User login
eConsult Data Shed Light on Care Coordination Decisions During the COVID-19 Pandemic
From the Multi-County eConsult Initiative, Rancho Cucamonga, CA.
The COVID-19 pandemic has forced many health care professionals and their patients to use telehealth and virtual care to address care needs in new ways.1 To shed light on care coordination decisions with respect to specialty resource access, we analyzed data collected from the Multi-County eConsult Initiative (MCeI)—the second-largest electronic consultation (eConsult) program in the United States—before and during the COVID-19 pandemic. Our analysis of these data suggests opportunities for improving access to care and reducing unnecessary costs in the health system nationally.
The Inland Empire Health Plan (IEHP) launched MCeI (econsultie.com) in 2018. The initiative is a partnership between IEHP, Arrowhead Regional Medical Center, and Riverside University Health System aimed at improving access to specialty care for the safety-net population across San Bernardino and Riverside counties. IEHP is 1 of the 10 largest Medicaid health plans and the largest not-for-profit Medicare-Medicaid plan in the country, serving more than 1.2 million members.2 Data from MCel reveal the impacts of COVID-19 on eConsult use and offer insights into specialty resource availability during and outside of a crisis.
At the time of this analysis, 86 IEHP clinics in rural and urban settings across 38 specialties used the eConsult process to provide and obtain virtual specialty care, as well as timely appointments for in-person specialty care.3 eConsults are facilitated through a HIPAA-secure web-based portal that enables communication and sharing of information between the primary care provider (PCP) and a specialist. eConsult gives PCPs virtual access to specialists to coordinate care for their patients and determine the need for in-person specialty visits. Through the PCP-specialist eConsult dialogue, patients gain virtual access to specialty care. If a PCP-specialist care team determines the patient needs an in-person visit, that specialty referral is automatically authorized by IEHP, without the need for further review. At IEHP, eConsult is the primary method used for obtaining outpatient specialty referrals.
To analyze eConsult utilization before and during the pandemic, we gathered data from the MCeI program for the periods February 20–March 19, 2020, and March 20–April 19, 2020. Measures included eConsult volume and outcomes of eConsults (eConsults closed as referrals for face-to-face specialist visits versus eConsults closed without resulting in referrals for face-to-face specialist visits). Statistical analysis using chi-square tests for independence was performed using IBM SPSS Statistics 25 (IBM, Armonk, NY).
The data show that after California’s stay-at-home order, issued on March 19, 2020,4 eConsult volumes initially decreased, reflecting a similar decrease in clinic visits and authorization requests submitted to IEHP. We observed a 4-week average of 1100 eConsults processed before the pandemic, and then a steep drop to a 4-week average of 500 eConsults processed after the stay-at-home order was issued. Despite the overall drop in the volume of eConsults submitted, demand for specialties like hematology and neurology remained high throughout the pandemic.
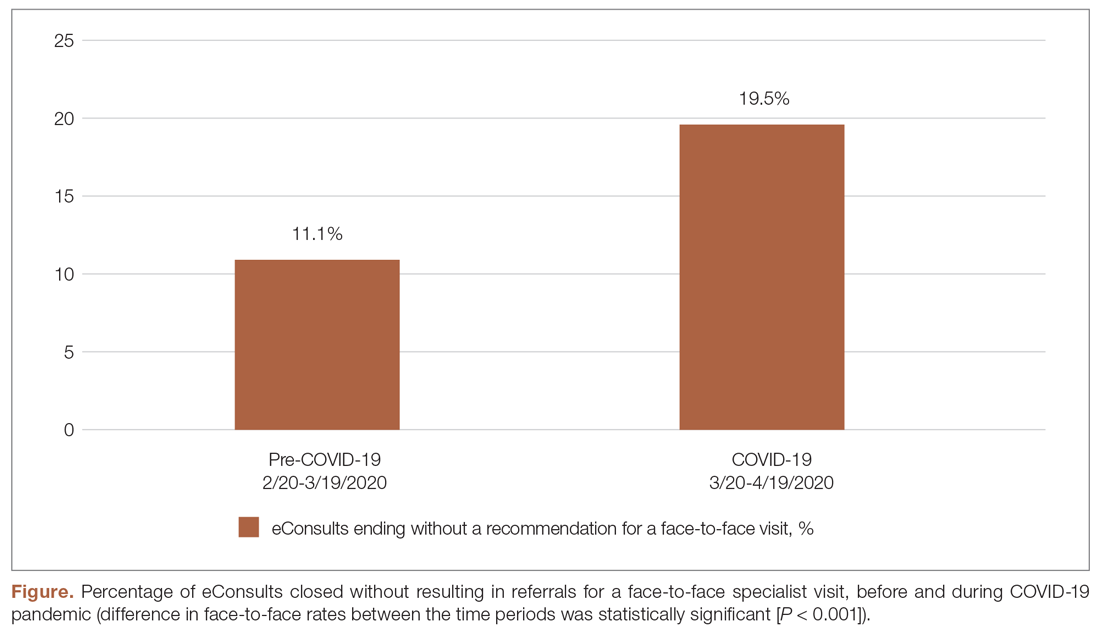
During the pandemic, certain specialties displayed rising rates of eConsults completed with specialists providing care recommendations to the PCP instead of resulting in a recommendation for a face-to-face (in-person or via telehealth) visit with a specialist (see Figure and Table). The trend of increasing eConsults that concluded without a face-to-face visit suggests newfound clinical consideration of limited medical resources, along with the desire to eliminate unnecessary risks of infection.
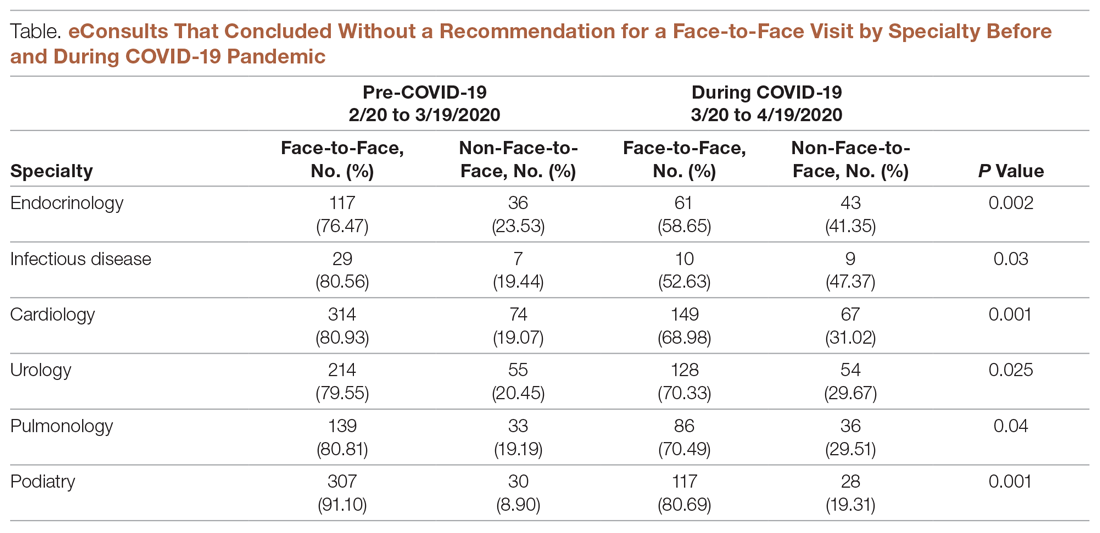
eConsults between PCPs and specialist reviewers via the IEHP portal resulted in higher rates of non-face-to-face recommendations. The specialist reviewers were able to provide treatment plans for PCPs to take care of patients without having to refer their patients to a specialist. This increase was significant across most of the specialties live on the MCeI program.
We believe that clinicians’ heightened awareness of the limitations of the US health care system should remain a key consideration and factor in medical decision-making about appropriate referrals after the pandemic has passed. The data demonstrate that the pandemic drove clinicians to make different decisions about referrals and care coordination. Physicians scrutinized individual cases more keenly and were not as quick to recommend a face-to-face visit. This awareness and consideration of specialty access before making a referral provides a valuable lesson. If this approach is applied to health care delivery post-pandemic, eConsults will help reduce unnecessary in-person specialist visits and will free up space and time for patients who genuinely do need in-person specialty care. In this way, eConsult will improve appropriate access to care for everyone and reduce unnecessary costs to the health care system at large.
An examination of eConsult utilization trends across Riverside and San Bernardino counties before and during the COVID-19 pandemic provides useful insights into how to reduce costs and improve access to care. Although the risk of exposure to COVID-19 currently presents a significant obstacle to obtaining in-person specialty care, pre-existing and long-standing barriers, such as long wait times and scarcity of specialists, remain critical issues to receiving care during and after the pandemic. The pandemic has proven eConsult’s value as a tool for effective care coordination. Leveraging provider-to-provider asynchronous communication offers an opportunity to reduce unnecessary utilization of scarce resources during and beyond the pandemic.
Corresponding author: Lisa Aubry, [email protected].
Financial disclosures: None.
Keywords: electronic consultation; care coordination; telehealth; telemedicine; virtual care.
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27:957-962.
2. Nash-Wong K. Inland Empire Health Plan Multi-county eConsult Initiative with Safety Net Connect improves specialty care for Southern California residents. [Press Release]. (July 24, 2019). www.businesswire.com/news/home/20190724005208/en/Inland-Empire-Health-Plan-Multi-county-eConsult-Initiative. Accessed July 16, 2020.
3. The Multi-County eConsult Initiative (March 2018). https://www.eConsultie.com. Accessed July 16, 2020.
4. Executive Department State of California. Exec. Order No. N-33-20 of March 19, 2020. Safer at Home, Stay at Home. www.gov.ca.gov. Accessed July 16, 2020.
From the Multi-County eConsult Initiative, Rancho Cucamonga, CA.
The COVID-19 pandemic has forced many health care professionals and their patients to use telehealth and virtual care to address care needs in new ways.1 To shed light on care coordination decisions with respect to specialty resource access, we analyzed data collected from the Multi-County eConsult Initiative (MCeI)—the second-largest electronic consultation (eConsult) program in the United States—before and during the COVID-19 pandemic. Our analysis of these data suggests opportunities for improving access to care and reducing unnecessary costs in the health system nationally.
The Inland Empire Health Plan (IEHP) launched MCeI (econsultie.com) in 2018. The initiative is a partnership between IEHP, Arrowhead Regional Medical Center, and Riverside University Health System aimed at improving access to specialty care for the safety-net population across San Bernardino and Riverside counties. IEHP is 1 of the 10 largest Medicaid health plans and the largest not-for-profit Medicare-Medicaid plan in the country, serving more than 1.2 million members.2 Data from MCel reveal the impacts of COVID-19 on eConsult use and offer insights into specialty resource availability during and outside of a crisis.
At the time of this analysis, 86 IEHP clinics in rural and urban settings across 38 specialties used the eConsult process to provide and obtain virtual specialty care, as well as timely appointments for in-person specialty care.3 eConsults are facilitated through a HIPAA-secure web-based portal that enables communication and sharing of information between the primary care provider (PCP) and a specialist. eConsult gives PCPs virtual access to specialists to coordinate care for their patients and determine the need for in-person specialty visits. Through the PCP-specialist eConsult dialogue, patients gain virtual access to specialty care. If a PCP-specialist care team determines the patient needs an in-person visit, that specialty referral is automatically authorized by IEHP, without the need for further review. At IEHP, eConsult is the primary method used for obtaining outpatient specialty referrals.
To analyze eConsult utilization before and during the pandemic, we gathered data from the MCeI program for the periods February 20–March 19, 2020, and March 20–April 19, 2020. Measures included eConsult volume and outcomes of eConsults (eConsults closed as referrals for face-to-face specialist visits versus eConsults closed without resulting in referrals for face-to-face specialist visits). Statistical analysis using chi-square tests for independence was performed using IBM SPSS Statistics 25 (IBM, Armonk, NY).
The data show that after California’s stay-at-home order, issued on March 19, 2020,4 eConsult volumes initially decreased, reflecting a similar decrease in clinic visits and authorization requests submitted to IEHP. We observed a 4-week average of 1100 eConsults processed before the pandemic, and then a steep drop to a 4-week average of 500 eConsults processed after the stay-at-home order was issued. Despite the overall drop in the volume of eConsults submitted, demand for specialties like hematology and neurology remained high throughout the pandemic.

During the pandemic, certain specialties displayed rising rates of eConsults completed with specialists providing care recommendations to the PCP instead of resulting in a recommendation for a face-to-face (in-person or via telehealth) visit with a specialist (see Figure and Table). The trend of increasing eConsults that concluded without a face-to-face visit suggests newfound clinical consideration of limited medical resources, along with the desire to eliminate unnecessary risks of infection.

