User login
Insights From the 2020-2021 Dermatology Residency Match
To the Editor:
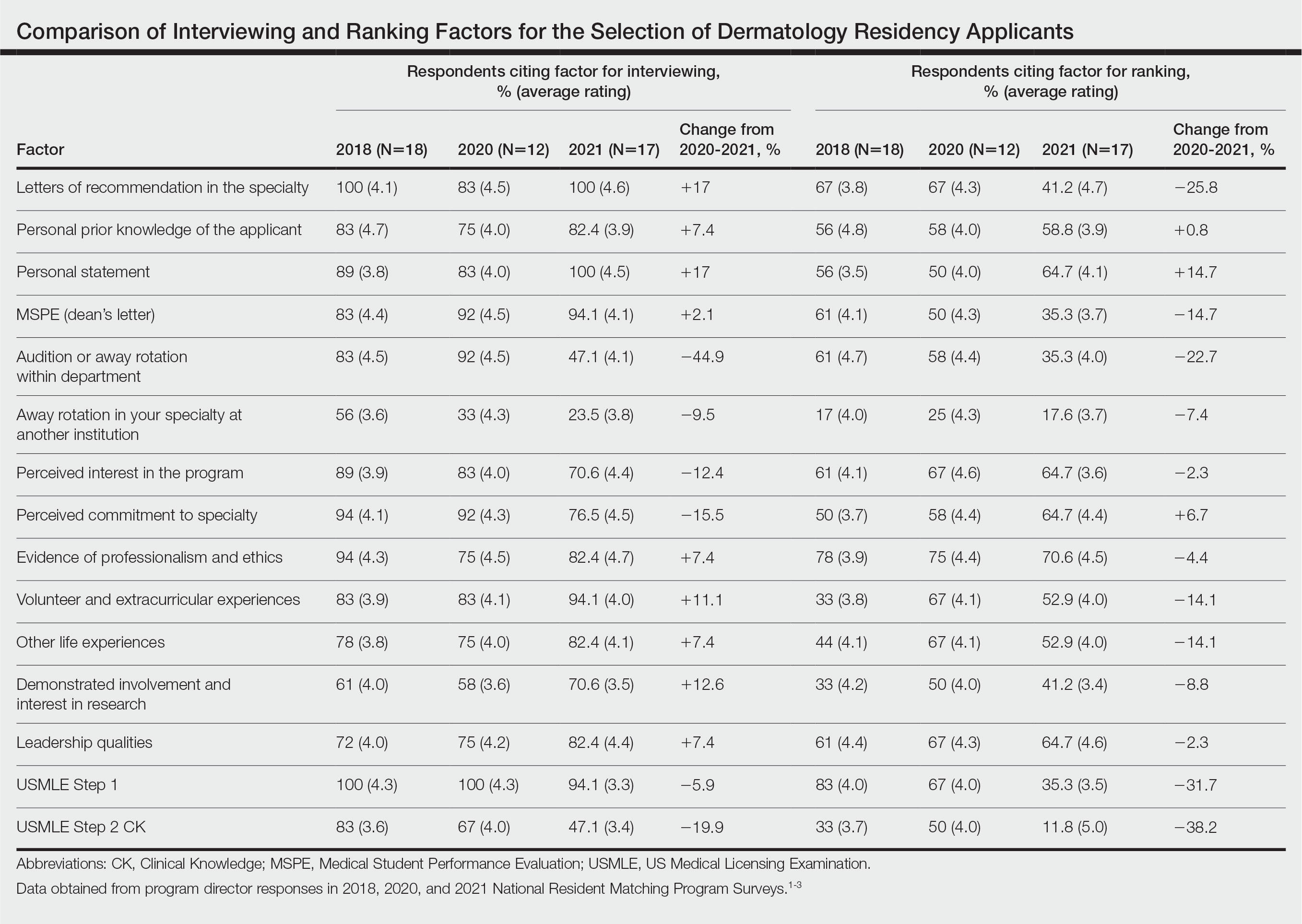
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.
We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
To the Editor:
Data from the program director survey of the National Resident Matching Program offer key insights into the 2021 dermatology application process.1,2 Examination of data from the 2020 (N=12) and 2021 (N=17) program director survey regarding interviewing applicants revealed that specialty-specific letters of recommendation (LORs), personal prior knowledge of an applicant, and personal statement increased in importance by 17%, 7.4%, and 17%, respectively, whereas away rotations within the department decreased in importance by 44.9% (Table).1,2 Interestingly, for ranking applicants, programs decreased their emphasis on specialty-specific LORs by 25.8% and away rotations within the department by 22.7% and increased emphasis on personal statements by 14.7% and personal prior knowledge of an applicant by 0.8% from 2020 to 2021 (Table).1,2 These findings align with the prior recommendation to limit away rotations; data are contradictory—when comparing factors for interviewing as compared to ranking applicants—for specialty-specific LORs.

We further compared data from the otolaryngology cycle, which implemented preference signaling by which an applicant can signal their interest in a particular residency program in the 2021 Match, to data from dermatology with no preference signaling. A 90% probability of matching is estimated to require approximately 8 or 9 interviews for dermatology or 12 interviews for otolaryngology for MD senior students in 2020.4 In prior dermatology application cycles, the most highly qualified candidates constituted 7% to 21% of all applicants but were estimated to receive half of all interviews, causing a maldistribution of interviews.5,6
For the 2021 otolaryngology match, the Society of University Otolaryngologists implemented a novel preference signaling system that allowed candidates to show interest in programs by sending 5 preferences, or tokens.7 Recent data reports from the otolaryngology cycle demonstrated at least a 2-fold increase in the rate of receiving an interview invitation for signaled programs compared to the closest nonsignaled program if applicants were provided an additional token.7 Regarding overall applicant competitiveness (ie, dividing participants into quartiles based on their competitiveness), the highest increase in the overall rate of interview invitations (3.5 [total invitations/total applications]) was demonstrated for fourth-quartile (ie, “lowest quartile”) applicants compared with the increase in the overall rate of interview invitations seen in other quartiles (first quartile, an increase of 2.3; second quartile, an increase of 2.6; and third quartile, an increase of 2.4).7 We look forward to seeing the impact of preference signaling on the results of the 2022 dermatology cycle.
Despite changes in the interviewing process to accommodate COVID-19 pandemic safety recommendations, the overall dermatology postgraduate year (PGY) 2 fill rate remained unchanged from 2018 (98.6%) to 2021 (98.7%). Zero PGY-1 positions and 5 PGY-2 positions were unfilled in the 2021 Main Residency Match compared to 1 unfilled PGY-1 position and 4 unfilled PGY-2 positions in 2018.8 The coordinated interview invitation release, holistic review of applications, increased number of rankings, and virtual interviews might have helped offset potential obstacles imparted by inability to complete away rotations, inability to obtain LORs, and conducting interviews virtually.5
A limitation of our analysis is the low response rate of program directors to National Resident Matching Program surveys.
These strategies—holistic application review and coordinated interview release—may be considered in future cycles given their convenience and negligible impact on the dermatology match rate. For example, virtual interviews relieve the financial and time burdens of in-person interviews—approximately $10,000 for each US senior applicant—thus potentially allowing for a more equitable matching process.3 Inversely, in-person interviews allow participants to effectively network and form more meaningful connections while obtaining a better understanding of facilities and surrounding locales. As such, the medical community should continue to come to a consensus on the optimal format to host interviews.
