User login
Closing your practice
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
A very strange place to find a tooth
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
A nose for the tooth
Have you ever had a stuffy nose that just wouldn’t go away? Those irritating head colds have nothing on the stuffy nose a man in New York recently had to go through. A stuffy nose to top all stuffy noses. One stuffy nose to rule them all, as it were.
This man went to a Mount Sinai clinic with difficulty breathing through his right nostril, a problem that had been going on for years. Let us repeat that: A stuffy nose that lasted for years. The exam revealed a white mass jutting through the back of the septum and a CT scan confirmed the diagnosis. Perhaps you’ve already guessed, since the headline does give things away. Yes, this man had a tooth growing into his nose.
The problem was a half-inch-long ectopic tooth. Ectopic teeth are rare, occurring in less than 1% of people, but an ectopic tooth growing backward into the nasal cavity? Well, that’s so uncommon that this man got a case report in the New England Journal of Medicine.
This story does have a happy ending. Not all ectopic teeth need to be treated, but this one really did have to go. The offending tooth was surgically removed and, at a 3-month follow-up, the stuffy nose issue was completely resolved. So our friend gets the best of both worlds: His issue gets cured and he gets a case report in a major medical publication. If that’s not living the dream, we don’t know what is, and that’s the tooth.
Lettuce recommend you a sleep aid
Lettuce is great for many things. The star in a salad? Of course. The fresh element in a BLT? Yep. A sleep aid? According to a TikTok hack with almost 5 million views, the pinch hitter in a sandwich is switching leagues to be used like a tea for faster sleep. But, does it really work? Researchers say yes and no, according to a recent report at Tyla.com.
Studies conducted in 2013 and 2017 pointed toward a compound called lactucin, which is found in the plant’s n-butanol fraction. In the 2013 study, mice that received n-butanol fraction fell asleep faster and stayed asleep longer. In 2017, researchers found that lettuce made mice sleep longer and helped protect against cell inflammation and damage.
OK, so it works on mice. But what about humans? In the TikTok video, user Shapla Hoque pours hot water on a few lettuce leaves in a mug with a peppermint tea bag (for flavor). After 10 minutes, when the leaves are soaked and soggy, she removes them and drinks the lettuce tea. By the end of the video she’s visibly drowsy and ready to crash. Does this hold water?
Here’s the no. Dr. Charlotte Norton of the Slimming Clinic told Tyla.com that yeah, there are some properties in lettuce that will help you fall asleep, such as lactucarium, which is prominent in romaine. But you would need a massive amount of lettuce to get any effect. The TikTok video, she said, is an example of the placebo effect.
Brains get a rise out of Viagra
A lot of medications are used off label. Antidepressants for COVID have taken the cake recently, but here’s a new one: Viagra for Alzheimer’s disease.
Although there’s no definite link yet between the two, neuron models derived from induced pluripotent stem cells from patients with Alzheimer’s suggest that sildenafil increases neurite growth and decreases phospho-tau expression, Jiansong Fang, PhD, of the Cleveland Clinic, and associates said in Nature Aging.
Their research is an attempt to find untapped sources of new treatments among existing drugs. They began the search with 1,600 approved drugs and focused on those that target the buildup of beta amyloid and tau proteins in the brain, according to the Daily Beast.
Since sildenafil is obviously for men, more research will need to be done on how this drug affects women. Don’t start stocking up just yet.
Omicron is not a social-distancing robot
COVID, safe to say, has not been your typical, run-of-the-mill pandemic. People have protested social distancing. People have protested lockdowns. People have protested mask mandates. People have protested vaccine mandates. People have protested people protesting vaccine mandates.
Someone used a fake arm to get a COVID vaccine card. People have tried to reverse their COVID vaccinations. People had COVID contamination parties.
The common denominator? People. Humans. Maybe what we need is a nonhuman intervention. To fight COVID, we need a hero. A robotic hero.
And where can we find such a hero? The University of Maryland, of course, where computer scientists and engineers are working on an autonomous mobile robot to enforce indoor social-distancing rules.
Their robot can detect lapses in social distancing using cameras, both thermal and visual, along with a LiDAR (Light Detection and Ranging) sensor. It then sorts the offenders into various groups depending on whether they are standing still or moving and predicts their future movement using a state-of-the-art hybrid collision avoidance method known as Frozone, Adarsh Jagan Sathyamoorthy and associates explained in PLOS One.
“Once it reaches the breach, the robot encourages people to move apart via text that appears on a mounted display,” ScienceDaily said.
Maybe you were expecting a Terminator-type robot coming to enforce social distancing requirements rather than a simple text message. Let’s just hope that all COVID guidelines are followed, including social distancing, so the pandemic will finally end and won’t “be back.”
Vaccine protection drops against Omicron, making boosters crucial
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
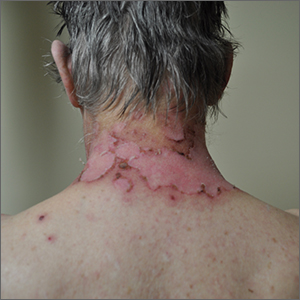
A sun distributed rash

The photo distribution and annular quality of this patient’s rash, combined with his positive autoimmune work-up, led to a diagnosis of subacute cutaneous lupus erythematosus (SCLE), a nonscarring subtype of cutaneous lupus erythematosus.
SCLE is a chronic and relapsing condition that may manifest as either a papulosquamous or annular eruption.1 It most commonly affects areas of sun exposure such as the shoulders, upper back, and extensor surfaces of the arms. This disorder typically affects young or middle-aged women between the ages of 30 and 40 years.
The differential diagnosis of this eruption includes dermatomyositis, polymorphous light eruption, psoriasis, tinea corporis, and other photodermatoses. The etiology of SCLE is multifactorial and may include a genetic susceptibility in combination with environmental triggers that provoke an autoimmune response to sunlight.1 There is strong evidence linking drug-induced SCLE with proton pump inhibitors, anticonvulsants, beta-blockers, terbinafine, and immune modulators.2
As many as 70% of patients with SCLE have positive anti-Ro/SSA autoantibodies, and this is most often associated with Sjogren syndrome.1 Interestingly, SCLE patients often exhibit symptoms that overlap with Sjogren syndrome. Systemic involvement is rare in SCLE, and if present, these symptoms are usually limited to arthritis and myalgia.
Treatment of SCLE includes photo-protective behaviors, topical corticosteroids/calcineurin inhibitors, and systemic therapies such as hydroxychloroquine (first-line), methotrexate, and mycophenolate mofetil (second-line).2
Our patient was started on hydroxychloroquine 200 mg orally bid, with complete resolution of the lesions at his 2 month–follow-up appointment. This case emphasizes the importance of distinguishing SCLE from other subtypes of lupus erythematosus as the prognostic course and treatment varies between these conditions.
Photos courtesy of Kriti Mishra, MD. Text courtesy of Jaimie Lin, BS, Kriti Mishra, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. https://doi.org/10.1016/j.berh.2013.07.008
2. Jatwani S, Hearth Holmes MP. Subacute cutaneous lupus erythematosus. 2021. StatPearls. StatPearls Publishing; 2021.

The photo distribution and annular quality of this patient’s rash, combined with his positive autoimmune work-up, led to a diagnosis of subacute cutaneous lupus erythematosus (SCLE), a nonscarring subtype of cutaneous lupus erythematosus.
SCLE is a chronic and relapsing condition that may manifest as either a papulosquamous or annular eruption.1 It most commonly affects areas of sun exposure such as the shoulders, upper back, and extensor surfaces of the arms. This disorder typically affects young or middle-aged women between the ages of 30 and 40 years.
The differential diagnosis of this eruption includes dermatomyositis, polymorphous light eruption, psoriasis, tinea corporis, and other photodermatoses. The etiology of SCLE is multifactorial and may include a genetic susceptibility in combination with environmental triggers that provoke an autoimmune response to sunlight.1 There is strong evidence linking drug-induced SCLE with proton pump inhibitors, anticonvulsants, beta-blockers, terbinafine, and immune modulators.2
As many as 70% of patients with SCLE have positive anti-Ro/SSA autoantibodies, and this is most often associated with Sjogren syndrome.1 Interestingly, SCLE patients often exhibit symptoms that overlap with Sjogren syndrome. Systemic involvement is rare in SCLE, and if present, these symptoms are usually limited to arthritis and myalgia.
Treatment of SCLE includes photo-protective behaviors, topical corticosteroids/calcineurin inhibitors, and systemic therapies such as hydroxychloroquine (first-line), methotrexate, and mycophenolate mofetil (second-line).2
Our patient was started on hydroxychloroquine 200 mg orally bid, with complete resolution of the lesions at his 2 month–follow-up appointment. This case emphasizes the importance of distinguishing SCLE from other subtypes of lupus erythematosus as the prognostic course and treatment varies between these conditions.
Photos courtesy of Kriti Mishra, MD. Text courtesy of Jaimie Lin, BS, Kriti Mishra, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The photo distribution and annular quality of this patient’s rash, combined with his positive autoimmune work-up, led to a diagnosis of subacute cutaneous lupus erythematosus (SCLE), a nonscarring subtype of cutaneous lupus erythematosus.
SCLE is a chronic and relapsing condition that may manifest as either a papulosquamous or annular eruption.1 It most commonly affects areas of sun exposure such as the shoulders, upper back, and extensor surfaces of the arms. This disorder typically affects young or middle-aged women between the ages of 30 and 40 years.
The differential diagnosis of this eruption includes dermatomyositis, polymorphous light eruption, psoriasis, tinea corporis, and other photodermatoses. The etiology of SCLE is multifactorial and may include a genetic susceptibility in combination with environmental triggers that provoke an autoimmune response to sunlight.1 There is strong evidence linking drug-induced SCLE with proton pump inhibitors, anticonvulsants, beta-blockers, terbinafine, and immune modulators.2
As many as 70% of patients with SCLE have positive anti-Ro/SSA autoantibodies, and this is most often associated with Sjogren syndrome.1 Interestingly, SCLE patients often exhibit symptoms that overlap with Sjogren syndrome. Systemic involvement is rare in SCLE, and if present, these symptoms are usually limited to arthritis and myalgia.
Treatment of SCLE includes photo-protective behaviors, topical corticosteroids/calcineurin inhibitors, and systemic therapies such as hydroxychloroquine (first-line), methotrexate, and mycophenolate mofetil (second-line).2
Our patient was started on hydroxychloroquine 200 mg orally bid, with complete resolution of the lesions at his 2 month–follow-up appointment. This case emphasizes the importance of distinguishing SCLE from other subtypes of lupus erythematosus as the prognostic course and treatment varies between these conditions.
Photos courtesy of Kriti Mishra, MD. Text courtesy of Jaimie Lin, BS, Kriti Mishra, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. https://doi.org/10.1016/j.berh.2013.07.008
2. Jatwani S, Hearth Holmes MP. Subacute cutaneous lupus erythematosus. 2021. StatPearls. StatPearls Publishing; 2021.
1. Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. https://doi.org/10.1016/j.berh.2013.07.008
2. Jatwani S, Hearth Holmes MP. Subacute cutaneous lupus erythematosus. 2021. StatPearls. StatPearls Publishing; 2021.
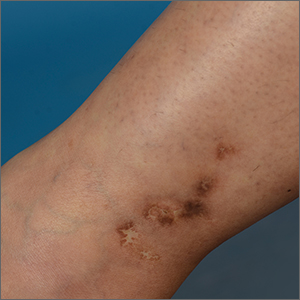
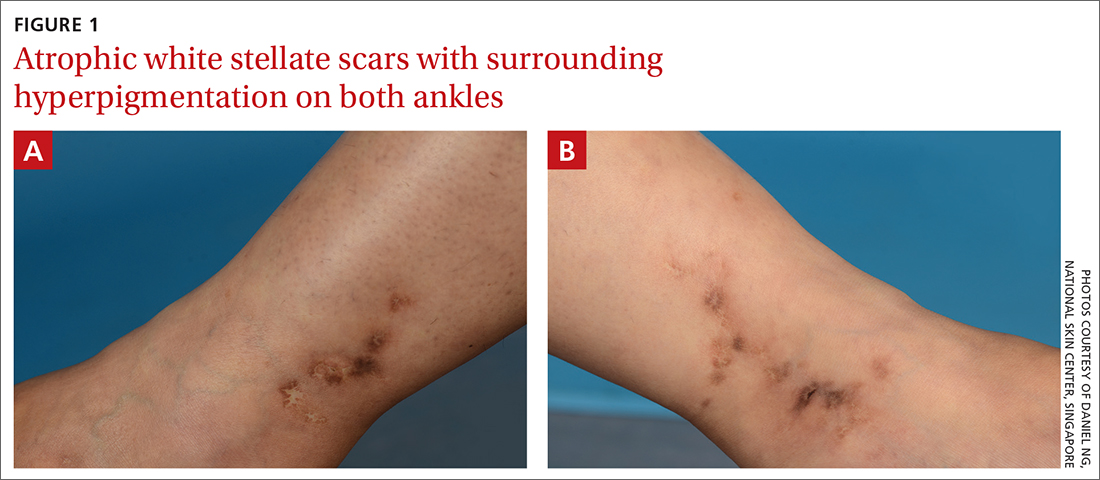
White ankle scars
A 42-year-old woman presented to our dermatology center with white scars on both of her ankles. She first noticed the lesions 2 years prior; they were initially erythematous and painful, even when she was at rest. Her past medical history included 3 spontaneous term miscarriages. She denied any prolonged standing or trauma.
On examination, atrophic porcelain-white stellate scars were visible with surrounding hyperpigmentation on the medial aspect of both ankles (FIGURE 1A & 1B). There were no tender erythematous nodules,
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophie blanche
Atrophie blanche is a morphologic feature described as porcelain-white stellate scars with surrounding telangiectasia and hyperpigmentation. The lesions are typically found over the peri-malleolar region and are sequelae of healed erythematous and painful ulcers. The lesions arise from upper dermal, small vessel, thrombotic vasculopathy leading to ischemic rest pain; if left untreated, atrophic white scars eventually develop.
A sign of venous insufficiency or thrombotic vasculopathy
Atrophie blanche may develop following healing of an ulcer due to venous insufficiency or small vessel thrombotic vasculopathy.1 The incidence of thrombotic vasculopathy is 1:100,000 with a female predominance, and up to 50% of cases are associated with procoagulant conditions.2 Thrombotic vasculopathy can be due to an inherited or acquired thrombophilia.1
Causes of hereditary thrombophilia include Factor V Leiden/prothrombin mutations, anti-thrombin III/protein C/protein S deficiencies, dysfibrinogenemia, and hyperhomocysteinemia.
Acquired thrombophilia arises from underlying prothrombotic states associated with the Virchow triad: hypercoagulability, blood flow stasis, and endothelial injury. The use of oral contraceptives or hormone replacement therapy, presence of malignancy, and antiphospholipid syndrome (APS) are causes of acquired thrombophilia.2
Obtaining a careful history is crucial
Thorough history-taking and physical examination are required to determine the underlying cause of atrophie blanche.
Continue to: Chronic venous insufficiency
Chronic venous insufficiency is more likely in patients with a history of prolonged standing, obesity, or previous injury/surgery to leg veins. Physical examination would reveal hyperpigmentation, telangiectasia, varicose veins, pedal edema, and venous ulcers.3
Inherited thrombophilia may be at work in patients with a family history of arterial and venous thrombosis (eg, stroke, acute coronary syndrome, or deep vein thromboses).
Acquired thrombophilia should be suspected if there is a history of recurrent miscarriages or malignancy.4 Given our patient’s history of miscarriages, we ordered further lab work and found that she had elevated anticardiolipin levels (> 40 U/mL) fulfilling the revised Sapporo criteria5 for APS.
Thrombophilia or chronic venous insufficiency? In a patient with a history suggestive of thrombophilia, further work-up should be done before attributing atrophie blanche to healed venous ulcers from chronic venous insufficiency. A skin lesion biopsy could reveal classic changes of thrombotic vasculopathy subjacent to the ulcer, including intraluminal thrombosis, endothelial proliferation, and subintimal hyaline degeneration, as opposed to dermal changes consistent with venous stasis, such as increased siderophages, hemosiderin deposition, erythrocyte extravasation, dermal fibrosis, and adipocytic damage.
Differential diagnosis includes atrophic scarring
The differential diagnosis for hypopigmented atrophic macules and plaques over the lower limbs include atrophic scarring from previous trauma, guttate morphea, extra-genital lichen sclerosus, and tuberculoid leprosy.
Continue to: Atrophic scarring
Atrophic scarring occurs only after trauma.
Guttate morphea lesions are sclerotic and may be depressed.
Extra-genital lichen sclerosus is characterized by polygonal, shiny, ivory-white sclerotic lesions with or without follicular plugging.
Tuberculoid leprosy involves loss of nociception, hypotrichosis, and palpable thickened regional nerves (eg, great auricular, sural, or ulnar nerve).
Treatment requires long-term anticoagulation
Our patient had APS and the mainstay of treatment is long-term systemic anticoagulation along with attentive wound care.6 Warfarin is preferred over a direct oral anticoagulant as it is more effective in the prevention of recurrent thrombosis in patients with APS.7
Our patient was started on warfarin. Since APS may occur as a primary condition or in the setting of a systemic disease, such as systemic lupus erythematosus, she was referred to a rheumatologist.
1. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27:518-24. doi: 10.1097/01.ASW.0000455098.98684.95
2. Di Giacomo TB, Hussein TP, Souza DG, et al. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24:1340-1346. doi: 10.1111/j.1468-3083.2010.03646.x
3. Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician. 2019;100:298-305.
4. Armstrong EM, Bellone JM, Hornsby LB, et al. Acquired thrombophilia. J Pharm Pract. 2014;27:234-242. doi: 10.1177/0897190014530424
5. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
7. Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426-e436. doi: 10.1016/S2352-3026(16)30079-5
A 42-year-old woman presented to our dermatology center with white scars on both of her ankles. She first noticed the lesions 2 years prior; they were initially erythematous and painful, even when she was at rest. Her past medical history included 3 spontaneous term miscarriages. She denied any prolonged standing or trauma.
On examination, atrophic porcelain-white stellate scars were visible with surrounding hyperpigmentation on the medial aspect of both ankles (FIGURE 1A & 1B). There were no tender erythematous nodules,

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophie blanche
Atrophie blanche is a morphologic feature described as porcelain-white stellate scars with surrounding telangiectasia and hyperpigmentation. The lesions are typically found over the peri-malleolar region and are sequelae of healed erythematous and painful ulcers. The lesions arise from upper dermal, small vessel, thrombotic vasculopathy leading to ischemic rest pain; if left untreated, atrophic white scars eventually develop.
A sign of venous insufficiency or thrombotic vasculopathy
Atrophie blanche may develop following healing of an ulcer due to venous insufficiency or small vessel thrombotic vasculopathy.1 The incidence of thrombotic vasculopathy is 1:100,000 with a female predominance, and up to 50% of cases are associated with procoagulant conditions.2 Thrombotic vasculopathy can be due to an inherited or acquired thrombophilia.1
Causes of hereditary thrombophilia include Factor V Leiden/prothrombin mutations, anti-thrombin III/protein C/protein S deficiencies, dysfibrinogenemia, and hyperhomocysteinemia.
Acquired thrombophilia arises from underlying prothrombotic states associated with the Virchow triad: hypercoagulability, blood flow stasis, and endothelial injury. The use of oral contraceptives or hormone replacement therapy, presence of malignancy, and antiphospholipid syndrome (APS) are causes of acquired thrombophilia.2
Obtaining a careful history is crucial
Thorough history-taking and physical examination are required to determine the underlying cause of atrophie blanche.
Continue to: Chronic venous insufficiency
Chronic venous insufficiency is more likely in patients with a history of prolonged standing, obesity, or previous injury/surgery to leg veins. Physical examination would reveal hyperpigmentation, telangiectasia, varicose veins, pedal edema, and venous ulcers.3
Inherited thrombophilia may be at work in patients with a family history of arterial and venous thrombosis (eg, stroke, acute coronary syndrome, or deep vein thromboses).
Acquired thrombophilia should be suspected if there is a history of recurrent miscarriages or malignancy.4 Given our patient’s history of miscarriages, we ordered further lab work and found that she had elevated anticardiolipin levels (> 40 U/mL) fulfilling the revised Sapporo criteria5 for APS.
Thrombophilia or chronic venous insufficiency? In a patient with a history suggestive of thrombophilia, further work-up should be done before attributing atrophie blanche to healed venous ulcers from chronic venous insufficiency. A skin lesion biopsy could reveal classic changes of thrombotic vasculopathy subjacent to the ulcer, including intraluminal thrombosis, endothelial proliferation, and subintimal hyaline degeneration, as opposed to dermal changes consistent with venous stasis, such as increased siderophages, hemosiderin deposition, erythrocyte extravasation, dermal fibrosis, and adipocytic damage.
Differential diagnosis includes atrophic scarring
The differential diagnosis for hypopigmented atrophic macules and plaques over the lower limbs include atrophic scarring from previous trauma, guttate morphea, extra-genital lichen sclerosus, and tuberculoid leprosy.
Continue to: Atrophic scarring
Atrophic scarring occurs only after trauma.
Guttate morphea lesions are sclerotic and may be depressed.
Extra-genital lichen sclerosus is characterized by polygonal, shiny, ivory-white sclerotic lesions with or without follicular plugging.
Tuberculoid leprosy involves loss of nociception, hypotrichosis, and palpable thickened regional nerves (eg, great auricular, sural, or ulnar nerve).
Treatment requires long-term anticoagulation
Our patient had APS and the mainstay of treatment is long-term systemic anticoagulation along with attentive wound care.6 Warfarin is preferred over a direct oral anticoagulant as it is more effective in the prevention of recurrent thrombosis in patients with APS.7
Our patient was started on warfarin. Since APS may occur as a primary condition or in the setting of a systemic disease, such as systemic lupus erythematosus, she was referred to a rheumatologist.
A 42-year-old woman presented to our dermatology center with white scars on both of her ankles. She first noticed the lesions 2 years prior; they were initially erythematous and painful, even when she was at rest. Her past medical history included 3 spontaneous term miscarriages. She denied any prolonged standing or trauma.
On examination, atrophic porcelain-white stellate scars were visible with surrounding hyperpigmentation on the medial aspect of both ankles (FIGURE 1A & 1B). There were no tender erythematous nodules,

