User login
AGA publishes clinical practice guidelines for gastric intestinal metaplasia
The American Gastroenterological Association (AGA) recently published clinical practice guidelines for managing gastric intestinal metaplasia (GIM).
The guidelines are the first of their kind to be published in the United States, according to lead author Samir Gupta, MD, of the University of California San Diego, and colleagues. The panelists suggested that the guidelines may help standardize decision making in a common clinical scenario.
“GIM has been considered as one specific marker to identify patients who might benefit from surveillance because it has been associated with increased risk for gastric cancer and is routinely encountered in clinical practice,” the panelists wrote in Gastroenterology.
The guideline panel was composed of three gastroenterologists, two guideline methodologist trainees, and three GRADE experts. Recommendations were based on the AGA guideline development process, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, best practices set forth by the Academy of Medicine, and a technical review.
“Given the paucity of robust direct data on GIM in the U.S., evidence from all regions of the world was considered relevant in the evidence-gathering phase,” the panelists wrote (Gastroenterology. 2019 Dec 6. doi: 10.1053/j.gastro.2019.12.003).
Based on available evidence, the expert panel developed three clinical recommendations.
First, the panelists recommended that clinicians test all patients with GIM for Helicobacter pylori, followed by eradication, over no testing and eradication. This recommendation was strong and based on moderate quality evidence from 22 studies, including 7 randomized controlled trials. These studies showed that, compared with placebo, eradication of H. pylori was associated with a 32% pooled relative risk reduction in gastric cancer and a 33% pooled relative risk reduction in gastric cancer mortality among patients with or without GIM. The pooled relative risk reduction rate was similar in analyses solely composed of individuals with GIM, the panelists noted, whereas mortality data restricted to individuals with GIM were lacking.
“Overall, the known strong association of H. pylori with risk for incident gastric cancer and the technical review’s findings, which reinforce the evidence of reduced risk for incident gastric cancer after H. pylori eradication, supports the AGA recommendation to test for and eradicate H. pylori,” the panelists wrote.
The second recommendation, which was conditional and based on very low quality evidence, advised against routine use of endoscopic surveillance for patients with GIM. Still, surveillance may be considered for patients with higher risk of gastric cancer, including those with incomplete and/or extensive GIM, a family history of gastric cancer, racial/ethnic minorities, and immigrants from high incidence regions, the panelists wrote.
“Although the technical review did not find evidence supporting increased risk for gastric cancer among racial/ethnic minorities or immigrants with documented GIM, an overall increased risk for gastric cancer (irrespective of presence/absence of GIM) has been established among these groups, and may be considered as part of decision making regarding surveillance,” the panelists wrote.
The third and final recommendation was also conditional and based on very weak evidence; the panelists recommended against routine short-interval repeat endoscopy for the purpose of risk stratification.
“The technical review found no direct evidence to support the impact of short-interval (less than 12 months) repeat upper endoscopy among patients with incidental GIM on patient-important outcomes,” the panelists wrote.
However, the guidelines note that patients with potentially elevated risk profiles, such as patients with a family history of gastric cancer, “may reasonably elect for repeat endoscopy within 1 year for risk stratification.”
Comparing these guidelines with those from other organizations, such as the European Society of Gastrointestinal Endoscopy (ESGE), the panelists concluded that recommendations across organizations are “generally similar.”
Finally, the panelists outlined relevant knowledge gaps and pointed to future research topics. For instance, data are scarce comparing outcomes in relation to surveillance versus no surveillance among patients with GIM; and biomarkers such as pepsinogen levels, which are used in Asian countries for risk stratification of gastric cancer, have been studied minimally in the United States.
Guideline development was funded by the AGA. The panelists disclosed no conflicts of interest.
The American Gastroenterological Association (AGA) recently published clinical practice guidelines for managing gastric intestinal metaplasia (GIM).
The guidelines are the first of their kind to be published in the United States, according to lead author Samir Gupta, MD, of the University of California San Diego, and colleagues. The panelists suggested that the guidelines may help standardize decision making in a common clinical scenario.
“GIM has been considered as one specific marker to identify patients who might benefit from surveillance because it has been associated with increased risk for gastric cancer and is routinely encountered in clinical practice,” the panelists wrote in Gastroenterology.
The guideline panel was composed of three gastroenterologists, two guideline methodologist trainees, and three GRADE experts. Recommendations were based on the AGA guideline development process, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, best practices set forth by the Academy of Medicine, and a technical review.
“Given the paucity of robust direct data on GIM in the U.S., evidence from all regions of the world was considered relevant in the evidence-gathering phase,” the panelists wrote (Gastroenterology. 2019 Dec 6. doi: 10.1053/j.gastro.2019.12.003).
Based on available evidence, the expert panel developed three clinical recommendations.
First, the panelists recommended that clinicians test all patients with GIM for Helicobacter pylori, followed by eradication, over no testing and eradication. This recommendation was strong and based on moderate quality evidence from 22 studies, including 7 randomized controlled trials. These studies showed that, compared with placebo, eradication of H. pylori was associated with a 32% pooled relative risk reduction in gastric cancer and a 33% pooled relative risk reduction in gastric cancer mortality among patients with or without GIM. The pooled relative risk reduction rate was similar in analyses solely composed of individuals with GIM, the panelists noted, whereas mortality data restricted to individuals with GIM were lacking.
“Overall, the known strong association of H. pylori with risk for incident gastric cancer and the technical review’s findings, which reinforce the evidence of reduced risk for incident gastric cancer after H. pylori eradication, supports the AGA recommendation to test for and eradicate H. pylori,” the panelists wrote.
The second recommendation, which was conditional and based on very low quality evidence, advised against routine use of endoscopic surveillance for patients with GIM. Still, surveillance may be considered for patients with higher risk of gastric cancer, including those with incomplete and/or extensive GIM, a family history of gastric cancer, racial/ethnic minorities, and immigrants from high incidence regions, the panelists wrote.
“Although the technical review did not find evidence supporting increased risk for gastric cancer among racial/ethnic minorities or immigrants with documented GIM, an overall increased risk for gastric cancer (irrespective of presence/absence of GIM) has been established among these groups, and may be considered as part of decision making regarding surveillance,” the panelists wrote.
The third and final recommendation was also conditional and based on very weak evidence; the panelists recommended against routine short-interval repeat endoscopy for the purpose of risk stratification.
“The technical review found no direct evidence to support the impact of short-interval (less than 12 months) repeat upper endoscopy among patients with incidental GIM on patient-important outcomes,” the panelists wrote.
However, the guidelines note that patients with potentially elevated risk profiles, such as patients with a family history of gastric cancer, “may reasonably elect for repeat endoscopy within 1 year for risk stratification.”
Comparing these guidelines with those from other organizations, such as the European Society of Gastrointestinal Endoscopy (ESGE), the panelists concluded that recommendations across organizations are “generally similar.”
Finally, the panelists outlined relevant knowledge gaps and pointed to future research topics. For instance, data are scarce comparing outcomes in relation to surveillance versus no surveillance among patients with GIM; and biomarkers such as pepsinogen levels, which are used in Asian countries for risk stratification of gastric cancer, have been studied minimally in the United States.
Guideline development was funded by the AGA. The panelists disclosed no conflicts of interest.
The American Gastroenterological Association (AGA) recently published clinical practice guidelines for managing gastric intestinal metaplasia (GIM).
The guidelines are the first of their kind to be published in the United States, according to lead author Samir Gupta, MD, of the University of California San Diego, and colleagues. The panelists suggested that the guidelines may help standardize decision making in a common clinical scenario.
“GIM has been considered as one specific marker to identify patients who might benefit from surveillance because it has been associated with increased risk for gastric cancer and is routinely encountered in clinical practice,” the panelists wrote in Gastroenterology.
The guideline panel was composed of three gastroenterologists, two guideline methodologist trainees, and three GRADE experts. Recommendations were based on the AGA guideline development process, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, best practices set forth by the Academy of Medicine, and a technical review.
“Given the paucity of robust direct data on GIM in the U.S., evidence from all regions of the world was considered relevant in the evidence-gathering phase,” the panelists wrote (Gastroenterology. 2019 Dec 6. doi: 10.1053/j.gastro.2019.12.003).
Based on available evidence, the expert panel developed three clinical recommendations.
First, the panelists recommended that clinicians test all patients with GIM for Helicobacter pylori, followed by eradication, over no testing and eradication. This recommendation was strong and based on moderate quality evidence from 22 studies, including 7 randomized controlled trials. These studies showed that, compared with placebo, eradication of H. pylori was associated with a 32% pooled relative risk reduction in gastric cancer and a 33% pooled relative risk reduction in gastric cancer mortality among patients with or without GIM. The pooled relative risk reduction rate was similar in analyses solely composed of individuals with GIM, the panelists noted, whereas mortality data restricted to individuals with GIM were lacking.
“Overall, the known strong association of H. pylori with risk for incident gastric cancer and the technical review’s findings, which reinforce the evidence of reduced risk for incident gastric cancer after H. pylori eradication, supports the AGA recommendation to test for and eradicate H. pylori,” the panelists wrote.
The second recommendation, which was conditional and based on very low quality evidence, advised against routine use of endoscopic surveillance for patients with GIM. Still, surveillance may be considered for patients with higher risk of gastric cancer, including those with incomplete and/or extensive GIM, a family history of gastric cancer, racial/ethnic minorities, and immigrants from high incidence regions, the panelists wrote.
“Although the technical review did not find evidence supporting increased risk for gastric cancer among racial/ethnic minorities or immigrants with documented GIM, an overall increased risk for gastric cancer (irrespective of presence/absence of GIM) has been established among these groups, and may be considered as part of decision making regarding surveillance,” the panelists wrote.
The third and final recommendation was also conditional and based on very weak evidence; the panelists recommended against routine short-interval repeat endoscopy for the purpose of risk stratification.
“The technical review found no direct evidence to support the impact of short-interval (less than 12 months) repeat upper endoscopy among patients with incidental GIM on patient-important outcomes,” the panelists wrote.
