User login
Velvety Plaques on the Abdomen and Extremities
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.
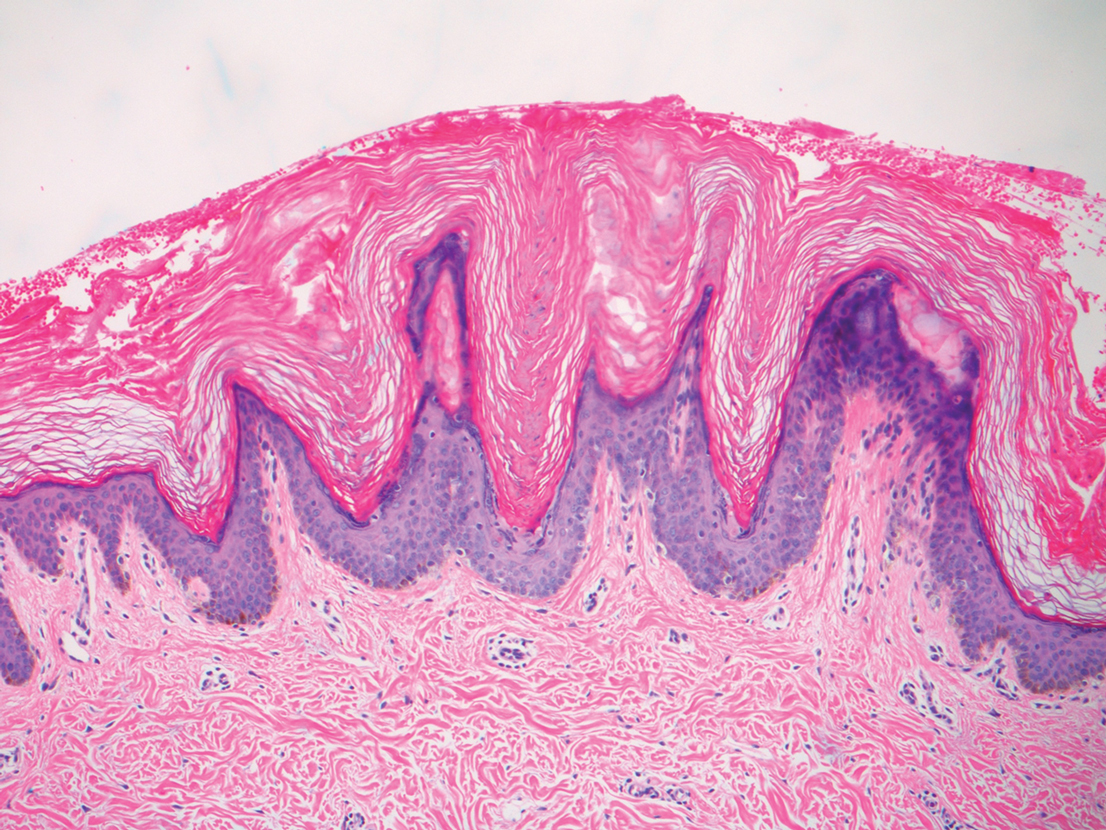
Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.

Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.

Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
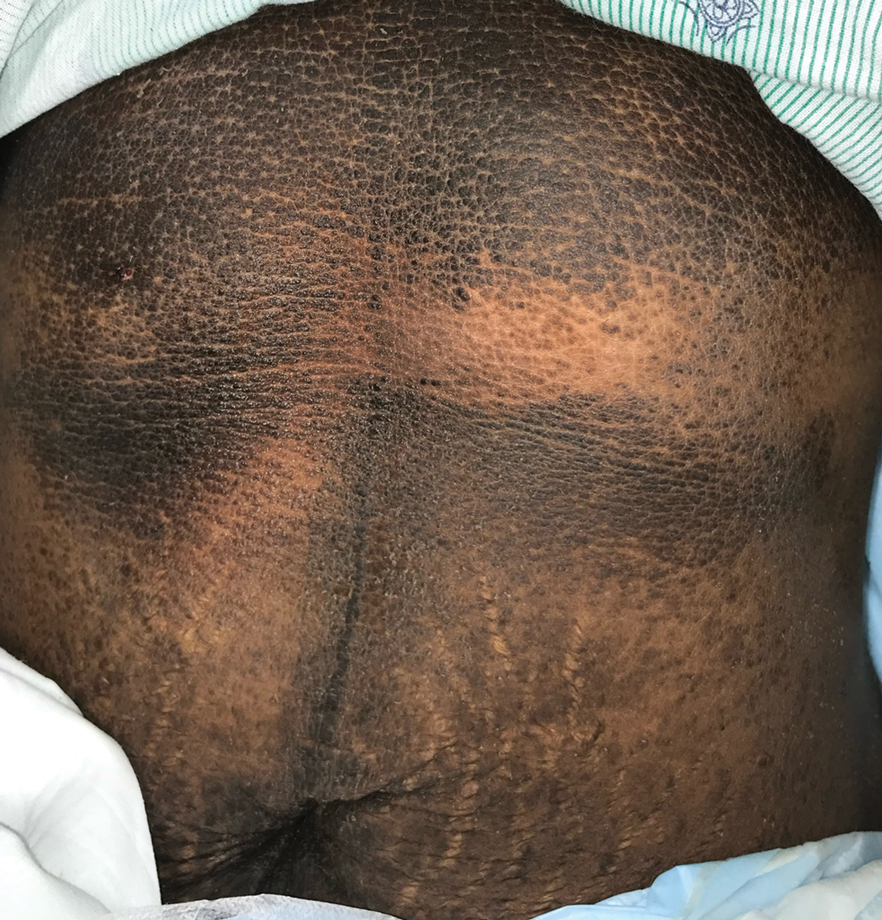
A 28-year-old woman was admitted to the medicine service with bilateral pedal numbness and ataxia, as well as an asymptomatic rash on the neck, chest, abdomen, and extremities of a few months’ duration. The patient was seen by an outside dermatologist for the same rash 1 month prior, at which time a punch biopsy of the right forearm was suggestive of psoriasiform dermatitis; however, the rash failed to improve with topical ammonium lactate and corticosteroids. During the current admission, the patient was found to have low methylmalonic acid and vitamin B1 levels; however, vitamin B12, thyroid studies, rapid plasma reagin test, and inflammatory markers, as well as central and peripheral imaging and nerve conduction studies were normal.
Dermatology was consulted. Physical examination revealed retention hyperkeratosis on the neck that was wipeable with 70% isopropyl alcohol, as well as nonwipeable, thin, reticulated plaques on the mid chest and thick velvety plaques on the abdomen and bilateral extremities. There was notable sparing of areas with natural occlusion such as the back and body folds. A punch biopsy of the abdomen was performed.
Adolescent immunizations and protecting our children from COVID-19
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
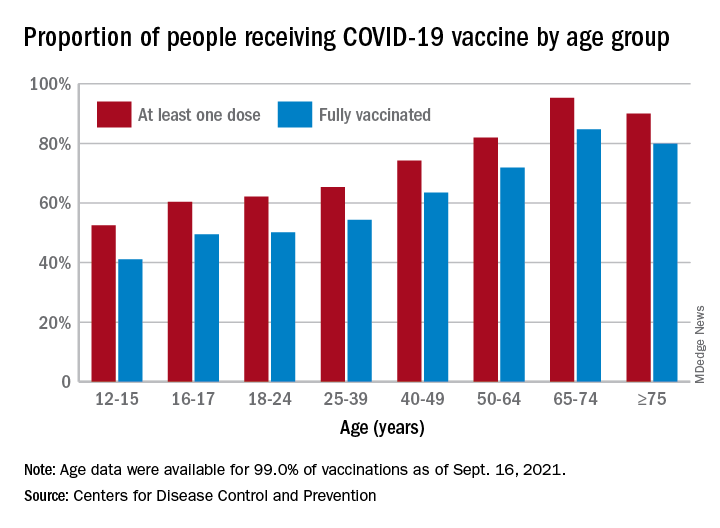
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)
It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)

It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
I began thinking of a topic for this column weeks ago determined to discuss anything except COVID-19. Yet, news reports from all sources blasted daily reminders of rising COVID-19 cases overall and specifically in children.
In August, school resumed for many of our patients and the battle over mandating masks for school attendance was in full swing. The fact that it is a Centers for Disease Control and Prevention recommendation supported by both the American Academy of Pediatrics and the Pediatric Infectious Disease Society fell on deaf ears. One day, I heard a report that over 25,000 students attending Texas public schools were diagnosed with COVID-19 between Aug. 23 and Aug. 29. This peak in activity occurred just 2 weeks after the start of school and led to the closure of 45 school districts. Texas does not have a monopoly on these rising cases. Delta, a more contagious variant, began circulating in June 2021 and by July it was the most predominant. Emergency department visits and hospitalizations have increased nationwide. During the latter 2 weeks of August 2021, COVID-19–related ED visits and hospitalizations for persons aged 0-17 years were 3.4 and 3.7 times higher in states with the lowest vaccination coverage, compared with states with high vaccination coverage (MMWR Morb Mortal Wkly Rep. 2021;70:1249-54). Specifically, the rates of hospitalization the week ending Aug. 14, 2021, were nearly 5 times the rates for the week ending June 26, 2021, for 0- to 17-year-olds and nearly 10 times the rates for children 0-4 years of age. Hospitalization rates were 10.1 times higher for unimmunized adolescents than for fully vaccinated ones (MMWR Morb Mortal Wkly Rep. 2021;70:1255-60).
Multiple elected state leaders have opposed interventions such as mandating masks in school, and our children are paying for it. These leaders have relinquished their responsibility to local school boards. Several have reinforced the no-mask mandate while others have had the courage and insight to ignore state government leaders and have established mask mandates.
How is this lack of enforcement of national recommendations affecting our patients? Let’s look at two neighboring school districts in Texas. School districts have COVID-19 dashboards that are updated daily and accessible to the general public. School District A requires masks for school entry. It serves 196,171 students and has 27,195 teachers and staff. Since school opened in August, 1,606 cumulative cases of COVID-19 in students (0.8%) and 282 in staff (1%) have been reported. Fifty-five percent of the student cases occurred in elementary schools. In contrast, School District B located in the adjacent county serves 64,517 students and has 3,906 teachers and staff with no mask mandate. Since August, there have been 4,506 cumulative COVID-19 cases in students (6.9%) and 578 (14.7%) in staff. Information regarding the specific school type was not provided; however, the dashboard indicates that 2,924 cases (64.8%) occurred in children younger than 11 years of age. County data indicate 62% of those older than 12 years of age were fully vaccinated in District A, compared with 54% of persons older than 12 years in District B. The county COVID-19 positivity rate in District A is 17.6% and in District B it is 20%. Both counties are experiencing increased COVID-19 activity yet have had strikingly different outcomes in the student/staff population. While supporting the case for wearing masks to prevent disease transmission, one can’t ignore the adolescents who were infected and vaccine eligible (District A: 706; District B: 1,582). Their vaccination status could not be determined.
As pediatricians we have played an integral part in the elimination of diseases through educating and administering vaccinations. Adolescents are relatively healthy, thus limiting the number of encounters with them. The majority complete the 11-year visit; however, many fail to return for the 16- to 18-year visit.
So how are we doing? CDC data from 10 U.S. jurisdictions demonstrated a substantial decrease in vaccine administration between March and May of 2020, compared with the same period in 2018 and 2019. A decline was anticipated because of the nationwide lockdown. Doses of HPV administered declined almost 64% and 71% for 9- to 12-year-olds and 13- to 17-year-olds, respectively. Tdap administration declined 66% and 61% for the same respective age groups. Although administered doses increased between June and September of 2020, it was not sufficient to achieve catch-up coverage. Compared to the same period in 2018-2019, administration of the HPV vaccine declined 12.8% and 28% (ages 9-12 and ages 13-17) and for Tdap it was 21% and 30% lower (ages 9-12 and ages 13-17) (MMWR Morb Mortal Wkly Rep. 2021;70:840-5).
Now, we have another adolescent vaccine to discuss and encourage our patients to receive. We also need to address their concerns and/or to at least direct them to a reliable source to obtain accurate information. For the first time, a recommended vaccine may not be available at their medical home. Many don’t know where to go to receive it (http://www.vaccines.gov). Results of a Kaiser Family Foundation COVID-19 survey (August 2021) indicated that parents trusted their pediatricians most often (78%) for vaccine advice. The respondents voiced concern about trusting the location where the child would be immunized and long-term effects especially related to fertility. Parents who received communications regarding the benefits of vaccination were twice as likely to have their adolescents immunized. Finally, remember: Like parent, like child. An immunized parent is more likely to immunize the adolescent. (See Fig. 1.)

It is beyond the scope of this column to discuss the psychosocial aspects of this disease: children experiencing the death of teachers, classmates, family members, and those viewing the vitriol between pro- and antimask proponents often exhibited on school premises. And let’s not forget the child who wants to wear a mask but may be ostracized or bullied for doing so.
Our job is to do our very best to advocate for and to protect our patients by promoting mandatory masks at schools and encouraging vaccination of adolescents as we patiently wait for vaccines to become available for all of our children.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
An update on COVID-19 vaccine recommendations for patients with IBD
In December 2019, cases of pulmonary infection secondary to a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2, were first identified in the city of Wuhan, China.
The clinical disease caused by the virus, COVID-19, has resulted in a worldwide pandemic that has portended significant morbidity and mortality throughout the United States. Three highly efficacious COVID-19 vaccines have received emergency use authorization (EUA) by the Food and Drug Administration to help prevent COVID-19, all of which are effective at preventing severe COVID-19.1-3 The Pfizer vaccine was given full FDA approval on Aug. 23, 2021.4
Patients with inflammatory bowel disease (IBD) are commonly treated with immune-modifying therapies that may increase their risk for serious and opportunistic infections. As such, there was concern at the beginning of the pandemic that patients with IBD may be at increased risk of contracting COVID-19 and/or developing severe disease (that is, ICU-level care, mechanical ventilation, and/or death). There is evidence that the incidence of COVID-19 in the IBD population is similar to that of the general population.5-7 Furthermore, most patients with IBD are not at increased risk of severe disease, including those on biologic therapies. Several studies demonstrated that those on corticosteroids are at increased risk of severe COVID-19, while those on other immune-modifying therapies such as tumor necrosis factor inhibitors (anti-TNFs) are not at increased risk.5,7-10 Patients with IBD with other well-known risk factors for severe disease include comorbidities such as diabetes and obesity.
It is known that patients with IBD on certain immune-modifying therapies such as anti-TNFs, especially those on combination therapy, may have a blunted immune response to certain vaccines.11 Neither patients with IBD nor patients on immunosuppressive therapy were included in phase 3 clinical trials for COVID-19 vaccine development, contributing to uncertainty regarding the safety and efficacy in our patient population. The risk of adverse events following COVID-19 vaccination in the IBD population has been found to be similar to that of the general population.12 It has also been reported that those who have had reactions to injectable therapies in the past may safely be vaccinated against COVID-19.13,14 With regard to vaccine efficacy, initial studies, including ICARUS, PREVENT-COVID, and CORALE-IBD, have demonstrated that patients with IBD do indeed mount a humoral immune response to the vaccine, including those on immune-modifying therapies.15-17 Nonhumoral aspects of immunity, such as cell-mediated immunity, have not yet been thoroughly evaluated. In addition, the risk of breakthrough COVID-19 infection after vaccination is low in patients with IBD, including those on immune-modifying therapy.14-18 While initial studies are reassuring that the vast majority of patients with IBD are able to mount a vaccine response, future studies are needed to determine the effects of immune-modifying therapy on sustained antibody concentrations and other correlates of immunity.
