User login
Gender and Patient Satisfaction in a Veterans Health Administration Outpatient Chemotherapy Unit
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
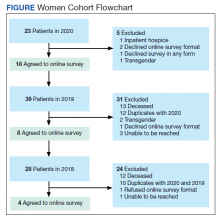
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
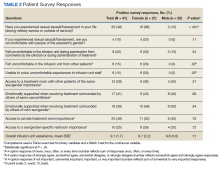
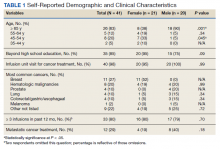
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
Gender differences in patient satisfaction with medical care have been evaluated in multiple settings; however, studies specific to the unique population of women veterans with cancer are lacking. Women are reported to value privacy, psychosocial support, and communication to a higher degree compared with men.1 Factors affecting satisfaction include the following: discomfort in sharing treatment rooms with the opposite gender, a desire for privacy with treatment and restroom use, anatomic or illness differences, and a personal history of abuse.2-4 Regrettably, up to 1 in 3 women in the United States are victims of sexual trauma in their lifetimes, and up to 1 in 4 women in the military are victims of military sexual trauma. Incidence in both settings is suspected to be higher due to underreporting.5,6
Chemotherapy treatment units are often uniquely designed as an open space, with several patients sharing a treatment area. The design reduces isolation and facilitates quick nurse-patient access during potentially toxic treatments known to have frequent adverse effects. Data suggest that nursing staff prefer open models to facilitate quick patient assessments and interventions as needed; however, patients and families prefer private treatment rooms, especially among women patients or those receiving longer infusions.7
The Veterans Health Administration (VHA) patient population is male predominant, comprised only of 10% female patients.8 Although the proportion of female patients in the VHA is expected to rise annually to about 16% by 2043, the low percentage of female veterans will persist for the foreseeable future.8 This low percentage of female veterans is reflected in the Veterans Affairs Portland Health Care System (VAPHCS) cancer patient population and in the use of the chemotherapy infusion unit, which is used for the ambulatory treatment of veterans undergoing cancer therapy.
The VHA has previously explored gender differences in health care, such as with cardiovascular disease, transgender care, and access to mental health.9-11 However, to the best of our knowledge, no analysis has explored gender differences within the outpatient cancer treatment experience. Patient satisfaction with outpatient cancer care may be magnified in the VHA setting due to the uniquely unequal gender populations, shared treatment space design, and high incidence of sexual abuse among women veterans. Given this, we aimed to identify gender-related preferences in outpatient cancer care in our chemotherapy infusion unit.
In our study, we used the terms male and female to reflect statistical data from the literature or labeled data from the electronic health record (EHR); whereas the terms men and women were used to describe and encompass the cultural implications and context of gender.12
Methods
This study was designated as a quality improvement (QI) project by the VAPHCS research office and Institutional Review Board in accordance with VHA policies.
The VAPHCS outpatient chemotherapy infusion unit is designed with 6 rooms for chemotherapy administration. One room is a large open space with 6 chairs for patients. The other rooms are smaller with glass dividers between the rooms, and 3 chairs inside each for patients. There are 2 private bathrooms, each gender neutral. Direct patient care is provided by physicians, nurse practitioners (NPs), infusion unit nurses, and nurse coordinators. Men represent the majority of hematology and oncology physicians (13 of 20 total: 5 women fellow physicians and 2 women attending physicians), and 2 of 4 NPs. Women represent 10 of 12 infusion unit and cancer coordinator nurses. We used the VHA Computerized Patient Record System (CPRS) EHR, to create a list of veterans treated at the VAPHCS outpatient chemotherapy infusion unit for a 2-year period (January 1, 2018, to December 31, 2020).
Male and female patient lists were first generated based on CPRS categorization. We identified all female veterans treated in the ambulatory infusion unit during the study period. Male patients were then chosen at random, recording the most recent names for each year until a matched number per year compared with the female cohort was reached. Patients were recorded only once even though they had multiple infusion unit visits. Patients were excluded who were deceased, on hospice care, lost to follow-up, could not be reached by phone, refused to take the survey, had undeliverable email addresses, or lacked internet or email access.
After filing the appropriate request through the VAPHCS Institutional Review Board committee in January 2021, patient records were reviewed for demographics data, contact information, and infusion treatment history. The survey was then conducted over a 2-week period during January and February 2021. Each patient was invited by phone to complete a 25-question anonymous online survey. The survey questions were created from patient-relayed experiences, then modeled into survey questions in a format similar to other patient satisfaction questionnaires described in cancer care and gender differences.2,13,14 The survey included self-identification of gender and was multiple choice for all except 2 questions, which allowed an open-ended response (Appendix). Only 1 answer per question was permitted. Only 1 survey link was sent to each veteran who gave permission for the survey. To protect anonymity for the small patient population, we excluded those identifying as gender nonbinary or transgender.
Statistical Analysis
Patient, disease, and treatment features are separated by male and female cohorts to reflect information from the EHR (Table 1). Survey percentages were calculated to reflect the affirmative response of the question asked (Table 2). Questions with answer options of not important, minimally important, important, or very important were calculated to reflect the sum of any importance in both cohorts. Questions with answer options of never, once, often, or every time were calculated to reflect any occurrence (sum of once, often, or every time) in both patient groups. Questions with answer options of strongly agree, somewhat agree, somewhat disagree, and strongly disagree were calculated to reflect any agreement (somewhat agree and strongly agree summed together) for both groups. Comparisons between cohorts were then conducted using a Fisher exact test. A Welch t test was used to calculate the significance of the continuous variable and overall ranking of the infusion unit experience between groups.
Results
In 2020, 414 individual patients were treated at the VAPAHCS outpatient infusion unit. Of these, 23 (5.6%) were female, and 18 agreed to take the survey. After deceased and duplicate names from 2020 were removed, another 14 eligible 2019 female patients were invited and 6 agreed to participate; 6 eligible 2018 female patients were invited and 4 agreed to take the survey (Figure). Thirty female veterans were sent a survey link and 21 (70%) responses were collected. Twenty-one male 2020 patients were contacted and 18 agreed to take the survey. After removing duplicate names and deceased individuals, 17 of 21 eligible 2019 male patients and 4 of 6 eligible 2018 patients agreed to take the survey. Five additional male veterans declined the online-based survey method. In total, 39 male veterans were reached who agreed to have the survey link emailed, and 20 (51%) total responses were collected.
Most respondents answered all questions in the survey. The most frequently skipped questions included 3 questions that were contingent on a yes answer to a prior question, and 2 openended questions asking for a write-in response. Percentages for female and male respondents were adjusted for number of responses when applicable.
Thirteen (62%) female patients were aged < 65 years, while 18 (90%) of male patients were aged ≥ 65 years. Education beyond high school was reported in 20 female and 15 male respondents. Almost all treatment administered in the infusion unit was for cancer-directed treatment, with only 1 reporting a noncancer treatment (IV iron). The most common malignancy among female patients was breast cancer (n = 11, 52%); for male patients prostate cancer (n = 4, 20%) and hematologic malignancy (n = 4, 20%) were most common. Four (19%) female and 8 (40%) male respondents reported having a metastatic diagnosis. Overall patient satisfaction ranked high with an average score of 9.1 on a 10-point scale. The mean (SD) satisfaction score for female respondents was 1 point lower than that for men: 8.7 (2.2) vs 9.6 (0.6) in men (P = .11).
Eighteen (86%) women reported a history of sexual abuse or harassment compared with 2 (10%) men (P < .001). The sexual abuse assailant was a different gender for 17 of 18 female respondents and of the same gender for both male respondents. Of those with sexual abuse history, 4 women reported feeling uncomfortable around their assailant’s gender vs no men (P = .11), but this difference was not statistically significant. Six women (29%) and 2 (10%) men reported feeling uncomfortable during clinical examinations from comments made by the clinician or during treatment administration (P = .24). Six (29%) women and no men reported that they “felt uncomfortable in the infusion unit by other patients” (P = .02). Six (29%) women and no men reported feeling unable to “voice uncomfortable experiences” to the infusion unit clinician (P = .02).
Ten (48%) women and 6 (30%) men reported emotional support when receiving treatments provided by staff of the same gender (P = .34). Eight (38%) women and 4 (20%) men noted that access to treatment with the same gender was important (P = .31). Six (29%) women and 4 (20%) men indicated that access to a sex or gender-specific restroom was important (P = .72). No gender preferences were identified in the survey questions regarding importance of private treatment room access and level of emotional support when receiving treatment with others of the same malignancy. These relationships were not statistically significant.
In addition, 2 open-ended questions were asked. Seventeen women and 14 men responded. Contact the corresponding author for more information on the questions and responses.
Discussion
Overall patient satisfaction was high among the men and women veterans with cancer who received treatment in our outpatient infusion unit; however, notable gender differences existed. Three items in the survey revealed statistically significant differences in the patient experience between men and women veterans: history of sexual abuse or harassment, uncomfortable feelings among other patients, and discomfort in relaying uncomfortable feelings to a clinician. Other items in the survey did not reach statistical significance; however, we have included discussion of the findings as they may highlight important trends and be of clinical significance.
We suspect differences among genders in patient satisfaction to be related to the high incidence of sexual abuse or harassment history reported by women, much higher at 86% than the one-third to one-fourth incidence rates estimated by the existing literature for civilian or military sexual abuse in women.5,6 These high sexual abuse or harassment rates are present in a majority of women who receive cancer-directed treatment toward a gender-specific breast malignancy, surrounded predominantly among men in a shared treatment space. Together, these factors are likely key reasons behind the differences in satisfaction observed. This sentiment is expressed in our cohort, where one-fifth of women with a sexual abuse or harassment history continue to remain uncomfortable around men, and 29% of women reporting some uncomfortable feelings during their treatment experience compared with none of the men. Additionally, 6 (29%) women vs no men felt uncomfortable in reporting an uncomfortable experience with a clinician; this represents a significant barrier in providing care for these patients.
A key gender preference among women included access to shared treatment rooms with other women and that sharing a treatment space with other women resulted in feeling more emotional support during treatments. Access to gender-specific restrooms was also preferred by women more than men. Key findings in both genders were that about half of men and women valued access to a private treatment room and would derive more emotional support when surrounded by others with the same cancer.
Prior studies on gender and patient satisfaction in general medical care and cancer care have found women value privacy more than men.1-3 Wessels and colleagues performed an analysis of 386 patients with cancer in Europe and found gender to be the strongest influence in patient preferences within cancer care. Specifically, the highest statically significant association in care preferences among women included privacy, support/counseling/rehabilitation access, and decreased wait times.2 These findings were most pronounced in those with breast cancer compared with other malignancy type and highlights that malignancy type and gender predominance impact care satisfaction.
Traditionally a shared treatment space design has been used in outpatient chemotherapy units, similar to the design of the VAPHCS. However, recent data report on the patient preference for a private treatment space, which was especially prominent among women and those receiving longer infusions.7 In another study that evaluated 225 patients with cancer preferences in sharing a treatment space with those of a different sexual orientation or gender identify, differences were found. Both men and women had a similar level of comfort in sharing a treatment room with someone of a different sexual orientation; however, more women reported discomfort in sharing a treatment space with a transgender woman compared with men who felt more comfortable sharing a space with a transgender man.4 We noted a gender preference may be present to explain the difference. Within our cohort, women valued access to treatment with other women and derived more emotional support when with other women; however, we did not inquire about feelings in sharing a treatment space among transgender individuals or differing sexual orientation.
Gender differences for privacy and in shared room preferences may result from the lasting impacts of prior sexual abuse or harassment. A history of sexual abuse negatively impacts later medical care access and use.15 Those veterans who experienced sexual abuse/harrassment reported higher feelings of lack of control, vulnerability, depression, and pursued less medical care.15,16 Within cancer care, these feelings are most pronounced among women with gender-specific malignancies, such as gynecologic cancers or breast cancer. Treatment, screening, and physical examinations by clinicians who are of the same gender as the sexual abuse/harassment assailant can recreate traumatic feelings.15,16
A majority of women (n = 18, 86%) in our cohort reported a history of sexual abuse or harassment and breast malignancy was the most common cancer among women. However women represent just 5.6% of the VAPHCS infusion unit treatment population. This combination of factors may explain the reasons for women veterans’ preference for privacy during treatments, access to gender-specific restrooms, and feeling more emotional support when surrounded by other women. Strategies to help patients with a history of abuse have been described and include discussions from the clinician asking about abuse history, allowing time for the patient to express fears with an examination or test, and training on how to deliver sensitive care for those with trauma.17,18
Quality Improvement
Project In the VAPHCS infusion unit, several low-cost interventions have been undertaken as a result of our survey findings. We presented our survey data to the VAPHCS Cancer Committee, accredited through the national American College of Surgeons Commission on Cancer. The committee awarded support for a yearlong QI project, including a formal framework of quarterly multidisciplinary meetings to discuss project updates, challenges, and resources. The QI project centers on education to raise awareness of survey results as well as specific interventions for improvement.
Education efforts have been applied through multiple department-wide emails, in-person education to our chemotherapy unit staff, abstract submission to national oncology conferences, and grand rounds department presentations at VAPHCS and at other VHA-affiliated university programs. Additionally, education to clinicians with specific contact information for psychology and women’s health to support mental health, trauma, and sexual abuse histories has been given to each clinician who cares for veterans in the chemotherapy unit.
We also have implemented a mandatory cancer care navigation consultation for all women veterans who have a new cancer or infusion need. The cancer care navigator has received specialized training in sensitive history-taking and provides women veterans with a direct number to reach the cancer care navigation nurse. Cancer care navigation also provides a continuum of support and referral access for psychosocial needs as indicated between infusion or health care visits. Our hope is that these resources may help offset the sentiment reflected in our cohort of women feeling unable to voice concerns to a clinician.
Other interventions underway include offering designated scheduling time each week to women so they can receive infusions in an area with other women. This may help mitigate the finding that women veterans felt more uncomfortable around other patients during infusion treatments compared with how men felt in the chemotherapy unit. We also have implemented gender-specific restrooms labeled with a sign on each bathroom door so men and women can have access to a designated restroom. Offering private or semiprivate treatment rooms is currently limited by space and capacity; however, these may offer the greatest opportunity to improve patient satisfaction, especially among women veterans. Working with the support of the VAPHCS Cancer Committee, we aim to reevaluate the impact of the education and QI efforts on gender differences and patient satisfaction at completion of the 1-year award.
Limitations
Limitations to our study include the overall small sample size. This is due to the combination of the low number of women treated at VAPHCS and many with advanced cancer who, unfortunately, have a limited overall survival and hinders accrual of a larger sample size. Other limitations included age as a possible confounder in our findings, with women representing a younger demographic compared with men. We did not collect responses on duration of infusion time, which also may impact overall satisfaction and patient experience. We also acknowledge that biologic male or female sex may not correspond to a specific individual’s gender. Use of CPRS to obtain a matched number of male and female patients through random selection relied on labeled data from the EHR. This potentially may have excluded male patients who identify as another gender that would have been captured on the anonymous survey.
Last, we restricted survey responses to online only, which excluded a small percentage who declined this approach.
Conclusions
Our findings may have broad applications to other VHA facilities and other cancer-directed treatment centers where the patient demographic and open shared infusion unit design may be similar. The study also may serve as a model of survey design and implementation from which other centers may consider improving patient satisfaction. We hope these survey results and interventions can provide insight and be used to improve patient satisfaction among all cancer patients at infusion units serving veterans and nonveterans.
Acknowledgments
We are very thankful to our cancer patients who took the time to take the survey. We also are very grateful to the VHA infusion unit nurses, staff, nurse practitioners, and physicians who have embraced this project and welcomed any changes that may positively impact treatment of veterans. Also, thank you to Tia Kohs for statistical support and Sophie West for gender discussions. Last, we specifically thank Barbara, for her pursuit of better care for women and for all veterans.
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
1. Clarke SA, Booth L, Velikova G, Hewison J. Social support: gender differences in cancer patients in the United Kingdom. Cancer Nurs. 2006;29(1):66-72. doi:10.1097/00002820-200601000-00012
2. Wessels H, de Graeff A, Wynia K, et al. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist. 2010;15(6):648-655. doi:10.1634/theoncologist.2009-0337
3. Hartigan SM, Bonnet K, Chisholm L, et al. Why do women not use the bathroom? Women’s attitudes and beliefs on using public restrooms. Int J Environ Res Public Health. 2020;17(6):2053. doi:10.3390/ijerph17062053
4. Alexander K, Walters CB, Banerjee SC. Oncology patients’ preferences regarding sexual orientation and gender identity (SOGI) disclosure and room sharing sharing. Patient Educ Couns. 2020;103(5):1041-1048. doi:10.1016/j.pec.2019.12.006
5. Centers for Disease Control and Prevention. Facts about sexual violence. Updated July 5, 2022. Accessed July 13, 2022. https://www.cdc.gov/injury/features /sexual-violence/index.html
6. US Department of Veterans Affairs. Military sexual trauma. Updated May 16, 2022. Accessed July 13, 2022. https:// www.mentalhealth.va.gov/mentalhealth/msthome/index.asp
7. Wang Z, Pukszta M. Private Rooms, Semi-open areas, or open areas for chemotherapy care: perspectives of cancer patients, families, and nursing staff. HERD. 2018;11(3):94- 108. doi:10.1177/1937586718758445
8. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Women veterans report: the past, present, and future of women veterans. Accessed July 13, 2022. https://www.va.gov/vetdata /docs/specialreports/women_veterans_2015_final.pdf
9. Driscoll MA, Higgins DM, Seng EK, et al. Trauma, social support, family conflict, and chronic pain in recent service veterans: does gender matter? Pain Med. 2015;16(6):1101- 1111. doi:10.1111/pme.12744
10. Fox AB, Meyer EC, Vogt D. Attitudes about the VA healthcare setting, mental illness, and mental health treatment and their relationship with VA mental health service use among female and male OEF/OIF veterans. Psychol Serv. 2015;12(1):49-58. doi:10.1037/a0038269
11. Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115(1):21-26. doi:10.1016/j.amjcard.2014.09.041
12. Tseng J. Sex, gender, and why the differences matter. Virtual Mentor. 2008;10(7):427-428. doi:10.1001/virtualmentor.2008.10.7.fred1-0807
13. Booij JC, Zegers M, Evers PMPJ, Hendricks M, Delnoij DMJ, Rademakers JJDJM. Improving cancer patient care: development of a generic cancer consumer quality index questionnaire for cancer patients. BMC Cancer. 2013;13(203). doi:10.1186/1471-2407-13-203
14. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer. 2008;113(12):3459-3466. doi:10.1002/cncr.23968 1
5. Schnur JB, Dillon MJ, Goldsmith RE, Montgomery GH. Cancer treatment experiences among survivors of childhood sexual abuse: a qualitative investigation of triggers and reactions to cumulative trauma. Palliat Support Care. 2018;16(6):767-776. doi:10.1017/S147895151700075X
16. Cadman L, Waller J, Ashdown-Barr L, Szarewski A. Barriers to cervical screening in women who have experienced sexual abuse: an exploratory study. J Fam Plann Reprod Health Care. 2012;38(4):214-220. doi:10.1136/jfprhc-2012-100378
17. Kelly S. The effects of childhood sexual abuse on women’s lives and their attitudes to cervical screening. J Fam Plann Reprod Health Care. 2012;38(4):212-213. doi:10.1136/jfprhc-2012-100418
18. McCloskey LA, Lichter E, Williams C, Gerber M, Wittenberg E, Ganz M. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Public Health Rep. 2006;121(4):435-444. doi:10.1177/003335490612100412
After cancer, abortion experience highlights post-Roe reality
The drive from Texas to the clinic in Albuquerque, N.M., took 10 hours. It was mid-April of this year. There wasn’t much to see along the mostly barren stretch, and there wasn’t much for Kailee DeSpain to do aside from think about where she was going and why.
Her husband was driving. She sensed his nervous glances toward the passenger seat where she sat struggling to quiet her thoughts.
No, she wasn’t having any pain, she told him. No, she wasn’t feeling like she did the last time or the two times before that.
This pregnancy was different. It was the first in which she feared for her own life. Her fetus – Finley – had triploidy, a rare chromosomal abnormality. Because of the condition, which affects 1%-3% of pregnancies, his heart, brain, and kidneys were not developing properly.
At 19 weeks, Finley was already struggling to draw breath from lungs squeezed inside an overcrowded chest cavity. Ms. DeSpain wanted nothing more than to carry Finley to term, hold him, meet him even for a moment before saying goodbye.
