User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Dietary flavonol intake linked to reduced risk of Alzheimer’s
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
FROM NEUROLOGY
High-dose chemo offers survival benefit only for highest-risk breast cancer
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
High-dose chemotherapy in the adjuvant setting offers a long-term survival advantage for women with very-high-risk stage III breast cancer, but does not improve survival odds for women with lower-risk cancers, an analysis of 20 years of follow-up data shows.
Among 885 women younger than 56 years at the time of treatment who had 4 or more involved axilliary lymph nodes, there was no overall survival difference over 2 decades between the total population of women randomized to receive adjuvant high-dose chemotherapy (HDCT) and those assigned to receive conventional-dose chemotherapy (CDCT).
However, women with 10 or more involved axilliary nodes and those with triple-negative breast cancer had an approximately 15% absolute improvement in 20-year overall survival with high-dose chemotherapy, although the difference for triple-negative disease fell just short of statistical significance, reported Tessa G. Steenbruggen, MD, from the Netherlands Cancer Institute in Amsterdam and colleagues.
“Our analysis confirms earlier results that HDCT has no significant overall survival benefit compared with CDCT for unselected patients with stage III [breast cancer]. However, we found a 14.6%improvement in 20-year OS estimates with HDCT in the predefined subgroup of patients with 10 or more involved [axilliary lymph nodes],” they wrote in JAMA Oncology.
And although other studies of chemotherapy regimens containing high doses of alkylating agents have shown increases in risk of late second malignancies and major cardiovascular events, there were no significant increases of either adverse event with HDCT in this study, the authors noted.
They reported 20-year follow-up results for 885 women who were enrolled in a 10-center randomized clinical trial conducted in the Netherlands from August 1, 1993, through July 31, 1999.
The participants were younger than age 56 years with breast cancer involving at least 4 axillary lymph nodes. All patients underwent surgery with complete axillary clearance and were then randomized to receive either conventional chemotherapy, which consisted of five cycles of fluorouracil 500mg/m2, epirubicin 90 mg/m2, and cyclophosphamide 500mg/m2 (FEC), or high-dose chemotherapy, with the first 4 cycles identical to conventional-dose chemotherapy but the fifth cycle consisting of cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2, and carboplatin 1600 mg/m2, supported with autologous hematopoietic stem cell transplant.
In addition, all patients received radiotherapy according to the local standard and 2 years of adjuvant tamoxifen.
After a median follow-up of 20.4 years, the 20-year overall survival (OS) rates were 45.3% for patients who had received high-dose chemotherapy and 41.5% for those who had received the conventional dose. This translated into a nonsignificant hazard ratio of 0.89.
However, for patients with 10 or more involved axillary nodes, the 20-year OS rates were 44.5% with HDCT and 29.9% with CDCT, translating into an absolute OS advantage for high-dose chemotherapy of 14.6% and an HR of 0.72 (P = .02).
Respective 20-year OS rates for women with triple-negative breast cancer were 52.9% and 37.5%, an absolute difference of 15.4% and a HR of 0.67, which fell just short of statistical significance, possibly because of the small number of patients with triple-negative breast cancer (140).
“In our 20-year follow-up analysis, there was no increase in cumulative risk for a second malignant neoplasm or for incidence of major cardiovascular events after HDCT,” the investigators wrote.
They noted that women randomized to high-dose chemotherapy had more frequent dysrhythmias, hypertension, and hypercholesterolemia, adding that the latter two adverse events may be partly attributable to a higher incidence of menopause induction among women who received HDCT.
The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
SOURCE: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
FROM JAMA ONCOLOGY
Key clinical point: High-dose chemotherapy offers a long-term breast cancer survival advantage only for women with very-high-risk disease.
Major finding: The absolute 20-year overall survival benefit for women with 10 or more involved lymph nodes was 14.6%.
Study details: Long-term, follow-up study of 885 women under age 56 years with stage III breast cancer treated with adjuvant high- or conventional-dose chemotherapy.
Disclosures: The study was sponsored by University Medical Center Groningen and the The Netherlands Cancer Institute. Dr. Steenbruggen reported receiving grants from the Dutch Health Insurance Council during the conduct of the study.
Source: Steenbruggen TG et al. JAMA Oncology. 2020 Jan 30. doi: 10.1001/jamaoncol.2019.6276.