However, the guidelines note that patients with potentially elevated risk profiles, such as patients with a family history of gastric cancer, “may reasonably elect for repeat endoscopy within 1 year for risk stratification.”
Comparing these guidelines with those from other organizations, such as the European Society of Gastrointestinal Endoscopy (ESGE), the panelists concluded that recommendations across organizations are “generally similar.”
Finally, the panelists outlined relevant knowledge gaps and pointed to future research topics. For instance, data are scarce comparing outcomes in relation to surveillance versus no surveillance among patients with GIM; and biomarkers such as pepsinogen levels, which are used in Asian countries for risk stratification of gastric cancer, have been studied minimally in the United States.
Guideline development was funded by the AGA. The panelists disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Pelvic insufficiency fractures are common after chemoradiotherapy for cervical cancer
CHICAGO – Radiation therapy for cervical cancer resulted in pelvic insufficiency fractures more frequently than previously thought, and many fractures were slow to heal, according to research presented at the annual meeting of the Radiological Society of North America.
“Pelvic insufficiency fractures had a prevalence of 38% on MRI follow-up” after chemoradiotherapy for locally advanced cervical cancer, said Alina Dragan, MD. This figure is more than double the previously reported prevalence of about 14%.
Dr. Dragan, a radiology resident at London North West Healthcare, National Health Service Trust, and coinvestigators also tracked the natural history of these fractures over time, to fill a knowledge gap about whether, and at what rate, these pelvic insufficiency fractures healed.
In the single-center retrospective study, the investigators found that just 14% of sacral fractures healed during the period of observation. For acetabular and pubic fractures, roughly one in three fractures had healed by the last MRI scan. About a third of all fractures remained stable across scans, while just over 10% of fractures were either fluctuant or worsened.
The study included 115 women with locally advanced cervical cancer who were treated with radical or adjuvant concurrent chemoradiotherapy over a 5-year period, and had MRI scans performed in-house; the follow-up protocol had patients receiving scans at 3, 12, and 24 months post treatment. From an initial pool of 197 patients, those who had previously had pelvic radiation or were receiving palliative treatment, as well as those with incomplete imaging follow-up and those with metal implants or prostheses that could affect radiation therapy delivery or imaging quality were excluded.
The chemoradiotherapy protocol involved five doses of weekly cisplatin at 400 mg/m2 of body surface area, as well as high–dose rate cervix brachytherapy. In practice, all but six participants received these treatments. Patients also received external beam radiotherapy with or without a simultaneous integrated boost to target affected lymph nodes, as clinically indicated.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema.
Patients were aged a median of 54 years, and 64 (56%) were postmenopausal. Most patients (n = 84; 73%) had never used tobacco. Participants’ median body mass index was 26 kg/m2.
Most patients (n = 73; 64%) were International Federation of Gynecology and Obstetrics stage 2b, and almost half (n = 55; 48%) had pelvic nodal involvement.
Patients were followed for a median of 12 months, with patients receiving a median of two MRIs curing that period. In all, 105 fractures were identified in 44 patients. A median of two fractures were identified among the group of patients who had pelvic insufficiency fractures.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema. In this schema, 41% of identified fractures were considered mild, while 32% were moderate and 12% were severe.
Although just over two-thirds of fractures (70%) were identified within 6 months of beginning surveillance, a quarter were not identified until 9-13 months post therapy, and 5% were found after more than 13 months.
Sacral fractures accounted for 72% of those identified, in keeping with previous findings, said Dr. Dragan. Acetabular and pubic fractures made up 16% and 10% of fractures, respectively. One fracture was seen at the ilium and one at the ischium.
Dr. Dragan and colleagues turned to multivariable analysis to look for risk factors for pelvic insufficiency fractures in this cohort of cervical cancer patients. Younger patients had a hazard ratio of 0.30 for fracture, compared with those over the age of 50 years (P less than .01). Similarly, being menopausal carried a hazard ratio of 2.25 for fracture. Higher radiation doses to the sacrum also boosted fracture risk (HR, 2.00; P = .03). Neither sacral volume and slope nor the receipt of simultaneous integrated boost were associated with increased fracture risk.
Dr. Dragan reported that she had no relevant conflicts of interest. She reported no outside sources of funding.
SOURCE: Dragan A et al. RSNA 2019, Presentation SSE25-03.
CHICAGO – Radiation therapy for cervical cancer resulted in pelvic insufficiency fractures more frequently than previously thought, and many fractures were slow to heal, according to research presented at the annual meeting of the Radiological Society of North America.
“Pelvic insufficiency fractures had a prevalence of 38% on MRI follow-up” after chemoradiotherapy for locally advanced cervical cancer, said Alina Dragan, MD. This figure is more than double the previously reported prevalence of about 14%.
Dr. Dragan, a radiology resident at London North West Healthcare, National Health Service Trust, and coinvestigators also tracked the natural history of these fractures over time, to fill a knowledge gap about whether, and at what rate, these pelvic insufficiency fractures healed.
In the single-center retrospective study, the investigators found that just 14% of sacral fractures healed during the period of observation. For acetabular and pubic fractures, roughly one in three fractures had healed by the last MRI scan. About a third of all fractures remained stable across scans, while just over 10% of fractures were either fluctuant or worsened.
The study included 115 women with locally advanced cervical cancer who were treated with radical or adjuvant concurrent chemoradiotherapy over a 5-year period, and had MRI scans performed in-house; the follow-up protocol had patients receiving scans at 3, 12, and 24 months post treatment. From an initial pool of 197 patients, those who had previously had pelvic radiation or were receiving palliative treatment, as well as those with incomplete imaging follow-up and those with metal implants or prostheses that could affect radiation therapy delivery or imaging quality were excluded.
The chemoradiotherapy protocol involved five doses of weekly cisplatin at 400 mg/m2 of body surface area, as well as high–dose rate cervix brachytherapy. In practice, all but six participants received these treatments. Patients also received external beam radiotherapy with or without a simultaneous integrated boost to target affected lymph nodes, as clinically indicated.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema.
Patients were aged a median of 54 years, and 64 (56%) were postmenopausal. Most patients (n = 84; 73%) had never used tobacco. Participants’ median body mass index was 26 kg/m2.
Most patients (n = 73; 64%) were International Federation of Gynecology and Obstetrics stage 2b, and almost half (n = 55; 48%) had pelvic nodal involvement.
Patients were followed for a median of 12 months, with patients receiving a median of two MRIs curing that period. In all, 105 fractures were identified in 44 patients. A median of two fractures were identified among the group of patients who had pelvic insufficiency fractures.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema. In this schema, 41% of identified fractures were considered mild, while 32% were moderate and 12% were severe.
Although just over two-thirds of fractures (70%) were identified within 6 months of beginning surveillance, a quarter were not identified until 9-13 months post therapy, and 5% were found after more than 13 months.
Sacral fractures accounted for 72% of those identified, in keeping with previous findings, said Dr. Dragan. Acetabular and pubic fractures made up 16% and 10% of fractures, respectively. One fracture was seen at the ilium and one at the ischium.
Dr. Dragan and colleagues turned to multivariable analysis to look for risk factors for pelvic insufficiency fractures in this cohort of cervical cancer patients. Younger patients had a hazard ratio of 0.30 for fracture, compared with those over the age of 50 years (P less than .01). Similarly, being menopausal carried a hazard ratio of 2.25 for fracture. Higher radiation doses to the sacrum also boosted fracture risk (HR, 2.00; P = .03). Neither sacral volume and slope nor the receipt of simultaneous integrated boost were associated with increased fracture risk.
Dr. Dragan reported that she had no relevant conflicts of interest. She reported no outside sources of funding.
SOURCE: Dragan A et al. RSNA 2019, Presentation SSE25-03.
CHICAGO – Radiation therapy for cervical cancer resulted in pelvic insufficiency fractures more frequently than previously thought, and many fractures were slow to heal, according to research presented at the annual meeting of the Radiological Society of North America.
“Pelvic insufficiency fractures had a prevalence of 38% on MRI follow-up” after chemoradiotherapy for locally advanced cervical cancer, said Alina Dragan, MD. This figure is more than double the previously reported prevalence of about 14%.
Dr. Dragan, a radiology resident at London North West Healthcare, National Health Service Trust, and coinvestigators also tracked the natural history of these fractures over time, to fill a knowledge gap about whether, and at what rate, these pelvic insufficiency fractures healed.
In the single-center retrospective study, the investigators found that just 14% of sacral fractures healed during the period of observation. For acetabular and pubic fractures, roughly one in three fractures had healed by the last MRI scan. About a third of all fractures remained stable across scans, while just over 10% of fractures were either fluctuant or worsened.
The study included 115 women with locally advanced cervical cancer who were treated with radical or adjuvant concurrent chemoradiotherapy over a 5-year period, and had MRI scans performed in-house; the follow-up protocol had patients receiving scans at 3, 12, and 24 months post treatment. From an initial pool of 197 patients, those who had previously had pelvic radiation or were receiving palliative treatment, as well as those with incomplete imaging follow-up and those with metal implants or prostheses that could affect radiation therapy delivery or imaging quality were excluded.
The chemoradiotherapy protocol involved five doses of weekly cisplatin at 400 mg/m2 of body surface area, as well as high–dose rate cervix brachytherapy. In practice, all but six participants received these treatments. Patients also received external beam radiotherapy with or without a simultaneous integrated boost to target affected lymph nodes, as clinically indicated.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema.
Patients were aged a median of 54 years, and 64 (56%) were postmenopausal. Most patients (n = 84; 73%) had never used tobacco. Participants’ median body mass index was 26 kg/m2.
Most patients (n = 73; 64%) were International Federation of Gynecology and Obstetrics stage 2b, and almost half (n = 55; 48%) had pelvic nodal involvement.
Patients were followed for a median of 12 months, with patients receiving a median of two MRIs curing that period. In all, 105 fractures were identified in 44 patients. A median of two fractures were identified among the group of patients who had pelvic insufficiency fractures.
