User login
Phase 1 study shows feasibility, safety, efficacy of STAR T cells for ALL
A phase 1 first-in-human study demonstrated synthetic T-cell receptor and antigen receptor (STAR) technical feasibility, clinical safety and efficacy in treating CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia (ALL), according to senior study author Peihua Lu, MD, Beijing Lu Daopei Institute of Hematology, Beijing, China. STAR T cells were found to be superior to conventional chimeric antigen receptor (CAR) T cells with respect to signaling capacity, cytokine production and antitumor potency in an animal model study, according to Dr. Lu’s presentation at the annual meeting of the American Society of Hematology.
Remission can be improved
While CAR T-cell therapy has demonstrated high response rates in patients with B-cell malignancies, remission durability and safety can be improved, Dr Lu said. Her team developed STAR, a novel double-chain chimeric receptor consisting of two protein modules, each containing an antibody light or heavy chain variable region, the T cell receptor (TCR) alpha or beta chain constant region fused to the OX-40 costimulatory domain. The 2 modules are linked by a self-cleaving Furin-p2A sequence that allows the modules to be proteolytically separated and reconstituted. In preclinical in vitro research, STAR-T-cells showed a much faster and stronger cell activation, compared with CAR T cells and superior target cell–killing ability, and higher levels of interferon-y after coculture with the CD19+ Raji cell. In a murine in vivo study, STAR-T cells had higher antileukemia activity, compared with CAR-T cells, and significantly inhibited tumor cell growth, Dr. Lu stated. All animals were sustainably tumor free 5 days after STAR-T cell injection.
The first-in-human study included 18 CD19+ relapsed/refractory B-cell ALL (median age 22.5 years) patients, with a median bone marrow blast level pre–CAR T of 15.3%.
The manufacture success rate was 100% and took about 9 days (7-13). Transduction efficacy was 57.4% (41.0%-78.2%). Subjects received a conditioning regimen of intravenous fludarabine (25mg/m2 per day) and cyclophosphamide (250mg/m2 per day) for 3 days followed by a single STAR T-cell infusion. Patients were given the option, after they achieved complete remission (CR), of proceeding to consolidation allogeneic hematopoietic stem cell transplantation (allo-HSCT).
100% MRD negative
On day 14 following transplant, 18/18 had achieved minimal residual disease–negative complete response/CRi (with incomplete hematologic recovery). One patient relapsed after allogeneic transplant, becoming minimal residual disease positive on day 28. After a median follow-up of 105 days, 11/18 bridged into allo-HSCT without relapse. Among the seven patients who did not undergo allo-HSCT, one relapsed on day 58 and died on day 63. The patient had CNS leukemia and 87% bone marrow blasts before receiving STAR T. The others, Dr. Lu said, remain in CR.
Mild cytokine release syndrome (CRS) occurred in only 10 patients (55.6%), with grade 1 CRS in 8 patients and grade 2 in 2 patients. Grade 3 neurotoxicity occurred in two patients.
Reporting cellular kinetics of STAR T cells in peripheral blood by fluorescence-activated cell sorting (FACS)/quantitative PCR showed the highest STAR-T proliferation ratio (STAR/CD3) of 88.1%. Median peak level was 4.9 x 104 copies number/mcg genomic DNA. The peak time was day 8.5 and the longest detection time was 6 months after STAR T infusion (STAR T ratio, 0.46%-1.85%). High in vivo proliferation and persistence was observed regardless of infusion dose.
STAR holds promise
Dr. Lu concluded: “The phase 1 first-in-human study demonstrated technical feasibility, clinical safety and efficacy of STAR T in treating CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia.” She noted also that long-term observation of these patients and studies of larger patient cohorts are warranted to evaluate a beneficial advantage of the STAR T over the conventional CAR T product.
Asked about future directions in the discussion period, Dr. Lu responded that “this product holds great promise, No. 1 because it is actually between a T-cell receptor and a CAR T, and so clearly has fewer side effects. It potentially can recognize and target the tumor intracellular antigen better than a conventional CAR T. It is easier to construct – and holds great promise for treating solid tumors.”
Dr. Lu reported that she had no relevant disclosures.
SOURCE: Lu P et al. ASH 2020, Abstract 270.
A phase 1 first-in-human study demonstrated synthetic T-cell receptor and antigen receptor (STAR) technical feasibility, clinical safety and efficacy in treating CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia (ALL), according to senior study author Peihua Lu, MD, Beijing Lu Daopei Institute of Hematology, Beijing, China. STAR T cells were found to be superior to conventional chimeric antigen receptor (CAR) T cells with respect to signaling capacity, cytokine production and antitumor potency in an animal model study, according to Dr. Lu’s presentation at the annual meeting of the American Society of Hematology.
Remission can be improved
While CAR T-cell therapy has demonstrated high response rates in patients with B-cell malignancies, remission durability and safety can be improved, Dr Lu said. Her team developed STAR, a novel double-chain chimeric receptor consisting of two protein modules, each containing an antibody light or heavy chain variable region, the T cell receptor (TCR) alpha or beta chain constant region fused to the OX-40 costimulatory domain. The 2 modules are linked by a self-cleaving Furin-p2A sequence that allows the modules to be proteolytically separated and reconstituted. In preclinical in vitro research, STAR-T-cells showed a much faster and stronger cell activation, compared with CAR T cells and superior target cell–killing ability, and higher levels of interferon-y after coculture with the CD19+ Raji cell. In a murine in vivo study, STAR-T cells had higher antileukemia activity, compared with CAR-T cells, and significantly inhibited tumor cell growth, Dr. Lu stated. All animals were sustainably tumor free 5 days after STAR-T cell injection.
The first-in-human study included 18 CD19+ relapsed/refractory B-cell ALL (median age 22.5 years) patients, with a median bone marrow blast level pre–CAR T of 15.3%.
The manufacture success rate was 100% and took about 9 days (7-13). Transduction efficacy was 57.4% (41.0%-78.2%). Subjects received a conditioning regimen of intravenous fludarabine (25mg/m2 per day) and cyclophosphamide (250mg/m2 per day) for 3 days followed by a single STAR T-cell infusion. Patients were given the option, after they achieved complete remission (CR), of proceeding to consolidation allogeneic hematopoietic stem cell transplantation (allo-HSCT).
100% MRD negative
On day 14 following transplant, 18/18 had achieved minimal residual disease–negative complete response/CRi (with incomplete hematologic recovery). One patient relapsed after allogeneic transplant, becoming minimal residual disease positive on day 28. After a median follow-up of 105 days, 11/18 bridged into allo-HSCT without relapse. Among the seven patients who did not undergo allo-HSCT, one relapsed on day 58 and died on day 63. The patient had CNS leukemia and 87% bone marrow blasts before receiving STAR T. The others, Dr. Lu said, remain in CR.
Mild cytokine release syndrome (CRS) occurred in only 10 patients (55.6%), with grade 1 CRS in 8 patients and grade 2 in 2 patients. Grade 3 neurotoxicity occurred in two patients.
Reporting cellular kinetics of STAR T cells in peripheral blood by fluorescence-activated cell sorting (FACS)/quantitative PCR showed the highest STAR-T proliferation ratio (STAR/CD3) of 88.1%. Median peak level was 4.9 x 104 copies number/mcg genomic DNA. The peak time was day 8.5 and the longest detection time was 6 months after STAR T infusion (STAR T ratio, 0.46%-1.85%). High in vivo proliferation and persistence was observed regardless of infusion dose.
STAR holds promise
Dr. Lu concluded: “The phase 1 first-in-human study demonstrated technical feasibility, clinical safety and efficacy of STAR T in treating CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia.” She noted also that long-term observation of these patients and studies of larger patient cohorts are warranted to evaluate a beneficial advantage of the STAR T over the conventional CAR T product.
Asked about future directions in the discussion period, Dr. Lu responded that “this product holds great promise, No. 1 because it is actually between a T-cell receptor and a CAR T, and so clearly has fewer side effects. It potentially can recognize and target the tumor intracellular antigen better than a conventional CAR T. It is easier to construct – and holds great promise for treating solid tumors.”
Dr. Lu reported that she had no relevant disclosures.
SOURCE: Lu P et al. ASH 2020, Abstract 270.
A phase 1 first-in-human study demonstrated synthetic T-cell receptor and antigen receptor (STAR) technical feasibility, clinical safety and efficacy in treating CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia (ALL), according to senior study author Peihua Lu, MD, Beijing Lu Daopei Institute of Hematology, Beijing, China. STAR T cells were found to be superior to conventional chimeric antigen receptor (CAR) T cells with respect to signaling capacity, cytokine production and antitumor potency in an animal model study, according to Dr. Lu’s presentation at the annual meeting of the American Society of Hematology.
