User login
Novel trastuzumab duocarmazine significantly improved survival in advanced HER2-positive breast cancer
Based on significant progression-free survival (PFS) benefits shown in the phase 3 TULIP trial, trastuzumab duocarmazine (SYD985) may provide a new treatment option among HER2-positive metastatic breast cancer patients, according to Cristina Saura, MD, head of the breast cancer program at Vall d’Hebron University Hospital, Barcelona. Dr. Saura presented the results of the TULIP trial (abstract LBA15) on Sept. 19 at the 2021 European Society for Medical Oncology Congress.
Trastuzumab duocarmazine is a novel HER2-targeting antibody-drug conjugate that consists of trastuzumab and a drug containing duocarmycin. Its three-way mechanism of action includes uptake of the antibody-drug conjugate by internalization and intracellular release of duocarmycin with two bystander effects: proteolytic cleavage and subsequent release of payload in the tumor microenvironment and diffusion of active payload to neighboring tumor cells.
While one physician described the results as encouraging, another said the treatment is not nearly ready for primetime.
“It is encouraging to observe clinically meaningful and potentially practice changing progression-free survival improvements in patients receiving treatment in the third line and beyond,” said Aditya Bardia, MD, Massachusetts General Hospital, Boston. “Several agents have been approved as treatments for HER2-positive metastatic breast cancer in recent years including T-DXd, neratinib, tucatinib, and margetuximab. Trastuzumab duocarmazine could eventually be another option.”
Fatima Cardoso, MD, director of the breast cancer unit at the Breast Cancer Research Foundation, New York, said: “At this time there is only a minor 2-month difference in progression-free survival and a nonsignificant overall difference. With the high incidence of ocular toxicity and four toxic deaths, we cannot recommend this drug for clinical practice, in my opinion.”
Two or more prior therapies for metastatic breast cancer
TULIP investigators enrolled 437 patients from 83 sites in 11 countries with HER2-positive locally advanced or metastatic breast cancer who had received two or more therapies for metastatic disease (treatment for brain metastases allowed). They were randomized 2:1 to trastuzumab duocarmazine (1.2 mg/kg every 21 days, 291 patients) or physician’s choice (146 patients) of one of three trastuzumab-containing combinations or lapatinib plus capecitabine. Treatment was continued until progression or unacceptable toxicity. The primary endpoint was centrally assessed PFS.
Longer PFS with trastuzumab duocarmazine
Median age was 57 years, and the median number of prior metastatic breast cancer regimens was 4.7. Centrally reviewed PFS was significantly longer in the trastuzumab duocarmazine group at 7.0 months versus 4.9 months for physicians choice treatment (hazard ratio, 0.64; 95% confidence interval, 0.49-0.84; P = .002). Subgroup analysis, also centrally reviewed, revealed numerical advantage for trastuzumab duocarmazine over physician’s choice across all categories (except for Eastern Cooperative Oncology Group status 2). Analysis of PFS by investigators showed a similar benefit for trastuzumab duocarmazine (6.9 months vs. 4.6 months; HR, 0.60; P < .001).
A first look at median overall survival showed a nonsignificant advantage for trastuzumab duocarmazine (20.4 months vs. 16.3 months (HR, 0.83; 95% CI, 0.62-1.09, P = .153). The overall response rate (partial or complete response) was similar between groups at 27.8% for trastuzumab duocarmazine and 29.5% for physician’s choice with reductions in target lesion measurement at 70.2% and 32.2% for trastuzumab duocarmazine and physician’s choice, respectively. The clinical benefit rates were 38.5% for trastuzumab duocarmazine and 32.2% for physician’s choice.
Ocular toxicity
Most patients had at least one treatment-related adverse event (96.5% SD985, 96.4% PC), and grade 3 or higher event rates were similar between groups (52.8% SYD985, 48.2% PC). The most frequently reported adverse events for trastuzumab duocarmazine were ocular toxicity, with conjunctivitis reported in 38.2%, and keratitis in 38.2%, with fatigue at 33.3%; for physician’s choice these were diarrhea (35.8%), nausea (31.4%) and fatigue (29.9%). Interstitial lung disease pneumonitis was reported for 7.6% (5.2% grade 1-2) of patients treated with trastuzumab duocarmazine, including two grade 5 events.
Eye toxicity led to discontinuations in 20.8% of trastuzumab duocarmazine patients, dose modifications in 22.9%, with dose modifications for interstitial lung disease/pneumonitis in 5.2% of trastuzumab duocarmazine patients. Six fatalities (2.1%) were reported in the trastuzumab duocarmazine group, with four attributed to treatment. Assessment of health-related quality of life showed no significant difference between groups.
Dr. Manich outlined risk mitigation strategies. Patients with prior keratitis were excluded and patients were given prophylactic lubricating eye drops and regular eye exams by ophthalmologists. Treatment was discontinued if grade 3 or higher keratitis developed, and was delayed if grade 3 conjunctivitis developed until it reduced to grade 2. Also, patients with prior pneumonitis were excluded and CT lung scans were evaluated for lung changes. New or worsening respiratory symptoms triggered a full diagnostic workup. Treatment was discontinued for grade 2 or higher pneumonitis and delayed until resolution for grade 1 pneumonitis.
TULIP was funded by Byondis. Dr. Saura disclosed numerous financial interests including support from AstraZeneca and Daiichi Sankyo.
Based on significant progression-free survival (PFS) benefits shown in the phase 3 TULIP trial, trastuzumab duocarmazine (SYD985) may provide a new treatment option among HER2-positive metastatic breast cancer patients, according to Cristina Saura, MD, head of the breast cancer program at Vall d’Hebron University Hospital, Barcelona. Dr. Saura presented the results of the TULIP trial (abstract LBA15) on Sept. 19 at the 2021 European Society for Medical Oncology Congress.
Trastuzumab duocarmazine is a novel HER2-targeting antibody-drug conjugate that consists of trastuzumab and a drug containing duocarmycin. Its three-way mechanism of action includes uptake of the antibody-drug conjugate by internalization and intracellular release of duocarmycin with two bystander effects: proteolytic cleavage and subsequent release of payload in the tumor microenvironment and diffusion of active payload to neighboring tumor cells.
While one physician described the results as encouraging, another said the treatment is not nearly ready for primetime.
“It is encouraging to observe clinically meaningful and potentially practice changing progression-free survival improvements in patients receiving treatment in the third line and beyond,” said Aditya Bardia, MD, Massachusetts General Hospital, Boston. “Several agents have been approved as treatments for HER2-positive metastatic breast cancer in recent years including T-DXd, neratinib, tucatinib, and margetuximab. Trastuzumab duocarmazine could eventually be another option.”
Fatima Cardoso, MD, director of the breast cancer unit at the Breast Cancer Research Foundation, New York, said: “At this time there is only a minor 2-month difference in progression-free survival and a nonsignificant overall difference. With the high incidence of ocular toxicity and four toxic deaths, we cannot recommend this drug for clinical practice, in my opinion.”
Two or more prior therapies for metastatic breast cancer
TULIP investigators enrolled 437 patients from 83 sites in 11 countries with HER2-positive locally advanced or metastatic breast cancer who had received two or more therapies for metastatic disease (treatment for brain metastases allowed). They were randomized 2:1 to trastuzumab duocarmazine (1.2 mg/kg every 21 days, 291 patients) or physician’s choice (146 patients) of one of three trastuzumab-containing combinations or lapatinib plus capecitabine. Treatment was continued until progression or unacceptable toxicity. The primary endpoint was centrally assessed PFS.
Longer PFS with trastuzumab duocarmazine
Median age was 57 years, and the median number of prior metastatic breast cancer regimens was 4.7. Centrally reviewed PFS was significantly longer in the trastuzumab duocarmazine group at 7.0 months versus 4.9 months for physicians choice treatment (hazard ratio, 0.64; 95% confidence interval, 0.49-0.84; P = .002). Subgroup analysis, also centrally reviewed, revealed numerical advantage for trastuzumab duocarmazine over physician’s choice across all categories (except for Eastern Cooperative Oncology Group status 2). Analysis of PFS by investigators showed a similar benefit for trastuzumab duocarmazine (6.9 months vs. 4.6 months; HR, 0.60; P < .001).
A first look at median overall survival showed a nonsignificant advantage for trastuzumab duocarmazine (20.4 months vs. 16.3 months (HR, 0.83; 95% CI, 0.62-1.09, P = .153). The overall response rate (partial or complete response) was similar between groups at 27.8% for trastuzumab duocarmazine and 29.5% for physician’s choice with reductions in target lesion measurement at 70.2% and 32.2% for trastuzumab duocarmazine and physician’s choice, respectively. The clinical benefit rates were 38.5% for trastuzumab duocarmazine and 32.2% for physician’s choice.
Ocular toxicity
Most patients had at least one treatment-related adverse event (96.5% SD985, 96.4% PC), and grade 3 or higher event rates were similar between groups (52.8% SYD985, 48.2% PC). The most frequently reported adverse events for trastuzumab duocarmazine were ocular toxicity, with conjunctivitis reported in 38.2%, and keratitis in 38.2%, with fatigue at 33.3%; for physician’s choice these were diarrhea (35.8%), nausea (31.4%) and fatigue (29.9%). Interstitial lung disease pneumonitis was reported for 7.6% (5.2% grade 1-2) of patients treated with trastuzumab duocarmazine, including two grade 5 events.
Eye toxicity led to discontinuations in 20.8% of trastuzumab duocarmazine patients, dose modifications in 22.9%, with dose modifications for interstitial lung disease/pneumonitis in 5.2% of trastuzumab duocarmazine patients. Six fatalities (2.1%) were reported in the trastuzumab duocarmazine group, with four attributed to treatment. Assessment of health-related quality of life showed no significant difference between groups.
Dr. Manich outlined risk mitigation strategies. Patients with prior keratitis were excluded and patients were given prophylactic lubricating eye drops and regular eye exams by ophthalmologists. Treatment was discontinued if grade 3 or higher keratitis developed, and was delayed if grade 3 conjunctivitis developed until it reduced to grade 2. Also, patients with prior pneumonitis were excluded and CT lung scans were evaluated for lung changes. New or worsening respiratory symptoms triggered a full diagnostic workup. Treatment was discontinued for grade 2 or higher pneumonitis and delayed until resolution for grade 1 pneumonitis.
TULIP was funded by Byondis. Dr. Saura disclosed numerous financial interests including support from AstraZeneca and Daiichi Sankyo.
Based on significant progression-free survival (PFS) benefits shown in the phase 3 TULIP trial, trastuzumab duocarmazine (SYD985) may provide a new treatment option among HER2-positive metastatic breast cancer patients, according to Cristina Saura, MD, head of the breast cancer program at Vall d’Hebron University Hospital, Barcelona. Dr. Saura presented the results of the TULIP trial (abstract LBA15) on Sept. 19 at the 2021 European Society for Medical Oncology Congress.
Trastuzumab duocarmazine is a novel HER2-targeting antibody-drug conjugate that consists of trastuzumab and a drug containing duocarmycin. Its three-way mechanism of action includes uptake of the antibody-drug conjugate by internalization and intracellular release of duocarmycin with two bystander effects: proteolytic cleavage and subsequent release of payload in the tumor microenvironment and diffusion of active payload to neighboring tumor cells.
While one physician described the results as encouraging, another said the treatment is not nearly ready for primetime.
“It is encouraging to observe clinically meaningful and potentially practice changing progression-free survival improvements in patients receiving treatment in the third line and beyond,” said Aditya Bardia, MD, Massachusetts General Hospital, Boston. “Several agents have been approved as treatments for HER2-positive metastatic breast cancer in recent years including T-DXd, neratinib, tucatinib, and margetuximab. Trastuzumab duocarmazine could eventually be another option.”
Fatima Cardoso, MD, director of the breast cancer unit at the Breast Cancer Research Foundation, New York, said: “At this time there is only a minor 2-month difference in progression-free survival and a nonsignificant overall difference. With the high incidence of ocular toxicity and four toxic deaths, we cannot recommend this drug for clinical practice, in my opinion.”
Two or more prior therapies for metastatic breast cancer
TULIP investigators enrolled 437 patients from 83 sites in 11 countries with HER2-positive locally advanced or metastatic breast cancer who had received two or more therapies for metastatic disease (treatment for brain metastases allowed). They were randomized 2:1 to trastuzumab duocarmazine (1.2 mg/kg every 21 days, 291 patients) or physician’s choice (146 patients) of one of three trastuzumab-containing combinations or lapatinib plus capecitabine. Treatment was continued until progression or unacceptable toxicity. The primary endpoint was centrally assessed PFS.
Longer PFS with trastuzumab duocarmazine
Median age was 57 years, and the median number of prior metastatic breast cancer regimens was 4.7. Centrally reviewed PFS was significantly longer in the trastuzumab duocarmazine group at 7.0 months versus 4.9 months for physicians choice treatment (hazard ratio, 0.64; 95% confidence interval, 0.49-0.84; P = .002). Subgroup analysis, also centrally reviewed, revealed numerical advantage for trastuzumab duocarmazine over physician’s choice across all categories (except for Eastern Cooperative Oncology Group status 2). Analysis of PFS by investigators showed a similar benefit for trastuzumab duocarmazine (6.9 months vs. 4.6 months; HR, 0.60; P < .001).
A first look at median overall survival showed a nonsignificant advantage for trastuzumab duocarmazine (20.4 months vs. 16.3 months (HR, 0.83; 95% CI, 0.62-1.09, P = .153). The overall response rate (partial or complete response) was similar between groups at 27.8% for trastuzumab duocarmazine and 29.5% for physician’s choice with reductions in target lesion measurement at 70.2% and 32.2% for trastuzumab duocarmazine and physician’s choice, respectively. The clinical benefit rates were 38.5% for trastuzumab duocarmazine and 32.2% for physician’s choice.
Ocular toxicity
Most patients had at least one treatment-related adverse event (96.5% SD985, 96.4% PC), and grade 3 or higher event rates were similar between groups (52.8% SYD985, 48.2% PC). The most frequently reported adverse events for trastuzumab duocarmazine were ocular toxicity, with conjunctivitis reported in 38.2%, and keratitis in 38.2%, with fatigue at 33.3%; for physician’s choice these were diarrhea (35.8%), nausea (31.4%) and fatigue (29.9%). Interstitial lung disease pneumonitis was reported for 7.6% (5.2% grade 1-2) of patients treated with trastuzumab duocarmazine, including two grade 5 events.
Eye toxicity led to discontinuations in 20.8% of trastuzumab duocarmazine patients, dose modifications in 22.9%, with dose modifications for interstitial lung disease/pneumonitis in 5.2% of trastuzumab duocarmazine patients. Six fatalities (2.1%) were reported in the trastuzumab duocarmazine group, with four attributed to treatment. Assessment of health-related quality of life showed no significant difference between groups.
