User login
COVID boosters help protect blood cancer patients, but some still vulnerable
particularly in those with B-cell malignancies, an observational study suggests.
The findings, based on a review of COVID-19 booster vaccine recipients with B cell–derived hematologic malignancies from the prospective Leukemia & Lymphoma Society National Registry, provide valuable information about booster vaccinations in this vulnerable population, according to LLS chief medical officer and lead study author Gwen Nichols, MD.
The LLS Registry data
Of 49 patients included in the review, 38 failed to make antispike (anti-S) antibodies after full vaccination, and of those, 21 seroconverted after booster vaccination and 11 experienced seroelevation after the booster.
The patients who did not seroconvert were seronegative after initial vaccination and experienced no change in antibody level after the booster vaccination. In contrast, the 21 who seroconverted had a median 23.1 AU/ml increase in antibody level, Dr. Nichols, along with LLS chief scientific officer Lee M. Greenberger, PhD,and their colleagues reported online Sept. 6, 2021, in Cancer Cell.
Similar proportions of seroconverted patients had low-level responses between 2.2 and 23.1 AU/mL and robust response between 125 and 2,500 AU/mL, they noted. The 11 patients with seroelevation were seropositive after full vaccination and demonstrated a median increase of 2,128 AU/mL in antibody level after the booster vaccination.
Therapy effects on vaccine response
Outcomes of the current analysis also confirmed the authors’ previous finding, which suggested that “both disease and therapies can affect the serological response to vaccination,” they wrote, explaining that, among the 12 patients who received no malignancy-targeted treatments in the past 2 years, only 1 was a nonresponder, 7 demonstrated seroconversion, and 4 demonstrated seroelevation.
“In contrast, among the 21 patients who completed therapy with anti-CD20 antibodies either alone or in combination with other therapies, 12 patients were nonresponders, 7 patients demonstrated seroconversion, and 2 patients demonstrated seroelevation,” they added.
The authors also noted that five of seven patients who completed anti-CD20 antibody therapy alone or in combination with chemotherapy at least 7 months prior to the booster vaccination demonstrated seroconversion or seroelevation, whereas many of the patients with recent or maintenance anti-CD20 antibody therapy before the booster vaccination failed to seroconvert afterward.
In light of previous findings showing B-cell recovery begins 6-9 months after rituximab therapy, these data suggest that recent anti-CD20 antibody-containing treatment regimens may suppress booster vaccination response, the authors wrote.
The current data also support the group’s prior finding that use of a Bruton tyrosine kinase inhibitor may suppress vaccine response: Of the patients in the current study who experienced seroelevation and were treated with a BTKi, two discontinued BTKi therapy 7-23 months prior to booster vaccination, one maintained a low dose of ibrutinib before booster vaccination, one maintained BTKi therapy continuously before and after the booster, and the two who experienced marked seroconversion after booster vaccination stopped BTKi therapy at least 4 months prior.
Conversely, five patients with a very weak seroconversion and two patients with moderate seroconversion maintained BTKi therapy during booster vaccination.
“These data suggest that BTKi therapy can interfere with a response to booster vaccination,” they wrote, noting, however, that “it is encouraging that seven patients ... maintained on a BTKi seroconverted or experienced seroelevation after booster vaccination and [this] is consistent with a previous report on one patient.”
Study limitations and lessons
Although the findings of this study are limited by the small number of patients, the fact that treatment and disease were patient reported, and a lack of data on “antibody responses, particularly to the delta variant, B-cell memory, or T-cell responses,” they nevertheless provide encouraging news, Dr. Nichols told this news organization.
“Many blood cancer patients are getting boosters and a good number are able to make antibody with an additional dose. This is giving us much needed information about boosters,” she said. “Through the LLS National Patient Registry, we anticipate having data on hundreds of more patients over the course of the next few months.”
The information is needed because data suggest that up to 25% of patients with hematologic malignancies fail to make anti-S antibodies after full COVID-19 vaccination and that seronegative patients may be especially vulnerable to breakthrough infections, she and her colleagues noted.
Patients with B-cell malignancies are at the highest risk, and this is particularly concerning as some patients with blood cancer who contracted COVID-19 in the prevaccine period of the pandemic had “prolonged, severe infections; generated variant strains; and demonstrated significantly higher mortality rates compared to the general population,” they said.
However, a recently published placebo-controlled trial that demonstrated a booster vaccination–mediated increase in anti-S antibodies and neutralizing antibodies in immunosuppressed patients, and the current review, which focused on patients who obtained booster vaccinations prior to Aug. 12, 2021 (when the Food and Drug Administration granted emergency use authorization for booster doses in immunocompromised people), offer findings that suggest these patients may benefit from receiving COVID-19 boosters.
“We conclude that some patients with hematologic malignancies who are seronegative after a full course of vaccination may benefit from a booster,” the authors wrote. They added a warning: “Regulators, patients, and health care providers should be aware that a sizable subset of patients with blood cancer may remain at risk of breakthrough COVID-19 infections after full vaccination followed by booster vaccination.”
Dr. Nichols stressed that the findings “do not in any way suggest that blood cancer patients should stop therapy to get an antibody response to the vaccinations.”
“LLS is encouraging blood cancer patients to get vaccinated and to continue taking preventive measures such as wearing masks, social distancing, hand washing, and avoiding crowds and poorly ventilated indoor spaces,” she said.
Sergio Giralt, MD, professor and deputy head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center, New York, further emphasized the importance of preventive measures.
“I think the extra booster will go a long way to protect our patients at this time but should not be used as a replacement for masking indoors and continued social distancing in this vulnerable patient population,” he said.
This study was supported by the LLS.
particularly in those with B-cell malignancies, an observational study suggests.
The findings, based on a review of COVID-19 booster vaccine recipients with B cell–derived hematologic malignancies from the prospective Leukemia & Lymphoma Society National Registry, provide valuable information about booster vaccinations in this vulnerable population, according to LLS chief medical officer and lead study author Gwen Nichols, MD.
The LLS Registry data
Of 49 patients included in the review, 38 failed to make antispike (anti-S) antibodies after full vaccination, and of those, 21 seroconverted after booster vaccination and 11 experienced seroelevation after the booster.
The patients who did not seroconvert were seronegative after initial vaccination and experienced no change in antibody level after the booster vaccination. In contrast, the 21 who seroconverted had a median 23.1 AU/ml increase in antibody level, Dr. Nichols, along with LLS chief scientific officer Lee M. Greenberger, PhD,and their colleagues reported online Sept. 6, 2021, in Cancer Cell.
Similar proportions of seroconverted patients had low-level responses between 2.2 and 23.1 AU/mL and robust response between 125 and 2,500 AU/mL, they noted. The 11 patients with seroelevation were seropositive after full vaccination and demonstrated a median increase of 2,128 AU/mL in antibody level after the booster vaccination.
Therapy effects on vaccine response
Outcomes of the current analysis also confirmed the authors’ previous finding, which suggested that “both disease and therapies can affect the serological response to vaccination,” they wrote, explaining that, among the 12 patients who received no malignancy-targeted treatments in the past 2 years, only 1 was a nonresponder, 7 demonstrated seroconversion, and 4 demonstrated seroelevation.
“In contrast, among the 21 patients who completed therapy with anti-CD20 antibodies either alone or in combination with other therapies, 12 patients were nonresponders, 7 patients demonstrated seroconversion, and 2 patients demonstrated seroelevation,” they added.
The authors also noted that five of seven patients who completed anti-CD20 antibody therapy alone or in combination with chemotherapy at least 7 months prior to the booster vaccination demonstrated seroconversion or seroelevation, whereas many of the patients with recent or maintenance anti-CD20 antibody therapy before the booster vaccination failed to seroconvert afterward.
In light of previous findings showing B-cell recovery begins 6-9 months after rituximab therapy, these data suggest that recent anti-CD20 antibody-containing treatment regimens may suppress booster vaccination response, the authors wrote.
The current data also support the group’s prior finding that use of a Bruton tyrosine kinase inhibitor may suppress vaccine response: Of the patients in the current study who experienced seroelevation and were treated with a BTKi, two discontinued BTKi therapy 7-23 months prior to booster vaccination, one maintained a low dose of ibrutinib before booster vaccination, one maintained BTKi therapy continuously before and after the booster, and the two who experienced marked seroconversion after booster vaccination stopped BTKi therapy at least 4 months prior.
Conversely, five patients with a very weak seroconversion and two patients with moderate seroconversion maintained BTKi therapy during booster vaccination.
“These data suggest that BTKi therapy can interfere with a response to booster vaccination,” they wrote, noting, however, that “it is encouraging that seven patients ... maintained on a BTKi seroconverted or experienced seroelevation after booster vaccination and [this] is consistent with a previous report on one patient.”
Study limitations and lessons
Although the findings of this study are limited by the small number of patients, the fact that treatment and disease were patient reported, and a lack of data on “antibody responses, particularly to the delta variant, B-cell memory, or T-cell responses,” they nevertheless provide encouraging news, Dr. Nichols told this news organization.
“Many blood cancer patients are getting boosters and a good number are able to make antibody with an additional dose. This is giving us much needed information about boosters,” she said. “Through the LLS National Patient Registry, we anticipate having data on hundreds of more patients over the course of the next few months.”
The information is needed because data suggest that up to 25% of patients with hematologic malignancies fail to make anti-S antibodies after full COVID-19 vaccination and that seronegative patients may be especially vulnerable to breakthrough infections, she and her colleagues noted.
Patients with B-cell malignancies are at the highest risk, and this is particularly concerning as some patients with blood cancer who contracted COVID-19 in the prevaccine period of the pandemic had “prolonged, severe infections; generated variant strains; and demonstrated significantly higher mortality rates compared to the general population,” they said.
However, a recently published placebo-controlled trial that demonstrated a booster vaccination–mediated increase in anti-S antibodies and neutralizing antibodies in immunosuppressed patients, and the current review, which focused on patients who obtained booster vaccinations prior to Aug. 12, 2021 (when the Food and Drug Administration granted emergency use authorization for booster doses in immunocompromised people), offer findings that suggest these patients may benefit from receiving COVID-19 boosters.
“We conclude that some patients with hematologic malignancies who are seronegative after a full course of vaccination may benefit from a booster,” the authors wrote. They added a warning: “Regulators, patients, and health care providers should be aware that a sizable subset of patients with blood cancer may remain at risk of breakthrough COVID-19 infections after full vaccination followed by booster vaccination.”
Dr. Nichols stressed that the findings “do not in any way suggest that blood cancer patients should stop therapy to get an antibody response to the vaccinations.”
“LLS is encouraging blood cancer patients to get vaccinated and to continue taking preventive measures such as wearing masks, social distancing, hand washing, and avoiding crowds and poorly ventilated indoor spaces,” she said.
Sergio Giralt, MD, professor and deputy head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center, New York, further emphasized the importance of preventive measures.
“I think the extra booster will go a long way to protect our patients at this time but should not be used as a replacement for masking indoors and continued social distancing in this vulnerable patient population,” he said.
This study was supported by the LLS.
particularly in those with B-cell malignancies, an observational study suggests.
The findings, based on a review of COVID-19 booster vaccine recipients with B cell–derived hematologic malignancies from the prospective Leukemia & Lymphoma Society National Registry, provide valuable information about booster vaccinations in this vulnerable population, according to LLS chief medical officer and lead study author Gwen Nichols, MD.
The LLS Registry data
Of 49 patients included in the review, 38 failed to make antispike (anti-S) antibodies after full vaccination, and of those, 21 seroconverted after booster vaccination and 11 experienced seroelevation after the booster.
The patients who did not seroconvert were seronegative after initial vaccination and experienced no change in antibody level after the booster vaccination. In contrast, the 21 who seroconverted had a median 23.1 AU/ml increase in antibody level, Dr. Nichols, along with LLS chief scientific officer Lee M. Greenberger, PhD,and their colleagues reported online Sept. 6, 2021, in Cancer Cell.
Similar proportions of seroconverted patients had low-level responses between 2.2 and 23.1 AU/mL and robust response between 125 and 2,500 AU/mL, they noted. The 11 patients with seroelevation were seropositive after full vaccination and demonstrated a median increase of 2,128 AU/mL in antibody level after the booster vaccination.
Therapy effects on vaccine response
Outcomes of the current analysis also confirmed the authors’ previous finding, which suggested that “both disease and therapies can affect the serological response to vaccination,” they wrote, explaining that, among the 12 patients who received no malignancy-targeted treatments in the past 2 years, only 1 was a nonresponder, 7 demonstrated seroconversion, and 4 demonstrated seroelevation.
“In contrast, among the 21 patients who completed therapy with anti-CD20 antibodies either alone or in combination with other therapies, 12 patients were nonresponders, 7 patients demonstrated seroconversion, and 2 patients demonstrated seroelevation,” they added.
The authors also noted that five of seven patients who completed anti-CD20 antibody therapy alone or in combination with chemotherapy at least 7 months prior to the booster vaccination demonstrated seroconversion or seroelevation, whereas many of the patients with recent or maintenance anti-CD20 antibody therapy before the booster vaccination failed to seroconvert afterward.
