD-L1-negative group] benefits in a significant way from the addition of atezolizumab,” he said. “I don’t see evidence for a clinical benefit, I see evidence to look for new biomarkers to identify a potential population who will benefit.”
The “absence of evidence is not evidence of absence,” he stressed, noting that it may be possible – with the right biomarkers – to identify PD-L1-negative patients who would benefit.
What the findings do show, however, is support for the FDA decision to approve the SP142 assay with an IC cutoff of 1% as a companion diagnostic tool, and that PD-L1 is ideally assessed using samples from both the primary and metastatic site, as the IMPassion130 data “do not inform whether PD-L1 assessment in primary and metastatic sites is equally informative,” he said.
In addition, Dr. Bianchini said the findings suggest that more information is needed about using different cutoffs for SP263 and 22C3, and he cautioned against “directly translating these finding to other disease settings or immune combinations.
“Defining new biomarkers to identify who within the PD-L1-negative group might benefit from this combination remains an unmet need,” he said. “For sure, I don’t see a space for the other tests to define this population,” he added.
KEYNOTE-119, KATE2, and future directions
Both the randomized, open-label, phase 3 KEYNOTE-119 study of the checkpoint inhibitor pembrolizumab vs. single-agent chemotherapy for mTNBC, and the phase 2 KATE2 trial of the antibody-drug conjugate trastuzumab emtansine (T-DM1) + either atezolizumab or placebo in previously treated HER2-positive breast cancer patients, failed to meet their respective primary study endpoints.
But the news isn’t all bad, Dr. Bianchini said.
For example, in KEYNOTE-119, second- or third-line pembrolizumab monotherapy did not significantly improve OS vs. chemotherapy for mTNBC, but the pembrolizumab treatment effect increased as PD-L1 enrichment increased, he explained.
Pembrolizumab showed promising antitumor activity and manageable safety in mTNBC in prior trials, and was therefore further assessed in the KEYNOTE-119 study of 601 patients with centrally confirmed TNBC, 1-2 prior systemic treatments for mTNBC, progression on the latest therapy, and a prior anthracycline or taxane, Javier Cortés, MD, PhD, of Instituto Oncológico, Madrid, reported at the congress.
Pembrolizumab was given at a dose of 200 mg every 3 weeks, and chemotherapy was physician’s choice of capecitabine, eribulin, gemcitabine, or vinorelbine.
At a median follow-up of 9.9 months in the pembrolizumab group and 10.9 months in the chemotherapy group, OS did not differ significantly between the groups; this was true overall, in patients with a CPS of 10 or greater, and in those with a CPS of 1 or greater.
In all-comers, the HR for OS was 0.97, compared with 0.78 in patients with CPS of 10 or greater, and 0.86 in those with CPS of 1 or greater, Dr. Cortés said.
“One of the most interesting exploratory analyses was OS in those patients with CPS of 20 or higher,” he said, noting that median OS in that group was 14.9 vs.12.5 months with pembrolizumab vs. with chemotherapy (HR, 0.58).
Pembrolizumab did not improve overall PFS, but again, the rates improved with higher CPS. Duration of response, however, was longer with pembrolizumab vs. chemotherapy (12.2 vs. 8.3 months overall; 12.2 vs. 6.5 months for CPS of 1 or greater; and not reached vs. 7.1 months for CPS of 10 or greater).
Grade 3-5 AEs occurred in 35% vs. 49% of patients in the pembrolizumab vs. chemotherapy groups, with nine deaths occurring in each, Dr. Cortés said, adding that treatment-related AEs occurred in 14% (with one death) and 36% (with two deaths), respectively, and grade 3-4 immune-mediated AEs and infusion reactions occurred in 3.2% vs. 1.0% (no deaths), respectively.
In the double-blind, signal-seeking KATE2 trial, as reported in 2018 at the San Antonio Breast Cancer Symposium, no overall PFS improvement was seen with atezolizumab + T-DM1 (median of 8.2 vs. 6.8 months; HR, 0.82; 12-month PFS 38% vs. 34%), but again, a possible benefit was seen in PD-L1-positive patients (8.5 vs. 4.1 months; HR, 0.60).
KATE2 included 202 patients with advanced HER2-positive breast cancer that progressed after treatment with T-DM1 and a taxane. They were randomized 2:1 to receive intravenous T-DM1 at a dose of 3.6 mg/kg plus atezolizumab (1,200 mg) or placebo every 3 weeks until loss of clinical benefit or intolerable toxicity.
The “overall survival and final safety results” show that at a median follow-up of 19.0 months in the atezolizumab arm and 18.2 months in the placebo arm, with 52 OS events reported, median OS was not reached in either arm and 1-year survival was similar in the two groups (89.1% and 89.0%), Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology and co-leader of the Hillman Cancer Immunology and Immunotherapy Program at Hillman Cancer Center, University of Pittsburgh Medical Center (UPMC) reported at the congress.
The 1-year OS rate in the PD-L1-positive subgroup, however, was numerically higher with vs. without atezolizumab (94.2% vs. 87.9%), said Dr. Emens, director of translational immunotherapy for the Women’s Cancer Research Center at UPMC.
Of note, all additional biomarkers of T-cell activation and quantity analyzed, including PD-L1 gene expression, CD8 gene expression, T effector signature gene expression, and stromal tumor infiltrating lymphocytes (TILs), were enriched in the PD-L1-positive subgroup vs. the PD-L1-negative patients.
Further, OS rates in other immune biomarker subgroups (those with PD-L1 RNA expression, CD8 RNA expression, and T effector signature at or below vs. above the median, and those with TILs less than 5% vs. 5% or greater) were consistent with those in the PD-L1 IC-positive subgroup, and the biggest difference between the atezolizumab and placebo arms related to stromal TILs, she said.
The safety profile in this final analysis was consistent with the known safety profile of each drug, she added, noting that grade 3 or greater AEs occurred in 52.6% vs. 44.8% of patients in the atezolizumab vs. placebo arms, and serious AEs – primarily pyrexia – occurred in 36.1% vs 20.9%, respectively.
The rate of grade 5 AEs was similar in the groups.
T-DM1 is indicated for the treatment of HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane, either separately or in combination, Dr. Emens said.
“In addition to its cytotoxic activity, T-DM1 may potentiate tumor immunity,” she explained, adding that KATE2 was designed to assess whether combining T-DM1 with atezolizumab, an anti-PD-L1 antibody that restores anti-tumor immunity, would result in greater clinical activity than either drug alone.
Although the number of OS events was small, the data suggest an OS benefit with the addition of atezolizumab to T-DM1, specifically in the PD-L1 IC-positive patients, but follow-up was short and the study lacked statistical power, therefore additional study of HER2-targeted agents with atezolizumab in previously treated HER2-positive, PD-L1 IC-positive advanced breast cancer is warranted, Dr. Emens concluded.
Indeed, the finding of improved OS in the PD-L1-positive subgroups of both KEYNOTE-119 and KATE2, is of interest, Dr. Bianchini said.
Both trials failed to meet their primary endpoints, but a closer look into KEYNOTE-119 shows that PD-L1 as a continuous biomarker (using CPS, 22C3) was associated with a “continuous and strong trend” toward improved ORR with the addition of pembrolizumab.
The ORR was 9.6% vs. 10.6% in unselected patients, compared with 26.3% vs. 11.5% in those with CPS of 20 or greater.
“And when you look at duration of response, you see an increase not just in the number ... but the quality of the response,” he said, noting that for PFS, as well, a trend toward superiority is seen “that is consistent with all the other endpoints.”
“So overall, the application of incrementally restrictive cut-off of CPS lends weight to the exploratory analysis showing better survival from pembrolizumab in tumors with CPS more than 20,” Dr. Bianchini said, noting that the “real question,” however, is whether the finding “is worth clinical implementation.
“We know a lot about the primary tumor and immune infiltration. We’ve learned ... that if you wait and look ahead at immune infiltration in the advanced stage, you find that the tumor becomes smart,” he said, explaining that tumor/immune co-evolution leads to increased immuno-editing and immune subversion and it becomes “much harder to just hit the tumor with PD-L1, because this is not the only mechanism of immune escape.”
A review of several studies shows that in similar populations defined by biomarkers, response rates in patients treated with checkpoint inhibitors decrease in the second- and third-line setting vs. the first-line setting, he said.
For example, pembrolizumab response rates in the first-line and second-line or greater setting in cohort B of the KEYNOTE-086 study were 21.4% and 5.7%, respectively, compared with 12.3% in the second- to third-line setting in KEYNOTE-119, he said.
Another consideration is whether monotherapy or combination therapy is preferable, and the data suggest that regardless of how PD-L1-positivity is defined (by CPS cutoff of 1 vs. 20, for example), most patients treated with monotherapy progress within the first 3 months, he said.
“I don’t see that this is a safe approach for the majority of these patients. So without better biomarkers, combinations should always be preferred, at least to avoid early progression,” Dr. Bianchini said, adding that the open question, then, is: “If we set the new standard in the first-line as the combination of nab-paclitaxel and atezolizumab for PD-L1-positive patients defined by the VENTANA [SP142 assay], should we continue with immune checkpoint [inhibition] using different combinations?”
“Of course, at the time the trial was designed, the results of IMpassion were not available, but it’s very important, because [the findings] add to the evidence that immunotherapy is extremely relevant for some patients,” he said.
KATE2 further demonstrated the importance of PD-L1 status, he said, adding that due to its limitations, including small sample size and short follow-up, longer follow-up is needed to better evaluate duration of response and PFS.
“Despite the trial limitations, the qualitative effect seen in all clinical endpoints – overall response rate, progression-free survival, overall survival – in PD-L1-positive tumors defined by SP142 ... provided strong and robust signals supporting the investigation of immune checkpoint inhibitors in HER-positive breast cancer,” he said, noting that “many trials are ongoing in the early setting and the advanced setting.”
In addition to the lessons of these trials with respect to the interchangeability of PD-L1 IHC assays and the value of PD-L1 assessment for identifying the likelihood of benefit from immune checkpoint inhibitors, the findings highlight the possibility that PD-L1-negative tumors require different immunotherapy approaches or alternative therapeutic strategies, and underscore that the benefit of immunotherapy in PD-L1-positive patients is still restricted to a minority.
“So new studies and approaches with immuno-oncology are needed, and we need more effective biomarkers, because we need to have precision oncology applied – we need to go in that direction,” he concluded.
Dr. Bianchini reported consultancy/honorarium and or advisory board activity associated with Roche, MSD, AstraZeneca, Pfizer, Chugai, EISAI, Lilly, Novartis, Amgen, Sanofi, Neopharm, and Genomic Health. The IMPassion30 trial was funded by F. Hoffmann-La Roche Ltd.; Dr. Rugo reported research grants, other funding, and or travel/accommodation/expenses from Pfizer, Novartis, Eli Lilly, Merck, OBI, EISAI, Plexxikon, Genentech/Roche, MacroGenics, PUMA, Mylan, Immunomedics, Daiichi Sankyo, and Celltrion. KEYNOTE-119 was funded by Merck Sharp & Dohme Corp.; Dr. Cortés and Dr. Emens reported numerous funding relationships but none with F. Hoffman-La Roche. KATE2 was funded by F. Hoffmann-La Roche.
Sources: IMPassion130; ESMO Abstract LBA20; KEYNOTE-119: ESMO Abstract LBA21; KATE2: ESMO Abstract 305O.
BARCELONA – Programmed death-ligand 1 (PD-L1) status in patients with advanced triple negative or HER2-positive breast cancer appears to identify distinct disease entities with varying likelihood of benefit from immune checkpoint inhibition, according to Giampaolo Bianchini, MD.
This observation, which contrasts with findings in other solid tumors and expands the road map to improved outcomes with immunotherapy for metastatic breast cancer, is based in part on new findings presented at the European Society for Medical Oncology Congress.
Among additional lessons from those findings: PD-L1 assays are not easily interchangeable, and studies with a “one size fits all” approach should be avoided, Dr. Bianchini, head of the Breast Cancer Group – Medical Oncology and clinical translational and immunotherapy research at Ospedale San Raffaele, Milan, said at the congress.
IMPassion130 and PD-L1 assays
In the phase 3 IMpassion130 trial assessing nanoparticle, albumin-bound (nab)-paclitaxel chemotherapy + either the anti-PD-L1 monoclonal antibody atezolizumab or placebo for the first-line treatment of metastatic triple-negative breast cancer (mTNBC), investigators used, and validated, the VENTANA PD-L1 SP142 assay to evaluate PD-L1 expression in immune cells (IC). PD-L1 positivity was defined using a 1% cutoff, meaning that PD-L1-stained IC encompassed at least 1% of the tumor area.
The trial demonstrated significantly improved progression-free survival (PFS) in the atezolizumab arm, both in the intention-to-treat (ITT) analysis (7.2 vs. 5.5 months in the placebo arm; hazard ratio, 0.80), and the PD-L1-positive subgroup (7.5 vs. 5.0 months; HR, 0.62), and the results were published in November 2018 (N Engl J Med. 2018; 379:2108-21).
“IMpassion130 is the first phase 3 trial demonstrating clinical benefit of cancer immunotherapy in patients with PD-L1-positive, metastatic triple-negative breast cancer,” Hope S. Rugo, MD, said at the congress. “The combination of atezolizumab and nab-paclitaxel is now approved in the United States and Europe for this indication.”
In addition, the SP142 antibody (which binds to PD-L1), at the 1% cutoff, predicted PFS and overall survival (OS) with atezolizumab + nab-paclitaxel, compared with nab-paclitaxel + placebo; the absolute improvement in OS in the PD-L1-positive population was 7 months (HR, 0.71), whereas no impact was seen in PFS or OS in patients who were PD-L1-negative using the SP142 assay, said Dr. Rugo, professor of hematology/oncology, and director of breast oncology and clinical trials education at the University of California, San Francisco.
Based on the IMPassion130 findings, the Food and Drug Administration approved the SP142 assay, using the 1% cutoff, as a “companion diagnostic device for selecting TNBC patients for atezolizumab.”
However, questions remain about how to best identify patients who could benefit from the atezolizumab + nab-paclitaxel combination, Dr. Rugo said.
Therefore, she and her colleagues performed a retrospective post hoc subgroup analysis of data from the trial to assess the performance and analytical concordance of the SP142 assay and two other commonly used PD-L1 immunohistochemistry (IHC) assays: the VENTANA SP263 IHC assay typically used as a companion diagnostic with durvalumab, and the Dako PD-L1 IHC 22C3 assay typically used with pembrolizumab.
In addition, the investigators assessed PD-L1 prevalence and clinical activity.
“We also included an evaluation of important factors related to PD-L1 testing and ... relationship to clinical outcome,” Dr. Rugo said.
In 614 biomarker-evaluable patients, representing 68% of the IMPassion130 ITT population, PD-L1-positive prevalence was 46% with the SP142 assay, 75% with the SP263 assay (also based on a 1% IC cutoff), and 81% with the 22C3 assay (with positivity defined as a combined positive score [CPS] of 1 or more based on an algorithm including both tumor and IC counts).
“Almost all SP142-positive cases are captured by either 22C3 or SP263. However, about a third of patients’ tumors were positive for PD-L1 using only one of the other two assays,” she noted, explaining that “this leads to suboptimal analytical concordance.”
The overall percentage agreement between SP142 and the other assays was only 64%-68%, she said.
Positive percentage agreement rates of 98% for both SP263 and 22C3 suggest that the patients identified as PD-L1 positive using the SP142 assay are captured by the other two assays. However, negative percentage agreement rates were less than 45%.
The HRs for PFS were 0.60 in SP142-positive patients, 0.64 in SP263-positive patients, and 0.68 in 22C3-positive patients, and the HRs for OS were 0.74, 0.75, and 0.78, respectively.
Subgroup analyses indicated that PFS and OS benefit with atezolizumab + nab-paclitaxel vs. nab-paclitaxel alone was greater in double-positive patients (those with SP142 positivity and either SP263 or 22C3 positivity) than in patients who were SP263-positive/SP142-negative or 22C3-positive/SP142-negative.
Dr. Rugo and her colleagues also found that the benefits with atezolizumab + nab-paclitaxel in PD-L1-positive patients were apparent regardless of the source of tissue for testing (breast or distant metastases).
They concluded that the findings of the assays are not equivalent; 22C3 and SP263 identified more patients as PD-L1 positive, and SP142-positivity was encompassed in positive tests for both.
“The clinical benefit in the 22C3-positive and the SP263-positive subgroups appear to be driven by the SP142-positive subgroup, and [SP142] identifies patients with the longest median progression-free and overall survival from the addition of atezolizumab to nab-paclitaxel,” she said “The SP142 assay with an IC cutoff of 1% or greater is the approved diagnostic test used to identify patients with metastatic triple-negative breast cancer who are most likely to benefit from the addition of the checkpoint inhibitor atezolizumab to nab-paclitaxel.”
As for whether the SP142 should be the assay of choice in other settings in which it hasn’t been validated, Dr. Rugo said it is advisable to use the assay that has been validated in a positive trial.
“That’s what we would generally do ... however, recognizing that some countries are not using SP142, and some sites may not have access, certainly you encompass that population in the patients whose tumors are positive by both other assays,” she said. “The risk is that you might overtreat, and the cost of treatment is greater.”
Excess toxicity is also a concern in that situation, she said, adding that “hopefully in the future we’ll be able to figure out ways to have even more patients benefit from the addition of immunotherapy so that won’t be an issue.”
“What this data shows is that you can feel secure that you are encompassing the patient population identified by the parent trial to benefit from the addition of atezolizumab by using either of the other two assays; you’re only missing 1% – so that’s very reasonable,” she said. “The risk is that you’re overtreating; it’s quite likely that there’s a population there that isn’t benefiting as much, but that’s a balance.”
The findings from IMPassion130 with regard to OS in the unselected population that included PD-L1-negative patients (18.7 vs. 21.0 months with vs. without atezolizumab; HR, 0.86) underscore the fact that “one size does not fit all” when it comes to immunotherapy benefit, Dr. Bianchini said.
This is further demonstrated by the post hoc analysis comparing IHC assays, he said, explaining that 63% of IMPassion130 patients who were considered PD-L1-negative based on the SP142 “actually scored as PD-L1-positive by the other tests.