CDC: Risk in U.S. from 2019-nCoV remains low
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
Costs are keeping Americans out of the doctor’s office
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.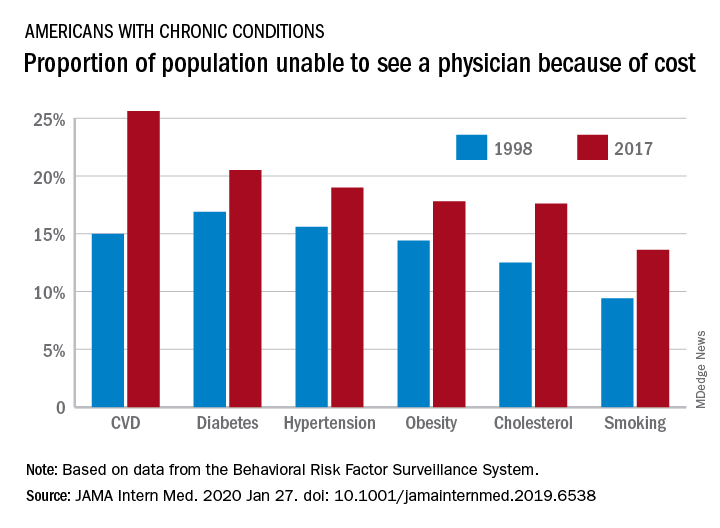
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
FROM JAMA INTERNAL MEDICINE
Psoriasis: A look back over the past 50 years, and forward to next steps
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Dual immunotherapy goes the distance in MSI-H colorectal cancer
SAN FRANCISCO – First-line dual immune checkpoint inhibitor therapy for microsatellite instability–high/DNA mismatch repair–deficient (MSI-H/dMMR) metastatic colorectal cancer has impressive durability, an update of the multicohort CheckMate 142 trial shows.
“We all know that MSI-H colorectal cancer patients have a poor prognosis,” said lead investigator Heinz-Josef Lenz, MD, codirector of the Colorectal Center and section head of the GI oncology program at the University of Southern California Norris Comprehensive Cancer Center, Los Angeles. First-line chemotherapy in this population yields median overall survival on the order of 20-22 months.
The cohort of 45 patients treated on the phase 2 trial received the programmed death–1 inhibitor nivolumab (Opdivo) every 2 weeks, plus a low dose of the CTLA4 inhibitor ipilimumab (Yervoy) every 6 weeks as first-line therapy for MSI-H metastatic colorectal cancer. (Nivolumab, with or without ipilimumab, has received Food and Drug Administration accelerated approval as second-line therapy based on data from other cohorts in the trial.)
Previously reported initial results, at a median follow-up of 13.8 months, showed that the investigator-assessed objective response rate was 60% (ESMO 2018, Abstract LBA18_PR). As of the update, now at a median follow-up of 19.9 months, that rate was 64%, according to data reported at the 2020 GI Cancers Symposium.
“Nivolumab and ipilimumab demonstrates clinically meaningful durable benefits and may present an option for first-line treatment for MSI-H metastatic colorectal cancer patients,” Dr. Lenz summarized.
“The incredible complete response rates and overall response rates seen are never seen with chemotherapy, and it’s very well tolerated, so if I have a choice, I would start with nivolumab-ipilimumab in first-line MSI-H,” he added. “The [National Comprehensive Cancer Network] guidelines recommend that you can start in first line with this combination if patients are not candidates for chemotherapy. So they leave this door open, that you have an opportunity to use immunotherapy in this patient population.”
Choosing single or dual immunotherapy
Given the so-called financial toxicity of dual nivolumab and ipilimumab therapy and lack of a randomized comparison against single-agent nivolumab, a session attendee said, can clinicians simply use the latter?
“Very importantly, in this clinical trial, ipilimumab was given every 6 weeks. As you know, the 2-week regimens are significantly more toxic,” Dr. Lenz noted. The combination using this low dose has safety similar to that seen with nivolumab alone. And in cross-trial comparisons, at least, “the combination seems to be a little bit more active, so I always choose both,” he said, adding that the financial aspects are beyond his purview.
However, session cochair Joseph J.Y. Sung, MD, PhD, MBBS, the Mok Hing Yiu Professor of Medicine in the department of medicine and therapeutics at the Chinese University of Hong Kong, had a different view.
“I think there is still room for a randomized study to prove the combination’s superiority against single immunotherapy,” he said in an interview. “If I’m treating an MSI patient, I would stick to single immunotherapy until I see more evidence that this combination has a substantial improvement in the outcome that is worth the money.”
Study details
The patients studied were unselected for baseline tumor expression of programmed death–ligand 1, with 27% having expression of 1% or greater and 58% having expression below that threshold.
As of the data cutoff for the update, 47% of patients were still on treatment, Dr. Lenz reported at the symposium, sponsored by the American Gastroenterological Association, American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The most common reason for stopping treatment was disease progression.
