User login
Targeting IL-23 could still be important for axial spondyloarthritis treatment
Interleukin (IL)–23 inhibition may still have a role to play in the treatment of patients with axial spondyloarthritis (SpA), suggests research presented at the 12th International Congress on Spondyloarthritides.
There is a strong rationale for using IL-23 inhibitors in patients with axial SpA, and the IL-23/IL-17 axis has been proposed as a critical player in the pathophysiology of the disease. But around 2018 it became clear from randomized, controlled trials that IL-23 inhibition was ineffective at improving key clinical outcomes, at least in patients with axial disease.
Although the overall results of a systematic review and meta-analysis that was presented at the meeting corroborated the negative results seen with IL-23–inhibiting agents in clinical trials, there were some data showing benefits of the IL-23 inhibitor risankizumab on secondary outcomes in one trial.
To look at the available evidence, Louise Vanhoutte, a 2nd-year internal medicine student at University Hospitals Leuven (Belgium) worked under the guidance of Rik Lories, MD, PhD, head of the division of rheumatology at University Hospitals Leuven. Together they searched known databases for randomized, controlled trials investigating the use of IL-23 and IL-17 inhibitors for the treatment of adults with axial SpA or psoriatic arthritis. Studies could be either phase 2 or phase 3, but had to have included a placebo and used the ASAS40 (40% Improvement in Assessment of SpondyloArthritis International Society Response criteria), ASAS20, Bath Ankylosing Spondylitis Disease Activity Index, or SPARCC (Spondyloarthritis Research Consortium of Canada) index score to assess outcomes.
The systematic review whittled the number of clinical trials in the meta-analysis to 12, which concerned the use of ustekinumab, an IL-12/23 inhibitor, and risankizumab, along with two IL-17 inhibitors, ixekizumab and secukinumab. Data for the IL-23 inhibitors guselkumab and tildrakizumab were not available.
“To no surprise, Forest plots showed that there was a lack of efficacy for IL-23 agents in the treatment of axial spondyloarthritis and a superior efficacy for IL-17 inhibitors in the treatment of axial spondyloarthritis,” Ms. Vanhoutte reported.
The respective odds ratios for IL-23 and IL-17 inhibitors in getting patients to meet ASAS40 response criteria in comparison to baseline were 1.51 (95% confidence interval, 0.98-2.31) and 2.54 (95% CI, 2.02-3.19).
“Does this mean it is a dead-end street for all IL-23 inhibition?” she asked. Not necessarily. In the meta-analysis, not only did risankizumab lower the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP) by a mean difference (MD) of –0.30 (95% CI, –0.41 to –0.19) from baseline values, but it also led to statistically significant reductions in SPARCC index score for the spine (MD, –3.10; 95% CI, –4.50 to –1.70) and high-sensitivity CRP (MD, –2.10; 95% CI, –2.56 to –1.64). The risankizumab findings might suggest there are potential disease-modifying properties for specifically targeting IL-23p19. There could also be a window of opportunity to use IL-23 inhibitors earlier.
“These are only results from one randomized, controlled trial in a small sample size where outcomes were reported as medians and interquartile ranges, so they had to be converted to means and standard deviations to have an odds ratio in the end,” she explained.
“Also, these were results from a radiographic axial spondyloarthritis population and not a nonradiographic axial spondyloarthritis population,” she added.
While that might limit the interpretation of the findings, “what we see here is both reduction in inflammation and reduction in structural disease progression as [measured] by SPARCC,” Ms. Vanhoutte said.
“Since IL-23 is an upstream molecule from IL-17 it’s probable that IL-23 is present in the prephase of the disease, in a prephase inflammation state,” she hypothesized. “This is especially interesting because there are very few randomized, controlled trials that examine therapeutic agents in nonradiographic axial spondyloarthritis,” she observed. Looking at IL-23 in radiographic, or established, disease therefore may not be as useful.
“I’m thinking you’re making actually a very important point for us,” commented Robert Landewé, MD, PhD, of Amsterdam University Medical Center.
“We are discussing whether or not IL-23 is important in inhibiting the disease activity of patients with axial spondyloarthritis, and we are surprised that it is not shown in RCTs.
“Why is it completely ineffective in axial spondyloarthritis? You show us that that is probably not the case,” Dr. Landewé suggested.
“What you make very clear here is that indeed there is some efficacy, and from a pathophysiological way of thinking it might be slightly different as compared with what most clinicians nowadays think.”
The study had no specific funding, and no disclosures were reported.
Interleukin (IL)–23 inhibition may still have a role to play in the treatment of patients with axial spondyloarthritis (SpA), suggests research presented at the 12th International Congress on Spondyloarthritides.
There is a strong rationale for using IL-23 inhibitors in patients with axial SpA, and the IL-23/IL-17 axis has been proposed as a critical player in the pathophysiology of the disease. But around 2018 it became clear from randomized, controlled trials that IL-23 inhibition was ineffective at improving key clinical outcomes, at least in patients with axial disease.
Although the overall results of a systematic review and meta-analysis that was presented at the meeting corroborated the negative results seen with IL-23–inhibiting agents in clinical trials, there were some data showing benefits of the IL-23 inhibitor risankizumab on secondary outcomes in one trial.
To look at the available evidence, Louise Vanhoutte, a 2nd-year internal medicine student at University Hospitals Leuven (Belgium) worked under the guidance of Rik Lories, MD, PhD, head of the division of rheumatology at University Hospitals Leuven. Together they searched known databases for randomized, controlled trials investigating the use of IL-23 and IL-17 inhibitors for the treatment of adults with axial SpA or psoriatic arthritis. Studies could be either phase 2 or phase 3, but had to have included a placebo and used the ASAS40 (40% Improvement in Assessment of SpondyloArthritis International Society Response criteria), ASAS20, Bath Ankylosing Spondylitis Disease Activity Index, or SPARCC (Spondyloarthritis Research Consortium of Canada) index score to assess outcomes.
The systematic review whittled the number of clinical trials in the meta-analysis to 12, which concerned the use of ustekinumab, an IL-12/23 inhibitor, and risankizumab, along with two IL-17 inhibitors, ixekizumab and secukinumab. Data for the IL-23 inhibitors guselkumab and tildrakizumab were not available.
“To no surprise, Forest plots showed that there was a lack of efficacy for IL-23 agents in the treatment of axial spondyloarthritis and a superior efficacy for IL-17 inhibitors in the treatment of axial spondyloarthritis,” Ms. Vanhoutte reported.
The respective odds ratios for IL-23 and IL-17 inhibitors in getting patients to meet ASAS40 response criteria in comparison to baseline were 1.51 (95% confidence interval, 0.98-2.31) and 2.54 (95% CI, 2.02-3.19).
“Does this mean it is a dead-end street for all IL-23 inhibition?” she asked. Not necessarily. In the meta-analysis, not only did risankizumab lower the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP) by a mean difference (MD) of –0.30 (95% CI, –0.41 to –0.19) from baseline values, but it also led to statistically significant reductions in SPARCC index score for the spine (MD, –3.10; 95% CI, –4.50 to –1.70) and high-sensitivity CRP (MD, –2.10; 95% CI, –2.56 to –1.64). The risankizumab findings might suggest there are potential disease-modifying properties for specifically targeting IL-23p19. There could also be a window of opportunity to use IL-23 inhibitors earlier.
“These are only results from one randomized, controlled trial in a small sample size where outcomes were reported as medians and interquartile ranges, so they had to be converted to means and standard deviations to have an odds ratio in the end,” she explained.
“Also, these were results from a radiographic axial spondyloarthritis population and not a nonradiographic axial spondyloarthritis population,” she added.
While that might limit the interpretation of the findings, “what we see here is both reduction in inflammation and reduction in structural disease progression as [measured] by SPARCC,” Ms. Vanhoutte said.
