User login
Could Resistin Predict Death and Disease Severity in PAH?
Resistin, a cytokine expressed in adipocytes, has been associated with poor clinical outcomes in heart failure and cardiovascular disease, Li Gao, MD, of Johns Hopkins University, Baltimore, Maryland, and colleagues wrote. While mouse studies have shown that human resistin drives pulmonary vascular remodeling and the development of PAH, the role of resistin as a biomarker for PAH remains unclear.
In a study published in Respiratory Research, the researchers reviewed biospecimens and clinical and genetic data from 1121 adults with PAH, 808 with idiopathic PAH (IPAH), and 313 with scleroderma-associated PAH (SSc-PAH). They examined the associations between serum resistin levels and PAH outcomes in multivariate regression models, using machine-learning algorithms to develop models to predict mortality.
Resistin levels were significantly higher in all patients with PAH and patients with the two subtypes than in control participants (all P < .0001). Resistin was also associated with significant discriminative properties, with area under the curve (AUC) measures of 0.84, 0.82, and 0.91 for PAH overall, IPAH, and SSc-PAH, respectively.
Elevated resistin levels (defined as > 4.54 ng/mL) were significantly associated with an increased risk for death (hazard ratio, 2.6; P < .0087) as well as with older age and shorter distance on the 6-minute walk test (P = .001 for both) and reduced cardiac capacity based on the New York Heart Association functional class (P < .014).
Survival models derived from machine learning confirmed the prognostic value of resistin for mortality in PAH as seen in the random forest model, with an AUC of 0.70. “When we used the AUC values of the ROC curve as criteria to evaluate how well resistin levels discerned the presence of PAH, all three tests had excellent discriminative ability (AUCs were 0.84, 0.82, and 0.91 for all PAH, IPAH, and SSc-PAH, respectively),” the researchers wrote.
The researchers also evaluated three RETN genetic variants (rs7408174, rs3219175, and rs3745367) for a specific association with serum resistin levels and measures of PAH severity. Resistin levels were highest among individuals who were carriers of either the rs3219175 or rs3745367 mutation, the researchers noted.
The findings were limited by several factors, including missing data on the 6-minute walk test from several centers, which led to the elimination of that item from the survival analysis. Other limitations included the inability to control for PAH therapy at the time of assessment and the collection of serum at a different time from other clinical variables.
However, “our study provides evidence to support the use of circulating biomarkers as objective and accessible tools for noninvasive PAH risk stratification,” the researchers said. Additional research is needed to strengthen the association, but the findings suggest that resistin represents a novel biomarker for PAH prognostication and risk stratification and may have implications for the development of new treatments.
Biomarker Research Expands Diagnosis and Treatment Horizons
“It is a dynamic time in PAH research and clinical management, given the recent approval and use of the BMP/TGF beta balancing agent sotatercept (Winrevair) as an effective agent to target the molecular origins of this disease,” Stephen Chan, MD, professor of medicine and director of the Vascular Medicine Institute at the University of Pittsburgh, Pittsburgh, Pennsylvania, said in an interview.
The growing number of medications that can be used to treat patients with PAH will likely be more effective if patients are identified and treated early, said Dr. Chan, who was not involved in the study.
However, the time to diagnosis for patients with PAH is still more than 3 years from the start of symptoms, he said. Factors contributing to the delay include the requirement of an invasive cardiac catheterization procedure to make the final diagnosis, the status of PAH as a borderline orphan disease, and the often nonspecific nature of the initial symptoms of PAH.
Consequently, “there is an unmet need to develop effective and preferably noninvasive tools to aid in early diagnosis of PAH,” Dr. Chan added.
The power of the study is in the number of patients included, as much of previous PAH research has involved small studies of patients that could not be replicated or did not generalize to the larger patient population, Dr. Chan said.
The use of the PAH Biobank allows researchers to access a larger population of patients with PAH. “With that in mind, it is not surprising that some markers would emerge as potentially powerful and clinically meaningful,” he said.
“Currently, we do not have a reliable blood-based biomarker that we use in clinical PAH practice, although there are emerging studies that suggest other markers such as metabolites, RNA molecules, and proteins that may serve in the same capacity. If these studies turn out to be reproducible, generalizable, and specific to PAH in larger populations, measuring resistin could be helpful in making early diagnosis, particularly in areas that do not have invasive catheterization facilities (and globally) and for nonspecialists who are puzzled about the nonspecificity of initial symptoms of PAH,” Dr. Chan said.
Resistin could also be incorporated into existing risk stratification scores, such as the REVEAL risk score, that are already used in PAH clinical practice as guidance for when and how to use currently approved medications, he added.
Limitations of the study included the focus only on resistin alone, not in combination with other molecules that might perform better. Also, no independent validation cohort was used, he noted. “While PAH Biobank certainly offered larger numbers than we typically see, we would have to see validation in large independent cohorts for us to be convinced that measurements of resistin should be used in clinical practice.”
Resistin is not specific to PAH, which makes interpretation of the results more complicated, said Dr. Chan. “In this study, the authors used a smaller healthy control cohort of 50 patients as a comparison to their PAH cohort. However, they did not compare their PAH cohort with other cohorts that represent these other ‘resistin-relevant diseases’ and thus do not know whether they can distinguish PAH from any of these other diseases based on simply the resistin levels.” The frequency of comorbidities in patients with PAH, such as obesity, other inflammatory diseases, and cardiovascular disease, could confound the resistin levels.
The study was supported by the National Institutes of Health. Neither the researchers nor Dr. Chan had financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Resistin, a cytokine expressed in adipocytes, has been associated with poor clinical outcomes in heart failure and cardiovascular disease, Li Gao, MD, of Johns Hopkins University, Baltimore, Maryland, and colleagues wrote. While mouse studies have shown that human resistin drives pulmonary vascular remodeling and the development of PAH, the role of resistin as a biomarker for PAH remains unclear.
In a study published in Respiratory Research, the researchers reviewed biospecimens and clinical and genetic data from 1121 adults with PAH, 808 with idiopathic PAH (IPAH), and 313 with scleroderma-associated PAH (SSc-PAH). They examined the associations between serum resistin levels and PAH outcomes in multivariate regression models, using machine-learning algorithms to develop models to predict mortality.
Resistin levels were significantly higher in all patients with PAH and patients with the two subtypes than in control participants (all P < .0001). Resistin was also associated with significant discriminative properties, with area under the curve (AUC) measures of 0.84, 0.82, and 0.91 for PAH overall, IPAH, and SSc-PAH, respectively.
Elevated resistin levels (defined as > 4.54 ng/mL) were significantly associated with an increased risk for death (hazard ratio, 2.6; P < .0087) as well as with older age and shorter distance on the 6-minute walk test (P = .001 for both) and reduced cardiac capacity based on the New York Heart Association functional class (P < .014).
Survival models derived from machine learning confirmed the prognostic value of resistin for mortality in PAH as seen in the random forest model, with an AUC of 0.70. “When we used the AUC values of the ROC curve as criteria to evaluate how well resistin levels discerned the presence of PAH, all three tests had excellent discriminative ability (AUCs were 0.84, 0.82, and 0.91 for all PAH, IPAH, and SSc-PAH, respectively),” the researchers wrote.
The researchers also evaluated three RETN genetic variants (rs7408174, rs3219175, and rs3745367) for a specific association with serum resistin levels and measures of PAH severity. Resistin levels were highest among individuals who were carriers of either the rs3219175 or rs3745367 mutation, the researchers noted.
The findings were limited by several factors, including missing data on the 6-minute walk test from several centers, which led to the elimination of that item from the survival analysis. Other limitations included the inability to control for PAH therapy at the time of assessment and the collection of serum at a different time from other clinical variables.
However, “our study provides evidence to support the use of circulating biomarkers as objective and accessible tools for noninvasive PAH risk stratification,” the researchers said. Additional research is needed to strengthen the association, but the findings suggest that resistin represents a novel biomarker for PAH prognostication and risk stratification and may have implications for the development of new treatments.
Biomarker Research Expands Diagnosis and Treatment Horizons
“It is a dynamic time in PAH research and clinical management, given the recent approval and use of the BMP/TGF beta balancing agent sotatercept (Winrevair) as an effective agent to target the molecular origins of this disease,” Stephen Chan, MD, professor of medicine and director of the Vascular Medicine Institute at the University of Pittsburgh, Pittsburgh, Pennsylvania, said in an interview.
The growing number of medications that can be used to treat patients with PAH will likely be more effective if patients are identified and treated early, said Dr. Chan, who was not involved in the study.
However, the time to diagnosis for patients with PAH is still more than 3 years from the start of symptoms, he said. Factors contributing to the delay include the requirement of an invasive cardiac catheterization procedure to make the final diagnosis, the status of PAH as a borderline orphan disease, and the often nonspecific nature of the initial symptoms of PAH.
Consequently, “there is an unmet need to develop effective and preferably noninvasive tools to aid in early diagnosis of PAH,” Dr. Chan added.