“So the very clinically important question is if there is any evidence from the data that [the PD-L1-negative group] benefits in a significant way from the addition of atezolizumab,” he said. “I don’t see evidence for a clinical benefit, I see evidence to look for new biomarkers to identify a potential population who will benefit.”
The “absence of evidence is not evidence of absence,” he stressed, noting that it may be possible – with the right biomarkers – to identify PD-L1-negative patients who would benefit.
What the findings do show, however, is support for the FDA decision to approve the SP142 assay with an IC cutoff of 1% as a companion diagnostic tool, and that PD-L1 is ideally assessed using samples from both the primary and metastatic site, as the IMPassion130 data “do not inform whether PD-L1 assessment in primary and metastatic sites is equally informative,” he said.
In addition, Dr. Bianchini said the findings suggest that more information is needed about using different cutoffs for SP263 and 22C3, and he cautioned against “directly translating these finding to other disease settings or immune combinations.
“Defining new biomarkers to identify who within the PD-L1-negative group might benefit from this combination remains an unmet need,” he said. “For sure, I don’t see a space for the other tests to define this population,” he added.
KEYNOTE-119, KATE2, and future directions
Both the randomized, open-label, phase 3 KEYNOTE-119 study of the checkpoint inhibitor pembrolizumab vs. single-agent chemotherapy for mTNBC, and the phase 2 KATE2 trial of the antibody-drug conjugate trastuzumab emtansine (T-DM1) + either atezolizumab or placebo in previously treated HER2-positive breast cancer patients, failed to meet their respective primary study endpoints.
But the news isn’t all bad, Dr. Bianchini said.
For example, in KEYNOTE-119, second- or third-line pembrolizumab monotherapy did not significantly improve OS vs. chemotherapy for mTNBC, but the pembrolizumab treatment effect increased as PD-L1 enrichment increased, he explained.
Pembrolizumab showed promising antitumor activity and manageable safety in mTNBC in prior trials, and was therefore further assessed in the KEYNOTE-119 study of 601 patients with centrally confirmed TNBC, 1-2 prior systemic treatments for mTNBC, progression on the latest therapy, and a prior anthracycline or taxane, Javier Cortés, MD, PhD, of Instituto Oncológico, Madrid, reported at the congress.
Pembrolizumab was given at a dose of 200 mg every 3 weeks, and chemotherapy was physician’s choice of capecitabine, eribulin, gemcitabine, or vinorelbine.
At a median follow-up of 9.9 months in the pembrolizumab group and 10.9 months in the chemotherapy group, OS did not differ significantly between the groups; this was true overall, in patients with a CPS of 10 or greater, and in those with a CPS of 1 or greater.
In all-comers, the HR for OS was 0.97, compared with 0.78 in patients with CPS of 10 or greater, and 0.86 in those with CPS of 1 or greater, Dr. Cortés said.
“One of the most interesting exploratory analyses was OS in those patients with CPS of 20 or higher,” he said, noting that median OS in that group was 14.9 vs.12.5 months with pembrolizumab vs. with chemotherapy (HR, 0.58).
Pembrolizumab did not improve overall PFS, but again, the rates improved with higher CPS. Duration of response, however, was longer with pembrolizumab vs. chemotherapy (12.2 vs. 8.3 months overall; 12.2 vs. 6.5 months for CPS of 1 or greater; and not reached vs. 7.1 months for CPS of 10 or greater).
Grade 3-5 AEs occurred in 35% vs. 49% of patients in the pembrolizumab vs. chemotherapy groups, with nine deaths occurring in each, Dr. Cortés said, adding that treatment-related AEs occurred in 14% (with one death) and 36% (with two deaths), respectively, and grade 3-4 immune-mediated AEs and infusion reactions occurred in 3.2% vs. 1.0% (no deaths), respectively.
In the double-blind, signal-seeking KATE2 trial, as reported in 2018 at the San Antonio Breast Cancer Symposium, no overall PFS improvement was seen with atezolizumab + T-DM1 (median of 8.2 vs. 6.8 months; HR, 0.82; 12-month PFS 38% vs. 34%), but again, a possible benefit was seen in PD-L1-positive patients (8.5 vs. 4.1 months; HR, 0.60).
KATE2 included 202 patients with advanced HER2-positive breast cancer that progressed after treatment with T-DM1 and a taxane. They were randomized 2:1 to receive intravenous T-DM1 at a dose of 3.6 mg/kg plus atezolizumab (1,200 mg) or placebo every 3 weeks until loss of clinical benefit or intolerable toxicity.
The “overall survival and final safety results” show that at a median follow-up of 19.0 months in the atezolizumab arm and 18.2 months in the placebo arm, with 52 OS events reported, median OS was not reached in either arm and 1-year survival was similar in the two groups (89.1% and 89.0%), Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology and co-leader of the Hillman Cancer Immunology and Immunotherapy Program at Hillman Cancer Center, University of Pittsburgh Medical Center (UPMC) reported at the congress.
The 1-year OS rate in the PD-L1-positive subgroup, however, was numerically higher with vs. without atezolizumab (94.2% vs. 87.9%), said Dr. Emens, director of translational immunotherapy for the Women’s Cancer Research Center at UPMC.
Of note, all additional biomarkers of T-cell activation and quantity analyzed, including PD-L1 gene expression, CD8 gene expression, T effector signature gene expression, and stromal tumor infiltrating lymphocytes (TILs), were enriched in the PD-L1-positive subgroup vs. the PD-L1-negative patients.
Further, OS rates in other immune biomarker subgroups (those with PD-L1 RNA expression, CD8 RNA expression, and T effector signature at or below vs. above the median, and those with TILs less than 5% vs. 5% or greater) were consistent with those in the PD-L1 IC-positive subgroup, and the biggest difference between the atezolizumab and placebo arms related to stromal TILs, she said.
The safety profile in this final analysis was consistent with the known safety profile of each drug, she added, noting that grade 3 or greater AEs occurred in 52.6% vs. 44.8% of patients in the atezolizumab vs. placebo arms, and serious AEs – primarily pyrexia – occurred in 36.1% vs 20.9%, respectively.
The rate of grade 5 AEs was similar in the groups.
T-DM1 is indicated for the treatment of HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane, either separately or in combination, Dr. Emens said.
“In addition to its cytotoxic activity, T-DM1 may potentiate tumor immunity,” she explained, adding that KATE2 was designed to assess whether combining T-DM1 with atezolizumab, an anti-PD-L1 antibody that restores anti-tumor immunity, would result in greater clinical activity than either drug alone.
Although the number of OS events was small, the data suggest an OS benefit with the addition of atezolizumab to T-DM1, specifically in the PD-L1 IC-positive patients, but follow-up was short and the study lacked statistical power, therefore additional study of HER2-targeted agents with atezolizumab in previously treated HER2-positive, PD-L1 IC-positive advanced breast cancer is warranted, Dr. Emens concluded.
Indeed, the finding of improved OS in the PD-L1-positive subgroups of both KEYNOTE-119 and KATE2, is of interest, Dr. Bianchini said.
Both trials failed to meet their primary endpoints, but a closer look into KEYNOTE-119 shows that PD-L1 as a continuous biomarker (using CPS, 22C3) was associated with a “continuous and strong trend” toward improved ORR with the addition of pembrolizumab.
The ORR was 9.6% vs. 10.6% in unselected patients, compared with 26.3% vs. 11.5% in those with CPS of 20 or greater.
“And when you look at duration of response, you see an increase not just in the number ... but the quality of the response,” he said, noting that for PFS, as well, a trend toward superiority is seen “that is consistent with all the other endpoints.”
“So overall, the application of incrementally restrictive cut-off of CPS lends weight to the exploratory analysis showing better survival from pembrolizumab in tumors with CPS more than 20,” Dr. Bianchini said, noting that the “real question,” however, is whether the finding “is worth clinical implementation.
“We know a lot about the primary tumor and immune infiltration. We’ve learned ... that if you wait and look ahead at immune infiltration in the advanced stage, you find that the tumor becomes smart,” he said, explaining that tumor/immune co-evolution leads to increased immuno-editing and immune subversion and it becomes “much harder to just hit the tumor with PD-L1, because this is not the only mechanism of immune escape.”
A review of several studies shows that in similar populations defined by biomarkers, response rates in patients treated with checkpoint inhibitors decrease in the second- and third-line setting vs. the first-line setting, he said.
For example, pembrolizumab response rates in the first-line and second-line or greater setting in cohort B of the KEYNOTE-086 study were 21.4% and 5.7%, respectively, compared with 12.3% in the second- to third-line setting in KEYNOTE-119, he said.
Another consideration is whether monotherapy or combination therapy is preferable, and the data suggest that regardless of how PD-L1-positivity is defined (by CPS cutoff of 1 vs. 20, for example), most patients treated with monotherapy progress within the first 3 months, he said.
“I don’t see that this is a safe approach for the majority of these patients. So without better biomarkers, combinations should always be preferred, at least to avoid early progression,” Dr. Bianchini said, adding that the open question, then, is: “If we set the new standard in the first-line as the combination of nab-paclitaxel and atezolizumab for PD-L1-positive patients defined by the VENTANA [SP142 assay], should we continue with immune checkpoint [inhibition] using different combinations?”
“Of course, at the time the trial was designed, the results of IMpassion were not available, but it’s very important, because [the findings] add to the evidence that immunotherapy is extremely relevant for some patients,” he said.
KATE2 further demonstrated the importance of PD-L1 status, he said, adding that due to its limitations, including small sample size and short follow-up, longer follow-up is needed to better evaluate duration of response and PFS.
“Despite the trial limitations, the qualitative effect seen in all clinical endpoints – overall response rate, progression-free survival, overall survival – in PD-L1-positive tumors defined by SP142 ... provided strong and robust signals supporting the investigation of immune checkpoint inhibitors in HER-positive breast cancer,” he said, noting that “many trials are ongoing in the early setting and the advanced setting.”
In addition to the lessons of these trials with respect to the interchangeability of PD-L1 IHC assays and the value of PD-L1 assessment for identifying the likelihood of benefit from immune checkpoint inhibitors, the findings highlight the possibility that PD-L1-negative tumors require different immunotherapy approaches or alternative therapeutic strategies, and underscore that the benefit of immunotherapy in PD-L1-positive patients is still restricted to a minority.
“So new studies and approaches with immuno-oncology are needed, and we need more effective biomarkers, because we need to have precision oncology applied – we need to go in that direction,” he concluded.
Dr. Bianchini reported consultancy/honorarium and or advisory board activity associated with Roche, MSD, AstraZeneca, Pfizer, Chugai, EISAI, Lilly, Novartis, Amgen, Sanofi, Neopharm, and Genomic Health. The IMPassion30 trial was funded by F. Hoffmann-La Roche Ltd.; Dr. Rugo reported research grants, other funding, and or travel/accommodation/expenses from Pfizer, Novartis, Eli Lilly, Merck, OBI, EISAI, Plexxikon, Genentech/Roche, MacroGenics, PUMA, Mylan, Immunomedics, Daiichi Sankyo, and Celltrion. KEYNOTE-119 was funded by Merck Sharp & Dohme Corp.; Dr. Cortés and Dr. Emens reported numerous funding relationships but none with F. Hoffman-La Roche. KATE2 was funded by F. Hoffmann-La Roche.
Sources: IMPassion130; ESMO Abstract LBA20; KEYNOTE-119: ESMO Abstract LBA21; KATE2: ESMO Abstract 305O.
BARCELONA – Programmed death-ligand 1 (PD-L1) status in patients with advanced triple negative or HER2-positive breast cancer appears to identify distinct disease entities with varying likelihood of benefit from immune checkpoint inhibition, according to Giampaolo Bianchini, MD.
This observation, which contrasts with findings in other solid tumors and expands the road map to improved outcomes with immunotherapy for metastatic breast cancer, is based in part on new findings presented at the European Society for Medical Oncology Congress.
Among additional lessons from those findings: PD-L1 assays are not easily interchangeable, and studies with a “one size fits all” approach should be avoided, Dr. Bianchini, head of the Breast Cancer Group – Medical Oncology and clinical translational and immunotherapy research at Ospedale San Raffaele, Milan, said at the congress.
IMPassion130 and PD-L1 assays
In the phase 3 IMpassion130 trial assessing nanoparticle, albumin-bound (nab)-paclitaxel chemotherapy + either the anti-PD-L1 monoclonal antibody atezolizumab or placebo for the first-line treatment of metastatic triple-negative breast cancer (mTNBC), investigators used, and validated, the VENTANA PD-L1 SP142 assay to evaluate PD-L1 expression in immune cells (IC). PD-L1 positivity was defined using a 1% cutoff, meaning that PD-L1-stained IC encompassed at least 1% of the tumor area.
The trial demonstrated significantly improved progression-free survival (PFS) in the atezolizumab arm, both in the intention-to-treat (ITT) analysis (7.2 vs. 5.5 months in the placebo arm; hazard ratio, 0.80), and the PD-L1-positive subgroup (7.5 vs. 5.0 months; HR, 0.62), and the results were published in November 2018 (N Engl J Med. 2018; 379:2108-21).
“IMpassion130 is the first phase 3 trial demonstrating clinical benefit of cancer immunotherapy in patients with PD-L1-positive, metastatic triple-negative breast cancer,” Hope S. Rugo, MD, said at the congress. “The combination of atezolizumab and nab-paclitaxel is now approved in the United States and Europe for this indication.”
In addition, the SP142 antibody (which binds to PD-L1), at the 1% cutoff, predicted PFS and overall survival (OS) with atezolizumab + nab-paclitaxel, compared with nab-paclitaxel + placebo; the absolute improvement in OS in the PD-L1-positive population was 7 months (HR, 0.71), whereas no impact was seen in PFS or OS in patients who were PD-L1-negative using the SP142 assay, said Dr. Rugo, professor of hematology/oncology, and director of breast oncology and clinical trials education at the University of California, San Francisco.
Based on the IMPassion130 findings, the Food and Drug Administration approved the SP142 assay, using the 1% cutoff, as a “companion diagnostic device for selecting TNBC patients for atezolizumab.”
However, questions remain about how to best identify patients who could benefit from the atezolizumab + nab-paclitaxel combination, Dr. Rugo said.
Therefore, she and her colleagues performed a retrospective post hoc subgroup analysis of data from the trial to assess the performance and analytical concordance of the SP142 assay and two other commonly used PD-L1 immunohistochemistry (IHC) assays: the VENTANA SP263 IHC assay typically used as a companion diagnostic with durvalumab, and the Dako PD-L1 IHC 22C3 assay typically used with pembrolizumab.
In addition, the investigators assessed PD-L1 prevalence and clinical activity.
“We also included an evaluation of important factors related to PD-L1 testing and ... relationship to clinical outcome,” Dr. Rugo said.
In 614 biomarker-evaluable patients, representing 68% of the IMPassion130 ITT population, PD-L1-positive prevalence was 46% with the SP142 assay, 75% with the SP263 assay (also based on a 1% IC cutoff), and 81% with the 22C3 assay (with positivity defined as a combined positive score [CPS] of 1 or more based on an algorithm including both tumor and IC counts).
“Almost all SP142-positive cases are captured by either 22C3 or SP263. However, about a third of patients’ tumors were positive for PD-L1 using only one of the other two assays,” she noted, explaining that “this leads to suboptimal analytical concordance.”
The overall percentage agreement between SP142 and the other assays was only 64%-68%, she said.
Positive percentage agreement rates of 98% for both SP263 and 22C3 suggest that the patients identified as PD-L1 positive using the SP142 assay are captured by the other two assays. However, negative percentage agreement rates were less than 45%.
The HRs for PFS were 0.60 in SP142-positive patients, 0.64 in SP263-positive patients, and 0.68 in 22C3-positive patients, and the HRs for OS were 0.74, 0.75, and 0.78, respectively.
Subgroup analyses indicated that PFS and OS benefit with atezolizumab + nab-paclitaxel vs. nab-paclitaxel alone was greater in double-positive patients (those with SP142 positivity and either SP263 or 22C3 positivity) than in patients who were SP263-positive/SP142-negative or 22C3-positive/SP142-negative.
Dr. Rugo and her colleagues also found that the benefits with atezolizumab + nab-paclitaxel in PD-L1-positive patients were apparent regardless of the source of tissue for testing (breast or distant metastases).
They concluded that the findings of the assays are not equivalent; 22C3 and SP263 identified more patients as PD-L1 positive, and SP142-positivity was encompassed in positive tests for both.
“The clinical benefit in the 22C3-positive and the SP263-positive subgroups appear to be driven by the SP142-positive subgroup, and [SP142] identifies patients with the longest median progression-free and overall survival from the addition of atezolizumab to nab-paclitaxel,” she said “The SP142 assay with an IC cutoff of 1% or greater is the approved diagnostic test used to identify patients with metastatic triple-negative breast cancer who are most likely to benefit from the addition of the checkpoint inhibitor atezolizumab to nab-paclitaxel.”
As for whether the SP142 should be the assay of choice in other settings in which it hasn’t been validated, Dr. Rugo said it is advisable to use the assay that has been validated in a positive trial.
“That’s what we would generally do ... however, recognizing that some countries are not using SP142, and some sites may not have access, certainly you encompass that population in the patients whose tumors are positive by both other assays,” she said. “The risk is that you might overtreat, and the cost of treatment is greater.”
Excess toxicity is also a concern in that situation, she said, adding that “hopefully in the future we’ll be able to figure out ways to have even more patients benefit from the addition of immunotherapy so that won’t be an issue.”
“What this data shows is that you can feel secure that you are encompassing the patient population identified by the parent trial to benefit from the addition of atezolizumab by using either of the other two assays; you’re only missing 1% – so that’s very reasonable,” she said. “The risk is that you’re overtreating; it’s quite likely that there’s a population there that isn’t benefiting as much, but that’s a balance.”
The findings from IMPassion130 with regard to OS in the unselected population that included PD-L1-negative patients (18.7 vs. 21.0 months with vs. without atezolizumab; HR, 0.86) underscore the fact that “one size does not fit all” when it comes to immunotherapy benefit, Dr. Bianchini said.
This is further demonstrated by the post hoc analysis comparing IHC assays, he said, explaining that 63% of IMPassion130 patients who were considered PD-L1-negative based on the SP142 “actually scored as PD-L1-positive by the other tests.
