User login
Ensifentrine for COPD: Out of reach for many?
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at an unreasonably high annual cost, authors of a cost and effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second within 0-12 hours of administration, compared with placebo. In addition, patients were reported to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High cost barrier
But as authors of the analysis from the Boston, Massachusetts–based Institute for Clinical and Economic Review (ICER) found, the therapeutic edge offered by ensifentrine is outweighed by the annual wholesale acquisition cost that its maker, Vernona Pharma, has established: $35,400, which far exceeds the estimated health-benefit price of $7,500-$12,700, according to ICER. ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides.”
As previously reported, as many as one in six persons with COPD in the United States miss or delay COPD medication doses because of high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher-volume sales, affording the drugmaker a comparable profit with the higher-cost but lower-volume option.
Good drug, high price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added healthcare costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug not yet available?
However, providers have not yet had direct experience with the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of Advance Practice Provider and Clinical Services for Colorado Springs Pulmonary Consultants, president and founder of the Association of Pulmonary Advance Practice Providers, and a member of the CHEST Physician Editorial Board.
She learned “they were going to release it to select specialty pharmacies in the 3rd quarter of 2024. But all the ones we call do not have it and no one knows who does. They haven’t sent any reps into the field in my area so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no relevant disclosures.
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at an unreasonably high annual cost, authors of a cost and effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second within 0-12 hours of administration, compared with placebo. In addition, patients were reported to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High cost barrier
But as authors of the analysis from the Boston, Massachusetts–based Institute for Clinical and Economic Review (ICER) found, the therapeutic edge offered by ensifentrine is outweighed by the annual wholesale acquisition cost that its maker, Vernona Pharma, has established: $35,400, which far exceeds the estimated health-benefit price of $7,500-$12,700, according to ICER. ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides.”
As previously reported, as many as one in six persons with COPD in the United States miss or delay COPD medication doses because of high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher-volume sales, affording the drugmaker a comparable profit with the higher-cost but lower-volume option.
Good drug, high price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added healthcare costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug not yet available?
However, providers have not yet had direct experience with the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of Advance Practice Provider and Clinical Services for Colorado Springs Pulmonary Consultants, president and founder of the Association of Pulmonary Advance Practice Providers, and a member of the CHEST Physician Editorial Board.
She learned “they were going to release it to select specialty pharmacies in the 3rd quarter of 2024. But all the ones we call do not have it and no one knows who does. They haven’t sent any reps into the field in my area so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no relevant disclosures.
Ensifentrine (Ohtuvayre), a novel medication for the treatment of chronic obstructive pulmonary disease (COPD) recently approved by the Food and Drug Administration, has been shown to reduce COPD exacerbations and may improve the quality of life for patients, but these potential benefits come at an unreasonably high annual cost, authors of a cost and effectiveness analysis say.
Ensifentrine is a first-in-class selective dual inhibitor of both phosphodiesterase 3 (PDE-3) and PDE-4, combining both bronchodilator and nonsteroidal anti-inflammatory effects in a single molecule. The drug is delivered through a standard jet nebulizer.
In the phase 3 ENHANCE 1 and 2 trials, ensifentrine significantly improved lung function based on the primary outcome of average forced expiratory volume in 1 second within 0-12 hours of administration, compared with placebo. In addition, patients were reported to tolerate the inhaled treatment well, with similar proportions of ensifentrine- and placebo-assigned patients reporting treatment-emergent adverse events. The most common treatment-emergent adverse events were nasopharyngitis, hypertension, and back pain, reported in < 3% of the ensifentrine group.
High cost barrier
But as authors of the analysis from the Boston, Massachusetts–based Institute for Clinical and Economic Review (ICER) found, the therapeutic edge offered by ensifentrine is outweighed by the annual wholesale acquisition cost that its maker, Vernona Pharma, has established: $35,400, which far exceeds the estimated health-benefit price of $7,500-$12,700, according to ICER. ICER is an independent, nonprofit research institute that conducts evidence-based reviews of healthcare interventions, including prescription drugs, other treatments, and diagnostic tests.
“Current evidence shows that ensifentrine decreases COPD exacerbations when used in combination with some current inhaled therapies, but there are uncertainties about how much benefit it may add to unstudied combinations of inhaled treatments,” said David Rind, MD, chief medical officer of ICER in a statement.
In an interview, Dr. Rind noted that the high price of ensifentrine may lead payers to restrict access to an otherwise promising new therapy. “Obviously many drugs in the US are overpriced, and this one, too, looks like it is overpriced. That causes ongoing financial toxicity for individual patients and it causes problems for the entire US health system, because when we pay too much for drugs we don’t have money for other things. So I’m worried about the fact that this price is too high compared to the benefit it provides.”
As previously reported, as many as one in six persons with COPD in the United States miss or delay COPD medication doses because of high drug costs. “I think that the pricing they chose is going to cause lots of barriers to people getting access and that insurance companies will throw up barriers. Primary care physicians like me won’t even try to get approval for a drug like this given the hoops we will be made to jump through, and so fewer people will get this drug,” Dr. Rind said. He pointed out that a lower wholesale acquisition cost could encourage higher-volume sales, affording the drugmaker a comparable profit with the higher-cost but lower-volume option.
Good drug, high price
An independent appraisal committee for ICER determined that “current evidence is adequate to demonstrate a net health benefit for ensifentrine added to maintenance therapy when compared to maintenance therapy alone.”
But ICER also issued an access and affordability alert “to signal to stakeholders and policymakers that the amount of added healthcare costs associated with a new service may be difficult for the health system to absorb over the short term without displacing other needed services.” ICER recommends that payers should include coverage for smoking cessation therapies, and that drug manufacturers “set prices that will foster affordability and good access for all patients by aligning prices with the patient-centered therapeutic value of their treatments.”
“This looks like a pretty good drug,” Dr. Rind said. “It looks quite safe and I think there will be a lot of patients, particularly those who are having frequent exacerbations, who this would be appropriate for, particularly once they’ve maxed out existing therapies, but maybe even earlier than that. And if the price comes down to the point that patients can really access this and providers can access it, people really should look at this as a potential therapy.”
Drug not yet available?
However, providers have not yet had direct experience with the new medication. “We haven’t been able to prescribe it yet,” said Corinne Young, MSN, FNP-C, FCCP, director of Advance Practice Provider and Clinical Services for Colorado Springs Pulmonary Consultants, president and founder of the Association of Pulmonary Advance Practice Providers, and a member of the CHEST Physician Editorial Board.
She learned “they were going to release it to select specialty pharmacies in the 3rd quarter of 2024. But all the ones we call do not have it and no one knows who does. They haven’t sent any reps into the field in my area so we don’t have any points of contact either,” she said.
Verona Pharma stated it anticipates ensifentrine to be available in the third quarter of 2024 “through an exclusive network of accredited specialty pharmacies.”
Funding for the ICER report came from nonprofit foundations. No funding came from health insurers, pharmacy benefit managers, or life science companies. Dr. Rind had no relevant disclosures.
The language of AI and its applications in health care
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.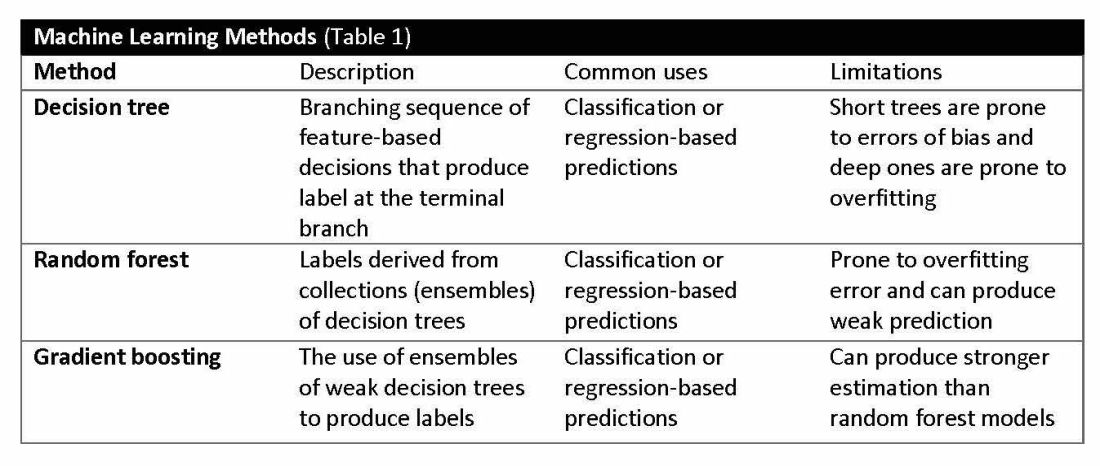
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
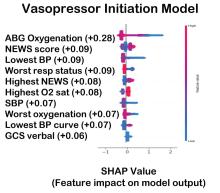
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
CHEST releases new guideline on management of central airway obstruction
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.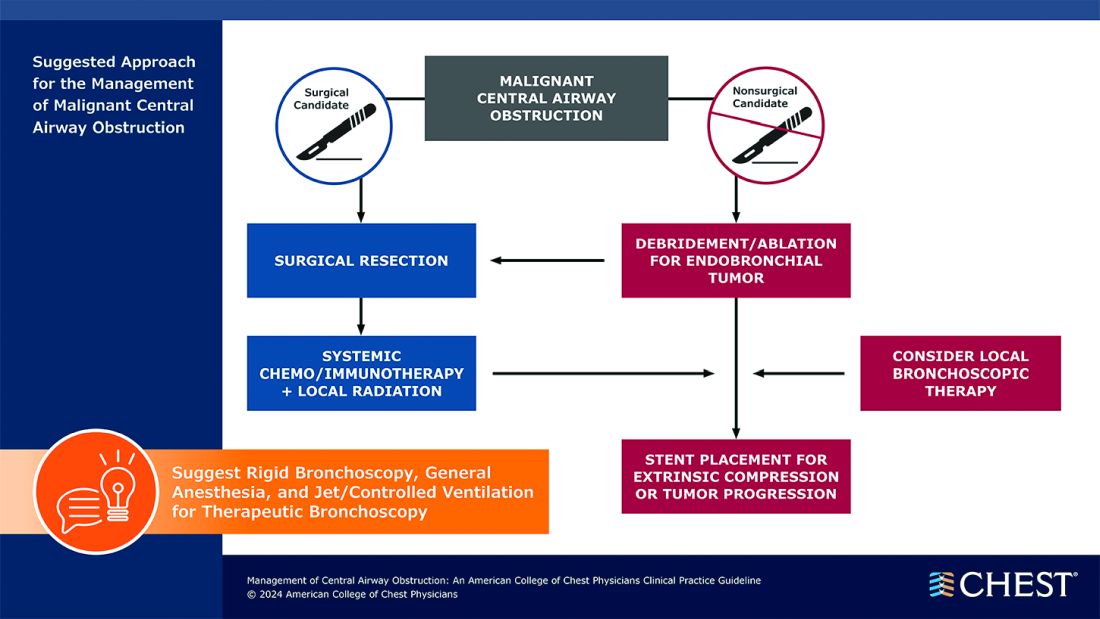
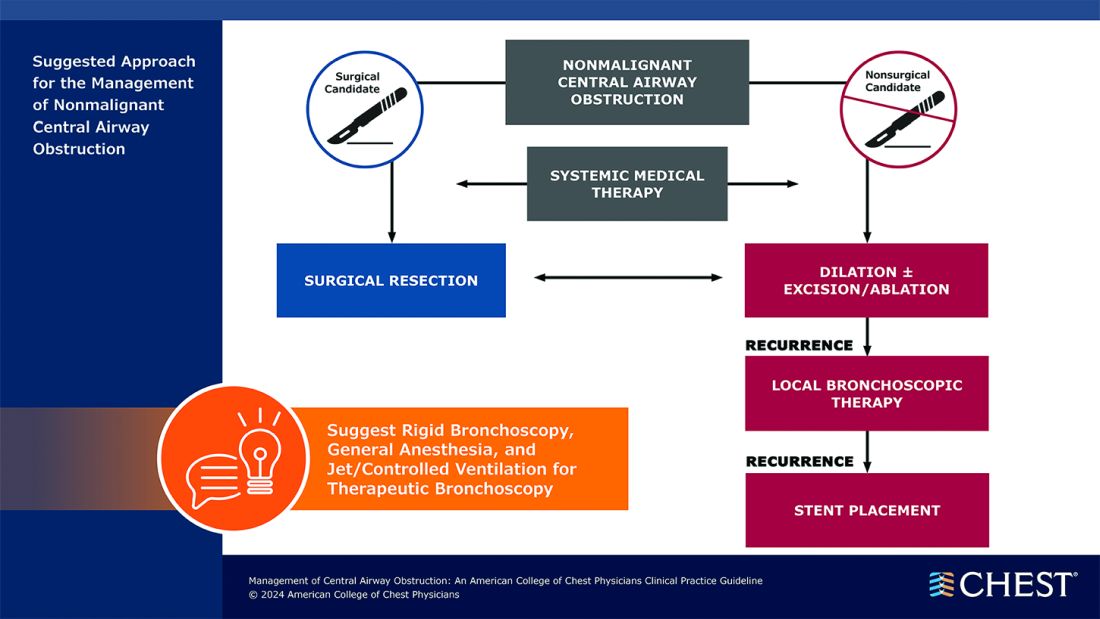
Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.

Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.

Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
Are Beta-Blockers Needed Post MI? No, Even After the ABYSS Trial
The ABYSS trial found that interruption of beta-blocker therapy in patients after myocardial infarction (MI) was not noninferior to continuing the drugs.
I will argue why I think it is okay to stop beta-blockers after MI — despite this conclusion. The results of ABYSS are, in fact, similar to REDUCE-AMI, which compared beta-blocker use or nonuse immediately after MI, and found no difference in a composite endpoint of death or MI.
The ABYSS Trial
ABYSS investigators randomly assigned nearly 3700 patients who had MI and were prescribed a beta-blocker to either continue (control arm) or stop (active arm) the drug at 1 year.
Patients had to have a left ventricular ejection fraction (LVEF) at least 40%; the median was 60%.
The composite primary endpoint included death, MI, stroke, or hospitalization for any cardiovascular reason. ABYSS authors chose a noninferiority design. The assumption must have been that the interruption arm offered an easier option for patients — eg, fewer pills.
Over 3 years, a primary endpoint occurred in 23.8% of the interruption group vs 21.1% in the continuation group.
In ABYSS, the noninferiority margin was set at a 3% absolute risk increase. The 2.7% absolute risk increase had an upper bound of the 95% CI (worst case) of 5.5% leading to the not-noninferior conclusion (5.5% exceeds the noninferiority margins).
More simply stated, the primary outcome event rate was higher in the interruption arm.
Does This Mean we Should Continue Beta-Blockers in Post-MI Patients?
This led some to conclude that we should continue beta-blockers. I disagree. To properly interpret the ABYSS trial, you must consider trial procedures, components of the primary endpoint, and then compare ABYSS with REDUCE-AMI.
It’s also reasonable to have extremely pessimistic prior beliefs about post-MI beta-blockade because the evidence establishing benefit comes from trials conducted before urgent revascularization became the standard therapy.
ABYSS was a pragmatic open-label trial. The core problem with this design is that one of the components of the primary outcome (hospitalization for cardiovascular reasons) requires clinical judgment — and is therefore susceptible to bias, particularly in an open-label trial.
This becomes apparent when we look at the components of the primary outcome in the two arms of the trial (interrupt vs continue):
- For death, the rates were 4.1 and 4.0%
- For MI, the rates were 2.5 and 2.4%
- For stroke, the rates were 1.0% in both arms
- For CV hospitalization, the rates were 18.9% vs 16.6%
The higher rate CV hospitalization alone drove the results of ABYSS. Death, MI, and stroke rates were nearly identical.
The most common reason for admission to the hospital in this category was for angiography. In fact, the rate of angiography was 2.3% higher in the interruption arm — identical to the rate increase in the CV hospitalization component of the primary endpoint.
The results of ABYSS, therefore, were driven by higher rates of angiography in the interrupt arm.
You need not imply malfeasance to speculate that patients who had their beta-blocker stopped might be treated differently regarding hospital admissions or angiography than those who stayed on beta-blockers. Researchers from Imperial College London called such a bias in unblinded trials “subtraction anxiety and faith healing.”
Had the ABYSS investigators chosen the simpler, less bias-prone endpoints of death, MI, or stroke, their results would have been the same as REDUCE-AMI.
My Final Two Conclusions
I would conclude that interruption of beta-blockers at 1 year vs continuation in post-MI patients did not lead to an increase in death, MI, or stroke.
ABYSS, therefore, is consistent with REDUCE-AMI. Taken together, along with the pessimistic priors, these are important findings because they allow us to stop a medicine and reduce the work of being a patient.
My second conclusion concerns ways of knowing in medicine. I’ve long felt that randomized controlled trials (RCTs) are the best way to sort out causation. This idea led me to the believe that medicine should have more RCTs rather than follow expert opinion or therapeutic fashion.
I’ve now modified my love of RCTs — a little. The ABYSS trial is yet another example of the need to be super careful with their design.
Something as seemingly simple as choosing what to measure can alter the way clinicians interpret and use the data.
So, let’s have (slightly) more trials, but we should be really careful in their design. Slow and careful is the best way to practice medicine. And it’s surely the best way to do research as well.
Dr. Mandrola, clinical electrophysiologist, Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The ABYSS trial found that interruption of beta-blocker therapy in patients after myocardial infarction (MI) was not noninferior to continuing the drugs.
I will argue why I think it is okay to stop beta-blockers after MI — despite this conclusion. The results of ABYSS are, in fact, similar to REDUCE-AMI, which compared beta-blocker use or nonuse immediately after MI, and found no difference in a composite endpoint of death or MI.
The ABYSS Trial
ABYSS investigators randomly assigned nearly 3700 patients who had MI and were prescribed a beta-blocker to either continue (control arm) or stop (active arm) the drug at 1 year.
Patients had to have a left ventricular ejection fraction (LVEF) at least 40%; the median was 60%.
The composite primary endpoint included death, MI, stroke, or hospitalization for any cardiovascular reason. ABYSS authors chose a noninferiority design. The assumption must have been that the interruption arm offered an easier option for patients — eg, fewer pills.
Over 3 years, a primary endpoint occurred in 23.8% of the interruption group vs 21.1% in the continuation group.
In ABYSS, the noninferiority margin was set at a 3% absolute risk increase. The 2.7% absolute risk increase had an upper bound of the 95% CI (worst case) of 5.5% leading to the not-noninferior conclusion (5.5% exceeds the noninferiority margins).
More simply stated, the primary outcome event rate was higher in the interruption arm.
Does This Mean we Should Continue Beta-Blockers in Post-MI Patients?
This led some to conclude that we should continue beta-blockers. I disagree. To properly interpret the ABYSS trial, you must consider trial procedures, components of the primary endpoint, and then compare ABYSS with REDUCE-AMI.
It’s also reasonable to have extremely pessimistic prior beliefs about post-MI beta-blockade because the evidence establishing benefit comes from trials conducted before urgent revascularization became the standard therapy.
ABYSS was a pragmatic open-label trial. The core problem with this design is that one of the components of the primary outcome (hospitalization for cardiovascular reasons) requires clinical judgment — and is therefore susceptible to bias, particularly in an open-label trial.
This becomes apparent when we look at the components of the primary outcome in the two arms of the trial (interrupt vs continue):
- For death, the rates were 4.1 and 4.0%
- For MI, the rates were 2.5 and 2.4%
- For stroke, the rates were 1.0% in both arms
- For CV hospitalization, the rates were 18.9% vs 16.6%
The higher rate CV hospitalization alone drove the results of ABYSS. Death, MI, and stroke rates were nearly identical.
The most common reason for admission to the hospital in this category was for angiography. In fact, the rate of angiography was 2.3% higher in the interruption arm — identical to the rate increase in the CV hospitalization component of the primary endpoint.
The results of ABYSS, therefore, were driven by higher rates of angiography in the interrupt arm.
You need not imply malfeasance to speculate that patients who had their beta-blocker stopped might be treated differently regarding hospital admissions or angiography than those who stayed on beta-blockers. Researchers from Imperial College London called such a bias in unblinded trials “subtraction anxiety and faith healing.”
Had the ABYSS investigators chosen the simpler, less bias-prone endpoints of death, MI, or stroke, their results would have been the same as REDUCE-AMI.
My Final Two Conclusions
I would conclude that interruption of beta-blockers at 1 year vs continuation in post-MI patients did not lead to an increase in death, MI, or stroke.
ABYSS, therefore, is consistent with REDUCE-AMI. Taken together, along with the pessimistic priors, these are important findings because they allow us to stop a medicine and reduce the work of being a patient.
My second conclusion concerns ways of knowing in medicine. I’ve long felt that randomized controlled trials (RCTs) are the best way to sort out causation. This idea led me to the believe that medicine should have more RCTs rather than follow expert opinion or therapeutic fashion.
I’ve now modified my love of RCTs — a little. The ABYSS trial is yet another example of the need to be super careful with their design.
Something as seemingly simple as choosing what to measure can alter the way clinicians interpret and use the data.
So, let’s have (slightly) more trials, but we should be really careful in their design. Slow and careful is the best way to practice medicine. And it’s surely the best way to do research as well.
Dr. Mandrola, clinical electrophysiologist, Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The ABYSS trial found that interruption of beta-blocker therapy in patients after myocardial infarction (MI) was not noninferior to continuing the drugs.
I will argue why I think it is okay to stop beta-blockers after MI — despite this conclusion. The results of ABYSS are, in fact, similar to REDUCE-AMI, which compared beta-blocker use or nonuse immediately after MI, and found no difference in a composite endpoint of death or MI.
The ABYSS Trial
ABYSS investigators randomly assigned nearly 3700 patients who had MI and were prescribed a beta-blocker to either continue (control arm) or stop (active arm) the drug at 1 year.
Patients had to have a left ventricular ejection fraction (LVEF) at least 40%; the median was 60%.
The composite primary endpoint included death, MI, stroke, or hospitalization for any cardiovascular reason. ABYSS authors chose a noninferiority design. The assumption must have been that the interruption arm offered an easier option for patients — eg, fewer pills.
Over 3 years, a primary endpoint occurred in 23.8% of the interruption group vs 21.1% in the continuation group.
In ABYSS, the noninferiority margin was set at a 3% absolute risk increase. The 2.7% absolute risk increase had an upper bound of the 95% CI (worst case) of 5.5% leading to the not-noninferior conclusion (5.5% exceeds the noninferiority margins).
More simply stated, the primary outcome event rate was higher in the interruption arm.
Does This Mean we Should Continue Beta-Blockers in Post-MI Patients?
This led some to conclude that we should continue beta-blockers. I disagree. To properly interpret the ABYSS trial, you must consider trial procedures, components of the primary endpoint, and then compare ABYSS with REDUCE-AMI.
It’s also reasonable to have extremely pessimistic prior beliefs about post-MI beta-blockade because the evidence establishing benefit comes from trials conducted before urgent revascularization became the standard therapy.
ABYSS was a pragmatic open-label trial. The core problem with this design is that one of the components of the primary outcome (hospitalization for cardiovascular reasons) requires clinical judgment — and is therefore susceptible to bias, particularly in an open-label trial.
This becomes apparent when we look at the components of the primary outcome in the two arms of the trial (interrupt vs continue):
- For death, the rates were 4.1 and 4.0%
- For MI, the rates were 2.5 and 2.4%
- For stroke, the rates were 1.0% in both arms
- For CV hospitalization, the rates were 18.9% vs 16.6%
The higher rate CV hospitalization alone drove the results of ABYSS. Death, MI, and stroke rates were nearly identical.
The most common reason for admission to the hospital in this category was for angiography. In fact, the rate of angiography was 2.3% higher in the interruption arm — identical to the rate increase in the CV hospitalization component of the primary endpoint.
The results of ABYSS, therefore, were driven by higher rates of angiography in the interrupt arm.
You need not imply malfeasance to speculate that patients who had their beta-blocker stopped might be treated differently regarding hospital admissions or angiography than those who stayed on beta-blockers. Researchers from Imperial College London called such a bias in unblinded trials “subtraction anxiety and faith healing.”
Had the ABYSS investigators chosen the simpler, less bias-prone endpoints of death, MI, or stroke, their results would have been the same as REDUCE-AMI.
