User login
Methotrexate gives durable remission from idiopathic granulomatous mastitis
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
REPORTING FROM ACR 2019
Researchers win funding for breast cancer studies
Five breast cancer researchers have won 3 years of funding from the National Comprehensive Cancer Network’s Oncology Research Program and Pfizer Global Medical Grants. The researchers will receive up to $1.4 million.
Allison Lipitz-Snyderman, PhD, and Erin Gillespie, MD, of Memorial Sloan Kettering Cancer Center in New York, have won funding for a project entitled, “Leveraging an academic-community partnership model to improve the quality of radiation treatment for metastatic breast cancer patients.”
Dr. Gillespie and Dr. Lipitz-Snyderman plan to use an existing partnership between Memorial Sloan Kettering and three community-based institutions to test a system for implementing best practices in radiation treatment. The system includes a web-based platform that disseminates expert recommendations as well as weekly conferences during which community radiation oncologists can consult with specialists on complex cases.
Aki Morikawa, MD, PhD, of the University of Michigan Rogel Cancer Center in Ann Arbor, won funding for a project entitled, “Personalized multi-care: A tailored approach to multidisciplinary care coordination delivery for metastatic breast cancer patients with central nervous system metastases.”
The goals of Dr. Morikawa’s project are to educate patients and providers on managing central nervous system metastases in the breast cancer setting, tailor care coordination and planning to patient and provider needs, and increase patient participation in studies.
Karen Lisa Smith, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, won funding for a project entitled, “The Johns Hopkins Metastatic Breast Cancer Partners Program: Collaborating to improve metastatic breast cancer care.”
The goal of the Metastatic Breast Cancer Partners Program is for Johns Hopkins and mid-Atlantic regional practices to fight metastatic breast cancer together. To that end, Dr. Smith plans to create a multidisciplinary clinic that offers supportive care and treatment recommendations, a database for patient tracking and trial screening, educational resources, and new opportunities for provider collaboration.
Laura Spring, MD, of Massachusetts General Hospital Cancer Center in Boston, won funding for a project entitled, “Expanding precision medicine for patients with metastatic breast cancer in the community: Leveraging academic strength and community partnership.”
The goal of Dr. Spring’s project is to extend academic resources to affiliated network sites. This will involve increasing access to tissue-based and blood-based tumor genotyping for patients with metastatic breast cancer, creating a virtual molecular and precision medicine clinic that provides interpretation of genomic data and treatment recommendations, and offering clinical trial matching to metastatic breast cancer patients treated at network sites.
Five breast cancer researchers have won 3 years of funding from the National Comprehensive Cancer Network’s Oncology Research Program and Pfizer Global Medical Grants. The researchers will receive up to $1.4 million.
Allison Lipitz-Snyderman, PhD, and Erin Gillespie, MD, of Memorial Sloan Kettering Cancer Center in New York, have won funding for a project entitled, “Leveraging an academic-community partnership model to improve the quality of radiation treatment for metastatic breast cancer patients.”
Dr. Gillespie and Dr. Lipitz-Snyderman plan to use an existing partnership between Memorial Sloan Kettering and three community-based institutions to test a system for implementing best practices in radiation treatment. The system includes a web-based platform that disseminates expert recommendations as well as weekly conferences during which community radiation oncologists can consult with specialists on complex cases.
Aki Morikawa, MD, PhD, of the University of Michigan Rogel Cancer Center in Ann Arbor, won funding for a project entitled, “Personalized multi-care: A tailored approach to multidisciplinary care coordination delivery for metastatic breast cancer patients with central nervous system metastases.”
The goals of Dr. Morikawa’s project are to educate patients and providers on managing central nervous system metastases in the breast cancer setting, tailor care coordination and planning to patient and provider needs, and increase patient participation in studies.
Karen Lisa Smith, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, won funding for a project entitled, “The Johns Hopkins Metastatic Breast Cancer Partners Program: Collaborating to improve metastatic breast cancer care.”
The goal of the Metastatic Breast Cancer Partners Program is for Johns Hopkins and mid-Atlantic regional practices to fight metastatic breast cancer together. To that end, Dr. Smith plans to create a multidisciplinary clinic that offers supportive care and treatment recommendations, a database for patient tracking and trial screening, educational resources, and new opportunities for provider collaboration.
Laura Spring, MD, of Massachusetts General Hospital Cancer Center in Boston, won funding for a project entitled, “Expanding precision medicine for patients with metastatic breast cancer in the community: Leveraging academic strength and community partnership.”
The goal of Dr. Spring’s project is to extend academic resources to affiliated network sites. This will involve increasing access to tissue-based and blood-based tumor genotyping for patients with metastatic breast cancer, creating a virtual molecular and precision medicine clinic that provides interpretation of genomic data and treatment recommendations, and offering clinical trial matching to metastatic breast cancer patients treated at network sites.
Five breast cancer researchers have won 3 years of funding from the National Comprehensive Cancer Network’s Oncology Research Program and Pfizer Global Medical Grants. The researchers will receive up to $1.4 million.
Allison Lipitz-Snyderman, PhD, and Erin Gillespie, MD, of Memorial Sloan Kettering Cancer Center in New York, have won funding for a project entitled, “Leveraging an academic-community partnership model to improve the quality of radiation treatment for metastatic breast cancer patients.”
Dr. Gillespie and Dr. Lipitz-Snyderman plan to use an existing partnership between Memorial Sloan Kettering and three community-based institutions to test a system for implementing best practices in radiation treatment. The system includes a web-based platform that disseminates expert recommendations as well as weekly conferences during which community radiation oncologists can consult with specialists on complex cases.
Aki Morikawa, MD, PhD, of the University of Michigan Rogel Cancer Center in Ann Arbor, won funding for a project entitled, “Personalized multi-care: A tailored approach to multidisciplinary care coordination delivery for metastatic breast cancer patients with central nervous system metastases.”
The goals of Dr. Morikawa’s project are to educate patients and providers on managing central nervous system metastases in the breast cancer setting, tailor care coordination and planning to patient and provider needs, and increase patient participation in studies.
Karen Lisa Smith, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, won funding for a project entitled, “The Johns Hopkins Metastatic Breast Cancer Partners Program: Collaborating to improve metastatic breast cancer care.”
The goal of the Metastatic Breast Cancer Partners Program is for Johns Hopkins and mid-Atlantic regional practices to fight metastatic breast cancer together. To that end, Dr. Smith plans to create a multidisciplinary clinic that offers supportive care and treatment recommendations, a database for patient tracking and trial screening, educational resources, and new opportunities for provider collaboration.
Laura Spring, MD, of Massachusetts General Hospital Cancer Center in Boston, won funding for a project entitled, “Expanding precision medicine for patients with metastatic breast cancer in the community: Leveraging academic strength and community partnership.”
The goal of Dr. Spring’s project is to extend academic resources to affiliated network sites. This will involve increasing access to tissue-based and blood-based tumor genotyping for patients with metastatic breast cancer, creating a virtual molecular and precision medicine clinic that provides interpretation of genomic data and treatment recommendations, and offering clinical trial matching to metastatic breast cancer patients treated at network sites.
Unnecessary pelvic exams, Pap tests common in young women
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
Pediatricians take on more mental health care
Assessment and treatment of many of the more common behavioral disorders in childhood, such as ADHD and anxiety, should be considered within a pediatrician’s scope of practice, a stance made very clear by a recent policy statement published by the American Academy of Pediatrics entitled “Mental health competencies for pediatric practice.”1 These competencies include medication treatment. As stated in the article, “certain disorders (ADHD, common anxiety disorders, depression), if associated with no more than moderate impairment, are amenable to primary care medication management because there are indicated medications with a well-established safety profile.”
