User login
3D Total Body Photography Shown to Decrease Biopsies, Improve Dx of Nonmelanoma Skin Cancers
ORLANDO, Fla. — A study of automated three-dimensional total-body photography (3D TBP) found that it improved “hit” rates of positive malignant biopsies and reduced unnecessary biopsies of skin lesions but left unanswered questions about the practicality of its widespread use and cost-effectiveness.
“We did observe improved biopsy practices and outcomes,” said Jordan Phillipps, MD, a dermatology resident at Mayo Clinic in Jacksonville, Florida, who reported the results of the study during a late-breaker session at the American Academy of Dermatology (AAD) 2025 Annual Meeting.
“We observed reduced unnecessary biopsies, which was driven by benign and premalignant, particularly actinic keratosis, lesions,” Phillipps said. “We observed improved malignancy detection, which was profoundly driven by nonmelanoma skin cancers.”
Study Design and Results
The retrospective study included 410 adult patients who had at least two sessions with the Vectra WB360 3D TBP imaging system at a dedicated 3D imaging clinic at Mayo Clinic in Rochester, Minnesota Patient eligibility for the 3D clinic requires a previous melanoma diagnosis. All study participants also underwent dermoscopy, Phillipps said. Their average age was 51.6 years, and 53% were women.
The study accounted for 5981 total patient encounters, including 1150 dedicated Vectra imaging sessions, Phillipps said. In this group, 3006 biopsies were performed, of which 56% were benign, 32% were malignant, and 12% were premalignant. The study also separately evaluated lesion type, focusing on keratinocytic and pigmented lesions.
Most of the keratinocytic lesions were nonmelanoma skin cancers, he said, whereas the pigmented lesions were mostly benign.
“The intervention did significantly reduce biopsies per encounter by 35%, and this was driven by benign lesions and premalignant lesions, particularly actinic keratosis lesions,” Phillipps said.
Previous studies of automated TBP have been hampered by small study populations, he said, and this is one of the largest studies of the Vectra WB360 device. “Nonmelanoma skin cancers are underreported,” Phillipps said, noting that most studies focus on melanoma and pigmented lesions. “Our aim was to assess the effect of Vectra implementation on biopsy practice and outcomes,” he explained.
For malignant lesions, the investigators observed an improvement in malignancy detection, a modest 1.6% increase in hit rates of positive malignant biopsies, and a modest 1.3% decrease in the number needed to biopsy, he said.
A subgroup analysis of pigmented and keratinocytic lesions demonstrated that improved malignancy detection is “profoundly driven by nonmelanoma skin cancers” of 71% per biopsy, Phillipps said, along with “sizable” increases in the hit rate (+17%) and a reduction in the number of biopsies (–14%).
Melanoma detection decreased by 62% per biopsy. Phillipps said the reduction was probably because of the study methodology, specifically the eligibility requirement of having had a previous melanoma diagnosis. “These patients typically develop only one primary melanoma,” Phillipps said. To test this, the investigators compared melanoma hit rates with a matched, unexposed cohort that did not have Vectra imaging. They found that the hit rates were similar. “So this was reassuring that we weren’t missing any melanomas,” Phillipps said.
The results also showed improved efficacy for detecting severely dysplastic nevi, for which the hit rate increased by 16% and the number needed to biopsy decreased by 13%. “Actinic keratoses lesions were biopsied less,” he said, noting a 50% decrease. Both benign keratinocytic lesions, predominantly seborrheic keratosis and benign lichenoid keratosis, and benign pigmented (benign nevi) lesions were biopsied less.
Limitations and Questions
The highly selective nature of the patient population was a limitation of the study, Phillipps noted, along with financial and logistical challenges that impede the generalizability of the findings. Overall, he said, the study emphasized that 3D TBP is effective in skin cancer screening and diagnosis, notably beyond pigmented lesions.
Kristina Callis Duffin, MD, MS, chair of dermatology at The University of Utah, Salt Lake City, Utah, called the findings “exciting” but noted that the study did not compare results to the gold standard of clinician-performed skin screenings. “That absolutely would be the important way to do it, through a randomized trial,” she said, “but that’s a hard study to do.”
The cost-effectiveness of total-body imaging also needs to be evaluated, Duffin said. “You really have to look at a number of factors in terms of protection compared to a human gold standard, the rates of biopsies,” she said. “There are a lot of things to unpack; that cost-effectiveness has to be balanced with a more accurate diagnosis and reduction of morbidity with multiple biopsies.”
Phillipps reported no relevant financial relationships. Duffin disclosed financial relationships with AbbVie, Alumis, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, FIDE, Janssen Pharmaceuticals, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
ORLANDO, Fla. — A study of automated three-dimensional total-body photography (3D TBP) found that it improved “hit” rates of positive malignant biopsies and reduced unnecessary biopsies of skin lesions but left unanswered questions about the practicality of its widespread use and cost-effectiveness.
“We did observe improved biopsy practices and outcomes,” said Jordan Phillipps, MD, a dermatology resident at Mayo Clinic in Jacksonville, Florida, who reported the results of the study during a late-breaker session at the American Academy of Dermatology (AAD) 2025 Annual Meeting.
“We observed reduced unnecessary biopsies, which was driven by benign and premalignant, particularly actinic keratosis, lesions,” Phillipps said. “We observed improved malignancy detection, which was profoundly driven by nonmelanoma skin cancers.”
Study Design and Results
The retrospective study included 410 adult patients who had at least two sessions with the Vectra WB360 3D TBP imaging system at a dedicated 3D imaging clinic at Mayo Clinic in Rochester, Minnesota Patient eligibility for the 3D clinic requires a previous melanoma diagnosis. All study participants also underwent dermoscopy, Phillipps said. Their average age was 51.6 years, and 53% were women.
The study accounted for 5981 total patient encounters, including 1150 dedicated Vectra imaging sessions, Phillipps said. In this group, 3006 biopsies were performed, of which 56% were benign, 32% were malignant, and 12% were premalignant. The study also separately evaluated lesion type, focusing on keratinocytic and pigmented lesions.
Most of the keratinocytic lesions were nonmelanoma skin cancers, he said, whereas the pigmented lesions were mostly benign.
“The intervention did significantly reduce biopsies per encounter by 35%, and this was driven by benign lesions and premalignant lesions, particularly actinic keratosis lesions,” Phillipps said.
Previous studies of automated TBP have been hampered by small study populations, he said, and this is one of the largest studies of the Vectra WB360 device. “Nonmelanoma skin cancers are underreported,” Phillipps said, noting that most studies focus on melanoma and pigmented lesions. “Our aim was to assess the effect of Vectra implementation on biopsy practice and outcomes,” he explained.
For malignant lesions, the investigators observed an improvement in malignancy detection, a modest 1.6% increase in hit rates of positive malignant biopsies, and a modest 1.3% decrease in the number needed to biopsy, he said.
A subgroup analysis of pigmented and keratinocytic lesions demonstrated that improved malignancy detection is “profoundly driven by nonmelanoma skin cancers” of 71% per biopsy, Phillipps said, along with “sizable” increases in the hit rate (+17%) and a reduction in the number of biopsies (–14%).
Melanoma detection decreased by 62% per biopsy. Phillipps said the reduction was probably because of the study methodology, specifically the eligibility requirement of having had a previous melanoma diagnosis. “These patients typically develop only one primary melanoma,” Phillipps said. To test this, the investigators compared melanoma hit rates with a matched, unexposed cohort that did not have Vectra imaging. They found that the hit rates were similar. “So this was reassuring that we weren’t missing any melanomas,” Phillipps said.
The results also showed improved efficacy for detecting severely dysplastic nevi, for which the hit rate increased by 16% and the number needed to biopsy decreased by 13%. “Actinic keratoses lesions were biopsied less,” he said, noting a 50% decrease. Both benign keratinocytic lesions, predominantly seborrheic keratosis and benign lichenoid keratosis, and benign pigmented (benign nevi) lesions were biopsied less.
Limitations and Questions
The highly selective nature of the patient population was a limitation of the study, Phillipps noted, along with financial and logistical challenges that impede the generalizability of the findings. Overall, he said, the study emphasized that 3D TBP is effective in skin cancer screening and diagnosis, notably beyond pigmented lesions.
Kristina Callis Duffin, MD, MS, chair of dermatology at The University of Utah, Salt Lake City, Utah, called the findings “exciting” but noted that the study did not compare results to the gold standard of clinician-performed skin screenings. “That absolutely would be the important way to do it, through a randomized trial,” she said, “but that’s a hard study to do.”
The cost-effectiveness of total-body imaging also needs to be evaluated, Duffin said. “You really have to look at a number of factors in terms of protection compared to a human gold standard, the rates of biopsies,” she said. “There are a lot of things to unpack; that cost-effectiveness has to be balanced with a more accurate diagnosis and reduction of morbidity with multiple biopsies.”
Phillipps reported no relevant financial relationships. Duffin disclosed financial relationships with AbbVie, Alumis, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, FIDE, Janssen Pharmaceuticals, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
ORLANDO, Fla. — A study of automated three-dimensional total-body photography (3D TBP) found that it improved “hit” rates of positive malignant biopsies and reduced unnecessary biopsies of skin lesions but left unanswered questions about the practicality of its widespread use and cost-effectiveness.
“We did observe improved biopsy practices and outcomes,” said Jordan Phillipps, MD, a dermatology resident at Mayo Clinic in Jacksonville, Florida, who reported the results of the study during a late-breaker session at the American Academy of Dermatology (AAD) 2025 Annual Meeting.
“We observed reduced unnecessary biopsies, which was driven by benign and premalignant, particularly actinic keratosis, lesions,” Phillipps said. “We observed improved malignancy detection, which was profoundly driven by nonmelanoma skin cancers.”
Study Design and Results
The retrospective study included 410 adult patients who had at least two sessions with the Vectra WB360 3D TBP imaging system at a dedicated 3D imaging clinic at Mayo Clinic in Rochester, Minnesota Patient eligibility for the 3D clinic requires a previous melanoma diagnosis. All study participants also underwent dermoscopy, Phillipps said. Their average age was 51.6 years, and 53% were women.
The study accounted for 5981 total patient encounters, including 1150 dedicated Vectra imaging sessions, Phillipps said. In this group, 3006 biopsies were performed, of which 56% were benign, 32% were malignant, and 12% were premalignant. The study also separately evaluated lesion type, focusing on keratinocytic and pigmented lesions.
Most of the keratinocytic lesions were nonmelanoma skin cancers, he said, whereas the pigmented lesions were mostly benign.
“The intervention did significantly reduce biopsies per encounter by 35%, and this was driven by benign lesions and premalignant lesions, particularly actinic keratosis lesions,” Phillipps said.
Previous studies of automated TBP have been hampered by small study populations, he said, and this is one of the largest studies of the Vectra WB360 device. “Nonmelanoma skin cancers are underreported,” Phillipps said, noting that most studies focus on melanoma and pigmented lesions. “Our aim was to assess the effect of Vectra implementation on biopsy practice and outcomes,” he explained.
For malignant lesions, the investigators observed an improvement in malignancy detection, a modest 1.6% increase in hit rates of positive malignant biopsies, and a modest 1.3% decrease in the number needed to biopsy, he said.
A subgroup analysis of pigmented and keratinocytic lesions demonstrated that improved malignancy detection is “profoundly driven by nonmelanoma skin cancers” of 71% per biopsy, Phillipps said, along with “sizable” increases in the hit rate (+17%) and a reduction in the number of biopsies (–14%).
Melanoma detection decreased by 62% per biopsy. Phillipps said the reduction was probably because of the study methodology, specifically the eligibility requirement of having had a previous melanoma diagnosis. “These patients typically develop only one primary melanoma,” Phillipps said. To test this, the investigators compared melanoma hit rates with a matched, unexposed cohort that did not have Vectra imaging. They found that the hit rates were similar. “So this was reassuring that we weren’t missing any melanomas,” Phillipps said.
The results also showed improved efficacy for detecting severely dysplastic nevi, for which the hit rate increased by 16% and the number needed to biopsy decreased by 13%. “Actinic keratoses lesions were biopsied less,” he said, noting a 50% decrease. Both benign keratinocytic lesions, predominantly seborrheic keratosis and benign lichenoid keratosis, and benign pigmented (benign nevi) lesions were biopsied less.
Limitations and Questions
The highly selective nature of the patient population was a limitation of the study, Phillipps noted, along with financial and logistical challenges that impede the generalizability of the findings. Overall, he said, the study emphasized that 3D TBP is effective in skin cancer screening and diagnosis, notably beyond pigmented lesions.
Kristina Callis Duffin, MD, MS, chair of dermatology at The University of Utah, Salt Lake City, Utah, called the findings “exciting” but noted that the study did not compare results to the gold standard of clinician-performed skin screenings. “That absolutely would be the important way to do it, through a randomized trial,” she said, “but that’s a hard study to do.”
The cost-effectiveness of total-body imaging also needs to be evaluated, Duffin said. “You really have to look at a number of factors in terms of protection compared to a human gold standard, the rates of biopsies,” she said. “There are a lot of things to unpack; that cost-effectiveness has to be balanced with a more accurate diagnosis and reduction of morbidity with multiple biopsies.”
Phillipps reported no relevant financial relationships. Duffin disclosed financial relationships with AbbVie, Alumis, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, FIDE, Janssen Pharmaceuticals, Novartis, and Pfizer.
A version of this article first appeared on Medscape.com.
FROM AAD 2025
ACG Issues First Guidance on Diagnosis and Management of Gastric Premalignant Conditions
including atrophic gastritis, gastric intestinal metaplasia, dysplasia, and certain gastric epithelial polyps, all of which have an increased risk of progressing to gastric cancer.
The guideline was published online in The American Journal of Gastroenterology.
GPMCs are “common in gastroenterology practices, but in the US, at least, we’ve not had concrete guidance,” first author Douglas Morgan, MD, MPH, AGAF, Division of Gastroenterology, The University of Alabama at Birmingham, noted in an interview.
With these guidelines, we hope there “will be a paradigm shift to finally establish surveillance in the stomach, much like we’ve been doing for decades in the colon and the esophagus,” Morgan said.
Gastric cancer is a common cancer in the United States with disproportionately higher incidence rates in immigrants from countries with a high incidence of gastric cancer and certain non-White populations.
In addition, the 5-year survival rate in the United States for gastric cancer is 36%, which falls short of global standards and is driven by the fact that only a small percentage of these cancers are diagnosed in the early, curable stage.
These guidelines will help address this marked disparity and the burden on minority and marginalized populations, the guideline authors wrote. “The overarching goals are to reduce [gastric cancer] incidence in the United States, increase the detection of early-stage disease (early gastric cancer), and to significantly increase the 5-year survival rates in the near term.”
Key Recommendations
The guideline includes recommendations on endoscopic surveillance for high-risk patients, the performance of high-quality endoscopy and image-enhanced endoscopy (IEE) for diagnosis and surveillance, GPMC histology criteria and reporting, endoscopic treatment of dysplasia, the role of Helicobacter pylori eradication, general risk reduction measures, and the management of autoimmune gastritis and gastric epithelial polyps.
In terms of screening, the guidelines recommend against routine upper endoscopy screening for gastric cancer and GPMC in the general US population (low quality of evidence; conditional recommendation).
They also noted that there is “insufficient” direct US evidence to make a recommendation on opportunistic endoscopy screening for gastric cancer and GPMC in patients deemed at high risk based on race/ethnicity and family history. In addition, they recommend against the use of noninvasive biomarkers for screening or surveillance of GPMC or gastric cancer.
In terms of endoscopic and histologic diagnosis of GPMC, high-quality endoscopic evaluation is crucial to detect premalignant conditions or gastric cancer, the authors said. This includes adequate mucosal cleansing and insufflation, and photodocumentation of anatomic landmarks, as well as use of high-definition white light endoscopy (HDWLE) and IEE.
Systematic gastric biopsies should follow the updated Sydney protocol, with at least two separate containers for antrum/incisura and corpus biopsies. Histology should document the subtype of gastric intestinal metaplasia — incomplete, complete, or mixed — and severity and extent of atrophic gastritis and metaplasia.
Morgan emphasized the importance of coordination between the gastroenterologist and pathologist. “Several of these measures are not routinely reported, so we need to be in conversations with our collaborating pathologists,” he told this news organization.
In terms of GPMC surveillance, the authors suggest surveillance endoscopy every 3 years in high-risk patients with gastric intestinal metaplasia who meet one of the following criteria: High-risk histology (incomplete vs complete subtype, extending into the corpus); family history of gastric cancer in a first-degree relative; foreign-born individuals from high-gastric cancer incidence nations; or high-risk race/ethnicity (East Asian, Latino/a, Black, American Indian, or Alaska Native).
Individuals with multiple risk factors for gastric cancer may be considered for shorter than 3-year intervals.
Low-risk gastric intestinal metaplasia (limited to antrum, mild atrophy, and complete gastric intestinal metaplasia subtype) does not require routine endoscopic surveillance.
In terms of endoscopic management of dysplastic GPMC, endoscopic resection is suggested for dysplasia with visible margins. If dysplasia is not visible, repeat endoscopy with HDWLE and IEE by an experienced endoscopist is advised.
In patients appropriate for endoscopic resection of dysplasia, particularly endoscopic submucosal dissection, referral to a high-volume center with appropriate expertise in the diagnosis and therapeutic resection of gastric neoplasia is strongly recommended.
In patients with confirmed complete resection of dysplasia, endoscopic surveillance is also strongly recommended. Surveillance examinations should be performed by an experienced endoscopist using HDWLE and IEE, with biopsies according to the systematic biopsy protocol in addition to targeted biopsies.
In terms of nonendoscopic GPMC management, testing for H pylori (and eradication treatment if possible) is strongly recommended for patients with GPMC and those with a history of resected early gastric cancer.
Aspirin, nonsteroidal anti-inflammatory drugs, cyclooxygenase 2 inhibitors, or antioxidants are not recommended for patients with GPMC for the purpose of preventing gastric cancer.
In patients with autoimmune gastritis, testing for H pylori with a nonserological test, eradication treatment if positive, and posttreatment testing to confirm eradication is advised.
There is not enough evidence to make a formal recommendation on endoscopic surveillance in those with autoimmune gastritis; surveillance should be individualized, considering the risk for neuroendocrine tumors and possibly gastric cancer.
In terms of gastric epithelial polyps, endoscopic resection of all gastric adenomas is recommended, regardless of size, to exclude or prevent dysplasia and early gastric cancer. Adenomas that are not amenable to endoscopic resection should be referred for surgical resection, if clinically appropriate.
Morgan noted that the ACG GPMC guideline aligns with the updated ACG/American Society for Gastrointestinal Endoscopy upper endoscopy quality indicators released earlier this year.
Implementation of the ACG GPMC guideline and “change in clinical practice will require concrete targets and include training and quality initiatives,” the authors said.
This research received no commercial support. Morgan disclosed research support from Panbela Therapeutics, Thorne, and American Molecular Laboratories.
A version of this article first appeared on Medscape.com.
including atrophic gastritis, gastric intestinal metaplasia, dysplasia, and certain gastric epithelial polyps, all of which have an increased risk of progressing to gastric cancer.
The guideline was published online in The American Journal of Gastroenterology.
GPMCs are “common in gastroenterology practices, but in the US, at least, we’ve not had concrete guidance,” first author Douglas Morgan, MD, MPH, AGAF, Division of Gastroenterology, The University of Alabama at Birmingham, noted in an interview.
With these guidelines, we hope there “will be a paradigm shift to finally establish surveillance in the stomach, much like we’ve been doing for decades in the colon and the esophagus,” Morgan said.
Gastric cancer is a common cancer in the United States with disproportionately higher incidence rates in immigrants from countries with a high incidence of gastric cancer and certain non-White populations.
In addition, the 5-year survival rate in the United States for gastric cancer is 36%, which falls short of global standards and is driven by the fact that only a small percentage of these cancers are diagnosed in the early, curable stage.
These guidelines will help address this marked disparity and the burden on minority and marginalized populations, the guideline authors wrote. “The overarching goals are to reduce [gastric cancer] incidence in the United States, increase the detection of early-stage disease (early gastric cancer), and to significantly increase the 5-year survival rates in the near term.”
Key Recommendations
The guideline includes recommendations on endoscopic surveillance for high-risk patients, the performance of high-quality endoscopy and image-enhanced endoscopy (IEE) for diagnosis and surveillance, GPMC histology criteria and reporting, endoscopic treatment of dysplasia, the role of Helicobacter pylori eradication, general risk reduction measures, and the management of autoimmune gastritis and gastric epithelial polyps.
In terms of screening, the guidelines recommend against routine upper endoscopy screening for gastric cancer and GPMC in the general US population (low quality of evidence; conditional recommendation).
They also noted that there is “insufficient” direct US evidence to make a recommendation on opportunistic endoscopy screening for gastric cancer and GPMC in patients deemed at high risk based on race/ethnicity and family history. In addition, they recommend against the use of noninvasive biomarkers for screening or surveillance of GPMC or gastric cancer.
In terms of endoscopic and histologic diagnosis of GPMC, high-quality endoscopic evaluation is crucial to detect premalignant conditions or gastric cancer, the authors said. This includes adequate mucosal cleansing and insufflation, and photodocumentation of anatomic landmarks, as well as use of high-definition white light endoscopy (HDWLE) and IEE.
Systematic gastric biopsies should follow the updated Sydney protocol, with at least two separate containers for antrum/incisura and corpus biopsies. Histology should document the subtype of gastric intestinal metaplasia — incomplete, complete, or mixed — and severity and extent of atrophic gastritis and metaplasia.
Morgan emphasized the importance of coordination between the gastroenterologist and pathologist. “Several of these measures are not routinely reported, so we need to be in conversations with our collaborating pathologists,” he told this news organization.
In terms of GPMC surveillance, the authors suggest surveillance endoscopy every 3 years in high-risk patients with gastric intestinal metaplasia who meet one of the following criteria: High-risk histology (incomplete vs complete subtype, extending into the corpus); family history of gastric cancer in a first-degree relative; foreign-born individuals from high-gastric cancer incidence nations; or high-risk race/ethnicity (East Asian, Latino/a, Black, American Indian, or Alaska Native).
Individuals with multiple risk factors for gastric cancer may be considered for shorter than 3-year intervals.
Low-risk gastric intestinal metaplasia (limited to antrum, mild atrophy, and complete gastric intestinal metaplasia subtype) does not require routine endoscopic surveillance.
In terms of endoscopic management of dysplastic GPMC, endoscopic resection is suggested for dysplasia with visible margins. If dysplasia is not visible, repeat endoscopy with HDWLE and IEE by an experienced endoscopist is advised.
In patients appropriate for endoscopic resection of dysplasia, particularly endoscopic submucosal dissection, referral to a high-volume center with appropriate expertise in the diagnosis and therapeutic resection of gastric neoplasia is strongly recommended.
In patients with confirmed complete resection of dysplasia, endoscopic surveillance is also strongly recommended. Surveillance examinations should be performed by an experienced endoscopist using HDWLE and IEE, with biopsies according to the systematic biopsy protocol in addition to targeted biopsies.
In terms of nonendoscopic GPMC management, testing for H pylori (and eradication treatment if possible) is strongly recommended for patients with GPMC and those with a history of resected early gastric cancer.
Aspirin, nonsteroidal anti-inflammatory drugs, cyclooxygenase 2 inhibitors, or antioxidants are not recommended for patients with GPMC for the purpose of preventing gastric cancer.
In patients with autoimmune gastritis, testing for H pylori with a nonserological test, eradication treatment if positive, and posttreatment testing to confirm eradication is advised.
There is not enough evidence to make a formal recommendation on endoscopic surveillance in those with autoimmune gastritis; surveillance should be individualized, considering the risk for neuroendocrine tumors and possibly gastric cancer.
In terms of gastric epithelial polyps, endoscopic resection of all gastric adenomas is recommended, regardless of size, to exclude or prevent dysplasia and early gastric cancer. Adenomas that are not amenable to endoscopic resection should be referred for surgical resection, if clinically appropriate.
Morgan noted that the ACG GPMC guideline aligns with the updated ACG/American Society for Gastrointestinal Endoscopy upper endoscopy quality indicators released earlier this year.
Implementation of the ACG GPMC guideline and “change in clinical practice will require concrete targets and include training and quality initiatives,” the authors said.
This research received no commercial support. Morgan disclosed research support from Panbela Therapeutics, Thorne, and American Molecular Laboratories.
A version of this article first appeared on Medscape.com.
including atrophic gastritis, gastric intestinal metaplasia, dysplasia, and certain gastric epithelial polyps, all of which have an increased risk of progressing to gastric cancer.
The guideline was published online in The American Journal of Gastroenterology.
GPMCs are “common in gastroenterology practices, but in the US, at least, we’ve not had concrete guidance,” first author Douglas Morgan, MD, MPH, AGAF, Division of Gastroenterology, The University of Alabama at Birmingham, noted in an interview.
With these guidelines, we hope there “will be a paradigm shift to finally establish surveillance in the stomach, much like we’ve been doing for decades in the colon and the esophagus,” Morgan said.
Gastric cancer is a common cancer in the United States with disproportionately higher incidence rates in immigrants from countries with a high incidence of gastric cancer and certain non-White populations.
In addition, the 5-year survival rate in the United States for gastric cancer is 36%, which falls short of global standards and is driven by the fact that only a small percentage of these cancers are diagnosed in the early, curable stage.
These guidelines will help address this marked disparity and the burden on minority and marginalized populations, the guideline authors wrote. “The overarching goals are to reduce [gastric cancer] incidence in the United States, increase the detection of early-stage disease (early gastric cancer), and to significantly increase the 5-year survival rates in the near term.”
