User login
PCI after TAVR mostly succeeds, some risks identified
Coronary angiography and percutaneous coronary interventions (PCI) can be performed successfully after transcatheter aortic valve replacement in most cases, according to data drawn from an international registry that has collected more than 400 such cases.
Overall, reaccess coronary angiography was successful in about 99% of cases with type of prosthesis identified as the most important variable in predicting success, according to a multicenter investigating team led by Won-Keun Kim, MD, director of structural heart disease, Kerckhoff Heart Center, Bad Nauheim, Germany.
By type of prosthesis, Dr. Kim was referring to long versus short stent-frame prostheses (SFP). In the case of angiography of the right coronary artery, for example, success was achieved in 99.6% of those with a short SFP and 95.9% of those with a long SFP (P = .005).
The study was published online in JACC: Cardiovascular Interventions.
Based on these and previous data, “prosthetic choice will be the main decisive factor that affects coronary reaccess, and this decision is in the hands of the TAVR operator,” said Dr. Kim in an interview.
This does not preclude use of a long SFP in TAVR. For patients with increased likelihood of eventually requiring a coronary intervention after TAVR, such as those undergoing the procedure at a relatively young age, a short device appears to be preferable, but Dr. Kim emphasized that it is not the only consideration.
When performing TAVR, “the highest priority is to accomplish a safe procedure with a good immediate outcome,” he said, pointing out that angiographic reaccess and PCI are successfully achieved in most patients whether fitted with a short or long SFP.
“If for any reason I assume that the immediate outcome [after TAVR] might be better using a long SFP, I would not hesitate to use a long SFP,” said Dr. Kim, giving such examples as a need for resheathing or precise positioning.
Coronary reaccess has low relative priority
“Coronary reaccess is an important issue and there is an increasing awareness of this, but it has a lower priority” than optimizing TAVR success,” Dr. Kim explained.
The analysis of coronary angiographic reaccess was based on 449 TAVR patients from 25 sites who required reaccess angiography. The indication in most cases was an acute coronary syndrome, mostly non–ST-elevation myocardial infarction (STEMI, 79%). Of the remaining patients, about half had STEMIs and half had other acute cardiovascular situations. The median time interval from TAVR to need for coronary angiography was 311 days.
In all but 2.7%, diagnostic catheterization was performed initially. It was successful in 98.3% of the procedures in the right coronary artery, 99.3% of the left coronary artery, and 97.3% overall.
Of the 60% who underwent PCI, 9% were considered unsuccessful. The reasons included lack of reflow in eight cases and coronary access issues in six cases. A variety of other issues accounted for the remaining seven cases.
Technical success was achieved in 91.4% of native arteries. In the six cases in which engagement of the culprit vessel with a guiding catheter failed, three were converted to urgent coronary bypass grafting and three died in the hospital. Neither selective versus unselective guiding-catheter engagement nor long versus short SFP related to PCI success, but PCI was performed less commonly in the native coronary arteries of TAVR patients with a long rather than short SFP (49% vs. 57%).
The 30-day all-cause mortality in this series was 12.2%. The independent predictors were a history of diabetes and the occurrence of cardiogenic shock. In the PCI subgroup, these factors plus PCI success predicted 30-day mortality.
Strategies to improve reaccess not resolved
When performing TAVR, other factors that might influence subsequent PCI success includes commissural alignment and positioning, according to Dr. Kim. But he cautioned that there are a number of potential controversies when weighing how to improve chances of post-TAVR angiographic reaccess without compromising the success of valve replacement.
“Lower positioning facilitates coronary access, but unfortunately will increase rates of conduction disturbances,” he noted.
Overall, one of the main messages from this analysis is that “the fear of impaired coronary access [after TAVR] may well be disproportionate to the reality,” according to Neal S. Kleiman, MD, an interventional cardiologist at Houston Methodist DeBakey Heart and Vascular Center. Dr. Kleiman wrote an editorial on the registry findings in the same issue of JACC: Cardiovascular Interventions).
Yet, he agreed that the issue of angiographic reaccess after TAVR cannot be ignored. Although reaccess after TAVR has so far been “surprisingly rare,” Dr. Kleiman expects cases to increase as more younger patients undergo TAVR. He suggested that interventionalists will need consider this issue when performing TAVR, a point he reemphasized in an interview.
“It is still a concern when recommending TAVR to a patient and still poses challenges to device manufacturers,” said Dr. Kleiman, suggesting that “a new set of skills” will be required to perform TAVR that will optimize subsequent angiographic access and PCI.
Dr. Kim agreed. Ultimately, other challenges, such as PCI performed after TAVR-in-TAVR placement, are likely to further complicate this issue, but he, too, is looking to new devices to minimize the problems.
“It would be desirable to modify the design, especially of long SFPs, to improve access for PCI, and there are ongoing efforts of the manufacturers to achieve this,” Dr. Kim said.
Dr. Kim reported financial relationships with Abbot, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Lifesciences. Dr. Kleiman reported financial relationships with Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic.
Coronary angiography and percutaneous coronary interventions (PCI) can be performed successfully after transcatheter aortic valve replacement in most cases, according to data drawn from an international registry that has collected more than 400 such cases.
Overall, reaccess coronary angiography was successful in about 99% of cases with type of prosthesis identified as the most important variable in predicting success, according to a multicenter investigating team led by Won-Keun Kim, MD, director of structural heart disease, Kerckhoff Heart Center, Bad Nauheim, Germany.
By type of prosthesis, Dr. Kim was referring to long versus short stent-frame prostheses (SFP). In the case of angiography of the right coronary artery, for example, success was achieved in 99.6% of those with a short SFP and 95.9% of those with a long SFP (P = .005).
The study was published online in JACC: Cardiovascular Interventions.
Based on these and previous data, “prosthetic choice will be the main decisive factor that affects coronary reaccess, and this decision is in the hands of the TAVR operator,” said Dr. Kim in an interview.
This does not preclude use of a long SFP in TAVR. For patients with increased likelihood of eventually requiring a coronary intervention after TAVR, such as those undergoing the procedure at a relatively young age, a short device appears to be preferable, but Dr. Kim emphasized that it is not the only consideration.
When performing TAVR, “the highest priority is to accomplish a safe procedure with a good immediate outcome,” he said, pointing out that angiographic reaccess and PCI are successfully achieved in most patients whether fitted with a short or long SFP.
“If for any reason I assume that the immediate outcome [after TAVR] might be better using a long SFP, I would not hesitate to use a long SFP,” said Dr. Kim, giving such examples as a need for resheathing or precise positioning.
Coronary reaccess has low relative priority
“Coronary reaccess is an important issue and there is an increasing awareness of this, but it has a lower priority” than optimizing TAVR success,” Dr. Kim explained.
The analysis of coronary angiographic reaccess was based on 449 TAVR patients from 25 sites who required reaccess angiography. The indication in most cases was an acute coronary syndrome, mostly non–ST-elevation myocardial infarction (STEMI, 79%). Of the remaining patients, about half had STEMIs and half had other acute cardiovascular situations. The median time interval from TAVR to need for coronary angiography was 311 days.
In all but 2.7%, diagnostic catheterization was performed initially. It was successful in 98.3% of the procedures in the right coronary artery, 99.3% of the left coronary artery, and 97.3% overall.
Of the 60% who underwent PCI, 9% were considered unsuccessful. The reasons included lack of reflow in eight cases and coronary access issues in six cases. A variety of other issues accounted for the remaining seven cases.
Technical success was achieved in 91.4% of native arteries. In the six cases in which engagement of the culprit vessel with a guiding catheter failed, three were converted to urgent coronary bypass grafting and three died in the hospital. Neither selective versus unselective guiding-catheter engagement nor long versus short SFP related to PCI success, but PCI was performed less commonly in the native coronary arteries of TAVR patients with a long rather than short SFP (49% vs. 57%).
The 30-day all-cause mortality in this series was 12.2%. The independent predictors were a history of diabetes and the occurrence of cardiogenic shock. In the PCI subgroup, these factors plus PCI success predicted 30-day mortality.
Strategies to improve reaccess not resolved
When performing TAVR, other factors that might influence subsequent PCI success includes commissural alignment and positioning, according to Dr. Kim. But he cautioned that there are a number of potential controversies when weighing how to improve chances of post-TAVR angiographic reaccess without compromising the success of valve replacement.
“Lower positioning facilitates coronary access, but unfortunately will increase rates of conduction disturbances,” he noted.
Overall, one of the main messages from this analysis is that “the fear of impaired coronary access [after TAVR] may well be disproportionate to the reality,” according to Neal S. Kleiman, MD, an interventional cardiologist at Houston Methodist DeBakey Heart and Vascular Center. Dr. Kleiman wrote an editorial on the registry findings in the same issue of JACC: Cardiovascular Interventions).
Yet, he agreed that the issue of angiographic reaccess after TAVR cannot be ignored. Although reaccess after TAVR has so far been “surprisingly rare,” Dr. Kleiman expects cases to increase as more younger patients undergo TAVR. He suggested that interventionalists will need consider this issue when performing TAVR, a point he reemphasized in an interview.
“It is still a concern when recommending TAVR to a patient and still poses challenges to device manufacturers,” said Dr. Kleiman, suggesting that “a new set of skills” will be required to perform TAVR that will optimize subsequent angiographic access and PCI.
Dr. Kim agreed. Ultimately, other challenges, such as PCI performed after TAVR-in-TAVR placement, are likely to further complicate this issue, but he, too, is looking to new devices to minimize the problems.
“It would be desirable to modify the design, especially of long SFPs, to improve access for PCI, and there are ongoing efforts of the manufacturers to achieve this,” Dr. Kim said.
Dr. Kim reported financial relationships with Abbot, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Lifesciences. Dr. Kleiman reported financial relationships with Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic.
Coronary angiography and percutaneous coronary interventions (PCI) can be performed successfully after transcatheter aortic valve replacement in most cases, according to data drawn from an international registry that has collected more than 400 such cases.
Overall, reaccess coronary angiography was successful in about 99% of cases with type of prosthesis identified as the most important variable in predicting success, according to a multicenter investigating team led by Won-Keun Kim, MD, director of structural heart disease, Kerckhoff Heart Center, Bad Nauheim, Germany.
By type of prosthesis, Dr. Kim was referring to long versus short stent-frame prostheses (SFP). In the case of angiography of the right coronary artery, for example, success was achieved in 99.6% of those with a short SFP and 95.9% of those with a long SFP (P = .005).
The study was published online in JACC: Cardiovascular Interventions.
Based on these and previous data, “prosthetic choice will be the main decisive factor that affects coronary reaccess, and this decision is in the hands of the TAVR operator,” said Dr. Kim in an interview.
This does not preclude use of a long SFP in TAVR. For patients with increased likelihood of eventually requiring a coronary intervention after TAVR, such as those undergoing the procedure at a relatively young age, a short device appears to be preferable, but Dr. Kim emphasized that it is not the only consideration.
When performing TAVR, “the highest priority is to accomplish a safe procedure with a good immediate outcome,” he said, pointing out that angiographic reaccess and PCI are successfully achieved in most patients whether fitted with a short or long SFP.
“If for any reason I assume that the immediate outcome [after TAVR] might be better using a long SFP, I would not hesitate to use a long SFP,” said Dr. Kim, giving such examples as a need for resheathing or precise positioning.
Coronary reaccess has low relative priority
“Coronary reaccess is an important issue and there is an increasing awareness of this, but it has a lower priority” than optimizing TAVR success,” Dr. Kim explained.
The analysis of coronary angiographic reaccess was based on 449 TAVR patients from 25 sites who required reaccess angiography. The indication in most cases was an acute coronary syndrome, mostly non–ST-elevation myocardial infarction (STEMI, 79%). Of the remaining patients, about half had STEMIs and half had other acute cardiovascular situations. The median time interval from TAVR to need for coronary angiography was 311 days.
In all but 2.7%, diagnostic catheterization was performed initially. It was successful in 98.3% of the procedures in the right coronary artery, 99.3% of the left coronary artery, and 97.3% overall.
Of the 60% who underwent PCI, 9% were considered unsuccessful. The reasons included lack of reflow in eight cases and coronary access issues in six cases. A variety of other issues accounted for the remaining seven cases.
Technical success was achieved in 91.4% of native arteries. In the six cases in which engagement of the culprit vessel with a guiding catheter failed, three were converted to urgent coronary bypass grafting and three died in the hospital. Neither selective versus unselective guiding-catheter engagement nor long versus short SFP related to PCI success, but PCI was performed less commonly in the native coronary arteries of TAVR patients with a long rather than short SFP (49% vs. 57%).
The 30-day all-cause mortality in this series was 12.2%. The independent predictors were a history of diabetes and the occurrence of cardiogenic shock. In the PCI subgroup, these factors plus PCI success predicted 30-day mortality.