In light of previous findings showing B-cell recovery begins 6-9 months after rituximab therapy, these data suggest that recent anti-CD20 antibody-containing treatment regimens may suppress booster vaccination response, the authors wrote.
The current data also support the group’s prior finding that use of a Bruton tyrosine kinase inhibitor may suppress vaccine response: Of the patients in the current study who experienced seroelevation and were treated with a BTKi, two discontinued BTKi therapy 7-23 months prior to booster vaccination, one maintained a low dose of ibrutinib before booster vaccination, one maintained BTKi therapy continuously before and after the booster, and the two who experienced marked seroconversion after booster vaccination stopped BTKi therapy at least 4 months prior.
Conversely, five patients with a very weak seroconversion and two patients with moderate seroconversion maintained BTKi therapy during booster vaccination.
“These data suggest that BTKi therapy can interfere with a response to booster vaccination,” they wrote, noting, however, that “it is encouraging that seven patients ... maintained on a BTKi seroconverted or experienced seroelevation after booster vaccination and [this] is consistent with a previous report on one patient.”
Study limitations and lessons
Although the findings of this study are limited by the small number of patients, the fact that treatment and disease were patient reported, and a lack of data on “antibody responses, particularly to the delta variant, B-cell memory, or T-cell responses,” they nevertheless provide encouraging news, Dr. Nichols told this news organization.
“Many blood cancer patients are getting boosters and a good number are able to make antibody with an additional dose. This is giving us much needed information about boosters,” she said. “Through the LLS National Patient Registry, we anticipate having data on hundreds of more patients over the course of the next few months.”
The information is needed because data suggest that up to 25% of patients with hematologic malignancies fail to make anti-S antibodies after full COVID-19 vaccination and that seronegative patients may be especially vulnerable to breakthrough infections, she and her colleagues noted.
Patients with B-cell malignancies are at the highest risk, and this is particularly concerning as some patients with blood cancer who contracted COVID-19 in the prevaccine period of the pandemic had “prolonged, severe infections; generated variant strains; and demonstrated significantly higher mortality rates compared to the general population,” they said.
However, a recently published placebo-controlled trial that demonstrated a booster vaccination–mediated increase in anti-S antibodies and neutralizing antibodies in immunosuppressed patients, and the current review, which focused on patients who obtained booster vaccinations prior to Aug. 12, 2021 (when the Food and Drug Administration granted emergency use authorization for booster doses in immunocompromised people), offer findings that suggest these patients may benefit from receiving COVID-19 boosters.
“We conclude that some patients with hematologic malignancies who are seronegative after a full course of vaccination may benefit from a booster,” the authors wrote. They added a warning: “Regulators, patients, and health care providers should be aware that a sizable subset of patients with blood cancer may remain at risk of breakthrough COVID-19 infections after full vaccination followed by booster vaccination.”
Dr. Nichols stressed that the findings “do not in any way suggest that blood cancer patients should stop therapy to get an antibody response to the vaccinations.”
“LLS is encouraging blood cancer patients to get vaccinated and to continue taking preventive measures such as wearing masks, social distancing, hand washing, and avoiding crowds and poorly ventilated indoor spaces,” she said.
Sergio Giralt, MD, professor and deputy head of the division of hematologic malignancies at Memorial Sloan Kettering Cancer Center, New York, further emphasized the importance of preventive measures.
“I think the extra booster will go a long way to protect our patients at this time but should not be used as a replacement for masking indoors and continued social distancing in this vulnerable patient population,” he said.
This study was supported by the LLS.
FROM CANCER CELL
Dr. Judy C. Washington shows URM physicians how to lead
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
For URM physicians, she also imparts a shared experience of being a minority in the field and helps prepare them for the challenges of facing racism or feeling marginalized or not equitably supported in academic life – and for making change.
While family medicine’s demographics have become more diverse over time, and more so than other specialties, they are not yet representative of the U.S. population. Within academia, male physicians who are Black or African American, or Hispanic or Latino, comprised about 4% and 5% of family medicine faculty, respectively, at the end of 2019, according to data from the Association of American Medical Colleges. For women, these numbers were about 9% and 4%, respectively. (Only those with an MD degree exclusively were included in the report.)
“When you have the privilege to serve in leadership, you have the responsibility to reach back and identify and help others who would not otherwise have the opportunity to be recognized,” Dr. Washington said.
Her mentorship work stems in large part from her long-time involvement and leadership roles in the Society of Teachers of Family Medicine (STFM) – roles she considers a pillar of her professional life. She currently serves as president of the STFM Foundation and is associate chief medical officer of the Atlantic Medical Group, a large multisite physician-led organization. She is also coordinator of women’s health for the Overlook Family Medicine Residency Program, which is affiliated with Atlantic Medical Group.
In Dr. Washington’s role as associate chief medical officer of Atlantic Medical Group in Summit, N.J., she focuses on physician engagement, satisfaction, and diversity. She also assists in areas such as population health. For the Overlook Family Medicine Residency Program also in Summit, she precepts residents in the obstetrics clinic and in the family medicine outpatient clinic.
Diana N. Carvajal, MD, MPH, one of Dr. Washington’s mentees, called her an “inspirational leader” for young academic faculty and said she is a familiar speaker at STFM meetings on topics of workforce diversity, equity, and leadership. She is “passionate” about mentorship, Dr. Carvajal said, and has understood “that URMs and women of color were not always getting [the mentorship they need to be successful].”
Guiding future leaders
Ivonne McLean, MD, assistant professor of family and community medicine at Icahn School of Medicine at Mount Sinai, New York, and an attending at a community health center in the Bronx, called Dr. Washington for advice a couple of years ago when she was considering her next career move.
“She took a genuine interest in me. She never said, this is what you should do. But the questions she asked and the examples she gave from her own life were incredibly helpful to me [in deciding to pursue a research fellowship] ... it was a pivotal conversation,” said Dr. McLean, associate director of a reproductive health fellowship and a research fellow in a New York State–funded program.
“From a lived experience angle, she also told me, here are some of the challenges you’ll have as a woman of color, and here are some of the ways you can approach that,” she said.
Dr. Carvajal, also a URM family physician, credits Dr. Washington’s mentorship with the development of a day-long workshop – held before the annual Society of Teachers of Family Medicine (STFM) meeting – on the low and declining rates of Black males in medicine. “We’d planned it as a presentation, and [she heard of it and] helped us expand it,” she said, calling Dr. Washington “warm, welcoming, and encouraging.
“That work and collaboration with her and the others she brought [into the process] have resulted in publications and more presentations and strategy building for diversifying the workforce,” said Dr. Carvajal, assistant professor, director of reproductive health education in family medicine, and codirector of the research section, all in the department of family and community medicine at the University of Maryland, Baltimore.
STFM involvement
Dr. Washington, who says that all or almost all of her mentees are now leaders in their academic institutions and communities, has been instrumental in developing STFM’s mentoring programming and in facilitating the organization’s multifaceted URM Initiative.
She has been active in STFM since the start of her academic career, and in 2009, while serving as assistant program director for the residency program in which she’d trained, she joined two other African American women, Monique Y. Davis-Smith, MD, and Joedrecka Brown-Speights, MD, in cochairing the society’s Group on Minority and Multicultural Health.
It was in this space, that Dr. Washington said she “heard people’s stories of being in major academic institutions and not feeling supported, not being given roadmaps to success, not getting assistance with publishing, or just kind of feeling like an outsider ... of not being pulled in.” Hispanic and African American females, in particular, “were feeling marginalized,” she said.
In 2018, having co-led development of the STFM Quality Mentoring Program for URM faculty, Dr. Washington was asked to join the STFM Foundation and subsequently led the STFM Foundation’s fundraising campaign for a new URM Initiative. She exceeded her goal, increasing support for URM participation in meetings and activities, and then participated in an STFM steering committee to create broader and longer-lasting support for URM faculty, community teachers, and medical students and residents going into academic family medicine.
Increasing the percentage of URM family medicine faculty in leadership positions – and raising awareness of structural barriers to achievement – is one of the current pillars of the URM Initiative.
Navigating the ‘minority tax’
As part of her mentoring, Dr. Washington helps URM physicians navigate the minority tax – a term referring to the uncompensated citizenship tasks that are more often assigned to Black and other URM physicians than to White physicians, and that take time away from scholarship, further perpetuating inequities.
“Some of our young faculty members find themselves thrust into being the diversity and inclusion leaders in their institutions at a level at which they feel little power and little buy-in from [leadership],” she noted.
A commentary written by Dr. Washington and several colleagues on the minority tax as it impacts women – and the need to build a “tax shelter” to make academic medicine a more just environment for URM women – was published earlier this year in the Journal of Women’s Health.
She also answers e-mails and fields phone calls from young URM faculty who are mulling career moves and facing other familiar challenges.
Physicians who are URM, and African American physicians in particular, tend to “get pulled into the [often underserved] communities, into the patient care and community service areas,” Dr. Washington explained. “But unless you convert these projects into scholarship and publications, and unless you serve on a national committee outside of your institution, you’re not going to be promoted.”
Dr. Washington helps junior faculty envision themselves 5-plus years down the road, find what she calls scholarly “passion projects,” and prepare themselves for their next steps.
She helps her mentees navigate other parts of the continuum of unconscious bias and racism as well, from microaggressions from colleagues to overt discrimination from patients.
“I spend countless minutes fielding texts and phone calls from those who need support,” she wrote in a blog post. “They are a constant reminder that I must continue to speak up when I get the opportunity to do so.”
A journey through family medicine, and through bias and racism
Dr. Washington’s early days in medicine included graduating from Meharry Medical College in 1983 and the Mountainside Family Practice Residency Program in 1990. Following 6 years of working in a private practice in rural Maryland, she moved to academia, spending 6 years at East Tennessee State University and 4 years at the UMDNJ–New Jersey Medical School in Newark as an assistant professor of family medicine.
As had happened in rural Maryland, bias and racism have too often lurked during her career as a physician.
“I grew up in Alabama so I was pretty much ready to deal with racism in the South,” Dr. Washington said. “What I was not ready for was coming to the Northeast and seeing that you’re marginalized because you’re not invited into the room. Or if you do go into spaces when you’re the only one, you often don’t feel as welcomed as you thought you might be.”
Her ideas and contributions were too often dismissed, she wrote in a 2020 blog entry posted on her LinkedIn page. And during contract negotiations, “I was not aware of all the information that my White colleagues had. They had the advantage of inside information.”
Dr. Washington says that “it took a village” to make her who she is today: teachers in her segregated schools in Alabama, one of her college professors, her best friend in medical school – and STFM, “where the list [of her own mentors] is long.”
Military sexual trauma tied to risk for hypertension
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
a new study suggests.
“Understanding a patient’s trauma history is invaluable for treating the whole person,” Allison E. Gaffey, PhD, Yale University, New Haven, Conn., and the Veterans Affairs Connecticut Healthcare System, told this news organization.
“Assessing men and women’s history of trauma, including sexual trauma, is critical for recognizing nontraditional factors that contribute to their cardiovascular risk and to gain a more comprehensive understanding of their mental and physical health,” Dr. Gaffey added.
She presented her research at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
Lasting impact on physical health
Dr. Gaffey and colleagues analyzed data from the VA for roughly 1.2 million veterans (mean age, 30.2 years; 12% female) who were discharged from the military after Sept. 30, 2001, and who received health care services at VA medical centers from 2001 to 2017.
All were screened for sexual harassment and assault, known as military sexual trauma (MST), when they first began receiving VA care.
During 16 years of follow-up, 33,881 veterans screened positive for MST (65% women), and 307,332 developed hypertension (15% women).
Overall, MST was associated with a 30% increase in risk for incident hypertension in unadjusted models (hazard ratio, 1.30; 95% confidence interval, 1.28-1.33; P < .001).
After adjustment for demographic characteristics, lifestyle factors, cardiovascular comorbidities, PTSD, anxiety, and depression, MST remained significantly associated with hypertension (adjusted HR, 1.10; 95% CI, 1.08-1.12; P < .001).
When women and men were examined separately, the link between MST and risk for hypertension remained for both groups, but was slightly stronger among women.
“Sexual trauma has been associated with autonomic dysfunction, inflammation, and dysregulation in the hypothalamic pituitary adrenal axis and renin-angiotensin-aldosterone system, which could lead to elevations in BP over time,” Dr. Gaffey told this news organization.
“These findings show that even many years after being discharged from military service, exposure to military sexual trauma can continue to significantly influence veterans’ physical health,” she added.
Dr. Gaffey said it will be important to determine if early treatment of MST improves hypertension risk, particularly among those showing elevated blood pressure or stage 1 hypertension.
Social determinants of health
Willie Lawrence Jr., MD, head of the AHA National Hypertension Control Initiative oversight committee, said the findings in this study are “in line with what we know about the impact of social determinants of health on high blood pressure.”
“There are studies that suggest that things that we historically don’t look at as risk factors for hypertension – lifelong racism, crime, mental health status – do in fact predict your risk of developing hypertension,” Dr. Lawrence, from Spectrum Health in Benton Harbor, Mich., told this news organization.
