User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA panel overwhelmingly backs emergency authorization for Pfizer COVID vaccine
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
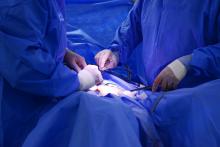
Radiofrequency ablation blocks hip, shoulder arthritis pain
Osteoarthritis patients report significant pain relief after treatment with cooled radiofrequency ablation, a new technique that “stuns” sensory nerves in shoulder and hip joints to reduce – and sometimes eliminate – pain.
“We send a small current to the sensory nerve to heat up the tissue and disrupt the fibers,” study lead author Felix Gonzalez, MD, of Emory University, Atlanta, said in an interview. “The effect is that the transmission of pain is significantly slowed or halted altogether.
“We damage something to fix something,” Dr. Gonzalez continued. “We target only the problematic nerve and get a very localized effect.”
Two-phase treatment
The treatment is performed in two phases. First, patients with shoulder pain are given an anesthetic to block their suprascapular, lateral pectoral, and axillary sensory articular nerves. Patients with hip pain have their obturator and femoral sensory articular nerves blocked.
A week or two later, the same nerves are treated with cooled radiofrequency ablation. Guided by x-ray imaging, a clinician heats up the affected nerve tissue using the tip of a needle, which is pointed at the nerve. “It’s a 22-gauge needle, slightly thicker than an acupuncture needle,” Dr. Gonzalez explained. “We heat up the nerve for about 2 minutes to about 60 degrees Celsius – it stuns the nerve,” he said.
“The result disrupts or slows down pain transmission while leaving the nerve intact.”
To test the efficacy of the technique, researchers treated 12 shoulders in patients with an average age of 61 years, and 11 hips in patients with an average age of 62 years.
Three months after treatment, patients with hip pain reported improvement in Hip Disability and Osteoarthritis Outcome Score (HOOS) from a baseline of 17.0 to 52.9 (P < .0001).
Shoulder pain was also reduced significantly. Using the American Shoulder and Elbow Surgeons (ASES) score, researchers reported an improvement from 17.2 (±6.6) at baseline to 65.7 (±5.9) at 3 months (P < .0001).
“We are targeting a subset of patients for this that don’t qualify for surgery,” Dr. Gonzalez noted. For patients with a body mass index above 35, or a history of hypertension, heart disease, or multiple strokes, opioids are the most common treatment, he said.
These patients “fall through the cracks,” he explained. Those who have mild to moderate pain are managed with physical therapy and injections, and those with severe pain go into surgery. “But what about the ones in the middle ... who are not eligible for surgery? They are at risk for opioid overuse,” he said. “So this treatment is a good option for them.”
Treats the symptoms, not the cause
“This study shows the efficacy of this method in taking care of shoulder and hip pain,” Luca Maria Sconfienza, MD, PhD, of Galeazzi Orthopedic Hospital in Milan, said in an interview. Dr. Sconfienza was not involved in Dr. Gonzalez’s study.
However, like corticosteroid injections, “the drawback of radiofrequency ablation is the fact that it only treats the symptoms and not the cause, and efficacy is usually limited over time,” she said.
Dr. Sconfienza said this study leaves her with three pertinent questions. “First, whether pain control extends beyond the 3-month follow-up reported by authors in the abstract; second, [what] is the efficacy of this method compared to other interventions (e.g., physical therapy, injections) or to doing nothing; and last, radiofrequency ablation is usually not a cheap treatment, thus a cost-efficacy analysis would be desirable, especially in comparison to other procedures.”
Dr. Gonzalez and Dr. Sconfienza have nothing relevant to disclose.
A version of this article originally appeared on Medscape.com.
Osteoarthritis patients report significant pain relief after treatment with cooled radiofrequency ablation, a new technique that “stuns” sensory nerves in shoulder and hip joints to reduce – and sometimes eliminate – pain.
“We send a small current to the sensory nerve to heat up the tissue and disrupt the fibers,” study lead author Felix Gonzalez, MD, of Emory University, Atlanta, said in an interview. “The effect is that the transmission of pain is significantly slowed or halted altogether.
“We damage something to fix something,” Dr. Gonzalez continued. “We target only the problematic nerve and get a very localized effect.”
Two-phase treatment
The treatment is performed in two phases. First, patients with shoulder pain are given an anesthetic to block their suprascapular, lateral pectoral, and axillary sensory articular nerves. Patients with hip pain have their obturator and femoral sensory articular nerves blocked.
