User login
Moderate alcohol intake may curb subsequent diabetes after gestational diabetes
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
Among women with a history of gestational diabetes, alcohol intake of half a drink to one drink daily was associated with a 55% lower risk for subsequent type 2 diabetes, based on data from approximately 4,700 women in the Nurses’ Health Study II cohort.
However, the findings must be considered in the context of other risks and benefits of alcohol consumption before making statements or clinical recommendations, wrote Stefanie N. Hinkle, PhD, of the National Institutes of Health, Bethesda, Md., and colleagues.
Women with a history of gestational diabetes remain at increased risk for developing type 2 diabetes, so modifiable diet and lifestyle factors deserve further study, the researchers noted. Previous research has shown an association between light to moderate alcohol consumption and reduced risk of type 2 diabetes among women in the general population, but data on a similar risk reduction for women with a history of gestational diabetes are lacking, they added.
In a study published in JAMA Network Open, the researchers reviewed data from 4,740 women enrolled in the Nurses’ Health Study II who reported a history of gestational diabetes. These women were followed from Jan. 1, 1991, to Dec. 31, 2017, as part of the Diabetes & Women’s Health Study; dietary intake, including alcohol intake, was assessed every 4 years via validated food frequency questionnaires.
The average age at baseline was 38 years, and the median follow-up time was 24 years, yielding a total of 78,328 person-years of follow-up. Alcohol consumption was divided into four categories: none; 0.1 g/day to 4.9 g/day; 5.0 to 14.9 g/day, and 15.0 g/day or higher.
A total of 897 incident cases of type 2 diabetes were reported during the study period. After adjustment for multiple dietary and lifestyle variables, including diet and physical activity, only alcohol consumption of 5.0-14.9 g/day (approximately half a drink to one drink) was associated with a significantly decreased risk for incident type 2 diabetes (hazard ratio, 0.45) compared with women who reported no alcohol consumption.
On further adjustment for body mass index, women who reported alcohol consumption in the 5.0-14.9 g/day range had a 41% lower risk for developing incident type 2 diabetes (HR, 0.59); alcohol consumption in the other ranges remained unassociated with type 2 diabetes risk, although the researchers noted that these estimates were attenuated.
The median daily intake for women who consumed alcohol was 2.3 g/day, approximately one drink per week. Beer was the most frequently consumed type of alcohol.
When the researchers analyzed the data by alcohol type, notably, “only beer consumption of 1 or more servings a week was associated with a lower risk for type 2 diabetes,” although previous studies have suggested a stronger association in diabetes risk reduction with wine consumption vs. beer, the researchers noted.
The study findings were the potential for confounding factors not included in the adjustment, potential underreporting of alcohol intake, and potential screening bias toward women who were more health conscious, the researchers noted. Other limitations were lack of generalizability given that most of the study participants were white women, and a lack of data on binge drinking and whether alcohol was consumed with meals, they added. The study strengths included the prospective design, large size, long-term follow-up, and use of validated questionnaires, they said.
The researchers cautioned that the results should not be interpreted without considering other health outcomes. “Consistent with the 2020 Dietary Guidelines for Americans, which recommend that adults who do not consume alcohol do not initiate drinking, it may not be prudent for those with a history of gestational diabetes who do not consume alcohol to initiate drinking alcohol solely to reduce their risk for type 2 diabetes,” they emphasized.
Risk/benefit ratio for alcohol includes many factors
“There is a relative paucity of data regarding women’s long-term health as it may relate to pregnancy and pregnancy outcomes,” Angela Bianco, MD, of Mount Sinai Hospital, New York, said in an interview.
Dr. Bianco said she was surprised by some of the study findings.
“Generally speaking, I consider alcohol to be of little to no nutritional value, and to have a high sugar content/glycemic index,” she said. “However, a reduced incidence of adult-onset diabetes has been observed among moderate drinkers in other large prospective studies as well,” she noted. “In contrast, some studies have shown an increased risk of diabetes among a proportion of subjects in the top alcohol consumption category, while other studies have found no association. Possible inconsistencies may be due to differences in drinking patterns and the types of beverages consumed,” Dr. Bianco explained.
A key point for clinicians to keep in mind is that “the study may be flawed based on the different criteria used to make a diagnosis of history of gestational diabetes, the fact that they excluded patients that did not return the questionnaires, and the fact that respondents may not have answered correctly due to recall bias” or other reasons, Dr. Bianco said. “Additionally, those who responded obviously had access to health care, which in and of itself is a confounder,” she noted.
