User login
Question 1
Q1. Correct answer: B. Expulsion of water-filled balloon in 3 minutes.
Rationale
The balloon expulsion test is highly suggestive of dyssynergia. A balloon expulsion time of greater than 2 minutes is abnormal. An absent RAIR can be seen in Hirschsprung's disease or megarectum. Defecation index equals maximum rectal pressure during attempted defecation divided by minimum anal residual pressure during attempted defecation. A normal defecation index is greater than 1.5. Decreased anal sphincter pressure during simulated defecation is normal and therefore not consistent with dyssynergic defecation.
References
Wald A et al. Am J Gastroenterol. 2014 Aug;109(8):1141-57.
Bharucha AE et al. Gastroenterology. 2013 Jan;144(1):218-38.
Q1. Correct answer: B. Expulsion of water-filled balloon in 3 minutes.
Rationale
The balloon expulsion test is highly suggestive of dyssynergia. A balloon expulsion time of greater than 2 minutes is abnormal. An absent RAIR can be seen in Hirschsprung's disease or megarectum. Defecation index equals maximum rectal pressure during attempted defecation divided by minimum anal residual pressure during attempted defecation. A normal defecation index is greater than 1.5. Decreased anal sphincter pressure during simulated defecation is normal and therefore not consistent with dyssynergic defecation.
References
Wald A et al. Am J Gastroenterol. 2014 Aug;109(8):1141-57.
Bharucha AE et al. Gastroenterology. 2013 Jan;144(1):218-38.
Q1. Correct answer: B. Expulsion of water-filled balloon in 3 minutes.
Rationale
The balloon expulsion test is highly suggestive of dyssynergia. A balloon expulsion time of greater than 2 minutes is abnormal. An absent RAIR can be seen in Hirschsprung's disease or megarectum. Defecation index equals maximum rectal pressure during attempted defecation divided by minimum anal residual pressure during attempted defecation. A normal defecation index is greater than 1.5. Decreased anal sphincter pressure during simulated defecation is normal and therefore not consistent with dyssynergic defecation.
References
Wald A et al. Am J Gastroenterol. 2014 Aug;109(8):1141-57.
Bharucha AE et al. Gastroenterology. 2013 Jan;144(1):218-38.
A 37-year-old woman with no significant past medical history presents for further evaluation of chronic constipation with straining. She denies significant abdominal pain, gastrointestinal bleeding, or weight loss. There is no family history of colorectal neoplasia or inflammatory bowel disease. She has not responded to many laxatives and undergoes anorectal manometry with balloon expulsion testing.
Hospital admissions of nursing home patients declined after ACA quality initiatives
Background: Following the ACA’s implementation, several measures were introduced to reduce unnecessary admissions of long-term nursing home residents to hospitals. These measures included an initiative to enhance a nursing home’s on-site capability to handle target populations; the accountable care organization payment model; and the Hospital Readmissions Reduction Program.
Study design: Cross-sectional study using the claims-based nationwide Minimum Data Set during 2011-2016.
Setting: Federally licensed nursing homes in the United States.
Synopsis: The authors examined the number of transfers between federally funded nursing homes and the hospital settings (EDs, observation, or inpatient hospitalizations) for greater than 460,000 long term–stay patients with advanced dementia, advanced heart failure, and/or advanced chronic obstructive pulmonary disease (COPD). A risk-adjusted model showed that, during 2011-2016, there were significant decreases in transfers rates for potentially avoidable conditions, measured as the mean number of transfers per person-year alive, for patients with advanced dementia (2.4 vs. 1.6), heart failure (8.5 vs. 6.7), and COPD (7.8 vs 5.5). Most of this decrease was linked to reductions in acute hospitalizations. Notably, hospice enrollment remained low throughout this time period, despite a high 1-year mortality.
Bottom line: During the 2011-2016 period, transfer rates for patients with advanced dementia, heart failure, and/or COPD from nursing homes to the hospital setting decreased.
Citation: McCarthy EP et al. Hospital transfer rates among U.S. nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2019 Dec 30. doi: 10.1001/jamainternmed.2019.6130.
Dr. Cool is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Following the ACA’s implementation, several measures were introduced to reduce unnecessary admissions of long-term nursing home residents to hospitals. These measures included an initiative to enhance a nursing home’s on-site capability to handle target populations; the accountable care organization payment model; and the Hospital Readmissions Reduction Program.
Study design: Cross-sectional study using the claims-based nationwide Minimum Data Set during 2011-2016.
Setting: Federally licensed nursing homes in the United States.
Synopsis: The authors examined the number of transfers between federally funded nursing homes and the hospital settings (EDs, observation, or inpatient hospitalizations) for greater than 460,000 long term–stay patients with advanced dementia, advanced heart failure, and/or advanced chronic obstructive pulmonary disease (COPD). A risk-adjusted model showed that, during 2011-2016, there were significant decreases in transfers rates for potentially avoidable conditions, measured as the mean number of transfers per person-year alive, for patients with advanced dementia (2.4 vs. 1.6), heart failure (8.5 vs. 6.7), and COPD (7.8 vs 5.5). Most of this decrease was linked to reductions in acute hospitalizations. Notably, hospice enrollment remained low throughout this time period, despite a high 1-year mortality.
Bottom line: During the 2011-2016 period, transfer rates for patients with advanced dementia, heart failure, and/or COPD from nursing homes to the hospital setting decreased.
Citation: McCarthy EP et al. Hospital transfer rates among U.S. nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2019 Dec 30. doi: 10.1001/jamainternmed.2019.6130.
Dr. Cool is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
Background: Following the ACA’s implementation, several measures were introduced to reduce unnecessary admissions of long-term nursing home residents to hospitals. These measures included an initiative to enhance a nursing home’s on-site capability to handle target populations; the accountable care organization payment model; and the Hospital Readmissions Reduction Program.
Study design: Cross-sectional study using the claims-based nationwide Minimum Data Set during 2011-2016.
Setting: Federally licensed nursing homes in the United States.
Synopsis: The authors examined the number of transfers between federally funded nursing homes and the hospital settings (EDs, observation, or inpatient hospitalizations) for greater than 460,000 long term–stay patients with advanced dementia, advanced heart failure, and/or advanced chronic obstructive pulmonary disease (COPD). A risk-adjusted model showed that, during 2011-2016, there were significant decreases in transfers rates for potentially avoidable conditions, measured as the mean number of transfers per person-year alive, for patients with advanced dementia (2.4 vs. 1.6), heart failure (8.5 vs. 6.7), and COPD (7.8 vs 5.5). Most of this decrease was linked to reductions in acute hospitalizations. Notably, hospice enrollment remained low throughout this time period, despite a high 1-year mortality.
Bottom line: During the 2011-2016 period, transfer rates for patients with advanced dementia, heart failure, and/or COPD from nursing homes to the hospital setting decreased.
Citation: McCarthy EP et al. Hospital transfer rates among U.S. nursing home residents with advanced illness before and after initiatives to reduce hospitalizations. JAMA Intern Med. 2019 Dec 30. doi: 10.1001/jamainternmed.2019.6130.
Dr. Cool is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, both in Boston.
High eradication, fewer adverse events with hybrid therapy for H. pylori
A 14-day course of hybrid therapy was as effective as 10-day bismuth quadruple therapy, but with fewer side effects, according to results of a randomized trial conducted in Taiwan.
“However, the former had fewer adverse events than the latter,” investigator Ping-I Hsu, MD, of An Nan Hospital, China Medical University, Taiwan said in a virtual presentation at the annual Digestive Disease Week® (DDW). Some patients in the trial received 14-day high-dose dual therapy, which also had a lower rate of adverse events but a lower eradication rate, compared with quadruple therapy, Dr. Hsu added.
This study confirms previous data showing that hybrid therapy has high eradication rates and a lower frequency of adverse events compared with bismuth quadruple therapy, noted Joseph Adrian L. Buensalido, MD, clinical associate professor of medicine in the division of infectious diseases at the University of the Philippines–Philippine General Hospital in Manila. “Clinicians and specialty/guideline groups may need to start looking at moving to first-line hybrid therapy as opposed to the traditional approach,” Dr. Buensalido said in an interview.
Current guidelines and data to date
An American College of Gastroenterology clinical guideline published in 2017 strongly recommends bismuth quadruple therapy, with a duration of 10-14 days, as a first-line treatment option. Hybrid therapy is conditionally recommended as a first-line option in the ACG guideline, while high-dose dual therapy is conditionally recommended as a salvage regimen.
In a prospective, randomized comparative study published in 2017, the eradication rates (93.9%) in patients receiving 14-day bismuth quadruple therapy (pantoprazole, bismuth subcitrate, tetracycline, and metronidazole) were comparable with eradication rates (92.8%) with 14-day hybrid therapy (dual therapy with pantoprazole plus amoxicillin for 7 days, followed by quadruple therapy with pantoprazole, amoxicillin, clarithromycin, and metronidazole for 7 days).
Quadruple therapy had a higher frequency of adverse events, at 55%, compared with 15.7% for hybrid therapy (P < .001). “Whether shortening the treatment duration of bismuth quadruple therapy from 14 days to 10 days can reduce the frequency of adverse effects remains unclear,” Dr. Hsu said in introductory comments to his study.
Comparing three approaches
In the multicenter, randomized, open-label superiority trial presented at DDW, Dr. Hsu and colleagues randomly assigned 600 Helicobacter pylori–infected participants in equal numbers to receive 14-day hybrid therapy, 14-day high-dose dual therapy, or 10-day bismuth quadruple therapy.
The hybrid therapy regimen consisted of rabeprazole 20 mg twice a day plus amoxicillin 1 g twice a day for 14 days, with clarithromycin 500 mg and metronidazole 500 mg twice a day in the final 7 days. The high-dose dual therapy regimen consisted of rabeprazole 20 mg and amoxicillin 750 mg four times a day for 14 days. The bismuth quadruple therapy regimen consisted of rabeprazole 20 mg twice a day, tripotassium dicitrato bismuthate 300 mg four times a day, tetracycline 500 mg twice a day, and metronidazole 250 mg four times a day for 10 days.
Investigators assessed H. pylori status 6 weeks following the end of therapy. In an intention-to-treat analysis, the hybrid therapy regimen yielded an eradication rate of 96.5%, which was comparable with the 93.5% eradication rate seen with bismuth quadruple therapy and was significantly higher than the 86.0% eradication rate seen with high-dose dual therapy (P < .001), according to Dr. Hsu. Similar efficacy outcomes were seen in per-protocol analysis.
The frequency of adverse events was lowest with high-dose dual therapy, at 13.0%, according to Dr. Hsu. That was significantly lower than the 25.5% frequency of adverse events with hybrid therapy. Bismuth quadruple therapy had a rate of 34.0%.