Strategies to improve reaccess not resolved
When performing TAVR, other factors that might influence subsequent PCI success includes commissural alignment and positioning, according to Dr. Kim. But he cautioned that there are a number of potential controversies when weighing how to improve chances of post-TAVR angiographic reaccess without compromising the success of valve replacement.
“Lower positioning facilitates coronary access, but unfortunately will increase rates of conduction disturbances,” he noted.
Overall, one of the main messages from this analysis is that “the fear of impaired coronary access [after TAVR] may well be disproportionate to the reality,” according to Neal S. Kleiman, MD, an interventional cardiologist at Houston Methodist DeBakey Heart and Vascular Center. Dr. Kleiman wrote an editorial on the registry findings in the same issue of JACC: Cardiovascular Interventions).
Yet, he agreed that the issue of angiographic reaccess after TAVR cannot be ignored. Although reaccess after TAVR has so far been “surprisingly rare,” Dr. Kleiman expects cases to increase as more younger patients undergo TAVR. He suggested that interventionalists will need consider this issue when performing TAVR, a point he reemphasized in an interview.
“It is still a concern when recommending TAVR to a patient and still poses challenges to device manufacturers,” said Dr. Kleiman, suggesting that “a new set of skills” will be required to perform TAVR that will optimize subsequent angiographic access and PCI.
Dr. Kim agreed. Ultimately, other challenges, such as PCI performed after TAVR-in-TAVR placement, are likely to further complicate this issue, but he, too, is looking to new devices to minimize the problems.
“It would be desirable to modify the design, especially of long SFPs, to improve access for PCI, and there are ongoing efforts of the manufacturers to achieve this,” Dr. Kim said.
Dr. Kim reported financial relationships with Abbot, Boston Scientific, Edwards Lifesciences, Medtronic, and Meril Lifesciences. Dr. Kleiman reported financial relationships with Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Pharmacologic and electrical cardioversion of acute Afib reduces hospital admissions
Background: Atrial fibrillation (Afib) is the most common arrhythmia requiring treatment in the ED. There is a paucity of literature regarding the management of acute (onset < 48 h) atrial fibrillation in this setting and no conclusive evidence exists regarding the superiority of pharmacologic vs. electrical cardioversion.
Study design: Multicenter, single-blind, randomized, placebo-controlled trial.
Setting: 11 Canadian academic medical centers.
Synopsis: In this trial of 396 patients with acute Afib, half were randomly assigned to pharmacologic cardioversion with procainamide infusion (followed by DC cardioversion, if unsuccessful), while half were given a placebo infusion then DC cardioversion. The primary outcome was conversion to sinus rhythm, with maintenance of sinus rhythm at 30 minutes. A secondary protocol evaluated the difference in efficacy between anterolateral (AL) and anteroposterior (AP) pad placement
The “drug-shock” group achieved and maintained sinus rhythm in 96% of cases, compared to 92% in the “placebo-shock” group (statistically insignificant difference). The procainamide infusion alone achieved and maintained sinus rhythm in 52% of recipients, who thereby avoided the need for procedural sedation and monitoring. Notably, only 2% of patients in the study required admission to the hospital. Pad placement was equally efficacious in the AL or AP positions. The most common adverse event observed was transient hypotension during infusion of procainamide. No strokes were observed in either arm. Follow-up ECGs obtained 14 days later showed that 95% of patients remained in sinus rhythm.
Bottom line: Pharmacologic cardioversion with procainamide infusion and/or electrical cardioversion is a safe and efficacious initial management strategy for acute atrial fibrillation, and all but eliminates the need for hospital admission.
Citation: Stiell IG et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomized trial. Lancet. 2020 Feb 1;395(10221):339-49.
Dr. Lawson is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Atrial fibrillation (Afib) is the most common arrhythmia requiring treatment in the ED. There is a paucity of literature regarding the management of acute (onset < 48 h) atrial fibrillation in this setting and no conclusive evidence exists regarding the superiority of pharmacologic vs. electrical cardioversion.
Study design: Multicenter, single-blind, randomized, placebo-controlled trial.
Setting: 11 Canadian academic medical centers.
Synopsis: In this trial of 396 patients with acute Afib, half were randomly assigned to pharmacologic cardioversion with procainamide infusion (followed by DC cardioversion, if unsuccessful), while half were given a placebo infusion then DC cardioversion. The primary outcome was conversion to sinus rhythm, with maintenance of sinus rhythm at 30 minutes. A secondary protocol evaluated the difference in efficacy between anterolateral (AL) and anteroposterior (AP) pad placement
The “drug-shock” group achieved and maintained sinus rhythm in 96% of cases, compared to 92% in the “placebo-shock” group (statistically insignificant difference). The procainamide infusion alone achieved and maintained sinus rhythm in 52% of recipients, who thereby avoided the need for procedural sedation and monitoring. Notably, only 2% of patients in the study required admission to the hospital. Pad placement was equally efficacious in the AL or AP positions. The most common adverse event observed was transient hypotension during infusion of procainamide. No strokes were observed in either arm. Follow-up ECGs obtained 14 days later showed that 95% of patients remained in sinus rhythm.
Bottom line: Pharmacologic cardioversion with procainamide infusion and/or electrical cardioversion is a safe and efficacious initial management strategy for acute atrial fibrillation, and all but eliminates the need for hospital admission.
Citation: Stiell IG et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomized trial. Lancet. 2020 Feb 1;395(10221):339-49.
Dr. Lawson is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Atrial fibrillation (Afib) is the most common arrhythmia requiring treatment in the ED. There is a paucity of literature regarding the management of acute (onset < 48 h) atrial fibrillation in this setting and no conclusive evidence exists regarding the superiority of pharmacologic vs. electrical cardioversion.
Study design: Multicenter, single-blind, randomized, placebo-controlled trial.
Setting: 11 Canadian academic medical centers.
Synopsis: In this trial of 396 patients with acute Afib, half were randomly assigned to pharmacologic cardioversion with procainamide infusion (followed by DC cardioversion, if unsuccessful), while half were given a placebo infusion then DC cardioversion. The primary outcome was conversion to sinus rhythm, with maintenance of sinus rhythm at 30 minutes. A secondary protocol evaluated the difference in efficacy between anterolateral (AL) and anteroposterior (AP) pad placement
The “drug-shock” group achieved and maintained sinus rhythm in 96% of cases, compared to 92% in the “placebo-shock” group (statistically insignificant difference). The procainamide infusion alone achieved and maintained sinus rhythm in 52% of recipients, who thereby avoided the need for procedural sedation and monitoring. Notably, only 2% of patients in the study required admission to the hospital. Pad placement was equally efficacious in the AL or AP positions. The most common adverse event observed was transient hypotension during infusion of procainamide. No strokes were observed in either arm. Follow-up ECGs obtained 14 days later showed that 95% of patients remained in sinus rhythm.
Bottom line: Pharmacologic cardioversion with procainamide infusion and/or electrical cardioversion is a safe and efficacious initial management strategy for acute atrial fibrillation, and all but eliminates the need for hospital admission.
Citation: Stiell IG et al. Electrical versus pharmacological cardioversion for emergency department patients with acute atrial fibrillation (RAFF2): a partial factorial randomized trial. Lancet. 2020 Feb 1;395(10221):339-49.
Dr. Lawson is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Animal-assisted therapy could boost patients’ mental health
For me, vacation planning brings with it a bit of anxiety and stress – particularly as we navigate the many uncertainties around COVID-19.
Not only must my husband and I think about our own safety, we also have to make sure that our beloved dog, Samson, gets the proper care while we are away.
My husband adopted Samson, an 11-year-old mixed-breed rescue, when he was just a year old. He’s an important part of our family.
So, when booking our hotel room and flights, we also had to find someone we trust to care for Samson in our absence. Family members are not always an option, so we often rely on pet-sitting apps. We looked through profile after profile, contacted sitters, and interrogated them as if we were looking for care for a tiny human.
Eventually, we found a service that allows owners to use a mobile app that provides updates about how their pets are faring. While we were away, the sitter sent daily photos and videos of Samson that put our minds at ease.
As a registered nurse who works in an ICU, my own anxiety about leaving Samson reminded me about my patients’ reservations about leaving their pets during hospitalizations. Many of them share the same kinds of anxieties when they are separated from their beloved pets. Hospital visits are rarely planned. I have cared for patients who expressed concerns about their pets being home alone and needing to coordinate pet care. In some cases – to alleviate those patients’ anxieties – I have helped them contact friends and family members to assist with care.
Pets’ popularity grows in U.S.
According to the 2019-2020 National Pet Owners Survey, about 67% of U.S. households own a pet – which translates to about 84.9 million homes. During the height of COVID, Americans also acquired a greater number of smaller pets.1 In addition, when social restrictions increased, the demand for dog adoptions and the desire to serve as foster owners rose significantly.2 Last Chance Animal Rescue of Waldorf, Md., reportedly saw the adoption of dogs rise from 30%-40% in 2020. Another animal rescue operation, Lucky Dog, of Arlington, Va., in 2020 helped about 3,385 pets find adoption, up from about 1,800 in 2019.3 About two-thirds of all American households and roughly half of elderly individuals own pets.4
I am not surprised by those numbers. In my nursing practice, I face many stress-related factors, such as alternating day and night shifts, 12-hour shifts, strenuous physical work, and the psychological strain of attending to ill and dying patients. Interacting with Samson helps relieve that stress. The motion of petting Samson helps calm my heart rate and decreases my anxiety. In addition, Samson makes me smile – and excites almost all the people I interact with while he’s around. Of course, I’m not objective, but I view Samson’s impact on people as a symbol of the power of animal-assisted therapy (AAT).
AAT, defined as “the positive interaction between an animal and a patient within a therapeutic framework,”has proven to be an effective intervention for adults with intellectual disabilities who experience anxiety in an observational study.5 The intervention also has helped reduce cortisol levels in a study of nurses in physical medicine, internal medicine, and long-term care.6 Since most patient hospital stays are unplanned, there is a need to introduce AAT into hospital care. This would lessen anxiety in patients concerning their pets’ welfare.
We know that long-term hospital stays often cause adverse psychosocial effects on patients. Such stays can result in “hospitalization syndrome,” which is characterized by a gradual loss of cognition and orientation, an unwillingness to maintain contact with others or to engage in group therapy, and a loss of interest in their surroundings.7 The common causes for this syndrome are infection, medication, isolation, response to surgery, and dehydration. A consequence can be a permanent change in cognitive function or psychological impairment. However, my experience of practicing nursing for years has led me to discover that pets as an external stimulus can prevent the syndrome’s onset. This is because a large percentage of hospitalized patients have pets, and contact with a pet reminds them of home and the memories they share at home.
Introducing animal therapy into health care facilities could boost patients’ mental health – and ease their anxiety – by acting as a bridge between their present circumstances and the lives they have outside the establishment.
References
1. American Pet Owners Association. Will the COVID Pet Spike Last? State of the industry presentation. 2021 Mar 24.
2. Morgan L et al. Humanit Soc Sci Comm. 2020 Nov 24;7(144). doi: 10.1057/S41599-020-00649-x.
3. Hedgpeth D. So many pets have been adopted during the pandemic that shelters are running out. Washington Post. 2021 Jan 6.
4. Cherniack EP and Cherniack AR. Curr Gerontol Geriatr Res. 2014. doi: 10.1155/2014/623203.
5. Giuliani F and Jacquemettaz M. Eur J Integ Med. 2017 Sep;14;13-9.
6. Machová K et al. Int J Environ Res and Public Health. 2019 Oct;16(19):3670.
7. Machová K et al. Int J Environ Res Public Health. 2012 Apr;16(8):1362.
Ms. Scott is a registered nurse specializing in critical care and also has experience in nursing leadership. She has 8 years of experience in cardiothoracic ICUs. Ms. Scott received a bachelor of science in nursing degree from Queens University of Charlotte (N.C.), and a master of business administration in health care administration from the University of North Alabama, Florence. She has no conflicts of interest.
For me, vacation planning brings with it a bit of anxiety and stress – particularly as we navigate the many uncertainties around COVID-19.
Not only must my husband and I think about our own safety, we also have to make sure that our beloved dog, Samson, gets the proper care while we are away.
My husband adopted Samson, an 11-year-old mixed-breed rescue, when he was just a year old. He’s an important part of our family.
So, when booking our hotel room and flights, we also had to find someone we trust to care for Samson in our absence. Family members are not always an option, so we often rely on pet-sitting apps. We looked through profile after profile, contacted sitters, and interrogated them as if we were looking for care for a tiny human.
Eventually, we found a service that allows owners to use a mobile app that provides updates about how their pets are faring. While we were away, the sitter sent daily photos and videos of Samson that put our minds at ease.
