User login
ASCO Releases Vaccination Guidelines for Adults With Cancer
TOPLINE:
“Optimizing vaccination status should be considered a key element in the care of patients with cancer,” according to the authors of newly released American of Clinical Oncology (ASCO) guidelines.
METHODOLOGY:
- “Infections are the second most common cause of noncancer-related mortality within the first year after a cancer diagnosis,” highlighting the need for oncologists to help ensure patients are up to date on key vaccines, an ASCO panel of experts wrote.
- The expert panel reviewed the existing evidence and made recommendations to guide vaccination of adults with solid tumors or hematologic malignancies, including those who received hematopoietic stem-cell transplantation (HSCT), chimeric antigen T-cell (CAR T-cell) therapy and B-cell-depleting therapy, as well as guide vaccination of their household contacts.
- The panel reviewed 102 publications, including 24 systematic reviews, 14 randomized controlled trials, and 64 nonrandomized studies.
- Vaccines evaluated included those for COVID-19, influenza, hepatitis A and B, respiratory syncytial virus, Tdap, human papillomavirus, inactivated polio, and rabies.
- The authors noted that patients’ underlying immune status and their cancer therapy could affect vaccination and revaccination strategies compared with recommendations for a general adult population without cancer.
TAKEAWAY:
- The first step is to determine patients’ vaccination status and ensure adults newly diagnosed with cancer (as well as their household contacts) are up to date on seasonal and age or risk-based vaccines before starting their cancer treatment. If there are gaps, patients would ideally receive their vaccinations 2-4 weeks before their cancer treatment begins; however, non-live vaccines can be given during or after treatment.
- The authors recommended complete revaccination of patients 6-12 months following HSCT to restore vaccine-induced immunity. The caveats: COVID-19, influenza, and pneumococcal vaccines can be given as early as 3 months after transplant, and patients should receive live and live attenuated vaccines only in the absence of active GVHD or immunosuppression and only ≥ 2 years following HSCT.
- After CAR T-cell therapy directed against B-cell antigens (CD19/BCMA), patients should not receive influenza and COVID-19 vaccines sooner than 3 months after completing therapy and nonlive vaccines should not be given before 6 months.
- After B-cell depleting therapy, revaccinate patients for COVID-19 only and no sooner than 6 months after completing treatment. Long-term survivors of hematologic cancer with or without active disease or those with long-standing B-cell dysfunction or hypogammaglobulinemia from therapy or B-cell lineage malignancies should receive the recommended nonlive vaccines.
- Adults with solid and hematologic cancers traveling to an area of risk should follow the CDC standard recommendations for the destination. Hepatitis A, intramuscular typhoid vaccine, inactivated polio, hepatitis B, rabies, meningococcal, and nonlive Japanese encephalitis vaccines are safe.
IN PRACTICE:
“Enhancing vaccine uptake against preventable illnesses will help the community and improve the quality of care for patients with cancer,” the authors said. “Clinicians play a critical role in helping the patient and caregiver to understand the potential benefits and risks of recommended vaccination[s]. In addition, clinicians should provide authoritative resources, such as fact-based vaccine informational handouts and Internet sites, to help patients and caregivers learn more about the topic.”
SOURCE:
Mini Kamboj, MD, with Memorial Sloan Kettering Cancer Center, New York City, and Elise Kohn, MD, with the National Cancer Institute, Rockville, Maryland, served as cochairs for the expert panel. The guideline was published March 18 in the Journal of Clinical Oncology.
LIMITATIONS:
The evidence for some vaccines in cancer patients continues to evolve, particularly for new vaccines like COVID-19 vaccines.
DISCLOSURES:
This research had no commercial funding. Disclosures for the guideline panel are available with the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
“Optimizing vaccination status should be considered a key element in the care of patients with cancer,” according to the authors of newly released American of Clinical Oncology (ASCO) guidelines.
METHODOLOGY:
- “Infections are the second most common cause of noncancer-related mortality within the first year after a cancer diagnosis,” highlighting the need for oncologists to help ensure patients are up to date on key vaccines, an ASCO panel of experts wrote.
- The expert panel reviewed the existing evidence and made recommendations to guide vaccination of adults with solid tumors or hematologic malignancies, including those who received hematopoietic stem-cell transplantation (HSCT), chimeric antigen T-cell (CAR T-cell) therapy and B-cell-depleting therapy, as well as guide vaccination of their household contacts.
- The panel reviewed 102 publications, including 24 systematic reviews, 14 randomized controlled trials, and 64 nonrandomized studies.
- Vaccines evaluated included those for COVID-19, influenza, hepatitis A and B, respiratory syncytial virus, Tdap, human papillomavirus, inactivated polio, and rabies.
- The authors noted that patients’ underlying immune status and their cancer therapy could affect vaccination and revaccination strategies compared with recommendations for a general adult population without cancer.
TAKEAWAY:
- The first step is to determine patients’ vaccination status and ensure adults newly diagnosed with cancer (as well as their household contacts) are up to date on seasonal and age or risk-based vaccines before starting their cancer treatment. If there are gaps, patients would ideally receive their vaccinations 2-4 weeks before their cancer treatment begins; however, non-live vaccines can be given during or after treatment.
- The authors recommended complete revaccination of patients 6-12 months following HSCT to restore vaccine-induced immunity. The caveats: COVID-19, influenza, and pneumococcal vaccines can be given as early as 3 months after transplant, and patients should receive live and live attenuated vaccines only in the absence of active GVHD or immunosuppression and only ≥ 2 years following HSCT.
- After CAR T-cell therapy directed against B-cell antigens (CD19/BCMA), patients should not receive influenza and COVID-19 vaccines sooner than 3 months after completing therapy and nonlive vaccines should not be given before 6 months.
- After B-cell depleting therapy, revaccinate patients for COVID-19 only and no sooner than 6 months after completing treatment. Long-term survivors of hematologic cancer with or without active disease or those with long-standing B-cell dysfunction or hypogammaglobulinemia from therapy or B-cell lineage malignancies should receive the recommended nonlive vaccines.
- Adults with solid and hematologic cancers traveling to an area of risk should follow the CDC standard recommendations for the destination. Hepatitis A, intramuscular typhoid vaccine, inactivated polio, hepatitis B, rabies, meningococcal, and nonlive Japanese encephalitis vaccines are safe.
IN PRACTICE:
“Enhancing vaccine uptake against preventable illnesses will help the community and improve the quality of care for patients with cancer,” the authors said. “Clinicians play a critical role in helping the patient and caregiver to understand the potential benefits and risks of recommended vaccination[s]. In addition, clinicians should provide authoritative resources, such as fact-based vaccine informational handouts and Internet sites, to help patients and caregivers learn more about the topic.”
SOURCE:
Mini Kamboj, MD, with Memorial Sloan Kettering Cancer Center, New York City, and Elise Kohn, MD, with the National Cancer Institute, Rockville, Maryland, served as cochairs for the expert panel. The guideline was published March 18 in the Journal of Clinical Oncology.
LIMITATIONS:
The evidence for some vaccines in cancer patients continues to evolve, particularly for new vaccines like COVID-19 vaccines.
