User login
Today’s top news highlights: Protests and COVID-19 risk, avoidable epilepsy deaths, and more
Here are the stories our MDedge editors across specialties think you need to know about today:
Mass protests could cause COVID-19 outbreaks
As mass protests continue throughout the country, officials expressed concern about a potential spike in coronavirus cases in the coming days.
“There’s going to be a lot of issues coming out of what’s happened in the last week, but one of them is going to be that chains of transmission will have become lit from these gatherings,” Scott Gottlieb, former FDA commissioner, said on the CBS News show “Face the Nation.”
The protests generally have started peacefully with some demonstrators following physical distancing rules. But they have evolved into sometimes violent gatherings of hundreds or thousands of people where standing six feet apart is impossible. Chanting, singing, and shouting may spread the virus through respiratory droplets.
“If you were out protesting last night, you probably need to go get a COVID test this week,” Atlanta Mayor Keisha Lance Bottoms said Saturday, according to The Associated Press. Read More.
Diabetes: 1 in 10 hospitalized for COVID-19 die within a week
More than 10% of people with diabetes who are hospitalized for COVID-19 die within a week, while nearly a third require mechanical ventilation, new research shows.
Data from the CORONADO study also revealed that body mass index was independently associated with death or intubation at 7 days, while A1c and use of renin-angiotensin-aldosterone system blockers and dipeptidyl peptidase–4 inhibitors were not.
The presence of diabetes-related complications and older age also increased the risk of death.
The findings were published online in Diabetologia.
Previous studies have linked diabetes to worse outcomes in COVID-19, but this is the first to examine specific characteristics before and at the time of hospital admission that predict worse outcomes among people with diabetes, study coauthor Samy Hadjadj, MD, PhD, said in an interview. Read more.
Most adult epilepsy-related deaths could be avoided
Almost 80% of epilepsy deaths among adults are potentially avoidable, results of a new study suggest.
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, of the University of Edinburgh, United Kingdom, said during a press briefing. The findings were presented at the virtual/online Congress of the European Academy of Neurology (EAN) 2020.
Dr. Mbizvo investigated adolescents and adults aged 16 years and older who died because of epilepsy from 2009 to 2016. He compared this group to patients of similar age who were living with epilepsy. A total of 2149 epilepsy-related deaths occurred.
The most common cause of death in the 16- to 54-year age group was sudden unexpected death in epilepsy, followed by respiratory disorders, such as aspiration pneumonia. “We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” Dr. Mbizvo said.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” Dr. Mbizvo said. Read More.
FDA approves combo treatment for hepatocellular cancer
The Food and Drug Administration has approved atezolizumab (Tecentriq) in combination with bevacizumab (Avastin) to treat patients with unresectable or metastatic hepatocellular carcinoma who have not received prior systemic therapy.
The approval was supported by results from the IMbrave150 trial (N Engl J Med 2020; 382:1894-1905). This phase 3 trial enrolled 501 patients with hepatocellular carcinoma who were randomized to receive either sorafenib or atezolizumab plus bevacizumab.
The median overall survival was not reached in patients who received atezolizumab plus bevacizumab, but it was 13.2 months in patients who received sorafenib.
The median progression-free survival was 6.8 months in patients who received atezolizumab plus bevacizumab and 4.3 months for those who received sorafenib. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Mass protests could cause COVID-19 outbreaks
As mass protests continue throughout the country, officials expressed concern about a potential spike in coronavirus cases in the coming days.
“There’s going to be a lot of issues coming out of what’s happened in the last week, but one of them is going to be that chains of transmission will have become lit from these gatherings,” Scott Gottlieb, former FDA commissioner, said on the CBS News show “Face the Nation.”
The protests generally have started peacefully with some demonstrators following physical distancing rules. But they have evolved into sometimes violent gatherings of hundreds or thousands of people where standing six feet apart is impossible. Chanting, singing, and shouting may spread the virus through respiratory droplets.
“If you were out protesting last night, you probably need to go get a COVID test this week,” Atlanta Mayor Keisha Lance Bottoms said Saturday, according to The Associated Press. Read More.
Diabetes: 1 in 10 hospitalized for COVID-19 die within a week
More than 10% of people with diabetes who are hospitalized for COVID-19 die within a week, while nearly a third require mechanical ventilation, new research shows.
Data from the CORONADO study also revealed that body mass index was independently associated with death or intubation at 7 days, while A1c and use of renin-angiotensin-aldosterone system blockers and dipeptidyl peptidase–4 inhibitors were not.
The presence of diabetes-related complications and older age also increased the risk of death.
The findings were published online in Diabetologia.
Previous studies have linked diabetes to worse outcomes in COVID-19, but this is the first to examine specific characteristics before and at the time of hospital admission that predict worse outcomes among people with diabetes, study coauthor Samy Hadjadj, MD, PhD, said in an interview. Read more.
Most adult epilepsy-related deaths could be avoided
Almost 80% of epilepsy deaths among adults are potentially avoidable, results of a new study suggest.
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, of the University of Edinburgh, United Kingdom, said during a press briefing. The findings were presented at the virtual/online Congress of the European Academy of Neurology (EAN) 2020.
Dr. Mbizvo investigated adolescents and adults aged 16 years and older who died because of epilepsy from 2009 to 2016. He compared this group to patients of similar age who were living with epilepsy. A total of 2149 epilepsy-related deaths occurred.
The most common cause of death in the 16- to 54-year age group was sudden unexpected death in epilepsy, followed by respiratory disorders, such as aspiration pneumonia. “We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” Dr. Mbizvo said.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” Dr. Mbizvo said. Read More.
FDA approves combo treatment for hepatocellular cancer
The Food and Drug Administration has approved atezolizumab (Tecentriq) in combination with bevacizumab (Avastin) to treat patients with unresectable or metastatic hepatocellular carcinoma who have not received prior systemic therapy.
The approval was supported by results from the IMbrave150 trial (N Engl J Med 2020; 382:1894-1905). This phase 3 trial enrolled 501 patients with hepatocellular carcinoma who were randomized to receive either sorafenib or atezolizumab plus bevacizumab.
The median overall survival was not reached in patients who received atezolizumab plus bevacizumab, but it was 13.2 months in patients who received sorafenib.
The median progression-free survival was 6.8 months in patients who received atezolizumab plus bevacizumab and 4.3 months for those who received sorafenib. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
Mass protests could cause COVID-19 outbreaks
As mass protests continue throughout the country, officials expressed concern about a potential spike in coronavirus cases in the coming days.
“There’s going to be a lot of issues coming out of what’s happened in the last week, but one of them is going to be that chains of transmission will have become lit from these gatherings,” Scott Gottlieb, former FDA commissioner, said on the CBS News show “Face the Nation.”
The protests generally have started peacefully with some demonstrators following physical distancing rules. But they have evolved into sometimes violent gatherings of hundreds or thousands of people where standing six feet apart is impossible. Chanting, singing, and shouting may spread the virus through respiratory droplets.
“If you were out protesting last night, you probably need to go get a COVID test this week,” Atlanta Mayor Keisha Lance Bottoms said Saturday, according to The Associated Press. Read More.
Diabetes: 1 in 10 hospitalized for COVID-19 die within a week
More than 10% of people with diabetes who are hospitalized for COVID-19 die within a week, while nearly a third require mechanical ventilation, new research shows.
Data from the CORONADO study also revealed that body mass index was independently associated with death or intubation at 7 days, while A1c and use of renin-angiotensin-aldosterone system blockers and dipeptidyl peptidase–4 inhibitors were not.
The presence of diabetes-related complications and older age also increased the risk of death.
The findings were published online in Diabetologia.
Previous studies have linked diabetes to worse outcomes in COVID-19, but this is the first to examine specific characteristics before and at the time of hospital admission that predict worse outcomes among people with diabetes, study coauthor Samy Hadjadj, MD, PhD, said in an interview. Read more.
Most adult epilepsy-related deaths could be avoided
Almost 80% of epilepsy deaths among adults are potentially avoidable, results of a new study suggest.
The research shows that such avoidable deaths “remain common and have not declined over time, despite advances in treatment,” Gashirai Mbizvo, MBChB, PhD, of the University of Edinburgh, United Kingdom, said during a press briefing. The findings were presented at the virtual/online Congress of the European Academy of Neurology (EAN) 2020.
Dr. Mbizvo investigated adolescents and adults aged 16 years and older who died because of epilepsy from 2009 to 2016. He compared this group to patients of similar age who were living with epilepsy. A total of 2149 epilepsy-related deaths occurred.
The most common cause of death in the 16- to 54-year age group was sudden unexpected death in epilepsy, followed by respiratory disorders, such as aspiration pneumonia. “We think this should be avoidable, in the sense that these are people that could perhaps be targeted early with, for example, antibiotics,” Dr. Mbizvo said.
The next most common cause of death was circulatory disease, largely cardiac arrest.