Remission can be improved
While CAR T-cell therapy has demonstrated high response rates in patients with B-cell malignancies, remission durability and safety can be improved, Dr Lu said. Her team developed STAR, a novel double-chain chimeric receptor consisting of two protein modules, each containing an antibody light or heavy chain variable region, the T cell receptor (TCR) alpha or beta chain constant region fused to the OX-40 costimulatory domain. The 2 modules are linked by a self-cleaving Furin-p2A sequence that allows the modules to be proteolytically separated and reconstituted. In preclinical in vitro research, STAR-T-cells showed a much faster and stronger cell activation, compared with CAR T cells and superior target cell–killing ability, and higher levels of interferon-y after coculture with the CD19+ Raji cell. In a murine in vivo study, STAR-T cells had higher antileukemia activity, compared with CAR-T cells, and significantly inhibited tumor cell growth, Dr. Lu stated. All animals were sustainably tumor free 5 days after STAR-T cell injection.
The first-in-human study included 18 CD19+ relapsed/refractory B-cell ALL (median age 22.5 years) patients, with a median bone marrow blast level pre–CAR T of 15.3%.
The manufacture success rate was 100% and took about 9 days (7-13). Transduction efficacy was 57.4% (41.0%-78.2%). Subjects received a conditioning regimen of intravenous fludarabine (25mg/m2 per day) and cyclophosphamide (250mg/m2 per day) for 3 days followed by a single STAR T-cell infusion. Patients were given the option, after they achieved complete remission (CR), of proceeding to consolidation allogeneic hematopoietic stem cell transplantation (allo-HSCT).
100% MRD negative
On day 14 following transplant, 18/18 had achieved minimal residual disease–negative complete response/CRi (with incomplete hematologic recovery). One patient relapsed after allogeneic transplant, becoming minimal residual disease positive on day 28. After a median follow-up of 105 days, 11/18 bridged into allo-HSCT without relapse. Among the seven patients who did not undergo allo-HSCT, one relapsed on day 58 and died on day 63. The patient had CNS leukemia and 87% bone marrow blasts before receiving STAR T. The others, Dr. Lu said, remain in CR.
Mild cytokine release syndrome (CRS) occurred in only 10 patients (55.6%), with grade 1 CRS in 8 patients and grade 2 in 2 patients. Grade 3 neurotoxicity occurred in two patients.
Reporting cellular kinetics of STAR T cells in peripheral blood by fluorescence-activated cell sorting (FACS)/quantitative PCR showed the highest STAR-T proliferation ratio (STAR/CD3) of 88.1%. Median peak level was 4.9 x 104 copies number/mcg genomic DNA. The peak time was day 8.5 and the longest detection time was 6 months after STAR T infusion (STAR T ratio, 0.46%-1.85%). High in vivo proliferation and persistence was observed regardless of infusion dose.
STAR holds promise
Dr. Lu concluded: “The phase 1 first-in-human study demonstrated technical feasibility, clinical safety and efficacy of STAR T in treating CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia.” She noted also that long-term observation of these patients and studies of larger patient cohorts are warranted to evaluate a beneficial advantage of the STAR T over the conventional CAR T product.
Asked about future directions in the discussion period, Dr. Lu responded that “this product holds great promise, No. 1 because it is actually between a T-cell receptor and a CAR T, and so clearly has fewer side effects. It potentially can recognize and target the tumor intracellular antigen better than a conventional CAR T. It is easier to construct – and holds great promise for treating solid tumors.”
Dr. Lu reported that she had no relevant disclosures.
SOURCE: Lu P et al. ASH 2020, Abstract 270.
FROM ASH 2020
Sac/val heart failure benefit extends to diabetes patients
The beneficial effects of sacubitril/valsartan on reverse cardiac remodeling in patients with heart failure and reduced ejection fraction have been well established, but those benefits haven’t been as clearly demonstrated to carry over to HFrEF patients who also have type 2 diabetes mellitus (T2DM).
Now, a post-hoc analysis of a pivotal clinical trial reports that those benefits do extend to patients with HFrEF and T2DM.
“It’s really not about a Sophie’s choice of whether you give this or that drug in these patients,” said corresponding author Javed Butler, MD, MPH, MBA. “We really ought to be giving all of these drugs – the angiotensin receptor neprilysin inhibitors (ARNIs) and sodium-glucose transporter 2 (SGLT-2) inhibitors – to our patients for the best outcomes.”
The post-hoc analysis, published in JACC: Heart Failure, evaluated 361 patients with T2DM who were enrolled in the PROVE-HF trial of sac/val therapy for HF, published in JAMA.
PROVE-HF evaluated biomarkers, myocardial remodeling, and outcomes through a year of treatment with sac/val. The primary endpoint was the level of changes in natriuretic peptide (NT-proBNP) concentrations, left-ventricle ejection fraction (LVEF) and overall Kansas City Cardiomyopathy Questionnaire (KCCQ)-23 scores through 12 months of treatment.
The post hoc study reported that baseline NT-proBNP concentrations were higher in the DM patients (854 pg/mL vs. 706 pg/mL), but at 12 months those levels were 513 and 441 pg/mL, respectively.
LVEF changed similarly from baseline to 12 months in both groups: from 28.3% to 37% in the DM patients and from 28.1% to 38.34% in non-DM patients. Overall KCCQ-23 scores improved similarly in both groups, but longitudinal analyses found modestly higher gains in the T2DM group, 9.3 vs. 8.6 points (P = .07).
“The real reason I wanted to do this study is that I’m a huge fan of all the SGLT-2 inhibitors, and I’m very involved in those trials, and there is right now so much momentum behind SGLT-2 inhibitors that I don’t want people to forget that ARNI is still the base therapy for HF,” said Dr. Butler, chair of cardiovascular research and the department of medicine at the University of Mississippi in Jackson.
He noted that the size of the diabetes cohort in PROVE-HF “is a nonissue” for evaluating power of the post hoc analysis because it tracked key measures in the study population continuously at eight intervals over the 12 months.
The analysis further demonstrates the synergistic effects of ARNI and SGLT-2 inhibitors in patients with T2DM and HF that were also reported in the PARADIGM-HF study, Dr. Butler said.
“We have sort of moved on, saying that SGLT-2 inhibitors have a benefit on the heart, but the reverse is also true: ARNIs are still heart failure drugs, and we don’t think of them as diabetes drugs, but the PARADIGM-HF data showed that there was a substantial reduction in hemoglobin A1c in those who had diabetes,” he said.
The researchers noted that an absence of a control group may contribute to an overestimation of reverse cardiac remodeling in the T2DM patients, and that the PROVE-HF study wasn’t prospectively powered to delineate differences in how sac/val therapy affected patients with or without diabetes. “Future investigations seeking to evaluate differences by T2DM status after sacubitril/valsartan initiation may use our findings to plan prospective sample sizes,” the researchers wrote.
Dr. Butler disclosed financial relationships with Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, StealthPeptide and Vifor. Lead author Muhammad Shahzeb Khan, MD, MSc, a professor at the University of Mississippi, has no relevant financial relationships to disclose.
SOURCE: Kahn MS et al. JACC: HF. 2020 Dec 9. doi: 10.1016/j.jchf.2020.09.014.
The beneficial effects of sacubitril/valsartan on reverse cardiac remodeling in patients with heart failure and reduced ejection fraction have been well established, but those benefits haven’t been as clearly demonstrated to carry over to HFrEF patients who also have type 2 diabetes mellitus (T2DM).
Now, a post-hoc analysis of a pivotal clinical trial reports that those benefits do extend to patients with HFrEF and T2DM.
“It’s really not about a Sophie’s choice of whether you give this or that drug in these patients,” said corresponding author Javed Butler, MD, MPH, MBA. “We really ought to be giving all of these drugs – the angiotensin receptor neprilysin inhibitors (ARNIs) and sodium-glucose transporter 2 (SGLT-2) inhibitors – to our patients for the best outcomes.”
The post-hoc analysis, published in JACC: Heart Failure, evaluated 361 patients with T2DM who were enrolled in the PROVE-HF trial of sac/val therapy for HF, published in JAMA.
PROVE-HF evaluated biomarkers, myocardial remodeling, and outcomes through a year of treatment with sac/val. The primary endpoint was the level of changes in natriuretic peptide (NT-proBNP) concentrations, left-ventricle ejection fraction (LVEF) and overall Kansas City Cardiomyopathy Questionnaire (KCCQ)-23 scores through 12 months of treatment.
The post hoc study reported that baseline NT-proBNP concentrations were higher in the DM patients (854 pg/mL vs. 706 pg/mL), but at 12 months those levels were 513 and 441 pg/mL, respectively.
