User login
FDA approves OTC antihistamine nasal spray
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, making it the first nasal antihistamine available over the counter in the United States.
The 0.15% strength of azelastine hydrochloride nasal spray is now approved for nonprescription treatment of seasonal and perennial allergic rhinitis in adults and children 6 years of age or older, the agency said. The 0.1% strength remains a prescription product that is indicated in younger children.
The “approval provides individuals an option for a safe and effective nasal antihistamine without requiring the assistance of a health care provider,” Theresa M. Michele, MD, director of the office of nonprescription drugs in the FDA’s Center for Drug Evaluation and Research, said in a prepared statement.
The FDA granted the nonprescription approval to Bayer Healthcare LLC, which said in a press release that the nasal spray would be available in national mass retail locations starting in the first quarter of 2022.
Oral antihistamines such as cetirizine (Zyrtec), loratadine (Claritin), and fexofenadine (Allegra) have been on store shelves for years. Azelastine 0.15% will be the first and only over-the-counter antihistamine for indoor and outdoor allergy relief in a nasal formulation, Bayer said.
An over-the-counter nasal antihistamine could be a better option for some allergy sufferers when compared with what is already over the counter, said Tracy Prematta, MD, a private practice allergist in Havertown, Pa.
“In general, I like the nasal antihistamines,” Dr. Prematta said in an interview. “They work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy relief are actually looking for something quick-acting.”
However, the cost of the over-the-counter azelastine may play a big role in whether patients go with the prescription or nonprescription option, according to Dr. Prematta.
Bayer has not yet set the price for nonprescription azelastine, a company spokesperson told this news organization.
The change in azelastine approval status happened through a regulatory process called an Rx-to-OTC switch. According to the FDA, products switched to nonprescription status need to have data demonstrating that they are safe and effective as self-medication when used as directed.
The product manufacturer has to show that consumers know how to use the drug safely and effectively without a health care professional supervising them, the FDA said.
The FDA considers the change in status for azelastine a partial Rx-to-OTC switch, since the 0.15% strength is now over the counter and the 0.1% strength remains a prescription product.
The 0.1% strength is indicated for perennial allergies in children 6 months to 6 years old, and seasonal allergies for children 2-6 years old, according to the FDA.
Drowsiness is a side effect of azelastine, the FDA said. According to prescribing information, consumers using the nasal spray need to be careful when driving or operating machinery, and should avoid alcohol.
Using the product with alcohol, sedatives, or tranquilizers may increase drowsiness, the agency added.
Sedation is also common with the oral antihistamines people take to treat their allergies, said Dr. Prematta, who added that patients may also complain of dry mouth, nose, or throat.
Although some allergy sufferers dislike the taste of antihistamine nasal spray, they can try to overcome that issue by tilting the head forward, pointing the tip of the nozzle toward the outside of the nose, and sniffing gently, Dr. Prematta said.
“That really minimizes what gets in the back of your throat, so taste becomes less of a problem,” she explained.
Dr. Prematta has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dynamic ultrasonography: An idea whose time has come
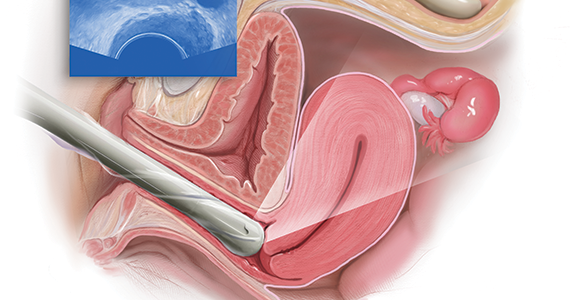
Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
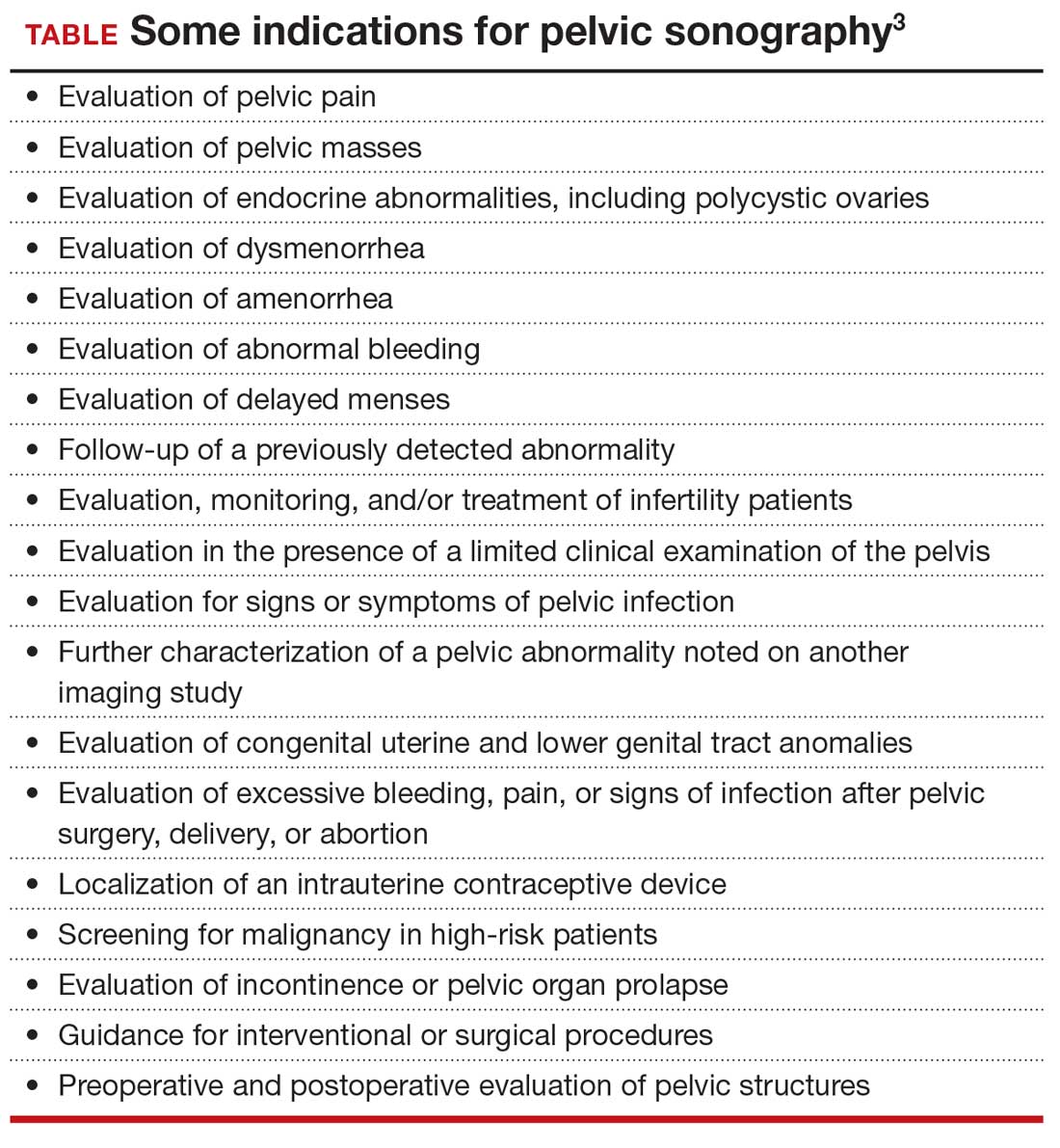
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3
Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
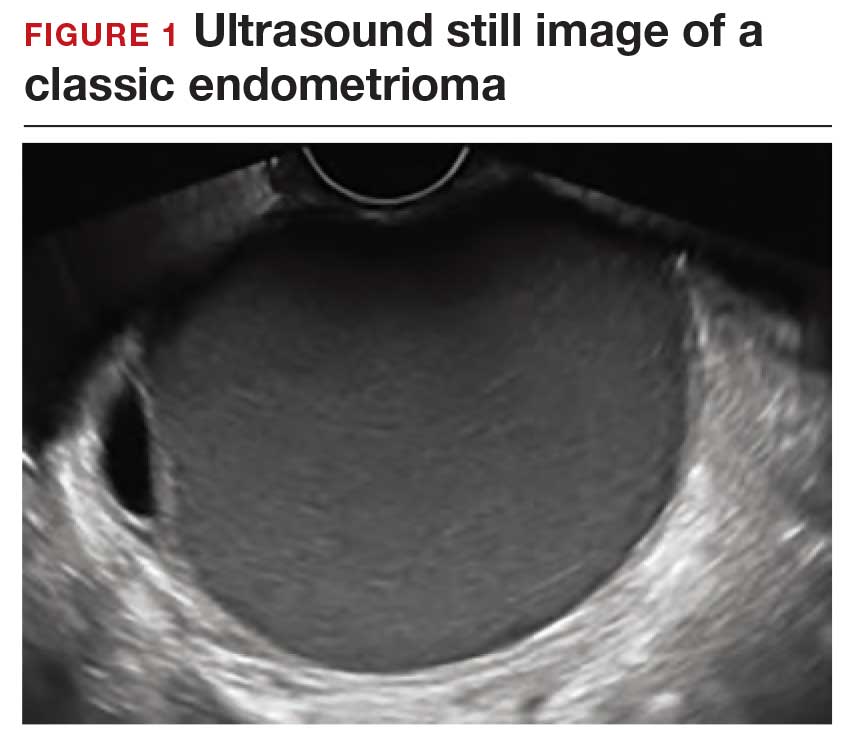
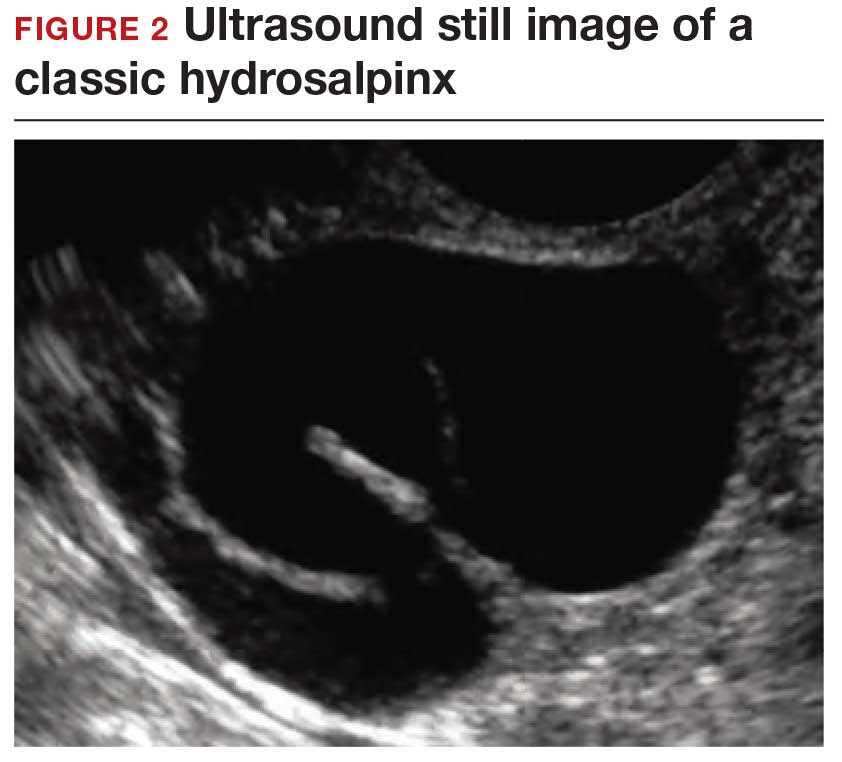
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.
Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation

Ultrasonography truly has revolutionized the practice of obstetrics and gynecology. Initially, transabdominal ultrasonography was mainly a tool of the obstetrician. Early linear array, real-time equipment had barely enough resolution to perform very limited assessments, such as measure biparietal diameter and identify vertex versus breech presentation, and anterior versus posterior placenta location. The introduction of transvaginal probes, which employ higher frequency and provide closer proximity to structures, yielded a degree of image magnification that was dubbed sonomicroscopy.1 In other words, we are seeing things with our naked eye that we could not see if we could hold them in our hand at arm’s length and squint at them. An example of this is the cardiac activity clearly visible in a 3-mm embryo at 45 days from the last menstrual period. One would not appreciate this without the low power magnification of the vaginal probe.