“It’s not just your genetics that will determine your health, and there are a lot of things that will affect your blood pressure. Your blood pressure is really just a barometer of everything that’s going on in your life and some of the things that have gone on in your life in the past,” added Dr. Lawrence, who wasn’t involved in the study.
Funding for the study was provided by the Department of Veterans Affairs. Dr. Gaffey and Dr. Lawrence have no relevant disclosures.
A version of this article first appeared on Medscape.com.
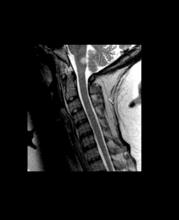
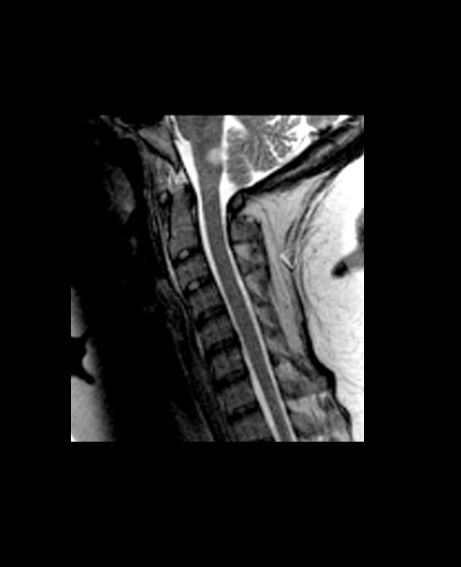
CVST after COVID-19 vaccine: New data confirm high mortality rate
, confirming the severity of the reaction and the associated high mortality rate.
The new series comes from an international registry of consecutive patients who experienced CVST within 28 days of COVID-19 vaccination between March 29 and June 18, 2021, from 81 hospitals in 19 countries.
The cases are described in an article published online on Sept. 28. in JAMA Neurology.
“This is a reliable description on the clinical condition of these patients with CVST associated with COVID-19 vaccination. It is striking that this a much worse condition than CVST not associated with COVID-19 vaccination, with a much higher rate of intracerebral hemorrhage and coma and a much higher mortality rate,” senior author Jonathan M. Coutinho, MD, Amsterdam University Medical Centers, told this news organization.
These data confirm the observations from an earlier U.K. cohort in which cases of cerebral venous thrombosis linked to COVID-19 vaccination occurred.
“This is the biggest series, and as an international series, it gives a broader perspective from a larger range of countries,” Dr. Coutinho said. “All the data together show that, although this side effect is rare, the consequences are very severe,” he added.
In the current study, the researchers regarded CVST as being linked to the vaccine if it was accompanied by thrombosis with thrombocytopenia syndrome (TTS), as evidenced by thrombosis and new-onset thrombocytopenia.
In the cohort of 116 patients with CVST after COVID-19 vaccination, 78 (67.2%) had thrombosis with TTS and were thus classified as having had a vaccine-related adverse event. These patients were frequently comatose at presentation (24%) and often had intracerebral hemorrhage (68%) and concomitant thromboembolism (36%); 47% died during hospitalization.
These patients were compared with the 38 patients in the same cohort who had CVST but in whom there was no indication of concomitant thrombosis and thrombocytopenia. The case patients were also compared with a control group of 207 patients with CVST who were included in a separate international registry before the COVID-19 pandemic.
Mortality rates were much higher among the patients deemed to have had a vaccine-related CVST. The in-hospital mortality rate was 47%, compared with 5% among the patients in the same cohort who did not have TTS and 3.9% among the prepandemic control group.
The mortality rate was even higher (61%) among patients in the TTS group for whom the diagnosis was made before the condition garnered attention in the scientific community. The mortality rate was 42% among patients diagnosed later.
Of the 78 patients in whom CVST and TTS occurred after COVID-19 vaccination in this cohort, 76 had received the AstraZeneca vaccine (in 75 patients, CVST and TTS occurred after the first vaccination; in one patient, they occurred after the second vaccination). One patient had received the Johnson & Johnson vaccine, and one had received the Pfizer vaccine.
“After more analysis, the case after the Pfizer vaccination is not believed to be caused by the vaccine,” Dr. Coutinho said. “In that case, the patient had a platelet count just below the lower limit and was taking an immunomodulator drug that is known to be associated with thrombocytopenia.”
For two patients who received the AstraZeneca vaccine, there was also an alternative explanation for the thrombocytopenia.
Dr. Coutinho also pointed out that the Johnson & Johnson vaccine has been used mainly in the United States, and these data were largely from other countries.
The median time from vaccination to CVST symptom onset was 9 days in the TTS group. The median platelet count at hospital admission among patients with postvaccination CVST-TTS was 45. Three patients presented with a normal platelet count and developed thrombocytopenia during admission; two patients presented with mild thrombocytopenia, 30 presented with moderate thrombocytopenia, and 43 presented with severe thrombocytopenia.
Antibodies against platelet factor 4 (PF4) were measured in 69 patients with TTS, of whom 63 (91%) tested positive (the one patient in whom TTS occurred after the patient received the Pfizer vaccine did not test positive). However, the researchers note that sensitivity varies among different PF4 ELISA tests. Findings of platelet activation assays were positive in all 36 tested patients.
In the TTS group, 52 patients (67%) received immunomodulation therapy, most often intravenous immunoglobulins (IVIG). Among patients treated with IVIG, the mortality rate was lower (28%).
Different from CVST linked to natural COVID-19 infection
Dr. Coutinho noted that CVST can occur in natural SARS-CoV-2 infection but that vaccine-associated CVST is very different.
“In natural COVID-19 infection, there is an increased risk of thrombosis, and some patients can get CVST as a part of this, but in these cases, this is not accompanied by thrombocytopenia. While the CVST in natural COVID-19 infection is also associated with a bad prognosis, this is more to do with the underlying disease. It is normally the very sick COVID patients who develop CVST, and these patients usually die from the underlying disease rather than the CVST itself,” he explained.
“Clinicians need to be aware of vaccine-related CVST, as it requires very specific and rapid treatment,” Dr. Coutinho stressed.
“Patients presenting with an extremely severe headache (unlike any headache they’ve had before) or with seizures or a focal deficit (weakness in arm or problems with speaking or vision) within 4 weeks of an adenovirus COVID-19 vaccination should ring alarm bells. It is important to do diagnostics quickly, with a platelet count the most important first step, and a rapid CT/MRI scan,” he said.
Other tests that should be conducted are D-dimer for thrombosis and the PF4 antibody test. But results for the PF4 antibody test can take days to come back, and clinicians shouldn’t wait for that, Dr. Coutinho notes.
“Specific treatment needs to be given immediately – with anticoagulation (preferably nonheparin) and immunomodulation with IVIG to stop the immune reaction. Platelets should not be given – that may seem counterintuitive in patients with a low platelet count, but giving platelets makes it worse,” he said.
Is there a geographic difference?
Dr. Coutinho pointed out that fewer cases of this vaccine-related CVST are being reported at the current time.
“We are not sure why this is the case. These adenovirus vaccines are not being used much now in Western countries, but our collaboration covers many less developed countries in South America and Asia, which are relying heavily on these vaccines. We are now shifting focus to these countries, but so far we have only seen a handful of cases from these areas,” he said.
He suggested that this may be because these countries started their vaccination programs later and are vaccinating their elderly (who are not so susceptible to this side effect) first, or it may be because of some environmental or genetic factor that has not yet been discovered.
“This is now an important research question – is the risk of vaccine-induced CVST the same in different countries or ethnicities? This could influence decisions on future vaccine strategies,” Dr. Coutinho said.
“So far, female sex is the strongest risk factor for vaccine-induced CVST. In our cohort, 81% of cases were in women. In addition, 95% were White, but that doesn’t allow us to conclude that this is a risk factor, as the majority of people who have been vaccinated are White. So, we have no clear insight into that yet,” he said.
In a comment for this news organization, the lead author of the previous U.K. report of a series of 70 cases of cerebral venous thrombosis linked to COVID-19 vaccination, Richard Perry, PhD, University College Hospital, London, described this new report as “an excellent study, with many of the same strengths and weaknesses as our study and has very similar results.”
Dr. Perry noted that the two studies used slightly different definitions of vaccine-induced thrombotic thrombocytopenia, but the cases reported appear to be very similar overall. “It is reassuring and gratifying to see that they have made such similar observations,” he said.
“And as they have drawn their cases from a broad range of countries whereas ours were all from the U.K., this provides evidence that the observations from both studies are reasonably generalizable,” he added.
Dr. Perry pointed out that this new report states that TTS occurred in one patient after the patient had received a second dose of the AstraZeneca vaccine. “I would like to know more about this case, because we didn’t see any cases after a second dose in our cohort,” he said.
Dr. Coutinho responded that he didn’t believe this was the first reported case after the second dose.
The study did not receive any specific funding. Dr. Coutinho has received grants paid to his institution from Boehringer Ingelheim and Bayer and payments paid to his institution for data safety monitoring board participation by Bayer.
A version of this article first appeared on Medscape.com.
, confirming the severity of the reaction and the associated high mortality rate.
The new series comes from an international registry of consecutive patients who experienced CVST within 28 days of COVID-19 vaccination between March 29 and June 18, 2021, from 81 hospitals in 19 countries.
The cases are described in an article published online on Sept. 28. in JAMA Neurology.
“This is a reliable description on the clinical condition of these patients with CVST associated with COVID-19 vaccination. It is striking that this a much worse condition than CVST not associated with COVID-19 vaccination, with a much higher rate of intracerebral hemorrhage and coma and a much higher mortality rate,” senior author Jonathan M. Coutinho, MD, Amsterdam University Medical Centers, told this news organization.
These data confirm the observations from an earlier U.K. cohort in which cases of cerebral venous thrombosis linked to COVID-19 vaccination occurred.
“This is the biggest series, and as an international series, it gives a broader perspective from a larger range of countries,” Dr. Coutinho said. “All the data together show that, although this side effect is rare, the consequences are very severe,” he added.
In the current study, the researchers regarded CVST as being linked to the vaccine if it was accompanied by thrombosis with thrombocytopenia syndrome (TTS), as evidenced by thrombosis and new-onset thrombocytopenia.
In the cohort of 116 patients with CVST after COVID-19 vaccination, 78 (67.2%) had thrombosis with TTS and were thus classified as having had a vaccine-related adverse event. These patients were frequently comatose at presentation (24%) and often had intracerebral hemorrhage (68%) and concomitant thromboembolism (36%); 47% died during hospitalization.
These patients were compared with the 38 patients in the same cohort who had CVST but in whom there was no indication of concomitant thrombosis and thrombocytopenia. The case patients were also compared with a control group of 207 patients with CVST who were included in a separate international registry before the COVID-19 pandemic.
Mortality rates were much higher among the patients deemed to have had a vaccine-related CVST. The in-hospital mortality rate was 47%, compared with 5% among the patients in the same cohort who did not have TTS and 3.9% among the prepandemic control group.
The mortality rate was even higher (61%) among patients in the TTS group for whom the diagnosis was made before the condition garnered attention in the scientific community. The mortality rate was 42% among patients diagnosed later.
Of the 78 patients in whom CVST and TTS occurred after COVID-19 vaccination in this cohort, 76 had received the AstraZeneca vaccine (in 75 patients, CVST and TTS occurred after the first vaccination; in one patient, they occurred after the second vaccination). One patient had received the Johnson & Johnson vaccine, and one had received the Pfizer vaccine.
“After more analysis, the case after the Pfizer vaccination is not believed to be caused by the vaccine,” Dr. Coutinho said. “In that case, the patient had a platelet count just below the lower limit and was taking an immunomodulator drug that is known to be associated with thrombocytopenia.”
For two patients who received the AstraZeneca vaccine, there was also an alternative explanation for the thrombocytopenia.
Dr. Coutinho also pointed out that the Johnson & Johnson vaccine has been used mainly in the United States, and these data were largely from other countries.
The median time from vaccination to CVST symptom onset was 9 days in the TTS group. The median platelet count at hospital admission among patients with postvaccination CVST-TTS was 45. Three patients presented with a normal platelet count and developed thrombocytopenia during admission; two patients presented with mild thrombocytopenia, 30 presented with moderate thrombocytopenia, and 43 presented with severe thrombocytopenia.
Antibodies against platelet factor 4 (PF4) were measured in 69 patients with TTS, of whom 63 (91%) tested positive (the one patient in whom TTS occurred after the patient received the Pfizer vaccine did not test positive). However, the researchers note that sensitivity varies among different PF4 ELISA tests. Findings of platelet activation assays were positive in all 36 tested patients.
In the TTS group, 52 patients (67%) received immunomodulation therapy, most often intravenous immunoglobulins (IVIG). Among patients treated with IVIG, the mortality rate was lower (28%).
Different from CVST linked to natural COVID-19 infection
Dr. Coutinho noted that CVST can occur in natural SARS-CoV-2 infection but that vaccine-associated CVST is very different.
“In natural COVID-19 infection, there is an increased risk of thrombosis, and some patients can get CVST as a part of this, but in these cases, this is not accompanied by thrombocytopenia. While the CVST in natural COVID-19 infection is also associated with a bad prognosis, this is more to do with the underlying disease. It is normally the very sick COVID patients who develop CVST, and these patients usually die from the underlying disease rather than the CVST itself,” he explained.