“So the very clinically important question is if there is any evidence from the data that [the PD-L1-negative group] benefits in a significant way from the addition of atezolizumab,” he said. “I don’t see evidence for a clinical benefit, I see evidence to look for new biomarkers to identify a potential population who will benefit.”
The “absence of evidence is not evidence of absence,” he stressed, noting that it may be possible – with the right biomarkers – to identify PD-L1-negative patients who would benefit.
What the findings do show, however, is support for the FDA decision to approve the SP142 assay with an IC cutoff of 1% as a companion diagnostic tool, and that PD-L1 is ideally assessed using samples from both the primary and metastatic site, as the IMPassion130 data “do not inform whether PD-L1 assessment in primary and metastatic sites is equally informative,” he said.
In addition, Dr. Bianchini said the findings suggest that more information is needed about using different cutoffs for SP263 and 22C3, and he cautioned against “directly translating these finding to other disease settings or immune combinations.
“Defining new biomarkers to identify who within the PD-L1-negative group might benefit from this combination remains an unmet need,” he said. “For sure, I don’t see a space for the other tests to define this population,” he added.
KEYNOTE-119, KATE2, and future directions
Both the randomized, open-label, phase 3 KEYNOTE-119 study of the checkpoint inhibitor pembrolizumab vs. single-agent chemotherapy for mTNBC, and the phase 2 KATE2 trial of the antibody-drug conjugate trastuzumab emtansine (T-DM1) + either atezolizumab or placebo in previously treated HER2-positive breast cancer patients, failed to meet their respective primary study endpoints.
But the news isn’t all bad, Dr. Bianchini said.
For example, in KEYNOTE-119, second- or third-line pembrolizumab monotherapy did not significantly improve OS vs. chemotherapy for mTNBC, but the pembrolizumab treatment effect increased as PD-L1 enrichment increased, he explained.
Pembrolizumab showed promising antitumor activity and manageable safety in mTNBC in prior trials, and was therefore further assessed in the KEYNOTE-119 study of 601 patients with centrally confirmed TNBC, 1-2 prior systemic treatments for mTNBC, progression on the latest therapy, and a prior anthracycline or taxane, Javier Cortés, MD, PhD, of Instituto Oncológico, Madrid, reported at the congress.
Pembrolizumab was given at a dose of 200 mg every 3 weeks, and chemotherapy was physician’s choice of capecitabine, eribulin, gemcitabine, or vinorelbine.
At a median follow-up of 9.9 months in the pembrolizumab group and 10.9 months in the chemotherapy group, OS did not differ significantly between the groups; this was true overall, in patients with a CPS of 10 or greater, and in those with a CPS of 1 or greater.
In all-comers, the HR for OS was 0.97, compared with 0.78 in patients with CPS of 10 or greater, and 0.86 in those with CPS of 1 or greater, Dr. Cortés said.
“One of the most interesting exploratory analyses was OS in those patients with CPS of 20 or higher,” he said, noting that median OS in that group was 14.9 vs.12.5 months with pembrolizumab vs. with chemotherapy (HR, 0.58).
Pembrolizumab did not improve overall PFS, but again, the rates improved with higher CPS. Duration of response, however, was longer with pembrolizumab vs. chemotherapy (12.2 vs. 8.3 months overall; 12.2 vs. 6.5 months for CPS of 1 or greater; and not reached vs. 7.1 months for CPS of 10 or greater).
Grade 3-5 AEs occurred in 35% vs. 49% of patients in the pembrolizumab vs. chemotherapy groups, with nine deaths occurring in each, Dr. Cortés said, adding that treatment-related AEs occurred in 14% (with one death) and 36% (with two deaths), respectively, and grade 3-4 immune-mediated AEs and infusion reactions occurred in 3.2% vs. 1.0% (no deaths), respectively.
In the double-blind, signal-seeking KATE2 trial, as reported in 2018 at the San Antonio Breast Cancer Symposium, no overall PFS improvement was seen with atezolizumab + T-DM1 (median of 8.2 vs. 6.8 months; HR, 0.82; 12-month PFS 38% vs. 34%), but again, a possible benefit was seen in PD-L1-positive patients (8.5 vs. 4.1 months; HR, 0.60).
KATE2 included 202 patients with advanced HER2-positive breast cancer that progressed after treatment with T-DM1 and a taxane. They were randomized 2:1 to receive intravenous T-DM1 at a dose of 3.6 mg/kg plus atezolizumab (1,200 mg) or placebo every 3 weeks until loss of clinical benefit or intolerable toxicity.
The “overall survival and final safety results” show that at a median follow-up of 19.0 months in the atezolizumab arm and 18.2 months in the placebo arm, with 52 OS events reported, median OS was not reached in either arm and 1-year survival was similar in the two groups (89.1% and 89.0%), Leisha A. Emens, MD, PhD, professor of medicine in hematology/oncology and co-leader of the Hillman Cancer Immunology and Immunotherapy Program at Hillman Cancer Center, University of Pittsburgh Medical Center (UPMC) reported at the congress.
The 1-year OS rate in the PD-L1-positive subgroup, however, was numerically higher with vs. without atezolizumab (94.2% vs. 87.9%), said Dr. Emens, director of translational immunotherapy for the Women’s Cancer Research Center at UPMC.
Of note, all additional biomarkers of T-cell activation and quantity analyzed, including PD-L1 gene expression, CD8 gene expression, T effector signature gene expression, and stromal tumor infiltrating lymphocytes (TILs), were enriched in the PD-L1-positive subgroup vs. the PD-L1-negative patients.
Further, OS rates in other immune biomarker subgroups (those with PD-L1 RNA expression, CD8 RNA expression, and T effector signature at or below vs. above the median, and those with TILs less than 5% vs. 5% or greater) were consistent with those in the PD-L1 IC-positive subgroup, and the biggest difference between the atezolizumab and placebo arms related to stromal TILs, she said.
The safety profile in this final analysis was consistent with the known safety profile of each drug, she added, noting that grade 3 or greater AEs occurred in 52.6% vs. 44.8% of patients in the atezolizumab vs. placebo arms, and serious AEs – primarily pyrexia – occurred in 36.1% vs 20.9%, respectively.
The rate of grade 5 AEs was similar in the groups.
T-DM1 is indicated for the treatment of HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane, either separately or in combination, Dr. Emens said.
“In addition to its cytotoxic activity, T-DM1 may potentiate tumor immunity,” she explained, adding that KATE2 was designed to assess whether combining T-DM1 with atezolizumab, an anti-PD-L1 antibody that restores anti-tumor immunity, would result in greater clinical activity than either drug alone.
Although the number of OS events was small, the data suggest an OS benefit with the addition of atezolizumab to T-DM1, specifically in the PD-L1 IC-positive patients, but follow-up was short and the study lacked statistical power, therefore additional study of HER2-targeted agents with atezolizumab in previously treated HER2-positive, PD-L1 IC-positive advanced breast cancer is warranted, Dr. Emens concluded.
Indeed, the finding of improved OS in the PD-L1-positive subgroups of both KEYNOTE-119 and KATE2, is of interest, Dr. Bianchini said.
Both trials failed to meet their primary endpoints, but a closer look into KEYNOTE-119 shows that PD-L1 as a continuous biomarker (using CPS, 22C3) was associated with a “continuous and strong trend” toward improved ORR with the addition of pembrolizumab.
The ORR was 9.6% vs. 10.6% in unselected patients, compared with 26.3% vs. 11.5% in those with CPS of 20 or greater.
“And when you look at duration of response, you see an increase not just in the number ... but the quality of the response,” he said, noting that for PFS, as well, a trend toward superiority is seen “that is consistent with all the other endpoints.”
“So overall, the application of incrementally restrictive cut-off of CPS lends weight to the exploratory analysis showing better survival from pembrolizumab in tumors with CPS more than 20,” Dr. Bianchini said, noting that the “real question,” however, is whether the finding “is worth clinical implementation.
“We know a lot about the primary tumor and immune infiltration. We’ve learned ... that if you wait and look ahead at immune infiltration in the advanced stage, you find that the tumor becomes smart,” he said, explaining that tumor/immune co-evolution leads to increased immuno-editing and immune subversion and it becomes “much harder to just hit the tumor with PD-L1, because this is not the only mechanism of immune escape.”
A review of several studies shows that in similar populations defined by biomarkers, response rates in patients treated with checkpoint inhibitors decrease in the second- and third-line setting vs. the first-line setting, he said.
For example, pembrolizumab response rates in the first-line and second-line or greater setting in cohort B of the KEYNOTE-086 study were 21.4% and 5.7%, respectively, compared with 12.3% in the second- to third-line setting in KEYNOTE-119, he said.
Another consideration is whether monotherapy or combination therapy is preferable, and the data suggest that regardless of how PD-L1-positivity is defined (by CPS cutoff of 1 vs. 20, for example), most patients treated with monotherapy progress within the first 3 months, he said.
“I don’t see that this is a safe approach for the majority of these patients. So without better biomarkers, combinations should always be preferred, at least to avoid early progression,” Dr. Bianchini said, adding that the open question, then, is: “If we set the new standard in the first-line as the combination of nab-paclitaxel and atezolizumab for PD-L1-positive patients defined by the VENTANA [SP142 assay], should we continue with immune checkpoint [inhibition] using different combinations?”
“Of course, at the time the trial was designed, the results of IMpassion were not available, but it’s very important, because [the findings] add to the evidence that immunotherapy is extremely relevant for some patients,” he said.
KATE2 further demonstrated the importance of PD-L1 status, he said, adding that due to its limitations, including small sample size and short follow-up, longer follow-up is needed to better evaluate duration of response and PFS.
“Despite the trial limitations, the qualitative effect seen in all clinical endpoints – overall response rate, progression-free survival, overall survival – in PD-L1-positive tumors defined by SP142 ... provided strong and robust signals supporting the investigation of immune checkpoint inhibitors in HER-positive breast cancer,” he said, noting that “many trials are ongoing in the early setting and the advanced setting.”
In addition to the lessons of these trials with respect to the interchangeability of PD-L1 IHC assays and the value of PD-L1 assessment for identifying the likelihood of benefit from immune checkpoint inhibitors, the findings highlight the possibility that PD-L1-negative tumors require different immunotherapy approaches or alternative therapeutic strategies, and underscore that the benefit of immunotherapy in PD-L1-positive patients is still restricted to a minority.
“So new studies and approaches with immuno-oncology are needed, and we need more effective biomarkers, because we need to have precision oncology applied – we need to go in that direction,” he concluded.
Dr. Bianchini reported consultancy/honorarium and or advisory board activity associated with Roche, MSD, AstraZeneca, Pfizer, Chugai, EISAI, Lilly, Novartis, Amgen, Sanofi, Neopharm, and Genomic Health. The IMPassion30 trial was funded by F. Hoffmann-La Roche Ltd.; Dr. Rugo reported research grants, other funding, and or travel/accommodation/expenses from Pfizer, Novartis, Eli Lilly, Merck, OBI, EISAI, Plexxikon, Genentech/Roche, MacroGenics, PUMA, Mylan, Immunomedics, Daiichi Sankyo, and Celltrion. KEYNOTE-119 was funded by Merck Sharp & Dohme Corp.; Dr. Cortés and Dr. Emens reported numerous funding relationships but none with F. Hoffman-La Roche. KATE2 was funded by F. Hoffmann-La Roche.
Sources: IMPassion130; ESMO Abstract LBA20; KEYNOTE-119: ESMO Abstract LBA21; KATE2: ESMO Abstract 305O.
REPORTING FROM ESMO 2019
Persistent vulvar itch: What is the diagnosis?
Genital lichen simplex chronicus
Lichen simplex chronicus (LSC) is an inflammatory skin condition that develops secondary to persistent rubbing or scratching of skin. Although LSC can occur anywhere on the body, genital LSC develops in association with genital itch, with the itch often described as intense and unrelenting. The itching sensation leads to scratching and rubbing of the area, which can provide temporary symptomatic relief.1,2 However, this action of rubbing and scratching stimulates local cutaneous nerves, inducing an even more intense itch sensation. This process, identified as the ‘itch-scratch cycle,’ plays a prominent role in all cases of LSC.1
On physical examination LSC appears as poorly defined, pink to red plaques with accentuated skin markings on bilateral labia majora. Less commonly, it can present as asymmetrical or unilateral plaques.3 LSC can extend onto labia minora, mons pubis, and medial thighs. However, the vagina is spared.1 Excoriations, marked by their geometric, angular appearance, often can be appreciated overlying plaques of LSC. Additionally, crusting, scale, broken hairs, hyperpigmentation, and scarring may be seen in LSC.2
In this case, white discharge was noted on vaginal examination, which was suspicious for vaginal candidiasis. Wet mount examination revealed multiple candida hyphae and spores (FIGURE 2), confirming vaginal candidiasis. This vulvovaginal fungal infection caused persistent vulvar pruritus, with subsequent development of LSC due to prolonged scratching. The patient was treated with both oral fluconazole and topical mometasone ointment, for vaginal candidiasis and vulvar LSC, respectively. Mometasone ointment is categorized as a class II (high potency) topical steroid. However, it is worth noting that mometasone cream is categorized as a class IV (medium potency) topical steroid.
FIGURE 2 Wet mount of vaginal discharge, revealing candida hyphae and spores
Treatment
Successful treatment of LSC requires addressing 4 elements, including recognizing and treating the underlying etiology, restoring barrier function, reducing inflammation, and interrupting the itch-scratch cycle.3
Identifying the underlying etiology. Knowing the etiology of vulvar pruritus is a key step in resolution of the condition because LSC is driven by repetitive rubbing and scratching behaviors in response to the itch. The differential diagnosis for vulvar pruritus is broad. Evaluation and workup should be tailored to suit each unique patient presentation. A review of past medical history and full-body skin examination can identify a contributing inflammatory skin disease, such as atopic dermatitis, psoriasis, lichen planus, lichen sclerosus, or autoimmune vesiculobullous disease (pemphigus).1,2 Careful review of products applied in the genital area can reveal an underlying irritant or allergic contact dermatitis. Scented soap or detergent commonly cause vulvar dermatitis.1 A speculum examination may suggest inflammatory vaginitis or atrophic vaginitis (genitourinary syndrome of menopause); run off of vaginal discharge onto the vulvar skin can result in vulvar pruritus. Vaginal wet mount can diagnose vulvovaginal candidiasis, trichomonas infection, and bacterial vaginitis.1 A skin scraping with mineral oil or potassium hydroxide can suggest scabies infestation or cutaneous dermatophyte infection, respectively.2 Treatment of vulvar pruritus should be initiated based on diagnosis.
Restoring barrier function. The repetitive scratching and rubbing behaviors disrupt the cutaneous barrier layer and lead to stimulation of the local nerves. This creates more itch and further traumatization to the barrier. Barrier function can be restored through soaking the area, with sitz baths or damp towels. Following 20- to 30-minute soaks, a lubricant, such as petroleum jelly, should be applied to the area.3
Reducing inflammation. To reduce inflammation, topical steroids should be applied to areas of LSC.3 In severe cases, high potency topical steroids should be prescribed. Examples of high potency topical steroids include:
- clobetasol propionate 0.05%
- betamethasone dipropionate 0.05%
- halobetasol propionate 0.05%.
Ointment is the choice vehicle because it is both more potent and associated with decreased stinging sensation. High potency steroid ointment should be applied twice daily for at least 2 to 4 weeks. The transition to lower potency topical steroids, such as triamcinolone acetonide 0.1% ointment, can be made as the LSC improves.2
Interrupting the itch-scratch cycle. As noted above, persistent rubbing and scratching generates increased itch sensation. Thus, breaking the itch-scratch cycle is essential. Nighttime scratching can be improved with hydroxyzine. The effective dosage ranges between 25 and 75 mg and should be titrated up slowly every 5 to 7 days. Sedation is a major adverse effect of hydroxyzine, limiting the treatment of daytime itching. Selective serotonin reuptake inhibitors (SSRIs), such as citalopram, also have been found to be effective. Over the counter, nonsedation antihistamines have not been found to be useful in breaking the itch-scratch cycle. The clinical course of LSC is chronic (as the name implies), waxing and waning, and sometimes can be challenging to treat—some patients require years-long continued follow-up and treatment.3
- Savas JA, Pichardo RO. Female genital itch. Dermatologic Clin. 2018;36:225-243.
- Chibnall R. Vulvar pruritus and lichen simplex chronicus. Obstet Gynecol Clin North Am. 2017;44:379-388.
- Lynch PJ. Lichen simplex chronicus (atopic/neurodermatitis) of the anogenital region. Dermatol Ther. 2004;17:8-19.
Genital lichen simplex chronicus
Lichen simplex chronicus (LSC) is an inflammatory skin condition that develops secondary to persistent rubbing or scratching of skin. Although LSC can occur anywhere on the body, genital LSC develops in association with genital itch, with the itch often described as intense and unrelenting. The itching sensation leads to scratching and rubbing of the area, which can provide temporary symptomatic relief.1,2 However, this action of rubbing and scratching stimulates local cutaneous nerves, inducing an even more intense itch sensation. This process, identified as the ‘itch-scratch cycle,’ plays a prominent role in all cases of LSC.1
On physical examination LSC appears as poorly defined, pink to red plaques with accentuated skin markings on bilateral labia majora. Less commonly, it can present as asymmetrical or unilateral plaques.3 LSC can extend onto labia minora, mons pubis, and medial thighs. However, the vagina is spared.1 Excoriations, marked by their geometric, angular appearance, often can be appreciated overlying plaques of LSC. Additionally, crusting, scale, broken hairs, hyperpigmentation, and scarring may be seen in LSC.2
In this case, white discharge was noted on vaginal examination, which was suspicious for vaginal candidiasis. Wet mount examination revealed multiple candida hyphae and spores (FIGURE 2), confirming vaginal candidiasis. This vulvovaginal fungal infection caused persistent vulvar pruritus, with subsequent development of LSC due to prolonged scratching. The patient was treated with both oral fluconazole and topical mometasone ointment, for vaginal candidiasis and vulvar LSC, respectively. Mometasone ointment is categorized as a class II (high potency) topical steroid. However, it is worth noting that mometasone cream is categorized as a class IV (medium potency) topical steroid.
FIGURE 2 Wet mount of vaginal discharge, revealing candida hyphae and spores