This shift to shared ownership when it comes to mental health care is likely coming from multiple sources, not the least of them being necessity and an acknowledgment that there simply aren’t enough psychiatrists to take over the mental health care of every youth with a diagnosable psychiatric disorder. While the number of child and adolescent psychiatrists remains relatively flat, the youth suicide rate is rising, as are the numbers presenting to emergency departments in crisis – all for reasons still to be fully understood. And these trends all are occurring as the medical community overall is appreciating more and more that good mental health is a cornerstone of all health.
The response from the pediatric community, whether it be because of personal conviction or simply a lack of options, largely has been to step up to the plate and take on these new responsibilities and challenges while trying to get up to speed with the latest information about mental health best practices. From my own experience doing evaluations and consultations from area primary care clinicians for over 15 years, the shift is noticeable. The typical patient now coming in has already seen a mental health counselor and tried at least one medication, while evaluations for diagnosis and treatment recommendations for things like uncomplicated and treatment-naive ADHD symptoms, for example, are becoming much more infrequent – although still far from extinct.
Nevertheless, there remain concerns about the extent of these new charges. Joe Nasca, MD, an experienced pediatrician who has been practicing in rural Vermont for decades, is worried that there is simply too much already for pediatricians to know and do to be able to add extensive mental health care. “There is so much to know in general peds [pediatrics] that I would guess a year or more of additional residency and experience would adequately prepare me to take this on,” he said in an interview. In comparing psychiatric care to other specialties, Dr. Nasca went on to say that, “I would not presume to treat chronic renal failure without the help of a nephrologist or a dilated aortic arch without a cardiologist.”
In a similar vein, however, it also is true that a significant percentage of children presenting to pediatricians for orthopedic problems, infections, asthma, and rashes are managed without referrals to specialists. The right balance, of course, will vary from clinician to clinician based on that pediatrician’s level of interest, experience, and available resources in the community. The AAP position papers don’t mandate or even encourage the notion that all pediatricians need to be at the same place when it comes to competency in assessment and treatment of mental health problems, although it is probably fair to say that there is a push for the pediatric community as a whole to raise the collective bar at least a notch or two.
In response, the mental health community has moved to support the primary care community in their expanded role. These efforts have taken many forms, most notably the model of integrated care, in which mental health clinicians of various types see patients in primary care offices rather than making patients come to them. There also are new consultation programs that provide easy access to a child psychiatrist or other mental health professional for case-related questions delivered by phone, email, or for single in-person consultations. Additional training and educational offerings also are now available for pediatricians either in training and for those already in practice. These initiatives are bolstered by research showing that, not only can good mental health care be delivered in pediatric settings, but there are cost savings that can be realized, particularly for nonpsychiatric medical care.2 Despite these promising leads, however, there will remain some for whom anything less than the increased availability of a psychiatrist to “take over” a patient’s mental health care will be seen as a falling short of the clinical need.
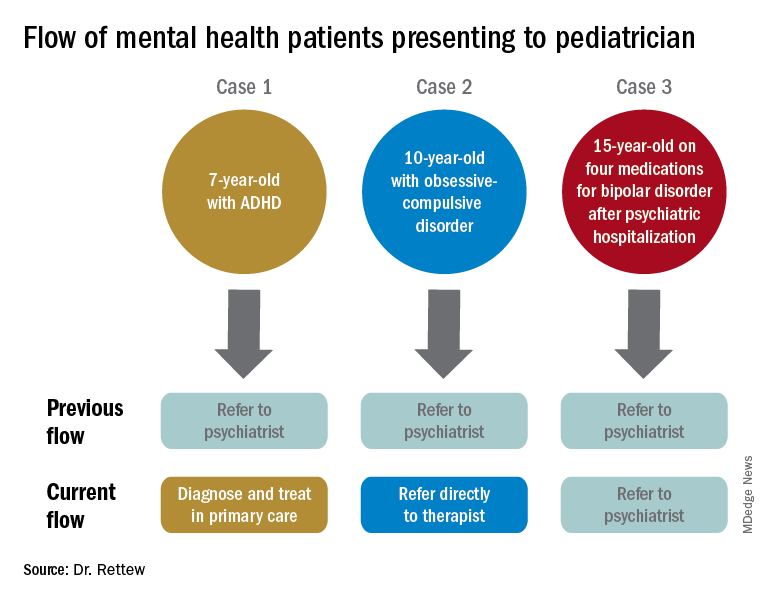
To illustrate how things have and continue to change, consider the following three common clinical scenarios that generally present to a pediatrician:
- New presentation of ADHD symptoms.
- Anxiety or obsessive-compulsive problems.
- Return of a patient who has been psychiatrically hospitalized and now is taking multiple medications.
In the past, all three cases often would have resulted in a referral to a psychiatrist. Today, however, it is quite likely that only one of these cases would be referred because ADHD could be well diagnosed and managed within the primary care setting, and problems like anxiety and obsessive-compulsive disorder are sent first not to a psychiatrist but to a non-MD psychotherapist.
Moving forward, today’s pediatricians are expected to do more for the mental health care of patients themselves instead of referring to a psychiatrist. Most already do, despite having had little in the way of formal training. As the evidence grows that the promotion of mental well-being can be a key to future overall health, as well as to the cost of future health care, there are many reasons to be optimistic that support for pediatricians and collaborative care models for clinicians trying to fulfill these new responsibilities will get only stronger.
References
1. Pediatrics. 2019 Nov;144(5). pii: e20192757.
2. Pediatrics. 2019 Jul;144(1). pii: e20183243.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Follow him on Twitter @PediPsych. Email him at [email protected]. Looking for more mental health training? Attend the 14th annual Child Psychiatry in Primary Care conference in Burlington on May 8, 2020 (http://www.med.uvm.edu/cme/conferences).
Assessment and treatment of many of the more common behavioral disorders in childhood, such as ADHD and anxiety, should be considered within a pediatrician’s scope of practice, a stance made very clear by a recent policy statement published by the American Academy of Pediatrics entitled “Mental health competencies for pediatric practice.”1 These competencies include medication treatment. As stated in the article, “certain disorders (ADHD, common anxiety disorders, depression), if associated with no more than moderate impairment, are amenable to primary care medication management because there are indicated medications with a well-established safety profile.”

This shift to shared ownership when it comes to mental health care is likely coming from multiple sources, not the least of them being necessity and an acknowledgment that there simply aren’t enough psychiatrists to take over the mental health care of every youth with a diagnosable psychiatric disorder. While the number of child and adolescent psychiatrists remains relatively flat, the youth suicide rate is rising, as are the numbers presenting to emergency departments in crisis – all for reasons still to be fully understood. And these trends all are occurring as the medical community overall is appreciating more and more that good mental health is a cornerstone of all health.
The response from the pediatric community, whether it be because of personal conviction or simply a lack of options, largely has been to step up to the plate and take on these new responsibilities and challenges while trying to get up to speed with the latest information about mental health best practices. From my own experience doing evaluations and consultations from area primary care clinicians for over 15 years, the shift is noticeable. The typical patient now coming in has already seen a mental health counselor and tried at least one medication, while evaluations for diagnosis and treatment recommendations for things like uncomplicated and treatment-naive ADHD symptoms, for example, are becoming much more infrequent – although still far from extinct.
Nevertheless, there remain concerns about the extent of these new charges. Joe Nasca, MD, an experienced pediatrician who has been practicing in rural Vermont for decades, is worried that there is simply too much already for pediatricians to know and do to be able to add extensive mental health care. “There is so much to know in general peds [pediatrics] that I would guess a year or more of additional residency and experience would adequately prepare me to take this on,” he said in an interview. In comparing psychiatric care to other specialties, Dr. Nasca went on to say that, “I would not presume to treat chronic renal failure without the help of a nephrologist or a dilated aortic arch without a cardiologist.”