The concept of dynamic imaging
As early as 1990, I realized that there is a difference between an ultrasound “examination” performed because of referral for imaging, which generated a report back to the referring health care provider, and “examining” one’s own patient with ultrasonography at the time of bimanual exam. I coined the phrase “the ultrasound-enhanced bimanual exam,” and I believed it should become a routine part of gynecologic care. I put forth this thesis in an article entitled, “Incorporating endovaginal ultrasonography into the overall gynecologic examination.”2 The idea is based on thinking: What exactly are we are trying to discern from a bimanual exam?
Clinicians perform the bimanual exam thousands of times. The bimanual examination consists of 2 components, an objective portion and a subjective portion. The objective component attempts to discern information that is totally objective, such as, Is the ovary enlarged? If so, is it cystic or solid? Is this uterus normal in shape and contour? If so, does it feel like leiomyomas or is it globularly enlarged as with adenomyosis? The subjective component of the bimanual examination attempts to determine whether or not tenderness is present or if there is normal mobility of the pelvic organs.
The objective component can be replaced by an image in very little time if the examiner has the equipment and the knowledge and skill. The subjective component, however, depends on the experience and often the nuance of the examiner. That was my original thought process. I wanted, and still want, the examining clinician to use imaging as part of the overall exam. But now, I want the imager to use examination as part of the overall imaging. (VIDEOS 1A and 1B.) This is the concept of dynamic imaging. It involves the liberal use of the abdominal hand as well as an in-and-out motion of the vaginal probe to ascertain aspects of the examination that in the past I deemed “subjective.” Mainly, this involves the aspects of mobility and/or tenderness.
Continue to: Guidelines concerning pelvic ultrasound do not consider dynamic imaging...
Guidelines concerning pelvic ultrasound do not consider dynamic imaging
Until now, most imagers take a myriad of pictures, mostly still snapshots, to illustrate anatomy. Most imaging physicians then look at a series of such pictures and may never even hold the transducer. This is increasingly true in instances of remote teleradiology. Even for the minority of imagers who utilize video clips (VIDEOS 2A–2C), these are still representations of anatomy .
One need look no further than the guidelines that underpin the expectation of those who scan the female pelvis. The American Institute of Ultrasound in Medicine (AIUM) published a practice parameter for the performance of ultrasonography of the female pelvis, developed in collaboration with the American College of Radiology, American College of Obstetricians and Gynecologists, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. 3 Nowhere does this document mention anything other than what images to obtain, where to look, and how to measure. Nowhere is there any mention of dynamic imaging—the concept of using one’s other hand on the abdomen, eliciting pain with the vaginal probe, checking for mobility, asking the patient to bear down. The document lists indications for pelvic sonography that include but are not limited to 19 different indications, such as pelvic pain, evaluation of dysmenorrhea, evaluation for signs or symptoms of pelvic infection, and evaluation of incontinence or pelvic organ prolapse (TABLE). 3

Dynamic ultrasonography can aid in the diagnosis of certain conditions
Specifically, what can dynamic ultrasonography add to anatomic imaging? The main considerations are pain, adhesions, endometriosis, and pelvic organ prolapse.
Pelvic pain or tenderness
How can you evaluate a patient’s pelvic pain with an anatomic image? Perhaps pain can be corroborated if there is a classic ovarian endometrioma (FIGURE 1) (VIDEOS 3A, 3B) or classic hydrosalpinx (FIGURE 2) (VIDEOS 4A–4C). But can we evaluate pelvic pain with only an anatomic image? No, absolutely not. Evaluating pain requires dynamic assessment. As described above, in a dynamic ultrasound assessment, liberal use of the abdominal hand and the tip of the vaginal probe can elicit where the patient’s pain exists and whether the pain can be recreated.


Adhesions
Pelvic adhesions can be a significant source of pelvic pain and, also, sometimes infertility. The adhesions themselves may not be visible on anatomic imaging. This is where the concept of the sliding organ sign is paramount, a concept first described by Dr. Ilan Timor-Tritsch in his book Transvaginal Sonography . 4 He stated, “Diagnosis of pelvic adhesions becomes possible by the ‘sliding organ sign.’ The transducer tip is pointed at the uterus, ovaries or any pelvic finding, and a gentle push-pull movement of several centimeters is started. If no adhesions are present, the organs will move freely in the pelvis. This displacement of organs is perceived on the screen as a sliding movement.” 4 Thus, if structures are in fact adherent, they will move in tandem with each other as evidenced by this dynamic assessment. If they are not adherent, they will move slightly but independently of each other ( VIDEOS 5A–5G ).
Continue to: Endometriosis...
Endometriosis
Dynamic ultrasonography can be a significant part of a nonlaparoscopic, presumptive diagnosis of endometriosis when there is no obvious ovarian endometrioma.5 The evidence for this comes from a classic paper by Okaro and colleagues, “The use of ultrasound‐based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy?”6 In that study, 120 consecutive women with chronic pelvic pain scheduled for laparoscopy underwent vaginal ultrasonography. Hard markers were defined as structural abnormalities, such as classic endometriomas or hydrosalpinges.
These markers demonstrated a 100% correlation (24 of 24 women) with laparoscopic findings, as one might have suspected. In addition, soft markers (VIDEOS 6A–6C) were defined as reduced ovarian mobility, site-specific pelvic tenderness, and the presence of loculated peritoneal fluid in the pelvis. These were predictive of pelvic pathology in 73% of these women (37 of 51).6
Thus, women who have soft markers on dynamic scanning but no obvious anatomic abnormalities can be treated with a high degree of sensitivity without the need for laparoscopic intervention.
Pelvic organ prolapse and incontinence
With the vaginal probe in place, and even a small amount of urine in the bladder, the patient can be asked to bear down (Valsalva maneuver), and cystocele (VIDEO 7) and/or hypermobility of the urethra (VIDEO 8) is easily discerned with dynamic ultrasonography. This information is not available on static anatomic imaging.
A tool that enhances patient care
Dynamic ultrasonography is an important and emerging topic in gynecologic imaging. Static images and even cine clips will yield only anatomic information. Increasingly, whoever holds the transducer—whether it be the gynecologist, radiologist, or sonographer—needs to examine the patient with the probe and include liberal use of the abdominal hand as well. Incorporating this concept will enhance the overall diagnostic input of ultrasound scanning, not just imaging, into better and more accurate patient care. ●
VIDEO 1A Liberal use of your nonscanning hand on dynamic scanning shows “wiggling” of debris classic of a hemorrhagic corpus luteum
VIDEO 1B Liberal use of your nonscanning hand helps identify a small postmenopausal ovary
VIDEO 2A Dynamic scanning can give the correct diagnosis even though clips were used! This clip appears to show a relatively normal uterus
VIDEO 2B Dynamic scanning can give the correct diagnosis even though clips were used! Same patient as in VIDEO 2A showing what appears to be a solid adnexal mass
VIDEO 2C Dynamic scan clearly shows the “mass” to be a pedunculated fibroid
VIDEO 3A Video clip of a classic endometrioma
VIDEO 3B Classic endometrioma showing no Doppler flow internally
VIDEO 4A Video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4B Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 4C Another example of video of dynamic assessment in a patient with pain symptoms with a hydrosalpinx
VIDEO 5A Sliding organ sign with normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5B Sliding sign showing adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5C Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5D Left ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5E Right ovary: Normal mobility (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5F Normal mobility even with a classic endometrioma (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 5G Adherent ovary (Courtesy of Dr. Ilan Timor-Tritsch)
VIDEO 6A Dynamic scanning shows the ovary to be “stuck” in the cul-de-sac in a patient with endometriosis
VIDEO 6B Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 6C Dynamic scanning in another patient with endometriosis showing markedly retroverted uterus with adherent bowel posteriorly
VIDEO 7 Cystocele or urethral lengthening are key elements for the diagnosis of incontinence with or without pelvic relaxation
VIDEO 8 Urethral lengthening is a key element for the diagnosis of incontinence with or without pelvic relaxation
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
- Goldstein SR. Pregnancy I: Embryo. In: Endovaginal Ultrasound. 2nd ed. Wiley-Liss; 1991:58.
- Goldstein SR. Incorporating endovaginal ultrasonography into the overall gynecologic examination. Am J Obstet Gynecol. 1990;162:625-632.
- AIUM practice parameter for the performance of an ultrasound examination of the female pelvis. J Ultrasound Med. 2020;39:E17-E23.
- Timor-Tritsch IE, Rottem S, Elgali S. How transvaginal sonography is done. In: Timor-Tritsch IE, Rottem S, eds. Transvaginal Sonography. Elsevier Science Publishing Company, Inc; 1988:24.
- Taylor HS, Adamson GD, Diamond MP, et al. An evidence-based approach to assessing surgical versus clinical diagnosis of symptomatic endometriosis. Int J Gynaecol Obstet. 2018;142:131-142.
- Okaro E, Condous G, Khalid A, et al. The use of ultrasound‐ based ‘soft markers’ for the prediction of pelvic pathology in women with chronic pelvic pain–can we reduce the need for laparoscopy? BJOG. 2006;113:251-256.
COVID-19 vaccines are safe and effective for patients with migraine
according to a presentation at the American Headache Society’s 2021 annual meeting.
Amy Gelfand, MD, director of pediatric headache at University of California, San Francisco, reviewed common concerns migraine patients or their clinicians might have related any of the three vaccines, starting with a review of how the vaccines work – by targeting the spike protein of the SARS-CoV-2 virus.
“The vaccines induce response to that protein, but only that protein, so there’s no reason to think they’re going to cause the body to produce neutralizing antibodies against any of our migraine therapeutics,” Dr. Gelfand said. She added that the phase 3 clinical trials included participants from a wide range of ages and comorbidities, so there were likely many people in the trials who have migraine, though no subgroup analyses have been performed for this group or are likely to be performed.
Common questions
The two treatments people have the most questions about concerning the COVID-19 vaccine are onabotulinumtoxinA and CGRP pathway monoclonal antibodies (mAbs), likely because both of these are injections, as is the vaccine, Dr. Gelfand said. First, she reminded attendees that onabotulinumtoxinA is not a dermal filler, since some reports following administration of the Moderna vaccine suggested that some people with dermal fillers had swelling in those areas after vaccination.
In addition, “there’s no reason to think the onabotulinumtoxinA would influence our body’s immune response to any vaccine, so there’s no need to retime the onabotulinumtoxinA injections around COVID-19 vaccine administration,” Dr. Gelfand said.
Regarding mAbs, she acknowledged that some white blood cells have CGRP receptors, which may have a pro- or anti-inflammatory role, but clinical trials of mAbs did not show any evidence of being immunosuppressive or myelosuppressive.
“The monoclonal antibodies themselves have undergone engineering so that they are just going after their one target,” Dr. Gelfand said. “They’re not going to be expected to bind to anything else outside of their targets, so I don’t think there’s anything there to make us retime the monoclonal antibody administration relative to the COVID-19 vaccine.”
She did note that patients who choose to get mAbs injections in their arm instead of their thigh or abdomen may want to receive it in the opposite arm than they one they have gotten or will get the vaccine in since the vaccine can cause discomfort.
The other common question patients may have is whether taking any NSAIDs or acetaminophen before getting the COVID-19 vaccine will reduce their immune response to the vaccination. This concern arises because of past evidence showing that some infants tended to have lower immunologic responses when they received acetaminophen after their primary vaccines’ series, but the clinical significance of those reduced responses is not clear since they still had strong responses. Further, this effect was not seen with booster shots, suggesting it’s an age-dependent effect.
