User login
Methotrexate’s impact on COVID-19 vaccination: New insights made
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who take methotrexate for a variety of immune-mediated inflammatory diseases and pause taking the drug following receipt of a COVID-19 vaccine dose did not have a higher risk of disease flare and had higher antireceptor binding domain (anti-RBD) antibody titers and increased immunogenicity when compared with continuing the drug, three recent studies suggest.
In one study, British researchers examined the effects of a 2-week break in methotrexate therapy on anti-RBD titers following receipt of a third COVID-19 vaccine dose. In their paper published in The Lancet: Respiratory Medicine, they reported results from a randomized, open-label, superiority trial that suggested pausing the drug improved immunogenicity, compared with no break.
In two trials presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 Congress, a team from India set out to determine whether holding methotrexate after receiving both doses of a COVID-19 vaccine, or holding it only after the second dose, was safe and effective. They found that pausing methotrexate only following the second dose contributed to a lower flare risk, and that patients had higher anti-RBD titers when holding methotrexate for 2 weeks following each dose.
Pausing methotrexate after booster
The 2-week methotrexate break and booster vaccine dose data in the Vaccine Response On Off Methotrexate (VROOM) trial showed that after a month, the geometric mean antispike 1 (S1)-RBD antibody titer was 10,798 U/mL (95% confidence interval [CI], 8,970-12,997) in the group that continued methotrexate and 22,750 U/mL (95% CI, 19,314-26,796) in the group that suspended methotrexate; the geometric mean ratio was 2.19 (P < .0001; mixed-effects model), reported Abhishek Abhishek, MD, PhD, professor of rheumatology at the University of Nottingham in Nottingham, England, and colleagues.
Prior research showed that stopping methotrexate therapy for 2 weeks following the seasonal influenza vaccine contributed to better vaccine immunity among patients with rheumatoid arthritis, but there was no impact of stopping the drug for up to 4 weeks before vaccination on vaccine-related immunity, the researchers noted.
It is crucial in maximizing long-lasting vaccine protection in people who are possibly susceptible through immune suppression at this point in the COVID-19 vaccination regimen, the study team noted.
“Evidence from this study will be useful for policymakers, national immunization advisory committees, and specialist societies formulating recommendations on the use of methotrexate around the time of COVID-19 vaccination. This evidence will help patients and clinicians make informed choices about the risks and benefits of interrupting methotrexate treatment around the time of COVID-19 vaccination, with implications for the potential to extend such approaches to other therapeutics,” they wrote.
In American College of Rheumatology (ACR) guidance for COVID-19 vaccination, the organization advised against using standard synthetic disease-modifying antirheumatic medicines such as methotrexate “for 1-2 weeks (as disease activity allows) after each COVID-19 vaccine dose,” given the at-risk population and public health concerns, Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine and associate physician at Brigham and Women’s Hospital and Harvard Medical School, Boston, and Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, noted in an accompanying editorial in The Lancet: Respiratory Medicine.
However, when the ACR developed this statement, there was only one trial involving patients with rheumatoid arthritis who paused methotrexate following seasonal influenza vaccination, the editorialists said.
“Although this finding adds to the evidence base to support interruption of methotrexate after vaccination, a shared decision process is needed to weigh the possible benefit of optimizing protection from COVID-19 and the possible risk of underlying disease flare,” they added.
Dr. Abhishek and colleagues assessed 254 patients with immune-mediated inflammatory disease from dermatology and rheumatology clinics across 26 hospitals in the United Kingdom. Participants had been diagnosed with systemic lupus erythematosus, rheumatoid arthritis, atopic dermatitis, polymyalgia rheumatica, axial spondyloarthritis, and psoriasis without or with arthritis. They had also been taking up to 25 mg of methotrexate per week for 3 months or longer and had received two doses of either the Pfizer/BioNTech BNT162b2 vaccine or AstraZeneca/Oxford viral vector vaccine. The booster dose was most often the Pfizer BNT162b2 vaccine (82%). The patients’ mean age was 59 years, with females comprising 61% of the cohort. Participants were randomly assigned 1:1 to either group.
Investigators performing laboratory analysis were masked to cohort assignment, and clinical research staff, data analysts, participants, and researchers were unmasked.
The elevated antibody response of patients who suspended methotrexate was the same across different kinds of immune-mediated inflammatory disease, primary vaccination platform, SARS-CoV-2 infection history, and age.
Notably, no intervention-associated adverse events were reported, the study team noted.
The conclusions that could be drawn from the booster-dose study were limited by the trial’s modest cohort size, the small number of patients in exploratory subgroup analyses, a lack of information about differences in prescription drug behavior, and early termination’s effect on the researchers’ ability to identify differences between subgroups and in secondary outcomes, the authors noted.
Other limitations included a lack of generalizability to patients with active disease who couldn’t stop therapy and were not included in the investigation, and participants were not blinded to what group they were in, the researchers said.
Expert commentary
This current study is consistent with other studies over the last several months showing that methotrexate harms both humoral and cell-mediated COVID-19 responses, noted Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study. “And so now the new wave of studies are like this one, where they are holding methotrexate experimentally and seeing if it makes a difference,” he said.
“The one shortcoming of this study – and so far, the studies to date – is that no one has looked at whether the experimental hold has resulted in a change in T-cell responses, which ... we are [now] recognizing [the importance of] more and more in long-term protection, particularly in severe disease. Theoretically, holding [methotrexate] might help enhance T-cell responses, but that hasn’t been shown experimentally.”
Dr. Winthrop pointed out that one might get the same benefit from holding methotrexate for 1 week instead of 2 and that there likely is a reduced risk of flare-up from underlying autoimmune disease.
It is still not certain that this benefit extends to other vaccines, Dr. Winthrop noted. “It is probably true for most vaccines that if you hold methotrexate for 1 or 2 weeks, you might see some short-term benefit in responsiveness, but you don’t know that there is any clinical meaningfulness of this. That’s going to take other long-term studies. You don’t know how long this benefit lasts.”
Pausing methotrexate during initial COVID vaccine doses
Patients with either rheumatoid arthritis or psoriatic arthritis had higher anti-RBD antibody titers when methotrexate was stopped after both doses of the AstraZeneca vaccine, or simply after the second dose, than when methotrexate was continued, according to results from two single-center, randomized controlled trials called MIVAC I and II, Anu Sreekanth, MD, of Sree Sudheendra Medical Mission in Kochi, Kerala, India, and colleagues reported at EULAR 2022.
Results from MIVAC I indicated that there was a higher flare rate when methotrexate was stopped after both vaccine doses, but there was no difference in flare rate in MIVAC II when methotrexate was stopped only after the second dose as opposed to stopping it after both doses.
In the MIVAC I trial, 158 unvaccinated patients were randomized 1:1 to a cohort in which methotrexate was held for 2 weeks after both doses and a cohort in which methotrexate was continued despite the vaccine. In MIVAC II, 157 patients continued methotrexate while receiving the first vaccine dose. These patients were subsequently randomized either to continue or to stop methotrexate for 2 weeks following the second dose.
The findings from MIVAC I demonstrated the flare rate was lower in the methotrexate-continue group than in the methotrexate-pause group (8% vs. 25%; P = .005) and that the median anti-RBD titer was significantly higher for the methotrexate-pause group than the methotrexate-continue group (2,484 vs. 1,147; P = .001).
The results from MIVAC II trial indicated that there was no difference in flare rates between the two study groups (7.9% vs. 11.8%; P = .15). Yet, the median anti-RBD titer was significantly higher in the methotrexate-pause cohort than in the methotrexate-continue cohort (2,553 vs. 990; P = .001).
The report suggests there is a flare risk when methotrexate is stopped, Dr. Sreekanth noted. “It appears more logical to hold only after the second dose, as comparable anti-RBD titers are generated” with either approach, Dr. Sreekanth said.
Expert commentary: MIVAC I and II
Inés Colmegna, MD, associate professor at McGill University in Montreal, noted that it was intriguing that the risk of flares in MIVAC II is half of that reported after each of the doses of MIVAC I. “It is also worth emphasizing that despite the reported frequency of flares, the actual disease activity [as measured by the Disease Activity Score in 28 joints] in patients who did or did not withhold methotrexate was similar.
“MIVAC I and II have practical implications as they help to adequately inform patients about the risk and benefit trade of withholding methotrexate post–COVID-19 vaccination,” Dr. Colmegna told this news organization.
“Additional information would help to [further] interpret the findings of these studies, including whether any of the participants were taking any other DMARDs; data on the severity of the flares and functional impact; analysis of factors that predict the risk of flares, such as higher doses of methotrexate; [and change in] disease activity scores pre- and postvaccination,” Dr. Colmegna concluded.
Dr. Abhishek disclosed relationships with Springer, UpTodate, Oxford, Immunotec, AstraZeneca, Inflazome, NGM Biopharmaceuticals, Menarini Pharmaceuticals, and Cadila Pharmaceuticals. Dr. Abhishek is cochair of the ACR/EULAR CPPD Classification Criteria Working Group and the OMERACT CPPD Working Group. Dr. Sparks disclosed relationships with Gilead, Boehringer Ingelheim, Amgen, Bristol-Myers Squibb, and AbbVie, unrelated to this study. Dr. Tedeschi disclosed relationships with ModernaTx and NGM Biopharmaceuticals. Dr. Winthrop disclosed a research grant and serving as a scientific consultant for Pfizer. Dr. Sreekanth and Dr. Colmegna have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Commentary: Reversal of Roe v. Wade affects adolescents
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
The Supreme Court decision to strike down the 50-year ruling on Roe v. Wade, which allowed legal abortion, will affect all patients and families seeking care in pediatric and adolescent medicine clinics. Regardless of how you view abortion, the reality is your adolescent female patients and their parents will seek your counsel.
The overturning of Roe has resulted in much confusion for both patients and providers. The overall effect of this decision in Wisconsin is yet to be known but currently we have had to create road maps to direct adolescent patients who experience an unplanned pregnancy and wish to abort. Unfortunately, these road maps include only resources out of state or online. Providing adolescents confidential care may be challenged as the teens may need to disclose the unplanned pregnancy to an adult to access resources.
Providers remain unsettled regarding their risk of assisting an adolescent who discloses an unplanned pregnancy. Recently, many questions arose regarding dispensing Plan B and the risk to prescribers. Communication was needed to assure providers that Plan B is contraception and at this time contraception remains legal in our state.
Daily I educate adolescent females on the risks of unplanned pregnancy and what the Supreme Court decision will mean to them if they become pregnant. Unfortunately, many teens do not understand the ruling and how this decision affects them personally. Education is needed today more than ever regarding pregnancy prevention.
The recent AAP policy statement reaffirms its position that the rights of adolescents to seek confidential care when considering abortion must be protected.1 It further reaffirms access to safe and legal abortion is a core tenant of sexual and reproductive health care.
A recent article published in AAP News by Elise D. Berlan, MD, “AAP’s teen reproductive health policies reaffirm right to comprehensive care,” further advises on the role of the pediatric provider.2 Pediatric providers should continue offering option counseling for pregnant adolescents, be prepared to provide accurate information regarding these options with awareness that some options such as the IUD may no longer be available, remain supportive of the decision they choose, and encourage discussion with a family member to support their decisions. It is imperative that we familiarize ourselves with the abortion policies in our states, advocate to prevent government interference with the patient-doctor relationship, and recognize the impact restrictive abortion has regarding marginalized individuals, she stated. Finally we must recognize our own bias regarding option counseling and refer appropriately to another professional if we are unable to confidently offer guidance.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee.
References
1. AAP Committee on Adolescence. Pediatrics. 2022. doi: 10.1542/peds.2022-058780.
2. Berlan ED. AAP’s teen reproductive health policies reaffirm right to comprehensive care. 2022. AAP News.
Five things most physicians don’t know about radiation oncology
As a field, radiation oncology is perhaps one of medicine’s best kept secrets. Sometimes, even our own colleagues don’t know where our department exists in the hospital or exactly what we do.
As two radiation oncologists who are, in fact, the children of radiation oncologists, we will admit that it’s possible we are a tiny bit biased. We cannot lie, though: Our field is a hidden gem.
What is well known is that radiation oncologists have a symbiotic relationship with our treatment technology. The evolution of treatment machines and radiation precision allows us to deliver patient-tailored treatment down to the millimeter. What may get lost in the discussions of isodose lines and penumbra, however, is that we’ve also got cutting-edge research and personalized patient care within a specialized team in the depths of the hospital.
Because the inner workings of what happens to patients as they come in and out of our office remains a mystery,
1. Nobody knows what goes on in the basement.
Misconceptions about our subspecialty are common, even among other oncologists. A frequent misconception is that a radiation oncologist’s involvement in patient care is limited, and radiation is delivered in a standardized manner. This essentially renders radiation oncologists technicians of expensive machines.
In reality however, radiation oncologists touch every aspect of a patient’s care, and customized radiation therapy may be indicated for virtually every cancer site in both curative and palliative settings. We strive to deliver precision medicine and practice truly patient-centered care.
To cure cancer, radiation may be used in the neoadjuvant (prior to local surgical resection), definitive (as the primary local therapy), and adjuvant (postsurgical) setting. In palliative cases, radiation can be used to treat areas of metastatic spread as well as primary unresectable tumors to alleviate obstruction and/or bleeding symptoms. Referral to radiation oncology, therefore, can be appropriate at many different points of time on the continuum of cancer care.
For many treating radiation oncologists, the close personal connections that we form with our patients is one of the primary reasons we went into this field. Not only are we making patient-centered clinical decisions during every step of the treatment plan evaluation and optimization but we also see our patients weekly for clinical visits and then ongoing in visits that may span many years of survivorship.
Our deep commitment to addressing patient needs as they are receiving treatment and responsibility for late radiation effects is absolutely an integral part of our training and lifelong practice.
2. We get down in the details.
The workflow from consultation to radiation delivery can be confusing for anyone outside our specialized field.
Once seen in consult and considered a good candidate for radiation, patients will enter the essential next step: the treatment planning imaging – or “simulation.”
The simulation scan – mostly CT, although occasionally fused MRI or PET – involves a separate appointment and another hour or so of arranging and scanning patients in the exact position that they will be treated. Given the precision of modern radiation, the simulation often includes making a customized mold so patients have minimal movement during treatment. These simulation images allow the radiation oncology team to create a treatment plan that is customized to each patient and precisely reproducible during their course of radiation treatments – what’s known as fractions.
Creating a treatment plan involves a radiation oncologist literally drawing – or contouring – on pictures of the patient’s internal anatomy in three dimensions. Radiation oncologists contour exactly where the cancer is – or where it was if the treatment is given postoperatively – and identify the surrounding organs so that the doses can be preferentially directed to the cancer target and minimize risk to nearby organs. This precision is within millimeters and accounts for microscopic disease, organ motion, and patient setup. Ultimately, we create colorful heat gradient volumes of the anticipated radiation dose delivery and optimize these to reflect our planning priorities.
We use advanced technologies to shape the beams of radiation to treat the tumor and avoid delivering high doses to the neighboring tissues with techniques such as intensity modulated radiation therapy (IMRT), stereotactic ablative radiation therapy (SABR or SBRT), and stereotactic radiosurgery. We can also take advantage of the unique properties of different modalities, such as proton therapy and electron therapy, to achieve these same goals if indicated. Radiation oncologists live for precision medicine in every aspect of their workflow.
3. We roll deep.
Radiation oncology is exemplary of “the art of medicine.” We fuse anatomy-based treatment design with advanced technology and orchestrate the daily functions of a large medical team.
But, the treatment plan and delivery would not be possible without the input and care given within a large multi- and intradisciplinary team of oncologists, medical physicists, dosimetrists, radiation therapists, nurses, social workers, and other support staff. Radiation oncologists participate in regular tumor boards with surgeons, medical oncologists, pathologists, and radiologists to optimize interdisciplinary management of complex patients, providing a thoughtful tumor localization and treatment plan, as well as to better understand an individual’s ongoing symptoms and well-being as a whole. Considering all aspects of what a patient may need involves communication with fellow physicians, nurse navigators, and social workers.
Within our own department, treatment plan creation and quality checking or verification can sometimes take over 2 weeks, with detailed input from dosimetrists and medical physicists. The actual treatment delivery involves daily communication with the radiation therapists who are dedicated to each treatment machine – like the linear accelerator – and symptom management with clinic nurses and supportive staff, such as physical therapists and registered dietitians.
This massive team effort is required to get each patient through daily radiation treatments that can last 7 weeks and may require rapid replanning if the tumor shrinks or the patient loses weight.
As part of this team, radiation oncologists are uniquely positioned to quarterback each play and guide the entire game strategy.
4. Radiation therapy takes a lot of heat.
Radiation therapy is often blamed for issues unrelated to the treatment. Irradiating the pelvis for prostate cancer, for instance, does not cause a headache or heartburn during or after treatment.
Other than fatigue, associated side effects are localized and related to the total radiation dose and fraction size – how much and how fast – that reaches the surrounding tissues.
Our colleagues often swap stories of the bizarre things radiation therapy has been blamed for, including dental problems in someone receiving vaginal cylinder treatment, heart dysfunction in someone treated for rectal cancer, and hip fracture in someone treated for breast cancer because the radiation “destroyed” their bones. At best, these are humorous stories, but at worst, they can delay diagnosis and treatment of what is truly causing someone’s symptoms.
5. We truly believe that less is more.
One of the most fundamental aspects of radiation oncology is our drive to optimize treatment delivery and continually improve patient care – sometimes at our own field’s economic detriment. We’re dedicated to showing that patients may get the same benefit from less and less radiation.
In the past 2 decades, the evolution and adoption of photon IMRT and proton therapy has allowed radiation plans to successfully spare surrounding tissue while improving our targeting. This evolution is coupled with technological and imaging advances that allow us to delivery of doses to certain tumors via SABR/SBRT in one to five total fractions.
A prime example: Treatment to eradicate lung or gastrointestinal tumors, which used to span up to 6 weeks, can now potentially be delivered in as little as 1 week.
For other common cancers, hypofractionation – slightly higher radiation doses per fraction at fewer total fractions overall – has revolutionized patient care, providing less radiation without impacting survival or increasing treatment toxicity.