Another key point is that “the effect of alcohol being consumed with or without a meal was not examined,” said Dr. Bianco. “Alcohol concentration is reduced if consumed with meals. Alcohol can lead to hypoglycemia (from reduced gluconeogenesis) during fasting states, but after meals (postprandial states) it can result in lower glucose disposal and higher blood glucose levels,” she said. “The available literature suggests that alcohol may improve insulin sensitivity and reduce resistance, but there is likely a U-shaped association between alcohol consumption and the risk of diabetes,” Dr. Bianco noted. “There is likely a delicate balance between benefits and risks of alcohol intake. The inherent benefit/risk ratio must take into account with other potential comorbidities including BMI, activity level, stress, and preexisting conditions,” she said.
“Additional long-term studies engaging patients with diverse ethnic and socioeconomic backgrounds with detailed information regarding the role of nutrition, alcohol intake, tobacco and drug use, environmental exposures, and medical comorbidities need to be performed,” Dr. Bianco concluded.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of General Medical Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases; the Nurses’ Health Study II was supported by the National Institutes of Health. Lead author Dr. Hinkle and coauthor Cuilin Zhang, MD, are employees of the U.S. federal government. The researchers and Dr. Bianco had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Overlapping Phenotypic Features of PTEN Hamartoma Tumor Syndrome and Birt-Hogg-Dubé Syndrome
To the Editor:
PTEN hamartoma tumor syndrome (PHTS) encompasses a spectrum of disorders that most commonly are caused by autosomal-dominant germline mutations in the phosphatase and tensin homolog, PTEN, tumor suppressor gene on chromosome 10q23. We describe a patient who presented with clinical features of PHTS and Birt-Hogg-Dubé syndrome (BHDS). Because the genetic mutations associated with both PHTS and BHDS result in altered mammalian target of rapamycin (mTOR) signaling, patients may have overlapping phenotypic features.
A 51-year-old man with a history of multiple carcinomas presented for evaluation of flesh-colored papules on the cheeks, nose, tongue, and hands, in addition to numerous skin tags on the neck, axillae, and lower abdomen bilaterally. His medical history was notable for several nasal and gastrointestinal tract polyps, chromophobe renal cell carcinoma, cutaneous lipomas, atypical carcinoid syndrome of the right lung, and a multinodular thyroid. His family history was notable for small cell lung cancer in his father, breast cancer and pancreatic cancer in his maternal aunt, esophageal cancer in his maternal grandfather, and celiac disease in his daughter.
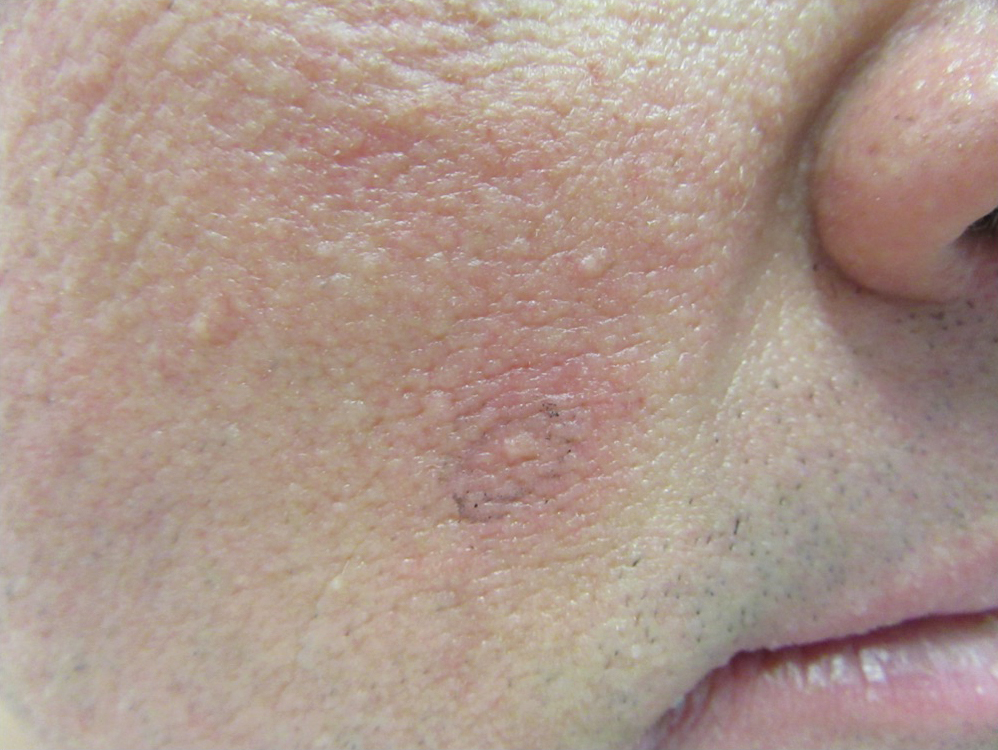
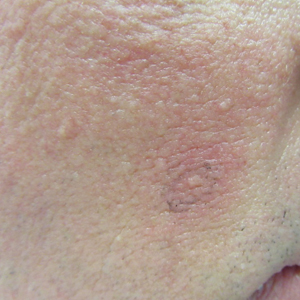
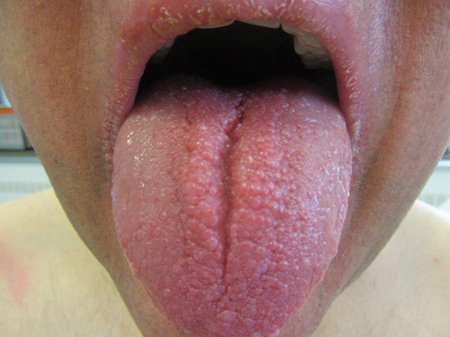
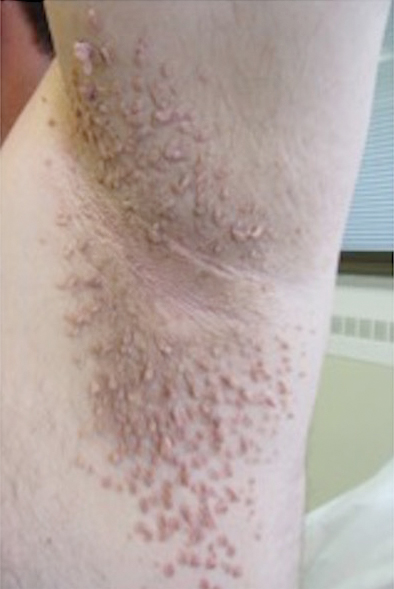
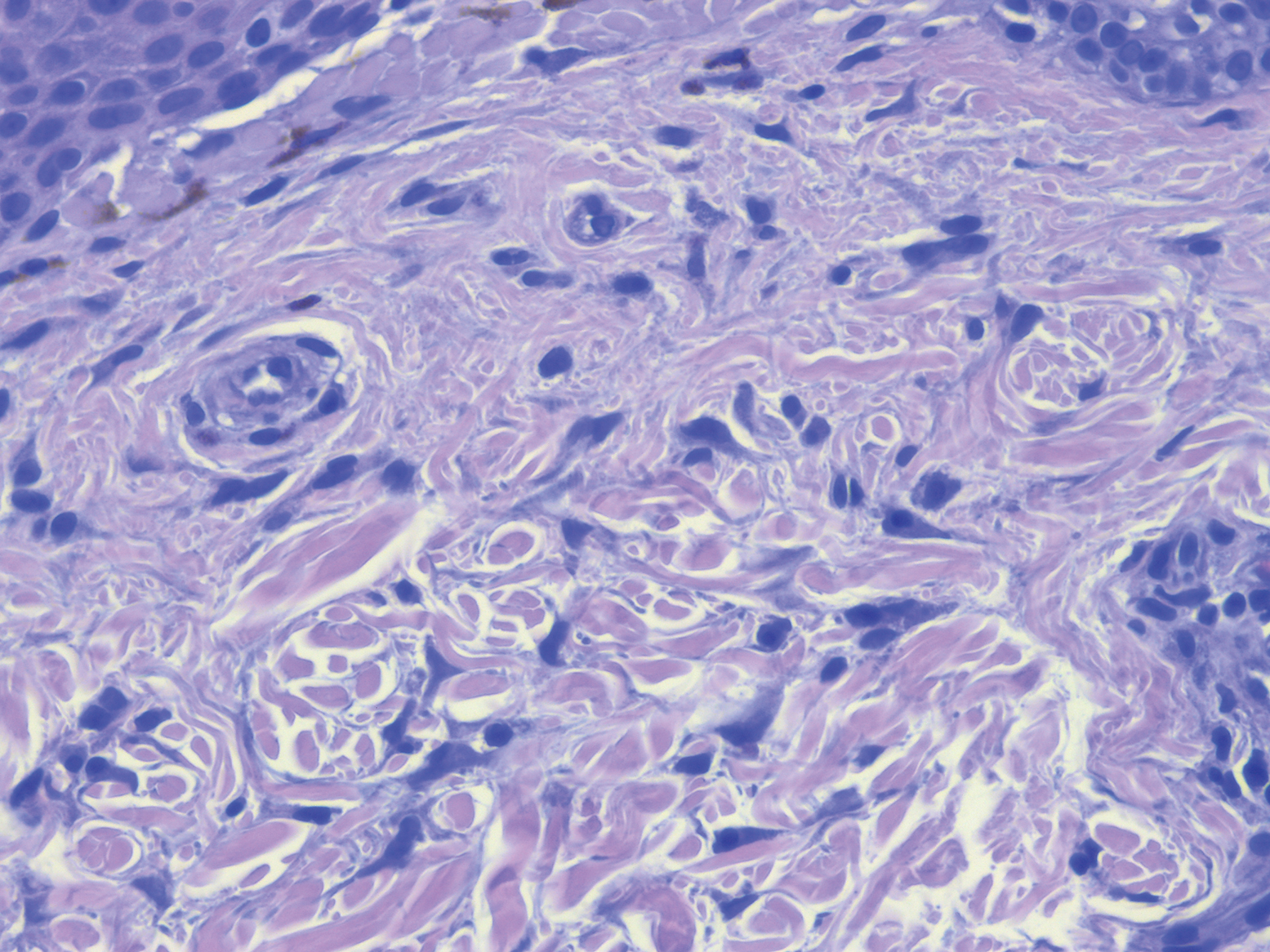
Clinical examination revealed flesh-colored, dome-shaped papules measuring 1 to 2 mm in diameter on the nose and cheeks (Figure 1). He had hyperkeratotic papules on the dorsal fingers, consistent with acral keratoses. Additionally, multiple flesh-colored papules with a cobblestonelike appearance were noted on the oral mucosa (Figure 2). Other findings included pedunculated papules on the neck, axillae, and lower abdomen bilaterally, consistent with fibroepithelial polyps, as well as hyperpigmented velvety plaques on the axillae, characteristic of acanthosis nigricans (Figure 3). A shave biopsy of a papule on the right cheek revealed a proliferation of plump stellate fibroblasts, small blood vessels, and thick collagen bundles, characteristic of a fibrous papule (Figure 4).
Differential diagnoses for our patient included BHDS and Cowden syndrome (CS). Due to the combination of extensive family history of multiorgan cancers as well as the clinical findings, he was referred to a geneticist for further evaluation. Genetic analysis was positive for a heterozygous mutation variant of uncertain significance in the PTEN gene.
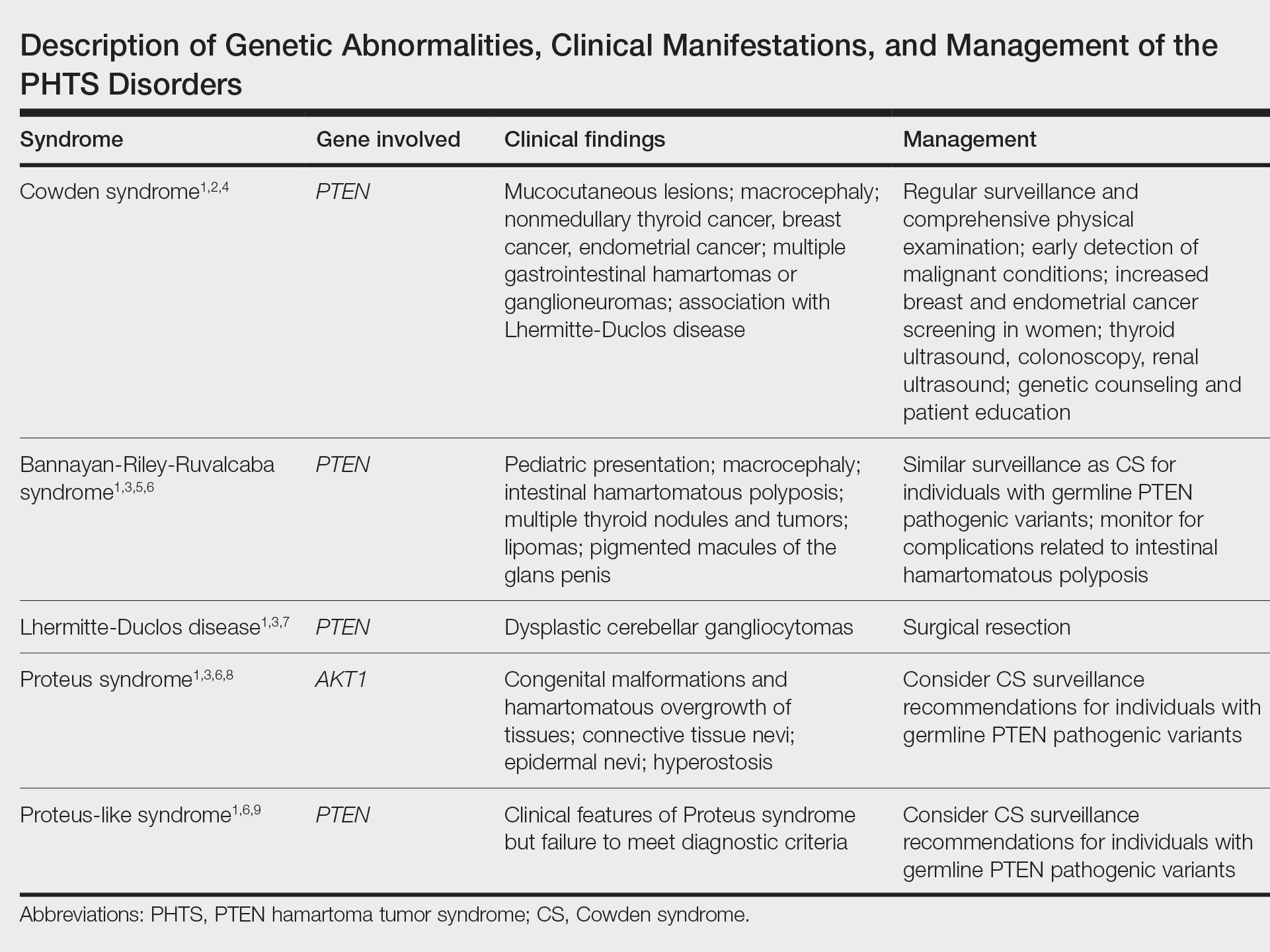
The PHTS disorders include CS, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, Proteus syndrome, and Proteus-like syndrome (Table).1-9 Our patient’s clinical findings were indicative of CS, a rare genodermatosis characterized by multiple hamartomas and neoplasms of ectodermal, mesodermal, and endodermal origin.1 Most CS patients develop trichilemmomas of the central face, mucocutaneous papillomatous papules, and acral and plantar keratoses by the third decade of life.1 Importantly, CS patients have an increased risk for breast, thyroid, renal, endometrial, and colorectal cancers, as well as melanoma, with estimated lifetime risks of 85%, 35%, 33%, 28%, 9%, and 6%, respectively.2,10
Regarding the pathophysiology of PHTS disorders, PTEN encodes a phosphatase that inhibits phosphoinositide 3-kinase/Akt and mTOR signaling pathways, thereby controlling cell proliferation, cell-cycle progression, and apoptosis.2,3 Loss of PTEN function, as seen in CS patients, results in an increased risk for cancer.2 Other genetic diseases, including juvenile polyposis syndrome, Proteus syndrome, tuberous sclerosis, and Peutz-Jeghers syndrome, have phenotypic similarities to PHTS.3 Specifically, loss-of-function mutations of TSC1 and TSC2, tumor suppressor genes associated with tuberous sclerosis, similarly result in dysregulation of mTOR signaling.
