User login
A Step-by-Step Guide for Diagnosing Cushing Syndrome
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
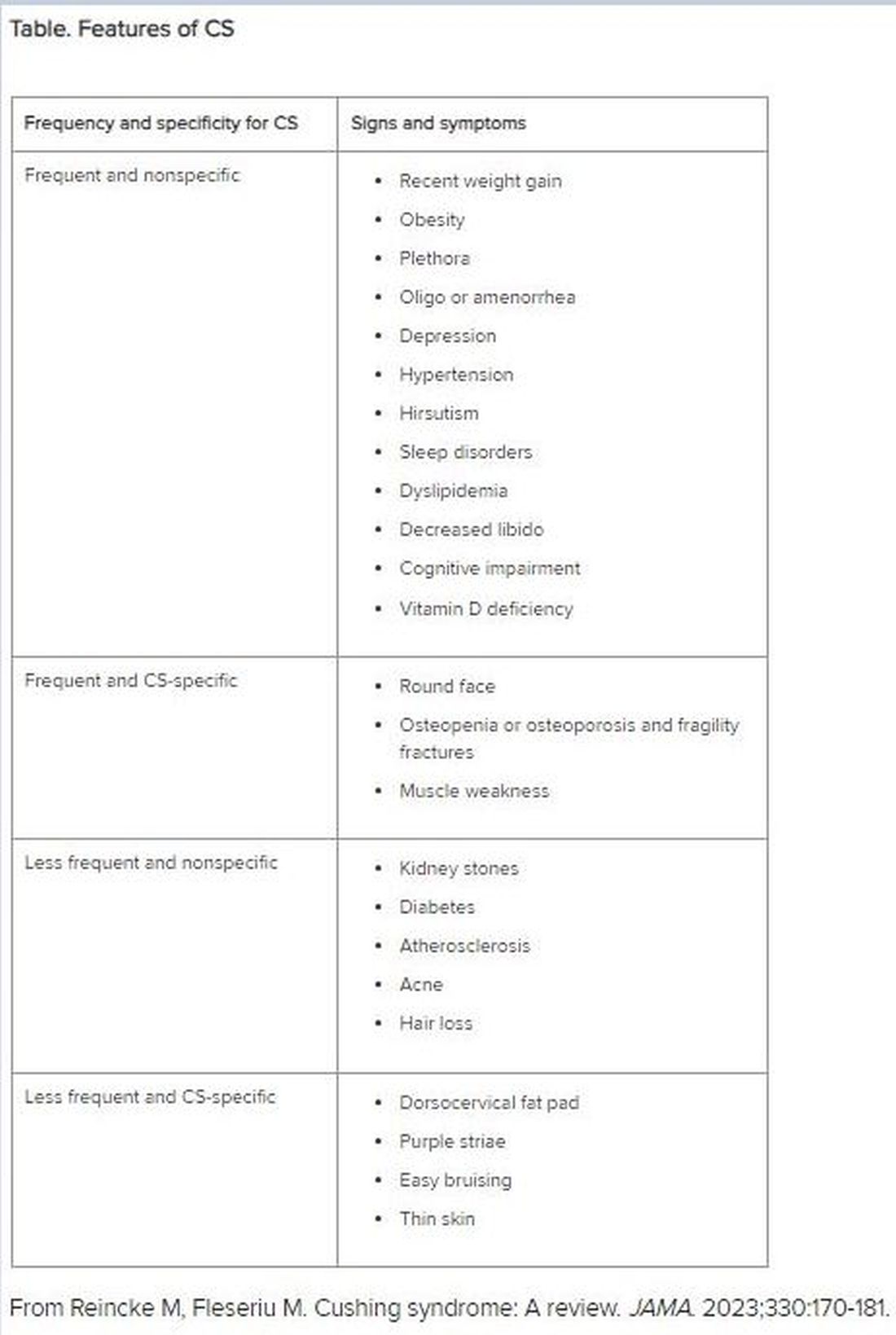
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
Physicians Lament Over Reliance on Relative Value Units: Survey
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
Most physicians oppose the way standardized relative value units (RVUs) are used to determine performance and compensation, according to Medscape’s 2024 Physicians and RVUs Report. About 6 in 10 survey respondents were unhappy with how RVUs affected them financially, while 7 in 10 said RVUs were poor measures of productivity.
The report analyzed 2024 survey data from 1005 practicing physicians who earn RVUs.
“I’m already mad that the medical field is controlled by health insurers and what they pay and authorize,” said an anesthesiologist in New York. “Then [that approach] is transferred to medical offices and hospitals, where physicians are paid by RVUs.”
Most physicians surveyed produced between 4000 and 8000 RVUs per year. Roughly one in six were high RVU generators, generating more than 10,000 annually.
In most cases, the metric influences earning potential — 42% of doctors surveyed said RVUs affect their salaries to some degree. One quarter said their salary was based entirely on RVUs. More than three fourths of physicians who received performance bonuses said they must meet RVU targets to do so.
“The current RVU system encourages unnecessary procedures, hurting patients,” said an orthopedic surgeon in Maine.
Nearly three fourths of practitioners surveyed said they occasionally to frequently felt pressure to take on more patients as a result of this system.
“I know numerous primary care doctors and specialists who have been forced to increase patient volume to meet RVU goals, and none is happy about it,” said Alok Patel, MD, a pediatric hospitalist with Stanford Hospital in Palo Alto, California. “Plus, patients are definitely not happy about being rushed.”
More than half of respondents said they occasionally or frequently felt compelled by their employer to use higher-level coding, which interferes with a physician’s ethical responsibility to the patient, said Arthur L. Caplan, PhD, a bioethicist at NYU Langone Medical Center in New York City.
“Rather than rewarding excellence or good outcomes, you’re kind of rewarding procedures and volume,” said Dr. Caplan. “It’s more than pressure; it’s expected.”
Nearly 6 in 10 physicians said that the method for calculating reimbursements was unfair. Almost half said that they weren’t happy with how their workplace uses RVUs.
A few respondents said that their RVU model, which is often based on what Dr. Patel called an “overly complicated algorithm,” did not account for the time spent on tasks or the fact that some patients miss appointments. RVUs also rely on factors outside the control of a physician, such as location and patient volume, said one doctor.
The model can also lower the level of care patients receive, Dr. Patel said.
“I know primary care doctors who work in RVU-based systems and simply cannot take the necessary time — even if it’s 30-45 minutes — to thoroughly assess a patient, when the model forces them to take on 15-minute encounters.”
Finally, over half of clinicians said alternatives to the RVU system would be more effective, and 77% suggested including qualitative data. One respondent recommended incorporating time spent doing paperwork and communicating with patients, complexity of conditions, and medication management.
A version of this article first appeared on Medscape.com.
After Remission Failure in Early RA, Adding Etanercept No Better Than Adding Leflunomide
TOPLINE:
Treatment with etanercept led to faster disease control initially in patients with early rheumatoid arthritis (RA) who had an insufficient early response to methotrexate and bridging glucocorticoids therapy, but more patients achieved disease control with leflunomide at 104 weeks.
METHODOLOGY:
- Researchers conducted CareRA2020, a randomized controlled trial including 276 patients with early RA who were initially treated with oral methotrexate 15 mg/wk and a step-down prednisone scheme, with early insufficient responders (n = 110) randomized to add etanercept 50 mg/wk or leflunomide 10 mg/d for 24 weeks.