The power of the study is in the number of patients included, as much of previous PAH research has involved small studies of patients that could not be replicated or did not generalize to the larger patient population, Dr. Chan said.
The use of the PAH Biobank allows researchers to access a larger population of patients with PAH. “With that in mind, it is not surprising that some markers would emerge as potentially powerful and clinically meaningful,” he said.
“Currently, we do not have a reliable blood-based biomarker that we use in clinical PAH practice, although there are emerging studies that suggest other markers such as metabolites, RNA molecules, and proteins that may serve in the same capacity. If these studies turn out to be reproducible, generalizable, and specific to PAH in larger populations, measuring resistin could be helpful in making early diagnosis, particularly in areas that do not have invasive catheterization facilities (and globally) and for nonspecialists who are puzzled about the nonspecificity of initial symptoms of PAH,” Dr. Chan said.
Resistin could also be incorporated into existing risk stratification scores, such as the REVEAL risk score, that are already used in PAH clinical practice as guidance for when and how to use currently approved medications, he added.
Limitations of the study included the focus only on resistin alone, not in combination with other molecules that might perform better. Also, no independent validation cohort was used, he noted. “While PAH Biobank certainly offered larger numbers than we typically see, we would have to see validation in large independent cohorts for us to be convinced that measurements of resistin should be used in clinical practice.”
Resistin is not specific to PAH, which makes interpretation of the results more complicated, said Dr. Chan. “In this study, the authors used a smaller healthy control cohort of 50 patients as a comparison to their PAH cohort. However, they did not compare their PAH cohort with other cohorts that represent these other ‘resistin-relevant diseases’ and thus do not know whether they can distinguish PAH from any of these other diseases based on simply the resistin levels.” The frequency of comorbidities in patients with PAH, such as obesity, other inflammatory diseases, and cardiovascular disease, could confound the resistin levels.
The study was supported by the National Institutes of Health. Neither the researchers nor Dr. Chan had financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Resistin, a cytokine expressed in adipocytes, has been associated with poor clinical outcomes in heart failure and cardiovascular disease, Li Gao, MD, of Johns Hopkins University, Baltimore, Maryland, and colleagues wrote. While mouse studies have shown that human resistin drives pulmonary vascular remodeling and the development of PAH, the role of resistin as a biomarker for PAH remains unclear.
In a study published in Respiratory Research, the researchers reviewed biospecimens and clinical and genetic data from 1121 adults with PAH, 808 with idiopathic PAH (IPAH), and 313 with scleroderma-associated PAH (SSc-PAH). They examined the associations between serum resistin levels and PAH outcomes in multivariate regression models, using machine-learning algorithms to develop models to predict mortality.
Resistin levels were significantly higher in all patients with PAH and patients with the two subtypes than in control participants (all P < .0001). Resistin was also associated with significant discriminative properties, with area under the curve (AUC) measures of 0.84, 0.82, and 0.91 for PAH overall, IPAH, and SSc-PAH, respectively.
Elevated resistin levels (defined as > 4.54 ng/mL) were significantly associated with an increased risk for death (hazard ratio, 2.6; P < .0087) as well as with older age and shorter distance on the 6-minute walk test (P = .001 for both) and reduced cardiac capacity based on the New York Heart Association functional class (P < .014).
Survival models derived from machine learning confirmed the prognostic value of resistin for mortality in PAH as seen in the random forest model, with an AUC of 0.70. “When we used the AUC values of the ROC curve as criteria to evaluate how well resistin levels discerned the presence of PAH, all three tests had excellent discriminative ability (AUCs were 0.84, 0.82, and 0.91 for all PAH, IPAH, and SSc-PAH, respectively),” the researchers wrote.
The researchers also evaluated three RETN genetic variants (rs7408174, rs3219175, and rs3745367) for a specific association with serum resistin levels and measures of PAH severity. Resistin levels were highest among individuals who were carriers of either the rs3219175 or rs3745367 mutation, the researchers noted.
The findings were limited by several factors, including missing data on the 6-minute walk test from several centers, which led to the elimination of that item from the survival analysis. Other limitations included the inability to control for PAH therapy at the time of assessment and the collection of serum at a different time from other clinical variables.
However, “our study provides evidence to support the use of circulating biomarkers as objective and accessible tools for noninvasive PAH risk stratification,” the researchers said. Additional research is needed to strengthen the association, but the findings suggest that resistin represents a novel biomarker for PAH prognostication and risk stratification and may have implications for the development of new treatments.
Biomarker Research Expands Diagnosis and Treatment Horizons
“It is a dynamic time in PAH research and clinical management, given the recent approval and use of the BMP/TGF beta balancing agent sotatercept (Winrevair) as an effective agent to target the molecular origins of this disease,” Stephen Chan, MD, professor of medicine and director of the Vascular Medicine Institute at the University of Pittsburgh, Pittsburgh, Pennsylvania, said in an interview.
The growing number of medications that can be used to treat patients with PAH will likely be more effective if patients are identified and treated early, said Dr. Chan, who was not involved in the study.
However, the time to diagnosis for patients with PAH is still more than 3 years from the start of symptoms, he said. Factors contributing to the delay include the requirement of an invasive cardiac catheterization procedure to make the final diagnosis, the status of PAH as a borderline orphan disease, and the often nonspecific nature of the initial symptoms of PAH.
Consequently, “there is an unmet need to develop effective and preferably noninvasive tools to aid in early diagnosis of PAH,” Dr. Chan added.
The power of the study is in the number of patients included, as much of previous PAH research has involved small studies of patients that could not be replicated or did not generalize to the larger patient population, Dr. Chan said.
The use of the PAH Biobank allows researchers to access a larger population of patients with PAH. “With that in mind, it is not surprising that some markers would emerge as potentially powerful and clinically meaningful,” he said.
“Currently, we do not have a reliable blood-based biomarker that we use in clinical PAH practice, although there are emerging studies that suggest other markers such as metabolites, RNA molecules, and proteins that may serve in the same capacity. If these studies turn out to be reproducible, generalizable, and specific to PAH in larger populations, measuring resistin could be helpful in making early diagnosis, particularly in areas that do not have invasive catheterization facilities (and globally) and for nonspecialists who are puzzled about the nonspecificity of initial symptoms of PAH,” Dr. Chan said.
Resistin could also be incorporated into existing risk stratification scores, such as the REVEAL risk score, that are already used in PAH clinical practice as guidance for when and how to use currently approved medications, he added.
Limitations of the study included the focus only on resistin alone, not in combination with other molecules that might perform better. Also, no independent validation cohort was used, he noted. “While PAH Biobank certainly offered larger numbers than we typically see, we would have to see validation in large independent cohorts for us to be convinced that measurements of resistin should be used in clinical practice.”
Resistin is not specific to PAH, which makes interpretation of the results more complicated, said Dr. Chan. “In this study, the authors used a smaller healthy control cohort of 50 patients as a comparison to their PAH cohort. However, they did not compare their PAH cohort with other cohorts that represent these other ‘resistin-relevant diseases’ and thus do not know whether they can distinguish PAH from any of these other diseases based on simply the resistin levels.” The frequency of comorbidities in patients with PAH, such as obesity, other inflammatory diseases, and cardiovascular disease, could confound the resistin levels.
The study was supported by the National Institutes of Health. Neither the researchers nor Dr. Chan had financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Almost 50% of Global Dementia Cases May Be Preventable
PHILADELPHIA – a report from the Lancet Commission on dementia prevention, intervention, and care.
The report adds two new modifiable risk factors for dementia — high cholesterol and vision loss — to the 12 risk factors identified in the 2020 Lancet Commission report, which were linked to about 40% of all dementia cases.
The original Lancet Commission report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one third of dementia cases.
“Our new report reveals that there is much more that can and should be done to reduce the risk of dementia. It’s never too early or too late to act, with opportunities to make an impact at any stage of life,” lead author Gill Livingston, MD, from University College London in England, said in a statement.
The 57-page report was published online in The Lancet Neurology (to coincide with its presentation at the 2024 Alzheimer’s Association International Conference (AAIC).
‘Compelling’ New Evidence
The 12 risk factors cited in the 2020 report are lower levels of education, hearing loss, hypertension, smoking, obesity, depression, physical inactivity, diabetes, excessive alcohol consumption, traumatic brain injury (TBI), air pollution, and social isolation.
According to the authors of the current report, there is “new compelling evidence” that untreated vision loss and elevated low-density lipoprotein (LDL) cholesterol are also risk factors for dementia.
These two added risk factors are associated with 9% of all dementia cases — with an estimated 7% of cases caused by high LDL cholesterol from about age 40 years, and 2% of cases caused by untreated vision loss in later life, the authors said.
Out of all 14 risk factors, those tied to the greatest proportion of dementia in the global population are hearing impairment and high LDL cholesterol (7% each), along with less education in early life, and social isolation in later life (5% each), the report estimates.
The new report also outlines 13 recommendations aimed at individuals and governments to help guard against dementia. They include preventing and treating hearing loss, vision loss, and depression; being cognitively active throughout life; using head protection in contact sports; reducing vascular risk factors (high cholesterol, diabetes, obesity, hypertension); improving air quality; and providing supportive community environments to increase social contact.