During the clinical trials of the AstraZeneca vaccine, several sites gave prophylactic paracetamol without any apparent detrimental effect on antibody response, Dr. Gelfand said. Further, the mRNA and adenovirus-vectored vaccines appear to induce antibodies far above what many believe is needed for protection.
“Even if there were a slight decrease, it’s not clear that that would have any kind of clinical significance for that person in terms of their level of protection against COVID-19,” she said. “Bottom line, it’s fine for patients to use either of these after administration of the COVID-19 vaccine.” The Centers for Disease Control and Prevention doesn’t recommend it prophylactically beforehand, but it’s fine to take it for a fever, aches or headache after getting the vaccine.
Migraine or vaccine reaction?
Dr. Gelfand then addressed whether it should affect physicians’ headache differential if seeing a patient who recently received an adenovirus-vectored vaccine, such as the Johnson & Johnson or AstraZeneca vaccines. The question relates to the discovery of a very rare potential adverse event from these vaccines: cerebral venous sinus thrombosis (CVST) with thrombocytopenia and thromboses in other major vessels, together called thrombosis thrombocytopenia syndrome (TTS). No TTS cases have been reported following mRNA vaccines.
TTS’s mechanism appears similar to autoimmune heparin-induced thrombocytopenia, where the body produces platelet-activating antibodies. TTS currently has three diagnostic criteria: new-onset thrombocytopenia (<150,000/microliter) without evidence of platelet clumping, venous or arterial thrombosis, and absence of prior exposure to heparin.
So far, TTS has been limited only to the vaccines that use an adenovirus vector. One male clinical trial participant experienced CVST with thrombocytopenia in Johnson & Johnson phase 3 trials, and 12 cases out of approximately 8 million Johnson & Johnson doses were reported to the Vaccine Adverse Event Reporting System between March 2 and April 21, 2021. Three TTS more cases followed these, resulting in 15 TTS events per 8 million doses.
In terms of clinical features, all 15 cases were females under age 60, mostly white, and all 11 who were tested were positive for the heparin-platelet factor 4 antibody test. TTS occurred 6-15 days after vaccination for these cases, and all but one had a headache. Their platelet count was 9,000-127,000. None were pregnant or postpartum.
“For us, as headache clinicians, the epidemiology of TTS overlaps with the epidemiology of migraine – they’re happening to the same group of patients,” Dr. Gelfand said. Most of the cases occurred in women aged 30-39 years, while the estimated incidence in women aged 50 or older is 0.9 cases per million doses.
The CDC has proceeded with the Johnson & Johnson vaccine because a risk-benefit analysis revealed that use of the vaccine will result in fewer hospitalization and deaths from COVID-19, compared with adverse events from the vaccine, Dr. Gelfand explained. However, the CDC notes that “women younger than 50 years old should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed.”
For clinicians, the existence of TTS raises a question when patients with a history of migraine call after having received the Johnson & Johnson vaccine, Dr. Gelfand said: “How do we know if this is a spontaneous attack, if it’s a headache provoked by receiving the vaccine, or they have one of these rare cases of [TTS]?”
Three things help with this differential, she said: timing, epidemiology, and headache phenotype. Headache after a vaccine is very common, but it usually happens within the first couple of hours or days after the vaccine. By day 4 after vaccination, few people had headaches in the clinical trials. Since TTS requires production of antibodies, a headache within a few hours of vaccination should not raise concerns about TTS. It should be considered, however, for patients who experience a headache within a week or 2 after vaccination.
Then consider the epidemiology: If it’s a woman between ages 18 and49 calling, the risk is higher than if it’s a male over age 50. Then consider whether there are any unusual headache features, positionality, encephalopathy, or clinical features that could suggest clots in other parts of the body, such as abdominal pain, shortness of breath, or pain in the legs.
“At the end of the day, if it’s a person who’s in this epidemiological window and they’re calling a week or 2 out from the Johnson & Johnson vaccine, we may just need to work it up and see,” Dr. Gelfand said. Work-up involves a CBC, a platelet count to see if they’re thrombocytopenic, and perhaps imaging, preferentially using MRI/MRV over CT since it’s a younger population. Treatment for CVST with thrombocytopenia is a nonheparin anticoagulant, and platelet transfusion should not occur before consulting with hematology.
Continue to vaccinate
“The big take home is that we should continue to vaccinate patients with migraine and that your current therapies do not interfere with the vaccine working and that the vaccine does not interact with our therapies,” Brian D. Loftus, MD, BSChE, immediate past president of the Southern Headache Society and a neurologist at Bellaire (Pa.) Neurology, said of the presentation. He also felt it was helpful to know that NSAIDs likely have no impact on the vaccines’ effectiveness as well.
“The most important new information for me was that the median onset of the CSVT was 8 days post vaccine,” Dr. Loftus said. “Typically, postvaccine headache is seen much sooner, within 1-2 days, so this is a useful clinical feature to separate out who needs to closer follow-up and possible neuroimaging.”
Given the epidemiology of those most likely to have TTS, Dr. Loftus said he would advise his female patients younger than 60 to simply get the Pfizer or Moderna vaccine since they appear safer for this demographic.
Dr. Gelfand is editor of the journal Headache but has no industry disclosures. Her spouse has received clinical trial grant support from Genentech and honoraria for editorial work from Dynamed Plus. Dr. Loftus has received grants or fees from Teva, Amgen, Abbvie, and Biohaven.
according to a presentation at the American Headache Society’s 2021 annual meeting.
Amy Gelfand, MD, director of pediatric headache at University of California, San Francisco, reviewed common concerns migraine patients or their clinicians might have related any of the three vaccines, starting with a review of how the vaccines work – by targeting the spike protein of the SARS-CoV-2 virus.
“The vaccines induce response to that protein, but only that protein, so there’s no reason to think they’re going to cause the body to produce neutralizing antibodies against any of our migraine therapeutics,” Dr. Gelfand said. She added that the phase 3 clinical trials included participants from a wide range of ages and comorbidities, so there were likely many people in the trials who have migraine, though no subgroup analyses have been performed for this group or are likely to be performed.
Common questions
The two treatments people have the most questions about concerning the COVID-19 vaccine are onabotulinumtoxinA and CGRP pathway monoclonal antibodies (mAbs), likely because both of these are injections, as is the vaccine, Dr. Gelfand said. First, she reminded attendees that onabotulinumtoxinA is not a dermal filler, since some reports following administration of the Moderna vaccine suggested that some people with dermal fillers had swelling in those areas after vaccination.
In addition, “there’s no reason to think the onabotulinumtoxinA would influence our body’s immune response to any vaccine, so there’s no need to retime the onabotulinumtoxinA injections around COVID-19 vaccine administration,” Dr. Gelfand said.
Regarding mAbs, she acknowledged that some white blood cells have CGRP receptors, which may have a pro- or anti-inflammatory role, but clinical trials of mAbs did not show any evidence of being immunosuppressive or myelosuppressive.
“The monoclonal antibodies themselves have undergone engineering so that they are just going after their one target,” Dr. Gelfand said. “They’re not going to be expected to bind to anything else outside of their targets, so I don’t think there’s anything there to make us retime the monoclonal antibody administration relative to the COVID-19 vaccine.”
She did note that patients who choose to get mAbs injections in their arm instead of their thigh or abdomen may want to receive it in the opposite arm than they one they have gotten or will get the vaccine in since the vaccine can cause discomfort.
The other common question patients may have is whether taking any NSAIDs or acetaminophen before getting the COVID-19 vaccine will reduce their immune response to the vaccination. This concern arises because of past evidence showing that some infants tended to have lower immunologic responses when they received acetaminophen after their primary vaccines’ series, but the clinical significance of those reduced responses is not clear since they still had strong responses. Further, this effect was not seen with booster shots, suggesting it’s an age-dependent effect.
During the clinical trials of the AstraZeneca vaccine, several sites gave prophylactic paracetamol without any apparent detrimental effect on antibody response, Dr. Gelfand said. Further, the mRNA and adenovirus-vectored vaccines appear to induce antibodies far above what many believe is needed for protection.
“Even if there were a slight decrease, it’s not clear that that would have any kind of clinical significance for that person in terms of their level of protection against COVID-19,” she said. “Bottom line, it’s fine for patients to use either of these after administration of the COVID-19 vaccine.” The Centers for Disease Control and Prevention doesn’t recommend it prophylactically beforehand, but it’s fine to take it for a fever, aches or headache after getting the vaccine.
Migraine or vaccine reaction?
Dr. Gelfand then addressed whether it should affect physicians’ headache differential if seeing a patient who recently received an adenovirus-vectored vaccine, such as the Johnson & Johnson or AstraZeneca vaccines. The question relates to the discovery of a very rare potential adverse event from these vaccines: cerebral venous sinus thrombosis (CVST) with thrombocytopenia and thromboses in other major vessels, together called thrombosis thrombocytopenia syndrome (TTS). No TTS cases have been reported following mRNA vaccines.
TTS’s mechanism appears similar to autoimmune heparin-induced thrombocytopenia, where the body produces platelet-activating antibodies. TTS currently has three diagnostic criteria: new-onset thrombocytopenia (<150,000/microliter) without evidence of platelet clumping, venous or arterial thrombosis, and absence of prior exposure to heparin.
So far, TTS has been limited only to the vaccines that use an adenovirus vector. One male clinical trial participant experienced CVST with thrombocytopenia in Johnson & Johnson phase 3 trials, and 12 cases out of approximately 8 million Johnson & Johnson doses were reported to the Vaccine Adverse Event Reporting System between March 2 and April 21, 2021. Three TTS more cases followed these, resulting in 15 TTS events per 8 million doses.
In terms of clinical features, all 15 cases were females under age 60, mostly white, and all 11 who were tested were positive for the heparin-platelet factor 4 antibody test. TTS occurred 6-15 days after vaccination for these cases, and all but one had a headache. Their platelet count was 9,000-127,000. None were pregnant or postpartum.
“For us, as headache clinicians, the epidemiology of TTS overlaps with the epidemiology of migraine – they’re happening to the same group of patients,” Dr. Gelfand said. Most of the cases occurred in women aged 30-39 years, while the estimated incidence in women aged 50 or older is 0.9 cases per million doses.
The CDC has proceeded with the Johnson & Johnson vaccine because a risk-benefit analysis revealed that use of the vaccine will result in fewer hospitalization and deaths from COVID-19, compared with adverse events from the vaccine, Dr. Gelfand explained. However, the CDC notes that “women younger than 50 years old should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed.”
For clinicians, the existence of TTS raises a question when patients with a history of migraine call after having received the Johnson & Johnson vaccine, Dr. Gelfand said: “How do we know if this is a spontaneous attack, if it’s a headache provoked by receiving the vaccine, or they have one of these rare cases of [TTS]?”
Three things help with this differential, she said: timing, epidemiology, and headache phenotype. Headache after a vaccine is very common, but it usually happens within the first couple of hours or days after the vaccine. By day 4 after vaccination, few people had headaches in the clinical trials. Since TTS requires production of antibodies, a headache within a few hours of vaccination should not raise concerns about TTS. It should be considered, however, for patients who experience a headache within a week or 2 after vaccination.
Then consider the epidemiology: If it’s a woman between ages 18 and49 calling, the risk is higher than if it’s a male over age 50. Then consider whether there are any unusual headache features, positionality, encephalopathy, or clinical features that could suggest clots in other parts of the body, such as abdominal pain, shortness of breath, or pain in the legs.
“At the end of the day, if it’s a person who’s in this epidemiological window and they’re calling a week or 2 out from the Johnson & Johnson vaccine, we may just need to work it up and see,” Dr. Gelfand said. Work-up involves a CBC, a platelet count to see if they’re thrombocytopenic, and perhaps imaging, preferentially using MRI/MRV over CT since it’s a younger population. Treatment for CVST with thrombocytopenia is a nonheparin anticoagulant, and platelet transfusion should not occur before consulting with hematology.
