User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Moderna COVID-19 vaccine wins decisive recommendation from FDA panel
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) put Moderna’s application before its Vaccines and Related Biological Products Advisory Committee. The panel voted 20-0 on this question: “Based on the totality of scientific evidence available, do the benefits of the Moderna COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?” There was one abstention.
The FDA is not bound to act on the recommendations of its advisers, but the agency usually takes the panel’s advice. The FDA cleared the similar Pfizer-BioNTech vaccine on December 11 through an emergency use authorization (EUA), following a positive vote for the product at a December 10 advisory committee meeting. In this case, the FDA staff appeared to be pushing for a broad endorsement of the Moderna vaccine, for which the agency appears likely to soon also grant an EUA.
Marion Gruber, PhD, director of the Office of Vaccines Research and Review at FDA’s Center for Biologics Evaluation and Research, earlier rebuffed attempts by some of the panelists to alter the voting question. Some panelists wanted to make tweaks, including a rephrasing to underscore the limited nature of an EUA, compared with a more complete approval through the biologics license application (BLA) process.
FDA panelist Michael Kurilla, MD, PhD, of the National Institutes of Health was the only panelist to abstain from voting. He said he was uncomfortable with the phrasing of the question.
“In the midst of a pandemic and with limited vaccine supply available, a blanket statement for individuals 18 years and older is just too broad,” he said. “I’m not convinced that for all of those age groups the benefits do actually outweigh the risks.”
In general, though, there was strong support for Moderna’s vaccine. FDA panelist James Hildreth Sr, MD, PhD, of Meharry Medical College in Nashville, Tennessee spoke of the “remarkable achievement” seen in having two vaccines ready for clearance by December for a virus that only emerged as a threat this year.
Study data indicate the primary efficacy endpoint demonstrated vaccine efficacy (VE) of 94.1% (95% CI, 89.3% - 96.8%) for the Moderna vaccine, with 11 COVID-19 cases in the vaccine group and 185 COVID-19 cases in the placebo group, the FDA staff noted during the meeting.
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines. Also of concern to the panel were early reports of allergic reactions seen with the Pfizer product.
Doran L. Fink, MD, PhD, an FDA official who has been closely involved with the COVID-19 vaccines, told the panel that two healthcare workers in Alaska had allergic reactions minutes after receiving the Pfizer vaccine, one of which was a case of anaphylactic reaction that resulted in hospitalization.
In the United Kingdom, there were two cases reported of notable allergic reactions, leading regulators there to issue a warning that people who have a history of significant allergic reactions should not currently receive the Pfizer-BioNTech vaccine.
The people involved in these incidents have recovered or are recovering, Fink said. But the FDA expects there will be additional reports of allergic reactions to COVID-19 vaccines.
“These cases underscores the need to remain vigilant during the early phase of the vaccination campaign,” Fink said. “To this end, FDA is working with Pfizer to further revise factsheets and prescribing information for their vaccine to draw attention to CDC guidelines for post- vaccination monitoring and management of immediate allergic reactions.”
mRNA vaccines in the lead
An FDA emergency clearance for Moderna’s product would be another vote of confidence in a new approach to making vaccines. Both the Pfizer-BioNTech and Moderna vaccines provide the immune system with a kind of blueprint in the form of genetic material, mRNA. The mRNA sets the stage for the synthesis of the signature spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells.
In a December 15 commentary for this news organization Michael E. Pichichero, MD, wrote that the “revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced.”
“This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab — and it can be done incredibly fast,” he wrote.
The FDA allowed one waiver for panelist James K. Hildreth in connection with his personal relationship to a trial participant and his university’s participation in vaccine testing.
This article first appeared on Medscape.com.
Vaccine rollout on track, expect 300 million doses through March: Feds
If the initial success of the Pfizer-BioNTech rollout continues, and emergency use authorization (EAU) is granted to Moderna and Johnson & Johnson vaccines in development, Operation Warp Speed officials expect to have 300 million doses of COVID-19 vaccines to distribute across the United States between now and March 31.
The initial rollout remains on track, said Alex Azar, US Department of Health and Human Services (HHS) secretary, during a media briefing today. “We continue to have good news to report. As of today, shipments of vaccine will have been delivered to every delivery site identified by public health jurisdictions for our first wave of shipments.”
Anomalies in shipments to California and Alabama arose when temperature monitors showed the Pfizer vaccine dropped lower than the recommended -80 ºC (-112 °F). These vaccine trays remained on delivery trucks and were returned to Pfizer for prompt replacement, said Operation Warp Speed Chief Operating Officer Gen. Gustave F. Perna.
Azar estimated another 2 million doses of the Pfizer vaccine will be available next week. “And if the Moderna vaccine is authorized by the FDA in the coming days, we have allocated nearly 5.9 million doses of that product.”
The Moderna vaccine data released this week look promising, said Moncef Slaoui, PhD, Operation Warp Speed chief scientific adviser. “In the short term, I expect the protection to be quite significant.”
The findings in the first 2 weeks after the first dose show up to 65% protection, he said, and predicted the second-dose efficacy data will be coming in the next few weeks.
Enrollment in the phase 3 Johnson & Johnson trial with nearly 44,000 participants is expected to end December 17. Initial efficacy results are anticipated by early January, with more complete efficacy numbers by late January, Slaoui said.
The AstraZeneca COVID-19 vaccine trial also is underway with enrollment continuing. “We expect accruement to end in late December or early next year, with first results expected probably in February,” Slaoui said.
Antibody treatments underutilized
The media briefing also addressed COVID-19 therapeutics. Azar reported low uptake of available antibody therapies. “I want to remind Americans that there are two authorized antibody treatments that Operation Warp Speed has supported. They can help prevent hospitalization in those patients with the highest risk for severe disease.”
The higher-risk group includes those who are 65 and older and people with comorbid conditions that put them at increased risk for COVID-19 hospitalization.
The federal government allocated more than 330,000 doses of these treatments and many states have product available, Azar said.
Slaoui agreed, saying there is a “disappointing level of usage of monoclonal antibody therapy in hospitals. We look forward to that improving.”
Up to 3 billion vaccine doses possible
“We now have more than 900 million doses of the vaccine we have contracted delivery for,” Azar said. The government has options to increase that to a total of 3 billion doses.
In addition to the 100 million Pfizer vaccine doses and 100 million Moderna doses already ordered, the government just took an option for another 100 million Moderna doses for the second quarter of 2021. Operation Warp Speed officials are negotiating with Pfizer for additional product as well.
Azar added that there are 100 million doses of the Johnson & Johnson vaccine in active production and expects AstraZeneca can provide 300 million doses of their product.
With the possibility of three or more vaccine products and with 330 million Americans, minus the 70 million or so children under age 16, “we believe we will actually have surplus supplies,” Azar said. Plans are to take the US surplus vaccine and surplus manufacturing capacity “and use that for the benefit of the world community.”
This article first appeared on Medscape.com.
If the initial success of the Pfizer-BioNTech rollout continues, and emergency use authorization (EAU) is granted to Moderna and Johnson & Johnson vaccines in development, Operation Warp Speed officials expect to have 300 million doses of COVID-19 vaccines to distribute across the United States between now and March 31.
The initial rollout remains on track, said Alex Azar, US Department of Health and Human Services (HHS) secretary, during a media briefing today. “We continue to have good news to report. As of today, shipments of vaccine will have been delivered to every delivery site identified by public health jurisdictions for our first wave of shipments.”
Anomalies in shipments to California and Alabama arose when temperature monitors showed the Pfizer vaccine dropped lower than the recommended -80 ºC (-112 °F). These vaccine trays remained on delivery trucks and were returned to Pfizer for prompt replacement, said Operation Warp Speed Chief Operating Officer Gen. Gustave F. Perna.
Azar estimated another 2 million doses of the Pfizer vaccine will be available next week. “And if the Moderna vaccine is authorized by the FDA in the coming days, we have allocated nearly 5.9 million doses of that product.”
The Moderna vaccine data released this week look promising, said Moncef Slaoui, PhD, Operation Warp Speed chief scientific adviser. “In the short term, I expect the protection to be quite significant.”
The findings in the first 2 weeks after the first dose show up to 65% protection, he said, and predicted the second-dose efficacy data will be coming in the next few weeks.
Enrollment in the phase 3 Johnson & Johnson trial with nearly 44,000 participants is expected to end December 17. Initial efficacy results are anticipated by early January, with more complete efficacy numbers by late January, Slaoui said.
The AstraZeneca COVID-19 vaccine trial also is underway with enrollment continuing. “We expect accruement to end in late December or early next year, with first results expected probably in February,” Slaoui said.
Antibody treatments underutilized
The media briefing also addressed COVID-19 therapeutics. Azar reported low uptake of available antibody therapies. “I want to remind Americans that there are two authorized antibody treatments that Operation Warp Speed has supported. They can help prevent hospitalization in those patients with the highest risk for severe disease.”
The higher-risk group includes those who are 65 and older and people with comorbid conditions that put them at increased risk for COVID-19 hospitalization.
The federal government allocated more than 330,000 doses of these treatments and many states have product available, Azar said.
Slaoui agreed, saying there is a “disappointing level of usage of monoclonal antibody therapy in hospitals. We look forward to that improving.”
Up to 3 billion vaccine doses possible
“We now have more than 900 million doses of the vaccine we have contracted delivery for,” Azar said. The government has options to increase that to a total of 3 billion doses.
In addition to the 100 million Pfizer vaccine doses and 100 million Moderna doses already ordered, the government just took an option for another 100 million Moderna doses for the second quarter of 2021. Operation Warp Speed officials are negotiating with Pfizer for additional product as well.
Azar added that there are 100 million doses of the Johnson & Johnson vaccine in active production and expects AstraZeneca can provide 300 million doses of their product.
With the possibility of three or more vaccine products and with 330 million Americans, minus the 70 million or so children under age 16, “we believe we will actually have surplus supplies,” Azar said. Plans are to take the US surplus vaccine and surplus manufacturing capacity “and use that for the benefit of the world community.”
This article first appeared on Medscape.com.
If the initial success of the Pfizer-BioNTech rollout continues, and emergency use authorization (EAU) is granted to Moderna and Johnson & Johnson vaccines in development, Operation Warp Speed officials expect to have 300 million doses of COVID-19 vaccines to distribute across the United States between now and March 31.
The initial rollout remains on track, said Alex Azar, US Department of Health and Human Services (HHS) secretary, during a media briefing today. “We continue to have good news to report. As of today, shipments of vaccine will have been delivered to every delivery site identified by public health jurisdictions for our first wave of shipments.”
