User login
New Biomarkers Identified for Treatment Response in IBD
BERLIN — presented at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Differences uncovered in multiple messenger RNAs, proteins, metabolites, and gut microbiota were associated with responders and nonresponders to biologics and Janus kinase inhibitors, suggesting the potential for predictive biomarkers in IBD, including Crohn’s disease (CD) and ulcerative colitis (UC).
“With further work we hope to confirm these findings and evaluate their clinical relevance in identifying patients most likely to respond to tailored therapeutic interventions,” said Montserrat Baldan-Martin, PhD, a researcher at the University Hospital of the Princess, Madrid, Spain.
The treatment of IBD is challenging because of the heterogeneity across clinical, immunological, molecular, genetic, and microbiologic features, with one third of patients failing to respond to any one treatment.
Baldan-Martin and colleagues wanted to find predictive biomarkers of response by examining the differences across multi-omics profiles relative to different therapies.
The study analyzed 127 patients with IBD (57 with CD and 70 with UC) before and after 14 weeks of treatment with one of the following: anti–tumor necrosis factors (TNFs), ustekinumab, vedolizumab, or tofacitinib. Patient response to treatment was evaluated using endoscopic criteria that categorized them as responders or nonresponders to the different therapies.
In addition, molecular data from various biologic samples — serum, urine, extracellular vesicles, intestinal biopsies, and stool — were tested using transcriptomics, proteomics, metabolomics, and metagenomics.
Clear Differences
“The most significant differences were seen in gene expression within intestinal tissue of responder and nonresponder patients with ulcerative colitis taking vedolizumab,” Baldan-Martin reported.
Proteomic analysis revealed that a total of 1377 proteins were identified across all groups (CD, UC, and the four drug classes/therapies). Responders and nonresponders for each therapy expressed different proteins in serum extracellular vesicles and intestinal tissues.
For example, patients with CD who responded to anti-TNF therapies had 138 different proteins from those of anti-TNF nonresponders, while patients with UC who responded to anti-TNF therapies had 218 different proteins from those of anti-TNF nonresponders, reported Baldan-Martin.
Also, we observed almost no proteins “in common between ulcerative colitis responders versus nonresponders for all treatments,” she noted. And we “saw only three proteins in common with Crohn’s disease patients [on different drugs].”
Metabolomic analysis identified deregulation of 24 serum lipoproteins in CD responders to ustekinumab, compared with nonresponders.
“We observed greater differences in the lipoproteins in serum than metabolites in serum and urine,” Baldan-Martin added.
Analysis of biologic pathways also highlighted enrichment in ketone and butyrate metabolism, mitochondrial electron transport chain activity, carnitine synthesis, and fatty acid oxidation pathways, while metagenomic analysis revealed the greatest microbial differences in UC responders and nonresponders to anti-TNF therapies.
Baldan-Martin said research was ongoing with a new cohort of patients that aims to validate some of the biomarkers and help identify the patients most likely to respond to tailored therapeutic interventions.
“One of the challenges is integrating results from different omics approaches to create a more holistic understanding of the disease,” she said, adding that she hopes the research “will potentially open doors for early detection through multi-panel biomarkers.”
Session moderator Mark Samaan, MD, consultant gastroenterologist at Guy’s and St Thomas’ NHS Foundation Trust, London, England, said that “the findings related to nonresponse to specific drugs in UC and CD were interesting. With longitudinal follow-up, we’d hope this might help us pick out patients less likely to respond and who show early nonresponse to specific drugs based on serum, urine, and fecal sampling.”
“It’s very helpful to know if someone is a nonresponder within 14 weeks because we can then move the patient on to something else relatively quickly,” he added.
Baldan-Martin and Samaan declared no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — presented at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Differences uncovered in multiple messenger RNAs, proteins, metabolites, and gut microbiota were associated with responders and nonresponders to biologics and Janus kinase inhibitors, suggesting the potential for predictive biomarkers in IBD, including Crohn’s disease (CD) and ulcerative colitis (UC).
“With further work we hope to confirm these findings and evaluate their clinical relevance in identifying patients most likely to respond to tailored therapeutic interventions,” said Montserrat Baldan-Martin, PhD, a researcher at the University Hospital of the Princess, Madrid, Spain.
The treatment of IBD is challenging because of the heterogeneity across clinical, immunological, molecular, genetic, and microbiologic features, with one third of patients failing to respond to any one treatment.
Baldan-Martin and colleagues wanted to find predictive biomarkers of response by examining the differences across multi-omics profiles relative to different therapies.
The study analyzed 127 patients with IBD (57 with CD and 70 with UC) before and after 14 weeks of treatment with one of the following: anti–tumor necrosis factors (TNFs), ustekinumab, vedolizumab, or tofacitinib. Patient response to treatment was evaluated using endoscopic criteria that categorized them as responders or nonresponders to the different therapies.
In addition, molecular data from various biologic samples — serum, urine, extracellular vesicles, intestinal biopsies, and stool — were tested using transcriptomics, proteomics, metabolomics, and metagenomics.
Clear Differences
“The most significant differences were seen in gene expression within intestinal tissue of responder and nonresponder patients with ulcerative colitis taking vedolizumab,” Baldan-Martin reported.
Proteomic analysis revealed that a total of 1377 proteins were identified across all groups (CD, UC, and the four drug classes/therapies). Responders and nonresponders for each therapy expressed different proteins in serum extracellular vesicles and intestinal tissues.
For example, patients with CD who responded to anti-TNF therapies had 138 different proteins from those of anti-TNF nonresponders, while patients with UC who responded to anti-TNF therapies had 218 different proteins from those of anti-TNF nonresponders, reported Baldan-Martin.
Also, we observed almost no proteins “in common between ulcerative colitis responders versus nonresponders for all treatments,” she noted. And we “saw only three proteins in common with Crohn’s disease patients [on different drugs].”
Metabolomic analysis identified deregulation of 24 serum lipoproteins in CD responders to ustekinumab, compared with nonresponders.
“We observed greater differences in the lipoproteins in serum than metabolites in serum and urine,” Baldan-Martin added.
Analysis of biologic pathways also highlighted enrichment in ketone and butyrate metabolism, mitochondrial electron transport chain activity, carnitine synthesis, and fatty acid oxidation pathways, while metagenomic analysis revealed the greatest microbial differences in UC responders and nonresponders to anti-TNF therapies.
Baldan-Martin said research was ongoing with a new cohort of patients that aims to validate some of the biomarkers and help identify the patients most likely to respond to tailored therapeutic interventions.
“One of the challenges is integrating results from different omics approaches to create a more holistic understanding of the disease,” she said, adding that she hopes the research “will potentially open doors for early detection through multi-panel biomarkers.”
Session moderator Mark Samaan, MD, consultant gastroenterologist at Guy’s and St Thomas’ NHS Foundation Trust, London, England, said that “the findings related to nonresponse to specific drugs in UC and CD were interesting. With longitudinal follow-up, we’d hope this might help us pick out patients less likely to respond and who show early nonresponse to specific drugs based on serum, urine, and fecal sampling.”
“It’s very helpful to know if someone is a nonresponder within 14 weeks because we can then move the patient on to something else relatively quickly,” he added.
Baldan-Martin and Samaan declared no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — presented at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Differences uncovered in multiple messenger RNAs, proteins, metabolites, and gut microbiota were associated with responders and nonresponders to biologics and Janus kinase inhibitors, suggesting the potential for predictive biomarkers in IBD, including Crohn’s disease (CD) and ulcerative colitis (UC).
“With further work we hope to confirm these findings and evaluate their clinical relevance in identifying patients most likely to respond to tailored therapeutic interventions,” said Montserrat Baldan-Martin, PhD, a researcher at the University Hospital of the Princess, Madrid, Spain.
The treatment of IBD is challenging because of the heterogeneity across clinical, immunological, molecular, genetic, and microbiologic features, with one third of patients failing to respond to any one treatment.
Baldan-Martin and colleagues wanted to find predictive biomarkers of response by examining the differences across multi-omics profiles relative to different therapies.
The study analyzed 127 patients with IBD (57 with CD and 70 with UC) before and after 14 weeks of treatment with one of the following: anti–tumor necrosis factors (TNFs), ustekinumab, vedolizumab, or tofacitinib. Patient response to treatment was evaluated using endoscopic criteria that categorized them as responders or nonresponders to the different therapies.
In addition, molecular data from various biologic samples — serum, urine, extracellular vesicles, intestinal biopsies, and stool — were tested using transcriptomics, proteomics, metabolomics, and metagenomics.
Clear Differences
“The most significant differences were seen in gene expression within intestinal tissue of responder and nonresponder patients with ulcerative colitis taking vedolizumab,” Baldan-Martin reported.
Proteomic analysis revealed that a total of 1377 proteins were identified across all groups (CD, UC, and the four drug classes/therapies). Responders and nonresponders for each therapy expressed different proteins in serum extracellular vesicles and intestinal tissues.
For example, patients with CD who responded to anti-TNF therapies had 138 different proteins from those of anti-TNF nonresponders, while patients with UC who responded to anti-TNF therapies had 218 different proteins from those of anti-TNF nonresponders, reported Baldan-Martin.
Also, we observed almost no proteins “in common between ulcerative colitis responders versus nonresponders for all treatments,” she noted. And we “saw only three proteins in common with Crohn’s disease patients [on different drugs].”
Metabolomic analysis identified deregulation of 24 serum lipoproteins in CD responders to ustekinumab, compared with nonresponders.
“We observed greater differences in the lipoproteins in serum than metabolites in serum and urine,” Baldan-Martin added.
Analysis of biologic pathways also highlighted enrichment in ketone and butyrate metabolism, mitochondrial electron transport chain activity, carnitine synthesis, and fatty acid oxidation pathways, while metagenomic analysis revealed the greatest microbial differences in UC responders and nonresponders to anti-TNF therapies.
Baldan-Martin said research was ongoing with a new cohort of patients that aims to validate some of the biomarkers and help identify the patients most likely to respond to tailored therapeutic interventions.
“One of the challenges is integrating results from different omics approaches to create a more holistic understanding of the disease,” she said, adding that she hopes the research “will potentially open doors for early detection through multi-panel biomarkers.”
Session moderator Mark Samaan, MD, consultant gastroenterologist at Guy’s and St Thomas’ NHS Foundation Trust, London, England, said that “the findings related to nonresponse to specific drugs in UC and CD were interesting. With longitudinal follow-up, we’d hope this might help us pick out patients less likely to respond and who show early nonresponse to specific drugs based on serum, urine, and fecal sampling.”
“It’s very helpful to know if someone is a nonresponder within 14 weeks because we can then move the patient on to something else relatively quickly,” he added.
Baldan-Martin and Samaan declared no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM ECCO 2025
Not All Plant-based Diets Are Equal in IBD Risk Mitigation
BERLIN — according to the results of a large cohort study.
The study, which included both Crohn’s disease (CD) and ulcerative colitis (UC), also showed that diet quality may affect disease progression and surgery risk for individuals already diagnosed with IBD.
“Not all plant-based foods are equal — they don’t all have the same effect on health outcomes,” said study researcher, Judith Wellens, MD, PhD, gastroenterology resident at Leuven University Hospital in Belgium.
“We need to look at what people are eating more carefully because it isn’t black and white, with all plant-based food being good and animal-based food being bad,” said Wellens, who presented the data at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Although she advocates for plant-based diets, Wellens stressed that “they need to be individualized to ensure the overall dietary quality is good. Just cutting out meat products is not very helpful. We think it is the unhealthy additions to some plant-based diets that drive the IBD risk.”
Is It the Plants or the Processed Ingredients?
“Preclinical studies have already taught us that plant-based diets alter the gut microbiota in a beneficial way. However, many diets promoted for IBD — for example the Crohn’s disease exclusion diet — contain ingredients that are animal based. This is confusing for patients and for clinicians,” said Wellens.
To look more closely at the question, she and her colleagues analyzed data for 187,888 participants from the UK Biobank and 341,539 participants from across eight European countries from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. None of the participants had IBD at baseline.
Based on participant 24-hour dietary recalls, the researchers constructed plant-based diet indices (PDIs) with diets categorized as healthy (eg, whole grains, fruits, vegetables, legumes, and vegetarian protein alternatives) or unhealthy (eg, emulsifiers, refined grains, fries, fruit juices, sweets, desserts, sugar-sweetened beverages, and processed foods).
The primary outcome was the incidence of IBD (either CD or UC), whereas the secondary outcome was IBD-related surgery, thereby marking disease progression. Cox regression analysis estimated IBD risk and progression. Incidences of IBD were similar between the two cohorts.