LVEF changed similarly from baseline to 12 months in both groups: from 28.3% to 37% in the DM patients and from 28.1% to 38.34% in non-DM patients. Overall KCCQ-23 scores improved similarly in both groups, but longitudinal analyses found modestly higher gains in the T2DM group, 9.3 vs. 8.6 points (P = .07).
“The real reason I wanted to do this study is that I’m a huge fan of all the SGLT-2 inhibitors, and I’m very involved in those trials, and there is right now so much momentum behind SGLT-2 inhibitors that I don’t want people to forget that ARNI is still the base therapy for HF,” said Dr. Butler, chair of cardiovascular research and the department of medicine at the University of Mississippi in Jackson.
He noted that the size of the diabetes cohort in PROVE-HF “is a nonissue” for evaluating power of the post hoc analysis because it tracked key measures in the study population continuously at eight intervals over the 12 months.
The analysis further demonstrates the synergistic effects of ARNI and SGLT-2 inhibitors in patients with T2DM and HF that were also reported in the PARADIGM-HF study, Dr. Butler said.
“We have sort of moved on, saying that SGLT-2 inhibitors have a benefit on the heart, but the reverse is also true: ARNIs are still heart failure drugs, and we don’t think of them as diabetes drugs, but the PARADIGM-HF data showed that there was a substantial reduction in hemoglobin A1c in those who had diabetes,” he said.
The researchers noted that an absence of a control group may contribute to an overestimation of reverse cardiac remodeling in the T2DM patients, and that the PROVE-HF study wasn’t prospectively powered to delineate differences in how sac/val therapy affected patients with or without diabetes. “Future investigations seeking to evaluate differences by T2DM status after sacubitril/valsartan initiation may use our findings to plan prospective sample sizes,” the researchers wrote.
Dr. Butler disclosed financial relationships with Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, StealthPeptide and Vifor. Lead author Muhammad Shahzeb Khan, MD, MSc, a professor at the University of Mississippi, has no relevant financial relationships to disclose.
SOURCE: Kahn MS et al. JACC: HF. 2020 Dec 9. doi: 10.1016/j.jchf.2020.09.014.
The beneficial effects of sacubitril/valsartan on reverse cardiac remodeling in patients with heart failure and reduced ejection fraction have been well established, but those benefits haven’t been as clearly demonstrated to carry over to HFrEF patients who also have type 2 diabetes mellitus (T2DM).
Now, a post-hoc analysis of a pivotal clinical trial reports that those benefits do extend to patients with HFrEF and T2DM.
“It’s really not about a Sophie’s choice of whether you give this or that drug in these patients,” said corresponding author Javed Butler, MD, MPH, MBA. “We really ought to be giving all of these drugs – the angiotensin receptor neprilysin inhibitors (ARNIs) and sodium-glucose transporter 2 (SGLT-2) inhibitors – to our patients for the best outcomes.”
The post-hoc analysis, published in JACC: Heart Failure, evaluated 361 patients with T2DM who were enrolled in the PROVE-HF trial of sac/val therapy for HF, published in JAMA.
PROVE-HF evaluated biomarkers, myocardial remodeling, and outcomes through a year of treatment with sac/val. The primary endpoint was the level of changes in natriuretic peptide (NT-proBNP) concentrations, left-ventricle ejection fraction (LVEF) and overall Kansas City Cardiomyopathy Questionnaire (KCCQ)-23 scores through 12 months of treatment.
The post hoc study reported that baseline NT-proBNP concentrations were higher in the DM patients (854 pg/mL vs. 706 pg/mL), but at 12 months those levels were 513 and 441 pg/mL, respectively.
LVEF changed similarly from baseline to 12 months in both groups: from 28.3% to 37% in the DM patients and from 28.1% to 38.34% in non-DM patients. Overall KCCQ-23 scores improved similarly in both groups, but longitudinal analyses found modestly higher gains in the T2DM group, 9.3 vs. 8.6 points (P = .07).
“The real reason I wanted to do this study is that I’m a huge fan of all the SGLT-2 inhibitors, and I’m very involved in those trials, and there is right now so much momentum behind SGLT-2 inhibitors that I don’t want people to forget that ARNI is still the base therapy for HF,” said Dr. Butler, chair of cardiovascular research and the department of medicine at the University of Mississippi in Jackson.
He noted that the size of the diabetes cohort in PROVE-HF “is a nonissue” for evaluating power of the post hoc analysis because it tracked key measures in the study population continuously at eight intervals over the 12 months.
The analysis further demonstrates the synergistic effects of ARNI and SGLT-2 inhibitors in patients with T2DM and HF that were also reported in the PARADIGM-HF study, Dr. Butler said.
“We have sort of moved on, saying that SGLT-2 inhibitors have a benefit on the heart, but the reverse is also true: ARNIs are still heart failure drugs, and we don’t think of them as diabetes drugs, but the PARADIGM-HF data showed that there was a substantial reduction in hemoglobin A1c in those who had diabetes,” he said.
The researchers noted that an absence of a control group may contribute to an overestimation of reverse cardiac remodeling in the T2DM patients, and that the PROVE-HF study wasn’t prospectively powered to delineate differences in how sac/val therapy affected patients with or without diabetes. “Future investigations seeking to evaluate differences by T2DM status after sacubitril/valsartan initiation may use our findings to plan prospective sample sizes,” the researchers wrote.
Dr. Butler disclosed financial relationships with Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, StealthPeptide and Vifor. Lead author Muhammad Shahzeb Khan, MD, MSc, a professor at the University of Mississippi, has no relevant financial relationships to disclose.
