User login
EMPEROR-Preserved: Empagliflozin’s HFpEF efficacy catalyzes a heart failure redefinition
Groundbreaking results from the EMPEROR-Preserved trial did more than establish for the first time that a drug, empagliflozin, has clearly proven efficacy for treating patients with heart failure with preserved ejection fraction (HFpEF). The results also helped catalyze a paradigm shift in how heart failure thought leaders think about the role of ejection fraction for making important distinctions among patients with heart failure.
EMPEROR-Preserved may also be the final nail in the coffin for defining patients with heart failure as having HFpEF or heart failure with reduced ejection fraction (HFrEF).
This new consensus essentially throws out left ventricular ejection fraction (EF) as the key metric for matching patients to heart failure treatments. Experts have instead begun suggesting a more unified treatment approach for all heart failure patients regardless of their EF.
‘Forget about ejection fraction’
“We encourage you to forget about ejection fraction,” declared Milton Packer, MD, during discussion at a session of the annual scientific meeting of the Heart Failure Society of America. “We certainly encourage you to forget about an ejection fraction of less than 40%” as having special significance,” added Dr. Packer, a lead investigator for both the EMPEROR-Reduced and EMPEROR-Preserved trials (which researchers combined in a unified analysis with a total of 9,718 patients with heart failure called EMPEROR-Pooled), and a heart failure researcher at Baylor University Medical Center in Dallas.
“The 40% ejection fraction divide is artificial. It was created in 2003 as part of a trial design, but it has no physiological significance,” Dr. Packer explained. A much better way to distinguish systolic and diastolic heart failure is by strain assessment rather than by ejection fraction. “Strain is a measure of myocardial shortening, a measure of what the heart does. Ejection fraction is a measure of volume,” said Dr. Packer. “Sign me up to get rid of ejection fraction,” he added.
“Ejection fraction is not as valuable as we thought for distinguishing the therapeutic benefit” of heart failure drugs, agreed Marvin A. Konstam, MD, professor of medicine at Tufts University and chief physician executive of the CardioVascular Center of Tufts Medical Center, both in Boston, who spoke during a different session at the meeting.
“It would easier if we didn’t spend time parsing this number,” ejection fraction, commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern Medicine in Chicago. “Wouldn’t it be easier if we said that every patient with heart failure needs to receive one agent from each of the four [pillar] drug classes, and put them in a polypill” at reduced dosages, he proposed, envisioning one potential consequence of jettisoning ejection fraction.
The four pillar drug classes, recently identified as essential for patients with HFrEF but until now not endorsed for patients with HFpEF, are the sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin (Jardiance); an angiotensin receptor blocker neprilysin inhibitor compound such as sacubitril/valsartan (Entresto); beta-blockers; and mineralocorticoid receptor antagonists such as spironolactone and eplerenone.
An opportunity for ‘simpler and easier’ treatments
“This is an opportunity to disrupt the way we’ve been doing things and think about something that is simpler and easier,” said Dr. Yancy, who chaired some of the panels serially formed by the American Heart Association and American College of Cardiology to write guidelines for treating heart failure. “An approach that would be easier to implement without worrying about staggering the start of each drug class and an incessant focus on titrating individual elements and taking 6 months to get to a certain place.”
Results from EMPEROR-Preserved and the combined EMPEROR-Pooled analysis triggered these paradigm-shifting sentiments by showing clear evidence that treatment with empagliflozin exerts consistent benefit – and is consistently safe – for patients with heart failure across a spectrum of EFs, from less than 25% to 64%, though its performance in patients with HFpEF and EFs of 65% or greater in the EMPEROR-Preserved trial remains unclear.
The consequence is that clinicians should feel comfortable prescribing empagliflozin to most patients with heart failure without regard to EF, even patients with EF values in the mid-60% range.
The EMPEROR-Preserved results showed a clear signal of attenuated benefit among patients with an EF of 65% or greater “on a population basis,” stressed Dr. Packer. “But on an individual basis, ejection fraction is not that reproducible, so measuring ejection fraction will not help you determine whom to treat or not treat. “
“There is significant variability” measuring EF using the most common modality, echocardiography, noted Javed Butler, MD, an EMPEROR coinvestigator who also spoke at the meeting session. A person with a measured EF of 65% could actually have a value that may be as low as 58% or as high as about 72%, noted Dr. Butler, who is professor and chair of medicine at the University of Mississippi, Jackson. The upshot is that any patient diagnosed with heart failure should receive an SGLT2 inhibitor “irrespective of their ejection fraction,” Dr. Butler advised.
“Ejection fraction is very crude, and probably not sufficient to identify a phenotype,” for treatment, said Dr. Yancy. “The real takeaway may be that we need to revisit what we call HFrEF, and then let that be the new standard for treatment.”
“Is [an EF of] 60% the new 40%?” asked Dr. Packer, implying that the answer was yes.
Results from several trials suggest redefining HFrEF
The idea that patients without traditionally defined HFrEF – an EF of 40% or less – could also benefit from other classes of heart failure drugs has been gestating for a while, and then rose to a new level with the August 2021 report of results from EMPEROR-Preserved. Two years ago, in September 2019, Dr. Butler, Dr. Packer, and a third colleague advanced the notion of redefining HFrEF by raising the ejection fraction ceiling in a published commentary.
They cited the experience with the angiotensin receptor blocker candesartan in a post hoc analysis of data collected in the CHARM-Preserved trial, which showed a strong signal of benefit in the subgroup of patients with EFs of 41%-49%, but not in those with an EF of 50% or higher. This finding prompted Dr. Konstam to express doubts about relying on EF to define heart failure subgroups in trials and guide management in a commentary published more than 3 years ago.
Another crack in the traditional EF framework came from analysis of results from the TOPCAT trial that tested spironolactone as a treatment for patients with HFpEF, according to the 2019 opinion published by Dr. Butler and Dr. Packer. Once again a post hoc analysis, this time using data from TOPCAT, suggested a benefit from the mineralocorticoid receptor antagonist spironolactone in patients with heart failure and an EF of 45%-49% (45% was the minimum EF for enrollment into the study).
Recently, data from a third trial that tested sacubitril/valsartan in patients with HFpEF, PARAGON-HF, showed benefit among patients with EFs below the study median of 57%. This finding led the Food and Drug Administration in February 2021 to amend its initial approval for sacubitril/valsartan by removing a specific EF ceiling from the drug’s indication and instead saying that patient’s receiving the drug should have a “below normal” EF.
Writing in a recent commentary, Dr. Yancy called the FDA’s action on sacubitril/valsartan “reasonable,” and that the subgroup assessment of data from the PARAGON-HF trial creates a “new, reasonably evidence-based therapy for HFpEF.” He also predicted that guideline-writing panels will “likely align with a permissive statement of indication” for sacubitril/valsartan in patients with HFpEF, especially those with EFs of less than 57%.
The idea of using an SGLT2 inhibitor like empagliflozin on all heart failure patients, and also adding agents like sacubitril/valsartan and spironolactone in patients with HFpEF and EFs in the mid-50% range or lower may take some time to catch on, but it already has one influential advocate.
“If a patient has HFpEF with an EF of less than 55%, use quadruple-class therapy,” summed up Dr. Butler during the HFSA session, while also suggesting prescribing an SGLT2 inhibitor to essentially all patients with heart failure regardless of their EF.
The EMPEROR-Preserved and EMPEROR-Reduced trials and the EMPEROR-Pooled analysis were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Packer has had financial relationships with BI and Lilly and numerous other companies. Dr. Konstam has served on data monitoring committees for trials funded by Boehringer Ingelheim and by Amgen, Luitpold, and Pfizer, and has been a consultant to Arena, LivaNova, Merck, SC Pharma, and Takeda. Dr. Yancy had no disclosures. Dr. Butler has had financial relationships with Boehringer Ingelheim and numerous other companies.
Groundbreaking results from the EMPEROR-Preserved trial did more than establish for the first time that a drug, empagliflozin, has clearly proven efficacy for treating patients with heart failure with preserved ejection fraction (HFpEF). The results also helped catalyze a paradigm shift in how heart failure thought leaders think about the role of ejection fraction for making important distinctions among patients with heart failure.
EMPEROR-Preserved may also be the final nail in the coffin for defining patients with heart failure as having HFpEF or heart failure with reduced ejection fraction (HFrEF).
This new consensus essentially throws out left ventricular ejection fraction (EF) as the key metric for matching patients to heart failure treatments. Experts have instead begun suggesting a more unified treatment approach for all heart failure patients regardless of their EF.
‘Forget about ejection fraction’
“We encourage you to forget about ejection fraction,” declared Milton Packer, MD, during discussion at a session of the annual scientific meeting of the Heart Failure Society of America. “We certainly encourage you to forget about an ejection fraction of less than 40%” as having special significance,” added Dr. Packer, a lead investigator for both the EMPEROR-Reduced and EMPEROR-Preserved trials (which researchers combined in a unified analysis with a total of 9,718 patients with heart failure called EMPEROR-Pooled), and a heart failure researcher at Baylor University Medical Center in Dallas.
“The 40% ejection fraction divide is artificial. It was created in 2003 as part of a trial design, but it has no physiological significance,” Dr. Packer explained. A much better way to distinguish systolic and diastolic heart failure is by strain assessment rather than by ejection fraction. “Strain is a measure of myocardial shortening, a measure of what the heart does. Ejection fraction is a measure of volume,” said Dr. Packer. “Sign me up to get rid of ejection fraction,” he added.
“Ejection fraction is not as valuable as we thought for distinguishing the therapeutic benefit” of heart failure drugs, agreed Marvin A. Konstam, MD, professor of medicine at Tufts University and chief physician executive of the CardioVascular Center of Tufts Medical Center, both in Boston, who spoke during a different session at the meeting.
“It would easier if we didn’t spend time parsing this number,” ejection fraction, commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern Medicine in Chicago. “Wouldn’t it be easier if we said that every patient with heart failure needs to receive one agent from each of the four [pillar] drug classes, and put them in a polypill” at reduced dosages, he proposed, envisioning one potential consequence of jettisoning ejection fraction.
The four pillar drug classes, recently identified as essential for patients with HFrEF but until now not endorsed for patients with HFpEF, are the sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin (Jardiance); an angiotensin receptor blocker neprilysin inhibitor compound such as sacubitril/valsartan (Entresto); beta-blockers; and mineralocorticoid receptor antagonists such as spironolactone and eplerenone.
An opportunity for ‘simpler and easier’ treatments
“This is an opportunity to disrupt the way we’ve been doing things and think about something that is simpler and easier,” said Dr. Yancy, who chaired some of the panels serially formed by the American Heart Association and American College of Cardiology to write guidelines for treating heart failure. “An approach that would be easier to implement without worrying about staggering the start of each drug class and an incessant focus on titrating individual elements and taking 6 months to get to a certain place.”
Results from EMPEROR-Preserved and the combined EMPEROR-Pooled analysis triggered these paradigm-shifting sentiments by showing clear evidence that treatment with empagliflozin exerts consistent benefit – and is consistently safe – for patients with heart failure across a spectrum of EFs, from less than 25% to 64%, though its performance in patients with HFpEF and EFs of 65% or greater in the EMPEROR-Preserved trial remains unclear.
The consequence is that clinicians should feel comfortable prescribing empagliflozin to most patients with heart failure without regard to EF, even patients with EF values in the mid-60% range.
The EMPEROR-Preserved results showed a clear signal of attenuated benefit among patients with an EF of 65% or greater “on a population basis,” stressed Dr. Packer. “But on an individual basis, ejection fraction is not that reproducible, so measuring ejection fraction will not help you determine whom to treat or not treat. “
“There is significant variability” measuring EF using the most common modality, echocardiography, noted Javed Butler, MD, an EMPEROR coinvestigator who also spoke at the meeting session. A person with a measured EF of 65% could actually have a value that may be as low as 58% or as high as about 72%, noted Dr. Butler, who is professor and chair of medicine at the University of Mississippi, Jackson. The upshot is that any patient diagnosed with heart failure should receive an SGLT2 inhibitor “irrespective of their ejection fraction,” Dr. Butler advised.
“Ejection fraction is very crude, and probably not sufficient to identify a phenotype,” for treatment, said Dr. Yancy. “The real takeaway may be that we need to revisit what we call HFrEF, and then let that be the new standard for treatment.”
