User login
Spesolimab speeds lesion clearance in generalized pustular psoriasis
GPP is a life-threatening skin condition involving the widespread eruption of sterile pustules, with a clinical course that “can be relapsing with recurrent flares or persistent with intermittent flares,” Hervé Bachelez, MD, of the Université de Paris and coauthors wrote. GPP patients are often hospitalized, and mortality ranges from 2% to 16% from causes that include sepsis and cardiorespiratory failure.
“The role of the interleukin-36 pathway in GPP is supported by the finding of loss-of-function mutations in the interleukin-36 receptor antagonist gene (IL36RN) and associated genes (CARD14, AP1S3, SERPINA3, and MPO) and by the overexpression of interleukin-36 cytokines in GPP skin lesions,” therefore, IL-36 is a potential treatment target to manage flares, they explained.
In the multicenter, double-blind trial, published in the New England Journal of Medicine, the researchers randomized 35 adults with GPP flares to a single 900-mg intravenous dose of spesolimab and 18 to placebo. Patients in both groups could receive an open-label dose of spesolimab after day 8; all patients were followed for 12 weeks.
The primary study endpoint was the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) pustulation subscore of 0 at 1 week after treatment. The GPPGA ranges from 0 (no visible pustules) to 4 (severe pustules). At baseline, 46% spesolimab patients and 39% placebo patients had a GPPGA pustulation subscore of 3, and 37% and 33%, respectively, had a pustulation subscore of 4.
After 1 week, 54% of the spesolimab patients had no visible pustules, compared with 6% of placebo patients; the difference was statistically significant (P < .001). The main secondary endpoint was a score of 0 or 1 (clear or almost clear skin) on the GPPGA total score after 1 week. Significantly more spesolimab patients had GPPGA total scores of 0 or 1, compared with placebo patients (43% vs. 11%, respectively; P = .02).
Overall, 6 of 35 spesolimab patients (17%) and 6% of those in the placebo groups developed infections during the first week, and 24 of 51 patients (47%) who had received spesolimab at any point during the study developed infections by week 12. Infections included urinary tract infections (three cases), influenza (three), otitis externa (two), folliculitis (two), upper respiratory tract infection (two), and pustule (two).
In the first week, 6% of spesolimab patients and none of the placebo patients reported serious adverse events; at week 12, 12% of patients who had received at least one spesolimab dose reported a serious adverse event. In addition, antidrug antibodies were identified in 23 (46%) of the 50 patients who received at least one dose of spesolimab.
“Symptoms that were observed in two patients who received spesolimab were reported as a drug reaction with eosinophilia and systemic symptoms (DRESS),” the authors noted. One patient had a RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions) score and the other had a score of 3; a score below 2 indicates no DRESS, and a score of 2 or 3 indicates “possible DRESS,” they added.
“Because 15 of the 18 patients who were assigned to the placebo group received open-label spesolimab, the effect of spesolimab as compared with that of placebo could not be determined after week 1,” the researchers noted.
The study findings were limited by several factors including the short randomization period and small study population, the researchers noted. However, the effect sizes for both the primary and secondary endpoints were large, which strengthened the results.
The results support data from previous studies suggesting a role for IL-36 in the pathogenesis of GPP, and support the need for longer and larger studies of the safety and effectiveness of spesolimab for GPP patients, they concluded.
No FDA-approved therapy
“GPP is a very rare but devastating life-threatening disease that presents with the sudden onset of pustules throughout the skin,” Joel Gelfand, MD, professor of dermatology and director of the psoriasis and phototherapy center at the University of Pennsylvania, Philadelphia, said in an interview. “Without rapid treatment, GPP can result in death. Currently there are no [Food and Drug Administration]–approved treatments for this orphan disease.”
Dr. Gelfand said he was surprised by the degree of efficacy and the speed of the patient response to spesolimab, compared with placebo, which he described as “truly remarkable.” Based on the current study results, “spesolimab offers a tremendous step forward for our patients,” he added.
Looking ahead, Dr. Gelfand noted that “longer-term studies with a comparator, such as a biologic that targets IL-17, would be helpful to more fully understand the safety, efficacy, and role that spesolimab will have in real-world patients.”
On Dec. 15, Boehringer Ingelheim announced that the FDA had granted priority review for spesolimab for treating GPP flares.
The study was supported by Boehringer Ingelheim. Lead author Dr. Bachelez had no financial conflicts to disclose. Several authors are employees of Boehringer Ingelheim. Dr. Gelfand is a consultant for the study sponsor Boehringer Ingelheim and has received research grants from Boehringer Ingelheim to his institution to support an investigator-initiated study. He also disclosed serving as a consultant and receiving research grants from other manufacturers of psoriasis products.
GPP is a life-threatening skin condition involving the widespread eruption of sterile pustules, with a clinical course that “can be relapsing with recurrent flares or persistent with intermittent flares,” Hervé Bachelez, MD, of the Université de Paris and coauthors wrote. GPP patients are often hospitalized, and mortality ranges from 2% to 16% from causes that include sepsis and cardiorespiratory failure.
“The role of the interleukin-36 pathway in GPP is supported by the finding of loss-of-function mutations in the interleukin-36 receptor antagonist gene (IL36RN) and associated genes (CARD14, AP1S3, SERPINA3, and MPO) and by the overexpression of interleukin-36 cytokines in GPP skin lesions,” therefore, IL-36 is a potential treatment target to manage flares, they explained.
In the multicenter, double-blind trial, published in the New England Journal of Medicine, the researchers randomized 35 adults with GPP flares to a single 900-mg intravenous dose of spesolimab and 18 to placebo. Patients in both groups could receive an open-label dose of spesolimab after day 8; all patients were followed for 12 weeks.
The primary study endpoint was the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) pustulation subscore of 0 at 1 week after treatment. The GPPGA ranges from 0 (no visible pustules) to 4 (severe pustules). At baseline, 46% spesolimab patients and 39% placebo patients had a GPPGA pustulation subscore of 3, and 37% and 33%, respectively, had a pustulation subscore of 4.
After 1 week, 54% of the spesolimab patients had no visible pustules, compared with 6% of placebo patients; the difference was statistically significant (P < .001). The main secondary endpoint was a score of 0 or 1 (clear or almost clear skin) on the GPPGA total score after 1 week. Significantly more spesolimab patients had GPPGA total scores of 0 or 1, compared with placebo patients (43% vs. 11%, respectively; P = .02).
Overall, 6 of 35 spesolimab patients (17%) and 6% of those in the placebo groups developed infections during the first week, and 24 of 51 patients (47%) who had received spesolimab at any point during the study developed infections by week 12. Infections included urinary tract infections (three cases), influenza (three), otitis externa (two), folliculitis (two), upper respiratory tract infection (two), and pustule (two).
In the first week, 6% of spesolimab patients and none of the placebo patients reported serious adverse events; at week 12, 12% of patients who had received at least one spesolimab dose reported a serious adverse event. In addition, antidrug antibodies were identified in 23 (46%) of the 50 patients who received at least one dose of spesolimab.
“Symptoms that were observed in two patients who received spesolimab were reported as a drug reaction with eosinophilia and systemic symptoms (DRESS),” the authors noted. One patient had a RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions) score and the other had a score of 3; a score below 2 indicates no DRESS, and a score of 2 or 3 indicates “possible DRESS,” they added.
“Because 15 of the 18 patients who were assigned to the placebo group received open-label spesolimab, the effect of spesolimab as compared with that of placebo could not be determined after week 1,” the researchers noted.
The study findings were limited by several factors including the short randomization period and small study population, the researchers noted. However, the effect sizes for both the primary and secondary endpoints were large, which strengthened the results.
The results support data from previous studies suggesting a role for IL-36 in the pathogenesis of GPP, and support the need for longer and larger studies of the safety and effectiveness of spesolimab for GPP patients, they concluded.
No FDA-approved therapy
“GPP is a very rare but devastating life-threatening disease that presents with the sudden onset of pustules throughout the skin,” Joel Gelfand, MD, professor of dermatology and director of the psoriasis and phototherapy center at the University of Pennsylvania, Philadelphia, said in an interview. “Without rapid treatment, GPP can result in death. Currently there are no [Food and Drug Administration]–approved treatments for this orphan disease.”
Dr. Gelfand said he was surprised by the degree of efficacy and the speed of the patient response to spesolimab, compared with placebo, which he described as “truly remarkable.” Based on the current study results, “spesolimab offers a tremendous step forward for our patients,” he added.
Looking ahead, Dr. Gelfand noted that “longer-term studies with a comparator, such as a biologic that targets IL-17, would be helpful to more fully understand the safety, efficacy, and role that spesolimab will have in real-world patients.”
On Dec. 15, Boehringer Ingelheim announced that the FDA had granted priority review for spesolimab for treating GPP flares.
The study was supported by Boehringer Ingelheim. Lead author Dr. Bachelez had no financial conflicts to disclose. Several authors are employees of Boehringer Ingelheim. Dr. Gelfand is a consultant for the study sponsor Boehringer Ingelheim and has received research grants from Boehringer Ingelheim to his institution to support an investigator-initiated study. He also disclosed serving as a consultant and receiving research grants from other manufacturers of psoriasis products.
GPP is a life-threatening skin condition involving the widespread eruption of sterile pustules, with a clinical course that “can be relapsing with recurrent flares or persistent with intermittent flares,” Hervé Bachelez, MD, of the Université de Paris and coauthors wrote. GPP patients are often hospitalized, and mortality ranges from 2% to 16% from causes that include sepsis and cardiorespiratory failure.
“The role of the interleukin-36 pathway in GPP is supported by the finding of loss-of-function mutations in the interleukin-36 receptor antagonist gene (IL36RN) and associated genes (CARD14, AP1S3, SERPINA3, and MPO) and by the overexpression of interleukin-36 cytokines in GPP skin lesions,” therefore, IL-36 is a potential treatment target to manage flares, they explained.