For those who received the Pfizer or Moderna vaccines, on Aug. 12, 2021, the FDA amended their EUA to allow for an additional dose in the initial vaccination series for certain immunocompromised individuals, specifically solid organ transplant recipients or those with conditions that make them equally immunocompromised.19 Based on evidence suggesting that certain solid organ transplant recipients do not mount an immune response after completing a two-dose series, the Advisory Committee on Immunization Practices, which advises the Centers for Disease Control and Prevention on how to use vaccines, recommended that moderate to severely immunocompromised individuals should complete a three-dose series, with the third dose being given at least 28 days after the second dose.20 This recommendation included those on high-dose corticosteroids defined as oral prednisone at least 20 mg/day, anti-TNFs and biosimilars, and antimetabolites such as azathioprine, mercaptopurine, and methotrexate.
It is worth noting that the role of the ACIP here was to consider the available evidence supporting the use of an additional dose and then make recommendations on which conditions may qualify; the ACIP was not able to provide recommendations for every disease state. At the time of writing this article, no recommendations have been made with regards to an additional dose of the Janssen vaccine. Likewise, in response to the ACIP recommendations, the Crohn’s & Colitis Foundation recommended an additional dose for patients with IBD on immune-modifying therapies.21,22
Less than one week after the EUA amendment for an additional dose, the Department of Health & Human Services announced that booster shots would likely become available to the general population as early as the week of Sept. 20, 2021 and starting 8 months after an individual’s second dose.23 Here, it is worth noting that an additional dose is distinct from a booster. An additional dose (or third dose here) refers to the initial vaccination series and is given when the standard schedule is thought to be insufficient in a certain patient population. In contrast, a booster dose is administered when the initial and sufficient immunity gained from a primary vaccination series has likely dissipated. The HHS acknowledged that boosters would likely be needed for those who received the Janssen vaccine but noted that further data and recommendations would be forthcoming.
To summarize, COVID-19 vaccines are safe and effective in the IBD population, and patients should be vaccinated at the earliest opportunity regardless of concurrent therapies. For those that received the Pfizer or Moderna vaccine, the ACIP recommended an additional dose in the initial vaccination series to be given at least 28 days after the second dose for those that are immunosuppressed. This recommendation was largely based off of transplant data. Reassuringly, the available data demonstrates a humoral immune response to a two-dose vaccination series in patients with IBD, including those on immune-modifying therapies. The Crohn’s & Colitis Foundation recommends that patients with IBD on immune-modifying therapy receive an additional dose (i.e., a three-dose series), which should be from the same manufacturer as the first two doses. In addition, at press time, HHS indicated that there will be a movement toward a booster dose for the general population in late September, which would also apply to patients with IBD. The ACIP has yet to comment on this change at the time of preparing this article, but the announcement indicated that a booster could be given “8 months after an individual’s second dose.” It is unclear how those who may receive a three-dose vaccination series will factor in, but it is possible that they would be eligible for a booster 8 months after their most recent dose. Gastroenterologists should also be aware that there is no role for serologic testing in the clinical setting because it has not been validated for such purposes and is primarily used in the research setting. Finally, it is paramount to emphasize that patients with IBD have historically had lower vaccination rates than the general population,24 and we must take an active role in ensuring that our patients are immunized by addressing their concerns, communicating the risks of COVID-19 and the benefits of vaccination, providing information on how to get vaccinated, and strongly recommending vaccination.
The following list also summarizes the recommendations:
- Patients with IBD should be vaccinated against COVID-19 regardless of concurrent therapies.
- Patients with IBD are not at increased risk of severe COVID-19.
- Patients with IBD, including those on immune-modifying therapies, mount a humoral immune response to the vaccine.
- Patients with IBD on immune-modifying therapies, who received either the Pfizer or Moderna vaccine, should receive a three-dose vaccination series, with the third dose at least 28 days after the second dose.
- Patients with IBD on biologic therapy can receive the third dose of the vaccine at any time point and should not interrupt biologic therapy.
- Boosters are likely to become available to the general public in September and would be given at least 8 months after an individual’s second dose.
- Recommendations regarding boosters for those who received a three-dose vaccination series are forthcoming.
- Recommendations regarding boosters and additional doses for those that received the Janssen vaccine are forthcoming.
- Gastroenterologists should take an active role in ensuring that their patients are vaccinated.
Dr. Schell is a second-year graduate student in the division of internal medicine at the University of Wisconsin–Madison. Dr. Caldera is an associate professor of medicine in the division of gastroenterology & hepatology at the University of Wisconsin–Madison. Dr. Schell has no conflicts of interest to disclose. Dr. Caldera has received research support from Takeda Pharmaceuticals and Sanofi. He has been a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene.
References
1. Sadoff J et al. N Engl J Med. 2021;384(23):2187-201.
2. Baden LR et al. N Engl J Med. 2021;384(5):403-16.
3. Polack FP et al. N Engl J Med. 2020;383:2603-15.
4. Johnson K et al. U.S. FDA aims to give full approval to Pfizer vaccine on Monday – NYT. Reuters. 2021 Aug 20. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-aims-give-full-nod-pfizers-covid-19-vaccine-monday-new-york-times-2021-08-20/.
5. Allocca M et al. J Clin Med. 2020 Oct;9(11):3533.
6. Monteleone G and Ardizzone S. J Crohns Colitis. 2020 Sep;14(9):1334-6.
7. Papa A et al. Am J Gastroenterol. 2020;115(10):1722-4.
8. Derikx LAAP et al. J Crohn’s Colitis. 2021 Apr 6;15(4):529-39.
9. Brenner EJ et al. Gastroenterology. 2020;159(2):481-91.
10. Ungaro RC et al. Gut. 2021;70(4):725-32.
11. Caldera F et al. Inflamm Bowel Dis. 2020;26(4):593-602.
12. Botwin GJ et al. Am J Gastroenterol. 2021. doi: 10.14309/ajg.0000000000001342.
13. Squire JD et al. Inflamm Bowel Dis. 2021 Jul 27;27(8):1358-60.
14. Hadi YB et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.014.
15. Wong S-Y et al. Gastroenterology. 2021;161:715-8.
16. Kappelman MD et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.016.
17. Pozdnyakova V et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.08.014.
18. Ben-Tov A et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.076.
19. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA News Release. 2021. Accessed 2021 Aug 18. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
20. Centers for Disease Control and Prevention. COVID-19 Vaccines for Moderately to Severely Immunocompromised People. 2021. Accessed 2021 Aug 18. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
21. Allocca M et al. J Clin Med. 2020 Oct 31;9(11):3533.
22. Crohn’s & Colitis Foundation. COVID-19 Vaccines: Position Statements. IBD & Coronavirus. 2021. Accessed 2021 Aug 20. https://www.crohnscolitisfoundation.org/coronavirus/vaccine-position-statements.
23. Centers for Disease Control and Prevention. Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html.
24. Caldera F et al. Inflamm Bowel Dis. 2021;27(1):123-133.
In December 2019, cases of pulmonary infection secondary to a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2, were first identified in the city of Wuhan, China.
The clinical disease caused by the virus, COVID-19, has resulted in a worldwide pandemic that has portended significant morbidity and mortality throughout the United States. Three highly efficacious COVID-19 vaccines have received emergency use authorization (EUA) by the Food and Drug Administration to help prevent COVID-19, all of which are effective at preventing severe COVID-19.1-3 The Pfizer vaccine was given full FDA approval on Aug. 23, 2021.4
Patients with inflammatory bowel disease (IBD) are commonly treated with immune-modifying therapies that may increase their risk for serious and opportunistic infections. As such, there was concern at the beginning of the pandemic that patients with IBD may be at increased risk of contracting COVID-19 and/or developing severe disease (that is, ICU-level care, mechanical ventilation, and/or death). There is evidence that the incidence of COVID-19 in the IBD population is similar to that of the general population.5-7 Furthermore, most patients with IBD are not at increased risk of severe disease, including those on biologic therapies. Several studies demonstrated that those on corticosteroids are at increased risk of severe COVID-19, while those on other immune-modifying therapies such as tumor necrosis factor inhibitors (anti-TNFs) are not at increased risk.5,7-10 Patients with IBD with other well-known risk factors for severe disease include comorbidities such as diabetes and obesity.
It is known that patients with IBD on certain immune-modifying therapies such as anti-TNFs, especially those on combination therapy, may have a blunted immune response to certain vaccines.11 Neither patients with IBD nor patients on immunosuppressive therapy were included in phase 3 clinical trials for COVID-19 vaccine development, contributing to uncertainty regarding the safety and efficacy in our patient population. The risk of adverse events following COVID-19 vaccination in the IBD population has been found to be similar to that of the general population.12 It has also been reported that those who have had reactions to injectable therapies in the past may safely be vaccinated against COVID-19.13,14 With regard to vaccine efficacy, initial studies, including ICARUS, PREVENT-COVID, and CORALE-IBD, have demonstrated that patients with IBD do indeed mount a humoral immune response to the vaccine, including those on immune-modifying therapies.15-17 Nonhumoral aspects of immunity, such as cell-mediated immunity, have not yet been thoroughly evaluated. In addition, the risk of breakthrough COVID-19 infection after vaccination is low in patients with IBD, including those on immune-modifying therapy.14-18 While initial studies are reassuring that the vast majority of patients with IBD are able to mount a vaccine response, future studies are needed to determine the effects of immune-modifying therapy on sustained antibody concentrations and other correlates of immunity.
For those who received the Pfizer or Moderna vaccines, on Aug. 12, 2021, the FDA amended their EUA to allow for an additional dose in the initial vaccination series for certain immunocompromised individuals, specifically solid organ transplant recipients or those with conditions that make them equally immunocompromised.19 Based on evidence suggesting that certain solid organ transplant recipients do not mount an immune response after completing a two-dose series, the Advisory Committee on Immunization Practices, which advises the Centers for Disease Control and Prevention on how to use vaccines, recommended that moderate to severely immunocompromised individuals should complete a three-dose series, with the third dose being given at least 28 days after the second dose.20 This recommendation included those on high-dose corticosteroids defined as oral prednisone at least 20 mg/day, anti-TNFs and biosimilars, and antimetabolites such as azathioprine, mercaptopurine, and methotrexate.
It is worth noting that the role of the ACIP here was to consider the available evidence supporting the use of an additional dose and then make recommendations on which conditions may qualify; the ACIP was not able to provide recommendations for every disease state. At the time of writing this article, no recommendations have been made with regards to an additional dose of the Janssen vaccine. Likewise, in response to the ACIP recommendations, the Crohn’s & Colitis Foundation recommended an additional dose for patients with IBD on immune-modifying therapies.21,22
Less than one week after the EUA amendment for an additional dose, the Department of Health & Human Services announced that booster shots would likely become available to the general population as early as the week of Sept. 20, 2021 and starting 8 months after an individual’s second dose.23 Here, it is worth noting that an additional dose is distinct from a booster. An additional dose (or third dose here) refers to the initial vaccination series and is given when the standard schedule is thought to be insufficient in a certain patient population. In contrast, a booster dose is administered when the initial and sufficient immunity gained from a primary vaccination series has likely dissipated. The HHS acknowledged that boosters would likely be needed for those who received the Janssen vaccine but noted that further data and recommendations would be forthcoming.
To summarize, COVID-19 vaccines are safe and effective in the IBD population, and patients should be vaccinated at the earliest opportunity regardless of concurrent therapies. For those that received the Pfizer or Moderna vaccine, the ACIP recommended an additional dose in the initial vaccination series to be given at least 28 days after the second dose for those that are immunosuppressed. This recommendation was largely based off of transplant data. Reassuringly, the available data demonstrates a humoral immune response to a two-dose vaccination series in patients with IBD, including those on immune-modifying therapies. The Crohn’s & Colitis Foundation recommends that patients with IBD on immune-modifying therapy receive an additional dose (i.e., a three-dose series), which should be from the same manufacturer as the first two doses. In addition, at press time, HHS indicated that there will be a movement toward a booster dose for the general population in late September, which would also apply to patients with IBD. The ACIP has yet to comment on this change at the time of preparing this article, but the announcement indicated that a booster could be given “8 months after an individual’s second dose.” It is unclear how those who may receive a three-dose vaccination series will factor in, but it is possible that they would be eligible for a booster 8 months after their most recent dose. Gastroenterologists should also be aware that there is no role for serologic testing in the clinical setting because it has not been validated for such purposes and is primarily used in the research setting. Finally, it is paramount to emphasize that patients with IBD have historically had lower vaccination rates than the general population,24 and we must take an active role in ensuring that our patients are immunized by addressing their concerns, communicating the risks of COVID-19 and the benefits of vaccination, providing information on how to get vaccinated, and strongly recommending vaccination.
The following list also summarizes the recommendations:
- Patients with IBD should be vaccinated against COVID-19 regardless of concurrent therapies.
- Patients with IBD are not at increased risk of severe COVID-19.
- Patients with IBD, including those on immune-modifying therapies, mount a humoral immune response to the vaccine.
- Patients with IBD on immune-modifying therapies, who received either the Pfizer or Moderna vaccine, should receive a three-dose vaccination series, with the third dose at least 28 days after the second dose.
- Patients with IBD on biologic therapy can receive the third dose of the vaccine at any time point and should not interrupt biologic therapy.
- Boosters are likely to become available to the general public in September and would be given at least 8 months after an individual’s second dose.
- Recommendations regarding boosters for those who received a three-dose vaccination series are forthcoming.
- Recommendations regarding boosters and additional doses for those that received the Janssen vaccine are forthcoming.
- Gastroenterologists should take an active role in ensuring that their patients are vaccinated.
Dr. Schell is a second-year graduate student in the division of internal medicine at the University of Wisconsin–Madison. Dr. Caldera is an associate professor of medicine in the division of gastroenterology & hepatology at the University of Wisconsin–Madison. Dr. Schell has no conflicts of interest to disclose. Dr. Caldera has received research support from Takeda Pharmaceuticals and Sanofi. He has been a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene.
References
1. Sadoff J et al. N Engl J Med. 2021;384(23):2187-201.
2. Baden LR et al. N Engl J Med. 2021;384(5):403-16.
3. Polack FP et al. N Engl J Med. 2020;383:2603-15.
4. Johnson K et al. U.S. FDA aims to give full approval to Pfizer vaccine on Monday – NYT. Reuters. 2021 Aug 20. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-aims-give-full-nod-pfizers-covid-19-vaccine-monday-new-york-times-2021-08-20/.
5. Allocca M et al. J Clin Med. 2020 Oct;9(11):3533.
6. Monteleone G and Ardizzone S. J Crohns Colitis. 2020 Sep;14(9):1334-6.
7. Papa A et al. Am J Gastroenterol. 2020;115(10):1722-4.
8. Derikx LAAP et al. J Crohn’s Colitis. 2021 Apr 6;15(4):529-39.
9. Brenner EJ et al. Gastroenterology. 2020;159(2):481-91.
10. Ungaro RC et al. Gut. 2021;70(4):725-32.
11. Caldera F et al. Inflamm Bowel Dis. 2020;26(4):593-602.
12. Botwin GJ et al. Am J Gastroenterol. 2021. doi: 10.14309/ajg.0000000000001342.
13. Squire JD et al. Inflamm Bowel Dis. 2021 Jul 27;27(8):1358-60.
14. Hadi YB et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.014.
15. Wong S-Y et al. Gastroenterology. 2021;161:715-8.
16. Kappelman MD et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.016.
17. Pozdnyakova V et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.08.014.
18. Ben-Tov A et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.076.
19. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA News Release. 2021. Accessed 2021 Aug 18. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
20. Centers for Disease Control and Prevention. COVID-19 Vaccines for Moderately to Severely Immunocompromised People. 2021. Accessed 2021 Aug 18. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
21. Allocca M et al. J Clin Med. 2020 Oct 31;9(11):3533.
22. Crohn’s & Colitis Foundation. COVID-19 Vaccines: Position Statements. IBD & Coronavirus. 2021. Accessed 2021 Aug 20. https://www.crohnscolitisfoundation.org/coronavirus/vaccine-position-statements.