Key Recommendations
The guideline includes recommendations on endoscopic surveillance for high-risk patients, the performance of high-quality endoscopy and image-enhanced endoscopy (IEE) for diagnosis and surveillance, GPMC histology criteria and reporting, endoscopic treatment of dysplasia, the role of Helicobacter pylori eradication, general risk reduction measures, and the management of autoimmune gastritis and gastric epithelial polyps.
In terms of screening, the guidelines recommend against routine upper endoscopy screening for gastric cancer and GPMC in the general US population (low quality of evidence; conditional recommendation).
They also noted that there is “insufficient” direct US evidence to make a recommendation on opportunistic endoscopy screening for gastric cancer and GPMC in patients deemed at high risk based on race/ethnicity and family history. In addition, they recommend against the use of noninvasive biomarkers for screening or surveillance of GPMC or gastric cancer.
In terms of endoscopic and histologic diagnosis of GPMC, high-quality endoscopic evaluation is crucial to detect premalignant conditions or gastric cancer, the authors said. This includes adequate mucosal cleansing and insufflation, and photodocumentation of anatomic landmarks, as well as use of high-definition white light endoscopy (HDWLE) and IEE.
Systematic gastric biopsies should follow the updated Sydney protocol, with at least two separate containers for antrum/incisura and corpus biopsies. Histology should document the subtype of gastric intestinal metaplasia — incomplete, complete, or mixed — and severity and extent of atrophic gastritis and metaplasia.
Morgan emphasized the importance of coordination between the gastroenterologist and pathologist. “Several of these measures are not routinely reported, so we need to be in conversations with our collaborating pathologists,” he told this news organization.
In terms of GPMC surveillance, the authors suggest surveillance endoscopy every 3 years in high-risk patients with gastric intestinal metaplasia who meet one of the following criteria: High-risk histology (incomplete vs complete subtype, extending into the corpus); family history of gastric cancer in a first-degree relative; foreign-born individuals from high-gastric cancer incidence nations; or high-risk race/ethnicity (East Asian, Latino/a, Black, American Indian, or Alaska Native).
Individuals with multiple risk factors for gastric cancer may be considered for shorter than 3-year intervals.
Low-risk gastric intestinal metaplasia (limited to antrum, mild atrophy, and complete gastric intestinal metaplasia subtype) does not require routine endoscopic surveillance.
In terms of endoscopic management of dysplastic GPMC, endoscopic resection is suggested for dysplasia with visible margins. If dysplasia is not visible, repeat endoscopy with HDWLE and IEE by an experienced endoscopist is advised.
In patients appropriate for endoscopic resection of dysplasia, particularly endoscopic submucosal dissection, referral to a high-volume center with appropriate expertise in the diagnosis and therapeutic resection of gastric neoplasia is strongly recommended.
In patients with confirmed complete resection of dysplasia, endoscopic surveillance is also strongly recommended. Surveillance examinations should be performed by an experienced endoscopist using HDWLE and IEE, with biopsies according to the systematic biopsy protocol in addition to targeted biopsies.
In terms of nonendoscopic GPMC management, testing for H pylori (and eradication treatment if possible) is strongly recommended for patients with GPMC and those with a history of resected early gastric cancer.
Aspirin, nonsteroidal anti-inflammatory drugs, cyclooxygenase 2 inhibitors, or antioxidants are not recommended for patients with GPMC for the purpose of preventing gastric cancer.
In patients with autoimmune gastritis, testing for H pylori with a nonserological test, eradication treatment if positive, and posttreatment testing to confirm eradication is advised.
There is not enough evidence to make a formal recommendation on endoscopic surveillance in those with autoimmune gastritis; surveillance should be individualized, considering the risk for neuroendocrine tumors and possibly gastric cancer.
In terms of gastric epithelial polyps, endoscopic resection of all gastric adenomas is recommended, regardless of size, to exclude or prevent dysplasia and early gastric cancer. Adenomas that are not amenable to endoscopic resection should be referred for surgical resection, if clinically appropriate.
Morgan noted that the ACG GPMC guideline aligns with the updated ACG/American Society for Gastrointestinal Endoscopy upper endoscopy quality indicators released earlier this year.
Implementation of the ACG GPMC guideline and “change in clinical practice will require concrete targets and include training and quality initiatives,” the authors said.
This research received no commercial support. Morgan disclosed research support from Panbela Therapeutics, Thorne, and American Molecular Laboratories.
A version of this article first appeared on Medscape.com.
AI-Enhanced Echocardiography: A Game-Changer for Opportunistic Liver Disease Detection?
An AI algorithm called EchoNet-Liver demonstrated strong performance for detecting cirrhosis and steatotic liver disease (SLD) from routinely acquired transthoracic echocardiography studies containing subcostal images of the liver, the developers reported in NEJM AI.
“We hope that this algorithm enables physicians to opportunistically screen for chronic liver disease to identify asymptomatic and undiagnosed patients, thus enabling us to treat comorbidities relevant to the patient’s cardiovascular and noncardiovascular health,” Alan C. Kwan, MD, assistant professor, Department of Cardiology, Smidt Heart Institute at Cedars-Sinai, Los Angeles, California, told this news organization.
Harnessing Echo to Reveal Liver Trouble
CLD affects over 1.5 billion people globally, with many cases remaining undiagnosed due to the asymptomatic nature of early disease and a lack of routine screening. Traditional diagnostic methods such as liver function tests, ultrasonography, and MRI are often limited by cost, availability, and patient access.
Echocardiography is a commonly performed imaging study that incidentally captures images of the liver but is not utilized for liver disease assessment.
EchoNet-Liver is an AI algorithm that can identify high-quality subcostal images from full echocardiography studies and detect the presence of cirrhosis and SLD.
Kwan and colleagues trained it using nearly 1.6 million echocardiogram videos from 66,922 studies and 24,276 adult patients at Cedars-Sinai Medical Center (CSMC). The model predictions were compared with diagnoses from clinical evaluations of paired abdominal ultrasound or MRI studies. External validation studies were conducted using similar data from Stanford Health Care.
In the “held-out” CSMC ultrasound dataset, EchoNet-Liver detected cirrhosis with an area under the receiver operating characteristic curve (AUROC) of 0.837 (95% CI, 0.828-0.848) and SLD with an AUROC of 0.799 (95% CI, 0.788-0.811).
The algorithm showed a sensitivity of 69.6% and a specificity of 84.6% for detecting cirrhosis, and a sensitivity of 74.1% and a specificity of 72.0% for detecting SLD.
In the Stanford Health Care external-validation test ultrasound cohort, the model detected cirrhosis with an AUROC of 0.830 (95% CI, 0.799-0.859) and SLD with an AUROC of 0.769 (95% CI, 0.733-0.813), with sensitivity and specificity of 80.0% and 70.9%, respectively, for cirrhosis and 66.7% and 78.0%, respectively, for SLD.
In the CSMC MRI-paired cohort, EchoNet-Liver detected cirrhosis with an AUROC of 0.704 (95% CI, 0.699-0.708) and SLD with an AUROC of 0.725 (95% CI, 0.707-0.762).
Identifying Subclinical Liver Disease to Improve Outcomes
“Across diverse populations and disease definitions, deep-learning-enhanced echocardiography enabled high-throughput, automated detection of CLD, which could enable opportunistic screening for asymptomatic liver disease,” the authors wrote.
“By improving the diagnosis of subclinical CLD, we may be able to limit or reverse disease progression and improve care by triaging patients toward appropriate clinical and diagnostic management,” they said.
By way of limitations, the researchers noted that the tool was developed using a cohort of patients who had both abdominal ultrasound and echocardiography within 30 days, and thus probably had a higher prevalence of liver disease compared with the general population receiving echocardiography. The true clinical utility of EchoNet-Liver will depend on whether its application to a general echocardiography population can efficiently detect undiagnosed CLD, they cautioned.
“While we developed this algorithm based on clinical data, the application within the clinic would typically require FDA approval, which we have not yet applied for,” Kwan told this news organization.
“We plan to prospectively validate this algorithm at multiple sites to ensure that application of this algorithm improves patient care without causing excess diagnostic testing, thus providing value to patients and the healthcare system as a whole,” Kwan said.
Funding was provided in part by KAKENHI (Japan Society for the Promotion of Science). Kwan reported receiving consulting fees from InVision.
A version of this article first appeared on Medscape.com.
An AI algorithm called EchoNet-Liver demonstrated strong performance for detecting cirrhosis and steatotic liver disease (SLD) from routinely acquired transthoracic echocardiography studies containing subcostal images of the liver, the developers reported in NEJM AI.
“We hope that this algorithm enables physicians to opportunistically screen for chronic liver disease to identify asymptomatic and undiagnosed patients, thus enabling us to treat comorbidities relevant to the patient’s cardiovascular and noncardiovascular health,” Alan C. Kwan, MD, assistant professor, Department of Cardiology, Smidt Heart Institute at Cedars-Sinai, Los Angeles, California, told this news organization.
Harnessing Echo to Reveal Liver Trouble
CLD affects over 1.5 billion people globally, with many cases remaining undiagnosed due to the asymptomatic nature of early disease and a lack of routine screening. Traditional diagnostic methods such as liver function tests, ultrasonography, and MRI are often limited by cost, availability, and patient access.
Echocardiography is a commonly performed imaging study that incidentally captures images of the liver but is not utilized for liver disease assessment.
EchoNet-Liver is an AI algorithm that can identify high-quality subcostal images from full echocardiography studies and detect the presence of cirrhosis and SLD.
Kwan and colleagues trained it using nearly 1.6 million echocardiogram videos from 66,922 studies and 24,276 adult patients at Cedars-Sinai Medical Center (CSMC). The model predictions were compared with diagnoses from clinical evaluations of paired abdominal ultrasound or MRI studies. External validation studies were conducted using similar data from Stanford Health Care.
In the “held-out” CSMC ultrasound dataset, EchoNet-Liver detected cirrhosis with an area under the receiver operating characteristic curve (AUROC) of 0.837 (95% CI, 0.828-0.848) and SLD with an AUROC of 0.799 (95% CI, 0.788-0.811).
The algorithm showed a sensitivity of 69.6% and a specificity of 84.6% for detecting cirrhosis, and a sensitivity of 74.1% and a specificity of 72.0% for detecting SLD.
In the Stanford Health Care external-validation test ultrasound cohort, the model detected cirrhosis with an AUROC of 0.830 (95% CI, 0.799-0.859) and SLD with an AUROC of 0.769 (95% CI, 0.733-0.813), with sensitivity and specificity of 80.0% and 70.9%, respectively, for cirrhosis and 66.7% and 78.0%, respectively, for SLD.
In the CSMC MRI-paired cohort, EchoNet-Liver detected cirrhosis with an AUROC of 0.704 (95% CI, 0.699-0.708) and SLD with an AUROC of 0.725 (95% CI, 0.707-0.762).
Identifying Subclinical Liver Disease to Improve Outcomes
“Across diverse populations and disease definitions, deep-learning-enhanced echocardiography enabled high-throughput, automated detection of CLD, which could enable opportunistic screening for asymptomatic liver disease,” the authors wrote.
“By improving the diagnosis of subclinical CLD, we may be able to limit or reverse disease progression and improve care by triaging patients toward appropriate clinical and diagnostic management,” they said.
By way of limitations, the researchers noted that the tool was developed using a cohort of patients who had both abdominal ultrasound and echocardiography within 30 days, and thus probably had a higher prevalence of liver disease compared with the general population receiving echocardiography. The true clinical utility of EchoNet-Liver will depend on whether its application to a general echocardiography population can efficiently detect undiagnosed CLD, they cautioned.
“While we developed this algorithm based on clinical data, the application within the clinic would typically require FDA approval, which we have not yet applied for,” Kwan told this news organization.
“We plan to prospectively validate this algorithm at multiple sites to ensure that application of this algorithm improves patient care without causing excess diagnostic testing, thus providing value to patients and the healthcare system as a whole,” Kwan said.
Funding was provided in part by KAKENHI (Japan Society for the Promotion of Science). Kwan reported receiving consulting fees from InVision.
A version of this article first appeared on Medscape.com.
An AI algorithm called EchoNet-Liver demonstrated strong performance for detecting cirrhosis and steatotic liver disease (SLD) from routinely acquired transthoracic echocardiography studies containing subcostal images of the liver, the developers reported in NEJM AI.
“We hope that this algorithm enables physicians to opportunistically screen for chronic liver disease to identify asymptomatic and undiagnosed patients, thus enabling us to treat comorbidities relevant to the patient’s cardiovascular and noncardiovascular health,” Alan C. Kwan, MD, assistant professor, Department of Cardiology, Smidt Heart Institute at Cedars-Sinai, Los Angeles, California, told this news organization.
Harnessing Echo to Reveal Liver Trouble
CLD affects over 1.5 billion people globally, with many cases remaining undiagnosed due to the asymptomatic nature of early disease and a lack of routine screening. Traditional diagnostic methods such as liver function tests, ultrasonography, and MRI are often limited by cost, availability, and patient access.
Echocardiography is a commonly performed imaging study that incidentally captures images of the liver but is not utilized for liver disease assessment.
EchoNet-Liver is an AI algorithm that can identify high-quality subcostal images from full echocardiography studies and detect the presence of cirrhosis and SLD.
Kwan and colleagues trained it using nearly 1.6 million echocardiogram videos from 66,922 studies and 24,276 adult patients at Cedars-Sinai Medical Center (CSMC). The model predictions were compared with diagnoses from clinical evaluations of paired abdominal ultrasound or MRI studies. External validation studies were conducted using similar data from Stanford Health Care.
In the “held-out” CSMC ultrasound dataset, EchoNet-Liver detected cirrhosis with an area under the receiver operating characteristic curve (AUROC) of 0.837 (95% CI, 0.828-0.848) and SLD with an AUROC of 0.799 (95% CI, 0.788-0.811).
The algorithm showed a sensitivity of 69.6% and a specificity of 84.6% for detecting cirrhosis, and a sensitivity of 74.1% and a specificity of 72.0% for detecting SLD.
In the Stanford Health Care external-validation test ultrasound cohort, the model detected cirrhosis with an AUROC of 0.830 (95% CI, 0.799-0.859) and SLD with an AUROC of 0.769 (95% CI, 0.733-0.813), with sensitivity and specificity of 80.0% and 70.9%, respectively, for cirrhosis and 66.7% and 78.0%, respectively, for SLD.
In the CSMC MRI-paired cohort, EchoNet-Liver detected cirrhosis with an AUROC of 0.704 (95% CI, 0.699-0.708) and SLD with an AUROC of 0.725 (95% CI, 0.707-0.762).
Identifying Subclinical Liver Disease to Improve Outcomes
“Across diverse populations and disease definitions, deep-learning-enhanced echocardiography enabled high-throughput, automated detection of CLD, which could enable opportunistic screening for asymptomatic liver disease,” the authors wrote.
“By improving the diagnosis of subclinical CLD, we may be able to limit or reverse disease progression and improve care by triaging patients toward appropriate clinical and diagnostic management,” they said.
By way of limitations, the researchers noted that the tool was developed using a cohort of patients who had both abdominal ultrasound and echocardiography within 30 days, and thus probably had a higher prevalence of liver disease compared with the general population receiving echocardiography. The true clinical utility of EchoNet-Liver will depend on whether its application to a general echocardiography population can efficiently detect undiagnosed CLD, they cautioned.
“While we developed this algorithm based on clinical data, the application within the clinic would typically require FDA approval, which we have not yet applied for,” Kwan told this news organization.
“We plan to prospectively validate this algorithm at multiple sites to ensure that application of this algorithm improves patient care without causing excess diagnostic testing, thus providing value to patients and the healthcare system as a whole,” Kwan said.
Funding was provided in part by KAKENHI (Japan Society for the Promotion of Science). Kwan reported receiving consulting fees from InVision.
A version of this article first appeared on Medscape.com.
Stool Test Detects Sensitivity to Food Additives
Diets in wealthier countries often include processed foods that contain additives, particularly emulsifiers. These additives are increasingly associated with the development of various diseases, including inflammatory bowel disease (IBD).
A research team led by Benoit Chassaing, PhD, research director at the French National Institute of Health and Medical Research (Inserm), focused on one such emulsifier — carboxymethylcellulose (CMC) — which is commonly found in processed baked goods, such as brioche and sandwich bread, and ice cream.
The study, published in the journal Gut, describes how the team developed a new method that uses a simple stool sample to predict an individual’s sensitivity to CMC.
Sensitivity Detection
In a previous clinical trial conducted on healthy volunteers, Chassaing and colleagues found that CMC consumption altered the gut microbiota and fecal metabolome in some healthy individuals. In mice, transplanting fecal microbiota from CMC-sensitive animals made other animals susceptible. This has led researchers to investigate the characteristics of sensitive microbiota.
To explore this, the researchers developed an in vitro microbiota model capable of replicating multiple healthy human microbiota. CMC sensitivity was tested using this model, and the findings were validated in vivo by transplanting microbiota classified as sensitive or resistant to mice. Only mice that received microbiota predicted to be CMC-sensitive developed severe colitis after consuming CMC.
Predictive Signature
Next, the team analyzed the stool metagenomes of individuals with microbiotas classified as sensitive or resistant to CMC. They identified a specific microbial signature that could predict whether a given microbiota would react negatively to emulsifiers. Using molecular analyses, this signature allows researchers to predict whether an individual’s microbiota is susceptible or resistant to CMC exposure.
For the research team, these findings open the possibility of determining whether an individual is sensitive to a particular emulsifier, allowing for personalized dietary recommendations. This is particularly relevant for patients with chronic IBD and may also help prevent these conditions in those not previously affected.
These findings could pave the way for personalized dietary recommendations, particularly for patients with chronic IBD.
To further validate these insights, the team is launching a cohort study in patients with Crohn’s to explore why some individuals are more susceptible to food additives than others.
This story was translated from Univadis France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Diets in wealthier countries often include processed foods that contain additives, particularly emulsifiers. These additives are increasingly associated with the development of various diseases, including inflammatory bowel disease (IBD).
A research team led by Benoit Chassaing, PhD, research director at the French National Institute of Health and Medical Research (Inserm), focused on one such emulsifier — carboxymethylcellulose (CMC) — which is commonly found in processed baked goods, such as brioche and sandwich bread, and ice cream.
The study, published in the journal Gut, describes how the team developed a new method that uses a simple stool sample to predict an individual’s sensitivity to CMC.
Sensitivity Detection
In a previous clinical trial conducted on healthy volunteers, Chassaing and colleagues found that CMC consumption altered the gut microbiota and fecal metabolome in some healthy individuals. In mice, transplanting fecal microbiota from CMC-sensitive animals made other animals susceptible. This has led researchers to investigate the characteristics of sensitive microbiota.
To explore this, the researchers developed an in vitro microbiota model capable of replicating multiple healthy human microbiota. CMC sensitivity was tested using this model, and the findings were validated in vivo by transplanting microbiota classified as sensitive or resistant to mice. Only mice that received microbiota predicted to be CMC-sensitive developed severe colitis after consuming CMC.
Predictive Signature
Next, the team analyzed the stool metagenomes of individuals with microbiotas classified as sensitive or resistant to CMC. They identified a specific microbial signature that could predict whether a given microbiota would react negatively to emulsifiers. Using molecular analyses, this signature allows researchers to predict whether an individual’s microbiota is susceptible or resistant to CMC exposure.
For the research team, these findings open the possibility of determining whether an individual is sensitive to a particular emulsifier, allowing for personalized dietary recommendations. This is particularly relevant for patients with chronic IBD and may also help prevent these conditions in those not previously affected.
These findings could pave the way for personalized dietary recommendations, particularly for patients with chronic IBD.
To further validate these insights, the team is launching a cohort study in patients with Crohn’s to explore why some individuals are more susceptible to food additives than others.
This story was translated from Univadis France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Diets in wealthier countries often include processed foods that contain additives, particularly emulsifiers. These additives are increasingly associated with the development of various diseases, including inflammatory bowel disease (IBD).
A research team led by Benoit Chassaing, PhD, research director at the French National Institute of Health and Medical Research (Inserm), focused on one such emulsifier — carboxymethylcellulose (CMC) — which is commonly found in processed baked goods, such as brioche and sandwich bread, and ice cream.
The study, published in the journal Gut, describes how the team developed a new method that uses a simple stool sample to predict an individual’s sensitivity to CMC.
Sensitivity Detection
In a previous clinical trial conducted on healthy volunteers, Chassaing and colleagues found that CMC consumption altered the gut microbiota and fecal metabolome in some healthy individuals. In mice, transplanting fecal microbiota from CMC-sensitive animals made other animals susceptible. This has led researchers to investigate the characteristics of sensitive microbiota.
To explore this, the researchers developed an in vitro microbiota model capable of replicating multiple healthy human microbiota. CMC sensitivity was tested using this model, and the findings were validated in vivo by transplanting microbiota classified as sensitive or resistant to mice. Only mice that received microbiota predicted to be CMC-sensitive developed severe colitis after consuming CMC.
Predictive Signature
Next, the team analyzed the stool metagenomes of individuals with microbiotas classified as sensitive or resistant to CMC. They identified a specific microbial signature that could predict whether a given microbiota would react negatively to emulsifiers. Using molecular analyses, this signature allows researchers to predict whether an individual’s microbiota is susceptible or resistant to CMC exposure.
For the research team, these findings open the possibility of determining whether an individual is sensitive to a particular emulsifier, allowing for personalized dietary recommendations. This is particularly relevant for patients with chronic IBD and may also help prevent these conditions in those not previously affected.
These findings could pave the way for personalized dietary recommendations, particularly for patients with chronic IBD.
To further validate these insights, the team is launching a cohort study in patients with Crohn’s to explore why some individuals are more susceptible to food additives than others.
This story was translated from Univadis France using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
FROM GUT
Better Prep, Better Scope: Task Force Updates Colonoscopy Bowel Prep Advice
The latest consensus recommendations emphasize the importance of verbal and written patient education, refine diet restrictions, update optimal purgative regimens, and advise tracking bowel prep adequacy rates at both the individual endoscopist and unit levels.
“Colorectal cancer remains the second most common cause of cancer death in the United States, and colonoscopy is considered the gold standard for evaluating the colon, including assessing causes of colon-related signs or symptoms and the detection of precancerous lesions. It is well recognized that the adequacy of bowel preparation is essential for optimal colonoscopy performance,” the task force wrote.
Choice of Prep, Dosing and Timing, and Dietary Restrictions
When choosing bowel preparation regimens, the task force recommends considering the individual’s medical history, medications, and, when available, the adequacy of bowel preparation reported from prior colonoscopies. Other considerations include patient preference, associated additional costs to the patient, and ease in obtaining and consuming any purgatives or adjuncts.
In terms of timing and dose, the task force now “suggests that lower-volume bowel preparation regimens, such as those that rely on only 2 liters of fluid compared to the traditional 4L, are acceptable options for individuals considered unlikely to have an inadequate bowel preparation. This assumes that the purgative is taken in a split-dose fashion (half the evening prior to colonoscopy and half the morning of the colonoscopy),” co–lead author Brian C. Jacobson, MD, MPH, AGAF, with Massachusetts General Hospital and Harvard Medical School, both in Boston, said in an interview.
The task force also states that a same-day bowel preparation regimen for afternoon, but not morning, colonoscopy is a “reasonable alternative to the now-common split-dose regimen,” Jacobson said.
The group did not find one bowel preparation purgative to be better than others, although table 7 in the document details characteristics of commonly used prep regimens including their side effects and contraindications.
Recommendations regarding dietary modifications depend upon the patient’s risk for inadequate bowel prep. For patients at low risk for inadequate bowel prep, the task force recommends limiting dietary restrictions to the day before a colonoscopy, relying on either clear liquids or low-fiber/low-residue diets for the early and midday meals. Table 5 in the document provides a list of low-residue foods and sample meals.
The task force also suggests the adjunctive use of oral simethicone (≥ 320 mg) to bowel prep as a way to potentially improve visualization, although they acknowledge that further research is needed.
How might these updated consensus recommendations change current clinical practice?
Jacobson said: “Some physicians may try to identify individuals who will do just as well with a more patient-friendly, easily tolerated bowel preparation regimen, including less stringent dietary restrictions leading up to colonoscopy.”
He noted that the task force prefers the term “guidance” to “guidelines.”
New Quality Benchmark
The task force recommends documenting bowel prep quality in the endoscopy report after all washing and suctioning have been completed using reliably understood descriptors that communicate the adequacy of the preparation.
They recommend the term “adequate bowel preparation” be used to indicate that standard screening or surveillance intervals can be assigned based on the findings of the colonoscopy.
Additionally, the task force recommends that endoscopy units and individual endoscopists track and aim for ≥ 90% adequacy rates in bowel preparation — up from the 85% benchmark contained in the prior recommendations.
Jacobson told this news organization it’s “currently unknown” how many individual endoscopists and endoscopy units track and meet the 90% benchmark at present.
David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, who wasn’t on the task force, said endoscopy units and providers “need to be accountable and should be tracking this quality metric.”
Johnson noted that bowel prep inadequacy has “intrinsic costs,” impacting lesion detection, CRC incidence, and patient outcomes. Inadequate prep leads to “increased risk for morbidity, mortality, longer appointment and wait times for rescheduling, and negative connotations that may deter patients from returning.”