Antibiotic resistance results
Antibiotic resistance was most common for metronidazole, seen in approximately 28% of the quadruple-therapy group, 34% of the hybrid group, and 37% of the high-dose therapy groups. Clarithromycin resistance occurred in about 23% of quadruple therapy recipients, 16% of hybrid recipients, and 16% of high-dose therapy recipients. Amoxicillin and tetracycline resistance was rare, occurring in approximately 0%-3% of groups.
In the quadruple therapy arm, metronidazole resistance was associated with H. pylori eradication failure, according to Dr. Hsu. The eradication rate was about 96% for those subjects with no metronidazole resistance, and 88% for those with resistance (P = .05). Amoxicillin resistance, although rare in the study, independently predicted eradication failure of high-dose dual therapy, Dr. Hsu said. The eradication rate with high-dose dual therapy was 87.6% for individuals without amoxicillin resistance, and 40.0% in individuals with resistance, according to presented data.
The authors reported no financial disclosures related to their research. Dr. Buensalido has been a speaker for Unilab, BSV Bioscience, and Philcare Pharma, and has received sponsorship from Pfizer.
A 14-day course of hybrid therapy was as effective as 10-day bismuth quadruple therapy, but with fewer side effects, according to results of a randomized trial conducted in Taiwan.
“However, the former had fewer adverse events than the latter,” investigator Ping-I Hsu, MD, of An Nan Hospital, China Medical University, Taiwan said in a virtual presentation at the annual Digestive Disease Week® (DDW). Some patients in the trial received 14-day high-dose dual therapy, which also had a lower rate of adverse events but a lower eradication rate, compared with quadruple therapy, Dr. Hsu added.
This study confirms previous data showing that hybrid therapy has high eradication rates and a lower frequency of adverse events compared with bismuth quadruple therapy, noted Joseph Adrian L. Buensalido, MD, clinical associate professor of medicine in the division of infectious diseases at the University of the Philippines–Philippine General Hospital in Manila. “Clinicians and specialty/guideline groups may need to start looking at moving to first-line hybrid therapy as opposed to the traditional approach,” Dr. Buensalido said in an interview.
Current guidelines and data to date
An American College of Gastroenterology clinical guideline published in 2017 strongly recommends bismuth quadruple therapy, with a duration of 10-14 days, as a first-line treatment option. Hybrid therapy is conditionally recommended as a first-line option in the ACG guideline, while high-dose dual therapy is conditionally recommended as a salvage regimen.
In a prospective, randomized comparative study published in 2017, the eradication rates (93.9%) in patients receiving 14-day bismuth quadruple therapy (pantoprazole, bismuth subcitrate, tetracycline, and metronidazole) were comparable with eradication rates (92.8%) with 14-day hybrid therapy (dual therapy with pantoprazole plus amoxicillin for 7 days, followed by quadruple therapy with pantoprazole, amoxicillin, clarithromycin, and metronidazole for 7 days).
Quadruple therapy had a higher frequency of adverse events, at 55%, compared with 15.7% for hybrid therapy (P < .001). “Whether shortening the treatment duration of bismuth quadruple therapy from 14 days to 10 days can reduce the frequency of adverse effects remains unclear,” Dr. Hsu said in introductory comments to his study.
Comparing three approaches
In the multicenter, randomized, open-label superiority trial presented at DDW, Dr. Hsu and colleagues randomly assigned 600 Helicobacter pylori–infected participants in equal numbers to receive 14-day hybrid therapy, 14-day high-dose dual therapy, or 10-day bismuth quadruple therapy.
The hybrid therapy regimen consisted of rabeprazole 20 mg twice a day plus amoxicillin 1 g twice a day for 14 days, with clarithromycin 500 mg and metronidazole 500 mg twice a day in the final 7 days. The high-dose dual therapy regimen consisted of rabeprazole 20 mg and amoxicillin 750 mg four times a day for 14 days. The bismuth quadruple therapy regimen consisted of rabeprazole 20 mg twice a day, tripotassium dicitrato bismuthate 300 mg four times a day, tetracycline 500 mg twice a day, and metronidazole 250 mg four times a day for 10 days.
Investigators assessed H. pylori status 6 weeks following the end of therapy. In an intention-to-treat analysis, the hybrid therapy regimen yielded an eradication rate of 96.5%, which was comparable with the 93.5% eradication rate seen with bismuth quadruple therapy and was significantly higher than the 86.0% eradication rate seen with high-dose dual therapy (P < .001), according to Dr. Hsu. Similar efficacy outcomes were seen in per-protocol analysis.
The frequency of adverse events was lowest with high-dose dual therapy, at 13.0%, according to Dr. Hsu. That was significantly lower than the 25.5% frequency of adverse events with hybrid therapy. Bismuth quadruple therapy had a rate of 34.0%.
Antibiotic resistance results
Antibiotic resistance was most common for metronidazole, seen in approximately 28% of the quadruple-therapy group, 34% of the hybrid group, and 37% of the high-dose therapy groups. Clarithromycin resistance occurred in about 23% of quadruple therapy recipients, 16% of hybrid recipients, and 16% of high-dose therapy recipients. Amoxicillin and tetracycline resistance was rare, occurring in approximately 0%-3% of groups.
In the quadruple therapy arm, metronidazole resistance was associated with H. pylori eradication failure, according to Dr. Hsu. The eradication rate was about 96% for those subjects with no metronidazole resistance, and 88% for those with resistance (P = .05). Amoxicillin resistance, although rare in the study, independently predicted eradication failure of high-dose dual therapy, Dr. Hsu said. The eradication rate with high-dose dual therapy was 87.6% for individuals without amoxicillin resistance, and 40.0% in individuals with resistance, according to presented data.
The authors reported no financial disclosures related to their research. Dr. Buensalido has been a speaker for Unilab, BSV Bioscience, and Philcare Pharma, and has received sponsorship from Pfizer.
A 14-day course of hybrid therapy was as effective as 10-day bismuth quadruple therapy, but with fewer side effects, according to results of a randomized trial conducted in Taiwan.
“However, the former had fewer adverse events than the latter,” investigator Ping-I Hsu, MD, of An Nan Hospital, China Medical University, Taiwan said in a virtual presentation at the annual Digestive Disease Week® (DDW). Some patients in the trial received 14-day high-dose dual therapy, which also had a lower rate of adverse events but a lower eradication rate, compared with quadruple therapy, Dr. Hsu added.
This study confirms previous data showing that hybrid therapy has high eradication rates and a lower frequency of adverse events compared with bismuth quadruple therapy, noted Joseph Adrian L. Buensalido, MD, clinical associate professor of medicine in the division of infectious diseases at the University of the Philippines–Philippine General Hospital in Manila. “Clinicians and specialty/guideline groups may need to start looking at moving to first-line hybrid therapy as opposed to the traditional approach,” Dr. Buensalido said in an interview.
Current guidelines and data to date
An American College of Gastroenterology clinical guideline published in 2017 strongly recommends bismuth quadruple therapy, with a duration of 10-14 days, as a first-line treatment option. Hybrid therapy is conditionally recommended as a first-line option in the ACG guideline, while high-dose dual therapy is conditionally recommended as a salvage regimen.
In a prospective, randomized comparative study published in 2017, the eradication rates (93.9%) in patients receiving 14-day bismuth quadruple therapy (pantoprazole, bismuth subcitrate, tetracycline, and metronidazole) were comparable with eradication rates (92.8%) with 14-day hybrid therapy (dual therapy with pantoprazole plus amoxicillin for 7 days, followed by quadruple therapy with pantoprazole, amoxicillin, clarithromycin, and metronidazole for 7 days).
Quadruple therapy had a higher frequency of adverse events, at 55%, compared with 15.7% for hybrid therapy (P < .001). “Whether shortening the treatment duration of bismuth quadruple therapy from 14 days to 10 days can reduce the frequency of adverse effects remains unclear,” Dr. Hsu said in introductory comments to his study.
Comparing three approaches
In the multicenter, randomized, open-label superiority trial presented at DDW, Dr. Hsu and colleagues randomly assigned 600 Helicobacter pylori–infected participants in equal numbers to receive 14-day hybrid therapy, 14-day high-dose dual therapy, or 10-day bismuth quadruple therapy.
The hybrid therapy regimen consisted of rabeprazole 20 mg twice a day plus amoxicillin 1 g twice a day for 14 days, with clarithromycin 500 mg and metronidazole 500 mg twice a day in the final 7 days. The high-dose dual therapy regimen consisted of rabeprazole 20 mg and amoxicillin 750 mg four times a day for 14 days. The bismuth quadruple therapy regimen consisted of rabeprazole 20 mg twice a day, tripotassium dicitrato bismuthate 300 mg four times a day, tetracycline 500 mg twice a day, and metronidazole 250 mg four times a day for 10 days.
Investigators assessed H. pylori status 6 weeks following the end of therapy. In an intention-to-treat analysis, the hybrid therapy regimen yielded an eradication rate of 96.5%, which was comparable with the 93.5% eradication rate seen with bismuth quadruple therapy and was significantly higher than the 86.0% eradication rate seen with high-dose dual therapy (P < .001), according to Dr. Hsu. Similar efficacy outcomes were seen in per-protocol analysis.
The frequency of adverse events was lowest with high-dose dual therapy, at 13.0%, according to Dr. Hsu. That was significantly lower than the 25.5% frequency of adverse events with hybrid therapy. Bismuth quadruple therapy had a rate of 34.0%.
Antibiotic resistance results
Antibiotic resistance was most common for metronidazole, seen in approximately 28% of the quadruple-therapy group, 34% of the hybrid group, and 37% of the high-dose therapy groups. Clarithromycin resistance occurred in about 23% of quadruple therapy recipients, 16% of hybrid recipients, and 16% of high-dose therapy recipients. Amoxicillin and tetracycline resistance was rare, occurring in approximately 0%-3% of groups.
In the quadruple therapy arm, metronidazole resistance was associated with H. pylori eradication failure, according to Dr. Hsu. The eradication rate was about 96% for those subjects with no metronidazole resistance, and 88% for those with resistance (P = .05). Amoxicillin resistance, although rare in the study, independently predicted eradication failure of high-dose dual therapy, Dr. Hsu said. The eradication rate with high-dose dual therapy was 87.6% for individuals without amoxicillin resistance, and 40.0% in individuals with resistance, according to presented data.
The authors reported no financial disclosures related to their research. Dr. Buensalido has been a speaker for Unilab, BSV Bioscience, and Philcare Pharma, and has received sponsorship from Pfizer.
FROM DDW 2021
Head-to-head trial compares ustekinumab with adalimumab in Crohn’s
For biologic-naive adults with moderate to severe Crohn’s disease, treatment with adalimumab or ustekinumab leads to similar outcomes, according to results of the head-to-head SEAVUE trial.