SOURCE: Kahn MS et al. JACC: HF. 2020 Dec 9. doi: 10.1016/j.jchf.2020.09.014.
FROM JACC: HEART FAILURE
Telangiectatic Patch on the Forehead
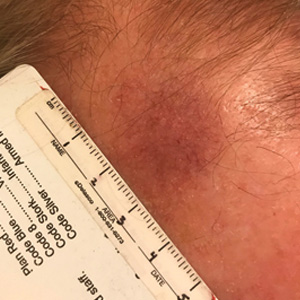
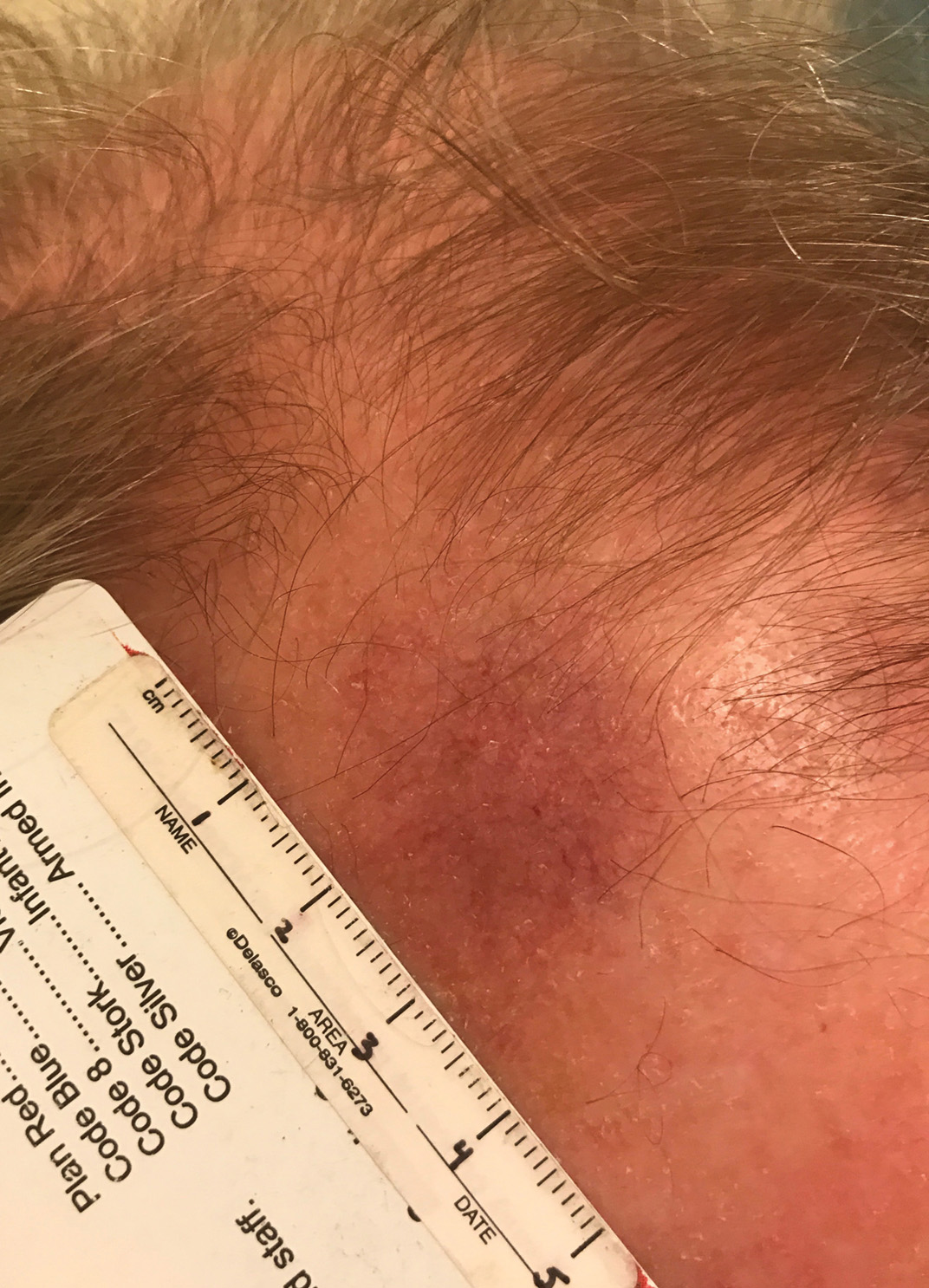
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3
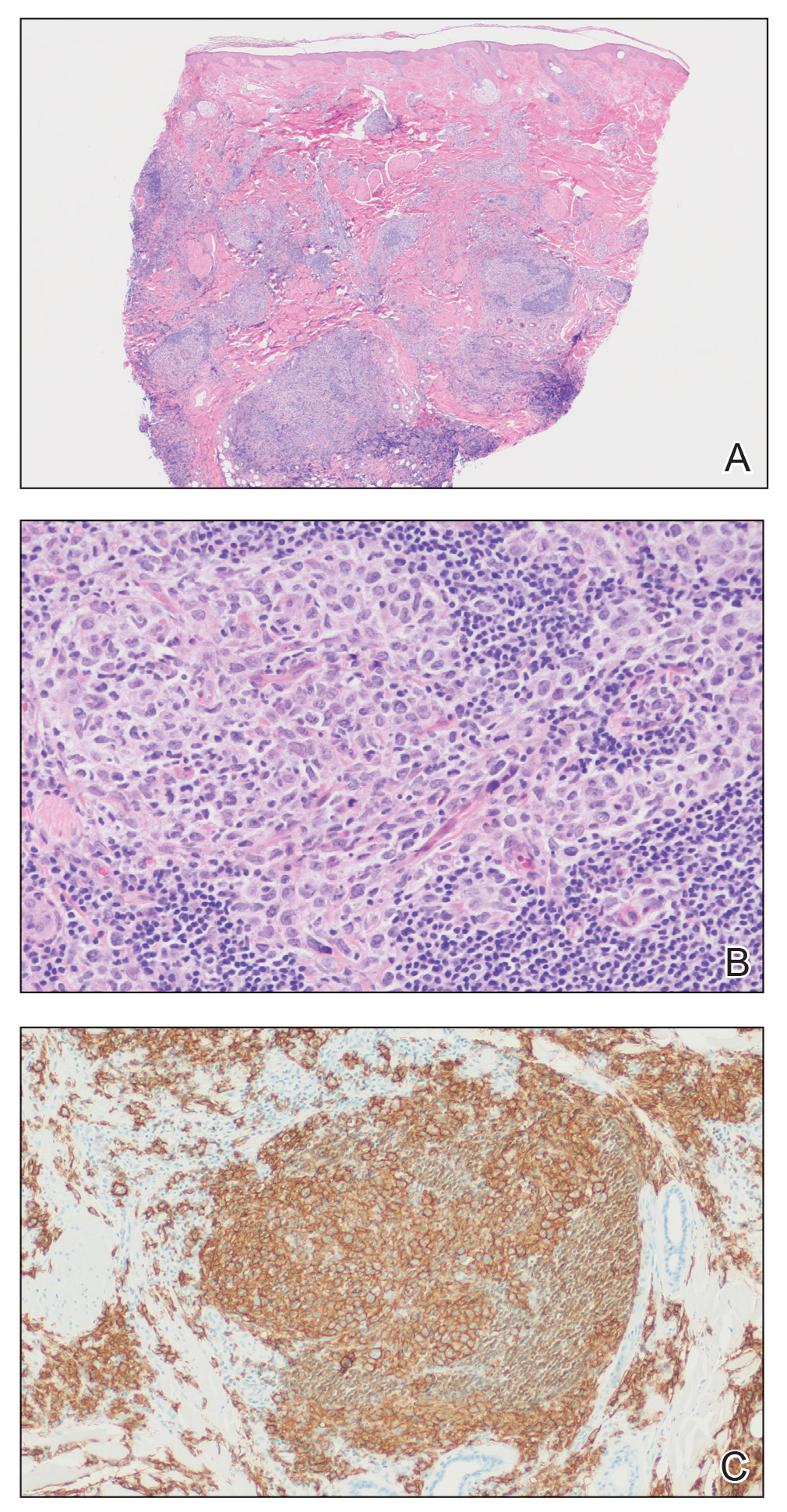
Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3

Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
The Diagnosis: Cutaneous B-cell Lymphoma
Histopathology was suggestive of cutaneous B-cell lymphoma (Figure). Further immunohistochemical studies including Bcl-6 positivity and Bcl-2 negativity in the large atypical cells supported a diagnosis of primary cutaneous follicle center lymphoma (PCFCL). The designation of primary cutaneous B-cell lymphoma includes several different types of lymphoma, including marginal zone lymphoma, diffuse large B-cell lymphoma, and intravascular lymphoma. To be considered a primary cutaneous lymphoma, there must be evidence of the lymphoma in the skin without concomitant evidence of systemic involvement, as determined through a full staging workup. Primary cutaneous follicle center lymphoma is an indolent lymphoma that most commonly presents as solitary or grouped, pink to plum-colored papules, plaques, nodules, and tumors on the scalp, forehead, or back.1 The lesions often are biopsied as suspected basal cell carcinomas or Merkel cell carcinomas (MCCs). Lesions on the face or scalp may easily evade diagnosis, as they initially may mimic rosacea or insect bites. Less common presentations include infiltrative lesions that cause rhinophymatous changes or scarring alopecia. Multifocal or disseminated lesions rarely can be observed. This case presentation is unique in its patchy appearance that clinically resembled angiosarcoma.2 When identified and treated, the disease-specific 5-year survival rate for PCFCL is greater than 95%.3

Merkel cell carcinoma was first described in 1972 and has been diagnosed with increasing frequency each year.4 It generally presents as an erythematous or violaceous, tender, indurated nodule on sun-exposed skin of the head or neck in elderly White men. However, other presentations have been reported, including papules, plaques, cystlike structures, pruritic tumors, pedunculated lesions, subcutaneous masses, and telangiectatic papules.5 Histopathologically, MCC is characterized by dermal nests and sheets of basaloid cells with finely granular salt and pepper-like chromatin. The histologic features can resemble other small blue cell tumors; therefore, the differential diagnosis can be broad.5 Immunohistochemistry that can confirm the diagnosis of MCC generally will be positive for cytokeratin 20 and neuroendocrine markers but negative for cytokeratin 7 and thyroid transcription factor 1. Merkel cell carcinoma is an aggressive tumor with a high risk for local recurrence and distant metastasis that carries a generally poor prognosis, especially when there is evidence of metastatic disease at presentation.5,6
Rosacea can appear as telangiectatic patches, though generally not as one discrete patch limited to the forehead, as in our patient. Histologic features vary based on the age of the lesion and clinical variant. In early lesions there is a mild perivascular lymphoplasmacytic infiltrate within the dermis, while older lesions can have a mixed infiltrate crowded around vessels and adnexal structures. Granulomas often are seen near hair follicles and interspersed throughout the dermis with ectatic vessels and dermal edema.7
Angiosarcoma is divided into 3 clinicopathological subtypes: idiopathic angiosarcoma of the head and neck, angiosarcoma in the setting of lymphedema, and postirradiation angiosarcoma.7 Idiopathic angiosarcoma most closely mimics PCFCL, as it can present as single or multifocal nodules, plaques, or patches. Histologically, the 3 groups appear similar with poorly circumscribed, infiltrative, dermal tumors. The neoplastic endothelial cells have large hyperchromatic nuclei that protrude into vascular lumens. The prognosis for idiopathic angiosarcoma of the head and neck is poor, with a 5-year survival rate of 15% to 34%, which often is due to delayed diagnosis.7
Pigmented purpuric dermatoses (PPDs) are chronic skin disorders characterized by purpura due to extravasation of blood from capillaries; the resulting hemosiderin deposition leads to pigmentation.7 There are various forms of PPD, which are classified into groups based on clinical appearance including Schamberg disease, purpura annularis telangiectodes of Majocchi, pigmented purpuric lichenoid dermatosis of Gougerot and Blum, lichen aureus, and others including eczematid and itching variants, which some consider to be distinct entities. Purpura annularis telangiectodes of Majocchi is the specific PPD that should be included in the clinical differential for PCFCL because it presents as annular patches with telangiectasias. Histologically, PPDs are characterized by a CD4+ lymphocytic infiltrate in the upper dermis with extravasated red blood cells and the presence of hemosiderin mostly within macrophages and a lack of true vasculitis. Clonality of the T cells has been shown, and there is some evidence that PPD may overlap with mycosis fungoides. However, this overlap mainly has been seen in patients with widespread lesions and would not apply to this case. In general, patients with PPD can be reassured of the benign process. In cases of widespread PPD, patients should be followed clinically to assess for progression to mycosis fungoides, though the likelihood is low.7
Our patient underwent a full staging workup, which confirmed the diagnosis of PCFCL. He was treated with radiation to the forehead that resulted in clearance of the lesion. Approximately 2 years after the initial diagnosis, the patient was alive and well with no evidence of recurrence of PCFCL.
