User login
Higher rates of PTSD, BPD in transgender vs. cisgender psych patients
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).
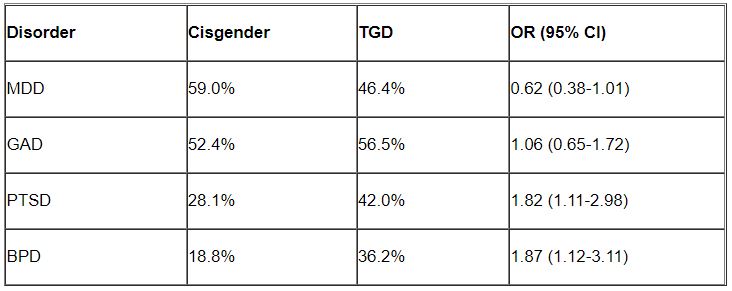
“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).

“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).

“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL PSYCHIATRY
Resection of infected sacrohysteropexy mesh
Pain in Cancer Survivors: Assess, Monitor, and Ask for Help
SAN DIEGO—As patients with cancer live longer, pain is going to become an even bigger challenge for clinicians, a palliative care specialist told cancer specialists in a presentation at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) in September, and decisions about treatment are becoming more complicated amid the opioid epidemic.
Fortunately, guidelines and clinical experience offer helpful insight into the best practices, said hematologist/oncologist Andrea Ruskin, MD, medical director of palliative care at Veterans Administration (VA) Connecticut Healthcare System (VACHS).
As Ruskin pointed out, two-thirds of newly diagnosed cancer patients are living for at least 5 years, “but with this progress comes challenges.” More than one-third (37%) of cancer survivors report cancer pain, 21% have noncancer pain, and 45% have both. About 5% to 8% of VA cancer survivors use opioids for the long term, she said, although there have been few studies in this population.
Among patients with head and neck cancer, specifically, chronic pain affects 45%, and severe pain affects 11%. Subclinical PTSD, depression, anxiety, and low quality of life are common in this population. “We may cure them, but they have a lot of issues going forward.”
One key strategy is to perform a comprehensive pain assessment at the first visit, and then address pain at every subsequent visit. She recommended a physician resource from the American Society of Clinical Oncology, and a template may be useful to provide helpful questions, Ruskin said.
At VACHS certain questions are routine. “Is pain interfering with your function? Sometimes people say it’s always a 10, but it’s not affecting function at all. Ask if the medicine is working. And how are they taking it? Sometimes they say, ‘I’m taking that for sleep,’ and we say ‘No, Mr. Smith, that is not a sleep medication.’”
Be aware that some patients may use nonmedical opioids, she said. And set expectations early on. “Safe opioid use starts with the very first prescription,” she said. “If I have somebody with myeloma or head and neck cancer, I make it very clear that my goal is that we want you off the opioids after the radiation or once the disease is in remission. I really make an effort at the very beginning to make sure that we're all on the same page.”
As you continue to see a patient, consider ordering urine tests, she said, not as a punitive measure but to make sure you’re offering the safest and most effective treatment. “We don’t do it to say ‘no, no, no.’ We do it for safety and to make sure they’re not getting meds elsewhere.”
What are the best practices when pain doesn’t go away? Should they stay on opioids? According to Ruskin, few evidence-based guidelines address the “more nuanced care” that patients need when their pain lasts for months or years.
But there are useful resources. Ruskin highlighted the National Comprehensive Cancer Network’s survivorship guidelines, and she summarized a few of the available painkiller options. “Opioids are great, and adjuvants are so-so. They work in some people, but we definitely have room for improvement.”
What if patients have persistent opioid use after cancer recovery? “I try to taper if I can, and I try to explain why I’m tapering. It could take months or years to taper patients,” she said. And consider transitioning the patient to buprenorphine, a drug that treats both pain and opioid use disorder, if appropriate. “You don’t need a waiver if you use it for pain. It’s definitely something we’re using more of.”
One important step is to bring in colleagues to help. Psychologists, chiropractors, physical therapists, physiatrists, and pain pharmacists can all be helpful, she said. “Learn about your VA resources and who can partner with you to help these complicated patients. They’re all at your fingertips.”
SAN DIEGO—As patients with cancer live longer, pain is going to become an even bigger challenge for clinicians, a palliative care specialist told cancer specialists in a presentation at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) in September, and decisions about treatment are becoming more complicated amid the opioid epidemic.
Fortunately, guidelines and clinical experience offer helpful insight into the best practices, said hematologist/oncologist Andrea Ruskin, MD, medical director of palliative care at Veterans Administration (VA) Connecticut Healthcare System (VACHS).
As Ruskin pointed out, two-thirds of newly diagnosed cancer patients are living for at least 5 years, “but with this progress comes challenges.” More than one-third (37%) of cancer survivors report cancer pain, 21% have noncancer pain, and 45% have both. About 5% to 8% of VA cancer survivors use opioids for the long term, she said, although there have been few studies in this population.
Among patients with head and neck cancer, specifically, chronic pain affects 45%, and severe pain affects 11%. Subclinical PTSD, depression, anxiety, and low quality of life are common in this population. “We may cure them, but they have a lot of issues going forward.”
One key strategy is to perform a comprehensive pain assessment at the first visit, and then address pain at every subsequent visit. She recommended a physician resource from the American Society of Clinical Oncology, and a template may be useful to provide helpful questions, Ruskin said.
At VACHS certain questions are routine. “Is pain interfering with your function? Sometimes people say it’s always a 10, but it’s not affecting function at all. Ask if the medicine is working. And how are they taking it? Sometimes they say, ‘I’m taking that for sleep,’ and we say ‘No, Mr. Smith, that is not a sleep medication.’”
Be aware that some patients may use nonmedical opioids, she said. And set expectations early on. “Safe opioid use starts with the very first prescription,” she said. “If I have somebody with myeloma or head and neck cancer, I make it very clear that my goal is that we want you off the opioids after the radiation or once the disease is in remission. I really make an effort at the very beginning to make sure that we're all on the same page.”
As you continue to see a patient, consider ordering urine tests, she said, not as a punitive measure but to make sure you’re offering the safest and most effective treatment. “We don’t do it to say ‘no, no, no.’ We do it for safety and to make sure they’re not getting meds elsewhere.”
What are the best practices when pain doesn’t go away? Should they stay on opioids? According to Ruskin, few evidence-based guidelines address the “more nuanced care” that patients need when their pain lasts for months or years.
But there are useful resources. Ruskin highlighted the National Comprehensive Cancer Network’s survivorship guidelines, and she summarized a few of the available painkiller options. “Opioids are great, and adjuvants are so-so. They work in some people, but we definitely have room for improvement.”
What if patients have persistent opioid use after cancer recovery? “I try to taper if I can, and I try to explain why I’m tapering. It could take months or years to taper patients,” she said. And consider transitioning the patient to buprenorphine, a drug that treats both pain and opioid use disorder, if appropriate. “You don’t need a waiver if you use it for pain. It’s definitely something we’re using more of.”
One important step is to bring in colleagues to help. Psychologists, chiropractors, physical therapists, physiatrists, and pain pharmacists can all be helpful, she said. “Learn about your VA resources and who can partner with you to help these complicated patients. They’re all at your fingertips.”
SAN DIEGO—As patients with cancer live longer, pain is going to become an even bigger challenge for clinicians, a palliative care specialist told cancer specialists in a presentation at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) in September, and decisions about treatment are becoming more complicated amid the opioid epidemic.
Fortunately, guidelines and clinical experience offer helpful insight into the best practices, said hematologist/oncologist Andrea Ruskin, MD, medical director of palliative care at Veterans Administration (VA) Connecticut Healthcare System (VACHS).
As Ruskin pointed out, two-thirds of newly diagnosed cancer patients are living for at least 5 years, “but with this progress comes challenges.” More than one-third (37%) of cancer survivors report cancer pain, 21% have noncancer pain, and 45% have both. About 5% to 8% of VA cancer survivors use opioids for the long term, she said, although there have been few studies in this population.
Among patients with head and neck cancer, specifically, chronic pain affects 45%, and severe pain affects 11%. Subclinical PTSD, depression, anxiety, and low quality of life are common in this population. “We may cure them, but they have a lot of issues going forward.”
One key strategy is to perform a comprehensive pain assessment at the first visit, and then address pain at every subsequent visit. She recommended a physician resource from the American Society of Clinical Oncology, and a template may be useful to provide helpful questions, Ruskin said.
At VACHS certain questions are routine. “Is pain interfering with your function? Sometimes people say it’s always a 10, but it’s not affecting function at all. Ask if the medicine is working. And how are they taking it? Sometimes they say, ‘I’m taking that for sleep,’ and we say ‘No, Mr. Smith, that is not a sleep medication.’”
Be aware that some patients may use nonmedical opioids, she said. And set expectations early on. “Safe opioid use starts with the very first prescription,” she said. “If I have somebody with myeloma or head and neck cancer, I make it very clear that my goal is that we want you off the opioids after the radiation or once the disease is in remission. I really make an effort at the very beginning to make sure that we're all on the same page.”
As you continue to see a patient, consider ordering urine tests, she said, not as a punitive measure but to make sure you’re offering the safest and most effective treatment. “We don’t do it to say ‘no, no, no.’ We do it for safety and to make sure they’re not getting meds elsewhere.”
What are the best practices when pain doesn’t go away? Should they stay on opioids? According to Ruskin, few evidence-based guidelines address the “more nuanced care” that patients need when their pain lasts for months or years.
But there are useful resources. Ruskin highlighted the National Comprehensive Cancer Network’s survivorship guidelines, and she summarized a few of the available painkiller options. “Opioids are great, and adjuvants are so-so. They work in some people, but we definitely have room for improvement.”
What if patients have persistent opioid use after cancer recovery? “I try to taper if I can, and I try to explain why I’m tapering. It could take months or years to taper patients,” she said. And consider transitioning the patient to buprenorphine, a drug that treats both pain and opioid use disorder, if appropriate. “You don’t need a waiver if you use it for pain. It’s definitely something we’re using more of.”
