User login
Long-term antidepressant use tied to an increase in CVD, mortality risk
The investigators drew on 10-year data from the UK Biobank on over 220,000 adults and compared the risk of developing adverse health outcomes among those taking antidepressants with the risk among those who were not taking antidepressants.
After adjusting for preexisting risk factors, they found that 10-year antidepressant use was associated with a twofold higher risk of CHD, an almost-twofold higher risk of CVD as well as CVD mortality, a higher risk of cerebrovascular disease, and more than double the risk of all-cause mortality.
On the other hand, at 10 years, antidepressant use was associated with a 23% lower risk of developing hypertension and a 32% lower risk of diabetes.
The main culprits were mirtazapine, venlafaxine, duloxetine, and trazodone, although SSRIs were also tied to increased risk.
“Our message for clinicians is that prescribing of antidepressants in the long term may not be harm free [and] we hope that this study will help doctors and patients have more informed conversations when they weigh up the potential risks and benefits of treatments for depression,” study investigator Narinder Bansal, MD, honorary research fellow, Centre for Academic Health and Centre for Academic Primary Care, University of Bristol (England), said in a news release.
“Regardless of whether the drugs are the underlying cause of these problems, our findings emphasize the importance of proactive cardiovascular monitoring and prevention in patients who have depression and are on antidepressants, given that both have been associated with higher risks,” she added.
The study was published online in the British Journal of Psychiatry Open.
Monitoring of CVD risk ‘critical’
Antidepressants are among the most widely prescribed drugs; 70 million prescriptions were dispensed in 2018 alone, representing a doubling of prescriptions for these agents in a decade, the investigators noted. “This striking rise in prescribing is attributed to long-term treatment rather than an increased incidence of depression.”
Most trials that have assessed antidepressant efficacy have been “poorly suited to examining adverse outcomes.” One reason for this is that many of the trials are short-term studies. Since depression is “strongly associated” with CVD risk factors, “careful assessment of the long-term cardiometabolic effects of antidepressant treatment is critical.”
Moreover, information about “a wide range of prospectively measured confounders ... is needed to provide robust estimates of the risks associated with long-term antidepressant use,” the authors noted.
The researchers examined the association between antidepressant use and four cardiometabolic morbidity outcomes – diabetes, hypertension, cerebrovascular disease, and CHD. In addition, they assessed two mortality outcomes – CVD mortality and all-cause mortality. Participants were divided into cohorts on the basis of outcome of interest.
The dataset contains detailed information on socioeconomic status, demographics, anthropometric, behavioral, and biochemical risk factors, disability, and health status and is linked to datasets of primary care records and deaths.
The study included 222,121 participants whose data had been linked to primary care records during 2018 (median age of participants, 56-57 years). About half were women, and 96% were of White ethnicity.
Participants were excluded if they had been prescribed antidepressants 12 months or less before baseline, if they had previously been diagnosed for the outcome of interest, if they had been previously prescribed psychotropic drugs, if they used cardiometabolic drugs at baseline, or if they had undergone treatment with antidepressant polytherapy.
Potential confounders included age, gender, body mass index, waist/hip ratio, smoking and alcohol intake status, physical activity, parental history of outcome, biochemical and hematologic biomarkers, socioeconomic status, and long-term illness, disability, or infirmity.
Mechanism unclear
By the end of the 5- and 10-year follow-up periods, an average of 8% and 6% of participants in each cohort, respectively, had been prescribed an antidepressant. SSRIs constituted the most commonly prescribed class (80%-82%), and citalopram was the most commonly prescribed SSRI (46%-47%). Mirtazapine was the most frequently prescribed non-SSRI antidepressant (44%-46%).
At 5 years, any antidepressant use was associated with an increased risk for diabetes, CHD, and all-cause mortality, but the findings were attenuated after further adjustment for confounders. In fact, SSRIs were associated with a reduced risk of diabetes at 5 years (hazard ratio, 0.64; 95% confidence interval, 0.49-0.83).
At 10 years, SSRIs were associated with an increased risk of cerebrovascular disease, CVD mortality, and all-cause mortality; non-SSRIs were associated with an increased risk of CHD, CVD, and all-cause mortality.
On the other hand, SSRIs were associated with a decrease in risk of diabetes and hypertension at 10 years (HR, 0.68; 95% CI, 0.53-0.87; and HR, 0.77; 95% CI, 0.66-0.89, respectively).
“While we have taken into account a wide range of pre-existing risk factors for cardiovascular disease, including those that are linked to depression such as excess weight, smoking, and low physical activity, it is difficult to fully control for the effects of depression in this kind of study, partly because there is considerable variability in the recording of depression severity in primary care,” said Dr. Bansal.
“This is important because many people taking antidepressants such as mirtazapine, venlafaxine, duloxetine and trazodone may have a more severe depression. This makes it difficult to fully separate the effects of the depression from the effects of medication,” she said.
Further research “is needed to assess whether the associations we have seen are genuinely due to the drugs; and, if so, why this might be,” she added.
Strengths, limitations
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the mood disorders psychopharmacology unit at the University of Toronto,, discussed the strengths and weaknesses of the study.
The UK Biobank is a “well-described, well-phenotyped dataset of good quality,” said Dr. McIntyre, chairperson and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study. Another strength is the “impressive number of variables the database contains, which enabled the authors to go much deeper into the topics.”
A “significant limitation” is the confounding that is inherent to the disorder itself – “people with depression have a much higher intrinsic risk of CVD, [cerebrovascular disease], and cardiovascular mortality,” Dr. McIntyre noted.
The researchers did not adjust for trauma or childhood maltreatment, “which are the biggest risk factors for both depression and CVD; and drug and alcohol misuse were also not accounted for.”
Additionally, “to determine whether something is an association or potentially causative, it must satisfy the Bradford-Hill criteria,” said Dr. McIntyre. “Since we’re moving more toward using these big databases and because we depend on them to give us long-term perspectives, we would want to see coherent, compelling Bradford-Hill criteria regarding causation. If you don’t have any, that’s fine too, but then it’s important to make clear that there is no clear causative line, just an association.”
The research was funded by the National Institute of Health Research School for Primary Care Research and was supported by the NI Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. Dr. McIntyre has received research grant support from CI/GACD/National Natural Science Foundation of China and the Milken Institute and speaker/consultation fees from numerous companies. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
The investigators drew on 10-year data from the UK Biobank on over 220,000 adults and compared the risk of developing adverse health outcomes among those taking antidepressants with the risk among those who were not taking antidepressants.
After adjusting for preexisting risk factors, they found that 10-year antidepressant use was associated with a twofold higher risk of CHD, an almost-twofold higher risk of CVD as well as CVD mortality, a higher risk of cerebrovascular disease, and more than double the risk of all-cause mortality.
On the other hand, at 10 years, antidepressant use was associated with a 23% lower risk of developing hypertension and a 32% lower risk of diabetes.
The main culprits were mirtazapine, venlafaxine, duloxetine, and trazodone, although SSRIs were also tied to increased risk.
“Our message for clinicians is that prescribing of antidepressants in the long term may not be harm free [and] we hope that this study will help doctors and patients have more informed conversations when they weigh up the potential risks and benefits of treatments for depression,” study investigator Narinder Bansal, MD, honorary research fellow, Centre for Academic Health and Centre for Academic Primary Care, University of Bristol (England), said in a news release.
“Regardless of whether the drugs are the underlying cause of these problems, our findings emphasize the importance of proactive cardiovascular monitoring and prevention in patients who have depression and are on antidepressants, given that both have been associated with higher risks,” she added.
The study was published online in the British Journal of Psychiatry Open.
Monitoring of CVD risk ‘critical’
Antidepressants are among the most widely prescribed drugs; 70 million prescriptions were dispensed in 2018 alone, representing a doubling of prescriptions for these agents in a decade, the investigators noted. “This striking rise in prescribing is attributed to long-term treatment rather than an increased incidence of depression.”
Most trials that have assessed antidepressant efficacy have been “poorly suited to examining adverse outcomes.” One reason for this is that many of the trials are short-term studies. Since depression is “strongly associated” with CVD risk factors, “careful assessment of the long-term cardiometabolic effects of antidepressant treatment is critical.”
Moreover, information about “a wide range of prospectively measured confounders ... is needed to provide robust estimates of the risks associated with long-term antidepressant use,” the authors noted.
The researchers examined the association between antidepressant use and four cardiometabolic morbidity outcomes – diabetes, hypertension, cerebrovascular disease, and CHD. In addition, they assessed two mortality outcomes – CVD mortality and all-cause mortality. Participants were divided into cohorts on the basis of outcome of interest.
The dataset contains detailed information on socioeconomic status, demographics, anthropometric, behavioral, and biochemical risk factors, disability, and health status and is linked to datasets of primary care records and deaths.
The study included 222,121 participants whose data had been linked to primary care records during 2018 (median age of participants, 56-57 years). About half were women, and 96% were of White ethnicity.
Participants were excluded if they had been prescribed antidepressants 12 months or less before baseline, if they had previously been diagnosed for the outcome of interest, if they had been previously prescribed psychotropic drugs, if they used cardiometabolic drugs at baseline, or if they had undergone treatment with antidepressant polytherapy.
Potential confounders included age, gender, body mass index, waist/hip ratio, smoking and alcohol intake status, physical activity, parental history of outcome, biochemical and hematologic biomarkers, socioeconomic status, and long-term illness, disability, or infirmity.
Mechanism unclear
By the end of the 5- and 10-year follow-up periods, an average of 8% and 6% of participants in each cohort, respectively, had been prescribed an antidepressant. SSRIs constituted the most commonly prescribed class (80%-82%), and citalopram was the most commonly prescribed SSRI (46%-47%). Mirtazapine was the most frequently prescribed non-SSRI antidepressant (44%-46%).
At 5 years, any antidepressant use was associated with an increased risk for diabetes, CHD, and all-cause mortality, but the findings were attenuated after further adjustment for confounders. In fact, SSRIs were associated with a reduced risk of diabetes at 5 years (hazard ratio, 0.64; 95% confidence interval, 0.49-0.83).
At 10 years, SSRIs were associated with an increased risk of cerebrovascular disease, CVD mortality, and all-cause mortality; non-SSRIs were associated with an increased risk of CHD, CVD, and all-cause mortality.
On the other hand, SSRIs were associated with a decrease in risk of diabetes and hypertension at 10 years (HR, 0.68; 95% CI, 0.53-0.87; and HR, 0.77; 95% CI, 0.66-0.89, respectively).
“While we have taken into account a wide range of pre-existing risk factors for cardiovascular disease, including those that are linked to depression such as excess weight, smoking, and low physical activity, it is difficult to fully control for the effects of depression in this kind of study, partly because there is considerable variability in the recording of depression severity in primary care,” said Dr. Bansal.
“This is important because many people taking antidepressants such as mirtazapine, venlafaxine, duloxetine and trazodone may have a more severe depression. This makes it difficult to fully separate the effects of the depression from the effects of medication,” she said.
Further research “is needed to assess whether the associations we have seen are genuinely due to the drugs; and, if so, why this might be,” she added.
Strengths, limitations
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the mood disorders psychopharmacology unit at the University of Toronto,, discussed the strengths and weaknesses of the study.
The UK Biobank is a “well-described, well-phenotyped dataset of good quality,” said Dr. McIntyre, chairperson and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study. Another strength is the “impressive number of variables the database contains, which enabled the authors to go much deeper into the topics.”
A “significant limitation” is the confounding that is inherent to the disorder itself – “people with depression have a much higher intrinsic risk of CVD, [cerebrovascular disease], and cardiovascular mortality,” Dr. McIntyre noted.
The researchers did not adjust for trauma or childhood maltreatment, “which are the biggest risk factors for both depression and CVD; and drug and alcohol misuse were also not accounted for.”
Additionally, “to determine whether something is an association or potentially causative, it must satisfy the Bradford-Hill criteria,” said Dr. McIntyre. “Since we’re moving more toward using these big databases and because we depend on them to give us long-term perspectives, we would want to see coherent, compelling Bradford-Hill criteria regarding causation. If you don’t have any, that’s fine too, but then it’s important to make clear that there is no clear causative line, just an association.”
The research was funded by the National Institute of Health Research School for Primary Care Research and was supported by the NI Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. Dr. McIntyre has received research grant support from CI/GACD/National Natural Science Foundation of China and the Milken Institute and speaker/consultation fees from numerous companies. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
The investigators drew on 10-year data from the UK Biobank on over 220,000 adults and compared the risk of developing adverse health outcomes among those taking antidepressants with the risk among those who were not taking antidepressants.
After adjusting for preexisting risk factors, they found that 10-year antidepressant use was associated with a twofold higher risk of CHD, an almost-twofold higher risk of CVD as well as CVD mortality, a higher risk of cerebrovascular disease, and more than double the risk of all-cause mortality.
On the other hand, at 10 years, antidepressant use was associated with a 23% lower risk of developing hypertension and a 32% lower risk of diabetes.
The main culprits were mirtazapine, venlafaxine, duloxetine, and trazodone, although SSRIs were also tied to increased risk.
“Our message for clinicians is that prescribing of antidepressants in the long term may not be harm free [and] we hope that this study will help doctors and patients have more informed conversations when they weigh up the potential risks and benefits of treatments for depression,” study investigator Narinder Bansal, MD, honorary research fellow, Centre for Academic Health and Centre for Academic Primary Care, University of Bristol (England), said in a news release.
“Regardless of whether the drugs are the underlying cause of these problems, our findings emphasize the importance of proactive cardiovascular monitoring and prevention in patients who have depression and are on antidepressants, given that both have been associated with higher risks,” she added.
The study was published online in the British Journal of Psychiatry Open.
Monitoring of CVD risk ‘critical’
Antidepressants are among the most widely prescribed drugs; 70 million prescriptions were dispensed in 2018 alone, representing a doubling of prescriptions for these agents in a decade, the investigators noted. “This striking rise in prescribing is attributed to long-term treatment rather than an increased incidence of depression.”
Most trials that have assessed antidepressant efficacy have been “poorly suited to examining adverse outcomes.” One reason for this is that many of the trials are short-term studies. Since depression is “strongly associated” with CVD risk factors, “careful assessment of the long-term cardiometabolic effects of antidepressant treatment is critical.”
Moreover, information about “a wide range of prospectively measured confounders ... is needed to provide robust estimates of the risks associated with long-term antidepressant use,” the authors noted.
The researchers examined the association between antidepressant use and four cardiometabolic morbidity outcomes – diabetes, hypertension, cerebrovascular disease, and CHD. In addition, they assessed two mortality outcomes – CVD mortality and all-cause mortality. Participants were divided into cohorts on the basis of outcome of interest.
The dataset contains detailed information on socioeconomic status, demographics, anthropometric, behavioral, and biochemical risk factors, disability, and health status and is linked to datasets of primary care records and deaths.
The study included 222,121 participants whose data had been linked to primary care records during 2018 (median age of participants, 56-57 years). About half were women, and 96% were of White ethnicity.
Participants were excluded if they had been prescribed antidepressants 12 months or less before baseline, if they had previously been diagnosed for the outcome of interest, if they had been previously prescribed psychotropic drugs, if they used cardiometabolic drugs at baseline, or if they had undergone treatment with antidepressant polytherapy.
Potential confounders included age, gender, body mass index, waist/hip ratio, smoking and alcohol intake status, physical activity, parental history of outcome, biochemical and hematologic biomarkers, socioeconomic status, and long-term illness, disability, or infirmity.
Mechanism unclear
By the end of the 5- and 10-year follow-up periods, an average of 8% and 6% of participants in each cohort, respectively, had been prescribed an antidepressant. SSRIs constituted the most commonly prescribed class (80%-82%), and citalopram was the most commonly prescribed SSRI (46%-47%). Mirtazapine was the most frequently prescribed non-SSRI antidepressant (44%-46%).
At 5 years, any antidepressant use was associated with an increased risk for diabetes, CHD, and all-cause mortality, but the findings were attenuated after further adjustment for confounders. In fact, SSRIs were associated with a reduced risk of diabetes at 5 years (hazard ratio, 0.64; 95% confidence interval, 0.49-0.83).
At 10 years, SSRIs were associated with an increased risk of cerebrovascular disease, CVD mortality, and all-cause mortality; non-SSRIs were associated with an increased risk of CHD, CVD, and all-cause mortality.
On the other hand, SSRIs were associated with a decrease in risk of diabetes and hypertension at 10 years (HR, 0.68; 95% CI, 0.53-0.87; and HR, 0.77; 95% CI, 0.66-0.89, respectively).
“While we have taken into account a wide range of pre-existing risk factors for cardiovascular disease, including those that are linked to depression such as excess weight, smoking, and low physical activity, it is difficult to fully control for the effects of depression in this kind of study, partly because there is considerable variability in the recording of depression severity in primary care,” said Dr. Bansal.
