User login
Medicaid spending on MS drugs rose despite introduction of generic glatiramer
Prescription pricing is a primary reason why Medicaid spending on multiple sclerosis disease-modifying therapies (DMTs) has more than doubled between 2011 and 2017 and the introduction of a generic glatiramer acetate is having nominal effect on pricing and utilization within the class, new research is showing.
“Gross spending on self-administered and infusible MS DMTs in the Medicaid program increased 2.9-fold from $453 million in 2011 to $1.32 billion in 2017,” Daniel Hartung, PharmD, of Oregon Health and Science University, Portland, and his colleagues wrote in a research report published Jan. 15 in Neurology. Net spending after accounting for rebates during this period showed a doubling of spending from $278 million per year to $600 million per year.
Use of MS DMTs during this period overall remained stable, but there was a shift from injectable DMTs to oral DMTs during this time window, the researchers found, with the plurality of utilization attributed to glatiramer acetate.
Sandoz began marketing a generic version of glatiramer acetate 20 mg in the second quarter of 2015, which led to an immediate increase in the cost per prescription of $441 for the branded version of glatiramer acetate 20 mg, although that cost has come down gradually by $52 per prescription over time. Other DMTs saw minimal price changes at that time, Dr. Hartung and his colleagues noted.
The researchers attributed the increased Medicaid spending to rising prices of DMTs.
“Although some of this increase is attributable to the 2014 Medicaid expansion, the primary driver was rising DMT costs per prescription, which doubled over the period,” the researchers wrote. “Thus, we assert that rising prices, not increasing use, are the primary driver of spending for DMTs in the Medicaid program.”
In addition, the introduction of the first generic DMT “appeared to have little effect on the overall trajectory of DMT costs,” they continued. “In fact, the cost of Teva’s 20-mg glatiramer acetate increased significantly following the release of Sandoz’s generic. ... The increase possibly signified efforts to both retain revenue and further push market share to the 40-mg version. Although the costs for generic glatiramer acetate declined over time, its introduction appears not to have fundamentally affected the overall trend in DMT costs.”
Indeed, the researchers’ examination of utilization trends found that Teva executed a successful preemptive strategy of converting 20-mg users of glatiramer acetate to 40-mg users, something that is not interchangeable with the generic product.
“Low generic penetration is also due to the fact that Sandoz’s product was only 15% less expensive than branded glatiramer acetate 20 mg and approximately the same cost as the 40-mg version at launch,” Dr. Hartung and his colleagues stated. “This difference may have been further diminished by rebates that Teva may have provided to maintain preferred status on state Medicaid formularies.”
These factors reflect an “urgent need for robust generic competition within the DMT class,” the authors wrote.
The study was supported by the National Multiple Sclerosis Society. Lead author Dr. Hartung reported receiving research support from AbbVie.
SOURCE: Hartung D et al. Neurology. Jan 15. doi: 10.1212/WNL.0000000000008936.
Prescription pricing is a primary reason why Medicaid spending on multiple sclerosis disease-modifying therapies (DMTs) has more than doubled between 2011 and 2017 and the introduction of a generic glatiramer acetate is having nominal effect on pricing and utilization within the class, new research is showing.
“Gross spending on self-administered and infusible MS DMTs in the Medicaid program increased 2.9-fold from $453 million in 2011 to $1.32 billion in 2017,” Daniel Hartung, PharmD, of Oregon Health and Science University, Portland, and his colleagues wrote in a research report published Jan. 15 in Neurology. Net spending after accounting for rebates during this period showed a doubling of spending from $278 million per year to $600 million per year.
Use of MS DMTs during this period overall remained stable, but there was a shift from injectable DMTs to oral DMTs during this time window, the researchers found, with the plurality of utilization attributed to glatiramer acetate.
Sandoz began marketing a generic version of glatiramer acetate 20 mg in the second quarter of 2015, which led to an immediate increase in the cost per prescription of $441 for the branded version of glatiramer acetate 20 mg, although that cost has come down gradually by $52 per prescription over time. Other DMTs saw minimal price changes at that time, Dr. Hartung and his colleagues noted.
The researchers attributed the increased Medicaid spending to rising prices of DMTs.
“Although some of this increase is attributable to the 2014 Medicaid expansion, the primary driver was rising DMT costs per prescription, which doubled over the period,” the researchers wrote. “Thus, we assert that rising prices, not increasing use, are the primary driver of spending for DMTs in the Medicaid program.”
In addition, the introduction of the first generic DMT “appeared to have little effect on the overall trajectory of DMT costs,” they continued. “In fact, the cost of Teva’s 20-mg glatiramer acetate increased significantly following the release of Sandoz’s generic. ... The increase possibly signified efforts to both retain revenue and further push market share to the 40-mg version. Although the costs for generic glatiramer acetate declined over time, its introduction appears not to have fundamentally affected the overall trend in DMT costs.”
Indeed, the researchers’ examination of utilization trends found that Teva executed a successful preemptive strategy of converting 20-mg users of glatiramer acetate to 40-mg users, something that is not interchangeable with the generic product.
“Low generic penetration is also due to the fact that Sandoz’s product was only 15% less expensive than branded glatiramer acetate 20 mg and approximately the same cost as the 40-mg version at launch,” Dr. Hartung and his colleagues stated. “This difference may have been further diminished by rebates that Teva may have provided to maintain preferred status on state Medicaid formularies.”
These factors reflect an “urgent need for robust generic competition within the DMT class,” the authors wrote.
The study was supported by the National Multiple Sclerosis Society. Lead author Dr. Hartung reported receiving research support from AbbVie.
SOURCE: Hartung D et al. Neurology. Jan 15. doi: 10.1212/WNL.0000000000008936.
Prescription pricing is a primary reason why Medicaid spending on multiple sclerosis disease-modifying therapies (DMTs) has more than doubled between 2011 and 2017 and the introduction of a generic glatiramer acetate is having nominal effect on pricing and utilization within the class, new research is showing.
“Gross spending on self-administered and infusible MS DMTs in the Medicaid program increased 2.9-fold from $453 million in 2011 to $1.32 billion in 2017,” Daniel Hartung, PharmD, of Oregon Health and Science University, Portland, and his colleagues wrote in a research report published Jan. 15 in Neurology. Net spending after accounting for rebates during this period showed a doubling of spending from $278 million per year to $600 million per year.
Use of MS DMTs during this period overall remained stable, but there was a shift from injectable DMTs to oral DMTs during this time window, the researchers found, with the plurality of utilization attributed to glatiramer acetate.
Sandoz began marketing a generic version of glatiramer acetate 20 mg in the second quarter of 2015, which led to an immediate increase in the cost per prescription of $441 for the branded version of glatiramer acetate 20 mg, although that cost has come down gradually by $52 per prescription over time. Other DMTs saw minimal price changes at that time, Dr. Hartung and his colleagues noted.
The researchers attributed the increased Medicaid spending to rising prices of DMTs.
“Although some of this increase is attributable to the 2014 Medicaid expansion, the primary driver was rising DMT costs per prescription, which doubled over the period,” the researchers wrote. “Thus, we assert that rising prices, not increasing use, are the primary driver of spending for DMTs in the Medicaid program.”
In addition, the introduction of the first generic DMT “appeared to have little effect on the overall trajectory of DMT costs,” they continued. “In fact, the cost of Teva’s 20-mg glatiramer acetate increased significantly following the release of Sandoz’s generic. ... The increase possibly signified efforts to both retain revenue and further push market share to the 40-mg version. Although the costs for generic glatiramer acetate declined over time, its introduction appears not to have fundamentally affected the overall trend in DMT costs.”
Indeed, the researchers’ examination of utilization trends found that Teva executed a successful preemptive strategy of converting 20-mg users of glatiramer acetate to 40-mg users, something that is not interchangeable with the generic product.
“Low generic penetration is also due to the fact that Sandoz’s product was only 15% less expensive than branded glatiramer acetate 20 mg and approximately the same cost as the 40-mg version at launch,” Dr. Hartung and his colleagues stated. “This difference may have been further diminished by rebates that Teva may have provided to maintain preferred status on state Medicaid formularies.”
These factors reflect an “urgent need for robust generic competition within the DMT class,” the authors wrote.
The study was supported by the National Multiple Sclerosis Society. Lead author Dr. Hartung reported receiving research support from AbbVie.
SOURCE: Hartung D et al. Neurology. Jan 15. doi: 10.1212/WNL.0000000000008936.
FROM NEUROLOGY
Key clinical point: Medicaid spending on MS DMTs continues to rise in spite of generic introduction.
Major finding: Cost is the major factor in spending as utilization has remained stable.
Study details: Researchers examined quarterly Medicaid State Drug Utilization Data from 2011 to 2017, examining spending, utilization and cost per prescription for 15 MS DMTs, including brand and generic versions of glatiramer acetate.
Disclosures: The study was supported by the National Multiple Sclerosis Society. Lead author Dr. Hartung reported receiving research support from AbbVie.
Source: Hartung D et al. Neurology. Jan 15. doi: 10.1212/WNL.0000000000008936.
Fewer interventions after sleeve gastrectomy vs. Roux-en-Y, real-world data show
Sleeve gastrectomy was associated with significantly fewer postsurgical interventions and operations than was Roux-en-Y gastric bypass over longer-term follow-up in a recent cohort study based on real-world clinical data, according to investigators.
Interventions, operations, and hospitalizations were “relatively common” for both procedures over the 5-year follow-up, though significantly less so with sleeve gastrectomy, which has become the most common bariatric operation, the investigators said in a recent report on the study.
The benefit of sleeve gastrectomy seemed to be most pronounced in patients with lower body mass index and those with fewer comorbidities, according to the authors of the report, led by bariatric and general surgeon Anita Courcoulas, MD, of the University of Pittsburgh Medical Center.
That finding is counterintuitive, since in clinical practice, sleeve gastrectomy is frequently thought of as the preferred procedure for individuals with higher BMI or more comorbidities, though Dr. Courcoulas and colleagues cautioned that the findings were “exploratory” and require further investigation. Historically, Roux-en-Y gastric bypass provided more weight loss.
Safety results from this study, balanced by weight loss and health outcomes data, will “help inform procedure-specific decision making for prospective patients and physicians,” said Dr. Courcoulas and coauthors of the report, which appears in JAMA Surgery.
The study included 33,560 adults who had undergone the Roux-en-Y procedure or sleeve gastrectomy, making it one of the largest bariatric cohort studies ever to be done, according to the investigators.
The majority of patients in the study (54%) underwent Roux-en-Y gastric bypass, though the number of sleeve gastrectomy procedures increased each year in the study, which included patients who underwent a primary bariatric surgery procedure between January 2005 and September 2015 at 1 of 10 sites in the National Patient-Centered Clinical Research Network.
Most of the patients were female (80%) and white (66%), and 26% were Hispanic, according to the researchers, who said their study was more racially diverse than other bariatric studies, and therefore potentially more generalizable to real-world clinical practice.
Over the 5-year follow-up period, patients who underwent sleeve gastrectomy were less likely to subsequently undergo operations involving the abdomen, or interventions for enteral access, such as placement of gastrostomy tubes, according to the investigators, who reported a hazard ratio (HR) of 0.72 (95% confidence interval [CI], 0.65-0.79; P less than .001).
The estimated cumulative rate of operations or interventions at 5 years was 8.94% (95% CI, 8.23%-9.65%) for the patients who underwent sleeve gastrectomy, compared with 12.27% (95% CI, 11.49%-13.05%) for those who underwent the Roux-en-Y procedure, according to the report.
Hospitalization was also less likely for sleeve gastrectomy versus Roux-en-Y gastric bypass, with a hazard ratio of 0.82 and estimated cumulative incidence rates of 32.79% and 38.33%, respectively. Likewise, endoscopy was less likely in the sleeve gastrectomy group.
All-cause mortality did not differ between the groups at this 5-year follow-up, the investigators said.
“The present data were gathered from clinical care in the real world, yet the results are comparable to controlled studies and therefore lend additional support to the findings of these other types of studies that operation and intervention occur less commonly after sleeve gastrectomy than after Roux-en-Y gastric bypass for up to 5 years,” Dr. Courcoulas and coauthors noted in a discussion of their results.
This work was funded by the Patient-Centered Outcomes Research Institute. Dr. Courcoulas reported receiving a grant from Allurion. Coauthors provided disclosures related to the National Institutes of Health, IFSO Latin America Chapter, and the Food and Drug Administration.
*This story was updated on January 16, 2020.
SOURCE: Courcoulas A et al. JAMA Surg. 2020 Jan 15. doi: 10.1001/jamasurg.2019.5470.
This study by Courcoulas and colleagues adds to an established body of data showing that bariatric surgery is safe, according to authors of an invited commentary on the study.
More specifically, the study provides data that sleeve gastrectomy – which has become the most common bariatric procedure in the United States – is safe in the long term, said Anne P. Ehlers, MD, MPH, and Amir A. Ghaferi, MD, MS.
Barriers to surgery need to come down in response to the worsening public health crisis that these procedures address, according to Dr. Ehlers and Dr. Ghaferi.
“It is time we stop questioning the safety or efficacy of surgery and begin advocating for comprehensive obesity care for patients,” they said in their invited commentary.
Less than 1% of patients eligible for bariatric surgery undergo this “life-saving” treatment, they said, adding that obesity bias delays surgical referrals, while some “continue to fear” surgical risks, and decades-old guidelines restrict procedures to patients with higher body mass index.
The present study shows that sleeve gastrectomy is at least as safe, if not safer, than the Roux-en-Y gastric bypass procedure, said Dr. Ehlers and Dr. Ghaferi.
The study also answers the question of whether sleeve gastrectomy has an “unacceptably high” rate of gastroesophageal reflux disease (GERD) prompting revision surgeries, they said.
On the contrary, sleeve gastrectomy was associated with lower rates of reoperation, and lower rates of endoscopies, which almost always come before reoperations related to GERD, they explained.