Our patient also had some clinical features characteristic of BHDS, such as flesh-colored facial papules, acrochordonlike lesions, and chromophobe renal cell carcinoma.11 Birt-Hogg-Dubé syndrome most often is caused by an autosomal-dominant germline mutation in FLCN, a tumor suppressor gene.11 Interestingly, FLCN interacts with AMP-activated protein kinase to help regulate mTOR signaling, which may explain phenotypic similarities seen in CS and BHDS.12
Because the PHTS disorders and BHDS result in similar functional consequences on the mTOR signaling pathway, patients can present with overlapping clinical features that may be diagnostically challenging. Management includes patient education regarding cancer risk, surveillance for early detection of malignancy, and genetic counseling for family members.2 It is important for clinicians to appreciate phenotypic similarities between PHTS and other disorders affecting mTOR signaling to prevent delays in diagnosis.
- Nosé V. Genodermatosis affecting the skin and mucosa of the head and neck: clinicopathologic, genetic, and molecular aspect—PTEN-hamartoma tumor syndrome/Cowden syndrome. Head Neck Pathol. 2016;10:131-138.
- Porto A, Roider E, Ruzicka T. Cowden syndrome: report of a case and brief review of literature. An Bras Dermatol. 2013;88(6 suppl 1):S52-S55.
- Leslie N, Longy M. Inherited PTEN mutations and the prediction of phenotype. Semin Cell Dev Biol. 2016;52:30-38.
- The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. genetic/familial high-risk assessment: breast and ovarian (version 1.2017). Published September 19, 2016. Accessed August 11, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Laury AR, Bongiovanni M, Tille J, et al. Thyroid pathology in PTEN-hamartoma tumor syndrome: characteristic findings of a distinct entity. Thyroid. 2011;21:135-144.
- Eng C. PTEN hamartoma tumor syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. University of Washington; 2001.
- Golden N, Tjokorda MGB, Sri M, et al. Management of unusual dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease) in a developing country: case report and review of the literature. Asian J Neurosurg. 2016;11:170.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Busa T, Milh M, Degardin N, et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol. 2015;19:188-192.
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400-407.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Baba M, Hong S, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552-15557.
To the Editor:
PTEN hamartoma tumor syndrome (PHTS) encompasses a spectrum of disorders that most commonly are caused by autosomal-dominant germline mutations in the phosphatase and tensin homolog, PTEN, tumor suppressor gene on chromosome 10q23. We describe a patient who presented with clinical features of PHTS and Birt-Hogg-Dubé syndrome (BHDS). Because the genetic mutations associated with both PHTS and BHDS result in altered mammalian target of rapamycin (mTOR) signaling, patients may have overlapping phenotypic features.
A 51-year-old man with a history of multiple carcinomas presented for evaluation of flesh-colored papules on the cheeks, nose, tongue, and hands, in addition to numerous skin tags on the neck, axillae, and lower abdomen bilaterally. His medical history was notable for several nasal and gastrointestinal tract polyps, chromophobe renal cell carcinoma, cutaneous lipomas, atypical carcinoid syndrome of the right lung, and a multinodular thyroid. His family history was notable for small cell lung cancer in his father, breast cancer and pancreatic cancer in his maternal aunt, esophageal cancer in his maternal grandfather, and celiac disease in his daughter.

Clinical examination revealed flesh-colored, dome-shaped papules measuring 1 to 2 mm in diameter on the nose and cheeks (Figure 1). He had hyperkeratotic papules on the dorsal fingers, consistent with acral keratoses. Additionally, multiple flesh-colored papules with a cobblestonelike appearance were noted on the oral mucosa (Figure 2). Other findings included pedunculated papules on the neck, axillae, and lower abdomen bilaterally, consistent with fibroepithelial polyps, as well as hyperpigmented velvety plaques on the axillae, characteristic of acanthosis nigricans (Figure 3). A shave biopsy of a papule on the right cheek revealed a proliferation of plump stellate fibroblasts, small blood vessels, and thick collagen bundles, characteristic of a fibrous papule (Figure 4).

Differential diagnoses for our patient included BHDS and Cowden syndrome (CS). Due to the combination of extensive family history of multiorgan cancers as well as the clinical findings, he was referred to a geneticist for further evaluation. Genetic analysis was positive for a heterozygous mutation variant of uncertain significance in the PTEN gene.

The PHTS disorders include CS, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, Proteus syndrome, and Proteus-like syndrome (Table).1-9 Our patient’s clinical findings were indicative of CS, a rare genodermatosis characterized by multiple hamartomas and neoplasms of ectodermal, mesodermal, and endodermal origin.1 Most CS patients develop trichilemmomas of the central face, mucocutaneous papillomatous papules, and acral and plantar keratoses by the third decade of life.1 Importantly, CS patients have an increased risk for breast, thyroid, renal, endometrial, and colorectal cancers, as well as melanoma, with estimated lifetime risks of 85%, 35%, 33%, 28%, 9%, and 6%, respectively.2,10

Regarding the pathophysiology of PHTS disorders, PTEN encodes a phosphatase that inhibits phosphoinositide 3-kinase/Akt and mTOR signaling pathways, thereby controlling cell proliferation, cell-cycle progression, and apoptosis.2,3 Loss of PTEN function, as seen in CS patients, results in an increased risk for cancer.2 Other genetic diseases, including juvenile polyposis syndrome, Proteus syndrome, tuberous sclerosis, and Peutz-Jeghers syndrome, have phenotypic similarities to PHTS.3 Specifically, loss-of-function mutations of TSC1 and TSC2, tumor suppressor genes associated with tuberous sclerosis, similarly result in dysregulation of mTOR signaling.

Our patient also had some clinical features characteristic of BHDS, such as flesh-colored facial papules, acrochordonlike lesions, and chromophobe renal cell carcinoma.11 Birt-Hogg-Dubé syndrome most often is caused by an autosomal-dominant germline mutation in FLCN, a tumor suppressor gene.11 Interestingly, FLCN interacts with AMP-activated protein kinase to help regulate mTOR signaling, which may explain phenotypic similarities seen in CS and BHDS.12
Because the PHTS disorders and BHDS result in similar functional consequences on the mTOR signaling pathway, patients can present with overlapping clinical features that may be diagnostically challenging. Management includes patient education regarding cancer risk, surveillance for early detection of malignancy, and genetic counseling for family members.2 It is important for clinicians to appreciate phenotypic similarities between PHTS and other disorders affecting mTOR signaling to prevent delays in diagnosis.
To the Editor:
PTEN hamartoma tumor syndrome (PHTS) encompasses a spectrum of disorders that most commonly are caused by autosomal-dominant germline mutations in the phosphatase and tensin homolog, PTEN, tumor suppressor gene on chromosome 10q23. We describe a patient who presented with clinical features of PHTS and Birt-Hogg-Dubé syndrome (BHDS). Because the genetic mutations associated with both PHTS and BHDS result in altered mammalian target of rapamycin (mTOR) signaling, patients may have overlapping phenotypic features.
A 51-year-old man with a history of multiple carcinomas presented for evaluation of flesh-colored papules on the cheeks, nose, tongue, and hands, in addition to numerous skin tags on the neck, axillae, and lower abdomen bilaterally. His medical history was notable for several nasal and gastrointestinal tract polyps, chromophobe renal cell carcinoma, cutaneous lipomas, atypical carcinoid syndrome of the right lung, and a multinodular thyroid. His family history was notable for small cell lung cancer in his father, breast cancer and pancreatic cancer in his maternal aunt, esophageal cancer in his maternal grandfather, and celiac disease in his daughter.

Clinical examination revealed flesh-colored, dome-shaped papules measuring 1 to 2 mm in diameter on the nose and cheeks (Figure 1). He had hyperkeratotic papules on the dorsal fingers, consistent with acral keratoses. Additionally, multiple flesh-colored papules with a cobblestonelike appearance were noted on the oral mucosa (Figure 2). Other findings included pedunculated papules on the neck, axillae, and lower abdomen bilaterally, consistent with fibroepithelial polyps, as well as hyperpigmented velvety plaques on the axillae, characteristic of acanthosis nigricans (Figure 3). A shave biopsy of a papule on the right cheek revealed a proliferation of plump stellate fibroblasts, small blood vessels, and thick collagen bundles, characteristic of a fibrous papule (Figure 4).

Differential diagnoses for our patient included BHDS and Cowden syndrome (CS). Due to the combination of extensive family history of multiorgan cancers as well as the clinical findings, he was referred to a geneticist for further evaluation. Genetic analysis was positive for a heterozygous mutation variant of uncertain significance in the PTEN gene.

The PHTS disorders include CS, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte-Duclos disease, Proteus syndrome, and Proteus-like syndrome (Table).1-9 Our patient’s clinical findings were indicative of CS, a rare genodermatosis characterized by multiple hamartomas and neoplasms of ectodermal, mesodermal, and endodermal origin.1 Most CS patients develop trichilemmomas of the central face, mucocutaneous papillomatous papules, and acral and plantar keratoses by the third decade of life.1 Importantly, CS patients have an increased risk for breast, thyroid, renal, endometrial, and colorectal cancers, as well as melanoma, with estimated lifetime risks of 85%, 35%, 33%, 28%, 9%, and 6%, respectively.2,10

Regarding the pathophysiology of PHTS disorders, PTEN encodes a phosphatase that inhibits phosphoinositide 3-kinase/Akt and mTOR signaling pathways, thereby controlling cell proliferation, cell-cycle progression, and apoptosis.2,3 Loss of PTEN function, as seen in CS patients, results in an increased risk for cancer.2 Other genetic diseases, including juvenile polyposis syndrome, Proteus syndrome, tuberous sclerosis, and Peutz-Jeghers syndrome, have phenotypic similarities to PHTS.3 Specifically, loss-of-function mutations of TSC1 and TSC2, tumor suppressor genes associated with tuberous sclerosis, similarly result in dysregulation of mTOR signaling.