When lead author Bruce E. Sands, MD, of Icahn School of Medicine at Mount Sinai, New York, compared treatment arms, patients had similar rates of clinical remission at one year. All major secondary endpoints, such as endoscopic remission, were comparable, as were safety profiles, Dr. Sands reported at the annual Digestive Disease Week® (DDW).
“From my perspective, this is an important study,” Dr. Sands wrote in a virtual chat following his presentation. “We need more head-to-head studies!”
Results from the SEAVUE trial come almost 2 years after Dr. Sands reported findings of another head-to-head IBD trial: VARSITY, which demonstrated the superiority of vedolizumab over adalimumab among patients with moderate to severe ulcerative colitis.
The multicenter, double-blinded SEAVUE trial involved 386 patients with biologic-naive Crohn’s disease who had failed corticosteroids or immunomodulators. All patients had Crohn’s Disease Activity Index (CDAI) scores ranging from 220 to 450 and had at least one ulcer detected at baseline ileocolonoscopy.
Participants were randomized in a 1:1 ratio to receive monotherapy with either subcutaneous adalimumab (citrate-free; 160 mg at baseline, 70 mg at week 2, then 40 mg every 2 weeks) or ustekinumab, which was given first intravenously at a dose of 6 mg/kg then subcutaneously at 90 mg every 8 weeks.
The primary endpoint was clinical remission at week 52, defined by a CDAI score less than 150. Major secondary endpoints included clinical response, corticosteroid-free remission, endoscopic remission, remission in patient-reported CDAI components, and clinical remission at week 16.
Results were statistically similar across all endpoints, with clinical remission at 1 year occurring in 64.9% and 61.0% of patients receiving ustekinumab and adalimumab, respectively (P = .417).
“Both treatments demonstrated rapid onset of action and robust endoscopy results,” Dr. Sands noted during his presentation; he reported comparable rates of endoscopic remission, at 28.5% and 30.7% for ustekinumab and adalimumab, respectively (P = .631).
Among secondary endpoints, ustekinumab demonstrated some superiority, with greater maintenance of clinical response at week 52 among patients with response at week 16 (88.6% vs. 78.0%; P = .016), greater reduction in liquid/soft stools in prior 7 days from baseline to week 52 (–19.9 vs. –16.2; P = .004), and greater reduction in sum number of liquid/soft stools and abdominal pain scores in prior 7 days from baseline to week 52 (–29.6 vs. –25.1; P = .013).
Safety metrics were similar between groups, and consistent with previous experience. Although the adalimumab group had a higher rate of discontinuation due to adverse events, this trend was not statistically significant (11.3% vs. 6.3%; P value not provided).
Don’t ignore discontinuation rates
Jordan E. Axelrad, MD, assistant professor of medicine at NYU and a clinician at the Inflammatory Bowel Disease Center at NYU Langone Health, New York, commended the SEAVUE trial for its head-to-head design, which is a first for biologics in Crohn’s disease.
“With newer drugs, there’s a critical need for head-to-head studies for us to understand where to position a lot of these agents,” he said in an interview. “[T]his was a good undifferentiated group to understand what’s the first biologic you should use in a patient with moderate-to-severe Crohn’s disease. The primary, major take-home is that [ustekinumab and adalimumab] are similarly effective.”
When asked about the slight superiority in minor secondary endpoints associated with ustekinumab, Dr. Axelrad suggested that rates of discontinuation deserve more attention.
“For me, maybe the major focus would be on the number of patients who stopped treatment,” Dr. Axelrad said, noting a higher rate of discontinuation in the adalimumab group. “Although that was just numerical, that to me is actually more important than [the minor secondary endpoints].” He also highlighted the lower injection burden associated with ustekinumab, which is given every 8 weeks, compared with every 2 weeks for adalimumab.
Ultimately, however, it’s unlikely that treatment sequencing will depend on these finer points, Dr. Axelrad suggested, and will instead come down to finances, especially with adalimumab biosimilars on the horizon, which may be the most cost-effective.
“A lot of the decision-making of where to position [ustekinumab in Crohn’s disease] is going to come down to the payer,” Dr. Axelrad said. “If there was a clear signal, providers such as myself would have a better leg to stand on, like we saw with VARSITY, where vedolizumab was clearly superior to adalimumab on multiple endpoints. We didn’t see that sort of robust signal here.”
The SEAVUE trial was supported by Janssen Scientific Affairs. Dr. Sands disclosed relationships with Janssen, AbbVie, Takeda, and others. Dr. Axelrad disclosed previous consulting fees from Janssen and research support from BioFire.
For biologic-naive adults with moderate to severe Crohn’s disease, treatment with adalimumab or ustekinumab leads to similar outcomes, according to results of the head-to-head SEAVUE trial.
When lead author Bruce E. Sands, MD, of Icahn School of Medicine at Mount Sinai, New York, compared treatment arms, patients had similar rates of clinical remission at one year. All major secondary endpoints, such as endoscopic remission, were comparable, as were safety profiles, Dr. Sands reported at the annual Digestive Disease Week® (DDW).
“From my perspective, this is an important study,” Dr. Sands wrote in a virtual chat following his presentation. “We need more head-to-head studies!”
Results from the SEAVUE trial come almost 2 years after Dr. Sands reported findings of another head-to-head IBD trial: VARSITY, which demonstrated the superiority of vedolizumab over adalimumab among patients with moderate to severe ulcerative colitis.
The multicenter, double-blinded SEAVUE trial involved 386 patients with biologic-naive Crohn’s disease who had failed corticosteroids or immunomodulators. All patients had Crohn’s Disease Activity Index (CDAI) scores ranging from 220 to 450 and had at least one ulcer detected at baseline ileocolonoscopy.
Participants were randomized in a 1:1 ratio to receive monotherapy with either subcutaneous adalimumab (citrate-free; 160 mg at baseline, 70 mg at week 2, then 40 mg every 2 weeks) or ustekinumab, which was given first intravenously at a dose of 6 mg/kg then subcutaneously at 90 mg every 8 weeks.
The primary endpoint was clinical remission at week 52, defined by a CDAI score less than 150. Major secondary endpoints included clinical response, corticosteroid-free remission, endoscopic remission, remission in patient-reported CDAI components, and clinical remission at week 16.
Results were statistically similar across all endpoints, with clinical remission at 1 year occurring in 64.9% and 61.0% of patients receiving ustekinumab and adalimumab, respectively (P = .417).
“Both treatments demonstrated rapid onset of action and robust endoscopy results,” Dr. Sands noted during his presentation; he reported comparable rates of endoscopic remission, at 28.5% and 30.7% for ustekinumab and adalimumab, respectively (P = .631).
Among secondary endpoints, ustekinumab demonstrated some superiority, with greater maintenance of clinical response at week 52 among patients with response at week 16 (88.6% vs. 78.0%; P = .016), greater reduction in liquid/soft stools in prior 7 days from baseline to week 52 (–19.9 vs. –16.2; P = .004), and greater reduction in sum number of liquid/soft stools and abdominal pain scores in prior 7 days from baseline to week 52 (–29.6 vs. –25.1; P = .013).
Safety metrics were similar between groups, and consistent with previous experience. Although the adalimumab group had a higher rate of discontinuation due to adverse events, this trend was not statistically significant (11.3% vs. 6.3%; P value not provided).
Don’t ignore discontinuation rates
Jordan E. Axelrad, MD, assistant professor of medicine at NYU and a clinician at the Inflammatory Bowel Disease Center at NYU Langone Health, New York, commended the SEAVUE trial for its head-to-head design, which is a first for biologics in Crohn’s disease.
“With newer drugs, there’s a critical need for head-to-head studies for us to understand where to position a lot of these agents,” he said in an interview. “[T]his was a good undifferentiated group to understand what’s the first biologic you should use in a patient with moderate-to-severe Crohn’s disease. The primary, major take-home is that [ustekinumab and adalimumab] are similarly effective.”
When asked about the slight superiority in minor secondary endpoints associated with ustekinumab, Dr. Axelrad suggested that rates of discontinuation deserve more attention.
“For me, maybe the major focus would be on the number of patients who stopped treatment,” Dr. Axelrad said, noting a higher rate of discontinuation in the adalimumab group. “Although that was just numerical, that to me is actually more important than [the minor secondary endpoints].” He also highlighted the lower injection burden associated with ustekinumab, which is given every 8 weeks, compared with every 2 weeks for adalimumab.
Ultimately, however, it’s unlikely that treatment sequencing will depend on these finer points, Dr. Axelrad suggested, and will instead come down to finances, especially with adalimumab biosimilars on the horizon, which may be the most cost-effective.
“A lot of the decision-making of where to position [ustekinumab in Crohn’s disease] is going to come down to the payer,” Dr. Axelrad said. “If there was a clear signal, providers such as myself would have a better leg to stand on, like we saw with VARSITY, where vedolizumab was clearly superior to adalimumab on multiple endpoints. We didn’t see that sort of robust signal here.”
The SEAVUE trial was supported by Janssen Scientific Affairs. Dr. Sands disclosed relationships with Janssen, AbbVie, Takeda, and others. Dr. Axelrad disclosed previous consulting fees from Janssen and research support from BioFire.
For biologic-naive adults with moderate to severe Crohn’s disease, treatment with adalimumab or ustekinumab leads to similar outcomes, according to results of the head-to-head SEAVUE trial.
When lead author Bruce E. Sands, MD, of Icahn School of Medicine at Mount Sinai, New York, compared treatment arms, patients had similar rates of clinical remission at one year. All major secondary endpoints, such as endoscopic remission, were comparable, as were safety profiles, Dr. Sands reported at the annual Digestive Disease Week® (DDW).
“From my perspective, this is an important study,” Dr. Sands wrote in a virtual chat following his presentation. “We need more head-to-head studies!”
Results from the SEAVUE trial come almost 2 years after Dr. Sands reported findings of another head-to-head IBD trial: VARSITY, which demonstrated the superiority of vedolizumab over adalimumab among patients with moderate to severe ulcerative colitis.
The multicenter, double-blinded SEAVUE trial involved 386 patients with biologic-naive Crohn’s disease who had failed corticosteroids or immunomodulators. All patients had Crohn’s Disease Activity Index (CDAI) scores ranging from 220 to 450 and had at least one ulcer detected at baseline ileocolonoscopy.
Participants were randomized in a 1:1 ratio to receive monotherapy with either subcutaneous adalimumab (citrate-free; 160 mg at baseline, 70 mg at week 2, then 40 mg every 2 weeks) or ustekinumab, which was given first intravenously at a dose of 6 mg/kg then subcutaneously at 90 mg every 8 weeks.