Continue to vaccinate
“The big take home is that we should continue to vaccinate patients with migraine and that your current therapies do not interfere with the vaccine working and that the vaccine does not interact with our therapies,” Brian D. Loftus, MD, BSChE, immediate past president of the Southern Headache Society and a neurologist at Bellaire (Pa.) Neurology, said of the presentation. He also felt it was helpful to know that NSAIDs likely have no impact on the vaccines’ effectiveness as well.
“The most important new information for me was that the median onset of the CSVT was 8 days post vaccine,” Dr. Loftus said. “Typically, postvaccine headache is seen much sooner, within 1-2 days, so this is a useful clinical feature to separate out who needs to closer follow-up and possible neuroimaging.”
Given the epidemiology of those most likely to have TTS, Dr. Loftus said he would advise his female patients younger than 60 to simply get the Pfizer or Moderna vaccine since they appear safer for this demographic.
Dr. Gelfand is editor of the journal Headache but has no industry disclosures. Her spouse has received clinical trial grant support from Genentech and honoraria for editorial work from Dynamed Plus. Dr. Loftus has received grants or fees from Teva, Amgen, Abbvie, and Biohaven.
according to a presentation at the American Headache Society’s 2021 annual meeting.
Amy Gelfand, MD, director of pediatric headache at University of California, San Francisco, reviewed common concerns migraine patients or their clinicians might have related any of the three vaccines, starting with a review of how the vaccines work – by targeting the spike protein of the SARS-CoV-2 virus.
“The vaccines induce response to that protein, but only that protein, so there’s no reason to think they’re going to cause the body to produce neutralizing antibodies against any of our migraine therapeutics,” Dr. Gelfand said. She added that the phase 3 clinical trials included participants from a wide range of ages and comorbidities, so there were likely many people in the trials who have migraine, though no subgroup analyses have been performed for this group or are likely to be performed.
Common questions
The two treatments people have the most questions about concerning the COVID-19 vaccine are onabotulinumtoxinA and CGRP pathway monoclonal antibodies (mAbs), likely because both of these are injections, as is the vaccine, Dr. Gelfand said. First, she reminded attendees that onabotulinumtoxinA is not a dermal filler, since some reports following administration of the Moderna vaccine suggested that some people with dermal fillers had swelling in those areas after vaccination.
In addition, “there’s no reason to think the onabotulinumtoxinA would influence our body’s immune response to any vaccine, so there’s no need to retime the onabotulinumtoxinA injections around COVID-19 vaccine administration,” Dr. Gelfand said.
Regarding mAbs, she acknowledged that some white blood cells have CGRP receptors, which may have a pro- or anti-inflammatory role, but clinical trials of mAbs did not show any evidence of being immunosuppressive or myelosuppressive.
“The monoclonal antibodies themselves have undergone engineering so that they are just going after their one target,” Dr. Gelfand said. “They’re not going to be expected to bind to anything else outside of their targets, so I don’t think there’s anything there to make us retime the monoclonal antibody administration relative to the COVID-19 vaccine.”
She did note that patients who choose to get mAbs injections in their arm instead of their thigh or abdomen may want to receive it in the opposite arm than they one they have gotten or will get the vaccine in since the vaccine can cause discomfort.
The other common question patients may have is whether taking any NSAIDs or acetaminophen before getting the COVID-19 vaccine will reduce their immune response to the vaccination. This concern arises because of past evidence showing that some infants tended to have lower immunologic responses when they received acetaminophen after their primary vaccines’ series, but the clinical significance of those reduced responses is not clear since they still had strong responses. Further, this effect was not seen with booster shots, suggesting it’s an age-dependent effect.
During the clinical trials of the AstraZeneca vaccine, several sites gave prophylactic paracetamol without any apparent detrimental effect on antibody response, Dr. Gelfand said. Further, the mRNA and adenovirus-vectored vaccines appear to induce antibodies far above what many believe is needed for protection.
“Even if there were a slight decrease, it’s not clear that that would have any kind of clinical significance for that person in terms of their level of protection against COVID-19,” she said. “Bottom line, it’s fine for patients to use either of these after administration of the COVID-19 vaccine.” The Centers for Disease Control and Prevention doesn’t recommend it prophylactically beforehand, but it’s fine to take it for a fever, aches or headache after getting the vaccine.
Migraine or vaccine reaction?
Dr. Gelfand then addressed whether it should affect physicians’ headache differential if seeing a patient who recently received an adenovirus-vectored vaccine, such as the Johnson & Johnson or AstraZeneca vaccines. The question relates to the discovery of a very rare potential adverse event from these vaccines: cerebral venous sinus thrombosis (CVST) with thrombocytopenia and thromboses in other major vessels, together called thrombosis thrombocytopenia syndrome (TTS). No TTS cases have been reported following mRNA vaccines.
TTS’s mechanism appears similar to autoimmune heparin-induced thrombocytopenia, where the body produces platelet-activating antibodies. TTS currently has three diagnostic criteria: new-onset thrombocytopenia (<150,000/microliter) without evidence of platelet clumping, venous or arterial thrombosis, and absence of prior exposure to heparin.
So far, TTS has been limited only to the vaccines that use an adenovirus vector. One male clinical trial participant experienced CVST with thrombocytopenia in Johnson & Johnson phase 3 trials, and 12 cases out of approximately 8 million Johnson & Johnson doses were reported to the Vaccine Adverse Event Reporting System between March 2 and April 21, 2021. Three TTS more cases followed these, resulting in 15 TTS events per 8 million doses.
In terms of clinical features, all 15 cases were females under age 60, mostly white, and all 11 who were tested were positive for the heparin-platelet factor 4 antibody test. TTS occurred 6-15 days after vaccination for these cases, and all but one had a headache. Their platelet count was 9,000-127,000. None were pregnant or postpartum.
“For us, as headache clinicians, the epidemiology of TTS overlaps with the epidemiology of migraine – they’re happening to the same group of patients,” Dr. Gelfand said. Most of the cases occurred in women aged 30-39 years, while the estimated incidence in women aged 50 or older is 0.9 cases per million doses.
The CDC has proceeded with the Johnson & Johnson vaccine because a risk-benefit analysis revealed that use of the vaccine will result in fewer hospitalization and deaths from COVID-19, compared with adverse events from the vaccine, Dr. Gelfand explained. However, the CDC notes that “women younger than 50 years old should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed.”
For clinicians, the existence of TTS raises a question when patients with a history of migraine call after having received the Johnson & Johnson vaccine, Dr. Gelfand said: “How do we know if this is a spontaneous attack, if it’s a headache provoked by receiving the vaccine, or they have one of these rare cases of [TTS]?”
Three things help with this differential, she said: timing, epidemiology, and headache phenotype. Headache after a vaccine is very common, but it usually happens within the first couple of hours or days after the vaccine. By day 4 after vaccination, few people had headaches in the clinical trials. Since TTS requires production of antibodies, a headache within a few hours of vaccination should not raise concerns about TTS. It should be considered, however, for patients who experience a headache within a week or 2 after vaccination.
Then consider the epidemiology: If it’s a woman between ages 18 and49 calling, the risk is higher than if it’s a male over age 50. Then consider whether there are any unusual headache features, positionality, encephalopathy, or clinical features that could suggest clots in other parts of the body, such as abdominal pain, shortness of breath, or pain in the legs.
“At the end of the day, if it’s a person who’s in this epidemiological window and they’re calling a week or 2 out from the Johnson & Johnson vaccine, we may just need to work it up and see,” Dr. Gelfand said. Work-up involves a CBC, a platelet count to see if they’re thrombocytopenic, and perhaps imaging, preferentially using MRI/MRV over CT since it’s a younger population. Treatment for CVST with thrombocytopenia is a nonheparin anticoagulant, and platelet transfusion should not occur before consulting with hematology.
Continue to vaccinate
“The big take home is that we should continue to vaccinate patients with migraine and that your current therapies do not interfere with the vaccine working and that the vaccine does not interact with our therapies,” Brian D. Loftus, MD, BSChE, immediate past president of the Southern Headache Society and a neurologist at Bellaire (Pa.) Neurology, said of the presentation. He also felt it was helpful to know that NSAIDs likely have no impact on the vaccines’ effectiveness as well.
“The most important new information for me was that the median onset of the CSVT was 8 days post vaccine,” Dr. Loftus said. “Typically, postvaccine headache is seen much sooner, within 1-2 days, so this is a useful clinical feature to separate out who needs to closer follow-up and possible neuroimaging.”
Given the epidemiology of those most likely to have TTS, Dr. Loftus said he would advise his female patients younger than 60 to simply get the Pfizer or Moderna vaccine since they appear safer for this demographic.
Dr. Gelfand is editor of the journal Headache but has no industry disclosures. Her spouse has received clinical trial grant support from Genentech and honoraria for editorial work from Dynamed Plus. Dr. Loftus has received grants or fees from Teva, Amgen, Abbvie, and Biohaven.
FROM AHS 2021
Reduced-intensity transplant benefits older patients with AML
Among older patients with acute myeloid leukemia (AML), survival is significantly better when they undergo reduced-intensity conditioning (RIC) before receiving an allogeneic hematopoietic cell transplant (HCT) at first remission. This improvement in survival is seen regardless of key factors such as genotype and the status of minimal residual disease (MRD) after initial chemotherapy, results from two large randomized trials show.
“Two consecutive trials of more than 1,500 older AML patients above 60 years of age demonstrate a consistent benefit for RIC transplant in first remission,” said first author Nigel Russell, MD, of Guy’s Hospital, London, and Nottingham University, England. “This benefit is seen independent of their post-course 1 MRD status,” he added.
Dr. Russell presented the new data at the European Hematology Association (EHA) 2021 Annual Meeting.
Commenting on the study, Charles Craddock, MD, said in an interview that the results “confirm the growing importance of RIC transplantation as a central treatment management strategy in high-risk AML and in this population high risk patients over 60.”
“[These findings] reinforce the evolving treatment paradigm that, in fit adults over 60 with AML, hematopoietic cell transplantation should be considered an essential component of their management plan,” said Dr. Craddock, academic director of the Center for Clinical Haematology, Queen Elizabeth Hospital, Birmingham, England.
Patients with AML who are older than 60 years can achieve complete remission with intensive chemotherapy alone; however, relapse is common, and only about 20% survive for 5 years, Dr. Russell explained.
HCT significantly improves survival outcomes, and the development of RIC has made transplantation accessible to high-risk patients by making the procedure more tolerable with lower toxicity in comparison with conventional conditioning regimens.
However, there is ongoing debate over the prognostic effect of key factors in pretransplant conditioning that may be predictive of the risk for post-transplant relapse – in particular, the presence of MRD after the first course of conditioning, he explained.
To more closely investigate those factors and the rate of survival of older patients with AML who undergo RIC transplant, Dr. Russell and his colleagues evaluated results from the National Cancer Research Institute’s (NCRI) AML16 trial, which was conducted from 2006 to 2012, and interim results from the NCRI AML18 trial, which started in 2015 and is ongoing.
Both trials employed double induction of daunorubicin and clofarabine or, in the AML16 trial, AraC ± gemtuzumab, and in the AML18 trial, daunorubicin and AraC (DA) + gemtuzumab.
In AML18, patients who were MRD positive after course 1 were randomly assigned to undergo either an intensification randomization after either FLAG-Ida or DA+cladribine or DA alone.
In AML16, of 983 patients in first complete response, 144 (15%) subsequently underwent RIC transplant. The median follow-up for survival from complete response was 45 months.
In the AML18 trial, of 847 patients, 648 patients achieved complete response. Among them, 201 (31%) underwent transplant. The median follow-up of survival was 45 months.
The results of both trials showed greater benefit with RIC transplant versus chemotherapy alone.
In the AML16 trial, among patients aged 60 to 70 who received RIC, survival at 5 years was significantly improved compared with chemotherapy alone (37% vs. 19%; hazard ratio, 0.65; 95% confidence interval, 0.52-0.82; P < .001).
In AML16, the higher survival benefit in comparison with chemotherapy alone was observed in the RIC group across subgroups of risk level, as stratified according to in the multivariate Wheatley risk group score. Subgroup stratification was based on age, cytogenics, and other factors (HR, 0.66; 95% CI, 0.53-0.83; P < .001).