In conclusion, it is imperative to identify unusual, macular, vascular-appearing patches, especially on the head and neck in older individuals. Because the clinical presentations of PCFCL, angiosarcoma, rosacea, MCC, and PPD can overlap with one another as well as with other entities, it is necessary to have a high level of suspicion and low threshold to biopsy these types of lesions, as outcomes can be drastically different.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Goyal A, LeBlanc RE, Carter JB. Cutaneous B-cell lymphoma. Hematol Oncol Clin North Am. 2019;33:149-161.
- Massone C, Fink-Puches R, Cerroni L. Atypical clinical presentation of primary and secondary cutaneous follicle center lymphoma (FCL) on the head characterized by macular lesions. J Am Acad Dermatol. 2016;75:1000-1006.
- Wilcox RA. Cutaneous B-cell lymphomas: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91:1052-1055.
- Conic RRZ, Ko J, Saridakis S, et al. Sentinel lymph node biopsy in Merkel cell carcinoma: predictors of sentinel lymph node positivity and association with overall survival. J Am Acad Dermatol. 2019;81:364-372
- Coggshall K, Tello TL, North JP, et al. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
- Tello TL, Coggshall K, Yom SS, et al. Merkel cell carcinoma: an update and review: current and future therapy. J Am Acad Dermatol. 2018;78:445-454.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
FDA gives guidance on allergy, pregnancy concerns for Pfizer COVID vaccine
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
stating that it is safe for people with any history of allergies, but not for those who might have a known history of severe allergic reaction to any component of the vaccine.
The warning is included in the FDA’s information sheet for health care providers, but questions are arising as to whether the vaccine – which was authorized for emergency use by the FDA on Friday – should not be given to anyone with a history of allergies.
Sara Oliver, MD, an epidemic intelligence service officer with the Centers for Disease Control and Prevention reported at a Dec. 11 meeting of the agency’s Advisory Committee on Immunization Practices that two U.K. health care workers with a history of significant allergic reactions had a reaction to the Pfizer vaccine. A third health care worker with no history of allergies developed tachycardia, Dr. Oliver said.
“I want to reassure the public that although there were these few reactions in Great Britain, these were not seen in the larger clinical trial datasets,” said Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA, during a press briefing on Dec. 12.
The Pfizer vaccine “is one that we’re comfortable giving to patients who have had other allergic reactions besides those other than severe allergic reactions to a vaccine or one of its components,” he said.
Dr. Marks suggested that individuals let their physicians know about any history of allergic reactions. He also noted that the federal government will be supplying vaccine administration sites, at least initially, with epinephrine, diphenhydramine, hydrocortisone, and other medications needed to manage allergic reactions.
The FDA is going to monitor side effects such as allergic reactions very closely, “but I think we still need to learn more and that’s why we’re going to be taking precautions. We may have to modify things as we move forward,” said Dr. Marks.
Dr. Oliver said that on Dec. 12 the CDC convened an external panel with experience in vaccine safety, immunology, and allergies “to collate expert knowledge regarding possible cases,” and that the FDA is getting more data from U.K. regulatory authorities.
Pregnancy concerns
Agency officials had little to say, however, about the safety or efficacy of the vaccine for pregnant or breastfeeding women.
The FDA’s information to health care professionals noted that “available data on Pfizer-BioNTech COVID-19 vaccine administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.”
Additionally, the agency stated, “data are not available to assess the effects of Pfizer-BioNTech COVID-19 vaccine on the breastfed infant or on milk production/excretion.”
Dr. Marks said that, for pregnant women and people who are immunocompromised, “it will be something that providers will need to consider on an individual basis.” He suggested that individuals consult with physicians to weigh the potential benefits and potential risks.
“Certainly, COVID-19 in a pregnant woman is not a good thing,” Dr. Marks said.
An individual might decide to go ahead with vaccination. “But that’s not something we’re recommending, that’s something we’re leaving up to the individual,” he said.
A version of this article originally appeared on Medscape.com.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
Diet and Skin: A Primer
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
Dermatologists frequently learn about skin conditions that are directly linked to diet. For example, we know that nutritional deficiencies can impact the hair, skin, and nails, and that celiac disease manifests with dermatitis herpetiformis of the skin. Patients commonly ask their dermatologists about the impact of diet on their skin. There are many outdated myths, but research on the subject is increasingly demonstrating important associations. Dermatologists must become familiar with the data on this topic so that we can provide informed counseling for our patients. This article reviews the current literature on associations between diet and 3 common cutaneous conditions—acne, psoriasis, and atopic dermatitis [AD]—and provides tips on how to best address our patients’ questions on this topic.
Acne
Studies increasingly support an association between a high glycemic diet (foods that lead to a spike in serum glucose) and acne; Bowe et al1 provided an excellent summary of the topic in 2010. This year, a large prospective cohort study of more than 24,000 participants demonstrated an association between adult acne and a diet high in milk, sugary beverages and foods, and fatty foods.2 In prospective cohort studies of more than 6000 adolescent girls and 4000 adolescent boys, Adebamowo et al3,4 demonstrated a correlation between skim milk consumption and acne. Whey protein supplementation also has been implicated in acne flares.5,6 The biological mechanism of the impact of high glycemic index foods and acne is believed to be mainly via activation of the insulinlike growth factor 1 (IGF-1) pathway, which promotes androgen synthesis and increases androgen bioavailability via decreased synthesis of sex hormone binding globulin.1,2 Insulinlike growth factor 1 also stimulates its downstream target, mammalian target of rapamycin (mTOR), leading to activation of antiapoptotic and proliferation signaling, ultimately resulting in oxidative stress and inflammation causing acne.2 Penso et al2 noted that patients with IGF-1 deficiency (Laron syndrome) never develop acne unless treated with exogenous IGF-1, further supporting its role in acne formation.7 There currently is a paucity of randomized controlled trials assessing the impact of diet on acne.
Psoriasis
The literature consistently shows that obesity is a predisposing factor for psoriasis. Additionally, weight gain may cause flares of existing psoriasis.8 Promotion of a healthy diet is an important factor in the management of obesity, alongside physical activity and, in some cases, medication and bariatric surgery.9 Patients with psoriasis who are overweight have been shown to experience improvement in their psoriasis after weight loss secondary to diet and exercise.8,10 The joint American Academy of Dermatology and National Psoriasis Foundation guidelines recommend that dermatologists advise patients to practice a healthy lifestyle including a healthy diet and communicate with a patient’s primary care provider so they can be appropriately evaluated and treated for comorbidities including metabolic syndrome, diabetes, and hyperlipidemia.11 In the NutriNet-Santé cohort study, investigators found an inverse correlation between psoriasis severity and adherence to a Mediterranean diet, which the authors conclude supports the hypothesis that this may slow the progression of psoriasis.12 In a single meta-analysis, it was reported that patients with psoriasis have a 3-fold increased risk for celiac disease compared to the general population.13 It remains unknown if these data are generalizable to the US population. Dermatologists should consider screening patients with psoriasis for celiac disease based on reported symptoms. When suspected, it is necessary to order appropriate serologies and consider referral to gastroenterology prior to recommending a gluten-free diet, as elimination of gluten prior to testing may lead to false-negative results.