My Final Two Conclusions
I would conclude that interruption of beta-blockers at 1 year vs continuation in post-MI patients did not lead to an increase in death, MI, or stroke.
ABYSS, therefore, is consistent with REDUCE-AMI. Taken together, along with the pessimistic priors, these are important findings because they allow us to stop a medicine and reduce the work of being a patient.
My second conclusion concerns ways of knowing in medicine. I’ve long felt that randomized controlled trials (RCTs) are the best way to sort out causation. This idea led me to the believe that medicine should have more RCTs rather than follow expert opinion or therapeutic fashion.
I’ve now modified my love of RCTs — a little. The ABYSS trial is yet another example of the need to be super careful with their design.
Something as seemingly simple as choosing what to measure can alter the way clinicians interpret and use the data.
So, let’s have (slightly) more trials, but we should be really careful in their design. Slow and careful is the best way to practice medicine. And it’s surely the best way to do research as well.
Dr. Mandrola, clinical electrophysiologist, Baptist Medical Associates, Louisville, Kentucky, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
First-Time Fathers Experience Period of High Psychological Risk
Anxiety and stress during fatherhood receive less research attention than do anxiety and stress during motherhood.
Longitudinal data tracking the evolution of men’s mental health following the birth of the first child are even rarer, especially in the French population. Only two studies of the subject have been conducted. They were dedicated solely to paternal depression and limited to the first 4 months post partum. Better understanding of the risk in the population can not only help identify public health issues, but also aid in defining targeted preventive approaches.
French researchers in epidemiology and public health sought to expand our knowledge of the mental health trajectories of new fathers using 9 years of data from the CONSTANCES cohort. Within this cohort, participants filled out self-administered questionnaires annually. They declared their parental status and the presence of mental illnesses. They also completed questionnaires to assess mental health, such as the Center for Epidemiologic Studies Depression Scale for depression and the General Health Questionnaire for depressive, anxious, and somatic disorders. Thresholds for each score were established to characterize the severity of symptoms. In addition, the researchers analyzed all factors (eg, sociodemographic, psychosocial, lifestyle, professional, family, or cultural) that potentially are associated with poor mental health and were available within the questionnaires.
The study included 6299 men who had their first child and for whom at least one mental health measure was collected during the follow-up period. These men had an average age of 38 years at inclusion, 88% lived with a partner, and 85% were employed. Overall, 7.9% of this male cohort self-reported a mental illness during the study, with 5.6% of illnesses occurring before the child’s birth and 9.7% after. Anxiety affected 6.5% of the cohort, and it was more pronounced after the birth than before (7.8% after vs 4.9% before).
The rate of clinically significant symptoms averaged 23.2% during the study period, increasing from 18.3% to 25.2% after the birth. The discrepancy between the self-declared diagnosis by new fathers and the symptom-related score highlights underreporting or insufficient awareness among men.
After conducting a latent class analysis, the researchers identified three homogeneous subgroups of men who had comparable mental health trajectories over time. The first group (90.3% of the cohort) maintained a constant and low risk for mental illnesses. The second (4.1%) presented a high and generally constant risk over time. Finally, 5.6% of the cohort had a temporarily high risk in the 2-4 years surrounding the birth.
The risk factors associated with being at a transiently high risk for mental illness were, in order of descending significance, not having a job, having had at least one negative experience during childhood, forgoing healthcare for financial reasons, and being aged 35-39 years (adjusted odds ratio [AOR] between 3.01 and 1.61). The risk factors associated with a high and constant mental illness risk were, in order of descending significance, being aged 60 years or older, not having a job, not living with a partner, being aged 40-44 years, and having other children in the following years (AOR between 3.79 and 1.85).
The authors noted that the risk factors for mental health challenges associated with fatherhood do not imply causality, the meaning of which would also need further study. They contended that French fathers, who on average are entitled to 2 weeks of paid paternity leave, may struggle to manage their time, professional responsibilities, and parenting duties. Consequently, they may experience dissatisfaction and difficulty seeking support, assistance, or a mental health diagnosis, especially in the face of a mental health risk to which they are less attuned than women.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Anxiety and stress during fatherhood receive less research attention than do anxiety and stress during motherhood.
Longitudinal data tracking the evolution of men’s mental health following the birth of the first child are even rarer, especially in the French population. Only two studies of the subject have been conducted. They were dedicated solely to paternal depression and limited to the first 4 months post partum. Better understanding of the risk in the population can not only help identify public health issues, but also aid in defining targeted preventive approaches.
French researchers in epidemiology and public health sought to expand our knowledge of the mental health trajectories of new fathers using 9 years of data from the CONSTANCES cohort. Within this cohort, participants filled out self-administered questionnaires annually. They declared their parental status and the presence of mental illnesses. They also completed questionnaires to assess mental health, such as the Center for Epidemiologic Studies Depression Scale for depression and the General Health Questionnaire for depressive, anxious, and somatic disorders. Thresholds for each score were established to characterize the severity of symptoms. In addition, the researchers analyzed all factors (eg, sociodemographic, psychosocial, lifestyle, professional, family, or cultural) that potentially are associated with poor mental health and were available within the questionnaires.
The study included 6299 men who had their first child and for whom at least one mental health measure was collected during the follow-up period. These men had an average age of 38 years at inclusion, 88% lived with a partner, and 85% were employed. Overall, 7.9% of this male cohort self-reported a mental illness during the study, with 5.6% of illnesses occurring before the child’s birth and 9.7% after. Anxiety affected 6.5% of the cohort, and it was more pronounced after the birth than before (7.8% after vs 4.9% before).
The rate of clinically significant symptoms averaged 23.2% during the study period, increasing from 18.3% to 25.2% after the birth. The discrepancy between the self-declared diagnosis by new fathers and the symptom-related score highlights underreporting or insufficient awareness among men.
After conducting a latent class analysis, the researchers identified three homogeneous subgroups of men who had comparable mental health trajectories over time. The first group (90.3% of the cohort) maintained a constant and low risk for mental illnesses. The second (4.1%) presented a high and generally constant risk over time. Finally, 5.6% of the cohort had a temporarily high risk in the 2-4 years surrounding the birth.
The risk factors associated with being at a transiently high risk for mental illness were, in order of descending significance, not having a job, having had at least one negative experience during childhood, forgoing healthcare for financial reasons, and being aged 35-39 years (adjusted odds ratio [AOR] between 3.01 and 1.61). The risk factors associated with a high and constant mental illness risk were, in order of descending significance, being aged 60 years or older, not having a job, not living with a partner, being aged 40-44 years, and having other children in the following years (AOR between 3.79 and 1.85).
The authors noted that the risk factors for mental health challenges associated with fatherhood do not imply causality, the meaning of which would also need further study. They contended that French fathers, who on average are entitled to 2 weeks of paid paternity leave, may struggle to manage their time, professional responsibilities, and parenting duties. Consequently, they may experience dissatisfaction and difficulty seeking support, assistance, or a mental health diagnosis, especially in the face of a mental health risk to which they are less attuned than women.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Anxiety and stress during fatherhood receive less research attention than do anxiety and stress during motherhood.
Longitudinal data tracking the evolution of men’s mental health following the birth of the first child are even rarer, especially in the French population. Only two studies of the subject have been conducted. They were dedicated solely to paternal depression and limited to the first 4 months post partum. Better understanding of the risk in the population can not only help identify public health issues, but also aid in defining targeted preventive approaches.
French researchers in epidemiology and public health sought to expand our knowledge of the mental health trajectories of new fathers using 9 years of data from the CONSTANCES cohort. Within this cohort, participants filled out self-administered questionnaires annually. They declared their parental status and the presence of mental illnesses. They also completed questionnaires to assess mental health, such as the Center for Epidemiologic Studies Depression Scale for depression and the General Health Questionnaire for depressive, anxious, and somatic disorders. Thresholds for each score were established to characterize the severity of symptoms. In addition, the researchers analyzed all factors (eg, sociodemographic, psychosocial, lifestyle, professional, family, or cultural) that potentially are associated with poor mental health and were available within the questionnaires.
The study included 6299 men who had their first child and for whom at least one mental health measure was collected during the follow-up period. These men had an average age of 38 years at inclusion, 88% lived with a partner, and 85% were employed. Overall, 7.9% of this male cohort self-reported a mental illness during the study, with 5.6% of illnesses occurring before the child’s birth and 9.7% after. Anxiety affected 6.5% of the cohort, and it was more pronounced after the birth than before (7.8% after vs 4.9% before).
The rate of clinically significant symptoms averaged 23.2% during the study period, increasing from 18.3% to 25.2% after the birth. The discrepancy between the self-declared diagnosis by new fathers and the symptom-related score highlights underreporting or insufficient awareness among men.
After conducting a latent class analysis, the researchers identified three homogeneous subgroups of men who had comparable mental health trajectories over time. The first group (90.3% of the cohort) maintained a constant and low risk for mental illnesses. The second (4.1%) presented a high and generally constant risk over time. Finally, 5.6% of the cohort had a temporarily high risk in the 2-4 years surrounding the birth.
The risk factors associated with being at a transiently high risk for mental illness were, in order of descending significance, not having a job, having had at least one negative experience during childhood, forgoing healthcare for financial reasons, and being aged 35-39 years (adjusted odds ratio [AOR] between 3.01 and 1.61). The risk factors associated with a high and constant mental illness risk were, in order of descending significance, being aged 60 years or older, not having a job, not living with a partner, being aged 40-44 years, and having other children in the following years (AOR between 3.79 and 1.85).
The authors noted that the risk factors for mental health challenges associated with fatherhood do not imply causality, the meaning of which would also need further study. They contended that French fathers, who on average are entitled to 2 weeks of paid paternity leave, may struggle to manage their time, professional responsibilities, and parenting duties. Consequently, they may experience dissatisfaction and difficulty seeking support, assistance, or a mental health diagnosis, especially in the face of a mental health risk to which they are less attuned than women.
This story was translated from Univadis France, which is part of the Medscape Professional Network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Setbacks Identified After Stopping Beta-Blockers
LONDON — It may not be advisable for patients with a history of myocardial infarction and preserved left ventricular function to discontinue long-term beta-blocker therapy, warn investigators.
In the randomized ABYSS trial, although there was no difference in death, MI, or stroke between patients who discontinued and those who continued taking beta-blockers, those who stopped taking the drugs had a higher rate of cardiovascular hospitalization.
Discontinuation was also associated with an increase in blood pressure and heart rate, without any improvement in quality of life.
The results, which were simultaneously published online in The New England Journal of Medicine, call into question current guidelines, which suggest that beta-blockers may be discontinued after 1 year in certain patient groups.
Beta-blockers have long been considered the standard of care for patients after MI, but trials showing the benefit of these drugs were conducted before the modern era of myocardial reperfusion and pharmacotherapy, which have led to sharp decreases in the risk for heart failure and for death after MI, Dr. Silvain explained.
This has led to questions about the add-on benefits of lifelong beta-blocker treatment for patients with MI and a preserved left ventricular ejection fraction and no other primary indication for beta-blocker therapy.
The ABYSS Trial
To explore this issue, the open-label, non-inferiority ABYSS trial randomly assigned 3698 patients with a history of MI to the discontinuation or continuation of beta-blocker treatment. All study participants had a left ventricular ejection fraction of at least 40%, were receiving long-term beta-blocker treatment, and had experienced no cardiovascular event in the previous 6 months.
At a median follow-up of 3 years, the primary endpoint — a composite of death, MI, stroke, and hospitalization for cardiovascular reasons — occurred more often in the discontinuation group than in the continuation group (23.8% vs 21.1%; hazard ratio, 1.16; 95% CI, 1.01-1.33). This did not meet the criteria for non-inferiority of discontinuation, compared with continuation, of beta-blocker therapy (P for non-inferiority = .44).
The difference in event rates between the two groups was driven by cardiovascular hospitalizations, which occurred more often in the discontinuation group than in the continuation group (18.9% vs 16.6%).
Other key results showed that there was no difference in quality of life between the two groups.
However, 6 months after randomization, there were increases in blood pressure and heart rate in the discontinuation group. Systolic blood pressure increased by 3.7 mm Hg and diastolic blood pressure increased by 3.9 mm Hg. Resting heart rate increased by 9.8 beats per minute.
“We were not able to show the non-inferiority of stopping beta-blockers in terms of cardiovascular events, [but we] showed a safety signal with this strategy of an increase in blood pressure and heart rate, with no improvement in quality of life,” Dr. Sylvain said.