Dr. Manich outlined risk mitigation strategies. Patients with prior keratitis were excluded and patients were given prophylactic lubricating eye drops and regular eye exams by ophthalmologists. Treatment was discontinued if grade 3 or higher keratitis developed, and was delayed if grade 3 conjunctivitis developed until it reduced to grade 2. Also, patients with prior pneumonitis were excluded and CT lung scans were evaluated for lung changes. New or worsening respiratory symptoms triggered a full diagnostic workup. Treatment was discontinued for grade 2 or higher pneumonitis and delayed until resolution for grade 1 pneumonitis.
TULIP was funded by Byondis. Dr. Saura disclosed numerous financial interests including support from AstraZeneca and Daiichi Sankyo.
FROM ESMO 2021
Worsening motor function tied to post COVID syndrome in Parkinson’s disease
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Pruritic blistering rash
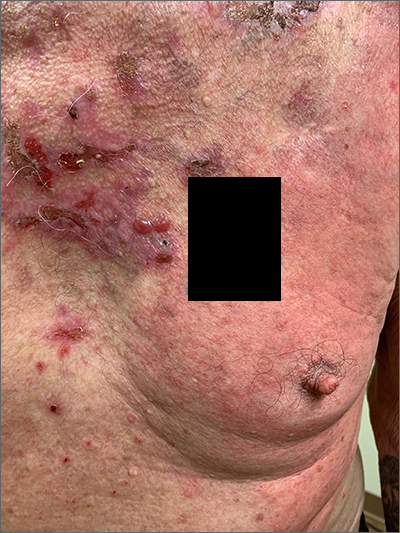
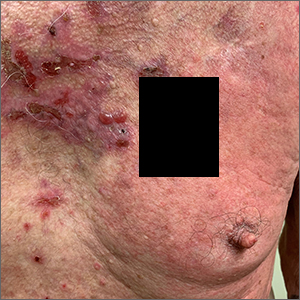
This patient was given a diagnosis of linear IgA bullous dermatosis (LABD) based on biopsy results. (Biopsy is extremely helpful in differentiating bullous disorders.) A shave biopsy of 1 of the mid-chest 3-mm vesicles and a perilesional punch biopsy sent in Michel media showed linear deposition of IgA and IgG along the basement membrane zone and subepidermal blister with neutrophils and eosinophils. The IgA appeared stronger in the direct immunofluorescence study and there were numerous neutrophils on histology, which confirmed the diagnosis.
LABD is one of the less common blistering disorders. It has a bimodal distribution occurring mostly in adults around 60 years of age and a lower peak incidence in young children.1 It often manifests with an acute onset of tense vesicles and bullae. The lesions can be extremely pruritic and can appear on mucous membranes, normal skin, or inflamed skin. Lesion formation is often sudden and manifests in clusters with an erythematous base on the trunk, extensor extremities, buttocks, and face—especially the area in and around the mouth.1
LABD is diagnosed by linear deposits of IgA at the dermo-epidermal interface by direct immunofluorescence. The mechanism for lesion formation is still not well known. It can occur spontaneously or can be drug-induced. In many individuals with LABD (such as this one), the precipitating event for the disease is unknown.
It is important to differentiate LABD from other blistering diseases that can also affect the oral mucosa. Bullous pemphigoid has tense vesicles, as well, but often has a prodromal phase before lesions appear in a nonclustered pattern on the skin.2 Pemphigus vulgaris, which is also in the differential, is characterized by soft blisters and almost always includes the oral mucosa, which is usually where lesions first develop.
Dapsone is first-line therapy. However, due to the risk of hemolysis with dapsone treatment in patients with glucose-6-phosphate dehydrogenase (G6DP) deficiency, the physician confirmed that the patient had normal levels of G6DP before starting the patient on dapsone 25 mg/d po. After starting dapsone, the patient reported unexplained syncopal episodes and falls and stopped the medication. (This was not an anticipated adverse effect.) The patient was then started on colchicine 0.6 mg orally tid. (Other second-line therapies include sulfapyridine and sulfamethoxypyridazine.1) Follow-up in 1 month was scheduled.
Image courtesy Daniel Stulberg, MD. Text courtesy of Riley Diehl, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy Daniel Stulberg, MD.
1. Hall III RP, Rao C. Linear IgA bullous dermatosis. Uptodate. Updated September 24, 2020. Accessed September 26, 2021. https://www.uptodate.com/contents/linear-iga-bullous-dermatosis#!
2. Leiferman K. Clinical features and diagnosis of bullous pemphigoid and mucous membrane pemphigoid. Uptodate. Updated June 30, 2021. Accessed September, 2021. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-bullous-pemphigoid-and-mucous-membrane-pemphigoid#!

This patient was given a diagnosis of linear IgA bullous dermatosis (LABD) based on biopsy results. (Biopsy is extremely helpful in differentiating bullous disorders.) A shave biopsy of 1 of the mid-chest 3-mm vesicles and a perilesional punch biopsy sent in Michel media showed linear deposition of IgA and IgG along the basement membrane zone and subepidermal blister with neutrophils and eosinophils. The IgA appeared stronger in the direct immunofluorescence study and there were numerous neutrophils on histology, which confirmed the diagnosis.
LABD is one of the less common blistering disorders. It has a bimodal distribution occurring mostly in adults around 60 years of age and a lower peak incidence in young children.1 It often manifests with an acute onset of tense vesicles and bullae. The lesions can be extremely pruritic and can appear on mucous membranes, normal skin, or inflamed skin. Lesion formation is often sudden and manifests in clusters with an erythematous base on the trunk, extensor extremities, buttocks, and face—especially the area in and around the mouth.1
LABD is diagnosed by linear deposits of IgA at the dermo-epidermal interface by direct immunofluorescence. The mechanism for lesion formation is still not well known. It can occur spontaneously or can be drug-induced. In many individuals with LABD (such as this one), the precipitating event for the disease is unknown.
It is important to differentiate LABD from other blistering diseases that can also affect the oral mucosa. Bullous pemphigoid has tense vesicles, as well, but often has a prodromal phase before lesions appear in a nonclustered pattern on the skin.2 Pemphigus vulgaris, which is also in the differential, is characterized by soft blisters and almost always includes the oral mucosa, which is usually where lesions first develop.
Dapsone is first-line therapy. However, due to the risk of hemolysis with dapsone treatment in patients with glucose-6-phosphate dehydrogenase (G6DP) deficiency, the physician confirmed that the patient had normal levels of G6DP before starting the patient on dapsone 25 mg/d po. After starting dapsone, the patient reported unexplained syncopal episodes and falls and stopped the medication. (This was not an anticipated adverse effect.) The patient was then started on colchicine 0.6 mg orally tid. (Other second-line therapies include sulfapyridine and sulfamethoxypyridazine.1) Follow-up in 1 month was scheduled.
Image courtesy Daniel Stulberg, MD. Text courtesy of Riley Diehl, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy Daniel Stulberg, MD.

This patient was given a diagnosis of linear IgA bullous dermatosis (LABD) based on biopsy results. (Biopsy is extremely helpful in differentiating bullous disorders.) A shave biopsy of 1 of the mid-chest 3-mm vesicles and a perilesional punch biopsy sent in Michel media showed linear deposition of IgA and IgG along the basement membrane zone and subepidermal blister with neutrophils and eosinophils. The IgA appeared stronger in the direct immunofluorescence study and there were numerous neutrophils on histology, which confirmed the diagnosis.
LABD is one of the less common blistering disorders. It has a bimodal distribution occurring mostly in adults around 60 years of age and a lower peak incidence in young children.1 It often manifests with an acute onset of tense vesicles and bullae. The lesions can be extremely pruritic and can appear on mucous membranes, normal skin, or inflamed skin. Lesion formation is often sudden and manifests in clusters with an erythematous base on the trunk, extensor extremities, buttocks, and face—especially the area in and around the mouth.1
LABD is diagnosed by linear deposits of IgA at the dermo-epidermal interface by direct immunofluorescence. The mechanism for lesion formation is still not well known. It can occur spontaneously or can be drug-induced. In many individuals with LABD (such as this one), the precipitating event for the disease is unknown.
It is important to differentiate LABD from other blistering diseases that can also affect the oral mucosa. Bullous pemphigoid has tense vesicles, as well, but often has a prodromal phase before lesions appear in a nonclustered pattern on the skin.2 Pemphigus vulgaris, which is also in the differential, is characterized by soft blisters and almost always includes the oral mucosa, which is usually where lesions first develop.
Dapsone is first-line therapy. However, due to the risk of hemolysis with dapsone treatment in patients with glucose-6-phosphate dehydrogenase (G6DP) deficiency, the physician confirmed that the patient had normal levels of G6DP before starting the patient on dapsone 25 mg/d po. After starting dapsone, the patient reported unexplained syncopal episodes and falls and stopped the medication. (This was not an anticipated adverse effect.) The patient was then started on colchicine 0.6 mg orally tid. (Other second-line therapies include sulfapyridine and sulfamethoxypyridazine.1) Follow-up in 1 month was scheduled.
Image courtesy Daniel Stulberg, MD. Text courtesy of Riley Diehl, MD, Department of Internal Medicine, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque. Photo courtesy Daniel Stulberg, MD.
1. Hall III RP, Rao C. Linear IgA bullous dermatosis. Uptodate. Updated September 24, 2020. Accessed September 26, 2021. https://www.uptodate.com/contents/linear-iga-bullous-dermatosis#!
2. Leiferman K. Clinical features and diagnosis of bullous pemphigoid and mucous membrane pemphigoid. Uptodate. Updated June 30, 2021. Accessed September, 2021. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-bullous-pemphigoid-and-mucous-membrane-pemphigoid#!
1. Hall III RP, Rao C. Linear IgA bullous dermatosis. Uptodate. Updated September 24, 2020. Accessed September 26, 2021. https://www.uptodate.com/contents/linear-iga-bullous-dermatosis#!
2. Leiferman K. Clinical features and diagnosis of bullous pemphigoid and mucous membrane pemphigoid. Uptodate. Updated June 30, 2021. Accessed September, 2021. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-bullous-pemphigoid-and-mucous-membrane-pemphigoid#!
Clinical Edge Journal Scan Commentary: Prostate Cancer October 2021
Radiation has long been a part of treatment for localized prostate cancer. However, radiation and radiation-delivery modalities continue to evolve to decrease toxicity without sacrificing efficacy, expand the roles of these modalities, and enhance efficacy of other concomitantly delivered treatment modalities. The accompanying studies describe efforts to those effects.
Hypofractionated delivery of radiation is now often considered preferred over conventionally fractionated radiation for localized prostate cancer to maintain efficacy while decreasing toxicity. To determine if the benefit of lower toxicity holds over time, Staffurth et al assessed patient-reported outcomes (PROs) in participants of the CHHiP trial where men were randomized to conventional radiation versus 2 alternative hypofraction schedules. At 5 years post-radiation, there were no significant differences amongst the 3 groups with respect to bowel bother, urinary bother, or sexual bother.
Evidence is emerging that stereotactic body radiation therapy (SBRT) has benefits for oligometastatic disease; however, studies are emerging that support the hypothesis that radiation may enhance immunotherapy in many cancers in the metastatic setting. Kwan et al evaluated the effects of a single fraction of SBRT administered to 1 or 2 disease sites before the first and second doses of avelumab (a PD-L1 antibody) in patients with metastatic castrate-resistant prostate cancer (with prior exposure to at least one second-generation androgen receptor inhibitor). In this single arm phase II study, the disease control rate was 48%, overall response rate was 31%, median radiographic progression-free survival (rPFS) was 8.4 months, and median overall survival (OS) was 14.1 months in this heavily pretreated group of 31 participants. As immunotherapy has been considered efficacious in select groups of prostate cancer (such as tumors with microsatellite instability), this study supports ongoing and new efforts to evaluate how to enhance immunotherapy against prostate cancer.
Radium-223 has been demonstrated to have efficacy in metastatic disease of the bone. Ongoing efforts to determine whether combined modality treatment in this setting may improve outcomes in metastatic disease, Maughn et al evaluated the combination of radium-223 and enzalutamide for safety and efficacy in a small and hypothesis-generating study 47 participants where 35 received enzalutamide plus radium-223 and 12 received enzlutamide alone. Of note, there were no increase in fractures (45 of the 47 participants received bone protective therapy, however), but there were no differences in the secondary endpoints of PSA-PFS, rPFS, or OS (primary endpoints of decline in bone metabolism markers were previously reported). This is a hypothesis generating study that supports the likely safety of this modality if bone protecting agents are utilized.
The 3 studies summarized here represent the ongoing evolution of radiation or radiation-delivery modalities in localized and metastatic prostate cancer. It is encouraging to see the ongoing evaluate of PROs over time, as long-term quality of life in patients potentially cured of disease is of utmost importance. In addition, efforts to evaluate radiation or radium-223 in widely metastatic disease as part of combination therapy may reveal not only situations with modest benefits in efficacy, but more importantly, may reveal molecular insights into newer treatment strategies.
Radiation has long been a part of treatment for localized prostate cancer. However, radiation and radiation-delivery modalities continue to evolve to decrease toxicity without sacrificing efficacy, expand the roles of these modalities, and enhance efficacy of other concomitantly delivered treatment modalities. The accompanying studies describe efforts to those effects.
Hypofractionated delivery of radiation is now often considered preferred over conventionally fractionated radiation for localized prostate cancer to maintain efficacy while decreasing toxicity. To determine if the benefit of lower toxicity holds over time, Staffurth et al assessed patient-reported outcomes (PROs) in participants of the CHHiP trial where men were randomized to conventional radiation versus 2 alternative hypofraction schedules. At 5 years post-radiation, there were no significant differences amongst the 3 groups with respect to bowel bother, urinary bother, or sexual bother.
Evidence is emerging that stereotactic body radiation therapy (SBRT) has benefits for oligometastatic disease; however, studies are emerging that support the hypothesis that radiation may enhance immunotherapy in many cancers in the metastatic setting. Kwan et al evaluated the effects of a single fraction of SBRT administered to 1 or 2 disease sites before the first and second doses of avelumab (a PD-L1 antibody) in patients with metastatic castrate-resistant prostate cancer (with prior exposure to at least one second-generation androgen receptor inhibitor). In this single arm phase II study, the disease control rate was 48%, overall response rate was 31%, median radiographic progression-free survival (rPFS) was 8.4 months, and median overall survival (OS) was 14.1 months in this heavily pretreated group of 31 participants. As immunotherapy has been considered efficacious in select groups of prostate cancer (such as tumors with microsatellite instability), this study supports ongoing and new efforts to evaluate how to enhance immunotherapy against prostate cancer.
