User login
REACH2: Ruxolitinib outperformed control treatment for refractory acute GVHD
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Ruxolitinib produced significantly better efficacy outcomes in patients with glucocorticoid-refractory acute graft-versus-host disease (GVHD), compared with investigator’s choice of control therapy, in the phase 3 REACH2 trial.
However, there was a higher incidence of thrombocytopenia with ruxolitinib than with control treatment, according to a report by Robert Zeiser, MD, of University of Freiburg (Germany) and colleagues on behalf of the REACH2 research group. The report was published in the New England Journal of Medicine.
The REACH2 trial (NCT02913261) is a randomized, open-label, phase 3 trial comparing the efficacy and safety of oral ruxolitinib (10 mg twice daily) with investigator’s choice of therapy for control treatment using a list of nine commonly used options.
Patients were 12 years of age or older with glucocorticoid-refractory acute GVHD after allogeneic stem cell transplant. A total of 154 patients were assigned to the ruxolitinib group, and 155 patients were in the control group.
Most patients – 152 in the ruxolitinib group and 150 in the control group – received at least one dose of trial treatment.
Treatment discontinuation occurred in 72% (111/154) of patients in the ruxolitinib group and in 85% (132/155) of those in the control group. The most common reason for discontinuation was lack of efficacy (in 21% and 44%, respectively).
Outcomes
The overall response at day 28 (the primary endpoint) was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; odds ratio, 2.64; P < .001). The durable overall response at day 56 was also significantly higher in the ruxolitinib group than in the control group (40% vs. 22%; OR, 2.38; P < .001).
The estimated cumulative incidence of loss of response at 6 months was 10% in the ruxolitinib group compared with 39% in the control group.
The median failure-free survival was considerably longer with ruxolitinib than with control treatment (5.0 months vs. 1.0 month; hazard ratio for relapse or progression of hematologic disease, non–relapse-related death, or the use of new systemic therapy for acute GVHD, 0.46).
The median overall survival was 11.1 months in the ruxolitinib group and 6.5 months in the control group (HR, 0.83).
Overall, 72 patients (47%) in the ruxolitinib group and 77 (51%) in the control group died by the data cutoff date. Most deaths were attributed to acute GVHD (22% in the ruxolitinib group and 25% in the control group).
The most common adverse events at day 28 (in the ruxolitinib and control arms, respectively) were thrombocytopenia (33% and 18%), anemia (30% and 28%), and cytomegalovirus infection (26% and 21%).
Praise for ‘successful’ randomized trial in GVHD
“The authors are to be congratulated for completing this successful randomized trial, which showed convincingly that ruxolitinib was more effective than the investigator’s choice of therapy ... in patients in whom glucocorticoid therapy had failed,” wrote Nelson Chao, MD, of Duke University in Durham, N.C., in his invited editorial.
He went on to speculate on the possible mechanism for ruxolitinib in these patients, discussing the possible role of the STAT3 and STAT1 signaling pathways.
Dr. Chao also found it “interesting that the incidence of infectious complications or relapse was apparently not greater with ruxolitinib than with control therapy,” but he noted that the total follow-up time was short.
“As with all good research, these observations raise important questions and set the stage for further work in this area,” he concluded.
The REACH2 trial was funded by Novartis. The study authors disclosed relationships with a variety of pharmaceutical companies, including Novartis. Dr. Chao reported having no relevant disclosures.
SOURCE: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Ruxolitinib was significantly more effective against acute graft-versus-host disease than was control treatment.
Major finding: The overall response at day 28 was significantly higher in the ruxolitinib group than in the control group (62% vs. 39%; P < .001).
Study details: Phase 3 trial of 154 patients randomized to ruxolitinib and 155 patients randomized to investigator’s choice of control therapy.
Disclosures: The trial was funded by Novartis. Authors disclosed relationships with a variety of pharmaceutical companies, including Novartis.
Source: Zeiser R et al. N Engl J Med. 2020. doi: 10.1056/NEJMoa1917635.
Double Masking and Decontamination: A Doctor's COVID-19 Routine

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.
Artisanal CBD may provide less seizure control than pharmaceutical CBD
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
preliminary results of a small study indicate.
Given the widespread use of artisanal CBD products, Nathan T. Cohen, MD, pediatric epilepsy fellow, Children’s National Hospital, Washington, DC, and his colleagues wanted to know how these products differ from pharmaceutical grade CBD with respect to seizure control.
“One of the challenges or questions we have is whether there is any information that would guide us and suggest patients transition from artisanal to pharmaceutical grade CBD,” Dr. Cohen, who is lead author of the study, told Medscape Medical News.
The findings were released February 27 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology. The AAN canceled the meeting and released abstracts and access to presenters for press coverage. The study received no outside funding.
In addition to helping relieve anxiety and stress, CBD, one of many constituents of Cannabis sativa, has antiseizure properties. The US Food and Drug Administration (FDA) has approved a pharmaceutical CBD (Epidiolex, GW Pharmaceuticals) for the management of seizures associated with Lennox-Gastaut syndrome (LGS) and Dravet syndrome.
This purified oral CBD prescription product does not contain tetrahydrocannabinol (THC), the component of marijuana that produces a “high.”
Popular products
Artisanal CBD, which has been around since the late 1970s, is manufactured using variable amounts of CBD and THC. Artisanal products, which typically come in the form of oils that are swallowed, are available in dispensaries and elsewhere, depending on the legal status in individual states.
These artisanal formulations are popular among patients with epilepsy and their families. On the basis of the advertising he sees, Dr. Cohen estimates there are at least 100 artisanal CBD products, but he was quick to stress he’s not an expert on artisanal CBD.
He noted that some families are “searching for an alternative treatment” to help control their child’s seizures, and if the seizure syndrome isn’t LGS or Dravet, “then technically, they don’t qualify for prescription-strength CBD,” said Dr. Cohen.
The current study was a retrospective chart review and included patients with epilepsy who underwent treatment with artisanal or pharmaceutical CBD for whom serum CBD levels were available.
In addition to CBD levels, the researchers had information on patients’ date of birth, gender, epilepsy diagnosis, artisanal or pharmaceutical CBD dose, seizure history, and side effects, among other things.
The analysis included 31 patients (48% female; mean age, about 10 years). Of these, 32% had LGS, 6% had Dravet, and the rest had other epilepsy syndromes.
Of the total, 22 patients participated in a pharmaceutical CBD expanded-access program. The remaining nine patients received artisanal CBD.
The mean serum CBD level was 30.1 ng/mL in the artisanal group and 124 ng/mL in the pharmaceutical group.
Dr. Cohen noted that artisanal products contain lower amounts of CBD because they’re not purified, and they may contain other compounds derived from marijuana.
At the last follow-up, which was a median of 11.8 months, patients who took artisanal CBD had a 70% increase in overall seizures. Dr. Cohen pointed out that some of the hundreds of compounds in marijuana could be “pro-convulsant.”
Some seizure free
The prescription CBD group experienced a 39% reduction in seizures. “Some of these kids had up to hundreds of seizures a day and went down to tens, and some kids became seizure free,” said Dr. Cohen.
Because the study was “looking back in time,” the investigators couldn’t determine whether age, type of epilepsy, or other factors affected seizure control in the two groups, said Dr. Cohen. “One of the limitations of a retrospective study is that we’re not able to control for those factors,” he said.
Eleven patients—all in the prescription CBD group—reported adverse effects, including somnolence, emesis, diarrhea, and diminished appetite; six discontinued CBD because of side effects.
Dr. Cohen said he’s not aware of any study that has compared artisanal products “head to head” with pharmaceutical grade CBD. “The whole point of this study was to ask the question, Is there a difference between the groups?, and these new data would suggest that there may be.”
The results appear to support giving encouragement to patients to transition from artisanal to pharmaceutical CBD if appropriate. “Anytime you’re giving your child a medication that has not been produced under the stringent guidelines that all pharmaceutical FDA-approved medications undergo, you don’t know exactly what’s in the product, and not knowing is a potential issue,” said Dr. Cohen.
The findings need to be studied in a more controlled setting “to make sure they’re valid,” said Dr. Cohen. Because this is “a very hot topic,” he’s keen to see what further research his colleagues would be interested in pursuing.
Commenting on the research, Joseph Sirven, MD, a neurologist in Scottsdale, Arizona, said this is an important study.
“It highlights one of the most common questions that I receive almost on a daily basis in my neurology practice,” he said.
Most people think that dispensary-based CBD is the same as prescription-based CBD, said Dr. Sirven. “Technically and theoretically, they certainly could be; however, what this study highlights is that in practice, they are not the same.”
He stressed that prescription CBD has to meet certain quality standards. “That means that whatever the ingredient list states about the concentration of CBD in the product has to be within the product, which is why the FDA approved it. It is, in essence, a quality control issue.”
A dispensary-based product does not need to meet such stringent standards and so “is subject to whatever the manufacturer chooses to put in the product,” said Dr. Sirven.
The study received no outside funding. Drs. Cohen and Sirven reported no relevant financial relationships.
This article first appeared on Medscape.com.
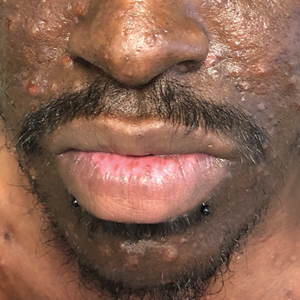
Pruritic Papules on the Face and Chest
The Diagnosis: Eosinophilic Folliculitis
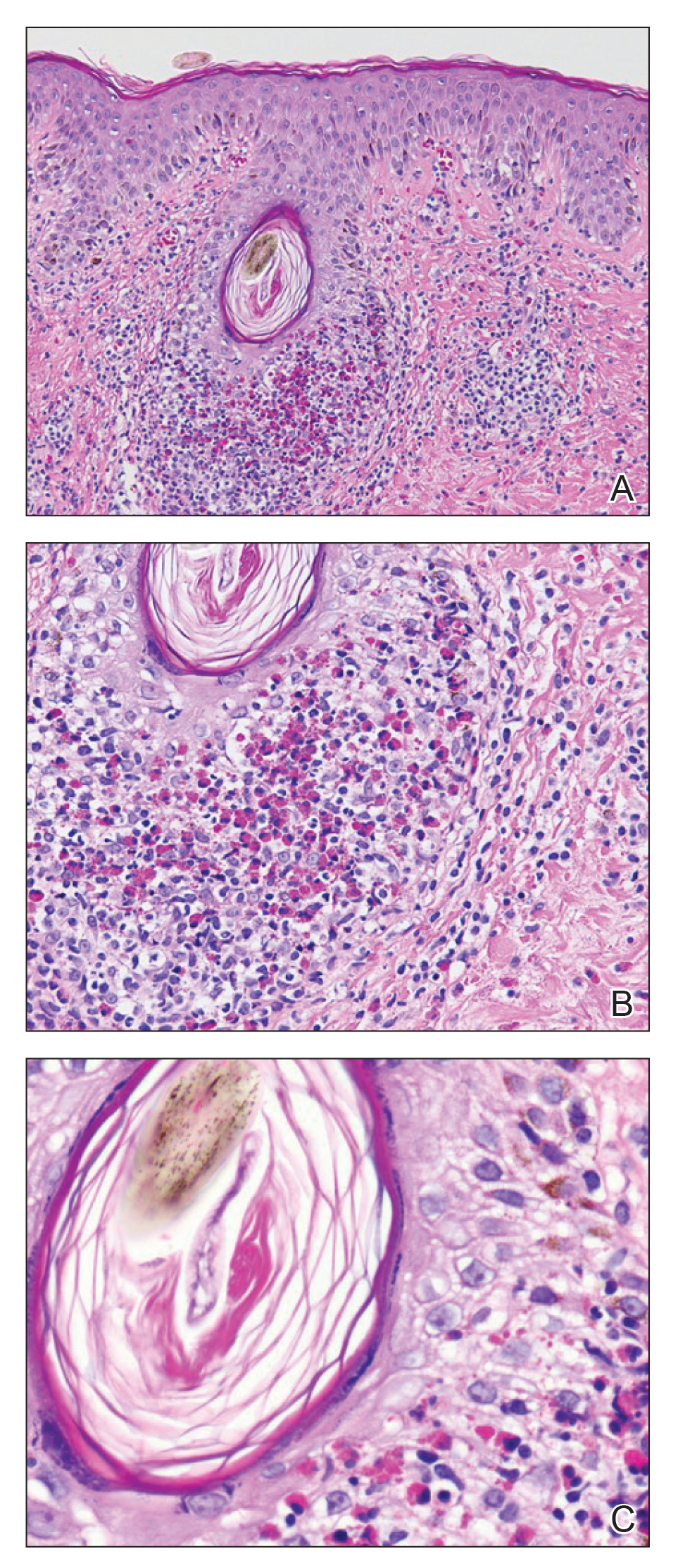
A shave biopsy specimen of an intact pustule on the left side of the chest was obtained. Histopathologic examination revealed follicular inflammation with copious eosinophils (Figure, A and B). Based on the histopathology and clinical presentation, a diagnosis of human immunodeficiency virus (HIV)-associated eosinophilic folliculitis (EF) was made.