In the UK Biobank cohort, 925 participants developed IBD over a median follow-up of 11.6 years. Participants who followed a healthy PDI had a 25% reduced IBD risk, whereas those who followed an unhealthy PDI had a 48% increased risk for disease development. Both CD and UC showed similar outcomes.
The EPIC cohort had a longer median follow-up time of 14.5 years, during which 548 people developed IBD. Healthy PDIs were linked to a 29% reduced risk for IBD, whereas unhealthy PDIs were associated with a 54% increased risk.
A healthy PDI halved the risk for surgery in participants from the UK Biobank, whereas an unhealthy PDI was associated with a twofold higher risk for surgery.
There were no significant associations between PDIs and other outcomes, such as cardiovascular disease, diabetes, or all-cause mortality.
The researchers also looked at the interactions between genetics and plant-based diets, but those results were not presented at the meeting.
However, Wellens said in an interview that people with a moderate to high risk for IBD based on their polygenetic risk score showed increased odds for IBD risk.
“We don’t test people for their genetic risk of IBD, but if people have close relatives with IBD, then there is probably an increased genetic risk of its development,” she added.
Commenting on the findings, James Lindsay, PhD, professor of inflammatory bowel disease, Queen Mary University of London in England, said that several recent epidemiological studies have highlighted “the negative impact of ultra-processed foods on increasing the risk of developing Crohn’s disease.”
Based on these studies, “one might assume that plant-based diets would be protective,” he said, however, the current study shows us “that plant-based diets are not all equal and there are unhealthy aspects to some.”
“Of course, showing that a diet is associated with an outcome is not the same as knowing that changing a diet will reduce the risk,” Lindsay added. “That requires a well-designed, carefully controlled trial.”
Wellens and Lindsay reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — according to the results of a large cohort study.
The study, which included both Crohn’s disease (CD) and ulcerative colitis (UC), also showed that diet quality may affect disease progression and surgery risk for individuals already diagnosed with IBD.
“Not all plant-based foods are equal — they don’t all have the same effect on health outcomes,” said study researcher, Judith Wellens, MD, PhD, gastroenterology resident at Leuven University Hospital in Belgium.
“We need to look at what people are eating more carefully because it isn’t black and white, with all plant-based food being good and animal-based food being bad,” said Wellens, who presented the data at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Although she advocates for plant-based diets, Wellens stressed that “they need to be individualized to ensure the overall dietary quality is good. Just cutting out meat products is not very helpful. We think it is the unhealthy additions to some plant-based diets that drive the IBD risk.”
Is It the Plants or the Processed Ingredients?
“Preclinical studies have already taught us that plant-based diets alter the gut microbiota in a beneficial way. However, many diets promoted for IBD — for example the Crohn’s disease exclusion diet — contain ingredients that are animal based. This is confusing for patients and for clinicians,” said Wellens.
To look more closely at the question, she and her colleagues analyzed data for 187,888 participants from the UK Biobank and 341,539 participants from across eight European countries from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. None of the participants had IBD at baseline.
Based on participant 24-hour dietary recalls, the researchers constructed plant-based diet indices (PDIs) with diets categorized as healthy (eg, whole grains, fruits, vegetables, legumes, and vegetarian protein alternatives) or unhealthy (eg, emulsifiers, refined grains, fries, fruit juices, sweets, desserts, sugar-sweetened beverages, and processed foods).
The primary outcome was the incidence of IBD (either CD or UC), whereas the secondary outcome was IBD-related surgery, thereby marking disease progression. Cox regression analysis estimated IBD risk and progression. Incidences of IBD were similar between the two cohorts.
In the UK Biobank cohort, 925 participants developed IBD over a median follow-up of 11.6 years. Participants who followed a healthy PDI had a 25% reduced IBD risk, whereas those who followed an unhealthy PDI had a 48% increased risk for disease development. Both CD and UC showed similar outcomes.
The EPIC cohort had a longer median follow-up time of 14.5 years, during which 548 people developed IBD. Healthy PDIs were linked to a 29% reduced risk for IBD, whereas unhealthy PDIs were associated with a 54% increased risk.
A healthy PDI halved the risk for surgery in participants from the UK Biobank, whereas an unhealthy PDI was associated with a twofold higher risk for surgery.
There were no significant associations between PDIs and other outcomes, such as cardiovascular disease, diabetes, or all-cause mortality.
The researchers also looked at the interactions between genetics and plant-based diets, but those results were not presented at the meeting.
However, Wellens said in an interview that people with a moderate to high risk for IBD based on their polygenetic risk score showed increased odds for IBD risk.
“We don’t test people for their genetic risk of IBD, but if people have close relatives with IBD, then there is probably an increased genetic risk of its development,” she added.
Commenting on the findings, James Lindsay, PhD, professor of inflammatory bowel disease, Queen Mary University of London in England, said that several recent epidemiological studies have highlighted “the negative impact of ultra-processed foods on increasing the risk of developing Crohn’s disease.”
Based on these studies, “one might assume that plant-based diets would be protective,” he said, however, the current study shows us “that plant-based diets are not all equal and there are unhealthy aspects to some.”
“Of course, showing that a diet is associated with an outcome is not the same as knowing that changing a diet will reduce the risk,” Lindsay added. “That requires a well-designed, carefully controlled trial.”
Wellens and Lindsay reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
BERLIN — according to the results of a large cohort study.
The study, which included both Crohn’s disease (CD) and ulcerative colitis (UC), also showed that diet quality may affect disease progression and surgery risk for individuals already diagnosed with IBD.
“Not all plant-based foods are equal — they don’t all have the same effect on health outcomes,” said study researcher, Judith Wellens, MD, PhD, gastroenterology resident at Leuven University Hospital in Belgium.
“We need to look at what people are eating more carefully because it isn’t black and white, with all plant-based food being good and animal-based food being bad,” said Wellens, who presented the data at the European Crohn’s and Colitis Organisation (ECCO) 2025 Congress.
Although she advocates for plant-based diets, Wellens stressed that “they need to be individualized to ensure the overall dietary quality is good. Just cutting out meat products is not very helpful. We think it is the unhealthy additions to some plant-based diets that drive the IBD risk.”
Is It the Plants or the Processed Ingredients?
“Preclinical studies have already taught us that plant-based diets alter the gut microbiota in a beneficial way. However, many diets promoted for IBD — for example the Crohn’s disease exclusion diet — contain ingredients that are animal based. This is confusing for patients and for clinicians,” said Wellens.
To look more closely at the question, she and her colleagues analyzed data for 187,888 participants from the UK Biobank and 341,539 participants from across eight European countries from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. None of the participants had IBD at baseline.
Based on participant 24-hour dietary recalls, the researchers constructed plant-based diet indices (PDIs) with diets categorized as healthy (eg, whole grains, fruits, vegetables, legumes, and vegetarian protein alternatives) or unhealthy (eg, emulsifiers, refined grains, fries, fruit juices, sweets, desserts, sugar-sweetened beverages, and processed foods).
The primary outcome was the incidence of IBD (either CD or UC), whereas the secondary outcome was IBD-related surgery, thereby marking disease progression. Cox regression analysis estimated IBD risk and progression. Incidences of IBD were similar between the two cohorts.
In the UK Biobank cohort, 925 participants developed IBD over a median follow-up of 11.6 years. Participants who followed a healthy PDI had a 25% reduced IBD risk, whereas those who followed an unhealthy PDI had a 48% increased risk for disease development. Both CD and UC showed similar outcomes.
The EPIC cohort had a longer median follow-up time of 14.5 years, during which 548 people developed IBD. Healthy PDIs were linked to a 29% reduced risk for IBD, whereas unhealthy PDIs were associated with a 54% increased risk.
A healthy PDI halved the risk for surgery in participants from the UK Biobank, whereas an unhealthy PDI was associated with a twofold higher risk for surgery.
There were no significant associations between PDIs and other outcomes, such as cardiovascular disease, diabetes, or all-cause mortality.
The researchers also looked at the interactions between genetics and plant-based diets, but those results were not presented at the meeting.
However, Wellens said in an interview that people with a moderate to high risk for IBD based on their polygenetic risk score showed increased odds for IBD risk.
“We don’t test people for their genetic risk of IBD, but if people have close relatives with IBD, then there is probably an increased genetic risk of its development,” she added.
Commenting on the findings, James Lindsay, PhD, professor of inflammatory bowel disease, Queen Mary University of London in England, said that several recent epidemiological studies have highlighted “the negative impact of ultra-processed foods on increasing the risk of developing Crohn’s disease.”
Based on these studies, “one might assume that plant-based diets would be protective,” he said, however, the current study shows us “that plant-based diets are not all equal and there are unhealthy aspects to some.”
“Of course, showing that a diet is associated with an outcome is not the same as knowing that changing a diet will reduce the risk,” Lindsay added. “That requires a well-designed, carefully controlled trial.”
Wellens and Lindsay reported no relevant financial disclosures.
A version of this article appeared on Medscape.com.
FROM ECCO 2025
ASCO Updates Treatment Guidance for Newly Diagnosed, Advanced Ovarian Cancer
The American Society of Clinical Oncology (ASCO) has released updated guidelines for neoadjuvant chemotherapy in newly diagnosed advanced ovarian cancer, introducing changes in patient selection and treatment strategies. The changes reflect emerging evidence on racial disparities, treatment outcomes, and quality of life considerations.
The publication of the new guidance follows dramatic shifts in treatment patterns over the past decade.
“There had been a big shift in how we were treating patients in the United States,” explained Stephanie Gaillard, MD, PhD, one of the authors of the updated guidelines. “We saw a substantial drop in the number of patients undergoing primary cytoreductive surgery for ovarian cancer from about 70% of patients in 2010 to only about 37% in 2021.”
The new guidelines maintain the recommendation for platinum/taxane-based neoadjuvant chemotherapy but introduce modifications regarding timing and duration.
“It’s still a recommendation that gynecologic oncologists are involved in determining whether someone is eligible for primary cytoreductive surgery or should undergo neoadjuvant chemotherapy first,” Gaillard noted. “We emphasize that patients who are eligible for primary cytoreductive surgery should undergo surgery as opposed to receiving neoadjuvant chemotherapy.”
Alexander Melamed, MD, MPH, a gynecologic oncologist at Massachusetts General Hospital, Boston, who was not involved in authoring the updated guidelines, noted that additional evidence-based guidance is needed to individualize treatment plans. He pointed to four completed trials comparing neoadjuvant chemotherapy with cytoreductive surgery, noting: “When these trials have been pooled together in meta-analyses, there was a higher risk of mortality associated with primary cytoreductive surgery and a higher risk of severe complications.”
The updated guidelines take this higher risk for mortality with primary cytoreductive surgery into consideration, and patients who are not eligible for primary surgery would receive neoadjuvant chemotherapy, Gaillard noted.
Changes in Patient Selection
The 2025 guidelines describe a more nuanced approach for selecting patients for neoadjuvant chemotherapy vs primary cytoreductive surgery. While the 2016 ASCO guidelines primarily focused on disease burden and surgical resectability when selecting patients for neoadjuvant chemotherapy, the new recommendations incorporate additional factors.
The guidelines discuss recent findings showing that Black patients experience a 38% lower likelihood of undergoing cytoreductive surgery than non-Black patients. In addition, compared with non-Hispanic White women, Asian and Black women more frequently receive neoadjuvant chemotherapy with interval debulking surgery rather than primary cytoreductive surgery. According to the authors, these differences persist even after accounting for clinical factors, suggesting that structural barriers to healthcare access may play a role.
The guidelines discuss how affordability, availability, and accessibility mediate racial disparities in ovarian cancer care. According to the authors, structural inequities in healthcare access influence treatment quality for minority patients. Non-White patients face greater challenges in accessing gynecologic oncology consultations and standard-of-care combination therapy, leading to poorer survival outcomes, the guidelines say.
According to Melamed, the guidelines serve as an important tool for promoting healthcare equity. “Having recommendations and standards is incredibly important for achieving equity because once there is consensus on a best practice, it doesn’t matter if you’re rich, poor, or a patient of a particular racial or ethnic group — if you have the disease, you ought to have access to that standard,” he said.