Importantly, the survival benefits were significantly greater with RIC transplant regardless of MRD-negative or MRD-positive status after course 1 (HR, 0.68; 95% CI, 0.54-0.85; P < .001).
Allograft transplant was also more favorable regardless of FLT3 ITD or NPM1 mutation status (P for heterogeneity by genetic subgroups, 0.61).
In AML16, no groups were found to have benefited more with RIC. Consequently, the criteria for transplant in AML18 trial were based on patients’ health status and donor availability.
An interim analysis of the ongoing AML 18 trial further underscored an overall benefit of RIC transplant. Rates of 3-year survival from remission were 48% with RIC transplant, versus 37.4% with chemotherapy alone (P = .027). The benefit was independent of MRD status after conditioning course 1, similar to the AML16 results (HR, 0.71; 95% CI, 0.54-0.95; P = .02).
Although the rate of transplantation in the AML18 trial was higher among patients who were MRD positive in comparison with those who were MRD negative (36% vs. 24.8%), the rates of post-transplant survival were not significantly different between those who were MRD positive and those who were MRD negative after course 1 (51.1% vs. 46.6% at 3 years; P = .84).
The authors evaluated the effects of a second conditioning course on transplant outcomes among patients who did not initially achieve an MRD-negative complete remission.
They found that 60% of patients did convert from MRD-positive to MRD-negative status after course 2. Among those patients, the survival versus chemotherapy alone was substantially higher (HR, 0.32; 95% CI, 0.11-0.92) compared to those who remained MRD-negative (HR 0.74; 95% CI, 0.32-1.72).
However, the authors note that, owing to a lack of heterogeneity, the results don’t necessarily mean that the patients who remained MRD positive did not also benefit from transplant.
“There was a significant benefit for transplant in those who converted to MRD negativity,” Dr. Russell said.
“With a hazard ratio of .32, this was far superior to those who remained MRD-positive post course 2,” he said.
“These results show that MRD status after course 1 is important information in terms of response to therapy and can alter your treatment strategy if you’re considering a transplant as an option for these patients,” Dr. Russell told this news organization.
In further commenting, Dr. Craddock said the research highlights the importance of randomized trials with regard to whether patients who are MRD-positive before transplant will benefit from an additional course of therapy to reduce the MRD load.
“Most get two courses, but the question is, if they are still MRD positive, should they get a third course, and if so, what should that look like?” he said.
“There are currently no randomized controlled trials to address that ongoing question, and they need to be done,” he added.
Dr. Russell has relationships with Pfizer, Astellas, and Jazz Pharma. Dr. Craddock has a relationship with Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Among older patients with acute myeloid leukemia (AML), survival is significantly better when they undergo reduced-intensity conditioning (RIC) before receiving an allogeneic hematopoietic cell transplant (HCT) at first remission. This improvement in survival is seen regardless of key factors such as genotype and the status of minimal residual disease (MRD) after initial chemotherapy, results from two large randomized trials show.
“Two consecutive trials of more than 1,500 older AML patients above 60 years of age demonstrate a consistent benefit for RIC transplant in first remission,” said first author Nigel Russell, MD, of Guy’s Hospital, London, and Nottingham University, England. “This benefit is seen independent of their post-course 1 MRD status,” he added.
Dr. Russell presented the new data at the European Hematology Association (EHA) 2021 Annual Meeting.
Commenting on the study, Charles Craddock, MD, said in an interview that the results “confirm the growing importance of RIC transplantation as a central treatment management strategy in high-risk AML and in this population high risk patients over 60.”
“[These findings] reinforce the evolving treatment paradigm that, in fit adults over 60 with AML, hematopoietic cell transplantation should be considered an essential component of their management plan,” said Dr. Craddock, academic director of the Center for Clinical Haematology, Queen Elizabeth Hospital, Birmingham, England.
Patients with AML who are older than 60 years can achieve complete remission with intensive chemotherapy alone; however, relapse is common, and only about 20% survive for 5 years, Dr. Russell explained.
HCT significantly improves survival outcomes, and the development of RIC has made transplantation accessible to high-risk patients by making the procedure more tolerable with lower toxicity in comparison with conventional conditioning regimens.
However, there is ongoing debate over the prognostic effect of key factors in pretransplant conditioning that may be predictive of the risk for post-transplant relapse – in particular, the presence of MRD after the first course of conditioning, he explained.
To more closely investigate those factors and the rate of survival of older patients with AML who undergo RIC transplant, Dr. Russell and his colleagues evaluated results from the National Cancer Research Institute’s (NCRI) AML16 trial, which was conducted from 2006 to 2012, and interim results from the NCRI AML18 trial, which started in 2015 and is ongoing.
Both trials employed double induction of daunorubicin and clofarabine or, in the AML16 trial, AraC ± gemtuzumab, and in the AML18 trial, daunorubicin and AraC (DA) + gemtuzumab.
In AML18, patients who were MRD positive after course 1 were randomly assigned to undergo either an intensification randomization after either FLAG-Ida or DA+cladribine or DA alone.
In AML16, of 983 patients in first complete response, 144 (15%) subsequently underwent RIC transplant. The median follow-up for survival from complete response was 45 months.
In the AML18 trial, of 847 patients, 648 patients achieved complete response. Among them, 201 (31%) underwent transplant. The median follow-up of survival was 45 months.
The results of both trials showed greater benefit with RIC transplant versus chemotherapy alone.
In the AML16 trial, among patients aged 60 to 70 who received RIC, survival at 5 years was significantly improved compared with chemotherapy alone (37% vs. 19%; hazard ratio, 0.65; 95% confidence interval, 0.52-0.82; P < .001).
In AML16, the higher survival benefit in comparison with chemotherapy alone was observed in the RIC group across subgroups of risk level, as stratified according to in the multivariate Wheatley risk group score. Subgroup stratification was based on age, cytogenics, and other factors (HR, 0.66; 95% CI, 0.53-0.83; P < .001).
Importantly, the survival benefits were significantly greater with RIC transplant regardless of MRD-negative or MRD-positive status after course 1 (HR, 0.68; 95% CI, 0.54-0.85; P < .001).
Allograft transplant was also more favorable regardless of FLT3 ITD or NPM1 mutation status (P for heterogeneity by genetic subgroups, 0.61).
In AML16, no groups were found to have benefited more with RIC. Consequently, the criteria for transplant in AML18 trial were based on patients’ health status and donor availability.
An interim analysis of the ongoing AML 18 trial further underscored an overall benefit of RIC transplant. Rates of 3-year survival from remission were 48% with RIC transplant, versus 37.4% with chemotherapy alone (P = .027). The benefit was independent of MRD status after conditioning course 1, similar to the AML16 results (HR, 0.71; 95% CI, 0.54-0.95; P = .02).
Although the rate of transplantation in the AML18 trial was higher among patients who were MRD positive in comparison with those who were MRD negative (36% vs. 24.8%), the rates of post-transplant survival were not significantly different between those who were MRD positive and those who were MRD negative after course 1 (51.1% vs. 46.6% at 3 years; P = .84).
The authors evaluated the effects of a second conditioning course on transplant outcomes among patients who did not initially achieve an MRD-negative complete remission.
They found that 60% of patients did convert from MRD-positive to MRD-negative status after course 2. Among those patients, the survival versus chemotherapy alone was substantially higher (HR, 0.32; 95% CI, 0.11-0.92) compared to those who remained MRD-negative (HR 0.74; 95% CI, 0.32-1.72).
However, the authors note that, owing to a lack of heterogeneity, the results don’t necessarily mean that the patients who remained MRD positive did not also benefit from transplant.
“There was a significant benefit for transplant in those who converted to MRD negativity,” Dr. Russell said.
“With a hazard ratio of .32, this was far superior to those who remained MRD-positive post course 2,” he said.
“These results show that MRD status after course 1 is important information in terms of response to therapy and can alter your treatment strategy if you’re considering a transplant as an option for these patients,” Dr. Russell told this news organization.
In further commenting, Dr. Craddock said the research highlights the importance of randomized trials with regard to whether patients who are MRD-positive before transplant will benefit from an additional course of therapy to reduce the MRD load.
“Most get two courses, but the question is, if they are still MRD positive, should they get a third course, and if so, what should that look like?” he said.
“There are currently no randomized controlled trials to address that ongoing question, and they need to be done,” he added.
Dr. Russell has relationships with Pfizer, Astellas, and Jazz Pharma. Dr. Craddock has a relationship with Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Among older patients with acute myeloid leukemia (AML), survival is significantly better when they undergo reduced-intensity conditioning (RIC) before receiving an allogeneic hematopoietic cell transplant (HCT) at first remission. This improvement in survival is seen regardless of key factors such as genotype and the status of minimal residual disease (MRD) after initial chemotherapy, results from two large randomized trials show.
“Two consecutive trials of more than 1,500 older AML patients above 60 years of age demonstrate a consistent benefit for RIC transplant in first remission,” said first author Nigel Russell, MD, of Guy’s Hospital, London, and Nottingham University, England. “This benefit is seen independent of their post-course 1 MRD status,” he added.
Dr. Russell presented the new data at the European Hematology Association (EHA) 2021 Annual Meeting.
Commenting on the study, Charles Craddock, MD, said in an interview that the results “confirm the growing importance of RIC transplantation as a central treatment management strategy in high-risk AML and in this population high risk patients over 60.”
“[These findings] reinforce the evolving treatment paradigm that, in fit adults over 60 with AML, hematopoietic cell transplantation should be considered an essential component of their management plan,” said Dr. Craddock, academic director of the Center for Clinical Haematology, Queen Elizabeth Hospital, Birmingham, England.
Patients with AML who are older than 60 years can achieve complete remission with intensive chemotherapy alone; however, relapse is common, and only about 20% survive for 5 years, Dr. Russell explained.
HCT significantly improves survival outcomes, and the development of RIC has made transplantation accessible to high-risk patients by making the procedure more tolerable with lower toxicity in comparison with conventional conditioning regimens.
However, there is ongoing debate over the prognostic effect of key factors in pretransplant conditioning that may be predictive of the risk for post-transplant relapse – in particular, the presence of MRD after the first course of conditioning, he explained.
To more closely investigate those factors and the rate of survival of older patients with AML who undergo RIC transplant, Dr. Russell and his colleagues evaluated results from the National Cancer Research Institute’s (NCRI) AML16 trial, which was conducted from 2006 to 2012, and interim results from the NCRI AML18 trial, which started in 2015 and is ongoing.
Both trials employed double induction of daunorubicin and clofarabine or, in the AML16 trial, AraC ± gemtuzumab, and in the AML18 trial, daunorubicin and AraC (DA) + gemtuzumab.
In AML18, patients who were MRD positive after course 1 were randomly assigned to undergo either an intensification randomization after either FLAG-Ida or DA+cladribine or DA alone.
In AML16, of 983 patients in first complete response, 144 (15%) subsequently underwent RIC transplant. The median follow-up for survival from complete response was 45 months.
In the AML18 trial, of 847 patients, 648 patients achieved complete response. Among them, 201 (31%) underwent transplant. The median follow-up of survival was 45 months.
The results of both trials showed greater benefit with RIC transplant versus chemotherapy alone.
In the AML16 trial, among patients aged 60 to 70 who received RIC, survival at 5 years was significantly improved compared with chemotherapy alone (37% vs. 19%; hazard ratio, 0.65; 95% confidence interval, 0.52-0.82; P < .001).
In AML16, the higher survival benefit in comparison with chemotherapy alone was observed in the RIC group across subgroups of risk level, as stratified according to in the multivariate Wheatley risk group score. Subgroup stratification was based on age, cytogenics, and other factors (HR, 0.66; 95% CI, 0.53-0.83; P < .001).
Importantly, the survival benefits were significantly greater with RIC transplant regardless of MRD-negative or MRD-positive status after course 1 (HR, 0.68; 95% CI, 0.54-0.85; P < .001).