The patient was started on triamcinolone ointment 0.1% twice daily to active lesions, oral cetirizine 10 mg in the morning, and oral hydroxyzine 25 mg at bedtime. Laboratory evaluation at the time of diagnosis showed eosinophilia with a peripheral blood eosinophil count of 0.5 K/μL (reference range, 0.03–0.48 K/μL).
Human immunodeficiency virus-associated EF is a pruritic follicular eruption that occurs in HIV-positive individuals with advanced disease. Clinically, it is characterized by intermittent, urticarial, red or flesh-colored, 2- to 5-mm papules with sparse pustules involving the head, neck, arms, and upper trunk.1,2 The cardinal clinical feature of the disorder is intense pruritus, with overlying crusts and excoriations present on physical examination.3
Patients usually have a CD4 count of less than 250 cells/mm3.2,3 Patients with HIV can develop an exacerbation of EF in the first 3 to 6 months after initiating antiretroviral therapy. This clinical pattern is believed to be due to the reconstituted immune system and increased circulation of inflammatory cells.4 Peripheral eosinophilia and elevated serum IgE levels are found in 25% to 50% of patients with HIV-associated EF.2,3
Clinically, the differential diagnosis of intensely pruritic papules with excoriations should include scabies.3 Other diagnoses to consider include opportunistic infections and papular urticaria.5 Acne vulgaris and Demodex folliculitis also may present with lesions similar to HIV-associated EF; however, these lesions tend not to be as intensely pruritic.1,5
The etiology of HIV-associated EF is unknown.3 One proposed mechanism involves a hypersensitivity reaction to Pityrosporum or Demodex mite fragments, as evidenced by studies that found fragments of these microorganisms in biopsied lesions of HIV-associated EF.3,6 In our patient's histopathology, it was noted that the afflicted hair follicle held a single Demodex mite (Figure, C).
The histopathology is characterized by a perifollicular inflammatory infiltrate of eosinophils and CD8+ lymphocytes with areas of sebaceous lysis.3,6 Spongiosis of the follicular epithelium is seen in early lesions of HIV-associated EF.6
The first-line treatment of HIV-associated EF includes antiretroviral therapy with topical steroids and antihistamines. Human immunodeficiency virus-associated EF improves as CD4 helper T-cell counts rise above 250 cells/mm3 with continued antiretroviral therapy, though it initially can cause a flare of the condition.4 High-potency steroids and antihistamines are added during this period to treat the severe pruritus.1,7 In particular, daily cetirizine has been shown to be effective, which may be due to its ability to block eosinophil migration in addition to H1-receptor antagonist properties.3,7
Various alternative therapies have been described in case reports and case series; however, there have been no controlled studies comparing therapies. Phototherapy with UVB light 3 times weekly for 3 to 6 weeks has been effective and curative in recalcitrant cases.7 Other frequently used treatments include oral metronidazole, oral itraconazole, and permethrin cream 5%. The effectiveness of the latter 2 treatments is believed to be related to the proposed role of Pityrosporum and Demodex in the pathogenesis.3
Acknowledgment
The authors thank Garth Fraga, MD (Kansas City, Kansas), for his help compiling the histopathological images and their diagnostic descriptions.
- Parker SR, Parker DC, McCall CO. Eosinophilic folliculitis in HIV-infected women: case series and review. Am J Clin Dermatol. 2006;7:193-200.
- Rosenthal D, LeBoit PE, Klumpp L, et al. Human immunodeficiency virus-associated eosinophilic folliculitis. a unique dermatosis associated with advanced human immunodeficiency virus infection. Arch Dermatol. 1991;127:206-209.
- Fearfield LA, Rowe A, Francis N, et al. Itchy folliculitis and human immunodeficiency virus infection: clinicopathological and immunological features, pathogenesis, and treatment. Br J Dermatol. 1999;141:3-11.
- Rajendran PM, Dolev JC, Heaphy MR, et al. Eosinophilic folliculitis: before and after the introduction of antiretroviral therapy. Arch Dermatol. 2005;141:1227-1231.
- Nervi SJ, Schwartz RA, Dmochowski M. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- McCalmont TH, Altemus D, Maurer T, et al. Eosinophilic folliculitis: the histological spectrum. Am J Dermatopathol. 1995;17:439-446.
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197.
The Diagnosis: Eosinophilic Folliculitis
A shave biopsy specimen of an intact pustule on the left side of the chest was obtained. Histopathologic examination revealed follicular inflammation with copious eosinophils (Figure, A and B). Based on the histopathology and clinical presentation, a diagnosis of human immunodeficiency virus (HIV)-associated eosinophilic folliculitis (EF) was made.

The patient was started on triamcinolone ointment 0.1% twice daily to active lesions, oral cetirizine 10 mg in the morning, and oral hydroxyzine 25 mg at bedtime. Laboratory evaluation at the time of diagnosis showed eosinophilia with a peripheral blood eosinophil count of 0.5 K/μL (reference range, 0.03–0.48 K/μL).
Human immunodeficiency virus-associated EF is a pruritic follicular eruption that occurs in HIV-positive individuals with advanced disease. Clinically, it is characterized by intermittent, urticarial, red or flesh-colored, 2- to 5-mm papules with sparse pustules involving the head, neck, arms, and upper trunk.1,2 The cardinal clinical feature of the disorder is intense pruritus, with overlying crusts and excoriations present on physical examination.3
Patients usually have a CD4 count of less than 250 cells/mm3.2,3 Patients with HIV can develop an exacerbation of EF in the first 3 to 6 months after initiating antiretroviral therapy. This clinical pattern is believed to be due to the reconstituted immune system and increased circulation of inflammatory cells.4 Peripheral eosinophilia and elevated serum IgE levels are found in 25% to 50% of patients with HIV-associated EF.2,3
Clinically, the differential diagnosis of intensely pruritic papules with excoriations should include scabies.3 Other diagnoses to consider include opportunistic infections and papular urticaria.5 Acne vulgaris and Demodex folliculitis also may present with lesions similar to HIV-associated EF; however, these lesions tend not to be as intensely pruritic.1,5
The etiology of HIV-associated EF is unknown.3 One proposed mechanism involves a hypersensitivity reaction to Pityrosporum or Demodex mite fragments, as evidenced by studies that found fragments of these microorganisms in biopsied lesions of HIV-associated EF.3,6 In our patient's histopathology, it was noted that the afflicted hair follicle held a single Demodex mite (Figure, C).
The histopathology is characterized by a perifollicular inflammatory infiltrate of eosinophils and CD8+ lymphocytes with areas of sebaceous lysis.3,6 Spongiosis of the follicular epithelium is seen in early lesions of HIV-associated EF.6
The first-line treatment of HIV-associated EF includes antiretroviral therapy with topical steroids and antihistamines. Human immunodeficiency virus-associated EF improves as CD4 helper T-cell counts rise above 250 cells/mm3 with continued antiretroviral therapy, though it initially can cause a flare of the condition.4 High-potency steroids and antihistamines are added during this period to treat the severe pruritus.1,7 In particular, daily cetirizine has been shown to be effective, which may be due to its ability to block eosinophil migration in addition to H1-receptor antagonist properties.3,7
Various alternative therapies have been described in case reports and case series; however, there have been no controlled studies comparing therapies. Phototherapy with UVB light 3 times weekly for 3 to 6 weeks has been effective and curative in recalcitrant cases.7 Other frequently used treatments include oral metronidazole, oral itraconazole, and permethrin cream 5%. The effectiveness of the latter 2 treatments is believed to be related to the proposed role of Pityrosporum and Demodex in the pathogenesis.3
Acknowledgment
The authors thank Garth Fraga, MD (Kansas City, Kansas), for his help compiling the histopathological images and their diagnostic descriptions.
The Diagnosis: Eosinophilic Folliculitis
A shave biopsy specimen of an intact pustule on the left side of the chest was obtained. Histopathologic examination revealed follicular inflammation with copious eosinophils (Figure, A and B). Based on the histopathology and clinical presentation, a diagnosis of human immunodeficiency virus (HIV)-associated eosinophilic folliculitis (EF) was made.

The patient was started on triamcinolone ointment 0.1% twice daily to active lesions, oral cetirizine 10 mg in the morning, and oral hydroxyzine 25 mg at bedtime. Laboratory evaluation at the time of diagnosis showed eosinophilia with a peripheral blood eosinophil count of 0.5 K/μL (reference range, 0.03–0.48 K/μL).
Human immunodeficiency virus-associated EF is a pruritic follicular eruption that occurs in HIV-positive individuals with advanced disease. Clinically, it is characterized by intermittent, urticarial, red or flesh-colored, 2- to 5-mm papules with sparse pustules involving the head, neck, arms, and upper trunk.1,2 The cardinal clinical feature of the disorder is intense pruritus, with overlying crusts and excoriations present on physical examination.3
Patients usually have a CD4 count of less than 250 cells/mm3.2,3 Patients with HIV can develop an exacerbation of EF in the first 3 to 6 months after initiating antiretroviral therapy. This clinical pattern is believed to be due to the reconstituted immune system and increased circulation of inflammatory cells.4 Peripheral eosinophilia and elevated serum IgE levels are found in 25% to 50% of patients with HIV-associated EF.2,3
Clinically, the differential diagnosis of intensely pruritic papules with excoriations should include scabies.3 Other diagnoses to consider include opportunistic infections and papular urticaria.5 Acne vulgaris and Demodex folliculitis also may present with lesions similar to HIV-associated EF; however, these lesions tend not to be as intensely pruritic.1,5
The etiology of HIV-associated EF is unknown.3 One proposed mechanism involves a hypersensitivity reaction to Pityrosporum or Demodex mite fragments, as evidenced by studies that found fragments of these microorganisms in biopsied lesions of HIV-associated EF.3,6 In our patient's histopathology, it was noted that the afflicted hair follicle held a single Demodex mite (Figure, C).