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
- Results of the 2021 NRMP Program Director Survey. National Resident Matching Program. August 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/11/2021-PD-Survey-Report-for-WWW.pdf
- Results of the 2020 NRMP Program Director Survey. National Resident Matching Program. August 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2022/01/2020-PD-Survey.pdf
- Rojek NW, Shinkai K, Fett N. Dermatology faculty and residents’ perspectives on the dermatology residency application process: a nationwide survey. J Am Acad Dermatol. 2018;79:157-159. doi:10.1016/j.jaad.2018.01.00
- Charting Outcomes in the Match: Senior Students of U.S. MD Medical Schools. National Resident Matching Program. July 2020. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/Charting-Outcomes-in-the-Match-2020_MD-Senior_final.pdf
- Thatiparthi A, Martin A, Liu J, et al. Preliminary outcomes of 2020-2021 dermatology residency application cycle and adverse effects of COVID-19. J Am Acad Dermatol. 2021;84:e263-e264. doi:10.1016/j.jaad.2021.03.034
- Hammoud MM, Standiford T, Carmody JB. Potential implications of COVID-19 for the 2020-2021 residency application cycle. JAMA. 2020;324:29-30. doi:10.1001/jama.2020.8911
- Interview offer rate with/without ENTSignaling. Society of University Otolaryngologists. Updated July 19, 2022. Accessed December 12, 2022. https://opdo-hns.org/mpage/signaling-updates
- Results and Data: 2021 Main Residency Match. National Resident Matching Program. May 2021. Accessed December 6, 2021. https://www.nrmp.org/wp-content/uploads/2021/08/MRM-Results_and-Data_2021.pdf
PRACTICE POINTS
- Although there have been numerous changes to the dermatology interview process due to the COVID-19 pandemic, the overall fill rate for postgraduate year 2 positions remained unchanged from 2018 (prepandemic) to 2021 (postpandemic).
- Strategies to accommodate new safety recommendations for interviews may reduce the financial burden (approximately $10,000 for each senior applicant) and time constraints on applicants. These strategies should be considered for implementation in future cycles.
Immune checkpoint inhibitor–related gastrointestinal adverse events
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10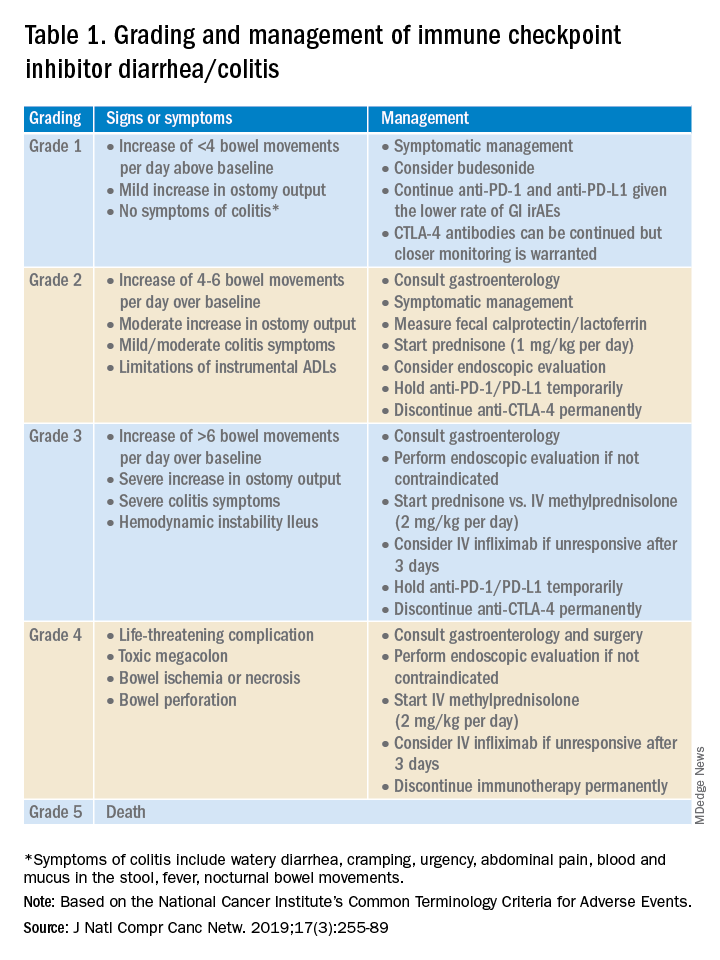
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.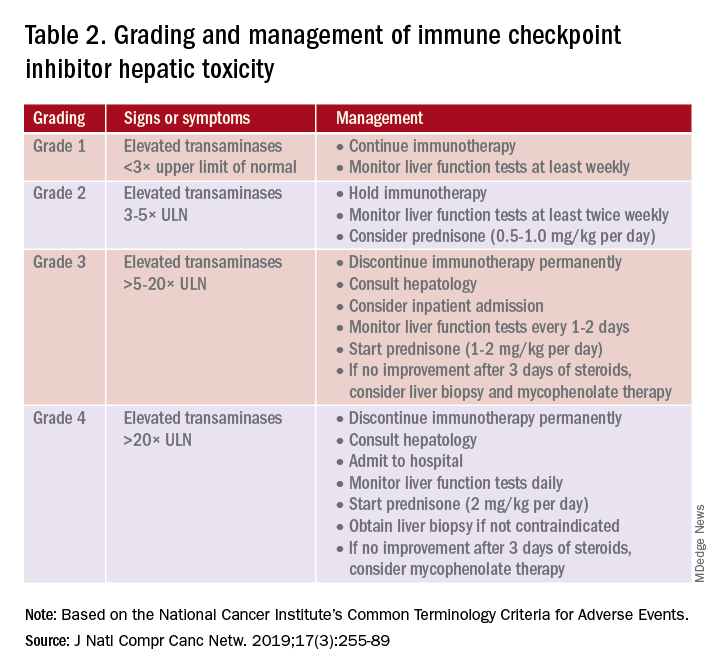
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14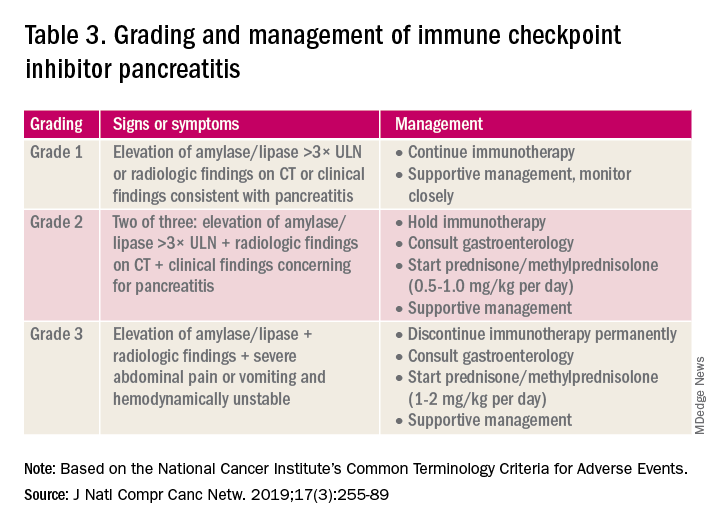
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Introduction
The field of cancer immunotherapy has exploded in recent years, with new therapies showing promising results for effective treatment of various cancer types. Immune checkpoint inhibitors (ICI) work by blocking checkpoint proteins that prevent breakdown of tumor cells by T-lymphocytes. Checkpoint proteins exist to prevent autoimmunity and destruction of healthy cells, but may allow tumor cells to grow unchallenged. Three checkpoint proteins – cytotoxic T-lymphocyte protein–4 (CTLA-4), programmed cell-death protein–1 (PD-1), and programmed cell-death protein ligand–1 (PDL-1) – are therapeutic targets for current ICIs.1
ICIs are used to treat various cancer types (e.g., lung, renal-cell, and Hodgkin’s lymphoma). Immune-related adverse events (irAE) are frequently seen with ICI use, ranging from 15% to 90%, and can occur at any point during, or even after, treatment.2
Immune checkpoint inhibitor–related gastrointestinal adverse reactions
GI adverse reactions are the second most common irAE, occurring in about 35%-50% of all reported irAEs.3 Anti-CTLA-4 medications have the highest association with GI irAE. The most common GI symptoms are diarrhea, abdominal pain, urgency, and nausea/vomiting. GI involvement can occur along the entirety of the GI tract – from the oral cavity to the colorectum. These are usually seen within 6-8 weeks of starting treatment, but can occur as early as 1 week after initiation or as late as 12 months after the last dose.2 Although colitis is the most common area of luminal inflammation, aphthous ulcers, esophagitis, gastritis, and enteritis can be seen. Anti-CTLA-4 antibodies have the highest associated rate of diarrhea (33%-50%) and colitis (7%-22%) of all ICIs.4 Computed tomography (CT) may show colonic wall thickening or fat stranding, indicating inflammation. Endoscopically, the colon can appear grossly normal or demonstrate erythema, erosions, ulcerations, and/or loss of vascular pattern.5 Inflammation can be patchy or continuous. Typical histology shows increased lamina propria cellularity, neutrophilic infiltration (intraepithelial or crypt abscesses), and increased crypt apoptosis.6
The liver, pancreas, gallbladder, and biliary tract can also be affected by irAE. The liver is most commonly involved (i.e. 5% of irAE), manifesting as asymptomatic liver chemistry elevation, particularly aminotransferases. This can progress to acute symptomatic hepatitis with jaundice, fever, or malaise, and rarely to fulminant hepatitis. ICI-associated hepatitis appears histologically similar to autoimmune hepatitis, with pan-lobular hepatitis and infiltrating CD8+ T lymphocytes seen on liver biopsy.7 Less commonly, pancreatic toxicity can occur (<2% of irAE), seen with anti-CTLA-4 therapy.8 While this typically results in asymptomatic lipase or amylase elevations (2.7%), acute pancreatitis (AP) can occur(1.9%). ICI-associated AP presents with classic symptoms and imaging changes, but can also manifest with exocrine or endocrine pancreatic insufficiency. An increase in rates of acute acalculous cholecystitis has been reported in patients receiving ICIs compared to patients receiving non-ICI chemotherapy.9 There are also rare reports of ICI-associated secondary sclerosing cholangitis.