Radium-223 has been demonstrated to have efficacy in metastatic disease of the bone. Ongoing efforts to determine whether combined modality treatment in this setting may improve outcomes in metastatic disease, Maughn et al evaluated the combination of radium-223 and enzalutamide for safety and efficacy in a small and hypothesis-generating study 47 participants where 35 received enzalutamide plus radium-223 and 12 received enzlutamide alone. Of note, there were no increase in fractures (45 of the 47 participants received bone protective therapy, however), but there were no differences in the secondary endpoints of PSA-PFS, rPFS, or OS (primary endpoints of decline in bone metabolism markers were previously reported). This is a hypothesis generating study that supports the likely safety of this modality if bone protecting agents are utilized.
The 3 studies summarized here represent the ongoing evolution of radiation or radiation-delivery modalities in localized and metastatic prostate cancer. It is encouraging to see the ongoing evaluate of PROs over time, as long-term quality of life in patients potentially cured of disease is of utmost importance. In addition, efforts to evaluate radiation or radium-223 in widely metastatic disease as part of combination therapy may reveal not only situations with modest benefits in efficacy, but more importantly, may reveal molecular insights into newer treatment strategies.
Radiation has long been a part of treatment for localized prostate cancer. However, radiation and radiation-delivery modalities continue to evolve to decrease toxicity without sacrificing efficacy, expand the roles of these modalities, and enhance efficacy of other concomitantly delivered treatment modalities. The accompanying studies describe efforts to those effects.
Hypofractionated delivery of radiation is now often considered preferred over conventionally fractionated radiation for localized prostate cancer to maintain efficacy while decreasing toxicity. To determine if the benefit of lower toxicity holds over time, Staffurth et al assessed patient-reported outcomes (PROs) in participants of the CHHiP trial where men were randomized to conventional radiation versus 2 alternative hypofraction schedules. At 5 years post-radiation, there were no significant differences amongst the 3 groups with respect to bowel bother, urinary bother, or sexual bother.
Evidence is emerging that stereotactic body radiation therapy (SBRT) has benefits for oligometastatic disease; however, studies are emerging that support the hypothesis that radiation may enhance immunotherapy in many cancers in the metastatic setting. Kwan et al evaluated the effects of a single fraction of SBRT administered to 1 or 2 disease sites before the first and second doses of avelumab (a PD-L1 antibody) in patients with metastatic castrate-resistant prostate cancer (with prior exposure to at least one second-generation androgen receptor inhibitor). In this single arm phase II study, the disease control rate was 48%, overall response rate was 31%, median radiographic progression-free survival (rPFS) was 8.4 months, and median overall survival (OS) was 14.1 months in this heavily pretreated group of 31 participants. As immunotherapy has been considered efficacious in select groups of prostate cancer (such as tumors with microsatellite instability), this study supports ongoing and new efforts to evaluate how to enhance immunotherapy against prostate cancer.
Radium-223 has been demonstrated to have efficacy in metastatic disease of the bone. Ongoing efforts to determine whether combined modality treatment in this setting may improve outcomes in metastatic disease, Maughn et al evaluated the combination of radium-223 and enzalutamide for safety and efficacy in a small and hypothesis-generating study 47 participants where 35 received enzalutamide plus radium-223 and 12 received enzlutamide alone. Of note, there were no increase in fractures (45 of the 47 participants received bone protective therapy, however), but there were no differences in the secondary endpoints of PSA-PFS, rPFS, or OS (primary endpoints of decline in bone metabolism markers were previously reported). This is a hypothesis generating study that supports the likely safety of this modality if bone protecting agents are utilized.
The 3 studies summarized here represent the ongoing evolution of radiation or radiation-delivery modalities in localized and metastatic prostate cancer. It is encouraging to see the ongoing evaluate of PROs over time, as long-term quality of life in patients potentially cured of disease is of utmost importance. In addition, efforts to evaluate radiation or radium-223 in widely metastatic disease as part of combination therapy may reveal not only situations with modest benefits in efficacy, but more importantly, may reveal molecular insights into newer treatment strategies.
Clinical Edge Journal Scan Commentary: HCC October 2021
Treatment of patients with hepatocellular carcinoma (HCC) requires a multidisciplinary approach. This month we will review articles that analyze outcomes after liver resection compared to percutaneous ablation, prediction of immunotherapy efficacy based on observed treatment-related side effects, and the risk of subsequent malignancies in patients with HCC.
Xie et al. reported their retrospective review of outcomes of 67 adults with resectable caudate HCC within Milan criteria. Out of these, 46 underwent hepatic resection and 21 underwent percutaneous ablation. Overall survival at 1, 3, and 5 years was 97.6%, 83.6%, and 71.5%, respectively, for the hepatic resection patients, vs 89.4%, 58.5%, and 48.8%, respectively, for the percutaneous ablation patients (P = 0.032). Recurrence-free survival at these time points was 77.6%, 47.9%, and 42.6%, respectively, for the hepatic resection group, and 40.5%, 23.2%, and 15.4%, respectively, for the percutaneous ablation group (P = 0.010). The investigators concluded that HCC patients who underwent hepatic resection had significantly higher rates of recurrence-free and overall survival compared to those who underwent percutaneous ablation.
For those patients with unresectable HCC, systemic immune checkpoint inhibitor therapy is now part of the standard of care. Treatment-associated adverse events occur in over half of patients treated with immunotherapy. Pinato et al. reviewed the outcomes of a cohort of 406 adults with unresectable or advanced HCC who were receiving immune checkpoint inhibitor therapy while enrolled in clinical trials that were submitted to the Food and Drug Administration. the development of adverse events was associated with longer overall survival and progression-free survival rates compared to patients who did not develop treatment-related adverse events (16.7 months vs 11.2 months; 5.5 months vs 2.2 months, respectively). The authors concluded that the development of treatment-related adverse events was significantly correlated with improve overall and progression-free survival in HCC patients treated with ICI monotherapy in clinical trials.
Finally, Kong et al. retrospectively looked at a cohort of 40,314 adult patients diagnosed with HCC between 2000 and 2014 in the SEER database, identifying the incidence of second primary cancers. Overall, the patients were followed for a median of 19 months following their HCC diagnosis. A total of 1,593 HCC patients (3.95%) developed secondary primary malignancies starting at 2 months after their initial HCC diagnosis. The 3-, 5-, and 10-year cumulative incidence of developing second primary malignancies were 2.35%, 3.12%, and 4.51%. The top five sites of the second primary malignancies were lung and bronchus, prostate, non-Hodgkin lymphoma, colon, and breast. The patients with poorer tumor-related characteristics such as larger tumor size, vascular invasion, positive AFP level, poorer tumor grade, and distant extension were associated with a decreased risk of developing second primary cancers, most probably because of their higher risk of dying from HCC. The authors developed a competing-risk nomogram for the purpose of improving guideline surveillance and further management of HCC survivors, concluding that HCC survivors should be monitored for evidence of secondary primary cancers.
Treatment of patients with hepatocellular carcinoma (HCC) requires a multidisciplinary approach. This month we will review articles that analyze outcomes after liver resection compared to percutaneous ablation, prediction of immunotherapy efficacy based on observed treatment-related side effects, and the risk of subsequent malignancies in patients with HCC.
Xie et al. reported their retrospective review of outcomes of 67 adults with resectable caudate HCC within Milan criteria. Out of these, 46 underwent hepatic resection and 21 underwent percutaneous ablation. Overall survival at 1, 3, and 5 years was 97.6%, 83.6%, and 71.5%, respectively, for the hepatic resection patients, vs 89.4%, 58.5%, and 48.8%, respectively, for the percutaneous ablation patients (P = 0.032). Recurrence-free survival at these time points was 77.6%, 47.9%, and 42.6%, respectively, for the hepatic resection group, and 40.5%, 23.2%, and 15.4%, respectively, for the percutaneous ablation group (P = 0.010). The investigators concluded that HCC patients who underwent hepatic resection had significantly higher rates of recurrence-free and overall survival compared to those who underwent percutaneous ablation.
For those patients with unresectable HCC, systemic immune checkpoint inhibitor therapy is now part of the standard of care. Treatment-associated adverse events occur in over half of patients treated with immunotherapy. Pinato et al. reviewed the outcomes of a cohort of 406 adults with unresectable or advanced HCC who were receiving immune checkpoint inhibitor therapy while enrolled in clinical trials that were submitted to the Food and Drug Administration. the development of adverse events was associated with longer overall survival and progression-free survival rates compared to patients who did not develop treatment-related adverse events (16.7 months vs 11.2 months; 5.5 months vs 2.2 months, respectively). The authors concluded that the development of treatment-related adverse events was significantly correlated with improve overall and progression-free survival in HCC patients treated with ICI monotherapy in clinical trials.
Finally, Kong et al. retrospectively looked at a cohort of 40,314 adult patients diagnosed with HCC between 2000 and 2014 in the SEER database, identifying the incidence of second primary cancers. Overall, the patients were followed for a median of 19 months following their HCC diagnosis. A total of 1,593 HCC patients (3.95%) developed secondary primary malignancies starting at 2 months after their initial HCC diagnosis. The 3-, 5-, and 10-year cumulative incidence of developing second primary malignancies were 2.35%, 3.12%, and 4.51%. The top five sites of the second primary malignancies were lung and bronchus, prostate, non-Hodgkin lymphoma, colon, and breast. The patients with poorer tumor-related characteristics such as larger tumor size, vascular invasion, positive AFP level, poorer tumor grade, and distant extension were associated with a decreased risk of developing second primary cancers, most probably because of their higher risk of dying from HCC. The authors developed a competing-risk nomogram for the purpose of improving guideline surveillance and further management of HCC survivors, concluding that HCC survivors should be monitored for evidence of secondary primary cancers.
Treatment of patients with hepatocellular carcinoma (HCC) requires a multidisciplinary approach. This month we will review articles that analyze outcomes after liver resection compared to percutaneous ablation, prediction of immunotherapy efficacy based on observed treatment-related side effects, and the risk of subsequent malignancies in patients with HCC.
Xie et al. reported their retrospective review of outcomes of 67 adults with resectable caudate HCC within Milan criteria. Out of these, 46 underwent hepatic resection and 21 underwent percutaneous ablation. Overall survival at 1, 3, and 5 years was 97.6%, 83.6%, and 71.5%, respectively, for the hepatic resection patients, vs 89.4%, 58.5%, and 48.8%, respectively, for the percutaneous ablation patients (P = 0.032). Recurrence-free survival at these time points was 77.6%, 47.9%, and 42.6%, respectively, for the hepatic resection group, and 40.5%, 23.2%, and 15.4%, respectively, for the percutaneous ablation group (P = 0.010). The investigators concluded that HCC patients who underwent hepatic resection had significantly higher rates of recurrence-free and overall survival compared to those who underwent percutaneous ablation.
For those patients with unresectable HCC, systemic immune checkpoint inhibitor therapy is now part of the standard of care. Treatment-associated adverse events occur in over half of patients treated with immunotherapy. Pinato et al. reviewed the outcomes of a cohort of 406 adults with unresectable or advanced HCC who were receiving immune checkpoint inhibitor therapy while enrolled in clinical trials that were submitted to the Food and Drug Administration. the development of adverse events was associated with longer overall survival and progression-free survival rates compared to patients who did not develop treatment-related adverse events (16.7 months vs 11.2 months; 5.5 months vs 2.2 months, respectively). The authors concluded that the development of treatment-related adverse events was significantly correlated with improve overall and progression-free survival in HCC patients treated with ICI monotherapy in clinical trials.
Finally, Kong et al. retrospectively looked at a cohort of 40,314 adult patients diagnosed with HCC between 2000 and 2014 in the SEER database, identifying the incidence of second primary cancers. Overall, the patients were followed for a median of 19 months following their HCC diagnosis. A total of 1,593 HCC patients (3.95%) developed secondary primary malignancies starting at 2 months after their initial HCC diagnosis. The 3-, 5-, and 10-year cumulative incidence of developing second primary malignancies were 2.35%, 3.12%, and 4.51%. The top five sites of the second primary malignancies were lung and bronchus, prostate, non-Hodgkin lymphoma, colon, and breast. The patients with poorer tumor-related characteristics such as larger tumor size, vascular invasion, positive AFP level, poorer tumor grade, and distant extension were associated with a decreased risk of developing second primary cancers, most probably because of their higher risk of dying from HCC. The authors developed a competing-risk nomogram for the purpose of improving guideline surveillance and further management of HCC survivors, concluding that HCC survivors should be monitored for evidence of secondary primary cancers.
COVID-19: Greater mortality among psych patients remains a mystery
Antipsychotics are not responsible for the increased COVID-related death rate among patients with serious mental illness (SMI), new research shows.
The significant increase in COVID-19 mortality that continues to be reported among those with schizophrenia and schizoaffective disorder “underscores the importance of protective interventions for this group, including priority vaccination,” study investigator Katlyn Nemani, MD, research assistant professor, department of psychiatry, New York University, told this news organization.
The study was published online September 22 in JAMA Psychiatry.
Threefold increase in death
Previous research has linked a diagnosis of a schizophrenia spectrum disorder, which includes schizophrenia and schizoaffective disorder, to an almost threefold increase in mortality among patients with COVID-19.
Some population-based research has also reported a link between antipsychotic medication use and increased risk for COVID-related mortality, but these studies did not take psychiatric diagnoses into account.
“This raised the question of whether the increased risk observed in this population is related to underlying psychiatric illness or its treatment,” said Dr. Nemani.
The retrospective cohort study included 464 adults (mean age, 53 years) who were diagnosed with COVID-19 between March 3, 2020, and Feb. 17, 2021, and who had previously been diagnosed with schizophrenia spectrum disorder or bipolar disorder. Of these, 42.2% were treated with an antipsychotic medication.
The primary endpoint was death within 60 days of COVID-19 diagnosis. Covariates included sociodemographic characteristics, such as patient-reported race and ethnicity, age, and insurance type, a psychiatric diagnosis, medical comorbidities, and smoking status.
Of the total, 41 patients (8.8%) died. The 60-day fatality rate was 13.7% among patients with a schizophrenia spectrum disorder (n = 182) and 5.7% among patients with bipolar disorder (n = 282).
Antipsychotic treatment was not significantly associated with mortality (odds ratio, 1.00; 95% confidence interval, 0.48-2.08; P = .99).
“This suggests that antipsychotic medication is unlikely to be responsible for the increased risk we’ve observed in this population, although this finding needs to be replicated,” said Dr. Nemani.
Surprise finding
A diagnosis of a schizophrenia spectrum disorder was associated with an almost threefold increased risk for mortality compared with bipolar disorder (OR, 2.88; 95% CI, 1.36-6.11; P = .006).
“This was a surprising finding,” said Dr. Nemani.
She noted that there is evidence suggesting the immune system may play a role in the pathogenesis of schizophrenia, and research has shown that pneumonia and infection are among the leading causes of premature mortality in this population.