“Clinicians need to be aware of vaccine-related CVST, as it requires very specific and rapid treatment,” Dr. Coutinho stressed.
“Patients presenting with an extremely severe headache (unlike any headache they’ve had before) or with seizures or a focal deficit (weakness in arm or problems with speaking or vision) within 4 weeks of an adenovirus COVID-19 vaccination should ring alarm bells. It is important to do diagnostics quickly, with a platelet count the most important first step, and a rapid CT/MRI scan,” he said.
Other tests that should be conducted are D-dimer for thrombosis and the PF4 antibody test. But results for the PF4 antibody test can take days to come back, and clinicians shouldn’t wait for that, Dr. Coutinho notes.
“Specific treatment needs to be given immediately – with anticoagulation (preferably nonheparin) and immunomodulation with IVIG to stop the immune reaction. Platelets should not be given – that may seem counterintuitive in patients with a low platelet count, but giving platelets makes it worse,” he said.
Is there a geographic difference?
Dr. Coutinho pointed out that fewer cases of this vaccine-related CVST are being reported at the current time.
“We are not sure why this is the case. These adenovirus vaccines are not being used much now in Western countries, but our collaboration covers many less developed countries in South America and Asia, which are relying heavily on these vaccines. We are now shifting focus to these countries, but so far we have only seen a handful of cases from these areas,” he said.
He suggested that this may be because these countries started their vaccination programs later and are vaccinating their elderly (who are not so susceptible to this side effect) first, or it may be because of some environmental or genetic factor that has not yet been discovered.
“This is now an important research question – is the risk of vaccine-induced CVST the same in different countries or ethnicities? This could influence decisions on future vaccine strategies,” Dr. Coutinho said.
“So far, female sex is the strongest risk factor for vaccine-induced CVST. In our cohort, 81% of cases were in women. In addition, 95% were White, but that doesn’t allow us to conclude that this is a risk factor, as the majority of people who have been vaccinated are White. So, we have no clear insight into that yet,” he said.
In a comment for this news organization, the lead author of the previous U.K. report of a series of 70 cases of cerebral venous thrombosis linked to COVID-19 vaccination, Richard Perry, PhD, University College Hospital, London, described this new report as “an excellent study, with many of the same strengths and weaknesses as our study and has very similar results.”
Dr. Perry noted that the two studies used slightly different definitions of vaccine-induced thrombotic thrombocytopenia, but the cases reported appear to be very similar overall. “It is reassuring and gratifying to see that they have made such similar observations,” he said.
“And as they have drawn their cases from a broad range of countries whereas ours were all from the U.K., this provides evidence that the observations from both studies are reasonably generalizable,” he added.
Dr. Perry pointed out that this new report states that TTS occurred in one patient after the patient had received a second dose of the AstraZeneca vaccine. “I would like to know more about this case, because we didn’t see any cases after a second dose in our cohort,” he said.
Dr. Coutinho responded that he didn’t believe this was the first reported case after the second dose.
The study did not receive any specific funding. Dr. Coutinho has received grants paid to his institution from Boehringer Ingelheim and Bayer and payments paid to his institution for data safety monitoring board participation by Bayer.
A version of this article first appeared on Medscape.com.
, confirming the severity of the reaction and the associated high mortality rate.
The new series comes from an international registry of consecutive patients who experienced CVST within 28 days of COVID-19 vaccination between March 29 and June 18, 2021, from 81 hospitals in 19 countries.
The cases are described in an article published online on Sept. 28. in JAMA Neurology.
“This is a reliable description on the clinical condition of these patients with CVST associated with COVID-19 vaccination. It is striking that this a much worse condition than CVST not associated with COVID-19 vaccination, with a much higher rate of intracerebral hemorrhage and coma and a much higher mortality rate,” senior author Jonathan M. Coutinho, MD, Amsterdam University Medical Centers, told this news organization.
These data confirm the observations from an earlier U.K. cohort in which cases of cerebral venous thrombosis linked to COVID-19 vaccination occurred.
“This is the biggest series, and as an international series, it gives a broader perspective from a larger range of countries,” Dr. Coutinho said. “All the data together show that, although this side effect is rare, the consequences are very severe,” he added.
In the current study, the researchers regarded CVST as being linked to the vaccine if it was accompanied by thrombosis with thrombocytopenia syndrome (TTS), as evidenced by thrombosis and new-onset thrombocytopenia.
In the cohort of 116 patients with CVST after COVID-19 vaccination, 78 (67.2%) had thrombosis with TTS and were thus classified as having had a vaccine-related adverse event. These patients were frequently comatose at presentation (24%) and often had intracerebral hemorrhage (68%) and concomitant thromboembolism (36%); 47% died during hospitalization.
These patients were compared with the 38 patients in the same cohort who had CVST but in whom there was no indication of concomitant thrombosis and thrombocytopenia. The case patients were also compared with a control group of 207 patients with CVST who were included in a separate international registry before the COVID-19 pandemic.
Mortality rates were much higher among the patients deemed to have had a vaccine-related CVST. The in-hospital mortality rate was 47%, compared with 5% among the patients in the same cohort who did not have TTS and 3.9% among the prepandemic control group.
The mortality rate was even higher (61%) among patients in the TTS group for whom the diagnosis was made before the condition garnered attention in the scientific community. The mortality rate was 42% among patients diagnosed later.
Of the 78 patients in whom CVST and TTS occurred after COVID-19 vaccination in this cohort, 76 had received the AstraZeneca vaccine (in 75 patients, CVST and TTS occurred after the first vaccination; in one patient, they occurred after the second vaccination). One patient had received the Johnson & Johnson vaccine, and one had received the Pfizer vaccine.
“After more analysis, the case after the Pfizer vaccination is not believed to be caused by the vaccine,” Dr. Coutinho said. “In that case, the patient had a platelet count just below the lower limit and was taking an immunomodulator drug that is known to be associated with thrombocytopenia.”
For two patients who received the AstraZeneca vaccine, there was also an alternative explanation for the thrombocytopenia.
Dr. Coutinho also pointed out that the Johnson & Johnson vaccine has been used mainly in the United States, and these data were largely from other countries.
The median time from vaccination to CVST symptom onset was 9 days in the TTS group. The median platelet count at hospital admission among patients with postvaccination CVST-TTS was 45. Three patients presented with a normal platelet count and developed thrombocytopenia during admission; two patients presented with mild thrombocytopenia, 30 presented with moderate thrombocytopenia, and 43 presented with severe thrombocytopenia.
Antibodies against platelet factor 4 (PF4) were measured in 69 patients with TTS, of whom 63 (91%) tested positive (the one patient in whom TTS occurred after the patient received the Pfizer vaccine did not test positive). However, the researchers note that sensitivity varies among different PF4 ELISA tests. Findings of platelet activation assays were positive in all 36 tested patients.
In the TTS group, 52 patients (67%) received immunomodulation therapy, most often intravenous immunoglobulins (IVIG). Among patients treated with IVIG, the mortality rate was lower (28%).
Different from CVST linked to natural COVID-19 infection
Dr. Coutinho noted that CVST can occur in natural SARS-CoV-2 infection but that vaccine-associated CVST is very different.
“In natural COVID-19 infection, there is an increased risk of thrombosis, and some patients can get CVST as a part of this, but in these cases, this is not accompanied by thrombocytopenia. While the CVST in natural COVID-19 infection is also associated with a bad prognosis, this is more to do with the underlying disease. It is normally the very sick COVID patients who develop CVST, and these patients usually die from the underlying disease rather than the CVST itself,” he explained.
“Clinicians need to be aware of vaccine-related CVST, as it requires very specific and rapid treatment,” Dr. Coutinho stressed.
“Patients presenting with an extremely severe headache (unlike any headache they’ve had before) or with seizures or a focal deficit (weakness in arm or problems with speaking or vision) within 4 weeks of an adenovirus COVID-19 vaccination should ring alarm bells. It is important to do diagnostics quickly, with a platelet count the most important first step, and a rapid CT/MRI scan,” he said.
Other tests that should be conducted are D-dimer for thrombosis and the PF4 antibody test. But results for the PF4 antibody test can take days to come back, and clinicians shouldn’t wait for that, Dr. Coutinho notes.
“Specific treatment needs to be given immediately – with anticoagulation (preferably nonheparin) and immunomodulation with IVIG to stop the immune reaction. Platelets should not be given – that may seem counterintuitive in patients with a low platelet count, but giving platelets makes it worse,” he said.
Is there a geographic difference?
Dr. Coutinho pointed out that fewer cases of this vaccine-related CVST are being reported at the current time.
“We are not sure why this is the case. These adenovirus vaccines are not being used much now in Western countries, but our collaboration covers many less developed countries in South America and Asia, which are relying heavily on these vaccines. We are now shifting focus to these countries, but so far we have only seen a handful of cases from these areas,” he said.
He suggested that this may be because these countries started their vaccination programs later and are vaccinating their elderly (who are not so susceptible to this side effect) first, or it may be because of some environmental or genetic factor that has not yet been discovered.
“This is now an important research question – is the risk of vaccine-induced CVST the same in different countries or ethnicities? This could influence decisions on future vaccine strategies,” Dr. Coutinho said.
“So far, female sex is the strongest risk factor for vaccine-induced CVST. In our cohort, 81% of cases were in women. In addition, 95% were White, but that doesn’t allow us to conclude that this is a risk factor, as the majority of people who have been vaccinated are White. So, we have no clear insight into that yet,” he said.
In a comment for this news organization, the lead author of the previous U.K. report of a series of 70 cases of cerebral venous thrombosis linked to COVID-19 vaccination, Richard Perry, PhD, University College Hospital, London, described this new report as “an excellent study, with many of the same strengths and weaknesses as our study and has very similar results.”
Dr. Perry noted that the two studies used slightly different definitions of vaccine-induced thrombotic thrombocytopenia, but the cases reported appear to be very similar overall. “It is reassuring and gratifying to see that they have made such similar observations,” he said.
“And as they have drawn their cases from a broad range of countries whereas ours were all from the U.K., this provides evidence that the observations from both studies are reasonably generalizable,” he added.
Dr. Perry pointed out that this new report states that TTS occurred in one patient after the patient had received a second dose of the AstraZeneca vaccine. “I would like to know more about this case, because we didn’t see any cases after a second dose in our cohort,” he said.
Dr. Coutinho responded that he didn’t believe this was the first reported case after the second dose.
The study did not receive any specific funding. Dr. Coutinho has received grants paid to his institution from Boehringer Ingelheim and Bayer and payments paid to his institution for data safety monitoring board participation by Bayer.
A version of this article first appeared on Medscape.com.
Boy with slightly impaired coordination
This young patient is probably presenting with pediatric multiple sclerosis (MS). It is estimated that MS onset before the age of 18 years accounts for 3%-5% of the general population of patients with this autoimmune disease. The condition represents the most common nontraumatic, disabling neurologic disorder among young adults. Disease prevalence is highest between the ages of 13 and 16. In children older than 10, a female predominance is seen, suggesting a hormonal role in pathogenesis. The vast majority (up to 98%) of children and adolescents with MS have a relapsing-remitting course. Overall, pediatric MS has a milder course than adult MS but can lead to significant disability at an early age. Although pediatric patients may experience more frequent relapses, data also suggest that children seem to recover more quickly from episodes than adults.
In children and adolescents, MS most typically manifests with sensory disturbances and impaired coordination. The most commonly reported symptoms in pediatric MS are sensory, motor, and brainstem dysfunction, though cognitive and emotional disorders can emerge over time.
Younger children will often show multifocal symptoms but with the onset of adolescence may begin to present with only a single focal symptom, as is often the case with adult patients.
Diagnosis of pediatric MS goes hand-in-hand with a diagnosis of clinically isolated syndrome (CIS) or sporadic acute disseminated encephalomyelitis (ADEM). CIS is diagnosed when symptoms last for over 24 hours with possible inflammatory demyelination but without encephalopathy. To confirm an MS diagnosis, two or more clinical episodes must occur at least 30 days apart. MRI can both confirm diagnosis and offer great value in monitoring disease progression in the brain and spinal cord. Of note, differentiating the first episode of juvenile MS from ADEM is a significant clinical challenge.
When it comes to treating relapses, the approach in children is similar to that of adults. Therapy may consist of an intravenous pulse of methylprednisolone (20-30 mg/kg/day for 3-5 days). In 2018, the FDA approved the use of the oral MS therapy Gilenya (fingolimod) for the treatment of patients 10 years of age or older with relapsing forms of MS. Providers can also adapt treatments approved for adults for pediatric patients.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
This young patient is probably presenting with pediatric multiple sclerosis (MS). It is estimated that MS onset before the age of 18 years accounts for 3%-5% of the general population of patients with this autoimmune disease. The condition represents the most common nontraumatic, disabling neurologic disorder among young adults. Disease prevalence is highest between the ages of 13 and 16. In children older than 10, a female predominance is seen, suggesting a hormonal role in pathogenesis. The vast majority (up to 98%) of children and adolescents with MS have a relapsing-remitting course. Overall, pediatric MS has a milder course than adult MS but can lead to significant disability at an early age. Although pediatric patients may experience more frequent relapses, data also suggest that children seem to recover more quickly from episodes than adults.