Management
Evaluation and management of GI irAEs are guided by severity, based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading classification (Table 1).10
A thorough history of GI and systemic symptoms should be obtained and compared to baseline bowel habits. Patients with mild symptoms should undergo studies to assess alternate etiologies for their symptoms. Bacterial stool cultures and testing for C. difficile should be performed. Erythrocyte sedimentation rate, C-reactive protein, fecal lactoferrin, and calprotectin can help assess the degree of intestinal inflammation and can be used to risk-stratify or assess treatment response. CT scans can assess for colitis and associated complications, including abdominal abscess, toxic megacolon, and bowel perforation.
Patients unresponsive to initial treatment for grade I irAE, with hematochezia, or with at least grade 2 diarrhea, should undergo GI consultation and endoscopic evaluation. Flexible sigmoidoscopy is the test of choice, as 95% of patients will have left-sided colonic inflammation.11 Patients with at least grade 3 diarrhea should be hospitalized for treatment. In cases of failed methylprednisolone and when infliximab is ineffective or contraindicated, vedolizumab is suggested, although evidence is limited.12
Patients responsive to systemic corticosteroids (complete resolution or improvement to grade 1) can continue a tapered regimen over 4-6 weeks. There is conflicting evidence on the effect that corticosteroids have on ICI-related antitumor response rates. While some studies report no change in antitumor response rates or survival, others report reduced overall survival.13 Regardless, given its unfavorable side-effect profile, steroids should be used only for short periods of time.
PD-1 and PD-L1 antibodies can be restarted after symptoms have resolved or improved to grade 1, having finished the corticosteroid taper. CTLA-4 antibodies should be discontinued permanently in the setting of grade 3 toxicity. All ICIs should be discontinued permanently in grade 4 toxicity.
A grading system also exists for ICI-associated hepatitis (Table 2) and AP (Table 3). Patients with elevated aminotransferases greater than 2x upper limit of normal (ULN) should have alternative etiologies excluded. A thorough medication reconciliation, including over-the-counter and nonpharmaceutical supplements, should be performed. All potentially-hepatotoxic drugs and substances (including alcohol) should be discontinued. Viral hepatitis serology (A,B,C), Epstein-Barr virus, and cytomegalovirus also should be performed. Additional tests, including prothrombin time and albumin, can help assess for liver synthetic dysfunction. Abdominal ultrasound or CT can assist in excluding biliary obstruction or metastatic disease. Magnetic resonance cholangiopancreatography (MRCP) can be considered for further evaluation of biliary obstruction in patients with hyperbilirubinemia and normal ultrasound.14
Table 2 reviews the grading system and management of ICI-associated hepatitis. Patients with grade 3 and above should be hospitalized for treatment. As with the management of colitis, patients responding to corticosteroids should be tapered off over 4-6 weeks. In steroid-refractory cases or if there is no improvement after 3 days, mycophenolate mofetil is used. Other immunomodulators such as azathioprine and tacrolimus also can be considered, although evidence is limited.15 ICI-associated cholangitis presenting with elevated bilirubin and alkaline phosphatase is approached similarly to ICI-associated hepatitis. Abnormal findings of biliary obstruction or sclerosing cholangitis should be further evaluated with endoscopic retrograde cholangiopancreatography.
Mild asymptomatic elevation in lipase and amylase <3x ULN can be managed with observation and ICIs can be safely continued. Symptomatic patients should have a diagnostic workup for other etiologies. As with hepatitis, a thorough history including alcohol intake and a medication reconciliation should be performed. In the absence of other etiologies, grade 2 ICI-associated AP is managed by holding immunotherapy, administering steroids, and managing AP with fluid resuscitation and analgesia.
Conclusions
Therapy with ICI is a rapidly expanding and changing field. Side effects of ICIs can affect nearly every organ system, and thus management should involve a multidisciplinary team of oncologists, pathologists, radiologists, pharmacists, and other specialists. Given that GI adverse effects are the second most commonly affected system, all gastroenterologists and hepatologists should be knowledgeable about the spectrum of GI adverse events, as well as with the respective clinical presentations, diagnostics, and management of these events.
Dr. Kwon is with the division of gastroenterology and hepatology, University of California Irvine, Orange. Dr. Kröner is with the division of advanced endoscopy, Riverside Health System, Newport News, Va. The authors certify that they have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product. Funding: None.
References
1. Webster RM. The immune checkpoint inhibitors: where are we now? Nature Reviews: Drug Discovery. 2014;13(12):883.
2. Thompson JA et al. NCCN guidelines insights: Management of immunotherapy-related toxicities, version 1.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18(3):230-41.
3. Bertrand A et al. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015 Sep 4;13:211.
4. Gupta A et al. Systematic review: Colitis associated with anti‐CTLA‐4 therapy. Aliment Pharmacol Ther. 2015;42(4):406-17.
5. Verschuren EC et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016;14(6):836-42.
6. Foppen MHG et al. Immune checkpoint inhibition–related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open. 2018;3(1):e000278.
7. Sanjeevaiah A et al. Approach and management of checkpoint inhibitor–related immune hepatitis. J Gastrointest Oncol. 2018;9(1):220.
8. Abu-Sbeih H et al. Clinical characteristics and outcomes of immune checkpoint inhibitor–induced pancreatic injury. J Immunother Cancer. 2019 Feb 6;7(1):31.
9. Abu-Sbeih H et al. Case series of cancer patients who developed cholecystitis related to immune checkpoint inhibitor treatment. J Immunother Cancer. 2019 May 3;7(1):118.
10. Thompson JA et al. Management of immunotherapy-related toxicities, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(3):255-89.
11. Marthey L et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016;10(4):395-401.
12. Abu-Sbeih H et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: A multicenter study. J Immunother Cancer. 2018 Dec 5;6(1):142.