“While recent guidelines suggest it may be reasonable to stop beta-blockers in this population, after these results, I will not be stopping these drugs if they are being well tolerated,” he said.
Sylvain said he was surprised that there was not an improvement in quality of life in the group that discontinued beta-blockers. “We are always told that beta-blockers have many side effects, so we expected to see an improvement in quality of life in the patients who stopped these drugs.”
One possible reason for the lack of improvement in quality of life is that the trial participants had been taking beta-blockers for several years. “We may have, therefore, selected patients who tolerate these drugs quite well. Those who had tolerance issues had probably already stopped taking them,” he explained.
In addition, the patient population had relatively high quality-of-life scores at baseline. “They were well treated and the therapies they were taking were well tolerated, so maybe it is difficult to improve quality of life further,” he said.
The REDUCE-AMI Trial
The ABYSS results appear at first to differ from results from the recent REDUCE-AMI trial, which failed to show the superiority of beta-blocker therapy, compared with no beta-blocker therapy, in acute MI patients with preserved ejection fraction.
But the REDUCE-AMI primary endpoint was a composite of death from any cause or new myocardial infarction; it did not include cardiovascular hospitalization, which was the main driver of the difference in outcomes in the ABYSS study, Dr. Sylvain pointed out.
“We showed an increase in coronary cases of hospitalization with stopping beta-blockers, and you have to remember that beta-blockers were developed to reduce coronary disease,” he said.
‘Slightly Inconclusive’
Jane Armitage, MBBS, University of Oxford, England, the ABYSS discussant for the ESC HOTLINE session, pointed out some limitations of the study, which led her to report that the result was “slightly inconclusive.”
The open-label design may have allowed some bias regarding the cardiovascular hospitalization endpoint, she said.
“The decision whether to admit a patient to [the] hospital is somewhat subjective and could be influenced by a physician’s knowledge of treatment allocation. That is why, ideally, we prefer blinded trials. I think there are questions there,” she explained.
She also questioned whether the non-inferiority margin could have been increased, given the higher-than-expected event rate.
More data on this issue will come from several trials that are currently ongoing, Dr. Armitage said.
The ABYSS and REDUCE-AMI trials together suggest that it is safe, with respect to serious cardiac events, to stop beta-blocker treatment in MI patients with preserved ejection fraction, writes Tomas Jernberg, MD, PhD, from the Karolinska Institute in Stockholm, Sweden, in an accompanying editorial.
However, “because of the anti-ischemic effects of beta-blockers, an interruption may increase the risk of recurrent angina and the need for rehospitalization,” he adds.
“It is prudent to wait for the results of additional ongoing trials of beta-blockers involving patients with MI and a preserved left ventricular ejection fraction before definitively updating guidelines,” Dr. Jernberg concludes.
The ABYSS trial was funded by the French Ministry of Health and the ACTION Study Group. Dr. Sylvain, Dr. Armitage, and Dr. Jernberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
LONDON — It may not be advisable for patients with a history of myocardial infarction and preserved left ventricular function to discontinue long-term beta-blocker therapy, warn investigators.
In the randomized ABYSS trial, although there was no difference in death, MI, or stroke between patients who discontinued and those who continued taking beta-blockers, those who stopped taking the drugs had a higher rate of cardiovascular hospitalization.
Discontinuation was also associated with an increase in blood pressure and heart rate, without any improvement in quality of life.
The results, which were simultaneously published online in The New England Journal of Medicine, call into question current guidelines, which suggest that beta-blockers may be discontinued after 1 year in certain patient groups.
Beta-blockers have long been considered the standard of care for patients after MI, but trials showing the benefit of these drugs were conducted before the modern era of myocardial reperfusion and pharmacotherapy, which have led to sharp decreases in the risk for heart failure and for death after MI, Dr. Silvain explained.
This has led to questions about the add-on benefits of lifelong beta-blocker treatment for patients with MI and a preserved left ventricular ejection fraction and no other primary indication for beta-blocker therapy.
The ABYSS Trial
To explore this issue, the open-label, non-inferiority ABYSS trial randomly assigned 3698 patients with a history of MI to the discontinuation or continuation of beta-blocker treatment. All study participants had a left ventricular ejection fraction of at least 40%, were receiving long-term beta-blocker treatment, and had experienced no cardiovascular event in the previous 6 months.
At a median follow-up of 3 years, the primary endpoint — a composite of death, MI, stroke, and hospitalization for cardiovascular reasons — occurred more often in the discontinuation group than in the continuation group (23.8% vs 21.1%; hazard ratio, 1.16; 95% CI, 1.01-1.33). This did not meet the criteria for non-inferiority of discontinuation, compared with continuation, of beta-blocker therapy (P for non-inferiority = .44).
The difference in event rates between the two groups was driven by cardiovascular hospitalizations, which occurred more often in the discontinuation group than in the continuation group (18.9% vs 16.6%).
Other key results showed that there was no difference in quality of life between the two groups.
However, 6 months after randomization, there were increases in blood pressure and heart rate in the discontinuation group. Systolic blood pressure increased by 3.7 mm Hg and diastolic blood pressure increased by 3.9 mm Hg. Resting heart rate increased by 9.8 beats per minute.
“We were not able to show the non-inferiority of stopping beta-blockers in terms of cardiovascular events, [but we] showed a safety signal with this strategy of an increase in blood pressure and heart rate, with no improvement in quality of life,” Dr. Sylvain said.
“While recent guidelines suggest it may be reasonable to stop beta-blockers in this population, after these results, I will not be stopping these drugs if they are being well tolerated,” he said.
Sylvain said he was surprised that there was not an improvement in quality of life in the group that discontinued beta-blockers. “We are always told that beta-blockers have many side effects, so we expected to see an improvement in quality of life in the patients who stopped these drugs.”
One possible reason for the lack of improvement in quality of life is that the trial participants had been taking beta-blockers for several years. “We may have, therefore, selected patients who tolerate these drugs quite well. Those who had tolerance issues had probably already stopped taking them,” he explained.
In addition, the patient population had relatively high quality-of-life scores at baseline. “They were well treated and the therapies they were taking were well tolerated, so maybe it is difficult to improve quality of life further,” he said.
The REDUCE-AMI Trial
The ABYSS results appear at first to differ from results from the recent REDUCE-AMI trial, which failed to show the superiority of beta-blocker therapy, compared with no beta-blocker therapy, in acute MI patients with preserved ejection fraction.
But the REDUCE-AMI primary endpoint was a composite of death from any cause or new myocardial infarction; it did not include cardiovascular hospitalization, which was the main driver of the difference in outcomes in the ABYSS study, Dr. Sylvain pointed out.
“We showed an increase in coronary cases of hospitalization with stopping beta-blockers, and you have to remember that beta-blockers were developed to reduce coronary disease,” he said.
‘Slightly Inconclusive’
Jane Armitage, MBBS, University of Oxford, England, the ABYSS discussant for the ESC HOTLINE session, pointed out some limitations of the study, which led her to report that the result was “slightly inconclusive.”
The open-label design may have allowed some bias regarding the cardiovascular hospitalization endpoint, she said.
“The decision whether to admit a patient to [the] hospital is somewhat subjective and could be influenced by a physician’s knowledge of treatment allocation. That is why, ideally, we prefer blinded trials. I think there are questions there,” she explained.
She also questioned whether the non-inferiority margin could have been increased, given the higher-than-expected event rate.
More data on this issue will come from several trials that are currently ongoing, Dr. Armitage said.
The ABYSS and REDUCE-AMI trials together suggest that it is safe, with respect to serious cardiac events, to stop beta-blocker treatment in MI patients with preserved ejection fraction, writes Tomas Jernberg, MD, PhD, from the Karolinska Institute in Stockholm, Sweden, in an accompanying editorial.
However, “because of the anti-ischemic effects of beta-blockers, an interruption may increase the risk of recurrent angina and the need for rehospitalization,” he adds.
“It is prudent to wait for the results of additional ongoing trials of beta-blockers involving patients with MI and a preserved left ventricular ejection fraction before definitively updating guidelines,” Dr. Jernberg concludes.
The ABYSS trial was funded by the French Ministry of Health and the ACTION Study Group. Dr. Sylvain, Dr. Armitage, and Dr. Jernberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
LONDON — It may not be advisable for patients with a history of myocardial infarction and preserved left ventricular function to discontinue long-term beta-blocker therapy, warn investigators.
In the randomized ABYSS trial, although there was no difference in death, MI, or stroke between patients who discontinued and those who continued taking beta-blockers, those who stopped taking the drugs had a higher rate of cardiovascular hospitalization.
Discontinuation was also associated with an increase in blood pressure and heart rate, without any improvement in quality of life.
The results, which were simultaneously published online in The New England Journal of Medicine, call into question current guidelines, which suggest that beta-blockers may be discontinued after 1 year in certain patient groups.
Beta-blockers have long been considered the standard of care for patients after MI, but trials showing the benefit of these drugs were conducted before the modern era of myocardial reperfusion and pharmacotherapy, which have led to sharp decreases in the risk for heart failure and for death after MI, Dr. Silvain explained.
This has led to questions about the add-on benefits of lifelong beta-blocker treatment for patients with MI and a preserved left ventricular ejection fraction and no other primary indication for beta-blocker therapy.
The ABYSS Trial
To explore this issue, the open-label, non-inferiority ABYSS trial randomly assigned 3698 patients with a history of MI to the discontinuation or continuation of beta-blocker treatment. All study participants had a left ventricular ejection fraction of at least 40%, were receiving long-term beta-blocker treatment, and had experienced no cardiovascular event in the previous 6 months.
At a median follow-up of 3 years, the primary endpoint — a composite of death, MI, stroke, and hospitalization for cardiovascular reasons — occurred more often in the discontinuation group than in the continuation group (23.8% vs 21.1%; hazard ratio, 1.16; 95% CI, 1.01-1.33). This did not meet the criteria for non-inferiority of discontinuation, compared with continuation, of beta-blocker therapy (P for non-inferiority = .44).
The difference in event rates between the two groups was driven by cardiovascular hospitalizations, which occurred more often in the discontinuation group than in the continuation group (18.9% vs 16.6%).
Other key results showed that there was no difference in quality of life between the two groups.
However, 6 months after randomization, there were increases in blood pressure and heart rate in the discontinuation group. Systolic blood pressure increased by 3.7 mm Hg and diastolic blood pressure increased by 3.9 mm Hg. Resting heart rate increased by 9.8 beats per minute.
“We were not able to show the non-inferiority of stopping beta-blockers in terms of cardiovascular events, [but we] showed a safety signal with this strategy of an increase in blood pressure and heart rate, with no improvement in quality of life,” Dr. Sylvain said.
“While recent guidelines suggest it may be reasonable to stop beta-blockers in this population, after these results, I will not be stopping these drugs if they are being well tolerated,” he said.
Sylvain said he was surprised that there was not an improvement in quality of life in the group that discontinued beta-blockers. “We are always told that beta-blockers have many side effects, so we expected to see an improvement in quality of life in the patients who stopped these drugs.”
One possible reason for the lack of improvement in quality of life is that the trial participants had been taking beta-blockers for several years. “We may have, therefore, selected patients who tolerate these drugs quite well. Those who had tolerance issues had probably already stopped taking them,” he explained.
In addition, the patient population had relatively high quality-of-life scores at baseline. “They were well treated and the therapies they were taking were well tolerated, so maybe it is difficult to improve quality of life further,” he said.
The REDUCE-AMI Trial
The ABYSS results appear at first to differ from results from the recent REDUCE-AMI trial, which failed to show the superiority of beta-blocker therapy, compared with no beta-blocker therapy, in acute MI patients with preserved ejection fraction.
But the REDUCE-AMI primary endpoint was a composite of death from any cause or new myocardial infarction; it did not include cardiovascular hospitalization, which was the main driver of the difference in outcomes in the ABYSS study, Dr. Sylvain pointed out.
“We showed an increase in coronary cases of hospitalization with stopping beta-blockers, and you have to remember that beta-blockers were developed to reduce coronary disease,” he said.
‘Slightly Inconclusive’
Jane Armitage, MBBS, University of Oxford, England, the ABYSS discussant for the ESC HOTLINE session, pointed out some limitations of the study, which led her to report that the result was “slightly inconclusive.”