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophie blanche
Atrophie blanche is a morphologic feature described as porcelain-white stellate scars with surrounding telangiectasia and hyperpigmentation. The lesions are typically found over the peri-malleolar region and are sequelae of healed erythematous and painful ulcers. The lesions arise from upper dermal, small vessel, thrombotic vasculopathy leading to ischemic rest pain; if left untreated, atrophic white scars eventually develop.
A sign of venous insufficiency or thrombotic vasculopathy
Atrophie blanche may develop following healing of an ulcer due to venous insufficiency or small vessel thrombotic vasculopathy.1 The incidence of thrombotic vasculopathy is 1:100,000 with a female predominance, and up to 50% of cases are associated with procoagulant conditions.2 Thrombotic vasculopathy can be due to an inherited or acquired thrombophilia.1
Causes of hereditary thrombophilia include Factor V Leiden/prothrombin mutations, anti-thrombin III/protein C/protein S deficiencies, dysfibrinogenemia, and hyperhomocysteinemia.
Acquired thrombophilia arises from underlying prothrombotic states associated with the Virchow triad: hypercoagulability, blood flow stasis, and endothelial injury. The use of oral contraceptives or hormone replacement therapy, presence of malignancy, and antiphospholipid syndrome (APS) are causes of acquired thrombophilia.2
Obtaining a careful history is crucial
Thorough history-taking and physical examination are required to determine the underlying cause of atrophie blanche.
Continue to: Chronic venous insufficiency
Chronic venous insufficiency is more likely in patients with a history of prolonged standing, obesity, or previous injury/surgery to leg veins. Physical examination would reveal hyperpigmentation, telangiectasia, varicose veins, pedal edema, and venous ulcers.3
Inherited thrombophilia may be at work in patients with a family history of arterial and venous thrombosis (eg, stroke, acute coronary syndrome, or deep vein thromboses).
Acquired thrombophilia should be suspected if there is a history of recurrent miscarriages or malignancy.4 Given our patient’s history of miscarriages, we ordered further lab work and found that she had elevated anticardiolipin levels (> 40 U/mL) fulfilling the revised Sapporo criteria5 for APS.
Thrombophilia or chronic venous insufficiency? In a patient with a history suggestive of thrombophilia, further work-up should be done before attributing atrophie blanche to healed venous ulcers from chronic venous insufficiency. A skin lesion biopsy could reveal classic changes of thrombotic vasculopathy subjacent to the ulcer, including intraluminal thrombosis, endothelial proliferation, and subintimal hyaline degeneration, as opposed to dermal changes consistent with venous stasis, such as increased siderophages, hemosiderin deposition, erythrocyte extravasation, dermal fibrosis, and adipocytic damage.
Differential diagnosis includes atrophic scarring
The differential diagnosis for hypopigmented atrophic macules and plaques over the lower limbs include atrophic scarring from previous trauma, guttate morphea, extra-genital lichen sclerosus, and tuberculoid leprosy.
Continue to: Atrophic scarring
Atrophic scarring occurs only after trauma.
Guttate morphea lesions are sclerotic and may be depressed.
Extra-genital lichen sclerosus is characterized by polygonal, shiny, ivory-white sclerotic lesions with or without follicular plugging.
Tuberculoid leprosy involves loss of nociception, hypotrichosis, and palpable thickened regional nerves (eg, great auricular, sural, or ulnar nerve).
Treatment requires long-term anticoagulation
Our patient had APS and the mainstay of treatment is long-term systemic anticoagulation along with attentive wound care.6 Warfarin is preferred over a direct oral anticoagulant as it is more effective in the prevention of recurrent thrombosis in patients with APS.7
Our patient was started on warfarin. Since APS may occur as a primary condition or in the setting of a systemic disease, such as systemic lupus erythematosus, she was referred to a rheumatologist.
1. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27:518-24. doi: 10.1097/01.ASW.0000455098.98684.95
2. Di Giacomo TB, Hussein TP, Souza DG, et al. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24:1340-1346. doi: 10.1111/j.1468-3083.2010.03646.x
3. Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician. 2019;100:298-305.
4. Armstrong EM, Bellone JM, Hornsby LB, et al. Acquired thrombophilia. J Pharm Pract. 2014;27:234-242. doi: 10.1177/0897190014530424
5. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
7. Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426-e436. doi: 10.1016/S2352-3026(16)30079-5
1. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27:518-24. doi: 10.1097/01.ASW.0000455098.98684.95
2. Di Giacomo TB, Hussein TP, Souza DG, et al. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24:1340-1346. doi: 10.1111/j.1468-3083.2010.03646.x
3. Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician. 2019;100:298-305.
4. Armstrong EM, Bellone JM, Hornsby LB, et al. Acquired thrombophilia. J Pharm Pract. 2014;27:234-242. doi: 10.1177/0897190014530424
5. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
7. Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426-e436. doi: 10.1016/S2352-3026(16)30079-5
25-hydroxyvitamin D concentration is key to analyzing vitamin D’s effects
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
The recent Practice Alert by Dr. Campos-Outcalt, “How to proceed when it comes to vitamin D” (J Fam Pract. 2021;70:289-292) claimed that the value of vitamin D supplements for prevention is nil or still unknown.1 Most of the references cited in support of this statement were centered on randomized controlled trials (RCTs) based on vitamin D dose rather than achieved 25-hydroxyvitamin D [25(OH)D] concentration. Since the health effects of vitamin D supplementation are correlated with 25(OH)D concentration, the latter should be used to evaluate the results of vitamin D RCTs—a point I made in my 2018 article on the topic.2
For example, in the Vitamin D and Type 2 Diabetes (D2d) Study, in which participants in the treatment arm received 4000 IU/d vitamin D3, there was no reduced rate of progression from prediabetes to diabetes. However, when 25(OH)D concentrations were analyzed for those in the vitamin D arm during the trial, the risk was found to be reduced by 25% (hazard ratio [HR] = 0.75; 95% CI, 0.68-0.82) per 10 ng/mL increase in 25(OH)D.3
Another trial, the Harvard-led VITamin D and OmegA-3 TriaL (VITAL), enrolled more than 25,000 participants, with the treatment arm receiving 2000 IU/d vitamin D3.4 There were no significant reductions in incidence of either cancer or cardiovascular disease for the entire group. The mean baseline 25(OH)D concentration for those for whom values were provided was 31 ng/mL (32.2 ng/mL for White participants, 24.9 ng/mL for Black participants). However, there were ~25% reductions in cancer risk among Black participants (who had lower 25(OH)D concentrations than White participants) and those with a body mass index < 25. A posthoc analysis suggested a possible benefit related to the rate of total cancer deaths.
A recent article reported the results of long-term vitamin D supplementation among Veterans Health Administration patients who had an initial 25(OH)D concentration of < 20 ng/mL.5 For those who were treated with vitamin D and achieved a 25(OH)D concentration of > 30 ng/mL (compared to those who were untreated and had an average concentration of < 20 ng/mL), the risk of myocardial infarction was 27% lower (HR = 0.73; 95% CI, 0.55-0.96) and the risk of all-cause mortality was reduced by 39% (HR = 0.61; 95% CI, 0.56-0.67).
An analysis of SARS-CoV-2 positivity examined data for more than 190,000 patients in the United States who had serum 25(OH)D concentration measurements taken up to 1 year prior to their SARS-CoV-2 test. Positivity rates were 12.5% (95% CI, 12.2%-12.8%) for those with a 25(OH)D concentration < 20 ng/mL vs 5.9% (95% CI, 5.5%-6.4%) for those with a 25(OH)D concentration ≥55 ng/mL.6
Thus, there are significant benefits of vitamin D supplementation to achieve a 25(OH)D concentration of 30 to 60 ng/mL for important health outcomes.
Continue to: Author's Response
Author's response
I appreciate the letter from Dr. Grant in response to my previous Practice Alert, as it provides an opportunity to make some important points about assessment of scientific evidence and drawing conclusions based on sound methodology. There is an overabundance of scientific literature published, much of which is of questionable quality, meaning a “study” or 2 can be found to support any preconceived point of view.
In 2011, the Institute of Medicine (now the National Academy of Medicine) published a series of recommendations on how trustworthy recommendations and guidelines should be produced.1,2 Key among the steps recommended is a full assessment of the totality of the literature on the subject by an independent, nonconflicted panel. This should be based on a systematic review that includes standard search methods to find all pertinent articles, an assessment of the quality of each study using standardized tools, and an overall assessment of the quality of the evidence. A high-quality systematic review meeting these standards was the basis for my review article on vitamin D.3
To challenge the findings of the unproven benefits of vitamin D, Dr. Grant cited 4 studies to support the purported benefit of achieving a specific serum 25(OH)D level to prevent cardiovascular disease, diabetes, cancer, and COVID-19. After reading these studies, I would not consider any of them a “game changer.”
The first study was restricted to those with prediabetes, had limited follow-up (mean of 2.5 years), and found different results for those with the same 25(OH)D concentrations in the placebo and treatment groups.4 The second study was a large, well-conducted clinical trial that found no benefit of vitamin D supplementation in preventing cancer and cardiovascular disease.5 While Dr. Grant claims that benefits were found for some subgroups, I could locate only the statistics on cancer incidence in Black participants, and the confidence intervals showed no statistically significant benefit. It is always questionable to look at multiple outcomes in multiple subgroups without a prior hypothesis because of the likely occurrence of chance findings in so many comparisons. The third was a retrospective observational study with all the potential biases and challenges to validity that such studies present.6 A single study, especially 1 with observational methods, almost never conclusively settles a point.
The role of vitamin D in the prevention or treatment of COVID-19 is an aspect that was not covered in the systematic review by the US Preventive Services Task Force. The study on this issuecited by Dr. Grant was a large retrospective observational study that found an inverse relationship between serum 25(OH)D levels and SARS-CoV-2 positivity rates.7 This is 1 observational study with interesting results. However, I believe the conclusion of the National Institutes of Health is currently still the correct one: “There is insufficient evidence to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19.”8
With time and further research, Dr. Grant may eventually prove to be correct on specific points. However, when challenging a high-quality systematic review, one must assess the quality of the studies used while also placing them in context of the totality of the literature.
Doug Campos-Outcalt, MD, MPA
Phoenix, AZ
References
1. Institute of Medicine. Finding What Works in Health Care. The National Academy Press, 2011.
2. Institute of Medicine. Clinical Practice Guidelines We Can Trust. The National Academy Press, 2011.
3. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults; updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463. doi: 10.1001/jama.2020.26498
4. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
5. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
6. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
7. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
8. National Institutes of Health. Vitamin D. COVID-19 treatment guidelines. Updated April 21, 2021. Accessed November 18, 2021. www.covid19treatmentguidelines.nih.gov/therapies/supplements/vitamin-d/
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
1. Campos-Outcalt D. How to proceed when it comes to vitamin D. J Fam Pract. 2021;70:289-292. doi: 10.12788/jfp.0215
2. Grant WB, Boucher BJ, Bhattoa HP, et al. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2018;177:266-269. doi: 10.1016/j.jsbmb.2017.08.009
3. Dawson-Hughes B, Staten MA, Knowler WC, et al. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: a secondary analysis from the Vitamin D and Type 2 Diabetes (D2d) Study. Diabetes Care. 2020;43:2916-2922. doi: 10.2337/dc20-1765
4. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33-44. doi: 10.1056/NEJMoa1809944
5. Acharya P, Dalia T, Ranka S, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. 2021;5:bvab124. doi: 10.1210/jendso/bvab124
6. Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. doi: 10.1371/journal.pone.0239252
Despite ‘getting it wrong’ we must continue to do what’s right
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
I have been wrong about the COVID-19 pandemic any number of times. During the early days of the pandemic, a colleague asked me if he should book his airline ticket to Chicago for our annual Essential Evidence conference. I told him to go ahead. The country shut down the next week.
In September of this year, I was ready to book my flight to Phoenix for a presentation at the Arizona Academy of Family Physicians annual meeting. I thought COVID-19 activity was winding down. I was wrong again. The conference was changed to virtual presentations.
And now, as I write this editorial late in November, I find myself wrong a third time. I figured the smoldering COVID-19 activity in Michigan, where I live, would wind down before Thanksgiving. But it is expanding wildly throughout the Midwest.
Wrong again, and again.
I figured most everyone would be vaccinated as soon as vaccines were available, given the dangerous nature of the virus and the benign nature of the vaccines. But here we are, more than 750,000 deaths later and, as a country, we still have not learned our lesson. I won’t get into the disinformation campaign against the existence of the pandemic and the effectiveness and safety of the vaccines; this disinformation campaign seems to be designed to kill as many Americans as possible.
The COVID-19 epidemic is personal for all of us. Not one of us has been immune to its effects. All of us have had a relative or friend die of COVID-19 infection. All of us have had to wear masks and be cautious about contacts with others. All of us have cancelled or restricted travel. My wife and I are debating whether or not we should gather for the holidays with our children and grandchildren in Michigan, despite the fact that all of us have been immunized. One of my sons has a mother-in-law with pulmonary fibrosis; he and his family will all be doing home testing for COVID-19 the day before visiting her.
When will this nightmare end? There is no question that everyone in the United States—and most likely, the entire world—will eventually get vaccinated against COVID-19 or get infected with it. We must continue urging everyone to make the smart, safe choice and get vaccinated.
There are still hundreds of thousands of lives to be saved.
Cervical cancer update: The latest on screening & management
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7
Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22
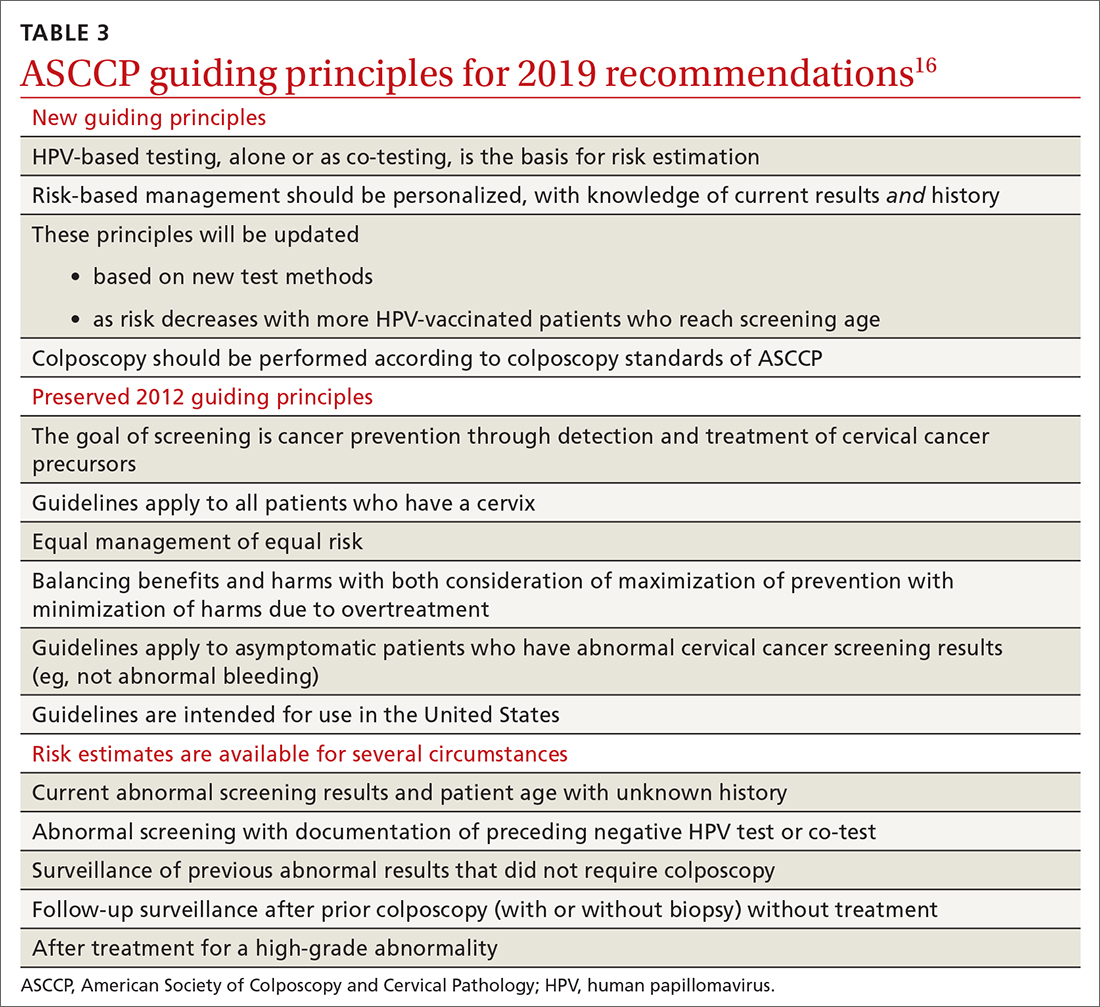
Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.
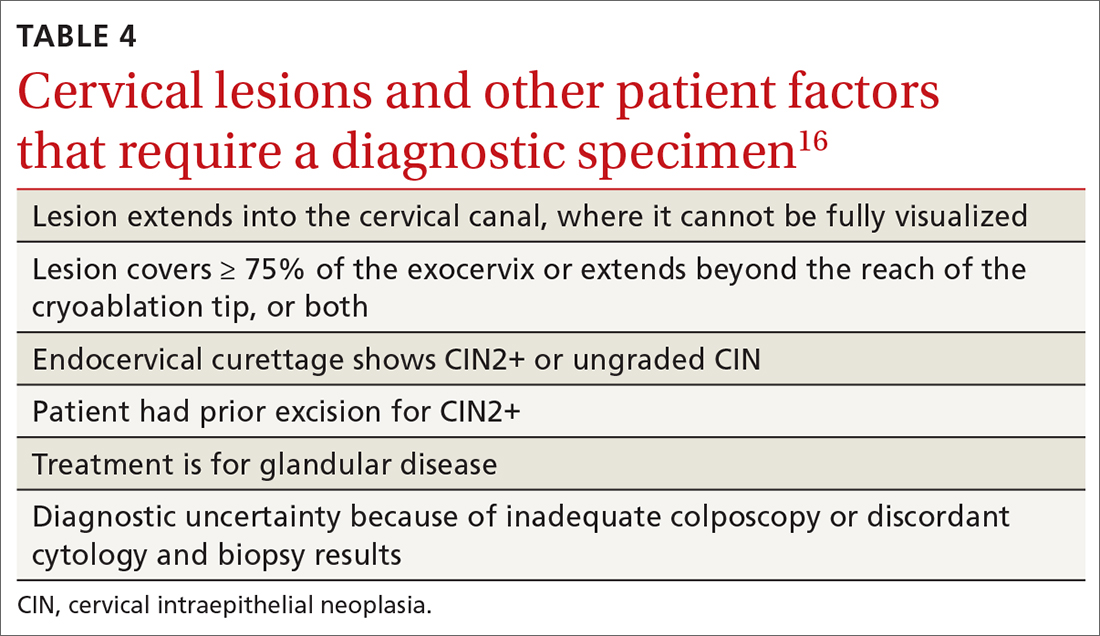
Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7

Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22

Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.

Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
The World Health Organization estimates that, in 2020, worldwide, there were 604,000 new cases of uterine cervical cancer and approximately 342,000 deaths, 84% of which occurred in developing countries.1 In the United States, as of 2018, the lifetime risk of death from cervical cancer was 2.2 for every 100,000
In this article, we summarize recent updates in the epidemiology, prevention, and treatment of cervical cancer. We emphasize recent information of value to family physicians, including updates in clinical guidelines and other pertinent national recommendations.
Spotlight continues to shine on HPV
It has been known for several decades that cervical cancer is caused by human papillomavirus (HPV). Of more than 100 known HPV types, 14 or 15 are classified as carcinogenic. HPV 16 is the most common oncogenic type, causing more than 60% of cases of cervical cancer3,4
HPV is the most common sexually transmitted infection, with as many as 80% of sexually active people becoming infected during their lifetime, generally before 50 years of age.5 HPV also causes other anogenital and oropharyngeal cancers; however, worldwide, more than 80% of HPV-associated cancers are cervical.6 Risk factors for cervical cancer are listed in TABLE 1.7 Cervical cancer is less common when partners are circumcised.7

Most cases of HPV infection clear in 1 or 2 years
At least 70% of cervical cancers are squamous cell carcinoma (SCC); 20% to 25% are adenocarcinoma (ADC); and < 3% to 5% are adenosquamous carcinoma.10 Almost 100% of cervical SCCs are HPV+, as are 86% of cervical ADCs. The most common reason for HPV-negative status in patients with cervical cancer is false-negative testing because of inadequate methods.
Primary prevention through vaccination
HPV vaccination was introduced in 2006 in the United States for girls,a and for boysa in 2011. The primary reason for vaccinating boys is to reduce the rates of HPV-related anal and oropharyngeal cancer. The only available HPV vaccine in the United States is Gardasil 9 (9-valent vaccine, recombinant; Merck), which provides coverage for 7 high-risk HPV types that account for approximately 90% of cervical cancers and 2 types (6 and 11) that are the principal causes of condylomata acuminata (genital warts). Future generations of prophylactic vaccines are expected to cover additional strains.
Continue to: Vaccine studies...
Vaccine studies have been summarized in a Cochrane review,11 showing that vaccination is highly effective for prevention of cervical dysplasia, especially when given to young girls and womena previously unexposed to the virus. It has not been fully established how long protection lasts, but vaccination appears to be 70% to 90% effective for ≥ 10 years.
Dosing schedule. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends a 2-dose schedule 6 to 15 months apart, for both girls and boys between 9 and 14 years of age.12 A third dose is indicated if the first and second doses were given less than 5 months apart, or the person is older than 15 years or is immunocompromised. No recommendation has been made for revaccination after the primary series.
In 2018, the US Food and Drug Administration approved Gardasil 9 for adults 27 to 45 years of age. In June 2019, ACIP recommended vaccination for mena as old as 26 years, and adopted a recommendation that unvaccinated men and women between 27 and 45 years discuss HPV vaccination with their physician.13
The adolescent HPV vaccination rate varies by state; however, all states lag behind the CDC’s Healthy People 2020 goal of 80%.14 Barriers to vaccination include cost, infrastructure limitations, and social stigma.
Secondary prevention: Screening and Tx of precancerous lesions
Cervical cancer screening identifies patients at increased risk of cervical cancer and reassures the great majority of them that their risk of cervical cancer is very low. There are 3 general approaches to cervical cancer screening:
- cytology-based screening, which has been implemented for decades in many countries
- primary testing for DNA or RNA markers of high-risk HPV types
- co-testing with cytology-based screening plus HPV testing.
Continue to: USPSTF guidance
USPSTF guidance. Recommendations of the US Preventive Services Task Force (USPSTF) for cervical cancer screening were updated in 2018 (TABLE 215). The recommendations state that high-risk HPV screening alone is a strategy that is amenable to patient self-sampling and self-mailing for processing—a protocol that has the potential to improve access

ASCCP guidance. The American Society of Colposcopy and Cervical Pathology (ASCCP) makes nearly the same recommendations for cervical cancer screening. An exception is that ASCCP guidelines allow for the possibility of screening using primary high-risk HPV testing for patients starting at 25 years of age.16
Screening programs that can be initiated at a later age and longer intervals should be possible once the adolescent vaccination rate is optimized and vaccination registries are widely implemented.
Cervical cytology protocol
Cervical cytologic abnormalities are reported using the Bethesda system. Specimen adequacy is the most important component of quality assurance,17 and is determined primarily by sufficient cellularity. However, any specimen containing abnormal squamous cells of undetermined significance (ASCUS) or atypical glandular cells (AGCs) is considered satisfactory, regardless of the number of cells. Obscuring factors that impair quality include excessive blood; inflammation; air-drying artifact; and an interfering substance, such as lubricant. The presence of reactive changes resulting from inflammation does not require further evaluation unless the patient is immunosuppressed.
Abnormalities are most often of squamous cells, of 2 categories: low-grade squamous intraepithelial lesions (LSILs) and high-grade squamous intraepithelial lesions (HSILs). HSILs are more likely to be associated with persistent HPV infection and higher risk of progression to cervical cancer.
Continue to: Cytologic findings...
Cytologic findings can be associated with histologic findings that are sometimes more, sometimes less, severe. LSIL cytology specimens that contain a few cells that are suspicious for HSIL, but that do not contain enough cells to be diagnostic, are reported as atypical squamous cells, and do not exclude a high-grade intraepithelial lesion.
Glandular-cell abnormalities usually originate from the glandular epithelium of the endocervix or the endometrium—most often, AGCs. Less frequent are AGCs, favor neoplasia; endocervical adenocarcinoma in situ; and ADC. Rarely, AGCs are associated with adenosquamous carcinoma. Endometrial polyps are a typical benign pathology that can be associated with AGCs.
In about 30% of cases, AGCs are associated with premalignant or malignant disease.18 The risk of malignancy in patients with AGCs increases with age, from < 2% among patients younger than 40 years to approximately 15% among those > 50 years.19 Endometrial malignancy is more common than cervical malignancy among patients > 40 years.
AGC cytology requires endocervical curettage, plus endometrial sampling for patients ≥ 35 years
Cytology-based screening has limitations. Sensitivity is relatively low and dependent on the expertise of the cytologist, although regular repeat testing has been used to overcome this limitation. A substantial subset of results are reported as equivocal—ie, ASCUS.
Continue to: Primary HPV screening
Primary HPV screening
Primary HPV testing was approved by the US Food and Drug Administration in 2015 and recommended as an appropriate screening option by professional societies
In contrast to cytology-based screening, HPV testing has high sensitivity (≥ 90%); the population-based negative likelihood ratio is near zero.20 This degree of sensitivity allows for extended screening intervals. However, primary HPV testing lacks specificity for persistent infection and high-grade or invasive lesions, which approximately doubles the number of patients who screen positive. The potential for excess patients to be referred for colposcopy led to the need for secondary triage.
Instituting secondary triage. Cytology is, currently, the primary method of secondary triage, reducing the number of referrals for colposcopy by nearly one-half, compared to referrals for all high-risk HPV results, and with better overall accuracy over cytology with high-risk HPV triage.21 When cytology shows ASCUS, or worse, refer the patient for colposcopy; alternatively, if so-called reflex testing for HPV types 16 and 18 is available and positive, direct referral to colposcopy without cytology is also appropriate.
In the future, secondary triage for cytology is likely to be replaced with improved technologies, such as immunostaining of the specimen for biomarkers associated with cervical precancer or cancer, or for viral genome methylation testing.22

Management of abnormal cervical cancer screening results
Routine screening applies to asymptomatic patients who do not require surveillance because they have not had prior abnormal screening results. In 2020, ASCCP published risk-based management consensus guidelines that were developed for abnormal cervical cancer screening tests and for cancer precursors.16 Guiding principles, and screening situations in which the guidelines can be applied, are summarized in TABLE 3
Continue to: ASCCP guidelines...
ASCCP guidelines provide a framework to incorporate new data and technologies without major revision
Some noteworthy scenarios in ASCCP risk-based management are:
- For unsatisfactory cytology with a negative HPV test or no HPV test, repeat age-based screening in 2 to 4 months. (Note: A negative HPV test might reflect an inadequate specimen; do not interpret this result as a true negative.)
- An absent transformation zone (ie, between glandular and squamous cervical cells) with an otherwise adequate specimen should be interpreted as satisfactory for screening in patients 21 to 29 years of age. For those ≥ 30 years and with no HPV testing in this circumstance, HPV testing is preferred; repeating cytology, in 3 years, is also acceptable.
- After a finding of LSIL/CIN1 without evidence of a high-grade abnormality, and after 2 negative annual screenings (including HPV testing), a return to 3-year (not 5-year) screening is recommended.
- A cytology result of an HSIL carries a risk of 26% for CIN3+, in which case colposcopy is recommended, regardless of HPV test results.
- For long-term management after treatment for CIN2+, continue surveillance testing every 3 years after 3 consecutive negative HPV tests or cytology findings, for at least 25 years. If the 25-year threshold is reached before 65 years of age, continuing surveillance every 3 years is optional, as long as the patient is in good health (ie, life expectancy ≥ 10 years).
- After hysterectomy for a high-grade abnormality, annual vaginal HPV testing is recommended until 3 negative tests are returned; after that, surveillance shifts to a 3-year interval until the 25-year threshold.
Treatment of cancer precursors
Treatment for cervical dysplasia is excisional or ablative.
Excisional therapy. In most cases, excisional therapy (either a loop electrosurgical excision procedure [LEEP; also known as large loop excision of the transformation zone, cold knife conization, and laser conization] or cone biopsy) is required, or preferred. Excisional treatment has the advantage of providing a diagnostic specimen.
The World Health Organization recommends LEEP over ablation in settings in which LEEP is available.23 ASCCP states that, in the relatively few cases in which treatment is needed and it is for CIN1, either excision or ablation is acceptable. TABLE 416 lists situations in which excisional treatment is required because a diagnostic specimen is needed.

Continue to: Ablative treatments
Ablative treatments are cryotherapy, CO2 laser ablation, and thermal ablation. Ablative therapy has the advantage of presenting less risk of adverse obstetric outcomes (eg, preterm birth); it can be used if the indication for therapy is:
- CIN1 or CIN2 and HPV type 16 or 18 positivity
- concordant cytology and histology
- satisfactory colposcopy
- negative endocervical curettage.
The most common ablative treatment is liquid nitrogen applied to a metal tip under local anesthesia.
Hysterectomy can be considered for patients with recurrent CIN2+ who have completed childbearing or for whom repeat excision is infeasible (eg, scarring or a short cervix), or both.
Cost, availability, and convenience might play a role in decision-making with regard to the treatment choice for cancer precursors.
Is care after treatment called for? Patients who continue to be at increased risk of (and thus mortality from) cervical and vaginal cancer require enhanced surveillance. The risk of cancer is more than triple for patients who were given their diagnosis, and treated, when they were > 60 years, compared to patients treated in their 30s.1 The excess period of risk covers at least 25 years after treatment, even among patients who have had 3 posttreatment screenings.
Continue to: Persistent HPV positivity...
Persistent HPV positivity is more challenging. Patients infected with HPV type 16 have an increased risk of residual disease.
Cancer management
Invasive cancer. Most cervical cancers (60%) occur among patients who have not been screened during the 5 years before their diagnosis.24 For patients who have a diagnosis of cancer, those detected through screening have a much better prognosis than those identified by symptoms (mean cure rate, 92% and 66%, respectively).25 The median 5-year survival for patients who were not screened during the 5 years before their diagnosis of cervical cancer is 66%.2
In unscreened patients, cervical cancer usually manifests as abnormal vaginal bleeding, especially postcoitally. In approximately 45% of cases, the patient has localized disease at diagnosis; in 36%, regional disease; and in 15%, distant metastases.26
For cancers marked by stromal invasion < 3 mm, appropriate treatment is cone biopsy or simple hysterectomy.27
Most patients with early-stage cervical cancer undergo modified radical hysterectomy. The ovaries are usually conserved, unless the cancer is adenocarcinoma. Sentinel-node dissection has become standard practice. Primary radiation therapy is most often used for patients who are a poor surgical candidate because of medical comorbidity or poor functional status. Antiangiogenic agents (eg, bevacizumab) can be used as adjuvant palliative therapy for advanced and recurrent disease
Continue to: After treatment for...
After treatment for invasive cervical cancer, the goal is early detection of recurrence, although there is no consensus on a protocol. Most recurrences are detected within the first 2 years.
Long-term sequelae after treatment for advanced cancer are considerable. Patients report significantly lower quality of life,
Hormone replacement therapy is generally considered acceptable after treatment of cervical cancer because it does not increase replication of HPV.
Recurrent or metastatic cancer. Recurrence or metastases will develop in 15% to 60% of patients,30 usually within the first 2 years after treatment.
Management depends on location and extent of disease, using mainly radiation therapy or surgical resection. Recurrence or metastasis is usually incurable.
Continue to: Last, there are promising...
Last, there are promising areas of research for more effective treatment for cervical cancer precursors and cancers, including gene editing tools31 and therapeutic
Prospects for better cervical cancer care
Prevention. HPV vaccination is likely to have a large impact on population-based risk of both cancer and cancer precursors in the next generation.
Screening in the foreseeable future will gravitate toward reliance on primary HPV screening, with a self-sampling option.
Surveillance after dysplastic disease. The 2019 ASCCP guidelines for surveillance and intervention decisions after abnormal cancer screening results will evolve to incorporate introduction of new technology into computerized algorithms.
Treatment. New biologic therapies, including monoclonal antibodies and therapeutic vaccines against HPV, will likely be introduced for treating cancer precursors and invasive cancer.
A NOTE FROM THE EDITORS The Editors of The Journal of Family Practice recognize the importance of addressing the reproductive health of gender-diverse individuals. In this article, we use the words “women,” “men,” “girls,” and “boys” in limited circumstances (1) for ease of reading and (2) to reflect the official language of the US Food and Drug Administration and the Advisory Committee on Immunization Practices. The reader should consider the information and guidance offered in this discussion of cervical cancer and other human papillomavirus-related cancers to speak to the care of people with a uterine cervix and people with a penis.
CORRESPONDENCE
Linda Speer, MD, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-249. doi: 10.3322/caac.21660
2. Cancer stat facts: cervical cancer. National Cancer Institute Surveillance, Epidemiology, and End Results [SEER] Program. Accessed November 14, 2021. https://seer.cancer.gov/statfacts/html/cervix.html
3. Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer 2012;131:2349-2359. doi: 10.1002/ijc.27485
4. Winer RL, Hughes JP, Feng Q, et al. Early history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011;20:699-707. doi: 10.1158/1055-9965.EPI-10-1108
5. Chesson HW, Dunne EF, Hariri F, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:660-664. doi: 10.1097/OLQ.0000000000000193
6. Human papillomavirus (HPV) and cervical cancer. Fact sheet. Geneva, Switzerland: World Health Organization; November 11, 2020. Accessed November 14, 2021. www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
7. International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120:885-891. doi: 10.1002/ijc.22357
8. McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical cancer neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008:9:425-434. doi: 10.1016/S1470-2045(08)70103-7
9. de Sanjose S, Quint WG, Alemany I, et al; . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective, cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056. doi: 10.1016/S1470-2045(10)70230-8
10. Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review 1975-2004. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2004/#citation
11. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3
12. Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016:65;1405-1408. doi: 10.15585/mmwr.mm6549a5
13. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
14. State-level data: Female adolescents receiving 2 or 3 doses of HPV vaccine by age 13-15 years (percent). HealthyPeople.gov. Accessed November 14, 2021. www.healthypeople.gov/2020/data/map/4657?year=2018
15. United States Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA 2018;320:674-686. doi: 10.1001/jama.2018.10897
16. Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
17. Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123;271-281. doi: 10.1002/cncy.21521
18. Schnatz PF, Guile M, O’Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701-708. doi: 10.1097/01.AOG.0000202401.29145.68
19. Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383-389. doi: 10.1016/j.ygyno.2009.05.019
20. Zazove P, Reed BD, Gregoire L, et al. Low false-negative rate of PCR analysis for detecting human papillomavirus-related cervical lesions. J Clin Microbiol. 1998;36:2708-2713. doi: 10.1128/JCM.36.9.2708-2713.1998
21. Richardson LA, El-Zein M, Ramankumar AV, et al; . HPV DNA testing with cytology triage in cervical cancer screening: influence of revealing HPV infection status. Cancer Cytopathol. 2015:123:745-754. doi: 10.1002/cncy.21596
22. Wentzensen N, Schiffman M, Palmer T, et al. Triage of HPV positive women in cervical cancer screening. J Clin Virol 2016;76:S49-S55. doi: 10.1016/j.jcv.2015.11.015
23. WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva, Switzerland: World Health Organization; 2011. Accessed November 14, 2021. www.ncbi.nlm.nih.gov/books/NBK138476/pdf/Bookshelf_NBK138476.pdf
24. Spence AR, Goggin P, Franco EL. Process of care failures in invasive cervical cancer: systematic review and meta-analysis. Prev Med. 2007:45:93-106. doi: 10.1016/j.ypmed.2007.06.007
25. Rositch AF, Nowak RG, Gravitt PE. Increased age and race-specific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000-2009. Cancer. 2014:120:2032-2038. doi: 10.1002/cncr.28548
26. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71:7-33. doi: 10.3322/caac.21654
27. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: cervical cancer. Accessed June 15, 2021. www.nccn.org/professionals/physician_gls/pdf/cervical.pdf
28. Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet. 2017;390:1654-1663. doi: 10.1016/S0140-6736(17)31607-0
29. Osann K, Hsieh S, Nelson EL, et al. Factors associated with poor quality of life among cervical cancer survivors: implications for clinical care and clinical trials. Gynecol Oncol. 2014;135:266-272. doi: 10.1016/j.ygyno.2014.08.036
30. Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975 to 2003. Bethesda, MD: National Cancer Institute; 2007. Accessed November 14, 2021. https://seer.cancer.gov/archive/csr/1975_2003/#citation
31. Hu Z, Ding M. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med. 2018;7:5217-5236. doi: 10.1002/cam4.1501
32. Wang R, Pan W, Jin L, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88-102. doi: 10.1016/j.canlet.2019.11.039
PRACTICE RECOMMENDATIONS
› Encourage eligible patients to be vaccinated against human papillomavirus (HPV) because the vaccine is highly effective for preventing cervical dysplasia, especially when given to patients previously unexposed to the virus. A
› Screen for cervical disease with either cytology plus HPV testing or primary HPV testing with secondary triage for cytology; both protocols are more accurate than screening with cervical cytology alone, and allow you to widen the screening interval. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Write an exercise Rx to improve patients' cardiorespiratory fitness
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.
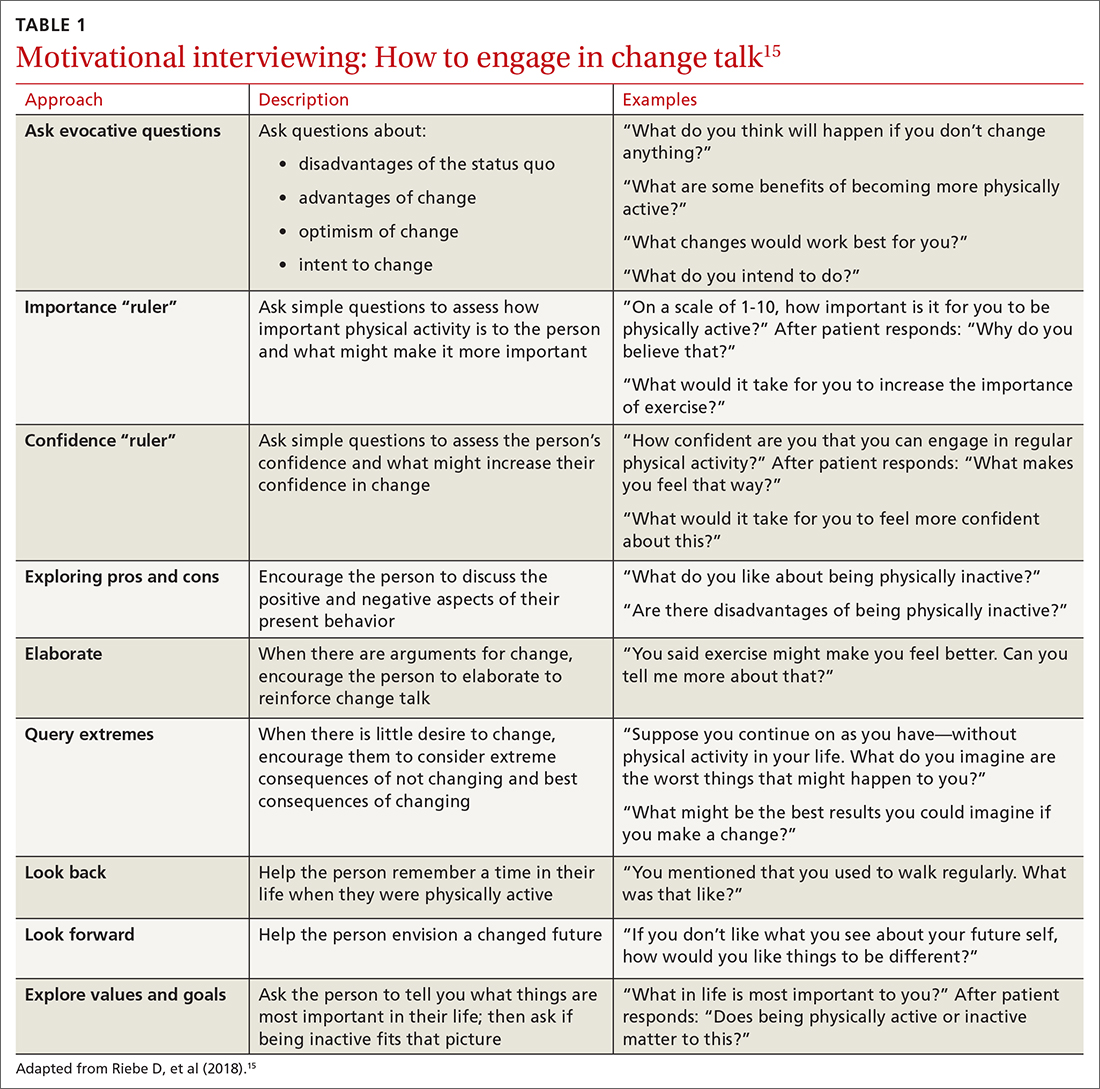
Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.
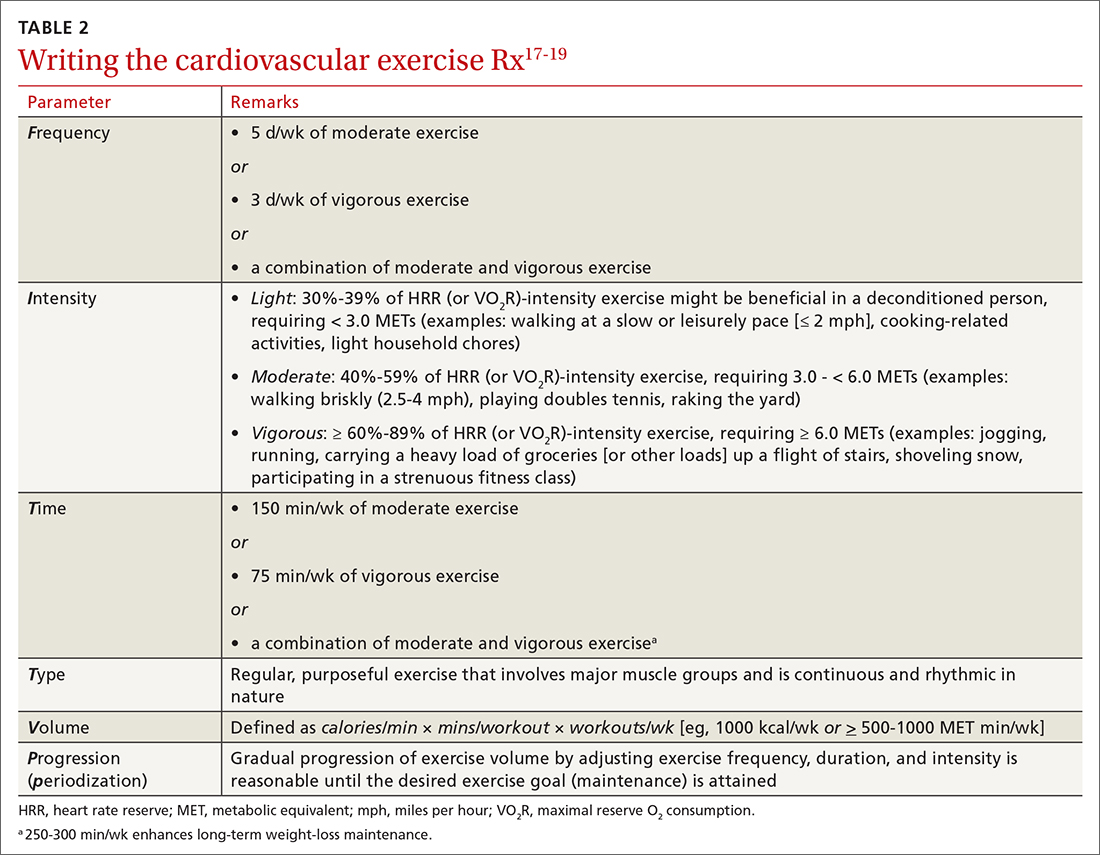
Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).
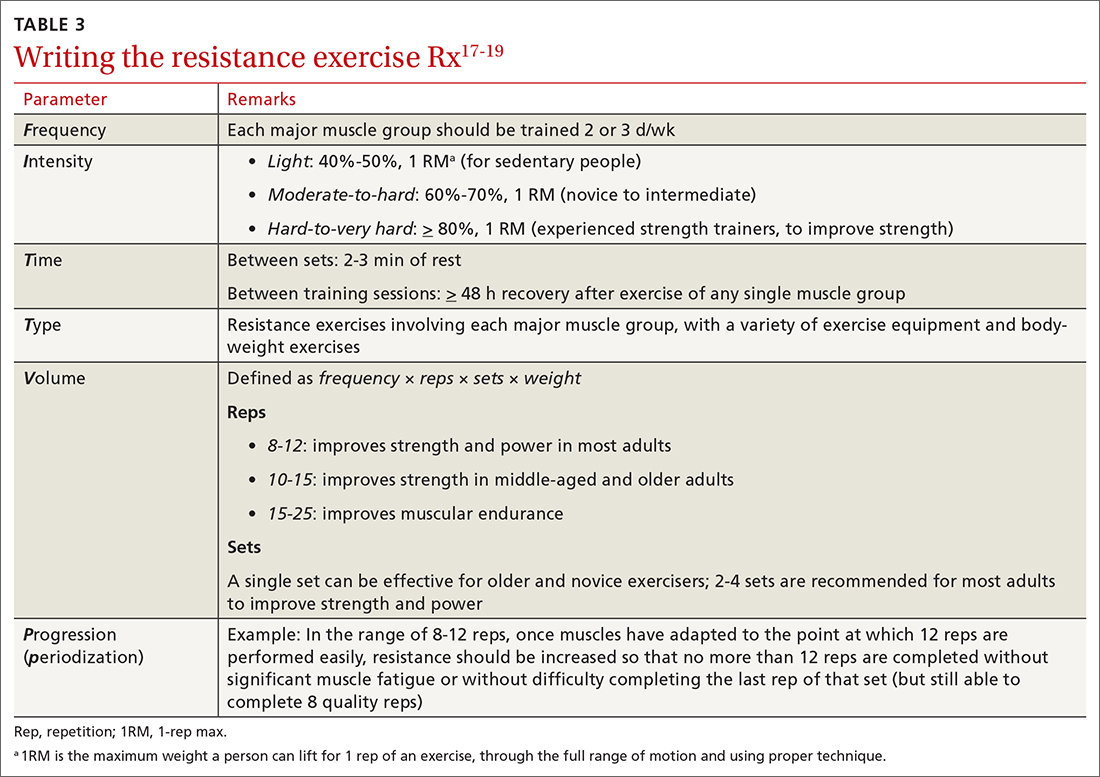
Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).
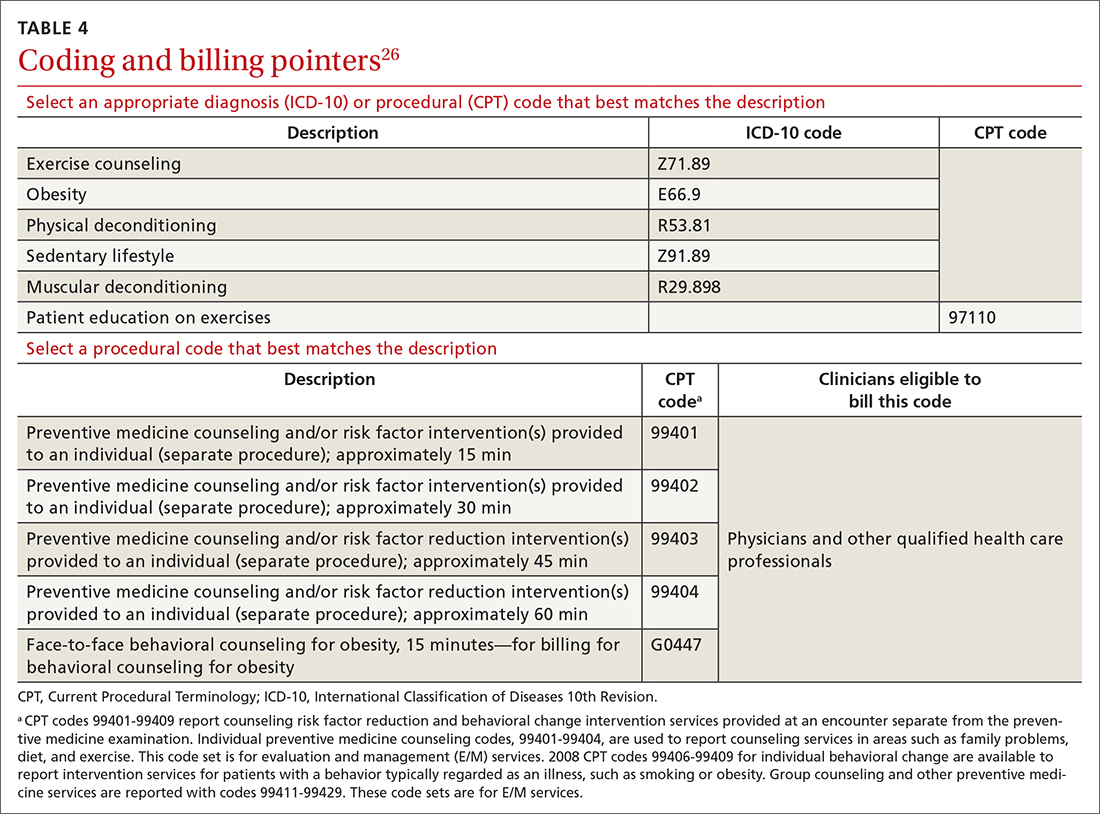
Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.

Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.

Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).

Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).

Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
It is well-known that per capita health care spending in the United States is more than twice the average in other developed countries1; nevertheless, the overall health care ranking of the US is near the bottom compared to other countries in this group.2 Much of the reason for this poor relative showing lies in the fact that the US has employed a somewhat traditional fee-for-service health care model that does not incentivize efforts to promote health and wellness or prevent chronic disease. The paradigm of promoting physical activity for its disease-preventing and treatment benefits has not been well-integrated in the US health care system.
In this article, we endeavor to provide better understanding of the barriers that keep family physicians from routinely promoting physical activity in clinical practice; define tools and resources that can be used in the clinical setting to promote physical activity; and delineate areas for future work.
Glaring hole in US physical activity education
Many primary care physicians feel underprepared to prescribe or motivate patients to exercise. The reason for that lack of preparedness likely relates to a medical education system that does not spend time preparing physicians to perform this critical task. A study showed that, on average, medical schools require only 8 hours of physical activity education in their curriculum during the 4 years of schooling.3 Likewise, the average primary care residency program offers only 3 hours of didactic training on physical activity, nutrition, and obesity.4 The problem extends to sports medicine fellowship training, in which a 2019 survey showed that 63% of fellows were never taught how to write an exercise prescription in their training program.5
Without education on physical activity, medical students, residents, and fellows are woefully underprepared to realize the therapeutic value of physical activity in patient care, comprehend current physical activity guidelines, appropriately motivate patients to engage in exercise, and competently discuss exercise prescriptions in different disease states. Throughout their training, it is imperative for medical professionals to be educated on the social determinants of health, which include the conditions in which people live, work, and play. These environmental variables can contribute to health inequities that create additional barriers to improvement in physical fitness.6
National guidelines on physical activity
The 2018 National Physical Activity Guidelines detail recommendations for children, adolescents, adults, and special populations.7 The guidelines define physical activity as bodily movement produced by skeletal muscles that result in energy expenditure above resting baseline levels, and includes all types, intensities, and domains of activity. Exercise is a subset of physical activity characterized as planned, structured, repetitive, and designed to improve or maintain physical fitness, physical performance, or health.
Highlights from the 2018 guidelines include7:
- Preschool-aged children (3 to 5 years of age) should be physically active throughout the day, with as much as 3 hours per day of physical activity of all intensities—light, moderate, and vigorous.
- Older children and adolescents (6 to 17 years) should accumulate 60 minutes per day of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening activities.
- Adults of all ages should achieve approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week, along with at least 2 days per week of muscle-strengthening activities. Other types of physical activity include flexibility, balance, bone-strengthening, and mind–body exercises.
3-step framework for enhancing physical activity counseling
Merely knowing that physical activity is healthy is not enough, during a patient encounter, to increase the level of physical activity. Therefore, it is imperative to learn and adopt a framework that has proved to yield successful outcomes. The Screening, Brief Intervention, and Referral to Treatment (SBIRT) framework, which has predominantly been used to change patient behavior related to alcohol and substance use, is now being utilized by some providers to promote physical activity.8 We apply the SBIRT approach in this article, although research is lacking on its clinical utility and outcome measures.
Continue to: SBIRT
SBIRT: Screening
An office visit provides an opportunity to understand a patient’s level of physical activity. Often, understanding a patient’s baseline level of activity is only asked during a thorough social history, which might not be performed during patient encounters. As physical activity is the primary determinant of cardiorespiratory fitness (CRF), some health care systems have begun delineating physical activity levels as a vital sign to ensure that the assessment of physical activity is a standard part of every clinical encounter. At a minimum, this serves as a prompt and provides an opportunity to start a conversation around improving physical activity levels when guidelines are not being met.
The exercise vital sign. Assessment and documentation of physical activity in the electronic health record are not yet standardized; however, Kaiser Permanente health plans have implemented the exercise vital sign, or EVS, in its HealthConnect (Epic Systems) electronic health record. The EVS incorporates information about a patient’s:
- days per week of moderate-to-strenuous exercise (eg, a brisk walk)
- minutes per day, on average, of exercise at this level.
The physical activity vital sign. Intermountain Healthcare implemented the physical activity vital sign, or PAVS, in its iCentra (Cerner Corp.) electronic health record. The 3-question PAVS assessment asks:
- On average, how many days of the week do you perform physical activity or exercise?
- On average, how many total minutes of physical activity or exercise do you perform on those days?
- How would you describe the intensity of your physical activity or exercise: Light (ie, a casual walk)? Moderate (a brisk walk)? Or vigorous (jogging)?
PAVS includes a fourth data point: The physician–user documents whether the patient was counseled to start, increase, maintain, or modify physical activity or exercise.
EVS and the PAVS have demonstrated validity.9-11
Continue to: Cardiorespiratory fitness as a vital sign
Cardiorespiratory fitness as a vital sign. In 2016, the American Heart Association (AHA) asserted the importance of assessing CRF as a clinical vital sign.12 CRF is commonly expressed as maximal oxygen consumption (VO2max = O2 mL/kg/min) and measured through cardiopulmonary exercise testing (CPET), considered the gold standard by combining conventional graded exercise testing with ventilatory expired gas analysis. CPET is more objective and precise than equations estimating CRF that are derived from peak work rate. AHA recommended that efforts to improve CRF should become standard in clinical encounters, explaining that even a small increase in CRF (eg, 1 or 2 metabolic equivalentsa [METs]) is associated with a considerably (10% to 30%) lower rate of adverse cardiovascular events.12
De Souza de Silva and colleagues revealed an association between each 1-MET increase in CRF and per-person annual health care cost savings (adjusted for age and presence of cardiovascular disease) of $3272 (normal-weight patients), $4252 (overweight), and $6103 (obese).13 In its 2016 scientific statement on CRF as a vital sign, AHA listed several methods of estimating CRF and concluded that, although CPET involves a higher level of training, proficiency, equipment, and, therefore, cost, the independent and additive information obtained justifies its use in many patients.12
CASE
Mary Q, 68 years of age, presents for an annual well-woman examination. Body mass index is 32; resting heart rate (HR), 73 bpm; and blood pressure, 126/74 mm Hg. She reports being inactive, except for light walking every day with her dog around the neighborhood, which takes them approximately 15 minutes. She denies any history or signs and symptoms of cardiovascular, metabolic, or renal disease.
You consider 3 questions before taking next steps regarding increasing Ms. Q’s activity level:
- What is her PAVS?
- Does she need medical clearance before starting an exercise program?
- What would an evidence-based cardiovascular exercise prescription for Ms. Q look like?
SBIRT: Brief intervention
When a patient does not meet the recommended level of physical activity, you have an opportunity to deliver a brief intervention. To do this effectively, you must have adequate understanding of the patient’s receptivity for change. The transtheoretical, or Stages of Change, model proposes that a person typically goes through 5 stages of growth—pre-contemplation, contemplation, preparation, action, and maintenance—in the process of lifestyle modification. This model highlights the different approaches to exercise adoption and maintenance that need to be taken, based on a given patient’s stage at the moment.
Continue to: Using this framework...
Using this framework, you can help patients realize intrinsic motivation that can facilitate progression through each stage, utilizing techniques such as motivational interviewing—so-called change talk—to increase self-efficacy.14TABLE 115 provides examples of motivational interviewing techniques that can be used during a patient encounter to improve health behaviors, such as physical activity.

Writing the exercise prescription
A patient who wants to increase their level of physical activity should be offered a formal exercise prescription, which has been shown to increase the level of physical activity, particularly in older patients. In fact, a study conducted in Spain in the practices of family physicians found that older patients who received a physical activity prescription increased their activity by 131 minutes per week; and compared to control patients, they doubled the minutes per week devoted to moderate or vigorous physical activity.16
FITT-VP. The basics of a cardiovascular exercise prescription can be found in the FITT-VP (Frequency, Intensity, Time, Type, Volume, and [monitoring of] Progression) framework (TABLE 217-19). For most patients, this model includes 3 to 5 days per week of moderate-to-vigorous physical activity for 30 to 60 minutes per session. For patients with established chronic disease, physical activity provides health benefits but might require modification. Disease-specific patient handouts for exercise can be downloaded, at no cost, through the American College of Sports Medicine (ACSM) “Exercise Is Medicine” program, which can be found at: www.exerciseismedicine.org/support_page.php/rx-for-health-series.

Determining intensity level. Although CPET is the gold standard for determining a patient’s target intensity level, such a test might be impracticable for a given patient. Surrogate markers of target intensity level can be obtained by measuring maximum HR (HRmax), using a well-known equation20:
HRmax = 220 – age
which is then multiplied by intensity range:
- light: 30%-39%
- moderate: 40%-59%
- vigorous: 60%-89%
or, more preferably, by calculating the HR training zone while accounting for HR at rest (HRrest). This is accomplished by calculating the HR reserve (HRR) (ie, HRR = HRmax – HRrest) and then calculating the target heart rate (THR)21:
THR = [HRR × %intensity] + HRrest
Continue to: The THR calculation...
The THR calculation is performed twice, once with a lower %intensity and again with a higher %intensity to develop a training zone based on HRR.
The HRR equation is more accurate than calculating HRmax from 220 – age, because HRR accounts for resting HR, which is often lower in people who are better conditioned.
Another method of calculating intensity for patients who are beginning a physical activity program is the rating of perceived exertion (RPE), which is graded on a scale of 6 to 20: Moderate exercise correlates with an RPE of 12 to 13 (“somewhat hard”); vigorous exercise correlates with an RPE of 14 to 16 (“hard”). By adding a zero to the rating on the RPE scale, the corresponding HR in a healthy adult can be estimated when they are performing an activity at that perceived intensity.22 Moderate exercise therefore correlates with a HR of 120 and 130 bpm.
The so-called talk test can also guide exercise intensity: Light-intensity activity correlates with an ability to sing; moderate-intensity physical activity likely allows the patient to still hold a conversation; and vigorous-intensity activity correlates with an inability to carry on a conversation while exercising.
An exercise prescription should be accompanied by a patient-derived goal, which can be reassessed during a follow-up visit. So-called SMART goals (Specific, Measurable, Achievable, Relevant, and Time-bound) are tools to help patients set personalized and realistic expectations for physical activity. Meeting the goal of approximately 150 to 300 minutes of moderate or 75 to 150 minutes of vigorous physical activity (or an equivalent combination) per week is ideal, but a patient needs to start where they are, at the moment, and gradually increase activity by setting what for them are realistic and sustainable goals.
Continue to: CASE
CASE
With a PAVS of 105 minutes (ie, 15 minutes per day × 7 days) of weekly light-to-moderate exercise walking her dog, Ms. Q does not satisfy current physical activity guidelines. She needs an exercise prescription to incorporate into her lifestyle (see “Cardiovascular exercise prescription,” at left).
First, based on ACSM pre-participation guidelines, Ms. Q does not need medical clearance before initiating light-to-moderate exercise and gradually progressing to vigorous-intensity exercise.
Second, in addition to walking the dog for 105 minutes a week, you:
- advise her to start walking for 10 minutes, 3 times per week, at a pace that keeps her HR at 97-104 bpm.
- encourage her to gradually increase the frequency or duration of her walks by no more than 10% per week.

SBIRT: Referral for treatment
When referring a patient to a fitness program or professional, it is essential to consider their preferences, resources, and environment.23 Community fitness partners are often an excellent referral option for a patient seeking guidance or structure for their exercise program. Using the ACSM ProFinder service, (www.acsm.org/get-stay-certified/find-a-pro) you can search for exercise professionals who have achieved the College’s Gold Standard credential.
Gym memberships or fitness programs might be part of the extra coverage offered by Medicare Advantage Plans, other Medicare health plans, or Medicare Supplement Insurance (Medigap) plans.24
Continue to: CASE
CASE
After providing Ms. Q with her exercise prescription, you refer her to a local gym that participates in the Silver Sneakers fitness and wellness program (for adults ≥ 65 years of age in eligible Medicare plans) to determine whether she qualifies to begin resistance and flexibility training, for which you will write a second exercise prescription (TABLE 317-19).

Pre-participation screening
Updated 2015 ACSM exercise pre-participation health screening recommendations attempt to decrease possible barriers to people who are becoming more physically active, by minimizing unnecessary referral to health care providers before they change their level of physical activity. ACSM recommendations on exercise clearance include this guidance25:
- For a patient who is asymptomatic and already physically active—regardless of whether they have known cardiovascular, metabolic, or renal disease—medical clearance is unnecessary for moderate-intensity exercise.
- Any patient who has been physically active and asymptomatic but who becomes symptomatic during exercise should immediately discontinue such activity and undergo medical evaluation.
- For a patient who is inactive, asymptomatic, and who does not have known cardiovascular, metabolic, or renal disease, medical clearance for light- or moderate-intensity exercise is unnecessary.
- For inactive, asymptomatic patients who have known cardiovascular, metabolic, or renal disease, medical clearance is recommended.
Digital health
Smartwatches and health apps (eg, CardioCoach, Fitbit, Garmin Connect, Nike Training Club, Strava, and Training Peaks) can provide workouts and offer patients the ability to collect information and even connect with other users through social media platforms. This information can be synced to Apple Health platforms for iPhones (www.apple.com/ios/health/) or through Google Fit (www.google.com/fit/) on Android devices. Primary care physicians who become familiar with health apps might find them useful for select patients who want to use technology to improve their physical activity level.
However, data on the value of using digital apps for increasing physical activity, in relation to their cost, are limited. Additional research is needed to assess their validity.
Billing and coding
For most patients, the physical activity assessment, prescription, and referral are performed in the context of treating another condition (eg, hypertension, type 2 diabetes, obesity, depression) or during a preventive health examination, and are typically covered without additional charge to the patient. An evaluation and management visit for an established patient could be used to bill if > 50% of the office visit was spent face-to-face with a physician, with patient counseling and coordination of care.
Continue to: Physicians and physical therapists...
Physicians and physical therapists can use the therapeutic exercise code (Current Procedural Terminology code 97110) when teaching patients exercises to develop muscle strength and endurance, joint range of motion, and flexibility26 (TABLE 426).