Allograft transplant was also more favorable regardless of FLT3 ITD or NPM1 mutation status (P for heterogeneity by genetic subgroups, 0.61).
In AML16, no groups were found to have benefited more with RIC. Consequently, the criteria for transplant in AML18 trial were based on patients’ health status and donor availability.
An interim analysis of the ongoing AML 18 trial further underscored an overall benefit of RIC transplant. Rates of 3-year survival from remission were 48% with RIC transplant, versus 37.4% with chemotherapy alone (P = .027). The benefit was independent of MRD status after conditioning course 1, similar to the AML16 results (HR, 0.71; 95% CI, 0.54-0.95; P = .02).
Although the rate of transplantation in the AML18 trial was higher among patients who were MRD positive in comparison with those who were MRD negative (36% vs. 24.8%), the rates of post-transplant survival were not significantly different between those who were MRD positive and those who were MRD negative after course 1 (51.1% vs. 46.6% at 3 years; P = .84).
The authors evaluated the effects of a second conditioning course on transplant outcomes among patients who did not initially achieve an MRD-negative complete remission.
They found that 60% of patients did convert from MRD-positive to MRD-negative status after course 2. Among those patients, the survival versus chemotherapy alone was substantially higher (HR, 0.32; 95% CI, 0.11-0.92) compared to those who remained MRD-negative (HR 0.74; 95% CI, 0.32-1.72).
However, the authors note that, owing to a lack of heterogeneity, the results don’t necessarily mean that the patients who remained MRD positive did not also benefit from transplant.
“There was a significant benefit for transplant in those who converted to MRD negativity,” Dr. Russell said.
“With a hazard ratio of .32, this was far superior to those who remained MRD-positive post course 2,” he said.
“These results show that MRD status after course 1 is important information in terms of response to therapy and can alter your treatment strategy if you’re considering a transplant as an option for these patients,” Dr. Russell told this news organization.
In further commenting, Dr. Craddock said the research highlights the importance of randomized trials with regard to whether patients who are MRD-positive before transplant will benefit from an additional course of therapy to reduce the MRD load.
“Most get two courses, but the question is, if they are still MRD positive, should they get a third course, and if so, what should that look like?” he said.
“There are currently no randomized controlled trials to address that ongoing question, and they need to be done,” he added.
Dr. Russell has relationships with Pfizer, Astellas, and Jazz Pharma. Dr. Craddock has a relationship with Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
Fact or fiction? Intravascular contrast and acute kidney injury
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.
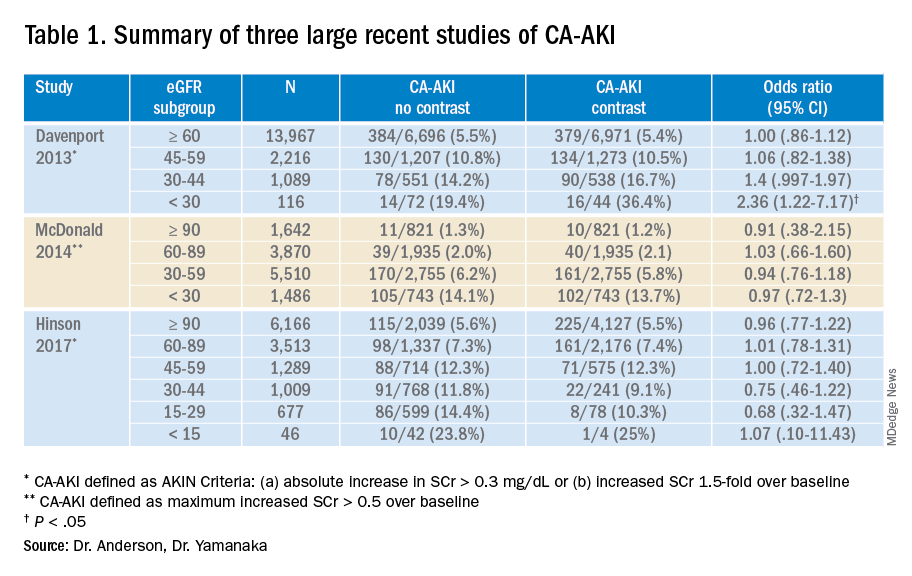
Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Withholding contrast may be the greater risk
Withholding contrast may be the greater risk
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Case
A 73-year-old man with stage III chronic kidney disease (CKD) presents to the emergency department with acute left–upper quadrant pain. Serum creatinine is 2.1mg/dL (eGFR 30 mL/min). Noncontrast computed tomography of the abdomen identifies small bowel inflammation and extensive atherosclerosis. Acute mesenteric ischemia is suspected, but further characterization requires intravenous contrast–enhanced images. He and his family worry about the safety of IV contrast and ask to speak with you.
Introduction
Intravenous iodinated contrast material enhances tissue conspicuity in CT imaging and improves its diagnostic performance. Several case reports published in the 1950s suggested that IV administration of high-osmolality contrast provoked acute kidney injury. An ensuing series of studies associated contrast utilization with renal impairment and additional data extrapolated from cardiology arteriography studies further amplified these concerns.
Contrast media use is often cited as a leading cause of hospital-acquired acute kidney injury.1 The associated fear of causing renal impairment or provoking the need for dialysis frequently leads clinicians to forgo contrast-enhanced CT studies or settle for suboptimal noncontrast imaging even in situations where these tests are clearly indicated. The potential for inadequate imaging to contribute to incomplete, delayed, or incorrect diagnoses represents an ongoing patient safety issue.
A growing body of literature suggests the risks of contrast-associated acute kidney injury are overstated, implying the truer danger lies with inadequate imaging, not contrast media utilization. This review discusses the definitions, risks, and incidence of contrast-associated acute kidney injury, informed by these recent studies.
Overview of the data
Definitions of contrast-induced renal dysfunction vary in clinical studies and range from a creatinine rise of 0.5-1 mg per deciliter or a 25%-50% increase from baseline within 2-5 days following contrast administration. In 2012, the Kidney Disease Improving Global Outcomes working group proposed the term “contrast-associated acute kidney injury” (CA-AKI) and defined it as a plasma creatinine rise of 0.3 mg/dL within 48 hours of contrast exposure, a creatinine increase by a factor of 1.5 over baseline within 7 days of contrast administration, or a urinary volume less than 0.5 mg per kg of body weight within 6 hours of contrast exposure (AKI Network or “AKIN” criteria for CA-AKI).2 Owing in part to inconsistent definitions and partly because of multiple potential confounders, the true incidence of contrast-associated acute kidney injury is uncertain.
The pathogenesis of CA-AKI is incompletely understood, but proposed mechanisms include direct tubular cytotoxic effects; reductions in intrarenal blood flow from contrast material–provoked arteriolar vasoconstriction and contrast-induced increases in blood viscosity; and renal microvascular thrombosis.
Risk factors for CA-AKI overlap with those for acute kidney injury in general. These include CKD, concurrent nephrotoxic medication use, advancing age, diabetes, hemodynamic disturbances to include intravascular volume depletion, systemic illness, and rapid arterial delivery of a large contrast volume.
Current American College of Radiology guidelines state that intravenous isotonic crystalloid volume expansion prior to contrast administration may provide some renal protection, although randomized clinical trial results are inconsistent. The largest clinical trials of N-acetylcysteine showed rates of CA-AKI, need for dialysis, and mortality were no different than placebo. Studies of intravenous sodium bicarbonate show outcomes similar to normal saline.
Introduced in the 1950s and used until the early 2000s, the osmolality of high-osmolality contrast material (HOCM) is roughly five times that of blood (1551 mOsm/kg H2O).3 The early case reports first identifying concern for contrast-induced renal damage were of HOCM used in angiography and pyelography testing. Multiple follow up clinical studies measured creatinine levels before and after contrast administration and classified the percentage of patients whose creatinine level rose above an arbitrary definition of renal injury as having contrast-induced renal injury. These studies formed the basis of the now longstanding concerns about contrast-associated renal dysfunction. Importantly, very few of these HOCM studies included a control group.
Following multiple studies demonstrating an improved safety profile with a similar image quality, the Food and Drug Administration approved low-osmolality contrast (LOCM, 413-796mOsm/kg H2O) in 1985. Early adoption was slow because of its significantly higher cost and incomplete Medicare reimbursement. Prices fell following generic LOCM introduction in 1995 and in 2005 Medicare approved universal reimbursement, leading to widespread use. The FDA approved an iso-osmolality contrast material (290 mOsm/kg H2O) in the mid-1990s; its safety profile and image quality is similar to LOCM. Both LOCM and iso-osmolality contrast material are used in CTs today. Iso-osmolality contrast is more viscous than LOCM and is currently more expensive. Iso-osmolality and LOCM have similar rates of CA-AKI.
A clinical series published in 2008 examined serum creatinine level variation over 5 consecutive days in 30,000 predominantly hospitalized patients who did not receive intravenous contrast material. Investigators simulated contrast administration between days 1 and 2, then observed creatinine changes over the subsequent days. The incidence of acute kidney injury following the simulated contrast dose closely resembled the rates identified in earlier studies that associated contrast exposure with renal injury.4 These results suggested that changes in renal function commonly attributed to contrast exposure may be because of other, concurrent, clinical factors.
A 2013 study compared 8,826 patients with stable renal function who received a low-osmolality contrast-enhanced CT with 8,826 patients who underwent a noncontrast study.5 After 1:1 propensity matching, they found higher rates of CA-AKI (as defined by AKIN criteria) among only those with baseline eGFR less than 30 mL/min. There was a trend towards higher rates of CA-AKI among those with baseline eGFR of 30-44 mL/min, and no difference among the bulk of patients with normal or near normal baseline renal function.
Another large propensity score–matched study published in 2014 compared 6,254 patients who underwent a contrast-enhanced CT with 6,254 patients who underwent a nonenhanced CT.
Investigators stratified this predominantly inpatient cohort by baseline eGFR. Results demonstrated similar rates of AKI between contrast material and non–contrast material cohorts. They concluded that intravenous contrast administration did not significantly affect the risk of acute kidney injury, even in patients with impaired renal function. The authors noted that the difference in contrast-mediated nephrotoxic risk in patients with eGFRless than 30 between their study and the Davenport study could be explained by their use of a different definition of CA-AKI, differences in propensity score calculation, and by enrolling greater numbers of patients with impaired kidney function in their study.6
Finally, a large single-center study published in 2017 included 16,801 ED patients divided into three groups; patients who received a contrast-enhanced CT, patients who underwent a noncontrast CT study, and a set of patients who did not undergo any CT imaging. Patients with creatinine levels under .4 mg/dL or over 4 mg/dL were excluded from initial analysis.
Investigators stratified each patient group by serum creatinine and eGFR and utilized both traditional contrast-induced nephropathy (serum creatinine increase of .5 mg/dL or a 25% increase over baseline serum creatinine level at 48-72 hours) and AKIN criteria to evaluate for acute kidney injury. Propensity score analyses comparing the contrast-enhanced group and two control groups failed to identify any significant change in AKI incidence. The authors concluded that, in situations where contrast-enhanced CT is indicated to avoid missing or delaying potential diagnoses, the risks of diagnostic failure outweigh any potential risks of contrast induced renal injury.7
While these three studies utilized control groups and propensity score matching, they are retrospective in nature and unknown or omitted confounding variables could be present. Together, though, they contribute to a growing body of literature suggesting that the risk of contrast-associated AKI relates less to the contrast itself and more to concurrent clinical factors affecting kidney function. Ethical concerns have to date prevented the conduct of a randomized trial of IV contrast in CT scanning. Table 1 summarizes the findings of these three studies.

Application of the data to the case
The patient presented with abdominal pain potentially attributable to acute mesenteric ischemia, where a delayed or missed diagnosis can be potentially fatal. He was counseled about the comparatively small risk of CA-AKI with IV contrast and underwent contrast-enhanced CT scanning without incident. The diagnosis of acute mesenteric ischemia was confirmed, and he was referred for urgent laparotomy.