Atopic Dermatitis
Patients and parents/guardians of children with AD often ask about the impact of diet on the condition. A small minority of patients may experience flares of AD due to ongoing, non–IgE-mediated allergen exposure.14 Diet as a trigger for flares should be suspected in children with persistent, moderate to severe AD. In these patients, allergen avoidance may lead to improvement but not resolution of AD. Allergens ordered from most common to least common are the following: eggs, milk, peanuts/tree nuts, shellfish, soy, and wheat.15 Additionally, it is important to note that children with AD are at higher risk for developing life-threatening, IgE-mediated food allergies compared to the general population (37% vs 6.8%).16,17 The LEAP (Learning Early about Peanut Allergy) study led to a paradigm shift in prevention of peanut allergies in high-risk children (ie, those with severe AD and/or egg allergy), providing data to support the idea that early introduction of allergenic foods such as peanuts may prevent severe allergies.18 Further studies are necessary to clarify the population in which allergen testing and recommendations on food avoidance are warranted vs early introduction.19
Conclusion
Early data support the relationship between diet and many common dermatologic conditions, including acne, psoriasis, and AD. Dermatologists should be familiar with the evidence supporting the relationship between diet and various skin conditions to best answer patients’ questions and counsel as appropriate. It is important for dermatologists to continue to stay up-to-date on the literature on this subject as new data emerge. Knowledge about the relationship between diet and skin allows dermatologists to not only support our patients’ skin health but their overall health as well.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
- Bowe WP, Joshi SS, Shalita AR. Diet and acne. J Am Acad Dermatol. 2010;63:124-141.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
- Cengiz FP, Cemil BC, Emiroglu N, et al. Acne located on the trunk, whey protein supplementation: is there any association? Health Promot Perspect. 2017;7:106-108.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Jensen P, Skov L. Psoriasis and obesity [published online February 23, 2017]. Dermatology. 2016;232:633-639.
- Extreme obesity, and what you can do. American Heart Association website. https://www.heart.org/en/healthy-living/healthy-eating/losing-weight/extreme-obesity-and-what-you-can-do. Updated April 18, 2014. Accessed November 30, 2020.
- Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170:634-642.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Silverberg NB, Lee-Wong M, Yosipovitch G. Diet and atopic dermatitis. Cutis. 2016;97:227-232.
- Bieber T, Bussmann C. Atopic dermatitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:203-218.
- Eigenmann PA, Sicherer SH, Borkowski TA, et al. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8.
- Age-adjusted percentages (with standard errors) of hay fever, respiratory allergies, food allergies, and skin allergies in the past 12 months for children under age 18 years, by selected characteristics: United States, 2016. CDC website. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_C-2.pdf. Accessed December 8, 2020.
- Du Toit G, Roberts G, Sayre PH, et al; LEAP study team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
- Sugita K, Akdis CA. Recent developments and advances in atopic dermatitis and food allergy [published online October 22, 2019]. Allergol Int. 2020;69:204-214.
Resident Pearls
- There are strong data on the relationship between dietary patterns and skin conditions.
- High glycemic index foods (eg, skim milk, whey protein, sugary beverages, fatty foods) are associated with acne vulgaris.
- Obesity is a risk factor for psoriasis; weight loss interventions such as improved dietary patterns can improve psoriasis.
- Children with atopic dermatitis (AD) are at higher risk for food allergies (both IgE and non–IgE-mediated allergies). A small subset may experience flares in their AD in relation to non–IgE-mediated food allergies.
IBD: Fecal calprotectin’s role in guiding treatment debated
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A version of this article originally appeared on Medscape.com.
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A version of this article originally appeared on Medscape.com.
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A version of this article originally appeared on Medscape.com.
COVID-19 neurologic fallout not limited to the severely ill
Serious neurologic complications in patients with COVID-19 are not limited to the severely ill, new research confirms.
“We found a range of neurologic diagnoses, including stroke and seizures, among hospitalized patients with COVID-19 and the majority were not critically ill, suggesting that these complications are not limited just to those patients who require ICU care or a ventilator,” study investigator Pria Anand, MD, division of neuro-infectious diseases, Boston University, said in an interview.
The study was published online Dec. 9 in Neurology Clinical Practice.
‘Moderately severe’ disability
For the study, the investigators reviewed the medical records of 74 adults (mean age, 64 years) who were hospitalized with COVID-19 and evaluated for neurologic conditions at Boston Medical Center, a safety-net hospital caring primarily for underserved, low-income, racial and ethnic minority populations.
The most common COVID-19 symptoms on arrival to the hospital were cough (39%), dyspnea (36%), and fever (34%). Eleven patients required intubation (15%) and 28 required some form of supplemental oxygen (38%). Thirty-four patients required intensive care (46%).
The most common neurologic COVID-19 symptoms at presentation were altered mental status (53%), myalgia (24%), fatigue (24%), and headache (18%).
After neurologic assessment, the most common final neurologic diagnosis was multifactorial or toxic-metabolic encephalopathy (35%), followed by seizure (20%), ischemic stroke (20%), primary movement disorder (9%), peripheral neuropathy (8%), and hemorrhagic stroke (4%).
Three patients (4%) suffered traumatic brain injuries after falling in their homes after developing COVID-19.
Ten (14%) patients died in the hospital. Survivors had “moderately severe” disability at discharge (median modified Rankin Scale score of 4 from a preadmission mRS score of 2) and many were discharged to nursing facilities or rehabilitation hospitals.
“Although we do not have data on their posthospital course, this suggests that patients with neurologic complications of COVID-19 are likely to require ongoing rehabilitation, even after they leave the hospital,” Dr. Anand, a member of the American Academy of Neurology, said in an interview.
“There are a diverse range of mechanisms by which COVID-19 can cause neurologic complications,” Dr. Anand said.
“These complications can result from the body’s immunological response to the virus (e.g., Guillain-Barré syndrome, an autoimmune disorder affecting the nerves), from having a systemic severe illness (e.g., brain injury as a result of insufficient oxygenation), from the increased tendency to form blood clots (e.g., stroke), from worsening of preexisting neurologic disorders, and possibly from involvement of the nervous system by the virus itself,” she explained.
The researchers said more study is needed to characterize the infectious and postinfectious neurologic complications of COVID-19 in diverse patient populations.
Lingering issues
In an interview, Kenneth L. Tyler, MD, chair of neurology, University of Colorado, Denver, noted that this is one of the larger series published to date of the neurologic complications associated with COVID-19, and the first to come from a U.S. safety-net hospital in a large metropolitan area.
“Overall, the types and categories of neurological complications reported including encephalopathy (35%) and acute cerebrovascular events (~20%) are similar to those reported elsewhere,” said Dr. Tyler.
However, the frequency of stroke (~20%) is higher than in some other reports, “likely reflecting the comorbidities such as diabetes, hypertension, limited access to care [that are] present in this population,” he said.
Dr. Tyler also noted that the “relatively high frequency” of primary movement disorders, notably myoclonus, “hasn’t been particularly well recognized or described, although one of the authors has written on this in COVID-19, so perhaps there is a bit of an ‘ascertainment bias’ – as they were looking harder for it?”
Finally, he noted, it’s important to understand that all the published studies “vary tremendously in the populations they examine, so direct comparisons can be difficult.”
Also weighing in on the report in an interview, Richard Temes, MD, director, Northwell Health’s Center for Neurocritical Care in Manhasset, N.Y., said neurologic problems have been noted since the start of COVID-19 and have been well described.
“It’s common for patients to present with very nonspecific neurological complaints like confusion, disorientation, altered mental status, lethargy, but also neurological disease such as strokes, brain hemorrhages, and seizures are quite common as well,” said Dr. Temes.
He also noted that a number of patients with COVID-19 will have “lingering effects, especially patients who are hospitalized, that can range from memory deficit, cognitive slowing, and trouble with activities of daily living and depression.
“These effects can occur with any patient who is hospitalized for a [significant] period of time, especially in the intensive care unit, so it’s hard to tease out whether or not this is truly from COVID itself or if it’s just being a survivor from a very severe, critical illness. We don’t know yet. We need more data on that,” he cautioned.
A version of this article originally appeared on Medscape.com.
Serious neurologic complications in patients with COVID-19 are not limited to the severely ill, new research confirms.
“We found a range of neurologic diagnoses, including stroke and seizures, among hospitalized patients with COVID-19 and the majority were not critically ill, suggesting that these complications are not limited just to those patients who require ICU care or a ventilator,” study investigator Pria Anand, MD, division of neuro-infectious diseases, Boston University, said in an interview.
The study was published online Dec. 9 in Neurology Clinical Practice.
‘Moderately severe’ disability
For the study, the investigators reviewed the medical records of 74 adults (mean age, 64 years) who were hospitalized with COVID-19 and evaluated for neurologic conditions at Boston Medical Center, a safety-net hospital caring primarily for underserved, low-income, racial and ethnic minority populations.
The most common COVID-19 symptoms on arrival to the hospital were cough (39%), dyspnea (36%), and fever (34%). Eleven patients required intubation (15%) and 28 required some form of supplemental oxygen (38%). Thirty-four patients required intensive care (46%).
The most common neurologic COVID-19 symptoms at presentation were altered mental status (53%), myalgia (24%), fatigue (24%), and headache (18%).