“Is [an EF of] 60% the new 40%?” asked Dr. Packer, implying that the answer was yes.
Results from several trials suggest redefining HFrEF
The idea that patients without traditionally defined HFrEF – an EF of 40% or less – could also benefit from other classes of heart failure drugs has been gestating for a while, and then rose to a new level with the August 2021 report of results from EMPEROR-Preserved. Two years ago, in September 2019, Dr. Butler, Dr. Packer, and a third colleague advanced the notion of redefining HFrEF by raising the ejection fraction ceiling in a published commentary.
They cited the experience with the angiotensin receptor blocker candesartan in a post hoc analysis of data collected in the CHARM-Preserved trial, which showed a strong signal of benefit in the subgroup of patients with EFs of 41%-49%, but not in those with an EF of 50% or higher. This finding prompted Dr. Konstam to express doubts about relying on EF to define heart failure subgroups in trials and guide management in a commentary published more than 3 years ago.
Another crack in the traditional EF framework came from analysis of results from the TOPCAT trial that tested spironolactone as a treatment for patients with HFpEF, according to the 2019 opinion published by Dr. Butler and Dr. Packer. Once again a post hoc analysis, this time using data from TOPCAT, suggested a benefit from the mineralocorticoid receptor antagonist spironolactone in patients with heart failure and an EF of 45%-49% (45% was the minimum EF for enrollment into the study).
Recently, data from a third trial that tested sacubitril/valsartan in patients with HFpEF, PARAGON-HF, showed benefit among patients with EFs below the study median of 57%. This finding led the Food and Drug Administration in February 2021 to amend its initial approval for sacubitril/valsartan by removing a specific EF ceiling from the drug’s indication and instead saying that patient’s receiving the drug should have a “below normal” EF.
Writing in a recent commentary, Dr. Yancy called the FDA’s action on sacubitril/valsartan “reasonable,” and that the subgroup assessment of data from the PARAGON-HF trial creates a “new, reasonably evidence-based therapy for HFpEF.” He also predicted that guideline-writing panels will “likely align with a permissive statement of indication” for sacubitril/valsartan in patients with HFpEF, especially those with EFs of less than 57%.
The idea of using an SGLT2 inhibitor like empagliflozin on all heart failure patients, and also adding agents like sacubitril/valsartan and spironolactone in patients with HFpEF and EFs in the mid-50% range or lower may take some time to catch on, but it already has one influential advocate.
“If a patient has HFpEF with an EF of less than 55%, use quadruple-class therapy,” summed up Dr. Butler during the HFSA session, while also suggesting prescribing an SGLT2 inhibitor to essentially all patients with heart failure regardless of their EF.
The EMPEROR-Preserved and EMPEROR-Reduced trials and the EMPEROR-Pooled analysis were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Packer has had financial relationships with BI and Lilly and numerous other companies. Dr. Konstam has served on data monitoring committees for trials funded by Boehringer Ingelheim and by Amgen, Luitpold, and Pfizer, and has been a consultant to Arena, LivaNova, Merck, SC Pharma, and Takeda. Dr. Yancy had no disclosures. Dr. Butler has had financial relationships with Boehringer Ingelheim and numerous other companies.
Groundbreaking results from the EMPEROR-Preserved trial did more than establish for the first time that a drug, empagliflozin, has clearly proven efficacy for treating patients with heart failure with preserved ejection fraction (HFpEF). The results also helped catalyze a paradigm shift in how heart failure thought leaders think about the role of ejection fraction for making important distinctions among patients with heart failure.
EMPEROR-Preserved may also be the final nail in the coffin for defining patients with heart failure as having HFpEF or heart failure with reduced ejection fraction (HFrEF).
This new consensus essentially throws out left ventricular ejection fraction (EF) as the key metric for matching patients to heart failure treatments. Experts have instead begun suggesting a more unified treatment approach for all heart failure patients regardless of their EF.
‘Forget about ejection fraction’
“We encourage you to forget about ejection fraction,” declared Milton Packer, MD, during discussion at a session of the annual scientific meeting of the Heart Failure Society of America. “We certainly encourage you to forget about an ejection fraction of less than 40%” as having special significance,” added Dr. Packer, a lead investigator for both the EMPEROR-Reduced and EMPEROR-Preserved trials (which researchers combined in a unified analysis with a total of 9,718 patients with heart failure called EMPEROR-Pooled), and a heart failure researcher at Baylor University Medical Center in Dallas.
“The 40% ejection fraction divide is artificial. It was created in 2003 as part of a trial design, but it has no physiological significance,” Dr. Packer explained. A much better way to distinguish systolic and diastolic heart failure is by strain assessment rather than by ejection fraction. “Strain is a measure of myocardial shortening, a measure of what the heart does. Ejection fraction is a measure of volume,” said Dr. Packer. “Sign me up to get rid of ejection fraction,” he added.
“Ejection fraction is not as valuable as we thought for distinguishing the therapeutic benefit” of heart failure drugs, agreed Marvin A. Konstam, MD, professor of medicine at Tufts University and chief physician executive of the CardioVascular Center of Tufts Medical Center, both in Boston, who spoke during a different session at the meeting.
“It would easier if we didn’t spend time parsing this number,” ejection fraction, commented Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern Medicine in Chicago. “Wouldn’t it be easier if we said that every patient with heart failure needs to receive one agent from each of the four [pillar] drug classes, and put them in a polypill” at reduced dosages, he proposed, envisioning one potential consequence of jettisoning ejection fraction.
The four pillar drug classes, recently identified as essential for patients with HFrEF but until now not endorsed for patients with HFpEF, are the sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin (Jardiance); an angiotensin receptor blocker neprilysin inhibitor compound such as sacubitril/valsartan (Entresto); beta-blockers; and mineralocorticoid receptor antagonists such as spironolactone and eplerenone.
An opportunity for ‘simpler and easier’ treatments
“This is an opportunity to disrupt the way we’ve been doing things and think about something that is simpler and easier,” said Dr. Yancy, who chaired some of the panels serially formed by the American Heart Association and American College of Cardiology to write guidelines for treating heart failure. “An approach that would be easier to implement without worrying about staggering the start of each drug class and an incessant focus on titrating individual elements and taking 6 months to get to a certain place.”
Results from EMPEROR-Preserved and the combined EMPEROR-Pooled analysis triggered these paradigm-shifting sentiments by showing clear evidence that treatment with empagliflozin exerts consistent benefit – and is consistently safe – for patients with heart failure across a spectrum of EFs, from less than 25% to 64%, though its performance in patients with HFpEF and EFs of 65% or greater in the EMPEROR-Preserved trial remains unclear.
The consequence is that clinicians should feel comfortable prescribing empagliflozin to most patients with heart failure without regard to EF, even patients with EF values in the mid-60% range.
The EMPEROR-Preserved results showed a clear signal of attenuated benefit among patients with an EF of 65% or greater “on a population basis,” stressed Dr. Packer. “But on an individual basis, ejection fraction is not that reproducible, so measuring ejection fraction will not help you determine whom to treat or not treat. “
“There is significant variability” measuring EF using the most common modality, echocardiography, noted Javed Butler, MD, an EMPEROR coinvestigator who also spoke at the meeting session. A person with a measured EF of 65% could actually have a value that may be as low as 58% or as high as about 72%, noted Dr. Butler, who is professor and chair of medicine at the University of Mississippi, Jackson. The upshot is that any patient diagnosed with heart failure should receive an SGLT2 inhibitor “irrespective of their ejection fraction,” Dr. Butler advised.
“Ejection fraction is very crude, and probably not sufficient to identify a phenotype,” for treatment, said Dr. Yancy. “The real takeaway may be that we need to revisit what we call HFrEF, and then let that be the new standard for treatment.”
“Is [an EF of] 60% the new 40%?” asked Dr. Packer, implying that the answer was yes.
Results from several trials suggest redefining HFrEF
The idea that patients without traditionally defined HFrEF – an EF of 40% or less – could also benefit from other classes of heart failure drugs has been gestating for a while, and then rose to a new level with the August 2021 report of results from EMPEROR-Preserved. Two years ago, in September 2019, Dr. Butler, Dr. Packer, and a third colleague advanced the notion of redefining HFrEF by raising the ejection fraction ceiling in a published commentary.
They cited the experience with the angiotensin receptor blocker candesartan in a post hoc analysis of data collected in the CHARM-Preserved trial, which showed a strong signal of benefit in the subgroup of patients with EFs of 41%-49%, but not in those with an EF of 50% or higher. This finding prompted Dr. Konstam to express doubts about relying on EF to define heart failure subgroups in trials and guide management in a commentary published more than 3 years ago.
Another crack in the traditional EF framework came from analysis of results from the TOPCAT trial that tested spironolactone as a treatment for patients with HFpEF, according to the 2019 opinion published by Dr. Butler and Dr. Packer. Once again a post hoc analysis, this time using data from TOPCAT, suggested a benefit from the mineralocorticoid receptor antagonist spironolactone in patients with heart failure and an EF of 45%-49% (45% was the minimum EF for enrollment into the study).
Recently, data from a third trial that tested sacubitril/valsartan in patients with HFpEF, PARAGON-HF, showed benefit among patients with EFs below the study median of 57%. This finding led the Food and Drug Administration in February 2021 to amend its initial approval for sacubitril/valsartan by removing a specific EF ceiling from the drug’s indication and instead saying that patient’s receiving the drug should have a “below normal” EF.
Writing in a recent commentary, Dr. Yancy called the FDA’s action on sacubitril/valsartan “reasonable,” and that the subgroup assessment of data from the PARAGON-HF trial creates a “new, reasonably evidence-based therapy for HFpEF.” He also predicted that guideline-writing panels will “likely align with a permissive statement of indication” for sacubitril/valsartan in patients with HFpEF, especially those with EFs of less than 57%.
The idea of using an SGLT2 inhibitor like empagliflozin on all heart failure patients, and also adding agents like sacubitril/valsartan and spironolactone in patients with HFpEF and EFs in the mid-50% range or lower may take some time to catch on, but it already has one influential advocate.
“If a patient has HFpEF with an EF of less than 55%, use quadruple-class therapy,” summed up Dr. Butler during the HFSA session, while also suggesting prescribing an SGLT2 inhibitor to essentially all patients with heart failure regardless of their EF.
The EMPEROR-Preserved and EMPEROR-Reduced trials and the EMPEROR-Pooled analysis were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). Dr. Packer has had financial relationships with BI and Lilly and numerous other companies. Dr. Konstam has served on data monitoring committees for trials funded by Boehringer Ingelheim and by Amgen, Luitpold, and Pfizer, and has been a consultant to Arena, LivaNova, Merck, SC Pharma, and Takeda. Dr. Yancy had no disclosures. Dr. Butler has had financial relationships with Boehringer Ingelheim and numerous other companies.
FROM HFSA 2021
Survey: Nursing shortages affect safety during labor and delivery
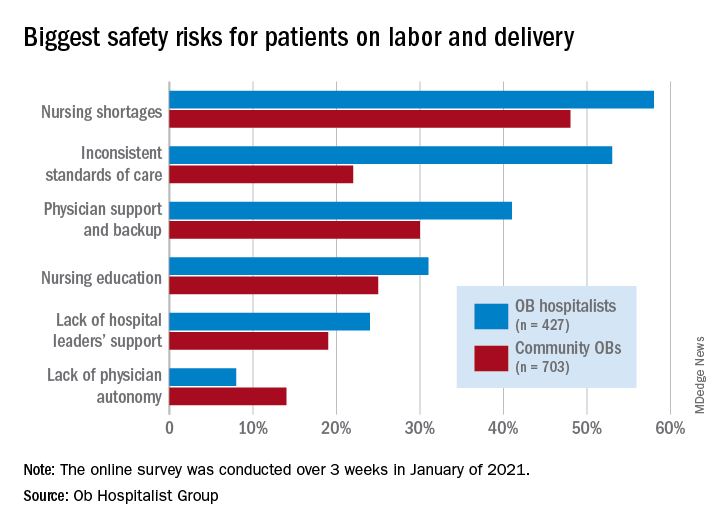
Just over 58% of the 1,130 respondents put nursing shortages ahead of physician support and backup (39.0%), inconsistent standards of care (38.5%), and nursing education (31.1%) as the most important challenge to patient safety, the Ob Hospitalist Group (OBHG) said in a new report.