One important step is to bring in colleagues to help. Psychologists, chiropractors, physical therapists, physiatrists, and pain pharmacists can all be helpful, she said. “Learn about your VA resources and who can partner with you to help these complicated patients. They’re all at your fingertips.”
HCC surveillance screening increased slightly with invitations, reminders
Mailing invitations for hepatocellular carcinoma (HCC) surveillance screening to patients with cirrhosis increased ultrasound uptake by 13 percentage points, but the majority of patients still did not receive the recommended semiannual screenings, according to findings published in Clinical Gastroenterology and Hepatology.
“These data highlight the need for more intensive interventions to further increase surveillance,” wrote Amit Singal, MD, of University of Texas Southwestern Medical Center and Parkland Health Hospital System in Dallas, and colleagues. “The underuse of HCC surveillance has been attributed to a combination of patient- and provider-level barriers, which can serve as future additional intervention targets.” These include transportation and financial barriers and possibly new blood-based screening modalities when they become available, thereby removing the need for a separate ultrasound appointment.
According to one study, more than 90% of hepatocellular carcinoma cases occur in people with chronic liver disease, and the cancer is a leading cause of death in those with compensated cirrhosis. Multiple medical associations therefore recommend an abdominal ultrasound every 6 months with or without alpha-fetoprotein (AFP) for surveillance in at-risk patients, including anyone with cirrhosis of any kind, but too few patients receive these surveillance ultrasounds, the authors write.
The researchers therefore conducted a pragmatic randomized clinical trial from March 2018 to September 2019 to compare surveillance ultrasound uptake for two groups of people with cirrhosis: 1,436 people who were mailed invitations to get a surveillance ultrasound and 1,436 people who received usual care, with surveillance recommended only at usual visits. The patients all received care at one of three health systems: a tertiary care referral center, a safety net health system, and a Veterans Affairs medical center. The primary outcome was semiannual surveillance in the patients over 1 year.
The researchers identified patients using ICD-9 and ICD-10 codes for cirrhosis and cirrhosis complications, as well as those with suspected but undocumented cirrhosis based on electronic medical record notes such as an elevated Fibrosis-4 index. They confirmed the diagnoses with chart review, confirmed that the patients had at least one outpatient visit in the previous year, and excluded those in whom surveillance is not recommended, who lacked contact information, or who spoke a language besides English or Spanish.
The mailing was a one-page letter in English and Spanish, written at a low literacy level, that explained hepatocellular carcinoma risk and recommended surveillance. Those who didn’t respond to the mailed invitation within 2 weeks received a reminder call to undergo surveillance, and those who scheduled an ultrasound received a reminder call about a week before the visit. Primary and subspecialty providers were blinded to the patients’ study arm assignments.
“We conducted the study as a pragmatic trial whereby patients in either arm could also be offered HCC surveillance by primary or specialty care providers during clinic visits,” the researchers wrote. “The frequency of the clinic visits and provider discussions regarding HCC surveillance were conducted per usual care and not dictated by the study protocol.”
Two-thirds of the patients (67.7%) were men, with a median age of 61.2 years. Just over a third (37.0%) were white, 31.9% were Hispanic, and 27.6% were Black. More than half the patients had hepatitis C (56.4%), 18.1% had alcohol-related liver disease, 14.5% had nonalcoholic fatty liver disease, and 2.4% had hepatitis B. Most of the patients had compensated cirrhosis, including 36.7% with ascites and 17.1% with hepatic encephalopathy.
Nearly a quarter of the patients in the outreach arm (23%) could not be contacted or lacked working phone numbers, but they remained in the intent-to-screen analysis. Just over a third of the patients who received mailed outreach (35.1%; 95% confidence interval, 32.6%-37.6%) received semiannual surveillance, compared to 21.9% (95% CI, 19.8%-24.2%) of the usual-care patients. The increased surveillance in the outreach group applied to most subgroups, including race/ethnicity and cirrhosis severity based on the Child-Turcotte-Pugh class.
“However, we observed site-level differences in the intervention effect, with significant increases in semiannual surveillance at the VA and safety net health systems (both P < .001) but not at the tertiary care referral center (P = .52),” the authors wrote. “In a post hoc subgroup analysis among patients with at least 1 primary care or gastroenterology outpatient visit during the study period, mailed outreach continued to increase semiannual surveillance, compared with usual care (46.8% vs. 32.7%; P < .001).”
Despite the improved rates from the intervention, the majority of patients still did not receive semiannual surveillance across all three sites, and almost 30% underwent no surveillance the entire year.
The research was funded by the National Cancer Institute, the Cancer Prevention Research Institute of Texas, and the Center for Innovations in Quality, Effectiveness and Safety. Dr. Singal has consulted for or served on the advisory boards of Bayer, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, and GRAIL. The other authors had no industry disclosures.
Hepatocellular carcinoma is a deadly cancer that is usually incurable unless detected at an early stage through regular surveillance. Current American guidelines support 6-monthly abdominal ultrasonography, with or without serum alpha-fetoprotein, for HCC surveillance in at-risk patients, such as those with cirrhosis. However, even in such a high-risk group, the uptake of and adherence to surveillance are far from satisfactory. This study by Dr. Singal and colleagues is therefore important and practical. Randomized controlled trials in HCC surveillance are rare. The authors clearly demonstrate that an outreach program comprising mail invitations followed by phone contacts if there was no response could increase the surveillance uptake by more than 10%.
None of these can work if chronic liver disease and cirrhosis are not diagnosed in the first place. Disease awareness, access to care (and racial discrepancies), and clinical care pathways are hurdles we need to overcome in order to make an impact on HCC mortality at the population level.
Vincent Wong, MD, is an academic hepatologist at the Chinese University of Hong Kong. He does not have relevant conflicts of interest in this article.
Hepatocellular carcinoma is a deadly cancer that is usually incurable unless detected at an early stage through regular surveillance. Current American guidelines support 6-monthly abdominal ultrasonography, with or without serum alpha-fetoprotein, for HCC surveillance in at-risk patients, such as those with cirrhosis. However, even in such a high-risk group, the uptake of and adherence to surveillance are far from satisfactory. This study by Dr. Singal and colleagues is therefore important and practical. Randomized controlled trials in HCC surveillance are rare. The authors clearly demonstrate that an outreach program comprising mail invitations followed by phone contacts if there was no response could increase the surveillance uptake by more than 10%.
None of these can work if chronic liver disease and cirrhosis are not diagnosed in the first place. Disease awareness, access to care (and racial discrepancies), and clinical care pathways are hurdles we need to overcome in order to make an impact on HCC mortality at the population level.
Vincent Wong, MD, is an academic hepatologist at the Chinese University of Hong Kong. He does not have relevant conflicts of interest in this article.
Hepatocellular carcinoma is a deadly cancer that is usually incurable unless detected at an early stage through regular surveillance. Current American guidelines support 6-monthly abdominal ultrasonography, with or without serum alpha-fetoprotein, for HCC surveillance in at-risk patients, such as those with cirrhosis. However, even in such a high-risk group, the uptake of and adherence to surveillance are far from satisfactory. This study by Dr. Singal and colleagues is therefore important and practical. Randomized controlled trials in HCC surveillance are rare. The authors clearly demonstrate that an outreach program comprising mail invitations followed by phone contacts if there was no response could increase the surveillance uptake by more than 10%.
None of these can work if chronic liver disease and cirrhosis are not diagnosed in the first place. Disease awareness, access to care (and racial discrepancies), and clinical care pathways are hurdles we need to overcome in order to make an impact on HCC mortality at the population level.
Vincent Wong, MD, is an academic hepatologist at the Chinese University of Hong Kong. He does not have relevant conflicts of interest in this article.
Mailing invitations for hepatocellular carcinoma (HCC) surveillance screening to patients with cirrhosis increased ultrasound uptake by 13 percentage points, but the majority of patients still did not receive the recommended semiannual screenings, according to findings published in Clinical Gastroenterology and Hepatology.
“These data highlight the need for more intensive interventions to further increase surveillance,” wrote Amit Singal, MD, of University of Texas Southwestern Medical Center and Parkland Health Hospital System in Dallas, and colleagues. “The underuse of HCC surveillance has been attributed to a combination of patient- and provider-level barriers, which can serve as future additional intervention targets.” These include transportation and financial barriers and possibly new blood-based screening modalities when they become available, thereby removing the need for a separate ultrasound appointment.
According to one study, more than 90% of hepatocellular carcinoma cases occur in people with chronic liver disease, and the cancer is a leading cause of death in those with compensated cirrhosis. Multiple medical associations therefore recommend an abdominal ultrasound every 6 months with or without alpha-fetoprotein (AFP) for surveillance in at-risk patients, including anyone with cirrhosis of any kind, but too few patients receive these surveillance ultrasounds, the authors write.
The researchers therefore conducted a pragmatic randomized clinical trial from March 2018 to September 2019 to compare surveillance ultrasound uptake for two groups of people with cirrhosis: 1,436 people who were mailed invitations to get a surveillance ultrasound and 1,436 people who received usual care, with surveillance recommended only at usual visits. The patients all received care at one of three health systems: a tertiary care referral center, a safety net health system, and a Veterans Affairs medical center. The primary outcome was semiannual surveillance in the patients over 1 year.
The researchers identified patients using ICD-9 and ICD-10 codes for cirrhosis and cirrhosis complications, as well as those with suspected but undocumented cirrhosis based on electronic medical record notes such as an elevated Fibrosis-4 index. They confirmed the diagnoses with chart review, confirmed that the patients had at least one outpatient visit in the previous year, and excluded those in whom surveillance is not recommended, who lacked contact information, or who spoke a language besides English or Spanish.
The mailing was a one-page letter in English and Spanish, written at a low literacy level, that explained hepatocellular carcinoma risk and recommended surveillance. Those who didn’t respond to the mailed invitation within 2 weeks received a reminder call to undergo surveillance, and those who scheduled an ultrasound received a reminder call about a week before the visit. Primary and subspecialty providers were blinded to the patients’ study arm assignments.
“We conducted the study as a pragmatic trial whereby patients in either arm could also be offered HCC surveillance by primary or specialty care providers during clinic visits,” the researchers wrote. “The frequency of the clinic visits and provider discussions regarding HCC surveillance were conducted per usual care and not dictated by the study protocol.”