“The idea is that electroexcitation – an abnormality in the brain – and the heart are related, and maybe that’s translating to a risk of death,” Dr. Mbizvo said. Read More.
FDA approves combo treatment for hepatocellular cancer
The Food and Drug Administration has approved atezolizumab (Tecentriq) in combination with bevacizumab (Avastin) to treat patients with unresectable or metastatic hepatocellular carcinoma who have not received prior systemic therapy.
The approval was supported by results from the IMbrave150 trial (N Engl J Med 2020; 382:1894-1905). This phase 3 trial enrolled 501 patients with hepatocellular carcinoma who were randomized to receive either sorafenib or atezolizumab plus bevacizumab.
The median overall survival was not reached in patients who received atezolizumab plus bevacizumab, but it was 13.2 months in patients who received sorafenib.
The median progression-free survival was 6.8 months in patients who received atezolizumab plus bevacizumab and 4.3 months for those who received sorafenib. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
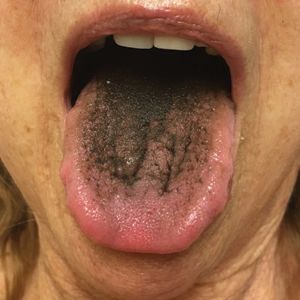
Asymptomatic Transient Lingual Hyperpigmentation
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
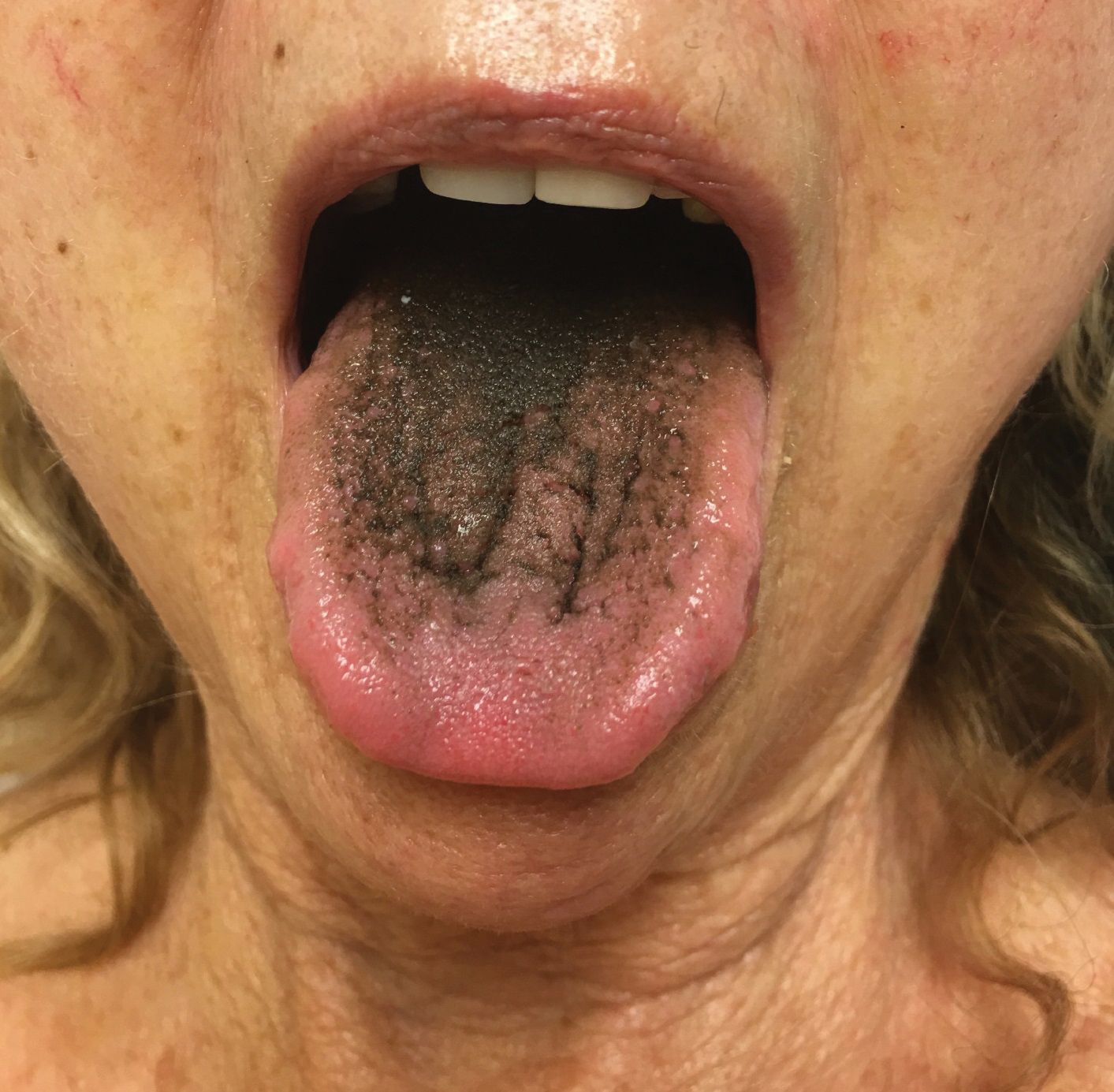
A 77-year-old woman incidentally was noted to have black discoloration of the tongue during a routine dermatologic examination. The patient was unaware of the tongue discoloration and reported that her tongue appeared normal the day prior. The tongue was asymptomatic. Clinical examination revealed black hyperpigmentation on the dorsal aspect of the tongue without appreciable hypertrophy or hyperkeratosis of the filiform papillae. The patient had a half-pack daily smoking habit for many years but had abstained from any smoking or tobacco use for the last 15 years. The patient endorsed good oral hygiene. Upon further questioning, the patient revealed that she had ingested 1 tablet of bismuth salicylate the prior night to relieve postprandial dyspepsia. A cotton-tipped applicator was rubbed gently against the affected area and removed some of the black pigment.
FDA approves ramucirumab-erlotinib combo for metastatic NSCLC
The approval was supported by results from the phase 3 RELAY trial (Lancet Oncol. 2019 Dec;20[12]:1655-69). The trial enrolled 449 patients with previously untreated, EGFR-mutated, metastatic NSCLC.
Patients received either ramucirumab at 10 mg/kg or placebo every 2 weeks as an intravenous infusion in combination with erlotinib at 150 mg orally once daily. Patients continued treatment until they progressed or developed unacceptable toxicity. The median progression-free survival was 19.4 months in the ramucirumab-erlotinib arm, compared with 12.4 months in the placebo-erlotinib arm (hazard ratio, 0.59; 95% confidence interval, 0.46-0.76; P < .0001). The overall response rate was 76% in the ramucirumab arm and 75% in the placebo arm. The median duration of response was 18.0 months and 11.1 months, respectively. Overall survival data were not mature at the final analysis.
Adverse events that were more common in the ramucirumab arm were infections, hypertension, stomatitis, proteinuria, alopecia, epistaxis, and peripheral edema. Full prescribing information is available on the FDA website.
The approval was supported by results from the phase 3 RELAY trial (Lancet Oncol. 2019 Dec;20[12]:1655-69). The trial enrolled 449 patients with previously untreated, EGFR-mutated, metastatic NSCLC.
Patients received either ramucirumab at 10 mg/kg or placebo every 2 weeks as an intravenous infusion in combination with erlotinib at 150 mg orally once daily. Patients continued treatment until they progressed or developed unacceptable toxicity. The median progression-free survival was 19.4 months in the ramucirumab-erlotinib arm, compared with 12.4 months in the placebo-erlotinib arm (hazard ratio, 0.59; 95% confidence interval, 0.46-0.76; P < .0001). The overall response rate was 76% in the ramucirumab arm and 75% in the placebo arm. The median duration of response was 18.0 months and 11.1 months, respectively. Overall survival data were not mature at the final analysis.
Adverse events that were more common in the ramucirumab arm were infections, hypertension, stomatitis, proteinuria, alopecia, epistaxis, and peripheral edema. Full prescribing information is available on the FDA website.
The approval was supported by results from the phase 3 RELAY trial (Lancet Oncol. 2019 Dec;20[12]:1655-69). The trial enrolled 449 patients with previously untreated, EGFR-mutated, metastatic NSCLC.
Patients received either ramucirumab at 10 mg/kg or placebo every 2 weeks as an intravenous infusion in combination with erlotinib at 150 mg orally once daily. Patients continued treatment until they progressed or developed unacceptable toxicity. The median progression-free survival was 19.4 months in the ramucirumab-erlotinib arm, compared with 12.4 months in the placebo-erlotinib arm (hazard ratio, 0.59; 95% confidence interval, 0.46-0.76; P < .0001). The overall response rate was 76% in the ramucirumab arm and 75% in the placebo arm. The median duration of response was 18.0 months and 11.1 months, respectively. Overall survival data were not mature at the final analysis.