As well, several potential risk factors disproportionately affect people with serious mental illness, including an increase in the prevalence of medical comorbidities such as cardiovascular disease and diabetes, socioeconomic disadvantages, and barriers to accessing timely care. Prior studies have also found that people with SMI are less likely to receive preventive care interventions, including vaccination, said Dr. Nemani.
However, these factors are unlikely to fully account for the increased risk found in the study, she said.
“Our study population was limited to people who had received treatment within the NYU Langone Health System. We took a comprehensive list of sociodemographic and medical risk factors into account, and our research was conducted prior to the availability of COVID-19 vaccines,” she said.
Further research is necessary to understand what underlies the increase in susceptibility to severe infection among patients with schizophrenia and to identify interventions that may mitigate risk, said Dr. Nemani.
“This includes evaluating systems-level factors, such as access to preventive interventions and treatment, as well as investigating underlying immune mechanisms that may contribute to severe and fatal infection,” she said.
The researchers could not validate psychiatric diagnoses or capture deaths not documented in the electronic health record. In addition, the limited sample size precluded analysis of the use of individual antipsychotic medications, which may differ in their associated effects.
“It’s possible individual antipsychotic medications may be associated with harmful or protective effects,” said Dr. Nemani.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antipsychotics are not responsible for the increased COVID-related death rate among patients with serious mental illness (SMI), new research shows.
The significant increase in COVID-19 mortality that continues to be reported among those with schizophrenia and schizoaffective disorder “underscores the importance of protective interventions for this group, including priority vaccination,” study investigator Katlyn Nemani, MD, research assistant professor, department of psychiatry, New York University, told this news organization.
The study was published online September 22 in JAMA Psychiatry.
Threefold increase in death
Previous research has linked a diagnosis of a schizophrenia spectrum disorder, which includes schizophrenia and schizoaffective disorder, to an almost threefold increase in mortality among patients with COVID-19.
Some population-based research has also reported a link between antipsychotic medication use and increased risk for COVID-related mortality, but these studies did not take psychiatric diagnoses into account.
“This raised the question of whether the increased risk observed in this population is related to underlying psychiatric illness or its treatment,” said Dr. Nemani.
The retrospective cohort study included 464 adults (mean age, 53 years) who were diagnosed with COVID-19 between March 3, 2020, and Feb. 17, 2021, and who had previously been diagnosed with schizophrenia spectrum disorder or bipolar disorder. Of these, 42.2% were treated with an antipsychotic medication.
The primary endpoint was death within 60 days of COVID-19 diagnosis. Covariates included sociodemographic characteristics, such as patient-reported race and ethnicity, age, and insurance type, a psychiatric diagnosis, medical comorbidities, and smoking status.
Of the total, 41 patients (8.8%) died. The 60-day fatality rate was 13.7% among patients with a schizophrenia spectrum disorder (n = 182) and 5.7% among patients with bipolar disorder (n = 282).
Antipsychotic treatment was not significantly associated with mortality (odds ratio, 1.00; 95% confidence interval, 0.48-2.08; P = .99).
“This suggests that antipsychotic medication is unlikely to be responsible for the increased risk we’ve observed in this population, although this finding needs to be replicated,” said Dr. Nemani.
Surprise finding
A diagnosis of a schizophrenia spectrum disorder was associated with an almost threefold increased risk for mortality compared with bipolar disorder (OR, 2.88; 95% CI, 1.36-6.11; P = .006).
“This was a surprising finding,” said Dr. Nemani.
She noted that there is evidence suggesting the immune system may play a role in the pathogenesis of schizophrenia, and research has shown that pneumonia and infection are among the leading causes of premature mortality in this population.
As well, several potential risk factors disproportionately affect people with serious mental illness, including an increase in the prevalence of medical comorbidities such as cardiovascular disease and diabetes, socioeconomic disadvantages, and barriers to accessing timely care. Prior studies have also found that people with SMI are less likely to receive preventive care interventions, including vaccination, said Dr. Nemani.
However, these factors are unlikely to fully account for the increased risk found in the study, she said.
“Our study population was limited to people who had received treatment within the NYU Langone Health System. We took a comprehensive list of sociodemographic and medical risk factors into account, and our research was conducted prior to the availability of COVID-19 vaccines,” she said.
Further research is necessary to understand what underlies the increase in susceptibility to severe infection among patients with schizophrenia and to identify interventions that may mitigate risk, said Dr. Nemani.
“This includes evaluating systems-level factors, such as access to preventive interventions and treatment, as well as investigating underlying immune mechanisms that may contribute to severe and fatal infection,” she said.
The researchers could not validate psychiatric diagnoses or capture deaths not documented in the electronic health record. In addition, the limited sample size precluded analysis of the use of individual antipsychotic medications, which may differ in their associated effects.
“It’s possible individual antipsychotic medications may be associated with harmful or protective effects,” said Dr. Nemani.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antipsychotics are not responsible for the increased COVID-related death rate among patients with serious mental illness (SMI), new research shows.
The significant increase in COVID-19 mortality that continues to be reported among those with schizophrenia and schizoaffective disorder “underscores the importance of protective interventions for this group, including priority vaccination,” study investigator Katlyn Nemani, MD, research assistant professor, department of psychiatry, New York University, told this news organization.
The study was published online September 22 in JAMA Psychiatry.
Threefold increase in death
Previous research has linked a diagnosis of a schizophrenia spectrum disorder, which includes schizophrenia and schizoaffective disorder, to an almost threefold increase in mortality among patients with COVID-19.
Some population-based research has also reported a link between antipsychotic medication use and increased risk for COVID-related mortality, but these studies did not take psychiatric diagnoses into account.
“This raised the question of whether the increased risk observed in this population is related to underlying psychiatric illness or its treatment,” said Dr. Nemani.
The retrospective cohort study included 464 adults (mean age, 53 years) who were diagnosed with COVID-19 between March 3, 2020, and Feb. 17, 2021, and who had previously been diagnosed with schizophrenia spectrum disorder or bipolar disorder. Of these, 42.2% were treated with an antipsychotic medication.
The primary endpoint was death within 60 days of COVID-19 diagnosis. Covariates included sociodemographic characteristics, such as patient-reported race and ethnicity, age, and insurance type, a psychiatric diagnosis, medical comorbidities, and smoking status.
Of the total, 41 patients (8.8%) died. The 60-day fatality rate was 13.7% among patients with a schizophrenia spectrum disorder (n = 182) and 5.7% among patients with bipolar disorder (n = 282).
Antipsychotic treatment was not significantly associated with mortality (odds ratio, 1.00; 95% confidence interval, 0.48-2.08; P = .99).
“This suggests that antipsychotic medication is unlikely to be responsible for the increased risk we’ve observed in this population, although this finding needs to be replicated,” said Dr. Nemani.
Surprise finding
A diagnosis of a schizophrenia spectrum disorder was associated with an almost threefold increased risk for mortality compared with bipolar disorder (OR, 2.88; 95% CI, 1.36-6.11; P = .006).
“This was a surprising finding,” said Dr. Nemani.
She noted that there is evidence suggesting the immune system may play a role in the pathogenesis of schizophrenia, and research has shown that pneumonia and infection are among the leading causes of premature mortality in this population.
As well, several potential risk factors disproportionately affect people with serious mental illness, including an increase in the prevalence of medical comorbidities such as cardiovascular disease and diabetes, socioeconomic disadvantages, and barriers to accessing timely care. Prior studies have also found that people with SMI are less likely to receive preventive care interventions, including vaccination, said Dr. Nemani.
However, these factors are unlikely to fully account for the increased risk found in the study, she said.
“Our study population was limited to people who had received treatment within the NYU Langone Health System. We took a comprehensive list of sociodemographic and medical risk factors into account, and our research was conducted prior to the availability of COVID-19 vaccines,” she said.
Further research is necessary to understand what underlies the increase in susceptibility to severe infection among patients with schizophrenia and to identify interventions that may mitigate risk, said Dr. Nemani.
“This includes evaluating systems-level factors, such as access to preventive interventions and treatment, as well as investigating underlying immune mechanisms that may contribute to severe and fatal infection,” she said.
The researchers could not validate psychiatric diagnoses or capture deaths not documented in the electronic health record. In addition, the limited sample size precluded analysis of the use of individual antipsychotic medications, which may differ in their associated effects.
“It’s possible individual antipsychotic medications may be associated with harmful or protective effects,” said Dr. Nemani.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fatigued absent of medical history
The patient is probably presenting with relapsing-remitting multiple sclerosis (RRMS). MS is characterized by symptomatic episodes that are heralded by symptoms of central nervous system involvement. These attacks last longer than 24 hours and may occur months or years apart and affect different anatomic locations. Consistent with other autoimmune conditions, MS is more common in women. Patients are usually diagnosed between the ages of 20 and 49 years. The condition presents differently from patient to patient; some experience cognitive changes or visual symptoms, while others may have numbness, ataxia, clumsiness, hemiparesis, paraparesis, depression, or seizures. Symptoms can also include fatigue, impaired mobility, mood diagnosed changes, elimination dysfunction, and pain.
Of the four disease courses identified in MS, the most common is RRMS, characterized by a cycle of relapse and remission. In the initial stages, RRMS is characterized largely by an inflammatory pathology which, over time, becomes largely neurodegenerative. Most cases of RRMS evolve to secondary progressive MS after about 15 years. Early in the spectrum of demyelinating disease is clinically isolated syndrome (CIS), defined by a single episode of neurologic symptoms and MRI showing more than two classic lesions seen in MS. CIS patients subsequently will present with a second episode or relapse, at which point the diagnosis of RRMS is usually confirmed.
MS is diagnosed on the basis of clinical findings, exclusion of mimickers, and supporting evidence from the workup, namely MRI of the brain and spinal cord as well as cerebrospinal fluid examination. From a clinical perspective, presentation must align with the constellation of neurologic deficits seen in MS. Typically, the duration of deficit is days to weeks, as seen in the present case. While MRI alone cannot be used to diagnose MS, imaging may confirm diagnosis and offer value in monitoring disease progression in the brain and spinal cord. New lesions on MRI usually occur with relapses in RRMS.
Treatment of MS encompasses immunomodulatory therapy to address the underlying immune disorder together with therapies to relieve symptoms. In general, disease-modifying therapy (DMT) should be considered for patients who have experienced a single demyelinating event and exhibit two or more brain lesions on MRI testing. This recommendation holds true even for patients with CIS or those who have experienced their first clinical event and have MRI features consistent with MS, so long as all other conditions in the differential are ruled out. Pivotal trials support the early initiation of DMT with CIS to delay disability.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
The patient is probably presenting with relapsing-remitting multiple sclerosis (RRMS). MS is characterized by symptomatic episodes that are heralded by symptoms of central nervous system involvement. These attacks last longer than 24 hours and may occur months or years apart and affect different anatomic locations. Consistent with other autoimmune conditions, MS is more common in women. Patients are usually diagnosed between the ages of 20 and 49 years. The condition presents differently from patient to patient; some experience cognitive changes or visual symptoms, while others may have numbness, ataxia, clumsiness, hemiparesis, paraparesis, depression, or seizures. Symptoms can also include fatigue, impaired mobility, mood diagnosed changes, elimination dysfunction, and pain.
Of the four disease courses identified in MS, the most common is RRMS, characterized by a cycle of relapse and remission. In the initial stages, RRMS is characterized largely by an inflammatory pathology which, over time, becomes largely neurodegenerative. Most cases of RRMS evolve to secondary progressive MS after about 15 years. Early in the spectrum of demyelinating disease is clinically isolated syndrome (CIS), defined by a single episode of neurologic symptoms and MRI showing more than two classic lesions seen in MS. CIS patients subsequently will present with a second episode or relapse, at which point the diagnosis of RRMS is usually confirmed.
MS is diagnosed on the basis of clinical findings, exclusion of mimickers, and supporting evidence from the workup, namely MRI of the brain and spinal cord as well as cerebrospinal fluid examination. From a clinical perspective, presentation must align with the constellation of neurologic deficits seen in MS. Typically, the duration of deficit is days to weeks, as seen in the present case. While MRI alone cannot be used to diagnose MS, imaging may confirm diagnosis and offer value in monitoring disease progression in the brain and spinal cord. New lesions on MRI usually occur with relapses in RRMS.
Treatment of MS encompasses immunomodulatory therapy to address the underlying immune disorder together with therapies to relieve symptoms. In general, disease-modifying therapy (DMT) should be considered for patients who have experienced a single demyelinating event and exhibit two or more brain lesions on MRI testing. This recommendation holds true even for patients with CIS or those who have experienced their first clinical event and have MRI features consistent with MS, so long as all other conditions in the differential are ruled out. Pivotal trials support the early initiation of DMT with CIS to delay disability.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
The patient is probably presenting with relapsing-remitting multiple sclerosis (RRMS). MS is characterized by symptomatic episodes that are heralded by symptoms of central nervous system involvement. These attacks last longer than 24 hours and may occur months or years apart and affect different anatomic locations. Consistent with other autoimmune conditions, MS is more common in women. Patients are usually diagnosed between the ages of 20 and 49 years. The condition presents differently from patient to patient; some experience cognitive changes or visual symptoms, while others may have numbness, ataxia, clumsiness, hemiparesis, paraparesis, depression, or seizures. Symptoms can also include fatigue, impaired mobility, mood diagnosed changes, elimination dysfunction, and pain.
Of the four disease courses identified in MS, the most common is RRMS, characterized by a cycle of relapse and remission. In the initial stages, RRMS is characterized largely by an inflammatory pathology which, over time, becomes largely neurodegenerative. Most cases of RRMS evolve to secondary progressive MS after about 15 years. Early in the spectrum of demyelinating disease is clinically isolated syndrome (CIS), defined by a single episode of neurologic symptoms and MRI showing more than two classic lesions seen in MS. CIS patients subsequently will present with a second episode or relapse, at which point the diagnosis of RRMS is usually confirmed.
MS is diagnosed on the basis of clinical findings, exclusion of mimickers, and supporting evidence from the workup, namely MRI of the brain and spinal cord as well as cerebrospinal fluid examination. From a clinical perspective, presentation must align with the constellation of neurologic deficits seen in MS. Typically, the duration of deficit is days to weeks, as seen in the present case. While MRI alone cannot be used to diagnose MS, imaging may confirm diagnosis and offer value in monitoring disease progression in the brain and spinal cord. New lesions on MRI usually occur with relapses in RRMS.
Treatment of MS encompasses immunomodulatory therapy to address the underlying immune disorder together with therapies to relieve symptoms. In general, disease-modifying therapy (DMT) should be considered for patients who have experienced a single demyelinating event and exhibit two or more brain lesions on MRI testing. This recommendation holds true even for patients with CIS or those who have experienced their first clinical event and have MRI features consistent with MS, so long as all other conditions in the differential are ruled out. Pivotal trials support the early initiation of DMT with CIS to delay disability.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
A 51-year-old woman reports that she has been feeling fatigued despite the absence of any significant medical history. Although she usually walks to work, lately she has not had the energy to participate in her daily routine. She notes that over the past 2 weeks, colleagues have asked her if she is feeling well due to unusual ocular symptoms. She explains that several months ago she felt similarly unwell, with fatigue and generalized weakness, but her symptoms seemed to resolve. Upon presentation, she has diplopia on lateral gaze. MRI reveals lesions with high T2 signal intensity.