“Since IL-23 is an upstream molecule from IL-17 it’s probable that IL-23 is present in the prephase of the disease, in a prephase inflammation state,” she hypothesized. “This is especially interesting because there are very few randomized, controlled trials that examine therapeutic agents in nonradiographic axial spondyloarthritis,” she observed. Looking at IL-23 in radiographic, or established, disease therefore may not be as useful.
“I’m thinking you’re making actually a very important point for us,” commented Robert Landewé, MD, PhD, of Amsterdam University Medical Center.
“We are discussing whether or not IL-23 is important in inhibiting the disease activity of patients with axial spondyloarthritis, and we are surprised that it is not shown in RCTs.
“Why is it completely ineffective in axial spondyloarthritis? You show us that that is probably not the case,” Dr. Landewé suggested.
“What you make very clear here is that indeed there is some efficacy, and from a pathophysiological way of thinking it might be slightly different as compared with what most clinicians nowadays think.”
The study had no specific funding, and no disclosures were reported.
Interleukin (IL)–23 inhibition may still have a role to play in the treatment of patients with axial spondyloarthritis (SpA), suggests research presented at the 12th International Congress on Spondyloarthritides.
There is a strong rationale for using IL-23 inhibitors in patients with axial SpA, and the IL-23/IL-17 axis has been proposed as a critical player in the pathophysiology of the disease. But around 2018 it became clear from randomized, controlled trials that IL-23 inhibition was ineffective at improving key clinical outcomes, at least in patients with axial disease.
Although the overall results of a systematic review and meta-analysis that was presented at the meeting corroborated the negative results seen with IL-23–inhibiting agents in clinical trials, there were some data showing benefits of the IL-23 inhibitor risankizumab on secondary outcomes in one trial.
To look at the available evidence, Louise Vanhoutte, a 2nd-year internal medicine student at University Hospitals Leuven (Belgium) worked under the guidance of Rik Lories, MD, PhD, head of the division of rheumatology at University Hospitals Leuven. Together they searched known databases for randomized, controlled trials investigating the use of IL-23 and IL-17 inhibitors for the treatment of adults with axial SpA or psoriatic arthritis. Studies could be either phase 2 or phase 3, but had to have included a placebo and used the ASAS40 (40% Improvement in Assessment of SpondyloArthritis International Society Response criteria), ASAS20, Bath Ankylosing Spondylitis Disease Activity Index, or SPARCC (Spondyloarthritis Research Consortium of Canada) index score to assess outcomes.
The systematic review whittled the number of clinical trials in the meta-analysis to 12, which concerned the use of ustekinumab, an IL-12/23 inhibitor, and risankizumab, along with two IL-17 inhibitors, ixekizumab and secukinumab. Data for the IL-23 inhibitors guselkumab and tildrakizumab were not available.
“To no surprise, Forest plots showed that there was a lack of efficacy for IL-23 agents in the treatment of axial spondyloarthritis and a superior efficacy for IL-17 inhibitors in the treatment of axial spondyloarthritis,” Ms. Vanhoutte reported.
The respective odds ratios for IL-23 and IL-17 inhibitors in getting patients to meet ASAS40 response criteria in comparison to baseline were 1.51 (95% confidence interval, 0.98-2.31) and 2.54 (95% CI, 2.02-3.19).
“Does this mean it is a dead-end street for all IL-23 inhibition?” she asked. Not necessarily. In the meta-analysis, not only did risankizumab lower the Ankylosing Spondylitis Disease Activity Score based on C-reactive protein (ASDAS-CRP) by a mean difference (MD) of –0.30 (95% CI, –0.41 to –0.19) from baseline values, but it also led to statistically significant reductions in SPARCC index score for the spine (MD, –3.10; 95% CI, –4.50 to –1.70) and high-sensitivity CRP (MD, –2.10; 95% CI, –2.56 to –1.64). The risankizumab findings might suggest there are potential disease-modifying properties for specifically targeting IL-23p19. There could also be a window of opportunity to use IL-23 inhibitors earlier.
“These are only results from one randomized, controlled trial in a small sample size where outcomes were reported as medians and interquartile ranges, so they had to be converted to means and standard deviations to have an odds ratio in the end,” she explained.
“Also, these were results from a radiographic axial spondyloarthritis population and not a nonradiographic axial spondyloarthritis population,” she added.
While that might limit the interpretation of the findings, “what we see here is both reduction in inflammation and reduction in structural disease progression as [measured] by SPARCC,” Ms. Vanhoutte said.
“Since IL-23 is an upstream molecule from IL-17 it’s probable that IL-23 is present in the prephase of the disease, in a prephase inflammation state,” she hypothesized. “This is especially interesting because there are very few randomized, controlled trials that examine therapeutic agents in nonradiographic axial spondyloarthritis,” she observed. Looking at IL-23 in radiographic, or established, disease therefore may not be as useful.
“I’m thinking you’re making actually a very important point for us,” commented Robert Landewé, MD, PhD, of Amsterdam University Medical Center.
“We are discussing whether or not IL-23 is important in inhibiting the disease activity of patients with axial spondyloarthritis, and we are surprised that it is not shown in RCTs.
“Why is it completely ineffective in axial spondyloarthritis? You show us that that is probably not the case,” Dr. Landewé suggested.
“What you make very clear here is that indeed there is some efficacy, and from a pathophysiological way of thinking it might be slightly different as compared with what most clinicians nowadays think.”
The study had no specific funding, and no disclosures were reported.
FROM THE 2021 SPA CONGRESS
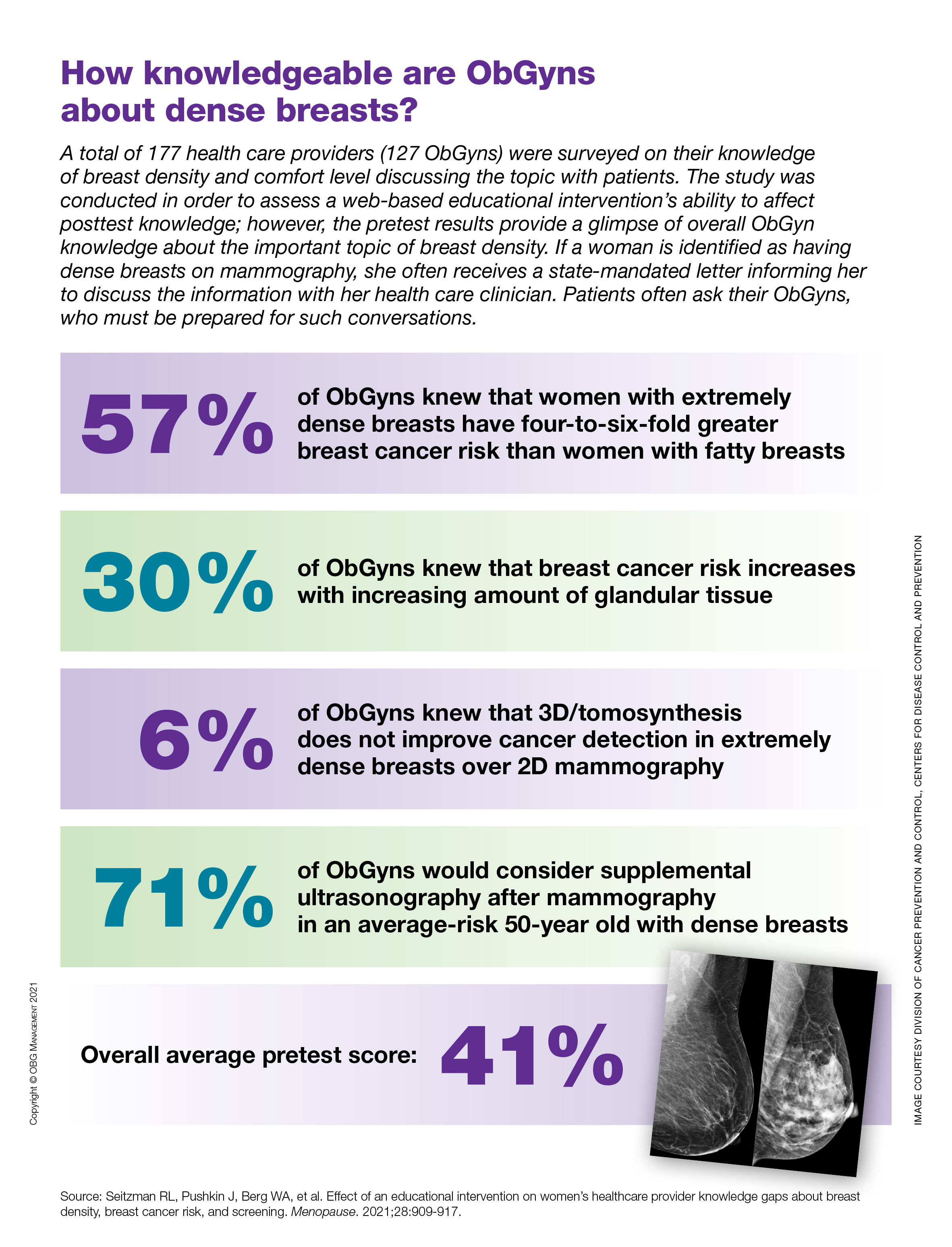
How knowledgeable are ObGyns about dense breasts?