The 2016 ASCO guidelines focused primarily on disease burden and surgical resectability, whereas the 2024 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for ovarian cancer focus more on oncologic outcomes and surgical considerations. Based on the NCCN guidelines, treatment selection for ovarian cancer is primarily determined by the histologic subtype, stage of disease, and whether the patient is a candidate for primary surgery. The 2025 ASCO guidelines, on the other hand, emphasize the importance of quality-of-life outcomes during treatment selection. The authors of the updated ASCO guidelines acknowledged that treatment decisions should consider both the duration and quality of life, particularly for elderly patients or those with multiple comorbidities.
Treatment Timing and Duration
The guidelines maintain the recommendations for platinum/taxane-based neoadjuvant chemotherapy described in the previous ASCO guidelines but introduce modifications regarding treatment timing and duration. The optimal window for interval cytoreductive surgery now falls after three to four chemotherapy cycles, allowing more individualized approaches based on patient response and tolerance.
In addition, postsurgical chemotherapy protocols have become more flexible. Rather than mandating a fixed number of cycles, the guidelines encourage tailoring treatment duration to individual patient factors including response assessment, performance status, and quality-of-life considerations.
The updated guidelines also emphasize the importance of genetic and molecular testing at diagnosis, which Melamed identifies as “absolutely central to treatment and deciding who receives maintenance therapy.” This is also recommended by the NCCN guidelines.
However, he highlighted the following practical challenge in molecular testing after neoadjuvant chemotherapy. “Probably 20% of patients have an exceptional response to neoadjuvant therapy, such that there is insufficient tissue at the time of their cytoreduction to do somatic testing,” he said.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
A notable difference between the 2016 and 2025 guidelines is the inclusion of HIPEC in the updated guidelines.
Commenting to this news organization, Gaillard explained the nuanced approach to HIPEC: “The committee discussed HIPEC extensively. We recognize that it may not be available at many centers and requires specially trained staff and dedicated resources. The reason for including HIPEC in the guidelines is to highlight that there have been studies that show a potential overall survival benefit.”
Melamed considers the recommendation of HIPEC to be one of the strongest aspects of the updated guidelines. “There have been two large trials and one smaller one that have shown that for patients treated with neoadjuvant chemotherapy, the addition of HIPEC appears to improve overall survival,” he explained.
Implementation Strategies
The authors acknowledged that barriers to healthcare delivery present significant challenges to the implementation of the guidelines. Limited access to gynecologic oncologists in rural areas, insurance coverage gaps, and varying surgical expertise across institutions complicate the delivery of optimal care. The guidelines also emphasize the need for solutions to ensure equitable access to recommended treatments.
Melamed noted that the decentralized structure of the healthcare system in the United States complicates the uniform adoption of guidelines, particularly in resource-limited settings, adding that “geographic region and local resources and expertise influence both access to treatment and outcomes.”
Although both the updated ASCO guidelines and NCCN guidelines emphasize the importance of evaluation by a gynecologic oncologist for determining the most appropriate treatment strategy, the scarcity of gynecologic oncologists is one of the most significant barriers to accessing optimal care, according to Gaillard. She proposes telemedicine consultations and enhanced communication between medical oncologists and gynecologic oncologists to ensure equitable access.
Gaillard also commented on the challenges in implementing a multidisciplinary treatment approach, the importance of which is emphasized in the updated guidelines.
“There can be a limited availability of the multidisciplinary team to be involved in this decision-making,” she said. “Ideally, patient assessment by a gynecologic oncologist would happen in person, but recognizing that availability is limited, it doesn’t necessarily have to. Sometimes, it can just be a conversation between a medical oncologist and a gynecologic oncologist detailing a treatment plan together.”
Looking Ahead
Gaillard noted that ovarian cancer is a very active field of research and that the guidelines may need to be updated again in the near future to incorporate novel treatment approaches.
“Newer and more effective targeted therapies based on tumor profiling are being developed,” she said. “These will hopefully move earlier in the treatment course for patients. Maybe we will not use chemotherapy in the future because we will have more directed and targeted therapies.”
She also emphasized the importance of early diagnosis in shaping future treatment guidelines for ovarian cancer.
“Neoadjuvant chemotherapy is predominantly used in situations where patients have very advanced disease and may not benefit from primary cytoreductive surgery,” she noted. “If we develop better diagnostic tools that will allow us to diagnose patients earlier, then we may not need to use neoadjuvant chemotherapy.”
All funding for the administration of the guideline development project was provided by ASCO. Gaillard reported receiving consulting or advisory fees from Verastem, Merck, AstraZeneca, and Compugen; research funding from AstraZeneca, Tesaro, Compugen, Genentech/Roche, Clovis Oncology, Tempest Therapeutics, Blueprint Pharmaceutic, Immunogen, Volastra Therapeutics, and Beigene; and patents, royalties, or other intellectual property from US Patent Nos 10,258,604 and 10,905,659, licensed by Duke University to Sermonix. Melamed reported receiving research funding from the National Cancer Institute and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
The American Society of Clinical Oncology (ASCO) has released updated guidelines for neoadjuvant chemotherapy in newly diagnosed advanced ovarian cancer, introducing changes in patient selection and treatment strategies. The changes reflect emerging evidence on racial disparities, treatment outcomes, and quality of life considerations.
The publication of the new guidance follows dramatic shifts in treatment patterns over the past decade.
“There had been a big shift in how we were treating patients in the United States,” explained Stephanie Gaillard, MD, PhD, one of the authors of the updated guidelines. “We saw a substantial drop in the number of patients undergoing primary cytoreductive surgery for ovarian cancer from about 70% of patients in 2010 to only about 37% in 2021.”
The new guidelines maintain the recommendation for platinum/taxane-based neoadjuvant chemotherapy but introduce modifications regarding timing and duration.
“It’s still a recommendation that gynecologic oncologists are involved in determining whether someone is eligible for primary cytoreductive surgery or should undergo neoadjuvant chemotherapy first,” Gaillard noted. “We emphasize that patients who are eligible for primary cytoreductive surgery should undergo surgery as opposed to receiving neoadjuvant chemotherapy.”
Alexander Melamed, MD, MPH, a gynecologic oncologist at Massachusetts General Hospital, Boston, who was not involved in authoring the updated guidelines, noted that additional evidence-based guidance is needed to individualize treatment plans. He pointed to four completed trials comparing neoadjuvant chemotherapy with cytoreductive surgery, noting: “When these trials have been pooled together in meta-analyses, there was a higher risk of mortality associated with primary cytoreductive surgery and a higher risk of severe complications.”
The updated guidelines take this higher risk for mortality with primary cytoreductive surgery into consideration, and patients who are not eligible for primary surgery would receive neoadjuvant chemotherapy, Gaillard noted.
Changes in Patient Selection
The 2025 guidelines describe a more nuanced approach for selecting patients for neoadjuvant chemotherapy vs primary cytoreductive surgery. While the 2016 ASCO guidelines primarily focused on disease burden and surgical resectability when selecting patients for neoadjuvant chemotherapy, the new recommendations incorporate additional factors.
The guidelines discuss recent findings showing that Black patients experience a 38% lower likelihood of undergoing cytoreductive surgery than non-Black patients. In addition, compared with non-Hispanic White women, Asian and Black women more frequently receive neoadjuvant chemotherapy with interval debulking surgery rather than primary cytoreductive surgery. According to the authors, these differences persist even after accounting for clinical factors, suggesting that structural barriers to healthcare access may play a role.
The guidelines discuss how affordability, availability, and accessibility mediate racial disparities in ovarian cancer care. According to the authors, structural inequities in healthcare access influence treatment quality for minority patients. Non-White patients face greater challenges in accessing gynecologic oncology consultations and standard-of-care combination therapy, leading to poorer survival outcomes, the guidelines say.
According to Melamed, the guidelines serve as an important tool for promoting healthcare equity. “Having recommendations and standards is incredibly important for achieving equity because once there is consensus on a best practice, it doesn’t matter if you’re rich, poor, or a patient of a particular racial or ethnic group — if you have the disease, you ought to have access to that standard,” he said.
The 2016 ASCO guidelines focused primarily on disease burden and surgical resectability, whereas the 2024 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for ovarian cancer focus more on oncologic outcomes and surgical considerations. Based on the NCCN guidelines, treatment selection for ovarian cancer is primarily determined by the histologic subtype, stage of disease, and whether the patient is a candidate for primary surgery. The 2025 ASCO guidelines, on the other hand, emphasize the importance of quality-of-life outcomes during treatment selection. The authors of the updated ASCO guidelines acknowledged that treatment decisions should consider both the duration and quality of life, particularly for elderly patients or those with multiple comorbidities.
Treatment Timing and Duration
The guidelines maintain the recommendations for platinum/taxane-based neoadjuvant chemotherapy described in the previous ASCO guidelines but introduce modifications regarding treatment timing and duration. The optimal window for interval cytoreductive surgery now falls after three to four chemotherapy cycles, allowing more individualized approaches based on patient response and tolerance.
In addition, postsurgical chemotherapy protocols have become more flexible. Rather than mandating a fixed number of cycles, the guidelines encourage tailoring treatment duration to individual patient factors including response assessment, performance status, and quality-of-life considerations.
The updated guidelines also emphasize the importance of genetic and molecular testing at diagnosis, which Melamed identifies as “absolutely central to treatment and deciding who receives maintenance therapy.” This is also recommended by the NCCN guidelines.
However, he highlighted the following practical challenge in molecular testing after neoadjuvant chemotherapy. “Probably 20% of patients have an exceptional response to neoadjuvant therapy, such that there is insufficient tissue at the time of their cytoreduction to do somatic testing,” he said.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
A notable difference between the 2016 and 2025 guidelines is the inclusion of HIPEC in the updated guidelines.
Commenting to this news organization, Gaillard explained the nuanced approach to HIPEC: “The committee discussed HIPEC extensively. We recognize that it may not be available at many centers and requires specially trained staff and dedicated resources. The reason for including HIPEC in the guidelines is to highlight that there have been studies that show a potential overall survival benefit.”
Melamed considers the recommendation of HIPEC to be one of the strongest aspects of the updated guidelines. “There have been two large trials and one smaller one that have shown that for patients treated with neoadjuvant chemotherapy, the addition of HIPEC appears to improve overall survival,” he explained.
Implementation Strategies
The authors acknowledged that barriers to healthcare delivery present significant challenges to the implementation of the guidelines. Limited access to gynecologic oncologists in rural areas, insurance coverage gaps, and varying surgical expertise across institutions complicate the delivery of optimal care. The guidelines also emphasize the need for solutions to ensure equitable access to recommended treatments.
Melamed noted that the decentralized structure of the healthcare system in the United States complicates the uniform adoption of guidelines, particularly in resource-limited settings, adding that “geographic region and local resources and expertise influence both access to treatment and outcomes.”
Although both the updated ASCO guidelines and NCCN guidelines emphasize the importance of evaluation by a gynecologic oncologist for determining the most appropriate treatment strategy, the scarcity of gynecologic oncologists is one of the most significant barriers to accessing optimal care, according to Gaillard. She proposes telemedicine consultations and enhanced communication between medical oncologists and gynecologic oncologists to ensure equitable access.
Gaillard also commented on the challenges in implementing a multidisciplinary treatment approach, the importance of which is emphasized in the updated guidelines.
“There can be a limited availability of the multidisciplinary team to be involved in this decision-making,” she said. “Ideally, patient assessment by a gynecologic oncologist would happen in person, but recognizing that availability is limited, it doesn’t necessarily have to. Sometimes, it can just be a conversation between a medical oncologist and a gynecologic oncologist detailing a treatment plan together.”
Looking Ahead
Gaillard noted that ovarian cancer is a very active field of research and that the guidelines may need to be updated again in the near future to incorporate novel treatment approaches.
“Newer and more effective targeted therapies based on tumor profiling are being developed,” she said. “These will hopefully move earlier in the treatment course for patients. Maybe we will not use chemotherapy in the future because we will have more directed and targeted therapies.”
She also emphasized the importance of early diagnosis in shaping future treatment guidelines for ovarian cancer.
“Neoadjuvant chemotherapy is predominantly used in situations where patients have very advanced disease and may not benefit from primary cytoreductive surgery,” she noted. “If we develop better diagnostic tools that will allow us to diagnose patients earlier, then we may not need to use neoadjuvant chemotherapy.”