Our patient also had some clinical features characteristic of BHDS, such as flesh-colored facial papules, acrochordonlike lesions, and chromophobe renal cell carcinoma.11 Birt-Hogg-Dubé syndrome most often is caused by an autosomal-dominant germline mutation in FLCN, a tumor suppressor gene.11 Interestingly, FLCN interacts with AMP-activated protein kinase to help regulate mTOR signaling, which may explain phenotypic similarities seen in CS and BHDS.12
Because the PHTS disorders and BHDS result in similar functional consequences on the mTOR signaling pathway, patients can present with overlapping clinical features that may be diagnostically challenging. Management includes patient education regarding cancer risk, surveillance for early detection of malignancy, and genetic counseling for family members.2 It is important for clinicians to appreciate phenotypic similarities between PHTS and other disorders affecting mTOR signaling to prevent delays in diagnosis.
- Nosé V. Genodermatosis affecting the skin and mucosa of the head and neck: clinicopathologic, genetic, and molecular aspect—PTEN-hamartoma tumor syndrome/Cowden syndrome. Head Neck Pathol. 2016;10:131-138.
- Porto A, Roider E, Ruzicka T. Cowden syndrome: report of a case and brief review of literature. An Bras Dermatol. 2013;88(6 suppl 1):S52-S55.
- Leslie N, Longy M. Inherited PTEN mutations and the prediction of phenotype. Semin Cell Dev Biol. 2016;52:30-38.
- The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. genetic/familial high-risk assessment: breast and ovarian (version 1.2017). Published September 19, 2016. Accessed August 11, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Laury AR, Bongiovanni M, Tille J, et al. Thyroid pathology in PTEN-hamartoma tumor syndrome: characteristic findings of a distinct entity. Thyroid. 2011;21:135-144.
- Eng C. PTEN hamartoma tumor syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. University of Washington; 2001.
- Golden N, Tjokorda MGB, Sri M, et al. Management of unusual dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease) in a developing country: case report and review of the literature. Asian J Neurosurg. 2016;11:170.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Busa T, Milh M, Degardin N, et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol. 2015;19:188-192.
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400-407.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Baba M, Hong S, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552-15557.
- Nosé V. Genodermatosis affecting the skin and mucosa of the head and neck: clinicopathologic, genetic, and molecular aspect—PTEN-hamartoma tumor syndrome/Cowden syndrome. Head Neck Pathol. 2016;10:131-138.
- Porto A, Roider E, Ruzicka T. Cowden syndrome: report of a case and brief review of literature. An Bras Dermatol. 2013;88(6 suppl 1):S52-S55.
- Leslie N, Longy M. Inherited PTEN mutations and the prediction of phenotype. Semin Cell Dev Biol. 2016;52:30-38.
- The National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. genetic/familial high-risk assessment: breast and ovarian (version 1.2017). Published September 19, 2016. Accessed August 11, 2021. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- Laury AR, Bongiovanni M, Tille J, et al. Thyroid pathology in PTEN-hamartoma tumor syndrome: characteristic findings of a distinct entity. Thyroid. 2011;21:135-144.
- Eng C. PTEN hamartoma tumor syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. University of Washington; 2001.
- Golden N, Tjokorda MGB, Sri M, et al. Management of unusual dysplastic gangliocytoma of the cerebellum (Lhermitte-Duclos disease) in a developing country: case report and review of the literature. Asian J Neurosurg. 2016;11:170.
- Biesecker LG, Happle R, Mulliken JB, et al. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389-395.
- Busa T, Milh M, Degardin N, et al. Clinical presentation of PTEN mutations in childhood in the absence of family history of Cowden syndrome. Eur J Paediatr Neurol. 2015;19:188-192.
- Tan MH, Mester JL, Ngeow J, et al. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400-407.
- Ponti G, Pellacani G, Seidenari S, et al. Cancer-associated genodermatoses: skin neoplasms as clues to hereditary tumor syndromes. Crit Rev Oncol Hematol. 2013;85:239-256.
- Baba M, Hong S, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci U S A. 2006;103:15552-15557.
PRACTICE POINTS
- PTEN hamartoma tumor syndrome (PHTS) represents a spectrum of disorders caused by autosomal-dominant germline mutations in PTEN.
- Our patient presented with phenotypic features of PHTS and Birt-Hogg-Dubé syndrome. Given that both syndromes cause alterations in mammalian target of rapamycin signaling, overlapping phenotypic features may be seen.
- Recognizing overlapping phenotypic features of these syndromes will allow for timely diagnosis and surveillance for malignancy.
Aloha
In July, 2021, JAMA published a study about physicians’ sartorial habits, basically saying that people prefer doctors to dress “professionally.” Even today the white coat still carries some weight.
And I still don’t care.
Today, like every workday since June 2006, I put on my standard patient-seeing attire: shorts, sneakers, and a Hawaiian shirt. The only significant change has been the addition of a face mask since March 2020.
I have no plans to change anytime between now and retirement. I live in Phoenix, the hottest major city in the U.S., and have no desire to be uncomfortable because someone doesn’t think I look professional. It’s even become, albeit unintentionally, a trademark of sorts.
Now, as always, I let my patients be the judge. If someone isn’t happy with my appearance, or feels it makes me less competent, they certainly have the right to feel that way. There are plenty of other neurologists here who dress to higher standards (though jackets and ties, outside of the Mayo Clinic down the road, are getting pretty hard to find).
This is one of the things I like about having a small solo practice. I can be who I am, not who some administrator or dress code specialist says I have to be.
I do my best for my patients, and those who know me are aware that my complete lack of fashion sense doesn’t represent (I hope) an equal lack of medical care. Most of them seem to come back, so I guess I’m doing something right.
But it brings up the question of what should a doctor look like? In a world of changing demographics the stereotype of a neatly-dressed middle-aged white male certainly isn’t it anymore.
Nor should there be. Medicine should be open to all with the drive, brains, and talent who want to follow to path of Hippocrates. Maybe I’m naive, but I still see this as a calling more than a job. Judging someone’s medical competence solely on their sex, race, appearance, or fashion sense is foolhardy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In July, 2021, JAMA published a study about physicians’ sartorial habits, basically saying that people prefer doctors to dress “professionally.” Even today the white coat still carries some weight.
And I still don’t care.
Today, like every workday since June 2006, I put on my standard patient-seeing attire: shorts, sneakers, and a Hawaiian shirt. The only significant change has been the addition of a face mask since March 2020.
I have no plans to change anytime between now and retirement. I live in Phoenix, the hottest major city in the U.S., and have no desire to be uncomfortable because someone doesn’t think I look professional. It’s even become, albeit unintentionally, a trademark of sorts.
Now, as always, I let my patients be the judge. If someone isn’t happy with my appearance, or feels it makes me less competent, they certainly have the right to feel that way. There are plenty of other neurologists here who dress to higher standards (though jackets and ties, outside of the Mayo Clinic down the road, are getting pretty hard to find).
This is one of the things I like about having a small solo practice. I can be who I am, not who some administrator or dress code specialist says I have to be.
I do my best for my patients, and those who know me are aware that my complete lack of fashion sense doesn’t represent (I hope) an equal lack of medical care. Most of them seem to come back, so I guess I’m doing something right.
But it brings up the question of what should a doctor look like? In a world of changing demographics the stereotype of a neatly-dressed middle-aged white male certainly isn’t it anymore.
Nor should there be. Medicine should be open to all with the drive, brains, and talent who want to follow to path of Hippocrates. Maybe I’m naive, but I still see this as a calling more than a job. Judging someone’s medical competence solely on their sex, race, appearance, or fashion sense is foolhardy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In July, 2021, JAMA published a study about physicians’ sartorial habits, basically saying that people prefer doctors to dress “professionally.” Even today the white coat still carries some weight.
And I still don’t care.
Today, like every workday since June 2006, I put on my standard patient-seeing attire: shorts, sneakers, and a Hawaiian shirt. The only significant change has been the addition of a face mask since March 2020.
I have no plans to change anytime between now and retirement. I live in Phoenix, the hottest major city in the U.S., and have no desire to be uncomfortable because someone doesn’t think I look professional. It’s even become, albeit unintentionally, a trademark of sorts.
Now, as always, I let my patients be the judge. If someone isn’t happy with my appearance, or feels it makes me less competent, they certainly have the right to feel that way. There are plenty of other neurologists here who dress to higher standards (though jackets and ties, outside of the Mayo Clinic down the road, are getting pretty hard to find).
This is one of the things I like about having a small solo practice. I can be who I am, not who some administrator or dress code specialist says I have to be.
I do my best for my patients, and those who know me are aware that my complete lack of fashion sense doesn’t represent (I hope) an equal lack of medical care. Most of them seem to come back, so I guess I’m doing something right.
But it brings up the question of what should a doctor look like? In a world of changing demographics the stereotype of a neatly-dressed middle-aged white male certainly isn’t it anymore.
Nor should there be. Medicine should be open to all with the drive, brains, and talent who want to follow to path of Hippocrates. Maybe I’m naive, but I still see this as a calling more than a job. Judging someone’s medical competence solely on their sex, race, appearance, or fashion sense is foolhardy.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
COVID-19 claims more than 675,000 U.S. lives, surpassing the 1918 flu
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
, according to data collected by Johns Hopkins University.
Although the raw numbers match, epidemiologists point out that 675,000 deaths in 1918 was a much greater proportion of the population. In 1918, the U.S. population was 105 million, less than one third of what it is today.
The AIDS pandemic of the 1980s remains the deadliest of the 20th Century, claiming the lives of 700,000 Americans. But at our current pace of 2,000 COVID deaths a day, we could quickly eclipse that death toll, too.
Even though the 1918 epidemic is often called the “Spanish Flu,” there is no universal consensus regarding where the virus originated, according to the Centers for Disease Control and Prevention.
Still, the almost incomprehensible loss harkens back to a time when medicine and technology were far less advanced than they are today.
In 1918, the United States didn’t have access to a vaccine, or near real-time tools to trace the spread and communicate the threat.
In some ways, the United States has failed to learn from the mistakes of the past.
There are many similarities between the two pandemics. In the spring of 1918, when the first wave of influenza hit, the United States and its allies were nearing victory in Europe in World War I. Just this summer the United States has ended its longest war, the conflict in Afghanistan, as COVID cases surge.
In both pandemics, hospitals and funeral homes were overrun and makeshift clinics were opened where space was available. Mask mandates were installed; schools, churches, and theaters closed; and social distancing was encouraged.