In a similar vein, however, it also is true that a significant percentage of children presenting to pediatricians for orthopedic problems, infections, asthma, and rashes are managed without referrals to specialists. The right balance, of course, will vary from clinician to clinician based on that pediatrician’s level of interest, experience, and available resources in the community. The AAP position papers don’t mandate or even encourage the notion that all pediatricians need to be at the same place when it comes to competency in assessment and treatment of mental health problems, although it is probably fair to say that there is a push for the pediatric community as a whole to raise the collective bar at least a notch or two.
In response, the mental health community has moved to support the primary care community in their expanded role. These efforts have taken many forms, most notably the model of integrated care, in which mental health clinicians of various types see patients in primary care offices rather than making patients come to them. There also are new consultation programs that provide easy access to a child psychiatrist or other mental health professional for case-related questions delivered by phone, email, or for single in-person consultations. Additional training and educational offerings also are now available for pediatricians either in training and for those already in practice. These initiatives are bolstered by research showing that, not only can good mental health care be delivered in pediatric settings, but there are cost savings that can be realized, particularly for nonpsychiatric medical care.2 Despite these promising leads, however, there will remain some for whom anything less than the increased availability of a psychiatrist to “take over” a patient’s mental health care will be seen as a falling short of the clinical need.
To illustrate how things have and continue to change, consider the following three common clinical scenarios that generally present to a pediatrician:
- New presentation of ADHD symptoms.
- Anxiety or obsessive-compulsive problems.
- Return of a patient who has been psychiatrically hospitalized and now is taking multiple medications.
In the past, all three cases often would have resulted in a referral to a psychiatrist. Today, however, it is quite likely that only one of these cases would be referred because ADHD could be well diagnosed and managed within the primary care setting, and problems like anxiety and obsessive-compulsive disorder are sent first not to a psychiatrist but to a non-MD psychotherapist.
Moving forward, today’s pediatricians are expected to do more for the mental health care of patients themselves instead of referring to a psychiatrist. Most already do, despite having had little in the way of formal training. As the evidence grows that the promotion of mental well-being can be a key to future overall health, as well as to the cost of future health care, there are many reasons to be optimistic that support for pediatricians and collaborative care models for clinicians trying to fulfill these new responsibilities will get only stronger.
References
1. Pediatrics. 2019 Nov;144(5). pii: e20192757.
2. Pediatrics. 2019 Jul;144(1). pii: e20183243.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Follow him on Twitter @PediPsych. Email him at [email protected]. Looking for more mental health training? Attend the 14th annual Child Psychiatry in Primary Care conference in Burlington on May 8, 2020 (http://www.med.uvm.edu/cme/conferences).
Assessment and treatment of many of the more common behavioral disorders in childhood, such as ADHD and anxiety, should be considered within a pediatrician’s scope of practice, a stance made very clear by a recent policy statement published by the American Academy of Pediatrics entitled “Mental health competencies for pediatric practice.”1 These competencies include medication treatment. As stated in the article, “certain disorders (ADHD, common anxiety disorders, depression), if associated with no more than moderate impairment, are amenable to primary care medication management because there are indicated medications with a well-established safety profile.”

This shift to shared ownership when it comes to mental health care is likely coming from multiple sources, not the least of them being necessity and an acknowledgment that there simply aren’t enough psychiatrists to take over the mental health care of every youth with a diagnosable psychiatric disorder. While the number of child and adolescent psychiatrists remains relatively flat, the youth suicide rate is rising, as are the numbers presenting to emergency departments in crisis – all for reasons still to be fully understood. And these trends all are occurring as the medical community overall is appreciating more and more that good mental health is a cornerstone of all health.
The response from the pediatric community, whether it be because of personal conviction or simply a lack of options, largely has been to step up to the plate and take on these new responsibilities and challenges while trying to get up to speed with the latest information about mental health best practices. From my own experience doing evaluations and consultations from area primary care clinicians for over 15 years, the shift is noticeable. The typical patient now coming in has already seen a mental health counselor and tried at least one medication, while evaluations for diagnosis and treatment recommendations for things like uncomplicated and treatment-naive ADHD symptoms, for example, are becoming much more infrequent – although still far from extinct.
Nevertheless, there remain concerns about the extent of these new charges. Joe Nasca, MD, an experienced pediatrician who has been practicing in rural Vermont for decades, is worried that there is simply too much already for pediatricians to know and do to be able to add extensive mental health care. “There is so much to know in general peds [pediatrics] that I would guess a year or more of additional residency and experience would adequately prepare me to take this on,” he said in an interview. In comparing psychiatric care to other specialties, Dr. Nasca went on to say that, “I would not presume to treat chronic renal failure without the help of a nephrologist or a dilated aortic arch without a cardiologist.”
In a similar vein, however, it also is true that a significant percentage of children presenting to pediatricians for orthopedic problems, infections, asthma, and rashes are managed without referrals to specialists. The right balance, of course, will vary from clinician to clinician based on that pediatrician’s level of interest, experience, and available resources in the community. The AAP position papers don’t mandate or even encourage the notion that all pediatricians need to be at the same place when it comes to competency in assessment and treatment of mental health problems, although it is probably fair to say that there is a push for the pediatric community as a whole to raise the collective bar at least a notch or two.
In response, the mental health community has moved to support the primary care community in their expanded role. These efforts have taken many forms, most notably the model of integrated care, in which mental health clinicians of various types see patients in primary care offices rather than making patients come to them. There also are new consultation programs that provide easy access to a child psychiatrist or other mental health professional for case-related questions delivered by phone, email, or for single in-person consultations. Additional training and educational offerings also are now available for pediatricians either in training and for those already in practice. These initiatives are bolstered by research showing that, not only can good mental health care be delivered in pediatric settings, but there are cost savings that can be realized, particularly for nonpsychiatric medical care.2 Despite these promising leads, however, there will remain some for whom anything less than the increased availability of a psychiatrist to “take over” a patient’s mental health care will be seen as a falling short of the clinical need.
To illustrate how things have and continue to change, consider the following three common clinical scenarios that generally present to a pediatrician:
- New presentation of ADHD symptoms.
- Anxiety or obsessive-compulsive problems.
- Return of a patient who has been psychiatrically hospitalized and now is taking multiple medications.
In the past, all three cases often would have resulted in a referral to a psychiatrist. Today, however, it is quite likely that only one of these cases would be referred because ADHD could be well diagnosed and managed within the primary care setting, and problems like anxiety and obsessive-compulsive disorder are sent first not to a psychiatrist but to a non-MD psychotherapist.
Moving forward, today’s pediatricians are expected to do more for the mental health care of patients themselves instead of referring to a psychiatrist. Most already do, despite having had little in the way of formal training. As the evidence grows that the promotion of mental well-being can be a key to future overall health, as well as to the cost of future health care, there are many reasons to be optimistic that support for pediatricians and collaborative care models for clinicians trying to fulfill these new responsibilities will get only stronger.
References
1. Pediatrics. 2019 Nov;144(5). pii: e20192757.
2. Pediatrics. 2019 Jul;144(1). pii: e20183243.
Dr. Rettew is a child and adolescent psychiatrist and associate professor of psychiatry and pediatrics at the University of Vermont, Burlington. Follow him on Twitter @PediPsych. Email him at [email protected]. Looking for more mental health training? Attend the 14th annual Child Psychiatry in Primary Care conference in Burlington on May 8, 2020 (http://www.med.uvm.edu/cme/conferences).
Score predicts locoregional recurrence of breast cancer
The 21-gene assay recurrence score can aid decisions about radiotherapy for postmenopausal patients with node-positive breast cancer, according to researchers.
The researchers analyzed patients who underwent mastectomy or breast-conserving surgery (excision and radiation) and received chemotherapy plus tamoxifen or tamoxifen alone. Results showed that patients with an intermediate or high recurrence score, according to the 21-gene assay OncotypeDX, were more likely to have locoregional recurrence (LRR).
“We believe that the recurrence score adds independent prognostic information that could be used with standard clinical factors for identifying LRR risk and making radiotherapy decisions,” Wendy A. Woodward, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and coauthors wrote in JAMA Oncology.