- Patients were classified as early insufficient responders if they did not achieve a 28-joint Disease Activity Score with C-reactive protein (DAS28-CRP) < 3.2 between weeks 8 and 32 or < 2.6 at week 32, despite an increase in methotrexate dose to 20 mg/wk.
- The primary outcome was the longitudinal disease activity measured by DAS28-CRP over 104 weeks.
- The secondary outcomes included disease control at 28 weeks post randomization and the use of biologic or targeted synthetic disease-modifying antirheumatic drugs at week 104.
TAKEAWAY:
- Early introduction of etanercept in patients with RA did not show long-term superiority over leflunomide in disease control over 2 years (P = .157).
- At 28 weeks post randomization, the percentage of patients who achieved a DAS28-CRP < 2.6 was higher in the etanercept group than in the leflunomide group (59% vs 44%).
- After stopping etanercept, disease activity scores worsened, and a lower proportion of patients achieved DAS28-CRP < 2.6 in the etanercept group than in the leflunomide group (55% vs 69%) at week 104.
- Even after treatment with etanercept or leflunomide, the 110 early insufficient responders never reached the same level of disease control as the 142 patients who responded to methotrexate and bridging glucocorticoids within weeks 8-32.
IN PRACTICE:
“The CareRA2020 trial did not completely solve the unmet need of patients responding insufficiently to conventional initial therapy for early RA, but it provides opportunities to further optimize the treatment approach in this population, for instance, by focusing on the identification of potential subgroups with different disease activity trajectories within the early insufficient responder group,” wrote the authors.
SOURCE:
The study was led by Delphine Bertrand of the Skeletal Biology and Engineering Research Center in the Department of Development and Regeneration at KU Leuven in Belgium, and was published online on August 7, 2024, in RMD Open.
LIMITATIONS:
The open-label design of the study may have introduced bias, as patients and investigators were aware of the treatment. The temporary administration of etanercept may not have reflected its long-term effects. The study was conducted in Belgium, which limited the generalizability of the findings to other populations.
DISCLOSURES:
The study was supported by the Belgian Health Care Knowledge Centre. Some authors reported serving as speakers or receiving grants, consulting fees, honoraria, or meeting or travel support from financial ties with Novartis, Pfizer, Amgen, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Treatment with etanercept led to faster disease control initially in patients with early rheumatoid arthritis (RA) who had an insufficient early response to methotrexate and bridging glucocorticoids therapy, but more patients achieved disease control with leflunomide at 104 weeks.
METHODOLOGY:
- Researchers conducted CareRA2020, a randomized controlled trial including 276 patients with early RA who were initially treated with oral methotrexate 15 mg/wk and a step-down prednisone scheme, with early insufficient responders (n = 110) randomized to add etanercept 50 mg/wk or leflunomide 10 mg/d for 24 weeks.
- Patients were classified as early insufficient responders if they did not achieve a 28-joint Disease Activity Score with C-reactive protein (DAS28-CRP) < 3.2 between weeks 8 and 32 or < 2.6 at week 32, despite an increase in methotrexate dose to 20 mg/wk.
- The primary outcome was the longitudinal disease activity measured by DAS28-CRP over 104 weeks.
- The secondary outcomes included disease control at 28 weeks post randomization and the use of biologic or targeted synthetic disease-modifying antirheumatic drugs at week 104.
TAKEAWAY:
- Early introduction of etanercept in patients with RA did not show long-term superiority over leflunomide in disease control over 2 years (P = .157).
- At 28 weeks post randomization, the percentage of patients who achieved a DAS28-CRP < 2.6 was higher in the etanercept group than in the leflunomide group (59% vs 44%).
- After stopping etanercept, disease activity scores worsened, and a lower proportion of patients achieved DAS28-CRP < 2.6 in the etanercept group than in the leflunomide group (55% vs 69%) at week 104.
- Even after treatment with etanercept or leflunomide, the 110 early insufficient responders never reached the same level of disease control as the 142 patients who responded to methotrexate and bridging glucocorticoids within weeks 8-32.
IN PRACTICE:
“The CareRA2020 trial did not completely solve the unmet need of patients responding insufficiently to conventional initial therapy for early RA, but it provides opportunities to further optimize the treatment approach in this population, for instance, by focusing on the identification of potential subgroups with different disease activity trajectories within the early insufficient responder group,” wrote the authors.
SOURCE:
The study was led by Delphine Bertrand of the Skeletal Biology and Engineering Research Center in the Department of Development and Regeneration at KU Leuven in Belgium, and was published online on August 7, 2024, in RMD Open.
LIMITATIONS:
The open-label design of the study may have introduced bias, as patients and investigators were aware of the treatment. The temporary administration of etanercept may not have reflected its long-term effects. The study was conducted in Belgium, which limited the generalizability of the findings to other populations.
DISCLOSURES:
The study was supported by the Belgian Health Care Knowledge Centre. Some authors reported serving as speakers or receiving grants, consulting fees, honoraria, or meeting or travel support from financial ties with Novartis, Pfizer, Amgen, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
Treatment with etanercept led to faster disease control initially in patients with early rheumatoid arthritis (RA) who had an insufficient early response to methotrexate and bridging glucocorticoids therapy, but more patients achieved disease control with leflunomide at 104 weeks.
METHODOLOGY:
- Researchers conducted CareRA2020, a randomized controlled trial including 276 patients with early RA who were initially treated with oral methotrexate 15 mg/wk and a step-down prednisone scheme, with early insufficient responders (n = 110) randomized to add etanercept 50 mg/wk or leflunomide 10 mg/d for 24 weeks.
- Patients were classified as early insufficient responders if they did not achieve a 28-joint Disease Activity Score with C-reactive protein (DAS28-CRP) < 3.2 between weeks 8 and 32 or < 2.6 at week 32, despite an increase in methotrexate dose to 20 mg/wk.
- The primary outcome was the longitudinal disease activity measured by DAS28-CRP over 104 weeks.
- The secondary outcomes included disease control at 28 weeks post randomization and the use of biologic or targeted synthetic disease-modifying antirheumatic drugs at week 104.
TAKEAWAY:
- Early introduction of etanercept in patients with RA did not show long-term superiority over leflunomide in disease control over 2 years (P = .157).
- At 28 weeks post randomization, the percentage of patients who achieved a DAS28-CRP < 2.6 was higher in the etanercept group than in the leflunomide group (59% vs 44%).
- After stopping etanercept, disease activity scores worsened, and a lower proportion of patients achieved DAS28-CRP < 2.6 in the etanercept group than in the leflunomide group (55% vs 69%) at week 104.
- Even after treatment with etanercept or leflunomide, the 110 early insufficient responders never reached the same level of disease control as the 142 patients who responded to methotrexate and bridging glucocorticoids within weeks 8-32.
IN PRACTICE:
“The CareRA2020 trial did not completely solve the unmet need of patients responding insufficiently to conventional initial therapy for early RA, but it provides opportunities to further optimize the treatment approach in this population, for instance, by focusing on the identification of potential subgroups with different disease activity trajectories within the early insufficient responder group,” wrote the authors.
SOURCE:
The study was led by Delphine Bertrand of the Skeletal Biology and Engineering Research Center in the Department of Development and Regeneration at KU Leuven in Belgium, and was published online on August 7, 2024, in RMD Open.
LIMITATIONS:
The open-label design of the study may have introduced bias, as patients and investigators were aware of the treatment. The temporary administration of etanercept may not have reflected its long-term effects. The study was conducted in Belgium, which limited the generalizability of the findings to other populations.