All funding for the administration of the guideline development project was provided by ASCO. Gaillard reported receiving consulting or advisory fees from Verastem, Merck, AstraZeneca, and Compugen; research funding from AstraZeneca, Tesaro, Compugen, Genentech/Roche, Clovis Oncology, Tempest Therapeutics, Blueprint Pharmaceutic, Immunogen, Volastra Therapeutics, and Beigene; and patents, royalties, or other intellectual property from US Patent Nos 10,258,604 and 10,905,659, licensed by Duke University to Sermonix. Melamed reported receiving research funding from the National Cancer Institute and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
The American Society of Clinical Oncology (ASCO) has released updated guidelines for neoadjuvant chemotherapy in newly diagnosed advanced ovarian cancer, introducing changes in patient selection and treatment strategies. The changes reflect emerging evidence on racial disparities, treatment outcomes, and quality of life considerations.
The publication of the new guidance follows dramatic shifts in treatment patterns over the past decade.
“There had been a big shift in how we were treating patients in the United States,” explained Stephanie Gaillard, MD, PhD, one of the authors of the updated guidelines. “We saw a substantial drop in the number of patients undergoing primary cytoreductive surgery for ovarian cancer from about 70% of patients in 2010 to only about 37% in 2021.”
The new guidelines maintain the recommendation for platinum/taxane-based neoadjuvant chemotherapy but introduce modifications regarding timing and duration.
“It’s still a recommendation that gynecologic oncologists are involved in determining whether someone is eligible for primary cytoreductive surgery or should undergo neoadjuvant chemotherapy first,” Gaillard noted. “We emphasize that patients who are eligible for primary cytoreductive surgery should undergo surgery as opposed to receiving neoadjuvant chemotherapy.”
Alexander Melamed, MD, MPH, a gynecologic oncologist at Massachusetts General Hospital, Boston, who was not involved in authoring the updated guidelines, noted that additional evidence-based guidance is needed to individualize treatment plans. He pointed to four completed trials comparing neoadjuvant chemotherapy with cytoreductive surgery, noting: “When these trials have been pooled together in meta-analyses, there was a higher risk of mortality associated with primary cytoreductive surgery and a higher risk of severe complications.”
The updated guidelines take this higher risk for mortality with primary cytoreductive surgery into consideration, and patients who are not eligible for primary surgery would receive neoadjuvant chemotherapy, Gaillard noted.
Changes in Patient Selection
The 2025 guidelines describe a more nuanced approach for selecting patients for neoadjuvant chemotherapy vs primary cytoreductive surgery. While the 2016 ASCO guidelines primarily focused on disease burden and surgical resectability when selecting patients for neoadjuvant chemotherapy, the new recommendations incorporate additional factors.
The guidelines discuss recent findings showing that Black patients experience a 38% lower likelihood of undergoing cytoreductive surgery than non-Black patients. In addition, compared with non-Hispanic White women, Asian and Black women more frequently receive neoadjuvant chemotherapy with interval debulking surgery rather than primary cytoreductive surgery. According to the authors, these differences persist even after accounting for clinical factors, suggesting that structural barriers to healthcare access may play a role.
The guidelines discuss how affordability, availability, and accessibility mediate racial disparities in ovarian cancer care. According to the authors, structural inequities in healthcare access influence treatment quality for minority patients. Non-White patients face greater challenges in accessing gynecologic oncology consultations and standard-of-care combination therapy, leading to poorer survival outcomes, the guidelines say.
According to Melamed, the guidelines serve as an important tool for promoting healthcare equity. “Having recommendations and standards is incredibly important for achieving equity because once there is consensus on a best practice, it doesn’t matter if you’re rich, poor, or a patient of a particular racial or ethnic group — if you have the disease, you ought to have access to that standard,” he said.
The 2016 ASCO guidelines focused primarily on disease burden and surgical resectability, whereas the 2024 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for ovarian cancer focus more on oncologic outcomes and surgical considerations. Based on the NCCN guidelines, treatment selection for ovarian cancer is primarily determined by the histologic subtype, stage of disease, and whether the patient is a candidate for primary surgery. The 2025 ASCO guidelines, on the other hand, emphasize the importance of quality-of-life outcomes during treatment selection. The authors of the updated ASCO guidelines acknowledged that treatment decisions should consider both the duration and quality of life, particularly for elderly patients or those with multiple comorbidities.
Treatment Timing and Duration
The guidelines maintain the recommendations for platinum/taxane-based neoadjuvant chemotherapy described in the previous ASCO guidelines but introduce modifications regarding treatment timing and duration. The optimal window for interval cytoreductive surgery now falls after three to four chemotherapy cycles, allowing more individualized approaches based on patient response and tolerance.
In addition, postsurgical chemotherapy protocols have become more flexible. Rather than mandating a fixed number of cycles, the guidelines encourage tailoring treatment duration to individual patient factors including response assessment, performance status, and quality-of-life considerations.
The updated guidelines also emphasize the importance of genetic and molecular testing at diagnosis, which Melamed identifies as “absolutely central to treatment and deciding who receives maintenance therapy.” This is also recommended by the NCCN guidelines.
However, he highlighted the following practical challenge in molecular testing after neoadjuvant chemotherapy. “Probably 20% of patients have an exceptional response to neoadjuvant therapy, such that there is insufficient tissue at the time of their cytoreduction to do somatic testing,” he said.
Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
A notable difference between the 2016 and 2025 guidelines is the inclusion of HIPEC in the updated guidelines.
Commenting to this news organization, Gaillard explained the nuanced approach to HIPEC: “The committee discussed HIPEC extensively. We recognize that it may not be available at many centers and requires specially trained staff and dedicated resources. The reason for including HIPEC in the guidelines is to highlight that there have been studies that show a potential overall survival benefit.”
Melamed considers the recommendation of HIPEC to be one of the strongest aspects of the updated guidelines. “There have been two large trials and one smaller one that have shown that for patients treated with neoadjuvant chemotherapy, the addition of HIPEC appears to improve overall survival,” he explained.
Implementation Strategies
The authors acknowledged that barriers to healthcare delivery present significant challenges to the implementation of the guidelines. Limited access to gynecologic oncologists in rural areas, insurance coverage gaps, and varying surgical expertise across institutions complicate the delivery of optimal care. The guidelines also emphasize the need for solutions to ensure equitable access to recommended treatments.
Melamed noted that the decentralized structure of the healthcare system in the United States complicates the uniform adoption of guidelines, particularly in resource-limited settings, adding that “geographic region and local resources and expertise influence both access to treatment and outcomes.”
Although both the updated ASCO guidelines and NCCN guidelines emphasize the importance of evaluation by a gynecologic oncologist for determining the most appropriate treatment strategy, the scarcity of gynecologic oncologists is one of the most significant barriers to accessing optimal care, according to Gaillard. She proposes telemedicine consultations and enhanced communication between medical oncologists and gynecologic oncologists to ensure equitable access.
Gaillard also commented on the challenges in implementing a multidisciplinary treatment approach, the importance of which is emphasized in the updated guidelines.
“There can be a limited availability of the multidisciplinary team to be involved in this decision-making,” she said. “Ideally, patient assessment by a gynecologic oncologist would happen in person, but recognizing that availability is limited, it doesn’t necessarily have to. Sometimes, it can just be a conversation between a medical oncologist and a gynecologic oncologist detailing a treatment plan together.”
Looking Ahead
Gaillard noted that ovarian cancer is a very active field of research and that the guidelines may need to be updated again in the near future to incorporate novel treatment approaches.
“Newer and more effective targeted therapies based on tumor profiling are being developed,” she said. “These will hopefully move earlier in the treatment course for patients. Maybe we will not use chemotherapy in the future because we will have more directed and targeted therapies.”
She also emphasized the importance of early diagnosis in shaping future treatment guidelines for ovarian cancer.
“Neoadjuvant chemotherapy is predominantly used in situations where patients have very advanced disease and may not benefit from primary cytoreductive surgery,” she noted. “If we develop better diagnostic tools that will allow us to diagnose patients earlier, then we may not need to use neoadjuvant chemotherapy.”
All funding for the administration of the guideline development project was provided by ASCO. Gaillard reported receiving consulting or advisory fees from Verastem, Merck, AstraZeneca, and Compugen; research funding from AstraZeneca, Tesaro, Compugen, Genentech/Roche, Clovis Oncology, Tempest Therapeutics, Blueprint Pharmaceutic, Immunogen, Volastra Therapeutics, and Beigene; and patents, royalties, or other intellectual property from US Patent Nos 10,258,604 and 10,905,659, licensed by Duke University to Sermonix. Melamed reported receiving research funding from the National Cancer Institute and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Choosing the Ideal Endoscopic Enteral Access Method: AGA Practice Update
At least 250,000 US hospitalized patients a year require enteral support using an artificial pathway into the gastrointestinal (GI) tract to deliver nutrition or medication. In light of this,
Covering indications, placement techniques, and management, the comprehensive document is a response to the increasing use of enteral access devices in chronic GI conditions. The update, published in Gastroenterology, addresses patient factors complicating placement decision-making such as thrombocytopenia, use of dual antiplatelet therapy, or performance of percutaneous access in the setting of cirrhosis.
“We provide clinical recommendations in these various scenarios understanding that the final decision-making is in the hands of the provider and care team,” said first author Dejan Micic, MD, a gastroenterologist and associate professor at University of Chicago Medical Center in Illinois at the time of the update (since relocated to Loyola University Medical Center in Chicago). “We hope this can serve a day-to-day purpose for clinical gastroenterologists and can be referenced as they encounter individuals with or needing an enteral access device.”
Traditionally, enteral access was reserved for patients with severe malnutrition or those unable to maintain oral intake. Recent recommendations emphasize early nutritional intervention including prehabilitation before major surgery, adjunctive therapy for oncology patients, and in specific inflammatory conditions such as Crohn’s disease. “These shifts recognize the role of enteral nutrition not only in preventing malnutrition but also as a therapeutic strategy,” Micic said in an interview.
There is, however, variability in the use of devices including the selection of appropriate units, technical aspects of placement, and subsequent management. “Such variability can lead to complications, suboptimal patient outcomes, and inefficiencies in care delivery,” Micic said.
He added that enteral access has been historically underemphasized in GI endoscopic training. “While procedural skill in placing devices such as percutaneous endoscopic gastrostomy, or PEG, tubes is often taught, a comprehensive understanding of the broader clinical context — such as proper patient selection, prevention of complications, and postplacement care — is not always thoroughly covered.”
The current update aims to bridge knowledge gaps with evidence-based-guidance. “It also underscores the importance of interdisciplinary collaboration with dietitians, nurses, and care givers to achieve the best outcomes for patients,” Micic said.
Commenting on the update but not involved with creating it, Shirley C. Paski, MD, MS, a gastroenterologist at the Cleveland Clinic, Ohio, called it timely, adding: “As GI training is becoming more subspecialized and interventional radiology has been able to provide enteral access, gastroenterology training in enteral access has declined to where some fellows are graduating with limited enteral access experience.”
Yet malnutrition remains a common consequence when GI disease is severe, chronic, or refractory to treatment, or in the setting of postsurgical anatomy, she added. “Enteral nutrition is increasingly being considered a therapeutic or adjunct treatment in some cases of Crohn’s disease or small intestinal bacterial overgrowth. Gastroenterologists need the endoscopic skill to secure enteral access tubes, particularly in more challenging anatomy.”
Also commenting on the document but not involved in it, Steven Shamah, MD, director of Endoscopy at Northwell Lenox Hill Hospital in New York City, said: “This should serve as a concise review for any general hospitalist or gastroenterologist to understand what we have and when we should offer the proper feeding tube options.” He stressed, however, that all gastroenterologists should be trained in the placing of all of tube options.
“The axiom ‘If the gut works, we should use it’ is something that I was taught when I was a medical student and it still holds true,” Shamah continued. “There’s been a jump in interventional procedures to assure continuity of the GI tract even in progressive malignancy. So there’s a rise in moving away from intravenous nutrition and a rise in tube-delivered enteral nutrition.” Options for reducing reflux and aspiration will likely take on more importance, he said.
Tubing Options