DISCLOSURES:
This research had no commercial funding. Disclosures for the guideline panel are available with the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
“Optimizing vaccination status should be considered a key element in the care of patients with cancer,” according to the authors of newly released American of Clinical Oncology (ASCO) guidelines.
METHODOLOGY:
- “Infections are the second most common cause of noncancer-related mortality within the first year after a cancer diagnosis,” highlighting the need for oncologists to help ensure patients are up to date on key vaccines, an ASCO panel of experts wrote.
- The expert panel reviewed the existing evidence and made recommendations to guide vaccination of adults with solid tumors or hematologic malignancies, including those who received hematopoietic stem-cell transplantation (HSCT), chimeric antigen T-cell (CAR T-cell) therapy and B-cell-depleting therapy, as well as guide vaccination of their household contacts.
- The panel reviewed 102 publications, including 24 systematic reviews, 14 randomized controlled trials, and 64 nonrandomized studies.
- Vaccines evaluated included those for COVID-19, influenza, hepatitis A and B, respiratory syncytial virus, Tdap, human papillomavirus, inactivated polio, and rabies.
- The authors noted that patients’ underlying immune status and their cancer therapy could affect vaccination and revaccination strategies compared with recommendations for a general adult population without cancer.
TAKEAWAY:
- The first step is to determine patients’ vaccination status and ensure adults newly diagnosed with cancer (as well as their household contacts) are up to date on seasonal and age or risk-based vaccines before starting their cancer treatment. If there are gaps, patients would ideally receive their vaccinations 2-4 weeks before their cancer treatment begins; however, non-live vaccines can be given during or after treatment.
- The authors recommended complete revaccination of patients 6-12 months following HSCT to restore vaccine-induced immunity. The caveats: COVID-19, influenza, and pneumococcal vaccines can be given as early as 3 months after transplant, and patients should receive live and live attenuated vaccines only in the absence of active GVHD or immunosuppression and only ≥ 2 years following HSCT.
- After CAR T-cell therapy directed against B-cell antigens (CD19/BCMA), patients should not receive influenza and COVID-19 vaccines sooner than 3 months after completing therapy and nonlive vaccines should not be given before 6 months.
- After B-cell depleting therapy, revaccinate patients for COVID-19 only and no sooner than 6 months after completing treatment. Long-term survivors of hematologic cancer with or without active disease or those with long-standing B-cell dysfunction or hypogammaglobulinemia from therapy or B-cell lineage malignancies should receive the recommended nonlive vaccines.
- Adults with solid and hematologic cancers traveling to an area of risk should follow the CDC standard recommendations for the destination. Hepatitis A, intramuscular typhoid vaccine, inactivated polio, hepatitis B, rabies, meningococcal, and nonlive Japanese encephalitis vaccines are safe.
IN PRACTICE:
“Enhancing vaccine uptake against preventable illnesses will help the community and improve the quality of care for patients with cancer,” the authors said. “Clinicians play a critical role in helping the patient and caregiver to understand the potential benefits and risks of recommended vaccination[s]. In addition, clinicians should provide authoritative resources, such as fact-based vaccine informational handouts and Internet sites, to help patients and caregivers learn more about the topic.”
SOURCE:
Mini Kamboj, MD, with Memorial Sloan Kettering Cancer Center, New York City, and Elise Kohn, MD, with the National Cancer Institute, Rockville, Maryland, served as cochairs for the expert panel. The guideline was published March 18 in the Journal of Clinical Oncology.
LIMITATIONS:
The evidence for some vaccines in cancer patients continues to evolve, particularly for new vaccines like COVID-19 vaccines.
DISCLOSURES:
This research had no commercial funding. Disclosures for the guideline panel are available with the original article.
A version of this article appeared on Medscape.com.
No Increased Stroke Risk After COVID-19 Bivalent Vaccine
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study of Medicare beneficiaries showed.
METHODOLOGY:
- The analysis included 5.4 million people age ≥ 65 years who received either the Pfizer-BioNTech COVID-19 bivalent vaccine or the Moderna bivalent vaccine, or the Pfizer vaccine and a high-dose or adjuvanted concomitant influenza vaccine (ie, administered on the same day).
- A total of 11,001 of the cohort experienced a stroke in the first 90 days after vaccination.
- The main outcome was stroke risk (nonhemorrhagic stroke, transient ischemic attack [TIA], or hemorrhagic stroke) during the 1- to 21-day or 22- to 42-day window after vaccination vs the 43- to 90-day control window.
- The mean age of participants was 74 years, and 56% were female.
TAKEAWAY:
- There was no statistically significant association with either brand of the COVID-19 bivalent vaccine or any of the stroke outcomes during the 1- to 21-day or 22- to 42-day risk window compared with the 43- to 90-day control window (incidence rate ratio [IRR] range, 0.72-1.12).
- Vaccination with COVID-19 bivalent vaccine plus a high-dose or adjuvanted influenza vaccine (n = 4596) was associated with a significantly greater risk for nonhemorrhagic stroke 22-42 days after vaccination with Pfizer-BioNTech (IRR, 1.20; risk difference/100,000 doses, 3.13) and an increase in TIA risk 1-21 days after vaccination with Moderna (IRR, 1.35; risk difference/100,000 doses, 3.33).
- There was a significant association between vaccination with a high-dose or adjuvanted influenza vaccine (n = 21,345) and nonhemorrhagic stroke 22-42 days after vaccination (IRR, 1.09; risk difference/100,000 doses, 1.65).
IN PRACTICE:
“The clinical significance of the risk of stroke after vaccination must be carefully considered together with the significant benefits of receiving an influenza vaccination,” the authors wrote. “Because the framework of the current self-controlled case series study does not compare the populations who were vaccinated vs those who were unvaccinated, it does not account for the reduced rate of severe influenza after vaccination. More studies are needed to better understand the association between high-dose or adjuvanted influenza vaccination and stroke.”
SOURCE:
Yun Lu, PhD, of the Center for Biologics Evaluation and Research, US Food and Drug Administration, Silver Spring, Maryland, was the lead and corresponding author of the study. It was published online on March 19 in JAMA.
LIMITATIONS:
Some stroke cases may have been missed or misclassified. The study included only vaccinated individuals — a population considered to have health-seeking behaviors — which may limit the generalizability of the findings. The study was conducted using COVID-19 bivalent vaccines, which are no longer available.
DISCLOSURES:
This work was funded by the US Food and Drug Administration through an interagency agreement with the Centers for Medicare & Medicaid Services. Dr. Lu reported no relevant financial relationships. The other authors’ disclosures are listed in the original paper.
A version of this article appeared on Medscape.com.
Could Regular, Daytime Naps Increase Glucose Levels?
TOPLINE:
Long naps of an hour or more, naps in the morning, or regular siestas may increase blood glucose levels in older people with type 2 diabetes (T2D).
METHODOLOGY:
- Napping is common in China and other cultures and may play a role in cardiometabolic health, but previous studies on the relationship between napping and glycemic control in T2D have reported conflicting results.
- In a cross-sectional study, the researchers assessed 226 individuals with T2D (median age, 67 years; about half women; mostly retired) from two community healthcare centers in China between May 2023 and July 2023.