DISCLOSURES:
The study was supported by the Belgian Health Care Knowledge Centre. Some authors reported serving as speakers or receiving grants, consulting fees, honoraria, or meeting or travel support from financial ties with Novartis, Pfizer, Amgen, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
The Most Misinterpreted Study in Medicine: Don’t be TRICCed
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients With Immune-Mediated Inflammatory Diseases, Type 2 Diabetes Reap GLP-1 Receptor Agonist Benefits, Too
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are associated with a lower risk for all-cause mortality and major adverse cardiovascular events (MACE) in patients with immune-mediated inflammatory diseases (IMIDs) and type 2 diabetes (T2D).
METHODOLOGY:
- GLP-1 RAs reduce the risk for all-cause mortality, cardiovascular mortality, and stroke in patients with diabetes. However, previous trials have excluded those with IMIDs, leaving a gap in understanding the cardioprotective effects of GLP-1 RAs in this population.
- Researchers conducted a population-based cohort study to assess if patients with an IMID derive greater benefits from GLP-1 RAs than DPP-4 inhibitors.
- They used administrative health data from British Columbia, Canada, to include 10,855 patients with IMIDs (rheumatoid arthritis, psoriatic disease, ankylosing spondylitis, inflammatory bowel disease, or systemic autoimmune rheumatic disease) and T2D who initiated either GLP-1 RA (n = 3570) or DPP-4 inhibitor (n = 7285).
- The mean follow-up was 1.46 and 1.88 years in the GLP-1 RA and DPP-4 inhibitor cohorts, respectively.
- The primary outcome was all-cause mortality, and the secondary outcome was MACE, including cardiovascular death, myocardial infarction, and ischemic stroke.
TAKEAWAY:
- The risk for all-cause mortality was 52% lower in patients who initiated GLP-1 RAs than in those who initiated DPP-4 inhibitors (weighted hazard ratio [HR], 0.48; 95% CI, 0.31-0.75).
- Additionally, patients initiating DPP-4 inhibitors.
- In the subgroup of patients with GLP-1 RAs had a significantly lower risk for MACE (weighted HR, 0.66; 95% CI, 0.50-0.88), particularly myocardial infarction (weighted HR, 0.62; 95% CI, 0.40-0.96), than those initiating rheumatoid arthritis and T2D, those who initiated GLP-1 RAs had a 55% lower risk for all-cause mortality and 61% lower risk for MACE than those who initiated DPP-4 inhibitors.
IN PRACTICE:
“This corresponds to nine fewer deaths and 11 fewer MACE per 1000 person-years, respectively, supporting the hypothesis that these agents have a cardioprotective effect in this high-risk population,” the authors wrote.
SOURCE:
This study was led by Derin Karacabeyli, MD, Division of Rheumatology, Department of Medicine, University of British Columbia, Vancouver, Canada, and was published online on August 8, 2024, in PLOS ONE.
LIMITATIONS:
The study’s dependence on administrative health data might have resulted in incomplete capture of comorbidities, particularly obesity. The mean follow-up period was relatively short, which might have limited the long-term applicability of these findings. The accuracy of the case definitions for IMIDs and T2D, according to International Classification of Diseases codes, could not be fully ascertained.
DISCLOSURES:
The study was supported by grants from the Canadian Institutes of Health Research. Two authors declared receiving research support, consulting fees, or participating in advisory boards outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Hearing Loss, Neuropathy Cut Survival in Older Adults
TOPLINE:
METHODOLOGY:
- Researchers analyzed 793 older adults recruited from primary care practices participating in the OKLAHOMA Studies in 1999.
- Participants completed a questionnaire and underwent a physical examination; timed gait assessments (50 ft); and tests for peripheral nerve function, balance, and hearing.
- Hearing thresholds were tested at 20, 25, and 40 dB, respectively, and at sound frequencies of 500, 1000, 2000, and 4000 Hz.
- Researchers tracked mortality data over 22 years.
TAKEAWAY:
- Overall, 83% participants experienced hearing loss. Regular use of hearing aids was low, reported in 19% and 55% of those with moderate and severe hearing loss, respectively.
- Hearing loss was linked to impaired balance (P = .0014), slower walking (P = .0024), and reduced survival time (P = .0001). Moderate to severe hearing loss was strongly associated with reduced survival time (odds ratio, 1.36; P = .001), independent of the use of hearing aids.
- Peripheral neuropathy was present in 32% participants. The condition also increased the risk for death over the study period (hazard ratio [HR], 1.32; P = .003). Participants with both hearing loss and peripheral neuropathy showed reduced balance and survival time compared with people with either condition alone (HR, 1.55; P < .0001).
IN PRACTICE:
“Like peripheral neuropathy, advanced-age hearing loss is associated with reduced life expectancy, probably mediated in part through an adverse impact on balance,” the authors wrote. “Greater appreciation for the serious impacts of hearing loss and peripheral neuropathy could lead to further efforts to understand their causes and improve prevention and treatment strategies.”
SOURCE:
The study was led by James W. Mold, MD, MPH, of the University of Oklahoma Health Sciences Center, Oklahoma City. It was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
The dataset was collected in 1999 and may not entirely represent the current cohorts of older primary care patients. The absence of soundproof rooms and the exclusion of some components of the standard audiometric evaluation may have affected low-frequency sound measurements. Furthermore, physical examination was a less accurate measure of peripheral neuropathy. Information on the duration or severity of predictors and causes of death was not available.
DISCLOSURES:
The study was funded by the Presbyterian Health Foundation. The authors did not disclose any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed 793 older adults recruited from primary care practices participating in the OKLAHOMA Studies in 1999.
- Participants completed a questionnaire and underwent a physical examination; timed gait assessments (50 ft); and tests for peripheral nerve function, balance, and hearing.
- Hearing thresholds were tested at 20, 25, and 40 dB, respectively, and at sound frequencies of 500, 1000, 2000, and 4000 Hz.
- Researchers tracked mortality data over 22 years.
TAKEAWAY:
- Overall, 83% participants experienced hearing loss. Regular use of hearing aids was low, reported in 19% and 55% of those with moderate and severe hearing loss, respectively.
- Hearing loss was linked to impaired balance (P = .0014), slower walking (P = .0024), and reduced survival time (P = .0001). Moderate to severe hearing loss was strongly associated with reduced survival time (odds ratio, 1.36; P = .001), independent of the use of hearing aids.
- Peripheral neuropathy was present in 32% participants. The condition also increased the risk for death over the study period (hazard ratio [HR], 1.32; P = .003). Participants with both hearing loss and peripheral neuropathy showed reduced balance and survival time compared with people with either condition alone (HR, 1.55; P < .0001).
IN PRACTICE:
“Like peripheral neuropathy, advanced-age hearing loss is associated with reduced life expectancy, probably mediated in part through an adverse impact on balance,” the authors wrote. “Greater appreciation for the serious impacts of hearing loss and peripheral neuropathy could lead to further efforts to understand their causes and improve prevention and treatment strategies.”
SOURCE:
The study was led by James W. Mold, MD, MPH, of the University of Oklahoma Health Sciences Center, Oklahoma City. It was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
The dataset was collected in 1999 and may not entirely represent the current cohorts of older primary care patients. The absence of soundproof rooms and the exclusion of some components of the standard audiometric evaluation may have affected low-frequency sound measurements. Furthermore, physical examination was a less accurate measure of peripheral neuropathy. Information on the duration or severity of predictors and causes of death was not available.