The open-label design may have allowed some bias regarding the cardiovascular hospitalization endpoint, she said.
“The decision whether to admit a patient to [the] hospital is somewhat subjective and could be influenced by a physician’s knowledge of treatment allocation. That is why, ideally, we prefer blinded trials. I think there are questions there,” she explained.
She also questioned whether the non-inferiority margin could have been increased, given the higher-than-expected event rate.
More data on this issue will come from several trials that are currently ongoing, Dr. Armitage said.
The ABYSS and REDUCE-AMI trials together suggest that it is safe, with respect to serious cardiac events, to stop beta-blocker treatment in MI patients with preserved ejection fraction, writes Tomas Jernberg, MD, PhD, from the Karolinska Institute in Stockholm, Sweden, in an accompanying editorial.
However, “because of the anti-ischemic effects of beta-blockers, an interruption may increase the risk of recurrent angina and the need for rehospitalization,” he adds.
“It is prudent to wait for the results of additional ongoing trials of beta-blockers involving patients with MI and a preserved left ventricular ejection fraction before definitively updating guidelines,” Dr. Jernberg concludes.
The ABYSS trial was funded by the French Ministry of Health and the ACTION Study Group. Dr. Sylvain, Dr. Armitage, and Dr. Jernberg report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Can Endurance Exercise Be Harmful?
In 490 BC, Pheidippides (or possibly Philippides) ran from Athens to Sparta to ask for military aid against the invading Persian army, then back to Athens, then off to the battlefield of Marathon, then back to Athens to announce the army’s victory, after which he promptly died. The story, if it is to be believed (there is some doubt among historians), raises an interesting question: Are some forms of exercise dangerous?
Running a marathon is a lot of work. The “worst parade ever,” as one spectator described it, is not without its risks. As a runner myself, I know that it doesn’t take much to generate a bloody sock at the end of a long run.
But when most people think about the risks of exercise, they mean the cardiovascular risks, such as sudden deaths during marathons, probably because of the aforementioned ancient Greek’s demise. The reality is more reassuring. An analysis of 10 years’ worth of data from US marathons and half-marathons found that out of 10.9 million runners, there were 59 cardiac arrests, an incidence rate of 0.54 per 100,000 participants. Others have found incidence rates in the same range. An analysis of the annual Marine Corps and Twin Cities marathons found a sudden death rate of 0.002%.
Marathon runners do sometimes require medical attention. In the Twin Cities cohort, 25 out of every 1000 finishers required medical attention, but 90% of their problems were mild. The majority included issues such as dehydration, vasovagal syncope, hyperthermia, and exhaustion. Musculoskeletal problems and skin abrasions made up the rest. Objectively, long distance running is fairly safe.
Running and Coronary Calcium
Then a study comes around suggesting that marathon runners have more coronary artery calcium (CAC). In 2008, German researchers compared 108 healthy male marathon runners over 50 years of age with Framingham risk–matched controls. The marathoners had a higher median CAC score (36 vs 12; P =.02), but scores across the board were quite low and not all studies were in agreement. The MESA study and another from Korea found an inverse relationship between physical activity and coronary calcium, but they compared sedentary people with vigorous exercisers, not specifically marathoners.
Two later studies, published in 2017, generally corroborated that endurance exercise was associated with higher calcium — with some caveats. A group from the Netherlands looked at lifelong exercise volume and compared men who accumulated > 2000 MET-min/week with those who exercised < 1000 MET-min/week. Again, the analysis was limited to men, and CAC scores, though statistically different, were still very low (9.4 vs 0; P =.02). Importantly, in men with coronary plaques, the more active group had less mixed plaque and more calcified plaque.
A UK study of middle-aged masters-level athletes at low cardiovascular risk had similar findings. Most of the study population (70%) were men, and 77% were runners (not all were marathoners). Overall, the male athletes had not only more plaque but more calcified plaque than their sedentary peers, even though most male athletes (60%) had a CAC score of zero.
The findings from these two studies were interpreted as reassuring. They confirmed that athletes are a generally low-risk group with low calcium scores, and although they might have more plaque and coronary calcium on average, it tends to be the more benign calcified type.
Masters at Heart
But the 2023 Master@Heart study challenged that assertion. It analyzed lifelong endurance athletes, late-onset endurance athletes (those who came to the game later in life), and healthy nonathletic controls. The study also found more coronary stenoses in lifelong athletes, but the breakdown of calcified vs noncalcified vs mixed plaques was the same across groups, thus contradicting the idea that exercise exerted its protective effect by calcifying and therefore stabilizing said plaques. The silver lining was fewer vulnerable plaques in the lifelong athletes (defined via high-risk features) but these were generally rare across the entire population.
Whether Master@Heart is groundbreaking or an outlier depends on your point of view. In 2024, a study from Portugal suggested that the relationship between exercise and coronary calcification is more complicated. Among 105 male veteran athletes, a high volume of exercise was associated with more coronary atherosclerosis in those at higher cardiovascular risk, but it tended to be protective in those deemed lower risk. In fact, the high-volume exercise group had fewer individuals with a CAC score > 100 (16% vs 4%; P =.029), though again, the vast majority had low CAC scores.
A limitation of all these studies is that they had cross-sectional designs, measuring coronary calcium at a single point in time and relying on questionnaires and patient recall to determine lifelong exposure to exercise. Recall bias could have been a problem, and exercise patterns vary over time. It’s not unreasonable to wonder whether people at higher cardiovascular risk should start exercising to mitigate that risk. Granted, they might not start running marathons, but many of these studies looked only at physical activity levels. A study that measured the increase (or stability) of coronary calcium over time would be more helpful.
Prior research (in men again) showed that high levels of physical activity were associated with more coronary calcium, but not with all-cause or cardiovascular mortality. But it too looked only at a single time point. The most recent study added to the body of evidence included data on nearly 9000 men and women and found that higher exercise volume did not correlate with CAC progression over the mean follow-up of 7.8 years. The study measured physical activity of any variety and included physically taxing sports like golf (without a cart). So it was not an assessment of the dangers of endurance exercise.
Outstanding Questions and Bananas
Ultimately, many questions remain. Is the lack of risk seen in women a spurious finding because they are underrepresented in most studies, or might exercise affect men and women differently? Is it valid to combine studies on endurance exercise with those looking at physical activity more generally? How accurate are self-reports of exercise? Could endurance exercisers be using performance-enhancing drugs that are confounding the associations? Are people who engage in more physical activity healthier or just trying to mitigate a higher baseline cardiovascular risk? Why do they give out bananas at the end of marathons given that there are better sources of potassium?
We have no randomized trials on the benefits and risks of endurance exercise. Even if you could get ethics approval, one imagines there would be few volunteers. In the end, we must make do with observational data and remember that coronary calcifications are a surrogate endpoint.
When it comes to hard endpoints, an analysis of French Tour de France participants found a lower risk for both cardiovascular and cancer deaths compared with the general male population. So perhaps the most important take-home message is one that has been said many times: Beware of surrogate endpoints. And for those contemplating running a marathon, I am forced to agree with the person who wrote the sign I saw during my first race. It does seem like a lot of work for a free banana.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In 490 BC, Pheidippides (or possibly Philippides) ran from Athens to Sparta to ask for military aid against the invading Persian army, then back to Athens, then off to the battlefield of Marathon, then back to Athens to announce the army’s victory, after which he promptly died. The story, if it is to be believed (there is some doubt among historians), raises an interesting question: Are some forms of exercise dangerous?
Running a marathon is a lot of work. The “worst parade ever,” as one spectator described it, is not without its risks. As a runner myself, I know that it doesn’t take much to generate a bloody sock at the end of a long run.
But when most people think about the risks of exercise, they mean the cardiovascular risks, such as sudden deaths during marathons, probably because of the aforementioned ancient Greek’s demise. The reality is more reassuring. An analysis of 10 years’ worth of data from US marathons and half-marathons found that out of 10.9 million runners, there were 59 cardiac arrests, an incidence rate of 0.54 per 100,000 participants. Others have found incidence rates in the same range. An analysis of the annual Marine Corps and Twin Cities marathons found a sudden death rate of 0.002%.
Marathon runners do sometimes require medical attention. In the Twin Cities cohort, 25 out of every 1000 finishers required medical attention, but 90% of their problems were mild. The majority included issues such as dehydration, vasovagal syncope, hyperthermia, and exhaustion. Musculoskeletal problems and skin abrasions made up the rest. Objectively, long distance running is fairly safe.
Running and Coronary Calcium
Then a study comes around suggesting that marathon runners have more coronary artery calcium (CAC). In 2008, German researchers compared 108 healthy male marathon runners over 50 years of age with Framingham risk–matched controls. The marathoners had a higher median CAC score (36 vs 12; P =.02), but scores across the board were quite low and not all studies were in agreement. The MESA study and another from Korea found an inverse relationship between physical activity and coronary calcium, but they compared sedentary people with vigorous exercisers, not specifically marathoners.
Two later studies, published in 2017, generally corroborated that endurance exercise was associated with higher calcium — with some caveats. A group from the Netherlands looked at lifelong exercise volume and compared men who accumulated > 2000 MET-min/week with those who exercised < 1000 MET-min/week. Again, the analysis was limited to men, and CAC scores, though statistically different, were still very low (9.4 vs 0; P =.02). Importantly, in men with coronary plaques, the more active group had less mixed plaque and more calcified plaque.
A UK study of middle-aged masters-level athletes at low cardiovascular risk had similar findings. Most of the study population (70%) were men, and 77% were runners (not all were marathoners). Overall, the male athletes had not only more plaque but more calcified plaque than their sedentary peers, even though most male athletes (60%) had a CAC score of zero.
The findings from these two studies were interpreted as reassuring. They confirmed that athletes are a generally low-risk group with low calcium scores, and although they might have more plaque and coronary calcium on average, it tends to be the more benign calcified type.
Masters at Heart
But the 2023 Master@Heart study challenged that assertion. It analyzed lifelong endurance athletes, late-onset endurance athletes (those who came to the game later in life), and healthy nonathletic controls. The study also found more coronary stenoses in lifelong athletes, but the breakdown of calcified vs noncalcified vs mixed plaques was the same across groups, thus contradicting the idea that exercise exerted its protective effect by calcifying and therefore stabilizing said plaques. The silver lining was fewer vulnerable plaques in the lifelong athletes (defined via high-risk features) but these were generally rare across the entire population.
Whether Master@Heart is groundbreaking or an outlier depends on your point of view. In 2024, a study from Portugal suggested that the relationship between exercise and coronary calcification is more complicated. Among 105 male veteran athletes, a high volume of exercise was associated with more coronary atherosclerosis in those at higher cardiovascular risk, but it tended to be protective in those deemed lower risk. In fact, the high-volume exercise group had fewer individuals with a CAC score > 100 (16% vs 4%; P =.029), though again, the vast majority had low CAC scores.
A limitation of all these studies is that they had cross-sectional designs, measuring coronary calcium at a single point in time and relying on questionnaires and patient recall to determine lifelong exposure to exercise. Recall bias could have been a problem, and exercise patterns vary over time. It’s not unreasonable to wonder whether people at higher cardiovascular risk should start exercising to mitigate that risk. Granted, they might not start running marathons, but many of these studies looked only at physical activity levels. A study that measured the increase (or stability) of coronary calcium over time would be more helpful.
Prior research (in men again) showed that high levels of physical activity were associated with more coronary calcium, but not with all-cause or cardiovascular mortality. But it too looked only at a single time point. The most recent study added to the body of evidence included data on nearly 9000 men and women and found that higher exercise volume did not correlate with CAC progression over the mean follow-up of 7.8 years. The study measured physical activity of any variety and included physically taxing sports like golf (without a cart). So it was not an assessment of the dangers of endurance exercise.
Outstanding Questions and Bananas
Ultimately, many questions remain. Is the lack of risk seen in women a spurious finding because they are underrepresented in most studies, or might exercise affect men and women differently? Is it valid to combine studies on endurance exercise with those looking at physical activity more generally? How accurate are self-reports of exercise? Could endurance exercisers be using performance-enhancing drugs that are confounding the associations? Are people who engage in more physical activity healthier or just trying to mitigate a higher baseline cardiovascular risk? Why do they give out bananas at the end of marathons given that there are better sources of potassium?