“The survey reveals some startling gaps in physician and patient support all along the care continuum in obstetrics and OB hospitalist programs filling them,” said Lenny Castiglione, the CEO of OBHG, a national network of over 1,000 obstetric hospitalists. “As pressure builds on ob. units to improve care and reduce costs, and as clinical resources are stretched in the continuing battle against COVID-19 and its variants, health systems need to take serious measures to fill these gaps through staff recruitment, retention, and training.”
The national shortage of nurses is expected to get worse over the coming years as Baby Boomers’ need for health care increases and the large population (over 1 million) of older registered nurses retires by 2030, the OBHG said based on projections from the American Association of Colleges of Nursing.
Ob. hospitalists were somewhat more likely to see the nursing shortage as a major problem, compared with community-based Obs (58% vs. 48%), but the biggest difference in perception of safety risk between the two groups (53% for hospitalists vs. 22% for community physicians) involved inconsistent standards of care. “This is likely due to the ob. hospitalists’ 24/7 presence on the unit, and their visibility into the care provided across the unit,” the report noted.
Priorities for the future
Participants also were asked to rank each of seven focus areas on a scale of 0 (lowest priority) to 3 (highest) by its importance in the next 5 years. Maternal mortality was identified as the highest priority by 59.2% of physicians, followed by gaps in access to care between patient populations (38.0%), rural health care (26.5%), and ob.gyn. shortage (26.4%), the OBHG said.
A number of respondents noted the increase in high-risk patients, many of whom are obese and/or older and have comorbidities. “We know that the risk of C-sections increases relative to maternal weight. We need to focus on maternal morbidity and mortality,” one physician wrote in the open-ended response section.
When compared with the community obs., the hospitalists were much more likely to assign top priority over the next 5 years to maternal mortality (73% vs. 50%) and to gaps in access between patient populations (51% vs. 30%), according to the OBHG survey, which was conducted in January of 2021.
How will practice change in 5 years?
As for changes coming to obstetrical care over the next 5 years, respondents gave their strongest prediction to increased use of telemedicine, with 81.2% saying it would increase and just 14.4% predicting no change. The focus on subspecialization is expected to increase by 79.4% of participants (16.5% said no change), and 75.7% said that use of mid-level providers would rise (20.6% said no change), the survey data show.
The move toward mid-level providers was noted in many of the open-ended responses. “There is nothing mid-level about the midwifery care my colleagues provide our patients. They are experts in their field,” one physician wrote, but another said, “just because I foresee a shift toward increasing utilization of mid-levels and primary care practitioners for women’s health does not mean I endorse this trend.”

Just over 58% of the 1,130 respondents put nursing shortages ahead of physician support and backup (39.0%), inconsistent standards of care (38.5%), and nursing education (31.1%) as the most important challenge to patient safety, the Ob Hospitalist Group (OBHG) said in a new report.
“The survey reveals some startling gaps in physician and patient support all along the care continuum in obstetrics and OB hospitalist programs filling them,” said Lenny Castiglione, the CEO of OBHG, a national network of over 1,000 obstetric hospitalists. “As pressure builds on ob. units to improve care and reduce costs, and as clinical resources are stretched in the continuing battle against COVID-19 and its variants, health systems need to take serious measures to fill these gaps through staff recruitment, retention, and training.”
The national shortage of nurses is expected to get worse over the coming years as Baby Boomers’ need for health care increases and the large population (over 1 million) of older registered nurses retires by 2030, the OBHG said based on projections from the American Association of Colleges of Nursing.
Ob. hospitalists were somewhat more likely to see the nursing shortage as a major problem, compared with community-based Obs (58% vs. 48%), but the biggest difference in perception of safety risk between the two groups (53% for hospitalists vs. 22% for community physicians) involved inconsistent standards of care. “This is likely due to the ob. hospitalists’ 24/7 presence on the unit, and their visibility into the care provided across the unit,” the report noted.
Priorities for the future
Participants also were asked to rank each of seven focus areas on a scale of 0 (lowest priority) to 3 (highest) by its importance in the next 5 years. Maternal mortality was identified as the highest priority by 59.2% of physicians, followed by gaps in access to care between patient populations (38.0%), rural health care (26.5%), and ob.gyn. shortage (26.4%), the OBHG said.
A number of respondents noted the increase in high-risk patients, many of whom are obese and/or older and have comorbidities. “We know that the risk of C-sections increases relative to maternal weight. We need to focus on maternal morbidity and mortality,” one physician wrote in the open-ended response section.
When compared with the community obs., the hospitalists were much more likely to assign top priority over the next 5 years to maternal mortality (73% vs. 50%) and to gaps in access between patient populations (51% vs. 30%), according to the OBHG survey, which was conducted in January of 2021.
How will practice change in 5 years?
As for changes coming to obstetrical care over the next 5 years, respondents gave their strongest prediction to increased use of telemedicine, with 81.2% saying it would increase and just 14.4% predicting no change. The focus on subspecialization is expected to increase by 79.4% of participants (16.5% said no change), and 75.7% said that use of mid-level providers would rise (20.6% said no change), the survey data show.
The move toward mid-level providers was noted in many of the open-ended responses. “There is nothing mid-level about the midwifery care my colleagues provide our patients. They are experts in their field,” one physician wrote, but another said, “just because I foresee a shift toward increasing utilization of mid-levels and primary care practitioners for women’s health does not mean I endorse this trend.”

Just over 58% of the 1,130 respondents put nursing shortages ahead of physician support and backup (39.0%), inconsistent standards of care (38.5%), and nursing education (31.1%) as the most important challenge to patient safety, the Ob Hospitalist Group (OBHG) said in a new report.
“The survey reveals some startling gaps in physician and patient support all along the care continuum in obstetrics and OB hospitalist programs filling them,” said Lenny Castiglione, the CEO of OBHG, a national network of over 1,000 obstetric hospitalists. “As pressure builds on ob. units to improve care and reduce costs, and as clinical resources are stretched in the continuing battle against COVID-19 and its variants, health systems need to take serious measures to fill these gaps through staff recruitment, retention, and training.”
The national shortage of nurses is expected to get worse over the coming years as Baby Boomers’ need for health care increases and the large population (over 1 million) of older registered nurses retires by 2030, the OBHG said based on projections from the American Association of Colleges of Nursing.
Ob. hospitalists were somewhat more likely to see the nursing shortage as a major problem, compared with community-based Obs (58% vs. 48%), but the biggest difference in perception of safety risk between the two groups (53% for hospitalists vs. 22% for community physicians) involved inconsistent standards of care. “This is likely due to the ob. hospitalists’ 24/7 presence on the unit, and their visibility into the care provided across the unit,” the report noted.
Priorities for the future
Participants also were asked to rank each of seven focus areas on a scale of 0 (lowest priority) to 3 (highest) by its importance in the next 5 years. Maternal mortality was identified as the highest priority by 59.2% of physicians, followed by gaps in access to care between patient populations (38.0%), rural health care (26.5%), and ob.gyn. shortage (26.4%), the OBHG said.
A number of respondents noted the increase in high-risk patients, many of whom are obese and/or older and have comorbidities. “We know that the risk of C-sections increases relative to maternal weight. We need to focus on maternal morbidity and mortality,” one physician wrote in the open-ended response section.
When compared with the community obs., the hospitalists were much more likely to assign top priority over the next 5 years to maternal mortality (73% vs. 50%) and to gaps in access between patient populations (51% vs. 30%), according to the OBHG survey, which was conducted in January of 2021.
How will practice change in 5 years?
As for changes coming to obstetrical care over the next 5 years, respondents gave their strongest prediction to increased use of telemedicine, with 81.2% saying it would increase and just 14.4% predicting no change. The focus on subspecialization is expected to increase by 79.4% of participants (16.5% said no change), and 75.7% said that use of mid-level providers would rise (20.6% said no change), the survey data show.
The move toward mid-level providers was noted in many of the open-ended responses. “There is nothing mid-level about the midwifery care my colleagues provide our patients. They are experts in their field,” one physician wrote, but another said, “just because I foresee a shift toward increasing utilization of mid-levels and primary care practitioners for women’s health does not mean I endorse this trend.”
Navigating parenthood as pediatricians
PHM 2021 session
The Baby at Work or the Baby at Home: Navigating Parenthood as Pediatricians
Presenters
Jessica Gold, MD; Dana Foradori, MD, MEd; Nivedita Srinivas, MD; Honora Burnett, MD; Julie Pantaleoni, MD; and Barrett Fromme, MD, MHPE
Session summary
A group of physician-mothers from multiple academic children’s hospitals came together in a storytelling format to discuss topics relating to being a parent and pediatric hospitalist. Through short and poignant stories, the presenters shared their experiences and reviewed recent literature and policy changes relating to the topic. This mini-plenary focused on three themes:
1. Easing the transition back to work after the birth of a child.
2. Coping with the tension between being a parent and pediatrician.
3. The role that divisions, departments, and institutions can play in supporting parents and promoting workplace engagement.
The session concluded with a robust question-and-answer portion where participants built upon the themes above and shared their own experiences as pediatric hospitalist parents.
Key takeaways
- “Use your voice.” Physicians who are parents must continue having conversations about the challenging aspects of being a parent and hospitalist and advocate for the changes they would like to see.
- There will always be tension as a physician parent, but we can learn to embrace it while also learning how to ask for help, set boundaries, and share when we are struggling.
- There are numerous challenges for hospitalists who are parents because of poor parental leave policies in the United States, but this is slowly changing. For example, starting in July 2021, the ACGME mandated 6 weeks of parental leave during training without having to extend training.
- “You are not alone.” The presenters emphasized that their reason for hosting this session was to shed light on this topic and let all pediatric hospitalist parents know that they are not alone in this experience.
Dr. Scott is a second-year pediatric hospital medicine fellow at New York–Presbyterian Columbia/Cornell. Her academic interests are in curriculum development and evaluation in medical education with a focus on telemedicine.
PHM 2021 session
The Baby at Work or the Baby at Home: Navigating Parenthood as Pediatricians
Presenters
Jessica Gold, MD; Dana Foradori, MD, MEd; Nivedita Srinivas, MD; Honora Burnett, MD; Julie Pantaleoni, MD; and Barrett Fromme, MD, MHPE
Session summary
A group of physician-mothers from multiple academic children’s hospitals came together in a storytelling format to discuss topics relating to being a parent and pediatric hospitalist. Through short and poignant stories, the presenters shared their experiences and reviewed recent literature and policy changes relating to the topic. This mini-plenary focused on three themes:
1. Easing the transition back to work after the birth of a child.
2. Coping with the tension between being a parent and pediatrician.
3. The role that divisions, departments, and institutions can play in supporting parents and promoting workplace engagement.
The session concluded with a robust question-and-answer portion where participants built upon the themes above and shared their own experiences as pediatric hospitalist parents.
Key takeaways
- “Use your voice.” Physicians who are parents must continue having conversations about the challenging aspects of being a parent and hospitalist and advocate for the changes they would like to see.
- There will always be tension as a physician parent, but we can learn to embrace it while also learning how to ask for help, set boundaries, and share when we are struggling.
- There are numerous challenges for hospitalists who are parents because of poor parental leave policies in the United States, but this is slowly changing. For example, starting in July 2021, the ACGME mandated 6 weeks of parental leave during training without having to extend training.
- “You are not alone.” The presenters emphasized that their reason for hosting this session was to shed light on this topic and let all pediatric hospitalist parents know that they are not alone in this experience.
Dr. Scott is a second-year pediatric hospital medicine fellow at New York–Presbyterian Columbia/Cornell. Her academic interests are in curriculum development and evaluation in medical education with a focus on telemedicine.
PHM 2021 session
The Baby at Work or the Baby at Home: Navigating Parenthood as Pediatricians
Presenters
Jessica Gold, MD; Dana Foradori, MD, MEd; Nivedita Srinivas, MD; Honora Burnett, MD; Julie Pantaleoni, MD; and Barrett Fromme, MD, MHPE
Session summary
A group of physician-mothers from multiple academic children’s hospitals came together in a storytelling format to discuss topics relating to being a parent and pediatric hospitalist. Through short and poignant stories, the presenters shared their experiences and reviewed recent literature and policy changes relating to the topic. This mini-plenary focused on three themes:
1. Easing the transition back to work after the birth of a child.
2. Coping with the tension between being a parent and pediatrician.
3. The role that divisions, departments, and institutions can play in supporting parents and promoting workplace engagement.