According to Micic and colleagues, recent data suggest a favorable safety profile of enteral feeding tubes placed endoscopically compared with surgical or radiologic placement. The illustrated AGA document outlines such approaches as synthetic flexible tubes placed into the stomach or small bowel via the oral (orogastric and oroenteral) or nasal routes (nasogastric [NG] and nasojejunal [NJ]) and percutaneous tubes accessing the stomach. The choice of tube, access point, delivery site, and feeding method varies with indication, expected duration of use, and patient anatomy, the authors stressed.
The update notes that NG and NJ tubes can be used immediately after confirmation of placement, most often with abdominal radiography. PEG tubes can be used immediately for medications and after 4 hours for tube feedings. A multidisciplinary team approach after placement provides improved patient care. “Dietitians assist with formula choice, volume, free water needs, and delivery method, and nurses and advanced practice clinicians assist with tube site assessment and troubleshooting,” the authors wrote.
Complications can occur but should be infrequent, Micic said. “Frankly, most complications can be predicted based on the duration of use and prevented with appropriate monitoring.” Common complications include tube dislodgement, clogging, site infections, buried bumper syndrome, and aspiration. “Minimizing these risks requires a thorough understanding of patient-specific factors, careful technique during placement, and ongoing monitoring after the device is in use,” he added.
Paski said the update aligns with established guidelines for enteral access but also offers suggestions to mitigate the risk of tube placement in patients in whom placement has traditionally been more challenging. “This is a helpful addition to the literature because if enteral access cannot be obtained in a patient unable to meet their needs orally, total paternal nutrition is the next and much more invasive step for nutrition support.”
She called the practice update a concise, comprehensive reference for trainees and experienced gastroenterologists to optimize placement conditions and reduce complication risk, noting that training in nutrition is suboptimal in many GI fellowships.
Becoming familiar with common and advanced enteral access techniques is within the armamentarium of all practicing gastroenterologists, the authors stated. Because malnutrition affects nearly all GI disorders, “understanding common routes of enteral access and the basic principles of nutrition support promotes the initiation of optimal enteral nutrition, mitigating the impact of malnutrition, and improving prognosis for patients at nutritional risk,” they wrote.
Micic served on the advisory board for Ironwood Pharmaceuticals and is on the speaker’s bureau for Takeda Pharmaceuticals. One coauthor served as a consultant for Merit Medical, Circa Scientific, and Aspero Medical. Paski and Shamah had disclosed no competing interests relevant to their comments.
A version of this article appeared on Medscape.com.
At least 250,000 US hospitalized patients a year require enteral support using an artificial pathway into the gastrointestinal (GI) tract to deliver nutrition or medication. In light of this,
Covering indications, placement techniques, and management, the comprehensive document is a response to the increasing use of enteral access devices in chronic GI conditions. The update, published in Gastroenterology, addresses patient factors complicating placement decision-making such as thrombocytopenia, use of dual antiplatelet therapy, or performance of percutaneous access in the setting of cirrhosis.
“We provide clinical recommendations in these various scenarios understanding that the final decision-making is in the hands of the provider and care team,” said first author Dejan Micic, MD, a gastroenterologist and associate professor at University of Chicago Medical Center in Illinois at the time of the update (since relocated to Loyola University Medical Center in Chicago). “We hope this can serve a day-to-day purpose for clinical gastroenterologists and can be referenced as they encounter individuals with or needing an enteral access device.”
Traditionally, enteral access was reserved for patients with severe malnutrition or those unable to maintain oral intake. Recent recommendations emphasize early nutritional intervention including prehabilitation before major surgery, adjunctive therapy for oncology patients, and in specific inflammatory conditions such as Crohn’s disease. “These shifts recognize the role of enteral nutrition not only in preventing malnutrition but also as a therapeutic strategy,” Micic said in an interview.
There is, however, variability in the use of devices including the selection of appropriate units, technical aspects of placement, and subsequent management. “Such variability can lead to complications, suboptimal patient outcomes, and inefficiencies in care delivery,” Micic said.
He added that enteral access has been historically underemphasized in GI endoscopic training. “While procedural skill in placing devices such as percutaneous endoscopic gastrostomy, or PEG, tubes is often taught, a comprehensive understanding of the broader clinical context — such as proper patient selection, prevention of complications, and postplacement care — is not always thoroughly covered.”
The current update aims to bridge knowledge gaps with evidence-based-guidance. “It also underscores the importance of interdisciplinary collaboration with dietitians, nurses, and care givers to achieve the best outcomes for patients,” Micic said.
Commenting on the update but not involved with creating it, Shirley C. Paski, MD, MS, a gastroenterologist at the Cleveland Clinic, Ohio, called it timely, adding: “As GI training is becoming more subspecialized and interventional radiology has been able to provide enteral access, gastroenterology training in enteral access has declined to where some fellows are graduating with limited enteral access experience.”
Yet malnutrition remains a common consequence when GI disease is severe, chronic, or refractory to treatment, or in the setting of postsurgical anatomy, she added. “Enteral nutrition is increasingly being considered a therapeutic or adjunct treatment in some cases of Crohn’s disease or small intestinal bacterial overgrowth. Gastroenterologists need the endoscopic skill to secure enteral access tubes, particularly in more challenging anatomy.”
Also commenting on the document but not involved in it, Steven Shamah, MD, director of Endoscopy at Northwell Lenox Hill Hospital in New York City, said: “This should serve as a concise review for any general hospitalist or gastroenterologist to understand what we have and when we should offer the proper feeding tube options.” He stressed, however, that all gastroenterologists should be trained in the placing of all of tube options.
“The axiom ‘If the gut works, we should use it’ is something that I was taught when I was a medical student and it still holds true,” Shamah continued. “There’s been a jump in interventional procedures to assure continuity of the GI tract even in progressive malignancy. So there’s a rise in moving away from intravenous nutrition and a rise in tube-delivered enteral nutrition.” Options for reducing reflux and aspiration will likely take on more importance, he said.
Tubing Options
According to Micic and colleagues, recent data suggest a favorable safety profile of enteral feeding tubes placed endoscopically compared with surgical or radiologic placement. The illustrated AGA document outlines such approaches as synthetic flexible tubes placed into the stomach or small bowel via the oral (orogastric and oroenteral) or nasal routes (nasogastric [NG] and nasojejunal [NJ]) and percutaneous tubes accessing the stomach. The choice of tube, access point, delivery site, and feeding method varies with indication, expected duration of use, and patient anatomy, the authors stressed.
The update notes that NG and NJ tubes can be used immediately after confirmation of placement, most often with abdominal radiography. PEG tubes can be used immediately for medications and after 4 hours for tube feedings. A multidisciplinary team approach after placement provides improved patient care. “Dietitians assist with formula choice, volume, free water needs, and delivery method, and nurses and advanced practice clinicians assist with tube site assessment and troubleshooting,” the authors wrote.
Complications can occur but should be infrequent, Micic said. “Frankly, most complications can be predicted based on the duration of use and prevented with appropriate monitoring.” Common complications include tube dislodgement, clogging, site infections, buried bumper syndrome, and aspiration. “Minimizing these risks requires a thorough understanding of patient-specific factors, careful technique during placement, and ongoing monitoring after the device is in use,” he added.
Paski said the update aligns with established guidelines for enteral access but also offers suggestions to mitigate the risk of tube placement in patients in whom placement has traditionally been more challenging. “This is a helpful addition to the literature because if enteral access cannot be obtained in a patient unable to meet their needs orally, total paternal nutrition is the next and much more invasive step for nutrition support.”
She called the practice update a concise, comprehensive reference for trainees and experienced gastroenterologists to optimize placement conditions and reduce complication risk, noting that training in nutrition is suboptimal in many GI fellowships.
Becoming familiar with common and advanced enteral access techniques is within the armamentarium of all practicing gastroenterologists, the authors stated. Because malnutrition affects nearly all GI disorders, “understanding common routes of enteral access and the basic principles of nutrition support promotes the initiation of optimal enteral nutrition, mitigating the impact of malnutrition, and improving prognosis for patients at nutritional risk,” they wrote.
Micic served on the advisory board for Ironwood Pharmaceuticals and is on the speaker’s bureau for Takeda Pharmaceuticals. One coauthor served as a consultant for Merit Medical, Circa Scientific, and Aspero Medical. Paski and Shamah had disclosed no competing interests relevant to their comments.
A version of this article appeared on Medscape.com.
At least 250,000 US hospitalized patients a year require enteral support using an artificial pathway into the gastrointestinal (GI) tract to deliver nutrition or medication. In light of this,
Covering indications, placement techniques, and management, the comprehensive document is a response to the increasing use of enteral access devices in chronic GI conditions. The update, published in Gastroenterology, addresses patient factors complicating placement decision-making such as thrombocytopenia, use of dual antiplatelet therapy, or performance of percutaneous access in the setting of cirrhosis.
“We provide clinical recommendations in these various scenarios understanding that the final decision-making is in the hands of the provider and care team,” said first author Dejan Micic, MD, a gastroenterologist and associate professor at University of Chicago Medical Center in Illinois at the time of the update (since relocated to Loyola University Medical Center in Chicago). “We hope this can serve a day-to-day purpose for clinical gastroenterologists and can be referenced as they encounter individuals with or needing an enteral access device.”
Traditionally, enteral access was reserved for patients with severe malnutrition or those unable to maintain oral intake. Recent recommendations emphasize early nutritional intervention including prehabilitation before major surgery, adjunctive therapy for oncology patients, and in specific inflammatory conditions such as Crohn’s disease. “These shifts recognize the role of enteral nutrition not only in preventing malnutrition but also as a therapeutic strategy,” Micic said in an interview.
There is, however, variability in the use of devices including the selection of appropriate units, technical aspects of placement, and subsequent management. “Such variability can lead to complications, suboptimal patient outcomes, and inefficiencies in care delivery,” Micic said.
He added that enteral access has been historically underemphasized in GI endoscopic training. “While procedural skill in placing devices such as percutaneous endoscopic gastrostomy, or PEG, tubes is often taught, a comprehensive understanding of the broader clinical context — such as proper patient selection, prevention of complications, and postplacement care — is not always thoroughly covered.”
The current update aims to bridge knowledge gaps with evidence-based-guidance. “It also underscores the importance of interdisciplinary collaboration with dietitians, nurses, and care givers to achieve the best outcomes for patients,” Micic said.
Commenting on the update but not involved with creating it, Shirley C. Paski, MD, MS, a gastroenterologist at the Cleveland Clinic, Ohio, called it timely, adding: “As GI training is becoming more subspecialized and interventional radiology has been able to provide enteral access, gastroenterology training in enteral access has declined to where some fellows are graduating with limited enteral access experience.”
Yet malnutrition remains a common consequence when GI disease is severe, chronic, or refractory to treatment, or in the setting of postsurgical anatomy, she added. “Enteral nutrition is increasingly being considered a therapeutic or adjunct treatment in some cases of Crohn’s disease or small intestinal bacterial overgrowth. Gastroenterologists need the endoscopic skill to secure enteral access tubes, particularly in more challenging anatomy.”
Also commenting on the document but not involved in it, Steven Shamah, MD, director of Endoscopy at Northwell Lenox Hill Hospital in New York City, said: “This should serve as a concise review for any general hospitalist or gastroenterologist to understand what we have and when we should offer the proper feeding tube options.” He stressed, however, that all gastroenterologists should be trained in the placing of all of tube options.
“The axiom ‘If the gut works, we should use it’ is something that I was taught when I was a medical student and it still holds true,” Shamah continued. “There’s been a jump in interventional procedures to assure continuity of the GI tract even in progressive malignancy. So there’s a rise in moving away from intravenous nutrition and a rise in tube-delivered enteral nutrition.” Options for reducing reflux and aspiration will likely take on more importance, he said.
Tubing Options
According to Micic and colleagues, recent data suggest a favorable safety profile of enteral feeding tubes placed endoscopically compared with surgical or radiologic placement. The illustrated AGA document outlines such approaches as synthetic flexible tubes placed into the stomach or small bowel via the oral (orogastric and oroenteral) or nasal routes (nasogastric [NG] and nasojejunal [NJ]) and percutaneous tubes accessing the stomach. The choice of tube, access point, delivery site, and feeding method varies with indication, expected duration of use, and patient anatomy, the authors stressed.
