User login
Top cases
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Junaid Beig, MBBS, FRACP wrote the following in “Subtherapeutic Azathioprine metabolites despite being adherent to medication”:
“I have an Ulcerative patient (Pancolitis) on Mesalazine and Azathioprine 150mg since 2018. His levels are subtherapeutic (6TGN 159 and 6MMP 70) despite being adherent to medication. He drinks 2 liters of wine per week.
“Questions: Is there any way we can find if he has high TPMT activity (Level is normal 6.1)? Does alcohol have an impact on TPMT activity? Does he warrant alternative treatment?”
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25109.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Junaid Beig, MBBS, FRACP wrote the following in “Subtherapeutic Azathioprine metabolites despite being adherent to medication”:
“I have an Ulcerative patient (Pancolitis) on Mesalazine and Azathioprine 150mg since 2018. His levels are subtherapeutic (6TGN 159 and 6MMP 70) despite being adherent to medication. He drinks 2 liters of wine per week.
“Questions: Is there any way we can find if he has high TPMT activity (Level is normal 6.1)? Does alcohol have an impact on TPMT activity? Does he warrant alternative treatment?”
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25109.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Junaid Beig, MBBS, FRACP wrote the following in “Subtherapeutic Azathioprine metabolites despite being adherent to medication”:
“I have an Ulcerative patient (Pancolitis) on Mesalazine and Azathioprine 150mg since 2018. His levels are subtherapeutic (6TGN 159 and 6MMP 70) despite being adherent to medication. He drinks 2 liters of wine per week.
“Questions: Is there any way we can find if he has high TPMT activity (Level is normal 6.1)? Does alcohol have an impact on TPMT activity? Does he warrant alternative treatment?”
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25109.
Fraudulent misbranding of PPE nets $22 million settlement
Avanos medical to pay $22 million to resolve criminal charge for fraudulent misbranding of PPE
A U.S.-based multinational medical device corporation will pay more than $22 million to resolve a criminal charge regarding fraudulent misbranding of their surgical gowns.
Avanos Medical Inc, which as its U.S. headquarters in Alpharetta, Georgia, is charged with one count of introducing misbranded surgical gowns into interstate commerce with the intent to defraud and mislead.
According to the Department of Justice, the company knowingly falsely labeled its MicroCool surgical gowns as providing AAMI Level 4 protection (the highest level) against fluid and virus penetration. Under the standards set by the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI), the highest protection level for surgical gowns is reserved for gowns intended to be used in surgeries and other high-risk medical procedures on patients suspected of having infectious diseases.
Avanos admitted to selling hundreds of thousands of MicroCool gowns that were falsely labeled as AAMI Level 4 between late 2014 and early 2015, as well as directly lying to customers about the gowns’ protective capacities. In total, Avanos sold almost $9 million of misbranded MicroCool gowns.
“The last thing health care workers should have to worry about is whether their personal protective equipment lives up to manufacturers’ claims,” said Acting U.S. Attorney Prerak Shah for the Northern District of Texas. “Misbranded PPE can pose serious risks to medical professionals and patients alike.”
Company pays $38.75 million to settle allegations of knowingly selling defective devices
Medical device manufacturers Alere and Alere San Diego (collectively, Alere) have agreed to pay almost $39 million to resolve allegations that they violated the False Claims Act by billing, and causing others to bill, the Medicare program for defective rapid point-of-care testing devices.
From 2008 to 2016, the Department of Justice alleges, Alere knowingly sold defective INRatio blood coagulation monitors used by Medicare beneficiaries who were taking anticoagulants. The software algorithms in the monitors contained a material defect, which Alere had found in their research, to cause inaccurate readings. Blood coagulation monitoring is essential for the safety of these patients, enabling them to maintain a safe dosage of their medications. Taking too much of an anticoagulant can cause major bleeding, while taking too little can cause blood clots that lead to strokes.
While Alere was aware that these devices were linked to over a dozen deaths and hundreds of injuries, the company continued to conceal the defect and billed Medicare for the devices.
In 2016, the product was taken off the market at the request of the FDA.
Mass. doctor, wife charged in international money laundering, fraud scheme
Massachusetts psychiatrist Rahim Shafa, MD, and his wife and office manager, Nahid Tormosi Shafa, are charged in connection to an international money laundering scheme involving importing illegal and misbranded drugs.
Through Shafa’s company, Novel Psychopharmacology, the two allegedly filed false and fraudulent Medicare reimbursement claims from 2016-2019, then deposited the money in their bank accounts, according to federal officials. From 2008-2018, the couple also engaged in an international money laundering scheme to purchase naltrexone pellet implants, disulfiram pellet implants, and injections from Hong Kong that were not approved by the FDA. According to officials, they falsified shipping documents, disguising the naltrexone pellet implants as “plastic beads in plastic tubes” to receive the drugs. They then offered to sell these drugs to patients of Novel Psychopharmacology.
Rahim Shafa was indicted on conspiracies of international money laundering, health care fraud, and defrauding the United States, as well as illegally importing merchandise and purposely delivering misbranded drugs. His wife was indicted on one count each of health care fraud conspiracy and international money laundering conspiracy.
Jury convicts medical equipment company owners of $27 million fraud
A federal jury in Texas convicted the owners of two durable medical equipment (DME) companies linked to a scheme to defraud Medicare.
Leah Hagen, 49, and Michael Hagen, 54, were convicted of one count of conspiracy to defraud the United States and to pay and receive health care kickbacks and one count of conspiracy to commit money laundering. The defendants owned and operated Metro DME Supply and Ortho Pain Solutions.
Ms. Hagen and Mr. Hagen paid a fixed rate per DME item in exchange for prescriptions and paperwork completed by telemedicine doctors that were used to submit false claims to Medicare, which totaled about $59 million. They were paid $27 million, and wired millions to their personal bank accounts. The defendants paid illegal bribes and kickbacks and wired money to their co-conspirator’s call center in the Philippines that provided signed doctor’s orders for orthotic braces.
At trial, evidence showed emails between Leah and Michael Hagen and their co-conspirators outlining a per-product pricing structure for orthotic braces, but not disclosing their agreement as one for marketing and other services.
At sentencing, the Hagens each face a maximum sentence of 25 years in prison.
A version of this article first appeared on Medscape.com.
Avanos medical to pay $22 million to resolve criminal charge for fraudulent misbranding of PPE
A U.S.-based multinational medical device corporation will pay more than $22 million to resolve a criminal charge regarding fraudulent misbranding of their surgical gowns.
Avanos Medical Inc, which as its U.S. headquarters in Alpharetta, Georgia, is charged with one count of introducing misbranded surgical gowns into interstate commerce with the intent to defraud and mislead.
According to the Department of Justice, the company knowingly falsely labeled its MicroCool surgical gowns as providing AAMI Level 4 protection (the highest level) against fluid and virus penetration. Under the standards set by the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI), the highest protection level for surgical gowns is reserved for gowns intended to be used in surgeries and other high-risk medical procedures on patients suspected of having infectious diseases.
Avanos admitted to selling hundreds of thousands of MicroCool gowns that were falsely labeled as AAMI Level 4 between late 2014 and early 2015, as well as directly lying to customers about the gowns’ protective capacities. In total, Avanos sold almost $9 million of misbranded MicroCool gowns.
“The last thing health care workers should have to worry about is whether their personal protective equipment lives up to manufacturers’ claims,” said Acting U.S. Attorney Prerak Shah for the Northern District of Texas. “Misbranded PPE can pose serious risks to medical professionals and patients alike.”
Company pays $38.75 million to settle allegations of knowingly selling defective devices
Medical device manufacturers Alere and Alere San Diego (collectively, Alere) have agreed to pay almost $39 million to resolve allegations that they violated the False Claims Act by billing, and causing others to bill, the Medicare program for defective rapid point-of-care testing devices.
From 2008 to 2016, the Department of Justice alleges, Alere knowingly sold defective INRatio blood coagulation monitors used by Medicare beneficiaries who were taking anticoagulants. The software algorithms in the monitors contained a material defect, which Alere had found in their research, to cause inaccurate readings. Blood coagulation monitoring is essential for the safety of these patients, enabling them to maintain a safe dosage of their medications. Taking too much of an anticoagulant can cause major bleeding, while taking too little can cause blood clots that lead to strokes.
While Alere was aware that these devices were linked to over a dozen deaths and hundreds of injuries, the company continued to conceal the defect and billed Medicare for the devices.
In 2016, the product was taken off the market at the request of the FDA.
Mass. doctor, wife charged in international money laundering, fraud scheme
Massachusetts psychiatrist Rahim Shafa, MD, and his wife and office manager, Nahid Tormosi Shafa, are charged in connection to an international money laundering scheme involving importing illegal and misbranded drugs.
Through Shafa’s company, Novel Psychopharmacology, the two allegedly filed false and fraudulent Medicare reimbursement claims from 2016-2019, then deposited the money in their bank accounts, according to federal officials. From 2008-2018, the couple also engaged in an international money laundering scheme to purchase naltrexone pellet implants, disulfiram pellet implants, and injections from Hong Kong that were not approved by the FDA. According to officials, they falsified shipping documents, disguising the naltrexone pellet implants as “plastic beads in plastic tubes” to receive the drugs. They then offered to sell these drugs to patients of Novel Psychopharmacology.
Rahim Shafa was indicted on conspiracies of international money laundering, health care fraud, and defrauding the United States, as well as illegally importing merchandise and purposely delivering misbranded drugs. His wife was indicted on one count each of health care fraud conspiracy and international money laundering conspiracy.
Jury convicts medical equipment company owners of $27 million fraud
A federal jury in Texas convicted the owners of two durable medical equipment (DME) companies linked to a scheme to defraud Medicare.
Leah Hagen, 49, and Michael Hagen, 54, were convicted of one count of conspiracy to defraud the United States and to pay and receive health care kickbacks and one count of conspiracy to commit money laundering. The defendants owned and operated Metro DME Supply and Ortho Pain Solutions.
Ms. Hagen and Mr. Hagen paid a fixed rate per DME item in exchange for prescriptions and paperwork completed by telemedicine doctors that were used to submit false claims to Medicare, which totaled about $59 million. They were paid $27 million, and wired millions to their personal bank accounts. The defendants paid illegal bribes and kickbacks and wired money to their co-conspirator’s call center in the Philippines that provided signed doctor’s orders for orthotic braces.
At trial, evidence showed emails between Leah and Michael Hagen and their co-conspirators outlining a per-product pricing structure for orthotic braces, but not disclosing their agreement as one for marketing and other services.
At sentencing, the Hagens each face a maximum sentence of 25 years in prison.
A version of this article first appeared on Medscape.com.
Avanos medical to pay $22 million to resolve criminal charge for fraudulent misbranding of PPE
A U.S.-based multinational medical device corporation will pay more than $22 million to resolve a criminal charge regarding fraudulent misbranding of their surgical gowns.