- Using questionnaires, the participants were evaluated for A1c levels, as well as frequency, duration (shorter or longer than 1 hour), timing, and type of napping behavior (restorative for lack of sleep vs appetitive by habit or for enjoyment).
- Multivariate analysis controlled for age, sex, body mass index, T2D treatment regimen, diabetes duration, cognitive impairment, depression, night sleep duration, and insomnia symptoms.
TAKEAWAY:
- Among 180 participants who reported napping, 61 (33.9%) took long naps of 60 minutes and more, 162 (90%) reported afternoon napping, and 131 (72.8%) displayed appetitive napping.
- Restorative napping was linked to lower A1c levels than appetitive napping (β, −0.176; P = 0.028).
- Napping frequency was not associated with A1c levels.
IN PRACTICE:
“In clinical practice, healthcare professionals may offer tips about napping, eg, taking a nap less than an hour, taking a nap in the afternoon instead of in the morning, avoiding appetitive napping,” the authors concluded.
SOURCE:
The study, from corresponding author Bingqian Zhu, PhD, of the Shanghai Jiao Tong University School of Nursing, Shanghai, was published in Frontiers in Endocrinology.
LIMITATIONS:
The participants were older individuals, mostly retired, who may have had less need for restorative napping and more time for appetitive napping, limiting generalizability. The sample size may have been too small to find a link to napping frequency. Self-reported data could introduce recall bias. Only A1c levels were used as a measure of glycemic control.
DISCLOSURES:
The study was supported by the National Natural Science Foundation of China and other sources. The authors declared no potential conflict of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Long naps of an hour or more, naps in the morning, or regular siestas may increase blood glucose levels in older people with type 2 diabetes (T2D).
METHODOLOGY:
- Napping is common in China and other cultures and may play a role in cardiometabolic health, but previous studies on the relationship between napping and glycemic control in T2D have reported conflicting results.
- In a cross-sectional study, the researchers assessed 226 individuals with T2D (median age, 67 years; about half women; mostly retired) from two community healthcare centers in China between May 2023 and July 2023.
- Using questionnaires, the participants were evaluated for A1c levels, as well as frequency, duration (shorter or longer than 1 hour), timing, and type of napping behavior (restorative for lack of sleep vs appetitive by habit or for enjoyment).
- Multivariate analysis controlled for age, sex, body mass index, T2D treatment regimen, diabetes duration, cognitive impairment, depression, night sleep duration, and insomnia symptoms.
TAKEAWAY:
- Among 180 participants who reported napping, 61 (33.9%) took long naps of 60 minutes and more, 162 (90%) reported afternoon napping, and 131 (72.8%) displayed appetitive napping.
- Restorative napping was linked to lower A1c levels than appetitive napping (β, −0.176; P = 0.028).
- Napping frequency was not associated with A1c levels.
IN PRACTICE:
“In clinical practice, healthcare professionals may offer tips about napping, eg, taking a nap less than an hour, taking a nap in the afternoon instead of in the morning, avoiding appetitive napping,” the authors concluded.
SOURCE:
The study, from corresponding author Bingqian Zhu, PhD, of the Shanghai Jiao Tong University School of Nursing, Shanghai, was published in Frontiers in Endocrinology.
LIMITATIONS:
The participants were older individuals, mostly retired, who may have had less need for restorative napping and more time for appetitive napping, limiting generalizability. The sample size may have been too small to find a link to napping frequency. Self-reported data could introduce recall bias. Only A1c levels were used as a measure of glycemic control.
DISCLOSURES:
The study was supported by the National Natural Science Foundation of China and other sources. The authors declared no potential conflict of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Long naps of an hour or more, naps in the morning, or regular siestas may increase blood glucose levels in older people with type 2 diabetes (T2D).
METHODOLOGY:
- Napping is common in China and other cultures and may play a role in cardiometabolic health, but previous studies on the relationship between napping and glycemic control in T2D have reported conflicting results.
- In a cross-sectional study, the researchers assessed 226 individuals with T2D (median age, 67 years; about half women; mostly retired) from two community healthcare centers in China between May 2023 and July 2023.
- Using questionnaires, the participants were evaluated for A1c levels, as well as frequency, duration (shorter or longer than 1 hour), timing, and type of napping behavior (restorative for lack of sleep vs appetitive by habit or for enjoyment).
- Multivariate analysis controlled for age, sex, body mass index, T2D treatment regimen, diabetes duration, cognitive impairment, depression, night sleep duration, and insomnia symptoms.
TAKEAWAY:
- Among 180 participants who reported napping, 61 (33.9%) took long naps of 60 minutes and more, 162 (90%) reported afternoon napping, and 131 (72.8%) displayed appetitive napping.
- Restorative napping was linked to lower A1c levels than appetitive napping (β, −0.176; P = 0.028).
- Napping frequency was not associated with A1c levels.
IN PRACTICE:
“In clinical practice, healthcare professionals may offer tips about napping, eg, taking a nap less than an hour, taking a nap in the afternoon instead of in the morning, avoiding appetitive napping,” the authors concluded.
SOURCE:
The study, from corresponding author Bingqian Zhu, PhD, of the Shanghai Jiao Tong University School of Nursing, Shanghai, was published in Frontiers in Endocrinology.
LIMITATIONS:
The participants were older individuals, mostly retired, who may have had less need for restorative napping and more time for appetitive napping, limiting generalizability. The sample size may have been too small to find a link to napping frequency. Self-reported data could introduce recall bias. Only A1c levels were used as a measure of glycemic control.
DISCLOSURES:
The study was supported by the National Natural Science Foundation of China and other sources. The authors declared no potential conflict of interest.