“This is important because many people taking antidepressants such as mirtazapine, venlafaxine, duloxetine and trazodone may have a more severe depression. This makes it difficult to fully separate the effects of the depression from the effects of medication,” she said.
Further research “is needed to assess whether the associations we have seen are genuinely due to the drugs; and, if so, why this might be,” she added.
Strengths, limitations
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology and head of the mood disorders psychopharmacology unit at the University of Toronto,, discussed the strengths and weaknesses of the study.
The UK Biobank is a “well-described, well-phenotyped dataset of good quality,” said Dr. McIntyre, chairperson and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study. Another strength is the “impressive number of variables the database contains, which enabled the authors to go much deeper into the topics.”
A “significant limitation” is the confounding that is inherent to the disorder itself – “people with depression have a much higher intrinsic risk of CVD, [cerebrovascular disease], and cardiovascular mortality,” Dr. McIntyre noted.
The researchers did not adjust for trauma or childhood maltreatment, “which are the biggest risk factors for both depression and CVD; and drug and alcohol misuse were also not accounted for.”
Additionally, “to determine whether something is an association or potentially causative, it must satisfy the Bradford-Hill criteria,” said Dr. McIntyre. “Since we’re moving more toward using these big databases and because we depend on them to give us long-term perspectives, we would want to see coherent, compelling Bradford-Hill criteria regarding causation. If you don’t have any, that’s fine too, but then it’s important to make clear that there is no clear causative line, just an association.”
The research was funded by the National Institute of Health Research School for Primary Care Research and was supported by the NI Biomedical Research Centre at University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. Dr. McIntyre has received research grant support from CI/GACD/National Natural Science Foundation of China and the Milken Institute and speaker/consultation fees from numerous companies. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF PSYCHIATRY OPEN
Critical Care Network
Palliative and End-of-Life Care Section
Time-limited trials of critical care
Many patients die in the ICU, often after long courses of aggressive interventions, with potentially nonbeneficial treatments. Surrogate decision makers are tasked with decisions to initiate or forgo treatments based on recommendations from clinicians in the face of prognostic uncertainty and emotional duress. A strategy that has been adopted by ICU clinicians to address this has been proposing a “time-limited trial” (TLT) of ICU-specific interventions. A TLT involves clinicians partnering with patients and their surrogate decision makers in a shared decision-making model, proposing initiation of treatments for a set time, evaluating for specific measures of what is considered beneficial, and deciding to continue treatment or stop if without benefit. with palliative care teams (Vink EE, et al. Intensive Care Med. 2018;44:1369). Recent research about TLT in the ICU has found that when executed well, TLTs can improve quality of care and provide patients with the care they desire and can benefit from (Vink, et al). Additionally, the use of an education intervention for ICU clinicians regarding protocolled TLT interventions was associated with improved quality of family meetings, and, importantly, a reduced intensity and duration of ICU treatments (Chang DW, et al. JAMA Intern Med. 2021;181[6]:786).
Bradley Hayward, MD
Member-at-Large
Palliative and End-of-Life Care Section
Time-limited trials of critical care
Many patients die in the ICU, often after long courses of aggressive interventions, with potentially nonbeneficial treatments. Surrogate decision makers are tasked with decisions to initiate or forgo treatments based on recommendations from clinicians in the face of prognostic uncertainty and emotional duress. A strategy that has been adopted by ICU clinicians to address this has been proposing a “time-limited trial” (TLT) of ICU-specific interventions. A TLT involves clinicians partnering with patients and their surrogate decision makers in a shared decision-making model, proposing initiation of treatments for a set time, evaluating for specific measures of what is considered beneficial, and deciding to continue treatment or stop if without benefit. with palliative care teams (Vink EE, et al. Intensive Care Med. 2018;44:1369). Recent research about TLT in the ICU has found that when executed well, TLTs can improve quality of care and provide patients with the care they desire and can benefit from (Vink, et al). Additionally, the use of an education intervention for ICU clinicians regarding protocolled TLT interventions was associated with improved quality of family meetings, and, importantly, a reduced intensity and duration of ICU treatments (Chang DW, et al. JAMA Intern Med. 2021;181[6]:786).
Bradley Hayward, MD
Member-at-Large
Palliative and End-of-Life Care Section
Time-limited trials of critical care
Many patients die in the ICU, often after long courses of aggressive interventions, with potentially nonbeneficial treatments. Surrogate decision makers are tasked with decisions to initiate or forgo treatments based on recommendations from clinicians in the face of prognostic uncertainty and emotional duress. A strategy that has been adopted by ICU clinicians to address this has been proposing a “time-limited trial” (TLT) of ICU-specific interventions. A TLT involves clinicians partnering with patients and their surrogate decision makers in a shared decision-making model, proposing initiation of treatments for a set time, evaluating for specific measures of what is considered beneficial, and deciding to continue treatment or stop if without benefit. with palliative care teams (Vink EE, et al. Intensive Care Med. 2018;44:1369). Recent research about TLT in the ICU has found that when executed well, TLTs can improve quality of care and provide patients with the care they desire and can benefit from (Vink, et al). Additionally, the use of an education intervention for ICU clinicians regarding protocolled TLT interventions was associated with improved quality of family meetings, and, importantly, a reduced intensity and duration of ICU treatments (Chang DW, et al. JAMA Intern Med. 2021;181[6]:786).
Bradley Hayward, MD
Member-at-Large
Expert makes the case for not subtyping patients with rosacea
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
. At least they should be, according to Julie C. Harper, MD.
“How many people with papules and pustules don’t also have redness?” Dr. Harper, who practices in Birmingham, Ala., said at Medscape Live’s annual Coastal Dermatology Symposium. “If we’re not careful, and we try to classify a person into a subtype of rosacea, we end up treating only part of their rosacea; we don’t treat all of it. We have seen this in the literature,” she added.
“The idea now is to take a phenotypic approach to rosacea. What we mean by that is that you look at the patient, you document every part of rosacea that you see, and you treat according to that,” she continued. “That person with papules and pustules may also have phyma and ocular disease. They may have telangiectasia and persistent background erythema. They may also have flushing.”
Dr. Harper incorporates the mnemonic “STOP” to her visits with rosacea patients.
S stands for: Identify signs and symptoms of the condition. “Listen to the patient for symptoms,” she advised. “We’ve learned to listen to darker skinned patients for what they tell us about erythema, for example, because we may not be able to see it, yet they are experiencing it. They may also have symptomatic burning, itching, and stinging.”
T stands for: Discuss triggers. “Ask patients, ‘what is it that makes your rosacea worse?’ That’s different for everyone,” she said.
O stands for: Agree on a treatment outcome. “Ask, ‘what is it that really bothers you? Are you bothered by the bumps? The redness?’ ” she said.
“The P stands for: Develop a plan that addresses all of that,” she said.
Different treatments for different rosacea symptoms
No one-size-fits-all treatment exists for rosacea. Options that work well for papules and pustules aren’t effective for redness. Similarly, products that work for redness don’t work for telangiectasia.
“Different lesions and signs of rosacea will likely require multiple modes of treatment,” Dr. Harper said. “So, when you evaluate your rosacea patients, if they’re doing great, don’t change their regimen. But if you see somebody who is not well controlled, is there an opportunity for you to come in and add something to that regimen that may make them better? Maybe so.”
Treatment options indicated for papules and pustules include ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, modified release doxycycline, minocycline foam, and encapsulated benzoyl peroxide.
Options indicated for persistent background erythema include brimonidine and oxymetazoline, while device-based treatments include the pulsed dye laser, the KTP laser, intense pulsed light, and electrosurgery.
Anti-inflammatory action for pustules and papules
A relatively new product indicated for pustules and papules is minocycline 1.5% foam, the only minocycline that is FDA approved to treat rosacea.
“There is no oral minocycline product approved for rosacea yet,” Dr. Harper said. “There is not a known bacterial pathogen in rosacea. Tetracyclines likely work in rosacea by inhibiting neutrophil chemotaxis, inhibiting MMP and thus KLK-5 and LL-37, inhibiting pro-inflammatory cytokines, downregulating reactive oxygen species, and inhibiting angiogenesis.”
In two 12-week, phase 3 randomized studies of 1,522 patients with moderate to severe rosacea, participants were assigned to receive minocycline 5% foam or a vehicle that contained mineral oil and coconut oil.
At week 12, about 50% of patients who received minocycline 5% foam were clear, compared with about 40% of those in the vehicle arm. Also, the reduction of lesion count was about 63% for patients in the treatment group, compared with a reduction of about 54% in the vehicle arm.
Dr. Harper characterized the 63% reduction as “pretty good, but is it good enough or fast enough? I don’t think so, so even with a great drug like this, I would use something else. You can use two medications sometimes to get people better faster. There’s room to bring in something for that background erythema.”
Minocycline 1.5% foam is colored yellow and may stain fabric. “It contains coconut oil, soybean oil, and light mineral oil,” she said. “Most people prefer to use this at bedtime, but you don’t have to.”
Another treatment option is 5% microencapsulated benzoyl peroxide cream, which is FDA approved for inflammatory lesions of rosacea.
“What’s the mechanism of action? Probably not being antimicrobial,” Dr. Harper said. “I think it’s probably at least in part anti-inflammatory, because we have some data to show that it’s killing Demodex [mites]. If Demodex [are] a trigger of inflammation, and we can lessen Demodex, then we could lessen the inflammatory response after that.”
The drug’s approval was based on data from two positive, identical phase 3 randomized, double-blind, multicenter, 12-week clinical trials that evaluated its safety compared with vehicle in 733 people with inflammatory lesions of rosacea (NCT03564119 and NCT03448939).
At week 12, inflammatory lesions of rosacea were reduced by nearly 70% in both trials among those who received 5% microencapsulated benzoyl peroxide cream, compared with 38%-46% among those who received the vehicle. Also, nearly 50% of subjects in the treatment groups were clear or almost clear at 12 weeks, compared with 38%-46% of those who received the vehicle.
Dr. Harper added that about one-quarter of patients in the treatment group of the trials were clear or almost clear by week 4. “That’s pretty fast,” she said, noting that the product’s microencapsulated shell acts as a fenestrated barrier. “It has little openings, which means that it takes a while for the drug to work itself out,” she said. “I think of it as being like a speed bump for benzoyl peroxide delivery. It has to get through this little maze before it lands on the skin. We think that is what has helped with tolerability.”
Oral sarecycline, a narrow spectrum tetracycline that was FDA approved for acne in 2018, may also benefit rosacea patients. In a 12-week, investigator-blinded pilot study, 72 patients with papulopustular rosacea were assigned to receive sarecycline, while 25 received a multivitamin.
By week 12, 75% of patients in the sarecycline group were clear, compared with 16% of those in the multivitamin group, while the inflammatory lesion counts dropped from baseline by 80% and 60%, respectively. Studies of sarecycline for acne have demonstrated similar rates of vertigo, dizziness, and sunburn to those of placebo.
“There were also low rates of gastrointestinal disturbances,” Dr. Harper said. “That’s important in rosacea, because there is no bacterial pathogen.”
Dr. Harper disclosed that she serves as an advisor or consultant for Almirall, BioPharmX, Cassiopeia, Cutanea, Cutera, Dermira, EPI, Galderma, LaRoche-Posay, Ortho, Vyne, Sol Gel, and Sun. She also serves as a speaker or member of a speakers bureau for Almirall, EPI, Galderma, Ortho, and Vyne.
Medscape Live and this news organization are owned by the same parent company.
FROM MEDSCAPE LIVE COASTAL DERM
Advances in Insulin Therapy for Type 2 Diabetes From EASD 2022
Dr Anne Peters, of the Keck School of Medicine of the University of Southern California, reports on the latest research on insulin therapy in adults with type 2 diabetes, presented at the European Association for the Study of Diabetes (EASD).
Dr Peters highlights a clinical study evaluating whether bedtime is optimal for the administration of neutral protamine Hagedorn (NPH) insulin, also known as isophane insulin. The results indicate that titration of NPH plays an important role in avoiding nocturnal hypoglycemia.
Next, Dr Peters discusses the ONWARDS 2 study, a phase 3a trial looking at once-weekly insulin icodec vs once-daily insulin degludec in basal insulin–treated type 2 diabetes. The trial found insulin icodec to be superior to insulin degludec in reducing A1c.
Dr Peters also examines the SoliMix trial, which compared iGlarLixi once daily to twice-daily premix BIAsp 30 in suboptimally controlled type 2 diabetes. The trial evaluated whether patients currently on a basal-bolus regimen would have an equal or more effective response to once-a-day combination therapy. Results showed that iGlarLixi provided better glycemic control and weight benefit than the twice-daily BlAsp 30.
Finally, Dr Peters evaluates a study that looked at switching from a basal-bolus insulin treatment to insulin degludec + liraglutide combination. The combination proved at least as effective as the basal-bolus approach.
--
Anne L. Peters, MD, Professor, Department of Clinical Medicine, Clinical Scholar, Keck School of Medicine of the University of Southern California; Director, USC Clinical Diabetes Programs, University of Southern California Westside Center for Diabetes, Los Angeles, California
Anne L. Peters, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or a trustee for: AstraZeneca; Lilly; NovoNordisk; Abbott; Vertex; Zealand; ShouTi
Received research grant from: Insulet; Dexcom; Abbott
Received income in an amount equal to or greater than $250 from: AstraZeneca; Lilly; NovoNordisk; Abbott; Vertex; Zealand; ShouTi; Insulet; Dexcom
Stock options from: Teladoc; Omada Health (not even close to 5% equity)
Dr Anne Peters, of the Keck School of Medicine of the University of Southern California, reports on the latest research on insulin therapy in adults with type 2 diabetes, presented at the European Association for the Study of Diabetes (EASD).
Dr Peters highlights a clinical study evaluating whether bedtime is optimal for the administration of neutral protamine Hagedorn (NPH) insulin, also known as isophane insulin. The results indicate that titration of NPH plays an important role in avoiding nocturnal hypoglycemia.
Next, Dr Peters discusses the ONWARDS 2 study, a phase 3a trial looking at once-weekly insulin icodec vs once-daily insulin degludec in basal insulin–treated type 2 diabetes. The trial found insulin icodec to be superior to insulin degludec in reducing A1c.
Dr Peters also examines the SoliMix trial, which compared iGlarLixi once daily to twice-daily premix BIAsp 30 in suboptimally controlled type 2 diabetes. The trial evaluated whether patients currently on a basal-bolus regimen would have an equal or more effective response to once-a-day combination therapy. Results showed that iGlarLixi provided better glycemic control and weight benefit than the twice-daily BlAsp 30.
Finally, Dr Peters evaluates a study that looked at switching from a basal-bolus insulin treatment to insulin degludec + liraglutide combination. The combination proved at least as effective as the basal-bolus approach.
--
Anne L. Peters, MD, Professor, Department of Clinical Medicine, Clinical Scholar, Keck School of Medicine of the University of Southern California; Director, USC Clinical Diabetes Programs, University of Southern California Westside Center for Diabetes, Los Angeles, California
Anne L. Peters, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or a trustee for: AstraZeneca; Lilly; NovoNordisk; Abbott; Vertex; Zealand; ShouTi
Received research grant from: Insulet; Dexcom; Abbott
Received income in an amount equal to or greater than $250 from: AstraZeneca; Lilly; NovoNordisk; Abbott; Vertex; Zealand; ShouTi; Insulet; Dexcom
Stock options from: Teladoc; Omada Health (not even close to 5% equity)
Dr Anne Peters, of the Keck School of Medicine of the University of Southern California, reports on the latest research on insulin therapy in adults with type 2 diabetes, presented at the European Association for the Study of Diabetes (EASD).
Dr Peters highlights a clinical study evaluating whether bedtime is optimal for the administration of neutral protamine Hagedorn (NPH) insulin, also known as isophane insulin. The results indicate that titration of NPH plays an important role in avoiding nocturnal hypoglycemia.
Next, Dr Peters discusses the ONWARDS 2 study, a phase 3a trial looking at once-weekly insulin icodec vs once-daily insulin degludec in basal insulin–treated type 2 diabetes. The trial found insulin icodec to be superior to insulin degludec in reducing A1c.
Dr Peters also examines the SoliMix trial, which compared iGlarLixi once daily to twice-daily premix BIAsp 30 in suboptimally controlled type 2 diabetes. The trial evaluated whether patients currently on a basal-bolus regimen would have an equal or more effective response to once-a-day combination therapy. Results showed that iGlarLixi provided better glycemic control and weight benefit than the twice-daily BlAsp 30.
Finally, Dr Peters evaluates a study that looked at switching from a basal-bolus insulin treatment to insulin degludec + liraglutide combination. The combination proved at least as effective as the basal-bolus approach.