Anne P. Ehlers, MD, MPH, and Amir A. Ghaferi, MD, MS, are with the department of surgery at the University of Michigan in Ann Arbor. They reported no conflicts of interests related to their invited commentary, which appears in JAMA Surgery .
This study by Courcoulas and colleagues adds to an established body of data showing that bariatric surgery is safe, according to authors of an invited commentary on the study.
More specifically, the study provides data that sleeve gastrectomy – which has become the most common bariatric procedure in the United States – is safe in the long term, said Anne P. Ehlers, MD, MPH, and Amir A. Ghaferi, MD, MS.
Barriers to surgery need to come down in response to the worsening public health crisis that these procedures address, according to Dr. Ehlers and Dr. Ghaferi.
“It is time we stop questioning the safety or efficacy of surgery and begin advocating for comprehensive obesity care for patients,” they said in their invited commentary.
Less than 1% of patients eligible for bariatric surgery undergo this “life-saving” treatment, they said, adding that obesity bias delays surgical referrals, while some “continue to fear” surgical risks, and decades-old guidelines restrict procedures to patients with higher body mass index.
The present study shows that sleeve gastrectomy is at least as safe, if not safer, than the Roux-en-Y gastric bypass procedure, said Dr. Ehlers and Dr. Ghaferi.
The study also answers the question of whether sleeve gastrectomy has an “unacceptably high” rate of gastroesophageal reflux disease (GERD) prompting revision surgeries, they said.
On the contrary, sleeve gastrectomy was associated with lower rates of reoperation, and lower rates of endoscopies, which almost always come before reoperations related to GERD, they explained.
Anne P. Ehlers, MD, MPH, and Amir A. Ghaferi, MD, MS, are with the department of surgery at the University of Michigan in Ann Arbor. They reported no conflicts of interests related to their invited commentary, which appears in JAMA Surgery .
This study by Courcoulas and colleagues adds to an established body of data showing that bariatric surgery is safe, according to authors of an invited commentary on the study.
More specifically, the study provides data that sleeve gastrectomy – which has become the most common bariatric procedure in the United States – is safe in the long term, said Anne P. Ehlers, MD, MPH, and Amir A. Ghaferi, MD, MS.
Barriers to surgery need to come down in response to the worsening public health crisis that these procedures address, according to Dr. Ehlers and Dr. Ghaferi.
“It is time we stop questioning the safety or efficacy of surgery and begin advocating for comprehensive obesity care for patients,” they said in their invited commentary.
Less than 1% of patients eligible for bariatric surgery undergo this “life-saving” treatment, they said, adding that obesity bias delays surgical referrals, while some “continue to fear” surgical risks, and decades-old guidelines restrict procedures to patients with higher body mass index.
The present study shows that sleeve gastrectomy is at least as safe, if not safer, than the Roux-en-Y gastric bypass procedure, said Dr. Ehlers and Dr. Ghaferi.
The study also answers the question of whether sleeve gastrectomy has an “unacceptably high” rate of gastroesophageal reflux disease (GERD) prompting revision surgeries, they said.
On the contrary, sleeve gastrectomy was associated with lower rates of reoperation, and lower rates of endoscopies, which almost always come before reoperations related to GERD, they explained.
Anne P. Ehlers, MD, MPH, and Amir A. Ghaferi, MD, MS, are with the department of surgery at the University of Michigan in Ann Arbor. They reported no conflicts of interests related to their invited commentary, which appears in JAMA Surgery .
Sleeve gastrectomy was associated with significantly fewer postsurgical interventions and operations than was Roux-en-Y gastric bypass over longer-term follow-up in a recent cohort study based on real-world clinical data, according to investigators.
Interventions, operations, and hospitalizations were “relatively common” for both procedures over the 5-year follow-up, though significantly less so with sleeve gastrectomy, which has become the most common bariatric operation, the investigators said in a recent report on the study.
The benefit of sleeve gastrectomy seemed to be most pronounced in patients with lower body mass index and those with fewer comorbidities, according to the authors of the report, led by bariatric and general surgeon Anita Courcoulas, MD, of the University of Pittsburgh Medical Center.
That finding is counterintuitive, since in clinical practice, sleeve gastrectomy is frequently thought of as the preferred procedure for individuals with higher BMI or more comorbidities, though Dr. Courcoulas and colleagues cautioned that the findings were “exploratory” and require further investigation. Historically, Roux-en-Y gastric bypass provided more weight loss.
Safety results from this study, balanced by weight loss and health outcomes data, will “help inform procedure-specific decision making for prospective patients and physicians,” said Dr. Courcoulas and coauthors of the report, which appears in JAMA Surgery.
The study included 33,560 adults who had undergone the Roux-en-Y procedure or sleeve gastrectomy, making it one of the largest bariatric cohort studies ever to be done, according to the investigators.
The majority of patients in the study (54%) underwent Roux-en-Y gastric bypass, though the number of sleeve gastrectomy procedures increased each year in the study, which included patients who underwent a primary bariatric surgery procedure between January 2005 and September 2015 at 1 of 10 sites in the National Patient-Centered Clinical Research Network.
Most of the patients were female (80%) and white (66%), and 26% were Hispanic, according to the researchers, who said their study was more racially diverse than other bariatric studies, and therefore potentially more generalizable to real-world clinical practice.
Over the 5-year follow-up period, patients who underwent sleeve gastrectomy were less likely to subsequently undergo operations involving the abdomen, or interventions for enteral access, such as placement of gastrostomy tubes, according to the investigators, who reported a hazard ratio (HR) of 0.72 (95% confidence interval [CI], 0.65-0.79; P less than .001).
The estimated cumulative rate of operations or interventions at 5 years was 8.94% (95% CI, 8.23%-9.65%) for the patients who underwent sleeve gastrectomy, compared with 12.27% (95% CI, 11.49%-13.05%) for those who underwent the Roux-en-Y procedure, according to the report.
Hospitalization was also less likely for sleeve gastrectomy versus Roux-en-Y gastric bypass, with a hazard ratio of 0.82 and estimated cumulative incidence rates of 32.79% and 38.33%, respectively. Likewise, endoscopy was less likely in the sleeve gastrectomy group.
All-cause mortality did not differ between the groups at this 5-year follow-up, the investigators said.
“The present data were gathered from clinical care in the real world, yet the results are comparable to controlled studies and therefore lend additional support to the findings of these other types of studies that operation and intervention occur less commonly after sleeve gastrectomy than after Roux-en-Y gastric bypass for up to 5 years,” Dr. Courcoulas and coauthors noted in a discussion of their results.
This work was funded by the Patient-Centered Outcomes Research Institute. Dr. Courcoulas reported receiving a grant from Allurion. Coauthors provided disclosures related to the National Institutes of Health, IFSO Latin America Chapter, and the Food and Drug Administration.
*This story was updated on January 16, 2020.
SOURCE: Courcoulas A et al. JAMA Surg. 2020 Jan 15. doi: 10.1001/jamasurg.2019.5470.
Sleeve gastrectomy was associated with significantly fewer postsurgical interventions and operations than was Roux-en-Y gastric bypass over longer-term follow-up in a recent cohort study based on real-world clinical data, according to investigators.
Interventions, operations, and hospitalizations were “relatively common” for both procedures over the 5-year follow-up, though significantly less so with sleeve gastrectomy, which has become the most common bariatric operation, the investigators said in a recent report on the study.
The benefit of sleeve gastrectomy seemed to be most pronounced in patients with lower body mass index and those with fewer comorbidities, according to the authors of the report, led by bariatric and general surgeon Anita Courcoulas, MD, of the University of Pittsburgh Medical Center.
That finding is counterintuitive, since in clinical practice, sleeve gastrectomy is frequently thought of as the preferred procedure for individuals with higher BMI or more comorbidities, though Dr. Courcoulas and colleagues cautioned that the findings were “exploratory” and require further investigation. Historically, Roux-en-Y gastric bypass provided more weight loss.
Safety results from this study, balanced by weight loss and health outcomes data, will “help inform procedure-specific decision making for prospective patients and physicians,” said Dr. Courcoulas and coauthors of the report, which appears in JAMA Surgery.
The study included 33,560 adults who had undergone the Roux-en-Y procedure or sleeve gastrectomy, making it one of the largest bariatric cohort studies ever to be done, according to the investigators.
The majority of patients in the study (54%) underwent Roux-en-Y gastric bypass, though the number of sleeve gastrectomy procedures increased each year in the study, which included patients who underwent a primary bariatric surgery procedure between January 2005 and September 2015 at 1 of 10 sites in the National Patient-Centered Clinical Research Network.
Most of the patients were female (80%) and white (66%), and 26% were Hispanic, according to the researchers, who said their study was more racially diverse than other bariatric studies, and therefore potentially more generalizable to real-world clinical practice.
Over the 5-year follow-up period, patients who underwent sleeve gastrectomy were less likely to subsequently undergo operations involving the abdomen, or interventions for enteral access, such as placement of gastrostomy tubes, according to the investigators, who reported a hazard ratio (HR) of 0.72 (95% confidence interval [CI], 0.65-0.79; P less than .001).
The estimated cumulative rate of operations or interventions at 5 years was 8.94% (95% CI, 8.23%-9.65%) for the patients who underwent sleeve gastrectomy, compared with 12.27% (95% CI, 11.49%-13.05%) for those who underwent the Roux-en-Y procedure, according to the report.
Hospitalization was also less likely for sleeve gastrectomy versus Roux-en-Y gastric bypass, with a hazard ratio of 0.82 and estimated cumulative incidence rates of 32.79% and 38.33%, respectively. Likewise, endoscopy was less likely in the sleeve gastrectomy group.
All-cause mortality did not differ between the groups at this 5-year follow-up, the investigators said.
“The present data were gathered from clinical care in the real world, yet the results are comparable to controlled studies and therefore lend additional support to the findings of these other types of studies that operation and intervention occur less commonly after sleeve gastrectomy than after Roux-en-Y gastric bypass for up to 5 years,” Dr. Courcoulas and coauthors noted in a discussion of their results.
This work was funded by the Patient-Centered Outcomes Research Institute. Dr. Courcoulas reported receiving a grant from Allurion. Coauthors provided disclosures related to the National Institutes of Health, IFSO Latin America Chapter, and the Food and Drug Administration.
*This story was updated on January 16, 2020.
SOURCE: Courcoulas A et al. JAMA Surg. 2020 Jan 15. doi: 10.1001/jamasurg.2019.5470.
FROM JAMA SURGERY
Synaptic pruning deficits may cause tremor in essential tremor
according to an investigation published January 15 in Science Translational Medicine. These synaptic pruning deficits result from insufficiency of glutamate receptor delta 2 (GluR[delta]2) protein. The findings indicate molecular, structural, physiological, and behavioral factors that contribute to tremor and might influence future treatment of essential tremor, the authors wrote.

Essential tremor has a complex etiology that includes genetic and environmental factors. Its pathophysiology is poorly understood. First author Ming-Kai Pan, MD, assistant professor of medical research and neurology at National Taiwan University Hospital in Taipei, and colleagues previously observed pruning deficits of CF-PC synapses in the cerebellum of deceased patients with essential tremor. An excess of CF-PC synapses are a prominent feature of essential tremor, but not of other cerebellar degenerative disorders. Researchers have observed this pathology consistently in patients with essential tremor who have diverse clinical features. Dr. Pan and colleagues therefore chose to examine these synaptic changes to clarify the pathophysiology of essential tremor.
Patients had more CF synapses than did controls
The investigators performed a pathological examination of postmortem cerebellar tissue from patients with essential tremor and controls to identify microstructural changes in essential tremor. Next, they applied these changes to mouse models of essential tremor and examined the corresponding structural, electrophysiologic, and behavioral changes. Finally, Dr. Pan and colleagues used cerebellar EEG to validate their findings in patients with essential tremor.
Compared with age-matched controls, patients with essential tremor had more CF synapses in the parallel-fiber synaptic territory on PC dendrites. Patients also had an approximately 75% reduction in mean GluR(delta)2 expression, compared with controls. The amount of GluR(delta)2 was inversely correlated with the percentage of CFs extending to parallel-fiber synaptic territory. The findings suggest that PC synaptic pathology in essential tremor might be related to reduced GluR(delta)2 expression, Dr. Pan and colleagues wrote.
The investigators examined a mouse model that produces 10% of full-length GluR(delta)2 protein. These mice had significant reduction of GluR(delta)2 in the cerebellar cortex and the PC dendrites. In addition, the mice consistently developed CF synapses innervating distal, thin PC dendrites. The investigators observed a 20-Hz tremor in the mice that occurred mainly during action and rarely during rest.
Dr. Pan and colleagues injected a virus containing GluR(delta)2 protein into the mice’s brains to test the protein’s relationship to tremor. Five days after the injection, the mice’s brains were expressing GluR(delta)2 protein reliably. By 4-6 days after injection, the mice’s tremor had been reduced. It returned to baseline levels at 12-14 days after injection. Injecting a control virus did not affect tremor.
Cerebellar oscillatory indexes were correlated with tremor scores
When the researchers examined local field potentials in mouse cerebellum, they found cerebellar oscillations at 20 Hz that were consistent with the observed tremor. “Putting the evidence together, GluR(delta)2 insufficiency causes CF synaptic pruning deficits, and the surplus CF-PC synaptic activity generates excessive cerebellar oscillations, which drive tremor,” Dr. Pan and colleagues reported.
Next, the researchers performed cerebellar EEG in 10 patients with essential tremor and 10 age-matched controls. Patients had cerebellar oscillations at 4-12 Hz, which are the human tremor frequencies. In an expanded cohort of 20 patients with essential tremor and 20 controls, the cerebellar oscillatory indexes were correlated with tremor scores in patients, which showed that the former could be an index of tremor severity. “Currently, diagnosis of essential tremor is based on pure clinical tremor phenomenology and direct tremor measurement, without a physiological marker indicating the underlying brain circuitry abnormalities,” they wrote. “Cerebellar oscillations can be a physiological signature and a therapeutic target for essential tremor.”