13. Das S and Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019 Nov 15;7(1):306.
14. Reddy HG et al. Immune checkpoint inhibitor–associated colitis and hepatitis. Clin Transl Gastroenterol. 2018 Sep 19;9(9):180.
15. Reynolds K et al. Diagnosis and management of hepatitis in patients on checkpoint blockade. Oncologist. 2018;23(9):991-7.
Kaposi’s sarcoma: Antiretroviral-related improvements in survival measured
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.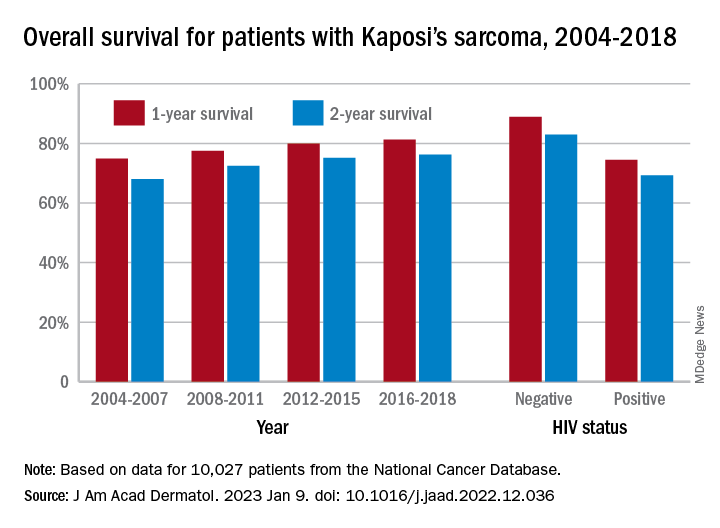
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