Adverse events that were more common in the ramucirumab arm were infections, hypertension, stomatitis, proteinuria, alopecia, epistaxis, and peripheral edema. Full prescribing information is available on the FDA website.
FDA approves mAb combo for hepatocellular carcinoma
The approval was supported by results from the IMbrave150 trial (N Engl J Med. 2020;382:1894-1905). This phase 3 trial enrolled 501 patients with hepatocellular carcinoma who were randomized to receive either sorafenib or atezolizumab plus bevacizumab.
The median overall survival was not reached in patients who received atezolizumab plus bevacizumab, but it was 13.2 months in patients who received sorafenib (hazard ratio, 0.58; 95% confidence interval, 0.42-0.79; P = .0006). The median progression-free survival was 6.8 months in patients who received atezolizumab plus bevacizumab and 4.3 months for those who received sorafenib.
The most common adverse events seen in the atezolizumab-bevacizumab arm were hypertension, fatigue, and proteinuria.
The recommended atezolizumab dose is 1,200 mg, followed by 15 mg/kg bevacizumab on the same day every 3 weeks.
The FDA collaborated with regulatory agencies from Canada, Australia, and Singapore on the review of the atezolizumab application, as part of Project Orbis. The FDA approved the application ahead of schedule. It is still under review for the other agencies.
The approval was supported by results from the IMbrave150 trial (N Engl J Med. 2020;382:1894-1905). This phase 3 trial enrolled 501 patients with hepatocellular carcinoma who were randomized to receive either sorafenib or atezolizumab plus bevacizumab.
The median overall survival was not reached in patients who received atezolizumab plus bevacizumab, but it was 13.2 months in patients who received sorafenib (hazard ratio, 0.58; 95% confidence interval, 0.42-0.79; P = .0006). The median progression-free survival was 6.8 months in patients who received atezolizumab plus bevacizumab and 4.3 months for those who received sorafenib.
The most common adverse events seen in the atezolizumab-bevacizumab arm were hypertension, fatigue, and proteinuria.
The recommended atezolizumab dose is 1,200 mg, followed by 15 mg/kg bevacizumab on the same day every 3 weeks.
The FDA collaborated with regulatory agencies from Canada, Australia, and Singapore on the review of the atezolizumab application, as part of Project Orbis. The FDA approved the application ahead of schedule. It is still under review for the other agencies.
The approval was supported by results from the IMbrave150 trial (N Engl J Med. 2020;382:1894-1905). This phase 3 trial enrolled 501 patients with hepatocellular carcinoma who were randomized to receive either sorafenib or atezolizumab plus bevacizumab.
The median overall survival was not reached in patients who received atezolizumab plus bevacizumab, but it was 13.2 months in patients who received sorafenib (hazard ratio, 0.58; 95% confidence interval, 0.42-0.79; P = .0006). The median progression-free survival was 6.8 months in patients who received atezolizumab plus bevacizumab and 4.3 months for those who received sorafenib.
The most common adverse events seen in the atezolizumab-bevacizumab arm were hypertension, fatigue, and proteinuria.
The recommended atezolizumab dose is 1,200 mg, followed by 15 mg/kg bevacizumab on the same day every 3 weeks.
The FDA collaborated with regulatory agencies from Canada, Australia, and Singapore on the review of the atezolizumab application, as part of Project Orbis. The FDA approved the application ahead of schedule. It is still under review for the other agencies.
Sleep burden index predicts recurrent stroke
A sleep burden index that considers multiple sleep-wake disturbances (SWDs) predicts subsequent cardiocerebrovascular events during the 2 years after a stroke, preliminary results on an ongoing study suggest.
The index, which combines sleep duration, sleep disordered breathing, restless leg syndrome (RLS), insomnia, and sleep duration, is a better predictor of new events than a single sleep disorder alone.
With further evidence of its usefulness, “the sleep burden index could be integrated into clinical routine,” Simone B. Duss, PhD, of the department of neurology at Bern (Switzerland) University Hospital, told a press briefing.
The findings were presented online at the Congress of the European Academy of Neurology 2020, which transitioned to a virtual meeting because of the COVID-19 pandemic.
Sleep-wake disorders are very common in stroke patients and may preexist or appear de novo as a consequence of brain damage, said Dr. Duss. “They may also be a result of medical, psychological, or environmental challenges these patients face after a stroke.”
Clear Evidence
There’s “clear evidence” that sleep disordered breathing is a risk factor for stroke, and negatively affects stroke outcome if left untreated, said Dr. Duss.
But for other SWDs, such as insomnia, RLS, and long and short sleep duration, “the evidence is less compelling,” she said. “However, some studies still suggest they influence stroke risk and outcome.”
Experts believe that sleep disturbances after a stroke lead to sleep fragmentation, as well as decreased slow wave sleep and REM sleep.
“This negatively affects inflammatory neuroprotective and synaptic plasticity processes during the recovery process of a stroke,” said Dr. Duss. “In the end, this results in worse outcomes with regard to recurrent events but also in activities of daily living and mood.”
The new analysis aimed to assess the impact of sleep-wake disturbances on recurrent events and outcomes following a stroke or transient ischemic attack (TIA). It included 438 patients with acute stroke (85%) or TIA (15%). The mean age of the study population was 65 years, and 64% were male.
Researchers used the National Institutes of Health Stroke Scale (NIHSS) to assess stroke severity. At admission, the mean NIHSS score was 4. Most strokes (77.2%) were supratentorial.
About one-fifth of stroke patients and one-third of TIA patients had experienced a previous event.
Researchers used functional outcome scores to assess the clinical course of the stroke or TIA. In addition, they regularly asked patients about recurrence of cardiocerebrovascular events.
Investigators assessed sleep disordered breathing during the acute phase of stroke, so within the first few days, using respirography. They collected information on the presence of other sleep-wake disturbances from questionnaires and clinical interviews at 1 month, 3 months, 1 year, and 2 years after the event.
About 26% of subjects showed severe sleep disordered breathing, “meaning that they had more than 20 apnea-hypopnea events per hour,” said Dr. Duss.
More than a quarter of patients reported subclinical symptoms of insomnia (measured using the Insomnia Severity Index), and up to 10% reported severe insomnia symptoms corresponding to the clinical diagnosis of insomnia, she said.
About 9% of patients in the acute phase of stroke, and 6% in the more chronic phase, fulfilled the diagnostic criteria of RLS.
More ‘skewed’
The results for sleep duration were relatively “skewed,” said Dr. Duss. More patients reported longer sleep duration (more than 9 hours) at 1 month than at month 3, and more reported shorter sleep duration (4.0 hours or less) at month 3 than at month 1.
The researchers built a sleep burden index for the combined impact of the various sleep-wake disturbances.
They used this index as a predictor of subsequent cardiocerebrovascular events within 3 months after an event. They used a composite outcome that included recurrent stroke or TIA, MI, heart failure, and urgent revascularization, as well as new cardiocerebrovascular events, from 3 to 24 months.
The analysis showed that the mean sleep burden index was higher for stroke patients with a recurrent event, compared with stroke or TIA patients without a recurrent event (P = .0002).
A multiple logistical regression model with the presence or absence of a recurrent event as an outcome showed that the sleep burden index is a significant predictor of recurrent events (odds ratio, 2.10; P = .001). This was true even after controlling for age, gender, and baseline stroke severity.
The baseline apnea/hypopnea index and sleep duration were also significant predictors of new events. Importantly, though, the sleep burden index remained a significant predictor of recurrent events even after excluding the apnea/hypopnea index component, said Dr. Duss. “So the predictive power of the sleep burden index is not only driven by the apnea-hypopnea index at the beginning of a stroke.”
Sleep-wake disturbances “should be more carefully assessed and considered in comprehensive treatment approaches,” not only in stroke patients, but in neurologic patients in general, said Dr. Duss
She noted that these are preliminary observations from an ongoing study. The results need to be confirmed and should be when the study is finalized, she said.
Researchers are also analyzing MRI data to assess whether certain brain lesions are associated with sleep disturbances.
Jesse Dawson, MD, professor of stroke medicine at the University of Glasgow, said the clinical scoring system the study included “will be a big help in design and conduct of clinical trials.”
Although he and other stroke experts are aware of the high prevalence of sleep disorders after stroke, “we don’t routinely look for them as we’re uncertain whether intervention is of benefit,” said Dr. Dawson.
This new study “suggests there is an association with adverse outcome,” he said.
The research was supported by grants from the Swiss National Science Foundation and the Swiss Heart Foundation. Dr. Duss and Dr. Dawson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A sleep burden index that considers multiple sleep-wake disturbances (SWDs) predicts subsequent cardiocerebrovascular events during the 2 years after a stroke, preliminary results on an ongoing study suggest.
The index, which combines sleep duration, sleep disordered breathing, restless leg syndrome (RLS), insomnia, and sleep duration, is a better predictor of new events than a single sleep disorder alone.