The primary endpoint was clinical remission at week 52, defined by a CDAI score less than 150. Major secondary endpoints included clinical response, corticosteroid-free remission, endoscopic remission, remission in patient-reported CDAI components, and clinical remission at week 16.
Results were statistically similar across all endpoints, with clinical remission at 1 year occurring in 64.9% and 61.0% of patients receiving ustekinumab and adalimumab, respectively (P = .417).
“Both treatments demonstrated rapid onset of action and robust endoscopy results,” Dr. Sands noted during his presentation; he reported comparable rates of endoscopic remission, at 28.5% and 30.7% for ustekinumab and adalimumab, respectively (P = .631).
Among secondary endpoints, ustekinumab demonstrated some superiority, with greater maintenance of clinical response at week 52 among patients with response at week 16 (88.6% vs. 78.0%; P = .016), greater reduction in liquid/soft stools in prior 7 days from baseline to week 52 (–19.9 vs. –16.2; P = .004), and greater reduction in sum number of liquid/soft stools and abdominal pain scores in prior 7 days from baseline to week 52 (–29.6 vs. –25.1; P = .013).
Safety metrics were similar between groups, and consistent with previous experience. Although the adalimumab group had a higher rate of discontinuation due to adverse events, this trend was not statistically significant (11.3% vs. 6.3%; P value not provided).
Don’t ignore discontinuation rates
Jordan E. Axelrad, MD, assistant professor of medicine at NYU and a clinician at the Inflammatory Bowel Disease Center at NYU Langone Health, New York, commended the SEAVUE trial for its head-to-head design, which is a first for biologics in Crohn’s disease.
“With newer drugs, there’s a critical need for head-to-head studies for us to understand where to position a lot of these agents,” he said in an interview. “[T]his was a good undifferentiated group to understand what’s the first biologic you should use in a patient with moderate-to-severe Crohn’s disease. The primary, major take-home is that [ustekinumab and adalimumab] are similarly effective.”
When asked about the slight superiority in minor secondary endpoints associated with ustekinumab, Dr. Axelrad suggested that rates of discontinuation deserve more attention.
“For me, maybe the major focus would be on the number of patients who stopped treatment,” Dr. Axelrad said, noting a higher rate of discontinuation in the adalimumab group. “Although that was just numerical, that to me is actually more important than [the minor secondary endpoints].” He also highlighted the lower injection burden associated with ustekinumab, which is given every 8 weeks, compared with every 2 weeks for adalimumab.
Ultimately, however, it’s unlikely that treatment sequencing will depend on these finer points, Dr. Axelrad suggested, and will instead come down to finances, especially with adalimumab biosimilars on the horizon, which may be the most cost-effective.
“A lot of the decision-making of where to position [ustekinumab in Crohn’s disease] is going to come down to the payer,” Dr. Axelrad said. “If there was a clear signal, providers such as myself would have a better leg to stand on, like we saw with VARSITY, where vedolizumab was clearly superior to adalimumab on multiple endpoints. We didn’t see that sort of robust signal here.”
The SEAVUE trial was supported by Janssen Scientific Affairs. Dr. Sands disclosed relationships with Janssen, AbbVie, Takeda, and others. Dr. Axelrad disclosed previous consulting fees from Janssen and research support from BioFire.
FROM DDW 2021
Microbiome therapeutic offers durable protection against C. difficile recurrence
SER-109, an oral microbiome therapeutic, safely protects against Clostridioides difficile recurrence for up to 24 weeks, according to a recent phase 3 trial. Three days of treatment with purified Firmicutes spores reduced risk of recurrence by 54%, suggesting a sustained, clinically meaningful response, according to a multicenter study presented at this year’s Digestive Disease Week® (DDW).
“Antibiotics targeted against C. difficile bacteria are necessary but insufficient to achieve a durable clinical response because they have no effect on C. difficile spores that germinate within a disrupted microbiome,” the investigators reported at the meeting.
“The manufacturing processes for SER-109 are designed to inactivate potential pathogens, while enriching for beneficial Firmicutes spores, which play a central role in inhibiting the cycle of C. difficile,” said Louis Y. Korman, MD, a gastroenterologist in Washington, who was lead author.
Extended data from ECOSPOR-III
The ECOSPOR-III trial involved 182 patients with at least three episodes of C. difficile infection in the previous 12 months. Patients underwent 10-21 days of antibiotic therapy with fidaxomicin or vancomycin to resolve symptoms before they were then randomized in a 1:1 ratio to receive either SER-109 (four capsules daily for 3 days) or placebo, with stratification by specific antibiotic and patient age (threshold of 65 years).
The primary objectives were safety and efficacy at 8 weeks. These results, which were previously reported at ACG 2020, showed a 68% relative risk reduction in the SER-109 group, and favorable safety data. The findings presented at DDW added to those earlier ones by providing safety and efficacy data extending to week 24. At this time point, patients treated with SER-109 had a 54% relative risk reduction in C. difficile recurrence. Recurrence rates were 21.3% and 47.3% for the treatment and placebo groups, respectively (P less than .001).
Patients 65 years and older benefited the most from SER-109 therapy, based on a relative risk reduction of 56% (P less than .001), versus a 49% relative risk reduction (lacking statistical significance) for patients younger than 65 years (P = .093). The specific antibiotic therapy patients received also appeared to impact outcomes. Patients treated with fidaxomicin had a 73% relative risk reduction (P = .009), compared with 48% for vancomycin (P = .006). Safety profiles were similar between study arms.
“By enriching for Firmicutes spores, SER-109 achieves high efficacy, while mitigating risk of transmitting infectious agents and represents a major paradigm shift in the clinical management of patients with recurrent C. difficile infection,” the investigators concluded, noting that “an open-label study for patients with recurrent C. difficile infection is currently enrolling.”
Microbiome restoration therapies
According to Sahil Khanna, MBBS, professor of medicine at Mayo Clinic, Rochester, Minn., these findings “advance the field” because they show a sustained response. “We know that microbiome restoration therapies help restore colonization resistance,” Dr. Khanna said in an interview, noting that they offer benefits comparable to fecal microbiota transplantation (FMT) without the downsides.
“The trouble with FMT is that it’s heterogenous – everybody does it differently … and also it’s an invasive procedure,” Dr. Khanna said. He noted that FMT may transmit infectious agents between donors and patients, which isn’t an issue with purified products such as SER-109.
Several other standardized microbiota restoration products are under development, Dr. Khanna said, including an enema form (RBX2660) in phase 3 testing, and two other capsules (CP101 and VE303) in phase 2 trials. “The hope would be that one or more of these products would be approved for clinical use in the near future and would probably replace the vast majority of FMT [procedures] that we do clinically,” Dr. Khanna said. “That’s where the field is headed.”
The investigators reported no conflicts of interest. Dr. Khanna disclosed research support from Finch, Rebiotix/Ferring, Vedanta, and Seres.
SER-109, an oral microbiome therapeutic, safely protects against Clostridioides difficile recurrence for up to 24 weeks, according to a recent phase 3 trial. Three days of treatment with purified Firmicutes spores reduced risk of recurrence by 54%, suggesting a sustained, clinically meaningful response, according to a multicenter study presented at this year’s Digestive Disease Week® (DDW).
“Antibiotics targeted against C. difficile bacteria are necessary but insufficient to achieve a durable clinical response because they have no effect on C. difficile spores that germinate within a disrupted microbiome,” the investigators reported at the meeting.
“The manufacturing processes for SER-109 are designed to inactivate potential pathogens, while enriching for beneficial Firmicutes spores, which play a central role in inhibiting the cycle of C. difficile,” said Louis Y. Korman, MD, a gastroenterologist in Washington, who was lead author.
Extended data from ECOSPOR-III
The ECOSPOR-III trial involved 182 patients with at least three episodes of C. difficile infection in the previous 12 months. Patients underwent 10-21 days of antibiotic therapy with fidaxomicin or vancomycin to resolve symptoms before they were then randomized in a 1:1 ratio to receive either SER-109 (four capsules daily for 3 days) or placebo, with stratification by specific antibiotic and patient age (threshold of 65 years).
The primary objectives were safety and efficacy at 8 weeks. These results, which were previously reported at ACG 2020, showed a 68% relative risk reduction in the SER-109 group, and favorable safety data. The findings presented at DDW added to those earlier ones by providing safety and efficacy data extending to week 24. At this time point, patients treated with SER-109 had a 54% relative risk reduction in C. difficile recurrence. Recurrence rates were 21.3% and 47.3% for the treatment and placebo groups, respectively (P less than .001).
Patients 65 years and older benefited the most from SER-109 therapy, based on a relative risk reduction of 56% (P less than .001), versus a 49% relative risk reduction (lacking statistical significance) for patients younger than 65 years (P = .093). The specific antibiotic therapy patients received also appeared to impact outcomes. Patients treated with fidaxomicin had a 73% relative risk reduction (P = .009), compared with 48% for vancomycin (P = .006). Safety profiles were similar between study arms.
“By enriching for Firmicutes spores, SER-109 achieves high efficacy, while mitigating risk of transmitting infectious agents and represents a major paradigm shift in the clinical management of patients with recurrent C. difficile infection,” the investigators concluded, noting that “an open-label study for patients with recurrent C. difficile infection is currently enrolling.”
Microbiome restoration therapies
According to Sahil Khanna, MBBS, professor of medicine at Mayo Clinic, Rochester, Minn., these findings “advance the field” because they show a sustained response. “We know that microbiome restoration therapies help restore colonization resistance,” Dr. Khanna said in an interview, noting that they offer benefits comparable to fecal microbiota transplantation (FMT) without the downsides.
“The trouble with FMT is that it’s heterogenous – everybody does it differently … and also it’s an invasive procedure,” Dr. Khanna said. He noted that FMT may transmit infectious agents between donors and patients, which isn’t an issue with purified products such as SER-109.
Several other standardized microbiota restoration products are under development, Dr. Khanna said, including an enema form (RBX2660) in phase 3 testing, and two other capsules (CP101 and VE303) in phase 2 trials. “The hope would be that one or more of these products would be approved for clinical use in the near future and would probably replace the vast majority of FMT [procedures] that we do clinically,” Dr. Khanna said. “That’s where the field is headed.”
The investigators reported no conflicts of interest. Dr. Khanna disclosed research support from Finch, Rebiotix/Ferring, Vedanta, and Seres.
SER-109, an oral microbiome therapeutic, safely protects against Clostridioides difficile recurrence for up to 24 weeks, according to a recent phase 3 trial. Three days of treatment with purified Firmicutes spores reduced risk of recurrence by 54%, suggesting a sustained, clinically meaningful response, according to a multicenter study presented at this year’s Digestive Disease Week® (DDW).