As is the case today, different jurisdictions took different steps to fight the pandemic and some were more successful than others.
According to History.com, in 1918, Philadelphia’s mayor said a popular annual parade could be held, and an estimated 200,000 people attended. In less than 2 weeks, more than 1,000 local residents were dead. But in St. Louis, public gatherings were banned, schools and theaters closed, and the death toll there was one eighth of Philadelphia’s.
Just as in 1918, America has at times continued to fan the flames of the epidemic by relaxing restrictions too quickly and relying on unproven treatments. Poor communication allowed younger people to feel that they wouldn’t necessarily face the worst consequences of the virus, contributing to a false sense of security in the age group that was fueling the spread.
“A lot of the mistakes that we definitely fell into in 1918, we hoped we wouldn’t fall into in 2020,” epidemiologist Stephen Kissler, PhD, of the Harvard T.H. Chan School of Public Health, told CNN. “We did.”
A version of this article first appeared on Medscape.com.
HPV vaccine safety concerns up 80% from 2015 to 2018
Despite a decrease in reported adverse events after receiving the human papillomavirus (HPV) vaccine, among parents of unvaccinated adolescents, concerns about the vaccine’s safety rose 80% from 2015 to 2018, according to research published September 17 in JAMA Network Open.
Since its approval in 2006 by the U.S. Food and Drug Administration, uptake of the HPV vaccine has consistently lagged behind that of other routine vaccinations. According to the most recent data from the Centers for Disease Control and Prevention, released September 3, 58.6% of adolescents were considered up to date with their HPV vaccinations in 2020.
Trials prior to the vaccine’s FDA approval as well as an abundance of clinical and observational evidence after it hit the market demonstrate the vaccine’s efficacy and safety, said lead author Kalyani Sonawane, PhD, an assistant professor of management, policy, and community health at the UTHealth School of Public Health, in Houston, Texas, in an interview. Still, recent research suggests that safety concerns are a main reason why parents are hesitant to have their children vaccinated, she noted.
In the study, Dr. Sonawane and colleagues analyzed data from National Immunization Survey-Teen (NIS-Teen) from 2015 through 2018. NIS-Teen is a random-digit-dialed telephone survey conducted annually by the CDC to monitor routine vaccination coverage among adolescents aged 13 to 17. The researchers identified 39,364 adolescents who had not received any HPV shots and reviewed the caregivers’ reasons for vaccine hesitancy. The research team also reviewed the Vaccine Adverse Event Reporting System (VAERS). They identified 16,621 reports that listed the HPV vaccine from 2015 through 2018.
The top five reasons caregivers cited for avoiding the HPV vaccine were the following:
- not needed or necessary
- safety concerns
- not recommended
- lack of knowledge
- not sexually active
Of these, safety concerns were the only factor that increased during the study period. They increased from 13.0% in 2015 to 23.4% in 2018. Concerns over vaccine safety rose in 30 states, with increases of over 200% in California, Hawaii, South Dakota, and Mississippi.
The proportion of unvaccinated adolescents whose caregivers thought the HPV vaccine was not needed or necessary remained steady at around 25%. Those whose caregivers listed “not recommended,” “lack of knowledge,” and “not sexually active” as reasons for avoiding vaccination decreased over the study period.
The reporting rate for adverse events following HPV vaccination decreased from 44.7 per 100,000 doses in 2015 to 29.4 per 100,000 doses in 2018. Of the reported 16,621 adverse events following HPV vaccination that occurred over the study period, 4.6% were serious, resulting in hospitalizations, disability, life-threatening events, or death. From 2015 through 2018, reporting rates for serious adverse events remained level at around 0.3 events per 100,000 doses.
This mismatch between increasing vaccine safety concerns and decreasing adverse events suggests that disinformation may be driving these concerns more than scientific fact, Nosayaba Osazuwa-Peters, PhD, MPH, an assistant professor in head and neck surgery and communication sciences at the Duke University School of Medicine, in Durham, North Carolina, told this news organization. He co-wrote an invited commentary on the study and was not involved with the research. Although there have always been people who are hesitant to receive vaccinations, he said, social media and the internet have undoubtedly played a role in spreading concern.
Dr. Sonawane agreed. Online, “there are a lot of antivaccine groups that are making unwarranted claims about the vaccine’s safety,” such as that the HPV vaccine causes autism or fertility problems in women, she said. “We believe that this growing antivaccine movement in the U.S. and across the globe – which the World Health Organization has declared as one of the biggest threats right now – is also contributing to safety concerns among U.S. parents, particularly HPV vaccine safety.”
Although the study did not address strategies to combat this misinformation, Dr. Osazuwa-Peters said clinicians need to improve their communication with parents and patients. One way to do that, he said, is by bolstering an online presence and by countering vaccine disinformation with evidence-based responses on the internet. Most people get their medical information online. “Many people are just afraid because they don’t trust the messages coming from health care,” he said. “So, we need to a better job of not just providing the facts but providing the facts in a way that the end users can understand and appreciate.”
Dr. Sonawane and Dr. Osazuwa-Peters report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Despite a decrease in reported adverse events after receiving the human papillomavirus (HPV) vaccine, among parents of unvaccinated adolescents, concerns about the vaccine’s safety rose 80% from 2015 to 2018, according to research published September 17 in JAMA Network Open.
Since its approval in 2006 by the U.S. Food and Drug Administration, uptake of the HPV vaccine has consistently lagged behind that of other routine vaccinations. According to the most recent data from the Centers for Disease Control and Prevention, released September 3, 58.6% of adolescents were considered up to date with their HPV vaccinations in 2020.
Trials prior to the vaccine’s FDA approval as well as an abundance of clinical and observational evidence after it hit the market demonstrate the vaccine’s efficacy and safety, said lead author Kalyani Sonawane, PhD, an assistant professor of management, policy, and community health at the UTHealth School of Public Health, in Houston, Texas, in an interview. Still, recent research suggests that safety concerns are a main reason why parents are hesitant to have their children vaccinated, she noted.
In the study, Dr. Sonawane and colleagues analyzed data from National Immunization Survey-Teen (NIS-Teen) from 2015 through 2018. NIS-Teen is a random-digit-dialed telephone survey conducted annually by the CDC to monitor routine vaccination coverage among adolescents aged 13 to 17. The researchers identified 39,364 adolescents who had not received any HPV shots and reviewed the caregivers’ reasons for vaccine hesitancy. The research team also reviewed the Vaccine Adverse Event Reporting System (VAERS). They identified 16,621 reports that listed the HPV vaccine from 2015 through 2018.
The top five reasons caregivers cited for avoiding the HPV vaccine were the following:
- not needed or necessary
- safety concerns
- not recommended
- lack of knowledge
- not sexually active
Of these, safety concerns were the only factor that increased during the study period. They increased from 13.0% in 2015 to 23.4% in 2018. Concerns over vaccine safety rose in 30 states, with increases of over 200% in California, Hawaii, South Dakota, and Mississippi.
The proportion of unvaccinated adolescents whose caregivers thought the HPV vaccine was not needed or necessary remained steady at around 25%. Those whose caregivers listed “not recommended,” “lack of knowledge,” and “not sexually active” as reasons for avoiding vaccination decreased over the study period.
The reporting rate for adverse events following HPV vaccination decreased from 44.7 per 100,000 doses in 2015 to 29.4 per 100,000 doses in 2018. Of the reported 16,621 adverse events following HPV vaccination that occurred over the study period, 4.6% were serious, resulting in hospitalizations, disability, life-threatening events, or death. From 2015 through 2018, reporting rates for serious adverse events remained level at around 0.3 events per 100,000 doses.
This mismatch between increasing vaccine safety concerns and decreasing adverse events suggests that disinformation may be driving these concerns more than scientific fact, Nosayaba Osazuwa-Peters, PhD, MPH, an assistant professor in head and neck surgery and communication sciences at the Duke University School of Medicine, in Durham, North Carolina, told this news organization. He co-wrote an invited commentary on the study and was not involved with the research. Although there have always been people who are hesitant to receive vaccinations, he said, social media and the internet have undoubtedly played a role in spreading concern.
Dr. Sonawane agreed. Online, “there are a lot of antivaccine groups that are making unwarranted claims about the vaccine’s safety,” such as that the HPV vaccine causes autism or fertility problems in women, she said. “We believe that this growing antivaccine movement in the U.S. and across the globe – which the World Health Organization has declared as one of the biggest threats right now – is also contributing to safety concerns among U.S. parents, particularly HPV vaccine safety.”
Although the study did not address strategies to combat this misinformation, Dr. Osazuwa-Peters said clinicians need to improve their communication with parents and patients. One way to do that, he said, is by bolstering an online presence and by countering vaccine disinformation with evidence-based responses on the internet. Most people get their medical information online. “Many people are just afraid because they don’t trust the messages coming from health care,” he said. “So, we need to a better job of not just providing the facts but providing the facts in a way that the end users can understand and appreciate.”
Dr. Sonawane and Dr. Osazuwa-Peters report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Despite a decrease in reported adverse events after receiving the human papillomavirus (HPV) vaccine, among parents of unvaccinated adolescents, concerns about the vaccine’s safety rose 80% from 2015 to 2018, according to research published September 17 in JAMA Network Open.
Since its approval in 2006 by the U.S. Food and Drug Administration, uptake of the HPV vaccine has consistently lagged behind that of other routine vaccinations. According to the most recent data from the Centers for Disease Control and Prevention, released September 3, 58.6% of adolescents were considered up to date with their HPV vaccinations in 2020.
Trials prior to the vaccine’s FDA approval as well as an abundance of clinical and observational evidence after it hit the market demonstrate the vaccine’s efficacy and safety, said lead author Kalyani Sonawane, PhD, an assistant professor of management, policy, and community health at the UTHealth School of Public Health, in Houston, Texas, in an interview. Still, recent research suggests that safety concerns are a main reason why parents are hesitant to have their children vaccinated, she noted.
In the study, Dr. Sonawane and colleagues analyzed data from National Immunization Survey-Teen (NIS-Teen) from 2015 through 2018. NIS-Teen is a random-digit-dialed telephone survey conducted annually by the CDC to monitor routine vaccination coverage among adolescents aged 13 to 17. The researchers identified 39,364 adolescents who had not received any HPV shots and reviewed the caregivers’ reasons for vaccine hesitancy. The research team also reviewed the Vaccine Adverse Event Reporting System (VAERS). They identified 16,621 reports that listed the HPV vaccine from 2015 through 2018.