In the multicenter, double-blind trial, published in the New England Journal of Medicine, the researchers randomized 35 adults with GPP flares to a single 900-mg intravenous dose of spesolimab and 18 to placebo. Patients in both groups could receive an open-label dose of spesolimab after day 8; all patients were followed for 12 weeks.
The primary study endpoint was the Generalized Pustular Psoriasis Physician Global Assessment (GPPGA) pustulation subscore of 0 at 1 week after treatment. The GPPGA ranges from 0 (no visible pustules) to 4 (severe pustules). At baseline, 46% spesolimab patients and 39% placebo patients had a GPPGA pustulation subscore of 3, and 37% and 33%, respectively, had a pustulation subscore of 4.
After 1 week, 54% of the spesolimab patients had no visible pustules, compared with 6% of placebo patients; the difference was statistically significant (P < .001). The main secondary endpoint was a score of 0 or 1 (clear or almost clear skin) on the GPPGA total score after 1 week. Significantly more spesolimab patients had GPPGA total scores of 0 or 1, compared with placebo patients (43% vs. 11%, respectively; P = .02).
Overall, 6 of 35 spesolimab patients (17%) and 6% of those in the placebo groups developed infections during the first week, and 24 of 51 patients (47%) who had received spesolimab at any point during the study developed infections by week 12. Infections included urinary tract infections (three cases), influenza (three), otitis externa (two), folliculitis (two), upper respiratory tract infection (two), and pustule (two).
In the first week, 6% of spesolimab patients and none of the placebo patients reported serious adverse events; at week 12, 12% of patients who had received at least one spesolimab dose reported a serious adverse event. In addition, antidrug antibodies were identified in 23 (46%) of the 50 patients who received at least one dose of spesolimab.
“Symptoms that were observed in two patients who received spesolimab were reported as a drug reaction with eosinophilia and systemic symptoms (DRESS),” the authors noted. One patient had a RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions) score and the other had a score of 3; a score below 2 indicates no DRESS, and a score of 2 or 3 indicates “possible DRESS,” they added.
“Because 15 of the 18 patients who were assigned to the placebo group received open-label spesolimab, the effect of spesolimab as compared with that of placebo could not be determined after week 1,” the researchers noted.
The study findings were limited by several factors including the short randomization period and small study population, the researchers noted. However, the effect sizes for both the primary and secondary endpoints were large, which strengthened the results.
The results support data from previous studies suggesting a role for IL-36 in the pathogenesis of GPP, and support the need for longer and larger studies of the safety and effectiveness of spesolimab for GPP patients, they concluded.
No FDA-approved therapy
“GPP is a very rare but devastating life-threatening disease that presents with the sudden onset of pustules throughout the skin,” Joel Gelfand, MD, professor of dermatology and director of the psoriasis and phototherapy center at the University of Pennsylvania, Philadelphia, said in an interview. “Without rapid treatment, GPP can result in death. Currently there are no [Food and Drug Administration]–approved treatments for this orphan disease.”
Dr. Gelfand said he was surprised by the degree of efficacy and the speed of the patient response to spesolimab, compared with placebo, which he described as “truly remarkable.” Based on the current study results, “spesolimab offers a tremendous step forward for our patients,” he added.
Looking ahead, Dr. Gelfand noted that “longer-term studies with a comparator, such as a biologic that targets IL-17, would be helpful to more fully understand the safety, efficacy, and role that spesolimab will have in real-world patients.”
On Dec. 15, Boehringer Ingelheim announced that the FDA had granted priority review for spesolimab for treating GPP flares.
The study was supported by Boehringer Ingelheim. Lead author Dr. Bachelez had no financial conflicts to disclose. Several authors are employees of Boehringer Ingelheim. Dr. Gelfand is a consultant for the study sponsor Boehringer Ingelheim and has received research grants from Boehringer Ingelheim to his institution to support an investigator-initiated study. He also disclosed serving as a consultant and receiving research grants from other manufacturers of psoriasis products.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Complaints of incomplete bladder emptying
Many patients with nonmetastatic prostate cancer are asymptomatic at the time of diagnosis due to widespread routine screening. When localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. An increasing proportion of patients with localized disease are asymptomatic, however; such signs and symptoms may well be related to age-associated prostate enlargement or other conditions. Nevertheless, men over the age of 50 years who present with urinary symptoms should be screened for prostate cancer using DRE and PSA. Benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA. Acute prostatitis, on the other hand, presents as a urinary tract infection.
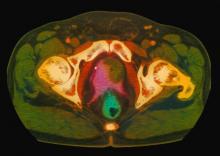
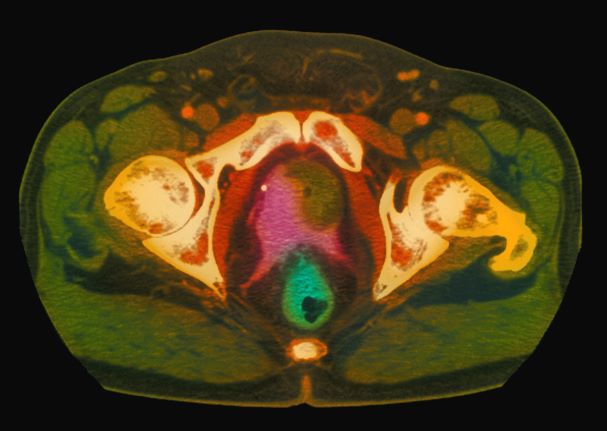
Because this patient showed elevated PSA levels, albeit with normal DRE findings, needle biopsy of the prostate is indicated for tissue diagnosis, usually performed with transrectal ultrasound. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density (amount of PSA per gram of prostate tissue) and PSA doubling time should be collected as well. As seen in the present case, MRI can be used to assess lesions concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging–Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. Imaging is also useful in staging and active surveillance. Staging is based on the tumor, node, and metastasis (TNM), with clinically localized prostate cancers including any T, N0, M0, NX, or MX cases. The clinician should pursue genetic testing to determine the presence of high-risk germline mutations.
The NCCN Guidelines recommend that for clinically localized prostate cancer, approaches include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. For asymptomatic patients who are older and/or have other serious comorbidities, active surveillance is often suggested. Radical prostatectomy is typically reserved for patients with a life expectancy of 10 years or more. Pelvic lymph node dissection may be performed on the basis of probability of nodal metastasis. Radiotherapy is also potentially curative in localized prostate cancer and may be delivered via brachytherapy, proton radiation, or external beam radiation therapy (EBRT). EBRT techniques include intensity-modulated radiation therapy (IMRT) and hypofractionated, image-guided stereotactic body radiation therapy (SBRT).
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Cvico Medical Solutions
Many patients with nonmetastatic prostate cancer are asymptomatic at the time of diagnosis due to widespread routine screening. When localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. An increasing proportion of patients with localized disease are asymptomatic, however; such signs and symptoms may well be related to age-associated prostate enlargement or other conditions. Nevertheless, men over the age of 50 years who present with urinary symptoms should be screened for prostate cancer using DRE and PSA. Benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA. Acute prostatitis, on the other hand, presents as a urinary tract infection.
Because this patient showed elevated PSA levels, albeit with normal DRE findings, needle biopsy of the prostate is indicated for tissue diagnosis, usually performed with transrectal ultrasound. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density (amount of PSA per gram of prostate tissue) and PSA doubling time should be collected as well. As seen in the present case, MRI can be used to assess lesions concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging–Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. Imaging is also useful in staging and active surveillance. Staging is based on the tumor, node, and metastasis (TNM), with clinically localized prostate cancers including any T, N0, M0, NX, or MX cases. The clinician should pursue genetic testing to determine the presence of high-risk germline mutations.
The NCCN Guidelines recommend that for clinically localized prostate cancer, approaches include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. For asymptomatic patients who are older and/or have other serious comorbidities, active surveillance is often suggested. Radical prostatectomy is typically reserved for patients with a life expectancy of 10 years or more. Pelvic lymph node dissection may be performed on the basis of probability of nodal metastasis. Radiotherapy is also potentially curative in localized prostate cancer and may be delivered via brachytherapy, proton radiation, or external beam radiation therapy (EBRT). EBRT techniques include intensity-modulated radiation therapy (IMRT) and hypofractionated, image-guided stereotactic body radiation therapy (SBRT).
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Cvico Medical Solutions
Many patients with nonmetastatic prostate cancer are asymptomatic at the time of diagnosis due to widespread routine screening. When localized symptoms do occur, they may include urinary frequency, decreased urine stream, urinary urgency, and hematuria. An increasing proportion of patients with localized disease are asymptomatic, however; such signs and symptoms may well be related to age-associated prostate enlargement or other conditions. Nevertheless, men over the age of 50 years who present with urinary symptoms should be screened for prostate cancer using DRE and PSA. Benign prostatic hyperplasia, for example, can manifest in urinary symptoms and even elevate PSA. Acute prostatitis, on the other hand, presents as a urinary tract infection.
Because this patient showed elevated PSA levels, albeit with normal DRE findings, needle biopsy of the prostate is indicated for tissue diagnosis, usually performed with transrectal ultrasound. A pathologic evaluation of the biopsy specimen will determine the patient's Gleason score. PSA density (amount of PSA per gram of prostate tissue) and PSA doubling time should be collected as well. As seen in the present case, MRI can be used to assess lesions concerning for prostate cancer prior to biopsy. Lesions are then assigned Prostate Imaging–Reporting and Data System (PI-RADS) scores depending on their location within the prostatic zones. Imaging is also useful in staging and active surveillance. Staging is based on the tumor, node, and metastasis (TNM), with clinically localized prostate cancers including any T, N0, M0, NX, or MX cases. The clinician should pursue genetic testing to determine the presence of high-risk germline mutations.