The research was funded by grants from the National Institutes of Health, the Parkinson’s Foundation, the International Essential Tremor Foundation, the Ministry of Science and Technology in Taiwan, and the National Taiwan University Hospital. The authors declared that they had no competing interests.
SOURCE: Pan M-K et al. Sci Transl Med. 2020;12:eaay1769. doi: 10.1126/scitranslmed.aay1769.
according to an investigation published January 15 in Science Translational Medicine. These synaptic pruning deficits result from insufficiency of glutamate receptor delta 2 (GluR[delta]2) protein. The findings indicate molecular, structural, physiological, and behavioral factors that contribute to tremor and might influence future treatment of essential tremor, the authors wrote.

Essential tremor has a complex etiology that includes genetic and environmental factors. Its pathophysiology is poorly understood. First author Ming-Kai Pan, MD, assistant professor of medical research and neurology at National Taiwan University Hospital in Taipei, and colleagues previously observed pruning deficits of CF-PC synapses in the cerebellum of deceased patients with essential tremor. An excess of CF-PC synapses are a prominent feature of essential tremor, but not of other cerebellar degenerative disorders. Researchers have observed this pathology consistently in patients with essential tremor who have diverse clinical features. Dr. Pan and colleagues therefore chose to examine these synaptic changes to clarify the pathophysiology of essential tremor.
Patients had more CF synapses than did controls
The investigators performed a pathological examination of postmortem cerebellar tissue from patients with essential tremor and controls to identify microstructural changes in essential tremor. Next, they applied these changes to mouse models of essential tremor and examined the corresponding structural, electrophysiologic, and behavioral changes. Finally, Dr. Pan and colleagues used cerebellar EEG to validate their findings in patients with essential tremor.
Compared with age-matched controls, patients with essential tremor had more CF synapses in the parallel-fiber synaptic territory on PC dendrites. Patients also had an approximately 75% reduction in mean GluR(delta)2 expression, compared with controls. The amount of GluR(delta)2 was inversely correlated with the percentage of CFs extending to parallel-fiber synaptic territory. The findings suggest that PC synaptic pathology in essential tremor might be related to reduced GluR(delta)2 expression, Dr. Pan and colleagues wrote.
The investigators examined a mouse model that produces 10% of full-length GluR(delta)2 protein. These mice had significant reduction of GluR(delta)2 in the cerebellar cortex and the PC dendrites. In addition, the mice consistently developed CF synapses innervating distal, thin PC dendrites. The investigators observed a 20-Hz tremor in the mice that occurred mainly during action and rarely during rest.
Dr. Pan and colleagues injected a virus containing GluR(delta)2 protein into the mice’s brains to test the protein’s relationship to tremor. Five days after the injection, the mice’s brains were expressing GluR(delta)2 protein reliably. By 4-6 days after injection, the mice’s tremor had been reduced. It returned to baseline levels at 12-14 days after injection. Injecting a control virus did not affect tremor.
Cerebellar oscillatory indexes were correlated with tremor scores
When the researchers examined local field potentials in mouse cerebellum, they found cerebellar oscillations at 20 Hz that were consistent with the observed tremor. “Putting the evidence together, GluR(delta)2 insufficiency causes CF synaptic pruning deficits, and the surplus CF-PC synaptic activity generates excessive cerebellar oscillations, which drive tremor,” Dr. Pan and colleagues reported.
Next, the researchers performed cerebellar EEG in 10 patients with essential tremor and 10 age-matched controls. Patients had cerebellar oscillations at 4-12 Hz, which are the human tremor frequencies. In an expanded cohort of 20 patients with essential tremor and 20 controls, the cerebellar oscillatory indexes were correlated with tremor scores in patients, which showed that the former could be an index of tremor severity. “Currently, diagnosis of essential tremor is based on pure clinical tremor phenomenology and direct tremor measurement, without a physiological marker indicating the underlying brain circuitry abnormalities,” they wrote. “Cerebellar oscillations can be a physiological signature and a therapeutic target for essential tremor.”
The research was funded by grants from the National Institutes of Health, the Parkinson’s Foundation, the International Essential Tremor Foundation, the Ministry of Science and Technology in Taiwan, and the National Taiwan University Hospital. The authors declared that they had no competing interests.
SOURCE: Pan M-K et al. Sci Transl Med. 2020;12:eaay1769. doi: 10.1126/scitranslmed.aay1769.
according to an investigation published January 15 in Science Translational Medicine. These synaptic pruning deficits result from insufficiency of glutamate receptor delta 2 (GluR[delta]2) protein. The findings indicate molecular, structural, physiological, and behavioral factors that contribute to tremor and might influence future treatment of essential tremor, the authors wrote.

Essential tremor has a complex etiology that includes genetic and environmental factors. Its pathophysiology is poorly understood. First author Ming-Kai Pan, MD, assistant professor of medical research and neurology at National Taiwan University Hospital in Taipei, and colleagues previously observed pruning deficits of CF-PC synapses in the cerebellum of deceased patients with essential tremor. An excess of CF-PC synapses are a prominent feature of essential tremor, but not of other cerebellar degenerative disorders. Researchers have observed this pathology consistently in patients with essential tremor who have diverse clinical features. Dr. Pan and colleagues therefore chose to examine these synaptic changes to clarify the pathophysiology of essential tremor.
Patients had more CF synapses than did controls
The investigators performed a pathological examination of postmortem cerebellar tissue from patients with essential tremor and controls to identify microstructural changes in essential tremor. Next, they applied these changes to mouse models of essential tremor and examined the corresponding structural, electrophysiologic, and behavioral changes. Finally, Dr. Pan and colleagues used cerebellar EEG to validate their findings in patients with essential tremor.
Compared with age-matched controls, patients with essential tremor had more CF synapses in the parallel-fiber synaptic territory on PC dendrites. Patients also had an approximately 75% reduction in mean GluR(delta)2 expression, compared with controls. The amount of GluR(delta)2 was inversely correlated with the percentage of CFs extending to parallel-fiber synaptic territory. The findings suggest that PC synaptic pathology in essential tremor might be related to reduced GluR(delta)2 expression, Dr. Pan and colleagues wrote.
The investigators examined a mouse model that produces 10% of full-length GluR(delta)2 protein. These mice had significant reduction of GluR(delta)2 in the cerebellar cortex and the PC dendrites. In addition, the mice consistently developed CF synapses innervating distal, thin PC dendrites. The investigators observed a 20-Hz tremor in the mice that occurred mainly during action and rarely during rest.
Dr. Pan and colleagues injected a virus containing GluR(delta)2 protein into the mice’s brains to test the protein’s relationship to tremor. Five days after the injection, the mice’s brains were expressing GluR(delta)2 protein reliably. By 4-6 days after injection, the mice’s tremor had been reduced. It returned to baseline levels at 12-14 days after injection. Injecting a control virus did not affect tremor.
Cerebellar oscillatory indexes were correlated with tremor scores
When the researchers examined local field potentials in mouse cerebellum, they found cerebellar oscillations at 20 Hz that were consistent with the observed tremor. “Putting the evidence together, GluR(delta)2 insufficiency causes CF synaptic pruning deficits, and the surplus CF-PC synaptic activity generates excessive cerebellar oscillations, which drive tremor,” Dr. Pan and colleagues reported.
Next, the researchers performed cerebellar EEG in 10 patients with essential tremor and 10 age-matched controls. Patients had cerebellar oscillations at 4-12 Hz, which are the human tremor frequencies. In an expanded cohort of 20 patients with essential tremor and 20 controls, the cerebellar oscillatory indexes were correlated with tremor scores in patients, which showed that the former could be an index of tremor severity. “Currently, diagnosis of essential tremor is based on pure clinical tremor phenomenology and direct tremor measurement, without a physiological marker indicating the underlying brain circuitry abnormalities,” they wrote. “Cerebellar oscillations can be a physiological signature and a therapeutic target for essential tremor.”
The research was funded by grants from the National Institutes of Health, the Parkinson’s Foundation, the International Essential Tremor Foundation, the Ministry of Science and Technology in Taiwan, and the National Taiwan University Hospital. The authors declared that they had no competing interests.
SOURCE: Pan M-K et al. Sci Transl Med. 2020;12:eaay1769. doi: 10.1126/scitranslmed.aay1769.
FROM SCIENCE TRANSLATIONAL MEDICINE
Sleep apnea’s got your tongue, and singin’ in the kerosene rain
On the tip of my tongue
The greatest risk factor for obstructive sleep apnea is obesity, and unsurprisingly, people who are obese and have sleep apnea very often improve their breathing when they lose weight.
But what if you’re secretly a hobbit named Peregrin Took, and you absolutely have to have both first and second breakfast? Is there any way to ease your sleep apnea?
According to a study published in the American Journal of Respiratory and Critical Care Medicine, weight loss in and of itself isn’t what improves sleep apnea symptoms. No, it’s something more targeted.
The secret to improving sleep apnea is ... tongue fat.
The patients in the study lost about 10% of their body weight over 6 months, and experienced a 31% improvement in sleep apnea scores. MRIs done before and after the intervention showed that, while reductions in pterygoid and pharyngeal lateral wall volumes helped, the reduction of tongue fat volume was the primary link between weight loss and sleep apnea improvement.
The Livin’ on the MDedge team eagerly awaits the dawning of the tongue weight-loss industry, thanks to this new research. Tongue diets. Tongue exercise. Pretty soon you’ll be able to buy “Sweatin’ to the Oldies” DVDs featuring tongues in bad ’80s Spandex flopping all over the place. Have your cake and eat it too – just don’t let your tongue know.
The rain falls mainly from the plane
Why does rain inspire music? Gene Kelly sang in it. Prince crooned about its purple hue. The Weather Girls gave vocal thanks for a downpour of men. And kids will joyfully create a symphony of mud in a summer shower.
But what if it rains on the playground? On a sunny day? Kids will definitely sing the blues, right?
Especially when the shower’s not water.
Shortly after takeoff from Los Angeles International Airport this week, Delta Air Lines Flight 89 to Shanghai developed engine problems. Which sent Flight 89 right back to LAX. Not wanting to land a distressed Boeing 777 with wings full of explosive aviation fuel, the pilot began dumping his Jet A-1 kerosene as he circled back to the airport.
Which fell to earth as a mist ... that blanketed five elementary schools in the middle of the school day.
The plane rain led to minor lung and skin irritation in 56 kids and adults below. But the Los Angeles County Fire Department said injuries were minor, and the drizzling jet fuel evaporated quickly.
Given the absence of serious injuries, it’s clear the Los Angeles students heeded at least one public health message during the kerosene shower: Nobody was engaged in outdoor underage smoking.
And the Inventing Oscar goes to ...
For many people, the new year means the announcement of the Oscar nominations.
We here at LOTME have been waiting for an announcement that comes at the beginning of each year, but it has nothing to do with who got snubbed by the Academy. We’re talking about something really big: the National Inventors Hall of Fame class of 2020.
As usual, we were not disappointed. The world of health care was well represented among this year’s inductees.
At the head of the class, at least alphabetically, is R. Rox Anderson, who developed groundbreaking laser technology (patent number 5,595,568) used in medical treatments and procedures. Then there’s James McEwen, who invented the first microprocessor-controlled automatic surgical tourniquet system (patent number 4,469,099).
Posthumous NIHF nominations went to Stewart Adams and John Nicholson, the codevelopers of 2-(4-isobutylphenyl) propionic acid, which we know as ibuprofen (patent number 3,228,831). Adams, a pharmacologist, and Nicholson, an organic chemist, worked for Boots Pure Drug Co. in England during the 1950s and 1960s while they collaborated on the drug’s creation.
Several other nominees have somewhat-less-direct medical connections. Edward W. Bullard invented the hard hat (patent number 1,699,133), which has undoubtedly saved lives and prevented injuries. Lisa Lindahl, Hinda Miller, and Polly Smith invented the sports bra (patent number 4,174,717), which “has enabled women’s participation in athletic activities and advanced women’s health and well-being,” the NIHF said in a written statement.
And finally – for those of you who thought this would never end – there’s Floyd Smith, the trapeze artist turned aviator who invented the modern parachute (patent numbers 1,340,423 and 1,462,456) and kept many sky divers out of the emergency department.

On the tip of my tongue
The greatest risk factor for obstructive sleep apnea is obesity, and unsurprisingly, people who are obese and have sleep apnea very often improve their breathing when they lose weight.
But what if you’re secretly a hobbit named Peregrin Took, and you absolutely have to have both first and second breakfast? Is there any way to ease your sleep apnea?
According to a study published in the American Journal of Respiratory and Critical Care Medicine, weight loss in and of itself isn’t what improves sleep apnea symptoms. No, it’s something more targeted.
The secret to improving sleep apnea is ... tongue fat.
The patients in the study lost about 10% of their body weight over 6 months, and experienced a 31% improvement in sleep apnea scores. MRIs done before and after the intervention showed that, while reductions in pterygoid and pharyngeal lateral wall volumes helped, the reduction of tongue fat volume was the primary link between weight loss and sleep apnea improvement.
The Livin’ on the MDedge team eagerly awaits the dawning of the tongue weight-loss industry, thanks to this new research. Tongue diets. Tongue exercise. Pretty soon you’ll be able to buy “Sweatin’ to the Oldies” DVDs featuring tongues in bad ’80s Spandex flopping all over the place. Have your cake and eat it too – just don’t let your tongue know.
The rain falls mainly from the plane
Why does rain inspire music? Gene Kelly sang in it. Prince crooned about its purple hue. The Weather Girls gave vocal thanks for a downpour of men. And kids will joyfully create a symphony of mud in a summer shower.
But what if it rains on the playground? On a sunny day? Kids will definitely sing the blues, right?
Especially when the shower’s not water.
Shortly after takeoff from Los Angeles International Airport this week, Delta Air Lines Flight 89 to Shanghai developed engine problems. Which sent Flight 89 right back to LAX. Not wanting to land a distressed Boeing 777 with wings full of explosive aviation fuel, the pilot began dumping his Jet A-1 kerosene as he circled back to the airport.