The investigator-assessed objective response rate, the primary endpoint, consisted of complete responses in 9% of patients and partial responses in 56%. (The rate as assessed by blinded independent central reviewers was 58%, consisting of complete responses in 18% and partial responses in 40%.)
“The high response rate was consistent among all evaluated subgroups,” Dr. Lenz noted, with no differences detected by presence or absence of Lynch syndrome and/or specific microsatellite-related mutations.
Median time to response was 2.6 months, and median duration of response was not reached. Median progression-free and overall survival were also not reached, but 15-month rates were 75% and 84%, respectively.
The rate of grade 3-4 treatment-related adverse events was 20%, and the rate of grade 3-4 serious treatment-related adverse events was 11%. Only 4% of patients discontinued treatment because of such events.
Ongoing trials may take immunotherapy even further in this patient population, according to Dr. Lenz. “We are all waiting for the treatments with the immunotherapy in combination with chemo and bevacizumab in first line. Will that change the efficacy and toxicity?” he elaborated. “You can imagine, in the MSI-H patients with the immunotherapy alone, we haven’t reached median progression-free and overall survival, so it may take a long time to get this data.”
Dr. Lenz reported receiving honoraria from Bayer, Boehringer Ingelheim, Merck Serono, and Roche; consulting or advising with Bayer, Merck Serono, Pfizer, and Roche; receiving travel expenses from Bayer, Merck Serono, and Roche. The trial was funded by Bristol-Myers Squibb. Dr. Sung reported that he had no relevant conflicts of interest.
SOURCE: Lenz H-J et al. 2020 GI Cancers Symposium, Abstract 11.
SAN FRANCISCO – First-line dual immune checkpoint inhibitor therapy for microsatellite instability–high/DNA mismatch repair–deficient (MSI-H/dMMR) metastatic colorectal cancer has impressive durability, an update of the multicohort CheckMate 142 trial shows.
“We all know that MSI-H colorectal cancer patients have a poor prognosis,” said lead investigator Heinz-Josef Lenz, MD, codirector of the Colorectal Center and section head of the GI oncology program at the University of Southern California Norris Comprehensive Cancer Center, Los Angeles. First-line chemotherapy in this population yields median overall survival on the order of 20-22 months.
The cohort of 45 patients treated on the phase 2 trial received the programmed death–1 inhibitor nivolumab (Opdivo) every 2 weeks, plus a low dose of the CTLA4 inhibitor ipilimumab (Yervoy) every 6 weeks as first-line therapy for MSI-H metastatic colorectal cancer. (Nivolumab, with or without ipilimumab, has received Food and Drug Administration accelerated approval as second-line therapy based on data from other cohorts in the trial.)
Previously reported initial results, at a median follow-up of 13.8 months, showed that the investigator-assessed objective response rate was 60% (ESMO 2018, Abstract LBA18_PR). As of the update, now at a median follow-up of 19.9 months, that rate was 64%, according to data reported at the 2020 GI Cancers Symposium.
“Nivolumab and ipilimumab demonstrates clinically meaningful durable benefits and may present an option for first-line treatment for MSI-H metastatic colorectal cancer patients,” Dr. Lenz summarized.
“The incredible complete response rates and overall response rates seen are never seen with chemotherapy, and it’s very well tolerated, so if I have a choice, I would start with nivolumab-ipilimumab in first-line MSI-H,” he added. “The [National Comprehensive Cancer Network] guidelines recommend that you can start in first line with this combination if patients are not candidates for chemotherapy. So they leave this door open, that you have an opportunity to use immunotherapy in this patient population.”
Choosing single or dual immunotherapy
Given the so-called financial toxicity of dual nivolumab and ipilimumab therapy and lack of a randomized comparison against single-agent nivolumab, a session attendee said, can clinicians simply use the latter?
“Very importantly, in this clinical trial, ipilimumab was given every 6 weeks. As you know, the 2-week regimens are significantly more toxic,” Dr. Lenz noted. The combination using this low dose has safety similar to that seen with nivolumab alone. And in cross-trial comparisons, at least, “the combination seems to be a little bit more active, so I always choose both,” he said, adding that the financial aspects are beyond his purview.
However, session cochair Joseph J.Y. Sung, MD, PhD, MBBS, the Mok Hing Yiu Professor of Medicine in the department of medicine and therapeutics at the Chinese University of Hong Kong, had a different view.