The session concluded with a robust question-and-answer portion where participants built upon the themes above and shared their own experiences as pediatric hospitalist parents.
Key takeaways
- “Use your voice.” Physicians who are parents must continue having conversations about the challenging aspects of being a parent and hospitalist and advocate for the changes they would like to see.
- There will always be tension as a physician parent, but we can learn to embrace it while also learning how to ask for help, set boundaries, and share when we are struggling.
- There are numerous challenges for hospitalists who are parents because of poor parental leave policies in the United States, but this is slowly changing. For example, starting in July 2021, the ACGME mandated 6 weeks of parental leave during training without having to extend training.
- “You are not alone.” The presenters emphasized that their reason for hosting this session was to shed light on this topic and let all pediatric hospitalist parents know that they are not alone in this experience.
Dr. Scott is a second-year pediatric hospital medicine fellow at New York–Presbyterian Columbia/Cornell. Her academic interests are in curriculum development and evaluation in medical education with a focus on telemedicine.
Maryland reduces cesarean delivery rates
A statewide educational initiative in Maryland was associated with a significant reduction in cesarean delivery rates over 30 months, although program implementation was widely variable across participating hospitals, according to investigators.
Cesarean deliveries dropped 1.6% among nulliparous, term, singleton, vertex births, falling short of the 3.2% reduction recently achieved by a similar program in California, reported lead author Jennifer A. Callaghan-Koru, PhD, of the University of Maryland, Baltimore County, and colleagues.
“Although cesarean delivery can be lifesaving, evidence suggests that there is no benefit to maternal health when national cesarean delivery rates are higher than 20 per 100 live births,” the investigators wrote in Obstetrics & Gynecology.
They noted that cesarean delivery rates in the United States rose dramatically between 1996 and 2006, from 20.7% to 32.9%, before falling back to 31.7% in 2019.
According to the investigators, high cesarean delivery rates have drawn action from a roster of stakeholders, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine (SMFM), the Department of Health and Human Services, the Joint Commission, and the Council on Patient Safety in Women’s Health Care.
The latter group created an evidence-based obstetric patient safety bundle that was used in the present study. The investigators recruited 31 out of 32 birthing hospitals in Maryland, and over the course of 30 months, educated the participating hospitals on the practices recommended by the bundle, via in-person and remote training.
To measure implementation and associated outcomes, the investigators used a data portal provided by the ACOG Alliance for Innovation in Maternal Health (AIM) program, which supports adoption of the council’s safety bundle nationwide. Data included number of births; number of cesarean births; overall cesarean delivery rates; cesarean rates among nulliparous, term, singleton, vertex births; cesarean rates among nulliparous, term, singleton, vertex inductions; as well as severe maternal morbidity rates.
Among the 26 safety practices in the bundle, hospitals reported current use of 7 (median) before the program began, with a range from 0 to 23. During the 30-month collaboration, hospitals added a median of 4 practices, ranging from 0 to 17.
Concurrently, cesarean delivery rates for nulliparous, term, singleton, vertex births dropped from 26.9% to 28.5% (P = .011), while rates for inductions dropped to a greater degree, from 36.1% to 31.3% (P less than .001). Further analysis showed that greater reductions in rates of cesarean delivery were associated with adoption of practices in the “Response” domain.
“The Response domain has the largest number of practices that standardize clinical care, including induction scheduling, diagnosis and treatment of labor dystocia and failed induction, and interpretation of fetal heart rate patterns,” the investigators wrote. “The important role of standardizing care is consistent with a Cochrane review that found moderately strong evidence that the implementation of clinical practice guidelines, alongside feedback to clinicians (e.g., second opinions, audit and feedback of rates), can reduce cesarean delivery.”
Dr. Callaghan-Koru and colleagues noted the high variability of implementation among hospitals, which could explain why statewide reductions weren’t more dramatic.
“Other evaluations of perinatal quality improvement collaboratives have also found that some hospitals get left behind in these efforts, without making considerable progress and improving outcomes,” they wrote. “Given that work by state perinatal quality improvement collaboratives represents a primary national strategy for reducing maternal morbidity and mortality, it is critically important to conduct further implementation research to identify determinants of success and strategies to support all participating hospitals to make improvements.”
According to Iris Krishna, MD, of Emory University, Atlanta, each state’s starting point may predict how successful similar programs will be.
“The safe reduction in the cesarean delivery rate will vary by state,” Dr. Krishna said in a written comment. “States that have a well-established perinatal quality collaborative that have the support on the provider, hospital, and statewide level are more likely to successfully implement strategies and see a statistically significant decrease.”
She went on to emphasize the importance of collaboration across multiple levels of organization, and across a variety of health care providers and administrators.
“Successful implementation requires a multidisciplinary team (physicians, nurses, quality improvement officers) and a multifaceted approach (statewide policies),” Dr. Krishna said. “Key stakeholders will need to ‘buy in’ or be willing to support the policy and practice change to ensure its success. To address obstacles, initiatives to support vaginal birth are important, such as provider training on labor and support techniques, criteria for diagnosis of and management of labor dystocia and arrest disorders, standard responses to abnormal fetal heart rate patterns, and availability of expertise to lessen the need for cesarean delivery, such as breech version, instrumented delivery, and twin delivery protocols. It is also important for hospitals to a have a mentor model of quality improvement and shared learning strategies that work.”
Dr. Krishna agreed with the investigators that more work is necessary to determine best strategies for future intervention.
“Research is needed in identifying determinants of success and sustainment,” she said.
Dr. Burke received funding from her employer, Trinity Health, to conduct a pilot study concerning blood loss.
A statewide educational initiative in Maryland was associated with a significant reduction in cesarean delivery rates over 30 months, although program implementation was widely variable across participating hospitals, according to investigators.
Cesarean deliveries dropped 1.6% among nulliparous, term, singleton, vertex births, falling short of the 3.2% reduction recently achieved by a similar program in California, reported lead author Jennifer A. Callaghan-Koru, PhD, of the University of Maryland, Baltimore County, and colleagues.
“Although cesarean delivery can be lifesaving, evidence suggests that there is no benefit to maternal health when national cesarean delivery rates are higher than 20 per 100 live births,” the investigators wrote in Obstetrics & Gynecology.
They noted that cesarean delivery rates in the United States rose dramatically between 1996 and 2006, from 20.7% to 32.9%, before falling back to 31.7% in 2019.
According to the investigators, high cesarean delivery rates have drawn action from a roster of stakeholders, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine (SMFM), the Department of Health and Human Services, the Joint Commission, and the Council on Patient Safety in Women’s Health Care.
The latter group created an evidence-based obstetric patient safety bundle that was used in the present study. The investigators recruited 31 out of 32 birthing hospitals in Maryland, and over the course of 30 months, educated the participating hospitals on the practices recommended by the bundle, via in-person and remote training.
To measure implementation and associated outcomes, the investigators used a data portal provided by the ACOG Alliance for Innovation in Maternal Health (AIM) program, which supports adoption of the council’s safety bundle nationwide. Data included number of births; number of cesarean births; overall cesarean delivery rates; cesarean rates among nulliparous, term, singleton, vertex births; cesarean rates among nulliparous, term, singleton, vertex inductions; as well as severe maternal morbidity rates.
Among the 26 safety practices in the bundle, hospitals reported current use of 7 (median) before the program began, with a range from 0 to 23. During the 30-month collaboration, hospitals added a median of 4 practices, ranging from 0 to 17.
Concurrently, cesarean delivery rates for nulliparous, term, singleton, vertex births dropped from 26.9% to 28.5% (P = .011), while rates for inductions dropped to a greater degree, from 36.1% to 31.3% (P less than .001). Further analysis showed that greater reductions in rates of cesarean delivery were associated with adoption of practices in the “Response” domain.
“The Response domain has the largest number of practices that standardize clinical care, including induction scheduling, diagnosis and treatment of labor dystocia and failed induction, and interpretation of fetal heart rate patterns,” the investigators wrote. “The important role of standardizing care is consistent with a Cochrane review that found moderately strong evidence that the implementation of clinical practice guidelines, alongside feedback to clinicians (e.g., second opinions, audit and feedback of rates), can reduce cesarean delivery.”
Dr. Callaghan-Koru and colleagues noted the high variability of implementation among hospitals, which could explain why statewide reductions weren’t more dramatic.
“Other evaluations of perinatal quality improvement collaboratives have also found that some hospitals get left behind in these efforts, without making considerable progress and improving outcomes,” they wrote. “Given that work by state perinatal quality improvement collaboratives represents a primary national strategy for reducing maternal morbidity and mortality, it is critically important to conduct further implementation research to identify determinants of success and strategies to support all participating hospitals to make improvements.”
According to Iris Krishna, MD, of Emory University, Atlanta, each state’s starting point may predict how successful similar programs will be.
“The safe reduction in the cesarean delivery rate will vary by state,” Dr. Krishna said in a written comment. “States that have a well-established perinatal quality collaborative that have the support on the provider, hospital, and statewide level are more likely to successfully implement strategies and see a statistically significant decrease.”
She went on to emphasize the importance of collaboration across multiple levels of organization, and across a variety of health care providers and administrators.
“Successful implementation requires a multidisciplinary team (physicians, nurses, quality improvement officers) and a multifaceted approach (statewide policies),” Dr. Krishna said. “Key stakeholders will need to ‘buy in’ or be willing to support the policy and practice change to ensure its success. To address obstacles, initiatives to support vaginal birth are important, such as provider training on labor and support techniques, criteria for diagnosis of and management of labor dystocia and arrest disorders, standard responses to abnormal fetal heart rate patterns, and availability of expertise to lessen the need for cesarean delivery, such as breech version, instrumented delivery, and twin delivery protocols. It is also important for hospitals to a have a mentor model of quality improvement and shared learning strategies that work.”
Dr. Krishna agreed with the investigators that more work is necessary to determine best strategies for future intervention.
“Research is needed in identifying determinants of success and sustainment,” she said.
Dr. Burke received funding from her employer, Trinity Health, to conduct a pilot study concerning blood loss.
A statewide educational initiative in Maryland was associated with a significant reduction in cesarean delivery rates over 30 months, although program implementation was widely variable across participating hospitals, according to investigators.
Cesarean deliveries dropped 1.6% among nulliparous, term, singleton, vertex births, falling short of the 3.2% reduction recently achieved by a similar program in California, reported lead author Jennifer A. Callaghan-Koru, PhD, of the University of Maryland, Baltimore County, and colleagues.
“Although cesarean delivery can be lifesaving, evidence suggests that there is no benefit to maternal health when national cesarean delivery rates are higher than 20 per 100 live births,” the investigators wrote in Obstetrics & Gynecology.
They noted that cesarean delivery rates in the United States rose dramatically between 1996 and 2006, from 20.7% to 32.9%, before falling back to 31.7% in 2019.
According to the investigators, high cesarean delivery rates have drawn action from a roster of stakeholders, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine (SMFM), the Department of Health and Human Services, the Joint Commission, and the Council on Patient Safety in Women’s Health Care.
The latter group created an evidence-based obstetric patient safety bundle that was used in the present study. The investigators recruited 31 out of 32 birthing hospitals in Maryland, and over the course of 30 months, educated the participating hospitals on the practices recommended by the bundle, via in-person and remote training.
To measure implementation and associated outcomes, the investigators used a data portal provided by the ACOG Alliance for Innovation in Maternal Health (AIM) program, which supports adoption of the council’s safety bundle nationwide. Data included number of births; number of cesarean births; overall cesarean delivery rates; cesarean rates among nulliparous, term, singleton, vertex births; cesarean rates among nulliparous, term, singleton, vertex inductions; as well as severe maternal morbidity rates.
Among the 26 safety practices in the bundle, hospitals reported current use of 7 (median) before the program began, with a range from 0 to 23. During the 30-month collaboration, hospitals added a median of 4 practices, ranging from 0 to 17.
Concurrently, cesarean delivery rates for nulliparous, term, singleton, vertex births dropped from 26.9% to 28.5% (P = .011), while rates for inductions dropped to a greater degree, from 36.1% to 31.3% (P less than .001). Further analysis showed that greater reductions in rates of cesarean delivery were associated with adoption of practices in the “Response” domain.