Anomalies in shipments to California and Alabama arose when temperature monitors showed the Pfizer vaccine dropped lower than the recommended -80 ºC (-112 °F). These vaccine trays remained on delivery trucks and were returned to Pfizer for prompt replacement, said Operation Warp Speed Chief Operating Officer Gen. Gustave F. Perna.
Azar estimated another 2 million doses of the Pfizer vaccine will be available next week. “And if the Moderna vaccine is authorized by the FDA in the coming days, we have allocated nearly 5.9 million doses of that product.”
The Moderna vaccine data released this week look promising, said Moncef Slaoui, PhD, Operation Warp Speed chief scientific adviser. “In the short term, I expect the protection to be quite significant.”
The findings in the first 2 weeks after the first dose show up to 65% protection, he said, and predicted the second-dose efficacy data will be coming in the next few weeks.
Enrollment in the phase 3 Johnson & Johnson trial with nearly 44,000 participants is expected to end December 17. Initial efficacy results are anticipated by early January, with more complete efficacy numbers by late January, Slaoui said.
The AstraZeneca COVID-19 vaccine trial also is underway with enrollment continuing. “We expect accruement to end in late December or early next year, with first results expected probably in February,” Slaoui said.
Antibody treatments underutilized
The media briefing also addressed COVID-19 therapeutics. Azar reported low uptake of available antibody therapies. “I want to remind Americans that there are two authorized antibody treatments that Operation Warp Speed has supported. They can help prevent hospitalization in those patients with the highest risk for severe disease.”
The higher-risk group includes those who are 65 and older and people with comorbid conditions that put them at increased risk for COVID-19 hospitalization.
The federal government allocated more than 330,000 doses of these treatments and many states have product available, Azar said.
Slaoui agreed, saying there is a “disappointing level of usage of monoclonal antibody therapy in hospitals. We look forward to that improving.”
Up to 3 billion vaccine doses possible
“We now have more than 900 million doses of the vaccine we have contracted delivery for,” Azar said. The government has options to increase that to a total of 3 billion doses.
In addition to the 100 million Pfizer vaccine doses and 100 million Moderna doses already ordered, the government just took an option for another 100 million Moderna doses for the second quarter of 2021. Operation Warp Speed officials are negotiating with Pfizer for additional product as well.
Azar added that there are 100 million doses of the Johnson & Johnson vaccine in active production and expects AstraZeneca can provide 300 million doses of their product.
With the possibility of three or more vaccine products and with 330 million Americans, minus the 70 million or so children under age 16, “we believe we will actually have surplus supplies,” Azar said. Plans are to take the US surplus vaccine and surplus manufacturing capacity “and use that for the benefit of the world community.”
This article first appeared on Medscape.com.
COVID-19 vaccines: Safe for immunocompromised patients?
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Coronavirus vaccines have become a reality, as they are now being approved and authorized for use in a growing number of countries including the United States. The U.S. Food and Drug Administration has just issued emergency authorization for the use of the COVID-19 vaccine produced by Pfizer and BioNTech. Close behind is the vaccine developed by Moderna, which has also applied to the FDA for emergency authorization.
The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, and the FDA has said that the 95% credible interval for the vaccine efficacy was 90.3%-97.6%. But as with many initial clinical trials, whether for drugs or vaccines, not all populations were represented in the trial cohort, including individuals who are immunocompromised. At the current time, it is largely unknown how safe or effective the vaccine may be in this large population, many of whom are at high risk for serious COVID-19 complications.
At a special session held during the recent annual meeting of the American Society of Hematology, Anthony Fauci, MD, the nation’s leading infectious disease expert, said that individuals with compromised immune systems, whether because of chemotherapy or a bone marrow transplant, should plan to be vaccinated when the opportunity arises.
In response to a question from ASH President Stephanie J. Lee, MD, of the Fred Hutchinson Cancer Center, Seattle, Dr. Fauci emphasized that, despite being excluded from clinical trials, this population should get vaccinated. “I think we should recommend that they get vaccinated,” he said. “I mean, it is clear that, if you are on immunosuppressive agents, history tells us that you’re not going to have as robust a response as if you had an intact immune system that was not being compromised. But some degree of immunity is better than no degree of immunity.”
That does seem to be the consensus among experts who spoke in interviews: that as long as these are not live attenuated vaccines, they hold no specific risk to an immunocompromised patient, other than any factors specific to the individual that could be a contraindication.
“Patients, family members, friends, and work contacts should be encouraged to receive the vaccine,” said William Stohl, MD, PhD, chief of the division of rheumatology at the University of Southern California, Los Angeles. “Clinicians should advise patients to obtain the vaccine sooner rather than later.”
Kevin C. Wang, MD, PhD, of the department of dermatology at Stanford (Calif.) University, agreed. “I am 100% with Dr. Fauci. Everyone should get the vaccine, even if it may not be as effective,” he said. “I would treat it exactly like the flu vaccines that we recommend folks get every year.”
Dr. Wang noted that he couldn’t think of any contraindications unless the immunosuppressed patients have a history of severe allergic reactions to prior vaccinations. “But I would even say patients with history of cancer, upon recommendation of their oncologists, are likely to be suitable candidates for the vaccine,” he added. “I would say clinicians should approach counseling the same way they counsel patients for the flu vaccine, and as far as I know, there are no concerns for systemic drugs commonly used in dermatology patients.”
However, guidance has not yet been issued from either the FDA or the Centers for Disease Control and Prevention regarding the use of the vaccine in immunocompromised individuals. Given the lack of data, the FDA has said that “it will be something that providers will need to consider on an individual basis,” and that individuals should consult with physicians to weigh the potential benefits and potential risks.
The CDC’s Advisory Committee on Immunization Practices has said that clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies. The CDC itself has not yet released its formal guidance on vaccine use.
COVID-19 vaccines
Vaccines typically require years of research and testing before reaching the clinic, but this year researchers embarked on a global effort to develop safe and effective coronavirus vaccines in record time. Both the Pfizer/BioNTech and Moderna vaccines have only a few months of phase 3 clinical trial data, so much remains unknown about them, including their duration of effect and any long-term safety signals. In addition to excluding immunocompromised individuals, the clinical trials did not include children or pregnant women, so data are lacking for several population subgroups.
But these will not be the only vaccines available, as the pipeline is already becoming crowded. U.S. clinical trial data from a vaccine jointly being developed by Oxford-AstraZeneca, could potentially be ready, along with a request for FDA emergency use authorization, by late January 2021.
In addition, China and Russia have released vaccines, and there are currently 61 vaccines being investigated in clinical trials and at least 85 preclinical products under active investigation.
The vaccine candidates are using both conventional and novel mechanisms of action to elicit an immune response in patients. Conventional methods include attenuated inactivated (killed) virus and recombinant viral protein vaccines to develop immunity. Novel approaches include replication-deficient, adenovirus vector-based vaccines that contain the viral protein, and mRNA-based vaccines, such as the Pfizer and Moderna vaccines, that encode for a SARS-CoV-2 spike protein.
“The special vaccine concern for immunocompromised individuals is introduction of a live virus,” Dr. Stohl said. “Neither the Moderna nor Pfizer vaccines are live viruses, so there should be no special contraindication for such individuals.”
Live vaccine should be avoided in immunocompromised patients, and currently, live SARS-CoV-2 vaccines are only being developed in India and Turkey.
It is not unusual for vaccine trials to begin with cohorts that exclude participants with various health conditions, including those who are immunocompromised. These groups are generally then evaluated in phase 4 trials, or postmarketing surveillance. While the precise number of immunosuppressed adults in the United States is not known, the numbers are believed to be rising because of increased life expectancy among immunosuppressed adults as a result of advances in treatment and new and wider indications for therapies that can affect the immune system.
According to data from the 2013 National Health Interview Survey, an estimated 2.7% of U.S. adults are immunosuppressed. This population covers a broad array of health conditions and medical specialties; people living with inflammatory or autoimmune conditions, such as inflammatory rheumatic diseases (rheumatoid arthritis, axial spondyloarthritis, lupus); inflammatory bowel disease (Crohn’s disease and ulcerative colitis); psoriasis; multiple sclerosis; organ transplant recipients; patients undergoing chemotherapy; and life-long immunosuppression attributable to HIV infection.
As the vaccines begin to roll out and become available, how should clinicians advise their patients, in the absence of any clinical trial data?
Risk vs. benefit
Gilaad Kaplan, MD, MPH, a gastroenterologist and professor of medicine at the University of Calgary (Alta.), noted that the inflammatory bowel disease (IBD) community has dealt with tremendous anxiety during the pandemic because many are immunocompromised because of the medications they use to treat their disease.
“For example, many patients with IBD are on biologics like anti-TNF [tumor necrosis factor] therapies, which are also used in other immune-mediated inflammatory diseases such as rheumatoid arthritis,” he said. “Understandably, individuals with IBD on immunosuppressive medications are concerned about the risk of severe complications due to COVID-19.”
The entire IBD community, along with the world, celebrated the announcement that multiple vaccines are protective against SARS-CoV-2, he noted. “Vaccines offer the potential to reduce the spread of COVID-19, allowing society to revert back to normalcy,” Dr. Kaplan said. “Moreover, for vulnerable populations, including those who are immunocompromised, vaccines offer the potential to directly protect them from the morbidity and mortality associated with COVID-19.”
That said, even though the news of vaccines are extremely promising, some cautions must be raised regarding their use in immunocompromised populations, such as persons with IBD. “The current trials, to my knowledge, did not include immunocompromised individuals and thus, we can only extrapolate from what we know from other trials of different vaccines,” he explained. “We know from prior vaccines studies that the immune response following vaccination is less robust in those who are immunocompromised as compared to a healthy control population.”
Dr. Kaplan also pointed to recent reports of allergic reactions that have been reported in healthy individuals. “We don’t know whether side effects, like allergic reactions, may be different in unstudied populations,” he said. “Thus, the medical and scientific community should prioritize clinical studies of safety and effectiveness of COVID-19 vaccines in immunocompromised populations.”
So, what does this mean for an individual with an immune-mediated inflammatory disease like Crohn’s disease or ulcerative colitis who is immunocompromised? Dr. Kaplan explained that it is a balance between the potential harm of being infected with COVID-19 and the uncertainty of receiving a vaccine in an understudied population. For those who are highly susceptible to dying from COVID-19, such as an older adult with IBD, or someone who faces high exposure, such as a health care worker, the potential protection of the vaccine greatly outweighs the uncertainty.
“However, for individuals who are at otherwise lower risk – for example, young and able to work from home – then waiting a few extra months for postmarketing surveillance studies in immunocompromised populations may be a reasonable approach, as long as these individuals are taking great care to avoid infection,” he said.
No waiting needed
Joel M. Gelfand, MD, MSCE, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, feels that the newly approved vaccine should be safe for most of his patients.