The fractures were graded as mild, moderate, or severe by the interpreting radiologist according to the course of the fracture line and corresponding bone edema. In this schema, 41% of identified fractures were considered mild, while 32% were moderate and 12% were severe.
Although just over two-thirds of fractures (70%) were identified within 6 months of beginning surveillance, a quarter were not identified until 9-13 months post therapy, and 5% were found after more than 13 months.
Sacral fractures accounted for 72% of those identified, in keeping with previous findings, said Dr. Dragan. Acetabular and pubic fractures made up 16% and 10% of fractures, respectively. One fracture was seen at the ilium and one at the ischium.
Dr. Dragan and colleagues turned to multivariable analysis to look for risk factors for pelvic insufficiency fractures in this cohort of cervical cancer patients. Younger patients had a hazard ratio of 0.30 for fracture, compared with those over the age of 50 years (P less than .01). Similarly, being menopausal carried a hazard ratio of 2.25 for fracture. Higher radiation doses to the sacrum also boosted fracture risk (HR, 2.00; P = .03). Neither sacral volume and slope nor the receipt of simultaneous integrated boost were associated with increased fracture risk.
Dr. Dragan reported that she had no relevant conflicts of interest. She reported no outside sources of funding.
SOURCE: Dragan A et al. RSNA 2019, Presentation SSE25-03.
REPORTING FROM RSNA 2019
Appeals court rules ACA’s individual mandate is unconstitutional
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
Learn more about AGA’s position on patient protections and access to care at https://www.gastro.org/
A version of this story first appeared on Medscape.com.
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
Learn more about AGA’s position on patient protections and access to care at https://www.gastro.org/
A version of this story first appeared on Medscape.com.
A federal appeals court ruled Dec. 18 that the individual mandate of the Affordable Care Act (ACA) is unconstitutional, but the panel sent the case back to a lower court to decide how much of the remainder of the law could topple along with it.
The three-judge panel of the New Orleans-based U.S. Fifth Circuit Court of Appeals said, “The individual mandate is unconstitutional because, under [a previous ruling, National Federation of Independent Business v Sebelius], it finds no constitutional footing in either the Interstate Commerce Clause or the Necessary and Proper Clause.”
The ruling upholds a December 2018 US District Court decision in which Judge Reed O’Connor found that the individual mandate that most Americans must have health insurance or pay a fine was unconstitutional and that without it the ACA itself was invalid.
In sending the case back to a Texas district court, however, the federal panel is asking for a central question to be resolved: Whether the individual mandate is “severable” from the rest of the law, while the rest of the law can be left intact.
If the district court eventually decides that the individual mandate cannot be severed from the rest of the ACA, the entire law will likely be ruled invalid, and some 24 million Americans could lose health coverage.
“Today’s ruling is the result of the Trump administration and congressional Republicans attempting to make dangerous health policy using the courts since they failed to succeed in Congress,” House Ways and Means Committee Chairman Richard E. Neal (D-Mass.) said in a statement. “This is a blow to our nation’s health care system and the millions of Americans who have gained coverage and protections under the Affordable Care Act. Democrats will continue to fight to protect Americans’ access to quality, affordable care.”
Some groups are applauding the decision, though. The Citizens’ Council for Health Freedom (CCHF), which filed an amicus brief with the Fifth Circuit arguing against the ACA, said it wants more.
“We are pleased with the Fifth Circuit Court of Appeals ruling, but it didn’t go far enough,” said Twila Brase, president and cofounder of CCHF, in a statement. “The individual mandate cannot be severed from the rest of the 2,700-page Affordable Care Act, thus the court should have ruled that the entire law is invalid, as the lower district court found.
“As the Court notes in the first paragraph of the ruling, we argued in our Amicus Brief, filed jointly with the Association of American Physicians and Surgeons, that the Act ‘has deprived patients nationwide of a competitive market for affordable high-deductible health insurance,’ leaving ‘patients with no alternative to ... skyrocketing premiums,’ “ Ms. Brase added. “Sending it back to the lower court, which already ruled the right way, continues to deprive citizens and patients of the affordable coverage that freedom from Obamacare would bring.”
Future uncertain
The ruling in Texas v Azar is not a surprise because, during oral arguments in July, as reported by Medscape Medical News, at least two of the three judges – Jennifer Walker Elrod, appointed by President George W. Bush in 2007, and Kurt Engelhardt, appointed by President Donald J. Trump in 2018 – appeared to be more receptive to the arguments of a group of 18 Republican states and two individuals seeking to invalidate the ACA.
Judge Carolyn Dineen King, appointed by President Jimmy Carter in 1979, did not comment during the hearing.
The Trump administration chose not to defend the ACA, but it does not seem entirely prepared for what might happen if the law is overturned. In a briefing before the Fifth Circuit hearing, the administration argued that, if ultimately the law is ruled unconstitutional, it should be struck down only in the states seeking to overturn the law.
“A lot of this has to get sorted out – it’s complicated,” said August E. Flentje, a U.S. Department of Justice lawyer, at the oral arguments in July.
For now, though, the ACA remains.
“In 2012, the Supreme Court upheld Obamacare, despite serious constitutional issues with the federal government forcing Americans to purchase a product from a private company. Until an ultimate decision is made by the Supreme Court or Congress decides otherwise, the Affordable Care Act will remain the law of the land,” Senate Finance Committee Chairman Chuck Grassley (R-Iowa), said in a statement.
And those who have led the court battle to keep the ACA intact plan to keep fighting. “For now, the President got the gift he wanted – uncertainty in the health care system and a pathway to repeal – so that the health care that seniors, workers, and families secured under the Affordable Care Act can be yanked from under them. This decision could take us to a dangerous and irresponsible place, not just for the 133 million Americans with pre-existing conditions, but for our seniors who use Medicare, our children under the age of 26, and the 20 million additional Americans covered directly through the ACA marketplace. California will move swiftly to challenge this decision because this could mean the difference between life and death for so many Americans and their families,” California Attorney General Xavier Becerra said in a statement.
Learn more about AGA’s position on patient protections and access to care at https://www.gastro.org/
A version of this story first appeared on Medscape.com.
New ustekinumab response predictor in Crohn’s called ‘brilliant’
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.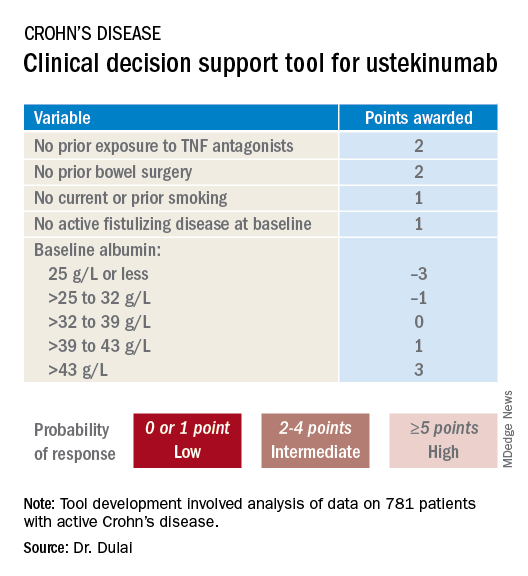
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
SAN ANTONIO – The probability of achieving clinical remission of Crohn’s disease in response to ustekinumab can now be readily estimated by using a clinical prediction tool, Parambir S. Dulai, MBBS, announced at the annual meeting of the American College of Gastroenterology.
This new clinical decision support tool also provides individualized stratification of the rapidity with which symptoms will be reduced in response to the anti-interleukin-12/23 biologic, added Dr. Dulai, a gastroenterologist at the University of California, San Diego.
He and his coinvestigators developed the prediction tool through analysis of detailed data on 781 patients with active Crohn’s disease treated with ustekinumab (Stelara) during both the induction and maintenance portions of the phase 3 UNITI randomized trials conducted in the biologic’s development program. The researchers identified a series of baseline features associated with clinical remission as defined by a Crohn’s Disease Activity Index (CDAI) score below 150 by week 16 of treatment. Through statistical manipulation, they transformed the data into a predictive model and then went one step further by turning the model into a decision support tool with points given for the individual predictive variables (see graphic).
Patients with 5 or more total points were categorized as having a high probability of week-16 clinical remission. Patients with 0 or 1 point were deemed low probability, and a score of 2-4 indicated an intermediate likelihood of clinical remission.
Next, the investigators applied their new clinical decision support tool to the 781 ustekinumab-treated patients included in the derivation analysis. The tool performed well: The high-probability group had a 57% clinical remission rate, significantly better than the 34% rate in the intermediate-probability group, which in turn was significantly better than the 21% rate of clinical remission in the group with a baseline score of 0 or 1.
In addition, onset of treatment benefit was significantly faster in the group having a score of 5 or more. They had a significantly higher clinical remission rate than the intermediate- and low-probability groups at all scheduled assessments, which were conducted at weeks 3, 6, 8, and 16. Indeed, by week 3 the high-probability group experienced a mean 69-point drop from baseline in CDAI and a 94-point drop by week 8, as compared with week-8 reductions of 54 and 40 points in the intermediate- and low-probability groups, respectively.
In an exploratory analysis involving the 122 patients who underwent week-8 endoscopy, endoscopic remission was documented in 12% of patients whose baseline scores placed them in the high-probability group, 10% in the intermediate group, and 8% of those in the low-probability group.
The high-probability group had significantly higher ustekinumab trough concentrations than did the intermediate- and low-probability groups when measured at weeks 3, 6, 8, and 16.
An external validation study conducted in a large cohort of Crohn’s disease patients seen in routine clinical practice has recently been completed, with the results now being analyzed, according to Dr. Dulai.
Miguel Requeiro, MD, chairman of gastroenterology and hepatology at the Cleveland Clinic, rose from the audience to declare the creation of the decision support tool to be “brilliant work.” He asked if it has changed clinical practice for Dr. Dulai and his coworkers.