The histopathology is characterized by a perifollicular inflammatory infiltrate of eosinophils and CD8+ lymphocytes with areas of sebaceous lysis.3,6 Spongiosis of the follicular epithelium is seen in early lesions of HIV-associated EF.6
The first-line treatment of HIV-associated EF includes antiretroviral therapy with topical steroids and antihistamines. Human immunodeficiency virus-associated EF improves as CD4 helper T-cell counts rise above 250 cells/mm3 with continued antiretroviral therapy, though it initially can cause a flare of the condition.4 High-potency steroids and antihistamines are added during this period to treat the severe pruritus.1,7 In particular, daily cetirizine has been shown to be effective, which may be due to its ability to block eosinophil migration in addition to H1-receptor antagonist properties.3,7
Various alternative therapies have been described in case reports and case series; however, there have been no controlled studies comparing therapies. Phototherapy with UVB light 3 times weekly for 3 to 6 weeks has been effective and curative in recalcitrant cases.7 Other frequently used treatments include oral metronidazole, oral itraconazole, and permethrin cream 5%. The effectiveness of the latter 2 treatments is believed to be related to the proposed role of Pityrosporum and Demodex in the pathogenesis.3
Acknowledgment
The authors thank Garth Fraga, MD (Kansas City, Kansas), for his help compiling the histopathological images and their diagnostic descriptions.
- Parker SR, Parker DC, McCall CO. Eosinophilic folliculitis in HIV-infected women: case series and review. Am J Clin Dermatol. 2006;7:193-200.
- Rosenthal D, LeBoit PE, Klumpp L, et al. Human immunodeficiency virus-associated eosinophilic folliculitis. a unique dermatosis associated with advanced human immunodeficiency virus infection. Arch Dermatol. 1991;127:206-209.
- Fearfield LA, Rowe A, Francis N, et al. Itchy folliculitis and human immunodeficiency virus infection: clinicopathological and immunological features, pathogenesis, and treatment. Br J Dermatol. 1999;141:3-11.
- Rajendran PM, Dolev JC, Heaphy MR, et al. Eosinophilic folliculitis: before and after the introduction of antiretroviral therapy. Arch Dermatol. 2005;141:1227-1231.
- Nervi SJ, Schwartz RA, Dmochowski M. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- McCalmont TH, Altemus D, Maurer T, et al. Eosinophilic folliculitis: the histological spectrum. Am J Dermatopathol. 1995;17:439-446.
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197.
- Parker SR, Parker DC, McCall CO. Eosinophilic folliculitis in HIV-infected women: case series and review. Am J Clin Dermatol. 2006;7:193-200.
- Rosenthal D, LeBoit PE, Klumpp L, et al. Human immunodeficiency virus-associated eosinophilic folliculitis. a unique dermatosis associated with advanced human immunodeficiency virus infection. Arch Dermatol. 1991;127:206-209.
- Fearfield LA, Rowe A, Francis N, et al. Itchy folliculitis and human immunodeficiency virus infection: clinicopathological and immunological features, pathogenesis, and treatment. Br J Dermatol. 1999;141:3-11.
- Rajendran PM, Dolev JC, Heaphy MR, et al. Eosinophilic folliculitis: before and after the introduction of antiretroviral therapy. Arch Dermatol. 2005;141:1227-1231.
- Nervi SJ, Schwartz RA, Dmochowski M. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- McCalmont TH, Altemus D, Maurer T, et al. Eosinophilic folliculitis: the histological spectrum. Am J Dermatopathol. 1995;17:439-446.
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197.

A 31-year-old man presented with a severely pruritic rash of 2 weeks' duration. Physical examination revealed numerous urticarial papules and rare erythematous pustules over the face (top), upper chest (bottom), and proximal arms; most lesions were excoriated. Additionally, there were numerous hyperpigmented papules with central hypopigmentation on the upper chest and arms. The lower half of the body was spared. His medical history was notable for human immunodeficiency virus/AIDS with a prior episode of Pneumocystis pneumonia. He had been noncompliant with antiretroviral therapy for the last 2 years but restarted therapy 3 weeks prior to presentation. Laboratory test results revealed a CD4 cell count of 13 cells/mm3 (reference range, 500-1500 cells/mm3) with a viral load of 179 copies/mL (reference range, undetectable).
Climate changes are leading to ‘eco-anxiety,’ trauma
It is difficult right now to contemplate issues other than battling COVID-19. However, we must not lose sight of another worldwide crisis that, unless we confront it head-on, will be with us long after the pandemic is behind us. That crisis is climate change. Increased susceptibility to pandemics is likely to be a consequence of it. Unlike pandemics, climate change poses an even more long-term and pervasive existential threat to both our mental and physical health, and our existences. Many more of us who live in Australia now fear that climate change is upon us and here to stay.
Droughts, no stranger to Australians, often are punctuated by dramatic floods, and we are now dealing with extended summer seasons filled with bushfires. We are experienced in managing them. These fires are usually limited to a few different states, so fire crews typically help one another out as they are controlled and extinguished. Australians pull together with great community spirit and resilience under these circumstances.
But the last two fire seasons have been different. They have become unseasonably long, more severe, and often uncontrollable and overwhelming. We have experienced two uncharacteristically prolonged droughts, more recently creeping across most of our continent. Last spring, wild fires took hold very early and were ubiquitous, increasing during the unusually high summer heat. Climate change already had worsened our accustomed pattern of droughts, fires, and floods.
Meanwhile, the Australian federal government repeatedly ignored advice from highly respected meteorological, environmental, scientific, and economic experts.1
Warnings from experts
The state fire commissioners had formally warned our government of increasing vulnerability via climate change to bushfires. This occurred in the context of government inaction, lack of national investment (for example, insufficient water bombing equipment), and the absence of national preparation for the predicted catastrophic fire season. Prime Minister Scott Morrison declined to meet with them, minimizing the role of climate change. He provided no extra resources, emphatically leaving the responsibility to state governments.2
Distinguished economist Ross Garnaut concluded that Australia could lead the world in renewable energy production and harness it for industries and employment, if only the government chose to invest in our ample renewable sources. Sadly, our conservative government and its corporate sponsors maintain an addiction to fossil fuels, arguing that they protect employment. Meanwhile, the economic “trickle-down” benefit from massive coal and gas exports has been illusory. Socioeconomic inequities have widened, with profits favoring the mega-rich, while mining automation takes jobs.
With the fire emergency crisis at its height, Mr. Morrison sent his energy minister to the U.N. Madrid Climate Change Conference with the goal of preventing meaningful CO2 reductions, in collaboration with Brazil, Saudi Arabia, and the United States.
The sustained drought and desiccated vegetation, the escalating fuel load growth, and early hot weather led to super-hot fires, with catapulted ember attacks and fireballs falling from the sky, which burned down thousands of homes and incinerated livestock. The fires led to numerous human fatalities and overloaded hospital burn units. The unprecedented fire season duration and uncontrollable fires exhausted voluntary fire crews. There have even been fires in cool damp rain forests – the usual refuge/reservoir of endangered flora and fauna species.
The simultaneous droughts, unusual heat, and pervasive smoke also badly affect major cities, and intense fires terrorized the entire nation. Consequently, regional firefighting teams were unable to help other regions. Huge, unquenchable fires created spiraling micro-weather systems, with thunderstorms spitting dry lightning, sparking new fires and twisters, tornadoes, and updrafts hurtling heavy fire trucks into the air, which caused terrible injuries and death to fire crews. Ultimately, the federal government had to supply large-scale sea and air evacuations, and call up military reservists for civic duties.
Mental health implications
In 2007, Australian Glenn Albrecht defined “solastalgia” as the emotional pain, existential distress, loss, and grieving derived from rapid and severe changes in one’s geophysical environment or familiar habitat.3 Studies now support its existence worldwide in communities suffering great environmental change, indicating its contribution to climate change’s psychosocial impacts.4 Mental health studies also recognize the reality of “eco-anxiety,” defined as “a chronic fear of ecological doom” for self, family, community, future generations, and our planet.5
Other climate-derived psychiatric consequences include trauma, which leads to lifelong consequences for survivors of fires; grief associated with lost lives, homes, and livelihoods; posttraumatic hyperarousal; hypervigilance, re-experiencing, and rekindling; anxiety; depression; substance misuse; and long-term cognitive impacts of poor air quality. These effects are all borne from anticipated and actual loss, uncertainty about the future, and distrust in the capacity of leadership to aid recovery or prevent future recurrences. The Australian government has announced commendable, but long overdue, funds for psychological first aid, counseling, telepsychiatry, and support for developing community cohesion and resilience for first responders, young people, and badly affected rural families and communities. However, those efforts do nothing to prevent the ongoing shift of resources away from rural community mental health services, which results in severe depletion of community mental health teams, often in the very locations and communities that are suffering most from bushfires. This forces affected communities to rely on less reliable and time-limited telehealth assessments and other online services conducted by strangers, rather than more familiar and engaging in-person services – thus betraying community expectations of continuity of care and support.
While we observe our country’s path to a fateful rendezvous with an rapidly accelerating climate emergency, we can only hope that Australia and the world beyond can awaken to its reality, immediacy, extremity, and persistence and to the compelling need for serious constructive responses. It is finally dawning on the easy-going and complacent Australian public that climate change is here to stay, fully formed, as a runaway, spiraling vicious cycle – unpredictable and uncontrolled. This is not “the new normal”: It can only get worse, unless and until the nations of the world move collaboratively beyond their denial to ensure the survival of the planet and our species.
So, rather than just exemplifying a tragic casualty of rampant climate change for the world, maybe we can transform this catastrophe into an opportunity to collectively wake us up. Only then, can Australia ultimately become a positive example of developing a full national awareness of the reality and severity of the threat. Hopefully, we Australians will then commit ourselves to a full share of the global effort needed to effectively address our climate’s dire last-ditch warnings to us all.
References
1. Easton S. “ ‘Ignored and trivialized’: Experts warned Australian government before catastrophic blazes.” NBCnews.com. 2020 Feb 9.
2. Rouse A. “Scott Morrison defends why he refused to meet former fire chiefs who warned him about horror season – as he defends his handling of bushfire crisis.” Daily Mail Australia. 2020 Jan 3.
3. Albrecht G et al. Australas Psychiatry. 2007;15 Supp1:S95-8.
4. Prescott SL et al. Int J Environ Res Public Health. 2019 Nov 5;16(21).
5. Usher K et al. Int J Ment Health Nurs. 2019. Dec;28(6):1233-4.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong, Australia. He also is a community psychiatrist in a remote region of New South Wales, Australia. Dr. Rosen has no conflicts of interest. In Part 2, he discusses the impact of the fires on Australia’s indigenous population.
It is difficult right now to contemplate issues other than battling COVID-19. However, we must not lose sight of another worldwide crisis that, unless we confront it head-on, will be with us long after the pandemic is behind us. That crisis is climate change. Increased susceptibility to pandemics is likely to be a consequence of it. Unlike pandemics, climate change poses an even more long-term and pervasive existential threat to both our mental and physical health, and our existences. Many more of us who live in Australia now fear that climate change is upon us and here to stay.