With further evidence of its usefulness, “the sleep burden index could be integrated into clinical routine,” Simone B. Duss, PhD, of the department of neurology at Bern (Switzerland) University Hospital, told a press briefing.
The findings were presented online at the Congress of the European Academy of Neurology 2020, which transitioned to a virtual meeting because of the COVID-19 pandemic.
Sleep-wake disorders are very common in stroke patients and may preexist or appear de novo as a consequence of brain damage, said Dr. Duss. “They may also be a result of medical, psychological, or environmental challenges these patients face after a stroke.”
Clear Evidence
There’s “clear evidence” that sleep disordered breathing is a risk factor for stroke, and negatively affects stroke outcome if left untreated, said Dr. Duss.
But for other SWDs, such as insomnia, RLS, and long and short sleep duration, “the evidence is less compelling,” she said. “However, some studies still suggest they influence stroke risk and outcome.”
Experts believe that sleep disturbances after a stroke lead to sleep fragmentation, as well as decreased slow wave sleep and REM sleep.
“This negatively affects inflammatory neuroprotective and synaptic plasticity processes during the recovery process of a stroke,” said Dr. Duss. “In the end, this results in worse outcomes with regard to recurrent events but also in activities of daily living and mood.”
The new analysis aimed to assess the impact of sleep-wake disturbances on recurrent events and outcomes following a stroke or transient ischemic attack (TIA). It included 438 patients with acute stroke (85%) or TIA (15%). The mean age of the study population was 65 years, and 64% were male.
Researchers used the National Institutes of Health Stroke Scale (NIHSS) to assess stroke severity. At admission, the mean NIHSS score was 4. Most strokes (77.2%) were supratentorial.
About one-fifth of stroke patients and one-third of TIA patients had experienced a previous event.
Researchers used functional outcome scores to assess the clinical course of the stroke or TIA. In addition, they regularly asked patients about recurrence of cardiocerebrovascular events.
Investigators assessed sleep disordered breathing during the acute phase of stroke, so within the first few days, using respirography. They collected information on the presence of other sleep-wake disturbances from questionnaires and clinical interviews at 1 month, 3 months, 1 year, and 2 years after the event.
About 26% of subjects showed severe sleep disordered breathing, “meaning that they had more than 20 apnea-hypopnea events per hour,” said Dr. Duss.
More than a quarter of patients reported subclinical symptoms of insomnia (measured using the Insomnia Severity Index), and up to 10% reported severe insomnia symptoms corresponding to the clinical diagnosis of insomnia, she said.
About 9% of patients in the acute phase of stroke, and 6% in the more chronic phase, fulfilled the diagnostic criteria of RLS.
More ‘skewed’
The results for sleep duration were relatively “skewed,” said Dr. Duss. More patients reported longer sleep duration (more than 9 hours) at 1 month than at month 3, and more reported shorter sleep duration (4.0 hours or less) at month 3 than at month 1.
The researchers built a sleep burden index for the combined impact of the various sleep-wake disturbances.
They used this index as a predictor of subsequent cardiocerebrovascular events within 3 months after an event. They used a composite outcome that included recurrent stroke or TIA, MI, heart failure, and urgent revascularization, as well as new cardiocerebrovascular events, from 3 to 24 months.
The analysis showed that the mean sleep burden index was higher for stroke patients with a recurrent event, compared with stroke or TIA patients without a recurrent event (P = .0002).
A multiple logistical regression model with the presence or absence of a recurrent event as an outcome showed that the sleep burden index is a significant predictor of recurrent events (odds ratio, 2.10; P = .001). This was true even after controlling for age, gender, and baseline stroke severity.
The baseline apnea/hypopnea index and sleep duration were also significant predictors of new events. Importantly, though, the sleep burden index remained a significant predictor of recurrent events even after excluding the apnea/hypopnea index component, said Dr. Duss. “So the predictive power of the sleep burden index is not only driven by the apnea-hypopnea index at the beginning of a stroke.”
Sleep-wake disturbances “should be more carefully assessed and considered in comprehensive treatment approaches,” not only in stroke patients, but in neurologic patients in general, said Dr. Duss
She noted that these are preliminary observations from an ongoing study. The results need to be confirmed and should be when the study is finalized, she said.
Researchers are also analyzing MRI data to assess whether certain brain lesions are associated with sleep disturbances.
Jesse Dawson, MD, professor of stroke medicine at the University of Glasgow, said the clinical scoring system the study included “will be a big help in design and conduct of clinical trials.”
Although he and other stroke experts are aware of the high prevalence of sleep disorders after stroke, “we don’t routinely look for them as we’re uncertain whether intervention is of benefit,” said Dr. Dawson.
This new study “suggests there is an association with adverse outcome,” he said.
The research was supported by grants from the Swiss National Science Foundation and the Swiss Heart Foundation. Dr. Duss and Dr. Dawson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A sleep burden index that considers multiple sleep-wake disturbances (SWDs) predicts subsequent cardiocerebrovascular events during the 2 years after a stroke, preliminary results on an ongoing study suggest.
The index, which combines sleep duration, sleep disordered breathing, restless leg syndrome (RLS), insomnia, and sleep duration, is a better predictor of new events than a single sleep disorder alone.
With further evidence of its usefulness, “the sleep burden index could be integrated into clinical routine,” Simone B. Duss, PhD, of the department of neurology at Bern (Switzerland) University Hospital, told a press briefing.
The findings were presented online at the Congress of the European Academy of Neurology 2020, which transitioned to a virtual meeting because of the COVID-19 pandemic.
Sleep-wake disorders are very common in stroke patients and may preexist or appear de novo as a consequence of brain damage, said Dr. Duss. “They may also be a result of medical, psychological, or environmental challenges these patients face after a stroke.”
Clear Evidence
There’s “clear evidence” that sleep disordered breathing is a risk factor for stroke, and negatively affects stroke outcome if left untreated, said Dr. Duss.
But for other SWDs, such as insomnia, RLS, and long and short sleep duration, “the evidence is less compelling,” she said. “However, some studies still suggest they influence stroke risk and outcome.”
Experts believe that sleep disturbances after a stroke lead to sleep fragmentation, as well as decreased slow wave sleep and REM sleep.
“This negatively affects inflammatory neuroprotective and synaptic plasticity processes during the recovery process of a stroke,” said Dr. Duss. “In the end, this results in worse outcomes with regard to recurrent events but also in activities of daily living and mood.”
The new analysis aimed to assess the impact of sleep-wake disturbances on recurrent events and outcomes following a stroke or transient ischemic attack (TIA). It included 438 patients with acute stroke (85%) or TIA (15%). The mean age of the study population was 65 years, and 64% were male.
Researchers used the National Institutes of Health Stroke Scale (NIHSS) to assess stroke severity. At admission, the mean NIHSS score was 4. Most strokes (77.2%) were supratentorial.
About one-fifth of stroke patients and one-third of TIA patients had experienced a previous event.
Researchers used functional outcome scores to assess the clinical course of the stroke or TIA. In addition, they regularly asked patients about recurrence of cardiocerebrovascular events.
Investigators assessed sleep disordered breathing during the acute phase of stroke, so within the first few days, using respirography. They collected information on the presence of other sleep-wake disturbances from questionnaires and clinical interviews at 1 month, 3 months, 1 year, and 2 years after the event.
About 26% of subjects showed severe sleep disordered breathing, “meaning that they had more than 20 apnea-hypopnea events per hour,” said Dr. Duss.
More than a quarter of patients reported subclinical symptoms of insomnia (measured using the Insomnia Severity Index), and up to 10% reported severe insomnia symptoms corresponding to the clinical diagnosis of insomnia, she said.
About 9% of patients in the acute phase of stroke, and 6% in the more chronic phase, fulfilled the diagnostic criteria of RLS.
More ‘skewed’
The results for sleep duration were relatively “skewed,” said Dr. Duss. More patients reported longer sleep duration (more than 9 hours) at 1 month than at month 3, and more reported shorter sleep duration (4.0 hours or less) at month 3 than at month 1.
The researchers built a sleep burden index for the combined impact of the various sleep-wake disturbances.
They used this index as a predictor of subsequent cardiocerebrovascular events within 3 months after an event. They used a composite outcome that included recurrent stroke or TIA, MI, heart failure, and urgent revascularization, as well as new cardiocerebrovascular events, from 3 to 24 months.
The analysis showed that the mean sleep burden index was higher for stroke patients with a recurrent event, compared with stroke or TIA patients without a recurrent event (P = .0002).
A multiple logistical regression model with the presence or absence of a recurrent event as an outcome showed that the sleep burden index is a significant predictor of recurrent events (odds ratio, 2.10; P = .001). This was true even after controlling for age, gender, and baseline stroke severity.
The baseline apnea/hypopnea index and sleep duration were also significant predictors of new events. Importantly, though, the sleep burden index remained a significant predictor of recurrent events even after excluding the apnea/hypopnea index component, said Dr. Duss. “So the predictive power of the sleep burden index is not only driven by the apnea-hypopnea index at the beginning of a stroke.”