Which fell to earth as a mist ... that blanketed five elementary schools in the middle of the school day.
The plane rain led to minor lung and skin irritation in 56 kids and adults below. But the Los Angeles County Fire Department said injuries were minor, and the drizzling jet fuel evaporated quickly.
Given the absence of serious injuries, it’s clear the Los Angeles students heeded at least one public health message during the kerosene shower: Nobody was engaged in outdoor underage smoking.
And the Inventing Oscar goes to ...
For many people, the new year means the announcement of the Oscar nominations.
We here at LOTME have been waiting for an announcement that comes at the beginning of each year, but it has nothing to do with who got snubbed by the Academy. We’re talking about something really big: the National Inventors Hall of Fame class of 2020.
As usual, we were not disappointed. The world of health care was well represented among this year’s inductees.
At the head of the class, at least alphabetically, is R. Rox Anderson, who developed groundbreaking laser technology (patent number 5,595,568) used in medical treatments and procedures. Then there’s James McEwen, who invented the first microprocessor-controlled automatic surgical tourniquet system (patent number 4,469,099).
Posthumous NIHF nominations went to Stewart Adams and John Nicholson, the codevelopers of 2-(4-isobutylphenyl) propionic acid, which we know as ibuprofen (patent number 3,228,831). Adams, a pharmacologist, and Nicholson, an organic chemist, worked for Boots Pure Drug Co. in England during the 1950s and 1960s while they collaborated on the drug’s creation.
Several other nominees have somewhat-less-direct medical connections. Edward W. Bullard invented the hard hat (patent number 1,699,133), which has undoubtedly saved lives and prevented injuries. Lisa Lindahl, Hinda Miller, and Polly Smith invented the sports bra (patent number 4,174,717), which “has enabled women’s participation in athletic activities and advanced women’s health and well-being,” the NIHF said in a written statement.
And finally – for those of you who thought this would never end – there’s Floyd Smith, the trapeze artist turned aviator who invented the modern parachute (patent numbers 1,340,423 and 1,462,456) and kept many sky divers out of the emergency department.

On the tip of my tongue
The greatest risk factor for obstructive sleep apnea is obesity, and unsurprisingly, people who are obese and have sleep apnea very often improve their breathing when they lose weight.
But what if you’re secretly a hobbit named Peregrin Took, and you absolutely have to have both first and second breakfast? Is there any way to ease your sleep apnea?
According to a study published in the American Journal of Respiratory and Critical Care Medicine, weight loss in and of itself isn’t what improves sleep apnea symptoms. No, it’s something more targeted.
The secret to improving sleep apnea is ... tongue fat.
The patients in the study lost about 10% of their body weight over 6 months, and experienced a 31% improvement in sleep apnea scores. MRIs done before and after the intervention showed that, while reductions in pterygoid and pharyngeal lateral wall volumes helped, the reduction of tongue fat volume was the primary link between weight loss and sleep apnea improvement.
The Livin’ on the MDedge team eagerly awaits the dawning of the tongue weight-loss industry, thanks to this new research. Tongue diets. Tongue exercise. Pretty soon you’ll be able to buy “Sweatin’ to the Oldies” DVDs featuring tongues in bad ’80s Spandex flopping all over the place. Have your cake and eat it too – just don’t let your tongue know.
The rain falls mainly from the plane
Why does rain inspire music? Gene Kelly sang in it. Prince crooned about its purple hue. The Weather Girls gave vocal thanks for a downpour of men. And kids will joyfully create a symphony of mud in a summer shower.
But what if it rains on the playground? On a sunny day? Kids will definitely sing the blues, right?
Especially when the shower’s not water.
Shortly after takeoff from Los Angeles International Airport this week, Delta Air Lines Flight 89 to Shanghai developed engine problems. Which sent Flight 89 right back to LAX. Not wanting to land a distressed Boeing 777 with wings full of explosive aviation fuel, the pilot began dumping his Jet A-1 kerosene as he circled back to the airport.
Which fell to earth as a mist ... that blanketed five elementary schools in the middle of the school day.
The plane rain led to minor lung and skin irritation in 56 kids and adults below. But the Los Angeles County Fire Department said injuries were minor, and the drizzling jet fuel evaporated quickly.
Given the absence of serious injuries, it’s clear the Los Angeles students heeded at least one public health message during the kerosene shower: Nobody was engaged in outdoor underage smoking.
And the Inventing Oscar goes to ...
For many people, the new year means the announcement of the Oscar nominations.
We here at LOTME have been waiting for an announcement that comes at the beginning of each year, but it has nothing to do with who got snubbed by the Academy. We’re talking about something really big: the National Inventors Hall of Fame class of 2020.
As usual, we were not disappointed. The world of health care was well represented among this year’s inductees.
At the head of the class, at least alphabetically, is R. Rox Anderson, who developed groundbreaking laser technology (patent number 5,595,568) used in medical treatments and procedures. Then there’s James McEwen, who invented the first microprocessor-controlled automatic surgical tourniquet system (patent number 4,469,099).
Posthumous NIHF nominations went to Stewart Adams and John Nicholson, the codevelopers of 2-(4-isobutylphenyl) propionic acid, which we know as ibuprofen (patent number 3,228,831). Adams, a pharmacologist, and Nicholson, an organic chemist, worked for Boots Pure Drug Co. in England during the 1950s and 1960s while they collaborated on the drug’s creation.
Several other nominees have somewhat-less-direct medical connections. Edward W. Bullard invented the hard hat (patent number 1,699,133), which has undoubtedly saved lives and prevented injuries. Lisa Lindahl, Hinda Miller, and Polly Smith invented the sports bra (patent number 4,174,717), which “has enabled women’s participation in athletic activities and advanced women’s health and well-being,” the NIHF said in a written statement.
And finally – for those of you who thought this would never end – there’s Floyd Smith, the trapeze artist turned aviator who invented the modern parachute (patent numbers 1,340,423 and 1,462,456) and kept many sky divers out of the emergency department.

Bariatric surgery is most effective early in the diabetes trajectory
LOS ANGELES – In the clinical experience of Kurt GMM Alberti, DPhil, FRCP, FRCPath, The problem is, far fewer people with diabetes are being referred for the surgery than would benefit from it.
At the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease, Dr. Alberti said that only about 1% of eligible patients in the United States undergo bariatric surgery, compared with just 0.22% of eligible patients in the United Kingdom.
“Obesity is an increasing problem,” said Dr. Alberti, a senior research investigator in the section of diabetes, endocrinology, and metabolism at Imperial College, London. “The United States is leading the field [in obesity], and it doesn’t seem to be going away. This is in parallel with diabetes. According to the 2019 International Diabetes Federation’s Diabetes Atlas, the diabetes prevalence worldwide is now 463 million. It’s projected to reach 578 million by 2030 and 700 million by 2045. We have a major problem.”
Lifestyle modification with diet and exercise have been the cornerstone of diabetes therapy for more than 100 years, with only modest success. “A range of oral agents have been added [and they] certainly improve glycemic control, but few achieve lasting success, and few achieve remission,” Dr. Alberti said. Findings from DiRECT (Lancet. 2018;391:541-51) and the Look AHEAD (Action for Health in Diabetes) study (N Engl J Med. 2013;369:145-54) have shown dramatic improvements in glycemia, but only in the minority of patients who achieved weight loss of 10 kg or more. “In this group, 80% achieved remission after 2 years in DiRECT,” he said. “It is uncertain whether this can be sustained long term in real life, and how many people will respond. Major community prevention programs are under way in the U.S. and [United Kingdom], but many target individuals with prediabetes. Currently, it is unknown how successful these programs will be.”
The 2018 American Diabetes Association/European Association for the Study of Diabetes clinical guidelines state that bariatric surgery is a recommended treatment option for adults with type 2 diabetes and a body mass index of either 40.0 kg/m2 or higher (or 37.5 kg/m2 or higher in people of Asian ancestry) or 35.0-39.9 kg/m2 (32.5-37.4 kg/m2 in people of Asian ancestry) and who do not achieve durable weight loss and improvement in comorbidities with reasonable nonsurgical methods. According to Dr. Alberti, surgery should be an accepted option in people who have type 2 diabetes and a body mass index of 30 kg/m2 or higher, and priority should be given to adolescents, young adults, and those with a shorter duration of diabetes.
The main suggestive evidence in favor of bariatric surgery having an impact on diabetes comes from the Swedish Obese Subjects Study, which had a median follow-up of 10 years (J Intern Med. 2013;273[3]:219-34). In patients with diabetes at baseline, 30% were still in remission at 15 years after surgery. After 18 years, cumulative microvascular disease had fallen from 42 to 21 per 1,000 person-years, and macrovascular complications had fallen by 25%. “This was not a randomized, controlled trial, however,” Dr. Alberti said. Several of these studies have now been performed, including the STAMPEDE trial (N Engl J Med. 2017;376:641-51) and recent studies led by Geltrude Mingrone, MD, PhD, which show major glycemic benefit (Lancet. 2015;386[9997]:P964-73) with bariatric surgery.
According to Dr. Alberti, laparoscopic adjustable gastric lap band is the easiest bariatric surgery to perform but it has fallen out of favor because of lower diabetes remission rates, compared with the other procedures. Roux-en-Y gastric bypass, sleeve gastrectomy, and biliary pancreatic diversion are all in use. Slightly higher remission rates have been shown with biliary pancreatic diversion. “There have also been consistent improvements in quality of life and cardiovascular risk factors,” he said. “Long-term follow-up is still [needed], but, in general, it seems that diabetic complications are lower than in medically treated control groups, and mortality may also be lower. The real question is, what is the impact on complications? There we have a problem, because we do not have any good randomized, controlled trials covering a 10- to 20-year period, which would give us clear evidence. There is reasonable evidence from cohort studies, though.”
Added benefits of bariatric surgery include improved quality of life, decreased blood pressure, less sleep apnea, improved cardiovascular risk factors, and better musculoskeletal function. For now, though, an unmet need persists. “The problem is that far fewer people are referred for bariatric surgery than would benefit,” Dr. Alberti said. “There are several barriers to greater use of bariatric surgery in those with diabetes. These include physician attitudes, inadequate referrals, patient perceptions, lack of awareness among patients, inadequate insurance coverage, and particularly health system capacity. There is also a lack of sympathy for overweight people in some places.”
Dr. Alberti reported having no financial disclosures.
LOS ANGELES – In the clinical experience of Kurt GMM Alberti, DPhil, FRCP, FRCPath, The problem is, far fewer people with diabetes are being referred for the surgery than would benefit from it.
At the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease, Dr. Alberti said that only about 1% of eligible patients in the United States undergo bariatric surgery, compared with just 0.22% of eligible patients in the United Kingdom.
“Obesity is an increasing problem,” said Dr. Alberti, a senior research investigator in the section of diabetes, endocrinology, and metabolism at Imperial College, London. “The United States is leading the field [in obesity], and it doesn’t seem to be going away. This is in parallel with diabetes. According to the 2019 International Diabetes Federation’s Diabetes Atlas, the diabetes prevalence worldwide is now 463 million. It’s projected to reach 578 million by 2030 and 700 million by 2045. We have a major problem.”
Lifestyle modification with diet and exercise have been the cornerstone of diabetes therapy for more than 100 years, with only modest success. “A range of oral agents have been added [and they] certainly improve glycemic control, but few achieve lasting success, and few achieve remission,” Dr. Alberti said. Findings from DiRECT (Lancet. 2018;391:541-51) and the Look AHEAD (Action for Health in Diabetes) study (N Engl J Med. 2013;369:145-54) have shown dramatic improvements in glycemia, but only in the minority of patients who achieved weight loss of 10 kg or more. “In this group, 80% achieved remission after 2 years in DiRECT,” he said. “It is uncertain whether this can be sustained long term in real life, and how many people will respond. Major community prevention programs are under way in the U.S. and [United Kingdom], but many target individuals with prediabetes. Currently, it is unknown how successful these programs will be.”
The 2018 American Diabetes Association/European Association for the Study of Diabetes clinical guidelines state that bariatric surgery is a recommended treatment option for adults with type 2 diabetes and a body mass index of either 40.0 kg/m2 or higher (or 37.5 kg/m2 or higher in people of Asian ancestry) or 35.0-39.9 kg/m2 (32.5-37.4 kg/m2 in people of Asian ancestry) and who do not achieve durable weight loss and improvement in comorbidities with reasonable nonsurgical methods. According to Dr. Alberti, surgery should be an accepted option in people who have type 2 diabetes and a body mass index of 30 kg/m2 or higher, and priority should be given to adolescents, young adults, and those with a shorter duration of diabetes.
The main suggestive evidence in favor of bariatric surgery having an impact on diabetes comes from the Swedish Obese Subjects Study, which had a median follow-up of 10 years (J Intern Med. 2013;273[3]:219-34). In patients with diabetes at baseline, 30% were still in remission at 15 years after surgery. After 18 years, cumulative microvascular disease had fallen from 42 to 21 per 1,000 person-years, and macrovascular complications had fallen by 25%. “This was not a randomized, controlled trial, however,” Dr. Alberti said. Several of these studies have now been performed, including the STAMPEDE trial (N Engl J Med. 2017;376:641-51) and recent studies led by Geltrude Mingrone, MD, PhD, which show major glycemic benefit (Lancet. 2015;386[9997]:P964-73) with bariatric surgery.
According to Dr. Alberti, laparoscopic adjustable gastric lap band is the easiest bariatric surgery to perform but it has fallen out of favor because of lower diabetes remission rates, compared with the other procedures. Roux-en-Y gastric bypass, sleeve gastrectomy, and biliary pancreatic diversion are all in use. Slightly higher remission rates have been shown with biliary pancreatic diversion. “There have also been consistent improvements in quality of life and cardiovascular risk factors,” he said. “Long-term follow-up is still [needed], but, in general, it seems that diabetic complications are lower than in medically treated control groups, and mortality may also be lower. The real question is, what is the impact on complications? There we have a problem, because we do not have any good randomized, controlled trials covering a 10- to 20-year period, which would give us clear evidence. There is reasonable evidence from cohort studies, though.”