23. Centers for Disease Control and Prevention. Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html.
24. Caldera F et al. Inflamm Bowel Dis. 2021;27(1):123-133.
In December 2019, cases of pulmonary infection secondary to a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2, were first identified in the city of Wuhan, China.
The clinical disease caused by the virus, COVID-19, has resulted in a worldwide pandemic that has portended significant morbidity and mortality throughout the United States. Three highly efficacious COVID-19 vaccines have received emergency use authorization (EUA) by the Food and Drug Administration to help prevent COVID-19, all of which are effective at preventing severe COVID-19.1-3 The Pfizer vaccine was given full FDA approval on Aug. 23, 2021.4
Patients with inflammatory bowel disease (IBD) are commonly treated with immune-modifying therapies that may increase their risk for serious and opportunistic infections. As such, there was concern at the beginning of the pandemic that patients with IBD may be at increased risk of contracting COVID-19 and/or developing severe disease (that is, ICU-level care, mechanical ventilation, and/or death). There is evidence that the incidence of COVID-19 in the IBD population is similar to that of the general population.5-7 Furthermore, most patients with IBD are not at increased risk of severe disease, including those on biologic therapies. Several studies demonstrated that those on corticosteroids are at increased risk of severe COVID-19, while those on other immune-modifying therapies such as tumor necrosis factor inhibitors (anti-TNFs) are not at increased risk.5,7-10 Patients with IBD with other well-known risk factors for severe disease include comorbidities such as diabetes and obesity.
It is known that patients with IBD on certain immune-modifying therapies such as anti-TNFs, especially those on combination therapy, may have a blunted immune response to certain vaccines.11 Neither patients with IBD nor patients on immunosuppressive therapy were included in phase 3 clinical trials for COVID-19 vaccine development, contributing to uncertainty regarding the safety and efficacy in our patient population. The risk of adverse events following COVID-19 vaccination in the IBD population has been found to be similar to that of the general population.12 It has also been reported that those who have had reactions to injectable therapies in the past may safely be vaccinated against COVID-19.13,14 With regard to vaccine efficacy, initial studies, including ICARUS, PREVENT-COVID, and CORALE-IBD, have demonstrated that patients with IBD do indeed mount a humoral immune response to the vaccine, including those on immune-modifying therapies.15-17 Nonhumoral aspects of immunity, such as cell-mediated immunity, have not yet been thoroughly evaluated. In addition, the risk of breakthrough COVID-19 infection after vaccination is low in patients with IBD, including those on immune-modifying therapy.14-18 While initial studies are reassuring that the vast majority of patients with IBD are able to mount a vaccine response, future studies are needed to determine the effects of immune-modifying therapy on sustained antibody concentrations and other correlates of immunity.
For those who received the Pfizer or Moderna vaccines, on Aug. 12, 2021, the FDA amended their EUA to allow for an additional dose in the initial vaccination series for certain immunocompromised individuals, specifically solid organ transplant recipients or those with conditions that make them equally immunocompromised.19 Based on evidence suggesting that certain solid organ transplant recipients do not mount an immune response after completing a two-dose series, the Advisory Committee on Immunization Practices, which advises the Centers for Disease Control and Prevention on how to use vaccines, recommended that moderate to severely immunocompromised individuals should complete a three-dose series, with the third dose being given at least 28 days after the second dose.20 This recommendation included those on high-dose corticosteroids defined as oral prednisone at least 20 mg/day, anti-TNFs and biosimilars, and antimetabolites such as azathioprine, mercaptopurine, and methotrexate.
It is worth noting that the role of the ACIP here was to consider the available evidence supporting the use of an additional dose and then make recommendations on which conditions may qualify; the ACIP was not able to provide recommendations for every disease state. At the time of writing this article, no recommendations have been made with regards to an additional dose of the Janssen vaccine. Likewise, in response to the ACIP recommendations, the Crohn’s & Colitis Foundation recommended an additional dose for patients with IBD on immune-modifying therapies.21,22
Less than one week after the EUA amendment for an additional dose, the Department of Health & Human Services announced that booster shots would likely become available to the general population as early as the week of Sept. 20, 2021 and starting 8 months after an individual’s second dose.23 Here, it is worth noting that an additional dose is distinct from a booster. An additional dose (or third dose here) refers to the initial vaccination series and is given when the standard schedule is thought to be insufficient in a certain patient population. In contrast, a booster dose is administered when the initial and sufficient immunity gained from a primary vaccination series has likely dissipated. The HHS acknowledged that boosters would likely be needed for those who received the Janssen vaccine but noted that further data and recommendations would be forthcoming.
To summarize, COVID-19 vaccines are safe and effective in the IBD population, and patients should be vaccinated at the earliest opportunity regardless of concurrent therapies. For those that received the Pfizer or Moderna vaccine, the ACIP recommended an additional dose in the initial vaccination series to be given at least 28 days after the second dose for those that are immunosuppressed. This recommendation was largely based off of transplant data. Reassuringly, the available data demonstrates a humoral immune response to a two-dose vaccination series in patients with IBD, including those on immune-modifying therapies. The Crohn’s & Colitis Foundation recommends that patients with IBD on immune-modifying therapy receive an additional dose (i.e., a three-dose series), which should be from the same manufacturer as the first two doses. In addition, at press time, HHS indicated that there will be a movement toward a booster dose for the general population in late September, which would also apply to patients with IBD. The ACIP has yet to comment on this change at the time of preparing this article, but the announcement indicated that a booster could be given “8 months after an individual’s second dose.” It is unclear how those who may receive a three-dose vaccination series will factor in, but it is possible that they would be eligible for a booster 8 months after their most recent dose. Gastroenterologists should also be aware that there is no role for serologic testing in the clinical setting because it has not been validated for such purposes and is primarily used in the research setting. Finally, it is paramount to emphasize that patients with IBD have historically had lower vaccination rates than the general population,24 and we must take an active role in ensuring that our patients are immunized by addressing their concerns, communicating the risks of COVID-19 and the benefits of vaccination, providing information on how to get vaccinated, and strongly recommending vaccination.
The following list also summarizes the recommendations:
- Patients with IBD should be vaccinated against COVID-19 regardless of concurrent therapies.
- Patients with IBD are not at increased risk of severe COVID-19.
- Patients with IBD, including those on immune-modifying therapies, mount a humoral immune response to the vaccine.
- Patients with IBD on immune-modifying therapies, who received either the Pfizer or Moderna vaccine, should receive a three-dose vaccination series, with the third dose at least 28 days after the second dose.
- Patients with IBD on biologic therapy can receive the third dose of the vaccine at any time point and should not interrupt biologic therapy.
- Boosters are likely to become available to the general public in September and would be given at least 8 months after an individual’s second dose.
- Recommendations regarding boosters for those who received a three-dose vaccination series are forthcoming.
- Recommendations regarding boosters and additional doses for those that received the Janssen vaccine are forthcoming.
- Gastroenterologists should take an active role in ensuring that their patients are vaccinated.
Dr. Schell is a second-year graduate student in the division of internal medicine at the University of Wisconsin–Madison. Dr. Caldera is an associate professor of medicine in the division of gastroenterology & hepatology at the University of Wisconsin–Madison. Dr. Schell has no conflicts of interest to disclose. Dr. Caldera has received research support from Takeda Pharmaceuticals and Sanofi. He has been a consultant for Takeda, Arena Pharmaceuticals, GSK, and Celgene.
References
1. Sadoff J et al. N Engl J Med. 2021;384(23):2187-201.
2. Baden LR et al. N Engl J Med. 2021;384(5):403-16.
3. Polack FP et al. N Engl J Med. 2020;383:2603-15.
4. Johnson K et al. U.S. FDA aims to give full approval to Pfizer vaccine on Monday – NYT. Reuters. 2021 Aug 20. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-aims-give-full-nod-pfizers-covid-19-vaccine-monday-new-york-times-2021-08-20/.
5. Allocca M et al. J Clin Med. 2020 Oct;9(11):3533.
6. Monteleone G and Ardizzone S. J Crohns Colitis. 2020 Sep;14(9):1334-6.
7. Papa A et al. Am J Gastroenterol. 2020;115(10):1722-4.
8. Derikx LAAP et al. J Crohn’s Colitis. 2021 Apr 6;15(4):529-39.
9. Brenner EJ et al. Gastroenterology. 2020;159(2):481-91.
10. Ungaro RC et al. Gut. 2021;70(4):725-32.
11. Caldera F et al. Inflamm Bowel Dis. 2020;26(4):593-602.
12. Botwin GJ et al. Am J Gastroenterol. 2021. doi: 10.14309/ajg.0000000000001342.
13. Squire JD et al. Inflamm Bowel Dis. 2021 Jul 27;27(8):1358-60.
14. Hadi YB et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.014.
15. Wong S-Y et al. Gastroenterology. 2021;161:715-8.
16. Kappelman MD et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.016.
17. Pozdnyakova V et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.08.014.
18. Ben-Tov A et al. Gastroenterology. 2021. doi: 10.1053/j.gastro.2021.06.076.
19. Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA News Release. 2021. Accessed 2021 Aug 18. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.
20. Centers for Disease Control and Prevention. COVID-19 Vaccines for Moderately to Severely Immunocompromised People. 2021. Accessed 2021 Aug 18. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html.
21. Allocca M et al. J Clin Med. 2020 Oct 31;9(11):3533.
22. Crohn’s & Colitis Foundation. COVID-19 Vaccines: Position Statements. IBD & Coronavirus. 2021. Accessed 2021 Aug 20. https://www.crohnscolitisfoundation.org/coronavirus/vaccine-position-statements.
23. Centers for Disease Control and Prevention. Joint Statement from HHS Public Health and Medical Experts on COVID-19 Booster Shots. https://www.cdc.gov/media/releases/2021/s0818-covid-19-booster-shots.html.
24. Caldera F et al. Inflamm Bowel Dis. 2021;27(1):123-133.
Residency programs need greater focus on BPD treatment
Borderline personality disorder (BPD) has suffered from underdiagnosis, in part because not enough clinicians know how to handle patients with BPD. “They don’t have the tools to know how to manage these situations effectively,” Lois W. Choi-Kain, MEd, MD, director of the Gunderson Personality Disorders Institute, McLean Hospital, Belmont, Mass., said in an interview.
As a result, the clinician avoids the BPD patient, who feels demeaned and never finds the capacity to get better.
Psychiatry training in residency tends to emphasize biomedical treatments and does not focus enough on learning psychotherapy and other psychosocial treatments, according to Eric M. Plakun, MD, DLFAPA, FACPsych, medical director/CEO of the Austen Riggs Center in Stockbridge, Mass.
“This is where I see the need for a greater psychotherapy teaching focus in residency, along with teaching of general principles for working with patients with BPD,” said Dr. Plakun.
In his last phase of his career, BPD pioneer John G. Gunderson, MD, worked with Dr. Choi-Kain to train clinicians on general psychiatric management (GPM), which employs a sensitive, nonattacking approach to diffuse and calm situations with BPD patients.
As interest grows in combining GPM with manual treatments, GPM alone offers a more accessible approach for therapist and patient, said Dr. Choi-Kain, who has been trying to promote its use and do research on its techniques.
“It’s trying to boil it down to make it simple,” she said. As much as evidence-based, manualized approaches have advanced the field, they’re just not that widely available, she said.
Orchestrating treatments such as dialectical behavior therapy and mentalization-based therapy takes a lot of specialization, noted Dr. Choi-Kain. “And because of the amount of work that it involves for both the clinician and the patient, it decreases the capacity that clinicians and systems have to offer treatment to a wider number of patients.”
Learning a manualized treatment for BPD is asking a lot from residents, agreed Dr. Plakun. “Those who want more immersion in treating these patients can pursue further training in residency electives, in postresidency graduate medical education programs or through psychoanalytic training.”
Borderline personality disorder (BPD) has suffered from underdiagnosis, in part because not enough clinicians know how to handle patients with BPD. “They don’t have the tools to know how to manage these situations effectively,” Lois W. Choi-Kain, MEd, MD, director of the Gunderson Personality Disorders Institute, McLean Hospital, Belmont, Mass., said in an interview.
As a result, the clinician avoids the BPD patient, who feels demeaned and never finds the capacity to get better.
Psychiatry training in residency tends to emphasize biomedical treatments and does not focus enough on learning psychotherapy and other psychosocial treatments, according to Eric M. Plakun, MD, DLFAPA, FACPsych, medical director/CEO of the Austen Riggs Center in Stockbridge, Mass.
“This is where I see the need for a greater psychotherapy teaching focus in residency, along with teaching of general principles for working with patients with BPD,” said Dr. Plakun.
In his last phase of his career, BPD pioneer John G. Gunderson, MD, worked with Dr. Choi-Kain to train clinicians on general psychiatric management (GPM), which employs a sensitive, nonattacking approach to diffuse and calm situations with BPD patients.
As interest grows in combining GPM with manual treatments, GPM alone offers a more accessible approach for therapist and patient, said Dr. Choi-Kain, who has been trying to promote its use and do research on its techniques.
“It’s trying to boil it down to make it simple,” she said. As much as evidence-based, manualized approaches have advanced the field, they’re just not that widely available, she said.
Orchestrating treatments such as dialectical behavior therapy and mentalization-based therapy takes a lot of specialization, noted Dr. Choi-Kain. “And because of the amount of work that it involves for both the clinician and the patient, it decreases the capacity that clinicians and systems have to offer treatment to a wider number of patients.”
Learning a manualized treatment for BPD is asking a lot from residents, agreed Dr. Plakun. “Those who want more immersion in treating these patients can pursue further training in residency electives, in postresidency graduate medical education programs or through psychoanalytic training.”
Borderline personality disorder (BPD) has suffered from underdiagnosis, in part because not enough clinicians know how to handle patients with BPD. “They don’t have the tools to know how to manage these situations effectively,” Lois W. Choi-Kain, MEd, MD, director of the Gunderson Personality Disorders Institute, McLean Hospital, Belmont, Mass., said in an interview.
As a result, the clinician avoids the BPD patient, who feels demeaned and never finds the capacity to get better.
Psychiatry training in residency tends to emphasize biomedical treatments and does not focus enough on learning psychotherapy and other psychosocial treatments, according to Eric M. Plakun, MD, DLFAPA, FACPsych, medical director/CEO of the Austen Riggs Center in Stockbridge, Mass.
“This is where I see the need for a greater psychotherapy teaching focus in residency, along with teaching of general principles for working with patients with BPD,” said Dr. Plakun.
In his last phase of his career, BPD pioneer John G. Gunderson, MD, worked with Dr. Choi-Kain to train clinicians on general psychiatric management (GPM), which employs a sensitive, nonattacking approach to diffuse and calm situations with BPD patients.
As interest grows in combining GPM with manual treatments, GPM alone offers a more accessible approach for therapist and patient, said Dr. Choi-Kain, who has been trying to promote its use and do research on its techniques.
“It’s trying to boil it down to make it simple,” she said. As much as evidence-based, manualized approaches have advanced the field, they’re just not that widely available, she said.
Orchestrating treatments such as dialectical behavior therapy and mentalization-based therapy takes a lot of specialization, noted Dr. Choi-Kain. “And because of the amount of work that it involves for both the clinician and the patient, it decreases the capacity that clinicians and systems have to offer treatment to a wider number of patients.”