Patient risk-benefit thresholds for antibiotic use in dermatologic surgery vary widely
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM DERMATOLOGIC SURGERY
European agency recommends two new adalimumab biosimilars
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended marketing authorization this week for two new adalimumab biosimilars, Hukyndra and Libmyris.
The biosimilars, both developed by STADA Arzneimittel AG, will be available as a 40-mg solution for injection in a pre-filled syringe and pre-filled pen and 80-mg solution for injection in a pre-filled syringe. Both biosimilars will have 15 indications:
- rheumatoid arthritis
- polyarticular juvenile idiopathic arthritis
- enthesitis-related arthritis
- ankylosing spondylitis
- axial spondyloarthritis without radiographic evidence of ankylosing spondylitis
- psoriatic arthritis
- chronic plaque psoriasis (adults and children)
- hidradenitis suppurativa
- Crohn’s disease (adults and children)
- ulcerative colitis (adults and children)
- uveitis (adults and children)
Data show that both Hukyndra and Libmyris are highly similar to the reference product Humira (adalimumab), a monoclonal antibody to tumor necrosis factor alpha, and have comparable quality, safety, and efficacy.
A version of this article first appeared on Medscape.com.
U.S. seniors’ pandemic care worst among wealthy nations: Survey
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults in the United States – particularly among Black and Latino/Hispanic populations – experienced worse access to health care for chronic conditions during the pandemic than older adults in 10 other wealthy countries, according to findings from The Commonwealth Fund’s 2021 International Health Policy Survey of Older Adults released today.
David Blumenthal, MD, president of The Commonwealth Fund, said during a press briefing that surveying the senior population in the United States is particularly insightful because it is the only group with the universal coverage of Medicare, which offers a more direct comparison with other countries’ universal health care coverage.
More than one-third (37%) of older U.S. adults with multiple chronic conditions reported pandemic-related disruptions in their care – higher than rates in Canada, the Netherlands, and U.K. In Germany, only 11% had canceled or postponed appointments.
The survey was conducted between March and June 2021 and included responses from 18,477 adults age 65 and older in Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and U.K., and U.S. adults age 60 and older.
Among older adults who need help with daily activities, those in the United States, Canada, U.K., and Australia were the most likely to say they did not receive needed services from professionals or family members.
In the United States, 23% of people who said they needed help with activities such as housework, meal preparation, and medication management experienced a disruption in care because services were canceled or very limited during the pandemic. For comparison, only 8% of seniors in Germany and 11% of seniors in the Netherlands did not receive help with basic daily activities.
Many U.S. seniors used up savings
“Nearly one in five older adults report that they used up their savings or lost their main source of income because of the pandemic. We see much lower rates in other countries like Germany, Switzerland, the Netherlands, and Sweden,” Reginald D. Williams, vice president for international health policy and practice innovations at The Commonwealth Fund, said during a briefing.
Older U.S. adults reported economic difficulties related to the pandemic at a rate of up to six times that of other countries, he said.
The differences by race were stark. While 19% of U.S. seniors overall experienced financial hardships related to the pandemic, 32% of Black seniors and 39% of Latino/Hispanic seniors in the United States experienced hardships. Germany had the lowest rate, at 3% overall.
“As the COVID-19 pandemic in the United States continues to evolve,” Mr. Williams said, “finding ways to reduce care barriers – affordability and connecting adults to usual sources of primary care, enhancing access to economic supports and social services – can help narrow the gaps.”
Dr. Blumenthal said that even though “Medicare is a critical lifeline,” it has flaws.
“Medicare plans have significant gaps that leave beneficiaries vulnerable to sizable out-of-pocket expenses,” he said.
Placing caps on out-of-pocket costs and covering more health services, such as dental, vision, and hearing care, could help make the population less vulnerable, Dr. Blumenthal said. “The chronic lack of security facing U.S. seniors, especially those who are Black or Hispanic, is exacerbating the pandemic’s devastating toll,” he added.
Dr. Blumenthal and Mr. Williams have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Korean siblings face high familial IBD risk
Among Asian-Pacific populations, the first-degree relatives (FDRs) of individuals with inflammatory bowel disease (IBD) have a significantly increased risk for IBD themselves, according to a large analysis of data from South Korea. The greatest risk was found in siblings and for Crohn’s disease (CD).
The analysis of the South Korean Health Insurance Database included a cohort of 21,940,795 individuals from about 12 million families, with data collected between 2002 and 2017.
Previous studies have examined risk of IBD and familial relationships with existing IBD patients, but they have been subject to biases and have been heterogeneous in design, according to the authors, led by co–first authors Hyun Jung Kim, MD, of Korea University in Seoul, South Korea, and Shailja C. Shah, MD, of Vanderbilt University in Nashville, Tenn. There are few true population-based studies that quantify specific risks for family members of IBD patients, and none that were conducted in non-Western populations.
There are concerns about extrapolating familial IBD risk estimates from Western European populations to Asian populations because new data suggest that there are both genetic and nongenetic disease risk factors that reflect geography and ethnicity, the authors noted.
The researchers identified 45,717 individuals with ulcerative colitis (UC) and 17,848 with CD. Mean annual incidence rates were 4.6 cases of UC and 3.2 cases of CD per 100,000 person-years, which was relatively stable across the study period.
In all, 3.8% of UC and 3.1% of CD diagnoses occurred in FDR’s of existing patients. Among those with an FDR with IBD, the incidence of UC and CD was 54.5 and 99.2 per 100,000 person-years, respectively. When compared with individuals who had no FDRs with IBD, subjects who had an FDR with CD were at a more than 20-fold increased risk of CD (incident rate ratio, 22.2; 95% confidence interval, 20.5-24.5), whereas individuals with an FDR with UC were at a little more than a 10-fold risk for UC (IRR, 10.2; 95% CI, 9.39-11.1).
Subjects with an FDR with CD were at higher risk of UC (IRR, 3.56; 95% CI, 2.77-4.50), and those with an FDR with UC were at higher risk of CD (IRR, 2.94; 95% CI, 2.45-3.49). After adjustment for smoking, having an FDR with IBD was associated with an almost eightfold increased risk of UC (IRR, 7.94; 95% CI, 6.98-9.03) and a nearly 20-fold increased risk of CD (IRR, 19.03; 95% CI, 15.58-23.25).