DISCLOSURES:
The study was funded by the Presbyterian Health Foundation. The authors did not disclose any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers analyzed 793 older adults recruited from primary care practices participating in the OKLAHOMA Studies in 1999.
- Participants completed a questionnaire and underwent a physical examination; timed gait assessments (50 ft); and tests for peripheral nerve function, balance, and hearing.
- Hearing thresholds were tested at 20, 25, and 40 dB, respectively, and at sound frequencies of 500, 1000, 2000, and 4000 Hz.
- Researchers tracked mortality data over 22 years.
TAKEAWAY:
- Overall, 83% participants experienced hearing loss. Regular use of hearing aids was low, reported in 19% and 55% of those with moderate and severe hearing loss, respectively.
- Hearing loss was linked to impaired balance (P = .0014), slower walking (P = .0024), and reduced survival time (P = .0001). Moderate to severe hearing loss was strongly associated with reduced survival time (odds ratio, 1.36; P = .001), independent of the use of hearing aids.
- Peripheral neuropathy was present in 32% participants. The condition also increased the risk for death over the study period (hazard ratio [HR], 1.32; P = .003). Participants with both hearing loss and peripheral neuropathy showed reduced balance and survival time compared with people with either condition alone (HR, 1.55; P < .0001).
IN PRACTICE:
“Like peripheral neuropathy, advanced-age hearing loss is associated with reduced life expectancy, probably mediated in part through an adverse impact on balance,” the authors wrote. “Greater appreciation for the serious impacts of hearing loss and peripheral neuropathy could lead to further efforts to understand their causes and improve prevention and treatment strategies.”
SOURCE:
The study was led by James W. Mold, MD, MPH, of the University of Oklahoma Health Sciences Center, Oklahoma City. It was published online in the Journal of the American Geriatrics Society.
LIMITATIONS:
The dataset was collected in 1999 and may not entirely represent the current cohorts of older primary care patients. The absence of soundproof rooms and the exclusion of some components of the standard audiometric evaluation may have affected low-frequency sound measurements. Furthermore, physical examination was a less accurate measure of peripheral neuropathy. Information on the duration or severity of predictors and causes of death was not available.
DISCLOSURES:
The study was funded by the Presbyterian Health Foundation. The authors did not disclose any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Necrotic Papules in a Pediatric Patient
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
A 7-year-old boy was referred to the dermatology clinic for evaluation of a diffuse pruritic rash of 3 months’ duration. The rash began as scant erythematous papules on the face, and crops of similar lesions later erupted on the trunk, arms, and legs. He was treated previously by a pediatrician for scabies with topical permethrin followed by 2 doses of oral ivermectin 200 μg/kg without improvement. Physical examination revealed innumerable erythematous macules and papules with centrally adherent scaling distributed on the trunk, arms, and legs, as well as scant necrotic papules with a hemorrhagic crust and a peripheral rim of scale.

Can Belzutifan Improve Outcomes in Advanced RCC?
TOPLINE:
At 18 months, 24% of participants on belzutifan were alive and progression free, compared with 8.3% on everolimus.
METHODOLOGY:
- The phase 3, multicenter, open-label, randomized, active-controlled LITESPARK-005 trial was conducted at 147 sites in six regions.
- The trial aimed to compare the efficacy and safety of belzutifan with everolimus in 746 participants with advanced clear cell renal cell carcinoma who had disease progression after receiving immune checkpoint and antiangiogenic therapies.
- Patients were randomly assigned to receive either 120 mg of belzutifan or 10 mg of everolimus orally once daily and were followed for a median of 18.4 months at the first interim analysis and of 25.7 months at the second interim analysis.
- The dual primary endpoints were progression-free survival and overall survival, with the key secondary endpoint having been a confirmed objective response, defined as a complete or partial response.
- Dose modifications were allowed to manage adverse events, with belzutifan doses reduced to 80 mg and then to 40 mg daily if needed.
TAKEAWAY:
- Belzutifan showed a significant benefit over everolimus in progression-free survival, with 24% of participants on belzutifan alive and progression free at 18 months, compared with 8.3% on everolimus (P = .002).
- The objective response rate was significantly higher in the belzutifan group (21.9%) compared with the everolimus group (3.5%) (P < .001).
- The median overall survival was 21.4 months for belzutifan and 18.1 months for everolimus, with a hazard ratio for death of 0.88 (95% CI, 0.73-1.07; P = .20).
- Grade 3 or higher adverse events occurred in 61.8% of participants in the belzutifan group and in 62.5% in the everolimus group, with adverse events leading to treatment discontinuation in 5.9% and 14.7% of participants, respectively.
IN PRACTICE:
“The LITESPARK-005 trial introduced [hypoxia-Inducible factor 2 alpha] inhibition as an active therapeutic mechanism and established belzutifan as a treatment option in patients with advanced renal-cell carcinoma after both immune checkpoint and antiangiogenic therapies,” the authors wrote.
SOURCE:
Corresponding author Toni K. Choueiri, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, was one of four authors who contributed equally to the article, which was published on August 21 in The New England Journal of Medicine.
LIMITATIONS:
The study’s limitations include the open-label design, which may introduce bias. The trial did not include a placebo group, and the follow-up period may not be sufficient to capture long-term outcomes. In addition, the study population was heavily pretreated, which may limit the generalizability of the findings to broader patient populations.
DISCLOSURES:
Dr. Choueiri disclosed receiving consultancy fees from Merck, the sponsor of the study, and other pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
At 18 months, 24% of participants on belzutifan were alive and progression free, compared with 8.3% on everolimus.
METHODOLOGY:
- The phase 3, multicenter, open-label, randomized, active-controlled LITESPARK-005 trial was conducted at 147 sites in six regions.
- The trial aimed to compare the efficacy and safety of belzutifan with everolimus in 746 participants with advanced clear cell renal cell carcinoma who had disease progression after receiving immune checkpoint and antiangiogenic therapies.
- Patients were randomly assigned to receive either 120 mg of belzutifan or 10 mg of everolimus orally once daily and were followed for a median of 18.4 months at the first interim analysis and of 25.7 months at the second interim analysis.
- The dual primary endpoints were progression-free survival and overall survival, with the key secondary endpoint having been a confirmed objective response, defined as a complete or partial response.
- Dose modifications were allowed to manage adverse events, with belzutifan doses reduced to 80 mg and then to 40 mg daily if needed.
TAKEAWAY:
- Belzutifan showed a significant benefit over everolimus in progression-free survival, with 24% of participants on belzutifan alive and progression free at 18 months, compared with 8.3% on everolimus (P = .002).
- The objective response rate was significantly higher in the belzutifan group (21.9%) compared with the everolimus group (3.5%) (P < .001).
- The median overall survival was 21.4 months for belzutifan and 18.1 months for everolimus, with a hazard ratio for death of 0.88 (95% CI, 0.73-1.07; P = .20).
- Grade 3 or higher adverse events occurred in 61.8% of participants in the belzutifan group and in 62.5% in the everolimus group, with adverse events leading to treatment discontinuation in 5.9% and 14.7% of participants, respectively.
IN PRACTICE:
“The LITESPARK-005 trial introduced [hypoxia-Inducible factor 2 alpha] inhibition as an active therapeutic mechanism and established belzutifan as a treatment option in patients with advanced renal-cell carcinoma after both immune checkpoint and antiangiogenic therapies,” the authors wrote.