But his condition meant he would likely suffocate in utero well before that. And Ms. DeSpain knew that carrying him longer would likely raise her risk of bleeding and of her blood pressure increasing to dangerous highs.
“This could kill you,” her husband told her. “Do you realize you could die bringing a baby into this world who is not going to live? I don’t want to lose you.’”
Unlike her other pregnancies, the timing of this one and the decision she faced to end it put her health in even greater danger.
Imminent danger
On Sept. 1, 2021, a bill went into effect in Texas that banned abortions from as early as 6 weeks’ gestation. Texas Senate Bill 8 (SB8) became one of the most restrictive abortion laws in the country. It prohibited abortions whenever a fetal heartbeat, defined by lawmakers, could be detected on an ultrasound, often before many women knew they were pregnant.
The Texas abortion law was hardly the last word on the topic. Ms. DeSpain didn’t know it on her drive to New Mexico in April, but the U.S. Supreme Court was weeks away from overturning the landmark Roe v. Wade decision.
On June 24, the Supreme Court delivered its 6-3 ruling overturning Roe v. Wade, the 1973 case that granted women the right to abortion.
This decision set in motion “trigger laws” in some states – laws that essentially fully banned abortions. Those states included Ms. DeSpain’s home state of Texas, where abortion is now a felony except when the life of the mother is in peril.
However, legal definitions of what qualifies as “life-threatening” remain murky.
The law is unclear, says Lisa Harris, MD, PhD, professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “What does the risk of death have to be, and how imminent must it be?” she asked in a recent editorial in the New England Journal of Medicine. Is 25% enough? 50%? Or does a woman have to be moments from dying?
“This whole thing makes me so angry,” says Shikha Jain, MD, a medical oncologist at University of Illinois Health, Chicago. “A patient may not be experiencing an emergency right now, but if we don’t take care of the situation, it may become an emergency in 2 hours or 2 days.”
Even before the Roe v. Wade decision, pregnancy had been a high-stakes endeavor for many women. In 2019, more than 750 women died from pregnancy-related events in the United States. In 2020, that number rose to 850. Each year dozens more suffer pregnancy-related events that require lifesaving interventions.
Now, in a post-Roe world, the number of maternal deaths will likely climb as more abortion bans take effect and fewer women have access to lifesaving care, experts say. A 2021 study that compared 2017 maternal mortality rates in states with different levels of abortion restrictions found that the rate of maternal mortality was almost two times higher in states that restricted abortion access compared with those that protected it – 28.5 per 100,000 women vs. 15.7.
Some women living in states with abortion bans won’t have the resources to cross state lines for care.
“This is just going to widen the health care disparities that are already so prevalent in this country,” Dr. Jain says.
Navigating a crossroads
Ms. DeSpain’s medical history reads like a checklist of pregnancy-related perils: chronic high blood pressure, persistent clotting problems, and a high risk of hemorrhage. She was also diagnosed with cervical cancer in 2020, which left her body more fragile.
Cardiovascular conditions, including hypertension and hemorrhage, are the leading causes of maternal mortality, responsible for more than one-third of pregnancy-related deaths. Preeclampsia, characterized by high blood pressure, accounts for more than 7% of maternal deaths in the United States. Although less common, genetic disorders, such as spinal muscular atrophy and triploidy, or cancer during pregnancy can put a mother and fetus at risk.
Cancer – which affects about 1 in 1,000 pregnant women and results in termination in as many as 28% of cases – brings sharp focus to the new dangers and complex decision-making patients and their doctors face as abortion bans take hold.
Before the Supreme Court decision, a pregnant woman with cancer was already facing great uncertainty. The decision to treat cancer during pregnancy involves “weighing the risk of exposing the fetus to medication vs. the risk to the mother’s untreated illness if you don’t expose the fetus to medication,” Elyce Cardonick, MD, an obstetrician at Cooper University Health Care, Camden, N.J., who specializes in high-risk pregnancies, told the National Cancer Institute.
Oncologists generally agree that it’s safe for pregnant women to receive chemotherapy during the second and third trimesters. But for women with aggressive cancers that are diagnosed in the first trimester, chemotherapy is dangerous. For women who need immunotherapy, the risks of treatment remain unclear.
In these cases, Alice S. Mims, MD, must broach the possibility of terminating the pregnancy.
“Cancer is a very urgent condition,” says Dr. Mims, a hematology specialist at the Ohio State University Comprehensive Cancer Center, Columbus, who sees patients who are pregnant. “These women may have other children at home, and they want to do their best to fight the disease so they can be around for their family long term.”
Now the changing legal landscape on abortion will put hundreds more pregnant women with cancer in danger. In a recent viewpoint article published in JAMA Oncology, Jordyn Silverstein and Katherine Van Loon, MD, MPH, estimate that during the next year, up to 420 pregnant women living in states with restricted abortion access will face threats to their cancer care and potentially their life.
“The repercussions of overturning Roe v. Wade – and the failure of the Supreme Court to provide any guidance on exceptions related to the life and health of the mother – are potentially catastrophic for a subset of women who face a life-threating diagnosis of [pregnancy-associated cancer],” they write.
The choice Ms. DeSpain faced after her cervical cancer diagnosis was different. She was not pregnant at the time, but she was at a crossroads.
Although it was caught early, the cancer was aggressive. Her oncologist recommended that she undergo a hysterectomy – the surgery that would give her the best chance for a cancer-free future. It would also mean she could no longer become pregnant.
With a less invasive procedure, on the other hand, she could still carry a child, but she would face a much greater chance that the cancer would come back.
At 27, Ms. DeSpain was not ready to close the pregnancy door. She opted for a surgery in which part of her cervix was removed, allowing her to try for another baby.
But she faced a ticking clock in the event her cancer returned.
If you want to have a baby, “try soon,” her doctor warned.
A dead end
After her cancer surgery and a third miscarriage, Ms. DeSpain and her husband were surprised and excited when in late 2021 she again became pregnant.
The first trimester seemed blissfully uneventful. As the weeks passed, Finley’s heart started to beat.
But the 16-week ultrasound signaled a turning point. The sonographer was too quiet.
“This is really bad, isn’t it?” Ms. DeSpain asked her sonographer.
The doctors told her he wouldn’t survive. Finley had no heart chambers. His heart couldn’t pump blood properly. He was missing one kidney, and his brain was split in the back. With almost no amniotic fluid, her doctor said he would likely die in utero, crushed to death without support from the protective liquid.
She fought for him anyway. She sought specialty care, followed bed rest orders, and traveled 3 hours to Houston to enroll in a clinical trial.
But every road was a dead end.
Ultimately, testing revealed Finley had triploidy, and all lines led to one point.
“There were too many things wrong, too much wrong for them to fix,” says Ms. DeSpain, recalling the news from her doctor in Houston. “I was in shock. My husband was just sitting with his hands flat on the table, staring at nothing, shaking a little bit.”
However, Finley still had a heartbeat, making an abortion after 6 weeks a felony in Texas. Even a compassionate induction was now out of the question unless her death was imminent.
Ms. DeSpain called the abortion clinic in Albuquerque and made an appointment. She would have to wait 2 weeks because of an influx of pregnant patients coming from Texas.
She welcomed the wait … just in case she changed her mind.
“At that point I wanted to carry him as far as I could,” she says.
For those 2 weeks, Ms. DeSpain remained on bed rest. She cried all day every day. She worried that Finley was experiencing pain.
Through this process, her doctor’s support helped keep her grounded.
“She cried with us in her office and said, ‘I wish that you didn’t have to go, but I think you’re doing the right thing, doing what keeps you safest,’ “ Ms. DeSpain recalls.
Ms. DeSpain declined to share the name of her doctor out of fear that even expressing compassion for a patient’s safety could put the physician in legal jeopardy and provoke harassment.
That fear is warranted. Some doctors will be forced to choose between doing what is legal – even though the law is vague – and doing what is right for patients, says law professor Jamie Abrams, who was recently diagnosed with breast cancer.
To live in a world where there’s talk of criminalizing doctors for taking care of their patients, where there’s “this national movement to position some women to be shunned and exiled for seeking care that’s right for them, their health, and might save their life is staggering and beyond comprehension,” says Ms. Abrams, professor of law at the American University Washington College of Law.
Ms. Abrams, who was diagnosed with hormone receptor–positive invasive breast cancer the same day she read the leaked Supreme Court draft on the decision to end of Roe v. Wade, said that “overnight, I became a person who would need an abortion if I became pregnant, because my treatment would compromise a healthy birth or delay necessary cancer care.” Ms. Abrams was also told she could no longer use hormonal contraception.
Dr. Harris’s advice to clinicians is to try to do what they feel is best for patients, including referring them to centers that have legal resources and protections regarding abortions.
Dr. Mims agrees and recommends that doctors reach out to those with more resources and legal backing for support. “I would advise doctors in [states with restrictive laws] to familiarize themselves with available resources and organizations taking action to deal with questionable cases,” Dr. Mims says.
‘Baby killers work here’
Following her 10-hour drive to Albuquerque, Ms. DeSpain encountered lines of protesters at the clinic. They were holding signs that said, “Abortion is murder,” and “Baby killers work here.”
“Please don’t kill your baby – we have resources for you,” a woman screeched through a megaphone as Ms. DeSpain, nearly 20 weeks’ pregnant, stepped out of the car to enter the clinic.
“I remember turning around, looking at her and making eye contact, and yelling back, ‘My baby has triploidy – he is dying! He is going to suffocate if I carry him full term. You don’t know what you’re talking about!’ “
A nurse held her hand during the procedure.
“He said, ‘You’re doing great, you’re okay,’ “ she recalls. She knew there was a chance that Finley’s face would be crushed by contractions during labor because of the lack of amniotic fluid, but she hoped not. Ms. DeSpain longed for a photo.
There was no photo to take home the next day, but Ms. DeSpain did receive Finley’s footprints, and his heartbeat – as captured by the specialty team in Houston – lives on in a stuffed giraffe.
His ashes arrived a few weeks later.
By then, the Supreme Court draft had been leaked. Ms. DeSpain knew her predicament in Texas would soon affect women across the United States and make any future pregnancy attempt for her even more risky.
The weeks and months that followed were a blur of grief, anger, and medical testing.
But she received some good news. A second triploidy pregnancy was extremely unlikely.
Several weeks later, Ms. DeSpain got more good news.
“I had a follow-up cancer appointment, and everything was completely clear,” she says.
She remains hopeful that she will be able to give birth, but her doctor cautioned that it’s no longer safe to become pregnant in Texas.
“I need you to understand that if you get pregnant and you have complications, we can’t intervene unless the baby doesn’t have a heartbeat, even if it would save your life,” Ms. DeSpain recalls her doctor saying.
If Texas remains a dangerous place to be pregnant, Ms. DeSpain and her husband will have to move.
For now, Ms. DeSpain wants people to know her story and to continue to fight for her right to govern her body.
In a public post to Facebook, she laid bare her pregnancy journey.
“No one should have to share a story like mine to justify abortion,” she wrote. “My choice is not yours to judge, and my rights are not yours to gleefully take away.”
Ms. Abrams, Ms. DeSpain, Dr. Harris, Dr. Jain, and Dr. Mims have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The drive from Texas to the clinic in Albuquerque, N.M., took 10 hours. It was mid-April of this year. There wasn’t much to see along the mostly barren stretch, and there wasn’t much for Kailee DeSpain to do aside from think about where she was going and why.
Her husband was driving. She sensed his nervous glances toward the passenger seat where she sat struggling to quiet her thoughts.
No, she wasn’t having any pain, she told him. No, she wasn’t feeling like she did the last time or the two times before that.
This pregnancy was different. It was the first in which she feared for her own life. Her fetus – Finley – had triploidy, a rare chromosomal abnormality. Because of the condition, which affects 1%-3% of pregnancies, his heart, brain, and kidneys were not developing properly.
At 19 weeks, Finley was already struggling to draw breath from lungs squeezed inside an overcrowded chest cavity. Ms. DeSpain wanted nothing more than to carry Finley to term, hold him, meet him even for a moment before saying goodbye.
But his condition meant he would likely suffocate in utero well before that. And Ms. DeSpain knew that carrying him longer would likely raise her risk of bleeding and of her blood pressure increasing to dangerous highs.
“This could kill you,” her husband told her. “Do you realize you could die bringing a baby into this world who is not going to live? I don’t want to lose you.’”
Unlike her other pregnancies, the timing of this one and the decision she faced to end it put her health in even greater danger.
Imminent danger
On Sept. 1, 2021, a bill went into effect in Texas that banned abortions from as early as 6 weeks’ gestation. Texas Senate Bill 8 (SB8) became one of the most restrictive abortion laws in the country. It prohibited abortions whenever a fetal heartbeat, defined by lawmakers, could be detected on an ultrasound, often before many women knew they were pregnant.
The Texas abortion law was hardly the last word on the topic. Ms. DeSpain didn’t know it on her drive to New Mexico in April, but the U.S. Supreme Court was weeks away from overturning the landmark Roe v. Wade decision.
On June 24, the Supreme Court delivered its 6-3 ruling overturning Roe v. Wade, the 1973 case that granted women the right to abortion.
This decision set in motion “trigger laws” in some states – laws that essentially fully banned abortions. Those states included Ms. DeSpain’s home state of Texas, where abortion is now a felony except when the life of the mother is in peril.
However, legal definitions of what qualifies as “life-threatening” remain murky.
The law is unclear, says Lisa Harris, MD, PhD, professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “What does the risk of death have to be, and how imminent must it be?” she asked in a recent editorial in the New England Journal of Medicine. Is 25% enough? 50%? Or does a woman have to be moments from dying?
“This whole thing makes me so angry,” says Shikha Jain, MD, a medical oncologist at University of Illinois Health, Chicago. “A patient may not be experiencing an emergency right now, but if we don’t take care of the situation, it may become an emergency in 2 hours or 2 days.”
Even before the Roe v. Wade decision, pregnancy had been a high-stakes endeavor for many women. In 2019, more than 750 women died from pregnancy-related events in the United States. In 2020, that number rose to 850. Each year dozens more suffer pregnancy-related events that require lifesaving interventions.
Now, in a post-Roe world, the number of maternal deaths will likely climb as more abortion bans take effect and fewer women have access to lifesaving care, experts say. A 2021 study that compared 2017 maternal mortality rates in states with different levels of abortion restrictions found that the rate of maternal mortality was almost two times higher in states that restricted abortion access compared with those that protected it – 28.5 per 100,000 women vs. 15.7.
Some women living in states with abortion bans won’t have the resources to cross state lines for care.
“This is just going to widen the health care disparities that are already so prevalent in this country,” Dr. Jain says.
Navigating a crossroads
Ms. DeSpain’s medical history reads like a checklist of pregnancy-related perils: chronic high blood pressure, persistent clotting problems, and a high risk of hemorrhage. She was also diagnosed with cervical cancer in 2020, which left her body more fragile.
Cardiovascular conditions, including hypertension and hemorrhage, are the leading causes of maternal mortality, responsible for more than one-third of pregnancy-related deaths. Preeclampsia, characterized by high blood pressure, accounts for more than 7% of maternal deaths in the United States. Although less common, genetic disorders, such as spinal muscular atrophy and triploidy, or cancer during pregnancy can put a mother and fetus at risk.
Cancer – which affects about 1 in 1,000 pregnant women and results in termination in as many as 28% of cases – brings sharp focus to the new dangers and complex decision-making patients and their doctors face as abortion bans take hold.
Before the Supreme Court decision, a pregnant woman with cancer was already facing great uncertainty. The decision to treat cancer during pregnancy involves “weighing the risk of exposing the fetus to medication vs. the risk to the mother’s untreated illness if you don’t expose the fetus to medication,” Elyce Cardonick, MD, an obstetrician at Cooper University Health Care, Camden, N.J., who specializes in high-risk pregnancies, told the National Cancer Institute.
Oncologists generally agree that it’s safe for pregnant women to receive chemotherapy during the second and third trimesters. But for women with aggressive cancers that are diagnosed in the first trimester, chemotherapy is dangerous. For women who need immunotherapy, the risks of treatment remain unclear.
In these cases, Alice S. Mims, MD, must broach the possibility of terminating the pregnancy.
“Cancer is a very urgent condition,” says Dr. Mims, a hematology specialist at the Ohio State University Comprehensive Cancer Center, Columbus, who sees patients who are pregnant. “These women may have other children at home, and they want to do their best to fight the disease so they can be around for their family long term.”
Now the changing legal landscape on abortion will put hundreds more pregnant women with cancer in danger. In a recent viewpoint article published in JAMA Oncology, Jordyn Silverstein and Katherine Van Loon, MD, MPH, estimate that during the next year, up to 420 pregnant women living in states with restricted abortion access will face threats to their cancer care and potentially their life.
“The repercussions of overturning Roe v. Wade – and the failure of the Supreme Court to provide any guidance on exceptions related to the life and health of the mother – are potentially catastrophic for a subset of women who face a life-threating diagnosis of [pregnancy-associated cancer],” they write.
The choice Ms. DeSpain faced after her cervical cancer diagnosis was different. She was not pregnant at the time, but she was at a crossroads.
Although it was caught early, the cancer was aggressive. Her oncologist recommended that she undergo a hysterectomy – the surgery that would give her the best chance for a cancer-free future. It would also mean she could no longer become pregnant.
With a less invasive procedure, on the other hand, she could still carry a child, but she would face a much greater chance that the cancer would come back.
At 27, Ms. DeSpain was not ready to close the pregnancy door. She opted for a surgery in which part of her cervix was removed, allowing her to try for another baby.
But she faced a ticking clock in the event her cancer returned.
If you want to have a baby, “try soon,” her doctor warned.
A dead end
After her cancer surgery and a third miscarriage, Ms. DeSpain and her husband were surprised and excited when in late 2021 she again became pregnant.
The first trimester seemed blissfully uneventful. As the weeks passed, Finley’s heart started to beat.
But the 16-week ultrasound signaled a turning point. The sonographer was too quiet.
“This is really bad, isn’t it?” Ms. DeSpain asked her sonographer.
The doctors told her he wouldn’t survive. Finley had no heart chambers. His heart couldn’t pump blood properly. He was missing one kidney, and his brain was split in the back. With almost no amniotic fluid, her doctor said he would likely die in utero, crushed to death without support from the protective liquid.
She fought for him anyway. She sought specialty care, followed bed rest orders, and traveled 3 hours to Houston to enroll in a clinical trial.
But every road was a dead end.
Ultimately, testing revealed Finley had triploidy, and all lines led to one point.
“There were too many things wrong, too much wrong for them to fix,” says Ms. DeSpain, recalling the news from her doctor in Houston. “I was in shock. My husband was just sitting with his hands flat on the table, staring at nothing, shaking a little bit.”
However, Finley still had a heartbeat, making an abortion after 6 weeks a felony in Texas. Even a compassionate induction was now out of the question unless her death was imminent.
Ms. DeSpain called the abortion clinic in Albuquerque and made an appointment. She would have to wait 2 weeks because of an influx of pregnant patients coming from Texas.
She welcomed the wait … just in case she changed her mind.
“At that point I wanted to carry him as far as I could,” she says.
For those 2 weeks, Ms. DeSpain remained on bed rest. She cried all day every day. She worried that Finley was experiencing pain.
Through this process, her doctor’s support helped keep her grounded.
“She cried with us in her office and said, ‘I wish that you didn’t have to go, but I think you’re doing the right thing, doing what keeps you safest,’ “ Ms. DeSpain recalls.
Ms. DeSpain declined to share the name of her doctor out of fear that even expressing compassion for a patient’s safety could put the physician in legal jeopardy and provoke harassment.
That fear is warranted. Some doctors will be forced to choose between doing what is legal – even though the law is vague – and doing what is right for patients, says law professor Jamie Abrams, who was recently diagnosed with breast cancer.
To live in a world where there’s talk of criminalizing doctors for taking care of their patients, where there’s “this national movement to position some women to be shunned and exiled for seeking care that’s right for them, their health, and might save their life is staggering and beyond comprehension,” says Ms. Abrams, professor of law at the American University Washington College of Law.
Ms. Abrams, who was diagnosed with hormone receptor–positive invasive breast cancer the same day she read the leaked Supreme Court draft on the decision to end of Roe v. Wade, said that “overnight, I became a person who would need an abortion if I became pregnant, because my treatment would compromise a healthy birth or delay necessary cancer care.” Ms. Abrams was also told she could no longer use hormonal contraception.
Dr. Harris’s advice to clinicians is to try to do what they feel is best for patients, including referring them to centers that have legal resources and protections regarding abortions.