“I think there is still room for a randomized study to prove the combination’s superiority against single immunotherapy,” he said in an interview. “If I’m treating an MSI patient, I would stick to single immunotherapy until I see more evidence that this combination has a substantial improvement in the outcome that is worth the money.”
Study details
The patients studied were unselected for baseline tumor expression of programmed death–ligand 1, with 27% having expression of 1% or greater and 58% having expression below that threshold.
As of the data cutoff for the update, 47% of patients were still on treatment, Dr. Lenz reported at the symposium, sponsored by the American Gastroenterological Association, American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The most common reason for stopping treatment was disease progression.
The investigator-assessed objective response rate, the primary endpoint, consisted of complete responses in 9% of patients and partial responses in 56%. (The rate as assessed by blinded independent central reviewers was 58%, consisting of complete responses in 18% and partial responses in 40%.)
“The high response rate was consistent among all evaluated subgroups,” Dr. Lenz noted, with no differences detected by presence or absence of Lynch syndrome and/or specific microsatellite-related mutations.
Median time to response was 2.6 months, and median duration of response was not reached. Median progression-free and overall survival were also not reached, but 15-month rates were 75% and 84%, respectively.
The rate of grade 3-4 treatment-related adverse events was 20%, and the rate of grade 3-4 serious treatment-related adverse events was 11%. Only 4% of patients discontinued treatment because of such events.
Ongoing trials may take immunotherapy even further in this patient population, according to Dr. Lenz. “We are all waiting for the treatments with the immunotherapy in combination with chemo and bevacizumab in first line. Will that change the efficacy and toxicity?” he elaborated. “You can imagine, in the MSI-H patients with the immunotherapy alone, we haven’t reached median progression-free and overall survival, so it may take a long time to get this data.”
Dr. Lenz reported receiving honoraria from Bayer, Boehringer Ingelheim, Merck Serono, and Roche; consulting or advising with Bayer, Merck Serono, Pfizer, and Roche; receiving travel expenses from Bayer, Merck Serono, and Roche. The trial was funded by Bristol-Myers Squibb. Dr. Sung reported that he had no relevant conflicts of interest.
SOURCE: Lenz H-J et al. 2020 GI Cancers Symposium, Abstract 11.
SAN FRANCISCO – First-line dual immune checkpoint inhibitor therapy for microsatellite instability–high/DNA mismatch repair–deficient (MSI-H/dMMR) metastatic colorectal cancer has impressive durability, an update of the multicohort CheckMate 142 trial shows.
“We all know that MSI-H colorectal cancer patients have a poor prognosis,” said lead investigator Heinz-Josef Lenz, MD, codirector of the Colorectal Center and section head of the GI oncology program at the University of Southern California Norris Comprehensive Cancer Center, Los Angeles. First-line chemotherapy in this population yields median overall survival on the order of 20-22 months.
The cohort of 45 patients treated on the phase 2 trial received the programmed death–1 inhibitor nivolumab (Opdivo) every 2 weeks, plus a low dose of the CTLA4 inhibitor ipilimumab (Yervoy) every 6 weeks as first-line therapy for MSI-H metastatic colorectal cancer. (Nivolumab, with or without ipilimumab, has received Food and Drug Administration accelerated approval as second-line therapy based on data from other cohorts in the trial.)
Previously reported initial results, at a median follow-up of 13.8 months, showed that the investigator-assessed objective response rate was 60% (ESMO 2018, Abstract LBA18_PR). As of the update, now at a median follow-up of 19.9 months, that rate was 64%, according to data reported at the 2020 GI Cancers Symposium.
“Nivolumab and ipilimumab demonstrates clinically meaningful durable benefits and may present an option for first-line treatment for MSI-H metastatic colorectal cancer patients,” Dr. Lenz summarized.
“The incredible complete response rates and overall response rates seen are never seen with chemotherapy, and it’s very well tolerated, so if I have a choice, I would start with nivolumab-ipilimumab in first-line MSI-H,” he added. “The [National Comprehensive Cancer Network] guidelines recommend that you can start in first line with this combination if patients are not candidates for chemotherapy. So they leave this door open, that you have an opportunity to use immunotherapy in this patient population.”
Choosing single or dual immunotherapy
Given the so-called financial toxicity of dual nivolumab and ipilimumab therapy and lack of a randomized comparison against single-agent nivolumab, a session attendee said, can clinicians simply use the latter?
“Very importantly, in this clinical trial, ipilimumab was given every 6 weeks. As you know, the 2-week regimens are significantly more toxic,” Dr. Lenz noted. The combination using this low dose has safety similar to that seen with nivolumab alone. And in cross-trial comparisons, at least, “the combination seems to be a little bit more active, so I always choose both,” he said, adding that the financial aspects are beyond his purview.