A version of this article appeared on Medscape.com.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
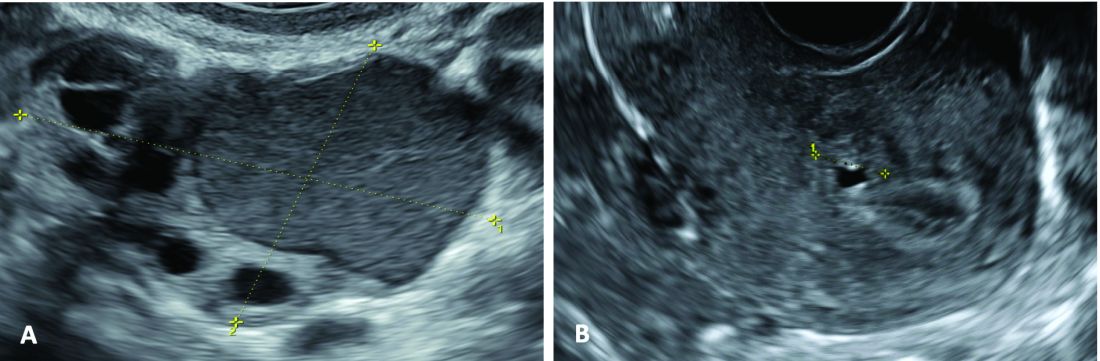
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
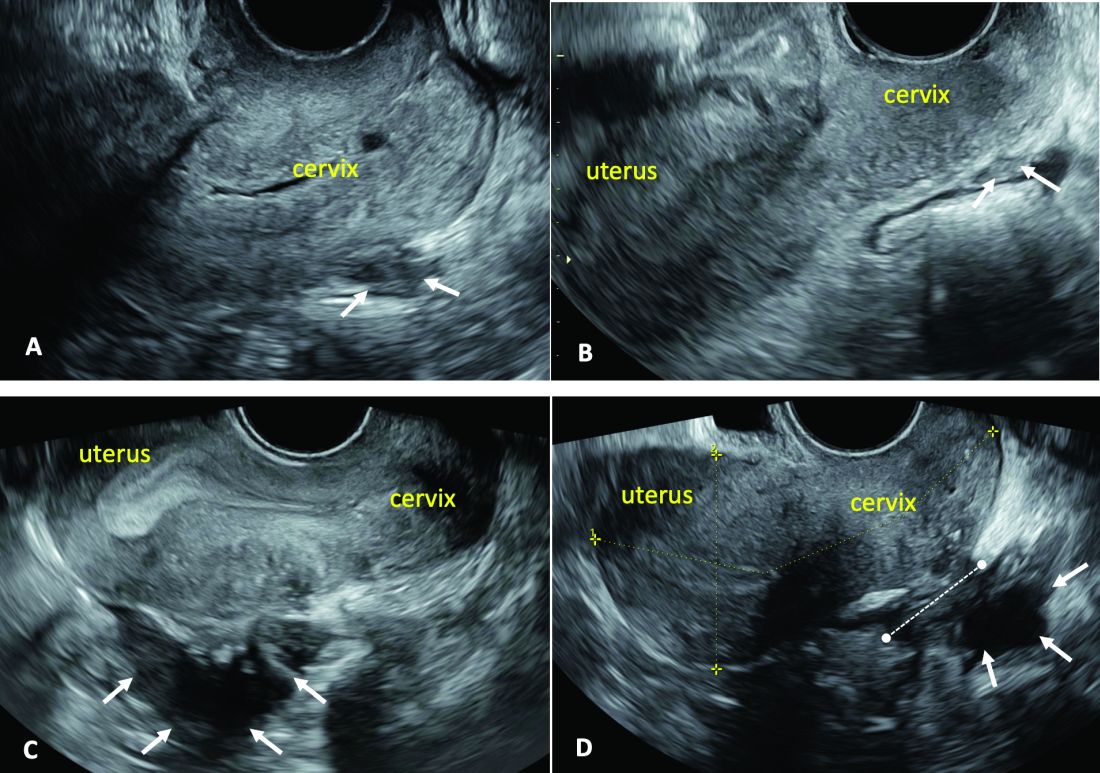
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).

(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).

(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
AI and Suicide Prevention in Primary Care: A Q&A
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Primary care physicians play a critical role in identifying patients at risk for serious mental health issues, including suicidality. But the ever-increasing demands on their clinical time can hinder the ability to identify emotional distress in time to intervene. Can artificial intelligence (AI) help?
This news organization spoke with Tom Zaubler, MD, a psychiatrist and chief medical officer of NeuroFlow, about how AI can improve the ability of primary care physicians and other clinicians to screen their patients for suicidal ideation and boost rates of treatment for mental health issues in their patients. This interview has been edited for clarity and length.
Question: How can AI help in suicide prevention and mental health screening in primary care?
Answer: Recent studies have demonstrated the potential of AI in mental health screening and suicide prevention. One method is natural language processing (NLP), which can analyze patients› journal entries for signs of suicidal thoughts or behaviors. This technology has shown promise in detecting suicidal ideation in patients who may not report such thoughts on traditional screening tools like the Patient Health Questionnaire-9 (PHQ-9). AI can be part of an integrated approach to identify and provide support to individuals at risk for suicide or those without a psychiatric history but who may still be at risk.
Q: A recent study by [Maria] Oquendo and colleagues found that one fifth of patients who attempt suicide do not meet the criteria for a mental health disorder.
Improved screening is obviously important, but in some ways it’s not the most important part of the problem. The lack of accessibility to specialized mental health care is a critical obstacle to treating patients with acute psychiatric needs.
How can primary care doctors effectively connect patients with mental health support, given the scarcity of mental health professionals?
A: Primary care doctors can leverage technology to extend mental health support. This includes using platforms for safety screening and providing patients with immediate access to local and national resources and digital interventions. Alerts can be sent to professionals within the practice or employed by technology companies to offer immediate support, including suicide safety planning and counseling. Users can hit a button to “Find a Therapist.” Also, if they acknowledge feelings of self-harm, these keywords are detected within the app by NLP. “Urgent alerts” are then sent to clinicians who are overseeing patient care. If someone is flagged, a social worker or member of a response services team intervenes and calls the person at risk to tailor care. These interventions do not always require a psychiatrist or masters-prepared clinician but can be effectively managed by trained paraprofessionals. These staff members can provide suicide safety planning and lethal-means-restriction counseling, and can assess the need for escalation of care.
Q: How is technology likely to manifest in physician practices in the near future to support mental health care?
A: Automated screening platforms for depression and anxiety, alerts for physicians when patients screen positively, and integration with collaborative care models are a few of the ways technology will become part of clinical practice. Additionally, advanced data analytics and predictive modeling using electronic health records and claims data will help identify high-risk patients. Technologies like voice recognition and machine learning can analyze patient journals and possibly, in the future, social media feeds to detect mental health issues. These technologies aim to extend and augment the capabilities of healthcare practices, improving the identification and management of patients at risk for mental health issues.
Q: Are these technologies as effective in pediatric populations, and are there any specific challenges?
A: Technologies for mental health screening and support are effective in pediatric populations, with certain age-specific considerations and legal restrictions on technology use. For adolescents and older children comfortable with technology, digital tools can significantly impact mental health care. For younger children, technology must facilitate information-gathering from various sources, including parents and teachers. Despite challenges, technology is crucial for early identification and intervention in pediatric mental health, potentially shortening the time to diagnosis and improving outcomes.
The statistics are horrifying. One third of adolescent girls have seriously thought about suicide over the past year; 13% attempt suicide. So there’s a need in the adolescent population and in the preadolescent population, too, because there’s an 8- to 10-year lag between onset of symptoms and diagnosis of mental illness. If we can shorten that lag, you see improved performance in schools; you see decreased truancy; you see greater economic achievement and so on. It makes such a profound difference. Not to mention it saves lives. So, yes, technology is critical in a pediatric population. It exists and it’s happening right now. There are challenges, but the goal can be met.
Q: A 2014 study found that 45% of people who completed suicide visited a primary care physician in the preceding month. And only 23% of people who attempt suicide have not seen a primary care physician within the past year. What does that say about the importance of screening at the primary care level?
A: The fact that a significant percentage of individuals who die by suicide have visited a primary care physician within a month or year prior to their death underscores the critical role of primary care in suicide prevention. This highlights the potential for primary care settings to identify and intervene with individuals at risk for suicide, making the case for the importance of integrating effective mental health screenings and support technologies in primary care practices.
Q: In other words, we’re not talking about a marginal benefit.