eConsults between PCPs and specialist reviewers via the IEHP portal resulted in higher rates of non-face-to-face recommendations. The specialist reviewers were able to provide treatment plans for PCPs to take care of patients without having to refer their patients to a specialist. This increase was significant across most of the specialties live on the MCeI program.
We believe that clinicians’ heightened awareness of the limitations of the US health care system should remain a key consideration and factor in medical decision-making about appropriate referrals after the pandemic has passed. The data demonstrate that the pandemic drove clinicians to make different decisions about referrals and care coordination. Physicians scrutinized individual cases more keenly and were not as quick to recommend a face-to-face visit. This awareness and consideration of specialty access before making a referral provides a valuable lesson. If this approach is applied to health care delivery post-pandemic, eConsults will help reduce unnecessary in-person specialist visits and will free up space and time for patients who genuinely do need in-person specialty care. In this way, eConsult will improve appropriate access to care for everyone and reduce unnecessary costs to the health care system at large.
An examination of eConsult utilization trends across Riverside and San Bernardino counties before and during the COVID-19 pandemic provides useful insights into how to reduce costs and improve access to care. Although the risk of exposure to COVID-19 currently presents a significant obstacle to obtaining in-person specialty care, pre-existing and long-standing barriers, such as long wait times and scarcity of specialists, remain critical issues to receiving care during and after the pandemic. The pandemic has proven eConsult’s value as a tool for effective care coordination. Leveraging provider-to-provider asynchronous communication offers an opportunity to reduce unnecessary utilization of scarce resources during and beyond the pandemic.
Corresponding author: Lisa Aubry, [email protected].
Financial disclosures: None.
Keywords: electronic consultation; care coordination; telehealth; telemedicine; virtual care.
From the Multi-County eConsult Initiative, Rancho Cucamonga, CA.
The COVID-19 pandemic has forced many health care professionals and their patients to use telehealth and virtual care to address care needs in new ways.1 To shed light on care coordination decisions with respect to specialty resource access, we analyzed data collected from the Multi-County eConsult Initiative (MCeI)—the second-largest electronic consultation (eConsult) program in the United States—before and during the COVID-19 pandemic. Our analysis of these data suggests opportunities for improving access to care and reducing unnecessary costs in the health system nationally.
The Inland Empire Health Plan (IEHP) launched MCeI (econsultie.com) in 2018. The initiative is a partnership between IEHP, Arrowhead Regional Medical Center, and Riverside University Health System aimed at improving access to specialty care for the safety-net population across San Bernardino and Riverside counties. IEHP is 1 of the 10 largest Medicaid health plans and the largest not-for-profit Medicare-Medicaid plan in the country, serving more than 1.2 million members.2 Data from MCel reveal the impacts of COVID-19 on eConsult use and offer insights into specialty resource availability during and outside of a crisis.
At the time of this analysis, 86 IEHP clinics in rural and urban settings across 38 specialties used the eConsult process to provide and obtain virtual specialty care, as well as timely appointments for in-person specialty care.3 eConsults are facilitated through a HIPAA-secure web-based portal that enables communication and sharing of information between the primary care provider (PCP) and a specialist. eConsult gives PCPs virtual access to specialists to coordinate care for their patients and determine the need for in-person specialty visits. Through the PCP-specialist eConsult dialogue, patients gain virtual access to specialty care. If a PCP-specialist care team determines the patient needs an in-person visit, that specialty referral is automatically authorized by IEHP, without the need for further review. At IEHP, eConsult is the primary method used for obtaining outpatient specialty referrals.
To analyze eConsult utilization before and during the pandemic, we gathered data from the MCeI program for the periods February 20–March 19, 2020, and March 20–April 19, 2020. Measures included eConsult volume and outcomes of eConsults (eConsults closed as referrals for face-to-face specialist visits versus eConsults closed without resulting in referrals for face-to-face specialist visits). Statistical analysis using chi-square tests for independence was performed using IBM SPSS Statistics 25 (IBM, Armonk, NY).
The data show that after California’s stay-at-home order, issued on March 19, 2020,4 eConsult volumes initially decreased, reflecting a similar decrease in clinic visits and authorization requests submitted to IEHP. We observed a 4-week average of 1100 eConsults processed before the pandemic, and then a steep drop to a 4-week average of 500 eConsults processed after the stay-at-home order was issued. Despite the overall drop in the volume of eConsults submitted, demand for specialties like hematology and neurology remained high throughout the pandemic.

During the pandemic, certain specialties displayed rising rates of eConsults completed with specialists providing care recommendations to the PCP instead of resulting in a recommendation for a face-to-face (in-person or via telehealth) visit with a specialist (see Figure and Table). The trend of increasing eConsults that concluded without a face-to-face visit suggests newfound clinical consideration of limited medical resources, along with the desire to eliminate unnecessary risks of infection.

eConsults between PCPs and specialist reviewers via the IEHP portal resulted in higher rates of non-face-to-face recommendations. The specialist reviewers were able to provide treatment plans for PCPs to take care of patients without having to refer their patients to a specialist. This increase was significant across most of the specialties live on the MCeI program.
We believe that clinicians’ heightened awareness of the limitations of the US health care system should remain a key consideration and factor in medical decision-making about appropriate referrals after the pandemic has passed. The data demonstrate that the pandemic drove clinicians to make different decisions about referrals and care coordination. Physicians scrutinized individual cases more keenly and were not as quick to recommend a face-to-face visit. This awareness and consideration of specialty access before making a referral provides a valuable lesson. If this approach is applied to health care delivery post-pandemic, eConsults will help reduce unnecessary in-person specialist visits and will free up space and time for patients who genuinely do need in-person specialty care. In this way, eConsult will improve appropriate access to care for everyone and reduce unnecessary costs to the health care system at large.
An examination of eConsult utilization trends across Riverside and San Bernardino counties before and during the COVID-19 pandemic provides useful insights into how to reduce costs and improve access to care. Although the risk of exposure to COVID-19 currently presents a significant obstacle to obtaining in-person specialty care, pre-existing and long-standing barriers, such as long wait times and scarcity of specialists, remain critical issues to receiving care during and after the pandemic. The pandemic has proven eConsult’s value as a tool for effective care coordination. Leveraging provider-to-provider asynchronous communication offers an opportunity to reduce unnecessary utilization of scarce resources during and beyond the pandemic.
Corresponding author: Lisa Aubry, [email protected].
Financial disclosures: None.
Keywords: electronic consultation; care coordination; telehealth; telemedicine; virtual care.
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27:957-962.
2. Nash-Wong K. Inland Empire Health Plan Multi-county eConsult Initiative with Safety Net Connect improves specialty care for Southern California residents. [Press Release]. (July 24, 2019). www.businesswire.com/news/home/20190724005208/en/Inland-Empire-Health-Plan-Multi-county-eConsult-Initiative. Accessed July 16, 2020.
3. The Multi-County eConsult Initiative (March 2018). https://www.eConsultie.com. Accessed July 16, 2020.
4. Executive Department State of California. Exec. Order No. N-33-20 of March 19, 2020. Safer at Home, Stay at Home. www.gov.ca.gov. Accessed July 16, 2020.
1. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc. 2020;27:957-962.
2. Nash-Wong K. Inland Empire Health Plan Multi-county eConsult Initiative with Safety Net Connect improves specialty care for Southern California residents. [Press Release]. (July 24, 2019). www.businesswire.com/news/home/20190724005208/en/Inland-Empire-Health-Plan-Multi-county-eConsult-Initiative. Accessed July 16, 2020.
3. The Multi-County eConsult Initiative (March 2018). https://www.eConsultie.com. Accessed July 16, 2020.
4. Executive Department State of California. Exec. Order No. N-33-20 of March 19, 2020. Safer at Home, Stay at Home. www.gov.ca.gov. Accessed July 16, 2020.
Dapagliflozin Improves Cardiovascular Outcomes in Patients With Heart Failure and Reduced Ejection Fraction
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
Study Overview
Objective. To evaluate the effects of dapagliflozin in patients with heart failure with reduced ejection fraction in the presence or absence of type 2 diabetes.
Design. Multicenter, international, double-blind, prospective, randomized, controlled trial.
Setting and participants. Adult patients with symptomatic heart failure with an ejection fraction of 40% or less and elevated heart failure biomarkers who were already on appropriate guideline-directed therapies were eligible for the study.
Intervention. A total of 4744 patients were randomly assigned to receive dapagliflozin (10 mg once daily) or placebo, in addition to recommended therapy. Randomization was stratified by the presence or absence of type 2 diabetes.
Main outcome measures. The primary outcome was the composite of a first episode of worsening heart failure (hospitalization or urgent intravenous therapy) or cardiovascular death.
Main results. Median follow-up was 18.2 months; during this time, the primary outcome occurred in 16.3% (386 of 2373) of patients in the dapagliflozin group and in 21.2% (502 of 2371) of patients in the placebo group (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.65-0.85; P < 0.001). In the dapagliflozin group, 237 patients (10.0%) experienced a first worsening heart failure event, as compared with 326 patients (13.7%) in the placebo group (HR, 0.70; 95% CI, 0.59-0.83). The dapagliflozin group hadlower rates of death from cardiovascular causes (9.6% vs 11.5%; HR, 0.82; 95% CI, 0.69-0.98) and from any causes (11.6% vs 13.9%; HR, 0.83; 95% CI, 0.71-0.97), compared to the placebo group. Findings in patients with diabetes were similar to those in patients without diabetes.
Conclusion. Among patients with heart failure and a reduced ejection fraction, the risk of worsening heart failure or death from cardiovascular causes was lower among those who received dapagliflozin than among those who received placebo, regardless of the presence or absence of diabetes.
Commentary
Inhibitors of sodium-glucose cotransporter 2 (SGLT-2) are a novel class of diabetic medication that decrease renal glucose reabsorption, thereby increasing urinary glucose excretion. In several large clinical trials of these medications for patients with diabetes, which were designed to meet the regulatory requirements for cardiovascular safety in novel diabetic agents, investigators unexpectedly found that SGLT-2 inhibitors were associated with a reduction in cardiovascular events, driven by a reduction in heart failure hospitalizations. The results of EMPA-REG OUTCOME, the first of these trials, showed significantly lower risks of both death from any cause and hospitalization for heart failure in patients treated with empagliflozin.1 This improvement in cardiovascular outcomes was subsequently confirmed as a class effect of SGLT-2 inhibitors in the CANVAS Program (canagliflozin) and DECLARE TIMI 58 (dapagliflozin) trials.2,3
While these trials were designed for patients with type 2 diabetes who had either established cardiovascular disease or multiple risk factors for it, most patients did not have heart failure at baseline. Accordingly, despite a signal toward benefit of SGLT-2 inhibitors in patients with heart failure, the trials were not powered to test the hypothesis that SGLT-2 inhibitors benefit patients with heart failure, regardless of diabetes status. Therefore, McMurray et al designed the DAPA-HF trial to investigate whether SGLT-2 inhibitors can improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction, with or without diabetes. The trial included 4744 patients with heart failure with reduced ejection fraction, who were randomly assigned to dapagliflozin 10 mg once daily or placebo, atop guideline-directed heart failure therapy, with randomization stratified by presence or absence of type 2 diabetes. Investigators found that the composite primary outcome, a first episode of worsening heart failure or cardiovascular death, occurred less frequently in patients in the dapagliflozin group compared to the placebo group (16.3% vs 21.2%; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). Individual components of the primary outcome and death from any cause were all significantly lower, and heart failure–related quality of life was significantly improved in the dapagliflozin group compared to placebo.
DAPA-HF was the first randomized study to investigate the effect of SGLT-2 inhibitors on patients with heart failure regardless of the presence of diabetes. In addition to the reduction in the above-mentioned primary and secondary endpoints, the study yielded other important findings worth noting. First, the consistent benefit of dapagliflozin on cardiovascular outcomes in patients with and without diabetes suggests that the cardioprotective effect of dapagliflozin is independent of its glucose-lowering effect. Prior studies have proposed alternative mechanisms, such as diuretic function and related hemodynamic actions, effects on myocardial metabolism, ion transporters, fibrosis, adipokines, vascular function, and the preservation of renal function. Future studies are needed to fully understand the likely pleiotropic effects of this class of medication on patients with heart failure. Second, there was no difference in the safety endpoints between the groups, including renal adverse events and major hypoglycemia, implying dapagliflozin is as safe as placebo.