Brian Sullivan, MD, MHS, assistant professor of medicine, division of gastroenterology, Duke University School of Medicine, Durham, North Carolina, who wasn’t on the task force, said the recommendation to target a 90% or higher bowel preparation adequacy rate is “appreciated.”
“This benchmark encourages practices to standardize measurement, tracking, and reporting of preparation quality at both the individual and unit levels. Specifically, it should motivate providers to critically evaluate their interpretation of preparation quality and ensure adequate cleansing before making determinations,” Sullivan said in an interview.
“At the unit level, this metric can identify whether there are opportunities for quality improvement, such as by implementing evidence-based initiatives (provided in the guidance) to enhance outpatient preparation processes,” Sullivan noted.
The task force emphasized that the majority of consensus recommendations focus on individuals at average risk for inadequate bowel prep. Patients at high risk for inadequate bowel prep (eg, diabetes, constipation, opioid use) should receive tailored instructions, including a more extended dietary prep and high-volume purgatives.
‘Timely and Important’ Updates
Sullivan said the updated consensus recommendations on optimizing bowel preparation quality for colonoscopy are both “timely and important.”
“Clear guidance facilitates dissemination and adoption, promoting flexible yet evidence-based approaches that enhance patient and provider satisfaction while potentially improving CRC prevention outcomes. For instance, surveys reveal that some practices still do not utilize split-dose bowel preparation, which is proven to improve preparation quality, particularly for the right-side of the colon. This gap underscores the need for standardized guidance to ensure high-quality colonoscopy and effective CRC screening,” Sullivan said.
He also noted that the inclusion of lower-volume bowel prep regimens and less intensive dietary modifications for selected patients is a “welcome update.”
“These options can improve patient adherence and satisfaction, which are critical not only for the quality of the index exam but also for ensuring patients return for future screenings, thereby supporting long-term CRC prevention efforts,” Sullivan said.
The task force includes representatives from the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy.
The consensus document was published online in the three societies’ respective scientific journals — Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endsocopy.
This research had no financial support. Jacobson is a consultant for Curis and Guardant Health. Sullivan had no disclosures. Johnson is an adviser to ISOThrive and a past president of the American College of Gastroenterology.
A version of this article first appeared on Medscape.com.
The latest consensus recommendations emphasize the importance of verbal and written patient education, refine diet restrictions, update optimal purgative regimens, and advise tracking bowel prep adequacy rates at both the individual endoscopist and unit levels.
“Colorectal cancer remains the second most common cause of cancer death in the United States, and colonoscopy is considered the gold standard for evaluating the colon, including assessing causes of colon-related signs or symptoms and the detection of precancerous lesions. It is well recognized that the adequacy of bowel preparation is essential for optimal colonoscopy performance,” the task force wrote.
Choice of Prep, Dosing and Timing, and Dietary Restrictions
When choosing bowel preparation regimens, the task force recommends considering the individual’s medical history, medications, and, when available, the adequacy of bowel preparation reported from prior colonoscopies. Other considerations include patient preference, associated additional costs to the patient, and ease in obtaining and consuming any purgatives or adjuncts.
In terms of timing and dose, the task force now “suggests that lower-volume bowel preparation regimens, such as those that rely on only 2 liters of fluid compared to the traditional 4L, are acceptable options for individuals considered unlikely to have an inadequate bowel preparation. This assumes that the purgative is taken in a split-dose fashion (half the evening prior to colonoscopy and half the morning of the colonoscopy),” co–lead author Brian C. Jacobson, MD, MPH, AGAF, with Massachusetts General Hospital and Harvard Medical School, both in Boston, said in an interview.
The task force also states that a same-day bowel preparation regimen for afternoon, but not morning, colonoscopy is a “reasonable alternative to the now-common split-dose regimen,” Jacobson said.
The group did not find one bowel preparation purgative to be better than others, although table 7 in the document details characteristics of commonly used prep regimens including their side effects and contraindications.
Recommendations regarding dietary modifications depend upon the patient’s risk for inadequate bowel prep. For patients at low risk for inadequate bowel prep, the task force recommends limiting dietary restrictions to the day before a colonoscopy, relying on either clear liquids or low-fiber/low-residue diets for the early and midday meals. Table 5 in the document provides a list of low-residue foods and sample meals.
The task force also suggests the adjunctive use of oral simethicone (≥ 320 mg) to bowel prep as a way to potentially improve visualization, although they acknowledge that further research is needed.
How might these updated consensus recommendations change current clinical practice?
Jacobson said: “Some physicians may try to identify individuals who will do just as well with a more patient-friendly, easily tolerated bowel preparation regimen, including less stringent dietary restrictions leading up to colonoscopy.”
He noted that the task force prefers the term “guidance” to “guidelines.”
New Quality Benchmark
The task force recommends documenting bowel prep quality in the endoscopy report after all washing and suctioning have been completed using reliably understood descriptors that communicate the adequacy of the preparation.
They recommend the term “adequate bowel preparation” be used to indicate that standard screening or surveillance intervals can be assigned based on the findings of the colonoscopy.
Additionally, the task force recommends that endoscopy units and individual endoscopists track and aim for ≥ 90% adequacy rates in bowel preparation — up from the 85% benchmark contained in the prior recommendations.
Jacobson told this news organization it’s “currently unknown” how many individual endoscopists and endoscopy units track and meet the 90% benchmark at present.
David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, who wasn’t on the task force, said endoscopy units and providers “need to be accountable and should be tracking this quality metric.”
Johnson noted that bowel prep inadequacy has “intrinsic costs,” impacting lesion detection, CRC incidence, and patient outcomes. Inadequate prep leads to “increased risk for morbidity, mortality, longer appointment and wait times for rescheduling, and negative connotations that may deter patients from returning.”
Brian Sullivan, MD, MHS, assistant professor of medicine, division of gastroenterology, Duke University School of Medicine, Durham, North Carolina, who wasn’t on the task force, said the recommendation to target a 90% or higher bowel preparation adequacy rate is “appreciated.”
“This benchmark encourages practices to standardize measurement, tracking, and reporting of preparation quality at both the individual and unit levels. Specifically, it should motivate providers to critically evaluate their interpretation of preparation quality and ensure adequate cleansing before making determinations,” Sullivan said in an interview.
“At the unit level, this metric can identify whether there are opportunities for quality improvement, such as by implementing evidence-based initiatives (provided in the guidance) to enhance outpatient preparation processes,” Sullivan noted.
The task force emphasized that the majority of consensus recommendations focus on individuals at average risk for inadequate bowel prep. Patients at high risk for inadequate bowel prep (eg, diabetes, constipation, opioid use) should receive tailored instructions, including a more extended dietary prep and high-volume purgatives.
‘Timely and Important’ Updates
Sullivan said the updated consensus recommendations on optimizing bowel preparation quality for colonoscopy are both “timely and important.”
“Clear guidance facilitates dissemination and adoption, promoting flexible yet evidence-based approaches that enhance patient and provider satisfaction while potentially improving CRC prevention outcomes. For instance, surveys reveal that some practices still do not utilize split-dose bowel preparation, which is proven to improve preparation quality, particularly for the right-side of the colon. This gap underscores the need for standardized guidance to ensure high-quality colonoscopy and effective CRC screening,” Sullivan said.
He also noted that the inclusion of lower-volume bowel prep regimens and less intensive dietary modifications for selected patients is a “welcome update.”
“These options can improve patient adherence and satisfaction, which are critical not only for the quality of the index exam but also for ensuring patients return for future screenings, thereby supporting long-term CRC prevention efforts,” Sullivan said.
The task force includes representatives from the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy.
The consensus document was published online in the three societies’ respective scientific journals — Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endsocopy.
This research had no financial support. Jacobson is a consultant for Curis and Guardant Health. Sullivan had no disclosures. Johnson is an adviser to ISOThrive and a past president of the American College of Gastroenterology.
A version of this article first appeared on Medscape.com.
The latest consensus recommendations emphasize the importance of verbal and written patient education, refine diet restrictions, update optimal purgative regimens, and advise tracking bowel prep adequacy rates at both the individual endoscopist and unit levels.
“Colorectal cancer remains the second most common cause of cancer death in the United States, and colonoscopy is considered the gold standard for evaluating the colon, including assessing causes of colon-related signs or symptoms and the detection of precancerous lesions. It is well recognized that the adequacy of bowel preparation is essential for optimal colonoscopy performance,” the task force wrote.
Choice of Prep, Dosing and Timing, and Dietary Restrictions
When choosing bowel preparation regimens, the task force recommends considering the individual’s medical history, medications, and, when available, the adequacy of bowel preparation reported from prior colonoscopies. Other considerations include patient preference, associated additional costs to the patient, and ease in obtaining and consuming any purgatives or adjuncts.
In terms of timing and dose, the task force now “suggests that lower-volume bowel preparation regimens, such as those that rely on only 2 liters of fluid compared to the traditional 4L, are acceptable options for individuals considered unlikely to have an inadequate bowel preparation. This assumes that the purgative is taken in a split-dose fashion (half the evening prior to colonoscopy and half the morning of the colonoscopy),” co–lead author Brian C. Jacobson, MD, MPH, AGAF, with Massachusetts General Hospital and Harvard Medical School, both in Boston, said in an interview.
The task force also states that a same-day bowel preparation regimen for afternoon, but not morning, colonoscopy is a “reasonable alternative to the now-common split-dose regimen,” Jacobson said.
The group did not find one bowel preparation purgative to be better than others, although table 7 in the document details characteristics of commonly used prep regimens including their side effects and contraindications.
Recommendations regarding dietary modifications depend upon the patient’s risk for inadequate bowel prep. For patients at low risk for inadequate bowel prep, the task force recommends limiting dietary restrictions to the day before a colonoscopy, relying on either clear liquids or low-fiber/low-residue diets for the early and midday meals. Table 5 in the document provides a list of low-residue foods and sample meals.
The task force also suggests the adjunctive use of oral simethicone (≥ 320 mg) to bowel prep as a way to potentially improve visualization, although they acknowledge that further research is needed.
How might these updated consensus recommendations change current clinical practice?
Jacobson said: “Some physicians may try to identify individuals who will do just as well with a more patient-friendly, easily tolerated bowel preparation regimen, including less stringent dietary restrictions leading up to colonoscopy.”
He noted that the task force prefers the term “guidance” to “guidelines.”
New Quality Benchmark
The task force recommends documenting bowel prep quality in the endoscopy report after all washing and suctioning have been completed using reliably understood descriptors that communicate the adequacy of the preparation.
They recommend the term “adequate bowel preparation” be used to indicate that standard screening or surveillance intervals can be assigned based on the findings of the colonoscopy.
Additionally, the task force recommends that endoscopy units and individual endoscopists track and aim for ≥ 90% adequacy rates in bowel preparation — up from the 85% benchmark contained in the prior recommendations.
Jacobson told this news organization it’s “currently unknown” how many individual endoscopists and endoscopy units track and meet the 90% benchmark at present.
David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, who wasn’t on the task force, said endoscopy units and providers “need to be accountable and should be tracking this quality metric.”
Johnson noted that bowel prep inadequacy has “intrinsic costs,” impacting lesion detection, CRC incidence, and patient outcomes. Inadequate prep leads to “increased risk for morbidity, mortality, longer appointment and wait times for rescheduling, and negative connotations that may deter patients from returning.”
Brian Sullivan, MD, MHS, assistant professor of medicine, division of gastroenterology, Duke University School of Medicine, Durham, North Carolina, who wasn’t on the task force, said the recommendation to target a 90% or higher bowel preparation adequacy rate is “appreciated.”
“This benchmark encourages practices to standardize measurement, tracking, and reporting of preparation quality at both the individual and unit levels. Specifically, it should motivate providers to critically evaluate their interpretation of preparation quality and ensure adequate cleansing before making determinations,” Sullivan said in an interview.
“At the unit level, this metric can identify whether there are opportunities for quality improvement, such as by implementing evidence-based initiatives (provided in the guidance) to enhance outpatient preparation processes,” Sullivan noted.
The task force emphasized that the majority of consensus recommendations focus on individuals at average risk for inadequate bowel prep. Patients at high risk for inadequate bowel prep (eg, diabetes, constipation, opioid use) should receive tailored instructions, including a more extended dietary prep and high-volume purgatives.
‘Timely and Important’ Updates
Sullivan said the updated consensus recommendations on optimizing bowel preparation quality for colonoscopy are both “timely and important.”
“Clear guidance facilitates dissemination and adoption, promoting flexible yet evidence-based approaches that enhance patient and provider satisfaction while potentially improving CRC prevention outcomes. For instance, surveys reveal that some practices still do not utilize split-dose bowel preparation, which is proven to improve preparation quality, particularly for the right-side of the colon. This gap underscores the need for standardized guidance to ensure high-quality colonoscopy and effective CRC screening,” Sullivan said.
He also noted that the inclusion of lower-volume bowel prep regimens and less intensive dietary modifications for selected patients is a “welcome update.”
“These options can improve patient adherence and satisfaction, which are critical not only for the quality of the index exam but also for ensuring patients return for future screenings, thereby supporting long-term CRC prevention efforts,” Sullivan said.
The task force includes representatives from the American Gastroenterological Association, the American College of Gastroenterology, and the American Society for Gastrointestinal Endoscopy.
The consensus document was published online in the three societies’ respective scientific journals — Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endsocopy.
This research had no financial support. Jacobson is a consultant for Curis and Guardant Health. Sullivan had no disclosures. Johnson is an adviser to ISOThrive and a past president of the American College of Gastroenterology.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
A Common Pancreatic Condition That Few Have Heard Of
— a disorder experienced by roughly one fifth of the world’s population. Although it is more common than type 2 diabetes, pancreatitis, and pancreatic cancer combined, it has remained relatively obscure.
By contrast, fatty liver — once called nonalcoholic fatty liver disease and recently renamed metabolic dysfunction–associated steatotic liver disease (MASLD) — is well-known.
“When it comes to diseases of the liver and pancreas, the liver is the big brother that has gotten all the attention, while the pancreas is the neglected little stepbrother that’s not sufficiently profiled in most medical textbooks and gets very little attention,” Max Petrov, MD, MPH, PhD, professor of pancreatology, University of Auckland, New Zealand, said in an interview. “The phenomenon of fatty pancreas has been observed for decades, but it is underappreciated and underrecognized.”
As early as 1926, fat depositions were identified during autopsies, but the condition remained relatively unknown, Mohammad Bilal, MD, associate professor of medicine-gastroenterology, University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Fortunately, FPD has recently been receiving more focus.”
Generally, healthy individuals have small amounts of fat in their pancreas. IPFD is defined as “the diffuse presence of fat in the pancreas, measured on a continuous scale,” and FPD refers to IPFD above the upper limit of normal. While there is no clear consensus as to what the normal range is, studies suggest it’s a pancreatic fat content ranging from 1.8% to 10.4%.
FPD’s “most important implication is that it can be a precursor for more challenging and burdensome diseases of the pancreas,” Petrov said.
Fatty changes in the pancreas affect both its endocrine and exocrine systems. FPD is associated with type 2 diabetes, the most common disease of the endocrine pancreas, as well as pancreatitis and pancreatic cancer, the most common diseases of the exocrine pancreas. It’s also implicated in the development of carotid atherosclerosis, pancreatic fistula following surgery, and exocrine pancreatic insufficiency (EPI).
A ‘Pandora’s Box’
Up to half of people with fatty pancreas are lean. The condition isn’t merely caused by an overflow of fat from the liver into the pancreas in people who consume more calories than they burn, Petrov said. Neither robust postmortem nor biopsy studies have found a statistically significant association between fatty deposition in the pancreas and liver fat.
Compared with the way people accumulate liver fat, the development of FPD is more complex, Petrov said.
“Hepatic fat is a relatively simple process: Lipid droplets accumulate in the hepatocytes; but, in the pancreas, there are several ways by which fat may accumulate,” he said.
One relates to the location of the pancreas within visceral, retroperitoneal fat, Petrov said. That fat can migrate and build up between pancreatic lobules.
Fat also can accumulate inside the lobes. This process can involve a buildup of fat droplets in acinar and stellate cells on the exocrine side and in the islets of Langerhans on the endocrine side. Additionally, when functional pancreatic cells die, particularly acinar cells, adult stem cells may replace them with adipocytes. Transformation of acinar cells into fat cells — a process called acinar-to-adipocyte transdifferentiation — also may be a way fat accumulates inside the lobes, Petrov said.
The accumulation of fat is a response to a wide array of insults to the pancreas over time. For example, obesity and metabolic syndrome lead to the accumulation of adipocytes and fat infiltration, whereas alcohol abuse and viral infections may lead to the death of acinar cells, which produce digestive enzymes.
Ultimately, the negative changes produced by excess fat in the pancreas are the origin of all common noninherited pancreatic diseases, bringing them under one umbrella, Petrov maintained. He dubbed this hypothesis PANcreatic Diseases Originating from intRapancreatic fAt (PANDORA).
The type of cells involved has implications for which disease may arise. For example, fat infiltration in stellate cells may promote pancreatic cancer, whereas its accumulation in the islets of Langerhans, which produce insulin and glucagon, is associated with type 2 diabetes.
The PANDORA hypothesis has eight foundational principles:
- Fatty pancreas is a key driver of pancreatic diseases in most people.
- Inflammation within the pancreatic microenvironment results from overwhelming lipotoxicity fueled by fatty pancreas.
- Aberrant communication between acinar cells involving lipid droplets drives acute pancreatitis.
- The pancreas responds to lipotoxicity with fibrosis and calcification — the hallmarks of chronic pancreatitis.
- Fat deposition affects signaling between stellate cells and other components of the microenvironment in ways that raise the risk for pancreatic cancer.
- The development of diabetes of the exocrine pancreas and EPI is affected by the presence of fatty pancreas.
- The higher risk for pancreatic disease in older adults is influenced by fatty pancreas.
- The multipronged nature of intrapancreatic fat deposition accounts for the common development of one pancreatic disease after another.
The idea that all common pancreatic diseases are the result of pathways emanating from FPD could “explain the bidirectional relationship between diabetes and pancreatitis or pancreatic cancer,” Petrov said.
Risk Factors, Symptoms, and Diagnosis
A variety of risk factors are involved in the accumulation of fat that may lead to pancreatic diseases, including aging, cholelithiasis, dyslipidemia, drugs/toxins (eg, steroids), genetic predisposition, iron overload, diet (eg, fatty foods, ultraprocessed foods), heavy alcohol use, overweight/obesity, pancreatic duct obstruction, tobacco use, viral infection (eg, hepatitis B, COVID-19), severe malnutrition, prediabetes, and dysglycemia.
Petrov described FPD as a “silent disease” that’s often asymptomatic, with its presence emerging as an incidental finding during abdominal ultrasonography for other reasons. However, patients may sometimes experience stomach pain or nausea if they have concurrent diseases of the pancreas, he said.
There are no currently available lab tests that can definitively detect the presence of FPD. Rather, the gold standard for a noninvasive diagnosis of FPD is MRI, with CT as the second-best choice, Petrov said.
In countries where advanced imaging is not available, a low-cost alternative might be a simple abdominal ultrasound, but it is not definitive, he said. “It’s operator-dependent and can be subjective.”
Some risk factors, such as derangements of glucose and lipid metabolism, especially in the presence of heavy alcohol use and a high-fat diet, can “be detected on lab tests,” Petrov said. “This, in combination with the abdominal ultrasound, might suggest the patients will benefit from deeper investigation, including MRI.”
Because the exocrine pancreas helps with digestion of fatty food, intralobular fatty deposits or replacement of pancreatic exocrine cells with adipose cells can lead to steatorrhea, Bilal said.
“Fat within the stool or oily diarrhea is a clue to the presence of FPD,” Bilal said.
Although this symptom isn’t unique to FPD and is found in other types of pancreatic conditions, its presence suggests that further investigation for FPD is warranted, he added.
Common-Sense Treatment Approaches
At present, there are no US Food and Drug Administration–approved treatments for FPD, Petrov said.
“What might be recommended is something along the lines of treatment of MASLD — appropriate diet and physical activity,” he said. Petrov hopes that as the disease entity garners more research attention, more clinical drug trials will be initiated, and new medications are found and approved.
Petrov suggested that there could be a “theoretical rationale” for the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) as a treatment, given their effectiveness in multiple conditions, including MASLD, but no human trials have robustly shown specific benefits of these drugs for FPD.
Petrov added that, to date, 12 classes of drugs have been investigated for reducing IPFD: biguanides, sulfonylureas, GLP-1 RAs, thiazolidinediones, dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 inhibitors, statins, fibrates, pancreatic lipase inhibitors, angiotensin II receptor blockers, somatostatin receptor agonists, and antioxidants.
Of these, most have shown promise in preclinical animal models. But only thiazolidinediones, GLP-1 RAs, DPP-4 inhibitors, and somatostatin receptor agonists have been investigated in randomized controlled trials in humans. The findings have been inconsistent, with the active treatment often not achieving statistically significant improvements.
“At this stage of our knowledge, we can’t recommend a specific pharmacotherapy,” Petrov said. But we can suggest dietary changes, such as saturated fat reduction, alcohol reduction, smoking cessation, reduction in consumption of ultraprocessed food, physical exercise, and addressing obesity and other drivers of metabolic disease.
Bilal, who is also a spokesperson for AGA, suggested that pancreatic enzyme replacement therapy, often used to treat pancreatic EPI, may treat some symptoms of FPD such as diarrhea.
Bariatric surgery has shown promise for FPD, in that it can decrease the patient’s body mass and potentially reduce the fat in the pancreas as well as it can improve metabolic diseases and hyperlipidemia. One study showed that it significantly decreased IPFD, fatty acid uptake, and blood flow, and these improvements were associated with more favorable glucose homeostasis and beta-cell function.
However, bariatric surgery is only appropriate for certain patients; is associated with potentially adverse sequelae including malnutrition, anemia, and digestive tract stenosis; and is currently not indicated for FPD.
Bilal advises clinicians to “keep an eye on FPD” if it’s detected incidentally and to screen patients more carefully for MASLD, metabolic disease, and diabetes.
“Although there are no consensus guidelines and recommendations for managing FPD at present, these common-sense approaches will benefit the patient’s overall health and hopefully will have a beneficial impact on pancreatic health as well,” he said.
Petrov reported no relevant financial relationships. Bilal reported being a consultant for Boston Scientific, Steris Endoscopy, and Cook Medical.
A version of this article first appeared on Medscape.com.
— a disorder experienced by roughly one fifth of the world’s population. Although it is more common than type 2 diabetes, pancreatitis, and pancreatic cancer combined, it has remained relatively obscure.
By contrast, fatty liver — once called nonalcoholic fatty liver disease and recently renamed metabolic dysfunction–associated steatotic liver disease (MASLD) — is well-known.
“When it comes to diseases of the liver and pancreas, the liver is the big brother that has gotten all the attention, while the pancreas is the neglected little stepbrother that’s not sufficiently profiled in most medical textbooks and gets very little attention,” Max Petrov, MD, MPH, PhD, professor of pancreatology, University of Auckland, New Zealand, said in an interview. “The phenomenon of fatty pancreas has been observed for decades, but it is underappreciated and underrecognized.”
As early as 1926, fat depositions were identified during autopsies, but the condition remained relatively unknown, Mohammad Bilal, MD, associate professor of medicine-gastroenterology, University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Fortunately, FPD has recently been receiving more focus.”
Generally, healthy individuals have small amounts of fat in their pancreas. IPFD is defined as “the diffuse presence of fat in the pancreas, measured on a continuous scale,” and FPD refers to IPFD above the upper limit of normal. While there is no clear consensus as to what the normal range is, studies suggest it’s a pancreatic fat content ranging from 1.8% to 10.4%.
FPD’s “most important implication is that it can be a precursor for more challenging and burdensome diseases of the pancreas,” Petrov said.
Fatty changes in the pancreas affect both its endocrine and exocrine systems. FPD is associated with type 2 diabetes, the most common disease of the endocrine pancreas, as well as pancreatitis and pancreatic cancer, the most common diseases of the exocrine pancreas. It’s also implicated in the development of carotid atherosclerosis, pancreatic fistula following surgery, and exocrine pancreatic insufficiency (EPI).
A ‘Pandora’s Box’
Up to half of people with fatty pancreas are lean. The condition isn’t merely caused by an overflow of fat from the liver into the pancreas in people who consume more calories than they burn, Petrov said. Neither robust postmortem nor biopsy studies have found a statistically significant association between fatty deposition in the pancreas and liver fat.