Droughts, no stranger to Australians, often are punctuated by dramatic floods, and we are now dealing with extended summer seasons filled with bushfires. We are experienced in managing them. These fires are usually limited to a few different states, so fire crews typically help one another out as they are controlled and extinguished. Australians pull together with great community spirit and resilience under these circumstances.
But the last two fire seasons have been different. They have become unseasonably long, more severe, and often uncontrollable and overwhelming. We have experienced two uncharacteristically prolonged droughts, more recently creeping across most of our continent. Last spring, wild fires took hold very early and were ubiquitous, increasing during the unusually high summer heat. Climate change already had worsened our accustomed pattern of droughts, fires, and floods.
Meanwhile, the Australian federal government repeatedly ignored advice from highly respected meteorological, environmental, scientific, and economic experts.1
Warnings from experts
The state fire commissioners had formally warned our government of increasing vulnerability via climate change to bushfires. This occurred in the context of government inaction, lack of national investment (for example, insufficient water bombing equipment), and the absence of national preparation for the predicted catastrophic fire season. Prime Minister Scott Morrison declined to meet with them, minimizing the role of climate change. He provided no extra resources, emphatically leaving the responsibility to state governments.2
Distinguished economist Ross Garnaut concluded that Australia could lead the world in renewable energy production and harness it for industries and employment, if only the government chose to invest in our ample renewable sources. Sadly, our conservative government and its corporate sponsors maintain an addiction to fossil fuels, arguing that they protect employment. Meanwhile, the economic “trickle-down” benefit from massive coal and gas exports has been illusory. Socioeconomic inequities have widened, with profits favoring the mega-rich, while mining automation takes jobs.
With the fire emergency crisis at its height, Mr. Morrison sent his energy minister to the U.N. Madrid Climate Change Conference with the goal of preventing meaningful CO2 reductions, in collaboration with Brazil, Saudi Arabia, and the United States.
The sustained drought and desiccated vegetation, the escalating fuel load growth, and early hot weather led to super-hot fires, with catapulted ember attacks and fireballs falling from the sky, which burned down thousands of homes and incinerated livestock. The fires led to numerous human fatalities and overloaded hospital burn units. The unprecedented fire season duration and uncontrollable fires exhausted voluntary fire crews. There have even been fires in cool damp rain forests – the usual refuge/reservoir of endangered flora and fauna species.
The simultaneous droughts, unusual heat, and pervasive smoke also badly affect major cities, and intense fires terrorized the entire nation. Consequently, regional firefighting teams were unable to help other regions. Huge, unquenchable fires created spiraling micro-weather systems, with thunderstorms spitting dry lightning, sparking new fires and twisters, tornadoes, and updrafts hurtling heavy fire trucks into the air, which caused terrible injuries and death to fire crews. Ultimately, the federal government had to supply large-scale sea and air evacuations, and call up military reservists for civic duties.
Mental health implications
In 2007, Australian Glenn Albrecht defined “solastalgia” as the emotional pain, existential distress, loss, and grieving derived from rapid and severe changes in one’s geophysical environment or familiar habitat.3 Studies now support its existence worldwide in communities suffering great environmental change, indicating its contribution to climate change’s psychosocial impacts.4 Mental health studies also recognize the reality of “eco-anxiety,” defined as “a chronic fear of ecological doom” for self, family, community, future generations, and our planet.5
Other climate-derived psychiatric consequences include trauma, which leads to lifelong consequences for survivors of fires; grief associated with lost lives, homes, and livelihoods; posttraumatic hyperarousal; hypervigilance, re-experiencing, and rekindling; anxiety; depression; substance misuse; and long-term cognitive impacts of poor air quality. These effects are all borne from anticipated and actual loss, uncertainty about the future, and distrust in the capacity of leadership to aid recovery or prevent future recurrences. The Australian government has announced commendable, but long overdue, funds for psychological first aid, counseling, telepsychiatry, and support for developing community cohesion and resilience for first responders, young people, and badly affected rural families and communities. However, those efforts do nothing to prevent the ongoing shift of resources away from rural community mental health services, which results in severe depletion of community mental health teams, often in the very locations and communities that are suffering most from bushfires. This forces affected communities to rely on less reliable and time-limited telehealth assessments and other online services conducted by strangers, rather than more familiar and engaging in-person services – thus betraying community expectations of continuity of care and support.
While we observe our country’s path to a fateful rendezvous with an rapidly accelerating climate emergency, we can only hope that Australia and the world beyond can awaken to its reality, immediacy, extremity, and persistence and to the compelling need for serious constructive responses. It is finally dawning on the easy-going and complacent Australian public that climate change is here to stay, fully formed, as a runaway, spiraling vicious cycle – unpredictable and uncontrolled. This is not “the new normal”: It can only get worse, unless and until the nations of the world move collaboratively beyond their denial to ensure the survival of the planet and our species.
So, rather than just exemplifying a tragic casualty of rampant climate change for the world, maybe we can transform this catastrophe into an opportunity to collectively wake us up. Only then, can Australia ultimately become a positive example of developing a full national awareness of the reality and severity of the threat. Hopefully, we Australians will then commit ourselves to a full share of the global effort needed to effectively address our climate’s dire last-ditch warnings to us all.
References
1. Easton S. “ ‘Ignored and trivialized’: Experts warned Australian government before catastrophic blazes.” NBCnews.com. 2020 Feb 9.
2. Rouse A. “Scott Morrison defends why he refused to meet former fire chiefs who warned him about horror season – as he defends his handling of bushfire crisis.” Daily Mail Australia. 2020 Jan 3.
3. Albrecht G et al. Australas Psychiatry. 2007;15 Supp1:S95-8.
4. Prescott SL et al. Int J Environ Res Public Health. 2019 Nov 5;16(21).
5. Usher K et al. Int J Ment Health Nurs. 2019. Dec;28(6):1233-4.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong, Australia. He also is a community psychiatrist in a remote region of New South Wales, Australia. Dr. Rosen has no conflicts of interest. In Part 2, he discusses the impact of the fires on Australia’s indigenous population.
It is difficult right now to contemplate issues other than battling COVID-19. However, we must not lose sight of another worldwide crisis that, unless we confront it head-on, will be with us long after the pandemic is behind us. That crisis is climate change. Increased susceptibility to pandemics is likely to be a consequence of it. Unlike pandemics, climate change poses an even more long-term and pervasive existential threat to both our mental and physical health, and our existences. Many more of us who live in Australia now fear that climate change is upon us and here to stay.
Droughts, no stranger to Australians, often are punctuated by dramatic floods, and we are now dealing with extended summer seasons filled with bushfires. We are experienced in managing them. These fires are usually limited to a few different states, so fire crews typically help one another out as they are controlled and extinguished. Australians pull together with great community spirit and resilience under these circumstances.
But the last two fire seasons have been different. They have become unseasonably long, more severe, and often uncontrollable and overwhelming. We have experienced two uncharacteristically prolonged droughts, more recently creeping across most of our continent. Last spring, wild fires took hold very early and were ubiquitous, increasing during the unusually high summer heat. Climate change already had worsened our accustomed pattern of droughts, fires, and floods.
Meanwhile, the Australian federal government repeatedly ignored advice from highly respected meteorological, environmental, scientific, and economic experts.1
Warnings from experts
The state fire commissioners had formally warned our government of increasing vulnerability via climate change to bushfires. This occurred in the context of government inaction, lack of national investment (for example, insufficient water bombing equipment), and the absence of national preparation for the predicted catastrophic fire season. Prime Minister Scott Morrison declined to meet with them, minimizing the role of climate change. He provided no extra resources, emphatically leaving the responsibility to state governments.2
Distinguished economist Ross Garnaut concluded that Australia could lead the world in renewable energy production and harness it for industries and employment, if only the government chose to invest in our ample renewable sources. Sadly, our conservative government and its corporate sponsors maintain an addiction to fossil fuels, arguing that they protect employment. Meanwhile, the economic “trickle-down” benefit from massive coal and gas exports has been illusory. Socioeconomic inequities have widened, with profits favoring the mega-rich, while mining automation takes jobs.
With the fire emergency crisis at its height, Mr. Morrison sent his energy minister to the U.N. Madrid Climate Change Conference with the goal of preventing meaningful CO2 reductions, in collaboration with Brazil, Saudi Arabia, and the United States.
The sustained drought and desiccated vegetation, the escalating fuel load growth, and early hot weather led to super-hot fires, with catapulted ember attacks and fireballs falling from the sky, which burned down thousands of homes and incinerated livestock. The fires led to numerous human fatalities and overloaded hospital burn units. The unprecedented fire season duration and uncontrollable fires exhausted voluntary fire crews. There have even been fires in cool damp rain forests – the usual refuge/reservoir of endangered flora and fauna species.
The simultaneous droughts, unusual heat, and pervasive smoke also badly affect major cities, and intense fires terrorized the entire nation. Consequently, regional firefighting teams were unable to help other regions. Huge, unquenchable fires created spiraling micro-weather systems, with thunderstorms spitting dry lightning, sparking new fires and twisters, tornadoes, and updrafts hurtling heavy fire trucks into the air, which caused terrible injuries and death to fire crews. Ultimately, the federal government had to supply large-scale sea and air evacuations, and call up military reservists for civic duties.
Mental health implications
In 2007, Australian Glenn Albrecht defined “solastalgia” as the emotional pain, existential distress, loss, and grieving derived from rapid and severe changes in one’s geophysical environment or familiar habitat.3 Studies now support its existence worldwide in communities suffering great environmental change, indicating its contribution to climate change’s psychosocial impacts.4 Mental health studies also recognize the reality of “eco-anxiety,” defined as “a chronic fear of ecological doom” for self, family, community, future generations, and our planet.5
Other climate-derived psychiatric consequences include trauma, which leads to lifelong consequences for survivors of fires; grief associated with lost lives, homes, and livelihoods; posttraumatic hyperarousal; hypervigilance, re-experiencing, and rekindling; anxiety; depression; substance misuse; and long-term cognitive impacts of poor air quality. These effects are all borne from anticipated and actual loss, uncertainty about the future, and distrust in the capacity of leadership to aid recovery or prevent future recurrences. The Australian government has announced commendable, but long overdue, funds for psychological first aid, counseling, telepsychiatry, and support for developing community cohesion and resilience for first responders, young people, and badly affected rural families and communities. However, those efforts do nothing to prevent the ongoing shift of resources away from rural community mental health services, which results in severe depletion of community mental health teams, often in the very locations and communities that are suffering most from bushfires. This forces affected communities to rely on less reliable and time-limited telehealth assessments and other online services conducted by strangers, rather than more familiar and engaging in-person services – thus betraying community expectations of continuity of care and support.
While we observe our country’s path to a fateful rendezvous with an rapidly accelerating climate emergency, we can only hope that Australia and the world beyond can awaken to its reality, immediacy, extremity, and persistence and to the compelling need for serious constructive responses. It is finally dawning on the easy-going and complacent Australian public that climate change is here to stay, fully formed, as a runaway, spiraling vicious cycle – unpredictable and uncontrolled. This is not “the new normal”: It can only get worse, unless and until the nations of the world move collaboratively beyond their denial to ensure the survival of the planet and our species.
So, rather than just exemplifying a tragic casualty of rampant climate change for the world, maybe we can transform this catastrophe into an opportunity to collectively wake us up. Only then, can Australia ultimately become a positive example of developing a full national awareness of the reality and severity of the threat. Hopefully, we Australians will then commit ourselves to a full share of the global effort needed to effectively address our climate’s dire last-ditch warnings to us all.