In children and adolescents, MS most typically manifests with sensory disturbances and impaired coordination. The most commonly reported symptoms in pediatric MS are sensory, motor, and brainstem dysfunction, though cognitive and emotional disorders can emerge over time.
Younger children will often show multifocal symptoms but with the onset of adolescence may begin to present with only a single focal symptom, as is often the case with adult patients.
Diagnosis of pediatric MS goes hand-in-hand with a diagnosis of clinically isolated syndrome (CIS) or sporadic acute disseminated encephalomyelitis (ADEM). CIS is diagnosed when symptoms last for over 24 hours with possible inflammatory demyelination but without encephalopathy. To confirm an MS diagnosis, two or more clinical episodes must occur at least 30 days apart. MRI can both confirm diagnosis and offer great value in monitoring disease progression in the brain and spinal cord. Of note, differentiating the first episode of juvenile MS from ADEM is a significant clinical challenge.
When it comes to treating relapses, the approach in children is similar to that of adults. Therapy may consist of an intravenous pulse of methylprednisolone (20-30 mg/kg/day for 3-5 days). In 2018, the FDA approved the use of the oral MS therapy Gilenya (fingolimod) for the treatment of patients 10 years of age or older with relapsing forms of MS. Providers can also adapt treatments approved for adults for pediatric patients.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
This young patient is probably presenting with pediatric multiple sclerosis (MS). It is estimated that MS onset before the age of 18 years accounts for 3%-5% of the general population of patients with this autoimmune disease. The condition represents the most common nontraumatic, disabling neurologic disorder among young adults. Disease prevalence is highest between the ages of 13 and 16. In children older than 10, a female predominance is seen, suggesting a hormonal role in pathogenesis. The vast majority (up to 98%) of children and adolescents with MS have a relapsing-remitting course. Overall, pediatric MS has a milder course than adult MS but can lead to significant disability at an early age. Although pediatric patients may experience more frequent relapses, data also suggest that children seem to recover more quickly from episodes than adults.
In children and adolescents, MS most typically manifests with sensory disturbances and impaired coordination. The most commonly reported symptoms in pediatric MS are sensory, motor, and brainstem dysfunction, though cognitive and emotional disorders can emerge over time.
Younger children will often show multifocal symptoms but with the onset of adolescence may begin to present with only a single focal symptom, as is often the case with adult patients.
Diagnosis of pediatric MS goes hand-in-hand with a diagnosis of clinically isolated syndrome (CIS) or sporadic acute disseminated encephalomyelitis (ADEM). CIS is diagnosed when symptoms last for over 24 hours with possible inflammatory demyelination but without encephalopathy. To confirm an MS diagnosis, two or more clinical episodes must occur at least 30 days apart. MRI can both confirm diagnosis and offer great value in monitoring disease progression in the brain and spinal cord. Of note, differentiating the first episode of juvenile MS from ADEM is a significant clinical challenge.
When it comes to treating relapses, the approach in children is similar to that of adults. Therapy may consist of an intravenous pulse of methylprednisolone (20-30 mg/kg/day for 3-5 days). In 2018, the FDA approved the use of the oral MS therapy Gilenya (fingolimod) for the treatment of patients 10 years of age or older with relapsing forms of MS. Providers can also adapt treatments approved for adults for pediatric patients.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme
A 10-year-old boy, typically active, presents with slightly impaired coordination and facial weakness. His parents noticed that his gait in particular seems impaired, though to his knowledge he had not been injured. His mother reports a history of meningoencephalitis. A sagittal T2-weighted MRI sequence shows a portion of the brainstem with a large demyelinating plaque in the dorsal part of the medulla and several other lesions in the periventricular regions of the brain. Spinal fluid is normal.
Two Colorado nurses admit to stealing drugs from hospital patients
, according to the US Attorney’s Office in Denver.
Alicia Nickel-Tangeman, 44, formerly of Woodland Park, Colo., pled guilty to four counts of obtaining controlled substances using fraud and deception. She gained access to rooms of patients who weren’t assigned to her and diverted drugs from their pain-on-demand devices, according to federal officials.
The defendant told patients she was conducting a study on the pumps that deliver drugs to relieve pain when the patient pushes a button, the officials stated. She would open the machine and would remove a portion of the drug with a syringe. She obtained drugs in this way from three patients on four occasions, a press release stated.
When questioned by law enforcement, Ms. Nickel-Tangeman continued to lie about her conduct and produced a false email address to substantiate her claims, the Department of Justice reported. She is scheduled to be sentenced November 30.
Ms. Nickel-Tangeman’s LinkedIn profile shows that she was a nurse with UCHealth in Colorado for 17 years, ending in May 2019.
Katie Muhs, 34, of Littleton, Colo., was convicted of a felony for using fraud and deception to divert fentanyl for her personal use while serving as an intensive care nurse.
The defendant admitted that between June and September 2019 she stole fentanyl by removing it from the IV bags of patients using a syringe. She also admitted to stealing fentanyl that remained in vials after fentanyl had been administered to patients. She would replace the stolen drug with saline and would “then have a fellow nurse witness her ‘waste,’ or dispose of the saline.”
U.S. District Judge Raymond Moore sentenced Ms. Muhs to 3 months of probation as a result of “the defendant’s confession and her cooperation in disclosing full information on her diversion, which is a matter potentially affecting the public health and the integrity of the health care system. The felony offense is punishable by up to four years of imprisonment and a fine of $250,000, per count.”
In pleading guilty to the single count in the case, Ms. Muhs admitted that on September 8, 2019, “she removed a bag of fentanyl from the automated medication control machine at the hospital under a different nurse’s login credentials. She then removed fentanyl from the IV bag for personal use.”
In April, the Colorado Court of Appeals denied her request for unemployment benefits. Court documents reveal that Ms. Muhs lost her job at St. Anthony Hospital after it was discovered that she stole and self-injected fentanyl while working as a registered nurse there.
The investigations in these cases were conducted by the U.S. Food and Drug Administration, the Office of Criminal Investigations, and the Drug Enforcement Administration (DEA).
“We want it to be known that healthcare professionals who take advantage of patients in need by stealing their medications will be held accountable to the law,” said Deanne Reuter, DEA Denver Field Division special agent in charge.
A version of this article first appeared on Medscape.com.
, according to the US Attorney’s Office in Denver.
Alicia Nickel-Tangeman, 44, formerly of Woodland Park, Colo., pled guilty to four counts of obtaining controlled substances using fraud and deception. She gained access to rooms of patients who weren’t assigned to her and diverted drugs from their pain-on-demand devices, according to federal officials.
The defendant told patients she was conducting a study on the pumps that deliver drugs to relieve pain when the patient pushes a button, the officials stated. She would open the machine and would remove a portion of the drug with a syringe. She obtained drugs in this way from three patients on four occasions, a press release stated.
When questioned by law enforcement, Ms. Nickel-Tangeman continued to lie about her conduct and produced a false email address to substantiate her claims, the Department of Justice reported. She is scheduled to be sentenced November 30.
Ms. Nickel-Tangeman’s LinkedIn profile shows that she was a nurse with UCHealth in Colorado for 17 years, ending in May 2019.
Katie Muhs, 34, of Littleton, Colo., was convicted of a felony for using fraud and deception to divert fentanyl for her personal use while serving as an intensive care nurse.
The defendant admitted that between June and September 2019 she stole fentanyl by removing it from the IV bags of patients using a syringe. She also admitted to stealing fentanyl that remained in vials after fentanyl had been administered to patients. She would replace the stolen drug with saline and would “then have a fellow nurse witness her ‘waste,’ or dispose of the saline.”
U.S. District Judge Raymond Moore sentenced Ms. Muhs to 3 months of probation as a result of “the defendant’s confession and her cooperation in disclosing full information on her diversion, which is a matter potentially affecting the public health and the integrity of the health care system. The felony offense is punishable by up to four years of imprisonment and a fine of $250,000, per count.”
In pleading guilty to the single count in the case, Ms. Muhs admitted that on September 8, 2019, “she removed a bag of fentanyl from the automated medication control machine at the hospital under a different nurse’s login credentials. She then removed fentanyl from the IV bag for personal use.”
In April, the Colorado Court of Appeals denied her request for unemployment benefits. Court documents reveal that Ms. Muhs lost her job at St. Anthony Hospital after it was discovered that she stole and self-injected fentanyl while working as a registered nurse there.
The investigations in these cases were conducted by the U.S. Food and Drug Administration, the Office of Criminal Investigations, and the Drug Enforcement Administration (DEA).
“We want it to be known that healthcare professionals who take advantage of patients in need by stealing their medications will be held accountable to the law,” said Deanne Reuter, DEA Denver Field Division special agent in charge.
A version of this article first appeared on Medscape.com.
, according to the US Attorney’s Office in Denver.
Alicia Nickel-Tangeman, 44, formerly of Woodland Park, Colo., pled guilty to four counts of obtaining controlled substances using fraud and deception. She gained access to rooms of patients who weren’t assigned to her and diverted drugs from their pain-on-demand devices, according to federal officials.
The defendant told patients she was conducting a study on the pumps that deliver drugs to relieve pain when the patient pushes a button, the officials stated. She would open the machine and would remove a portion of the drug with a syringe. She obtained drugs in this way from three patients on four occasions, a press release stated.
When questioned by law enforcement, Ms. Nickel-Tangeman continued to lie about her conduct and produced a false email address to substantiate her claims, the Department of Justice reported. She is scheduled to be sentenced November 30.
Ms. Nickel-Tangeman’s LinkedIn profile shows that she was a nurse with UCHealth in Colorado for 17 years, ending in May 2019.
Katie Muhs, 34, of Littleton, Colo., was convicted of a felony for using fraud and deception to divert fentanyl for her personal use while serving as an intensive care nurse.
The defendant admitted that between June and September 2019 she stole fentanyl by removing it from the IV bags of patients using a syringe. She also admitted to stealing fentanyl that remained in vials after fentanyl had been administered to patients. She would replace the stolen drug with saline and would “then have a fellow nurse witness her ‘waste,’ or dispose of the saline.”
U.S. District Judge Raymond Moore sentenced Ms. Muhs to 3 months of probation as a result of “the defendant’s confession and her cooperation in disclosing full information on her diversion, which is a matter potentially affecting the public health and the integrity of the health care system. The felony offense is punishable by up to four years of imprisonment and a fine of $250,000, per count.”
In pleading guilty to the single count in the case, Ms. Muhs admitted that on September 8, 2019, “she removed a bag of fentanyl from the automated medication control machine at the hospital under a different nurse’s login credentials. She then removed fentanyl from the IV bag for personal use.”
In April, the Colorado Court of Appeals denied her request for unemployment benefits. Court documents reveal that Ms. Muhs lost her job at St. Anthony Hospital after it was discovered that she stole and self-injected fentanyl while working as a registered nurse there.
The investigations in these cases were conducted by the U.S. Food and Drug Administration, the Office of Criminal Investigations, and the Drug Enforcement Administration (DEA).
“We want it to be known that healthcare professionals who take advantage of patients in need by stealing their medications will be held accountable to the law,” said Deanne Reuter, DEA Denver Field Division special agent in charge.
A version of this article first appeared on Medscape.com.
Improving quality and return-on-investment: Provider onboarding
Physician and advanced practice provider (APP) (collectively, “provider”) onboarding into health care delivery settings requires careful planning and systematic integration. Assimilation into health care settings and cultures necessitates more than a 1- or 2-day orientation. Rather, an intentional, longitudinal onboarding program (starting with orientation) needs to be designed to assimilate providers into the unique culture of a medical practice.
Establishing mutual expectations
Communication concerning mutual expectations is a vital component of the agreement between provider and practice. Items that should be included in provider onboarding (likely addressed in either the practice visit or amplified in a contract) include the following:
- Committees: Committee orientation should include a discussion of provider preferences/expectations and why getting the new provider involved in the business of the practice is a priority of the group.
- Operations: Key clinical operations details should be reviewed with the incoming provider and reinforced through follow-up discussions with a physician mentor/coach (for example, call distribution; role of the senior nonclinical leadership team/accountants, fellow practice/group partners, and IT support; role definitions and expectations for duties, transitioning call, and EHR charting; revenue-sharing; supplies/preferences/adaptability to scope type).
- Interests: Specific provider interests (for example, clinical research, infusion, hemorrhoidal banding, weight loss/nutrition, inflammatory bowel disease, irritable bowel disease, pathology) and productivity expectations (for example, number of procedures, number of new and return patient visits per day) should be communicated.
- Miscellaneous: Discussion about marketing the practice, importance of growing satellite programs and nuance of major referral groups to the practice are also key components of the assimilation process.