--
Anne L. Peters, MD, Professor, Department of Clinical Medicine, Clinical Scholar, Keck School of Medicine of the University of Southern California; Director, USC Clinical Diabetes Programs, University of Southern California Westside Center for Diabetes, Los Angeles, California
Anne L. Peters, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or a trustee for: AstraZeneca; Lilly; NovoNordisk; Abbott; Vertex; Zealand; ShouTi
Received research grant from: Insulet; Dexcom; Abbott
Received income in an amount equal to or greater than $250 from: AstraZeneca; Lilly; NovoNordisk; Abbott; Vertex; Zealand; ShouTi; Insulet; Dexcom
Stock options from: Teladoc; Omada Health (not even close to 5% equity)

PCCM diversity grant recipient looks to inhibit platelet endothelial interactions via NEDD9 to improve acute lung injury
In February, The American College of Chest Physicians (CHEST), the American Thoracic Society, and the American Lung Association announced a partnership with the prestigious Harold Amos Medical Faculty Development Program (AMFDP), a Robert Wood Johnson Foundation initiative, to sponsor a scholar in pulmonary and critical care medicine.
George Alba, MD, is a pulmonary and critical care physician investigator at Massachusetts General Hospital. Dr. Alba studied English Literature and Biology as an undergraduate at Washington University in St. Louis, where he worked in a developmental biology laboratory; earned his MD at the Mount Sinai School of Medicine, where he graduated AOA with Distinction in Medical Education; and then completed both Internal Medicine and Pulmonary and Critical Care Medicine training at Massachusetts General Hospital.
During his fellowship, Dr. Alba specialized in pulmonary and critical care medicine because he appreciated the variety that comes with working in the intensive care unit.
“I love the medical complexity, the physiology, and the decision-making,” said Dr. Alba. “I’ve always enjoyed all aspects of clinical medicine, so it was hard to choose a path, but the benefit of the ICU is that it allows me to take care of a spectrum of medical illness across all subspecialties.”
He continued, “What I loved about pulmonary, specifically, was that I could see patients in the hospital and in the ICU, perform procedures, and still have a longitudinal relationship with patients in the clinic, which gave me a very flexible, wide grasp of medicine.”
Growing up in a close-knit Cuban family and community, Dr. Alba was raised speaking Spanish at home and learned English primarily in school. Being bilingual helped him in medicine greatly: in clinic, in the hospital, and in the ICU, he is able to communicate directly with Spanish-speaking patients and their families. This became critically important during the COVID-19 pandemic when Chelsea, a primarily Hispanic community in Boston, was disproportionately impacted. The patients greatly benefited from Spanish-speaking clinicians to communicate with their family members who were unable to visit due to the infection control policies in place.
As an instructor of medicine at Harvard Medical School and pulmonary and critical care physician at Massachusetts General, Dr. Alba is actively engaged in clinical care, teaching, and research focusing primarily on mechanisms of pulmonary vascular dysfunction in lung disease.
Dr. Alba’s AMFDP award project is titled “Pulmonary Endothelial NEDD9 and Acute Lung Injury,” and through the proposed scientific aims, he looks to advance NEDD9 antagonism as a potential therapeutic target in acute respiratory distress syndrome (ARDS.) He is being co-mentored by Bradley Maron, MD, a pulmonary vascular disease researcher at Brigham and Women’s Hospital, and Eric Schmidt, MD, an endothelial biologist and expert in animal models of acute lung injury at Massachusetts General Hospital.
This is especially relevant research during the COVID-19 pandemic, as patients with severe lung injury frequently develop clotting in the lung blood vessels. Dr. Alba’s prior work demonstrated that NEDD9 is a pulmonary endothelial protein that is upregulated by hypoxia, that it binds to activated platelets to promote platelet adhesion and clotting, and that inhibition of NEDD9-platelet interactions with a custom antibody can decrease clotting in the lungs of animals. He recently showed that pulmonary endothelial NEDD9 is increased in patients with ARDS who demonstrate blood vessel clotting.
Now, Dr. Alba seeks to use a custom-made anti-NEDD9 antibody to block platelet adhesion in animal models of ARDS to decrease the extent of lung injury. While aspirin and anticoagulants have been unhelpful in treating ARDS in prior trials, Dr. Alba believes that circulating pulmonary endothelial protein NEDD9 can serve as a biomarker to identify subgroups of ARDS who may benefit from earlier targeted antithrombotic therapy.
Dr. Alba hopes that one day the anti-NEDD9 antibody may become one such therapeutic option for patients. The AMFDP will help support his ongoing work.
“Growing up, I saw through my father’s example how education unlocks opportunities. Our community came together to help him on this path. Now a retired doctor of osteopathy in neonatology, he inspired me to pursue a career in medicine,” said Dr. Alba. “This award comes at a critical time in my junior faculty career: It allows me to continue pursuing my research in a meaningful way while also gaining new skills that will be critical for my ongoing career development.”
Dr. Alba continued, “Programs like the Robert Wood Johnson Foundation initiative that specifically try to increase the number of individuals traditionally underrepresented in academia are key and would not be possible without the support of groups like CHEST, the American Lung Association, and the American Thoracic Society.
These programs help folks who may have other external barriers to being in academia, including socioeconomic pressures, lack of resources
Dr. Alba is also committed to paying it forward: “I want to ensure that the type of invested mentorship I experienced to help get me this far is not a matter of serendipity for the fortunate few, but rather a standard for all students and trainees, especially those from underrepresented backgrounds.”
In February, The American College of Chest Physicians (CHEST), the American Thoracic Society, and the American Lung Association announced a partnership with the prestigious Harold Amos Medical Faculty Development Program (AMFDP), a Robert Wood Johnson Foundation initiative, to sponsor a scholar in pulmonary and critical care medicine.
George Alba, MD, is a pulmonary and critical care physician investigator at Massachusetts General Hospital. Dr. Alba studied English Literature and Biology as an undergraduate at Washington University in St. Louis, where he worked in a developmental biology laboratory; earned his MD at the Mount Sinai School of Medicine, where he graduated AOA with Distinction in Medical Education; and then completed both Internal Medicine and Pulmonary and Critical Care Medicine training at Massachusetts General Hospital.
During his fellowship, Dr. Alba specialized in pulmonary and critical care medicine because he appreciated the variety that comes with working in the intensive care unit.
“I love the medical complexity, the physiology, and the decision-making,” said Dr. Alba. “I’ve always enjoyed all aspects of clinical medicine, so it was hard to choose a path, but the benefit of the ICU is that it allows me to take care of a spectrum of medical illness across all subspecialties.”
He continued, “What I loved about pulmonary, specifically, was that I could see patients in the hospital and in the ICU, perform procedures, and still have a longitudinal relationship with patients in the clinic, which gave me a very flexible, wide grasp of medicine.”
Growing up in a close-knit Cuban family and community, Dr. Alba was raised speaking Spanish at home and learned English primarily in school. Being bilingual helped him in medicine greatly: in clinic, in the hospital, and in the ICU, he is able to communicate directly with Spanish-speaking patients and their families. This became critically important during the COVID-19 pandemic when Chelsea, a primarily Hispanic community in Boston, was disproportionately impacted. The patients greatly benefited from Spanish-speaking clinicians to communicate with their family members who were unable to visit due to the infection control policies in place.
As an instructor of medicine at Harvard Medical School and pulmonary and critical care physician at Massachusetts General, Dr. Alba is actively engaged in clinical care, teaching, and research focusing primarily on mechanisms of pulmonary vascular dysfunction in lung disease.
Dr. Alba’s AMFDP award project is titled “Pulmonary Endothelial NEDD9 and Acute Lung Injury,” and through the proposed scientific aims, he looks to advance NEDD9 antagonism as a potential therapeutic target in acute respiratory distress syndrome (ARDS.) He is being co-mentored by Bradley Maron, MD, a pulmonary vascular disease researcher at Brigham and Women’s Hospital, and Eric Schmidt, MD, an endothelial biologist and expert in animal models of acute lung injury at Massachusetts General Hospital.
This is especially relevant research during the COVID-19 pandemic, as patients with severe lung injury frequently develop clotting in the lung blood vessels. Dr. Alba’s prior work demonstrated that NEDD9 is a pulmonary endothelial protein that is upregulated by hypoxia, that it binds to activated platelets to promote platelet adhesion and clotting, and that inhibition of NEDD9-platelet interactions with a custom antibody can decrease clotting in the lungs of animals. He recently showed that pulmonary endothelial NEDD9 is increased in patients with ARDS who demonstrate blood vessel clotting.
Now, Dr. Alba seeks to use a custom-made anti-NEDD9 antibody to block platelet adhesion in animal models of ARDS to decrease the extent of lung injury. While aspirin and anticoagulants have been unhelpful in treating ARDS in prior trials, Dr. Alba believes that circulating pulmonary endothelial protein NEDD9 can serve as a biomarker to identify subgroups of ARDS who may benefit from earlier targeted antithrombotic therapy.
Dr. Alba hopes that one day the anti-NEDD9 antibody may become one such therapeutic option for patients. The AMFDP will help support his ongoing work.
“Growing up, I saw through my father’s example how education unlocks opportunities. Our community came together to help him on this path. Now a retired doctor of osteopathy in neonatology, he inspired me to pursue a career in medicine,” said Dr. Alba. “This award comes at a critical time in my junior faculty career: It allows me to continue pursuing my research in a meaningful way while also gaining new skills that will be critical for my ongoing career development.”
Dr. Alba continued, “Programs like the Robert Wood Johnson Foundation initiative that specifically try to increase the number of individuals traditionally underrepresented in academia are key and would not be possible without the support of groups like CHEST, the American Lung Association, and the American Thoracic Society.
These programs help folks who may have other external barriers to being in academia, including socioeconomic pressures, lack of resources
Dr. Alba is also committed to paying it forward: “I want to ensure that the type of invested mentorship I experienced to help get me this far is not a matter of serendipity for the fortunate few, but rather a standard for all students and trainees, especially those from underrepresented backgrounds.”
In February, The American College of Chest Physicians (CHEST), the American Thoracic Society, and the American Lung Association announced a partnership with the prestigious Harold Amos Medical Faculty Development Program (AMFDP), a Robert Wood Johnson Foundation initiative, to sponsor a scholar in pulmonary and critical care medicine.
George Alba, MD, is a pulmonary and critical care physician investigator at Massachusetts General Hospital. Dr. Alba studied English Literature and Biology as an undergraduate at Washington University in St. Louis, where he worked in a developmental biology laboratory; earned his MD at the Mount Sinai School of Medicine, where he graduated AOA with Distinction in Medical Education; and then completed both Internal Medicine and Pulmonary and Critical Care Medicine training at Massachusetts General Hospital.
During his fellowship, Dr. Alba specialized in pulmonary and critical care medicine because he appreciated the variety that comes with working in the intensive care unit.
“I love the medical complexity, the physiology, and the decision-making,” said Dr. Alba. “I’ve always enjoyed all aspects of clinical medicine, so it was hard to choose a path, but the benefit of the ICU is that it allows me to take care of a spectrum of medical illness across all subspecialties.”
He continued, “What I loved about pulmonary, specifically, was that I could see patients in the hospital and in the ICU, perform procedures, and still have a longitudinal relationship with patients in the clinic, which gave me a very flexible, wide grasp of medicine.”
Growing up in a close-knit Cuban family and community, Dr. Alba was raised speaking Spanish at home and learned English primarily in school. Being bilingual helped him in medicine greatly: in clinic, in the hospital, and in the ICU, he is able to communicate directly with Spanish-speaking patients and their families. This became critically important during the COVID-19 pandemic when Chelsea, a primarily Hispanic community in Boston, was disproportionately impacted. The patients greatly benefited from Spanish-speaking clinicians to communicate with their family members who were unable to visit due to the infection control policies in place.
As an instructor of medicine at Harvard Medical School and pulmonary and critical care physician at Massachusetts General, Dr. Alba is actively engaged in clinical care, teaching, and research focusing primarily on mechanisms of pulmonary vascular dysfunction in lung disease.
Dr. Alba’s AMFDP award project is titled “Pulmonary Endothelial NEDD9 and Acute Lung Injury,” and through the proposed scientific aims, he looks to advance NEDD9 antagonism as a potential therapeutic target in acute respiratory distress syndrome (ARDS.) He is being co-mentored by Bradley Maron, MD, a pulmonary vascular disease researcher at Brigham and Women’s Hospital, and Eric Schmidt, MD, an endothelial biologist and expert in animal models of acute lung injury at Massachusetts General Hospital.
This is especially relevant research during the COVID-19 pandemic, as patients with severe lung injury frequently develop clotting in the lung blood vessels. Dr. Alba’s prior work demonstrated that NEDD9 is a pulmonary endothelial protein that is upregulated by hypoxia, that it binds to activated platelets to promote platelet adhesion and clotting, and that inhibition of NEDD9-platelet interactions with a custom antibody can decrease clotting in the lungs of animals. He recently showed that pulmonary endothelial NEDD9 is increased in patients with ARDS who demonstrate blood vessel clotting.
Now, Dr. Alba seeks to use a custom-made anti-NEDD9 antibody to block platelet adhesion in animal models of ARDS to decrease the extent of lung injury. While aspirin and anticoagulants have been unhelpful in treating ARDS in prior trials, Dr. Alba believes that circulating pulmonary endothelial protein NEDD9 can serve as a biomarker to identify subgroups of ARDS who may benefit from earlier targeted antithrombotic therapy.
Dr. Alba hopes that one day the anti-NEDD9 antibody may become one such therapeutic option for patients. The AMFDP will help support his ongoing work.
“Growing up, I saw through my father’s example how education unlocks opportunities. Our community came together to help him on this path. Now a retired doctor of osteopathy in neonatology, he inspired me to pursue a career in medicine,” said Dr. Alba. “This award comes at a critical time in my junior faculty career: It allows me to continue pursuing my research in a meaningful way while also gaining new skills that will be critical for my ongoing career development.”
Dr. Alba continued, “Programs like the Robert Wood Johnson Foundation initiative that specifically try to increase the number of individuals traditionally underrepresented in academia are key and would not be possible without the support of groups like CHEST, the American Lung Association, and the American Thoracic Society.
These programs help folks who may have other external barriers to being in academia, including socioeconomic pressures, lack of resources
Dr. Alba is also committed to paying it forward: “I want to ensure that the type of invested mentorship I experienced to help get me this far is not a matter of serendipity for the fortunate few, but rather a standard for all students and trainees, especially those from underrepresented backgrounds.”