Sleep-wake disturbances “should be more carefully assessed and considered in comprehensive treatment approaches,” not only in stroke patients, but in neurologic patients in general, said Dr. Duss
She noted that these are preliminary observations from an ongoing study. The results need to be confirmed and should be when the study is finalized, she said.
Researchers are also analyzing MRI data to assess whether certain brain lesions are associated with sleep disturbances.
Jesse Dawson, MD, professor of stroke medicine at the University of Glasgow, said the clinical scoring system the study included “will be a big help in design and conduct of clinical trials.”
Although he and other stroke experts are aware of the high prevalence of sleep disorders after stroke, “we don’t routinely look for them as we’re uncertain whether intervention is of benefit,” said Dr. Dawson.
This new study “suggests there is an association with adverse outcome,” he said.
The research was supported by grants from the Swiss National Science Foundation and the Swiss Heart Foundation. Dr. Duss and Dr. Dawson disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
10% with diabetes hospitalized for COVID-19 die within a week
Data from the CORONADO (French Coronavirus SARS-CoV-2 and Diabetes Outcomes) study also revealed that body mass index (BMI) was independently associated with death or intubation at 7 days, while A1c and use of renin-angiotensin-aldosterone system (RAAS) blockers and dipeptidyl peptidase–4 inhibitors were not.
The presence of diabetes-related complications and older age also increased the risk of death.
The findings were published online Diabetologia by Bertrand Cariou, MD, PhD, of the department of endocrinology at the Hôpital Guillaume et René Laennec in Nantes, France, and colleagues.
First study to examine specific characteristics at time of admission
Previous studies have linked diabetes to worse outcomes in COVID-19, but this is the first to examine specific characteristics before and at the time of hospital admission that predict worse outcomes among people with diabetes, study coauthor Samy Hadjadj, MD, PhD, said in an interview.
“Before the CORONADO study it was ‘all diabetes [patients] are the same.’ Now we can surely consider more precisely the risk, taking age, sex, BMI, complications, and [obstructive sleep apnea] as clear ‘very high-risk situations,’” said Dr. Hadjadj, of the same institution as Dr. Cariou.
Another clinical message, Dr. Hadjadj said, is that, “even in diabetes, each increase in BMI is associated with an increase in the risk of intubation and/or death in the 7 days following admission for COVID-19. So let’s target this population as a really important population to keep social distancing and stay alert on avoiding the virus.”
But he urged caution regarding the A1c finding. “A1c might be associated with admission to hospital but other factors far beyond A1c drive the prognosis as soon as a patient is admitted. It’s surprising but reasonable speculation can explain this.”
And Dr. Hadjadj said that no obvious signals were identified with regard to medication use.
“Insulin is not suspected of having adverse effects closely related to COVID-19. RAAS blockers are not deleterious but indicative of hypertension, which is a comorbidity even in diabetes patients,” he said. (None of the patients studied were taking sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide receptor agonists.)
Yet again, high BMI emerges as a major risk factor
The study included 1,317 patients with diabetes and confirmed COVID-19 admitted to 53 French hospitals during March 10-31, 2020. Participants included 88.5% with type 2 diabetes, 3% with type 1 diabetes, and 3.1% newly diagnosed on admission. Mean age was 69.8 years.
Diabetes-related disorders on admission were reported in 11.1% of participants overall. These included 132 episodes of severe hyperglycemia, including 40 of ketosis, of which 19 were ketoacidosis, and 14 hypoglycemic events. Severe anorexia was reported in 6.3%.
The composite primary endpoint, tracheal intubation for mechanical ventilation and/or death within 7 days of admission, occurred in 29% of patients (n = 382).
Of the secondary outcomes, 31.1% (n = 410) were admitted to ICUs within 7 days of hospital admission, including 20.3% (n = 267) who required tracheal intubation for mechanical ventilation.
On day 7, 10.6% (n = 140) had died and 18.0% (n = 237) were discharged.
In the univariate analysis, the primary outcome was more frequent in men (69.1% vs. 63.2%; P = .0420) and those taking RAAS blockers (61.5% vs. 55.3%; P = .0386). Median BMI was significantly higher in those in whom the primary outcome occurred (29.1 vs 28.1 kg/m2; P = .0009),
Other characteristics prior to admission associated with risk of death on day 7 included age, hypertension, micro- and macrovascular diabetes-related complications, and comorbidities such as heart failure and treated obstructive sleep apnea.
Over 40% of those admitted had such complications. Of the patients analyzed, microvascular complications (eye, kidney, and neuropathy) were present in 47% and macrovascular complications (arteries of the heart, brain, and legs) were present in 41%.
Encouragingly, there were no deaths in patients aged under 65 years with type 1 diabetes, but only 39 participants had type 1 diabetes. Other work is ongoing to establish the effect of COVID-19 in this specific population, the researchers wrote.
Among prior medications, metformin use was lower in people who died, while insulin use, RAAS blockers, beta-blockers, loop diuretics, and mineralocorticoid-receptor antagonists were associated with death on day 7. The medication findings didn’t reach statistical significance, however.
When asked about the hint of a protective effect of metformin (odds ratio, 0.80; P = .4532), given that some experts have advised stopping it in the setting of COVID-19 because of the risk of lactic acidosis, Dr. Hadjadj said he wouldn’t necessarily stop it in all patients with COVID-19, but said, “let’s stop it in cases of severe condition.”
Analysis ongoing, ‘some new messages might pop up’
After adjustment for age and sex, BMI was significantly and positively associated with the primary outcome (P = .0001) but not with death on day 7 (P = .1488), and A1c wasn’t associated with either outcome.
In a multivariable analysis that included characteristics prior to admission, BMI remained the only independent preadmission predictor associated with the primary outcome (adjusted odds ratio, 1.28), while factors independently associated with risk of death on day 7 included age, diabetes complication history, and treated obstructive sleep apnea.
And after adjustment for age and sex, admission plasma glucose level was significantly and positively associated with both the primary outcome (P = .0001) and death on day 7 (P = .0059).
In the multivariate analysis, admission characteristics that predicted the primary outcome were dyspnea, lymphopenia, increased AST, and increased C-reactive protein.
Dr. Hadjadj said his team is now “focusing on specific risk factors such as obesity, age, vascular complications, medications ... to perform some deeper analyses.”
“We look forward to analyzing the data on in-hospital stay up to day 28 after admission. Some new messages might well pop up,” he added.
But in the meantime, “Elderly populations with long-term diabetes with advanced diabetes-related complications and/or treated obstructive sleep apnea were particularly at risk of early death and might require specific management to avoid infection with the novel coronavirus,” the researchers stressed.
The study received funding from the Fondation Francophone de Recherche sur le Diabète and was supported by Novo Nordisk, MSD, Abbott, AstraZeneca, Lilly, and the Fédération Française des Diabétiques; Société Francophone du Diabète; and Air Liquide Healthcare International. Dr. Hadjadj reported receiving grants, personal fees, and/or nonfinancial support from AstraZeneca, Bayer, Boehringer Ingelheim, Dinno Santé, Eli Lilly, LVL, MSD, Novartis, Pierre Fabre Santé, Sanofi, Servier, and Valbiotis.
A version of this article originally appeared on Medscape.com.
Data from the CORONADO (French Coronavirus SARS-CoV-2 and Diabetes Outcomes) study also revealed that body mass index (BMI) was independently associated with death or intubation at 7 days, while A1c and use of renin-angiotensin-aldosterone system (RAAS) blockers and dipeptidyl peptidase–4 inhibitors were not.
The presence of diabetes-related complications and older age also increased the risk of death.
The findings were published online Diabetologia by Bertrand Cariou, MD, PhD, of the department of endocrinology at the Hôpital Guillaume et René Laennec in Nantes, France, and colleagues.
First study to examine specific characteristics at time of admission
Previous studies have linked diabetes to worse outcomes in COVID-19, but this is the first to examine specific characteristics before and at the time of hospital admission that predict worse outcomes among people with diabetes, study coauthor Samy Hadjadj, MD, PhD, said in an interview.
“Before the CORONADO study it was ‘all diabetes [patients] are the same.’ Now we can surely consider more precisely the risk, taking age, sex, BMI, complications, and [obstructive sleep apnea] as clear ‘very high-risk situations,’” said Dr. Hadjadj, of the same institution as Dr. Cariou.
Another clinical message, Dr. Hadjadj said, is that, “even in diabetes, each increase in BMI is associated with an increase in the risk of intubation and/or death in the 7 days following admission for COVID-19. So let’s target this population as a really important population to keep social distancing and stay alert on avoiding the virus.”
But he urged caution regarding the A1c finding. “A1c might be associated with admission to hospital but other factors far beyond A1c drive the prognosis as soon as a patient is admitted. It’s surprising but reasonable speculation can explain this.”