Bottom line
The absolute risk of CA-AKI varies according to baseline renal function and is not clearly linked to the receipt of IV contrast. The risks of withholding contrast may be greater than the risk of CA-AKI. Clinicians should counsel patients accordingly.
Dr. Anderson is national lead, VHA Hospital Medicine, and associate professor of medicine at the Minneapolis VA Health Care System. Dr. Yamanaka is a hospitalist at the Minneapolis VA Medical Center and an assistant professor of medicine at the University of Minnesota.
References
1. Nash K et al. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39(5):930-6. doi: 10.1053/ajkd.2002.32766.
2. Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69-88. doi: 10.1038/kisup.2011.34.
3. Wilmot A et al. The adoption of low-osmolar contrast agents in the United States: Historical analysis of health policy and clinical practice. AJR Am J Roentgenol. 2012;199(5):1049-53. doi: 10.2214/AJR.11.8426.
4. Newhouse JH et al. Frequency of serum creatinine changes in the absence of iodinated contrast material: Implications for studies of contrast nephrotoxicity. AJR Am J Roentgenol. 2008;191(2):376-82. doi: 10.2214/AJR.07.3280.
5. Davenport MS et al. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: Risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268(3):719-28. doi: 10.1148/radiol.13122276.
6. McDonald JS et al. Risk of intravenous contrast material-mediated acute kidney injury: A propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014;271(1):65-73. doi: 10.1148/radiol.13130775.
7. Hinson JS et al. Risk of acute kidney injury after intravenous contrast media administration. Ann Emerg Med. 2017;69(5):577-86. doi: 10.1016/j.annemergmed.2016.11.021.
Key points
- Early studies suggesting an association between IV contrast and AKI used an older formulation of contrast media not routinely used today. Importantly, these studies did not use control groups.
- Results from multiple recent large trials comparing IV contrast patients with controls suggest that AKI is not clearly linked to the receipt of IV contrast and that it varies according to baseline renal function.
- Randomized controlled trials of prophylactic normal saline or sodium bicarbonate to prevent CA-AKI show mixed results. Clinical trials comparing N-acetylcysteine with placebo showed no difference in the rates of AKI, dialysis initiation, or mortality.
Quiz
Which of the following is not clearly associated with acute kidney injury in hospitalized patients?
A. Decreased baseline glomerular filtration rate
B. Angiotensin-converting enzyme (ACE) inhibitor use
C. Hemodynamic instability
D. Intravenous contrast administration
Answer: D
While decreased baseline renal function, ACE inhibitors, and hemodynamic instability are known risk factors for hospital-associated renal injury, a growing body of literature suggests that intravenous contrast used in computed tomography studies does not precipitate acute kidney injury.
Further reading
McDonald JS et al. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013;267(1):119-128. doi: 10.1148/radiol.12121460.
McDonald RJ et al. Behind the numbers: Propensity score analysis – a primer for the diagnostic radiologist. Radiology. 2013;269(3):640-5. doi: 10.1148/radiol.13131465.
Luk L et al. Intravenous contrast-induced nephropathy – the rise and fall of a threatening idea. Adv Chronic Kidney Dis. 2017;24(3):169-75. doi: 10.1053/j.ackd.2017.03.001.
Mehran R et al. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146-55. doi: 10.1056/NEJMra1805256.
Is event-driven PrEP dosing for HIV as effective as daily dosing?
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
EVIDENCE SUMMARY
Event-driven PrEP is effective for prevention of HIV transmission
An RCT evaluating the effectiveness of event-driven PrEP in 400 patients at high risk for HIV found that it reduced HIV incidence by 86% compared to placebo. Researchers recruited HIV-negative men or transgender women who had sex with men, who’d had condomless anal sex with at least 2 partners in the previous 6 months, and followed them for a median of 9.3 months for HIV acquisition.1
Patients randomized to event-driven PrEP took tenofovir-emtricitabine (300-200 mg) on the following schedule: 2 pills 2 to 24 hours before intercourse (or 1 pill if they had taken it within the past week), followed by a third pill 24 hours later, and a fourth pill 24 hours after that. When patients had multiple consecutive episodes of intercourse, daily use was continued until 2 days after the last episode. Patients in the control group took placebo pills.1
Event-driven PrEP reduced HIV incidence vs placebo (2 infections vs 14 infections; 0.91 vs 6.6 per 100 person-years; relative risk [RR] = 0.86; P = .002). PrEP produced more gastrointestinal (14% vs 5%; P = .002) and renal (18% vs 10%; P = .03) adverse effects than placebo. Participants took a median of 15 pills per month.1
A post-hoc analysis of the above study, evaluating 270 patients, found that event-driven PrEP reduced HIV incidence by 100% during periods of less frequent sexual encounters. Selected participants had a median of 5 sexual encounters per month (range, 2-10), used a median of 9.5 pills per month (range, 6-13), and represented 134 person-years of follow-up. No HIV infections (0 per 100 person-years; 95% CI, 0-5; P = .013) were diagnosed in the PrEP group and 6 HIV infections (9.2 per 100 person-years; 95% CI, 3.4-20.1) were diagnosed in the placebo group, with a relative reduction of HIV incidence of 100% (95% CI, 39-100).2
For comparison, 2 large open-label trials evaluating daily PrEP found that it reduced HIV incidence by 44%3 and 86%4 vs placebo.
Adherence is better with daily PrEPthan event-driven PrEP
Three prospective cohort trials evaluated PrEP adherence (extent that participants were taking PrEP at the time of sexual encounters) with different dosing regimens and found that event-driven PrEP tended to have lower adherence than daily PrEP. An open-label trial in Bangkok and Harlem (New York City) randomized 357 at-risk patients to 1 of 3 regimens: event-driven (1 tablet before and after sex), time-driven (1 tablet twice weekly with a postsex dose), and daily. Overall, patients with event-driven PrEP had lower adherence than those with daily PrEP (67% event-driven vs 97% daily; P < 0.0001).5
Continue to: In an open-label...
In an open-label prospective cohort trial in Belgium, at-risk patients chose between using event-driven (N = 44) and daily (N = 135) PrEP. Analysis was conducted for both high-risk HIV exposure days (defined as condomless anal receptive intercourse with a new or HIV-positive steady partner with a detectable viral load) and low-risk HIV exposure days (consistent condom use or condomless anal intercourse with a steady partner who is HIV-negative). Over 18 months, lower adherence was demonstrated with event-driven PrEP than with daily PrEP for high-risk days (88% [95% CI, 86%-90%] vs 97.5% [95% CI, 97%-98%]; P < .0001) and also for low-risk days (42% [95% CI, 40%-45%] vs 96% [95% CI, 95%-96%]; P < .0001).6 Researchers diagnosed no new HIV infections in any participant, and the incidence of STIs was the same in both groups.
A third open-label trial evaluated adherence among 178 South African women randomized to event-driven or daily PrEP and found lower sexual event coverage with event-driven PrEP (52% vs 75%; odds ratio = 2.76; 95% CI, 1.68-4.53; P < 0.0006). Four women in each group seroconverted to HIV positive.7
Drug costs, patient preferences, and STI risk are important considerations
Several of the above trials reported use of fewer pills in the event-driven groups, with lower drug costs.2,5,7 A large prospective cohort trial of men who have sex with men (N = 1049) with an average of 10 sexual partners found that most (76%) opted for event-driven PrEP.8 Researchers also reported no difference in STI rates (RR = 1.24 for “at least 1 bacterial STI”; 95% CI, 0.84 to 1.81).8 However, a smaller, open-label prospective cohort trial (N = 200) found that more participants chose daily PrEP than event-driven PrEP (76.5% vs 23.5%), although almost all said they would change their dosing regimen in the next year.9
Recommendations from others
In 2019, the World Health Organization recommended oral PrEP as an additional prevention choice for people at substantial risk for HIV infection and stated that different dosing strategies offer users flexibility, choice, and convenience.10 Also in 2019, the US Preventive Services Task Force published a recommendation that clinicians offer PrEP with effective antiretroviral therapy to patients at high risk for HIV acquisition. They did not specify which regimen to offer.11
Editor’s takeaway
While there are theoretical reasons why event-driven PrEP might not work as well as daily PrEP, we have 1 RCT that suggests the real-world outcomes are similar. Given the apparent effectiveness of either option, the best choice is the one the patient will use. JFP
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
- Molina JM, Capitant C, Spire B, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. NEJM. 2015;373:2237-2246.
- Antoni G, Tremblay C, Delaugerre C, et al. On-demand pre-exposure prophylaxis with tenofovir disoproxil fumarate plus emtricitabine among men who have sex with men with less frequent sexual intercourse: a post-hoc analysis of the ANRS IPERGAY trial. Lancet HIV. 2020;7:e113-e120.
- Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis in men who have sex with men. NEJM. 2010;363:2587-2599.
- McCormack S, Dunn DT, Desai M, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot of a pragmatic open-label randomized trial. Lancet. 2016;387:53-60.
- Grant RM, Mannheimer S, Hughes JP, et al. Daily and nondaily oral preexposure prophylaxis in men and transgender women who have sex with men: the Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT study. Clin Infect Dis. 2018;66:1712-1721.
 Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.
Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behavior and STI incidence. J Intl AIDS Soc. 2019;22:e25407.- Bekker LG, Roux S, Sebastien E, et al. Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomized, open-label, phase 2 trial. Lancet HIV. 2018;5:e68-e78.
- Noret M, Balavoine S, Pintado C, et al. Daily or on-demand oral tenofovir disoproxil fumarate/emtricitabine for HIV pre-exposure prophylaxis: experience from a hospital-based clinic in France. AIDS. 2018;32:2161-2169.
- Reyniers T, Nöstlinger C, Laga M, et al. Choosing between daily and event-driven pre-exposure prophylaxis: results of a Belgian PrEP demonstration project. J Acquir Immune Defic Syndr. 2018;79:186-194.
- WHO. What’s the 2+1+1? Event-driven oral pre-exposure prophylaxis to prevent HIV in men who have sex with men: update to WHO’s recommendation on oral PrEP [technical brief]. Published July 2019. Accessed May 14, 2021. https://who.int/hiv/pub/prep/211/en
- US Preventive Services Task Force. Prevention of human immunodeficiency virus (HIV) infection: preexposure prophylaxis [evidence summary]. Published June 11, 2019. Accessed May 14, 2021. www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
EVIDENCE-BASED ANSWER:
Probably, although there are no head-to-head trials comparing the 2 dosing regimens. Event-driven pre-exposure prophylaxis (PrEP) dosing reduces HIV conversion by 86% compared to placebo (strength of recommendation [SOR]: B, large randomized controlled trial [RCT]). Daily PrEP reduces HIV conversion by 44% to 86% (SOR: B, based on open-label RCTs).
Event-driven PrEP regimens may be associated with lower adherence when compared with daily PrEP regimens (average of 70% for event-driven PrEP vs average of 92% for daily PrEP) (SOR: B, based on open-label and cohort trials). Event-driven PrEP regimens have lower medication costs, and they are associated with no difference in the rate of sexually transmitted infections (STIs) (SOR: B, based on prospective cohort studies). Patients may prefer them to daily regimens (75% choose event-driven PrEP vs 25% choose daily PrEP) (SOR: B, based on the preponderance of prospective cohort studies with conflicting results).
Breast cancer: Young women likely to receive guideline-concordant care
Key clinical point: A high number of young women with breast cancer receive guideline-concordant care (GCC).
Major finding: GCC was given to 81.7% of the patients. Patients with stage III vs. stage I or II disease (93.4% vs. 88.4%) received GCC more frequently in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-positive or HR-negative/HER-positive subtypes. In women with HR-negative/HER2-negative or HR-positive/HER2-negative tumors, a higher proportion of patients with stage II vs stage I or III disease received GCC (91.8% vs. 83.7%).
Study details: A retrospective study of 1,295 young women with invasive breast cancer diagnosed in 2013.
Disclosures: This study was supported by the National Cancer Institute. Dr. AW Kurian received research funding from Myriad Genetics and served on the board of directors of a patient advocacy group outside this work. The other authors reported no conflicts of interest.