The investigators also performed an analysis based on type of relative, with matching relations with unaffected relatives as the reference for each comparison. The highest risk for incident CD was with twin siblings (IRR, 336.2; 95% CI, 235.0-481.1) followed by nontwin siblings (IRR, 27.6; 95% CI, 24.6-30.9). The risk of CD among offspring of an affected father was 9.40 (95% CI, 6.81-13.0) and 6.54 (95% CI, 4.17-10.3) for offspring of affected mothers. There was a similar pattern for UC, although the magnitude was smaller: 163.7 for twin siblings (95% CI, 105.6-253.9), 13.1 for nontwin siblings (95% CI, 11.4-15.0), 7.11 for offspring of affected fathers (95% CI, 6.10-8.29), and 8.77 for offspring of affected mothers (95% CI, 7.46-10.3).
The researchers found no evidence of a birth cohort effect. Family history and IBD risk is a complicated relationship. Family history includes shared genetics as well as similar environmental exposures, and gene-environment interactions can add another layer of uncertainty. Previous studies have found that asymptomatic family members of IBD patients sometimes have preclinical signs such as changes in intestinal permeability, immune function, the microbiome, and biomarker levels.
IBD has emerged recently among Asian-Pacific populations as a serious health concern, with a recent rapid increase. This may reflect a shift in potentially modifiable environmental triggers. “Precisely quantifying familial risk and patterns might enable more accurate risk counseling and better-targeted clinical surveillance for earlier diagnosis and treatment among FDRs. Moreover, an accurate definition of familial IBD risk across populations also might inform subsequent investigations untangling the various shared environmental and genetic contributions,” the authors wrote.
Although genetic susceptibility is generally accepted as the predominant driver in familial trends for IBD, the authors noted their “study was not designed to determine the contribution of genetic vs. nongenetic determinants to familial IBD risk, and future well-designed dedicated investigations are needed to provide this clarity.”
The study is limited by the relatively short follow-up period, which may not have captured all IBD cases within patients’ families.
The authors have no relevant financial disclosures.
One of the hallmarks of effective IBD management is early disease intervention to modify the natural history. This work will be instrumental in counseling patients’ families on the need to monitor for subclinical red flag or early warning signs, and it will be important to recognize that male and female IBD patients will both need to be counseled equally on the risk of offspring developing IBD. Further work will be needed to understand whether modifiable risk factors can be identified to help prevent the development of IBD in these at-risk individuals and whether specific mutations are responsible for multilineage IBD syndromes affecting several generations or multiple first-degree relatives.
Parambir S. Dulai, MD, is an assistant professor in the division of gastroenterology and hepatology at University of California, San Diego. He has no relevant conflicts of interest.
One of the hallmarks of effective IBD management is early disease intervention to modify the natural history. This work will be instrumental in counseling patients’ families on the need to monitor for subclinical red flag or early warning signs, and it will be important to recognize that male and female IBD patients will both need to be counseled equally on the risk of offspring developing IBD. Further work will be needed to understand whether modifiable risk factors can be identified to help prevent the development of IBD in these at-risk individuals and whether specific mutations are responsible for multilineage IBD syndromes affecting several generations or multiple first-degree relatives.
Parambir S. Dulai, MD, is an assistant professor in the division of gastroenterology and hepatology at University of California, San Diego. He has no relevant conflicts of interest.
One of the hallmarks of effective IBD management is early disease intervention to modify the natural history. This work will be instrumental in counseling patients’ families on the need to monitor for subclinical red flag or early warning signs, and it will be important to recognize that male and female IBD patients will both need to be counseled equally on the risk of offspring developing IBD. Further work will be needed to understand whether modifiable risk factors can be identified to help prevent the development of IBD in these at-risk individuals and whether specific mutations are responsible for multilineage IBD syndromes affecting several generations or multiple first-degree relatives.
Parambir S. Dulai, MD, is an assistant professor in the division of gastroenterology and hepatology at University of California, San Diego. He has no relevant conflicts of interest.
Among Asian-Pacific populations, the first-degree relatives (FDRs) of individuals with inflammatory bowel disease (IBD) have a significantly increased risk for IBD themselves, according to a large analysis of data from South Korea. The greatest risk was found in siblings and for Crohn’s disease (CD).
The analysis of the South Korean Health Insurance Database included a cohort of 21,940,795 individuals from about 12 million families, with data collected between 2002 and 2017.
Previous studies have examined risk of IBD and familial relationships with existing IBD patients, but they have been subject to biases and have been heterogeneous in design, according to the authors, led by co–first authors Hyun Jung Kim, MD, of Korea University in Seoul, South Korea, and Shailja C. Shah, MD, of Vanderbilt University in Nashville, Tenn. There are few true population-based studies that quantify specific risks for family members of IBD patients, and none that were conducted in non-Western populations.
There are concerns about extrapolating familial IBD risk estimates from Western European populations to Asian populations because new data suggest that there are both genetic and nongenetic disease risk factors that reflect geography and ethnicity, the authors noted.
The researchers identified 45,717 individuals with ulcerative colitis (UC) and 17,848 with CD. Mean annual incidence rates were 4.6 cases of UC and 3.2 cases of CD per 100,000 person-years, which was relatively stable across the study period.
In all, 3.8% of UC and 3.1% of CD diagnoses occurred in FDR’s of existing patients. Among those with an FDR with IBD, the incidence of UC and CD was 54.5 and 99.2 per 100,000 person-years, respectively. When compared with individuals who had no FDRs with IBD, subjects who had an FDR with CD were at a more than 20-fold increased risk of CD (incident rate ratio, 22.2; 95% confidence interval, 20.5-24.5), whereas individuals with an FDR with UC were at a little more than a 10-fold risk for UC (IRR, 10.2; 95% CI, 9.39-11.1).
Subjects with an FDR with CD were at higher risk of UC (IRR, 3.56; 95% CI, 2.77-4.50), and those with an FDR with UC were at higher risk of CD (IRR, 2.94; 95% CI, 2.45-3.49). After adjustment for smoking, having an FDR with IBD was associated with an almost eightfold increased risk of UC (IRR, 7.94; 95% CI, 6.98-9.03) and a nearly 20-fold increased risk of CD (IRR, 19.03; 95% CI, 15.58-23.25).
The investigators also performed an analysis based on type of relative, with matching relations with unaffected relatives as the reference for each comparison. The highest risk for incident CD was with twin siblings (IRR, 336.2; 95% CI, 235.0-481.1) followed by nontwin siblings (IRR, 27.6; 95% CI, 24.6-30.9). The risk of CD among offspring of an affected father was 9.40 (95% CI, 6.81-13.0) and 6.54 (95% CI, 4.17-10.3) for offspring of affected mothers. There was a similar pattern for UC, although the magnitude was smaller: 163.7 for twin siblings (95% CI, 105.6-253.9), 13.1 for nontwin siblings (95% CI, 11.4-15.0), 7.11 for offspring of affected fathers (95% CI, 6.10-8.29), and 8.77 for offspring of affected mothers (95% CI, 7.46-10.3).
The researchers found no evidence of a birth cohort effect. Family history and IBD risk is a complicated relationship. Family history includes shared genetics as well as similar environmental exposures, and gene-environment interactions can add another layer of uncertainty. Previous studies have found that asymptomatic family members of IBD patients sometimes have preclinical signs such as changes in intestinal permeability, immune function, the microbiome, and biomarker levels.
IBD has emerged recently among Asian-Pacific populations as a serious health concern, with a recent rapid increase. This may reflect a shift in potentially modifiable environmental triggers. “Precisely quantifying familial risk and patterns might enable more accurate risk counseling and better-targeted clinical surveillance for earlier diagnosis and treatment among FDRs. Moreover, an accurate definition of familial IBD risk across populations also might inform subsequent investigations untangling the various shared environmental and genetic contributions,” the authors wrote.
Although genetic susceptibility is generally accepted as the predominant driver in familial trends for IBD, the authors noted their “study was not designed to determine the contribution of genetic vs. nongenetic determinants to familial IBD risk, and future well-designed dedicated investigations are needed to provide this clarity.”