Dr. Mims agrees and recommends that doctors reach out to those with more resources and legal backing for support. “I would advise doctors in [states with restrictive laws] to familiarize themselves with available resources and organizations taking action to deal with questionable cases,” Dr. Mims says.
‘Baby killers work here’
Following her 10-hour drive to Albuquerque, Ms. DeSpain encountered lines of protesters at the clinic. They were holding signs that said, “Abortion is murder,” and “Baby killers work here.”
“Please don’t kill your baby – we have resources for you,” a woman screeched through a megaphone as Ms. DeSpain, nearly 20 weeks’ pregnant, stepped out of the car to enter the clinic.
“I remember turning around, looking at her and making eye contact, and yelling back, ‘My baby has triploidy – he is dying! He is going to suffocate if I carry him full term. You don’t know what you’re talking about!’ “
A nurse held her hand during the procedure.
“He said, ‘You’re doing great, you’re okay,’ “ she recalls. She knew there was a chance that Finley’s face would be crushed by contractions during labor because of the lack of amniotic fluid, but she hoped not. Ms. DeSpain longed for a photo.
There was no photo to take home the next day, but Ms. DeSpain did receive Finley’s footprints, and his heartbeat – as captured by the specialty team in Houston – lives on in a stuffed giraffe.
His ashes arrived a few weeks later.
By then, the Supreme Court draft had been leaked. Ms. DeSpain knew her predicament in Texas would soon affect women across the United States and make any future pregnancy attempt for her even more risky.
The weeks and months that followed were a blur of grief, anger, and medical testing.
But she received some good news. A second triploidy pregnancy was extremely unlikely.
Several weeks later, Ms. DeSpain got more good news.
“I had a follow-up cancer appointment, and everything was completely clear,” she says.
She remains hopeful that she will be able to give birth, but her doctor cautioned that it’s no longer safe to become pregnant in Texas.
“I need you to understand that if you get pregnant and you have complications, we can’t intervene unless the baby doesn’t have a heartbeat, even if it would save your life,” Ms. DeSpain recalls her doctor saying.
If Texas remains a dangerous place to be pregnant, Ms. DeSpain and her husband will have to move.
For now, Ms. DeSpain wants people to know her story and to continue to fight for her right to govern her body.
In a public post to Facebook, she laid bare her pregnancy journey.
“No one should have to share a story like mine to justify abortion,” she wrote. “My choice is not yours to judge, and my rights are not yours to gleefully take away.”
Ms. Abrams, Ms. DeSpain, Dr. Harris, Dr. Jain, and Dr. Mims have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The drive from Texas to the clinic in Albuquerque, N.M., took 10 hours. It was mid-April of this year. There wasn’t much to see along the mostly barren stretch, and there wasn’t much for Kailee DeSpain to do aside from think about where she was going and why.
Her husband was driving. She sensed his nervous glances toward the passenger seat where she sat struggling to quiet her thoughts.
No, she wasn’t having any pain, she told him. No, she wasn’t feeling like she did the last time or the two times before that.
This pregnancy was different. It was the first in which she feared for her own life. Her fetus – Finley – had triploidy, a rare chromosomal abnormality. Because of the condition, which affects 1%-3% of pregnancies, his heart, brain, and kidneys were not developing properly.
At 19 weeks, Finley was already struggling to draw breath from lungs squeezed inside an overcrowded chest cavity. Ms. DeSpain wanted nothing more than to carry Finley to term, hold him, meet him even for a moment before saying goodbye.
But his condition meant he would likely suffocate in utero well before that. And Ms. DeSpain knew that carrying him longer would likely raise her risk of bleeding and of her blood pressure increasing to dangerous highs.
“This could kill you,” her husband told her. “Do you realize you could die bringing a baby into this world who is not going to live? I don’t want to lose you.’”
Unlike her other pregnancies, the timing of this one and the decision she faced to end it put her health in even greater danger.
Imminent danger
On Sept. 1, 2021, a bill went into effect in Texas that banned abortions from as early as 6 weeks’ gestation. Texas Senate Bill 8 (SB8) became one of the most restrictive abortion laws in the country. It prohibited abortions whenever a fetal heartbeat, defined by lawmakers, could be detected on an ultrasound, often before many women knew they were pregnant.
The Texas abortion law was hardly the last word on the topic. Ms. DeSpain didn’t know it on her drive to New Mexico in April, but the U.S. Supreme Court was weeks away from overturning the landmark Roe v. Wade decision.
On June 24, the Supreme Court delivered its 6-3 ruling overturning Roe v. Wade, the 1973 case that granted women the right to abortion.
This decision set in motion “trigger laws” in some states – laws that essentially fully banned abortions. Those states included Ms. DeSpain’s home state of Texas, where abortion is now a felony except when the life of the mother is in peril.
However, legal definitions of what qualifies as “life-threatening” remain murky.
The law is unclear, says Lisa Harris, MD, PhD, professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “What does the risk of death have to be, and how imminent must it be?” she asked in a recent editorial in the New England Journal of Medicine. Is 25% enough? 50%? Or does a woman have to be moments from dying?
“This whole thing makes me so angry,” says Shikha Jain, MD, a medical oncologist at University of Illinois Health, Chicago. “A patient may not be experiencing an emergency right now, but if we don’t take care of the situation, it may become an emergency in 2 hours or 2 days.”
Even before the Roe v. Wade decision, pregnancy had been a high-stakes endeavor for many women. In 2019, more than 750 women died from pregnancy-related events in the United States. In 2020, that number rose to 850. Each year dozens more suffer pregnancy-related events that require lifesaving interventions.
Now, in a post-Roe world, the number of maternal deaths will likely climb as more abortion bans take effect and fewer women have access to lifesaving care, experts say. A 2021 study that compared 2017 maternal mortality rates in states with different levels of abortion restrictions found that the rate of maternal mortality was almost two times higher in states that restricted abortion access compared with those that protected it – 28.5 per 100,000 women vs. 15.7.
Some women living in states with abortion bans won’t have the resources to cross state lines for care.
“This is just going to widen the health care disparities that are already so prevalent in this country,” Dr. Jain says.
Navigating a crossroads
Ms. DeSpain’s medical history reads like a checklist of pregnancy-related perils: chronic high blood pressure, persistent clotting problems, and a high risk of hemorrhage. She was also diagnosed with cervical cancer in 2020, which left her body more fragile.
Cardiovascular conditions, including hypertension and hemorrhage, are the leading causes of maternal mortality, responsible for more than one-third of pregnancy-related deaths. Preeclampsia, characterized by high blood pressure, accounts for more than 7% of maternal deaths in the United States. Although less common, genetic disorders, such as spinal muscular atrophy and triploidy, or cancer during pregnancy can put a mother and fetus at risk.
Cancer – which affects about 1 in 1,000 pregnant women and results in termination in as many as 28% of cases – brings sharp focus to the new dangers and complex decision-making patients and their doctors face as abortion bans take hold.
Before the Supreme Court decision, a pregnant woman with cancer was already facing great uncertainty. The decision to treat cancer during pregnancy involves “weighing the risk of exposing the fetus to medication vs. the risk to the mother’s untreated illness if you don’t expose the fetus to medication,” Elyce Cardonick, MD, an obstetrician at Cooper University Health Care, Camden, N.J., who specializes in high-risk pregnancies, told the National Cancer Institute.
Oncologists generally agree that it’s safe for pregnant women to receive chemotherapy during the second and third trimesters. But for women with aggressive cancers that are diagnosed in the first trimester, chemotherapy is dangerous. For women who need immunotherapy, the risks of treatment remain unclear.
In these cases, Alice S. Mims, MD, must broach the possibility of terminating the pregnancy.
“Cancer is a very urgent condition,” says Dr. Mims, a hematology specialist at the Ohio State University Comprehensive Cancer Center, Columbus, who sees patients who are pregnant. “These women may have other children at home, and they want to do their best to fight the disease so they can be around for their family long term.”
Now the changing legal landscape on abortion will put hundreds more pregnant women with cancer in danger. In a recent viewpoint article published in JAMA Oncology, Jordyn Silverstein and Katherine Van Loon, MD, MPH, estimate that during the next year, up to 420 pregnant women living in states with restricted abortion access will face threats to their cancer care and potentially their life.
“The repercussions of overturning Roe v. Wade – and the failure of the Supreme Court to provide any guidance on exceptions related to the life and health of the mother – are potentially catastrophic for a subset of women who face a life-threating diagnosis of [pregnancy-associated cancer],” they write.
The choice Ms. DeSpain faced after her cervical cancer diagnosis was different. She was not pregnant at the time, but she was at a crossroads.
Although it was caught early, the cancer was aggressive. Her oncologist recommended that she undergo a hysterectomy – the surgery that would give her the best chance for a cancer-free future. It would also mean she could no longer become pregnant.
With a less invasive procedure, on the other hand, she could still carry a child, but she would face a much greater chance that the cancer would come back.
At 27, Ms. DeSpain was not ready to close the pregnancy door. She opted for a surgery in which part of her cervix was removed, allowing her to try for another baby.
But she faced a ticking clock in the event her cancer returned.
If you want to have a baby, “try soon,” her doctor warned.
A dead end
After her cancer surgery and a third miscarriage, Ms. DeSpain and her husband were surprised and excited when in late 2021 she again became pregnant.
The first trimester seemed blissfully uneventful. As the weeks passed, Finley’s heart started to beat.
But the 16-week ultrasound signaled a turning point. The sonographer was too quiet.
“This is really bad, isn’t it?” Ms. DeSpain asked her sonographer.
The doctors told her he wouldn’t survive. Finley had no heart chambers. His heart couldn’t pump blood properly. He was missing one kidney, and his brain was split in the back. With almost no amniotic fluid, her doctor said he would likely die in utero, crushed to death without support from the protective liquid.
She fought for him anyway. She sought specialty care, followed bed rest orders, and traveled 3 hours to Houston to enroll in a clinical trial.
But every road was a dead end.
Ultimately, testing revealed Finley had triploidy, and all lines led to one point.
“There were too many things wrong, too much wrong for them to fix,” says Ms. DeSpain, recalling the news from her doctor in Houston. “I was in shock. My husband was just sitting with his hands flat on the table, staring at nothing, shaking a little bit.”
However, Finley still had a heartbeat, making an abortion after 6 weeks a felony in Texas. Even a compassionate induction was now out of the question unless her death was imminent.
Ms. DeSpain called the abortion clinic in Albuquerque and made an appointment. She would have to wait 2 weeks because of an influx of pregnant patients coming from Texas.
She welcomed the wait … just in case she changed her mind.
“At that point I wanted to carry him as far as I could,” she says.
For those 2 weeks, Ms. DeSpain remained on bed rest. She cried all day every day. She worried that Finley was experiencing pain.
Through this process, her doctor’s support helped keep her grounded.
“She cried with us in her office and said, ‘I wish that you didn’t have to go, but I think you’re doing the right thing, doing what keeps you safest,’ “ Ms. DeSpain recalls.
Ms. DeSpain declined to share the name of her doctor out of fear that even expressing compassion for a patient’s safety could put the physician in legal jeopardy and provoke harassment.
That fear is warranted. Some doctors will be forced to choose between doing what is legal – even though the law is vague – and doing what is right for patients, says law professor Jamie Abrams, who was recently diagnosed with breast cancer.
To live in a world where there’s talk of criminalizing doctors for taking care of their patients, where there’s “this national movement to position some women to be shunned and exiled for seeking care that’s right for them, their health, and might save their life is staggering and beyond comprehension,” says Ms. Abrams, professor of law at the American University Washington College of Law.
Ms. Abrams, who was diagnosed with hormone receptor–positive invasive breast cancer the same day she read the leaked Supreme Court draft on the decision to end of Roe v. Wade, said that “overnight, I became a person who would need an abortion if I became pregnant, because my treatment would compromise a healthy birth or delay necessary cancer care.” Ms. Abrams was also told she could no longer use hormonal contraception.
Dr. Harris’s advice to clinicians is to try to do what they feel is best for patients, including referring them to centers that have legal resources and protections regarding abortions.
Dr. Mims agrees and recommends that doctors reach out to those with more resources and legal backing for support. “I would advise doctors in [states with restrictive laws] to familiarize themselves with available resources and organizations taking action to deal with questionable cases,” Dr. Mims says.
‘Baby killers work here’
Following her 10-hour drive to Albuquerque, Ms. DeSpain encountered lines of protesters at the clinic. They were holding signs that said, “Abortion is murder,” and “Baby killers work here.”
“Please don’t kill your baby – we have resources for you,” a woman screeched through a megaphone as Ms. DeSpain, nearly 20 weeks’ pregnant, stepped out of the car to enter the clinic.
“I remember turning around, looking at her and making eye contact, and yelling back, ‘My baby has triploidy – he is dying! He is going to suffocate if I carry him full term. You don’t know what you’re talking about!’ “
A nurse held her hand during the procedure.
“He said, ‘You’re doing great, you’re okay,’ “ she recalls. She knew there was a chance that Finley’s face would be crushed by contractions during labor because of the lack of amniotic fluid, but she hoped not. Ms. DeSpain longed for a photo.
There was no photo to take home the next day, but Ms. DeSpain did receive Finley’s footprints, and his heartbeat – as captured by the specialty team in Houston – lives on in a stuffed giraffe.
His ashes arrived a few weeks later.
By then, the Supreme Court draft had been leaked. Ms. DeSpain knew her predicament in Texas would soon affect women across the United States and make any future pregnancy attempt for her even more risky.
The weeks and months that followed were a blur of grief, anger, and medical testing.
But she received some good news. A second triploidy pregnancy was extremely unlikely.
Several weeks later, Ms. DeSpain got more good news.
“I had a follow-up cancer appointment, and everything was completely clear,” she says.
She remains hopeful that she will be able to give birth, but her doctor cautioned that it’s no longer safe to become pregnant in Texas.
“I need you to understand that if you get pregnant and you have complications, we can’t intervene unless the baby doesn’t have a heartbeat, even if it would save your life,” Ms. DeSpain recalls her doctor saying.
If Texas remains a dangerous place to be pregnant, Ms. DeSpain and her husband will have to move.
For now, Ms. DeSpain wants people to know her story and to continue to fight for her right to govern her body.
In a public post to Facebook, she laid bare her pregnancy journey.
“No one should have to share a story like mine to justify abortion,” she wrote. “My choice is not yours to judge, and my rights are not yours to gleefully take away.”
Ms. Abrams, Ms. DeSpain, Dr. Harris, Dr. Jain, and Dr. Mims have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Incomplete recovery common 6 months after mild TBI
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Abrocitinib evaluated in patients with and without prior dupilumab treatment
an industry-sponsored study reports.
“In this post hoc analysis, both the efficacy and the safety profiles of abrocitinib were consistent in patients with moderate-to-severe atopic dermatitis, regardless of prior biologic therapy use,” lead author Melinda Gooderham, MD, medical director of the SKiN Centre for Dermatology, Peterborough, Ont., said during an oral presentation at the Society for Investigative Dermatology (SID) 2022 Annual Meeting.
“These results ... support the use of abrocitinib in patients who might have received biologic therapy prior,” she added.
“Prior biologic use did not reveal any new safety signals ... keeping in mind the key limitation of this analysis is that it was done post hoc,” she noted.
Guidelines for moderate-to-severe atopic dermatitis refractory to topical or systemic therapy include systemic immunosuppressants and dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 cytokine-induced responses, Dr. Gooderham said.
The Food and Drug Administration recently approved abrocitinib, an oral once-a-day Janus kinase 1 (JAK1) inhibitor, to treat the disease. The approval came with a boxed warning about increased risk for serious infections, mortality, malignancy, and lymphoproliferative disorders, major adverse cardiovascular events, thrombosis, and laboratory abnormalities.
Comparing the bio-experienced with the bio-naive
Dr. Gooderham and colleagues investigated whether patients who’d been treated with a biologic would respond to abrocitinib differently than patients who had not received prior biologic treatment.
Researchers pooled data from two phase 3 placebo-controlled trials of abrocitinib that led to approval and an earlier phase 2b study. They identified 67 patients previously treated with dupilumab and 867 patients who were bio-naive. They repeated their analysis using data from another phase 3 study of abrocitinib on 86 patients previously treated with dupilumab and 1,147 who were bio-naive. On average, the bio-experienced patients were in their mid-30s to early 40s, and the bio-naive group was several years younger.
In the pooled phase 2b and phase 3 JADE MONO-1 and JADE MONO-2 monotherapy trials, patients received once-daily abrocitinib 100 or 200 mg or placebo for 12 weeks. In the phase 3 JADE REGIMEN, which they analyzed separately, eligible patients were enrolled in a 12-week open-label run-in period during which they received an induction treatment of abrocitinib 200 mg once a day.
Researchers compared results of two assessments: the IGA (Investigator Global Assessment) and EASI-75 (Eczema Area and Severity Index, 75% or greater improvement from baseline).
- At week 12, IGA 0/1 dose-dependent response rates were similar in the pooled groups, regardless of whether they had received prior biologic therapy. With abrocitinib 200 mg, 43.5% of those with prior dupilumab therapy responded versus 41.4% of bio-naive patients; with abrocitinib 100 mg, 24.1% versus 26.7% responded. In JADE REGIMEN, corresponding response rates with abrocitinib 200 mg were 53.5% versus 66.9%, respectively.
- At week 12, EASI-75 responses were also comparable. In the pooled groups by dose, with abrocitinib 200 mg, EASI-75 response rates were 65.2% in patients with prior dupilumab therapy versus 62.4% in those without; at abrocitinib 100 mg, 34.5% versus 42.7% responded. Corresponding rates in JADE REGIMEN were 64.0% versus 76.4%, respectively.
- Treatment-emergent adverse event rates among patients with versus without prior biologic therapy were, respectively, 71.7% versus 69.9% (abrocitinib 200 mg + 100 mg groups) in the pooled population. Rates in JADE REGIMEN with abrocitinib 200 mg were, respectively, 66.3% versus 66.5%.
- Abrocitinib efficacy and safety were consistent in patients with moderate-to-severe atopic dermatitis, regardless of prior biologic therapy. Adverse events in the pooled monotherapy trials and in JADE REGIMEN included acne, atopic dermatitis, diarrhea, headache, nasopharyngitis, nausea, upper abdominal pain, and upper respiratory tract infection.
The authors acknowledge that the post hoc study design is a limitation and recommend confirming these findings in a large, long-term prospective study.
JAK inhibitors expand treatment options
The results will help doctors treat their patients, Jami L. Miller, MD, associate professor of dermatology and dermatology clinic medical director at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
“Because JAK inhibitors have potentially more side effects than inhibitors of interleukin-4 and interleukin-13, in clinical practice most dermatologists are more likely to treat patients first with dupilumab or similar meds and step up to a JAK inhibitor if they do not respond,” she added in an email.
“With more meds coming out to meet the needs of this population, this is an exciting time for patients with moderate-to-severe atopic dermatitis,” she commented.
Lindsay C. Strowd, MD, associate professor and vice chair of the department of dermatology at Wake Forest University, Winston-Salem, N.C., said JAK inhibitors are increasingly being studied and approved for use in various dermatologic diseases.
An oral JAK inhibitor (upadacitinib) is currently FDA approved for moderate-to-severe atopic dermatitis, and a topical JAK inhibitor (ruxolitinib) is also approved for use in atopic dermatitis, Dr. Strowd noted.
“The study results give providers important practical information,” added Dr. Strowd, who also was not involved with the study. “Those of us who care for patients with severe atopic dermatitis need to know how patients with prior biologic exposure will respond as newer agents come to market and the options for biologic use in atopic dermatitis continue to grow.”
The study was sponsored by Pfizer. All study authors have reported relevant financial relationships with, and several authors are employees of, Pfizer, the developer of abrocitinib. Dr. Strowd and Dr. Miller have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
an industry-sponsored study reports.
“In this post hoc analysis, both the efficacy and the safety profiles of abrocitinib were consistent in patients with moderate-to-severe atopic dermatitis, regardless of prior biologic therapy use,” lead author Melinda Gooderham, MD, medical director of the SKiN Centre for Dermatology, Peterborough, Ont., said during an oral presentation at the Society for Investigative Dermatology (SID) 2022 Annual Meeting.
“These results ... support the use of abrocitinib in patients who might have received biologic therapy prior,” she added.
“Prior biologic use did not reveal any new safety signals ... keeping in mind the key limitation of this analysis is that it was done post hoc,” she noted.
Guidelines for moderate-to-severe atopic dermatitis refractory to topical or systemic therapy include systemic immunosuppressants and dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 cytokine-induced responses, Dr. Gooderham said.