Avanos Medical Inc, which as its U.S. headquarters in Alpharetta, Georgia, is charged with one count of introducing misbranded surgical gowns into interstate commerce with the intent to defraud and mislead.
According to the Department of Justice, the company knowingly falsely labeled its MicroCool surgical gowns as providing AAMI Level 4 protection (the highest level) against fluid and virus penetration. Under the standards set by the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI), the highest protection level for surgical gowns is reserved for gowns intended to be used in surgeries and other high-risk medical procedures on patients suspected of having infectious diseases.
Avanos admitted to selling hundreds of thousands of MicroCool gowns that were falsely labeled as AAMI Level 4 between late 2014 and early 2015, as well as directly lying to customers about the gowns’ protective capacities. In total, Avanos sold almost $9 million of misbranded MicroCool gowns.
“The last thing health care workers should have to worry about is whether their personal protective equipment lives up to manufacturers’ claims,” said Acting U.S. Attorney Prerak Shah for the Northern District of Texas. “Misbranded PPE can pose serious risks to medical professionals and patients alike.”
Company pays $38.75 million to settle allegations of knowingly selling defective devices
Medical device manufacturers Alere and Alere San Diego (collectively, Alere) have agreed to pay almost $39 million to resolve allegations that they violated the False Claims Act by billing, and causing others to bill, the Medicare program for defective rapid point-of-care testing devices.
From 2008 to 2016, the Department of Justice alleges, Alere knowingly sold defective INRatio blood coagulation monitors used by Medicare beneficiaries who were taking anticoagulants. The software algorithms in the monitors contained a material defect, which Alere had found in their research, to cause inaccurate readings. Blood coagulation monitoring is essential for the safety of these patients, enabling them to maintain a safe dosage of their medications. Taking too much of an anticoagulant can cause major bleeding, while taking too little can cause blood clots that lead to strokes.
While Alere was aware that these devices were linked to over a dozen deaths and hundreds of injuries, the company continued to conceal the defect and billed Medicare for the devices.
In 2016, the product was taken off the market at the request of the FDA.
Mass. doctor, wife charged in international money laundering, fraud scheme
Massachusetts psychiatrist Rahim Shafa, MD, and his wife and office manager, Nahid Tormosi Shafa, are charged in connection to an international money laundering scheme involving importing illegal and misbranded drugs.
Through Shafa’s company, Novel Psychopharmacology, the two allegedly filed false and fraudulent Medicare reimbursement claims from 2016-2019, then deposited the money in their bank accounts, according to federal officials. From 2008-2018, the couple also engaged in an international money laundering scheme to purchase naltrexone pellet implants, disulfiram pellet implants, and injections from Hong Kong that were not approved by the FDA. According to officials, they falsified shipping documents, disguising the naltrexone pellet implants as “plastic beads in plastic tubes” to receive the drugs. They then offered to sell these drugs to patients of Novel Psychopharmacology.
Rahim Shafa was indicted on conspiracies of international money laundering, health care fraud, and defrauding the United States, as well as illegally importing merchandise and purposely delivering misbranded drugs. His wife was indicted on one count each of health care fraud conspiracy and international money laundering conspiracy.
Jury convicts medical equipment company owners of $27 million fraud
A federal jury in Texas convicted the owners of two durable medical equipment (DME) companies linked to a scheme to defraud Medicare.
Leah Hagen, 49, and Michael Hagen, 54, were convicted of one count of conspiracy to defraud the United States and to pay and receive health care kickbacks and one count of conspiracy to commit money laundering. The defendants owned and operated Metro DME Supply and Ortho Pain Solutions.
Ms. Hagen and Mr. Hagen paid a fixed rate per DME item in exchange for prescriptions and paperwork completed by telemedicine doctors that were used to submit false claims to Medicare, which totaled about $59 million. They were paid $27 million, and wired millions to their personal bank accounts. The defendants paid illegal bribes and kickbacks and wired money to their co-conspirator’s call center in the Philippines that provided signed doctor’s orders for orthotic braces.
At trial, evidence showed emails between Leah and Michael Hagen and their co-conspirators outlining a per-product pricing structure for orthotic braces, but not disclosing their agreement as one for marketing and other services.
At sentencing, the Hagens each face a maximum sentence of 25 years in prison.
A version of this article first appeared on Medscape.com.
Once daily poziotinib shows efficacy in non–small cell lung cancer
Tumor reductions, stated lead author Robin Cornelissen, PhD, MD, Erasmus University, Rotterdam, the Netherlands, were seen in 88% of patients.
EGFR and HER2 exon 20 insertion mutations are rare subsets accounting for about 10% each of all mutations and 2%-4% each in NSCLC. “There is no approved therapy for either treatment-naive or previously treated NSCLC with HER2 exon 20 mutations,” Dr. Cornelissen said in a virtual oral presentation (abstract LBA46) on Sept. 18. While chemotherapy agents with or without checkpoint inhibitors and tyrosine kinase inhibitors (TKIs) are currently utilized, none are specific to exon 20 mutations, and historical response rates from mostly small uncontrolled studies vary widely from about 6.9%-35%, with median progression-free survival (PFS) ranging from 3 to 7 months. Poziotinib is a potent oral pan-HER TKI with activity in patients with EGFR or HER2 exon 20–mutated NSCLC.
Dr. Cornelissen presented preliminary safety and efficacy data from the phase 2 ZENITH20, a seven-cohort global clinical trial, specifically from cohort 4 (daily dosing) which included 48 HER2 exon 20 insertion NSCLC patients (median age, 60.5 years; female/male, 26/22) treated first-line with oral daily poziotinib (16 mg). The majority were White (75%), female (54%), and nonsmokers (69%) with an Eastern Cooperative Oncology Group performance status of 1 (65%).The primary endpoint was objective response rate evaluated centrally by an independent image review committee using RECIST 1.1 criteria.
All patients have experienced treatment-related adverse events (TRAEs) with 10% considered serious, and permanent discontinuation in 13%. About 83% of patients had dose interruptions and 76% had dose reductions. The most common adverse events were diarrhea (any grade, 83%; grade 3, 15%), rash (any grade, 69%; grade 3, 35%), stomatitis/mucosal inflammation (any grade, 81%; grade 3, 21%), and paronychia (any grade, 46%; grade 3 8%). Pneumonitis occurred in two patients (4%), with one grade 3 (2%). No grade 4/5 TRAEs were reported.
Discontinuations in 44 patients (92%), Dr. Cornelissen said, are attributed to death (5/10%), disease progression (30/63%), adverse events (1/2%), and other (8/17%), with treatment ongoing in 4 patients (8%).
The rate for the primary endpoint of ORR was 43.8% (n = 21) (95% confidence interval, 29.5%-58.8%).Tumor reductions have been observed in 42/48 patients (88%) with a median reduction of 35%. One complete response was reported (2.1%), with partial responses in 20 (41.7%), stable disease in 15 (31.3%), progressive disease in 7 (14.6%), and 5 (10.4%) not evaluable. The disease control rate was 75.0%.
Among secondary endpoints, median duration of response (DoR) was 5.4 months (range, 2.8 to >19.1), with 42% of patients having response duration greater than6 months and 24% greater than 12 months. Median PFS was 5.6 months (range, 0 to >20.2), with progression-free survival duration greater than6 months in 42% and duration greater than12 months in 26%.
Dr. Cornelissen concluded: “Poziotinib shows clinically meaningful efficacy for treatment-naive NSCLC HER2 exon 20 mutations with [daily] dosing.” The toxicity profile, he added, is manageable and in line with previous poziotinib studies and other second-generation EGFR TKIs.
Noting that improved tolerability and antitumor activity have been observed in the cohort 5 (8 mg b.i.d.) interim analysis, Dr. Cornelissen said that cohort 4 is ongoing with patients enrolling at 8-mg b.i.d. dosing.
HER2 mutations represents 1.7%-2.2% of NSCLC, with high-sequence homology with EGFR mutation, observed ESMO-appointed discussant Daniel S.W. Tan, PhD, National Cancer Center in Singapore. He pointed out that, while HER2 antibody drug conjugates and TKIs have gained approval in other cancer types (e.g., breast, gastric), currently no HER2 therapies are approved in NSCLC. Reviewing ZENITH20 findings (risk ratio, 43.8%; DoR, 5.4 months; PFS, 5.6 months), Dr. Tan stated that poziotinib is an active agent in HER2 mutated NSCLC. “One concern that remains for me is the safety profile that will require further evaluation in order to determine optimal dosing,” he said.
Potential combinations, he added, need to be explored to improve the durability of response. “Until we can properly characterize this and other important aspects such as CNS activity, we need to be cautious about transitioning to a frontline setting. Also, we do need to give due consideration to strategies to improve HER2 testing rates in order to expand on clinical experience. This argues for the importance of broad upfront next generation sequencing testing in NSCLC.”
The study was funded by Spectrum Pharmaceuticals. Other authors associated with the research disclosed full or part-time employment with Spectrum.
Tumor reductions, stated lead author Robin Cornelissen, PhD, MD, Erasmus University, Rotterdam, the Netherlands, were seen in 88% of patients.
EGFR and HER2 exon 20 insertion mutations are rare subsets accounting for about 10% each of all mutations and 2%-4% each in NSCLC. “There is no approved therapy for either treatment-naive or previously treated NSCLC with HER2 exon 20 mutations,” Dr. Cornelissen said in a virtual oral presentation (abstract LBA46) on Sept. 18. While chemotherapy agents with or without checkpoint inhibitors and tyrosine kinase inhibitors (TKIs) are currently utilized, none are specific to exon 20 mutations, and historical response rates from mostly small uncontrolled studies vary widely from about 6.9%-35%, with median progression-free survival (PFS) ranging from 3 to 7 months. Poziotinib is a potent oral pan-HER TKI with activity in patients with EGFR or HER2 exon 20–mutated NSCLC.
Dr. Cornelissen presented preliminary safety and efficacy data from the phase 2 ZENITH20, a seven-cohort global clinical trial, specifically from cohort 4 (daily dosing) which included 48 HER2 exon 20 insertion NSCLC patients (median age, 60.5 years; female/male, 26/22) treated first-line with oral daily poziotinib (16 mg). The majority were White (75%), female (54%), and nonsmokers (69%) with an Eastern Cooperative Oncology Group performance status of 1 (65%).The primary endpoint was objective response rate evaluated centrally by an independent image review committee using RECIST 1.1 criteria.
All patients have experienced treatment-related adverse events (TRAEs) with 10% considered serious, and permanent discontinuation in 13%. About 83% of patients had dose interruptions and 76% had dose reductions. The most common adverse events were diarrhea (any grade, 83%; grade 3, 15%), rash (any grade, 69%; grade 3, 35%), stomatitis/mucosal inflammation (any grade, 81%; grade 3, 21%), and paronychia (any grade, 46%; grade 3 8%). Pneumonitis occurred in two patients (4%), with one grade 3 (2%). No grade 4/5 TRAEs were reported.