The study is limited by the relatively short follow-up period, which may not have captured all IBD cases within patients’ families.
The authors have no relevant financial disclosures.
Among Asian-Pacific populations, the first-degree relatives (FDRs) of individuals with inflammatory bowel disease (IBD) have a significantly increased risk for IBD themselves, according to a large analysis of data from South Korea. The greatest risk was found in siblings and for Crohn’s disease (CD).
The analysis of the South Korean Health Insurance Database included a cohort of 21,940,795 individuals from about 12 million families, with data collected between 2002 and 2017.
Previous studies have examined risk of IBD and familial relationships with existing IBD patients, but they have been subject to biases and have been heterogeneous in design, according to the authors, led by co–first authors Hyun Jung Kim, MD, of Korea University in Seoul, South Korea, and Shailja C. Shah, MD, of Vanderbilt University in Nashville, Tenn. There are few true population-based studies that quantify specific risks for family members of IBD patients, and none that were conducted in non-Western populations.
There are concerns about extrapolating familial IBD risk estimates from Western European populations to Asian populations because new data suggest that there are both genetic and nongenetic disease risk factors that reflect geography and ethnicity, the authors noted.
The researchers identified 45,717 individuals with ulcerative colitis (UC) and 17,848 with CD. Mean annual incidence rates were 4.6 cases of UC and 3.2 cases of CD per 100,000 person-years, which was relatively stable across the study period.
In all, 3.8% of UC and 3.1% of CD diagnoses occurred in FDR’s of existing patients. Among those with an FDR with IBD, the incidence of UC and CD was 54.5 and 99.2 per 100,000 person-years, respectively. When compared with individuals who had no FDRs with IBD, subjects who had an FDR with CD were at a more than 20-fold increased risk of CD (incident rate ratio, 22.2; 95% confidence interval, 20.5-24.5), whereas individuals with an FDR with UC were at a little more than a 10-fold risk for UC (IRR, 10.2; 95% CI, 9.39-11.1).
Subjects with an FDR with CD were at higher risk of UC (IRR, 3.56; 95% CI, 2.77-4.50), and those with an FDR with UC were at higher risk of CD (IRR, 2.94; 95% CI, 2.45-3.49). After adjustment for smoking, having an FDR with IBD was associated with an almost eightfold increased risk of UC (IRR, 7.94; 95% CI, 6.98-9.03) and a nearly 20-fold increased risk of CD (IRR, 19.03; 95% CI, 15.58-23.25).
The investigators also performed an analysis based on type of relative, with matching relations with unaffected relatives as the reference for each comparison. The highest risk for incident CD was with twin siblings (IRR, 336.2; 95% CI, 235.0-481.1) followed by nontwin siblings (IRR, 27.6; 95% CI, 24.6-30.9). The risk of CD among offspring of an affected father was 9.40 (95% CI, 6.81-13.0) and 6.54 (95% CI, 4.17-10.3) for offspring of affected mothers. There was a similar pattern for UC, although the magnitude was smaller: 163.7 for twin siblings (95% CI, 105.6-253.9), 13.1 for nontwin siblings (95% CI, 11.4-15.0), 7.11 for offspring of affected fathers (95% CI, 6.10-8.29), and 8.77 for offspring of affected mothers (95% CI, 7.46-10.3).
The researchers found no evidence of a birth cohort effect. Family history and IBD risk is a complicated relationship. Family history includes shared genetics as well as similar environmental exposures, and gene-environment interactions can add another layer of uncertainty. Previous studies have found that asymptomatic family members of IBD patients sometimes have preclinical signs such as changes in intestinal permeability, immune function, the microbiome, and biomarker levels.
IBD has emerged recently among Asian-Pacific populations as a serious health concern, with a recent rapid increase. This may reflect a shift in potentially modifiable environmental triggers. “Precisely quantifying familial risk and patterns might enable more accurate risk counseling and better-targeted clinical surveillance for earlier diagnosis and treatment among FDRs. Moreover, an accurate definition of familial IBD risk across populations also might inform subsequent investigations untangling the various shared environmental and genetic contributions,” the authors wrote.
Although genetic susceptibility is generally accepted as the predominant driver in familial trends for IBD, the authors noted their “study was not designed to determine the contribution of genetic vs. nongenetic determinants to familial IBD risk, and future well-designed dedicated investigations are needed to provide this clarity.”
The study is limited by the relatively short follow-up period, which may not have captured all IBD cases within patients’ families.
The authors have no relevant financial disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Expert advice
I once answered online skin questions. The most popular one was, “Is my penis supposed to look like that?”
Then the site was bought by an entrepreneur with a corporate sensibility. He opened two forums: for 15 bucks, you could access the Medical Forum and ask a doctor. For 10, you could join the Community Forum and ask anybody with an opinion. One guess about which forum was more popular.
Years later, a colleague referred a fellow who had run a poison ivy website for a decade and wanted to interview a doctor. He had never spoken with one before, “because it never occurred to me.” His site featured the usual folklore: that blister fluid spreads the poison, that you can catch it from your dog. His website had many pictures. Some were in focus, and a few actually showed poison ivy.
I checked a year later and found that he had never uploaded our interview to his website. When I emailed to ask how come, he said he’d been busy, and did I want him to? I told him I was OK.
What made me think of these old episodes was a phone chat I had the other day with an IT guy about my laptop.
After I told him my problem, he said, “Since you’re a doctor, could I ask you a medical question?”
“Sure.”
“Is the COVID vaccine safe?” he asked.
“I had two shots myself,” I said, “and I’m planning a third. Does that tell you what I think about how safe it is?”
He didn’t answer, and we got back to the laptop.
Five minutes later he said, “I just wonder whether we should mess with vaccines. Maybe we should let nature take its course.”
“How about polio and diphtheria?” I said. “Should we let nature take its course with them?”
He thought for a moment and said, “If you don’t get vaccinated, can you spread the virus to other people?”
“Yes, you can,” I said. “It’s not just that you can get sick, but you can make other people sick, and possibly die if they’re old or vulnerable.”
Again, no response. We finished up with the laptop.
“Thanks for your medical advice,” he said. “I get conflicting information from so many sources.”
Yes, he does. He and everybody else always have. When the issues are poison ivy and genital blotchiness, the stakes are not high enough for anyone to talk about. To a large extent, people have always made their minds up about things based on what their friends think and tell them.
If your friends all wear masks, they will stare at you if you don’t. If your friends don’t wear masks, they will stare at you if you do. Or more than that. Very few people like to be stared at. Or worse.
or another: social media disinformation, distrust of the establishment, personal freedom. When the stakes are low, no reasons are needed. Who cares why someone blames Fido for his poison ivy?
Addressing the reasons people give for their positions, or the reasons others assign to them, may sometimes help people reconsider. For all those other times, the old adage applies: You cannot reason someone out of what he never reasoned himself into.
When it comes to contact dermatitis or penile blotches, you can try to straighten people out, but it doesn’t matter much if you fail. When the people you are trying to convince are spreading disease, filling up ICUs, or dying, it matters a great deal, which does not necessarily increase your odds of succeeding.
There have always been “Medical Forums” – where you ask a professional with official credentials – and “Community Forums” – where you ask Jerry next door or Hortense on Instagram. There always will be. Most of the time this is a curiosity of little general interest. Though not always.
Of course I believe in expert advice. I spent my whole career dispensing it.
Still, modesty is proper. Knowledge may be evolving and tentative, and sensible advice often ignored.
As Hippocrates said a long time ago: Life is short, and art long, opportunity fleeting, experimentation perilous, and judgment difficult.
They all still are.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now retired, after more than 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His latest book, “Doctoring from the Outside In,” was recently published. This is his last column for Dermatology News. Write to him at [email protected].
I once answered online skin questions. The most popular one was, “Is my penis supposed to look like that?”