“We’ve begun doing two things differently,” Dr. Dulai replied. “First, we’ve built a similar model for vedolizumab and Crohn’s. That means we can use both tools together to discriminate between a patient who should get vedolizumab versus ustekinumab because the variables and their weighting differ between the two. And the other thing we’ve been able to do is argue with payers for positioning of the treatments when we have evidence to support that we can use them earlier in the treatment course to optimize outcomes.”
Another audience member, David T. Rubin, MD, also praised the decision support tool as “brilliant” and “definitely needed.”
“Thank you for hitting the sweet spot of what we’ve all been waiting for,” added Dr. Rubin, professor of medicine and codirector of the Digestive Diseases Center at the University of Chicago.
Dr. Dulai reported receiving a research grant for the project from Janssen, which markets ustekinumab.
Help your patients better understand their Crohn’s disease treatment options by sharing AGA patient education at https://www.gastro.org/
REPORTING FROM ACG 2019
Experts call to revise the Uniform Determination of Death Act
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
, according to an editorial published online Dec. 24, 2019, in Annals of Internal Medicine. Proposed revisions would identify the standards for determining death by neurologic criteria and address the question of whether consent is required to make this determination. If accepted, the revisions would enhance public trust in the determination of death by neurologic criteria, the authors said.
“There is a disconnect between the medical and legal standards for brain death,” said Ariane K. Lewis, MD, associate professor of neurology and neurosurgery at New York University and lead author of the editorial. The discrepancy must be remedied because it has led to lawsuits and has proved to be problematic from a societal standpoint, she added.
“We defend changing the law to match medical practice, rather than changing medical practice to match the law,” said Thaddeus Mason Pope, JD, PhD, director of the Health Law Institute at Mitchell Hamline School of Law in Saint Paul, Minnesota, and an author of the editorial.
Accepted medical standards are unclear
The UDDA was drafted in 1981 to establish a uniform legal standard for death by neurologic criteria. A person with “irreversible cessation of all functions of the entire brain, including the brainstem,” is dead, according to the statute. A determination of death, it adds, “must be made in accordance with accepted medical standards.”
But the medical standards used to determine death by neurologic cause have not been uniform. In 2015, the Supreme Court of Nevada ruled that it was not clear that the standard published by the American Academy of Neurology (AAN), which had been used in the case at issue, was the “accepted medical standard.” An AAN summit later affirmed that the accepted medical standards for determination of death by neurologic cause are the 2010 AAN standard for determination of brain death in adults and the 2011 Society of Critical Care Medicine (SCCM), American Academy of Pediatrics (AAP), and Child Neurology Society (CNS) standard for determination of brain death in children. The Nevada legislature amended the state UDDA to identify these standards as the accepted standards. A revised UDDA also should identify these standards and grant an administrative agency (i.e., the board of medicine) the power to review and update the accepted medical standards as needed, according to the editorial.
To the extent that hospitals are not following the AAN or SCCM/AAP/CNS standards for determining death by neurologic cause, “enshrining” these standards in a revised UDDA “should increase uniformity and consistency” in hospitals’ policies on brain death, Dr. Pope said.
The question of hormonal function
Lawsuits in California and Nevada raised the question of whether the pituitary gland and hypothalamus are parts of the brain. If so, then the accepted medical standards for death by neurologic cause are not consistent with the statutory requirements for the determination of death, since the former do not test for cessation of hormonal function.
The current edition of the adult standards for determining death by neurologic cause were published in 2010. “Whenever we measure brain death, we’re not measuring the cessation of all functions of the entire brain,” Dr. Pope said. “That’s not a new thing; that’s been the case for a long time.”
To address the discrepancy between medical practice and the legal statute, Dr. Lewis and colleagues proposed that the UDDA’s reference to “irreversible cessation of functions of the entire brain” be followed by the following clause: “including the brainstem, leading to unresponsive coma with loss of capacity for consciousness, brainstem areflexia, and the inability to breathe spontaneously.” An alternative revision would be to add the briefer phrase “... with the exception of hormonal function.”
Authors say consent is not required for testing
Other complications have arisen from the UDDA’s failure to specify whether consent is required for a determination of death by neurologic cause. Court rulings on this question have not been consistent. Dr. Lewis and colleagues propose adding the following text to the UDDA: “Reasonable efforts should be made to notify a patient’s legally authorized decision-maker before performing a determination of death by neurologic criteria, but consent is not required to initiate such an evaluation.”
The proposed revisions to the UDDA “might give [clinicians] more confidence to proceed with brain death testing, because it would clarify that they don’t need the parents’ [or the patient’s legally authorized decision-maker] consent to do the tests,” said Dr. Pope. “If anything, they might even have a duty to do the tests.”
The final problem with the UDDA that Dr. Lewis and colleagues cited is that it does not provide clear guidance about how to respond to religious objections to discontinuation of organ support after a determination of death by neurologic cause. “Because the issue is rather complicated, we have not advocated for a singular position related to this [question] in our revised UDDA,” Dr. Lewis said. “Rather, we recommended the need for a multidisciplinary group to come together to determine what is the best approach. In an ideal world, this [approach] would be universal throughout the country.”
Although a revised UDDA would provide greater clarity to physicians and promote uniformity of practice, it would not resolve ongoing theological and philosophical debates about whether brain death is biological death, Dr. Pope said. “The key thing is that it would give clinicians a green light or certainty and clarity that they may proceed to do the test in the first place. If the tests are positive and the patient really is dead, then they could proceed to organ procurement or to move to the morgue.”
Dr. Lewis is a member of various AAN committees and working groups but receives no compensation for her role. A coauthor received personal fees from the AAN that were unrelated to the editorial.
SOURCE: Lewis A et al. Ann Intern Med. 2019 Dec 24. doi: 10.7326/M19-2731.
FROM ANNALS OF INTERNAL MEDICINE
Patient poaching: High on practices’ dirty tricks list
Years ago, I wrote about patient poaching – the practice of stealing another practice’s patients without the patient or other physician realizing what’s going on.
This is high on the dirty tricks list of some practices. It’s certainly not illegal, but seems pretty unethical. Fortunately, it’s infrequent (at least where I work), but still occurs.
Recently, I encountered a particularly egregious example.
One of my patients, a nice lady in her 70s, had a syncopal event and landed in a hospital I don’t cover. A neurologist who saw her there did a brain MRI and head/neck MR angiogram, which were fine. A cardiology evaluation was also fine, so she was sent home. The neurologist there told her to follow-up with him at his office.
As my nurse says, “some people just do whatever they’re told, they don’t want to make a doctor angry.” So my patient did, and at the other doctor’s office had a four-limb electromyogram test and nerve conduction study, carotid Dopplers, transcranial Dopplers, and an EEG. He also made changes in her medications.
The first time I found out about it was when the patient scheduled an unrelated procedure, and I got a clearance request to take her off a medication in advance of it. Since I hadn’t started her on the medication (or was even aware she was on it) I refused, saying they’d have to contact the physician who prescribed it.
This got back to the patient, who was under the impression I’d been aware of all this the whole time, and she called the other neurologist to have his records sent to me.
When I got them, I was surprised to find he’d documented that I’d asked him to assume her outpatient care and do these studies for me. I have no recollection of such a conversation, and I would not have agreed to such a thing unless the patient had informed me she was transferring care to him (in which case it’s no longer my concern). Unless I was in a coma at the time this conversation occurred, I’m pretty sure it didn’t happen.
Basically, what the other doctor did was perform a walletectomy (or, in this case insurance-ectomy) on the patient under the guise (to her) that he was doing this as a favor to me.
How do you look yourself in mirror each day when you do stuff like this? Apparently, it’s easier for some doctors than it is for me.
I’ll do my best to keep it that way, too. I can’t change others, but I can do my best to take the high road.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Years ago, I wrote about patient poaching – the practice of stealing another practice’s patients without the patient or other physician realizing what’s going on.
This is high on the dirty tricks list of some practices. It’s certainly not illegal, but seems pretty unethical. Fortunately, it’s infrequent (at least where I work), but still occurs.
Recently, I encountered a particularly egregious example.
One of my patients, a nice lady in her 70s, had a syncopal event and landed in a hospital I don’t cover. A neurologist who saw her there did a brain MRI and head/neck MR angiogram, which were fine. A cardiology evaluation was also fine, so she was sent home. The neurologist there told her to follow-up with him at his office.
As my nurse says, “some people just do whatever they’re told, they don’t want to make a doctor angry.” So my patient did, and at the other doctor’s office had a four-limb electromyogram test and nerve conduction study, carotid Dopplers, transcranial Dopplers, and an EEG. He also made changes in her medications.
The first time I found out about it was when the patient scheduled an unrelated procedure, and I got a clearance request to take her off a medication in advance of it. Since I hadn’t started her on the medication (or was even aware she was on it) I refused, saying they’d have to contact the physician who prescribed it.
This got back to the patient, who was under the impression I’d been aware of all this the whole time, and she called the other neurologist to have his records sent to me.
When I got them, I was surprised to find he’d documented that I’d asked him to assume her outpatient care and do these studies for me. I have no recollection of such a conversation, and I would not have agreed to such a thing unless the patient had informed me she was transferring care to him (in which case it’s no longer my concern). Unless I was in a coma at the time this conversation occurred, I’m pretty sure it didn’t happen.
Basically, what the other doctor did was perform a walletectomy (or, in this case insurance-ectomy) on the patient under the guise (to her) that he was doing this as a favor to me.
How do you look yourself in mirror each day when you do stuff like this? Apparently, it’s easier for some doctors than it is for me.
I’ll do my best to keep it that way, too. I can’t change others, but I can do my best to take the high road.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Years ago, I wrote about patient poaching – the practice of stealing another practice’s patients without the patient or other physician realizing what’s going on.
This is high on the dirty tricks list of some practices. It’s certainly not illegal, but seems pretty unethical. Fortunately, it’s infrequent (at least where I work), but still occurs.
Recently, I encountered a particularly egregious example.
One of my patients, a nice lady in her 70s, had a syncopal event and landed in a hospital I don’t cover. A neurologist who saw her there did a brain MRI and head/neck MR angiogram, which were fine. A cardiology evaluation was also fine, so she was sent home. The neurologist there told her to follow-up with him at his office.