LDL cholesterol triglycerides ‘robust’ ASCVD risk marker
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Spikes out: A COVID mystery
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.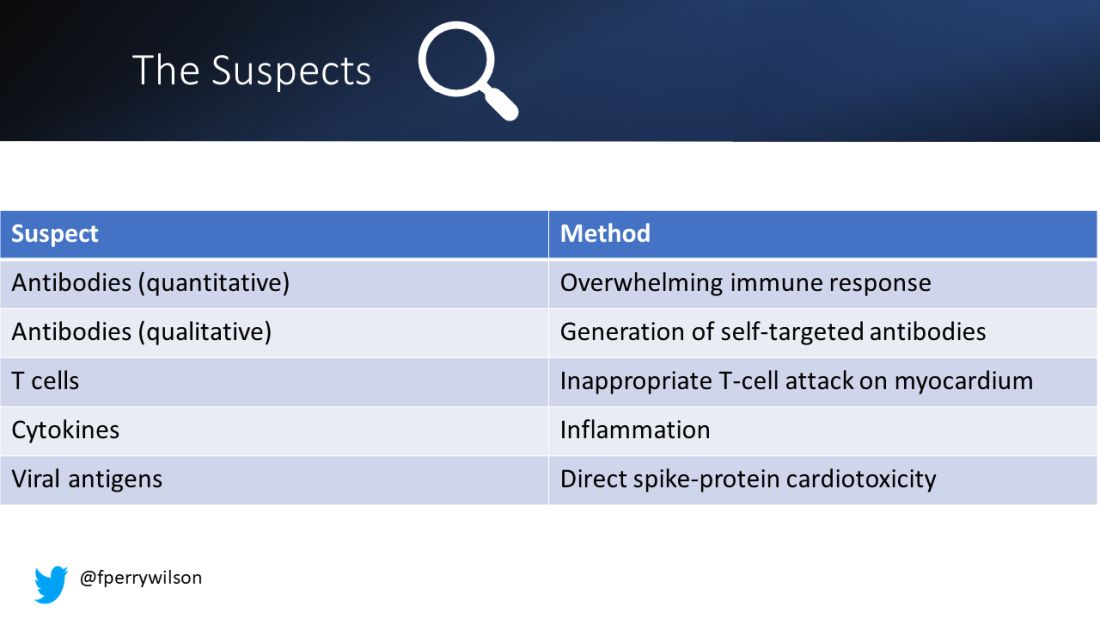
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.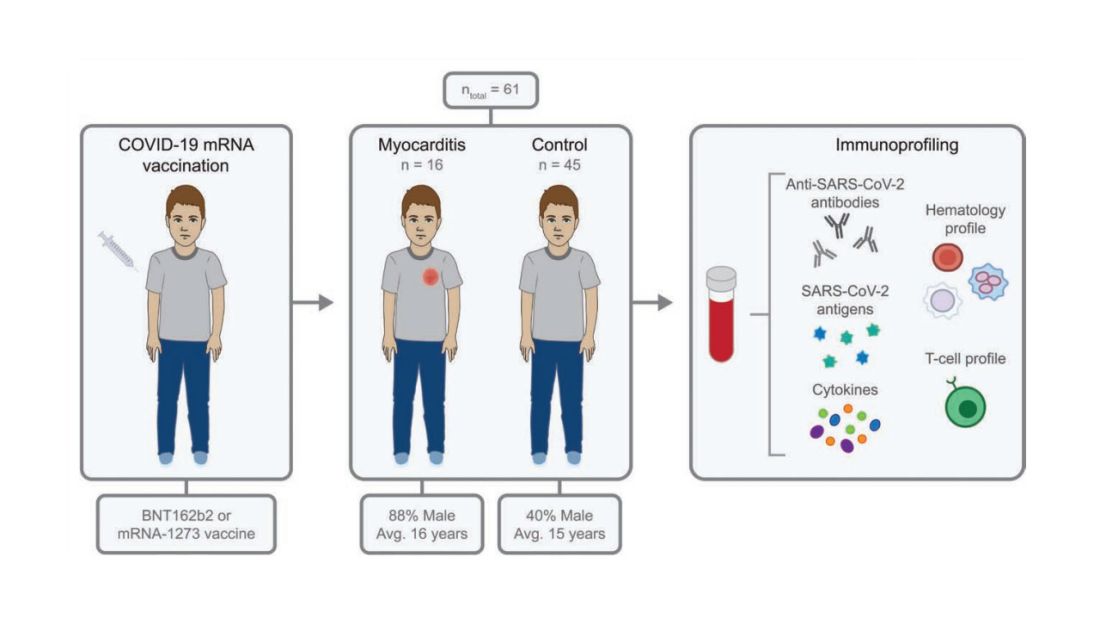
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.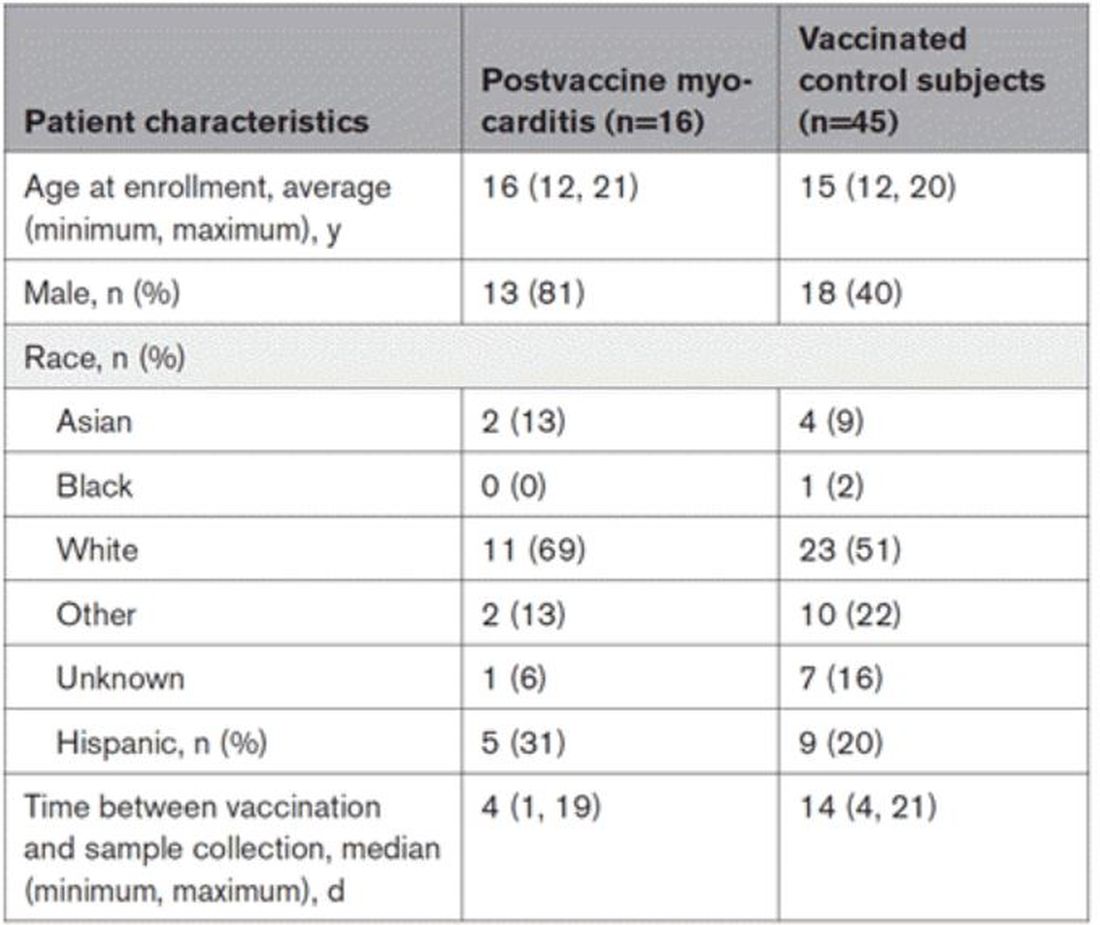
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.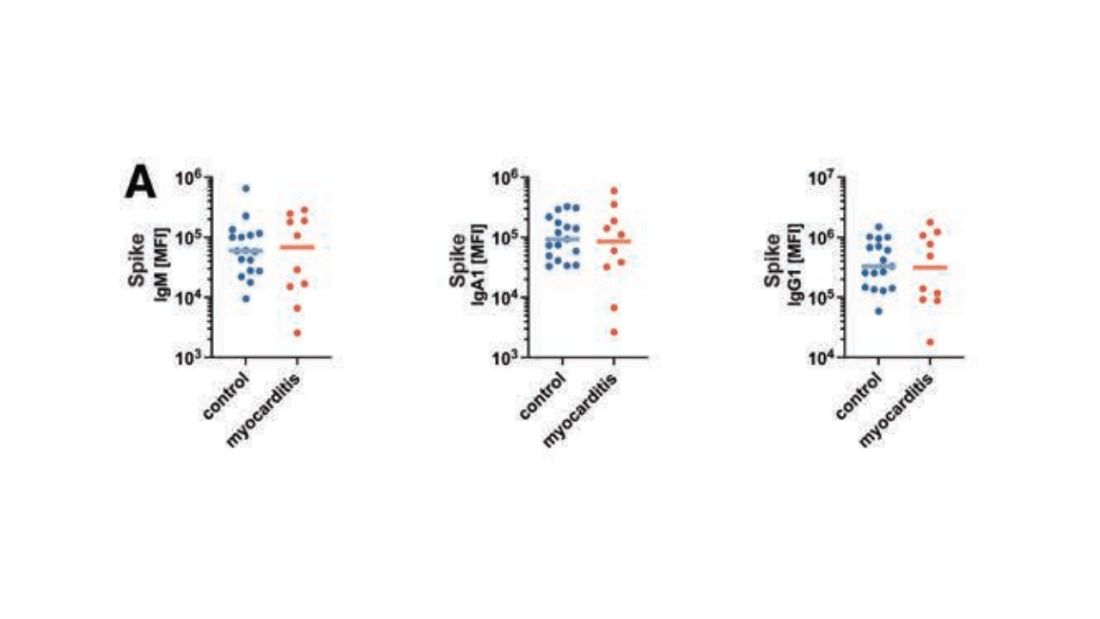
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.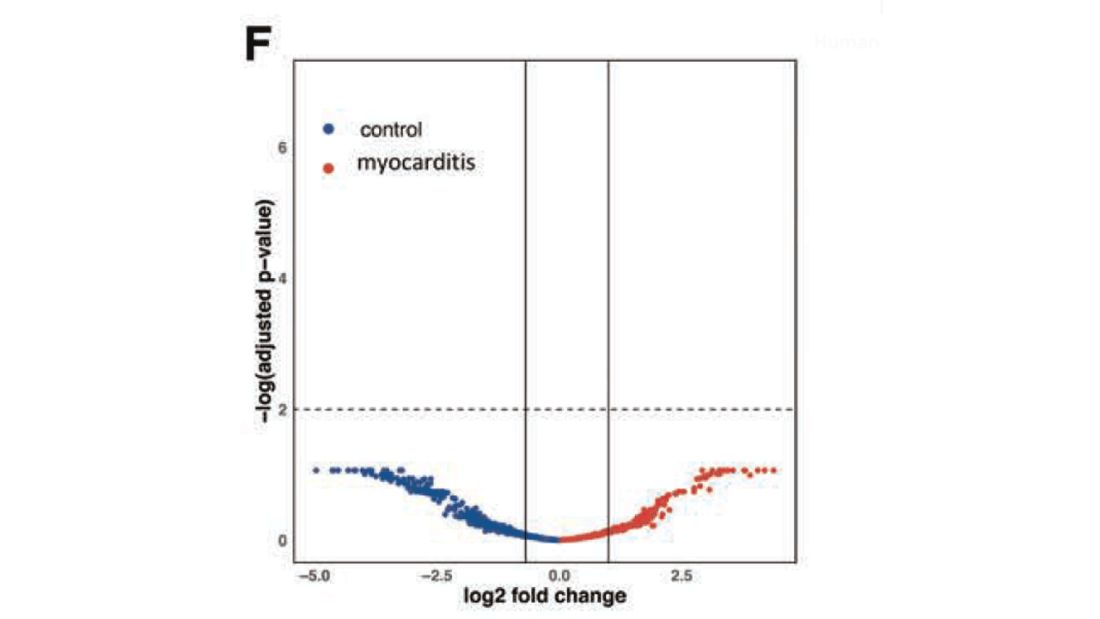