As a registered nurse who works in an ICU, my own anxiety about leaving Samson reminded me about my patients’ reservations about leaving their pets during hospitalizations. Many of them share the same kinds of anxieties when they are separated from their beloved pets. Hospital visits are rarely planned. I have cared for patients who expressed concerns about their pets being home alone and needing to coordinate pet care. In some cases – to alleviate those patients’ anxieties – I have helped them contact friends and family members to assist with care.
Pets’ popularity grows in U.S.
According to the 2019-2020 National Pet Owners Survey, about 67% of U.S. households own a pet – which translates to about 84.9 million homes. During the height of COVID, Americans also acquired a greater number of smaller pets.1 In addition, when social restrictions increased, the demand for dog adoptions and the desire to serve as foster owners rose significantly.2 Last Chance Animal Rescue of Waldorf, Md., reportedly saw the adoption of dogs rise from 30%-40% in 2020. Another animal rescue operation, Lucky Dog, of Arlington, Va., in 2020 helped about 3,385 pets find adoption, up from about 1,800 in 2019.3 About two-thirds of all American households and roughly half of elderly individuals own pets.4
I am not surprised by those numbers. In my nursing practice, I face many stress-related factors, such as alternating day and night shifts, 12-hour shifts, strenuous physical work, and the psychological strain of attending to ill and dying patients. Interacting with Samson helps relieve that stress. The motion of petting Samson helps calm my heart rate and decreases my anxiety. In addition, Samson makes me smile – and excites almost all the people I interact with while he’s around. Of course, I’m not objective, but I view Samson’s impact on people as a symbol of the power of animal-assisted therapy (AAT).
AAT, defined as “the positive interaction between an animal and a patient within a therapeutic framework,”has proven to be an effective intervention for adults with intellectual disabilities who experience anxiety in an observational study.5 The intervention also has helped reduce cortisol levels in a study of nurses in physical medicine, internal medicine, and long-term care.6 Since most patient hospital stays are unplanned, there is a need to introduce AAT into hospital care. This would lessen anxiety in patients concerning their pets’ welfare.
We know that long-term hospital stays often cause adverse psychosocial effects on patients. Such stays can result in “hospitalization syndrome,” which is characterized by a gradual loss of cognition and orientation, an unwillingness to maintain contact with others or to engage in group therapy, and a loss of interest in their surroundings.7 The common causes for this syndrome are infection, medication, isolation, response to surgery, and dehydration. A consequence can be a permanent change in cognitive function or psychological impairment. However, my experience of practicing nursing for years has led me to discover that pets as an external stimulus can prevent the syndrome’s onset. This is because a large percentage of hospitalized patients have pets, and contact with a pet reminds them of home and the memories they share at home.
Introducing animal therapy into health care facilities could boost patients’ mental health – and ease their anxiety – by acting as a bridge between their present circumstances and the lives they have outside the establishment.
References
1. American Pet Owners Association. Will the COVID Pet Spike Last? State of the industry presentation. 2021 Mar 24.
2. Morgan L et al. Humanit Soc Sci Comm. 2020 Nov 24;7(144). doi: 10.1057/S41599-020-00649-x.
3. Hedgpeth D. So many pets have been adopted during the pandemic that shelters are running out. Washington Post. 2021 Jan 6.
4. Cherniack EP and Cherniack AR. Curr Gerontol Geriatr Res. 2014. doi: 10.1155/2014/623203.
5. Giuliani F and Jacquemettaz M. Eur J Integ Med. 2017 Sep;14;13-9.
6. Machová K et al. Int J Environ Res and Public Health. 2019 Oct;16(19):3670.
7. Machová K et al. Int J Environ Res Public Health. 2012 Apr;16(8):1362.
Ms. Scott is a registered nurse specializing in critical care and also has experience in nursing leadership. She has 8 years of experience in cardiothoracic ICUs. Ms. Scott received a bachelor of science in nursing degree from Queens University of Charlotte (N.C.), and a master of business administration in health care administration from the University of North Alabama, Florence. She has no conflicts of interest.
For me, vacation planning brings with it a bit of anxiety and stress – particularly as we navigate the many uncertainties around COVID-19.
Not only must my husband and I think about our own safety, we also have to make sure that our beloved dog, Samson, gets the proper care while we are away.
My husband adopted Samson, an 11-year-old mixed-breed rescue, when he was just a year old. He’s an important part of our family.
So, when booking our hotel room and flights, we also had to find someone we trust to care for Samson in our absence. Family members are not always an option, so we often rely on pet-sitting apps. We looked through profile after profile, contacted sitters, and interrogated them as if we were looking for care for a tiny human.
Eventually, we found a service that allows owners to use a mobile app that provides updates about how their pets are faring. While we were away, the sitter sent daily photos and videos of Samson that put our minds at ease.
As a registered nurse who works in an ICU, my own anxiety about leaving Samson reminded me about my patients’ reservations about leaving their pets during hospitalizations. Many of them share the same kinds of anxieties when they are separated from their beloved pets. Hospital visits are rarely planned. I have cared for patients who expressed concerns about their pets being home alone and needing to coordinate pet care. In some cases – to alleviate those patients’ anxieties – I have helped them contact friends and family members to assist with care.
Pets’ popularity grows in U.S.
According to the 2019-2020 National Pet Owners Survey, about 67% of U.S. households own a pet – which translates to about 84.9 million homes. During the height of COVID, Americans also acquired a greater number of smaller pets.1 In addition, when social restrictions increased, the demand for dog adoptions and the desire to serve as foster owners rose significantly.2 Last Chance Animal Rescue of Waldorf, Md., reportedly saw the adoption of dogs rise from 30%-40% in 2020. Another animal rescue operation, Lucky Dog, of Arlington, Va., in 2020 helped about 3,385 pets find adoption, up from about 1,800 in 2019.3 About two-thirds of all American households and roughly half of elderly individuals own pets.4
I am not surprised by those numbers. In my nursing practice, I face many stress-related factors, such as alternating day and night shifts, 12-hour shifts, strenuous physical work, and the psychological strain of attending to ill and dying patients. Interacting with Samson helps relieve that stress. The motion of petting Samson helps calm my heart rate and decreases my anxiety. In addition, Samson makes me smile – and excites almost all the people I interact with while he’s around. Of course, I’m not objective, but I view Samson’s impact on people as a symbol of the power of animal-assisted therapy (AAT).
AAT, defined as “the positive interaction between an animal and a patient within a therapeutic framework,”has proven to be an effective intervention for adults with intellectual disabilities who experience anxiety in an observational study.5 The intervention also has helped reduce cortisol levels in a study of nurses in physical medicine, internal medicine, and long-term care.6 Since most patient hospital stays are unplanned, there is a need to introduce AAT into hospital care. This would lessen anxiety in patients concerning their pets’ welfare.
We know that long-term hospital stays often cause adverse psychosocial effects on patients. Such stays can result in “hospitalization syndrome,” which is characterized by a gradual loss of cognition and orientation, an unwillingness to maintain contact with others or to engage in group therapy, and a loss of interest in their surroundings.7 The common causes for this syndrome are infection, medication, isolation, response to surgery, and dehydration. A consequence can be a permanent change in cognitive function or psychological impairment. However, my experience of practicing nursing for years has led me to discover that pets as an external stimulus can prevent the syndrome’s onset. This is because a large percentage of hospitalized patients have pets, and contact with a pet reminds them of home and the memories they share at home.
Introducing animal therapy into health care facilities could boost patients’ mental health – and ease their anxiety – by acting as a bridge between their present circumstances and the lives they have outside the establishment.
References
1. American Pet Owners Association. Will the COVID Pet Spike Last? State of the industry presentation. 2021 Mar 24.
2. Morgan L et al. Humanit Soc Sci Comm. 2020 Nov 24;7(144). doi: 10.1057/S41599-020-00649-x.
3. Hedgpeth D. So many pets have been adopted during the pandemic that shelters are running out. Washington Post. 2021 Jan 6.
4. Cherniack EP and Cherniack AR. Curr Gerontol Geriatr Res. 2014. doi: 10.1155/2014/623203.
5. Giuliani F and Jacquemettaz M. Eur J Integ Med. 2017 Sep;14;13-9.
6. Machová K et al. Int J Environ Res and Public Health. 2019 Oct;16(19):3670.
7. Machová K et al. Int J Environ Res Public Health. 2012 Apr;16(8):1362.
Ms. Scott is a registered nurse specializing in critical care and also has experience in nursing leadership. She has 8 years of experience in cardiothoracic ICUs. Ms. Scott received a bachelor of science in nursing degree from Queens University of Charlotte (N.C.), and a master of business administration in health care administration from the University of North Alabama, Florence. She has no conflicts of interest.
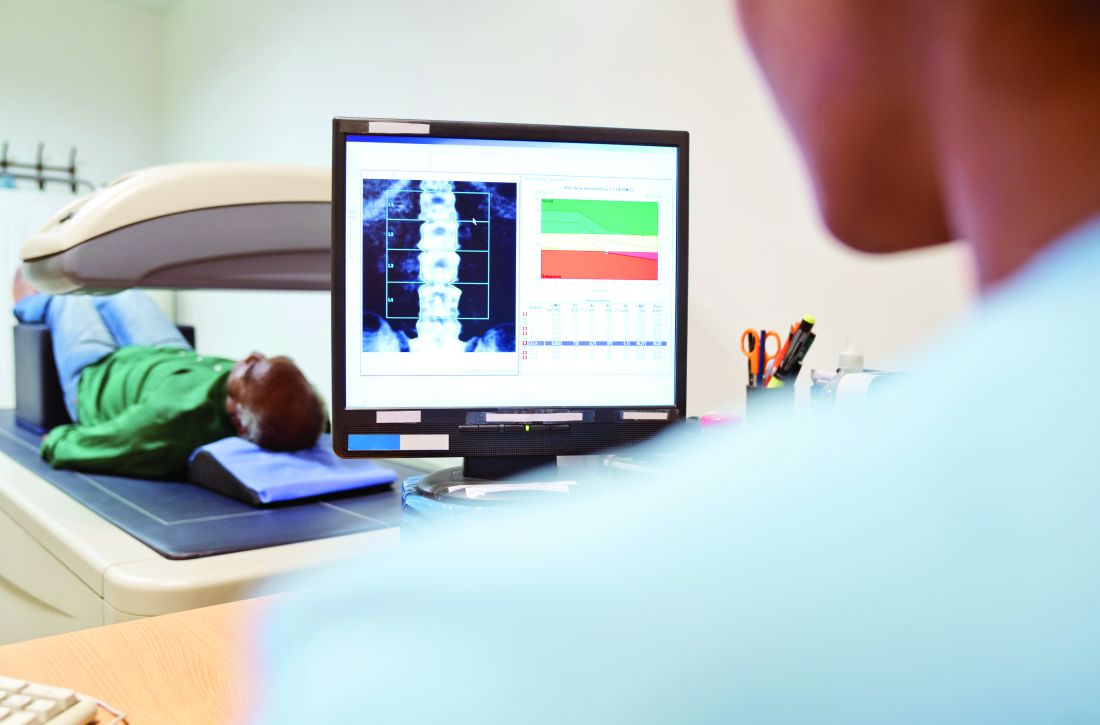
Vertebral fractures still a risk with low-dose oral glucocorticoids for RA
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
Patients with rheumatoid arthritis currently being treated with low doses of oral glucocorticoids (GCs) had a 59% increased risk of sustaining a vertebral fracture when compared with past users, results of a retrospective cohort study have shown.
Although the overall risk of an osteoporotic fracture was not increased when comparing current and past GC users, with a hazard ratio of 1.14 (95% confidence interval, 0.98-1.33), the HR for sustaining a spinal fracture was 1.59 (95% CI, 1.11-2.29).
“Clinicians should be aware that, even in RA patients who receive low daily glucocorticoid doses, the risk of clinical vertebral fracture is increased,” Shahab Abtahi, MD, of Maastricht (the Netherlands) University and coauthors reported in Rheumatology.
This is important considering around a quarter of RA patients are treated with GCs in the United Kingdom in accordance with European recommendations, they observed.
Conflicting randomized and observational findings on whether or not osteoporotic fractures might be linked to the use of low-dose GCs prompted Dr. Abtahi and associates to see if there were any signals in real-world data. To do so, they used data one of the world’s largest primary care databases – the Clinical Practice Research Datalink (CPRD), which consists of anonymized patient data from a network of primary care practices across the United Kingdom.
Altogether, the records of more than 15,000 patients with RA aged 50 years and older who were held in the CRPD between 1997 and 2017 were pulled for analysis, and just half (n = 7,039) were receiving or had received GC therapy. Low-dose GC therapy was defined as a prednisolone equivalent dose (PED) of 7.5 mg or less per day.
The use of low-dose GCs use during three key time periods was considered: within the past 6 months (current users), within the past 7-12 months (recent users), and within the past year (past users).