Dr. Woodward and colleagues analyzed data from a phase 3 trial (NCT00929591) of postmenopausal women with estrogen or progesterone receptor–positive, node-positive breast cancer. There were 367 patients who received tamoxifen alone (n = 148) or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen (n = 219).
Of the 367 patients, 316 were included in the primary analysis. This includes 252 patients who underwent mastectomy without radiotherapy and 64 patients who underwent breast-conserving surgery with radiotherapy.
The researchers defined LRR as a recurrence in the breast; chest wall; or axillary, infraclavicular, supraclavicular, or internal mammary lymph nodes.
The LRR incidence was 5.8% (7/121) among patients with a low recurrence score and 13.8% (27/195) among patients with an intermediate or high recurrence score. The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
The researchers conducted a multivariable analysis for LRR, which included the recurrence score, randomized treatment (combination regimen vs. tamoxifen alone), number of positive nodes (three or fewer vs. four or more), and type of surgery (mastectomy vs. excision and radiation).
Having intermediate or high recurrence scores was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). Having four or more involved nodes was a significant predictor of LRR as well (hazard ratio, 3.37; P = .001). Randomized treatment and surgery were not significantly associated with LRR.
The researchers also conducted an exploratory analysis and found that a recurrence score of 18 was the optimal cutoff for the association of recurrence score and LRR.
“This study found that higher recurrence scores were associated with increased LRR after adjustment for treatment, type of surgical procedure, and number of positive nodes,” Dr. Woodward and colleagues wrote. “This finding suggests that the recurrence score may be used, along with accepted clinical variables, to assess the risk of LRR during radiotherapy decision making. We recommend considering the recurrence score, when available, as one of the factors in selecting patients for postmastectomy radiotherapy.”
This research was funded by the National Cancer Institute of Canada, Canadian Cancer Society, and Genomic Health, which markets the 21-gene assay OncotypeDX. Dr. Woodward disclosed receiving personal fees from Genomic Health outside this research as well as an advisory fee from Merck.
SOURCE: Woodward WA et al. JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559.
The 21-gene assay recurrence score can aid decisions about radiotherapy for postmenopausal patients with node-positive breast cancer, according to researchers.
The researchers analyzed patients who underwent mastectomy or breast-conserving surgery (excision and radiation) and received chemotherapy plus tamoxifen or tamoxifen alone. Results showed that patients with an intermediate or high recurrence score, according to the 21-gene assay OncotypeDX, were more likely to have locoregional recurrence (LRR).
“We believe that the recurrence score adds independent prognostic information that could be used with standard clinical factors for identifying LRR risk and making radiotherapy decisions,” Wendy A. Woodward, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and coauthors wrote in JAMA Oncology.
Dr. Woodward and colleagues analyzed data from a phase 3 trial (NCT00929591) of postmenopausal women with estrogen or progesterone receptor–positive, node-positive breast cancer. There were 367 patients who received tamoxifen alone (n = 148) or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen (n = 219).
Of the 367 patients, 316 were included in the primary analysis. This includes 252 patients who underwent mastectomy without radiotherapy and 64 patients who underwent breast-conserving surgery with radiotherapy.
The researchers defined LRR as a recurrence in the breast; chest wall; or axillary, infraclavicular, supraclavicular, or internal mammary lymph nodes.
The LRR incidence was 5.8% (7/121) among patients with a low recurrence score and 13.8% (27/195) among patients with an intermediate or high recurrence score. The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
The researchers conducted a multivariable analysis for LRR, which included the recurrence score, randomized treatment (combination regimen vs. tamoxifen alone), number of positive nodes (three or fewer vs. four or more), and type of surgery (mastectomy vs. excision and radiation).
Having intermediate or high recurrence scores was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). Having four or more involved nodes was a significant predictor of LRR as well (hazard ratio, 3.37; P = .001). Randomized treatment and surgery were not significantly associated with LRR.
The researchers also conducted an exploratory analysis and found that a recurrence score of 18 was the optimal cutoff for the association of recurrence score and LRR.
“This study found that higher recurrence scores were associated with increased LRR after adjustment for treatment, type of surgical procedure, and number of positive nodes,” Dr. Woodward and colleagues wrote. “This finding suggests that the recurrence score may be used, along with accepted clinical variables, to assess the risk of LRR during radiotherapy decision making. We recommend considering the recurrence score, when available, as one of the factors in selecting patients for postmastectomy radiotherapy.”
This research was funded by the National Cancer Institute of Canada, Canadian Cancer Society, and Genomic Health, which markets the 21-gene assay OncotypeDX. Dr. Woodward disclosed receiving personal fees from Genomic Health outside this research as well as an advisory fee from Merck.
SOURCE: Woodward WA et al. JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559.
The 21-gene assay recurrence score can aid decisions about radiotherapy for postmenopausal patients with node-positive breast cancer, according to researchers.
The researchers analyzed patients who underwent mastectomy or breast-conserving surgery (excision and radiation) and received chemotherapy plus tamoxifen or tamoxifen alone. Results showed that patients with an intermediate or high recurrence score, according to the 21-gene assay OncotypeDX, were more likely to have locoregional recurrence (LRR).
“We believe that the recurrence score adds independent prognostic information that could be used with standard clinical factors for identifying LRR risk and making radiotherapy decisions,” Wendy A. Woodward, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and coauthors wrote in JAMA Oncology.
Dr. Woodward and colleagues analyzed data from a phase 3 trial (NCT00929591) of postmenopausal women with estrogen or progesterone receptor–positive, node-positive breast cancer. There were 367 patients who received tamoxifen alone (n = 148) or cyclophosphamide, doxorubicin, and fluorouracil followed by tamoxifen (n = 219).
Of the 367 patients, 316 were included in the primary analysis. This includes 252 patients who underwent mastectomy without radiotherapy and 64 patients who underwent breast-conserving surgery with radiotherapy.
The researchers defined LRR as a recurrence in the breast; chest wall; or axillary, infraclavicular, supraclavicular, or internal mammary lymph nodes.
The LRR incidence was 5.8% (7/121) among patients with a low recurrence score and 13.8% (27/195) among patients with an intermediate or high recurrence score. The estimated 10-year cumulative LRR incidence rates were 9.7% and 16.5%, respectively (P = .02).
The researchers conducted a multivariable analysis for LRR, which included the recurrence score, randomized treatment (combination regimen vs. tamoxifen alone), number of positive nodes (three or fewer vs. four or more), and type of surgery (mastectomy vs. excision and radiation).
Having intermediate or high recurrence scores was a significant predictor of LRR, with a hazard ratio of 2.36 (P = .04). Having four or more involved nodes was a significant predictor of LRR as well (hazard ratio, 3.37; P = .001). Randomized treatment and surgery were not significantly associated with LRR.
The researchers also conducted an exploratory analysis and found that a recurrence score of 18 was the optimal cutoff for the association of recurrence score and LRR.
“This study found that higher recurrence scores were associated with increased LRR after adjustment for treatment, type of surgical procedure, and number of positive nodes,” Dr. Woodward and colleagues wrote. “This finding suggests that the recurrence score may be used, along with accepted clinical variables, to assess the risk of LRR during radiotherapy decision making. We recommend considering the recurrence score, when available, as one of the factors in selecting patients for postmastectomy radiotherapy.”
This research was funded by the National Cancer Institute of Canada, Canadian Cancer Society, and Genomic Health, which markets the 21-gene assay OncotypeDX. Dr. Woodward disclosed receiving personal fees from Genomic Health outside this research as well as an advisory fee from Merck.
SOURCE: Woodward WA et al. JAMA Oncol. 2020 Jan 9. doi: 10.1001/jamaoncol.2019.5559.