The NCCN Guidelines recommend that for clinically localized prostate cancer, approaches include watchful waiting, active surveillance, radical prostatectomy, and radiation therapy. For asymptomatic patients who are older and/or have other serious comorbidities, active surveillance is often suggested. Radical prostatectomy is typically reserved for patients with a life expectancy of 10 years or more. Pelvic lymph node dissection may be performed on the basis of probability of nodal metastasis. Radiotherapy is also potentially curative in localized prostate cancer and may be delivered via brachytherapy, proton radiation, or external beam radiation therapy (EBRT). EBRT techniques include intensity-modulated radiation therapy (IMRT) and hypofractionated, image-guided stereotactic body radiation therapy (SBRT).
Chad R. Tracy, MD, Professor; Director, Minimally Invasive Surgery, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, Iowa
Chad R. Tracy, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Cvico Medical Solutions
A 61-year-old man presents with complaints of frequent urination and incomplete bladder emptying. He also has been feeling fatigued but cannot tell if this is because his symptoms are worse at night and he has not been sleeping well. Despite a family history of atrial fibrillation, he reports no significant medical history beyond appendicitis many years ago. The patient underwent a prostate cancer screening about 18 months ago, which was normal. During a recent office visit, digital rectal examination (DRE) was normal, but prostate-specific antigen (PSA) levels were elevated at 10.2 ng/mL. An MRI is performed as part of the workup.
Are we failing to diagnose and treat the many faces of catatonia?
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
I had seen many new and exciting presentations of psychopathology during my intern year, yet one patient was uniquely memorable. When stable, he worked as a counselor, though for any number of reasons (eg, missing a dose of medication, smoking marijuana) his manic symptoms would emerge quickly, the disease rearing its ugly head within hours. He would become extremely hyperactive, elated, disinhibited (running naked in the streets), and grandiose (believing he was working for the president). He would be escorted to our psychiatric emergency department (ED) by police, who would have to resort to handcuffing him. His symptoms were described by ED and inpatient nursing staff and residents as “disorganized,” “psychotic,” “agitated,”’ or “combative.” He would receive large doses of intramuscular (IM) haloperidol, chlorpromazine, and diphenhydramine in desperate attempts to rein in his mania. Frustratingly—and paradoxically— this would make him more confused, disoriented, restless, and hyperactive, and often led to the need for restraints.
This behavior persisted for days until an attending I was working with assessed him. The attending observed that the patient did not know his current location, day of the week or month, or how he ended up in the hospital. He observed this patient intermittently staring, making abnormal repetitive movements with his arms and hands, occasionally freezing, making impulsive movements, and becoming combative without provocation. His heart rate and temperature were elevated; he was diaphoretic, especially after receiving parenteral antipsychotics. The attending, a pupil of Max Fink, made the diagnosis: delirious mania, a form of catatonia.1,2 Resolution was quick and complete after 6 bilateral electroconvulsive therapy (ECT) sessions.
Catatonia, a neuropsychiatric phenomenon characterized by abnormal speech, movement, and affect, has undergone numerous paradigm shifts since it was recognized by Karl Ludwig Kahlbaum in 1874.3 Shortly after Kahlbaum, Emil Kraepelin held the belief that catatonia was a subtype of dementia praecox, or what is now known as schizophrenia.4 Due to this, patients were likely receiving less-than-optimal treatments, because their catatonia was being diagnosed as acute psychosis. Finally, in DSM-5, catatonia was unshackled from the constraints of schizophrenia and is now an entity of its own.5 However, catatonia is often met with incertitude (despite being present in up to 15% of inpatients),1 with its treatment typically delayed or not even pursued. This is amplified because many forms of catatonia are often misdiagnosed as disorders that are more common or better understood.
Potential catatonia presentations
Delirious mania. Patients with delirious mania typically present with acute delirium, severe paranoia, hyperactivity, and visual/auditory hallucinations.2,6,7 They usually have excited catatonic signs, such as excessive movement, combativeness, impulsivity, stereotypy, and echophenomena. Unfortunately, the catatonia is overshadowed by extreme psychotic and manic symptoms, or delirium (for which an underlying medical cause is usually not found). As was the case for the patient I described earlier, large doses of IM antipsychotics usually are administered, which can cause neuroleptic malignant syndrome (NMS) or precipitate seizures.8
Neuroleptic malignant syndrome. NMS is marked by fever, elevated blood pressure and heart rate, lead-pipe rigidity, parkinsonian features, altered mental status, and lab abnormalities (elevated liver enzymes or creatinine phosphokinase). This syndrome is preceded by the administration of an antipsychotic. It has features of catatonia that include mutism, negativism, and posturing.9 NMS is commonly interpreted as a subtype of malignant catatonia. Some argue that the diagnosis of malignant catatonia yields a more favorable outcome because it leads to more effective treatments (ie, benzodiazepines and ECT as opposed to dopamine agonists and dantrolene).10 Because NMS has much overlap with serotonin syndrome and drug-induced parkinsonism, initiation of benzodiazepines and ECT often is delayed.11
Retarded catatonia. This version of catatonia usually is well recognized. The typical presentation is a patient who does not speak (mutism) or move (stupor), stares, becomes withdrawn (does not eat or drink), or maintains abnormal posturing. Retarded catatonia can be confused with a major depressive episode or hypoactive delirium.
Catatonia in autism spectrum disorder. Historically, co-occurring catatonia and autism spectrum disorder (ASD) was believed to be extremely rare. However, recent retrospective studies have found that up to 17% of patients with ASD older than age 15 have catatonia.12 Many pediatric psychiatrists fail to recognize catatonia; in 1 study, only 2 patients (of 18) were correctly identified as having catatonia.13 The catatonic signs may vary, but the core features include withdrawal (children may need a feeding tube), decreased communication and/or worsening psychomotor slowing, agitation, or stereotypical movements, which can manifest as worsening self-injurious behavior.14,15
An approach to treatment
Regardless of the etiology or presentation, first-line treatment for catatonia is benzodiazepines and/or ECT. A lorazepam challenge is used for diagnostic clarification; if effective, lorazepam can be titrated until symptoms fully resolve.16,17 Doses >20 mg have been reported as effective and well-tolerated, without the feared sedation and respiratory depression.6 An unsuccessful lorazepam challenge does not rule out catatonia. If benzodiazepine therapy fails or the patient requires immediate symptom relief, ECT is the most effective treatment. Many clinicians use a bilateral electrode placement with high-energy dosing and frequent sessions until the catatonia resolves.1,18
In my experience, catatonia in all its forms remains poorly recognized, with its treatment questioned. Residents—especially those in psychiatry—must understand that catatonia can result in systemic illness or death.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
1. Fink M. Expanding the catatonia tent: recognizing electroconvulsive therapy responsive syndromes. J ECT. 2021;37(2):77-79.
2. Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54-60.
3. Starkstein SE, Goldar JC, Hodgkiss A. Karl Ludwig Kahlbaum’s concept of catatonia. Hist Psychiatry. 1995;6(22 Pt 2):201-207.
4. Jain A, Mitra P. Catatonic schizophrenia. StatPearls Publishing. Last updated July 31, 2021. Accessed December 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK563222/
5. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
6. Karmacharya R, England ML, Ongür D. Delirious mania: clinical features and treatment response. J Affect Disord. 2008;109(3):312-316.
7. Jacobowski NL, Heckers S, Bobo WV. Delirious mania: detection, diagnosis, and clinical management in the acute setting. J Psychiatr Pract. 2013;19(1):15-28.
8. Fink M. Electroconvulsive Therapy: A Guide for Professionals and Their Patients. Oxford University Press; 2009.
9. Francis A, Yacoub A. Catatonia and neuroleptic malignant syndrome. Ann Clin Psychiatry. 2008:231; author reply 232-233.
10. Fink M. Hidden in plain sight: catatonia in pediatrics: “An editorial comment to Shorter E. “Making childhood catatonia visible (Separate from competing diagnoses”, (1) Dhossche D, Ross CA, Stoppelbein L. ‘The role of deprivation, abuse, and trauma in pediatric catatonia without a clear medical cause’, (2) Ghaziuddin N, Dhossche D, Marcotte K. ‘Retrospective chart review of catatonia in child and adolescent psychiatric patients’ (3)”. Acta Psychiatr Scand. 2012;125(1):11-12.
11. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
12. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000;176:357-362.
13. Ghaziuddin N, Dhossche D, Marcotte K. Retrospective chart review of catatonia in child and adolescent psychiatric patients. Acta Psychiatr Scand. 2012;125(1):33-38.
14. Wachtel LE, Hermida A, Dhossche DM. Maintenance electroconvulsive therapy in autistic catatonia: a case series review. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):581-587.
15. Wachtel LE. The multiple faces of catatonia in autism spectrum disorders: descriptive clinical experience of 22 patients over 12 years. Eur Child Adolesc Psychiatry. 2019;28(4):471-480.
16. Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129-136.
17. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93(2):137-143.
18. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
Boy presents with abdominal cramping
Ulcerative colitis (UC) is an autoimmune-related inflammatory bowel disease (IBD). It typically develops in the rectum and extends to involve the large intestine. Pediatric UC can have a more severe phenotype than adult disease and may affect a child's pubertal development, bone mineral density, nutrition levels, and social life. It is currently theorized that the age at diagnosis and sex of the patient do not predict disease activity.
UC disease can announce itself as mild, moderate, or severe, and the Pediatric Ulcerative Colitis Activity Index (PUCAI) endoscopic grading is used as a clinical scoring system. The most common presenting symptoms are rectal bleeding, diarrhea, and abdominal pain; among children, the presentation can vary.