The analyses involved time-dependent Cox proportional-hazards models to look for associations between GC use and all types of osteoporotic fracture, including the risk for incident hip, vertebral, humeral, forearm, pelvis, and rib fractures. They were adjusted for various lifestyle parameters, comorbidities, and the use of other medications.
“Current GC use was further broken down into subcategories based on average daily and cumulative dose,” Dr. Abtahi observed. As might be expected, doses even lower than 7.5 mg or less PED did not increase the chance of any osteoporotic fracture but there was an increased risk for some types with higher average daily doses, notably at the hip and pelvis, as well as the spine.
“Low-dose oral GC therapy was associated with an increased risk of clinical vertebral fracture, while the risk of other individual OP fracture sites was not increased,” said the team, adding that the main results remained unchanged regardless of short- or long-term use.
“We know that vertebral fracture risk is markedly increased in RA, and it is well known that GC therapy in particular affects trabecular bone, which is abundantly present in lumbar vertebrae,” Dr. Abtahi wrote.
“Therefore, we can hypothesize that the beneficial effect of low-dose GC therapy on suppressing the background inflammation of RA could probably be enough to offset its negative effect on bone synthesis in most fracture sites but not in vertebrae,” they suggested.
One of the limitations of the study is that the researchers lacked data on the disease activity of the patients or if they were being treated with biologic therapy. This means that confounding by disease severity might be an issue with only those with higher disease activity being treated with GCs and thus were at higher risk for fractures.
“Another limitation was a potential misclassification of exposure with oral GCs, as we had only prescribing information from CPRD, which is roughly two steps behind actual drug use by patients,” the researchers conceded. The average duration of GC use was estimated at 3.7 years, which is an indication of actual use.
A detection bias may also be involved with regard to vertebral fractures, with complaints of back pain maybe being discussed more often when prescribing GCs, leading to more referrals for possible fracture assessment.
Dr. Abtahi and a fellow coauthor disclosed receiving research and other funding from several pharmaceutical companies unrelated to this study. All other coauthors had no conflicts of interest.
FROM RHEUMATOLOGY
Cycling linked to longer life in people with type 2 diabetes
Bicycle riding may help people with diabetes live longer, new research suggests.
Among more than 7,000 adults with diabetes in 10 Western European countries followed for about 15 years, those who cycled regularly were significantly less likely to die of any cause or of cardiovascular causes, even after accounting for differences in factors such as sex, age, educational level, diet, comorbidities, and other physical activities.
“The association between cycling and all-cause and CVD [cardiovascular disease] mortality in this study of person[s] with diabetes was of the same magnitude and direction as observed in the healthy population,” wrote Mathias Ried-Larsen, PhD, of the Centre for Physical Activity Research, Rigshospitalet, Copenhagen, and colleagues. The findings were published online July 19, 2021, in JAMA Internal Medicine.
In an accompanying Editor’s Note, JAMA Internal Medicine editor Rita F. Redberg, MD, and two deputy editors said that the new data add to previous studies showing benefits of cycling, compared with other physical activities. “The analysis from Ried-Larsen and colleagues strengthens the epidemiologic data on cycling and strongly suggests that it may contribute directly to longer and healthier lives,” they wrote.
Dr. Redberg, of the University of California, San Francisco, told this news organization: “I think the number of cyclists grew greatly during pandemic, when there was little auto traffic, and people did not want to take public transportation. Cities that add bike lanes, especially protected bike lanes, see an increase in cyclists. I think Americans can cycle more, would enjoy cycling more, and would live longer [by] cycling, to work and for pleasure.”
Dr. Redberg disclosed that she is “an avid cyclist and am currently on a bike ride in Glacier National Park. ... This group [Climate Ride] raises money for more bike lanes, promotes climate change awareness, has paid for solar panels at Glacier, and more.”
However, Dr. Redberg and colleagues also “recognize that cycling requires fitness, a good sense of balance, and the means to purchase a bicycle. We also understand that regular cycling requires living in an area where it is reasonably safe, and we celebrate the installation of more bike lanes, particularly protected lanes, in many cities around the world.”
But, despite the limitations of an observational study and possible selection bias of people who are able to cycle, “it is important to share this evidence for the potentially large health benefits of cycling, which almost surely generalize to persons without diabetes.”
Cycling tied to lower all-cause and CVD mortality
The prospective cohort study included 7,459 adults with diabetes from the European Prospective Investigation into Cancer and Nutrition. All were assessed during 1992-1998 and again in 1996-2011, with a mean follow-up of roughly 15 years. During that time, there were 1,673 deaths from all causes, with 811 attributed to CVD.
Compared with no cycling, those who reported any cycling had a 24% lower risk of death from any cause over a 5-year period, after adjustment for confounders and for other physical activity. The greatest risk reduction was seen in those who reported cycling between 150-299 minutes per week, particularly in CVD mortality.
In a subanalysis of 5,423 individuals with 10.7 years of follow-up, there were 975 all-cause deaths and 429 from CVD. Individuals who began or continued cycling during follow-up experienced reductions of about 35% for both all-cause and CVD mortality, compared with those who never cycled.
Dr. Redberg and colleagues added that “there are environmental benefits to increasing the use of cycling for commuting and other transport because cycling helps to decrease the adverse environmental and health effects of automobile exhaust.”
They concluded: “As avid and/or aspiring cyclists ourselves, we are sold on the mental and physical benefits of getting to work and seeing the world on two wheels, self-propelled, and think it is well worth a try.”
The study work was supported by the Health Research Fund of Instituto de Salud Carlos III; the Spanish regional governments of Andalucía, Asturias, Basque Country, Murcia, and Navarra; and the Catalan Institute of Oncology. The Centre for Physical Activity Research is supported by a grant from TrygFonden. Dr. Ried-Larsen reported personal fees from Novo Nordisk. Dr. Redberg reported receiving grants from Arnold Ventures; the Greenwall Foundation; and the National Heart, Lung, and Blood Institute.
Bicycle riding may help people with diabetes live longer, new research suggests.
Among more than 7,000 adults with diabetes in 10 Western European countries followed for about 15 years, those who cycled regularly were significantly less likely to die of any cause or of cardiovascular causes, even after accounting for differences in factors such as sex, age, educational level, diet, comorbidities, and other physical activities.
“The association between cycling and all-cause and CVD [cardiovascular disease] mortality in this study of person[s] with diabetes was of the same magnitude and direction as observed in the healthy population,” wrote Mathias Ried-Larsen, PhD, of the Centre for Physical Activity Research, Rigshospitalet, Copenhagen, and colleagues. The findings were published online July 19, 2021, in JAMA Internal Medicine.
In an accompanying Editor’s Note, JAMA Internal Medicine editor Rita F. Redberg, MD, and two deputy editors said that the new data add to previous studies showing benefits of cycling, compared with other physical activities. “The analysis from Ried-Larsen and colleagues strengthens the epidemiologic data on cycling and strongly suggests that it may contribute directly to longer and healthier lives,” they wrote.
Dr. Redberg, of the University of California, San Francisco, told this news organization: “I think the number of cyclists grew greatly during pandemic, when there was little auto traffic, and people did not want to take public transportation. Cities that add bike lanes, especially protected bike lanes, see an increase in cyclists. I think Americans can cycle more, would enjoy cycling more, and would live longer [by] cycling, to work and for pleasure.”
Dr. Redberg disclosed that she is “an avid cyclist and am currently on a bike ride in Glacier National Park. ... This group [Climate Ride] raises money for more bike lanes, promotes climate change awareness, has paid for solar panels at Glacier, and more.”
However, Dr. Redberg and colleagues also “recognize that cycling requires fitness, a good sense of balance, and the means to purchase a bicycle. We also understand that regular cycling requires living in an area where it is reasonably safe, and we celebrate the installation of more bike lanes, particularly protected lanes, in many cities around the world.”
But, despite the limitations of an observational study and possible selection bias of people who are able to cycle, “it is important to share this evidence for the potentially large health benefits of cycling, which almost surely generalize to persons without diabetes.”
Cycling tied to lower all-cause and CVD mortality
The prospective cohort study included 7,459 adults with diabetes from the European Prospective Investigation into Cancer and Nutrition. All were assessed during 1992-1998 and again in 1996-2011, with a mean follow-up of roughly 15 years. During that time, there were 1,673 deaths from all causes, with 811 attributed to CVD.
Compared with no cycling, those who reported any cycling had a 24% lower risk of death from any cause over a 5-year period, after adjustment for confounders and for other physical activity. The greatest risk reduction was seen in those who reported cycling between 150-299 minutes per week, particularly in CVD mortality.
In a subanalysis of 5,423 individuals with 10.7 years of follow-up, there were 975 all-cause deaths and 429 from CVD. Individuals who began or continued cycling during follow-up experienced reductions of about 35% for both all-cause and CVD mortality, compared with those who never cycled.
Dr. Redberg and colleagues added that “there are environmental benefits to increasing the use of cycling for commuting and other transport because cycling helps to decrease the adverse environmental and health effects of automobile exhaust.”
They concluded: “As avid and/or aspiring cyclists ourselves, we are sold on the mental and physical benefits of getting to work and seeing the world on two wheels, self-propelled, and think it is well worth a try.”
The study work was supported by the Health Research Fund of Instituto de Salud Carlos III; the Spanish regional governments of Andalucía, Asturias, Basque Country, Murcia, and Navarra; and the Catalan Institute of Oncology. The Centre for Physical Activity Research is supported by a grant from TrygFonden. Dr. Ried-Larsen reported personal fees from Novo Nordisk. Dr. Redberg reported receiving grants from Arnold Ventures; the Greenwall Foundation; and the National Heart, Lung, and Blood Institute.
Bicycle riding may help people with diabetes live longer, new research suggests.
Among more than 7,000 adults with diabetes in 10 Western European countries followed for about 15 years, those who cycled regularly were significantly less likely to die of any cause or of cardiovascular causes, even after accounting for differences in factors such as sex, age, educational level, diet, comorbidities, and other physical activities.
“The association between cycling and all-cause and CVD [cardiovascular disease] mortality in this study of person[s] with diabetes was of the same magnitude and direction as observed in the healthy population,” wrote Mathias Ried-Larsen, PhD, of the Centre for Physical Activity Research, Rigshospitalet, Copenhagen, and colleagues. The findings were published online July 19, 2021, in JAMA Internal Medicine.
In an accompanying Editor’s Note, JAMA Internal Medicine editor Rita F. Redberg, MD, and two deputy editors said that the new data add to previous studies showing benefits of cycling, compared with other physical activities. “The analysis from Ried-Larsen and colleagues strengthens the epidemiologic data on cycling and strongly suggests that it may contribute directly to longer and healthier lives,” they wrote.
Dr. Redberg, of the University of California, San Francisco, told this news organization: “I think the number of cyclists grew greatly during pandemic, when there was little auto traffic, and people did not want to take public transportation. Cities that add bike lanes, especially protected bike lanes, see an increase in cyclists. I think Americans can cycle more, would enjoy cycling more, and would live longer [by] cycling, to work and for pleasure.”
Dr. Redberg disclosed that she is “an avid cyclist and am currently on a bike ride in Glacier National Park. ... This group [Climate Ride] raises money for more bike lanes, promotes climate change awareness, has paid for solar panels at Glacier, and more.”
However, Dr. Redberg and colleagues also “recognize that cycling requires fitness, a good sense of balance, and the means to purchase a bicycle. We also understand that regular cycling requires living in an area where it is reasonably safe, and we celebrate the installation of more bike lanes, particularly protected lanes, in many cities around the world.”
But, despite the limitations of an observational study and possible selection bias of people who are able to cycle, “it is important to share this evidence for the potentially large health benefits of cycling, which almost surely generalize to persons without diabetes.”
Cycling tied to lower all-cause and CVD mortality
The prospective cohort study included 7,459 adults with diabetes from the European Prospective Investigation into Cancer and Nutrition. All were assessed during 1992-1998 and again in 1996-2011, with a mean follow-up of roughly 15 years. During that time, there were 1,673 deaths from all causes, with 811 attributed to CVD.
Compared with no cycling, those who reported any cycling had a 24% lower risk of death from any cause over a 5-year period, after adjustment for confounders and for other physical activity. The greatest risk reduction was seen in those who reported cycling between 150-299 minutes per week, particularly in CVD mortality.
In a subanalysis of 5,423 individuals with 10.7 years of follow-up, there were 975 all-cause deaths and 429 from CVD. Individuals who began or continued cycling during follow-up experienced reductions of about 35% for both all-cause and CVD mortality, compared with those who never cycled.
Dr. Redberg and colleagues added that “there are environmental benefits to increasing the use of cycling for commuting and other transport because cycling helps to decrease the adverse environmental and health effects of automobile exhaust.”
They concluded: “As avid and/or aspiring cyclists ourselves, we are sold on the mental and physical benefits of getting to work and seeing the world on two wheels, self-propelled, and think it is well worth a try.”