There are a few limitations of this trial. First, as the authors point out, the study included mostly white males—less than 5% of participants were African Americans—and the finding may not be generalizable to all patient populations. Second, although all patients were already treated with guideline-directed heart failure therapy, only 10% of patients were on sacubitril–valsartan, which is more effective than renin–angiotensin system blockade alone at reducing the incidence of hospitalization for heart failure and death from cardiovascular causes. Also, mineralocorticoid receptor blockers were used in only 70% of the population. Finally, since the doses were not provided, whether patients were on the maximal tolerated dose of heart failure therapy prior to enrollment is unclear.
Based on the results of the DAPA-HF trial, the Food and Drug Administration approved dapagliflozin for the treatment of heart failure with reduced ejection fraction on May 5, 2020. This is the first diabetic drug approved for the treatment of heart failure.
Applications for Clinical Practice
SGLT-2 inhibitors represent a fourth class of medication that patients with heart failure with reduced ejection fraction should be initiated on, in addition to beta blocker, ACE inhibitor/angiotensin receptor blocker/neprilysin inhibitor, and mineralocorticoid receptor blocker. SGLT-2 inhibitors may be especially applicable in patients with heart failure with reduced ejection fraction and relative hypotension, as these agents are not associated with a significant blood-pressure-lowering effect, which can often limit our ability to initiate or uptitrate the other main 3 classes of guideline-directed medical therapy.
—Rie Hirai, MD, Fukui Kosei Hospital, Fukui, Japan
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO
—Timothy Fendler, MD, St. Luke’s Mid America Heart Institute, Kansas City, MO
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
An Advance Care Planning Video Program in Nursing Homes Did Not Reduce Hospital Transfer and Burdensome Treatment in Long-Stay Residents
Study Overview
Objective. To examine the effect of an advance care planning video intervention in nursing homes on resident outcomes of hospital transfer, burdensome treatment, and hospice enrollment.
Design. Pragmatic cluster randomized controlled trial.
Setting and participants. The study was conducted in 360 nursing homes located in 32 states across the United States. The facilities were owned by 2 for-profit nursing home chains; facilities with more than 50 beds were eligible to be included in the study. Facilities deemed by corporate leaders to have serious organizational problems or that lacked the ability to transfer electronic health records were excluded. The facilities, stratified by the primary outcome hospitalizations per 1000 person-days, were then randomized to intervention and control in a 1:2 ratio. Leaders from facilities in the intervention group received letters describing their selection to participate in the advance care planning video program, and all facilities invited agreed to participate. Participants (residents in nursing homes) were enrolled from February 1, 2016, to May 31, 2018. Each participant was followed for 12 months after enrollment. All residents living in intervention facilities were offered the opportunity to watch intervention videos. The target population of the study was residents with advanced illness, including advanced dementia or advanced cardiopulmonary disease, as defined by the Minimum Data Set (MDS) variables, who were aged 65 and older, were long-stay residents (100 days or more), and were enrolled as Medicare fee-for-service beneficiaries. Secondary analysis included residents without advanced illness meeting other criteria.
Intervention. The intervention consisted of a selection of 5 short videos (6 to 10 minutes each), which had been previously developed and tested in smaller randomized trials. These videos cover the topics of general goals of care, goals of care for advanced dementia, hospice, hospitalization, and advance care planning for healthy patients, and use narration and images of typical treatments representing intensive medical care, basic medical care, and comfort care. The video for goals of care for advanced dementia targeted proxies of residents rather than residents themselves.
The implementation strategy for the video program included using a program manager to oversee the organization of the program’s rollout (a manager for each for-profit nursing home chain) and 2 champions at each facility (typically social workers were tasked with showing videos to patients and families). Champions received training from the study investigators and the manager and were asked to choose and offer selected videos to residents or proxies within 7 days of admission or readmission, every 6 months during a resident’s stay, and when specific decisions occurred, such as transition to hospice care, and on special occasions, such as out-of-town family visits.
Video offering and use were captured through documentation by a facility champion using a report tool embedded in the facility’s electronic health record. Champions met with the facility’s program manager and study team to review reports of video use, identify residents who had not been shown a video, and problem-solve on how to reach these residents. Facilities in the control group used their usual procedures for advance care planning.
Main outcome measures. Study outcomes included hospitalization transfers per 1000 person-days alive among long-stay residents with advanced illness (primary outcome); proportion of residents with at least 1 hospital transfer; proportion of residents with at least 1 burdensome treatment; and hospice enrollment (secondary outcomes). Secondary outcomes also included hospitalization transfers for long-stay residents without advanced illness. Hospital transfers were identified using Medicare claims for admissions, emergency department visits, and observation stays. Burdensome treatments were identified from Medicare claims and MDS, including tube feeding, parenteral therapy, invasive mechanical intervention, and intensive care unit admission. Fidelity to video intervention was measured by the proportion of residents offered the videos and the proportion of residents shown the videos at least once during the study period.
Main results. A total of 360 facilities were included in the study, 119 intervention and 241 control facilities. For the primary outcome, 4171 residents with advanced illness were included in the intervention group and 8308 residents with advanced illness were included in the control group. The average age was 83.6 years in both groups. In the intervention and control groups, respectively, 71.2% and 70.5% were female, 78.4% and 81.5% were White, 68.6% and 70.1% had advanced dementia at baseline, and 35.4% and 33.4% had advanced congestive heart failure or chronic obstructive pulmonary disease at baseline. Approximately 34% of residents received hospice care at baseline. In the intervention and control groups, 43.9% and 45.3% of residents died during follow-up, and the average length of follow-up in each group was 253.1 days and 252.6 days, respectively.
For the primary outcome of hospital transfers per 1000 person-days alive, there were 3.7 episodes (standard error 0.2) in the intervention group and 3.9 episodes in the control group (standard error 0.3); the difference was not statistically significant. For residents without advanced illness, there also was no difference in the hospital transfer rate. For other secondary outcomes, the proportion of residents in the intervention and control groups with 1 or more hospital transfer was 40.9% and 41.6%, respectively; the proportion with 1 or more burdensome treatment was 9.6% and 10.7%; and hospice enrollment was 24.9% and 25.5%. None of these differences was statistically significant. In the intervention group, 55.6% of residents or proxies were offered the video intervention and 21.9% were shown the videos at least once. There was substantial variability in the proportion of residents in the intervention group who were shown videos.
Conclusion. The advance planning video program did not lead to a reduction in hospital transfer, burdensome treatment, or changes in hospice enrollment. Acceptance of the intervention by residents was variable, and this may have contributed to the null finding.
Commentary
Nursing home residents often have advanced illness and limited functional ability. Hospital transfers may be burdensome and of limited clinical benefit for these patients, particularly for those with advanced illness and limited life expectancy, and are associated with markers of poor quality of end-of-life care, such as increased rates of stage IV decubitus ulcer and feeding-tube use towards the end of life.1 Advance care planning is associated with less aggressive care towards the end of life for persons with advanced illness,2 which ultimately improves the quality of end-of-life care for these individuals. Prior interventions to improve advance care planning have had variable effects, while video-based interventions to improve advance care planning have shown promise.3
This pragmatic randomized trial assessed the effect of an advance care planning video program on important clinical outcomes for nursing home residents, particularly those with advanced illness. The results, however, are disappointing, as the video intervention failed to improve hospital transfer rate and burdensome treatment in this population. The negative results could be attributed to the limited adoption of the video intervention in the study, as only 21.9% of residents in the intervention group were actually exposed to the intervention. What is not reported, and is difficult to assess, is whether the video intervention led to advance care planning, as would be demonstrated by advance directive documentation and acceptance of goals of care of comfort. A per-protocol analysis may be considered to demonstrate if there is an effect on residents who were exposed to the intervention. Nonetheless, the low adoption rate of the intervention may prompt further investigation of factors limiting adoption and perhaps lead to a redesigned trial aimed at enhancing adoption, with consideration of use of implementation trial designs.
As pointed out by the study investigators, other changes to nursing home practices, specifically on hospital transfer, likely occurred during the study period. A number of national initiatives to reduce unnecessary hospital transfer from nursing homes have been introduced, and a reduction in hospital transfers occurred between 2011 and 20174; these initiatives could have impacted staff priorities and adoption of the study intervention relative to other co-occurring initiatives.
Applications for Clinical Practice
The authors of this study reported negative trial results, but their findings highlight important issues in conducting trials in the nursing home setting. Additional demonstration of actual effect on advance care planning discussions and documentation will further enhance our understanding of whether the intervention, as tested, yields changes in practice on advance care planning in nursing homes. The pragmatic clinical trial design used in this study accounts for real-world settings, but may have limited the study’s ability to account for and adjust for differences in staff, settings, and other conditions and factors that may impact adoption of and fidelity to the intervention. Quality improvement approaches, such as INTERACT, have targeted unnecessary hospital transfers and may yield positive results.5 Quality improvement approaches like INTERACT allow for a high degree of adaptation to local procedures and settings, which in clinical trials is difficult to do. However, in a real-world setting, such approaches may be necessary to improve care.
–William W. Hung, MD, MPH
1. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212-1221
2. Nichols LH, Bynum J, Iwashyna TJ, et al. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33:667-674.
3. Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: randomized controlled trial. BMJ. 2009;338:b2159.
4. McCarthy EP, Ogarek JA, Loomer L, et al. Hospital transfer rates among US nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2020;180:385-394.
5. Rantz MJ, Popejoy L, Vogelsmeier, A et al. Successfully reducing hospitalizations of nursing home residents: results of the Missouri Quality Initiative. JAMA. 2017:18;960-966.
Study Overview
Objective. To examine the effect of an advance care planning video intervention in nursing homes on resident outcomes of hospital transfer, burdensome treatment, and hospice enrollment.
Design. Pragmatic cluster randomized controlled trial.
Setting and participants. The study was conducted in 360 nursing homes located in 32 states across the United States. The facilities were owned by 2 for-profit nursing home chains; facilities with more than 50 beds were eligible to be included in the study. Facilities deemed by corporate leaders to have serious organizational problems or that lacked the ability to transfer electronic health records were excluded. The facilities, stratified by the primary outcome hospitalizations per 1000 person-days, were then randomized to intervention and control in a 1:2 ratio. Leaders from facilities in the intervention group received letters describing their selection to participate in the advance care planning video program, and all facilities invited agreed to participate. Participants (residents in nursing homes) were enrolled from February 1, 2016, to May 31, 2018. Each participant was followed for 12 months after enrollment. All residents living in intervention facilities were offered the opportunity to watch intervention videos. The target population of the study was residents with advanced illness, including advanced dementia or advanced cardiopulmonary disease, as defined by the Minimum Data Set (MDS) variables, who were aged 65 and older, were long-stay residents (100 days or more), and were enrolled as Medicare fee-for-service beneficiaries. Secondary analysis included residents without advanced illness meeting other criteria.
Intervention. The intervention consisted of a selection of 5 short videos (6 to 10 minutes each), which had been previously developed and tested in smaller randomized trials. These videos cover the topics of general goals of care, goals of care for advanced dementia, hospice, hospitalization, and advance care planning for healthy patients, and use narration and images of typical treatments representing intensive medical care, basic medical care, and comfort care. The video for goals of care for advanced dementia targeted proxies of residents rather than residents themselves.
The implementation strategy for the video program included using a program manager to oversee the organization of the program’s rollout (a manager for each for-profit nursing home chain) and 2 champions at each facility (typically social workers were tasked with showing videos to patients and families). Champions received training from the study investigators and the manager and were asked to choose and offer selected videos to residents or proxies within 7 days of admission or readmission, every 6 months during a resident’s stay, and when specific decisions occurred, such as transition to hospice care, and on special occasions, such as out-of-town family visits.
Video offering and use were captured through documentation by a facility champion using a report tool embedded in the facility’s electronic health record. Champions met with the facility’s program manager and study team to review reports of video use, identify residents who had not been shown a video, and problem-solve on how to reach these residents. Facilities in the control group used their usual procedures for advance care planning.
Main outcome measures. Study outcomes included hospitalization transfers per 1000 person-days alive among long-stay residents with advanced illness (primary outcome); proportion of residents with at least 1 hospital transfer; proportion of residents with at least 1 burdensome treatment; and hospice enrollment (secondary outcomes). Secondary outcomes also included hospitalization transfers for long-stay residents without advanced illness. Hospital transfers were identified using Medicare claims for admissions, emergency department visits, and observation stays. Burdensome treatments were identified from Medicare claims and MDS, including tube feeding, parenteral therapy, invasive mechanical intervention, and intensive care unit admission. Fidelity to video intervention was measured by the proportion of residents offered the videos and the proportion of residents shown the videos at least once during the study period.