Take breast cancer care: 50 years ago, virtually all patients with breast cancer received a mastectomy and lymph node dissections. Today, surgical techniques for lumpectomy paired with radiation therapy to the whole breast now allows us to preserve disease-free survival for those who elected to keep their breasts.
Over the past 20 years, the standards of care have shifted from 6-7 weeks of treatment to 3-4 weeks using a hypofractionated model that involves daily whole-breast radiation. The most recent clinical trials have shown that whole-breast radiation can be delivered safely and effectively for select women in as few as five fractions with either whole or partial breast targeting. Additional research driven by the idea of “right sizing” radiation treatment has even shown that certain women may not need radiation at all.
This evolution in radiation therapy illustrates how our subspecialty is constantly working to improve survival and patient well-being, form deep connections with our patients, and push the boundaries of medical innovations.
We are proud to be radiation oncologists and happy to share more. Want to know more about what goes on in the basement? Come on down, we’re happy to show you around.
Dr. Giap is a resident in the department of radiation oncology at the University of Florida, Gainesville. Dr. Chino is an assistant attending in the department of radiation oncology at Memorial Sloan Kettering Cancer Center, New York. Neither reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
As a field, radiation oncology is perhaps one of medicine’s best kept secrets. Sometimes, even our own colleagues don’t know where our department exists in the hospital or exactly what we do.
As two radiation oncologists who are, in fact, the children of radiation oncologists, we will admit that it’s possible we are a tiny bit biased. We cannot lie, though: Our field is a hidden gem.
What is well known is that radiation oncologists have a symbiotic relationship with our treatment technology. The evolution of treatment machines and radiation precision allows us to deliver patient-tailored treatment down to the millimeter. What may get lost in the discussions of isodose lines and penumbra, however, is that we’ve also got cutting-edge research and personalized patient care within a specialized team in the depths of the hospital.
Because the inner workings of what happens to patients as they come in and out of our office remains a mystery,
1. Nobody knows what goes on in the basement.
Misconceptions about our subspecialty are common, even among other oncologists. A frequent misconception is that a radiation oncologist’s involvement in patient care is limited, and radiation is delivered in a standardized manner. This essentially renders radiation oncologists technicians of expensive machines.
In reality however, radiation oncologists touch every aspect of a patient’s care, and customized radiation therapy may be indicated for virtually every cancer site in both curative and palliative settings. We strive to deliver precision medicine and practice truly patient-centered care.
To cure cancer, radiation may be used in the neoadjuvant (prior to local surgical resection), definitive (as the primary local therapy), and adjuvant (postsurgical) setting. In palliative cases, radiation can be used to treat areas of metastatic spread as well as primary unresectable tumors to alleviate obstruction and/or bleeding symptoms. Referral to radiation oncology, therefore, can be appropriate at many different points of time on the continuum of cancer care.
For many treating radiation oncologists, the close personal connections that we form with our patients is one of the primary reasons we went into this field. Not only are we making patient-centered clinical decisions during every step of the treatment plan evaluation and optimization but we also see our patients weekly for clinical visits and then ongoing in visits that may span many years of survivorship.
Our deep commitment to addressing patient needs as they are receiving treatment and responsibility for late radiation effects is absolutely an integral part of our training and lifelong practice.
2. We get down in the details.
The workflow from consultation to radiation delivery can be confusing for anyone outside our specialized field.
Once seen in consult and considered a good candidate for radiation, patients will enter the essential next step: the treatment planning imaging – or “simulation.”
The simulation scan – mostly CT, although occasionally fused MRI or PET – involves a separate appointment and another hour or so of arranging and scanning patients in the exact position that they will be treated. Given the precision of modern radiation, the simulation often includes making a customized mold so patients have minimal movement during treatment. These simulation images allow the radiation oncology team to create a treatment plan that is customized to each patient and precisely reproducible during their course of radiation treatments – what’s known as fractions.
Creating a treatment plan involves a radiation oncologist literally drawing – or contouring – on pictures of the patient’s internal anatomy in three dimensions. Radiation oncologists contour exactly where the cancer is – or where it was if the treatment is given postoperatively – and identify the surrounding organs so that the doses can be preferentially directed to the cancer target and minimize risk to nearby organs. This precision is within millimeters and accounts for microscopic disease, organ motion, and patient setup. Ultimately, we create colorful heat gradient volumes of the anticipated radiation dose delivery and optimize these to reflect our planning priorities.
We use advanced technologies to shape the beams of radiation to treat the tumor and avoid delivering high doses to the neighboring tissues with techniques such as intensity modulated radiation therapy (IMRT), stereotactic ablative radiation therapy (SABR or SBRT), and stereotactic radiosurgery. We can also take advantage of the unique properties of different modalities, such as proton therapy and electron therapy, to achieve these same goals if indicated. Radiation oncologists live for precision medicine in every aspect of their workflow.
3. We roll deep.
Radiation oncology is exemplary of “the art of medicine.” We fuse anatomy-based treatment design with advanced technology and orchestrate the daily functions of a large medical team.
But, the treatment plan and delivery would not be possible without the input and care given within a large multi- and intradisciplinary team of oncologists, medical physicists, dosimetrists, radiation therapists, nurses, social workers, and other support staff. Radiation oncologists participate in regular tumor boards with surgeons, medical oncologists, pathologists, and radiologists to optimize interdisciplinary management of complex patients, providing a thoughtful tumor localization and treatment plan, as well as to better understand an individual’s ongoing symptoms and well-being as a whole. Considering all aspects of what a patient may need involves communication with fellow physicians, nurse navigators, and social workers.
Within our own department, treatment plan creation and quality checking or verification can sometimes take over 2 weeks, with detailed input from dosimetrists and medical physicists. The actual treatment delivery involves daily communication with the radiation therapists who are dedicated to each treatment machine – like the linear accelerator – and symptom management with clinic nurses and supportive staff, such as physical therapists and registered dietitians.
This massive team effort is required to get each patient through daily radiation treatments that can last 7 weeks and may require rapid replanning if the tumor shrinks or the patient loses weight.
As part of this team, radiation oncologists are uniquely positioned to quarterback each play and guide the entire game strategy.
4. Radiation therapy takes a lot of heat.
Radiation therapy is often blamed for issues unrelated to the treatment. Irradiating the pelvis for prostate cancer, for instance, does not cause a headache or heartburn during or after treatment.
Other than fatigue, associated side effects are localized and related to the total radiation dose and fraction size – how much and how fast – that reaches the surrounding tissues.
Our colleagues often swap stories of the bizarre things radiation therapy has been blamed for, including dental problems in someone receiving vaginal cylinder treatment, heart dysfunction in someone treated for rectal cancer, and hip fracture in someone treated for breast cancer because the radiation “destroyed” their bones. At best, these are humorous stories, but at worst, they can delay diagnosis and treatment of what is truly causing someone’s symptoms.
5. We truly believe that less is more.
One of the most fundamental aspects of radiation oncology is our drive to optimize treatment delivery and continually improve patient care – sometimes at our own field’s economic detriment. We’re dedicated to showing that patients may get the same benefit from less and less radiation.
In the past 2 decades, the evolution and adoption of photon IMRT and proton therapy has allowed radiation plans to successfully spare surrounding tissue while improving our targeting. This evolution is coupled with technological and imaging advances that allow us to delivery of doses to certain tumors via SABR/SBRT in one to five total fractions.
A prime example: Treatment to eradicate lung or gastrointestinal tumors, which used to span up to 6 weeks, can now potentially be delivered in as little as 1 week.
For other common cancers, hypofractionation – slightly higher radiation doses per fraction at fewer total fractions overall – has revolutionized patient care, providing less radiation without impacting survival or increasing treatment toxicity.
Take breast cancer care: 50 years ago, virtually all patients with breast cancer received a mastectomy and lymph node dissections. Today, surgical techniques for lumpectomy paired with radiation therapy to the whole breast now allows us to preserve disease-free survival for those who elected to keep their breasts.
Over the past 20 years, the standards of care have shifted from 6-7 weeks of treatment to 3-4 weeks using a hypofractionated model that involves daily whole-breast radiation. The most recent clinical trials have shown that whole-breast radiation can be delivered safely and effectively for select women in as few as five fractions with either whole or partial breast targeting. Additional research driven by the idea of “right sizing” radiation treatment has even shown that certain women may not need radiation at all.
This evolution in radiation therapy illustrates how our subspecialty is constantly working to improve survival and patient well-being, form deep connections with our patients, and push the boundaries of medical innovations.
We are proud to be radiation oncologists and happy to share more. Want to know more about what goes on in the basement? Come on down, we’re happy to show you around.
Dr. Giap is a resident in the department of radiation oncology at the University of Florida, Gainesville. Dr. Chino is an assistant attending in the department of radiation oncology at Memorial Sloan Kettering Cancer Center, New York. Neither reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
As a field, radiation oncology is perhaps one of medicine’s best kept secrets. Sometimes, even our own colleagues don’t know where our department exists in the hospital or exactly what we do.
As two radiation oncologists who are, in fact, the children of radiation oncologists, we will admit that it’s possible we are a tiny bit biased. We cannot lie, though: Our field is a hidden gem.
What is well known is that radiation oncologists have a symbiotic relationship with our treatment technology. The evolution of treatment machines and radiation precision allows us to deliver patient-tailored treatment down to the millimeter. What may get lost in the discussions of isodose lines and penumbra, however, is that we’ve also got cutting-edge research and personalized patient care within a specialized team in the depths of the hospital.
Because the inner workings of what happens to patients as they come in and out of our office remains a mystery,
1. Nobody knows what goes on in the basement.
Misconceptions about our subspecialty are common, even among other oncologists. A frequent misconception is that a radiation oncologist’s involvement in patient care is limited, and radiation is delivered in a standardized manner. This essentially renders radiation oncologists technicians of expensive machines.
In reality however, radiation oncologists touch every aspect of a patient’s care, and customized radiation therapy may be indicated for virtually every cancer site in both curative and palliative settings. We strive to deliver precision medicine and practice truly patient-centered care.
To cure cancer, radiation may be used in the neoadjuvant (prior to local surgical resection), definitive (as the primary local therapy), and adjuvant (postsurgical) setting. In palliative cases, radiation can be used to treat areas of metastatic spread as well as primary unresectable tumors to alleviate obstruction and/or bleeding symptoms. Referral to radiation oncology, therefore, can be appropriate at many different points of time on the continuum of cancer care.
For many treating radiation oncologists, the close personal connections that we form with our patients is one of the primary reasons we went into this field. Not only are we making patient-centered clinical decisions during every step of the treatment plan evaluation and optimization but we also see our patients weekly for clinical visits and then ongoing in visits that may span many years of survivorship.
Our deep commitment to addressing patient needs as they are receiving treatment and responsibility for late radiation effects is absolutely an integral part of our training and lifelong practice.
2. We get down in the details.
The workflow from consultation to radiation delivery can be confusing for anyone outside our specialized field.
Once seen in consult and considered a good candidate for radiation, patients will enter the essential next step: the treatment planning imaging – or “simulation.”
The simulation scan – mostly CT, although occasionally fused MRI or PET – involves a separate appointment and another hour or so of arranging and scanning patients in the exact position that they will be treated. Given the precision of modern radiation, the simulation often includes making a customized mold so patients have minimal movement during treatment. These simulation images allow the radiation oncology team to create a treatment plan that is customized to each patient and precisely reproducible during their course of radiation treatments – what’s known as fractions.
Creating a treatment plan involves a radiation oncologist literally drawing – or contouring – on pictures of the patient’s internal anatomy in three dimensions. Radiation oncologists contour exactly where the cancer is – or where it was if the treatment is given postoperatively – and identify the surrounding organs so that the doses can be preferentially directed to the cancer target and minimize risk to nearby organs. This precision is within millimeters and accounts for microscopic disease, organ motion, and patient setup. Ultimately, we create colorful heat gradient volumes of the anticipated radiation dose delivery and optimize these to reflect our planning priorities.
We use advanced technologies to shape the beams of radiation to treat the tumor and avoid delivering high doses to the neighboring tissues with techniques such as intensity modulated radiation therapy (IMRT), stereotactic ablative radiation therapy (SABR or SBRT), and stereotactic radiosurgery. We can also take advantage of the unique properties of different modalities, such as proton therapy and electron therapy, to achieve these same goals if indicated. Radiation oncologists live for precision medicine in every aspect of their workflow.
3. We roll deep.
Radiation oncology is exemplary of “the art of medicine.” We fuse anatomy-based treatment design with advanced technology and orchestrate the daily functions of a large medical team.
But, the treatment plan and delivery would not be possible without the input and care given within a large multi- and intradisciplinary team of oncologists, medical physicists, dosimetrists, radiation therapists, nurses, social workers, and other support staff. Radiation oncologists participate in regular tumor boards with surgeons, medical oncologists, pathologists, and radiologists to optimize interdisciplinary management of complex patients, providing a thoughtful tumor localization and treatment plan, as well as to better understand an individual’s ongoing symptoms and well-being as a whole. Considering all aspects of what a patient may need involves communication with fellow physicians, nurse navigators, and social workers.
Within our own department, treatment plan creation and quality checking or verification can sometimes take over 2 weeks, with detailed input from dosimetrists and medical physicists. The actual treatment delivery involves daily communication with the radiation therapists who are dedicated to each treatment machine – like the linear accelerator – and symptom management with clinic nurses and supportive staff, such as physical therapists and registered dietitians.
This massive team effort is required to get each patient through daily radiation treatments that can last 7 weeks and may require rapid replanning if the tumor shrinks or the patient loses weight.
As part of this team, radiation oncologists are uniquely positioned to quarterback each play and guide the entire game strategy.
4. Radiation therapy takes a lot of heat.
Radiation therapy is often blamed for issues unrelated to the treatment. Irradiating the pelvis for prostate cancer, for instance, does not cause a headache or heartburn during or after treatment.
Other than fatigue, associated side effects are localized and related to the total radiation dose and fraction size – how much and how fast – that reaches the surrounding tissues.
Our colleagues often swap stories of the bizarre things radiation therapy has been blamed for, including dental problems in someone receiving vaginal cylinder treatment, heart dysfunction in someone treated for rectal cancer, and hip fracture in someone treated for breast cancer because the radiation “destroyed” their bones. At best, these are humorous stories, but at worst, they can delay diagnosis and treatment of what is truly causing someone’s symptoms.
5. We truly believe that less is more.
One of the most fundamental aspects of radiation oncology is our drive to optimize treatment delivery and continually improve patient care – sometimes at our own field’s economic detriment. We’re dedicated to showing that patients may get the same benefit from less and less radiation.
In the past 2 decades, the evolution and adoption of photon IMRT and proton therapy has allowed radiation plans to successfully spare surrounding tissue while improving our targeting. This evolution is coupled with technological and imaging advances that allow us to delivery of doses to certain tumors via SABR/SBRT in one to five total fractions.
A prime example: Treatment to eradicate lung or gastrointestinal tumors, which used to span up to 6 weeks, can now potentially be delivered in as little as 1 week.
For other common cancers, hypofractionation – slightly higher radiation doses per fraction at fewer total fractions overall – has revolutionized patient care, providing less radiation without impacting survival or increasing treatment toxicity.
Take breast cancer care: 50 years ago, virtually all patients with breast cancer received a mastectomy and lymph node dissections. Today, surgical techniques for lumpectomy paired with radiation therapy to the whole breast now allows us to preserve disease-free survival for those who elected to keep their breasts.
Over the past 20 years, the standards of care have shifted from 6-7 weeks of treatment to 3-4 weeks using a hypofractionated model that involves daily whole-breast radiation. The most recent clinical trials have shown that whole-breast radiation can be delivered safely and effectively for select women in as few as five fractions with either whole or partial breast targeting. Additional research driven by the idea of “right sizing” radiation treatment has even shown that certain women may not need radiation at all.
This evolution in radiation therapy illustrates how our subspecialty is constantly working to improve survival and patient well-being, form deep connections with our patients, and push the boundaries of medical innovations.
We are proud to be radiation oncologists and happy to share more. Want to know more about what goes on in the basement? Come on down, we’re happy to show you around.
Dr. Giap is a resident in the department of radiation oncology at the University of Florida, Gainesville. Dr. Chino is an assistant attending in the department of radiation oncology at Memorial Sloan Kettering Cancer Center, New York. Neither reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Plan B vending machine in Boston goes viral
A Plan B vending machine in Boston is gaining attention as reproductive rights have come into question since the Supreme Court overturned Roe v. Wade.
A group of students at Boston University installed the vending machine to dispense emergency contraception at a lower cost for students, according to NBC Boston. Plan B, also known as the morning-after pill, is a form of emergency contraception that can prevent pregnancy after unprotected sex or when another birth control method may have failed.
The vending machine is next to other vending machines filled with drinks and snacks in the basement of the student union at Boston University, NBC Boston reported. The machine contains boxes of levonorgestrel, a generic version of Plan B.
The boxes sell for $7.25, and the machine accepts all major credit cards. The charges are listed as “vending and snacks” on bank statements.
The Students for Reproductive Freedom decided to install the machine after seeing a similar one at Brandeis University, the news outlet reported. The vending machine was installed in March and has sold more than 1,000 emergency contraception pills. Students can also access emergency contraception through the university’s Student Health Services, which orders the contraception for the machine.
“We just wanted something that was low-cost and easy to access,” Charlotte Beatty, former copresident of Students for Reproductive Freedom, told NBC Boston.
“You don’t need to take a train across town. You don’t need to call a doctor,” she said. “It’s right there, and you can get it as soon as you need it.”
The demand for emergency contraception has increased since the Supreme Court overturned Roe. Some retailers have placed limits on how many units can be purchased at one time.
“The overturning of Roe made us even more proud to offer this service to people in our community,” Molly Baker, the group’s other former copresident, told NBC Boston.
Pictures of the vending machine have recently gone viral on social media.
“It’s going viral because people are scared, and this is a solution,” Rebecca Hart Holder, executive director of Reproductive Equity Now, told the news station.