Tara Spires-Jones, PhD, president of the British Neuroscience Association, emphasized that, while this research doesn’t directly link specific factors to dementia, it supports evidence that a healthy lifestyle — encompassing education, social activities, exercise, cognitive engagement, and avoiding head injuries and harmful factors for heart and lung health — can enhance brain resilience and prevent dementia.
In an interview, Heather M. Snyder, PhD, senior vice president of medical and scientific relations, Alzheimer’s Association, said: “Our brains are complex and what happens throughout our lives may increase or decrease our risk for dementia as we age. Protecting brain health as we age requires a comprehensive approach that includes discussions on diet, exercise, heart health, hearing, and vision.”
Also weighing in on the new report, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, said the addition of high cholesterol is “particularly noteworthy as it reinforces the intricate connection between vascular health and brain health — a link we’ve long suspected but can now target more effectively.”
As for vision loss, “it’s not just a matter of seeing clearly; it’s a matter of thinking clearly. Untreated vision loss can lead to social isolation, reduced physical activity, and cognitive decline,” said Dr. Lakhan.
Dementia Is Not Inevitable
In his view, “the potential to prevent or delay nearly half of dementia cases by addressing these risk factors is nothing short of revolutionary. It shifts our perspective from viewing dementia as an inevitable part of aging to seeing it as a condition we can actively work to prevent,” Dr. Lakhan added.
He said the report’s emphasis on health equity is also important.
“Dementia risk factors disproportionately affect socioeconomically disadvantaged groups and low- and middle-income countries. Addressing these disparities isn’t just a matter of fairness in the fight against dementia, equality in prevention is as important as equality in treatment,” Dr. Lakhan commented.
While the report offers hope, it also presents a challenge, he said.
Implementing the recommended preventive measures requires a “coordinated effort from individuals, healthcare systems, and policymakers. The potential benefits, both in terms of quality of life and economic savings, make this effort not just worthwhile but imperative. Preventing dementia is not just a medical imperative — it’s an economic and humanitarian one,” Dr. Lakhan said.
Masud Husain, PhD, with the University of Oxford in England, agreed.
The conclusions in this report are “very important for all of us, but particularly for health policy makers and government,” he said.
“If we did simple things well such as screening for some of the factors identified in this report, with adequate resources to perform this, we have the potential to prevent dementia on a national scale. This would be far more cost effective than developing high-tech treatments, which so far have been disappointing in their impacts on people with established dementia,” Dr. Husain said.
The Lancet Commission was funded by University College London, Alzheimer’s Society, Alzheimer’s Research UK, and the Economic and Social Research Council. A complete list of author disclosures is available with the original article. Dr. Snyder, Dr. Lakhan, Dr. Husain and Dr. Spires-Jones have no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA – a report from the Lancet Commission on dementia prevention, intervention, and care.
The report adds two new modifiable risk factors for dementia — high cholesterol and vision loss — to the 12 risk factors identified in the 2020 Lancet Commission report, which were linked to about 40% of all dementia cases.
The original Lancet Commission report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one third of dementia cases.
“Our new report reveals that there is much more that can and should be done to reduce the risk of dementia. It’s never too early or too late to act, with opportunities to make an impact at any stage of life,” lead author Gill Livingston, MD, from University College London in England, said in a statement.
The 57-page report was published online in The Lancet Neurology (to coincide with its presentation at the 2024 Alzheimer’s Association International Conference (AAIC).
‘Compelling’ New Evidence
The 12 risk factors cited in the 2020 report are lower levels of education, hearing loss, hypertension, smoking, obesity, depression, physical inactivity, diabetes, excessive alcohol consumption, traumatic brain injury (TBI), air pollution, and social isolation.
According to the authors of the current report, there is “new compelling evidence” that untreated vision loss and elevated low-density lipoprotein (LDL) cholesterol are also risk factors for dementia.
These two added risk factors are associated with 9% of all dementia cases — with an estimated 7% of cases caused by high LDL cholesterol from about age 40 years, and 2% of cases caused by untreated vision loss in later life, the authors said.
Out of all 14 risk factors, those tied to the greatest proportion of dementia in the global population are hearing impairment and high LDL cholesterol (7% each), along with less education in early life, and social isolation in later life (5% each), the report estimates.
The new report also outlines 13 recommendations aimed at individuals and governments to help guard against dementia. They include preventing and treating hearing loss, vision loss, and depression; being cognitively active throughout life; using head protection in contact sports; reducing vascular risk factors (high cholesterol, diabetes, obesity, hypertension); improving air quality; and providing supportive community environments to increase social contact.
Tara Spires-Jones, PhD, president of the British Neuroscience Association, emphasized that, while this research doesn’t directly link specific factors to dementia, it supports evidence that a healthy lifestyle — encompassing education, social activities, exercise, cognitive engagement, and avoiding head injuries and harmful factors for heart and lung health — can enhance brain resilience and prevent dementia.
In an interview, Heather M. Snyder, PhD, senior vice president of medical and scientific relations, Alzheimer’s Association, said: “Our brains are complex and what happens throughout our lives may increase or decrease our risk for dementia as we age. Protecting brain health as we age requires a comprehensive approach that includes discussions on diet, exercise, heart health, hearing, and vision.”
Also weighing in on the new report, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, said the addition of high cholesterol is “particularly noteworthy as it reinforces the intricate connection between vascular health and brain health — a link we’ve long suspected but can now target more effectively.”
As for vision loss, “it’s not just a matter of seeing clearly; it’s a matter of thinking clearly. Untreated vision loss can lead to social isolation, reduced physical activity, and cognitive decline,” said Dr. Lakhan.
Dementia Is Not Inevitable
In his view, “the potential to prevent or delay nearly half of dementia cases by addressing these risk factors is nothing short of revolutionary. It shifts our perspective from viewing dementia as an inevitable part of aging to seeing it as a condition we can actively work to prevent,” Dr. Lakhan added.
He said the report’s emphasis on health equity is also important.
“Dementia risk factors disproportionately affect socioeconomically disadvantaged groups and low- and middle-income countries. Addressing these disparities isn’t just a matter of fairness in the fight against dementia, equality in prevention is as important as equality in treatment,” Dr. Lakhan commented.
While the report offers hope, it also presents a challenge, he said.
Implementing the recommended preventive measures requires a “coordinated effort from individuals, healthcare systems, and policymakers. The potential benefits, both in terms of quality of life and economic savings, make this effort not just worthwhile but imperative. Preventing dementia is not just a medical imperative — it’s an economic and humanitarian one,” Dr. Lakhan said.
Masud Husain, PhD, with the University of Oxford in England, agreed.
The conclusions in this report are “very important for all of us, but particularly for health policy makers and government,” he said.
“If we did simple things well such as screening for some of the factors identified in this report, with adequate resources to perform this, we have the potential to prevent dementia on a national scale. This would be far more cost effective than developing high-tech treatments, which so far have been disappointing in their impacts on people with established dementia,” Dr. Husain said.
The Lancet Commission was funded by University College London, Alzheimer’s Society, Alzheimer’s Research UK, and the Economic and Social Research Council. A complete list of author disclosures is available with the original article. Dr. Snyder, Dr. Lakhan, Dr. Husain and Dr. Spires-Jones have no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA – a report from the Lancet Commission on dementia prevention, intervention, and care.
The report adds two new modifiable risk factors for dementia — high cholesterol and vision loss — to the 12 risk factors identified in the 2020 Lancet Commission report, which were linked to about 40% of all dementia cases.
The original Lancet Commission report, published in 2017, identified nine modifiable risk factors that were estimated to be responsible for one third of dementia cases.
“Our new report reveals that there is much more that can and should be done to reduce the risk of dementia. It’s never too early or too late to act, with opportunities to make an impact at any stage of life,” lead author Gill Livingston, MD, from University College London in England, said in a statement.
The 57-page report was published online in The Lancet Neurology (to coincide with its presentation at the 2024 Alzheimer’s Association International Conference (AAIC).
‘Compelling’ New Evidence
The 12 risk factors cited in the 2020 report are lower levels of education, hearing loss, hypertension, smoking, obesity, depression, physical inactivity, diabetes, excessive alcohol consumption, traumatic brain injury (TBI), air pollution, and social isolation.
According to the authors of the current report, there is “new compelling evidence” that untreated vision loss and elevated low-density lipoprotein (LDL) cholesterol are also risk factors for dementia.
These two added risk factors are associated with 9% of all dementia cases — with an estimated 7% of cases caused by high LDL cholesterol from about age 40 years, and 2% of cases caused by untreated vision loss in later life, the authors said.
Out of all 14 risk factors, those tied to the greatest proportion of dementia in the global population are hearing impairment and high LDL cholesterol (7% each), along with less education in early life, and social isolation in later life (5% each), the report estimates.