Two-thirds of the patients (67.7%) were men, with a median age of 61.2 years. Just over a third (37.0%) were white, 31.9% were Hispanic, and 27.6% were Black. More than half the patients had hepatitis C (56.4%), 18.1% had alcohol-related liver disease, 14.5% had nonalcoholic fatty liver disease, and 2.4% had hepatitis B. Most of the patients had compensated cirrhosis, including 36.7% with ascites and 17.1% with hepatic encephalopathy.
Nearly a quarter of the patients in the outreach arm (23%) could not be contacted or lacked working phone numbers, but they remained in the intent-to-screen analysis. Just over a third of the patients who received mailed outreach (35.1%; 95% confidence interval, 32.6%-37.6%) received semiannual surveillance, compared to 21.9% (95% CI, 19.8%-24.2%) of the usual-care patients. The increased surveillance in the outreach group applied to most subgroups, including race/ethnicity and cirrhosis severity based on the Child-Turcotte-Pugh class.
“However, we observed site-level differences in the intervention effect, with significant increases in semiannual surveillance at the VA and safety net health systems (both P < .001) but not at the tertiary care referral center (P = .52),” the authors wrote. “In a post hoc subgroup analysis among patients with at least 1 primary care or gastroenterology outpatient visit during the study period, mailed outreach continued to increase semiannual surveillance, compared with usual care (46.8% vs. 32.7%; P < .001).”
Despite the improved rates from the intervention, the majority of patients still did not receive semiannual surveillance across all three sites, and almost 30% underwent no surveillance the entire year.
The research was funded by the National Cancer Institute, the Cancer Prevention Research Institute of Texas, and the Center for Innovations in Quality, Effectiveness and Safety. Dr. Singal has consulted for or served on the advisory boards of Bayer, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, and GRAIL. The other authors had no industry disclosures.
Mailing invitations for hepatocellular carcinoma (HCC) surveillance screening to patients with cirrhosis increased ultrasound uptake by 13 percentage points, but the majority of patients still did not receive the recommended semiannual screenings, according to findings published in Clinical Gastroenterology and Hepatology.
“These data highlight the need for more intensive interventions to further increase surveillance,” wrote Amit Singal, MD, of University of Texas Southwestern Medical Center and Parkland Health Hospital System in Dallas, and colleagues. “The underuse of HCC surveillance has been attributed to a combination of patient- and provider-level barriers, which can serve as future additional intervention targets.” These include transportation and financial barriers and possibly new blood-based screening modalities when they become available, thereby removing the need for a separate ultrasound appointment.
According to one study, more than 90% of hepatocellular carcinoma cases occur in people with chronic liver disease, and the cancer is a leading cause of death in those with compensated cirrhosis. Multiple medical associations therefore recommend an abdominal ultrasound every 6 months with or without alpha-fetoprotein (AFP) for surveillance in at-risk patients, including anyone with cirrhosis of any kind, but too few patients receive these surveillance ultrasounds, the authors write.
The researchers therefore conducted a pragmatic randomized clinical trial from March 2018 to September 2019 to compare surveillance ultrasound uptake for two groups of people with cirrhosis: 1,436 people who were mailed invitations to get a surveillance ultrasound and 1,436 people who received usual care, with surveillance recommended only at usual visits. The patients all received care at one of three health systems: a tertiary care referral center, a safety net health system, and a Veterans Affairs medical center. The primary outcome was semiannual surveillance in the patients over 1 year.
The researchers identified patients using ICD-9 and ICD-10 codes for cirrhosis and cirrhosis complications, as well as those with suspected but undocumented cirrhosis based on electronic medical record notes such as an elevated Fibrosis-4 index. They confirmed the diagnoses with chart review, confirmed that the patients had at least one outpatient visit in the previous year, and excluded those in whom surveillance is not recommended, who lacked contact information, or who spoke a language besides English or Spanish.
The mailing was a one-page letter in English and Spanish, written at a low literacy level, that explained hepatocellular carcinoma risk and recommended surveillance. Those who didn’t respond to the mailed invitation within 2 weeks received a reminder call to undergo surveillance, and those who scheduled an ultrasound received a reminder call about a week before the visit. Primary and subspecialty providers were blinded to the patients’ study arm assignments.
“We conducted the study as a pragmatic trial whereby patients in either arm could also be offered HCC surveillance by primary or specialty care providers during clinic visits,” the researchers wrote. “The frequency of the clinic visits and provider discussions regarding HCC surveillance were conducted per usual care and not dictated by the study protocol.”
Two-thirds of the patients (67.7%) were men, with a median age of 61.2 years. Just over a third (37.0%) were white, 31.9% were Hispanic, and 27.6% were Black. More than half the patients had hepatitis C (56.4%), 18.1% had alcohol-related liver disease, 14.5% had nonalcoholic fatty liver disease, and 2.4% had hepatitis B. Most of the patients had compensated cirrhosis, including 36.7% with ascites and 17.1% with hepatic encephalopathy.
Nearly a quarter of the patients in the outreach arm (23%) could not be contacted or lacked working phone numbers, but they remained in the intent-to-screen analysis. Just over a third of the patients who received mailed outreach (35.1%; 95% confidence interval, 32.6%-37.6%) received semiannual surveillance, compared to 21.9% (95% CI, 19.8%-24.2%) of the usual-care patients. The increased surveillance in the outreach group applied to most subgroups, including race/ethnicity and cirrhosis severity based on the Child-Turcotte-Pugh class.
“However, we observed site-level differences in the intervention effect, with significant increases in semiannual surveillance at the VA and safety net health systems (both P < .001) but not at the tertiary care referral center (P = .52),” the authors wrote. “In a post hoc subgroup analysis among patients with at least 1 primary care or gastroenterology outpatient visit during the study period, mailed outreach continued to increase semiannual surveillance, compared with usual care (46.8% vs. 32.7%; P < .001).”
Despite the improved rates from the intervention, the majority of patients still did not receive semiannual surveillance across all three sites, and almost 30% underwent no surveillance the entire year.
The research was funded by the National Cancer Institute, the Cancer Prevention Research Institute of Texas, and the Center for Innovations in Quality, Effectiveness and Safety. Dr. Singal has consulted for or served on the advisory boards of Bayer, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, and GRAIL. The other authors had no industry disclosures.
REPORTING FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Should health care be a right?
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Sex-linked IL-22 activity may affect NAFLD outcomes
Interleukin-22 may mitigate nonalcoholic fatty liver disease (NAFLD)–related fibrosis in females but not males, suggesting a sex-linked hepatoprotective pathway, according to investigators.
These differences between men and women should be considered when conducting clinical trials for IL-22–targeting therapies, reported lead author Mohamed N. Abdelnabi, MSc, of the Centre de Recherche du Centre Hospitalier de l’Université de Montréal and colleagues.
“IL-22 is a pleiotropic cytokine with both inflammatory and protective effects during injury and repair in various tissues including the liver,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, noting that IL-22 activity has been linked with both antifibrotic and profibrotic outcomes in previous preclinical studies. “These different observations highlight the dual nature of IL-22 that likely is dictated by multiple factors including the tissue involved, pathologic environment, endogenous vs. exogenous IL-22 level, and the time of exposure.”
Prior research has left some questions unanswered, the investigators noted, because many studies have relied on exogenous administration of IL-22 in mouse models, some of which lack all the metabolic abnormalities observed in human disease. Furthermore, these mice were all male, which has prevented detection of possible sex-linked differences in IL-22–related pathophysiology, they added.
To address these gaps, the investigators conducted a series of experiments involving men and women with NAFLD, plus mice of both sexes with NAFLD induced by a high-fat diet, both wild-type and with knock-out of the IL-22 receptor.
Human data
To characterize IL-22 activity in men versus women with NAFLD, the investigators first analyzed two publicly available microarray datasets. These revealed notably increased expression of hepatic IL-22 mRNA in the livers of females compared with males. Supporting this finding, liver biopsies from 11 men and 9 women with NAFLD with similar levels of fibrosis showed significantly increased IL-22–producing cells in female patients compared with male patients.
“These results suggest a sexual dimorphic expression of IL-22 in the context of NAFLD,” the investigators wrote.
Mouse data
Echoing the human data, the livers of female wild-type mice with NAFLD had significantly greater IL-22 expression than male mice at both mRNA and protein levels.
Next, the investigators explored the effects of IL-22–receptor knockout. In addition to NAFLD, these knockout mice developed weight gain and metabolic alterations, especially insulin resistance, supporting previous work that highlighted the protective role of IL-22 against these outcomes. More relevant to the present study, female knockout mice had significantly worse hepatic liver injury, apoptosis, inflammation, and fibrosis than male knockout mice, suggesting that IL-22 signaling confers hepatoprotection in females but not males.
“These observations may suggest a regulation of IL-22 expression by the female sex hormone estrogen,” the investigators wrote. “Indeed, estrogen is known to modulate inflammatory responses in NAFLD, but the underlying mechanisms remain undefined. ... Further in vivo studies are warranted to investigate whether endogenous estrogen regulates hepatic IL-22 expression in the context of NAFLD.”
In the meantime, the present data may steer drug development.
“These findings should be considered in clinical trials testing IL-22–based therapeutic approaches in treatment of female vs. male subjects with NAFLD,” the investigators concluded.
The study was partially funded by the Canadian Liver Foundation and the Canadian Institutes of Health Research, the Bourse d’Exemption des Droits de Scolarité Supplémentaires from the Université de Montréal, the Canadian Network on Hepatitis, and others. The investigators disclosed no competing interests.
The cytokine interleukin-22 has potential as a therapeutic for nonalcoholic fatty liver disease, as it has been shown to decrease fat accumulation in hepatocytes and has various other liver protective effects such as prevention of cell death, enhancement of proliferation, and, importantly, reduction of liver fibrosis progression. Indeed, a recombinant derivative of IL22 has been studied in a clinical trial of alcoholic liver disease and has been found to be safe. However, the beneficial effect of this cytokine is context dependent. High levels of IL22 increased inflammation or fibrosis in hepatitis B infection and in toxic injury models in mouse models.