Reproductive Equity Now, a reproductive health care nonprofit in Boston, recently honored the Boston University student group at its annual gala. Although emergency contraception is still legal, people are concerned about the effect that overturning Roe may have on future contraception access cases, Ms. Hart Holder said.
“We have to be fighting and planning for a nation that would restrict access to birth control, which is a terrifying thing to say,” she said.
The Boston University student group is now helping students at other schools who want a Plan B vending machine, and they published a resource guide to help others. They hope to install more machines on their campus and stock them with different types of medication in the future.
Plan B contains a high dose of progestin, a synthetic form of the hormone progesterone, which helps to regulate the menstrual cycle, according to Today. The pill works by inhibiting or delaying ovulation and can be taken within 72 hours after unprotected sex, though it’s most effective when taken within 24 hours. Plan B doesn’t cause an abortion and has no effect on an existing pregnancy.
Plan B and its generic versions can be purchased over the counter at most pharmacies and ordered online from major retailers. Plan B typically costs $40-$50, while generic versions cost $11-$45.
A version of this article first appeared on WebMD.com.
A Plan B vending machine in Boston is gaining attention as reproductive rights have come into question since the Supreme Court overturned Roe v. Wade.
A group of students at Boston University installed the vending machine to dispense emergency contraception at a lower cost for students, according to NBC Boston. Plan B, also known as the morning-after pill, is a form of emergency contraception that can prevent pregnancy after unprotected sex or when another birth control method may have failed.
The vending machine is next to other vending machines filled with drinks and snacks in the basement of the student union at Boston University, NBC Boston reported. The machine contains boxes of levonorgestrel, a generic version of Plan B.
The boxes sell for $7.25, and the machine accepts all major credit cards. The charges are listed as “vending and snacks” on bank statements.
The Students for Reproductive Freedom decided to install the machine after seeing a similar one at Brandeis University, the news outlet reported. The vending machine was installed in March and has sold more than 1,000 emergency contraception pills. Students can also access emergency contraception through the university’s Student Health Services, which orders the contraception for the machine.
“We just wanted something that was low-cost and easy to access,” Charlotte Beatty, former copresident of Students for Reproductive Freedom, told NBC Boston.
“You don’t need to take a train across town. You don’t need to call a doctor,” she said. “It’s right there, and you can get it as soon as you need it.”
The demand for emergency contraception has increased since the Supreme Court overturned Roe. Some retailers have placed limits on how many units can be purchased at one time.
“The overturning of Roe made us even more proud to offer this service to people in our community,” Molly Baker, the group’s other former copresident, told NBC Boston.
Pictures of the vending machine have recently gone viral on social media.
“It’s going viral because people are scared, and this is a solution,” Rebecca Hart Holder, executive director of Reproductive Equity Now, told the news station.
Reproductive Equity Now, a reproductive health care nonprofit in Boston, recently honored the Boston University student group at its annual gala. Although emergency contraception is still legal, people are concerned about the effect that overturning Roe may have on future contraception access cases, Ms. Hart Holder said.
“We have to be fighting and planning for a nation that would restrict access to birth control, which is a terrifying thing to say,” she said.
The Boston University student group is now helping students at other schools who want a Plan B vending machine, and they published a resource guide to help others. They hope to install more machines on their campus and stock them with different types of medication in the future.
Plan B contains a high dose of progestin, a synthetic form of the hormone progesterone, which helps to regulate the menstrual cycle, according to Today. The pill works by inhibiting or delaying ovulation and can be taken within 72 hours after unprotected sex, though it’s most effective when taken within 24 hours. Plan B doesn’t cause an abortion and has no effect on an existing pregnancy.
Plan B and its generic versions can be purchased over the counter at most pharmacies and ordered online from major retailers. Plan B typically costs $40-$50, while generic versions cost $11-$45.
A version of this article first appeared on WebMD.com.
A Plan B vending machine in Boston is gaining attention as reproductive rights have come into question since the Supreme Court overturned Roe v. Wade.
A group of students at Boston University installed the vending machine to dispense emergency contraception at a lower cost for students, according to NBC Boston. Plan B, also known as the morning-after pill, is a form of emergency contraception that can prevent pregnancy after unprotected sex or when another birth control method may have failed.
The vending machine is next to other vending machines filled with drinks and snacks in the basement of the student union at Boston University, NBC Boston reported. The machine contains boxes of levonorgestrel, a generic version of Plan B.
The boxes sell for $7.25, and the machine accepts all major credit cards. The charges are listed as “vending and snacks” on bank statements.
The Students for Reproductive Freedom decided to install the machine after seeing a similar one at Brandeis University, the news outlet reported. The vending machine was installed in March and has sold more than 1,000 emergency contraception pills. Students can also access emergency contraception through the university’s Student Health Services, which orders the contraception for the machine.
“We just wanted something that was low-cost and easy to access,” Charlotte Beatty, former copresident of Students for Reproductive Freedom, told NBC Boston.
“You don’t need to take a train across town. You don’t need to call a doctor,” she said. “It’s right there, and you can get it as soon as you need it.”
The demand for emergency contraception has increased since the Supreme Court overturned Roe. Some retailers have placed limits on how many units can be purchased at one time.
“The overturning of Roe made us even more proud to offer this service to people in our community,” Molly Baker, the group’s other former copresident, told NBC Boston.
Pictures of the vending machine have recently gone viral on social media.
“It’s going viral because people are scared, and this is a solution,” Rebecca Hart Holder, executive director of Reproductive Equity Now, told the news station.
Reproductive Equity Now, a reproductive health care nonprofit in Boston, recently honored the Boston University student group at its annual gala. Although emergency contraception is still legal, people are concerned about the effect that overturning Roe may have on future contraception access cases, Ms. Hart Holder said.
“We have to be fighting and planning for a nation that would restrict access to birth control, which is a terrifying thing to say,” she said.
The Boston University student group is now helping students at other schools who want a Plan B vending machine, and they published a resource guide to help others. They hope to install more machines on their campus and stock them with different types of medication in the future.
Plan B contains a high dose of progestin, a synthetic form of the hormone progesterone, which helps to regulate the menstrual cycle, according to Today. The pill works by inhibiting or delaying ovulation and can be taken within 72 hours after unprotected sex, though it’s most effective when taken within 24 hours. Plan B doesn’t cause an abortion and has no effect on an existing pregnancy.
Plan B and its generic versions can be purchased over the counter at most pharmacies and ordered online from major retailers. Plan B typically costs $40-$50, while generic versions cost $11-$45.
A version of this article first appeared on WebMD.com.
Think of pediatric morphea as a systemic, chronic disease, expert advises
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
INDIANAPOLIS – In the opinion of Elena Pope, MD, MSc,
“There is no correlation between the extent and activity of skin lesions and the presence, severity, and activity of extracutaneous manifestations,” Dr. Pope, professor of pediatrics at the University of Toronto and division head of pediatric dermatology at the Hospital for Sick Children in Toronto, said during the annual meeting of the Society for Pediatric Dermatology. “Treatment needs to be tailored to the extent of cutaneous manifestations, and I think we need to be aware of and address the impact on patients’ quality of life,” she added. There is also a need for more research “on targeted and better-tolerated therapies to put a stop to the progression of disease.”
Congenital morphea is a form of localized scleroderma that presents at birth but can be confused with port wine stain. Results from a multicenter retrospective review of 25 cases conducted by Dr. Pope and colleagues found that the median age at diagnosis was 2.9 years and 76% had linear-type lesions. In addition, 48% had extracutaneous involvement (all of these patients had linear morphea), most commonly of the central nervous system.
“It’s important to realize these lesions may become active over time,” Dr. Pope said. “In my experience, there are two different courses. Either you have innocuous lesions when the patients are born and they may become active around 3-4 years of age, or you have early intrauterine involvement, with lesions inactive at birth but with potential for significant damage in utero.”
She cautioned against treating a suspected port wine stain lesion with laser until congenital morphea is ruled out. “I’m aware of at least one lawsuit of a child where someone used a laser in a child who had progression with significant sclerosis,” she said. “The parents assumed it was the use of the laser that led to the progression, not the actual disease.”
Extracutaneous manifestations are common in morphea patients. A multicenter study of 750 patients with juvenile scleroderma found that 22% had extracutaneous manifestations. Almost half of patients (47%) had arthritis, but 17% had neurologic findings such as seizures and headaches, 9% had vascular manifestations, and 8% had uveitis. Subsequent studies found that neurological disease affects between 11% and 19% of cases, especially in those involving the head and neck.
“There is a wide range of manifestations from headache and neuropsychiatric changes to brain atrophy, seizures, and CNS cavernoma,” Dr. Pope said. “There also can be orthodental involvement such as malocclusion. It’s important to do a brain MRI, eye exam for uveitis, and don’t forget the orthodental assessment.”
She recalled a 10-year-old boy who presented to the Hospital for Sick Children with tissue loss on the forehead and eyebrow and eyelashes. He had no other congenital morphea symptoms and the MRI was normal, but the eye exam revealed uveitis. “It’s important to remember that uveitis is asymptomatic, so unless you look for it, you’re not going to find it,” she said.
According to unpublished data in 42 congenital morphea patients with lesions limited to the head and neck, who underwent MRI imaging at the Hospital for Sick Children, 57% had CNS changes that were ipsilateral in 68% of cases. “White matter changes were the most common, and to our surprise, there were patients who had progressive CNS disease, including CNS vasculitis, new lesions, and enhancement of prior stable lesions,” Dr. Pope said.
She recalled the case of an 8-year-old boy who presented to the hospital with intractable seizures. Upon completion of the MRI, one of the radiologists noted that the imaging showed subtle thinning of the forehead, and he was referred to Dr. Pope and colleagues for assessment. In the span of 4 years, despite aggressive treatment, the boy’s CNS disease progressed. “There was more enhancement, more tissue loss, his seizures are very hard to control, and he has many neurodevelopmental changes,” she recalled. “What I learned from this case is that skin activity does not correlate with imaging. Don’t assume that just because the skin is burnt out that the CNS will be the same. Also, the extent of skin disease does not predict involvement or progression of the CNS.”
Linear lesions on the lower extremities are a harbinger of orthopedic complications, which can occur in about half of patients. Joint contractures in this subset of patients are seen in about 81% of cases, while other sequelae can include arthritis, limb atrophy, leg-leg discrepancy, and angular deformity. “About 14% of patients require intervention,” Dr. Pope said. “In terms of working those patients up, you need to do an MRI and assess the extent of muscle and fascial involvement. Early physiotherapy and an orthopedic evaluation are also recommended.”
As for possible markers of morphea, antinuclear antibody is positive in 22%-68% of cases and correlates with disease severity, extracutaneous manifestations, and disease flare-up. Antihistone antibodies (AHA) are positive in about 47% of cases, “and that tends to correlate with the extent of skin and muscle involvement,” Dr. Pope said. “Anti–double-stranded DNA correlates with extent of disease, but the only known biomarker to date that correlates with disease activity is CXCL9/10. This has been documented in the skin as well as in the blood. So, this marker may help us determine if the patient needs to be treated or not.”
Treatments
For treatment of active localized disease, topical medications are helpful in some cases. Options include topical steroids, calcipotriol with or without betamethasone, imiquimod, and tacrolimus. “In my experience the combination of calcipotriol with betamethasone is best,” she said. “It really shuts down the activity fairly soon, and you can scale down to calcipotriol alone. I don’t find imiquimod very helpful for active lesions, although it has a role for inactive lesions.”
For patients with linear or generalized/mixed disease, “the combination of methotrexate and corticosteroids or methotrexate alone is probably the way to go,” Dr. Pope said. “The addition of steroids really depends on where the lesion is and how worried you are about other problems.”
According to the best available literature, 88% of patients should respond to treatment with methotrexate (MTX) and/or steroids within 3-6 months, and 74% within 3 months. “If they don’t, you have to wonder if the patient’s taking the medication, or you need to think about other alternative treatments,” she said. “Complete remission is possible in most of the patients, and the longer you treat the more you will see that. On average, most of us treat patients for about 3 years, but there are treatment failures as well. This can occur in up to 16% of patients.”
As for second-line treatment agents for congenital morphea, clinicians often turn to mycophenolate mofetil (MMF). Results from a retrospective longitudinal study of juvenile localized scleroderma patients found that after a mean of 9 years 91% of patients on MMF and 100% of patients on MTX had inactive disease. “There were no differences in relapse rates, although MMF seems to have a more sustained long-term effect and overall is better tolerated,” said Dr. Pope, who was not involved with the study. “However, it’s more immunosuppressive than MTX, which is important, especially in the era of COVID-19. You also need to think about the potential for more hematological suppression with MMF use.” If standard therapy fails, there is anecdotal data supporting the use of abatacept (which suppresses the T-cell activity in affected patients), tofacitinib (which inhibits transforming growth factor–beta), or dupilumab (which inhibits interleukin-4).
Dr. Pope emphasized the effect congenital morphea has on quality of life. Remarks from patients with facial morphea and their parents who participated in a focus group on the topic organized by the Hospital for Sick Children included, “You just want to stay inside because you are afraid of what people will say,” “They laugh at her. They make fun of her, and it’s terrible,” and “MTX makes me feel weird. I would throw up, feel dizzy.”
“You have to take that into consideration, because we cannot make the treatment worse than the disease,” Dr. Pope said. “There are many domains where patients could be affected, including skin symptoms, physical functioning, body image and social support, side effects of medication, and presence of extracutaneous manifestations. Predictors of poor quality of life include female sex and involvement of hands and feet.”
Dr. Pope disclosed that she has received grants/research support from AbbVie, Centocor, and Amgen. She has also received consulting fees from AbbVie, Sanofi, Novartis, Boehringer-Ingelheim, Phoenix, Amryt Pharma, and Timber Pharmaceuticals.
AT SPD 2022
Her ex-husband is suing a clinic over the abortion she had 4 years ago
A judge allowed the woman’s ex-husband to establish an estate for the embryo, which had been aborted in its seventh week of development. The ex-husband filed a wrongful death lawsuit against the clinic and its doctors in 2020, alleging that physicians failed to obtain proper informed consent from the woman as required by Arizona law.
Across the United States, people have sued for negligence in the death of a fetus or embryo in cases where a pregnant person has been killed in a car crash or a pregnancy was lost because of alleged wrongdoing by a physician. But a court action claiming the wrongful death of an aborted embryo or fetus is a more novel strategy, legal experts said.
The experts said this rare tactic could become more common, as anti-abortion groups have signaled their desire to further limit reproductive rights following the U.S. Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade. The Arizona lawsuit and others that may follow could also be an attempt to discourage and intimidate providers and harass plaintiffs’ former romantic partners, experts said.
Lucinda Finley, a law professor at the University at Buffalo who specializes in tort law and reproductive rights, said the Arizona case is a “harbinger of things to come” and called it “troubling for the future.”
Ms. Finley said she expects state lawmakers and anti-abortion groups to use “unprecedented strategies” to try to prevent people from traveling to obtain abortions or block them from obtaining information on where to seek one.
Perhaps the most extreme example is in Texas, where the Texas Heartbeat Act, signed into law in May 2021 and upheld by the U.S. Supreme Court in December, allows private citizens to sue a person who performs or aids in an abortion.
“It’s much bigger than these wrongful death suits,” Ms. Finley said.
Civia Tamarkin, president of the National Council of Jewish Women Arizona, which advocates for reproductive rights, said the Arizona lawsuit is part of a larger agenda that anti-abortion advocates are working toward.
“It’s a lawsuit that appears to be a trial balloon to see how far the attorney and the plaintiff can push the limits of the law, the limits of reason, the limits of science and medicine,” Ms. Tamarkin said.
In July 2018, the ex-husband, Mario Villegas, accompanied his then-wife to three medical appointments – a consultation, the abortion, and a follow-up. The woman, who ProPublica is not identifying for privacy reasons, said in a deposition in the wrongful death suit that at the time of the procedure the two were already talking about obtaining a divorce, which was finalized later that year.
“We were not happy together at all,” she said.
Mr. Villegas, a former Marine from Globe, Ariz., a mining town east of Phoenix, had been married twice before and has other children. He has since moved out of state.
In a form his then-wife filled out at the clinic, she said she was seeking an abortion because she was not ready to be a parent and her relationship with Mr. Villegas was unstable, according to court records. She also checked a box affirming that “I am comfortable with my decision to terminate this pregnancy.” The woman declined to speak on the record with ProPublica out of fear for her safety.
The following year, in 2019, Mr. Villegas learned about an Alabama man who hadn’t wanted his ex-girlfriend to have an abortion and sued the Alabama Women’s Center for Reproductive Alternatives in Huntsville on behalf of an embryo that was aborted at six weeks.
To sue on behalf of the embryo, the would-be father, Ryan Magers, went to probate court where he asked a judge to appoint him as the personal representative of the estate. In probate court, a judge may appoint someone to represent the estate of a person who has died without a will. That representative then has the authority to distribute the estate’s assets to beneficiaries.
When Mr. Magers filed to open an estate for the embryo, his attorney cited various Alabama court rulings involving pregnant people and a 2018 amendment to the Alabama Constitution recognizing the “sanctity of unborn life and the rights of unborn children.”
A probate judge appointed Mr. Magers representative of the estate, giving him legal standing to sue for damages in the wrongful death claim. The case, believed to be the first instance in which an aborted embryo was given legal rights, made national headlines.
It’s unclear how many states allow an estate to be opened on behalf of an embryo or fetus. Some states, like Arizona, don’t explicitly define what counts as a deceased person in their probate code, leaving it to a judge to decide. In a handful of states, laws define embryos and fetuses as a person at conception, which could allow for an estate, but it’s rare.
An Alabama circuit court judge eventually dismissed Mr. Magers’ wrongful death lawsuit, stating that the claims were “precluded by State and Federal laws.”
Mr. Villegas contacted Mr. Magers’ attorney, Brent Helms, about pursuing a similar action in Arizona and was referred to J. Stanley Martineau, an Arizona attorney who had flown to Alabama to talk to Mr. Helms about Mr. Magers’ case.
In August 2020, Mr. Villegas filed a petition to be appointed personal representative of the estate of “Baby Villegas.” His ex-wife opposed the action and contacted a legal advocacy organization focused on reproductive justice, which helped her obtain a lawyer.