Compared with the way people accumulate liver fat, the development of FPD is more complex, Petrov said.
“Hepatic fat is a relatively simple process: Lipid droplets accumulate in the hepatocytes; but, in the pancreas, there are several ways by which fat may accumulate,” he said.
One relates to the location of the pancreas within visceral, retroperitoneal fat, Petrov said. That fat can migrate and build up between pancreatic lobules.
Fat also can accumulate inside the lobes. This process can involve a buildup of fat droplets in acinar and stellate cells on the exocrine side and in the islets of Langerhans on the endocrine side. Additionally, when functional pancreatic cells die, particularly acinar cells, adult stem cells may replace them with adipocytes. Transformation of acinar cells into fat cells — a process called acinar-to-adipocyte transdifferentiation — also may be a way fat accumulates inside the lobes, Petrov said.
The accumulation of fat is a response to a wide array of insults to the pancreas over time. For example, obesity and metabolic syndrome lead to the accumulation of adipocytes and fat infiltration, whereas alcohol abuse and viral infections may lead to the death of acinar cells, which produce digestive enzymes.
Ultimately, the negative changes produced by excess fat in the pancreas are the origin of all common noninherited pancreatic diseases, bringing them under one umbrella, Petrov maintained. He dubbed this hypothesis PANcreatic Diseases Originating from intRapancreatic fAt (PANDORA).
The type of cells involved has implications for which disease may arise. For example, fat infiltration in stellate cells may promote pancreatic cancer, whereas its accumulation in the islets of Langerhans, which produce insulin and glucagon, is associated with type 2 diabetes.
The PANDORA hypothesis has eight foundational principles:
- Fatty pancreas is a key driver of pancreatic diseases in most people.
- Inflammation within the pancreatic microenvironment results from overwhelming lipotoxicity fueled by fatty pancreas.
- Aberrant communication between acinar cells involving lipid droplets drives acute pancreatitis.
- The pancreas responds to lipotoxicity with fibrosis and calcification — the hallmarks of chronic pancreatitis.
- Fat deposition affects signaling between stellate cells and other components of the microenvironment in ways that raise the risk for pancreatic cancer.
- The development of diabetes of the exocrine pancreas and EPI is affected by the presence of fatty pancreas.
- The higher risk for pancreatic disease in older adults is influenced by fatty pancreas.
- The multipronged nature of intrapancreatic fat deposition accounts for the common development of one pancreatic disease after another.
The idea that all common pancreatic diseases are the result of pathways emanating from FPD could “explain the bidirectional relationship between diabetes and pancreatitis or pancreatic cancer,” Petrov said.
Risk Factors, Symptoms, and Diagnosis
A variety of risk factors are involved in the accumulation of fat that may lead to pancreatic diseases, including aging, cholelithiasis, dyslipidemia, drugs/toxins (eg, steroids), genetic predisposition, iron overload, diet (eg, fatty foods, ultraprocessed foods), heavy alcohol use, overweight/obesity, pancreatic duct obstruction, tobacco use, viral infection (eg, hepatitis B, COVID-19), severe malnutrition, prediabetes, and dysglycemia.
Petrov described FPD as a “silent disease” that’s often asymptomatic, with its presence emerging as an incidental finding during abdominal ultrasonography for other reasons. However, patients may sometimes experience stomach pain or nausea if they have concurrent diseases of the pancreas, he said.
There are no currently available lab tests that can definitively detect the presence of FPD. Rather, the gold standard for a noninvasive diagnosis of FPD is MRI, with CT as the second-best choice, Petrov said.
In countries where advanced imaging is not available, a low-cost alternative might be a simple abdominal ultrasound, but it is not definitive, he said. “It’s operator-dependent and can be subjective.”
Some risk factors, such as derangements of glucose and lipid metabolism, especially in the presence of heavy alcohol use and a high-fat diet, can “be detected on lab tests,” Petrov said. “This, in combination with the abdominal ultrasound, might suggest the patients will benefit from deeper investigation, including MRI.”
Because the exocrine pancreas helps with digestion of fatty food, intralobular fatty deposits or replacement of pancreatic exocrine cells with adipose cells can lead to steatorrhea, Bilal said.
“Fat within the stool or oily diarrhea is a clue to the presence of FPD,” Bilal said.
Although this symptom isn’t unique to FPD and is found in other types of pancreatic conditions, its presence suggests that further investigation for FPD is warranted, he added.
Common-Sense Treatment Approaches
At present, there are no US Food and Drug Administration–approved treatments for FPD, Petrov said.
“What might be recommended is something along the lines of treatment of MASLD — appropriate diet and physical activity,” he said. Petrov hopes that as the disease entity garners more research attention, more clinical drug trials will be initiated, and new medications are found and approved.
Petrov suggested that there could be a “theoretical rationale” for the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) as a treatment, given their effectiveness in multiple conditions, including MASLD, but no human trials have robustly shown specific benefits of these drugs for FPD.
Petrov added that, to date, 12 classes of drugs have been investigated for reducing IPFD: biguanides, sulfonylureas, GLP-1 RAs, thiazolidinediones, dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 inhibitors, statins, fibrates, pancreatic lipase inhibitors, angiotensin II receptor blockers, somatostatin receptor agonists, and antioxidants.
Of these, most have shown promise in preclinical animal models. But only thiazolidinediones, GLP-1 RAs, DPP-4 inhibitors, and somatostatin receptor agonists have been investigated in randomized controlled trials in humans. The findings have been inconsistent, with the active treatment often not achieving statistically significant improvements.
“At this stage of our knowledge, we can’t recommend a specific pharmacotherapy,” Petrov said. But we can suggest dietary changes, such as saturated fat reduction, alcohol reduction, smoking cessation, reduction in consumption of ultraprocessed food, physical exercise, and addressing obesity and other drivers of metabolic disease.
Bilal, who is also a spokesperson for AGA, suggested that pancreatic enzyme replacement therapy, often used to treat pancreatic EPI, may treat some symptoms of FPD such as diarrhea.
Bariatric surgery has shown promise for FPD, in that it can decrease the patient’s body mass and potentially reduce the fat in the pancreas as well as it can improve metabolic diseases and hyperlipidemia. One study showed that it significantly decreased IPFD, fatty acid uptake, and blood flow, and these improvements were associated with more favorable glucose homeostasis and beta-cell function.
However, bariatric surgery is only appropriate for certain patients; is associated with potentially adverse sequelae including malnutrition, anemia, and digestive tract stenosis; and is currently not indicated for FPD.
Bilal advises clinicians to “keep an eye on FPD” if it’s detected incidentally and to screen patients more carefully for MASLD, metabolic disease, and diabetes.
“Although there are no consensus guidelines and recommendations for managing FPD at present, these common-sense approaches will benefit the patient’s overall health and hopefully will have a beneficial impact on pancreatic health as well,” he said.
Petrov reported no relevant financial relationships. Bilal reported being a consultant for Boston Scientific, Steris Endoscopy, and Cook Medical.
A version of this article first appeared on Medscape.com.
— a disorder experienced by roughly one fifth of the world’s population. Although it is more common than type 2 diabetes, pancreatitis, and pancreatic cancer combined, it has remained relatively obscure.
By contrast, fatty liver — once called nonalcoholic fatty liver disease and recently renamed metabolic dysfunction–associated steatotic liver disease (MASLD) — is well-known.
“When it comes to diseases of the liver and pancreas, the liver is the big brother that has gotten all the attention, while the pancreas is the neglected little stepbrother that’s not sufficiently profiled in most medical textbooks and gets very little attention,” Max Petrov, MD, MPH, PhD, professor of pancreatology, University of Auckland, New Zealand, said in an interview. “The phenomenon of fatty pancreas has been observed for decades, but it is underappreciated and underrecognized.”
As early as 1926, fat depositions were identified during autopsies, but the condition remained relatively unknown, Mohammad Bilal, MD, associate professor of medicine-gastroenterology, University of Colorado Anschutz Medical Campus, Aurora, said in an interview. “Fortunately, FPD has recently been receiving more focus.”
Generally, healthy individuals have small amounts of fat in their pancreas. IPFD is defined as “the diffuse presence of fat in the pancreas, measured on a continuous scale,” and FPD refers to IPFD above the upper limit of normal. While there is no clear consensus as to what the normal range is, studies suggest it’s a pancreatic fat content ranging from 1.8% to 10.4%.
FPD’s “most important implication is that it can be a precursor for more challenging and burdensome diseases of the pancreas,” Petrov said.
Fatty changes in the pancreas affect both its endocrine and exocrine systems. FPD is associated with type 2 diabetes, the most common disease of the endocrine pancreas, as well as pancreatitis and pancreatic cancer, the most common diseases of the exocrine pancreas. It’s also implicated in the development of carotid atherosclerosis, pancreatic fistula following surgery, and exocrine pancreatic insufficiency (EPI).
A ‘Pandora’s Box’
Up to half of people with fatty pancreas are lean. The condition isn’t merely caused by an overflow of fat from the liver into the pancreas in people who consume more calories than they burn, Petrov said. Neither robust postmortem nor biopsy studies have found a statistically significant association between fatty deposition in the pancreas and liver fat.
Compared with the way people accumulate liver fat, the development of FPD is more complex, Petrov said.
“Hepatic fat is a relatively simple process: Lipid droplets accumulate in the hepatocytes; but, in the pancreas, there are several ways by which fat may accumulate,” he said.
One relates to the location of the pancreas within visceral, retroperitoneal fat, Petrov said. That fat can migrate and build up between pancreatic lobules.
Fat also can accumulate inside the lobes. This process can involve a buildup of fat droplets in acinar and stellate cells on the exocrine side and in the islets of Langerhans on the endocrine side. Additionally, when functional pancreatic cells die, particularly acinar cells, adult stem cells may replace them with adipocytes. Transformation of acinar cells into fat cells — a process called acinar-to-adipocyte transdifferentiation — also may be a way fat accumulates inside the lobes, Petrov said.
The accumulation of fat is a response to a wide array of insults to the pancreas over time. For example, obesity and metabolic syndrome lead to the accumulation of adipocytes and fat infiltration, whereas alcohol abuse and viral infections may lead to the death of acinar cells, which produce digestive enzymes.
Ultimately, the negative changes produced by excess fat in the pancreas are the origin of all common noninherited pancreatic diseases, bringing them under one umbrella, Petrov maintained. He dubbed this hypothesis PANcreatic Diseases Originating from intRapancreatic fAt (PANDORA).
The type of cells involved has implications for which disease may arise. For example, fat infiltration in stellate cells may promote pancreatic cancer, whereas its accumulation in the islets of Langerhans, which produce insulin and glucagon, is associated with type 2 diabetes.
The PANDORA hypothesis has eight foundational principles:
- Fatty pancreas is a key driver of pancreatic diseases in most people.
- Inflammation within the pancreatic microenvironment results from overwhelming lipotoxicity fueled by fatty pancreas.
- Aberrant communication between acinar cells involving lipid droplets drives acute pancreatitis.
- The pancreas responds to lipotoxicity with fibrosis and calcification — the hallmarks of chronic pancreatitis.
- Fat deposition affects signaling between stellate cells and other components of the microenvironment in ways that raise the risk for pancreatic cancer.
- The development of diabetes of the exocrine pancreas and EPI is affected by the presence of fatty pancreas.
- The higher risk for pancreatic disease in older adults is influenced by fatty pancreas.
- The multipronged nature of intrapancreatic fat deposition accounts for the common development of one pancreatic disease after another.
The idea that all common pancreatic diseases are the result of pathways emanating from FPD could “explain the bidirectional relationship between diabetes and pancreatitis or pancreatic cancer,” Petrov said.
Risk Factors, Symptoms, and Diagnosis
A variety of risk factors are involved in the accumulation of fat that may lead to pancreatic diseases, including aging, cholelithiasis, dyslipidemia, drugs/toxins (eg, steroids), genetic predisposition, iron overload, diet (eg, fatty foods, ultraprocessed foods), heavy alcohol use, overweight/obesity, pancreatic duct obstruction, tobacco use, viral infection (eg, hepatitis B, COVID-19), severe malnutrition, prediabetes, and dysglycemia.
Petrov described FPD as a “silent disease” that’s often asymptomatic, with its presence emerging as an incidental finding during abdominal ultrasonography for other reasons. However, patients may sometimes experience stomach pain or nausea if they have concurrent diseases of the pancreas, he said.
There are no currently available lab tests that can definitively detect the presence of FPD. Rather, the gold standard for a noninvasive diagnosis of FPD is MRI, with CT as the second-best choice, Petrov said.
In countries where advanced imaging is not available, a low-cost alternative might be a simple abdominal ultrasound, but it is not definitive, he said. “It’s operator-dependent and can be subjective.”
Some risk factors, such as derangements of glucose and lipid metabolism, especially in the presence of heavy alcohol use and a high-fat diet, can “be detected on lab tests,” Petrov said. “This, in combination with the abdominal ultrasound, might suggest the patients will benefit from deeper investigation, including MRI.”
Because the exocrine pancreas helps with digestion of fatty food, intralobular fatty deposits or replacement of pancreatic exocrine cells with adipose cells can lead to steatorrhea, Bilal said.
“Fat within the stool or oily diarrhea is a clue to the presence of FPD,” Bilal said.
Although this symptom isn’t unique to FPD and is found in other types of pancreatic conditions, its presence suggests that further investigation for FPD is warranted, he added.
Common-Sense Treatment Approaches
At present, there are no US Food and Drug Administration–approved treatments for FPD, Petrov said.
“What might be recommended is something along the lines of treatment of MASLD — appropriate diet and physical activity,” he said. Petrov hopes that as the disease entity garners more research attention, more clinical drug trials will be initiated, and new medications are found and approved.
Petrov suggested that there could be a “theoretical rationale” for the use of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) as a treatment, given their effectiveness in multiple conditions, including MASLD, but no human trials have robustly shown specific benefits of these drugs for FPD.
Petrov added that, to date, 12 classes of drugs have been investigated for reducing IPFD: biguanides, sulfonylureas, GLP-1 RAs, thiazolidinediones, dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 inhibitors, statins, fibrates, pancreatic lipase inhibitors, angiotensin II receptor blockers, somatostatin receptor agonists, and antioxidants.
Of these, most have shown promise in preclinical animal models. But only thiazolidinediones, GLP-1 RAs, DPP-4 inhibitors, and somatostatin receptor agonists have been investigated in randomized controlled trials in humans. The findings have been inconsistent, with the active treatment often not achieving statistically significant improvements.
“At this stage of our knowledge, we can’t recommend a specific pharmacotherapy,” Petrov said. But we can suggest dietary changes, such as saturated fat reduction, alcohol reduction, smoking cessation, reduction in consumption of ultraprocessed food, physical exercise, and addressing obesity and other drivers of metabolic disease.
Bilal, who is also a spokesperson for AGA, suggested that pancreatic enzyme replacement therapy, often used to treat pancreatic EPI, may treat some symptoms of FPD such as diarrhea.
Bariatric surgery has shown promise for FPD, in that it can decrease the patient’s body mass and potentially reduce the fat in the pancreas as well as it can improve metabolic diseases and hyperlipidemia. One study showed that it significantly decreased IPFD, fatty acid uptake, and blood flow, and these improvements were associated with more favorable glucose homeostasis and beta-cell function.
However, bariatric surgery is only appropriate for certain patients; is associated with potentially adverse sequelae including malnutrition, anemia, and digestive tract stenosis; and is currently not indicated for FPD.
Bilal advises clinicians to “keep an eye on FPD” if it’s detected incidentally and to screen patients more carefully for MASLD, metabolic disease, and diabetes.
“Although there are no consensus guidelines and recommendations for managing FPD at present, these common-sense approaches will benefit the patient’s overall health and hopefully will have a beneficial impact on pancreatic health as well,” he said.
Petrov reported no relevant financial relationships. Bilal reported being a consultant for Boston Scientific, Steris Endoscopy, and Cook Medical.
A version of this article first appeared on Medscape.com.
HCV Screening Rates in Women Remain Low in the US
“We found that screening rates were higher and rose more steeply in pregnant individuals compared to nonpregnant reproductive age females after this guidance.” However overall, HCV screening in women still remained low by the end of 2022, authors Roshni Singh, MD, and Rachel Epstein, MD, MSc, with the section of infectious diseases, Boston Medical Center, noted in an email to this news organization.
The study was published online in JAMA.
The researchers leveraged TriNetX LIVE electronic health record data to compare HCV screening rates from 68 US healthcare organizations covering more than 115 million patients.
Using a multiple-group interrupted time series analysis, they compared HCV screening rates for pregnant and nonpregnant women for each 6-month period before (January 2014 to December 2019) and after (July 2020 to December 2022) the 2020 guidelines. January to June 2020 was considered a washout period to account for the COVID-19 pandemic peak and guideline dissemination.
For the entire 9-year study period (2014-2022), a total of 79,231 incident HCV tests occurred among pregnant women and 678,951 occurred among nonpregnant women.
In the 6 months before the guidance, HCV screening per 1000 person-years increased from 52 to 117 tests among pregnant women and 16 to 24 tests among nonpregnant women.
In the 6 months after the guidance, screening per 1000 person-years increased from 141 to 253 among pregnant women and from 29 to 37 among nonpregnant women.
Yet by the end of 2022, only 38.7% of women with a pregnancy and 8.7% of nonpregnant women were ever tested for the HCV.
How to Boost HCV Screening
These results suggest that “innovative strategies are needed to improve HCV diagnosis and treatment,” the authors wrote.
“Several interventions have been demonstrated to be effective in increasing screening in general, including electronic medical record alerts for opt-out testing, routine test offer by nonclinician office staff, offering testing in nontraditional spaces, including substance use treatment programs, harm reduction centers, STI clinics, and mobile health units,” Singh and Epstein told this news organization.
“A key step is educating primary care providers in addition to addiction medicine and emergency medicine clinicians about the updated guidelines as they interface with a large number of at-risk individuals,” they said. And the most important measure is creating clear work flows that respond to positive results to link people to treatment and cure.
“Clinicians need to feel empowered that their work screening a patient can make a meaningful difference in both the patient’s life and in helping end this epidemic,” the two researchers explained.
Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America, who wasn’t involved in the study, told this news organization that the low HCV screening rates are not surprising.
“We tend not to do well with screening. It’s not necessarily anybody’s fault, but patients don’t necessarily want to be screened. Sometimes physicians are very busy. Sometimes screening is not the most important thing for them to do. Sometimes there are processes in place that fall through,” said Glatt, chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau, Oceanside, New York.
“We tend to do a better job of screening in pregnant than nonpregnant women because pregnancy is a focus and there is 9 months that you can be following-up, so there is more opportunity. A healthy nonpregnant woman may not see her doctor for another year,” Glatt noted.
“I think that many physicians are very good at screening for hepatitis C in patients that are clearly at risk,” he added. “We’re not so good at screening for people” that don’t have a clear risk but do “have risk factors.”
The study had no commercial funding. Singh, Epstein, and Glatt had no relevant disclosures.
A version of this article first appeared on Medscape.com.
“We found that screening rates were higher and rose more steeply in pregnant individuals compared to nonpregnant reproductive age females after this guidance.” However overall, HCV screening in women still remained low by the end of 2022, authors Roshni Singh, MD, and Rachel Epstein, MD, MSc, with the section of infectious diseases, Boston Medical Center, noted in an email to this news organization.
The study was published online in JAMA.
The researchers leveraged TriNetX LIVE electronic health record data to compare HCV screening rates from 68 US healthcare organizations covering more than 115 million patients.
Using a multiple-group interrupted time series analysis, they compared HCV screening rates for pregnant and nonpregnant women for each 6-month period before (January 2014 to December 2019) and after (July 2020 to December 2022) the 2020 guidelines. January to June 2020 was considered a washout period to account for the COVID-19 pandemic peak and guideline dissemination.
For the entire 9-year study period (2014-2022), a total of 79,231 incident HCV tests occurred among pregnant women and 678,951 occurred among nonpregnant women.
In the 6 months before the guidance, HCV screening per 1000 person-years increased from 52 to 117 tests among pregnant women and 16 to 24 tests among nonpregnant women.
In the 6 months after the guidance, screening per 1000 person-years increased from 141 to 253 among pregnant women and from 29 to 37 among nonpregnant women.
Yet by the end of 2022, only 38.7% of women with a pregnancy and 8.7% of nonpregnant women were ever tested for the HCV.
How to Boost HCV Screening
These results suggest that “innovative strategies are needed to improve HCV diagnosis and treatment,” the authors wrote.
“Several interventions have been demonstrated to be effective in increasing screening in general, including electronic medical record alerts for opt-out testing, routine test offer by nonclinician office staff, offering testing in nontraditional spaces, including substance use treatment programs, harm reduction centers, STI clinics, and mobile health units,” Singh and Epstein told this news organization.
“A key step is educating primary care providers in addition to addiction medicine and emergency medicine clinicians about the updated guidelines as they interface with a large number of at-risk individuals,” they said. And the most important measure is creating clear work flows that respond to positive results to link people to treatment and cure.
“Clinicians need to feel empowered that their work screening a patient can make a meaningful difference in both the patient’s life and in helping end this epidemic,” the two researchers explained.
Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America, who wasn’t involved in the study, told this news organization that the low HCV screening rates are not surprising.
“We tend not to do well with screening. It’s not necessarily anybody’s fault, but patients don’t necessarily want to be screened. Sometimes physicians are very busy. Sometimes screening is not the most important thing for them to do. Sometimes there are processes in place that fall through,” said Glatt, chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau, Oceanside, New York.
“We tend to do a better job of screening in pregnant than nonpregnant women because pregnancy is a focus and there is 9 months that you can be following-up, so there is more opportunity. A healthy nonpregnant woman may not see her doctor for another year,” Glatt noted.
“I think that many physicians are very good at screening for hepatitis C in patients that are clearly at risk,” he added. “We’re not so good at screening for people” that don’t have a clear risk but do “have risk factors.”
The study had no commercial funding. Singh, Epstein, and Glatt had no relevant disclosures.
A version of this article first appeared on Medscape.com.
“We found that screening rates were higher and rose more steeply in pregnant individuals compared to nonpregnant reproductive age females after this guidance.” However overall, HCV screening in women still remained low by the end of 2022, authors Roshni Singh, MD, and Rachel Epstein, MD, MSc, with the section of infectious diseases, Boston Medical Center, noted in an email to this news organization.
The study was published online in JAMA.
The researchers leveraged TriNetX LIVE electronic health record data to compare HCV screening rates from 68 US healthcare organizations covering more than 115 million patients.
Using a multiple-group interrupted time series analysis, they compared HCV screening rates for pregnant and nonpregnant women for each 6-month period before (January 2014 to December 2019) and after (July 2020 to December 2022) the 2020 guidelines. January to June 2020 was considered a washout period to account for the COVID-19 pandemic peak and guideline dissemination.
For the entire 9-year study period (2014-2022), a total of 79,231 incident HCV tests occurred among pregnant women and 678,951 occurred among nonpregnant women.
In the 6 months before the guidance, HCV screening per 1000 person-years increased from 52 to 117 tests among pregnant women and 16 to 24 tests among nonpregnant women.
In the 6 months after the guidance, screening per 1000 person-years increased from 141 to 253 among pregnant women and from 29 to 37 among nonpregnant women.
Yet by the end of 2022, only 38.7% of women with a pregnancy and 8.7% of nonpregnant women were ever tested for the HCV.
How to Boost HCV Screening
These results suggest that “innovative strategies are needed to improve HCV diagnosis and treatment,” the authors wrote.
“Several interventions have been demonstrated to be effective in increasing screening in general, including electronic medical record alerts for opt-out testing, routine test offer by nonclinician office staff, offering testing in nontraditional spaces, including substance use treatment programs, harm reduction centers, STI clinics, and mobile health units,” Singh and Epstein told this news organization.
“A key step is educating primary care providers in addition to addiction medicine and emergency medicine clinicians about the updated guidelines as they interface with a large number of at-risk individuals,” they said. And the most important measure is creating clear work flows that respond to positive results to link people to treatment and cure.
“Clinicians need to feel empowered that their work screening a patient can make a meaningful difference in both the patient’s life and in helping end this epidemic,” the two researchers explained.
Aaron Glatt, MD, a spokesperson for the Infectious Diseases Society of America, who wasn’t involved in the study, told this news organization that the low HCV screening rates are not surprising.
“We tend not to do well with screening. It’s not necessarily anybody’s fault, but patients don’t necessarily want to be screened. Sometimes physicians are very busy. Sometimes screening is not the most important thing for them to do. Sometimes there are processes in place that fall through,” said Glatt, chief of infectious diseases and hospital epidemiologist at Mount Sinai South Nassau, Oceanside, New York.
“We tend to do a better job of screening in pregnant than nonpregnant women because pregnancy is a focus and there is 9 months that you can be following-up, so there is more opportunity. A healthy nonpregnant woman may not see her doctor for another year,” Glatt noted.
“I think that many physicians are very good at screening for hepatitis C in patients that are clearly at risk,” he added. “We’re not so good at screening for people” that don’t have a clear risk but do “have risk factors.”
The study had no commercial funding. Singh, Epstein, and Glatt had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.
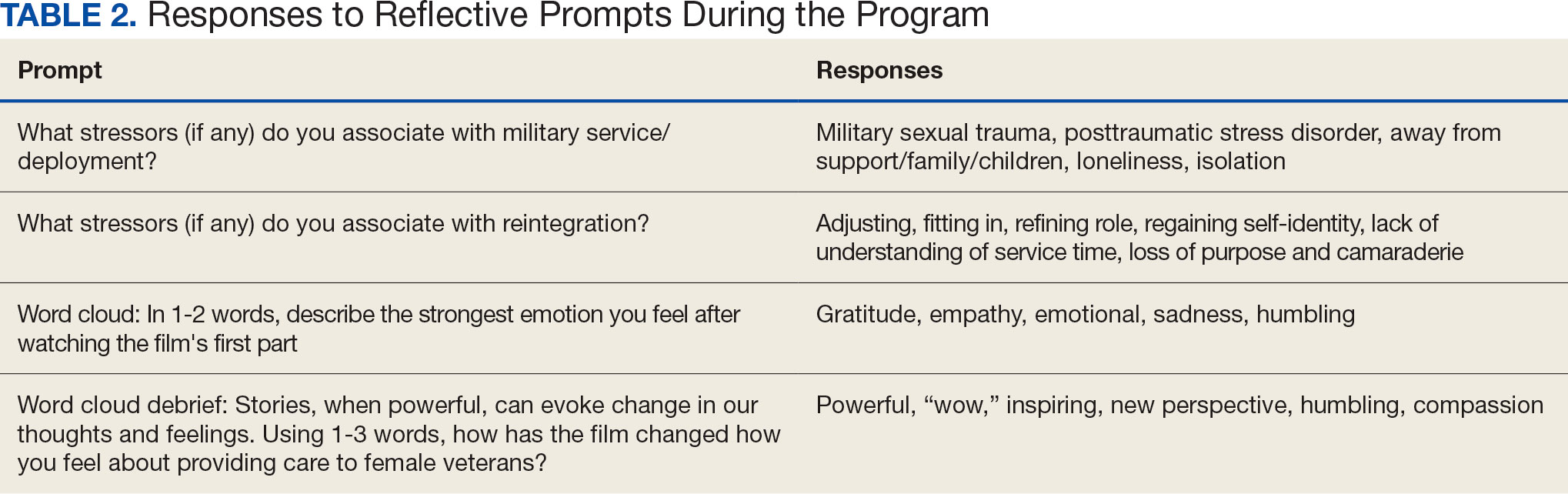
DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.

DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
The COVID-19 pandemic presented stressors for patients and health care professionals alike, and the prevalence of health care practitioner burnout and dissatisfaction has risen dramatically.1,2 This, in combination with an increasingly virtual interface between patients and care teams, has the potential to lead to increased depersonalization, anxiety, distress, and diminished overall well-being among clinicians.1,3 Within the Veterans Health Administration (VHA), women’s health primary care practitioners (PCPs) are specially trained clinicians thatprovide comprehensive care to women veterans. Data suggest that women’s health PCPs may experience higher rates of burnout and attrition (14% per year) compared to general PCPs in VHA.4 Burnout among PCPs, especially those working at VHA, is well known and likely related to poor interdisciplinary team structure, limited administrative time, high patient complexity, and isolation from additional resources (eg, rural settings).4-7 Increased clinician burnout is associated with poorer quality of care and worsening quality of the doctor-patient relationship.8
The medical humanities can act as a countermeasure to clinician burnout.9,10 Studies have demonstrated that physicians who participate in the medical humanities are more empathic and experience less burnout.11,12 Engaging with patient stories through listening and writing has been a source of fulfillment for clinicians.13 Despite the benefits of narrative medicine, programs are often limited in scope in small face-to-face group settings during elective time or outside work hours.14 The COVID-19 pandemic presented significant challenges to implementing such programming. The VHA is a large health care system with many rural locations, which further limits the availability of traditional small-group and face-to-face trainings. Few studies describe large-scale medical humanities training in virtual learning environments.
NARRATIVE MEDICINE EVENT
To improve satisfaction and engagement among PCPs who care for women veterans, we developed, implemented, and evaluated a large-scale, virtual, interprofessional narrative medicine event aimed at achieving the following: (1) gain a deeper appreciation of the impact of deployments on women veterans; (2) describe the social and emotional challenges faced by women veterans returning from deployment (reintegration); (3) identify strategies to support veterans during reintegration; (4) apply narrative medicine techniques on a large-scale, virtual platform; and (5) assess clinician engagement and satisfaction following participation. We hypothesized that clinician satisfaction and appreciation would improve with a better understanding of the unique complexities of deployment and reintegration faced by women veterans. Utilizing a novel, humanities-based intervention would lead to strong engagement and interaction from participants.
Setting
A 3-hour virtual session was conducted on November 15, 2022, for an interdisciplinary audience. This included physicians and trainees in medicine and behavioral health, nurse practitioners, social workers, dieticians, nurses, and clinical support staff. The training was advertised via emails through established mailing lists and newsletters, reaching a large interdisciplinary VHA audience 90 days prior to the event. This allowed potential participants to dedicate time to attend the session. The training was open to all VHA employees, with no inclusion or exclusion criteria for either the training or the evaluation. The training was delivered within existing space utilized for continuing medical education in women’s health.
For the session, the 93-minute documentary Journey to Normal (jtninc.org) was chosen because it focused on the impact of deployment on women veterans and their experiences when returning home. The film follows the stories of several women veterans through combat and reintegration. The screening was split into 2 segments given the emotional impact and length of the documentary.
A facilitator opened the session by reading a series of reflective prompts centered on women veteran deployment, reintegration, and the stressors surrounding these transitions. The initial prompt served to familiarize participants with the session’s interactive components. Additional prompts were interspersed and discussed in real time and were chosen to mirror the major themes of the documentary: the emotional and psychological impact of deployment and reintegration for women veterans. Short responses and word cloud generation were used and debriefed synchronously to encourage ongoing engagement. Participants responded to prompts through anonymous polling and the chat function of the virtual platform.
During intermission, we introduced My Life, My Story (MLMS). MLMS is a VHA initiative started in 2013 that, with the veteran’s permission, shares a piece of a veteran’s life story with their health care practitioner in their medical chart.15 Evaluation of MLMS has demonstrated positive impacts on assessments of patient-clinician connection.16 The MLMS goal to improve patient-centered care competencies by learning stories of veterans aligned with the overarching goals of this program. Following the film, participants were given 10 minutes to respond to a final reflective prompt. The session ended with a review of existing VHA resources to support returning veterans, followed by a question-and-answer session conducted via chat.
We used the Brightcove virtual platform to stream this program, which facilitated significant interaction between participants and facilitators, as well as between participants themselves. In addition to posing questions to the session leaders, participants could directly respond to each other’s comments within the chat function and also upvote/downvote or emphasize others’ comments.
Evaluation
The evaluation schema was 2-fold. Because this session was presented as a part of the national VA Women’s Health webinar series, a standard evaluation was dictated by the VHA Employee Education System. This survey was electronically disseminated and included questions on occupational category and overall satisfaction, plus 9 standard evaluation questions and 4 program-specific questions tied to the workshop objectives. The standard evaluation questions assessed participant satisfaction with the training, satisfaction with the training environment, and appropriateness of the content. The programspecific questions asked the participants whether the session met the stated learning objectives. All questions used a 5-point Likert scale (1, strongly disagree; 5, strongly agree). Descriptive statistics were used for analysis. Individual chat messages and spontaneous replies were analyzed as a surrogate measures of audience engagement. A qualitative analysis of participants’ final reflections to assess for attitudes related to patient care, empathy, and burnout following participation in this curriculum is forthcoming.
A total of 876 participants attended the virtual setting and 525 (59.9%) completed the immediate postevaluation survey. Respondents represented a variety of disciplines, including 179 nurses (34.1%), 100 social workers (19.0%), 65 physicians (12.4%), and 10 physician assistants (1.9%), with < 10% comprising counselors, dentists, dietitians, pharmacists, physical therapists, and psychologists. Nearly all participants reported satisfaction with the learning activity, would recommend it to others, and felt it advanced their knowledge, attitudes, and skills to better contribute to their VHA interprofessional team for patient care (Table 1). Similarly, participants reported a highlevel of agreement that the program satisfied the session-specific objectives. In response to an open-ended question on the standard VA evaluation regarding overall perceptions of the training, free-text responses included such statements as, “I think this should be mandatory training for all VA [clinicians]”; and “This webinar [opened] my mind to the various struggles women veterans may encounter when [they] return to civilian life and [increased] my understanding of how I could support.”