The study work was supported by the Health Research Fund of Instituto de Salud Carlos III; the Spanish regional governments of Andalucía, Asturias, Basque Country, Murcia, and Navarra; and the Catalan Institute of Oncology. The Centre for Physical Activity Research is supported by a grant from TrygFonden. Dr. Ried-Larsen reported personal fees from Novo Nordisk. Dr. Redberg reported receiving grants from Arnold Ventures; the Greenwall Foundation; and the National Heart, Lung, and Blood Institute.
FROM JAMA INTERNAL MEDICINE
Long COVID seen in patients with severe and mild disease
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.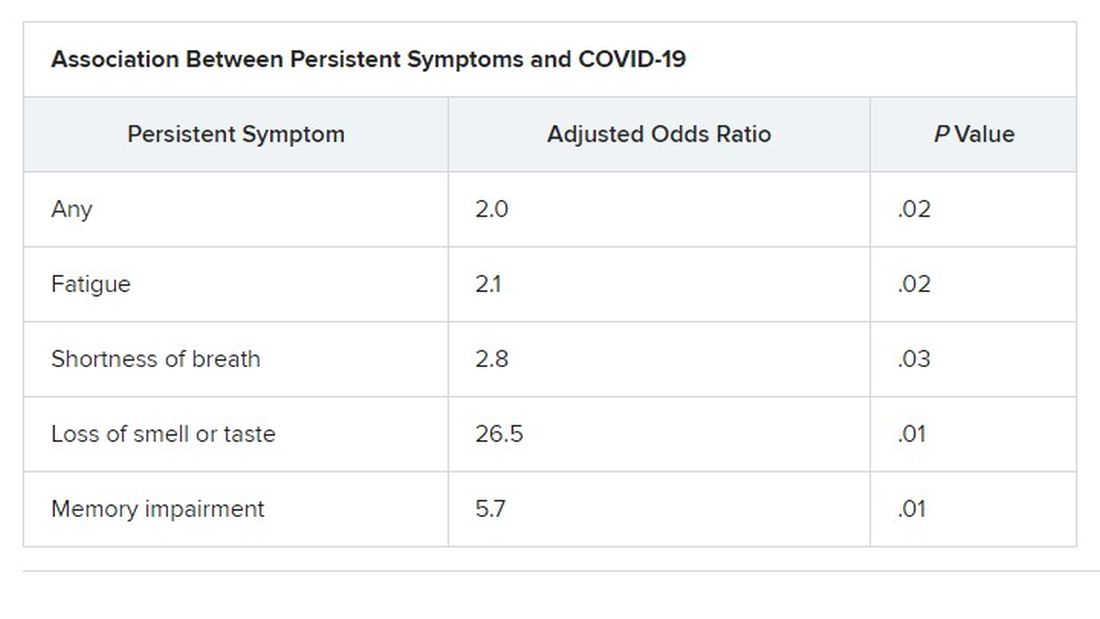
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Findings from the cohort, composed of 113 COVID-19 survivors who developed ARDS after admission to a single center before to April 16, 2020, were presented online at the 31st European Congress of Clinical Microbiology & Infectious Diseases by Judit Aranda, MD, from Complex Hospitalari Moisés Broggi in Barcelona.
Median age of the participants was 64 years, and 70% were male. At least one persistent symptom was experienced during follow-up by 81% of the cohort, with 45% reporting shortness of breath, 50% reporting muscle pain, 43% reporting memory impairment, and 46% reporting physical weakness of at least 5 on a 10-point scale.
Of the 104 participants who completed a 6-minute walk test, 30% had a decrease in oxygen saturation level of at least 4%, and 5% had an initial or final level below 88%. Of the 46 participants who underwent a pulmonary function test, 15% had a forced expiratory volume in 1 second below 70%.
And of the 49% of participants with pathologic findings on chest x-ray, most were bilateral interstitial infiltrates (88%).
In addition, more than 90% of participants developed depression, anxiety, or PTSD, Dr. Aranda reported.
Not the whole picture
This study shows that sicker people – “those in intensive care units with acute respiratory distress syndrome” – are “more likely to be struggling with more severe symptoms,” said Christopher Terndrup, MD, from the division of general internal medicine and geriatrics at Oregon Health & Science University, Portland.
But a Swiss study, also presented at the meeting, “shows how even mild COVID cases can lead to debilitating symptoms,” Dr. Terndrup said in an interview.
The investigation of long-term COVID symptoms in outpatients was presented online by Florian Desgranges, MD, from Lausanne (Switzerland) University Hospital. He and his colleagues found that more than half of those with a mild to moderate disease had persistent symptoms at least 3 months after diagnosis.
The prevalence of long COVID has varied in previous research, from 15% in a study of health care workers, to 46% in a study of patients with mild COVID, 52% in a study of young COVID outpatients, and 76% in a study of patients hospitalized with COVID.
Dr. Desgranges and colleagues evaluated patients seen in an ED or outpatient clinic from February to April 2020.
The 418 patients with a confirmed COVID-19 diagnosis were compared with a control group of 89 patients who presented to the same centers during the same time frame with similar symptoms – cough, shortness of breath, or fever – but had a negative SARS-CoV-2 test.
The number of patients with comorbidities was similar in the COVID and control groups (34% vs. 36%), as was median age (41 vs. 36 years) and the prevalence of women (62% vs 64%), but the proportion of health care workers was lower in the COVID group (64% vs 82%; P =.006).
Symptoms that persisted for at least 3 months were more common in the COVID than in the control group (53% vs. 37%). And patients in the COVID group reported more symptoms than those in the control group after adjustment for age, gender, smoking status, comorbidities, and timing of the survey phone call.
Levels of sleeping problems and headache were similar in the two groups.
“We have to remember that with COVID-19 came the psychosocial changes of the pandemic situation” Dr. Desgranges said.
This study suggests that some long-COVID symptoms – such as the fatigue, headache, and sleep disorders reported in the control group – could be related to the pandemic itself, which has caused psychosocial distress, Dr. Terndrup said.
Another study that looked at outpatients “has some fantastic long-term follow-up data, and shows that many patients are still engaging in rehabilitation programs nearly a year after their diagnosis,” he explained.
The COVID HOME study
That prospective longitudinal COVID HOME study, which assessed long-term symptoms in people who were never hospitalized for COVID, was presented online by Adriana Tami, MD, PhD, from the University Medical Center Groningen (the Netherlands).
The researchers visited the homes of patients to collect data, blood samples, and perform polymerase chain reaction (PCR) testing 1, 2, and 3 weeks after a diagnosis of COVID-19. If their PCR test was still positive, testing continued until week 6 or a negative test. In addition, participants completed questionnaires at week 2 and at months 3, 6 and 12 to assess fatigue, quality of life, and symptoms of depression and anxiety.
Three-month follow-up data were available for 134 of the 276 people initially enrolled in the study. Questionnaires were completed by 85 participants at 3 months, 62 participants at 6 months, and 10 participants at 12 months.
At least 40% of participants reported long-lasting symptoms at some point during follow-up, and at least 30% said they didn’t feel fully recovered at 12 months. The most common symptom was persistent fatigue, reported at 3, 6, and 12 months by at least 44% of participants. Other common symptoms – reported by at least 20% of respondents at 3, 6, and 12 months – were headache, mental or neurologic symptoms, and sleep disorders, shortness of breath, lack of smell or taste, and severe fatigue.
“We have a high proportion of nonhospitalized individuals who suffer from long COVID after more than 12 months,” Dr. Tami concluded, adding that the study is ongoing. “We have other variables that we want to look at, including duration viral shedding and serological results and variants.”
“These cohort studies are very helpful, but they can lead to inaccurate conclusions,” Dr. Terndrup cautioned.
They only provide pieces of the big picture, but they “do add to a growing body of knowledge about a significant portion of COVID patients still struggling with symptoms long after their initial infection. The symptoms can be quite variable but are dominated by both physical and mental fatigue, and tend to be worse in patients who were sicker at initial infection,” he said in an interview.
As a whole, these studies reinforce the need for treatment programs to help patients who suffer from long COVID, he added, but “I advise caution to folks suffering out there who seek ‘miracle cures’; across the world, we are collaborating to find solutions that are safe and effective.”
We are in desperate need of an equity lens in these studies.
“There is still a great deal to learn about long COVID,” said Dr. Terndrup. Data on underrepresented populations – such as Black, Indigenous, and people of color – are lacking from these and others studies, he explained. “We are in desperate need of an equity lens in these studies,” particularly in the United States, where there are “significant disparities” in the treatment of different populations.
However, “I do hope that this work can lead to a better understanding of how other viral infections can cause long-lasting symptoms,” said Dr. Terndrup.
“We have long proposed that after acute presentation, some microbes can cause chronic symptoms, like fatigue and widespread pain. Perhaps we can learn how to better care for these patients after learning from COVID’s significant impact on our societies across the globe.”
Dr. Aranda and Dr. Desgranges have disclosed no relevant financial relationships or study funding. The study by Dr. Tami’s team was funded by the University Medical Center Groningen Organization for Health Research and Development, and Connecting European Cohorts to Increase Common and Effective Response to SARS-CoV-2 Pandemic. Dr. Terndrup disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN CONGRESS OF CLINICAL MICROBIOLOGY & INFECTIOUS DISEASES
Socioeconomic disparities persist in hysterectomy access
Black women undergoing hysterectomies were significantly more likely to be treated by low-volume surgeons than high-volume surgeons, and to experience perioperative complications as a result, based on data from more than 300,000 patients.
“Outcomes for hysterectomy, for both benign and malignant disease, are improved when the procedure is performed at high-volume hospitals and by high-volume surgeons,” Anne Knisely, MD, of Columbia University, New York, and colleagues wrote.
Historically, Black patients have been less likely to be referred to high-volume hospitals, the researchers noted. Recent efforts to regionalize surgical procedures to high-volume hospitals aim to reduce disparities and improve care for all patients, but the data on disparities in care within high-volume hospitals are limited, they said.
In a study published in Obstetrics & Gynecology, the researchers identified 300,586 women who underwent hysterectomy in New York state between 2000 and 2014. The researchers divided surgeons at these hospitals into volume groups based on average annual hysterectomy volume.
The women were treated by 5,505 surgeons at 59 hospitals. Overall, Black women comprised significantly more of the patients treated by low-volume surgeons compared with high-volume surgeons (19.4% vs. 14.3%; adjusted odds ratio, 1.26), and more women treated by low-volume surgeons had Medicare insurance compared with those treated by high-volume surgeons (20.6% vs. 14.5%; aOR, 1.22).
A majority of the patients (262,005 patients) were treated by a total of 1,377 high-volume surgeons, while 2,105 low-volume surgeons treated 2,900 patients. Abdominal hysterectomies accounted for 57.5% of the procedures, followed by laparoscopic (23.9%), vaginal (13.2%), and robotic assisted (5.3%). Approximately two-thirds (64.4%) of the patients were aged 40-59 years; 63.7% were White, 15.1% were Black, and 8.5% were Hispanic.
The overall complication rate was significantly higher in patients treated by low-volume surgeons, compared with high-volume surgeons (31.0% vs. 10.3%), including intraoperative complications, surgical-site complications, medical complications, and transfusions. The perioperative mortality rate also was significantly higher for patients of low-volume surgeons compared with high-volume surgeons (2.2% vs. 0.2%).
Low-volume surgeons were more likely to perform urgent or emergent procedures, compared with high-volume surgeons (26.1% vs 6.4%), and to perform abdominal hysterectomy versus minimally invasive hysterectomy compared with high-volume surgeons (77.8% vs. 54.7%), the researchers added.
The study findings were limited by several factors, including the observational design and possible undercoding of outcomes, inclusion only of New York state patients, lack of data on clinical characteristics such as surgical history and complexity, lack of data on surgeon characteristics, and changing practice patterns over time, the researchers noted.
However, “this study demonstrates increased perioperative morbidity and mortality for patients who underwent hysterectomy by low-volume surgeons, in comparison with high-volume surgeons, at high-volume hospitals,” and that Black patients were more likely to be treated by low-volume surgeons, they said. “Although centralization of complex surgical care to higher-volume hospitals may have benefit, there are additional surgeon-level factors that must be considered to address disparities in access to high-quality care for patients undergoing hysterectomy.”
Explore range of issues to improve access
“It is always beneficial to review morbidity and mortality statistics,” Constance Bohon, MD, a gynecologist in private practice in Washington, D.C., said in an interview. “With a heightened awareness of equity and equality, now is a good time to review the data with that focus in mind. Hospital committees review the data on a regular basis, but they may not have looked closely at demographics in the past.