Discontinuations in 44 patients (92%), Dr. Cornelissen said, are attributed to death (5/10%), disease progression (30/63%), adverse events (1/2%), and other (8/17%), with treatment ongoing in 4 patients (8%).
The rate for the primary endpoint of ORR was 43.8% (n = 21) (95% confidence interval, 29.5%-58.8%).Tumor reductions have been observed in 42/48 patients (88%) with a median reduction of 35%. One complete response was reported (2.1%), with partial responses in 20 (41.7%), stable disease in 15 (31.3%), progressive disease in 7 (14.6%), and 5 (10.4%) not evaluable. The disease control rate was 75.0%.
Among secondary endpoints, median duration of response (DoR) was 5.4 months (range, 2.8 to >19.1), with 42% of patients having response duration greater than6 months and 24% greater than 12 months. Median PFS was 5.6 months (range, 0 to >20.2), with progression-free survival duration greater than6 months in 42% and duration greater than12 months in 26%.
Dr. Cornelissen concluded: “Poziotinib shows clinically meaningful efficacy for treatment-naive NSCLC HER2 exon 20 mutations with [daily] dosing.” The toxicity profile, he added, is manageable and in line with previous poziotinib studies and other second-generation EGFR TKIs.
Noting that improved tolerability and antitumor activity have been observed in the cohort 5 (8 mg b.i.d.) interim analysis, Dr. Cornelissen said that cohort 4 is ongoing with patients enrolling at 8-mg b.i.d. dosing.
HER2 mutations represents 1.7%-2.2% of NSCLC, with high-sequence homology with EGFR mutation, observed ESMO-appointed discussant Daniel S.W. Tan, PhD, National Cancer Center in Singapore. He pointed out that, while HER2 antibody drug conjugates and TKIs have gained approval in other cancer types (e.g., breast, gastric), currently no HER2 therapies are approved in NSCLC. Reviewing ZENITH20 findings (risk ratio, 43.8%; DoR, 5.4 months; PFS, 5.6 months), Dr. Tan stated that poziotinib is an active agent in HER2 mutated NSCLC. “One concern that remains for me is the safety profile that will require further evaluation in order to determine optimal dosing,” he said.
Potential combinations, he added, need to be explored to improve the durability of response. “Until we can properly characterize this and other important aspects such as CNS activity, we need to be cautious about transitioning to a frontline setting. Also, we do need to give due consideration to strategies to improve HER2 testing rates in order to expand on clinical experience. This argues for the importance of broad upfront next generation sequencing testing in NSCLC.”
The study was funded by Spectrum Pharmaceuticals. Other authors associated with the research disclosed full or part-time employment with Spectrum.
Tumor reductions, stated lead author Robin Cornelissen, PhD, MD, Erasmus University, Rotterdam, the Netherlands, were seen in 88% of patients.
EGFR and HER2 exon 20 insertion mutations are rare subsets accounting for about 10% each of all mutations and 2%-4% each in NSCLC. “There is no approved therapy for either treatment-naive or previously treated NSCLC with HER2 exon 20 mutations,” Dr. Cornelissen said in a virtual oral presentation (abstract LBA46) on Sept. 18. While chemotherapy agents with or without checkpoint inhibitors and tyrosine kinase inhibitors (TKIs) are currently utilized, none are specific to exon 20 mutations, and historical response rates from mostly small uncontrolled studies vary widely from about 6.9%-35%, with median progression-free survival (PFS) ranging from 3 to 7 months. Poziotinib is a potent oral pan-HER TKI with activity in patients with EGFR or HER2 exon 20–mutated NSCLC.
Dr. Cornelissen presented preliminary safety and efficacy data from the phase 2 ZENITH20, a seven-cohort global clinical trial, specifically from cohort 4 (daily dosing) which included 48 HER2 exon 20 insertion NSCLC patients (median age, 60.5 years; female/male, 26/22) treated first-line with oral daily poziotinib (16 mg). The majority were White (75%), female (54%), and nonsmokers (69%) with an Eastern Cooperative Oncology Group performance status of 1 (65%).The primary endpoint was objective response rate evaluated centrally by an independent image review committee using RECIST 1.1 criteria.
All patients have experienced treatment-related adverse events (TRAEs) with 10% considered serious, and permanent discontinuation in 13%. About 83% of patients had dose interruptions and 76% had dose reductions. The most common adverse events were diarrhea (any grade, 83%; grade 3, 15%), rash (any grade, 69%; grade 3, 35%), stomatitis/mucosal inflammation (any grade, 81%; grade 3, 21%), and paronychia (any grade, 46%; grade 3 8%). Pneumonitis occurred in two patients (4%), with one grade 3 (2%). No grade 4/5 TRAEs were reported.
Discontinuations in 44 patients (92%), Dr. Cornelissen said, are attributed to death (5/10%), disease progression (30/63%), adverse events (1/2%), and other (8/17%), with treatment ongoing in 4 patients (8%).
The rate for the primary endpoint of ORR was 43.8% (n = 21) (95% confidence interval, 29.5%-58.8%).Tumor reductions have been observed in 42/48 patients (88%) with a median reduction of 35%. One complete response was reported (2.1%), with partial responses in 20 (41.7%), stable disease in 15 (31.3%), progressive disease in 7 (14.6%), and 5 (10.4%) not evaluable. The disease control rate was 75.0%.
Among secondary endpoints, median duration of response (DoR) was 5.4 months (range, 2.8 to >19.1), with 42% of patients having response duration greater than6 months and 24% greater than 12 months. Median PFS was 5.6 months (range, 0 to >20.2), with progression-free survival duration greater than6 months in 42% and duration greater than12 months in 26%.
Dr. Cornelissen concluded: “Poziotinib shows clinically meaningful efficacy for treatment-naive NSCLC HER2 exon 20 mutations with [daily] dosing.” The toxicity profile, he added, is manageable and in line with previous poziotinib studies and other second-generation EGFR TKIs.
Noting that improved tolerability and antitumor activity have been observed in the cohort 5 (8 mg b.i.d.) interim analysis, Dr. Cornelissen said that cohort 4 is ongoing with patients enrolling at 8-mg b.i.d. dosing.
HER2 mutations represents 1.7%-2.2% of NSCLC, with high-sequence homology with EGFR mutation, observed ESMO-appointed discussant Daniel S.W. Tan, PhD, National Cancer Center in Singapore. He pointed out that, while HER2 antibody drug conjugates and TKIs have gained approval in other cancer types (e.g., breast, gastric), currently no HER2 therapies are approved in NSCLC. Reviewing ZENITH20 findings (risk ratio, 43.8%; DoR, 5.4 months; PFS, 5.6 months), Dr. Tan stated that poziotinib is an active agent in HER2 mutated NSCLC. “One concern that remains for me is the safety profile that will require further evaluation in order to determine optimal dosing,” he said.
Potential combinations, he added, need to be explored to improve the durability of response. “Until we can properly characterize this and other important aspects such as CNS activity, we need to be cautious about transitioning to a frontline setting. Also, we do need to give due consideration to strategies to improve HER2 testing rates in order to expand on clinical experience. This argues for the importance of broad upfront next generation sequencing testing in NSCLC.”
The study was funded by Spectrum Pharmaceuticals. Other authors associated with the research disclosed full or part-time employment with Spectrum.
FROM ESMO 2021
Children and COVID: New cases topped 200,000 after 3 weeks of declines
Weekly COVID-19 cases in children dropped again, but the count remained above 200,000 for the fifth consecutive week, according to the American Academy of Pediatrics and the Children’s Hospital Association.
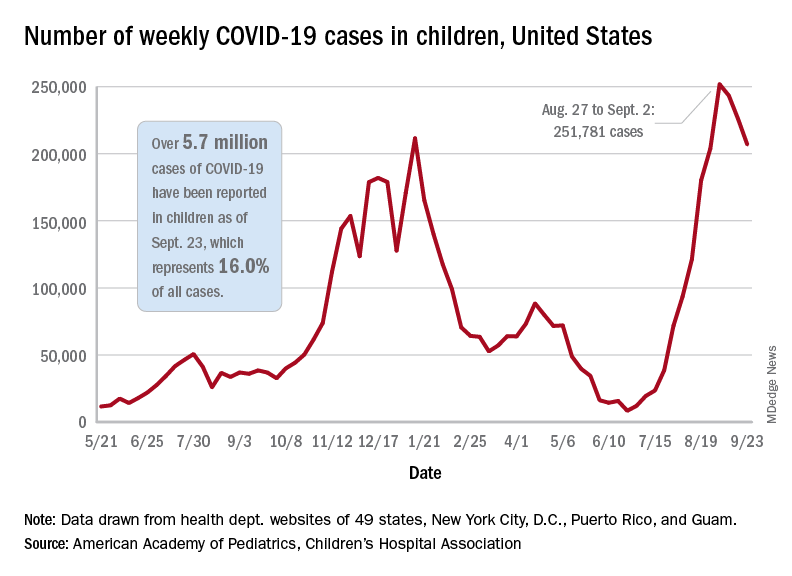
based on the data in the AAP/CHA joint weekly report on COVID in children.
In the most recent week, Sept. 17-23, there were almost 207,000 new cases of COVID-19 in children, which represented 26.7% of all cases reported in the 46 states that are currently posting data by age on their COVID dashboards, the AAP and CHA said. (New York has never reported such data by age, and Alabama, Nebraska, and Texas have not updated their websites since July 29, June 24, and Aug. 26, respectively.)
The decline in new vaccinations among children, however, began before the summer surge in new cases hit its peak – 251,781 during the week of Aug. 27 to Sept. 2 – and has continued for 7 straight weeks in children aged 12-17 years, based on data from the Centers for Disease Control and Prevention.
There were about 172,000 COVID vaccine initiations in children aged 12-17 for the week of Sept. 21-27, the lowest number since April, before it was approved for use in 12- to 15-year-olds. That figure is down by almost a third from the previous week and by more than two-thirds since early August, just before the decline in vaccinations began, according to the CDC’s COVID Data Tracker.
The cumulative vaccine situation looks like this: Just over 13 million children under age 18 years have received at least one dose as of Sept. 27, and almost 10.6 million are fully vaccinated. By age group, 53.9% of 12- to 15-year-olds and 61.6% of 16- to 17-year-olds have received at least one dose, with corresponding figures of 43.3% and 51.3% for full vaccination, the CDC said.
COVID-related hospital admissions also continue to fall after peaking at 0.51 children aged 0-17 per 100,000 population on Sept. 4. The admission rate was down to 0.45 per 100,000 as of Sept. 17, and the latest 7-day average (Sept. 19-25) was 258 admissions, compared with a peak of 371 for the week of Aug. 29 to Sept. 4, the CDC reported.