The Food and Drug Administration recently approved abrocitinib, an oral once-a-day Janus kinase 1 (JAK1) inhibitor, to treat the disease. The approval came with a boxed warning about increased risk for serious infections, mortality, malignancy, and lymphoproliferative disorders, major adverse cardiovascular events, thrombosis, and laboratory abnormalities.
Comparing the bio-experienced with the bio-naive
Dr. Gooderham and colleagues investigated whether patients who’d been treated with a biologic would respond to abrocitinib differently than patients who had not received prior biologic treatment.
Researchers pooled data from two phase 3 placebo-controlled trials of abrocitinib that led to approval and an earlier phase 2b study. They identified 67 patients previously treated with dupilumab and 867 patients who were bio-naive. They repeated their analysis using data from another phase 3 study of abrocitinib on 86 patients previously treated with dupilumab and 1,147 who were bio-naive. On average, the bio-experienced patients were in their mid-30s to early 40s, and the bio-naive group was several years younger.
In the pooled phase 2b and phase 3 JADE MONO-1 and JADE MONO-2 monotherapy trials, patients received once-daily abrocitinib 100 or 200 mg or placebo for 12 weeks. In the phase 3 JADE REGIMEN, which they analyzed separately, eligible patients were enrolled in a 12-week open-label run-in period during which they received an induction treatment of abrocitinib 200 mg once a day.
Researchers compared results of two assessments: the IGA (Investigator Global Assessment) and EASI-75 (Eczema Area and Severity Index, 75% or greater improvement from baseline).
- At week 12, IGA 0/1 dose-dependent response rates were similar in the pooled groups, regardless of whether they had received prior biologic therapy. With abrocitinib 200 mg, 43.5% of those with prior dupilumab therapy responded versus 41.4% of bio-naive patients; with abrocitinib 100 mg, 24.1% versus 26.7% responded. In JADE REGIMEN, corresponding response rates with abrocitinib 200 mg were 53.5% versus 66.9%, respectively.
- At week 12, EASI-75 responses were also comparable. In the pooled groups by dose, with abrocitinib 200 mg, EASI-75 response rates were 65.2% in patients with prior dupilumab therapy versus 62.4% in those without; at abrocitinib 100 mg, 34.5% versus 42.7% responded. Corresponding rates in JADE REGIMEN were 64.0% versus 76.4%, respectively.
- Treatment-emergent adverse event rates among patients with versus without prior biologic therapy were, respectively, 71.7% versus 69.9% (abrocitinib 200 mg + 100 mg groups) in the pooled population. Rates in JADE REGIMEN with abrocitinib 200 mg were, respectively, 66.3% versus 66.5%.
- Abrocitinib efficacy and safety were consistent in patients with moderate-to-severe atopic dermatitis, regardless of prior biologic therapy. Adverse events in the pooled monotherapy trials and in JADE REGIMEN included acne, atopic dermatitis, diarrhea, headache, nasopharyngitis, nausea, upper abdominal pain, and upper respiratory tract infection.
The authors acknowledge that the post hoc study design is a limitation and recommend confirming these findings in a large, long-term prospective study.
JAK inhibitors expand treatment options
The results will help doctors treat their patients, Jami L. Miller, MD, associate professor of dermatology and dermatology clinic medical director at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
“Because JAK inhibitors have potentially more side effects than inhibitors of interleukin-4 and interleukin-13, in clinical practice most dermatologists are more likely to treat patients first with dupilumab or similar meds and step up to a JAK inhibitor if they do not respond,” she added in an email.
“With more meds coming out to meet the needs of this population, this is an exciting time for patients with moderate-to-severe atopic dermatitis,” she commented.
Lindsay C. Strowd, MD, associate professor and vice chair of the department of dermatology at Wake Forest University, Winston-Salem, N.C., said JAK inhibitors are increasingly being studied and approved for use in various dermatologic diseases.
An oral JAK inhibitor (upadacitinib) is currently FDA approved for moderate-to-severe atopic dermatitis, and a topical JAK inhibitor (ruxolitinib) is also approved for use in atopic dermatitis, Dr. Strowd noted.
“The study results give providers important practical information,” added Dr. Strowd, who also was not involved with the study. “Those of us who care for patients with severe atopic dermatitis need to know how patients with prior biologic exposure will respond as newer agents come to market and the options for biologic use in atopic dermatitis continue to grow.”
The study was sponsored by Pfizer. All study authors have reported relevant financial relationships with, and several authors are employees of, Pfizer, the developer of abrocitinib. Dr. Strowd and Dr. Miller have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
an industry-sponsored study reports.
“In this post hoc analysis, both the efficacy and the safety profiles of abrocitinib were consistent in patients with moderate-to-severe atopic dermatitis, regardless of prior biologic therapy use,” lead author Melinda Gooderham, MD, medical director of the SKiN Centre for Dermatology, Peterborough, Ont., said during an oral presentation at the Society for Investigative Dermatology (SID) 2022 Annual Meeting.
“These results ... support the use of abrocitinib in patients who might have received biologic therapy prior,” she added.
“Prior biologic use did not reveal any new safety signals ... keeping in mind the key limitation of this analysis is that it was done post hoc,” she noted.
Guidelines for moderate-to-severe atopic dermatitis refractory to topical or systemic therapy include systemic immunosuppressants and dupilumab, a monoclonal antibody that inhibits interleukin-4 and interleukin-13 cytokine-induced responses, Dr. Gooderham said.
The Food and Drug Administration recently approved abrocitinib, an oral once-a-day Janus kinase 1 (JAK1) inhibitor, to treat the disease. The approval came with a boxed warning about increased risk for serious infections, mortality, malignancy, and lymphoproliferative disorders, major adverse cardiovascular events, thrombosis, and laboratory abnormalities.
Comparing the bio-experienced with the bio-naive
Dr. Gooderham and colleagues investigated whether patients who’d been treated with a biologic would respond to abrocitinib differently than patients who had not received prior biologic treatment.
Researchers pooled data from two phase 3 placebo-controlled trials of abrocitinib that led to approval and an earlier phase 2b study. They identified 67 patients previously treated with dupilumab and 867 patients who were bio-naive. They repeated their analysis using data from another phase 3 study of abrocitinib on 86 patients previously treated with dupilumab and 1,147 who were bio-naive. On average, the bio-experienced patients were in their mid-30s to early 40s, and the bio-naive group was several years younger.
In the pooled phase 2b and phase 3 JADE MONO-1 and JADE MONO-2 monotherapy trials, patients received once-daily abrocitinib 100 or 200 mg or placebo for 12 weeks. In the phase 3 JADE REGIMEN, which they analyzed separately, eligible patients were enrolled in a 12-week open-label run-in period during which they received an induction treatment of abrocitinib 200 mg once a day.
Researchers compared results of two assessments: the IGA (Investigator Global Assessment) and EASI-75 (Eczema Area and Severity Index, 75% or greater improvement from baseline).
- At week 12, IGA 0/1 dose-dependent response rates were similar in the pooled groups, regardless of whether they had received prior biologic therapy. With abrocitinib 200 mg, 43.5% of those with prior dupilumab therapy responded versus 41.4% of bio-naive patients; with abrocitinib 100 mg, 24.1% versus 26.7% responded. In JADE REGIMEN, corresponding response rates with abrocitinib 200 mg were 53.5% versus 66.9%, respectively.
- At week 12, EASI-75 responses were also comparable. In the pooled groups by dose, with abrocitinib 200 mg, EASI-75 response rates were 65.2% in patients with prior dupilumab therapy versus 62.4% in those without; at abrocitinib 100 mg, 34.5% versus 42.7% responded. Corresponding rates in JADE REGIMEN were 64.0% versus 76.4%, respectively.
- Treatment-emergent adverse event rates among patients with versus without prior biologic therapy were, respectively, 71.7% versus 69.9% (abrocitinib 200 mg + 100 mg groups) in the pooled population. Rates in JADE REGIMEN with abrocitinib 200 mg were, respectively, 66.3% versus 66.5%.
- Abrocitinib efficacy and safety were consistent in patients with moderate-to-severe atopic dermatitis, regardless of prior biologic therapy. Adverse events in the pooled monotherapy trials and in JADE REGIMEN included acne, atopic dermatitis, diarrhea, headache, nasopharyngitis, nausea, upper abdominal pain, and upper respiratory tract infection.
The authors acknowledge that the post hoc study design is a limitation and recommend confirming these findings in a large, long-term prospective study.
JAK inhibitors expand treatment options
The results will help doctors treat their patients, Jami L. Miller, MD, associate professor of dermatology and dermatology clinic medical director at Vanderbilt University Medical Center, Nashville, Tenn., told this news organization.
“Because JAK inhibitors have potentially more side effects than inhibitors of interleukin-4 and interleukin-13, in clinical practice most dermatologists are more likely to treat patients first with dupilumab or similar meds and step up to a JAK inhibitor if they do not respond,” she added in an email.
“With more meds coming out to meet the needs of this population, this is an exciting time for patients with moderate-to-severe atopic dermatitis,” she commented.
Lindsay C. Strowd, MD, associate professor and vice chair of the department of dermatology at Wake Forest University, Winston-Salem, N.C., said JAK inhibitors are increasingly being studied and approved for use in various dermatologic diseases.
An oral JAK inhibitor (upadacitinib) is currently FDA approved for moderate-to-severe atopic dermatitis, and a topical JAK inhibitor (ruxolitinib) is also approved for use in atopic dermatitis, Dr. Strowd noted.
“The study results give providers important practical information,” added Dr. Strowd, who also was not involved with the study. “Those of us who care for patients with severe atopic dermatitis need to know how patients with prior biologic exposure will respond as newer agents come to market and the options for biologic use in atopic dermatitis continue to grow.”
The study was sponsored by Pfizer. All study authors have reported relevant financial relationships with, and several authors are employees of, Pfizer, the developer of abrocitinib. Dr. Strowd and Dr. Miller have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Commentary: Psychiatric Comorbidity, the Microbiome, and IBS, September 2022
Fecal and mucosal microbiota have been the focus of much research. A study by Hou and colleagues that showed distinct differences in the intestinal and fecal microbiomes of patients with constipation-predominant or diarrhea-predominant IBS, compared with healthy controls, highlights the importance of a balanced and diverse microbiome to maintain a healthy gut. The article notes specific genera of microbiota that were associated with intestinal pain. When microbiota diversity is limited, the incidence of IBS is increased. This reinforces the importance of promoting a healthy microbiome in all patients and appreciating the correlation of the microbiome with the development of IBS.
Internet-based cognitive-behavioral therapy may make it easier for patients to obtain therapy services. Kim and colleagues have shown this to be a cost-effective and efficacious way to deliver care. This care improves the quality of life for patients with IBS and is an effective intervention that is readily available. During pandemic times, the forum of online care also provides a safe way to deliver therapy services without the burden of the patient needing to commute to clinic and potentially be exposed to infectious disease. Given the relationship between IBS and psychiatric diagnoses noted by Creed and colleagues, it seems important to extend the availability of therapeutic interventions to as many patients with IBS as possible.
Fecal and mucosal microbiota have been the focus of much research. A study by Hou and colleagues that showed distinct differences in the intestinal and fecal microbiomes of patients with constipation-predominant or diarrhea-predominant IBS, compared with healthy controls, highlights the importance of a balanced and diverse microbiome to maintain a healthy gut. The article notes specific genera of microbiota that were associated with intestinal pain. When microbiota diversity is limited, the incidence of IBS is increased. This reinforces the importance of promoting a healthy microbiome in all patients and appreciating the correlation of the microbiome with the development of IBS.
Internet-based cognitive-behavioral therapy may make it easier for patients to obtain therapy services. Kim and colleagues have shown this to be a cost-effective and efficacious way to deliver care. This care improves the quality of life for patients with IBS and is an effective intervention that is readily available. During pandemic times, the forum of online care also provides a safe way to deliver therapy services without the burden of the patient needing to commute to clinic and potentially be exposed to infectious disease. Given the relationship between IBS and psychiatric diagnoses noted by Creed and colleagues, it seems important to extend the availability of therapeutic interventions to as many patients with IBS as possible.
Fecal and mucosal microbiota have been the focus of much research. A study by Hou and colleagues that showed distinct differences in the intestinal and fecal microbiomes of patients with constipation-predominant or diarrhea-predominant IBS, compared with healthy controls, highlights the importance of a balanced and diverse microbiome to maintain a healthy gut. The article notes specific genera of microbiota that were associated with intestinal pain. When microbiota diversity is limited, the incidence of IBS is increased. This reinforces the importance of promoting a healthy microbiome in all patients and appreciating the correlation of the microbiome with the development of IBS.
Internet-based cognitive-behavioral therapy may make it easier for patients to obtain therapy services. Kim and colleagues have shown this to be a cost-effective and efficacious way to deliver care. This care improves the quality of life for patients with IBS and is an effective intervention that is readily available. During pandemic times, the forum of online care also provides a safe way to deliver therapy services without the burden of the patient needing to commute to clinic and potentially be exposed to infectious disease. Given the relationship between IBS and psychiatric diagnoses noted by Creed and colleagues, it seems important to extend the availability of therapeutic interventions to as many patients with IBS as possible.
How does not getting enough sleep affect the developing brain?
Children who do not get enough sleep for one night can be cranky, groggy, or meltdown prone the next day.
Over time, though, insufficient sleep may impair neurodevelopment in ways that can be measured on brain scans and tests long term, a new study shows.
Research published in The Lancet Child & Adolescent Health found that 9- and 10-year-olds who do not get at least 9 hours of sleep most nights tend to have less gray matter and smaller areas of the brain responsible for attention, memory, and inhibition control, relative to children who do get enough sleep.
The researchers also found a relationship between insufficient sleep and disrupted connections between the basal ganglia and cortical regions of the brain. These disruptions appeared to be linked to depression, thought problems, and impairments in crystallized intelligence, a type of intelligence that depends on memory.
The overall patterns persisted 2 years later, even as those who got enough sleep at baseline gradually slept less over time, while those who were not getting enough sleep to begin with continued to sleep about the same amount, the researchers reported.
The results bolster the case for delaying school start times, as California recently did, according one researcher who was not involved in the study.
The ABCD Study
To examine how insufficient sleep affects children’s mental health, cognition, brain function, and brain structure over 2 years, Ze Wang, PhD, professor of diagnostic radiology and nuclear medicine at the University of Maryland, Baltimore, and colleagues analyzed data from the ongoing Adolescent Brain Cognitive Development (ABCD) Study. The ABCD Study is tracking the biologic and behavioral development of more than 11,000 children in the United States who were recruited for the study when they were 9 or 10 years old.
For their new analysis, Dr. Wang’s group focused on 6,042 participants: 3,021 children with insufficient sleep who were matched with an equal number of participants who were similar in many respects, including sex, socioeconomic status, and puberty status, except they got at least 9 hours of sleep. They also looked at outcomes 2 years later from 749 of the matched pairs who had results available.
The investigators determined sleep duration based on how parents answered the question: “How many hours of sleep does your child get on most nights in the past 6 months?” Possible answers included at least 9 hours, 8-9 hours, 7-8 hours, 5-7 hours, or less than 5 hours. They also looked at functional and structural MRI scans, test results, and responses to questionnaires.
Negative effects of inadequate sleep were spread over “several different domains including brain structure, function, cognition, behavior, and mental health,” Dr. Wang said.
The strength of the relationship between sleep duration and the various outcomes was “modest” and based on group averages, he said. So, a given child who does not sleep for 9 hours most nights won’t necessarily perform worse than a child who gets enough sleep.
Still, modest effects may accumulate and have lasting consequences, Dr. Wang said.
Crystallized intelligence
The researchers looked at 42 behavioral outcomes, 32 of which were significantly different between the groups. Four outcomes in particular – depression, thought problems, performance on a picture-vocabulary test, and crystallized intelligence – were areas where insufficient sleep seemed to have a larger negative effect.
Sleep duration’s relationship with crystallized intelligence was twice that for fluid intelligence, which does not depend on memory.
“Sleep affects memory,” Dr. Wang said. “Crystallized intelligence depends on learned skills and knowledge, which are memory. In this sense, sleep is related to crystallized intelligence.”
One limitation of the study is that some parents may not accurately report how much sleep their child gets, Dr. Wang acknowledged. Children may be awake when parents think they are asleep, for example.
And although the results show getting 9 hours of sleep may help neurocognitive development, it’s also possible that excessive amounts of sleep could be problematic, the study authors wrote.
Further experiments are needed to prove that insufficient sleep – and not some other, unaccounted for factor – causes the observed impairments in neurodevelopment.
To promote healthy sleep, parents should keep a strict routine for their children, such as a regular bedtime and no electronic devices in the bedroom, Dr. Wang suggested. More physical activity during the day also should help.
If children have high levels of stress and depression, “finding the source is critical,” he said. Likewise, clinicians should consider how mental health can affect their patients’ sleep.
More to healthy sleep than duration
“This study both aligns with and advances existing research on the importance of sufficient sleep for child well-being,” said Ariel A. Williamson, PhD, DBSM, a psychologist and pediatric sleep expert in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia and assistant professor of psychiatry and pediatrics at University of Pennsylvania, also in Philadelphia.
The researchers used rigorous propensity score matching, longitudinal data, and brain imaging, which are “innovative methods that provide more evidence on potential mechanisms linking insufficient sleep and child outcomes,” said Dr. Williamson, who was not involved in the study.
While the investigators focused on sleep duration, child sleep health is multidimensional and includes other elements like timing and perception of sleep quality, Dr. Williamson noted. “For example, some research shows that having a sleep schedule that varies night to night is linked to poor child outcomes.”
Dr. Williamson tells families and clinicians that “sleep is a pillar of health,” equal to diet and exercise. That said, sleep recommendations need to fit within a family’s life – taking into account after school activities and late-night homework sessions. But extending sleep by just “20-30 minutes can make a meaningful difference for daytime functioning,” Dr. Williamson said.
Start school later?
Researchers have only relatively recently begun to understand how insufficient sleep affects adolescent neurocognitive development long term, and this study provides “crucial evidence” about the consequences, Lydia Gabriela Speyer, PhD, said in an editorial published with the study. Dr. Speyer is affiliated with the department of psychology at the University of Cambridge (England).
“Given the novel finding that insufficient sleep is associated with changes in brain structure and connectivity that are long-lasting, early intervention is crucial because such neural changes are probably not reversible and might consequently affect adolescents’ development into adulthood,” Dr. Speyer wrote.
Delaying school start times could be one way to help kids get more sleep. The American Academy of Pediatrics and the American Academy of Sleep Medicine recommend that middle schools and high schools start no earlier than 8:30 a.m. to better align with students’ circadian rhythm, Dr. Speyer noted.
As it is in the United States, most schools start closer to 8 a.m. In California, though, a law that went into effect on July 1 prohibits high schools from starting before 8:30 a.m. Other states are weighing similar legislation.
The research was supported by the National Institutes of Health. Dr. Wang and his coauthors and Dr. Speyer had no conflict of interest disclosures. Dr. Williamson is a sleep expert for the Pediatric Sleep Council (www.babysleep.com), which provides free information about early childhood sleep, but she does not receive compensation for this role.
Children who do not get enough sleep for one night can be cranky, groggy, or meltdown prone the next day.
Over time, though, insufficient sleep may impair neurodevelopment in ways that can be measured on brain scans and tests long term, a new study shows.
Research published in The Lancet Child & Adolescent Health found that 9- and 10-year-olds who do not get at least 9 hours of sleep most nights tend to have less gray matter and smaller areas of the brain responsible for attention, memory, and inhibition control, relative to children who do get enough sleep.
The researchers also found a relationship between insufficient sleep and disrupted connections between the basal ganglia and cortical regions of the brain. These disruptions appeared to be linked to depression, thought problems, and impairments in crystallized intelligence, a type of intelligence that depends on memory.
The overall patterns persisted 2 years later, even as those who got enough sleep at baseline gradually slept less over time, while those who were not getting enough sleep to begin with continued to sleep about the same amount, the researchers reported.
The results bolster the case for delaying school start times, as California recently did, according one researcher who was not involved in the study.
The ABCD Study
To examine how insufficient sleep affects children’s mental health, cognition, brain function, and brain structure over 2 years, Ze Wang, PhD, professor of diagnostic radiology and nuclear medicine at the University of Maryland, Baltimore, and colleagues analyzed data from the ongoing Adolescent Brain Cognitive Development (ABCD) Study. The ABCD Study is tracking the biologic and behavioral development of more than 11,000 children in the United States who were recruited for the study when they were 9 or 10 years old.