References
1. Easton S. “ ‘Ignored and trivialized’: Experts warned Australian government before catastrophic blazes.” NBCnews.com. 2020 Feb 9.
2. Rouse A. “Scott Morrison defends why he refused to meet former fire chiefs who warned him about horror season – as he defends his handling of bushfire crisis.” Daily Mail Australia. 2020 Jan 3.
3. Albrecht G et al. Australas Psychiatry. 2007;15 Supp1:S95-8.
4. Prescott SL et al. Int J Environ Res Public Health. 2019 Nov 5;16(21).
5. Usher K et al. Int J Ment Health Nurs. 2019. Dec;28(6):1233-4.
Dr. Rosen, an officer of the Order of Australia and a Fellow of the Royal Australian and New Zealand College of Psychiatrists, is affiliated with the Brain & Mind Centre, University of Sydney, and the Institute of Mental Health at the University of Wollongong, Australia. He also is a community psychiatrist in a remote region of New South Wales, Australia. Dr. Rosen has no conflicts of interest. In Part 2, he discusses the impact of the fires on Australia’s indigenous population.
Implementing Physical Distancing in the Hospital: A Key Strategy to Prevent Nosocomial Transmission of COVID-19
Hospitalists serve as frontline healthcare professionals caring for the increasing number of COVID-19 patients in the United States. The safety of hospitalists and other frontline healthcare workers is paramount to preventing high nosocomial transmission as has been reported in several other countries. Much effort to date has rightly focused on ensuring healthcare workers have appropriate personal protective equipment (PPE) given the known increased risk of nosocomial infection to healthcare workers. However, another important strategy to prevent nosocomial transmission is to implement “social distancing,” or avoiding close contact with others. While this approach has received considerable press with regards to implementation in communities, social, or physical, distancing in the hospital is also a critical way to prevent nosocomial transmission and ensure the health and welfare of our workforce to meet the challenge. The Centers for Disease Control and Prevention (CDC) defines close contact as less than 6 feet away for over 10 minutes.1 Given the myriad clinical interactions that occur within teams in the hospital, such distancing can prove challenging.
At the University of Chicago Medicine in Illinois, our hospitalist group was an early adopter of implementing several strategies to facilitate physical distancing in the context of clinical care to minimize community transmission of COVID-19 among healthcare professionals. We describe how to implement physical distancing effectively in specific hospital settings, including some challenges and strategies to surmount them.
EDUCATIONAL CONFERENCES AND ADMINISTRATIVE MEETINGS
Educational conferences and administrative meetings need to be transitioned to virtual meetings. While it may be easy to broadcast a conference in lieu of meeting in a conference room, it is critical that hospital clinicians do not “huddle close together” in front of a computer, which would defeat the purpose of physical distancing. While “flipping the classroom” in preclinical and higher education is common, this method can be effective to deliver standard education followed by a virtual question and answer session or chat room.2
Educational discussions can also occur asynchronously through learning management systems, such as Canvas, or even closed social media channels, such as Slack, that enable discussions. These tools require training to work, so it is important to invest in education on the chosen platform to ensure that it functions smoothly. It is equally important that administrators become familiar with these tools while working remotely and can facilitate administrative meetings without difficulty. We created a one-page tip sheet to help ease the transition for department administrators. The tip sheet highlighted how to start a virtual meeting and meeting etiquette (eg, mute upon entry into the meeting, mute when not talking, announce yourself when talking) as well as ensuring that dial-ins could easily access the meeting by including one-touch options, when available, on calendar invites in addition to the weblink. A daily email update can be an important adjunct to administrative meetings to ensure critical updates are reaching all clinicians in a group and also preserves meeting time for clarifying questions.
CLINICAL WORKROOMS
Perhaps the biggest challenge is how many clinical workrooms in hospitals today are crowded with computers next to each other. Ventilation can also be poor, making conditions riskier. This makes implemention of social distancing extremely challenging, but also critical, given how much time hospital-based clinicians spend on computers and in their workrooms. The first step to achieving social distancing in the workroom is to take an inventory of how many people work there and get a log of the number of computers. Consider whether existing computers can be rearranged with a goal of keeping people 6 feet apart. For particularly cramped workrooms, this may require assigning computer spaces to physicians across a floor or several floors, using computers out on a unit, or using mobile computers to limit the number of people in the workroom at one time. We suggest working with physical plant leaders and Information Technology to reallocate mobile workstations, laptops, or desktops to conference rooms, patient visiting areas, and offices that are not being used. Because coronavirus can survive on surfaces for several hours, it is also important to stock work rooms with disinfectants to clean surfaces such as keyboards and desktops frequently. One other important thing to consider is whether computers can be assigned to specific teams or people to limit the use of a computer by multiple people.
ROUNDING, SIGN-OUT, AND MULTIDISCIPLINARY ROUNDS
Rounding
Perhaps one of the most fundamental hardships with physical distancing is how to conduct routine clinical care such as rounds, sign-out, or multidisciplinary rounds. Rounds on teaching services are particularly challenging given the number of people. At many teaching institutions, medical students are no longer on clinical rotations, which immediately reduces the number of people on teaching teams. The other thing to consider is how rounds are conducted. As opposed to a large team walking together, assign one person from the team as the liaison for the patient, which also has the added benefit of conserving precious PPE. Virtual rounding enables clinicians, including residents and attendings, to work together and decide the plan for the day without first crowding into a patient room. This is perhaps the most important cultural hurdle that one may face.
Another administrative hurdle and common concern is how to bill for such interactions. While federal guidance evolves, our institution created smartphrases for this type of virtual rounding whereby attendings attest to resident notes even if they did not physically see the patient. Additional information may be obtained from patients by calling them on their patient-room phones or by using telemedicine as some hospitals are implementing.3 For large “mega” teams, split the team into smaller groups to facilitate continuity and easier conversations.
Sign-out
When feasible, it is important to transition to phone sign-out supplemented with viewing an updated shared sign-out, ideally electronically, for shift change. When using phone sign-out, it is ideal to implement a verbal read-back to ensure understanding and to keep your sign-out updated. Because using the telephone is not the most effective communication channel for sign-out, it is key to be vigilant with other sign-out best practices, such as using a standard template like IPASS4 or another framework, prioritizing sick patients, and ensuring a focus on to-do and if/then items that are critical for the receiver to ensure understanding.5
Multidisciplinary Rounds
As multidisciplinary rounds typically occur either at the bedside or in a conference room, it is key to ensure that these occur virtually whenever possible. One option is to use conference calls or video chat (eg, Zoom) for multidisciplinary rounds whenever possible. Calendar invites or paging reminders can be used to prompt teams when to call in to discuss patients. Because multiple people are entering a virtual room at once, it is important to establish an order or have a leader orchestrate who is next. In addition, given the importance of multiple people contributing to the discussion, it is also equally important for those speaking always to announce who they are and their role (eg, social worker, case manager, physical therapist) since it may not be possible to recognize people’s voices alone. This is where visual recognition can be helpful through use of institutional video conferencing that enables hearing and seeing someone. Further, it is important to ensure that the platform being used is HIPAA compliant.
CALL ROOMS
Call rooms in hospitals can be particularly challenging if they are shared. Finding additional call rooms may require use of cots or reallocation of patient rooms. It is also possible for hospitalists to consider air mattresses in their offices or other private spaces to avoid sharing call rooms. Consider assigning the same call room to the same few people over the course of a rotation or period to avoid many people sharing one room. If a hospital is converting units to group patients under investigation or those who are COVID-19 positive, reallocating call rooms may be necessary to accommodate new teams. Lastly, it is important to communicate proactively with environmental services staff to make sure all call rooms are equipped with cleaning supplies and hand sanitizer and are cleaned daily to avoid nosocomial transmission.
CONCLUSION
/section>Containing nosocomial spread of coronavirus is particularly challenging for hospitals because of how contagious the virus is, the extreme shortage of PPE, and lack of mass testing to identify those who are sick. Therefore, physical distancing in the hospital is critical to ensure the health and well-being of the health professional workforce during the pandemic.
1. Centers for Disease Control and Prevention. Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 2, 2020.
2. Stephenson CR, Wang AT, Szostek JH, et al. Flipping the continuing medical education classroom: validating a measure of attendees’ perceptions. J Contin Educ Health Prof. 2016;36(4):256-262. https://doi.org/10.1097/CEH.0000000000000113.
3. Doshi A, Platt Y, Dressen JR, K Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(4):xxx-xxxx. https://doi.org/10.12788/jhm.3419.
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
5. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The modified, multi-patient observed simulated handoff experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
Hospitalists serve as frontline healthcare professionals caring for the increasing number of COVID-19 patients in the United States. The safety of hospitalists and other frontline healthcare workers is paramount to preventing high nosocomial transmission as has been reported in several other countries. Much effort to date has rightly focused on ensuring healthcare workers have appropriate personal protective equipment (PPE) given the known increased risk of nosocomial infection to healthcare workers. However, another important strategy to prevent nosocomial transmission is to implement “social distancing,” or avoiding close contact with others. While this approach has received considerable press with regards to implementation in communities, social, or physical, distancing in the hospital is also a critical way to prevent nosocomial transmission and ensure the health and welfare of our workforce to meet the challenge. The Centers for Disease Control and Prevention (CDC) defines close contact as less than 6 feet away for over 10 minutes.1 Given the myriad clinical interactions that occur within teams in the hospital, such distancing can prove challenging.
At the University of Chicago Medicine in Illinois, our hospitalist group was an early adopter of implementing several strategies to facilitate physical distancing in the context of clinical care to minimize community transmission of COVID-19 among healthcare professionals. We describe how to implement physical distancing effectively in specific hospital settings, including some challenges and strategies to surmount them.
EDUCATIONAL CONFERENCES AND ADMINISTRATIVE MEETINGS
Educational conferences and administrative meetings need to be transitioned to virtual meetings. While it may be easy to broadcast a conference in lieu of meeting in a conference room, it is critical that hospital clinicians do not “huddle close together” in front of a computer, which would defeat the purpose of physical distancing. While “flipping the classroom” in preclinical and higher education is common, this method can be effective to deliver standard education followed by a virtual question and answer session or chat room.2
Educational discussions can also occur asynchronously through learning management systems, such as Canvas, or even closed social media channels, such as Slack, that enable discussions. These tools require training to work, so it is important to invest in education on the chosen platform to ensure that it functions smoothly. It is equally important that administrators become familiar with these tools while working remotely and can facilitate administrative meetings without difficulty. We created a one-page tip sheet to help ease the transition for department administrators. The tip sheet highlighted how to start a virtual meeting and meeting etiquette (eg, mute upon entry into the meeting, mute when not talking, announce yourself when talking) as well as ensuring that dial-ins could easily access the meeting by including one-touch options, when available, on calendar invites in addition to the weblink. A daily email update can be an important adjunct to administrative meetings to ensure critical updates are reaching all clinicians in a group and also preserves meeting time for clarifying questions.