As my nurse says, “some people just do whatever they’re told, they don’t want to make a doctor angry.” So my patient did, and at the other doctor’s office had a four-limb electromyogram test and nerve conduction study, carotid Dopplers, transcranial Dopplers, and an EEG. He also made changes in her medications.
The first time I found out about it was when the patient scheduled an unrelated procedure, and I got a clearance request to take her off a medication in advance of it. Since I hadn’t started her on the medication (or was even aware she was on it) I refused, saying they’d have to contact the physician who prescribed it.
This got back to the patient, who was under the impression I’d been aware of all this the whole time, and she called the other neurologist to have his records sent to me.
When I got them, I was surprised to find he’d documented that I’d asked him to assume her outpatient care and do these studies for me. I have no recollection of such a conversation, and I would not have agreed to such a thing unless the patient had informed me she was transferring care to him (in which case it’s no longer my concern). Unless I was in a coma at the time this conversation occurred, I’m pretty sure it didn’t happen.
Basically, what the other doctor did was perform a walletectomy (or, in this case insurance-ectomy) on the patient under the guise (to her) that he was doing this as a favor to me.
How do you look yourself in mirror each day when you do stuff like this? Apparently, it’s easier for some doctors than it is for me.
I’ll do my best to keep it that way, too. I can’t change others, but I can do my best to take the high road.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
2020 Directory of VA and DoD Facilities
Click here to access The 2020 Directory of VA and DoD Facilities
Table of Contents
- Letter From the Publisher
- Explanatory Notes and Abbreviation Key
- Veterans Integrated Service Network (VISN) Guide
- Department of Veterans Affairs Health Care Facilities
- TRICARE Region Guide
- Department of Defense Health Care Facilities

Click here to access The 2020 Directory of VA and DoD Facilities
Table of Contents
- Letter From the Publisher
- Explanatory Notes and Abbreviation Key
- Veterans Integrated Service Network (VISN) Guide
- Department of Veterans Affairs Health Care Facilities
- TRICARE Region Guide
- Department of Defense Health Care Facilities

Click here to access The 2020 Directory of VA and DoD Facilities
Table of Contents
- Letter From the Publisher
- Explanatory Notes and Abbreviation Key
- Veterans Integrated Service Network (VISN) Guide
- Department of Veterans Affairs Health Care Facilities
- TRICARE Region Guide
- Department of Defense Health Care Facilities

FDA targets flavored cartridge-based e-cigarettes, but says it is not a ‘ban’
but states it is not a “ban.”
On Jan. 2, the agency issued enforcement guidance alerting companies that manufacture, distribute, and sell unauthorized flavored cartridge-based e-cigarettes within the next 30 days will risk FDA enforcement action.
FDA has had the authority to require premarket authorization of all e-cigarettes and other electronic nicotine delivery systems (ENDS) since August 2016, but thus far has exercised enforcement discretion regarding the need for premarket authorization for these types of products.
“By prioritizing enforcement against the products that are most widely used by children, our action today seeks to strike the right public health balance by maintaining e-cigarettes as a potential off-ramp for adults using combustible tobacco while ensuring these products don’t provide an on-ramp to nicotine addiction for our youth,” Department of Health & Human Services Secretary Alex Azar said in a statement.
The action comes in the wake of more than 2,500 vaping-related injuries being reported, including more than 50 deaths associated with vaping reported by the Centers for Disease Control and Prevention (although many are related to the use of tetrahydrocannabinol [THC] within vaping products) and a continued rise in youth use of e-cigarettes noted in government surveys.
The agency noted in a Jan. 2 statement announcing the enforcement action that, to date, no ENDS products have received a premarket authorization, “meaning that all ENDS products currently on the market are considered illegally marketed and are subject to enforcement, at any time, in the FDA’s discretion.”
FDA said it is prioritizing enforcement in 30 days against:
- Any flavored, cartridge-based ENDS product, other than those with a tobacco or menthol flavoring.
- All other ENDS products for which manufacturers are failing to take adequate measures to prevent access by minors.
- Any ENDS product that is targeted to minors or is likely to promote use by minors.
In the last category, this might include labeling or advertising resembling “kid-friendly food and drinks such as juice boxes or kid-friendly cereal; products marketed directly to minors by promoting ease of concealing the product or disguising it as another product; and products marketed with characters designed to appeal to youth,” according to the FDA statement.
As of May 12, FDA also will prioritize enforcement against any ENDS product for which the manufacturer has not submitted a premarket application. The agency will continue to exercise enforcement discretion for up to 1 year on these products if an application has been submitted, pending the review of that application.
“By not prioritizing enforcement against other flavored ENDS products in the same way as flavored cartridge-based ENDS products, the FDA has attempted to balance the public health concerns related to youth use of ENDS products with consideration regarding addicted adult cigarette smokers who may try to use ENDS products to transition away from combustible tobacco products,” the agency stated, adding that cartridge-based ENDS products are most commonly used among youth.
The FDA statement noted that the enforcement priorities outlined in the guidance document were not a “ban” on flavored or cartridge-based ENDS, noting the agency “has already accepted and begun review of several premarket applications for flavored ENDS products through the pathway that Congress established in the Tobacco Control Act. ... If a company can demonstrate to the FDA that a specific product meets the applicable standard set forth by Congress, including considering how the marketing of the product may affect youth initiation and use, then the FDA could authorize that product for sale.”
“Coupled with the recently signed legislation increasing the minimum age of sale of tobacco to 21, we believe this policy balances the urgency with which we must address the public health threat of youth use of e-cigarette products with the potential role that e-cigarettes may play in helping adult smokers transition completely away from combustible tobacco to a potentially less risky form of nicotine delivery,” FDA Commissioner Stephen Hahn, MD, said in a statement. “While we expect that responsible members of industry will comply with premarket requirements, we’re ready to take action against any unauthorized e-cigarette products as outlined in our priorities. We’ll also closely monitor the use rates of all e-cigarette products and take additional steps to address youth use as necessary.”
The American Medical Association criticized the action as not going far enough, even though it was a step in the right direction.
“The AMA is disappointed that menthol flavors, one of the most popular, will still be allowed, and that flavored e-liquids will remain on the market, leaving young people with easy access to alternative flavored e-cigarette products,” AMA President Patrice A. Harris, MD, said in a statement. “If we are serious about tackling this epidemic and keeping these harmful products out of the hands of young people, a total ban on all flavored e-cigarettes, in all forms and at all locations, is prudent and urgently needed. We are pleased the administration committed today to closely monitoring the situation and trends in e-cigarette use among young people, and to taking further action if needed.”
but states it is not a “ban.”
On Jan. 2, the agency issued enforcement guidance alerting companies that manufacture, distribute, and sell unauthorized flavored cartridge-based e-cigarettes within the next 30 days will risk FDA enforcement action.
FDA has had the authority to require premarket authorization of all e-cigarettes and other electronic nicotine delivery systems (ENDS) since August 2016, but thus far has exercised enforcement discretion regarding the need for premarket authorization for these types of products.
“By prioritizing enforcement against the products that are most widely used by children, our action today seeks to strike the right public health balance by maintaining e-cigarettes as a potential off-ramp for adults using combustible tobacco while ensuring these products don’t provide an on-ramp to nicotine addiction for our youth,” Department of Health & Human Services Secretary Alex Azar said in a statement.
The action comes in the wake of more than 2,500 vaping-related injuries being reported, including more than 50 deaths associated with vaping reported by the Centers for Disease Control and Prevention (although many are related to the use of tetrahydrocannabinol [THC] within vaping products) and a continued rise in youth use of e-cigarettes noted in government surveys.
The agency noted in a Jan. 2 statement announcing the enforcement action that, to date, no ENDS products have received a premarket authorization, “meaning that all ENDS products currently on the market are considered illegally marketed and are subject to enforcement, at any time, in the FDA’s discretion.”
FDA said it is prioritizing enforcement in 30 days against:
- Any flavored, cartridge-based ENDS product, other than those with a tobacco or menthol flavoring.
- All other ENDS products for which manufacturers are failing to take adequate measures to prevent access by minors.
- Any ENDS product that is targeted to minors or is likely to promote use by minors.
In the last category, this might include labeling or advertising resembling “kid-friendly food and drinks such as juice boxes or kid-friendly cereal; products marketed directly to minors by promoting ease of concealing the product or disguising it as another product; and products marketed with characters designed to appeal to youth,” according to the FDA statement.
As of May 12, FDA also will prioritize enforcement against any ENDS product for which the manufacturer has not submitted a premarket application. The agency will continue to exercise enforcement discretion for up to 1 year on these products if an application has been submitted, pending the review of that application.
“By not prioritizing enforcement against other flavored ENDS products in the same way as flavored cartridge-based ENDS products, the FDA has attempted to balance the public health concerns related to youth use of ENDS products with consideration regarding addicted adult cigarette smokers who may try to use ENDS products to transition away from combustible tobacco products,” the agency stated, adding that cartridge-based ENDS products are most commonly used among youth.
The FDA statement noted that the enforcement priorities outlined in the guidance document were not a “ban” on flavored or cartridge-based ENDS, noting the agency “has already accepted and begun review of several premarket applications for flavored ENDS products through the pathway that Congress established in the Tobacco Control Act. ... If a company can demonstrate to the FDA that a specific product meets the applicable standard set forth by Congress, including considering how the marketing of the product may affect youth initiation and use, then the FDA could authorize that product for sale.”
“Coupled with the recently signed legislation increasing the minimum age of sale of tobacco to 21, we believe this policy balances the urgency with which we must address the public health threat of youth use of e-cigarette products with the potential role that e-cigarettes may play in helping adult smokers transition completely away from combustible tobacco to a potentially less risky form of nicotine delivery,” FDA Commissioner Stephen Hahn, MD, said in a statement. “While we expect that responsible members of industry will comply with premarket requirements, we’re ready to take action against any unauthorized e-cigarette products as outlined in our priorities. We’ll also closely monitor the use rates of all e-cigarette products and take additional steps to address youth use as necessary.”