In court filings, Mr. Villegas said he prefers to think of “Baby Villegas” as a girl, although the sex of the embryo was never determined, and his lawyer argued that there isn’t an Arizona case that explicitly defines a deceased person, “so the issue appears to be an open one in Arizona.”
In a 2021 motion arguing for dismissal, the ex-wife’s attorney, Louis Silverman, argued that Arizona’s probate code doesn’t authorize the appointment of a personal representative for an embryo, and that granting Mr. Villegas’ request would violate a woman’s constitutional right to decide whether to carry a pregnancy to term.
“U.S. Supreme Court precedent has long protected the constitutional right of a woman to obtain an abortion, including that the decision whether to do so belongs to the woman alone – even where her partner, spouse, or ex-spouse disagrees with that decision,” Mr. Silverman said last year.
Gila County Superior Court Judge Bryan B. Chambers said in an order denying the motion that his decision allows Mr. Villegas to make the argument that the embryo is a person in a wrongful death lawsuit, but that he has not reached that conclusion at this stage. Mr. Villegas was later appointed the personal representative of the estate.
As states determine what is legal in the wake of Dobbs and legislators propose new abortion laws, anti-abortion groups such as the National Right to Life Committee see civil suits as a way to enforce abortion bans and have released model legislation they hope sympathetic legislators will duplicate in statehouses nationwide.
“In addition to criminal penalties and medical license revocation, civil remedies will be critical to ensure that unborn lives are protected from illegal abortions,” the group wrote in a June 15 letter to its state affiliates that included the model legislation.
James Bopp Jr., general counsel for the committee, said in an interview with ProPublica that such actions will be necessary because some “radical Democrat” prosecutors have signaled they won’t enforce criminal abortion bans. Last month, 90 prosecutors from across the country indicated that they would not prosecute those who seek abortions.
“The civil remedies follow what the criminal law makes unlawful,” he said. “And that’s what we’re doing.”
The National Right to Life Committee’s model legislation, which advocates prohibiting abortion except to prevent the death of the pregnant person, recommends that states permit civil actions against people or entities that violate abortion laws “to prevent future violations.” It also suggests that people who have had or have sought to have an illegal abortion, as well as the expectant father and the parents of a pregnant minor, be allowed to pursue wrongful death actions.
Under the legislation, an action for wrongful death of an “unborn child” would be treated like that of a child who died after being born.
In one regard, Arizona has already implemented a piece of this model legislation as the state’s lawmakers have chipped away at access to abortion and enacted a myriad of regulations on doctors who provide the procedure.
The state’s “informed consent” statute for abortion, first signed into law by then-Gov. Jan Brewer in 2009, mandated an in-person counseling session and a 24-hour waiting period before an abortion. It allows a pregnant person, their husband or a maternal grandparent of a minor to sue if a physician does not properly obtain the pregnant person’s informed consent, and to receive damages for psychological, emotional and physical injuries, statutory damages and attorney fees.
The informed consent laws, which have changed over time, mandate that the patient be told about the “probable anatomical and physiological characteristics” of the embryo or fetus and the “immediate and long-term medical risks” associated with abortion, as well as alternatives to the procedure. Some abortion-rights groups and medical professionals have criticized informed consent processes, arguing the materials can be misleading and personify the embryo or fetus. A 2018 review of numerous studies concluded that having an abortion does not increase a person’s risk of infertility in their next pregnancy, nor is it linked to a higher risk of breast cancer or preterm birth, among other issues.
The wrongful death suit comes at a time of extraordinary confusion over abortion law in Arizona.
Until Roe v. Wade was handed down in 1973, establishing a constitutional right to abortion, a law dating to before statehood had banned the procedure. In March, Gov. Doug Ducey, a Republican who has called Arizona “the most pro-life state in the country,” signed into law a bill outlawing abortions after 15 weeks, and said that law would supersede the pre-statehood ban if Roe were overturned. But now that Roe has been overturned, Arizona Attorney General Mark Brnovich, another Republican, said he intends to enforce the pre-statehood ban, which outlawed abortion except to preserve the life of the person seeking the procedure. On July 14, he filed a motion to lift an injunction on the law, which would make it enforceable.
Adding to the muddle, a U.S. district court judge on July 11 blocked part of a 2021 Arizona law that would classify fertilized eggs, embryos and fetuses as people starting at conception, ruling that the attorney general cannot use the so-called personhood law against abortion providers. Following the Supreme Court decision in Dobbs, eight of the state’s nine abortion providers – all located in three Arizona counties – halted abortion services, but following the emergency injunction some are again offering them.
In the wrongful death claim, Mr. Martineau argued that the woman’s consent was invalidated because the doctors didn’t follow the informed consent statute. Although the woman signed four consent documents, the suit claims that “evidence shows that in her rush to maximize profits,” the clinic’s owner, Dr. Gabrielle Goodrick, “cut corners.” Mr. Martineau alleged that Dr. Goodrick and another doctor didn’t inform the woman of the loss of “maternal-fetal” attachment, about the alternatives to abortion or that if not for the abortion, the embryo would likely have been “delivered to term,” among other violations.
Tom Slutes, Dr. Goodrick’s lawyer, called the lawsuit “ridiculous.”
“They didn’t cut any corners,” he said, adding that the woman “clearly knew what was going to happen and definitely, strongly” wanted the abortion. Regardless of the information the woman received, she wouldn’t have changed her mind, Mr. Slutes said. Mr. Slutes referenced the deposition, where the woman said she “felt completely informed.”
Mr. Martineau said in an interview that Mr. Villegas isn’t motivated by collecting money from the lawsuit.
“He has no desire to harass” his ex-wife, Mr. Martineau said. “All he wants to do is make sure it doesn’t happen to another father.”
In a deposition, Mr. Villegas’ ex-wife said that he was emotionally abusive during their marriage, which lasted nearly 5 years. At first, she said, Mr. Villegas seemed like the “greatest guy I’ve ever met in my life,” taking her to California for a week as a birthday gift. But as the marriage progressed, she said, there were times he wouldn’t allow her to get a job or leave the house unless she was with him.
The woman alleged that Mr. Villegas made fake social media profiles, hacked into her social media accounts and threatened to “blackmail” her if she left him during his failed campaign to be a justice of the peace in Gila County, outside of Phoenix.
Mr. Villegas denied the allegations about his relationship but declined to comment further for this story, Mr. Martineau said.
Carliss Chatman, an associate law professor at Washington and Lee University in Virginia, said certain civil remedies can also be a mechanism for men to continue to abuse their former partners through the court system.
“What happens if the father who is suing on behalf of the fetus is your rapist or your abuser? It’s another way to torture a woman,” Ms. Chatman said.
Ms. Chatman added that these legal actions can be a deterrent for physicians in states where abortion is banned after a certain gestational period, because the threat of civil suits makes it harder for doctors to get insurance.
The lawsuit has added to the stresses on Dr. Goodrick, who has been performing abortions in Arizona since the mid-1990s, and her practice. She said that, since the lawsuit was filed, the annual cost of her medical malpractice insurance has risen from $32,000 to $67,000.
Before providers in Arizona halted abortions following the Supreme Court decision, people would begin lining up outside Dr. Goodrick’s clinic at 6 a.m., sometimes with lawn chairs in hand, like “a concert line,” Dr. Goodrick said.
“Every year there’s something and we never know what it’s going to be,” Dr. Goodrick said recently at her Phoenix clinic. “I’m kind of desensitized to it all.”
Nicole Santa Cruz is a reporter covering issues of inequality in the Southwest.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A judge allowed the woman’s ex-husband to establish an estate for the embryo, which had been aborted in its seventh week of development. The ex-husband filed a wrongful death lawsuit against the clinic and its doctors in 2020, alleging that physicians failed to obtain proper informed consent from the woman as required by Arizona law.
Across the United States, people have sued for negligence in the death of a fetus or embryo in cases where a pregnant person has been killed in a car crash or a pregnancy was lost because of alleged wrongdoing by a physician. But a court action claiming the wrongful death of an aborted embryo or fetus is a more novel strategy, legal experts said.
The experts said this rare tactic could become more common, as anti-abortion groups have signaled their desire to further limit reproductive rights following the U.S. Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade. The Arizona lawsuit and others that may follow could also be an attempt to discourage and intimidate providers and harass plaintiffs’ former romantic partners, experts said.
Lucinda Finley, a law professor at the University at Buffalo who specializes in tort law and reproductive rights, said the Arizona case is a “harbinger of things to come” and called it “troubling for the future.”
Ms. Finley said she expects state lawmakers and anti-abortion groups to use “unprecedented strategies” to try to prevent people from traveling to obtain abortions or block them from obtaining information on where to seek one.
Perhaps the most extreme example is in Texas, where the Texas Heartbeat Act, signed into law in May 2021 and upheld by the U.S. Supreme Court in December, allows private citizens to sue a person who performs or aids in an abortion.
“It’s much bigger than these wrongful death suits,” Ms. Finley said.
Civia Tamarkin, president of the National Council of Jewish Women Arizona, which advocates for reproductive rights, said the Arizona lawsuit is part of a larger agenda that anti-abortion advocates are working toward.
“It’s a lawsuit that appears to be a trial balloon to see how far the attorney and the plaintiff can push the limits of the law, the limits of reason, the limits of science and medicine,” Ms. Tamarkin said.
In July 2018, the ex-husband, Mario Villegas, accompanied his then-wife to three medical appointments – a consultation, the abortion, and a follow-up. The woman, who ProPublica is not identifying for privacy reasons, said in a deposition in the wrongful death suit that at the time of the procedure the two were already talking about obtaining a divorce, which was finalized later that year.
“We were not happy together at all,” she said.
Mr. Villegas, a former Marine from Globe, Ariz., a mining town east of Phoenix, had been married twice before and has other children. He has since moved out of state.
In a form his then-wife filled out at the clinic, she said she was seeking an abortion because she was not ready to be a parent and her relationship with Mr. Villegas was unstable, according to court records. She also checked a box affirming that “I am comfortable with my decision to terminate this pregnancy.” The woman declined to speak on the record with ProPublica out of fear for her safety.
The following year, in 2019, Mr. Villegas learned about an Alabama man who hadn’t wanted his ex-girlfriend to have an abortion and sued the Alabama Women’s Center for Reproductive Alternatives in Huntsville on behalf of an embryo that was aborted at six weeks.
To sue on behalf of the embryo, the would-be father, Ryan Magers, went to probate court where he asked a judge to appoint him as the personal representative of the estate. In probate court, a judge may appoint someone to represent the estate of a person who has died without a will. That representative then has the authority to distribute the estate’s assets to beneficiaries.
When Mr. Magers filed to open an estate for the embryo, his attorney cited various Alabama court rulings involving pregnant people and a 2018 amendment to the Alabama Constitution recognizing the “sanctity of unborn life and the rights of unborn children.”
A probate judge appointed Mr. Magers representative of the estate, giving him legal standing to sue for damages in the wrongful death claim. The case, believed to be the first instance in which an aborted embryo was given legal rights, made national headlines.
It’s unclear how many states allow an estate to be opened on behalf of an embryo or fetus. Some states, like Arizona, don’t explicitly define what counts as a deceased person in their probate code, leaving it to a judge to decide. In a handful of states, laws define embryos and fetuses as a person at conception, which could allow for an estate, but it’s rare.
An Alabama circuit court judge eventually dismissed Mr. Magers’ wrongful death lawsuit, stating that the claims were “precluded by State and Federal laws.”
Mr. Villegas contacted Mr. Magers’ attorney, Brent Helms, about pursuing a similar action in Arizona and was referred to J. Stanley Martineau, an Arizona attorney who had flown to Alabama to talk to Mr. Helms about Mr. Magers’ case.
In August 2020, Mr. Villegas filed a petition to be appointed personal representative of the estate of “Baby Villegas.” His ex-wife opposed the action and contacted a legal advocacy organization focused on reproductive justice, which helped her obtain a lawyer.
In court filings, Mr. Villegas said he prefers to think of “Baby Villegas” as a girl, although the sex of the embryo was never determined, and his lawyer argued that there isn’t an Arizona case that explicitly defines a deceased person, “so the issue appears to be an open one in Arizona.”
In a 2021 motion arguing for dismissal, the ex-wife’s attorney, Louis Silverman, argued that Arizona’s probate code doesn’t authorize the appointment of a personal representative for an embryo, and that granting Mr. Villegas’ request would violate a woman’s constitutional right to decide whether to carry a pregnancy to term.
“U.S. Supreme Court precedent has long protected the constitutional right of a woman to obtain an abortion, including that the decision whether to do so belongs to the woman alone – even where her partner, spouse, or ex-spouse disagrees with that decision,” Mr. Silverman said last year.
Gila County Superior Court Judge Bryan B. Chambers said in an order denying the motion that his decision allows Mr. Villegas to make the argument that the embryo is a person in a wrongful death lawsuit, but that he has not reached that conclusion at this stage. Mr. Villegas was later appointed the personal representative of the estate.
As states determine what is legal in the wake of Dobbs and legislators propose new abortion laws, anti-abortion groups such as the National Right to Life Committee see civil suits as a way to enforce abortion bans and have released model legislation they hope sympathetic legislators will duplicate in statehouses nationwide.
“In addition to criminal penalties and medical license revocation, civil remedies will be critical to ensure that unborn lives are protected from illegal abortions,” the group wrote in a June 15 letter to its state affiliates that included the model legislation.
James Bopp Jr., general counsel for the committee, said in an interview with ProPublica that such actions will be necessary because some “radical Democrat” prosecutors have signaled they won’t enforce criminal abortion bans. Last month, 90 prosecutors from across the country indicated that they would not prosecute those who seek abortions.
“The civil remedies follow what the criminal law makes unlawful,” he said. “And that’s what we’re doing.”
The National Right to Life Committee’s model legislation, which advocates prohibiting abortion except to prevent the death of the pregnant person, recommends that states permit civil actions against people or entities that violate abortion laws “to prevent future violations.” It also suggests that people who have had or have sought to have an illegal abortion, as well as the expectant father and the parents of a pregnant minor, be allowed to pursue wrongful death actions.
Under the legislation, an action for wrongful death of an “unborn child” would be treated like that of a child who died after being born.
In one regard, Arizona has already implemented a piece of this model legislation as the state’s lawmakers have chipped away at access to abortion and enacted a myriad of regulations on doctors who provide the procedure.
The state’s “informed consent” statute for abortion, first signed into law by then-Gov. Jan Brewer in 2009, mandated an in-person counseling session and a 24-hour waiting period before an abortion. It allows a pregnant person, their husband or a maternal grandparent of a minor to sue if a physician does not properly obtain the pregnant person’s informed consent, and to receive damages for psychological, emotional and physical injuries, statutory damages and attorney fees.
The informed consent laws, which have changed over time, mandate that the patient be told about the “probable anatomical and physiological characteristics” of the embryo or fetus and the “immediate and long-term medical risks” associated with abortion, as well as alternatives to the procedure. Some abortion-rights groups and medical professionals have criticized informed consent processes, arguing the materials can be misleading and personify the embryo or fetus. A 2018 review of numerous studies concluded that having an abortion does not increase a person’s risk of infertility in their next pregnancy, nor is it linked to a higher risk of breast cancer or preterm birth, among other issues.
The wrongful death suit comes at a time of extraordinary confusion over abortion law in Arizona.
Until Roe v. Wade was handed down in 1973, establishing a constitutional right to abortion, a law dating to before statehood had banned the procedure. In March, Gov. Doug Ducey, a Republican who has called Arizona “the most pro-life state in the country,” signed into law a bill outlawing abortions after 15 weeks, and said that law would supersede the pre-statehood ban if Roe were overturned. But now that Roe has been overturned, Arizona Attorney General Mark Brnovich, another Republican, said he intends to enforce the pre-statehood ban, which outlawed abortion except to preserve the life of the person seeking the procedure. On July 14, he filed a motion to lift an injunction on the law, which would make it enforceable.
Adding to the muddle, a U.S. district court judge on July 11 blocked part of a 2021 Arizona law that would classify fertilized eggs, embryos and fetuses as people starting at conception, ruling that the attorney general cannot use the so-called personhood law against abortion providers. Following the Supreme Court decision in Dobbs, eight of the state’s nine abortion providers – all located in three Arizona counties – halted abortion services, but following the emergency injunction some are again offering them.
In the wrongful death claim, Mr. Martineau argued that the woman’s consent was invalidated because the doctors didn’t follow the informed consent statute. Although the woman signed four consent documents, the suit claims that “evidence shows that in her rush to maximize profits,” the clinic’s owner, Dr. Gabrielle Goodrick, “cut corners.” Mr. Martineau alleged that Dr. Goodrick and another doctor didn’t inform the woman of the loss of “maternal-fetal” attachment, about the alternatives to abortion or that if not for the abortion, the embryo would likely have been “delivered to term,” among other violations.
Tom Slutes, Dr. Goodrick’s lawyer, called the lawsuit “ridiculous.”
“They didn’t cut any corners,” he said, adding that the woman “clearly knew what was going to happen and definitely, strongly” wanted the abortion. Regardless of the information the woman received, she wouldn’t have changed her mind, Mr. Slutes said. Mr. Slutes referenced the deposition, where the woman said she “felt completely informed.”
Mr. Martineau said in an interview that Mr. Villegas isn’t motivated by collecting money from the lawsuit.
“He has no desire to harass” his ex-wife, Mr. Martineau said. “All he wants to do is make sure it doesn’t happen to another father.”
In a deposition, Mr. Villegas’ ex-wife said that he was emotionally abusive during their marriage, which lasted nearly 5 years. At first, she said, Mr. Villegas seemed like the “greatest guy I’ve ever met in my life,” taking her to California for a week as a birthday gift. But as the marriage progressed, she said, there were times he wouldn’t allow her to get a job or leave the house unless she was with him.
The woman alleged that Mr. Villegas made fake social media profiles, hacked into her social media accounts and threatened to “blackmail” her if she left him during his failed campaign to be a justice of the peace in Gila County, outside of Phoenix.