CLINICAL WORKROOMS
Perhaps the biggest challenge is how many clinical workrooms in hospitals today are crowded with computers next to each other. Ventilation can also be poor, making conditions riskier. This makes implemention of social distancing extremely challenging, but also critical, given how much time hospital-based clinicians spend on computers and in their workrooms. The first step to achieving social distancing in the workroom is to take an inventory of how many people work there and get a log of the number of computers. Consider whether existing computers can be rearranged with a goal of keeping people 6 feet apart. For particularly cramped workrooms, this may require assigning computer spaces to physicians across a floor or several floors, using computers out on a unit, or using mobile computers to limit the number of people in the workroom at one time. We suggest working with physical plant leaders and Information Technology to reallocate mobile workstations, laptops, or desktops to conference rooms, patient visiting areas, and offices that are not being used. Because coronavirus can survive on surfaces for several hours, it is also important to stock work rooms with disinfectants to clean surfaces such as keyboards and desktops frequently. One other important thing to consider is whether computers can be assigned to specific teams or people to limit the use of a computer by multiple people.
ROUNDING, SIGN-OUT, AND MULTIDISCIPLINARY ROUNDS
Rounding
Perhaps one of the most fundamental hardships with physical distancing is how to conduct routine clinical care such as rounds, sign-out, or multidisciplinary rounds. Rounds on teaching services are particularly challenging given the number of people. At many teaching institutions, medical students are no longer on clinical rotations, which immediately reduces the number of people on teaching teams. The other thing to consider is how rounds are conducted. As opposed to a large team walking together, assign one person from the team as the liaison for the patient, which also has the added benefit of conserving precious PPE. Virtual rounding enables clinicians, including residents and attendings, to work together and decide the plan for the day without first crowding into a patient room. This is perhaps the most important cultural hurdle that one may face.
Another administrative hurdle and common concern is how to bill for such interactions. While federal guidance evolves, our institution created smartphrases for this type of virtual rounding whereby attendings attest to resident notes even if they did not physically see the patient. Additional information may be obtained from patients by calling them on their patient-room phones or by using telemedicine as some hospitals are implementing.3 For large “mega” teams, split the team into smaller groups to facilitate continuity and easier conversations.
Sign-out
When feasible, it is important to transition to phone sign-out supplemented with viewing an updated shared sign-out, ideally electronically, for shift change. When using phone sign-out, it is ideal to implement a verbal read-back to ensure understanding and to keep your sign-out updated. Because using the telephone is not the most effective communication channel for sign-out, it is key to be vigilant with other sign-out best practices, such as using a standard template like IPASS4 or another framework, prioritizing sick patients, and ensuring a focus on to-do and if/then items that are critical for the receiver to ensure understanding.5
Multidisciplinary Rounds
As multidisciplinary rounds typically occur either at the bedside or in a conference room, it is key to ensure that these occur virtually whenever possible. One option is to use conference calls or video chat (eg, Zoom) for multidisciplinary rounds whenever possible. Calendar invites or paging reminders can be used to prompt teams when to call in to discuss patients. Because multiple people are entering a virtual room at once, it is important to establish an order or have a leader orchestrate who is next. In addition, given the importance of multiple people contributing to the discussion, it is also equally important for those speaking always to announce who they are and their role (eg, social worker, case manager, physical therapist) since it may not be possible to recognize people’s voices alone. This is where visual recognition can be helpful through use of institutional video conferencing that enables hearing and seeing someone. Further, it is important to ensure that the platform being used is HIPAA compliant.
CALL ROOMS
Call rooms in hospitals can be particularly challenging if they are shared. Finding additional call rooms may require use of cots or reallocation of patient rooms. It is also possible for hospitalists to consider air mattresses in their offices or other private spaces to avoid sharing call rooms. Consider assigning the same call room to the same few people over the course of a rotation or period to avoid many people sharing one room. If a hospital is converting units to group patients under investigation or those who are COVID-19 positive, reallocating call rooms may be necessary to accommodate new teams. Lastly, it is important to communicate proactively with environmental services staff to make sure all call rooms are equipped with cleaning supplies and hand sanitizer and are cleaned daily to avoid nosocomial transmission.
CONCLUSION
/section>Containing nosocomial spread of coronavirus is particularly challenging for hospitals because of how contagious the virus is, the extreme shortage of PPE, and lack of mass testing to identify those who are sick. Therefore, physical distancing in the hospital is critical to ensure the health and well-being of the health professional workforce during the pandemic.
Hospitalists serve as frontline healthcare professionals caring for the increasing number of COVID-19 patients in the United States. The safety of hospitalists and other frontline healthcare workers is paramount to preventing high nosocomial transmission as has been reported in several other countries. Much effort to date has rightly focused on ensuring healthcare workers have appropriate personal protective equipment (PPE) given the known increased risk of nosocomial infection to healthcare workers. However, another important strategy to prevent nosocomial transmission is to implement “social distancing,” or avoiding close contact with others. While this approach has received considerable press with regards to implementation in communities, social, or physical, distancing in the hospital is also a critical way to prevent nosocomial transmission and ensure the health and welfare of our workforce to meet the challenge. The Centers for Disease Control and Prevention (CDC) defines close contact as less than 6 feet away for over 10 minutes.1 Given the myriad clinical interactions that occur within teams in the hospital, such distancing can prove challenging.
At the University of Chicago Medicine in Illinois, our hospitalist group was an early adopter of implementing several strategies to facilitate physical distancing in the context of clinical care to minimize community transmission of COVID-19 among healthcare professionals. We describe how to implement physical distancing effectively in specific hospital settings, including some challenges and strategies to surmount them.
EDUCATIONAL CONFERENCES AND ADMINISTRATIVE MEETINGS
Educational conferences and administrative meetings need to be transitioned to virtual meetings. While it may be easy to broadcast a conference in lieu of meeting in a conference room, it is critical that hospital clinicians do not “huddle close together” in front of a computer, which would defeat the purpose of physical distancing. While “flipping the classroom” in preclinical and higher education is common, this method can be effective to deliver standard education followed by a virtual question and answer session or chat room.2
Educational discussions can also occur asynchronously through learning management systems, such as Canvas, or even closed social media channels, such as Slack, that enable discussions. These tools require training to work, so it is important to invest in education on the chosen platform to ensure that it functions smoothly. It is equally important that administrators become familiar with these tools while working remotely and can facilitate administrative meetings without difficulty. We created a one-page tip sheet to help ease the transition for department administrators. The tip sheet highlighted how to start a virtual meeting and meeting etiquette (eg, mute upon entry into the meeting, mute when not talking, announce yourself when talking) as well as ensuring that dial-ins could easily access the meeting by including one-touch options, when available, on calendar invites in addition to the weblink. A daily email update can be an important adjunct to administrative meetings to ensure critical updates are reaching all clinicians in a group and also preserves meeting time for clarifying questions.
CLINICAL WORKROOMS
Perhaps the biggest challenge is how many clinical workrooms in hospitals today are crowded with computers next to each other. Ventilation can also be poor, making conditions riskier. This makes implemention of social distancing extremely challenging, but also critical, given how much time hospital-based clinicians spend on computers and in their workrooms. The first step to achieving social distancing in the workroom is to take an inventory of how many people work there and get a log of the number of computers. Consider whether existing computers can be rearranged with a goal of keeping people 6 feet apart. For particularly cramped workrooms, this may require assigning computer spaces to physicians across a floor or several floors, using computers out on a unit, or using mobile computers to limit the number of people in the workroom at one time. We suggest working with physical plant leaders and Information Technology to reallocate mobile workstations, laptops, or desktops to conference rooms, patient visiting areas, and offices that are not being used. Because coronavirus can survive on surfaces for several hours, it is also important to stock work rooms with disinfectants to clean surfaces such as keyboards and desktops frequently. One other important thing to consider is whether computers can be assigned to specific teams or people to limit the use of a computer by multiple people.
ROUNDING, SIGN-OUT, AND MULTIDISCIPLINARY ROUNDS
Rounding
Perhaps one of the most fundamental hardships with physical distancing is how to conduct routine clinical care such as rounds, sign-out, or multidisciplinary rounds. Rounds on teaching services are particularly challenging given the number of people. At many teaching institutions, medical students are no longer on clinical rotations, which immediately reduces the number of people on teaching teams. The other thing to consider is how rounds are conducted. As opposed to a large team walking together, assign one person from the team as the liaison for the patient, which also has the added benefit of conserving precious PPE. Virtual rounding enables clinicians, including residents and attendings, to work together and decide the plan for the day without first crowding into a patient room. This is perhaps the most important cultural hurdle that one may face.
Another administrative hurdle and common concern is how to bill for such interactions. While federal guidance evolves, our institution created smartphrases for this type of virtual rounding whereby attendings attest to resident notes even if they did not physically see the patient. Additional information may be obtained from patients by calling them on their patient-room phones or by using telemedicine as some hospitals are implementing.3 For large “mega” teams, split the team into smaller groups to facilitate continuity and easier conversations.
Sign-out
When feasible, it is important to transition to phone sign-out supplemented with viewing an updated shared sign-out, ideally electronically, for shift change. When using phone sign-out, it is ideal to implement a verbal read-back to ensure understanding and to keep your sign-out updated. Because using the telephone is not the most effective communication channel for sign-out, it is key to be vigilant with other sign-out best practices, such as using a standard template like IPASS4 or another framework, prioritizing sick patients, and ensuring a focus on to-do and if/then items that are critical for the receiver to ensure understanding.5
Multidisciplinary Rounds
As multidisciplinary rounds typically occur either at the bedside or in a conference room, it is key to ensure that these occur virtually whenever possible. One option is to use conference calls or video chat (eg, Zoom) for multidisciplinary rounds whenever possible. Calendar invites or paging reminders can be used to prompt teams when to call in to discuss patients. Because multiple people are entering a virtual room at once, it is important to establish an order or have a leader orchestrate who is next. In addition, given the importance of multiple people contributing to the discussion, it is also equally important for those speaking always to announce who they are and their role (eg, social worker, case manager, physical therapist) since it may not be possible to recognize people’s voices alone. This is where visual recognition can be helpful through use of institutional video conferencing that enables hearing and seeing someone. Further, it is important to ensure that the platform being used is HIPAA compliant.
CALL ROOMS
Call rooms in hospitals can be particularly challenging if they are shared. Finding additional call rooms may require use of cots or reallocation of patient rooms. It is also possible for hospitalists to consider air mattresses in their offices or other private spaces to avoid sharing call rooms. Consider assigning the same call room to the same few people over the course of a rotation or period to avoid many people sharing one room. If a hospital is converting units to group patients under investigation or those who are COVID-19 positive, reallocating call rooms may be necessary to accommodate new teams. Lastly, it is important to communicate proactively with environmental services staff to make sure all call rooms are equipped with cleaning supplies and hand sanitizer and are cleaned daily to avoid nosocomial transmission.
CONCLUSION
/section>Containing nosocomial spread of coronavirus is particularly challenging for hospitals because of how contagious the virus is, the extreme shortage of PPE, and lack of mass testing to identify those who are sick. Therefore, physical distancing in the hospital is critical to ensure the health and well-being of the health professional workforce during the pandemic.
1. Centers for Disease Control and Prevention. Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 2, 2020.
2. Stephenson CR, Wang AT, Szostek JH, et al. Flipping the continuing medical education classroom: validating a measure of attendees’ perceptions. J Contin Educ Health Prof. 2016;36(4):256-262. https://doi.org/10.1097/CEH.0000000000000113.
3. Doshi A, Platt Y, Dressen JR, K Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(4):xxx-xxxx. https://doi.org/10.12788/jhm.3419.
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
5. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The modified, multi-patient observed simulated handoff experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
1. Centers for Disease Control and Prevention. Interim U.S. Guidance for Risk Assessment and Public Health Management of Healthcare Personnel with Potential Exposure in a Healthcare Setting to Patients with Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 2, 2020.