A: No, the potential benefit is huge. The United States Preventive Services Task Force did not endorse universal screening for suicide in its 2023 recommendations; they felt — and I accept that conclusion — there wasn›t enough evidence [at the time] to really support that recommendation. I think when you talk to a lot of suicide researchers, what you will hear is that providing suicide assessments as far upstream as possible is critical, especially when you start seeing more and more research showing that 20% of the population who die by suicide are not likely to have any psychiatric pathology at all. I believe the evidence base will soon support a recommendation for universal screening for adults. I believe it is especially important to screen for suicidal ideation in kids, given the high rates of suicide in this population.
Dr. Zaubler has disclosed the following relevant financial relationships: chief medical officer, NeuroFlow.
A version of this article appeared on Medscape.com.
Florida Legislature Passes Free Skin Cancer Screening Requirement
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
By this summer, state employees in Florida covered by state group health insurance plans should have access to free annual skin cancer screenings.
On March 1, 2024, legislation was unanimously passed by both chambers of the state legislature that will provide for the free screenings for this group as of July 1. Some 321,000 state employees would be eligible, at a cost of about $357,000 per year, according to a legislative analysis. Gov. Ron DeSantis (R) has received and is expected to sign the bill.
The analysis concluded that the bill would have a “significant negative fiscal impact on the state employee group health plan,” as screenings will ultimately reduce cancer incidence and related morbidity and mortality.
The screenings aim to provide access to patients who may think they might not be able to afford a visit or who may have other perceived or real barriers to going for a skin check, said Sima Jain, MD, president of the Florida Academy of Dermatology. “It’s really meant to give patients access who need it,” said Dr. Jain, a dermatologist in private practice in Orlando.
The goal is early detection. “If I do a simple excision on a melanoma and we catch it early, it’s done, it’s cured,” Dr. Jain told this news organization. “It’s a win-win. We catch it early and insurance companies pay less money,” she said.
An effort to have all insurers in the state provide free screenings failed in 2023.
From 2016 to 2020, Florida had a higher overall incidence of melanoma at 25.4 per 100,000 than the national average of 22.5, according to the National Cancer Institute. The state had some 7500 cases of melanoma each year during that period. The incidence rate in some Florida counties is as high as 32.7-45.6 per 100,000.
The Florida legislation will allow physician assistants and advanced practice nurses who operate under the supervision of a dermatologist to conduct the screenings.
It’s not clear how many state employees will access the free skin checks. “I don’t expect to see a flood of skin cancer screenings,” said Dr. Jain, noting that she hopes that it attracts primarily those at highest risk.
Once the bill is signed by the governor, Florida will be the second state to cover skin cancer screenings in some way. Illinois has required free skin cancer screening for all insured residents since 2020.
A version of this article appeared on Medscape.com .
Commentary: Gut Dysbiosis, DMARD, Joint Involvement, and MACE in PsA, April 2024
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
After PsA onset, early diagnosis and management leads to better long-term outcomes. These prior observations were confirmed in a study by Snoeck Henkemans and colleagues that included 708 newly diagnosed patients with PsA naive to disease-modifying antirheumatic drugs (DMARD) who were followed up for 3 years or more. Patients with a short (<12 weeks) vs long delay (>1 year) in PsA diagnosis after symptom onset were more likely to achieve minimum disease activity (OR 2.55; 95% CI 1.37-4.76). Thus, longer delay in diagnosing PsA is associated with worse clinical outcomes.
Bimekizumab is a novel biologic therapy that inhibits interleukins (IL)-17A and -17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. However, the effectiveness in PsA vis-à-vis other IL-17A inhibitors is not known. In the absence of a formal head-to-head study, matching-adjusted indirect comparisons is a method to evaluate comparative effectiveness. Such a study by Mease and colleagues included the data of patients with PsA who were biological DMARD–naive or who had an inadequate response to tumor necrosis factor inhibitors (TNFi-IR), and who received bimekizumab from the BE OPTIMAL (n = 236) and BE COMPLETE (n = 146) trials and secukinumab from the FUTURE 2 trial (n = 200). They demonstrated that, in the biological DMARD–naive subgroup, the probability of achieving at least 70% improvement in American College of Rheumatology (ACR) response was two times higher with bimekizumab (160 mg every 4 weeks) vs secukinumab (150 mg or 300 mg every 4 weeks) at week 52. In the TNFi-IR subgroup, bimekizumab had a greater likelihood of response compared with 150 mg secukinumab for ACR20, ACR70, and minimal disease activity outcomes and a greater likelihood of response compared with 300 mg secukinumab for ACR50 and minimal disease activity. Thus, bimekizumab is at least as effective as secukinumab in PsA. Formal head-to-head studies comparing bimekizumab with other IL-17A inhibitors are required.
Distal interphalangeal (DIP) joint involvement is an important manifestation of PsA and is closely related to nail dystrophy in the adjacent nail. Ixekizumab is another biologic that targets IL-17A. In a post hoc analysis of the SPIRIT-H2H study, McGonagle and colleagues confirmed that over 96% of patients with PsA and simultaneous DIP joint involvement reported adjacent nail psoriasis. When compared with adalimumab, ixekizumab led to greater improvements in DIP involvement and adjacent nail psoriasis as early as week 12 (38.8% vs 28.4%; P < .0001), with improvements sustained up to week 52 (64.9% vs 57.5%; P = .0055). This probably reflects a greater effectiveness of IL-17A inhibition in treating skin and nail psoriasis compared with TNFi.
Finally, in a population-based retrospective cohort study that included 13,905 patients with PsA (n = 1672) or rheumatoid arthritis (n = 12,233) who did not have any previous history of major adverse cardiovascular events (MACE), Meng and colleagues showed that the incidence rates of MACE were similar in patients with PsA and rheumatoid arthritis. Thus, cardiovascular risk management should be similarly aggressive in patients with PsA and rheumatoid arthritis.
Commentary: MRI Surveillance and Risk Factors in Breast Cancer, April 2024
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
A positive family history of cancer and obesity are established risk factors for development of breast cancer among women.[4,5] A population-based cohort study that included 15,055 Chinese women evaluated the association and interaction between body mass index (BMI) and family history of cancer on the risk for breast cancer (Cao et al). The incidence risk for breast cancer was highest in the group with obesity vs the group with normal weight (adjusted HR 2.09; 95% CI 1.42-3.07), and those with a family history of cancer also had an increased risk vs those without a family history of cancer (adjusted HR 1.63; 95% CI 1.22-2.49). Furthermore, women with a BMI ≥ 24 and family history of cancer had a higher risk for breast cancer development compared with women with a BMI < 24 and no family history of cancer (adjusted HR 2.06; 95% CI 1.39-3.06). This study indicates a heightened breast cancer risk when cancer family history and obesity coexist, suggesting the importance of addressing modifiable risk factors and targeting lifestyle interventions in this population.