Learning a manualized treatment for BPD is asking a lot from residents, agreed Dr. Plakun. “Those who want more immersion in treating these patients can pursue further training in residency electives, in postresidency graduate medical education programs or through psychoanalytic training.”
A new name for BPD?
Michael A. Cummings, MD, has never liked the term “borderline personality disorder” (BPD). In his view, it’s a misnomer and needs to be changed.
“What is it bordering on? It’s not bordering on something, it’s a disorder on its own,” said Dr. Cummings of the department of psychiatry at the University of California, Riverside, and a psychopharmacology consultant with the California Department of State Hospitals’ Psychopharmacology Resource Network.
BPD grew out of the concept that patients were bordering on something, perhaps becoming bipolar. “In many ways, I don’t think it is even a personality disorder. It appears to be an inherent temperament that evolves into an inability to regulate mood.”
In his view, this puts it in the category of a mood dysregulation disorder.
Changing the label would not necessarily improve treatment, he added. However, transitioning from a pejorative to a more neutral label could make it easier for people to say, “this is just a type of mood disorder. It’s not necessarily easy, but it’s workable,” said Dr. Cummings.
Others in the field contend that the term fits the condition. BPD “describes how it encompasses a lot of complex psychological difficulties, undermining functioning of patients in a specific way,” said Lois W. Choi-Kain, MD, MEd, director of the Gunderson Personality Disorders Institute, McLean Hospital, Belmont, Mass. The disorder was identified because of its relationship with other known psychiatric disorders, said Dr. Choi-Kain. “There’s an element of BPD that borders on mood disorders because moods are so unstable with BPD. It also borders on trauma-related disorders. It borders on psychotic disorders because there’s sometimes stress-induced experiences of losing contact with realistic thinking.”
If anything needs to change, it’s the attitude toward the disorder, not the name. “I don’t think the term itself is pejorative. But I think that associations with the term have been very stigmatizing. For a long time, there was an attitude that these patients could not be treated or had negative therapeutic reactions.”
Data suggest that these patients are highly prevalent in clinical settings. “And I interpret that as them seeking the care that they need rather than resisting care or not responding to care,” said Dr. Choi-Kain.
Michael A. Cummings, MD, has never liked the term “borderline personality disorder” (BPD). In his view, it’s a misnomer and needs to be changed.
“What is it bordering on? It’s not bordering on something, it’s a disorder on its own,” said Dr. Cummings of the department of psychiatry at the University of California, Riverside, and a psychopharmacology consultant with the California Department of State Hospitals’ Psychopharmacology Resource Network.
BPD grew out of the concept that patients were bordering on something, perhaps becoming bipolar. “In many ways, I don’t think it is even a personality disorder. It appears to be an inherent temperament that evolves into an inability to regulate mood.”
In his view, this puts it in the category of a mood dysregulation disorder.
Changing the label would not necessarily improve treatment, he added. However, transitioning from a pejorative to a more neutral label could make it easier for people to say, “this is just a type of mood disorder. It’s not necessarily easy, but it’s workable,” said Dr. Cummings.
Others in the field contend that the term fits the condition. BPD “describes how it encompasses a lot of complex psychological difficulties, undermining functioning of patients in a specific way,” said Lois W. Choi-Kain, MD, MEd, director of the Gunderson Personality Disorders Institute, McLean Hospital, Belmont, Mass. The disorder was identified because of its relationship with other known psychiatric disorders, said Dr. Choi-Kain. “There’s an element of BPD that borders on mood disorders because moods are so unstable with BPD. It also borders on trauma-related disorders. It borders on psychotic disorders because there’s sometimes stress-induced experiences of losing contact with realistic thinking.”
If anything needs to change, it’s the attitude toward the disorder, not the name. “I don’t think the term itself is pejorative. But I think that associations with the term have been very stigmatizing. For a long time, there was an attitude that these patients could not be treated or had negative therapeutic reactions.”
Data suggest that these patients are highly prevalent in clinical settings. “And I interpret that as them seeking the care that they need rather than resisting care or not responding to care,” said Dr. Choi-Kain.
Michael A. Cummings, MD, has never liked the term “borderline personality disorder” (BPD). In his view, it’s a misnomer and needs to be changed.
“What is it bordering on? It’s not bordering on something, it’s a disorder on its own,” said Dr. Cummings of the department of psychiatry at the University of California, Riverside, and a psychopharmacology consultant with the California Department of State Hospitals’ Psychopharmacology Resource Network.
BPD grew out of the concept that patients were bordering on something, perhaps becoming bipolar. “In many ways, I don’t think it is even a personality disorder. It appears to be an inherent temperament that evolves into an inability to regulate mood.”
In his view, this puts it in the category of a mood dysregulation disorder.
Changing the label would not necessarily improve treatment, he added. However, transitioning from a pejorative to a more neutral label could make it easier for people to say, “this is just a type of mood disorder. It’s not necessarily easy, but it’s workable,” said Dr. Cummings.
Others in the field contend that the term fits the condition. BPD “describes how it encompasses a lot of complex psychological difficulties, undermining functioning of patients in a specific way,” said Lois W. Choi-Kain, MD, MEd, director of the Gunderson Personality Disorders Institute, McLean Hospital, Belmont, Mass. The disorder was identified because of its relationship with other known psychiatric disorders, said Dr. Choi-Kain. “There’s an element of BPD that borders on mood disorders because moods are so unstable with BPD. It also borders on trauma-related disorders. It borders on psychotic disorders because there’s sometimes stress-induced experiences of losing contact with realistic thinking.”
If anything needs to change, it’s the attitude toward the disorder, not the name. “I don’t think the term itself is pejorative. But I think that associations with the term have been very stigmatizing. For a long time, there was an attitude that these patients could not be treated or had negative therapeutic reactions.”
Data suggest that these patients are highly prevalent in clinical settings. “And I interpret that as them seeking the care that they need rather than resisting care or not responding to care,” said Dr. Choi-Kain.
Trust is key in treating borderline personality disorder
Difficulties associated with treating borderline personality disorder (BPD) make for an uneasy alliance between patient and clinician. Deep-seated anxiety and trust issues often lead to patients skipping visits or raging at those who treat them, leaving clinicians frustrated and ready to give up or relying on a pill to make the patient better.
John M. Oldham, MD, MS, recalls one patient he almost lost, a woman who was struggling with aggressive behavior. Initially cooperative and punctual, the patient gradually became distrustful, grilling Dr. Oldham on his training and credentials. “As the questions continued, she slipped from being very cooperative to being enraged and attacking me,” said Dr. Oldham, Distinguished Emeritus Professor in the Menninger department of psychiatry and behavioral sciences at Baylor College in Houston.
Dr. Oldham eventually drew her back in by earning her trust. “There’s no magic to this,” he acknowledged. “You try to be as alert and informed and vigilant for anything you say that produces a negative or concerning reaction in the patient.”
This interactive approach to BPD treatment has been gaining traction in a profession that often looks to medications to alleviate specific symptoms. It’s so effective that it sometimes even surprises the patient, Dr. Oldham noted. “When you approach them like this, they can begin to settle down,” which was the case with the female patient he once treated.
About 1.4% of the U.S. population has BPD, according to the National Institute of Mental Health. Conceptualized by the late John G. Gunderson, MD, BPD initially was seen as floating on the borderline between psychosis and neurosis. Clinicians now understand that this isn’t the case. The patients need, as Dr. Gunderson once pointed out, constant vigilance because of attachment issues and childhood trauma.
A stable therapeutic alliance between patient and physician, sometimes in combination with evidence-based therapies, is a formula for success, some experts say.
A misunderstood condition
Although there is some degree of heritable risk, BPD patients are often the product of an invalidating environment in childhood. “As kids, we’re guided and nurtured by caring adults to provide models of reasonable, trustworthy behavior. If those role models are missing or just so inconsistent and unpredictable, the patient doesn’t end up with a sturdy self-image. Instead, they’re adrift, trying to figure out who will be helpful and be a meaningful, trustworthy companion and adviser,” Dr. Oldham said.
Emotional or affective instability and impulsivity, sometimes impulsive aggression, often characterize their condition. “Brain-imaging studies have revealed that certain nerve pathways that are necessary to regulate emotions are impoverished in patients with BPD,” Dr. Oldham said.
An analogy is a car going too fast, with a runaway engine that’s running too hot – and the brakes don’t work, he added.
“People think these patients are trying to create big drama, that they’re putting on a big show. That’s not accurate,” he continued. These patients don’t have the ability to stop the trigger that leads to their emotional storms. They also don’t have the ability to regulate themselves. “We may say, it’s a beautiful day outside, but I still have to go to work. Someone with BPD may say: It’s a beautiful day; I’m going to the beach,” Dr. Oldham explained.
A person with BPD might sound coherent when arguing with someone else. But their words are driven by the storm they can’t turn off.
This can lead to their own efforts to turn off the intensity. They might become self-injurious or push other people away. It’s one of the ironies of this condition because BPD patients desperately want to trust others but are scared to do so. “They look for any little signal – that someone else will hurt, disappoint, or leave them. Eventually their relationships unravel,” Dr. Oldham saod.
For some, suicide is sometimes a final solution.
Those traits make it difficult for a therapist to connect with a patient. “This is a very difficult group of people to treat and to establish treatment,” said Michael A. Cummings, MD, of the department of psychiatry at University of California, Riverside, and a psychopharmacology consultant with the California Department of State Hospitals’ Psychopharmacology Resource Network.
BPD patients tend to idealize people who are attempting to help them. When they become frustrated or disappointed in some way, “they then devalue the caregiver or the treatment and not infrequently, fall out of treatment,” Dr. Cummings said. It can be a very taxing experience, particularly for younger, less experienced therapists.
Medication only goes so far
Psychiatrists tend to look at BPD patients as receptor sites for molecules, assessing symptoms they can prescribe for, Eric M. Plakun, MD, DLFAPA, FACPsych, medical director/CEO of the Austen Riggs Center in Stockbridge, Mass., said in an interview.
Yet, BPD is not a molecular problem, principally. It’s an interpersonal disorder. When BPD is a co-occurring disorder, as is often the case, the depressive, anxiety, or other disorder can mask the BPD, he added, citing his 2018 paper on tensions in psychiatry between the biomedical and biopsychosocial models (Psychiatr Clin North Am. 2018 Jun;41[2]:237-48).
In one longitudinal study (J Pers Disord. 2005 Oct;19[5]:487-504), the presence of BPD strongly predicted the persistence of depression. BPD comorbid with depression is often a recipe for treatment-resistant depression, which results in higher costs, more utilization of resources, and higher suicide rates. Too often, psychiatrists diagnose the depression but miss the BPD. They keep trying molecular approaches with prescription drugs – even though it’s really the interpersonal issues of BPD that need to be addressed, said Dr. Plakun, who is a member of the Group for the Advancement of Psychiatry’s Psychotherapy Committee, and founder and past leader of the American Psychiatric Association’s Psychotherapy Caucus.
Medication can be helpful as a short-term adjunctive therapy. Long term, it’s not a sustainable approach, said Dr. Oldham. “If a patient is in a particularly stressful period, in the middle of a stormy breakup or having a depressive episode or talking about suicide, a time-limited course of an antidepressant may be helpful,” he said. They could also benefit from an anxiety-related drug or medication to help them sleep.
What you don’t want is for the patient to start relying on medications to help them feel better. The problem is, many are suffering so much that they’ll go to their primary care doctor and say, “I’m suffering from anxiety,” and get an antianxiety drug. Or they’re depressed or in pain and end up with a cocktail of medications. “And that’s just going to make matters worse,” Dr. Oldham said.
Psychotherapy as a first-line approach
APA practice guidelines and others worldwide have all come to the same conclusion about BPD. , who chaired an APA committee that developed an evidence-based practice guideline for patients with BPD.
Psychotherapy keeps the patient from firing you, he asserted. “Because of the lack of trust, they push away. They’re very scared, and this fear also applies to therapist. The goal is to help the patient learn to trust you. To do that, you need to develop a strong therapeutic alliance.”
In crafting the APA’s practice guideline, Dr. Oldham and his colleagues studied a variety of approaches, including mentalization-based therapy (MBT) and dialectical behavior therapy (DBT), which was developed by Marsha Linehan, PhD. Since then, other approaches have demonstrated efficacy in randomized clinical trials, including schema-based therapy (SBT), cognitive-behavioral therapy (CBT), and transference-focused psychotherapy (TFP).
Those treatments might complement the broader goal of establishing a strong alliance with the patient, Dr. Oldham said. Manualized approaches can help prepackage a program that allows clinicians and patients to look at their problems in an objective, nonpejorative way, Lois W. Choi-Kain, MD, MEd, director of the Gunderson Personality Disorders Institute at McLean Hospital in Belmont, Mass., said in an interview. DBT, for example, focuses on emotion dysregulation. MBT addresses how the patient sees themselves through others and their interactions with others. “It destigmatizes a problem as a clinical entity rather than an interpersonal problem between the patient and the clinician,” Dr. Choi-Kain said.
The choice of approach depends on several factors: the patient’s needs and preferences, and the therapist’s skills and experience, said Dr. Oldham. Some patients don’t do well with DBT because it involves a lot of homework and didactic work. Others do better with TFP because they want to understand what drives their behavior.
Dr. Cummings recalled how one of his patients used TFP to look inward and heal.
He first met the patient when she was in her early 30s. “She had made some progress, but I remember she was still struggling mightily with relationship issues and with identifying her role in relationships,” he said. The patient was becoming increasingly aware that she was going to end up alone and didn’t want that as an outcome.
Adapting to a TFP model, “she worked very hard trying to understand herself as she related to other people, understanding her own emotional volatility, and some of her proneness to behavioral problems,” Dr. Cummings said. The patient also had to learn how to negotiate her relationships to the point where she didn’t end up destroying them and alienating people.
Customizing the treatment
Physicians can choose from one of these manualized forms of treatment to see what’s appropriate and what works for the patient. “You can individualize the treatment, borrowing from these approaches and shaping it based on what your patient needs,” Dr. Oldham recommended.
Recently, the field of psychiatry has seen the benefits of combining manualized, evidence-based approaches with general psychiatric management (GPM), a method conceived by Dr. Gunderson. GPM “reflects a sensitive understanding of mental illness, offering ‘non attacking’ or collaborative work with the patient and a sensitive recognition of appropriate interventions or corrections to help the patient stay in treatment,” said Dr. Oldham.
It aims to conceptualize BPD in a clinically objective way, medicalizing the disorder so it’s something that the patient has, rather than something he or she is, explained Dr. Choi-Kain, who worked with Dr. Gunderson to train clinicians on using this approach. Using a framework that’s compatible with good medical practices, the clinician tries to define the problem together with the patient, “really assessing whether or not the treatment works, setting goals, managing safety, and trying to promote functioning, something we need to pay more attention to with BPD,” she said.
For these patients, the goal is to have positive, corrective experiences in the real world, reinforcing their hopes and what they’re capable of, and an interface with the world that makes them feel like contributors, she said.
Cycle of rupture and repair
Many people with BPD struggle with the desire to find and feel love, but also deal with their rage and hate. Hence, therapists must prepare themselves for the experience of sometimes being hated, said Dr. Plakun. The patient needs to feel they’re in a safe enough space to express those feelings, activating a cycle of “rupture and repair,” he continued.
The key in working with these patients is to avoid any language that will make them feel attacked or criticized, said Dr. Oldham.
A patient may get furious and say “I don’t know what you’re talking about. I didn’t say that.” When in truth, the psychiatrist is flat accurate about what the patient said. Instead of arguing with the patient, a physician can back up and say: “Help me understand what you’re feeling right now. What did I say that made you feel that you couldn’t trust me? Help me understand you. I may have made a mistake,” he advised.