Source: White DP. Cancer. 2021 Jun 1. doi: 10.1002/cncr.33652.
Key clinical point: A high number of young women with breast cancer receive guideline-concordant care (GCC).
Major finding: GCC was given to 81.7% of the patients. Patients with stage III vs. stage I or II disease (93.4% vs. 88.4%) received GCC more frequently in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-positive or HR-negative/HER-positive subtypes. In women with HR-negative/HER2-negative or HR-positive/HER2-negative tumors, a higher proportion of patients with stage II vs stage I or III disease received GCC (91.8% vs. 83.7%).
Study details: A retrospective study of 1,295 young women with invasive breast cancer diagnosed in 2013.
Disclosures: This study was supported by the National Cancer Institute. Dr. AW Kurian received research funding from Myriad Genetics and served on the board of directors of a patient advocacy group outside this work. The other authors reported no conflicts of interest.
Source: White DP. Cancer. 2021 Jun 1. doi: 10.1002/cncr.33652.
Key clinical point: A high number of young women with breast cancer receive guideline-concordant care (GCC).
Major finding: GCC was given to 81.7% of the patients. Patients with stage III vs. stage I or II disease (93.4% vs. 88.4%) received GCC more frequently in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-positive or HR-negative/HER-positive subtypes. In women with HR-negative/HER2-negative or HR-positive/HER2-negative tumors, a higher proportion of patients with stage II vs stage I or III disease received GCC (91.8% vs. 83.7%).
Study details: A retrospective study of 1,295 young women with invasive breast cancer diagnosed in 2013.
Disclosures: This study was supported by the National Cancer Institute. Dr. AW Kurian received research funding from Myriad Genetics and served on the board of directors of a patient advocacy group outside this work. The other authors reported no conflicts of interest.
Source: White DP. Cancer. 2021 Jun 1. doi: 10.1002/cncr.33652.
TNBC: Trop-2 expression is a potential biomarker for sacituzumab govitecan activity
Key clinical point: High or medium human trophoblast cell-surface antigen-2 (Trop-2) expression is associated with numerically higher survival with sacituzumab govitecan in patients with metastatic triple-negative breast cancer (TNBC).
Major finding: The median progression-free survival in the sacituzumab govitecan group was 6.9, 5.6, and 2.7 months in the patients with high, medium, and low Trop-2 H-scores, respectively. The median overall survival with sacituzumab govitecan was 14.2, 14.9, and 9.3 months in patients with high, medium, and low Trop-2 scores, respectively. The germline BRCA1/2 mutation status did not affect the outcomes in the sacituzumab govitecan vs. chemotherapy group.
Study details: A prespecified, exploratory biomarker analysis from the ASCENT trial evaluated the association between tumor Trop-2 expression and germline BRCA1/2 mutation status with clinical outcomes in patients with metastatic TNBC. The patients were randomly assigned to sacituzumab govitecan or chemotherapy.
Disclosures: This study was sponsored by Immunomedics, Inc. The authors received consulting/advisory fees, research funding, travel/accommodations/expenses, speaker fees, nonfinancial support, and/or declared intellectual property rights, patents, and royalties from various companies/organizations. Dr. K Kalinsky reported spouse employment at Array Biopharma. The authors disclosed no other potential conflicts of interest.
Source: Bardia A et al. Ann Oncol. 2021 Jun 8. doi: 10.1016/j.annonc.2021.06.002.
Key clinical point: High or medium human trophoblast cell-surface antigen-2 (Trop-2) expression is associated with numerically higher survival with sacituzumab govitecan in patients with metastatic triple-negative breast cancer (TNBC).
Major finding: The median progression-free survival in the sacituzumab govitecan group was 6.9, 5.6, and 2.7 months in the patients with high, medium, and low Trop-2 H-scores, respectively. The median overall survival with sacituzumab govitecan was 14.2, 14.9, and 9.3 months in patients with high, medium, and low Trop-2 scores, respectively. The germline BRCA1/2 mutation status did not affect the outcomes in the sacituzumab govitecan vs. chemotherapy group.
Study details: A prespecified, exploratory biomarker analysis from the ASCENT trial evaluated the association between tumor Trop-2 expression and germline BRCA1/2 mutation status with clinical outcomes in patients with metastatic TNBC. The patients were randomly assigned to sacituzumab govitecan or chemotherapy.
Disclosures: This study was sponsored by Immunomedics, Inc. The authors received consulting/advisory fees, research funding, travel/accommodations/expenses, speaker fees, nonfinancial support, and/or declared intellectual property rights, patents, and royalties from various companies/organizations. Dr. K Kalinsky reported spouse employment at Array Biopharma. The authors disclosed no other potential conflicts of interest.
Source: Bardia A et al. Ann Oncol. 2021 Jun 8. doi: 10.1016/j.annonc.2021.06.002.
Key clinical point: High or medium human trophoblast cell-surface antigen-2 (Trop-2) expression is associated with numerically higher survival with sacituzumab govitecan in patients with metastatic triple-negative breast cancer (TNBC).
Major finding: The median progression-free survival in the sacituzumab govitecan group was 6.9, 5.6, and 2.7 months in the patients with high, medium, and low Trop-2 H-scores, respectively. The median overall survival with sacituzumab govitecan was 14.2, 14.9, and 9.3 months in patients with high, medium, and low Trop-2 scores, respectively. The germline BRCA1/2 mutation status did not affect the outcomes in the sacituzumab govitecan vs. chemotherapy group.
Study details: A prespecified, exploratory biomarker analysis from the ASCENT trial evaluated the association between tumor Trop-2 expression and germline BRCA1/2 mutation status with clinical outcomes in patients with metastatic TNBC. The patients were randomly assigned to sacituzumab govitecan or chemotherapy.
Disclosures: This study was sponsored by Immunomedics, Inc. The authors received consulting/advisory fees, research funding, travel/accommodations/expenses, speaker fees, nonfinancial support, and/or declared intellectual property rights, patents, and royalties from various companies/organizations. Dr. K Kalinsky reported spouse employment at Array Biopharma. The authors disclosed no other potential conflicts of interest.
Source: Bardia A et al. Ann Oncol. 2021 Jun 8. doi: 10.1016/j.annonc.2021.06.002.
HER-2-negative BRCA-mutated breast cancer: Olaparib effective in real world
Key clinical point: Olaparib monotherapy is effective in germline BRCA-mutated, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer in a real-world setting.
Major finding: The median progression-free survival was 8.11 months, and the clinical response rate was 48.6%. The grade 3 or higher treatment-related adverse event rate was 25.4%. There were no new safety signals.
Study details: An interim analysis of an open-label, single-arm, phase 3b LUCY trial including 252 previously treated patients with HER2-negative metastatic breast cancer with a germline BRCA mutation who received olaparib.
Disclosures: The study was funded by AstraZeneca. Dr. S McCutcheon was an employee and stockholder of AstraZeneca LP. Dr. J Bennett and Dr. G Walker were contractors for AstraZeneca LP. The authors received consulting/speaker fees or research support from various sources.
Source: Gelmon KA et al. Eur J Cancer. 2021 Jun 1. doi: 10.1016/j.ejca.2021.03.029.
Key clinical point: Olaparib monotherapy is effective in germline BRCA-mutated, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer in a real-world setting.
Major finding: The median progression-free survival was 8.11 months, and the clinical response rate was 48.6%. The grade 3 or higher treatment-related adverse event rate was 25.4%. There were no new safety signals.
Study details: An interim analysis of an open-label, single-arm, phase 3b LUCY trial including 252 previously treated patients with HER2-negative metastatic breast cancer with a germline BRCA mutation who received olaparib.
Disclosures: The study was funded by AstraZeneca. Dr. S McCutcheon was an employee and stockholder of AstraZeneca LP. Dr. J Bennett and Dr. G Walker were contractors for AstraZeneca LP. The authors received consulting/speaker fees or research support from various sources.
Source: Gelmon KA et al. Eur J Cancer. 2021 Jun 1. doi: 10.1016/j.ejca.2021.03.029.
Key clinical point: Olaparib monotherapy is effective in germline BRCA-mutated, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer in a real-world setting.
Major finding: The median progression-free survival was 8.11 months, and the clinical response rate was 48.6%. The grade 3 or higher treatment-related adverse event rate was 25.4%. There were no new safety signals.
Study details: An interim analysis of an open-label, single-arm, phase 3b LUCY trial including 252 previously treated patients with HER2-negative metastatic breast cancer with a germline BRCA mutation who received olaparib.
Disclosures: The study was funded by AstraZeneca. Dr. S McCutcheon was an employee and stockholder of AstraZeneca LP. Dr. J Bennett and Dr. G Walker were contractors for AstraZeneca LP. The authors received consulting/speaker fees or research support from various sources.
Source: Gelmon KA et al. Eur J Cancer. 2021 Jun 1. doi: 10.1016/j.ejca.2021.03.029.
Internet-based interventions do not reduce fear of breast cancer recurrence
Key clinical point: The Internet–based-targeted psychological interventions fail to reduce fear of recurrence among early breast cancer survivors.
Major finding: The Fear of Cancer Recurrence Inventory (FCRI) scores significantly decreased at 8 weeks from baseline in all groups (P less than .001). The magnitude of reduction in FCRI scores was similar in cognitive-behavioral interventions and attention controls.
Study details: The randomized controlled FoRtitude study of breast cancer survivors who completed primary treatment. The survivors were randomly assigned to 4 Internet-based interventions or controls. The 4 interventions given for 4 weeks consisted of 3 cognitive behavioral interventions (relaxation, cognitive restructuring, and worry practice vs. attention controls) and telecoaching (motivational interviewing to improve adherence vs. no telecoaching).
Disclosures: This work was supported by the National Cancer Institute at the National Institutes of Health and the ECOG-ACRIN Medical Research Foundation. The authors did not disclose any conflict of interest.
Source: Wagner LI. J Natl Cancer Inst. 2021 May 31. doi: 10.1093/jnci/djab100.
Key clinical point: The Internet–based-targeted psychological interventions fail to reduce fear of recurrence among early breast cancer survivors.
Major finding: The Fear of Cancer Recurrence Inventory (FCRI) scores significantly decreased at 8 weeks from baseline in all groups (P less than .001). The magnitude of reduction in FCRI scores was similar in cognitive-behavioral interventions and attention controls.
Study details: The randomized controlled FoRtitude study of breast cancer survivors who completed primary treatment. The survivors were randomly assigned to 4 Internet-based interventions or controls. The 4 interventions given for 4 weeks consisted of 3 cognitive behavioral interventions (relaxation, cognitive restructuring, and worry practice vs. attention controls) and telecoaching (motivational interviewing to improve adherence vs. no telecoaching).
Disclosures: This work was supported by the National Cancer Institute at the National Institutes of Health and the ECOG-ACRIN Medical Research Foundation. The authors did not disclose any conflict of interest.
Source: Wagner LI. J Natl Cancer Inst. 2021 May 31. doi: 10.1093/jnci/djab100.
Key clinical point: The Internet–based-targeted psychological interventions fail to reduce fear of recurrence among early breast cancer survivors.
Major finding: The Fear of Cancer Recurrence Inventory (FCRI) scores significantly decreased at 8 weeks from baseline in all groups (P less than .001). The magnitude of reduction in FCRI scores was similar in cognitive-behavioral interventions and attention controls.
Study details: The randomized controlled FoRtitude study of breast cancer survivors who completed primary treatment. The survivors were randomly assigned to 4 Internet-based interventions or controls. The 4 interventions given for 4 weeks consisted of 3 cognitive behavioral interventions (relaxation, cognitive restructuring, and worry practice vs. attention controls) and telecoaching (motivational interviewing to improve adherence vs. no telecoaching).
Disclosures: This work was supported by the National Cancer Institute at the National Institutes of Health and the ECOG-ACRIN Medical Research Foundation. The authors did not disclose any conflict of interest.
Source: Wagner LI. J Natl Cancer Inst. 2021 May 31. doi: 10.1093/jnci/djab100.