“It was always my understanding that for many procedures, including surgery, volume impacts outcome, so the finding that low-volume surgeons had worse outcomes than high-volume surgeons was not particularly surprising,” said Dr. Bohon. However, the question of how hospitals might address disparities in access to high-volume surgeons “is a difficult question, because there are a variety of issues that may not be caused by disparities,” she added. “It may be that the high-volume surgeons do not take Medicare. It may be that some of the emergent/urgent surgeries come from patients seen in the ED and the high-volume surgeons may not take call or see new patients in the ED. There may be a difference in the preop testing done that may be more extensive with the high-volume surgeons as compared with the low-volume surgeons. It may be that it is easier to get an appointment with a low-volume rather than a high-volume surgeon.
“Additional research is needed to determine whether there is an algorithm that can be created to determine risk for morbidity or mortality based on factors such as the number of years in practice, the number of hysterectomies per year, and the age of the physician,” Dr. Bohon explained. “The patient data could include preexisting risk factors such as weight, preexisting medical conditions, prior surgeries, and current medications, along with demographics. It would be interesting to determine whether low-risk patients have similar outcomes with low- as compared with high-volume surgeons while high-risk patients do not. The demographics could then be evaluated to determine if disparities exist for both low- and high-risk patients.”
The study received no outside funding. One coauthor disclosed serving as a consultant for Clovis Oncology, receiving research funding from Merck, and receiving royalties from UpToDate. Lead author Dr. Knisely had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose, but serves on the Ob.Gyn. News editorial advisory board.
Black women undergoing hysterectomies were significantly more likely to be treated by low-volume surgeons than high-volume surgeons, and to experience perioperative complications as a result, based on data from more than 300,000 patients.
“Outcomes for hysterectomy, for both benign and malignant disease, are improved when the procedure is performed at high-volume hospitals and by high-volume surgeons,” Anne Knisely, MD, of Columbia University, New York, and colleagues wrote.
Historically, Black patients have been less likely to be referred to high-volume hospitals, the researchers noted. Recent efforts to regionalize surgical procedures to high-volume hospitals aim to reduce disparities and improve care for all patients, but the data on disparities in care within high-volume hospitals are limited, they said.
In a study published in Obstetrics & Gynecology, the researchers identified 300,586 women who underwent hysterectomy in New York state between 2000 and 2014. The researchers divided surgeons at these hospitals into volume groups based on average annual hysterectomy volume.
The women were treated by 5,505 surgeons at 59 hospitals. Overall, Black women comprised significantly more of the patients treated by low-volume surgeons compared with high-volume surgeons (19.4% vs. 14.3%; adjusted odds ratio, 1.26), and more women treated by low-volume surgeons had Medicare insurance compared with those treated by high-volume surgeons (20.6% vs. 14.5%; aOR, 1.22).
A majority of the patients (262,005 patients) were treated by a total of 1,377 high-volume surgeons, while 2,105 low-volume surgeons treated 2,900 patients. Abdominal hysterectomies accounted for 57.5% of the procedures, followed by laparoscopic (23.9%), vaginal (13.2%), and robotic assisted (5.3%). Approximately two-thirds (64.4%) of the patients were aged 40-59 years; 63.7% were White, 15.1% were Black, and 8.5% were Hispanic.
The overall complication rate was significantly higher in patients treated by low-volume surgeons, compared with high-volume surgeons (31.0% vs. 10.3%), including intraoperative complications, surgical-site complications, medical complications, and transfusions. The perioperative mortality rate also was significantly higher for patients of low-volume surgeons compared with high-volume surgeons (2.2% vs. 0.2%).
Low-volume surgeons were more likely to perform urgent or emergent procedures, compared with high-volume surgeons (26.1% vs 6.4%), and to perform abdominal hysterectomy versus minimally invasive hysterectomy compared with high-volume surgeons (77.8% vs. 54.7%), the researchers added.
The study findings were limited by several factors, including the observational design and possible undercoding of outcomes, inclusion only of New York state patients, lack of data on clinical characteristics such as surgical history and complexity, lack of data on surgeon characteristics, and changing practice patterns over time, the researchers noted.
However, “this study demonstrates increased perioperative morbidity and mortality for patients who underwent hysterectomy by low-volume surgeons, in comparison with high-volume surgeons, at high-volume hospitals,” and that Black patients were more likely to be treated by low-volume surgeons, they said. “Although centralization of complex surgical care to higher-volume hospitals may have benefit, there are additional surgeon-level factors that must be considered to address disparities in access to high-quality care for patients undergoing hysterectomy.”
Explore range of issues to improve access
“It is always beneficial to review morbidity and mortality statistics,” Constance Bohon, MD, a gynecologist in private practice in Washington, D.C., said in an interview. “With a heightened awareness of equity and equality, now is a good time to review the data with that focus in mind. Hospital committees review the data on a regular basis, but they may not have looked closely at demographics in the past.
“It was always my understanding that for many procedures, including surgery, volume impacts outcome, so the finding that low-volume surgeons had worse outcomes than high-volume surgeons was not particularly surprising,” said Dr. Bohon. However, the question of how hospitals might address disparities in access to high-volume surgeons “is a difficult question, because there are a variety of issues that may not be caused by disparities,” she added. “It may be that the high-volume surgeons do not take Medicare. It may be that some of the emergent/urgent surgeries come from patients seen in the ED and the high-volume surgeons may not take call or see new patients in the ED. There may be a difference in the preop testing done that may be more extensive with the high-volume surgeons as compared with the low-volume surgeons. It may be that it is easier to get an appointment with a low-volume rather than a high-volume surgeon.
“Additional research is needed to determine whether there is an algorithm that can be created to determine risk for morbidity or mortality based on factors such as the number of years in practice, the number of hysterectomies per year, and the age of the physician,” Dr. Bohon explained. “The patient data could include preexisting risk factors such as weight, preexisting medical conditions, prior surgeries, and current medications, along with demographics. It would be interesting to determine whether low-risk patients have similar outcomes with low- as compared with high-volume surgeons while high-risk patients do not. The demographics could then be evaluated to determine if disparities exist for both low- and high-risk patients.”
The study received no outside funding. One coauthor disclosed serving as a consultant for Clovis Oncology, receiving research funding from Merck, and receiving royalties from UpToDate. Lead author Dr. Knisely had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose, but serves on the Ob.Gyn. News editorial advisory board.
Black women undergoing hysterectomies were significantly more likely to be treated by low-volume surgeons than high-volume surgeons, and to experience perioperative complications as a result, based on data from more than 300,000 patients.
“Outcomes for hysterectomy, for both benign and malignant disease, are improved when the procedure is performed at high-volume hospitals and by high-volume surgeons,” Anne Knisely, MD, of Columbia University, New York, and colleagues wrote.
Historically, Black patients have been less likely to be referred to high-volume hospitals, the researchers noted. Recent efforts to regionalize surgical procedures to high-volume hospitals aim to reduce disparities and improve care for all patients, but the data on disparities in care within high-volume hospitals are limited, they said.
In a study published in Obstetrics & Gynecology, the researchers identified 300,586 women who underwent hysterectomy in New York state between 2000 and 2014. The researchers divided surgeons at these hospitals into volume groups based on average annual hysterectomy volume.
The women were treated by 5,505 surgeons at 59 hospitals. Overall, Black women comprised significantly more of the patients treated by low-volume surgeons compared with high-volume surgeons (19.4% vs. 14.3%; adjusted odds ratio, 1.26), and more women treated by low-volume surgeons had Medicare insurance compared with those treated by high-volume surgeons (20.6% vs. 14.5%; aOR, 1.22).
A majority of the patients (262,005 patients) were treated by a total of 1,377 high-volume surgeons, while 2,105 low-volume surgeons treated 2,900 patients. Abdominal hysterectomies accounted for 57.5% of the procedures, followed by laparoscopic (23.9%), vaginal (13.2%), and robotic assisted (5.3%). Approximately two-thirds (64.4%) of the patients were aged 40-59 years; 63.7% were White, 15.1% were Black, and 8.5% were Hispanic.
The overall complication rate was significantly higher in patients treated by low-volume surgeons, compared with high-volume surgeons (31.0% vs. 10.3%), including intraoperative complications, surgical-site complications, medical complications, and transfusions. The perioperative mortality rate also was significantly higher for patients of low-volume surgeons compared with high-volume surgeons (2.2% vs. 0.2%).
Low-volume surgeons were more likely to perform urgent or emergent procedures, compared with high-volume surgeons (26.1% vs 6.4%), and to perform abdominal hysterectomy versus minimally invasive hysterectomy compared with high-volume surgeons (77.8% vs. 54.7%), the researchers added.
The study findings were limited by several factors, including the observational design and possible undercoding of outcomes, inclusion only of New York state patients, lack of data on clinical characteristics such as surgical history and complexity, lack of data on surgeon characteristics, and changing practice patterns over time, the researchers noted.
However, “this study demonstrates increased perioperative morbidity and mortality for patients who underwent hysterectomy by low-volume surgeons, in comparison with high-volume surgeons, at high-volume hospitals,” and that Black patients were more likely to be treated by low-volume surgeons, they said. “Although centralization of complex surgical care to higher-volume hospitals may have benefit, there are additional surgeon-level factors that must be considered to address disparities in access to high-quality care for patients undergoing hysterectomy.”
Explore range of issues to improve access
“It is always beneficial to review morbidity and mortality statistics,” Constance Bohon, MD, a gynecologist in private practice in Washington, D.C., said in an interview. “With a heightened awareness of equity and equality, now is a good time to review the data with that focus in mind. Hospital committees review the data on a regular basis, but they may not have looked closely at demographics in the past.
“It was always my understanding that for many procedures, including surgery, volume impacts outcome, so the finding that low-volume surgeons had worse outcomes than high-volume surgeons was not particularly surprising,” said Dr. Bohon. However, the question of how hospitals might address disparities in access to high-volume surgeons “is a difficult question, because there are a variety of issues that may not be caused by disparities,” she added. “It may be that the high-volume surgeons do not take Medicare. It may be that some of the emergent/urgent surgeries come from patients seen in the ED and the high-volume surgeons may not take call or see new patients in the ED. There may be a difference in the preop testing done that may be more extensive with the high-volume surgeons as compared with the low-volume surgeons. It may be that it is easier to get an appointment with a low-volume rather than a high-volume surgeon.
“Additional research is needed to determine whether there is an algorithm that can be created to determine risk for morbidity or mortality based on factors such as the number of years in practice, the number of hysterectomies per year, and the age of the physician,” Dr. Bohon explained. “The patient data could include preexisting risk factors such as weight, preexisting medical conditions, prior surgeries, and current medications, along with demographics. It would be interesting to determine whether low-risk patients have similar outcomes with low- as compared with high-volume surgeons while high-risk patients do not. The demographics could then be evaluated to determine if disparities exist for both low- and high-risk patients.”
The study received no outside funding. One coauthor disclosed serving as a consultant for Clovis Oncology, receiving research funding from Merck, and receiving royalties from UpToDate. Lead author Dr. Knisely had no financial conflicts to disclose. Dr. Bohon had no financial conflicts to disclose, but serves on the Ob.Gyn. News editorial advisory board.
FROM OBSTETRICS & GYNECOLOGY
Resistant TB: Adjustments to BPaL regimen reduce AEs, not efficacy
Lower doses of linezolid in the BPaL drug regimen (bedaquiline, pretomanid, and linezolid) significantly reduce the adverse events associated with the treatment for patients with highly drug-resistant tuberculosis (TB) without compromising its high efficacy, new research shows.
“The ZeNix trial shows that reduced doses and/or shorter durations of linezolid appear to have high efficacy and improved safety,” said first author Francesca Conradie, MB, BCh, of the clinical HIV research unit, faculty of health sciences, University of Witwatersrand, Johannesburg, South Africa, in presenting the findings at the virtual meeting of the International AIDS Society conference.
As recently reported in the pivotal Nix-TB trial, the BPaL regimen yielded a 90% treatment success rate among people with highly drug-resistant forms of TB.
However, a 6-month regimen that included linezolid 1,200 mg resulted in toxic effects: 81% of patients in the study experienced peripheral neuropathy, and myelosuppression occurred in 48%. These effects often led to dose reductions or treatment interruption.
Adjustments in the dose of linezolid in the new ZeNix trial substantially reduced peripheral neuropathy to 13% and myelosuppression to 7%, with no significant reduction in the treatment response.
Importantly, the results were similar among patients with and those without HIV. This is of note because TB is the leading cause of death among patients with HIV.
“In the ZeNix trial, only 20% of patients were HIV infected, but in the [previous] Nix-TB trial, 30% were infected, so we have experience now in about 70 patients who were infected, and the outcomes were no different,” Dr. Conradie said in an interview.
Experts say the findings represent an important turn in the steep challenge of tackling highly resistant TB.
“In our opinion, these are exciting results that could change treatment guidelines for highly drug-resistant tuberculosis, with real benefits for the patients,” said Hendrik Streeck, MD, International AIDS Society cochair and director of the Institute of Virology and the Institute for HIV Research at the University Bonn (Germany), in a press conference.