The update notes that NG and NJ tubes can be used immediately after confirmation of placement, most often with abdominal radiography. PEG tubes can be used immediately for medications and after 4 hours for tube feedings. A multidisciplinary team approach after placement provides improved patient care. “Dietitians assist with formula choice, volume, free water needs, and delivery method, and nurses and advanced practice clinicians assist with tube site assessment and troubleshooting,” the authors wrote.
Complications can occur but should be infrequent, Micic said. “Frankly, most complications can be predicted based on the duration of use and prevented with appropriate monitoring.” Common complications include tube dislodgement, clogging, site infections, buried bumper syndrome, and aspiration. “Minimizing these risks requires a thorough understanding of patient-specific factors, careful technique during placement, and ongoing monitoring after the device is in use,” he added.
Paski said the update aligns with established guidelines for enteral access but also offers suggestions to mitigate the risk of tube placement in patients in whom placement has traditionally been more challenging. “This is a helpful addition to the literature because if enteral access cannot be obtained in a patient unable to meet their needs orally, total paternal nutrition is the next and much more invasive step for nutrition support.”
She called the practice update a concise, comprehensive reference for trainees and experienced gastroenterologists to optimize placement conditions and reduce complication risk, noting that training in nutrition is suboptimal in many GI fellowships.
Becoming familiar with common and advanced enteral access techniques is within the armamentarium of all practicing gastroenterologists, the authors stated. Because malnutrition affects nearly all GI disorders, “understanding common routes of enteral access and the basic principles of nutrition support promotes the initiation of optimal enteral nutrition, mitigating the impact of malnutrition, and improving prognosis for patients at nutritional risk,” they wrote.
Micic served on the advisory board for Ironwood Pharmaceuticals and is on the speaker’s bureau for Takeda Pharmaceuticals. One coauthor served as a consultant for Merit Medical, Circa Scientific, and Aspero Medical. Paski and Shamah had disclosed no competing interests relevant to their comments.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Open Clinical Trials for Patients With Chronic Obstructive Pulmonary Disease
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
The clinical trials listed below are open as of February 21, 2025; have ≥ 1 US Department of Veterans Affairs (VA) medical center (VAMC) or US Department of Defense (DoD) military treatment facility location recruiting patients; and are focused on treatments for chronic obstructive pulmonary disease (COPD). For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
Actively Recruiting
The Effect of Interval Exercise on Functional Outcomes in Veterans With COPD and OSA
The term overlap syndrome (OS) is used to describe the presence of both COPD and obstructive sleep apnea (OSA) in a single patient. Due to premature aging, patients with OS are prone to developing functional decline up to 20 years earlier than the general population. The International Classification of Functioning, Disability and Health (ICF) evaluates functional status in chronic pulmonary disease globally in 5 domains. The investigators propose to study validated outcomes in 3 of these domains: (1) participation in life situations; (2) physical activity; and (3) cardiovascular health. The investigators’ long-term goal is to develop an exercise strategy tailored to veterans with OS which will reduce the risk of functional decline through increased physical activity.
ID: NCT05254431
Sponsor; Collaborator: VA Office of Research and Development; Madalina Macrea, MD, PhD
Location: Salem VA Medical Center, Virginia
The Development of an Integrated Physical Activity and Mental Health Intervention for Veterans With COPD, Emotion Distress, and Low Physical Activity
COPD is a prevalent and debilitating chronic disease in veterans. COPD is highly comorbid with depression and anxiety, conferring greater morbidity and mortality risk. Physical activity is a modifiable behavior that can improve COPD outcomes. However, to date, interventions targeting physical activity have not addressed the high comorbidity between COPD and depression and/or anxiety symptoms (emotional distress) despite emotional distress predicting poorer response to physical activity interventions. This CDA-2 proposal will develop and test the acceptability and feasibility of an integrative physical activity and mental health intervention for veterans with COPD, emotional distress, and low physical activity. The intervention will be delivered via VA Video Connect enabling access to care among veterans with substantial barriers to hospital-based outpatient care.
ID: NCT04953806
Sponsor; Collaborator: VA Office of Research and Development; Patricia Bamonti, PhD
Location: VA Boston Healthcare System, Jamaica Plain Campus
Neurocognitive and Health Impact of Sleep Apnea in Elderly Veterans With Comorbid COPD
Cognitive dysfunction in the aging veteran population is a growing health concern in the Veterans Health System. It is not known whether OSA coexisting with COPD will enhance the risk for cognitive dysfunction. The investigators sought to investigate whether these two highly prevalent diseases that often coexist as 'overlap syndrome' combine to enhance cognitive impairment in the elderly veteran population. Thus, the investigators will study whether elderly patients with overlap syndrome have increased cognitive deficits compared with OSA or COPD alone. Additionally, treatment of OSA with positive airway pressure (PAP) has been shown to improve neurocognitive function in moderate-to-severe OSA while cognitive decline in COPD may be reversible through treatment with long-term oxygen therapy. The investigators will also study whether treatment with PAP and supplemental oxygen vs PAP alone will improve cognitive function and improve quality of life of elderly veterans.
ID: NCT02703207
Sponsor; Investigators: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: John D. Dingell VA Medical Center, Detroit
The Effect of a Technology-Mediated Integrated Walking and Tai Chi Intervention on Physical Function in Veterans With COPD and Chronic Musculoskeletal Pain (WATCH for Pain)
Persons with COPD benefit from being physically active, but they are often limited by chronic musculoskeletal pain. This project will determine whether a non-pharmacologic, integrated, technology-mediated walking and tai chi mindfulness intervention can improve physical function in veterans with COPD and chronic musculoskeletal pain. The proposed research addresses VA Rehabilitation R&D Service's high priority area of improving health-related quality of life by reducing disease burden and maximizing function in veterans with chronic disease.
ID: NCT05701982
Sponsor; Investigator: VA Office of Research and Development; Marilyn L. Moy, MD; University of Michigan, Beth Israel Deaconess Medical Center
Location: VA Boston Healthcare System
Internet-based Cognitive-behavioral Treatment for Insomnia in COPD Patients Undergoing Pulmonary Rehabilitation
This study is a randomized controlled trial (RCT) to compare sleep and health-related functioning in veterans with COPD and insomnia receiving an Internet-based behavioral treatment for insomnia vs online insomnia patient education. Participants will undergo a sleep and health assessment that will be performed at baseline, post-treatment, and 3 months later. Participants will be randomly assigned to either Internet-based behavioral treatment for insomnia or online insomnia patient education.
ID: NCT04700098
Sponsor; Collaborators: VA Office of Research and Development; Faith S. Luyster, PhD
Locations: VA Pittsburgh Healthcare System; John D. Dingell VA Medical Center, Detroit
Breathe Easier With Tadalafil Therapy for Dyspnea in COPD-PH (BETTER COPD-PH)
The investigators will study whether the drug tadalafil improves shortness of breath in 126 veterans with COPD and high blood pressure in the lungs. The investigators will also assess whether tadalafil improves quality of life, home daily physical activity, exercise endurance, the frequency of acute flares of COPD, blood pressure in the lungs, and lung function. Veterans who enroll in the trial will be allocated by chance to either active tadalafil or an inactive identical capsule (placebo). Neither the veteran nor the investigator will know whether the veteran is taking tadalafil or placebo. Veterans will be followed closely in clinic or by telephone at 1, 2, 3, 4, 5, and 6 months, with attention to side effects and safety. At 1,3, and 6 months the investigators will repeat the questionnaires and testing of blood pressures in the lung and lung function. The investigators anticipate that the results of this study will determine whether tadalafil improves shortness of breath when added to usual medications for COPD.
ID: NCT05937854
Sponsor; Collaborator: VA Office of Research and Development; Sharon I. Rounds, MD
Locations: Rocky Mountain Regional VA Medical Center, Colorado; Joseph Maxwell Cleland Atlanta VA Medical Center ; VA Boston Healthcare System Jamaica Plain Campus; VA Nebraska-Western Iowa Health Care System; Providence VA Medical Center
Impact of Positive Airway Pressure Therapy on Clinical Outcomes in Older Veterans With Chronic Obstructive Pulmonary Disease and Comorbid Obstructive Sleep Apnea (Overlap Syndrome)
Obstructive sleep apnea (OSA) and COPD are highly prevalent chronic respiratory diseases in the veteran population. OSA co-occurring with COPD, known as overlap syndrome (OVS), is a complex chronic medical condition associated with grave consequences. OVS is highly prevalent in veterans. Veterans with OVS may be at increased risk for cognitive deficits, poor sleep quality as well as a reduced quality of life (QoL). The overall objective is to study the effects of positive airway pressure therapy on clinical outcomes in patients with OVS.
ID: NCT04179981
Sponsor; Investigator: VA Office of Research and Development; Susmita Chowdhuri, MD, MS
Locations: VA Ann Arbor Healthcare System; John D. Dingell VA Medical Center, Detroit
Developing an Intervention to Optimize Virtual Care Adoption for COPD Management (VC-OPTIONS)
VA is a leader in virtual care (VC), including the patient portal, mobile apps, and telehealth programs. VC has great utility for managing chronic conditions like COPD. However, adoption of many VC services has been slow. Lack of awareness about these services is one of the most prominent patient- and health care team-facing barriers to adopting VC. This study will develop, refine, and pilot a stakeholder-informed multicomponent implementation strategy to support adoption of VC, referred to as VC-OPTIONS (Virtual Care for Chronic Obstructive Pulmonary Disease Adoption Support). This feasibility trial will pilot the VC-OPTIONS implementation strategy to assess feasibility and acceptability and gather preliminary effectiveness data to inform a larger hybrid effectiveness-implementation trial. The core component of VC-OPTIONS will be the provision of information via VA's Annie texting program to empower patients with knowledge about the array of VC services and how they can be used to support COPD management. It is hypothesized that this strategy will be acceptable and feasible. This work will improve patient and team awareness of and communication about VC services, and support patient access to VC services for COPD management.
ID: NCT05986214
Sponsor; Collaborators: VA Office of Research and Development; Stephanie Robinson, PhD
Location: VA Bedford Healthcare System, Massachusetts; VA Boston Healthcare System Jamaica Plain Campus
Chronic Lung Disease and COVID-19: Understanding Severity, Recovery and Rehabilitation Needs (LAUREL)
This study is comprised of 3 approaches. First, the investigators will conduct a retrospective cohort study to determine factors associated with COVID-19 severity and complications and understand COVID-19 outcomes, including all-cause mortality, postdischarge events, and impacts of rehabilitation services (third aim). The second aim is a mixed-method study and follows COVID-19 patients with repeated surveys to determine patient-reported functional outcomes, health recovery, and rehabilitation needs after COVID-19. The investigators will recruit patients and their informal caregivers for interviews to assess their function and rehabilitation needs.
ID: NCT04628039
Sponsor; Collaborators: VA Office of Research and Development; Kristina A. Crothers, MD
Locations: VA Ann Arbor Healthcare System; VA Puget Sound Health Care System, Washington
Accessing Mobility Using Wearable Sensors
This study will examine whether wearable sensors can be used to track changes in cognitive-motor performance in response to a disease or an intervention. The investigators specific aims are twofold, first aim to explore whether and how a clinical condition such as chronic obstructive pulmonary disease (COPD) or congestive heart failure (CHF) may impact motor-cognitive performance measurable using validated wearable devices (eg, LEGSys, BalanSENS, and Frailty Meter). Second, the investigators will explore whether an exercise intervention provided via telemedicine (telerehabilitation) can enhance motor-cognitive performance.
ID: NCT04306588
Sponsor; Collaborators: Baylor College of Medicine, Bijan Najafi, PhD
Locations: Michael E. DeBakey Veterans Affairs Medical Center, Houston
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).
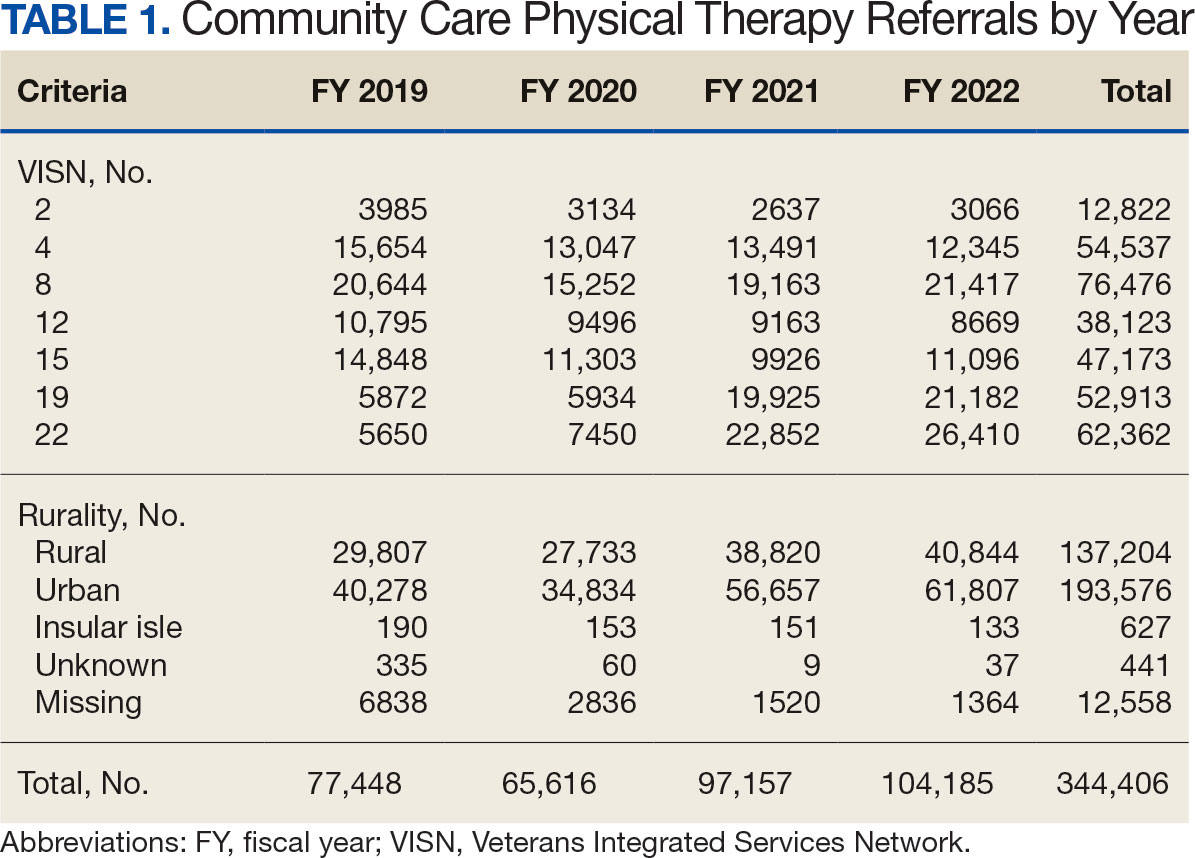
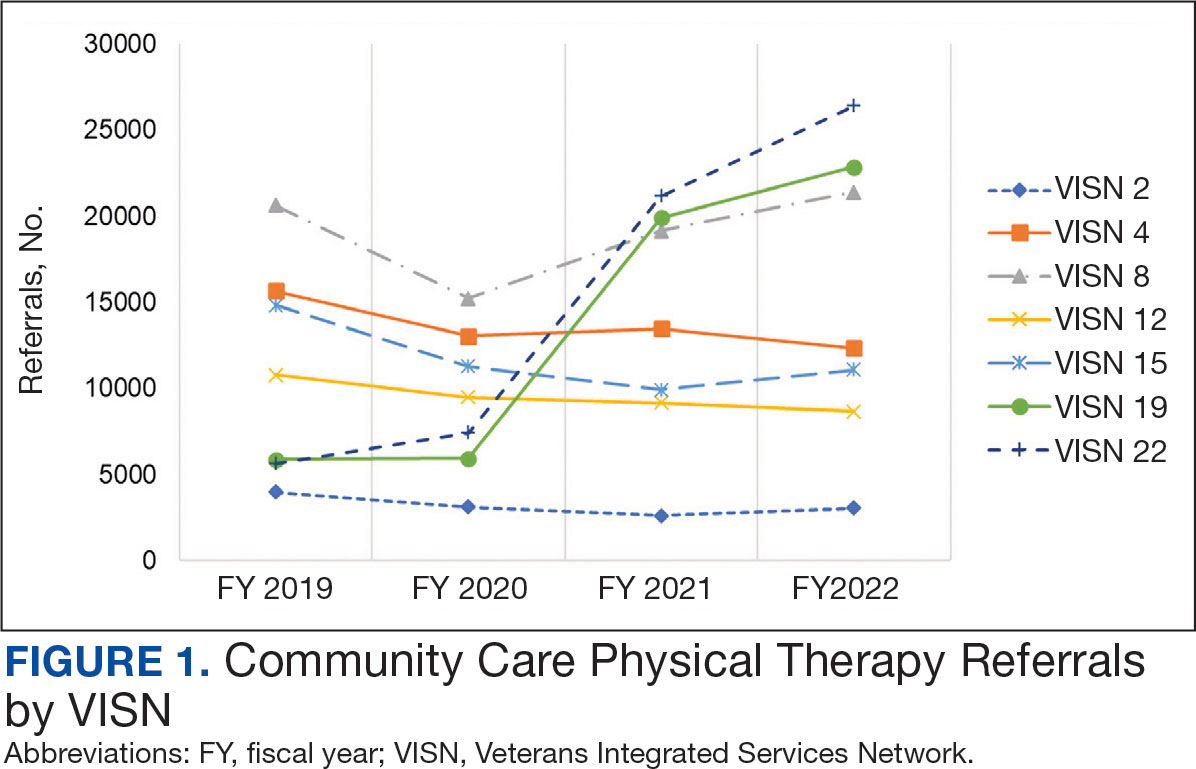
For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).
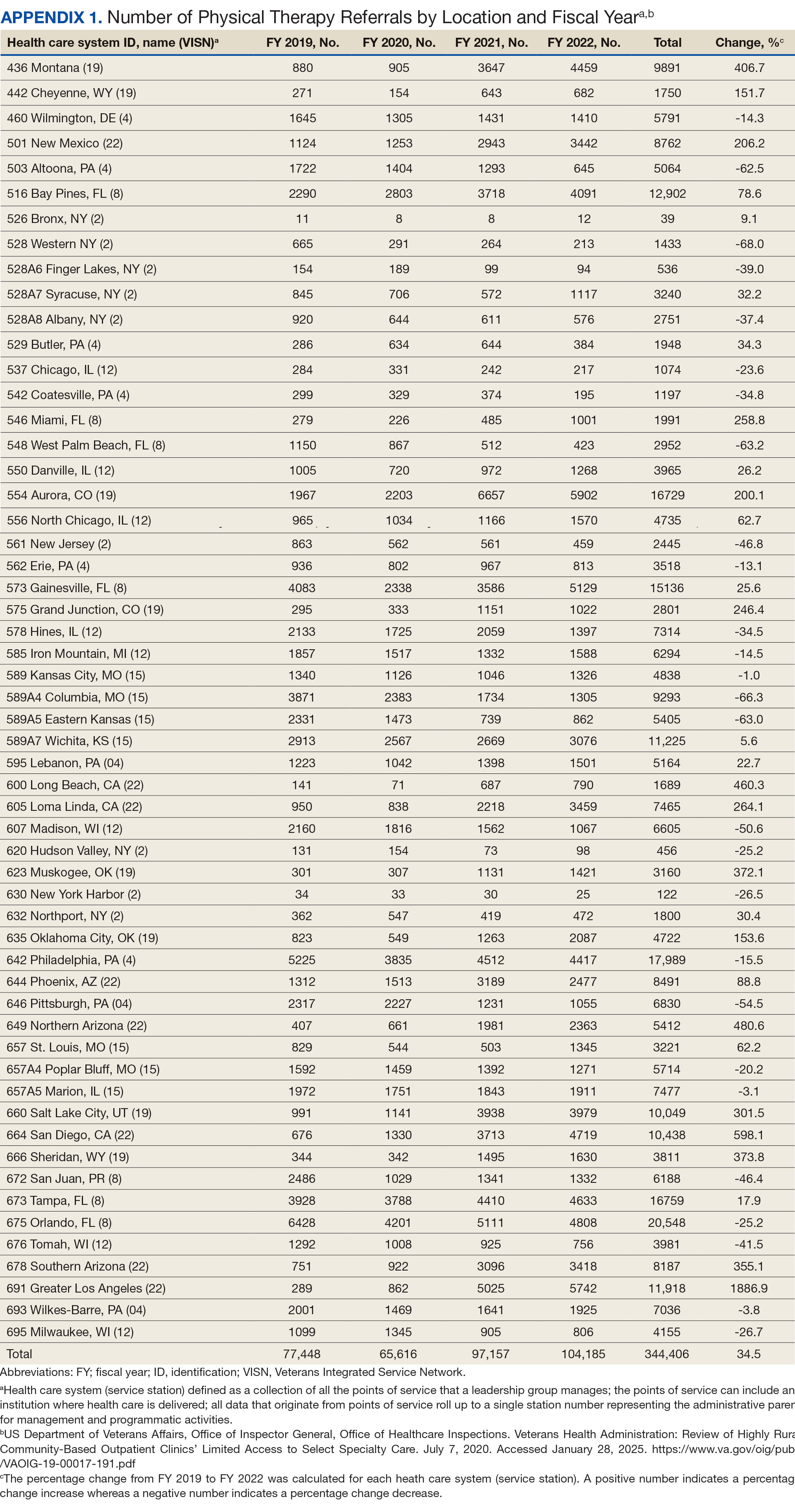
Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).
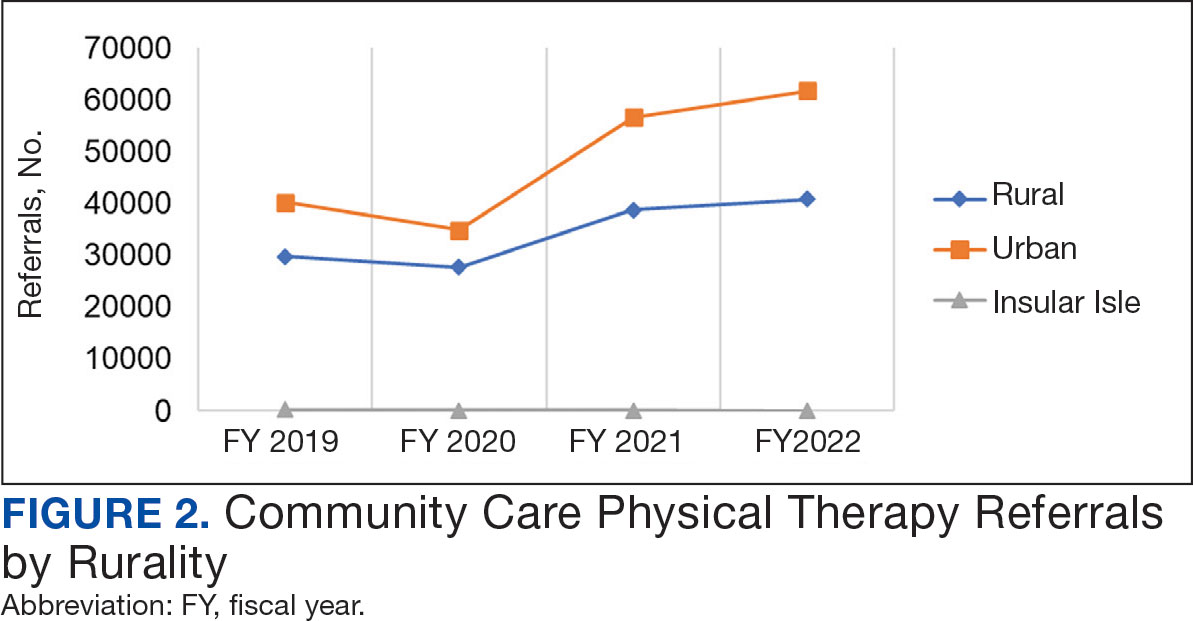
There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).
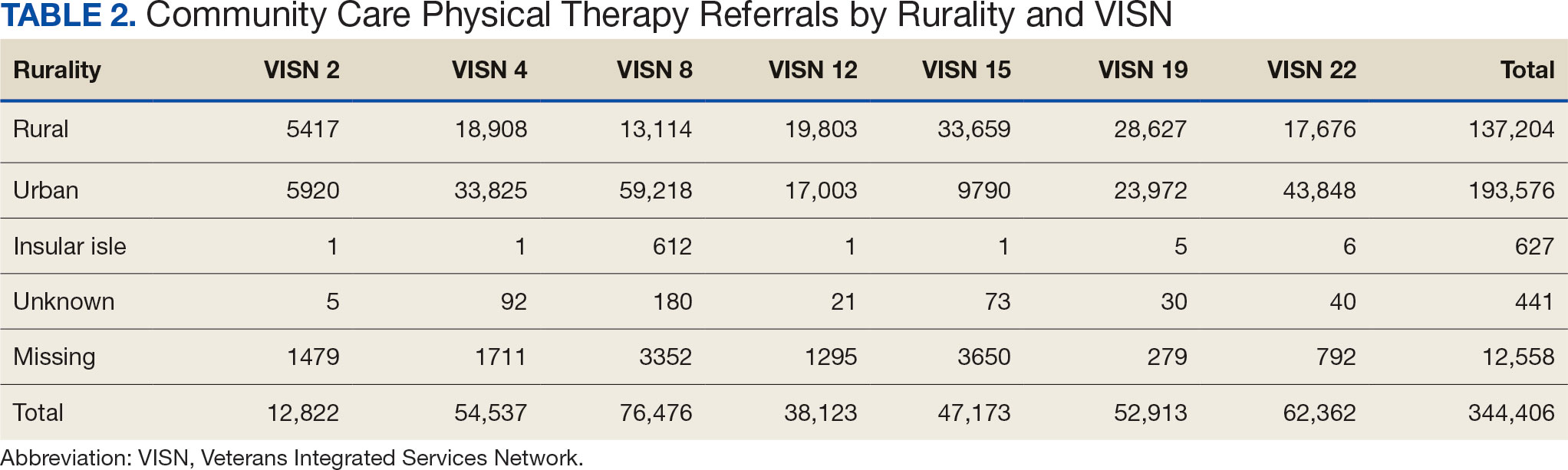
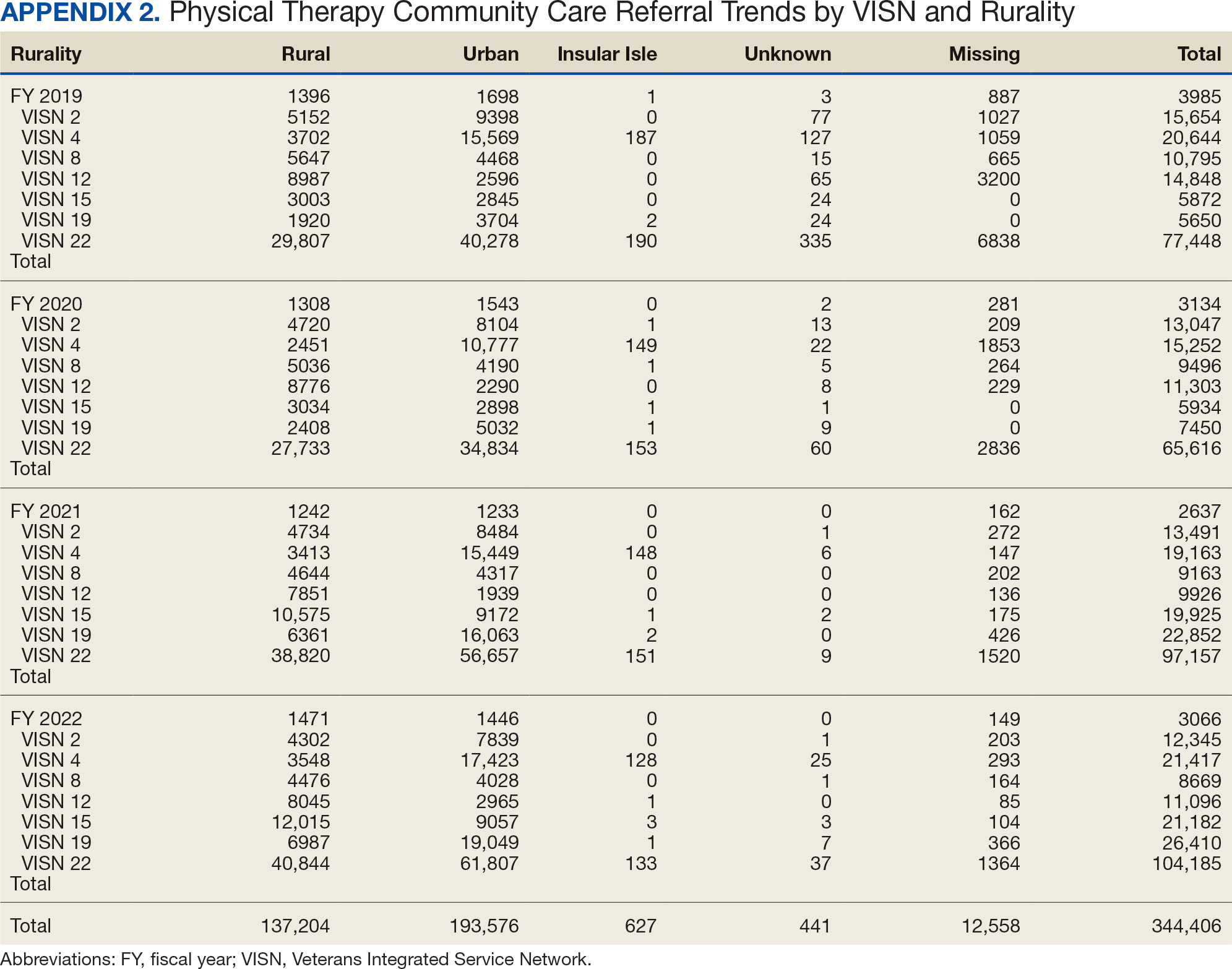
Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.
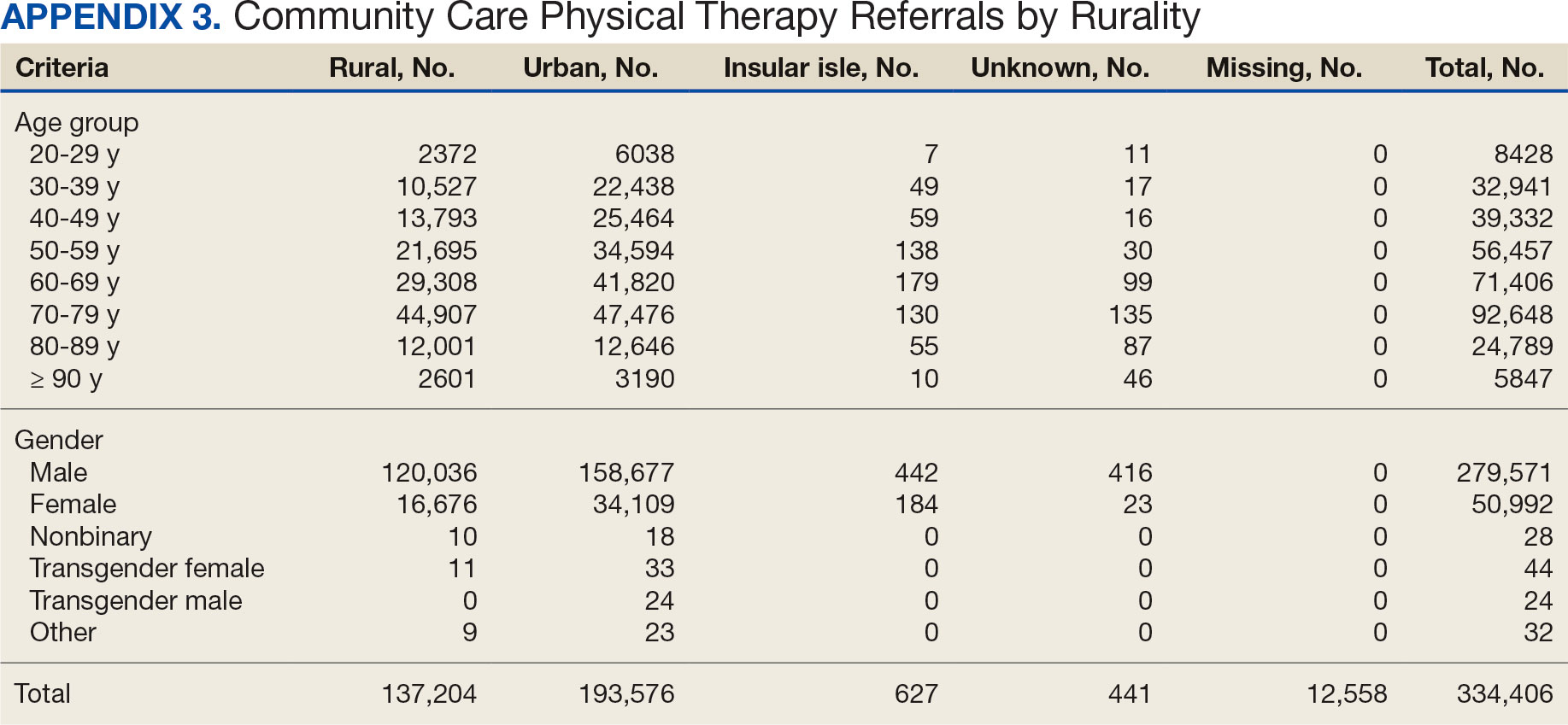
Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).
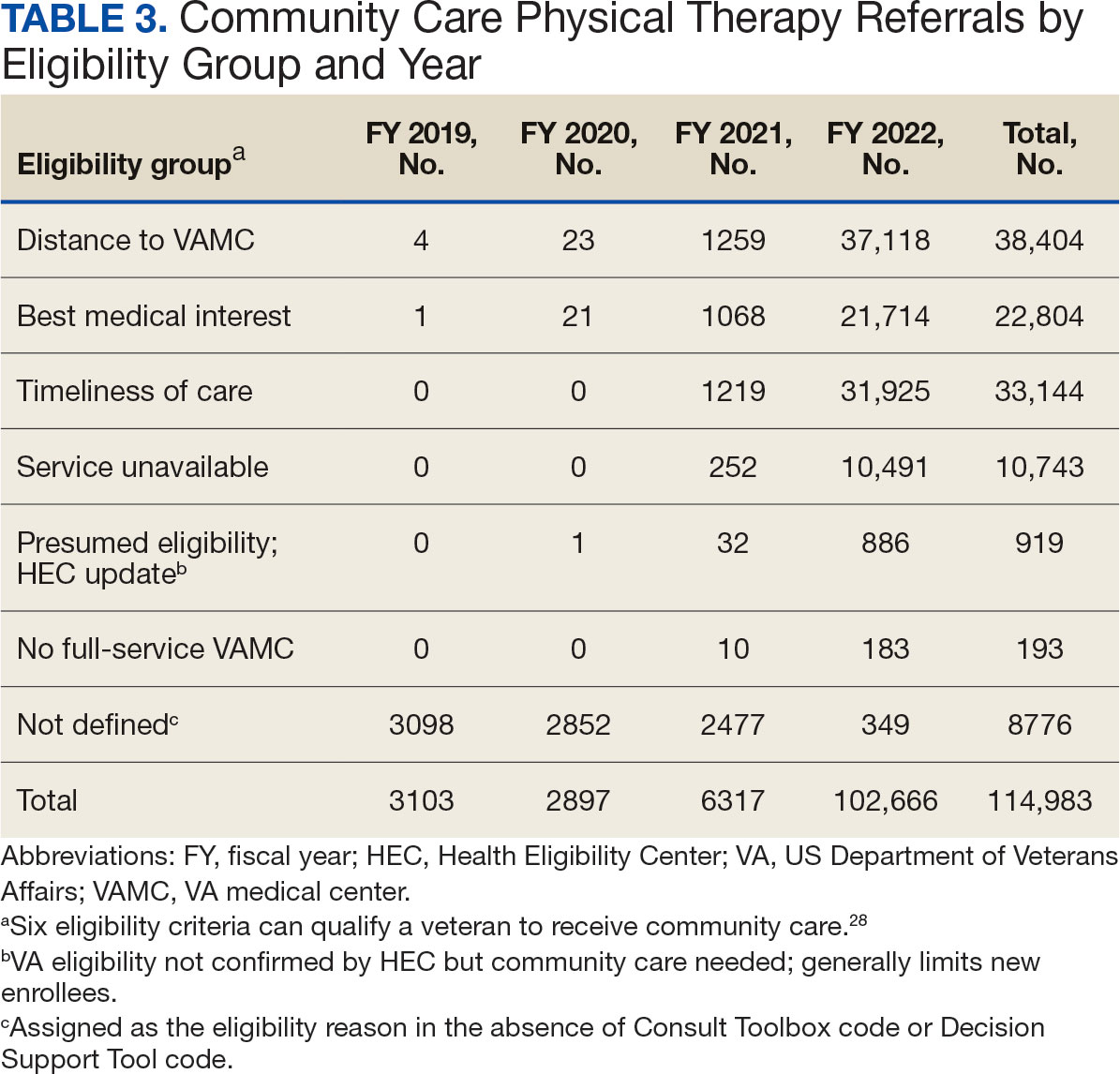
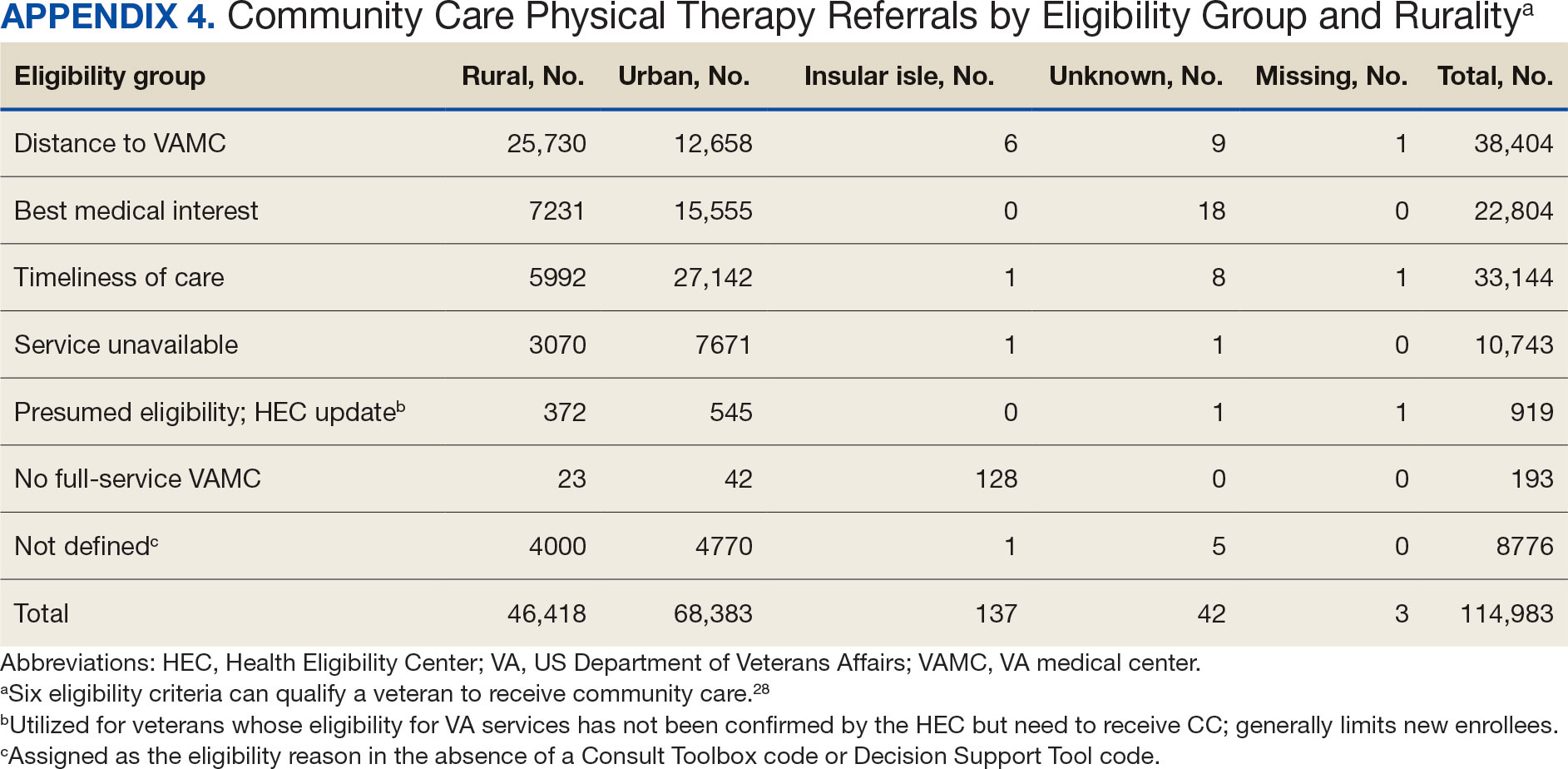
Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.
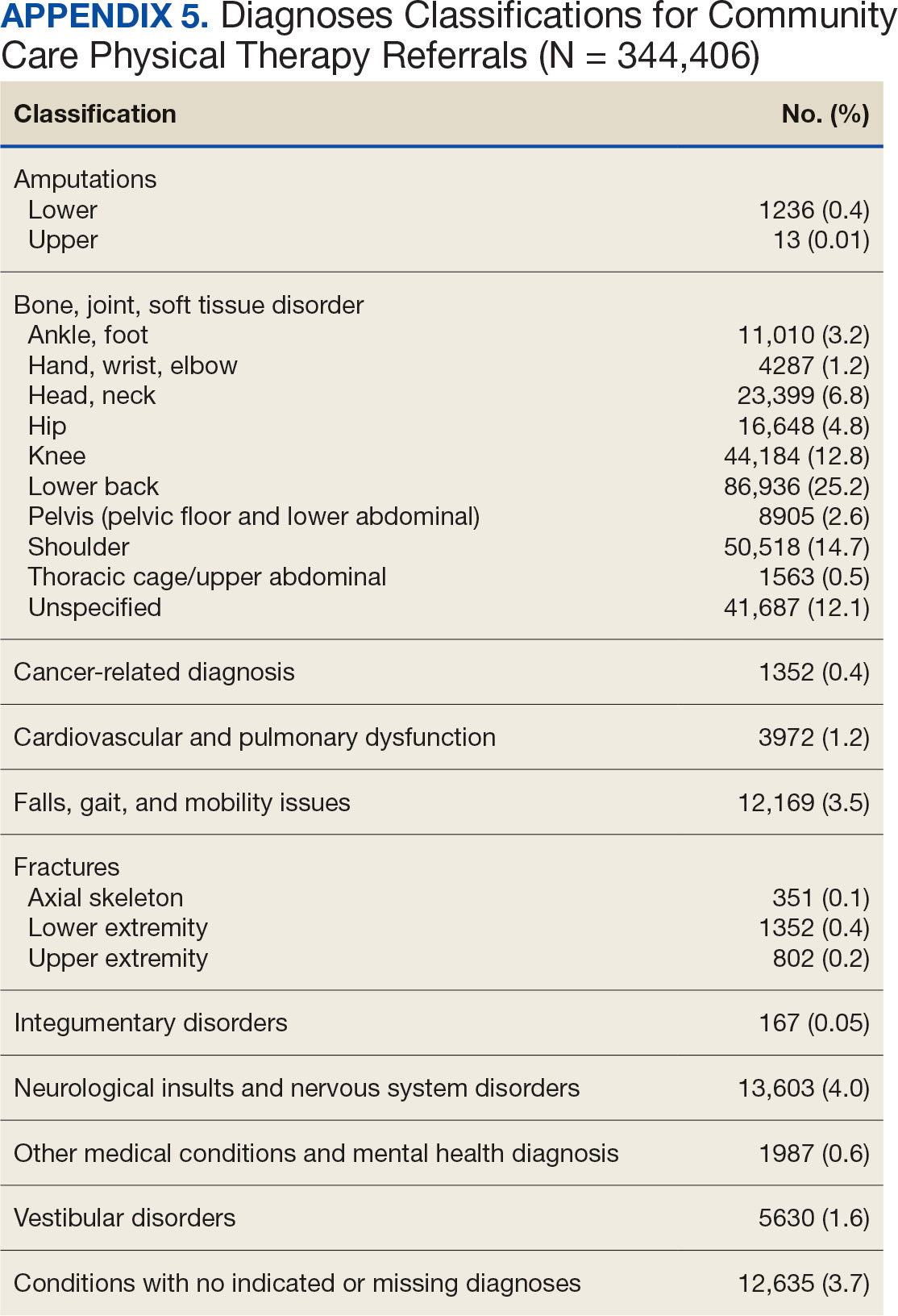
Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).
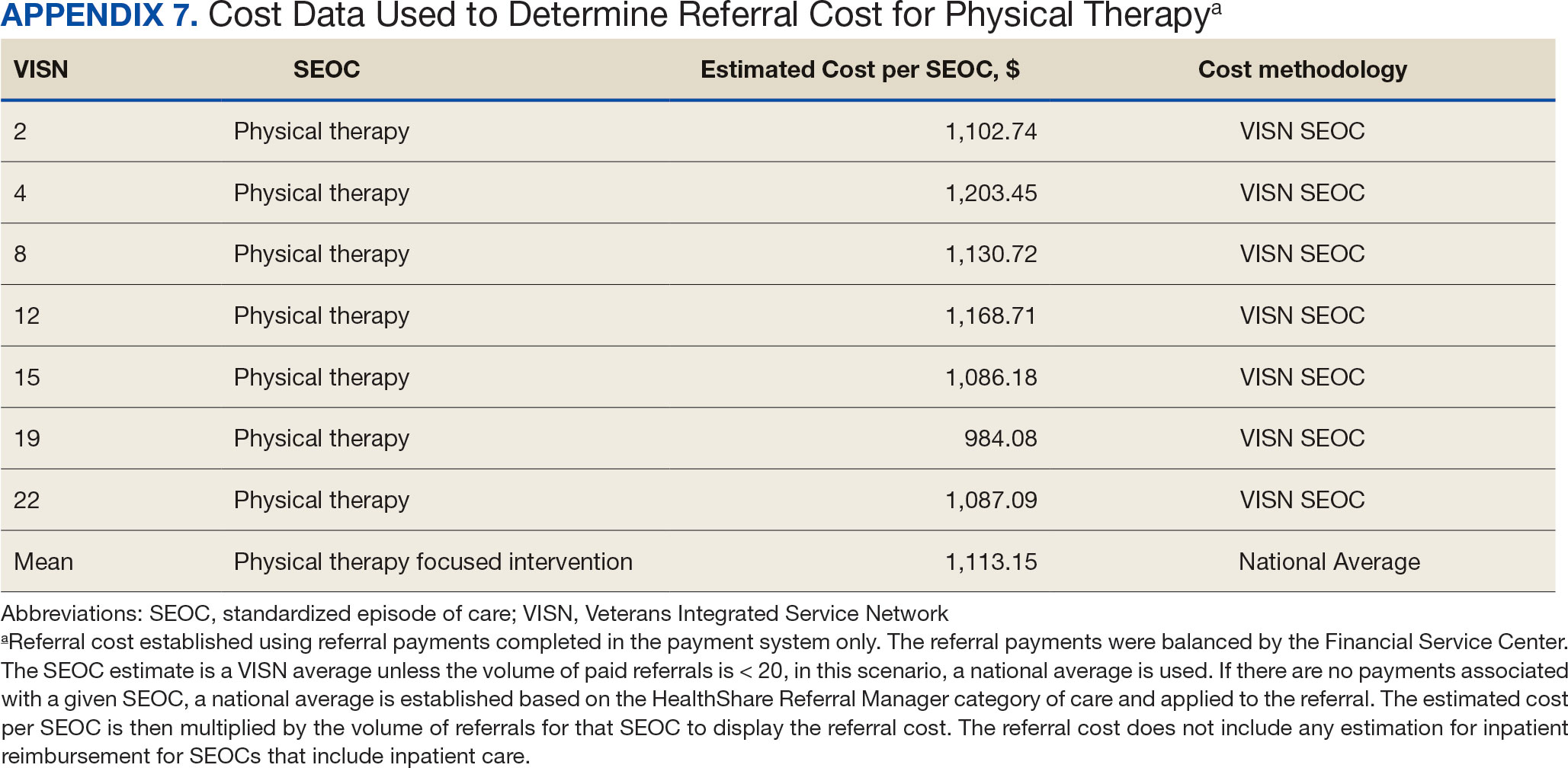
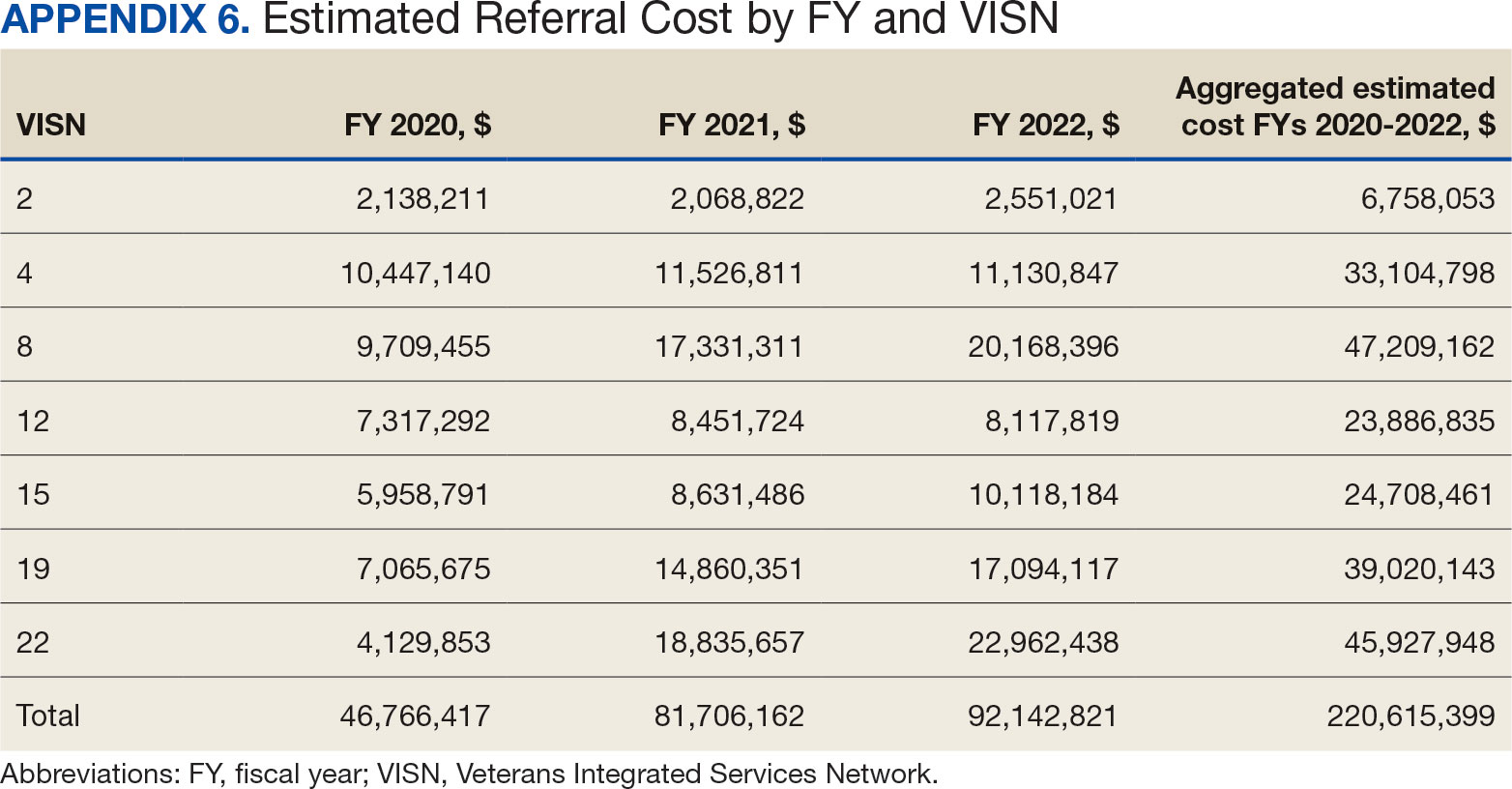
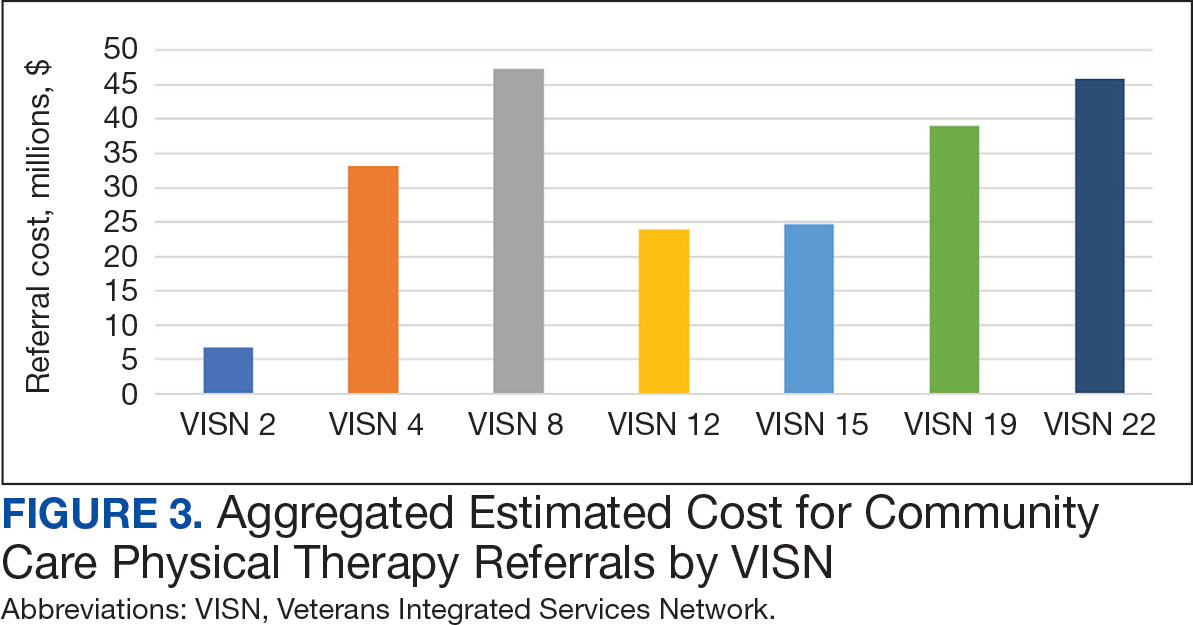
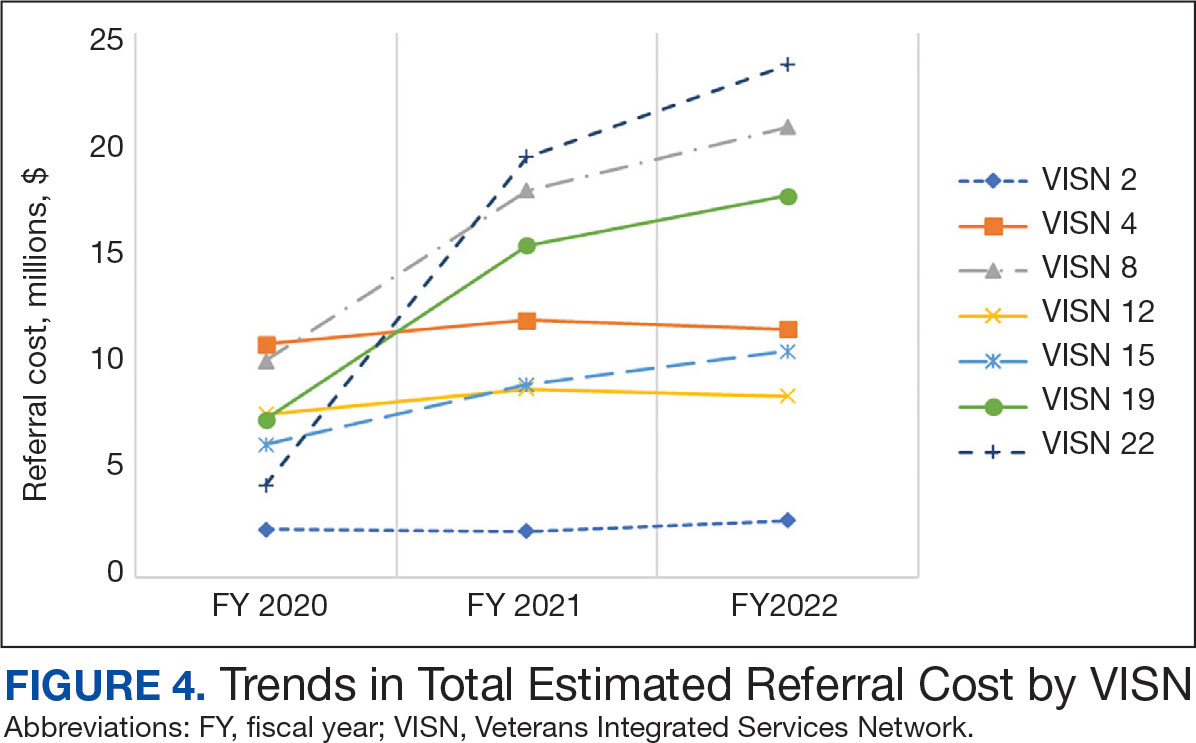
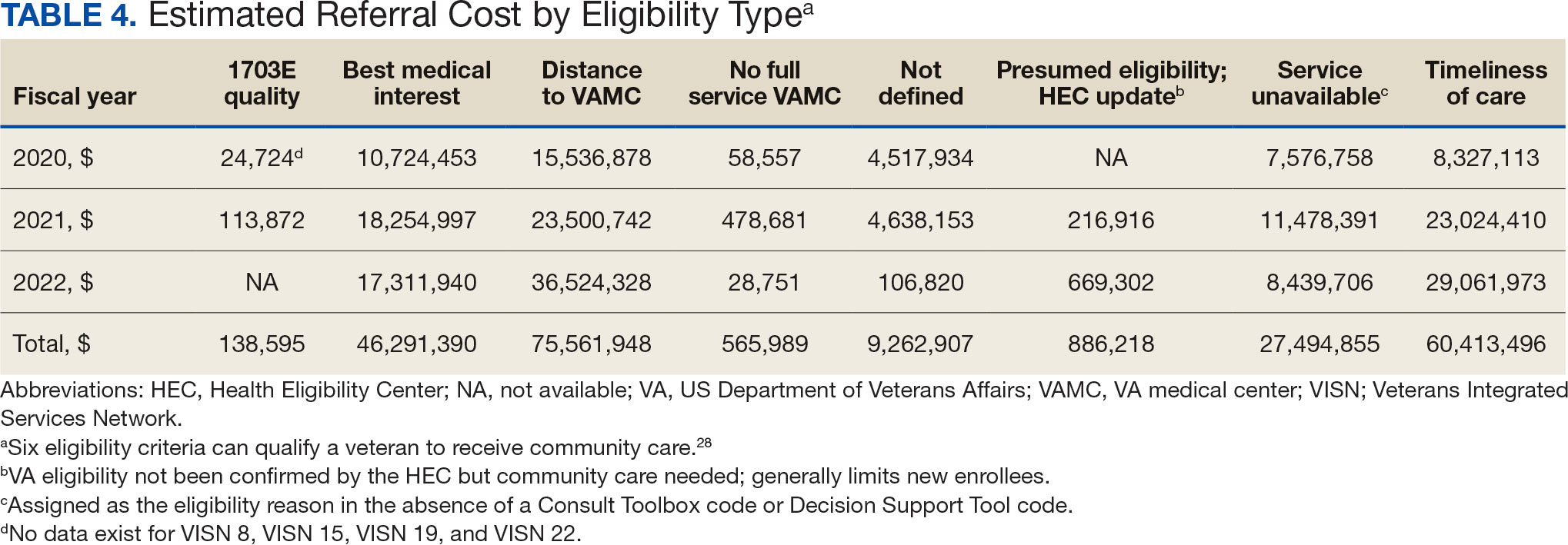
Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.
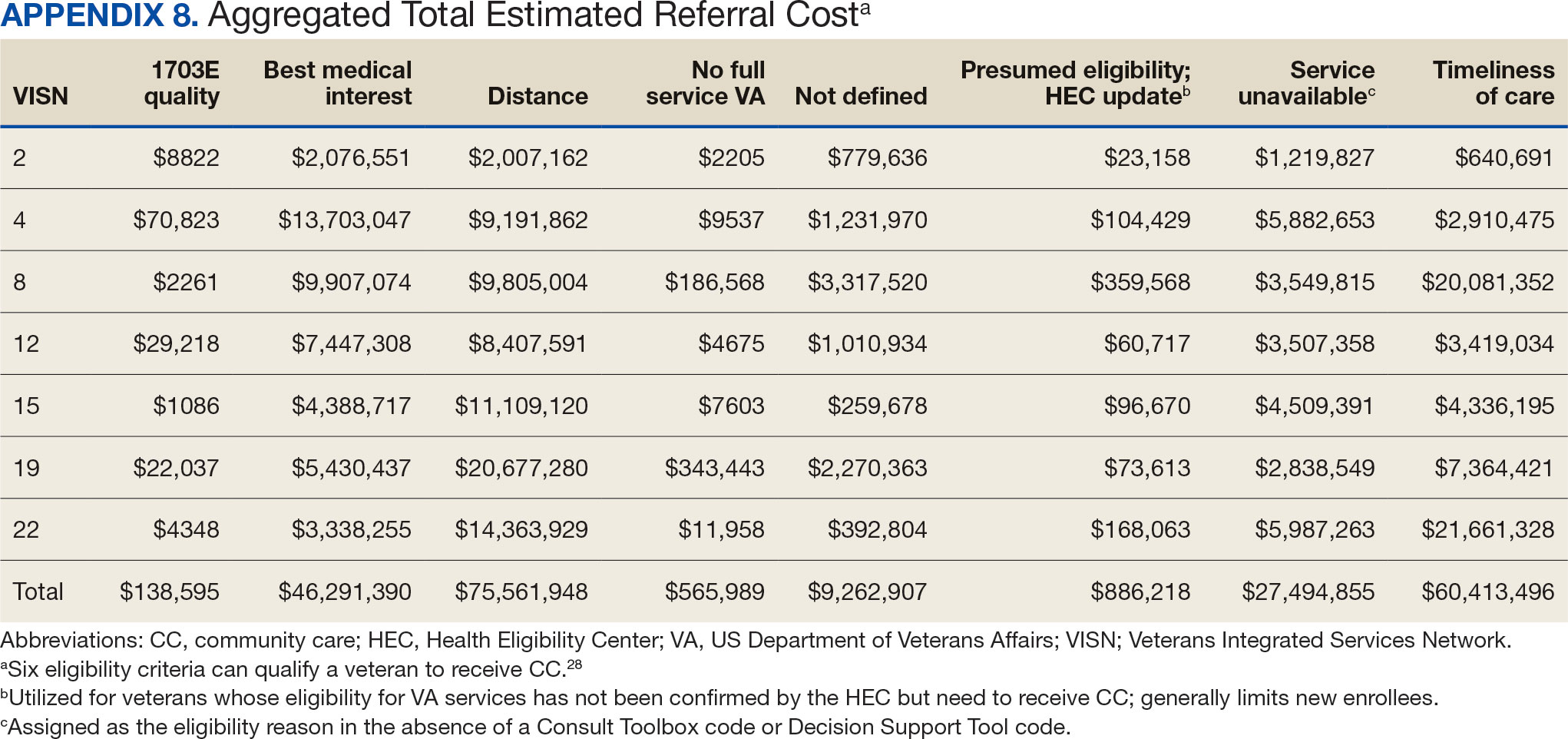
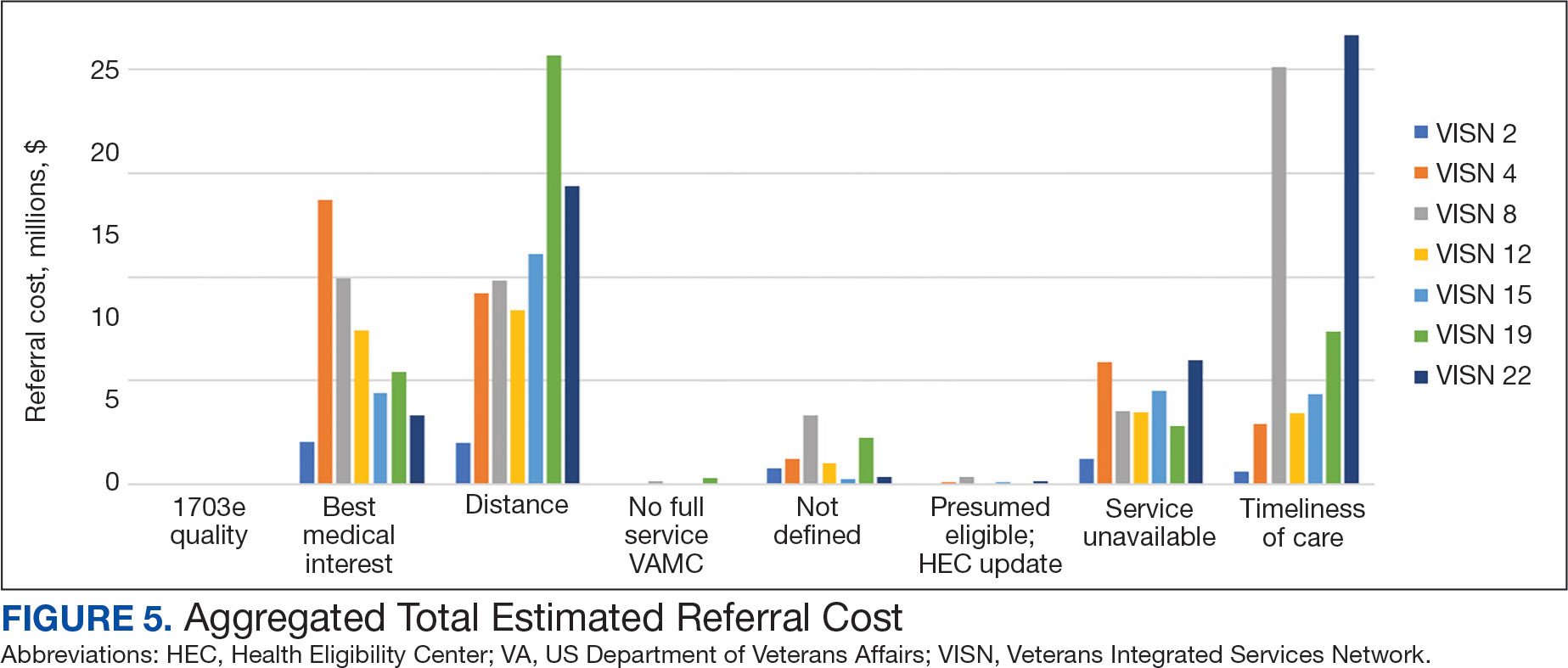
Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).