This is in line with observations that progression to cirrhosis in NAFLD is greater after menopause. On the other hand, women are more likely to develop cirrhosis than men despite higher levels of IL22, indicating more factors are at play in the progression of NAFLD. Overall, this report should alert investigators to consider the sex-specific effects of emerging therapies for NAFLD. Future IL22-based trials must include sex-based subgroup analyses.
Kirk Wangensteen, MD, PhD, is with the department of medicine, division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. He declares no relevant conflicts of interest.
The cytokine interleukin-22 has potential as a therapeutic for nonalcoholic fatty liver disease, as it has been shown to decrease fat accumulation in hepatocytes and has various other liver protective effects such as prevention of cell death, enhancement of proliferation, and, importantly, reduction of liver fibrosis progression. Indeed, a recombinant derivative of IL22 has been studied in a clinical trial of alcoholic liver disease and has been found to be safe. However, the beneficial effect of this cytokine is context dependent. High levels of IL22 increased inflammation or fibrosis in hepatitis B infection and in toxic injury models in mouse models.
This is in line with observations that progression to cirrhosis in NAFLD is greater after menopause. On the other hand, women are more likely to develop cirrhosis than men despite higher levels of IL22, indicating more factors are at play in the progression of NAFLD. Overall, this report should alert investigators to consider the sex-specific effects of emerging therapies for NAFLD. Future IL22-based trials must include sex-based subgroup analyses.
Kirk Wangensteen, MD, PhD, is with the department of medicine, division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. He declares no relevant conflicts of interest.
The cytokine interleukin-22 has potential as a therapeutic for nonalcoholic fatty liver disease, as it has been shown to decrease fat accumulation in hepatocytes and has various other liver protective effects such as prevention of cell death, enhancement of proliferation, and, importantly, reduction of liver fibrosis progression. Indeed, a recombinant derivative of IL22 has been studied in a clinical trial of alcoholic liver disease and has been found to be safe. However, the beneficial effect of this cytokine is context dependent. High levels of IL22 increased inflammation or fibrosis in hepatitis B infection and in toxic injury models in mouse models.
This is in line with observations that progression to cirrhosis in NAFLD is greater after menopause. On the other hand, women are more likely to develop cirrhosis than men despite higher levels of IL22, indicating more factors are at play in the progression of NAFLD. Overall, this report should alert investigators to consider the sex-specific effects of emerging therapies for NAFLD. Future IL22-based trials must include sex-based subgroup analyses.
Kirk Wangensteen, MD, PhD, is with the department of medicine, division of gastroenterology and hepatology at the Mayo Clinic in Rochester, Minn. He declares no relevant conflicts of interest.
Interleukin-22 may mitigate nonalcoholic fatty liver disease (NAFLD)–related fibrosis in females but not males, suggesting a sex-linked hepatoprotective pathway, according to investigators.
These differences between men and women should be considered when conducting clinical trials for IL-22–targeting therapies, reported lead author Mohamed N. Abdelnabi, MSc, of the Centre de Recherche du Centre Hospitalier de l’Université de Montréal and colleagues.
“IL-22 is a pleiotropic cytokine with both inflammatory and protective effects during injury and repair in various tissues including the liver,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, noting that IL-22 activity has been linked with both antifibrotic and profibrotic outcomes in previous preclinical studies. “These different observations highlight the dual nature of IL-22 that likely is dictated by multiple factors including the tissue involved, pathologic environment, endogenous vs. exogenous IL-22 level, and the time of exposure.”
Prior research has left some questions unanswered, the investigators noted, because many studies have relied on exogenous administration of IL-22 in mouse models, some of which lack all the metabolic abnormalities observed in human disease. Furthermore, these mice were all male, which has prevented detection of possible sex-linked differences in IL-22–related pathophysiology, they added.
To address these gaps, the investigators conducted a series of experiments involving men and women with NAFLD, plus mice of both sexes with NAFLD induced by a high-fat diet, both wild-type and with knock-out of the IL-22 receptor.
Human data
To characterize IL-22 activity in men versus women with NAFLD, the investigators first analyzed two publicly available microarray datasets. These revealed notably increased expression of hepatic IL-22 mRNA in the livers of females compared with males. Supporting this finding, liver biopsies from 11 men and 9 women with NAFLD with similar levels of fibrosis showed significantly increased IL-22–producing cells in female patients compared with male patients.
“These results suggest a sexual dimorphic expression of IL-22 in the context of NAFLD,” the investigators wrote.
Mouse data
Echoing the human data, the livers of female wild-type mice with NAFLD had significantly greater IL-22 expression than male mice at both mRNA and protein levels.
Next, the investigators explored the effects of IL-22–receptor knockout. In addition to NAFLD, these knockout mice developed weight gain and metabolic alterations, especially insulin resistance, supporting previous work that highlighted the protective role of IL-22 against these outcomes. More relevant to the present study, female knockout mice had significantly worse hepatic liver injury, apoptosis, inflammation, and fibrosis than male knockout mice, suggesting that IL-22 signaling confers hepatoprotection in females but not males.
“These observations may suggest a regulation of IL-22 expression by the female sex hormone estrogen,” the investigators wrote. “Indeed, estrogen is known to modulate inflammatory responses in NAFLD, but the underlying mechanisms remain undefined. ... Further in vivo studies are warranted to investigate whether endogenous estrogen regulates hepatic IL-22 expression in the context of NAFLD.”
In the meantime, the present data may steer drug development.
“These findings should be considered in clinical trials testing IL-22–based therapeutic approaches in treatment of female vs. male subjects with NAFLD,” the investigators concluded.
The study was partially funded by the Canadian Liver Foundation and the Canadian Institutes of Health Research, the Bourse d’Exemption des Droits de Scolarité Supplémentaires from the Université de Montréal, the Canadian Network on Hepatitis, and others. The investigators disclosed no competing interests.
Interleukin-22 may mitigate nonalcoholic fatty liver disease (NAFLD)–related fibrosis in females but not males, suggesting a sex-linked hepatoprotective pathway, according to investigators.
These differences between men and women should be considered when conducting clinical trials for IL-22–targeting therapies, reported lead author Mohamed N. Abdelnabi, MSc, of the Centre de Recherche du Centre Hospitalier de l’Université de Montréal and colleagues.
“IL-22 is a pleiotropic cytokine with both inflammatory and protective effects during injury and repair in various tissues including the liver,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, noting that IL-22 activity has been linked with both antifibrotic and profibrotic outcomes in previous preclinical studies. “These different observations highlight the dual nature of IL-22 that likely is dictated by multiple factors including the tissue involved, pathologic environment, endogenous vs. exogenous IL-22 level, and the time of exposure.”
Prior research has left some questions unanswered, the investigators noted, because many studies have relied on exogenous administration of IL-22 in mouse models, some of which lack all the metabolic abnormalities observed in human disease. Furthermore, these mice were all male, which has prevented detection of possible sex-linked differences in IL-22–related pathophysiology, they added.
To address these gaps, the investigators conducted a series of experiments involving men and women with NAFLD, plus mice of both sexes with NAFLD induced by a high-fat diet, both wild-type and with knock-out of the IL-22 receptor.
Human data
To characterize IL-22 activity in men versus women with NAFLD, the investigators first analyzed two publicly available microarray datasets. These revealed notably increased expression of hepatic IL-22 mRNA in the livers of females compared with males. Supporting this finding, liver biopsies from 11 men and 9 women with NAFLD with similar levels of fibrosis showed significantly increased IL-22–producing cells in female patients compared with male patients.
“These results suggest a sexual dimorphic expression of IL-22 in the context of NAFLD,” the investigators wrote.
Mouse data
Echoing the human data, the livers of female wild-type mice with NAFLD had significantly greater IL-22 expression than male mice at both mRNA and protein levels.
Next, the investigators explored the effects of IL-22–receptor knockout. In addition to NAFLD, these knockout mice developed weight gain and metabolic alterations, especially insulin resistance, supporting previous work that highlighted the protective role of IL-22 against these outcomes. More relevant to the present study, female knockout mice had significantly worse hepatic liver injury, apoptosis, inflammation, and fibrosis than male knockout mice, suggesting that IL-22 signaling confers hepatoprotection in females but not males.
“These observations may suggest a regulation of IL-22 expression by the female sex hormone estrogen,” the investigators wrote. “Indeed, estrogen is known to modulate inflammatory responses in NAFLD, but the underlying mechanisms remain undefined. ... Further in vivo studies are warranted to investigate whether endogenous estrogen regulates hepatic IL-22 expression in the context of NAFLD.”
In the meantime, the present data may steer drug development.
“These findings should be considered in clinical trials testing IL-22–based therapeutic approaches in treatment of female vs. male subjects with NAFLD,” the investigators concluded.
The study was partially funded by the Canadian Liver Foundation and the Canadian Institutes of Health Research, the Bourse d’Exemption des Droits de Scolarité Supplémentaires from the Université de Montréal, the Canadian Network on Hepatitis, and others. The investigators disclosed no competing interests.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Children and COVID: Weekly cases can’t sustain downward trend
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.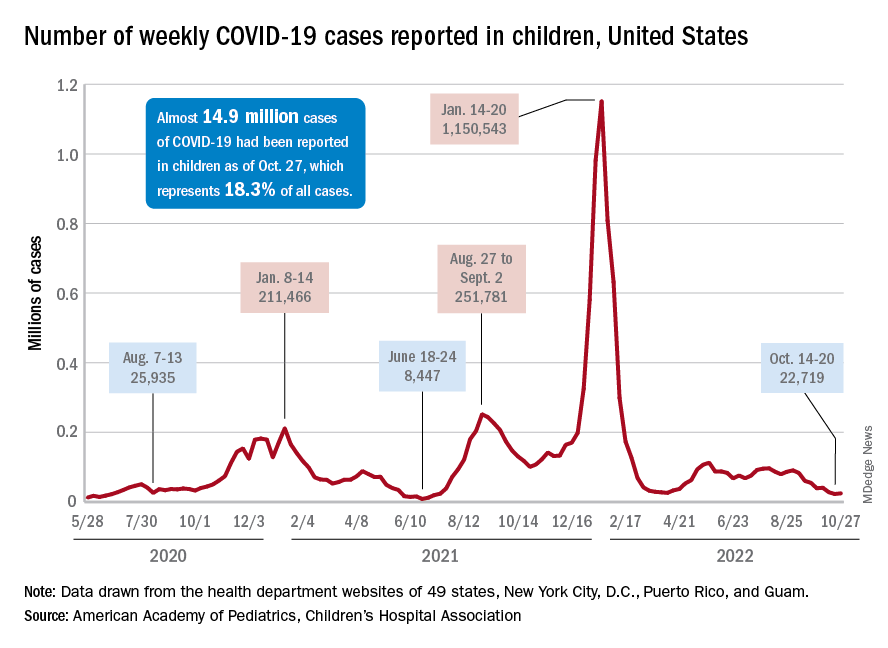
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
Oral FMT on par with colonic FMT for recurrent C. difficile
A real-world analysis confirms that fecal microbiota transplantation (FMT) is highly effective for recurrent Clostridioides difficile infection (rCDI) – and there is no difference between delivery by capsule (cap-FMT) and colonoscopy (colo-FMT).