SOURCE:
Corresponding author Toni K. Choueiri, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, was one of four authors who contributed equally to the article, which was published on August 21 in The New England Journal of Medicine.
LIMITATIONS:
The study’s limitations include the open-label design, which may introduce bias. The trial did not include a placebo group, and the follow-up period may not be sufficient to capture long-term outcomes. In addition, the study population was heavily pretreated, which may limit the generalizability of the findings to broader patient populations.
DISCLOSURES:
Dr. Choueiri disclosed receiving consultancy fees from Merck, the sponsor of the study, and other pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
At 18 months, 24% of participants on belzutifan were alive and progression free, compared with 8.3% on everolimus.
METHODOLOGY:
- The phase 3, multicenter, open-label, randomized, active-controlled LITESPARK-005 trial was conducted at 147 sites in six regions.
- The trial aimed to compare the efficacy and safety of belzutifan with everolimus in 746 participants with advanced clear cell renal cell carcinoma who had disease progression after receiving immune checkpoint and antiangiogenic therapies.
- Patients were randomly assigned to receive either 120 mg of belzutifan or 10 mg of everolimus orally once daily and were followed for a median of 18.4 months at the first interim analysis and of 25.7 months at the second interim analysis.
- The dual primary endpoints were progression-free survival and overall survival, with the key secondary endpoint having been a confirmed objective response, defined as a complete or partial response.
- Dose modifications were allowed to manage adverse events, with belzutifan doses reduced to 80 mg and then to 40 mg daily if needed.
TAKEAWAY:
- Belzutifan showed a significant benefit over everolimus in progression-free survival, with 24% of participants on belzutifan alive and progression free at 18 months, compared with 8.3% on everolimus (P = .002).
- The objective response rate was significantly higher in the belzutifan group (21.9%) compared with the everolimus group (3.5%) (P < .001).
- The median overall survival was 21.4 months for belzutifan and 18.1 months for everolimus, with a hazard ratio for death of 0.88 (95% CI, 0.73-1.07; P = .20).
- Grade 3 or higher adverse events occurred in 61.8% of participants in the belzutifan group and in 62.5% in the everolimus group, with adverse events leading to treatment discontinuation in 5.9% and 14.7% of participants, respectively.
IN PRACTICE:
“The LITESPARK-005 trial introduced [hypoxia-Inducible factor 2 alpha] inhibition as an active therapeutic mechanism and established belzutifan as a treatment option in patients with advanced renal-cell carcinoma after both immune checkpoint and antiangiogenic therapies,” the authors wrote.
SOURCE:
Corresponding author Toni K. Choueiri, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, was one of four authors who contributed equally to the article, which was published on August 21 in The New England Journal of Medicine.
LIMITATIONS:
The study’s limitations include the open-label design, which may introduce bias. The trial did not include a placebo group, and the follow-up period may not be sufficient to capture long-term outcomes. In addition, the study population was heavily pretreated, which may limit the generalizability of the findings to broader patient populations.
DISCLOSURES:
Dr. Choueiri disclosed receiving consultancy fees from Merck, the sponsor of the study, and other pharmaceutical companies. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Multiple Myeloma: New Treatments Aid Patient Subgroups
“The introduction of treatments such as elranatamab (Elrexfio) is allowing patients with multiple myeloma, which is still incurable for now, to have different options and achieve long periods of remission, thus improving their survival,” she added. “This therapeutic innovation is highly effective and well tolerated in patients with relapse or refractory multiple myeloma.” The overall response rate is “up to 61%, early, deep, and long-lasting.”
In an interview with El Médico Interactivo, Dr. Mateos explained the new approaches to multiple myeloma. She highlighted the effectiveness of new treatments and reviewed the latest data on this disease, which were presented at the recent European Hematology Association Congress.
What is the incidence rate of multiple myeloma in the Spanish population?
Multiple myeloma has an incidence of approximately 4-5 new cases per 100,000 inhabitants per year. This means that around 3000 new cases are diagnosed each year in Spain. As with most tumors, multiple myeloma is generally slightly more common in males than females. It is the third most frequent hematologic cancer in men (1757 new cases) and women (1325 new cases), behind lymphoma and leukemias.
At what age is it most often diagnosed?
It affects older people, with recent reports indicating around 68-69 years as the median age. Although more young people are being diagnosed with multiple myeloma, analyses of how this hematologic cancer affects the general population show that it generally impacts patients over age 65 years.
What is the typical survival prognosis?
Thanks to research and therapeutic innovation, the prognosis has changed significantly over the past 20-25 years. Today, if a patient with multiple myeloma receives a diagnosis and does not exhibit poor prognostic characteristics (and this description fits approximately 70%-80% of patients with multiple myeloma), it is realistic to expect a survival exceeding 10 years. A few years ago, this outcome was unimaginable, but a significant amount of therapeutic innovation has made it possible. That’s why I emphasize that it is realistic to provide these data with such a positive outlook.
Is multiple myeloma a refractory type of cancer?
It was a refractory type of cancer. Twenty years ago, there were no treatment options, and therefore survival was around 2-3 years, because treatment mainly consisted of using alkylating agents and corticosteroids. This is what made it refractory.
With the emergence of new therapeutic innovations, patients have been responding better and their responses are lasting longer. Although there is still a group of patients, about 10%-15%, with a poor prognosis and refractory disease, those with standard risk are responding better to different therapies.
Although most patients will eventually exhaust the treatments, which until now were primarily triple-drug regimens (such as proteasome inhibitors, immunomodulators, and antiCD38 antibodies), the introduction of new therapies is extending the duration of responses.
Is the risk for relapse high?
It is very high, in the sense that almost all patients with multiple myeloma eventually relapse. However, we hope that there soon will be some patients who do not relapse.
What are the typical pathologic manifestations of this cancer? Does it affect everyone equally, or in specific ways in each person?
In multiple myeloma, we often say there are multiple myelomas. Clinically, the disease presents in most patients, around 80%, with two clinical manifestations: anemia and bone lesions. Less frequently, patients may also have kidney failure, hypercalcemia, and a higher tendency toward infection. Behind this rather common symptomatology, from a molecular and genetic perspective, each myeloma is practically unique, adding complexity to its treatment. Therefore, ultimately, myelomas end up being refractory.
Elranatamab is a new therapeutic tool. For which patients is it recommended?
It is a bispecific monoclonal antibody that corresponds to the new monotherapy strategies we have for treating patients with multiple myeloma. On the one hand, it targets damaged plasma cells, which are the patient’s tumor cells, and on the other, it binds the patient’s T cells and redirects them to the tumor niche. When this happens, the T cell activates and destroys the tumor cell.
This medication has been approved for patients with relapsed myeloma who have received traditional drugs for their treatment. We know well that patients who have already received proteasome inhibitors, immunomodulators, and anti-CD38 antibodies typically need something new after treatment. Before, there were no other options, and we would reuse what had been previously used. Now we have elranatamab, a bispecific monoclonal antibody targeting a new receptor that has shown significant responses as monotherapy.
More than 60% of patients respond, and more than 30% achieve complete remission. The key is the response duration and progression-free survival of almost a year and a half. This is the longest progression-free survival we have seen to date in previous lines. Therefore, it fills the needs we had for these relapsed or refractory myeloma patients.
What advantages does this new treatment offer?
It represents a therapeutic innovation because, as mentioned, it achieves a response in more than 60% of patients, and around 35% achieve complete remission. The median response duration has not been reached yet. Progression-free survival is 17.2 months, almost a year and a half, and overall survival is almost two years.