For their new analysis, Dr. Wang’s group focused on 6,042 participants: 3,021 children with insufficient sleep who were matched with an equal number of participants who were similar in many respects, including sex, socioeconomic status, and puberty status, except they got at least 9 hours of sleep. They also looked at outcomes 2 years later from 749 of the matched pairs who had results available.
The investigators determined sleep duration based on how parents answered the question: “How many hours of sleep does your child get on most nights in the past 6 months?” Possible answers included at least 9 hours, 8-9 hours, 7-8 hours, 5-7 hours, or less than 5 hours. They also looked at functional and structural MRI scans, test results, and responses to questionnaires.
Negative effects of inadequate sleep were spread over “several different domains including brain structure, function, cognition, behavior, and mental health,” Dr. Wang said.
The strength of the relationship between sleep duration and the various outcomes was “modest” and based on group averages, he said. So, a given child who does not sleep for 9 hours most nights won’t necessarily perform worse than a child who gets enough sleep.
Still, modest effects may accumulate and have lasting consequences, Dr. Wang said.
Crystallized intelligence
The researchers looked at 42 behavioral outcomes, 32 of which were significantly different between the groups. Four outcomes in particular – depression, thought problems, performance on a picture-vocabulary test, and crystallized intelligence – were areas where insufficient sleep seemed to have a larger negative effect.
Sleep duration’s relationship with crystallized intelligence was twice that for fluid intelligence, which does not depend on memory.
“Sleep affects memory,” Dr. Wang said. “Crystallized intelligence depends on learned skills and knowledge, which are memory. In this sense, sleep is related to crystallized intelligence.”
One limitation of the study is that some parents may not accurately report how much sleep their child gets, Dr. Wang acknowledged. Children may be awake when parents think they are asleep, for example.
And although the results show getting 9 hours of sleep may help neurocognitive development, it’s also possible that excessive amounts of sleep could be problematic, the study authors wrote.
Further experiments are needed to prove that insufficient sleep – and not some other, unaccounted for factor – causes the observed impairments in neurodevelopment.
To promote healthy sleep, parents should keep a strict routine for their children, such as a regular bedtime and no electronic devices in the bedroom, Dr. Wang suggested. More physical activity during the day also should help.
If children have high levels of stress and depression, “finding the source is critical,” he said. Likewise, clinicians should consider how mental health can affect their patients’ sleep.
More to healthy sleep than duration
“This study both aligns with and advances existing research on the importance of sufficient sleep for child well-being,” said Ariel A. Williamson, PhD, DBSM, a psychologist and pediatric sleep expert in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia and assistant professor of psychiatry and pediatrics at University of Pennsylvania, also in Philadelphia.
The researchers used rigorous propensity score matching, longitudinal data, and brain imaging, which are “innovative methods that provide more evidence on potential mechanisms linking insufficient sleep and child outcomes,” said Dr. Williamson, who was not involved in the study.
While the investigators focused on sleep duration, child sleep health is multidimensional and includes other elements like timing and perception of sleep quality, Dr. Williamson noted. “For example, some research shows that having a sleep schedule that varies night to night is linked to poor child outcomes.”
Dr. Williamson tells families and clinicians that “sleep is a pillar of health,” equal to diet and exercise. That said, sleep recommendations need to fit within a family’s life – taking into account after school activities and late-night homework sessions. But extending sleep by just “20-30 minutes can make a meaningful difference for daytime functioning,” Dr. Williamson said.
Start school later?
Researchers have only relatively recently begun to understand how insufficient sleep affects adolescent neurocognitive development long term, and this study provides “crucial evidence” about the consequences, Lydia Gabriela Speyer, PhD, said in an editorial published with the study. Dr. Speyer is affiliated with the department of psychology at the University of Cambridge (England).
“Given the novel finding that insufficient sleep is associated with changes in brain structure and connectivity that are long-lasting, early intervention is crucial because such neural changes are probably not reversible and might consequently affect adolescents’ development into adulthood,” Dr. Speyer wrote.
Delaying school start times could be one way to help kids get more sleep. The American Academy of Pediatrics and the American Academy of Sleep Medicine recommend that middle schools and high schools start no earlier than 8:30 a.m. to better align with students’ circadian rhythm, Dr. Speyer noted.
As it is in the United States, most schools start closer to 8 a.m. In California, though, a law that went into effect on July 1 prohibits high schools from starting before 8:30 a.m. Other states are weighing similar legislation.
The research was supported by the National Institutes of Health. Dr. Wang and his coauthors and Dr. Speyer had no conflict of interest disclosures. Dr. Williamson is a sleep expert for the Pediatric Sleep Council (www.babysleep.com), which provides free information about early childhood sleep, but she does not receive compensation for this role.
Children who do not get enough sleep for one night can be cranky, groggy, or meltdown prone the next day.
Over time, though, insufficient sleep may impair neurodevelopment in ways that can be measured on brain scans and tests long term, a new study shows.
Research published in The Lancet Child & Adolescent Health found that 9- and 10-year-olds who do not get at least 9 hours of sleep most nights tend to have less gray matter and smaller areas of the brain responsible for attention, memory, and inhibition control, relative to children who do get enough sleep.
The researchers also found a relationship between insufficient sleep and disrupted connections between the basal ganglia and cortical regions of the brain. These disruptions appeared to be linked to depression, thought problems, and impairments in crystallized intelligence, a type of intelligence that depends on memory.
The overall patterns persisted 2 years later, even as those who got enough sleep at baseline gradually slept less over time, while those who were not getting enough sleep to begin with continued to sleep about the same amount, the researchers reported.
The results bolster the case for delaying school start times, as California recently did, according one researcher who was not involved in the study.
The ABCD Study
To examine how insufficient sleep affects children’s mental health, cognition, brain function, and brain structure over 2 years, Ze Wang, PhD, professor of diagnostic radiology and nuclear medicine at the University of Maryland, Baltimore, and colleagues analyzed data from the ongoing Adolescent Brain Cognitive Development (ABCD) Study. The ABCD Study is tracking the biologic and behavioral development of more than 11,000 children in the United States who were recruited for the study when they were 9 or 10 years old.
For their new analysis, Dr. Wang’s group focused on 6,042 participants: 3,021 children with insufficient sleep who were matched with an equal number of participants who were similar in many respects, including sex, socioeconomic status, and puberty status, except they got at least 9 hours of sleep. They also looked at outcomes 2 years later from 749 of the matched pairs who had results available.
The investigators determined sleep duration based on how parents answered the question: “How many hours of sleep does your child get on most nights in the past 6 months?” Possible answers included at least 9 hours, 8-9 hours, 7-8 hours, 5-7 hours, or less than 5 hours. They also looked at functional and structural MRI scans, test results, and responses to questionnaires.
Negative effects of inadequate sleep were spread over “several different domains including brain structure, function, cognition, behavior, and mental health,” Dr. Wang said.
The strength of the relationship between sleep duration and the various outcomes was “modest” and based on group averages, he said. So, a given child who does not sleep for 9 hours most nights won’t necessarily perform worse than a child who gets enough sleep.
Still, modest effects may accumulate and have lasting consequences, Dr. Wang said.
Crystallized intelligence
The researchers looked at 42 behavioral outcomes, 32 of which were significantly different between the groups. Four outcomes in particular – depression, thought problems, performance on a picture-vocabulary test, and crystallized intelligence – were areas where insufficient sleep seemed to have a larger negative effect.
Sleep duration’s relationship with crystallized intelligence was twice that for fluid intelligence, which does not depend on memory.
“Sleep affects memory,” Dr. Wang said. “Crystallized intelligence depends on learned skills and knowledge, which are memory. In this sense, sleep is related to crystallized intelligence.”
One limitation of the study is that some parents may not accurately report how much sleep their child gets, Dr. Wang acknowledged. Children may be awake when parents think they are asleep, for example.
And although the results show getting 9 hours of sleep may help neurocognitive development, it’s also possible that excessive amounts of sleep could be problematic, the study authors wrote.
Further experiments are needed to prove that insufficient sleep – and not some other, unaccounted for factor – causes the observed impairments in neurodevelopment.
To promote healthy sleep, parents should keep a strict routine for their children, such as a regular bedtime and no electronic devices in the bedroom, Dr. Wang suggested. More physical activity during the day also should help.
If children have high levels of stress and depression, “finding the source is critical,” he said. Likewise, clinicians should consider how mental health can affect their patients’ sleep.
More to healthy sleep than duration
“This study both aligns with and advances existing research on the importance of sufficient sleep for child well-being,” said Ariel A. Williamson, PhD, DBSM, a psychologist and pediatric sleep expert in the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia and assistant professor of psychiatry and pediatrics at University of Pennsylvania, also in Philadelphia.
The researchers used rigorous propensity score matching, longitudinal data, and brain imaging, which are “innovative methods that provide more evidence on potential mechanisms linking insufficient sleep and child outcomes,” said Dr. Williamson, who was not involved in the study.
While the investigators focused on sleep duration, child sleep health is multidimensional and includes other elements like timing and perception of sleep quality, Dr. Williamson noted. “For example, some research shows that having a sleep schedule that varies night to night is linked to poor child outcomes.”
Dr. Williamson tells families and clinicians that “sleep is a pillar of health,” equal to diet and exercise. That said, sleep recommendations need to fit within a family’s life – taking into account after school activities and late-night homework sessions. But extending sleep by just “20-30 minutes can make a meaningful difference for daytime functioning,” Dr. Williamson said.
Start school later?
Researchers have only relatively recently begun to understand how insufficient sleep affects adolescent neurocognitive development long term, and this study provides “crucial evidence” about the consequences, Lydia Gabriela Speyer, PhD, said in an editorial published with the study. Dr. Speyer is affiliated with the department of psychology at the University of Cambridge (England).
“Given the novel finding that insufficient sleep is associated with changes in brain structure and connectivity that are long-lasting, early intervention is crucial because such neural changes are probably not reversible and might consequently affect adolescents’ development into adulthood,” Dr. Speyer wrote.
Delaying school start times could be one way to help kids get more sleep. The American Academy of Pediatrics and the American Academy of Sleep Medicine recommend that middle schools and high schools start no earlier than 8:30 a.m. to better align with students’ circadian rhythm, Dr. Speyer noted.
As it is in the United States, most schools start closer to 8 a.m. In California, though, a law that went into effect on July 1 prohibits high schools from starting before 8:30 a.m. Other states are weighing similar legislation.
The research was supported by the National Institutes of Health. Dr. Wang and his coauthors and Dr. Speyer had no conflict of interest disclosures. Dr. Williamson is a sleep expert for the Pediatric Sleep Council (www.babysleep.com), which provides free information about early childhood sleep, but she does not receive compensation for this role.
FROM THE LANCET CHILD & ADOLESCENT HEALTH
Adult ADHD improved by home-based, noninvasive brain stimulation
Results from the sham-controlled trial also showed that the tDCS treatment was both safe and well tolerated.
Overall, the findings suggest that the device could be a nondrug alternative for treating this patient population, Douglas Teixeira Leffa, MD, PhD, department of psychiatry, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and colleagues note.
“This is particularly relevant since a vast body of literature describes low long-term adherence rates and persistence to pharmacological treatment in patients with ADHD,” they write.
The findings were published online in JAMA Psychiatry.
Avoiding office visits
A noninvasive technique that is easy to use and relatively inexpensive, tDCS involves applying a low-intensity current over the scalp to modulate cortical excitability and induce neuroplasticity. Home-use tDCS devices, which avoid the need for daily office visits for stimulation sessions, have been validated in previous clinical samples.
The current study included 64 adults with ADHD who are not taking stimulants. They had moderate or severe symptoms of inattention, with an inattention score of 21 or higher on the clinician-administered Adult ADHD Self-Report Scale version 1.1 (CASRS).
The CASRS includes nine questions related to inattention symptoms (CASRS-I) and nine related to hyperactivity-impulsivity symptoms (CASRS-HI). The score can vary from 0 to 36 for each domain, with higher scores indicating increased symptoms.
Researchers randomly assigned participants to receive either active or sham stimulation.
The tDCS device used in the study delivered a current with 35-cm2 electrodes (7 cm by 5 cm). The anodal and cathodal electrodes were positioned corresponding to the right and left dorsolateral prefrontal cortex (DLPFC), respectively.
The investigators note that decreased activation in the right DLPFC has been reported before in patients with ADHD during tasks that require attention.
After learning to use the device, participants underwent 30-minute daily sessions of tDCS (2-mA direct constant current) for 4 weeks for a total of 28 sessions.
Devices programmed for sham treatment delivered a 30-second ramp-up (0-2 mA) stimulation followed by a 30-second ramp-down (2-0 mA) at the beginning, middle, and end of the application. This mimicked the tactile sensations reported with tDCS and has been shown to be a reliable sham protocol.
Participants were encouraged to perform the stimulation sessions at the same time of day. To improve adherence, they received daily text message reminders.
Nine patients discontinued treatment, two in the sham group and seven in the active group. However, patients who finished the trial completed a mean 25 of 28 sessions.
Window of opportunity?
The mean inattention score on CASRS-I at week 4, the primary outcome, was 18.88 in the active tDCS group vs. 23.63 in the sham tDCS group. There was a statistically significant treatment by time interaction for CASRS-I (beta interaction, –3.18; 95% confidence interval, –4.60 to –1.75; P < .001), showing decreased inattention symptoms in the active vs. sham groups.
The estimated Cohen’s d was 1.23 (95% CI, .67-1.78), indicating at least a moderate effect. This effect was similar to that reported with trigeminal nerve stimulation (TNS), the first approved device-based therapy for ADHD, and to that of atomoxetine, the second-line treatment for ADHD, the researchers note.
About one-third of patients (34.3%) in the active tDCS group achieved a 30% reduction in CASRS-I score, compared with 6.2% in the sham tDCS group.
There was no statistically significant difference in the secondary outcome of hyperactivity-impulsivity symptoms evaluated with the CASRS-HI. This may be because hyperactivity-impulsivity in ADHD is associated with a hypoactivation in the right inferior frontal cortex rather than the right DLPFC, the investigators write.
There were also no significant group differences in other secondary outcomes, including depression, anxiety, and executive function.
Adverse events (AE) were mostly mild and included skin redness and scalp burn. There were no severe or serious AEs.
Using a home-based tDCS device allows for considerably more sessions, with 28 being the highest number so far applied to patients with ADHD. This, the researchers note, is important because evidence suggests increased efficacy of tDCS with extended periods of treatment.
The home-based device “opens a new window of opportunity, especially for participants who live in geographically remote areas or have physical or cognitive disabilities that may hinder access to clinical centers,” they write.
Although a study limitation was the relatively high dropout rate in the active group, which might bias interpretation of the findings, only two of seven dropouts in the active group left because of an AE, the investigators note.
Patients received training in using the device, but there was no remote monitoring of sessions. In addition, the study population, which was relatively homogeneous with participants having no moderate to severe symptoms of depression or anxiety, differed from the usual patients with ADHD who are treated in clinical centers, the researchers point out.
As well, the study included only patients not taking pharmacologic treatment for ADHD – so the findings might not be generalizable to other patients, they add.
‘Just a first step’
Commenting on the study, Mark George, MD, distinguished professor of psychiatry, radiology, and neurology, Medical University of South Carolina, Charleston, noted that although this was a single-center study with a relatively small sample size, it is still important.
Showing it is possible to do high-quality tDCS studies at home “is a huge advance,” said Dr. George, who was not involved with the research.
“Home treatment is cheaper and easier for patients and allows many people to get treatment who would not be able to make it to the clinic daily for treatment,” he added.
He noted the study showed “a clear improvement in ADHD,” which is important because better treatments are needed.
However, he cautioned that this is “just a first step” and more studies are needed. For example, he said, it is not clear whether improvements persist and if patients need to self-treat forever, as they would with a medication.
Dr. George also noted that although the study used “a pioneering research device” with several safety features, many home-based tDCS devices on the market do not have those.
“I don’t advise patients to do this now. Further studies are needed for FDA approval and general public use,” he said.
The study was funded by the National Council for Scientific and Technological Development, the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul, the Brain & Behavior Research Foundation, Fundação de Amparo à Pesquisa do Estado de São Paulo, and the Brazilian Innovation Agency. Dr. Leffa reported having received grants from the Brain & Behavior Research Foundation, the National Council for Scientific and Technological Development, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul during the conduction of the study. Dr. George reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the sham-controlled trial also showed that the tDCS treatment was both safe and well tolerated.
Overall, the findings suggest that the device could be a nondrug alternative for treating this patient population, Douglas Teixeira Leffa, MD, PhD, department of psychiatry, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and colleagues note.
“This is particularly relevant since a vast body of literature describes low long-term adherence rates and persistence to pharmacological treatment in patients with ADHD,” they write.
The findings were published online in JAMA Psychiatry.
Avoiding office visits
A noninvasive technique that is easy to use and relatively inexpensive, tDCS involves applying a low-intensity current over the scalp to modulate cortical excitability and induce neuroplasticity. Home-use tDCS devices, which avoid the need for daily office visits for stimulation sessions, have been validated in previous clinical samples.
The current study included 64 adults with ADHD who are not taking stimulants. They had moderate or severe symptoms of inattention, with an inattention score of 21 or higher on the clinician-administered Adult ADHD Self-Report Scale version 1.1 (CASRS).
The CASRS includes nine questions related to inattention symptoms (CASRS-I) and nine related to hyperactivity-impulsivity symptoms (CASRS-HI). The score can vary from 0 to 36 for each domain, with higher scores indicating increased symptoms.
Researchers randomly assigned participants to receive either active or sham stimulation.
The tDCS device used in the study delivered a current with 35-cm2 electrodes (7 cm by 5 cm). The anodal and cathodal electrodes were positioned corresponding to the right and left dorsolateral prefrontal cortex (DLPFC), respectively.
The investigators note that decreased activation in the right DLPFC has been reported before in patients with ADHD during tasks that require attention.
After learning to use the device, participants underwent 30-minute daily sessions of tDCS (2-mA direct constant current) for 4 weeks for a total of 28 sessions.
Devices programmed for sham treatment delivered a 30-second ramp-up (0-2 mA) stimulation followed by a 30-second ramp-down (2-0 mA) at the beginning, middle, and end of the application. This mimicked the tactile sensations reported with tDCS and has been shown to be a reliable sham protocol.
Participants were encouraged to perform the stimulation sessions at the same time of day. To improve adherence, they received daily text message reminders.
Nine patients discontinued treatment, two in the sham group and seven in the active group. However, patients who finished the trial completed a mean 25 of 28 sessions.
Window of opportunity?
The mean inattention score on CASRS-I at week 4, the primary outcome, was 18.88 in the active tDCS group vs. 23.63 in the sham tDCS group. There was a statistically significant treatment by time interaction for CASRS-I (beta interaction, –3.18; 95% confidence interval, –4.60 to –1.75; P < .001), showing decreased inattention symptoms in the active vs. sham groups.
The estimated Cohen’s d was 1.23 (95% CI, .67-1.78), indicating at least a moderate effect. This effect was similar to that reported with trigeminal nerve stimulation (TNS), the first approved device-based therapy for ADHD, and to that of atomoxetine, the second-line treatment for ADHD, the researchers note.
About one-third of patients (34.3%) in the active tDCS group achieved a 30% reduction in CASRS-I score, compared with 6.2% in the sham tDCS group.
There was no statistically significant difference in the secondary outcome of hyperactivity-impulsivity symptoms evaluated with the CASRS-HI. This may be because hyperactivity-impulsivity in ADHD is associated with a hypoactivation in the right inferior frontal cortex rather than the right DLPFC, the investigators write.
There were also no significant group differences in other secondary outcomes, including depression, anxiety, and executive function.
Adverse events (AE) were mostly mild and included skin redness and scalp burn. There were no severe or serious AEs.
Using a home-based tDCS device allows for considerably more sessions, with 28 being the highest number so far applied to patients with ADHD. This, the researchers note, is important because evidence suggests increased efficacy of tDCS with extended periods of treatment.
The home-based device “opens a new window of opportunity, especially for participants who live in geographically remote areas or have physical or cognitive disabilities that may hinder access to clinical centers,” they write.
Although a study limitation was the relatively high dropout rate in the active group, which might bias interpretation of the findings, only two of seven dropouts in the active group left because of an AE, the investigators note.
Patients received training in using the device, but there was no remote monitoring of sessions. In addition, the study population, which was relatively homogeneous with participants having no moderate to severe symptoms of depression or anxiety, differed from the usual patients with ADHD who are treated in clinical centers, the researchers point out.
As well, the study included only patients not taking pharmacologic treatment for ADHD – so the findings might not be generalizable to other patients, they add.