The new report also outlines 13 recommendations aimed at individuals and governments to help guard against dementia. They include preventing and treating hearing loss, vision loss, and depression; being cognitively active throughout life; using head protection in contact sports; reducing vascular risk factors (high cholesterol, diabetes, obesity, hypertension); improving air quality; and providing supportive community environments to increase social contact.
Tara Spires-Jones, PhD, president of the British Neuroscience Association, emphasized that, while this research doesn’t directly link specific factors to dementia, it supports evidence that a healthy lifestyle — encompassing education, social activities, exercise, cognitive engagement, and avoiding head injuries and harmful factors for heart and lung health — can enhance brain resilience and prevent dementia.
In an interview, Heather M. Snyder, PhD, senior vice president of medical and scientific relations, Alzheimer’s Association, said: “Our brains are complex and what happens throughout our lives may increase or decrease our risk for dementia as we age. Protecting brain health as we age requires a comprehensive approach that includes discussions on diet, exercise, heart health, hearing, and vision.”
Also weighing in on the new report, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, said the addition of high cholesterol is “particularly noteworthy as it reinforces the intricate connection between vascular health and brain health — a link we’ve long suspected but can now target more effectively.”
As for vision loss, “it’s not just a matter of seeing clearly; it’s a matter of thinking clearly. Untreated vision loss can lead to social isolation, reduced physical activity, and cognitive decline,” said Dr. Lakhan.
Dementia Is Not Inevitable
In his view, “the potential to prevent or delay nearly half of dementia cases by addressing these risk factors is nothing short of revolutionary. It shifts our perspective from viewing dementia as an inevitable part of aging to seeing it as a condition we can actively work to prevent,” Dr. Lakhan added.
He said the report’s emphasis on health equity is also important.
“Dementia risk factors disproportionately affect socioeconomically disadvantaged groups and low- and middle-income countries. Addressing these disparities isn’t just a matter of fairness in the fight against dementia, equality in prevention is as important as equality in treatment,” Dr. Lakhan commented.
While the report offers hope, it also presents a challenge, he said.
Implementing the recommended preventive measures requires a “coordinated effort from individuals, healthcare systems, and policymakers. The potential benefits, both in terms of quality of life and economic savings, make this effort not just worthwhile but imperative. Preventing dementia is not just a medical imperative — it’s an economic and humanitarian one,” Dr. Lakhan said.
Masud Husain, PhD, with the University of Oxford in England, agreed.
The conclusions in this report are “very important for all of us, but particularly for health policy makers and government,” he said.
“If we did simple things well such as screening for some of the factors identified in this report, with adequate resources to perform this, we have the potential to prevent dementia on a national scale. This would be far more cost effective than developing high-tech treatments, which so far have been disappointing in their impacts on people with established dementia,” Dr. Husain said.
The Lancet Commission was funded by University College London, Alzheimer’s Society, Alzheimer’s Research UK, and the Economic and Social Research Council. A complete list of author disclosures is available with the original article. Dr. Snyder, Dr. Lakhan, Dr. Husain and Dr. Spires-Jones have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAIC 2024
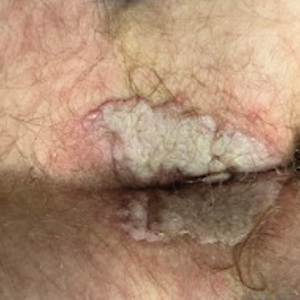
Painful Anal Lesions in a Patient With HIV
The Diagnosis: Condyloma Latum
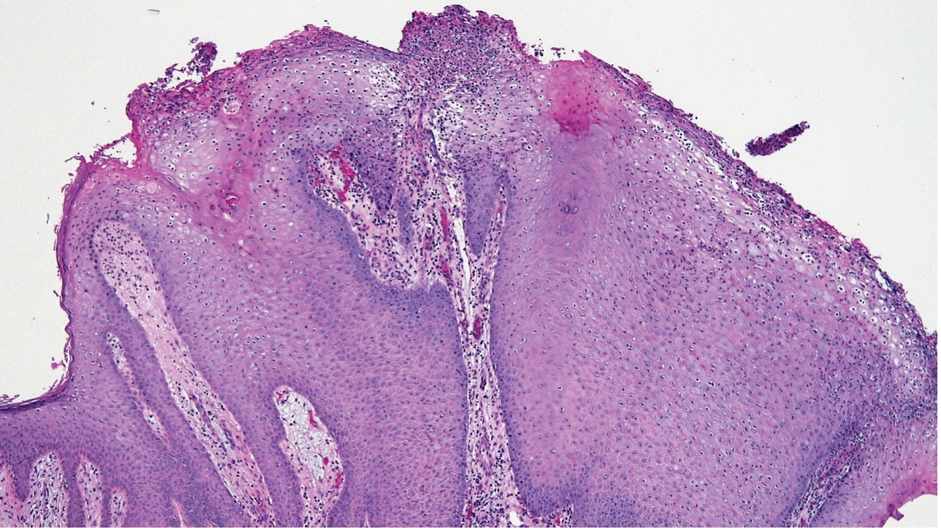
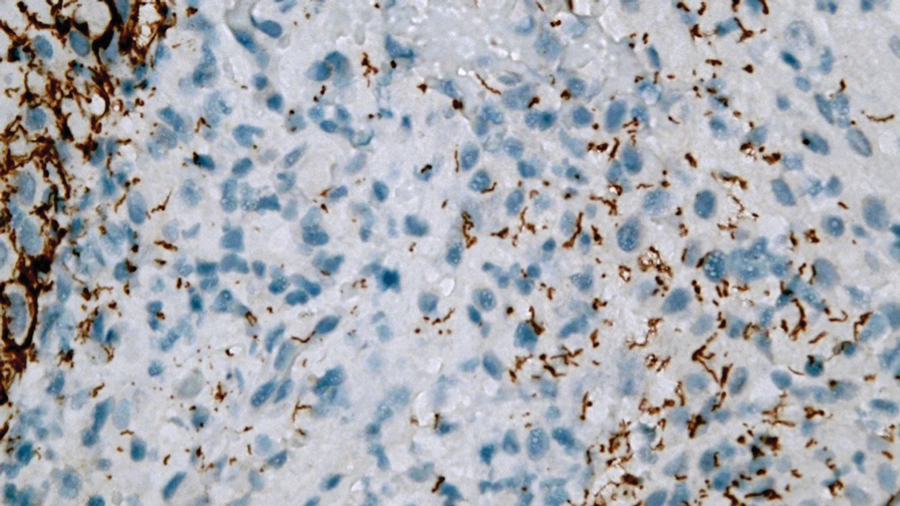
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.
Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
The Diagnosis: Condyloma Latum
Laboratory test results were notable for a rapid plasma reagin titer of 1:512, a positive Treponema pallidum particle agglutination test, negative rectal nucleic acid amplification tests for gonorrhea and chlamydia, and a negative herpes simplex virus polymerase chain reaction. A VDRL test of cerebrospinal fluid from a lumbar puncture was negative. Histopathology of the punch biopsy sample revealed marked verrucous epidermal hyperplasia without keratinocytic atypia and with mixed inflammation (Figure 1), while immunohistochemical staining showed numerus T pallidum organisms (Figure 2). A diagnosis of condyloma latum was made based on the laboratory, lumbar puncture, and punch biopsy results. Due to a penicillin allergy, the patient was treated with oral doxycycline for 14 days. On follow-up at day 12 of therapy, he reported cessation of rectal pain, and resolution of anal lesions was noted on physical examination.


Condylomata lata are highly infectious cutaneous lesions that can manifest during secondary syphilis.1 They typically are described as white or gray, raised, flatappearing plaques and occur in moist areas or skin folds including the anus, scrotum, and vulva. However, these lesions also have been reported in the axillae, umbilicus, nasolabial folds, and other anatomic areas.1,2 The lesions can be painful and often manifest in multiples, especially in patients living with HIV.3
Condylomata lata can have a verrucous appearance and may mimic other anogenital lesions, such as condylomata acuminata, genital herpes, and malignant tumors, leading to an initial misdiagnosis.1,2 Condylomata lata should always be included in the differential when evaluating anogenital lesions. Other conditions in the differential diagnosis include psoriasis, typically manifesting as erythematous plaques with silver scale, and molluscum contagiosum, appearing as small umbilicated papules on physical examination.
Condylomata lata have been reported to occur in 6% to 23% of patients with secondary syphilis.1 Although secondary syphilis more typically manifests with a diffuse maculopapular rash, condylomata lata may be the sole dermatologic manifestation.4
Histopathology of condylomata lata consists of epithelial hyperplasia as well as lymphocytic and plasma cell infiltrates. It is diagnosed by serologic testing as well as immunohistochemical staining or dark-field microscopy.
First-line treatment of secondary syphilis is a single dose of benzathine penicillin G administered intramuscularly.5 However, a 14-day course of oral doxycycline can be used in patients with a penicillin allergy. When compliance and follow-up cannot be guaranteed, penicillin desensitization and treatment with benzathine penicillin G is recommended. Clinical evaluation and repeat serologic testing should be performed at 6 and 12 months follow-up, or more frequently if clinically indicated.5
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21. doi:10.1016/j.jdcr.2021.01.025
- Liu Z, Wang L, Zhang G, et al. Warty mucosal lesions: oral condyloma lata of secondary syphilis. Indian J Dermatol Venereol Leprol. 2017;83:277. doi:10.4103/0378-6323.191129
- Rompalo AM, Joesoef MR, O’Donnell JA, et al; Syphilis and HIV Study Group. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis.2001;28:158-165.