Leadership self-awareness and cultural alignment
Leadership self-awareness is a key element of provider onboarding. Physicians and APPs are trained to think independently and may be challenged to share decision-making and rely on others. The following are some no-cost self-assessment and awareness resources:
- Myers-Briggs Personality Profile Preferences:
- VIA Strengths:
- VARK Analysis:
Cultural alignment is also a critical consideration to ensure orderly assimilation into the practice/health care setting and with stakeholders. A shared commitment to embed a culture with shared values has relevance to merging cultures – not only when organizations come together – but with individuals as well. Time spent developing a better understanding of the customs, culture and traditions of the practice will be helpful if a practice must change its trajectory based on meeting an unmovable obstruction (for example, market forces requiring practice consolidation).
Improved quality
Transitioning a new provider into an existing practice culture can have a ripple effect on support staff and patient satisfaction and is, therefore, an important consideration in provider onboarding. Written standards, procedures, expectations, and practices are always advisable when possible. Attention to the demographics of the recruited physician is also important with shifts in interests and priorities from a practice. Millennials will constitute most of the workforce by 2025 and arrive with a mindset that the tenure in a role will be shorter than providers before them. Accordingly, the intentionality of the relationship is critical for successful bonding.
If current physician leaders want to achieve simultaneous succession planning and maintain the legacy of a patient-centric and resilient practice, these leaders must consider bridging the “cultural knowledge acumen gap.” James S. Hernandez, MD, MS, FCAP, and colleagues suggest a “connector” role between new and experienced providers. Reverse mentoring/distance/reciprocal mentoring is also mentioned as a two-way learning process between mentor and mentee.
Process structure considerations
Each new hire affects the culture of the practice. Best practices for the onboarding and orientation process should be followed. A written project master list with a timeline for completion of onboarding tasks with responsible and accountable persons, target dates for completion, and measurement should be established. Establishing mutual expectations up front can help practices tailor committee roles and clinical responsibilities to maximize provider engagement and longevity. A robust onboarding process may take up to 2 years depending on the size of the practice and the complexity of its structure and associated duties.
Desired outcomes
The desired outcome of the onboarding process is a satisfied provider whose passion and enthusiasm for quality patient care is demonstrated objectively through excellent performance on clinical quality measures and metrics of patient and referral source satisfaction.
Periodic reviews of how the onboarding process is progressing should be undertaken. These reviews can be modeled after the After-Action Review (AAR) process used in the military for measuring progress. Simply stated, what items went well with onboarding and why? What items did not go well with onboarding and why not? (Consider something like the Center for Medicare & Medicaid Services’ “5 Whys” assessment to determine root cause for items that need correction.) What elements of the onboarding process could be further improved? Using a Delphi method during the AAR session is an excellent way for the group to hear from all participants ranging from senior partners to recently recruited providers.
Conclusion
Medical practices must recognize that assimilating a new provider into the practice through a robust onboarding process is not lost effort but rather a force multiplier. Effective provider onboarding gives the incoming provider a sense of purpose and resolve, which results in optimized clinical productivity and engagement because the new provider is invested in the future of the practice. Once successfully onboarded and integrated into the practice, new providers need to understand that the work effort invested in their onboarding comes with a “pay it forward” obligation for the next provider recruited by the group. Group members also need to realize that the baseline is always changing–the provider onboarding process needs to continually evolve and adapt as the practice changes and new providers are hired.
Mr. Rudnick is a visiting professor and program director healthcare quality, innovation, and strategy at St Thomas University, Miami. Mr. Turner is regional vice president for the Midatlantic market of Covenant Physician Partners.
References
“Best practices for onboarding physicians.” The Rheumatologist. 2019 Sep 17. Accessed 2021 Sep 6. https://www.the-rheumatologist.org/article/best-practices-for-onboarding-new-physicians/
Centers for Medicare & Medicaid Services. Five Whys Tool for Root Cause Analysis: QAPI. 2021. Accessed 2021 Sep 6. https://www.cms.gov/medicare/provider-enrollment-and-certification/qapi/downloads/fivewhys.pdf.
DeIuliis ET, Saylor E. Open J Occup Ther. 2021;9(1):1-13.
Hernandez JS et al. “Discussion: Mentoring millennials for future leadership.” Physician Leadership Journal. 2018 May 14. Accessed 2021 Sep 6. https://www.physicianleaders.org/news/discussion-mentoring-millennials-future-leadership
Moore L et al. J Trauma Acute Care Surg. 2015 Jun;78(6):1168-75..
Klein CJ et al. West J Nurs Res. 2021 Feb;43(2):105-114.
Weinburger T, Gordon J. Health Prog. Nov-Dec 2013;94(6):76-9.
Wentlandt K et al. Healthc Q. 2016;18(4):36-41.
Physician and advanced practice provider (APP) (collectively, “provider”) onboarding into health care delivery settings requires careful planning and systematic integration. Assimilation into health care settings and cultures necessitates more than a 1- or 2-day orientation. Rather, an intentional, longitudinal onboarding program (starting with orientation) needs to be designed to assimilate providers into the unique culture of a medical practice.
Establishing mutual expectations
Communication concerning mutual expectations is a vital component of the agreement between provider and practice. Items that should be included in provider onboarding (likely addressed in either the practice visit or amplified in a contract) include the following:
- Committees: Committee orientation should include a discussion of provider preferences/expectations and why getting the new provider involved in the business of the practice is a priority of the group.
- Operations: Key clinical operations details should be reviewed with the incoming provider and reinforced through follow-up discussions with a physician mentor/coach (for example, call distribution; role of the senior nonclinical leadership team/accountants, fellow practice/group partners, and IT support; role definitions and expectations for duties, transitioning call, and EHR charting; revenue-sharing; supplies/preferences/adaptability to scope type).
- Interests: Specific provider interests (for example, clinical research, infusion, hemorrhoidal banding, weight loss/nutrition, inflammatory bowel disease, irritable bowel disease, pathology) and productivity expectations (for example, number of procedures, number of new and return patient visits per day) should be communicated.
- Miscellaneous: Discussion about marketing the practice, importance of growing satellite programs and nuance of major referral groups to the practice are also key components of the assimilation process.
Leadership self-awareness and cultural alignment
Leadership self-awareness is a key element of provider onboarding. Physicians and APPs are trained to think independently and may be challenged to share decision-making and rely on others. The following are some no-cost self-assessment and awareness resources:
- Myers-Briggs Personality Profile Preferences:
- VIA Strengths:
- VARK Analysis:
Cultural alignment is also a critical consideration to ensure orderly assimilation into the practice/health care setting and with stakeholders. A shared commitment to embed a culture with shared values has relevance to merging cultures – not only when organizations come together – but with individuals as well. Time spent developing a better understanding of the customs, culture and traditions of the practice will be helpful if a practice must change its trajectory based on meeting an unmovable obstruction (for example, market forces requiring practice consolidation).
Improved quality
Transitioning a new provider into an existing practice culture can have a ripple effect on support staff and patient satisfaction and is, therefore, an important consideration in provider onboarding. Written standards, procedures, expectations, and practices are always advisable when possible. Attention to the demographics of the recruited physician is also important with shifts in interests and priorities from a practice. Millennials will constitute most of the workforce by 2025 and arrive with a mindset that the tenure in a role will be shorter than providers before them. Accordingly, the intentionality of the relationship is critical for successful bonding.
If current physician leaders want to achieve simultaneous succession planning and maintain the legacy of a patient-centric and resilient practice, these leaders must consider bridging the “cultural knowledge acumen gap.” James S. Hernandez, MD, MS, FCAP, and colleagues suggest a “connector” role between new and experienced providers. Reverse mentoring/distance/reciprocal mentoring is also mentioned as a two-way learning process between mentor and mentee.
Process structure considerations
Each new hire affects the culture of the practice. Best practices for the onboarding and orientation process should be followed. A written project master list with a timeline for completion of onboarding tasks with responsible and accountable persons, target dates for completion, and measurement should be established. Establishing mutual expectations up front can help practices tailor committee roles and clinical responsibilities to maximize provider engagement and longevity. A robust onboarding process may take up to 2 years depending on the size of the practice and the complexity of its structure and associated duties.
Desired outcomes
The desired outcome of the onboarding process is a satisfied provider whose passion and enthusiasm for quality patient care is demonstrated objectively through excellent performance on clinical quality measures and metrics of patient and referral source satisfaction.
Periodic reviews of how the onboarding process is progressing should be undertaken. These reviews can be modeled after the After-Action Review (AAR) process used in the military for measuring progress. Simply stated, what items went well with onboarding and why? What items did not go well with onboarding and why not? (Consider something like the Center for Medicare & Medicaid Services’ “5 Whys” assessment to determine root cause for items that need correction.) What elements of the onboarding process could be further improved? Using a Delphi method during the AAR session is an excellent way for the group to hear from all participants ranging from senior partners to recently recruited providers.
Conclusion
Medical practices must recognize that assimilating a new provider into the practice through a robust onboarding process is not lost effort but rather a force multiplier. Effective provider onboarding gives the incoming provider a sense of purpose and resolve, which results in optimized clinical productivity and engagement because the new provider is invested in the future of the practice. Once successfully onboarded and integrated into the practice, new providers need to understand that the work effort invested in their onboarding comes with a “pay it forward” obligation for the next provider recruited by the group. Group members also need to realize that the baseline is always changing–the provider onboarding process needs to continually evolve and adapt as the practice changes and new providers are hired.
Mr. Rudnick is a visiting professor and program director healthcare quality, innovation, and strategy at St Thomas University, Miami. Mr. Turner is regional vice president for the Midatlantic market of Covenant Physician Partners.
References
“Best practices for onboarding physicians.” The Rheumatologist. 2019 Sep 17. Accessed 2021 Sep 6. https://www.the-rheumatologist.org/article/best-practices-for-onboarding-new-physicians/
Centers for Medicare & Medicaid Services. Five Whys Tool for Root Cause Analysis: QAPI. 2021. Accessed 2021 Sep 6. https://www.cms.gov/medicare/provider-enrollment-and-certification/qapi/downloads/fivewhys.pdf.
DeIuliis ET, Saylor E. Open J Occup Ther. 2021;9(1):1-13.
Hernandez JS et al. “Discussion: Mentoring millennials for future leadership.” Physician Leadership Journal. 2018 May 14. Accessed 2021 Sep 6. https://www.physicianleaders.org/news/discussion-mentoring-millennials-future-leadership
Moore L et al. J Trauma Acute Care Surg. 2015 Jun;78(6):1168-75..
Klein CJ et al. West J Nurs Res. 2021 Feb;43(2):105-114.
Weinburger T, Gordon J. Health Prog. Nov-Dec 2013;94(6):76-9.
Wentlandt K et al. Healthc Q. 2016;18(4):36-41.
Physician and advanced practice provider (APP) (collectively, “provider”) onboarding into health care delivery settings requires careful planning and systematic integration. Assimilation into health care settings and cultures necessitates more than a 1- or 2-day orientation. Rather, an intentional, longitudinal onboarding program (starting with orientation) needs to be designed to assimilate providers into the unique culture of a medical practice.
Establishing mutual expectations
Communication concerning mutual expectations is a vital component of the agreement between provider and practice. Items that should be included in provider onboarding (likely addressed in either the practice visit or amplified in a contract) include the following:
- Committees: Committee orientation should include a discussion of provider preferences/expectations and why getting the new provider involved in the business of the practice is a priority of the group.
- Operations: Key clinical operations details should be reviewed with the incoming provider and reinforced through follow-up discussions with a physician mentor/coach (for example, call distribution; role of the senior nonclinical leadership team/accountants, fellow practice/group partners, and IT support; role definitions and expectations for duties, transitioning call, and EHR charting; revenue-sharing; supplies/preferences/adaptability to scope type).
- Interests: Specific provider interests (for example, clinical research, infusion, hemorrhoidal banding, weight loss/nutrition, inflammatory bowel disease, irritable bowel disease, pathology) and productivity expectations (for example, number of procedures, number of new and return patient visits per day) should be communicated.
- Miscellaneous: Discussion about marketing the practice, importance of growing satellite programs and nuance of major referral groups to the practice are also key components of the assimilation process.
Leadership self-awareness and cultural alignment
Leadership self-awareness is a key element of provider onboarding. Physicians and APPs are trained to think independently and may be challenged to share decision-making and rely on others. The following are some no-cost self-assessment and awareness resources:
- Myers-Briggs Personality Profile Preferences:
- VIA Strengths:
- VARK Analysis:
Cultural alignment is also a critical consideration to ensure orderly assimilation into the practice/health care setting and with stakeholders. A shared commitment to embed a culture with shared values has relevance to merging cultures – not only when organizations come together – but with individuals as well. Time spent developing a better understanding of the customs, culture and traditions of the practice will be helpful if a practice must change its trajectory based on meeting an unmovable obstruction (for example, market forces requiring practice consolidation).
Improved quality
Transitioning a new provider into an existing practice culture can have a ripple effect on support staff and patient satisfaction and is, therefore, an important consideration in provider onboarding. Written standards, procedures, expectations, and practices are always advisable when possible. Attention to the demographics of the recruited physician is also important with shifts in interests and priorities from a practice. Millennials will constitute most of the workforce by 2025 and arrive with a mindset that the tenure in a role will be shorter than providers before them. Accordingly, the intentionality of the relationship is critical for successful bonding.