Triple-negative breast cancer (TNBC), although exhibiting its own heterogeneity, has various features that differentiate this subtype from luminal breast cancers. For example, TNBC generally has a more aggressive course, increased responsiveness to chemotherapy, and earlier pattern of recurrence compared with hormone receptor–positive disease. Prior studies have also shown that established breast cancer risk factors reflect those for the luminal A subtype, whereas those for TNBC are less consistent.[6] A meta-analysis that included 33 studies evaluated the association between traditional breast cancer risk factors and TNBC incidence (Kumar et al). Family history (odds ratio [OR] 1.55; 95% CI 1.34-1.81; P < .001), longer duration of oral contraceptive use (OR 1.29; 95% CI 1.08-1.55; P < .001), and higher breast density (OR 2.19; 95% CI 1.67-2.88; P < .001) were significantly associated with an increased risk for TNBC. Factors including later age at menarche, later age at first birth, and breastfeeding were associated with reduced risk for TNBC. Furthermore, there was no significant association with parity, menopausal hormone therapy, alcohol, smoking, and BMI. This study highlights distinct risk factors that may contribute to a higher risk for TNBC, and future research will be valuable to better elucidate the mechanisms at play and to further understand the differences within this subtype itself.
Additional References
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 3.2024. Source
- Saadatmand S, Geuzinge HA, Rutgers EJT, et al; on behalf of the FaMRIsc study group. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): A multicentre, randomised, controlled trial. Lancet Oncol. 2019;20:1136-1147. doi: 10.1016/S1470-2045(19)30275-X Source
- Warner E, Zhu S, Plewes DB, et al. Breast cancer mortality among women with a BRCA1 or BRCA2 mutation in a magnetic resonance imaging plus mammography screening program. Cancers (Basel). 2020;12:3479. doi: 10.3390/cancers12113479 Source
- Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378-397. doi: 10.3322/caac.21405 Source
- Engmann NJ, Golmakani MK, Miglioretti DL, et al; for the Breast Cancer Surveillance Consortium. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228-1236. doi: 10.1001/jamaoncol.2016.6326 Source
- Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta Rev Cancer. 2015;1856:73-85. doi: 10.1016/j.bbcan.2015.0002 Source
Tarlatamab Shows Promise in Tackling Previously Treated SCLC
The investigational bispecific T-cell engager tarlatamab achieved durable responses and clinically meaningful survival outcomes in patients with small-cell lung cancer (SCLC), particularly at lower doses, according to a follow-up analysis of the phase 1 DeLLphi-300 trial.
Most patients with central nervous system tumors also sustained tumor shrinkage long after receiving radiotherapy, providing “encouraging evidence” of the new agent’s intracranial activity, said study presenter Horst-Dieter Hummel, MD, Comprehensive Cancer Center Mainfranken, Würzburg, Germany.
The research was presented at the European Lung Cancer Congress 2024 on March 22.
Data from the phase 1 and phase 2 DeLLphi trials, published last year, showed the compound achieved “encouraging clinical activity” in pretreated patients, said Dr. Hummel.
The initial phase 1 DeLLphi study found that after a median follow-up of 8.7 months, the immunotherapy led to a disease control rate of 51.4%, a median progression-free survival of 3.7 months, and median overall survival of 13.2 months.
At the meeting, Dr. Hummel reported longer-term outcomes from the phase 1 study over a median of 12.1 months as well as intracranial activity in patients who received clinically relevant doses of tarlatamab, defined as ≥ 10 mg.
The 152 patients included in the analysis had a median of two prior lines of therapy; 76.3% had undergone radiotherapy, and 63.2% had received immunotherapy. Liver metastases were present in 42.1% of patients, and 25.0% had brain metastases.
Doses varied among participants, with 76 patients (50.0%) receiving 100 mg, 32 (21.0%) receiving 100 mg via extended intravenous infusion, 17 (11.2%) receiving 10 mg, and 8 (5.3%) receiving 30 mg.
The overall objective response rate was 25.0%, with a median duration of response of 11.2 months. Among patients given the 10-mg dose, the objective response rate was higher, at 35.3%, as was the median duration of response, at 14.9 months.
Tarlatamab was associated with a median overall survival of 17.5 months, with 57.9% of patients alive at 12 months. Patients receiving the 10 mg dose had a better median overall survival of 20.3 months.
Of the 16 patients with analyzable central nervous system tumors, 62.5% experienced tumor shrinkage by ≥ 30% and 87.5% experienced intracranial disease control, which lasted for a median of 7.4 months.
In this follow-up study, tarlatamab demonstrated “clinically meaningful survival outcomes in patients with previously treated SCLC, particularly with the 10 mg dose,” Dr. Hummel concluded in his presentation.
No new safety signals emerged, though almost all patients did experience tarlatamab-related adverse events (94.8% for doses > 10 mg and 100% of patients with 10 mg doses). Overall, 66.4% of the total cohort experienced cytokine release syndrome of any grade, and 11.8% developed immune effector cell-associated neurotoxicity syndrome.
Discontinuation due to treatment-related adverse events occurred in 9 patients overall, and adverse events that led to dose interruption or reduction occurred in 32 patients overall.
“After many efforts at DLL3 targeting, we finally have an agent that shows activity and efficacy, and with convincing data,” said Jessica Menis, MD, a medical oncologist at the oncology department of the University Hospital of Verona, Italy, who was not involved in the study. The intracranial activity of tarlatamab “needs to be further evaluated in untreated patients,” Dr. Menis noted, because the study included only patients with stable, treated brain metastases.
And given the high rates of adverse events, Dr. Menis cautioned that adverse event management “will be a challenge.”
On X (Twitter), Tom Newsom-Davis, MBBS, PhD, a consultant in medical oncology at Chelsea and Westminster Hospital, London, said that tarlatamab is “not a straightforward drug to use,” highlighting the occurrence of cytokine release syndrome.
“But in this significantly pretreated population and in this hard-to-treat tumor type,” the rate and duration of responses seen with the extended follow-up are ‘impressive’,” he added.
DeLLphi-300, 301, and 304 were funded by Amgen Inc. Dr. Hummel declared relationships with several companies, including Amgen, Bristol Myers Squibb, AstraZeneca, Celgene, Merck, Novartis, Daiichi Sankyo, and Roche. Dr. Menis declared relationships with AstraZeneca, BMS, MSD, Roche, and Novartis.
A version of this article appeared on Medscape.com.
The investigational bispecific T-cell engager tarlatamab achieved durable responses and clinically meaningful survival outcomes in patients with small-cell lung cancer (SCLC), particularly at lower doses, according to a follow-up analysis of the phase 1 DeLLphi-300 trial.
Most patients with central nervous system tumors also sustained tumor shrinkage long after receiving radiotherapy, providing “encouraging evidence” of the new agent’s intracranial activity, said study presenter Horst-Dieter Hummel, MD, Comprehensive Cancer Center Mainfranken, Würzburg, Germany.
The research was presented at the European Lung Cancer Congress 2024 on March 22.
Data from the phase 1 and phase 2 DeLLphi trials, published last year, showed the compound achieved “encouraging clinical activity” in pretreated patients, said Dr. Hummel.
The initial phase 1 DeLLphi study found that after a median follow-up of 8.7 months, the immunotherapy led to a disease control rate of 51.4%, a median progression-free survival of 3.7 months, and median overall survival of 13.2 months.
At the meeting, Dr. Hummel reported longer-term outcomes from the phase 1 study over a median of 12.1 months as well as intracranial activity in patients who received clinically relevant doses of tarlatamab, defined as ≥ 10 mg.