Added benefits of bariatric surgery include improved quality of life, decreased blood pressure, less sleep apnea, improved cardiovascular risk factors, and better musculoskeletal function. For now, though, an unmet need persists. “The problem is that far fewer people are referred for bariatric surgery than would benefit,” Dr. Alberti said. “There are several barriers to greater use of bariatric surgery in those with diabetes. These include physician attitudes, inadequate referrals, patient perceptions, lack of awareness among patients, inadequate insurance coverage, and particularly health system capacity. There is also a lack of sympathy for overweight people in some places.”
Dr. Alberti reported having no financial disclosures.
LOS ANGELES – In the clinical experience of Kurt GMM Alberti, DPhil, FRCP, FRCPath, The problem is, far fewer people with diabetes are being referred for the surgery than would benefit from it.
At the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease, Dr. Alberti said that only about 1% of eligible patients in the United States undergo bariatric surgery, compared with just 0.22% of eligible patients in the United Kingdom.
“Obesity is an increasing problem,” said Dr. Alberti, a senior research investigator in the section of diabetes, endocrinology, and metabolism at Imperial College, London. “The United States is leading the field [in obesity], and it doesn’t seem to be going away. This is in parallel with diabetes. According to the 2019 International Diabetes Federation’s Diabetes Atlas, the diabetes prevalence worldwide is now 463 million. It’s projected to reach 578 million by 2030 and 700 million by 2045. We have a major problem.”
Lifestyle modification with diet and exercise have been the cornerstone of diabetes therapy for more than 100 years, with only modest success. “A range of oral agents have been added [and they] certainly improve glycemic control, but few achieve lasting success, and few achieve remission,” Dr. Alberti said. Findings from DiRECT (Lancet. 2018;391:541-51) and the Look AHEAD (Action for Health in Diabetes) study (N Engl J Med. 2013;369:145-54) have shown dramatic improvements in glycemia, but only in the minority of patients who achieved weight loss of 10 kg or more. “In this group, 80% achieved remission after 2 years in DiRECT,” he said. “It is uncertain whether this can be sustained long term in real life, and how many people will respond. Major community prevention programs are under way in the U.S. and [United Kingdom], but many target individuals with prediabetes. Currently, it is unknown how successful these programs will be.”
The 2018 American Diabetes Association/European Association for the Study of Diabetes clinical guidelines state that bariatric surgery is a recommended treatment option for adults with type 2 diabetes and a body mass index of either 40.0 kg/m2 or higher (or 37.5 kg/m2 or higher in people of Asian ancestry) or 35.0-39.9 kg/m2 (32.5-37.4 kg/m2 in people of Asian ancestry) and who do not achieve durable weight loss and improvement in comorbidities with reasonable nonsurgical methods. According to Dr. Alberti, surgery should be an accepted option in people who have type 2 diabetes and a body mass index of 30 kg/m2 or higher, and priority should be given to adolescents, young adults, and those with a shorter duration of diabetes.
The main suggestive evidence in favor of bariatric surgery having an impact on diabetes comes from the Swedish Obese Subjects Study, which had a median follow-up of 10 years (J Intern Med. 2013;273[3]:219-34). In patients with diabetes at baseline, 30% were still in remission at 15 years after surgery. After 18 years, cumulative microvascular disease had fallen from 42 to 21 per 1,000 person-years, and macrovascular complications had fallen by 25%. “This was not a randomized, controlled trial, however,” Dr. Alberti said. Several of these studies have now been performed, including the STAMPEDE trial (N Engl J Med. 2017;376:641-51) and recent studies led by Geltrude Mingrone, MD, PhD, which show major glycemic benefit (Lancet. 2015;386[9997]:P964-73) with bariatric surgery.
According to Dr. Alberti, laparoscopic adjustable gastric lap band is the easiest bariatric surgery to perform but it has fallen out of favor because of lower diabetes remission rates, compared with the other procedures. Roux-en-Y gastric bypass, sleeve gastrectomy, and biliary pancreatic diversion are all in use. Slightly higher remission rates have been shown with biliary pancreatic diversion. “There have also been consistent improvements in quality of life and cardiovascular risk factors,” he said. “Long-term follow-up is still [needed], but, in general, it seems that diabetic complications are lower than in medically treated control groups, and mortality may also be lower. The real question is, what is the impact on complications? There we have a problem, because we do not have any good randomized, controlled trials covering a 10- to 20-year period, which would give us clear evidence. There is reasonable evidence from cohort studies, though.”
Added benefits of bariatric surgery include improved quality of life, decreased blood pressure, less sleep apnea, improved cardiovascular risk factors, and better musculoskeletal function. For now, though, an unmet need persists. “The problem is that far fewer people are referred for bariatric surgery than would benefit,” Dr. Alberti said. “There are several barriers to greater use of bariatric surgery in those with diabetes. These include physician attitudes, inadequate referrals, patient perceptions, lack of awareness among patients, inadequate insurance coverage, and particularly health system capacity. There is also a lack of sympathy for overweight people in some places.”
Dr. Alberti reported having no financial disclosures.
EXPERT ANALYSIS FROM WCIRDC 2019
Administrative burden and burnout
In May 2019, SHM sent a letter to U.S. Senators Tina Smith and Bill Cassidy in support of the Reducing Administrative Costs and Burdens in Health Care Act of 2019. In excerpts from the letter below, the society details the link between administrative burdens and physician burnout.
Providers and hospital systems expend countless resources, both time and dollars, adhering to unnecessary and excessive administrative burdens instead of investing those resources in providing quality patient care. National data suggests that more than 50 percent of the physician workforce is burned out. Excessive administrative burden is a major contributor to physician burnout, which negatively affects quality and safety within the hospital and further increases health care costs. Notably, the Reducing Administrative Costs and Burdens in Health Care Act calls for a 50% reduction of unnecessary administrative costs from the Department of Health and Human Services within the next ten years.
Hospitalists are front-line clinicians in America's acute care hospitals whose professional focus is the general medical care of hospitalized patients. Their unique position in the healthcare system affords hospitalists a distinct perspective and systems-based approach to confronting and solving challenges at the individual provider and overall institutional level of the hospital. In this capacity, hospitalists experience multiple examples of administrative requirements directly detracting from patient care and redirecting finite resources away from care to meet compliance demands.
By way of example, navigating the administrative rules around inpatient admissions and outpatient observation care, for example, requires a significant shift of healthcare resources away from patient care. While patients admitted under observation receive nearly identical care to those admitted as an inpatient, hospitalists report that, in addition to themselves as the direct healthcare provider, status determinations between inpatient admissions and outpatient observation care require the input of a myriad of staff including nursing, coding/compliance teams, utilization review, case managers and external review organizations. A recent study in the Journal of Hospital Medicine indicated that an average of 5.1 full time employees, not including case managers, are required to navigate the audit and appeals process associated with hospital stay status determinations. These are resources that should be directly used for patient care, but are redirected towards regulation compliance, increasing cost of care without increasing quality.
To read the entire letter, visit https://www.hospitalmedicine.org/policy--advocacy/letters/shm-supports-the-reducing-administrative-costs-and-burdens-in-health-care-act-of-2019/.
In May 2019, SHM sent a letter to U.S. Senators Tina Smith and Bill Cassidy in support of the Reducing Administrative Costs and Burdens in Health Care Act of 2019. In excerpts from the letter below, the society details the link between administrative burdens and physician burnout.
Providers and hospital systems expend countless resources, both time and dollars, adhering to unnecessary and excessive administrative burdens instead of investing those resources in providing quality patient care. National data suggests that more than 50 percent of the physician workforce is burned out. Excessive administrative burden is a major contributor to physician burnout, which negatively affects quality and safety within the hospital and further increases health care costs. Notably, the Reducing Administrative Costs and Burdens in Health Care Act calls for a 50% reduction of unnecessary administrative costs from the Department of Health and Human Services within the next ten years.
Hospitalists are front-line clinicians in America's acute care hospitals whose professional focus is the general medical care of hospitalized patients. Their unique position in the healthcare system affords hospitalists a distinct perspective and systems-based approach to confronting and solving challenges at the individual provider and overall institutional level of the hospital. In this capacity, hospitalists experience multiple examples of administrative requirements directly detracting from patient care and redirecting finite resources away from care to meet compliance demands.
By way of example, navigating the administrative rules around inpatient admissions and outpatient observation care, for example, requires a significant shift of healthcare resources away from patient care. While patients admitted under observation receive nearly identical care to those admitted as an inpatient, hospitalists report that, in addition to themselves as the direct healthcare provider, status determinations between inpatient admissions and outpatient observation care require the input of a myriad of staff including nursing, coding/compliance teams, utilization review, case managers and external review organizations. A recent study in the Journal of Hospital Medicine indicated that an average of 5.1 full time employees, not including case managers, are required to navigate the audit and appeals process associated with hospital stay status determinations. These are resources that should be directly used for patient care, but are redirected towards regulation compliance, increasing cost of care without increasing quality.
To read the entire letter, visit https://www.hospitalmedicine.org/policy--advocacy/letters/shm-supports-the-reducing-administrative-costs-and-burdens-in-health-care-act-of-2019/.
In May 2019, SHM sent a letter to U.S. Senators Tina Smith and Bill Cassidy in support of the Reducing Administrative Costs and Burdens in Health Care Act of 2019. In excerpts from the letter below, the society details the link between administrative burdens and physician burnout.
Providers and hospital systems expend countless resources, both time and dollars, adhering to unnecessary and excessive administrative burdens instead of investing those resources in providing quality patient care. National data suggests that more than 50 percent of the physician workforce is burned out. Excessive administrative burden is a major contributor to physician burnout, which negatively affects quality and safety within the hospital and further increases health care costs. Notably, the Reducing Administrative Costs and Burdens in Health Care Act calls for a 50% reduction of unnecessary administrative costs from the Department of Health and Human Services within the next ten years.
Hospitalists are front-line clinicians in America's acute care hospitals whose professional focus is the general medical care of hospitalized patients. Their unique position in the healthcare system affords hospitalists a distinct perspective and systems-based approach to confronting and solving challenges at the individual provider and overall institutional level of the hospital. In this capacity, hospitalists experience multiple examples of administrative requirements directly detracting from patient care and redirecting finite resources away from care to meet compliance demands.
By way of example, navigating the administrative rules around inpatient admissions and outpatient observation care, for example, requires a significant shift of healthcare resources away from patient care. While patients admitted under observation receive nearly identical care to those admitted as an inpatient, hospitalists report that, in addition to themselves as the direct healthcare provider, status determinations between inpatient admissions and outpatient observation care require the input of a myriad of staff including nursing, coding/compliance teams, utilization review, case managers and external review organizations. A recent study in the Journal of Hospital Medicine indicated that an average of 5.1 full time employees, not including case managers, are required to navigate the audit and appeals process associated with hospital stay status determinations. These are resources that should be directly used for patient care, but are redirected towards regulation compliance, increasing cost of care without increasing quality.
To read the entire letter, visit https://www.hospitalmedicine.org/policy--advocacy/letters/shm-supports-the-reducing-administrative-costs-and-burdens-in-health-care-act-of-2019/.
Community pediatric care is diminishing
The mantra of community hospital administrators is that pediatric care does not pay. Neonatal intensive care pays. For pediatrics, it is similar to how football programs (Medicare patients) support minor sports (pediatrics and obstetrics) at colleges. However, fewer even mildly sick newborns are cared for at community hospitals, which has led to a centralization of neonatal and pediatric care and a loss of pediatric expertise at the affected hospitals.
Pediatric hospitalists are hired to cover the pediatric floor, the emergency department, and labor and delivery, then fired over empty pediatric beds. The rationale expressed is that pediatricians have done such a good job in preventive care that children rarely need hospitalization, so why have a pediatric inpatient unit? It is true that preventive care has been an integral part of primary care for children. Significantly less that 1% of child office visits result in hospitalization.
Advocate Health Care has closed inpatient pediatric units at Illinois Masonic, on Chicago’s North Side, Good Samaritan in Downers Grove, and Good Shepherd in Barrington. Units also have been closed at Mount Sinai in North Lawndale, Norwegian American on Chicago’s West Side, Little Company of Mary in Evergreen Park, and Alexian Brothers in Elk Grove.
As a Chicago-area pediatrician for more than 30 years, I have learned several things about community-based pediatric care:
1. Pediatrics is a geographic specialty. Parents will travel to shop, but would rather walk or have a short ride to their children’s medical providers. Secondary care should be community based, and hospitalization, if necessary, should be close by as well.
2. Hospitals that ceased delivering pediatric inpatient care lost their child-friendliness and pediatric competence, becoming uncomfortable delivering almost any care for children (e.g., sedated MRIs and EEGs, x-rays and ultrasounds, ECGs and echocardiograms, and emergency care).
3. In almost all hospitals, after pediatrics was gone eventually so passed obstetrics (another less remunerative specialty). Sick newborns need immediate, competent care. Most pediatric hospitalizations are short term, often overnight. Delaying newborn care is a medicolegal nightmare. and exposes the child and his or her family to a potentially dangerous drive or helicopter ride.
4. As pediatric subspecialty care becomes more centralized, parents are asked to travel for hours to see a pediatric specialist. There are times when that is necessary (e.g., cardiovascular surgery). Pediatric subspecialists, such as pediatric otolaryngologists, then leave community hospitals, forcing even minor surgeries (e.g., ear ventilation tubes) to be done at a center. In rural areas, this could mean hours of travel, lost work days, and family disruption.
5. Children’s hospitals get uninsured and publicly insured children sent hundreds of miles, because there were no subspecialists in the community who would care for these children.
What is the solution, in our profit-focused health care system?
1. Hospitals’ Certificates of Need could include a mandate for pediatric care.
2. Children’s hospitals could be made responsible for community-based care within their geographic catchment areas.
3. The state or the federal government could mandate and financially support community-based hospital care.
4. Deciding what level of care might be appropriate for each community could depend upon closeness to a pediatric hospital, health problems in the community, and the availability of pediatric specialists.