FROM JAMA ONCOLOGY
Treatment of heart failure with preserved ejection fraction is a work in progress
LOS ANGELES – When it comes to the optimal treatment of patients with heart failure with preserved ejection fraction and diabetes, cardiologists like Mark T. Kearney, MB ChB, MD, remain stumped.
“Over the years, the diagnosis of heart failure with preserved ejection fraction has been notoriously difficult [to treat], controversial, and ultimately involves aggressive catheterization of the heart to assess diastolic dysfunction, complex echocardiography, and invasive tests,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease. “These patients have an ejection fraction of over 50% and classic signs and symptoms of heart failure. Studies of beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers have been unsuccessful in this group of patients. We’re at the beginning of a journey in understanding this disorder, and it’s important, because more and more patients present to us with signs and symptoms of heart failure with an ejection fraction greater than 50%.”
In a recent analysis of 1,797 patients with chronic heart failure, Dr. Kearney, British Heart Foundation Professor of Cardiovascular and Diabetes Research at the Leeds (England) Institute of Cardiovascular and Metabolic Medicine, and colleagues examined whether beta-blockers and ACE inhibitors were associated with differential effects on mortality in patients with and without diabetes (Diabetes Care. 2018;41:136-42). Mean follow-up was 4 years.
For the ACE inhibitor component of the trial, the researchers correlated the dose of ramipril to outcomes and found that each milligram increase of ramipril reduced the risk of death by about 3%. “In the nondiabetic patients who did not receive an ACE inhibitor, mortality was about 60% – worse than most cancers,” Dr. Kearney said. “In patients with diabetes, there was a similar pattern. If you didn’t get an ACE inhibitor, mortality was 70%. So, if you get patients on an optimal dose of an ACE inhibitor, you improve their mortality substantially, whether they have diabetes or not.”
The beta-blocker component of the trial yielded similar results. “Among patients who did not receive a beta-blocker, the mortality was about 70% at 5 years – really terrible,” he said. “Every milligram of bisoprolol was associated with a reduction in mortality of about 9%. So, if a patient gets on an optimal dose of a beta-blocker and they have diabetes, it’s associated with prolongation of life over a year.”
Dr. Kearney said that patients often do not want to take an increased dose of a beta-blocker because of concerns about side effects, such as tiredness. “They ask me what the side effects of an increased dose would be. My answer is: ‘It will make you live longer.’ Usually, they’ll respond by agreeing to have a little bit more of the beta-blocker. The message here is, if you have a patient with ejection fraction heart failure and diabetes, get them on the optimal dose of a beta-blocker, even at the expense of an ACE inhibitor.”
In 2016, the European Society of Cardiology introduced guidelines for physicians to make a diagnosis of heart failure with preserved ejection fraction. The guidelines mandate that a diagnosis requires signs and symptoms of heart failure, elevated levels of natriuretic peptide, and echocardiographic abnormalities of cardiac structure and/or function in the presence of a left ventricular ejection fraction of 50% or more (Eur J Heart Fail. 2016;18[8]:891-975).
“Signs and symptoms of heart failure, elevated BNP [brain natriuretic peptide], and echocardiography allow us to make a diagnosis of heart failure with preserved ejection fraction,” Dr. Kearney, who is also dean of the Leeds University School of Medicine. “But we don’t know the outcome of these patients, we don’t know how to treat them, and we don’t know the impact on hospitalizations.”
In a large, unpublished cohort study conducted at Leeds, Dr. Kearney and colleagues evaluated how many patients met criteria for heart failure with reduced ejection fraction or heart failure with preserved ejection fraction after undergoing a BNP measurement. Ultimately, 959 patients met criteria. After assessment, 23% had no heart failure, 44% had heart failure with preserved ejection fraction, and 33% had heart failure with reduced ejection fraction. They found that patients with preserved ejection fraction were older (mean age, 84 years); were more likely to be female; and had less ischemia, less diabetes, and more hypertension. In addition, patients with preserved ejection fraction had significantly better survival than patients with reduced ejection fraction over 5 years follow-up.
“What was really interesting were the findings related to hospitalization,” he said. “All 959 patients accounted for 20,517 days in the hospital over 5 years, which is the equivalent of 1 patient occupying a hospital bed for 56 years. This disorder [heart failure with preserved ejection fraction], despite having a lower mortality than heart failure with reduced ejection fraction, leads to a significant burden on health care systems.”
Among patients with preserved ejection fraction, 82% were hospitalized for a noncardiovascular cause, 6.9% because of heart failure, and 11% were caused by other cardiovascular causes. Most of the hospital admissions were because of chest infections, falls, and other frailty-linked causes. “This link between systemic frailty and heart failure with preserved ejection fraction warrants further investigation,” Dr. Kearney said. “This is a major burden on patient hospital care.”
When the researchers examined outcomes in patients with and without diabetes, those with diabetes were younger, more likely to be male, and have a higher body mass index. They found that, in the presence of diabetes, mortality was increased in heart failure with preserved and reduced ejection fraction. “So, even at the age of 81 or 82, diabetes changes the pathophysiology of mortality in what was previously believed to be a benign disease,” he said.
In a subset analysis of patients with and without diabetes who were not taking a beta-blocker, there did not seem to be increased sympathetic activation in the patients with diabetes and heart failure with preserved ejection fraction, nor a difference in heart rate between the nondiabetic patients and patients with diabetes. However, among patients with heart failure with reduced ejection fraction, those with diabetes had an increased heart rate.
“Is heart failure with preserved ejection fraction in diabetes benign? I think the answer is no,” Dr. Kearney said. “It increases hospitalization and is a major burden on health care systems. What should we do? We deal with comorbidity and fall risk. It’s good old-fashioned doctoring, really. We address frailty and respiratory tract infections, but the key thing here is that we need more research.”
Dr. Kearney reported having no relevant financial disclosures.
LOS ANGELES – When it comes to the optimal treatment of patients with heart failure with preserved ejection fraction and diabetes, cardiologists like Mark T. Kearney, MB ChB, MD, remain stumped.
“Over the years, the diagnosis of heart failure with preserved ejection fraction has been notoriously difficult [to treat], controversial, and ultimately involves aggressive catheterization of the heart to assess diastolic dysfunction, complex echocardiography, and invasive tests,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease. “These patients have an ejection fraction of over 50% and classic signs and symptoms of heart failure. Studies of beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers have been unsuccessful in this group of patients. We’re at the beginning of a journey in understanding this disorder, and it’s important, because more and more patients present to us with signs and symptoms of heart failure with an ejection fraction greater than 50%.”
In a recent analysis of 1,797 patients with chronic heart failure, Dr. Kearney, British Heart Foundation Professor of Cardiovascular and Diabetes Research at the Leeds (England) Institute of Cardiovascular and Metabolic Medicine, and colleagues examined whether beta-blockers and ACE inhibitors were associated with differential effects on mortality in patients with and without diabetes (Diabetes Care. 2018;41:136-42). Mean follow-up was 4 years.
For the ACE inhibitor component of the trial, the researchers correlated the dose of ramipril to outcomes and found that each milligram increase of ramipril reduced the risk of death by about 3%. “In the nondiabetic patients who did not receive an ACE inhibitor, mortality was about 60% – worse than most cancers,” Dr. Kearney said. “In patients with diabetes, there was a similar pattern. If you didn’t get an ACE inhibitor, mortality was 70%. So, if you get patients on an optimal dose of an ACE inhibitor, you improve their mortality substantially, whether they have diabetes or not.”
The beta-blocker component of the trial yielded similar results. “Among patients who did not receive a beta-blocker, the mortality was about 70% at 5 years – really terrible,” he said. “Every milligram of bisoprolol was associated with a reduction in mortality of about 9%. So, if a patient gets on an optimal dose of a beta-blocker and they have diabetes, it’s associated with prolongation of life over a year.”