Margin Size for Unique Skin Tumors Treated With Mohs Micrographic Surgery: A Survey of Practice Patterns
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
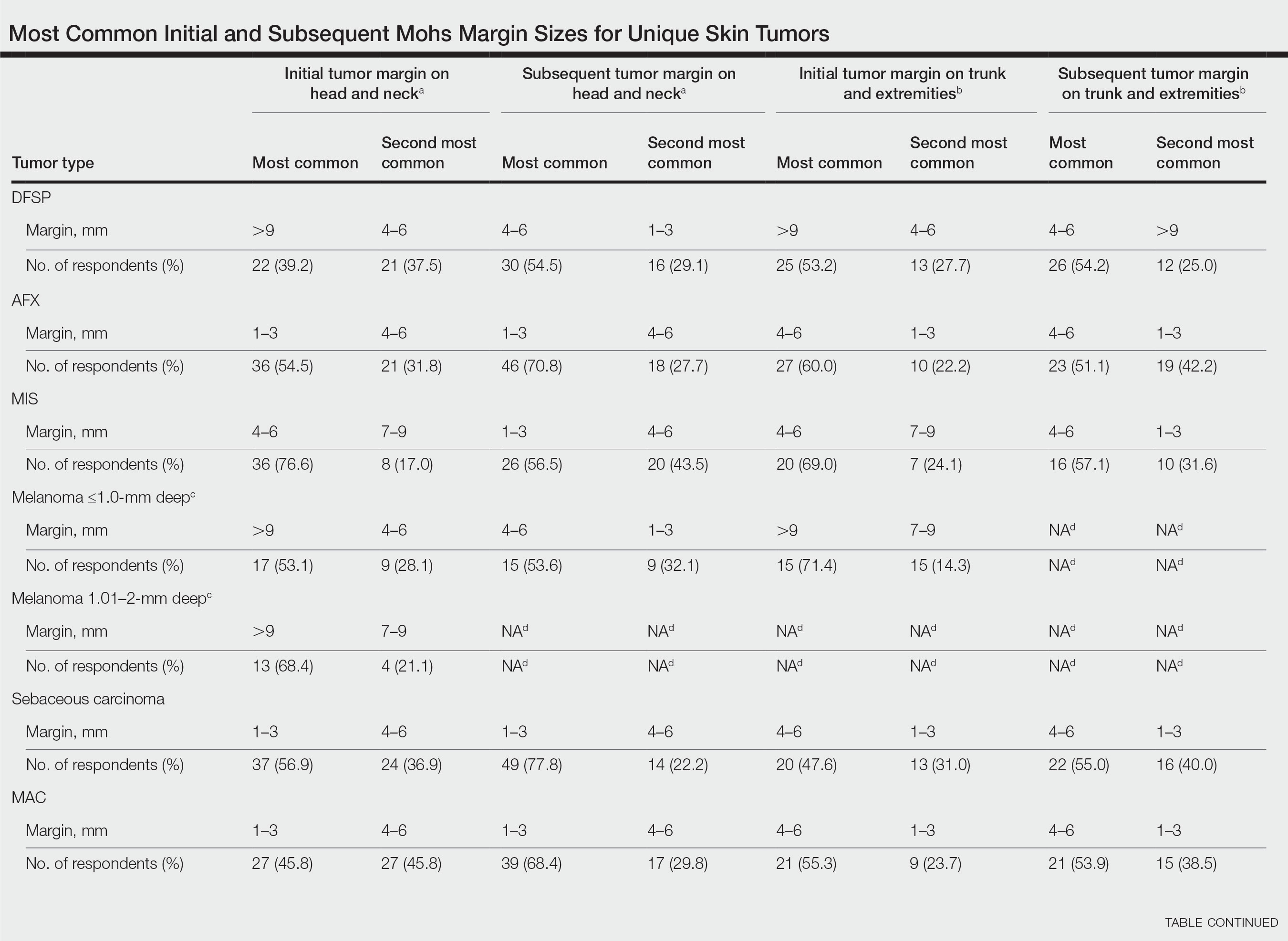
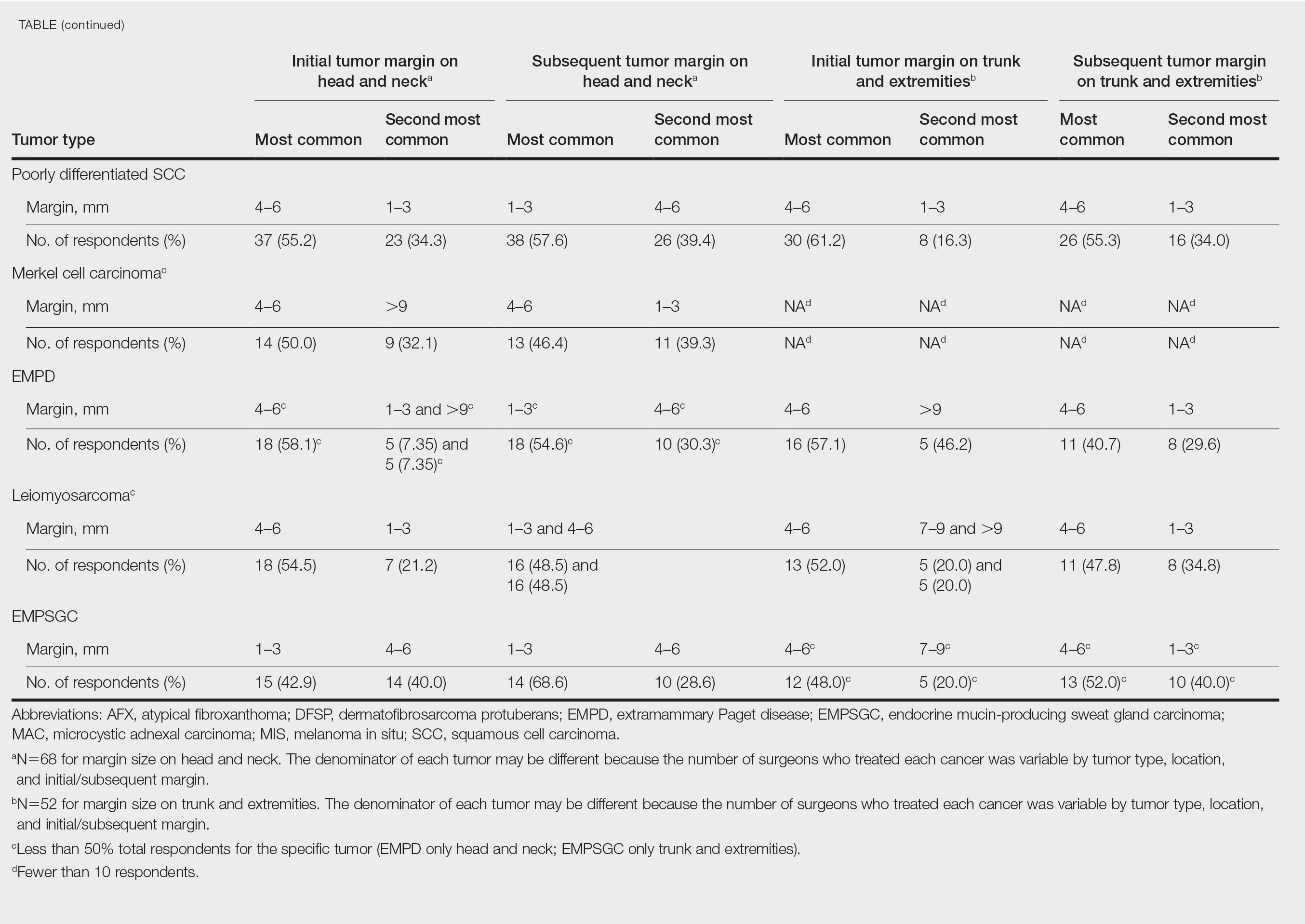
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.
Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.


Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
Mohs micrographic surgery (MMS) is most commonly used for the surgical management of squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) in high-risk locations. The ability for 100% margin evaluation with MMS also has shown lower recurrence rates compared with wide local excision for less common and/or more aggressive tumors. However, there is a lack of standardization on initial and subsequent margin size when treating these less common skin tumors, such as dermatofibrosarcoma protuberans (DFSP), atypical fibroxanthoma (AFX), and sebaceous carcinoma.
Because Mohs surgeons must balance normal tissue preservation with the importance of tumor clearance in the context of comprehensive margin control, we aimed to assess the practice patterns of Mohs surgeons regarding margin size for these unique tumors. The average margin size for each Mohs layer has been reported to be 1 to 3 mm for BCC compared with 3 to 6 mm or larger for other skin cancers, such as melanoma in situ (MIS).1-3 We hypothesized that the initial margin size would vary among surgeons and likely be greater for more aggressive and rarer malignancies as well as for lesions on the trunk and extremities.
Methods
A descriptive survey was created using SurveyMonkey and distributed to members of the American College of Mohs Surgery (ACMS). Survey participants and their responses were anonymous. Demographic information on survey participants was collected in addition to initial and subsequent MMS margin size for DFSP, AFX, MIS, invasive melanoma, sebaceous carcinoma, microcystic adnexal carcinoma (MAC), poorly differentiated SCC, Merkel cell carcinoma, extramammary Paget disease, leiomyosarcoma, and endocrine mucin-producing sweat gland carcinoma. Survey participants were asked to choose from a range of margin sizes: 1 to 3 mm, 4 to 6 mm, 7 to 9 mm, and greater than 9 mm. This study was approved by the University of Texas Southwest Medical Center (Dallas, Texas) institutional review board.
Results
Eighty-seven respondents from the ACMS listserve completed the survey (response rate <10%). Of these, 58 respondents (66.7%) reported practicing for more than 5 years, and 58 (66.7%) were male. Practice setting was primarily private/community (71.3% [62/87]), and survey respondents were located across the United States. More than 50% of survey respondents treated the following tumors on the head and neck in their respective practices: DFSP (80.9% [55/68]), AFX (95.6% [65/68]), MIS (67.7% [46/68]), sebaceous carcinoma (92.7% [63/68]), MAC (83.8% [57/68]), poorly differentiated SCC (97.1% [66/68]), and endocrine mucin-producing sweat gland carcinoma (51.5% [35/68]). More than 50% of survey respondents treated the following tumors on the trunk and extremities: DFSP (90.3% [47/52]), AFX (86.4% [45/52]), MIS (55.8% [29/52]), sebaceous carcinoma (80.8% [42/52]), MAC (73.1% [38/52]), poorly differentiated SCC (94.2% [49/52]), and extramammary Paget disease (53.9% [28/52]). Invasive melanoma, Merkel cell carcinoma, and leiomyosarcoma were overall less commonly treated.
In general, respondent Mohs surgeons were more likely to take larger initial and subsequent margins for tumors treated on the trunk and extremities compared with the head and neck (Table). In addition, initial margin size often was larger than the 1- to 3-mm margin commonly used in Mohs surgery for BCCs and less aggressive SCCs (Table). A larger initial margin size (>9 mm) and subsequent margin size (4–6 mm) was more commonly reported for certain tumors known to be more aggressive and/or have extensive subclinical extension, such as DFSP and invasive melanoma. Of note, most respondents performed 4- to 6-mm margins (37/67 [55.2%]) for poorly differentiated SCC. Overall, there was a high range of margin size variability among Mohs surgeons for these unique and/or more aggressive skin tumors.


Comment
Given that no guidelines exist on margins with MMS for less commonly treated skin tumors, this study helps give Mohs surgeons perspective on current practice patterns for both initial and subsequent Mohs margin sizes. High margin-size variability among Mohs surgeons is expected, as surgeons also need to account for high-risk features of the tumor or specific locations where tissue sparing is critical. Overall, Mohs surgeons are more likely to take larger initial margins for these less common skin tumors compared with BCCs or SCCs. Initial margin size was consistently larger on the trunk and extremities where tissue sparing often is less critical.
Our survey was limited by a small sample size and incomplete response of the ACMS membership. In addition, most respondents practiced in a private/community setting, which may have led to bias, as academic centers may manage rare malignancies more commonly and/or have increased access to immunostains and multispecialty care. Future registries for rare skin malignancies will hopefully be developed that will allow for further consensus on standardized margins. Additional studies on the average number of stages required to clear these less common tumors also are warranted.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
- Muller FM, Dawe RS, Moseley H, et al. Randomized comparison of Mohs micrographic surgery and surgical excision for small nodular basal cell carcinoma: tissue‐sparing outcome. Dermatol Surg. 2009;35:1349-1354.
- van Loo E, Mosterd K, Krekels GA, et al. Surgical excision versus Mohs’ micrographic surgery for basal cell carcinoma of the face: a randomised clinical trial with 10 year follow-up. Eur J Cancer. 2014;50:3011-3020.
- Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81:767-774.
Practice Points
- It is common for initial margin size for uncommon skin tumors to be larger than the 1 to 3 mm commonly used in Mohs surgery for basal cell carcinomas and less aggressive squamous cell carcinomas.
- Mohs surgeons commonly take larger starting and subsequent margins for uncommon skin tumors treated on the trunk and extremities compared with the head and neck.
Early FMT shows promise for preventing recurrent C. difficile
Fecal microbiota transplantation (FMT) is safe and highly effective as first-line therapy for patients with first or second Clostridioides difficile infection, according to the first randomized, double-blind, placebo-controlled trial of its kind.
Study enrollment was halted after an interim analysis revealed significantly better outcomes among patients who received vancomycin plus FMT versus vancomycin alone, reported lead author Simon Mark Dahl Baunwall, MD, of Aarhus (Denmark) University Hospital and colleagues in The Lancet Gastroenterology & Hepatology.
The investigators noted that the participants represented a real-world patient population, so the data support FMT “as a necessary, effective first-line option” in routine management of C. difficile infection.
“Previous studies have demonstrated clinical cure rates [with FMT] of up to 92%,” Dr. Baunwall and colleagues wrote. “Early use of FMT for first or second C. difficile infection has therapeutic potential, but no formal randomized trials to support use of the approach as a first-line therapy have been done.”
The present trial, conducted at a university hospital in Denmark, involved 42 adult patients with first or second C. difficile infection. Patients were randomized in a 1:1 ratio to receive either vancomycin alone or vancomycin plus FMT. All patients received 125 mg oral vancomycin four times daily for a minimum of 10 days after diagnosis. On day 1 after completion of vancomycin therapy and again between day 3 and 7, patients received either oral FMT or matching placebo, depending on their group. After completing the protocol, patients were followed for 8 weeks or C. difficile recurrence to evaluate resolution of C. difficile–associated diarrhea.
“In this trial, patients were treated with two sequential FMT procedures on separate days,” the investigators noted. “This practice might have overtreated some patients and differs from previous trials. It remains unknown whether optimal effect is achieved by one or two treatments.”
The trial design called for 84 patients, but enrollment was halted after an interim analysis of the above cohort of 42 patients because of significantly lower rate resolution in the placebo group. At the 2-month mark, 90% (95% confidence interval, 70%-99%) of patients in the FMT group had resolution, compared with only 33% (95% CI, 15%-57%) of patients in the placebo group (P = .0003), constituting a 57% (95% CI, 33%-81%) absolute risk reduction.
Most patients experienced adverse events, including 20 in the FMT group and all 21 in the placebo group, although most were transient and nonserious. The most common adverse events were diarrhea, which occurred more frequently in the FMT group (23 vs. 14 events), followed by abdominal pain(14 vs. 11 events) and nausea (12 vs. 5 events).
One limitation of the study was its single-center design with regional uptake; the authors noted that, despite having high statistical power for the clinical effect, the study’s premature termination and low patient number prevent inferences regarding mortality, time to effect, and cost.
“The results of this trial highlight how the use of fecal microbiota transplantation as a first-line treatment can effectively prevent C. difficile recurrence and suggests that microbiota restoration might be necessary to obtain sustained resolution,” the investigators wrote. “At present, only 10% of patients with multiple, recurrent C. difficile infection and indication for FMT receive it. International initiatives address the unmet need, but logistic and regulatory obstacles remain unsolved.”
Encouraging findings, lingering concerns
Nicholas Turner, MD, assistant professor in the division of infectious diseases at Duke University, Durham, N.C., praised the study for “pushing the boundaries for FMT,” and noted that the methodology appeared sound. Results in the placebo group, however, cast doubt on the generalizability of the findings, he said.
“If you look at the group that received vancomycin plus placebo, their failure rate was really astoundingly high,” Dr. Turner said in an interview, referring to the 67% failure rate in the control group; he noted previous studies had reported failure rates closer to 10%. “I think that just calls into question just a little bit what happened with that control group.”
Dr. Turner said his confidence would go “way, way up” if the findings were reproduced in a larger study. Ideally, these future trials would use fidaxomicin, he added, which is becoming the preferred option over vancomycin for treating C. difficile.
John Y. Kao, MD, professor of medicine and codirector of the FMT program at University of Michigan Medicine, Ann Arbor, offered a different perspective, suggesting that the control group findings shouldn’t overshadow the efficacy of FMT.
“I agree that historical data would tell us that the placebo population should see a much higher response,” Dr. Kao said in an interview. “In my mind though, the success rate of FMT over placebo is what I would expect. The message of the study should be upheld: that FMT is an effective therapy whether it’s given early or, as the way we give it now, as a sort of rescue therapy.”
Despite this confidence in FMT as an efficacious first-line option, Dr. Kao said it is unlikely to be routinely used in this way anytime soon, even if a larger trial echoes the present results.
“We don’t know the long-term risks of FMT therapy, although we’ve been doing this now probably close to 20 years,” Dr. Kao said.
Specifically, Dr. Kao was most concerned about the long-term risk of colon cancer, as mouse models suggest that microbiome characteristics may affect risk level, and risk may vary based on host-microbiome relationships. In other words, an organism may pose no risk in the gut of the donor, but the same may not be true for the recipient.
While increased rates of colon cancer or other serious illnesses have not been detected in humans who have undergone FMT over the past 2 decades, Dr. Kao said that these findings cannot be extrapolated over a patient’s entire lifetime, especially for younger individuals.
“In a patient that’s 80, you would say, yeah, let’s go ahead and treat you [with FMT] as first-line therapy, whereas someone who’s 20, and has maybe another 50 or 60 years longevity, you may not want to give FMT as first-line therapy,” Dr. Kao said.
This study was supported by Innovation Fund Denmark. The investigators disclosed no competing interests. Dr. Turner previously performed statistical analyses for a Merck study comparing vancomycin, fidaxomicin, and metronidazole for C. difficile infection. Dr. Kao disclosed no relevant conflicts of interest.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center (www.gastro.org/Cdiff).
Fecal microbiota transplantation (FMT) is safe and highly effective as first-line therapy for patients with first or second Clostridioides difficile infection, according to the first randomized, double-blind, placebo-controlled trial of its kind.
Study enrollment was halted after an interim analysis revealed significantly better outcomes among patients who received vancomycin plus FMT versus vancomycin alone, reported lead author Simon Mark Dahl Baunwall, MD, of Aarhus (Denmark) University Hospital and colleagues in The Lancet Gastroenterology & Hepatology.
The investigators noted that the participants represented a real-world patient population, so the data support FMT “as a necessary, effective first-line option” in routine management of C. difficile infection.