Trust is a key ingredient in an alliance-based intervention for suicidal patients with BPD that Dr. Plakun has frequently written about. A bond he had with a deeply suicidal patient helped her overcome her grief and come to terms with an abusive childhood.
“She had a horrible history of abuse and had BPD and bipolar disorder. Even controlled with medications her life was still awful. She contemplated suicide relentlessly.” Working through her history of sexual abuse, the patient discovered that much of what she and clinicians thought of as a depressive illness was in fact intense grief about the irreparable damage that had taken place during childhood.
Through their work she was able to mourn, and her depression and BPD improved.
Developing a trusting relationship with the patient isn’t a starting point; it’s the goal, he emphasized.
“You don’t prescribe trust to someone. It’s earned.” Through the shared journey of therapy, as the patient suffers from inevitable injuries and ruptures and as the therapist reveals his or her imperfections, opportunities arise to nonjudgmentally examine and repair ruptures. This lead to gains in trust, he said.
It’s not just about genes
Many in the psychiatric and psychological communities tend to develop a very nihilistic view of BPD patients, observed Dr. Cummings. “They’ll say: ‘Oh, well, it’s hopeless. There’s nothing that can be done.’ That isn’t true,” he said.
Epidemiologic studies of these individuals have shown that many of these patients no longer meet the diagnostic criteria for BPD by the time they reach middle age. This means they get better over time, noted Dr. Cummings.
Dr. Plakun’s hope is that the field will evolve in a direction that recognizes the importance of psychosocial treatments like psychotherapy, in addition to biomedical treatments. The drive to medicate still exists, which can contribute to underdiagnosis and undertreatment of BPD, he said. “Although there are manualized, evidence-based treatments, few clinicians learn even one of these for BPD, not to mention those for other disorders.”
In 1996, Francis S. Collins, MD, PhD, the current director of the National Institutes of Health, predicted that the decoding of the human genome would transform treatment of medical and mental disorders [and] “that we would discover the ways in which genes equal disease,” said Dr. Plakun. What the science has since shown, is genes by environmental interaction lead to disease and health.
Nature and nurture both matter. “And I don’t think we’re paying enough attention to the nurture side,” Dr. Plakun said.
The solution is a return to a biopsychosocial model, recognizing that psychotherapy is an essential part of treatment of BPD and other conditions, and an essential clinician skill, he said.
Dr. Oldham is coeditor of the “Textbook of Personality Disorders”, 3rd edition (Washington: American Psychiatric Association Publishing, 2021).Dr. Choi-Kain is coeditor with Dr. Gunderson of “Applications of Good Psychiatric Management for Borderline Personality Disorder: A Practical Guide” (Washington: American Psychiatric Association Publishing, 2019).
Dr. Cummings and Dr. Plakun had no disclosures.
Difficulties associated with treating borderline personality disorder (BPD) make for an uneasy alliance between patient and clinician. Deep-seated anxiety and trust issues often lead to patients skipping visits or raging at those who treat them, leaving clinicians frustrated and ready to give up or relying on a pill to make the patient better.
John M. Oldham, MD, MS, recalls one patient he almost lost, a woman who was struggling with aggressive behavior. Initially cooperative and punctual, the patient gradually became distrustful, grilling Dr. Oldham on his training and credentials. “As the questions continued, she slipped from being very cooperative to being enraged and attacking me,” said Dr. Oldham, Distinguished Emeritus Professor in the Menninger department of psychiatry and behavioral sciences at Baylor College in Houston.
Dr. Oldham eventually drew her back in by earning her trust. “There’s no magic to this,” he acknowledged. “You try to be as alert and informed and vigilant for anything you say that produces a negative or concerning reaction in the patient.”
This interactive approach to BPD treatment has been gaining traction in a profession that often looks to medications to alleviate specific symptoms. It’s so effective that it sometimes even surprises the patient, Dr. Oldham noted. “When you approach them like this, they can begin to settle down,” which was the case with the female patient he once treated.
About 1.4% of the U.S. population has BPD, according to the National Institute of Mental Health. Conceptualized by the late John G. Gunderson, MD, BPD initially was seen as floating on the borderline between psychosis and neurosis. Clinicians now understand that this isn’t the case. The patients need, as Dr. Gunderson once pointed out, constant vigilance because of attachment issues and childhood trauma.
A stable therapeutic alliance between patient and physician, sometimes in combination with evidence-based therapies, is a formula for success, some experts say.
A misunderstood condition
Although there is some degree of heritable risk, BPD patients are often the product of an invalidating environment in childhood. “As kids, we’re guided and nurtured by caring adults to provide models of reasonable, trustworthy behavior. If those role models are missing or just so inconsistent and unpredictable, the patient doesn’t end up with a sturdy self-image. Instead, they’re adrift, trying to figure out who will be helpful and be a meaningful, trustworthy companion and adviser,” Dr. Oldham said.
Emotional or affective instability and impulsivity, sometimes impulsive aggression, often characterize their condition. “Brain-imaging studies have revealed that certain nerve pathways that are necessary to regulate emotions are impoverished in patients with BPD,” Dr. Oldham said.
An analogy is a car going too fast, with a runaway engine that’s running too hot – and the brakes don’t work, he added.
“People think these patients are trying to create big drama, that they’re putting on a big show. That’s not accurate,” he continued. These patients don’t have the ability to stop the trigger that leads to their emotional storms. They also don’t have the ability to regulate themselves. “We may say, it’s a beautiful day outside, but I still have to go to work. Someone with BPD may say: It’s a beautiful day; I’m going to the beach,” Dr. Oldham explained.
A person with BPD might sound coherent when arguing with someone else. But their words are driven by the storm they can’t turn off.
This can lead to their own efforts to turn off the intensity. They might become self-injurious or push other people away. It’s one of the ironies of this condition because BPD patients desperately want to trust others but are scared to do so. “They look for any little signal – that someone else will hurt, disappoint, or leave them. Eventually their relationships unravel,” Dr. Oldham saod.
For some, suicide is sometimes a final solution.
Those traits make it difficult for a therapist to connect with a patient. “This is a very difficult group of people to treat and to establish treatment,” said Michael A. Cummings, MD, of the department of psychiatry at University of California, Riverside, and a psychopharmacology consultant with the California Department of State Hospitals’ Psychopharmacology Resource Network.
BPD patients tend to idealize people who are attempting to help them. When they become frustrated or disappointed in some way, “they then devalue the caregiver or the treatment and not infrequently, fall out of treatment,” Dr. Cummings said. It can be a very taxing experience, particularly for younger, less experienced therapists.
Medication only goes so far
Psychiatrists tend to look at BPD patients as receptor sites for molecules, assessing symptoms they can prescribe for, Eric M. Plakun, MD, DLFAPA, FACPsych, medical director/CEO of the Austen Riggs Center in Stockbridge, Mass., said in an interview.
Yet, BPD is not a molecular problem, principally. It’s an interpersonal disorder. When BPD is a co-occurring disorder, as is often the case, the depressive, anxiety, or other disorder can mask the BPD, he added, citing his 2018 paper on tensions in psychiatry between the biomedical and biopsychosocial models (Psychiatr Clin North Am. 2018 Jun;41[2]:237-48).
In one longitudinal study (J Pers Disord. 2005 Oct;19[5]:487-504), the presence of BPD strongly predicted the persistence of depression. BPD comorbid with depression is often a recipe for treatment-resistant depression, which results in higher costs, more utilization of resources, and higher suicide rates. Too often, psychiatrists diagnose the depression but miss the BPD. They keep trying molecular approaches with prescription drugs – even though it’s really the interpersonal issues of BPD that need to be addressed, said Dr. Plakun, who is a member of the Group for the Advancement of Psychiatry’s Psychotherapy Committee, and founder and past leader of the American Psychiatric Association’s Psychotherapy Caucus.
Medication can be helpful as a short-term adjunctive therapy. Long term, it’s not a sustainable approach, said Dr. Oldham. “If a patient is in a particularly stressful period, in the middle of a stormy breakup or having a depressive episode or talking about suicide, a time-limited course of an antidepressant may be helpful,” he said. They could also benefit from an anxiety-related drug or medication to help them sleep.
What you don’t want is for the patient to start relying on medications to help them feel better. The problem is, many are suffering so much that they’ll go to their primary care doctor and say, “I’m suffering from anxiety,” and get an antianxiety drug. Or they’re depressed or in pain and end up with a cocktail of medications. “And that’s just going to make matters worse,” Dr. Oldham said.
Psychotherapy as a first-line approach
APA practice guidelines and others worldwide have all come to the same conclusion about BPD. , who chaired an APA committee that developed an evidence-based practice guideline for patients with BPD.
Psychotherapy keeps the patient from firing you, he asserted. “Because of the lack of trust, they push away. They’re very scared, and this fear also applies to therapist. The goal is to help the patient learn to trust you. To do that, you need to develop a strong therapeutic alliance.”
In crafting the APA’s practice guideline, Dr. Oldham and his colleagues studied a variety of approaches, including mentalization-based therapy (MBT) and dialectical behavior therapy (DBT), which was developed by Marsha Linehan, PhD. Since then, other approaches have demonstrated efficacy in randomized clinical trials, including schema-based therapy (SBT), cognitive-behavioral therapy (CBT), and transference-focused psychotherapy (TFP).
Those treatments might complement the broader goal of establishing a strong alliance with the patient, Dr. Oldham said. Manualized approaches can help prepackage a program that allows clinicians and patients to look at their problems in an objective, nonpejorative way, Lois W. Choi-Kain, MD, MEd, director of the Gunderson Personality Disorders Institute at McLean Hospital in Belmont, Mass., said in an interview. DBT, for example, focuses on emotion dysregulation. MBT addresses how the patient sees themselves through others and their interactions with others. “It destigmatizes a problem as a clinical entity rather than an interpersonal problem between the patient and the clinician,” Dr. Choi-Kain said.
The choice of approach depends on several factors: the patient’s needs and preferences, and the therapist’s skills and experience, said Dr. Oldham. Some patients don’t do well with DBT because it involves a lot of homework and didactic work. Others do better with TFP because they want to understand what drives their behavior.
Dr. Cummings recalled how one of his patients used TFP to look inward and heal.
He first met the patient when she was in her early 30s. “She had made some progress, but I remember she was still struggling mightily with relationship issues and with identifying her role in relationships,” he said. The patient was becoming increasingly aware that she was going to end up alone and didn’t want that as an outcome.
Adapting to a TFP model, “she worked very hard trying to understand herself as she related to other people, understanding her own emotional volatility, and some of her proneness to behavioral problems,” Dr. Cummings said. The patient also had to learn how to negotiate her relationships to the point where she didn’t end up destroying them and alienating people.
Customizing the treatment
Physicians can choose from one of these manualized forms of treatment to see what’s appropriate and what works for the patient. “You can individualize the treatment, borrowing from these approaches and shaping it based on what your patient needs,” Dr. Oldham recommended.
Recently, the field of psychiatry has seen the benefits of combining manualized, evidence-based approaches with general psychiatric management (GPM), a method conceived by Dr. Gunderson. GPM “reflects a sensitive understanding of mental illness, offering ‘non attacking’ or collaborative work with the patient and a sensitive recognition of appropriate interventions or corrections to help the patient stay in treatment,” said Dr. Oldham.
It aims to conceptualize BPD in a clinically objective way, medicalizing the disorder so it’s something that the patient has, rather than something he or she is, explained Dr. Choi-Kain, who worked with Dr. Gunderson to train clinicians on using this approach. Using a framework that’s compatible with good medical practices, the clinician tries to define the problem together with the patient, “really assessing whether or not the treatment works, setting goals, managing safety, and trying to promote functioning, something we need to pay more attention to with BPD,” she said.
For these patients, the goal is to have positive, corrective experiences in the real world, reinforcing their hopes and what they’re capable of, and an interface with the world that makes them feel like contributors, she said.
Cycle of rupture and repair
Many people with BPD struggle with the desire to find and feel love, but also deal with their rage and hate. Hence, therapists must prepare themselves for the experience of sometimes being hated, said Dr. Plakun. The patient needs to feel they’re in a safe enough space to express those feelings, activating a cycle of “rupture and repair,” he continued.
The key in working with these patients is to avoid any language that will make them feel attacked or criticized, said Dr. Oldham.
A patient may get furious and say “I don’t know what you’re talking about. I didn’t say that.” When in truth, the psychiatrist is flat accurate about what the patient said. Instead of arguing with the patient, a physician can back up and say: “Help me understand what you’re feeling right now. What did I say that made you feel that you couldn’t trust me? Help me understand you. I may have made a mistake,” he advised.
Trust is a key ingredient in an alliance-based intervention for suicidal patients with BPD that Dr. Plakun has frequently written about. A bond he had with a deeply suicidal patient helped her overcome her grief and come to terms with an abusive childhood.
“She had a horrible history of abuse and had BPD and bipolar disorder. Even controlled with medications her life was still awful. She contemplated suicide relentlessly.” Working through her history of sexual abuse, the patient discovered that much of what she and clinicians thought of as a depressive illness was in fact intense grief about the irreparable damage that had taken place during childhood.
Through their work she was able to mourn, and her depression and BPD improved.
Developing a trusting relationship with the patient isn’t a starting point; it’s the goal, he emphasized.
“You don’t prescribe trust to someone. It’s earned.” Through the shared journey of therapy, as the patient suffers from inevitable injuries and ruptures and as the therapist reveals his or her imperfections, opportunities arise to nonjudgmentally examine and repair ruptures. This lead to gains in trust, he said.
It’s not just about genes
Many in the psychiatric and psychological communities tend to develop a very nihilistic view of BPD patients, observed Dr. Cummings. “They’ll say: ‘Oh, well, it’s hopeless. There’s nothing that can be done.’ That isn’t true,” he said.
Epidemiologic studies of these individuals have shown that many of these patients no longer meet the diagnostic criteria for BPD by the time they reach middle age. This means they get better over time, noted Dr. Cummings.
Dr. Plakun’s hope is that the field will evolve in a direction that recognizes the importance of psychosocial treatments like psychotherapy, in addition to biomedical treatments. The drive to medicate still exists, which can contribute to underdiagnosis and undertreatment of BPD, he said. “Although there are manualized, evidence-based treatments, few clinicians learn even one of these for BPD, not to mention those for other disorders.”
In 1996, Francis S. Collins, MD, PhD, the current director of the National Institutes of Health, predicted that the decoding of the human genome would transform treatment of medical and mental disorders [and] “that we would discover the ways in which genes equal disease,” said Dr. Plakun. What the science has since shown, is genes by environmental interaction lead to disease and health.
Nature and nurture both matter. “And I don’t think we’re paying enough attention to the nurture side,” Dr. Plakun said.
The solution is a return to a biopsychosocial model, recognizing that psychotherapy is an essential part of treatment of BPD and other conditions, and an essential clinician skill, he said.
Dr. Oldham is coeditor of the “Textbook of Personality Disorders”, 3rd edition (Washington: American Psychiatric Association Publishing, 2021).Dr. Choi-Kain is coeditor with Dr. Gunderson of “Applications of Good Psychiatric Management for Borderline Personality Disorder: A Practical Guide” (Washington: American Psychiatric Association Publishing, 2019).
Dr. Cummings and Dr. Plakun had no disclosures.
Difficulties associated with treating borderline personality disorder (BPD) make for an uneasy alliance between patient and clinician. Deep-seated anxiety and trust issues often lead to patients skipping visits or raging at those who treat them, leaving clinicians frustrated and ready to give up or relying on a pill to make the patient better.
John M. Oldham, MD, MS, recalls one patient he almost lost, a woman who was struggling with aggressive behavior. Initially cooperative and punctual, the patient gradually became distrustful, grilling Dr. Oldham on his training and credentials. “As the questions continued, she slipped from being very cooperative to being enraged and attacking me,” said Dr. Oldham, Distinguished Emeritus Professor in the Menninger department of psychiatry and behavioral sciences at Baylor College in Houston.