“We present one of the largest cohorts involving people who received capsule FMT. Byron Vaughn, MD, with the division of gastroenterology, hepatology, and nutrition, University of Minnesota, Minneapolis, said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
The Food and Drug Administration allows FMT to be used for patients who have failed standard treatment for rCDI under a policy of enforcement discretion.
The past decade has seen an increase in the use of FMT in clinical practice, owing to an increase in cases of rCDI after failure of standard antibiotic therapy.
Unlike antibiotics, which perpetuate and worsen intestinal dysbiosis, FMT restores the diversity and function of host microbiota, effectively breaking the cycle of rCDI, the authors of the study noted. But it’s been unclear whether the efficacy and safety of FMT vary by route of administration.
Effective without procedural risks
To investigate, Dr. Vaughn and colleagues evaluated clinical outcomes and adverse events in 170 patients with rCDI who underwent cap-FMT and 96 peers who underwent colo-FMT.
FMT was performed using one of two standardized formulations of microbiota manufactured by the University of Minnesota microbiota therapeutics program: freeze-dried/encapsulated or frozen-thawed/liquid.
Overall, the cure rates of CDI were 86% at 1 month and 81% at 2 months. There was no statistically significant difference at either time between cap-FMT and colo-FMT.
The 1-month cure rate was 84% with cap-FMT and 91% with colo-FMT; at 2 months, the cure rates were 81% and 83%, respectively.
Cap-FMT has a safety and effectiveness profile similar to that of colo-FMT, without the procedural risks of colonoscopy, the researchers concluded.
They cautioned that, although FMT is highly effective overall, patient selection is a key factor to optimizing FMT success.
Older age and hemodialysis were associated with FMT failure by 2 months on multivariate logistic regression.
“These risk factors can help determine if a patient should receive FMT or an alternative therapy for rCDI. This is not to say FMT should be avoided in older patients or those on dialysis, but clinicians should be aware of these associations in light of other options for rCDI,” Dr. Vaughn said.
Confirming prior studies, antibiotic use after FMT was a major factor in its failure. Patient selection for FMT should include an assessment of the potential need for antibiotics after transplant, the researchers noted.
One serious adverse event (aspiration pneumonia) was related to colonoscopy; otherwise, no new safety signals were identified.
As reported in other studies, changes in bowel function, including diarrhea, constipation, gas, and bloating were common, although it’s tough to disentangle gastrointestinal symptoms related to FMT from those after CDI, the researchers said. Importantly, no transmission of an infectious agent related to FMT was identified.
Two good options
The researchers said their findings are “highly generalizable” because the population reflects all FMT use by participating institutions and contains a mix of academic centers and private practices.
Many patients included in the study would not have been eligible for a clinical trial, owing to their having many comorbid conditions, including immune compromise and inflammatory bowel disease, the authors noted.
“FMT is recommended by major gastroenterology and infectious disease society guidelines,” Dr. Vaughn said. “Our group, and others, have consistently found strategies that incorporate FMT as cost-effective strategies for treating rCDI.”
However, lack of access to FMT products often is a barrier to treatment, he said.
“A stool banking model, similar to the nonprofit blood banking model, may be a useful solution to ensure equitable access to FMT to all who need it,” Dr. Vaughn added.
Reached for comment, Majdi Osman, MD, MPH, told this news organization that the study is valuable, “as it nicely shows in a real-world setting that capsules and colonoscopy are good options for patients who need this.”
Dr. Osman is chief medical officer of OpenBiome, a nonprofit organization that operates a public stool bank and is the major FMT source in the United States. The organization has provided over 63,000 FMT treatments to over 1,200 hospitals in the United States.
“FMT has become standard of care for patients who failed antibiotic therapy, and certainly is being used widely as a treatment option for these patients who have often run out of existing options,” Dr. Osman said.
Support for the study was provided by a donation from Achieving Cures Together, a nonprofit organization dedicated to advancing microbiome-based research. Dr. Vaughn receives grant support from Takeda, Roche, Celgene, and Diasorin and has received consulting fees from Prometheus and AbbVie. Dr. Osman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A real-world analysis confirms that fecal microbiota transplantation (FMT) is highly effective for recurrent Clostridioides difficile infection (rCDI) – and there is no difference between delivery by capsule (cap-FMT) and colonoscopy (colo-FMT).
“We present one of the largest cohorts involving people who received capsule FMT. Byron Vaughn, MD, with the division of gastroenterology, hepatology, and nutrition, University of Minnesota, Minneapolis, said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
The Food and Drug Administration allows FMT to be used for patients who have failed standard treatment for rCDI under a policy of enforcement discretion.
The past decade has seen an increase in the use of FMT in clinical practice, owing to an increase in cases of rCDI after failure of standard antibiotic therapy.
Unlike antibiotics, which perpetuate and worsen intestinal dysbiosis, FMT restores the diversity and function of host microbiota, effectively breaking the cycle of rCDI, the authors of the study noted. But it’s been unclear whether the efficacy and safety of FMT vary by route of administration.
Effective without procedural risks
To investigate, Dr. Vaughn and colleagues evaluated clinical outcomes and adverse events in 170 patients with rCDI who underwent cap-FMT and 96 peers who underwent colo-FMT.
FMT was performed using one of two standardized formulations of microbiota manufactured by the University of Minnesota microbiota therapeutics program: freeze-dried/encapsulated or frozen-thawed/liquid.
Overall, the cure rates of CDI were 86% at 1 month and 81% at 2 months. There was no statistically significant difference at either time between cap-FMT and colo-FMT.
The 1-month cure rate was 84% with cap-FMT and 91% with colo-FMT; at 2 months, the cure rates were 81% and 83%, respectively.
Cap-FMT has a safety and effectiveness profile similar to that of colo-FMT, without the procedural risks of colonoscopy, the researchers concluded.
They cautioned that, although FMT is highly effective overall, patient selection is a key factor to optimizing FMT success.
Older age and hemodialysis were associated with FMT failure by 2 months on multivariate logistic regression.
“These risk factors can help determine if a patient should receive FMT or an alternative therapy for rCDI. This is not to say FMT should be avoided in older patients or those on dialysis, but clinicians should be aware of these associations in light of other options for rCDI,” Dr. Vaughn said.
Confirming prior studies, antibiotic use after FMT was a major factor in its failure. Patient selection for FMT should include an assessment of the potential need for antibiotics after transplant, the researchers noted.
One serious adverse event (aspiration pneumonia) was related to colonoscopy; otherwise, no new safety signals were identified.
As reported in other studies, changes in bowel function, including diarrhea, constipation, gas, and bloating were common, although it’s tough to disentangle gastrointestinal symptoms related to FMT from those after CDI, the researchers said. Importantly, no transmission of an infectious agent related to FMT was identified.
Two good options
The researchers said their findings are “highly generalizable” because the population reflects all FMT use by participating institutions and contains a mix of academic centers and private practices.
Many patients included in the study would not have been eligible for a clinical trial, owing to their having many comorbid conditions, including immune compromise and inflammatory bowel disease, the authors noted.
“FMT is recommended by major gastroenterology and infectious disease society guidelines,” Dr. Vaughn said. “Our group, and others, have consistently found strategies that incorporate FMT as cost-effective strategies for treating rCDI.”
However, lack of access to FMT products often is a barrier to treatment, he said.
“A stool banking model, similar to the nonprofit blood banking model, may be a useful solution to ensure equitable access to FMT to all who need it,” Dr. Vaughn added.
Reached for comment, Majdi Osman, MD, MPH, told this news organization that the study is valuable, “as it nicely shows in a real-world setting that capsules and colonoscopy are good options for patients who need this.”
Dr. Osman is chief medical officer of OpenBiome, a nonprofit organization that operates a public stool bank and is the major FMT source in the United States. The organization has provided over 63,000 FMT treatments to over 1,200 hospitals in the United States.
“FMT has become standard of care for patients who failed antibiotic therapy, and certainly is being used widely as a treatment option for these patients who have often run out of existing options,” Dr. Osman said.
Support for the study was provided by a donation from Achieving Cures Together, a nonprofit organization dedicated to advancing microbiome-based research. Dr. Vaughn receives grant support from Takeda, Roche, Celgene, and Diasorin and has received consulting fees from Prometheus and AbbVie. Dr. Osman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A real-world analysis confirms that fecal microbiota transplantation (FMT) is highly effective for recurrent Clostridioides difficile infection (rCDI) – and there is no difference between delivery by capsule (cap-FMT) and colonoscopy (colo-FMT).
“We present one of the largest cohorts involving people who received capsule FMT. Byron Vaughn, MD, with the division of gastroenterology, hepatology, and nutrition, University of Minnesota, Minneapolis, said in an interview.
The study was published online in Clinical Gastroenterology and Hepatology.
The Food and Drug Administration allows FMT to be used for patients who have failed standard treatment for rCDI under a policy of enforcement discretion.
The past decade has seen an increase in the use of FMT in clinical practice, owing to an increase in cases of rCDI after failure of standard antibiotic therapy.