Mr. Villegas denied the allegations about his relationship but declined to comment further for this story, Mr. Martineau said.
Carliss Chatman, an associate law professor at Washington and Lee University in Virginia, said certain civil remedies can also be a mechanism for men to continue to abuse their former partners through the court system.
“What happens if the father who is suing on behalf of the fetus is your rapist or your abuser? It’s another way to torture a woman,” Ms. Chatman said.
Ms. Chatman added that these legal actions can be a deterrent for physicians in states where abortion is banned after a certain gestational period, because the threat of civil suits makes it harder for doctors to get insurance.
The lawsuit has added to the stresses on Dr. Goodrick, who has been performing abortions in Arizona since the mid-1990s, and her practice. She said that, since the lawsuit was filed, the annual cost of her medical malpractice insurance has risen from $32,000 to $67,000.
Before providers in Arizona halted abortions following the Supreme Court decision, people would begin lining up outside Dr. Goodrick’s clinic at 6 a.m., sometimes with lawn chairs in hand, like “a concert line,” Dr. Goodrick said.
“Every year there’s something and we never know what it’s going to be,” Dr. Goodrick said recently at her Phoenix clinic. “I’m kind of desensitized to it all.”
Nicole Santa Cruz is a reporter covering issues of inequality in the Southwest.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
A judge allowed the woman’s ex-husband to establish an estate for the embryo, which had been aborted in its seventh week of development. The ex-husband filed a wrongful death lawsuit against the clinic and its doctors in 2020, alleging that physicians failed to obtain proper informed consent from the woman as required by Arizona law.
Across the United States, people have sued for negligence in the death of a fetus or embryo in cases where a pregnant person has been killed in a car crash or a pregnancy was lost because of alleged wrongdoing by a physician. But a court action claiming the wrongful death of an aborted embryo or fetus is a more novel strategy, legal experts said.
The experts said this rare tactic could become more common, as anti-abortion groups have signaled their desire to further limit reproductive rights following the U.S. Supreme Court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade. The Arizona lawsuit and others that may follow could also be an attempt to discourage and intimidate providers and harass plaintiffs’ former romantic partners, experts said.
Lucinda Finley, a law professor at the University at Buffalo who specializes in tort law and reproductive rights, said the Arizona case is a “harbinger of things to come” and called it “troubling for the future.”
Ms. Finley said she expects state lawmakers and anti-abortion groups to use “unprecedented strategies” to try to prevent people from traveling to obtain abortions or block them from obtaining information on where to seek one.
Perhaps the most extreme example is in Texas, where the Texas Heartbeat Act, signed into law in May 2021 and upheld by the U.S. Supreme Court in December, allows private citizens to sue a person who performs or aids in an abortion.
“It’s much bigger than these wrongful death suits,” Ms. Finley said.
Civia Tamarkin, president of the National Council of Jewish Women Arizona, which advocates for reproductive rights, said the Arizona lawsuit is part of a larger agenda that anti-abortion advocates are working toward.
“It’s a lawsuit that appears to be a trial balloon to see how far the attorney and the plaintiff can push the limits of the law, the limits of reason, the limits of science and medicine,” Ms. Tamarkin said.
In July 2018, the ex-husband, Mario Villegas, accompanied his then-wife to three medical appointments – a consultation, the abortion, and a follow-up. The woman, who ProPublica is not identifying for privacy reasons, said in a deposition in the wrongful death suit that at the time of the procedure the two were already talking about obtaining a divorce, which was finalized later that year.
“We were not happy together at all,” she said.
Mr. Villegas, a former Marine from Globe, Ariz., a mining town east of Phoenix, had been married twice before and has other children. He has since moved out of state.
In a form his then-wife filled out at the clinic, she said she was seeking an abortion because she was not ready to be a parent and her relationship with Mr. Villegas was unstable, according to court records. She also checked a box affirming that “I am comfortable with my decision to terminate this pregnancy.” The woman declined to speak on the record with ProPublica out of fear for her safety.
The following year, in 2019, Mr. Villegas learned about an Alabama man who hadn’t wanted his ex-girlfriend to have an abortion and sued the Alabama Women’s Center for Reproductive Alternatives in Huntsville on behalf of an embryo that was aborted at six weeks.
To sue on behalf of the embryo, the would-be father, Ryan Magers, went to probate court where he asked a judge to appoint him as the personal representative of the estate. In probate court, a judge may appoint someone to represent the estate of a person who has died without a will. That representative then has the authority to distribute the estate’s assets to beneficiaries.
When Mr. Magers filed to open an estate for the embryo, his attorney cited various Alabama court rulings involving pregnant people and a 2018 amendment to the Alabama Constitution recognizing the “sanctity of unborn life and the rights of unborn children.”
A probate judge appointed Mr. Magers representative of the estate, giving him legal standing to sue for damages in the wrongful death claim. The case, believed to be the first instance in which an aborted embryo was given legal rights, made national headlines.
It’s unclear how many states allow an estate to be opened on behalf of an embryo or fetus. Some states, like Arizona, don’t explicitly define what counts as a deceased person in their probate code, leaving it to a judge to decide. In a handful of states, laws define embryos and fetuses as a person at conception, which could allow for an estate, but it’s rare.
An Alabama circuit court judge eventually dismissed Mr. Magers’ wrongful death lawsuit, stating that the claims were “precluded by State and Federal laws.”
Mr. Villegas contacted Mr. Magers’ attorney, Brent Helms, about pursuing a similar action in Arizona and was referred to J. Stanley Martineau, an Arizona attorney who had flown to Alabama to talk to Mr. Helms about Mr. Magers’ case.
In August 2020, Mr. Villegas filed a petition to be appointed personal representative of the estate of “Baby Villegas.” His ex-wife opposed the action and contacted a legal advocacy organization focused on reproductive justice, which helped her obtain a lawyer.
In court filings, Mr. Villegas said he prefers to think of “Baby Villegas” as a girl, although the sex of the embryo was never determined, and his lawyer argued that there isn’t an Arizona case that explicitly defines a deceased person, “so the issue appears to be an open one in Arizona.”
In a 2021 motion arguing for dismissal, the ex-wife’s attorney, Louis Silverman, argued that Arizona’s probate code doesn’t authorize the appointment of a personal representative for an embryo, and that granting Mr. Villegas’ request would violate a woman’s constitutional right to decide whether to carry a pregnancy to term.
“U.S. Supreme Court precedent has long protected the constitutional right of a woman to obtain an abortion, including that the decision whether to do so belongs to the woman alone – even where her partner, spouse, or ex-spouse disagrees with that decision,” Mr. Silverman said last year.
Gila County Superior Court Judge Bryan B. Chambers said in an order denying the motion that his decision allows Mr. Villegas to make the argument that the embryo is a person in a wrongful death lawsuit, but that he has not reached that conclusion at this stage. Mr. Villegas was later appointed the personal representative of the estate.
As states determine what is legal in the wake of Dobbs and legislators propose new abortion laws, anti-abortion groups such as the National Right to Life Committee see civil suits as a way to enforce abortion bans and have released model legislation they hope sympathetic legislators will duplicate in statehouses nationwide.
“In addition to criminal penalties and medical license revocation, civil remedies will be critical to ensure that unborn lives are protected from illegal abortions,” the group wrote in a June 15 letter to its state affiliates that included the model legislation.
James Bopp Jr., general counsel for the committee, said in an interview with ProPublica that such actions will be necessary because some “radical Democrat” prosecutors have signaled they won’t enforce criminal abortion bans. Last month, 90 prosecutors from across the country indicated that they would not prosecute those who seek abortions.
“The civil remedies follow what the criminal law makes unlawful,” he said. “And that’s what we’re doing.”
The National Right to Life Committee’s model legislation, which advocates prohibiting abortion except to prevent the death of the pregnant person, recommends that states permit civil actions against people or entities that violate abortion laws “to prevent future violations.” It also suggests that people who have had or have sought to have an illegal abortion, as well as the expectant father and the parents of a pregnant minor, be allowed to pursue wrongful death actions.
Under the legislation, an action for wrongful death of an “unborn child” would be treated like that of a child who died after being born.
In one regard, Arizona has already implemented a piece of this model legislation as the state’s lawmakers have chipped away at access to abortion and enacted a myriad of regulations on doctors who provide the procedure.
The state’s “informed consent” statute for abortion, first signed into law by then-Gov. Jan Brewer in 2009, mandated an in-person counseling session and a 24-hour waiting period before an abortion. It allows a pregnant person, their husband or a maternal grandparent of a minor to sue if a physician does not properly obtain the pregnant person’s informed consent, and to receive damages for psychological, emotional and physical injuries, statutory damages and attorney fees.
The informed consent laws, which have changed over time, mandate that the patient be told about the “probable anatomical and physiological characteristics” of the embryo or fetus and the “immediate and long-term medical risks” associated with abortion, as well as alternatives to the procedure. Some abortion-rights groups and medical professionals have criticized informed consent processes, arguing the materials can be misleading and personify the embryo or fetus. A 2018 review of numerous studies concluded that having an abortion does not increase a person’s risk of infertility in their next pregnancy, nor is it linked to a higher risk of breast cancer or preterm birth, among other issues.
The wrongful death suit comes at a time of extraordinary confusion over abortion law in Arizona.
Until Roe v. Wade was handed down in 1973, establishing a constitutional right to abortion, a law dating to before statehood had banned the procedure. In March, Gov. Doug Ducey, a Republican who has called Arizona “the most pro-life state in the country,” signed into law a bill outlawing abortions after 15 weeks, and said that law would supersede the pre-statehood ban if Roe were overturned. But now that Roe has been overturned, Arizona Attorney General Mark Brnovich, another Republican, said he intends to enforce the pre-statehood ban, which outlawed abortion except to preserve the life of the person seeking the procedure. On July 14, he filed a motion to lift an injunction on the law, which would make it enforceable.
Adding to the muddle, a U.S. district court judge on July 11 blocked part of a 2021 Arizona law that would classify fertilized eggs, embryos and fetuses as people starting at conception, ruling that the attorney general cannot use the so-called personhood law against abortion providers. Following the Supreme Court decision in Dobbs, eight of the state’s nine abortion providers – all located in three Arizona counties – halted abortion services, but following the emergency injunction some are again offering them.
In the wrongful death claim, Mr. Martineau argued that the woman’s consent was invalidated because the doctors didn’t follow the informed consent statute. Although the woman signed four consent documents, the suit claims that “evidence shows that in her rush to maximize profits,” the clinic’s owner, Dr. Gabrielle Goodrick, “cut corners.” Mr. Martineau alleged that Dr. Goodrick and another doctor didn’t inform the woman of the loss of “maternal-fetal” attachment, about the alternatives to abortion or that if not for the abortion, the embryo would likely have been “delivered to term,” among other violations.
Tom Slutes, Dr. Goodrick’s lawyer, called the lawsuit “ridiculous.”
“They didn’t cut any corners,” he said, adding that the woman “clearly knew what was going to happen and definitely, strongly” wanted the abortion. Regardless of the information the woman received, she wouldn’t have changed her mind, Mr. Slutes said. Mr. Slutes referenced the deposition, where the woman said she “felt completely informed.”
Mr. Martineau said in an interview that Mr. Villegas isn’t motivated by collecting money from the lawsuit.
“He has no desire to harass” his ex-wife, Mr. Martineau said. “All he wants to do is make sure it doesn’t happen to another father.”
In a deposition, Mr. Villegas’ ex-wife said that he was emotionally abusive during their marriage, which lasted nearly 5 years. At first, she said, Mr. Villegas seemed like the “greatest guy I’ve ever met in my life,” taking her to California for a week as a birthday gift. But as the marriage progressed, she said, there were times he wouldn’t allow her to get a job or leave the house unless she was with him.
The woman alleged that Mr. Villegas made fake social media profiles, hacked into her social media accounts and threatened to “blackmail” her if she left him during his failed campaign to be a justice of the peace in Gila County, outside of Phoenix.
Mr. Villegas denied the allegations about his relationship but declined to comment further for this story, Mr. Martineau said.
Carliss Chatman, an associate law professor at Washington and Lee University in Virginia, said certain civil remedies can also be a mechanism for men to continue to abuse their former partners through the court system.
“What happens if the father who is suing on behalf of the fetus is your rapist or your abuser? It’s another way to torture a woman,” Ms. Chatman said.
Ms. Chatman added that these legal actions can be a deterrent for physicians in states where abortion is banned after a certain gestational period, because the threat of civil suits makes it harder for doctors to get insurance.
The lawsuit has added to the stresses on Dr. Goodrick, who has been performing abortions in Arizona since the mid-1990s, and her practice. She said that, since the lawsuit was filed, the annual cost of her medical malpractice insurance has risen from $32,000 to $67,000.
Before providers in Arizona halted abortions following the Supreme Court decision, people would begin lining up outside Dr. Goodrick’s clinic at 6 a.m., sometimes with lawn chairs in hand, like “a concert line,” Dr. Goodrick said.
“Every year there’s something and we never know what it’s going to be,” Dr. Goodrick said recently at her Phoenix clinic. “I’m kind of desensitized to it all.”
Nicole Santa Cruz is a reporter covering issues of inequality in the Southwest.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Fungated Eroded Plaque on the Arm
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
A 39-year-old man from Ohio presented with a tender, 10×6-cm, fungated, eroded plaque on the right medial upper arm that developed over the last 4 years. He initially noticed a firm lump under the right arm 4 years prior that was diagnosed as possible cellulitis at an outside clinic and treated with trimethoprim-sulfamethoxazole. The lesion then began to erode and became a chronic nonhealing wound. Approximately 1 year prior to the current presentation, the patient recalled unloading a truckload of soil around the same time the wound began to enlarge in diameter and depth. He denied any prior or current respiratory or systemic symptoms including fevers, chills, or weight loss.
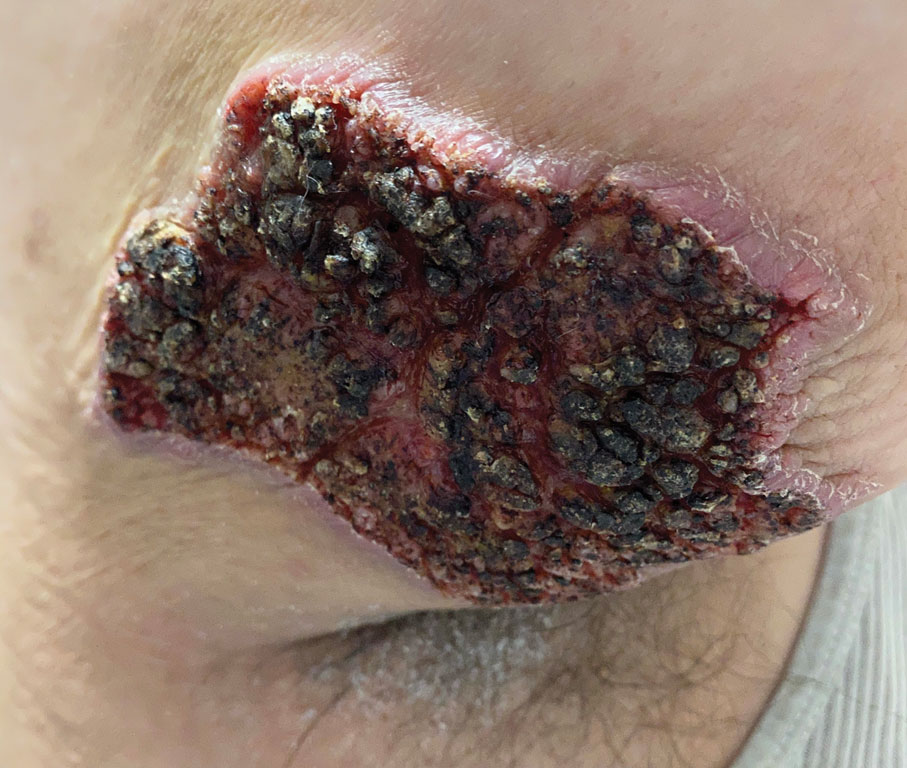
27-year-old man • muscle weakness • fatigue • electrolyte abnormalities • Dx?
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).