Treatment
Successful treatment of LSC requires addressing 4 elements, including recognizing and treating the underlying etiology, restoring barrier function, reducing inflammation, and interrupting the itch-scratch cycle.3
Identifying the underlying etiology. Knowing the etiology of vulvar pruritus is a key step in resolution of the condition because LSC is driven by repetitive rubbing and scratching behaviors in response to the itch. The differential diagnosis for vulvar pruritus is broad. Evaluation and workup should be tailored to suit each unique patient presentation. A review of past medical history and full-body skin examination can identify a contributing inflammatory skin disease, such as atopic dermatitis, psoriasis, lichen planus, lichen sclerosus, or autoimmune vesiculobullous disease (pemphigus).1,2 Careful review of products applied in the genital area can reveal an underlying irritant or allergic contact dermatitis. Scented soap or detergent commonly cause vulvar dermatitis.1 A speculum examination may suggest inflammatory vaginitis or atrophic vaginitis (genitourinary syndrome of menopause); run off of vaginal discharge onto the vulvar skin can result in vulvar pruritus. Vaginal wet mount can diagnose vulvovaginal candidiasis, trichomonas infection, and bacterial vaginitis.1 A skin scraping with mineral oil or potassium hydroxide can suggest scabies infestation or cutaneous dermatophyte infection, respectively.2 Treatment of vulvar pruritus should be initiated based on diagnosis.
Restoring barrier function. The repetitive scratching and rubbing behaviors disrupt the cutaneous barrier layer and lead to stimulation of the local nerves. This creates more itch and further traumatization to the barrier. Barrier function can be restored through soaking the area, with sitz baths or damp towels. Following 20- to 30-minute soaks, a lubricant, such as petroleum jelly, should be applied to the area.3
Reducing inflammation. To reduce inflammation, topical steroids should be applied to areas of LSC.3 In severe cases, high potency topical steroids should be prescribed. Examples of high potency topical steroids include:
- clobetasol propionate 0.05%
- betamethasone dipropionate 0.05%
- halobetasol propionate 0.05%.
Ointment is the choice vehicle because it is both more potent and associated with decreased stinging sensation. High potency steroid ointment should be applied twice daily for at least 2 to 4 weeks. The transition to lower potency topical steroids, such as triamcinolone acetonide 0.1% ointment, can be made as the LSC improves.2
Interrupting the itch-scratch cycle. As noted above, persistent rubbing and scratching generates increased itch sensation. Thus, breaking the itch-scratch cycle is essential. Nighttime scratching can be improved with hydroxyzine. The effective dosage ranges between 25 and 75 mg and should be titrated up slowly every 5 to 7 days. Sedation is a major adverse effect of hydroxyzine, limiting the treatment of daytime itching. Selective serotonin reuptake inhibitors (SSRIs), such as citalopram, also have been found to be effective. Over the counter, nonsedation antihistamines have not been found to be useful in breaking the itch-scratch cycle. The clinical course of LSC is chronic (as the name implies), waxing and waning, and sometimes can be challenging to treat—some patients require years-long continued follow-up and treatment.3
Genital lichen simplex chronicus
Lichen simplex chronicus (LSC) is an inflammatory skin condition that develops secondary to persistent rubbing or scratching of skin. Although LSC can occur anywhere on the body, genital LSC develops in association with genital itch, with the itch often described as intense and unrelenting. The itching sensation leads to scratching and rubbing of the area, which can provide temporary symptomatic relief.1,2 However, this action of rubbing and scratching stimulates local cutaneous nerves, inducing an even more intense itch sensation. This process, identified as the ‘itch-scratch cycle,’ plays a prominent role in all cases of LSC.1
On physical examination LSC appears as poorly defined, pink to red plaques with accentuated skin markings on bilateral labia majora. Less commonly, it can present as asymmetrical or unilateral plaques.3 LSC can extend onto labia minora, mons pubis, and medial thighs. However, the vagina is spared.1 Excoriations, marked by their geometric, angular appearance, often can be appreciated overlying plaques of LSC. Additionally, crusting, scale, broken hairs, hyperpigmentation, and scarring may be seen in LSC.2
In this case, white discharge was noted on vaginal examination, which was suspicious for vaginal candidiasis. Wet mount examination revealed multiple candida hyphae and spores (FIGURE 2), confirming vaginal candidiasis. This vulvovaginal fungal infection caused persistent vulvar pruritus, with subsequent development of LSC due to prolonged scratching. The patient was treated with both oral fluconazole and topical mometasone ointment, for vaginal candidiasis and vulvar LSC, respectively. Mometasone ointment is categorized as a class II (high potency) topical steroid. However, it is worth noting that mometasone cream is categorized as a class IV (medium potency) topical steroid.
FIGURE 2 Wet mount of vaginal discharge, revealing candida hyphae and spores