A week or two later, the same nerves are treated with cooled radiofrequency ablation. Guided by x-ray imaging, a clinician heats up the affected nerve tissue using the tip of a needle, which is pointed at the nerve. “It’s a 22-gauge needle, slightly thicker than an acupuncture needle,” Dr. Gonzalez explained. “We heat up the nerve for about 2 minutes to about 60 degrees Celsius – it stuns the nerve,” he said.
“The result disrupts or slows down pain transmission while leaving the nerve intact.”
To test the efficacy of the technique, researchers treated 12 shoulders in patients with an average age of 61 years, and 11 hips in patients with an average age of 62 years.
Three months after treatment, patients with hip pain reported improvement in Hip Disability and Osteoarthritis Outcome Score (HOOS) from a baseline of 17.0 to 52.9 (P < .0001).
Shoulder pain was also reduced significantly. Using the American Shoulder and Elbow Surgeons (ASES) score, researchers reported an improvement from 17.2 (±6.6) at baseline to 65.7 (±5.9) at 3 months (P < .0001).
“We are targeting a subset of patients for this that don’t qualify for surgery,” Dr. Gonzalez noted. For patients with a body mass index above 35, or a history of hypertension, heart disease, or multiple strokes, opioids are the most common treatment, he said.
These patients “fall through the cracks,” he explained. Those who have mild to moderate pain are managed with physical therapy and injections, and those with severe pain go into surgery. “But what about the ones in the middle ... who are not eligible for surgery? They are at risk for opioid overuse,” he said. “So this treatment is a good option for them.”
Treats the symptoms, not the cause
“This study shows the efficacy of this method in taking care of shoulder and hip pain,” Luca Maria Sconfienza, MD, PhD, of Galeazzi Orthopedic Hospital in Milan, said in an interview. Dr. Sconfienza was not involved in Dr. Gonzalez’s study.
However, like corticosteroid injections, “the drawback of radiofrequency ablation is the fact that it only treats the symptoms and not the cause, and efficacy is usually limited over time,” she said.
Dr. Sconfienza said this study leaves her with three pertinent questions. “First, whether pain control extends beyond the 3-month follow-up reported by authors in the abstract; second, [what] is the efficacy of this method compared to other interventions (e.g., physical therapy, injections) or to doing nothing; and last, radiofrequency ablation is usually not a cheap treatment, thus a cost-efficacy analysis would be desirable, especially in comparison to other procedures.”
Dr. Gonzalez and Dr. Sconfienza have nothing relevant to disclose.
A version of this article originally appeared on Medscape.com.
Osteoarthritis patients report significant pain relief after treatment with cooled radiofrequency ablation, a new technique that “stuns” sensory nerves in shoulder and hip joints to reduce – and sometimes eliminate – pain.
“We send a small current to the sensory nerve to heat up the tissue and disrupt the fibers,” study lead author Felix Gonzalez, MD, of Emory University, Atlanta, said in an interview. “The effect is that the transmission of pain is significantly slowed or halted altogether.
“We damage something to fix something,” Dr. Gonzalez continued. “We target only the problematic nerve and get a very localized effect.”
Two-phase treatment
The treatment is performed in two phases. First, patients with shoulder pain are given an anesthetic to block their suprascapular, lateral pectoral, and axillary sensory articular nerves. Patients with hip pain have their obturator and femoral sensory articular nerves blocked.
A week or two later, the same nerves are treated with cooled radiofrequency ablation. Guided by x-ray imaging, a clinician heats up the affected nerve tissue using the tip of a needle, which is pointed at the nerve. “It’s a 22-gauge needle, slightly thicker than an acupuncture needle,” Dr. Gonzalez explained. “We heat up the nerve for about 2 minutes to about 60 degrees Celsius – it stuns the nerve,” he said.
“The result disrupts or slows down pain transmission while leaving the nerve intact.”
To test the efficacy of the technique, researchers treated 12 shoulders in patients with an average age of 61 years, and 11 hips in patients with an average age of 62 years.
Three months after treatment, patients with hip pain reported improvement in Hip Disability and Osteoarthritis Outcome Score (HOOS) from a baseline of 17.0 to 52.9 (P < .0001).
Shoulder pain was also reduced significantly. Using the American Shoulder and Elbow Surgeons (ASES) score, researchers reported an improvement from 17.2 (±6.6) at baseline to 65.7 (±5.9) at 3 months (P < .0001).