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.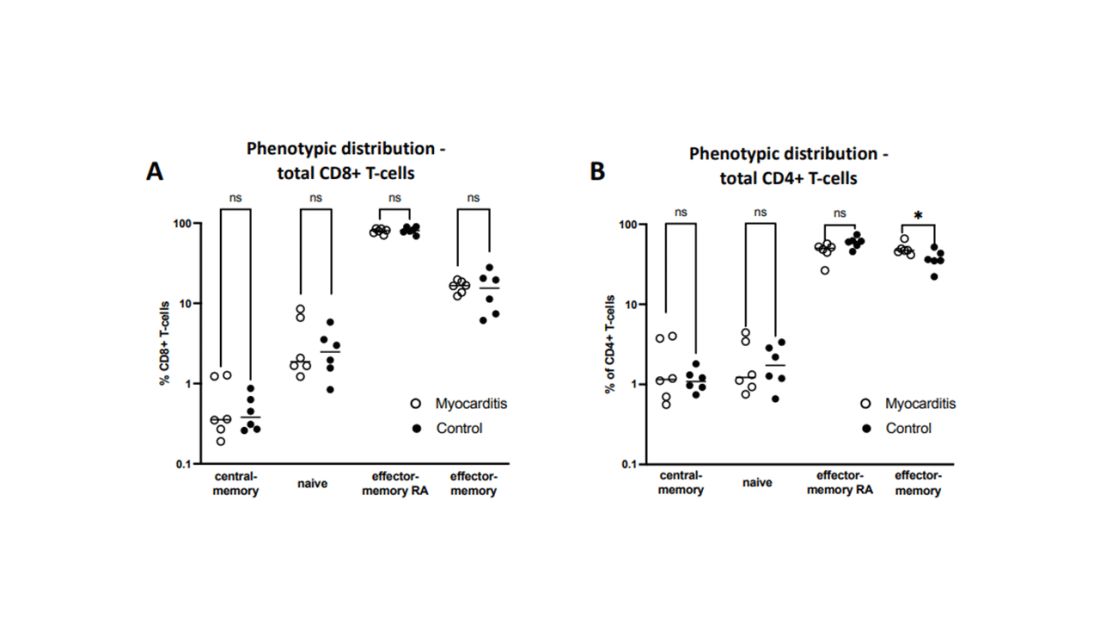
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.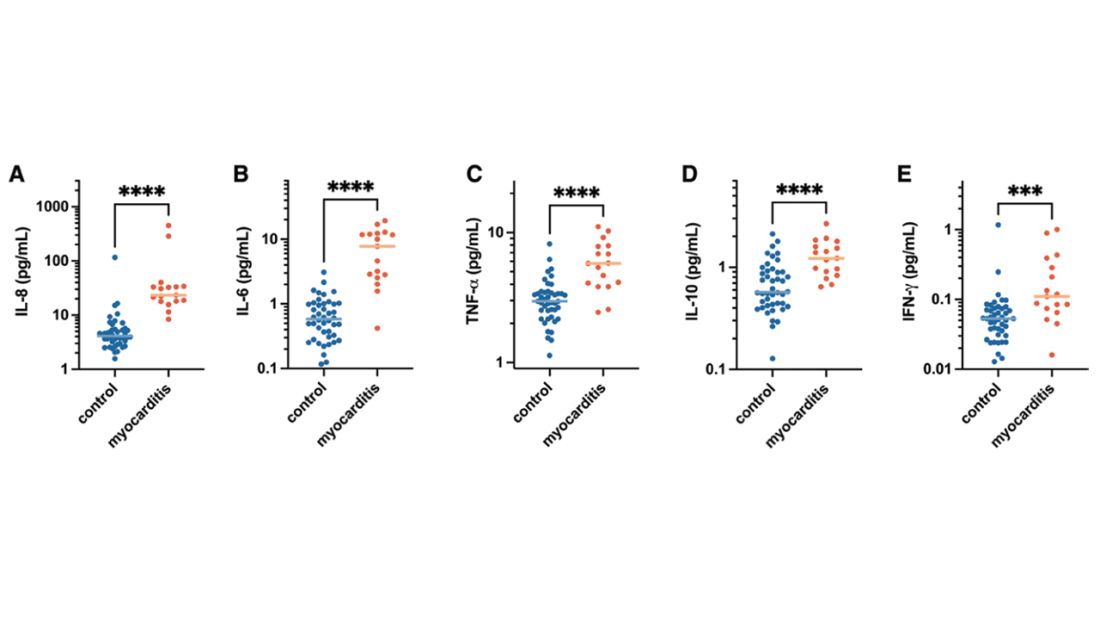
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.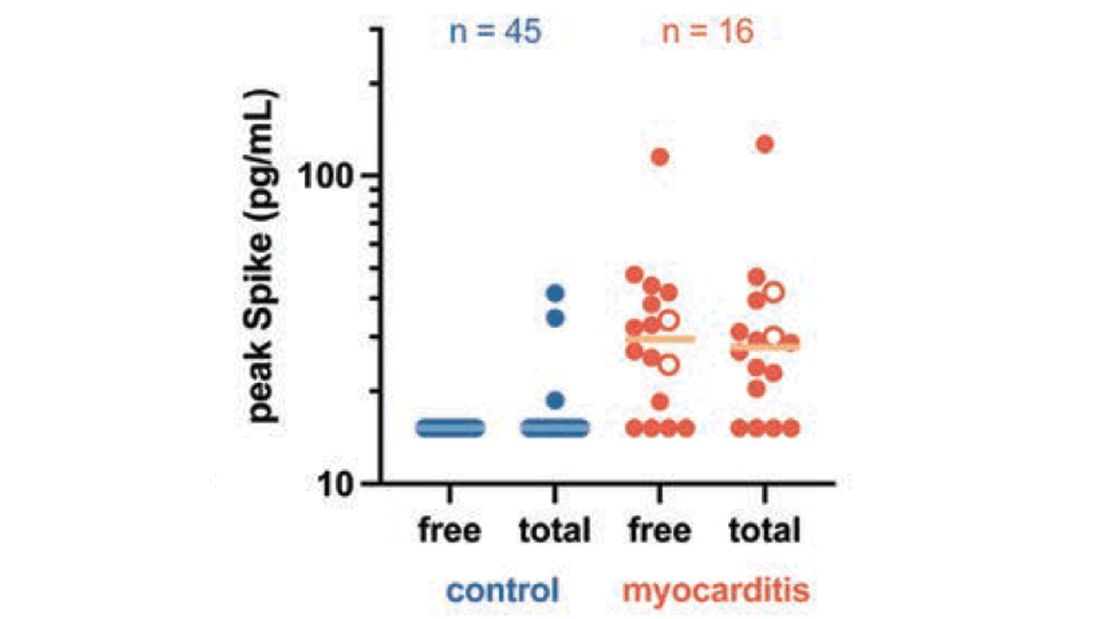
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!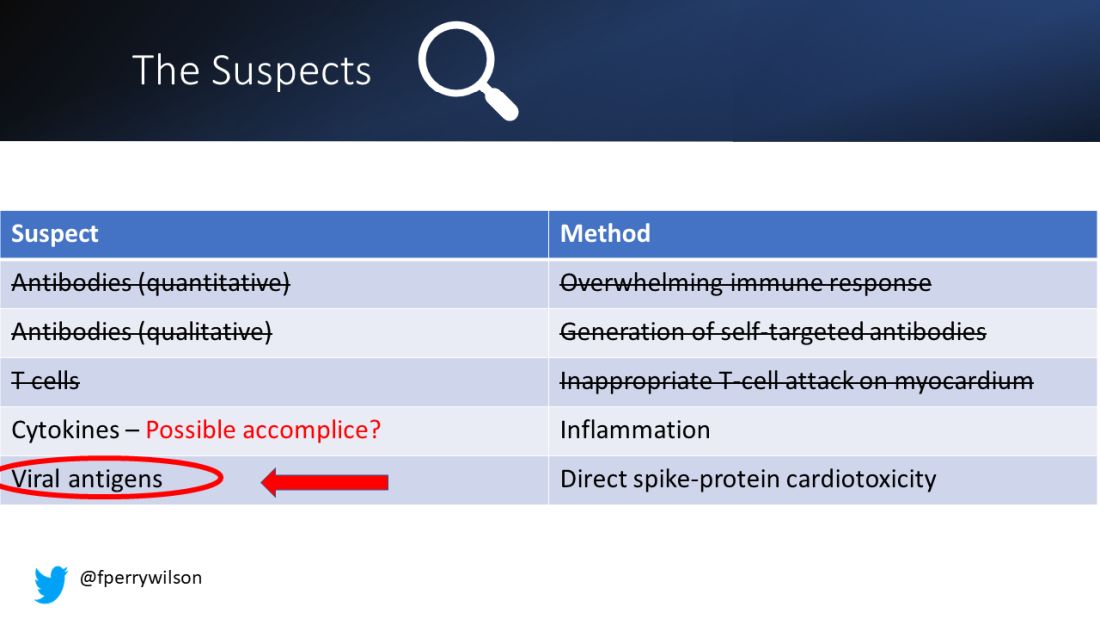
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
To date, it has been a mystery, like “Glass Onion.” And in the spirit of all the great mysteries, to get to the bottom of this, we’ll need to round up the usual suspects.