2. Stephenson CR, Wang AT, Szostek JH, et al. Flipping the continuing medical education classroom: validating a measure of attendees’ perceptions. J Contin Educ Health Prof. 2016;36(4):256-262. https://doi.org/10.1097/CEH.0000000000000113.
3. Doshi A, Platt Y, Dressen JR, K Mathews BK, Siy JC. Keep calm and log on: telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15(4):xxx-xxxx. https://doi.org/10.12788/jhm.3419.
4. Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. https://doi.org/10.1056/NEJMsa1405556.
5. Gaffney S, Farnan JM, Hirsch K, McGinty M, Arora VM. The modified, multi-patient observed simulated handoff experience (M-OSHE): assessment and feedback for entering residents on handoff performance. J Gen Intern Med. 2016;31(4):438-441. https://doi.org/10.1007/s11606-016-3591-8.
© 2020 Society of Hospital Medicine
Expert outlines strategies for managing migraine in women
Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

‘Encouraging’ results with pre-op therapy for DCIS
First-of-its-kind findings suggest that hormonal therapy could be the primary treatment instead of surgery for women who are diagnosed with a precursor of breast cancer, ductal carcinoma in situ (DCIS).
New results show that pre-operative endocrine therapy produced measurable radiographic changes in a cohort of postmenopausal women with estrogen receptor (ER)–positive disease. Additionally, 15% had pathologic complete responses, which were assessed after surgery as part of the study design.
The study was published in the Journal of Clinical Oncology.
Before the current results, the benefit of endocrine therapy in the absence of surgery for DCIS has been “largely unknown,” say the authors, led by Shelley Hwang, MD, of Duke University, Durham, North Carolina.
The data “represent an important step forward” in developing nonsurgical regimens for breast cancer risk reduction, say Matteo Lazzeroni, MD, and Andrea De Censi, MD, of the European Institute of Oncology, Milan, Italy, in an accompanying editorial.
They explain that primary endocrine treatment and/or active surveillance are not generally offered in DCIS because it’s hard to tell which lesions are indolent and which are likely to progress to invasive disease.
However, the editorialists point out that two phase 3 studies, including the Comparison of Operative versus Monitoring and Endocrine Therapy (COMET) trial in the United States, are examining endocrine therapy as a long-term alternative to surgery for low-risk DCIS.
In the meantime, clinicians can look at the data from Hwang and colleagues and find “encouraging” results, the Italian duo say.
Complete response in 15%
The new study is a phase 2, single-arm, multicenter US cooperative-group trial, known as Cancer and Leukemia Group B (CALGB) 40903, now part of the Alliance for Clinical Trials in Oncology. The trial involved 79 postmenopausal patients diagnosed with ER-positive DCIS without invasion. Patients were treated with letrozole 2.5 mg per day for 6 months before surgery.
The primary end point was the change in 6-month tumor volume from baseline (as assessed by magnetic resonance imaging [MRI]).
A total of 67 patients completed the 6 months of drug therapy and had all of the necessary imaging; most had intermediate- or high-grade disease (90.6%).
Median reduction from baseline MRI volume (1.4 cm3) was 0.8 cm3 (71.7%) at 6 months (P < .001).
Of the 59 patients who underwent surgery per study protocol, DCIS remained in 50 patients (85%), invasive cancer was detected in six patients (10%), and no residual DCIS or invasive cancer was seen in nine patients (15%), report the authors.
That only 10% of patients were upgraded after surgery was a surprise, say the editorialists.
That suggests “a possible down-staging effect of letrozole for concurrent invasive cancer at baseline,” they speculate, adding that such an effect would be an important potential benefit of this nonsurgical treatment of DCIS.
The authors highlighted the fact that nine patients (15%) had no disease present at surgery. These pathologic complete responses included three of the five patients in the cohort with low-grade DCIS. These outcomes occurred despite calcifications that ranged from 15 mm to 59 mm among the nine patients, they comment.
“Although this [complete response] finding is not definitive because of the small sample size and short duration of treatment, it nevertheless is provocative and suggests some women with DCIS may in the future be candidates for primary endocrine therapy alone,” write Hwang and team.
The authors also acknowledge that MRI has known limitations for assessing DCIS, “including variable concordance with pathologic size.” Notably, about 25% of the baseline MRIs in the study did not meet study criteria.
Nevertheless, the authors observe that two other histologies with a high risk of subsequent breast cancer, lobular carcinoma in situ and atypical hyperplasia, are treated preventively with primary endocrine therapy.
So this treatment approach exists and “should be studied more in DCIS as a long-term to surgery for low-risk DCIS,” the authors conclude.
Change is needed, suggest the editorialists.
DCIS of the breast accounts for up to 25% of all breast cancers in the era of screening mammography, they point out.
However, while DCIS incidence has increased by more than seven times from 1980 to 2007, its treatment has not translated into a decreased incidence of invasive breast cancer during this period.
The study was supported the National Cancer Institute. Hwang has disclosed no relevant financial relationships, but multiple study authors have ties to pharmaceutical companies. The editorial was supported by grants from the Italian Ministry of Health, Italian Association for Cancer Research, and the Italian League Against Cancer; and partially supported by the Italian Ministry of Health. Lazzeroni reported no relevant financial relationships, but De Censi disclosed research funding from Ente Ospedaliero Ospedali Galliera and nonfinancial ties to Novartis.
This article first appeared on Medscape.com.
First-of-its-kind findings suggest that hormonal therapy could be the primary treatment instead of surgery for women who are diagnosed with a precursor of breast cancer, ductal carcinoma in situ (DCIS).
New results show that pre-operative endocrine therapy produced measurable radiographic changes in a cohort of postmenopausal women with estrogen receptor (ER)–positive disease. Additionally, 15% had pathologic complete responses, which were assessed after surgery as part of the study design.
The study was published in the Journal of Clinical Oncology.
Before the current results, the benefit of endocrine therapy in the absence of surgery for DCIS has been “largely unknown,” say the authors, led by Shelley Hwang, MD, of Duke University, Durham, North Carolina.
The data “represent an important step forward” in developing nonsurgical regimens for breast cancer risk reduction, say Matteo Lazzeroni, MD, and Andrea De Censi, MD, of the European Institute of Oncology, Milan, Italy, in an accompanying editorial.
They explain that primary endocrine treatment and/or active surveillance are not generally offered in DCIS because it’s hard to tell which lesions are indolent and which are likely to progress to invasive disease.
However, the editorialists point out that two phase 3 studies, including the Comparison of Operative versus Monitoring and Endocrine Therapy (COMET) trial in the United States, are examining endocrine therapy as a long-term alternative to surgery for low-risk DCIS.
In the meantime, clinicians can look at the data from Hwang and colleagues and find “encouraging” results, the Italian duo say.
Complete response in 15%
The new study is a phase 2, single-arm, multicenter US cooperative-group trial, known as Cancer and Leukemia Group B (CALGB) 40903, now part of the Alliance for Clinical Trials in Oncology. The trial involved 79 postmenopausal patients diagnosed with ER-positive DCIS without invasion. Patients were treated with letrozole 2.5 mg per day for 6 months before surgery.
The primary end point was the change in 6-month tumor volume from baseline (as assessed by magnetic resonance imaging [MRI]).
A total of 67 patients completed the 6 months of drug therapy and had all of the necessary imaging; most had intermediate- or high-grade disease (90.6%).
Median reduction from baseline MRI volume (1.4 cm3) was 0.8 cm3 (71.7%) at 6 months (P < .001).
Of the 59 patients who underwent surgery per study protocol, DCIS remained in 50 patients (85%), invasive cancer was detected in six patients (10%), and no residual DCIS or invasive cancer was seen in nine patients (15%), report the authors.
That only 10% of patients were upgraded after surgery was a surprise, say the editorialists.
That suggests “a possible down-staging effect of letrozole for concurrent invasive cancer at baseline,” they speculate, adding that such an effect would be an important potential benefit of this nonsurgical treatment of DCIS.
The authors highlighted the fact that nine patients (15%) had no disease present at surgery. These pathologic complete responses included three of the five patients in the cohort with low-grade DCIS. These outcomes occurred despite calcifications that ranged from 15 mm to 59 mm among the nine patients, they comment.
“Although this [complete response] finding is not definitive because of the small sample size and short duration of treatment, it nevertheless is provocative and suggests some women with DCIS may in the future be candidates for primary endocrine therapy alone,” write Hwang and team.
The authors also acknowledge that MRI has known limitations for assessing DCIS, “including variable concordance with pathologic size.” Notably, about 25% of the baseline MRIs in the study did not meet study criteria.
Nevertheless, the authors observe that two other histologies with a high risk of subsequent breast cancer, lobular carcinoma in situ and atypical hyperplasia, are treated preventively with primary endocrine therapy.
So this treatment approach exists and “should be studied more in DCIS as a long-term to surgery for low-risk DCIS,” the authors conclude.
Change is needed, suggest the editorialists.
DCIS of the breast accounts for up to 25% of all breast cancers in the era of screening mammography, they point out.
However, while DCIS incidence has increased by more than seven times from 1980 to 2007, its treatment has not translated into a decreased incidence of invasive breast cancer during this period.
The study was supported the National Cancer Institute. Hwang has disclosed no relevant financial relationships, but multiple study authors have ties to pharmaceutical companies. The editorial was supported by grants from the Italian Ministry of Health, Italian Association for Cancer Research, and the Italian League Against Cancer; and partially supported by the Italian Ministry of Health. Lazzeroni reported no relevant financial relationships, but De Censi disclosed research funding from Ente Ospedaliero Ospedali Galliera and nonfinancial ties to Novartis.
This article first appeared on Medscape.com.
First-of-its-kind findings suggest that hormonal therapy could be the primary treatment instead of surgery for women who are diagnosed with a precursor of breast cancer, ductal carcinoma in situ (DCIS).
New results show that pre-operative endocrine therapy produced measurable radiographic changes in a cohort of postmenopausal women with estrogen receptor (ER)–positive disease. Additionally, 15% had pathologic complete responses, which were assessed after surgery as part of the study design.
The study was published in the Journal of Clinical Oncology.
Before the current results, the benefit of endocrine therapy in the absence of surgery for DCIS has been “largely unknown,” say the authors, led by Shelley Hwang, MD, of Duke University, Durham, North Carolina.
The data “represent an important step forward” in developing nonsurgical regimens for breast cancer risk reduction, say Matteo Lazzeroni, MD, and Andrea De Censi, MD, of the European Institute of Oncology, Milan, Italy, in an accompanying editorial.
They explain that primary endocrine treatment and/or active surveillance are not generally offered in DCIS because it’s hard to tell which lesions are indolent and which are likely to progress to invasive disease.
However, the editorialists point out that two phase 3 studies, including the Comparison of Operative versus Monitoring and Endocrine Therapy (COMET) trial in the United States, are examining endocrine therapy as a long-term alternative to surgery for low-risk DCIS.
In the meantime, clinicians can look at the data from Hwang and colleagues and find “encouraging” results, the Italian duo say.
Complete response in 15%
The new study is a phase 2, single-arm, multicenter US cooperative-group trial, known as Cancer and Leukemia Group B (CALGB) 40903, now part of the Alliance for Clinical Trials in Oncology. The trial involved 79 postmenopausal patients diagnosed with ER-positive DCIS without invasion. Patients were treated with letrozole 2.5 mg per day for 6 months before surgery.