Dr. Kearney said that patients often do not want to take an increased dose of a beta-blocker because of concerns about side effects, such as tiredness. “They ask me what the side effects of an increased dose would be. My answer is: ‘It will make you live longer.’ Usually, they’ll respond by agreeing to have a little bit more of the beta-blocker. The message here is, if you have a patient with ejection fraction heart failure and diabetes, get them on the optimal dose of a beta-blocker, even at the expense of an ACE inhibitor.”
In 2016, the European Society of Cardiology introduced guidelines for physicians to make a diagnosis of heart failure with preserved ejection fraction. The guidelines mandate that a diagnosis requires signs and symptoms of heart failure, elevated levels of natriuretic peptide, and echocardiographic abnormalities of cardiac structure and/or function in the presence of a left ventricular ejection fraction of 50% or more (Eur J Heart Fail. 2016;18[8]:891-975).
“Signs and symptoms of heart failure, elevated BNP [brain natriuretic peptide], and echocardiography allow us to make a diagnosis of heart failure with preserved ejection fraction,” Dr. Kearney, who is also dean of the Leeds University School of Medicine. “But we don’t know the outcome of these patients, we don’t know how to treat them, and we don’t know the impact on hospitalizations.”
In a large, unpublished cohort study conducted at Leeds, Dr. Kearney and colleagues evaluated how many patients met criteria for heart failure with reduced ejection fraction or heart failure with preserved ejection fraction after undergoing a BNP measurement. Ultimately, 959 patients met criteria. After assessment, 23% had no heart failure, 44% had heart failure with preserved ejection fraction, and 33% had heart failure with reduced ejection fraction. They found that patients with preserved ejection fraction were older (mean age, 84 years); were more likely to be female; and had less ischemia, less diabetes, and more hypertension. In addition, patients with preserved ejection fraction had significantly better survival than patients with reduced ejection fraction over 5 years follow-up.
“What was really interesting were the findings related to hospitalization,” he said. “All 959 patients accounted for 20,517 days in the hospital over 5 years, which is the equivalent of 1 patient occupying a hospital bed for 56 years. This disorder [heart failure with preserved ejection fraction], despite having a lower mortality than heart failure with reduced ejection fraction, leads to a significant burden on health care systems.”
Among patients with preserved ejection fraction, 82% were hospitalized for a noncardiovascular cause, 6.9% because of heart failure, and 11% were caused by other cardiovascular causes. Most of the hospital admissions were because of chest infections, falls, and other frailty-linked causes. “This link between systemic frailty and heart failure with preserved ejection fraction warrants further investigation,” Dr. Kearney said. “This is a major burden on patient hospital care.”
When the researchers examined outcomes in patients with and without diabetes, those with diabetes were younger, more likely to be male, and have a higher body mass index. They found that, in the presence of diabetes, mortality was increased in heart failure with preserved and reduced ejection fraction. “So, even at the age of 81 or 82, diabetes changes the pathophysiology of mortality in what was previously believed to be a benign disease,” he said.
In a subset analysis of patients with and without diabetes who were not taking a beta-blocker, there did not seem to be increased sympathetic activation in the patients with diabetes and heart failure with preserved ejection fraction, nor a difference in heart rate between the nondiabetic patients and patients with diabetes. However, among patients with heart failure with reduced ejection fraction, those with diabetes had an increased heart rate.
“Is heart failure with preserved ejection fraction in diabetes benign? I think the answer is no,” Dr. Kearney said. “It increases hospitalization and is a major burden on health care systems. What should we do? We deal with comorbidity and fall risk. It’s good old-fashioned doctoring, really. We address frailty and respiratory tract infections, but the key thing here is that we need more research.”
Dr. Kearney reported having no relevant financial disclosures.
LOS ANGELES – When it comes to the optimal treatment of patients with heart failure with preserved ejection fraction and diabetes, cardiologists like Mark T. Kearney, MB ChB, MD, remain stumped.
“Over the years, the diagnosis of heart failure with preserved ejection fraction has been notoriously difficult [to treat], controversial, and ultimately involves aggressive catheterization of the heart to assess diastolic dysfunction, complex echocardiography, and invasive tests,” Dr. Kearney said at the World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease. “These patients have an ejection fraction of over 50% and classic signs and symptoms of heart failure. Studies of beta-blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers have been unsuccessful in this group of patients. We’re at the beginning of a journey in understanding this disorder, and it’s important, because more and more patients present to us with signs and symptoms of heart failure with an ejection fraction greater than 50%.”
In a recent analysis of 1,797 patients with chronic heart failure, Dr. Kearney, British Heart Foundation Professor of Cardiovascular and Diabetes Research at the Leeds (England) Institute of Cardiovascular and Metabolic Medicine, and colleagues examined whether beta-blockers and ACE inhibitors were associated with differential effects on mortality in patients with and without diabetes (Diabetes Care. 2018;41:136-42). Mean follow-up was 4 years.
For the ACE inhibitor component of the trial, the researchers correlated the dose of ramipril to outcomes and found that each milligram increase of ramipril reduced the risk of death by about 3%. “In the nondiabetic patients who did not receive an ACE inhibitor, mortality was about 60% – worse than most cancers,” Dr. Kearney said. “In patients with diabetes, there was a similar pattern. If you didn’t get an ACE inhibitor, mortality was 70%. So, if you get patients on an optimal dose of an ACE inhibitor, you improve their mortality substantially, whether they have diabetes or not.”
The beta-blocker component of the trial yielded similar results. “Among patients who did not receive a beta-blocker, the mortality was about 70% at 5 years – really terrible,” he said. “Every milligram of bisoprolol was associated with a reduction in mortality of about 9%. So, if a patient gets on an optimal dose of a beta-blocker and they have diabetes, it’s associated with prolongation of life over a year.”
Dr. Kearney said that patients often do not want to take an increased dose of a beta-blocker because of concerns about side effects, such as tiredness. “They ask me what the side effects of an increased dose would be. My answer is: ‘It will make you live longer.’ Usually, they’ll respond by agreeing to have a little bit more of the beta-blocker. The message here is, if you have a patient with ejection fraction heart failure and diabetes, get them on the optimal dose of a beta-blocker, even at the expense of an ACE inhibitor.”
In 2016, the European Society of Cardiology introduced guidelines for physicians to make a diagnosis of heart failure with preserved ejection fraction. The guidelines mandate that a diagnosis requires signs and symptoms of heart failure, elevated levels of natriuretic peptide, and echocardiographic abnormalities of cardiac structure and/or function in the presence of a left ventricular ejection fraction of 50% or more (Eur J Heart Fail. 2016;18[8]:891-975).
“Signs and symptoms of heart failure, elevated BNP [brain natriuretic peptide], and echocardiography allow us to make a diagnosis of heart failure with preserved ejection fraction,” Dr. Kearney, who is also dean of the Leeds University School of Medicine. “But we don’t know the outcome of these patients, we don’t know how to treat them, and we don’t know the impact on hospitalizations.”
In a large, unpublished cohort study conducted at Leeds, Dr. Kearney and colleagues evaluated how many patients met criteria for heart failure with reduced ejection fraction or heart failure with preserved ejection fraction after undergoing a BNP measurement. Ultimately, 959 patients met criteria. After assessment, 23% had no heart failure, 44% had heart failure with preserved ejection fraction, and 33% had heart failure with reduced ejection fraction. They found that patients with preserved ejection fraction were older (mean age, 84 years); were more likely to be female; and had less ischemia, less diabetes, and more hypertension. In addition, patients with preserved ejection fraction had significantly better survival than patients with reduced ejection fraction over 5 years follow-up.
“What was really interesting were the findings related to hospitalization,” he said. “All 959 patients accounted for 20,517 days in the hospital over 5 years, which is the equivalent of 1 patient occupying a hospital bed for 56 years. This disorder [heart failure with preserved ejection fraction], despite having a lower mortality than heart failure with reduced ejection fraction, leads to a significant burden on health care systems.”