Conclusion
Physical activity and CRF are strong predictors of premature mortality, even compared to other risk factors, such as cigarette smoking, hypertension, hypercholesterolemia, and type 2 diabetes.27 Brief physical activity assessment and counseling is an efficient, effective, and cost-effective means to increase physical activity, and presents a unique opportunity for you to encourage lifestyle-based strategies for reducing cardiovascular risk.28
However, it is essential to meet patients where they are before trying to have them progress; it is therefore imperative to assess the individual patient’s level of activity using PAVS. With that information in hand, you can personalize physical activity advice; determine readiness for change and potential barriers for change; assist the patient in setting SMART goals; and arrange follow-up to assess adherence to the exercise prescription. Encourage the patient to call their health insurance plan to determine whether a gym membership or fitness program is covered.
Research is needed to evaluate the value of using digital apps, in light of their cost, to increase physical activity and improve CRF in a clinical setting. Prospective trials should be initiated to determine how routine implementation of CRF assessment in primary care alters the trajectory of clinical care. It is hoped that future research will answer the question: Would such an approach improve clinical outcomes and reduce health care expenditures?12
a Defined as O2 consumed while sitting at rest; equivalent to 3.5 mL of O2 × kg of body weight × min.
CORRESPONDENCE
Matthew Kampert, DO, MS, Sports Medicine, 5555 Transportation Boulevard, Cleveland, OH 44125; [email protected]
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
1. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319:1024-1039. doi: 10.1001/jama.2018.1150
2. Tikkanen R, Abrams MK. U.S. health care from a global perspective, 2019: higher spending, worse outcomes? The Commonwealth Fund Website. January 30, 2020. Accessed November 16, 2021. www.commonwealthfund.org/publications/issue-briefs/2020/jan/us-health-care-global-perspective-2019
3. Stoutenberg M, Stasi S, Stamatakis E, et al. Physical activity training in US medical schools: preparing future physicians to engage in primary prevention. Phys Sportsmed. 2015;43:388-394. doi: 10.1080/00913847.2015.1084868
4. Antognoli EL, Seeholzer EL, Gullett H, et al. Primary care resident training for obesity, nutrition, and physical activity counseling: a mixed-methods study. Health Promot Pract. 2017;18:672-680. doi: 10.1177/1524839916658025
5. Asif IM, Drezner JA. Sports and exercise medicine education in the USA: call to action. Br J Sports Med. 2020;54:195-196. doi: 10.1136/bjsports-2019-101104
6. Douglas JA, Briones MD, Bauer EZ, et al. Social and environmental determinants of physical activity in urban parks: testing a neighborhood disorder model. Prev Med. 2018;109:119-124. doi: 10.1016/j.ypmed.2018.01.013
7. 2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health & Human Services; 2018. Accessed November 15, 2021. https://health.gov/sites/default/files/2019-09/PAG_Advisory_Committee_Report.pdf
8. Avis JL, Cave AL, Donaldson S, et al. Working with parents to prevent childhood obesity: protocol for a primary care-based ehealth study. JMIR Res Protoc. 2015;4:e35. doi:10.2196/resprot.4147
9. Ball TJ, Joy EA, Gren LH, et al. Concurrent validity of a self-reported physical activity ‘vital sign’ questionnaire with adult primary care patients. Prev Chronic Dis. 2016;13:e16. doi: 10.5888/pcd13.150228
10. Ball TJ, Joy EA, Gren LH, et al. Predictive validity of an adult physical activity “vital sign” recorded in electronic health records. J Phys Act Health. 2016;13:403-408. doi: 10.1123/jpah.2015-0210
11. Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071-2076. doi: 10.1249/MSS.0b013e3182630ec1
12. Ross R, Blair SN, Arena R, et al; ; ; ; ; ; . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653-e699. doi: 10.1161/CIR.0000000000000461
13. de Souza de Silva CG, Kokkinos PP, Doom R, et al. Association between cardiorespiratory fitness, obesity, and health care costs: The Veterans Exercise Testing Study. Int J Obes (Lond). 2019;43:2225-2232. doi: 10.1038/s41366-018-0257-0
14. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38-48. doi: 10.4278/0890-1171-12.1.38
15. Riebe D, Ehrman JK, Liguori G, et al. Methods for evoking change talk. In: ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer; 2018.
16. Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
17. McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: a review of concepts and evidence. Soc Sci Med. 2006;63:1011-1022. doi: 10.1016/j.socscimed.2006.03.012
18. Garber CE, Blissmer BE, Deschenes MR, et al; American College of Sports Medicine. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Position stand. Med Sci Sport Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb
19. Donnelly JE, Blair SN, Jakicic JM, et al; American College of Sports Medicine. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Position stand. Med Sci Sport Exerc. 2009;41:459-471. doi: 10.1249/MSS.0b013e3181949333
20. Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3:404-432.
21. Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307-315.
22. The Borg RPE scale. In: Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; 1998:29-38.
23. Ratamess NA, Alvar BA, Evetoch TK, et al; American College of Sports Medicine. Progression models in resistance training for healthy adults. Position stand. Med Sci Sport Exerc. 2009;41:687-708. doi: 10.1249/MSS.0b013e3181915670
24. Gym memberships & fitness programs. Medicare.gov. Baltimore, MD: US Centers for Medicare and Medicaid Services. Accessed November 16, 2021. www.medicare.gov/coverage/gym-memberships-fitness-programs
25. Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM’s recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473-2479. doi: 10.1249/MSS.0000000000000664
26. Physical Activity Related Current Procedural Terminology (CPT®) Codes. Physical Activity Alliance website. Accessed November 16, 2021. https://paamovewithus.org/wp-content/uploads/2020/11/PAA-Physical-Activity-CPT-Codes-Nov-2020-AMA-Approved-Final-1.pdf
27. Blair SN. Physical inactivity: the biggest public health problem of the 21st century Br J Sports Med. 2009;43:1-2.
28. Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88:1446-1461. doi: 10.1016/j.mayocp.2013.08.020
PRACTICE RECOMMENDATIONS
› Encourage children and adolescents (6 to 17 years of age) to engage in 60 min of moderate-to-vigorous physical activity, including aerobic, muscle-strengthening, and bone-strengthening endeavors on most, if not all, days of the week. A
› Encourage adults to perform approximately 150 to 300 min of moderate or 75 to 150 min of vigorous physical activity (or an equivalent combination) per week, along with moderate-intensity muscle-strengthening activities on ≥ 2 days per week. A
› Counsel patients that even a small (eg, 1-2 metabolic equivalents) increase in cardiorespiratory fitness is associated with a 10% to 30% lower rate of adverse events. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Functional medicine: Focusing on imbalances in core metabolic processes
Could screening patients for cytokine markers help direct interventions to prevent quality-of-life deterioration? What evidence is there that a patient’s methylenetetrahydrofolate reductase (MTHFR) genotype and baseline folate level can determine whether folate therapy will be needed to prevent stroke?
Favorable findings in these areas and others—eg, that specific probiotics benefit those with various gastrointestinal, respiratory, and lipid disorders—are strengthening support for the clinical approach of functional medicine (FM), which focuses on core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
In this article, we describe the paradigm of FM, review its origins, and present the evidence base of selected topics: diagnostic testing, nutrition and supplements, probiotics, and chelation. As FM’s popularity increases, a better understanding of it will help us educate our patients on this approach and implement some of its evidence-backed practices. In preparing this review we used keyword searches of PubMed, Embase, Web of Science, Cochrane Library, University of Florida Health Science Center Library eJournals, the Institute for Functional Medicine website,1 and Lifestyle Medicine textbook.2
The core of functional medicine
Complementary and alternative medicine (CAM) refers to medical practices diverging from standards of care and not generally taught at US medical schools or available at US hospitals.3 Integrative Medicine encompasses evidence-informed CAM, conventional Western medicine, and whole systems like FM.4
FM aims to identify root causes of disease, emphasizing function as a dynamic process that can move back and forth on a continuum between health and disorder.5,6 There are 7 defining characteristics of FM: (1) patient centered vs disease centered; (2) systems biology approach acknowledging web-like interconnections of physiologic factors; (3) dynamic balance of gene–environment interactions; (4) personalized care based on biochemical individuality; (5) promotion of organ reserve and sustained health span; (6) health as a positive vitality—not merely the absence of disease; and (7) function vs pathology focused.5
The concept of FM is not new. Its origins can be traced to the 19th century. Jeffrey Bland, PhD, credits the term’s first use to Sir Willoughby F. Wade, MD, in an 1871 Lancet editorial, “Clinical lecture on functional medicine.” Bland formulated the FM paradigm and in 1991 founded the Institute for Functional Medicine (the Institute), its main educational and certifying organization.7 The Institute certifies masters- or doctorate-prepared health professionals from both conventional and complementary health fields who complete 7 courses (Applying FM in Clinical Practice, Gastrointestinal, Environmental Health, Immune, Hormone, Cardiometabolic, and Bioenergetics), present 1 case report, and pass a written exam. In a retrospective cohort study, researchers found that the FM model was associated with greater improvements in patient-reported, health-related quality of life (QOL) compared with usual care.8
Clinical model
FM uses a comprehensive yet practical matrix for obtaining patient histories and for guiding diagnostic testing.9 Tools that support history-taking include a timeline; weeklong dietary survey; daily activity log; exercise, sleep and self-care questionnaires; and an environmental risk assessment. Instead of a review of symptoms arranged by organ system as is typical with conventional Western medicine, FM assesses the balance of core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
Continue to: Within the matrix...
Within the matrix, FM also recognizes 5 modifiable personal lifestyle factors: sleep and relaxation, exercise and movement, nutrition, stress, and relationships. When these lifestyle elements are influenced by specific predisposing factors (antecedents), discrete events precipitating illness (triggers), and ongoing physiologic processes (mediators), fundamental imbalances eventually result in the signs and symptoms characterizing diagnosable diseases.
The essence of the FM therapeutic plan is a discussion of lifestyle changes, personal strengths, and potential adherence challenges so the clinician can better offer assistive resources, which may include multidisciplinary referrals to personalized counselors such as a nutritionist, health coach, mind-body therapist, personal trainer, exercise physiologist, or physical therapist. While these professionals are not unique to FM, they are more frequently used by FM practitioners as part of the health care team.
Diagnostic testing through a different lens
A proposed solution to complex diseases recalcitrant to conventional modern treatments is a systems biology approach to health care.10 Current laboratory testing such as liver enzymes, C-reactive protein (CRP), and leukocyte count provide some information on organ system homeostasis and molecular pathology, but even high-sensitivity CRP, which measures inflammation across the human system, is not adequate in cardiovascular disease (CVD) risk assessment.11 Genome-wide association studies suggest an association between biological pathways, genes, molecular markers, and QOL domains, and screening patients for cytokine markers may help provide prophylactic interventions to prevent QOL deterioration.12
With the purpose of being more systems-biology centric rather than disease centric,13 FM practitioners use tests to guide dietary recommendations and supplement selection, the evidence for which is found mostly in symptom-specific case series.14-16 Commonly used tests include MTHFR genotyping, comprehensive stool profiles, hormone and heavy metal panels, allergy panels, lactulose breath testing, micronutrient and advanced lipid panels, omega 3:6 ratios, and oxidative stress tests.
Some of these tests are performed by standard labs and are covered by insurance. Certain assays are primarily performed by specialized functional laboratories, such as comprehensive stool profiles, provoked heavy metal tests, non-IgE allergy panels, and oxidative stress tests. These laboratories also frequently offer direct-to-consumer orders. The latter situation raises a potential conflict of interest from supplement sales linked to laboratory testing and makes it imperative for health care professionals to understand the reliability and clinical utility of these tests in order to counsel patients accordingly.
Continue to: The remainder of this article...
The remainder of this article focuses on the evidence behind a subset of FM treatments, which typically include various dietary interventions (elimination, cardiometabolic, detox) and multidomain lifestyle modifications. The practitioner’s selection of dietary interventions, nutraceuticals (vitamins, minerals, essential fatty acids, botanicals), and probiotics is informed by results of different diagnostic tests.
Nutrition and supplements
Nearly one-third of older Americans are affected by at least 1 vitamin deficiency or anemia, and even those consuming an adequate diet have a substantial risk of any deficiency (16%), although less so than those with an inadequate diet (57%).17 The Western diet is known to be nutrient deficient, particularly in vitamin D, thiamine, riboflavin, calcium, magnesium, and selenium.18 Dietary supplement nonusers have the highest risk of any deficiency (40%) compared with users of “full-spectrum multivitamin-multimineral supplements” (14%) and other dietary supplement users (28%).17
Nevertheless, the US Preventive Services Task Force (USPSTF) concluded in 2014 that there are not enough data to make a recommendation for or against taking vitamins A, C, or E; multivitamins with folic acid; or combinations of these vitamins for the primary prevention of CVD or cancer.19 USPSTF also does not recommend daily vitamin D and calcium supplementation in community-dwelling, postmenopausal women for primary prevention of fracture.20 Notwithstanding the lack of supplement recommendations for primary prevention, their benefits in patients with chronic disease is still being investigated. For example, a polyphenol-rich antioxidant may reduce cardiovascular complications in those with diabetes.21
Rethinking how nutrition studies are designed. Drawing on studies to determine the benefits of nutrition in chronic disease has been challenging. Factors that must be taken into account include the types of vitamin and mineral supplements patients use, nutrient absorption and utilization, and differing dietary assessment methods and reference values used.18 For example, vitamin and mineral absorption work best with whole food or fortified diets wherein specific nutrients are consumed together (eg, vitamin D and vitamin E with fat; non-heme iron with vitamin C).22-24 Foods with competing nutrients or “inhibitors” may even require absorption enhancers at minimum molar ratios.24
Adding to the complexity of vitamin and mineral absorption, botanical dietary supplements have their own modifying effects on micronutrient absorption.25 These are just some of the reasons randomized controlled trials (RCTs) are fundamentally limited when investigating the health outcomes of diet and supplementation, and alternative study methods should be considered for future nutrition clinical trials to better inform clinicians who are prescribing supplements.26 In the meantime, nutrition plans can be individualized to patients’ biological and cultural needs, ideally in conjunction with a multidisplinary team that includes dietitians, behaviorists, and exercise specialists.27
Continue to: Nutrition is one of the most...
Nutrition is one of the most important environmental factors modulating genes and phenotypes (nutrigenomics),28,29 and recent studies on single nucleotide polymorphisms (SNPs) have reinforced the importance of considering the effect of genetic variation on dietary response (nutrigenetics).29,30 While nutrigenomics and nutrigenetics have the potential to improve the health of large populations by personalizing dietary advice based on genotype and phenotype, genetic variation occurring within a specific biochemical pathway makes generalizing genotype-based dietary advice to other populations complex.
For instance, Greenlandic Inuit, the indigenous people inhabiting the Arctic regions of Greenland, have a low incidence of CVD, largely due to “non-European” genetic variants that lower their LDL, protecting them from the oxidative stress of their diet high in polyunsaturated fats.31,32 In a study of European adults, phenotypic and phenotypic-plus-genotypic information did not enhance the effectiveness of the personalized nutrition advice, demonstrating that more research is needed in larger populations.33
Another specific, complex example of gene-nutrient interaction is the pathophysiologic outcome of polymorphisms in the MTHFR gene.30 MTHFR is a rate-limiting enzyme involved in folate and homocysteine metabolism, DNA and RNA biosynthesis, and DNA and protein methylation. MTHFR polymorphisms are common in otherwise healthy people, but some have been reported to increase chronic disease susceptibility via MTHFR deficiency. The most common MTHFR variant is the SNP rs1801133 that reduces enzyme activity to ~30% in homozygotes (677TT), which can lead to reduced folate bioavailability and mild-to-moderate hyperhomocysteinemia depending on one’s dietary folate intake and food fortification.
One FM focus at variance with current recommendations. FM’s approach to homocysteine and MTHFR genotyping for nutritional assessment, dietary counseling, and supplement advice contrasts with recommendations from meta-analyses34-36 and guidelines from the American Heart Association and USPSTF.37,38 While USPSTF does recommend all women planning or capable of pregnancy take 0.4 to 0.8 mg of folic acid daily to prevent neural tube defects,39MTHFR polymorphism screening to guide supplementation is not recommended during pregnancy given conflicting studies and uncertain clinical significance.40 Moreover, while it is generally agreed that the MTHFR C677T polymorphism, and in particular the homozygous MTHFR 677TT genotype, is an independent risk factor for hyperhomocysteinemia, neither variant genotype was an independent coronary artery disease risk factor in several large studies.41-43
However, study populations with generally high folate consumption or fortified foods may be a main confounding variable.44 For example, in a recent meta-analysis of an elderly population, the T-allele of the MTHFR C677T variant increased pooled stroke risk. While this increased stroke risk is highest in homozygotes, the association was statistically significant only in the Chinese cohort—a group that generally has poor dietary intake of folate and B12.45 Therefore, both MTHFR genotype and baseline folate level are important determinants of folate therapy efficacy in stroke prevention, and this may explain why US studies have not clearly identified a patient subset most likely to benefit.46 Future high-quality studies should measure baseline folate, target populations with moderate-to-severe hyperhomocysteinemia with specific MTHFR polymorphisms, and compare high- and low-dose B vitamins or use 5-methylfolate (the active form of folate).
Continue to: Probiotics
Probiotics
Probiotics are used extensively in FM, and there are very few fields in conventional Western medicine where probiotics have not been researched. Interestingly, gut microbiota (microflora) change rapidly and individually to therapeutic dietary changes, both in composition (community) and function (metabolic plasticity), implicating gut microbiota as a mediator of dietary impact on host metabolism.47 This highlights the potential for tailored probiotics to transform dietary nonresponders (eg, those who do not routinely consume a high-fiber diet) into responders whose metabolism becomes enabled to counter such conditions as obesity and type 2 diabetes.48
Caveats with probiotic administration. While the strength of recommendation for probiotic therapy is increased by their safe use in pregnancy, infants, and immunocompromised populations,49-51 various harms (ranging from mild to severe) may be underreported.52-54 In clinical practice, the effective probiotic strain(s), formulation, and dosage will vary not only by disease, but also from patient to patient, and may be dependent on nutritional factors such as vitamin D,55 diet, and even epigenetics.56
As we come to more fully recognize commensal microbiota as a major player in overall health with crosstalk in signaling pathways between intestinal bacterial and epithelial immune cells, future research is needed to help optimize probiotic dose, duration, route of administration, and whether microbial communities outperform single-species probiotics.50 Furthermore, much of a bacterial community’s effect on the host is through its metabolites, and since certain species can be used interchangeably given similar metabolic activity and function, studies to understand how prebiotics and probiotics affect the host must analyze the metabolome, in addition to the bacterial community composition.51
Alternative research methods could be informative. Similar to their limited evaluation of health outcomes in nutrition and supplement research, many RCTs examining the health benefits of probiotics often yield ambiguous results or fall short of valid conclusions because the underlying presuppositions are not met.26 For example, assuming the RCT uses a well-defined probiotic formulation, efficacy and generalizability can still be confounded by patient microbiota, diet, mucosa, immune system, and emotional status, which all affect probiotic activity/potency.50 Under these circumstances, other research methods may be more suitable or supplemental—eg, Phase II trials, epidemiologic studies, single-case experiments (n-of-1 trials) and their meta-analyses, using historical controls, preclinical studies for conceptual and theoretical development, and clinical experience.26,57-59
It is currently unknown how nonvaginal microbiota affect the health of menopausal women;63,64 and while a multicenter RCT is underway to examine a novel probiotic’s effect on menopausal symptoms and bone health, the supplemental study methods listed above could be employed as well.
Continue to: Evidence of probiotic effectiveness
Evidence of probiotic effectiveness. Positive evidence in RCTs and meta-analyses suggests that strain-specific probiotics are beneficial for infectious gastroenteritis, persistent antibiotic-associated diarrhea, infant colic,50 irritable bowel syndrome (IBS),50,65Clostridioides difficile prevention,66 inflammatory bowel disease (IBD),67 radiation-induced diarrhea,68,69 and nongastrointestinal conditions such as multiple sclerosis,70 upper respiratory infections,50 atopic dermatitis,50,71,72 surgical site infections,73,74 hyperlipidemia,50,75,76 and nonalcoholic steatohepatitis.77
However, because of knowledge gaps and low-quality evidence, the American Gastroenterological Association’s (AGA) 2020 Clinical Practice Guidelines recommend “conditional” use of strain-specific probiotics only for necrotizing enterocolitis prevention (in pre-term, low-birth-weight infants), antibiotic-induced C difficile prevention, and pouchitis.53,54 Additionally, the AGA’s guidelines state that probiotics should only be used for C difficile infection, IBD, or IBS in a clinical trial and not used at all for infectious gastroenteritis.53,54
Chelation
Chelation therapy is thought to inhibit metal-catalyzed oxidation reactions and inflammatory processes in tissues that lead to, and result from, accumulation of oxidative damage. While many FM practitioners recommend some form of chelation therapy, there is much controversy surrounding its use. Well-designed, large-scale clinical trials continue to emerge for what appears to be an old intervention with still uncertain clinical applications.
The first chelation agent, disodium ethylenediaminetetraacetic acid (EDTA), was synthesized in the early 1930s, but it was not until World War II that chelation therapy began with use of dimercaprol, a potent antidote for the arsenical chemical weapon lewisite. Disodium EDTA infusions were first used to treat angina pectoris in the mid-20th century,78 and use accelerated in the early 21st century without a clear indication to treat CVD besides decades of anecdotes and case reports.79 Strong epidemiologic data support a causal association between heavy metals (arsenic, lead, cadmium, mercury, and copper) and CVD,80,81 but a distinct mechanism of action is not yet known.
Disodium EDTA was FDA approved in the 1950s for use in patients with hypercalcemia or ventricular arrhythmias from digitalis toxicity, but it was eventually withdrawn from the market in 2008, in part due to safety concerns following 3 deaths from severe hypocalcemia.82 These deaths were caused by inappropriate use related to rapid infusions, pediatric use, and in one case inadvertently administering disodium EDTA to a child instead of calcium EDTA (which can safely be bolused to treat pediatric lead toxicity).82-85