“We are targeting a subset of patients for this that don’t qualify for surgery,” Dr. Gonzalez noted. For patients with a body mass index above 35, or a history of hypertension, heart disease, or multiple strokes, opioids are the most common treatment, he said.
These patients “fall through the cracks,” he explained. Those who have mild to moderate pain are managed with physical therapy and injections, and those with severe pain go into surgery. “But what about the ones in the middle ... who are not eligible for surgery? They are at risk for opioid overuse,” he said. “So this treatment is a good option for them.”
Treats the symptoms, not the cause
“This study shows the efficacy of this method in taking care of shoulder and hip pain,” Luca Maria Sconfienza, MD, PhD, of Galeazzi Orthopedic Hospital in Milan, said in an interview. Dr. Sconfienza was not involved in Dr. Gonzalez’s study.
However, like corticosteroid injections, “the drawback of radiofrequency ablation is the fact that it only treats the symptoms and not the cause, and efficacy is usually limited over time,” she said.
Dr. Sconfienza said this study leaves her with three pertinent questions. “First, whether pain control extends beyond the 3-month follow-up reported by authors in the abstract; second, [what] is the efficacy of this method compared to other interventions (e.g., physical therapy, injections) or to doing nothing; and last, radiofrequency ablation is usually not a cheap treatment, thus a cost-efficacy analysis would be desirable, especially in comparison to other procedures.”
Dr. Gonzalez and Dr. Sconfienza have nothing relevant to disclose.
A version of this article originally appeared on Medscape.com.
Prioritize COVID-19 vaccination in both types of diabetes, say docs
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The risk for increased COVID-19 severity in people with type 1 diabetes appears similar to that of type 2 diabetes, contrary to some official advice from the Centers for Disease Control and Prevention. The new finding indicates that people with both types should be priority for receiving a vaccine, investigators say.
The study is the first to prospectively evaluate both inpatients and outpatients and to examine COVID-19 severity factors in addition to death in people with type 1 and type 2 diabetes separately, and was published online Dec. 2 in Diabetes Care.
Among the patients, who were seen at Vanderbilt University Medical Center in Nashville, Tenn., between March and August of 2020, those with both type 1 and type 2 diabetes had between a three- and fourfold greater risk for COVID-19 hospitalization and greater illness severity compared with people without diabetes after adjustments for age, race, and a number of other risk factors.
This finding is important since as of Dec. 1, 2020, the CDC has classified the diabetes types differently in terms of underlying medical conditions that increase the risk for severe COVID-19.
Adults of any age with type 2 diabetes are considered “at increased risk of severe illness” from the virus that causes COVID-19 whereas the CDC says those with type 1 “might be at an increased risk.”
Lead author of the new paper Justin M. Gregory, MD, said in an interview: “I think this needs revision based on the current evidence. I think the data presented in our study and that of Barron et al. in Lancet Endocrinology 2020 indicate the need to place type 1 diabetes at parity with type 2 diabetes.
“These studies indicate both conditions carry an adjusted odds ratio of three to four when compared with people without diabetes for hospitalization, illness severity, and mortality,” he stressed.
Vaccines look promising for patients with diabetes
There were no phase 3 vaccine data available for the vaccine at the time that Dr. Gregory, of the Ian M. Burr Division of Pediatric Endocrinology and Diabetes, Vanderbilt University, Nashville, Tenn., and colleagues were writing their manuscript in late summer, so the article does not mention this.
But now, Dr. Gregory said, “Based on the initial press releases from Pfizer and Moderna, I am now optimistic that these vaccines might mitigate the excess morbidity and mortality from COVID-19 experienced by patients with diabetes.
“I am eager to see what we learn on December 10 and 17 [the scheduled dates for the meetings of the Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee to review the Pfizer and Moderna vaccines, respectively].”
But with the winter pandemic surge in the meantime, “Our investigation suggests that as COVID-19 hospitalizations rise, patients with both type 1 and 2 diabetes will comprise a disproportionately higher number of those admissions and, once hospitalized, demonstrate a greater degree of illness severity,” he and his colleagues said.
“In light of these data, we call on our colleagues to emphasize the importance of social distancing measures and hand hygiene, with particular emphasis on patients with diabetes, including those in the most vulnerable communities whom our study affirms will face the most severe impact.”