The top five reasons caregivers cited for avoiding the HPV vaccine were the following:
- not needed or necessary
- safety concerns
- not recommended
- lack of knowledge
- not sexually active
Of these, safety concerns were the only factor that increased during the study period. They increased from 13.0% in 2015 to 23.4% in 2018. Concerns over vaccine safety rose in 30 states, with increases of over 200% in California, Hawaii, South Dakota, and Mississippi.
The proportion of unvaccinated adolescents whose caregivers thought the HPV vaccine was not needed or necessary remained steady at around 25%. Those whose caregivers listed “not recommended,” “lack of knowledge,” and “not sexually active” as reasons for avoiding vaccination decreased over the study period.
The reporting rate for adverse events following HPV vaccination decreased from 44.7 per 100,000 doses in 2015 to 29.4 per 100,000 doses in 2018. Of the reported 16,621 adverse events following HPV vaccination that occurred over the study period, 4.6% were serious, resulting in hospitalizations, disability, life-threatening events, or death. From 2015 through 2018, reporting rates for serious adverse events remained level at around 0.3 events per 100,000 doses.
This mismatch between increasing vaccine safety concerns and decreasing adverse events suggests that disinformation may be driving these concerns more than scientific fact, Nosayaba Osazuwa-Peters, PhD, MPH, an assistant professor in head and neck surgery and communication sciences at the Duke University School of Medicine, in Durham, North Carolina, told this news organization. He co-wrote an invited commentary on the study and was not involved with the research. Although there have always been people who are hesitant to receive vaccinations, he said, social media and the internet have undoubtedly played a role in spreading concern.
Dr. Sonawane agreed. Online, “there are a lot of antivaccine groups that are making unwarranted claims about the vaccine’s safety,” such as that the HPV vaccine causes autism or fertility problems in women, she said. “We believe that this growing antivaccine movement in the U.S. and across the globe – which the World Health Organization has declared as one of the biggest threats right now – is also contributing to safety concerns among U.S. parents, particularly HPV vaccine safety.”
Although the study did not address strategies to combat this misinformation, Dr. Osazuwa-Peters said clinicians need to improve their communication with parents and patients. One way to do that, he said, is by bolstering an online presence and by countering vaccine disinformation with evidence-based responses on the internet. Most people get their medical information online. “Many people are just afraid because they don’t trust the messages coming from health care,” he said. “So, we need to a better job of not just providing the facts but providing the facts in a way that the end users can understand and appreciate.”
Dr. Sonawane and Dr. Osazuwa-Peters report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sublingual film well tolerated for Parkinson ‘off’ episodes
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.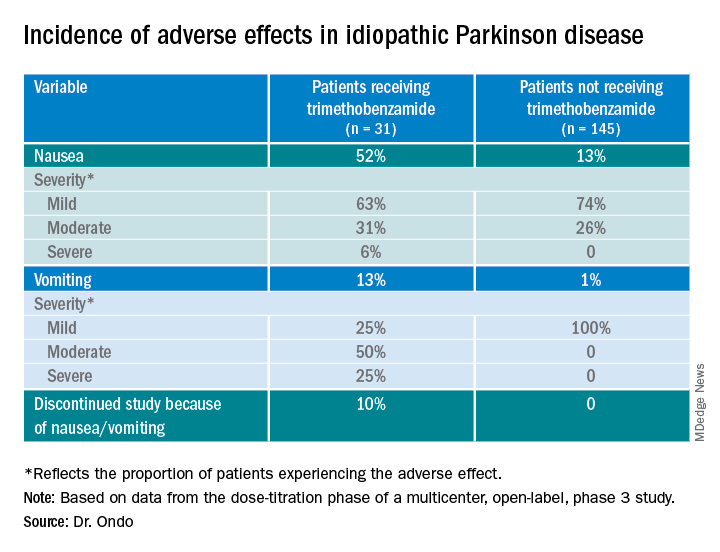
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
“The bottom line was that the majority of patients did not have dose-limiting nausea or vomiting,” said coinvestigator William Ondo, MD, from Houston Methodist Neurological Institute. “And although it really did not compare in a prospective, placebo-controlled manner use of [trimethobenzamide antiemetic] ... versus not using [it], anecdotally and based on historic data, nausea really seemed to be about the same even without the antinausea medication.”
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
This study was the dose-titration phase to determine the effective and tolerable dose of the drug as part of a longer study looking at safety and efficacy.
Only 13% of patients experienced nausea and/or vomiting, and of those, 74% cases were of mild severity and 26% were of moderate severity. These rates of nausea/vomiting were lower than those seen when trimethobenzamide (Tigan, Pfizer) was needed to be administered during the titration period, at the discretion of the investigator.
This multicenter, ongoing, open-label, phase 3 study enrolled 176 patients (mean age, 64.4 years) who had idiopathic Parkinson’s disease for a mean of 8.0 years and had no prior exposure to SL-apo, with modified Hoehn and Yahr stage 1-3 disease (83% stage 2 or 2.5 during “on” time).
Study participants had Mini-Mental State Examination scores greater than 25, were receiving stable doses of levodopa/carbidopa, and had 1 or more (mean, 4.2) “off” episodes per day with a total daily “off” time of 2 hours or more. Patients with mouth cankers or sores within 30 days of screening were excluded.
Open-label dose titration occurred during sequential office visits while patients were “off,” with escalating doses of 10-35 mg in 5-mg increments to determine a tolerable dose leading to a full “on” period within 45 minutes. Patients self-administered this achieved dose of SL-apo for up to five “off” episodes per day with a minimum of 2 hours between doses for the full 48-week study period.
The study protocol prohibited antiemetic use except when clinically warranted at the investigator’s discretion. Of the 176 patients, 31 (18%) received the antiemetic trimethobenzamide and 145 (82%) did not.
Of the 176 patients, 76% received their effective and tolerated dose within the first three doses. Just over half (55%) received 10 mg or 15 mg. Only 24% received the highest doses of 25 mg or 30 mg.
About 52%of patients who received trimethobenzamide experienced treatment-related nausea and 13% experienced vomiting; in comparison, 13% not receiving trimethobenzamide had nausea and 1% had vomiting. About 10%of patients in the former group and none in the latter discontinued the study because of nausea and/or vomiting.
The apomorphine sublingual film has “the advantage of ease of use compared to the injectable form,” Dr. Ondo said. “I think the injectable form, purely based on anecdotal experience, might start to work a minute or 2 faster than the sublingual form, but overall I would say efficacy as far as potency of turning ‘on’ and consistency of turning ‘on’ is comparable.”
In addition to the known adverse effects of nausea, vomiting, and hypotension with the use of any apomorphine, he said that long-term use of the sublingual form can lead to gingival irritation. Two recommendations are to place the film in a different site and to use a more basic toothpaste, such as one containing baking powder, because irritation may result from the acidity of the apomorphine.
Good news
Commenting on the study, Ludy Shih, MD, MMSc, from Boston University, noted that the drug label reports that “13%-15% had oropharyngeal soft tissue swelling or pain ... and 7% had oral ulcers and stomatitis.”
In addition, oral trimethobenzamide has been discontinued, although an injectable form is still available. This situation may present a problem, she said. “Most antinausea drugs block dopamine, so ... I would say they’re contraindicated for treating people with Parkinson’s disease. But trimethobenzamide in particular is one that we often reach for. ... But that appears to be constrained and may, in fact, be expensive for patients.”
Turning to the study findings, she said they suggest that “not everyone needs prophylactic use of trimethobenzamide before they take the apomorphine sublingual film, which is good news that helps doctors try to decide whether or not it’s reasonable to recommend people trying it without the trimethobenzamide.”
Although some patients did experience mild nausea, she said the fact that no needle is involved may attract some patients. Moreover, taking this medication may be easier than administering an injection during an “off” episode.
Dr. Ondo is a consultant for Sunovion Pharmaceuticals, which sponsored the study. Dr. Shih had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Friedreich’s ataxia treatment shows extended benefit
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
according to results of a clinical trial presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders.
The study, labeled the Delayed-Start Study, is an extension study of the two-part MOXIE phase 2 trial of omaveloxolone.
“This study shows two things,” said David Lynch, MD, PhD, of Children’s Hospital of Philadelphia. “It doesn’t matter when you started omaveloxolone for you to see a benefit; and that the benefit that the active group saw in the first part of the study was maintained as they went into the delayed-start part. So in fact omaveloxolone does modify the long-term behavior of the disease.”
Friedreich’s ataxia only affects about 22,000 people worldwide, and children typically present between the ages of 5 and 15, Dr. Lynch said.
The extension study included 73 patients who completed either of the first two parts of the MOXIe trial. The MOXIe trial randomized patients on a 3:1 basis to either omaveloxolone 2.5-300 mg or placebo for 12 weeks in the first part. The second part was a double-blind trial of 103 patients randomized on a 1:1 basis to 150 mg omaveloxolone or placebo for 48 weeks. Participants had a baseline modified Friedreich’s ataxia scale (mFARS) of 20-80 and were aged 16-40 years.
Patients in the extension study did not have severe pes cavus. The extension study was a 72-week evaluation of patients who were in either the treatment or placebo groups in the first two parts. There was a 4-week off-treatment period between the end of MOXIe part 2 and the beginning of the extension study, in which all patients received omaveloxolone.
At the end of the placebo-controlled study, patients taking omaveloxolone showed a –2.18-point (±0.96) difference in improvement in mFARS score (P = .027), compared with the placebo group, which was preserved at the end of the delayed-start period, with a –2.92-point (±2.13) improvement (P = .179), Dr. Lynch said.
In the extension study, former placebo patients who went on omaveloxolone had annualized mFARS slopes similar to the previously treated patients – 0.29 (±0.68) and 0.17 (±0.61), respectively (P = .85) – from weeks 48 to 144, Dr. Lynch said.
“This study showed that, when analyzed in a delayed-start fashion, it does not matter when you start omaveloxolone to see a benefit: Each cohort benefited almost equally once they started the drug,” Dr. Lynch said in an interview. “Also, in both groups, once they started omaveloxolone, they changed slower than people in natural history studies.”
A clinically meaningful difference?
Reached for comment, Massimo Pandolfo, MD, a neurologist at McGill University, Montreal, noted that the Delayed-Start Study included only patients without pes cavus, an indication that the patients had less severe disease. “It would be important to see how overall patients with Friedreich’s ataxia would have responded to the medication without this kind of selection,” Dr. Pandolfo said in an interview.