5. A condition for medical licensure might be that a community-based pediatric subspecialist is required to care for a proportion of the uninsured or publicly insured children in his or her area.
6. Reimbursements for pediatric care need to rise enough to make caring for children worth it.
The major decision point regarding care for children cannot be financial, but must instead embrace the needs of each affected community. If quality health care is a right, and not a privilege, then it is time to stop closing pediatric inpatient units, and, instead, look for creative ways to better care for our children.
This process has led to pediatric care being available only in designated centers. The centralization of pediatric care has progressed from 30 years ago, when most community hospitals had inpatient pediatric units, to the search for innovative ways to fill pediatric beds in the mid-90s (sick day care, flex- or shared pediatric units), to the wholesale closure of community pediatric inpatient beds, from 2000 to the present. I have, unfortunately, seen this firsthand, watching the rise of pediatric mega-hospitals and the demise of community pediatrics. It is a simple financial argument. Care for children simply does not pay nearly as well as does care for adults, especially Medicaid patients. Pediatricians are the poorest paid practicing doctors (public health doctors are paid less).
It is true that pediatricians always have been at the forefront of preventive medicine, and that pediatric patients almost always get better, in spite of our best-intentioned interventions. So community-based pediatricians admit very few patients.
With the loss of pediatric units, community hospitals lose their comfort caring for children. This includes phlebotomy, x-ray, trauma, surgery, and behavioral health. And eroding community hospital pediatric expertise has catastrophic implications for rural hospitals, where parents may have to drive for hours to find a child-friendly emergency department.
Is there an answer?
1. Hospitals are responsible for the patients they serve, including children. Why should a hospital be able to close pediatric services so easily?
2. Every hospital that sees children, through the emergency department, needs to have a pediatrician available to evaluate a child, 24/7.
3. There needs to be an observation unit for children, with pediatric staffing, for overnight stays.
4. Pediatric hospitalists should be staffing community hospitals.
5. Pediatric behavioral health resources need to be available, e.g., inpatient psychiatry, partial hospitalization programs, intensive outpatient programs.
6. Telehealth communication is not adequate to address acute care problems, because the hospital caring for the child has to have the proper equipment and adequate expertise to carry out the recommendations of the teleconsultant.
If we accept that our children will shape the future, we must allow them to survive and thrive. Is health care a right or a privilege, and is it just for adults or for children, too?
Dr. Ochs is in private practice at Ravenswood Pediatrics in Chicago. He said he had no relevant financial disclosures. Email him at [email protected].
The mantra of community hospital administrators is that pediatric care does not pay. Neonatal intensive care pays. For pediatrics, it is similar to how football programs (Medicare patients) support minor sports (pediatrics and obstetrics) at colleges. However, fewer even mildly sick newborns are cared for at community hospitals, which has led to a centralization of neonatal and pediatric care and a loss of pediatric expertise at the affected hospitals.
Pediatric hospitalists are hired to cover the pediatric floor, the emergency department, and labor and delivery, then fired over empty pediatric beds. The rationale expressed is that pediatricians have done such a good job in preventive care that children rarely need hospitalization, so why have a pediatric inpatient unit? It is true that preventive care has been an integral part of primary care for children. Significantly less that 1% of child office visits result in hospitalization.
Advocate Health Care has closed inpatient pediatric units at Illinois Masonic, on Chicago’s North Side, Good Samaritan in Downers Grove, and Good Shepherd in Barrington. Units also have been closed at Mount Sinai in North Lawndale, Norwegian American on Chicago’s West Side, Little Company of Mary in Evergreen Park, and Alexian Brothers in Elk Grove.
As a Chicago-area pediatrician for more than 30 years, I have learned several things about community-based pediatric care:
1. Pediatrics is a geographic specialty. Parents will travel to shop, but would rather walk or have a short ride to their children’s medical providers. Secondary care should be community based, and hospitalization, if necessary, should be close by as well.
2. Hospitals that ceased delivering pediatric inpatient care lost their child-friendliness and pediatric competence, becoming uncomfortable delivering almost any care for children (e.g., sedated MRIs and EEGs, x-rays and ultrasounds, ECGs and echocardiograms, and emergency care).
3. In almost all hospitals, after pediatrics was gone eventually so passed obstetrics (another less remunerative specialty). Sick newborns need immediate, competent care. Most pediatric hospitalizations are short term, often overnight. Delaying newborn care is a medicolegal nightmare. and exposes the child and his or her family to a potentially dangerous drive or helicopter ride.
4. As pediatric subspecialty care becomes more centralized, parents are asked to travel for hours to see a pediatric specialist. There are times when that is necessary (e.g., cardiovascular surgery). Pediatric subspecialists, such as pediatric otolaryngologists, then leave community hospitals, forcing even minor surgeries (e.g., ear ventilation tubes) to be done at a center. In rural areas, this could mean hours of travel, lost work days, and family disruption.
5. Children’s hospitals get uninsured and publicly insured children sent hundreds of miles, because there were no subspecialists in the community who would care for these children.
What is the solution, in our profit-focused health care system?
1. Hospitals’ Certificates of Need could include a mandate for pediatric care.
2. Children’s hospitals could be made responsible for community-based care within their geographic catchment areas.
3. The state or the federal government could mandate and financially support community-based hospital care.
4. Deciding what level of care might be appropriate for each community could depend upon closeness to a pediatric hospital, health problems in the community, and the availability of pediatric specialists.
5. A condition for medical licensure might be that a community-based pediatric subspecialist is required to care for a proportion of the uninsured or publicly insured children in his or her area.
6. Reimbursements for pediatric care need to rise enough to make caring for children worth it.
The major decision point regarding care for children cannot be financial, but must instead embrace the needs of each affected community. If quality health care is a right, and not a privilege, then it is time to stop closing pediatric inpatient units, and, instead, look for creative ways to better care for our children.
This process has led to pediatric care being available only in designated centers. The centralization of pediatric care has progressed from 30 years ago, when most community hospitals had inpatient pediatric units, to the search for innovative ways to fill pediatric beds in the mid-90s (sick day care, flex- or shared pediatric units), to the wholesale closure of community pediatric inpatient beds, from 2000 to the present. I have, unfortunately, seen this firsthand, watching the rise of pediatric mega-hospitals and the demise of community pediatrics. It is a simple financial argument. Care for children simply does not pay nearly as well as does care for adults, especially Medicaid patients. Pediatricians are the poorest paid practicing doctors (public health doctors are paid less).
It is true that pediatricians always have been at the forefront of preventive medicine, and that pediatric patients almost always get better, in spite of our best-intentioned interventions. So community-based pediatricians admit very few patients.
With the loss of pediatric units, community hospitals lose their comfort caring for children. This includes phlebotomy, x-ray, trauma, surgery, and behavioral health. And eroding community hospital pediatric expertise has catastrophic implications for rural hospitals, where parents may have to drive for hours to find a child-friendly emergency department.
Is there an answer?
1. Hospitals are responsible for the patients they serve, including children. Why should a hospital be able to close pediatric services so easily?
2. Every hospital that sees children, through the emergency department, needs to have a pediatrician available to evaluate a child, 24/7.
3. There needs to be an observation unit for children, with pediatric staffing, for overnight stays.
4. Pediatric hospitalists should be staffing community hospitals.
5. Pediatric behavioral health resources need to be available, e.g., inpatient psychiatry, partial hospitalization programs, intensive outpatient programs.
6. Telehealth communication is not adequate to address acute care problems, because the hospital caring for the child has to have the proper equipment and adequate expertise to carry out the recommendations of the teleconsultant.
If we accept that our children will shape the future, we must allow them to survive and thrive. Is health care a right or a privilege, and is it just for adults or for children, too?
Dr. Ochs is in private practice at Ravenswood Pediatrics in Chicago. He said he had no relevant financial disclosures. Email him at [email protected].
The mantra of community hospital administrators is that pediatric care does not pay. Neonatal intensive care pays. For pediatrics, it is similar to how football programs (Medicare patients) support minor sports (pediatrics and obstetrics) at colleges. However, fewer even mildly sick newborns are cared for at community hospitals, which has led to a centralization of neonatal and pediatric care and a loss of pediatric expertise at the affected hospitals.
Pediatric hospitalists are hired to cover the pediatric floor, the emergency department, and labor and delivery, then fired over empty pediatric beds. The rationale expressed is that pediatricians have done such a good job in preventive care that children rarely need hospitalization, so why have a pediatric inpatient unit? It is true that preventive care has been an integral part of primary care for children. Significantly less that 1% of child office visits result in hospitalization.
Advocate Health Care has closed inpatient pediatric units at Illinois Masonic, on Chicago’s North Side, Good Samaritan in Downers Grove, and Good Shepherd in Barrington. Units also have been closed at Mount Sinai in North Lawndale, Norwegian American on Chicago’s West Side, Little Company of Mary in Evergreen Park, and Alexian Brothers in Elk Grove.
As a Chicago-area pediatrician for more than 30 years, I have learned several things about community-based pediatric care:
1. Pediatrics is a geographic specialty. Parents will travel to shop, but would rather walk or have a short ride to their children’s medical providers. Secondary care should be community based, and hospitalization, if necessary, should be close by as well.
2. Hospitals that ceased delivering pediatric inpatient care lost their child-friendliness and pediatric competence, becoming uncomfortable delivering almost any care for children (e.g., sedated MRIs and EEGs, x-rays and ultrasounds, ECGs and echocardiograms, and emergency care).
3. In almost all hospitals, after pediatrics was gone eventually so passed obstetrics (another less remunerative specialty). Sick newborns need immediate, competent care. Most pediatric hospitalizations are short term, often overnight. Delaying newborn care is a medicolegal nightmare. and exposes the child and his or her family to a potentially dangerous drive or helicopter ride.
4. As pediatric subspecialty care becomes more centralized, parents are asked to travel for hours to see a pediatric specialist. There are times when that is necessary (e.g., cardiovascular surgery). Pediatric subspecialists, such as pediatric otolaryngologists, then leave community hospitals, forcing even minor surgeries (e.g., ear ventilation tubes) to be done at a center. In rural areas, this could mean hours of travel, lost work days, and family disruption.
5. Children’s hospitals get uninsured and publicly insured children sent hundreds of miles, because there were no subspecialists in the community who would care for these children.
What is the solution, in our profit-focused health care system?
1. Hospitals’ Certificates of Need could include a mandate for pediatric care.
2. Children’s hospitals could be made responsible for community-based care within their geographic catchment areas.
3. The state or the federal government could mandate and financially support community-based hospital care.
4. Deciding what level of care might be appropriate for each community could depend upon closeness to a pediatric hospital, health problems in the community, and the availability of pediatric specialists.
5. A condition for medical licensure might be that a community-based pediatric subspecialist is required to care for a proportion of the uninsured or publicly insured children in his or her area.
6. Reimbursements for pediatric care need to rise enough to make caring for children worth it.
The major decision point regarding care for children cannot be financial, but must instead embrace the needs of each affected community. If quality health care is a right, and not a privilege, then it is time to stop closing pediatric inpatient units, and, instead, look for creative ways to better care for our children.
This process has led to pediatric care being available only in designated centers. The centralization of pediatric care has progressed from 30 years ago, when most community hospitals had inpatient pediatric units, to the search for innovative ways to fill pediatric beds in the mid-90s (sick day care, flex- or shared pediatric units), to the wholesale closure of community pediatric inpatient beds, from 2000 to the present. I have, unfortunately, seen this firsthand, watching the rise of pediatric mega-hospitals and the demise of community pediatrics. It is a simple financial argument. Care for children simply does not pay nearly as well as does care for adults, especially Medicaid patients. Pediatricians are the poorest paid practicing doctors (public health doctors are paid less).
It is true that pediatricians always have been at the forefront of preventive medicine, and that pediatric patients almost always get better, in spite of our best-intentioned interventions. So community-based pediatricians admit very few patients.
With the loss of pediatric units, community hospitals lose their comfort caring for children. This includes phlebotomy, x-ray, trauma, surgery, and behavioral health. And eroding community hospital pediatric expertise has catastrophic implications for rural hospitals, where parents may have to drive for hours to find a child-friendly emergency department.
Is there an answer?
1. Hospitals are responsible for the patients they serve, including children. Why should a hospital be able to close pediatric services so easily?
2. Every hospital that sees children, through the emergency department, needs to have a pediatrician available to evaluate a child, 24/7.
3. There needs to be an observation unit for children, with pediatric staffing, for overnight stays.
4. Pediatric hospitalists should be staffing community hospitals.
5. Pediatric behavioral health resources need to be available, e.g., inpatient psychiatry, partial hospitalization programs, intensive outpatient programs.
6. Telehealth communication is not adequate to address acute care problems, because the hospital caring for the child has to have the proper equipment and adequate expertise to carry out the recommendations of the teleconsultant.
If we accept that our children will shape the future, we must allow them to survive and thrive. Is health care a right or a privilege, and is it just for adults or for children, too?
Dr. Ochs is in private practice at Ravenswood Pediatrics in Chicago. He said he had no relevant financial disclosures. Email him at [email protected].
RECOVERY: Early SAVR benefits asymptomatic severe AS patients
PHILADELPHIA – Early aortic valve replacement surgery for patients with asymptomatic, severe aortic stenosis has been a controversial strategy, but the first randomized trial comparing early surgery with conservative management found that early-surgery patients had a 17-fold improved survival at 8 years, according to trial results reported at the American Heart Association scientific sessions.
Albeit small – 145 patients randomized to early surgery or conservative therapy – the RECOVERY trial results provide important evidence to support early preemptive aortic valve replacement for patients with asymptomatic but severe aortic stenosis, said Duk-Hyun Kang, MD, of the division of cardiology, Asan Medical Center in Seoul, South Korea.