After adjustments, excess severity risk similar for both diabetes types
The new study data came from electronic health records at Vanderbilt University Medical Center, comprising 137 primary care, urgent care, and hospital facilities where patients were tested for SARS-CoV-2 regardless of the reason for their visit.
Between March 17 and August 7, 2020, 6,451 patients tested positive for COVID-19. Of those, 273 had type 2 diabetes and 40 had type 1 diabetes.
Children younger than 18 years accounted for 20% of those with type 1 diabetes and 9.4% of those without diabetes, but none of the type 2 group. The group with type 2 diabetes was considerably older than the type 1 diabetes and no-diabetes groups, 58 years versus 37 and 33 years, respectively.
Before adjustment for baseline characteristics that differed between groups, patients with type 1 diabetes appeared to have a risk for hospitalization and greater illness severity that was intermediate between the group with no diabetes and the group with type 2 diabetes, the researchers said.
But after adjustment for age, race, sex, hypertension, smoking, and body mass index, people with type 1 diabetes had odds ratios of 3.90 for hospitalization and 3.35 for greater illness severity, which was similar to risk in type 2 diabetes (3.36 and 3.42, respectively), compared to those without diabetes.
Deep dive explores COVID-19 severity risk factors in type 1 diabetes
The investigators then conducted a detailed chart review for 37 of the 40 patients with type 1 diabetes and phone surveys with 15 of them.
The majority (28) had not been hospitalized, and only one was hospitalized for diabetic ketoacidosis (DKA) within 14 days of positive SARS-CoV-2 testing.
This contrasts with a report from the T1D Exchange, in which nearly half of 33 patients with type 1 diabetes and COVID-19 had been hospitalized with DKA. The reason for the discrepancy may be that more severe patients would more likely be referred to the T1D Exchange Registry, Dr. Gregory and colleagues hypothesized.
Clinical factors associated with COVID-19 severity (P < .05) in their study included a prior hypertension diagnosis, higher hemoglobin A1c, at least one prior DKA admission in the past year, and not using a continuous glucose monitor (CGM).
Hospitalizations were twice as likely and illness severity nearly twice as great among those with type 1 diabetes who were Black versus White. Just 8% of those with private insurance were hospitalized, compared with 60% of those with public insurance and 67% with no insurance (P = .001).
“Whereas previous reports have indicated proportionally higher rates of hospitalizations from COVID-19 among Black patients and those with public insurance, this study is the first to show a similar finding in the population with type 1 diabetes,” Dr. Gregory and colleagues wrote.
Only 9% of patients using a CGM were hospitalized versus 47% who used blood glucose meters (P < .016). Similarly, hospitalizations occurred in 6% using an insulin pump versus 33% using multiple daily injections (P < .085).
“Our analysis cannot exclude the possibility that greater amounts of diabetes technology use are a surrogate for higher socioeconomic status,” they noted.
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, JDRF, and the Appleby Foundation. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New residency matching sets record, says NRMP
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
beginning in 2021, the NRMP reported.
“Specifically, the 2020 MSMP included 6,847 applicants submitting certified rank order lists (an 8.9% increase), 2042 programs submitting certified rank order lists (a 4.3% increase), 5,734 positions (a 2.8% increase), and 5,208 positions filled (a 6.1% increase),” according to a news release.
The MSMP now includes 14 internal medicine subspecialties and four sub-subspecialties. The MSMP offered 5,734 positions this year, and 5,208 (90.8%) were successfully filled. That represents an increase of almost 3 percentage points, compared with last year’s results.
Among those subspecialties that offered 30 positions or more, the most competitive were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, gastroenterology, hematology and oncology, and pulmonary/critical care. Each of those filled at least 95% of available slots. More than half of the positions were filled by U.S. MDs.
By contrast, the least competitive subspecialties were geriatric medicine and nephrology. Programs in these two fields filled less than 75% of positions offered. Less than 45% were filled by U.S. MDs.
More than 76% of the 6,847 applicants who submitted rank order lists (5,208) matched into residency programs.
The number of U.S. MDs in this category increased nearly 7% over last year, with a total of 2,935. The number of DO graduates increased as well, with a total of 855, which was 9.6% more than the previous year.
More U.S. citizens who graduated from international medical schools matched this year as well; 1,087 placed into subspecialty residency, a 9% increase, compared with last year.
A version of this article originally appeared on Medscape.com.