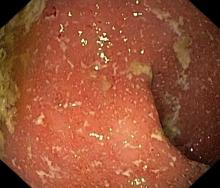
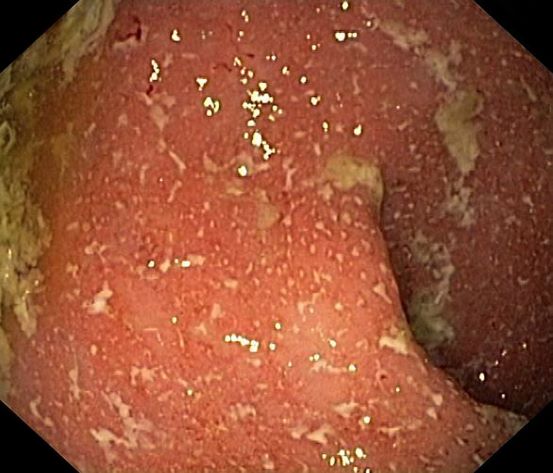
Crohns disease, another IBD, must be carefully ruled out of the differential. Colonoscopy represents the first-line approach in the diagnosis of IBD. The findings that would suggest Crohns disease are sparing of the rectal mucosa, aphthous ulceration, and noncontiguous (or skip) lesions. Micronutrient and vitamin levels are usually low in Crohns disease. And although weight loss, perineal disease, fistulae, and obstruction are commonly seen in the context of Crohns disease, they are uncommon or rare in UC. Bleeding is observed much more frequently in UC.
During UC workup, elevated erythrocyte sedimentation rate and C-reactive protein level often serve as markers of disease activity. Antineutrophil cytoplasmic antibody (ANCA) test is frequently used with suspected UC (though this measure may not correlate with disease activity). In addition, a broad metabolic panel should be performed, along with stool cultures, to rule out infection.
The goals of pediatric UC management are to maintain control of the disease, extend periods of remission, and reduce long-term damage caused by inflammation, all while potentially allowing the patient to function as normally as possible. Anti-inflammatory therapy with 5-aminosalicylic acid agents, such as sulfasalazine and mesalamine, is foundational to treatment. Acute flares of UC in the pediatric population are usually responsive to corticosteroids, but these regimens should be short-term only. Immunomodulatory agents, tumor necrosis factor inhibitors, and newer therapies such as monoclonal antibodies are also used during flares, but only a minority of patients will require these therapies. These are also considered treatment alternatives for patients who are steroid-dependent or steroid-refractory.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships
Ulcerative colitis (UC) is an autoimmune-related inflammatory bowel disease (IBD). It typically develops in the rectum and extends to involve the large intestine. Pediatric UC can have a more severe phenotype than adult disease and may affect a child's pubertal development, bone mineral density, nutrition levels, and social life. It is currently theorized that the age at diagnosis and sex of the patient do not predict disease activity.
UC disease can announce itself as mild, moderate, or severe, and the Pediatric Ulcerative Colitis Activity Index (PUCAI) endoscopic grading is used as a clinical scoring system. The most common presenting symptoms are rectal bleeding, diarrhea, and abdominal pain; among children, the presentation can vary.
Crohns disease, another IBD, must be carefully ruled out of the differential. Colonoscopy represents the first-line approach in the diagnosis of IBD. The findings that would suggest Crohns disease are sparing of the rectal mucosa, aphthous ulceration, and noncontiguous (or skip) lesions. Micronutrient and vitamin levels are usually low in Crohns disease. And although weight loss, perineal disease, fistulae, and obstruction are commonly seen in the context of Crohns disease, they are uncommon or rare in UC. Bleeding is observed much more frequently in UC.
During UC workup, elevated erythrocyte sedimentation rate and C-reactive protein level often serve as markers of disease activity. Antineutrophil cytoplasmic antibody (ANCA) test is frequently used with suspected UC (though this measure may not correlate with disease activity). In addition, a broad metabolic panel should be performed, along with stool cultures, to rule out infection.
The goals of pediatric UC management are to maintain control of the disease, extend periods of remission, and reduce long-term damage caused by inflammation, all while potentially allowing the patient to function as normally as possible. Anti-inflammatory therapy with 5-aminosalicylic acid agents, such as sulfasalazine and mesalamine, is foundational to treatment. Acute flares of UC in the pediatric population are usually responsive to corticosteroids, but these regimens should be short-term only. Immunomodulatory agents, tumor necrosis factor inhibitors, and newer therapies such as monoclonal antibodies are also used during flares, but only a minority of patients will require these therapies. These are also considered treatment alternatives for patients who are steroid-dependent or steroid-refractory.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships
Ulcerative colitis (UC) is an autoimmune-related inflammatory bowel disease (IBD). It typically develops in the rectum and extends to involve the large intestine. Pediatric UC can have a more severe phenotype than adult disease and may affect a child's pubertal development, bone mineral density, nutrition levels, and social life. It is currently theorized that the age at diagnosis and sex of the patient do not predict disease activity.
UC disease can announce itself as mild, moderate, or severe, and the Pediatric Ulcerative Colitis Activity Index (PUCAI) endoscopic grading is used as a clinical scoring system. The most common presenting symptoms are rectal bleeding, diarrhea, and abdominal pain; among children, the presentation can vary.
Crohns disease, another IBD, must be carefully ruled out of the differential. Colonoscopy represents the first-line approach in the diagnosis of IBD. The findings that would suggest Crohns disease are sparing of the rectal mucosa, aphthous ulceration, and noncontiguous (or skip) lesions. Micronutrient and vitamin levels are usually low in Crohns disease. And although weight loss, perineal disease, fistulae, and obstruction are commonly seen in the context of Crohns disease, they are uncommon or rare in UC. Bleeding is observed much more frequently in UC.
During UC workup, elevated erythrocyte sedimentation rate and C-reactive protein level often serve as markers of disease activity. Antineutrophil cytoplasmic antibody (ANCA) test is frequently used with suspected UC (though this measure may not correlate with disease activity). In addition, a broad metabolic panel should be performed, along with stool cultures, to rule out infection.
The goals of pediatric UC management are to maintain control of the disease, extend periods of remission, and reduce long-term damage caused by inflammation, all while potentially allowing the patient to function as normally as possible. Anti-inflammatory therapy with 5-aminosalicylic acid agents, such as sulfasalazine and mesalamine, is foundational to treatment. Acute flares of UC in the pediatric population are usually responsive to corticosteroids, but these regimens should be short-term only. Immunomodulatory agents, tumor necrosis factor inhibitors, and newer therapies such as monoclonal antibodies are also used during flares, but only a minority of patients will require these therapies. These are also considered treatment alternatives for patients who are steroid-dependent or steroid-refractory.
Bhupinder S. Anand, MD, Professor, Department of Medicine, Baylor College of Medicine, Houston, TX
Bhupinder S. Anand, MD, has disclosed no relevant financial relationships
A 5-year-old boy presents with abdominal cramping and bloody stools over the course of 2 days. His mother explains that the onset of diarrhea was insidious. Because the patient has a sensitive stomach, she tries to keep his diet relatively bland, but she worries about what he eats at school. He is slightly underweight for his age group. The family has not traveled recently. The patient does not have a fever, but skin turgor is decreased. There is no evidence of fistulae or abscesses. His complete blood cell count is 10.6 g/dL.
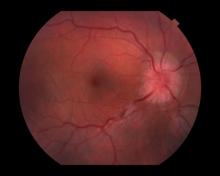
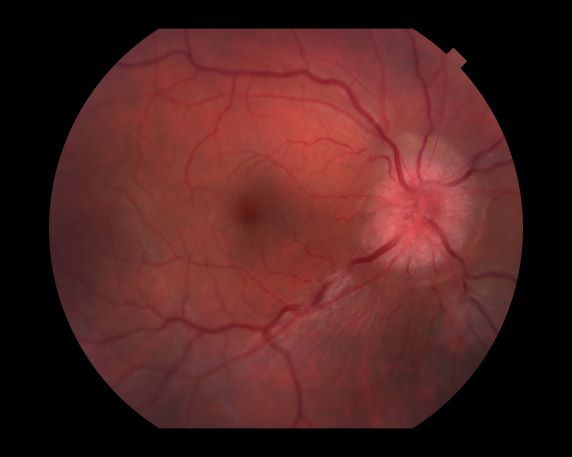
Decreased visual acuity and paresthesia
All of the above conditions can have ophthalmic manifestations, but the majority of optic neuritis cases seen in clinical practice are either sporadic or MS related. Optic neuritis is the first demyelinating event in approximately 20% of patients with MS. It develops in approximately 40% of MS patients during the course of their disease.
Optic neuritis is characterized by loss of vision (or loss of color vision) in the affected eye and pain on movement of the eye (painful ophthalmoplegia). Less often, patients with optic neuritis may describe phosphenes (transient flashes of light or black squares) lasting from hours to months. Phosphenes may occur before or during an optic neuritis event or even several months after recovery.
The diagnosis of optic neuritis is usually made clinically, with direct imaging of the optic nerves showing evidence of optic disc swelling with blurred margins. The real contribution of imaging in the setting of optic neuritis, however, is made by imaging of the brain, not of the optic nerves themselves. MRI of the brain provides information that can change the management of optic neuritis and yields prognostic information regarding the patient's future risk of developing MS. The most valuable predictor of the development of subsequent MS is the presence of white matter abnormalities. Between 27% and 70% of patients (in various studies) with isolated optic neuritis showed abnormal MRI brain findings, as defined by the presence of two or more white matter lesions on T2-weighted images. Patients with two or more lesions may have up to an 80% chance of meeting criteria for MS within the next 5 years.
A gradual recovery of visual acuity with time is characteristic of optic neuritis, although permanent residual deficits in color vision and contrast and brightness sensitivity are common. The symptoms of optic neuritis will usually resolve without medical treatment, although continuing to take regular MS disease-modulating medication is usually helpful. An intravenous steroid or oral prednisone is sometimes recommended to speed recovery. A 3- to 5-day course of high-dose (1 g) IV methylprednisolone, followed by a rapid oral taper of prednisone, has been shown to provide rapid recovery of symptoms in the acute phase. However, IV steroids do little to affect the ultimate visual acuity in patients with optic neuritis.