Treatment
Successful treatment of LSC requires addressing 4 elements, including recognizing and treating the underlying etiology, restoring barrier function, reducing inflammation, and interrupting the itch-scratch cycle.3
Identifying the underlying etiology. Knowing the etiology of vulvar pruritus is a key step in resolution of the condition because LSC is driven by repetitive rubbing and scratching behaviors in response to the itch. The differential diagnosis for vulvar pruritus is broad. Evaluation and workup should be tailored to suit each unique patient presentation. A review of past medical history and full-body skin examination can identify a contributing inflammatory skin disease, such as atopic dermatitis, psoriasis, lichen planus, lichen sclerosus, or autoimmune vesiculobullous disease (pemphigus).1,2 Careful review of products applied in the genital area can reveal an underlying irritant or allergic contact dermatitis. Scented soap or detergent commonly cause vulvar dermatitis.1 A speculum examination may suggest inflammatory vaginitis or atrophic vaginitis (genitourinary syndrome of menopause); run off of vaginal discharge onto the vulvar skin can result in vulvar pruritus. Vaginal wet mount can diagnose vulvovaginal candidiasis, trichomonas infection, and bacterial vaginitis.1 A skin scraping with mineral oil or potassium hydroxide can suggest scabies infestation or cutaneous dermatophyte infection, respectively.2 Treatment of vulvar pruritus should be initiated based on diagnosis.
Restoring barrier function. The repetitive scratching and rubbing behaviors disrupt the cutaneous barrier layer and lead to stimulation of the local nerves. This creates more itch and further traumatization to the barrier. Barrier function can be restored through soaking the area, with sitz baths or damp towels. Following 20- to 30-minute soaks, a lubricant, such as petroleum jelly, should be applied to the area.3
Reducing inflammation. To reduce inflammation, topical steroids should be applied to areas of LSC.3 In severe cases, high potency topical steroids should be prescribed. Examples of high potency topical steroids include:
- clobetasol propionate 0.05%
- betamethasone dipropionate 0.05%
- halobetasol propionate 0.05%.
Ointment is the choice vehicle because it is both more potent and associated with decreased stinging sensation. High potency steroid ointment should be applied twice daily for at least 2 to 4 weeks. The transition to lower potency topical steroids, such as triamcinolone acetonide 0.1% ointment, can be made as the LSC improves.2
Interrupting the itch-scratch cycle. As noted above, persistent rubbing and scratching generates increased itch sensation. Thus, breaking the itch-scratch cycle is essential. Nighttime scratching can be improved with hydroxyzine. The effective dosage ranges between 25 and 75 mg and should be titrated up slowly every 5 to 7 days. Sedation is a major adverse effect of hydroxyzine, limiting the treatment of daytime itching. Selective serotonin reuptake inhibitors (SSRIs), such as citalopram, also have been found to be effective. Over the counter, nonsedation antihistamines have not been found to be useful in breaking the itch-scratch cycle. The clinical course of LSC is chronic (as the name implies), waxing and waning, and sometimes can be challenging to treat—some patients require years-long continued follow-up and treatment.3
- Savas JA, Pichardo RO. Female genital itch. Dermatologic Clin. 2018;36:225-243.
- Chibnall R. Vulvar pruritus and lichen simplex chronicus. Obstet Gynecol Clin North Am. 2017;44:379-388.
- Lynch PJ. Lichen simplex chronicus (atopic/neurodermatitis) of the anogenital region. Dermatol Ther. 2004;17:8-19.
- Savas JA, Pichardo RO. Female genital itch. Dermatologic Clin. 2018;36:225-243.
- Chibnall R. Vulvar pruritus and lichen simplex chronicus. Obstet Gynecol Clin North Am. 2017;44:379-388.
- Lynch PJ. Lichen simplex chronicus (atopic/neurodermatitis) of the anogenital region. Dermatol Ther. 2004;17:8-19.
CASE Lingering vulvar pruritus developed during traveling
A 48-year-old premenopausal Hispanic woman with past medical history of breast cancer presents to a dermatologist with the chief complaint of persistent vulvar pruritus. The vulvar itching began while traveling and has continued for 6 months. Previous treatments have been trialed, including over-the-counter feminine hygiene products, wipes, and hydrocortisone ointment.
Physical examination reveals pink, symmetric, bilateral lichenified plaques on the labia majora, without evidence of atrophy or scarring ( FIGURE 1 ). Scant white vaginal discharge is also noted.
FIGURE 1 Bilateral labia majora show lichenification
Figure caption: On bilateral labia majora, symmetric, pink plaques with accentuated skin markings (lichenification) noted on physical examination. Scant white vaginal discharge was noted on exam but is inconspicuous in photo.
Current Controversies in Mohs Micrographic Surgery
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
Resident Pearl:
• Further investigation is needed to elucidate and optimize solutions to current controversies in Mohs micrographic surgery.
Get ready for changes in polypharmacy quality ratings
NATIONAL HARBOR, MD. – and managed care organizations should start preparing now for the shift.
Panelists at an Oct. 30 session at the annual meeting of the Academy of Managed Care Pharmacy presented strategies for addressing the three areas of polypharmacy that will be tracked in the new rating system, which will replace the current high-risk medication measurement that is being retired this year.
Anticholinergic medications
The first area presented by the panelists was polypharmacy use of multiple anticholinergic medications in older adults (Poly-ACH). The new quality measure will examine the percentage of members aged 65 years or older who are using two or more anticholinergic medications concurrently.
“We know that anticholinergic burden increases the risk of cognitive decline in particular, but it’s also associated with a higher risk of falls, an increased number of hospitalizations, and [diminished] physical function,” said Marti Groeneweg, PharmD, supervisor of clinical pharmacy services at Kaiser Permanente.
Dr. Groeneweg noted that, in addition to using multiple drugs in this class, patients can also benefit from a decrease in the dosage of their drugs, so that should also be considered in managing the medication of beneficiaries.
She highlighted a program Kaiser Permanente started in the Northwest United States to reduce the concurrent use of these drugs. The program targeted tricyclic antidepressants – nortriptyline, in particular.
The company instituted a multipronged approach that included provider detailing of the risks of using multiple drugs and how they could taper schedules, as well as providing them with other supporting resources and a list of safer, alternative drugs. It also reached out to patients to educate them about the risks of their medications and why it was important for them to taper their medications. The third part of the approach was to use the EHR to provide doctors with the best-available information at the point of prescribing. And finally, there was a pharmacist review process put in place for more complex cases.
Dr. Groeneweg emphasized that this information was incorporated into existing programs.
The intervention, which is fairly new, has not been in place long enough to know exactly how well it is working, but early indicators suggest “we are on the right track,” she said, noting that to date there has been a decrease of 28% in the number of tricyclic antidepressant prescriptions per 1,000 Medicare members per month.
CNS medications
The second area the panelists addressed was the polypharmacy use of multiple CNS-active medications in older adults (Poly-CNS).
Rainelle Gaddy, PharmD, Rx clinical programs pharmacy lead at Humana Pharmacy Solutions, , noted that the clinical rationale for this measure was the “increased risk of falls and fractures when these medications are taken concurrently.”
She pointed out that taking one or more of the CNS medications can result in a 1.5-fold increase in the risk for falls, and that risk increases to 2.5-fold if two or more drugs are taken. In addition, a high-dose of these medications can lead to a threefold increase in risk of recurrent falls.
Dr. Gaddy highlighted a number of interventions that could be implemented when the managed care organization is not integrated in the way Kaiser Permanente is.
“Pharmacists can pay a pivotal role [in helping] patients who are receiving these Poly-CNS medications because they are able to interact and talk through the actual patient picture for all their medications ... because pharmacists have always been seen as being a trusted source,” she said.
Dr. Gaddy added that health plans can take a more direct role in reaching out to patients, for example, through telephone outreach, as well as direct mail, email, and newsletters.
“We want to make sure that members have as much information as possible,” she said.
She added that it is very important to include physicians and other prescribers in this process through faxes and information included in EHRs.
Opioids and benzodiazepines
The final measure highlighted during the session was the one measuring the concurrent use of opioids and benzodiazepines.
Dr. Gaddy noted that taking the two concurrently is associated with a fourfold increase in risk of opioid overdose and death, compared with opioid use without a benzodiazepine.
She noted that a black box warning on the risks of concurrent use was added to both opioids and benzodiazepines in August 2016 and that resulted in a 10% decrease in the concurrent use.
“This new measure is intended to ensure that the downward trend continues. CMS has indicated as such,” Dr. Gaddy said.
Most of the intervention strategies she highlighted were similar to those for the Poly-CNS category, including the use of medication therapy management programs and targeted interventions, telephone outreach to members, and provider detailing and outreach.
“Provider detailing is really key,” Dr. Gaddy said. “On any given day, it’s so easy for physicians to see 30 patients. The great thing about the provider detailing is that you are able to give the provider a ‘packet’ of their members, you can identify and/or aid in showing them the risk assessment associated with members taking these medications, and then equip them with pocket guides and [materials so they can] streamline the medications.”
NATIONAL HARBOR, MD. – and managed care organizations should start preparing now for the shift.
Panelists at an Oct. 30 session at the annual meeting of the Academy of Managed Care Pharmacy presented strategies for addressing the three areas of polypharmacy that will be tracked in the new rating system, which will replace the current high-risk medication measurement that is being retired this year.
Anticholinergic medications
The first area presented by the panelists was polypharmacy use of multiple anticholinergic medications in older adults (Poly-ACH). The new quality measure will examine the percentage of members aged 65 years or older who are using two or more anticholinergic medications concurrently.
“We know that anticholinergic burden increases the risk of cognitive decline in particular, but it’s also associated with a higher risk of falls, an increased number of hospitalizations, and [diminished] physical function,” said Marti Groeneweg, PharmD, supervisor of clinical pharmacy services at Kaiser Permanente.
Dr. Groeneweg noted that, in addition to using multiple drugs in this class, patients can also benefit from a decrease in the dosage of their drugs, so that should also be considered in managing the medication of beneficiaries.
She highlighted a program Kaiser Permanente started in the Northwest United States to reduce the concurrent use of these drugs. The program targeted tricyclic antidepressants – nortriptyline, in particular.
The company instituted a multipronged approach that included provider detailing of the risks of using multiple drugs and how they could taper schedules, as well as providing them with other supporting resources and a list of safer, alternative drugs. It also reached out to patients to educate them about the risks of their medications and why it was important for them to taper their medications. The third part of the approach was to use the EHR to provide doctors with the best-available information at the point of prescribing. And finally, there was a pharmacist review process put in place for more complex cases.
Dr. Groeneweg emphasized that this information was incorporated into existing programs.
The intervention, which is fairly new, has not been in place long enough to know exactly how well it is working, but early indicators suggest “we are on the right track,” she said, noting that to date there has been a decrease of 28% in the number of tricyclic antidepressant prescriptions per 1,000 Medicare members per month.
CNS medications
The second area the panelists addressed was the polypharmacy use of multiple CNS-active medications in older adults (Poly-CNS).
Rainelle Gaddy, PharmD, Rx clinical programs pharmacy lead at Humana Pharmacy Solutions, , noted that the clinical rationale for this measure was the “increased risk of falls and fractures when these medications are taken concurrently.”
She pointed out that taking one or more of the CNS medications can result in a 1.5-fold increase in the risk for falls, and that risk increases to 2.5-fold if two or more drugs are taken. In addition, a high-dose of these medications can lead to a threefold increase in risk of recurrent falls.
Dr. Gaddy highlighted a number of interventions that could be implemented when the managed care organization is not integrated in the way Kaiser Permanente is.
“Pharmacists can pay a pivotal role [in helping] patients who are receiving these Poly-CNS medications because they are able to interact and talk through the actual patient picture for all their medications ... because pharmacists have always been seen as being a trusted source,” she said.
Dr. Gaddy added that health plans can take a more direct role in reaching out to patients, for example, through telephone outreach, as well as direct mail, email, and newsletters.
“We want to make sure that members have as much information as possible,” she said.
She added that it is very important to include physicians and other prescribers in this process through faxes and information included in EHRs.
Opioids and benzodiazepines
The final measure highlighted during the session was the one measuring the concurrent use of opioids and benzodiazepines.
Dr. Gaddy noted that taking the two concurrently is associated with a fourfold increase in risk of opioid overdose and death, compared with opioid use without a benzodiazepine.
She noted that a black box warning on the risks of concurrent use was added to both opioids and benzodiazepines in August 2016 and that resulted in a 10% decrease in the concurrent use.
“This new measure is intended to ensure that the downward trend continues. CMS has indicated as such,” Dr. Gaddy said.
Most of the intervention strategies she highlighted were similar to those for the Poly-CNS category, including the use of medication therapy management programs and targeted interventions, telephone outreach to members, and provider detailing and outreach.
“Provider detailing is really key,” Dr. Gaddy said. “On any given day, it’s so easy for physicians to see 30 patients. The great thing about the provider detailing is that you are able to give the provider a ‘packet’ of their members, you can identify and/or aid in showing them the risk assessment associated with members taking these medications, and then equip them with pocket guides and [materials so they can] streamline the medications.”
NATIONAL HARBOR, MD. – and managed care organizations should start preparing now for the shift.
Panelists at an Oct. 30 session at the annual meeting of the Academy of Managed Care Pharmacy presented strategies for addressing the three areas of polypharmacy that will be tracked in the new rating system, which will replace the current high-risk medication measurement that is being retired this year.
Anticholinergic medications
The first area presented by the panelists was polypharmacy use of multiple anticholinergic medications in older adults (Poly-ACH). The new quality measure will examine the percentage of members aged 65 years or older who are using two or more anticholinergic medications concurrently.
“We know that anticholinergic burden increases the risk of cognitive decline in particular, but it’s also associated with a higher risk of falls, an increased number of hospitalizations, and [diminished] physical function,” said Marti Groeneweg, PharmD, supervisor of clinical pharmacy services at Kaiser Permanente.
Dr. Groeneweg noted that, in addition to using multiple drugs in this class, patients can also benefit from a decrease in the dosage of their drugs, so that should also be considered in managing the medication of beneficiaries.
She highlighted a program Kaiser Permanente started in the Northwest United States to reduce the concurrent use of these drugs. The program targeted tricyclic antidepressants – nortriptyline, in particular.
The company instituted a multipronged approach that included provider detailing of the risks of using multiple drugs and how they could taper schedules, as well as providing them with other supporting resources and a list of safer, alternative drugs. It also reached out to patients to educate them about the risks of their medications and why it was important for them to taper their medications. The third part of the approach was to use the EHR to provide doctors with the best-available information at the point of prescribing. And finally, there was a pharmacist review process put in place for more complex cases.
Dr. Groeneweg emphasized that this information was incorporated into existing programs.
The intervention, which is fairly new, has not been in place long enough to know exactly how well it is working, but early indicators suggest “we are on the right track,” she said, noting that to date there has been a decrease of 28% in the number of tricyclic antidepressant prescriptions per 1,000 Medicare members per month.
CNS medications
The second area the panelists addressed was the polypharmacy use of multiple CNS-active medications in older adults (Poly-CNS).
Rainelle Gaddy, PharmD, Rx clinical programs pharmacy lead at Humana Pharmacy Solutions, , noted that the clinical rationale for this measure was the “increased risk of falls and fractures when these medications are taken concurrently.”
She pointed out that taking one or more of the CNS medications can result in a 1.5-fold increase in the risk for falls, and that risk increases to 2.5-fold if two or more drugs are taken. In addition, a high-dose of these medications can lead to a threefold increase in risk of recurrent falls.
Dr. Gaddy highlighted a number of interventions that could be implemented when the managed care organization is not integrated in the way Kaiser Permanente is.
“Pharmacists can pay a pivotal role [in helping] patients who are receiving these Poly-CNS medications because they are able to interact and talk through the actual patient picture for all their medications ... because pharmacists have always been seen as being a trusted source,” she said.
Dr. Gaddy added that health plans can take a more direct role in reaching out to patients, for example, through telephone outreach, as well as direct mail, email, and newsletters.
“We want to make sure that members have as much information as possible,” she said.
She added that it is very important to include physicians and other prescribers in this process through faxes and information included in EHRs.
Opioids and benzodiazepines
The final measure highlighted during the session was the one measuring the concurrent use of opioids and benzodiazepines.
Dr. Gaddy noted that taking the two concurrently is associated with a fourfold increase in risk of opioid overdose and death, compared with opioid use without a benzodiazepine.
She noted that a black box warning on the risks of concurrent use was added to both opioids and benzodiazepines in August 2016 and that resulted in a 10% decrease in the concurrent use.
“This new measure is intended to ensure that the downward trend continues. CMS has indicated as such,” Dr. Gaddy said.
Most of the intervention strategies she highlighted were similar to those for the Poly-CNS category, including the use of medication therapy management programs and targeted interventions, telephone outreach to members, and provider detailing and outreach.
“Provider detailing is really key,” Dr. Gaddy said. “On any given day, it’s so easy for physicians to see 30 patients. The great thing about the provider detailing is that you are able to give the provider a ‘packet’ of their members, you can identify and/or aid in showing them the risk assessment associated with members taking these medications, and then equip them with pocket guides and [materials so they can] streamline the medications.”
REPORTING FROM AMCP NEXUS 2019
Vaping-linked lung injury cases near 1,900
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.

Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.

Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
according to the latest update provided by the Centers for Disease Control and Prevention. Thirty-seven deaths have been confirmed.

Deaths have occurred in 24 states and the District of Columbia: Alabama, California (3), Connecticut, Delaware, Florida, Georgia (3), Illinois (2), Indiana (3), Kansas (2), Massachusetts, Michigan, Minnesota (3), Mississippi, Missouri, Montana, Nebraska, New Jersey, New York, Oregon (2), Pennsylvania, Tennessee (2), Texas, Utah, and Virginia. As on Oct. 28, the median age of deceased patients was 49 years and ranged from 17 to 75 years.
The CDC is now doing additional testing on available samples for chemical in the bronchoalveolar lavage fluid, blood, or urine, as well as lung biopsy or autopsy specimens. It also is validating methods for aerosol emission testing of case-associated product samples from vaping products and e-liquids.
For more information and resources visit For the Public, For Healthcare Providers, and For State and Local Health Departments pages, as well as the CDC’s Publications and Resources page.
Synchronizing refills saves money, improves outcomes
NATIONAL HARBOR, MD. – according to research presented at the annual meeting of the Academy of Managed Care Pharmacy.
Investigators with Pharmacy Quality Alliance (PQA) used data from Truven MarketScan Research Databases to conduct a retrospective cohort study of more than 20,000 patients eligible for inclusion in PQA’s diabetes medication adherence measure. To be included, patients needed to have two or more prescriptions for diabetes medications (excluding insulin), statins, or renin-angiotensin system antagonists. About 80% of patients were commercially insured and 20% came from Medicare supplement insurance (Medigap) plans.
Commercially insured patients whose medication refills were synchronized had better medication adherence than did matched controls (67.7% vs. 57.4%) and lower median health care expenditures ($3,687 vs. $7,480).
The same was true for patients with Medicare supplemental insurance. Synchronized patients in this group also had better medication adherence than controls, at 86.5% vs. 70.4% and lower median health care expenditures ($7,353 vs. $10,592).
Based on their findings in diabetes patients, “I think we should synchronize refills,” Matthew K. Pickering, PharmD, senior director of research and quality strategies at PQA, said. “However, there are populations that were not represented in this, like COPD [chronic obstructive pulmonary disease]. That’s another high-comorbidity, high-cost population that should be studied.”
Session moderator Laura Happe, PharmD, editor in chief of the Journal of Managed Care and Specialty Pharmacy, questioned Dr. Pickering about the barriers to medication synchronization.
In previous research, “we discovered that some patients were resistant to synchronizing their medication refills because of the copays – having all of their copays at one time, rather than spreading them out over the month,” Dr. Happe said.
“Certainly, patients may not be able to afford all their copays at one time, so that can be a barrier,” Dr. Pickering said. “With medication synchronization programs, there’s a lot of variation across the board. Patients can choose which medication to synchronize in some programs. Others only synchronize the three-star medication, etc. But there are real barriers and they should be explored.”
Pharmacy Quality Alliance is a nonprofit public-private partnership that develops pharmacy quality measures in collaboration with the Centers for Medicare & Medicaid Services.
Dr. Pickering disclosed no relevant conflicts of interest.
NATIONAL HARBOR, MD. – according to research presented at the annual meeting of the Academy of Managed Care Pharmacy.
Investigators with Pharmacy Quality Alliance (PQA) used data from Truven MarketScan Research Databases to conduct a retrospective cohort study of more than 20,000 patients eligible for inclusion in PQA’s diabetes medication adherence measure. To be included, patients needed to have two or more prescriptions for diabetes medications (excluding insulin), statins, or renin-angiotensin system antagonists. About 80% of patients were commercially insured and 20% came from Medicare supplement insurance (Medigap) plans.
Commercially insured patients whose medication refills were synchronized had better medication adherence than did matched controls (67.7% vs. 57.4%) and lower median health care expenditures ($3,687 vs. $7,480).
The same was true for patients with Medicare supplemental insurance. Synchronized patients in this group also had better medication adherence than controls, at 86.5% vs. 70.4% and lower median health care expenditures ($7,353 vs. $10,592).
Based on their findings in diabetes patients, “I think we should synchronize refills,” Matthew K. Pickering, PharmD, senior director of research and quality strategies at PQA, said. “However, there are populations that were not represented in this, like COPD [chronic obstructive pulmonary disease]. That’s another high-comorbidity, high-cost population that should be studied.”
Session moderator Laura Happe, PharmD, editor in chief of the Journal of Managed Care and Specialty Pharmacy, questioned Dr. Pickering about the barriers to medication synchronization.
In previous research, “we discovered that some patients were resistant to synchronizing their medication refills because of the copays – having all of their copays at one time, rather than spreading them out over the month,” Dr. Happe said.
“Certainly, patients may not be able to afford all their copays at one time, so that can be a barrier,” Dr. Pickering said. “With medication synchronization programs, there’s a lot of variation across the board. Patients can choose which medication to synchronize in some programs. Others only synchronize the three-star medication, etc. But there are real barriers and they should be explored.”
Pharmacy Quality Alliance is a nonprofit public-private partnership that develops pharmacy quality measures in collaboration with the Centers for Medicare & Medicaid Services.
Dr. Pickering disclosed no relevant conflicts of interest.
NATIONAL HARBOR, MD. – according to research presented at the annual meeting of the Academy of Managed Care Pharmacy.
Investigators with Pharmacy Quality Alliance (PQA) used data from Truven MarketScan Research Databases to conduct a retrospective cohort study of more than 20,000 patients eligible for inclusion in PQA’s diabetes medication adherence measure. To be included, patients needed to have two or more prescriptions for diabetes medications (excluding insulin), statins, or renin-angiotensin system antagonists. About 80% of patients were commercially insured and 20% came from Medicare supplement insurance (Medigap) plans.
Commercially insured patients whose medication refills were synchronized had better medication adherence than did matched controls (67.7% vs. 57.4%) and lower median health care expenditures ($3,687 vs. $7,480).
The same was true for patients with Medicare supplemental insurance. Synchronized patients in this group also had better medication adherence than controls, at 86.5% vs. 70.4% and lower median health care expenditures ($7,353 vs. $10,592).
Based on their findings in diabetes patients, “I think we should synchronize refills,” Matthew K. Pickering, PharmD, senior director of research and quality strategies at PQA, said. “However, there are populations that were not represented in this, like COPD [chronic obstructive pulmonary disease]. That’s another high-comorbidity, high-cost population that should be studied.”
Session moderator Laura Happe, PharmD, editor in chief of the Journal of Managed Care and Specialty Pharmacy, questioned Dr. Pickering about the barriers to medication synchronization.
In previous research, “we discovered that some patients were resistant to synchronizing their medication refills because of the copays – having all of their copays at one time, rather than spreading them out over the month,” Dr. Happe said.
“Certainly, patients may not be able to afford all their copays at one time, so that can be a barrier,” Dr. Pickering said. “With medication synchronization programs, there’s a lot of variation across the board. Patients can choose which medication to synchronize in some programs. Others only synchronize the three-star medication, etc. But there are real barriers and they should be explored.”
Pharmacy Quality Alliance is a nonprofit public-private partnership that develops pharmacy quality measures in collaboration with the Centers for Medicare & Medicaid Services.
Dr. Pickering disclosed no relevant conflicts of interest.
REPORTING FROM AMCP NEXUS 2019
2019 Update on minimally invasive gynecologic surgery
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).
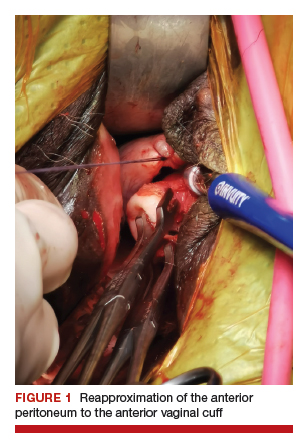
2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).
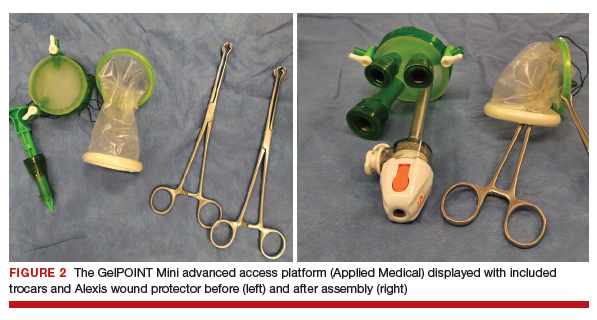