We have no randomized trials on the benefits and risks of endurance exercise. Even if you could get ethics approval, one imagines there would be few volunteers. In the end, we must make do with observational data and remember that coronary calcifications are a surrogate endpoint.
When it comes to hard endpoints, an analysis of French Tour de France participants found a lower risk for both cardiovascular and cancer deaths compared with the general male population. So perhaps the most important take-home message is one that has been said many times: Beware of surrogate endpoints. And for those contemplating running a marathon, I am forced to agree with the person who wrote the sign I saw during my first race. It does seem like a lot of work for a free banana.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In 490 BC, Pheidippides (or possibly Philippides) ran from Athens to Sparta to ask for military aid against the invading Persian army, then back to Athens, then off to the battlefield of Marathon, then back to Athens to announce the army’s victory, after which he promptly died. The story, if it is to be believed (there is some doubt among historians), raises an interesting question: Are some forms of exercise dangerous?
Running a marathon is a lot of work. The “worst parade ever,” as one spectator described it, is not without its risks. As a runner myself, I know that it doesn’t take much to generate a bloody sock at the end of a long run.
But when most people think about the risks of exercise, they mean the cardiovascular risks, such as sudden deaths during marathons, probably because of the aforementioned ancient Greek’s demise. The reality is more reassuring. An analysis of 10 years’ worth of data from US marathons and half-marathons found that out of 10.9 million runners, there were 59 cardiac arrests, an incidence rate of 0.54 per 100,000 participants. Others have found incidence rates in the same range. An analysis of the annual Marine Corps and Twin Cities marathons found a sudden death rate of 0.002%.
Marathon runners do sometimes require medical attention. In the Twin Cities cohort, 25 out of every 1000 finishers required medical attention, but 90% of their problems were mild. The majority included issues such as dehydration, vasovagal syncope, hyperthermia, and exhaustion. Musculoskeletal problems and skin abrasions made up the rest. Objectively, long distance running is fairly safe.
Running and Coronary Calcium
Then a study comes around suggesting that marathon runners have more coronary artery calcium (CAC). In 2008, German researchers compared 108 healthy male marathon runners over 50 years of age with Framingham risk–matched controls. The marathoners had a higher median CAC score (36 vs 12; P =.02), but scores across the board were quite low and not all studies were in agreement. The MESA study and another from Korea found an inverse relationship between physical activity and coronary calcium, but they compared sedentary people with vigorous exercisers, not specifically marathoners.
Two later studies, published in 2017, generally corroborated that endurance exercise was associated with higher calcium — with some caveats. A group from the Netherlands looked at lifelong exercise volume and compared men who accumulated > 2000 MET-min/week with those who exercised < 1000 MET-min/week. Again, the analysis was limited to men, and CAC scores, though statistically different, were still very low (9.4 vs 0; P =.02). Importantly, in men with coronary plaques, the more active group had less mixed plaque and more calcified plaque.
A UK study of middle-aged masters-level athletes at low cardiovascular risk had similar findings. Most of the study population (70%) were men, and 77% were runners (not all were marathoners). Overall, the male athletes had not only more plaque but more calcified plaque than their sedentary peers, even though most male athletes (60%) had a CAC score of zero.
The findings from these two studies were interpreted as reassuring. They confirmed that athletes are a generally low-risk group with low calcium scores, and although they might have more plaque and coronary calcium on average, it tends to be the more benign calcified type.
Masters at Heart
But the 2023 Master@Heart study challenged that assertion. It analyzed lifelong endurance athletes, late-onset endurance athletes (those who came to the game later in life), and healthy nonathletic controls. The study also found more coronary stenoses in lifelong athletes, but the breakdown of calcified vs noncalcified vs mixed plaques was the same across groups, thus contradicting the idea that exercise exerted its protective effect by calcifying and therefore stabilizing said plaques. The silver lining was fewer vulnerable plaques in the lifelong athletes (defined via high-risk features) but these were generally rare across the entire population.
Whether Master@Heart is groundbreaking or an outlier depends on your point of view. In 2024, a study from Portugal suggested that the relationship between exercise and coronary calcification is more complicated. Among 105 male veteran athletes, a high volume of exercise was associated with more coronary atherosclerosis in those at higher cardiovascular risk, but it tended to be protective in those deemed lower risk. In fact, the high-volume exercise group had fewer individuals with a CAC score > 100 (16% vs 4%; P =.029), though again, the vast majority had low CAC scores.
A limitation of all these studies is that they had cross-sectional designs, measuring coronary calcium at a single point in time and relying on questionnaires and patient recall to determine lifelong exposure to exercise. Recall bias could have been a problem, and exercise patterns vary over time. It’s not unreasonable to wonder whether people at higher cardiovascular risk should start exercising to mitigate that risk. Granted, they might not start running marathons, but many of these studies looked only at physical activity levels. A study that measured the increase (or stability) of coronary calcium over time would be more helpful.
Prior research (in men again) showed that high levels of physical activity were associated with more coronary calcium, but not with all-cause or cardiovascular mortality. But it too looked only at a single time point. The most recent study added to the body of evidence included data on nearly 9000 men and women and found that higher exercise volume did not correlate with CAC progression over the mean follow-up of 7.8 years. The study measured physical activity of any variety and included physically taxing sports like golf (without a cart). So it was not an assessment of the dangers of endurance exercise.
Outstanding Questions and Bananas
Ultimately, many questions remain. Is the lack of risk seen in women a spurious finding because they are underrepresented in most studies, or might exercise affect men and women differently? Is it valid to combine studies on endurance exercise with those looking at physical activity more generally? How accurate are self-reports of exercise? Could endurance exercisers be using performance-enhancing drugs that are confounding the associations? Are people who engage in more physical activity healthier or just trying to mitigate a higher baseline cardiovascular risk? Why do they give out bananas at the end of marathons given that there are better sources of potassium?
We have no randomized trials on the benefits and risks of endurance exercise. Even if you could get ethics approval, one imagines there would be few volunteers. In the end, we must make do with observational data and remember that coronary calcifications are a surrogate endpoint.
When it comes to hard endpoints, an analysis of French Tour de France participants found a lower risk for both cardiovascular and cancer deaths compared with the general male population. So perhaps the most important take-home message is one that has been said many times: Beware of surrogate endpoints. And for those contemplating running a marathon, I am forced to agree with the person who wrote the sign I saw during my first race. It does seem like a lot of work for a free banana.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, Quebec, Canada. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New AFib Guidelines Address Underlying Illness, Comorbidities
LONDON — Updated guidelines for the management of atrial fibrillation released by the European Society of Cardiology are revamping the approach to care for this complex, multifactorial disease.
, Isabelle Van Gelder, MD, PhD, professor of cardiology at the University Medical Center in Groningen, the Netherlands, explained at the European Society of Cardiology (ESC) Congress.
It is not just appropriate to place the same emphasis on the control of comorbidities as on the rhythm disturbance, it is critical, said Dr. Van Gelder, who served as chair of the ESC-AF guidelines task force.
Comorbidities are the drivers of both the onset and recurrence of atrial fibrillation, and a dynamic approach to comorbidities is “central for the success of AF management.”
Class I Recommendation
In fact, on the basis of overwhelming evidence, a class I recommendation has been issued for a large number of goals in the comorbidity and risk factor management step of atrial fibrillation management, including those for hypertension, components of heart failure, obesity, diabetes, alcohol consumption, and exercise.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors “should be offered to all patients with AF,” according to Dr. Van Gelder, who identified this as a new class I recommendation.
Patients who are not managed aggressively for the listed comorbidities ultimately face “treatment failure, poor patient outcomes, and a waste of healthcare resources,” she said.
Control of sleep apnea is also noted as a key target, although Van Gelder acknowledged that the supporting evidence only allows for a class IIb recommendation.
Control of comorbidities is not a new idea. In the 2023 joint guideline, led by a consortium of professional groups, including the American Heart Association (AHA) and the American College of Cardiology (ACC), the control of comorbidities, including most of those identified in the new ESC guidelines, was second in a list of 10 key take-home messages.
However, the new ESC guidelines have prioritized comorbidity management by listing it first in each of the specific patient-care pathways developed to define optimized care.
These pathways, defined in algorithms for newly diagnosed AF, paroxysmal AF, and persistent AF, always start with the assessment of comorbidities, followed by step A — avoiding stroke — largely with anticoagulation.
Direct oral anticoagulants should be used, “except in those with a mechanical valve or mitral stenosis,” Dr. Van Gelder said. This includes, essentially, all patients with a CHA2DS2-VASc score of 2 or greater, and it should be “considered” in those with a score of 1.
The ESC framework has been identified with the acronym AF-CARE, in which the C stands for comorbidities.
In the A step of the framework, identifying and treating all modifiable bleeding risk factors in AF patients is a class I recommendation. On the basis of a class III recommendation, she cautioned against withholding anticoagulants because of CHA2DS2-VASc risk factors alone. Rather, Dr. Van Gelder called the decision to administer or withhold anticoagulation — like all decisions — one that should be individualized in consultation with the patient.
For reducing AF symptoms and rhythm control, the specific pathways diverge for newly diagnosed AF, paroxysmal AF, and persistent AF. Like all of the guidelines, the specific options for symptom management and AF ablation are color coded, with green signifying level 1 evidence.
The evaluation and dynamic reassessment step refers to the need to periodically assess patients for new modifiable risk factors related to comorbidities, risk for stroke, risk for bleeding, and risk for AF.
The management of risk factors for AF has long been emphasized in guidelines, but a previous focus on AF with attention to comorbidities has been replaced by a focus on comorbidities with an expectation of more durable AF control. The success of this pivot is based on multidisciplinary care, chosen in collaboration with the patient, to reduce or eliminate the triggers of AF and the risks of its complications.
Pathways Are Appropriate for All Patients
A very important recommendation — and this is new — is “to treat all our patients with atrial fibrillation, whether they are young or old, men or women, Black or White, or at high or low risk, according to our patient-centered integrated AF-CARE approach,” Dr. Van Gelder said.
The changes reflect a shared appreciation for the tight relation between the control of comorbidities and the control of AF, according to José A. Joglar, MD, professor of cardiac electrophysiologic research at the University of Texas Southwestern Medical Center in Dallas. Dr. Joglar was chair of the writing committee for the joint 2023 AF guidelines released by the AHA, ACC, the American College of Clinical Pharmacy, and the Heart Rhythm Society.
“It is increasingly clear that AF in many cases is the consequence of underlying risk factors and comorbidities, which cannot be separated from AF alone,” Dr. Joglar explained in an interview.
This was placed first “to emphasize the importance of viewing AFib as a complex disease that requires a holistic, multidisciplinary approach to care, as opposed to being viewed just as a rhythm abnormality,” he said.
A version of this article first appeared on Medscape.com.
LONDON — Updated guidelines for the management of atrial fibrillation released by the European Society of Cardiology are revamping the approach to care for this complex, multifactorial disease.
, Isabelle Van Gelder, MD, PhD, professor of cardiology at the University Medical Center in Groningen, the Netherlands, explained at the European Society of Cardiology (ESC) Congress.
It is not just appropriate to place the same emphasis on the control of comorbidities as on the rhythm disturbance, it is critical, said Dr. Van Gelder, who served as chair of the ESC-AF guidelines task force.
Comorbidities are the drivers of both the onset and recurrence of atrial fibrillation, and a dynamic approach to comorbidities is “central for the success of AF management.”
Class I Recommendation
In fact, on the basis of overwhelming evidence, a class I recommendation has been issued for a large number of goals in the comorbidity and risk factor management step of atrial fibrillation management, including those for hypertension, components of heart failure, obesity, diabetes, alcohol consumption, and exercise.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors “should be offered to all patients with AF,” according to Dr. Van Gelder, who identified this as a new class I recommendation.
Patients who are not managed aggressively for the listed comorbidities ultimately face “treatment failure, poor patient outcomes, and a waste of healthcare resources,” she said.
Control of sleep apnea is also noted as a key target, although Van Gelder acknowledged that the supporting evidence only allows for a class IIb recommendation.
Control of comorbidities is not a new idea. In the 2023 joint guideline, led by a consortium of professional groups, including the American Heart Association (AHA) and the American College of Cardiology (ACC), the control of comorbidities, including most of those identified in the new ESC guidelines, was second in a list of 10 key take-home messages.
However, the new ESC guidelines have prioritized comorbidity management by listing it first in each of the specific patient-care pathways developed to define optimized care.