Main results. A total of 360 facilities were included in the study, 119 intervention and 241 control facilities. For the primary outcome, 4171 residents with advanced illness were included in the intervention group and 8308 residents with advanced illness were included in the control group. The average age was 83.6 years in both groups. In the intervention and control groups, respectively, 71.2% and 70.5% were female, 78.4% and 81.5% were White, 68.6% and 70.1% had advanced dementia at baseline, and 35.4% and 33.4% had advanced congestive heart failure or chronic obstructive pulmonary disease at baseline. Approximately 34% of residents received hospice care at baseline. In the intervention and control groups, 43.9% and 45.3% of residents died during follow-up, and the average length of follow-up in each group was 253.1 days and 252.6 days, respectively.
For the primary outcome of hospital transfers per 1000 person-days alive, there were 3.7 episodes (standard error 0.2) in the intervention group and 3.9 episodes in the control group (standard error 0.3); the difference was not statistically significant. For residents without advanced illness, there also was no difference in the hospital transfer rate. For other secondary outcomes, the proportion of residents in the intervention and control groups with 1 or more hospital transfer was 40.9% and 41.6%, respectively; the proportion with 1 or more burdensome treatment was 9.6% and 10.7%; and hospice enrollment was 24.9% and 25.5%. None of these differences was statistically significant. In the intervention group, 55.6% of residents or proxies were offered the video intervention and 21.9% were shown the videos at least once. There was substantial variability in the proportion of residents in the intervention group who were shown videos.
Conclusion. The advance planning video program did not lead to a reduction in hospital transfer, burdensome treatment, or changes in hospice enrollment. Acceptance of the intervention by residents was variable, and this may have contributed to the null finding.
Commentary
Nursing home residents often have advanced illness and limited functional ability. Hospital transfers may be burdensome and of limited clinical benefit for these patients, particularly for those with advanced illness and limited life expectancy, and are associated with markers of poor quality of end-of-life care, such as increased rates of stage IV decubitus ulcer and feeding-tube use towards the end of life.1 Advance care planning is associated with less aggressive care towards the end of life for persons with advanced illness,2 which ultimately improves the quality of end-of-life care for these individuals. Prior interventions to improve advance care planning have had variable effects, while video-based interventions to improve advance care planning have shown promise.3
This pragmatic randomized trial assessed the effect of an advance care planning video program on important clinical outcomes for nursing home residents, particularly those with advanced illness. The results, however, are disappointing, as the video intervention failed to improve hospital transfer rate and burdensome treatment in this population. The negative results could be attributed to the limited adoption of the video intervention in the study, as only 21.9% of residents in the intervention group were actually exposed to the intervention. What is not reported, and is difficult to assess, is whether the video intervention led to advance care planning, as would be demonstrated by advance directive documentation and acceptance of goals of care of comfort. A per-protocol analysis may be considered to demonstrate if there is an effect on residents who were exposed to the intervention. Nonetheless, the low adoption rate of the intervention may prompt further investigation of factors limiting adoption and perhaps lead to a redesigned trial aimed at enhancing adoption, with consideration of use of implementation trial designs.
As pointed out by the study investigators, other changes to nursing home practices, specifically on hospital transfer, likely occurred during the study period. A number of national initiatives to reduce unnecessary hospital transfer from nursing homes have been introduced, and a reduction in hospital transfers occurred between 2011 and 20174; these initiatives could have impacted staff priorities and adoption of the study intervention relative to other co-occurring initiatives.
Applications for Clinical Practice
The authors of this study reported negative trial results, but their findings highlight important issues in conducting trials in the nursing home setting. Additional demonstration of actual effect on advance care planning discussions and documentation will further enhance our understanding of whether the intervention, as tested, yields changes in practice on advance care planning in nursing homes. The pragmatic clinical trial design used in this study accounts for real-world settings, but may have limited the study’s ability to account for and adjust for differences in staff, settings, and other conditions and factors that may impact adoption of and fidelity to the intervention. Quality improvement approaches, such as INTERACT, have targeted unnecessary hospital transfers and may yield positive results.5 Quality improvement approaches like INTERACT allow for a high degree of adaptation to local procedures and settings, which in clinical trials is difficult to do. However, in a real-world setting, such approaches may be necessary to improve care.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine the effect of an advance care planning video intervention in nursing homes on resident outcomes of hospital transfer, burdensome treatment, and hospice enrollment.
Design. Pragmatic cluster randomized controlled trial.
Setting and participants. The study was conducted in 360 nursing homes located in 32 states across the United States. The facilities were owned by 2 for-profit nursing home chains; facilities with more than 50 beds were eligible to be included in the study. Facilities deemed by corporate leaders to have serious organizational problems or that lacked the ability to transfer electronic health records were excluded. The facilities, stratified by the primary outcome hospitalizations per 1000 person-days, were then randomized to intervention and control in a 1:2 ratio. Leaders from facilities in the intervention group received letters describing their selection to participate in the advance care planning video program, and all facilities invited agreed to participate. Participants (residents in nursing homes) were enrolled from February 1, 2016, to May 31, 2018. Each participant was followed for 12 months after enrollment. All residents living in intervention facilities were offered the opportunity to watch intervention videos. The target population of the study was residents with advanced illness, including advanced dementia or advanced cardiopulmonary disease, as defined by the Minimum Data Set (MDS) variables, who were aged 65 and older, were long-stay residents (100 days or more), and were enrolled as Medicare fee-for-service beneficiaries. Secondary analysis included residents without advanced illness meeting other criteria.
Intervention. The intervention consisted of a selection of 5 short videos (6 to 10 minutes each), which had been previously developed and tested in smaller randomized trials. These videos cover the topics of general goals of care, goals of care for advanced dementia, hospice, hospitalization, and advance care planning for healthy patients, and use narration and images of typical treatments representing intensive medical care, basic medical care, and comfort care. The video for goals of care for advanced dementia targeted proxies of residents rather than residents themselves.
The implementation strategy for the video program included using a program manager to oversee the organization of the program’s rollout (a manager for each for-profit nursing home chain) and 2 champions at each facility (typically social workers were tasked with showing videos to patients and families). Champions received training from the study investigators and the manager and were asked to choose and offer selected videos to residents or proxies within 7 days of admission or readmission, every 6 months during a resident’s stay, and when specific decisions occurred, such as transition to hospice care, and on special occasions, such as out-of-town family visits.
Video offering and use were captured through documentation by a facility champion using a report tool embedded in the facility’s electronic health record. Champions met with the facility’s program manager and study team to review reports of video use, identify residents who had not been shown a video, and problem-solve on how to reach these residents. Facilities in the control group used their usual procedures for advance care planning.
Main outcome measures. Study outcomes included hospitalization transfers per 1000 person-days alive among long-stay residents with advanced illness (primary outcome); proportion of residents with at least 1 hospital transfer; proportion of residents with at least 1 burdensome treatment; and hospice enrollment (secondary outcomes). Secondary outcomes also included hospitalization transfers for long-stay residents without advanced illness. Hospital transfers were identified using Medicare claims for admissions, emergency department visits, and observation stays. Burdensome treatments were identified from Medicare claims and MDS, including tube feeding, parenteral therapy, invasive mechanical intervention, and intensive care unit admission. Fidelity to video intervention was measured by the proportion of residents offered the videos and the proportion of residents shown the videos at least once during the study period.
Main results. A total of 360 facilities were included in the study, 119 intervention and 241 control facilities. For the primary outcome, 4171 residents with advanced illness were included in the intervention group and 8308 residents with advanced illness were included in the control group. The average age was 83.6 years in both groups. In the intervention and control groups, respectively, 71.2% and 70.5% were female, 78.4% and 81.5% were White, 68.6% and 70.1% had advanced dementia at baseline, and 35.4% and 33.4% had advanced congestive heart failure or chronic obstructive pulmonary disease at baseline. Approximately 34% of residents received hospice care at baseline. In the intervention and control groups, 43.9% and 45.3% of residents died during follow-up, and the average length of follow-up in each group was 253.1 days and 252.6 days, respectively.
For the primary outcome of hospital transfers per 1000 person-days alive, there were 3.7 episodes (standard error 0.2) in the intervention group and 3.9 episodes in the control group (standard error 0.3); the difference was not statistically significant. For residents without advanced illness, there also was no difference in the hospital transfer rate. For other secondary outcomes, the proportion of residents in the intervention and control groups with 1 or more hospital transfer was 40.9% and 41.6%, respectively; the proportion with 1 or more burdensome treatment was 9.6% and 10.7%; and hospice enrollment was 24.9% and 25.5%. None of these differences was statistically significant. In the intervention group, 55.6% of residents or proxies were offered the video intervention and 21.9% were shown the videos at least once. There was substantial variability in the proportion of residents in the intervention group who were shown videos.
Conclusion. The advance planning video program did not lead to a reduction in hospital transfer, burdensome treatment, or changes in hospice enrollment. Acceptance of the intervention by residents was variable, and this may have contributed to the null finding.
Commentary
Nursing home residents often have advanced illness and limited functional ability. Hospital transfers may be burdensome and of limited clinical benefit for these patients, particularly for those with advanced illness and limited life expectancy, and are associated with markers of poor quality of end-of-life care, such as increased rates of stage IV decubitus ulcer and feeding-tube use towards the end of life.1 Advance care planning is associated with less aggressive care towards the end of life for persons with advanced illness,2 which ultimately improves the quality of end-of-life care for these individuals. Prior interventions to improve advance care planning have had variable effects, while video-based interventions to improve advance care planning have shown promise.3
This pragmatic randomized trial assessed the effect of an advance care planning video program on important clinical outcomes for nursing home residents, particularly those with advanced illness. The results, however, are disappointing, as the video intervention failed to improve hospital transfer rate and burdensome treatment in this population. The negative results could be attributed to the limited adoption of the video intervention in the study, as only 21.9% of residents in the intervention group were actually exposed to the intervention. What is not reported, and is difficult to assess, is whether the video intervention led to advance care planning, as would be demonstrated by advance directive documentation and acceptance of goals of care of comfort. A per-protocol analysis may be considered to demonstrate if there is an effect on residents who were exposed to the intervention. Nonetheless, the low adoption rate of the intervention may prompt further investigation of factors limiting adoption and perhaps lead to a redesigned trial aimed at enhancing adoption, with consideration of use of implementation trial designs.
As pointed out by the study investigators, other changes to nursing home practices, specifically on hospital transfer, likely occurred during the study period. A number of national initiatives to reduce unnecessary hospital transfer from nursing homes have been introduced, and a reduction in hospital transfers occurred between 2011 and 20174; these initiatives could have impacted staff priorities and adoption of the study intervention relative to other co-occurring initiatives.
Applications for Clinical Practice
The authors of this study reported negative trial results, but their findings highlight important issues in conducting trials in the nursing home setting. Additional demonstration of actual effect on advance care planning discussions and documentation will further enhance our understanding of whether the intervention, as tested, yields changes in practice on advance care planning in nursing homes. The pragmatic clinical trial design used in this study accounts for real-world settings, but may have limited the study’s ability to account for and adjust for differences in staff, settings, and other conditions and factors that may impact adoption of and fidelity to the intervention. Quality improvement approaches, such as INTERACT, have targeted unnecessary hospital transfers and may yield positive results.5 Quality improvement approaches like INTERACT allow for a high degree of adaptation to local procedures and settings, which in clinical trials is difficult to do. However, in a real-world setting, such approaches may be necessary to improve care.
–William W. Hung, MD, MPH
1. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212-1221
2. Nichols LH, Bynum J, Iwashyna TJ, et al. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33:667-674.
3. Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: randomized controlled trial. BMJ. 2009;338:b2159.
4. McCarthy EP, Ogarek JA, Loomer L, et al. Hospital transfer rates among US nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2020;180:385-394.
5. Rantz MJ, Popejoy L, Vogelsmeier, A et al. Successfully reducing hospitalizations of nursing home residents: results of the Missouri Quality Initiative. JAMA. 2017:18;960-966.
1. Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365:1212-1221
2. Nichols LH, Bynum J, Iwashyna TJ, et al. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33:667-674.
3. Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: randomized controlled trial. BMJ. 2009;338:b2159.
4. McCarthy EP, Ogarek JA, Loomer L, et al. Hospital transfer rates among US nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2020;180:385-394.
5. Rantz MJ, Popejoy L, Vogelsmeier, A et al. Successfully reducing hospitalizations of nursing home residents: results of the Missouri Quality Initiative. JAMA. 2017:18;960-966.