“The Response domain has the largest number of practices that standardize clinical care, including induction scheduling, diagnosis and treatment of labor dystocia and failed induction, and interpretation of fetal heart rate patterns,” the investigators wrote. “The important role of standardizing care is consistent with a Cochrane review that found moderately strong evidence that the implementation of clinical practice guidelines, alongside feedback to clinicians (e.g., second opinions, audit and feedback of rates), can reduce cesarean delivery.”
Dr. Callaghan-Koru and colleagues noted the high variability of implementation among hospitals, which could explain why statewide reductions weren’t more dramatic.
“Other evaluations of perinatal quality improvement collaboratives have also found that some hospitals get left behind in these efforts, without making considerable progress and improving outcomes,” they wrote. “Given that work by state perinatal quality improvement collaboratives represents a primary national strategy for reducing maternal morbidity and mortality, it is critically important to conduct further implementation research to identify determinants of success and strategies to support all participating hospitals to make improvements.”
According to Iris Krishna, MD, of Emory University, Atlanta, each state’s starting point may predict how successful similar programs will be.
“The safe reduction in the cesarean delivery rate will vary by state,” Dr. Krishna said in a written comment. “States that have a well-established perinatal quality collaborative that have the support on the provider, hospital, and statewide level are more likely to successfully implement strategies and see a statistically significant decrease.”
She went on to emphasize the importance of collaboration across multiple levels of organization, and across a variety of health care providers and administrators.
“Successful implementation requires a multidisciplinary team (physicians, nurses, quality improvement officers) and a multifaceted approach (statewide policies),” Dr. Krishna said. “Key stakeholders will need to ‘buy in’ or be willing to support the policy and practice change to ensure its success. To address obstacles, initiatives to support vaginal birth are important, such as provider training on labor and support techniques, criteria for diagnosis of and management of labor dystocia and arrest disorders, standard responses to abnormal fetal heart rate patterns, and availability of expertise to lessen the need for cesarean delivery, such as breech version, instrumented delivery, and twin delivery protocols. It is also important for hospitals to a have a mentor model of quality improvement and shared learning strategies that work.”
Dr. Krishna agreed with the investigators that more work is necessary to determine best strategies for future intervention.
“Research is needed in identifying determinants of success and sustainment,” she said.
Dr. Burke received funding from her employer, Trinity Health, to conduct a pilot study concerning blood loss.
FROM OBSTETRICS & GYNECOLOGY
A new weight loss threshold for T2d remission after bariatric surgery?
Patients with type 2 diabetes who underwent bariatric surgery commonly experienced remission, but there was little increase in rates of remission above a threshold of 20% total weight loss (TWL), according to a retrospective analysis of 5,928 patients with diabetes in an integrated health care system in Southern California.
The findings should reassure physicians and patients that surgery will be beneficial, according to lead author Karen Coleman, PhD, professor of health systems science at Kaiser Permanente Southern California.
Dr. Coleman has heard from many physicians saying they recommend against bariatric surgery because of concerns that patients gain weight back and therefore won’t get a long-term benefit, but this is not supported by the literature. “Hundreds of articles at this point show that this simply is not true. In addition, providers seem to think about bariatric surgery as an ‘all or none’ treatment. Gaining any weight back means that patients ‘fail.’ Weight regain is a normal part of massive weight loss; however, maintaining a certain amount of weight loss still provides benefits for patients, especially those with cardiovascular conditions like diabetes,” said Dr. Coleman.
Most patients lose 20%-30% of their body weight after bariatric surgery, but they don’t have to lose that much to see an improvement in type 2 diabetes (T2D). In addition, if patients lose that much or more, and then gain some weight back, it doesn’t eliminate benefit. “Although we did not measure weight regain, a corollary statement is that patients can regain some of the weight they lose, but if they stay around 20% of their total weight lost, then their diabetes still remits,” said Dr. Coleman.
In the past, some standards to treat severe weight loss and metabolic disease called for 50% or more TWL. More recent standards target a 30% threshold. “We want physicians to understand that they need to have more reasonable expectations of weight loss with surgery and that these reasonable expectations still result in profound improvements in cardiovascular risk, death, and quality of life. A 20% TWL threshold is easier for these patients to get to, and like other patients, they still get the benefit. So even if these patients may not have as much weight loss they can still benefit from the surgery for their diabetes,” Dr. Coleman added.
Physicians have long assumed that the effect of bariatric surgery on T2D remission is tied to weight loss, but this has been tested only recently. Previous studies found a link and suggested that 25% TWL may be the needed threshold, but more data are needed, especially for sleeve gastrectomy.
In the current study, published in Diabetes Care, 73% of patients were female. Mean age was 49.8 years, and mean body mass index was 43.8 kg/m2. Fifty-seven percent underwent Roux-en-Y gastric bypass (RYGB). Follow-up averaged 5.9 years. Overall, 71% of patients had an initial remission of their diabetes (72% RYGB, 70% sleeve). The average time to remission was 1.0 years. The researchers categorized participants by percentage TWL. Compared with the 0%-5% group, each 5% increase in TWL was linked with a greater likelihood of achieving remission: 5%-10%, hazard ratio 1.22 (P = .23); 10%-15%, HR 1.97 (95% confidence interval, 1.47-2.64); 15%-20%, HR 2.33 (95% CI, 1.74-3.11); 20%-25%, HR 2.81 (95% CI, 2.11-3.75); 25%-30%, HR 2.88 (95% CI, 2.16-3.83); >30%, HR, 2.92 (95% CI, 2.19-3.88). Categories above 25% TWL had remission rates similar to those of the 20%-25% group. Those in the over 20% TWL group who were taking insulin at the time of surgery had better odds of T2D remission than did those in the 0%-5% TWL group who were not taking insulin (HR, 2.18; 95% CI, 1.64-2.88).
The study is a useful addition to the literature on the topic, according to W. Timothy Garvey, MD, director of the diabetes research center at the University of Alabama at Birmingham. “This tends to quantify it a little bit more than people might have had before,” he said.
Dr. Garvey noted that there were wide error bars in the outcomes grouped by TWL, and suggested that individual results of surgery may vary widely. “There are plenty of individuals in each of those bins that will require more weight loss for remission or less weight loss. That’s just the average of people in that weight loss category. So if a clinician is going to use this information, they need to take it with a grain of salt and realize that, just because they reach that 20% weight loss threshold, it doesn’t mean that their patient is going to go into remission. As a loose guide, as something to shoot for, I think this is valuable,” he added.
Dr. Coleman recommended that physicians not wait too long to suggest bariatric surgery, since patients are likely to have better outcomes if they are healthier going in. “Bariatric surgery is by far the most effective long-term treatment we have for severe obesity and we should be treating it as a secondary prevention strategy, not a last resort to save people’s lives. Bariatric surgery cannot regrow the cells in the pancreas that make insulin. So if we wait until patients with type 2 diabetes are insulin dependent to offer bariatric surgery, we are compromising the great effect surgery can have for them,” said Dr. Coleman.
Patients with type 2 diabetes who underwent bariatric surgery commonly experienced remission, but there was little increase in rates of remission above a threshold of 20% total weight loss (TWL), according to a retrospective analysis of 5,928 patients with diabetes in an integrated health care system in Southern California.
The findings should reassure physicians and patients that surgery will be beneficial, according to lead author Karen Coleman, PhD, professor of health systems science at Kaiser Permanente Southern California.
Dr. Coleman has heard from many physicians saying they recommend against bariatric surgery because of concerns that patients gain weight back and therefore won’t get a long-term benefit, but this is not supported by the literature. “Hundreds of articles at this point show that this simply is not true. In addition, providers seem to think about bariatric surgery as an ‘all or none’ treatment. Gaining any weight back means that patients ‘fail.’ Weight regain is a normal part of massive weight loss; however, maintaining a certain amount of weight loss still provides benefits for patients, especially those with cardiovascular conditions like diabetes,” said Dr. Coleman.
Most patients lose 20%-30% of their body weight after bariatric surgery, but they don’t have to lose that much to see an improvement in type 2 diabetes (T2D). In addition, if patients lose that much or more, and then gain some weight back, it doesn’t eliminate benefit. “Although we did not measure weight regain, a corollary statement is that patients can regain some of the weight they lose, but if they stay around 20% of their total weight lost, then their diabetes still remits,” said Dr. Coleman.
In the past, some standards to treat severe weight loss and metabolic disease called for 50% or more TWL. More recent standards target a 30% threshold. “We want physicians to understand that they need to have more reasonable expectations of weight loss with surgery and that these reasonable expectations still result in profound improvements in cardiovascular risk, death, and quality of life. A 20% TWL threshold is easier for these patients to get to, and like other patients, they still get the benefit. So even if these patients may not have as much weight loss they can still benefit from the surgery for their diabetes,” Dr. Coleman added.
Physicians have long assumed that the effect of bariatric surgery on T2D remission is tied to weight loss, but this has been tested only recently. Previous studies found a link and suggested that 25% TWL may be the needed threshold, but more data are needed, especially for sleeve gastrectomy.
In the current study, published in Diabetes Care, 73% of patients were female. Mean age was 49.8 years, and mean body mass index was 43.8 kg/m2. Fifty-seven percent underwent Roux-en-Y gastric bypass (RYGB). Follow-up averaged 5.9 years. Overall, 71% of patients had an initial remission of their diabetes (72% RYGB, 70% sleeve). The average time to remission was 1.0 years. The researchers categorized participants by percentage TWL. Compared with the 0%-5% group, each 5% increase in TWL was linked with a greater likelihood of achieving remission: 5%-10%, hazard ratio 1.22 (P = .23); 10%-15%, HR 1.97 (95% confidence interval, 1.47-2.64); 15%-20%, HR 2.33 (95% CI, 1.74-3.11); 20%-25%, HR 2.81 (95% CI, 2.11-3.75); 25%-30%, HR 2.88 (95% CI, 2.16-3.83); >30%, HR, 2.92 (95% CI, 2.19-3.88). Categories above 25% TWL had remission rates similar to those of the 20%-25% group. Those in the over 20% TWL group who were taking insulin at the time of surgery had better odds of T2D remission than did those in the 0%-5% TWL group who were not taking insulin (HR, 2.18; 95% CI, 1.64-2.88).
The study is a useful addition to the literature on the topic, according to W. Timothy Garvey, MD, director of the diabetes research center at the University of Alabama at Birmingham. “This tends to quantify it a little bit more than people might have had before,” he said.
Dr. Garvey noted that there were wide error bars in the outcomes grouped by TWL, and suggested that individual results of surgery may vary widely. “There are plenty of individuals in each of those bins that will require more weight loss for remission or less weight loss. That’s just the average of people in that weight loss category. So if a clinician is going to use this information, they need to take it with a grain of salt and realize that, just because they reach that 20% weight loss threshold, it doesn’t mean that their patient is going to go into remission. As a loose guide, as something to shoot for, I think this is valuable,” he added.
Dr. Coleman recommended that physicians not wait too long to suggest bariatric surgery, since patients are likely to have better outcomes if they are healthier going in. “Bariatric surgery is by far the most effective long-term treatment we have for severe obesity and we should be treating it as a secondary prevention strategy, not a last resort to save people’s lives. Bariatric surgery cannot regrow the cells in the pancreas that make insulin. So if we wait until patients with type 2 diabetes are insulin dependent to offer bariatric surgery, we are compromising the great effect surgery can have for them,” said Dr. Coleman.
Patients with type 2 diabetes who underwent bariatric surgery commonly experienced remission, but there was little increase in rates of remission above a threshold of 20% total weight loss (TWL), according to a retrospective analysis of 5,928 patients with diabetes in an integrated health care system in Southern California.
The findings should reassure physicians and patients that surgery will be beneficial, according to lead author Karen Coleman, PhD, professor of health systems science at Kaiser Permanente Southern California.
Dr. Coleman has heard from many physicians saying they recommend against bariatric surgery because of concerns that patients gain weight back and therefore won’t get a long-term benefit, but this is not supported by the literature. “Hundreds of articles at this point show that this simply is not true. In addition, providers seem to think about bariatric surgery as an ‘all or none’ treatment. Gaining any weight back means that patients ‘fail.’ Weight regain is a normal part of massive weight loss; however, maintaining a certain amount of weight loss still provides benefits for patients, especially those with cardiovascular conditions like diabetes,” said Dr. Coleman.