- Kumar P, Das A, Mondal A. Secondary syphilis: an unusual presentation. Indian J Sex Transm Dis AIDS. 2017;38:98-99. doi:10.4103/0253-7184.194318
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
A 24-year-old man presented to the emergency department with rectal pain and lesions of 3 weeks’ duration that were progressively worsening. He had a medical history of poorly controlled HIV, cerebral toxoplasmosis, and genital herpes, as well as a social history of sexual activity with other men.
He had been diagnosed with HIV 7 years prior and had been off therapy until 1 year prior to the current presentation, when he was hospitalized with encephalopathy (CD4 count, <50 cells/mm3). A diagnosis of cerebral toxoplasmosis was made, and he began a treatment regimen of sulfadiazine, pyrimethamine, and leucovorin, as well as bictegravir, emtricitabine, and tenofovir alafenamide. Since then, the patient admitted to difficulty with medication adherence.
Rapid plasma reagin, gonorrhea, and chlamydia testing were negative during a routine workup 6 months prior to the current presentation. He initially presented to an urgent care clinic for evaluation of the rectal pain and lesions and was treated empirically with topical podofilox. He presented to the emergency department 1 week later (3 weeks after symptom onset) with anal warts and apparent vesicular lesions. Empiric treatment with oral valacyclovir was prescribed.
Despite these treatments, the rectal pain became severe—especially upon sitting, defecation, and physical exertion—prompting further evaluation. Physical examination revealed soft, flat-topped, moist-appearing, gray plaques with minimal surrounding erythema at the anus. Laboratory test results demonstrated a CD4 count of 161 cells/mm3 and an HIV viral load of 137 copies/mL.
New Study Says Your Sedentary Lifestyle Is Killing You
TOPLINE:
METHODOLOGY:
- Researchers evaluated the association between PA and ST with the risk for mortality in 5836 middle-aged and older Australian adults (mean age, 56.4 years; 45% men) from the Australian Diabetes, Obesity and Lifestyle Study.
- The Physical Activity and Sitting Time Balance Index (PASTBI) was calculated by dividing the total duration of daily PA by the duration of daily ST.
- Participants were categorized into quartiles on the basis of their PASTBI score, ranging from low PA/high ST to high PA/low ST.
- The primary outcome was all-cause mortality.
TAKEAWAY:
- During a median follow-up time of 14.3 years, 885 (15%) all-cause deaths were reported.
- The risk for all-cause mortality was 47% higher in participants with lower engagement in PA and higher ST (low PASTBI) than those with higher engagement in PA and lower ST (high PASTBI; adjusted hazard ratio, 1.47; 95% confidence interval, 1.21-1.79).
IN PRACTICE:
“The utility of the PASTBI in identifying relationships with mortality risk further highlights the importance of achieving a healthier balance in the dual health behaviors of PA [physical activity] and ST [sitting time],” the authors wrote.
SOURCE:
The study was led by Roslin Botlero, MBBS, MPH, PhD, of the School of Public Health and Preventive Medicine at Monash University in Melbourne, Australia. It was published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study relied on self-reported data for PA and ST, which may have introduced recall or reporting bias. The generalizability of the findings is restricted to a specific set of self-reported questionnaires. Even after adjustment for several potential confounders, other unmeasured or unknown confounders may have influenced the association between PASTBI and all-cause mortality.
DISCLOSURES:
The Australian Diabetes, Obesity and Lifestyle Study was sponsored by the National Health and Medical Research Council, the Australian Government Department of Health and Aged Care, and others. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated the association between PA and ST with the risk for mortality in 5836 middle-aged and older Australian adults (mean age, 56.4 years; 45% men) from the Australian Diabetes, Obesity and Lifestyle Study.
- The Physical Activity and Sitting Time Balance Index (PASTBI) was calculated by dividing the total duration of daily PA by the duration of daily ST.
- Participants were categorized into quartiles on the basis of their PASTBI score, ranging from low PA/high ST to high PA/low ST.
- The primary outcome was all-cause mortality.
TAKEAWAY:
- During a median follow-up time of 14.3 years, 885 (15%) all-cause deaths were reported.
- The risk for all-cause mortality was 47% higher in participants with lower engagement in PA and higher ST (low PASTBI) than those with higher engagement in PA and lower ST (high PASTBI; adjusted hazard ratio, 1.47; 95% confidence interval, 1.21-1.79).
IN PRACTICE:
“The utility of the PASTBI in identifying relationships with mortality risk further highlights the importance of achieving a healthier balance in the dual health behaviors of PA [physical activity] and ST [sitting time],” the authors wrote.
SOURCE:
The study was led by Roslin Botlero, MBBS, MPH, PhD, of the School of Public Health and Preventive Medicine at Monash University in Melbourne, Australia. It was published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study relied on self-reported data for PA and ST, which may have introduced recall or reporting bias. The generalizability of the findings is restricted to a specific set of self-reported questionnaires. Even after adjustment for several potential confounders, other unmeasured or unknown confounders may have influenced the association between PASTBI and all-cause mortality.
DISCLOSURES:
The Australian Diabetes, Obesity and Lifestyle Study was sponsored by the National Health and Medical Research Council, the Australian Government Department of Health and Aged Care, and others. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers evaluated the association between PA and ST with the risk for mortality in 5836 middle-aged and older Australian adults (mean age, 56.4 years; 45% men) from the Australian Diabetes, Obesity and Lifestyle Study.
- The Physical Activity and Sitting Time Balance Index (PASTBI) was calculated by dividing the total duration of daily PA by the duration of daily ST.
- Participants were categorized into quartiles on the basis of their PASTBI score, ranging from low PA/high ST to high PA/low ST.
- The primary outcome was all-cause mortality.
TAKEAWAY:
- During a median follow-up time of 14.3 years, 885 (15%) all-cause deaths were reported.
- The risk for all-cause mortality was 47% higher in participants with lower engagement in PA and higher ST (low PASTBI) than those with higher engagement in PA and lower ST (high PASTBI; adjusted hazard ratio, 1.47; 95% confidence interval, 1.21-1.79).
IN PRACTICE:
“The utility of the PASTBI in identifying relationships with mortality risk further highlights the importance of achieving a healthier balance in the dual health behaviors of PA [physical activity] and ST [sitting time],” the authors wrote.
SOURCE:
The study was led by Roslin Botlero, MBBS, MPH, PhD, of the School of Public Health and Preventive Medicine at Monash University in Melbourne, Australia. It was published online in the American Journal of Preventive Medicine.
LIMITATIONS:
The study relied on self-reported data for PA and ST, which may have introduced recall or reporting bias. The generalizability of the findings is restricted to a specific set of self-reported questionnaires. Even after adjustment for several potential confounders, other unmeasured or unknown confounders may have influenced the association between PASTBI and all-cause mortality.