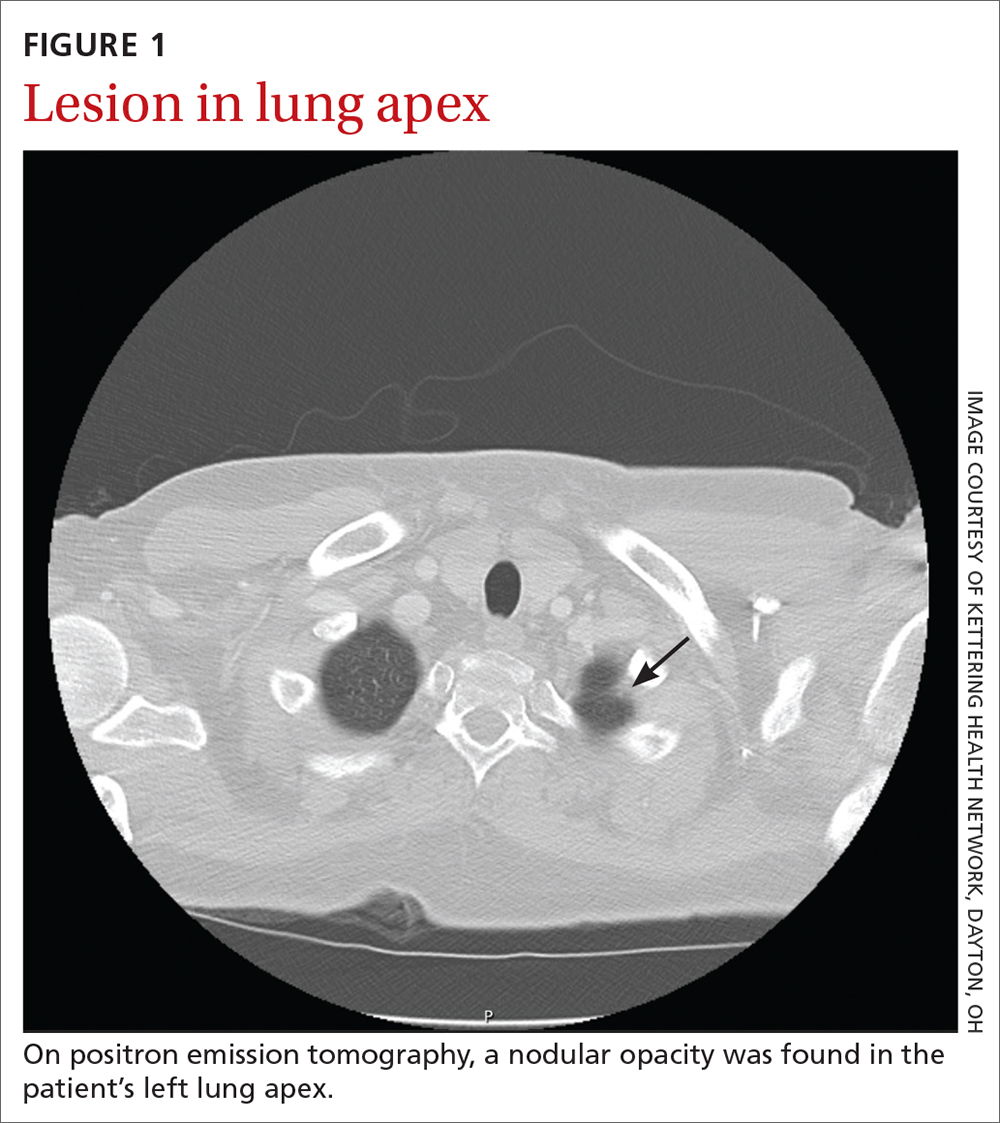
THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2
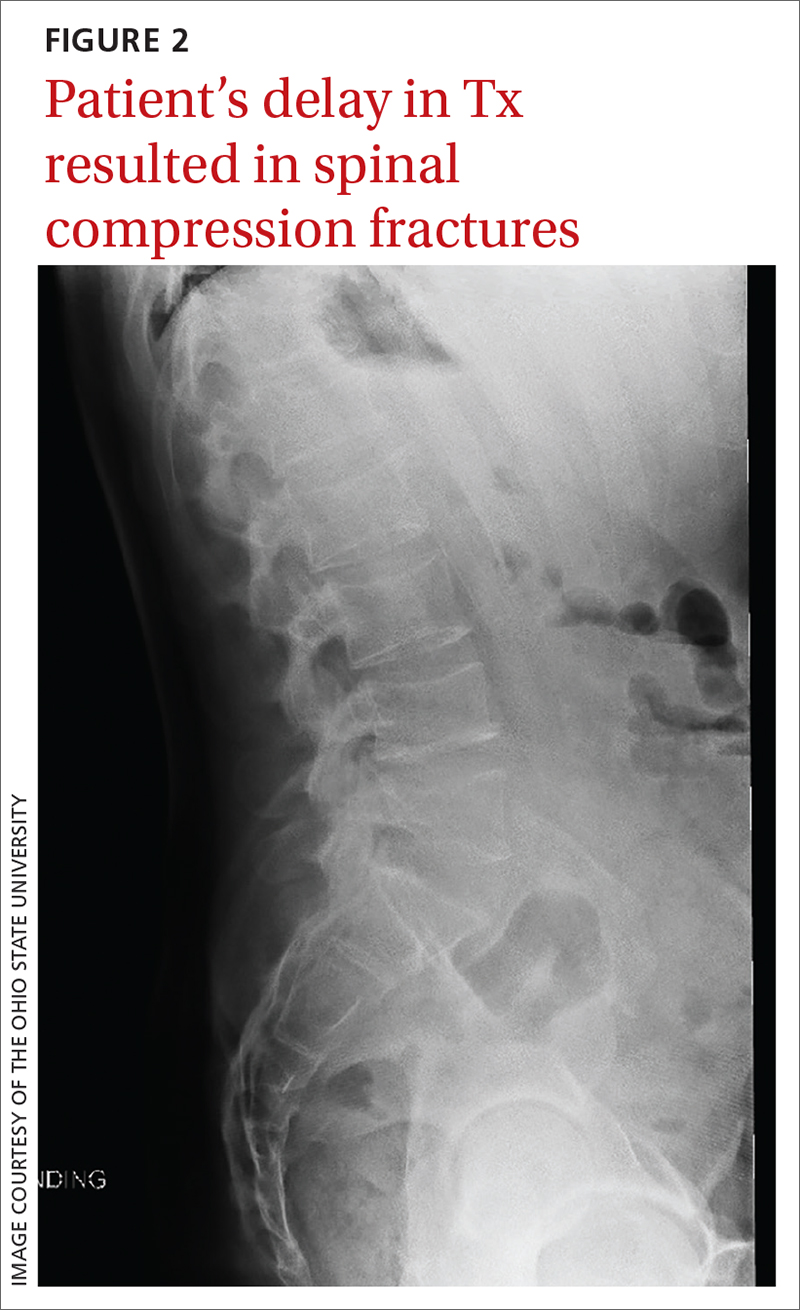
Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).

THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2

Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
THE CASE
A 27-year-old man with no past medical history presented to his primary care physician (PCP) for a routine physical. He reported experiencing muscle weakness and fatigue for the previous 1 to 2 months. Two blood pressure measurements were recorded: 138/80 mm Hg and 142/95 mm Hg. The patient was given a diagnosis of hypertension and started on triamterene/hydrochlorothiazide. Labwork was ordered, including a complete metabolic panel, lipid panel, urinalysis, thyroid-stimulating hormone (TSH) plus thyroxine (T4), HIV antibodies, and a complete blood count.
The samples were drawn 1 week later, and the results were notable for low-normal TSH with a T4 of 0.8 ng/dL (normal range, 0.9-2.3 ng/dL); sodium, 151 mmol/L (normal range, 136-145 mmol/L); potassium, 3.4 mmol/L (normal range, 3.6-5.2 mmol/L); and white blood cell count, 13.8 x 103/mcL. The electrolyte abnormalities were attributed to the triamterene/hydrochlorothiazide, which was stopped. One week later, repeat labs showed a persistent potassium level of 3.0 mmol/L; sodium, 141 mmol/L; and glucose, 310 mg/dL. Follow-up A1C was measured at 7.4%.
At the next appointment (2 weeks after initial evaluation), the patient received a diagnosis of type 2 diabetes in addition to new-onset essential hypertension. He expressed surprise at his diagnoses, as he said he primarily ate a balanced diet with plenty of vegetables and lots of healthy home-cooked meals. His body mass index (BMI) was in normal range, and he said he exercised regularly.
The patient was started on metformin 500 mg/d and referred to Endocrinology. After seeing the endocrinologist, who agreed with metformin for initial management, the patient contacted his PCP with concerns about worsening “muscle wasting.” Based on these ongoing symptoms, the patient was advised to go to the emergency department (ED).
In the ED, the patient reported muscle aches and weakness, weight gain, dyspnea on exertion, and polyuria. He also said that his face had widened with his weight gain, and his weakness was greatest in his thighs compared to his distal lower extremities. Labs drawn in the ED indicated hyperglycemia (glucose, 334 mg/dL) and severe hypokalemia (potassium, 2.2 mmol/L).
THE DIAGNOSIS
The patient was admitted in the afternoon for further evaluation, and a random serum cortisol measurement was ordered. The results showed an elevated cortisol level (55.2 mcg/dL; normal range, 3-20 mcg/dL). This was followed by a profoundly positive low-dose dexamethasone suppression test with a morning cortisol level of 75.9 mcg/dL (normal range, < 1.8 mcg/dL). With these findings, the diagnosis of Cushing syndrome was made and the focus of the evaluation shifted to localization.
An adrenocorticotropic hormone (ACTH) measurement was ordered, as well as magnetic resonance imaging (MRI) of the pituitary gland and of the abdomen to assess the adrenal glands. Both MRIs were negative, prompting a high-dose 8-mg dexamethasone suppression test to be performed. The patient’s morning cortisol level remained elevated (69.9 mcg/dL), confirming the diagnosis of Cushing syndrome.
Continue to: Based on the results...
Based on the results of the dexamethasone suppression test, a pituitary adenoma was unlikely (as they are often suppressed to < 5 mcg/dL with this test). The patient’s morning ACTH results came back as elevated (356.6 pg/mL; normal range, 10-60 pg/mL), suggesting inappropriate ACTH secretion, which most often has an ectopic source. However, a nuclear medicine octreotide scan and multiple computed tomography scans failed to locate such a source.
The patient eventually underwent bilateral petrosal venous sinus sampling to definitively rule out a pituitary source. Lastly, he underwent nuclear medicine positron emission tomography, which identified a nodular opacity in the anterior left lung apex, demonstrating moderate radiotracer activity (FIGURE 1).

THE DISCUSSION
Cushing syndrome is rarely encountered—it is estimated to affect 2% of patients with uncontrolled diabetes1 and 1% of those with uncontrolled hypertension2—and requires a high level of clinical suspicion. This case highlights the importance of considering secondary causes of diabetes in patients who present atypically. This patient presented with symptoms consistent with Cushing syndrome that went unrecognized initially; these included high blood pressure, rounded face, weak muscles, hypokalemia, and intermittent hypernatremia in addition to new-onset hyperglycemia.2-5 Despite the atypical findings, evaluation for diabetes and potential secondary causes was neglected until an ED evaluation 1 month after initial presentation. The work-up for possible Cushing syndrome was completed in the hospital but could easily have been conducted in the outpatient setting.
Making the diagnosis. When Cushing syndrome is suspected, consider consultation with Endocrinology. It is important to exclude exogenous glucocorticoid exposure through a thorough review of the patient’s medications.2 The Endocrine Society2 recommends that one of the following tests be performed:
- 24-hour urine free cortisol (≥ 2 tests)
- Overnight 1-mg dexamethasone suppression test
- Late-night salivary cortisol test.
Results within normal range make Cushing syndrome an unlikely diagnosis; however, for patients with suggestive clinical features, further work-up may be warranted.
Continue to: Any abnormal result...
Any abnormal result is an indication to exclude a physiologic cause of hypercortisolism by repeating at least 1 of the previous studies. As with the initial testing, normal results may rule out Cushing syndrome, while abnormal results would be confirmatory. (Conflicting results require additional evaluation.)
Morbidity and mortality. Finding the etiology of Cushing syndrome can present a challenge but is also rewarding due to the reversible nature of most of the abnormalities. That said, Cushing syndrome can have a significant impact on morbidity and mortality.
Morbidity. The case patient developed compression fractures throughout his thoracic and lumbar spine, with a loss of 4 inches in height, attributed to the delay in curative treatment (FIGURE 2); these were identified about 2 months after his initial presentation to a health care facility. In addition to bone mineral density, cognitive function and quality of life can be impacted by untreated hypercortisolism and Cushing syndrome.2

Mortality. In the earliest studies6,7 (from the 1930s-1950s), the average survival rate was about 4.6 years and the 5-year survival was just 50%—and yet, outcomes data from modern treatment modalities are scant. While there is limited data on outcomes in untreated disease, the Endocrine Society states that treatment of moderate-to-severe cases “clearly reduces mortality and morbidity” while early identification and treatment of mild cases “would reduce the risk of residual morbidity.”2
Our patient underwent video-assisted thoracoscopic surgery, during which a nodule in the anterior lingula was removed. In addition, lymph node dissection was performed. Two lymph nodes were positive for atypical well-differentiated carcinoid tumor. After surgical removal, the patient’s cortisol levels normalized and his diabetes resolved.
THE TAKEAWAY
In primary care, the frequency at which we evaluate and diagnose type 2 diabetes without secondary cause can lead to cognitive biases, such as anchoring bias, that impact patient care. In this case, the atypical secondary nature of the diabetes was missed at 3 outpatient appointments prior to presentation at the hospital ED. In an active patient who has a normal BMI and a healthy diet—but systemic symptoms—it is critical to consider secondary causes of diabetes, such as Cushing syndrome.
CORRESPONDENCE
Anna Murley Squibb, MD, 2145 North Fairfield Road, Suite 100, Beavercreek, OH 45385; [email protected]
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
1. Bulow B, Jansson S, Juhlin C, et al. Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol. 2006;154:419-423. doi: 10.1530/eje.1.02110
2. Nieman LK, Biller BMK, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526-1540. doi: 10.1210/jc.2008-0125
3. Juszczak A, Morris DG, Grossman AB, et al. Chapter 13: Cushing’s syndrome. In: Jameson JL, De Groot LJ. Endocrinology: Adult and Pediatric. 7th ed. Elsevier Saunders; 2016:227-255.e11. https://doi.org/10.1016/B978-0-323-18907-1.00013-5
4. Lacroix A, Feelders RA, Stratakis CA, et al. Cushing’s syndrome. Lancet. 2015;386:913-927. doi: 10.1016/S0140-6736(14)61375-1
5. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593-5602. doi: 10.1210/jc.2003-030871
6. Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp. 1932;50:137-195. doi: 10.1002/j.1550-8528.1994.tb00097.x
7. Plotz CM, Knowlton AI, Ragan C. The natural history of Cushing’s syndrome. Am J Med. 1952;13:597-614. doi: 10.1016/0002-9343(52)90027-2
Colchicine may decrease cardiovascular events in patients with coronary artery disease
ILLUSTRATIVE CASE
A 62-year-old woman with a past medical history of type 2 diabetes, hyperlipidemia, hypertension, and remote myocardial infarction (MI) presents to her primary care office for a preventive visit. She is a nonsmoker and has been taking her daily medications as prescribed, including an angiotensin-converting enzyme inhibitor, high-intensity statin, and aspirin. Her diabetes is well controlled. What else would you consider recommending to decrease this patient’s risk for future CVEs?
Cardiovascular disease (CVD) is a major contributor to morbidity and mortality, affecting more than 50% of patients older than 60.2 Despite control of risk factors with standard treatment modalities, patients with established CVD remain at high risk for future events, which makes elucidating and targeting other causative pathways essential.3
Inflammation has been identified as a key player in the development and progression of atherosclerosis and its downstream effects, with increased inflammatory markers correlating with increased risk for CVEs.4 Due to these findings, anti-inflammatory treatments have been under investigation as agents to further reduce risk for CVEs. In 1 such trial, the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS), patients with MI and elevated C-reactive protein levels treated with the interleukin-1 beta inhibitor canakinumab showed reduced risk for future CVEs compared to those receiving placebo.5 However, due to canakinumab’s high cost, inconvenient subcutaneous administration, and increased incidence of fatal infections, other agents are under investigation.
Colchicine is a potent anti-inflammatory agent, with approval in the United States for treatment of gout and familial Mediterranean fever. It works broadly to reduce inflammation by disrupting tubulin polymerization.6,7 Colchicine decreases interleukin-1 beta production through inactivation of the NLRP3 inflammasome pathway, which has been associated with the inflammatory component driving atherosclerotic plaque progression and instability.5,8 Colchicine’s oral administration, relative cost-effectiveness, and safety profile make it an attractive option for potential use in secondary prevention of CVEs.
The Low-Dose Colchicine (LoDoCo) trial, published in 2013, demonstrated a reduction in CVEs in those with CVD taking guideline-directed medical therapy (GDMT) plus colchicine 0.5 mg/d, compared with those taking GDMT alone.9 However, the LoDoCo study enrolled only 532 patients and was not placebo controlled. The Colchicine Cardiovascular Outcomes Trial (COLCOT), published in 2019, was a randomized, double-blind, placebo-controlled trial that aimed to further evaluate the effects of colchicine on CVEs on a larger scale and to assess its longer-term safety.10 In this study, the colchicine group had a significantly lower risk of CVEs vs placebo, with a comparable safety profile.10
STUDY SUMMARY
Fewer CVEs occurred when colchicine was added to the regimen
The randomized, multicenter, double-blind Low Dose Colchicine 2 (LoDoCo2) trial evaluated whether colchicine 0.5 mg daily reduces CV death, spontaneous (nonprocedural) MI, ischemic stroke, or ischemia-driven coronary revascularization in patients with chronic CAD (composite primary endpoint). This trial included 5522 patients, ages 35 to 82, in Australia and the Netherlands. Patients were eligible to participate if they had evidence of CAD by invasive coronary angiography, coronary calcium score, or computed tomography angiography, as well as evidence of clinical stability for 6 months. Exclusion criteria included moderate-to-severe renal impairment, severe heart failure, severe valvular disease, or intolerance to colchicine.
Patients (N = 6528) took colchicine 0.5 mg daily as part of a 1-month, open-label run-in phase; 1006 patients stopped taking colchicine during this time. Perceived adverse effects were observed in 611 of these patients, the most common being gastrointestinal (GI) upset (437 patients). After the run-in phase, the remaining 5522 patients were randomized to either the colchicine or placebo group. Both groups continued to receive GDMT for CVD, including antiplatelet therapy, anticoagulants, and hypertensive therapy as indicated. Lipid-lowering therapies were continued in 96.7% of the colchicine group and 96.6% of the placebo group. These patients were then followed for a minimum of 1 year (median duration, 28.6 months).
Continue to: The primary endpoint...
The primary endpoint occurred less frequently in the colchicine group than in the placebo group (6.8% vs 9.6%; P < .001; number needed to treat = 36). The incidence rates for 2 of the individual outcomes in the composite, MI (hazard ratio [HR] = 0.7; 95% CI, 0.53-0.93) and ischemia-driven coronary revascularization (HR = 0.75; 95% CI, 0.60-0.94), were significantly lower in the colchicine group. The other outcomes were no different from placebo.1
There was a similar incidence of serious adverse events, such as noncardiovascular death, cancer diagnosis, and hospitalization for infection, pneumonia, or GI issues. High-dose statins were used by 3413 patients (61.8%). Myalgia (data collected only from the Netherlands cohort) was reported more commonly in the colchicine group than the placebo group (21.2% vs 18.5%; cumulative incidence ratio = 1.15; 95% CI, 1.01-1.31). Myotoxic effects were rare in both groups.1
WHAT’S NEW
RCT supports potential for anti-inflammatory therapy in CAD
This large RCT demonstrated that the addition of daily colchicine reduces CVE risk in patients with known CAD while maintaining a good safety profile.1
CAVEATS
Watch for potential drug interactions in patients with renal dysfunction
Prescribers should be aware of potential drug interactions, especially in those with renal or hepatic dysfunction, when prescribing colchicine, as it is metabolized through cytochrome P450 3A4 (CYP3A4) and excreted via the P-glycoprotein transport system, by which many statins are also metabolized and act as a competitive substrate.7 In addition, simvastatin, and to a lesser degree atorvastatin, are CYP3A4 inhibitors.