Cook your amphibians before you eat them
Novel food for thought
When you were growing up, your parents probably told you to brush your teeth before you went to bed, warned you not to run with the scissors or play with matches, and punished you whenever you used the neighbor children to play Schrödinger’s cat.
They did those things for your own good, of course, and now the nation’s mother – the Centers for Disease Control and Prevention – is doing the same by warning us about novel outbreak–associated foods. As in, “Put down that novel outbreak–associated food! You don’t know where it’s been!”
Seriously, you don’t know where it’s been. CDC investigators identified 28 novel foods that were linked to 36 foodborne-disease outbreaks that occurred during 2007-2016, including moringa leaf (herb/spice), tempeh (grain), frog, sprouted nut butter, and skate.
The novel foods implicated in these outbreaks were more likely to be imported, compared with 14,216 outbreaks that occurred from 1973 to 2016, and about half didn’t require refrigeration. Two-thirds did not need to be cooked after purchase. Another thing your parents wouldn’t like: Some can’t be washed, like sheep milk, sugar cane, or the aforementioned nut butter.
We wanted to get a food expert to comment on these novel foods, but our editor said that the assistant manager of our local Burger King wasn’t expert enough, so we’ve commandeered someone else’s expert. Cynthia Sears, MD, of Johns Hopkins University in Baltimore, told Today.com all about the dangers of frogs: “Essentially all amphibians are contaminated, often with salmonella. Eating any amphibian that is not thoroughly cooked is a risk.”
Be sure to cook your amphibians before you eat them. Advice that your parents would be proud to share.
Dieters should stay away from diet drinks
When a drink is labeled “diet” many assume that the calorie-free beverage is the best choice. However, one of the largest studies to date on artificial sweeteners is out to set the record straight.
Artificial sweeteners, or nonnutritive sweeteners (NNS), are used in most if not all diet products to give the illusion of sweetness without the caloric guilt. Some studies say they help with weight loss for that very reason, but others say they can contribute to weight gain. So which is it?
Researchers at the University of Southern California sought to add some clarity to the research already out there.
They looked at an even-gendered split of 74 participants who drank 300 mL of drinks sweetened with NNS, table sugar, or water. The researchers then used functional MRI to see how parts of the brain responsible for appetite and cravings responded to images of high-calorie foods. They also looked at glucose, insulin, and other metabolic hormone levels, as well as how much food the participants ate at their free buffet. (In the participants’ defense, who can say no to a free buffet?)
The researchers made some interesting observations:
- Women who drank the NNS drink ate more than did the table-sugar group, but all men ate the same.
- Images of those calorie-packed goodies increased cravings and appetite for obese men and women in the NNS group, compared with the table-sugar group.
- For all participants who drank the NNS drink, there was a decrease in the hormone that tells the body it’s full.
“By studying different groups we were able to show that females and people with obesity may be more sensitive to artificial sweeteners. For these groups, drinking artificially sweetened drinks may trick the brain into feeling hungry, which may in turn result in more calories being consumed,” Kathleen Page, MD, the study’s corresponding author, said in a separate statement.
Today’s lesson? Don’t believe every label you read.
Instagram vegetables and the triumph of peer pressure
You and your family are sitting down for dinner. You’ve taken the time to prepare a healthy, nutritious meal. Vegetables, rice, seafood – all the right things. But the children around you refuse to partake. What can you do? Why, show them a highly liked photo of broccoli on Instagram!
In reality, kids will probably never like to eat their vegetables, but according to a study published in Appetite, viewing highly liked images on social media can compel adults to eat theirs.
The investigators recruited a group of 169 adults aged 18-28 (average age, 21) and showed them a series of mock Instagram posts of all sorts of food, everything from Brussels sprouts to chocolate cake, as well as nonfood images to act as a baseline. The images had a varying amount of likes. After viewing the images, study participants were offered a snack buffet consisting of grapes and cookies.
The results were a triumph of peer pressure. Those who viewed highly liked images of nutritious foods ate a significantly larger proportion of grapes, compared with those who saw highly liked images of unhealthy food or nonfood.
The authors cautioned that more research is needed, but they said that they’re onto something in the eternal struggle of getting people to eat better. If Mikey liked it, maybe you should, too. Just as long as you don’t try to encourage the eating of peas. That is a dark road none should take, and no one should ever be subjected to that cursed food.
It’s nice to share … hypertension?
You may have heard that, over time, you begin to resemble your spouse. You may have also heard that, as time goes by, your pet might start to resemble you, but that is a story for another time.
A lot of the time, it’s human nature that people partner with someone who is similar to them in physical and environmental status. If you like to go jogging at 5 a.m., you might want a spouse who does the same. A study done using data from couples in Japan and the Netherlands found that couples who had the same lifestyle had similar levels of blood pressure, cholesterol, and triglycerides. They also had similar illnesses such as hypertension and diabetes.
It’s important to note that many of the couples were not very genetically similar but had similar lifestyles. Encourage your partner to have a healthier lifestyle, so you can live on for many years to come!
Novel food for thought
When you were growing up, your parents probably told you to brush your teeth before you went to bed, warned you not to run with the scissors or play with matches, and punished you whenever you used the neighbor children to play Schrödinger’s cat.
They did those things for your own good, of course, and now the nation’s mother – the Centers for Disease Control and Prevention – is doing the same by warning us about novel outbreak–associated foods. As in, “Put down that novel outbreak–associated food! You don’t know where it’s been!”
Seriously, you don’t know where it’s been. CDC investigators identified 28 novel foods that were linked to 36 foodborne-disease outbreaks that occurred during 2007-2016, including moringa leaf (herb/spice), tempeh (grain), frog, sprouted nut butter, and skate.
The novel foods implicated in these outbreaks were more likely to be imported, compared with 14,216 outbreaks that occurred from 1973 to 2016, and about half didn’t require refrigeration. Two-thirds did not need to be cooked after purchase. Another thing your parents wouldn’t like: Some can’t be washed, like sheep milk, sugar cane, or the aforementioned nut butter.
We wanted to get a food expert to comment on these novel foods, but our editor said that the assistant manager of our local Burger King wasn’t expert enough, so we’ve commandeered someone else’s expert. Cynthia Sears, MD, of Johns Hopkins University in Baltimore, told Today.com all about the dangers of frogs: “Essentially all amphibians are contaminated, often with salmonella. Eating any amphibian that is not thoroughly cooked is a risk.”
Be sure to cook your amphibians before you eat them. Advice that your parents would be proud to share.
Dieters should stay away from diet drinks
When a drink is labeled “diet” many assume that the calorie-free beverage is the best choice. However, one of the largest studies to date on artificial sweeteners is out to set the record straight.
Artificial sweeteners, or nonnutritive sweeteners (NNS), are used in most if not all diet products to give the illusion of sweetness without the caloric guilt. Some studies say they help with weight loss for that very reason, but others say they can contribute to weight gain. So which is it?
Researchers at the University of Southern California sought to add some clarity to the research already out there.
They looked at an even-gendered split of 74 participants who drank 300 mL of drinks sweetened with NNS, table sugar, or water. The researchers then used functional MRI to see how parts of the brain responsible for appetite and cravings responded to images of high-calorie foods. They also looked at glucose, insulin, and other metabolic hormone levels, as well as how much food the participants ate at their free buffet. (In the participants’ defense, who can say no to a free buffet?)
The researchers made some interesting observations:
- Women who drank the NNS drink ate more than did the table-sugar group, but all men ate the same.
- Images of those calorie-packed goodies increased cravings and appetite for obese men and women in the NNS group, compared with the table-sugar group.
- For all participants who drank the NNS drink, there was a decrease in the hormone that tells the body it’s full.
“By studying different groups we were able to show that females and people with obesity may be more sensitive to artificial sweeteners. For these groups, drinking artificially sweetened drinks may trick the brain into feeling hungry, which may in turn result in more calories being consumed,” Kathleen Page, MD, the study’s corresponding author, said in a separate statement.
Today’s lesson? Don’t believe every label you read.
Instagram vegetables and the triumph of peer pressure
You and your family are sitting down for dinner. You’ve taken the time to prepare a healthy, nutritious meal. Vegetables, rice, seafood – all the right things. But the children around you refuse to partake. What can you do? Why, show them a highly liked photo of broccoli on Instagram!
In reality, kids will probably never like to eat their vegetables, but according to a study published in Appetite, viewing highly liked images on social media can compel adults to eat theirs.
The investigators recruited a group of 169 adults aged 18-28 (average age, 21) and showed them a series of mock Instagram posts of all sorts of food, everything from Brussels sprouts to chocolate cake, as well as nonfood images to act as a baseline. The images had a varying amount of likes. After viewing the images, study participants were offered a snack buffet consisting of grapes and cookies.
The results were a triumph of peer pressure. Those who viewed highly liked images of nutritious foods ate a significantly larger proportion of grapes, compared with those who saw highly liked images of unhealthy food or nonfood.
The authors cautioned that more research is needed, but they said that they’re onto something in the eternal struggle of getting people to eat better. If Mikey liked it, maybe you should, too. Just as long as you don’t try to encourage the eating of peas. That is a dark road none should take, and no one should ever be subjected to that cursed food.
It’s nice to share … hypertension?
You may have heard that, over time, you begin to resemble your spouse. You may have also heard that, as time goes by, your pet might start to resemble you, but that is a story for another time.
A lot of the time, it’s human nature that people partner with someone who is similar to them in physical and environmental status. If you like to go jogging at 5 a.m., you might want a spouse who does the same. A study done using data from couples in Japan and the Netherlands found that couples who had the same lifestyle had similar levels of blood pressure, cholesterol, and triglycerides. They also had similar illnesses such as hypertension and diabetes.
It’s important to note that many of the couples were not very genetically similar but had similar lifestyles. Encourage your partner to have a healthier lifestyle, so you can live on for many years to come!
Novel food for thought
When you were growing up, your parents probably told you to brush your teeth before you went to bed, warned you not to run with the scissors or play with matches, and punished you whenever you used the neighbor children to play Schrödinger’s cat.
They did those things for your own good, of course, and now the nation’s mother – the Centers for Disease Control and Prevention – is doing the same by warning us about novel outbreak–associated foods. As in, “Put down that novel outbreak–associated food! You don’t know where it’s been!”
Seriously, you don’t know where it’s been. CDC investigators identified 28 novel foods that were linked to 36 foodborne-disease outbreaks that occurred during 2007-2016, including moringa leaf (herb/spice), tempeh (grain), frog, sprouted nut butter, and skate.
The novel foods implicated in these outbreaks were more likely to be imported, compared with 14,216 outbreaks that occurred from 1973 to 2016, and about half didn’t require refrigeration. Two-thirds did not need to be cooked after purchase. Another thing your parents wouldn’t like: Some can’t be washed, like sheep milk, sugar cane, or the aforementioned nut butter.
We wanted to get a food expert to comment on these novel foods, but our editor said that the assistant manager of our local Burger King wasn’t expert enough, so we’ve commandeered someone else’s expert. Cynthia Sears, MD, of Johns Hopkins University in Baltimore, told Today.com all about the dangers of frogs: “Essentially all amphibians are contaminated, often with salmonella. Eating any amphibian that is not thoroughly cooked is a risk.”
Be sure to cook your amphibians before you eat them. Advice that your parents would be proud to share.
Dieters should stay away from diet drinks
When a drink is labeled “diet” many assume that the calorie-free beverage is the best choice. However, one of the largest studies to date on artificial sweeteners is out to set the record straight.
Artificial sweeteners, or nonnutritive sweeteners (NNS), are used in most if not all diet products to give the illusion of sweetness without the caloric guilt. Some studies say they help with weight loss for that very reason, but others say they can contribute to weight gain. So which is it?
Researchers at the University of Southern California sought to add some clarity to the research already out there.
They looked at an even-gendered split of 74 participants who drank 300 mL of drinks sweetened with NNS, table sugar, or water. The researchers then used functional MRI to see how parts of the brain responsible for appetite and cravings responded to images of high-calorie foods. They also looked at glucose, insulin, and other metabolic hormone levels, as well as how much food the participants ate at their free buffet. (In the participants’ defense, who can say no to a free buffet?)
The researchers made some interesting observations:
- Women who drank the NNS drink ate more than did the table-sugar group, but all men ate the same.
- Images of those calorie-packed goodies increased cravings and appetite for obese men and women in the NNS group, compared with the table-sugar group.
- For all participants who drank the NNS drink, there was a decrease in the hormone that tells the body it’s full.
“By studying different groups we were able to show that females and people with obesity may be more sensitive to artificial sweeteners. For these groups, drinking artificially sweetened drinks may trick the brain into feeling hungry, which may in turn result in more calories being consumed,” Kathleen Page, MD, the study’s corresponding author, said in a separate statement.
Today’s lesson? Don’t believe every label you read.
Instagram vegetables and the triumph of peer pressure
You and your family are sitting down for dinner. You’ve taken the time to prepare a healthy, nutritious meal. Vegetables, rice, seafood – all the right things. But the children around you refuse to partake. What can you do? Why, show them a highly liked photo of broccoli on Instagram!
In reality, kids will probably never like to eat their vegetables, but according to a study published in Appetite, viewing highly liked images on social media can compel adults to eat theirs.
The investigators recruited a group of 169 adults aged 18-28 (average age, 21) and showed them a series of mock Instagram posts of all sorts of food, everything from Brussels sprouts to chocolate cake, as well as nonfood images to act as a baseline. The images had a varying amount of likes. After viewing the images, study participants were offered a snack buffet consisting of grapes and cookies.
The results were a triumph of peer pressure. Those who viewed highly liked images of nutritious foods ate a significantly larger proportion of grapes, compared with those who saw highly liked images of unhealthy food or nonfood.
The authors cautioned that more research is needed, but they said that they’re onto something in the eternal struggle of getting people to eat better. If Mikey liked it, maybe you should, too. Just as long as you don’t try to encourage the eating of peas. That is a dark road none should take, and no one should ever be subjected to that cursed food.
It’s nice to share … hypertension?
You may have heard that, over time, you begin to resemble your spouse. You may have also heard that, as time goes by, your pet might start to resemble you, but that is a story for another time.
A lot of the time, it’s human nature that people partner with someone who is similar to them in physical and environmental status. If you like to go jogging at 5 a.m., you might want a spouse who does the same. A study done using data from couples in Japan and the Netherlands found that couples who had the same lifestyle had similar levels of blood pressure, cholesterol, and triglycerides. They also had similar illnesses such as hypertension and diabetes.