DISCLOSURES:
The Australian Diabetes, Obesity and Lifestyle Study was sponsored by the National Health and Medical Research Council, the Australian Government Department of Health and Aged Care, and others. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Changing the tumor board conversation: Immunotherapy in resectable NSCLC
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4
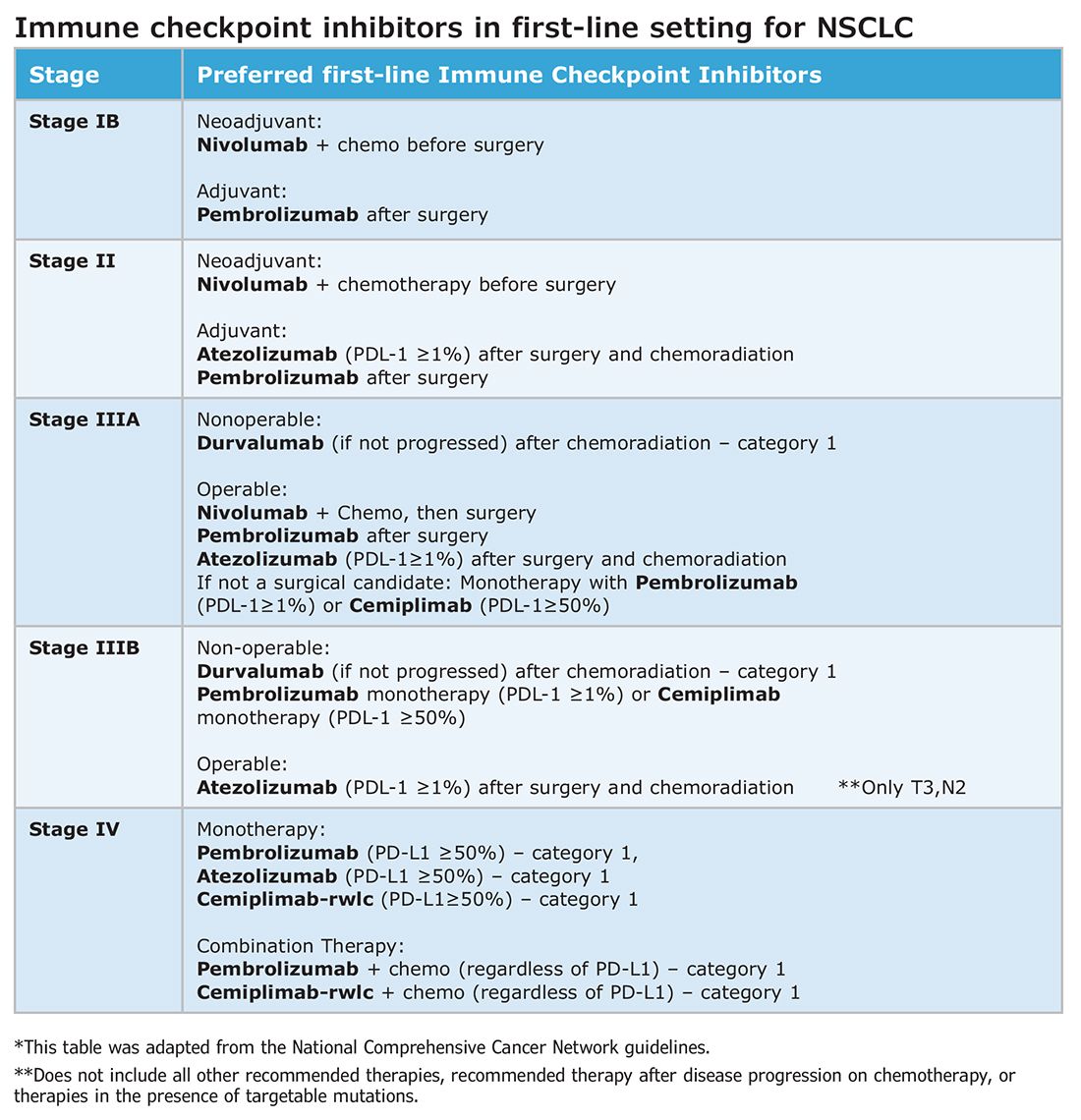
Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
CMS Proposes Maternal-Health Conditions-of-Participation Standards
Federal officials intend to compel US hospitals to improve obstetrical services, with a plan that could result in a potential loss of Medicare and Medicaid funds for institutions that fail to comply with the demands.
The Centers for Medicare and Medicaid Services (CMS) on July 10 announced this proposal, tucking its plan for new conditions of participation (COP) for obstetrician services into the draft 2025 rule on Medicare payments for outpatient hospital services.
The COP requirements are considered the most powerful tool CMS has for trying to improve the quality of medical care. With the new obstetric COP requirement, CMS said it intends to address what it sees as potential shortfalls in training, staffing, transfer protocols, and emergency services readiness.
In practice, hospitals, CMS, and accrediting bodies such as the Joint Commission usually try to address deficiencies to prevent what would be a devastating financial loss for a hospital.
“CMS is using all of our tools to improve the safety, quality, and timeliness of the care that hospitals provide to pregnant women,” Dora Hughes, MD, MPH, acting chief medical officer of the agency, said in a press release about the proposal.
CMS estimated the proposal may add new annual expenses of $70,671 per hospital. For comparison, this figure would represent far less than 1% of the total $1.4 trillion spent on hospital care in the United States in 2022.
CMS said it is trying to address the reasons women in the United States face more risk in giving birth than those in other nations. There were 22 maternal deaths for every 100,000 live births in this country in 2022, compared with 8.6 deaths per 100,000 live births or lower that year in Canada, France, the United Kingdom, Germany, and Japan, CMS said.
But CMS is seeking to impose this new requirement at a time amid growing concerns about “maternity care deserts.”
Reasonable Asks?
Between 2011 and 2021, one out of every four rural hospitals in America stopped providing obstetrics services, Senate Finance Chairman Ron Wyden (D-Ore.) said at a May hearing. Mr. Wyden last year was part of a fight to try to prevent the closure of a birthing center in Baker City in rural eastern Oregon.
The federal government should focus first on helping hospitals keep obstetrical facilities open, said Elizabeth Powers, MD, MHA, the health services officer of the Winding Waters Clinic in Enterprise, Oregon.
“Until we can ensure access to services, we can’t even work on quality,” Dr. Powers told this news organization. “If you’re thinking about a Maslow’s hierarchy of achieving health outcomes, access is your foundation, and without a shift in payment, that foundation is eroded.”
In the draft rule, CMS sketched broad mandates about staffing and training. For example, the agency proposes requiring if a hospital offers obstetrical services, “the services must be well organized and provided in accordance with nationally recognized acceptable standards of practice.”
That means CMS likely will need to provide further guidance for hospitals if it proceeds with this plan for obstetric COP requirements, said Soumi Saha, PharmD, JD, senior vice president of government affairs at Premier Inc., a healthcare consultancy and purchasing organization.
Premier is among the many groups, including the American Hospital Association, that oppose the COP proposal.
Dr. Saha said a better approach would be to consolidate the work being done through the US Department of Health and Human Services (HHS), including earlier CMS projects, to address maternal health in a cohesive way. The Centers for Disease Control and Prevention has programs, as does the HHS Office on Women’s Health.
“How do we really get to a holistic, national, unified approach to addressing this issue that is led by HHS at the top level as the top agency and trickles down consistently versus having all of these kinds of disparate programs in place?” she said.
In recent years, the federal and state governments have taken many steps to try to improve maternal healthcare.
These include the extension of Medicaid benefits to new mothers out to 12 months following delivery in most states. CMS also has encouraged hospitals to participate in voluntary statewide or national programs to improve the quality of perinatal care. Last year the agency launched a “Birthing-Friendly” designation icon for qualifying hospitals on its Care Compare online tool.
Support and Opposition
CMS is accepting comments on the draft 2025 hospital outpatient rule, which includes the obstetric COP proposal, through September 9.
Supporters of the obstetric COP approach included the American Nurses Association (ANA), which urged CMS to consider how staffing shortages can undermine patient care in creating COP requirements.
“Nurses are professionals providing critical healthcare services to patients; they should not have to fight for allotted breaks and other challenges created by antiquated views of the profession and payment policies that disincentivize adequate nurse staffing,” Debbie Hatmaker, PhD, RN, ANA’s chief nursing officer, wrote in a June 7 comment to CMS.
The American College of Obstetricians and Gynecologists (ACOG) and the Association of American Medical Colleges (AAMC) also objected to the prospect of new COP for maternal healthcare. They detailed their concerns in separate comments submitted in June 2024.
ACOG said it feared many hospitals might opt to close labor and delivery (L&D) units due to new CMS COP requirements, especially if these take effect “without important and direct stakeholder engagement and buy-in.” More than 200 rural hospitals across the United States stopped providing L&D services in the last decade, Christopher M. Zahn, MD, ACOG’s interim chief executive officer, wrote in a comment to CMS.
“The reason for these closures is varied. Many rural hospitals that still have L&D units continue to lose money on patient services overall, and their ability to continue to deliver maternity care is at risk,” Dr. Zahn wrote.
The AAMC urged CMS to focus on using other strategies such as quality measures to try to improve maternal health and to drop the COP approach. CMS must consider how many clinicians play a role in successful births, including those who see patients during their pregnancies, Jonathan Jaffery, MD, MS, AAMC’s chief healthcare officer, wrote in a comment to the agency.
“Hospitals do have a critical role in improving maternal healthcare equity, especially for labor and delivery outcomes,” he wrote, “but cannot be held solely responsible for implementing much-needed improvements and solutions.”
A version of this article first appeared on Medscape.com.
Federal officials intend to compel US hospitals to improve obstetrical services, with a plan that could result in a potential loss of Medicare and Medicaid funds for institutions that fail to comply with the demands.
The Centers for Medicare and Medicaid Services (CMS) on July 10 announced this proposal, tucking its plan for new conditions of participation (COP) for obstetrician services into the draft 2025 rule on Medicare payments for outpatient hospital services.
The COP requirements are considered the most powerful tool CMS has for trying to improve the quality of medical care. With the new obstetric COP requirement, CMS said it intends to address what it sees as potential shortfalls in training, staffing, transfer protocols, and emergency services readiness.
In practice, hospitals, CMS, and accrediting bodies such as the Joint Commission usually try to address deficiencies to prevent what would be a devastating financial loss for a hospital.
“CMS is using all of our tools to improve the safety, quality, and timeliness of the care that hospitals provide to pregnant women,” Dora Hughes, MD, MPH, acting chief medical officer of the agency, said in a press release about the proposal.
CMS estimated the proposal may add new annual expenses of $70,671 per hospital. For comparison, this figure would represent far less than 1% of the total $1.4 trillion spent on hospital care in the United States in 2022.