‘Excellent short-term outcomes’ seen in HCV+ liver transplants to HCV– recipients
Liver transplantation using hepatitis C virus (HCV)-seropositive grafts to HCV-seronegative recipients resulted in “excellent short-term outcomes,” according to the results of a prospective, multicenter study reported in the Journal of Hepatology.
A total of 34 HCV– liver transplantation recipients received grafts from HCV+ donors (20 HCV viremic and 14 nonviremic) from January 2018 to September 2019, according to Bashar Aqel, MD, of the Mayo Clinic, Phoenix, Ariz., and colleagues.
Seven of the grafts were obtained from donation after cardiac death (DCD). Six recipients underwent simultaneous liver/kidney (SLK) transplant, and four patients were repeat liver transplants.
Sustained viral response
None of the recipients of an HCV nonviremic graft developed HCV viremia. However, all 20 patients who received HCV viremic grafts had HCV viremia confirmed within 3 days after liver transplant. Direct-acting antiviral (DAA) treatment was started at the median time of 27.5 days in these patients.
All 20 patients successfully completed the treatment and achieved a sustained viral response. In addition, the DAA treatment was well tolerated with minimal adverse events, according to the researchers.
However, one patient died, having developed HCV-related acute membranous nephropathy that resulted in end-stage kidney disease. In addition, a recipient of an HCV nonviremic graft died with acute myocardial infarction 610 days post liver transplant, the authors reported.
“This multicenter study demonstrated LT [liver transplantation] using HCV-seropositive grafts to HCV-seronegative recipients resulted in acceptable short-term outcomes even with the use of DCD grafts and expansion into SLK or repeat LT. However, a careful ongoing assessment regarding patient and graft selection, complications, and the timing of treatment is required,” the researchers concluded.
The study was funded in part by the McIver Estate Young Investigator Benefactor Award. The authors reported they had no potential conflicts.
SOURCE: Aqel B et al. J Hepatol. 2020, Nov 11. doi: 10.1016/j.jhep.2020.11.005.
Liver transplantation using hepatitis C virus (HCV)-seropositive grafts to HCV-seronegative recipients resulted in “excellent short-term outcomes,” according to the results of a prospective, multicenter study reported in the Journal of Hepatology.
A total of 34 HCV– liver transplantation recipients received grafts from HCV+ donors (20 HCV viremic and 14 nonviremic) from January 2018 to September 2019, according to Bashar Aqel, MD, of the Mayo Clinic, Phoenix, Ariz., and colleagues.
Seven of the grafts were obtained from donation after cardiac death (DCD). Six recipients underwent simultaneous liver/kidney (SLK) transplant, and four patients were repeat liver transplants.
Sustained viral response
None of the recipients of an HCV nonviremic graft developed HCV viremia. However, all 20 patients who received HCV viremic grafts had HCV viremia confirmed within 3 days after liver transplant. Direct-acting antiviral (DAA) treatment was started at the median time of 27.5 days in these patients.
All 20 patients successfully completed the treatment and achieved a sustained viral response. In addition, the DAA treatment was well tolerated with minimal adverse events, according to the researchers.
However, one patient died, having developed HCV-related acute membranous nephropathy that resulted in end-stage kidney disease. In addition, a recipient of an HCV nonviremic graft died with acute myocardial infarction 610 days post liver transplant, the authors reported.
“This multicenter study demonstrated LT [liver transplantation] using HCV-seropositive grafts to HCV-seronegative recipients resulted in acceptable short-term outcomes even with the use of DCD grafts and expansion into SLK or repeat LT. However, a careful ongoing assessment regarding patient and graft selection, complications, and the timing of treatment is required,” the researchers concluded.
The study was funded in part by the McIver Estate Young Investigator Benefactor Award. The authors reported they had no potential conflicts.
SOURCE: Aqel B et al. J Hepatol. 2020, Nov 11. doi: 10.1016/j.jhep.2020.11.005.
Liver transplantation using hepatitis C virus (HCV)-seropositive grafts to HCV-seronegative recipients resulted in “excellent short-term outcomes,” according to the results of a prospective, multicenter study reported in the Journal of Hepatology.
A total of 34 HCV– liver transplantation recipients received grafts from HCV+ donors (20 HCV viremic and 14 nonviremic) from January 2018 to September 2019, according to Bashar Aqel, MD, of the Mayo Clinic, Phoenix, Ariz., and colleagues.
Seven of the grafts were obtained from donation after cardiac death (DCD). Six recipients underwent simultaneous liver/kidney (SLK) transplant, and four patients were repeat liver transplants.