“Patients with psoriatic disease should get the mRNA-based COVID-19 vaccine as soon as possible based on eligibility as determined by the CDC and local public health officials,” he said. “It is not a live vaccine, and therefore patients on biologics or other immune-modulating or immune-suppressing treatment can receive it.”
However, the impact of psoriasis treatment on immune response to the mRNA-based vaccines is not known. Dr. Gelfand noted that, extrapolating from the vaccine literature, there is some evidence that methotrexate reduces response to the influenza vaccine. “However, the clinical significance of this finding is not clear,” he said. “Since the mRNA vaccine needs to be taken twice, a few weeks apart, I do not recommend interrupting or delaying treatment for psoriatic disease while undergoing vaccination for COVID-19.”
Given the reports of allergic reactions, he added that it is advisable for patients with a history of life-threatening allergic reactions such as anaphylaxis or who have been advised to carry an epinephrine autoinjector, to talk with their health care provider to determine if COVID-19 vaccination is medically appropriate.
The National Psoriasis Foundation has issued guidance on COVID-19, explained Steven R. Feldman, MD, PhD, professor of dermatology, pathology, and social sciences & health policy at Wake Forest University, Winston-Salem, N.C., who is also a member of the committee that is working on those guidelines and keeping them up to date. “We are in the process of updating the guidelines with information on COVID vaccines,” he said.
He agreed that there are no contraindications for psoriasis patients to receive the vaccine, regardless of whether they are on immunosuppressive treatment, even though definitive data are lacking. “Fortunately, there’s a lot of good data coming out of Italy that patients with psoriasis on biologics do not appear to be at increased risk of getting COVID or of having worse outcomes from COVID,” he said.
Patients are going to ask about the vaccines, and when counseling them, clinicians should discuss the available data, the residual uncertainty, and patients’ concerns should be considered, Dr. Feldman explained. “There may be some concern that steroids and cyclosporine would reduce the effectiveness of vaccines, but there is no concern that any of the drugs would cause increased risk from nonlive vaccines.”
He added that there is evidence that “patients on biologics who receive nonlive vaccines do develop antibody responses and are immunized.”
Boosting efficacy
Even prior to making their announcement, the American College of Rheumatology had said that they would endorse the vaccine for all patients, explained rheumatologist Brett Smith, DO, from Blount Memorial Physicians Group and East Tennessee Children’s Hospital, Alcoa. “The vaccine is safe for all patients, but the problem may be that it’s not as effective,” he said. “But we don’t know that because it hasn’t been tested.”
With other vaccines, biologic medicines are held for 2 weeks before and afterwards, to get the best response. “But some patients don’t want to stop the medication,” Dr. Smith said. “They are afraid that their symptoms will return.”
As for counseling patients as to whether they should receive this vaccine, he explained that he typically doesn’t try to sway patients one way or another until they are really high risk. “When I counsel, it really depends on the individual situation. And for this vaccine, we have to be open to the fact that many people have already made up their mind.”
There are a lot of questions regarding the vaccine. One is the short time frame of development. “Vaccines typically take 6-10 years to come on the market, and this one is now available after a 3-month study,” Dr. Smith said. “Some have already decided that it’s too new for them.”
The process is also new, and patients need to understand that it doesn’t contain an active virus and “you can’t catch coronavirus from it.”
Dr. Smith also explained that, because the vaccine may be less effective in a person using biologic therapies, there is currently no information available on repeat vaccination. “These are all unanswered questions,” he said. “If the antibodies wane in a short time, can we be revaccinated and in what time frame? We just don’t know that yet.”
Marcelo Bonomi, MD, a medical oncologist from The Ohio State University Comprehensive Cancer Center, Columbus, explained that one way to ensure a more optimal response to the vaccine would be to wait until the patient has finished chemotherapy.* “The vaccine can be offered at that time, and in the meantime, they can take other steps to avoid infection,” he said. “If they are very immunosuppressed, it isn’t worth trying to give the vaccine.”
Cancer patients should be encouraged to stay as healthy as possible, and to wear masks and social distance. “It’s a comprehensive approach. Eat healthy, avoid alcohol and tobacco, and exercise. [These things] will help boost the immune system,” Dr. Bonomi said. “Family members should be encouraged to get vaccinated, which will help them avoid infection and exposing the patient.”
Jim Boonyaratanakornkit, MD, PhD, an infectious disease specialist who cares for cancer patients at the Fred Hutchinson Cancer Research Center, agreed. “Giving a vaccine right after a transplant is a futile endeavor,” he said. “We need to wait 6 months to have an immune response.”
He pointed out there may be a continuing higher number of cases, with high levels peaking in Washington in February and March. “Close friends and family should be vaccinated if possible,” he said, “which will help interrupt transmission.”
The vaccines are using new platforms that are totally different, and there is no clear data as to how long the antibodies will persist. “We know that they last for at least 4 months,” said Dr. Boonyaratanakornkit. “We don’t know what level of antibody will protect them from COVID-19 infection. Current studies are being conducted, but we don’t have that information for anyone yet.”
*Correction, 1/7/21: An earlier version of this article misattributed quotes from Dr. Marcelo Bonomi.
Six big changes coming for office-visit coding
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).
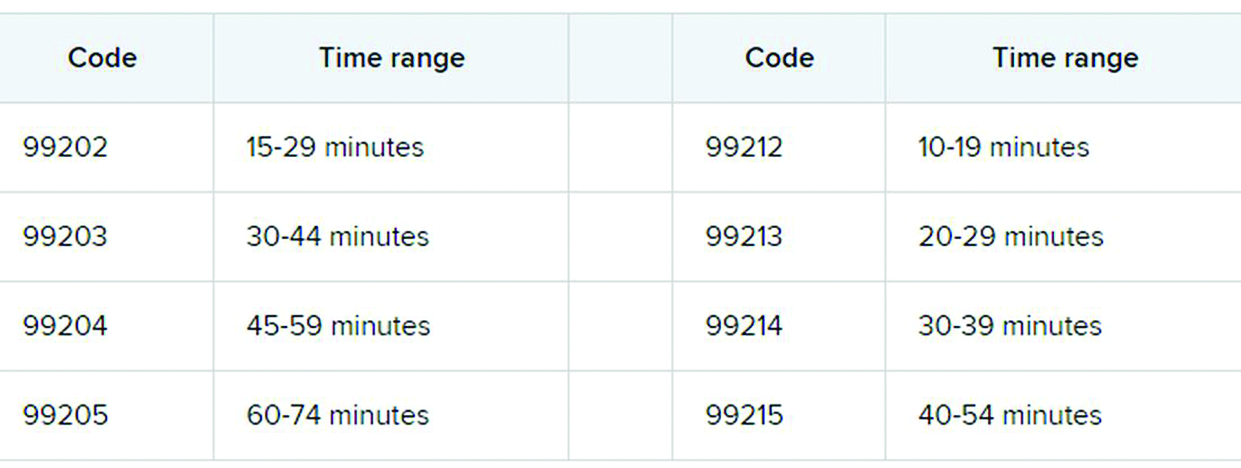
3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).

3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Betsy Nicoletti, MS, a nationally recognized coding expert, will take your coding questions via email and provide guidance on how to code properly to maximize reimbursement. Have a question about coding? Send it to [email protected].
thanks to the American Medical Association.
The first major changes to the definitions for E/M services will be in effect as of Jan. 1, 2021, with all payers expected to adopt these new guidelines. In particular, the AMA has revised the definitions for E/M codes 99202-99215 in the Current Procedural Terminology (CPT) 2021 codebook. The existing guidelines were developed in 1995 and 1997 and remain in effect for all other E/M services determined by history, exam, and medical decision-making (MDM).
What do the new changes mean to you? In 2021, for new and established office and other outpatient services reported with codes 99202-99215, a clinician may select the code on the basis of time or MDM.
There are three elements in MDM, and two of three are required. These elements are the number and complexity of problems addressed, amount and/or complexity of data to be reviewed and analyzed, and risk of complications and/or morbidity or mortality of patient management.
Make sure you familiarize yourself with these six big changes. It may take a bit of time to integrate these new processes into your daily routine, but wrapping your head around them as soon as possible can help boost your bottom line:
1. History and exam don’t count toward level of service
Physicians, advanced practice registered nurses, and physician assistants won’t use history or exam to select what level of code to bill for office visits 99202-99215, as they did in the past. They need only document a medically appropriate history and exam. The history may be obtained by staff members and reviewed by the billing practitioner.
While specific history and exam requirements disappear for office visit codes, they remain for all other types of visits, selected on the basis of history, exam, and MDM, such as hospital services, nursing facility services, and home and domiciliary care. So, say goodbye to “all other systems reviewed and negative” in office notes, but keep it handy for those other E/M codes.
2. All time spent caring for the patient on a particular day counts
This includes all time spent on the day of service, including preparing to see the patient, seeing the patient, phone calls or other work done after the visit (if not billed with a care management or other CPT code), and documenting in the medical record. The AMA developed new guidelines for using time for office and other outpatient services. For codes 99202-99215, count all of the face-to-face and non–face-to-face time spent by the billing clinician on the day of the visit. Counseling does not need to be more than 50% of the total time.
Do not include any staff time or time spent on any days before or after the visit. This allows clinicians to capture the work when a significant amount of it takes place before or after the visit with the patient, and to bill for it on the day of the visit.
According to the 2021 CPT codebook, physician or other qualified health care professional time includes the following activities:
- Preparing to see the patient (e.g., review of tests).
- Obtaining and/or reviewing separately obtained history.
- Performing a medically appropriate examination and/or evaluation.
- Counseling and educating the patient/family/caregiver.
- Ordering medications, tests, or procedures.
- Referring and communicating with other health care professionals (when not separately reported).
- Documenting clinical information in the electronic or other health record.
- Independently interpreting results (not separately reported) and communicating results to the patient/family/caregiver.
- Care coordination (not separately reported).

3. Soon to be gone: ‘new to the examiner’ and ‘workup planned’
The current guidelines don’t differentiate between a new problem to the clinician or an established problem to the clinician. So it doesn’t matter whether you’re hearing about a particular problem for the first time or the fifth time. The new office and outpatient services guidelines define problems only as they relate to the patient. For example, when selecting a level of service, a chronic problem with a mild exacerbation is the same level whether it’s the primary care physician seeing the patient for the 10th time to help manage her diabetes or the endocrinologist seeing the patient for the first time.
In the current guidelines (1995 and 1997), additional weight is given in selecting the level of MDM for a problem that’s new to the examiner with a workup planned, yet when the diagnostic test couldn’t be completed at the visit. This concept is gone from element of number and complexity of new problems. Ordering diagnostic tests is part of the second element, the amount and/or complexity of data to be reviewed.