Appearing in Circulation, a new study does a great job of systematically evaluating multiple hypotheses linking vaccination to myocarditis, and eliminating them, Poirot-style, one by one until only one remains. We’ll get there.
But first, let’s review the suspects. Why do the mRNA vaccines cause myocarditis in a small subset of people?
There are a few leading candidates.
Number one: antibody responses. There are two flavors here. The quantitative hypothesis suggests that some people simply generate too many antibodies to the vaccine, leading to increased inflammation and heart damage.
The qualitative hypothesis suggests that maybe it’s the nature of the antibodies generated rather than the amount; they might cross-react with some protein on the surface of heart cells for instance.
Or maybe it is driven by T-cell responses, which, of course, are independent of antibody levels.
There’s the idea that myocarditis is due to excessive cytokine release – sort of like what we see in the multisystem inflammatory syndrome in children.
Or it could be due to the viral antigens themselves – the spike protein the mRNA codes for that is generated after vaccination.
To tease all these possibilities apart, researchers led by Lael Yonker at Mass General performed a case-control study. Sixteen children with postvaccine myocarditis were matched by age to 45 control children who had been vaccinated without complications.
The matching was OK, but as you can see here, there were more boys in the myocarditis group, and the time from vaccination was a bit shorter in that group as well. We’ll keep that in mind as we go through the results.
OK, let’s start eliminating suspects.
First, quantitative antibodies. Seems unlikely. Absolute antibody titers were really no different in the myocarditis vs. the control group.
What about the quality of the antibodies? Would the kids with myocarditis have more self-recognizing antibodies present? It doesn’t appear so. Autoantibody levels were similar in the two groups.
Take antibodies off the list.
T-cell responses come next, and, again, no major differences here, save for one specific T-cell subtype that was moderately elevated in the myocarditis group. Not what I would call a smoking gun, frankly.
Cytokines give us a bit more to chew on. Levels of interleukin (IL)-8, IL-6, tumor necrosis factor (TNF)-alpha, and IL-10 were all substantially higher in the kids with myocarditis.
But the thing about cytokines is that they are not particularly specific. OK, kids with myocarditis have more systemic inflammation than kids without; that’s not really surprising. It still leaves us with the question of what is causing all this inflammation? Who is the arch-villain? The kingpin? The don?
It’s the analyses of antigens – the protein products of vaccination – that may hold the key here.
In 12 out of 16 kids with myocarditis, the researchers were able to measure free spike protein in the blood – that is to say spike protein, not bound by antispike antibodies.
These free spikes were present in – wait for it – zero of the 45 control patients. That makes spike protein itself our prime suspect. J’accuse free spike protein!
Of course, all good detectives need to wrap up the case with a good story: How was it all done?
And here’s where we could use Agatha Christie’s help. How could this all work? The vaccine gets injected; mRNA is taken up into cells, where spike protein is generated and released, generating antibody and T-cell responses all the while. Those responses rapidly clear that spike protein from the system – this has been demonstrated in multiple studies – in adults, at least. But in some small number of people, apparently, spike protein is not cleared. Why? It makes no damn sense. Compels me, though. Some have suggested that inadvertent intravenous injection of vaccine, compared with the appropriate intramuscular route, might distribute the vaccine to sites with less immune surveillance. But that is definitely not proven yet.
We are on the path for sure, but this is, as Benoit Blanc would say, a twisted web – and we are not finished untangling it. Not yet.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available for preorder now. He reports no conflicts of interest.
A version of this article first appeared on Medscape.com.
Measles
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
I received a call late one night from a colleague in the emergency department of the children’s hospital. “This 2-year-old has a fever, cough, red eyes, and an impressive rash. I’ve personally never seen a case of measles, but I’m worried given that this child has never received the MMR vaccine.”
By the end of the call, I was worried too. Measles is a febrile respiratory illness classically accompanied by cough, coryza, conjunctivitis, and a characteristic maculopapular rash that begins on the face and spreads to the trunk and limbs. It is also highly contagious: 90% percent of susceptible, exposed individuals become infected.
Admittedly, measles is rare. Just 118 cases were reported in the United States in 2022, but 83 of those were in Columbus just 3 hours from where my colleague and I live and work. According to City of Columbus officials, the outbreak occurred almost exclusively in unimmunized children, the majority of whom were 5 years and younger. An unexpectedly high number of children were hospitalized. Typically, one in five people with measles will require hospitalization. In this outbreak, 33 children have been hospitalized as of Jan. 10.
Public health experts warn that 2023 could be much worse unless we increase measles immunization rates in the United States and globally. Immunization of around 95% of eligible people with two doses of measles-containing vaccine is associated with herd immunity. Globally, we’re falling short. Only 81% of the world’s children have received their first measle vaccine dose and only 71% have received the second dose. These are the lowest coverage rates for measles vaccine since 2008.
A 2022 joint press release from the Centers for Disease Control and Prevention and the World Health Organization noted that “measles anywhere is a threat everywhere, as the virus can quickly spread to multiple communities and across international borders.” Some prior measles outbreaks in the United States have started with a case in an international traveler or a U.S. resident who contracted measles during travel abroad.
In the United States, the number of children immunized with multiple routine vaccines has fallen in the last couple of years, in part because of pandemic-related disruptions in health care delivery. Increasing vaccine hesitancy, fueled by debates over the COVID-19 vaccine, may be slowing catch-up immunization in kids who fell behind.
Investigators from Emory University, Atlanta, and Marshfield Clinic Research Institute recently estimated that 9,145,026 U.S. children are susceptible to measles. If pandemic-level immunization rates continue without effective catch-up immunization, that number could rise to more than 15 million.
School vaccination requirements support efforts to ensure that kids are protected against vaccine-preventable diseases, but some data suggest that opposition to requiring MMR vaccine to attend public school is growing. According to a 2022 Kaiser Family Foundation Vaccine Monitor survey, 28% of U.S. adults – and 35% of parents of children under 18 – now say that parents should be able to decide to not vaccinate their children for measles, mumps, and rubella. That’s up from 16% of adults and 23% of parents in a 2019 Pew Research Center poll.
Public confidence in the benefits of MMR has also dropped modestly. About 85% of adults surveyed said that the benefits of MMR vaccine outweigh the risk, down from 88% in 2019. Among adults not vaccinated against COVID-19, only 70% said that benefits of these vaccines outweigh the risks.
While the WHO ramps up efforts to improve measles vaccination globally, pediatric clinicians can take steps now to mitigate the risk of measles outbreaks in their own communities. Query health records to understand how many eligible children in your practice have not yet received MMR vaccine. Notify families that vaccination is strongly recommended and make scheduling an appointment to receive vaccine easy. Some practices may have the bandwidth to offer evening and weekend hours for vaccine catch-up visits.
Curious about immunization rates in your state? The American Academy of Pediatrics has an interactive map that reports immunization coverage levels by state and provides comparisons to national rates and goals.
Prompt recognition and isolation of individuals with measles, along with prophylaxis of susceptible contacts, can limit community transmission. Measles can resemble other illnesses associated with fever and rash. Washington state has developed a screening tool to assist with recognition of measles. The CDC also has a measles outbreak toolkit that includes resources that outline clinical features and diagnoses, as well as strategies for talking to parents about vaccines.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant disclosed that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected].
Study spotlights clinicopathologic features, survival outcomes of pediatric melanoma
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
ADHD beyond medications
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
Attention-deficit/hyperactivity disorder (ADHD) is often a very challenging condition for parents to manage, both because of the “gleeful mayhem” children with ADHD manifest and because of the nature of effective treatments. Multiple randomized controlled studies and meta-analyses have demonstrated that stimulant medication with behavioral interventions is the optimal first-line treatment for children with both subtypes of ADHD, and that medications alone are superior to behavioral interventions alone. By improving attention and impulse control, the medications effectively decrease the many negative interactions with teachers, peers, and parents, aiding development and healthy self-esteem.