It’s important to note that many of the couples were not very genetically similar but had similar lifestyles. Encourage your partner to have a healthier lifestyle, so you can live on for many years to come!
Treatment shows 'important improvements' in triple-negative breast cancer
But as a subanalysis of data from the randomized, phase 3 study showed, sacituzumab govitecan was associated with significantly better progression-free survival (PFS) and overall survival among patients without an initial TNBC diagnosis, with efficacy similar to that seen in the overall study population and in patients without known brain metastases, reported Joyce O’Shaughnessy, MD, at the 2021 European Society for Medical Oncology Congress. In her presentation (abstract 258P), Dr. O’Shaughnessy, who is an oncologist with the Texas Oncology–Baylor Charles A. Sammons Cancer Center in Dallas, said that it’s not uncommon to see acquired triple-negative breast cancer in patients who have hormone receptor–positive disease initially and then later on, develop triple-negative breast cancer.
“That is why at the time of metastatic diagnosis we always say that patients should have repeat receptor [testing], because it’s been well described that a subset of hormone receptor–positive breast can become triple negative under pressure from endocrine therapy,” said ASCENT coinvestigator Aditya Bardia, MD, MPH, Massachusetts General Hospital, Boston, in an interview
Coinvestigator Kevine Punie, MD, from University Hospitals Leuven (Belgium), said that “triple-negative breast cancer is a diagnosis of exclusion. This is a heterogenous disease where several biological subsets are merged.”
“It was important to perform this subgroup analysis to reassure us that the treatment effect we see from [sacituzumab govitecan] in the overall population is also observed in this specific subgroup of patients where biologically we’re treating a different disease,” he said in an interview.
Antibody-drug conjugate
Sacituzumab govitecan consists of an antibody targeted to the trophoblast antigen 2 cell surface receptor found on most breast cancer cells, plus the topoisomerase-1 inhibitor SN-38 as its toxic payload.
In the primary intent-to-treat (ITT) analysis of ASCENT, sacituzumab significantly prolonged PFS with a median of 4.8 versus 1.7 months for patients treated with the physician’s choice of either capecitabine, eribulin, vinorelbine, or gemcitabine. The difference translated into an HR for progression with the antibody-drug conjugate of 0.41 (P < .00001).
The subgroup analysis looked at outcomes for 70 patients randomized to sacituzumab govitecan and 78 randomized to single-agent chemotherapy who did not have an initial diagnosis of TNBC.
In all, 27% of patients in this subgroup in the sacituzumab arm and 29% in the chemotherapy arm had received a prior line of treatment with a CDK4/6 inhibitor.
At the time of data cutoff in March 2020, four patients (6%) in the sacituzumab arm remained on treatment versus no patient in the chemotherapy arm. The most common reason for treatment discontinuation was disease progression, which occurred in 84% and 72% of patients, respectively.
The median treatment duration was 5.1 months in the sacituzumab and 1.2 months in the chemotherapy arm. The median duration of follow-up was 10.6 and 6.1 months, respectively.
Progression-free survival, overall survival results
For all patients without an initial TNBC diagnosis, the median PFS was 4.6 months with sacituzumab versus 2.3 months with chemotherapy, which translated into a hazard ratio for progression of 0.48 (95% confidence interval, 0.32-0.72). The progression-free benefit with the antibody-drug conjugate was similar to that seen in the intent-to-treat population, as noted before, and among the overall population of patients in the study with no known brain metastases (HR, 5.6 vs. 1.7, respectively; P < .001).
The objective response rates were 21% in the sacituzumab and 5% in the chemotherapy arm. Objective responses were not affected by the prior use of CDK 4/6 inhibitors.
Median overall survival in this subgroup was 12.4 months with the antibody-drug conjugate versus 6.7 months with chemotherapy (95% CI, 0.30-0.64).
The investigators reported that the safety profile was manageable in this subgroup of patients, with a low rate of treatment discontinuations because of adverse events (5%) and no treatment-related deaths with sacituzumab govitecan.
Health-related quality of life
In a separate poster (abstract 257P) presented at the meeting, the ASCENT investigators reported health-related quality of life results in the overall population.
The mean European Organization for the Research and Treatment of Cancer quality of life questionnaire subscale scores at baseline were similar between treatment arms.
For the major domains of global health status, physical and emotional functioning as well as lower symptomatic impact of fatigue, pain, dyspnea, and insomnia, sacituzumab govitecan showed “significantly and meaningfully greater improvement” than the physician’s choice of chemotherapy, the investigators found.
Of individual symptoms, only diarrhea was worse with the antibody-drug conjugate.
“These were important improvements,” Dr. Punie said, “because we saw improvements in the primary health-related quality of life domains such as global health status. We also saw that the time to clinically meaningful deterioration of quality of life domains were significantly longer for patients treated with [sacituzumab govitecan].”
“Patients who received sacituzumab govitecan had better health-related quality of life as compared to the control arm, and that’s a very important result, because in the metastatic setting our goal is twofold: We want to prolong survival, and we want patients to have improved quality of life,” Dr. Bardia said.
The Ascent trial is sponsored by Gilead Sciences. Dr. Punie disclosed relationships with multiple companies/organizations, not including Gilead. Dr. Bardia disclosed contracted research for Gilead and others, as well as other relationships.
But as a subanalysis of data from the randomized, phase 3 study showed, sacituzumab govitecan was associated with significantly better progression-free survival (PFS) and overall survival among patients without an initial TNBC diagnosis, with efficacy similar to that seen in the overall study population and in patients without known brain metastases, reported Joyce O’Shaughnessy, MD, at the 2021 European Society for Medical Oncology Congress. In her presentation (abstract 258P), Dr. O’Shaughnessy, who is an oncologist with the Texas Oncology–Baylor Charles A. Sammons Cancer Center in Dallas, said that it’s not uncommon to see acquired triple-negative breast cancer in patients who have hormone receptor–positive disease initially and then later on, develop triple-negative breast cancer.
“That is why at the time of metastatic diagnosis we always say that patients should have repeat receptor [testing], because it’s been well described that a subset of hormone receptor–positive breast can become triple negative under pressure from endocrine therapy,” said ASCENT coinvestigator Aditya Bardia, MD, MPH, Massachusetts General Hospital, Boston, in an interview
Coinvestigator Kevine Punie, MD, from University Hospitals Leuven (Belgium), said that “triple-negative breast cancer is a diagnosis of exclusion. This is a heterogenous disease where several biological subsets are merged.”
“It was important to perform this subgroup analysis to reassure us that the treatment effect we see from [sacituzumab govitecan] in the overall population is also observed in this specific subgroup of patients where biologically we’re treating a different disease,” he said in an interview.
Antibody-drug conjugate
Sacituzumab govitecan consists of an antibody targeted to the trophoblast antigen 2 cell surface receptor found on most breast cancer cells, plus the topoisomerase-1 inhibitor SN-38 as its toxic payload.
In the primary intent-to-treat (ITT) analysis of ASCENT, sacituzumab significantly prolonged PFS with a median of 4.8 versus 1.7 months for patients treated with the physician’s choice of either capecitabine, eribulin, vinorelbine, or gemcitabine. The difference translated into an HR for progression with the antibody-drug conjugate of 0.41 (P < .00001).
The subgroup analysis looked at outcomes for 70 patients randomized to sacituzumab govitecan and 78 randomized to single-agent chemotherapy who did not have an initial diagnosis of TNBC.
In all, 27% of patients in this subgroup in the sacituzumab arm and 29% in the chemotherapy arm had received a prior line of treatment with a CDK4/6 inhibitor.
At the time of data cutoff in March 2020, four patients (6%) in the sacituzumab arm remained on treatment versus no patient in the chemotherapy arm. The most common reason for treatment discontinuation was disease progression, which occurred in 84% and 72% of patients, respectively.
The median treatment duration was 5.1 months in the sacituzumab and 1.2 months in the chemotherapy arm. The median duration of follow-up was 10.6 and 6.1 months, respectively.
Progression-free survival, overall survival results
For all patients without an initial TNBC diagnosis, the median PFS was 4.6 months with sacituzumab versus 2.3 months with chemotherapy, which translated into a hazard ratio for progression of 0.48 (95% confidence interval, 0.32-0.72). The progression-free benefit with the antibody-drug conjugate was similar to that seen in the intent-to-treat population, as noted before, and among the overall population of patients in the study with no known brain metastases (HR, 5.6 vs. 1.7, respectively; P < .001).
The objective response rates were 21% in the sacituzumab and 5% in the chemotherapy arm. Objective responses were not affected by the prior use of CDK 4/6 inhibitors.
Median overall survival in this subgroup was 12.4 months with the antibody-drug conjugate versus 6.7 months with chemotherapy (95% CI, 0.30-0.64).
The investigators reported that the safety profile was manageable in this subgroup of patients, with a low rate of treatment discontinuations because of adverse events (5%) and no treatment-related deaths with sacituzumab govitecan.
Health-related quality of life
In a separate poster (abstract 257P) presented at the meeting, the ASCENT investigators reported health-related quality of life results in the overall population.
The mean European Organization for the Research and Treatment of Cancer quality of life questionnaire subscale scores at baseline were similar between treatment arms.
For the major domains of global health status, physical and emotional functioning as well as lower symptomatic impact of fatigue, pain, dyspnea, and insomnia, sacituzumab govitecan showed “significantly and meaningfully greater improvement” than the physician’s choice of chemotherapy, the investigators found.
Of individual symptoms, only diarrhea was worse with the antibody-drug conjugate.
“These were important improvements,” Dr. Punie said, “because we saw improvements in the primary health-related quality of life domains such as global health status. We also saw that the time to clinically meaningful deterioration of quality of life domains were significantly longer for patients treated with [sacituzumab govitecan].”
“Patients who received sacituzumab govitecan had better health-related quality of life as compared to the control arm, and that’s a very important result, because in the metastatic setting our goal is twofold: We want to prolong survival, and we want patients to have improved quality of life,” Dr. Bardia said.
The Ascent trial is sponsored by Gilead Sciences. Dr. Punie disclosed relationships with multiple companies/organizations, not including Gilead. Dr. Bardia disclosed contracted research for Gilead and others, as well as other relationships.
But as a subanalysis of data from the randomized, phase 3 study showed, sacituzumab govitecan was associated with significantly better progression-free survival (PFS) and overall survival among patients without an initial TNBC diagnosis, with efficacy similar to that seen in the overall study population and in patients without known brain metastases, reported Joyce O’Shaughnessy, MD, at the 2021 European Society for Medical Oncology Congress. In her presentation (abstract 258P), Dr. O’Shaughnessy, who is an oncologist with the Texas Oncology–Baylor Charles A. Sammons Cancer Center in Dallas, said that it’s not uncommon to see acquired triple-negative breast cancer in patients who have hormone receptor–positive disease initially and then later on, develop triple-negative breast cancer.
“That is why at the time of metastatic diagnosis we always say that patients should have repeat receptor [testing], because it’s been well described that a subset of hormone receptor–positive breast can become triple negative under pressure from endocrine therapy,” said ASCENT coinvestigator Aditya Bardia, MD, MPH, Massachusetts General Hospital, Boston, in an interview
Coinvestigator Kevine Punie, MD, from University Hospitals Leuven (Belgium), said that “triple-negative breast cancer is a diagnosis of exclusion. This is a heterogenous disease where several biological subsets are merged.”
“It was important to perform this subgroup analysis to reassure us that the treatment effect we see from [sacituzumab govitecan] in the overall population is also observed in this specific subgroup of patients where biologically we’re treating a different disease,” he said in an interview.
Antibody-drug conjugate
Sacituzumab govitecan consists of an antibody targeted to the trophoblast antigen 2 cell surface receptor found on most breast cancer cells, plus the topoisomerase-1 inhibitor SN-38 as its toxic payload.
In the primary intent-to-treat (ITT) analysis of ASCENT, sacituzumab significantly prolonged PFS with a median of 4.8 versus 1.7 months for patients treated with the physician’s choice of either capecitabine, eribulin, vinorelbine, or gemcitabine. The difference translated into an HR for progression with the antibody-drug conjugate of 0.41 (P < .00001).
The subgroup analysis looked at outcomes for 70 patients randomized to sacituzumab govitecan and 78 randomized to single-agent chemotherapy who did not have an initial diagnosis of TNBC.
In all, 27% of patients in this subgroup in the sacituzumab arm and 29% in the chemotherapy arm had received a prior line of treatment with a CDK4/6 inhibitor.
At the time of data cutoff in March 2020, four patients (6%) in the sacituzumab arm remained on treatment versus no patient in the chemotherapy arm. The most common reason for treatment discontinuation was disease progression, which occurred in 84% and 72% of patients, respectively.
The median treatment duration was 5.1 months in the sacituzumab and 1.2 months in the chemotherapy arm. The median duration of follow-up was 10.6 and 6.1 months, respectively.
Progression-free survival, overall survival results
For all patients without an initial TNBC diagnosis, the median PFS was 4.6 months with sacituzumab versus 2.3 months with chemotherapy, which translated into a hazard ratio for progression of 0.48 (95% confidence interval, 0.32-0.72). The progression-free benefit with the antibody-drug conjugate was similar to that seen in the intent-to-treat population, as noted before, and among the overall population of patients in the study with no known brain metastases (HR, 5.6 vs. 1.7, respectively; P < .001).
The objective response rates were 21% in the sacituzumab and 5% in the chemotherapy arm. Objective responses were not affected by the prior use of CDK 4/6 inhibitors.
Median overall survival in this subgroup was 12.4 months with the antibody-drug conjugate versus 6.7 months with chemotherapy (95% CI, 0.30-0.64).
The investigators reported that the safety profile was manageable in this subgroup of patients, with a low rate of treatment discontinuations because of adverse events (5%) and no treatment-related deaths with sacituzumab govitecan.
Health-related quality of life
In a separate poster (abstract 257P) presented at the meeting, the ASCENT investigators reported health-related quality of life results in the overall population.
The mean European Organization for the Research and Treatment of Cancer quality of life questionnaire subscale scores at baseline were similar between treatment arms.
For the major domains of global health status, physical and emotional functioning as well as lower symptomatic impact of fatigue, pain, dyspnea, and insomnia, sacituzumab govitecan showed “significantly and meaningfully greater improvement” than the physician’s choice of chemotherapy, the investigators found.
Of individual symptoms, only diarrhea was worse with the antibody-drug conjugate.
“These were important improvements,” Dr. Punie said, “because we saw improvements in the primary health-related quality of life domains such as global health status. We also saw that the time to clinically meaningful deterioration of quality of life domains were significantly longer for patients treated with [sacituzumab govitecan].”
“Patients who received sacituzumab govitecan had better health-related quality of life as compared to the control arm, and that’s a very important result, because in the metastatic setting our goal is twofold: We want to prolong survival, and we want patients to have improved quality of life,” Dr. Bardia said.