Also of note, the 0.5-mg colchicine tablet is not available in some countries—including the United States, where only 0.6-mg tablets are available. The 0.6-mg dose would likely have the same benefit and similar adverse effect profile but was not included in the study.
CHALLENGES TO IMPLEMENTATION
GI tolerability may be an issue
Colchicine is widely available and relatively low in cost, at approximately $32 per month for the 0.6-mg daily tablets. A major limitation is lack of tolerability, as adverse effects such as nausea, vomiting, diarrhea, and abdominal pain are frequently reported.
1. Nidorf SM, Fiolet ATL, Mosterd A, et al; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838-1847. doi: 10.1056/NEJMoa2021372
2. Laslett LJ, Alagona P Jr, Clark BA III, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl):S1-S49. doi: 10.1016/j.jacc.2012.11.002
3. Bhatt DL, Eagle KA, Ohman EM, et al; REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350-1357. doi: 10.1001/jama.2010.13224. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-1695. doi: 10.1056/NEJMra043430
5. Ridker PM, Everett BM, Thuren T, et al; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119-1131. doi: 10.1056/NEJMoa1707914
6. Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112:2012-2016. doi: 10.1161/CIRCULATIONAHA.105.542738
7. Angelidis C, Kotsialou Z, Kossyvakis C, et al. Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des. 2018;24:659-663. doi: 10.2174/1381612824666180123110042
8. Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262-271. doi: 10.1016/j.atherosclerosis.2017.12.027
9. Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404-410. doi: 10.1016/j.jacc.2012.10.027
10. Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497-2505. doi: 10.1056/NEJMoa1912388
ILLUSTRATIVE CASE
A 62-year-old woman with a past medical history of type 2 diabetes, hyperlipidemia, hypertension, and remote myocardial infarction (MI) presents to her primary care office for a preventive visit. She is a nonsmoker and has been taking her daily medications as prescribed, including an angiotensin-converting enzyme inhibitor, high-intensity statin, and aspirin. Her diabetes is well controlled. What else would you consider recommending to decrease this patient’s risk for future CVEs?
Cardiovascular disease (CVD) is a major contributor to morbidity and mortality, affecting more than 50% of patients older than 60.2 Despite control of risk factors with standard treatment modalities, patients with established CVD remain at high risk for future events, which makes elucidating and targeting other causative pathways essential.3
Inflammation has been identified as a key player in the development and progression of atherosclerosis and its downstream effects, with increased inflammatory markers correlating with increased risk for CVEs.4 Due to these findings, anti-inflammatory treatments have been under investigation as agents to further reduce risk for CVEs. In 1 such trial, the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS), patients with MI and elevated C-reactive protein levels treated with the interleukin-1 beta inhibitor canakinumab showed reduced risk for future CVEs compared to those receiving placebo.5 However, due to canakinumab’s high cost, inconvenient subcutaneous administration, and increased incidence of fatal infections, other agents are under investigation.
Colchicine is a potent anti-inflammatory agent, with approval in the United States for treatment of gout and familial Mediterranean fever. It works broadly to reduce inflammation by disrupting tubulin polymerization.6,7 Colchicine decreases interleukin-1 beta production through inactivation of the NLRP3 inflammasome pathway, which has been associated with the inflammatory component driving atherosclerotic plaque progression and instability.5,8 Colchicine’s oral administration, relative cost-effectiveness, and safety profile make it an attractive option for potential use in secondary prevention of CVEs.
The Low-Dose Colchicine (LoDoCo) trial, published in 2013, demonstrated a reduction in CVEs in those with CVD taking guideline-directed medical therapy (GDMT) plus colchicine 0.5 mg/d, compared with those taking GDMT alone.9 However, the LoDoCo study enrolled only 532 patients and was not placebo controlled. The Colchicine Cardiovascular Outcomes Trial (COLCOT), published in 2019, was a randomized, double-blind, placebo-controlled trial that aimed to further evaluate the effects of colchicine on CVEs on a larger scale and to assess its longer-term safety.10 In this study, the colchicine group had a significantly lower risk of CVEs vs placebo, with a comparable safety profile.10
STUDY SUMMARY
Fewer CVEs occurred when colchicine was added to the regimen
The randomized, multicenter, double-blind Low Dose Colchicine 2 (LoDoCo2) trial evaluated whether colchicine 0.5 mg daily reduces CV death, spontaneous (nonprocedural) MI, ischemic stroke, or ischemia-driven coronary revascularization in patients with chronic CAD (composite primary endpoint). This trial included 5522 patients, ages 35 to 82, in Australia and the Netherlands. Patients were eligible to participate if they had evidence of CAD by invasive coronary angiography, coronary calcium score, or computed tomography angiography, as well as evidence of clinical stability for 6 months. Exclusion criteria included moderate-to-severe renal impairment, severe heart failure, severe valvular disease, or intolerance to colchicine.
Patients (N = 6528) took colchicine 0.5 mg daily as part of a 1-month, open-label run-in phase; 1006 patients stopped taking colchicine during this time. Perceived adverse effects were observed in 611 of these patients, the most common being gastrointestinal (GI) upset (437 patients). After the run-in phase, the remaining 5522 patients were randomized to either the colchicine or placebo group. Both groups continued to receive GDMT for CVD, including antiplatelet therapy, anticoagulants, and hypertensive therapy as indicated. Lipid-lowering therapies were continued in 96.7% of the colchicine group and 96.6% of the placebo group. These patients were then followed for a minimum of 1 year (median duration, 28.6 months).
Continue to: The primary endpoint...
The primary endpoint occurred less frequently in the colchicine group than in the placebo group (6.8% vs 9.6%; P < .001; number needed to treat = 36). The incidence rates for 2 of the individual outcomes in the composite, MI (hazard ratio [HR] = 0.7; 95% CI, 0.53-0.93) and ischemia-driven coronary revascularization (HR = 0.75; 95% CI, 0.60-0.94), were significantly lower in the colchicine group. The other outcomes were no different from placebo.1
There was a similar incidence of serious adverse events, such as noncardiovascular death, cancer diagnosis, and hospitalization for infection, pneumonia, or GI issues. High-dose statins were used by 3413 patients (61.8%). Myalgia (data collected only from the Netherlands cohort) was reported more commonly in the colchicine group than the placebo group (21.2% vs 18.5%; cumulative incidence ratio = 1.15; 95% CI, 1.01-1.31). Myotoxic effects were rare in both groups.1
WHAT’S NEW
RCT supports potential for anti-inflammatory therapy in CAD
This large RCT demonstrated that the addition of daily colchicine reduces CVE risk in patients with known CAD while maintaining a good safety profile.1
CAVEATS
Watch for potential drug interactions in patients with renal dysfunction
Prescribers should be aware of potential drug interactions, especially in those with renal or hepatic dysfunction, when prescribing colchicine, as it is metabolized through cytochrome P450 3A4 (CYP3A4) and excreted via the P-glycoprotein transport system, by which many statins are also metabolized and act as a competitive substrate.7 In addition, simvastatin, and to a lesser degree atorvastatin, are CYP3A4 inhibitors.
Also of note, the 0.5-mg colchicine tablet is not available in some countries—including the United States, where only 0.6-mg tablets are available. The 0.6-mg dose would likely have the same benefit and similar adverse effect profile but was not included in the study.
CHALLENGES TO IMPLEMENTATION
GI tolerability may be an issue
Colchicine is widely available and relatively low in cost, at approximately $32 per month for the 0.6-mg daily tablets. A major limitation is lack of tolerability, as adverse effects such as nausea, vomiting, diarrhea, and abdominal pain are frequently reported.
ILLUSTRATIVE CASE
A 62-year-old woman with a past medical history of type 2 diabetes, hyperlipidemia, hypertension, and remote myocardial infarction (MI) presents to her primary care office for a preventive visit. She is a nonsmoker and has been taking her daily medications as prescribed, including an angiotensin-converting enzyme inhibitor, high-intensity statin, and aspirin. Her diabetes is well controlled. What else would you consider recommending to decrease this patient’s risk for future CVEs?
Cardiovascular disease (CVD) is a major contributor to morbidity and mortality, affecting more than 50% of patients older than 60.2 Despite control of risk factors with standard treatment modalities, patients with established CVD remain at high risk for future events, which makes elucidating and targeting other causative pathways essential.3
Inflammation has been identified as a key player in the development and progression of atherosclerosis and its downstream effects, with increased inflammatory markers correlating with increased risk for CVEs.4 Due to these findings, anti-inflammatory treatments have been under investigation as agents to further reduce risk for CVEs. In 1 such trial, the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS), patients with MI and elevated C-reactive protein levels treated with the interleukin-1 beta inhibitor canakinumab showed reduced risk for future CVEs compared to those receiving placebo.5 However, due to canakinumab’s high cost, inconvenient subcutaneous administration, and increased incidence of fatal infections, other agents are under investigation.
Colchicine is a potent anti-inflammatory agent, with approval in the United States for treatment of gout and familial Mediterranean fever. It works broadly to reduce inflammation by disrupting tubulin polymerization.6,7 Colchicine decreases interleukin-1 beta production through inactivation of the NLRP3 inflammasome pathway, which has been associated with the inflammatory component driving atherosclerotic plaque progression and instability.5,8 Colchicine’s oral administration, relative cost-effectiveness, and safety profile make it an attractive option for potential use in secondary prevention of CVEs.
The Low-Dose Colchicine (LoDoCo) trial, published in 2013, demonstrated a reduction in CVEs in those with CVD taking guideline-directed medical therapy (GDMT) plus colchicine 0.5 mg/d, compared with those taking GDMT alone.9 However, the LoDoCo study enrolled only 532 patients and was not placebo controlled. The Colchicine Cardiovascular Outcomes Trial (COLCOT), published in 2019, was a randomized, double-blind, placebo-controlled trial that aimed to further evaluate the effects of colchicine on CVEs on a larger scale and to assess its longer-term safety.10 In this study, the colchicine group had a significantly lower risk of CVEs vs placebo, with a comparable safety profile.10
STUDY SUMMARY
Fewer CVEs occurred when colchicine was added to the regimen
The randomized, multicenter, double-blind Low Dose Colchicine 2 (LoDoCo2) trial evaluated whether colchicine 0.5 mg daily reduces CV death, spontaneous (nonprocedural) MI, ischemic stroke, or ischemia-driven coronary revascularization in patients with chronic CAD (composite primary endpoint). This trial included 5522 patients, ages 35 to 82, in Australia and the Netherlands. Patients were eligible to participate if they had evidence of CAD by invasive coronary angiography, coronary calcium score, or computed tomography angiography, as well as evidence of clinical stability for 6 months. Exclusion criteria included moderate-to-severe renal impairment, severe heart failure, severe valvular disease, or intolerance to colchicine.
Patients (N = 6528) took colchicine 0.5 mg daily as part of a 1-month, open-label run-in phase; 1006 patients stopped taking colchicine during this time. Perceived adverse effects were observed in 611 of these patients, the most common being gastrointestinal (GI) upset (437 patients). After the run-in phase, the remaining 5522 patients were randomized to either the colchicine or placebo group. Both groups continued to receive GDMT for CVD, including antiplatelet therapy, anticoagulants, and hypertensive therapy as indicated. Lipid-lowering therapies were continued in 96.7% of the colchicine group and 96.6% of the placebo group. These patients were then followed for a minimum of 1 year (median duration, 28.6 months).
Continue to: The primary endpoint...
The primary endpoint occurred less frequently in the colchicine group than in the placebo group (6.8% vs 9.6%; P < .001; number needed to treat = 36). The incidence rates for 2 of the individual outcomes in the composite, MI (hazard ratio [HR] = 0.7; 95% CI, 0.53-0.93) and ischemia-driven coronary revascularization (HR = 0.75; 95% CI, 0.60-0.94), were significantly lower in the colchicine group. The other outcomes were no different from placebo.1
There was a similar incidence of serious adverse events, such as noncardiovascular death, cancer diagnosis, and hospitalization for infection, pneumonia, or GI issues. High-dose statins were used by 3413 patients (61.8%). Myalgia (data collected only from the Netherlands cohort) was reported more commonly in the colchicine group than the placebo group (21.2% vs 18.5%; cumulative incidence ratio = 1.15; 95% CI, 1.01-1.31). Myotoxic effects were rare in both groups.1
WHAT’S NEW
RCT supports potential for anti-inflammatory therapy in CAD
This large RCT demonstrated that the addition of daily colchicine reduces CVE risk in patients with known CAD while maintaining a good safety profile.1
CAVEATS
Watch for potential drug interactions in patients with renal dysfunction
Prescribers should be aware of potential drug interactions, especially in those with renal or hepatic dysfunction, when prescribing colchicine, as it is metabolized through cytochrome P450 3A4 (CYP3A4) and excreted via the P-glycoprotein transport system, by which many statins are also metabolized and act as a competitive substrate.7 In addition, simvastatin, and to a lesser degree atorvastatin, are CYP3A4 inhibitors.
Also of note, the 0.5-mg colchicine tablet is not available in some countries—including the United States, where only 0.6-mg tablets are available. The 0.6-mg dose would likely have the same benefit and similar adverse effect profile but was not included in the study.
CHALLENGES TO IMPLEMENTATION
GI tolerability may be an issue
Colchicine is widely available and relatively low in cost, at approximately $32 per month for the 0.6-mg daily tablets. A major limitation is lack of tolerability, as adverse effects such as nausea, vomiting, diarrhea, and abdominal pain are frequently reported.
1. Nidorf SM, Fiolet ATL, Mosterd A, et al; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838-1847. doi: 10.1056/NEJMoa2021372
2. Laslett LJ, Alagona P Jr, Clark BA III, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl):S1-S49. doi: 10.1016/j.jacc.2012.11.002
3. Bhatt DL, Eagle KA, Ohman EM, et al; REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350-1357. doi: 10.1001/jama.2010.13224. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-1695. doi: 10.1056/NEJMra043430
5. Ridker PM, Everett BM, Thuren T, et al; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119-1131. doi: 10.1056/NEJMoa1707914
6. Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112:2012-2016. doi: 10.1161/CIRCULATIONAHA.105.542738
7. Angelidis C, Kotsialou Z, Kossyvakis C, et al. Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des. 2018;24:659-663. doi: 10.2174/1381612824666180123110042
8. Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262-271. doi: 10.1016/j.atherosclerosis.2017.12.027
9. Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404-410. doi: 10.1016/j.jacc.2012.10.027
10. Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497-2505. doi: 10.1056/NEJMoa1912388
1. Nidorf SM, Fiolet ATL, Mosterd A, et al; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838-1847. doi: 10.1056/NEJMoa2021372
2. Laslett LJ, Alagona P Jr, Clark BA III, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(suppl):S1-S49. doi: 10.1016/j.jacc.2012.11.002
3. Bhatt DL, Eagle KA, Ohman EM, et al; REACH Registry Investigators. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350-1357. doi: 10.1001/jama.2010.13224. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685-1695. doi: 10.1056/NEJMra043430
5. Ridker PM, Everett BM, Thuren T, et al; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119-1131. doi: 10.1056/NEJMoa1707914
6. Imazio M, Bobbio M, Cecchi E, et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112:2012-2016. doi: 10.1161/CIRCULATIONAHA.105.542738
7. Angelidis C, Kotsialou Z, Kossyvakis C, et al. Colchicine pharmacokinetics and mechanism of action. Curr Pharm Des. 2018;24:659-663. doi: 10.2174/1381612824666180123110042
8. Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262-271. doi: 10.1016/j.atherosclerosis.2017.12.027
9. Nidorf SM, Eikelboom JW, Budgeon CA, et al. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404-410. doi: 10.1016/j.jacc.2012.10.027
10. Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497-2505. doi: 10.1056/NEJMoa1912388
PRACTICE CHANGER
Consider prescribing colchicine 0.5 mg daily as an addition to current standard-of-care therapies for patients with coronary artery disease (CAD) to prevent further cardiovascular events (CVEs).
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT).1
Nidorf SM, Fiolet ATL, Mosterd A, et al; LoDoCo2 Trial Investigators. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838-1847.
How to overcome hesitancy for COVID-19 and other vaccines
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.
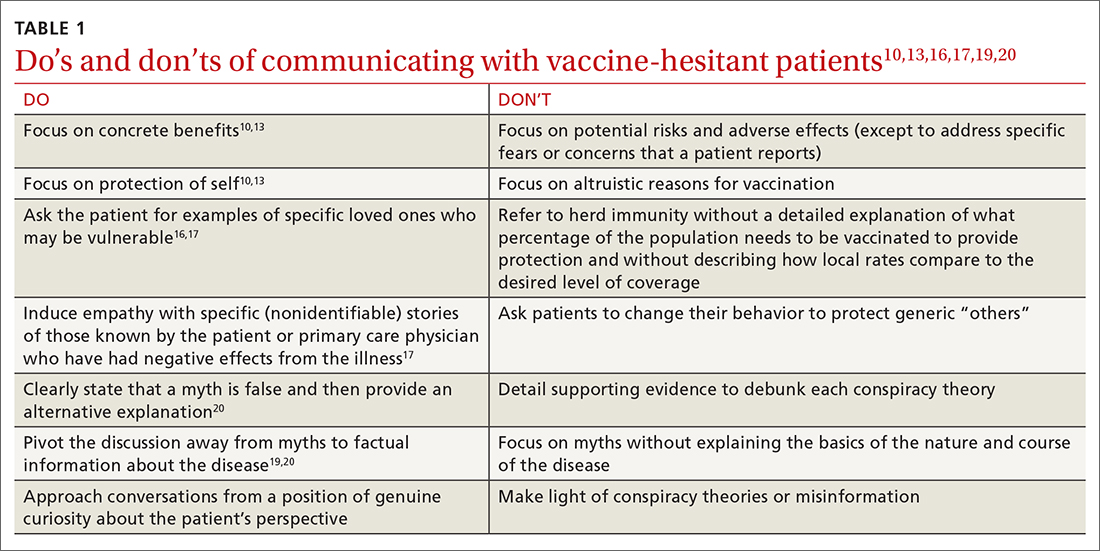
Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.

Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
The World Health Organization (WHO) named vaccine hesitancy as one of the top 10 threats to public health as of 2019.1 Although the COVID-19 vaccines manufactured by Pfizer-BioNTech and Moderna, first authorized for use in November 2020 and fully approved in August 2021,2 are widely available in most countries, vaccination uptake is insufficient.3
As of June 2022, 78% of the US population had received at least 1 vaccine dose and 66.8% were fully vaccinated against COVID-19.4 High confidence in vaccines is associated with greater uptake; thus, engendering confidence in patients is a critical area of intervention for increasing uptake of COVID-19 and other vaccines.5 Despite the steady increase in vaccine acceptance observed following the release of the COVID-19 vaccine, acceptance remains suboptimal.2,6
Demographic characteristics associated with lower vaccine acceptance include younger age, female sex, lower education and/or income, and Black race or Hispanic/Latinx ethnicity (compared to white or Asian non-Hispanic).6,7 Moreover, patients who are skeptical of vaccine safety and efficacy are associated with lower intentions to vaccinate. In contrast, patients with a history of receiving influenza vaccinations and those with a greater concern about COVID-19 and their risk of infection have increased vaccine intentions.6
Numerous strategies exist to increase vaccine acceptance; however, there does not appear to be a single “best” method to overcome individual or parental vaccine hesitancy for COVID-19 or other vaccines.8,9 There are no large-scale randomized controlled trials (RCTs) demonstrating one strategy as more effective than another. In this review, we outline a variety of evidenced-based strategies to help patients overcome vaccine hesitancy for COVID-19 and other vaccines, with a focus on practical tips for primary care physicians (PCPs).
Which talking points are likely to resonate with your patients?
Intervention strategies promote vaccine acceptance by communicating personal benefit, collective benefit, or both to vaccine-hesitant patients. In a study sample of US undergraduate students, Kim and colleagues10 found that providing information about the benefits and risks of influenza vaccines resulted in significantly less vaccine intent compared to communicating information only on the benefits. Similarly, Shim and colleagues11 investigated how game theory (acting to maximize personal payoff regardless of payoff to others) and altruism affect influenza vaccination decisions. Through a survey-based study of 427 US university employees, researchers found altruistic motivation had a significant impact on the decision to vaccinate against influenza, resulting in a shift from self-interest to that of the good of the community.11
A German trial on COVID-19 vaccine acceptance by Sprengholz and colleagues12 found that communications about the benefits of vaccination, availability of financial compensation for vaccination, or a combination of both, did not increase a person’s willingness to get vaccinated. This trial, however, did not separate out individual vs collective benefit, and it was conducted prior to widespread COVID-19 vaccine availability.
In an online RCT conducted in early 2021, Freeman and colleagues13 randomized UK adults to 1 of 10 different “information conditions.” Participants read from 1 of 10 vaccine scripts that varied by the talking points they addressed. The topics that researchers drew from for these scripts included the personal or collective benefit from the COVID-19 vaccine, safety and effectiveness of the vaccine, and the seriousness of the pandemic. They found communications emphasizing personal benefit from vaccination and safety concerns were more effective in participants identified as being strongly hesitant (defined as those who said they would avoid getting the COVID-19 vaccine for as long as possible or who said they’d never get it). However, none of the information arms in this study decreased vaccine hesitancy among those who were doubtful of vaccination (defined as those who said they would delay vaccination or who didn’t know if they would get vaccinated).13
Continue to: When encountering patients who are strongly...
When encountering patients who are strongly hesitant to vaccination, an approach emphasizing concrete personal benefit may prove more effective than one stressing protection of others from illness. It is important to note, though, that findings from other countries may not be relevant to US patients due to differences in demographic factors, individual beliefs, and political climate.
It helps to explain herd immunity by providing concrete examples
Among the collective benefits of vaccination is the decreased risk of transmitting the disease to others (eg, family, friends, neighbors, colleagues), a quicker “return to normalcy,” and herd immunity.13 While individual health benefits may more strongly motivate people to get vaccinated than collective benefits, this may be due to a lack of understanding about herd immunity among the general public. The optimal method of communicating information on herd immunity is not known.14
Betsch and colleagues15 found that explaining herd immunity using interactive simulations increased vaccine intent, especially in countries that prioritize the self (rather than prioritizing the group over the individual). In addition to educating study participants about herd immunity, telling them how local vaccine coverage compared to the desired level of coverage helped to increase (influenza) vaccine intent among those who were least informed about herd immunity.16
Providing concrete examples of the collective benefits of vaccination (eg, protecting grandparents, children too young to be vaccinated, and those at increased risk for severe illness) or sharing stories about how other patients suffered from the disease in question may increase the likelihood of vaccination. One recent trial by Pfattheicher and colleagues17 found that empathy for those most vulnerable to COVID-19 and increased knowledge about herd immunity were 2 factors associated with greater vaccine intentions.
In this study, the authors induced empathy and increased COVID-19 vaccination intention by having participants read a short story about 2 close siblings who worked together in a nursing facility. In the story, participants learned that both siblings were given a diagnosis of COVID-19 at the same time but only 1 survived.17
Continue to: Try this 3-pronged approach
Try this 3-pronged approach. Consider explaining herd immunity to vaccine-hesitant patients, pairing this concept with information about local vaccine uptake, and appealing to the patient’s sense of empathy. You might share de-identified information on other patients in your practice or personal network who experienced severe illness, had long-term effects, or died from COVID-19 infection. Such concrete examples may help to increase motivation to vaccinate more than a general appeal to altruism.
Initiate the discussion by emphasizing that community immunity protects those who are vulnerable and lack immunity while providing specific empathetic examples (eg, newborns, cancer survivors) and asking patients to consider friends and family who might be at risk. Additionally, it is essential to explain that although community immunity can decrease the spread of infection, it can only be achieved when enough people are vaccinated.
Proceed with caution: Addressing conspiracy theories can backfire
Accurate information is critical to improving vaccine intentions; belief in conspiracy theories or misinformation related to COVID-19 is associated with reduced vaccine intentions and uptake.6 For example, a study by Loomba and colleagues18 showed that after exposure to misinformation, US and UK adults reported reduced intentions to vaccinate against COVID-19 once a vaccine became available.
Unfortunately, addressing myths about vaccines can sometimes backfire and unintentionally reinforce vaccine misperceptions.19,20 This is especially true for patients with the highest levels of concern or mistrust in vaccines. Nyhan and colleagues21,22 observed the backfire effect in 2 US studies looking at influenza and measles, mumps, and rubella vaccine misperceptions. Although corrective information significantly reduced belief in vaccine myths, they found individuals with the most concerns more strongly endorsed misperceptions when their beliefs were challenged.21,22
An Australian randomized study by Steffens and colleagues23 found repeating myths about childhood vaccines, followed by corrective text, to parents of children ages 0 to 5 years had no difference on parental intent to vaccinate their children compared to providing vaccine information as a statement or in a question/answer format. Furthermore, an RCT in Brazil by Carey and colleagues24 found that myth-correction messages about Zika virus failed to reduce misperceptions about the virus and actually reduced the belief in factual information about Zika—regardless of baseline beliefs in conspiracies. However, a similar experiment in the same study showed that myth-correction messages reduced false beliefs about yellow fever.
Continue to: The authors speculated...
The authors speculated that this may be because Zika is a relatively new virus when compared to yellow fever, and participants may have more pre-existing knowledge about yellow fever.24 These findings are important to keep in mind when addressing misinformation regarding COVID-19. When addressing myth perceptions with patients, consider pivoting the conversation from vaccine myths to the disease itself, focusing on the disease risk and severity of symptoms.19,20
Other studies have had positive results when addressing misinformation, including a digital RCT of older adults in the Netherlands by Yousuf and colleagues.25 In this study, participants were randomized to view 1 of 2 versions of an information video on vaccination featuring an informative discussion by celebrity scientists, government officials, and a cardiologist. Video 1 did not include debunking strategies, only information about vaccination; Video 2 provided the same information about vaccines but also described the myths surrounding vaccines and reiterated the truth to debunk the myths.
Findings demonstrated that a significantly higher number of participants in the Video 2 group overcame vaccination myths related to influenza and COVID-19.25 Notably, this study took place prior to the widespread availability of COVID-19 vaccines and did not measure intent to vaccinate against COVID-19.
Taken together, strategies for correcting vaccine misinformation may vary by population as well as type of vaccine; however, placing emphasis on facts delivered by trusted sources appears to be beneficial. When addressing misinformation, PCPs should first focus on key details (not all supporting information) and clearly explain why the misinformation is false before pointing out the actual myth and providing an alternative explanation.20 When caring for patients who express strong concerns over the vaccine in question or have avid beliefs in certain myths or conspiracy theories, it’s best to pivot the conversation back to the disease rather than address the misinformation to avoid a potential backfire effect.

Utilize these effective communication techniques
TABLE 110,13,16,17,19,20 summarizes the “do’s and don’ts” of communicating with vaccine-hesitant patients. PCPs should provide strong recommendations for vaccination, approaching it presumptively—ie, framing it as normative behavior.19,26 This approach is critical to building patient trust so that vaccine-hesitant patients feel the PCP is truly listening to them and addressing their concerns.27 Additionally, implementing motivational interviewing (MI) and self-determination theory (SDT)28 techniques when discussing vaccinations with patients can improve intentions and uptake.19,29TABLE 219,29 outlines specific techniques based on SDT and MI that PCPs may utilize to communicate with vaccine-hesitant individuals or parents.

Continue to: The takeaway
The takeaway
Strategies for increasing vaccine intentions include educating hesitant patients about the benefits and risks of vaccines, addressing misinformation, and explaining the personal and collective benefits of vaccination. These strategies appear to be more effective when delivered by a trusted source, such as a health care provider (HCP). Care should be taken when implementing vaccine-acceptance strategies to ensure that they are tailored to specific populations and vaccines.
At this stage in the COVID-19 pandemic, when several vaccines have been widely available for more than a year, we expect that the majority of patients desiring vaccination (ie, those with the greatest vaccine intent) have already received them. With the recent approval of COVID-19 vaccines for children younger than 5 years, we must now advocate for our patients to vaccinate not only themselves, but their children. Patients who remain unvaccinated may be hesitant or outright reject vaccination for a number of reasons, including fear or skepticism over the safety and efficacy of the vaccine, belief in conspiracy theories, belief that COVID-19 is not real or not severe, or mistrust of the government.6 Vaccine hesitation or rejection is also often political in nature.
Based on the studies included in this review, we have identified several strategies for reducing vaccine hesitancy, which can be used with vaccine-hesitant patients and parents. We suggest emphasizing the personal benefit of vaccination and focusing on specific disease risks. If time allows, you can also explain the collective benefit of vaccination through herd immunity, including the current levels of local vaccine uptake compared to the desired level for community immunity. Communicating the collective benefits of vaccination may be more effective when paired with a strategy intended to increase empathy and altruism, such as sharing actual stories about those who have suffered from a vaccine-preventable disease.
Addressing myths and misinformation related to COVID-19 and other vaccines, with emphasis placed on the correct information delivered by trusted sources may be beneficial for those who are uncertain but not strongly against vaccination. For those who remain staunchly hesitant against vaccination, we recommend focusing on the personal benefits of vaccination with a focus on delivering facts about the risk of the disease in question, rather than trying to refute misinformation.
COVID-19 vaccine acceptance in the United States is disturbingly low among health care workers, particularly nurses, technicians, and those in nonclinical roles, compared to physicians.6,30 Many of the strategies for addressing vaccine hesitancy among the general population can also apply to health care personnel (eg, vaccine education, addressing misinformation, delivering information from a trusted source). Health care personnel may also be subject to vaccine mandates by their employers, which have demonstrated increases in vaccination rates for influenza.31 Given that COVID-19 vaccination recommendations made by HCPs are associated with greater vaccine intentions and uptake,6 reducing hesitancy among health care workers is a critical first step to achieving optimal implementation.
CORRESPONDENCE
Nicole Mayo, PhD, 236 Pearl Street, Rochester, NY 14607; [email protected]
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
1. Ten threats to global health in 2019. World Health Organization. Accessed June 17, 2022. www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
2. FDA approves first COVID-19 vaccine. US Food and Drug Administration. August 23, 2021. Accessed June 17, 2022. www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
3. Mathieu E, Ritchie H, Ortiz-Ospina E, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947-953. doi: 10.1038/s41562-021-01122-8.
4. Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our world in data. Accessed June 17, 2022. https://ourworldindata.org/covid-vaccinations?country=USA
5. de Figueiredo A, Simas C, Karafillakis E, et al. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet. 2020;396:898-908. doi: 10.1016/S0140-6736(20)31558-0
6. Wang Y, Liu Y. Multilevel determinants of COVID-19 vaccination hesitancy in the United States: a rapid systematic review. Prev Med Rep. 2021;25:101673. doi: 10.1016/j.pmedr.2021.101673
7. Robinson E, Jones A, Lesser I, et al. International estimates of intended uptake and refusal of COVID-19 vaccines: a rapid systematic review and meta-analysis of large nationally representative samples. Vaccine. 2021;39:2024-2034. doi: 10.1016/j.vaccine.2021.02.005
8. Dubé E, Gagnon D, MacDonald NE; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33:4191-4203. doi: 10.1016/j.vaccine.2015.04.041
9. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-4304. doi: 10.1016/j.vaccine.2013.07.013
10. Kim S, Pjesivac I, Jin Y. Effects of message framing on influenza vaccination: understanding the role of risk disclosure, perceived vaccine efficacy, and felt ambivalence. Health Commun. 2019;34:21-30. doi: 10.1080/10410236.2017.1384353
11. Shim E, Chapman GB, Townsend JP, et al. The influence of altruism on influenza vaccination decisions. J R Soc Interface. 2012;9:2234-2243. doi: 10.1098/rsif.2012.0115
12. Sprengholz P, Eitze S, Felgendreff L, et al. Money is not everything: experimental evidence that payments do not increase willingness to be vaccinated against COVID-19. J Med Ethics. 2021;47:547-548. doi: 10.1136/medethics-2020-107122
13. Freeman D, Loe BS, Yu LM, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416-e427. doi: 10.1016/S2468-2667(21)00096-7
14. Hakim H, Provencher T, Chambers CT, et al. Interventions to help people understand community immunity: a systematic review. Vaccine. 2019;37:235-247. doi: 10.1016/j.vaccine.2018.11.016
15. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1:1-6. doi: 10.1038/s41562-017-0056
16. Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36:4118-4125. doi: 10.1016/j.vaccine.2018.05.037
17. Pfattheicher S, Petersen MB, Böhm R. Information about herd immunity through vaccination and empathy promote COVID-19 vaccination intentions. Health Psychol. 2022;41:85-93. doi: 10.1037/hea0001096
18. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5:337-348. doi: 10.1038/s41562-021-01056-1
19. Limaye RJ, Opel DJ, Dempsey A, et al. Communicating with vaccine-hesitant parents: a narrative review. Acad Pediatr. 2021;21:S24-S29. doi: 10.1016/j.acap.2021.01.018
20. Omer SB, Amin AB, Limaye RJ. Communicating about vaccines in a fact-resistant world. JAMA Pediatr. 2017;171:929-930. doi: 10.1001/jamapediatrics.2017.2219
21. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464. doi: 10.1016/j.vaccine.2014.11.017
22. Nyhan B, Reifler J, Richey S, et al. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133:e835-e842. doi: 10.1542/peds.2013-2365
23. Steffens MS, Dunn AG, Marques MD, et al. Addressing myths and vaccine hesitancy: a randomized trial. Pediatrics. 2021;148:e2020049304. doi: 10.1542/peds.2020-049304
24. Carey JM, Chi V, Flynn DJ, et al. The effects of corrective information about disease epidemics and outbreaks: evidence from Zika and yellow fever in Brazil. Sci Adv. 2020;6:eaaw7449. doi: 10.1126/sciadv.aaw7449
25. Yousuf H, van der Linden S, Bredius L, et al. A media intervention applying debunking versus non-debunking content to combat vaccine misinformation in elderly in the Netherlands: a digital randomised trial. EClinicalMedicine. 2021;35:100881. doi: 10.1016/j.eclinm.2021.100881
26. Cambon L, Schwarzinger M, Alla F. Increasing acceptance of a vaccination program for coronavirus disease 2019 in France: a challenge for one of the world’s most vaccine-hesitant countries. Vaccine. 2022;40:178-182. doi: 10.1016/j.vaccine.2021.11.023
27. Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154
28. Martela F, Hankonen N, Ryan RM, et al. Motivating voluntary compliance to behavioural restrictions: self-determination theory–based checklist of principles for COVID-19 and other emergency communications. Eur Rev Soc Psychol. 2021:305-347. doi: 10.1080/10463283.2020.1857082
29. Boness CL, Nelson M, Douaihy AB. Motivational interviewing strategies for addressing COVID-19 vaccine hesitancy. J Am Board Fam Med. 2022;35:420-426. doi: 10.3122/jabfm.2022.02.210327
30. Salomoni MG, Di Valerio Z, Gabrielli E, et al. Hesitant or not hesitant? A systematic review on global COVID-19 vaccine acceptance in different populations. Vaccines (Basel). 2021;9:873. doi: 10.3390/vaccines9080873
31. Pitts SI, Maruthur NM, Millar KR, et al. A systematic review of mandatory influenza vaccination in healthcare personnel. Am J Prev Med. 2014;47:330-340. doi:
PRACTICE RECOMMENDATIONS
› Focus on personal benefits of vaccination with patients who express strong hesitancy and endorse vaccine myths; refocus the conversation away from myths and back to disease facts. C
› Emphasize personal and collective benefit to patients who are uncertain about vaccination; provide education about herd immunity and local vaccine coverage. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series