If current physician leaders want to achieve simultaneous succession planning and maintain the legacy of a patient-centric and resilient practice, these leaders must consider bridging the “cultural knowledge acumen gap.” James S. Hernandez, MD, MS, FCAP, and colleagues suggest a “connector” role between new and experienced providers. Reverse mentoring/distance/reciprocal mentoring is also mentioned as a two-way learning process between mentor and mentee.
Process structure considerations
Each new hire affects the culture of the practice. Best practices for the onboarding and orientation process should be followed. A written project master list with a timeline for completion of onboarding tasks with responsible and accountable persons, target dates for completion, and measurement should be established. Establishing mutual expectations up front can help practices tailor committee roles and clinical responsibilities to maximize provider engagement and longevity. A robust onboarding process may take up to 2 years depending on the size of the practice and the complexity of its structure and associated duties.
Desired outcomes
The desired outcome of the onboarding process is a satisfied provider whose passion and enthusiasm for quality patient care is demonstrated objectively through excellent performance on clinical quality measures and metrics of patient and referral source satisfaction.
Periodic reviews of how the onboarding process is progressing should be undertaken. These reviews can be modeled after the After-Action Review (AAR) process used in the military for measuring progress. Simply stated, what items went well with onboarding and why? What items did not go well with onboarding and why not? (Consider something like the Center for Medicare & Medicaid Services’ “5 Whys” assessment to determine root cause for items that need correction.) What elements of the onboarding process could be further improved? Using a Delphi method during the AAR session is an excellent way for the group to hear from all participants ranging from senior partners to recently recruited providers.
Conclusion
Medical practices must recognize that assimilating a new provider into the practice through a robust onboarding process is not lost effort but rather a force multiplier. Effective provider onboarding gives the incoming provider a sense of purpose and resolve, which results in optimized clinical productivity and engagement because the new provider is invested in the future of the practice. Once successfully onboarded and integrated into the practice, new providers need to understand that the work effort invested in their onboarding comes with a “pay it forward” obligation for the next provider recruited by the group. Group members also need to realize that the baseline is always changing–the provider onboarding process needs to continually evolve and adapt as the practice changes and new providers are hired.
Mr. Rudnick is a visiting professor and program director healthcare quality, innovation, and strategy at St Thomas University, Miami. Mr. Turner is regional vice president for the Midatlantic market of Covenant Physician Partners.
References
“Best practices for onboarding physicians.” The Rheumatologist. 2019 Sep 17. Accessed 2021 Sep 6. https://www.the-rheumatologist.org/article/best-practices-for-onboarding-new-physicians/
Centers for Medicare & Medicaid Services. Five Whys Tool for Root Cause Analysis: QAPI. 2021. Accessed 2021 Sep 6. https://www.cms.gov/medicare/provider-enrollment-and-certification/qapi/downloads/fivewhys.pdf.
DeIuliis ET, Saylor E. Open J Occup Ther. 2021;9(1):1-13.
Hernandez JS et al. “Discussion: Mentoring millennials for future leadership.” Physician Leadership Journal. 2018 May 14. Accessed 2021 Sep 6. https://www.physicianleaders.org/news/discussion-mentoring-millennials-future-leadership
Moore L et al. J Trauma Acute Care Surg. 2015 Jun;78(6):1168-75..
Klein CJ et al. West J Nurs Res. 2021 Feb;43(2):105-114.
Weinburger T, Gordon J. Health Prog. Nov-Dec 2013;94(6):76-9.
Wentlandt K et al. Healthc Q. 2016;18(4):36-41.
Booster shot back-and-forth creates uncertainty, confusion
Many people are confused — patients and healthcare providers alike — in the wake of the U.S. Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) announcements about who is authorized to get a third or ‘booster’ shot of the Pfizer/BioNTech COVID-19 vaccine.
, healthcare providers report. At the same time, the uncertainty from changing federal messages about boosters is causing some chaos, especially in the form of vaccine misinformation.
The confusion started, in part, with the August 13 announcement that immunocompromised Americans were eligible for a booster shot. Next came the initial Biden administration intention to provide most U.S. adults with a third shot starting September 20 — an announcement later rolled back — followed by the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) limiting boosters to select groups last week.
“It was only 3% of the population that was going to be getting a third dose, then it was back to everyone being able to get the booster, and then it’s back to a select crew,” Louito Edje, MD, a family physician in private practice in Cincinnati, said in an interview with this news organization.
This kind of mixed messaging is generating more questions than answers.
“Even though that is following the science, translating the science into policy, it’s really fraught with confusion for patients, especially,” added Dr. Edje, professor educator in the departments of medical education and family and community medicine at UC Health and a fellow of the American Academy of Family Physicians.
When asked if he’s seeing more uncertainty about boosters, community pharmacist Brian Caswell, RPh, said: “I’m going to have to say yes because I’ve been confused myself at times.”
“Yes, there is a lot of confusion,” added Mr. Caswell, owner or co-owner of four pharmacies in Kansas and Missouri and president of the National Community Pharmacists Association.
Boosting misinformation?
“Unfortunately, confusion leads to an acceleration of misinformation,” Mr. Caswell said.
Dr. Edje shared an example. “The folks who have been hesitant to even get the first vaccine appear now a little less likely to want to go ahead and get vaccinated.”
These patients point to breakthrough COVID-19 cases of the Delta variant, which “reinforces that they don’t need to get vaccinated in the first place,” Dr. Edje said.
“That’s unfortunate because it’s a complete fallacy.”
Clearer communication from the federal government could help alleviate confusion, Mr. Caswell said. “I would like to see an official CDC chart that states who is eligible as of a certain date. Something that is accessible through their webpage or a social media source that can be updated. That would help all of us.”
“For myself, I’ve got patients from Kansas, Oklahoma, and Missouri that might be operating under different guidelines. That makes it even more confusing,” he said.
More clarity is needed for individuals seeking boosters as well. “It would help to be very clear with the general public, who are becoming very knowledgeable within this vaccine realm,” Mr. Caswell said.
‘Gaming the system’
Although most people seeking a booster shot at one of Caswell’s pharmacies are following official recommendations, there are some who remain ineligible but nonetheless come in for an additional vaccine.
“Even before this announcement last Friday, in the latter part of August when the CDC talked about a booster for immunocompromised, we had interest from people who did not meet the criteria,” Mr. Caswell said.
To the ineligible, he and his staff explain the approval process, why certain decisions are made, and point out that the number of eligible Americans is likely to expand in the future.
“The vast majority of them are understanding,” Mr. Caswell said. “But we’ve had some people who really didn’t want to accept the information, and I don’t know what they’ve done.”
“Some people are gaming the system to get their booster or second shot of J&J,” he said.
For example, Mr. Caswell had a patient who crossed over state lines from Missouri seeking a vaccine booster at Wolkar Drug, a pharmacy in Baxter Springs, Kan. “We found out later he had a J&J shot at a facility or provider in Missouri. He came over to Kansas, signed up for it and got a booster with Moderna.”
“We called and asked him if he was aware of it. He said, ‘yes.’ When we questioned him more about it, he hung up.”
Dr. Edje is likewise seeing interest from some ineligible patients, she said.
Crossing a liability line?
Mr. Caswell has asked for advice from lawyers and the State Board of Pharmacy on potential liability if a pharmacist gives a booster to a patient not eligible under the official FDA and CDC guidance.
“We ask patients direct questions about whether they’ve had the COVID vaccine, COVID, and a whole litany of questions they must answer. And we’re assuming they are going to be honest and forthright,” he said. “The pharmacist needs to make sure they make every effort to get that information from the patient.”
Normally, healthcare providers like Mr. Caswell report each COVID-19 vaccination to the state registry after administration. “We have not gone through a police action and checked the registry first,” he said.
But, if people continue to try ‘gaming the system,’ he said, he might have to start checking the state registry before giving someone a booster.
The American Academy of Family Physicians offers advice from the CDC about legal protections for providers.
“As outlined by CDC, any off-label use of the Comirnaty/Pfizer-BioNTech COVID-19 vaccine is not authorized at this time and may not be covered under the PREP Act or the PREP Act declaration. This means that clinicians providing the vaccine outside of the authorized/approved use may not have immunity from claims,” the AAFP website states.
“Per CDC, individuals who receive a third dose may not be eligible for compensation after a possible adverse event. Such use would be in violation of the CDC COVID-19 vaccination program provider agreement and therefore may not be reimbursable and may impact the ability of a provider to remain in the CDC program, in addition to other potential sanctions. Administration fees for off-label doses may not be reimbursed by payers.”
Despite confusion, demand is up
Even amid all the uncertainty, there appears to be a jump in enthusiasm for the booster shots.
“The requests have gone up quite a bit. We’ve seen a number of requests from people in person and over the phone looking to get a booster,” Mr. Caswell said. “Since the discussion at the federal level...there has been a lot of interest in the third shot booster, itself, as well as about a booster for J&J.”
“There is quite a bit of excitement out there,” he said.
Dr. Edje agreed: “I take care of a fair number of folks...including the elderly and healthcare professionals. They are already asking for the booster.”
Interestingly, Dr. Edje would like to get a booster herself but is not eligible for the Pfizer third shot. She is a participant in a Moderna vaccine trial and can only receive additional immunization as part of the study.
‘Walk, don’t run’
To quell any potential early rush to get a third shot, U.S. health officials are reminding booster-ineligible people that they still have some protection against COVID-19.
“If you’re a person who ultimately might get a booster that will make you optimally protected, you don’t necessarily need to get it tomorrow,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases told CNN.
CDC Director Rochelle Walensky, MD, also weighed in. She told ABC that boosters for people who received a Moderna or Johnson & Johnson vaccine will be addressed with urgency.
“I want to reiterate that this is a very slow wane. There is no urgency here to go and get your booster immediately. You know, walk don’t run to your booster appointment,” she said.
“We will come and look at the data for Moderna and J&J in very short order.”
Dr. Edje and Mr. Caswell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many people are confused — patients and healthcare providers alike — in the wake of the U.S. Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) announcements about who is authorized to get a third or ‘booster’ shot of the Pfizer/BioNTech COVID-19 vaccine.
, healthcare providers report. At the same time, the uncertainty from changing federal messages about boosters is causing some chaos, especially in the form of vaccine misinformation.
The confusion started, in part, with the August 13 announcement that immunocompromised Americans were eligible for a booster shot. Next came the initial Biden administration intention to provide most U.S. adults with a third shot starting September 20 — an announcement later rolled back — followed by the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) limiting boosters to select groups last week.
“It was only 3% of the population that was going to be getting a third dose, then it was back to everyone being able to get the booster, and then it’s back to a select crew,” Louito Edje, MD, a family physician in private practice in Cincinnati, said in an interview with this news organization.
This kind of mixed messaging is generating more questions than answers.
“Even though that is following the science, translating the science into policy, it’s really fraught with confusion for patients, especially,” added Dr. Edje, professor educator in the departments of medical education and family and community medicine at UC Health and a fellow of the American Academy of Family Physicians.
When asked if he’s seeing more uncertainty about boosters, community pharmacist Brian Caswell, RPh, said: “I’m going to have to say yes because I’ve been confused myself at times.”
“Yes, there is a lot of confusion,” added Mr. Caswell, owner or co-owner of four pharmacies in Kansas and Missouri and president of the National Community Pharmacists Association.
Boosting misinformation?
“Unfortunately, confusion leads to an acceleration of misinformation,” Mr. Caswell said.
Dr. Edje shared an example. “The folks who have been hesitant to even get the first vaccine appear now a little less likely to want to go ahead and get vaccinated.”
These patients point to breakthrough COVID-19 cases of the Delta variant, which “reinforces that they don’t need to get vaccinated in the first place,” Dr. Edje said.
“That’s unfortunate because it’s a complete fallacy.”
Clearer communication from the federal government could help alleviate confusion, Mr. Caswell said. “I would like to see an official CDC chart that states who is eligible as of a certain date. Something that is accessible through their webpage or a social media source that can be updated. That would help all of us.”
“For myself, I’ve got patients from Kansas, Oklahoma, and Missouri that might be operating under different guidelines. That makes it even more confusing,” he said.
More clarity is needed for individuals seeking boosters as well. “It would help to be very clear with the general public, who are becoming very knowledgeable within this vaccine realm,” Mr. Caswell said.
‘Gaming the system’
Although most people seeking a booster shot at one of Caswell’s pharmacies are following official recommendations, there are some who remain ineligible but nonetheless come in for an additional vaccine.
“Even before this announcement last Friday, in the latter part of August when the CDC talked about a booster for immunocompromised, we had interest from people who did not meet the criteria,” Mr. Caswell said.
To the ineligible, he and his staff explain the approval process, why certain decisions are made, and point out that the number of eligible Americans is likely to expand in the future.
“The vast majority of them are understanding,” Mr. Caswell said. “But we’ve had some people who really didn’t want to accept the information, and I don’t know what they’ve done.”
“Some people are gaming the system to get their booster or second shot of J&J,” he said.
For example, Mr. Caswell had a patient who crossed over state lines from Missouri seeking a vaccine booster at Wolkar Drug, a pharmacy in Baxter Springs, Kan. “We found out later he had a J&J shot at a facility or provider in Missouri. He came over to Kansas, signed up for it and got a booster with Moderna.”