After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).
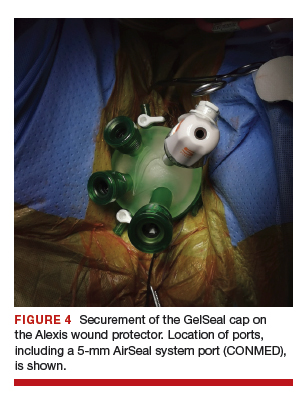
Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).
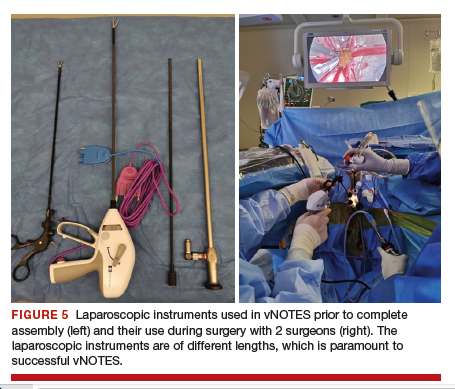
vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).
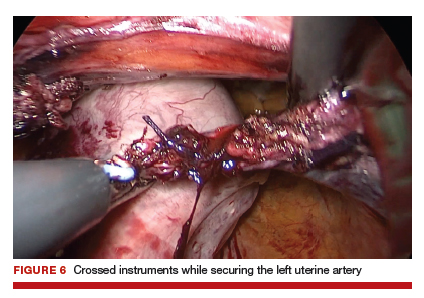
This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.
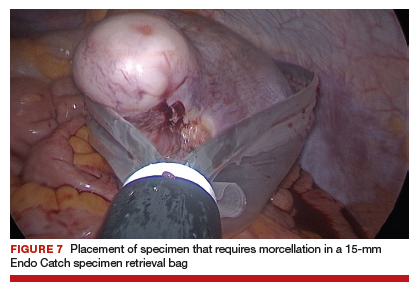
Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).

2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).


After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).

Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).

vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).

This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.


Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
Through the years, the surgical approach to hysterectomy has expanded from its early beginnings of being performed only through an abdominal or transvaginal route with traditional surgical clamps and suture. The late 1980s saw the advent of the laparoscopic-assisted vaginal hysterectomy (LAVH), and from that point forward several additional hysterectomy methods evolved, including today’s robotic approaches.
Although clinical evidence and societal endorsements support vaginal hysterectomy as a superior high-value modality, it remains one of the least performed among all available routes.1-3 In an analysis of inpatient hysterectomies published by Wright and colleagues in 2013, 16.7% of hysterectomies were performed vaginally, a number that essentially has remained steady throughout the ensuing years.4
Attempts to improve the application of vaginal hysterectomy have been made.5 These include the development of various curriculum and simulation-based medical education programs on vaginal surgical skills training and acquisition in the hopes of improving utilization.6 An interesting recent development is the rethinking of vaginal hysterectomy by several surgeons globally who are applying facets of the various hysterectomy methods to a transvaginal approach known as vaginal natural orifice transluminal endoscopic surgery (vNOTES).7,8 Unique to this thinking is the incorporation of conventional laparoscopic instrumentation.
Although I have not yet incorporated this approach in my surgical armamentarium at Columbia University Medical Center/New York–Presbyterian Hospital, I am intrigued by the possibility that this technique may serve as a rescue for vaginal hysterectomies that are at risk of conversion or of not being performed at all.9
At this time, vNOTES is not a standard of care and should be performed only by highly specialized surgeons. However, in the spirit of this Update on minimally invasive surgery and to keep our readers abreast of burgeoning techniques, I am delighted to bring you this overview by Dr. Xiaoming Guan, one of the pioneers of this surgical approach, and Dr. Tamisa Koythong and Dr. Juan Liu. I hope you find this recent development in hysterectomy of interest.
—Arnold P. Advincula, MD
Continue to: Development and evolution of NOTES...
Development and evolution of NOTES
Over the past few decades, emphasis has shifted from laparotomy to minimally invasive surgery because of its proven significant advantages in patient care, such as improved cosmesis, shorter hospital stay, shorter postoperative recovery, and decreased postoperative pain and blood loss.10 Advances in laparoendoscopic surgery and instrumentation, including robot-assisted laparoscopy (RAL), single-incision laparoscopic surgery (SILS), and most recently natural orifice transluminal endoscopic surgery (NOTES), reflect ongoing innovative developments in the field of minimally invasive surgery.
Here, we provide a brief literature review of the NOTES technique, focus on its application in gynecologic surgery, and describe how we perform NOTES at our institution.
NOTES application in gynecology
With NOTES, peritoneal access is gained through a natural orifice (such as the mouth, vagina, urethra, or anus) to perform endoscopic surgery, occasionally without requiring an abdominal incision. First described in 2004, transgastric peritoneoscopy was performed in a porcine model, and shortly thereafter the first transgastric appendectomy was performed in humans.11,12 The technique has further been adopted in cholecystectomy, appendectomy, gastrectomy, and nephrectomy procedures.13
Given rapid interest in a possible paradigm shift in the field of minimally invasive surgery, the Natural Orifice Surgery Consortiumfor Assessment and Research (NOSCAR) was formed, and the group published an article on potential barriers to accepted practice and adoption of NOTES as a realistic alternative to traditional laparoscopic surgery.14
While transgastric and transanal access to the peritoneum were initially more popular, the risk of anastomotic leaks associated with incomplete closure and subsequent infection were thought to be prohibitively high.15 Transvaginal access was considered a safer and simpler alternative, allowing for complete closure without increased risk of infection, and this is now the route through which the majority of NOTES procedures are completed.16,17
The eventual application of NOTES in the field of gynecology seemed inevitable. The American College of Obstetricians and Gynecologists stated that transvaginal surgery is the most minimally invasive and preferred surgical route in the management of patients with benign gynecologic diseases.18 However, performing it can be challenging at times due to limited visualization and lack of the required skills for single-site surgery. NOTES allows a gynecologic surgeon to improve visualization through the use of laparoendoscopic instruments and to complete surgery through a transvaginal route.
In 2012, Ahn and colleagues demonstrated the feasibility of the NOTES technique in gynecologic surgery after using it to successfully complete benign adnexal surgery in 10 patients.19 Vaginal NOTES (vNOTES) has since been further developed to include successful hysterectomy, myomectomy, sacrocolpopexy, tubal anastomosis, and even lymphadenectomy in the treatment of early- stage endometrial carcinoma.20-26 vNOTES also can be considered a rescue approach for traditional vaginal hysterectomy in instances in which it is necessary to evaluate adnexal pathology.9 Most recently, vNOTES hysterectomy has been reported with da Vinci Si or Xi robotic platforms.27,28
Continue to: Operative time, post-op stay shorter in NAOC-treated patients...
Operative time, post-op stay shorter in NAOC-treated patients
Few studies have compared outcomes with vNOTES to those with traditional laparoscopy. In 2016, Wang and colleagues compared surgical outcomes between NOTES-assisted ovarian cystectomy (NAOC) and laparoscopic ovarian cystectomy (LOC) in a case-matched study that included 277 patients.29 Although mean (SD) blood loss in patients who underwent LOC was significantly less compared with those who underwent NAOC (21.4 [14.7] mL vs 31.6 [24.1] mL; P = .028), absolute blood loss in both groups was deemed minimal. Additionally, mean (SD) operative time and postoperative stay were significantly less in patients undergoing NAOC compared with those having LOC (38.23 [10.19] minutes vs 53.82 [18.61] minutes; P≤.001; and 1.38 [0.55] days vs 1.82 [0.52] days; P≤.001; respectively).29
How vNOTES hysterectomy stacked up against TLH
In 2018, Baekelandt and colleagues compared outcomes between vNOTES hysterectomy and total laparoscopic hysterectomy (TLH) in a noninferiority single-blinded trial of 70 women.8 Compared with TLH, vNOTES hysterectomy was associated with shorter operative time (41 vs 75 minutes; P<.001), shorter hospital stay (0.8 vs 1.3 days; P = .004), and lower postoperative analgesic requirement (8 vs 14 U; P = .006). Additionally, there were no differences between the 2 groups in postoperative infection rate, intraoperative complications, or hospital readmissions within 6 weeks.8
Clearly, vNOTES is the next exciting development in minimally invasive surgery, improving patient outcomes and satisfaction with truly scarless surgery. Compared with traditional transvaginal surgery, vNOTES has the advantage of improved visualization with laparoendoscopic guidance, and it may be beneficial even for patients previously thought to have relative contraindications to successful completion of transvaginal surgery, such as nulliparity or a narrow introitus.
Approach for performing vNOTES procedures
At our institution, Baylor College of Medicine, the majority of gynecologic surgeries are performed via either transumbilical robot-assisted single-incision laparoscopy or vNOTES. Preoperative selection of appropriate candidates for vNOTES includes:
- low suspicion for or prior diagnosis of endometriosis with obliteration of the posterior cul-de-sac
- no surgical history suggestive of severe adhesive disease, and
- adequate vaginal sidewall access and sufficient descent for instrumentation for entry into the peritoneal cavity.
In general, a key concept in vNOTES is "vaginal pull, laparoscopic push," which means that the surgeon must pull the cervix while performing vaginal entry and then push the uterus back in the peritoneal cavity to increase surgical space during laparoscopic surgery.
Continue to: Overview of vNOTES steps...
Overview of vNOTES steps
Below we break down a description of vNOTES in 6 sections. Our patients are always placed in dorsal lithotomy position with TrenGuard (D.A. Surgical) Trendelenburg restraint. We prep the abdomen in case we need to convert to transabdominal surgery via transumbilical single-incision laparoscopic surgery or traditional laparoscopic surgery.
1. Vaginal entry
Accessing the peritoneal cavity through the vagina initially proceeds like a vaginal hysterectomy. We inject dilute vasopressin (20 U in 20 mL of normal saline) circumferentially in the cervix (for hysterectomy) or in the posterior cervix in the cervicovaginal junction (for adnexal surgery without hysterectomy) for vasoconstriction and hydrodissection.
We then incise the vaginal mucosa circumferentially with electrosurgical cautery and follow with posterior colpotomy. We find that reapproximating the posterior peritoneum to the posterior vagina with either figure-of-8 stitches or a running stitch of polyglactin 910 suture (2-0 Vicryl) assists in port placement, bleeding at the peritoneal edge, and closure of the cuff or colpotomy at the end of the case. We tag this suture with a curved hemostat.
Depending on whether a hysterectomy is being performed, anterior colpotomy is made. Again, the anterior peritoneum is then tagged to the anterior vaginal cuff in similar fashion, and this suture is tagged with a different instrument; we typically use a straight hemostat or Sarot clamp (FIGURE 1).

2. Traditional vaginal hysterectomy
After colpotomy, we prefer to perform progressive clamping of the broad ligament from the uterosacral and cardinal ligaments to the level of uterine artery as in traditional vaginal hysterectomy, if feasible.
3. Single-site port placement
The assembled GelPOINT Mini advanced access platform (Applied Medical) (FIGURE 2) is introduced through the vagina after the Alexis wound protector (included with the kit) is first placed through the colpotomy with assistance of Babcock clamps (FIGURE 3).


After ensuring that the green rigid ring of the Alexis wound protector is contained and completely expanded within the peritoneal cavity, we cross our previously tagged sutures as we find this helps with preventing the GelPOINT Mini access platform from inadvertently shifting out of the peritoneal cavity during surgery. The GelSeal cap is then secured and pneumoperitoneum is established (FIGURE 4).

Continue to: 4. Laparoendoscopic surgery...
4. Laparoendoscopic surgery
Instruments used in our surgeries include a 10-mm rigid 30° 43-cm working length laparoscope; a 44-cm LigaSure device (Medtronic); a 5-mm, 37-cm laparoscopic cobra grasping forceps and fenestrated grasper (Karl Storz); and a 5-mm, 45-cm laparoscopic suction with hydrodissection tip (Stryker) (FIGURE 5).

vNOTES allows a gynecologic surgeon the unique ability to survey the upper abdomen. The remainder of the surgery proceeds using basic laparoscopic single-site skills.
During vNOTES, as with all single-site surgical procedures, understanding the optimal placement of crossed instruments is important for successful completion. For example, when securing the right uterine artery, the surgeon needs to push the cervix toward the patient's left and slightly into the peritoneal cavity using a laparoscopic cobra grasper with his or her left hand while then securing the uterine pedicle using the LigaSure device with his or her right hand. This is then reversed when securing the left uterine artery, where the assistant surgeon pushes the cervix toward the patient's right while the surgeon secures the pedicle ("vaginal pull, laparoscopic push") (FIGURE 6).

This again is reiterated in securing the ovarian pedicles, which are pushed into the peritoneal cavity while being secured with the LigaSure device.
5. Specimen removal
For large uteri or specimens that need morcellation, a 15-mm Endo Catch specimen retrieval bag (Medtronic) is introduced through the GelPOINT Mini system. The specimen is then placed in the bag and delivered to the vagina, where contained bag morcellation is performed in standard fashion (FIGURES 7 AND 8). We utilized the "big C" technique by first grasping the specimen with a penetrating clamp. The clamp is then held in our nondominant hand and a No. 10 blade scalpel is used to create a reverse c-incision, keeping one surface of the specimen intact. This is continued until the specimen can be completely delivered through the vagina.


Specimens that do not require morcellation can be grasped laparoscopically, brought to the GelPOINT Mini port, which is quickly disassembled, and delivered. The GelSeal cap is then reassembled.
6. Vaginal cuff closure
The colpotomy or vaginal cuff is closed with barbed suture continuously, as in traditional vaginal hysterectomy cuff closure. Uterosacral ligament suspension should be performed for vaginal cuff support.
vNOTES is the most recent innovative development in the field of minimally invasive surgery, and it has demonstrated feasibility and safety in the fields of general surgery, urology, and gynecology. Adopting vNOTES in clinical practice can improve patient satisfaction and cosmesis as well as surgical outcomes. Gynecologic surgeons can think of vNOTES hysterectomy as "placing an eye" in the vagina while performing transvaginal hysterectomy. The surgical principle of "vaginal pull, laparoscopic push" facilitates the learning process.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.
1. ACOG Committee on Gynecologic Practice. Committee opinion no. 444. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114:1156-1158.
2. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL position statement: route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18:1-3.
3. Whiteside JL, Kaeser CT, Ridgeway B. Achieving high value in the surgical approach to hysterectomy. Am J Obstet Gynecol. 2019;220:242-245.
4. Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233-241.
5. Moen M, Walter A, Harmanli O, et al. Considerations to improve the evidence-based use of vaginal hysterectomy in benign gynecology. Obstet Gynecol. 2014;124:585-588.
6. Balgobin S, Owens DM, Florian-Rodriguez ME, et al. Vaginal hysterectomy suturing skills training model and curriculum. Obstet Gynecol. 2019;134:553-558.
7. Baekelandt J. Total vaginal NOTES hysterectomy: a new approach to hysterectomy. J Minim Invasive Gynecol. 2015;22:1088-1094.
8. Baekelandt JF, De Mulder PA, Le Roy I, et al. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG. 2019;126:105-113.
9. Guan X, Bardawil E, Liu J, et al. Transvaginal natural orifice transluminal endoscopic surgery as a rescue for total vaginal hysterectomy. J Minim Invasive Gynecol. 2018;25:1135-1136.
10. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677.
11. Kalloo AN, Singh VK, Jagannath SB, et al. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest Endosc. 2004;60:114-117.
12. Reddy N, Rao P. Per oral transgastric endoscopic appendectomy in human. Paper Presented at: 45th Annual Conference of the Society of Gastrointestinal Endoscopy of India; February 28-29, 2004; Jaipur, India.
13. Clark MP, Qayed ES, Kooby DA, et al. Natural orifice translumenal endoscopic surgery in humans: a review. Minim Invasive Surg. 2012;189296.
14. Rattner D, Kalloo A; ASGE/SAGES Working Group. ASGE/ SAGES Working Group on natural orifice translumenal endoscopic surgery, October 2005. Surg Endosc. 2006;20:329-333.
15. Autorino R, Yakoubi R, White WM, et al. Natural orifice transluminal endoscopic surgery (NOTES): where are we going? A bibliometric assessment. BJU Int. 2013;111:11-16.
16. Santos BF, Hungness ES. Natural orifice transluminal endoscopic surgery: progress in humans since the white paper. World J Gastroenterol. 2011;17:1655-1665.
17. Tolcher MC, Kalogera E, Hopkins MR, et al. Safety of culdotomy as a surgical approach: implications for natural orifice transluminal endoscopic surgery. JSLS. 2012;16:413-420.
18. ACOG Committee on Gynecologic Practice. Committee opinion no. 701. Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2017:129:e155-e159.
19. Ahn KH, Song JY, Kim SH, et al. Transvaginal single-port natural orifice transluminal endoscopic surgery for benign uterine adnexal pathologies. J Minim Invasive Gynecol. 2012;19:631-635.
20. Liu J, Kohn J, Sun B, et al. Transvaginal natural orifice transluminal endoscopic surgery sacrocolpopexy: tips and tricks. Minim Invasive Gynecol. 2019;26:38-39.
21. Liu J, Kohn J, Fu H, et al. Transvaginal natural orifice transluminal endoscopic surgery for sacrocolpopexy: a pilot study of 26 cases. J Minim Invasive Gynecol. 2019;26:748-753.
22. Su H, Yen CF, Wu KY, et al. Hysterectomy via transvaginal natural orifice transluminal endoscopic surgery (NOTES): feasibility of an innovative approach. Taiwan J Obstet Gynecol. 2012;51:217-221.
23. Lee CL, Huang CY, Wu KY, et al. Natural orifice transvaginal endoscopic surgery myomectomy: an innovative approach to myomectomy. Gynecol Minim Invasive Ther. 2014;3:127-130.
24. Chen Y, Li J, Zhang Y, et al. Transvaginal single-port laparoscopy sacrocolpopexy. J Minim Invasive Gynecol. 2018;25:585- 588.
25. Lee CL, Wu KY, Tsao FY, et al. Natural orifice transvaginal endoscopic surgery for endometrial cancer. Gynecol Minim Invasive Ther. 2014;3:89-92.
26. Leblanc E, Narducci F, Bresson L, et al. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol Oncol. 2016;143:686-687.
27. Lee CL, Wu KY, Su H, et al. Robot-assisted natural orifice transluminal endoscopic surgery for hysterectomy. Taiwan J Obstet Gynecol. 2015;54:761-765.
28. Rezai S, Giovane RA, Johnson SN, et al. Robotic natural orifice transluminal endoscopic surgery (R-NOTES) in gynecologic surgeries, a case report and review of literature. Obstet Gynecol Int J. 2019;10:287-289.
29. Wang CJ, Wu PY, Kuo HH, et al. Natural orifice transluminal endoscopic surgery-assisted versus laparoscopic ovarian cystectomy (NAOC vs. LOC): a case-matched study. Surg Endosc. 2016;30:1227-1234.