Payam Nahid, MD, MPH, director of the Center for Tuberculosis at theUniversity of California, San Francisco, agreed.
“The results of this trial will impact global practices in treating drug-resistant TB as well as the design and conduct of future TB clinical trials,” Dr. Nahid said in an interview.
ZeNix trial
The phase 3 ZeNix trial included 181 patients with highly resistant TB in South Africa, Russia, Georgia, and Moldova. The mean age of the patients was 37 years; 67.4% were men, 63.5% were White, and 19.9% were HIV positive.
All patients were treated for 6 months with bedaquiline 200 mg daily for 8 weeks followed by 100 mg daily for 18 weeks, as well as pretomanid 200 mg daily.
The patients were randomly assigned to receive one of four daily doses of linezolid: 1,200 mg for 6 months (the original dose from the Nix-TB trial; n = 45) or 2 months (n = 46), or 600 mg for 6 or 2 months (45 patients each).
Percentages of patients with HIV were equal among the four groups, at about 20% each.
The primary outcomes – resolution of clinical disease and a negative culture status after 6 months – were observed across all linezolid dose groups. The success rate was 93% for those receiving 1,200 mg for 6 months, 89% for those receiving 1,200 mg for 2 months, 91% for those receiving 600 mg for 6 months, and 84% for those receiving 600 mg for 2 months.
With regard to the key adverse events of peripheral neuropathy and myelosuppression, manifested as anemia, the highest rates were among those who received linezolid 1,200 mg for 6 month, at 38% and 22%, respectively, compared with 24% and 17.4% among those who received 1,200 mg for 2 months, 24% and 2% among those who received 600 mg for 6 months, and 13% and 6.7% among those who received 600 mg for 2 months.
Four cases of optic neuropathy occurred among those who received 1,200 mg for 6 months; all cases resolved.
Patients who received 1,200 mg for 6 months required the highest number of linezolid dose modifications; 51% required changes that included reduction, interruption, or discontinuation, compared with 28% among those who received 1,200 mg for 2 months and 13% each in the other two groups.
On the basis of these results, “my personal opinion is that 600 mg at 6 months [of linezolid] is most likely the best strategy for the treatment of this highly resistant treatment population group,” Dr. Conradie told this news organization.
Findings represent ‘great news’ in addressing concerns
Dr. Nahid further commented that the results are highly encouraging in light of the ongoing concerns about the effects of linezolid in the BPaL regimen.
“This is great news,” he said. “The ZeNix trial addresses a key concern that providers and patients have had regarding the safety and tolerability of taking 6 months of linezolid at 1200 mg/d as part of the BPaL regimen.
“The findings that doses lower and durations shorter than the current 1,200 mg linezolid daily for 6 months will significantly expand the usability of the BPaL regimen worldwide.”
The inclusion of patients with HIV was essential in the trial, he noted.
“There are drug-drug interactions to be considered, among other factors that impact drug exposure,” Dr. Nahid said.
“Inclusion of patients living with HIV in this study means that any modifications to the BPaL regimen considered by the WHO [World Health Organization] and other policy decision makers will include data from this key population,” he said. “Of course, more data are needed on safety, tolerability, and efficacy on BPaL in general, and there are international cohorts and demonstration projects underway that will enhance our understanding of the regimen in HIV and in other special populations.”
The authors, Dr. Streeck, and Dr. Nahid have disclosed no relevant financial relationships.
This article was updated 7/21/21.
Lower doses of linezolid in the BPaL drug regimen (bedaquiline, pretomanid, and linezolid) significantly reduce the adverse events associated with the treatment for patients with highly drug-resistant tuberculosis (TB) without compromising its high efficacy, new research shows.
“The ZeNix trial shows that reduced doses and/or shorter durations of linezolid appear to have high efficacy and improved safety,” said first author Francesca Conradie, MB, BCh, of the clinical HIV research unit, faculty of health sciences, University of Witwatersrand, Johannesburg, South Africa, in presenting the findings at the virtual meeting of the International AIDS Society conference.
As recently reported in the pivotal Nix-TB trial, the BPaL regimen yielded a 90% treatment success rate among people with highly drug-resistant forms of TB.
However, a 6-month regimen that included linezolid 1,200 mg resulted in toxic effects: 81% of patients in the study experienced peripheral neuropathy, and myelosuppression occurred in 48%. These effects often led to dose reductions or treatment interruption.
Adjustments in the dose of linezolid in the new ZeNix trial substantially reduced peripheral neuropathy to 13% and myelosuppression to 7%, with no significant reduction in the treatment response.
Importantly, the results were similar among patients with and those without HIV. This is of note because TB is the leading cause of death among patients with HIV.
“In the ZeNix trial, only 20% of patients were HIV infected, but in the [previous] Nix-TB trial, 30% were infected, so we have experience now in about 70 patients who were infected, and the outcomes were no different,” Dr. Conradie said in an interview.
Experts say the findings represent an important turn in the steep challenge of tackling highly resistant TB.
“In our opinion, these are exciting results that could change treatment guidelines for highly drug-resistant tuberculosis, with real benefits for the patients,” said Hendrik Streeck, MD, International AIDS Society cochair and director of the Institute of Virology and the Institute for HIV Research at the University Bonn (Germany), in a press conference.
Payam Nahid, MD, MPH, director of the Center for Tuberculosis at theUniversity of California, San Francisco, agreed.
“The results of this trial will impact global practices in treating drug-resistant TB as well as the design and conduct of future TB clinical trials,” Dr. Nahid said in an interview.
ZeNix trial
The phase 3 ZeNix trial included 181 patients with highly resistant TB in South Africa, Russia, Georgia, and Moldova. The mean age of the patients was 37 years; 67.4% were men, 63.5% were White, and 19.9% were HIV positive.
All patients were treated for 6 months with bedaquiline 200 mg daily for 8 weeks followed by 100 mg daily for 18 weeks, as well as pretomanid 200 mg daily.
The patients were randomly assigned to receive one of four daily doses of linezolid: 1,200 mg for 6 months (the original dose from the Nix-TB trial; n = 45) or 2 months (n = 46), or 600 mg for 6 or 2 months (45 patients each).
Percentages of patients with HIV were equal among the four groups, at about 20% each.
The primary outcomes – resolution of clinical disease and a negative culture status after 6 months – were observed across all linezolid dose groups. The success rate was 93% for those receiving 1,200 mg for 6 months, 89% for those receiving 1,200 mg for 2 months, 91% for those receiving 600 mg for 6 months, and 84% for those receiving 600 mg for 2 months.
With regard to the key adverse events of peripheral neuropathy and myelosuppression, manifested as anemia, the highest rates were among those who received linezolid 1,200 mg for 6 month, at 38% and 22%, respectively, compared with 24% and 17.4% among those who received 1,200 mg for 2 months, 24% and 2% among those who received 600 mg for 6 months, and 13% and 6.7% among those who received 600 mg for 2 months.
Four cases of optic neuropathy occurred among those who received 1,200 mg for 6 months; all cases resolved.
Patients who received 1,200 mg for 6 months required the highest number of linezolid dose modifications; 51% required changes that included reduction, interruption, or discontinuation, compared with 28% among those who received 1,200 mg for 2 months and 13% each in the other two groups.
On the basis of these results, “my personal opinion is that 600 mg at 6 months [of linezolid] is most likely the best strategy for the treatment of this highly resistant treatment population group,” Dr. Conradie told this news organization.
Findings represent ‘great news’ in addressing concerns
Dr. Nahid further commented that the results are highly encouraging in light of the ongoing concerns about the effects of linezolid in the BPaL regimen.
“This is great news,” he said. “The ZeNix trial addresses a key concern that providers and patients have had regarding the safety and tolerability of taking 6 months of linezolid at 1200 mg/d as part of the BPaL regimen.
“The findings that doses lower and durations shorter than the current 1,200 mg linezolid daily for 6 months will significantly expand the usability of the BPaL regimen worldwide.”
The inclusion of patients with HIV was essential in the trial, he noted.
“There are drug-drug interactions to be considered, among other factors that impact drug exposure,” Dr. Nahid said.
“Inclusion of patients living with HIV in this study means that any modifications to the BPaL regimen considered by the WHO [World Health Organization] and other policy decision makers will include data from this key population,” he said. “Of course, more data are needed on safety, tolerability, and efficacy on BPaL in general, and there are international cohorts and demonstration projects underway that will enhance our understanding of the regimen in HIV and in other special populations.”
The authors, Dr. Streeck, and Dr. Nahid have disclosed no relevant financial relationships.
This article was updated 7/21/21.
Lower doses of linezolid in the BPaL drug regimen (bedaquiline, pretomanid, and linezolid) significantly reduce the adverse events associated with the treatment for patients with highly drug-resistant tuberculosis (TB) without compromising its high efficacy, new research shows.
“The ZeNix trial shows that reduced doses and/or shorter durations of linezolid appear to have high efficacy and improved safety,” said first author Francesca Conradie, MB, BCh, of the clinical HIV research unit, faculty of health sciences, University of Witwatersrand, Johannesburg, South Africa, in presenting the findings at the virtual meeting of the International AIDS Society conference.
As recently reported in the pivotal Nix-TB trial, the BPaL regimen yielded a 90% treatment success rate among people with highly drug-resistant forms of TB.
However, a 6-month regimen that included linezolid 1,200 mg resulted in toxic effects: 81% of patients in the study experienced peripheral neuropathy, and myelosuppression occurred in 48%. These effects often led to dose reductions or treatment interruption.
Adjustments in the dose of linezolid in the new ZeNix trial substantially reduced peripheral neuropathy to 13% and myelosuppression to 7%, with no significant reduction in the treatment response.
Importantly, the results were similar among patients with and those without HIV. This is of note because TB is the leading cause of death among patients with HIV.
“In the ZeNix trial, only 20% of patients were HIV infected, but in the [previous] Nix-TB trial, 30% were infected, so we have experience now in about 70 patients who were infected, and the outcomes were no different,” Dr. Conradie said in an interview.
Experts say the findings represent an important turn in the steep challenge of tackling highly resistant TB.
“In our opinion, these are exciting results that could change treatment guidelines for highly drug-resistant tuberculosis, with real benefits for the patients,” said Hendrik Streeck, MD, International AIDS Society cochair and director of the Institute of Virology and the Institute for HIV Research at the University Bonn (Germany), in a press conference.
Payam Nahid, MD, MPH, director of the Center for Tuberculosis at theUniversity of California, San Francisco, agreed.
“The results of this trial will impact global practices in treating drug-resistant TB as well as the design and conduct of future TB clinical trials,” Dr. Nahid said in an interview.
ZeNix trial
The phase 3 ZeNix trial included 181 patients with highly resistant TB in South Africa, Russia, Georgia, and Moldova. The mean age of the patients was 37 years; 67.4% were men, 63.5% were White, and 19.9% were HIV positive.
All patients were treated for 6 months with bedaquiline 200 mg daily for 8 weeks followed by 100 mg daily for 18 weeks, as well as pretomanid 200 mg daily.
The patients were randomly assigned to receive one of four daily doses of linezolid: 1,200 mg for 6 months (the original dose from the Nix-TB trial; n = 45) or 2 months (n = 46), or 600 mg for 6 or 2 months (45 patients each).
Percentages of patients with HIV were equal among the four groups, at about 20% each.
The primary outcomes – resolution of clinical disease and a negative culture status after 6 months – were observed across all linezolid dose groups. The success rate was 93% for those receiving 1,200 mg for 6 months, 89% for those receiving 1,200 mg for 2 months, 91% for those receiving 600 mg for 6 months, and 84% for those receiving 600 mg for 2 months.
With regard to the key adverse events of peripheral neuropathy and myelosuppression, manifested as anemia, the highest rates were among those who received linezolid 1,200 mg for 6 month, at 38% and 22%, respectively, compared with 24% and 17.4% among those who received 1,200 mg for 2 months, 24% and 2% among those who received 600 mg for 6 months, and 13% and 6.7% among those who received 600 mg for 2 months.
Four cases of optic neuropathy occurred among those who received 1,200 mg for 6 months; all cases resolved.
Patients who received 1,200 mg for 6 months required the highest number of linezolid dose modifications; 51% required changes that included reduction, interruption, or discontinuation, compared with 28% among those who received 1,200 mg for 2 months and 13% each in the other two groups.
On the basis of these results, “my personal opinion is that 600 mg at 6 months [of linezolid] is most likely the best strategy for the treatment of this highly resistant treatment population group,” Dr. Conradie told this news organization.
Findings represent ‘great news’ in addressing concerns
Dr. Nahid further commented that the results are highly encouraging in light of the ongoing concerns about the effects of linezolid in the BPaL regimen.