Sustained viral response
None of the recipients of an HCV nonviremic graft developed HCV viremia. However, all 20 patients who received HCV viremic grafts had HCV viremia confirmed within 3 days after liver transplant. Direct-acting antiviral (DAA) treatment was started at the median time of 27.5 days in these patients.
All 20 patients successfully completed the treatment and achieved a sustained viral response. In addition, the DAA treatment was well tolerated with minimal adverse events, according to the researchers.
However, one patient died, having developed HCV-related acute membranous nephropathy that resulted in end-stage kidney disease. In addition, a recipient of an HCV nonviremic graft died with acute myocardial infarction 610 days post liver transplant, the authors reported.
“This multicenter study demonstrated LT [liver transplantation] using HCV-seropositive grafts to HCV-seronegative recipients resulted in acceptable short-term outcomes even with the use of DCD grafts and expansion into SLK or repeat LT. However, a careful ongoing assessment regarding patient and graft selection, complications, and the timing of treatment is required,” the researchers concluded.
The study was funded in part by the McIver Estate Young Investigator Benefactor Award. The authors reported they had no potential conflicts.
SOURCE: Aqel B et al. J Hepatol. 2020, Nov 11. doi: 10.1016/j.jhep.2020.11.005.
FROM JOURNAL OF HEPATOLOGY
Biden chooses California Attorney General Xavier Becerra to head HHS
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
If confirmed by the US Senate, Becerra will face the challenge of overseeing the federal agency charged with protecting the health of all Americans in the midst of the COVID-19 pandemic. At the time of the announcement, nearly 15 million Americans had tested positive for COVID-19 and more than 280,000 had died.
Becerra served 12 terms in Congress, representing the Los Angeles area. Although his public health experience is limited, he served on the Congressional Ways and Means Committee overseeing health-related issues. Becerra is known as an advocate for the health and well-being of women in particular.
The American College of Physicians, American Academy of Pediatrics, American College of Obstetricians & Gynecologists, American Academy of Family Physicians, and the American Psychiatric Association wrote a letter to Biden on December 3 urging him to select leaders with medical and healthcare expertise, in particular physicians.
“We believe that your administration and the country would be well-served by the appointment of qualified physicians to serve in key positions critical to advancing the health of our nation,” they wrote. “Therefore, our organizations, which represent more than 400,000 front-line physicians practicing in the United States, write to request that you identify and appoint physicians to healthcare leadership positions within your administration.”
Recent advocacy
Becerra has worked with Republican attorneys general to lobby HHS to increase access to remdesivir to treat people with COVID-19.
As attorney general, Becerra filed more than 100 lawsuits against the Trump administration. In November, he also represented more than 20 states in arguments supporting the Affordable Care Act before the Supreme Court.
On December 4, Becerra joined with attorneys general from 23 states and the District of Columbia opposing a proposed rule from the outgoing Trump administration. The rule would deregulate HHS and “sunset”many agency provisions before Trump leaves office next month.
Becerra will be the first Latino appointed as HHS secretary, which furthers Biden’s goal to create a diverse cabinet. Becerra has been attorney general of California since 2017, replacing Vice President-elect Kamala Harris when she became senator.
Biden’s choice of Becerra was unexpected, according to The New York Times, and he was not the only candidate. Speculation was that Biden initially considered Vivek Murthy, MD, later chosen as the next US surgeon general, as well New Mexico Gov. Michelle Lujan Grisham and Rhode Island Gov. Gina Raimondo.
A huge undertaking
As HHS secretary, Becerra would oversee a wide range of federal agencies, including the US Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Centers for Medicare & Medicaid Services.
The fiscal year 2021 budget proposed for HHS includes $94.5 billion in discretionary budget authority and $1.3 trillion in mandatory funding. Overall, HHS controls nearly one quarter of all federal expenditures and provides more grant money than all other federal agencies combined.
Becerra, 62, grew up in Sacramento, California. He was the first in his family to graduate from college. He received his undergraduate and law degrees from Stanford University.
This article first appeared on Medscape.com.
Obesity, hypoxia predict severity in children with COVID-19
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
FROM THE JOURNAL OF PEDIATRICS
Cervical cancer recurrence patterns differ after laparoscopic and open hysterectomy
When cervical cancer recurs after radical hysterectomy, the likelihood of recurrence at certain sites and the timing of recurrence may be associated with the surgical approach, according to a retrospective study.
according to a propensity-matched analysis of data from 105 patients with recurrence.