Most patients lose 20%-30% of their body weight after bariatric surgery, but they don’t have to lose that much to see an improvement in type 2 diabetes (T2D). In addition, if patients lose that much or more, and then gain some weight back, it doesn’t eliminate benefit. “Although we did not measure weight regain, a corollary statement is that patients can regain some of the weight they lose, but if they stay around 20% of their total weight lost, then their diabetes still remits,” said Dr. Coleman.
In the past, some standards to treat severe weight loss and metabolic disease called for 50% or more TWL. More recent standards target a 30% threshold. “We want physicians to understand that they need to have more reasonable expectations of weight loss with surgery and that these reasonable expectations still result in profound improvements in cardiovascular risk, death, and quality of life. A 20% TWL threshold is easier for these patients to get to, and like other patients, they still get the benefit. So even if these patients may not have as much weight loss they can still benefit from the surgery for their diabetes,” Dr. Coleman added.
Physicians have long assumed that the effect of bariatric surgery on T2D remission is tied to weight loss, but this has been tested only recently. Previous studies found a link and suggested that 25% TWL may be the needed threshold, but more data are needed, especially for sleeve gastrectomy.
In the current study, published in Diabetes Care, 73% of patients were female. Mean age was 49.8 years, and mean body mass index was 43.8 kg/m2. Fifty-seven percent underwent Roux-en-Y gastric bypass (RYGB). Follow-up averaged 5.9 years. Overall, 71% of patients had an initial remission of their diabetes (72% RYGB, 70% sleeve). The average time to remission was 1.0 years. The researchers categorized participants by percentage TWL. Compared with the 0%-5% group, each 5% increase in TWL was linked with a greater likelihood of achieving remission: 5%-10%, hazard ratio 1.22 (P = .23); 10%-15%, HR 1.97 (95% confidence interval, 1.47-2.64); 15%-20%, HR 2.33 (95% CI, 1.74-3.11); 20%-25%, HR 2.81 (95% CI, 2.11-3.75); 25%-30%, HR 2.88 (95% CI, 2.16-3.83); >30%, HR, 2.92 (95% CI, 2.19-3.88). Categories above 25% TWL had remission rates similar to those of the 20%-25% group. Those in the over 20% TWL group who were taking insulin at the time of surgery had better odds of T2D remission than did those in the 0%-5% TWL group who were not taking insulin (HR, 2.18; 95% CI, 1.64-2.88).
The study is a useful addition to the literature on the topic, according to W. Timothy Garvey, MD, director of the diabetes research center at the University of Alabama at Birmingham. “This tends to quantify it a little bit more than people might have had before,” he said.
Dr. Garvey noted that there were wide error bars in the outcomes grouped by TWL, and suggested that individual results of surgery may vary widely. “There are plenty of individuals in each of those bins that will require more weight loss for remission or less weight loss. That’s just the average of people in that weight loss category. So if a clinician is going to use this information, they need to take it with a grain of salt and realize that, just because they reach that 20% weight loss threshold, it doesn’t mean that their patient is going to go into remission. As a loose guide, as something to shoot for, I think this is valuable,” he added.
Dr. Coleman recommended that physicians not wait too long to suggest bariatric surgery, since patients are likely to have better outcomes if they are healthier going in. “Bariatric surgery is by far the most effective long-term treatment we have for severe obesity and we should be treating it as a secondary prevention strategy, not a last resort to save people’s lives. Bariatric surgery cannot regrow the cells in the pancreas that make insulin. So if we wait until patients with type 2 diabetes are insulin dependent to offer bariatric surgery, we are compromising the great effect surgery can have for them,” said Dr. Coleman.
FROM DIABETES CARE
Moderna vaccine more effective than Pfizer and J&J
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
New sarcoidosis treatment guideline bringing light to the darkness
Nothing about sarcoidosis is easy. First identified in 1877, it is quite common. In the United States, lifetime risk is 2.4% and 0.85% for African American persons and White persons, respectively. Still, it remains an enigma. Despite study of its genetics and immunopathology, we don’t know its cause. Diagnosis is challenging because noncaseating granulomas, the tissue finding associated with sarcoidosis, aren’t specific for the disease. With the exception of Löfgren syndrome, a well-described sarcoid presentation that portends an excellent prognosis, initial signs and symptoms are variable and disease course is unpredictable.
The inherent heterogeneity of sarcoid makes it challenging to study. In the modern era of evidence-based medicine, it’s hard to say much about it with certainty. The American Thoracic Society (ATS) is one of just a few, premier organizations that creates respiratory medicine guidelines. In 1999, they published a sarcoid consensus statement with the European Respiratory Society (ERS), another outstanding and influential respiratory medicine organization, and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG). For the past 20 years, I’ve been referring trainees to this document for guidance on managing their patients with sarcoid.
Twenty years later, sarcoid remains frustrating and mysterious, but much has changed. Our methods for evaluating evidence and creating guidelines are now based on the GRADE criteria. Now that we have easy access to advanced technologies such as endobronchial ultrasound, obtaining tissue for diagnosis is easier. Our study of sarcoid itself has advanced, with large cohorts providing data on phenotyping, new immunosuppressants being used for treatment, and an improved understanding of cardiac sarcoidosis. In short, we’re in need of a sarcoidosis guideline for the 21st century.
Within in the past 18 months, the ATS and ERS have delivered updated guidelines for diagnosis and treatment. Despite the advancements cited above, sarcoid remains difficult to study. So predictably, neither document issues earth-shattering conclusions. Truth be told, well-done guidelines rarely do. They do provide several important updates that physicians managing patients with sarcoid should note.
The guideline on diagnosis provides recommendations for routine monitoring after diagnosis. Many practicing clinicians took from the 1999 ATS/ERS/WASOG consensus statement that all patients with sarcoid needed to be seen annually. At pulmonary clinics where I’ve worked, we’ve defaulted to annual follow-up for everyone, usually with chest radiography, lab testing, electrocardiography, and referral to ophthalmology. Because a majority of patients with sarcoid will remain asymptomatic or experience spontaneous remission, this practice never really seemed cost-effective or clinically efficient. The new guidelines are far more proscriptive on what monitoring is required and grade requirements at specific levels of certainty and often advise symptom-based assessments in lieu of reflexive annual testing.
The ERS guideline on treatment provides a thoughtful discussion of corticosteroid indications and dosing, broken down by underlying disease severity (assessed by lung function abnormalities and imaging). It also recognizes that two of the most common sarcoid symptoms are fatigue and dyspnea, which are both inherently nonspecific. In practice, proving these symptoms are directly attributable to sarcoid is challenging. The treatment guideline allows for flexibility in these cases, with shared decision-making and trials of low-dose steroids recommended. This seems an excellent hedge against overtreatment with immunosuppressive medications that have harmful side effects.
The ATS and ERS guidelines are not without controversy. Their approach to cardiac sarcoid differs slightly from that recommended by a commonly cited Heart Rhythm Society consensus statement, and despite discussing treatment options, the section on fatigue is quite limited. These two facts and other limitations largely reflect differing interpretations of the limited data; they do not detract from the overall importance of the ATS and ERS guidelines. Sarcoid remains an enigma, but little by little the outstanding academic physicians at the ATS and ERS are providing clarity.
Dr. Holley is an associate professor, department of medicine, Uniformed Services University (USU); program director, pulmonary and critical care medical fellowship, department of medicine, Walter Reed National Military Medical Center, Bethesda, Maryland. He has received a research grant from Fisher-Paykel and income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Nothing about sarcoidosis is easy. First identified in 1877, it is quite common. In the United States, lifetime risk is 2.4% and 0.85% for African American persons and White persons, respectively. Still, it remains an enigma. Despite study of its genetics and immunopathology, we don’t know its cause. Diagnosis is challenging because noncaseating granulomas, the tissue finding associated with sarcoidosis, aren’t specific for the disease. With the exception of Löfgren syndrome, a well-described sarcoid presentation that portends an excellent prognosis, initial signs and symptoms are variable and disease course is unpredictable.
The inherent heterogeneity of sarcoid makes it challenging to study. In the modern era of evidence-based medicine, it’s hard to say much about it with certainty. The American Thoracic Society (ATS) is one of just a few, premier organizations that creates respiratory medicine guidelines. In 1999, they published a sarcoid consensus statement with the European Respiratory Society (ERS), another outstanding and influential respiratory medicine organization, and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG). For the past 20 years, I’ve been referring trainees to this document for guidance on managing their patients with sarcoid.
Twenty years later, sarcoid remains frustrating and mysterious, but much has changed. Our methods for evaluating evidence and creating guidelines are now based on the GRADE criteria. Now that we have easy access to advanced technologies such as endobronchial ultrasound, obtaining tissue for diagnosis is easier. Our study of sarcoid itself has advanced, with large cohorts providing data on phenotyping, new immunosuppressants being used for treatment, and an improved understanding of cardiac sarcoidosis. In short, we’re in need of a sarcoidosis guideline for the 21st century.
Within in the past 18 months, the ATS and ERS have delivered updated guidelines for diagnosis and treatment. Despite the advancements cited above, sarcoid remains difficult to study. So predictably, neither document issues earth-shattering conclusions. Truth be told, well-done guidelines rarely do. They do provide several important updates that physicians managing patients with sarcoid should note.
The guideline on diagnosis provides recommendations for routine monitoring after diagnosis. Many practicing clinicians took from the 1999 ATS/ERS/WASOG consensus statement that all patients with sarcoid needed to be seen annually. At pulmonary clinics where I’ve worked, we’ve defaulted to annual follow-up for everyone, usually with chest radiography, lab testing, electrocardiography, and referral to ophthalmology. Because a majority of patients with sarcoid will remain asymptomatic or experience spontaneous remission, this practice never really seemed cost-effective or clinically efficient. The new guidelines are far more proscriptive on what monitoring is required and grade requirements at specific levels of certainty and often advise symptom-based assessments in lieu of reflexive annual testing.
The ERS guideline on treatment provides a thoughtful discussion of corticosteroid indications and dosing, broken down by underlying disease severity (assessed by lung function abnormalities and imaging). It also recognizes that two of the most common sarcoid symptoms are fatigue and dyspnea, which are both inherently nonspecific. In practice, proving these symptoms are directly attributable to sarcoid is challenging. The treatment guideline allows for flexibility in these cases, with shared decision-making and trials of low-dose steroids recommended. This seems an excellent hedge against overtreatment with immunosuppressive medications that have harmful side effects.
The ATS and ERS guidelines are not without controversy. Their approach to cardiac sarcoid differs slightly from that recommended by a commonly cited Heart Rhythm Society consensus statement, and despite discussing treatment options, the section on fatigue is quite limited. These two facts and other limitations largely reflect differing interpretations of the limited data; they do not detract from the overall importance of the ATS and ERS guidelines. Sarcoid remains an enigma, but little by little the outstanding academic physicians at the ATS and ERS are providing clarity.
Dr. Holley is an associate professor, department of medicine, Uniformed Services University (USU); program director, pulmonary and critical care medical fellowship, department of medicine, Walter Reed National Military Medical Center, Bethesda, Maryland. He has received a research grant from Fisher-Paykel and income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Nothing about sarcoidosis is easy. First identified in 1877, it is quite common. In the United States, lifetime risk is 2.4% and 0.85% for African American persons and White persons, respectively. Still, it remains an enigma. Despite study of its genetics and immunopathology, we don’t know its cause. Diagnosis is challenging because noncaseating granulomas, the tissue finding associated with sarcoidosis, aren’t specific for the disease. With the exception of Löfgren syndrome, a well-described sarcoid presentation that portends an excellent prognosis, initial signs and symptoms are variable and disease course is unpredictable.
The inherent heterogeneity of sarcoid makes it challenging to study. In the modern era of evidence-based medicine, it’s hard to say much about it with certainty. The American Thoracic Society (ATS) is one of just a few, premier organizations that creates respiratory medicine guidelines. In 1999, they published a sarcoid consensus statement with the European Respiratory Society (ERS), another outstanding and influential respiratory medicine organization, and the World Association of Sarcoidosis and other Granulomatous Disorders (WASOG). For the past 20 years, I’ve been referring trainees to this document for guidance on managing their patients with sarcoid.