He also noted that the seemingly modest improvement in mFARS score could be an issue. “It’s a very difficult question: What is a clinically meaningful difference in this kind of rating scale? I would argue that probably 2 points is not a huge difference by itself, but it may be meaningful and one indicator of that is that if it was accompanied by also a significant difference in activities of daily living scale.”
In any event, Dr. Pandolfo said this is the first medication for Friedreich’s ataxia that has “survived” a randomized clinical trial.
Dr. Lynch said the study sponsor, Reata, may prepare a new drug application for omaveloxolone in patients ages 16 and older. “That would leave a need for investigation in younger FA patients.”
Dr. Lynch disclosed that his institution receives a grant from trial sponsor Reata to conduct the MOXIe trial. Dr. Pandolfo reports financial relationships with Design Therapeutics, Exicure and Voyager Therapeutics.
FROM MDS VIRTUAL CONGRESS 2021
Survey identifies clinicians’ unease with genetic testing
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
Before getting to work on developing guidelines for genetic testing in Parkinson’s disease, a task force of the Movement Disorders Society surveyed members worldwide to identify concerns they have about using genetic testing in practice. In results presented as a late-breaking abstract at the International Congress of Parkinson’s Disease and Movement Disorders,
“Some of the major outstanding issues are the clinical actionability of genetic testing – and this was highlighted by some survey participants,” senior study author Rachel Saunders-Pullman, MD, MPH, professor of neurology at the Icahn School of Medicine at Mount Sinai, New York, said in an interview. The issue is “dynamic,” and will change even more radically when genetic therapies for Parkinson’s disease become available. “It is planned that, in the development of the MDS Task Force guidelines, scenarios which outline the changes in consideration of testing will depend on the availability of clinically actionable data,” she said.
Barriers to genetic testing
The MDS Task Force for Genetic Testing in Parkinson Disease conducted the survey, completed online by 568 MDS members. Respondents were from the four regions from which the MDS draws members: Africa, Europe, Asia/Oceania, and Pan-America. Half of the respondents considered themselves movement disorder specialists and 31% as general neurologists, said Maggie Markgraf, research coordinator at Mount Sinai Beth Israel in New York, who presented the survey findings.
Barriers to genetic testing that the clinicians cited included cost (57%), lack of availability of genetic counseling (37%), time for testing (20%) or time for counseling (17%). About 14%also cited a lack of knowledge, and only 8.5 % said they saw no barriers for genetic testing. Other concerns included a lack of therapeutic options if tests are positive and low overall positivity rates.
“Perceived barriers for general neurologists differed slightly, with limited knowledge being the most widely reported barrier, followed closely by cost and access to testing and genetic counseling,” Ms. Markgraf said.
Respondents were also asked to identify what they thought their patients perceived as barriers to genetic testing. The major one was cost (65%), followed by limited knowledge about genetics (43%), lack of access to genetic counseling (34%), and lack of access to testing separate from cost (30%). “Across all MDS regions, the perceived level of a patient’s knowledge about genetic testing is considered to be exceedingly low,” Ms. Markgraf said.
Europe had the highest availability to genetic tests, with 41.8% saying they’re accessible to general neurologists, followed by Asia/Oceania (31%) and Pan-America (30%).
“The area of most unmet need when it comes to PD genetic testing was cost for each MDS region, although the intertwined issue of access was also high, and over 50% reported that knowledge was an unmet need in their region,” Dr. Saunders-Pullman said.
Insurance coverage was another issue the survey respondents identified. In Europe, 53.6% said insurance or government programs cover genetic testing for PD, while only 14% in Pan-America and 10.3% in Asia/Oceania (and 0% in Africa) said such coverage was available.
“While there are limitations to this study, greater awareness of availability and barriers to genetic testing and counseling across different regions, as well as disparities among regions, will help inform development of the MDS Task Force guidelines,” Dr. Saunders-Pullman said.
Unmet needs
Connie Marras, MD, PhD, a professor of neurology at the University of Toronto, noted the survey suggested neurologists exhibit a “lack of comfort or lack of time” with genetic testing and counseling for Parkinson’s disease. “Even if we make genetic testing more widely available, we need health care providers that are comfortable and available to counsel patients before and after the testing, and clearly these are unmet needs,” Dr. Marras said in an interview.
“To date, pharmacologic treatment of Parkinson’s disease did not depend on genetics,” Dr. Marras said. “This may well change in the near future with treatments specifically targeting mechanisms related to two of the most common genetic risk factors for PD: LRRK2 and GBA gene variants being in clinical trials.” These developments may soon raise the urgency to reduce barriers to genetic testing.
Dr. Saunders-Pullman and Dr. Marras have no relevant relationships to disclose.
FROM MDS VIRTUAL CONGRESS 2021
Medicare payments for most skin procedures dropped over last 15 years
according to a new analysis.
Large increases in mean reimbursement for skin biopsies (+30.3%) and shave removal (+24.6%) were not enough to offset lower rates for procedures such as simple repair (–38.7%), premalignant destruction (–19.6%), Mohs micrographic surgery (–14.4%), and flap repair (–14.1%), Rishabh S. Mazmudar, BS, of Case Western Reserve University, Cleveland, and associates said.
“Given Medicare’s contribution to a large proportion of health care expenditures, changes in Medicare reimbursement rates may result in parallel changes in private insurance reimbursement,” they wrote in JAMA Dermatology.
A recently published study showed that Medicare reimbursement for 20 dermatologic service codes had fallen by 10% between the two comparison years, 2000 and 2020. The current study expanded the number of procedures to 46 (divided into nine categories) and used historical Medicare fee schedules to examine annual trends over a 15-year period, Mr. Mazmudar and associates explained.
Other specialties have seen reimbursement fall by more than 4.8%, including emergency medicine (–21.2% from 2000 to 2020) and general surgery (–24.4% from 2000 to 2018), but “these comparisons are likely skewed by the disproportionate increase in reimbursement rates for biopsies and shave removals during this time period,” the investigators said.
If those two procedure categories were excluded from the analysis, the mean change in overall reimbursement for the remaining dermatologic procedures would be −9.6%, they noted.
Detailed payment information provided for 15 of the 46 procedures shows that only intermediate repair (+4.5%) and benign destruction (+2.3%) joined biopsies and shave excisions with increased reimbursement from 2007 to 2021. The smallest drop among the other procedures was –1.9% for malignant destruction, the research team reported.
The inflation-adjusted, year-by-year analysis showed that reimbursement for the nine procedure categories has gradually declined since peaking in 2011, with the most notable exceptions being biopsies and shave excisions. Both had been following the trend until 2013, when reimbursement for shave removals jumped by almost 30 percentage points, and 2019, when rates for biopsies soared by more than 30 percentage points, according to the investigators.
The increase for skin biopsies followed the split of the original CPT code into three categories, but “the jump in reimbursement for shave removals in 2013 requires further investigation,” Mr. Mazmudar and associates wrote.
The investigators did not disclose any conflicts of interest.
according to a new analysis.

Large increases in mean reimbursement for skin biopsies (+30.3%) and shave removal (+24.6%) were not enough to offset lower rates for procedures such as simple repair (–38.7%), premalignant destruction (–19.6%), Mohs micrographic surgery (–14.4%), and flap repair (–14.1%), Rishabh S. Mazmudar, BS, of Case Western Reserve University, Cleveland, and associates said.
“Given Medicare’s contribution to a large proportion of health care expenditures, changes in Medicare reimbursement rates may result in parallel changes in private insurance reimbursement,” they wrote in JAMA Dermatology.
A recently published study showed that Medicare reimbursement for 20 dermatologic service codes had fallen by 10% between the two comparison years, 2000 and 2020. The current study expanded the number of procedures to 46 (divided into nine categories) and used historical Medicare fee schedules to examine annual trends over a 15-year period, Mr. Mazmudar and associates explained.
Other specialties have seen reimbursement fall by more than 4.8%, including emergency medicine (–21.2% from 2000 to 2020) and general surgery (–24.4% from 2000 to 2018), but “these comparisons are likely skewed by the disproportionate increase in reimbursement rates for biopsies and shave removals during this time period,” the investigators said.
If those two procedure categories were excluded from the analysis, the mean change in overall reimbursement for the remaining dermatologic procedures would be −9.6%, they noted.
Detailed payment information provided for 15 of the 46 procedures shows that only intermediate repair (+4.5%) and benign destruction (+2.3%) joined biopsies and shave excisions with increased reimbursement from 2007 to 2021. The smallest drop among the other procedures was –1.9% for malignant destruction, the research team reported.
The inflation-adjusted, year-by-year analysis showed that reimbursement for the nine procedure categories has gradually declined since peaking in 2011, with the most notable exceptions being biopsies and shave excisions. Both had been following the trend until 2013, when reimbursement for shave removals jumped by almost 30 percentage points, and 2019, when rates for biopsies soared by more than 30 percentage points, according to the investigators.
The increase for skin biopsies followed the split of the original CPT code into three categories, but “the jump in reimbursement for shave removals in 2013 requires further investigation,” Mr. Mazmudar and associates wrote.
The investigators did not disclose any conflicts of interest.
according to a new analysis.

Large increases in mean reimbursement for skin biopsies (+30.3%) and shave removal (+24.6%) were not enough to offset lower rates for procedures such as simple repair (–38.7%), premalignant destruction (–19.6%), Mohs micrographic surgery (–14.4%), and flap repair (–14.1%), Rishabh S. Mazmudar, BS, of Case Western Reserve University, Cleveland, and associates said.
“Given Medicare’s contribution to a large proportion of health care expenditures, changes in Medicare reimbursement rates may result in parallel changes in private insurance reimbursement,” they wrote in JAMA Dermatology.
A recently published study showed that Medicare reimbursement for 20 dermatologic service codes had fallen by 10% between the two comparison years, 2000 and 2020. The current study expanded the number of procedures to 46 (divided into nine categories) and used historical Medicare fee schedules to examine annual trends over a 15-year period, Mr. Mazmudar and associates explained.
Other specialties have seen reimbursement fall by more than 4.8%, including emergency medicine (–21.2% from 2000 to 2020) and general surgery (–24.4% from 2000 to 2018), but “these comparisons are likely skewed by the disproportionate increase in reimbursement rates for biopsies and shave removals during this time period,” the investigators said.