More than 1700 individual chat messages and > 80 spontaneous replies between participants were recorded during the interactive session (Table 2). Spontaneous quotes written in the chat included: “This is the best film representing the female veteran I have ever seen;” “Powerful and perspective changing;” “Thank you for sharing this incredible film;” and “I needed this to remind me to focus on woman veterans. Although our female veteran population is small it will remind me daily of their dedication, recognizing that there are so many facets of making the ultimate sacrifice.” Several participants said such programming should be a mandatory component of VA new employee orientation.

DISCUSSION
Clinician burnout diminishes empathetic patient-physician engagement. Patients’ stories are a known, powerful way to evoke empathy. This session provides one of the first examples of a straightforward approach to delivering a medical humanities intervention to a large audience via virtual platform. As measured by its high engagement, participant satisfaction, and narrative evaluations, this model was successful in evoking empathy and reinforcing the core VHA values for patient care: integrity, commitment, advocacy, respect, and excellence.
Rates of burnout and disengagement among PCPs are high and increased during the COVID-19 pandemic.2 This curriculum used a synchronous, narrative-based approach during work hours to address burnout. Lack of empathy is a cause and consequence of burnout and disengagement. Narrative approaches, especially those evoking patients’ stories can evoke empathy and help counteract such burnout. This curriculum demonstrates one of the first large-scale, narrative-based, virtual-platform approaches to utilizing patients’ stories for positive clinician impact, as evidenced by the extensive participation, engagement, and satisfaction of participants.
Individuals interested in implementing a similar program should consider common barriers, including time constraints, advertising, and clinician buy-in. Several key factors led to the successful implementation of this program. First, partnering with established educational efforts related to improving care for veterans provided time to implement the program and establish mechanisms for advertising. The VHA is a mission-driven organization; directly tying this intervention to the mission likely contributed to participant buy-in and programmatic success. Further, by partnering with established educational efforts, this session was conducted during business hours, allowing for widespread participation.
A diverse group of VHA clinicians were actively engaged throughout the session. Chat data demonstrated not only numerous responses to directed prompts, but also a larger extemporaneous conversation among participants. Additionally, it is clear participants were deeply engaged with the material. The quality of participant responses demonstrates the impact of narrative stories and included a new respect for our shared patients, a sense of humbleness as it relates to the women veteran experience, and a sense of pride in both the VHA mission and their roles as a part of the organization.
This session did not end with traditional take-home skills or reference handout resources typical of continuing education. This was intentional; the intended take-home message was the evoked emotional response and resultant perspective shift. The impact of this session on patient care will be examined in a forthcoming qualitative analysis of participants written reflections.
Limitations
Some participants noted that the chat could be distracting from the film. Others described that virtually attending the session allowed increased opportunity for interruption by ongoing patient care responsibilities, resulting in diverted attention. Many participants were granted protected time to attend this continuing education session; however, this was not always the case. Additionally, this evaluation is limited, as 40% of participants elected to not complete the postevent survey. The individuals who choose to respond may have been more engaged with the content or felt more strongly about the impact of the session. However, the volume of chat engagement during the session suggests strong participant involvement. The analysis was also limited by an electronic survey which did not allow more granular assessment of the data.
This session also raised an ethical consideration. The film evoked very strong emotional responses which, for some, were challenging to attend to personally in a large-scale virtual environment. Established clinician resources were highlighted during the session that were available for any participant who needed additional support. Participants were also encouraged to step away and process their emotions, if needed. Future interactions of this session might consider improved interparticipant chat management and upfront warnings about the emotional impact of the film accompanied by proactive dissemination of resources for participant support. One example of such resources includes breakout rooms facilitated by trained counselors. Prompts might also be adjusted to allow for more guided interparticipant engagement; facilitation can be brief as participants’ responses often carry the conversation.
CONCLUSIONS
This study shows that a large-scale, virtual medical humanities intervention is not only possible but well received, as evidenced by both quantity and quality of participant responses and engagement. The narrative approach of hearing patients’ stories, as portrayed in Journey to Normal, was found to be satisfying and appreciated by participants. Such an intervention has the potential to evoke empathy and help counteract burnout and disengagement among clinicians. This study directly aligned to the greater mission of the VHA: to improve quality medical care for all veterans, including women veterans, a subset population that is often overlooked. Organizations beyond the VHA may wish to leverage virtual learning as a mechanism to offer medical humanities to a wider audience. To optimize success, future programs should be tied to organizational missions, highlight patient voices and stories, and utilize platforms that allow for participant interactivity. Through virtual platforms, the medical humanities can reach a broader audience without detracting from its impact.
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
- Van Wert MJ, Gandhi S, Gupta I, et al. Healthcare worker mental health after the initial peak of the COVID- 19 pandemic: a US medical center cross-sectional survey. J Gen Intern Med. 2022;37(5):1169-1176. doi:10.1007/s11606-021-07251-0
- Centers for Disease Control and Prevention. Vital Signs. Health workers face a mental health crisis: workers report harassment, burnout, and poor mental health; supportive workplaces can help. Updated October 24, 2023. Accessed February 18, 2025. https://www.cdc.gov/vitalsigns/health-worker-mental-health/index.html
- Holmgren AJ, Downing NL, Tang M, Sharp C, Longhurst C, Huckman RS. Assessing the impact of the COVID-19 pandemic on clinician ambulatory electronic health record use. J Am Med Inform Assoc. 2022;29(3):453-460. doi:10.1093/jamia/ocab268
- Apaydin EA, Mohr DC, Hamilton AB, Rose DE, Haskell S, Yano EM. Differences in burnout and intent to leave between women’s health and general primary care providers in the Veterans Health Administration. J Gen Intern Med. 2022;37(10):2382-2389. doi:10.1007/s11606-021-07133-5
- Willard-Grace R, Knox M, Huang B, Hammer H, Kivlahan C, Grumbach K. Burnout and health care workforce turnover. Ann Fam Med. 2019;17(1):36-41. doi:10.1370/afm.2338
- Rinne ST, Mohr DC, Swamy L, Blok AC, Wong ES, Charns MP. National burnout trends among physicians working in the department of veterans affairs. J Gen Intern Med. 2020;35(5):1382-1388. doi:10.1007/s11606-019-05582-7
- Spinelli WM, Fernstrom KM, Galos DL, Britt HR. Extending our understanding of burnout and its associated factors: providers and staff in primary care clinics. Eval Health Prof. 2016;39(3):282-298. doi:10.1177/0163278716637900
- Abraham CM, Zheng K, Poghosyan L. Predictors and outcomes of burnout among primary care providers in the United States: a systematic review. Med Care Res Rev. 2020;77(5):387-401. doi:10.1177/1077558719888427
- Charon R, Williams P. Introduction: the humanities and medical education. Acad Med. 1995;70(9):758-760.
- Winkel AF, Yingling S, Jones A-A, Nicholson J. Reflection as a learning tool in graduate medical education: a systematic review. J Grad Med Educ. 2017;9(4):430-439. doi:10.4300/JGME-D-16-00500.1
- Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286(15):1897-1902. doi:10.1001/jama.286.15.1897
- DasGupta S, Charon R. Personal illness narratives: using reflective writing to teach empathy. Acad Med. 2004; 79(4):351-356. doi:10.1097/00001888-200404000-00013
- Liao JM, Secemsky BJ. The value of narrative medical writing in internal medicine residency. J Gen Intern Med. 2015;30(11):1707-1710. doi:10.1007/s11606-015-3460-x
- Branch WT, Kern D, Haidet P, et al. The patient-physician relationship. Teaching the human dimensions of care in clinical settings. JAMA. 2001;286(9):1067-1074. doi:10.1001/jama.286.9.1067
- Roberts TJ, Ringler T, Krahn D, Ahearn E. The my life, my story program: sustained impact of veterans’ personal narratives on healthcare providers 5 years after implementation. Health Commun. 2021;36(7):829-836. doi:10.1080/10410236.2020.1719316
- Lam JA, Feingold-Link M, Noguchi J, et al. My life, my story: integrating a life story narrative component into medical student curricula. MedEdPORTAL. 2022;18:11211. doi:10.15766/mep_2374-8265.11211
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Hearing Patient Stories: Use of Medical Humanities on a Large-Scale, Virtual Platform to Improve Clinician Engagement
Safety Profile of GLP-1s ‘Reassuring’ in Upper Endoscopy
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).
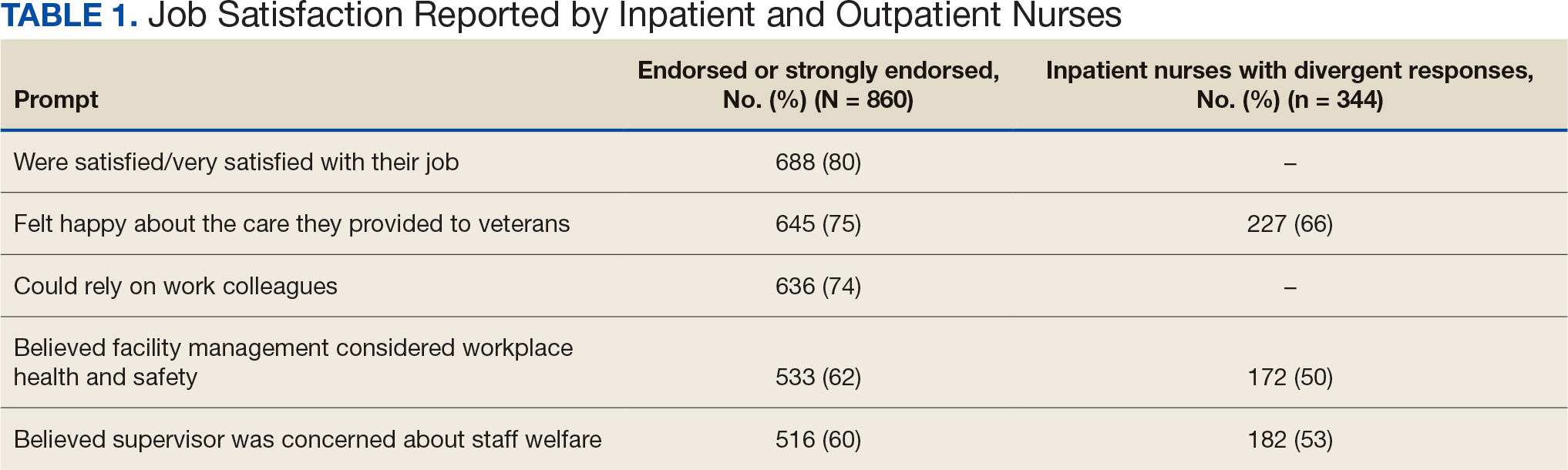
Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).
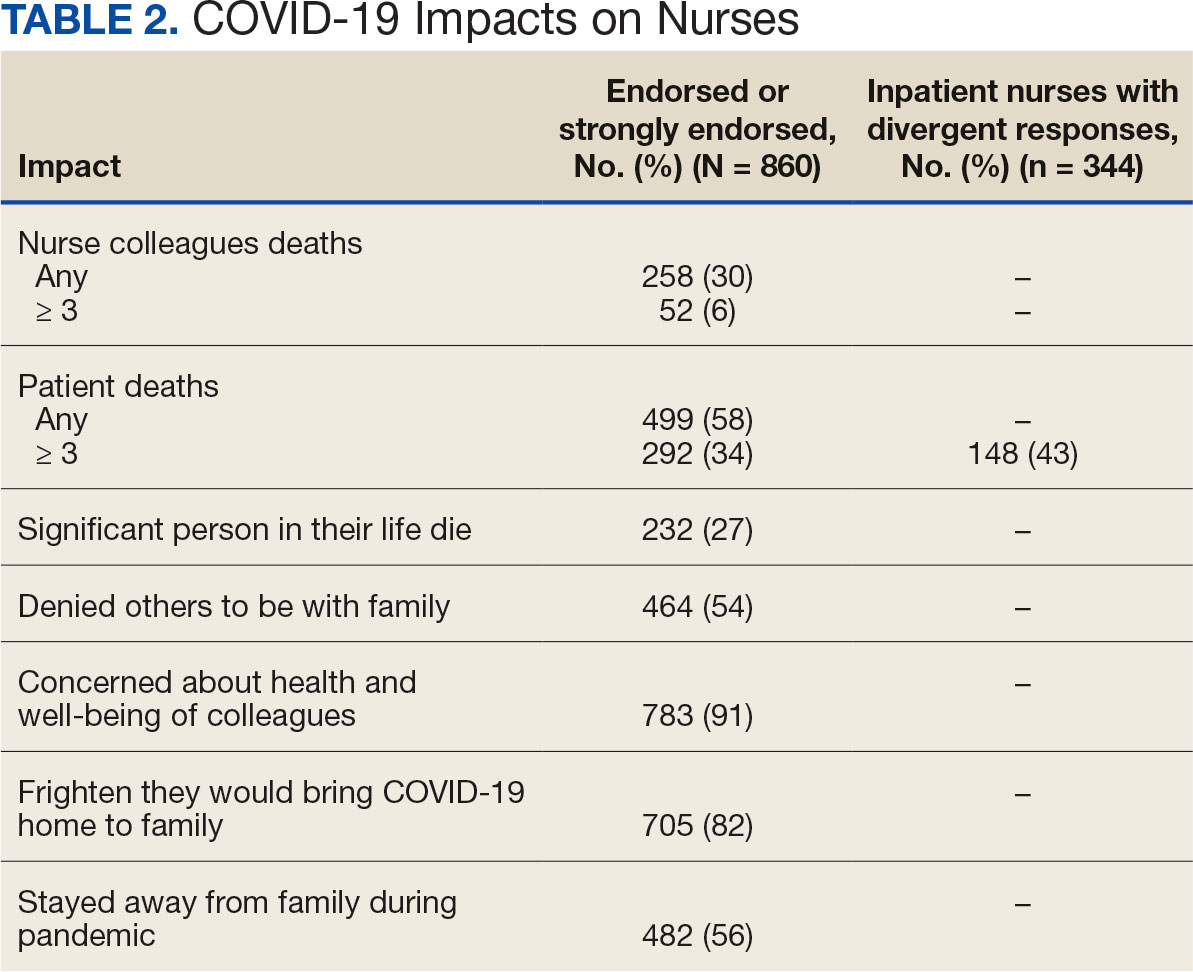
A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).
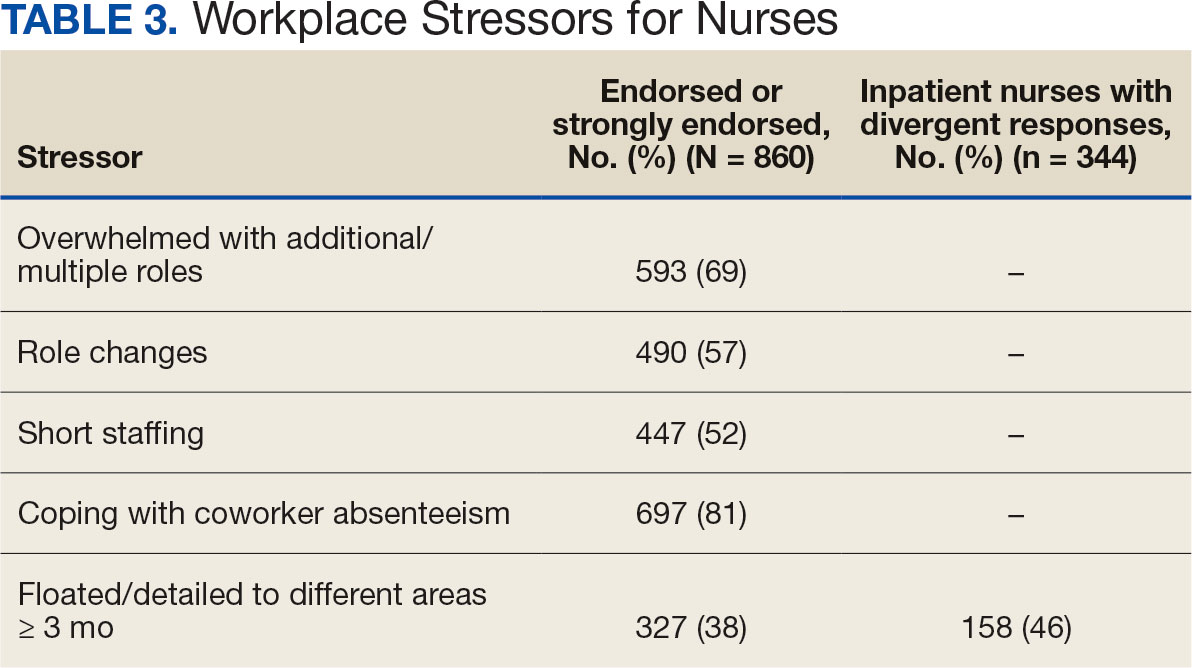
Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).
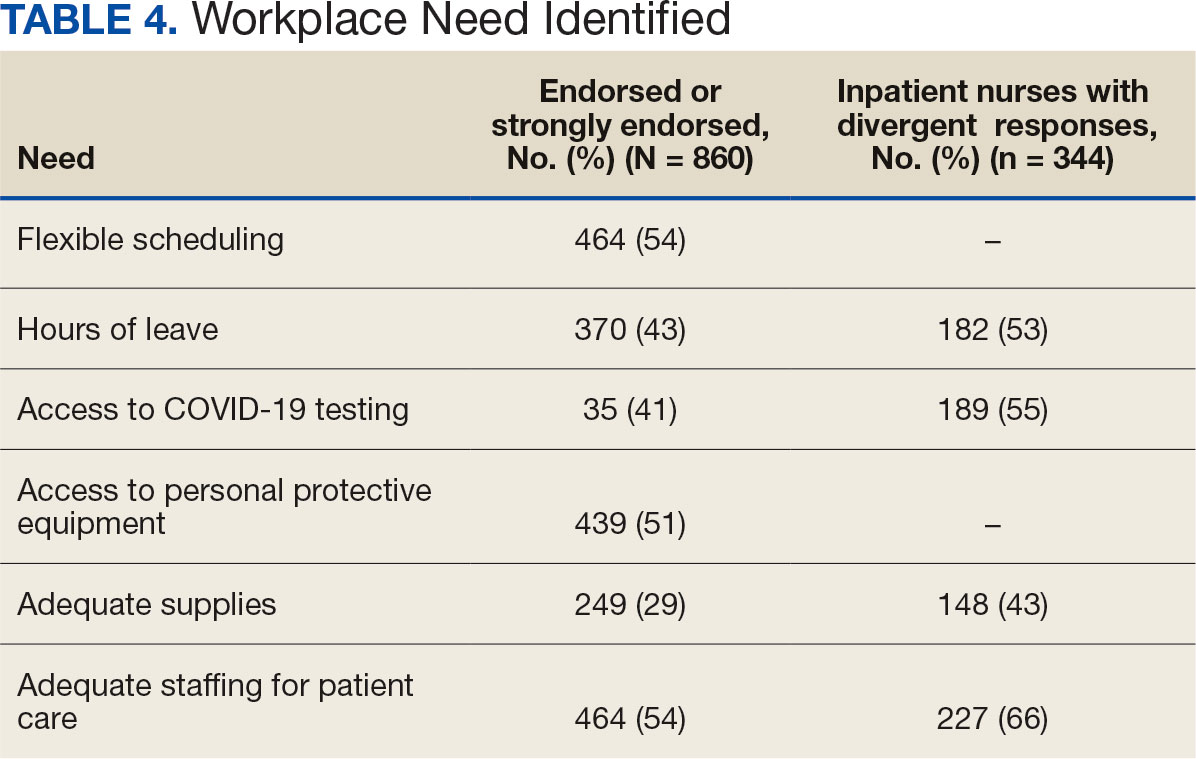
Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.