Twenty years later, sarcoid remains frustrating and mysterious, but much has changed. Our methods for evaluating evidence and creating guidelines are now based on the GRADE criteria. Now that we have easy access to advanced technologies such as endobronchial ultrasound, obtaining tissue for diagnosis is easier. Our study of sarcoid itself has advanced, with large cohorts providing data on phenotyping, new immunosuppressants being used for treatment, and an improved understanding of cardiac sarcoidosis. In short, we’re in need of a sarcoidosis guideline for the 21st century.
Within in the past 18 months, the ATS and ERS have delivered updated guidelines for diagnosis and treatment. Despite the advancements cited above, sarcoid remains difficult to study. So predictably, neither document issues earth-shattering conclusions. Truth be told, well-done guidelines rarely do. They do provide several important updates that physicians managing patients with sarcoid should note.
The guideline on diagnosis provides recommendations for routine monitoring after diagnosis. Many practicing clinicians took from the 1999 ATS/ERS/WASOG consensus statement that all patients with sarcoid needed to be seen annually. At pulmonary clinics where I’ve worked, we’ve defaulted to annual follow-up for everyone, usually with chest radiography, lab testing, electrocardiography, and referral to ophthalmology. Because a majority of patients with sarcoid will remain asymptomatic or experience spontaneous remission, this practice never really seemed cost-effective or clinically efficient. The new guidelines are far more proscriptive on what monitoring is required and grade requirements at specific levels of certainty and often advise symptom-based assessments in lieu of reflexive annual testing.
The ERS guideline on treatment provides a thoughtful discussion of corticosteroid indications and dosing, broken down by underlying disease severity (assessed by lung function abnormalities and imaging). It also recognizes that two of the most common sarcoid symptoms are fatigue and dyspnea, which are both inherently nonspecific. In practice, proving these symptoms are directly attributable to sarcoid is challenging. The treatment guideline allows for flexibility in these cases, with shared decision-making and trials of low-dose steroids recommended. This seems an excellent hedge against overtreatment with immunosuppressive medications that have harmful side effects.
The ATS and ERS guidelines are not without controversy. Their approach to cardiac sarcoid differs slightly from that recommended by a commonly cited Heart Rhythm Society consensus statement, and despite discussing treatment options, the section on fatigue is quite limited. These two facts and other limitations largely reflect differing interpretations of the limited data; they do not detract from the overall importance of the ATS and ERS guidelines. Sarcoid remains an enigma, but little by little the outstanding academic physicians at the ATS and ERS are providing clarity.
Dr. Holley is an associate professor, department of medicine, Uniformed Services University (USU); program director, pulmonary and critical care medical fellowship, department of medicine, Walter Reed National Military Medical Center, Bethesda, Maryland. He has received a research grant from Fisher-Paykel and income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
COVID vaccine is safe, effective for children aged 5-11, Pfizer says
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
With record numbers of COVID-19 cases being reported in kids, Pfizer and its partner BioNTech have announced that their mRNA vaccine for COVID-19 is safe and appears to generate a protective immune response in children as young as 5.
The companies have been testing a lower dose of the vaccine -- just 10 milligrams -- in children between the ages of 5 and 11. That’s one-third the dose given to adults.
In a clinical trial that included more than 2,200 children, Pfizer says two doses of the vaccines given 3 weeks apart generated a high level of neutralizing antibodies, comparable to the level seen in older children who get a higher dose of the vaccine.
On the advice of its vaccine advisory committee, the Food and Drug Administration asked vaccine makers to include more children in these studies earlier this year.
Rather than testing whether the vaccines are preventing COVID-19 illness in children, as they did in adults, the pharmaceutical companies that make the COVID-19 vaccines are looking at the antibody levels generated by the vaccines instead. The FDA has approved the approach in hopes of speeding vaccines to children, who are now back in school full time in most parts of the United States.
With that in mind, Evan Anderson, MD, a doctor with Children’s Healthcare of Atlanta who is an investigator for the trial — and is therefore kept in the dark about its results — said it’s important to keep in mind that the company didn’t share any efficacy data today.
“We don’t know whether there were cases of COVID-19 among children that were enrolled in the study and how those compared in those who received placebo versus those that received vaccine,” he said.
The company says side effects seen in the trial are comparable to those seen in older children. The company said there were no cases of heart inflammation called myocarditis observed. Pfizer says they plan to send their data to the FDA as soon as possible.
The company says side effects seen in the trial are comparable to those seen in older children. Pfizer says they plan to send their data to the FDA as soon as possible.
“We are pleased to be able to submit data to regulatory authorities for this group of school-aged children before the start of the winter season,” Ugur Sahin, MD, CEO and co-founder of BioNTech, said in a news release. “The safety profile and immunogenicity data in children aged 5 to 11 years vaccinated at a lower dose are consistent with those we have observed with our vaccine in other older populations at a higher dose.”
When asked how soon the FDA might act on Pfizer’s application, Anderson said others had speculated about timelines of 4 to 6 weeks, but he also noted that the FDA could still exercise its authority to ask the company for more information, which could slow the process down.
“As a parent myself, I would love to see that timeline occurring quickly. However, I do want the FDA to fully review the data and ask the necessary questions,” he said. “It’s a little speculative to get too definitive with timelines.”
A version of this article first appeared on WebMD.com.
Beware patients’ health plans that skirt state laws on specialty drug access
There is a dangerous trend in our country in which employers, seeking to reduce health plan costs they pay, enter into agreements with small third-party administrators that “carve out” specialty drug benefits from their self-funded health insurance plan. What employers are not told is that these spending reductions are accomplished by risking the health of their employees. It is the self-funded businesses that are being preyed upon by these administrators because there is a lot of money to be made by carving out the specialty drugs in their self-funded health plan.
Let’s start with a little primer on “fully insured” versus “self-funded” health plans. As a small business owner, I understand the need to make sure that expenses don’t outpace revenue if I want to keep my doors open. One of the largest expenses for any business is health insurance. My private rheumatology practice uses a fully insured health plan. In a fully insured plan, the insurer is the party taking the financial risk. We pay the premiums, and the insurance company pays the bills after the deductible is met. It may cost more in premiums than a self-funded plan, but if an employee has an accident or severe illness, our practice is not responsible for the cost of care.
On the other hand, large and small businesses that are self-funded cover the health costs of their employees themselves. These businesses will hire a third-party administrator to pay the bills out of an account that is supplied with money from the business owner. Looking at the insurance card of your patient is one way to tell if they are covered by a fully insured or self-funded plan. If the insurance card says the plan is “administered by” the insurer or “administrative services only,” it is most likely a self-funded plan. If their insurance card states “underwritten by” the insurer on the card then it is likely a fully insured plan. This becomes important because self-funded plans are not subject to the jurisdiction of state laws such as utilization management reform. These state laws are preempted from applying to self-funded plans by the Employee Retirement Income Security Act of 1974. The Rutledge v. Pharmaceutical Care Management Association Supreme Court case took up the question of whether certain state laws impermissibly applied or were connected to self-funded plans. The ruling in favor of Rutledge opens the door that certain state legislation may one day apply to self-funded plans.
Specialty drug benefit carve outs are not in best interests of employees, employers
This piece is not about Rutledge but about the small third-party administrators that are convincing self-funded businesses to let them “carve out” specialty drug benefits from the larger administrator of the plan by promising huge savings in the employer’s specialty drug spending. Two such companies that have come to the attention of the Coalition of State Rheumatology Organizations are Vivio Health and Archimedes. CSRO has received numerous complaints from rheumatologists regarding interference from these two entities with their clinical decision making and disregard for standard of care.
Vivio’s website reveals a disturbing approach to cost reduction. The website states that Vivio profiles physicians through ProPublica and Open Payments to determine if they are prescribing for the right reason and not for self-interest. This serves as an attempt to set up mistrust of the physician by the employer. Vivio’s website also states that the Food and Drug Administration has declining standards for approval of drugs, and consequently many approved drugs should be considered “experimental.” They say that business owners should not have to pay for “experimental” treatments. Through conversation with Vivio, it appears they believe that oncologists could be replaced by a primary care physician with the right algorithm.
Many of Vivio’s egregious behaviors are enumerated in our letter to Vivio: outrageous nonmedical switches, mandatory biologic tapering, and site of care changes. In all of the complaints that we received, Vivio attempted to switch patients to the same infusible medication, Renflexis, and also mandated white bagging, which means the payer has a specialty pharmacy ship a patient’s medication directly to the physician’s office for administration. This switch was made regardless of the mechanism of action or route of administration of the drug that had stabilized the patient. Peer-to-peer reviews with a retired radiologist led to routinely denied appeals and would even force the patient to a different site of care if the rheumatologist refused the new treatment or the mandated white bagging.
Our letter resulted in two conversations with Vivio. Vivio insisted that it was using American College of Rheumatology guidelines and comparisons between drug studies to make these decisions. The company stated that patients can be switched to any drug that has the same ACR 20, 50, and 70 response criteria outcomes as the drug that they are presently taking, even though these sorts of comparisons of results across completely different studies are invalid for a number of reasons, including because they do not have the same patient populations, protocols, and inclusion/exclusion criteria. These are dangerous policies and thus far we have not been able to find any rheumatologists who have gone along with these demands.
Companies such as Vivio are spreading, and employers are unaware that their policies are only paying lip service to “individualized care” while maintaining an approach to patient care that is focused only on cost cutting. Indeed, Archimedes represents one such metastasis. Their practices are similar to those of Vivio and of which CSRO has received complaints. Archimedes has similarly attempted to mandate white-bagging for the enrollees it manages and switch stable patients for nonmedical reasons to an entirely different molecule and mechanism of action.
Business owners do not understand the harm that these policies can cause their employees. This harm increases downstream medical spending as a result of loss of control of disease activity.
This is a call to action for all advocates and advocacy groups to get in the room with employer/business groups and explain how these third-party administrators, carving out specialty drug benefits, can ultimately cause physical harm to employees and increase monetary cost to the employer in the long run. Rheumatology as a specialty needs to educate employers and work out ways to save money for them and, at the same time, maintain excellence in care for their employees. CSRO has a letter it used successfully with the human resources department of Edward Jones to effect a change in its policies on this matter, which you are welcome to use to craft your own to businesses.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
There is a dangerous trend in our country in which employers, seeking to reduce health plan costs they pay, enter into agreements with small third-party administrators that “carve out” specialty drug benefits from their self-funded health insurance plan. What employers are not told is that these spending reductions are accomplished by risking the health of their employees. It is the self-funded businesses that are being preyed upon by these administrators because there is a lot of money to be made by carving out the specialty drugs in their self-funded health plan.
Let’s start with a little primer on “fully insured” versus “self-funded” health plans. As a small business owner, I understand the need to make sure that expenses don’t outpace revenue if I want to keep my doors open. One of the largest expenses for any business is health insurance. My private rheumatology practice uses a fully insured health plan. In a fully insured plan, the insurer is the party taking the financial risk. We pay the premiums, and the insurance company pays the bills after the deductible is met. It may cost more in premiums than a self-funded plan, but if an employee has an accident or severe illness, our practice is not responsible for the cost of care.
On the other hand, large and small businesses that are self-funded cover the health costs of their employees themselves. These businesses will hire a third-party administrator to pay the bills out of an account that is supplied with money from the business owner. Looking at the insurance card of your patient is one way to tell if they are covered by a fully insured or self-funded plan. If the insurance card says the plan is “administered by” the insurer or “administrative services only,” it is most likely a self-funded plan. If their insurance card states “underwritten by” the insurer on the card then it is likely a fully insured plan. This becomes important because self-funded plans are not subject to the jurisdiction of state laws such as utilization management reform. These state laws are preempted from applying to self-funded plans by the Employee Retirement Income Security Act of 1974. The Rutledge v. Pharmaceutical Care Management Association Supreme Court case took up the question of whether certain state laws impermissibly applied or were connected to self-funded plans. The ruling in favor of Rutledge opens the door that certain state legislation may one day apply to self-funded plans.
Specialty drug benefit carve outs are not in best interests of employees, employers
This piece is not about Rutledge but about the small third-party administrators that are convincing self-funded businesses to let them “carve out” specialty drug benefits from the larger administrator of the plan by promising huge savings in the employer’s specialty drug spending. Two such companies that have come to the attention of the Coalition of State Rheumatology Organizations are Vivio Health and Archimedes. CSRO has received numerous complaints from rheumatologists regarding interference from these two entities with their clinical decision making and disregard for standard of care.