The trial randomized 73 patients to early surgical aortic valve replacement (SAVR) and 72 to conventional treatment. Baseline characteristics were similar between the two groups, with a mean EuroSCORE II of 0.9, peak aortic-valve jet velocity (Vmax) of 5.1 m/sec, and mean aortic-valve area of 0.63 cm2. Fifty-three conventional treatment patients went on to have SAVR when symptoms developed during follow-up. There were no operation-related deaths in either group, although one early surgery patient had a stroke and one conventional treatment patients had an MI during the operative period.
At a median follow-up of 6.2 years, 1 early-surgery patient (1.4%) and 11 conventional-therapy patients (15.3%) died from operative or cardiovascular death, Dr. Kang said (P = 0.003). “The number needed to treat to prevent one cardiovascular death within 4 years was 20 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidences of operative CV or death were 1.4% for both in the early-surgery group and 5.7% and 25.5% in the conventional-treatment group, Dr. Kang said (P = 0.003).
Rates of all-cause mortality were 6.8% and 20.8% (P = 0.030) in the respective groups. “The number needed to treat to save one life in 4 years was 16 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidence of any-cause death was 4.1% and 10.2% in the early-surgery patients and 9.7% and 31.8% in the conventional group (P = 0.018).
Among the trial limitations Dr. Kang acknowledged were that the population had severe AS with aortic velocity of 4.5 m/sec or greater. “The benefit of early surgery may be relatively smaller in asymptomatic patients with less severe aortic stenosis,” he said.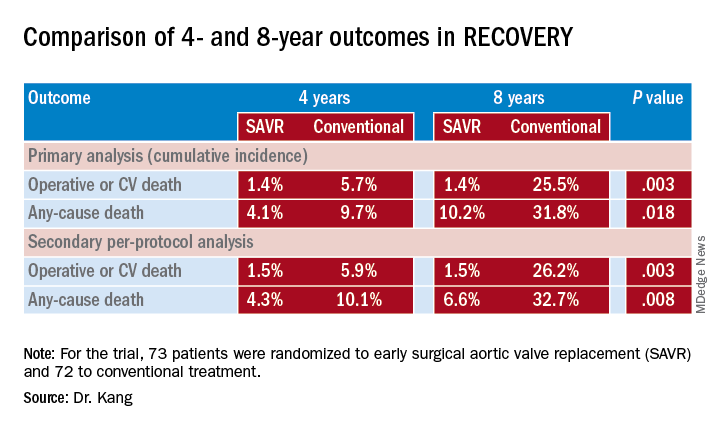
Not all patients had an exercise test to confirm their asymptomatic status, but as discussant Robert Bonow, MD, of Northwestern University in Chicago pointed out, “this is what we’re dealing with clinically.” Also the mean age of the patient population – 64.9 years – is relatively young, and they had a high incidence of bicuspid aortic valve, few comorbidities, and low operative risk, Dr. Kang said. “Thus, our study population is quite different from the populations enrolled in the TAVR [transcatheter AVR] trials, and the results of our study cannot be directly applied to early TAVR for asymptomatic severe aortic stenosis,” he said.
The mean age in both the PARTNER 3 (N Engl J Med. 2019;380:1695-705) and EVOLUT (N Engl J Med. 2019;380:1706-15) trials comparing TAVR and SAVR in low-risk patients was 73.6 years, and they had higher rates of comorbidities than did the RECOVERY patients.
Dr. Bonow said the RECOVERY findings add to the PARTNER 3 and EVOLUT findings and raise the question about rethinking guideline indications for AVR in asymptomatic severe AS patients. “Should we be talking about moving our Vmax threshold down to 4.5 m/sec, or maybe increasing the Class IIa indications to Class I?” he said. “I think we need to wait to see more data to support these excellent results.”
However, most of these asymptomatic patients do eventually have surgery “in a very short period of time,” Dr. Bonow said. “So from a clinical management point of view, I think we already have the data suggesting that we could move the ball forward, and now we have these excellent outcome data from Korea as well.”
Results were published simultaneously in the New England Journal of Medicine (2019 Nov 16. doi: 10.1056/NEJMoa1912846).
Dr. Kang and Dr. Bonow had no financial relationships to disclose.
PHILADELPHIA – Early aortic valve replacement surgery for patients with asymptomatic, severe aortic stenosis has been a controversial strategy, but the first randomized trial comparing early surgery with conservative management found that early-surgery patients had a 17-fold improved survival at 8 years, according to trial results reported at the American Heart Association scientific sessions.
Albeit small – 145 patients randomized to early surgery or conservative therapy – the RECOVERY trial results provide important evidence to support early preemptive aortic valve replacement for patients with asymptomatic but severe aortic stenosis, said Duk-Hyun Kang, MD, of the division of cardiology, Asan Medical Center in Seoul, South Korea.
The trial randomized 73 patients to early surgical aortic valve replacement (SAVR) and 72 to conventional treatment. Baseline characteristics were similar between the two groups, with a mean EuroSCORE II of 0.9, peak aortic-valve jet velocity (Vmax) of 5.1 m/sec, and mean aortic-valve area of 0.63 cm2. Fifty-three conventional treatment patients went on to have SAVR when symptoms developed during follow-up. There were no operation-related deaths in either group, although one early surgery patient had a stroke and one conventional treatment patients had an MI during the operative period.
At a median follow-up of 6.2 years, 1 early-surgery patient (1.4%) and 11 conventional-therapy patients (15.3%) died from operative or cardiovascular death, Dr. Kang said (P = 0.003). “The number needed to treat to prevent one cardiovascular death within 4 years was 20 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidences of operative CV or death were 1.4% for both in the early-surgery group and 5.7% and 25.5% in the conventional-treatment group, Dr. Kang said (P = 0.003).
Rates of all-cause mortality were 6.8% and 20.8% (P = 0.030) in the respective groups. “The number needed to treat to save one life in 4 years was 16 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidence of any-cause death was 4.1% and 10.2% in the early-surgery patients and 9.7% and 31.8% in the conventional group (P = 0.018).
Among the trial limitations Dr. Kang acknowledged were that the population had severe AS with aortic velocity of 4.5 m/sec or greater. “The benefit of early surgery may be relatively smaller in asymptomatic patients with less severe aortic stenosis,” he said.
Not all patients had an exercise test to confirm their asymptomatic status, but as discussant Robert Bonow, MD, of Northwestern University in Chicago pointed out, “this is what we’re dealing with clinically.” Also the mean age of the patient population – 64.9 years – is relatively young, and they had a high incidence of bicuspid aortic valve, few comorbidities, and low operative risk, Dr. Kang said. “Thus, our study population is quite different from the populations enrolled in the TAVR [transcatheter AVR] trials, and the results of our study cannot be directly applied to early TAVR for asymptomatic severe aortic stenosis,” he said.
The mean age in both the PARTNER 3 (N Engl J Med. 2019;380:1695-705) and EVOLUT (N Engl J Med. 2019;380:1706-15) trials comparing TAVR and SAVR in low-risk patients was 73.6 years, and they had higher rates of comorbidities than did the RECOVERY patients.
Dr. Bonow said the RECOVERY findings add to the PARTNER 3 and EVOLUT findings and raise the question about rethinking guideline indications for AVR in asymptomatic severe AS patients. “Should we be talking about moving our Vmax threshold down to 4.5 m/sec, or maybe increasing the Class IIa indications to Class I?” he said. “I think we need to wait to see more data to support these excellent results.”
However, most of these asymptomatic patients do eventually have surgery “in a very short period of time,” Dr. Bonow said. “So from a clinical management point of view, I think we already have the data suggesting that we could move the ball forward, and now we have these excellent outcome data from Korea as well.”
Results were published simultaneously in the New England Journal of Medicine (2019 Nov 16. doi: 10.1056/NEJMoa1912846).
Dr. Kang and Dr. Bonow had no financial relationships to disclose.
PHILADELPHIA – Early aortic valve replacement surgery for patients with asymptomatic, severe aortic stenosis has been a controversial strategy, but the first randomized trial comparing early surgery with conservative management found that early-surgery patients had a 17-fold improved survival at 8 years, according to trial results reported at the American Heart Association scientific sessions.
Albeit small – 145 patients randomized to early surgery or conservative therapy – the RECOVERY trial results provide important evidence to support early preemptive aortic valve replacement for patients with asymptomatic but severe aortic stenosis, said Duk-Hyun Kang, MD, of the division of cardiology, Asan Medical Center in Seoul, South Korea.
The trial randomized 73 patients to early surgical aortic valve replacement (SAVR) and 72 to conventional treatment. Baseline characteristics were similar between the two groups, with a mean EuroSCORE II of 0.9, peak aortic-valve jet velocity (Vmax) of 5.1 m/sec, and mean aortic-valve area of 0.63 cm2. Fifty-three conventional treatment patients went on to have SAVR when symptoms developed during follow-up. There were no operation-related deaths in either group, although one early surgery patient had a stroke and one conventional treatment patients had an MI during the operative period.
At a median follow-up of 6.2 years, 1 early-surgery patient (1.4%) and 11 conventional-therapy patients (15.3%) died from operative or cardiovascular death, Dr. Kang said (P = 0.003). “The number needed to treat to prevent one cardiovascular death within 4 years was 20 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidences of operative CV or death were 1.4% for both in the early-surgery group and 5.7% and 25.5% in the conventional-treatment group, Dr. Kang said (P = 0.003).
Rates of all-cause mortality were 6.8% and 20.8% (P = 0.030) in the respective groups. “The number needed to treat to save one life in 4 years was 16 patients,” Dr. Kang said. At 4 and 8 years, the cumulative incidence of any-cause death was 4.1% and 10.2% in the early-surgery patients and 9.7% and 31.8% in the conventional group (P = 0.018).
Among the trial limitations Dr. Kang acknowledged were that the population had severe AS with aortic velocity of 4.5 m/sec or greater. “The benefit of early surgery may be relatively smaller in asymptomatic patients with less severe aortic stenosis,” he said.
Not all patients had an exercise test to confirm their asymptomatic status, but as discussant Robert Bonow, MD, of Northwestern University in Chicago pointed out, “this is what we’re dealing with clinically.” Also the mean age of the patient population – 64.9 years – is relatively young, and they had a high incidence of bicuspid aortic valve, few comorbidities, and low operative risk, Dr. Kang said. “Thus, our study population is quite different from the populations enrolled in the TAVR [transcatheter AVR] trials, and the results of our study cannot be directly applied to early TAVR for asymptomatic severe aortic stenosis,” he said.
The mean age in both the PARTNER 3 (N Engl J Med. 2019;380:1695-705) and EVOLUT (N Engl J Med. 2019;380:1706-15) trials comparing TAVR and SAVR in low-risk patients was 73.6 years, and they had higher rates of comorbidities than did the RECOVERY patients.
Dr. Bonow said the RECOVERY findings add to the PARTNER 3 and EVOLUT findings and raise the question about rethinking guideline indications for AVR in asymptomatic severe AS patients. “Should we be talking about moving our Vmax threshold down to 4.5 m/sec, or maybe increasing the Class IIa indications to Class I?” he said. “I think we need to wait to see more data to support these excellent results.”
However, most of these asymptomatic patients do eventually have surgery “in a very short period of time,” Dr. Bonow said. “So from a clinical management point of view, I think we already have the data suggesting that we could move the ball forward, and now we have these excellent outcome data from Korea as well.”
Results were published simultaneously in the New England Journal of Medicine (2019 Nov 16. doi: 10.1056/NEJMoa1912846).
Dr. Kang and Dr. Bonow had no financial relationships to disclose.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Promising early efficacy of venetoclax/navitoclax in r/r acute lymphoblastic leukemia
ORLANDO – A combination of venetoclax (Venclexta) and the experimental BCL-2 inhibitor navitoclax showed good activity and acceptable safety in both children and adults with relapsed or refractory acute lymphoblastic leukemia or lymphoblastic lymphoma, investigators in a phase 1 trial reported.
The overall rate of combined complete response (CR), CR with incomplete marrow recovery (CRi) or incomplete platelet recovery (CRp) was 49% among 45 patients: 24 with acute lymphoblastic leukemia of B-cell lineage (B-ALL), 18 with T-cell lineage ALL (T-ALL), and 3 with lymphoblastic lymphoma, reported Norman J. Lacayo, MD, from Stanford (Calif.) University.
“Venetoclax, navitoclax, and chemotherapy is well tolerated with few discontinuations for dose reductions due to adverse events in patients with relapsed/refractory ALL or lymphoblastic lymphoma. The preliminary efficacy was promising in this heavily pretreated population of patients, including pediatric patients and patients with prior stem cell transplantation of CAR [chimeric antigen receptor] T-cell therapy,” he said at the annual meeting of the American Society of Hematology.
Venetoclax is a highly selective inhibitor of the B-cell lymphoma 2 (BCL-2) pathway. Navitoclax inhibits BCL-2, the BCL–extra large (BCL-XL) transmembrane molecule, and the apoptotic protein BCL-W, but was associated with dose-limiting toxicities when used at standard doses in monotherapy, he noted.
To see whether adding venetoclax to low-dose navitoclax could be safe and have synergistic activity against BCL-2, Dr. Lacayo and colleagues are conducting a phase 1, open-label, dose-escalation study of patients aged 4 years and older with relapsed/refractory ALL or lymphoblastic lymphoma.
Patients receive the weight-adjusted equivalent of 200 mg venetoclax on day 1, and 400 mg equivalent daily thereafter. Beginning on day 3, patients receive oral navitoclax daily at doses of 25, 50, or 100 mg for patients weighing 45 kg or more, or 25 or 50 mg for patients weighing from 20 to less than 45 kg.
Patients can also receive two cycles of chemotherapy with asparaginase, vincristine, and dexamethasone, with additional cycles allowed at the investigators’ discretion.
The patients reported on at ASH 2019 had received a median of 4 prior lines of therapy (range, 1-10).
After a median time on study of 8 months, preliminary efficacy – a secondary endpoint of this phase 1 trial – was promising, Dr. Lacayo said. The CR rate was 25% among patients with B-ALL, 11% in patients with T-ALL, and 67% (two of three patients) with lymphoblastic lymphoma. The respective CRi rates were 13%, 17%, and 0%, and respective CRp rates were 17%, 11%, and 0%.