“Previous studies have demonstrated clinical cure rates [with FMT] of up to 92%,” Dr. Baunwall and colleagues wrote. “Early use of FMT for first or second C. difficile infection has therapeutic potential, but no formal randomized trials to support use of the approach as a first-line therapy have been done.”
The present trial, conducted at a university hospital in Denmark, involved 42 adult patients with first or second C. difficile infection. Patients were randomized in a 1:1 ratio to receive either vancomycin alone or vancomycin plus FMT. All patients received 125 mg oral vancomycin four times daily for a minimum of 10 days after diagnosis. On day 1 after completion of vancomycin therapy and again between day 3 and 7, patients received either oral FMT or matching placebo, depending on their group. After completing the protocol, patients were followed for 8 weeks or C. difficile recurrence to evaluate resolution of C. difficile–associated diarrhea.
“In this trial, patients were treated with two sequential FMT procedures on separate days,” the investigators noted. “This practice might have overtreated some patients and differs from previous trials. It remains unknown whether optimal effect is achieved by one or two treatments.”
The trial design called for 84 patients, but enrollment was halted after an interim analysis of the above cohort of 42 patients because of significantly lower rate resolution in the placebo group. At the 2-month mark, 90% (95% confidence interval, 70%-99%) of patients in the FMT group had resolution, compared with only 33% (95% CI, 15%-57%) of patients in the placebo group (P = .0003), constituting a 57% (95% CI, 33%-81%) absolute risk reduction.
Most patients experienced adverse events, including 20 in the FMT group and all 21 in the placebo group, although most were transient and nonserious. The most common adverse events were diarrhea, which occurred more frequently in the FMT group (23 vs. 14 events), followed by abdominal pain(14 vs. 11 events) and nausea (12 vs. 5 events).
One limitation of the study was its single-center design with regional uptake; the authors noted that, despite having high statistical power for the clinical effect, the study’s premature termination and low patient number prevent inferences regarding mortality, time to effect, and cost.
“The results of this trial highlight how the use of fecal microbiota transplantation as a first-line treatment can effectively prevent C. difficile recurrence and suggests that microbiota restoration might be necessary to obtain sustained resolution,” the investigators wrote. “At present, only 10% of patients with multiple, recurrent C. difficile infection and indication for FMT receive it. International initiatives address the unmet need, but logistic and regulatory obstacles remain unsolved.”
Encouraging findings, lingering concerns
Nicholas Turner, MD, assistant professor in the division of infectious diseases at Duke University, Durham, N.C., praised the study for “pushing the boundaries for FMT,” and noted that the methodology appeared sound. Results in the placebo group, however, cast doubt on the generalizability of the findings, he said.
“If you look at the group that received vancomycin plus placebo, their failure rate was really astoundingly high,” Dr. Turner said in an interview, referring to the 67% failure rate in the control group; he noted previous studies had reported failure rates closer to 10%. “I think that just calls into question just a little bit what happened with that control group.”
Dr. Turner said his confidence would go “way, way up” if the findings were reproduced in a larger study. Ideally, these future trials would use fidaxomicin, he added, which is becoming the preferred option over vancomycin for treating C. difficile.
John Y. Kao, MD, professor of medicine and codirector of the FMT program at University of Michigan Medicine, Ann Arbor, offered a different perspective, suggesting that the control group findings shouldn’t overshadow the efficacy of FMT.
“I agree that historical data would tell us that the placebo population should see a much higher response,” Dr. Kao said in an interview. “In my mind though, the success rate of FMT over placebo is what I would expect. The message of the study should be upheld: that FMT is an effective therapy whether it’s given early or, as the way we give it now, as a sort of rescue therapy.”
Despite this confidence in FMT as an efficacious first-line option, Dr. Kao said it is unlikely to be routinely used in this way anytime soon, even if a larger trial echoes the present results.
“We don’t know the long-term risks of FMT therapy, although we’ve been doing this now probably close to 20 years,” Dr. Kao said.
Specifically, Dr. Kao was most concerned about the long-term risk of colon cancer, as mouse models suggest that microbiome characteristics may affect risk level, and risk may vary based on host-microbiome relationships. In other words, an organism may pose no risk in the gut of the donor, but the same may not be true for the recipient.
While increased rates of colon cancer or other serious illnesses have not been detected in humans who have undergone FMT over the past 2 decades, Dr. Kao said that these findings cannot be extrapolated over a patient’s entire lifetime, especially for younger individuals.
“In a patient that’s 80, you would say, yeah, let’s go ahead and treat you [with FMT] as first-line therapy, whereas someone who’s 20, and has maybe another 50 or 60 years longevity, you may not want to give FMT as first-line therapy,” Dr. Kao said.
This study was supported by Innovation Fund Denmark. The investigators disclosed no competing interests. Dr. Turner previously performed statistical analyses for a Merck study comparing vancomycin, fidaxomicin, and metronidazole for C. difficile infection. Dr. Kao disclosed no relevant conflicts of interest.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center (www.gastro.org/Cdiff).
Fecal microbiota transplantation (FMT) is safe and highly effective as first-line therapy for patients with first or second Clostridioides difficile infection, according to the first randomized, double-blind, placebo-controlled trial of its kind.
Study enrollment was halted after an interim analysis revealed significantly better outcomes among patients who received vancomycin plus FMT versus vancomycin alone, reported lead author Simon Mark Dahl Baunwall, MD, of Aarhus (Denmark) University Hospital and colleagues in The Lancet Gastroenterology & Hepatology.
The investigators noted that the participants represented a real-world patient population, so the data support FMT “as a necessary, effective first-line option” in routine management of C. difficile infection.
“Previous studies have demonstrated clinical cure rates [with FMT] of up to 92%,” Dr. Baunwall and colleagues wrote. “Early use of FMT for first or second C. difficile infection has therapeutic potential, but no formal randomized trials to support use of the approach as a first-line therapy have been done.”
The present trial, conducted at a university hospital in Denmark, involved 42 adult patients with first or second C. difficile infection. Patients were randomized in a 1:1 ratio to receive either vancomycin alone or vancomycin plus FMT. All patients received 125 mg oral vancomycin four times daily for a minimum of 10 days after diagnosis. On day 1 after completion of vancomycin therapy and again between day 3 and 7, patients received either oral FMT or matching placebo, depending on their group. After completing the protocol, patients were followed for 8 weeks or C. difficile recurrence to evaluate resolution of C. difficile–associated diarrhea.
“In this trial, patients were treated with two sequential FMT procedures on separate days,” the investigators noted. “This practice might have overtreated some patients and differs from previous trials. It remains unknown whether optimal effect is achieved by one or two treatments.”
The trial design called for 84 patients, but enrollment was halted after an interim analysis of the above cohort of 42 patients because of significantly lower rate resolution in the placebo group. At the 2-month mark, 90% (95% confidence interval, 70%-99%) of patients in the FMT group had resolution, compared with only 33% (95% CI, 15%-57%) of patients in the placebo group (P = .0003), constituting a 57% (95% CI, 33%-81%) absolute risk reduction.
Most patients experienced adverse events, including 20 in the FMT group and all 21 in the placebo group, although most were transient and nonserious. The most common adverse events were diarrhea, which occurred more frequently in the FMT group (23 vs. 14 events), followed by abdominal pain(14 vs. 11 events) and nausea (12 vs. 5 events).
One limitation of the study was its single-center design with regional uptake; the authors noted that, despite having high statistical power for the clinical effect, the study’s premature termination and low patient number prevent inferences regarding mortality, time to effect, and cost.
“The results of this trial highlight how the use of fecal microbiota transplantation as a first-line treatment can effectively prevent C. difficile recurrence and suggests that microbiota restoration might be necessary to obtain sustained resolution,” the investigators wrote. “At present, only 10% of patients with multiple, recurrent C. difficile infection and indication for FMT receive it. International initiatives address the unmet need, but logistic and regulatory obstacles remain unsolved.”
Encouraging findings, lingering concerns
Nicholas Turner, MD, assistant professor in the division of infectious diseases at Duke University, Durham, N.C., praised the study for “pushing the boundaries for FMT,” and noted that the methodology appeared sound. Results in the placebo group, however, cast doubt on the generalizability of the findings, he said.
“If you look at the group that received vancomycin plus placebo, their failure rate was really astoundingly high,” Dr. Turner said in an interview, referring to the 67% failure rate in the control group; he noted previous studies had reported failure rates closer to 10%. “I think that just calls into question just a little bit what happened with that control group.”
Dr. Turner said his confidence would go “way, way up” if the findings were reproduced in a larger study. Ideally, these future trials would use fidaxomicin, he added, which is becoming the preferred option over vancomycin for treating C. difficile.
John Y. Kao, MD, professor of medicine and codirector of the FMT program at University of Michigan Medicine, Ann Arbor, offered a different perspective, suggesting that the control group findings shouldn’t overshadow the efficacy of FMT.
“I agree that historical data would tell us that the placebo population should see a much higher response,” Dr. Kao said in an interview. “In my mind though, the success rate of FMT over placebo is what I would expect. The message of the study should be upheld: that FMT is an effective therapy whether it’s given early or, as the way we give it now, as a sort of rescue therapy.”
Despite this confidence in FMT as an efficacious first-line option, Dr. Kao said it is unlikely to be routinely used in this way anytime soon, even if a larger trial echoes the present results.
“We don’t know the long-term risks of FMT therapy, although we’ve been doing this now probably close to 20 years,” Dr. Kao said.
Specifically, Dr. Kao was most concerned about the long-term risk of colon cancer, as mouse models suggest that microbiome characteristics may affect risk level, and risk may vary based on host-microbiome relationships. In other words, an organism may pose no risk in the gut of the donor, but the same may not be true for the recipient.
While increased rates of colon cancer or other serious illnesses have not been detected in humans who have undergone FMT over the past 2 decades, Dr. Kao said that these findings cannot be extrapolated over a patient’s entire lifetime, especially for younger individuals.
“In a patient that’s 80, you would say, yeah, let’s go ahead and treat you [with FMT] as first-line therapy, whereas someone who’s 20, and has maybe another 50 or 60 years longevity, you may not want to give FMT as first-line therapy,” Dr. Kao said.
This study was supported by Innovation Fund Denmark. The investigators disclosed no competing interests. Dr. Turner previously performed statistical analyses for a Merck study comparing vancomycin, fidaxomicin, and metronidazole for C. difficile infection. Dr. Kao disclosed no relevant conflicts of interest.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center (www.gastro.org/Cdiff).
FROM THE LANCET GASTROENTEROLOGY & HEPATOLOGY
The price we pay for an MD
It is no secret that medical school is expensive. Depending on where in the world you live, the cost of a medical degree varies. In the United States, it is well known that medical students end up with an average of $241,600 in debt after at least 8 years of education; attending a public, private, Ivy League or non–Ivy League school may cause this to differ among students.
In the United Kingdom, however, the true cost of medical training isn’t as obvious. All students pay the same amount of tuition, which is usually paid for by a government loan for the entirety of their course. For those who need it, a maintenance loan is also available for living expenses. Naturally, those from lower income households receive higher maintenance loans and therefore, have a higher debt burden by the end of their studies – debt you don’t think about until your first real paycheck post medical school. (It is also important to note that these loans are completely optional – throughout my time in medical school, I’ve encountered students who didn’t need all or any of their student loans to pay for their medical school tuition or living expenses.)
when embarking on our journey through medical school – for example, the cost of a stethoscope, revision resources, transport, and housing. In addition, as the commitment of a medical degree intensifies, there is less and less time for students to work-part time outside of their studies, meaning less income coming in while expenses increase and student loans accumulate interest.
Furthermore, it is common knowledge that a higher proportion of students from affluent backgrounds are accepted into medical school and that socioeconomic status and one’s finances create a huge disparity in achievement and general well-being during medical school. Indeed, studies show that higher levels of debt are negatively correlated with mental well-being and academic performance; students from lower socioeconomic backgrounds have higher debts and as a result, worry more about money.
Four years into my medical education, I have experienced and now understand the financial strain of a medical degree. The path to becoming a doctor truly is one of life-long sacrifice, so why doesn’t our society look after those who make it their life’s work to look after them? It is incredibly unfortunate that there is a lack of financial support and assistance for medical trainees, especially those from lower socioeconomic backgrounds. In fact, according to a BMJ study, about 5% of students were considering dropping out of medical school because of financial hardship alone and have cut back on simple living essentials such as heating and food.
Unfortunately, these are the students that are more representative of the patient population; the average patient that we see is not one had that a private school education or had affluent parents. Although more people from diverse backgrounds are enrolling in medical school, finances act as yet another barrier preventing them from completing this degree; such factors are why we see such limited diversity in medicine.
Talking about finances appears to be a taboo in the medical field, but doing so is important, for it not only raises awareness and creates a voice for those 5% of students who are struggling financially but also helps premed students have a realistic understanding of the financial sacrifice of pursuing medicine, allowing them to make more informed choices about their future careers. In the meantime, as we create this new culture, it will be important for institutions to consider supporting students financially through bursaries and scholarships (and reducing tuition prices) throughout their time at university. In this way, we can create a more equitable and encouraging environments for all medical trainees.
Ms. Ntorinkansah is a medical student at the University of Nottingham (England). She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
It is no secret that medical school is expensive. Depending on where in the world you live, the cost of a medical degree varies. In the United States, it is well known that medical students end up with an average of $241,600 in debt after at least 8 years of education; attending a public, private, Ivy League or non–Ivy League school may cause this to differ among students.
In the United Kingdom, however, the true cost of medical training isn’t as obvious. All students pay the same amount of tuition, which is usually paid for by a government loan for the entirety of their course. For those who need it, a maintenance loan is also available for living expenses. Naturally, those from lower income households receive higher maintenance loans and therefore, have a higher debt burden by the end of their studies – debt you don’t think about until your first real paycheck post medical school. (It is also important to note that these loans are completely optional – throughout my time in medical school, I’ve encountered students who didn’t need all or any of their student loans to pay for their medical school tuition or living expenses.)
when embarking on our journey through medical school – for example, the cost of a stethoscope, revision resources, transport, and housing. In addition, as the commitment of a medical degree intensifies, there is less and less time for students to work-part time outside of their studies, meaning less income coming in while expenses increase and student loans accumulate interest.
Furthermore, it is common knowledge that a higher proportion of students from affluent backgrounds are accepted into medical school and that socioeconomic status and one’s finances create a huge disparity in achievement and general well-being during medical school. Indeed, studies show that higher levels of debt are negatively correlated with mental well-being and academic performance; students from lower socioeconomic backgrounds have higher debts and as a result, worry more about money.
Four years into my medical education, I have experienced and now understand the financial strain of a medical degree. The path to becoming a doctor truly is one of life-long sacrifice, so why doesn’t our society look after those who make it their life’s work to look after them? It is incredibly unfortunate that there is a lack of financial support and assistance for medical trainees, especially those from lower socioeconomic backgrounds. In fact, according to a BMJ study, about 5% of students were considering dropping out of medical school because of financial hardship alone and have cut back on simple living essentials such as heating and food.
Unfortunately, these are the students that are more representative of the patient population; the average patient that we see is not one had that a private school education or had affluent parents. Although more people from diverse backgrounds are enrolling in medical school, finances act as yet another barrier preventing them from completing this degree; such factors are why we see such limited diversity in medicine.
Talking about finances appears to be a taboo in the medical field, but doing so is important, for it not only raises awareness and creates a voice for those 5% of students who are struggling financially but also helps premed students have a realistic understanding of the financial sacrifice of pursuing medicine, allowing them to make more informed choices about their future careers. In the meantime, as we create this new culture, it will be important for institutions to consider supporting students financially through bursaries and scholarships (and reducing tuition prices) throughout their time at university. In this way, we can create a more equitable and encouraging environments for all medical trainees.
Ms. Ntorinkansah is a medical student at the University of Nottingham (England). She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
It is no secret that medical school is expensive. Depending on where in the world you live, the cost of a medical degree varies. In the United States, it is well known that medical students end up with an average of $241,600 in debt after at least 8 years of education; attending a public, private, Ivy League or non–Ivy League school may cause this to differ among students.
In the United Kingdom, however, the true cost of medical training isn’t as obvious. All students pay the same amount of tuition, which is usually paid for by a government loan for the entirety of their course. For those who need it, a maintenance loan is also available for living expenses. Naturally, those from lower income households receive higher maintenance loans and therefore, have a higher debt burden by the end of their studies – debt you don’t think about until your first real paycheck post medical school. (It is also important to note that these loans are completely optional – throughout my time in medical school, I’ve encountered students who didn’t need all or any of their student loans to pay for their medical school tuition or living expenses.)
when embarking on our journey through medical school – for example, the cost of a stethoscope, revision resources, transport, and housing. In addition, as the commitment of a medical degree intensifies, there is less and less time for students to work-part time outside of their studies, meaning less income coming in while expenses increase and student loans accumulate interest.