Furthermore, it is administered as subcutaneous monotherapy weekly for the first six cycles and then every 15 days. It has a good safety profile, although some adverse events are known, so we have strategies to combat or mitigate them, making the treatment generally well tolerated.
What side effects are being observed?
They are manageable. When the drug is first administered, patients may experience what we call a cytokine release syndrome, which is a result of the treatment’s mechanism. However, we can predict very well when it occurs, usually 2 days after the first doses, and we have strategies to mitigate it.
The second most common adverse event we need to be cautious about is infection. Nowadays, before starting treatment, patients update their vaccination schedule, receive antiviral prophylaxis, and receive prophylaxis against certain germs, resulting in reduced infections. However, infections are probably the adverse events we need to be most careful about when treating the patient.
We must ensure that prophylaxis is performed, and if fever occurs and an infection is suspected, cultures and all kinds of studies must be done to identify and treat it properly.
How does elranatamab change the treatment of an incurable disease? Does it bring us closer to a cure or to making multiple myeloma a manageable chronic disease?
With the already approved elranatamab, the most important aspect is that it adds another treatment option for patients with myeloma. With the progression-free survival data I indicated, life expectancy is increased, with a good quality of life and acceptable safety.
Obviously, elranatamab is still under study and development, even in early lines, including in patients with newly diagnosed myeloma. When we are choosing first-line therapy, we select the best patients by combining traditional drugs with these new immunotherapies, such as elranatamab, it is likely that we are much closer to offering a cure to specific subgroups.
Although it won’t happen in all cases, I believe it will be applicable to a significant subgroup of patients, making chronicity of the disease a reality we are already approaching. Each day, we encounter more patients receiving different lines of treatment and ultimately meeting their life expectancy with myeloma. Even though some may die, it is often due to causes not related to myeloma. This is the most important contribution of these innovations, such as elranatamab.
Dr. Mateos reported receiving honoraria from Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Stemline, Oncopeptides, and Kite for delivering lectures and for participating in advisory boards.
This story was translated from El Médico Interactivo, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
“The introduction of treatments such as elranatamab (Elrexfio) is allowing patients with multiple myeloma, which is still incurable for now, to have different options and achieve long periods of remission, thus improving their survival,” she added. “This therapeutic innovation is highly effective and well tolerated in patients with relapse or refractory multiple myeloma.” The overall response rate is “up to 61%, early, deep, and long-lasting.”
In an interview with El Médico Interactivo, Dr. Mateos explained the new approaches to multiple myeloma. She highlighted the effectiveness of new treatments and reviewed the latest data on this disease, which were presented at the recent European Hematology Association Congress.
What is the incidence rate of multiple myeloma in the Spanish population?
Multiple myeloma has an incidence of approximately 4-5 new cases per 100,000 inhabitants per year. This means that around 3000 new cases are diagnosed each year in Spain. As with most tumors, multiple myeloma is generally slightly more common in males than females. It is the third most frequent hematologic cancer in men (1757 new cases) and women (1325 new cases), behind lymphoma and leukemias.
At what age is it most often diagnosed?
It affects older people, with recent reports indicating around 68-69 years as the median age. Although more young people are being diagnosed with multiple myeloma, analyses of how this hematologic cancer affects the general population show that it generally impacts patients over age 65 years.
What is the typical survival prognosis?
Thanks to research and therapeutic innovation, the prognosis has changed significantly over the past 20-25 years. Today, if a patient with multiple myeloma receives a diagnosis and does not exhibit poor prognostic characteristics (and this description fits approximately 70%-80% of patients with multiple myeloma), it is realistic to expect a survival exceeding 10 years. A few years ago, this outcome was unimaginable, but a significant amount of therapeutic innovation has made it possible. That’s why I emphasize that it is realistic to provide these data with such a positive outlook.
Is multiple myeloma a refractory type of cancer?
It was a refractory type of cancer. Twenty years ago, there were no treatment options, and therefore survival was around 2-3 years, because treatment mainly consisted of using alkylating agents and corticosteroids. This is what made it refractory.
With the emergence of new therapeutic innovations, patients have been responding better and their responses are lasting longer. Although there is still a group of patients, about 10%-15%, with a poor prognosis and refractory disease, those with standard risk are responding better to different therapies.
Although most patients will eventually exhaust the treatments, which until now were primarily triple-drug regimens (such as proteasome inhibitors, immunomodulators, and antiCD38 antibodies), the introduction of new therapies is extending the duration of responses.
Is the risk for relapse high?
It is very high, in the sense that almost all patients with multiple myeloma eventually relapse. However, we hope that there soon will be some patients who do not relapse.
What are the typical pathologic manifestations of this cancer? Does it affect everyone equally, or in specific ways in each person?
In multiple myeloma, we often say there are multiple myelomas. Clinically, the disease presents in most patients, around 80%, with two clinical manifestations: anemia and bone lesions. Less frequently, patients may also have kidney failure, hypercalcemia, and a higher tendency toward infection. Behind this rather common symptomatology, from a molecular and genetic perspective, each myeloma is practically unique, adding complexity to its treatment. Therefore, ultimately, myelomas end up being refractory.
Elranatamab is a new therapeutic tool. For which patients is it recommended?
It is a bispecific monoclonal antibody that corresponds to the new monotherapy strategies we have for treating patients with multiple myeloma. On the one hand, it targets damaged plasma cells, which are the patient’s tumor cells, and on the other, it binds the patient’s T cells and redirects them to the tumor niche. When this happens, the T cell activates and destroys the tumor cell.
This medication has been approved for patients with relapsed myeloma who have received traditional drugs for their treatment. We know well that patients who have already received proteasome inhibitors, immunomodulators, and anti-CD38 antibodies typically need something new after treatment. Before, there were no other options, and we would reuse what had been previously used. Now we have elranatamab, a bispecific monoclonal antibody targeting a new receptor that has shown significant responses as monotherapy.
More than 60% of patients respond, and more than 30% achieve complete remission. The key is the response duration and progression-free survival of almost a year and a half. This is the longest progression-free survival we have seen to date in previous lines. Therefore, it fills the needs we had for these relapsed or refractory myeloma patients.
What advantages does this new treatment offer?
It represents a therapeutic innovation because, as mentioned, it achieves a response in more than 60% of patients, and around 35% achieve complete remission. The median response duration has not been reached yet. Progression-free survival is 17.2 months, almost a year and a half, and overall survival is almost two years.
Furthermore, it is administered as subcutaneous monotherapy weekly for the first six cycles and then every 15 days. It has a good safety profile, although some adverse events are known, so we have strategies to combat or mitigate them, making the treatment generally well tolerated.
What side effects are being observed?
They are manageable. When the drug is first administered, patients may experience what we call a cytokine release syndrome, which is a result of the treatment’s mechanism. However, we can predict very well when it occurs, usually 2 days after the first doses, and we have strategies to mitigate it.
The second most common adverse event we need to be cautious about is infection. Nowadays, before starting treatment, patients update their vaccination schedule, receive antiviral prophylaxis, and receive prophylaxis against certain germs, resulting in reduced infections. However, infections are probably the adverse events we need to be most careful about when treating the patient.
We must ensure that prophylaxis is performed, and if fever occurs and an infection is suspected, cultures and all kinds of studies must be done to identify and treat it properly.
How does elranatamab change the treatment of an incurable disease? Does it bring us closer to a cure or to making multiple myeloma a manageable chronic disease?