“Although we have seen slight improvements in COVID-19 volumes in the past week, we are at the beginning of an anticipated increase in” multi-inflammatory syndrome in children, Margaret Rush, MD, president of Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said at a recent hearing of the House Committee on Energy and Commerce’s Oversight subcommittee. That increase would be expected to produce “a secondary wave of seriously ill children 3-6 weeks after acute infection peaks in the community,” the American Hospital Association said.
Meanwhile, Dr. Rush noted, there are signs that seasonal viruses are coming into play. “With the emergence of the Delta variant, we’ve experienced a steep increase in COVID-19 hospitalizations among children on top of an early surge of [respiratory syncytial virus], a serious respiratory illness we usually see in the winter months,” she said in a prepared statement before her testimony.
Weekly COVID-19 cases in children dropped again, but the count remained above 200,000 for the fifth consecutive week, according to the American Academy of Pediatrics and the Children’s Hospital Association.

based on the data in the AAP/CHA joint weekly report on COVID in children.
In the most recent week, Sept. 17-23, there were almost 207,000 new cases of COVID-19 in children, which represented 26.7% of all cases reported in the 46 states that are currently posting data by age on their COVID dashboards, the AAP and CHA said. (New York has never reported such data by age, and Alabama, Nebraska, and Texas have not updated their websites since July 29, June 24, and Aug. 26, respectively.)
The decline in new vaccinations among children, however, began before the summer surge in new cases hit its peak – 251,781 during the week of Aug. 27 to Sept. 2 – and has continued for 7 straight weeks in children aged 12-17 years, based on data from the Centers for Disease Control and Prevention.
There were about 172,000 COVID vaccine initiations in children aged 12-17 for the week of Sept. 21-27, the lowest number since April, before it was approved for use in 12- to 15-year-olds. That figure is down by almost a third from the previous week and by more than two-thirds since early August, just before the decline in vaccinations began, according to the CDC’s COVID Data Tracker.
The cumulative vaccine situation looks like this: Just over 13 million children under age 18 years have received at least one dose as of Sept. 27, and almost 10.6 million are fully vaccinated. By age group, 53.9% of 12- to 15-year-olds and 61.6% of 16- to 17-year-olds have received at least one dose, with corresponding figures of 43.3% and 51.3% for full vaccination, the CDC said.
COVID-related hospital admissions also continue to fall after peaking at 0.51 children aged 0-17 per 100,000 population on Sept. 4. The admission rate was down to 0.45 per 100,000 as of Sept. 17, and the latest 7-day average (Sept. 19-25) was 258 admissions, compared with a peak of 371 for the week of Aug. 29 to Sept. 4, the CDC reported.
“Although we have seen slight improvements in COVID-19 volumes in the past week, we are at the beginning of an anticipated increase in” multi-inflammatory syndrome in children, Margaret Rush, MD, president of Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said at a recent hearing of the House Committee on Energy and Commerce’s Oversight subcommittee. That increase would be expected to produce “a secondary wave of seriously ill children 3-6 weeks after acute infection peaks in the community,” the American Hospital Association said.
Meanwhile, Dr. Rush noted, there are signs that seasonal viruses are coming into play. “With the emergence of the Delta variant, we’ve experienced a steep increase in COVID-19 hospitalizations among children on top of an early surge of [respiratory syncytial virus], a serious respiratory illness we usually see in the winter months,” she said in a prepared statement before her testimony.
Weekly COVID-19 cases in children dropped again, but the count remained above 200,000 for the fifth consecutive week, according to the American Academy of Pediatrics and the Children’s Hospital Association.

based on the data in the AAP/CHA joint weekly report on COVID in children.
In the most recent week, Sept. 17-23, there were almost 207,000 new cases of COVID-19 in children, which represented 26.7% of all cases reported in the 46 states that are currently posting data by age on their COVID dashboards, the AAP and CHA said. (New York has never reported such data by age, and Alabama, Nebraska, and Texas have not updated their websites since July 29, June 24, and Aug. 26, respectively.)
The decline in new vaccinations among children, however, began before the summer surge in new cases hit its peak – 251,781 during the week of Aug. 27 to Sept. 2 – and has continued for 7 straight weeks in children aged 12-17 years, based on data from the Centers for Disease Control and Prevention.
There were about 172,000 COVID vaccine initiations in children aged 12-17 for the week of Sept. 21-27, the lowest number since April, before it was approved for use in 12- to 15-year-olds. That figure is down by almost a third from the previous week and by more than two-thirds since early August, just before the decline in vaccinations began, according to the CDC’s COVID Data Tracker.
The cumulative vaccine situation looks like this: Just over 13 million children under age 18 years have received at least one dose as of Sept. 27, and almost 10.6 million are fully vaccinated. By age group, 53.9% of 12- to 15-year-olds and 61.6% of 16- to 17-year-olds have received at least one dose, with corresponding figures of 43.3% and 51.3% for full vaccination, the CDC said.
COVID-related hospital admissions also continue to fall after peaking at 0.51 children aged 0-17 per 100,000 population on Sept. 4. The admission rate was down to 0.45 per 100,000 as of Sept. 17, and the latest 7-day average (Sept. 19-25) was 258 admissions, compared with a peak of 371 for the week of Aug. 29 to Sept. 4, the CDC reported.
“Although we have seen slight improvements in COVID-19 volumes in the past week, we are at the beginning of an anticipated increase in” multi-inflammatory syndrome in children, Margaret Rush, MD, president of Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said at a recent hearing of the House Committee on Energy and Commerce’s Oversight subcommittee. That increase would be expected to produce “a secondary wave of seriously ill children 3-6 weeks after acute infection peaks in the community,” the American Hospital Association said.
Meanwhile, Dr. Rush noted, there are signs that seasonal viruses are coming into play. “With the emergence of the Delta variant, we’ve experienced a steep increase in COVID-19 hospitalizations among children on top of an early surge of [respiratory syncytial virus], a serious respiratory illness we usually see in the winter months,” she said in a prepared statement before her testimony.
Polyethylene glycol linked to rare allergic reactions seen with mRNA COVID-19 vaccines
A common inert ingredient may be the culprit behind the rare allergic reactions reported among individuals who have received mRNA COVID-19 vaccines, according to investigators at a large regional health center that was among the first to administer the shots.
Blood samples from 10 of 11 individuals with suspected allergic reactions reacted to polyethylene glycol (PEG), a component of both the Pfizer and Moderna mRNA vaccines, according to a report in JAMA Network Open.
In total, only 22 individuals had suspected allergic reactions out of nearly 39,000 mRNA COVID-19 vaccine doses administered, the investigators reported, noting that the reactions were generally mild and all fully resolved.
Those findings should be reassuring to individuals who are reticent to sign up for a COVID-19 vaccine because of fear of an allergic reaction, said study senior author Kari Nadeau, MD, PhD, director of the Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
“We’re hoping that this word will get out and then that the companies could also think about making vaccines that have other products in them that don’t include polyethylene glycol,” Dr. Nadeau said in an interview.
PEG is a compound used in many products, including pharmaceuticals, cosmetics, and food. In the mRNA COVID-19 vaccines, PEG serves to stabilize the lipid nanoparticles that help protect and transport mRNA. However, its use in this setting has been linked to allergic reactions in this and previous studies.
No immunoglobulin E (IgE) antibodies to PEG were detected among the 22 individuals with suspected allergic reactions to mRNA COVID-19 vaccine, but PEG immunoglobulin G (IgG) was present. That suggests non-IgE mediated allergic reactions to PEG may be implicated for the majority of cases, Dr. Nadeau said.
This case series provides interesting new evidence to confirm previous reports that a mechanism other than the classic IgE-mediated allergic response is behind the suspected allergic reactions that are occurring after mRNA COVID-19 vaccine, said Aleena Banerji, MD, associate professor at Harvard Medical School, Boston, and clinical director of the Drug Allergy Program at Massachusetts General Hospital.
“We need to further understand the mechanism of these reactions, but what we know is that IGE mediated allergy to excipients like PEG is probably not the main cause,” Dr. Banerji, who was not involved in the study, said in an interview.
In a recent research letter published in JAMA Internal Medicine, Dr. Banerji and coauthors reported that all individuals with immediate suspected allergic reactions to mRNA COVID-19 vaccine went on to tolerate the second dose, with mild symptoms reported in the minority of patients (32 out of 159, or about 20%).
“Again, that is very consistent with not having an IgE-mediated allergy, so it seems to all be fitting with that picture,” Dr. Banerji said.
The case series by Dr. Nadeau and coauthors was based on review of nearly 39,000 mRNA COVID-19 vaccine doses administered between December 18, 2020 and January 26, 2021. Most mRNA vaccine recipients were Stanford-affiliated health care workers, according to the report.
Among recipients of those doses, they identified 148 individuals who had anaphylaxis-related ICD-10 codes recorded over the same time period. In a review of medical records, investigators pinpointed 22 individuals as having suspected allergy and invited them to participate in follow-up allergy testing.
A total of 11 individuals underwent skin prick testing, but none of them tested positive to PEG or to polysorbate 80, another excipient that has been linked to vaccine-related allergic reactions. One of the patients tested positive to the same mRNA vaccine they had previously received, according to the report.
Those same 11 individuals also underwent basophil activation testing (BAT). In contrast to the skin testing results, BAT results were positive for PEG in 10 of 11 cases (or 91%) and positive for their administered vaccine in all 11 cases, the report shows.
High levels of IgG to PEG were identified in blood samples of individuals with an allergy to the vaccine. Investigators said it’s possible that the BAT results were activated due to IgG via complement activation–related pseudoallergy, or CARPA, as has been hypothesized by some other investigators.
The negative skin prick testing results for PEG, which contrast with the positive BAT results to PEG, suggest that the former may not be appropriate for use as a predictive marker of potential vaccine allergy, according to Dr. Nadeau.
“The take-home message for doctors is to be careful,” she said. “Don’t assume that just because the person skin-tests negative to PEG or to the vaccine itself that you’re out of the woods, because the skin test would be often negative in those scenarios.”
The study was supported by a grants from the Asthma and Allergic Diseases Cooperative Research Centers, a grant from the National Institutes of Health, the National Institute of Allergy and Infectious Disease SARS Vaccine study, the Parker Foundation, the Crown Foundation, and the Sunshine Foundation. Dr. Nadeau reports numerous conflicts with various sources in the industry. Dr. Banerji has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A common inert ingredient may be the culprit behind the rare allergic reactions reported among individuals who have received mRNA COVID-19 vaccines, according to investigators at a large regional health center that was among the first to administer the shots.
Blood samples from 10 of 11 individuals with suspected allergic reactions reacted to polyethylene glycol (PEG), a component of both the Pfizer and Moderna mRNA vaccines, according to a report in JAMA Network Open.
In total, only 22 individuals had suspected allergic reactions out of nearly 39,000 mRNA COVID-19 vaccine doses administered, the investigators reported, noting that the reactions were generally mild and all fully resolved.