CMS said it is trying to address the reasons women in the United States face more risk in giving birth than those in other nations. There were 22 maternal deaths for every 100,000 live births in this country in 2022, compared with 8.6 deaths per 100,000 live births or lower that year in Canada, France, the United Kingdom, Germany, and Japan, CMS said.
But CMS is seeking to impose this new requirement at a time amid growing concerns about “maternity care deserts.”
Reasonable Asks?
Between 2011 and 2021, one out of every four rural hospitals in America stopped providing obstetrics services, Senate Finance Chairman Ron Wyden (D-Ore.) said at a May hearing. Mr. Wyden last year was part of a fight to try to prevent the closure of a birthing center in Baker City in rural eastern Oregon.
The federal government should focus first on helping hospitals keep obstetrical facilities open, said Elizabeth Powers, MD, MHA, the health services officer of the Winding Waters Clinic in Enterprise, Oregon.
“Until we can ensure access to services, we can’t even work on quality,” Dr. Powers told this news organization. “If you’re thinking about a Maslow’s hierarchy of achieving health outcomes, access is your foundation, and without a shift in payment, that foundation is eroded.”
In the draft rule, CMS sketched broad mandates about staffing and training. For example, the agency proposes requiring if a hospital offers obstetrical services, “the services must be well organized and provided in accordance with nationally recognized acceptable standards of practice.”
That means CMS likely will need to provide further guidance for hospitals if it proceeds with this plan for obstetric COP requirements, said Soumi Saha, PharmD, JD, senior vice president of government affairs at Premier Inc., a healthcare consultancy and purchasing organization.
Premier is among the many groups, including the American Hospital Association, that oppose the COP proposal.
Dr. Saha said a better approach would be to consolidate the work being done through the US Department of Health and Human Services (HHS), including earlier CMS projects, to address maternal health in a cohesive way. The Centers for Disease Control and Prevention has programs, as does the HHS Office on Women’s Health.
“How do we really get to a holistic, national, unified approach to addressing this issue that is led by HHS at the top level as the top agency and trickles down consistently versus having all of these kinds of disparate programs in place?” she said.
In recent years, the federal and state governments have taken many steps to try to improve maternal healthcare.
These include the extension of Medicaid benefits to new mothers out to 12 months following delivery in most states. CMS also has encouraged hospitals to participate in voluntary statewide or national programs to improve the quality of perinatal care. Last year the agency launched a “Birthing-Friendly” designation icon for qualifying hospitals on its Care Compare online tool.
Support and Opposition
CMS is accepting comments on the draft 2025 hospital outpatient rule, which includes the obstetric COP proposal, through September 9.
Supporters of the obstetric COP approach included the American Nurses Association (ANA), which urged CMS to consider how staffing shortages can undermine patient care in creating COP requirements.
“Nurses are professionals providing critical healthcare services to patients; they should not have to fight for allotted breaks and other challenges created by antiquated views of the profession and payment policies that disincentivize adequate nurse staffing,” Debbie Hatmaker, PhD, RN, ANA’s chief nursing officer, wrote in a June 7 comment to CMS.
The American College of Obstetricians and Gynecologists (ACOG) and the Association of American Medical Colleges (AAMC) also objected to the prospect of new COP for maternal healthcare. They detailed their concerns in separate comments submitted in June 2024.
ACOG said it feared many hospitals might opt to close labor and delivery (L&D) units due to new CMS COP requirements, especially if these take effect “without important and direct stakeholder engagement and buy-in.” More than 200 rural hospitals across the United States stopped providing L&D services in the last decade, Christopher M. Zahn, MD, ACOG’s interim chief executive officer, wrote in a comment to CMS.
“The reason for these closures is varied. Many rural hospitals that still have L&D units continue to lose money on patient services overall, and their ability to continue to deliver maternity care is at risk,” Dr. Zahn wrote.
The AAMC urged CMS to focus on using other strategies such as quality measures to try to improve maternal health and to drop the COP approach. CMS must consider how many clinicians play a role in successful births, including those who see patients during their pregnancies, Jonathan Jaffery, MD, MS, AAMC’s chief healthcare officer, wrote in a comment to the agency.
“Hospitals do have a critical role in improving maternal healthcare equity, especially for labor and delivery outcomes,” he wrote, “but cannot be held solely responsible for implementing much-needed improvements and solutions.”
A version of this article first appeared on Medscape.com.
Federal officials intend to compel US hospitals to improve obstetrical services, with a plan that could result in a potential loss of Medicare and Medicaid funds for institutions that fail to comply with the demands.
The Centers for Medicare and Medicaid Services (CMS) on July 10 announced this proposal, tucking its plan for new conditions of participation (COP) for obstetrician services into the draft 2025 rule on Medicare payments for outpatient hospital services.
The COP requirements are considered the most powerful tool CMS has for trying to improve the quality of medical care. With the new obstetric COP requirement, CMS said it intends to address what it sees as potential shortfalls in training, staffing, transfer protocols, and emergency services readiness.
In practice, hospitals, CMS, and accrediting bodies such as the Joint Commission usually try to address deficiencies to prevent what would be a devastating financial loss for a hospital.
“CMS is using all of our tools to improve the safety, quality, and timeliness of the care that hospitals provide to pregnant women,” Dora Hughes, MD, MPH, acting chief medical officer of the agency, said in a press release about the proposal.
CMS estimated the proposal may add new annual expenses of $70,671 per hospital. For comparison, this figure would represent far less than 1% of the total $1.4 trillion spent on hospital care in the United States in 2022.
CMS said it is trying to address the reasons women in the United States face more risk in giving birth than those in other nations. There were 22 maternal deaths for every 100,000 live births in this country in 2022, compared with 8.6 deaths per 100,000 live births or lower that year in Canada, France, the United Kingdom, Germany, and Japan, CMS said.
But CMS is seeking to impose this new requirement at a time amid growing concerns about “maternity care deserts.”
Reasonable Asks?
Between 2011 and 2021, one out of every four rural hospitals in America stopped providing obstetrics services, Senate Finance Chairman Ron Wyden (D-Ore.) said at a May hearing. Mr. Wyden last year was part of a fight to try to prevent the closure of a birthing center in Baker City in rural eastern Oregon.
The federal government should focus first on helping hospitals keep obstetrical facilities open, said Elizabeth Powers, MD, MHA, the health services officer of the Winding Waters Clinic in Enterprise, Oregon.
“Until we can ensure access to services, we can’t even work on quality,” Dr. Powers told this news organization. “If you’re thinking about a Maslow’s hierarchy of achieving health outcomes, access is your foundation, and without a shift in payment, that foundation is eroded.”
In the draft rule, CMS sketched broad mandates about staffing and training. For example, the agency proposes requiring if a hospital offers obstetrical services, “the services must be well organized and provided in accordance with nationally recognized acceptable standards of practice.”
That means CMS likely will need to provide further guidance for hospitals if it proceeds with this plan for obstetric COP requirements, said Soumi Saha, PharmD, JD, senior vice president of government affairs at Premier Inc., a healthcare consultancy and purchasing organization.
Premier is among the many groups, including the American Hospital Association, that oppose the COP proposal.
Dr. Saha said a better approach would be to consolidate the work being done through the US Department of Health and Human Services (HHS), including earlier CMS projects, to address maternal health in a cohesive way. The Centers for Disease Control and Prevention has programs, as does the HHS Office on Women’s Health.
“How do we really get to a holistic, national, unified approach to addressing this issue that is led by HHS at the top level as the top agency and trickles down consistently versus having all of these kinds of disparate programs in place?” she said.
In recent years, the federal and state governments have taken many steps to try to improve maternal healthcare.
These include the extension of Medicaid benefits to new mothers out to 12 months following delivery in most states. CMS also has encouraged hospitals to participate in voluntary statewide or national programs to improve the quality of perinatal care. Last year the agency launched a “Birthing-Friendly” designation icon for qualifying hospitals on its Care Compare online tool.
Support and Opposition
CMS is accepting comments on the draft 2025 hospital outpatient rule, which includes the obstetric COP proposal, through September 9.
Supporters of the obstetric COP approach included the American Nurses Association (ANA), which urged CMS to consider how staffing shortages can undermine patient care in creating COP requirements.
“Nurses are professionals providing critical healthcare services to patients; they should not have to fight for allotted breaks and other challenges created by antiquated views of the profession and payment policies that disincentivize adequate nurse staffing,” Debbie Hatmaker, PhD, RN, ANA’s chief nursing officer, wrote in a June 7 comment to CMS.
The American College of Obstetricians and Gynecologists (ACOG) and the Association of American Medical Colleges (AAMC) also objected to the prospect of new COP for maternal healthcare. They detailed their concerns in separate comments submitted in June 2024.
ACOG said it feared many hospitals might opt to close labor and delivery (L&D) units due to new CMS COP requirements, especially if these take effect “without important and direct stakeholder engagement and buy-in.” More than 200 rural hospitals across the United States stopped providing L&D services in the last decade, Christopher M. Zahn, MD, ACOG’s interim chief executive officer, wrote in a comment to CMS.