And recurrence in the pelvic cavity and peritoneal carcinomatosis were more common after laparoscopic hysterectomy than after open surgery. Overall survival was similar between the groups, however.
The different patterns of recurrence may relate to dissemination of the disease during colpotomy, but the reasons are unknown, study author Giorgio Bogani, MD, PhD, said at the meeting sponsored by AAGL.
To examine patterns of recurrence after laparoscopic and open abdominal radical hysterectomy for cervical cancer, Dr. Bogani of the department of gynecologic surgery at the National Cancer Institute in Milan and colleagues analyzed data from patients with cervical cancer who developed recurrence after surgery at two oncologic referral centers between 1990 and 2018 (Int J Gynecol Cancer. 2020 Jul. doi: 10.1136/ijgc-2020-001381).
The investigators applied a propensity-matching algorithm to reduce possible confounding factors. They matched 35 patients who had recurrence after laparoscopic hysterectomy to 70 patients who had recurrence after open surgery. The groups had similar baseline characteristics.
As in the Laparoscopic Approach to Cervical Cancer (LACC) trial, patients who had minimally invasive surgery were more likely to have a worse disease-free survival, compared with patients who had open surgery, Dr. Bogani said. Patients who underwent laparoscopic radical hysterectomy had a median progression-free survival of 8 months, whereas patients who underwent open abdominal procedures had a median progression-free survival of 15.8 months.
Although vaginal, lymphatic, and distant recurrences were similar between the groups, a greater percentage of patients in the laparoscopic hysterectomy group had recurrence in the pelvic cavity (74% vs. 34%) and peritoneal carcinomatosis (17% vs. 1.5%).
The LACC trial, which found significantly lower disease-free and overall survival with laparoscopic hysterectomy, sent a “shockwave through the gynecologic oncology community” when it was published in 2018, said Masoud Azodi, MD, in a discussion following Dr. Bogani’s presentation.
Researchers have raised questions about that trial’s design and validity, noted Dr. Azodi, director of minimally invasive and robotic surgery at Yale University in New Haven, Conn.
It could be that local recurrences are attributable to surgical technique, rather than to the minimally invasive approach in itself, Dr. Azodi said. Prior studies of laparoscopic hysterectomy for cervical cancer had indicated better surgical outcomes and equivalent oncologic results, relative to open surgery.
Before the LACC trial, Dr. Bogani used the minimally invasive approach for almost all surgeries. Since then, he has performed open surgeries. If he were to use a minimally invasive approach now, it would be in the context of a clinical trial, Dr. Bogani said.
Dr. Bogani and Dr. Azodi had no relevant financial disclosures.
SOURCE: Bogani G et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.069.
When cervical cancer recurs after radical hysterectomy, the likelihood of recurrence at certain sites and the timing of recurrence may be associated with the surgical approach, according to a retrospective study.
according to a propensity-matched analysis of data from 105 patients with recurrence.
And recurrence in the pelvic cavity and peritoneal carcinomatosis were more common after laparoscopic hysterectomy than after open surgery. Overall survival was similar between the groups, however.
The different patterns of recurrence may relate to dissemination of the disease during colpotomy, but the reasons are unknown, study author Giorgio Bogani, MD, PhD, said at the meeting sponsored by AAGL.
To examine patterns of recurrence after laparoscopic and open abdominal radical hysterectomy for cervical cancer, Dr. Bogani of the department of gynecologic surgery at the National Cancer Institute in Milan and colleagues analyzed data from patients with cervical cancer who developed recurrence after surgery at two oncologic referral centers between 1990 and 2018 (Int J Gynecol Cancer. 2020 Jul. doi: 10.1136/ijgc-2020-001381).
The investigators applied a propensity-matching algorithm to reduce possible confounding factors. They matched 35 patients who had recurrence after laparoscopic hysterectomy to 70 patients who had recurrence after open surgery. The groups had similar baseline characteristics.
As in the Laparoscopic Approach to Cervical Cancer (LACC) trial, patients who had minimally invasive surgery were more likely to have a worse disease-free survival, compared with patients who had open surgery, Dr. Bogani said. Patients who underwent laparoscopic radical hysterectomy had a median progression-free survival of 8 months, whereas patients who underwent open abdominal procedures had a median progression-free survival of 15.8 months.