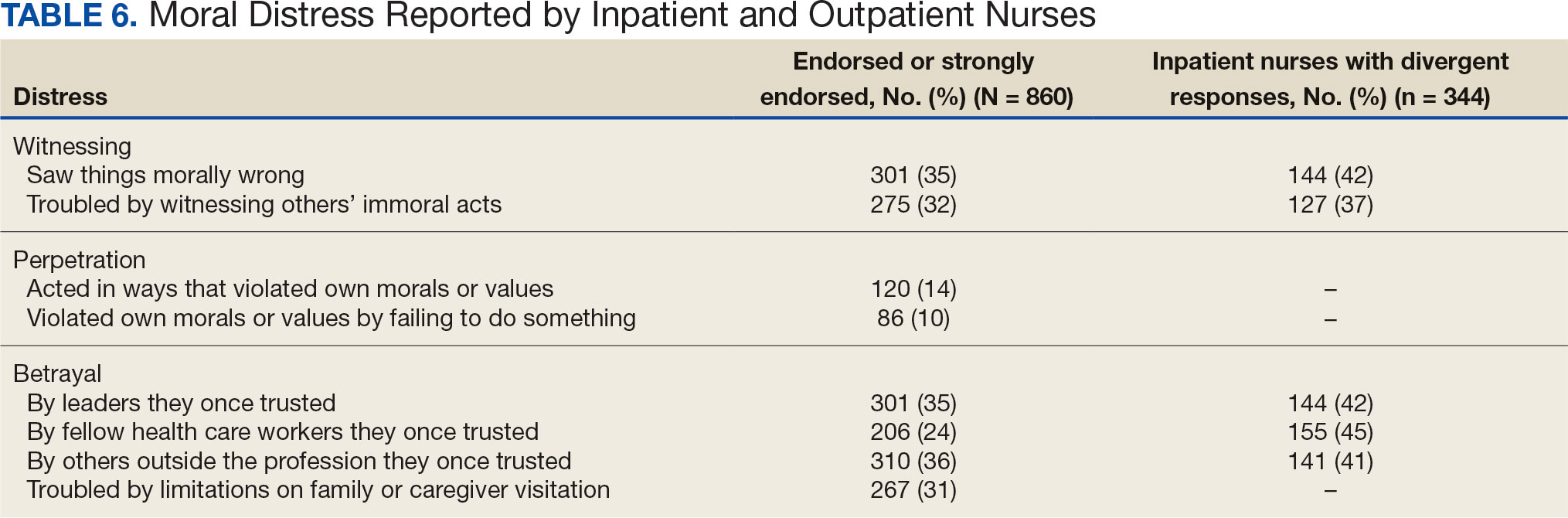

DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).

Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).

A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).

Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).

Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.



DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
On March 11, 2020, the World Health Organization designated COVID- 19 as a pandemic.1 Pandemics have historically impacted physical and mental health across all populations, but especially health care workers (HCWs).2 Nurses and other HCWs were profoundly impacted by the pandemic.3-8
Throughout the pandemic, nurses continued to provide care while working in short-staffed workplaces, facing increased exposure to COVID-19, and witnessing COVID—19–related morbidity and mortality.9 Many nurses were mandated to cross-train in unfamiliar clinical settings and adjust to new and prolonged shift schedules. Physical and emotional exhaustion associated with managing care for individuals with COVID-19, shortage of personal protective equipment (PPE), risk of infection, fear of secondary transmission to family members, feelings of being rejected by others, and social isolation, led to HCWs’ increased vulnerability to psychological impacts of the pandemic.8,10
A meta-analysis of 65 studies with > 79,000 participants found HCWs experienced significant levels of anxiety, depression, stress, insomnia, and other mental health issues, such as posttraumatic stress disorder (PTSD). Female HCWs, nurses, and frontline responders experienced a higher incidence of psychological impact.11 Other meta-analyses revealed that nurses’ compassion satisfaction, compassion fatigue, and burnout levels were significantly impacted with increased levels of burnout among nurses who had a friend or family member diagnosed with COVID- 19 or experienced prolonged threat of exposure to the virus.12,13 A study of 350 nurses found high rates of perceived transgressions by others, and betrayal.8 Nurse leaders and staff nurses had to persevere as moral distress became pervasive among nursing staff, which led to complex and often unsustainable circumstances. 14 The themes identified in the literature about the pandemic’s impact as well as witnessing nurse colleagues’ distress with patient mortality and death of coworkers during the early phase of the COVID-19 pandemic compelled a group of Veterans Health Administration (VHA) nurses to form a research team to understand the scope of impact and identify possible solutions.
Since published studies on the impact of pandemics on HCWs, including nurses, primarily focused on inpatient settings, the investigators of this study sought to capture the experiences of outpatient and inpatient nurses providing care in the US Department of Veterans Affairs (VA) Sierra Pacific Network (Veterans Integrated Service Network [VISN] 21), which has facilities in northern California, Hawaii, and Nevada.15-19 The purpose of this study was to identify the impact of COVID-19 on nurses caring for veterans in both outpatient and inpatient settings at VISN 21 facilities from March 2020 to September 2022, to inform leadership about the extent the virus affected nurses, and identify strategies that address current and future impacts of pandemics.
METHODS
This retrospective descriptive survey adapted the Pandemic Impact Survey by Purcell et al, which included the Moral Injury Events Scale, Primary Care PTSD Screener, the Patient Health Questionnaire-2 for depression, and a modified burnout scale.20-24 The survey of 70 Likert-scale questions was intended to measure nurses’ needs, burnout, moral distress, depression and stress symptoms, work-related factors, and intent to remain working in their current position. A nurse was defined broadly and included those employed as licensed vocational nurses (LVN), licensed practical nurses (LPN), registered nurses (RN), nurses with advanced degrees, advanced practice registered nurses (APRNs), and nurses with other certifications or licenses.
The VA Pacific Islands Research and Development Committee reviewed and approved the institutional review board-exempted study. The VISN 21 union was notified; only limited demographic information and broad VA tenure categories were collected to protect privacy. The principal investigator redacted facility identifier data after each facility had participated.
The survey was placed in REDCAP and a confidential link was emailed to all VISN 21 inpatient and outpatient nurses during March 2023. Because a comprehensive VISN 21 list of nurse email addresses was unavailable, the email was distributed by nursing leadership at each facility. Nurses received an email reminder at the 2-week halfway point, prompting them to complete the survey. The email indicated the purpose and voluntary nature of the study and cautioned nurses that they might experience stress while answering survey questions. Stress management resources were provided.
Descriptive statistics were used to report the results. Data were aggregated for analyzing and reporting purposes.
RESULTS
In March 2023, 860 of 5586 nurses (15%) responded to the survey. Respondents included 344 clinical inpatient nurses (40%) and 516 clinical outpatient nurses (60%); 688 (80%) were RNs, 129 (15%) were LPNs/LVNs, and 43 (5%) were APRNs. Of 849 respondents to provide their age, 15 (2%) were < 30 years, 163 (19%) were 30 to 39 years, 232 (27%) were 40 to 49 years, 259 (30%) were 50 to 59 years, and 180 (21%) were ≥ 60 years.
The survey found that 688 nurses reported job satisfaction (80%) and 75% of all respondents (66% among inpatient nurses) reported feeling happy with the care they delivered. Both inpatient and outpatient nurses indicated they could rely on staff. Sixty percent (n = 516) of the nurses indicated that facility management considered workplace health and safety and supervisors showed concern for subordinates, although inpatient nurses reported a lower percentage (Table 1).

Two hundred fifty-eight nurses (30%) reported having nurse colleagues who died and 52 (6%) had ≥ 3 colleagues who died. Among respondents, 292 had ≥ 3 patients who died after contracting COVID-19 and 232 (27%) had a significant person in their life die. More than one-half (54%; n = 464) of nurses had to limit contact with a family member who had COVID-19. Most nurses reported concerns about their colleagues (91%), were concerned about bringing COVID-19 home (82%), and stayed away from family during the pandemic (56%) (Table 2).