But many parents feel anxious about treating their young children with stimulants. Importantly, how children with ADHD will fare as adults is not predicted by their symptom level, but instead by the quality of their relationships with their parents, their ability to perform at school, and their social skills. Bring this framework to parents as you listen to their questions and help them decide on the best approach for their family. To assist you in these conversations, we will review the evidence for (or against) several of the most common alternatives to medication that parents are likely to ask about.
Diets and supplements
Dietary modifications are among the most popular “natural” approaches to managing ADHD in children. Diets that eliminate processed sugars or food additives (particularly artificial food coloring) are among the most common approaches discussed in the lay press. These diets are usually very time-consuming and disruptive for families to follow, and there is no evidence to support their general use in ADHD management. Those studies that rigorously examined them suggest that, for children with severe impairment who have failed to respond to medications for ADHD, a workup for food intolerance or nutritional deficits may reveal a different problem underlying their behavioral difficulties.1
Similarly, supplementation with high-dose omega-3 fatty acids is modestly helpful only in a subset of children with ADHD symptoms, and not nearly as effective as medications or behavioral interventions. Spending time on an exacting diet or buying expensive supplements is very unlikely to relieve the children’s symptoms and may only add to their stress at home. The “sugar high” parents note may be the rare joy of eating a candy bar and not sugar causing ADHD. Offer parents the guidance to focus on a healthy diet, high in fruits and vegetables, whole grains, and healthy protein, and on meals that emphasize family time instead of struggles around food.
Neurofeedback
Neurofeedback is an approach that grew out of the observation that many adults with ADHD had resting patterns of brain wave activity different from those of neurotypical adults. In neurofeedback, patients learn strategies that amplify the brain waves associated with focused mental activity, rather than listless or hyperactive states. Businesses market this service for all sorts of illnesses and challenges, ADHD chief among them. Despite the marketing, there are very few randomized controlled studies of this intervention for ADHD in youth, and those have shown only the possibility of a benefit.
While there is no evidence of serious side effects, these treatments are time-consuming and expensive and unlikely to be covered by any insurance. You might suggest to parents that they could achieve some of the same theoretical benefits by looking for hobbies that invite sustained focus in their children. That is, they should think about activities that interest the children, such as music lessons or karate, that they could practice in classes and at home. If the children find these activities even somewhat interesting (or just enjoy the reward of their parents’ or teachers’ attention), regular practice will be supporting their developing attention while building social skills and authentic self-confidence, rather than the activities feeling like a treatment for an illness or condition.
Sleep and exercise
There are not many businesses or books selling worried and exhausted parents a quick nonmedication solution for their children’s ADHD in the form of healthy sleep and exercise habits. But these are safe and healthy ways to reduce symptoms and support development. Children with ADHD often enjoy and benefit from participating in a sport, and daily exercise can help with sleep and regulating their energy. They also often have difficulty with sleep initiation, and commonly do not get adequate or restful sleep. Inadequate sleep exacerbates inattention, distractibility, and irritability. Children with untreated ADHD also often spend a lot of time on screens, as it is difficult for them to shift away from rewarding activities, and parents can find screen time to be a welcome break from hyperactivity and negative interactions. But excessive screen time, especially close to bedtime, can worsen irritability and make sleep more difficult. Talk with parents about the value of establishing a routine around screen time, modest daily physical activity, and sleep that everyone can follow. If their family life is currently marked by late bedtimes and long hours in front of video games, this change will take effort. But within a few weeks, it could lead to significant improvements in energy, attention, and interactions at home.
Behavioral treatments
Effective behavioral treatments for ADHD do not change ADHD symptoms, but they do help children learn how to manage them. In “parent management training,” younger children and parents learn together how to avoid negative cycles of behavior (i.e., temper outbursts) by focusing on consistent routines and consequences that support children calmly learning to manage their impulses. The only other evidence-based treatment focuses on helping school age and older children develop executive functions – their planning, organization, and time management skills – with a range of age-appropriate tools. Both of these therapies may be more effective if the children are also receiving medication, but medication is not necessary for them to be helpful. It is important to note that play therapy and other evidence-based psychotherapies are not effective for management of ADHD, although they may treat comorbid problems.
Parent treatment
You may have diagnosed children with ADHD only to hear their parents respond by saying that they suspect (or know) that they (or their spouses) also have ADHD. This would not be surprising, as ADHD has one of the highest rates of heritability of psychiatric disorders, at 80%. Somewhere between 25% and 50% of parents of children with ADHD have ADHD themselves.2 Screening for adults with ADHD, such as the Adult ADHD Self-Report Scale, is widely available and free. Speak with parents about the fact that behavioral treatments for their children’s ADHD are demanding. Such treatments require patience, calm, organization, and consistency.
If parents have ADHD, it may be very helpful for them to prioritize their own effective treatments, so that their attention and impulse control will support their parenting. They may be interested in learning about how treatment might also improve their performance at work and even the quality of their relationships. While there is some evidence that their children’s treatment outcome will hinge on the parents’ treatment,3 they deserve good care independent of the expectations of parenting.
Families benefit from a comprehensive “ADHD plan” for their children. This would start with an assessment of the severity of their children’s symptoms, specifying their impairment at home, school, and in social relationships. It would include their nonacademic performance, exploration of interests, and developing self-confidence. All of these considerations lead to setting reasonable expectations so the children can feel successful. Parents should think about how best to structure their children’s schedules to promote healthy sleep, exercise, and nutrition, and to expand opportunities for building their frustration tolerance, social skills, and executive function.
Parents will need to consider what kind of supports they themselves need to offer this structure. There are good resources available online for information and support, including Children and Adults with ADHD (chadd.org) and the ADHD Resource Center from the American Academy of Child and Adolescent Psychiatry (aacap.org). This approach may help parents to evaluate the potential risks and benefits of medications as a component of treatment. Most of the quick fixes for childhood ADHD on the market will take a family’s time and money without providing meaningful improvement. Parents should focus instead on the tried-and-true routines and supports that will help them to create the setting at home that will enable their children to flourish.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Millichap JG and Yee MM. Pediatrics. 2012 Feb;129(2):330-7.
2. Grimm O et al. Curr Psychiatry Rep. 2020 Feb 27;22(4):18.
3. Chronis-Tuscano A et al. J Abnorm Child Psychol. 2017 Apr;45(3):501-7.
New Omicron subvariant is ‘crazy infectious,’ COVID expert warns
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
Autopsies show COVID virus invades entire body
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
A study on the subject was published in the journal Nature. The researchers completed autopsies from April 2020 to March 2021 of 44 unvaccinated people who had severe COVID-19. The median age was 62.5 years old, and 30% were female. Extensive brain sampling was done for 11 cases.
Because of its nature as a respiratory illness, SARS-CoV-2 was most widespread in the respiratory system such as in the lungs. But it was also found in 79 other body locations, including the heart, kidneys, liver, muscles, nerves, reproductive tract, and eyes.
The researchers said their work shows the SARS-CoV-2 “is capable of infecting and replicating within the human brain.” They also said their results indicate the virus spreads via the blood early during infection, which “seeds the virus throughout the body following infection of the respiratory tract.”
The authors noted that, while the virus was found outside the respiratory tract, they did not find signs of inflammation beyond the respiratory system.
The results will help narrow down treatments for long COVID, and particularly support the idea of using the antiviral drug Paxlovid to treat long COVID, according to a blog post from the National Institute of Allergy and Infectious Diseases. A clinical trial is already underway examining the treatment, and results are expected in January 2024.
A version of this article first appeared on WebMD.com.
FROM NATURE