The American Medical Association criticized the action as not going far enough, even though it was a step in the right direction.
“The AMA is disappointed that menthol flavors, one of the most popular, will still be allowed, and that flavored e-liquids will remain on the market, leaving young people with easy access to alternative flavored e-cigarette products,” AMA President Patrice A. Harris, MD, said in a statement. “If we are serious about tackling this epidemic and keeping these harmful products out of the hands of young people, a total ban on all flavored e-cigarettes, in all forms and at all locations, is prudent and urgently needed. We are pleased the administration committed today to closely monitoring the situation and trends in e-cigarette use among young people, and to taking further action if needed.”
but states it is not a “ban.”
On Jan. 2, the agency issued enforcement guidance alerting companies that manufacture, distribute, and sell unauthorized flavored cartridge-based e-cigarettes within the next 30 days will risk FDA enforcement action.
FDA has had the authority to require premarket authorization of all e-cigarettes and other electronic nicotine delivery systems (ENDS) since August 2016, but thus far has exercised enforcement discretion regarding the need for premarket authorization for these types of products.
“By prioritizing enforcement against the products that are most widely used by children, our action today seeks to strike the right public health balance by maintaining e-cigarettes as a potential off-ramp for adults using combustible tobacco while ensuring these products don’t provide an on-ramp to nicotine addiction for our youth,” Department of Health & Human Services Secretary Alex Azar said in a statement.
The action comes in the wake of more than 2,500 vaping-related injuries being reported, including more than 50 deaths associated with vaping reported by the Centers for Disease Control and Prevention (although many are related to the use of tetrahydrocannabinol [THC] within vaping products) and a continued rise in youth use of e-cigarettes noted in government surveys.
The agency noted in a Jan. 2 statement announcing the enforcement action that, to date, no ENDS products have received a premarket authorization, “meaning that all ENDS products currently on the market are considered illegally marketed and are subject to enforcement, at any time, in the FDA’s discretion.”
FDA said it is prioritizing enforcement in 30 days against:
- Any flavored, cartridge-based ENDS product, other than those with a tobacco or menthol flavoring.
- All other ENDS products for which manufacturers are failing to take adequate measures to prevent access by minors.
- Any ENDS product that is targeted to minors or is likely to promote use by minors.
In the last category, this might include labeling or advertising resembling “kid-friendly food and drinks such as juice boxes or kid-friendly cereal; products marketed directly to minors by promoting ease of concealing the product or disguising it as another product; and products marketed with characters designed to appeal to youth,” according to the FDA statement.
As of May 12, FDA also will prioritize enforcement against any ENDS product for which the manufacturer has not submitted a premarket application. The agency will continue to exercise enforcement discretion for up to 1 year on these products if an application has been submitted, pending the review of that application.
“By not prioritizing enforcement against other flavored ENDS products in the same way as flavored cartridge-based ENDS products, the FDA has attempted to balance the public health concerns related to youth use of ENDS products with consideration regarding addicted adult cigarette smokers who may try to use ENDS products to transition away from combustible tobacco products,” the agency stated, adding that cartridge-based ENDS products are most commonly used among youth.
The FDA statement noted that the enforcement priorities outlined in the guidance document were not a “ban” on flavored or cartridge-based ENDS, noting the agency “has already accepted and begun review of several premarket applications for flavored ENDS products through the pathway that Congress established in the Tobacco Control Act. ... If a company can demonstrate to the FDA that a specific product meets the applicable standard set forth by Congress, including considering how the marketing of the product may affect youth initiation and use, then the FDA could authorize that product for sale.”
“Coupled with the recently signed legislation increasing the minimum age of sale of tobacco to 21, we believe this policy balances the urgency with which we must address the public health threat of youth use of e-cigarette products with the potential role that e-cigarettes may play in helping adult smokers transition completely away from combustible tobacco to a potentially less risky form of nicotine delivery,” FDA Commissioner Stephen Hahn, MD, said in a statement. “While we expect that responsible members of industry will comply with premarket requirements, we’re ready to take action against any unauthorized e-cigarette products as outlined in our priorities. We’ll also closely monitor the use rates of all e-cigarette products and take additional steps to address youth use as necessary.”
The American Medical Association criticized the action as not going far enough, even though it was a step in the right direction.
“The AMA is disappointed that menthol flavors, one of the most popular, will still be allowed, and that flavored e-liquids will remain on the market, leaving young people with easy access to alternative flavored e-cigarette products,” AMA President Patrice A. Harris, MD, said in a statement. “If we are serious about tackling this epidemic and keeping these harmful products out of the hands of young people, a total ban on all flavored e-cigarettes, in all forms and at all locations, is prudent and urgently needed. We are pleased the administration committed today to closely monitoring the situation and trends in e-cigarette use among young people, and to taking further action if needed.”
Down syndrome arthritis: Distinct from JIA and missed in the clinic
ATLANTA – Pediatric Down syndrome arthritis is more aggressive and severe than juvenile idiopathic arthritis (JIA), but it’s underrecognized and undertreated, according to reports at the annual meeting of the American College of Rheumatology.
“The vast majority of parents don’t know their kids are at risk for arthritis,” and a lot of doctors don’t realize it, either. Meanwhile, children show up in the clinic a year or more into the process with irreversible joint damage, said pediatric rheumatologist Jordan Jones, DO, an assistant professor at the University of Missouri, Kansas City, and the lead investigator on a review of 36 children with Down syndrome (DS) in the national Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
One solution is to add routine musculoskeletal exams to American Academy of Pediatrics DS guidelines, something Dr. Jones said he and his colleagues are hoping to do.
Part of the problem is that children with DS have a hard time articulating and localizing pain, and it’s easy to attribute functional issues to DS itself. Charlene Foley, MD, PhD, from the National Centre for Paediatric Rheumatology in Dublin, said she’s seen “loads of cases” in which parents were told that their children were acting up, probably because of the DS, when they didn’t want to walk down stairs anymore or hold their parent’s hand.
She was the lead investigator on an Irish program that screened 503 DS children, about one-third of the country’s pediatric DS population, for arthritis; 33 cases were identified, including 18 new ones. Most of the children had polyarticular, rheumatoid factor–negative arthritis, and all of them were antinuclear antibody negative.
A key take-home from the work is that DS arthritis preferentially attacks the hands and wrists and was present exclusively in the hands and wrists of about one-third of the Irish cohort. “So, if you only have a second to examine a child or you can’t get them to sit still, just go straight for the hands, and have a low threshold for imaging,” Dr. Foley said.
DS arthritis is often considered a subtype of JIA, but findings from the studies call that into question and suggest the need for novel therapeutic targets, the investigators said.
The Irish team found that 42% of their subjects (14 of 33) had joint erosions, far more than the 14% of JIA children (3 of 21) who served as controls, and Dr. Foley and colleagues didn’t think that was solely because of delayed diagnosis. Also, at about 20 cases per 1,000, they estimated that arthritis was far more prevalent in DS than was JIA in the general pediatrics population.
Disease onset was at a mean of 7.1 years in Dr. Jones’ CARRA registry review, and mean delay to diagnosis was 11.5 months. The 36 children presented with an average of four affected joints. Only 22% (8 of 36) had elevated inflammatory markers; just one-third were positive for antinuclear antibody, and 17% for human leukocyte antigen B27. It means that “these kids can present with normal labs, even with very aggressive disease. The threshold of concern for arthritis has to be very high when you evaluate these children,” Dr. Jones said.
Treatment was initiated with disease-modifying antirheumatic drugs (DMARDs) in two-thirds of the registry children, often with a concomitant biologic, most commonly etanercept. Over half had at least one switch during a mean follow-up of 4.5 years; methotrexate was a leading culprit, frequently discontinued because of nausea and other problems, and biologics were changed for lack of effect. Active joint counts and physician assessments improved, but there were no significant changes in limited joint counts and health assessments.
In short, “the current therapies for JIA appear to be poorly tolerated, more toxic, and less effective in patients with Down syndrome. These kids don’t respond the same. They have a very high disease burden despite being treated aggressively,” Dr. Jones said.
That finding adds additional weight to the idea that DS arthritis is a distinct disease entity, with unique therapeutic targets. “Down syndrome has a lot of immunologic issues associated with it; maybe that’s it. I think in the next few years, we will be able to show that this is a different disease,” Dr. Jones said.
There was a boost in that direction from benchwork, also led and presented by Dr. Foley, that found significant immunologic, histologic, and genetic differences between JIA and DS arthritis, including lower CD19- and CD20-positive B-cell counts in DS arthritis and higher interferon-gamma and tumor necrosis factor–alpha production, greater synovial lining hyperplasia, and different minor allele frequencies.
There was no industry funding for the studies, and the investigators didn’t have any industry disclosures.
SOURCES: Jones J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2722; Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1817; and Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 781
ATLANTA – Pediatric Down syndrome arthritis is more aggressive and severe than juvenile idiopathic arthritis (JIA), but it’s underrecognized and undertreated, according to reports at the annual meeting of the American College of Rheumatology.
“The vast majority of parents don’t know their kids are at risk for arthritis,” and a lot of doctors don’t realize it, either. Meanwhile, children show up in the clinic a year or more into the process with irreversible joint damage, said pediatric rheumatologist Jordan Jones, DO, an assistant professor at the University of Missouri, Kansas City, and the lead investigator on a review of 36 children with Down syndrome (DS) in the national Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
One solution is to add routine musculoskeletal exams to American Academy of Pediatrics DS guidelines, something Dr. Jones said he and his colleagues are hoping to do.
Part of the problem is that children with DS have a hard time articulating and localizing pain, and it’s easy to attribute functional issues to DS itself. Charlene Foley, MD, PhD, from the National Centre for Paediatric Rheumatology in Dublin, said she’s seen “loads of cases” in which parents were told that their children were acting up, probably because of the DS, when they didn’t want to walk down stairs anymore or hold their parent’s hand.