The 152 patients included in the analysis had a median of two prior lines of therapy; 76.3% had undergone radiotherapy, and 63.2% had received immunotherapy. Liver metastases were present in 42.1% of patients, and 25.0% had brain metastases.
Doses varied among participants, with 76 patients (50.0%) receiving 100 mg, 32 (21.0%) receiving 100 mg via extended intravenous infusion, 17 (11.2%) receiving 10 mg, and 8 (5.3%) receiving 30 mg.
The overall objective response rate was 25.0%, with a median duration of response of 11.2 months. Among patients given the 10-mg dose, the objective response rate was higher, at 35.3%, as was the median duration of response, at 14.9 months.
Tarlatamab was associated with a median overall survival of 17.5 months, with 57.9% of patients alive at 12 months. Patients receiving the 10 mg dose had a better median overall survival of 20.3 months.
Of the 16 patients with analyzable central nervous system tumors, 62.5% experienced tumor shrinkage by ≥ 30% and 87.5% experienced intracranial disease control, which lasted for a median of 7.4 months.
In this follow-up study, tarlatamab demonstrated “clinically meaningful survival outcomes in patients with previously treated SCLC, particularly with the 10 mg dose,” Dr. Hummel concluded in his presentation.
No new safety signals emerged, though almost all patients did experience tarlatamab-related adverse events (94.8% for doses > 10 mg and 100% of patients with 10 mg doses). Overall, 66.4% of the total cohort experienced cytokine release syndrome of any grade, and 11.8% developed immune effector cell-associated neurotoxicity syndrome.
Discontinuation due to treatment-related adverse events occurred in 9 patients overall, and adverse events that led to dose interruption or reduction occurred in 32 patients overall.
“After many efforts at DLL3 targeting, we finally have an agent that shows activity and efficacy, and with convincing data,” said Jessica Menis, MD, a medical oncologist at the oncology department of the University Hospital of Verona, Italy, who was not involved in the study. The intracranial activity of tarlatamab “needs to be further evaluated in untreated patients,” Dr. Menis noted, because the study included only patients with stable, treated brain metastases.
And given the high rates of adverse events, Dr. Menis cautioned that adverse event management “will be a challenge.”
On X (Twitter), Tom Newsom-Davis, MBBS, PhD, a consultant in medical oncology at Chelsea and Westminster Hospital, London, said that tarlatamab is “not a straightforward drug to use,” highlighting the occurrence of cytokine release syndrome.
“But in this significantly pretreated population and in this hard-to-treat tumor type,” the rate and duration of responses seen with the extended follow-up are ‘impressive’,” he added.
DeLLphi-300, 301, and 304 were funded by Amgen Inc. Dr. Hummel declared relationships with several companies, including Amgen, Bristol Myers Squibb, AstraZeneca, Celgene, Merck, Novartis, Daiichi Sankyo, and Roche. Dr. Menis declared relationships with AstraZeneca, BMS, MSD, Roche, and Novartis.
A version of this article appeared on Medscape.com.
The investigational bispecific T-cell engager tarlatamab achieved durable responses and clinically meaningful survival outcomes in patients with small-cell lung cancer (SCLC), particularly at lower doses, according to a follow-up analysis of the phase 1 DeLLphi-300 trial.
Most patients with central nervous system tumors also sustained tumor shrinkage long after receiving radiotherapy, providing “encouraging evidence” of the new agent’s intracranial activity, said study presenter Horst-Dieter Hummel, MD, Comprehensive Cancer Center Mainfranken, Würzburg, Germany.
The research was presented at the European Lung Cancer Congress 2024 on March 22.
Data from the phase 1 and phase 2 DeLLphi trials, published last year, showed the compound achieved “encouraging clinical activity” in pretreated patients, said Dr. Hummel.
The initial phase 1 DeLLphi study found that after a median follow-up of 8.7 months, the immunotherapy led to a disease control rate of 51.4%, a median progression-free survival of 3.7 months, and median overall survival of 13.2 months.
At the meeting, Dr. Hummel reported longer-term outcomes from the phase 1 study over a median of 12.1 months as well as intracranial activity in patients who received clinically relevant doses of tarlatamab, defined as ≥ 10 mg.
The 152 patients included in the analysis had a median of two prior lines of therapy; 76.3% had undergone radiotherapy, and 63.2% had received immunotherapy. Liver metastases were present in 42.1% of patients, and 25.0% had brain metastases.
Doses varied among participants, with 76 patients (50.0%) receiving 100 mg, 32 (21.0%) receiving 100 mg via extended intravenous infusion, 17 (11.2%) receiving 10 mg, and 8 (5.3%) receiving 30 mg.
The overall objective response rate was 25.0%, with a median duration of response of 11.2 months. Among patients given the 10-mg dose, the objective response rate was higher, at 35.3%, as was the median duration of response, at 14.9 months.
Tarlatamab was associated with a median overall survival of 17.5 months, with 57.9% of patients alive at 12 months. Patients receiving the 10 mg dose had a better median overall survival of 20.3 months.
Of the 16 patients with analyzable central nervous system tumors, 62.5% experienced tumor shrinkage by ≥ 30% and 87.5% experienced intracranial disease control, which lasted for a median of 7.4 months.
In this follow-up study, tarlatamab demonstrated “clinically meaningful survival outcomes in patients with previously treated SCLC, particularly with the 10 mg dose,” Dr. Hummel concluded in his presentation.
No new safety signals emerged, though almost all patients did experience tarlatamab-related adverse events (94.8% for doses > 10 mg and 100% of patients with 10 mg doses). Overall, 66.4% of the total cohort experienced cytokine release syndrome of any grade, and 11.8% developed immune effector cell-associated neurotoxicity syndrome.
Discontinuation due to treatment-related adverse events occurred in 9 patients overall, and adverse events that led to dose interruption or reduction occurred in 32 patients overall.
“After many efforts at DLL3 targeting, we finally have an agent that shows activity and efficacy, and with convincing data,” said Jessica Menis, MD, a medical oncologist at the oncology department of the University Hospital of Verona, Italy, who was not involved in the study. The intracranial activity of tarlatamab “needs to be further evaluated in untreated patients,” Dr. Menis noted, because the study included only patients with stable, treated brain metastases.
And given the high rates of adverse events, Dr. Menis cautioned that adverse event management “will be a challenge.”
On X (Twitter), Tom Newsom-Davis, MBBS, PhD, a consultant in medical oncology at Chelsea and Westminster Hospital, London, said that tarlatamab is “not a straightforward drug to use,” highlighting the occurrence of cytokine release syndrome.
“But in this significantly pretreated population and in this hard-to-treat tumor type,” the rate and duration of responses seen with the extended follow-up are ‘impressive’,” he added.
DeLLphi-300, 301, and 304 were funded by Amgen Inc. Dr. Hummel declared relationships with several companies, including Amgen, Bristol Myers Squibb, AstraZeneca, Celgene, Merck, Novartis, Daiichi Sankyo, and Roche. Dr. Menis declared relationships with AstraZeneca, BMS, MSD, Roche, and Novartis.
A version of this article appeared on Medscape.com.