Typically, patients begin to recover 2-4 weeks after the onset of the vision loss. The optic nerve may take up to 6-12 months to heal completely, but most patients recover as much vision as they are going to within the first few months.
For patients with optic neuritis whose brain lesions on MRI indicate a high risk of developing clinically definite MS, treatment with immunomodulators may be considered. IV immunoglobulin treatment of acute optic neuritis has been shown to have no beneficial effect. In severe cases, plasma exchange may be considered.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ.
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme.
All of the above conditions can have ophthalmic manifestations, but the majority of optic neuritis cases seen in clinical practice are either sporadic or MS related. Optic neuritis is the first demyelinating event in approximately 20% of patients with MS. It develops in approximately 40% of MS patients during the course of their disease.
Optic neuritis is characterized by loss of vision (or loss of color vision) in the affected eye and pain on movement of the eye (painful ophthalmoplegia). Less often, patients with optic neuritis may describe phosphenes (transient flashes of light or black squares) lasting from hours to months. Phosphenes may occur before or during an optic neuritis event or even several months after recovery.
The diagnosis of optic neuritis is usually made clinically, with direct imaging of the optic nerves showing evidence of optic disc swelling with blurred margins. The real contribution of imaging in the setting of optic neuritis, however, is made by imaging of the brain, not of the optic nerves themselves. MRI of the brain provides information that can change the management of optic neuritis and yields prognostic information regarding the patient's future risk of developing MS. The most valuable predictor of the development of subsequent MS is the presence of white matter abnormalities. Between 27% and 70% of patients (in various studies) with isolated optic neuritis showed abnormal MRI brain findings, as defined by the presence of two or more white matter lesions on T2-weighted images. Patients with two or more lesions may have up to an 80% chance of meeting criteria for MS within the next 5 years.
A gradual recovery of visual acuity with time is characteristic of optic neuritis, although permanent residual deficits in color vision and contrast and brightness sensitivity are common. The symptoms of optic neuritis will usually resolve without medical treatment, although continuing to take regular MS disease-modulating medication is usually helpful. An intravenous steroid or oral prednisone is sometimes recommended to speed recovery. A 3- to 5-day course of high-dose (1 g) IV methylprednisolone, followed by a rapid oral taper of prednisone, has been shown to provide rapid recovery of symptoms in the acute phase. However, IV steroids do little to affect the ultimate visual acuity in patients with optic neuritis.
Typically, patients begin to recover 2-4 weeks after the onset of the vision loss. The optic nerve may take up to 6-12 months to heal completely, but most patients recover as much vision as they are going to within the first few months.
For patients with optic neuritis whose brain lesions on MRI indicate a high risk of developing clinically definite MS, treatment with immunomodulators may be considered. IV immunoglobulin treatment of acute optic neuritis has been shown to have no beneficial effect. In severe cases, plasma exchange may be considered.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ.
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme.
All of the above conditions can have ophthalmic manifestations, but the majority of optic neuritis cases seen in clinical practice are either sporadic or MS related. Optic neuritis is the first demyelinating event in approximately 20% of patients with MS. It develops in approximately 40% of MS patients during the course of their disease.
Optic neuritis is characterized by loss of vision (or loss of color vision) in the affected eye and pain on movement of the eye (painful ophthalmoplegia). Less often, patients with optic neuritis may describe phosphenes (transient flashes of light or black squares) lasting from hours to months. Phosphenes may occur before or during an optic neuritis event or even several months after recovery.
The diagnosis of optic neuritis is usually made clinically, with direct imaging of the optic nerves showing evidence of optic disc swelling with blurred margins. The real contribution of imaging in the setting of optic neuritis, however, is made by imaging of the brain, not of the optic nerves themselves. MRI of the brain provides information that can change the management of optic neuritis and yields prognostic information regarding the patient's future risk of developing MS. The most valuable predictor of the development of subsequent MS is the presence of white matter abnormalities. Between 27% and 70% of patients (in various studies) with isolated optic neuritis showed abnormal MRI brain findings, as defined by the presence of two or more white matter lesions on T2-weighted images. Patients with two or more lesions may have up to an 80% chance of meeting criteria for MS within the next 5 years.
A gradual recovery of visual acuity with time is characteristic of optic neuritis, although permanent residual deficits in color vision and contrast and brightness sensitivity are common. The symptoms of optic neuritis will usually resolve without medical treatment, although continuing to take regular MS disease-modulating medication is usually helpful. An intravenous steroid or oral prednisone is sometimes recommended to speed recovery. A 3- to 5-day course of high-dose (1 g) IV methylprednisolone, followed by a rapid oral taper of prednisone, has been shown to provide rapid recovery of symptoms in the acute phase. However, IV steroids do little to affect the ultimate visual acuity in patients with optic neuritis.
Typically, patients begin to recover 2-4 weeks after the onset of the vision loss. The optic nerve may take up to 6-12 months to heal completely, but most patients recover as much vision as they are going to within the first few months.
For patients with optic neuritis whose brain lesions on MRI indicate a high risk of developing clinically definite MS, treatment with immunomodulators may be considered. IV immunoglobulin treatment of acute optic neuritis has been shown to have no beneficial effect. In severe cases, plasma exchange may be considered.
Krupa Pandey, MD, Director, Multiple Sclerosis Center, Department of Neurology & Neuroscience Institute, Hackensack University Medical Center; Neurologist, Department of Neurology, Hackensack Meridian Health, Hackensack, NJ.
Krupa Pandey, MD, has serve(d) as a speaker or a member of a speakers bureau for: Bristol-Myers Squibb; Biogen; Alexion; Genentech; Sanofi-Genzyme.
A 44-year-old woman presents with decreased visual acuity, painful ophthalmoplegia, photophobia, and paresthesia of the left hand. The patient's ocular history was unremarkable. Her medical history was significant only for recurrent urinary tract infections. She did not have a history of neurologic problems and reported that she did not have dizziness, tingling, tremors, sensory changes, speech changes, or focal weaknesses. Besides current use of naproxen, she said she was not taking any other medications. Her family ocular history was significant for glaucoma in her father and paternal grandfather. Her maternal grandfather died at age 58 of multiple sclerosis (MS).
How do digital technologies affect young people’s mental health?
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
For almost all of us, “screen time”—time spent using a device with a screen such as a smartphone, computer, television, or video game console—has become a large part of our daily lives. This is very much the case for children and adolescents. In the United States, children ages 8 to 12 years spend an average of 4 to 6 hours each day watching or using screens, and teens spend up to 9 hours.1 Because young people are continually adopting newer forms of entertainment and technologies, new digital technologies are an ongoing source of concern for parents and clinicians alike.2 Studies have suggested that excessive screen time is associated with numerous psychiatric symptoms and disorders, including poor sleep, weight gain, anxiety, depression, and attention-deficit/hyperactive disorder.3,4 However, a recent systematic review and meta-analysis found that individuals’ self-reports of media use were rarely an accurate reflection of their actual, logged media use, and that measures of problematic media use had an even weaker association with usage logs.5 Therefore, it is crucial to have an accurate understanding of how children and adolescents are affected by new technologies. In this article, we discuss a recent study that investigated variations in adolescents’ mental health over time, and the association of their mental health and their use of digital technologies.
Results were mixed
Vuorre et al6 conducted a study to examine a possible shift in the associations between adolescents’ technology use and mental health outcomes. To investigate whether technology engagement and mental health outcomes changed over time, these researchers evaluated the impact not only of smartphones and social media, but also of television, which in the mid- to late-20th century elicited comparable levels of academic, public, and policy concern about its potential impact on child development. They analyzed data from 3 large-scale studies of adolescents living in the United States (Monitoring the Future and Youth Risk Behavior Surveillance System) and the United Kingdom (Understanding Society) that included a total of 430,561 participants.
The results were mixed across types of technology and mental health outcomes. Television and social media were found to have a direct correlation with conduct problems and emotional problems. Suicidal ideation and behavior were associated with digital device use; however, no correlation was found between depression and technology use. Regarding social media use, researchers found that its association with conduct problems remained stable, decreased with depression, and increased with emotional problems. The magnitudes of the observed changes over time were small. These researchers concluded there is “little evidence for increases in the associations between adolescents’ technology engagement and mental health [problems]” and “drawing firm conclusions about changes in ... associations with mental health may be premature.”6
Future directions
The study by Vuorre et al6 has opened the door to better analysis of the association between screen use and mental health outcomes. More robust, detailed studies are required to fully understand the varying impact of technologies on the lives of children and adolescents. Collaborative efforts by technology companies and researchers can help to determine the impact of technology on young people’s mental health.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
1. American Academy of Child & Adolescent Psychiatry. Screen time and children. Updated February 2020. Accessed October 7, 2021. http://www.aacap.org/AACAP/Families_and_Youth/Facts_for_Families/FFF-Guide/Children-And-Watching-TV-054.aspx
2. Orben A. The Sisyphean cycle of technology panics. Perspect Psychol Sci. 2020;15(5):1143-1157.
3. Paulich KN, Ross JM, Lessem JM, et al. Screen time and early adolescent mental health, academic, and social outcomes in 9- and 10-year old children: utilizing the Adolescent Brain Cognitive Development (ABCD) Study. PLoS One. 2021;16(9):e0256591. doi: 10.1371/journal.pone.0256591
4. Twenge JM, Campbell WK. Associations between screen time and lower psychological well-being among children and adolescents: evidence from a population-based study. Prev Med Rep. 2018;12:271-283. doi: 10.1016/j.pmedr.2018.10.003
5. Parry DA, Davidson BI, Sewall CJR, et al. A systematic review and meta-analysis of discrepancies between logged and self-reported digital media use. Nat Hum Behav. 2021;5(11):1535-1547.