Continue to: The Trial to Assess Chelation Therapy...
The Trial to Assess Chelation Therapy (TACT) was the first large, double-blinded, placebo-controlled RCT to test post-myocardial infarction (MI) disodium EDTA infusions (weekly 3-hour infusions for 30 weeks followed by 10 infusions 2 to 8 weeks apart).86 Despite the chelation group having a modest reduction in the primary composite endpoint (hazard ratio [HR] = 0.82; 95% CI, 0.69-0.99; P = .035),86 especially in those with diabetes (HR = 0.59; 95% CI, 0.44-0.79; P < .001; number needed to treat [NNT] = 6.5),87 the authors concluded these results were insufficient to support routine disodium EDTA chelation post MI. Moreover, the 2014 Guidelines for Chronic Ischemic Heart Disease only upgraded chelation therapy from “not recommended” to “uncertain.”88
Nevertheless, to put into perspective the magnitude of chelation’s treatment effect post MI in patients with diabetes, it can be compared to standard-of-care statin RCTs for secondary prevention, reported as follows. The absolute risk reduction (ARR) of coronary events with statin therapy in a high genetic risk group (the group with the greatest ARR) was 6.03% in the CARE trial and 6.87% in the PROVE IT-TIMI 22 trial, corresponding to a calculated NNT (1/ARR) of 16.6 and 14.6 respectively.89
In order to understand the hesitancy to accept chelation therapy as a reasonable CVD treatment,59 one must balance the modest effect demonstrated by TACT with its limitations, the greatest of which was the unusually large proportion of patients who withdrew their consent or were lost to follow-up (~18%).86 However, it is important to note that some patients withdrew after experiencing a primary endpoint and that at least 50% more were from the placebo group than the chelation group,86 rather than the reverse that was erroneously reported by Fihn et al.88 Unblinding was possible, but there were no significant differences in serious adverse events between groups.
Furthermore, since withdrawn subjects had similar CVD risk profiles, more withdrawals in the placebo arm increases the likelihood of more unrecorded primary outcome events in the placebo arm than treatment arm. One possibility is that these could have accentuated the benefit of chelation therapy, and that missing data reduced the reported efficacy. Because of TACT’s profound findings for those with diabetes post MI, the TACT2 Phase 3 clinical trial underway through 2022 will further clarify chelation’s utility for treating stable ischemic heart disease specifically in this group.
Interestingly, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldose reductase inhibitors themselves inhibit advanced glycation end products (AGE) formation in diabetes, most likely via chelation. Increased protein glycation and accumulation of AGEs on tissue proteins during hyperglycemia (the Maillard reaction) are hypothesized to be fundamental in the pathogenesis of diabetic vascular complications. Consequently, chronic, low-dose chelation therapy may have a role in preventing and treating diabetic CVD and nephropathy.90
ACKNOWLEDGEMENT
The authors thank Ariel Pomputius, MLIS, for contributing to the literature searches during manuscript preparation.
CORRESPONDENCE
Frank A. Orlando, MD, UF Health Family Medicine – Springhill, 4197 NW 86th Terrace; [email protected]
1. The Institute for Functional Medicine. 2020. Accessed November 19, 2021. www.ifm.org/
2. Rippe JM, ed. Lifestyle Medicine. 3rd ed. CRC Press, Taylor & Francis Group; 2019.
3. Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-252.
4. Ali A, Katz DL. Disease prevention and health promotion: how integrative medicine fits. Am J Prev Med. 2015;49(5 suppl 3):S230-S240.
5. Bland J. Defining function in the functional medicine model. Integr Med (Encinitas). 2017;16:22-25.
6. ABPS. Integrative medicine examination description. 2020. Accessed November 19, 2021. www.abpsus.org/integrative-medicine-description/
7. Bland JS. The natural roots of functional medicine. Integr Med (Encinitas). 2018;17:12-17.
8. Beidelschies M, Alejandro-Rodriguez M, Ji X, et al. Association of the functional medicine model of care with patient-reported health-related quality-of-life outcomes. JAMA Netw Open. 2019;2:e1914017.
9. The Institute for Functional Medicine. Functional medicine matrix: organizing clinical imbalances. 2020. Accessed November 19, 2021. www.ifm.org/news-insights/toolkit-functional-medicine-matrix/
10. Schadt EE, Björkegren JL. NEW: Network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4:115rv1.
11. Curry SJ, Krist AH, Owens DK, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:272-280.
12. Sprangers MA, Thong MS, Bartels M, et al. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: an update. Qual Life Res. 2014;23:1997-2013.
13. Bland J. Functional medicine: an operating system for integrative medicine. Integr Med (Encinitas). 2015;14:18-20.
14. Cutshall SM, Bergstrom LR, Kalish DJ. Evaluation of a functional medicine approach to treating fatigue, stress, and digestive issues in women. Complement Ther Clin Pract. 2016;23:75-81.
15. Jaffe R. First line comprehensive care. Part II: Anthropogenic xenobiotics in functional medicine. Managing persisting bioaccumulating pollutants: toxic minerals, biocides, hormone mimics, solvents, and chemical disruptors. Semin Integr Med. 2005;3:79-92.
16. Muran PJ, Muran SY, Beseler CL, et al. Breast health and reducing breast cancer risk: a functional medicine approach. J Altern Complement Med. 2015;21:321-326.
17. Bird JK, Murphy RA, Ciappio ED, et al. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9:655.
18. ter Borg S, Verlaan S, Hemsworth J, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195-1206.
19. Moyer VA, on behalf of the USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558-564.
20. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319:1592-1599.
21. Fenercioglu AK, Saler T, Genc E, et al. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33:118-124.
22. Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: A systematic review. Nutr Rev. 2018;76:60-76.
23. Schmölz L, Birringer M, Lorkowski S, et al. Complexity of vitamin E metabolism. World J Biol Chem. 2016;7:14-43.
24. Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403-419.
25. Gurley BJ, Tonsing-Carter A, Thomas SL, et al. Clinically relevant herb-micronutrient interactions: when botanicals, minerals, and vitamins collide. Adv Nutr. 2018;9:524s-532s.
26. Zeilstra D, Younes JA, Brummer RJ, et al. Perspective: fundamental limitations of the randomized controlled trial method in nutritional research: the example of probiotics. Adv Nutr. 2018;9:561-571.
27. Kimokoti RW, Millen BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. 2016;100:1185-1198.
28. Tucker KL, Smith CE, Lai CQ, et al. Quantifying diet for nutrigenomic studies. Annu Rev Nutr. 2013;33:349-371.
29. Fenech M, El-Sohemy A, Cahill L, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69-89.
30. van Ommen B, van den Broek T, de Hoogh I, et al. Systems biology of personalized nutrition. Nutr Rev. 2017;75:579-599.
31. Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343-1347.
32. Mathieson I, Lazaridis I, Rohland N, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499-503.
33. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578-588.
34. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54.
35. Li Y, Huang T, Zheng Y, et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768.
36. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612.
37. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73.
38. USPSTF. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
39. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. JAMA. 2017;317:183-189.
40. Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. 2016;25:901-911.
41. Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17:191.
42. Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177.
43. Kölling K, Ndrepepa G, Koch W, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms, plasma homocysteine, folate, and vitamin B12 levels and the extent of coronary artery disease. Am J Cardiol. 2004;93:1201-1206.
44. Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet. 2011;378:584-594.
45. Chang G, Kuai Z, Wang J, et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr. 2019;19:331.
46. Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325-1335.
47. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30:17-25.
48. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64.
49. Van den Nieuwboer M, Brummer RJ, Guarner F, et al. The administration of probiotics and synbiotics in immune compromised adults: Is it safe? Benef Microbes. 2015;6:3-17.
50. Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58(suppl 10):S164-S179.
51. O’Connell TM. The application of metabolomics to probiotic and prebiotic interventions in human clinical studies. Metabolites. 2020;10:120.
52. Lerner A, Shoenfeld Y, Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms. 2019;7:104.
53. Su G, Ko C, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterol. 2020;159:697-705.
54. Preidis GA, Weizman AV, Kashyap PC, et al. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159:708-738.e4.
55. Charoenngam N, Shirvani A, Kalajian TA, et al. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer Res. 2020;40:551-556.
56. Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, et al. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10(suppl1):S17-S30.
57. APA. Clay RA. More than one way to measure. Monitor Psychol. 2010;4:52. Accessed November 19, 2021. www.apa.org/monitor/2010/09/trials
58. Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2-21.
59. Bauchner H, Fontanarosa PB, Golub RM. Evaluation of the trial to assess chelation therapy (TACT): the scientific process, peer review, and editorial scrutiny. JAMA. 2013;309:1291-1292.
60. Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20-40.
61. Matsumoto M, Kitada Y, Naito Y. Endothelial function is improved by inducing microbial polyamine production in the gut: a randomized placebo-controlled trial. Nutrients. 2019;11:1188.
62. Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496.
63. Vieira AT, Castelo PM, Ribeiro DA, et al. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. 2017;8:1884.
64. Ribeiro AE, Monteiro NES, Moraes AVG, et al. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2018;26:643-652.
65. Hong YS, Hong KS, Park MH, et al. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415-425.
66. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterol. 2017;152:1889-1900.e9.
67. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091-2103.
68. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 2019;11:638-647.
69. Liu M-M, Li S-T, Shu Y, et al. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0178870.
70. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245-1249.
71. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37-43.
72. Wang HT, Anvari S, Anagnostou K. The role of probiotics in preventing allergic disease. Children (Basel). 2019;6:24.
73. Kasatpibal N, Whitney JD, Saokaew S, et al. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl2):S153-S160.
74. Liu PC, Yan YK, Ma YJ, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075.
75. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr. 2018;37:532-541.
76. Wu Y, Zhang Q, Ren Y, et al. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12:e0178868.
77. Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients. 2016;8:397.
78. Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232:654-666.
79. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1-23.
80. Chowdhury R, Ramond A, O’Keeffe LM, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310.
81. Zhuang X, Ni A, Liao L, et al. Environment-wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis. 2018;269:172-177.
82. Wax PM. Current use of chelation in American health care. J Med Toxicol. 2013:9;303-307.
83. CDC. Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006;55:204-207.
84. Atwood KC, Woeckner E. In pediatric fatality, edetate disodium was no accident. Clin Toxicol (Phila). 2009;47:256.
85. Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol (Phila). 2008;46:1083-1084.
86. Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241-1250.
87. Escolar E, Lamas GA, Mark DB, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15-24.
88. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949.
89. Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264-2271.
90. Nagai R, Murray DB, Metz TO, et al. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549-559.
Could screening patients for cytokine markers help direct interventions to prevent quality-of-life deterioration? What evidence is there that a patient’s methylenetetrahydrofolate reductase (MTHFR) genotype and baseline folate level can determine whether folate therapy will be needed to prevent stroke?
Favorable findings in these areas and others—eg, that specific probiotics benefit those with various gastrointestinal, respiratory, and lipid disorders—are strengthening support for the clinical approach of functional medicine (FM), which focuses on core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
In this article, we describe the paradigm of FM, review its origins, and present the evidence base of selected topics: diagnostic testing, nutrition and supplements, probiotics, and chelation. As FM’s popularity increases, a better understanding of it will help us educate our patients on this approach and implement some of its evidence-backed practices. In preparing this review we used keyword searches of PubMed, Embase, Web of Science, Cochrane Library, University of Florida Health Science Center Library eJournals, the Institute for Functional Medicine website,1 and Lifestyle Medicine textbook.2
The core of functional medicine
Complementary and alternative medicine (CAM) refers to medical practices diverging from standards of care and not generally taught at US medical schools or available at US hospitals.3 Integrative Medicine encompasses evidence-informed CAM, conventional Western medicine, and whole systems like FM.4
FM aims to identify root causes of disease, emphasizing function as a dynamic process that can move back and forth on a continuum between health and disorder.5,6 There are 7 defining characteristics of FM: (1) patient centered vs disease centered; (2) systems biology approach acknowledging web-like interconnections of physiologic factors; (3) dynamic balance of gene–environment interactions; (4) personalized care based on biochemical individuality; (5) promotion of organ reserve and sustained health span; (6) health as a positive vitality—not merely the absence of disease; and (7) function vs pathology focused.5
The concept of FM is not new. Its origins can be traced to the 19th century. Jeffrey Bland, PhD, credits the term’s first use to Sir Willoughby F. Wade, MD, in an 1871 Lancet editorial, “Clinical lecture on functional medicine.” Bland formulated the FM paradigm and in 1991 founded the Institute for Functional Medicine (the Institute), its main educational and certifying organization.7 The Institute certifies masters- or doctorate-prepared health professionals from both conventional and complementary health fields who complete 7 courses (Applying FM in Clinical Practice, Gastrointestinal, Environmental Health, Immune, Hormone, Cardiometabolic, and Bioenergetics), present 1 case report, and pass a written exam. In a retrospective cohort study, researchers found that the FM model was associated with greater improvements in patient-reported, health-related quality of life (QOL) compared with usual care.8
Clinical model
FM uses a comprehensive yet practical matrix for obtaining patient histories and for guiding diagnostic testing.9 Tools that support history-taking include a timeline; weeklong dietary survey; daily activity log; exercise, sleep and self-care questionnaires; and an environmental risk assessment. Instead of a review of symptoms arranged by organ system as is typical with conventional Western medicine, FM assesses the balance of core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
Continue to: Within the matrix...
Within the matrix, FM also recognizes 5 modifiable personal lifestyle factors: sleep and relaxation, exercise and movement, nutrition, stress, and relationships. When these lifestyle elements are influenced by specific predisposing factors (antecedents), discrete events precipitating illness (triggers), and ongoing physiologic processes (mediators), fundamental imbalances eventually result in the signs and symptoms characterizing diagnosable diseases.
The essence of the FM therapeutic plan is a discussion of lifestyle changes, personal strengths, and potential adherence challenges so the clinician can better offer assistive resources, which may include multidisciplinary referrals to personalized counselors such as a nutritionist, health coach, mind-body therapist, personal trainer, exercise physiologist, or physical therapist. While these professionals are not unique to FM, they are more frequently used by FM practitioners as part of the health care team.
Diagnostic testing through a different lens
A proposed solution to complex diseases recalcitrant to conventional modern treatments is a systems biology approach to health care.10 Current laboratory testing such as liver enzymes, C-reactive protein (CRP), and leukocyte count provide some information on organ system homeostasis and molecular pathology, but even high-sensitivity CRP, which measures inflammation across the human system, is not adequate in cardiovascular disease (CVD) risk assessment.11 Genome-wide association studies suggest an association between biological pathways, genes, molecular markers, and QOL domains, and screening patients for cytokine markers may help provide prophylactic interventions to prevent QOL deterioration.12
With the purpose of being more systems-biology centric rather than disease centric,13 FM practitioners use tests to guide dietary recommendations and supplement selection, the evidence for which is found mostly in symptom-specific case series.14-16 Commonly used tests include MTHFR genotyping, comprehensive stool profiles, hormone and heavy metal panels, allergy panels, lactulose breath testing, micronutrient and advanced lipid panels, omega 3:6 ratios, and oxidative stress tests.
Some of these tests are performed by standard labs and are covered by insurance. Certain assays are primarily performed by specialized functional laboratories, such as comprehensive stool profiles, provoked heavy metal tests, non-IgE allergy panels, and oxidative stress tests. These laboratories also frequently offer direct-to-consumer orders. The latter situation raises a potential conflict of interest from supplement sales linked to laboratory testing and makes it imperative for health care professionals to understand the reliability and clinical utility of these tests in order to counsel patients accordingly.
Continue to: The remainder of this article...
The remainder of this article focuses on the evidence behind a subset of FM treatments, which typically include various dietary interventions (elimination, cardiometabolic, detox) and multidomain lifestyle modifications. The practitioner’s selection of dietary interventions, nutraceuticals (vitamins, minerals, essential fatty acids, botanicals), and probiotics is informed by results of different diagnostic tests.
Nutrition and supplements
Nearly one-third of older Americans are affected by at least 1 vitamin deficiency or anemia, and even those consuming an adequate diet have a substantial risk of any deficiency (16%), although less so than those with an inadequate diet (57%).17 The Western diet is known to be nutrient deficient, particularly in vitamin D, thiamine, riboflavin, calcium, magnesium, and selenium.18 Dietary supplement nonusers have the highest risk of any deficiency (40%) compared with users of “full-spectrum multivitamin-multimineral supplements” (14%) and other dietary supplement users (28%).17
Nevertheless, the US Preventive Services Task Force (USPSTF) concluded in 2014 that there are not enough data to make a recommendation for or against taking vitamins A, C, or E; multivitamins with folic acid; or combinations of these vitamins for the primary prevention of CVD or cancer.19 USPSTF also does not recommend daily vitamin D and calcium supplementation in community-dwelling, postmenopausal women for primary prevention of fracture.20 Notwithstanding the lack of supplement recommendations for primary prevention, their benefits in patients with chronic disease is still being investigated. For example, a polyphenol-rich antioxidant may reduce cardiovascular complications in those with diabetes.21
Rethinking how nutrition studies are designed. Drawing on studies to determine the benefits of nutrition in chronic disease has been challenging. Factors that must be taken into account include the types of vitamin and mineral supplements patients use, nutrient absorption and utilization, and differing dietary assessment methods and reference values used.18 For example, vitamin and mineral absorption work best with whole food or fortified diets wherein specific nutrients are consumed together (eg, vitamin D and vitamin E with fat; non-heme iron with vitamin C).22-24 Foods with competing nutrients or “inhibitors” may even require absorption enhancers at minimum molar ratios.24
Adding to the complexity of vitamin and mineral absorption, botanical dietary supplements have their own modifying effects on micronutrient absorption.25 These are just some of the reasons randomized controlled trials (RCTs) are fundamentally limited when investigating the health outcomes of diet and supplementation, and alternative study methods should be considered for future nutrition clinical trials to better inform clinicians who are prescribing supplements.26 In the meantime, nutrition plans can be individualized to patients’ biological and cultural needs, ideally in conjunction with a multidisplinary team that includes dietitians, behaviorists, and exercise specialists.27
Continue to: Nutrition is one of the most...
Nutrition is one of the most important environmental factors modulating genes and phenotypes (nutrigenomics),28,29 and recent studies on single nucleotide polymorphisms (SNPs) have reinforced the importance of considering the effect of genetic variation on dietary response (nutrigenetics).29,30 While nutrigenomics and nutrigenetics have the potential to improve the health of large populations by personalizing dietary advice based on genotype and phenotype, genetic variation occurring within a specific biochemical pathway makes generalizing genotype-based dietary advice to other populations complex.
For instance, Greenlandic Inuit, the indigenous people inhabiting the Arctic regions of Greenland, have a low incidence of CVD, largely due to “non-European” genetic variants that lower their LDL, protecting them from the oxidative stress of their diet high in polyunsaturated fats.31,32 In a study of European adults, phenotypic and phenotypic-plus-genotypic information did not enhance the effectiveness of the personalized nutrition advice, demonstrating that more research is needed in larger populations.33
Another specific, complex example of gene-nutrient interaction is the pathophysiologic outcome of polymorphisms in the MTHFR gene.30 MTHFR is a rate-limiting enzyme involved in folate and homocysteine metabolism, DNA and RNA biosynthesis, and DNA and protein methylation. MTHFR polymorphisms are common in otherwise healthy people, but some have been reported to increase chronic disease susceptibility via MTHFR deficiency. The most common MTHFR variant is the SNP rs1801133 that reduces enzyme activity to ~30% in homozygotes (677TT), which can lead to reduced folate bioavailability and mild-to-moderate hyperhomocysteinemia depending on one’s dietary folate intake and food fortification.
One FM focus at variance with current recommendations. FM’s approach to homocysteine and MTHFR genotyping for nutritional assessment, dietary counseling, and supplement advice contrasts with recommendations from meta-analyses34-36 and guidelines from the American Heart Association and USPSTF.37,38 While USPSTF does recommend all women planning or capable of pregnancy take 0.4 to 0.8 mg of folic acid daily to prevent neural tube defects,39MTHFR polymorphism screening to guide supplementation is not recommended during pregnancy given conflicting studies and uncertain clinical significance.40 Moreover, while it is generally agreed that the MTHFR C677T polymorphism, and in particular the homozygous MTHFR 677TT genotype, is an independent risk factor for hyperhomocysteinemia, neither variant genotype was an independent coronary artery disease risk factor in several large studies.41-43
However, study populations with generally high folate consumption or fortified foods may be a main confounding variable.44 For example, in a recent meta-analysis of an elderly population, the T-allele of the MTHFR C677T variant increased pooled stroke risk. While this increased stroke risk is highest in homozygotes, the association was statistically significant only in the Chinese cohort—a group that generally has poor dietary intake of folate and B12.45 Therefore, both MTHFR genotype and baseline folate level are important determinants of folate therapy efficacy in stroke prevention, and this may explain why US studies have not clearly identified a patient subset most likely to benefit.46 Future high-quality studies should measure baseline folate, target populations with moderate-to-severe hyperhomocysteinemia with specific MTHFR polymorphisms, and compare high- and low-dose B vitamins or use 5-methylfolate (the active form of folate).
Continue to: Probiotics
Probiotics