Then the site was bought by an entrepreneur with a corporate sensibility. He opened two forums: for 15 bucks, you could access the Medical Forum and ask a doctor. For 10, you could join the Community Forum and ask anybody with an opinion. One guess about which forum was more popular.
Years later, a colleague referred a fellow who had run a poison ivy website for a decade and wanted to interview a doctor. He had never spoken with one before, “because it never occurred to me.” His site featured the usual folklore: that blister fluid spreads the poison, that you can catch it from your dog. His website had many pictures. Some were in focus, and a few actually showed poison ivy.
I checked a year later and found that he had never uploaded our interview to his website. When I emailed to ask how come, he said he’d been busy, and did I want him to? I told him I was OK.
What made me think of these old episodes was a phone chat I had the other day with an IT guy about my laptop.
After I told him my problem, he said, “Since you’re a doctor, could I ask you a medical question?”
“Sure.”
“Is the COVID vaccine safe?” he asked.
“I had two shots myself,” I said, “and I’m planning a third. Does that tell you what I think about how safe it is?”
He didn’t answer, and we got back to the laptop.
Five minutes later he said, “I just wonder whether we should mess with vaccines. Maybe we should let nature take its course.”
“How about polio and diphtheria?” I said. “Should we let nature take its course with them?”
He thought for a moment and said, “If you don’t get vaccinated, can you spread the virus to other people?”
“Yes, you can,” I said. “It’s not just that you can get sick, but you can make other people sick, and possibly die if they’re old or vulnerable.”
Again, no response. We finished up with the laptop.
“Thanks for your medical advice,” he said. “I get conflicting information from so many sources.”
Yes, he does. He and everybody else always have. When the issues are poison ivy and genital blotchiness, the stakes are not high enough for anyone to talk about. To a large extent, people have always made their minds up about things based on what their friends think and tell them.
If your friends all wear masks, they will stare at you if you don’t. If your friends don’t wear masks, they will stare at you if you do. Or more than that. Very few people like to be stared at. Or worse.
or another: social media disinformation, distrust of the establishment, personal freedom. When the stakes are low, no reasons are needed. Who cares why someone blames Fido for his poison ivy?
Addressing the reasons people give for their positions, or the reasons others assign to them, may sometimes help people reconsider. For all those other times, the old adage applies: You cannot reason someone out of what he never reasoned himself into.
When it comes to contact dermatitis or penile blotches, you can try to straighten people out, but it doesn’t matter much if you fail. When the people you are trying to convince are spreading disease, filling up ICUs, or dying, it matters a great deal, which does not necessarily increase your odds of succeeding.
There have always been “Medical Forums” – where you ask a professional with official credentials – and “Community Forums” – where you ask Jerry next door or Hortense on Instagram. There always will be. Most of the time this is a curiosity of little general interest. Though not always.
Of course I believe in expert advice. I spent my whole career dispensing it.
Still, modesty is proper. Knowledge may be evolving and tentative, and sensible advice often ignored.
As Hippocrates said a long time ago: Life is short, and art long, opportunity fleeting, experimentation perilous, and judgment difficult.
They all still are.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now retired, after more than 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His latest book, “Doctoring from the Outside In,” was recently published. This is his last column for Dermatology News. Write to him at [email protected].
I once answered online skin questions. The most popular one was, “Is my penis supposed to look like that?”
Then the site was bought by an entrepreneur with a corporate sensibility. He opened two forums: for 15 bucks, you could access the Medical Forum and ask a doctor. For 10, you could join the Community Forum and ask anybody with an opinion. One guess about which forum was more popular.
Years later, a colleague referred a fellow who had run a poison ivy website for a decade and wanted to interview a doctor. He had never spoken with one before, “because it never occurred to me.” His site featured the usual folklore: that blister fluid spreads the poison, that you can catch it from your dog. His website had many pictures. Some were in focus, and a few actually showed poison ivy.
I checked a year later and found that he had never uploaded our interview to his website. When I emailed to ask how come, he said he’d been busy, and did I want him to? I told him I was OK.
What made me think of these old episodes was a phone chat I had the other day with an IT guy about my laptop.
After I told him my problem, he said, “Since you’re a doctor, could I ask you a medical question?”
“Sure.”
“Is the COVID vaccine safe?” he asked.
“I had two shots myself,” I said, “and I’m planning a third. Does that tell you what I think about how safe it is?”
He didn’t answer, and we got back to the laptop.
Five minutes later he said, “I just wonder whether we should mess with vaccines. Maybe we should let nature take its course.”
“How about polio and diphtheria?” I said. “Should we let nature take its course with them?”
He thought for a moment and said, “If you don’t get vaccinated, can you spread the virus to other people?”
“Yes, you can,” I said. “It’s not just that you can get sick, but you can make other people sick, and possibly die if they’re old or vulnerable.”
Again, no response. We finished up with the laptop.
“Thanks for your medical advice,” he said. “I get conflicting information from so many sources.”
Yes, he does. He and everybody else always have. When the issues are poison ivy and genital blotchiness, the stakes are not high enough for anyone to talk about. To a large extent, people have always made their minds up about things based on what their friends think and tell them.
If your friends all wear masks, they will stare at you if you don’t. If your friends don’t wear masks, they will stare at you if you do. Or more than that. Very few people like to be stared at. Or worse.
or another: social media disinformation, distrust of the establishment, personal freedom. When the stakes are low, no reasons are needed. Who cares why someone blames Fido for his poison ivy?
Addressing the reasons people give for their positions, or the reasons others assign to them, may sometimes help people reconsider. For all those other times, the old adage applies: You cannot reason someone out of what he never reasoned himself into.
When it comes to contact dermatitis or penile blotches, you can try to straighten people out, but it doesn’t matter much if you fail. When the people you are trying to convince are spreading disease, filling up ICUs, or dying, it matters a great deal, which does not necessarily increase your odds of succeeding.
There have always been “Medical Forums” – where you ask a professional with official credentials – and “Community Forums” – where you ask Jerry next door or Hortense on Instagram. There always will be. Most of the time this is a curiosity of little general interest. Though not always.
Of course I believe in expert advice. I spent my whole career dispensing it.
Still, modesty is proper. Knowledge may be evolving and tentative, and sensible advice often ignored.
As Hippocrates said a long time ago: Life is short, and art long, opportunity fleeting, experimentation perilous, and judgment difficult.
They all still are.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now retired, after more than 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His latest book, “Doctoring from the Outside In,” was recently published. This is his last column for Dermatology News. Write to him at [email protected].
Nonopioid med promising for neuropathic pain
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Top-line results from a phase 2 study suggest vixotrigine (BIIB074, Biogen), a nonopioid investigational oral pain medication, reduces chronic neuropathic pain caused by small fiber neuropathy (SFN) and is generally well tolerated.
“We are encouraged by the overall results of the CONVEY study, especially given the significant unmet medical need for additional agents to treat chronic painful neuropathy,” Katherine Dawson, MD, senior vice president and head of the therapeutics development unit at Biogen, said in a news release.
Vixotrigine (BIIB074) is a peripherally and centrally acting, orally administered, voltage- and use-dependent voltage-gated sodium channel blocker.
CONVEY was a phase 2, placebo-controlled, double-blind, randomized withdrawal study of 265 patients experiencing pain from confirmed idiopathic or diabetes-associated SFN.
Following a 4-week open-label run-in period, 123 responders to vixotrigine were randomly allocated to 200 mg or 350 mg vixotrigine or placebo twice daily for 12 weeks in the double-blind portion of the study.
At week 12, vixotrigine 200 mg twice daily met the primary endpoint of a statistically significant reduction from baseline in the mean average daily pain (ADP) score versus placebo (P = .0501).
A subgroup analysis showed a treatment effect in patients with diabetes-associated SFN but not in the smaller subgroup of patients with idiopathic SFN.
The 200-mg dose also led to a significant improvement over placebo in mean worst daily pain score at 12 weeks (P = .0455).