Those findings should be reassuring to individuals who are reticent to sign up for a COVID-19 vaccine because of fear of an allergic reaction, said study senior author Kari Nadeau, MD, PhD, director of the Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
“We’re hoping that this word will get out and then that the companies could also think about making vaccines that have other products in them that don’t include polyethylene glycol,” Dr. Nadeau said in an interview.
PEG is a compound used in many products, including pharmaceuticals, cosmetics, and food. In the mRNA COVID-19 vaccines, PEG serves to stabilize the lipid nanoparticles that help protect and transport mRNA. However, its use in this setting has been linked to allergic reactions in this and previous studies.
No immunoglobulin E (IgE) antibodies to PEG were detected among the 22 individuals with suspected allergic reactions to mRNA COVID-19 vaccine, but PEG immunoglobulin G (IgG) was present. That suggests non-IgE mediated allergic reactions to PEG may be implicated for the majority of cases, Dr. Nadeau said.
This case series provides interesting new evidence to confirm previous reports that a mechanism other than the classic IgE-mediated allergic response is behind the suspected allergic reactions that are occurring after mRNA COVID-19 vaccine, said Aleena Banerji, MD, associate professor at Harvard Medical School, Boston, and clinical director of the Drug Allergy Program at Massachusetts General Hospital.
“We need to further understand the mechanism of these reactions, but what we know is that IGE mediated allergy to excipients like PEG is probably not the main cause,” Dr. Banerji, who was not involved in the study, said in an interview.
In a recent research letter published in JAMA Internal Medicine, Dr. Banerji and coauthors reported that all individuals with immediate suspected allergic reactions to mRNA COVID-19 vaccine went on to tolerate the second dose, with mild symptoms reported in the minority of patients (32 out of 159, or about 20%).
“Again, that is very consistent with not having an IgE-mediated allergy, so it seems to all be fitting with that picture,” Dr. Banerji said.
The case series by Dr. Nadeau and coauthors was based on review of nearly 39,000 mRNA COVID-19 vaccine doses administered between December 18, 2020 and January 26, 2021. Most mRNA vaccine recipients were Stanford-affiliated health care workers, according to the report.
Among recipients of those doses, they identified 148 individuals who had anaphylaxis-related ICD-10 codes recorded over the same time period. In a review of medical records, investigators pinpointed 22 individuals as having suspected allergy and invited them to participate in follow-up allergy testing.
A total of 11 individuals underwent skin prick testing, but none of them tested positive to PEG or to polysorbate 80, another excipient that has been linked to vaccine-related allergic reactions. One of the patients tested positive to the same mRNA vaccine they had previously received, according to the report.
Those same 11 individuals also underwent basophil activation testing (BAT). In contrast to the skin testing results, BAT results were positive for PEG in 10 of 11 cases (or 91%) and positive for their administered vaccine in all 11 cases, the report shows.
High levels of IgG to PEG were identified in blood samples of individuals with an allergy to the vaccine. Investigators said it’s possible that the BAT results were activated due to IgG via complement activation–related pseudoallergy, or CARPA, as has been hypothesized by some other investigators.
The negative skin prick testing results for PEG, which contrast with the positive BAT results to PEG, suggest that the former may not be appropriate for use as a predictive marker of potential vaccine allergy, according to Dr. Nadeau.
“The take-home message for doctors is to be careful,” she said. “Don’t assume that just because the person skin-tests negative to PEG or to the vaccine itself that you’re out of the woods, because the skin test would be often negative in those scenarios.”
The study was supported by a grants from the Asthma and Allergic Diseases Cooperative Research Centers, a grant from the National Institutes of Health, the National Institute of Allergy and Infectious Disease SARS Vaccine study, the Parker Foundation, the Crown Foundation, and the Sunshine Foundation. Dr. Nadeau reports numerous conflicts with various sources in the industry. Dr. Banerji has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A common inert ingredient may be the culprit behind the rare allergic reactions reported among individuals who have received mRNA COVID-19 vaccines, according to investigators at a large regional health center that was among the first to administer the shots.
Blood samples from 10 of 11 individuals with suspected allergic reactions reacted to polyethylene glycol (PEG), a component of both the Pfizer and Moderna mRNA vaccines, according to a report in JAMA Network Open.
In total, only 22 individuals had suspected allergic reactions out of nearly 39,000 mRNA COVID-19 vaccine doses administered, the investigators reported, noting that the reactions were generally mild and all fully resolved.
Those findings should be reassuring to individuals who are reticent to sign up for a COVID-19 vaccine because of fear of an allergic reaction, said study senior author Kari Nadeau, MD, PhD, director of the Parker Center for Allergy and Asthma Research at Stanford (Calif.) University.
“We’re hoping that this word will get out and then that the companies could also think about making vaccines that have other products in them that don’t include polyethylene glycol,” Dr. Nadeau said in an interview.
PEG is a compound used in many products, including pharmaceuticals, cosmetics, and food. In the mRNA COVID-19 vaccines, PEG serves to stabilize the lipid nanoparticles that help protect and transport mRNA. However, its use in this setting has been linked to allergic reactions in this and previous studies.
No immunoglobulin E (IgE) antibodies to PEG were detected among the 22 individuals with suspected allergic reactions to mRNA COVID-19 vaccine, but PEG immunoglobulin G (IgG) was present. That suggests non-IgE mediated allergic reactions to PEG may be implicated for the majority of cases, Dr. Nadeau said.
This case series provides interesting new evidence to confirm previous reports that a mechanism other than the classic IgE-mediated allergic response is behind the suspected allergic reactions that are occurring after mRNA COVID-19 vaccine, said Aleena Banerji, MD, associate professor at Harvard Medical School, Boston, and clinical director of the Drug Allergy Program at Massachusetts General Hospital.
“We need to further understand the mechanism of these reactions, but what we know is that IGE mediated allergy to excipients like PEG is probably not the main cause,” Dr. Banerji, who was not involved in the study, said in an interview.
In a recent research letter published in JAMA Internal Medicine, Dr. Banerji and coauthors reported that all individuals with immediate suspected allergic reactions to mRNA COVID-19 vaccine went on to tolerate the second dose, with mild symptoms reported in the minority of patients (32 out of 159, or about 20%).
“Again, that is very consistent with not having an IgE-mediated allergy, so it seems to all be fitting with that picture,” Dr. Banerji said.
The case series by Dr. Nadeau and coauthors was based on review of nearly 39,000 mRNA COVID-19 vaccine doses administered between December 18, 2020 and January 26, 2021. Most mRNA vaccine recipients were Stanford-affiliated health care workers, according to the report.
Among recipients of those doses, they identified 148 individuals who had anaphylaxis-related ICD-10 codes recorded over the same time period. In a review of medical records, investigators pinpointed 22 individuals as having suspected allergy and invited them to participate in follow-up allergy testing.
A total of 11 individuals underwent skin prick testing, but none of them tested positive to PEG or to polysorbate 80, another excipient that has been linked to vaccine-related allergic reactions. One of the patients tested positive to the same mRNA vaccine they had previously received, according to the report.
Those same 11 individuals also underwent basophil activation testing (BAT). In contrast to the skin testing results, BAT results were positive for PEG in 10 of 11 cases (or 91%) and positive for their administered vaccine in all 11 cases, the report shows.
High levels of IgG to PEG were identified in blood samples of individuals with an allergy to the vaccine. Investigators said it’s possible that the BAT results were activated due to IgG via complement activation–related pseudoallergy, or CARPA, as has been hypothesized by some other investigators.
The negative skin prick testing results for PEG, which contrast with the positive BAT results to PEG, suggest that the former may not be appropriate for use as a predictive marker of potential vaccine allergy, according to Dr. Nadeau.
“The take-home message for doctors is to be careful,” she said. “Don’t assume that just because the person skin-tests negative to PEG or to the vaccine itself that you’re out of the woods, because the skin test would be often negative in those scenarios.”
The study was supported by a grants from the Asthma and Allergic Diseases Cooperative Research Centers, a grant from the National Institutes of Health, the National Institute of Allergy and Infectious Disease SARS Vaccine study, the Parker Foundation, the Crown Foundation, and the Sunshine Foundation. Dr. Nadeau reports numerous conflicts with various sources in the industry. Dr. Banerji has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Why Texas Senate Bill 8 will negatively affect LGBTQ patients
On Sept. 1, Texas enacted astonishing legislation that effectively bans abortion after a fetal heartbeat is detected. In addition, it further empowers private citizens to sue anyone “aiding and abetting” patients who seek abortion services. Many organizations, including Planned Parenthood and the American College of Obstetricians and Gynecologists, have issued formalized statements condemning the bill. While we as obstetrician/gynecologists try to remain as nonpartisan as humanly possible in our patient care, unfortunately our specialty is inarguably one of the few in the medical field that is routinely significantly affected by federal and state politics.
It is no secret that Texas Senate Bill 8, otherwise referred to the “Texas Heartbeat Act,” will have devastating consequences for women, particularly women of color, but it will also have potentially catastrophic repercussions for patients who identify as LGBTQ. Overall, the LGBTQ population faces higher rates of poverty, unemployment, insurance coverage barriers, and provider discrimination because of their gender identity or sexual orientation, which can make access to abortion services challenging. Furthermore, they are more susceptible to hate-motivated violence and sexual assault and as a result, may seek to terminate pregnancies that result from these traumatic experiences.
A survey conducted by the Centers for Disease Control and Prevention examining rates of intimate partner violence and sexual violence found that 44% of lesbians and 61% of bisexual women experience rape and physical violence, compared with 35% of straight women.1 A separate survey revealed that 47% of transgender people are sexually assaulted at some point in their lifetime, with rates reaching as high as 65% among transgender people of color.2 Furthermore, many members of the LGBTQ population are misinformed or have misconceptions regarding their need for contraceptives and experience unintended pregnancies. As discussed in a previous column, one-third of pregnancies in transgender men were unplanned, and 20% of those patients were amenorrheic on testosterone at the time of conception.3
Current studies estimate that approximately 25% of all cisgender women will have an abortion. No corresponding data exist to describe the abortion rates of transgender and gender diverse patients.4,5 Bills such as Texas SB8 make accessing safe abortions for patients virtually impossible and interferes with the ability for physicians to provide patients with much needed health care services. It further delegitimizes rape and incest victims and is almost punitive in requiring such victims to carry the unintended pregnancies resulting from these heinous acts to term.
Regardless of a provider’s feelings toward abortion or even gender-affirming care, it is undeniable that access to these services is necessary and should be readily available to patients seeking them. As we all took an oath in medical school to “do no harm,” we must not only abide by that solemn decree in everyday patient interactions, but also live by those words to advocate for our patients when politics prohibit appropriate care. While discussions surrounding abortion are often limited to cisgender, heterosexual patients, providers must also be aware that abortion access spans across a wider spectrum that includes the LGBTQ community. Our patients, and all patients, deserve equal access to abortion. This harmful law sets a dangerous precedent that could continue to threaten these services with detrimental effects to our patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
3. Abern L, Maguire K. Obstet Gynecol 2018;131:65S.
4. Jones RK et al. Abortion incidence and service availability in the Unites States, 2017. New York, NY: Guttmacher Institute: 2019.