With the already approved elranatamab, the most important aspect is that it adds another treatment option for patients with myeloma. With the progression-free survival data I indicated, life expectancy is increased, with a good quality of life and acceptable safety.
Obviously, elranatamab is still under study and development, even in early lines, including in patients with newly diagnosed myeloma. When we are choosing first-line therapy, we select the best patients by combining traditional drugs with these new immunotherapies, such as elranatamab, it is likely that we are much closer to offering a cure to specific subgroups.
Although it won’t happen in all cases, I believe it will be applicable to a significant subgroup of patients, making chronicity of the disease a reality we are already approaching. Each day, we encounter more patients receiving different lines of treatment and ultimately meeting their life expectancy with myeloma. Even though some may die, it is often due to causes not related to myeloma. This is the most important contribution of these innovations, such as elranatamab.
Dr. Mateos reported receiving honoraria from Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Stemline, Oncopeptides, and Kite for delivering lectures and for participating in advisory boards.
This story was translated from El Médico Interactivo, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
“The introduction of treatments such as elranatamab (Elrexfio) is allowing patients with multiple myeloma, which is still incurable for now, to have different options and achieve long periods of remission, thus improving their survival,” she added. “This therapeutic innovation is highly effective and well tolerated in patients with relapse or refractory multiple myeloma.” The overall response rate is “up to 61%, early, deep, and long-lasting.”
In an interview with El Médico Interactivo, Dr. Mateos explained the new approaches to multiple myeloma. She highlighted the effectiveness of new treatments and reviewed the latest data on this disease, which were presented at the recent European Hematology Association Congress.
What is the incidence rate of multiple myeloma in the Spanish population?
Multiple myeloma has an incidence of approximately 4-5 new cases per 100,000 inhabitants per year. This means that around 3000 new cases are diagnosed each year in Spain. As with most tumors, multiple myeloma is generally slightly more common in males than females. It is the third most frequent hematologic cancer in men (1757 new cases) and women (1325 new cases), behind lymphoma and leukemias.
At what age is it most often diagnosed?
It affects older people, with recent reports indicating around 68-69 years as the median age. Although more young people are being diagnosed with multiple myeloma, analyses of how this hematologic cancer affects the general population show that it generally impacts patients over age 65 years.
What is the typical survival prognosis?
Thanks to research and therapeutic innovation, the prognosis has changed significantly over the past 20-25 years. Today, if a patient with multiple myeloma receives a diagnosis and does not exhibit poor prognostic characteristics (and this description fits approximately 70%-80% of patients with multiple myeloma), it is realistic to expect a survival exceeding 10 years. A few years ago, this outcome was unimaginable, but a significant amount of therapeutic innovation has made it possible. That’s why I emphasize that it is realistic to provide these data with such a positive outlook.
Is multiple myeloma a refractory type of cancer?
It was a refractory type of cancer. Twenty years ago, there were no treatment options, and therefore survival was around 2-3 years, because treatment mainly consisted of using alkylating agents and corticosteroids. This is what made it refractory.
With the emergence of new therapeutic innovations, patients have been responding better and their responses are lasting longer. Although there is still a group of patients, about 10%-15%, with a poor prognosis and refractory disease, those with standard risk are responding better to different therapies.
Although most patients will eventually exhaust the treatments, which until now were primarily triple-drug regimens (such as proteasome inhibitors, immunomodulators, and antiCD38 antibodies), the introduction of new therapies is extending the duration of responses.
Is the risk for relapse high?
It is very high, in the sense that almost all patients with multiple myeloma eventually relapse. However, we hope that there soon will be some patients who do not relapse.
What are the typical pathologic manifestations of this cancer? Does it affect everyone equally, or in specific ways in each person?
In multiple myeloma, we often say there are multiple myelomas. Clinically, the disease presents in most patients, around 80%, with two clinical manifestations: anemia and bone lesions. Less frequently, patients may also have kidney failure, hypercalcemia, and a higher tendency toward infection. Behind this rather common symptomatology, from a molecular and genetic perspective, each myeloma is practically unique, adding complexity to its treatment. Therefore, ultimately, myelomas end up being refractory.
Elranatamab is a new therapeutic tool. For which patients is it recommended?
It is a bispecific monoclonal antibody that corresponds to the new monotherapy strategies we have for treating patients with multiple myeloma. On the one hand, it targets damaged plasma cells, which are the patient’s tumor cells, and on the other, it binds the patient’s T cells and redirects them to the tumor niche. When this happens, the T cell activates and destroys the tumor cell.
This medication has been approved for patients with relapsed myeloma who have received traditional drugs for their treatment. We know well that patients who have already received proteasome inhibitors, immunomodulators, and anti-CD38 antibodies typically need something new after treatment. Before, there were no other options, and we would reuse what had been previously used. Now we have elranatamab, a bispecific monoclonal antibody targeting a new receptor that has shown significant responses as monotherapy.
More than 60% of patients respond, and more than 30% achieve complete remission. The key is the response duration and progression-free survival of almost a year and a half. This is the longest progression-free survival we have seen to date in previous lines. Therefore, it fills the needs we had for these relapsed or refractory myeloma patients.
What advantages does this new treatment offer?
It represents a therapeutic innovation because, as mentioned, it achieves a response in more than 60% of patients, and around 35% achieve complete remission. The median response duration has not been reached yet. Progression-free survival is 17.2 months, almost a year and a half, and overall survival is almost two years.
Furthermore, it is administered as subcutaneous monotherapy weekly for the first six cycles and then every 15 days. It has a good safety profile, although some adverse events are known, so we have strategies to combat or mitigate them, making the treatment generally well tolerated.
What side effects are being observed?
They are manageable. When the drug is first administered, patients may experience what we call a cytokine release syndrome, which is a result of the treatment’s mechanism. However, we can predict very well when it occurs, usually 2 days after the first doses, and we have strategies to mitigate it.
The second most common adverse event we need to be cautious about is infection. Nowadays, before starting treatment, patients update their vaccination schedule, receive antiviral prophylaxis, and receive prophylaxis against certain germs, resulting in reduced infections. However, infections are probably the adverse events we need to be most careful about when treating the patient.
We must ensure that prophylaxis is performed, and if fever occurs and an infection is suspected, cultures and all kinds of studies must be done to identify and treat it properly.
How does elranatamab change the treatment of an incurable disease? Does it bring us closer to a cure or to making multiple myeloma a manageable chronic disease?
With the already approved elranatamab, the most important aspect is that it adds another treatment option for patients with myeloma. With the progression-free survival data I indicated, life expectancy is increased, with a good quality of life and acceptable safety.
Obviously, elranatamab is still under study and development, even in early lines, including in patients with newly diagnosed myeloma. When we are choosing first-line therapy, we select the best patients by combining traditional drugs with these new immunotherapies, such as elranatamab, it is likely that we are much closer to offering a cure to specific subgroups.
Although it won’t happen in all cases, I believe it will be applicable to a significant subgroup of patients, making chronicity of the disease a reality we are already approaching. Each day, we encounter more patients receiving different lines of treatment and ultimately meeting their life expectancy with myeloma. Even though some may die, it is often due to causes not related to myeloma. This is the most important contribution of these innovations, such as elranatamab.
Dr. Mateos reported receiving honoraria from Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Stemline, Oncopeptides, and Kite for delivering lectures and for participating in advisory boards.