These pathways, defined in algorithms for newly diagnosed AF, paroxysmal AF, and persistent AF, always start with the assessment of comorbidities, followed by step A — avoiding stroke — largely with anticoagulation.
Direct oral anticoagulants should be used, “except in those with a mechanical valve or mitral stenosis,” Dr. Van Gelder said. This includes, essentially, all patients with a CHA2DS2-VASc score of 2 or greater, and it should be “considered” in those with a score of 1.
The ESC framework has been identified with the acronym AF-CARE, in which the C stands for comorbidities.
In the A step of the framework, identifying and treating all modifiable bleeding risk factors in AF patients is a class I recommendation. On the basis of a class III recommendation, she cautioned against withholding anticoagulants because of CHA2DS2-VASc risk factors alone. Rather, Dr. Van Gelder called the decision to administer or withhold anticoagulation — like all decisions — one that should be individualized in consultation with the patient.
For reducing AF symptoms and rhythm control, the specific pathways diverge for newly diagnosed AF, paroxysmal AF, and persistent AF. Like all of the guidelines, the specific options for symptom management and AF ablation are color coded, with green signifying level 1 evidence.
The evaluation and dynamic reassessment step refers to the need to periodically assess patients for new modifiable risk factors related to comorbidities, risk for stroke, risk for bleeding, and risk for AF.
The management of risk factors for AF has long been emphasized in guidelines, but a previous focus on AF with attention to comorbidities has been replaced by a focus on comorbidities with an expectation of more durable AF control. The success of this pivot is based on multidisciplinary care, chosen in collaboration with the patient, to reduce or eliminate the triggers of AF and the risks of its complications.
Pathways Are Appropriate for All Patients
A very important recommendation — and this is new — is “to treat all our patients with atrial fibrillation, whether they are young or old, men or women, Black or White, or at high or low risk, according to our patient-centered integrated AF-CARE approach,” Dr. Van Gelder said.
The changes reflect a shared appreciation for the tight relation between the control of comorbidities and the control of AF, according to José A. Joglar, MD, professor of cardiac electrophysiologic research at the University of Texas Southwestern Medical Center in Dallas. Dr. Joglar was chair of the writing committee for the joint 2023 AF guidelines released by the AHA, ACC, the American College of Clinical Pharmacy, and the Heart Rhythm Society.
“It is increasingly clear that AF in many cases is the consequence of underlying risk factors and comorbidities, which cannot be separated from AF alone,” Dr. Joglar explained in an interview.
This was placed first “to emphasize the importance of viewing AFib as a complex disease that requires a holistic, multidisciplinary approach to care, as opposed to being viewed just as a rhythm abnormality,” he said.
A version of this article first appeared on Medscape.com.
LONDON — Updated guidelines for the management of atrial fibrillation released by the European Society of Cardiology are revamping the approach to care for this complex, multifactorial disease.
, Isabelle Van Gelder, MD, PhD, professor of cardiology at the University Medical Center in Groningen, the Netherlands, explained at the European Society of Cardiology (ESC) Congress.
It is not just appropriate to place the same emphasis on the control of comorbidities as on the rhythm disturbance, it is critical, said Dr. Van Gelder, who served as chair of the ESC-AF guidelines task force.
Comorbidities are the drivers of both the onset and recurrence of atrial fibrillation, and a dynamic approach to comorbidities is “central for the success of AF management.”
Class I Recommendation
In fact, on the basis of overwhelming evidence, a class I recommendation has been issued for a large number of goals in the comorbidity and risk factor management step of atrial fibrillation management, including those for hypertension, components of heart failure, obesity, diabetes, alcohol consumption, and exercise.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors “should be offered to all patients with AF,” according to Dr. Van Gelder, who identified this as a new class I recommendation.
Patients who are not managed aggressively for the listed comorbidities ultimately face “treatment failure, poor patient outcomes, and a waste of healthcare resources,” she said.
Control of sleep apnea is also noted as a key target, although Van Gelder acknowledged that the supporting evidence only allows for a class IIb recommendation.
Control of comorbidities is not a new idea. In the 2023 joint guideline, led by a consortium of professional groups, including the American Heart Association (AHA) and the American College of Cardiology (ACC), the control of comorbidities, including most of those identified in the new ESC guidelines, was second in a list of 10 key take-home messages.
However, the new ESC guidelines have prioritized comorbidity management by listing it first in each of the specific patient-care pathways developed to define optimized care.
These pathways, defined in algorithms for newly diagnosed AF, paroxysmal AF, and persistent AF, always start with the assessment of comorbidities, followed by step A — avoiding stroke — largely with anticoagulation.
Direct oral anticoagulants should be used, “except in those with a mechanical valve or mitral stenosis,” Dr. Van Gelder said. This includes, essentially, all patients with a CHA2DS2-VASc score of 2 or greater, and it should be “considered” in those with a score of 1.
The ESC framework has been identified with the acronym AF-CARE, in which the C stands for comorbidities.
In the A step of the framework, identifying and treating all modifiable bleeding risk factors in AF patients is a class I recommendation. On the basis of a class III recommendation, she cautioned against withholding anticoagulants because of CHA2DS2-VASc risk factors alone. Rather, Dr. Van Gelder called the decision to administer or withhold anticoagulation — like all decisions — one that should be individualized in consultation with the patient.
For reducing AF symptoms and rhythm control, the specific pathways diverge for newly diagnosed AF, paroxysmal AF, and persistent AF. Like all of the guidelines, the specific options for symptom management and AF ablation are color coded, with green signifying level 1 evidence.
The evaluation and dynamic reassessment step refers to the need to periodically assess patients for new modifiable risk factors related to comorbidities, risk for stroke, risk for bleeding, and risk for AF.
The management of risk factors for AF has long been emphasized in guidelines, but a previous focus on AF with attention to comorbidities has been replaced by a focus on comorbidities with an expectation of more durable AF control. The success of this pivot is based on multidisciplinary care, chosen in collaboration with the patient, to reduce or eliminate the triggers of AF and the risks of its complications.
Pathways Are Appropriate for All Patients
A very important recommendation — and this is new — is “to treat all our patients with atrial fibrillation, whether they are young or old, men or women, Black or White, or at high or low risk, according to our patient-centered integrated AF-CARE approach,” Dr. Van Gelder said.
The changes reflect a shared appreciation for the tight relation between the control of comorbidities and the control of AF, according to José A. Joglar, MD, professor of cardiac electrophysiologic research at the University of Texas Southwestern Medical Center in Dallas. Dr. Joglar was chair of the writing committee for the joint 2023 AF guidelines released by the AHA, ACC, the American College of Clinical Pharmacy, and the Heart Rhythm Society.
“It is increasingly clear that AF in many cases is the consequence of underlying risk factors and comorbidities, which cannot be separated from AF alone,” Dr. Joglar explained in an interview.
This was placed first “to emphasize the importance of viewing AFib as a complex disease that requires a holistic, multidisciplinary approach to care, as opposed to being viewed just as a rhythm abnormality,” he said.
A version of this article first appeared on Medscape.com.
FROM ESC 2024
New Blood Pressure Guidelines Simplified, Lower Treatment Target
LONDON — Simplified and more aggressive targets are among the significant changes to the updated hypertension guidelines released by the European Society of Cardiology.
Although the updated guidelines, presented here at the ESC Congress, continue to define hypertension as a systolic BP of at least 140 mm Hg and a diastolic BP of at least 90 mm Hg, there is a new category — elevated BP. This is defined as a systolic BP of 120 mm Hg to 139 mm Hg or a diastolic BP of 70 mm Hg to 89 mm Hg, and cardiovascular risk assessment is advised to guide treatment, particularly in patients with a BP of at least 130/80 mm Hg.
The guidelines also introduce new recommendations for lifestyle options to help lower BP, including changes to exercise advice and the addition of potassium supplementation. And for the first time, the ESC guidelines provide recommendations for the use of renal denervation to treat hypertension in certain circumstances.
The guidelines were produced by an international panel, led by Bill McEvoy, MB BCh, from the University of Galway, Ireland, and Rhian Touyz, MB BCh, PhD, from McGill University in Montreal.
Three Categories of Blood Pressure
There are now three categories for BP classification — non-elevated (< 120/70 mm Hg), elevated (120 mm Hg to139 mm Hg/70 mm Hg to 89 mm Hg), and hypertension (≥ 140/90 mm Hg) — Dr. McEvoy reported during a session on the new guidelines here at ESC.
The emphasis on out-of-office BP measurement is stronger than in previous guidelines, but office measurement will still be used, he said.
All patients in the hypertension category qualify for treatment, whereas those in the new elevated BP category will be subject to cardiovascular risk stratification before a treatment decision is made.
Patients in the elevated BP category who also have moderate or severe chronic kidney disease, established cardiovascular disease, diabetes, or familial hypercholesterolemia are among those considered at increased risk for cardiovascular disease, as are patients with an estimated 10-year cardiovascular risk of 10% or higher. In such patients with a confirmed BP of at least 130/80 mm Hg, after 3 months of lifestyle intervention, pharmacologic treatment is recommended.
“This new category of elevated blood pressure recognizes that people do not go from normal blood pressure to hypertensive overnight,” Dr. McEvoy said. “It is, in most cases, a steady gradient of change, and different subgroups of patients — for example, those at a higher risk of developing cardiovascular disease — could benefit from more intensive treatment before their blood pressure reaches the traditional threshold of hypertension.”
New Lower Target
The major change in target pressures in these guidelines is based on new clinical trial data that confirm that lower pressures lead to lower cardiovascular event rates, resulting in the new systolic BP target of 120 mm Hg to 129 mm Hg for most patients receiving antihypertensive medications.
This systolic target represents a major change from previous European guidelines, Dr. McEvoy said, which have generally recommended that patients be treated to a target of less than 140/90 mm Hg and, only after that has been reached, then treated to a target of less than 130/80 mm Hg (a two-step approach).
“This change is driven by new trial evidence confirming that more intensive blood pressure treatment targets reduce cardiovascular outcomes across a broad spectrum of eligible patients,” Dr. McEvoy said.
There are, however, several caveats to this recommendation, including the requirement that treatment to this target be well tolerated; more lenient targets can be considered in people with symptomatic orthostatic hypotension, those 85 years and older, and those with moderate to severe frailty or a limited life expectancy. For these patients, the guidelines recommend a target “that is as low as reasonably achievable.”
More in Line With US Guidelines
The new European guidelines are now more in line with the American guidelines, said Eugene Yang, MD, from the University of Washington in Seattle, who is chair of the Hypertension Writing Group at the American College of Cardiology.
“These new European guidelines have thoughtfully used the latest study data to simplify recommendations for a specific lower blood pressure target. This is a step forward. There is now a greater alignment of European and US guidelines. This is good to reduce confusion and build consensus across the world,” he said.
Both sets of guidelines now recommend a BP target of less than 130/80 mm Hg for most people.
“I think the Europeans have now embraced this more aggressive target because there are many more studies now showing that these lower blood pressure levels do lead to a reduction in cardiovascular events,” Dr. Yang explained. “When the last European guidelines came out, there was only SPRINT. Now there are several more studies showing similar results.”
New Lifestyle Advice
The updated recommendation of 75 minutes of vigorous-intensity aerobic exercise per week has been added as an alternative to the previous recommendation of at least 2.5 hours per week of moderate-intensity aerobic exercise. This should be complemented with low- or moderate-intensity dynamic or isometric resistance training two to three times a week.
It is also recommended that people with hypertension, but without moderate or advanced chronic kidney disease, increase potassium intake with salt substitutes or diets rich in fruits and vegetables.
Renal Denervation Included for First Time
For the first time, the guidelines include the option of renal denervation for the treatment of hypertension — at medium- to high-volume centers — for patients with resistant hypertension that is uncontrolled despite a three-drug combination.
However, renal denervation is not recommended as a first-line treatment because of the lack of evidence of a benefit in cardiovascular outcomes. It is also not recommended for patients with highly impaired renal function or secondary causes of hypertension.
Dr. Yang said he approves of the inclusion of a frailty assessment in the new guidelines and less aggressive targets for people who are in poor health and older than age 85 years, but added that, “on the whole, they have less age-specific stratification than before, which is a significant change, and a good one in my view.”