‘Just a first step’
Commenting on the study, Mark George, MD, distinguished professor of psychiatry, radiology, and neurology, Medical University of South Carolina, Charleston, noted that although this was a single-center study with a relatively small sample size, it is still important.
Showing it is possible to do high-quality tDCS studies at home “is a huge advance,” said Dr. George, who was not involved with the research.
“Home treatment is cheaper and easier for patients and allows many people to get treatment who would not be able to make it to the clinic daily for treatment,” he added.
He noted the study showed “a clear improvement in ADHD,” which is important because better treatments are needed.
However, he cautioned that this is “just a first step” and more studies are needed. For example, he said, it is not clear whether improvements persist and if patients need to self-treat forever, as they would with a medication.
Dr. George also noted that although the study used “a pioneering research device” with several safety features, many home-based tDCS devices on the market do not have those.
“I don’t advise patients to do this now. Further studies are needed for FDA approval and general public use,” he said.
The study was funded by the National Council for Scientific and Technological Development, the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul, the Brain & Behavior Research Foundation, Fundação de Amparo à Pesquisa do Estado de São Paulo, and the Brazilian Innovation Agency. Dr. Leffa reported having received grants from the Brain & Behavior Research Foundation, the National Council for Scientific and Technological Development, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul during the conduction of the study. Dr. George reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the sham-controlled trial also showed that the tDCS treatment was both safe and well tolerated.
Overall, the findings suggest that the device could be a nondrug alternative for treating this patient population, Douglas Teixeira Leffa, MD, PhD, department of psychiatry, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, and colleagues note.
“This is particularly relevant since a vast body of literature describes low long-term adherence rates and persistence to pharmacological treatment in patients with ADHD,” they write.
The findings were published online in JAMA Psychiatry.
Avoiding office visits
A noninvasive technique that is easy to use and relatively inexpensive, tDCS involves applying a low-intensity current over the scalp to modulate cortical excitability and induce neuroplasticity. Home-use tDCS devices, which avoid the need for daily office visits for stimulation sessions, have been validated in previous clinical samples.
The current study included 64 adults with ADHD who are not taking stimulants. They had moderate or severe symptoms of inattention, with an inattention score of 21 or higher on the clinician-administered Adult ADHD Self-Report Scale version 1.1 (CASRS).
The CASRS includes nine questions related to inattention symptoms (CASRS-I) and nine related to hyperactivity-impulsivity symptoms (CASRS-HI). The score can vary from 0 to 36 for each domain, with higher scores indicating increased symptoms.
Researchers randomly assigned participants to receive either active or sham stimulation.
The tDCS device used in the study delivered a current with 35-cm2 electrodes (7 cm by 5 cm). The anodal and cathodal electrodes were positioned corresponding to the right and left dorsolateral prefrontal cortex (DLPFC), respectively.
The investigators note that decreased activation in the right DLPFC has been reported before in patients with ADHD during tasks that require attention.
After learning to use the device, participants underwent 30-minute daily sessions of tDCS (2-mA direct constant current) for 4 weeks for a total of 28 sessions.
Devices programmed for sham treatment delivered a 30-second ramp-up (0-2 mA) stimulation followed by a 30-second ramp-down (2-0 mA) at the beginning, middle, and end of the application. This mimicked the tactile sensations reported with tDCS and has been shown to be a reliable sham protocol.
Participants were encouraged to perform the stimulation sessions at the same time of day. To improve adherence, they received daily text message reminders.
Nine patients discontinued treatment, two in the sham group and seven in the active group. However, patients who finished the trial completed a mean 25 of 28 sessions.
Window of opportunity?
The mean inattention score on CASRS-I at week 4, the primary outcome, was 18.88 in the active tDCS group vs. 23.63 in the sham tDCS group. There was a statistically significant treatment by time interaction for CASRS-I (beta interaction, –3.18; 95% confidence interval, –4.60 to –1.75; P < .001), showing decreased inattention symptoms in the active vs. sham groups.
The estimated Cohen’s d was 1.23 (95% CI, .67-1.78), indicating at least a moderate effect. This effect was similar to that reported with trigeminal nerve stimulation (TNS), the first approved device-based therapy for ADHD, and to that of atomoxetine, the second-line treatment for ADHD, the researchers note.
About one-third of patients (34.3%) in the active tDCS group achieved a 30% reduction in CASRS-I score, compared with 6.2% in the sham tDCS group.
There was no statistically significant difference in the secondary outcome of hyperactivity-impulsivity symptoms evaluated with the CASRS-HI. This may be because hyperactivity-impulsivity in ADHD is associated with a hypoactivation in the right inferior frontal cortex rather than the right DLPFC, the investigators write.
There were also no significant group differences in other secondary outcomes, including depression, anxiety, and executive function.
Adverse events (AE) were mostly mild and included skin redness and scalp burn. There were no severe or serious AEs.
Using a home-based tDCS device allows for considerably more sessions, with 28 being the highest number so far applied to patients with ADHD. This, the researchers note, is important because evidence suggests increased efficacy of tDCS with extended periods of treatment.
The home-based device “opens a new window of opportunity, especially for participants who live in geographically remote areas or have physical or cognitive disabilities that may hinder access to clinical centers,” they write.
Although a study limitation was the relatively high dropout rate in the active group, which might bias interpretation of the findings, only two of seven dropouts in the active group left because of an AE, the investigators note.
Patients received training in using the device, but there was no remote monitoring of sessions. In addition, the study population, which was relatively homogeneous with participants having no moderate to severe symptoms of depression or anxiety, differed from the usual patients with ADHD who are treated in clinical centers, the researchers point out.
As well, the study included only patients not taking pharmacologic treatment for ADHD – so the findings might not be generalizable to other patients, they add.
‘Just a first step’
Commenting on the study, Mark George, MD, distinguished professor of psychiatry, radiology, and neurology, Medical University of South Carolina, Charleston, noted that although this was a single-center study with a relatively small sample size, it is still important.
Showing it is possible to do high-quality tDCS studies at home “is a huge advance,” said Dr. George, who was not involved with the research.
“Home treatment is cheaper and easier for patients and allows many people to get treatment who would not be able to make it to the clinic daily for treatment,” he added.
He noted the study showed “a clear improvement in ADHD,” which is important because better treatments are needed.
However, he cautioned that this is “just a first step” and more studies are needed. For example, he said, it is not clear whether improvements persist and if patients need to self-treat forever, as they would with a medication.
Dr. George also noted that although the study used “a pioneering research device” with several safety features, many home-based tDCS devices on the market do not have those.
“I don’t advise patients to do this now. Further studies are needed for FDA approval and general public use,” he said.
The study was funded by the National Council for Scientific and Technological Development, the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul, the Brain & Behavior Research Foundation, Fundação de Amparo à Pesquisa do Estado de São Paulo, and the Brazilian Innovation Agency. Dr. Leffa reported having received grants from the Brain & Behavior Research Foundation, the National Council for Scientific and Technological Development, and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul during the conduction of the study. Dr. George reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Patients with cancer get valuable second opinion for free
Halfway through her first round of chemotherapy, with hair falling out, weight down, and her brain in a fog, Carolyn Hackett sat down for a Zoom meeting with a team of oncologists.
She didn’t even want a second opinion, but a friend had insisted that she get one, and she had agreed for the sake of their friendship.
But that Zoom meeting likely saved her life. The team of five specialists who had been reviewing her case for a week explained that she had been misdiagnosed. The original pathologist was mistaken. She didn’t have Hodgkin lymphoma; her cancer was really non-Hodgkin T-cell lymphoma. And the chemotherapy regimen she was in the middle of would do nothing to stop it.
The panel of doctors who populated Hackett’s computer screen during that December 2020 Zoom call were all volunteers with an organization called thesecondopinion, based in San Francisco.
The group, organized in 1969, currently offers free second opinions to at least three California patients with cancer each week. Patients meet for an average of 30-40 minutes – though there is really no limit – with a panel of doctors who have expertise in their specific case.
More than 70 cancer-related specialists, both current and retired, make up the roster of volunteers. A paid staffer rounds up a patient’s medical records, imaging and pathology slides. And a team of four to five doctors spends a week reviewing each case.
Then they meet directly with the patient and their doctor to answer questions and deliver their consensus.
Ms. Hackett was in disbelief after her meeting with thesecondopinion. Her diagnosis had gone from bad to worse, but the new information had changed her life. Without it, she would have surely continued chemotherapy and died.
On top of the new acccurate diagnosis, Ms. Hackett said it was a relief and reassurance when the team of volunteer doctors honored and included her oncologist. “I’m a nurse,” she said, and so she knows through experience that medicine comes with egos and frequent risk of lawsuits. But the team from thesecondopinion never criticized her doctor – after all, it wasn’t his mistake anyway – nor did they suggest legal action. Instead, they immediately included him as part of the team and sent him a letter detailing the panel concensus, she said, which made her feel safe.
Second opinions are big business
“And rightly so,” says Alan Venook, MD, a professor of medicine at UCSF with experience and expertise in gastrointestinal malignancies who was approached for comment but is not associated with thesecondopinion. Cancer is an increasingly a sub-specialized area, and every patient should get a second opinion, he said in an interview.
What is unique to an appointment with thesecondopinion, however, is the price tag – $0.
By comparison, a virtual second opinion at the Cleveland Clinic comes at a flat rate of $1,850, and at the Dana-Farber Cancer Institute, Boston, the cost is $2,400.
At UCSF, a second opinion from Dr. Venook and his colleagues will cost patients a couple thousand dollars out-of-pocket, he said. “Many patients don’t have the luxury of paying for a second opinion,” he said.
More than looking for misdiagnosis
Research shows that getting a second opinion can significantly change the course of a patient’s disease and treatment. A 2017 study by the Mayo Clinic found that at their institution, around 22% of second opinions changed the diagnosis, and 66% of patients received a refined or redefined diagnosis.
However, a misdiagnosis – such the case presented by Ms. Hackett – is a rare occurrence at thesecondopinion, said Howard Kleckner, MD, a medical oncologist and the organization’s medical director.
“We aren’t in the business to look for mistakes,” he said.
More often, thesecondopinion panels are about clarifying and helping patients understand the disease and options they have.”People with cancer need to make a peace with it and make peace with the treatment,” Dr. Kleckner said.
He estimates that 90% of the patients that come to the group already have the right diagnosis and treatment plan because, he says, there are “very good doctors in the state and in the Bay Area in particular.”
And even in the case of the remaining 10% of patients whose second opinion differs from their first, it’s largely a case of differences in the staging the disease or treatment options, Dr. Kleckner said.
“We aren’t coming up with brilliant suggestions. Often we are agreeing with what’s already been said,” said David Lakes, MD, a retired medical oncologist who has been volunteering with thesecondopinion for more than 30 years. “But we often see people understand for the first time.”
Both Dr. Kleckner and Dr. Lakes say that the organization attracts a certain kind of doctor, who tends to be an excellent communicator and really cares about helping the patients.
Many of these doctors are retired, but they want to keep doing the work, and they understand which pieces of information are most important for patients to know, Dr. Kleckner told this news organization. They are also willing to do this kind of work even when they won’t get paid, Dr. Kleckner said.
Part of that comes with gray hair and storied careers, Dr. Lakes added. “Retired people have experience and judgment and communication skills that a lot of younger doctors don’t have,” he commented. They often have more experience with some of the tough stuff, like exploring the goals of treatment, discontinuing treatment, and end-of-life care, and they also have more time to engage patients on their emotional health, he said.
Dr. Venook said that the services provided free-of-charge by these volunteers is “laudable,” and their thorough review of all the diagnostic information is “to their credit.” But he questions whether every second opinion provided by the organization is an expert one, since the doctors are no longer practicing. Oncology is a particularly fast-moving field, with many new developments and novel drugs launched in recent years.
“Second opinions are incredibly helpful but [have] to be [provided] by a knowledgeable expert who gets all the details and gives it serious thought,” Dr. Venook said.
Dr. Lakes says that he is constantly evaluating whether he is entitled to keep offering second opinions when he isn’t practicing; so far the answer remains a yes. Although he now has to spend more time researching treatment options like biologics, he still feels adept at engaging with patients and helping patients understand where they are in their illness and the potential benefits of fourth- or fifth-line treatments.
Another strength of thesecondopinion model lies in numbers. Most of the time second opinions are given by one doctor, Dr. Kleckner pointed out. In contrast, thesecondopinion provides the patient with access to a whole team of specialists.
“Sometimes people on the panels don’t completely agree,” Dr. Lakes said. So, before meeting with the patient on Zoom, the doctors review the case together for about half an hour and come up with a consensus. This way there’s no mixed messaging and as little anxiety for the patient as possible, he said.
The fact that patients have direct access to the panel of experts who review their cases is unique in itself, Dr. Kleckner said.
Many hospitals have tumor boards, but they are reserved for physicians, he pointed out. Patients get to hear the board’s opinion secondhand through their primary doctor or oncologist.
But at thesecondopinion, the patient gets to engage with the doctors directly. There is time to review up to four questions that the patient has submitted before the meeting and also time for any additional questions that may have arisen during the course of the meeting.
In oncology, and medicine in general, patients are often shuffled from one specialist to another, Dr. Lakes said. But often, there is no one who takes a steps back to see the whole picture.
That’s part of what thesecondopinion offers, he said. “We have the time, the experience, and no skin in the game. We can think about [the cases] in an intellectual way without feeling like we are in the hot seat or in charge.”
Thanks to her second opinion, Ms. Hackett was able to get connected with an oncologist in San Francisco who specializes in the type of cancer she actually had. She went on to receive nine rounds of a monoclonal antibody treatment formulated for her specific cancer. She is now in remission 6 months after finishing that therapy.
Scans in late July showed that she was cancer-free and doing really well. “I’m so grateful to them. I’m so impressed with thesecondopinion, I can’t believe it,” she said. “I’m alive because of them.”
A version of this article first appeared on Medscape.com.
Halfway through her first round of chemotherapy, with hair falling out, weight down, and her brain in a fog, Carolyn Hackett sat down for a Zoom meeting with a team of oncologists.
She didn’t even want a second opinion, but a friend had insisted that she get one, and she had agreed for the sake of their friendship.
But that Zoom meeting likely saved her life. The team of five specialists who had been reviewing her case for a week explained that she had been misdiagnosed. The original pathologist was mistaken. She didn’t have Hodgkin lymphoma; her cancer was really non-Hodgkin T-cell lymphoma. And the chemotherapy regimen she was in the middle of would do nothing to stop it.
The panel of doctors who populated Hackett’s computer screen during that December 2020 Zoom call were all volunteers with an organization called thesecondopinion, based in San Francisco.
The group, organized in 1969, currently offers free second opinions to at least three California patients with cancer each week. Patients meet for an average of 30-40 minutes – though there is really no limit – with a panel of doctors who have expertise in their specific case.
More than 70 cancer-related specialists, both current and retired, make up the roster of volunteers. A paid staffer rounds up a patient’s medical records, imaging and pathology slides. And a team of four to five doctors spends a week reviewing each case.
Then they meet directly with the patient and their doctor to answer questions and deliver their consensus.
Ms. Hackett was in disbelief after her meeting with thesecondopinion. Her diagnosis had gone from bad to worse, but the new information had changed her life. Without it, she would have surely continued chemotherapy and died.
On top of the new acccurate diagnosis, Ms. Hackett said it was a relief and reassurance when the team of volunteer doctors honored and included her oncologist. “I’m a nurse,” she said, and so she knows through experience that medicine comes with egos and frequent risk of lawsuits. But the team from thesecondopinion never criticized her doctor – after all, it wasn’t his mistake anyway – nor did they suggest legal action. Instead, they immediately included him as part of the team and sent him a letter detailing the panel concensus, she said, which made her feel safe.
Second opinions are big business
“And rightly so,” says Alan Venook, MD, a professor of medicine at UCSF with experience and expertise in gastrointestinal malignancies who was approached for comment but is not associated with thesecondopinion. Cancer is an increasingly a sub-specialized area, and every patient should get a second opinion, he said in an interview.
What is unique to an appointment with thesecondopinion, however, is the price tag – $0.
By comparison, a virtual second opinion at the Cleveland Clinic comes at a flat rate of $1,850, and at the Dana-Farber Cancer Institute, Boston, the cost is $2,400.
At UCSF, a second opinion from Dr. Venook and his colleagues will cost patients a couple thousand dollars out-of-pocket, he said. “Many patients don’t have the luxury of paying for a second opinion,” he said.
More than looking for misdiagnosis
Research shows that getting a second opinion can significantly change the course of a patient’s disease and treatment. A 2017 study by the Mayo Clinic found that at their institution, around 22% of second opinions changed the diagnosis, and 66% of patients received a refined or redefined diagnosis.
However, a misdiagnosis – such the case presented by Ms. Hackett – is a rare occurrence at thesecondopinion, said Howard Kleckner, MD, a medical oncologist and the organization’s medical director.
“We aren’t in the business to look for mistakes,” he said.
More often, thesecondopinion panels are about clarifying and helping patients understand the disease and options they have.”People with cancer need to make a peace with it and make peace with the treatment,” Dr. Kleckner said.
He estimates that 90% of the patients that come to the group already have the right diagnosis and treatment plan because, he says, there are “very good doctors in the state and in the Bay Area in particular.”
And even in the case of the remaining 10% of patients whose second opinion differs from their first, it’s largely a case of differences in the staging the disease or treatment options, Dr. Kleckner said.
“We aren’t coming up with brilliant suggestions. Often we are agreeing with what’s already been said,” said David Lakes, MD, a retired medical oncologist who has been volunteering with thesecondopinion for more than 30 years. “But we often see people understand for the first time.”
Both Dr. Kleckner and Dr. Lakes say that the organization attracts a certain kind of doctor, who tends to be an excellent communicator and really cares about helping the patients.
Many of these doctors are retired, but they want to keep doing the work, and they understand which pieces of information are most important for patients to know, Dr. Kleckner told this news organization. They are also willing to do this kind of work even when they won’t get paid, Dr. Kleckner said.
Part of that comes with gray hair and storied careers, Dr. Lakes added. “Retired people have experience and judgment and communication skills that a lot of younger doctors don’t have,” he commented. They often have more experience with some of the tough stuff, like exploring the goals of treatment, discontinuing treatment, and end-of-life care, and they also have more time to engage patients on their emotional health, he said.
Dr. Venook said that the services provided free-of-charge by these volunteers is “laudable,” and their thorough review of all the diagnostic information is “to their credit.” But he questions whether every second opinion provided by the organization is an expert one, since the doctors are no longer practicing. Oncology is a particularly fast-moving field, with many new developments and novel drugs launched in recent years.
“Second opinions are incredibly helpful but [have] to be [provided] by a knowledgeable expert who gets all the details and gives it serious thought,” Dr. Venook said.
Dr. Lakes says that he is constantly evaluating whether he is entitled to keep offering second opinions when he isn’t practicing; so far the answer remains a yes. Although he now has to spend more time researching treatment options like biologics, he still feels adept at engaging with patients and helping patients understand where they are in their illness and the potential benefits of fourth- or fifth-line treatments.
Another strength of thesecondopinion model lies in numbers. Most of the time second opinions are given by one doctor, Dr. Kleckner pointed out. In contrast, thesecondopinion provides the patient with access to a whole team of specialists.
“Sometimes people on the panels don’t completely agree,” Dr. Lakes said. So, before meeting with the patient on Zoom, the doctors review the case together for about half an hour and come up with a consensus. This way there’s no mixed messaging and as little anxiety for the patient as possible, he said.
The fact that patients have direct access to the panel of experts who review their cases is unique in itself, Dr. Kleckner said.
Many hospitals have tumor boards, but they are reserved for physicians, he pointed out. Patients get to hear the board’s opinion secondhand through their primary doctor or oncologist.
But at thesecondopinion, the patient gets to engage with the doctors directly. There is time to review up to four questions that the patient has submitted before the meeting and also time for any additional questions that may have arisen during the course of the meeting.
In oncology, and medicine in general, patients are often shuffled from one specialist to another, Dr. Lakes said. But often, there is no one who takes a steps back to see the whole picture.
That’s part of what thesecondopinion offers, he said. “We have the time, the experience, and no skin in the game. We can think about [the cases] in an intellectual way without feeling like we are in the hot seat or in charge.”
Thanks to her second opinion, Ms. Hackett was able to get connected with an oncologist in San Francisco who specializes in the type of cancer she actually had. She went on to receive nine rounds of a monoclonal antibody treatment formulated for her specific cancer. She is now in remission 6 months after finishing that therapy.