4. Different guidelines if you need a history from a parent or other source
The new guidelines recognize the additional work required by the clinician when the patient is unable to give a history or when the practitioner doesn’t find the history to be reliable.
For example, in the case of a baby or child who is unable to give a history, the parent counts as an “independent historian,” according to the new guidelines. Likewise, for a patient with dementia, the caregiver counts as a historian. Note, however, that the criteria is not met simply because the patient is accompanied by another person. The additional weight in selecting the level of service is based on the patient being unable to give a reliable history.
Bottom line: In cases where patients are unable to communicate clearly, physicians or other providers should document the necessity of getting a complete history and who provided it.
5. A new spin on social determinants of health (SDoH)
In the risk of morbidity and/or mortality element, conditions described as “social determinants of health” are considered moderate complexity. SDoH are social and environmental factors that affect a patient’s health and medical outcomes. These include homelessness, inability to afford medications, food insecurity, and occupational exposure to risk factors. These circumstances are reported with codes in categories Z55-Z65.
In the past, physicians often documented this information in their office notes but rarely added a diagnosis code that described the patient’s situation. The ICD-10-CM code set includes codes that describe these factors. Using them allows the practice to track patients who have increased needs, and it communicates to payers the complexity of caring for these patients.
6. Risks related to surgery are defined
The current guidelines assign different levels of risk to minor and major surgery. They also include differentiation for “minor surgery with no identified risk factors,” “minor surgery with identified risk factors,” “elective major surgery with no identified risk factors,” and “elective major surgery with identified risk factors.” The old guidelines didn’t state whether the risk factors pertained to the patient – such as smoking, heart disease, or high body mass index – or to the procedure itself.
The new guidelines specifically say that it’s both. In the risk column, “decision regarding minor surgery with identified patient or procedure risk factors” and “decision regarding elective major surgery without patient or procedure risk factors” are both considered moderate. “Decision regarding elective major surgery with identified patient or procedure risk factors” and “decision regarding emergency major surgery” are in the high complexity column for risk.
Keep in mind that two of three elements are required: the number and complexity of problems, amount of data, and morbidity/mortality risk. Risk of morbidity/mortality alone doesn’t count as the basis for selecting the code. Of course, when surgeons see this, they ask, “What major procedures don’t have identified risk factors?”
Note, too, that these new CPT guidelines do not define the terms “minor” and “major” surgery. For payment reasons related to the postop period, the Centers for Medicare & Medicaid Services defines minor surgery as a procedure with 0-10 global days and a major surgery as a procedure with 90 global days. However, there are many procedures with 0 global days (endoscopy, cardiac catheterization) that are not minor procedures. Hopefully, the AMA will clarify this in 2021.
What’s the take-away for clinicians?
There are sure to be shifts in coding patterns based on these new guidelines. Some specialties will find that not being able to select a service based on history and exam alone will lower the level of service for which they can bill. Some practices, on the other hand, will be able to code for more high-level visits, without the need for a complete review of systems or a comprehensive exam.
The biggest challenge will be for practices that provide services both in the hospital and in the office, because they’ll have to use both sets of guidelines, depending on which type of service they’re performing.
For more details on what’s coming your way beginning on New Year’s Day, you may want to read the 16-page AMA document .
A version of this article first appeared on Medscape.com.
Etonogestrel implants may be bent, fractured by trauma or during sports
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
In 2017, Global Pediatric Health published a case report series associated with the use of long-acting reversible contraceptives, specifically the etonogestrel implant.
In November 2020, the makers of the etonogestrel implant (Merck) recommended a change in practice with the release of a notice to health care providers certified in the training of this product. This mass marketing blast included an updated warning and cautions for prescribers as well as patient information on the potential risks of migration, fracture, and bent devices attributable to trauma or sports. “Broken or Bent Implant (Section 5.16). The addition of the following underlined language: “There have been reports of broken or bent implants, which may be related to external forces (e.g., manipulation of the implant or contact sports) while in the patient’s arm. There have also been reports of migration of a broken implant fragment within the arm.”
Clearly the etonogestrel subdermal hormonal implant is an effective form of contraception and particularly beneficial in nonadherent sexually active teens who struggle to remember oral contraceptives. But it is important to be aware of this alert. Little is known about the type of trauma or rate of external force required to cause migration, fracture, or bend implants. This update requires adequate counseling of potential risks and complications of the etonogestrel implant, including the risk of migration, fracture, or bent devices specifically in the event of contact sports and trauma.
Ms. Thew is medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She had no relevant financial disclosures. Email Ms. Thew at [email protected].
COVID-related harm to HCWs must be tracked more rigorously: NAS panel
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
A panel of scientific experts is urging the nation to do more to track morbidity and mortality among health care workers (HCWs), given the large and disproportionate number who have been infected with or died from SARS-CoV-2.
The National Academies of Sciences, Engineering, and Medicine’s Standing Committee on Emerging Infectious Diseases and 21st Century Health Threats issued a 10-page “rapid expert consultation” on what is known about deaths and mental health problems among HCWs associated with the COVID-19 pandemic and how to protect workers.
“The absence of a uniform national framework and inconsistent requirements across states for collecting, recording, and reporting HCW mortality and morbidity data associated with COVID-19 impairs anyone’s ability to make comparisons, do combined analyses, or draw conclusions about the scale of the problem,” says the panel in the report.
Mental health, in particular, needs to be examined, it says. Although the data are still limited, the prevalence of burnout and suicide “points to a serious concern,” according to the report.
“As with mortality due to COVID-19, there are currently no national systems nor reporting standards for morbidity measures related to the pandemic, such as mental health status, provider well-being, and other psychological effects on HCWs,” the report says.
A more robust national system that collected data on circumstances and interventions that may raise or lower risk, as well as on where the infection occurred, “would support the adoption of effective mitigation strategies,” says the report. It would also facilitate epidemiologic studies on risk factors, such as face-to-face contact with COVID-19 patients and the availability and use of personal protective equipment (PPE). Studies could also examine the impact of institutional requirements for masking.
Studies have consistently shown that universal mask wearing and access to appropriate PPE support the physical safety and mental health of HCWs, says the report.
Track scale of crisis
The committee cited many gaps in the current system. The Occupational Safety and Health Administration, for instance, doesn’t count deaths from occupationally acquired infection. Many states don’t report COVID-19 deaths by profession. The Centers for Disease Control and Prevention (CDC) relies on case report forms from local health departments for all COVID-19 cases, which typically are lacking in specifics, such as occupation or job setting, says the committee’s report.
As of Nov. 3, the CDC had reported 786 deaths among HCWs that were attributable to COVID-19 – a far lower number than other sources have reported.
The committee notes that much could be done immediately. A National Academy of Medicine panel on clinician well-being and resilience in August recommended that the CDC establish a national epidemiologic tracking program to measure HCWs’ well-being, assess the acute and long-term effects of COVID-19 on those workers, and report on the outcomes of interventions.
Such a program “is needed to comprehensively acknowledge the scale of the COVID-19 crisis and protect the health care workforce that is already stretched to the breaking point in many locations,” the committee says in its report.
A version of this article originally appeared on Medscape.com.
Medicare payments could get tougher for docs
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
More than 40 value-based payment models – from direct contracting to bundled payments – have been introduced into the Medicare program in the past 10 years, with the goal of improving care while lowering costs. Hopes were high that they would be successful.
Physicians could suffer a huge blow to their income.
Many of the value-based care models simply did not work as expected, said Seema Verma, head of the Centers for Medicare & Medicaid Services, at a recent HLTH Conference. “They are not producing the types of savings the taxpayers deserve,” Ms. Verma said.
The Medicare Payment Advisory Commission (MedPac) concluded that, while dozens of payment models were tested, most failed to generate net savings for Medicare. Even the most successful of the models produced only modest savings. MedPac elaborated: “The track record raises the question of whether changes to particular models or CMMI’s [Center for Medicare & Medicaid Innovation’s] broader strategies might be warranted.”
What will happen now, as government officials admit that their value-based programs haven’t worked? The value-based programs could become more stringent. Here’s what physicians will have to contend with.
More risk. Experts agree that risk – financial risk – will be a component of future programs. Two-sided risk is likely to be the norm. This means that both parties – the provider and the insurer – are at financial risk for the patients covered by the program.
For example, a plan with 50,000 beneficiary patients would estimate the cost of caring for those patients on the basis of multiple variables. If the actual cost is lower than anticipated, both parties share in the savings. However, both share in the loss if the cost of caring for their patient population exceeds expectations.
This may compel physicians to enhance efficiency and potentially limit the services provided to patients. Typically, however, the strategy is to make efforts to prevent services like ED visits and admissions by focusing on health maintenance.
In contrast to most current value-based models, which feature little to no downside risk for physicians, double-sided risk means physicians could lose money. The loss may incorporate a cap – 5%, for example – but programs may differ. Experts concur that double-sided risk will be a hallmark of future programs.
Better data. The majority of health care services are rendered via fee-for-service: Patients receive services and physicians are paid, yet little or no information about outcomes is exchanged between insurers and physicians.
Penny Noyes, president of Health Business Navigators and contract negotiator for physicians, is not a fan of the current crop of value-based programs and feels that data transparency is positive. Sound metrics can lead to improvement, she said, adding: “It’s not money that drives physicians to make decisions; it’s what’s in the best interest of their patients and their patients’ long-term care.”
Value-based programs can work but only if applicable data are developed and given to physicians so that they can better understand their current performance and how to improve.
Mandated participation. Participation in value-based programs has been voluntary, but that may have skewed the results, which were better than what typical practice would have shown. Acknowledging this may lead CMS to call for mandated participation as a component of future programs. Physicians may be brought into programs, if only to determine whether the models really work. To date, participation in the programs has been voluntary, but that may change in the future.
Innovation. The private insurance market may end up as a key player. Over the past 6 months, health insurers have either consolidated partnerships with telemedicine companies to provide no-cost care to beneficiaries or have launched their own initiatives.
Others are focused on bringing together patients and providers operating outside of the traditional health care system, such as Aetna’s merger with CVS which now offers retail-based acute care (MinuteClinic) and chronic care (HealthHUB). Still other payers are gambling with physician practice ownership, as in the case of United Healthcare’s OptumHealth, which now boasts around 50,000 physicians throughout the country.
New practice models are emerging in private practices as well. Physicians are embracing remote care, proactively managing care transitions, and seeking out more methods to keep patients healthy and at home.
Not much was expected from value-based plans
Many are not surprised that the value-based models did not produce impressive results. Ms. Noyes doubted that positive outcomes will be achieved for physicians in comparison with what could have been attained under fee-for-service arrangements with lower administrative costs.