6. Vuorre M, Orben A, Przybylski AK. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin Psychol Sci. 2021;9(5):823-835.
Woman with dyspnea and persistent cough
On the basis of the patient's presentation and imaging results, the likely diagnosis is non–small cell cancer (NSCLC) of an adenocarcinoma subtype. NSCLC makes up about 80% of all lung cancer cases. Adenocarcinoma in particular is the most common type of lung cancer in the United States, accounting for about 40% of cases, and it is the most common histology among nonsmokers. Women are more likely to develop this subtype of NSCLC and are generally younger when they present with symptoms. This type of cancer arises from the bronchial mucosal glands and usually develops in a peripheral location within the lung.
In the course of workup, immunohistochemical (IHC) analyses are used to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether ALK inhibitor therapy or programmed cell death protein ligand 1 (PD-L1) inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. NCCN guidelines advise that all patients with adenocarcinoma should be tested for EGFR mutations, and DNA mutational analysis is the preferred method for assessment. Patients should also undergo routine biomarker testing, with an eye toward ALK, RET, and ROS1 rearrangements, BRAF mutations, c-MET and exon 14 skipping mutations, and PD-L1 expression levels. For patients with metastatic NSCLC, PD-L1 IHC testing is recommended.
Most cases of lung cancer are diagnosed at a late stage, when symptoms have already begun to manifest. Of note, however, women with adenocarcinoma are more likely to present with localized disease. Treatment is largely influenced by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes.
For patients who are EGFR mutation positive (exon 10 deletion or L858R), osimertinib is the recommended first-line therapy. For patients who are positive for the EGFR exon 20 insertion mutation, initial systemic therapy options for adenocarcinoma are appropriate; the preferred regimen being pembrolizumab-carboplatin-pemetrexed if there are no contraindications to programmed cell death protein 1 (PD-1) or PD-L1 inhibitors.
KRAS mutations, unlike EGFR mutations, are associated with smoking. Because overlapping targetable alterations are uncommon, identification of KRAS mutations suggests that these patients will not benefit from additional molecular testing. Again, initial systemic therapy options for adenocarcinoma are appropriate, but the presence of KRAS mutation predicts a poor response to EGFR tyrosine kinase inhibitors. The FDA approved a KRAS inhibitor in June 2021 and immune checkpoint inhibitors appear to be beneficial in this population.
Maurie Markman, MD, President, Department of Medical Oncology, Cancer Treatment Centers of America.
Maurie Markman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Merck
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novis; Glaxo Smith Kline
Received research grant from: AstraZeneca; Novis; GSK; Merck
On the basis of the patient's presentation and imaging results, the likely diagnosis is non–small cell cancer (NSCLC) of an adenocarcinoma subtype. NSCLC makes up about 80% of all lung cancer cases. Adenocarcinoma in particular is the most common type of lung cancer in the United States, accounting for about 40% of cases, and it is the most common histology among nonsmokers. Women are more likely to develop this subtype of NSCLC and are generally younger when they present with symptoms. This type of cancer arises from the bronchial mucosal glands and usually develops in a peripheral location within the lung.
In the course of workup, immunohistochemical (IHC) analyses are used to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether ALK inhibitor therapy or programmed cell death protein ligand 1 (PD-L1) inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. NCCN guidelines advise that all patients with adenocarcinoma should be tested for EGFR mutations, and DNA mutational analysis is the preferred method for assessment. Patients should also undergo routine biomarker testing, with an eye toward ALK, RET, and ROS1 rearrangements, BRAF mutations, c-MET and exon 14 skipping mutations, and PD-L1 expression levels. For patients with metastatic NSCLC, PD-L1 IHC testing is recommended.
Most cases of lung cancer are diagnosed at a late stage, when symptoms have already begun to manifest. Of note, however, women with adenocarcinoma are more likely to present with localized disease. Treatment is largely influenced by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes.
For patients who are EGFR mutation positive (exon 10 deletion or L858R), osimertinib is the recommended first-line therapy. For patients who are positive for the EGFR exon 20 insertion mutation, initial systemic therapy options for adenocarcinoma are appropriate; the preferred regimen being pembrolizumab-carboplatin-pemetrexed if there are no contraindications to programmed cell death protein 1 (PD-1) or PD-L1 inhibitors.
KRAS mutations, unlike EGFR mutations, are associated with smoking. Because overlapping targetable alterations are uncommon, identification of KRAS mutations suggests that these patients will not benefit from additional molecular testing. Again, initial systemic therapy options for adenocarcinoma are appropriate, but the presence of KRAS mutation predicts a poor response to EGFR tyrosine kinase inhibitors. The FDA approved a KRAS inhibitor in June 2021 and immune checkpoint inhibitors appear to be beneficial in this population.
Maurie Markman, MD, President, Department of Medical Oncology, Cancer Treatment Centers of America.
Maurie Markman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Merck
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novis; Glaxo Smith Kline
Received research grant from: AstraZeneca; Novis; GSK; Merck
On the basis of the patient's presentation and imaging results, the likely diagnosis is non–small cell cancer (NSCLC) of an adenocarcinoma subtype. NSCLC makes up about 80% of all lung cancer cases. Adenocarcinoma in particular is the most common type of lung cancer in the United States, accounting for about 40% of cases, and it is the most common histology among nonsmokers. Women are more likely to develop this subtype of NSCLC and are generally younger when they present with symptoms. This type of cancer arises from the bronchial mucosal glands and usually develops in a peripheral location within the lung.
In the course of workup, immunohistochemical (IHC) analyses are used to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether ALK inhibitor therapy or programmed cell death protein ligand 1 (PD-L1) inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. NCCN guidelines advise that all patients with adenocarcinoma should be tested for EGFR mutations, and DNA mutational analysis is the preferred method for assessment. Patients should also undergo routine biomarker testing, with an eye toward ALK, RET, and ROS1 rearrangements, BRAF mutations, c-MET and exon 14 skipping mutations, and PD-L1 expression levels. For patients with metastatic NSCLC, PD-L1 IHC testing is recommended.
Most cases of lung cancer are diagnosed at a late stage, when symptoms have already begun to manifest. Of note, however, women with adenocarcinoma are more likely to present with localized disease. Treatment is largely influenced by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes.
For patients who are EGFR mutation positive (exon 10 deletion or L858R), osimertinib is the recommended first-line therapy. For patients who are positive for the EGFR exon 20 insertion mutation, initial systemic therapy options for adenocarcinoma are appropriate; the preferred regimen being pembrolizumab-carboplatin-pemetrexed if there are no contraindications to programmed cell death protein 1 (PD-1) or PD-L1 inhibitors.
KRAS mutations, unlike EGFR mutations, are associated with smoking. Because overlapping targetable alterations are uncommon, identification of KRAS mutations suggests that these patients will not benefit from additional molecular testing. Again, initial systemic therapy options for adenocarcinoma are appropriate, but the presence of KRAS mutation predicts a poor response to EGFR tyrosine kinase inhibitors. The FDA approved a KRAS inhibitor in June 2021 and immune checkpoint inhibitors appear to be beneficial in this population.
Maurie Markman, MD, President, Department of Medical Oncology, Cancer Treatment Centers of America.
Maurie Markman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Merck
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novis; Glaxo Smith Kline
Received research grant from: AstraZeneca; Novis; GSK; Merck
A 52-year-old woman presents with dyspnea and a persistent cough. She is 5 ft 5 in and weighs 155 lb, with no recent significant weight loss. She has been experiencing symptoms for a few months, which she originally thought might be related to her history of GERD. She reports that she was a light smoker before she had children but has not smoked regularly in about 20 years. Because of the patient's respiratory symptoms, chest radiography is ordered.
This frontal projection chest radiography clearly demonstrates a mass in the upper lobe of the right lung that represents the appearance of lung cancer (malignancy).
Is anosognosia a delusion, a negative symptom, or a cognitive deficit?
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.
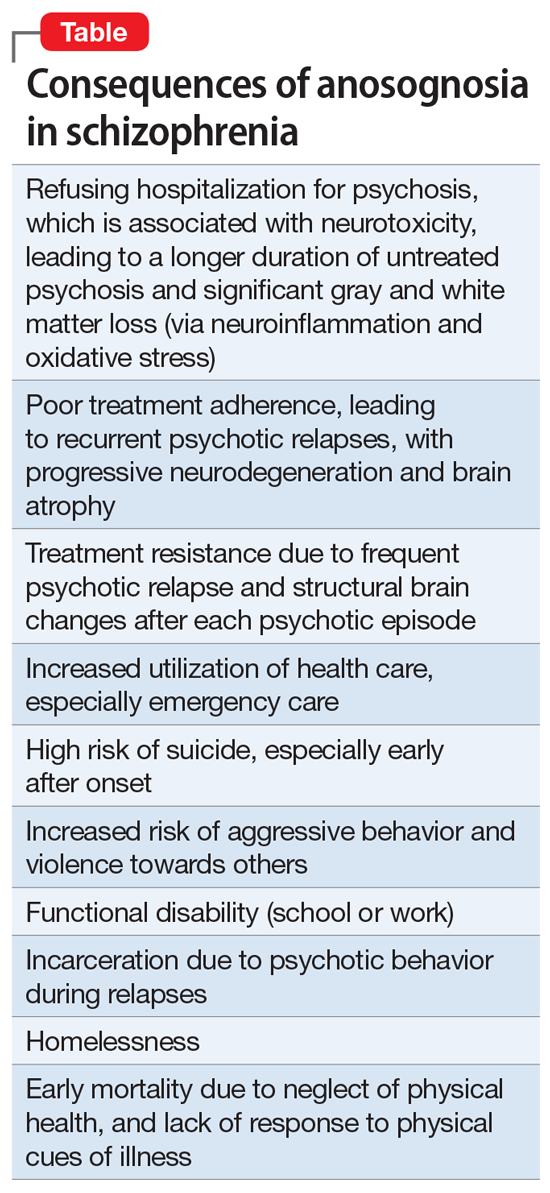
Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
Anosognosia is the lack of awareness of a disabling physical or mental illness. The term was coined by Joseph Babinski in 1914 following his observations that patients with left-side paralysis due to right hemisphere stroke do not recognize their hemiplegia and strongly deny that there is anything physically wrong with their body, or that they need treatment or rehabilitation.