Among patients with preserved ejection fraction, 82% were hospitalized for a noncardiovascular cause, 6.9% because of heart failure, and 11% were caused by other cardiovascular causes. Most of the hospital admissions were because of chest infections, falls, and other frailty-linked causes. “This link between systemic frailty and heart failure with preserved ejection fraction warrants further investigation,” Dr. Kearney said. “This is a major burden on patient hospital care.”
When the researchers examined outcomes in patients with and without diabetes, those with diabetes were younger, more likely to be male, and have a higher body mass index. They found that, in the presence of diabetes, mortality was increased in heart failure with preserved and reduced ejection fraction. “So, even at the age of 81 or 82, diabetes changes the pathophysiology of mortality in what was previously believed to be a benign disease,” he said.
In a subset analysis of patients with and without diabetes who were not taking a beta-blocker, there did not seem to be increased sympathetic activation in the patients with diabetes and heart failure with preserved ejection fraction, nor a difference in heart rate between the nondiabetic patients and patients with diabetes. However, among patients with heart failure with reduced ejection fraction, those with diabetes had an increased heart rate.
“Is heart failure with preserved ejection fraction in diabetes benign? I think the answer is no,” Dr. Kearney said. “It increases hospitalization and is a major burden on health care systems. What should we do? We deal with comorbidity and fall risk. It’s good old-fashioned doctoring, really. We address frailty and respiratory tract infections, but the key thing here is that we need more research.”
Dr. Kearney reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM WCIRDC 2019
Beware nerve injuries in laparoscopic surgery
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Recognize early window of opportunity in hidradenitis suppurativa
MADRID – Antonio Martorell, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
This distinction is critical in recognizing a window of opportunity in the management of hidradenitis suppurativa (HS): The period early in the disease course when medical therapy alone can be life-changing.
“The window of opportunity is an old concept in gastroenterology, but it’s a new idea in dermatology: It’s the moment in which the patient can have the best results with medical control of inflammation, before progression to tissue scarring has occurred,” explained Dr. Martorell, a dermatologist at the Hospital of Manises in Valencia, Spain, and coauthor of recent HS treatment recommendations by an international expert panel (J Eur Acad Dermatol Venereol. 2019 Jan;33[1]:19-31).
Symptoms in patients with HS can be caused by either dynamic or static lesions. Dynamic lesions arise directly from acute inflammation that can be treated with antibiotics and immunomodulatory therapy. Static lesions are associated with tissue scarring secondary to inflammatory activity and generally benefit only from surgery.
Dynamic lesions consist of nodules, abscesses, and some but not all fistulae. Although the traditional view among dermatologists has been that fistulae simply don’t respond to medical therapy and must be treated surgically, Dr. Martorell and coinvestigators have recently demonstrated in a retrospective study of 117 fistulae in 40 patients that ultrasound was useful in distinguishing four fistular subtypes, two of which responded reasonably well to medical management.
What the investigators call Type A or dermal fistulae are by definition not connected to tunnels. In Dr. Martorell’s study, they had a 95% complete resolution rate after 6 months of various medications. Dermoepidermal fistulae tunnel through the dermis to the epidermis; they had a 65% complete resolution rate. In contrast, Type C or complex fistulae, identified by the ultrasound finding of multiple tunnels extending through the dermis into underlying fat tissue, had no significant response to medical management. Neither did Type D fistulae, which are essentially Type C lesions with scarring (Dermatol Surg. 2019 Oct;45[10]:1237-44).
Thus, ultrasound can have an important impact on patient management and the decision to opt for a combined medical/surgical approach.
“It’s important to apply the HS severity scores and complete the clinical exam, but it’s also important to use ultrasound or another imaging technique,” Dr. Martorell concluded.
MADRID – Antonio Martorell, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
This distinction is critical in recognizing a window of opportunity in the management of hidradenitis suppurativa (HS): The period early in the disease course when medical therapy alone can be life-changing.
“The window of opportunity is an old concept in gastroenterology, but it’s a new idea in dermatology: It’s the moment in which the patient can have the best results with medical control of inflammation, before progression to tissue scarring has occurred,” explained Dr. Martorell, a dermatologist at the Hospital of Manises in Valencia, Spain, and coauthor of recent HS treatment recommendations by an international expert panel (J Eur Acad Dermatol Venereol. 2019 Jan;33[1]:19-31).
Symptoms in patients with HS can be caused by either dynamic or static lesions. Dynamic lesions arise directly from acute inflammation that can be treated with antibiotics and immunomodulatory therapy. Static lesions are associated with tissue scarring secondary to inflammatory activity and generally benefit only from surgery.
Dynamic lesions consist of nodules, abscesses, and some but not all fistulae. Although the traditional view among dermatologists has been that fistulae simply don’t respond to medical therapy and must be treated surgically, Dr. Martorell and coinvestigators have recently demonstrated in a retrospective study of 117 fistulae in 40 patients that ultrasound was useful in distinguishing four fistular subtypes, two of which responded reasonably well to medical management.
What the investigators call Type A or dermal fistulae are by definition not connected to tunnels. In Dr. Martorell’s study, they had a 95% complete resolution rate after 6 months of various medications. Dermoepidermal fistulae tunnel through the dermis to the epidermis; they had a 65% complete resolution rate. In contrast, Type C or complex fistulae, identified by the ultrasound finding of multiple tunnels extending through the dermis into underlying fat tissue, had no significant response to medical management. Neither did Type D fistulae, which are essentially Type C lesions with scarring (Dermatol Surg. 2019 Oct;45[10]:1237-44).
Thus, ultrasound can have an important impact on patient management and the decision to opt for a combined medical/surgical approach.
“It’s important to apply the HS severity scores and complete the clinical exam, but it’s also important to use ultrasound or another imaging technique,” Dr. Martorell concluded.
MADRID – Antonio Martorell, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
This distinction is critical in recognizing a window of opportunity in the management of hidradenitis suppurativa (HS): The period early in the disease course when medical therapy alone can be life-changing.
“The window of opportunity is an old concept in gastroenterology, but it’s a new idea in dermatology: It’s the moment in which the patient can have the best results with medical control of inflammation, before progression to tissue scarring has occurred,” explained Dr. Martorell, a dermatologist at the Hospital of Manises in Valencia, Spain, and coauthor of recent HS treatment recommendations by an international expert panel (J Eur Acad Dermatol Venereol. 2019 Jan;33[1]:19-31).
Symptoms in patients with HS can be caused by either dynamic or static lesions. Dynamic lesions arise directly from acute inflammation that can be treated with antibiotics and immunomodulatory therapy. Static lesions are associated with tissue scarring secondary to inflammatory activity and generally benefit only from surgery.
Dynamic lesions consist of nodules, abscesses, and some but not all fistulae. Although the traditional view among dermatologists has been that fistulae simply don’t respond to medical therapy and must be treated surgically, Dr. Martorell and coinvestigators have recently demonstrated in a retrospective study of 117 fistulae in 40 patients that ultrasound was useful in distinguishing four fistular subtypes, two of which responded reasonably well to medical management.
What the investigators call Type A or dermal fistulae are by definition not connected to tunnels. In Dr. Martorell’s study, they had a 95% complete resolution rate after 6 months of various medications. Dermoepidermal fistulae tunnel through the dermis to the epidermis; they had a 65% complete resolution rate. In contrast, Type C or complex fistulae, identified by the ultrasound finding of multiple tunnels extending through the dermis into underlying fat tissue, had no significant response to medical management. Neither did Type D fistulae, which are essentially Type C lesions with scarring (Dermatol Surg. 2019 Oct;45[10]:1237-44).
Thus, ultrasound can have an important impact on patient management and the decision to opt for a combined medical/surgical approach.