Probiotics are used extensively in FM, and there are very few fields in conventional Western medicine where probiotics have not been researched. Interestingly, gut microbiota (microflora) change rapidly and individually to therapeutic dietary changes, both in composition (community) and function (metabolic plasticity), implicating gut microbiota as a mediator of dietary impact on host metabolism.47 This highlights the potential for tailored probiotics to transform dietary nonresponders (eg, those who do not routinely consume a high-fiber diet) into responders whose metabolism becomes enabled to counter such conditions as obesity and type 2 diabetes.48
Caveats with probiotic administration. While the strength of recommendation for probiotic therapy is increased by their safe use in pregnancy, infants, and immunocompromised populations,49-51 various harms (ranging from mild to severe) may be underreported.52-54 In clinical practice, the effective probiotic strain(s), formulation, and dosage will vary not only by disease, but also from patient to patient, and may be dependent on nutritional factors such as vitamin D,55 diet, and even epigenetics.56
As we come to more fully recognize commensal microbiota as a major player in overall health with crosstalk in signaling pathways between intestinal bacterial and epithelial immune cells, future research is needed to help optimize probiotic dose, duration, route of administration, and whether microbial communities outperform single-species probiotics.50 Furthermore, much of a bacterial community’s effect on the host is through its metabolites, and since certain species can be used interchangeably given similar metabolic activity and function, studies to understand how prebiotics and probiotics affect the host must analyze the metabolome, in addition to the bacterial community composition.51
Alternative research methods could be informative. Similar to their limited evaluation of health outcomes in nutrition and supplement research, many RCTs examining the health benefits of probiotics often yield ambiguous results or fall short of valid conclusions because the underlying presuppositions are not met.26 For example, assuming the RCT uses a well-defined probiotic formulation, efficacy and generalizability can still be confounded by patient microbiota, diet, mucosa, immune system, and emotional status, which all affect probiotic activity/potency.50 Under these circumstances, other research methods may be more suitable or supplemental—eg, Phase II trials, epidemiologic studies, single-case experiments (n-of-1 trials) and their meta-analyses, using historical controls, preclinical studies for conceptual and theoretical development, and clinical experience.26,57-59
It is currently unknown how nonvaginal microbiota affect the health of menopausal women;63,64 and while a multicenter RCT is underway to examine a novel probiotic’s effect on menopausal symptoms and bone health, the supplemental study methods listed above could be employed as well.
Continue to: Evidence of probiotic effectiveness
Evidence of probiotic effectiveness. Positive evidence in RCTs and meta-analyses suggests that strain-specific probiotics are beneficial for infectious gastroenteritis, persistent antibiotic-associated diarrhea, infant colic,50 irritable bowel syndrome (IBS),50,65Clostridioides difficile prevention,66 inflammatory bowel disease (IBD),67 radiation-induced diarrhea,68,69 and nongastrointestinal conditions such as multiple sclerosis,70 upper respiratory infections,50 atopic dermatitis,50,71,72 surgical site infections,73,74 hyperlipidemia,50,75,76 and nonalcoholic steatohepatitis.77
However, because of knowledge gaps and low-quality evidence, the American Gastroenterological Association’s (AGA) 2020 Clinical Practice Guidelines recommend “conditional” use of strain-specific probiotics only for necrotizing enterocolitis prevention (in pre-term, low-birth-weight infants), antibiotic-induced C difficile prevention, and pouchitis.53,54 Additionally, the AGA’s guidelines state that probiotics should only be used for C difficile infection, IBD, or IBS in a clinical trial and not used at all for infectious gastroenteritis.53,54
Chelation
Chelation therapy is thought to inhibit metal-catalyzed oxidation reactions and inflammatory processes in tissues that lead to, and result from, accumulation of oxidative damage. While many FM practitioners recommend some form of chelation therapy, there is much controversy surrounding its use. Well-designed, large-scale clinical trials continue to emerge for what appears to be an old intervention with still uncertain clinical applications.
The first chelation agent, disodium ethylenediaminetetraacetic acid (EDTA), was synthesized in the early 1930s, but it was not until World War II that chelation therapy began with use of dimercaprol, a potent antidote for the arsenical chemical weapon lewisite. Disodium EDTA infusions were first used to treat angina pectoris in the mid-20th century,78 and use accelerated in the early 21st century without a clear indication to treat CVD besides decades of anecdotes and case reports.79 Strong epidemiologic data support a causal association between heavy metals (arsenic, lead, cadmium, mercury, and copper) and CVD,80,81 but a distinct mechanism of action is not yet known.
Disodium EDTA was FDA approved in the 1950s for use in patients with hypercalcemia or ventricular arrhythmias from digitalis toxicity, but it was eventually withdrawn from the market in 2008, in part due to safety concerns following 3 deaths from severe hypocalcemia.82 These deaths were caused by inappropriate use related to rapid infusions, pediatric use, and in one case inadvertently administering disodium EDTA to a child instead of calcium EDTA (which can safely be bolused to treat pediatric lead toxicity).82-85
Continue to: The Trial to Assess Chelation Therapy...
The Trial to Assess Chelation Therapy (TACT) was the first large, double-blinded, placebo-controlled RCT to test post-myocardial infarction (MI) disodium EDTA infusions (weekly 3-hour infusions for 30 weeks followed by 10 infusions 2 to 8 weeks apart).86 Despite the chelation group having a modest reduction in the primary composite endpoint (hazard ratio [HR] = 0.82; 95% CI, 0.69-0.99; P = .035),86 especially in those with diabetes (HR = 0.59; 95% CI, 0.44-0.79; P < .001; number needed to treat [NNT] = 6.5),87 the authors concluded these results were insufficient to support routine disodium EDTA chelation post MI. Moreover, the 2014 Guidelines for Chronic Ischemic Heart Disease only upgraded chelation therapy from “not recommended” to “uncertain.”88
Nevertheless, to put into perspective the magnitude of chelation’s treatment effect post MI in patients with diabetes, it can be compared to standard-of-care statin RCTs for secondary prevention, reported as follows. The absolute risk reduction (ARR) of coronary events with statin therapy in a high genetic risk group (the group with the greatest ARR) was 6.03% in the CARE trial and 6.87% in the PROVE IT-TIMI 22 trial, corresponding to a calculated NNT (1/ARR) of 16.6 and 14.6 respectively.89
In order to understand the hesitancy to accept chelation therapy as a reasonable CVD treatment,59 one must balance the modest effect demonstrated by TACT with its limitations, the greatest of which was the unusually large proportion of patients who withdrew their consent or were lost to follow-up (~18%).86 However, it is important to note that some patients withdrew after experiencing a primary endpoint and that at least 50% more were from the placebo group than the chelation group,86 rather than the reverse that was erroneously reported by Fihn et al.88 Unblinding was possible, but there were no significant differences in serious adverse events between groups.
Furthermore, since withdrawn subjects had similar CVD risk profiles, more withdrawals in the placebo arm increases the likelihood of more unrecorded primary outcome events in the placebo arm than treatment arm. One possibility is that these could have accentuated the benefit of chelation therapy, and that missing data reduced the reported efficacy. Because of TACT’s profound findings for those with diabetes post MI, the TACT2 Phase 3 clinical trial underway through 2022 will further clarify chelation’s utility for treating stable ischemic heart disease specifically in this group.
Interestingly, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldose reductase inhibitors themselves inhibit advanced glycation end products (AGE) formation in diabetes, most likely via chelation. Increased protein glycation and accumulation of AGEs on tissue proteins during hyperglycemia (the Maillard reaction) are hypothesized to be fundamental in the pathogenesis of diabetic vascular complications. Consequently, chronic, low-dose chelation therapy may have a role in preventing and treating diabetic CVD and nephropathy.90
ACKNOWLEDGEMENT
The authors thank Ariel Pomputius, MLIS, for contributing to the literature searches during manuscript preparation.
CORRESPONDENCE
Frank A. Orlando, MD, UF Health Family Medicine – Springhill, 4197 NW 86th Terrace; [email protected]
Could screening patients for cytokine markers help direct interventions to prevent quality-of-life deterioration? What evidence is there that a patient’s methylenetetrahydrofolate reductase (MTHFR) genotype and baseline folate level can determine whether folate therapy will be needed to prevent stroke?
Favorable findings in these areas and others—eg, that specific probiotics benefit those with various gastrointestinal, respiratory, and lipid disorders—are strengthening support for the clinical approach of functional medicine (FM), which focuses on core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
In this article, we describe the paradigm of FM, review its origins, and present the evidence base of selected topics: diagnostic testing, nutrition and supplements, probiotics, and chelation. As FM’s popularity increases, a better understanding of it will help us educate our patients on this approach and implement some of its evidence-backed practices. In preparing this review we used keyword searches of PubMed, Embase, Web of Science, Cochrane Library, University of Florida Health Science Center Library eJournals, the Institute for Functional Medicine website,1 and Lifestyle Medicine textbook.2
The core of functional medicine
Complementary and alternative medicine (CAM) refers to medical practices diverging from standards of care and not generally taught at US medical schools or available at US hospitals.3 Integrative Medicine encompasses evidence-informed CAM, conventional Western medicine, and whole systems like FM.4
FM aims to identify root causes of disease, emphasizing function as a dynamic process that can move back and forth on a continuum between health and disorder.5,6 There are 7 defining characteristics of FM: (1) patient centered vs disease centered; (2) systems biology approach acknowledging web-like interconnections of physiologic factors; (3) dynamic balance of gene–environment interactions; (4) personalized care based on biochemical individuality; (5) promotion of organ reserve and sustained health span; (6) health as a positive vitality—not merely the absence of disease; and (7) function vs pathology focused.5
The concept of FM is not new. Its origins can be traced to the 19th century. Jeffrey Bland, PhD, credits the term’s first use to Sir Willoughby F. Wade, MD, in an 1871 Lancet editorial, “Clinical lecture on functional medicine.” Bland formulated the FM paradigm and in 1991 founded the Institute for Functional Medicine (the Institute), its main educational and certifying organization.7 The Institute certifies masters- or doctorate-prepared health professionals from both conventional and complementary health fields who complete 7 courses (Applying FM in Clinical Practice, Gastrointestinal, Environmental Health, Immune, Hormone, Cardiometabolic, and Bioenergetics), present 1 case report, and pass a written exam. In a retrospective cohort study, researchers found that the FM model was associated with greater improvements in patient-reported, health-related quality of life (QOL) compared with usual care.8
Clinical model
FM uses a comprehensive yet practical matrix for obtaining patient histories and for guiding diagnostic testing.9 Tools that support history-taking include a timeline; weeklong dietary survey; daily activity log; exercise, sleep and self-care questionnaires; and an environmental risk assessment. Instead of a review of symptoms arranged by organ system as is typical with conventional Western medicine, FM assesses the balance of core functional processes: assimilation, defense and repair, energy, biotransformation and elimination, transport, communication, and structural integrity.
Continue to: Within the matrix...
Within the matrix, FM also recognizes 5 modifiable personal lifestyle factors: sleep and relaxation, exercise and movement, nutrition, stress, and relationships. When these lifestyle elements are influenced by specific predisposing factors (antecedents), discrete events precipitating illness (triggers), and ongoing physiologic processes (mediators), fundamental imbalances eventually result in the signs and symptoms characterizing diagnosable diseases.
The essence of the FM therapeutic plan is a discussion of lifestyle changes, personal strengths, and potential adherence challenges so the clinician can better offer assistive resources, which may include multidisciplinary referrals to personalized counselors such as a nutritionist, health coach, mind-body therapist, personal trainer, exercise physiologist, or physical therapist. While these professionals are not unique to FM, they are more frequently used by FM practitioners as part of the health care team.
Diagnostic testing through a different lens
A proposed solution to complex diseases recalcitrant to conventional modern treatments is a systems biology approach to health care.10 Current laboratory testing such as liver enzymes, C-reactive protein (CRP), and leukocyte count provide some information on organ system homeostasis and molecular pathology, but even high-sensitivity CRP, which measures inflammation across the human system, is not adequate in cardiovascular disease (CVD) risk assessment.11 Genome-wide association studies suggest an association between biological pathways, genes, molecular markers, and QOL domains, and screening patients for cytokine markers may help provide prophylactic interventions to prevent QOL deterioration.12
With the purpose of being more systems-biology centric rather than disease centric,13 FM practitioners use tests to guide dietary recommendations and supplement selection, the evidence for which is found mostly in symptom-specific case series.14-16 Commonly used tests include MTHFR genotyping, comprehensive stool profiles, hormone and heavy metal panels, allergy panels, lactulose breath testing, micronutrient and advanced lipid panels, omega 3:6 ratios, and oxidative stress tests.
Some of these tests are performed by standard labs and are covered by insurance. Certain assays are primarily performed by specialized functional laboratories, such as comprehensive stool profiles, provoked heavy metal tests, non-IgE allergy panels, and oxidative stress tests. These laboratories also frequently offer direct-to-consumer orders. The latter situation raises a potential conflict of interest from supplement sales linked to laboratory testing and makes it imperative for health care professionals to understand the reliability and clinical utility of these tests in order to counsel patients accordingly.
Continue to: The remainder of this article...
The remainder of this article focuses on the evidence behind a subset of FM treatments, which typically include various dietary interventions (elimination, cardiometabolic, detox) and multidomain lifestyle modifications. The practitioner’s selection of dietary interventions, nutraceuticals (vitamins, minerals, essential fatty acids, botanicals), and probiotics is informed by results of different diagnostic tests.
Nutrition and supplements
Nearly one-third of older Americans are affected by at least 1 vitamin deficiency or anemia, and even those consuming an adequate diet have a substantial risk of any deficiency (16%), although less so than those with an inadequate diet (57%).17 The Western diet is known to be nutrient deficient, particularly in vitamin D, thiamine, riboflavin, calcium, magnesium, and selenium.18 Dietary supplement nonusers have the highest risk of any deficiency (40%) compared with users of “full-spectrum multivitamin-multimineral supplements” (14%) and other dietary supplement users (28%).17
Nevertheless, the US Preventive Services Task Force (USPSTF) concluded in 2014 that there are not enough data to make a recommendation for or against taking vitamins A, C, or E; multivitamins with folic acid; or combinations of these vitamins for the primary prevention of CVD or cancer.19 USPSTF also does not recommend daily vitamin D and calcium supplementation in community-dwelling, postmenopausal women for primary prevention of fracture.20 Notwithstanding the lack of supplement recommendations for primary prevention, their benefits in patients with chronic disease is still being investigated. For example, a polyphenol-rich antioxidant may reduce cardiovascular complications in those with diabetes.21
Rethinking how nutrition studies are designed. Drawing on studies to determine the benefits of nutrition in chronic disease has been challenging. Factors that must be taken into account include the types of vitamin and mineral supplements patients use, nutrient absorption and utilization, and differing dietary assessment methods and reference values used.18 For example, vitamin and mineral absorption work best with whole food or fortified diets wherein specific nutrients are consumed together (eg, vitamin D and vitamin E with fat; non-heme iron with vitamin C).22-24 Foods with competing nutrients or “inhibitors” may even require absorption enhancers at minimum molar ratios.24
Adding to the complexity of vitamin and mineral absorption, botanical dietary supplements have their own modifying effects on micronutrient absorption.25 These are just some of the reasons randomized controlled trials (RCTs) are fundamentally limited when investigating the health outcomes of diet and supplementation, and alternative study methods should be considered for future nutrition clinical trials to better inform clinicians who are prescribing supplements.26 In the meantime, nutrition plans can be individualized to patients’ biological and cultural needs, ideally in conjunction with a multidisplinary team that includes dietitians, behaviorists, and exercise specialists.27
Continue to: Nutrition is one of the most...
Nutrition is one of the most important environmental factors modulating genes and phenotypes (nutrigenomics),28,29 and recent studies on single nucleotide polymorphisms (SNPs) have reinforced the importance of considering the effect of genetic variation on dietary response (nutrigenetics).29,30 While nutrigenomics and nutrigenetics have the potential to improve the health of large populations by personalizing dietary advice based on genotype and phenotype, genetic variation occurring within a specific biochemical pathway makes generalizing genotype-based dietary advice to other populations complex.
For instance, Greenlandic Inuit, the indigenous people inhabiting the Arctic regions of Greenland, have a low incidence of CVD, largely due to “non-European” genetic variants that lower their LDL, protecting them from the oxidative stress of their diet high in polyunsaturated fats.31,32 In a study of European adults, phenotypic and phenotypic-plus-genotypic information did not enhance the effectiveness of the personalized nutrition advice, demonstrating that more research is needed in larger populations.33
Another specific, complex example of gene-nutrient interaction is the pathophysiologic outcome of polymorphisms in the MTHFR gene.30 MTHFR is a rate-limiting enzyme involved in folate and homocysteine metabolism, DNA and RNA biosynthesis, and DNA and protein methylation. MTHFR polymorphisms are common in otherwise healthy people, but some have been reported to increase chronic disease susceptibility via MTHFR deficiency. The most common MTHFR variant is the SNP rs1801133 that reduces enzyme activity to ~30% in homozygotes (677TT), which can lead to reduced folate bioavailability and mild-to-moderate hyperhomocysteinemia depending on one’s dietary folate intake and food fortification.
One FM focus at variance with current recommendations. FM’s approach to homocysteine and MTHFR genotyping for nutritional assessment, dietary counseling, and supplement advice contrasts with recommendations from meta-analyses34-36 and guidelines from the American Heart Association and USPSTF.37,38 While USPSTF does recommend all women planning or capable of pregnancy take 0.4 to 0.8 mg of folic acid daily to prevent neural tube defects,39MTHFR polymorphism screening to guide supplementation is not recommended during pregnancy given conflicting studies and uncertain clinical significance.40 Moreover, while it is generally agreed that the MTHFR C677T polymorphism, and in particular the homozygous MTHFR 677TT genotype, is an independent risk factor for hyperhomocysteinemia, neither variant genotype was an independent coronary artery disease risk factor in several large studies.41-43
However, study populations with generally high folate consumption or fortified foods may be a main confounding variable.44 For example, in a recent meta-analysis of an elderly population, the T-allele of the MTHFR C677T variant increased pooled stroke risk. While this increased stroke risk is highest in homozygotes, the association was statistically significant only in the Chinese cohort—a group that generally has poor dietary intake of folate and B12.45 Therefore, both MTHFR genotype and baseline folate level are important determinants of folate therapy efficacy in stroke prevention, and this may explain why US studies have not clearly identified a patient subset most likely to benefit.46 Future high-quality studies should measure baseline folate, target populations with moderate-to-severe hyperhomocysteinemia with specific MTHFR polymorphisms, and compare high- and low-dose B vitamins or use 5-methylfolate (the active form of folate).
Continue to: Probiotics
Probiotics
Probiotics are used extensively in FM, and there are very few fields in conventional Western medicine where probiotics have not been researched. Interestingly, gut microbiota (microflora) change rapidly and individually to therapeutic dietary changes, both in composition (community) and function (metabolic plasticity), implicating gut microbiota as a mediator of dietary impact on host metabolism.47 This highlights the potential for tailored probiotics to transform dietary nonresponders (eg, those who do not routinely consume a high-fiber diet) into responders whose metabolism becomes enabled to counter such conditions as obesity and type 2 diabetes.48
Caveats with probiotic administration. While the strength of recommendation for probiotic therapy is increased by their safe use in pregnancy, infants, and immunocompromised populations,49-51 various harms (ranging from mild to severe) may be underreported.52-54 In clinical practice, the effective probiotic strain(s), formulation, and dosage will vary not only by disease, but also from patient to patient, and may be dependent on nutritional factors such as vitamin D,55 diet, and even epigenetics.56
As we come to more fully recognize commensal microbiota as a major player in overall health with crosstalk in signaling pathways between intestinal bacterial and epithelial immune cells, future research is needed to help optimize probiotic dose, duration, route of administration, and whether microbial communities outperform single-species probiotics.50 Furthermore, much of a bacterial community’s effect on the host is through its metabolites, and since certain species can be used interchangeably given similar metabolic activity and function, studies to understand how prebiotics and probiotics affect the host must analyze the metabolome, in addition to the bacterial community composition.51
Alternative research methods could be informative. Similar to their limited evaluation of health outcomes in nutrition and supplement research, many RCTs examining the health benefits of probiotics often yield ambiguous results or fall short of valid conclusions because the underlying presuppositions are not met.26 For example, assuming the RCT uses a well-defined probiotic formulation, efficacy and generalizability can still be confounded by patient microbiota, diet, mucosa, immune system, and emotional status, which all affect probiotic activity/potency.50 Under these circumstances, other research methods may be more suitable or supplemental—eg, Phase II trials, epidemiologic studies, single-case experiments (n-of-1 trials) and their meta-analyses, using historical controls, preclinical studies for conceptual and theoretical development, and clinical experience.26,57-59
It is currently unknown how nonvaginal microbiota affect the health of menopausal women;63,64 and while a multicenter RCT is underway to examine a novel probiotic’s effect on menopausal symptoms and bone health, the supplemental study methods listed above could be employed as well.
Continue to: Evidence of probiotic effectiveness
Evidence of probiotic effectiveness. Positive evidence in RCTs and meta-analyses suggests that strain-specific probiotics are beneficial for infectious gastroenteritis, persistent antibiotic-associated diarrhea, infant colic,50 irritable bowel syndrome (IBS),50,65Clostridioides difficile prevention,66 inflammatory bowel disease (IBD),67 radiation-induced diarrhea,68,69 and nongastrointestinal conditions such as multiple sclerosis,70 upper respiratory infections,50 atopic dermatitis,50,71,72 surgical site infections,73,74 hyperlipidemia,50,75,76 and nonalcoholic steatohepatitis.77
However, because of knowledge gaps and low-quality evidence, the American Gastroenterological Association’s (AGA) 2020 Clinical Practice Guidelines recommend “conditional” use of strain-specific probiotics only for necrotizing enterocolitis prevention (in pre-term, low-birth-weight infants), antibiotic-induced C difficile prevention, and pouchitis.53,54 Additionally, the AGA’s guidelines state that probiotics should only be used for C difficile infection, IBD, or IBS in a clinical trial and not used at all for infectious gastroenteritis.53,54