“Antibiotics targeted against C. difficile bacteria are necessary but insufficient to achieve a durable clinical response because they have no effect on C. difficile spores that germinate within a disrupted microbiome,” the investigators reported at the meeting.
“The manufacturing processes for SER-109 are designed to inactivate potential pathogens, while enriching for beneficial Firmicutes spores, which play a central role in inhibiting the cycle of C. difficile,” said Louis Y. Korman, MD, a gastroenterologist in Washington, who was lead author.
Extended data from ECOSPOR-III
The ECOSPOR-III trial involved 182 patients with at least three episodes of C. difficile infection in the previous 12 months. Patients underwent 10-21 days of antibiotic therapy with fidaxomicin or vancomycin to resolve symptoms before they were then randomized in a 1:1 ratio to receive either SER-109 (four capsules daily for 3 days) or placebo, with stratification by specific antibiotic and patient age (threshold of 65 years).
The primary objectives were safety and efficacy at 8 weeks. These results, which were previously reported at ACG 2020, showed a 68% relative risk reduction in the SER-109 group, and favorable safety data. The findings presented at DDW added to those earlier ones by providing safety and efficacy data extending to week 24. At this time point, patients treated with SER-109 had a 54% relative risk reduction in C. difficile recurrence. Recurrence rates were 21.3% and 47.3% for the treatment and placebo groups, respectively (P less than .001).
Patients 65 years and older benefited the most from SER-109 therapy, based on a relative risk reduction of 56% (P less than .001), versus a 49% relative risk reduction (lacking statistical significance) for patients younger than 65 years (P = .093). The specific antibiotic therapy patients received also appeared to impact outcomes. Patients treated with fidaxomicin had a 73% relative risk reduction (P = .009), compared with 48% for vancomycin (P = .006). Safety profiles were similar between study arms.
“By enriching for Firmicutes spores, SER-109 achieves high efficacy, while mitigating risk of transmitting infectious agents and represents a major paradigm shift in the clinical management of patients with recurrent C. difficile infection,” the investigators concluded, noting that “an open-label study for patients with recurrent C. difficile infection is currently enrolling.”
Microbiome restoration therapies
According to Sahil Khanna, MBBS, professor of medicine at Mayo Clinic, Rochester, Minn., these findings “advance the field” because they show a sustained response. “We know that microbiome restoration therapies help restore colonization resistance,” Dr. Khanna said in an interview, noting that they offer benefits comparable to fecal microbiota transplantation (FMT) without the downsides.
“The trouble with FMT is that it’s heterogenous – everybody does it differently … and also it’s an invasive procedure,” Dr. Khanna said. He noted that FMT may transmit infectious agents between donors and patients, which isn’t an issue with purified products such as SER-109.
Several other standardized microbiota restoration products are under development, Dr. Khanna said, including an enema form (RBX2660) in phase 3 testing, and two other capsules (CP101 and VE303) in phase 2 trials. “The hope would be that one or more of these products would be approved for clinical use in the near future and would probably replace the vast majority of FMT [procedures] that we do clinically,” Dr. Khanna said. “That’s where the field is headed.”
The investigators reported no conflicts of interest. Dr. Khanna disclosed research support from Finch, Rebiotix/Ferring, Vedanta, and Seres.
FROM DDW 2021
FDA panel endorses teplizumab for delaying type 1 diabetes
in at-risk individuals.
The 10-7 vote of the FDA’s endocrinologic and metabolic drugs advisory committee on May 27 reflected a difficult decision-making process on the part of many members to weigh the benefits of a potential 2-year delay in the onset of type 1 diabetes against both observed and theoretical risks, as well as what most considered to be insufficient data.
Regardless of their vote, nearly all panel members advised the FDA that the company should be required to conduct at least one additional larger long-term efficacy and safety trial to satisfy what they felt were major gaps in the data. Some advised that use of the drug be restricted to a very narrow group of recipients until efficacy and safety can be better established.
If approved, teplizumab, which interferes with T cell–mediated autoimmune destruction of pancreatic beta cells, would be the first disease-modifying therapy for impeding progression of type 1 diabetes. The proposed indication is for individuals who have two or more type 1 diabetes-associated autoantibodies and subclinical dysglycemia.
That “stage 2” or “at-risk” condition is associated with a nearly 100% lifetime risk of progression to clinical (“stage 3”) type 1 diabetes and a 75% risk of developing the disease within 5 years. As of now, most such individuals are first-degree relatives of people with type 1 diabetes identified through TrialNet.
What’s the evidence to support approval so far?
In 2019, a pivotal phase 2 randomized, placebo-controlled TN-10 trial involving 76 at-risk children and adults ages 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes, compared with 32 who received placebo. Further follow-up data continue to show that fewer patients who received teplizumab have progressed to clinical type 1 diabetes.
While most advisory panelists agreed that the TN-10 study demonstrated efficacy, several also said that the sample size was insufficient and at least one additional randomized trial should be conducted to replicate the findings.
Although the FDA typically requires companies to demonstrate a drug’s effectiveness with at least two separate clinical trials, the agency allows companies to substitute other forms of data for a second randomized clinical trial, such as study results for the drug in a closely related condition, mechanistic data, or knowledge of other drugs from the same class.
In this case, Provention’s submission included as “confirmatory” evidence a meta-analysis of data from five earlier randomized trials (three placebo controlled, two open label) of a total 942 individuals with newly diagnosed type 1 diabetes (“stage 3”) who received either one or two 14-day teplizumab courses (n = 729) or placebo. These showed consistent preservation of C-peptide, a surrogate marker of beta-cell function, along with lower mean insulin use.
Several panel members expressed dissatisfaction with those confirmatory data, noting the patient population was different from those for which the company is currently seeking the indication, and that C-peptide is an inadequate endpoint for demonstrating efficacy.
Safety: Adverse events mostly transient, but unanswered questions
Adverse events reported in at least 10% of teplizumab recipients included lymphopenia (76.8% vs. 9.4% placebo; relative risk, 8.2), leukopenia (82.1% vs. 24.1%; RR, 3.4), and rash (44.5% vs. 9.0%; RR, 4.9).
“Most adverse events related to teplizumab were mechanism-based, predictable, transient, and manageable,” Chief Medical Officer of Provention Bio, Eleanor Ramos, MD, said.
Among other safety issues that concerned the panel, diabetic ketoacidosis (DKA) was seen in 2.3% of 773 teplizumab recipients with new-onset type 1 diabetes versus just 1% among the 245 controls, a significant, nearly sixfold increase. No DKA occurred in the TN-10 trial. No clear explanation was offered for the imbalance in the meta-analysis.
Cytokine release syndrome occurred in 0.6% of patients who received teplizumab versus no controls, and infections in 3.4% versus 2.0%, respectively.
Approximately 10% of patients were not able to complete the treatment course because of protocol-directed withdrawal criteria, which included elevations in bilirubin or liver enzymes, or drops in platelet count, neutrophils, or hemoglobin, FDA reviewer Lauren Wood Heickman, MD, noted.
There was only one malignancy, a melanoma in a patient with a preexisting lesion, but malignancy is a theoretical concern with long-term immunosuppression, Dr. Heickman said.
Despite the concerns about the data, panel members expressed unanimous appreciation for the 18 people who spoke during public comments attesting to the lifelong burdens involved in living with type 1 diabetes who urged the FDA to approve teplizumab.
Many of them noted that even a 2-year reprieve from the burden of constant attention to managing blood glucose can make a major difference in the life of a young person. The speakers included physicians, parents of children with type 1 diabetes, adults who have the condition themselves and who worry about their children getting it, and researchers in the field.
Panel members describe ‘struggle’ with vote decision
Panel member Michael Blaha, MD, of Johns Hopkins University, Baltimore, voted in favor of teplizumab approval. However, he said, “I was very conflicted on this one and my ‘yes’ is very qualified. In my opinion the risk-benefit is very narrow, and I would only approve this drug for the exact indication of the trial. ... Patients who don’t fit the criteria could hopefully be enrolled in a second confirmatory trial.”
He also advised an extensive Risk Evaluation and Mitigation Strategies program to look for both short- and long-term adverse effects.
“My overall take on this is that I do think it’s a promising paradigm-shifting therapy that really needs to move forward, at least scientifically. I’m excited about it, but I have a lot of skepticism about the entire body of data to make any more than the most narrow of approval,” Dr. Blaha said.
Susan S. Ellenberg, PhD, professor of biostatistics, medical ethics, and health policy at the University of Pennsylvania, Philadelphia, voted yes but also with difficulty.
“I really struggled with it. ... I was pushed by the very encouraging results of what is admittedly a very small study and something I can’t feel is completely definitive. But I would not like to deny the kind of people that we heard from today the opportunity to weigh their own risks and benefits to try this. And I would certainly agree that a very, very rigorous postmarketing program, preferably including another controlled trial, should be carried out.”
But David M. Nathan, MD, director of the Diabetes Center and Clinical Research Center, Massachusetts General Hospital, Boston, voted no.
“I struggled with this vote, tremendously, having listened carefully to the patients with type 1 diabetes ... but that said, having done clinical research for 40 years in type 1 diabetes, I think we need more data, both in terms of efficacy and of safety. I would hate a number of years down the road to figure out that we actually caused more harm than good, especially keeping in mind that the treatment of type 1 diabetes is evolving rapidly.”
A different perspective came from Mara L. Becker, MD, vice chair of the department of pediatric rheumatology at Duke University, Durham, N.C. She voted yes, pointing out that she’s accustomed to prescribing biologics for chronic conditions in children.
“I was unconflicted in my vote, which was yes. I thought the data ... were convincing and the need is great. I would support a label for children [aged 8 years] and older with at least stage 2 disease ... and I would require postmarketing safety surveillance to understand what the long-term side effects could be, but I would still be in favor of it.”
FDA advisory panel committee members are vetted for conflicts of interest and waivers granted for participation if necessary; none were granted for this meeting.
A version of this article first appeared on Medscape.com.
in at-risk individuals.
The 10-7 vote of the FDA’s endocrinologic and metabolic drugs advisory committee on May 27 reflected a difficult decision-making process on the part of many members to weigh the benefits of a potential 2-year delay in the onset of type 1 diabetes against both observed and theoretical risks, as well as what most considered to be insufficient data.
Regardless of their vote, nearly all panel members advised the FDA that the company should be required to conduct at least one additional larger long-term efficacy and safety trial to satisfy what they felt were major gaps in the data. Some advised that use of the drug be restricted to a very narrow group of recipients until efficacy and safety can be better established.
If approved, teplizumab, which interferes with T cell–mediated autoimmune destruction of pancreatic beta cells, would be the first disease-modifying therapy for impeding progression of type 1 diabetes. The proposed indication is for individuals who have two or more type 1 diabetes-associated autoantibodies and subclinical dysglycemia.