And Dr. Hadjadj said that no obvious signals were identified with regard to medication use.
“Insulin is not suspected of having adverse effects closely related to COVID-19. RAAS blockers are not deleterious but indicative of hypertension, which is a comorbidity even in diabetes patients,” he said. (None of the patients studied were taking sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide receptor agonists.)
Yet again, high BMI emerges as a major risk factor
The study included 1,317 patients with diabetes and confirmed COVID-19 admitted to 53 French hospitals during March 10-31, 2020. Participants included 88.5% with type 2 diabetes, 3% with type 1 diabetes, and 3.1% newly diagnosed on admission. Mean age was 69.8 years.
Diabetes-related disorders on admission were reported in 11.1% of participants overall. These included 132 episodes of severe hyperglycemia, including 40 of ketosis, of which 19 were ketoacidosis, and 14 hypoglycemic events. Severe anorexia was reported in 6.3%.
The composite primary endpoint, tracheal intubation for mechanical ventilation and/or death within 7 days of admission, occurred in 29% of patients (n = 382).
Of the secondary outcomes, 31.1% (n = 410) were admitted to ICUs within 7 days of hospital admission, including 20.3% (n = 267) who required tracheal intubation for mechanical ventilation.
On day 7, 10.6% (n = 140) had died and 18.0% (n = 237) were discharged.
In the univariate analysis, the primary outcome was more frequent in men (69.1% vs. 63.2%; P = .0420) and those taking RAAS blockers (61.5% vs. 55.3%; P = .0386). Median BMI was significantly higher in those in whom the primary outcome occurred (29.1 vs 28.1 kg/m2; P = .0009),
Other characteristics prior to admission associated with risk of death on day 7 included age, hypertension, micro- and macrovascular diabetes-related complications, and comorbidities such as heart failure and treated obstructive sleep apnea.
Over 40% of those admitted had such complications. Of the patients analyzed, microvascular complications (eye, kidney, and neuropathy) were present in 47% and macrovascular complications (arteries of the heart, brain, and legs) were present in 41%.
Encouragingly, there were no deaths in patients aged under 65 years with type 1 diabetes, but only 39 participants had type 1 diabetes. Other work is ongoing to establish the effect of COVID-19 in this specific population, the researchers wrote.
Among prior medications, metformin use was lower in people who died, while insulin use, RAAS blockers, beta-blockers, loop diuretics, and mineralocorticoid-receptor antagonists were associated with death on day 7. The medication findings didn’t reach statistical significance, however.
When asked about the hint of a protective effect of metformin (odds ratio, 0.80; P = .4532), given that some experts have advised stopping it in the setting of COVID-19 because of the risk of lactic acidosis, Dr. Hadjadj said he wouldn’t necessarily stop it in all patients with COVID-19, but said, “let’s stop it in cases of severe condition.”
Analysis ongoing, ‘some new messages might pop up’
After adjustment for age and sex, BMI was significantly and positively associated with the primary outcome (P = .0001) but not with death on day 7 (P = .1488), and A1c wasn’t associated with either outcome.
In a multivariable analysis that included characteristics prior to admission, BMI remained the only independent preadmission predictor associated with the primary outcome (adjusted odds ratio, 1.28), while factors independently associated with risk of death on day 7 included age, diabetes complication history, and treated obstructive sleep apnea.
And after adjustment for age and sex, admission plasma glucose level was significantly and positively associated with both the primary outcome (P = .0001) and death on day 7 (P = .0059).
In the multivariate analysis, admission characteristics that predicted the primary outcome were dyspnea, lymphopenia, increased AST, and increased C-reactive protein.
Dr. Hadjadj said his team is now “focusing on specific risk factors such as obesity, age, vascular complications, medications ... to perform some deeper analyses.”
“We look forward to analyzing the data on in-hospital stay up to day 28 after admission. Some new messages might well pop up,” he added.
But in the meantime, “Elderly populations with long-term diabetes with advanced diabetes-related complications and/or treated obstructive sleep apnea were particularly at risk of early death and might require specific management to avoid infection with the novel coronavirus,” the researchers stressed.
The study received funding from the Fondation Francophone de Recherche sur le Diabète and was supported by Novo Nordisk, MSD, Abbott, AstraZeneca, Lilly, and the Fédération Française des Diabétiques; Société Francophone du Diabète; and Air Liquide Healthcare International. Dr. Hadjadj reported receiving grants, personal fees, and/or nonfinancial support from AstraZeneca, Bayer, Boehringer Ingelheim, Dinno Santé, Eli Lilly, LVL, MSD, Novartis, Pierre Fabre Santé, Sanofi, Servier, and Valbiotis.
A version of this article originally appeared on Medscape.com.
Data from the CORONADO (French Coronavirus SARS-CoV-2 and Diabetes Outcomes) study also revealed that body mass index (BMI) was independently associated with death or intubation at 7 days, while A1c and use of renin-angiotensin-aldosterone system (RAAS) blockers and dipeptidyl peptidase–4 inhibitors were not.
The presence of diabetes-related complications and older age also increased the risk of death.
The findings were published online Diabetologia by Bertrand Cariou, MD, PhD, of the department of endocrinology at the Hôpital Guillaume et René Laennec in Nantes, France, and colleagues.
First study to examine specific characteristics at time of admission
Previous studies have linked diabetes to worse outcomes in COVID-19, but this is the first to examine specific characteristics before and at the time of hospital admission that predict worse outcomes among people with diabetes, study coauthor Samy Hadjadj, MD, PhD, said in an interview.
“Before the CORONADO study it was ‘all diabetes [patients] are the same.’ Now we can surely consider more precisely the risk, taking age, sex, BMI, complications, and [obstructive sleep apnea] as clear ‘very high-risk situations,’” said Dr. Hadjadj, of the same institution as Dr. Cariou.
Another clinical message, Dr. Hadjadj said, is that, “even in diabetes, each increase in BMI is associated with an increase in the risk of intubation and/or death in the 7 days following admission for COVID-19. So let’s target this population as a really important population to keep social distancing and stay alert on avoiding the virus.”
But he urged caution regarding the A1c finding. “A1c might be associated with admission to hospital but other factors far beyond A1c drive the prognosis as soon as a patient is admitted. It’s surprising but reasonable speculation can explain this.”
And Dr. Hadjadj said that no obvious signals were identified with regard to medication use.
“Insulin is not suspected of having adverse effects closely related to COVID-19. RAAS blockers are not deleterious but indicative of hypertension, which is a comorbidity even in diabetes patients,” he said. (None of the patients studied were taking sodium-glucose cotransporter 2 inhibitors or glucagonlike peptide receptor agonists.)
Yet again, high BMI emerges as a major risk factor
The study included 1,317 patients with diabetes and confirmed COVID-19 admitted to 53 French hospitals during March 10-31, 2020. Participants included 88.5% with type 2 diabetes, 3% with type 1 diabetes, and 3.1% newly diagnosed on admission. Mean age was 69.8 years.
Diabetes-related disorders on admission were reported in 11.1% of participants overall. These included 132 episodes of severe hyperglycemia, including 40 of ketosis, of which 19 were ketoacidosis, and 14 hypoglycemic events. Severe anorexia was reported in 6.3%.
The composite primary endpoint, tracheal intubation for mechanical ventilation and/or death within 7 days of admission, occurred in 29% of patients (n = 382).
Of the secondary outcomes, 31.1% (n = 410) were admitted to ICUs within 7 days of hospital admission, including 20.3% (n = 267) who required tracheal intubation for mechanical ventilation.
On day 7, 10.6% (n = 140) had died and 18.0% (n = 237) were discharged.
In the univariate analysis, the primary outcome was more frequent in men (69.1% vs. 63.2%; P = .0420) and those taking RAAS blockers (61.5% vs. 55.3%; P = .0386). Median BMI was significantly higher in those in whom the primary outcome occurred (29.1 vs 28.1 kg/m2; P = .0009),
Other characteristics prior to admission associated with risk of death on day 7 included age, hypertension, micro- and macrovascular diabetes-related complications, and comorbidities such as heart failure and treated obstructive sleep apnea.
Over 40% of those admitted had such complications. Of the patients analyzed, microvascular complications (eye, kidney, and neuropathy) were present in 47% and macrovascular complications (arteries of the heart, brain, and legs) were present in 41%.
Encouragingly, there were no deaths in patients aged under 65 years with type 1 diabetes, but only 39 participants had type 1 diabetes. Other work is ongoing to establish the effect of COVID-19 in this specific population, the researchers wrote.
Among prior medications, metformin use was lower in people who died, while insulin use, RAAS blockers, beta-blockers, loop diuretics, and mineralocorticoid-receptor antagonists were associated with death on day 7. The medication findings didn’t reach statistical significance, however.