If those two procedure categories were excluded from the analysis, the mean change in overall reimbursement for the remaining dermatologic procedures would be −9.6%, they noted.
Detailed payment information provided for 15 of the 46 procedures shows that only intermediate repair (+4.5%) and benign destruction (+2.3%) joined biopsies and shave excisions with increased reimbursement from 2007 to 2021. The smallest drop among the other procedures was –1.9% for malignant destruction, the research team reported.
The inflation-adjusted, year-by-year analysis showed that reimbursement for the nine procedure categories has gradually declined since peaking in 2011, with the most notable exceptions being biopsies and shave excisions. Both had been following the trend until 2013, when reimbursement for shave removals jumped by almost 30 percentage points, and 2019, when rates for biopsies soared by more than 30 percentage points, according to the investigators.
The increase for skin biopsies followed the split of the original CPT code into three categories, but “the jump in reimbursement for shave removals in 2013 requires further investigation,” Mr. Mazmudar and associates wrote.
The investigators did not disclose any conflicts of interest.
FROM JAMA DERMATOLOGY
Guideline gives weak support to trying oral medical cannabis for chronic pain
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
“Evidence alone is not sufficient for clinical decision-making, particularly in chronic pain,” said Jason Busse, DC, PhD, director of Michael G. DeGroote Centre for Medicinal Cannabis Research at McMaster University, Hamilton, Ont., and lead author of a newly released rapid guideline on medical cannabis or cannabinoids for chronic pain.
The recommendations, published online Sept. 9, 2021 in the British Medical Journal, suggest that providers offer patients with chronic pain a trial of noninhaled medical cannabis or cannabinoids if standard care or management is ineffective. However, the “weak” rating attached to the recommendation may compel some clinicians to automatically write off the panel’s recommendations.
“Because of the close balance between benefits and harms and wide variability in patient attitudes, the panel came to the conclusion that [some] patients presented with the current best evidence would likely choose to engage in a trial of medicinal cannabis, if their current care was felt to be suboptimal,” Dr. Busse explained in an interview.
But more importantly, “the recommendation allows for shared decision making to occur, and for different patients to make different decisions based on individual preferences and circumstances,” he said.
Evidence supports improved pain and sleep quality, physical functioning
Evidence supporting the use of medical cannabis in chronic pain is derived from a rigorous systematic review and meta-analysis of 32 studies enrolling 5,174 patients randomized to oral (capsule, spray, sublingual drops) or topical (transdermal cream) medical cannabis or placebo. Of note, three types of cannabinoids were represented: phytocannabinoids, synthetic, and endocannabinoids.
The studies included both patients with chronic noncancer pain (28 studies, n = 3,812) and chronic cancer pain not receiving palliative care (4 studies, n = 1,362). On average, baseline pain scores were a median 6.28 cm on a 10-cm visual analog scale (VAS), and median participant age was 53 years. 60% of trials reporting sex differences enrolled female participants. Overall, patients were followed for roughly 2 months (median, 50 days).
Findings (27 studies, n = 3,939) showed that, compared with placebo, medical cannabis resulted in a small, albeit important, improvement in the proportion of patients experiencing pain relief at or above the minimally important difference (MID) (moderate-certainty evidence, 10% modeled risk difference [RD; 95% confidence interval, 5%-15%] for achieving at least the MID of 1 cm).
Medical cannabis (15 studies, n = 2,425) also provided a small increase in the proportion of patients experiencing improvements in physical functioning at or above the MID (high certainty evidence, 4% modeled RD [95% CI, 0.1%-8%] for achieving at least a MID of 10 points).
Additionally, participants experienced significant improvements in sleep quality, compared with placebo (16 studies, 3,124 participants, high-quality evidence), demonstrating a weighted mean difference of –0.53 cm on a 10-cm VAS (95% CI, –0.75 to –0.30 cm). A total of nine larger trials (n = 2,652, high-certainty evidence) saw a small increase in the proportion of patients experiencing improved sleep quality at or above the MID: 6% modeled RD (95% CI, 2%-9%).
On the other hand, benefits did not extend to emotional, role, or social functioning (high-certainty evidence).
First do no harm: Start low, go slow
While these findings provide a rationale for medical cannabis in chronic pain, exploring options with patients can be challenging. Studies on medical cannabis consistently note that patients want information, but data also show that many providers express a lack of knowledge to provide adequate counseling.
There are also legal hurdles. Despite the authorization of medicinal cannabis across a majority of states and territories, cannabis is still a schedule I substance under the Federal Controlled Substances Act. In addition, the absence of standards around formulations, potency, and dosing has also been cited as a major barrier to recommending medical cannabis, as have concerns about adverse events (AEs), especially with inhaled and tetrahydrocannabinol (THC)-predominant formulations.
Like most medications, medical cannabis dosing should be individualized depending on product, patient, and ability to titrate the dose, but the guidelines provide a general rule of thumb. Providers considering therapeutic noninhaled medical cannabis trials are encouraged to start with a low-dose cannabidiol (CBD) oral tablet, spray, or sublingual oil drops 5 mg twice daily, increasing it by 10 mg every 2-3 days depending on the clinical response (to a maximum daily dose of 40 mg/day). If patient response is unsatisfactory, they should consider adding 1-2.5 mg THC/daily, titrated every 2-7 days to a maximum of 40 mg/day.
Still, an important caveat is whether or not adjunctive CBD alone is effective for chronic pain.
“While we know that one out of seven U.S. adults are using cannabidiol, we know very little about its therapeutic effects when given by itself for pain,” Ziva Cooper, PhD, director of the Cannabis Research Initiative at the University of California, Los Angeles, and an associate professor at-large of psychology and behavioral science, said in an interview. (Dr. Cooper was not involved in the guideline development.)
“But patients tend to self-report that CBD is helpful, and at low doses, we know that it is unlikely to have adverse effects of any significant concern,” Dr. Cooper noted.
Depending on its components, medical cannabis is associated with a wide range of AEs. Studies comprising the evidence base for the guideline reported transient cognitive impairment (relative risk, 2.39; 95% CI, 1.06-5.38), vomiting (RR, 1.46; 95% CI, 1.07-1.99), and drowsiness (RR, 2.14; 95% CI, 1.55-2.95), attention impairment (RR, 4.04; 95% CI, 1.67-9.74), and nausea (RR, 1.59; 95% CI, 1.28-1.99). Of note, findings of a subgroup analysis showed that the risk of dizziness increased with treatment duration, starting at 3 months (test of interaction P = .002).
However, Dr. Cooper explained that, because the included studies were inconsistent in terms of cannabis type (e.g., some looked at synthetic THC or THC-like substances where others looked at a THC/CBD combination) and formulation (capsules, oral mucosal sprays), it’s difficult to tease out component-specific AEs.
“These are really important things to note, especially when you think about different populations that might be using these types of medicines moving forward,” she said.
Toward that end, the guideline specifically states that there is “no reason why the expected benefits would be systematically different among adolescents and emerging adults.”
Among children with cancer, prior study findings reinforce the conclusion that benefits are similar to adults, but studies in this area are limited to end-of-life treatment, childhood cancer with primarily palliative intent, or progressive or relapsed cancer. Because THC’s safety profile is less certain in children, it’s also important to consider adverse neurocognitive effects before initiating a medical cannabis trial in this population.
Navigating the landscape
Although promising, the medical cannabis landscape is undoubtedly difficult to navigate, with land mines ranging from a limited inability to simply pick up a prescribing pad to quality control.
With the exception of three Food and Drug Administration–approved products – dronabinol, cannabidiol Rx, and nabilone – U.S. providers are only able to ‘certify,’ not prescribe, medical cannabis for chronic pain, and only if it is included within the state cannabis board’s list of eligible conditions. (A state-by-state guide is available.)
Quality control also varies by product but is critical. “You want to look for certificates of quality assurance,” Jenny Wilkerson, PhD, a research assistant professor of pharmacodynamics at the University of Florida, Gainesville, said in an interview. (Dr. Wilkerson was not involved in the guideline development.)
“A good dispensary should have that information or at least be willing to get that information, but generally speaking, that is something that patients need to ask for,” she emphasized, noting that “most available mass readouts are not divided by lots.”
Initial counseling and AE monitoring and regular follow-up is important, especially among patients who’ve never tried medical cannabis (or older patients whose prior experience may be limited to weaker recreational marijuana).
Notably, the reliance on medical dispensaries to deliver the right information at the right time may prove to be faulty. While recent data show that frontline dispensary workers regularly provide information to customers on their medical conditions and available products, they rarely, if ever, base recommendations on provider input, and never or rarely discuss potential AEs and other risks.
Per the new guideline, inexperienced patients should be seen monthly until a stable dose is achieved; longer times between visits can be considered in those who are more experienced. Still, patients should be advised to contact their provider when pain relief or other goals are insufficient, or when response or problematic AEs occur. This facilitates down-titration to a previously tolerated dose, up-titration in CBD and/or THC, or a different route of administration/formulation altogether.
Dr. Wilkerson pointed out that follow-up visits also provide an opportunity to do a blood draw and ask the lab to conduct pharmacokinetic analysis.
If possible, “ask patients to [ensure that they] take a standard dose before the visit so that the lab can assess the blood percentage of primary compounds and metabolites in the product that they are using,” she explained, noting that the information is helping to determine how “the different ratios may be affecting therapeutic response in individual patients.”
Granted, the guideline is only a start. But it is a good one.
“A lot of physicians want to be able to hang their hat on evidence of the safety and efficacy of these products, and the analysis that was leveraged for this guideline was very rigorous,” Dr. Cooper said.
Not only do they reinforce that “oral cannabinoids can produce small improvements in pain and provide a dosing structure that minimizes risk to the patient, [but they] should be able to help educate physicians who [are looking] for a sense of what the literature tells us at this time,” she added.
“With chronic pain, we often find that different treatments will show small potential benefits and they have a certain risk profile,” Dr. Busse said.
“It’s almost impossible to know what patients think about this option unless you present them with the evidence and ask them to make a decision based on their values and preferences,” he said.
The Michael G. DeGroote Centre for Medicinal Cannabis Research funded the MAGIC Evidence Ecosystem Foundation to support the creation of the guideline. The center receives no funding from industry Dr. Busse, Dr. Cooper, and Dr. Wilkerson reported having no relevant financial relationships.
FROM THE BMJ