Unlike antibiotics, which perpetuate and worsen intestinal dysbiosis, FMT restores the diversity and function of host microbiota, effectively breaking the cycle of rCDI, the authors of the study noted. But it’s been unclear whether the efficacy and safety of FMT vary by route of administration.
Effective without procedural risks
To investigate, Dr. Vaughn and colleagues evaluated clinical outcomes and adverse events in 170 patients with rCDI who underwent cap-FMT and 96 peers who underwent colo-FMT.
FMT was performed using one of two standardized formulations of microbiota manufactured by the University of Minnesota microbiota therapeutics program: freeze-dried/encapsulated or frozen-thawed/liquid.
Overall, the cure rates of CDI were 86% at 1 month and 81% at 2 months. There was no statistically significant difference at either time between cap-FMT and colo-FMT.
The 1-month cure rate was 84% with cap-FMT and 91% with colo-FMT; at 2 months, the cure rates were 81% and 83%, respectively.
Cap-FMT has a safety and effectiveness profile similar to that of colo-FMT, without the procedural risks of colonoscopy, the researchers concluded.
They cautioned that, although FMT is highly effective overall, patient selection is a key factor to optimizing FMT success.
Older age and hemodialysis were associated with FMT failure by 2 months on multivariate logistic regression.
“These risk factors can help determine if a patient should receive FMT or an alternative therapy for rCDI. This is not to say FMT should be avoided in older patients or those on dialysis, but clinicians should be aware of these associations in light of other options for rCDI,” Dr. Vaughn said.
Confirming prior studies, antibiotic use after FMT was a major factor in its failure. Patient selection for FMT should include an assessment of the potential need for antibiotics after transplant, the researchers noted.
One serious adverse event (aspiration pneumonia) was related to colonoscopy; otherwise, no new safety signals were identified.
As reported in other studies, changes in bowel function, including diarrhea, constipation, gas, and bloating were common, although it’s tough to disentangle gastrointestinal symptoms related to FMT from those after CDI, the researchers said. Importantly, no transmission of an infectious agent related to FMT was identified.
Two good options
The researchers said their findings are “highly generalizable” because the population reflects all FMT use by participating institutions and contains a mix of academic centers and private practices.
Many patients included in the study would not have been eligible for a clinical trial, owing to their having many comorbid conditions, including immune compromise and inflammatory bowel disease, the authors noted.
“FMT is recommended by major gastroenterology and infectious disease society guidelines,” Dr. Vaughn said. “Our group, and others, have consistently found strategies that incorporate FMT as cost-effective strategies for treating rCDI.”
However, lack of access to FMT products often is a barrier to treatment, he said.
“A stool banking model, similar to the nonprofit blood banking model, may be a useful solution to ensure equitable access to FMT to all who need it,” Dr. Vaughn added.
Reached for comment, Majdi Osman, MD, MPH, told this news organization that the study is valuable, “as it nicely shows in a real-world setting that capsules and colonoscopy are good options for patients who need this.”
Dr. Osman is chief medical officer of OpenBiome, a nonprofit organization that operates a public stool bank and is the major FMT source in the United States. The organization has provided over 63,000 FMT treatments to over 1,200 hospitals in the United States.
“FMT has become standard of care for patients who failed antibiotic therapy, and certainly is being used widely as a treatment option for these patients who have often run out of existing options,” Dr. Osman said.
Support for the study was provided by a donation from Achieving Cures Together, a nonprofit organization dedicated to advancing microbiome-based research. Dr. Vaughn receives grant support from Takeda, Roche, Celgene, and Diasorin and has received consulting fees from Prometheus and AbbVie. Dr. Osman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Don’t wait for patients to bring up their GI symptoms
Nearly three-quarters of Americans would wait before discussing GI symptoms with a health care provider if their bowel frequency or symptoms changed, with more than a quarter overall waiting for symptoms to become severe, according to a new survey from the American Gastroenterological Association.
Nearly 40% of people said GI symptoms had disrupted everyday activities such as exercising, running errands, and spending time with family or friends, but despite these disruptions, 30% of people said they would only discuss their bowel-related concerns if their doctor brought it up first. In response, the AGA launched “Trust Your Gut,” an awareness campaign aimed at shortening the time from the onset of bowel symptoms to discussions with health care providers.
“So many patients are either fearful or embarrassed about discussing their digestive symptoms such that they delay care unless the health care provider brings it up,” said Rajeev Jain, MD, a gastroenterologist with Texas Digestive Disease Consultants, AGA patient education adviser and a Trust Your Gut spokesperson.
“This potential delay could be detrimental in some cases, such as bleeding related to colon cancer,” he said. “If diagnosed sooner, an operation or chemotherapy could lead to treatment and a cure in those cases, versus advanced cancer that may be incurable.”
The AGA Trust Your Gut survey, conducted by Kelton Global during May 9-11, 2022, included 1,010 respondents from a nationally representative sample of U.S. adults.
Struggling with the issue
About 28% of respondents said they would see a clinician immediately if their bowel frequency or symptoms changed. However, 72% said they would wait, and on top of that, 27% said they would wait until the condition became severe or didn’t resolve over time. Women were more likely than men to say they would wait, at 72% versus 64%.
Overall, 39% of respondents said bowel issues have stopped them from doing some type of activity in the past year. Men were more likely than women to say that bowel issues have affected their ability to do an activity, at 44% versus 35%.
“Typically, when it comes to functional or motility disorders or bowel dysfunction, we tend to see a higher prevalence in women, so this was somewhat surprising to see,” said Andrea Shin, MD, a gastroenterology specialist and assistant professor of medicine at Indiana University, Indianapolis, and AGA patient education adviser designate.
“Part of this difference may be related to the communication barrier and how sex or gender affects that relationship between a clinician and a patient,” she said.
The reasons for patients’ reluctance varies, but themes of uncertainty and embarrassment are prevalent. About 33% said they’re not sure whether the symptoms are a problem, 31% said they hope the symptoms improve on their own, 23% said it’s embarrassing, and 12% don’t know what to tell the doctor. Men were more likely than women to say they don’t know what to say to a doctor about their symptoms, at 15% versus 9%.
Starting the conversation
From a young age, many respondents were raised to avoid the topic of bowel issues. About 23% said their parents encouraged them not to mention bathroom-related health issues, and 10% said they didn’t talk about bowel issues at all. Another 32% said they could talk about it but had to use code words, such as “go to the bathroom” or “potty.”
“What this highlights is that patients are culturally taught not to talk about their digestive tract, or they’re embarrassed or uncertain,” Dr. Jain said. “At the end of the day, we need to destigmatize discussions about digestive function and normalize it as part of overall health.”
The survey respondents said they’d feel most comfortable talking about bowel issues with doctors (63%) and nurses (41%), as well as a significant other (44%), parent (32%), or friend (27%). Women were more likely than men to feel comfortable turning to a nurse practitioner or physician’s assistant (47% versus 35%) or a friend (30% versus 24%).
To feel more comfortable with these conversations, 42% of survey participants said they would like their doctor or clinician to describe what’s normal. About 30% want to know the appropriate terms to describe their situation.
Health care providers should also consider the cultural and social factors that may affect a patient’s disease experience, as well as how they interact with the health care system, Shin said.
“Understanding these differences might help us to better engage with a community that is diverse,” she said. “In general, we also need to be more proactive about drawing these conversations out of patients, who may not mention it unless we ask because they find it so personal.”
The AGA Trust Your Gut campaign is supported by a sponsorship from Janssen. Dr. Jain and Dr. Shin reported to relevant disclosures.
Help your patients learn more by encouraging them to visit https://patient.gastro.org/trust-your-gut/.
Nearly three-quarters of Americans would wait before discussing GI symptoms with a health care provider if their bowel frequency or symptoms changed, with more than a quarter overall waiting for symptoms to become severe, according to a new survey from the American Gastroenterological Association.
Nearly 40% of people said GI symptoms had disrupted everyday activities such as exercising, running errands, and spending time with family or friends, but despite these disruptions, 30% of people said they would only discuss their bowel-related concerns if their doctor brought it up first. In response, the AGA launched “Trust Your Gut,” an awareness campaign aimed at shortening the time from the onset of bowel symptoms to discussions with health care providers.
“So many patients are either fearful or embarrassed about discussing their digestive symptoms such that they delay care unless the health care provider brings it up,” said Rajeev Jain, MD, a gastroenterologist with Texas Digestive Disease Consultants, AGA patient education adviser and a Trust Your Gut spokesperson.
“This potential delay could be detrimental in some cases, such as bleeding related to colon cancer,” he said. “If diagnosed sooner, an operation or chemotherapy could lead to treatment and a cure in those cases, versus advanced cancer that may be incurable.”
The AGA Trust Your Gut survey, conducted by Kelton Global during May 9-11, 2022, included 1,010 respondents from a nationally representative sample of U.S. adults.
Struggling with the issue
About 28% of respondents said they would see a clinician immediately if their bowel frequency or symptoms changed. However, 72% said they would wait, and on top of that, 27% said they would wait until the condition became severe or didn’t resolve over time. Women were more likely than men to say they would wait, at 72% versus 64%.
Overall, 39% of respondents said bowel issues have stopped them from doing some type of activity in the past year. Men were more likely than women to say that bowel issues have affected their ability to do an activity, at 44% versus 35%.
“Typically, when it comes to functional or motility disorders or bowel dysfunction, we tend to see a higher prevalence in women, so this was somewhat surprising to see,” said Andrea Shin, MD, a gastroenterology specialist and assistant professor of medicine at Indiana University, Indianapolis, and AGA patient education adviser designate.
“Part of this difference may be related to the communication barrier and how sex or gender affects that relationship between a clinician and a patient,” she said.
The reasons for patients’ reluctance varies, but themes of uncertainty and embarrassment are prevalent. About 33% said they’re not sure whether the symptoms are a problem, 31% said they hope the symptoms improve on their own, 23% said it’s embarrassing, and 12% don’t know what to tell the doctor. Men were more likely than women to say they don’t know what to say to a doctor about their symptoms, at 15% versus 9%.