While the Affordable Care Act attempted to encourage alternative reimbursement, it limits the maximum medical loss ratio (MLR) a payer could achieve. For many plans, that maximum was 85%. Simply put, at least $0.85 of each premium collected had to be paid in claims; the remaining $0.15 went to margin, claims, and other administrative costs. A payer with an 82% MLR then would have to rebate the 3% difference to enrollees.
But that’s not what occurred, according to Ms. Noyes. Because value-based payments to providers are considered a claims expense, an MLR ratio of 82% allowed the payer to distribute the 3% difference to providers as value-based payments. Ms. Noyes said: “That may sound good for the provider, but the result was essentially a freeze on the provider’s fee-for-service reimbursement with the prospect of getting value-based payments like ‘shared savings.’
“When the providers tried to increase their base fee-for-service rates just to match inflation, payers often advised that any future raises had to be earned through value-based programs,” Ms. Noyes added. The value-based formulas confuse providers because payments are often made for periods as far back as 18 months, and providers do not have data systems to reconcile their payer report cards retrospectively. The result is that providers tended to accept whatever amount the payer distributed.
Executives at Lumeris, a company that helps health systems participate successfully in value-based care, see potential in a newer approach to alternative payments, such as CMS’ Direct Contracting initiative. This voluntary payment model offers options tailored to several types of organizations that aim to reduce costs while preserving or enhancing the quality of care for Medicare fee-for-service beneficiaries.
Jeff Smith, chief commercial officer for population health at Lumeris, explained that the Direct Contracting initiative can provide physicians with a more attractive option than prior value-based models because it adjusts for the complexity and fragility of patients with complex and chronic conditions. By allowing providers to participate in the savings generated, the initiative stands in stark contrast to what Mr. Smith described as the “shared savings to nothingness” experienced by providers in earlier-stage alternative payment models.
Physicians engaged with value-based programs like Direct Contracting are investing in nurses to aid with initiatives regarding health promotion and transitions of care. When a patient is discharged, for example, the nurse contacts the patient to discuss medications, schedule follow-up appointments, and so forth – tasks typically left to the patient (or caregiver) to navigate in the traditional system.
The initiative recognizes the importance of managing high-risk patients, those whom physicians identify as having an extraordinary number of ED visits and admissions. These patients, as well as so-called “rising-risk” patients, are targeted by nurses who proactively communicate with patients (and caregivers) to address patient’s needs, including social determinants of health.
Physicians who have a large load of patients in value-based programs are hiring social workers, pharmacists, and behavioral health experts to help. Of course, these personnel are costly, but that’s what the value-based programs aim to reimburse.
Still, the road ahead to value based is rocky and may not gain momentum for some time. Johns Hopkins University’s Doug Hough, PhD, an economist, recounts a government research study that sought to assess the university’s health system participation in a value-based payment program. While there were positive impacts on the program’s target population, Hough and his team discovered that the returns achieved by the optional model didn’t justify the health system’s financial support for it. The increasingly indebted health system ultimately decided to drop the optional program.
Dr. Hough indicated that the health system – Johns Hopkins Medicine – likely would have continued its support for the program had the government at least allowed it to break even. Although the payment program under study was a 3-year project, the bigger challenge, declared Dr. Hough, is that “we can’t turn an aircraft carrier that quickly.”
“Three years won’t show whether value-based care is really working,” Dr. Hough said.
Robert Zipper, MD, a hospitalist and senior policy advisor for Sound Physicians, a company that works to improve outcomes in acute care, agreed with Dr. Hough that performance tends to improve with time. Yet, Dr. Zipper doesn’t see much change in the near term, because “after all, there is nothing to replace them [the programs].”
The problem gets even stickier for private payers because patients may be on an insurance panel for as little as a year or 2. Thanks to this rapid churn of beneficiaries, even the best-designed value-based program will have little time to prove its worth.
Dr. Zipper is among the many who don’t expect significant changes in the near term, asserting that “President Biden will want to get a few policy wins first, and health care is not the easiest place to start.”
But it’s likely that payers and others will want to see more emphasis on value-based programs despite these programs’ possible value to patients, physicians, and health systems alike.
A version of this article originally appeared on Medscape.com.
Understanding messenger RNA and other SARS-CoV-2 vaccines
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.
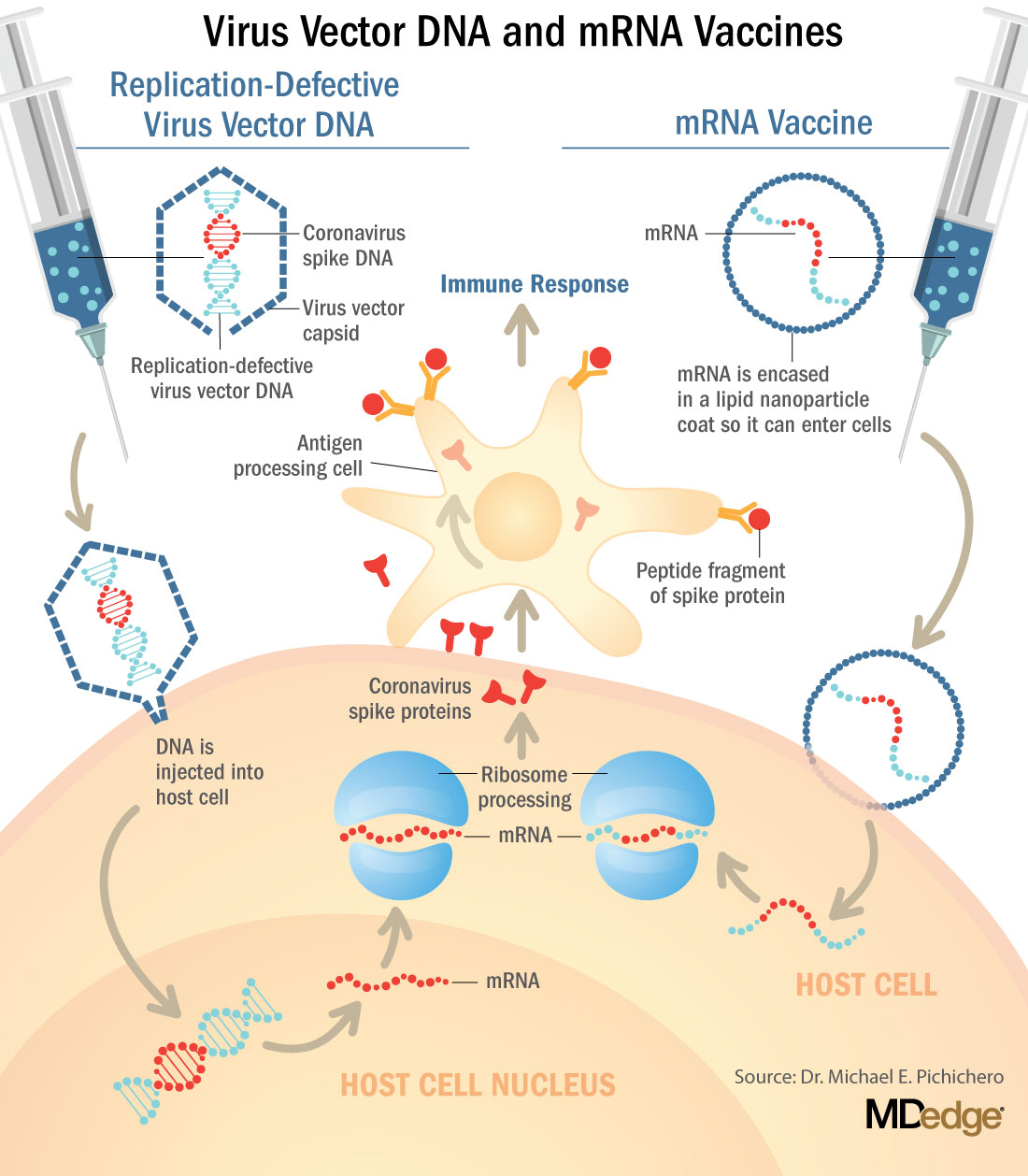
These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
In mid-November, Pfizer/BioNTech were the first with surprising positive protection interim data for their coronavirus vaccine, BNT162b2. A week later, Moderna released interim efficacy results showing its coronavirus vaccine, mRNA-1273, also protected patients from developing SARS-CoV-2 infections. Both studies included mostly healthy adults. A diverse ethnic and racial vaccinated population was included. A reasonable number of persons aged over 65 years, and persons with stable compromising medical conditions were included. Adolescents aged 16 years and over were included. Younger adolescents have been vaccinated or such studies are in the planning or early implementation stage as 2020 came to a close.

These are new and revolutionary vaccines, although the ability to inject mRNA into animals dates back to 1990, technological advances today make it a reality.1 Traditional vaccines typically involve injection with antigens such as purified proteins or polysaccharides or inactivated/attenuated viruses. In the case of Pfizer’s and Moderna’s vaccines, the mRNA provides the genetic information to synthesize the spike protein that the SARS-CoV-2 virus uses to attach to and infect human cells. Each type of vaccine is packaged in proprietary lipid nanoparticles to protect the mRNA from rapid degradation, and the nanoparticles serve as an adjuvant to attract immune cells to the site of injection. (The properties of the respective lipid nanoparticle packaging may be the factor that impacts storage requirements discussed below.) When injected into muscle (myocyte), the lipid nanoparticles containing the mRNA inside are taken into muscle cells, where the cytoplasmic ribosomes detect and decode the mRNA resulting in the production of the spike protein antigen. It should be noted that the mRNA does not enter the nucleus, where the genetic information (DNA) of a cell is located, and can’t be reproduced or integrated into the DNA. The antigen is exported to the myocyte cell surface where the immune system’s antigen presenting cells detect the protein, ingest it, and take it to regional lymph nodes where interactions with T cells and B cells results in antibodies, T cell–mediated immunity, and generation of immune memory T cells and B cells. A particular subset of T cells – cytotoxic or killer T cells – destroy cells that have been infected by a pathogen. The SARS-CoV-2 mRNA vaccine from Pfizer was reported to induce powerful cytotoxic T-cell responses. Results for Moderna’s vaccine had not been reported at the time this column was prepared, but I anticipate the same positive results.
The revolutionary aspect of mRNA vaccines is the speed at which they can be designed and produced. This is why they lead the pack among the SARS-CoV-2 vaccine candidates and why the National Institute of Allergy and Infectious Diseases provided financial, technical, and/or clinical support. Indeed, once the amino acid sequence of a protein can be determined (a relatively easy task these days) it’s straightforward to synthesize mRNA in the lab – and it can be done incredibly fast. It is reported that the mRNA code for the vaccine by Moderna was made in 2 days and production development was completed in about 2 months.2
A 2007 World Health Organization report noted that infectious diseases are emerging at “the historically unprecedented rate of one per year.”3 Severe acute respiratory syndrome (SARS), Zika, Ebola, and avian and swine flu are recent examples. For most vaccines against emerging diseases, the challenge is about speed: developing and manufacturing a vaccine and getting it to persons who need it as quickly as possible. The current seasonal flu vaccine takes about 6 months to develop; it takes years for most of the traditional vaccines. That’s why once the infrastructure is in place, mRNA vaccines may prove to offer a big advantage as vaccines against emerging pathogens.