Vivio’s website reveals a disturbing approach to cost reduction. The website states that Vivio profiles physicians through ProPublica and Open Payments to determine if they are prescribing for the right reason and not for self-interest. This serves as an attempt to set up mistrust of the physician by the employer. Vivio’s website also states that the Food and Drug Administration has declining standards for approval of drugs, and consequently many approved drugs should be considered “experimental.” They say that business owners should not have to pay for “experimental” treatments. Through conversation with Vivio, it appears they believe that oncologists could be replaced by a primary care physician with the right algorithm.
Many of Vivio’s egregious behaviors are enumerated in our letter to Vivio: outrageous nonmedical switches, mandatory biologic tapering, and site of care changes. In all of the complaints that we received, Vivio attempted to switch patients to the same infusible medication, Renflexis, and also mandated white bagging, which means the payer has a specialty pharmacy ship a patient’s medication directly to the physician’s office for administration. This switch was made regardless of the mechanism of action or route of administration of the drug that had stabilized the patient. Peer-to-peer reviews with a retired radiologist led to routinely denied appeals and would even force the patient to a different site of care if the rheumatologist refused the new treatment or the mandated white bagging.
Our letter resulted in two conversations with Vivio. Vivio insisted that it was using American College of Rheumatology guidelines and comparisons between drug studies to make these decisions. The company stated that patients can be switched to any drug that has the same ACR 20, 50, and 70 response criteria outcomes as the drug that they are presently taking, even though these sorts of comparisons of results across completely different studies are invalid for a number of reasons, including because they do not have the same patient populations, protocols, and inclusion/exclusion criteria. These are dangerous policies and thus far we have not been able to find any rheumatologists who have gone along with these demands.
Companies such as Vivio are spreading, and employers are unaware that their policies are only paying lip service to “individualized care” while maintaining an approach to patient care that is focused only on cost cutting. Indeed, Archimedes represents one such metastasis. Their practices are similar to those of Vivio and of which CSRO has received complaints. Archimedes has similarly attempted to mandate white-bagging for the enrollees it manages and switch stable patients for nonmedical reasons to an entirely different molecule and mechanism of action.
Business owners do not understand the harm that these policies can cause their employees. This harm increases downstream medical spending as a result of loss of control of disease activity.
This is a call to action for all advocates and advocacy groups to get in the room with employer/business groups and explain how these third-party administrators, carving out specialty drug benefits, can ultimately cause physical harm to employees and increase monetary cost to the employer in the long run. Rheumatology as a specialty needs to educate employers and work out ways to save money for them and, at the same time, maintain excellence in care for their employees. CSRO has a letter it used successfully with the human resources department of Edward Jones to effect a change in its policies on this matter, which you are welcome to use to craft your own to businesses.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
There is a dangerous trend in our country in which employers, seeking to reduce health plan costs they pay, enter into agreements with small third-party administrators that “carve out” specialty drug benefits from their self-funded health insurance plan. What employers are not told is that these spending reductions are accomplished by risking the health of their employees. It is the self-funded businesses that are being preyed upon by these administrators because there is a lot of money to be made by carving out the specialty drugs in their self-funded health plan.
Let’s start with a little primer on “fully insured” versus “self-funded” health plans. As a small business owner, I understand the need to make sure that expenses don’t outpace revenue if I want to keep my doors open. One of the largest expenses for any business is health insurance. My private rheumatology practice uses a fully insured health plan. In a fully insured plan, the insurer is the party taking the financial risk. We pay the premiums, and the insurance company pays the bills after the deductible is met. It may cost more in premiums than a self-funded plan, but if an employee has an accident or severe illness, our practice is not responsible for the cost of care.
On the other hand, large and small businesses that are self-funded cover the health costs of their employees themselves. These businesses will hire a third-party administrator to pay the bills out of an account that is supplied with money from the business owner. Looking at the insurance card of your patient is one way to tell if they are covered by a fully insured or self-funded plan. If the insurance card says the plan is “administered by” the insurer or “administrative services only,” it is most likely a self-funded plan. If their insurance card states “underwritten by” the insurer on the card then it is likely a fully insured plan. This becomes important because self-funded plans are not subject to the jurisdiction of state laws such as utilization management reform. These state laws are preempted from applying to self-funded plans by the Employee Retirement Income Security Act of 1974. The Rutledge v. Pharmaceutical Care Management Association Supreme Court case took up the question of whether certain state laws impermissibly applied or were connected to self-funded plans. The ruling in favor of Rutledge opens the door that certain state legislation may one day apply to self-funded plans.
Specialty drug benefit carve outs are not in best interests of employees, employers
This piece is not about Rutledge but about the small third-party administrators that are convincing self-funded businesses to let them “carve out” specialty drug benefits from the larger administrator of the plan by promising huge savings in the employer’s specialty drug spending. Two such companies that have come to the attention of the Coalition of State Rheumatology Organizations are Vivio Health and Archimedes. CSRO has received numerous complaints from rheumatologists regarding interference from these two entities with their clinical decision making and disregard for standard of care.
Vivio’s website reveals a disturbing approach to cost reduction. The website states that Vivio profiles physicians through ProPublica and Open Payments to determine if they are prescribing for the right reason and not for self-interest. This serves as an attempt to set up mistrust of the physician by the employer. Vivio’s website also states that the Food and Drug Administration has declining standards for approval of drugs, and consequently many approved drugs should be considered “experimental.” They say that business owners should not have to pay for “experimental” treatments. Through conversation with Vivio, it appears they believe that oncologists could be replaced by a primary care physician with the right algorithm.
Many of Vivio’s egregious behaviors are enumerated in our letter to Vivio: outrageous nonmedical switches, mandatory biologic tapering, and site of care changes. In all of the complaints that we received, Vivio attempted to switch patients to the same infusible medication, Renflexis, and also mandated white bagging, which means the payer has a specialty pharmacy ship a patient’s medication directly to the physician’s office for administration. This switch was made regardless of the mechanism of action or route of administration of the drug that had stabilized the patient. Peer-to-peer reviews with a retired radiologist led to routinely denied appeals and would even force the patient to a different site of care if the rheumatologist refused the new treatment or the mandated white bagging.
Our letter resulted in two conversations with Vivio. Vivio insisted that it was using American College of Rheumatology guidelines and comparisons between drug studies to make these decisions. The company stated that patients can be switched to any drug that has the same ACR 20, 50, and 70 response criteria outcomes as the drug that they are presently taking, even though these sorts of comparisons of results across completely different studies are invalid for a number of reasons, including because they do not have the same patient populations, protocols, and inclusion/exclusion criteria. These are dangerous policies and thus far we have not been able to find any rheumatologists who have gone along with these demands.
Companies such as Vivio are spreading, and employers are unaware that their policies are only paying lip service to “individualized care” while maintaining an approach to patient care that is focused only on cost cutting. Indeed, Archimedes represents one such metastasis. Their practices are similar to those of Vivio and of which CSRO has received complaints. Archimedes has similarly attempted to mandate white-bagging for the enrollees it manages and switch stable patients for nonmedical reasons to an entirely different molecule and mechanism of action.
Business owners do not understand the harm that these policies can cause their employees. This harm increases downstream medical spending as a result of loss of control of disease activity.
This is a call to action for all advocates and advocacy groups to get in the room with employer/business groups and explain how these third-party administrators, carving out specialty drug benefits, can ultimately cause physical harm to employees and increase monetary cost to the employer in the long run. Rheumatology as a specialty needs to educate employers and work out ways to save money for them and, at the same time, maintain excellence in care for their employees. CSRO has a letter it used successfully with the human resources department of Edward Jones to effect a change in its policies on this matter, which you are welcome to use to craft your own to businesses.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is President of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Parent-led intervention linked with decreased autism symptoms in at-risk infants
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
These findings, which were published in JAMA Pediatrics, were the first evidence worldwide that a preemptive intervention during infancy could lead to such a significant improvement in children’s social development, resulting in “three times fewer diagnoses of autism at age 3,” said lead author Andrew Whitehouse, PhD, in a statement.
“No trial of a preemptive infant intervention, applied prior to diagnosis, has to date shown such an effect to impact diagnostic outcomes – until now,” he said.
Study intervention is a nontraditonal approach
Dr. Whitehouse, who is professor of Autism Research at Telethon Kids and University of Western Australia, and Director of CliniKids in Perth, said the intervention is a departure from traditional approaches. “Traditionally, therapy seeks to train children to learn ‘typical’ behaviors,” he said in an email. “The difference of this therapy is that we help parents understand the unique abilities of their baby, and to use these strengths as a foundation for future development.”
Dr. Whitehouse’s study included 103 children (aged approximately 12 months), who displayed at least three of five behaviors indicating a high likelihood of ASD as defined by the Social Attention and Communication Surveillance–Revised (SACS-R) 12-month checklist. The infants were randomized to receive either usual care or the intervention, which is called the iBASIS–Video Interaction to Promote Positive Parenting (iBASIS-VIPP). Usual care was delivered by community physicians, whereas the intervention involved 10 sessions delivered at home by a trained therapist.
“The iBASIS-VIPP uses video-feedback as a means of helping parents recognize their baby’s communication cues so they can respond in a way that builds their social communication development,” Dr. Whitehouse explained in an interview. “The therapist then provides guidance to the parent as to how their baby is communicating with them, and they can communicate back to have back-and-forth conversations.”
“We know these back-and-forth conversations are crucial to support early social communication development, and are a precursor to more complex skills, such as verbal language,” he added.
Reassessment of the children at age 3 years showed a “small but enduring” benefit of the intervention, noted the authors. Children in the intervention group had a reduction in ASD symptom severity (P = .04), and reduced odds of ASD classification, compared with children receiving usual care (6.7% vs. 20.5%; odds ratio, 0.18; P = .02).
The findings provide “initial evidence of efficacy for a new clinical model that uses a specific developmentally focused intervention,” noted the authors. “The children falling below the diagnostic threshold still had developmental difficulties, but by working with each child’s unique differences, rather than trying to counter them, the therapy has effectively supported their development through the early childhood years,” noted Dr. Whitehouse in a statement.
Other research has shown benefits of new study approach
This is a “solid” study, “but, as acknowledged by the authors, the main effects are small in magnitude, and longer-term outcomes will be important to capture,” said Jessica Brian, PhD, C Psych, associate professor in the department of pediatrics at the University of Toronto, colead at the Autism Research Centre, and psychologist and clinician-investigator at Holland Bloorview Kids Rehab Hospital in Toronto.
Dr. Brian said she and her coauthors of a paper published in Autism Research and others have shown that the kind of approach used in the new study can be helpful for enhancing different areas of toddler development, but “the specific finding of reduced likelihood of a clinical ASD diagnosis is a bit different.”
The goal of reducing the likelihood of an ASD diagnosis “needs to be considered carefully, from the perspective of autism acceptance,” she added. “From an acceptance lens, the primary objective of early intervention in ASD might be better positioned as aiming to enhance or support a young child’s development, help them make developmental progress, build on their strengths, optimize outcomes, or reduce impairment. … I think the authors do a good job of balancing this perspective.”
New study shows value of parent-mediated interventions
Overall, Dr. Brian, who coauthored the Canadian Paediatric Society’s position statement on ASD diagnosis, lauded the findings as good news.
“It shows the value of using parent-mediated interventions, which are far less costly and are more resource-efficient than most therapist-delivered models.”
“In cases where parent-mediated approaches are made available to families prior to diagnosis, there is potential for strong effects, when the brain is most amenable to learning. Such models may also be an ideal fit before diagnosis, since they are less resource-intensive than therapist-delivered models, which may only be funded by governments once a diagnosis is confirmed,” she said.
“Finally, parent-mediated programs have the potential to support parents during what, for many families, is a particularly challenging time as they identify their child’s developmental differences or receive a diagnosis. Such programs have potential to increase parents’ confidence in parenting a young child with unique learning needs.”
What Dr. Brian thought was missing from the paper was acknowledgment that, “despite early developmental gains from parent-mediated interventions, it is likely that most children with ASD will need additional supports throughout development.”
This study was sponsored by the Telethon Kids Institute. Dr. Whitehouse reported no conflicts of interest. Dr. Brian codeveloped a parent-mediated intervention for toddlers with probable or confirmed ASD (the Social ABCs), for which she does not receive any royalties.
FROM JAMA PEDIATRICS