In addition, 3 of 24 patients (13%) with B-ALL had a partial response, as did 1 patient with lymphoblastic lymphoma.
The median time to first response was about 1.1 months. The median duration of response was 9.1 months for patients with B-ALL, 4.2 months for patients with T-ALL, and had not been reached among patients with lymphoblastic leukemia.
The median overall survival was 9.7 months for patients with B-ALL, 6.8 months for those with T-ALL, and not reached for those with lymphoblastic leukemia.
In all, 6 of 24 patients with B-ALL, 3 of 18 with T-ALL, and 2 of 3 with lymphoblastic leukemia survived long enough to proceed to stem cell transplantation or CAR T-cell therapy.
Analysis of the combination’s safety, the primary endpoint, showed that 58% of patients had grade 3-4 adverse events (AEs) related to venetoclax, and 42% had grade 3-4 AEs related to navitoclax. Four patients had to discontinue the combination because of treatment-related AEs.
Dose-limiting toxicities, which occurred in seven patients, included delayed count recovery, drug-induced liver injury, intestinal ischemia, and increase in serum bilirubin.
One patient died from an intestinal ischemic event deemed to be related to the combination, and seven other patients had fatal adverse events considered not related to the study drugs. The causes of death included sepsis, septic shock, cardiac arrest, and neurotoxicity.
The investigators are enrolling an expansion cohort to see whether a 21-day dosing schedule of venetoclax with 50 mg navitoclax, or 25 mg for patients under 45 kg, could improve count recovery time.
The study was funded by AbbVie. Dr. Lacayo reported having no conflict of interests to disclose. Several coauthors are AbbVie employees.
SOURCE: Lacayo NJ et al. ASH 2019, Abstract 285.
ORLANDO – A combination of venetoclax (Venclexta) and the experimental BCL-2 inhibitor navitoclax showed good activity and acceptable safety in both children and adults with relapsed or refractory acute lymphoblastic leukemia or lymphoblastic lymphoma, investigators in a phase 1 trial reported.
The overall rate of combined complete response (CR), CR with incomplete marrow recovery (CRi) or incomplete platelet recovery (CRp) was 49% among 45 patients: 24 with acute lymphoblastic leukemia of B-cell lineage (B-ALL), 18 with T-cell lineage ALL (T-ALL), and 3 with lymphoblastic lymphoma, reported Norman J. Lacayo, MD, from Stanford (Calif.) University.
“Venetoclax, navitoclax, and chemotherapy is well tolerated with few discontinuations for dose reductions due to adverse events in patients with relapsed/refractory ALL or lymphoblastic lymphoma. The preliminary efficacy was promising in this heavily pretreated population of patients, including pediatric patients and patients with prior stem cell transplantation of CAR [chimeric antigen receptor] T-cell therapy,” he said at the annual meeting of the American Society of Hematology.
Venetoclax is a highly selective inhibitor of the B-cell lymphoma 2 (BCL-2) pathway. Navitoclax inhibits BCL-2, the BCL–extra large (BCL-XL) transmembrane molecule, and the apoptotic protein BCL-W, but was associated with dose-limiting toxicities when used at standard doses in monotherapy, he noted.
To see whether adding venetoclax to low-dose navitoclax could be safe and have synergistic activity against BCL-2, Dr. Lacayo and colleagues are conducting a phase 1, open-label, dose-escalation study of patients aged 4 years and older with relapsed/refractory ALL or lymphoblastic lymphoma.
Patients receive the weight-adjusted equivalent of 200 mg venetoclax on day 1, and 400 mg equivalent daily thereafter. Beginning on day 3, patients receive oral navitoclax daily at doses of 25, 50, or 100 mg for patients weighing 45 kg or more, or 25 or 50 mg for patients weighing from 20 to less than 45 kg.
Patients can also receive two cycles of chemotherapy with asparaginase, vincristine, and dexamethasone, with additional cycles allowed at the investigators’ discretion.
The patients reported on at ASH 2019 had received a median of 4 prior lines of therapy (range, 1-10).
After a median time on study of 8 months, preliminary efficacy – a secondary endpoint of this phase 1 trial – was promising, Dr. Lacayo said. The CR rate was 25% among patients with B-ALL, 11% in patients with T-ALL, and 67% (two of three patients) with lymphoblastic lymphoma. The respective CRi rates were 13%, 17%, and 0%, and respective CRp rates were 17%, 11%, and 0%.
In addition, 3 of 24 patients (13%) with B-ALL had a partial response, as did 1 patient with lymphoblastic lymphoma.
The median time to first response was about 1.1 months. The median duration of response was 9.1 months for patients with B-ALL, 4.2 months for patients with T-ALL, and had not been reached among patients with lymphoblastic leukemia.
The median overall survival was 9.7 months for patients with B-ALL, 6.8 months for those with T-ALL, and not reached for those with lymphoblastic leukemia.
In all, 6 of 24 patients with B-ALL, 3 of 18 with T-ALL, and 2 of 3 with lymphoblastic leukemia survived long enough to proceed to stem cell transplantation or CAR T-cell therapy.
Analysis of the combination’s safety, the primary endpoint, showed that 58% of patients had grade 3-4 adverse events (AEs) related to venetoclax, and 42% had grade 3-4 AEs related to navitoclax. Four patients had to discontinue the combination because of treatment-related AEs.
Dose-limiting toxicities, which occurred in seven patients, included delayed count recovery, drug-induced liver injury, intestinal ischemia, and increase in serum bilirubin.
One patient died from an intestinal ischemic event deemed to be related to the combination, and seven other patients had fatal adverse events considered not related to the study drugs. The causes of death included sepsis, septic shock, cardiac arrest, and neurotoxicity.
The investigators are enrolling an expansion cohort to see whether a 21-day dosing schedule of venetoclax with 50 mg navitoclax, or 25 mg for patients under 45 kg, could improve count recovery time.
The study was funded by AbbVie. Dr. Lacayo reported having no conflict of interests to disclose. Several coauthors are AbbVie employees.
SOURCE: Lacayo NJ et al. ASH 2019, Abstract 285.
ORLANDO – A combination of venetoclax (Venclexta) and the experimental BCL-2 inhibitor navitoclax showed good activity and acceptable safety in both children and adults with relapsed or refractory acute lymphoblastic leukemia or lymphoblastic lymphoma, investigators in a phase 1 trial reported.
The overall rate of combined complete response (CR), CR with incomplete marrow recovery (CRi) or incomplete platelet recovery (CRp) was 49% among 45 patients: 24 with acute lymphoblastic leukemia of B-cell lineage (B-ALL), 18 with T-cell lineage ALL (T-ALL), and 3 with lymphoblastic lymphoma, reported Norman J. Lacayo, MD, from Stanford (Calif.) University.
“Venetoclax, navitoclax, and chemotherapy is well tolerated with few discontinuations for dose reductions due to adverse events in patients with relapsed/refractory ALL or lymphoblastic lymphoma. The preliminary efficacy was promising in this heavily pretreated population of patients, including pediatric patients and patients with prior stem cell transplantation of CAR [chimeric antigen receptor] T-cell therapy,” he said at the annual meeting of the American Society of Hematology.
Venetoclax is a highly selective inhibitor of the B-cell lymphoma 2 (BCL-2) pathway. Navitoclax inhibits BCL-2, the BCL–extra large (BCL-XL) transmembrane molecule, and the apoptotic protein BCL-W, but was associated with dose-limiting toxicities when used at standard doses in monotherapy, he noted.
To see whether adding venetoclax to low-dose navitoclax could be safe and have synergistic activity against BCL-2, Dr. Lacayo and colleagues are conducting a phase 1, open-label, dose-escalation study of patients aged 4 years and older with relapsed/refractory ALL or lymphoblastic lymphoma.
Patients receive the weight-adjusted equivalent of 200 mg venetoclax on day 1, and 400 mg equivalent daily thereafter. Beginning on day 3, patients receive oral navitoclax daily at doses of 25, 50, or 100 mg for patients weighing 45 kg or more, or 25 or 50 mg for patients weighing from 20 to less than 45 kg.
Patients can also receive two cycles of chemotherapy with asparaginase, vincristine, and dexamethasone, with additional cycles allowed at the investigators’ discretion.
The patients reported on at ASH 2019 had received a median of 4 prior lines of therapy (range, 1-10).
After a median time on study of 8 months, preliminary efficacy – a secondary endpoint of this phase 1 trial – was promising, Dr. Lacayo said. The CR rate was 25% among patients with B-ALL, 11% in patients with T-ALL, and 67% (two of three patients) with lymphoblastic lymphoma. The respective CRi rates were 13%, 17%, and 0%, and respective CRp rates were 17%, 11%, and 0%.
In addition, 3 of 24 patients (13%) with B-ALL had a partial response, as did 1 patient with lymphoblastic lymphoma.
The median time to first response was about 1.1 months. The median duration of response was 9.1 months for patients with B-ALL, 4.2 months for patients with T-ALL, and had not been reached among patients with lymphoblastic leukemia.
The median overall survival was 9.7 months for patients with B-ALL, 6.8 months for those with T-ALL, and not reached for those with lymphoblastic leukemia.
In all, 6 of 24 patients with B-ALL, 3 of 18 with T-ALL, and 2 of 3 with lymphoblastic leukemia survived long enough to proceed to stem cell transplantation or CAR T-cell therapy.
Analysis of the combination’s safety, the primary endpoint, showed that 58% of patients had grade 3-4 adverse events (AEs) related to venetoclax, and 42% had grade 3-4 AEs related to navitoclax. Four patients had to discontinue the combination because of treatment-related AEs.
Dose-limiting toxicities, which occurred in seven patients, included delayed count recovery, drug-induced liver injury, intestinal ischemia, and increase in serum bilirubin.
One patient died from an intestinal ischemic event deemed to be related to the combination, and seven other patients had fatal adverse events considered not related to the study drugs. The causes of death included sepsis, septic shock, cardiac arrest, and neurotoxicity.
The investigators are enrolling an expansion cohort to see whether a 21-day dosing schedule of venetoclax with 50 mg navitoclax, or 25 mg for patients under 45 kg, could improve count recovery time.
The study was funded by AbbVie. Dr. Lacayo reported having no conflict of interests to disclose. Several coauthors are AbbVie employees.
SOURCE: Lacayo NJ et al. ASH 2019, Abstract 285.
REPORTING FROM ASH 2019
Testosterone gel increases LV mass in older men
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
PHILADELPHIA – Testosterone gel for treatment of hypogonadism in older men boosted their left ventricular mass by 3.5% in a single year in the multicenter, double-blind, placebo-controlled Testosterone Cardiovascular Trial, although the clinical implications of this impressive increase remain unclear, Elizabeth Hutchins, MD, reported at the American Heart Association scientific sessions.
“I do think these results should be considered as part of the safety profile for testosterone gel and also represent an interesting and understudied area for future research,” said Dr. Hutchins, a hospitalist affiliated with the Los Angeles Biomedical Research Center at Harbor-UCLA Medical Center.
The Testosterone Cardiovascular Trial was one of seven coordinated placebo-controlled, double-blind clinical trials of the impact of raising serum testosterone levels in older men with low testosterone. Some results of what are known as the TTrials have previously been reported (Endocr Rev. 2018 Jun 1;39[3]:369-86).
Dr. Hutchins presented new findings on the effect of treatment with 1% topical testosterone gel on body surface area–indexed left ventricular mass. The trial utilized a widely prescribed, commercially available product known as AndroGel. The study included 123 men over age 65 with low serum testosterone and coronary CT angiography images obtained at baseline and again after 1 year of double-blind testosterone gel or placebo. More than 80% of the men were above age 75, half were obese, more than two-thirds had hypertension, and 30% had diabetes.
The men initially applied 5 g of the testosterone gel daily, providing 15 mg/day of testosterone, with subsequent dosing adjustments as needed based on serum testosterone levels measured at a central laboratory. Participants were evaluated in office visits with serum testosterone measurements every 3 months. Testosterone levels in the men assigned to active treatment quickly rose to normal range and stayed there for the full 12 months, while the placebo-treated controls continued to have below-normal testosterone throughout the trial.
The key study finding was that LV mass indexed to body surface area rose significantly in the testosterone gel group, from an average of 71.5 g/m2 at baseline to 74.8 g/m2 at 1 year. That’s a statistically significant 3.5% increase. In contrast, LV mass remained flat across the year in controls: 73.8 g/m2 at baseline and 73.3 g/m2 at 12 months.
There was, however, no change over time in left or right atrial or ventricular chamber volumes in the testosterone gel recipients, nor in the controls.
Session comoderator Eric D. Peterson, MD, professor of medicine and a cardiologist at Duke University in Durham, N.C., said that “this is a very important topic,” then posed a provocative question to Dr. Hutchins: “If the intervention had been running instead of testosterone gel, would the results have looked similar, and would you be concluding that there should be a warning around the use of running?”
Dr. Hutchins replied that she’s given that question much thought.
“Of course, exercise leads to LV hypertrophy and we consider that to be good muscle, and high blood pressure leads to LV hypertrophy and we consider that bad muscle. So which one is it in this case? From what I can find in the literature, it seems that incremental increases in LV mass in the absence of being an athlete are deleterious. But I think we would need outcomes-based research to really answer that question,” she said.
Dr. Hutchins noted that this was the first-ever randomized controlled trial to measure the effect of testosterone therapy on LV mass in humans. The documented increase achieved with 1 year of testosterone gel doesn’t come close to reaching the threshold of LV hypertrophy, which is about 125 g/m2 for men. But evidence from animal and observational human studies suggests that even in the absence of LV hypertrophy, increases in LV mass are associated with increased mortality, she added.
She reported having no financial conflicts regarding her study, sponsored by the National Institutes of Health.
SOURCE: Hutchins E. AHA 2019, Session FS.AOS.04.
REPORTING FROM AHA 2019


