Early efficacy results have been surprising
Both vaccines were reported to produce about 95% efficacy in the final analysis. That was unexpectedly high because most vaccines for respiratory illness achieve efficacy of 60%-80%, e.g., flu vaccines. However, the efficacy rate may drop as time goes by because stimulation of short-term immunity would be in the earliest reported results.
Preventing SARS-CoV-2 cases is an important aspect of a coronavirus vaccine, but preventing severe illness is especially important considering that severe cases can result in prolonged intubation/artificial ventilation, prolonged disability and death. Pfizer/BioNTech had not released any data on the breakdown of severe cases as this column was finalized. In Moderna’s clinical trial, a secondary endpoint analyzed severe cases of COVID-19 and included 30 severe cases (as defined in the study protocol) in this analysis. All 30 cases occurred in the placebo group and none in the mRNA-1273–vaccinated group. In the Pfizer/BioNTech trial there were too few cases of severe illness to calculate efficacy.
Duration of immunity and need to revaccinate after initial primary vaccination are unknowns. Study of induction of B- and T-cell memory and levels of long-term protection have not been reported thus far.
Could mRNA COVID-19 vaccines be dangerous in the long term?
These will be the first-ever mRNA vaccines brought to market for humans. In order to receive Food and Drug Administration approval, the companies had to prove there were no immediate or short-term negative adverse effects from the vaccines. The companies reported that their independent data-monitoring committees hadn’t “reported any serious safety concerns.” However, fairly significant local reactions at the site of injection, fever, malaise, and fatigue occur with modest frequency following vaccinations with these products, reportedly in 10%-15% of vaccinees. Overall, the immediate reaction profile appears to be more severe than what occurs following seasonal influenza vaccination. When mass inoculations with these completely new and revolutionary vaccines begins, we will know virtually nothing about their long-term side effects. The possibility of systemic inflammatory responses that could lead to autoimmune conditions, persistence of the induced immunogen expression, development of autoreactive antibodies, and toxic effects of delivery components have been raised as theoretical concerns.4-6 None of these theoretical risks have been observed to date and postmarketing phase 4 safety monitoring studies are in place from the Centers for Disease Control and Prevention and the companies that produce the vaccines. This is a risk public health authorities are willing to take because the risk to benefit calculation strongly favors taking theoretical risks, compared with clear benefits in preventing severe illnesses and death.
What about availability?
Pfizer/BioNTech expects to be able to produce up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. Moderna expects to produce 20 million doses by the end of 2020, and 500 million to 1 billion doses in 2021. Storage requirements are inherent to the composition of the vaccines with their differing lipid nanoparticle delivery systems. Pfizer/BioNTech’s BNT162b2 has to be stored and transported at –80° C, which requires specialized freezers, which most doctors’ offices and pharmacies are unlikely to have on site, or dry ice containers. Once the vaccine is thawed, it can only remain in the refrigerator for 24 hours. Moderna’s mRNA-1273 will be much easier to distribute. The vaccine is stable in a standard freezer at –20° C for up to 6 months, in a refrigerator for up to 30 days within that 6-month shelf life, and at room temperature for up to 12 hours.
Timelines and testing other vaccines
Strong efficacy data from the two leading SARS-CoV-2 vaccines and emergency-use authorization Food and Drug Administration approval suggest the window for testing additional vaccine candidates in the United States could soon start to close. Of the more than 200 vaccines in development for SARS-CoV-2, at least 7 have a chance of gathering pivotal data before the front-runners become broadly available.
Testing diverse vaccine candidates, based on different technologies, is important for ensuring sufficient supply and could lead to products with tolerability and safety profiles that make them better suited, or more attractive, to subsets of the population. Different vaccine antigens and technologies also may yield different durations of protection, a question that will not be answered until long after the first products are on the market.
AstraZeneca enrolled about 23,000 subjects into its two phase 3 trials of AZD1222 (ChAdOx1 nCoV-19): a 40,000-subject U.S. trial and a 10,000-subject study in Brazil. AstraZeneca’s AZD1222, developed with the University of Oxford (England), uses a replication defective simian adenovirus vector called ChAdOx1.AZD1222 which encodes the SARS-CoV-2 spike protein. After injection, the viral vector delivers recombinant DNA that is decoded to mRNA, followed by mRNA decoding to become a protein. A serendipitous manufacturing error for the first 3,000 doses resulted in a half dose for those subjects before the error was discovered. Full doses were given to those subjects on second injections and those subjects showed 90% efficacy. Subjects who received 2 full doses showed 62% efficacy. A vaccine cannot be licensed based on 3,000 subjects so AstraZeneca has started a new phase 3 trial involving many more subjects to receive the combination lower dose followed by the full dose.
Johnson and Johnson (J&J) started its phase 3 trial evaluating a single dose of JNJ-78436735 in September. Phase 3 data may be reported by the end of2020. In November, J&J announced it was starting a second phase 3 trial to test two doses of the candidate. J&J’s JNJ-78436735 encodes the SARS-CoV-2 spike protein in an adenovirus serotype 26 (Ad26) vector, which is one of the two adenovirus vectors used in Sputnik V, the Russian vaccine reported to have 90% efficacy at an early interim analysis.
Sanofi and Novavax are both developing protein-based vaccines, a proven modality. Sanofi, in partnership with GlaxoSmithKline started a phase 1/2 clinical trial in the Fall 2020 with plans to commence a phase 3 trial in late December. Sanofi developed the protein ingredients and GlaxoSmithKline added one of their novel adjuvants. Novavax expects data from a U.K. phase 3 trial of NVX-CoV2373 in early 2021 and began a U.S. phase 3 study in late November. NVX-CoV2373 was created using Novavax’ recombinant nanoparticle technology to generate antigen derived from the coronavirus spike protein and contains Novavax’s patented saponin-based Matrix-M adjuvant.
Inovio Pharmaceuticals was gearing up to start a U.S. phase 2/3 trial of DNA vaccine INO-4800 by the end of 2020.
After Moderna and Pfizer-BioNTech, CureVac has the next most advanced mRNA vaccine. It was planned that a phase 2b/3 trial of CVnCoV would be conducted in Europe, Latin America, Africa, and Asia. Sanofi is also developing a mRNA vaccine as a second product in addition to its protein vaccine.
Vaxxinity planned to begin phase 3 testing of UB-612, a multitope peptide–based vaccine, in Brazil by the end of 2020.
However, emergency-use authorizations for the Pfizer and Moderna vaccines could hinder trial recruitment in at least two ways. Given the gravity of the pandemic, some stakeholders believe it would be ethical to unblind ongoing trials to give subjects the opportunity to switch to a vaccine proven to be effective. Even if unblinding doesn’t occur, as the two authorized vaccines start to become widely available, volunteering for clinical trials may become less attractive.
Dr. Pichichero is a specialist in pediatric infectious diseases, and director of the Research Institute at Rochester (N.Y.) General Hospital. He said he has no relevant financial disclosures. Email Dr. Pichichero at [email protected].
References
1. Wolff JA et al. Science. 1990 Mar 23. doi: 10.1126/science.1690918.
2. Jackson LA et al. N Engl J Med. 2020 Nov 12. doi: 10.1056/NEJMoa2022483.
3. Prentice T and Reinders LT. The world health report 2007. (Geneva Switzerland: World Health Organization, 2007).
4. Peck KM and Lauring AS. J Virol. 2018. doi: 10.1128/JVI.01031-17.
5. Pepini T et al. J Immunol. 2017 May 15. doi: 10.4049/jimmunol.1601877.
6. Theofilopoulos AN et al. Annu Rev Immunol. 2005. doi: 10.1146/annurev.immunol.23.021704.115843.
CDC panel recommends Pfizer’s COVID-19 vaccine for people 16 and over
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
stating they found it was safe and effective.
The agency said it will quickly issue guidance to clinicians so they can determine when and when not to give the vaccine, and to help them communicate the risks and benefits to patients.
CDC staff gave a preview of those clinical considerations at the agency’s Advisory Committee on Immunization Practices (ACIP) meeting on December 12 and said it would be holding calls with clinicians on December 13 and 14.
The CDC will also issue guidance December 13 on how organizations can handle the workforce problems that might arise as health care workers experience side effects from vaccination.
ACIP voted 11-0, with three recusals, to recommend use of the Pfizer-BioNTech mRNA vaccine in individuals 16 years or older according to the guidelines of the Food and Drug Administration’s (FDA’s) emergency use authorization issued December 11.
The panel also voted unanimously to include the vaccine in 2021 immunization schedules. All panel members said the recommendation should go hand-in-hand with ACIP’s previous recommendation on December 1 that allocation of the vaccine be phased-in, with health care workers and residents and staff of long-term care facilities in phase 1a.
Allergies, pregnant women?
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women, the immunocompromised, or those who have a history of allergies.
The FDA health care provider information sheet said there is not enough data to recommend vaccinating those women or the immunocompromised, and also advises against giving the vaccine to individuals who have a history of serious allergic reaction to any component of the vaccine.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologic Evaluation and Research (CBER) clarified this in a briefing on December 12, noting that women who are pregnant or lactating can make the decision in consultation with their physician. And, he said, patients with any other history of allergy should be able to safely get the vaccine.
The CDC — in its soon-to-be-released guidance — will make the same recommendations. For any woman considering vaccination, she should consider the level of COVID-19 in the community, her personal risk of contracting the virus, the risks to her or her fetus of developing the disease, and the vaccine’s known side effects, Sarah Mbaeyi, MD, MPH, a medical officer at the agency, said during the panel meeting December 12.
She added that the CDC will also urge physicians to advise women to take acetaminophen if they develop a fever after vaccination — to protect the developing fetus from fever.
Sandra Fryhofer, MD, representing the American Medical Association, commended the CDC for these recommendations. But she also called on Pfizer, the FDA, and the CDC to make data from the developmental and reproductive toxicity (DART) studies public as soon as possible.
“We really need to put those results on warp speed and get them out there to give our physicians and pregnant women more information,” said Fryhofer, an adjunct associate professor of medicine at Emory University School of Medicine in Atlanta, Georgia.
The American College of Obstetricians and Gynecologists (ACOG) will also soon release guidance for vaccinating pregnant and breastfeeding women, said Linda Eckert, MD, FACOG, an ACOG representative on the panel.