Scans in late July showed that she was cancer-free and doing really well. “I’m so grateful to them. I’m so impressed with thesecondopinion, I can’t believe it,” she said. “I’m alive because of them.”
A version of this article first appeared on Medscape.com.
Halfway through her first round of chemotherapy, with hair falling out, weight down, and her brain in a fog, Carolyn Hackett sat down for a Zoom meeting with a team of oncologists.
She didn’t even want a second opinion, but a friend had insisted that she get one, and she had agreed for the sake of their friendship.
But that Zoom meeting likely saved her life. The team of five specialists who had been reviewing her case for a week explained that she had been misdiagnosed. The original pathologist was mistaken. She didn’t have Hodgkin lymphoma; her cancer was really non-Hodgkin T-cell lymphoma. And the chemotherapy regimen she was in the middle of would do nothing to stop it.
The panel of doctors who populated Hackett’s computer screen during that December 2020 Zoom call were all volunteers with an organization called thesecondopinion, based in San Francisco.
The group, organized in 1969, currently offers free second opinions to at least three California patients with cancer each week. Patients meet for an average of 30-40 minutes – though there is really no limit – with a panel of doctors who have expertise in their specific case.
More than 70 cancer-related specialists, both current and retired, make up the roster of volunteers. A paid staffer rounds up a patient’s medical records, imaging and pathology slides. And a team of four to five doctors spends a week reviewing each case.
Then they meet directly with the patient and their doctor to answer questions and deliver their consensus.
Ms. Hackett was in disbelief after her meeting with thesecondopinion. Her diagnosis had gone from bad to worse, but the new information had changed her life. Without it, she would have surely continued chemotherapy and died.
On top of the new acccurate diagnosis, Ms. Hackett said it was a relief and reassurance when the team of volunteer doctors honored and included her oncologist. “I’m a nurse,” she said, and so she knows through experience that medicine comes with egos and frequent risk of lawsuits. But the team from thesecondopinion never criticized her doctor – after all, it wasn’t his mistake anyway – nor did they suggest legal action. Instead, they immediately included him as part of the team and sent him a letter detailing the panel concensus, she said, which made her feel safe.
Second opinions are big business
“And rightly so,” says Alan Venook, MD, a professor of medicine at UCSF with experience and expertise in gastrointestinal malignancies who was approached for comment but is not associated with thesecondopinion. Cancer is an increasingly a sub-specialized area, and every patient should get a second opinion, he said in an interview.
What is unique to an appointment with thesecondopinion, however, is the price tag – $0.
By comparison, a virtual second opinion at the Cleveland Clinic comes at a flat rate of $1,850, and at the Dana-Farber Cancer Institute, Boston, the cost is $2,400.
At UCSF, a second opinion from Dr. Venook and his colleagues will cost patients a couple thousand dollars out-of-pocket, he said. “Many patients don’t have the luxury of paying for a second opinion,” he said.
More than looking for misdiagnosis
Research shows that getting a second opinion can significantly change the course of a patient’s disease and treatment. A 2017 study by the Mayo Clinic found that at their institution, around 22% of second opinions changed the diagnosis, and 66% of patients received a refined or redefined diagnosis.
However, a misdiagnosis – such the case presented by Ms. Hackett – is a rare occurrence at thesecondopinion, said Howard Kleckner, MD, a medical oncologist and the organization’s medical director.
“We aren’t in the business to look for mistakes,” he said.
More often, thesecondopinion panels are about clarifying and helping patients understand the disease and options they have.”People with cancer need to make a peace with it and make peace with the treatment,” Dr. Kleckner said.
He estimates that 90% of the patients that come to the group already have the right diagnosis and treatment plan because, he says, there are “very good doctors in the state and in the Bay Area in particular.”
And even in the case of the remaining 10% of patients whose second opinion differs from their first, it’s largely a case of differences in the staging the disease or treatment options, Dr. Kleckner said.
“We aren’t coming up with brilliant suggestions. Often we are agreeing with what’s already been said,” said David Lakes, MD, a retired medical oncologist who has been volunteering with thesecondopinion for more than 30 years. “But we often see people understand for the first time.”
Both Dr. Kleckner and Dr. Lakes say that the organization attracts a certain kind of doctor, who tends to be an excellent communicator and really cares about helping the patients.
Many of these doctors are retired, but they want to keep doing the work, and they understand which pieces of information are most important for patients to know, Dr. Kleckner told this news organization. They are also willing to do this kind of work even when they won’t get paid, Dr. Kleckner said.
Part of that comes with gray hair and storied careers, Dr. Lakes added. “Retired people have experience and judgment and communication skills that a lot of younger doctors don’t have,” he commented. They often have more experience with some of the tough stuff, like exploring the goals of treatment, discontinuing treatment, and end-of-life care, and they also have more time to engage patients on their emotional health, he said.
Dr. Venook said that the services provided free-of-charge by these volunteers is “laudable,” and their thorough review of all the diagnostic information is “to their credit.” But he questions whether every second opinion provided by the organization is an expert one, since the doctors are no longer practicing. Oncology is a particularly fast-moving field, with many new developments and novel drugs launched in recent years.
“Second opinions are incredibly helpful but [have] to be [provided] by a knowledgeable expert who gets all the details and gives it serious thought,” Dr. Venook said.
Dr. Lakes says that he is constantly evaluating whether he is entitled to keep offering second opinions when he isn’t practicing; so far the answer remains a yes. Although he now has to spend more time researching treatment options like biologics, he still feels adept at engaging with patients and helping patients understand where they are in their illness and the potential benefits of fourth- or fifth-line treatments.
Another strength of thesecondopinion model lies in numbers. Most of the time second opinions are given by one doctor, Dr. Kleckner pointed out. In contrast, thesecondopinion provides the patient with access to a whole team of specialists.
“Sometimes people on the panels don’t completely agree,” Dr. Lakes said. So, before meeting with the patient on Zoom, the doctors review the case together for about half an hour and come up with a consensus. This way there’s no mixed messaging and as little anxiety for the patient as possible, he said.
The fact that patients have direct access to the panel of experts who review their cases is unique in itself, Dr. Kleckner said.
Many hospitals have tumor boards, but they are reserved for physicians, he pointed out. Patients get to hear the board’s opinion secondhand through their primary doctor or oncologist.
But at thesecondopinion, the patient gets to engage with the doctors directly. There is time to review up to four questions that the patient has submitted before the meeting and also time for any additional questions that may have arisen during the course of the meeting.
In oncology, and medicine in general, patients are often shuffled from one specialist to another, Dr. Lakes said. But often, there is no one who takes a steps back to see the whole picture.
That’s part of what thesecondopinion offers, he said. “We have the time, the experience, and no skin in the game. We can think about [the cases] in an intellectual way without feeling like we are in the hot seat or in charge.”
Thanks to her second opinion, Ms. Hackett was able to get connected with an oncologist in San Francisco who specializes in the type of cancer she actually had. She went on to receive nine rounds of a monoclonal antibody treatment formulated for her specific cancer. She is now in remission 6 months after finishing that therapy.
Scans in late July showed that she was cancer-free and doing really well. “I’m so grateful to them. I’m so impressed with thesecondopinion, I can’t believe it,” she said. “I’m alive because of them.”
A version of this article first appeared on Medscape.com.
Will monkeypox be the ‘syphilis of the 21st century’?
PARIS – France is boosting its vaccination campaign in response to the increase in cases of monkeypox. After a sluggish start, newly appointed French health minister François Braun has announced the release of 42,000 vaccine doses. At the same time, medical students will be able to lend a helping hand at vaccination sites. However, some experts have criticized the measures taken as being too lax to combat what the World Health Organization has designated a global health emergency.
For Benjamin Davido, MD, MSc, PhD, an infectious disease specialist at the Raymond-Poincaré Hospital (Paris Public Hospital Trust, AP-HP, Garches region), the risks of this disease have been minimized and the measures taken are not adequate, despite the ready availability of the tools needed to manage the epidemic. We must remain alert to the risks posed by this monkeypox epidemic, which seems different from the sporadic outbreaks that usually crop up in Central and West Africa, he said. Dr. Davido recently shared his opinions in an interview.
Question: What do you think about the monkeypox vaccination campaign currently underway in France?
Dr. Davido: It doesn’t go far enough, and I am surprised by the lack of a concrete and specific objective. It seems we have to wait until the fire is out of control before we can call the fire department. We should have been more reactive and taken a more drastic approach from the get-go. In France, as in other countries affected by this epidemic, we are still, unfortunately, in a phase of observation, reassuring ourselves that this will surely not become another pandemic, as that would be really bad luck.
Yet we find ourselves in an unprecedented situation: We have known about the disease in question for a long time, the target population has been identified, and we have a vaccine immediately available. So, we have all the tools and knowledge acquired from the COVID-19 pandemic at our disposal, yet we are choosing to wait and see. We have clearly underestimated the risks of failing after a stalled start to the vaccination campaign.
Question: What exactly are the risks, in your opinion? Should we already be worried about how the epidemic is progressing?
Dr. Davido: The situation is definitely worrying. I personally am convinced that this disease will be the syphilis of the 21st century. Although the risk is low, it is not beyond the bounds of possibility that this could be the start of a new pandemic. For the time being, its spread is limited to at-risk populations, mainly men who have sex with other men and who have multiple partners, which accounts for around 300,000 people in France. However, the risk for heterosexuals must not be minimized; we must not forget that this disease can also be transmitted through contact with an infected person and by respiratory droplets from people living in the same household. There have been recent cases of women and children infected with monkeypox. If monkeypox starts to spread in the community, rather than being a sexually transmitted infection, the epidemic could spread to the rest of the population. With the rise in cases, scientists are also concerned about transmission to animals. Monkeypox could become endemic like it is in Africa, where rodents are the main reservoir of the virus.
Question: What do we know about the dynamics of this epidemic? What can be done to effectively improve the situation?
Dr. Davido: Experience gained from African countries affected by monkeypox, as well as from the spate of cases that occurred in the United States in 2003, has shown us that the epidemic can be controlled once the cases have been contained. It is hoped that further waves of the epidemic can be avoided, providing the monkeypox vaccine achieves its objectives.
But we need to give ourselves the means to do so. The expansion of the vaccination program to the most at-risk populations in early July was the right decision. We have seen that ring vaccination targeting close-contact cases does not work with monkeypox. The current problem is that this vaccine is nearly exclusively restricted to hospital settings. We are making the same mistakes as [we did] at the start of the COVID-19 epidemic. We don’t have the right infrastructure in place for this vaccination program. We need to get doctors, paramedics, pharmacists, etc., involved. And cut back on the red tape. After embracing digital procedures during COVID-19, we find ourselves having to complete paper copies of documents for every single person attending a vaccination site. It just doesn’t make sense!
Question: You highlighted the lack of a clear objective with this vaccination campaign. What should we be aiming for?
Dr. Davido: During the COVID-19 vaccination campaign, there was a set number of people to be vaccinated within a given time frame. The approach demanded a fast pace and a desired outcome. Yes, it was an ambitious target from the get-go, but it was one that we stuck to. Currently, no figure, no target, has been set for the monkeypox vaccination program. Ideally, we would have completed the vaccination campaign before the start of the new school year to limit new infections.
As it stands now, only 10% of the target population has received the vaccine. There is talk of the summer period not being favorable. Yet I remember that last year, the COVID-19 vaccination program was strengthened in the middle of August. If the monkeypox vaccination campaign is not given a boost by the end of the summer, we run the risk of encouraging transmission of the virus between close contacts when different groups mix after being on holiday at the start of the new school year. I think that, first and foremost, we must make general practitioners aware of the disease and train them in how to diagnose it so that patients can be isolated and vaccinated as quickly as possible.
Question: There has also been talk of increasing the set 28-day period between the two doses, or even getting rid of it entirely. Would this perhaps lead to better vaccine uptake?
Dr. Davido: The United Kingdom has chosen to give a single dose and recommends a second dose after exposure. I am not sure that this is the best strategy. Although the efficacy data are still limited, the results are not as good after a single dose. According to initial data from the French National Agency for the Safety of Medicines and Health Products (the ANSM), the rate of seroconversion after one dose rises from 10% to 56% on D28 in healthy volunteers, but is between 77% and 89% 2 weeks after the second dose administered on D28.
So, the second dose is needed, especially as immunological memory seems to drop 2 years after the first injection. The U.S. Centers for Disease Control and Prevention proposes leaving 35 days between the two doses. I think this is a reasonable time frame. So, delaying the second dose makes administration of the first dose even easier because the second often fell in the middle of the holiday period and so we also save precious doses. If the time between doses is longer, we risk vaccinated individuals becoming lax and possibly being tempted to skip the “optional” booster or simply forgetting about it.
Question: Are people who have already had the smallpox vaccine better protected against monkeypox?
Dr. Davido: The efficacy of this vaccine against monkeypox is not perfect on a very long-term basis and, to be honest, we don’t really know the level of protection afforded by first-generation vaccines after 20 years. We must not forget that 20% of people infected with monkeypox were vaccinated against smallpox before mandatory vaccination for this disease was abolished [Editor’s note: The requirement of an initial dose of smallpox vaccine was lifted in 1979, once smallpox had been eradicated].
It is hoped that, as a minimum, this vaccine protects against serious illness. Yet in my department, we regularly see severe cases of monkeypox with widespread lesions in the over 45s, who are said to be vaccinated against smallpox.
Question: By comparison, is it likely that a third-generation vaccine would afford better protection against severe illness?
Dr. Davido: We still don’t have enough data or hindsight to assess the real-world impact of third-generation vaccines. This vaccine has a better tolerance profile than its predecessors, but we currently don’t know if it protects against severe forms of monkeypox. We also need to learn more about the disease causing the current epidemic, since it seems different from the sporadic outbreaks that usually crop up in Central and West Africa. The lesions seen are notably milder. The WHO has given this vaccine an efficacy level of 85% against infection by the monkeypox virus, but we must remain cautious: This figure is based on data from Africa. The epidemic in which we find ourselves is not the same. Overall, we must be wary of overly optimistic rhetoric around this new epidemic.
A version of this article appeared on Medscape.com. The article was translated from the Medscape French edition.
PARIS – France is boosting its vaccination campaign in response to the increase in cases of monkeypox. After a sluggish start, newly appointed French health minister François Braun has announced the release of 42,000 vaccine doses. At the same time, medical students will be able to lend a helping hand at vaccination sites. However, some experts have criticized the measures taken as being too lax to combat what the World Health Organization has designated a global health emergency.
For Benjamin Davido, MD, MSc, PhD, an infectious disease specialist at the Raymond-Poincaré Hospital (Paris Public Hospital Trust, AP-HP, Garches region), the risks of this disease have been minimized and the measures taken are not adequate, despite the ready availability of the tools needed to manage the epidemic. We must remain alert to the risks posed by this monkeypox epidemic, which seems different from the sporadic outbreaks that usually crop up in Central and West Africa, he said. Dr. Davido recently shared his opinions in an interview.
Question: What do you think about the monkeypox vaccination campaign currently underway in France?
Dr. Davido: It doesn’t go far enough, and I am surprised by the lack of a concrete and specific objective. It seems we have to wait until the fire is out of control before we can call the fire department. We should have been more reactive and taken a more drastic approach from the get-go. In France, as in other countries affected by this epidemic, we are still, unfortunately, in a phase of observation, reassuring ourselves that this will surely not become another pandemic, as that would be really bad luck.
Yet we find ourselves in an unprecedented situation: We have known about the disease in question for a long time, the target population has been identified, and we have a vaccine immediately available. So, we have all the tools and knowledge acquired from the COVID-19 pandemic at our disposal, yet we are choosing to wait and see. We have clearly underestimated the risks of failing after a stalled start to the vaccination campaign.
Question: What exactly are the risks, in your opinion? Should we already be worried about how the epidemic is progressing?
Dr. Davido: The situation is definitely worrying. I personally am convinced that this disease will be the syphilis of the 21st century. Although the risk is low, it is not beyond the bounds of possibility that this could be the start of a new pandemic. For the time being, its spread is limited to at-risk populations, mainly men who have sex with other men and who have multiple partners, which accounts for around 300,000 people in France. However, the risk for heterosexuals must not be minimized; we must not forget that this disease can also be transmitted through contact with an infected person and by respiratory droplets from people living in the same household. There have been recent cases of women and children infected with monkeypox. If monkeypox starts to spread in the community, rather than being a sexually transmitted infection, the epidemic could spread to the rest of the population. With the rise in cases, scientists are also concerned about transmission to animals. Monkeypox could become endemic like it is in Africa, where rodents are the main reservoir of the virus.
Question: What do we know about the dynamics of this epidemic? What can be done to effectively improve the situation?
Dr. Davido: Experience gained from African countries affected by monkeypox, as well as from the spate of cases that occurred in the United States in 2003, has shown us that the epidemic can be controlled once the cases have been contained. It is hoped that further waves of the epidemic can be avoided, providing the monkeypox vaccine achieves its objectives.
But we need to give ourselves the means to do so. The expansion of the vaccination program to the most at-risk populations in early July was the right decision. We have seen that ring vaccination targeting close-contact cases does not work with monkeypox. The current problem is that this vaccine is nearly exclusively restricted to hospital settings. We are making the same mistakes as [we did] at the start of the COVID-19 epidemic. We don’t have the right infrastructure in place for this vaccination program. We need to get doctors, paramedics, pharmacists, etc., involved. And cut back on the red tape. After embracing digital procedures during COVID-19, we find ourselves having to complete paper copies of documents for every single person attending a vaccination site. It just doesn’t make sense!
Question: You highlighted the lack of a clear objective with this vaccination campaign. What should we be aiming for?
Dr. Davido: During the COVID-19 vaccination campaign, there was a set number of people to be vaccinated within a given time frame. The approach demanded a fast pace and a desired outcome. Yes, it was an ambitious target from the get-go, but it was one that we stuck to. Currently, no figure, no target, has been set for the monkeypox vaccination program. Ideally, we would have completed the vaccination campaign before the start of the new school year to limit new infections.
As it stands now, only 10% of the target population has received the vaccine. There is talk of the summer period not being favorable. Yet I remember that last year, the COVID-19 vaccination program was strengthened in the middle of August. If the monkeypox vaccination campaign is not given a boost by the end of the summer, we run the risk of encouraging transmission of the virus between close contacts when different groups mix after being on holiday at the start of the new school year. I think that, first and foremost, we must make general practitioners aware of the disease and train them in how to diagnose it so that patients can be isolated and vaccinated as quickly as possible.
Question: There has also been talk of increasing the set 28-day period between the two doses, or even getting rid of it entirely. Would this perhaps lead to better vaccine uptake?
Dr. Davido: The United Kingdom has chosen to give a single dose and recommends a second dose after exposure. I am not sure that this is the best strategy. Although the efficacy data are still limited, the results are not as good after a single dose. According to initial data from the French National Agency for the Safety of Medicines and Health Products (the ANSM), the rate of seroconversion after one dose rises from 10% to 56% on D28 in healthy volunteers, but is between 77% and 89% 2 weeks after the second dose administered on D28.
So, the second dose is needed, especially as immunological memory seems to drop 2 years after the first injection. The U.S. Centers for Disease Control and Prevention proposes leaving 35 days between the two doses. I think this is a reasonable time frame. So, delaying the second dose makes administration of the first dose even easier because the second often fell in the middle of the holiday period and so we also save precious doses. If the time between doses is longer, we risk vaccinated individuals becoming lax and possibly being tempted to skip the “optional” booster or simply forgetting about it.
Question: Are people who have already had the smallpox vaccine better protected against monkeypox?
Dr. Davido: The efficacy of this vaccine against monkeypox is not perfect on a very long-term basis and, to be honest, we don’t really know the level of protection afforded by first-generation vaccines after 20 years. We must not forget that 20% of people infected with monkeypox were vaccinated against smallpox before mandatory vaccination for this disease was abolished [Editor’s note: The requirement of an initial dose of smallpox vaccine was lifted in 1979, once smallpox had been eradicated].
It is hoped that, as a minimum, this vaccine protects against serious illness. Yet in my department, we regularly see severe cases of monkeypox with widespread lesions in the over 45s, who are said to be vaccinated against smallpox.
Question: By comparison, is it likely that a third-generation vaccine would afford better protection against severe illness?