That “stage 2” or “at-risk” condition is associated with a nearly 100% lifetime risk of progression to clinical (“stage 3”) type 1 diabetes and a 75% risk of developing the disease within 5 years. As of now, most such individuals are first-degree relatives of people with type 1 diabetes identified through TrialNet.
What’s the evidence to support approval so far?
In 2019, a pivotal phase 2 randomized, placebo-controlled TN-10 trial involving 76 at-risk children and adults ages 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes, compared with 32 who received placebo. Further follow-up data continue to show that fewer patients who received teplizumab have progressed to clinical type 1 diabetes.
While most advisory panelists agreed that the TN-10 study demonstrated efficacy, several also said that the sample size was insufficient and at least one additional randomized trial should be conducted to replicate the findings.
Although the FDA typically requires companies to demonstrate a drug’s effectiveness with at least two separate clinical trials, the agency allows companies to substitute other forms of data for a second randomized clinical trial, such as study results for the drug in a closely related condition, mechanistic data, or knowledge of other drugs from the same class.
In this case, Provention’s submission included as “confirmatory” evidence a meta-analysis of data from five earlier randomized trials (three placebo controlled, two open label) of a total 942 individuals with newly diagnosed type 1 diabetes (“stage 3”) who received either one or two 14-day teplizumab courses (n = 729) or placebo. These showed consistent preservation of C-peptide, a surrogate marker of beta-cell function, along with lower mean insulin use.
Several panel members expressed dissatisfaction with those confirmatory data, noting the patient population was different from those for which the company is currently seeking the indication, and that C-peptide is an inadequate endpoint for demonstrating efficacy.
Safety: Adverse events mostly transient, but unanswered questions
Adverse events reported in at least 10% of teplizumab recipients included lymphopenia (76.8% vs. 9.4% placebo; relative risk, 8.2), leukopenia (82.1% vs. 24.1%; RR, 3.4), and rash (44.5% vs. 9.0%; RR, 4.9).
“Most adverse events related to teplizumab were mechanism-based, predictable, transient, and manageable,” Chief Medical Officer of Provention Bio, Eleanor Ramos, MD, said.
Among other safety issues that concerned the panel, diabetic ketoacidosis (DKA) was seen in 2.3% of 773 teplizumab recipients with new-onset type 1 diabetes versus just 1% among the 245 controls, a significant, nearly sixfold increase. No DKA occurred in the TN-10 trial. No clear explanation was offered for the imbalance in the meta-analysis.
Cytokine release syndrome occurred in 0.6% of patients who received teplizumab versus no controls, and infections in 3.4% versus 2.0%, respectively.
Approximately 10% of patients were not able to complete the treatment course because of protocol-directed withdrawal criteria, which included elevations in bilirubin or liver enzymes, or drops in platelet count, neutrophils, or hemoglobin, FDA reviewer Lauren Wood Heickman, MD, noted.
There was only one malignancy, a melanoma in a patient with a preexisting lesion, but malignancy is a theoretical concern with long-term immunosuppression, Dr. Heickman said.
Despite the concerns about the data, panel members expressed unanimous appreciation for the 18 people who spoke during public comments attesting to the lifelong burdens involved in living with type 1 diabetes who urged the FDA to approve teplizumab.
Many of them noted that even a 2-year reprieve from the burden of constant attention to managing blood glucose can make a major difference in the life of a young person. The speakers included physicians, parents of children with type 1 diabetes, adults who have the condition themselves and who worry about their children getting it, and researchers in the field.
Panel members describe ‘struggle’ with vote decision
Panel member Michael Blaha, MD, of Johns Hopkins University, Baltimore, voted in favor of teplizumab approval. However, he said, “I was very conflicted on this one and my ‘yes’ is very qualified. In my opinion the risk-benefit is very narrow, and I would only approve this drug for the exact indication of the trial. ... Patients who don’t fit the criteria could hopefully be enrolled in a second confirmatory trial.”
He also advised an extensive Risk Evaluation and Mitigation Strategies program to look for both short- and long-term adverse effects.
“My overall take on this is that I do think it’s a promising paradigm-shifting therapy that really needs to move forward, at least scientifically. I’m excited about it, but I have a lot of skepticism about the entire body of data to make any more than the most narrow of approval,” Dr. Blaha said.
Susan S. Ellenberg, PhD, professor of biostatistics, medical ethics, and health policy at the University of Pennsylvania, Philadelphia, voted yes but also with difficulty.
“I really struggled with it. ... I was pushed by the very encouraging results of what is admittedly a very small study and something I can’t feel is completely definitive. But I would not like to deny the kind of people that we heard from today the opportunity to weigh their own risks and benefits to try this. And I would certainly agree that a very, very rigorous postmarketing program, preferably including another controlled trial, should be carried out.”
But David M. Nathan, MD, director of the Diabetes Center and Clinical Research Center, Massachusetts General Hospital, Boston, voted no.
“I struggled with this vote, tremendously, having listened carefully to the patients with type 1 diabetes ... but that said, having done clinical research for 40 years in type 1 diabetes, I think we need more data, both in terms of efficacy and of safety. I would hate a number of years down the road to figure out that we actually caused more harm than good, especially keeping in mind that the treatment of type 1 diabetes is evolving rapidly.”
A different perspective came from Mara L. Becker, MD, vice chair of the department of pediatric rheumatology at Duke University, Durham, N.C. She voted yes, pointing out that she’s accustomed to prescribing biologics for chronic conditions in children.
“I was unconflicted in my vote, which was yes. I thought the data ... were convincing and the need is great. I would support a label for children [aged 8 years] and older with at least stage 2 disease ... and I would require postmarketing safety surveillance to understand what the long-term side effects could be, but I would still be in favor of it.”
FDA advisory panel committee members are vetted for conflicts of interest and waivers granted for participation if necessary; none were granted for this meeting.
A version of this article first appeared on Medscape.com.
in at-risk individuals.
The 10-7 vote of the FDA’s endocrinologic and metabolic drugs advisory committee on May 27 reflected a difficult decision-making process on the part of many members to weigh the benefits of a potential 2-year delay in the onset of type 1 diabetes against both observed and theoretical risks, as well as what most considered to be insufficient data.
Regardless of their vote, nearly all panel members advised the FDA that the company should be required to conduct at least one additional larger long-term efficacy and safety trial to satisfy what they felt were major gaps in the data. Some advised that use of the drug be restricted to a very narrow group of recipients until efficacy and safety can be better established.
If approved, teplizumab, which interferes with T cell–mediated autoimmune destruction of pancreatic beta cells, would be the first disease-modifying therapy for impeding progression of type 1 diabetes. The proposed indication is for individuals who have two or more type 1 diabetes-associated autoantibodies and subclinical dysglycemia.
That “stage 2” or “at-risk” condition is associated with a nearly 100% lifetime risk of progression to clinical (“stage 3”) type 1 diabetes and a 75% risk of developing the disease within 5 years. As of now, most such individuals are first-degree relatives of people with type 1 diabetes identified through TrialNet.
What’s the evidence to support approval so far?
In 2019, a pivotal phase 2 randomized, placebo-controlled TN-10 trial involving 76 at-risk children and adults ages 8 years and older showed that a single 14-day treatment of daily intravenous infusions of teplizumab in 44 patients resulted in a significant median 2-year delay to onset of clinical type 1 diabetes, compared with 32 who received placebo. Further follow-up data continue to show that fewer patients who received teplizumab have progressed to clinical type 1 diabetes.
While most advisory panelists agreed that the TN-10 study demonstrated efficacy, several also said that the sample size was insufficient and at least one additional randomized trial should be conducted to replicate the findings.
Although the FDA typically requires companies to demonstrate a drug’s effectiveness with at least two separate clinical trials, the agency allows companies to substitute other forms of data for a second randomized clinical trial, such as study results for the drug in a closely related condition, mechanistic data, or knowledge of other drugs from the same class.
In this case, Provention’s submission included as “confirmatory” evidence a meta-analysis of data from five earlier randomized trials (three placebo controlled, two open label) of a total 942 individuals with newly diagnosed type 1 diabetes (“stage 3”) who received either one or two 14-day teplizumab courses (n = 729) or placebo. These showed consistent preservation of C-peptide, a surrogate marker of beta-cell function, along with lower mean insulin use.
Several panel members expressed dissatisfaction with those confirmatory data, noting the patient population was different from those for which the company is currently seeking the indication, and that C-peptide is an inadequate endpoint for demonstrating efficacy.
Safety: Adverse events mostly transient, but unanswered questions
Adverse events reported in at least 10% of teplizumab recipients included lymphopenia (76.8% vs. 9.4% placebo; relative risk, 8.2), leukopenia (82.1% vs. 24.1%; RR, 3.4), and rash (44.5% vs. 9.0%; RR, 4.9).
“Most adverse events related to teplizumab were mechanism-based, predictable, transient, and manageable,” Chief Medical Officer of Provention Bio, Eleanor Ramos, MD, said.
Among other safety issues that concerned the panel, diabetic ketoacidosis (DKA) was seen in 2.3% of 773 teplizumab recipients with new-onset type 1 diabetes versus just 1% among the 245 controls, a significant, nearly sixfold increase. No DKA occurred in the TN-10 trial. No clear explanation was offered for the imbalance in the meta-analysis.
Cytokine release syndrome occurred in 0.6% of patients who received teplizumab versus no controls, and infections in 3.4% versus 2.0%, respectively.
Approximately 10% of patients were not able to complete the treatment course because of protocol-directed withdrawal criteria, which included elevations in bilirubin or liver enzymes, or drops in platelet count, neutrophils, or hemoglobin, FDA reviewer Lauren Wood Heickman, MD, noted.
There was only one malignancy, a melanoma in a patient with a preexisting lesion, but malignancy is a theoretical concern with long-term immunosuppression, Dr. Heickman said.
Despite the concerns about the data, panel members expressed unanimous appreciation for the 18 people who spoke during public comments attesting to the lifelong burdens involved in living with type 1 diabetes who urged the FDA to approve teplizumab.
Many of them noted that even a 2-year reprieve from the burden of constant attention to managing blood glucose can make a major difference in the life of a young person. The speakers included physicians, parents of children with type 1 diabetes, adults who have the condition themselves and who worry about their children getting it, and researchers in the field.
Panel members describe ‘struggle’ with vote decision
Panel member Michael Blaha, MD, of Johns Hopkins University, Baltimore, voted in favor of teplizumab approval. However, he said, “I was very conflicted on this one and my ‘yes’ is very qualified. In my opinion the risk-benefit is very narrow, and I would only approve this drug for the exact indication of the trial. ... Patients who don’t fit the criteria could hopefully be enrolled in a second confirmatory trial.”
He also advised an extensive Risk Evaluation and Mitigation Strategies program to look for both short- and long-term adverse effects.