FROM ELCC 2024
FDA OKs First-in-Class Agent for Pulmonary Arterial Hypertension
The US Food and Drug Administration (FDA) has approved sotatercept (Winrevair, Merck), for the treatment of adults with pulmonary arterial hypertension (PAH), World Health Organization (WHO) Group 1, to increase exercise capacity, improve WHO functional class, and reduce the risk for clinical worsening events.
“Sotatercept added to background therapy has the potential to become a new standard-of-care option for patients with pulmonary arterial hypertension,” added coinvestigator Aaron B. Waxman, MD, PhD, executive director of the Center for Pulmonary Heart Diseases at Brigham and Women’s Hospital, Boston.
The approval was based on results of the phase 3 STELLAR study, a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which, 323 patients with PAH (WHO Group 1, functional class II or III) were randomly assigned 1:1 to add sotatercept or placebo to stable background therapy.
The results showed that sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved average 6-minute walk distance from baseline by a significant and clinically meaningful 40.8 meters compared with placebo for the trial’s primary efficacy endpoint (P < .001).
Sotatercept also led to significant improvement in multiple secondary outcome measures, including:
- Reduction in the risk for death from any cause or PAH clinical worsening events by 84% vs background therapy alone (number of events: 9 vs 42; hazard ratio [HR], 0.16; P < .001)
- Improvement in FC from baseline at 24 weeks in 29% of patients compared with 14% of patients treated with placebo (P < .001)
- Improvement in pulmonary vascular resistance (PVR), with an average 235 dyn/sec/cm5 reduction in PVR from baseline (P < .001)
- Improvement from baseline in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels. The median treatment difference in NT-proBNP between sotatercept and placebo was -442 pg/mL (P < .001)
The results were reported last year at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, with simultaneous publication in The New England Journal of Medicine.
Sotatercept injection may be administered by patients or caregivers with guidance, training, and follow-up from a healthcare provider. The recommended starting dose is 0.3 mg/kg. The recommended target dose is 0.7 mg/kg every 3 weeks.
Sotatercept may increase hemoglobin, may lead to erythrocytosis, and may decrease platelet count and lead to severe thrombocytopenia. Treatment should not be initiated if platelet count is < 50,000/mm3.
Hemoglobin and platelets should be monitored before each dose of sotatercept for the first five doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required.
Full prescribing information is available online.
Merck estimates that sotatercept will be available for dispensing by select specialty pharmacies in the United States by the end of April 2024.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sotatercept (Winrevair, Merck), for the treatment of adults with pulmonary arterial hypertension (PAH), World Health Organization (WHO) Group 1, to increase exercise capacity, improve WHO functional class, and reduce the risk for clinical worsening events.
“Sotatercept added to background therapy has the potential to become a new standard-of-care option for patients with pulmonary arterial hypertension,” added coinvestigator Aaron B. Waxman, MD, PhD, executive director of the Center for Pulmonary Heart Diseases at Brigham and Women’s Hospital, Boston.
The approval was based on results of the phase 3 STELLAR study, a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which, 323 patients with PAH (WHO Group 1, functional class II or III) were randomly assigned 1:1 to add sotatercept or placebo to stable background therapy.
The results showed that sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved average 6-minute walk distance from baseline by a significant and clinically meaningful 40.8 meters compared with placebo for the trial’s primary efficacy endpoint (P < .001).
Sotatercept also led to significant improvement in multiple secondary outcome measures, including:
- Reduction in the risk for death from any cause or PAH clinical worsening events by 84% vs background therapy alone (number of events: 9 vs 42; hazard ratio [HR], 0.16; P < .001)
- Improvement in FC from baseline at 24 weeks in 29% of patients compared with 14% of patients treated with placebo (P < .001)
- Improvement in pulmonary vascular resistance (PVR), with an average 235 dyn/sec/cm5 reduction in PVR from baseline (P < .001)
- Improvement from baseline in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels. The median treatment difference in NT-proBNP between sotatercept and placebo was -442 pg/mL (P < .001)
The results were reported last year at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, with simultaneous publication in The New England Journal of Medicine.
Sotatercept injection may be administered by patients or caregivers with guidance, training, and follow-up from a healthcare provider. The recommended starting dose is 0.3 mg/kg. The recommended target dose is 0.7 mg/kg every 3 weeks.
Sotatercept may increase hemoglobin, may lead to erythrocytosis, and may decrease platelet count and lead to severe thrombocytopenia. Treatment should not be initiated if platelet count is < 50,000/mm3.
Hemoglobin and platelets should be monitored before each dose of sotatercept for the first five doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required.
Full prescribing information is available online.
Merck estimates that sotatercept will be available for dispensing by select specialty pharmacies in the United States by the end of April 2024.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved sotatercept (Winrevair, Merck), for the treatment of adults with pulmonary arterial hypertension (PAH), World Health Organization (WHO) Group 1, to increase exercise capacity, improve WHO functional class, and reduce the risk for clinical worsening events.
“Sotatercept added to background therapy has the potential to become a new standard-of-care option for patients with pulmonary arterial hypertension,” added coinvestigator Aaron B. Waxman, MD, PhD, executive director of the Center for Pulmonary Heart Diseases at Brigham and Women’s Hospital, Boston.
The approval was based on results of the phase 3 STELLAR study, a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which, 323 patients with PAH (WHO Group 1, functional class II or III) were randomly assigned 1:1 to add sotatercept or placebo to stable background therapy.
The results showed that sotatercept, administered subcutaneously every 3 weeks for 24 weeks, improved average 6-minute walk distance from baseline by a significant and clinically meaningful 40.8 meters compared with placebo for the trial’s primary efficacy endpoint (P < .001).
Sotatercept also led to significant improvement in multiple secondary outcome measures, including:
- Reduction in the risk for death from any cause or PAH clinical worsening events by 84% vs background therapy alone (number of events: 9 vs 42; hazard ratio [HR], 0.16; P < .001)
- Improvement in FC from baseline at 24 weeks in 29% of patients compared with 14% of patients treated with placebo (P < .001)
- Improvement in pulmonary vascular resistance (PVR), with an average 235 dyn/sec/cm5 reduction in PVR from baseline (P < .001)
- Improvement from baseline in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels. The median treatment difference in NT-proBNP between sotatercept and placebo was -442 pg/mL (P < .001)
The results were reported last year at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, with simultaneous publication in The New England Journal of Medicine.
Sotatercept injection may be administered by patients or caregivers with guidance, training, and follow-up from a healthcare provider. The recommended starting dose is 0.3 mg/kg. The recommended target dose is 0.7 mg/kg every 3 weeks.
Sotatercept may increase hemoglobin, may lead to erythrocytosis, and may decrease platelet count and lead to severe thrombocytopenia. Treatment should not be initiated if platelet count is < 50,000/mm3.
Hemoglobin and platelets should be monitored before each dose of sotatercept for the first five doses, or longer if values are unstable, and periodically thereafter to determine if dose adjustments are required.
Full prescribing information is available online.
Merck estimates that sotatercept will be available for dispensing by select specialty pharmacies in the United States by the end of April 2024.
A version of this article appeared on Medscape.com.