After neurologic assessment, the most common final neurologic diagnosis was multifactorial or toxic-metabolic encephalopathy (35%), followed by seizure (20%), ischemic stroke (20%), primary movement disorder (9%), peripheral neuropathy (8%), and hemorrhagic stroke (4%).
Three patients (4%) suffered traumatic brain injuries after falling in their homes after developing COVID-19.
Ten (14%) patients died in the hospital. Survivors had “moderately severe” disability at discharge (median modified Rankin Scale score of 4 from a preadmission mRS score of 2) and many were discharged to nursing facilities or rehabilitation hospitals.
“Although we do not have data on their posthospital course, this suggests that patients with neurologic complications of COVID-19 are likely to require ongoing rehabilitation, even after they leave the hospital,” Dr. Anand, a member of the American Academy of Neurology, said in an interview.
“There are a diverse range of mechanisms by which COVID-19 can cause neurologic complications,” Dr. Anand said.
“These complications can result from the body’s immunological response to the virus (e.g., Guillain-Barré syndrome, an autoimmune disorder affecting the nerves), from having a systemic severe illness (e.g., brain injury as a result of insufficient oxygenation), from the increased tendency to form blood clots (e.g., stroke), from worsening of preexisting neurologic disorders, and possibly from involvement of the nervous system by the virus itself,” she explained.
The researchers said more study is needed to characterize the infectious and postinfectious neurologic complications of COVID-19 in diverse patient populations.
Lingering issues
In an interview, Kenneth L. Tyler, MD, chair of neurology, University of Colorado, Denver, noted that this is one of the larger series published to date of the neurologic complications associated with COVID-19, and the first to come from a U.S. safety-net hospital in a large metropolitan area.
“Overall, the types and categories of neurological complications reported including encephalopathy (35%) and acute cerebrovascular events (~20%) are similar to those reported elsewhere,” said Dr. Tyler.
However, the frequency of stroke (~20%) is higher than in some other reports, “likely reflecting the comorbidities such as diabetes, hypertension, limited access to care [that are] present in this population,” he said.
Dr. Tyler also noted that the “relatively high frequency” of primary movement disorders, notably myoclonus, “hasn’t been particularly well recognized or described, although one of the authors has written on this in COVID-19, so perhaps there is a bit of an ‘ascertainment bias’ – as they were looking harder for it?”
Finally, he noted, it’s important to understand that all the published studies “vary tremendously in the populations they examine, so direct comparisons can be difficult.”
Also weighing in on the report in an interview, Richard Temes, MD, director, Northwell Health’s Center for Neurocritical Care in Manhasset, N.Y., said neurologic problems have been noted since the start of COVID-19 and have been well described.
“It’s common for patients to present with very nonspecific neurological complaints like confusion, disorientation, altered mental status, lethargy, but also neurological disease such as strokes, brain hemorrhages, and seizures are quite common as well,” said Dr. Temes.
He also noted that a number of patients with COVID-19 will have “lingering effects, especially patients who are hospitalized, that can range from memory deficit, cognitive slowing, and trouble with activities of daily living and depression.
“These effects can occur with any patient who is hospitalized for a [significant] period of time, especially in the intensive care unit, so it’s hard to tease out whether or not this is truly from COVID itself or if it’s just being a survivor from a very severe, critical illness. We don’t know yet. We need more data on that,” he cautioned.
A version of this article originally appeared on Medscape.com.
Serious neurologic complications in patients with COVID-19 are not limited to the severely ill, new research confirms.
“We found a range of neurologic diagnoses, including stroke and seizures, among hospitalized patients with COVID-19 and the majority were not critically ill, suggesting that these complications are not limited just to those patients who require ICU care or a ventilator,” study investigator Pria Anand, MD, division of neuro-infectious diseases, Boston University, said in an interview.
The study was published online Dec. 9 in Neurology Clinical Practice.
‘Moderately severe’ disability
For the study, the investigators reviewed the medical records of 74 adults (mean age, 64 years) who were hospitalized with COVID-19 and evaluated for neurologic conditions at Boston Medical Center, a safety-net hospital caring primarily for underserved, low-income, racial and ethnic minority populations.
The most common COVID-19 symptoms on arrival to the hospital were cough (39%), dyspnea (36%), and fever (34%). Eleven patients required intubation (15%) and 28 required some form of supplemental oxygen (38%). Thirty-four patients required intensive care (46%).
The most common neurologic COVID-19 symptoms at presentation were altered mental status (53%), myalgia (24%), fatigue (24%), and headache (18%).
After neurologic assessment, the most common final neurologic diagnosis was multifactorial or toxic-metabolic encephalopathy (35%), followed by seizure (20%), ischemic stroke (20%), primary movement disorder (9%), peripheral neuropathy (8%), and hemorrhagic stroke (4%).
Three patients (4%) suffered traumatic brain injuries after falling in their homes after developing COVID-19.
Ten (14%) patients died in the hospital. Survivors had “moderately severe” disability at discharge (median modified Rankin Scale score of 4 from a preadmission mRS score of 2) and many were discharged to nursing facilities or rehabilitation hospitals.
“Although we do not have data on their posthospital course, this suggests that patients with neurologic complications of COVID-19 are likely to require ongoing rehabilitation, even after they leave the hospital,” Dr. Anand, a member of the American Academy of Neurology, said in an interview.
“There are a diverse range of mechanisms by which COVID-19 can cause neurologic complications,” Dr. Anand said.
“These complications can result from the body’s immunological response to the virus (e.g., Guillain-Barré syndrome, an autoimmune disorder affecting the nerves), from having a systemic severe illness (e.g., brain injury as a result of insufficient oxygenation), from the increased tendency to form blood clots (e.g., stroke), from worsening of preexisting neurologic disorders, and possibly from involvement of the nervous system by the virus itself,” she explained.
The researchers said more study is needed to characterize the infectious and postinfectious neurologic complications of COVID-19 in diverse patient populations.
Lingering issues
In an interview, Kenneth L. Tyler, MD, chair of neurology, University of Colorado, Denver, noted that this is one of the larger series published to date of the neurologic complications associated with COVID-19, and the first to come from a U.S. safety-net hospital in a large metropolitan area.
“Overall, the types and categories of neurological complications reported including encephalopathy (35%) and acute cerebrovascular events (~20%) are similar to those reported elsewhere,” said Dr. Tyler.
However, the frequency of stroke (~20%) is higher than in some other reports, “likely reflecting the comorbidities such as diabetes, hypertension, limited access to care [that are] present in this population,” he said.
Dr. Tyler also noted that the “relatively high frequency” of primary movement disorders, notably myoclonus, “hasn’t been particularly well recognized or described, although one of the authors has written on this in COVID-19, so perhaps there is a bit of an ‘ascertainment bias’ – as they were looking harder for it?”
Finally, he noted, it’s important to understand that all the published studies “vary tremendously in the populations they examine, so direct comparisons can be difficult.”
Also weighing in on the report in an interview, Richard Temes, MD, director, Northwell Health’s Center for Neurocritical Care in Manhasset, N.Y., said neurologic problems have been noted since the start of COVID-19 and have been well described.
“It’s common for patients to present with very nonspecific neurological complaints like confusion, disorientation, altered mental status, lethargy, but also neurological disease such as strokes, brain hemorrhages, and seizures are quite common as well,” said Dr. Temes.
He also noted that a number of patients with COVID-19 will have “lingering effects, especially patients who are hospitalized, that can range from memory deficit, cognitive slowing, and trouble with activities of daily living and depression.
“These effects can occur with any patient who is hospitalized for a [significant] period of time, especially in the intensive care unit, so it’s hard to tease out whether or not this is truly from COVID itself or if it’s just being a survivor from a very severe, critical illness. We don’t know yet. We need more data on that,” he cautioned.
A version of this article originally appeared on Medscape.com.
Baricitinib combo for COVID-19 accelerates recovery, study shows
according to trial results published Dec. 11 in the New England Journal of Medicine.
Median time to recovery was 7 days for patients who received baricitinib versus 8 days for patients who received placebo.
The difference was greater in patients who required high-flow oxygen or noninvasive ventilation during their hospitalization. In this group, baricitinib shortened median time to recovery from 18 days to 10 days.
“Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status, notably among patients receiving high-flow oxygen or noninvasive mechanical ventilation,” reported Andre C. Kalil, MD, MPH, from the University of Nebraska Medical Center, Omaha, and colleagues. In addition, the combination was associated with fewer adverse events.
The study details data from the ACTT-2 trial that the Food and Drug Administration used to issue an emergency-use authorization for baricitinib in combination with remdesivir on Nov. 19.
Under the emergency-use authorization, baricitinib (Olumiant, Eli Lilly), a Janus kinase inhibitor approved for the treatment of rheumatoid arthritis, may be used in combination with remdesivir (Veklury, Gilead), an antiviral, for treating hospitalized adults and children aged at least 2 years with suspected or confirmed COVID-19.