For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).

Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).

There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).


Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.

Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).


Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.

Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).





Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.


Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).


For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).

Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).

There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).


Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.

Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).


Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.

Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).





Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.


Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Navigating Esophageal Dysfunction in Immune and Infectious Disorders: AGA Clinical Practice Update
“Many different disorders can lead to esophageal dysfunction, which is characterized by symptoms including dysphagia, odynophagia, chest pain and heartburn. These symptoms can be caused either by immune or infectious conditions and can either be localized to the esophagus or part of a larger systemic process,” co–first author Emily McGowan, MD, PhD, with the division of allergy and immunology, University of Virginia School of Medicine, Charlottesville, said in an AGA podcast.
However, without a “high index of suspicion,” these conditions can be overlooked, leading to delays in diagnosis and unnecessary procedures. “With this clinical practice update, we wanted to help providers more readily recognize these conditions so that patients can be diagnosed and treated earlier in the course of their disease,” McGowan explained.
“This is a fantastic review that highlights how many different systemic disorders can affect the esophagus,” Scott Gabbard, MD, gastroenterologist and section head at the Center for Neurogastroenterology and Motility, Cleveland Clinic, Ohio, who wasn’t involved in the review, said in an interview.
“Honestly, for the practicing gastroenterologist, this is one of those reviews that I could envision someone either saving to his or her desktop for reference or printing it and pinning it next to his or her desk,” Gabbard said.
Best Practice Advice
The clinical practice update is published in Clinical Gastroenterology and Hepatology. It includes 10 “best practice advice” statements and a table highlighting “important” considerations when evaluating patients with esophageal dysfunction.
The review authors note that esophageal dysfunction may result from localized infections — most commonly Candida, herpes simplex virus, and cytomegalovirus — or systemic immune-mediated diseases, such as systemic sclerosis (SSc), mixed connective tissue disease (MCTD), and eosinophilic esophagitis (EoE).
They advise clinicians to identify if there are risks for inflammatory or infectious possibilities for a patient’s esophageal symptoms and investigate for these disorders as a potential cause of esophageal dysfunction.
Once esophageal infection is identified, it’s important to identify whether accompanying signs and symptoms point to immunocompromise leading to a more systemic infection. Consultation with an infectious disease expert is recommended to guide appropriate treatment, the authors said.
If symptoms fail to improve after therapy for infectious esophagitis, the patient should be evaluated for refractory infection or additional underlying sources of esophageal and immunologic dysfunction is advised.
It’s also important to recognize that patients with EoE who continue to have symptoms of esophageal dysfunction despite histologic and endoscopic disease remission, may develop a motility disorder and evaluation of esophageal motility may be warranted, the authors said.
In patients with histologic and endoscopic features of lymphocytic esophagitis, treatment of lymphocytic-related inflammation with proton-pump inhibitor (PPI) therapy or swallowed topical corticosteroids and esophageal dilation as needed should be considered.
In patients who present with esophageal symptoms in the setting of hypereosinophilia (absolute eosinophil count > 1500 cells/uL), the authors advise further workup of non-EoE eosinophilic gastrointestinal disease, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis should be considered, with consultation with an allergy/immunology specialist if helpful.
In patients with rheumatologic diseases, especially SSc and MCTD, it’s important to be aware that esophageal symptoms can occur because of involvement of the esophageal muscle layer, resulting in dysmotility and/or incompetence of the lower esophageal sphincter, they said.
In the setting of Crohn’s disease, some patients can develop esophageal involvement from inflammation, stricturing, or fistulizing changes with granulomas seen histologically. Esophageal manifestations of Crohn’s disease tend to occur in patients with active intestinal disease.
In patients with dermatologic diseases of lichen planus or bullous disorders, dysphagia can occur because of endoscopically visible esophageal mucosal involvement. Esophageal lichen planus, in particular, can occur without skin involvement and can be difficult to define on esophageal histopathology.
The authors also advise clinicians to consider infectious and inflammatory causes of secondary achalasia during initial evaluation.
“Achalasia and EoE might coexist more commonly than what gastroenterologists think, especially in younger patients,” co–first author Chanakyaram Reddy, MD, a gastroenterologist with Baylor University Medical Center, Dallas, Texas, said in the AGA podcast.
He noted that in a recent population-based study, the estimated relative risk of EoE was over 30-fold higher in patients with achalasia aged ≤ 40 years.
“In any suspected achalasia case, it would be wise to obtain biopsies throughout the entire esophagus when the patient is off confounding medications such as PPI therapy to establish if significant esophageal eosinophilia is coexistent,” Reddy said.
“If EoE-level eosinophilia is found, it would be reasonable to consider treating medically for EoE prior to committing to achalasia-specific interventions, which often involve permanent disruption of the esophageal muscle layer,” he added.
Gabbard said this review helps the clinician think beyond gastroesophageal reflux disease (GERD) — the most common cause of esophageal dysfunction — and consider other causes for esophageal dysfunction.
“We are seeing more complex disorders affect the esophagus. It’s not just GERD and you absolutely need a high index of suspicion because you can find varying disorders to blame for many esophageal symptoms that could otherwise be thought to be just reflux,” he said.
This research had no commercial funding. Disclosures for the authors are listed with the original article. Gabbard had no relevant disclosures.
A version of this article appeared on Medscape.com.
“Many different disorders can lead to esophageal dysfunction, which is characterized by symptoms including dysphagia, odynophagia, chest pain and heartburn. These symptoms can be caused either by immune or infectious conditions and can either be localized to the esophagus or part of a larger systemic process,” co–first author Emily McGowan, MD, PhD, with the division of allergy and immunology, University of Virginia School of Medicine, Charlottesville, said in an AGA podcast.
However, without a “high index of suspicion,” these conditions can be overlooked, leading to delays in diagnosis and unnecessary procedures. “With this clinical practice update, we wanted to help providers more readily recognize these conditions so that patients can be diagnosed and treated earlier in the course of their disease,” McGowan explained.
“This is a fantastic review that highlights how many different systemic disorders can affect the esophagus,” Scott Gabbard, MD, gastroenterologist and section head at the Center for Neurogastroenterology and Motility, Cleveland Clinic, Ohio, who wasn’t involved in the review, said in an interview.
“Honestly, for the practicing gastroenterologist, this is one of those reviews that I could envision someone either saving to his or her desktop for reference or printing it and pinning it next to his or her desk,” Gabbard said.
Best Practice Advice
The clinical practice update is published in Clinical Gastroenterology and Hepatology. It includes 10 “best practice advice” statements and a table highlighting “important” considerations when evaluating patients with esophageal dysfunction.
The review authors note that esophageal dysfunction may result from localized infections — most commonly Candida, herpes simplex virus, and cytomegalovirus — or systemic immune-mediated diseases, such as systemic sclerosis (SSc), mixed connective tissue disease (MCTD), and eosinophilic esophagitis (EoE).
They advise clinicians to identify if there are risks for inflammatory or infectious possibilities for a patient’s esophageal symptoms and investigate for these disorders as a potential cause of esophageal dysfunction.
Once esophageal infection is identified, it’s important to identify whether accompanying signs and symptoms point to immunocompromise leading to a more systemic infection. Consultation with an infectious disease expert is recommended to guide appropriate treatment, the authors said.
If symptoms fail to improve after therapy for infectious esophagitis, the patient should be evaluated for refractory infection or additional underlying sources of esophageal and immunologic dysfunction is advised.
It’s also important to recognize that patients with EoE who continue to have symptoms of esophageal dysfunction despite histologic and endoscopic disease remission, may develop a motility disorder and evaluation of esophageal motility may be warranted, the authors said.
In patients with histologic and endoscopic features of lymphocytic esophagitis, treatment of lymphocytic-related inflammation with proton-pump inhibitor (PPI) therapy or swallowed topical corticosteroids and esophageal dilation as needed should be considered.
In patients who present with esophageal symptoms in the setting of hypereosinophilia (absolute eosinophil count > 1500 cells/uL), the authors advise further workup of non-EoE eosinophilic gastrointestinal disease, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis should be considered, with consultation with an allergy/immunology specialist if helpful.
In patients with rheumatologic diseases, especially SSc and MCTD, it’s important to be aware that esophageal symptoms can occur because of involvement of the esophageal muscle layer, resulting in dysmotility and/or incompetence of the lower esophageal sphincter, they said.
In the setting of Crohn’s disease, some patients can develop esophageal involvement from inflammation, stricturing, or fistulizing changes with granulomas seen histologically. Esophageal manifestations of Crohn’s disease tend to occur in patients with active intestinal disease.
In patients with dermatologic diseases of lichen planus or bullous disorders, dysphagia can occur because of endoscopically visible esophageal mucosal involvement. Esophageal lichen planus, in particular, can occur without skin involvement and can be difficult to define on esophageal histopathology.
The authors also advise clinicians to consider infectious and inflammatory causes of secondary achalasia during initial evaluation.
“Achalasia and EoE might coexist more commonly than what gastroenterologists think, especially in younger patients,” co–first author Chanakyaram Reddy, MD, a gastroenterologist with Baylor University Medical Center, Dallas, Texas, said in the AGA podcast.
He noted that in a recent population-based study, the estimated relative risk of EoE was over 30-fold higher in patients with achalasia aged ≤ 40 years.
“In any suspected achalasia case, it would be wise to obtain biopsies throughout the entire esophagus when the patient is off confounding medications such as PPI therapy to establish if significant esophageal eosinophilia is coexistent,” Reddy said.
“If EoE-level eosinophilia is found, it would be reasonable to consider treating medically for EoE prior to committing to achalasia-specific interventions, which often involve permanent disruption of the esophageal muscle layer,” he added.
Gabbard said this review helps the clinician think beyond gastroesophageal reflux disease (GERD) — the most common cause of esophageal dysfunction — and consider other causes for esophageal dysfunction.
“We are seeing more complex disorders affect the esophagus. It’s not just GERD and you absolutely need a high index of suspicion because you can find varying disorders to blame for many esophageal symptoms that could otherwise be thought to be just reflux,” he said.
This research had no commercial funding. Disclosures for the authors are listed with the original article. Gabbard had no relevant disclosures.
A version of this article appeared on Medscape.com.
“Many different disorders can lead to esophageal dysfunction, which is characterized by symptoms including dysphagia, odynophagia, chest pain and heartburn. These symptoms can be caused either by immune or infectious conditions and can either be localized to the esophagus or part of a larger systemic process,” co–first author Emily McGowan, MD, PhD, with the division of allergy and immunology, University of Virginia School of Medicine, Charlottesville, said in an AGA podcast.
However, without a “high index of suspicion,” these conditions can be overlooked, leading to delays in diagnosis and unnecessary procedures. “With this clinical practice update, we wanted to help providers more readily recognize these conditions so that patients can be diagnosed and treated earlier in the course of their disease,” McGowan explained.
“This is a fantastic review that highlights how many different systemic disorders can affect the esophagus,” Scott Gabbard, MD, gastroenterologist and section head at the Center for Neurogastroenterology and Motility, Cleveland Clinic, Ohio, who wasn’t involved in the review, said in an interview.
“Honestly, for the practicing gastroenterologist, this is one of those reviews that I could envision someone either saving to his or her desktop for reference or printing it and pinning it next to his or her desk,” Gabbard said.
Best Practice Advice
The clinical practice update is published in Clinical Gastroenterology and Hepatology. It includes 10 “best practice advice” statements and a table highlighting “important” considerations when evaluating patients with esophageal dysfunction.
The review authors note that esophageal dysfunction may result from localized infections — most commonly Candida, herpes simplex virus, and cytomegalovirus — or systemic immune-mediated diseases, such as systemic sclerosis (SSc), mixed connective tissue disease (MCTD), and eosinophilic esophagitis (EoE).
They advise clinicians to identify if there are risks for inflammatory or infectious possibilities for a patient’s esophageal symptoms and investigate for these disorders as a potential cause of esophageal dysfunction.
Once esophageal infection is identified, it’s important to identify whether accompanying signs and symptoms point to immunocompromise leading to a more systemic infection. Consultation with an infectious disease expert is recommended to guide appropriate treatment, the authors said.
If symptoms fail to improve after therapy for infectious esophagitis, the patient should be evaluated for refractory infection or additional underlying sources of esophageal and immunologic dysfunction is advised.
It’s also important to recognize that patients with EoE who continue to have symptoms of esophageal dysfunction despite histologic and endoscopic disease remission, may develop a motility disorder and evaluation of esophageal motility may be warranted, the authors said.
In patients with histologic and endoscopic features of lymphocytic esophagitis, treatment of lymphocytic-related inflammation with proton-pump inhibitor (PPI) therapy or swallowed topical corticosteroids and esophageal dilation as needed should be considered.
In patients who present with esophageal symptoms in the setting of hypereosinophilia (absolute eosinophil count > 1500 cells/uL), the authors advise further workup of non-EoE eosinophilic gastrointestinal disease, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis should be considered, with consultation with an allergy/immunology specialist if helpful.
In patients with rheumatologic diseases, especially SSc and MCTD, it’s important to be aware that esophageal symptoms can occur because of involvement of the esophageal muscle layer, resulting in dysmotility and/or incompetence of the lower esophageal sphincter, they said.
In the setting of Crohn’s disease, some patients can develop esophageal involvement from inflammation, stricturing, or fistulizing changes with granulomas seen histologically. Esophageal manifestations of Crohn’s disease tend to occur in patients with active intestinal disease.
In patients with dermatologic diseases of lichen planus or bullous disorders, dysphagia can occur because of endoscopically visible esophageal mucosal involvement. Esophageal lichen planus, in particular, can occur without skin involvement and can be difficult to define on esophageal histopathology.
The authors also advise clinicians to consider infectious and inflammatory causes of secondary achalasia during initial evaluation.
“Achalasia and EoE might coexist more commonly than what gastroenterologists think, especially in younger patients,” co–first author Chanakyaram Reddy, MD, a gastroenterologist with Baylor University Medical Center, Dallas, Texas, said in the AGA podcast.
He noted that in a recent population-based study, the estimated relative risk of EoE was over 30-fold higher in patients with achalasia aged ≤ 40 years.
“In any suspected achalasia case, it would be wise to obtain biopsies throughout the entire esophagus when the patient is off confounding medications such as PPI therapy to establish if significant esophageal eosinophilia is coexistent,” Reddy said.
“If EoE-level eosinophilia is found, it would be reasonable to consider treating medically for EoE prior to committing to achalasia-specific interventions, which often involve permanent disruption of the esophageal muscle layer,” he added.
Gabbard said this review helps the clinician think beyond gastroesophageal reflux disease (GERD) — the most common cause of esophageal dysfunction — and consider other causes for esophageal dysfunction.
“We are seeing more complex disorders affect the esophagus. It’s not just GERD and you absolutely need a high index of suspicion because you can find varying disorders to blame for many esophageal symptoms that could otherwise be thought to be just reflux,” he said.
This research had no commercial funding. Disclosures for the authors are listed with the original article. Gabbard had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Best Practices When Using POEM to Treat Achalasia: AGA Clinical Update
The American Gastroenterological Association (AGA) has released
“Any patient suspected to have achalasia, or difficulty swallowing for that matter, should undergo a comprehensive diagnostic workup, and that should include clinical history, review of medication, as well as tests. The diagnosis should not be based on isolated tests but on the clinical picture as a whole,” first author Dennis Yang, MD, AGAF, with the Center for Interventional Endoscopy, AdventHealth, Orlando, Florida, noted in an AGA podcast about the update.
The clinical practice update, published in Gastroenterology, includes 12 “best practice advice” statements.
Since its introduction to clinical practice more than a decade ago, POEM has matured and gained widespread acceptance because of its efficacy and safety profile.
POEM has at least similar outcomes to laparoscopic Heller myotomy and pneumatic dilation for type I and type II achalasia with better results for those with type III achalasia, Yang noted.
“However, besides disease phenotype, we need to remember that choosing the right treatment for the patient is going to be based on multiple factors including patient characteristics as well as local expertise,” Yang added.
In terms of technical considerations, the update states that both anterior and posterior tunnel approaches demonstrate comparable success and postprocedure reflux rates. Tunnel orientation should be tailored to the patient’s surgical history and endoscopist’s preference.
It further states that optimal length of the myotomy in the esophagus and cardia, as it pertains to treatment efficacy and risk for postprocedure reflux, remains to be determined.
Adjunct techniques, including real-time intraprocedure functional luminal impedance planimetry, may be considered to tailor or confirm the adequacy of the myotomy.
Same-day discharge after POEM can be considered in select patients who meet discharge criteria. Patients with advanced age, significant comorbidities, poor social support, and/or access to specialized care should be considered for hospital admission, irrespective of symptoms.
The update notes that specific guidelines on the role and extent of antibiotic prophylaxis before and after POEM are lacking. A single dose of antibiotics at the time of POEM “may be sufficient” for antibiotic prophylaxis.
In terms of immediate post-POEM care, the update notes that the clinical impact of routine esophagram or endoscopy immediately post-POEM remains unclear. Testing can be considered based on local practice preferences and in cases in which intraprocedural events or postprocedural findings warrant further evaluation.
Proton pump inhibitors are recommended immediately following POEM, as gastroesophageal reflux disease (GERD) is common following POEM, occurring in up to 65% of cases.
Routine endoscopic surveillance is advised to monitor GERD, disease progression, and esophageal cancer risk, which is significantly higher in achalasia patients.
“Just like diabetes and hypertension, we need to remember that achalasia is a chronic disease and long-term postprocedural surveillance is strongly encouraged to monitor disease progression as well as potential complications of reflux,” Yang said.
He noted that surveillance should be considered irrespective of patient symptoms because many of these patients may remain asymptomatic.
“Primary gastroenterologists should have a very low threshold in referring the patient back to the POEM endoscopist or any specialized esophageal center because the ideology of symptoms in these patients can be quite difficult to tease out and often require comprehensive diagnostic workup,” Yang said.
Evidence for POEM in esophagogastric outflow obstruction and other nonachalasia spastic motility disorders is limited and should only be considered on a case-by-case basis after other less invasive approaches have been exhausted, the update states.
For perspective on the POEM clinical practice update, this news organization spoke with Mouen Khashab, MD, director of therapeutic endoscopy, Johns Hopkins University, Baltimore, Maryland.
“The document is very well written and comprehensive,” Khashab said.
However, Khashab said he would have liked to see greater emphasis on the value or role of a short myotomy in the esophagus and cardia.
“There is level I evidence that the short esophageal myotomy is equivalent to a long esophageal myotomy for type I and II achalasia. When you do a short myotomy, you save procedure time and there is potentially a lower incidence of blown-out myotomy or BOM,” Khashab said.
Khashab also noted that a long myotomy on the gastric side “likely increases the risk of reflux disease, and therefore a limited myotomy on the gastric side likely also is advantageous.”
This research had no commercial funding. Yang serves as a consultant for Boston Scientific, Olympus, FujiFilm, Microtech, Medtronic, 3D-Matrix, and Neptune Medical, and has received research support from Microtech and 3D-Matrix. Khashab had no relevant disclosures.
A version of this article appeared on Medscape.com .
The American Gastroenterological Association (AGA) has released
“Any patient suspected to have achalasia, or difficulty swallowing for that matter, should undergo a comprehensive diagnostic workup, and that should include clinical history, review of medication, as well as tests. The diagnosis should not be based on isolated tests but on the clinical picture as a whole,” first author Dennis Yang, MD, AGAF, with the Center for Interventional Endoscopy, AdventHealth, Orlando, Florida, noted in an AGA podcast about the update.
The clinical practice update, published in Gastroenterology, includes 12 “best practice advice” statements.
Since its introduction to clinical practice more than a decade ago, POEM has matured and gained widespread acceptance because of its efficacy and safety profile.
POEM has at least similar outcomes to laparoscopic Heller myotomy and pneumatic dilation for type I and type II achalasia with better results for those with type III achalasia, Yang noted.
“However, besides disease phenotype, we need to remember that choosing the right treatment for the patient is going to be based on multiple factors including patient characteristics as well as local expertise,” Yang added.
In terms of technical considerations, the update states that both anterior and posterior tunnel approaches demonstrate comparable success and postprocedure reflux rates. Tunnel orientation should be tailored to the patient’s surgical history and endoscopist’s preference.
It further states that optimal length of the myotomy in the esophagus and cardia, as it pertains to treatment efficacy and risk for postprocedure reflux, remains to be determined.
Adjunct techniques, including real-time intraprocedure functional luminal impedance planimetry, may be considered to tailor or confirm the adequacy of the myotomy.
Same-day discharge after POEM can be considered in select patients who meet discharge criteria. Patients with advanced age, significant comorbidities, poor social support, and/or access to specialized care should be considered for hospital admission, irrespective of symptoms.
The update notes that specific guidelines on the role and extent of antibiotic prophylaxis before and after POEM are lacking. A single dose of antibiotics at the time of POEM “may be sufficient” for antibiotic prophylaxis.
In terms of immediate post-POEM care, the update notes that the clinical impact of routine esophagram or endoscopy immediately post-POEM remains unclear. Testing can be considered based on local practice preferences and in cases in which intraprocedural events or postprocedural findings warrant further evaluation.
Proton pump inhibitors are recommended immediately following POEM, as gastroesophageal reflux disease (GERD) is common following POEM, occurring in up to 65% of cases.
Routine endoscopic surveillance is advised to monitor GERD, disease progression, and esophageal cancer risk, which is significantly higher in achalasia patients.
“Just like diabetes and hypertension, we need to remember that achalasia is a chronic disease and long-term postprocedural surveillance is strongly encouraged to monitor disease progression as well as potential complications of reflux,” Yang said.
He noted that surveillance should be considered irrespective of patient symptoms because many of these patients may remain asymptomatic.
“Primary gastroenterologists should have a very low threshold in referring the patient back to the POEM endoscopist or any specialized esophageal center because the ideology of symptoms in these patients can be quite difficult to tease out and often require comprehensive diagnostic workup,” Yang said.
Evidence for POEM in esophagogastric outflow obstruction and other nonachalasia spastic motility disorders is limited and should only be considered on a case-by-case basis after other less invasive approaches have been exhausted, the update states.
For perspective on the POEM clinical practice update, this news organization spoke with Mouen Khashab, MD, director of therapeutic endoscopy, Johns Hopkins University, Baltimore, Maryland.
“The document is very well written and comprehensive,” Khashab said.
However, Khashab said he would have liked to see greater emphasis on the value or role of a short myotomy in the esophagus and cardia.
“There is level I evidence that the short esophageal myotomy is equivalent to a long esophageal myotomy for type I and II achalasia. When you do a short myotomy, you save procedure time and there is potentially a lower incidence of blown-out myotomy or BOM,” Khashab said.
Khashab also noted that a long myotomy on the gastric side “likely increases the risk of reflux disease, and therefore a limited myotomy on the gastric side likely also is advantageous.”
This research had no commercial funding. Yang serves as a consultant for Boston Scientific, Olympus, FujiFilm, Microtech, Medtronic, 3D-Matrix, and Neptune Medical, and has received research support from Microtech and 3D-Matrix. Khashab had no relevant disclosures.
A version of this article appeared on Medscape.com .
The American Gastroenterological Association (AGA) has released
“Any patient suspected to have achalasia, or difficulty swallowing for that matter, should undergo a comprehensive diagnostic workup, and that should include clinical history, review of medication, as well as tests. The diagnosis should not be based on isolated tests but on the clinical picture as a whole,” first author Dennis Yang, MD, AGAF, with the Center for Interventional Endoscopy, AdventHealth, Orlando, Florida, noted in an AGA podcast about the update.
The clinical practice update, published in Gastroenterology, includes 12 “best practice advice” statements.
Since its introduction to clinical practice more than a decade ago, POEM has matured and gained widespread acceptance because of its efficacy and safety profile.
POEM has at least similar outcomes to laparoscopic Heller myotomy and pneumatic dilation for type I and type II achalasia with better results for those with type III achalasia, Yang noted.
“However, besides disease phenotype, we need to remember that choosing the right treatment for the patient is going to be based on multiple factors including patient characteristics as well as local expertise,” Yang added.
In terms of technical considerations, the update states that both anterior and posterior tunnel approaches demonstrate comparable success and postprocedure reflux rates. Tunnel orientation should be tailored to the patient’s surgical history and endoscopist’s preference.
It further states that optimal length of the myotomy in the esophagus and cardia, as it pertains to treatment efficacy and risk for postprocedure reflux, remains to be determined.
Adjunct techniques, including real-time intraprocedure functional luminal impedance planimetry, may be considered to tailor or confirm the adequacy of the myotomy.
Same-day discharge after POEM can be considered in select patients who meet discharge criteria. Patients with advanced age, significant comorbidities, poor social support, and/or access to specialized care should be considered for hospital admission, irrespective of symptoms.
The update notes that specific guidelines on the role and extent of antibiotic prophylaxis before and after POEM are lacking. A single dose of antibiotics at the time of POEM “may be sufficient” for antibiotic prophylaxis.
In terms of immediate post-POEM care, the update notes that the clinical impact of routine esophagram or endoscopy immediately post-POEM remains unclear. Testing can be considered based on local practice preferences and in cases in which intraprocedural events or postprocedural findings warrant further evaluation.
Proton pump inhibitors are recommended immediately following POEM, as gastroesophageal reflux disease (GERD) is common following POEM, occurring in up to 65% of cases.
Routine endoscopic surveillance is advised to monitor GERD, disease progression, and esophageal cancer risk, which is significantly higher in achalasia patients.
“Just like diabetes and hypertension, we need to remember that achalasia is a chronic disease and long-term postprocedural surveillance is strongly encouraged to monitor disease progression as well as potential complications of reflux,” Yang said.
He noted that surveillance should be considered irrespective of patient symptoms because many of these patients may remain asymptomatic.
“Primary gastroenterologists should have a very low threshold in referring the patient back to the POEM endoscopist or any specialized esophageal center because the ideology of symptoms in these patients can be quite difficult to tease out and often require comprehensive diagnostic workup,” Yang said.
Evidence for POEM in esophagogastric outflow obstruction and other nonachalasia spastic motility disorders is limited and should only be considered on a case-by-case basis after other less invasive approaches have been exhausted, the update states.
For perspective on the POEM clinical practice update, this news organization spoke with Mouen Khashab, MD, director of therapeutic endoscopy, Johns Hopkins University, Baltimore, Maryland.
“The document is very well written and comprehensive,” Khashab said.
However, Khashab said he would have liked to see greater emphasis on the value or role of a short myotomy in the esophagus and cardia.
“There is level I evidence that the short esophageal myotomy is equivalent to a long esophageal myotomy for type I and II achalasia. When you do a short myotomy, you save procedure time and there is potentially a lower incidence of blown-out myotomy or BOM,” Khashab said.
Khashab also noted that a long myotomy on the gastric side “likely increases the risk of reflux disease, and therefore a limited myotomy on the gastric side likely also is advantageous.”
This research had no commercial funding. Yang serves as a consultant for Boston Scientific, Olympus, FujiFilm, Microtech, Medtronic, 3D-Matrix, and Neptune Medical, and has received research support from Microtech and 3D-Matrix. Khashab had no relevant disclosures.
A version of this article appeared on Medscape.com .
FROM GASTROENTEROLOGY
Improving Care for Sexual and Gender Minority Patients with Disorders of Gut-Brain Interaction
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.
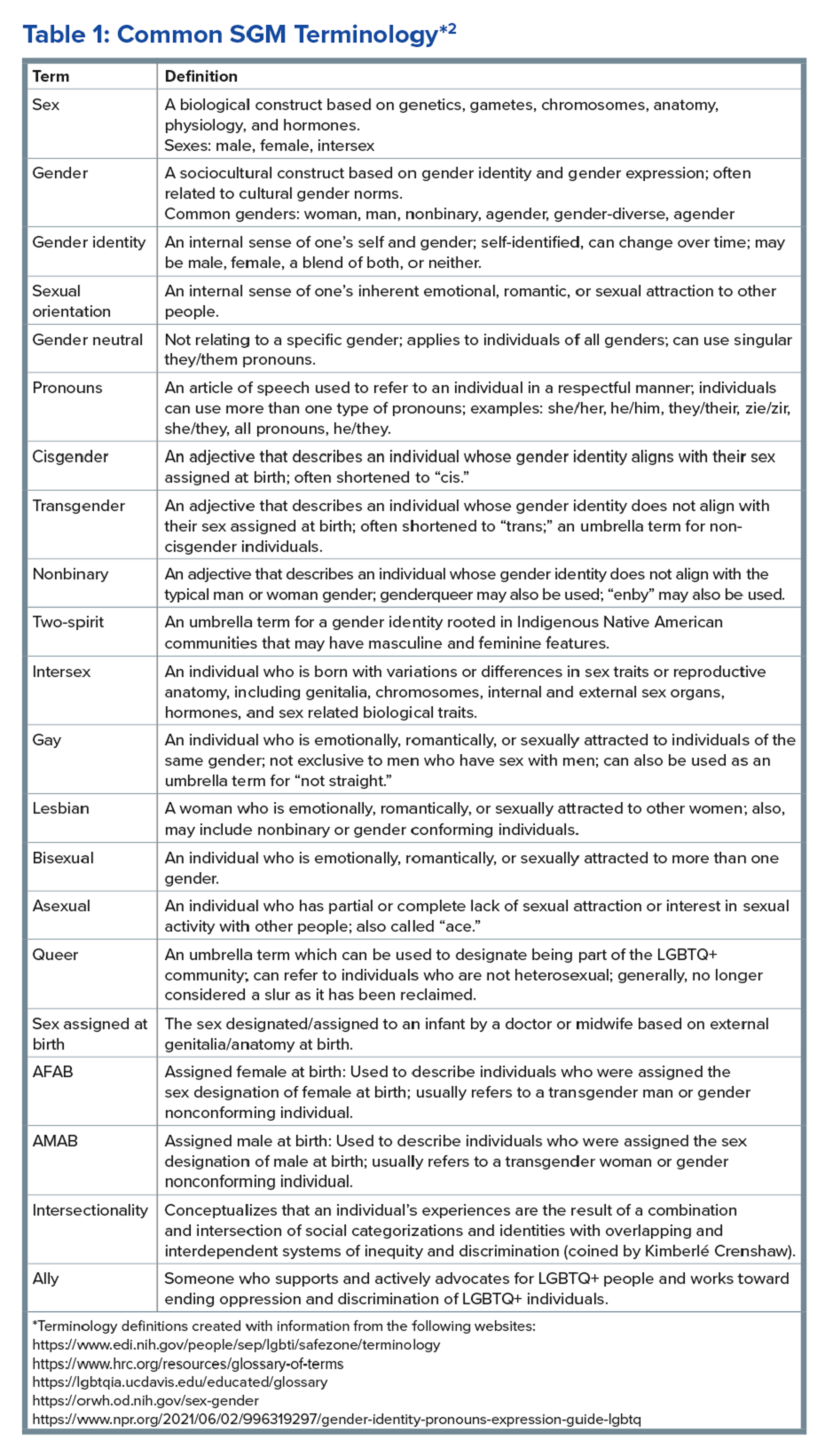
Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.
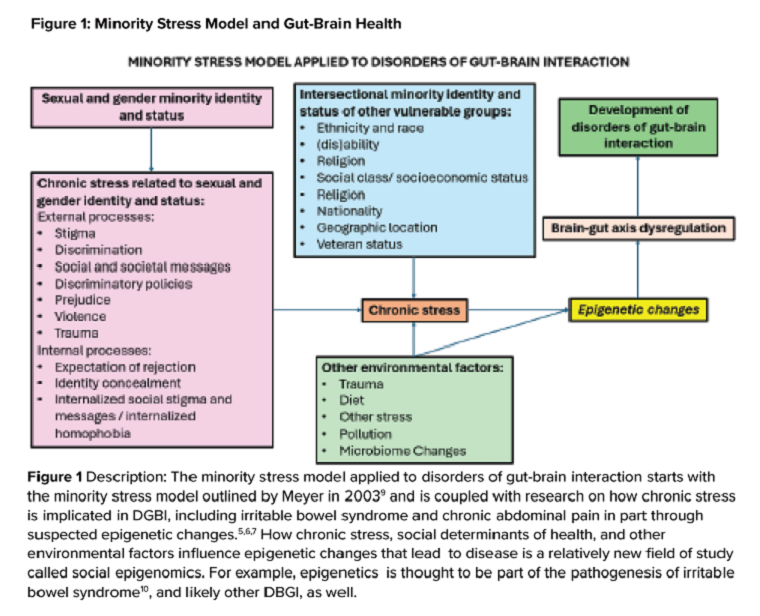
Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.
Brief Introduction to the SGM Communities
The sexual and gender minority (SGM) communities (see Table 1), also termed “LGBTQIA+ community” (lesbian, gay, bisexual, transgender, queer, intersex, asexual, plus — including two spirit) are historically minoritized with unique risks for inequities in gastrointestinal health outcomes.1 These potential disparities remain largely uninvestigated because of continued systemic discrimination and inadequate collection of sexual orientation and gender identity (SOGI) data,2 with the National Institutes of Health Sexual & Gender Minority Research Office (SGMRO) having been instructed to address these failures. There is increased SGM self-identification (7.1% of all people in the United States and 20.8% of generation Z).3 Given the high worldwide prevalence of disorders of gut-brain interaction (DGBIs)and the influence of biopsychosocial determinants of health in DGBI incidence,4 it becomes increasingly likely that research in DGBI-related factors in SGM people will be fruitful.