“My overall take on this is that I do think it’s a promising paradigm-shifting therapy that really needs to move forward, at least scientifically. I’m excited about it, but I have a lot of skepticism about the entire body of data to make any more than the most narrow of approval,” Dr. Blaha said.
Susan S. Ellenberg, PhD, professor of biostatistics, medical ethics, and health policy at the University of Pennsylvania, Philadelphia, voted yes but also with difficulty.
“I really struggled with it. ... I was pushed by the very encouraging results of what is admittedly a very small study and something I can’t feel is completely definitive. But I would not like to deny the kind of people that we heard from today the opportunity to weigh their own risks and benefits to try this. And I would certainly agree that a very, very rigorous postmarketing program, preferably including another controlled trial, should be carried out.”
But David M. Nathan, MD, director of the Diabetes Center and Clinical Research Center, Massachusetts General Hospital, Boston, voted no.
“I struggled with this vote, tremendously, having listened carefully to the patients with type 1 diabetes ... but that said, having done clinical research for 40 years in type 1 diabetes, I think we need more data, both in terms of efficacy and of safety. I would hate a number of years down the road to figure out that we actually caused more harm than good, especially keeping in mind that the treatment of type 1 diabetes is evolving rapidly.”
A different perspective came from Mara L. Becker, MD, vice chair of the department of pediatric rheumatology at Duke University, Durham, N.C. She voted yes, pointing out that she’s accustomed to prescribing biologics for chronic conditions in children.
“I was unconflicted in my vote, which was yes. I thought the data ... were convincing and the need is great. I would support a label for children [aged 8 years] and older with at least stage 2 disease ... and I would require postmarketing safety surveillance to understand what the long-term side effects could be, but I would still be in favor of it.”
FDA advisory panel committee members are vetted for conflicts of interest and waivers granted for participation if necessary; none were granted for this meeting.
A version of this article first appeared on Medscape.com.
NSCLC survival on immunotherapy much lower in ‘real world’
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Dermatologists took 2020’s income drop in stride
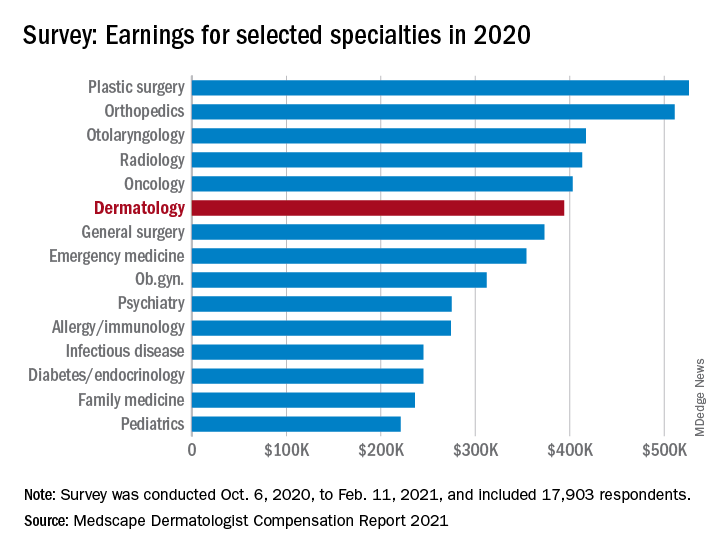
The numbers look like this: Average income was $394,000 in 2020, compared with $411,000 in 2019 – a drop of 4.1% – but 67% of dermatologists said they felt fairly compensated in 2020, compared with 65% in 2019, Medscape said in its 2021 Dermatologist Compensation Report. Only 3 of the 29 participating specialties had a more favorable reaction: oncology (79%), psychiatry (69%), and plastic surgery (68%).
“Most dermatologists who saw a drop in income cited COVID-19–related issues, such as job loss, fewer hours, and fewer patients,” Keith L. Martin wrote in the annual report, while also noting that 45% of dermatologist respondents “said that the pandemic did not cause them financial or practice-related harm.”
For the dermatologists who did see such negative effects, just over half (54%) said that they expect income to return to pre–COVID-19 levels in the next year, while 31% believe it will take 2-3 years and 12% said that their income would never return to normal. For all specialists included in the survey, the corresponding numbers were 42%, 41%, and 12%, with primary care physicians coming in at 39%, 43%, and 10%, the report said.
Among all participating specialties, plastic surgeons reported the highest average earnings at $526,000, with orthopedists ($511,000) and cardiologists ($459,000) next. Pediatricians had not just the lowest average income ($221,000) for 2020, but also the largest decline in patients seen per week (18%), according to the results of the survey, which was conducted from Oct. 6, 2020, to Feb. 11, 2021, and involved 17,903 physicians.
Dermatologists also experienced a larger-than-average decline (16%) in patient traffic – only the pediatricians had a larger drop – as their weekly patient count fell from 141 before the pandemic to the current 119. Despite that drop, though, average hours worked per week remained at 45, as time is now being spent on office safety protocols and other issues involving COVID-19, Medscape pointed out.
Dermatologists also spent more time on paperwork and administration in 2020 than in 2019: 14.6 hours per week versus 13.2 hours. Their 2020 average, however, was still lower than that of all physicians, 16.3 hours, and much lower than that of the infectious disease physicians, who topped the survey with an average of 24.2 hours per week, the Medscape data show.
One area where dermatologists did lead the survey was in their commitment to their specialty: 96% said they would choose dermatology again if given the chance, which was equaled by orthopedics and oncology, Medscape said.

The numbers look like this: Average income was $394,000 in 2020, compared with $411,000 in 2019 – a drop of 4.1% – but 67% of dermatologists said they felt fairly compensated in 2020, compared with 65% in 2019, Medscape said in its 2021 Dermatologist Compensation Report. Only 3 of the 29 participating specialties had a more favorable reaction: oncology (79%), psychiatry (69%), and plastic surgery (68%).
“Most dermatologists who saw a drop in income cited COVID-19–related issues, such as job loss, fewer hours, and fewer patients,” Keith L. Martin wrote in the annual report, while also noting that 45% of dermatologist respondents “said that the pandemic did not cause them financial or practice-related harm.”
For the dermatologists who did see such negative effects, just over half (54%) said that they expect income to return to pre–COVID-19 levels in the next year, while 31% believe it will take 2-3 years and 12% said that their income would never return to normal. For all specialists included in the survey, the corresponding numbers were 42%, 41%, and 12%, with primary care physicians coming in at 39%, 43%, and 10%, the report said.
Among all participating specialties, plastic surgeons reported the highest average earnings at $526,000, with orthopedists ($511,000) and cardiologists ($459,000) next. Pediatricians had not just the lowest average income ($221,000) for 2020, but also the largest decline in patients seen per week (18%), according to the results of the survey, which was conducted from Oct. 6, 2020, to Feb. 11, 2021, and involved 17,903 physicians.
Dermatologists also experienced a larger-than-average decline (16%) in patient traffic – only the pediatricians had a larger drop – as their weekly patient count fell from 141 before the pandemic to the current 119. Despite that drop, though, average hours worked per week remained at 45, as time is now being spent on office safety protocols and other issues involving COVID-19, Medscape pointed out.
Dermatologists also spent more time on paperwork and administration in 2020 than in 2019: 14.6 hours per week versus 13.2 hours. Their 2020 average, however, was still lower than that of all physicians, 16.3 hours, and much lower than that of the infectious disease physicians, who topped the survey with an average of 24.2 hours per week, the Medscape data show.
One area where dermatologists did lead the survey was in their commitment to their specialty: 96% said they would choose dermatology again if given the chance, which was equaled by orthopedics and oncology, Medscape said.

The numbers look like this: Average income was $394,000 in 2020, compared with $411,000 in 2019 – a drop of 4.1% – but 67% of dermatologists said they felt fairly compensated in 2020, compared with 65% in 2019, Medscape said in its 2021 Dermatologist Compensation Report. Only 3 of the 29 participating specialties had a more favorable reaction: oncology (79%), psychiatry (69%), and plastic surgery (68%).
“Most dermatologists who saw a drop in income cited COVID-19–related issues, such as job loss, fewer hours, and fewer patients,” Keith L. Martin wrote in the annual report, while also noting that 45% of dermatologist respondents “said that the pandemic did not cause them financial or practice-related harm.”
For the dermatologists who did see such negative effects, just over half (54%) said that they expect income to return to pre–COVID-19 levels in the next year, while 31% believe it will take 2-3 years and 12% said that their income would never return to normal. For all specialists included in the survey, the corresponding numbers were 42%, 41%, and 12%, with primary care physicians coming in at 39%, 43%, and 10%, the report said.
Among all participating specialties, plastic surgeons reported the highest average earnings at $526,000, with orthopedists ($511,000) and cardiologists ($459,000) next. Pediatricians had not just the lowest average income ($221,000) for 2020, but also the largest decline in patients seen per week (18%), according to the results of the survey, which was conducted from Oct. 6, 2020, to Feb. 11, 2021, and involved 17,903 physicians.
Dermatologists also experienced a larger-than-average decline (16%) in patient traffic – only the pediatricians had a larger drop – as their weekly patient count fell from 141 before the pandemic to the current 119. Despite that drop, though, average hours worked per week remained at 45, as time is now being spent on office safety protocols and other issues involving COVID-19, Medscape pointed out.
Dermatologists also spent more time on paperwork and administration in 2020 than in 2019: 14.6 hours per week versus 13.2 hours. Their 2020 average, however, was still lower than that of all physicians, 16.3 hours, and much lower than that of the infectious disease physicians, who topped the survey with an average of 24.2 hours per week, the Medscape data show.
One area where dermatologists did lead the survey was in their commitment to their specialty: 96% said they would choose dermatology again if given the chance, which was equaled by orthopedics and oncology, Medscape said.
New Editor in Chief: Ebrahim Barkoudah, MD, MPH
With another long winter officially in the rearview mirror and spring sunshine displaying new signs of life outdoors, I am excited to share some of the changes happening inside the offices of the Journal of Clinical Outcomes Management (JCOM). It is my pleasure to introduce Ebrahim Barkoudah, MD, MPH, as the journal’s new physician Editor in Chief. Dr. Barkoudah’s extensive experience in education and his work to improve patient outcomes will be assets to JCOM.
Specializing in both internal medicine and hospital medicine, Dr. Barkoudah is the Associate Director of the Hospital Medicine Unit and a Medical Director in the Department of Medicine at Brigham and Women’s Hospital in Boston. He is also Assistant Professor of Medicine at Harvard Medical School, where he led the school’s international education efforts.
Dr. Barkoudah serves patients with a range of complex clinical disorders, managing their care and seeking innovative treatment options. His research interest is in health care outcomes as well as clinical trials of therapeutic interventions. Dr. Barkoudah also serves on numerous clinical innovation committees at Brigham Health and national task forces.