“This is great news,” he said. “The ZeNix trial addresses a key concern that providers and patients have had regarding the safety and tolerability of taking 6 months of linezolid at 1200 mg/d as part of the BPaL regimen.
“The findings that doses lower and durations shorter than the current 1,200 mg linezolid daily for 6 months will significantly expand the usability of the BPaL regimen worldwide.”
The inclusion of patients with HIV was essential in the trial, he noted.
“There are drug-drug interactions to be considered, among other factors that impact drug exposure,” Dr. Nahid said.
“Inclusion of patients living with HIV in this study means that any modifications to the BPaL regimen considered by the WHO [World Health Organization] and other policy decision makers will include data from this key population,” he said. “Of course, more data are needed on safety, tolerability, and efficacy on BPaL in general, and there are international cohorts and demonstration projects underway that will enhance our understanding of the regimen in HIV and in other special populations.”
The authors, Dr. Streeck, and Dr. Nahid have disclosed no relevant financial relationships.
This article was updated 7/21/21.
FROM IAS 2021
Recent trend: Melanoma mortality declining rapidly
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
according to an annual report by several national organizations.
“Death rates for cutaneous melanoma have declined rapidly in recent years following introduction of new therapies, including targeted and immune checkpoint inhibitors, the first of which was approved by the [Food and Drug Administration] in early 2011,” Farhad Islami, MD, PhD, of the American Cancer Society, and associates wrote in the Journal of the National Cancer Institute.
The American Cancer Society, along with the Centers for Disease Control and Prevention, the National Cancer Institute, and the North American Association of Central Cancer Registries, issue a joint report each year to update the incidence and mortality of the most common cancers and analyze short- and long-term trends since 2001.
Long-term melanoma mortality gets divided into two trends: First a slow decline over about a decade, then an accelerated decline until the end of the study period, although the timing is slightly different between males and females. For men, the death rate fell by an average of 0.9% a year from 2001 to 2009, compared with 5.7% per year in 2013-2018. For women, the average annual change went from –0.3% for 2001-2012 to –4.4% in 2012-2018.
The incidence of melanoma, however, headed in the opposite direction, rising 1.9% per year for females and 2.2% for males from 2001 to 2017, without the notable change in trend seen with death rates, Dr. Islami and associates said.
Incidence by race/ethnicity, reported for 2013-2017, shows that melanoma is much more common among white non-Hispanics: 37.4 per 100,000 standard population for males and 24.5 for females. Non-Hispanic American Indians/Alaska Natives were next at 10.8 (men) and 6.7 (women), followed by Hispanics (5.1/4.5), non-Hispanic Asians/Pacific Islanders (1.6/1.3), and non-Hispanic Blacks (1.2/1.0), they reported.
Death rates for melanoma, reported for 2014-2018, follow a similar pattern. White males (4.2 per 100,000) and females (1.8 per 100,000) had the highest mortality, then American Indians/Alaska Natives (1.0/0.5) and Hispanics (0.9/0.5), but rates were the same for Blacks and Asians/Pacific Islanders (0.4/0.3), the investigators said.
The accelerated decline in death rates in more recent years reflects “a substantial increase in survival for metastatic melanoma,” the participating organizations noted in a joint statement.
Increases in 2-year survival in distant-stage disease averaged 3.1% per year for those diagnosed during 2009-2014, which “slightly preceded the FDA approval of new therapies, likely because of the administration of these therapies through clinical trials and the FDA expanded access programs prior to the approval,” Dr. Islami and associates wrote.
The 2-year relative survival for those with nonmetastatic melanoma also improved over the study period, but the increases were much smaller: 0.4% per year for regional-stage disease and just 0.03% localized-stage cases diagnosed in 2001-2014, they reported.
The report was funded by the four participating groups. Six of the 12 investigators are employees of the American Cancer Society whose salaries are solely paid by the society; the other authors had no conflicts of interest to disclose.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
Fungemia, other fungal infections associated with s. Boulardii probiotics
Life-threatening fungal bloodstream infections associated with probiotic supplements have been reported in the journal Emerging Infectious Diseases by a group of researchers in Finland. While individuals consume these mixtures of bacteria and yeast in the hopes of “balancing” their microbiome or preventing diarrhea from antibiotic use, some died or developed yeast infections requiring prolonged antifungal treatment.
In a retrospective registry study at five university hospitals in Finland, the researchers found 46 patients between 2009 and 2018 with Saccharomyces sp. of yeast in their blood associated with ingesting probiotics. At least 20 (43%) had been using S. cerevisiae var. boulardii as a probiotic, with the organism then causing a bloodstream infection. Overall, 37% of the fungemic patients died.
Juha Rannikko, MD, lead author and infectious disease faculty member at Tampere University Hospital, Finland, said in an interview that there were an additional 1,153 nonblood isolates of Saccharomyces. He expressed surprise at the large number of nonblood isolates, saying: “If extrapolated ... it is about 10 nonblood Saccharomyces boulardii–associated findings for each Saccharomyces boulardii–associated fungemia.”
Most of the yeast infections (59%) occurred in patients with underlying gastrointestinal disease. Prior studies suggested that patients receiving enteral nutrition might become ill from translocation of the yeast from the inflamed GI tract.
If there were positive cultures for yeast from sites other than blood, physicians changed the antibiotics in 38% of patients.
Conventional wisdom has been that patients receiving broad-spectrum antibiotics should also receive an S. cerevisiae var. boulardii probiotic to prevent Clostridioides difficile infections. Dr. Rannikko and coauthors questioned this, noting results of such studies of prophylaxis were equivocal. “There is not enough evidence that clinicians should use Saccharomyces (probiotics) alongside antibiotics,” Dr. Rannikko concluded.
Laila Woc-Colburn, MD, associate professor at the Emory University School of Medicine, Atlanta, told this news organization that although the study was well done and was published in Emerging Infectious Diseases, the findings do not represent an “emerging” infectious disease. “We have known this for a while – since the 1990s,” she said. Warnings about probiotics are part of the standard advice Dr. Woc-Colburn gives transplant, chemotherapy, or immunosuppressed patients. “Don’t do these probiotics, because this is what’s going to happen,” she tells them. And she told this news organization, “If I see this in the blood, the first question I’m going to ask my patients is ... what probiotic were you drinking?”
Dr. Woc-Colburn said the Finnish researchers “did their due diligence” when conducting the study. “They were clear on their limitations. And they came out to the same conclusion as the 2005 Muñoz paper: That if we have some GI disruption, we should not be taking probiotics.”
She acknowledged that the Emerging Infectious Diseases study adds a substantial number of cases to those previously reported in the literature and confirms previous findings and recommendations to avoid probiotics if immunosuppressed or acutely ill.
Dr. Rannikko and Dr. Woc-Coburn have reported no relevant financial relationships. Dr. Rannikko has received a lecture fee from Novo Nordisk and a virtual congress attendance fee from Roche.
A version of this article first appeared on Medscape.com.
Life-threatening fungal bloodstream infections associated with probiotic supplements have been reported in the journal Emerging Infectious Diseases by a group of researchers in Finland. While individuals consume these mixtures of bacteria and yeast in the hopes of “balancing” their microbiome or preventing diarrhea from antibiotic use, some died or developed yeast infections requiring prolonged antifungal treatment.
In a retrospective registry study at five university hospitals in Finland, the researchers found 46 patients between 2009 and 2018 with Saccharomyces sp. of yeast in their blood associated with ingesting probiotics. At least 20 (43%) had been using S. cerevisiae var. boulardii as a probiotic, with the organism then causing a bloodstream infection. Overall, 37% of the fungemic patients died.
Juha Rannikko, MD, lead author and infectious disease faculty member at Tampere University Hospital, Finland, said in an interview that there were an additional 1,153 nonblood isolates of Saccharomyces. He expressed surprise at the large number of nonblood isolates, saying: “If extrapolated ... it is about 10 nonblood Saccharomyces boulardii–associated findings for each Saccharomyces boulardii–associated fungemia.”
Most of the yeast infections (59%) occurred in patients with underlying gastrointestinal disease. Prior studies suggested that patients receiving enteral nutrition might become ill from translocation of the yeast from the inflamed GI tract.
If there were positive cultures for yeast from sites other than blood, physicians changed the antibiotics in 38% of patients.
Conventional wisdom has been that patients receiving broad-spectrum antibiotics should also receive an S. cerevisiae var. boulardii probiotic to prevent Clostridioides difficile infections. Dr. Rannikko and coauthors questioned this, noting results of such studies of prophylaxis were equivocal. “There is not enough evidence that clinicians should use Saccharomyces (probiotics) alongside antibiotics,” Dr. Rannikko concluded.
Laila Woc-Colburn, MD, associate professor at the Emory University School of Medicine, Atlanta, told this news organization that although the study was well done and was published in Emerging Infectious Diseases, the findings do not represent an “emerging” infectious disease. “We have known this for a while – since the 1990s,” she said. Warnings about probiotics are part of the standard advice Dr. Woc-Colburn gives transplant, chemotherapy, or immunosuppressed patients. “Don’t do these probiotics, because this is what’s going to happen,” she tells them. And she told this news organization, “If I see this in the blood, the first question I’m going to ask my patients is ... what probiotic were you drinking?”
Dr. Woc-Colburn said the Finnish researchers “did their due diligence” when conducting the study. “They were clear on their limitations. And they came out to the same conclusion as the 2005 Muñoz paper: That if we have some GI disruption, we should not be taking probiotics.”
She acknowledged that the Emerging Infectious Diseases study adds a substantial number of cases to those previously reported in the literature and confirms previous findings and recommendations to avoid probiotics if immunosuppressed or acutely ill.
Dr. Rannikko and Dr. Woc-Coburn have reported no relevant financial relationships. Dr. Rannikko has received a lecture fee from Novo Nordisk and a virtual congress attendance fee from Roche.
A version of this article first appeared on Medscape.com.
Life-threatening fungal bloodstream infections associated with probiotic supplements have been reported in the journal Emerging Infectious Diseases by a group of researchers in Finland. While individuals consume these mixtures of bacteria and yeast in the hopes of “balancing” their microbiome or preventing diarrhea from antibiotic use, some died or developed yeast infections requiring prolonged antifungal treatment.
In a retrospective registry study at five university hospitals in Finland, the researchers found 46 patients between 2009 and 2018 with Saccharomyces sp. of yeast in their blood associated with ingesting probiotics. At least 20 (43%) had been using S. cerevisiae var. boulardii as a probiotic, with the organism then causing a bloodstream infection. Overall, 37% of the fungemic patients died.
Juha Rannikko, MD, lead author and infectious disease faculty member at Tampere University Hospital, Finland, said in an interview that there were an additional 1,153 nonblood isolates of Saccharomyces. He expressed surprise at the large number of nonblood isolates, saying: “If extrapolated ... it is about 10 nonblood Saccharomyces boulardii–associated findings for each Saccharomyces boulardii–associated fungemia.”
Most of the yeast infections (59%) occurred in patients with underlying gastrointestinal disease. Prior studies suggested that patients receiving enteral nutrition might become ill from translocation of the yeast from the inflamed GI tract.
If there were positive cultures for yeast from sites other than blood, physicians changed the antibiotics in 38% of patients.
Conventional wisdom has been that patients receiving broad-spectrum antibiotics should also receive an S. cerevisiae var. boulardii probiotic to prevent Clostridioides difficile infections. Dr. Rannikko and coauthors questioned this, noting results of such studies of prophylaxis were equivocal. “There is not enough evidence that clinicians should use Saccharomyces (probiotics) alongside antibiotics,” Dr. Rannikko concluded.
Laila Woc-Colburn, MD, associate professor at the Emory University School of Medicine, Atlanta, told this news organization that although the study was well done and was published in Emerging Infectious Diseases, the findings do not represent an “emerging” infectious disease. “We have known this for a while – since the 1990s,” she said. Warnings about probiotics are part of the standard advice Dr. Woc-Colburn gives transplant, chemotherapy, or immunosuppressed patients. “Don’t do these probiotics, because this is what’s going to happen,” she tells them. And she told this news organization, “If I see this in the blood, the first question I’m going to ask my patients is ... what probiotic were you drinking?”
Dr. Woc-Colburn said the Finnish researchers “did their due diligence” when conducting the study. “They were clear on their limitations. And they came out to the same conclusion as the 2005 Muñoz paper: That if we have some GI disruption, we should not be taking probiotics.”
She acknowledged that the Emerging Infectious Diseases study adds a substantial number of cases to those previously reported in the literature and confirms previous findings and recommendations to avoid probiotics if immunosuppressed or acutely ill.
Dr. Rannikko and Dr. Woc-Coburn have reported no relevant financial relationships. Dr. Rannikko has received a lecture fee from Novo Nordisk and a virtual congress attendance fee from Roche.
A version of this article first appeared on Medscape.com.