ACOG and the CDC met the morning of December 12 to discuss risks and benefits with experts in immunology, placental pathology, and vaccine kinetics, she said.
“The overall complete consensus was that we don’t see biological plausibility at this time for placental transfer of the mRNA and that we see that direct fetal exposure or the possibility of fetal inflammatory response is extremely unlikely,” said Eckert, professor of obstetrics and gynecology at the University of Washington, Seattle. “Clearly we are waiting on the data.”
A Pfizer official told the ACIP panel that preliminary data “show no indication of either developmental or reproductive toxicity,” and that the company plans to send the final DART data to the FDA at the end of December.
On the potential for allergic reactions, the CDC concurred with the FDA that the vaccine should not be given to people with a history of serious reactions. The agency added that the category should include anyone who has had a reaction to any vaccine or injectable drug product because injectables may contain the same ingredients as the Pfizer vaccine, said Mbaeyi.
The CDC will also urge clinicians to observe patients with a history of anaphylaxis for 30 minutes after vaccination and all patients for at least 15 minutes afterward.
Should teens be a special population?
At least one ACIP panel member — Henry Bernstein, DO, MHCM, FAAP — said he was concerned that backing use of the vaccine in 16- and 17-year-olds was a leap of faith, given that Pfizer had extremely limited data on this cohort.
Bernstein, professor of pediatrics at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, also said that systemic reactions were more common in that age group.
He argued for making the 16- and 17-year-olds a “special population” that would get specific attention and guidance for vaccination from the federal agencies and professional societies.
Bernstein said he did not want to sow any more doubts in parents’ minds about vaccination, noting that hesitancy was a growing concern. “A successful pediatric vaccination program depends on creating and sustaining parental confidence in both the safety and effectiveness of this vaccine,” he said.
Many panelists, however, noted that there has been no evidence to suggest that the vaccine is not safe or less effective in that younger age group.
Yvonne Maldonado, MD, the American Academy of Pediatrics representative on the panel, said that this age group should not be denied the vaccine as they often have essential or front-line jobs that put them at higher risk for infection.
“I am very concerned about this message being sent out that this vaccine will not be safe in children,” said Maldonado, professor of pediatrics and health research and policy at Stanford University School of Medicine in California.
“We currently have no evidence that that is the case,” she said, adding there is also no indication younger children are biologically or physiologically different in their response or safety risk than 18-year-olds.
Vaccine = hope
Committee members breathed a sigh of relief at the end of the 2-day meeting, saying that although the Pfizer vaccine is not perfect, it represents a scientific milestone and a significant advance against the continuing march of the SARS-CoV-2 pandemic.
“This vaccine and future vaccines do provide a promise for a lot of progress in the future,” said panelist Beth P. Bell, MD, MPH, clinical professor of global health at the University of Washington School of Public Health in Seattle.
Peter Szilagyi, MD, MPH, executive vice-chair and vice-chair for research at the University of California, Los Angeles pediatrics department, said, “I’m really hopeful that this is the beginning of the end of the coronavirus pandemic.”
“The need for this vaccine is profound,” said Veronica McNally, president and CEO of the Franny Strong Foundation in West Bloomfield, Michigan.
The ACIP panel also made the argument that while the at least $10 billion spent on vaccine development by the federal government’s Operation Warp Speed alone has been a good investment, more spending is needed to actually get Americans vaccinated.
The imbalance between the two is “shocking and needs to be corrected,” said Bell. “We are not going to be able to protect the American public if we don’t have a way to deliver the vaccine to them.”
This article first appeared on Medscape.com.
FDA OKs emergency use of Pfizer COVID-19 vaccine
The much-anticipated emergency use authorization (EUA) of this vaccine — the first such approval in the United States — was greeted with optimism by infectious disease and pulmonary experts, although unanswered questions remain regarding use in people with allergic hypersensitivity, safety in pregnant women, and how smooth distribution will be.
“I am delighted. This is a first, firm step on a long path to getting this COVID pandemic under control,” William Schaffner, MD, professor of infectious diseases at the Vanderbilt University School of Medicine in Nashville, Tennessee, said in an interview.
The FDA gave the green light after the December 10 recommendation from the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. The committee voted 17-4 in favor of the emergency authorization.
The COVID-19 vaccine is “going to have a major impact here in the US. I’m very optimistic about it,” Dial Hewlett, MD, a spokesperson for the Infectious Diseases Society of American (IDSA), told this news organization.
Daniel Culver, DO, chair of medicine at the Cleveland Clinic in Ohio, is likewise hopeful. “My understanding is that supplies of the vaccine are already in place in hubs and will be shipped relatively quickly. The hope would be we can start vaccinating people as early as next week.”
Allergic reactions reported in the UK
After vaccinations with the Pfizer vaccine began in the UK on December 8, reports surfaced of two healthcare workers who experienced allergic reactions. They have since recovered, but officials warned that people with a history of severe allergic reactions should not receive the Pfizer vaccine at this time.
“For the moment, they are asking people who have had notable allergic reactions to step aside while this is investigated. It shows you that the system is working,” Schaffner said.
Both vaccine recipients who experienced anaphylaxis carried EpiPens, as they were at high risk for allergic reactions, Hewlett said. Also, if other COVID-19 vaccines are approved for use in the future, people allergic to the Pfizer vaccine might have another option, he added.
Reassuring role models
Schaffner supports the CDC Advisory Committee on Immunization Practices (ACIP) decision to start vaccinations with healthcare workers and residents of long-term care facilities.
“Vaccinating healthcare workers, in particular, will be a model for the general public,” said Schaffner, who is also a former member of the IDSA board of directors. “If they see those of us in white coats and blue scrubs lining up for the vaccine, that will provide confidence.”
To further increase acceptance of the COVID-19 vaccine, public health officials need to provide information and reassure the general public, Schaffner said.
Hewlett agreed. “I know there are a lot of people in the population who are very hesitant about vaccines. As infection disease specialists and people in public health, we are trying to allay a lot of concerns people have.”
Reassurance will be especially important in minority communities. “They have been disproportionately affected by the virus, and they have a traditional history of not being optimally vaccinated,” Schaffner said. “We need to reach them in particular with good information and reassurance…so they can make good decisions for themselves and their families.”
No vaccine is 100% effective or completely free of side effects. “There is always a chance there can be adverse reactions, but we think for the most part this is going to be a safe and effective vaccine,” said Hewlett, medical director at the Division of Disease Control and deputy to commissioner of health at the Westchester County Department of Health in White Plains, New York.
Distribution: Smooth or full of strife?
In addition to the concern that some people will not take advantage of vaccination against COVID-19, there could be vaccine supply issues down the road, Schaffner said.
Culver agreed. “In the early phases, I expect that there will be some kinks to work out, but because the numbers are relatively small, this should be okay,” he said.
“I think when we start to get into larger-scale vaccination programs — the supply chain, transport, and storage will be a Herculean undertaking,” Culver added. “It will take careful coordination between healthcare providers, distributors, suppliers, and public health officials to pull this off.”
Planning and distribution also should focus beyond US borders. Any issues in vaccine distribution or administration in the United States “will only be multiplied in several other parts of the world,” Culver said. Because COVID-19 is a pandemic, “we need to think about vaccinating globally.”
Investigating adverse events
Adverse events common to vaccinations in general — injection site pain, headaches, and fever — would not be unexpected with the COVID-19 vaccines. However, experts remain concerned that other, unrelated adverse events might be erroneously attributed to vaccination. For example, if a fall, heart attack, or death occurs within days of immunization, some might immediately blame the vaccine product.
“It’s important to remember that any new, highly touted medical therapy like this will receive a lot of scrutiny, so it would be unusual not to hear about something happening to somebody,” Culver said. Vaccine companies and health agencies will be carefully evaluating any reported adverse events to ensure no safety signal was missed in the trials.
“Fortunately, there are systems in place to investigate these events immediately,” Schaffner said.
Pregnancy recommendations pending
One question still looms: Is the COVID-19 vaccination safe for pregnant women? This isn’t just a question for the general public, either, Schaffner said. He estimated that about 70 percent of healthcare workers are women, and data suggests about 300,000 of these healthcare workers are pregnant.
“The CDC’s Advisory Committee on Immunization Practices will speak to that just as soon as the EUA is issued,” he added.
Patients are asking Culver about the priority order for vaccination. He said it’s difficult to provide firm guidance at this point.
People also have “lingering skepticism” about whether vaccine development was done in a prudent way, Culver said. Some people question whether the Pfizer vaccine and others were rushed to market. “So we try to spend time with the patients, reassuring them that all the usual safety evaluations were carefully done,” he said.
Another concern is whether mRNA vaccines can interact with human DNA. “The quick, short, and definitive answer is no,” Schaffner said. The m stands for messenger — the vaccines transmit information. "Once it gets into a cell, the mRNA does not go anywhere near the DNA, and once it transmits its information to the cell appropriately, it gets metabolized, and we excrete all the remnants."
Hewlett pointed out that investigations and surveillance will continue. Because this is an EUA and not full approval, “that essentially means they will still be obligated to collect a lot more data than they would ordinarily,” he said.
How long immunoprotection will last also remains an unknown. “The big question left on the table now is the durability,” Culver said. “Of course, we won’t know the answer to that for quite some time.”
Schaffner and Culver have disclosed no relevant financial relationships. Hewlett was an employee of Pfizer until mid-2019. His previous work as Pfizer’s senior medical director of global medical product evaluation was not associated with development of the COVID-19 vaccine.
This article first appeared on Medscape.com.
The much-anticipated emergency use authorization (EUA) of this vaccine — the first such approval in the United States — was greeted with optimism by infectious disease and pulmonary experts, although unanswered questions remain regarding use in people with allergic hypersensitivity, safety in pregnant women, and how smooth distribution will be.
“I am delighted. This is a first, firm step on a long path to getting this COVID pandemic under control,” William Schaffner, MD, professor of infectious diseases at the Vanderbilt University School of Medicine in Nashville, Tennessee, said in an interview.
The FDA gave the green light after the December 10 recommendation from the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. The committee voted 17-4 in favor of the emergency authorization.
The COVID-19 vaccine is “going to have a major impact here in the US. I’m very optimistic about it,” Dial Hewlett, MD, a spokesperson for the Infectious Diseases Society of American (IDSA), told this news organization.
Daniel Culver, DO, chair of medicine at the Cleveland Clinic in Ohio, is likewise hopeful. “My understanding is that supplies of the vaccine are already in place in hubs and will be shipped relatively quickly. The hope would be we can start vaccinating people as early as next week.”