The combination is intended for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.
Combo treatment favored
It is unclear how baricitinib compares with dexamethasone, which improved survival and led to a 1-day shorter hospital stay in another trial. There are differences between the drugs and trial designs, and only a “head-to-head comparison ... will allow the efficacy and safety differences between these two approaches to be fully understood,” Dr. Kalil and coauthors wrote.
“Dexamethasone has a long half-life, acts on glucocorticoid receptors, and reduces inflammation through a broad-pathway approach that has been associated with immunosuppression, hospital-acquired infections, gastrointestinal bleeding, hyperglycemia, and neuromuscular weakness, even with short courses,” they wrote. “Baricitinib has a short half-life, acts on targeted critical pathways to reduce inflammation while minimizing biologic redundancy with less immunosuppression, and may have antiviral activity.”
The ACTT-2 trial started in May and enrolled 1,033 patients in eight countries. Participants were randomly assigned to receive oral baricitinib tablets plus intravenous remdesivir or oral placebo tablets plus remdesivir.
Participants who received both drugs had significantly improved clinical status at day 15. Patients who received both treatments also had fewer serious adverse events.
“Although ACTT-2 was not powered to detect a difference in mortality between the two groups, both the survival rate and the time-to-death analyses favored combination treatment,” the researchers wrote.
The trial was sponsored by the National Institute of Allergy and Infectious Diseases. Some of the authors disclosed funding from government grants and financial ties to Eli Lilly, Gilead, and other companies.
A version of this article originally appeared on Medscape.com.
according to trial results published Dec. 11 in the New England Journal of Medicine.
Median time to recovery was 7 days for patients who received baricitinib versus 8 days for patients who received placebo.
The difference was greater in patients who required high-flow oxygen or noninvasive ventilation during their hospitalization. In this group, baricitinib shortened median time to recovery from 18 days to 10 days.
“Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status, notably among patients receiving high-flow oxygen or noninvasive mechanical ventilation,” reported Andre C. Kalil, MD, MPH, from the University of Nebraska Medical Center, Omaha, and colleagues. In addition, the combination was associated with fewer adverse events.
The study details data from the ACTT-2 trial that the Food and Drug Administration used to issue an emergency-use authorization for baricitinib in combination with remdesivir on Nov. 19.
Under the emergency-use authorization, baricitinib (Olumiant, Eli Lilly), a Janus kinase inhibitor approved for the treatment of rheumatoid arthritis, may be used in combination with remdesivir (Veklury, Gilead), an antiviral, for treating hospitalized adults and children aged at least 2 years with suspected or confirmed COVID-19.
The combination is intended for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.
Combo treatment favored
It is unclear how baricitinib compares with dexamethasone, which improved survival and led to a 1-day shorter hospital stay in another trial. There are differences between the drugs and trial designs, and only a “head-to-head comparison ... will allow the efficacy and safety differences between these two approaches to be fully understood,” Dr. Kalil and coauthors wrote.
“Dexamethasone has a long half-life, acts on glucocorticoid receptors, and reduces inflammation through a broad-pathway approach that has been associated with immunosuppression, hospital-acquired infections, gastrointestinal bleeding, hyperglycemia, and neuromuscular weakness, even with short courses,” they wrote. “Baricitinib has a short half-life, acts on targeted critical pathways to reduce inflammation while minimizing biologic redundancy with less immunosuppression, and may have antiviral activity.”
The ACTT-2 trial started in May and enrolled 1,033 patients in eight countries. Participants were randomly assigned to receive oral baricitinib tablets plus intravenous remdesivir or oral placebo tablets plus remdesivir.
Participants who received both drugs had significantly improved clinical status at day 15. Patients who received both treatments also had fewer serious adverse events.
“Although ACTT-2 was not powered to detect a difference in mortality between the two groups, both the survival rate and the time-to-death analyses favored combination treatment,” the researchers wrote.
The trial was sponsored by the National Institute of Allergy and Infectious Diseases. Some of the authors disclosed funding from government grants and financial ties to Eli Lilly, Gilead, and other companies.
A version of this article originally appeared on Medscape.com.
according to trial results published Dec. 11 in the New England Journal of Medicine.
Median time to recovery was 7 days for patients who received baricitinib versus 8 days for patients who received placebo.
The difference was greater in patients who required high-flow oxygen or noninvasive ventilation during their hospitalization. In this group, baricitinib shortened median time to recovery from 18 days to 10 days.
“Baricitinib plus remdesivir was superior to remdesivir alone in reducing recovery time and accelerating improvement in clinical status, notably among patients receiving high-flow oxygen or noninvasive mechanical ventilation,” reported Andre C. Kalil, MD, MPH, from the University of Nebraska Medical Center, Omaha, and colleagues. In addition, the combination was associated with fewer adverse events.
The study details data from the ACTT-2 trial that the Food and Drug Administration used to issue an emergency-use authorization for baricitinib in combination with remdesivir on Nov. 19.
Under the emergency-use authorization, baricitinib (Olumiant, Eli Lilly), a Janus kinase inhibitor approved for the treatment of rheumatoid arthritis, may be used in combination with remdesivir (Veklury, Gilead), an antiviral, for treating hospitalized adults and children aged at least 2 years with suspected or confirmed COVID-19.
The combination is intended for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation.
Combo treatment favored
It is unclear how baricitinib compares with dexamethasone, which improved survival and led to a 1-day shorter hospital stay in another trial. There are differences between the drugs and trial designs, and only a “head-to-head comparison ... will allow the efficacy and safety differences between these two approaches to be fully understood,” Dr. Kalil and coauthors wrote.
“Dexamethasone has a long half-life, acts on glucocorticoid receptors, and reduces inflammation through a broad-pathway approach that has been associated with immunosuppression, hospital-acquired infections, gastrointestinal bleeding, hyperglycemia, and neuromuscular weakness, even with short courses,” they wrote. “Baricitinib has a short half-life, acts on targeted critical pathways to reduce inflammation while minimizing biologic redundancy with less immunosuppression, and may have antiviral activity.”
The ACTT-2 trial started in May and enrolled 1,033 patients in eight countries. Participants were randomly assigned to receive oral baricitinib tablets plus intravenous remdesivir or oral placebo tablets plus remdesivir.
Participants who received both drugs had significantly improved clinical status at day 15. Patients who received both treatments also had fewer serious adverse events.
“Although ACTT-2 was not powered to detect a difference in mortality between the two groups, both the survival rate and the time-to-death analyses favored combination treatment,” the researchers wrote.
The trial was sponsored by the National Institute of Allergy and Infectious Diseases. Some of the authors disclosed funding from government grants and financial ties to Eli Lilly, Gilead, and other companies.
A version of this article originally appeared on Medscape.com.
Quick Byte: Global health before COVID-19
How quickly things change. On September 23, 2019 – months before the COVID-19 pandemic struck – at a UN High-Level Meeting on Universal Health Coverage, heads of state from around the world pledged to achieve universal health coverage by 2030.
“This will be an unprecedented moment in public health: according to the declaration being negotiated by member states, this commitment is being made globally ‘for the first time.’ Whether or not the new commitment succeeds will depend on a large degree of advocacy at the national level.”
Reference
1. Carter M, Emmel A. The Global Community Has Pledged To Achieve Universal Health Coverage: What’s It Going To Take? Health Affairs Blog, 2019 Sept 23. doi: 10.1377/hblog20190920.827005.
How quickly things change. On September 23, 2019 – months before the COVID-19 pandemic struck – at a UN High-Level Meeting on Universal Health Coverage, heads of state from around the world pledged to achieve universal health coverage by 2030.
“This will be an unprecedented moment in public health: according to the declaration being negotiated by member states, this commitment is being made globally ‘for the first time.’ Whether or not the new commitment succeeds will depend on a large degree of advocacy at the national level.”
Reference
1. Carter M, Emmel A. The Global Community Has Pledged To Achieve Universal Health Coverage: What’s It Going To Take? Health Affairs Blog, 2019 Sept 23. doi: 10.1377/hblog20190920.827005.
How quickly things change. On September 23, 2019 – months before the COVID-19 pandemic struck – at a UN High-Level Meeting on Universal Health Coverage, heads of state from around the world pledged to achieve universal health coverage by 2030.
“This will be an unprecedented moment in public health: according to the declaration being negotiated by member states, this commitment is being made globally ‘for the first time.’ Whether or not the new commitment succeeds will depend on a large degree of advocacy at the national level.”
Reference
1. Carter M, Emmel A. The Global Community Has Pledged To Achieve Universal Health Coverage: What’s It Going To Take? Health Affairs Blog, 2019 Sept 23. doi: 10.1377/hblog20190920.827005.