Dr. Oldham eventually drew her back in by earning her trust. “There’s no magic to this,” he acknowledged. “You try to be as alert and informed and vigilant for anything you say that produces a negative or concerning reaction in the patient.”
This interactive approach to BPD treatment has been gaining traction in a profession that often looks to medications to alleviate specific symptoms. It’s so effective that it sometimes even surprises the patient, Dr. Oldham noted. “When you approach them like this, they can begin to settle down,” which was the case with the female patient he once treated.
About 1.4% of the U.S. population has BPD, according to the National Institute of Mental Health. Conceptualized by the late John G. Gunderson, MD, BPD initially was seen as floating on the borderline between psychosis and neurosis. Clinicians now understand that this isn’t the case. The patients need, as Dr. Gunderson once pointed out, constant vigilance because of attachment issues and childhood trauma.
A stable therapeutic alliance between patient and physician, sometimes in combination with evidence-based therapies, is a formula for success, some experts say.
A misunderstood condition
Although there is some degree of heritable risk, BPD patients are often the product of an invalidating environment in childhood. “As kids, we’re guided and nurtured by caring adults to provide models of reasonable, trustworthy behavior. If those role models are missing or just so inconsistent and unpredictable, the patient doesn’t end up with a sturdy self-image. Instead, they’re adrift, trying to figure out who will be helpful and be a meaningful, trustworthy companion and adviser,” Dr. Oldham said.
Emotional or affective instability and impulsivity, sometimes impulsive aggression, often characterize their condition. “Brain-imaging studies have revealed that certain nerve pathways that are necessary to regulate emotions are impoverished in patients with BPD,” Dr. Oldham said.
An analogy is a car going too fast, with a runaway engine that’s running too hot – and the brakes don’t work, he added.
“People think these patients are trying to create big drama, that they’re putting on a big show. That’s not accurate,” he continued. These patients don’t have the ability to stop the trigger that leads to their emotional storms. They also don’t have the ability to regulate themselves. “We may say, it’s a beautiful day outside, but I still have to go to work. Someone with BPD may say: It’s a beautiful day; I’m going to the beach,” Dr. Oldham explained.
A person with BPD might sound coherent when arguing with someone else. But their words are driven by the storm they can’t turn off.
This can lead to their own efforts to turn off the intensity. They might become self-injurious or push other people away. It’s one of the ironies of this condition because BPD patients desperately want to trust others but are scared to do so. “They look for any little signal – that someone else will hurt, disappoint, or leave them. Eventually their relationships unravel,” Dr. Oldham saod.
For some, suicide is sometimes a final solution.
Those traits make it difficult for a therapist to connect with a patient. “This is a very difficult group of people to treat and to establish treatment,” said Michael A. Cummings, MD, of the department of psychiatry at University of California, Riverside, and a psychopharmacology consultant with the California Department of State Hospitals’ Psychopharmacology Resource Network.
BPD patients tend to idealize people who are attempting to help them. When they become frustrated or disappointed in some way, “they then devalue the caregiver or the treatment and not infrequently, fall out of treatment,” Dr. Cummings said. It can be a very taxing experience, particularly for younger, less experienced therapists.
Medication only goes so far
Psychiatrists tend to look at BPD patients as receptor sites for molecules, assessing symptoms they can prescribe for, Eric M. Plakun, MD, DLFAPA, FACPsych, medical director/CEO of the Austen Riggs Center in Stockbridge, Mass., said in an interview.
Yet, BPD is not a molecular problem, principally. It’s an interpersonal disorder. When BPD is a co-occurring disorder, as is often the case, the depressive, anxiety, or other disorder can mask the BPD, he added, citing his 2018 paper on tensions in psychiatry between the biomedical and biopsychosocial models (Psychiatr Clin North Am. 2018 Jun;41[2]:237-48).
In one longitudinal study (J Pers Disord. 2005 Oct;19[5]:487-504), the presence of BPD strongly predicted the persistence of depression. BPD comorbid with depression is often a recipe for treatment-resistant depression, which results in higher costs, more utilization of resources, and higher suicide rates. Too often, psychiatrists diagnose the depression but miss the BPD. They keep trying molecular approaches with prescription drugs – even though it’s really the interpersonal issues of BPD that need to be addressed, said Dr. Plakun, who is a member of the Group for the Advancement of Psychiatry’s Psychotherapy Committee, and founder and past leader of the American Psychiatric Association’s Psychotherapy Caucus.
Medication can be helpful as a short-term adjunctive therapy. Long term, it’s not a sustainable approach, said Dr. Oldham. “If a patient is in a particularly stressful period, in the middle of a stormy breakup or having a depressive episode or talking about suicide, a time-limited course of an antidepressant may be helpful,” he said. They could also benefit from an anxiety-related drug or medication to help them sleep.
What you don’t want is for the patient to start relying on medications to help them feel better. The problem is, many are suffering so much that they’ll go to their primary care doctor and say, “I’m suffering from anxiety,” and get an antianxiety drug. Or they’re depressed or in pain and end up with a cocktail of medications. “And that’s just going to make matters worse,” Dr. Oldham said.
Psychotherapy as a first-line approach
APA practice guidelines and others worldwide have all come to the same conclusion about BPD. , who chaired an APA committee that developed an evidence-based practice guideline for patients with BPD.
Psychotherapy keeps the patient from firing you, he asserted. “Because of the lack of trust, they push away. They’re very scared, and this fear also applies to therapist. The goal is to help the patient learn to trust you. To do that, you need to develop a strong therapeutic alliance.”
In crafting the APA’s practice guideline, Dr. Oldham and his colleagues studied a variety of approaches, including mentalization-based therapy (MBT) and dialectical behavior therapy (DBT), which was developed by Marsha Linehan, PhD. Since then, other approaches have demonstrated efficacy in randomized clinical trials, including schema-based therapy (SBT), cognitive-behavioral therapy (CBT), and transference-focused psychotherapy (TFP).
Those treatments might complement the broader goal of establishing a strong alliance with the patient, Dr. Oldham said. Manualized approaches can help prepackage a program that allows clinicians and patients to look at their problems in an objective, nonpejorative way, Lois W. Choi-Kain, MD, MEd, director of the Gunderson Personality Disorders Institute at McLean Hospital in Belmont, Mass., said in an interview. DBT, for example, focuses on emotion dysregulation. MBT addresses how the patient sees themselves through others and their interactions with others. “It destigmatizes a problem as a clinical entity rather than an interpersonal problem between the patient and the clinician,” Dr. Choi-Kain said.
The choice of approach depends on several factors: the patient’s needs and preferences, and the therapist’s skills and experience, said Dr. Oldham. Some patients don’t do well with DBT because it involves a lot of homework and didactic work. Others do better with TFP because they want to understand what drives their behavior.
Dr. Cummings recalled how one of his patients used TFP to look inward and heal.
He first met the patient when she was in her early 30s. “She had made some progress, but I remember she was still struggling mightily with relationship issues and with identifying her role in relationships,” he said. The patient was becoming increasingly aware that she was going to end up alone and didn’t want that as an outcome.
Adapting to a TFP model, “she worked very hard trying to understand herself as she related to other people, understanding her own emotional volatility, and some of her proneness to behavioral problems,” Dr. Cummings said. The patient also had to learn how to negotiate her relationships to the point where she didn’t end up destroying them and alienating people.
Customizing the treatment
Physicians can choose from one of these manualized forms of treatment to see what’s appropriate and what works for the patient. “You can individualize the treatment, borrowing from these approaches and shaping it based on what your patient needs,” Dr. Oldham recommended.
Recently, the field of psychiatry has seen the benefits of combining manualized, evidence-based approaches with general psychiatric management (GPM), a method conceived by Dr. Gunderson. GPM “reflects a sensitive understanding of mental illness, offering ‘non attacking’ or collaborative work with the patient and a sensitive recognition of appropriate interventions or corrections to help the patient stay in treatment,” said Dr. Oldham.
It aims to conceptualize BPD in a clinically objective way, medicalizing the disorder so it’s something that the patient has, rather than something he or she is, explained Dr. Choi-Kain, who worked with Dr. Gunderson to train clinicians on using this approach. Using a framework that’s compatible with good medical practices, the clinician tries to define the problem together with the patient, “really assessing whether or not the treatment works, setting goals, managing safety, and trying to promote functioning, something we need to pay more attention to with BPD,” she said.
For these patients, the goal is to have positive, corrective experiences in the real world, reinforcing their hopes and what they’re capable of, and an interface with the world that makes them feel like contributors, she said.
Cycle of rupture and repair
Many people with BPD struggle with the desire to find and feel love, but also deal with their rage and hate. Hence, therapists must prepare themselves for the experience of sometimes being hated, said Dr. Plakun. The patient needs to feel they’re in a safe enough space to express those feelings, activating a cycle of “rupture and repair,” he continued.
The key in working with these patients is to avoid any language that will make them feel attacked or criticized, said Dr. Oldham.
A patient may get furious and say “I don’t know what you’re talking about. I didn’t say that.” When in truth, the psychiatrist is flat accurate about what the patient said. Instead of arguing with the patient, a physician can back up and say: “Help me understand what you’re feeling right now. What did I say that made you feel that you couldn’t trust me? Help me understand you. I may have made a mistake,” he advised.
Trust is a key ingredient in an alliance-based intervention for suicidal patients with BPD that Dr. Plakun has frequently written about. A bond he had with a deeply suicidal patient helped her overcome her grief and come to terms with an abusive childhood.
“She had a horrible history of abuse and had BPD and bipolar disorder. Even controlled with medications her life was still awful. She contemplated suicide relentlessly.” Working through her history of sexual abuse, the patient discovered that much of what she and clinicians thought of as a depressive illness was in fact intense grief about the irreparable damage that had taken place during childhood.
Through their work she was able to mourn, and her depression and BPD improved.
Developing a trusting relationship with the patient isn’t a starting point; it’s the goal, he emphasized.
“You don’t prescribe trust to someone. It’s earned.” Through the shared journey of therapy, as the patient suffers from inevitable injuries and ruptures and as the therapist reveals his or her imperfections, opportunities arise to nonjudgmentally examine and repair ruptures. This lead to gains in trust, he said.
It’s not just about genes
Many in the psychiatric and psychological communities tend to develop a very nihilistic view of BPD patients, observed Dr. Cummings. “They’ll say: ‘Oh, well, it’s hopeless. There’s nothing that can be done.’ That isn’t true,” he said.
Epidemiologic studies of these individuals have shown that many of these patients no longer meet the diagnostic criteria for BPD by the time they reach middle age. This means they get better over time, noted Dr. Cummings.
Dr. Plakun’s hope is that the field will evolve in a direction that recognizes the importance of psychosocial treatments like psychotherapy, in addition to biomedical treatments. The drive to medicate still exists, which can contribute to underdiagnosis and undertreatment of BPD, he said. “Although there are manualized, evidence-based treatments, few clinicians learn even one of these for BPD, not to mention those for other disorders.”
In 1996, Francis S. Collins, MD, PhD, the current director of the National Institutes of Health, predicted that the decoding of the human genome would transform treatment of medical and mental disorders [and] “that we would discover the ways in which genes equal disease,” said Dr. Plakun. What the science has since shown, is genes by environmental interaction lead to disease and health.
Nature and nurture both matter. “And I don’t think we’re paying enough attention to the nurture side,” Dr. Plakun said.
The solution is a return to a biopsychosocial model, recognizing that psychotherapy is an essential part of treatment of BPD and other conditions, and an essential clinician skill, he said.
Dr. Oldham is coeditor of the “Textbook of Personality Disorders”, 3rd edition (Washington: American Psychiatric Association Publishing, 2021).Dr. Choi-Kain is coeditor with Dr. Gunderson of “Applications of Good Psychiatric Management for Borderline Personality Disorder: A Practical Guide” (Washington: American Psychiatric Association Publishing, 2019).
Dr. Cummings and Dr. Plakun had no disclosures.
Patients panic as docs cut off breast cancer drug
The discontinuance appears to be in reaction to an announcement by the manufacturer (Genentech) in late August that it has voluntarily withdrawn its application for accelerated approval of the drug for use in metastatic triple-negative breast cancer (mTNBC).
However, experts stress that discontinuing atezolizumab is not advised if a patient is responding to or is stable on the immune checkpoint inhibitor.
“I think the Genentech announcement has been misinterpreted,” Maryam Lustberg, MD, of Yale Cancer Center, New Haven, Conn., said in an interview. “The consensus opinion from all academic breast oncologists is that people should not be switching off atezolizumab if they are responding. They should not be changing their immunotherapy.”
Dr. Lustberg said the announcement had two major points: “don’t start a new patient on atezolizumab,” and the company is “committed” to supplying the drug to patients whose conditions are stable or responding.
Nevertheless, some patients with mTNBC were recently in a state of escalating emotional upset, said one patient advocate.
“The level of panic among those currently on & responding well to Atezo is growing quickly,” tweeted Janice Cowden on Sept. 5, a former nurse living with mTNBC in Bradenton, Fla.
Ms. Cowden explained that “at least 10-20 patients” were “pulled [off the drug by their oncologists] this past week who have been stable/no evidence of disease/no evidence of disease activity on Tecentriq.”
She estimated that as many as 50 patients in the 2,200-member Triple Negative BC Stage 4 Facebook group who have been responding to the drug were abruptly de-prescribed atezolizumab since the Aug. 27 announcement from Genentech.
Many women learned of the change via patient portals or text messaging, not directly from their physicians, Cowden told Medscape Medical News.
Some of the women had been taking atezolizumab for 2-3 years, including those with no evidence of disease, she said. “Finding out that their oncologist was discontinuing a treatment that was working for them has been driving so much anxiety and stress,” Ms. Cowden emphasized.
Most market withdrawals of drugs are related to safety, but that is not the case with atezolizumab, said Sara Horton, MD, of Howard University, Washington. She was speaking at the recent Facebook webinar on atezolizumab and mTNBC that was sponsored by the TNBC Foundation and the Young Survivors Coalition.
In the case of atezolizumab, it was a question about efficacy that prompted the withdrawal. After the indication was granted an accelerated approval on the basis of response data, a confirmatory trial set out to show clinical benefit. However, the confirmatory phase 3 IMpassion131 trial did not do so: it found that atezolizumab plus paclitaxel did not significantly reduce the risk for cancer progression and death in comparison with paclitaxel plus placebo among patients with TNBC with tumors that were positive for programmed cell death protein–1 (PD-L1), as reported by Medscape Medical News.
These results were discussed by the Food and Drug Administration on the first day of a historic 3-day meeting on accelerated approvals in April 2021. Despite the failure of confirmation of clinical benefit, the advisory panel voted 7-2 in favor of keeping the approval in place for atezolizumab in TNBC. At the same time, it urged Genentech to carry out more studies to show that the drug works in this patient population.
The company apparently decided not to do that and instead voluntarily withdrew the application for the indication some 4 months later.
During the recent TNBC Foundation webinar, Genentech official Lauren Davis said that the company sent letters about this decision to atezolizumab-prescribing physicians and included another letter that was to be shared with patients. Ms. Davis had not responded to this news organization’s request to review the communications at the time this article was published.
At the webinar, Ms. Davis did clarify that current atezolizumab patients (who are responding to the drug), who have commercial insurance, and who benefit from Genentech’s copay program will continue to receive the benefit until June 2022.
In its August announcement, Genentech said it decided to withdraw the atezolizumab approval on the basis of the FDA’s assessment of the “current mTNBC treatment landscape and in accordance with the requirements of the accelerated approval program.”