Again, this is like the American guidelines, which have no age cutoffs and a target of less than 130/80 mm Hg for all, with the caveat that clinical judgment may be needed for individuals who are institutionalized, he added.
Dr. Yang said he was not as keen on the requirement for a cardiovascular risk assessment to guide treatment decisions for people with a systolic BP in the 130 mm Hg to 139 mm Hg range, although this is also included in the current American guidelines.
“As a clinician, I think this complicates things a bit too much and, as such, will be a barrier to treatment. In my view, blood pressure treatment recommendations need to be as simple as possible, so I think we still have some work to do there,” he said.
A version of this article first appeared on Medscape.com.
LONDON — Simplified and more aggressive targets are among the significant changes to the updated hypertension guidelines released by the European Society of Cardiology.
Although the updated guidelines, presented here at the ESC Congress, continue to define hypertension as a systolic BP of at least 140 mm Hg and a diastolic BP of at least 90 mm Hg, there is a new category — elevated BP. This is defined as a systolic BP of 120 mm Hg to 139 mm Hg or a diastolic BP of 70 mm Hg to 89 mm Hg, and cardiovascular risk assessment is advised to guide treatment, particularly in patients with a BP of at least 130/80 mm Hg.
The guidelines also introduce new recommendations for lifestyle options to help lower BP, including changes to exercise advice and the addition of potassium supplementation. And for the first time, the ESC guidelines provide recommendations for the use of renal denervation to treat hypertension in certain circumstances.
The guidelines were produced by an international panel, led by Bill McEvoy, MB BCh, from the University of Galway, Ireland, and Rhian Touyz, MB BCh, PhD, from McGill University in Montreal.
Three Categories of Blood Pressure
There are now three categories for BP classification — non-elevated (< 120/70 mm Hg), elevated (120 mm Hg to139 mm Hg/70 mm Hg to 89 mm Hg), and hypertension (≥ 140/90 mm Hg) — Dr. McEvoy reported during a session on the new guidelines here at ESC.
The emphasis on out-of-office BP measurement is stronger than in previous guidelines, but office measurement will still be used, he said.
All patients in the hypertension category qualify for treatment, whereas those in the new elevated BP category will be subject to cardiovascular risk stratification before a treatment decision is made.
Patients in the elevated BP category who also have moderate or severe chronic kidney disease, established cardiovascular disease, diabetes, or familial hypercholesterolemia are among those considered at increased risk for cardiovascular disease, as are patients with an estimated 10-year cardiovascular risk of 10% or higher. In such patients with a confirmed BP of at least 130/80 mm Hg, after 3 months of lifestyle intervention, pharmacologic treatment is recommended.
“This new category of elevated blood pressure recognizes that people do not go from normal blood pressure to hypertensive overnight,” Dr. McEvoy said. “It is, in most cases, a steady gradient of change, and different subgroups of patients — for example, those at a higher risk of developing cardiovascular disease — could benefit from more intensive treatment before their blood pressure reaches the traditional threshold of hypertension.”
New Lower Target
The major change in target pressures in these guidelines is based on new clinical trial data that confirm that lower pressures lead to lower cardiovascular event rates, resulting in the new systolic BP target of 120 mm Hg to 129 mm Hg for most patients receiving antihypertensive medications.
This systolic target represents a major change from previous European guidelines, Dr. McEvoy said, which have generally recommended that patients be treated to a target of less than 140/90 mm Hg and, only after that has been reached, then treated to a target of less than 130/80 mm Hg (a two-step approach).
“This change is driven by new trial evidence confirming that more intensive blood pressure treatment targets reduce cardiovascular outcomes across a broad spectrum of eligible patients,” Dr. McEvoy said.
There are, however, several caveats to this recommendation, including the requirement that treatment to this target be well tolerated; more lenient targets can be considered in people with symptomatic orthostatic hypotension, those 85 years and older, and those with moderate to severe frailty or a limited life expectancy. For these patients, the guidelines recommend a target “that is as low as reasonably achievable.”
More in Line With US Guidelines
The new European guidelines are now more in line with the American guidelines, said Eugene Yang, MD, from the University of Washington in Seattle, who is chair of the Hypertension Writing Group at the American College of Cardiology.
“These new European guidelines have thoughtfully used the latest study data to simplify recommendations for a specific lower blood pressure target. This is a step forward. There is now a greater alignment of European and US guidelines. This is good to reduce confusion and build consensus across the world,” he said.
Both sets of guidelines now recommend a BP target of less than 130/80 mm Hg for most people.
“I think the Europeans have now embraced this more aggressive target because there are many more studies now showing that these lower blood pressure levels do lead to a reduction in cardiovascular events,” Dr. Yang explained. “When the last European guidelines came out, there was only SPRINT. Now there are several more studies showing similar results.”
New Lifestyle Advice
The updated recommendation of 75 minutes of vigorous-intensity aerobic exercise per week has been added as an alternative to the previous recommendation of at least 2.5 hours per week of moderate-intensity aerobic exercise. This should be complemented with low- or moderate-intensity dynamic or isometric resistance training two to three times a week.
It is also recommended that people with hypertension, but without moderate or advanced chronic kidney disease, increase potassium intake with salt substitutes or diets rich in fruits and vegetables.
Renal Denervation Included for First Time
For the first time, the guidelines include the option of renal denervation for the treatment of hypertension — at medium- to high-volume centers — for patients with resistant hypertension that is uncontrolled despite a three-drug combination.
However, renal denervation is not recommended as a first-line treatment because of the lack of evidence of a benefit in cardiovascular outcomes. It is also not recommended for patients with highly impaired renal function or secondary causes of hypertension.
Dr. Yang said he approves of the inclusion of a frailty assessment in the new guidelines and less aggressive targets for people who are in poor health and older than age 85 years, but added that, “on the whole, they have less age-specific stratification than before, which is a significant change, and a good one in my view.”
Again, this is like the American guidelines, which have no age cutoffs and a target of less than 130/80 mm Hg for all, with the caveat that clinical judgment may be needed for individuals who are institutionalized, he added.
Dr. Yang said he was not as keen on the requirement for a cardiovascular risk assessment to guide treatment decisions for people with a systolic BP in the 130 mm Hg to 139 mm Hg range, although this is also included in the current American guidelines.
“As a clinician, I think this complicates things a bit too much and, as such, will be a barrier to treatment. In my view, blood pressure treatment recommendations need to be as simple as possible, so I think we still have some work to do there,” he said.
A version of this article first appeared on Medscape.com.
LONDON — Simplified and more aggressive targets are among the significant changes to the updated hypertension guidelines released by the European Society of Cardiology.
Although the updated guidelines, presented here at the ESC Congress, continue to define hypertension as a systolic BP of at least 140 mm Hg and a diastolic BP of at least 90 mm Hg, there is a new category — elevated BP. This is defined as a systolic BP of 120 mm Hg to 139 mm Hg or a diastolic BP of 70 mm Hg to 89 mm Hg, and cardiovascular risk assessment is advised to guide treatment, particularly in patients with a BP of at least 130/80 mm Hg.
The guidelines also introduce new recommendations for lifestyle options to help lower BP, including changes to exercise advice and the addition of potassium supplementation. And for the first time, the ESC guidelines provide recommendations for the use of renal denervation to treat hypertension in certain circumstances.
The guidelines were produced by an international panel, led by Bill McEvoy, MB BCh, from the University of Galway, Ireland, and Rhian Touyz, MB BCh, PhD, from McGill University in Montreal.
Three Categories of Blood Pressure
There are now three categories for BP classification — non-elevated (< 120/70 mm Hg), elevated (120 mm Hg to139 mm Hg/70 mm Hg to 89 mm Hg), and hypertension (≥ 140/90 mm Hg) — Dr. McEvoy reported during a session on the new guidelines here at ESC.
The emphasis on out-of-office BP measurement is stronger than in previous guidelines, but office measurement will still be used, he said.
All patients in the hypertension category qualify for treatment, whereas those in the new elevated BP category will be subject to cardiovascular risk stratification before a treatment decision is made.
Patients in the elevated BP category who also have moderate or severe chronic kidney disease, established cardiovascular disease, diabetes, or familial hypercholesterolemia are among those considered at increased risk for cardiovascular disease, as are patients with an estimated 10-year cardiovascular risk of 10% or higher. In such patients with a confirmed BP of at least 130/80 mm Hg, after 3 months of lifestyle intervention, pharmacologic treatment is recommended.
“This new category of elevated blood pressure recognizes that people do not go from normal blood pressure to hypertensive overnight,” Dr. McEvoy said. “It is, in most cases, a steady gradient of change, and different subgroups of patients — for example, those at a higher risk of developing cardiovascular disease — could benefit from more intensive treatment before their blood pressure reaches the traditional threshold of hypertension.”
New Lower Target
The major change in target pressures in these guidelines is based on new clinical trial data that confirm that lower pressures lead to lower cardiovascular event rates, resulting in the new systolic BP target of 120 mm Hg to 129 mm Hg for most patients receiving antihypertensive medications.
This systolic target represents a major change from previous European guidelines, Dr. McEvoy said, which have generally recommended that patients be treated to a target of less than 140/90 mm Hg and, only after that has been reached, then treated to a target of less than 130/80 mm Hg (a two-step approach).
“This change is driven by new trial evidence confirming that more intensive blood pressure treatment targets reduce cardiovascular outcomes across a broad spectrum of eligible patients,” Dr. McEvoy said.
There are, however, several caveats to this recommendation, including the requirement that treatment to this target be well tolerated; more lenient targets can be considered in people with symptomatic orthostatic hypotension, those 85 years and older, and those with moderate to severe frailty or a limited life expectancy. For these patients, the guidelines recommend a target “that is as low as reasonably achievable.”
More in Line With US Guidelines
The new European guidelines are now more in line with the American guidelines, said Eugene Yang, MD, from the University of Washington in Seattle, who is chair of the Hypertension Writing Group at the American College of Cardiology.
“These new European guidelines have thoughtfully used the latest study data to simplify recommendations for a specific lower blood pressure target. This is a step forward. There is now a greater alignment of European and US guidelines. This is good to reduce confusion and build consensus across the world,” he said.
Both sets of guidelines now recommend a BP target of less than 130/80 mm Hg for most people.
“I think the Europeans have now embraced this more aggressive target because there are many more studies now showing that these lower blood pressure levels do lead to a reduction in cardiovascular events,” Dr. Yang explained. “When the last European guidelines came out, there was only SPRINT. Now there are several more studies showing similar results.”
New Lifestyle Advice
The updated recommendation of 75 minutes of vigorous-intensity aerobic exercise per week has been added as an alternative to the previous recommendation of at least 2.5 hours per week of moderate-intensity aerobic exercise. This should be complemented with low- or moderate-intensity dynamic or isometric resistance training two to three times a week.
It is also recommended that people with hypertension, but without moderate or advanced chronic kidney disease, increase potassium intake with salt substitutes or diets rich in fruits and vegetables.
Renal Denervation Included for First Time
For the first time, the guidelines include the option of renal denervation for the treatment of hypertension — at medium- to high-volume centers — for patients with resistant hypertension that is uncontrolled despite a three-drug combination.
However, renal denervation is not recommended as a first-line treatment because of the lack of evidence of a benefit in cardiovascular outcomes. It is also not recommended for patients with highly impaired renal function or secondary causes of hypertension.
Dr. Yang said he approves of the inclusion of a frailty assessment in the new guidelines and less aggressive targets for people who are in poor health and older than age 85 years, but added that, “on the whole, they have less age-specific stratification than before, which is a significant change, and a good one in my view.”
Again, this is like the American guidelines, which have no age cutoffs and a target of less than 130/80 mm Hg for all, with the caveat that clinical judgment may be needed for individuals who are institutionalized, he added.
Dr. Yang said he was not as keen on the requirement for a cardiovascular risk assessment to guide treatment decisions for people with a systolic BP in the 130 mm Hg to 139 mm Hg range, although this is also included in the current American guidelines.
“As a clinician, I think this complicates things a bit too much and, as such, will be a barrier to treatment. In my view, blood pressure treatment recommendations need to be as simple as possible, so I think we still have some work to do there,” he said.
A version of this article first appeared on Medscape.com.
FROM ESC 2024
Acute Tender Papules on the Arms and Legs
The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).
Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.
Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.
Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9
Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
A 66-year-old man presented with new tender erythematous papules scattered over the arms and legs. A biopsy of a lesion on the left thigh was performed.