“The reason for these closures is varied. Many rural hospitals that still have L&D units continue to lose money on patient services overall, and their ability to continue to deliver maternity care is at risk,” Dr. Zahn wrote.
The AAMC urged CMS to focus on using other strategies such as quality measures to try to improve maternal health and to drop the COP approach. CMS must consider how many clinicians play a role in successful births, including those who see patients during their pregnancies, Jonathan Jaffery, MD, MS, AAMC’s chief healthcare officer, wrote in a comment to the agency.
“Hospitals do have a critical role in improving maternal healthcare equity, especially for labor and delivery outcomes,” he wrote, “but cannot be held solely responsible for implementing much-needed improvements and solutions.”
A version of this article first appeared on Medscape.com.
HDL Cholesterol Increases Kidney Disease Risk in T2D
TOPLINE:
Very high and very low levels of high-density lipoprotein cholesterol (HDL-C) are linked to a higher risk for kidney disease in women with type 2 diabetes (T2D), but not in men.
METHODOLOGY:
- Studies have reported a strong association between low HDL-C levels and the risk for diabetic kidney disease, but whether higher HDL-C levels can influence the risk for diabetic kidney disease remains unclear.
- Researchers conducted a cross-sectional observational study of 936 patients with T2D (mean age, about 60 years; 41% women; 33% with diabetic kidney disease) from the Endocrinology Department at the Jinhua Hospital between September 2020 and July 2021.
- To examine the relationship between HDL-C levels and the risk for diabetic kidney disease, researchers used logistic regression to assess the continuous and categorical associations and a restricted cubic spline curve to assess the nonlinear association.
- HDL-C levels were categorized into four groups, with 0.40-0.96 mmol/L corresponding to the lowest quartile and 1.32-6.27 mmol/L corresponding to the highest quartile.
- The researchers observed a U-shaped association between HDL-C levels and the risk for diabetic kidney disease (Pnonlinear = .010) and selected two threshold values of 0.95 and 1.54 mmol/L.
TAKEAWAY:
- The risk for diabetic kidney disease was higher when the HDL-C levels were < 0.95 mmol/L or > 1.54 mmol/L.
- Compared with patients with HDL-C levels in the range of 0.95-1.54 mmol/L, those with very high and very low HDL-C levels had a 128% and 77% increased risk for diabetic kidney disease, respectively.
- The association was significant in women (P = .006) and not in men (P = .054), after adjusting for confounding factors.
- HDL-C level as a continuous variable was not associated with the risk for kidney disease (P = .902).
IN PRACTICE:
“Although HDL-C is generally considered a cardiovascular protective factor, at very high levels, this protective effect does not seem to hold true and may be associated with an increased DKD [diabetic kidney disease] risk,” the authors wrote.
SOURCE:
This study was led by Huabin Wang, from the Department of Clinical Laboratory, Jinhua Hospital, Zhejiang University School of Medicine, Jinhua, China, and was published online in Scientific Reports.
LIMITATIONS:
The cross-sectional nature of the study limited the ability to establish a causal relationship between high HDL-C levels and the risk for diabetic kidney disease. The sample size of the study was relatively small at the higher end of the HDL-C concentration spectrum. Moreover, the study did not consider other potential confounding factors such as diet, sedentary lifestyle, obesity, genetic diseases, drug effects on HDL-C levels, and fluctuating estrogen levels, which could affect the overall findings.
DISCLOSURES:
The study was funded by the Department of Science and Technology of Zhejiang Province, China, and The Science and Technology Bureau of Jinhua City. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
Very high and very low levels of high-density lipoprotein cholesterol (HDL-C) are linked to a higher risk for kidney disease in women with type 2 diabetes (T2D), but not in men.
METHODOLOGY:
- Studies have reported a strong association between low HDL-C levels and the risk for diabetic kidney disease, but whether higher HDL-C levels can influence the risk for diabetic kidney disease remains unclear.
- Researchers conducted a cross-sectional observational study of 936 patients with T2D (mean age, about 60 years; 41% women; 33% with diabetic kidney disease) from the Endocrinology Department at the Jinhua Hospital between September 2020 and July 2021.
- To examine the relationship between HDL-C levels and the risk for diabetic kidney disease, researchers used logistic regression to assess the continuous and categorical associations and a restricted cubic spline curve to assess the nonlinear association.
- HDL-C levels were categorized into four groups, with 0.40-0.96 mmol/L corresponding to the lowest quartile and 1.32-6.27 mmol/L corresponding to the highest quartile.
- The researchers observed a U-shaped association between HDL-C levels and the risk for diabetic kidney disease (Pnonlinear = .010) and selected two threshold values of 0.95 and 1.54 mmol/L.
TAKEAWAY:
- The risk for diabetic kidney disease was higher when the HDL-C levels were < 0.95 mmol/L or > 1.54 mmol/L.
- Compared with patients with HDL-C levels in the range of 0.95-1.54 mmol/L, those with very high and very low HDL-C levels had a 128% and 77% increased risk for diabetic kidney disease, respectively.
- The association was significant in women (P = .006) and not in men (P = .054), after adjusting for confounding factors.
- HDL-C level as a continuous variable was not associated with the risk for kidney disease (P = .902).
IN PRACTICE:
“Although HDL-C is generally considered a cardiovascular protective factor, at very high levels, this protective effect does not seem to hold true and may be associated with an increased DKD [diabetic kidney disease] risk,” the authors wrote.
SOURCE:
This study was led by Huabin Wang, from the Department of Clinical Laboratory, Jinhua Hospital, Zhejiang University School of Medicine, Jinhua, China, and was published online in Scientific Reports.
LIMITATIONS:
The cross-sectional nature of the study limited the ability to establish a causal relationship between high HDL-C levels and the risk for diabetic kidney disease. The sample size of the study was relatively small at the higher end of the HDL-C concentration spectrum. Moreover, the study did not consider other potential confounding factors such as diet, sedentary lifestyle, obesity, genetic diseases, drug effects on HDL-C levels, and fluctuating estrogen levels, which could affect the overall findings.
DISCLOSURES:
The study was funded by the Department of Science and Technology of Zhejiang Province, China, and The Science and Technology Bureau of Jinhua City. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
TOPLINE:
Very high and very low levels of high-density lipoprotein cholesterol (HDL-C) are linked to a higher risk for kidney disease in women with type 2 diabetes (T2D), but not in men.
METHODOLOGY:
- Studies have reported a strong association between low HDL-C levels and the risk for diabetic kidney disease, but whether higher HDL-C levels can influence the risk for diabetic kidney disease remains unclear.
- Researchers conducted a cross-sectional observational study of 936 patients with T2D (mean age, about 60 years; 41% women; 33% with diabetic kidney disease) from the Endocrinology Department at the Jinhua Hospital between September 2020 and July 2021.
- To examine the relationship between HDL-C levels and the risk for diabetic kidney disease, researchers used logistic regression to assess the continuous and categorical associations and a restricted cubic spline curve to assess the nonlinear association.
- HDL-C levels were categorized into four groups, with 0.40-0.96 mmol/L corresponding to the lowest quartile and 1.32-6.27 mmol/L corresponding to the highest quartile.
- The researchers observed a U-shaped association between HDL-C levels and the risk for diabetic kidney disease (Pnonlinear = .010) and selected two threshold values of 0.95 and 1.54 mmol/L.
TAKEAWAY:
- The risk for diabetic kidney disease was higher when the HDL-C levels were < 0.95 mmol/L or > 1.54 mmol/L.
- Compared with patients with HDL-C levels in the range of 0.95-1.54 mmol/L, those with very high and very low HDL-C levels had a 128% and 77% increased risk for diabetic kidney disease, respectively.
- The association was significant in women (P = .006) and not in men (P = .054), after adjusting for confounding factors.
- HDL-C level as a continuous variable was not associated with the risk for kidney disease (P = .902).
IN PRACTICE:
“Although HDL-C is generally considered a cardiovascular protective factor, at very high levels, this protective effect does not seem to hold true and may be associated with an increased DKD [diabetic kidney disease] risk,” the authors wrote.
SOURCE:
This study was led by Huabin Wang, from the Department of Clinical Laboratory, Jinhua Hospital, Zhejiang University School of Medicine, Jinhua, China, and was published online in Scientific Reports.
LIMITATIONS:
The cross-sectional nature of the study limited the ability to establish a causal relationship between high HDL-C levels and the risk for diabetic kidney disease. The sample size of the study was relatively small at the higher end of the HDL-C concentration spectrum. Moreover, the study did not consider other potential confounding factors such as diet, sedentary lifestyle, obesity, genetic diseases, drug effects on HDL-C levels, and fluctuating estrogen levels, which could affect the overall findings.
DISCLOSURES:
The study was funded by the Department of Science and Technology of Zhejiang Province, China, and The Science and Technology Bureau of Jinhua City. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
Trauma epidemiology and the organization of trauma care in the US
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
On 5 Ps: PSG, PM, PPG, PulseOx, and PAT
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
On thoughtful selection of medications in the acute critical care setting
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399