That landscape presumably includes pembrolizumab (Keytruda), which received a full approval for a TNBC indication similar to that of atezolizumab in July. That full approval was based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen in comparison with neoadjuvant chemotherapy alone for previously untreated stage II or III TNBC. Details of these clinical data will be presented at the upcoming annual meeting of the European Society of Medical Oncology.
Switching the immunotherapy?
Some U.S. oncologists have been telling patients with mTNBC that the atezolizumab withdrawal is “not an issue” because the new full approval of pembrolizumab in this setting will allow prescriptions to be switched, said patient advocate Ms. Cowden.
However, experts have said that no patient who is responding to or whose condition is stable with atezolizumab should switch immunotherapies. “This is a very aggressive disease,” reminded Dr. Lustberg.
Switching the immunotherapies is complicated by the difference in the respective drugs’ companion biomarker assays used to establish the presence of PD-L1.
Dr. Lustberg explained that patients who are not responding to atezolizumab and who now want to try pembrolizumab will have to be assessed with the CTS assay.
“About 22% of the patients who are positive for the atezolizumab biomarker assay SP-142 are not going to be positive for the CTS,” she said.
In other words, about one in four patients with mTNBC who are taking atezolizumab will not qualify for treatment with pembrolizumab.
Rebecca Shatsky, MD, of the University of California, San Diego, echoed those comments in an email to this news organization – and emphatically discouraged switching off atezolizumab (and going on pembrolizumab) if a patient is having success (i.e., stable disease or positive response).
“The two groups don’t always overlap, so it isn’t an easy switch. That’s why if they are already responding, I would NOT have them stop the drug,” she said.
Not every mTNBC patient receiving – and responding to – atezolizumab has had the unfortunate experience of having their prescription canceled.
Johanna Rauhala, of San Francisco, who is a former middle-school teacher and who writes the blog Pink Stinks, has been taking atezolizumab for 2 years. She has had a partial response and now, after taking the immunotherapy in combination with chemotherapy (gemcitabine and carboplatin), has stable disease. Currently, she is taking single-agent atezolizumab..
Ms. Rauhala has been living with mTNBC for 5 years. She said in an interview that she was “very surprised and concerned” to learn about Genentech’s withdrawal of its accelerated approval. She said that at her next treatment appointment, she was “probably going to ask the oncology nurse first [about the atezolizumab withdrawal] – because they are the front line, and I will then follow-up with my doctor. But I can’t imagine that they will take away something that is working.”
Dr. Shatsky, Dr. Horton, and Dr. Lunsberg report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
The discontinuance appears to be in reaction to an announcement by the manufacturer (Genentech) in late August that it has voluntarily withdrawn its application for accelerated approval of the drug for use in metastatic triple-negative breast cancer (mTNBC).
However, experts stress that discontinuing atezolizumab is not advised if a patient is responding to or is stable on the immune checkpoint inhibitor.
“I think the Genentech announcement has been misinterpreted,” Maryam Lustberg, MD, of Yale Cancer Center, New Haven, Conn., said in an interview. “The consensus opinion from all academic breast oncologists is that people should not be switching off atezolizumab if they are responding. They should not be changing their immunotherapy.”
Dr. Lustberg said the announcement had two major points: “don’t start a new patient on atezolizumab,” and the company is “committed” to supplying the drug to patients whose conditions are stable or responding.
Nevertheless, some patients with mTNBC were recently in a state of escalating emotional upset, said one patient advocate.
“The level of panic among those currently on & responding well to Atezo is growing quickly,” tweeted Janice Cowden on Sept. 5, a former nurse living with mTNBC in Bradenton, Fla.
Ms. Cowden explained that “at least 10-20 patients” were “pulled [off the drug by their oncologists] this past week who have been stable/no evidence of disease/no evidence of disease activity on Tecentriq.”
She estimated that as many as 50 patients in the 2,200-member Triple Negative BC Stage 4 Facebook group who have been responding to the drug were abruptly de-prescribed atezolizumab since the Aug. 27 announcement from Genentech.
Many women learned of the change via patient portals or text messaging, not directly from their physicians, Cowden told Medscape Medical News.
Some of the women had been taking atezolizumab for 2-3 years, including those with no evidence of disease, she said. “Finding out that their oncologist was discontinuing a treatment that was working for them has been driving so much anxiety and stress,” Ms. Cowden emphasized.
Most market withdrawals of drugs are related to safety, but that is not the case with atezolizumab, said Sara Horton, MD, of Howard University, Washington. She was speaking at the recent Facebook webinar on atezolizumab and mTNBC that was sponsored by the TNBC Foundation and the Young Survivors Coalition.
In the case of atezolizumab, it was a question about efficacy that prompted the withdrawal. After the indication was granted an accelerated approval on the basis of response data, a confirmatory trial set out to show clinical benefit. However, the confirmatory phase 3 IMpassion131 trial did not do so: it found that atezolizumab plus paclitaxel did not significantly reduce the risk for cancer progression and death in comparison with paclitaxel plus placebo among patients with TNBC with tumors that were positive for programmed cell death protein–1 (PD-L1), as reported by Medscape Medical News.
These results were discussed by the Food and Drug Administration on the first day of a historic 3-day meeting on accelerated approvals in April 2021. Despite the failure of confirmation of clinical benefit, the advisory panel voted 7-2 in favor of keeping the approval in place for atezolizumab in TNBC. At the same time, it urged Genentech to carry out more studies to show that the drug works in this patient population.
The company apparently decided not to do that and instead voluntarily withdrew the application for the indication some 4 months later.
During the recent TNBC Foundation webinar, Genentech official Lauren Davis said that the company sent letters about this decision to atezolizumab-prescribing physicians and included another letter that was to be shared with patients. Ms. Davis had not responded to this news organization’s request to review the communications at the time this article was published.
At the webinar, Ms. Davis did clarify that current atezolizumab patients (who are responding to the drug), who have commercial insurance, and who benefit from Genentech’s copay program will continue to receive the benefit until June 2022.
In its August announcement, Genentech said it decided to withdraw the atezolizumab approval on the basis of the FDA’s assessment of the “current mTNBC treatment landscape and in accordance with the requirements of the accelerated approval program.”
That landscape presumably includes pembrolizumab (Keytruda), which received a full approval for a TNBC indication similar to that of atezolizumab in July. That full approval was based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen in comparison with neoadjuvant chemotherapy alone for previously untreated stage II or III TNBC. Details of these clinical data will be presented at the upcoming annual meeting of the European Society of Medical Oncology.
Switching the immunotherapy?
Some U.S. oncologists have been telling patients with mTNBC that the atezolizumab withdrawal is “not an issue” because the new full approval of pembrolizumab in this setting will allow prescriptions to be switched, said patient advocate Ms. Cowden.
However, experts have said that no patient who is responding to or whose condition is stable with atezolizumab should switch immunotherapies. “This is a very aggressive disease,” reminded Dr. Lustberg.
Switching the immunotherapies is complicated by the difference in the respective drugs’ companion biomarker assays used to establish the presence of PD-L1.
Dr. Lustberg explained that patients who are not responding to atezolizumab and who now want to try pembrolizumab will have to be assessed with the CTS assay.
“About 22% of the patients who are positive for the atezolizumab biomarker assay SP-142 are not going to be positive for the CTS,” she said.
In other words, about one in four patients with mTNBC who are taking atezolizumab will not qualify for treatment with pembrolizumab.
Rebecca Shatsky, MD, of the University of California, San Diego, echoed those comments in an email to this news organization – and emphatically discouraged switching off atezolizumab (and going on pembrolizumab) if a patient is having success (i.e., stable disease or positive response).
“The two groups don’t always overlap, so it isn’t an easy switch. That’s why if they are already responding, I would NOT have them stop the drug,” she said.
Not every mTNBC patient receiving – and responding to – atezolizumab has had the unfortunate experience of having their prescription canceled.
Johanna Rauhala, of San Francisco, who is a former middle-school teacher and who writes the blog Pink Stinks, has been taking atezolizumab for 2 years. She has had a partial response and now, after taking the immunotherapy in combination with chemotherapy (gemcitabine and carboplatin), has stable disease. Currently, she is taking single-agent atezolizumab..
Ms. Rauhala has been living with mTNBC for 5 years. She said in an interview that she was “very surprised and concerned” to learn about Genentech’s withdrawal of its accelerated approval. She said that at her next treatment appointment, she was “probably going to ask the oncology nurse first [about the atezolizumab withdrawal] – because they are the front line, and I will then follow-up with my doctor. But I can’t imagine that they will take away something that is working.”
Dr. Shatsky, Dr. Horton, and Dr. Lunsberg report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
The discontinuance appears to be in reaction to an announcement by the manufacturer (Genentech) in late August that it has voluntarily withdrawn its application for accelerated approval of the drug for use in metastatic triple-negative breast cancer (mTNBC).
However, experts stress that discontinuing atezolizumab is not advised if a patient is responding to or is stable on the immune checkpoint inhibitor.
“I think the Genentech announcement has been misinterpreted,” Maryam Lustberg, MD, of Yale Cancer Center, New Haven, Conn., said in an interview. “The consensus opinion from all academic breast oncologists is that people should not be switching off atezolizumab if they are responding. They should not be changing their immunotherapy.”
Dr. Lustberg said the announcement had two major points: “don’t start a new patient on atezolizumab,” and the company is “committed” to supplying the drug to patients whose conditions are stable or responding.
Nevertheless, some patients with mTNBC were recently in a state of escalating emotional upset, said one patient advocate.
“The level of panic among those currently on & responding well to Atezo is growing quickly,” tweeted Janice Cowden on Sept. 5, a former nurse living with mTNBC in Bradenton, Fla.
Ms. Cowden explained that “at least 10-20 patients” were “pulled [off the drug by their oncologists] this past week who have been stable/no evidence of disease/no evidence of disease activity on Tecentriq.”
She estimated that as many as 50 patients in the 2,200-member Triple Negative BC Stage 4 Facebook group who have been responding to the drug were abruptly de-prescribed atezolizumab since the Aug. 27 announcement from Genentech.
Many women learned of the change via patient portals or text messaging, not directly from their physicians, Cowden told Medscape Medical News.
Some of the women had been taking atezolizumab for 2-3 years, including those with no evidence of disease, she said. “Finding out that their oncologist was discontinuing a treatment that was working for them has been driving so much anxiety and stress,” Ms. Cowden emphasized.
Most market withdrawals of drugs are related to safety, but that is not the case with atezolizumab, said Sara Horton, MD, of Howard University, Washington. She was speaking at the recent Facebook webinar on atezolizumab and mTNBC that was sponsored by the TNBC Foundation and the Young Survivors Coalition.
In the case of atezolizumab, it was a question about efficacy that prompted the withdrawal. After the indication was granted an accelerated approval on the basis of response data, a confirmatory trial set out to show clinical benefit. However, the confirmatory phase 3 IMpassion131 trial did not do so: it found that atezolizumab plus paclitaxel did not significantly reduce the risk for cancer progression and death in comparison with paclitaxel plus placebo among patients with TNBC with tumors that were positive for programmed cell death protein–1 (PD-L1), as reported by Medscape Medical News.
These results were discussed by the Food and Drug Administration on the first day of a historic 3-day meeting on accelerated approvals in April 2021. Despite the failure of confirmation of clinical benefit, the advisory panel voted 7-2 in favor of keeping the approval in place for atezolizumab in TNBC. At the same time, it urged Genentech to carry out more studies to show that the drug works in this patient population.
The company apparently decided not to do that and instead voluntarily withdrew the application for the indication some 4 months later.
During the recent TNBC Foundation webinar, Genentech official Lauren Davis said that the company sent letters about this decision to atezolizumab-prescribing physicians and included another letter that was to be shared with patients. Ms. Davis had not responded to this news organization’s request to review the communications at the time this article was published.
At the webinar, Ms. Davis did clarify that current atezolizumab patients (who are responding to the drug), who have commercial insurance, and who benefit from Genentech’s copay program will continue to receive the benefit until June 2022.
In its August announcement, Genentech said it decided to withdraw the atezolizumab approval on the basis of the FDA’s assessment of the “current mTNBC treatment landscape and in accordance with the requirements of the accelerated approval program.”
That landscape presumably includes pembrolizumab (Keytruda), which received a full approval for a TNBC indication similar to that of atezolizumab in July. That full approval was based on findings from the randomized, phase 3 KEYNOTE-522 trial, which showed significantly prolonged event-free survival with the pembrolizumab regimen in comparison with neoadjuvant chemotherapy alone for previously untreated stage II or III TNBC. Details of these clinical data will be presented at the upcoming annual meeting of the European Society of Medical Oncology.
Switching the immunotherapy?
Some U.S. oncologists have been telling patients with mTNBC that the atezolizumab withdrawal is “not an issue” because the new full approval of pembrolizumab in this setting will allow prescriptions to be switched, said patient advocate Ms. Cowden.
However, experts have said that no patient who is responding to or whose condition is stable with atezolizumab should switch immunotherapies. “This is a very aggressive disease,” reminded Dr. Lustberg.
Switching the immunotherapies is complicated by the difference in the respective drugs’ companion biomarker assays used to establish the presence of PD-L1.
Dr. Lustberg explained that patients who are not responding to atezolizumab and who now want to try pembrolizumab will have to be assessed with the CTS assay.
“About 22% of the patients who are positive for the atezolizumab biomarker assay SP-142 are not going to be positive for the CTS,” she said.
In other words, about one in four patients with mTNBC who are taking atezolizumab will not qualify for treatment with pembrolizumab.
Rebecca Shatsky, MD, of the University of California, San Diego, echoed those comments in an email to this news organization – and emphatically discouraged switching off atezolizumab (and going on pembrolizumab) if a patient is having success (i.e., stable disease or positive response).
“The two groups don’t always overlap, so it isn’t an easy switch. That’s why if they are already responding, I would NOT have them stop the drug,” she said.
Not every mTNBC patient receiving – and responding to – atezolizumab has had the unfortunate experience of having their prescription canceled.
Johanna Rauhala, of San Francisco, who is a former middle-school teacher and who writes the blog Pink Stinks, has been taking atezolizumab for 2 years. She has had a partial response and now, after taking the immunotherapy in combination with chemotherapy (gemcitabine and carboplatin), has stable disease. Currently, she is taking single-agent atezolizumab..
Ms. Rauhala has been living with mTNBC for 5 years. She said in an interview that she was “very surprised and concerned” to learn about Genentech’s withdrawal of its accelerated approval. She said that at her next treatment appointment, she was “probably going to ask the oncology nurse first [about the atezolizumab withdrawal] – because they are the front line, and I will then follow-up with my doctor. But I can’t imagine that they will take away something that is working.”
Dr. Shatsky, Dr. Horton, and Dr. Lunsberg report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Flurry of cancer drug endorsements from EU panel
The CHMP recommended the granting of a conditional marketing authorization for pralsetinib (Gavreto) for the treatment of non–small cell lung cancer (NSCLC).
Specifically, pralsetinib is indicated as monotherapy for the treatment of adult patients with rearranged during transfection (RET) fusion-positive advanced NSCLC not previously treated with a RET inhibitor.
Available as 100 mg capsules, pralsetinib is a RET-receptor tyrosine kinase inhibitor, targeting oncogenic RET fusion proteins (KIF5B-RET and CCDC6-RET).
Pralsetinib’s benefits are its objective response rate and response duration in patients with RET-fusion positive NSCLC, as observed in a pivotal phase 1/2, open-label, multi-cohort, single-arm study.
The most common side effects are anemia, increased aspartate aminotransferase, neutropenia, constipation, musculoskeletal pain, fatigue, leukopenia, increased alanine aminotransferase, and hypertension.