The Ascent trial is sponsored by Gilead Sciences. Dr. Punie disclosed relationships with multiple companies/organizations, not including Gilead. Dr. Bardia disclosed contracted research for Gilead and others, as well as other relationships.
FROM ESMO 2021
MDs doing wrong-site surgery: Why is it still happening?
In July 2021, University Hospitals, in Cleveland, announced that its staff had transplanted a kidney into the wrong patient. Although the patient who received the kidney was recovering well, the patient who was supposed to have received the kidney was skipped over. As a result of the error, two employees were placed on administrative leave and the incident was being investigated, the hospital announced.
In April 2020, an interventional radiologist at Boca Raton Regional Hospital, in Boca Raton, Fla., was sued for allegedly placing a stent into the wrong kidney of an 80-year-old patient. Using fluoroscopic guidance, the doctor removed an old stent from the right side but incorrectly replaced it with a new stent on the left side, according to an interview conducted by this news organization with the patient’s lawyers at Searcy Law, in West Palm Beach.
“The problem is that it is so rare that doctors don’t focus on it,” says Mary R. Kwaan, MD, a colorectal surgeon at UCLA Medical Center, Los Angeles.
A 2006 study in which Kwaan was the lead author concluded that there was one wrong-site surgery for every 112,994 surgeries. Those mistakes can add up. A 2006 study estimated that 25 to 52 wrong-site surgeries were performed each week in the United States.
“Many surgeons don’t think it can happen to them, so they don’t take extra precautions,” says David Mayer, MD, executive director of the MedStar Institute for Quality and Safety, in Washington, DC. “When they make a wrong-site error, usually the first thing they say is, ‘I never thought this would happen to me,’ ” he says.
Wrong-site surgeries are considered sentinel events -- the worst kinds of medical errors. The Sullivan Group, a patient safety consultancy based in Colorado, reports that in 2013, 2.7% of patients who were involved in wrong-site surgeries died and 41% experienced some type of permanent injury. The mean malpractice payment was $127,000.
Some malpractice payments are much higher. In 2013, a Maryland ob.gyn paid a $1.42 million malpractice award for removing the wrong ovary from a woman in 2009. In 2017, a Pennsylvania urologist paid $870,000 for removing the wrong testicle from a man in 2013.
Wrong-site surgery often involves experienced surgeons
One might think that wrong-site surgeries usually involve younger or less-experienced surgeons, but that’s not the case; two thirds of the surgeons who perform wrong-site surgeries are in their 40s and 50s, compared with fewer than 25% younger than 40.
In a rather chilling statistic, in a 2013 survey, 12.4% of doctors who were involved in sentinel events in general had claims for more than one event.
These errors are more common in certain specialties. In a study reported in the Journal of Neurology, Neurosurgery and Spine, 25% of orthopedic surgeons reported performing at least one wrong-site surgery during their career.
Within orthopedics, spine surgery is ground zero for wrong-site surgery. “Finding the site in spine surgery can be more difficult than in common left-right orthopedic procedures,” says Joseph A. Bosco III, a New York City orthopedist.
A 2007 study found that 25% of neurosurgeons had performed wrong-site surgeries. In Missouri in 2013, for example, a 53-year-old patient who was scheduled to undergo a left-sided craniotomy bypass allegedly underwent a right-sided craniotomy and was unable to speak after surgery.
Wrong-site surgeries are also performed by general surgeons, urologists, cardiologists, otolaryngologists, and ophthalmologists. A 2021 lawsuit accused a Tampa urologist of removing the patient’s wrong testicle. And a 2019 lawsuit accused a Chicago ophthalmologist of operating on the wrong eye to remove a cyst.
It’s not just the surgeon’s mistake
Mistakes are not only made by the surgeon in the operating room (OR). They can be made by staff when scheduling a surgery, radiologists and pathologists when writing their reports for surgery, and by team members in the OR.
Many people are prone to confusing left and right. A 2020 study found that 14.9% of people had difficulty distinguishing left from right; other studies have shown higher rates. Distractions increase the likelihood of mistakes. In a 2015 study, background noise in a hospital ward made it more difficult for medical students to make left-right judgments.
OR personnel can be confused when patients are turned around. “To operate on the back of someone’s leg, the surgeon may turn the patient from supine to prone, and so left becomes right,” says Samuel C. Seiden, MD, an anesthesiologist in Roseville, Calif., who has studied wrong-site surgery.
Operative site markings that are drawn on the skin can be rubbed off when surgical prep is applied, and markings aren’t usually possible for procedures such as spine surgeries. Surgical draping can make it harder to distinguish the patient’s left and right, and a busy surgeon relying on memory may confuse cases and perform wrong-patient surgery.
A push to eliminate wrong-site surgery
In 2004, the Joint Commission, which accredits hospitals and many surgery centers, decided to do something about wrong-site surgery and related surgical errors. It released a universal protocol, which requires hospitals to take three steps to prevent errors: perform preoperative verification that is based on patient care documents; mark the operative site; and take a time-out just before surgery, during which the team should consider whether a mistake is about to be made.
Two years after the Joint Commission published its protocol, Dr. Seiden led a study to determine what effect it had had. The investigators found that wrong-site cases had decreased by only about one third. Preventing wrong-site surgery “turns out to be more complicated to eradicate than anybody thought,” Mark Chassin, MD, president of the Joint Commission, stated a few years later.
Why did the protocol have only a limited effect? Dr. Seiden says that it has been hard to change doctors’ traditional attitudes against standardization. “Some have had an attitude that checklists are for dummies, but that is changing,” he says.
For instance, some surgical teams were not paying attention during time-outs. “The time-out should be like the invocation of the National Anthem,” an orthopedic surgeon from Iowa wrote. “All other activities should stop.”
Even had surgeons followed the universal protocol, about one third of wrong-site surgeries would not have been identified, according to Dr. Kwaan’s study, which was published in the same year as Dr. Seiden’s. As an example, when the wrong kidney was removed at Methodist Hospital, in St. Louis Park, Minn., the hospital said it was following a protocol set by the Minnesota Hospital Association.
Redoubling efforts
In 2009, the Joint Commission decided to take another tack. It encouraged hospitals to make root-cause analyses not only of wrong-site surgeries but also of near misses, which are much more plentiful. It used the insights gained to change surgical routines and protocols.
The Safe Surgery Project, a collaboration between the Joint Commission’s Center for Transforming Healthcare and eight hospitals and surgery centers, reduced the number of errors and near misses by 46% in the scheduling area, 63% in pre-op, and 51% in the OR area.
From that project, the center developed the Targeted Solutions Tool, which basically uses the same methodology that the project used. The center told this news organization that 79 healthcare organizations have used the tool and have reduced the number of errors and near misses by 56% in scheduling, 24% in pre-op, and 48% in the OR.
For this approach to work, however, surgical teams must report their errors to the hospital, which had not been done before. A 2008 study by the Office of the Inspector General of the U.S. Department of Health and Human Services found that surgical staff did not report 86% of adverse events to their hospitals. Reasons given included lack of time, fear of punitive action, and skepticism that reporting would do any good.
Unlike some other adverse events, it’s hard to keep wrong-site surgeries secret from patients, because they can usually see the scars from it, but some surgeons invent ways to cover it up from patients, too, Dr. Mayer says. One wrong-side hernia repair was corrected in mid operation. Afterward, the surgeon told the patient that he had found another hernia on the other side and had fixed that one, too.
Changing the culture
Reformers argue that wrong-site surgeries can be prevented by changing the culture of the hospital or surgery center. “We have to think of wrong-site surgeries as a failure of the system, not of the individual,” says Ron Savrin, MD, a general surgeon in Chagrin Falls, Ohio, who is a surgery subject matter expert for the Sullivan Group. “It should never be only up to one individual to stop an error from occurring.”
Seeing oneself as part of a team can reduce errors. Although other people can introduce errors that make a person look bad, they can also stop the errors that might otherwise have occurred. Punishing individuals for making errors does little good in stopping errors.
“It’s human nature to want to punish somebody for making a mistake, and it’s hard to change that mentality,” Dr. Savrin says. He recalls that when he was a resident, “the morbidity and mortality conferences could be very difficult for anyone who made a mistake, but I think that attitude is changing.”
Studies have found wide variation in the number of wrong-site surgeries among hospitals. A recent Pennsylvania study found an average of one wrong-site surgery or near miss per hospital per year, but about one third of hospitals did not report any.
Wrong-site surgeries are often concentrated in certain hospitals -- even prestigious teaching hospitals are not immune. A decade ago, Rhode Island Hospital had five wrong-site surgeries in 2 years, and Boston’s Beth Israel Deaconess Medical Center had three wrong-spine surgeries within 2 months.
Other ways to reduce errors
Dr. Seiden thinks reform efforts should take a page from his own specialty. Anesthesiology has developed a variety of forcing functions, which are simple changes in technology that can stop errors. An example is the use of a valve that will not deliver a drug unless certain steps are followed.
The StartBox System, a new way to prevent surgical errors, delivers the surgery blade only after all safety information has been provided. Tested by 11 orthopedic surgeons performing 487 procedures, the system identified 17 near misses.
Another approach is to film time-outs so as to enforce compliance with protocols and help with root-cause analyses. NYU-Langone Medical Center, in New York City, not only films the time-out but also grades OR teams on compliance, says Dr. Bosco, who is vice chair of clinical affairs in the department of orthopedic surgery at the hospital.
In addition, more states are requiring hospitals to report adverse events, including wrong-site surgeries. According to the National Academy for State Health Policy, 28 states require the reporting of adverse events. However, only six states identify facilities in public reports; 16 states publish only aggregate data; and five states do not report error data to the public.
The goal is zero errors
Are there fewer wrong-site surgeries now? “My sense is that surgeons, hospitals, and surgery centers are taking wrong-site errors more seriously,” Dr. Savrin says.
Because reported information is spotty and no major studies on incidence have been conducted in recent years, “we don’t have a clear idea,” he says, “but my best guess is that the rate is declining.
“Absolute zero preventable errors has to be our goal,” Dr. Savrin says “We might not get there, but we can’t stop trying.”
A version of this article first appeared on Medscape.com.
In July 2021, University Hospitals, in Cleveland, announced that its staff had transplanted a kidney into the wrong patient. Although the patient who received the kidney was recovering well, the patient who was supposed to have received the kidney was skipped over. As a result of the error, two employees were placed on administrative leave and the incident was being investigated, the hospital announced.
In April 2020, an interventional radiologist at Boca Raton Regional Hospital, in Boca Raton, Fla., was sued for allegedly placing a stent into the wrong kidney of an 80-year-old patient. Using fluoroscopic guidance, the doctor removed an old stent from the right side but incorrectly replaced it with a new stent on the left side, according to an interview conducted by this news organization with the patient’s lawyers at Searcy Law, in West Palm Beach.
“The problem is that it is so rare that doctors don’t focus on it,” says Mary R. Kwaan, MD, a colorectal surgeon at UCLA Medical Center, Los Angeles.
A 2006 study in which Kwaan was the lead author concluded that there was one wrong-site surgery for every 112,994 surgeries. Those mistakes can add up. A 2006 study estimated that 25 to 52 wrong-site surgeries were performed each week in the United States.
“Many surgeons don’t think it can happen to them, so they don’t take extra precautions,” says David Mayer, MD, executive director of the MedStar Institute for Quality and Safety, in Washington, DC. “When they make a wrong-site error, usually the first thing they say is, ‘I never thought this would happen to me,’ ” he says.
Wrong-site surgeries are considered sentinel events -- the worst kinds of medical errors. The Sullivan Group, a patient safety consultancy based in Colorado, reports that in 2013, 2.7% of patients who were involved in wrong-site surgeries died and 41% experienced some type of permanent injury. The mean malpractice payment was $127,000.
Some malpractice payments are much higher. In 2013, a Maryland ob.gyn paid a $1.42 million malpractice award for removing the wrong ovary from a woman in 2009. In 2017, a Pennsylvania urologist paid $870,000 for removing the wrong testicle from a man in 2013.
Wrong-site surgery often involves experienced surgeons
One might think that wrong-site surgeries usually involve younger or less-experienced surgeons, but that’s not the case; two thirds of the surgeons who perform wrong-site surgeries are in their 40s and 50s, compared with fewer than 25% younger than 40.
In a rather chilling statistic, in a 2013 survey, 12.4% of doctors who were involved in sentinel events in general had claims for more than one event.
These errors are more common in certain specialties. In a study reported in the Journal of Neurology, Neurosurgery and Spine, 25% of orthopedic surgeons reported performing at least one wrong-site surgery during their career.
Within orthopedics, spine surgery is ground zero for wrong-site surgery. “Finding the site in spine surgery can be more difficult than in common left-right orthopedic procedures,” says Joseph A. Bosco III, a New York City orthopedist.
A 2007 study found that 25% of neurosurgeons had performed wrong-site surgeries. In Missouri in 2013, for example, a 53-year-old patient who was scheduled to undergo a left-sided craniotomy bypass allegedly underwent a right-sided craniotomy and was unable to speak after surgery.
Wrong-site surgeries are also performed by general surgeons, urologists, cardiologists, otolaryngologists, and ophthalmologists. A 2021 lawsuit accused a Tampa urologist of removing the patient’s wrong testicle. And a 2019 lawsuit accused a Chicago ophthalmologist of operating on the wrong eye to remove a cyst.
It’s not just the surgeon’s mistake
Mistakes are not only made by the surgeon in the operating room (OR). They can be made by staff when scheduling a surgery, radiologists and pathologists when writing their reports for surgery, and by team members in the OR.
Many people are prone to confusing left and right. A 2020 study found that 14.9% of people had difficulty distinguishing left from right; other studies have shown higher rates. Distractions increase the likelihood of mistakes. In a 2015 study, background noise in a hospital ward made it more difficult for medical students to make left-right judgments.
OR personnel can be confused when patients are turned around. “To operate on the back of someone’s leg, the surgeon may turn the patient from supine to prone, and so left becomes right,” says Samuel C. Seiden, MD, an anesthesiologist in Roseville, Calif., who has studied wrong-site surgery.
Operative site markings that are drawn on the skin can be rubbed off when surgical prep is applied, and markings aren’t usually possible for procedures such as spine surgeries. Surgical draping can make it harder to distinguish the patient’s left and right, and a busy surgeon relying on memory may confuse cases and perform wrong-patient surgery.
A push to eliminate wrong-site surgery
In 2004, the Joint Commission, which accredits hospitals and many surgery centers, decided to do something about wrong-site surgery and related surgical errors. It released a universal protocol, which requires hospitals to take three steps to prevent errors: perform preoperative verification that is based on patient care documents; mark the operative site; and take a time-out just before surgery, during which the team should consider whether a mistake is about to be made.