Allergic reactions reported in the UK
After vaccinations with the Pfizer vaccine began in the UK on December 8, reports surfaced of two healthcare workers who experienced allergic reactions. They have since recovered, but officials warned that people with a history of severe allergic reactions should not receive the Pfizer vaccine at this time.
“For the moment, they are asking people who have had notable allergic reactions to step aside while this is investigated. It shows you that the system is working,” Schaffner said.
Both vaccine recipients who experienced anaphylaxis carried EpiPens, as they were at high risk for allergic reactions, Hewlett said. Also, if other COVID-19 vaccines are approved for use in the future, people allergic to the Pfizer vaccine might have another option, he added.
Reassuring role models
Schaffner supports the CDC Advisory Committee on Immunization Practices (ACIP) decision to start vaccinations with healthcare workers and residents of long-term care facilities.
“Vaccinating healthcare workers, in particular, will be a model for the general public,” said Schaffner, who is also a former member of the IDSA board of directors. “If they see those of us in white coats and blue scrubs lining up for the vaccine, that will provide confidence.”
To further increase acceptance of the COVID-19 vaccine, public health officials need to provide information and reassure the general public, Schaffner said.
Hewlett agreed. “I know there are a lot of people in the population who are very hesitant about vaccines. As infection disease specialists and people in public health, we are trying to allay a lot of concerns people have.”
Reassurance will be especially important in minority communities. “They have been disproportionately affected by the virus, and they have a traditional history of not being optimally vaccinated,” Schaffner said. “We need to reach them in particular with good information and reassurance…so they can make good decisions for themselves and their families.”
No vaccine is 100% effective or completely free of side effects. “There is always a chance there can be adverse reactions, but we think for the most part this is going to be a safe and effective vaccine,” said Hewlett, medical director at the Division of Disease Control and deputy to commissioner of health at the Westchester County Department of Health in White Plains, New York.
Distribution: Smooth or full of strife?
In addition to the concern that some people will not take advantage of vaccination against COVID-19, there could be vaccine supply issues down the road, Schaffner said.
Culver agreed. “In the early phases, I expect that there will be some kinks to work out, but because the numbers are relatively small, this should be okay,” he said.
“I think when we start to get into larger-scale vaccination programs — the supply chain, transport, and storage will be a Herculean undertaking,” Culver added. “It will take careful coordination between healthcare providers, distributors, suppliers, and public health officials to pull this off.”
Planning and distribution also should focus beyond US borders. Any issues in vaccine distribution or administration in the United States “will only be multiplied in several other parts of the world,” Culver said. Because COVID-19 is a pandemic, “we need to think about vaccinating globally.”
Investigating adverse events
Adverse events common to vaccinations in general — injection site pain, headaches, and fever — would not be unexpected with the COVID-19 vaccines. However, experts remain concerned that other, unrelated adverse events might be erroneously attributed to vaccination. For example, if a fall, heart attack, or death occurs within days of immunization, some might immediately blame the vaccine product.
“It’s important to remember that any new, highly touted medical therapy like this will receive a lot of scrutiny, so it would be unusual not to hear about something happening to somebody,” Culver said. Vaccine companies and health agencies will be carefully evaluating any reported adverse events to ensure no safety signal was missed in the trials.
“Fortunately, there are systems in place to investigate these events immediately,” Schaffner said.
Pregnancy recommendations pending
One question still looms: Is the COVID-19 vaccination safe for pregnant women? This isn’t just a question for the general public, either, Schaffner said. He estimated that about 70 percent of healthcare workers are women, and data suggests about 300,000 of these healthcare workers are pregnant.
“The CDC’s Advisory Committee on Immunization Practices will speak to that just as soon as the EUA is issued,” he added.
Patients are asking Culver about the priority order for vaccination. He said it’s difficult to provide firm guidance at this point.
People also have “lingering skepticism” about whether vaccine development was done in a prudent way, Culver said. Some people question whether the Pfizer vaccine and others were rushed to market. “So we try to spend time with the patients, reassuring them that all the usual safety evaluations were carefully done,” he said.
Another concern is whether mRNA vaccines can interact with human DNA. “The quick, short, and definitive answer is no,” Schaffner said. The m stands for messenger — the vaccines transmit information. "Once it gets into a cell, the mRNA does not go anywhere near the DNA, and once it transmits its information to the cell appropriately, it gets metabolized, and we excrete all the remnants."
Hewlett pointed out that investigations and surveillance will continue. Because this is an EUA and not full approval, “that essentially means they will still be obligated to collect a lot more data than they would ordinarily,” he said.
How long immunoprotection will last also remains an unknown. “The big question left on the table now is the durability,” Culver said. “Of course, we won’t know the answer to that for quite some time.”
Schaffner and Culver have disclosed no relevant financial relationships. Hewlett was an employee of Pfizer until mid-2019. His previous work as Pfizer’s senior medical director of global medical product evaluation was not associated with development of the COVID-19 vaccine.
This article first appeared on Medscape.com.
The much-anticipated emergency use authorization (EUA) of this vaccine — the first such approval in the United States — was greeted with optimism by infectious disease and pulmonary experts, although unanswered questions remain regarding use in people with allergic hypersensitivity, safety in pregnant women, and how smooth distribution will be.
“I am delighted. This is a first, firm step on a long path to getting this COVID pandemic under control,” William Schaffner, MD, professor of infectious diseases at the Vanderbilt University School of Medicine in Nashville, Tennessee, said in an interview.
The FDA gave the green light after the December 10 recommendation from the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting. The committee voted 17-4 in favor of the emergency authorization.
The COVID-19 vaccine is “going to have a major impact here in the US. I’m very optimistic about it,” Dial Hewlett, MD, a spokesperson for the Infectious Diseases Society of American (IDSA), told this news organization.
Daniel Culver, DO, chair of medicine at the Cleveland Clinic in Ohio, is likewise hopeful. “My understanding is that supplies of the vaccine are already in place in hubs and will be shipped relatively quickly. The hope would be we can start vaccinating people as early as next week.”
Allergic reactions reported in the UK
After vaccinations with the Pfizer vaccine began in the UK on December 8, reports surfaced of two healthcare workers who experienced allergic reactions. They have since recovered, but officials warned that people with a history of severe allergic reactions should not receive the Pfizer vaccine at this time.
“For the moment, they are asking people who have had notable allergic reactions to step aside while this is investigated. It shows you that the system is working,” Schaffner said.
Both vaccine recipients who experienced anaphylaxis carried EpiPens, as they were at high risk for allergic reactions, Hewlett said. Also, if other COVID-19 vaccines are approved for use in the future, people allergic to the Pfizer vaccine might have another option, he added.
Reassuring role models
Schaffner supports the CDC Advisory Committee on Immunization Practices (ACIP) decision to start vaccinations with healthcare workers and residents of long-term care facilities.
“Vaccinating healthcare workers, in particular, will be a model for the general public,” said Schaffner, who is also a former member of the IDSA board of directors. “If they see those of us in white coats and blue scrubs lining up for the vaccine, that will provide confidence.”
To further increase acceptance of the COVID-19 vaccine, public health officials need to provide information and reassure the general public, Schaffner said.
Hewlett agreed. “I know there are a lot of people in the population who are very hesitant about vaccines. As infection disease specialists and people in public health, we are trying to allay a lot of concerns people have.”
Reassurance will be especially important in minority communities. “They have been disproportionately affected by the virus, and they have a traditional history of not being optimally vaccinated,” Schaffner said. “We need to reach them in particular with good information and reassurance…so they can make good decisions for themselves and their families.”
No vaccine is 100% effective or completely free of side effects. “There is always a chance there can be adverse reactions, but we think for the most part this is going to be a safe and effective vaccine,” said Hewlett, medical director at the Division of Disease Control and deputy to commissioner of health at the Westchester County Department of Health in White Plains, New York.
Distribution: Smooth or full of strife?
In addition to the concern that some people will not take advantage of vaccination against COVID-19, there could be vaccine supply issues down the road, Schaffner said.
Culver agreed. “In the early phases, I expect that there will be some kinks to work out, but because the numbers are relatively small, this should be okay,” he said.
“I think when we start to get into larger-scale vaccination programs — the supply chain, transport, and storage will be a Herculean undertaking,” Culver added. “It will take careful coordination between healthcare providers, distributors, suppliers, and public health officials to pull this off.”
Planning and distribution also should focus beyond US borders. Any issues in vaccine distribution or administration in the United States “will only be multiplied in several other parts of the world,” Culver said. Because COVID-19 is a pandemic, “we need to think about vaccinating globally.”
Investigating adverse events
Adverse events common to vaccinations in general — injection site pain, headaches, and fever — would not be unexpected with the COVID-19 vaccines. However, experts remain concerned that other, unrelated adverse events might be erroneously attributed to vaccination. For example, if a fall, heart attack, or death occurs within days of immunization, some might immediately blame the vaccine product.
“It’s important to remember that any new, highly touted medical therapy like this will receive a lot of scrutiny, so it would be unusual not to hear about something happening to somebody,” Culver said. Vaccine companies and health agencies will be carefully evaluating any reported adverse events to ensure no safety signal was missed in the trials.
“Fortunately, there are systems in place to investigate these events immediately,” Schaffner said.
Pregnancy recommendations pending
One question still looms: Is the COVID-19 vaccination safe for pregnant women? This isn’t just a question for the general public, either, Schaffner said. He estimated that about 70 percent of healthcare workers are women, and data suggests about 300,000 of these healthcare workers are pregnant.
“The CDC’s Advisory Committee on Immunization Practices will speak to that just as soon as the EUA is issued,” he added.
Patients are asking Culver about the priority order for vaccination. He said it’s difficult to provide firm guidance at this point.
People also have “lingering skepticism” about whether vaccine development was done in a prudent way, Culver said. Some people question whether the Pfizer vaccine and others were rushed to market. “So we try to spend time with the patients, reassuring them that all the usual safety evaluations were carefully done,” he said.
Another concern is whether mRNA vaccines can interact with human DNA. “The quick, short, and definitive answer is no,” Schaffner said. The m stands for messenger — the vaccines transmit information. "Once it gets into a cell, the mRNA does not go anywhere near the DNA, and once it transmits its information to the cell appropriately, it gets metabolized, and we excrete all the remnants."
Hewlett pointed out that investigations and surveillance will continue. Because this is an EUA and not full approval, “that essentially means they will still be obligated to collect a lot more data than they would ordinarily,” he said.
How long immunoprotection will last also remains an unknown. “The big question left on the table now is the durability,” Culver said. “Of course, we won’t know the answer to that for quite some time.”
Schaffner and Culver have disclosed no relevant financial relationships. Hewlett was an employee of Pfizer until mid-2019. His previous work as Pfizer’s senior medical director of global medical product evaluation was not associated with development of the COVID-19 vaccine.
This article first appeared on Medscape.com.