Dr. Davido: We still don’t have enough data or hindsight to assess the real-world impact of third-generation vaccines. This vaccine has a better tolerance profile than its predecessors, but we currently don’t know if it protects against severe forms of monkeypox. We also need to learn more about the disease causing the current epidemic, since it seems different from the sporadic outbreaks that usually crop up in Central and West Africa. The lesions seen are notably milder. The WHO has given this vaccine an efficacy level of 85% against infection by the monkeypox virus, but we must remain cautious: This figure is based on data from Africa. The epidemic in which we find ourselves is not the same. Overall, we must be wary of overly optimistic rhetoric around this new epidemic.
A version of this article appeared on Medscape.com. The article was translated from the Medscape French edition.
PARIS – France is boosting its vaccination campaign in response to the increase in cases of monkeypox. After a sluggish start, newly appointed French health minister François Braun has announced the release of 42,000 vaccine doses. At the same time, medical students will be able to lend a helping hand at vaccination sites. However, some experts have criticized the measures taken as being too lax to combat what the World Health Organization has designated a global health emergency.
For Benjamin Davido, MD, MSc, PhD, an infectious disease specialist at the Raymond-Poincaré Hospital (Paris Public Hospital Trust, AP-HP, Garches region), the risks of this disease have been minimized and the measures taken are not adequate, despite the ready availability of the tools needed to manage the epidemic. We must remain alert to the risks posed by this monkeypox epidemic, which seems different from the sporadic outbreaks that usually crop up in Central and West Africa, he said. Dr. Davido recently shared his opinions in an interview.
Question: What do you think about the monkeypox vaccination campaign currently underway in France?
Dr. Davido: It doesn’t go far enough, and I am surprised by the lack of a concrete and specific objective. It seems we have to wait until the fire is out of control before we can call the fire department. We should have been more reactive and taken a more drastic approach from the get-go. In France, as in other countries affected by this epidemic, we are still, unfortunately, in a phase of observation, reassuring ourselves that this will surely not become another pandemic, as that would be really bad luck.
Yet we find ourselves in an unprecedented situation: We have known about the disease in question for a long time, the target population has been identified, and we have a vaccine immediately available. So, we have all the tools and knowledge acquired from the COVID-19 pandemic at our disposal, yet we are choosing to wait and see. We have clearly underestimated the risks of failing after a stalled start to the vaccination campaign.
Question: What exactly are the risks, in your opinion? Should we already be worried about how the epidemic is progressing?
Dr. Davido: The situation is definitely worrying. I personally am convinced that this disease will be the syphilis of the 21st century. Although the risk is low, it is not beyond the bounds of possibility that this could be the start of a new pandemic. For the time being, its spread is limited to at-risk populations, mainly men who have sex with other men and who have multiple partners, which accounts for around 300,000 people in France. However, the risk for heterosexuals must not be minimized; we must not forget that this disease can also be transmitted through contact with an infected person and by respiratory droplets from people living in the same household. There have been recent cases of women and children infected with monkeypox. If monkeypox starts to spread in the community, rather than being a sexually transmitted infection, the epidemic could spread to the rest of the population. With the rise in cases, scientists are also concerned about transmission to animals. Monkeypox could become endemic like it is in Africa, where rodents are the main reservoir of the virus.
Question: What do we know about the dynamics of this epidemic? What can be done to effectively improve the situation?
Dr. Davido: Experience gained from African countries affected by monkeypox, as well as from the spate of cases that occurred in the United States in 2003, has shown us that the epidemic can be controlled once the cases have been contained. It is hoped that further waves of the epidemic can be avoided, providing the monkeypox vaccine achieves its objectives.
But we need to give ourselves the means to do so. The expansion of the vaccination program to the most at-risk populations in early July was the right decision. We have seen that ring vaccination targeting close-contact cases does not work with monkeypox. The current problem is that this vaccine is nearly exclusively restricted to hospital settings. We are making the same mistakes as [we did] at the start of the COVID-19 epidemic. We don’t have the right infrastructure in place for this vaccination program. We need to get doctors, paramedics, pharmacists, etc., involved. And cut back on the red tape. After embracing digital procedures during COVID-19, we find ourselves having to complete paper copies of documents for every single person attending a vaccination site. It just doesn’t make sense!
Question: You highlighted the lack of a clear objective with this vaccination campaign. What should we be aiming for?
Dr. Davido: During the COVID-19 vaccination campaign, there was a set number of people to be vaccinated within a given time frame. The approach demanded a fast pace and a desired outcome. Yes, it was an ambitious target from the get-go, but it was one that we stuck to. Currently, no figure, no target, has been set for the monkeypox vaccination program. Ideally, we would have completed the vaccination campaign before the start of the new school year to limit new infections.
As it stands now, only 10% of the target population has received the vaccine. There is talk of the summer period not being favorable. Yet I remember that last year, the COVID-19 vaccination program was strengthened in the middle of August. If the monkeypox vaccination campaign is not given a boost by the end of the summer, we run the risk of encouraging transmission of the virus between close contacts when different groups mix after being on holiday at the start of the new school year. I think that, first and foremost, we must make general practitioners aware of the disease and train them in how to diagnose it so that patients can be isolated and vaccinated as quickly as possible.
Question: There has also been talk of increasing the set 28-day period between the two doses, or even getting rid of it entirely. Would this perhaps lead to better vaccine uptake?
Dr. Davido: The United Kingdom has chosen to give a single dose and recommends a second dose after exposure. I am not sure that this is the best strategy. Although the efficacy data are still limited, the results are not as good after a single dose. According to initial data from the French National Agency for the Safety of Medicines and Health Products (the ANSM), the rate of seroconversion after one dose rises from 10% to 56% on D28 in healthy volunteers, but is between 77% and 89% 2 weeks after the second dose administered on D28.
So, the second dose is needed, especially as immunological memory seems to drop 2 years after the first injection. The U.S. Centers for Disease Control and Prevention proposes leaving 35 days between the two doses. I think this is a reasonable time frame. So, delaying the second dose makes administration of the first dose even easier because the second often fell in the middle of the holiday period and so we also save precious doses. If the time between doses is longer, we risk vaccinated individuals becoming lax and possibly being tempted to skip the “optional” booster or simply forgetting about it.
Question: Are people who have already had the smallpox vaccine better protected against monkeypox?
Dr. Davido: The efficacy of this vaccine against monkeypox is not perfect on a very long-term basis and, to be honest, we don’t really know the level of protection afforded by first-generation vaccines after 20 years. We must not forget that 20% of people infected with monkeypox were vaccinated against smallpox before mandatory vaccination for this disease was abolished [Editor’s note: The requirement of an initial dose of smallpox vaccine was lifted in 1979, once smallpox had been eradicated].
It is hoped that, as a minimum, this vaccine protects against serious illness. Yet in my department, we regularly see severe cases of monkeypox with widespread lesions in the over 45s, who are said to be vaccinated against smallpox.
Question: By comparison, is it likely that a third-generation vaccine would afford better protection against severe illness?
Dr. Davido: We still don’t have enough data or hindsight to assess the real-world impact of third-generation vaccines. This vaccine has a better tolerance profile than its predecessors, but we currently don’t know if it protects against severe forms of monkeypox. We also need to learn more about the disease causing the current epidemic, since it seems different from the sporadic outbreaks that usually crop up in Central and West Africa. The lesions seen are notably milder. The WHO has given this vaccine an efficacy level of 85% against infection by the monkeypox virus, but we must remain cautious: This figure is based on data from Africa. The epidemic in which we find ourselves is not the same. Overall, we must be wary of overly optimistic rhetoric around this new epidemic.
A version of this article appeared on Medscape.com. The article was translated from the Medscape French edition.
‘I missed it’: Coping with medical error
Thursday night
It was 9 o’clock at night when my phone rang. I didn’t recognize the number but decided to answer it anyway. It was my doctor.
“Chase, I got your labs back and you have a critically low level. I spoke with someone at the hospital, I think I know what is happening, but I need you to go to the pharmacy right now and get a medicine.” She explained further and as I listened electric currents ran through my thighs until I could barely feel my legs.
“I’m so sorry, Chase. I missed it. It was low the last time we did your labs 9 months ago, and I missed it.”
In disbelief, I continued to listen as she instructed me about the next steps I was to take and prepared me for what was to come the next day.
“If you notice any changes overnight, go straight to the ED.”
My chest tingled and I could barely breathe. My mind struggled to comprehend what was happening. I looked at my husband sitting close by on the couch. He looked concerned. I tuned back in and heard her say: “Is your husband there? Can I talk to him?”
“Yes,” is all I could manage, and I handed him the phone. I sat while he listened and asked his questions. My breathing came back under my control, my legs felt wiry, and restlessness set in. “I have to get out of here,” I thought. “I have to go and pick up this medicine.”
Monday afternoon
I am sitting across from a PGY3 resident I have been treating since his intern year, as part of his treatment plan for managing a chronic mental illness that began in medical school. Earlier in the day, I received an urgent message from him requesting an emergency appointment.
Within a few minutes of sitting down, the story from his weekend call shift tumbled out of him. His speech became pressured, and his eyes welled with tears as he recounted in detail the steps he had taken to care for a very sick patient overnight.
“I missed it.” The dam broke and he sat sobbing in front of me, his body trembling.
I sat silently across from him. Willing him to breathe.
In time, his breathing came back under his control, and he slowly regained his composure. He continued: “I got the imaging, and I missed a bleed.”
Failure and shame
I can recall memorable moments from my training when I came to understand that what I initially perceived to be a mistake was instead part of the work. An example from our practice involves a patient whom I was comanaging with her primary care provider (PCP). She was not doing well following a critical work event. When I met with her after the event, she admitted having thoughts of suicide, refused a voluntary inpatient admission, and would not have met criteria for an involuntary admission. My hands were tied.
Together we created a plan to keep her safe, which included paging her PCP after hours if needed. I told her PCP before leaving that night that he might hear from her and that if she reached out, she would require hospitalization.
I arrived at work the following day, and her PCP shared with me that our patient had overdosed on medication, paged him, and was admitted to the unit.
He seemed forlorn.
I was both relieved by the news and confused by his reaction. I had hoped that she would choose a higher level of care than what we could provide her as an outpatient. I said: “This is good. She followed the plan.”
Her overdose was, of course, not part of the plan. She was struggling with several internal conflicts, including having mixed feelings about coming into the hospital; but, when the critical moment happened and she was faced with a decision to call for help or possibly die, she chose to call her PCP and have him paged as we had talked about.
I looked at her PCP. “You helped get her to where she needed to be.”
In the years of working side by side with medically trained colleagues, I have time and again needed to reframe for them that what they perceive to be a “failure” or a “crisis” is often a catalyst for change. The patient I comanaged with the PCP was a highly skilled caregiver and, as such, had been having a hard time asking for help. The hospitalization that her PCP facilitated allowed her to receive the care she needed and created an opportunity for family and friends to show up for her. Their support fed her, and she only made gains from that point on.
My training had taught me that respecting a patient’s autonomy was of the utmost importance. This instills confidence in patients as the authority in their lives. For a clinician to do this, a certain amount of helplessness must be tolerated. As I became better at identifying these moments of helplessness, feelings of failure and shame transformed.
Medical error
Sitting across from the PGY3 resident who I had met with weekly for the past 3 years, I thought about his error.
I thought about my phone call 4 nights earlier. My doctor was called at home by a lab technician, who never met their patients but was simply following protocol and alerted my doctor to the worsening number that she should have been aware of 9 months earlier.
Just like my doctor’s lapse of attention, my patient’s error was not a moment of helplessness to be tolerated. These were mistakes, and there was no way around it.
“People make mistakes.” I said simply.
We sat silently for a time.
I don’t remember who broke the silence. The conversation that followed was centered on our humanity and our capability for both compassion and fallibility. Afterward, I wondered who my doctor confided in and hoped she had a similar conversation.
Dr. Levesque is a clinical psychologist and clinical assistant professor of psychiatry at the Geisel School of Medicine at Dartmouth, Hanover, N.H., where she also serves on the Committee for a Respectful Learning Environment.
A version of this article first appeared on Medscape.com.
Thursday night
It was 9 o’clock at night when my phone rang. I didn’t recognize the number but decided to answer it anyway. It was my doctor.
“Chase, I got your labs back and you have a critically low level. I spoke with someone at the hospital, I think I know what is happening, but I need you to go to the pharmacy right now and get a medicine.” She explained further and as I listened electric currents ran through my thighs until I could barely feel my legs.
“I’m so sorry, Chase. I missed it. It was low the last time we did your labs 9 months ago, and I missed it.”
In disbelief, I continued to listen as she instructed me about the next steps I was to take and prepared me for what was to come the next day.
“If you notice any changes overnight, go straight to the ED.”
My chest tingled and I could barely breathe. My mind struggled to comprehend what was happening. I looked at my husband sitting close by on the couch. He looked concerned. I tuned back in and heard her say: “Is your husband there? Can I talk to him?”
“Yes,” is all I could manage, and I handed him the phone. I sat while he listened and asked his questions. My breathing came back under my control, my legs felt wiry, and restlessness set in. “I have to get out of here,” I thought. “I have to go and pick up this medicine.”
Monday afternoon
I am sitting across from a PGY3 resident I have been treating since his intern year, as part of his treatment plan for managing a chronic mental illness that began in medical school. Earlier in the day, I received an urgent message from him requesting an emergency appointment.
Within a few minutes of sitting down, the story from his weekend call shift tumbled out of him. His speech became pressured, and his eyes welled with tears as he recounted in detail the steps he had taken to care for a very sick patient overnight.
“I missed it.” The dam broke and he sat sobbing in front of me, his body trembling.
I sat silently across from him. Willing him to breathe.
In time, his breathing came back under his control, and he slowly regained his composure. He continued: “I got the imaging, and I missed a bleed.”
Failure and shame
I can recall memorable moments from my training when I came to understand that what I initially perceived to be a mistake was instead part of the work. An example from our practice involves a patient whom I was comanaging with her primary care provider (PCP). She was not doing well following a critical work event. When I met with her after the event, she admitted having thoughts of suicide, refused a voluntary inpatient admission, and would not have met criteria for an involuntary admission. My hands were tied.
Together we created a plan to keep her safe, which included paging her PCP after hours if needed. I told her PCP before leaving that night that he might hear from her and that if she reached out, she would require hospitalization.
I arrived at work the following day, and her PCP shared with me that our patient had overdosed on medication, paged him, and was admitted to the unit.
He seemed forlorn.
I was both relieved by the news and confused by his reaction. I had hoped that she would choose a higher level of care than what we could provide her as an outpatient. I said: “This is good. She followed the plan.”
Her overdose was, of course, not part of the plan. She was struggling with several internal conflicts, including having mixed feelings about coming into the hospital; but, when the critical moment happened and she was faced with a decision to call for help or possibly die, she chose to call her PCP and have him paged as we had talked about.
I looked at her PCP. “You helped get her to where she needed to be.”
In the years of working side by side with medically trained colleagues, I have time and again needed to reframe for them that what they perceive to be a “failure” or a “crisis” is often a catalyst for change. The patient I comanaged with the PCP was a highly skilled caregiver and, as such, had been having a hard time asking for help. The hospitalization that her PCP facilitated allowed her to receive the care she needed and created an opportunity for family and friends to show up for her. Their support fed her, and she only made gains from that point on.
My training had taught me that respecting a patient’s autonomy was of the utmost importance. This instills confidence in patients as the authority in their lives. For a clinician to do this, a certain amount of helplessness must be tolerated. As I became better at identifying these moments of helplessness, feelings of failure and shame transformed.
Medical error
Sitting across from the PGY3 resident who I had met with weekly for the past 3 years, I thought about his error.
I thought about my phone call 4 nights earlier. My doctor was called at home by a lab technician, who never met their patients but was simply following protocol and alerted my doctor to the worsening number that she should have been aware of 9 months earlier.
Just like my doctor’s lapse of attention, my patient’s error was not a moment of helplessness to be tolerated. These were mistakes, and there was no way around it.
“People make mistakes.” I said simply.
We sat silently for a time.
I don’t remember who broke the silence. The conversation that followed was centered on our humanity and our capability for both compassion and fallibility. Afterward, I wondered who my doctor confided in and hoped she had a similar conversation.
Dr. Levesque is a clinical psychologist and clinical assistant professor of psychiatry at the Geisel School of Medicine at Dartmouth, Hanover, N.H., where she also serves on the Committee for a Respectful Learning Environment.
A version of this article first appeared on Medscape.com.
Thursday night
It was 9 o’clock at night when my phone rang. I didn’t recognize the number but decided to answer it anyway. It was my doctor.
“Chase, I got your labs back and you have a critically low level. I spoke with someone at the hospital, I think I know what is happening, but I need you to go to the pharmacy right now and get a medicine.” She explained further and as I listened electric currents ran through my thighs until I could barely feel my legs.
“I’m so sorry, Chase. I missed it. It was low the last time we did your labs 9 months ago, and I missed it.”
In disbelief, I continued to listen as she instructed me about the next steps I was to take and prepared me for what was to come the next day.
“If you notice any changes overnight, go straight to the ED.”
My chest tingled and I could barely breathe. My mind struggled to comprehend what was happening. I looked at my husband sitting close by on the couch. He looked concerned. I tuned back in and heard her say: “Is your husband there? Can I talk to him?”
“Yes,” is all I could manage, and I handed him the phone. I sat while he listened and asked his questions. My breathing came back under my control, my legs felt wiry, and restlessness set in. “I have to get out of here,” I thought. “I have to go and pick up this medicine.”
Monday afternoon
I am sitting across from a PGY3 resident I have been treating since his intern year, as part of his treatment plan for managing a chronic mental illness that began in medical school. Earlier in the day, I received an urgent message from him requesting an emergency appointment.
Within a few minutes of sitting down, the story from his weekend call shift tumbled out of him. His speech became pressured, and his eyes welled with tears as he recounted in detail the steps he had taken to care for a very sick patient overnight.
“I missed it.” The dam broke and he sat sobbing in front of me, his body trembling.
I sat silently across from him. Willing him to breathe.
In time, his breathing came back under his control, and he slowly regained his composure. He continued: “I got the imaging, and I missed a bleed.”
Failure and shame
I can recall memorable moments from my training when I came to understand that what I initially perceived to be a mistake was instead part of the work. An example from our practice involves a patient whom I was comanaging with her primary care provider (PCP). She was not doing well following a critical work event. When I met with her after the event, she admitted having thoughts of suicide, refused a voluntary inpatient admission, and would not have met criteria for an involuntary admission. My hands were tied.
Together we created a plan to keep her safe, which included paging her PCP after hours if needed. I told her PCP before leaving that night that he might hear from her and that if she reached out, she would require hospitalization.
I arrived at work the following day, and her PCP shared with me that our patient had overdosed on medication, paged him, and was admitted to the unit.
He seemed forlorn.
I was both relieved by the news and confused by his reaction. I had hoped that she would choose a higher level of care than what we could provide her as an outpatient. I said: “This is good. She followed the plan.”
Her overdose was, of course, not part of the plan. She was struggling with several internal conflicts, including having mixed feelings about coming into the hospital; but, when the critical moment happened and she was faced with a decision to call for help or possibly die, she chose to call her PCP and have him paged as we had talked about.
I looked at her PCP. “You helped get her to where she needed to be.”
In the years of working side by side with medically trained colleagues, I have time and again needed to reframe for them that what they perceive to be a “failure” or a “crisis” is often a catalyst for change. The patient I comanaged with the PCP was a highly skilled caregiver and, as such, had been having a hard time asking for help. The hospitalization that her PCP facilitated allowed her to receive the care she needed and created an opportunity for family and friends to show up for her. Their support fed her, and she only made gains from that point on.
My training had taught me that respecting a patient’s autonomy was of the utmost importance. This instills confidence in patients as the authority in their lives. For a clinician to do this, a certain amount of helplessness must be tolerated. As I became better at identifying these moments of helplessness, feelings of failure and shame transformed.
Medical error
Sitting across from the PGY3 resident who I had met with weekly for the past 3 years, I thought about his error.
I thought about my phone call 4 nights earlier. My doctor was called at home by a lab technician, who never met their patients but was simply following protocol and alerted my doctor to the worsening number that she should have been aware of 9 months earlier.
Just like my doctor’s lapse of attention, my patient’s error was not a moment of helplessness to be tolerated. These were mistakes, and there was no way around it.
“People make mistakes.” I said simply.
We sat silently for a time.
I don’t remember who broke the silence. The conversation that followed was centered on our humanity and our capability for both compassion and fallibility. Afterward, I wondered who my doctor confided in and hoped she had a similar conversation.
Dr. Levesque is a clinical psychologist and clinical assistant professor of psychiatry at the Geisel School of Medicine at Dartmouth, Hanover, N.H., where she also serves on the Committee for a Respectful Learning Environment.
A version of this article first appeared on Medscape.com.