A total of 593 nurses (69%) reported feeling overwhelmed from the workload associated with the pandemic, 490 (57%) felt frustrated with role changes, 447 (52%) were stressed because of short staffing, and 327 (38%) felt stressed because of being assigned or floated to different patient care areas. Among inpatient nurses, 158 (46%) reported stress related to being floated. Coworker absenteeism caused challenges for 697 nurses (81%) (Table 3).

Nurses suggested a number of changes that could improve working conditions, including flexible scheduling (54%) and more hours of leave, which was requested by 43% of outpatient/inpatient nurses and 53% of inpatient alone nurses. Access to COVID-19 testing and PPE was endorsed as a workplace need by 439 nurses; the need for access to PPE was reported by 43% of inpatient-only nurses vs 29% of outpatient/inpatient nurses. The need for adequate staffing was reported by 54% of nurses although the rate was higher among those working inpatient settings (66%) (Table 4).

Four hundred sixty-four nurses (54%) felt tense and irritable at home because of work and 447 had ≥ 1 symptoms of burnout (Table 5). In terms of moral distress, > 30% of nurses witnessed morally incongruent situations, 10% felt their own moral code was violated, and > 30% felt betrayed by others (Table 6). Among respondents, 16% to 21% of nurses reported depressive symptoms (eAppendix). About 50% of nurses intended to stay in their current position while 20% indicated an intention to leave for another VA position.



DISCUSSION
This study identified the impact of COVID-19 on nurses who work in VISN 21. The survey included a significant number of nurses who work in outpatient settings, which differed from most other published studies to date.15-19 This study found that inpatient and outpatient nurses were similarly impacted by the COVID-19 pandemic, although there were differences. A high percentage of nurses reported job satisfaction despite the personal and professional impact of the pandemic.
Caring for veterans can result in a therapeutic relationship with a deep appreciation of veterans’ service and sensitivity to their needs.25 Some nurses reported that they feel it is a privilege to care for veterans.
Most nurses who participated in this study felt they could rely on their colleagues and were concerned about their health and wellbeing. Kissel et al explored protective factors for nurses during the pandemic and found participants often reported that their coworkers were positive safeguards.17 At least 50% of respondents reported that management considered workplace safety and was concerned about their welfare. Previous research has found that a positive working organization that promoted safety and concern for staff were protective factors against stress among HCWs.26 A literature review of 3 coronavirus outbreaks illustrated the support from supervisors and colleagues promoted resiliency and reduced stress disorders.3
Similar to other studies, study respondents experienced profound losses, including the deaths of colleagues, patients, and family. In 2021 Howell reported that HCWs experienced increased stress, fear, anxiety, and other negative emotions following news of colleagues’ deaths from COVID-19.27 Kissel et al reported that nurses frequently described pandemic-related physical and psychological harm and witnessing distress that they had not been previously exposed to.17
Our findings illustrate the tightrope nurses walked while caring for patients and concerns about the health of their colleagues and family. Consistent with our findings, Howell found that HCWs were afraid of contracting the infection at work and then unknowingly giving it to others such as patients, coworkers, and household members. 27 Murat et al reported that some nurses chose to live separately during the pandemic to avoid spreading COVID-19 to relatives.19 Several researchers found that concerns about family and children were prevalent and led to fear, anxiety, and burnout among nurses.18,28,29 Shah et al suggested that nurses experiencing death in the workplace and within their family may have resulted in fear and anxiety about returning to work.29 Garcia and Calvo argued that nurses may have been stigmatized as carriers of COVID-19.16 In addition, the loss of prepandemic workplace rituals may have impacted performance, team connection, and functioning, and led to increased turnover and decreased attachment to the organization.30
This study described the significant workplace issues nurses endured during the pandemic, including being overwhelmed with additional and/or multiple roles and frustrated and stressed with role changes and short staffing. Nurses endorsed workplace challenges in the context of coworker absenteeism and reassignments to different areas, such as intensive care units (ICUs).17 Researchers also reported that displaced team members experienced loneliness and isolation when they were removed from their usual place of work and experienced distress caring for patients beyond their perceived competency or comfort.17,31 Nurses also experienced rapid organizational changes, resource scarcity, high patient-to-nurse ratios, inconsistent or limited communications, and the absence of protocols for prolonged mass casualty events.17 These challenges, such as significant uncertainty and rapidly changing working conditions, were shared experiences suggested to be similar to “tumbling into chaos,” and likened to the overwhelming situations faced during patient surges to a medical “war zone.”17
Study respondents indicated that nurses wanted better access to critical supplies, PPE, and COVID-19 testing; more flexible scheduling; longer leave times; and staffing that was appropriate to the patient volumes. These findings aligned with previous research. Howell found that HCWs, especially nurses, worried about childcare because of school closures and increased work hours.27 Nurses felt that hospital support was inaccessible or inadequate and worried about access to essential resources.17-19,27 Studies also found excessive workloads, and many nurses needed mental or financial assistance from the hospital in addition to more rest and less work.18,28 An editorial highlighted the potential adverse effects that a lack of PPE could have on staff ’s mental health because of perceptions of institutional betrayal, which occurs when trusted and powerful organizations seemingly act in ways that can harm those dependent on them for safety and well-being.32
Consistent with other research, this study found that a majority of nurses experienced significant burnout symptoms. The number of nurses reporting symptoms of burnout increased during the pandemic with ICU nurses reporting the highest levels.17,33 Soto-Rubio et al emphasized that working conditions experienced by nurses, such as interpersonal conflict, lack of trust in administration, workload, and role conflict, contributed to burnout during COVID-19.34 Other studies found that nurses experienced burnout caused by uncertainty, intense work, and extra duties contributed to higher burnout scores.18,19 It is not surprising that researchers have indicated that nurses experiencing burnout might display depressive and stress-related symptoms, insomnia, and concentration and memory problems.19
The results of this study indicate that one-third of participating nurses were experiencing moral distress. Burton et al described COVID-19 as an environment in which nurses witnessed, experienced, and at times had to participate in acts that involved ethical violations in care, institutional betrayal, and traumatic strain.9 Of note, our findings revealed that both inpatient and outpatient nurses experienced moral distress. Interestingly, Mantri et al found that COVID-19 increased moral injury but not burnout among health professionals, which differed from the results of this study.35
The findings of this study indicate that many nurses experienced depressive symptoms. A systematic review found a similar percentage of HCWs experienced depression while caring for patients with COVID- 19, though a Chinese study found a higher percentage.36,37 Previous research also found that the most difficult aspect of the COVID- 19 pandemic for nurses was coping with mental disorders such as depression, and that many experienced difficulty sleeping/ had poor sleep quality, believed a similar disaster would occur in the future, were irritated or angered easily, and experienced emotional exhaustion.15,19 The long-term mental and physical ramifications of caring for individuals with COVID-19 remain unknown. However, previous research suggests a high prevalence of depression, insomnia, anxiety, and distress, which could impair nurses’ professional performance.29
This study reported that a majority of nurses intended to stay in their current position and about 20% intended to leave for another position within the VA. Similar findings conducted early in the pandemic indicated that most participants did not intend to quit nursing.19
This study’s findings suggest the COVID-19 pandemic had an adverse impact on VISN 21 nurses. It is critical to develop, implement, and adopt adequate measures as early as possible to support the health care system, especially nurses.18
Implications
Before the COVID-19 pandemic, discussing burnout and moral anguish was common, primarily in critical care.14 However, these experiences became more widespread throughout nursing settings during the pandemic. Nurse leaders have been identified as responsible for ensuring the environmental safety and personal well-being of their colleagues during and after pandemics.14
Studies of HCW experiences during COVID-19 provide many insights into future preparedness, strategies to best handle another pandemic during its acute stage, and techniques to address issues that might persist. This study and others suggest that comprehensive interventions in preparation for, during, and after a pandemic are needed. We break down strategies into pandemic and postpandemic interventions based on a synthesis of the literature and the research team’s knowledge and expertise.3,14-16,27,29,36,38-44
Pandemic interventions. During a pandemic, it is important that nurses are adequately cared for to ensure they can continue to provide quality care for others. Resources supporting emotional well-being and addressing moral distress offered during a pandemic are essential. Implementing meaningful strategies could enhance nurses’ health and wellbeing. It is essential that leaders provide nurses a safe work environment/experience during a pandemic by instituting meaningful resources. In addition, developing best practices for leadership are critical.
Postpandemic interventions. Personal experiences of depression, burnout, and moral distress have not spontaneously resolved as the pandemic receded. Providing postpandemic interventions to lessen ongoing and lingering depressive, burnout, and moral distress symptoms experienced by frontline workers are critical. These interventions might prevent long-term health issues and the exodus of nurses.
Postpandemic interventions should include the integration of pandemic planning into new or existing educational or training programs for staff. Promotion and support of mental health services by health system leadership for nursing personnel implemented as a usual service will play an important role in preparing for future pandemics. A key role in preparation is developing and maintaining cooperation and ongoing mutual understanding, respect, and communication between leadership and nursing staff.
Future Research
This study’s findings inform VHA leadership and society about how a large group of nurses were impacted by COVID-19 while caring for patients in inpatient and outpatient settings and could provide a basis for extending this research to other groups of nurses or health care personnel. Future research might be helpful in identifying the impact of COVID-19 on nursing leadership. During conversations with nursing leadership, a common theme identified was that nurses did not feel that leadership was fully prepared for the level of emergency the pandemic created both personally and professionally; leadership expressed experiences similar to nurses providing direct care and felt powerless to help their nursing staff. Other areas of research could include identifying underlying factors contributing to burnout and moral distress and describing nurses’ expectations of or needs from leadership to best manage burnout and moral distress.
Limitations
Experiences of nurses who stopped working were not captured and information about their experiences might have different results. The survey distribution was limited to 2 emails (an initial email and a second at midpoint) sent at the discretion of the nurse executive of each facility. The study timeline was long because of complex regulatory protective processes inherent in the VHA system for researchers to include initial institutional review board review process, union notifications, and each facility’s response to the survey. Although 860 nurses participated, this was 15% of the 5586 VISN 21 nurses at the time of the study. Many clinical inpatient nurses do not have regular access to email, which might have impacted participation rate.
CONCLUSIONS
This study identified the impact COVID-19 had on nurses who worked in a large hospital system. The research team outlined strategies to be employed during and after the pandemic, such as preplanning for future pandemics to provide a framework for a comprehensive pandemic response protocol.
This study adds to generalized knowledge because it captured voices of inpatient and outpatient nurses, the latter had not been previously studied. As nurses and health care organizations move beyond the pandemic with a significant number of nurses continuing to experience effects, there is a need to institute interventions to assist nurses in healing and begin preparations for future pandemics.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
- Liu X, Kakade M, Fuller CJ, et al. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53(1):15-23. doi:10.1016/j.comppsych.2011.02.003
- Carmassi C, Foghi C, Dell’Oste V, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: What can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312. doi:10.1016/j.psychres.2020.113312
- De Kock JH, Latham HA, Leslie SJ, et al. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21(1):104. doi:10.1186/s12889-020-10070-3
- Gualano MR, Sinigaglia T, Lo Moro G, et al. The burden of burnout among healthcare professionals of intensive care units and emergency departments during the covid-19 pandemic: a systematic review. Int J Environ Res Public Health. 2021;18(15):8172. doi:10.3390/ijerph18158172
- Sirois FM, Owens J. Factors associated with psychological distress in health-care workers during an infectious disease outbreak: a rapid systematic review of the evidence. Front Psychiatry. 2020;11;589545. doi:10.3389/fpsyt.2020.589545
- Talevi D, Socci V, Carai M, et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiatr. 2020;55(3);137-144. doi:10.1708/3382.33569
- Amsalem D, Lazarov A, Markowitz JC, et al. Psychiatric symptoms and moral injury among US healthcare workers in the COVID-19 era. BMC Psychiatry. 2021;21(1):546. doi:10.1186/s12888-021-03565-9
- Burton CW, Jenkins DK, Chan G.K, Zellner KL, Zalta AK. A mixed methods study of moral distress among frontline nurses during the COVID-19 pandemic. Psychol Trauma. 2023;16(4):568-575. doi:10.1037/tra0001493
- Stawicki SP, Jeanmonod R, Miller AC, et al. The 2019- 2020 novel coronavirus (Severe acute respiratory syndrome coronavirus 2) Pandemic:a Joint American College of Academic International Medicine-World Academic Council of Emergency Medicine Multidisciplinary COVID-19 Working Group consensus paper. J Glob Infect Dis. 2020;12(2):47- 93. doi:10.4103/jgid.jgid_86_20
- Batra K, Singh TP, Sharma M, Batra R, Schvaneveldt N. Investigating the psychological impact of COVID- 19 among healthcare workers: a meta-analysis. Int J Environ Res Public Health. 2020;17(23):9096. doi:10.3390/ijerph17239096
- Xie W, Chen L, Feng F, et al. The prevalence of compassion satisfaction and compassion fatigue among nurses: a systematic review and meta-analysis. Int J Nurs Stud. 2021;120:103973. doi:10.1016/j.ijnurstu.2021.103973
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Nurses’ burnout and associated risk factors during the COVID-19 pandemic: a systematic review and meta-analysis. J Adv Nurs. 2021;77(8):3286-3302. doi:10.1111/jan.14839
- Hofmeyer A, Taylor R. Strategies and resources for nurse leaders to use to lead with empathy and prudence so they understand and address sources of anxiety among nurses practicing in the era of COVID-19. J Clin Nurs. 2021;30(1- 2):298-305. doi:10.1111/jocn.15520
- Chen R, Sun C, Chen JJ, et al. A large-scale survey on trauma, burnout, and posttraumatic growth among nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(1):102-116. doi:10.1111/inm.12796
- García G, Calvo J. The threat of COVID-19 and its influence on nursing staff burnout. J Adv Nurs. 2021;77(2):832-844. doi:10.1111/jan.14642
- Kissel KA, Filipek C, Jenkins J. Impact of the COVID- 19 pandemic on nurses working in intensive care units: a scoping review. Crit Care Nurse. 2023;43(2):55-63. doi:10.4037/ccn2023196
- Lin YY, Pan YA, Hsieh YL, et al. COVID-19 pandemic is associated with an adverse impact on burnout and mood disorder in healthcare professionals. Int J Environ Res and Public Health. 2021;18(7):3654. doi:10.3390/ijerph18073654
- Murat M, Köse S, Savas¸er S. Determination of stress, depression and burnout levels of front-line nurses during the COVID-19 pandemic. Int J Ment Health Nurs. 2021;30(2):533-543. doi:10.1111/inm.12818
- Purcell N, Bertenthal D, Usman H, et al. Moral injury and mental health in healthcare workers are linked to organizational culture and modifiable workplace conditions: results of a national, mixed-methods study conducted at Veterans Affairs (VA) medical centers during the COVID- 19 pandemic. PLOS Ment Health. 2024;1(7):e0000085. doi:10.1371/journal.pmen.0000085
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the Moral Injury Events Scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Prins A, Bovin MJ, Smolenski DJ, et al. The Primary Care PTSD Screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016;31(10):1206-1211. doi:10.1007/s11606-016-3703-5
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284-1292. doi:10.1097/01.MLR.0000093487.78664.3C
- Rohland BM, Kruse GR, Rohrer JE. Validation of a single- item measure of burnout against the Maslach Burnout Inventory among physicians. Stress and Health. 2004;20(2):75-79. doi:10.1002/smi.1002
- Carlson J. Baccalaureate nursing faculty competencies and teaching strategies to enhance the care of the veteran population: perspectives of Veteran Affairs Nursing Academy (VANA) faculty. J Prof Nurs. 2016;32(4):314-323. doi:10.1016/j.profnurs.2016.01.006
- Denning M, Goh ET, Tan B, et al. Determinants of burnout and other aspects of psychological well-being in healthcare workers during the Covid-19 pandemic: a multinational cross-sectional study. PloS One. 2021;16(4):e0238666. doi:10.1371/journal.pone.0238666
- Howell BAM. Battling burnout at the frontlines of health care amid COVID-19. AACN Adv Crit Care. 2021;32(2):195- 203. doi:10.4037/aacnacc2021454
- Afshari D, Nourollahi-Darabad M, Chinisaz N. Demographic predictors of resilience among nurses during the COVID-19 pandemic. Work. 2021;68(2):297-303. doi:10.3233/WOR-203376
- Shah M, Roggenkamp M, Ferrer L, Burger V, Brassil KJ. Mental health and COVID-19: the psychological implications of a pandemic for nurses. Clin J Oncol Nurs. 2021;25(1), 69-75. doi:10.1188/21.CJON.69-75
- Griner T, Souza M, Girard A, Hain P, High H, Williams M. COVID-19’s impact on nurses’ workplace rituals. Nurs Lead. 2021;19(4):425-430. doi:10.1016/j.mnl.2021.06.008
- Koren A, Alam MAU, Koneru S, DeVito A, Abdallah L, Liu B. Nursing perspectives on the impacts of COVID- 19: social media content analysis. JMIR Form Res. 2021;5(12):e31358. doi:10.2196/31358
- Gold JA. Covid-19: adverse mental health outcomes for healthcare workers. BMJ. 2020;5:369:m1815. doi: 10.1136/bmj.m1815. doi:10.1136/bmj.m1815
- Slusarz R, Cwiekala-Lewis K, Wysokinski M, Filipska- Blejder K, Fidecki W, Biercewicz M. Characteristics of occupational burnout among nurses of various specialties and in the time of the COVID-19 pandemic-review. Int J Environ Res Public Health. 2022;19(21):13775. doi:10.3390/ijerph192113775
- Soto-Rubio A, Giménez-Espert MDC, Prado-Gascó V. Effect of emotional intelligence and psychosocial risks on burnout, job satisfaction, and nurses’ health during the COVID-19 pandemic. Int J Environ Res Public Health. 2020;17(21):7998. doi:10.3390/ijerph17217998
- Mantri S, Song YK, Lawson JM, Berger EJ, Koenig HG. Moral injury and burnout in health care professionals during the COVID-19 pandemic. J Nerv Ment Dis. 2021;209(10):720-726. doi:10.1097/NMD.0000000000001367
- Salari N, Khazaie H, Hosseinian-Far A, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health 2020;18(1):100. doi:10.1186/s12960-020-00544-1
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976
- Chesak SS, Cutshall SM, Bowe CL, Montanari KM, Bhagra A. Stress management interventions for nurses: critical literature review. J Holist Nurs. 2019;37(3):288-295. doi:10.1177/0898010119842693
- Cooper AL, Brown JA, Leslie GD. Nurse resilience for clinical practice: an integrative review. J Adv Nurs. 2021;77(6):2623-2640. doi:10.1111/jan.14763
- Melnyk BM, Kelly SA, Stephens J, et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am J Health Promot. 2020;34(8):929-941. doi:10.1177/0890117120920451
- Cho H, Sagherian K, Steege LM. Hospital staff nurse perceptions of resources and resource needs during the COVID-19 pandemic. Nurs Outlook. 2023;71(3):101984. doi:10.1016/j.outlook.2023.101984
- Bachem R, Tsur N, Levin Y, Abu-Raiya H, Maercker A. Negative affect, fatalism, and perceived institutional betrayal in times of the coronavirus pandemic: a cross-cultural investigation of control beliefs. Front Psychiatry. 2020;11:589914. doi:10.3389/fpsyt.2020.589914
- Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323(21):2133. doi:10.1001/jama.2020.5893
- Schuster M, Dwyer PA. Post-traumatic stress disorder in nurses: an integrative review. J Clin Nurs. 2020;29(15- 16):2769-2787. doi:10.1111/jocn.15288
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey
COVID-19 Impact on Veterans Health Administration Nurses: A Retrospective Survey