Furthermore, it is common knowledge that a higher proportion of students from affluent backgrounds are accepted into medical school and that socioeconomic status and one’s finances create a huge disparity in achievement and general well-being during medical school. Indeed, studies show that higher levels of debt are negatively correlated with mental well-being and academic performance; students from lower socioeconomic backgrounds have higher debts and as a result, worry more about money.
Four years into my medical education, I have experienced and now understand the financial strain of a medical degree. The path to becoming a doctor truly is one of life-long sacrifice, so why doesn’t our society look after those who make it their life’s work to look after them? It is incredibly unfortunate that there is a lack of financial support and assistance for medical trainees, especially those from lower socioeconomic backgrounds. In fact, according to a BMJ study, about 5% of students were considering dropping out of medical school because of financial hardship alone and have cut back on simple living essentials such as heating and food.
Unfortunately, these are the students that are more representative of the patient population; the average patient that we see is not one had that a private school education or had affluent parents. Although more people from diverse backgrounds are enrolling in medical school, finances act as yet another barrier preventing them from completing this degree; such factors are why we see such limited diversity in medicine.
Talking about finances appears to be a taboo in the medical field, but doing so is important, for it not only raises awareness and creates a voice for those 5% of students who are struggling financially but also helps premed students have a realistic understanding of the financial sacrifice of pursuing medicine, allowing them to make more informed choices about their future careers. In the meantime, as we create this new culture, it will be important for institutions to consider supporting students financially through bursaries and scholarships (and reducing tuition prices) throughout their time at university. In this way, we can create a more equitable and encouraging environments for all medical trainees.
Ms. Ntorinkansah is a medical student at the University of Nottingham (England). She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Too old to practice medicine?
Unlike for many other professions, there is no age limit for practicing medicine. According to international standards, airplane pilots, for example, who are responsible for the safety of many human lives, must retire by the age of 60 if they work alone, or 65 if they have a copilot. In Brazil, however, this age limit does not exist for pilots or physicians.
The only restriction on professional practice within the medical context is the mandatory retirement imposed on medical professors who teach at public (state and federal) universities, starting at the age of 75. Nevertheless, these professionals can continue practicing administrative and research-related activities. After “expulsion,” as this mandatory retirement is often called, professors who stood out or contributed to the institution and science may receive the title of professor emeritus.
In the private sector, age limits are not formally set, but the hiring of middle-aged professionals is limited.
At the Heart Institute of the University of São Paulo (Brazil) School of Medicine Clinical Hospital (InCor/HCFMUSP), one of the world’s largest teaching and research centers for cardiovascular and pulmonary diseases, several octogenarian specialists lead studies and teams. One of these is Noedir Stolf, MD, an 82-year-old cardiovascular surgeon who operates almost every day and coordinates studies on transplants, mechanical circulatory support, and aortic surgery. There is also Protásio Lemos da Luz, MD, an 82-year-old clinical cardiologist who guides research on subjects including atherosclerosis, the endothelium, microbiota, and diabetes. The protective effect of wine on atherosclerosis is one of his best-known studies.
No longer working is also not in the cards for Angelita Habr-Gama, MD, who, at 89 years old, is one of the oldest physicians in current practice. With a career spanning more than 7 decades, she is a world reference in coloproctology. She was the first woman to become a surgical resident at the HCFMUSP, where she later founded the coloproctology specialty and created the first residency program for the specialty. In April 2022, Dr. Habr-Gama joined the ranks of the 100 most influential scientists in the world, nominated by researchers at Stanford (Calif.) University, and published in PLOS Biology.
In 2020, she was sedated, intubated, and hospitalized in the intensive care unit of the Oswaldo Cruz German Hospital for 54 days because of a SARS-CoV-2 infection. After her discharge, she went back to work in less than 10 days – and added chess classes to her routine. “To get up and go to work makes me very happy. Work is my greatest hobby. No one has ever heard me complain about my life,” Dr. Habr-Gama told this news organization after having rescheduled the interview twice because of emergency surgeries.
“Doctors have a professional longevity that does not exist for other professions in which the person retires and stops practicing their profession or goes on to do something else for entertainment. Doctors can retire from one place of employment or public practice and continue practicing medicine in the office as an administrator or consultant,” Ângelo Vattimo, first secretary of the state of São Paulo Regional Board of Medicine (CREMESP), stated. The board regularly organizes a ceremony to honor professionals who have been practicing for 50 years, awarding them a certificate and engraved medal. “Many of them are around 80 years old, working and teaching. This always makes us very happy. What profession has such exceptional compliance for so long?” said Mr. Vattimo.
In the medical field, the older the age range, the smaller the number of women. According to the 2020 Medical Demographics in Brazil survey, only 2 out of 10 practicing professionals older than 70 are women.
Not everyone over 80 has Dr. Habr-Gama’s vitality, because the impact of aging is not equal. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” stated Mark Katlic, MD, chief of surgery at LifeBridge Health System in the United States, who has dedicated his life to studying the subject. Dr. Katlic spoke on the subject in an interview that was published in the article “How Old Is Too Old to Work as a Doctor?” published by this news organization in April of 2022. The article discusses the evaluations of elderly physicians’ skills and competences that U.S. companies conduct. The subject has been leading to profound debate.
Dr. Katlic defends screening programs for elderly physicians, which already are in effect at the company for which he works, LifeBridge Health, and various others in the United States. “We do [screen elderly physicians at LifeBridge Health], and so do a few dozen other [U.S. institutions], but there are hundreds [of health care institutions] that do not conduct this screening,” he pointed out.
Age-related assessment faces great resistance in the United States. One physician who is against the initiative is Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist affiliated with Stanford (Calif.) University Health. “It’s age discrimination ... Physicians [in the United States] receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as physicians reach a certain age,” Dr. Stockdale told this news organization.
The U.S. initiative of instituting physician assessment programs for those of a certain age has even been tested in court. According to an article published in Medscape, “in New Haven, Connecticut, for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory late career practitioner policy.”
Also, according to the article, a similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
In Brazil, the subject is of interest to more than 34,571 physicians between 65 and 69 years of age and 34,237 physicians older than 70. In all, this population represents approximately 14.3% of the country’s active workforce, according to the 2020 Medical Demographics in Brazil survey.
The significant participation of health care professionals over age 50 in a survey conducted by this news organization to learn what physicians think about the age limit for practicing their professions is evidence that the subject is a present concern. Of a total of 1,641 participants, 57% were age 60 or older, 17% were between 50 and 59 years, and 12% were between 40 and 49 years. Among all participants, 51% were against these limitations, 17% approved of the idea for all specialties, and 32% believed the restriction was appropriate only for some specialties. Regarding the possibility of older physicians undergoing regular assessments, the opinions were divided: Thirty-one percent thought they should be assessed in all specialties. Furthermore, 31% believed that cognitive abilities should be regularly tested in all specialties, 31% thought this should take place for some specialties, and 38% were against this approach.
Professionals want to know, for example, how (and whether) advanced age can interfere with performance, what are the competences required to practice their activities, and if the criteria vary by specialty. “A psychiatrist doesn’t have to have perfect visual acuity, as required from a dermatologist, but it is important that they have good hearing, for example,” argued Clóvis Constantino, MD, former president of the São Paulo Regional Medical Board (CRM-SP) and former vice president of the Brazilian Federal Medical Board (CFM). “However, a surgeon has to stand for several hours in positions that may be uncomfortable. It’s not easy,” he told this news organization.
In the opinion of 82-year-old Henrique Klajner, MD, the oldest pediatrician in practice at the Albert Einstein Israeli Hospital in São Paulo, the physician cannot be subjected to the types of evaluations that have been applied in the United States. “Physicians should conduct constant self-evaluations to see if they have the competences and skills needed to practice their profession ... Moreover, this is not a matter of age. It is a matter of ethics,” said Dr. Klajner.
The ability to adapt to change and implement innovation is critical to professional longevity, he said. “Nowadays, when I admit patients, I no longer do hospital rounds, which requires a mobility equal to physical abuse for me. Therefore, I work with physicians who take care of my hospitalized patients.”
Dr. Klajner also feels there is a distinction between innovations learned through studies and what can be offered safely to patients. “If I have to care for a hospitalized patient with severe pneumonia, for example, since I am not up to date in this specialty, I am going to call upon a pulmonologist I trust and forgo my honorarium for this admission. But I will remain on the team, monitoring the patient’s progression,” he said.
During the COVID-19 pandemic, Dr. Klajner stopped seeing patients in person under the recommendation of his son, Sidney Klajner, MD, also a physician. The elder Dr. Klajner began exploring telemedicine, which opened a whole new world of possibilities. “I have conducted several online visits to provide educational instruction to mothers returning home post delivery, for example,” he told this news organization. The time to stop is not something that concerns Dr. Klajner. “I’m only going to stop when I have a really important reason to do so. For example, if I can no longer write or study, reading and rereading an article without being able to understand what is being said. At this time, none of that is happening.”
In the United States, as well as in Brazil, physicians rarely provide information to human resources departments on colleagues showing signs of cognitive or motor decline affecting their professional performance. “The expectation is that health care professionals will report colleagues with cognitive impairments, but that often does not happen,” Dr. Katlic said.
It is also not common for professionals to report their own deficits to their institutions. In large part, this is caused by a lack of well-defined policies for dealing with this issue. This news organization sought out several public and private hospitals in Brazil to see if there is any guidance on professional longevity: Most said that there is not. Only the A. C. Camargo Cancer Center reported, through its public relations team, that a committee is discussing the subject but that it is still in the early stages.
Brazilian specialist associations do not offer guidelines or instructions on the various aspects of professional longevity. Dr. Constantino tried to put the subject on the agenda during the years in which he was an administrator with the CFM. “We tried to open up discussions regarding truly elderly physicians, but the subject was not well received. I believe that it is precisely because there is a tradition of physicians working until they are no longer able that this is more difficult in Brazil ... No one exactly knows what to do in this respect.” Dr. Constantino is against the use of age as a criterion for quitting practice.
“Of course, this is a point that has to be considered, but I always defended the need for regular assessment of physicians, regardless of age range. And, although assessments are always welcome, in any profession, I also believe this would not be well received in Brazil.” He endorses an assessment of one’s knowledge and not of physical abilities, which are generally assessed through investigation when needed.
The absence of guidelines increases individual responsibility, as well as vulnerability. “Consciously, physicians will not put patients at risk if they do not have the competence to care for them or to perform a surgical procedure,” said Clystenes Odyr Soares Silva, MD, PhD, adjunct professor of pulmonology of the Federal University of São Paulo (Brazil) School of Medicine (UNIFESP). “Your peers will tell you if you are no longer able,” he added. The problem is that physicians rarely admit to or talk about their colleagues’ deficits, especially if they are in the spotlight because of advanced age. In this situation, the observation and opinion of family members regarding the health care professional’s competences and skills will hold more weight.
In case of health-related physical impairment, such as partial loss of hand movement, for example, “it is expected that this will set off an ethical warning in the person,” said Dr. Constantino. When this warning does not occur naturally, patients or colleagues can report the professional, and this may lead to the opening of an administrative investigation. If the report is found to be true, this investigation is used to suspend physicians who do not have the physical or mental ability to continue practicing medicine.
“If it’s something very serious, the physician’s license can be temporarily suspended while [the physician] is treated by a psychiatrist, with follow-up by the professional board. When discharged, the physician will get his or her [professional] license back and can go back to work,” Dr. Constantino explained. If an expert evaluation is needed, the physician will then be assessed by a forensic psychiatrist. One of the most in-demand forensic psychiatrists in Brazil is Guido Arturo Palomba, MD, 73 years old. “I have assessed some physicians for actions reported to see if they were normal people or not, but never for circumstances related to age,” Dr. Palomba said.
In practice, Brazilian medical entities do not have policies or programs to guide physicians who wish to grow old while they work or those who have started to notice they are not performing as they used to. “We have never lived as long; therefore, the quality of life in old age, as well as the concept of aging, are some of the most relevant questions of our time. These are subjects requiring additional discussion, broadening understanding and awareness in this regard,” observed Mr. Vattimo.
Dr. Constantino and Dr. Silva, who are completely against age-based assessments, believe that recertification of the specialist license every 5 years is the best path to confirming whether the physician is still able to practice. “A knowledge-based test every 5 years to recertify the specialist license has often been a topic of conversation. I think it’s an excellent idea. The person would provide a dossier of all they have done in terms of courses, conferences, and other activities, present it, and receive a score,” said Dr. Silva.
In practice, recertification of the specialist license is a topic of discussion that has been raised for years, and it is an idea that the Brazilian Medical Association (AMB) defends. In conjunction with the CFM, the association is studying a way to best implement this assessment. “It’s important to emphasize that this measure would not be retroactive at first. Instead, it would only be in effect for professionals licensed after the recertification requirement is established,” the AMB pointed out in a note sent to this news organization. Even so, the measure has faced significant resistance from a faction of the profession, and its enactment does not seem to be imminent.
The debate regarding professional longevity is taking place in various countries. In 2021, the American Medical Association Council on Medical Education released a report with a set of guidelines for the screening and assessment of physicians. The document is the product of a committee created in 2015 to study the subject. The AMA recommends that the assessment of elderly physicians be based on evidence and ethical, relevant, fair, equitable, transparent, verifiable, nonexhaustive principles, contemplating support and protecting against legal proceedings. In April of this year, a new AMA document highlighted the same principles.
Also in the United States, one of oldest initiatives created to support physicians in the process of recycling, the University of California San Diego Physician Assessment and Clinical Education Program (PACE), has a section focusing on the extended practice of medicine (Practicing Medicine Longer). For those wanting to learn more about discussions on this subject, there are online presentations on experiences in Quebec and Ontario with assessing aging physicians, neuropsychological perspectives on the aging medical population, and what to expect of healthy aging, among other subjects.
Created in 1996, PACE mostly provides services to physicians who need to address requirements of the state medical boards. Few physicians enroll on their own.
The first part of the program assesses knowledge and skills over approximately 2 days. In the second phase, the physician participates in a series of activities in a corresponding residency program. Depending on the results, the physician may have to go through a remedial program with varying activities to deal with performance deficiencies to clinical experiences at the residency level.
A version of this article first appeared on Medscape.com.
Unlike for many other professions, there is no age limit for practicing medicine. According to international standards, airplane pilots, for example, who are responsible for the safety of many human lives, must retire by the age of 60 if they work alone, or 65 if they have a copilot. In Brazil, however, this age limit does not exist for pilots or physicians.
The only restriction on professional practice within the medical context is the mandatory retirement imposed on medical professors who teach at public (state and federal) universities, starting at the age of 75. Nevertheless, these professionals can continue practicing administrative and research-related activities. After “expulsion,” as this mandatory retirement is often called, professors who stood out or contributed to the institution and science may receive the title of professor emeritus.
In the private sector, age limits are not formally set, but the hiring of middle-aged professionals is limited.
At the Heart Institute of the University of São Paulo (Brazil) School of Medicine Clinical Hospital (InCor/HCFMUSP), one of the world’s largest teaching and research centers for cardiovascular and pulmonary diseases, several octogenarian specialists lead studies and teams. One of these is Noedir Stolf, MD, an 82-year-old cardiovascular surgeon who operates almost every day and coordinates studies on transplants, mechanical circulatory support, and aortic surgery. There is also Protásio Lemos da Luz, MD, an 82-year-old clinical cardiologist who guides research on subjects including atherosclerosis, the endothelium, microbiota, and diabetes. The protective effect of wine on atherosclerosis is one of his best-known studies.
No longer working is also not in the cards for Angelita Habr-Gama, MD, who, at 89 years old, is one of the oldest physicians in current practice. With a career spanning more than 7 decades, she is a world reference in coloproctology. She was the first woman to become a surgical resident at the HCFMUSP, where she later founded the coloproctology specialty and created the first residency program for the specialty. In April 2022, Dr. Habr-Gama joined the ranks of the 100 most influential scientists in the world, nominated by researchers at Stanford (Calif.) University, and published in PLOS Biology.
In 2020, she was sedated, intubated, and hospitalized in the intensive care unit of the Oswaldo Cruz German Hospital for 54 days because of a SARS-CoV-2 infection. After her discharge, she went back to work in less than 10 days – and added chess classes to her routine. “To get up and go to work makes me very happy. Work is my greatest hobby. No one has ever heard me complain about my life,” Dr. Habr-Gama told this news organization after having rescheduled the interview twice because of emergency surgeries.
“Doctors have a professional longevity that does not exist for other professions in which the person retires and stops practicing their profession or goes on to do something else for entertainment. Doctors can retire from one place of employment or public practice and continue practicing medicine in the office as an administrator or consultant,” Ângelo Vattimo, first secretary of the state of São Paulo Regional Board of Medicine (CREMESP), stated. The board regularly organizes a ceremony to honor professionals who have been practicing for 50 years, awarding them a certificate and engraved medal. “Many of them are around 80 years old, working and teaching. This always makes us very happy. What profession has such exceptional compliance for so long?” said Mr. Vattimo.