5. Moseson H et al. Am J Obstet Gynecol 2021;224:376.e1-11.
On Sept. 1, Texas enacted astonishing legislation that effectively bans abortion after a fetal heartbeat is detected. In addition, it further empowers private citizens to sue anyone “aiding and abetting” patients who seek abortion services. Many organizations, including Planned Parenthood and the American College of Obstetricians and Gynecologists, have issued formalized statements condemning the bill. While we as obstetrician/gynecologists try to remain as nonpartisan as humanly possible in our patient care, unfortunately our specialty is inarguably one of the few in the medical field that is routinely significantly affected by federal and state politics.
It is no secret that Texas Senate Bill 8, otherwise referred to the “Texas Heartbeat Act,” will have devastating consequences for women, particularly women of color, but it will also have potentially catastrophic repercussions for patients who identify as LGBTQ. Overall, the LGBTQ population faces higher rates of poverty, unemployment, insurance coverage barriers, and provider discrimination because of their gender identity or sexual orientation, which can make access to abortion services challenging. Furthermore, they are more susceptible to hate-motivated violence and sexual assault and as a result, may seek to terminate pregnancies that result from these traumatic experiences.
A survey conducted by the Centers for Disease Control and Prevention examining rates of intimate partner violence and sexual violence found that 44% of lesbians and 61% of bisexual women experience rape and physical violence, compared with 35% of straight women.1 A separate survey revealed that 47% of transgender people are sexually assaulted at some point in their lifetime, with rates reaching as high as 65% among transgender people of color.2 Furthermore, many members of the LGBTQ population are misinformed or have misconceptions regarding their need for contraceptives and experience unintended pregnancies. As discussed in a previous column, one-third of pregnancies in transgender men were unplanned, and 20% of those patients were amenorrheic on testosterone at the time of conception.3
Current studies estimate that approximately 25% of all cisgender women will have an abortion. No corresponding data exist to describe the abortion rates of transgender and gender diverse patients.4,5 Bills such as Texas SB8 make accessing safe abortions for patients virtually impossible and interferes with the ability for physicians to provide patients with much needed health care services. It further delegitimizes rape and incest victims and is almost punitive in requiring such victims to carry the unintended pregnancies resulting from these heinous acts to term.
Regardless of a provider’s feelings toward abortion or even gender-affirming care, it is undeniable that access to these services is necessary and should be readily available to patients seeking them. As we all took an oath in medical school to “do no harm,” we must not only abide by that solemn decree in everyday patient interactions, but also live by those words to advocate for our patients when politics prohibit appropriate care. While discussions surrounding abortion are often limited to cisgender, heterosexual patients, providers must also be aware that abortion access spans across a wider spectrum that includes the LGBTQ community. Our patients, and all patients, deserve equal access to abortion. This harmful law sets a dangerous precedent that could continue to threaten these services with detrimental effects to our patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
3. Abern L, Maguire K. Obstet Gynecol 2018;131:65S.
4. Jones RK et al. Abortion incidence and service availability in the Unites States, 2017. New York, NY: Guttmacher Institute: 2019.
5. Moseson H et al. Am J Obstet Gynecol 2021;224:376.e1-11.
On Sept. 1, Texas enacted astonishing legislation that effectively bans abortion after a fetal heartbeat is detected. In addition, it further empowers private citizens to sue anyone “aiding and abetting” patients who seek abortion services. Many organizations, including Planned Parenthood and the American College of Obstetricians and Gynecologists, have issued formalized statements condemning the bill. While we as obstetrician/gynecologists try to remain as nonpartisan as humanly possible in our patient care, unfortunately our specialty is inarguably one of the few in the medical field that is routinely significantly affected by federal and state politics.
It is no secret that Texas Senate Bill 8, otherwise referred to the “Texas Heartbeat Act,” will have devastating consequences for women, particularly women of color, but it will also have potentially catastrophic repercussions for patients who identify as LGBTQ. Overall, the LGBTQ population faces higher rates of poverty, unemployment, insurance coverage barriers, and provider discrimination because of their gender identity or sexual orientation, which can make access to abortion services challenging. Furthermore, they are more susceptible to hate-motivated violence and sexual assault and as a result, may seek to terminate pregnancies that result from these traumatic experiences.
A survey conducted by the Centers for Disease Control and Prevention examining rates of intimate partner violence and sexual violence found that 44% of lesbians and 61% of bisexual women experience rape and physical violence, compared with 35% of straight women.1 A separate survey revealed that 47% of transgender people are sexually assaulted at some point in their lifetime, with rates reaching as high as 65% among transgender people of color.2 Furthermore, many members of the LGBTQ population are misinformed or have misconceptions regarding their need for contraceptives and experience unintended pregnancies. As discussed in a previous column, one-third of pregnancies in transgender men were unplanned, and 20% of those patients were amenorrheic on testosterone at the time of conception.3
Current studies estimate that approximately 25% of all cisgender women will have an abortion. No corresponding data exist to describe the abortion rates of transgender and gender diverse patients.4,5 Bills such as Texas SB8 make accessing safe abortions for patients virtually impossible and interferes with the ability for physicians to provide patients with much needed health care services. It further delegitimizes rape and incest victims and is almost punitive in requiring such victims to carry the unintended pregnancies resulting from these heinous acts to term.
Regardless of a provider’s feelings toward abortion or even gender-affirming care, it is undeniable that access to these services is necessary and should be readily available to patients seeking them. As we all took an oath in medical school to “do no harm,” we must not only abide by that solemn decree in everyday patient interactions, but also live by those words to advocate for our patients when politics prohibit appropriate care. While discussions surrounding abortion are often limited to cisgender, heterosexual patients, providers must also be aware that abortion access spans across a wider spectrum that includes the LGBTQ community. Our patients, and all patients, deserve equal access to abortion. This harmful law sets a dangerous precedent that could continue to threaten these services with detrimental effects to our patients.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Black MC et al. The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011.
2. James SE et al. The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality; 2016.
3. Abern L, Maguire K. Obstet Gynecol 2018;131:65S.
4. Jones RK et al. Abortion incidence and service availability in the Unites States, 2017. New York, NY: Guttmacher Institute: 2019.
5. Moseson H et al. Am J Obstet Gynecol 2021;224:376.e1-11.
Novel drug effective for essential tremor, but with significant side effects
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.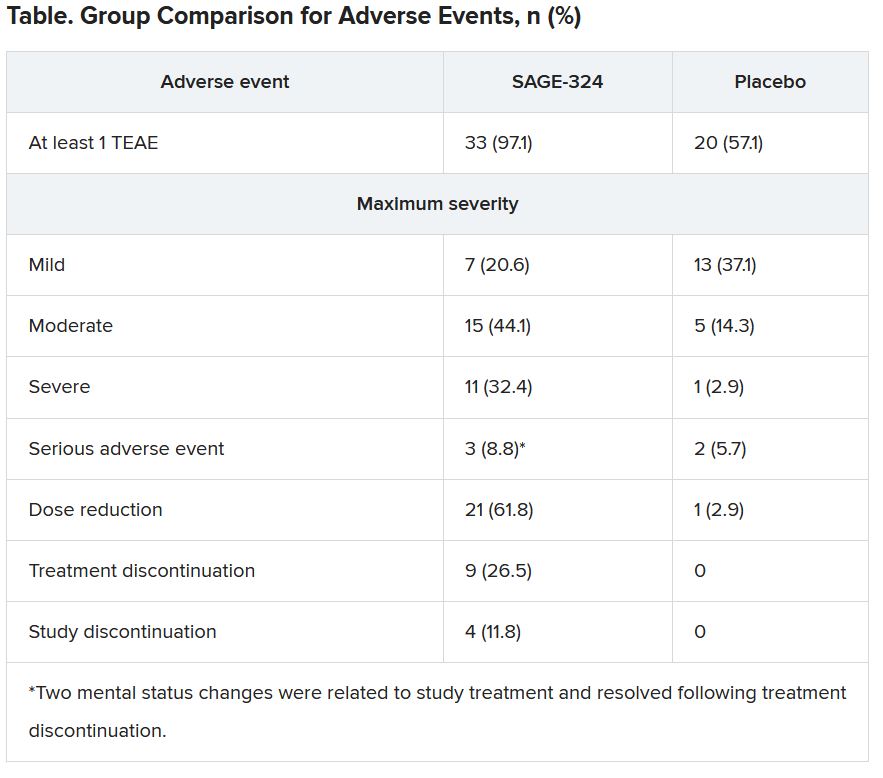
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
VA to Provide Services to Veterans With ‘Don’t Ask, Don’t Tell’ Discharges
The US Department of Veterans Affairs (VA) has issued a new policy statement to help ensure that active-duty service members who were discharged for their sexual orientation under the Don’t Ask, Don’t Tell policy will be able to receive full VA benefits.
“[A] great injustice was remedied and a tremendous weight was finally lifted off the shoulders of tens of thousands of dedicated American service members,” President Biden said on September 20 as the country commemorated the 10th anniversary of the repeal of “Don’t Ask, Don’t Tell,” the policy that barred lesbian, gay, bisexual, transgender, and queer (LGBTQ+) service members from serving openly.
Prior to DADT, if active-duty service members spoke out about their sexual orientation, they ran the risk of being hounded, shunned, in some cases assaulted, and discharged. If they kept it a secret, they felt they were living a lie, unable to be their whole selves. More than 100,000 were discharged because of their sexual orientation or gender identity.
Although Don’t Ask, Don’t Tell was a “compromise” law that purposed to protect them, LGBTQ+ service members were still at risk for harassment and abuse. Nor did the law protect them from discharge. Some 14,000 LGBTQ+ service members were discharged while DADT was in effect. And those who received “other than honorable” (OTH) discharges could be excluded from receiving services and benefits.
The 2011 repeal followed a “hard-fought battle,” said a release from the Human Rights Campaign, which led a coalition of members, supporters, elected officials, 70+ organizations, and 20,000 veterans to get the law overturned. HRC staff coordinated grassroots efforts and sent 19 million e-mails to members and supporters, in turn generating an “unprecedented” 625,000 e-mails and 50,000 handwritten letters to members of Congress.
After the repeal, the VA began the long process of inclusion for LGBTQ+ veterans. “At VA, we continuously work not only to meet the needs of LGBTQ+ veterans, but also to address ongoing issues that LGBTQ+ veterans face as a result of the military’s decades-long official policy of homophobia and transphobia,” Kayla Williams, assistant secretary for public affairs in VA’s Office of Public and Intergovernmental Affairs wrote in the Secretary’s blog.
The VA “recognizes that the trauma caused by the military’s decades-long policy of discrimination against LGBTQ+ people cannot be undone in a few short months,” she continued, but the Biden administration and Secretary McDonough are “taking the steps necessary to begin addressing the pain that such policies have created.”