Disorders of Gut-Brain Interaction and the Potential Minority Stress Link in SGM People
DGBIs are gastrointestinal conditions that occur because of brain-gut axis dysregulation. There is evidence that chronic stress and trauma negatively influence brain-gut interaction, which likely results in minority communities who face increased levels of trauma, stress, discrimination, and social injustice being at higher risk of DGBI development.5-7 Given increased rates of trauma in the SGM community, practicing trauma-informed care is essential to increase patient comfort and decrease the chance of retraumatization in medical settings.8 Trauma-informed care focuses on how trauma influences a patient’s life and response to medical care. To practice trauma-informed care, screening for trauma when appropriate, actively creating a supportive environment with active listening and communication, with informing the patient of planned actions prior to doing them, like physical exams, is key.
Trauma-Informed Care: Examples of Verbiage
Asking about Identity
- Begin by introducing yourself with your pronouns to create a safe environment for patient disclosure. Example: “Hello, I am Dr. Kara Jencks, and my pronouns are she/her. I am one of the gastroenterologists here at XYZ Clinic. How would you prefer to be addressed?”
- You can also wear a pronoun lapel pin or a pronoun button on your ID badge to indicate you are someone who your patient can be themselves around.
- The easiest way to obtain sexual orientation and gender identity is through intake forms. Below are examples of how to ask these questions on intake forms. It is important to offer the option to select more than one option when applicable and to opt out of answering if the patient is not comfortable answering these questions.
Sample Questions for Intake Forms
1. What is your sex assigned at birth? (Select one)
- Female
- Male
- Intersex
- Do not know
- Prefer not to disclose
2. What is your gender identity? (Select all that apply)
- Nonbinary
- Gender queer
- Woman
- Man
- Transwoman
- Transman
- Gender fluid
- Two-spirit
- Agender
- Intersex
- Other: type in response
- Prefer not to disclose
3. What are your pronouns? (Select all that apply)
- They/them/theirs
- She/her/hers
- He/him/his
- Zie/zir/zirs
- Other: type in response
- Prefer not to disclose
4. What is your sexual orientation? (Select all that apply)
- Bisexual
- Pansexual
- Queer
- Lesbian
- Gay
- Asexual
- Demisexual
- Heterosexual or straight
- Other: type in response
- Prefer not to disclose
Screening for Trauma
While there are questionnaires that exist to ask about trauma history, if time allows, it can be helpful to screen verbally with the patient. See reference number 8, for additional prompts and actions to practice trauma-informed care.
- Example: “Many patients with gastrointestinal symptoms and disorders have experienced trauma in the past. We do our best to ensure we are keeping you as comfortable as possible while caring for you. Are you comfortable sharing this information? [if yes->] Do you have a history of trauma, including physical, emotional, or sexual abuse? ... Have these experiences impacted the way in which you navigate your healthcare? ... Is there anything we can do to make you more comfortable today?”
General Physical Examination
Provide details for what you are going to do before you do it. Ask for permission for the examination. Here are two examples:
- “I would like to perform a physical exam to help better understand your symptoms. Is that okay with you?”
- “I would like to examine your abdomen with my stethoscope and my hands. Here is a sheet that we can use to help with your privacy. Please let me know if and when you feel any tenderness or pain.”
Rectal Physical Examination
Let the patient know why it would be helpful to perform a rectal exam, what the rectal exam will entail, and the benefits and risks to doing a rectal exam. An example follows:
- “Based on the symptoms you are describing, I think it would be helpful to perform a rectal exam to make sure you don’t have any fissures or hemorrhoids on the outside around the anus, any blockages or major issues inside the rectum, and to assess the strength and ability of your nerves and muscles or the pelvic floor to coordinate bowel movements. There are no risks aside from discomfort. If it is painful, and you would like me to stop, you tell me to stop, and I will stop right away. What questions do you have? Are we okay to proceed with the rectal exam?”
- “Please pull down your undergarments and your pants to either midthigh, your ankles, or all the way off, whatever your preference is, lie down on the left side on the exam table, and cover yourself with this sheet. In the meantime, I will be getting a chaperone to keep us safe and serve as a patient advocate during the procedure.”
- Upon returning to the exam room: “Here is Sara, who will be chaperoning today. Let myself or Sara know if you are uncomfortable or having pain during this exam. I will be lifting up the sheet to get a good look around the anus. [lifts up sheet] You will feel my hand helping to spread apart the buttocks. I am looking around the anus, and I do not see any fissures, hemorrhoids, or anything else concerning. Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. Okay, now you may feel some cold gel around the anus, and you will feel my finger go inside. Take a deep breath in. Do you feel any pain as I palpate? Please squeeze in like you are trying to hold in gas. Please bear down like you are trying to have a bowel movement or let out gas. I will be stopping the exam now.”
- You would then wash your hands and allow the patient to get dressed, and then disclose the exam findings and the rest of your visit.
Ilan H. Meyer coined the minority stress model when discussing mental health disorders in SGM patients in the early 2000s.9 With it being well known that DGBIs can overlap with (but are not necessarily caused by) mental health disorders, this model can easily apply to unify multiple individual and societal factors that can combine to result in disorders of brain-gut interaction (see Figure 1) in SGM communities. Let us keep this framework in mind when evaluating the following cases.

Case Presentations
Case 1
A 56-year-old man (pronouns: he/him) assigned male sex at birth, who identifies as gay, presents to your gastroenterology clinic for treatment-refractory constipation-predominant irritable bowel syndrome. It has impacted his sexual function. Outside hospital records report a normal colonoscopy 1 year ago and an unremarkable abdominal computerized tomography 4 months ago, aside from increased stool burden in the entire colon. He has tried to use enemas prior to sex, though these do not always help. Fiber-rich diet and fermentable food avoidance has not been successful. He is currently taking two capfuls of polyethylene glycol 3350 twice per day, as well as senna at night and continues to have a bowel movement every 2-3 days that is Bristol stool form scale type 1-2 unless he uses enemas. How do you counsel this patient about his IBS-C and rectal discomfort?
After assessing for sexual violence or other potential trauma-related factors, your digital rectal examination suggests that an anorectal defecatory disorder is less likely with normal relaxation and perineal movement. You recommend linaclotide. He notices improvement within 1 week, with improved comfort during anoreceptive sex.
Case 2
A 30-year-old woman (pronouns: she/her) assigned male sex at birth who has sex with men underwent vaginoplasty 2 years ago and is referred to the gastroenterology clinic for fecal incontinence and diarrhea. On review of her anatomic inventory, her vaginoplasty was a penile inversion vaginoplasty (no intestinal tissue was used for creation), and her prostate was left intact. The vaginal vault was created in between the urethra and rectum, similar to the pelvic floor anatomy of a woman assigned female sex at birth. Blood, imaging, and endoscopic workup has been negative. She is also not taking any medications associated with diarrhea, only taking estrogen and spironolactone. The diarrhea is not daily, but when present, about once per week, can be up to 10 episodes per day, and she has a sense of incomplete evacuation regularly. She notes having a rectal exam in the past but is not sure if her pelvic floor muscles have ever been assessed. How do you manage this patient?
To complete her evaluation in the office, you perform a trauma-informed rectal exam which reveals a decreased resting anal sphincter tone and paradoxical defecatory maneuvers without tenderness to the puborectalis muscle. Augmentation of the squeeze is also weak. Given her pelvic floor related surgical history, her symptoms, and her rectal exam, you recommend anorectal manometry which is abnormal and send her for anorectal biofeedback pelvic floor physical therapy, which improves her symptoms significantly.
Case 3
A 36-year-old woman (pronouns: she/her) assigned female sex at birth, who identifies as a lesbian, has a history of posttraumatic stress disorder and chronic nausea and vomiting that has begun to affect her quality of life. She notes the nausea and vomiting used to be managed well with evening cannabis gummies, though in the past 3 months, the nausea and vomiting has worsened, and she has lost 20 pounds as a result. As symptom predated cannabis usage, cannabis hyperemesis syndrome (CHS) was less likely (an important point as she has been stigmatized during prior encounters for her cannabis usage). Her primary care physician recommended a gastroscopy which was normal, aside from some residual solid food material in the stomach. Her bowel movements are normal, and she doesn’t have other gastrointestinal symptoms. She and her wife are considering having a third child, so she is worried about medications that may affect pregnancy or breast-feeding. How do you manage her nausea and vomiting?
After validating her concerns and performing a trauma-informed physical exam and encounter, you recommend a 4-hour gastric emptying test with a standard radiolabeled egg meal. Her gastric emptying does reveal significantly delayed gastric emptying at 2 and 4 hours. You discuss the risks and benefits of lifestyle modification (smaller frequent meals), initiating medications (erythromycin and metoclopramide) or cessation of cannabis (despite low likelihood of CHS). Desiring to avoid starting medications around initiation of pregnancy, she opts for the dietary approach and cessation of cannabis. You see her at a follow-up visit in 6 months, and her nausea is now only once a month, and she is excited to begin planning for a pregnancy using assisted reproductive technology.
Case 4
A 20-year-old nonbinary intersex individual (pronouns: he/they) (incorrectly assigned female at birth — is intersex with congenital adrenal hyperplasia) presents to the gastroenterology clinic with 8 years of heartburn, acid reflux, postprandial bloating, alternating diarrhea and constipation, nausea, and vomiting, complicated by avoidant restrictive food intake disorder. They have a history of bipolar II disorder with prior suicidal ideation. He has not yet had diagnostic workup as he previously had a bad encounter with a gastroenterologist where the gastroenterologist blamed his symptoms on his gender-affirming therapy, misgendered the patient, and told the patient their symptoms were “all in her [sic] head.”
You recognize that affirming their gender and using proper pronouns is the best first way to start rapport and help break the cycle of medicalized trauma. You then recommend a holistic work up with interdisciplinary management because of the complexity of his symptoms. For testing, you recommend a colonoscopy, upper endoscopy, a gastric emptying test with a 48-hour transit scintigraphy test, anorectal manometry, a dietitian referral, and a gastrointestinal psychology referral. Their anorectal manometry is consistent with an evacuation disorder. The rest of the work up is unremarkable. You diagnose them with anorectal pelvic floor dysfunction and functional dyspepsia, recommending biofeedback pelvic floor physical therapy, a proton-pump inhibitor, and neuromodulation in coordination with psychiatry and psychology to start with a plan for follow-up. The patient appreciates you for helping them and listening to their symptoms.
Discussion
When approaching DGBIs in the SGM community, it is vital to validate their concerns and be inclusive with diagnostic and treatment modalities. The diagnostic tools and treatments for DGBI are not different for patients in the SGM community. Like with other patients, trauma-informed care should be utilized, particularly given higher rates of trauma and discrimination in this community. Importantly, their DGBI is not a result of their sexual orientation or gender identity, and hormone therapy is not the cause of their DGBI. Recommending cessation of gender-affirming care or recommending lifestyle measures against their identity is generally not appropriate or necessary. among members of the SGM communities.
Dr. Jencks (@karajencks) is based in the division of gastroenterology and hepatology, Mayo Clinic, Rochester, Minnesota. Dr. Vélez (@Chris_Velez_MD) is based in the division of gastroenterology, Massachusetts General Hospital and Harvard Medical School, both in Boston. Both authors do not have any conflicts of interest for this article.
References
1. Duong N et al. 2023 Apr. doi: 10.1016/S2468-1253(23)00005-5.
2. Vélez C et al. Am J Gastroenterol. 2022 Jun. doi: 10.14309/ajg.0000000000001804.
3. Jones JM. Gallup. LGBTQ+ identification in U.S. now at 7.6%. 2024 Mar 13. https://news.gallup.com/poll/611864/lgbtq-identification.aspx
4. Sperber AD et al. Gastroenterology. 2021 Jan. doi: 10.1053/j.gastro.2020.04.014.
5. Wiley JW et al. Neurogastroenterol Motil. 2016 Jan. doi: 10.1111/nmo.12706.
6. Labanski A et al. Psychoneuroendocrinology. 2020 Jan. doi: 10.1016/j.psyneuen.2019.104501.
7. Khlevner J et al. Gastroenterol Clin North Am. 2018 Dec. doi: 10.1016/j.gtc.2018.07.002.
8. Jagielski CH and Harer KN. Gastroenterol Clin North Am. 2022 Dec. doi: 10.1016/j.gtc.2022.07.012.
9. Meyer IH. Psychol Bull. 2003 Sep. doi: 10.1037/0033-2909.129.5.674.
10. Mahurkar-Joshi S and Chang L. Front Psychiatry. 2020 Aug. doi: 10.3389/fpsyt.2020.00805.