CHMP also recommended ripretinib (Qinlock) for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with three or more kinase inhibitors, including imatinib (Gleevec).
Available as 50 mg tablets, ripretinib is a protein kinase inhibitor designed to selectively block the oncogenic KIT and PDGFRA kinases by inhibiting their active conformation.
Ripretinib improved progression-free survival in patients with GIST.
The most common side effects are fatigue, alopecia, nausea, myalgia, constipation, diarrhea, palmar-plantar erythrodysesthesia syndrome, weight loss, and vomiting.
The third drug recommended for approval was zanubrutinib (Brukinsa) for the treatment of adult patients with Waldenström’s macroglobulinemia who have received at least one prior therapy or who are to receive the drug as first-line treatment (and are unsuitable for chemo-immunotherapy).
Available as 80 mg capsules, zanubrutinib is a Bruton’s tyrosine kinase inhibitor that blocks the activity of BTK, inactivating the pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion.
Zanubrutinib has demonstrated a clinically meaningful rate of very good partial response and/or complete response.
The most common side effects are neutropenia, thrombocytopenia, upper respiratory tract infection, hemorrhage/hematoma, rash, bruising, anemia, musculoskeletal pain, diarrhea, pneumonia, and cough.
Two new indications for already marketed drugs
CHMP also recommended an extension of the indications for two immunotherapies.
Pembrolizumab (Keytruda) will now also have an indication for use in combination with chemotherapy for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumors express PD-L1 with a CPS greater than or equal to 10 and who have not received prior chemotherapy for metastatic disease
Nivolumab (Opdivo) received an extension of indication to include use, in combination with fluoropyrimidine- and platinum-based combination chemotherapy, in the firstline treatment of adult patients with HER2 negative advanced or metastatic gastric, gastroesophageal junction, or esophageal adenocarcinoma whose tumors express PD-L1 with a combined positive score (CPS) greater than or equal to 5.
A version of this article first appeared on Medscape.com.
The CHMP recommended the granting of a conditional marketing authorization for pralsetinib (Gavreto) for the treatment of non–small cell lung cancer (NSCLC).
Specifically, pralsetinib is indicated as monotherapy for the treatment of adult patients with rearranged during transfection (RET) fusion-positive advanced NSCLC not previously treated with a RET inhibitor.
Available as 100 mg capsules, pralsetinib is a RET-receptor tyrosine kinase inhibitor, targeting oncogenic RET fusion proteins (KIF5B-RET and CCDC6-RET).
Pralsetinib’s benefits are its objective response rate and response duration in patients with RET-fusion positive NSCLC, as observed in a pivotal phase 1/2, open-label, multi-cohort, single-arm study.
The most common side effects are anemia, increased aspartate aminotransferase, neutropenia, constipation, musculoskeletal pain, fatigue, leukopenia, increased alanine aminotransferase, and hypertension.
CHMP also recommended ripretinib (Qinlock) for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with three or more kinase inhibitors, including imatinib (Gleevec).
Available as 50 mg tablets, ripretinib is a protein kinase inhibitor designed to selectively block the oncogenic KIT and PDGFRA kinases by inhibiting their active conformation.
Ripretinib improved progression-free survival in patients with GIST.
The most common side effects are fatigue, alopecia, nausea, myalgia, constipation, diarrhea, palmar-plantar erythrodysesthesia syndrome, weight loss, and vomiting.
The third drug recommended for approval was zanubrutinib (Brukinsa) for the treatment of adult patients with Waldenström’s macroglobulinemia who have received at least one prior therapy or who are to receive the drug as first-line treatment (and are unsuitable for chemo-immunotherapy).
Available as 80 mg capsules, zanubrutinib is a Bruton’s tyrosine kinase inhibitor that blocks the activity of BTK, inactivating the pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion.
Zanubrutinib has demonstrated a clinically meaningful rate of very good partial response and/or complete response.
The most common side effects are neutropenia, thrombocytopenia, upper respiratory tract infection, hemorrhage/hematoma, rash, bruising, anemia, musculoskeletal pain, diarrhea, pneumonia, and cough.
Two new indications for already marketed drugs
CHMP also recommended an extension of the indications for two immunotherapies.
Pembrolizumab (Keytruda) will now also have an indication for use in combination with chemotherapy for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumors express PD-L1 with a CPS greater than or equal to 10 and who have not received prior chemotherapy for metastatic disease
Nivolumab (Opdivo) received an extension of indication to include use, in combination with fluoropyrimidine- and platinum-based combination chemotherapy, in the firstline treatment of adult patients with HER2 negative advanced or metastatic gastric, gastroesophageal junction, or esophageal adenocarcinoma whose tumors express PD-L1 with a combined positive score (CPS) greater than or equal to 5.
A version of this article first appeared on Medscape.com.
The CHMP recommended the granting of a conditional marketing authorization for pralsetinib (Gavreto) for the treatment of non–small cell lung cancer (NSCLC).
Specifically, pralsetinib is indicated as monotherapy for the treatment of adult patients with rearranged during transfection (RET) fusion-positive advanced NSCLC not previously treated with a RET inhibitor.
Available as 100 mg capsules, pralsetinib is a RET-receptor tyrosine kinase inhibitor, targeting oncogenic RET fusion proteins (KIF5B-RET and CCDC6-RET).
Pralsetinib’s benefits are its objective response rate and response duration in patients with RET-fusion positive NSCLC, as observed in a pivotal phase 1/2, open-label, multi-cohort, single-arm study.
The most common side effects are anemia, increased aspartate aminotransferase, neutropenia, constipation, musculoskeletal pain, fatigue, leukopenia, increased alanine aminotransferase, and hypertension.
CHMP also recommended ripretinib (Qinlock) for the treatment of adult patients with advanced gastrointestinal stromal tumor (GIST) who have received prior treatment with three or more kinase inhibitors, including imatinib (Gleevec).
Available as 50 mg tablets, ripretinib is a protein kinase inhibitor designed to selectively block the oncogenic KIT and PDGFRA kinases by inhibiting their active conformation.
Ripretinib improved progression-free survival in patients with GIST.
The most common side effects are fatigue, alopecia, nausea, myalgia, constipation, diarrhea, palmar-plantar erythrodysesthesia syndrome, weight loss, and vomiting.
The third drug recommended for approval was zanubrutinib (Brukinsa) for the treatment of adult patients with Waldenström’s macroglobulinemia who have received at least one prior therapy or who are to receive the drug as first-line treatment (and are unsuitable for chemo-immunotherapy).
Available as 80 mg capsules, zanubrutinib is a Bruton’s tyrosine kinase inhibitor that blocks the activity of BTK, inactivating the pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion.
Zanubrutinib has demonstrated a clinically meaningful rate of very good partial response and/or complete response.
The most common side effects are neutropenia, thrombocytopenia, upper respiratory tract infection, hemorrhage/hematoma, rash, bruising, anemia, musculoskeletal pain, diarrhea, pneumonia, and cough.
Two new indications for already marketed drugs
CHMP also recommended an extension of the indications for two immunotherapies.
Pembrolizumab (Keytruda) will now also have an indication for use in combination with chemotherapy for the treatment of locally recurrent unresectable or metastatic triple negative breast cancer in adults whose tumors express PD-L1 with a CPS greater than or equal to 10 and who have not received prior chemotherapy for metastatic disease
Nivolumab (Opdivo) received an extension of indication to include use, in combination with fluoropyrimidine- and platinum-based combination chemotherapy, in the firstline treatment of adult patients with HER2 negative advanced or metastatic gastric, gastroesophageal junction, or esophageal adenocarcinoma whose tumors express PD-L1 with a combined positive score (CPS) greater than or equal to 5.
A version of this article first appeared on Medscape.com.
FDA approves first oral drug for NSCLC with EGFR Exon 20 insertion
The drug is limited to use in patients whose disease has progressed on or after platinum-based chemotherapy and who have had the EGFR Exon 20 insertion mutation detected on an FDA-approved test.
Mobocertinib is the first oral tyrosine kinase inhibitor (TKI) specifically designed to target these mutations, which are less common than the more predominant EGFR mutations in this lung cancer.
“EGFR Exon 20 insertion+ NSCLC is an underserved cancer that we have been unable to target effectively with traditional EGFR TKIs,” said Pasi Jänne, MD, PhD, of the Dana Farber Cancer Institute, Boston, in a press statement from the maker, Takeda.
“The approval of [mobocertinib] marks another important step forward that provides physicians and their patients with a new targeted oral therapy specifically designed for this patient population that has shown clinically meaningful and sustained responses,” Dr. Jänne added.
According to the company, EGFR Exon 20 insertion+ NSCLC makes up approximately 1%-2% of patients with NSCLC and is more common in Asian populations compared with Western populations.
The new approval is based on overall response rate (ORR) and duration of response (DoR) results from a phase 1/2 trial consisting of 114 patients with EGFR Exon 20 insertion+ NSCLC who received prior platinum-based therapy and were treated with the 160-mg dose.
Per an independent review committee, mobocertinib demonstrated a confirmed ORR of 28% and a median DoR of 17.5 months.
Median overall survival was 24 months and median progression-free survival was 7.3 months.
The FDA-approved next-generation sequencing (NGS) companion diagnostic for mobocertinib is Thermo Fisher Scientific’s Oncomine Dx Target Test, which identifies NSCLC patients with EGFR Exon 20 insertions.
“NGS testing is critical for these patients, as it can enable more accurate diagnoses compared to polymerase chain reaction (PCR) testing, which detects less than 50% of EGFR Exon 20 insertions,” according to the company.
Results from the phase 1/2 trial used in the FDA approval were presented at the 2021 American Society of Clinical Oncology Annual Meeting.
The most common adverse reactions (greater than 20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain, according to the company.
The prescribing information includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings and precautions for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
“Patients with EGFR Exon 20 insertion+ NSCLC have historically faced a unique set of challenges living with a very rare lung cancer that is not only underdiagnosed but also lacking targeted treatment options that can improve response rates,” said Marcia Horn, executive director, Exon 20 Group at the International Cancer Advocacy Network, in the press statement.
The FDA review was conducted under Project Orbis, an FDA initiative that enables concurrent submission and review of oncology products among international partners.
The new drug was also granted priority review and received breakthrough therapy, fast track, and orphan drug designations from the FDA.
A version of this article first appeared on Medscape.com.
The drug is limited to use in patients whose disease has progressed on or after platinum-based chemotherapy and who have had the EGFR Exon 20 insertion mutation detected on an FDA-approved test.
Mobocertinib is the first oral tyrosine kinase inhibitor (TKI) specifically designed to target these mutations, which are less common than the more predominant EGFR mutations in this lung cancer.
“EGFR Exon 20 insertion+ NSCLC is an underserved cancer that we have been unable to target effectively with traditional EGFR TKIs,” said Pasi Jänne, MD, PhD, of the Dana Farber Cancer Institute, Boston, in a press statement from the maker, Takeda.
“The approval of [mobocertinib] marks another important step forward that provides physicians and their patients with a new targeted oral therapy specifically designed for this patient population that has shown clinically meaningful and sustained responses,” Dr. Jänne added.
According to the company, EGFR Exon 20 insertion+ NSCLC makes up approximately 1%-2% of patients with NSCLC and is more common in Asian populations compared with Western populations.
The new approval is based on overall response rate (ORR) and duration of response (DoR) results from a phase 1/2 trial consisting of 114 patients with EGFR Exon 20 insertion+ NSCLC who received prior platinum-based therapy and were treated with the 160-mg dose.
Per an independent review committee, mobocertinib demonstrated a confirmed ORR of 28% and a median DoR of 17.5 months.
Median overall survival was 24 months and median progression-free survival was 7.3 months.
The FDA-approved next-generation sequencing (NGS) companion diagnostic for mobocertinib is Thermo Fisher Scientific’s Oncomine Dx Target Test, which identifies NSCLC patients with EGFR Exon 20 insertions.
“NGS testing is critical for these patients, as it can enable more accurate diagnoses compared to polymerase chain reaction (PCR) testing, which detects less than 50% of EGFR Exon 20 insertions,” according to the company.
Results from the phase 1/2 trial used in the FDA approval were presented at the 2021 American Society of Clinical Oncology Annual Meeting.
The most common adverse reactions (greater than 20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain, according to the company.
The prescribing information includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings and precautions for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
“Patients with EGFR Exon 20 insertion+ NSCLC have historically faced a unique set of challenges living with a very rare lung cancer that is not only underdiagnosed but also lacking targeted treatment options that can improve response rates,” said Marcia Horn, executive director, Exon 20 Group at the International Cancer Advocacy Network, in the press statement.
The FDA review was conducted under Project Orbis, an FDA initiative that enables concurrent submission and review of oncology products among international partners.
The new drug was also granted priority review and received breakthrough therapy, fast track, and orphan drug designations from the FDA.
A version of this article first appeared on Medscape.com.
The drug is limited to use in patients whose disease has progressed on or after platinum-based chemotherapy and who have had the EGFR Exon 20 insertion mutation detected on an FDA-approved test.
Mobocertinib is the first oral tyrosine kinase inhibitor (TKI) specifically designed to target these mutations, which are less common than the more predominant EGFR mutations in this lung cancer.
“EGFR Exon 20 insertion+ NSCLC is an underserved cancer that we have been unable to target effectively with traditional EGFR TKIs,” said Pasi Jänne, MD, PhD, of the Dana Farber Cancer Institute, Boston, in a press statement from the maker, Takeda.
“The approval of [mobocertinib] marks another important step forward that provides physicians and their patients with a new targeted oral therapy specifically designed for this patient population that has shown clinically meaningful and sustained responses,” Dr. Jänne added.
According to the company, EGFR Exon 20 insertion+ NSCLC makes up approximately 1%-2% of patients with NSCLC and is more common in Asian populations compared with Western populations.
The new approval is based on overall response rate (ORR) and duration of response (DoR) results from a phase 1/2 trial consisting of 114 patients with EGFR Exon 20 insertion+ NSCLC who received prior platinum-based therapy and were treated with the 160-mg dose.
Per an independent review committee, mobocertinib demonstrated a confirmed ORR of 28% and a median DoR of 17.5 months.
Median overall survival was 24 months and median progression-free survival was 7.3 months.
The FDA-approved next-generation sequencing (NGS) companion diagnostic for mobocertinib is Thermo Fisher Scientific’s Oncomine Dx Target Test, which identifies NSCLC patients with EGFR Exon 20 insertions.
“NGS testing is critical for these patients, as it can enable more accurate diagnoses compared to polymerase chain reaction (PCR) testing, which detects less than 50% of EGFR Exon 20 insertions,” according to the company.
Results from the phase 1/2 trial used in the FDA approval were presented at the 2021 American Society of Clinical Oncology Annual Meeting.
The most common adverse reactions (greater than 20%) were diarrhea, rash, nausea, stomatitis, vomiting, decreased appetite, paronychia, fatigue, dry skin, and musculoskeletal pain, according to the company.
The prescribing information includes a boxed warning for QTc prolongation and Torsades de Pointes, and warnings and precautions for interstitial lung disease/pneumonitis, cardiac toxicity, and diarrhea.
“Patients with EGFR Exon 20 insertion+ NSCLC have historically faced a unique set of challenges living with a very rare lung cancer that is not only underdiagnosed but also lacking targeted treatment options that can improve response rates,” said Marcia Horn, executive director, Exon 20 Group at the International Cancer Advocacy Network, in the press statement.
The FDA review was conducted under Project Orbis, an FDA initiative that enables concurrent submission and review of oncology products among international partners.
The new drug was also granted priority review and received breakthrough therapy, fast track, and orphan drug designations from the FDA.
A version of this article first appeared on Medscape.com.
Immunotherapy for cancer patients with poor PS needs a rethink
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.