Dr. Barkoudah is an active member of several professional societies including the American College of Physicians, Society of Hospital Medicine, American Heart Association, Massachusetts Medical Society, among others. He was the Institutional Administration Fellow of the Safety and Quality Fellowship Program at the Institution for Healthcare Improvement.
On behalf of the JCOM Editorial Review Board, I want to extend a special thank you to outgoing editor Lori Tishler, MD, MPH. Dr. Tishler’s impact on the journal cannot be overstated, and we are indebted to the time and expertise she shared with the journal during her tenure.
—Eric Seger
With another long winter officially in the rearview mirror and spring sunshine displaying new signs of life outdoors, I am excited to share some of the changes happening inside the offices of the Journal of Clinical Outcomes Management (JCOM). It is my pleasure to introduce Ebrahim Barkoudah, MD, MPH, as the journal’s new physician Editor in Chief. Dr. Barkoudah’s extensive experience in education and his work to improve patient outcomes will be assets to JCOM.
Specializing in both internal medicine and hospital medicine, Dr. Barkoudah is the Associate Director of the Hospital Medicine Unit and a Medical Director in the Department of Medicine at Brigham and Women’s Hospital in Boston. He is also Assistant Professor of Medicine at Harvard Medical School, where he led the school’s international education efforts.
Dr. Barkoudah serves patients with a range of complex clinical disorders, managing their care and seeking innovative treatment options. His research interest is in health care outcomes as well as clinical trials of therapeutic interventions. Dr. Barkoudah also serves on numerous clinical innovation committees at Brigham Health and national task forces.
Dr. Barkoudah is an active member of several professional societies including the American College of Physicians, Society of Hospital Medicine, American Heart Association, Massachusetts Medical Society, among others. He was the Institutional Administration Fellow of the Safety and Quality Fellowship Program at the Institution for Healthcare Improvement.
On behalf of the JCOM Editorial Review Board, I want to extend a special thank you to outgoing editor Lori Tishler, MD, MPH. Dr. Tishler’s impact on the journal cannot be overstated, and we are indebted to the time and expertise she shared with the journal during her tenure.
—Eric Seger
With another long winter officially in the rearview mirror and spring sunshine displaying new signs of life outdoors, I am excited to share some of the changes happening inside the offices of the Journal of Clinical Outcomes Management (JCOM). It is my pleasure to introduce Ebrahim Barkoudah, MD, MPH, as the journal’s new physician Editor in Chief. Dr. Barkoudah’s extensive experience in education and his work to improve patient outcomes will be assets to JCOM.
Specializing in both internal medicine and hospital medicine, Dr. Barkoudah is the Associate Director of the Hospital Medicine Unit and a Medical Director in the Department of Medicine at Brigham and Women’s Hospital in Boston. He is also Assistant Professor of Medicine at Harvard Medical School, where he led the school’s international education efforts.
Dr. Barkoudah serves patients with a range of complex clinical disorders, managing their care and seeking innovative treatment options. His research interest is in health care outcomes as well as clinical trials of therapeutic interventions. Dr. Barkoudah also serves on numerous clinical innovation committees at Brigham Health and national task forces.
Dr. Barkoudah is an active member of several professional societies including the American College of Physicians, Society of Hospital Medicine, American Heart Association, Massachusetts Medical Society, among others. He was the Institutional Administration Fellow of the Safety and Quality Fellowship Program at the Institution for Healthcare Improvement.
On behalf of the JCOM Editorial Review Board, I want to extend a special thank you to outgoing editor Lori Tishler, MD, MPH. Dr. Tishler’s impact on the journal cannot be overstated, and we are indebted to the time and expertise she shared with the journal during her tenure.
—Eric Seger
Differences in Palliative Care Delivery Among Adults With Cancer and With Terminal Noncancer Illness in Their Last Year of Life
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
Study Overview
Objective. To examine the patterns in palliative care delivery in the last year of life among adults with cancer compared with adults with a noncancer terminal diagnosis.
Design. Population-based cohort study in Ontario, Canada, using linked administrative and clinical databases. The study included all adults ages 18 and over who died of cancer or noncancer terminal illnesses and received physician-delivered palliative care that was initiated in the last year of life between January 2010 and December 2017. These palliative care services are identified through the use of claims fee codes by physicians that account for delivery of palliative care, such as symptom management and counseling, that are intended to be palliative rather than curative. Exclusion criteria include patients who had 2 or more palliative care service claims the year prior to the last year of life, which may indicate existing palliative care services rather than initiation of new palliative care services in the last year of life. Other patients who were excluded from the study had palliative care services initiated within 7 days of death, as it is less likely that services and support would be arranged prior to death given the short time frame. The types of noncancer illnesses included heart failure, chronic obstructive pulmonary disease, end-stage renal disease, cirrhosis, stroke, and dementia. For the comparison of palliative care services, types of illnesses were divided into cancer, chronic organ failure (heart failure, chronic pulmonary disease, end-stage renal disease, cirrhosis, or stroke), and dementia, as they may represent different trajectories of illnesses and needs.
Setting and participants. The study included 145 709 adults who died during the study period, among 351 941 adults who died from illnesses described above. Another 105 587 were excluded because there were no palliative care services before death, 48 525 were excluded because of existing palliative care services prior to the last year of life, and 44 164 were excluded because palliative care was initiated within 7 days of death. Among the study population included, 21 054 died of chronic organ failure, 14 033 died of dementia, and 110 622 died of cancer. The median age of the study population was 78 years, with an interquartile range of 67 to 86 years, and 50.7% were female. Approximately 12.8% of the study population reside in rural areas; median frailty score (hospital frailty risk score) among those who died of chronic organ failure was 10, and the score among those who died of dementia was 13. The frailty score among those who died of cancer was 3, indicating less frailty. Those who died of organ failure and dementia also had a high mean number of prescription medications (18 and 16, respectively) compared with those with cancer (11).
Main outcome measures. Study outcome measures include the timing of palliative care initiation (primary outcome), categorized into time frames of ≤ 30 days, 31 to 90 days, and > 90 days before death; location of initiation of palliative care services, categorized into clinic, home, hospital, subacute care, and case management; models of care, categorized as generalist, consultative, or specialist palliative care; total number of palliative care visits before death; and location of death. The models of palliative care delivery were categorized based on the proportion of palliative care fee codes claimed by physicians. Physicians whose annual billing included more than 10% of palliative care service codes were considered palliative care specialists. Using this designation, models of palliative care were categorized into those delivered by palliative care specialists, generalists (nonpalliative care specialists), or both.
Main results. The study found that the timing of palliative care initiation was earlier among those who died of cancer compared with those with organ failure or dementia (28.9% vs 15.9% and 15.3%, respectively). After adjustment, those who died of organ failure and those who died of dementia were less likely to have palliative care services initiated > 90 days prior to death (odds ratio [OR] 0.48 and 0.42, respectively) and between 31 to 90 days prior to death (OR 0.77 and 0.60, respectively), when compared with those who died of cancer (who served as the reference group). Regarding location of palliative care initiation, adults who died of cancer were less likely to have palliative care services initiated at home (14.5%) compared with those who died of organ failure (32.8%) or dementia (27.9%). Overall, those who died of cancer received more palliative care visits from initiation to death (median of 11 visits) compared with those who died oforgan failure (median 4 visits) and dementia (median 4 visits). Regarding models of palliative care delivery, a higher proportion of palliative care was delivered by palliative care specialists rather than generalists among cancer patients (72.9%) compared with those with organ failure (43.3%) or dementia (40.1%). The proportion of patients with cancer who died at home was 62.6%, which was higher than those with organ failure (53.3%) but lower than those with dementia (75%).
Conclusion. There are differences in the delivery of palliative care among patients with cancer and other noncancer terminal illnesses, including timing of initiation of palliative care services, location of services, number of visits, and delivery by types of practitioners of palliative care. Understanding these disparities and targeting them are potentially important steps to ensuring appropriate access to palliative care across settings and disease types.
Commentary
Palliative care improves the quality of life of patients with serious illnesses and reduces symptom burden, and results in better satisfaction and less burdensome care.1 Although palliative care approaches have been championed for cancer management, there is increasing evidence that palliative care also improves outcomes for patients with noncancer illnesses such as heart failure.2 This study highlights the differences in palliative care delivery for patients who have cancer and noncancer diagnoses, demonstrating that timing, location, and care delivery models differ among patients with different diagnoses. The finding that noncancer terminal illness often has later palliative care initiation is a significant one, as early palliative care has been associated with improved patient outcomes3; thus, efforts to initiate palliative care earlier in the course of illness may benefit these patients.
A particular challenge in determining when to initiate palliative care lies in predicting outcomes,4 particularly for different types of illnesses, which may have different trajectories of advancing disease and functional change. Recent research has tested novel prognostic approaches, such as using machine learning to generate mortality estimates and integrating them into clinical decision support.5 These approaches may have the potential to enhance palliative care delivery and may be adapted to be used in managing patients with noncancer illnesses as well. The study also found that patients with cancer were more likely to receive palliative care from specialists rather than generalists, although this could be due to how palliative care is integrated in hospitals, clinics, and systems of care that serve patients with cancer. Identifying approaches that yield better palliative care models and delivery may help to further enhance care for patients with noncancer illnesses.
Applications for Clinical Practice
Identifying differences in patterns of palliative care delivery among those with cancer and other diagnoses may be an important step towards identifying gaps and avenues to improve palliative care delivery. The underlying reasons for these differences could be targeted so that patients across settings and diagnoses may have equal access to palliative care to improve their symptoms and quality of life. Policy makers and health system leaders may consider learning from how palliative care has been integrated into oncology care, to help transform care delivery for other noncancer terminal illnesses. It may also involve broadening education to providers in different specialties, so that the value and importance of palliative care may be recognized beyond oncological care.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.
1. Kavalieratos D, Corbelli J, Zhang D, et al. Association Between Palliative Care and Patient and Caregiver Outcomes: A Systematic Review and Meta-analysis. JAMA. 2016;316(20):2104-2114.
2. Quinn KL, Stukel T, Stall NM, et al. Association between palliative care and healthcare outcomes among adults with terminal non-cancer illness: population based matched cohort study. BMJ. 2020;370:m2257.
3. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733-742.
4. White N, Reid F, Harris A, et al. A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407.
5. Manz CR, Parikh RB, Small DS, et al. Effect of Integrating Machine Learning Mortality Estimates With Behavioral Nudges to Clinicians on Serious Illness Conversations Among Patients With Cancer: A Stepped-Wedge Cluster Randomized Clinical Trial. JAMA Oncol. 2020;6(12):e204759.