“We called and asked him if he was aware of it. He said, ‘yes.’ When we questioned him more about it, he hung up.”
Dr. Edje is likewise seeing interest from some ineligible patients, she said.
Crossing a liability line?
Mr. Caswell has asked for advice from lawyers and the State Board of Pharmacy on potential liability if a pharmacist gives a booster to a patient not eligible under the official FDA and CDC guidance.
“We ask patients direct questions about whether they’ve had the COVID vaccine, COVID, and a whole litany of questions they must answer. And we’re assuming they are going to be honest and forthright,” he said. “The pharmacist needs to make sure they make every effort to get that information from the patient.”
Normally, healthcare providers like Mr. Caswell report each COVID-19 vaccination to the state registry after administration. “We have not gone through a police action and checked the registry first,” he said.
But, if people continue to try ‘gaming the system,’ he said, he might have to start checking the state registry before giving someone a booster.
The American Academy of Family Physicians offers advice from the CDC about legal protections for providers.
“As outlined by CDC, any off-label use of the Comirnaty/Pfizer-BioNTech COVID-19 vaccine is not authorized at this time and may not be covered under the PREP Act or the PREP Act declaration. This means that clinicians providing the vaccine outside of the authorized/approved use may not have immunity from claims,” the AAFP website states.
“Per CDC, individuals who receive a third dose may not be eligible for compensation after a possible adverse event. Such use would be in violation of the CDC COVID-19 vaccination program provider agreement and therefore may not be reimbursable and may impact the ability of a provider to remain in the CDC program, in addition to other potential sanctions. Administration fees for off-label doses may not be reimbursed by payers.”
Despite confusion, demand is up
Even amid all the uncertainty, there appears to be a jump in enthusiasm for the booster shots.
“The requests have gone up quite a bit. We’ve seen a number of requests from people in person and over the phone looking to get a booster,” Mr. Caswell said. “Since the discussion at the federal level...there has been a lot of interest in the third shot booster, itself, as well as about a booster for J&J.”
“There is quite a bit of excitement out there,” he said.
Dr. Edje agreed: “I take care of a fair number of folks...including the elderly and healthcare professionals. They are already asking for the booster.”
Interestingly, Dr. Edje would like to get a booster herself but is not eligible for the Pfizer third shot. She is a participant in a Moderna vaccine trial and can only receive additional immunization as part of the study.
‘Walk, don’t run’
To quell any potential early rush to get a third shot, U.S. health officials are reminding booster-ineligible people that they still have some protection against COVID-19.
“If you’re a person who ultimately might get a booster that will make you optimally protected, you don’t necessarily need to get it tomorrow,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases told CNN.
CDC Director Rochelle Walensky, MD, also weighed in. She told ABC that boosters for people who received a Moderna or Johnson & Johnson vaccine will be addressed with urgency.
“I want to reiterate that this is a very slow wane. There is no urgency here to go and get your booster immediately. You know, walk don’t run to your booster appointment,” she said.
“We will come and look at the data for Moderna and J&J in very short order.”
Dr. Edje and Mr. Caswell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many people are confused — patients and healthcare providers alike — in the wake of the U.S. Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) announcements about who is authorized to get a third or ‘booster’ shot of the Pfizer/BioNTech COVID-19 vaccine.
, healthcare providers report. At the same time, the uncertainty from changing federal messages about boosters is causing some chaos, especially in the form of vaccine misinformation.
The confusion started, in part, with the August 13 announcement that immunocompromised Americans were eligible for a booster shot. Next came the initial Biden administration intention to provide most U.S. adults with a third shot starting September 20 — an announcement later rolled back — followed by the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) limiting boosters to select groups last week.
“It was only 3% of the population that was going to be getting a third dose, then it was back to everyone being able to get the booster, and then it’s back to a select crew,” Louito Edje, MD, a family physician in private practice in Cincinnati, said in an interview with this news organization.
This kind of mixed messaging is generating more questions than answers.
“Even though that is following the science, translating the science into policy, it’s really fraught with confusion for patients, especially,” added Dr. Edje, professor educator in the departments of medical education and family and community medicine at UC Health and a fellow of the American Academy of Family Physicians.
When asked if he’s seeing more uncertainty about boosters, community pharmacist Brian Caswell, RPh, said: “I’m going to have to say yes because I’ve been confused myself at times.”
“Yes, there is a lot of confusion,” added Mr. Caswell, owner or co-owner of four pharmacies in Kansas and Missouri and president of the National Community Pharmacists Association.
Boosting misinformation?
“Unfortunately, confusion leads to an acceleration of misinformation,” Mr. Caswell said.
Dr. Edje shared an example. “The folks who have been hesitant to even get the first vaccine appear now a little less likely to want to go ahead and get vaccinated.”
These patients point to breakthrough COVID-19 cases of the Delta variant, which “reinforces that they don’t need to get vaccinated in the first place,” Dr. Edje said.
“That’s unfortunate because it’s a complete fallacy.”
Clearer communication from the federal government could help alleviate confusion, Mr. Caswell said. “I would like to see an official CDC chart that states who is eligible as of a certain date. Something that is accessible through their webpage or a social media source that can be updated. That would help all of us.”
“For myself, I’ve got patients from Kansas, Oklahoma, and Missouri that might be operating under different guidelines. That makes it even more confusing,” he said.
More clarity is needed for individuals seeking boosters as well. “It would help to be very clear with the general public, who are becoming very knowledgeable within this vaccine realm,” Mr. Caswell said.
‘Gaming the system’
Although most people seeking a booster shot at one of Caswell’s pharmacies are following official recommendations, there are some who remain ineligible but nonetheless come in for an additional vaccine.
“Even before this announcement last Friday, in the latter part of August when the CDC talked about a booster for immunocompromised, we had interest from people who did not meet the criteria,” Mr. Caswell said.
To the ineligible, he and his staff explain the approval process, why certain decisions are made, and point out that the number of eligible Americans is likely to expand in the future.
“The vast majority of them are understanding,” Mr. Caswell said. “But we’ve had some people who really didn’t want to accept the information, and I don’t know what they’ve done.”
“Some people are gaming the system to get their booster or second shot of J&J,” he said.
For example, Mr. Caswell had a patient who crossed over state lines from Missouri seeking a vaccine booster at Wolkar Drug, a pharmacy in Baxter Springs, Kan. “We found out later he had a J&J shot at a facility or provider in Missouri. He came over to Kansas, signed up for it and got a booster with Moderna.”
“We called and asked him if he was aware of it. He said, ‘yes.’ When we questioned him more about it, he hung up.”
Dr. Edje is likewise seeing interest from some ineligible patients, she said.
Crossing a liability line?
Mr. Caswell has asked for advice from lawyers and the State Board of Pharmacy on potential liability if a pharmacist gives a booster to a patient not eligible under the official FDA and CDC guidance.
“We ask patients direct questions about whether they’ve had the COVID vaccine, COVID, and a whole litany of questions they must answer. And we’re assuming they are going to be honest and forthright,” he said. “The pharmacist needs to make sure they make every effort to get that information from the patient.”
Normally, healthcare providers like Mr. Caswell report each COVID-19 vaccination to the state registry after administration. “We have not gone through a police action and checked the registry first,” he said.
But, if people continue to try ‘gaming the system,’ he said, he might have to start checking the state registry before giving someone a booster.
The American Academy of Family Physicians offers advice from the CDC about legal protections for providers.
“As outlined by CDC, any off-label use of the Comirnaty/Pfizer-BioNTech COVID-19 vaccine is not authorized at this time and may not be covered under the PREP Act or the PREP Act declaration. This means that clinicians providing the vaccine outside of the authorized/approved use may not have immunity from claims,” the AAFP website states.
“Per CDC, individuals who receive a third dose may not be eligible for compensation after a possible adverse event. Such use would be in violation of the CDC COVID-19 vaccination program provider agreement and therefore may not be reimbursable and may impact the ability of a provider to remain in the CDC program, in addition to other potential sanctions. Administration fees for off-label doses may not be reimbursed by payers.”
Despite confusion, demand is up
Even amid all the uncertainty, there appears to be a jump in enthusiasm for the booster shots.
“The requests have gone up quite a bit. We’ve seen a number of requests from people in person and over the phone looking to get a booster,” Mr. Caswell said. “Since the discussion at the federal level...there has been a lot of interest in the third shot booster, itself, as well as about a booster for J&J.”
“There is quite a bit of excitement out there,” he said.
Dr. Edje agreed: “I take care of a fair number of folks...including the elderly and healthcare professionals. They are already asking for the booster.”
Interestingly, Dr. Edje would like to get a booster herself but is not eligible for the Pfizer third shot. She is a participant in a Moderna vaccine trial and can only receive additional immunization as part of the study.
‘Walk, don’t run’
To quell any potential early rush to get a third shot, U.S. health officials are reminding booster-ineligible people that they still have some protection against COVID-19.
“If you’re a person who ultimately might get a booster that will make you optimally protected, you don’t necessarily need to get it tomorrow,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases told CNN.
CDC Director Rochelle Walensky, MD, also weighed in. She told ABC that boosters for people who received a Moderna or Johnson & Johnson vaccine will be addressed with urgency.
“I want to reiterate that this is a very slow wane. There is no urgency here to go and get your booster immediately. You know, walk don’t run to your booster appointment,” she said.
“We will come and look at the data for Moderna and J&J in very short order.”
Dr. Edje and Mr. Caswell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
USPSTF updates diabetes recs, lowers screening age
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
REFERENCES
- US Preventive Services Task Force. Screening for prediabetes and type 2 diabetes. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(suppl 1):S15-S33. https://doi.org/10.2337/dc21-S002
SARS-CoV-2 Delta variant may double the risk for hospitalization
Key clinical point: Infection with the SARS-CoV-2 Delta (B.1.617.2) variant carries a significantly higher risk for hospitalization and attending hospital for emergency care than the Alpha (B.1.1.7) variant.
Major finding: The Delta variant was associated with more than twice the risk of being admitted to hospital (adjusted hazard ratio [aHR], 2·26; 95% confidence interval [CI], 1·32-3·89) and nearly 1.5 times the risk of seeking emergency care (aHR, 1·45; 95% CI, 1·08-1·95) compared with the Alpha variant.
Study details: A cohort study included 43,338 COVID-19-positive cases in England who were found to be infected with either the Alpha or Delta SARS-CoV-2 variant through whole-genome sequencing.
Disclosures: The study was funded by Medical Research Council, UK Research and Innovation, Department of Health and Social Care, and National Institute for Health Research. GD's employer, Public Health England was funded by GlaxoSmithKline for a research project related to seasonal influenza and antiviral treatment but had no relation to COVID-19. The remaining authors declared no conflict of interests.
Source: Twohig KA et al. Lancet Infect Dis. 2021 Aug 27. doi: 10.1016/S1473-3099(21)00475-8.
Key clinical point: Infection with the SARS-CoV-2 Delta (B.1.617.2) variant carries a significantly higher risk for hospitalization and attending hospital for emergency care than the Alpha (B.1.1.7) variant.
Major finding: The Delta variant was associated with more than twice the risk of being admitted to hospital (adjusted hazard ratio [aHR], 2·26; 95% confidence interval [CI], 1·32-3·89) and nearly 1.5 times the risk of seeking emergency care (aHR, 1·45; 95% CI, 1·08-1·95) compared with the Alpha variant.
Study details: A cohort study included 43,338 COVID-19-positive cases in England who were found to be infected with either the Alpha or Delta SARS-CoV-2 variant through whole-genome sequencing.
Disclosures: The study was funded by Medical Research Council, UK Research and Innovation, Department of Health and Social Care, and National Institute for Health Research. GD's employer, Public Health England was funded by GlaxoSmithKline for a research project related to seasonal influenza and antiviral treatment but had no relation to COVID-19. The remaining authors declared no conflict of interests.
Source: Twohig KA et al. Lancet Infect Dis. 2021 Aug 27. doi: 10.1016/S1473-3099(21)00475-8.
Key clinical point: Infection with the SARS-CoV-2 Delta (B.1.617.2) variant carries a significantly higher risk for hospitalization and attending hospital for emergency care than the Alpha (B.1.1.7) variant.
Major finding: The Delta variant was associated with more than twice the risk of being admitted to hospital (adjusted hazard ratio [aHR], 2·26; 95% confidence interval [CI], 1·32-3·89) and nearly 1.5 times the risk of seeking emergency care (aHR, 1·45; 95% CI, 1·08-1·95) compared with the Alpha variant.
Study details: A cohort study included 43,338 COVID-19-positive cases in England who were found to be infected with either the Alpha or Delta SARS-CoV-2 variant through whole-genome sequencing.
Disclosures: The study was funded by Medical Research Council, UK Research and Innovation, Department of Health and Social Care, and National Institute for Health Research. GD's employer, Public Health England was funded by GlaxoSmithKline for a research project related to seasonal influenza and antiviral treatment but had no relation to COVID-19. The remaining authors declared no conflict of interests.
Source: Twohig KA et al. Lancet Infect Dis. 2021 Aug 27. doi: 10.1016/S1473-3099(21)00475-8.