“It’s important to apply the HS severity scores and complete the clinical exam, but it’s also important to use ultrasound or another imaging technique,” Dr. Martorell concluded.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Uterine balloon tamponade found safe in postpartum hemorrhage
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
ID consult for Candida bloodstream infections can reduce mortality risk
findings from a large retrospective study suggest.
Mortality attributable to Candida bloodstream infection ranges between 15% and 47%, and delay in initiation of appropriate treatment has been associated with increased mortality. Previous small studies showed that ID consultation has conferred benefits to patients with Candida bloodstream infections. Carlos Mejia-Chew, MD, and colleagues from Washington University, St. Louis, sought to explore this further by performing a retrospective, single-center cohort study of 1,691 patients aged 18 years or older with Candida bloodstream infection from 2002 to 2015. They analyzed demographics, comorbidities, predisposing factors, all-cause mortality, antifungal use, central-line removal, and ophthalmological and echocardiographic evaluation in order to compare 90-day all-cause mortality between individuals with and without an ID consultation.
They found that those patients who received an ID consult for a Candida bloodstream infection had a significantly lower 90-day mortality rate than did those who did not (29% vs. 51%).
With a model using inverse weighting by the propensity score, they found that ID consultation was associated with a hazard ratio of 0.81 for mortality (95% confidence interval, 0.73-0.91; P less than .0001). In the ID consultation group, the median duration of antifungal therapy was significantly longer (18 vs. 14 days; P less than .0001); central-line removal was significantly more common (76% vs. 59%; P less than .0001); echocardiography use was more frequent (57% vs. 33%; P less than .0001); and ophthalmological examinations were performed more often (53% vs. 17%; P less than .0001). Importantly, fewer patients in the ID consultation group were untreated (2% vs. 14%; P less than .0001).
In an accompanying commentary, Katrien Lagrou, MD, and Eric Van Wijngaerden, MD, of the department of microbiology, immunology and transplantation, University Hospitals Leuven (Belgium) stated: “We think that the high proportion of patients (14%) with a Candida bloodstream infection who did not receive any antifungal treatment and did not have an infectious disease consultation is a particularly alarming finding. ... Ninety-day mortality in these untreated patients was high (67%).”
“We believe every hospital should have an expert management strategy addressing all individual cases of candidaemia. The need for such expert management should be incorporated in all future candidaemia management guidelines,” they concluded.
The study was funded by the Astellas Global Development Pharma, the Washington University Institute of Clinical and Translational Sciences, and the Agency for Healthcare Research and Quality. Several of the authors had financial connections to Astellas Global Development or other pharmaceutical companies. Dr. Lagrou and Dr. Van Wijngaerden both reported receiving personal fees and nonfinancial support from a number of pharmaceutical companies, but all outside the scope of the study.
SOURCE: Mejia-Chew C et al. Lancet Infect Dis. 2019;19:1336-44.
findings from a large retrospective study suggest.
Mortality attributable to Candida bloodstream infection ranges between 15% and 47%, and delay in initiation of appropriate treatment has been associated with increased mortality. Previous small studies showed that ID consultation has conferred benefits to patients with Candida bloodstream infections. Carlos Mejia-Chew, MD, and colleagues from Washington University, St. Louis, sought to explore this further by performing a retrospective, single-center cohort study of 1,691 patients aged 18 years or older with Candida bloodstream infection from 2002 to 2015. They analyzed demographics, comorbidities, predisposing factors, all-cause mortality, antifungal use, central-line removal, and ophthalmological and echocardiographic evaluation in order to compare 90-day all-cause mortality between individuals with and without an ID consultation.
They found that those patients who received an ID consult for a Candida bloodstream infection had a significantly lower 90-day mortality rate than did those who did not (29% vs. 51%).
With a model using inverse weighting by the propensity score, they found that ID consultation was associated with a hazard ratio of 0.81 for mortality (95% confidence interval, 0.73-0.91; P less than .0001). In the ID consultation group, the median duration of antifungal therapy was significantly longer (18 vs. 14 days; P less than .0001); central-line removal was significantly more common (76% vs. 59%; P less than .0001); echocardiography use was more frequent (57% vs. 33%; P less than .0001); and ophthalmological examinations were performed more often (53% vs. 17%; P less than .0001). Importantly, fewer patients in the ID consultation group were untreated (2% vs. 14%; P less than .0001).
In an accompanying commentary, Katrien Lagrou, MD, and Eric Van Wijngaerden, MD, of the department of microbiology, immunology and transplantation, University Hospitals Leuven (Belgium) stated: “We think that the high proportion of patients (14%) with a Candida bloodstream infection who did not receive any antifungal treatment and did not have an infectious disease consultation is a particularly alarming finding. ... Ninety-day mortality in these untreated patients was high (67%).”
“We believe every hospital should have an expert management strategy addressing all individual cases of candidaemia. The need for such expert management should be incorporated in all future candidaemia management guidelines,” they concluded.
The study was funded by the Astellas Global Development Pharma, the Washington University Institute of Clinical and Translational Sciences, and the Agency for Healthcare Research and Quality. Several of the authors had financial connections to Astellas Global Development or other pharmaceutical companies. Dr. Lagrou and Dr. Van Wijngaerden both reported receiving personal fees and nonfinancial support from a number of pharmaceutical companies, but all outside the scope of the study.
SOURCE: Mejia-Chew C et al. Lancet Infect Dis. 2019;19:1336-44.
findings from a large retrospective study suggest.
Mortality attributable to Candida bloodstream infection ranges between 15% and 47%, and delay in initiation of appropriate treatment has been associated with increased mortality. Previous small studies showed that ID consultation has conferred benefits to patients with Candida bloodstream infections. Carlos Mejia-Chew, MD, and colleagues from Washington University, St. Louis, sought to explore this further by performing a retrospective, single-center cohort study of 1,691 patients aged 18 years or older with Candida bloodstream infection from 2002 to 2015. They analyzed demographics, comorbidities, predisposing factors, all-cause mortality, antifungal use, central-line removal, and ophthalmological and echocardiographic evaluation in order to compare 90-day all-cause mortality between individuals with and without an ID consultation.
They found that those patients who received an ID consult for a Candida bloodstream infection had a significantly lower 90-day mortality rate than did those who did not (29% vs. 51%).
With a model using inverse weighting by the propensity score, they found that ID consultation was associated with a hazard ratio of 0.81 for mortality (95% confidence interval, 0.73-0.91; P less than .0001). In the ID consultation group, the median duration of antifungal therapy was significantly longer (18 vs. 14 days; P less than .0001); central-line removal was significantly more common (76% vs. 59%; P less than .0001); echocardiography use was more frequent (57% vs. 33%; P less than .0001); and ophthalmological examinations were performed more often (53% vs. 17%; P less than .0001). Importantly, fewer patients in the ID consultation group were untreated (2% vs. 14%; P less than .0001).
In an accompanying commentary, Katrien Lagrou, MD, and Eric Van Wijngaerden, MD, of the department of microbiology, immunology and transplantation, University Hospitals Leuven (Belgium) stated: “We think that the high proportion of patients (14%) with a Candida bloodstream infection who did not receive any antifungal treatment and did not have an infectious disease consultation is a particularly alarming finding. ... Ninety-day mortality in these untreated patients was high (67%).”
“We believe every hospital should have an expert management strategy addressing all individual cases of candidaemia. The need for such expert management should be incorporated in all future candidaemia management guidelines,” they concluded.
The study was funded by the Astellas Global Development Pharma, the Washington University Institute of Clinical and Translational Sciences, and the Agency for Healthcare Research and Quality. Several of the authors had financial connections to Astellas Global Development or other pharmaceutical companies. Dr. Lagrou and Dr. Van Wijngaerden both reported receiving personal fees and nonfinancial support from a number of pharmaceutical companies, but all outside the scope of the study.
SOURCE: Mejia-Chew C et al. Lancet Infect Dis. 2019;19:1336-44.
FROM LANCET: INFECTIOUS DISEASES