Psychiatrists have long observed anosognosia in patients with acute psychoses such as schizophrenia or mania who vehemently deny that there is anything wrong with them, despite experiencing hallucinations, delusions, and/or bizarre behavior. They adamantly refuse medical care and often have to be involuntarily hospitalized to receive urgently needed medications they don’t believe they need.
So is anosognosia in schizophrenia a fixed false belief (delusion), a negative symptom, or a cognitive deficit? Arguments can be made for any of those 3 options, but the evidence suggests that anosognosia is a disorder of consciousness, a “meta-cognitive” deficit, or, as I referred to it in a previous publication, the loss of self-proprioception.1
Anosognosia in neurologic brain disorders
Although right hemispheric stroke is the most common disease state associated with anosognosia,2 other neurologic disorders can be associated with anosognosia, including Anton’s syndrome of cortical blindness,3 traumatic brain injury,4 Wernicke’s aphasia,5 mild cognitive impairment,6 and Alzheimer’s disease.7 In addition to anosognosia, those disorders can be accompanied by indifference to the deficit, which is referred to as “anosodiaphoria.”
The neuroanatomy of anosognosia generally implicates right hemisphere deficits, especially the frontal cortex, the right parietal lobe, the temporoparietal cortex, and the thalamus. It can be conceptualized as a disturbance of “body schema” because all motor and sensory functions of the body have a “representation” in brain structure.
Anosognosia in psychiatric brain disorders
Although schizophrenia is most frequently associated with anosognosia, other psychiatric disorders also exhibit this absence of insight. They include delusional disorder,8 bipolar disorder,9 intellectual disability,10 and personality disorders.11 In all those psychiatric disorders, there is a lack of self-reflection (metacognition). At the neuroanatomical level, most studies have focused on schizophrenia, and abnormalities have been described in the frontal and parietal regions. Significant pathology in the inferior parietal lobe has been identified in schizophrenia.12 However, the right insula, which is connected to multiple neural circuits,13 appears to be intimately associated with anosognosia when impaired. The insula also regulates interoception and a “sense of self.”14 The loss of cortical gray matter in schizophrenia is most pronounced in the insula bilaterally. Another neurologic mechanism associated with anosognosia in schizophrenia is the default mode network (DMN). The DMN, which usually is overactive at rest and is deactivated during a focused activity, is involved in both insight and social cognition.15
Measurement of anosognosia
Several rating scales are used to measure the severity of anosognosia and the loss of insight. They include:
- The Insight and Treatment Attitude Questionnaire16
- The Scale to Assess Unawareness of Mental Disorder17
- The Beck Cognitive Insight Scale,18 the only self-administered scale that measures a patient’s ability to evaluate their psychiatric beliefs and possibly modify them
- The Positive and Negative Syndrome Scale,19 which is the gold standard for measuring the overall severity of schizophrenia, has only 1 item related to insight within the 16-item General Subscale (G12: Lack of judgement and insight).
Continue to: Consequences of anosognosia...
Consequences of anosognosia
Patients with anosognosia neglect themselves both mentally and physically and fail to seek or accept medical attention. Thus, schizophrenia is associated with many serious and damaging consequences due to the lack of self-monitoring or appraising their health needs. The Table summarizes the multiple consequences of anosognosia.

Is anosognosia treatable or irreversible?
Schizophrenia is well established to be a heterogeneous syndrome with hundreds of biotypes that share a similar phenotype of positive, negative, cognitive, mood, and neuromotor symptoms of variable severities.20 This includes anosognosia, which has been reported in 57% to 98% of patients in various studies.21,22
So what happens to anosognosia with antipsychotic therapy? In the first study that used a long-acting injectable (LAI) second-generation antipsychotic (SGA) in first-episode psychosis to ensure full adherence, Emsley et al23 reported a 64% remission rate after 2 years of treatment, and observed that many patients regained their insight after several months of uninterrupted antipsychotic pharmacotherapy. This suggests that avoiding psychotic relapse with uninterrupted antipsychotic therapy with LAIs may help restore insight. I have personally witnessed reversal of anosognosia in patients with first-episode schizophrenia whom I treated with LAI SGAs continuously for several years; these patients not only regained insight into their illness but were able to return to college or to work.
There is also evidence that stroke patients with left-side hemiplegia, or patients with cortical blindness (due to calcarine cortex damage secondary to posterior cerebral artery infarct), who paradoxically deny being blind due to anosognosia, do regain their insight after several months. Cognitive-behavioral therapy (CBT) and adherence therapy, as well as psychoeducation, can help in reversing anosognosia. Bilateral electroconvulsive therapy has been reported to improve insight in schizophrenia. Transcranial magnetic stimulation over the posterior parietal cortex has been reported to restore insight in patients with visuospatial neglect due to a stroke. However, more research targeting anosognosia along with psychotic symptoms is needed. It should be noted that patients with bipolar disorder who have anosognosia during the manic phase of their illness do have insight when they switch to a depressed phase,9 which suggests that anosognosia is reversible in bipolar disorder and is phase-dependent (ie, a state, not a trait, variable).
A symptom of impaired consciousness
A large body of evidence links lesions in the right hemisphere to delusion and to anosognosia.24 Gazzaniga and Miller25 published a book chapter with the provocative title “the left hemisphere does not miss the right hemisphere.” Such right-hemisphere lesions can lead to a disruption of consciousness, leading to anosognosia. Schizophrenia is a pervasive brain syndrome involving multiple brain regions and a wide range of clinical symptoms ranging across psychotic as well as negative and cognitive domains. Anosognosia can be conceptualized as a psychotic symptom (delusion), a negative symptom (self-monitoring deficit), or a cognitive failure. However, anosognosia in schizophrenia can be best understood as a symptom of impaired consciousness and self-pathology,26 where the brain fails to process and recognize one’s mental function, which culminates in faulty reality testing.
Schizophrenia is a neurologic syndrome associated with numerous psychiatric manifestations, and anosognosia is one of its fundamental initial symptoms.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
1. Nasrallah HA. Impaired mental proprioception in schizophrenia. Current Psychiatry. 2012;11(8):4-5.
2. Kirsch LP, Mathys C, Papadaki C, et al. Updating beliefs beyond the here-and-now: the counter-factual self in anosognosia for hemiplegia. Brain Commun. 2021;3(2):fcab098. doi: 10.1093/braincomms/fcab098
3. Das JM, Nagvi IA. Anton syndrome. StatPearls Publishing. Updated April 10, 2021. Accessed December 13, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538155/
4. Steward KA, Kretzmer T. Anosognosia in moderate-to-severe traumatic brain injury: a review of prevalence, clinical correlates, and diversity considerations. Clin Neuropsychol. 2021:1-20.
5. Klarendié M, Gorišek VR, Granda G, et al. Auditory agnosia with anosognosia. Cortex. 2021;137:255-270.
6. Bastin C, Giacomelli F, Miévis F, et al. Anosognosia in mild cognitive impairment: lack of awareness of memory difficulties characterizes prodromal Alzheimer’s disease. Front Psychiatry. 202;12:631518.
7. Chen S, Song Y, Xu W, et al; Alzheimer’s Disease Neuroimaging Initiative. Impaired memory awareness and loss integration in self-referential network across the progression of Alzheimer’s disease spectrum. J Alzheimers Dis. 2021;83(1):111-126.
8. Turnbull OH, Fotopoulou A, Solms M. Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex. 2014;61:18-29.
9. Ibrahim SU, Kalyanasundaram VB, Ramanathan SA, et al. Trajectory of insight on various dimensions among bipolar disorder in-patients. Ind Psychiatry J. 2020;29(2):285-292.
10. Levine DN. Unawareness of visual and sensorimotor defects: a hypothesis. Brain Cogn. 1990;13(2):233-281.
11. Pourmohammad P, Imani M, Goodarzi MA, et al. Impaired complex theory of mind and low emotional self-awareness in outpatients with borderline personality disorder compared to healthy controls: a cross-sectional study. J Psychiatr Res. 2021;143:445-450.
12. Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophr Res. 2007;97(1-3):215-225.
13. Dionisio S, Mayoglou L, Cho SM, et al. Connectivity of the human insula: a cortico-cortical evoked potential (CCEP) study. Cortex. 2019;120:419-442.
14. Nord CL, Lawson RP, Dalgleish T. Disrupted dorsal mid-insula activation during interoception across psychiatric disorders. Am J Psychiatry. 2021;178(8):761-770.
15. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64(9):774-781.
16. McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48-51.
17. Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150(6):873-879.
18. Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68(2-3):319-329.
19. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Nasrallah HA. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
21. Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21(2):129-141.
22. Lehrer DS, Lorenz J. Anosognosia in schizophrenia: hidden in plain sight. Innov Clin Neurosci. 2014;11(5-6):101-107.
23. Emsley R, Medori R, Koen L, et al. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28(2):210-213.
24. Gurin L, Blum S. Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci. 2017;29(3):225-235.
25. Gazzaniga MS, Miller M. The left hemisphere does not miss the right hemisphere. In: Laureys S, Tononi G (eds). The Neurology of Consciousness. Cognitive Neuroscience and Neuropathology. Academic Press; 2008:261-270.