In the medical field, the older the age range, the smaller the number of women. According to the 2020 Medical Demographics in Brazil survey, only 2 out of 10 practicing professionals older than 70 are women.
Not everyone over 80 has Dr. Habr-Gama’s vitality, because the impact of aging is not equal. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” stated Mark Katlic, MD, chief of surgery at LifeBridge Health System in the United States, who has dedicated his life to studying the subject. Dr. Katlic spoke on the subject in an interview that was published in the article “How Old Is Too Old to Work as a Doctor?” published by this news organization in April of 2022. The article discusses the evaluations of elderly physicians’ skills and competences that U.S. companies conduct. The subject has been leading to profound debate.
Dr. Katlic defends screening programs for elderly physicians, which already are in effect at the company for which he works, LifeBridge Health, and various others in the United States. “We do [screen elderly physicians at LifeBridge Health], and so do a few dozen other [U.S. institutions], but there are hundreds [of health care institutions] that do not conduct this screening,” he pointed out.
Age-related assessment faces great resistance in the United States. One physician who is against the initiative is Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist affiliated with Stanford (Calif.) University Health. “It’s age discrimination ... Physicians [in the United States] receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as physicians reach a certain age,” Dr. Stockdale told this news organization.
The U.S. initiative of instituting physician assessment programs for those of a certain age has even been tested in court. According to an article published in Medscape, “in New Haven, Connecticut, for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory late career practitioner policy.”
Also, according to the article, a similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
In Brazil, the subject is of interest to more than 34,571 physicians between 65 and 69 years of age and 34,237 physicians older than 70. In all, this population represents approximately 14.3% of the country’s active workforce, according to the 2020 Medical Demographics in Brazil survey.
The significant participation of health care professionals over age 50 in a survey conducted by this news organization to learn what physicians think about the age limit for practicing their professions is evidence that the subject is a present concern. Of a total of 1,641 participants, 57% were age 60 or older, 17% were between 50 and 59 years, and 12% were between 40 and 49 years. Among all participants, 51% were against these limitations, 17% approved of the idea for all specialties, and 32% believed the restriction was appropriate only for some specialties. Regarding the possibility of older physicians undergoing regular assessments, the opinions were divided: Thirty-one percent thought they should be assessed in all specialties. Furthermore, 31% believed that cognitive abilities should be regularly tested in all specialties, 31% thought this should take place for some specialties, and 38% were against this approach.
Professionals want to know, for example, how (and whether) advanced age can interfere with performance, what are the competences required to practice their activities, and if the criteria vary by specialty. “A psychiatrist doesn’t have to have perfect visual acuity, as required from a dermatologist, but it is important that they have good hearing, for example,” argued Clóvis Constantino, MD, former president of the São Paulo Regional Medical Board (CRM-SP) and former vice president of the Brazilian Federal Medical Board (CFM). “However, a surgeon has to stand for several hours in positions that may be uncomfortable. It’s not easy,” he told this news organization.
In the opinion of 82-year-old Henrique Klajner, MD, the oldest pediatrician in practice at the Albert Einstein Israeli Hospital in São Paulo, the physician cannot be subjected to the types of evaluations that have been applied in the United States. “Physicians should conduct constant self-evaluations to see if they have the competences and skills needed to practice their profession ... Moreover, this is not a matter of age. It is a matter of ethics,” said Dr. Klajner.
The ability to adapt to change and implement innovation is critical to professional longevity, he said. “Nowadays, when I admit patients, I no longer do hospital rounds, which requires a mobility equal to physical abuse for me. Therefore, I work with physicians who take care of my hospitalized patients.”
Dr. Klajner also feels there is a distinction between innovations learned through studies and what can be offered safely to patients. “If I have to care for a hospitalized patient with severe pneumonia, for example, since I am not up to date in this specialty, I am going to call upon a pulmonologist I trust and forgo my honorarium for this admission. But I will remain on the team, monitoring the patient’s progression,” he said.
During the COVID-19 pandemic, Dr. Klajner stopped seeing patients in person under the recommendation of his son, Sidney Klajner, MD, also a physician. The elder Dr. Klajner began exploring telemedicine, which opened a whole new world of possibilities. “I have conducted several online visits to provide educational instruction to mothers returning home post delivery, for example,” he told this news organization. The time to stop is not something that concerns Dr. Klajner. “I’m only going to stop when I have a really important reason to do so. For example, if I can no longer write or study, reading and rereading an article without being able to understand what is being said. At this time, none of that is happening.”
In the United States, as well as in Brazil, physicians rarely provide information to human resources departments on colleagues showing signs of cognitive or motor decline affecting their professional performance. “The expectation is that health care professionals will report colleagues with cognitive impairments, but that often does not happen,” Dr. Katlic said.
It is also not common for professionals to report their own deficits to their institutions. In large part, this is caused by a lack of well-defined policies for dealing with this issue. This news organization sought out several public and private hospitals in Brazil to see if there is any guidance on professional longevity: Most said that there is not. Only the A. C. Camargo Cancer Center reported, through its public relations team, that a committee is discussing the subject but that it is still in the early stages.
Brazilian specialist associations do not offer guidelines or instructions on the various aspects of professional longevity. Dr. Constantino tried to put the subject on the agenda during the years in which he was an administrator with the CFM. “We tried to open up discussions regarding truly elderly physicians, but the subject was not well received. I believe that it is precisely because there is a tradition of physicians working until they are no longer able that this is more difficult in Brazil ... No one exactly knows what to do in this respect.” Dr. Constantino is against the use of age as a criterion for quitting practice.
“Of course, this is a point that has to be considered, but I always defended the need for regular assessment of physicians, regardless of age range. And, although assessments are always welcome, in any profession, I also believe this would not be well received in Brazil.” He endorses an assessment of one’s knowledge and not of physical abilities, which are generally assessed through investigation when needed.
The absence of guidelines increases individual responsibility, as well as vulnerability. “Consciously, physicians will not put patients at risk if they do not have the competence to care for them or to perform a surgical procedure,” said Clystenes Odyr Soares Silva, MD, PhD, adjunct professor of pulmonology of the Federal University of São Paulo (Brazil) School of Medicine (UNIFESP). “Your peers will tell you if you are no longer able,” he added. The problem is that physicians rarely admit to or talk about their colleagues’ deficits, especially if they are in the spotlight because of advanced age. In this situation, the observation and opinion of family members regarding the health care professional’s competences and skills will hold more weight.
In case of health-related physical impairment, such as partial loss of hand movement, for example, “it is expected that this will set off an ethical warning in the person,” said Dr. Constantino. When this warning does not occur naturally, patients or colleagues can report the professional, and this may lead to the opening of an administrative investigation. If the report is found to be true, this investigation is used to suspend physicians who do not have the physical or mental ability to continue practicing medicine.
“If it’s something very serious, the physician’s license can be temporarily suspended while [the physician] is treated by a psychiatrist, with follow-up by the professional board. When discharged, the physician will get his or her [professional] license back and can go back to work,” Dr. Constantino explained. If an expert evaluation is needed, the physician will then be assessed by a forensic psychiatrist. One of the most in-demand forensic psychiatrists in Brazil is Guido Arturo Palomba, MD, 73 years old. “I have assessed some physicians for actions reported to see if they were normal people or not, but never for circumstances related to age,” Dr. Palomba said.
In practice, Brazilian medical entities do not have policies or programs to guide physicians who wish to grow old while they work or those who have started to notice they are not performing as they used to. “We have never lived as long; therefore, the quality of life in old age, as well as the concept of aging, are some of the most relevant questions of our time. These are subjects requiring additional discussion, broadening understanding and awareness in this regard,” observed Mr. Vattimo.
Dr. Constantino and Dr. Silva, who are completely against age-based assessments, believe that recertification of the specialist license every 5 years is the best path to confirming whether the physician is still able to practice. “A knowledge-based test every 5 years to recertify the specialist license has often been a topic of conversation. I think it’s an excellent idea. The person would provide a dossier of all they have done in terms of courses, conferences, and other activities, present it, and receive a score,” said Dr. Silva.
In practice, recertification of the specialist license is a topic of discussion that has been raised for years, and it is an idea that the Brazilian Medical Association (AMB) defends. In conjunction with the CFM, the association is studying a way to best implement this assessment. “It’s important to emphasize that this measure would not be retroactive at first. Instead, it would only be in effect for professionals licensed after the recertification requirement is established,” the AMB pointed out in a note sent to this news organization. Even so, the measure has faced significant resistance from a faction of the profession, and its enactment does not seem to be imminent.
The debate regarding professional longevity is taking place in various countries. In 2021, the American Medical Association Council on Medical Education released a report with a set of guidelines for the screening and assessment of physicians. The document is the product of a committee created in 2015 to study the subject. The AMA recommends that the assessment of elderly physicians be based on evidence and ethical, relevant, fair, equitable, transparent, verifiable, nonexhaustive principles, contemplating support and protecting against legal proceedings. In April of this year, a new AMA document highlighted the same principles.
Also in the United States, one of oldest initiatives created to support physicians in the process of recycling, the University of California San Diego Physician Assessment and Clinical Education Program (PACE), has a section focusing on the extended practice of medicine (Practicing Medicine Longer). For those wanting to learn more about discussions on this subject, there are online presentations on experiences in Quebec and Ontario with assessing aging physicians, neuropsychological perspectives on the aging medical population, and what to expect of healthy aging, among other subjects.
Created in 1996, PACE mostly provides services to physicians who need to address requirements of the state medical boards. Few physicians enroll on their own.
The first part of the program assesses knowledge and skills over approximately 2 days. In the second phase, the physician participates in a series of activities in a corresponding residency program. Depending on the results, the physician may have to go through a remedial program with varying activities to deal with performance deficiencies to clinical experiences at the residency level.
A version of this article first appeared on Medscape.com.
Unlike for many other professions, there is no age limit for practicing medicine. According to international standards, airplane pilots, for example, who are responsible for the safety of many human lives, must retire by the age of 60 if they work alone, or 65 if they have a copilot. In Brazil, however, this age limit does not exist for pilots or physicians.
The only restriction on professional practice within the medical context is the mandatory retirement imposed on medical professors who teach at public (state and federal) universities, starting at the age of 75. Nevertheless, these professionals can continue practicing administrative and research-related activities. After “expulsion,” as this mandatory retirement is often called, professors who stood out or contributed to the institution and science may receive the title of professor emeritus.
In the private sector, age limits are not formally set, but the hiring of middle-aged professionals is limited.
At the Heart Institute of the University of São Paulo (Brazil) School of Medicine Clinical Hospital (InCor/HCFMUSP), one of the world’s largest teaching and research centers for cardiovascular and pulmonary diseases, several octogenarian specialists lead studies and teams. One of these is Noedir Stolf, MD, an 82-year-old cardiovascular surgeon who operates almost every day and coordinates studies on transplants, mechanical circulatory support, and aortic surgery. There is also Protásio Lemos da Luz, MD, an 82-year-old clinical cardiologist who guides research on subjects including atherosclerosis, the endothelium, microbiota, and diabetes. The protective effect of wine on atherosclerosis is one of his best-known studies.
No longer working is also not in the cards for Angelita Habr-Gama, MD, who, at 89 years old, is one of the oldest physicians in current practice. With a career spanning more than 7 decades, she is a world reference in coloproctology. She was the first woman to become a surgical resident at the HCFMUSP, where she later founded the coloproctology specialty and created the first residency program for the specialty. In April 2022, Dr. Habr-Gama joined the ranks of the 100 most influential scientists in the world, nominated by researchers at Stanford (Calif.) University, and published in PLOS Biology.
In 2020, she was sedated, intubated, and hospitalized in the intensive care unit of the Oswaldo Cruz German Hospital for 54 days because of a SARS-CoV-2 infection. After her discharge, she went back to work in less than 10 days – and added chess classes to her routine. “To get up and go to work makes me very happy. Work is my greatest hobby. No one has ever heard me complain about my life,” Dr. Habr-Gama told this news organization after having rescheduled the interview twice because of emergency surgeries.
“Doctors have a professional longevity that does not exist for other professions in which the person retires and stops practicing their profession or goes on to do something else for entertainment. Doctors can retire from one place of employment or public practice and continue practicing medicine in the office as an administrator or consultant,” Ângelo Vattimo, first secretary of the state of São Paulo Regional Board of Medicine (CREMESP), stated. The board regularly organizes a ceremony to honor professionals who have been practicing for 50 years, awarding them a certificate and engraved medal. “Many of them are around 80 years old, working and teaching. This always makes us very happy. What profession has such exceptional compliance for so long?” said Mr. Vattimo.
In the medical field, the older the age range, the smaller the number of women. According to the 2020 Medical Demographics in Brazil survey, only 2 out of 10 practicing professionals older than 70 are women.
Not everyone over 80 has Dr. Habr-Gama’s vitality, because the impact of aging is not equal. “If you look at a group of 80-year-olds, there will be much more variability than within a group of 40-year-olds,” stated Mark Katlic, MD, chief of surgery at LifeBridge Health System in the United States, who has dedicated his life to studying the subject. Dr. Katlic spoke on the subject in an interview that was published in the article “How Old Is Too Old to Work as a Doctor?” published by this news organization in April of 2022. The article discusses the evaluations of elderly physicians’ skills and competences that U.S. companies conduct. The subject has been leading to profound debate.
Dr. Katlic defends screening programs for elderly physicians, which already are in effect at the company for which he works, LifeBridge Health, and various others in the United States. “We do [screen elderly physicians at LifeBridge Health], and so do a few dozen other [U.S. institutions], but there are hundreds [of health care institutions] that do not conduct this screening,” he pointed out.
Age-related assessment faces great resistance in the United States. One physician who is against the initiative is Frank Stockdale, MD, PhD, an 86-year-old practicing oncologist affiliated with Stanford (Calif.) University Health. “It’s age discrimination ... Physicians [in the United States] receive assessments throughout their careers as part of the accreditation process – there’s no need to change that as physicians reach a certain age,” Dr. Stockdale told this news organization.
The U.S. initiative of instituting physician assessment programs for those of a certain age has even been tested in court. According to an article published in Medscape, “in New Haven, Connecticut, for instance, the U.S. Equal Employment Opportunity Commission (EEOC) filed a suit in 2020 on behalf of the Yale New Haven Hospital staff, alleging a discriminatory late career practitioner policy.”
Also, according to the article, a similar case in Minnesota reached a settlement in 2021, providing monetary relief to staff impacted by out-of-pocket costs for the assessment, in addition to requiring that the hospital in question report to the EEOC any complaints related to age discrimination.
In Brazil, the subject is of interest to more than 34,571 physicians between 65 and 69 years of age and 34,237 physicians older than 70. In all, this population represents approximately 14.3% of the country’s active workforce, according to the 2020 Medical Demographics in Brazil survey.
The significant participation of health care professionals over age 50 in a survey conducted by this news organization to learn what physicians think about the age limit for practicing their professions is evidence that the subject is a present concern. Of a total of 1,641 participants, 57% were age 60 or older, 17% were between 50 and 59 years, and 12% were between 40 and 49 years. Among all participants, 51% were against these limitations, 17% approved of the idea for all specialties, and 32% believed the restriction was appropriate only for some specialties. Regarding the possibility of older physicians undergoing regular assessments, the opinions were divided: Thirty-one percent thought they should be assessed in all specialties. Furthermore, 31% believed that cognitive abilities should be regularly tested in all specialties, 31% thought this should take place for some specialties, and 38% were against this approach.
Professionals want to know, for example, how (and whether) advanced age can interfere with performance, what are the competences required to practice their activities, and if the criteria vary by specialty. “A psychiatrist doesn’t have to have perfect visual acuity, as required from a dermatologist, but it is important that they have good hearing, for example,” argued Clóvis Constantino, MD, former president of the São Paulo Regional Medical Board (CRM-SP) and former vice president of the Brazilian Federal Medical Board (CFM). “However, a surgeon has to stand for several hours in positions that may be uncomfortable. It’s not easy,” he told this news organization.
In the opinion of 82-year-old Henrique Klajner, MD, the oldest pediatrician in practice at the Albert Einstein Israeli Hospital in São Paulo, the physician cannot be subjected to the types of evaluations that have been applied in the United States. “Physicians should conduct constant self-evaluations to see if they have the competences and skills needed to practice their profession ... Moreover, this is not a matter of age. It is a matter of ethics,” said Dr. Klajner.
The ability to adapt to change and implement innovation is critical to professional longevity, he said. “Nowadays, when I admit patients, I no longer do hospital rounds, which requires a mobility equal to physical abuse for me. Therefore, I work with physicians who take care of my hospitalized patients.”