Two years after the Joint Commission published its protocol, Dr. Seiden led a study to determine what effect it had had. The investigators found that wrong-site cases had decreased by only about one third. Preventing wrong-site surgery “turns out to be more complicated to eradicate than anybody thought,” Mark Chassin, MD, president of the Joint Commission, stated a few years later.
Why did the protocol have only a limited effect? Dr. Seiden says that it has been hard to change doctors’ traditional attitudes against standardization. “Some have had an attitude that checklists are for dummies, but that is changing,” he says.
For instance, some surgical teams were not paying attention during time-outs. “The time-out should be like the invocation of the National Anthem,” an orthopedic surgeon from Iowa wrote. “All other activities should stop.”
Even had surgeons followed the universal protocol, about one third of wrong-site surgeries would not have been identified, according to Dr. Kwaan’s study, which was published in the same year as Dr. Seiden’s. As an example, when the wrong kidney was removed at Methodist Hospital, in St. Louis Park, Minn., the hospital said it was following a protocol set by the Minnesota Hospital Association.
Redoubling efforts
In 2009, the Joint Commission decided to take another tack. It encouraged hospitals to make root-cause analyses not only of wrong-site surgeries but also of near misses, which are much more plentiful. It used the insights gained to change surgical routines and protocols.
The Safe Surgery Project, a collaboration between the Joint Commission’s Center for Transforming Healthcare and eight hospitals and surgery centers, reduced the number of errors and near misses by 46% in the scheduling area, 63% in pre-op, and 51% in the OR area.
From that project, the center developed the Targeted Solutions Tool, which basically uses the same methodology that the project used. The center told this news organization that 79 healthcare organizations have used the tool and have reduced the number of errors and near misses by 56% in scheduling, 24% in pre-op, and 48% in the OR.
For this approach to work, however, surgical teams must report their errors to the hospital, which had not been done before. A 2008 study by the Office of the Inspector General of the U.S. Department of Health and Human Services found that surgical staff did not report 86% of adverse events to their hospitals. Reasons given included lack of time, fear of punitive action, and skepticism that reporting would do any good.
Unlike some other adverse events, it’s hard to keep wrong-site surgeries secret from patients, because they can usually see the scars from it, but some surgeons invent ways to cover it up from patients, too, Dr. Mayer says. One wrong-side hernia repair was corrected in mid operation. Afterward, the surgeon told the patient that he had found another hernia on the other side and had fixed that one, too.
Changing the culture
Reformers argue that wrong-site surgeries can be prevented by changing the culture of the hospital or surgery center. “We have to think of wrong-site surgeries as a failure of the system, not of the individual,” says Ron Savrin, MD, a general surgeon in Chagrin Falls, Ohio, who is a surgery subject matter expert for the Sullivan Group. “It should never be only up to one individual to stop an error from occurring.”
Seeing oneself as part of a team can reduce errors. Although other people can introduce errors that make a person look bad, they can also stop the errors that might otherwise have occurred. Punishing individuals for making errors does little good in stopping errors.
“It’s human nature to want to punish somebody for making a mistake, and it’s hard to change that mentality,” Dr. Savrin says. He recalls that when he was a resident, “the morbidity and mortality conferences could be very difficult for anyone who made a mistake, but I think that attitude is changing.”
Studies have found wide variation in the number of wrong-site surgeries among hospitals. A recent Pennsylvania study found an average of one wrong-site surgery or near miss per hospital per year, but about one third of hospitals did not report any.
Wrong-site surgeries are often concentrated in certain hospitals -- even prestigious teaching hospitals are not immune. A decade ago, Rhode Island Hospital had five wrong-site surgeries in 2 years, and Boston’s Beth Israel Deaconess Medical Center had three wrong-spine surgeries within 2 months.
Other ways to reduce errors
Dr. Seiden thinks reform efforts should take a page from his own specialty. Anesthesiology has developed a variety of forcing functions, which are simple changes in technology that can stop errors. An example is the use of a valve that will not deliver a drug unless certain steps are followed.
The StartBox System, a new way to prevent surgical errors, delivers the surgery blade only after all safety information has been provided. Tested by 11 orthopedic surgeons performing 487 procedures, the system identified 17 near misses.
Another approach is to film time-outs so as to enforce compliance with protocols and help with root-cause analyses. NYU-Langone Medical Center, in New York City, not only films the time-out but also grades OR teams on compliance, says Dr. Bosco, who is vice chair of clinical affairs in the department of orthopedic surgery at the hospital.
In addition, more states are requiring hospitals to report adverse events, including wrong-site surgeries. According to the National Academy for State Health Policy, 28 states require the reporting of adverse events. However, only six states identify facilities in public reports; 16 states publish only aggregate data; and five states do not report error data to the public.
The goal is zero errors
Are there fewer wrong-site surgeries now? “My sense is that surgeons, hospitals, and surgery centers are taking wrong-site errors more seriously,” Dr. Savrin says.
Because reported information is spotty and no major studies on incidence have been conducted in recent years, “we don’t have a clear idea,” he says, “but my best guess is that the rate is declining.
“Absolute zero preventable errors has to be our goal,” Dr. Savrin says “We might not get there, but we can’t stop trying.”
A version of this article first appeared on Medscape.com.
In July 2021, University Hospitals, in Cleveland, announced that its staff had transplanted a kidney into the wrong patient. Although the patient who received the kidney was recovering well, the patient who was supposed to have received the kidney was skipped over. As a result of the error, two employees were placed on administrative leave and the incident was being investigated, the hospital announced.
In April 2020, an interventional radiologist at Boca Raton Regional Hospital, in Boca Raton, Fla., was sued for allegedly placing a stent into the wrong kidney of an 80-year-old patient. Using fluoroscopic guidance, the doctor removed an old stent from the right side but incorrectly replaced it with a new stent on the left side, according to an interview conducted by this news organization with the patient’s lawyers at Searcy Law, in West Palm Beach.
“The problem is that it is so rare that doctors don’t focus on it,” says Mary R. Kwaan, MD, a colorectal surgeon at UCLA Medical Center, Los Angeles.
A 2006 study in which Kwaan was the lead author concluded that there was one wrong-site surgery for every 112,994 surgeries. Those mistakes can add up. A 2006 study estimated that 25 to 52 wrong-site surgeries were performed each week in the United States.
“Many surgeons don’t think it can happen to them, so they don’t take extra precautions,” says David Mayer, MD, executive director of the MedStar Institute for Quality and Safety, in Washington, DC. “When they make a wrong-site error, usually the first thing they say is, ‘I never thought this would happen to me,’ ” he says.
Wrong-site surgeries are considered sentinel events -- the worst kinds of medical errors. The Sullivan Group, a patient safety consultancy based in Colorado, reports that in 2013, 2.7% of patients who were involved in wrong-site surgeries died and 41% experienced some type of permanent injury. The mean malpractice payment was $127,000.
Some malpractice payments are much higher. In 2013, a Maryland ob.gyn paid a $1.42 million malpractice award for removing the wrong ovary from a woman in 2009. In 2017, a Pennsylvania urologist paid $870,000 for removing the wrong testicle from a man in 2013.
Wrong-site surgery often involves experienced surgeons
One might think that wrong-site surgeries usually involve younger or less-experienced surgeons, but that’s not the case; two thirds of the surgeons who perform wrong-site surgeries are in their 40s and 50s, compared with fewer than 25% younger than 40.
In a rather chilling statistic, in a 2013 survey, 12.4% of doctors who were involved in sentinel events in general had claims for more than one event.
These errors are more common in certain specialties. In a study reported in the Journal of Neurology, Neurosurgery and Spine, 25% of orthopedic surgeons reported performing at least one wrong-site surgery during their career.
Within orthopedics, spine surgery is ground zero for wrong-site surgery. “Finding the site in spine surgery can be more difficult than in common left-right orthopedic procedures,” says Joseph A. Bosco III, a New York City orthopedist.
A 2007 study found that 25% of neurosurgeons had performed wrong-site surgeries. In Missouri in 2013, for example, a 53-year-old patient who was scheduled to undergo a left-sided craniotomy bypass allegedly underwent a right-sided craniotomy and was unable to speak after surgery.
Wrong-site surgeries are also performed by general surgeons, urologists, cardiologists, otolaryngologists, and ophthalmologists. A 2021 lawsuit accused a Tampa urologist of removing the patient’s wrong testicle. And a 2019 lawsuit accused a Chicago ophthalmologist of operating on the wrong eye to remove a cyst.
It’s not just the surgeon’s mistake
Mistakes are not only made by the surgeon in the operating room (OR). They can be made by staff when scheduling a surgery, radiologists and pathologists when writing their reports for surgery, and by team members in the OR.
Many people are prone to confusing left and right. A 2020 study found that 14.9% of people had difficulty distinguishing left from right; other studies have shown higher rates. Distractions increase the likelihood of mistakes. In a 2015 study, background noise in a hospital ward made it more difficult for medical students to make left-right judgments.
OR personnel can be confused when patients are turned around. “To operate on the back of someone’s leg, the surgeon may turn the patient from supine to prone, and so left becomes right,” says Samuel C. Seiden, MD, an anesthesiologist in Roseville, Calif., who has studied wrong-site surgery.
Operative site markings that are drawn on the skin can be rubbed off when surgical prep is applied, and markings aren’t usually possible for procedures such as spine surgeries. Surgical draping can make it harder to distinguish the patient’s left and right, and a busy surgeon relying on memory may confuse cases and perform wrong-patient surgery.
A push to eliminate wrong-site surgery
In 2004, the Joint Commission, which accredits hospitals and many surgery centers, decided to do something about wrong-site surgery and related surgical errors. It released a universal protocol, which requires hospitals to take three steps to prevent errors: perform preoperative verification that is based on patient care documents; mark the operative site; and take a time-out just before surgery, during which the team should consider whether a mistake is about to be made.
Two years after the Joint Commission published its protocol, Dr. Seiden led a study to determine what effect it had had. The investigators found that wrong-site cases had decreased by only about one third. Preventing wrong-site surgery “turns out to be more complicated to eradicate than anybody thought,” Mark Chassin, MD, president of the Joint Commission, stated a few years later.
Why did the protocol have only a limited effect? Dr. Seiden says that it has been hard to change doctors’ traditional attitudes against standardization. “Some have had an attitude that checklists are for dummies, but that is changing,” he says.
For instance, some surgical teams were not paying attention during time-outs. “The time-out should be like the invocation of the National Anthem,” an orthopedic surgeon from Iowa wrote. “All other activities should stop.”
Even had surgeons followed the universal protocol, about one third of wrong-site surgeries would not have been identified, according to Dr. Kwaan’s study, which was published in the same year as Dr. Seiden’s. As an example, when the wrong kidney was removed at Methodist Hospital, in St. Louis Park, Minn., the hospital said it was following a protocol set by the Minnesota Hospital Association.
Redoubling efforts
In 2009, the Joint Commission decided to take another tack. It encouraged hospitals to make root-cause analyses not only of wrong-site surgeries but also of near misses, which are much more plentiful. It used the insights gained to change surgical routines and protocols.
The Safe Surgery Project, a collaboration between the Joint Commission’s Center for Transforming Healthcare and eight hospitals and surgery centers, reduced the number of errors and near misses by 46% in the scheduling area, 63% in pre-op, and 51% in the OR area.
From that project, the center developed the Targeted Solutions Tool, which basically uses the same methodology that the project used. The center told this news organization that 79 healthcare organizations have used the tool and have reduced the number of errors and near misses by 56% in scheduling, 24% in pre-op, and 48% in the OR.
For this approach to work, however, surgical teams must report their errors to the hospital, which had not been done before. A 2008 study by the Office of the Inspector General of the U.S. Department of Health and Human Services found that surgical staff did not report 86% of adverse events to their hospitals. Reasons given included lack of time, fear of punitive action, and skepticism that reporting would do any good.
Unlike some other adverse events, it’s hard to keep wrong-site surgeries secret from patients, because they can usually see the scars from it, but some surgeons invent ways to cover it up from patients, too, Dr. Mayer says. One wrong-side hernia repair was corrected in mid operation. Afterward, the surgeon told the patient that he had found another hernia on the other side and had fixed that one, too.
Changing the culture
Reformers argue that wrong-site surgeries can be prevented by changing the culture of the hospital or surgery center. “We have to think of wrong-site surgeries as a failure of the system, not of the individual,” says Ron Savrin, MD, a general surgeon in Chagrin Falls, Ohio, who is a surgery subject matter expert for the Sullivan Group. “It should never be only up to one individual to stop an error from occurring.”
Seeing oneself as part of a team can reduce errors. Although other people can introduce errors that make a person look bad, they can also stop the errors that might otherwise have occurred. Punishing individuals for making errors does little good in stopping errors.
“It’s human nature to want to punish somebody for making a mistake, and it’s hard to change that mentality,” Dr. Savrin says. He recalls that when he was a resident, “the morbidity and mortality conferences could be very difficult for anyone who made a mistake, but I think that attitude is changing.”
Studies have found wide variation in the number of wrong-site surgeries among hospitals. A recent Pennsylvania study found an average of one wrong-site surgery or near miss per hospital per year, but about one third of hospitals did not report any.
Wrong-site surgeries are often concentrated in certain hospitals -- even prestigious teaching hospitals are not immune. A decade ago, Rhode Island Hospital had five wrong-site surgeries in 2 years, and Boston’s Beth Israel Deaconess Medical Center had three wrong-spine surgeries within 2 months.
Other ways to reduce errors
Dr. Seiden thinks reform efforts should take a page from his own specialty. Anesthesiology has developed a variety of forcing functions, which are simple changes in technology that can stop errors. An example is the use of a valve that will not deliver a drug unless certain steps are followed.
The StartBox System, a new way to prevent surgical errors, delivers the surgery blade only after all safety information has been provided. Tested by 11 orthopedic surgeons performing 487 procedures, the system identified 17 near misses.
Another approach is to film time-outs so as to enforce compliance with protocols and help with root-cause analyses. NYU-Langone Medical Center, in New York City, not only films the time-out but also grades OR teams on compliance, says Dr. Bosco, who is vice chair of clinical affairs in the department of orthopedic surgery at the hospital.
In addition, more states are requiring hospitals to report adverse events, including wrong-site surgeries. According to the National Academy for State Health Policy, 28 states require the reporting of adverse events. However, only six states identify facilities in public reports; 16 states publish only aggregate data; and five states do not report error data to the public.
The goal is zero errors
Are there fewer wrong-site surgeries now? “My sense is that surgeons, hospitals, and surgery centers are taking wrong-site errors more seriously,” Dr. Savrin says.
Because reported information is spotty and no major studies on incidence have been conducted in recent years, “we don’t have a clear idea,” he says, “but my best guess is that the rate is declining.
“Absolute zero preventable errors has to be our goal,” Dr. Savrin says “We might not get there, but we can’t stop trying.”
A version of this article first appeared on Medscape.com.