Starting the conversation
From a young age, many respondents were raised to avoid the topic of bowel issues. About 23% said their parents encouraged them not to mention bathroom-related health issues, and 10% said they didn’t talk about bowel issues at all. Another 32% said they could talk about it but had to use code words, such as “go to the bathroom” or “potty.”
“What this highlights is that patients are culturally taught not to talk about their digestive tract, or they’re embarrassed or uncertain,” Dr. Jain said. “At the end of the day, we need to destigmatize discussions about digestive function and normalize it as part of overall health.”
The survey respondents said they’d feel most comfortable talking about bowel issues with doctors (63%) and nurses (41%), as well as a significant other (44%), parent (32%), or friend (27%). Women were more likely than men to feel comfortable turning to a nurse practitioner or physician’s assistant (47% versus 35%) or a friend (30% versus 24%).
To feel more comfortable with these conversations, 42% of survey participants said they would like their doctor or clinician to describe what’s normal. About 30% want to know the appropriate terms to describe their situation.
Health care providers should also consider the cultural and social factors that may affect a patient’s disease experience, as well as how they interact with the health care system, Shin said.
“Understanding these differences might help us to better engage with a community that is diverse,” she said. “In general, we also need to be more proactive about drawing these conversations out of patients, who may not mention it unless we ask because they find it so personal.”
The AGA Trust Your Gut campaign is supported by a sponsorship from Janssen. Dr. Jain and Dr. Shin reported to relevant disclosures.
Help your patients learn more by encouraging them to visit https://patient.gastro.org/trust-your-gut/.
Nearly three-quarters of Americans would wait before discussing GI symptoms with a health care provider if their bowel frequency or symptoms changed, with more than a quarter overall waiting for symptoms to become severe, according to a new survey from the American Gastroenterological Association.
Nearly 40% of people said GI symptoms had disrupted everyday activities such as exercising, running errands, and spending time with family or friends, but despite these disruptions, 30% of people said they would only discuss their bowel-related concerns if their doctor brought it up first. In response, the AGA launched “Trust Your Gut,” an awareness campaign aimed at shortening the time from the onset of bowel symptoms to discussions with health care providers.
“So many patients are either fearful or embarrassed about discussing their digestive symptoms such that they delay care unless the health care provider brings it up,” said Rajeev Jain, MD, a gastroenterologist with Texas Digestive Disease Consultants, AGA patient education adviser and a Trust Your Gut spokesperson.
“This potential delay could be detrimental in some cases, such as bleeding related to colon cancer,” he said. “If diagnosed sooner, an operation or chemotherapy could lead to treatment and a cure in those cases, versus advanced cancer that may be incurable.”
The AGA Trust Your Gut survey, conducted by Kelton Global during May 9-11, 2022, included 1,010 respondents from a nationally representative sample of U.S. adults.
Struggling with the issue
About 28% of respondents said they would see a clinician immediately if their bowel frequency or symptoms changed. However, 72% said they would wait, and on top of that, 27% said they would wait until the condition became severe or didn’t resolve over time. Women were more likely than men to say they would wait, at 72% versus 64%.
Overall, 39% of respondents said bowel issues have stopped them from doing some type of activity in the past year. Men were more likely than women to say that bowel issues have affected their ability to do an activity, at 44% versus 35%.
“Typically, when it comes to functional or motility disorders or bowel dysfunction, we tend to see a higher prevalence in women, so this was somewhat surprising to see,” said Andrea Shin, MD, a gastroenterology specialist and assistant professor of medicine at Indiana University, Indianapolis, and AGA patient education adviser designate.
“Part of this difference may be related to the communication barrier and how sex or gender affects that relationship between a clinician and a patient,” she said.
The reasons for patients’ reluctance varies, but themes of uncertainty and embarrassment are prevalent. About 33% said they’re not sure whether the symptoms are a problem, 31% said they hope the symptoms improve on their own, 23% said it’s embarrassing, and 12% don’t know what to tell the doctor. Men were more likely than women to say they don’t know what to say to a doctor about their symptoms, at 15% versus 9%.
Starting the conversation
From a young age, many respondents were raised to avoid the topic of bowel issues. About 23% said their parents encouraged them not to mention bathroom-related health issues, and 10% said they didn’t talk about bowel issues at all. Another 32% said they could talk about it but had to use code words, such as “go to the bathroom” or “potty.”
“What this highlights is that patients are culturally taught not to talk about their digestive tract, or they’re embarrassed or uncertain,” Dr. Jain said. “At the end of the day, we need to destigmatize discussions about digestive function and normalize it as part of overall health.”
The survey respondents said they’d feel most comfortable talking about bowel issues with doctors (63%) and nurses (41%), as well as a significant other (44%), parent (32%), or friend (27%). Women were more likely than men to feel comfortable turning to a nurse practitioner or physician’s assistant (47% versus 35%) or a friend (30% versus 24%).
To feel more comfortable with these conversations, 42% of survey participants said they would like their doctor or clinician to describe what’s normal. About 30% want to know the appropriate terms to describe their situation.
Health care providers should also consider the cultural and social factors that may affect a patient’s disease experience, as well as how they interact with the health care system, Shin said.
“Understanding these differences might help us to better engage with a community that is diverse,” she said. “In general, we also need to be more proactive about drawing these conversations out of patients, who may not mention it unless we ask because they find it so personal.”
The AGA Trust Your Gut campaign is supported by a sponsorship from Janssen. Dr. Jain and Dr. Shin reported to relevant disclosures.
Help your patients learn more by encouraging them to visit https://patient.gastro.org/trust-your-gut/.
Scientists identify new genetic links to dyslexia
Dyslexia occurs in 5%-17% of the general population, depending on the diagnostic criteria, and has been linked with speech and language disorders, as well as ADHD, Catherine Doust, PhD, of the University of Edinburgh and colleagues wrote.
However, previous studies of the genetics of dyslexia are limited, corresponding author Michelle Luciano, PhD, said in an interview. “So much progress has been made in understanding the genetics of behavior and health, but only a small genomewide study of dyslexia existed before ours.”
Currently, genetic testing for dyslexia alone is not done.
“You couldn’t order a genetic test for dyslexia unless it were part of another genetic panel,” according to Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y.
There are also known associations with some genes and autism, but none are definitive, and testing requires a workup of which a genetic panel may be a part. Such tests are expensive, and rarely covered by insurance, the pediatrician explained.
Experts recommend genetic screening for every child with developmental delay, but most insurance won’t cover it, Dr. Lessin continued.
In the new genomewide association study published in Nature Genetics, the researchers reviewed data from 51,800 adults aged 18 years and older with a self-reported dyslexia diagnosis and 1,087,070 controls. All study participants are enrolled in ongoing research with 23andMe, the personal genetics company.
The researchers investigated the genetic correlations with reading and related skills and evaluated evidence for genes previously associated with dyslexia. The mean ages of the dyslexia cases and controls were 49.6 years and 51.7 years, respectively.
The researchers identified 42 independent genetic variants (genomewide significant loci) associated with dyslexia; 15 of these loci were in genes previously associated with cognitive ability and educational attainment, and 27 were newly identified as specifically associated with dyslexia. The researchers further determined that 12 of the newly identified genes were associated with proficiency in reading and spelling in English and European languages, and 1 in a Chinese-language population.
A polygenic risk score is a way to characterize an individual’s risk of developing a disease, based on the total number of genetic changes related to the disease; the researchers used this score to validate their results. Dyslexia polygenic scores were used to predict reading and spelling in additional population-based and reading disorder–enriched samples outside of the study population; these genetic measures explained up to 6% of variance in reading traits, the researchers noted. Ultimately, these scores may be a tool to help identify children with a predisposition for dyslexia so reading skills support can begin early.
The researchers also found that many of the genes associated with dyslexia are also associated with ADHD, (24% of dyslexia patients reporting ADHD vs. 9% of controls), and with a moderate correlation, which suggests possible shared genetic components for deficits in working memory and attention.
The study findings were limited by the inability to prove causality, and by the potential bias in the study sample, but were strengthened by the large study population, the researchers noted.
Potential implications for reading and spelling
“We were surprised that none of the previous dyslexia candidate genes were genomewide significant in our study; all of our discoveries were in new genes that had not been previously implicated in dyslexia,” Dr. Luciano said in an interview. “Some of these genes have been found to be associated with general cognitive ability, but most were novel and may represent genes specifically related to cognitive processes dominant in reading and spelling.
“We were also surprised that there was little genetic correlation (or overlap) with brain MRI variables, given that brain regions have been linked to reading skill. This suggests that the link is environmental in origin,” she added.
“Our results do not directly feed into clinical practice,” said Dr. Luciano. However, “the moderate genetic overlap with ADHD suggests that broader assessments of behavior are important when a child presents with dyslexia, as co-occurrence with other conditions might influence the intervention chosen. Asking about family history of dyslexia might also help in identification.
With more research, genetic studies may find a place in the clinical setting, said Dr. Luciano.
“As genomewide association studies become larger and the findings more stable, genetic information might be used as an adjunct to what is known about the child’s environment and their performance on standardized tests of reading. The key advantage of genetic information is that it could allow much earlier identification of children who would benefit from extra learning support,” she said.
More research is needed to understand the interaction between genes and the environment, Dr. Luciano said. “It is essential that we understand what environmental learning support can minimize genetic predisposition to dyslexia.”
Too soon for clinical utility
The study findings are an important foundation for additional research, but not yet clinically useful, Dr. Lessin said in an interview.
“Dyslexia is a tough diagnosis,” that requires assessment by a developmental pediatrician or a pediatric neurologist and these specialists are often not accessible to many parents, Dr. Lessin noted.
In the current study, the researchers found a number of genes potentially associated with dyslexia, but the study does not prove causality, he emphasized. The findings simply mean that some of these genes may have something to do with dyslexia, and further research might identify a genetic cause.
“No one is going to make a diagnosis of dyslexia based on genes just yet,” said Dr. Lessin. In the meantime, clinicians should be aware that good research is being conducted, and that the genetic foundations for dyslexia are being explored.