She was the lead investigator on an Irish program that screened 503 DS children, about one-third of the country’s pediatric DS population, for arthritis; 33 cases were identified, including 18 new ones. Most of the children had polyarticular, rheumatoid factor–negative arthritis, and all of them were antinuclear antibody negative.
A key take-home from the work is that DS arthritis preferentially attacks the hands and wrists and was present exclusively in the hands and wrists of about one-third of the Irish cohort. “So, if you only have a second to examine a child or you can’t get them to sit still, just go straight for the hands, and have a low threshold for imaging,” Dr. Foley said.
DS arthritis is often considered a subtype of JIA, but findings from the studies call that into question and suggest the need for novel therapeutic targets, the investigators said.
The Irish team found that 42% of their subjects (14 of 33) had joint erosions, far more than the 14% of JIA children (3 of 21) who served as controls, and Dr. Foley and colleagues didn’t think that was solely because of delayed diagnosis. Also, at about 20 cases per 1,000, they estimated that arthritis was far more prevalent in DS than was JIA in the general pediatrics population.
Disease onset was at a mean of 7.1 years in Dr. Jones’ CARRA registry review, and mean delay to diagnosis was 11.5 months. The 36 children presented with an average of four affected joints. Only 22% (8 of 36) had elevated inflammatory markers; just one-third were positive for antinuclear antibody, and 17% for human leukocyte antigen B27. It means that “these kids can present with normal labs, even with very aggressive disease. The threshold of concern for arthritis has to be very high when you evaluate these children,” Dr. Jones said.
Treatment was initiated with disease-modifying antirheumatic drugs (DMARDs) in two-thirds of the registry children, often with a concomitant biologic, most commonly etanercept. Over half had at least one switch during a mean follow-up of 4.5 years; methotrexate was a leading culprit, frequently discontinued because of nausea and other problems, and biologics were changed for lack of effect. Active joint counts and physician assessments improved, but there were no significant changes in limited joint counts and health assessments.
In short, “the current therapies for JIA appear to be poorly tolerated, more toxic, and less effective in patients with Down syndrome. These kids don’t respond the same. They have a very high disease burden despite being treated aggressively,” Dr. Jones said.
That finding adds additional weight to the idea that DS arthritis is a distinct disease entity, with unique therapeutic targets. “Down syndrome has a lot of immunologic issues associated with it; maybe that’s it. I think in the next few years, we will be able to show that this is a different disease,” Dr. Jones said.
There was a boost in that direction from benchwork, also led and presented by Dr. Foley, that found significant immunologic, histologic, and genetic differences between JIA and DS arthritis, including lower CD19- and CD20-positive B-cell counts in DS arthritis and higher interferon-gamma and tumor necrosis factor–alpha production, greater synovial lining hyperplasia, and different minor allele frequencies.
There was no industry funding for the studies, and the investigators didn’t have any industry disclosures.
SOURCES: Jones J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2722; Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1817; and Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 781
ATLANTA – Pediatric Down syndrome arthritis is more aggressive and severe than juvenile idiopathic arthritis (JIA), but it’s underrecognized and undertreated, according to reports at the annual meeting of the American College of Rheumatology.
“The vast majority of parents don’t know their kids are at risk for arthritis,” and a lot of doctors don’t realize it, either. Meanwhile, children show up in the clinic a year or more into the process with irreversible joint damage, said pediatric rheumatologist Jordan Jones, DO, an assistant professor at the University of Missouri, Kansas City, and the lead investigator on a review of 36 children with Down syndrome (DS) in the national Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
One solution is to add routine musculoskeletal exams to American Academy of Pediatrics DS guidelines, something Dr. Jones said he and his colleagues are hoping to do.
Part of the problem is that children with DS have a hard time articulating and localizing pain, and it’s easy to attribute functional issues to DS itself. Charlene Foley, MD, PhD, from the National Centre for Paediatric Rheumatology in Dublin, said she’s seen “loads of cases” in which parents were told that their children were acting up, probably because of the DS, when they didn’t want to walk down stairs anymore or hold their parent’s hand.
She was the lead investigator on an Irish program that screened 503 DS children, about one-third of the country’s pediatric DS population, for arthritis; 33 cases were identified, including 18 new ones. Most of the children had polyarticular, rheumatoid factor–negative arthritis, and all of them were antinuclear antibody negative.
A key take-home from the work is that DS arthritis preferentially attacks the hands and wrists and was present exclusively in the hands and wrists of about one-third of the Irish cohort. “So, if you only have a second to examine a child or you can’t get them to sit still, just go straight for the hands, and have a low threshold for imaging,” Dr. Foley said.
DS arthritis is often considered a subtype of JIA, but findings from the studies call that into question and suggest the need for novel therapeutic targets, the investigators said.
The Irish team found that 42% of their subjects (14 of 33) had joint erosions, far more than the 14% of JIA children (3 of 21) who served as controls, and Dr. Foley and colleagues didn’t think that was solely because of delayed diagnosis. Also, at about 20 cases per 1,000, they estimated that arthritis was far more prevalent in DS than was JIA in the general pediatrics population.
Disease onset was at a mean of 7.1 years in Dr. Jones’ CARRA registry review, and mean delay to diagnosis was 11.5 months. The 36 children presented with an average of four affected joints. Only 22% (8 of 36) had elevated inflammatory markers; just one-third were positive for antinuclear antibody, and 17% for human leukocyte antigen B27. It means that “these kids can present with normal labs, even with very aggressive disease. The threshold of concern for arthritis has to be very high when you evaluate these children,” Dr. Jones said.
Treatment was initiated with disease-modifying antirheumatic drugs (DMARDs) in two-thirds of the registry children, often with a concomitant biologic, most commonly etanercept. Over half had at least one switch during a mean follow-up of 4.5 years; methotrexate was a leading culprit, frequently discontinued because of nausea and other problems, and biologics were changed for lack of effect. Active joint counts and physician assessments improved, but there were no significant changes in limited joint counts and health assessments.
In short, “the current therapies for JIA appear to be poorly tolerated, more toxic, and less effective in patients with Down syndrome. These kids don’t respond the same. They have a very high disease burden despite being treated aggressively,” Dr. Jones said.
That finding adds additional weight to the idea that DS arthritis is a distinct disease entity, with unique therapeutic targets. “Down syndrome has a lot of immunologic issues associated with it; maybe that’s it. I think in the next few years, we will be able to show that this is a different disease,” Dr. Jones said.
There was a boost in that direction from benchwork, also led and presented by Dr. Foley, that found significant immunologic, histologic, and genetic differences between JIA and DS arthritis, including lower CD19- and CD20-positive B-cell counts in DS arthritis and higher interferon-gamma and tumor necrosis factor–alpha production, greater synovial lining hyperplasia, and different minor allele frequencies.
There was no industry funding for the studies, and the investigators didn’t have any industry disclosures.
SOURCES: Jones J et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2722; Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 1817; and Foley C et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 781
REPORTING FROM ACR 2019
Numerous Flesh-Colored Nodules on the Trunk
The Diagnosis: Steatocystoma Multiplex
The punch biopsy of an abdominal lesion demonstrated a folded cyst wall with a wavy eosinophilic cuticle (Figure), characteristics consistent with steatocystoma multiplex (SM).
Also known as eruptive steatocystoma, SM consists of numerous flesh-colored, dome-shaped papules and nodules that most commonly arise during adolescence, with a median age of onset of 26 years.1 These hamartomatous nevoid malformations arise in areas with well-developed pilosebaceous units, such as the upper extremities, neck, axillae, and trunk.1,2 They occur less commonly on the scalp, face, and acral surfaces.2-5 The lesions range in size from 2 to 30 mm6 and usually are asymptomatic.1 Occasionally, steatocystomas become tender or can rupture.7
Steatocystoma multiplex may arise sporadically or may be inherited in an autosomal-dominant fashion. Mutations in exon 1 of the keratin 17 gene, KRT17, have been identified in autosomal-dominant SM.6,8KRT17 mutations also are responsible for pachyonychia congenita type 2, which is associated with SM.9 Some patients with pachyonychia congenita type 2 who have prominent SM and mild nail findings may be misdiagnosed as having pure SM.2
The histopathologic features of SM were described in a study by Cho and colleagues1 of 64 patients. Steatocystomas have cyst walls that may be either intricately folded or round/oval, comprised of an average of 4.9 epithelial cell layers. In most cases, the cyst wall contains sebaceous lobules. In all cases, an acellular eosinophilic cuticle was present, and no granular layer was seen. Few vellus hairs may be observed in the cystic cavity.1
The differential diagnosis of SM includes eruptive vellus hair cysts, lipomas, Muir-Torre syndrome, and Gardner syndrome. Some have suggested that eruptive vellus hair cysts and SM exist on a disease spectrum because of their similar clinical presentation.10 In contrast to SM, however, eruptive vellus hair cysts originate in the infundibulum of the hair shaft rather than the sebaceous duct, and more numerous vellus hair shafts are seen on histopathology.1
Various treatment modalities have been described, including isotretinoin for inflamed lesions,11 cryotherapy for noninflamed lesions,11 aspiration of lesions smallerthan 1 cm,12 and electrocautery combined with topical retinoids.13 Laser treatment has been described, with a 1450-nm diode laser used to target the abnormal sebaceous glands and a 1550-nm fractionated erbium-doped fiber laser used to target the dermal cysts.14 Carbon dioxide lasers also may be used to open the cyst for drainage.15 Surgical excision or mini-incision also may be performed.16,17
Acknowledgment
The authors thank Garth Fraga, MD (Kansas City, Kansas), for his assistance with interpretation of the dermatopathology in this case.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Rollins T, Levin RM, Heymann WR. Acral steatocystoma multiplex.J Am Acad Dermatol. 2000;43(2, pt 2):396-399.
- Setoyama M, Mizoguchi S, Usuki K, et al. Steatocystoma multiplex: a case with unusual clinical and histological manifestation. Am J Dermatopathol. 1997;19:89-92.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- McLean WH, Rugg EL, Lunny DP, et al. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat Genet. 1995;9:273-278.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sato K, Shibuya K, Taguchi H, et al. Aspiration therapy in steatocystoma multiplex. Arch Dermatol. 1993;129:35-37.