Dr. Klajner also feels there is a distinction between innovations learned through studies and what can be offered safely to patients. “If I have to care for a hospitalized patient with severe pneumonia, for example, since I am not up to date in this specialty, I am going to call upon a pulmonologist I trust and forgo my honorarium for this admission. But I will remain on the team, monitoring the patient’s progression,” he said.
During the COVID-19 pandemic, Dr. Klajner stopped seeing patients in person under the recommendation of his son, Sidney Klajner, MD, also a physician. The elder Dr. Klajner began exploring telemedicine, which opened a whole new world of possibilities. “I have conducted several online visits to provide educational instruction to mothers returning home post delivery, for example,” he told this news organization. The time to stop is not something that concerns Dr. Klajner. “I’m only going to stop when I have a really important reason to do so. For example, if I can no longer write or study, reading and rereading an article without being able to understand what is being said. At this time, none of that is happening.”
In the United States, as well as in Brazil, physicians rarely provide information to human resources departments on colleagues showing signs of cognitive or motor decline affecting their professional performance. “The expectation is that health care professionals will report colleagues with cognitive impairments, but that often does not happen,” Dr. Katlic said.
It is also not common for professionals to report their own deficits to their institutions. In large part, this is caused by a lack of well-defined policies for dealing with this issue. This news organization sought out several public and private hospitals in Brazil to see if there is any guidance on professional longevity: Most said that there is not. Only the A. C. Camargo Cancer Center reported, through its public relations team, that a committee is discussing the subject but that it is still in the early stages.
Brazilian specialist associations do not offer guidelines or instructions on the various aspects of professional longevity. Dr. Constantino tried to put the subject on the agenda during the years in which he was an administrator with the CFM. “We tried to open up discussions regarding truly elderly physicians, but the subject was not well received. I believe that it is precisely because there is a tradition of physicians working until they are no longer able that this is more difficult in Brazil ... No one exactly knows what to do in this respect.” Dr. Constantino is against the use of age as a criterion for quitting practice.
“Of course, this is a point that has to be considered, but I always defended the need for regular assessment of physicians, regardless of age range. And, although assessments are always welcome, in any profession, I also believe this would not be well received in Brazil.” He endorses an assessment of one’s knowledge and not of physical abilities, which are generally assessed through investigation when needed.
The absence of guidelines increases individual responsibility, as well as vulnerability. “Consciously, physicians will not put patients at risk if they do not have the competence to care for them or to perform a surgical procedure,” said Clystenes Odyr Soares Silva, MD, PhD, adjunct professor of pulmonology of the Federal University of São Paulo (Brazil) School of Medicine (UNIFESP). “Your peers will tell you if you are no longer able,” he added. The problem is that physicians rarely admit to or talk about their colleagues’ deficits, especially if they are in the spotlight because of advanced age. In this situation, the observation and opinion of family members regarding the health care professional’s competences and skills will hold more weight.
In case of health-related physical impairment, such as partial loss of hand movement, for example, “it is expected that this will set off an ethical warning in the person,” said Dr. Constantino. When this warning does not occur naturally, patients or colleagues can report the professional, and this may lead to the opening of an administrative investigation. If the report is found to be true, this investigation is used to suspend physicians who do not have the physical or mental ability to continue practicing medicine.
“If it’s something very serious, the physician’s license can be temporarily suspended while [the physician] is treated by a psychiatrist, with follow-up by the professional board. When discharged, the physician will get his or her [professional] license back and can go back to work,” Dr. Constantino explained. If an expert evaluation is needed, the physician will then be assessed by a forensic psychiatrist. One of the most in-demand forensic psychiatrists in Brazil is Guido Arturo Palomba, MD, 73 years old. “I have assessed some physicians for actions reported to see if they were normal people or not, but never for circumstances related to age,” Dr. Palomba said.
In practice, Brazilian medical entities do not have policies or programs to guide physicians who wish to grow old while they work or those who have started to notice they are not performing as they used to. “We have never lived as long; therefore, the quality of life in old age, as well as the concept of aging, are some of the most relevant questions of our time. These are subjects requiring additional discussion, broadening understanding and awareness in this regard,” observed Mr. Vattimo.
Dr. Constantino and Dr. Silva, who are completely against age-based assessments, believe that recertification of the specialist license every 5 years is the best path to confirming whether the physician is still able to practice. “A knowledge-based test every 5 years to recertify the specialist license has often been a topic of conversation. I think it’s an excellent idea. The person would provide a dossier of all they have done in terms of courses, conferences, and other activities, present it, and receive a score,” said Dr. Silva.
In practice, recertification of the specialist license is a topic of discussion that has been raised for years, and it is an idea that the Brazilian Medical Association (AMB) defends. In conjunction with the CFM, the association is studying a way to best implement this assessment. “It’s important to emphasize that this measure would not be retroactive at first. Instead, it would only be in effect for professionals licensed after the recertification requirement is established,” the AMB pointed out in a note sent to this news organization. Even so, the measure has faced significant resistance from a faction of the profession, and its enactment does not seem to be imminent.
The debate regarding professional longevity is taking place in various countries. In 2021, the American Medical Association Council on Medical Education released a report with a set of guidelines for the screening and assessment of physicians. The document is the product of a committee created in 2015 to study the subject. The AMA recommends that the assessment of elderly physicians be based on evidence and ethical, relevant, fair, equitable, transparent, verifiable, nonexhaustive principles, contemplating support and protecting against legal proceedings. In April of this year, a new AMA document highlighted the same principles.
Also in the United States, one of oldest initiatives created to support physicians in the process of recycling, the University of California San Diego Physician Assessment and Clinical Education Program (PACE), has a section focusing on the extended practice of medicine (Practicing Medicine Longer). For those wanting to learn more about discussions on this subject, there are online presentations on experiences in Quebec and Ontario with assessing aging physicians, neuropsychological perspectives on the aging medical population, and what to expect of healthy aging, among other subjects.
Created in 1996, PACE mostly provides services to physicians who need to address requirements of the state medical boards. Few physicians enroll on their own.
The first part of the program assesses knowledge and skills over approximately 2 days. In the second phase, the physician participates in a series of activities in a corresponding residency program. Depending on the results, the physician may have to go through a remedial program with varying activities to deal with performance deficiencies to clinical experiences at the residency level.
A version of this article first appeared on Medscape.com.
Eating earlier offers health benefits, studies say
New research suggests there may be better times during the day for eating and fasting.
Eating earlier in the day may help you lose weight, and eating meals within a 10-hour window could improve blood sugar and cholesterol levels, according to two new studies published in Cell Metabolism.
“You have this internal biological clock that makes you better at doing different things at different times of the day,” Courtney Peterson, PhD, an associate professor of nutrition sciences at the University of Alabama at Birmingham, told NBC News. Dr. Peterson wasn’t involved with the studies.
“It seems like the best time for your metabolism, in most people, is the mid to late morning,” she said.
In one study, researchers found that eating later in the day made people hungrier during a 24-hour period, as compared with eating the same meals earlier in the day. Combined, the changes may increase the risk for obesity, the study authors found.
In another study, among firefighters as shift workers, researchers found that eating meals within a 10-hour window decreased the size of bad cholesterol particles, which could reduce risk factors for heart disease. The 10-hour eating window also improved blood pressure and blood sugar levels among those with health conditions such as diabetes, high blood pressure, and high cholesterol.
The two new studies confirm findings from previous studies that indicate humans may have an ideal eating window based on the body’s circadian rhythms, which regulate sleep and wake cycles and can affect appetite, metabolism, and blood sugar levels.
In the firefighter study, for instance, the 10-hour window appears to be a “sweet spot” for the body, the authors found. More severe restrictions, as found with many intermittent fasting diets, could be difficult for the body to maintain.
“When we think about 6 or 8 hours, you might see a benefit, but people might not stick to it for a long time,” Satchidananda Panda, PhD, one of the study authors and a professor at the Salk Institute, La Jolla, Calif., told NBC News.
The new studies had small sample sizes, though they offer insight for future research. In the first study, 16 people who were overweight or obese tried two eating plans for 24-hour periods. Some of them began eating an hour after their natural wake-up time, and others waited to begin eating until about 5 hours after waking up. They ate the same meals with the same calories and nutrients.
The researchers measured their hormone levels and found that eating later decreased the levels of leptin, which helps people to feel full. Eating later also doubled the odds that people felt hungry throughout the day. Those in the study who ate later in the day also had more cravings for starchy or salty foods, as well as meat and dairy, which are energy-dense foods.
The research team also found changes in fat tissue, which could lead to a higher chance of building up new fat cells and a lower chance of burning fat. Late eaters burned about 60 fewer calories than early eaters during the day.
“Your body processes calories differently when you eat late in the day. It tips the scale in favor of weight gain and fat gain,” Dr. Peterson said. “From this study, we can get pretty clear recommendations that people shouldn’t skip breakfast.”
The second study followed 137 firefighters in San Diego who ate a Mediterranean diet with fish, vegetables, fruit, and olive oil for 12 weeks. Among those, 70 firefighters ate during a 10-hour window, and the rest ate during a longer window, generally about 13 hours. They logged their meals in an app and wore devices to track blood sugar levels.
In the 10-hour group, most firefighters ate between 8 a.m. or 9 a.m. and 6 p.m. or 7 p.m. The time-restricted eating appeared to be linked with health benefits, such as less harmful cholesterol buildup and reduced heart disease.
Among firefighters with risk factors for heart disease, such as high blood pressure and high blood sugar, the time-restricted eating decreased their blood pressure and blood sugar levels.
The restricted window appears to allow the body to break down toxins and get rid of sodium and other things that can drive up blood pressure and blood sugar, the authors wrote.
During periods of fasting, “organs get some rest from digesting food so they can divert their energy toward repairing cells,” Dr. Panda said.
A version of this article first appeared on WebMD.com.
New research suggests there may be better times during the day for eating and fasting.
Eating earlier in the day may help you lose weight, and eating meals within a 10-hour window could improve blood sugar and cholesterol levels, according to two new studies published in Cell Metabolism.
“You have this internal biological clock that makes you better at doing different things at different times of the day,” Courtney Peterson, PhD, an associate professor of nutrition sciences at the University of Alabama at Birmingham, told NBC News. Dr. Peterson wasn’t involved with the studies.
“It seems like the best time for your metabolism, in most people, is the mid to late morning,” she said.
In one study, researchers found that eating later in the day made people hungrier during a 24-hour period, as compared with eating the same meals earlier in the day. Combined, the changes may increase the risk for obesity, the study authors found.
In another study, among firefighters as shift workers, researchers found that eating meals within a 10-hour window decreased the size of bad cholesterol particles, which could reduce risk factors for heart disease. The 10-hour eating window also improved blood pressure and blood sugar levels among those with health conditions such as diabetes, high blood pressure, and high cholesterol.
The two new studies confirm findings from previous studies that indicate humans may have an ideal eating window based on the body’s circadian rhythms, which regulate sleep and wake cycles and can affect appetite, metabolism, and blood sugar levels.
In the firefighter study, for instance, the 10-hour window appears to be a “sweet spot” for the body, the authors found. More severe restrictions, as found with many intermittent fasting diets, could be difficult for the body to maintain.
“When we think about 6 or 8 hours, you might see a benefit, but people might not stick to it for a long time,” Satchidananda Panda, PhD, one of the study authors and a professor at the Salk Institute, La Jolla, Calif., told NBC News.
The new studies had small sample sizes, though they offer insight for future research. In the first study, 16 people who were overweight or obese tried two eating plans for 24-hour periods. Some of them began eating an hour after their natural wake-up time, and others waited to begin eating until about 5 hours after waking up. They ate the same meals with the same calories and nutrients.
The researchers measured their hormone levels and found that eating later decreased the levels of leptin, which helps people to feel full. Eating later also doubled the odds that people felt hungry throughout the day. Those in the study who ate later in the day also had more cravings for starchy or salty foods, as well as meat and dairy, which are energy-dense foods.
The research team also found changes in fat tissue, which could lead to a higher chance of building up new fat cells and a lower chance of burning fat. Late eaters burned about 60 fewer calories than early eaters during the day.
“Your body processes calories differently when you eat late in the day. It tips the scale in favor of weight gain and fat gain,” Dr. Peterson said. “From this study, we can get pretty clear recommendations that people shouldn’t skip breakfast.”
The second study followed 137 firefighters in San Diego who ate a Mediterranean diet with fish, vegetables, fruit, and olive oil for 12 weeks. Among those, 70 firefighters ate during a 10-hour window, and the rest ate during a longer window, generally about 13 hours. They logged their meals in an app and wore devices to track blood sugar levels.
In the 10-hour group, most firefighters ate between 8 a.m. or 9 a.m. and 6 p.m. or 7 p.m. The time-restricted eating appeared to be linked with health benefits, such as less harmful cholesterol buildup and reduced heart disease.
Among firefighters with risk factors for heart disease, such as high blood pressure and high blood sugar, the time-restricted eating decreased their blood pressure and blood sugar levels.
The restricted window appears to allow the body to break down toxins and get rid of sodium and other things that can drive up blood pressure and blood sugar, the authors wrote.
During periods of fasting, “organs get some rest from digesting food so they can divert their energy toward repairing cells,” Dr. Panda said.
A version of this article first appeared on WebMD.com.
New research suggests there may be better times during the day for eating and fasting.
Eating earlier in the day may help you lose weight, and eating meals within a 10-hour window could improve blood sugar and cholesterol levels, according to two new studies published in Cell Metabolism.
“You have this internal biological clock that makes you better at doing different things at different times of the day,” Courtney Peterson, PhD, an associate professor of nutrition sciences at the University of Alabama at Birmingham, told NBC News. Dr. Peterson wasn’t involved with the studies.
“It seems like the best time for your metabolism, in most people, is the mid to late morning,” she said.
In one study, researchers found that eating later in the day made people hungrier during a 24-hour period, as compared with eating the same meals earlier in the day. Combined, the changes may increase the risk for obesity, the study authors found.
In another study, among firefighters as shift workers, researchers found that eating meals within a 10-hour window decreased the size of bad cholesterol particles, which could reduce risk factors for heart disease. The 10-hour eating window also improved blood pressure and blood sugar levels among those with health conditions such as diabetes, high blood pressure, and high cholesterol.
The two new studies confirm findings from previous studies that indicate humans may have an ideal eating window based on the body’s circadian rhythms, which regulate sleep and wake cycles and can affect appetite, metabolism, and blood sugar levels.
In the firefighter study, for instance, the 10-hour window appears to be a “sweet spot” for the body, the authors found. More severe restrictions, as found with many intermittent fasting diets, could be difficult for the body to maintain.
“When we think about 6 or 8 hours, you might see a benefit, but people might not stick to it for a long time,” Satchidananda Panda, PhD, one of the study authors and a professor at the Salk Institute, La Jolla, Calif., told NBC News.
The new studies had small sample sizes, though they offer insight for future research. In the first study, 16 people who were overweight or obese tried two eating plans for 24-hour periods. Some of them began eating an hour after their natural wake-up time, and others waited to begin eating until about 5 hours after waking up. They ate the same meals with the same calories and nutrients.
The researchers measured their hormone levels and found that eating later decreased the levels of leptin, which helps people to feel full. Eating later also doubled the odds that people felt hungry throughout the day. Those in the study who ate later in the day also had more cravings for starchy or salty foods, as well as meat and dairy, which are energy-dense foods.
The research team also found changes in fat tissue, which could lead to a higher chance of building up new fat cells and a lower chance of burning fat. Late eaters burned about 60 fewer calories than early eaters during the day.
“Your body processes calories differently when you eat late in the day. It tips the scale in favor of weight gain and fat gain,” Dr. Peterson said. “From this study, we can get pretty clear recommendations that people shouldn’t skip breakfast.”
The second study followed 137 firefighters in San Diego who ate a Mediterranean diet with fish, vegetables, fruit, and olive oil for 12 weeks. Among those, 70 firefighters ate during a 10-hour window, and the rest ate during a longer window, generally about 13 hours. They logged their meals in an app and wore devices to track blood sugar levels.
In the 10-hour group, most firefighters ate between 8 a.m. or 9 a.m. and 6 p.m. or 7 p.m. The time-restricted eating appeared to be linked with health benefits, such as less harmful cholesterol buildup and reduced heart disease.
Among firefighters with risk factors for heart disease, such as high blood pressure and high blood sugar, the time-restricted eating decreased their blood pressure and blood sugar levels.
The restricted window appears to allow the body to break down toxins and get rid of sodium and other things that can drive up blood pressure and blood sugar, the authors wrote.
During periods of fasting, “organs get some rest from digesting food so they can divert their energy toward repairing cells,” Dr. Panda said.
A version of this article first appeared on WebMD.com.
FROM CELL METABOLISM