Tackling unhealthy substance use using USPSTF guidance and a 1-question tool
References
- US Preventive Services Task Force. Unhealthy drug use: screening [final recommendation statement]. Published June 9, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening. Accessed July 28, 2020.
- US Preventive Services Task Force. Illicit drug use in children, adolescents, and young adults: primary care-based interventions [final recommendation statement]. Published May 26, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents. Accessed July 28, 2020.
- US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions [final recommendation statement]. Published April 28, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 28, 2020.
- National Institute on Drug Abuse. NIDA Quick Screen v 1.0. www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed July 28, 2020.
- US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions [update in progress]. Published September 21, 2015. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed July 28, 2020.
References
- US Preventive Services Task Force. Unhealthy drug use: screening [final recommendation statement]. Published June 9, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening. Accessed July 28, 2020.
- US Preventive Services Task Force. Illicit drug use in children, adolescents, and young adults: primary care-based interventions [final recommendation statement]. Published May 26, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents. Accessed July 28, 2020.
- US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions [final recommendation statement]. Published April 28, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 28, 2020.
- National Institute on Drug Abuse. NIDA Quick Screen v 1.0. www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed July 28, 2020.
- US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions [update in progress]. Published September 21, 2015. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed July 28, 2020.
References
- US Preventive Services Task Force. Unhealthy drug use: screening [final recommendation statement]. Published June 9, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening. Accessed July 28, 2020.
- US Preventive Services Task Force. Illicit drug use in children, adolescents, and young adults: primary care-based interventions [final recommendation statement]. Published May 26, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents. Accessed July 28, 2020.
- US Preventive Services Task Force. Prevention and cessation of tobacco use in children and adolescents: primary care interventions [final recommendation statement]. Published April 28, 2020. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions. Accessed July 28, 2020.
- National Institute on Drug Abuse. NIDA Quick Screen v 1.0. www.drugabuse.gov/sites/default/files/pdf/nmassist.pdf. Accessed July 28, 2020.
- US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: behavioral and pharmacotherapy interventions [update in progress]. Published September 21, 2015. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions. Accessed July 28, 2020.
Oral Relugolix Yields Superior Testosterone Suppression and Decreased Cardiovascular Events Compared With GnRH Agonist
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
Study Overview
Objective. To evaluate the safety and efficacy of the highly selective oral gonadotropin-releasing hormone (GnRH) antagonist relugolix in men with advanced prostate cancer.
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 2:1 ratio to receive either relugolix 120 mg once daily after receiving a single loading dose of 360 mg, or 22.5 mg of leuprolide acetate every 3 months. Patients in Japan and Taiwan received 11.25 mg of leuprolide. The randomization was stratified by age (> 75 years or ≤ 75 years), metastatic disease status, and geographic region (Asia, Europe, North and South America). The intervention period was 48 weeks.
Setting and participants. 1327 patients were screened, and 934 patients underwent randomization: 622 patients to the relugolix group and 308 to the leuprolide group. Patients had histologically or cytologically confirmed adenocarcinoma of the prostate. All patients had to have 1 of the following: evidence of biochemical or clinical relapse after primary curative therapy, newly diagnosed hormone-sensitive metastatic disease, or advance localized disease unlikely to be cured by local primary intervention. The patients with disease progression or rising prostate-specific antigen (PSA) had the option to receive enzalutamide or docetaxel after the confirmation of progression. Patients were excluded if they had a major cardiovascular event within 6 months of enrollment.
Main outcome measures. The primary endpoint was sustained castration rate, defined as the cumulative probability of testosterone suppression to ≤ 50 ng/dL while on study treatment from week 5 through week 48. Secondary endpoints included noninferiority of relugolix to leuprolide in regard to sustained castration rate. Superiority testing was performed if the noninferiority margin of –10 percentage points was met. Additional secondary endpoints were probability of testosterone suppression to ≤ 50 ng/dL on day 4 and day 15 and the percentage of patients with a > 50% decrease in PSA at day 15 and follicle-stimulating hormone (FSH) levels at the end of week 24.
Main results. The baseline characteristics were well balanced between the treatment groups. Approximately 30% of the patients in each group had metastatic disease. Approximately 50% of patients enrolled had biochemical recurrence following primary treatment for prostate cancer. The mean PSA was 104.2 ng/mL in the relugolix group and 68.6 ng/mL in the leuprolide group. The majority of patients had at least 1 cardiovascular risk factor (ie, tobacco use, obesity, diabetes, hypertension, or a history of a major adverse cardiac event [MACE]). Adherence to oral therapy was reported as 99% in both groups. The median follow-up time was 52 weeks; 90% of patients in the relugolix arm and 89% in the leuprolide arm completed 48 weeks of treatment.
Sustained testosterone suppression to ≤ 50 ng/dL from day 29 through week 48 was seen in 96.7% of patients in the relugolix group and 88.8% in the leuprolide group, which was determined to be noninferior. Additionally, relugolix was also found to be superior to leuprolide in regard to sustained testosterone suppression (P < 0.001). These results were consistent across all subgroups. Relugolix was also found to be superior to leuprolide for all secondary endpoints, including cumulative probability of castration on day 4 (56% vs 0%) and day 15 (98.7% vs 12%) and testosterone suppression to ≤ 20 ng/dL on day 15 (78.4% vs 1%). Confirmed PSA response on day 15 was seen in 79.4% of patients in the relugolix arm and in 19.8% in the leuprolide arm (P < 0.001). FSH suppression was greater in the relugolix arm compared with the leuprolide arm by the end of week 24. An increase of testosterone levels from baseline was noted in the leuprolide patients at day 4, with the level decreasing to castrate level by day 29. In contrast, relugolix patients maintained castrate testosterone levels from day 4 throughout the intervention period. Testosterone recovery at 90 days was seen in 54% of patients in the relugolix group compared with 3% in the leuprolide group (P = 0.002).
The most frequent adverse event seen in both groups was hot flashes (54.3% in the relugolix group and 51.6% in the leuprolide group). The second most common adverse event report was fatigue, which occurred in 21.5% of patients in the relugolix arm and 18.5% in the leuprolide arm. Diarrhea was reported more frequently with relugolix than with leuprolide (12.2% vs 6.8%); however, diarrhea did not lead to discontinuation of therapy in any patient. Fatal events were reported more frequently in the leuprolide group (2.9%) compared with the relugolix group (1.1%). MACE were defined as nonfatal myocardial infarction, stroke, and death from any cause. After completing the intervention period of 48 weeks, the relugolix group had a 2.9% incidence of major cardiovascular events, compared with 6.2% in the leuprolide group. In patients having a medical history of cardiovascular events, the adverse event rate during the trial period was 3.6% in the relugolix group and 17.8% in leuprolide group. This translated into a 54% lower risk of MACE in the relugolix arm compared with the leuprolide arm.
Conclusion. The use of relugolix in advanced prostate cancer led to rapid, sustained suppression and faster recovery of testosterone level compared with leuprolide. Relugolix appeared safer to use for men with a medical history of cardiovascular events and showed a 54% lower risk of MACE than leuprolide.
Commentary
Relugolix is a highly selective oral GnRH antagonist that rapidly inhibits pituitary release of luteinizing hormone and FSH. The current phase 3 HERO trial highlights the efficacy of relugolix in regard to testosterone suppression, adding to potential therapeutic options for these men. Relugolix yielded superior sustained testosterone suppression to less than 50 ng/dL throughout the 48-week study period, meeting its primary endpoint. Additionally, relugolix showed superiority in all secondary endpoints across all subgroups of patients. To date, the only GnRH antagonist on the market is degarelix, which is given as a monthly subcutaneous injection.1 Injection-site reactions remain an issue with this formulation.
Cardiovascular disease is the leading cause of death in the United States, and it is known that men with prostate cancer have a higher incidence of cardiovascular disease.2 While data regarding adverse cardiac outcomes with androgen deprivation therapy have been mixed, it is thought that this therapy increases the risk for MACE. There is mounting evidence that GnRH antagonists may have a less detrimental effect on cardiovascular outcomes compared with GnRH agonists. For example, a pooled analysis of 6 phase 3 trials showed a lower incidence of cardiovascular events in men with preexisting cardiovascular disease using the GnRH antagonist degarelix compared with GnRH agonists after 12 months of treatment.3 Furthermore, a more recent phase 2 randomized trial showed that 20% of patients treated with a GnRH agonist developed cardiovascular events, compared to 3% in the GnRH antagonist group. The absolute risk reduction of cardiovascular events at 12 months was 18%.4 The results of the current trial support such findings, showing a 54% reduction in MACE after 48 weeks of therapy when compared with leuprolide (2.9% in relugolix arm vs 6.2% in leuprolide arm). More importantly perhaps, in the subgroup of men with preexisting cardiovascular disease, the benefit was even greater, with a MACE incidence of 3.6% with relugolix compared with 17.8% with leuprolide.
Studies have also shown that second-generation antiandrogens such as enzalutamide are associated with an increased risk of death from cardiovascular causes. For example, data from the recently updated PROSPER trial, which evaluated the use of enzalutamide in men with nonmetastatic, castration-resistant prostate cancer, showed an increased risk of adverse events, including falls, fatigue, hypertension, and death from cardiovascular events.5 Furthermore, adding second-generation antiandrogens to GnRH-agonist therapy is associated with a high risk of cardiovascular events in men with preexisting cardiovascular disease.3 These results were noted in all of the trials of second-generation antiandrogens, including enzalutamide, apalutamide, and darolutamide, in combination with GnRH agonists.6-8 Taken together, one might consider whether the use of a GnRH antagonist would result in improved cardiovascular outcomes in high-risk patients.
In light of the efficacy of relugolix in regard to testosterone suppression highlighted in the current trial, it is likely that its efficacy in regard to cancer outcomes will be similar; however, to date there is no level 1 evidence to support this. Nevertheless, there is a clear association of adverse cardiovascular outcomes in men treated with GnRH agonists, and the notable 54% risk reduction seen in the current trial certainly would support considering the use of a GnRH antagonist for the subgroup of patients with preexisting cardiovascular disease or those at high risk for MACE. Further work is needed to define the role of GnRH antagonists in conjunction with second-generation antiandrogens to help mitigate cardiovascular toxicities.
Clinical Implications
The use of GnRH antagonists should be considered in men with advanced prostate cancer who have underlying cardiovascular disease to help mitigate the risk of MACE. Currently, degarelix is the only commercially available agent; however, pending regulatory approval, oral relugolix may be considered an appropriate oral option in such patients, with data supporting superior testosterone suppressive effects. Further follow-up will be needed.
–Saud Alsubait, MD, Michigan State University, East Lansing, MI
–Daniel Isaac, MD, MS
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
1. Barkin J, Burton S, Lambert C. Optimizing subcutaneous injection of the gonadotropin-releasing hormone receptor antagonist degarelix. Can J Urol. 2016;23:8179-8183.
2. Higano CS. Cardiovascular disease and androgen axis-targeted drugs for prostate cancer. N Engl J Med. 2020;382:2257-2259.
3. Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565-573.
4. Margel D, Peer A, Ber Y, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. J Urol. 2019;202:1199-1208.
5. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197-2206.
6. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418.
7. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380:1235-1246.
MIS-C is a serious immune-mediated response to COVID-19 infection
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
One of the take-away messages from a review of multisystem inflammatory syndrome in children (MIS-C) is that clinicians treating this condition “need to be comfortable with uncertainty,” Melissa Hazen, MD, said at a synthesis of multiple published case series and personal experience summarized at the virtual Pediatric Hospital Medicine meeting.
She emphasized MIS-C patient care “requires flexibility,” and she advised clinicians managing these patients to open the lines of communication with the many specialists who often are required to deal with complications affecting an array of organ systems.
MIS-C might best be understood as the most serious manifestation of an immune-mediated response to COVID-19 infection that ranges from transient mild symptoms to the life-threatening multiple organ involvement that characterizes this newly recognized threat. Although “most children who encounter this pathogen only develop mild disease,” the spectrum of the disease can move in a subset of patients to a “Kawasaki-like illness” without hemodynamic instability and then to MIS-C “with highly elevated systemic inflammatory markers and multiple organ involvement,” explained Dr. Hazen, an attending physician in the rheumatology program at Boston Children’s Hospital.
most of which have only recently reached publication, according to Dr. Hazen. In general, the description of the most common symptoms and their course has been relatively consistent.