President Biden, in his remarks, noted that as a US Senator, he had supported allowing service members to serve openly and, as Vice President, championed the repeal. He said, “I am honored to be Commander-in-Chief of the strongest and most inclusive military in our nation’s history. Today, our military doesn’t just welcome LGBTQ+ service members—it is led at the highest levels by brave LGBTQ+ veterans, including Under Secretary of the Air Force Gina Ortiz Jones and Assistant Secretary of Defense for Readiness Shawn Skelly, who served under Don’t Ask, Don’t Tell. I was gratified to appoint the first openly gay Senate-confirmed Cabinet member, Secretary Pete Buttigieg, a lieutenant in the U.S. Navy Reserve and Afghanistan veteran who joined the military under the Don’t Ask, Don’t Tell policy. And during my first week in office, I proudly delivered on my pledge to repeal the discriminatory ban on open service by patriotic transgender service members.”
In early September, Rep. Chris Pappas (D-NH), a member of the House Veterans Affairs Committee and Co-Chair of the Equality Caucus, reintroduced the SERVE (Securing the Rights our Veterans Earned) Act. The act, which is co-sponsored by Reps. Mike Levin (D-CA), Kathleen Rice (D-NY), Anthony Brown (D-MD), and Jackie Speier (D-CA), would ensure LGBTQ+ veterans who received an OTH or Entry-Level Separation discharge solely due to sexual orientation or gender identity are afforded the VA benefits they rightfully earned, including access to VA healthcare, and education, burial and memorial services, and home loans. The act includes veterans who were issued “blue discharges” during World War II and veterans discharged under former President Trump’s ban on transgender service members. The legislation has been endorsed by the Congressional LGBTQ+ Equality Caucus and is supported by more than 45 representatives.
There are processes for those veterans through which they can have their discharge papers and separation statuses reviewed and modified, Pappas said in a release, but “it can take months for the changes to take effect, and many of these veterans do not even realize they are eligible.”
Earlier this year, Veterans Affairs Secretary Denis McDonough made it a priority to ensure that LGBTQ+ veterans have the same level of access to VA care and services that all other veterans have. Among other things, he established a task force to examine how VA policies hinder or prohibit access to care and services and remove barriers that transgender veterans face in accessing gender-affirming care.
The VA is also taking steps, Williams noted, to clarify VA policy for veterans who were given OTH discharges based on homosexual conduct, gender identity or HIV status. Under this newly issued guidance, VA adjudicators shall find that all discharged service members whose separation was due to sexual orientation, gender identity or HIV status are considered “Veterans” who may be eligible for VA benefits, like VR&E, home loan guaranty, compensation and pension, health care, homeless program and/or burial benefits, so long as the record does not implicate a statutory or regulatory bar to benefits.
The policy statement does not represent a change in law, as veterans who were discharged under DADT alone have been generally eligible for benefits under current statute and regulation. However, it reiterates what constitutes eligibility for benefits under law. In addition, every Character of Discharge case that is initially considered for denial will also get a second look before that action is taken.
“Given that large numbers of LGBTQ+ veterans who were affected by previous homophobic and transphobic policies have not applied for a discharge upgrade due to the perception that the process could be onerous,” Williams wrote, “we are hopeful that this policy statement encourages more of them to contact VA to determine their eligibility for care and services.”
Today, according to the Human Right Campaign, nearly 6.1% of US military personnel self-identify as LGBTQ. Williams, herself a bisexual veteran, said she chose to present as straight during the push to repeal DADT. “I could talk credibly about how the lack of sufficient Arabic linguists harmed our effectiveness downrange, and my own identity seemed irrelevant. It took many years for me to shed the toxic legacy of having served under DADT and come back out of the closet; I’m proud to recognize this anniversary as my authentic self.”
The US Department of Veterans Affairs (VA) has issued a new policy statement to help ensure that active-duty service members who were discharged for their sexual orientation under the Don’t Ask, Don’t Tell policy will be able to receive full VA benefits.
“[A] great injustice was remedied and a tremendous weight was finally lifted off the shoulders of tens of thousands of dedicated American service members,” President Biden said on September 20 as the country commemorated the 10th anniversary of the repeal of “Don’t Ask, Don’t Tell,” the policy that barred lesbian, gay, bisexual, transgender, and queer (LGBTQ+) service members from serving openly.
Prior to DADT, if active-duty service members spoke out about their sexual orientation, they ran the risk of being hounded, shunned, in some cases assaulted, and discharged. If they kept it a secret, they felt they were living a lie, unable to be their whole selves. More than 100,000 were discharged because of their sexual orientation or gender identity.
Although Don’t Ask, Don’t Tell was a “compromise” law that purposed to protect them, LGBTQ+ service members were still at risk for harassment and abuse. Nor did the law protect them from discharge. Some 14,000 LGBTQ+ service members were discharged while DADT was in effect. And those who received “other than honorable” (OTH) discharges could be excluded from receiving services and benefits.
The 2011 repeal followed a “hard-fought battle,” said a release from the Human Rights Campaign, which led a coalition of members, supporters, elected officials, 70+ organizations, and 20,000 veterans to get the law overturned. HRC staff coordinated grassroots efforts and sent 19 million e-mails to members and supporters, in turn generating an “unprecedented” 625,000 e-mails and 50,000 handwritten letters to members of Congress.
After the repeal, the VA began the long process of inclusion for LGBTQ+ veterans. “At VA, we continuously work not only to meet the needs of LGBTQ+ veterans, but also to address ongoing issues that LGBTQ+ veterans face as a result of the military’s decades-long official policy of homophobia and transphobia,” Kayla Williams, assistant secretary for public affairs in VA’s Office of Public and Intergovernmental Affairs wrote in the Secretary’s blog.
The VA “recognizes that the trauma caused by the military’s decades-long policy of discrimination against LGBTQ+ people cannot be undone in a few short months,” she continued, but the Biden administration and Secretary McDonough are “taking the steps necessary to begin addressing the pain that such policies have created.”
President Biden, in his remarks, noted that as a US Senator, he had supported allowing service members to serve openly and, as Vice President, championed the repeal. He said, “I am honored to be Commander-in-Chief of the strongest and most inclusive military in our nation’s history. Today, our military doesn’t just welcome LGBTQ+ service members—it is led at the highest levels by brave LGBTQ+ veterans, including Under Secretary of the Air Force Gina Ortiz Jones and Assistant Secretary of Defense for Readiness Shawn Skelly, who served under Don’t Ask, Don’t Tell. I was gratified to appoint the first openly gay Senate-confirmed Cabinet member, Secretary Pete Buttigieg, a lieutenant in the U.S. Navy Reserve and Afghanistan veteran who joined the military under the Don’t Ask, Don’t Tell policy. And during my first week in office, I proudly delivered on my pledge to repeal the discriminatory ban on open service by patriotic transgender service members.”
In early September, Rep. Chris Pappas (D-NH), a member of the House Veterans Affairs Committee and Co-Chair of the Equality Caucus, reintroduced the SERVE (Securing the Rights our Veterans Earned) Act. The act, which is co-sponsored by Reps. Mike Levin (D-CA), Kathleen Rice (D-NY), Anthony Brown (D-MD), and Jackie Speier (D-CA), would ensure LGBTQ+ veterans who received an OTH or Entry-Level Separation discharge solely due to sexual orientation or gender identity are afforded the VA benefits they rightfully earned, including access to VA healthcare, and education, burial and memorial services, and home loans. The act includes veterans who were issued “blue discharges” during World War II and veterans discharged under former President Trump’s ban on transgender service members. The legislation has been endorsed by the Congressional LGBTQ+ Equality Caucus and is supported by more than 45 representatives.
There are processes for those veterans through which they can have their discharge papers and separation statuses reviewed and modified, Pappas said in a release, but “it can take months for the changes to take effect, and many of these veterans do not even realize they are eligible.”
Earlier this year, Veterans Affairs Secretary Denis McDonough made it a priority to ensure that LGBTQ+ veterans have the same level of access to VA care and services that all other veterans have. Among other things, he established a task force to examine how VA policies hinder or prohibit access to care and services and remove barriers that transgender veterans face in accessing gender-affirming care.
The VA is also taking steps, Williams noted, to clarify VA policy for veterans who were given OTH discharges based on homosexual conduct, gender identity or HIV status. Under this newly issued guidance, VA adjudicators shall find that all discharged service members whose separation was due to sexual orientation, gender identity or HIV status are considered “Veterans” who may be eligible for VA benefits, like VR&E, home loan guaranty, compensation and pension, health care, homeless program and/or burial benefits, so long as the record does not implicate a statutory or regulatory bar to benefits.
The policy statement does not represent a change in law, as veterans who were discharged under DADT alone have been generally eligible for benefits under current statute and regulation. However, it reiterates what constitutes eligibility for benefits under law. In addition, every Character of Discharge case that is initially considered for denial will also get a second look before that action is taken.
“Given that large numbers of LGBTQ+ veterans who were affected by previous homophobic and transphobic policies have not applied for a discharge upgrade due to the perception that the process could be onerous,” Williams wrote, “we are hopeful that this policy statement encourages more of them to contact VA to determine their eligibility for care and services.”
Today, according to the Human Right Campaign, nearly 6.1% of US military personnel self-identify as LGBTQ. Williams, herself a bisexual veteran, said she chose to present as straight during the push to repeal DADT. “I could talk credibly about how the lack of sufficient Arabic linguists harmed our effectiveness downrange, and my own identity seemed irrelevant. It took many years for me to shed the toxic legacy of having served under DADT and come back out of the closet; I’m proud to recognize this anniversary as my authentic self.”
The US Department of Veterans Affairs (VA) has issued a new policy statement to help ensure that active-duty service members who were discharged for their sexual orientation under the Don’t Ask, Don’t Tell policy will be able to receive full VA benefits.
“[A] great injustice was remedied and a tremendous weight was finally lifted off the shoulders of tens of thousands of dedicated American service members,” President Biden said on September 20 as the country commemorated the 10th anniversary of the repeal of “Don’t Ask, Don’t Tell,” the policy that barred lesbian, gay, bisexual, transgender, and queer (LGBTQ+) service members from serving openly.
Prior to DADT, if active-duty service members spoke out about their sexual orientation, they ran the risk of being hounded, shunned, in some cases assaulted, and discharged. If they kept it a secret, they felt they were living a lie, unable to be their whole selves. More than 100,000 were discharged because of their sexual orientation or gender identity.
Although Don’t Ask, Don’t Tell was a “compromise” law that purposed to protect them, LGBTQ+ service members were still at risk for harassment and abuse. Nor did the law protect them from discharge. Some 14,000 LGBTQ+ service members were discharged while DADT was in effect. And those who received “other than honorable” (OTH) discharges could be excluded from receiving services and benefits.