This story was translated from El Médico Interactivo, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Hearing Loss, Hearing Aids, and Dementia Risk: What to Tell Your Patients
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In addition, some studies suggest that wearing hearing aids may help prevent dementia, though one study was recently voluntarily retracted due to methodological errors.
Given the overall evidence, how robust are these associations? And what guidance should clinicians provide to their patients?
Frank Lin, MD, PhD, a clinician and professor of otolaryngology and epidemiology at Johns Hopkins University School of Medicine, Baltimore, emphasized that the evidence from the past 10-15 years strongly links hearing loss to cognitive decline.
While quantifying the exact increase in risk is challenging, Dr. Lin said, “there’s no doubt about it; it’s not trivial.”
With respect to the potential link between hearing aids and dementia prevention, Dr. Lin is involved in the ongoing ACHIEVE randomized trial. Results presented at the 2023 Alzheimer’s Association International Conference and simultaneously published in The Lancet revealed participants who used hearing aids experienced a significant slowing of cognitive decline compared with those who received health education.
“It’s a no-risk intervention that can benefit social function, and for people at risk for cognitive decline, it can actually benefit cognitive health,” Dr. Lin said.
Potential Mechanisms
Dr. Lin pointed out that the Lancet Commission on Dementia identifies hearing impairment as one of the most significant risk factors for dementia. Overall, the consensus from most studies is that hearing loss definitely increases the risk for cognitive decline and dementia, he said.
Several hypotheses may explain this connection, and Dr. Lin believes that a combination of three key mechanisms is likely to be central to understanding this link.
The first theory focuses on cognitive load. As people experience age-related hearing changes, “the inner ear is no longer sending signals clearly to the brain,” Dr. Lin explained. This forces the brain to work harder, increasing its cognitive load as it reallocates resources to assist with hearing.
Dr. Lin emphasized that this is a hypothesis and does not prove hearing loss directly causes cognitive decline or dementia. Rather, it suggests that hearing loss accelerates the “unmasking” of cognitive issues. Brain resources that might otherwise buffer against dementia’s pathologic triggers are consumed earlier due to the demands of managing hearing loss.
The second potential mechanism suggests that hearing loss may have detrimental effects on brain structure and function over time — a theory supported by several recent studies.
These studies show that individuals with more severe hearing loss experience faster rates of brain atrophy. The reduced stimulation from poor auditory signals accelerates brain atrophy, Dr. Lin explained.
The third hypothesis focuses on social isolation. Individuals with hearing loss may engage less in social activities, reducing cognitive stimulation and overall social interaction. It’s well-known that social engagement and cognitive stimulation are crucial for maintaining cognitive health over time, Dr. Lin said.
Overall, Dr. Lin believes that the association between hearing loss and an increased risk for cognitive decline likely involves a combination of all three potential mechanisms. It’s not a matter of one theory being right and the others being wrong, he said.
The Role of Hearing Aids
However, the jury is out on the role of hearing aids in preventing dementia.
A large observational study published in 2023 in Lancet Public Health was hailed by its investigators as providing “the best evidence to date” that hearing aids could mitigate the impact of hearing loss on dementia (Lancet Public Health. 2023 May;8[5]:e329-e338. doi: 10.1016/S2468-2667[23]00048-8). However, the authors voluntarily retracted the paper in December 2023 due to a coding error.
Despite this, a large meta-analysis published in JAMA Neurology suggested that hearing aids might reduce cognitive decline and dementia risk and even enhance short-term cognitive function.
Additionally, the ACHIEVE study, the first randomized trial to investigate these issues, included nearly 1000 older participants from two populations — those from the ARIC study and healthy volunteers. Participants were randomly assigned to receive either a hearing intervention or education on healthy aging.
Although the primary endpoint of change in standardized neurocognitive scores at year 3 showed no significant difference between the hearing intervention and health education groups, the ARIC cohort experienced a notable 48% reduction in cognitive decline with hearing aids compared with education.
Dr. Lin explained that, due to the study’s design, the control group was healthier than the ARIC cohort, which was at higher risk for cognitive decline due to factors such as age and diabetes. This is where they observed a strong effect of hearing intervention in reducing cognitive decline within just 3 years, Dr. Lin said.
Conversely, the hearing aids had minimal impact on the healthy controls, likely because they had not experienced cognitive decline to begin with. Essentially, the benefits of hearing aids were more apparent once cognitive issues were already present.
“It seems sort of obvious. In a group of people who aren’t at risk for cognitive decline, a hearing intervention isn’t going to benefit their cognition” in the short term, Dr. Lin noted. That said, the investigators are continuing to follow the healthy controls to determine whether hearing aids lower dementia risk over the long term.
Which Comes First?
Some experts have questioned the directionality of the link between hearing aids and dementia — do hearing aids reduce dementia risk or are individuals with dementia simply less likely to use them?
Dr. Lin noted that observational studies often have confounders. For instance, people who use hearing aids are often healthier and better educated. This makes it difficult to distinguish the effect of the intervention from the factors that led people to use it, he said.
In contrast, the ACHIEVE trial, a randomized study, was designed to separate these factors from the hearing intervention, Dr. Lin explained.
However, he added that ACHIEVE was not specifically powered to assess dementia development, focusing instead on cognitive decline. The investigators plan long-term follow-up of participants to evaluate the impact on dementia in the future.
So, given the current evidence, what should clinicians tell their patients?
Because all people experience some degree of hearing changes as they age, which can gradually affect communication and social engagement, it’s important for everyone to be aware of their hearing health, Dr. Lin said.
He noted there are apps available that allow individuals to measure their hearing with their phones, including determining their “hearing number.”
With respect to hearing aids, Dr. Lin noted that if individuals have trouble participating in everyday activities, addressing hearing issues and considering a hearing intervention is crucial.
There’s no medical risk associated with hearing aids, he said. Even if they only improve social activities and engagement, that’s a benefit. If they also have potential positive effects on cognitive health, “even better,” he added.
Dr. Lin noted that as of 2022, hearing aids are now available over the counter, a move that has improved accessibility. In addition, new technologies, such as stylish “hearing aid glasses,” are being developed to offer more appealing options and reduce the stigma associated with traditional devices.
People often view hearing loss as a significant life event and are reluctant to admit they need hearing aids. However, focusing on “what’s your hearing?” as a neutral tracking metric could make it easier to adopt new technologies in the future, Lin said.
Alzheimer’s Association Weighs in
Heather Snyder, PhD, vice president, Medical & Scientific Relations at the Alzheimer’s Association, echoed Dr. Lin, noting that there has been substantial research showing a link between hearing loss and cognitive decline.
“This association is something that we have seen repeated and replicated in a number of different studies. What we don’t know is the cause and effect,” Dr. Snyder said.
She noted it is unknown whether there is a causal link between hearing loss and cognitive decline and/or whether cognitive decline may contribute to hearing loss. These are some of the “big questions” that remain, said Dr. Snyder.
Still, she noted that hearing health is an important part of quality of life and overall brain health and “should be part of the conversation” between clinicians and their patients.
Discussing the results of the ACHIEVE study, Dr. Snyder highlighted that while the subgroup at higher risk for cognitive decline did experience significant improvement, the overall population did not show a benefit from the intervention.
The brain “is complex,” and it’s unlikely that a single intervention or target will provide all the benefits, Dr. Snyder said.
She emphasized that addressing hearing loss with hearing aids, combined with managing other modifiable risk factors — such as heart and metabolic health, physical activity, and a balanced diet — appears to offer the greatest potential for synergy and preserving cognition.
Drs. Lin and Snyder reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.