In 186 cases of MIS-C collected in a study funded by the Centers for Disease Control and Prevention, 148 (80%) were admitted to intensive care, 90 patients (48%) received vasoactive support, 37 (20%) received mechanical ventilation, and 4 (2%) died.1 The median age was 8 years (range, 3-13 years) in this study. The case definition was fever for at least 24 hours, laboratory evidence of inflammation, multisystem organ involvement, and evidence of COVID-19 infection. In this cohort of 186 children, 92% had gastrointestinal, 80% had cardiovascular, 76% had hematologic, and 70% had respiratory system involvement.
In a different series of 95 cases collected in New York State, 79 (80%) were admitted to intensive care, 61 (62%) received vasoactive support, 10 (10%) received mechanical ventilation, 4 (4%) received extracorporeal membrane oxygenation (ECMO), and 2 (2%) died. 2 Thirty-one percent patients were aged 0-5 years, 42% were 6-12 years, and 26% were 13-20 years of age. In that series, for which the case definition was elevation of two or more inflammatory markers, virologic evidence of COVID-19 infection, 80% had gastrointestinal system involvement, and 53% had evidence of myocarditis.
In both of these series, as well as others published and unpublished, the peak in MIS-C cases has occurred about 3 to 4 weeks after peak COVID-19 activity, according to Diana Lee, MD, a pediatrician at Icahn School of Medicine at Mount Sinai, New York. This pattern, reported by others, was observed in New York State, where 230 cases of MIS-C were collected from the beginning of May until the end of June, which reflected this 3- to 4-week delay in peak incidence.
“This does seem to be a rare syndrome since this [group of] 230 cases is amongst the entire population of children in New York State. So, yes, we should be keeping this in mind in our differential, but we should not forget all the other reasons that children can have a fever,” she said.
Both Dr. Hazen and Dr. Lee cautioned that MIS-C, despite a general consistency among published studies, remains a moving target in regard to how it is being characterized. In a 2-day period in May, the CDC, the World Health Organization, and New York State all issued descriptions of MIS-C, employing compatible but slightly different terminology and diagnostic criteria. Many questions regarding optimal methods of diagnosis, treatment, and follow-up remain unanswered.
Questions regarding the risk to the cardiovascular system, one of the organs most commonly affected in MIS-C, are among the most urgent. It is not now clear how best to monitor cardiovascular involvement, how to intervene, and how to follow patients in the postinfection period, according to Kevin G. Friedman, MD, a pediatrician at Harvard Medical School, Boston, and an attending physician in the department of cardiology at Boston Children’s Hospital.
“The most frequent complication we have seen is ventricular dysfunction, which occurs in about half of these patients,” he reported. “Usually it is in the mild to moderate range, but occasionally patients have an ejection fraction of less than 40%.”
Coronary abnormalities, typically in the form of dilations or small aneurysms, occur in 10%-20% of children with MIS-C, according to Dr. Friedman. Giant aneurysms have been reported.
“Some of these findings can progress including in both the acute phase and, particularly for the coronary aneurysms, in the subacute phase. We recommend echocardiograms and EKGs at diagnosis and at 1-2 weeks to recheck coronary size or sooner if there are clinical indications,” Dr. Friedman advised.
Protocols like these are constantly under review as more information becomes available. There are as yet no guidelines, and practice differs across institutions, according to the investigators summarizing this information.
None of the speakers had any relevant financial disclosures.
References
1. Feldstein LR et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334-46.
2. Dufort EM et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med 2020;383:347-58.
FROM PHM20 VIRTUAL
Physician recruitment drops by 30% because of pandemic
the firm reported.
“Rather than having many practice opportunities to choose from, physicians now may have to compete to secure practice opportunities that meet their needs,” the authors wrote in Merritt Hawkins’ report on the impact of COVID-19.
Most of the report concerns physician recruitment from April 1, 2019, to March 31, 2020. The data were mostly derived from searches that Merritt Hawkins conducted before the effects of the pandemic was fully felt.
Family medicine was again the most sought-after specialty, as it has been for the past 14 years. But demand for primary care doctors – including family physicians, internists, and pediatricians – leveled off, and average starting salaries for primary care doctors dropped during 2019-2020. In contrast, the number of searches conducted for nurse practitioners (NPs) and physician assistants (PAs) increased by 54%, and their salaries increased slightly.
To explain the lackluster prospects for primary care before the pandemic, the authors cited research showing that patients were turning away from the traditional office visit model. At the same time, there was a rise in visits to NPs and PAs, including those in urgent care centers and retail clinics.
As a result of decreased demand for primary care physicians and the rising prevalence of telehealth, Merritt Hawkins expects primary care salaries to drop overall. With telehealth generating a larger portion of revenues, “it is uncertain whether primary care physicians will be able to sustain levels of reimbursement that were prevalent pre-COVID even at such time as the economy is improved and utilization increases,” the authors reported.
Demand for specialists was increasing prior to the COVID-19 crisis, partly as a result of the aging of the population. Seventy-eight percent of all searches were for medical specialists, compared with 67% 5 years ago. However, the pandemic has set back specialist searches. “Demand and compensation for specialists also will change as a result of COVID-19 in response to declines in the volume of medical procedures,” according to the authors.
In contrast, the recruitment of doctors who are on the front line of COVID-19 care is expected to increase. Among the fields anticipated to be in demand are emergency department specialists, infectious disease specialists, and pulmonology/critical care physicians. Travis Singleton, executive vice president of Merritt Hawkins, said in an interview that this trend is already happening and will accelerate as COVID-19 hot spots arise across the country.
Specialists in different fields received either higher or lower offers than during the previous year. Starting salaries for noninvasive cardiologists, for example, dropped 7.3%; gastroenterologists earned 7.7% less; and neurologists, 6.9% less. In contrast, orthopedic surgeons saw offers surge 16.7%; radiologists, 9.3%; and pulmonologists/critical care specialists, 7.7%.
Physicians were offered salaries plus bonuses in three-quarters of searches. Relative value unit–based production remained the most common basis for bonuses. Quality/value-based metrics were used in computing 64% of bonuses – up from 56% the previous year – but still determined only 11% of total physician compensation.
Pandemic outlook
Whereas health care helped drive the U.S. economy in 2018-2019, the pace of job growth in health care has decreased since March. As a result of the pandemic, health care spending in the United States declined by 18% in the first quarter of 2020. Physician practice revenue dropped by 55% during the first quarter, and many small and solo practices are still struggling.
In a 2018 Merritt Hawkins survey, 18% of physicians said they had used telehealth to treat patients. Because of the pandemic, that percentage jumped to 48% in April 2020. But telehealth hasn’t made up for the loss of patient revenue from in-office procedures, tests, and other services, and it still isn’t being reimbursed at the same level as in-office visits.
With practices under severe financial strain, the authors explained, “A majority of private practices have curtailed most physician recruiting activity since the virus emerged.”
In some states, many specialty practices have been adversely affected by the suspension of elective procedures, and specialty practices that rely on nonessential procedures are unlikely to recruit additional physicians.
One-third of practices could close
The survival of many private practices is now in question. “Based on the losses physician practices have sustained as a result of COVID-19, some markets could lose up to 35% or more of their most vulnerable group practices while a large percent of others will be acquired,” the authors wrote.
Hospitals and health systems will acquire the bulk of these practices, in many cases at fire-sale prices, Mr. Singleton predicted. This enormous shift from private practice to employment, he added, “will have as much to do with the [physician] income levels we’re going to see as the demand for the specialties themselves.”
Right now, he said, Merritt Hawkins is fielding a huge number of requests from doctors seeking employment, but there aren’t many jobs out there. “We haven’t seen an employer-friendly market like this since the 1970s,” he noted. “Before the pandemic, a physician might have had five to 10 jobs to choose from. Now it’s the opposite: We have one job, and 5 to 10 physicians are applying for it.”
Singleton believes the market will adjust by the second quarter of next year. Even if the pandemic worsens, he said, the system will have made the necessary corrections and adjustments “because we have to start seeing patients again, both in terms of demand and economics. So these doctors will be in demand again and will have work.”
Contingent employment
Although the COVID-related falloff in revenue has hit private practices the hardest, some employed physicians have also found themselves in a bind. According to a Merritt Hawkins/Physicians Foundation survey conducted in April, 21% of physicians said they had been furloughed or had taken a pay cut.
Mr. Singleton views this trend as part of hospitals’ reassessment of how they’re going to deal with labor going forward. To cope with utilization ebbs and flows in response to the virus, hospitals are now considering what the report calls a “contingent labor/flex staffing model.”
Under this type of arrangement, which some hospitals have already adopted, physicians may no longer work full time in a single setting, Mr. Singleton said. They may be asked to conduct telehealth visits on nights and weekends and work 20 hours a week in the clinic, or they may have shifts in multiple hospitals or clinics.
“You can make as much or more on a temporary basis as on a permanent basis,” he said. “But you have to be more flexible. You may have to travel or do a different scope of work, or work in different settings.”
A version of this article originally appeared on Medscape.com.
the firm reported.
“Rather than having many practice opportunities to choose from, physicians now may have to compete to secure practice opportunities that meet their needs,” the authors wrote in Merritt Hawkins’ report on the impact of COVID-19.
Most of the report concerns physician recruitment from April 1, 2019, to March 31, 2020. The data were mostly derived from searches that Merritt Hawkins conducted before the effects of the pandemic was fully felt.
Family medicine was again the most sought-after specialty, as it has been for the past 14 years. But demand for primary care doctors – including family physicians, internists, and pediatricians – leveled off, and average starting salaries for primary care doctors dropped during 2019-2020. In contrast, the number of searches conducted for nurse practitioners (NPs) and physician assistants (PAs) increased by 54%, and their salaries increased slightly.
To explain the lackluster prospects for primary care before the pandemic, the authors cited research showing that patients were turning away from the traditional office visit model. At the same time, there was a rise in visits to NPs and PAs, including those in urgent care centers and retail clinics.
As a result of decreased demand for primary care physicians and the rising prevalence of telehealth, Merritt Hawkins expects primary care salaries to drop overall. With telehealth generating a larger portion of revenues, “it is uncertain whether primary care physicians will be able to sustain levels of reimbursement that were prevalent pre-COVID even at such time as the economy is improved and utilization increases,” the authors reported.
Demand for specialists was increasing prior to the COVID-19 crisis, partly as a result of the aging of the population. Seventy-eight percent of all searches were for medical specialists, compared with 67% 5 years ago. However, the pandemic has set back specialist searches. “Demand and compensation for specialists also will change as a result of COVID-19 in response to declines in the volume of medical procedures,” according to the authors.
In contrast, the recruitment of doctors who are on the front line of COVID-19 care is expected to increase. Among the fields anticipated to be in demand are emergency department specialists, infectious disease specialists, and pulmonology/critical care physicians. Travis Singleton, executive vice president of Merritt Hawkins, said in an interview that this trend is already happening and will accelerate as COVID-19 hot spots arise across the country.
Specialists in different fields received either higher or lower offers than during the previous year. Starting salaries for noninvasive cardiologists, for example, dropped 7.3%; gastroenterologists earned 7.7% less; and neurologists, 6.9% less. In contrast, orthopedic surgeons saw offers surge 16.7%; radiologists, 9.3%; and pulmonologists/critical care specialists, 7.7%.
Physicians were offered salaries plus bonuses in three-quarters of searches. Relative value unit–based production remained the most common basis for bonuses. Quality/value-based metrics were used in computing 64% of bonuses – up from 56% the previous year – but still determined only 11% of total physician compensation.
Pandemic outlook
Whereas health care helped drive the U.S. economy in 2018-2019, the pace of job growth in health care has decreased since March. As a result of the pandemic, health care spending in the United States declined by 18% in the first quarter of 2020. Physician practice revenue dropped by 55% during the first quarter, and many small and solo practices are still struggling.
In a 2018 Merritt Hawkins survey, 18% of physicians said they had used telehealth to treat patients. Because of the pandemic, that percentage jumped to 48% in April 2020. But telehealth hasn’t made up for the loss of patient revenue from in-office procedures, tests, and other services, and it still isn’t being reimbursed at the same level as in-office visits.
With practices under severe financial strain, the authors explained, “A majority of private practices have curtailed most physician recruiting activity since the virus emerged.”
In some states, many specialty practices have been adversely affected by the suspension of elective procedures, and specialty practices that rely on nonessential procedures are unlikely to recruit additional physicians.
One-third of practices could close
The survival of many private practices is now in question. “Based on the losses physician practices have sustained as a result of COVID-19, some markets could lose up to 35% or more of their most vulnerable group practices while a large percent of others will be acquired,” the authors wrote.
Hospitals and health systems will acquire the bulk of these practices, in many cases at fire-sale prices, Mr. Singleton predicted. This enormous shift from private practice to employment, he added, “will have as much to do with the [physician] income levels we’re going to see as the demand for the specialties themselves.”