A numeric advantage of 200 mg vixotrigine over placebo was observed in additional secondary endpoints, including the proportion of patients with at least a 2-point improvement in ADP score and the proportion with at least a 30% reduction in ADP at week 12, but these failed to reach statistical significance.
Vixotrigine 350 mg twice daily did not meet the primary endpoint of mean change in ADP at 12 weeks.
However, treatment at the higher dose led to a significant increase in the proportion of patients who reported being “very much improved” or “much improved” over baseline (P = .0580), Biogen reported.
In addition, a numeric advantage of 350 mg over placebo was observed in the proportion of patients with a 2-point or greater improvement in ADP score and the proportion with at least a 30% reduction in ADP at 12 weeks, but these also did not reach statistical significance.
Both doses of vixotrigine were “generally well tolerated and the safety profile was consistent with previous studies of vixotrigine with no evidence of abuse potential,” the company said.
In the open-label period, common adverse events seen in at least 2.5% of patients were dizziness, headache, vertigo, and nausea; adverse events led 5.3% of patients to discontinue the open-label portion of the study. Across the entire study, most adverse events were mild or moderate in severity.
“The totality of data from the vixotrigine program will inform potential doses for study in future phase 3 clinical trials,” the company said.
A version of this article first appeared on Medscape.com.
Transfusions, readmissions higher for patients with CLL after cardiac surgery
Patients with chronic lymphocytic leukemia have similar outcomes following cardiac operations as patients without CLL, but commonly require more blood transfusions, according to the results of retrospective cohort study using the 2010-2017 Nationwide Readmissions Database (NRD).
The researchers assessed all adult patients undergoing elective coronary artery bypass grafting, valve repair, or valve replacement as identified using the NRD.
Patients were stratified by history of CLL and the incidence of in-hospital mortality, perioperative complications, blood transfusions, and readmission within 90 days were examined. A 3:1 nearest-neighbor matching was performed between patients with and without CLL for all primary and secondary outcomes of interest, according to the report, published online in Annals of Thoracic Surgery.
Comparable results
A total of 1,250,882 patients in the database were found who underwent cardiac operations. Of these, 0.23% had a diagnosis of CLL. Among 11,237 propensity-matched patients, those with CLL had similar rates of in-hospital mortality (3.8% vs. 2.6%, P = .08) and perioperative complications (33.4% vs. 33.6%, P = .92), compared with their non-CLL counterparts. However, the incidence of infection was comparable (8.5% vs. 9.4%, P = .38).
However, patients with CLL required blood transfusions more frequently (33.7% vs. 28.4%, P = .003) than did patients without CLL. In addition, patients with CLL were more likely to be readmitted within 90 days of discharge, compared with their counterparts, and “respiratory reasons, including pneumonia, contributed significantly to the readmission burden in this cohort,” the researchers, led by Josef Madrigal, BS, of the University of California, Los Angeles, stated.
“The inherent risk of transfusion and the possible benefits of blood conservation interventions must be considered in this patient population. Increased risk of rehospitalization in patients with CLL suggests the need for measures aimed at mitigating the risk of respiratory complications,” the researchers concluded.
There were no conflicts of interest reported in the article.
Patients with chronic lymphocytic leukemia have similar outcomes following cardiac operations as patients without CLL, but commonly require more blood transfusions, according to the results of retrospective cohort study using the 2010-2017 Nationwide Readmissions Database (NRD).
The researchers assessed all adult patients undergoing elective coronary artery bypass grafting, valve repair, or valve replacement as identified using the NRD.
Patients were stratified by history of CLL and the incidence of in-hospital mortality, perioperative complications, blood transfusions, and readmission within 90 days were examined. A 3:1 nearest-neighbor matching was performed between patients with and without CLL for all primary and secondary outcomes of interest, according to the report, published online in Annals of Thoracic Surgery.
Comparable results
A total of 1,250,882 patients in the database were found who underwent cardiac operations. Of these, 0.23% had a diagnosis of CLL. Among 11,237 propensity-matched patients, those with CLL had similar rates of in-hospital mortality (3.8% vs. 2.6%, P = .08) and perioperative complications (33.4% vs. 33.6%, P = .92), compared with their non-CLL counterparts. However, the incidence of infection was comparable (8.5% vs. 9.4%, P = .38).
However, patients with CLL required blood transfusions more frequently (33.7% vs. 28.4%, P = .003) than did patients without CLL. In addition, patients with CLL were more likely to be readmitted within 90 days of discharge, compared with their counterparts, and “respiratory reasons, including pneumonia, contributed significantly to the readmission burden in this cohort,” the researchers, led by Josef Madrigal, BS, of the University of California, Los Angeles, stated.
“The inherent risk of transfusion and the possible benefits of blood conservation interventions must be considered in this patient population. Increased risk of rehospitalization in patients with CLL suggests the need for measures aimed at mitigating the risk of respiratory complications,” the researchers concluded.
There were no conflicts of interest reported in the article.
Patients with chronic lymphocytic leukemia have similar outcomes following cardiac operations as patients without CLL, but commonly require more blood transfusions, according to the results of retrospective cohort study using the 2010-2017 Nationwide Readmissions Database (NRD).
The researchers assessed all adult patients undergoing elective coronary artery bypass grafting, valve repair, or valve replacement as identified using the NRD.
Patients were stratified by history of CLL and the incidence of in-hospital mortality, perioperative complications, blood transfusions, and readmission within 90 days were examined. A 3:1 nearest-neighbor matching was performed between patients with and without CLL for all primary and secondary outcomes of interest, according to the report, published online in Annals of Thoracic Surgery.
Comparable results
A total of 1,250,882 patients in the database were found who underwent cardiac operations. Of these, 0.23% had a diagnosis of CLL. Among 11,237 propensity-matched patients, those with CLL had similar rates of in-hospital mortality (3.8% vs. 2.6%, P = .08) and perioperative complications (33.4% vs. 33.6%, P = .92), compared with their non-CLL counterparts. However, the incidence of infection was comparable (8.5% vs. 9.4%, P = .38).
However, patients with CLL required blood transfusions more frequently (33.7% vs. 28.4%, P = .003) than did patients without CLL. In addition, patients with CLL were more likely to be readmitted within 90 days of discharge, compared with their counterparts, and “respiratory reasons, including pneumonia, contributed significantly to the readmission burden in this cohort,” the researchers, led by Josef Madrigal, BS, of the University of California, Los Angeles, stated.
“The inherent risk of transfusion and the possible benefits of blood conservation interventions must be considered in this patient population. Increased risk of rehospitalization in patients with CLL suggests the need for measures aimed at mitigating the risk of respiratory complications,” the researchers concluded.
There were no conflicts of interest reported in the article.
FROM THE ANNALS OF THORACIC SURGERY
Durvalumab combos beat monotherapy for unresectable stage 3 NSCLC
Both combinations – durvalumab plus either the anti-CD73 monoclonal antibody oleclumab or the anti-NKG2A mAb monalizumab – also numerically improved objective response rate. “Safety profiles were consistent across arms and no new safety signals were identified,” said Alexandre Martinez-Marti, MD, the lead investigator on the phase 2 trial, dubbed COAST, which he presented (abstract LBA42) at the 2021 European Society for Medical Oncology Congress on Sept. 17.
“These data support further evaluations of these combinations,” said Dr. Martinez-Marti, also a thoracic medical oncologist at the Vall d’Hebron Institute of Oncology in Barcelona.
Durvalumab is already established as a standard of care option for patients with unresectable stage 3 NSCLC who don’t progress after concurrent chemoradiation. There’s been preliminary data suggesting the benefits might be greater with oleclumab or monalizumab, so the study team looked into the issue.
They randomized 66 patients with unresectable stage 3 NSCLC and no progression after chemoradiotherapy to durvalumab 1,500 mg IV every 4 weeks; 59 others to durvalumab at the same dosage plus oleclumab 3,000 mg IV every 2 weeks for the first two cycles then every 4 weeks, and 61 were randomized to durvalumab plus monalizumab 750 mg IV every 2 weeks.