The primary end point was the change in 6-month tumor volume from baseline (as assessed by magnetic resonance imaging [MRI]).
A total of 67 patients completed the 6 months of drug therapy and had all of the necessary imaging; most had intermediate- or high-grade disease (90.6%).
Median reduction from baseline MRI volume (1.4 cm3) was 0.8 cm3 (71.7%) at 6 months (P < .001).
Of the 59 patients who underwent surgery per study protocol, DCIS remained in 50 patients (85%), invasive cancer was detected in six patients (10%), and no residual DCIS or invasive cancer was seen in nine patients (15%), report the authors.
That only 10% of patients were upgraded after surgery was a surprise, say the editorialists.
That suggests “a possible down-staging effect of letrozole for concurrent invasive cancer at baseline,” they speculate, adding that such an effect would be an important potential benefit of this nonsurgical treatment of DCIS.
The authors highlighted the fact that nine patients (15%) had no disease present at surgery. These pathologic complete responses included three of the five patients in the cohort with low-grade DCIS. These outcomes occurred despite calcifications that ranged from 15 mm to 59 mm among the nine patients, they comment.
“Although this [complete response] finding is not definitive because of the small sample size and short duration of treatment, it nevertheless is provocative and suggests some women with DCIS may in the future be candidates for primary endocrine therapy alone,” write Hwang and team.
The authors also acknowledge that MRI has known limitations for assessing DCIS, “including variable concordance with pathologic size.” Notably, about 25% of the baseline MRIs in the study did not meet study criteria.
Nevertheless, the authors observe that two other histologies with a high risk of subsequent breast cancer, lobular carcinoma in situ and atypical hyperplasia, are treated preventively with primary endocrine therapy.
So this treatment approach exists and “should be studied more in DCIS as a long-term to surgery for low-risk DCIS,” the authors conclude.
Change is needed, suggest the editorialists.
DCIS of the breast accounts for up to 25% of all breast cancers in the era of screening mammography, they point out.
However, while DCIS incidence has increased by more than seven times from 1980 to 2007, its treatment has not translated into a decreased incidence of invasive breast cancer during this period.
The study was supported the National Cancer Institute. Hwang has disclosed no relevant financial relationships, but multiple study authors have ties to pharmaceutical companies. The editorial was supported by grants from the Italian Ministry of Health, Italian Association for Cancer Research, and the Italian League Against Cancer; and partially supported by the Italian Ministry of Health. Lazzeroni reported no relevant financial relationships, but De Censi disclosed research funding from Ente Ospedaliero Ospedali Galliera and nonfinancial ties to Novartis.
This article first appeared on Medscape.com.
Signature STEMI sign may be less diagnostic in the COVID-19 age
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
The signature electrocardiographic sign indicating ST-segment-elevation MI may be a less-consistent indicator of actual STEMI at a time when patients with COVID-19 have come to overwhelm many hospital ICUs.
Many of the 18 such patients identified at six New York City hospitals who showed ST-segment elevation on their 12-lead ECG in the city’s first month of fighting the pandemic turned out to be free of either obstructive coronary artery disease by angiography or of regional wall-motion abnormalities (RWMA) by ECG, according to a letter published in the New England Journal of Medicine.
Those 10 patients in the 18-case series were said to have noncoronary myocardial injury, perhaps from myocarditis – a prevalent feature of severe COVID-19 – and the remaining 8 patients with obstructive coronary artery disease, RWMA, or both were diagnosed with STEMI. Of the latter patients, six went to the cath lab and five of those underwent percutaneous coronary intervention, Sripal Bangalore, MD, MHA, of New York University, and colleagues reported.
In an interview, Dr. Bangalore framed the case-series report as a caution against substituting fibrinolytic therapy for primary percutaneous coronary intervention in patients with STE while hospitals are unusually burdened by the COVID-19 pandemic and invasive procedures intensify the threat of SARS-CoV-2 exposure to clinicians.
The strategy was recently advanced as an option for highly selected patients in a statement from the American College of Cardiology and Society for Cardiovascular Angiography and Interventions (SCAI).
“During the COVID-19 pandemic, one of the main reasons fibrinolytic therapy has been pushed is to reduce the exposure to the cath-lab staff,” Dr. Bangalore observed. “But if you pursue that route, it’s problematic because more than half may not have obstructive disease and fibrinolytic therapy may not help. And if you give them fibrinolytics, you’re potentially increasing their risk of bleeding complications.
“The take-home from these 18 patients is that it’s very difficult to guess who is going to have obstructive disease and who is going to have nonobstructive disease,” Dr. Bangalore said. “Maybe we should assess these patients with not just an ECG but with a quick echo, then make a decision. Our practice so far has been to take these patients to the cath lab.”
The ACC/SCAI statement proposed that “fibrinolysis can be considered an option for the relatively stable STEMI patient with active COVID-19” after careful consideration of possible patient benefit versus the risks of cath-lab personnel exposure to the virus.
Only six patients in the current series, including five in the STEMI group, are reported to have had chest pain at about the time of STE, observed Michael J. Blaha, MD, MPH, of Johns Hopkins Hospital, Baltimore.
So, he said in an interview, “one of their points is that you have to take ST elevations with a grain of salt in this [COVID-19] era, because there are a lot of people presenting with ST elevations in the absence of chest pain.”
That, and the high prevalence of nonobstructive disease in the series, indeed argues against the use of fibrinolytic therapy in such patients, Dr. Blaha said.
Normally, when there is STE, “the pretest probability of STEMI is so high, and if you can’t make it to the cath lab for some reason, sure, it makes sense to give lytics.” However, he said, “COVID-19 is changing the clinical landscape. Now, with a variety of virus-mediated myocardial injury presentations, including myocarditis, the pretest probability of MI is lower.”
The current report “confirms that, in the COVID era, ST elevations are not diagnostic for MI and must be considered within the totality of clinical evidence, and a conservative approach to going to the cath lab is probably warranted,” Dr. Blaha said in an interview.
However, with the reduced pretest probability of STE for STEMI, he agreed, “I almost don’t see any scenario where I’d be comfortable, based on ECG changes alone, giving lytics at this time.”
Dr. Bangalore pointed out that all of the 18 patients in the series had elevated levels of the fibrin degradation product D-dimer, a biomarker that reflects ongoing hemostatic activation. Levels were higher in the 8 patients who ultimately received a STEMI diagnosis than in the remaining 10 patients.
But COVID-19 patients in general may have elevated D-dimer and “a lot of microthrombi,” he said. “So the question is, are those microthrombi also causal for any of the ECG changes we are also seeing?”
Aside from microthrombi, global hypoxia and myocarditis could be other potential causes of STE in COVID-19 patients in the absence of STEMI, Dr. Bangalore proposed. “At this point we just generally don’t know.”
Dr. Bangalore reported no conflicts; disclosures for the other authors are available at nejm.org. Dr. Blaha disclosed receiving grants from Amgen and serving on advisory boards for Amgen and other pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
Management of infants born to mothers with COVID-19
Initial guidance for pediatric hospitalists
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Initial guidance for pediatric hospitalists
Initial guidance for pediatric hospitalists
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Clinical question: How should we care for newborns born to mothers with COVID-19?
Background: Around the United States, the SARS-CoV-2 virus is infecting pregnant mothers and causing COVID-19. Current limited data demonstrates that children under the age of 1 year are at risk for severe disease. Clinicians are caring for infants born to mothers with COVID-19 during the pandemic with minimal guidance.
Study design: Clinical practice guidelines.
Synopsis: The American Academy of Pediatrics’ Committee on Fetus and Newborn, Section on Neonatal and Perinatal Medicine and Committee of Infectious Diseases developed guidelines of care for infants born to COVID-19 mothers to help clinicians care for newborns using limited data published before March 30, 2020.
- Neonates should be considered persons under investigation (PUIs) if they are born to mothers with diagnosed COVID-19 or with COVID-19 tests pending at the time of delivery.
- Neonatal clinicians should attend deliveries based on their center’s policies. If clinicians are required to perform stabilization they should use airborne, droplet, and contact personal protective equipment (PPE). This includes, gown, gloves, eye protection (goggles or face shield), and N95 respirator mask or an air-purifying respirator.
- Mother and newborn should be separated to minimize the infant’s risk of postnatal infection.
- Well newborns born at or near term may be admitted to areas physically separated from newborns unaffected by maternal COVID-19. Alternatively, a mother may room-in with her infant with 6 feet of separation between mother and infant. Newborn PUIs should be bathed as soon as possible.
- Newborns requiring intensive care should be admitted to a single negative-pressure room. Alternatively, COVID-19–exposed infants should be grouped with a minimum of 6 feet of separation, or placed in air temperature-controlled isolettes.
- Until the newborn PUI’s virologic status is known, clinical staff caring for the infant should use droplet and contact PPE. This includes gown, gloves, eye protection (goggles or face shield), and a standard surgical mask. Airborne, droplet, and contact precautions should be used for infants requiring CPAP or any form of mechanical ventilation.
- COVID-19–positive mothers who want to breastfeed may feed expressed breast milk using proper breast and hand hygiene or directly breastfeed their infants wearing a mask while practicing proper breast and hand hygiene.
- If testing is available, newborns should be tested for SARS-CoV-2 using molecular arrays. If testing is unavailable, clinicians may monitor newborns clinically. Infants should be tested if they require prolonged intensive care.
- Optimal timing and extent of testing is unknown. Tests should be performed around 24 hours of life and 48 hours of life. If discharge is planned for a well appearing infant before 48 hours of life, the clinician may choose not to do the 48-hour test. A single swab should be taken from the throat followed by the nasopharynx to perform the test.
- Newborns should receive all newborn care, including circumcision if requested.
- Infants who are asymptomatic with positive or pending SARS-CoV-2 tests may be discharged home with plans for frequent outpatient follow-up through 14 days after birth. Infants with negative SARS-CoV-2 testing should be discharged to the care of a noninfected caregiver. If the mother lives in the same household, she must keep a distance of 6 feet as often as possible. When not possible, the mother should wear a mask and practice hand hygiene. The mother may resume caring for her infant normally when she has been afebrile for more than 72 hours (without antipyretics) and has been asymptomatic for 7 days. Alternatively, the mother may resume care if she has two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
- Visitation to infants requiring intensive care should be limited for mothers with COVID-19 until her fever has resolved for more than 72 hours and has improvement of respiratory symptoms and has had two consecutive negative SARS-CoV-2 nasopharyngeal swabs taken more than 24 hours apart.
Bottom line: Clinicians should protect themselves with contact and droplet PPE at all times until the infant’s viral status is known. Clinicians should use airborne, contact, and droplet PPE when resuscitating the infant and/or when using CPAP/mechanical ventilation. Mothers should be encouraged to feed their infants expressed breast milk while practicing proper hygiene or directly breastfeed while wearing a mask and practicing proper hygiene. Viral testing of every infant born to a mother with COVID-19 should be performed after the infant is 24 hours old. Mothers should resume caring for their infants normally after they have met criteria suggesting they are no longer actively infected.
Article citation: Puopolo KM, Hudak ML, Kimberlin DW, Cummings J. Initial Guidance: Management of Infants born to Mothers with COVID-19. 2020 Apr 2. https://downloads.aap.org/AAP/PDF/COVID%2019%20Initial%20Newborn%20Guidance.pdf. Accessed Apr 2, 2020.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.