Right now, he said, Merritt Hawkins is fielding a huge number of requests from doctors seeking employment, but there aren’t many jobs out there. “We haven’t seen an employer-friendly market like this since the 1970s,” he noted. “Before the pandemic, a physician might have had five to 10 jobs to choose from. Now it’s the opposite: We have one job, and 5 to 10 physicians are applying for it.”
Singleton believes the market will adjust by the second quarter of next year. Even if the pandemic worsens, he said, the system will have made the necessary corrections and adjustments “because we have to start seeing patients again, both in terms of demand and economics. So these doctors will be in demand again and will have work.”
Contingent employment
Although the COVID-related falloff in revenue has hit private practices the hardest, some employed physicians have also found themselves in a bind. According to a Merritt Hawkins/Physicians Foundation survey conducted in April, 21% of physicians said they had been furloughed or had taken a pay cut.
Mr. Singleton views this trend as part of hospitals’ reassessment of how they’re going to deal with labor going forward. To cope with utilization ebbs and flows in response to the virus, hospitals are now considering what the report calls a “contingent labor/flex staffing model.”
Under this type of arrangement, which some hospitals have already adopted, physicians may no longer work full time in a single setting, Mr. Singleton said. They may be asked to conduct telehealth visits on nights and weekends and work 20 hours a week in the clinic, or they may have shifts in multiple hospitals or clinics.
“You can make as much or more on a temporary basis as on a permanent basis,” he said. “But you have to be more flexible. You may have to travel or do a different scope of work, or work in different settings.”
A version of this article originally appeared on Medscape.com.
the firm reported.
“Rather than having many practice opportunities to choose from, physicians now may have to compete to secure practice opportunities that meet their needs,” the authors wrote in Merritt Hawkins’ report on the impact of COVID-19.
Most of the report concerns physician recruitment from April 1, 2019, to March 31, 2020. The data were mostly derived from searches that Merritt Hawkins conducted before the effects of the pandemic was fully felt.
Family medicine was again the most sought-after specialty, as it has been for the past 14 years. But demand for primary care doctors – including family physicians, internists, and pediatricians – leveled off, and average starting salaries for primary care doctors dropped during 2019-2020. In contrast, the number of searches conducted for nurse practitioners (NPs) and physician assistants (PAs) increased by 54%, and their salaries increased slightly.
To explain the lackluster prospects for primary care before the pandemic, the authors cited research showing that patients were turning away from the traditional office visit model. At the same time, there was a rise in visits to NPs and PAs, including those in urgent care centers and retail clinics.
As a result of decreased demand for primary care physicians and the rising prevalence of telehealth, Merritt Hawkins expects primary care salaries to drop overall. With telehealth generating a larger portion of revenues, “it is uncertain whether primary care physicians will be able to sustain levels of reimbursement that were prevalent pre-COVID even at such time as the economy is improved and utilization increases,” the authors reported.
Demand for specialists was increasing prior to the COVID-19 crisis, partly as a result of the aging of the population. Seventy-eight percent of all searches were for medical specialists, compared with 67% 5 years ago. However, the pandemic has set back specialist searches. “Demand and compensation for specialists also will change as a result of COVID-19 in response to declines in the volume of medical procedures,” according to the authors.
In contrast, the recruitment of doctors who are on the front line of COVID-19 care is expected to increase. Among the fields anticipated to be in demand are emergency department specialists, infectious disease specialists, and pulmonology/critical care physicians. Travis Singleton, executive vice president of Merritt Hawkins, said in an interview that this trend is already happening and will accelerate as COVID-19 hot spots arise across the country.
Specialists in different fields received either higher or lower offers than during the previous year. Starting salaries for noninvasive cardiologists, for example, dropped 7.3%; gastroenterologists earned 7.7% less; and neurologists, 6.9% less. In contrast, orthopedic surgeons saw offers surge 16.7%; radiologists, 9.3%; and pulmonologists/critical care specialists, 7.7%.
Physicians were offered salaries plus bonuses in three-quarters of searches. Relative value unit–based production remained the most common basis for bonuses. Quality/value-based metrics were used in computing 64% of bonuses – up from 56% the previous year – but still determined only 11% of total physician compensation.
Pandemic outlook
Whereas health care helped drive the U.S. economy in 2018-2019, the pace of job growth in health care has decreased since March. As a result of the pandemic, health care spending in the United States declined by 18% in the first quarter of 2020. Physician practice revenue dropped by 55% during the first quarter, and many small and solo practices are still struggling.
In a 2018 Merritt Hawkins survey, 18% of physicians said they had used telehealth to treat patients. Because of the pandemic, that percentage jumped to 48% in April 2020. But telehealth hasn’t made up for the loss of patient revenue from in-office procedures, tests, and other services, and it still isn’t being reimbursed at the same level as in-office visits.
With practices under severe financial strain, the authors explained, “A majority of private practices have curtailed most physician recruiting activity since the virus emerged.”
In some states, many specialty practices have been adversely affected by the suspension of elective procedures, and specialty practices that rely on nonessential procedures are unlikely to recruit additional physicians.
One-third of practices could close
The survival of many private practices is now in question. “Based on the losses physician practices have sustained as a result of COVID-19, some markets could lose up to 35% or more of their most vulnerable group practices while a large percent of others will be acquired,” the authors wrote.
Hospitals and health systems will acquire the bulk of these practices, in many cases at fire-sale prices, Mr. Singleton predicted. This enormous shift from private practice to employment, he added, “will have as much to do with the [physician] income levels we’re going to see as the demand for the specialties themselves.”
Right now, he said, Merritt Hawkins is fielding a huge number of requests from doctors seeking employment, but there aren’t many jobs out there. “We haven’t seen an employer-friendly market like this since the 1970s,” he noted. “Before the pandemic, a physician might have had five to 10 jobs to choose from. Now it’s the opposite: We have one job, and 5 to 10 physicians are applying for it.”
Singleton believes the market will adjust by the second quarter of next year. Even if the pandemic worsens, he said, the system will have made the necessary corrections and adjustments “because we have to start seeing patients again, both in terms of demand and economics. So these doctors will be in demand again and will have work.”
Contingent employment
Although the COVID-related falloff in revenue has hit private practices the hardest, some employed physicians have also found themselves in a bind. According to a Merritt Hawkins/Physicians Foundation survey conducted in April, 21% of physicians said they had been furloughed or had taken a pay cut.
Mr. Singleton views this trend as part of hospitals’ reassessment of how they’re going to deal with labor going forward. To cope with utilization ebbs and flows in response to the virus, hospitals are now considering what the report calls a “contingent labor/flex staffing model.”
Under this type of arrangement, which some hospitals have already adopted, physicians may no longer work full time in a single setting, Mr. Singleton said. They may be asked to conduct telehealth visits on nights and weekends and work 20 hours a week in the clinic, or they may have shifts in multiple hospitals or clinics.
“You can make as much or more on a temporary basis as on a permanent basis,” he said. “But you have to be more flexible. You may have to travel or do a different scope of work, or work in different settings.”
A version of this article originally appeared on Medscape.com.
Speaking Up, Questioning Assumptions About Racism
Let me start with these 3 words that really should never have to be said: Black Lives Matter.
It was hard to sit down to write this piece—not just because it’s a sunny Sunday morning, but because I’m still afraid I’ll get it wrong, show my white privilege, offend someone. George Floyd’s murder has been a reckoning for Black Americans, for the police, for the nation (maybe the world), and for me. I live in a multi-racial household, and we have redoubled our efforts to talk about racism and bias and question our assumptions as part of our daily conversations. After Mr. Floyd was killed, I decided that I would try to be less afraid of getting it wrong and be more outspoken about my support for Black Lives Matter and for the work that we need to do in this country, and in ourselves, to become more antiracist.
Here are some things that I know: I know that study after study has shown that health care and health outcomes are worse for Black people than for White people. I know that people of color are sickening and dying with COVID-19 before our eyes, just as other pandemics, such as HIV, differentially affect communities of color. I know, too, that a Black physician executive who lives around the corner from me has been stopped by our local police more than 10 times; I have been stopped by our local police exactly once.
I don’t know how to fix it. But I do know that my silence won’t help. Here are some things I am trying to do at home and at work: I am educating myself about race and racism. I’m not asking my Black peers, patients, or colleagues to teach me, but I am listening to what they tell me, when they want to tell me. I am reading books like Ibram Kendi’s How to Be Antiracist and Bernadine Evaristo’s Girl, Woman, Other. I challenge myself to read articles that I might have skipped over—because they were simply too painful. People of color don’t have a choice about facing their pain. I have that choice—it’s a privilege—and I choose to be an ally.
I’m speaking up even when I’m afraid that I might say the wrong thing. This can take several forms—questioning assumptions about race and racism when it comes up, which is often, in medicine. It also means amplifying the voices that don’t always get heard—asking a young person of color her opinion in a meeting, retweeting the thoughts of a Black colleague, thanking someone publicly or personally for a comment, an idea, or the kernel of something important. I ask people to correct me, and I try to be humble in accepting criticism or correction.
Being a better ally also means putting our money where our mouth is, supporting Black-owned businesses and restaurants, and donating to causes that support equality and justice. We can diversify our social media feeds. We have to be willing to be excluded from the conversation—if you’re white or straight or cis-gendered, it’s not about you—and be ready to feel uncomfortable. We can encourag
Black Lives Matter. I’m looking forward to a day when that is so obvious that we don’t have to say it. Until then, I’m going to be hard at work with my head, my ears, and my whole heart.
Let me start with these 3 words that really should never have to be said: Black Lives Matter.
It was hard to sit down to write this piece—not just because it’s a sunny Sunday morning, but because I’m still afraid I’ll get it wrong, show my white privilege, offend someone. George Floyd’s murder has been a reckoning for Black Americans, for the police, for the nation (maybe the world), and for me. I live in a multi-racial household, and we have redoubled our efforts to talk about racism and bias and question our assumptions as part of our daily conversations. After Mr. Floyd was killed, I decided that I would try to be less afraid of getting it wrong and be more outspoken about my support for Black Lives Matter and for the work that we need to do in this country, and in ourselves, to become more antiracist.
Here are some things that I know: I know that study after study has shown that health care and health outcomes are worse for Black people than for White people. I know that people of color are sickening and dying with COVID-19 before our eyes, just as other pandemics, such as HIV, differentially affect communities of color. I know, too, that a Black physician executive who lives around the corner from me has been stopped by our local police more than 10 times; I have been stopped by our local police exactly once.
I don’t know how to fix it. But I do know that my silence won’t help. Here are some things I am trying to do at home and at work: I am educating myself about race and racism. I’m not asking my Black peers, patients, or colleagues to teach me, but I am listening to what they tell me, when they want to tell me. I am reading books like Ibram Kendi’s How to Be Antiracist and Bernadine Evaristo’s Girl, Woman, Other. I challenge myself to read articles that I might have skipped over—because they were simply too painful. People of color don’t have a choice about facing their pain. I have that choice—it’s a privilege—and I choose to be an ally.
I’m speaking up even when I’m afraid that I might say the wrong thing. This can take several forms—questioning assumptions about race and racism when it comes up, which is often, in medicine. It also means amplifying the voices that don’t always get heard—asking a young person of color her opinion in a meeting, retweeting the thoughts of a Black colleague, thanking someone publicly or personally for a comment, an idea, or the kernel of something important. I ask people to correct me, and I try to be humble in accepting criticism or correction.
Being a better ally also means putting our money where our mouth is, supporting Black-owned businesses and restaurants, and donating to causes that support equality and justice. We can diversify our social media feeds. We have to be willing to be excluded from the conversation—if you’re white or straight or cis-gendered, it’s not about you—and be ready to feel uncomfortable. We can encourag
Black Lives Matter. I’m looking forward to a day when that is so obvious that we don’t have to say it. Until then, I’m going to be hard at work with my head, my ears, and my whole heart.
Let me start with these 3 words that really should never have to be said: Black Lives Matter.
It was hard to sit down to write this piece—not just because it’s a sunny Sunday morning, but because I’m still afraid I’ll get it wrong, show my white privilege, offend someone. George Floyd’s murder has been a reckoning for Black Americans, for the police, for the nation (maybe the world), and for me. I live in a multi-racial household, and we have redoubled our efforts to talk about racism and bias and question our assumptions as part of our daily conversations. After Mr. Floyd was killed, I decided that I would try to be less afraid of getting it wrong and be more outspoken about my support for Black Lives Matter and for the work that we need to do in this country, and in ourselves, to become more antiracist.