- Papakonstantinou E, Franke I, Gollnick H. Facial steatocystoma multiplex combined with eruptive vellus hair cysts: a hybrid? J Eur Acad Dermatol Venereol. 2015;29:2051-2053.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7, pt 1):1104-1106.
- Rossi R, Cappugi P, Battini M, et al. CO2 laser therapy in a case of steatocystoma multiplex with prominent nodules on the face and neck. Int J Dermatol. 2003;42:302-304.
- Schmook T, Burg G, Hafner J. Surgical pearl: mini-incisions for the extraction of steatocystoma multiplex. J Am Acad Dermatol. 2001;44:1041-1042.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
The Diagnosis: Steatocystoma Multiplex
The punch biopsy of an abdominal lesion demonstrated a folded cyst wall with a wavy eosinophilic cuticle (Figure), characteristics consistent with steatocystoma multiplex (SM).
Also known as eruptive steatocystoma, SM consists of numerous flesh-colored, dome-shaped papules and nodules that most commonly arise during adolescence, with a median age of onset of 26 years.1 These hamartomatous nevoid malformations arise in areas with well-developed pilosebaceous units, such as the upper extremities, neck, axillae, and trunk.1,2 They occur less commonly on the scalp, face, and acral surfaces.2-5 The lesions range in size from 2 to 30 mm6 and usually are asymptomatic.1 Occasionally, steatocystomas become tender or can rupture.7
Steatocystoma multiplex may arise sporadically or may be inherited in an autosomal-dominant fashion. Mutations in exon 1 of the keratin 17 gene, KRT17, have been identified in autosomal-dominant SM.6,8KRT17 mutations also are responsible for pachyonychia congenita type 2, which is associated with SM.9 Some patients with pachyonychia congenita type 2 who have prominent SM and mild nail findings may be misdiagnosed as having pure SM.2
The histopathologic features of SM were described in a study by Cho and colleagues1 of 64 patients. Steatocystomas have cyst walls that may be either intricately folded or round/oval, comprised of an average of 4.9 epithelial cell layers. In most cases, the cyst wall contains sebaceous lobules. In all cases, an acellular eosinophilic cuticle was present, and no granular layer was seen. Few vellus hairs may be observed in the cystic cavity.1
The differential diagnosis of SM includes eruptive vellus hair cysts, lipomas, Muir-Torre syndrome, and Gardner syndrome. Some have suggested that eruptive vellus hair cysts and SM exist on a disease spectrum because of their similar clinical presentation.10 In contrast to SM, however, eruptive vellus hair cysts originate in the infundibulum of the hair shaft rather than the sebaceous duct, and more numerous vellus hair shafts are seen on histopathology.1
Various treatment modalities have been described, including isotretinoin for inflamed lesions,11 cryotherapy for noninflamed lesions,11 aspiration of lesions smallerthan 1 cm,12 and electrocautery combined with topical retinoids.13 Laser treatment has been described, with a 1450-nm diode laser used to target the abnormal sebaceous glands and a 1550-nm fractionated erbium-doped fiber laser used to target the dermal cysts.14 Carbon dioxide lasers also may be used to open the cyst for drainage.15 Surgical excision or mini-incision also may be performed.16,17
Acknowledgment
The authors thank Garth Fraga, MD (Kansas City, Kansas), for his assistance with interpretation of the dermatopathology in this case.
The Diagnosis: Steatocystoma Multiplex
The punch biopsy of an abdominal lesion demonstrated a folded cyst wall with a wavy eosinophilic cuticle (Figure), characteristics consistent with steatocystoma multiplex (SM).
Also known as eruptive steatocystoma, SM consists of numerous flesh-colored, dome-shaped papules and nodules that most commonly arise during adolescence, with a median age of onset of 26 years.1 These hamartomatous nevoid malformations arise in areas with well-developed pilosebaceous units, such as the upper extremities, neck, axillae, and trunk.1,2 They occur less commonly on the scalp, face, and acral surfaces.2-5 The lesions range in size from 2 to 30 mm6 and usually are asymptomatic.1 Occasionally, steatocystomas become tender or can rupture.7
Steatocystoma multiplex may arise sporadically or may be inherited in an autosomal-dominant fashion. Mutations in exon 1 of the keratin 17 gene, KRT17, have been identified in autosomal-dominant SM.6,8KRT17 mutations also are responsible for pachyonychia congenita type 2, which is associated with SM.9 Some patients with pachyonychia congenita type 2 who have prominent SM and mild nail findings may be misdiagnosed as having pure SM.2
The histopathologic features of SM were described in a study by Cho and colleagues1 of 64 patients. Steatocystomas have cyst walls that may be either intricately folded or round/oval, comprised of an average of 4.9 epithelial cell layers. In most cases, the cyst wall contains sebaceous lobules. In all cases, an acellular eosinophilic cuticle was present, and no granular layer was seen. Few vellus hairs may be observed in the cystic cavity.1
The differential diagnosis of SM includes eruptive vellus hair cysts, lipomas, Muir-Torre syndrome, and Gardner syndrome. Some have suggested that eruptive vellus hair cysts and SM exist on a disease spectrum because of their similar clinical presentation.10 In contrast to SM, however, eruptive vellus hair cysts originate in the infundibulum of the hair shaft rather than the sebaceous duct, and more numerous vellus hair shafts are seen on histopathology.1
Various treatment modalities have been described, including isotretinoin for inflamed lesions,11 cryotherapy for noninflamed lesions,11 aspiration of lesions smallerthan 1 cm,12 and electrocautery combined with topical retinoids.13 Laser treatment has been described, with a 1450-nm diode laser used to target the abnormal sebaceous glands and a 1550-nm fractionated erbium-doped fiber laser used to target the dermal cysts.14 Carbon dioxide lasers also may be used to open the cyst for drainage.15 Surgical excision or mini-incision also may be performed.16,17
Acknowledgment
The authors thank Garth Fraga, MD (Kansas City, Kansas), for his assistance with interpretation of the dermatopathology in this case.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Rollins T, Levin RM, Heymann WR. Acral steatocystoma multiplex.J Am Acad Dermatol. 2000;43(2, pt 2):396-399.
- Setoyama M, Mizoguchi S, Usuki K, et al. Steatocystoma multiplex: a case with unusual clinical and histological manifestation. Am J Dermatopathol. 1997;19:89-92.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- McLean WH, Rugg EL, Lunny DP, et al. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat Genet. 1995;9:273-278.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sato K, Shibuya K, Taguchi H, et al. Aspiration therapy in steatocystoma multiplex. Arch Dermatol. 1993;129:35-37.
- Papakonstantinou E, Franke I, Gollnick H. Facial steatocystoma multiplex combined with eruptive vellus hair cysts: a hybrid? J Eur Acad Dermatol Venereol. 2015;29:2051-2053.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7, pt 1):1104-1106.
- Rossi R, Cappugi P, Battini M, et al. CO2 laser therapy in a case of steatocystoma multiplex with prominent nodules on the face and neck. Int J Dermatol. 2003;42:302-304.
- Schmook T, Burg G, Hafner J. Surgical pearl: mini-incisions for the extraction of steatocystoma multiplex. J Am Acad Dermatol. 2001;44:1041-1042.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Rollins T, Levin RM, Heymann WR. Acral steatocystoma multiplex.J Am Acad Dermatol. 2000;43(2, pt 2):396-399.
- Setoyama M, Mizoguchi S, Usuki K, et al. Steatocystoma multiplex: a case with unusual clinical and histological manifestation. Am J Dermatopathol. 1997;19:89-92.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Marzano AV, Tavecchio S, Balice Y, et al. Acral subcutaneous steatocystoma multiplex: a distinct subtype of the disease? Australas J Dermatol. 2012;53:198-201.
- Liu Q, Wu W, Lu J, et al. Steatocystoma multiplex is associated with the R94C mutation in the KRTl7 gene. Mol Med Rep. 2015;12:5072-5076.
- Egbert BM, Price NM, Segal RJ. Steatocystoma multiplex. Report of a florid case and a review. Arch Dermatol. 1979;115:334-335.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480.
- McLean WH, Rugg EL, Lunny DP, et al. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat Genet. 1995;9:273-278.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Apaydin R, Bilen N, Bayramgurler D, et al. Steatocystoma multiplex suppurativum: oral isotretinoin treatment combined with cryotherapy. Australas J Dermatol. 2000;41:98-100.
- Sato K, Shibuya K, Taguchi H, et al. Aspiration therapy in steatocystoma multiplex. Arch Dermatol. 1993;129:35-37.
- Papakonstantinou E, Franke I, Gollnick H. Facial steatocystoma multiplex combined with eruptive vellus hair cysts: a hybrid? J Eur Acad Dermatol Venereol. 2015;29:2051-2053.
- Moody MN, Landau JM, Goldberg LH, et al. 1,450-nm diode laser in combination with the 1550-nm fractionated erbium-doped fiber laser for the treatment of steatocystoma multiplex: a case report. Dermatol Surg. 2012;38(7, pt 1):1104-1106.
- Rossi R, Cappugi P, Battini M, et al. CO2 laser therapy in a case of steatocystoma multiplex with prominent nodules on the face and neck. Int J Dermatol. 2003;42:302-304.
- Schmook T, Burg G, Hafner J. Surgical pearl: mini-incisions for the extraction of steatocystoma multiplex. J Am Acad Dermatol. 2001;44:1041-1042.
- Adams BB, Mutasim DF, Nordlund JJ. Steatocystoma multiplex: a quick removal technique. Cutis. 1999;64:127-130.
A 33-year-old woman presented with numerous firm, noncompressible, flesh-colored nodules that measured 3 to 4 mm and were distributed across the abdomen, chest, back, and neck. The lesions had been present for approximately 10 years. The patient denied any lesion-associated pain, itching, or bleeding, and there was no family history of similar lesions. A punch biopsy of a lesion on the central abdomen was obtained.