When asked about the hint of a protective effect of metformin (odds ratio, 0.80; P = .4532), given that some experts have advised stopping it in the setting of COVID-19 because of the risk of lactic acidosis, Dr. Hadjadj said he wouldn’t necessarily stop it in all patients with COVID-19, but said, “let’s stop it in cases of severe condition.”
Analysis ongoing, ‘some new messages might pop up’
After adjustment for age and sex, BMI was significantly and positively associated with the primary outcome (P = .0001) but not with death on day 7 (P = .1488), and A1c wasn’t associated with either outcome.
In a multivariable analysis that included characteristics prior to admission, BMI remained the only independent preadmission predictor associated with the primary outcome (adjusted odds ratio, 1.28), while factors independently associated with risk of death on day 7 included age, diabetes complication history, and treated obstructive sleep apnea.
And after adjustment for age and sex, admission plasma glucose level was significantly and positively associated with both the primary outcome (P = .0001) and death on day 7 (P = .0059).
In the multivariate analysis, admission characteristics that predicted the primary outcome were dyspnea, lymphopenia, increased AST, and increased C-reactive protein.
Dr. Hadjadj said his team is now “focusing on specific risk factors such as obesity, age, vascular complications, medications ... to perform some deeper analyses.”
“We look forward to analyzing the data on in-hospital stay up to day 28 after admission. Some new messages might well pop up,” he added.
But in the meantime, “Elderly populations with long-term diabetes with advanced diabetes-related complications and/or treated obstructive sleep apnea were particularly at risk of early death and might require specific management to avoid infection with the novel coronavirus,” the researchers stressed.
The study received funding from the Fondation Francophone de Recherche sur le Diabète and was supported by Novo Nordisk, MSD, Abbott, AstraZeneca, Lilly, and the Fédération Française des Diabétiques; Société Francophone du Diabète; and Air Liquide Healthcare International. Dr. Hadjadj reported receiving grants, personal fees, and/or nonfinancial support from AstraZeneca, Bayer, Boehringer Ingelheim, Dinno Santé, Eli Lilly, LVL, MSD, Novartis, Pierre Fabre Santé, Sanofi, Servier, and Valbiotis.
A version of this article originally appeared on Medscape.com.
Mass protests could cause COVID-19 outbreaks
“There’s going to be a lot of issues coming out of what’s happened in the last week, but one of them is going to be that chains of transmission will have become lit from these gatherings,” said Scott Gottlieb, former FDA commissioner, on the CBS News show “Face the Nation.”
In Minnesota, he noted, COVID-19 cases and hospitalizations increased in recent days – even before the protests started.
“We still have pockets of spread in communities that aren’t under good control,” he said.
The protests generally have started peacefully with some demonstrators following physical distancing rules. But they have evolved into sometimes violent gatherings of hundreds or thousands of people where standing 6 feet apart is impossible.
Chanting, singing, and shouting may spread the virus through respiratory droplets. In addition, people who have the virus but don’t show symptoms may infect others without knowing it.
“If you were out protesting last night, you probably need to go get a COVID test this week,” Atlanta Mayor Keisha Lance Bottoms said Saturday, according to the Associated Press.
Gottlieb and Bottoms also spoke Sunday about the disproportionate effect of the coronavirus on black and Hispanic people, who are contracting and dying from the virus at higher rates. Socioeconomic factors such as low incomes, limited health care access, underlying conditions and overcrowded housing play a role in the greater risk, Gottlieb said.
“It’s a symptom of broader racial inequities in our country that we need to work to resolve,” he said.
Protests against racial injustice, sparked by the death of George Floyd in Minneapolis last week, could harm those communities experiencing the most severe outcomes of the coronavirus, Bottoms added.
“We know what’s already happening in our community with this virus. We’re going to see the other side of this in a couple of weeks,” Bottoms said on CNN’s “State of the Union.”
The protests may affect the pandemic in other ways. Los Angeles Mayor Eric Garcetti said the city’s coronavirus testing centers were closed on Saturday because of “safety worries across the city,” according to KTLA.
“We need to make sure, especially in communities that have less power, that we are able to make sure people don’t disproportionately die because of the color of their skin,” he said. “We can’t do that when the city breaks down.”
This article first appeared on WebMD.com.
“There’s going to be a lot of issues coming out of what’s happened in the last week, but one of them is going to be that chains of transmission will have become lit from these gatherings,” said Scott Gottlieb, former FDA commissioner, on the CBS News show “Face the Nation.”
In Minnesota, he noted, COVID-19 cases and hospitalizations increased in recent days – even before the protests started.
“We still have pockets of spread in communities that aren’t under good control,” he said.
The protests generally have started peacefully with some demonstrators following physical distancing rules. But they have evolved into sometimes violent gatherings of hundreds or thousands of people where standing 6 feet apart is impossible.
Chanting, singing, and shouting may spread the virus through respiratory droplets. In addition, people who have the virus but don’t show symptoms may infect others without knowing it.
“If you were out protesting last night, you probably need to go get a COVID test this week,” Atlanta Mayor Keisha Lance Bottoms said Saturday, according to the Associated Press.
Gottlieb and Bottoms also spoke Sunday about the disproportionate effect of the coronavirus on black and Hispanic people, who are contracting and dying from the virus at higher rates. Socioeconomic factors such as low incomes, limited health care access, underlying conditions and overcrowded housing play a role in the greater risk, Gottlieb said.
“It’s a symptom of broader racial inequities in our country that we need to work to resolve,” he said.
Protests against racial injustice, sparked by the death of George Floyd in Minneapolis last week, could harm those communities experiencing the most severe outcomes of the coronavirus, Bottoms added.
“We know what’s already happening in our community with this virus. We’re going to see the other side of this in a couple of weeks,” Bottoms said on CNN’s “State of the Union.”
The protests may affect the pandemic in other ways. Los Angeles Mayor Eric Garcetti said the city’s coronavirus testing centers were closed on Saturday because of “safety worries across the city,” according to KTLA.
“We need to make sure, especially in communities that have less power, that we are able to make sure people don’t disproportionately die because of the color of their skin,” he said. “We can’t do that when the city breaks down.”
This article first appeared on WebMD.com.
“There’s going to be a lot of issues coming out of what’s happened in the last week, but one of them is going to be that chains of transmission will have become lit from these gatherings,” said Scott Gottlieb, former FDA commissioner, on the CBS News show “Face the Nation.”
In Minnesota, he noted, COVID-19 cases and hospitalizations increased in recent days – even before the protests started.
“We still have pockets of spread in communities that aren’t under good control,” he said.
The protests generally have started peacefully with some demonstrators following physical distancing rules. But they have evolved into sometimes violent gatherings of hundreds or thousands of people where standing 6 feet apart is impossible.
Chanting, singing, and shouting may spread the virus through respiratory droplets. In addition, people who have the virus but don’t show symptoms may infect others without knowing it.
“If you were out protesting last night, you probably need to go get a COVID test this week,” Atlanta Mayor Keisha Lance Bottoms said Saturday, according to the Associated Press.
Gottlieb and Bottoms also spoke Sunday about the disproportionate effect of the coronavirus on black and Hispanic people, who are contracting and dying from the virus at higher rates. Socioeconomic factors such as low incomes, limited health care access, underlying conditions and overcrowded housing play a role in the greater risk, Gottlieb said.
“It’s a symptom of broader racial inequities in our country that we need to work to resolve,” he said.
Protests against racial injustice, sparked by the death of George Floyd in Minneapolis last week, could harm those communities experiencing the most severe outcomes of the coronavirus, Bottoms added.
“We know what’s already happening in our community with this virus. We’re going to see the other side of this in a couple of weeks,” Bottoms said on CNN’s “State of the Union.”
The protests may affect the pandemic in other ways. Los Angeles Mayor Eric Garcetti said the city’s coronavirus testing centers were closed on Saturday because of “safety worries across the city,” according to KTLA.
“We need to make sure, especially in communities that have less power, that we are able to make sure people don’t disproportionately die because of the color of their skin,” he said. “We can’t do that when the city breaks down.”
This article first appeared on WebMD.com.
Telerehabilitation may be effective in MS
Telerehabilitation also saves time and travel cost, compared with outpatient rehabilitation.
“This model of home-based telerehabilitation offers a safe and cost-effective method for improving function and quality of life for MS patients with mobility deficits,” said Heather Barksdale, DPT, a neurological clinical specialist at UF Health Jacksonville (Florida).
The study was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The Centers for Medicare & Medicaid Services do not reimburse for telerehabilitation services. Patients with MS have difficulty accessing rehabilitation specialists because of impaired mobility and lack of access to transportation. “We are based in Jacksonville, Fla., and often have patients who have to travel from Tallahassee, Panama City, Daytona Beach, and Brunswick, Ga., to receive specialty services,” said Dr. Barksdale. “Telerehabilitation would allow these patients to get access to high-quality rehab services with clinicians that specialize in MS.”