The 2011 repeal followed a “hard-fought battle,” said a release from the Human Rights Campaign, which led a coalition of members, supporters, elected officials, 70+ organizations, and 20,000 veterans to get the law overturned. HRC staff coordinated grassroots efforts and sent 19 million e-mails to members and supporters, in turn generating an “unprecedented” 625,000 e-mails and 50,000 handwritten letters to members of Congress.
After the repeal, the VA began the long process of inclusion for LGBTQ+ veterans. “At VA, we continuously work not only to meet the needs of LGBTQ+ veterans, but also to address ongoing issues that LGBTQ+ veterans face as a result of the military’s decades-long official policy of homophobia and transphobia,” Kayla Williams, assistant secretary for public affairs in VA’s Office of Public and Intergovernmental Affairs wrote in the Secretary’s blog.
The VA “recognizes that the trauma caused by the military’s decades-long policy of discrimination against LGBTQ+ people cannot be undone in a few short months,” she continued, but the Biden administration and Secretary McDonough are “taking the steps necessary to begin addressing the pain that such policies have created.”
President Biden, in his remarks, noted that as a US Senator, he had supported allowing service members to serve openly and, as Vice President, championed the repeal. He said, “I am honored to be Commander-in-Chief of the strongest and most inclusive military in our nation’s history. Today, our military doesn’t just welcome LGBTQ+ service members—it is led at the highest levels by brave LGBTQ+ veterans, including Under Secretary of the Air Force Gina Ortiz Jones and Assistant Secretary of Defense for Readiness Shawn Skelly, who served under Don’t Ask, Don’t Tell. I was gratified to appoint the first openly gay Senate-confirmed Cabinet member, Secretary Pete Buttigieg, a lieutenant in the U.S. Navy Reserve and Afghanistan veteran who joined the military under the Don’t Ask, Don’t Tell policy. And during my first week in office, I proudly delivered on my pledge to repeal the discriminatory ban on open service by patriotic transgender service members.”
In early September, Rep. Chris Pappas (D-NH), a member of the House Veterans Affairs Committee and Co-Chair of the Equality Caucus, reintroduced the SERVE (Securing the Rights our Veterans Earned) Act. The act, which is co-sponsored by Reps. Mike Levin (D-CA), Kathleen Rice (D-NY), Anthony Brown (D-MD), and Jackie Speier (D-CA), would ensure LGBTQ+ veterans who received an OTH or Entry-Level Separation discharge solely due to sexual orientation or gender identity are afforded the VA benefits they rightfully earned, including access to VA healthcare, and education, burial and memorial services, and home loans. The act includes veterans who were issued “blue discharges” during World War II and veterans discharged under former President Trump’s ban on transgender service members. The legislation has been endorsed by the Congressional LGBTQ+ Equality Caucus and is supported by more than 45 representatives.
There are processes for those veterans through which they can have their discharge papers and separation statuses reviewed and modified, Pappas said in a release, but “it can take months for the changes to take effect, and many of these veterans do not even realize they are eligible.”
Earlier this year, Veterans Affairs Secretary Denis McDonough made it a priority to ensure that LGBTQ+ veterans have the same level of access to VA care and services that all other veterans have. Among other things, he established a task force to examine how VA policies hinder or prohibit access to care and services and remove barriers that transgender veterans face in accessing gender-affirming care.
The VA is also taking steps, Williams noted, to clarify VA policy for veterans who were given OTH discharges based on homosexual conduct, gender identity or HIV status. Under this newly issued guidance, VA adjudicators shall find that all discharged service members whose separation was due to sexual orientation, gender identity or HIV status are considered “Veterans” who may be eligible for VA benefits, like VR&E, home loan guaranty, compensation and pension, health care, homeless program and/or burial benefits, so long as the record does not implicate a statutory or regulatory bar to benefits.
The policy statement does not represent a change in law, as veterans who were discharged under DADT alone have been generally eligible for benefits under current statute and regulation. However, it reiterates what constitutes eligibility for benefits under law. In addition, every Character of Discharge case that is initially considered for denial will also get a second look before that action is taken.
“Given that large numbers of LGBTQ+ veterans who were affected by previous homophobic and transphobic policies have not applied for a discharge upgrade due to the perception that the process could be onerous,” Williams wrote, “we are hopeful that this policy statement encourages more of them to contact VA to determine their eligibility for care and services.”
Today, according to the Human Right Campaign, nearly 6.1% of US military personnel self-identify as LGBTQ. Williams, herself a bisexual veteran, said she chose to present as straight during the push to repeal DADT. “I could talk credibly about how the lack of sufficient Arabic linguists harmed our effectiveness downrange, and my own identity seemed irrelevant. It took many years for me to shed the toxic legacy of having served under DADT and come back out of the closet; I’m proud to recognize this anniversary as my authentic self.”
Antipsychotic effective for bipolar depression in phase 3 trial
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Results of a phase 3 study show that treatment with lumateperone (Caplyta) significantly improved depressive symptoms for patients with major depressive episodes associated with both bipolar I and bipolar II disorders.
“Bipolar depression represents the most prevalent and debilitating presentation of bipolar disorder. There is a critical need for more treatments that are effective and have favorable safety profiles,” study investigator Gary S. Sachs, MD, associate clinical professor in psychiatry, Harvard Medical School, Boston, said in a company news release.
“The strong efficacy and impressive safety results reported in this trial for a broad patient population position lumateperone as a potentially important advancement in the treatment of this disorder,” said Dr. Sachs, who is also founding director of the Bipolar Clinic and Research Program at Massachusetts General Hospital, Boston.
The findings were published online September 23 in the American Journal of Psychiatry.
First-in-class antipsychotic
Lumateperone is a first-in-class antipsychotic that acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems.
It was approved by the U.S. Food and Drug Administration in late 2019 for the treatment of adults with schizophrenia, as reported at the time by this news organization.
All were randomly allocated in a 1:1 ratio to receive 6 weeks of lumateperone monotherapy at 42 mg/d or matching placebo.
At day 43, lumateperone treatment was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale (MADRS) score in comparison with placebo (drug-placebo difference, -4.6 points; P < .0001; effect size = -0.56), which met the study’s primary endpoint.
The study drug led to significant improvement in MADRS total score as early as the first week, which was the first time point measured. Improvement continued throughout the study.
Treatment with lumateperone also led to significantly greater improvement in the key secondary endpoints of total score on the severity scale of the Clinical Global Impressions Scale–Bipolar Version (CGI-BP-S) (P < .0001; effect size = -0.46) and the CGI-BP-S depression score (P < .001; effect size = -50).
In addition, it was superior to placebo both for patients with bipolar I disorder and those with bipolar II disorder.
Somnolence and nausea were the most commonly reported adverse events associated with lumateperone. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. These findings are in line with previous studies involving patients with schizophrenia.
The incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
The company has submitted a supplemental new drug application for lumateperone for the treatment of bipolar depression, which is currently under review with the FDA. The target action date is December 17.
A version of this article first appeared on Medscape.com.
Predictive Models for In-Hospital Deterioration in Ward Patients
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
Adults admitted to general medical-surgical wards who experience in-hospital deterioration have a disproportionate effect on hospital mortality and length of stay.1 Not long ago, systematic electronic capture of vital signs—arguably the most important predictors of impending deterioration—was restricted to intensive care units (ICUs). Deployment of comprehensive electronic health records (EHRs) and handheld charting tools have made vital signs data more accessible, expanding the possibilities of early detection.
In this issue, Peelen et al2 report their scoping review of contemporary EHR-based predictive models for identifying ward patients at risk for deterioration. They identified 22 publications suitable for review. Impressively, some studies report extraordinary statistical performance, with positive predictive values (PPVs) exceeding 50% and with 12- to 24-hour lead times to prepare a clinician response. However, only five algorithms were implemented in an EHR and only three were used clinically. Peelen et al also quantified 48 barriers to and 54 facilitators of the implementation and use of these models. Improved statistical performance (higher PPVs) compared to manually assigned scores were the most important facilitators, while implementation in the context of daily practice (alarm fatigue, integration with existing workflows) were the most important barriers.
These reports invite an obvious question: If the models are this good, why have we not seen more reports of improved patient outcomes? Based on our own recent experience successfully deploying and evaluating the Advance Alert Monitor Program for early detection in a 21-hospital system,3 we suspect that there are several factors at play. Despite the relative computational ease of developing high-performing predictive models, it can be very challenging to create the right dataset (extracting and formatting data, standardizing variable definitions across different EHR builds). Investigators may also underestimate the difficulty of what can be implemented—and sustained—in real-world clinical practice. We encountered substantial difficulty, for example, around alarm fatigue mitigation and the relationship of alerts to end-of-life decisions. Greater attention to implementation is necessary to advance the field.
We suggest that four critical questions be considered when creating in-hospital predictive models. First, what are the statistical characteristics of a model around the likely clinical decision point? Simply having a high C-statistic is insufficient—what matters is the alert’s PPV at a clinically actionable threshold.4 Second, workflow burden—how many alerts per day at my hospital—must be measured, including other processes potentially affected by the new system. Third, will the extra work identify a meaningful proportion of the avoidable bad outcomes? Finally, how will model use affect care of patients near the end of life? Alerts for these patients may not make clinical sense and might even interfere with overall care (eg, by triggering an unwanted ICU transfer).
Implementation requires more than data scientists. Consideration must be given to system governance, predictive model maintenance (models can actually decalibrate over time!), and financing (not just the computation side—someone needs to pay for training clinicians and ensuring proper staffing of the clinical response).
Last, rigorous model evaluation must be undertaken. Given the increasing capabilities of comprehensive EHRs, patient-level randomization is becoming more feasible. But even randomized deployments present challenges. Since ward patients are a heterogeneous population, quantifying process-outcome relationships may be difficult. Alternative approaches to quantification of the impact of bundled interventions may need to be considered—not just for initial deployment, but on an ongoing basis. Peelen et al2 have effectively summarized the state of published predictive models, which hold the tantalizing possibility of meaningful improvement: saved lives, decreased morbidity. Now, we must work together to address the identified gaps so that, one day, implementation of real-time models is routine, and the promise of in-hospital predictive analytics is fulfilled.
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
1. Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS). J Hosp Med. 2011;6(2):74-80. https://doi.org/10.1002/jhm.817
2. Peelen REY, Koeneman M, van de Belt T, van Goor H, Bredie S. Predicting algorithms for clinical deterioration on the general ward. J Hosp Med. 2021;16(9):612-619. https://doi.org/10.12788/jhm.3675
3. Escobar GJ, Liu VX, Schuler A, Lawson B, Greene JD, Kipnis P. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020;383(20):1951-1960. https://doi.org/10.1056/NEJMsa2001090
4. Romero-Brufau S, Huddleston JM, Escobar GJ, Liebow M. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):285. https://doi.org/10.1186/s13054-015-0999-1
© 2021 Society of Hospital Medicine