Patients were treated for up to 12 months, and they started treatment no later than 42 days after completing chemoradiation.
Over a median follow-up of 11.5 months, median progression-free survival (PFS) was 6.3 months in the durvalumab arm, but 15.1 months with the monalizumab combination (PFS hazard ratio versus durvalumab alone, 0.65; 95% CI, 0.49-0.85), and not reached in the oleclumab arm (PFS HR, 0.44; 95% CI, 0.26-0.75).
There were only a few complete responders across the study groups. Partial responses rates were 22.4% of the durvalumab alone arm, 36.7% in the oleclumab group, and 32.3% in the monalizumab arm.
The investigator assessed objective response rate was 25.4% with durvalumab alone, 38.3% in the oleclumab group, and 37.1% with the monalizumab combination. Curves started to separate from durvalumab monotherapy at around 2-4 months.
“Overall, the safety profiles of the two combinations were generally similar to the safety profile of durvalumab alone,” Dr. Martinez-Marti said. The rate of grade 3 or higher treatment-emergent events incidence was 39.4% with durvalumab, 40.7% the oleclumab combination, and 27.9% with monalizumab.
The most common grade 3/4 events were pneumonia (5.9%) and decreased lymphocyte count (3.2%); both were less common with the monalizumab combination.
Combined rates of pneumonitis and radiation pneumonitis of any grade were 21.2% with durvalumab, 28.8% in the oleclumab group, and 21.3% with monalizumab.
The groups were generally well balanced at baseline. The majority of subjects were men, White, and former smokers. Most subjects had stage 3A or 3B disease.
The work was funded by AstraZeneca. The investigators disclosed numerous ties to the company, including Dr. Martinez-Marti, who reported personal fees, travel expenses, and other connections.
This article was updated 9/24/21.
Both combinations – durvalumab plus either the anti-CD73 monoclonal antibody oleclumab or the anti-NKG2A mAb monalizumab – also numerically improved objective response rate. “Safety profiles were consistent across arms and no new safety signals were identified,” said Alexandre Martinez-Marti, MD, the lead investigator on the phase 2 trial, dubbed COAST, which he presented (abstract LBA42) at the 2021 European Society for Medical Oncology Congress on Sept. 17.
“These data support further evaluations of these combinations,” said Dr. Martinez-Marti, also a thoracic medical oncologist at the Vall d’Hebron Institute of Oncology in Barcelona.
Durvalumab is already established as a standard of care option for patients with unresectable stage 3 NSCLC who don’t progress after concurrent chemoradiation. There’s been preliminary data suggesting the benefits might be greater with oleclumab or monalizumab, so the study team looked into the issue.
They randomized 66 patients with unresectable stage 3 NSCLC and no progression after chemoradiotherapy to durvalumab 1,500 mg IV every 4 weeks; 59 others to durvalumab at the same dosage plus oleclumab 3,000 mg IV every 2 weeks for the first two cycles then every 4 weeks, and 61 were randomized to durvalumab plus monalizumab 750 mg IV every 2 weeks.
Patients were treated for up to 12 months, and they started treatment no later than 42 days after completing chemoradiation.
Over a median follow-up of 11.5 months, median progression-free survival (PFS) was 6.3 months in the durvalumab arm, but 15.1 months with the monalizumab combination (PFS hazard ratio versus durvalumab alone, 0.65; 95% CI, 0.49-0.85), and not reached in the oleclumab arm (PFS HR, 0.44; 95% CI, 0.26-0.75).
There were only a few complete responders across the study groups. Partial responses rates were 22.4% of the durvalumab alone arm, 36.7% in the oleclumab group, and 32.3% in the monalizumab arm.
The investigator assessed objective response rate was 25.4% with durvalumab alone, 38.3% in the oleclumab group, and 37.1% with the monalizumab combination. Curves started to separate from durvalumab monotherapy at around 2-4 months.
“Overall, the safety profiles of the two combinations were generally similar to the safety profile of durvalumab alone,” Dr. Martinez-Marti said. The rate of grade 3 or higher treatment-emergent events incidence was 39.4% with durvalumab, 40.7% the oleclumab combination, and 27.9% with monalizumab.
The most common grade 3/4 events were pneumonia (5.9%) and decreased lymphocyte count (3.2%); both were less common with the monalizumab combination.
Combined rates of pneumonitis and radiation pneumonitis of any grade were 21.2% with durvalumab, 28.8% in the oleclumab group, and 21.3% with monalizumab.
The groups were generally well balanced at baseline. The majority of subjects were men, White, and former smokers. Most subjects had stage 3A or 3B disease.
The work was funded by AstraZeneca. The investigators disclosed numerous ties to the company, including Dr. Martinez-Marti, who reported personal fees, travel expenses, and other connections.
This article was updated 9/24/21.
Both combinations – durvalumab plus either the anti-CD73 monoclonal antibody oleclumab or the anti-NKG2A mAb monalizumab – also numerically improved objective response rate. “Safety profiles were consistent across arms and no new safety signals were identified,” said Alexandre Martinez-Marti, MD, the lead investigator on the phase 2 trial, dubbed COAST, which he presented (abstract LBA42) at the 2021 European Society for Medical Oncology Congress on Sept. 17.
“These data support further evaluations of these combinations,” said Dr. Martinez-Marti, also a thoracic medical oncologist at the Vall d’Hebron Institute of Oncology in Barcelona.
Durvalumab is already established as a standard of care option for patients with unresectable stage 3 NSCLC who don’t progress after concurrent chemoradiation. There’s been preliminary data suggesting the benefits might be greater with oleclumab or monalizumab, so the study team looked into the issue.
They randomized 66 patients with unresectable stage 3 NSCLC and no progression after chemoradiotherapy to durvalumab 1,500 mg IV every 4 weeks; 59 others to durvalumab at the same dosage plus oleclumab 3,000 mg IV every 2 weeks for the first two cycles then every 4 weeks, and 61 were randomized to durvalumab plus monalizumab 750 mg IV every 2 weeks.
Patients were treated for up to 12 months, and they started treatment no later than 42 days after completing chemoradiation.
Over a median follow-up of 11.5 months, median progression-free survival (PFS) was 6.3 months in the durvalumab arm, but 15.1 months with the monalizumab combination (PFS hazard ratio versus durvalumab alone, 0.65; 95% CI, 0.49-0.85), and not reached in the oleclumab arm (PFS HR, 0.44; 95% CI, 0.26-0.75).
There were only a few complete responders across the study groups. Partial responses rates were 22.4% of the durvalumab alone arm, 36.7% in the oleclumab group, and 32.3% in the monalizumab arm.
The investigator assessed objective response rate was 25.4% with durvalumab alone, 38.3% in the oleclumab group, and 37.1% with the monalizumab combination. Curves started to separate from durvalumab monotherapy at around 2-4 months.
“Overall, the safety profiles of the two combinations were generally similar to the safety profile of durvalumab alone,” Dr. Martinez-Marti said. The rate of grade 3 or higher treatment-emergent events incidence was 39.4% with durvalumab, 40.7% the oleclumab combination, and 27.9% with monalizumab.
The most common grade 3/4 events were pneumonia (5.9%) and decreased lymphocyte count (3.2%); both were less common with the monalizumab combination.
Combined rates of pneumonitis and radiation pneumonitis of any grade were 21.2% with durvalumab, 28.8% in the oleclumab group, and 21.3% with monalizumab.
The groups were generally well balanced at baseline. The majority of subjects were men, White, and former smokers. Most subjects had stage 3A or 3B disease.
The work was funded by AstraZeneca. The investigators disclosed numerous ties to the company, including Dr. Martinez-Marti, who reported personal fees, travel expenses, and other connections.
This article was updated 9/24/21.
FROM ESMO CONGRESS 2021