26. Cooney JW, Gazzaniga MS. Neurological disorders and the structure of human consciousness. Trends Cogn Sci. 2003;7(4):161-165.
Formaldehyde exposure tied to cognitive impairment
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term exposure to formaldehyde on the job is linked to cognitive impairment down the road, new research suggests.
In a large observational study of adults aged 45-70 years, researchers found a 17% higher risk for cognitive problems in those with occupational formaldehyde exposure – and higher risks for those with longer duration of exposure.
“The effect of formaldehyde on the brain has been previously shown mainly in animal experiments, but very few studies have been done on humans,” lead author Noemie Letellier, PhD, Institute for Neurosciences of Montpellier, University of Montpellier (France), said in an interview.
“Our results show that being or having been occupationally exposed to formaldehyde is associated with cognitive impairment in a relatively young population,” Dr. Letellier said.
The findings were published online Dec. 22, 2021, in the journal Neurology.
Dose-effect relationship
The investigators assessed a representative sample of 75,322 adults in France (median age, 57.5 years; 53% women). All were part of the CONSTANCES cohort, an observational cohort with a focus on occupational and environmental factors.
A total of 6,026 participants (8%) were exposed to formaldehyde during their careers. Their occupations included nurses, caregivers, medical technicians, workers in the textile, chemistry and metal industries, carpenters, and cleaners.
The researchers calculated lifetime formaldehyde exposure using a French job-exposure matrix created to estimate a person’s exposure to potential health hazards in different occupations.
Individuals were divided into three equal groups according to their years of exposure to formaldehyde. “Low” was considered to be 6 or fewer years of exposure, “medium” was 7-21 years, and “high” was 22 or more years.
Participants were also split into three groups according to their cumulative exposure (total lifetime formaldehyde exposure based on the probability, intensity, and frequency of exposure).
Prevention efforts needed
After adjusting for age, sex, education and other confounders, participants exposed to formaldehyde were at higher risk for global cognitive impairment (adjusted relative risk, 1.17; 95% confidence interval, 1.1-1.2).
Longer duration of exposure and high cumulative lifetime exposure were associated with worse cognitive impairment, “with a dose-effect relationship for exposure duration,” the researchers reported.
Those exposed to formaldehyde for 22 years or more had a 21% higher risk of global cognitive impairment and workers with the highest cumulative exposure had a 19% higher risk of cognitive impairment, compared with workers with no exposure.
Although workers with recent exposure showed higher cognitive impairment, “time may not fully attenuate formaldehyde-associated cognitive deficits, especially in highly exposed but also in moderately exposed workers,” the researchers wrote.
They caution that their findings only show an association and does not prove that exposure to formaldehyde causes cognitive impairment.
Nonetheless, Dr. Letellier encourages health care providers to “be aware of lifetime occupational exposure to target prevention efforts to the identified occupational groups.” This especially includes the care sector where the most people are exposed to formaldehyde, such as nurses, caregivers, and medical technicians.
“Despite the restrictions on the use of formaldehyde due to the better knowledge of its toxicity, especially its carcinogenic effect, formaldehyde is still widely used in many sectors. These results encourage prevention efforts to further limit worker exposure to formaldehyde,” Dr. Letellier said.
Relevant to health care workers
Commenting on the study, Shaheen E. Lakhan, MD, PhD, a neurologist in Newton, Mass., said in an interview that exposure to some degree of formaldehyde is found in every home and workplace, “from the floors to furniture.”
“If you have cigarette smoke in the environment, your exposure rises sharply. When limiting your exposure, it’s not only cancer that you are preventing, but also your brain health,” added Dr. Lakhan, who was not involved with the research.
He said the disturbances in cognitive function noted in the current study were “particularly relevant to health care workers, given the use of formaldehyde in sterilization, tissue pathology processing, and embalming.”
“Interestingly, with only past exposure, there seems to be some degree of cognitive recovery,” but it does not return to a level before any exposure when corrected for age and other factors, Dr. Lakhan said.
Some caveats should also be noted, he pointed out. The study included a French population, but regulators such as the U.S. Occupational Safety and Health Administration and the California Office of Environmental Health Hazard Assessment have strict standards on formaldehyde use in a variety of work settings.
On the flip side, given the COVID-19 pandemic, there has been greater use of chemical disinfectants in and out the workplace, some of which contain formaldehyde, Dr. Lakhan said.
In addition, he noted the study assessed data from 1950 to 2018, so prepandemic.
“A word of advice from a brain doc: Check with your employer on the level of occupational exposure to formaldehyde, heavy metals, and other toxic substances – and cross-reference with your local environmental standards,” Dr. Lakhan concluded.
The research was supported by a grant from the French Agency for Food, Environmental, and Occupational Health & Safety. The investigators and Dr. Lakhan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Remdesivir may keep unvaccinated out of the hospital: Study
The antiviral remdesivir, an intravenous drug given mostly to seriously ill COVID-19 patients in hospitals, could keep unvaccinated people who become infected out of the hospital if given on an outpatient basis, a new study says.
Researchers studied 562 unvaccinated people from September 2020 to April 2021, according to the study published in the New England Journal of Medicine. The study determined the risk of hospitalization or death was 87% lower in study participants who were given remdesivir than participants who received a placebo.
All participants were at high risk of developing severe COVID-19 because of their age – they were over 60 – or because they had an underlying medical condition such as diabetes or obesity.
An important caveat: The findings are based on data collected before the Delta variant surged in the summer of 2021 or the Omicron variant surged late in the year, the Washington Post reported.
The new study says the drug could be helpful in keeping vaccinated as well as unvaccinated people out of the hospital – an important factor as the Omicron surge threatens to overwhelm health systems around the world.
Remdesivir could be a boon for COVID-19 patients in parts of the world that don’t have vaccines or for patients with immunocompromised systems.
“These data provide evidence that a 3-day course of remdesivir could play a critical role in helping COVID-19 patients stay out of the hospital,” Robert L. Gottlieb, MD, PhD, the therapeutic lead for COVID-19 research at Baylor Scott & White Health in Dallas, said in a news release from Gilead Pharmaceuticals. “While our hospitals are ready to assist patients in need, prevention and early intervention are preferable to reduce the risk of disease progression and allow patients not requiring oxygen to recover from home when appropriate.”
Remdesivir was the first antiviral for COVID-19 authorized by the Food and Drug Administration. It was given to then-President Donald Trump when he was hospitalized with COVID-19 in October 2020.
Gilead released the study findings in September.
A version of this article first appeared on WebMD.com.
The antiviral remdesivir, an intravenous drug given mostly to seriously ill COVID-19 patients in hospitals, could keep unvaccinated people who become infected out of the hospital if given on an outpatient basis, a new study says.
Researchers studied 562 unvaccinated people from September 2020 to April 2021, according to the study published in the New England Journal of Medicine. The study determined the risk of hospitalization or death was 87% lower in study participants who were given remdesivir than participants who received a placebo.
All participants were at high risk of developing severe COVID-19 because of their age – they were over 60 – or because they had an underlying medical condition such as diabetes or obesity.
An important caveat: The findings are based on data collected before the Delta variant surged in the summer of 2021 or the Omicron variant surged late in the year, the Washington Post reported.
The new study says the drug could be helpful in keeping vaccinated as well as unvaccinated people out of the hospital – an important factor as the Omicron surge threatens to overwhelm health systems around the world.
Remdesivir could be a boon for COVID-19 patients in parts of the world that don’t have vaccines or for patients with immunocompromised systems.
“These data provide evidence that a 3-day course of remdesivir could play a critical role in helping COVID-19 patients stay out of the hospital,” Robert L. Gottlieb, MD, PhD, the therapeutic lead for COVID-19 research at Baylor Scott & White Health in Dallas, said in a news release from Gilead Pharmaceuticals. “While our hospitals are ready to assist patients in need, prevention and early intervention are preferable to reduce the risk of disease progression and allow patients not requiring oxygen to recover from home when appropriate.”
Remdesivir was the first antiviral for COVID-19 authorized by the Food and Drug Administration. It was given to then-President Donald Trump when he was hospitalized with COVID-19 in October 2020.
Gilead released the study findings in September.
A version of this article first appeared on WebMD.com.
The antiviral remdesivir, an intravenous drug given mostly to seriously ill COVID-19 patients in hospitals, could keep unvaccinated people who become infected out of the hospital if given on an outpatient basis, a new study says.
Researchers studied 562 unvaccinated people from September 2020 to April 2021, according to the study published in the New England Journal of Medicine. The study determined the risk of hospitalization or death was 87% lower in study participants who were given remdesivir than participants who received a placebo.
All participants were at high risk of developing severe COVID-19 because of their age – they were over 60 – or because they had an underlying medical condition such as diabetes or obesity.
An important caveat: The findings are based on data collected before the Delta variant surged in the summer of 2021 or the Omicron variant surged late in the year, the Washington Post reported.
The new study says the drug could be helpful in keeping vaccinated as well as unvaccinated people out of the hospital – an important factor as the Omicron surge threatens to overwhelm health systems around the world.
Remdesivir could be a boon for COVID-19 patients in parts of the world that don’t have vaccines or for patients with immunocompromised systems.
“These data provide evidence that a 3-day course of remdesivir could play a critical role in helping COVID-19 patients stay out of the hospital,” Robert L. Gottlieb, MD, PhD, the therapeutic lead for COVID-19 research at Baylor Scott & White Health in Dallas, said in a news release from Gilead Pharmaceuticals. “While our hospitals are ready to assist patients in need, prevention and early intervention are preferable to reduce the risk of disease progression and allow patients not requiring oxygen to recover from home when appropriate.”
Remdesivir was the first antiviral for COVID-19 authorized by the Food and Drug Administration. It was given to then-President Donald Trump when he was hospitalized with COVID-19 in October 2020.
Gilead released the study findings in September.
A version of this article first appeared on WebMD.com.