Although vaginal, lymphatic, and distant recurrences were similar between the groups, a greater percentage of patients in the laparoscopic hysterectomy group had recurrence in the pelvic cavity (74% vs. 34%) and peritoneal carcinomatosis (17% vs. 1.5%).
The LACC trial, which found significantly lower disease-free and overall survival with laparoscopic hysterectomy, sent a “shockwave through the gynecologic oncology community” when it was published in 2018, said Masoud Azodi, MD, in a discussion following Dr. Bogani’s presentation.
Researchers have raised questions about that trial’s design and validity, noted Dr. Azodi, director of minimally invasive and robotic surgery at Yale University in New Haven, Conn.
It could be that local recurrences are attributable to surgical technique, rather than to the minimally invasive approach in itself, Dr. Azodi said. Prior studies of laparoscopic hysterectomy for cervical cancer had indicated better surgical outcomes and equivalent oncologic results, relative to open surgery.
Before the LACC trial, Dr. Bogani used the minimally invasive approach for almost all surgeries. Since then, he has performed open surgeries. If he were to use a minimally invasive approach now, it would be in the context of a clinical trial, Dr. Bogani said.
Dr. Bogani and Dr. Azodi had no relevant financial disclosures.
SOURCE: Bogani G et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.069.
When cervical cancer recurs after radical hysterectomy, the likelihood of recurrence at certain sites and the timing of recurrence may be associated with the surgical approach, according to a retrospective study.
according to a propensity-matched analysis of data from 105 patients with recurrence.
And recurrence in the pelvic cavity and peritoneal carcinomatosis were more common after laparoscopic hysterectomy than after open surgery. Overall survival was similar between the groups, however.
The different patterns of recurrence may relate to dissemination of the disease during colpotomy, but the reasons are unknown, study author Giorgio Bogani, MD, PhD, said at the meeting sponsored by AAGL.
To examine patterns of recurrence after laparoscopic and open abdominal radical hysterectomy for cervical cancer, Dr. Bogani of the department of gynecologic surgery at the National Cancer Institute in Milan and colleagues analyzed data from patients with cervical cancer who developed recurrence after surgery at two oncologic referral centers between 1990 and 2018 (Int J Gynecol Cancer. 2020 Jul. doi: 10.1136/ijgc-2020-001381).
The investigators applied a propensity-matching algorithm to reduce possible confounding factors. They matched 35 patients who had recurrence after laparoscopic hysterectomy to 70 patients who had recurrence after open surgery. The groups had similar baseline characteristics.
As in the Laparoscopic Approach to Cervical Cancer (LACC) trial, patients who had minimally invasive surgery were more likely to have a worse disease-free survival, compared with patients who had open surgery, Dr. Bogani said. Patients who underwent laparoscopic radical hysterectomy had a median progression-free survival of 8 months, whereas patients who underwent open abdominal procedures had a median progression-free survival of 15.8 months.
Although vaginal, lymphatic, and distant recurrences were similar between the groups, a greater percentage of patients in the laparoscopic hysterectomy group had recurrence in the pelvic cavity (74% vs. 34%) and peritoneal carcinomatosis (17% vs. 1.5%).
The LACC trial, which found significantly lower disease-free and overall survival with laparoscopic hysterectomy, sent a “shockwave through the gynecologic oncology community” when it was published in 2018, said Masoud Azodi, MD, in a discussion following Dr. Bogani’s presentation.
Researchers have raised questions about that trial’s design and validity, noted Dr. Azodi, director of minimally invasive and robotic surgery at Yale University in New Haven, Conn.
It could be that local recurrences are attributable to surgical technique, rather than to the minimally invasive approach in itself, Dr. Azodi said. Prior studies of laparoscopic hysterectomy for cervical cancer had indicated better surgical outcomes and equivalent oncologic results, relative to open surgery.
Before the LACC trial, Dr. Bogani used the minimally invasive approach for almost all surgeries. Since then, he has performed open surgeries. If he were to use a minimally invasive approach now, it would be in the context of a clinical trial, Dr. Bogani said.
Dr. Bogani and Dr. Azodi had no relevant financial disclosures.
SOURCE: Bogani G et al. J Minim Invasive Gynecol. 2020 Nov. doi: 10.1016/j.jmig.2020.08.069.
FROM AAGL GLOBAL CONGRESS
Medicare finalizes 2021 physician pay rule with E/M changes
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
CDC shortens COVID-19 quarantine time to 10 or 7 days, with conditions
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.