Chelation
Chelation therapy is thought to inhibit metal-catalyzed oxidation reactions and inflammatory processes in tissues that lead to, and result from, accumulation of oxidative damage. While many FM practitioners recommend some form of chelation therapy, there is much controversy surrounding its use. Well-designed, large-scale clinical trials continue to emerge for what appears to be an old intervention with still uncertain clinical applications.
The first chelation agent, disodium ethylenediaminetetraacetic acid (EDTA), was synthesized in the early 1930s, but it was not until World War II that chelation therapy began with use of dimercaprol, a potent antidote for the arsenical chemical weapon lewisite. Disodium EDTA infusions were first used to treat angina pectoris in the mid-20th century,78 and use accelerated in the early 21st century without a clear indication to treat CVD besides decades of anecdotes and case reports.79 Strong epidemiologic data support a causal association between heavy metals (arsenic, lead, cadmium, mercury, and copper) and CVD,80,81 but a distinct mechanism of action is not yet known.
Disodium EDTA was FDA approved in the 1950s for use in patients with hypercalcemia or ventricular arrhythmias from digitalis toxicity, but it was eventually withdrawn from the market in 2008, in part due to safety concerns following 3 deaths from severe hypocalcemia.82 These deaths were caused by inappropriate use related to rapid infusions, pediatric use, and in one case inadvertently administering disodium EDTA to a child instead of calcium EDTA (which can safely be bolused to treat pediatric lead toxicity).82-85
Continue to: The Trial to Assess Chelation Therapy...
The Trial to Assess Chelation Therapy (TACT) was the first large, double-blinded, placebo-controlled RCT to test post-myocardial infarction (MI) disodium EDTA infusions (weekly 3-hour infusions for 30 weeks followed by 10 infusions 2 to 8 weeks apart).86 Despite the chelation group having a modest reduction in the primary composite endpoint (hazard ratio [HR] = 0.82; 95% CI, 0.69-0.99; P = .035),86 especially in those with diabetes (HR = 0.59; 95% CI, 0.44-0.79; P < .001; number needed to treat [NNT] = 6.5),87 the authors concluded these results were insufficient to support routine disodium EDTA chelation post MI. Moreover, the 2014 Guidelines for Chronic Ischemic Heart Disease only upgraded chelation therapy from “not recommended” to “uncertain.”88
Nevertheless, to put into perspective the magnitude of chelation’s treatment effect post MI in patients with diabetes, it can be compared to standard-of-care statin RCTs for secondary prevention, reported as follows. The absolute risk reduction (ARR) of coronary events with statin therapy in a high genetic risk group (the group with the greatest ARR) was 6.03% in the CARE trial and 6.87% in the PROVE IT-TIMI 22 trial, corresponding to a calculated NNT (1/ARR) of 16.6 and 14.6 respectively.89
In order to understand the hesitancy to accept chelation therapy as a reasonable CVD treatment,59 one must balance the modest effect demonstrated by TACT with its limitations, the greatest of which was the unusually large proportion of patients who withdrew their consent or were lost to follow-up (~18%).86 However, it is important to note that some patients withdrew after experiencing a primary endpoint and that at least 50% more were from the placebo group than the chelation group,86 rather than the reverse that was erroneously reported by Fihn et al.88 Unblinding was possible, but there were no significant differences in serious adverse events between groups.
Furthermore, since withdrawn subjects had similar CVD risk profiles, more withdrawals in the placebo arm increases the likelihood of more unrecorded primary outcome events in the placebo arm than treatment arm. One possibility is that these could have accentuated the benefit of chelation therapy, and that missing data reduced the reported efficacy. Because of TACT’s profound findings for those with diabetes post MI, the TACT2 Phase 3 clinical trial underway through 2022 will further clarify chelation’s utility for treating stable ischemic heart disease specifically in this group.
Interestingly, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldose reductase inhibitors themselves inhibit advanced glycation end products (AGE) formation in diabetes, most likely via chelation. Increased protein glycation and accumulation of AGEs on tissue proteins during hyperglycemia (the Maillard reaction) are hypothesized to be fundamental in the pathogenesis of diabetic vascular complications. Consequently, chronic, low-dose chelation therapy may have a role in preventing and treating diabetic CVD and nephropathy.90
ACKNOWLEDGEMENT
The authors thank Ariel Pomputius, MLIS, for contributing to the literature searches during manuscript preparation.
CORRESPONDENCE
Frank A. Orlando, MD, UF Health Family Medicine – Springhill, 4197 NW 86th Terrace; [email protected]
1. The Institute for Functional Medicine. 2020. Accessed November 19, 2021. www.ifm.org/
2. Rippe JM, ed. Lifestyle Medicine. 3rd ed. CRC Press, Taylor & Francis Group; 2019.
3. Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-252.
4. Ali A, Katz DL. Disease prevention and health promotion: how integrative medicine fits. Am J Prev Med. 2015;49(5 suppl 3):S230-S240.
5. Bland J. Defining function in the functional medicine model. Integr Med (Encinitas). 2017;16:22-25.
6. ABPS. Integrative medicine examination description. 2020. Accessed November 19, 2021. www.abpsus.org/integrative-medicine-description/
7. Bland JS. The natural roots of functional medicine. Integr Med (Encinitas). 2018;17:12-17.
8. Beidelschies M, Alejandro-Rodriguez M, Ji X, et al. Association of the functional medicine model of care with patient-reported health-related quality-of-life outcomes. JAMA Netw Open. 2019;2:e1914017.
9. The Institute for Functional Medicine. Functional medicine matrix: organizing clinical imbalances. 2020. Accessed November 19, 2021. www.ifm.org/news-insights/toolkit-functional-medicine-matrix/
10. Schadt EE, Björkegren JL. NEW: Network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4:115rv1.
11. Curry SJ, Krist AH, Owens DK, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:272-280.
12. Sprangers MA, Thong MS, Bartels M, et al. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: an update. Qual Life Res. 2014;23:1997-2013.
13. Bland J. Functional medicine: an operating system for integrative medicine. Integr Med (Encinitas). 2015;14:18-20.
14. Cutshall SM, Bergstrom LR, Kalish DJ. Evaluation of a functional medicine approach to treating fatigue, stress, and digestive issues in women. Complement Ther Clin Pract. 2016;23:75-81.
15. Jaffe R. First line comprehensive care. Part II: Anthropogenic xenobiotics in functional medicine. Managing persisting bioaccumulating pollutants: toxic minerals, biocides, hormone mimics, solvents, and chemical disruptors. Semin Integr Med. 2005;3:79-92.
16. Muran PJ, Muran SY, Beseler CL, et al. Breast health and reducing breast cancer risk: a functional medicine approach. J Altern Complement Med. 2015;21:321-326.
17. Bird JK, Murphy RA, Ciappio ED, et al. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9:655.
18. ter Borg S, Verlaan S, Hemsworth J, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195-1206.
19. Moyer VA, on behalf of the USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558-564.
20. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319:1592-1599.
21. Fenercioglu AK, Saler T, Genc E, et al. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33:118-124.
22. Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: A systematic review. Nutr Rev. 2018;76:60-76.
23. Schmölz L, Birringer M, Lorkowski S, et al. Complexity of vitamin E metabolism. World J Biol Chem. 2016;7:14-43.
24. Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403-419.
25. Gurley BJ, Tonsing-Carter A, Thomas SL, et al. Clinically relevant herb-micronutrient interactions: when botanicals, minerals, and vitamins collide. Adv Nutr. 2018;9:524s-532s.
26. Zeilstra D, Younes JA, Brummer RJ, et al. Perspective: fundamental limitations of the randomized controlled trial method in nutritional research: the example of probiotics. Adv Nutr. 2018;9:561-571.
27. Kimokoti RW, Millen BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. 2016;100:1185-1198.
28. Tucker KL, Smith CE, Lai CQ, et al. Quantifying diet for nutrigenomic studies. Annu Rev Nutr. 2013;33:349-371.
29. Fenech M, El-Sohemy A, Cahill L, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69-89.
30. van Ommen B, van den Broek T, de Hoogh I, et al. Systems biology of personalized nutrition. Nutr Rev. 2017;75:579-599.
31. Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343-1347.
32. Mathieson I, Lazaridis I, Rohland N, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499-503.
33. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578-588.
34. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54.
35. Li Y, Huang T, Zheng Y, et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768.
36. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612.
37. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73.
38. USPSTF. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
39. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. JAMA. 2017;317:183-189.
40. Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. 2016;25:901-911.
41. Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17:191.
42. Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177.
43. Kölling K, Ndrepepa G, Koch W, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms, plasma homocysteine, folate, and vitamin B12 levels and the extent of coronary artery disease. Am J Cardiol. 2004;93:1201-1206.
44. Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet. 2011;378:584-594.
45. Chang G, Kuai Z, Wang J, et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr. 2019;19:331.
46. Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325-1335.
47. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30:17-25.
48. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64.
49. Van den Nieuwboer M, Brummer RJ, Guarner F, et al. The administration of probiotics and synbiotics in immune compromised adults: Is it safe? Benef Microbes. 2015;6:3-17.
50. Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58(suppl 10):S164-S179.
51. O’Connell TM. The application of metabolomics to probiotic and prebiotic interventions in human clinical studies. Metabolites. 2020;10:120.
52. Lerner A, Shoenfeld Y, Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms. 2019;7:104.
53. Su G, Ko C, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterol. 2020;159:697-705.
54. Preidis GA, Weizman AV, Kashyap PC, et al. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159:708-738.e4.
55. Charoenngam N, Shirvani A, Kalajian TA, et al. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer Res. 2020;40:551-556.
56. Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, et al. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10(suppl1):S17-S30.
57. APA. Clay RA. More than one way to measure. Monitor Psychol. 2010;4:52. Accessed November 19, 2021. www.apa.org/monitor/2010/09/trials
58. Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2-21.
59. Bauchner H, Fontanarosa PB, Golub RM. Evaluation of the trial to assess chelation therapy (TACT): the scientific process, peer review, and editorial scrutiny. JAMA. 2013;309:1291-1292.
60. Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20-40.
61. Matsumoto M, Kitada Y, Naito Y. Endothelial function is improved by inducing microbial polyamine production in the gut: a randomized placebo-controlled trial. Nutrients. 2019;11:1188.
62. Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496.
63. Vieira AT, Castelo PM, Ribeiro DA, et al. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. 2017;8:1884.
64. Ribeiro AE, Monteiro NES, Moraes AVG, et al. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2018;26:643-652.
65. Hong YS, Hong KS, Park MH, et al. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415-425.
66. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterol. 2017;152:1889-1900.e9.
67. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091-2103.
68. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 2019;11:638-647.
69. Liu M-M, Li S-T, Shu Y, et al. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0178870.
70. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245-1249.
71. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37-43.
72. Wang HT, Anvari S, Anagnostou K. The role of probiotics in preventing allergic disease. Children (Basel). 2019;6:24.
73. Kasatpibal N, Whitney JD, Saokaew S, et al. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl2):S153-S160.
74. Liu PC, Yan YK, Ma YJ, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075.
75. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr. 2018;37:532-541.
76. Wu Y, Zhang Q, Ren Y, et al. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12:e0178868.
77. Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients. 2016;8:397.
78. Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232:654-666.
79. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1-23.
80. Chowdhury R, Ramond A, O’Keeffe LM, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310.
81. Zhuang X, Ni A, Liao L, et al. Environment-wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis. 2018;269:172-177.
82. Wax PM. Current use of chelation in American health care. J Med Toxicol. 2013:9;303-307.
83. CDC. Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006;55:204-207.
84. Atwood KC, Woeckner E. In pediatric fatality, edetate disodium was no accident. Clin Toxicol (Phila). 2009;47:256.
85. Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol (Phila). 2008;46:1083-1084.
86. Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241-1250.
87. Escolar E, Lamas GA, Mark DB, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15-24.
88. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949.
89. Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264-2271.
90. Nagai R, Murray DB, Metz TO, et al. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549-559.
1. The Institute for Functional Medicine. 2020. Accessed November 19, 2021. www.ifm.org/
2. Rippe JM, ed. Lifestyle Medicine. 3rd ed. CRC Press, Taylor & Francis Group; 2019.
3. Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246-252.
4. Ali A, Katz DL. Disease prevention and health promotion: how integrative medicine fits. Am J Prev Med. 2015;49(5 suppl 3):S230-S240.
5. Bland J. Defining function in the functional medicine model. Integr Med (Encinitas). 2017;16:22-25.
6. ABPS. Integrative medicine examination description. 2020. Accessed November 19, 2021. www.abpsus.org/integrative-medicine-description/
7. Bland JS. The natural roots of functional medicine. Integr Med (Encinitas). 2018;17:12-17.
8. Beidelschies M, Alejandro-Rodriguez M, Ji X, et al. Association of the functional medicine model of care with patient-reported health-related quality-of-life outcomes. JAMA Netw Open. 2019;2:e1914017.
9. The Institute for Functional Medicine. Functional medicine matrix: organizing clinical imbalances. 2020. Accessed November 19, 2021. www.ifm.org/news-insights/toolkit-functional-medicine-matrix/
10. Schadt EE, Björkegren JL. NEW: Network-enabled wisdom in biology, medicine, and health care. Sci Transl Med. 2012;4:115rv1.
11. Curry SJ, Krist AH, Owens DK, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:272-280.
12. Sprangers MA, Thong MS, Bartels M, et al. Biological pathways, candidate genes, and molecular markers associated with quality-of-life domains: an update. Qual Life Res. 2014;23:1997-2013.
13. Bland J. Functional medicine: an operating system for integrative medicine. Integr Med (Encinitas). 2015;14:18-20.
14. Cutshall SM, Bergstrom LR, Kalish DJ. Evaluation of a functional medicine approach to treating fatigue, stress, and digestive issues in women. Complement Ther Clin Pract. 2016;23:75-81.
15. Jaffe R. First line comprehensive care. Part II: Anthropogenic xenobiotics in functional medicine. Managing persisting bioaccumulating pollutants: toxic minerals, biocides, hormone mimics, solvents, and chemical disruptors. Semin Integr Med. 2005;3:79-92.
16. Muran PJ, Muran SY, Beseler CL, et al. Breast health and reducing breast cancer risk: a functional medicine approach. J Altern Complement Med. 2015;21:321-326.
17. Bird JK, Murphy RA, Ciappio ED, et al. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9:655.
18. ter Borg S, Verlaan S, Hemsworth J, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113:1195-1206.
19. Moyer VA, on behalf of the USPSTF. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:558-564.
20. Grossman DC, Curry SJ, Owens DK, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319:1592-1599.
21. Fenercioglu AK, Saler T, Genc E, et al. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J Endocrinol Invest. 2010;33:118-124.
22. Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: A systematic review. Nutr Rev. 2018;76:60-76.
23. Schmölz L, Birringer M, Lorkowski S, et al. Complexity of vitamin E metabolism. World J Biol Chem. 2016;7:14-43.
24. Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403-419.
25. Gurley BJ, Tonsing-Carter A, Thomas SL, et al. Clinically relevant herb-micronutrient interactions: when botanicals, minerals, and vitamins collide. Adv Nutr. 2018;9:524s-532s.
26. Zeilstra D, Younes JA, Brummer RJ, et al. Perspective: fundamental limitations of the randomized controlled trial method in nutritional research: the example of probiotics. Adv Nutr. 2018;9:561-571.
27. Kimokoti RW, Millen BE. Nutrition for the prevention of chronic diseases. Med Clin North Am. 2016;100:1185-1198.
28. Tucker KL, Smith CE, Lai CQ, et al. Quantifying diet for nutrigenomic studies. Annu Rev Nutr. 2013;33:349-371.
29. Fenech M, El-Sohemy A, Cahill L, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69-89.
30. van Ommen B, van den Broek T, de Hoogh I, et al. Systems biology of personalized nutrition. Nutr Rev. 2017;75:579-599.
31. Fumagalli M, Moltke I, Grarup N, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343-1347.
32. Mathieson I, Lazaridis I, Rohland N, et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature. 2015;528:499-503.
33. Celis-Morales C, Livingstone KM, Marsaux CF, et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol. 2017;46:578-588.
34. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009;60:39-54.
35. Li Y, Huang T, Zheng Y, et al. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5:e003768.
36. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612.
37. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S49-S73.
38. USPSTF. Using nontraditional risk factors in coronary heart disease risk assessment: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:474-482.
39. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic acid supplementation for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. JAMA. 2017;317:183-189.
40. Levin BL, Varga E. MTHFR: addressing genetic counseling dilemmas using evidence-based literature. J Genet Couns. 2016;25:901-911.
41. Luo Z, Lu Z, Muhammad I, et al. Associations of the MTHFR rs1801133 polymorphism with coronary artery disease and lipid levels: a systematic review and updated meta-analysis. Lipids Health Dis. 2018;17:191.
42. Clarke R, Bennett DA, Parish S, et al. Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias. PLoS Med. 2012;9:e1001177.
43. Kölling K, Ndrepepa G, Koch W, et al. Methylenetetrahydrofolate reductase gene C677T and A1298C polymorphisms, plasma homocysteine, folate, and vitamin B12 levels and the extent of coronary artery disease. Am J Cardiol. 2004;93:1201-1206.
44. Holmes MV, Newcombe P, Hubacek JA, et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: a meta-analysis of genetic studies and randomised trials. Lancet. 2011;378:584-594.
45. Chang G, Kuai Z, Wang J, et al. The association of MTHFR C677T variant with increased risk of ischemic stroke in the elderly population: a meta-analysis of observational studies. BMC Geriatr. 2019;19:331.
46. Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325-1335.
47. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30:17-25.
48. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64.
49. Van den Nieuwboer M, Brummer RJ, Guarner F, et al. The administration of probiotics and synbiotics in immune compromised adults: Is it safe? Benef Microbes. 2015;6:3-17.
50. Liu Y, Tran DQ, Rhoads JM. Probiotics in disease prevention and treatment. J Clin Pharmacol. 2018;58(suppl 10):S164-S179.
51. O’Connell TM. The application of metabolomics to probiotic and prebiotic interventions in human clinical studies. Metabolites. 2020;10:120.
52. Lerner A, Shoenfeld Y, Matthias T. Probiotics: if it does not help it does not do any harm. Really? Microorganisms. 2019;7:104.
53. Su G, Ko C, Bercik P, et al. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterol. 2020;159:697-705.
54. Preidis GA, Weizman AV, Kashyap PC, et al. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159:708-738.e4.
55. Charoenngam N, Shirvani A, Kalajian TA, et al. The effect of various doses of oral vitamin D3 supplementation on gut microbiota in healthy adults: A randomized, double-blinded, dose-response study. Anticancer Res. 2020;40:551-556.
56. Cuevas-Sierra A, Ramos-Lopez O, Riezu-Boj JI, et al. Diet, gut microbiota, and obesity: links with host genetics and epigenetics and potential applications. Adv Nutr. 2019;10(suppl1):S17-S30.
57. APA. Clay RA. More than one way to measure. Monitor Psychol. 2010;4:52. Accessed November 19, 2021. www.apa.org/monitor/2010/09/trials
58. Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2-21.
59. Bauchner H, Fontanarosa PB, Golub RM. Evaluation of the trial to assess chelation therapy (TACT): the scientific process, peer review, and editorial scrutiny. JAMA. 2013;309:1291-1292.
60. Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216:20-40.
61. Matsumoto M, Kitada Y, Naito Y. Endothelial function is improved by inducing microbial polyamine production in the gut: a randomized placebo-controlled trial. Nutrients. 2019;11:1188.
62. Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes. 2018;9:486-496.
63. Vieira AT, Castelo PM, Ribeiro DA, et al. Influence of oral and gut microbiota in the health of menopausal women. Front Microbiol. 2017;8:1884.
64. Ribeiro AE, Monteiro NES, Moraes AVG, et al. Can the use of probiotics in association with isoflavone improve the symptoms of genitourinary syndrome of menopause? Results from a randomized controlled trial. Menopause. 2018;26:643-652.
65. Hong YS, Hong KS, Park MH, et al. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45:415-425.
66. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterol. 2017;152:1889-1900.e9.
67. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. 2018;233:2091-2103.
68. Linn YH, Thu KK, Win NHH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics Antimicrob Proteins. 2019;11:638-647.
69. Liu M-M, Li S-T, Shu Y, et al. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0178870.
70. Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245-1249.
71. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2018;154:37-43.
72. Wang HT, Anvari S, Anagnostou K. The role of probiotics in preventing allergic disease. Children (Basel). 2019;6:24.
73. Kasatpibal N, Whitney JD, Saokaew S, et al. Effectiveness of probiotic, prebiotic, and synbiotic therapies in reducing postoperative complications: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl2):S153-S160.
74. Liu PC, Yan YK, Ma YJ, et al. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: a systematic review and meta-analysis. Gastroenterol Res Pract. 2017;2017:6029075.
75. Hendijani F, Akbari V. Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: a systematic review and meta-analysis. Clin Nutr. 2018;37:532-541.
76. Wu Y, Zhang Q, Ren Y, et al. Effect of probiotic Lactobacillus on lipid profile: a systematic review and meta-analysis of randomized, controlled trials. PLoS One. 2017;12:e0178868.
77. Ferolla SM, Couto CA, Costa-Silva L, et al. Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with nonalcoholic steatohepatitis. Nutrients. 2016;8:397.
78. Clarke CN, Clarke NE, Mosher RE. Treatment of angina pectoris with disodium ethylene diamine tetraacetic acid. Am J Med Sci. 1956;232:654-666.
79. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008:1-23.
80. Chowdhury R, Ramond A, O’Keeffe LM, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310.
81. Zhuang X, Ni A, Liao L, et al. Environment-wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis. 2018;269:172-177.
82. Wax PM. Current use of chelation in American health care. J Med Toxicol. 2013:9;303-307.
83. CDC. Deaths associated with hypocalcemia from chelation therapy—Texas, Pennsylvania, and Oregon, 2003-2005. MMWR Morb Mortal Wkly Rep. 2006;55:204-207.
84. Atwood KC, Woeckner E. In pediatric fatality, edetate disodium was no accident. Clin Toxicol (Phila). 2009;47:256.
85. Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol (Phila). 2008;46:1083-1084.
86. Lamas GA, Goertz C, Boineau R, et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA. 2013;309:1241-1250.
87. Escolar E, Lamas GA, Mark DB, et al. The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circ Cardiovasc Qual Outcomes. 2014;7:15-24.
88. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929-1949.
89. Mega JL, Stitziel NO, Smith JG, et al. Genetic risk, coronary heart disease events, and the clinical benefit of statin therapy: an analysis of primary and secondary prevention trials. Lancet. 2015;385:2264-2271.
90. Nagai R, Murray DB, Metz TO, et al. Chelation: a fundamental mechanism of action of AGE inhibitors, AGE breakers, and other inhibitors of diabetes complications. Diabetes. 2012;61:549-559.