Here are some things that I know: I know that study after study has shown that health care and health outcomes are worse for Black people than for White people. I know that people of color are sickening and dying with COVID-19 before our eyes, just as other pandemics, such as HIV, differentially affect communities of color. I know, too, that a Black physician executive who lives around the corner from me has been stopped by our local police more than 10 times; I have been stopped by our local police exactly once.
I don’t know how to fix it. But I do know that my silence won’t help. Here are some things I am trying to do at home and at work: I am educating myself about race and racism. I’m not asking my Black peers, patients, or colleagues to teach me, but I am listening to what they tell me, when they want to tell me. I am reading books like Ibram Kendi’s How to Be Antiracist and Bernadine Evaristo’s Girl, Woman, Other. I challenge myself to read articles that I might have skipped over—because they were simply too painful. People of color don’t have a choice about facing their pain. I have that choice—it’s a privilege—and I choose to be an ally.
I’m speaking up even when I’m afraid that I might say the wrong thing. This can take several forms—questioning assumptions about race and racism when it comes up, which is often, in medicine. It also means amplifying the voices that don’t always get heard—asking a young person of color her opinion in a meeting, retweeting the thoughts of a Black colleague, thanking someone publicly or personally for a comment, an idea, or the kernel of something important. I ask people to correct me, and I try to be humble in accepting criticism or correction.
Being a better ally also means putting our money where our mouth is, supporting Black-owned businesses and restaurants, and donating to causes that support equality and justice. We can diversify our social media feeds. We have to be willing to be excluded from the conversation—if you’re white or straight or cis-gendered, it’s not about you—and be ready to feel uncomfortable. We can encourag
Black Lives Matter. I’m looking forward to a day when that is so obvious that we don’t have to say it. Until then, I’m going to be hard at work with my head, my ears, and my whole heart.
Tamoxifen can reduce bleeding in women with contraceptive implants
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
FROM OBSTETRICS & GYNECOLOGY
NFL’s only physician player opts out of 2020 season over COVID
Canadian-born Duvernay-Tardif, right guard for the Kansas City Chiefs, announced on Twitter on July 24 what he called “one of the most difficult decisions I have had to make in my life.”
“There is no doubt in my mind the Chiefs’ medical staff have put together a strong plan to minimize the health risks associated with COVID-19, but some risks will remain,” he posted.
“Being at the frontline during this offseason has given me a different perspective on this pandemic and the stress it puts on individuals and our healthcare system. I cannot allow myself to potentially transmit the virus in our communities simply to play the sport that I love. If I am to take risks, I will do it caring for patients.”
According to CNN, Duvernay-Tardif, less than 3 months after helping the Chiefs win the Super Bowl in February, began working at a long-term care facility near Montreal in what he described as a “nursing role.”
Duvernay-Tardif wrote recently in an article for Sports Illustrated that he has not completed his residency and is not yet licensed to practice.
“My first day back in the hospital was April 24,” Duvernay-Tardif wrote. “I felt nervous the night before, but a good nervous, like before a game.”
Duvernay-Tardif has also served on the NFL Players’ Association COVID-19 task force, according to Yahoo News .
A spokesperson for Duvernay-Tardif told Medscape Medical News he was unavailable to comment about the announcement.
Starting His Dual Career
Duvernay-Tardif, 29, was drafted in the sixth round by the Chiefs in 2014.
According to Forbes , he spent 8 years (2010-2018) pursuing his medical degree while still playing college football for McGill University in Montreal. Duvernay-Tardif played offensive tackle for the Redmen and in his senior year (2013) won the Metras Trophy as most outstanding lineman in Canadian college football.
He explained in a previous Medscape interview how he managed his dual career; as a doctor he said he would like to focus on emergency medicine:
“I would say that at around 16-17 years of age, I was pretty convinced that medicine was for me,” he told Medscape.
“I was lucky that I didn’t have to do an undergrad program,” he continued. “In Canada, they have a fast-track program where instead of doing a full undergrad before getting into medical school, you can do a 1-year program where you can do all your physiology and biology classes all together.
“I had the chance to get into that program, and that’s how I was able to manage football and medicine at the same time. There’s no way I could have finished my med school doing part-time med school like I did for the past 4 years.”
ESPN explained the opt-out option: “According to an agreement approved by both the league and the union on [July 24], players considered high risk for COVID-19 can earn $350,000 and an accrued NFL season if they choose to opt out of the 2020 season. Players without risk can earn $150,000 for opting out. Duvernay-Tardif was scheduled to make $2.75 million this season.”
The danger of COVID-19 in professional sports has already been seen in Major League Baseball.
According to USA Today, the Miami Marlins have at least 14 players and staff who have tested positive for COVID-19, and major league baseball Commissioner Rob Manfred must decide whether to further delay the shortened season, cancel it, or allow it to continue.
MLB postponed the Marlins’ home opener July 27 against the Baltimore Orioles as well as the New York Yankees game in Philadelphia against the Phillies.
COVID-19 also shut down professional, college, high school, and recreational sports throughout much of the country beginning in March.
Medicine, Football Intersect
In the previous Medscape interview, Duvernay-Tardif talked about how medicine influenced his football career.
“For me, medicine was really helpful in the sense that I was better able to build a routine and question what works for me and what doesn’t. It gave me the ability to structure my work in order to optimize my time and to make sure that it’s pertinent.
“Another thing is the psychology and the sports psychology. I think there’s a little bit of a stigma around mental health issues in professional sports and everywhere, actually. I think because of medicine, I was more willing to question myself and more willing to use different tools in order to be a better football player.”
A version of this article first appeared on Medscape.com.
Canadian-born Duvernay-Tardif, right guard for the Kansas City Chiefs, announced on Twitter on July 24 what he called “one of the most difficult decisions I have had to make in my life.”
“There is no doubt in my mind the Chiefs’ medical staff have put together a strong plan to minimize the health risks associated with COVID-19, but some risks will remain,” he posted.
“Being at the frontline during this offseason has given me a different perspective on this pandemic and the stress it puts on individuals and our healthcare system. I cannot allow myself to potentially transmit the virus in our communities simply to play the sport that I love. If I am to take risks, I will do it caring for patients.”
According to CNN, Duvernay-Tardif, less than 3 months after helping the Chiefs win the Super Bowl in February, began working at a long-term care facility near Montreal in what he described as a “nursing role.”
Duvernay-Tardif wrote recently in an article for Sports Illustrated that he has not completed his residency and is not yet licensed to practice.
“My first day back in the hospital was April 24,” Duvernay-Tardif wrote. “I felt nervous the night before, but a good nervous, like before a game.”
Duvernay-Tardif has also served on the NFL Players’ Association COVID-19 task force, according to Yahoo News .
A spokesperson for Duvernay-Tardif told Medscape Medical News he was unavailable to comment about the announcement.
Starting His Dual Career
Duvernay-Tardif, 29, was drafted in the sixth round by the Chiefs in 2014.
According to Forbes , he spent 8 years (2010-2018) pursuing his medical degree while still playing college football for McGill University in Montreal. Duvernay-Tardif played offensive tackle for the Redmen and in his senior year (2013) won the Metras Trophy as most outstanding lineman in Canadian college football.
He explained in a previous Medscape interview how he managed his dual career; as a doctor he said he would like to focus on emergency medicine:
“I would say that at around 16-17 years of age, I was pretty convinced that medicine was for me,” he told Medscape.
“I was lucky that I didn’t have to do an undergrad program,” he continued. “In Canada, they have a fast-track program where instead of doing a full undergrad before getting into medical school, you can do a 1-year program where you can do all your physiology and biology classes all together.
“I had the chance to get into that program, and that’s how I was able to manage football and medicine at the same time. There’s no way I could have finished my med school doing part-time med school like I did for the past 4 years.”
ESPN explained the opt-out option: “According to an agreement approved by both the league and the union on [July 24], players considered high risk for COVID-19 can earn $350,000 and an accrued NFL season if they choose to opt out of the 2020 season. Players without risk can earn $150,000 for opting out. Duvernay-Tardif was scheduled to make $2.75 million this season.”
The danger of COVID-19 in professional sports has already been seen in Major League Baseball.
According to USA Today, the Miami Marlins have at least 14 players and staff who have tested positive for COVID-19, and major league baseball Commissioner Rob Manfred must decide whether to further delay the shortened season, cancel it, or allow it to continue.
MLB postponed the Marlins’ home opener July 27 against the Baltimore Orioles as well as the New York Yankees game in Philadelphia against the Phillies.
COVID-19 also shut down professional, college, high school, and recreational sports throughout much of the country beginning in March.
Medicine, Football Intersect
In the previous Medscape interview, Duvernay-Tardif talked about how medicine influenced his football career.
“For me, medicine was really helpful in the sense that I was better able to build a routine and question what works for me and what doesn’t. It gave me the ability to structure my work in order to optimize my time and to make sure that it’s pertinent.
“Another thing is the psychology and the sports psychology. I think there’s a little bit of a stigma around mental health issues in professional sports and everywhere, actually. I think because of medicine, I was more willing to question myself and more willing to use different tools in order to be a better football player.”
A version of this article first appeared on Medscape.com.
Canadian-born Duvernay-Tardif, right guard for the Kansas City Chiefs, announced on Twitter on July 24 what he called “one of the most difficult decisions I have had to make in my life.”
“There is no doubt in my mind the Chiefs’ medical staff have put together a strong plan to minimize the health risks associated with COVID-19, but some risks will remain,” he posted.
“Being at the frontline during this offseason has given me a different perspective on this pandemic and the stress it puts on individuals and our healthcare system. I cannot allow myself to potentially transmit the virus in our communities simply to play the sport that I love. If I am to take risks, I will do it caring for patients.”
According to CNN, Duvernay-Tardif, less than 3 months after helping the Chiefs win the Super Bowl in February, began working at a long-term care facility near Montreal in what he described as a “nursing role.”
Duvernay-Tardif wrote recently in an article for Sports Illustrated that he has not completed his residency and is not yet licensed to practice.
“My first day back in the hospital was April 24,” Duvernay-Tardif wrote. “I felt nervous the night before, but a good nervous, like before a game.”
Duvernay-Tardif has also served on the NFL Players’ Association COVID-19 task force, according to Yahoo News .
A spokesperson for Duvernay-Tardif told Medscape Medical News he was unavailable to comment about the announcement.
Starting His Dual Career
Duvernay-Tardif, 29, was drafted in the sixth round by the Chiefs in 2014.
According to Forbes , he spent 8 years (2010-2018) pursuing his medical degree while still playing college football for McGill University in Montreal. Duvernay-Tardif played offensive tackle for the Redmen and in his senior year (2013) won the Metras Trophy as most outstanding lineman in Canadian college football.
He explained in a previous Medscape interview how he managed his dual career; as a doctor he said he would like to focus on emergency medicine:
“I would say that at around 16-17 years of age, I was pretty convinced that medicine was for me,” he told Medscape.
“I was lucky that I didn’t have to do an undergrad program,” he continued. “In Canada, they have a fast-track program where instead of doing a full undergrad before getting into medical school, you can do a 1-year program where you can do all your physiology and biology classes all together.
“I had the chance to get into that program, and that’s how I was able to manage football and medicine at the same time. There’s no way I could have finished my med school doing part-time med school like I did for the past 4 years.”
ESPN explained the opt-out option: “According to an agreement approved by both the league and the union on [July 24], players considered high risk for COVID-19 can earn $350,000 and an accrued NFL season if they choose to opt out of the 2020 season. Players without risk can earn $150,000 for opting out. Duvernay-Tardif was scheduled to make $2.75 million this season.”
The danger of COVID-19 in professional sports has already been seen in Major League Baseball.
According to USA Today, the Miami Marlins have at least 14 players and staff who have tested positive for COVID-19, and major league baseball Commissioner Rob Manfred must decide whether to further delay the shortened season, cancel it, or allow it to continue.
MLB postponed the Marlins’ home opener July 27 against the Baltimore Orioles as well as the New York Yankees game in Philadelphia against the Phillies.
COVID-19 also shut down professional, college, high school, and recreational sports throughout much of the country beginning in March.
Medicine, Football Intersect
In the previous Medscape interview, Duvernay-Tardif talked about how medicine influenced his football career.
“For me, medicine was really helpful in the sense that I was better able to build a routine and question what works for me and what doesn’t. It gave me the ability to structure my work in order to optimize my time and to make sure that it’s pertinent.
“Another thing is the psychology and the sports psychology. I think there’s a little bit of a stigma around mental health issues in professional sports and everywhere, actually. I think because of medicine, I was more willing to question myself and more willing to use different tools in order to be a better football player.”
A version of this article first appeared on Medscape.com.