Lead author Dr. Doust and corresponding author Dr. Luciano had no financial conflicts to disclose. Several coauthors disclosed support from the Max Planck Society (Germany), the National Natural Science Foundation of China, Funds for Humanities and Social Sciences Research of the Ministry of Education, and General Project of Shaanxi Natural Science Basic Research Program. Two coauthors are employed by and hold stock or stock options in 23andMe. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Dyslexia occurs in 5%-17% of the general population, depending on the diagnostic criteria, and has been linked with speech and language disorders, as well as ADHD, Catherine Doust, PhD, of the University of Edinburgh and colleagues wrote.
However, previous studies of the genetics of dyslexia are limited, corresponding author Michelle Luciano, PhD, said in an interview. “So much progress has been made in understanding the genetics of behavior and health, but only a small genomewide study of dyslexia existed before ours.”
Currently, genetic testing for dyslexia alone is not done.
“You couldn’t order a genetic test for dyslexia unless it were part of another genetic panel,” according to Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y.
There are also known associations with some genes and autism, but none are definitive, and testing requires a workup of which a genetic panel may be a part. Such tests are expensive, and rarely covered by insurance, the pediatrician explained.
Experts recommend genetic screening for every child with developmental delay, but most insurance won’t cover it, Dr. Lessin continued.
In the new genomewide association study published in Nature Genetics, the researchers reviewed data from 51,800 adults aged 18 years and older with a self-reported dyslexia diagnosis and 1,087,070 controls. All study participants are enrolled in ongoing research with 23andMe, the personal genetics company.
The researchers investigated the genetic correlations with reading and related skills and evaluated evidence for genes previously associated with dyslexia. The mean ages of the dyslexia cases and controls were 49.6 years and 51.7 years, respectively.
The researchers identified 42 independent genetic variants (genomewide significant loci) associated with dyslexia; 15 of these loci were in genes previously associated with cognitive ability and educational attainment, and 27 were newly identified as specifically associated with dyslexia. The researchers further determined that 12 of the newly identified genes were associated with proficiency in reading and spelling in English and European languages, and 1 in a Chinese-language population.
A polygenic risk score is a way to characterize an individual’s risk of developing a disease, based on the total number of genetic changes related to the disease; the researchers used this score to validate their results. Dyslexia polygenic scores were used to predict reading and spelling in additional population-based and reading disorder–enriched samples outside of the study population; these genetic measures explained up to 6% of variance in reading traits, the researchers noted. Ultimately, these scores may be a tool to help identify children with a predisposition for dyslexia so reading skills support can begin early.
The researchers also found that many of the genes associated with dyslexia are also associated with ADHD, (24% of dyslexia patients reporting ADHD vs. 9% of controls), and with a moderate correlation, which suggests possible shared genetic components for deficits in working memory and attention.
The study findings were limited by the inability to prove causality, and by the potential bias in the study sample, but were strengthened by the large study population, the researchers noted.
Potential implications for reading and spelling
“We were surprised that none of the previous dyslexia candidate genes were genomewide significant in our study; all of our discoveries were in new genes that had not been previously implicated in dyslexia,” Dr. Luciano said in an interview. “Some of these genes have been found to be associated with general cognitive ability, but most were novel and may represent genes specifically related to cognitive processes dominant in reading and spelling.
“We were also surprised that there was little genetic correlation (or overlap) with brain MRI variables, given that brain regions have been linked to reading skill. This suggests that the link is environmental in origin,” she added.
“Our results do not directly feed into clinical practice,” said Dr. Luciano. However, “the moderate genetic overlap with ADHD suggests that broader assessments of behavior are important when a child presents with dyslexia, as co-occurrence with other conditions might influence the intervention chosen. Asking about family history of dyslexia might also help in identification.
With more research, genetic studies may find a place in the clinical setting, said Dr. Luciano.
“As genomewide association studies become larger and the findings more stable, genetic information might be used as an adjunct to what is known about the child’s environment and their performance on standardized tests of reading. The key advantage of genetic information is that it could allow much earlier identification of children who would benefit from extra learning support,” she said.
More research is needed to understand the interaction between genes and the environment, Dr. Luciano said. “It is essential that we understand what environmental learning support can minimize genetic predisposition to dyslexia.”
Too soon for clinical utility
The study findings are an important foundation for additional research, but not yet clinically useful, Dr. Lessin said in an interview.
“Dyslexia is a tough diagnosis,” that requires assessment by a developmental pediatrician or a pediatric neurologist and these specialists are often not accessible to many parents, Dr. Lessin noted.
In the current study, the researchers found a number of genes potentially associated with dyslexia, but the study does not prove causality, he emphasized. The findings simply mean that some of these genes may have something to do with dyslexia, and further research might identify a genetic cause.
“No one is going to make a diagnosis of dyslexia based on genes just yet,” said Dr. Lessin. In the meantime, clinicians should be aware that good research is being conducted, and that the genetic foundations for dyslexia are being explored.
Lead author Dr. Doust and corresponding author Dr. Luciano had no financial conflicts to disclose. Several coauthors disclosed support from the Max Planck Society (Germany), the National Natural Science Foundation of China, Funds for Humanities and Social Sciences Research of the Ministry of Education, and General Project of Shaanxi Natural Science Basic Research Program. Two coauthors are employed by and hold stock or stock options in 23andMe. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
Dyslexia occurs in 5%-17% of the general population, depending on the diagnostic criteria, and has been linked with speech and language disorders, as well as ADHD, Catherine Doust, PhD, of the University of Edinburgh and colleagues wrote.
However, previous studies of the genetics of dyslexia are limited, corresponding author Michelle Luciano, PhD, said in an interview. “So much progress has been made in understanding the genetics of behavior and health, but only a small genomewide study of dyslexia existed before ours.”
Currently, genetic testing for dyslexia alone is not done.
“You couldn’t order a genetic test for dyslexia unless it were part of another genetic panel,” according to Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y.
There are also known associations with some genes and autism, but none are definitive, and testing requires a workup of which a genetic panel may be a part. Such tests are expensive, and rarely covered by insurance, the pediatrician explained.
Experts recommend genetic screening for every child with developmental delay, but most insurance won’t cover it, Dr. Lessin continued.
In the new genomewide association study published in Nature Genetics, the researchers reviewed data from 51,800 adults aged 18 years and older with a self-reported dyslexia diagnosis and 1,087,070 controls. All study participants are enrolled in ongoing research with 23andMe, the personal genetics company.
The researchers investigated the genetic correlations with reading and related skills and evaluated evidence for genes previously associated with dyslexia. The mean ages of the dyslexia cases and controls were 49.6 years and 51.7 years, respectively.
The researchers identified 42 independent genetic variants (genomewide significant loci) associated with dyslexia; 15 of these loci were in genes previously associated with cognitive ability and educational attainment, and 27 were newly identified as specifically associated with dyslexia. The researchers further determined that 12 of the newly identified genes were associated with proficiency in reading and spelling in English and European languages, and 1 in a Chinese-language population.
A polygenic risk score is a way to characterize an individual’s risk of developing a disease, based on the total number of genetic changes related to the disease; the researchers used this score to validate their results. Dyslexia polygenic scores were used to predict reading and spelling in additional population-based and reading disorder–enriched samples outside of the study population; these genetic measures explained up to 6% of variance in reading traits, the researchers noted. Ultimately, these scores may be a tool to help identify children with a predisposition for dyslexia so reading skills support can begin early.
The researchers also found that many of the genes associated with dyslexia are also associated with ADHD, (24% of dyslexia patients reporting ADHD vs. 9% of controls), and with a moderate correlation, which suggests possible shared genetic components for deficits in working memory and attention.
The study findings were limited by the inability to prove causality, and by the potential bias in the study sample, but were strengthened by the large study population, the researchers noted.
Potential implications for reading and spelling
“We were surprised that none of the previous dyslexia candidate genes were genomewide significant in our study; all of our discoveries were in new genes that had not been previously implicated in dyslexia,” Dr. Luciano said in an interview. “Some of these genes have been found to be associated with general cognitive ability, but most were novel and may represent genes specifically related to cognitive processes dominant in reading and spelling.
“We were also surprised that there was little genetic correlation (or overlap) with brain MRI variables, given that brain regions have been linked to reading skill. This suggests that the link is environmental in origin,” she added.
“Our results do not directly feed into clinical practice,” said Dr. Luciano. However, “the moderate genetic overlap with ADHD suggests that broader assessments of behavior are important when a child presents with dyslexia, as co-occurrence with other conditions might influence the intervention chosen. Asking about family history of dyslexia might also help in identification.
With more research, genetic studies may find a place in the clinical setting, said Dr. Luciano.
“As genomewide association studies become larger and the findings more stable, genetic information might be used as an adjunct to what is known about the child’s environment and their performance on standardized tests of reading. The key advantage of genetic information is that it could allow much earlier identification of children who would benefit from extra learning support,” she said.
More research is needed to understand the interaction between genes and the environment, Dr. Luciano said. “It is essential that we understand what environmental learning support can minimize genetic predisposition to dyslexia.”
Too soon for clinical utility
The study findings are an important foundation for additional research, but not yet clinically useful, Dr. Lessin said in an interview.
“Dyslexia is a tough diagnosis,” that requires assessment by a developmental pediatrician or a pediatric neurologist and these specialists are often not accessible to many parents, Dr. Lessin noted.
In the current study, the researchers found a number of genes potentially associated with dyslexia, but the study does not prove causality, he emphasized. The findings simply mean that some of these genes may have something to do with dyslexia, and further research might identify a genetic cause.
“No one is going to make a diagnosis of dyslexia based on genes just yet,” said Dr. Lessin. In the meantime, clinicians should be aware that good research is being conducted, and that the genetic foundations for dyslexia are being explored.
Lead author Dr. Doust and corresponding author Dr. Luciano had no financial conflicts to disclose. Several coauthors disclosed support from the Max Planck Society (Germany), the National Natural Science Foundation of China, Funds for Humanities and Social Sciences Research of the Ministry of Education, and General Project of Shaanxi Natural Science Basic Research Program. Two coauthors are employed by and hold stock or stock options in 23andMe. Dr. Lessin had no financial conflicts to disclose, but serves on the editorial advisory board of Pediatric News.
FROM NATURE GENETICS