Dr. Barksdale and colleagues conducted a pilot study to evaluate the feasibility of a physical therapy–guided telerehabilitation program for people with mobility impairments resulting from confirmed MS. The investigators enrolled patients at the MS Center of Excellence at University of Florida Health Jacksonville into a telerehabilitation group. A board-certified neurologist and a physical therapist specializing in MS examined participants in person at baseline. The latter underwent an 8-week program of physical therapy–guided telerehabilitation that used the Jintronix software platform and a kinetic tracking system.
By reviewing charts during January 2018–September 2019, Dr. Barksdale and colleagues selected patients with MS who were seen on an outpatient basis by the same physical therapists who were administering telerehabilitation. This outpatient comparison group was matched to the telerehabilitation group on duration of treatment and outcome measures completed. Dr. Barksdale and colleagues reviewed the data for the effects of the two interventions on mobility and travel.
Eight patients completed the telerehabilitation program, and all had improvements in fatigue, quality of life, or mobility measures. The investigators did not observe any adverse events during or after the intervention. The total savings in projected travel costs for all eight participants was $8,487.23, compared with the outpatient group. Participants in the telerehabilitation and outpatient groups achieved minimal detectable changes in the outcome measures examined at equivalent rates.
“The game-based model with virtual visits by a physical therapist can be modified to include exercises specific for other motor, coordination, spasticity, and movement dysfunctions and may be useful for other chronic and progressive dysfunction seen in Parkinson’s disease, stroke, and other movement and neuromuscular disorders,” said Dr. Barksdale.
“Future studies are needed to further establish guidelines for patient selection and mode of delivery, as well as design of future telerehabilitation programs,” she added. “Duration of treatment and types of exercises to be included should also be examined. Further research into use of telerehabilitation for the treatment of upper-extremity, cognitive, speech, and swallowing dysfunction should also be examined.”
The investigators conducted their study without outside funding and reported no disclosures.
SOURCE: Barksdale H et al. CMSC 2020. Abstract REH11.
Telerehabilitation also saves time and travel cost, compared with outpatient rehabilitation.
“This model of home-based telerehabilitation offers a safe and cost-effective method for improving function and quality of life for MS patients with mobility deficits,” said Heather Barksdale, DPT, a neurological clinical specialist at UF Health Jacksonville (Florida).
The study was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The Centers for Medicare & Medicaid Services do not reimburse for telerehabilitation services. Patients with MS have difficulty accessing rehabilitation specialists because of impaired mobility and lack of access to transportation. “We are based in Jacksonville, Fla., and often have patients who have to travel from Tallahassee, Panama City, Daytona Beach, and Brunswick, Ga., to receive specialty services,” said Dr. Barksdale. “Telerehabilitation would allow these patients to get access to high-quality rehab services with clinicians that specialize in MS.”
Dr. Barksdale and colleagues conducted a pilot study to evaluate the feasibility of a physical therapy–guided telerehabilitation program for people with mobility impairments resulting from confirmed MS. The investigators enrolled patients at the MS Center of Excellence at University of Florida Health Jacksonville into a telerehabilitation group. A board-certified neurologist and a physical therapist specializing in MS examined participants in person at baseline. The latter underwent an 8-week program of physical therapy–guided telerehabilitation that used the Jintronix software platform and a kinetic tracking system.
By reviewing charts during January 2018–September 2019, Dr. Barksdale and colleagues selected patients with MS who were seen on an outpatient basis by the same physical therapists who were administering telerehabilitation. This outpatient comparison group was matched to the telerehabilitation group on duration of treatment and outcome measures completed. Dr. Barksdale and colleagues reviewed the data for the effects of the two interventions on mobility and travel.
Eight patients completed the telerehabilitation program, and all had improvements in fatigue, quality of life, or mobility measures. The investigators did not observe any adverse events during or after the intervention. The total savings in projected travel costs for all eight participants was $8,487.23, compared with the outpatient group. Participants in the telerehabilitation and outpatient groups achieved minimal detectable changes in the outcome measures examined at equivalent rates.
“The game-based model with virtual visits by a physical therapist can be modified to include exercises specific for other motor, coordination, spasticity, and movement dysfunctions and may be useful for other chronic and progressive dysfunction seen in Parkinson’s disease, stroke, and other movement and neuromuscular disorders,” said Dr. Barksdale.
“Future studies are needed to further establish guidelines for patient selection and mode of delivery, as well as design of future telerehabilitation programs,” she added. “Duration of treatment and types of exercises to be included should also be examined. Further research into use of telerehabilitation for the treatment of upper-extremity, cognitive, speech, and swallowing dysfunction should also be examined.”
The investigators conducted their study without outside funding and reported no disclosures.
SOURCE: Barksdale H et al. CMSC 2020. Abstract REH11.
Telerehabilitation also saves time and travel cost, compared with outpatient rehabilitation.
“This model of home-based telerehabilitation offers a safe and cost-effective method for improving function and quality of life for MS patients with mobility deficits,” said Heather Barksdale, DPT, a neurological clinical specialist at UF Health Jacksonville (Florida).
The study was presented at the virtual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
The Centers for Medicare & Medicaid Services do not reimburse for telerehabilitation services. Patients with MS have difficulty accessing rehabilitation specialists because of impaired mobility and lack of access to transportation. “We are based in Jacksonville, Fla., and often have patients who have to travel from Tallahassee, Panama City, Daytona Beach, and Brunswick, Ga., to receive specialty services,” said Dr. Barksdale. “Telerehabilitation would allow these patients to get access to high-quality rehab services with clinicians that specialize in MS.”
Dr. Barksdale and colleagues conducted a pilot study to evaluate the feasibility of a physical therapy–guided telerehabilitation program for people with mobility impairments resulting from confirmed MS. The investigators enrolled patients at the MS Center of Excellence at University of Florida Health Jacksonville into a telerehabilitation group. A board-certified neurologist and a physical therapist specializing in MS examined participants in person at baseline. The latter underwent an 8-week program of physical therapy–guided telerehabilitation that used the Jintronix software platform and a kinetic tracking system.
By reviewing charts during January 2018–September 2019, Dr. Barksdale and colleagues selected patients with MS who were seen on an outpatient basis by the same physical therapists who were administering telerehabilitation. This outpatient comparison group was matched to the telerehabilitation group on duration of treatment and outcome measures completed. Dr. Barksdale and colleagues reviewed the data for the effects of the two interventions on mobility and travel.
Eight patients completed the telerehabilitation program, and all had improvements in fatigue, quality of life, or mobility measures. The investigators did not observe any adverse events during or after the intervention. The total savings in projected travel costs for all eight participants was $8,487.23, compared with the outpatient group. Participants in the telerehabilitation and outpatient groups achieved minimal detectable changes in the outcome measures examined at equivalent rates.
“The game-based model with virtual visits by a physical therapist can be modified to include exercises specific for other motor, coordination, spasticity, and movement dysfunctions and may be useful for other chronic and progressive dysfunction seen in Parkinson’s disease, stroke, and other movement and neuromuscular disorders,” said Dr. Barksdale.
“Future studies are needed to further establish guidelines for patient selection and mode of delivery, as well as design of future telerehabilitation programs,” she added. “Duration of treatment and types of exercises to be included should also be examined. Further research into use of telerehabilitation for the treatment of upper-extremity, cognitive, speech, and swallowing dysfunction should also be examined.”
The investigators conducted their study without outside funding and reported no disclosures.
SOURCE: Barksdale H et al. CMSC 2020. Abstract REH11.
REPORTING FROM CMSC 2020
FDA approves ixekizumab for nonradiographic axSpA
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.
The Food and Drug Administration has extended approval of ixekizumab (Taltz) to the treatment of nonradiographic axial spondyloarthritis (nr-axSpA), according to a press release from its manufacturer, Eli Lilly. Specifically, this supplemental biologics license application refers to nr-axSpA with objective signs of inflammation.
The monoclonal interleukin-17A antagonist has three other indications, including ankylosing spondylitis in adults, psoriatic arthritis in adults, and plaque psoriasis in adults and children aged 6 years and older. It is the first IL-17A antagonist to receive FDA approval for nr-axSpA.
Approval for this indication was based on the phase 3, randomized, double-blind COAST-X trial, which put 96 nr-axSpA patients on 80-mg injections of ixekizumab every 4 weeks and 105 on placebo. After 52 weeks, ixekizumab was superior on the trial’s primary endpoint: 30% of patients had achieved a 40% improvement in Assessment of Spondyloarthritis International Society response criteria (ASAS 40), compared with 13% of patients on placebo (P = .0045).
Warnings and precautions for ixekizumab include considering potentially increased risk of infection and inflammatory bowel disease, as well as evaluating patients for tuberculosis before treatment. The most common adverse reactions (≥1%) are injection-site reactions, upper respiratory tract infections, nausea, and tinea infections. The safety profile for ixekizumab among nr-axSpA patients is mostly consistent with that seen among patients receiving it for other indications, according to Lilly. The full prescribing information is available on Lilly’s website.