However, session cochair Joseph J.Y. Sung, MD, PhD, MBBS, the Mok Hing Yiu Professor of Medicine in the department of medicine and therapeutics at the Chinese University of Hong Kong, had a different view.
“I think there is still room for a randomized study to prove the combination’s superiority against single immunotherapy,” he said in an interview. “If I’m treating an MSI patient, I would stick to single immunotherapy until I see more evidence that this combination has a substantial improvement in the outcome that is worth the money.”
Study details
The patients studied were unselected for baseline tumor expression of programmed death–ligand 1, with 27% having expression of 1% or greater and 58% having expression below that threshold.
As of the data cutoff for the update, 47% of patients were still on treatment, Dr. Lenz reported at the symposium, sponsored by the American Gastroenterological Association, American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology. The most common reason for stopping treatment was disease progression.
The investigator-assessed objective response rate, the primary endpoint, consisted of complete responses in 9% of patients and partial responses in 56%. (The rate as assessed by blinded independent central reviewers was 58%, consisting of complete responses in 18% and partial responses in 40%.)
“The high response rate was consistent among all evaluated subgroups,” Dr. Lenz noted, with no differences detected by presence or absence of Lynch syndrome and/or specific microsatellite-related mutations.
Median time to response was 2.6 months, and median duration of response was not reached. Median progression-free and overall survival were also not reached, but 15-month rates were 75% and 84%, respectively.
The rate of grade 3-4 treatment-related adverse events was 20%, and the rate of grade 3-4 serious treatment-related adverse events was 11%. Only 4% of patients discontinued treatment because of such events.
Ongoing trials may take immunotherapy even further in this patient population, according to Dr. Lenz. “We are all waiting for the treatments with the immunotherapy in combination with chemo and bevacizumab in first line. Will that change the efficacy and toxicity?” he elaborated. “You can imagine, in the MSI-H patients with the immunotherapy alone, we haven’t reached median progression-free and overall survival, so it may take a long time to get this data.”
Dr. Lenz reported receiving honoraria from Bayer, Boehringer Ingelheim, Merck Serono, and Roche; consulting or advising with Bayer, Merck Serono, Pfizer, and Roche; receiving travel expenses from Bayer, Merck Serono, and Roche. The trial was funded by Bristol-Myers Squibb. Dr. Sung reported that he had no relevant conflicts of interest.
SOURCE: Lenz H-J et al. 2020 GI Cancers Symposium, Abstract 11.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM
What 2019’s top five CAD trials tell us
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – A repeated theme threading through much of one prominent interventional cardiologist’s personal list of the top five coronary artery disease (CAD) trials of the past year is that aspirin is very often more trouble than it’s worth.
“For some years I’ve been concerned that the only thing that aspirin does [in patients after percutaneous coronary intervention] is increase your risk of bleeding. It doesn’t really provide any additional ischemic protection,” Malcolm R. Bell, MBBS, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“I’ll remind you that, when we go back to the early stent days, observed Dr. Bell, professor of medicine and vice chair of the department of cardiovascular medicine at the Mayo Clinic in Rochester, Minn.
Here are the key takeaway messages from his five most important randomized trials in CAD during the last year.
AUGUSTUS
For years, cardiologists have grappled with how to best manage high-cardiovascular-risk patients with atrial fibrillation who seem like they might benefit from triple-antithrombotic therapy. AUGUSTUS supplied the answer: Don’t do it. Skip the aspirin and turn instead to a P2Y12 inhibitor plus a non–vitamin K antagonist oral anticoagulant (NOAC), rather than warfarin.
“I would like you to think of triple therapy as a triple threat. That’s really what triple therapy is all about”– a three-pronged threat to patient safety, Dr. Bell commented.
In AUGUSTUS, 4,614 patients with atrial fibrillation and CAD with an acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI) in 33 countries were placed on a P2Y12 inhibitor – most often clopidogrel – and randomized double blind to either apixaban (Eliquis) or warfarin, and further to aspirin or placebo, for 6 months of antithrombotic therapy. The strategy of a P2Y12 inhibitor and apixaban without aspirin was the clear winner, resulting in significantly less major bleeding, mortality, and hospitalizations than treatment with a P2Y12 inhibitor and warfarin, with or without aspirin. Most importantly, ischemic event rates didn’t differ between the apixaban and warfarin groups. And patients randomized to aspirin had rates of ischemic events and death or hospitalization similar to placebo-treated controls, meaning aspirin accomplished nothing (N Engl J Med. 2019 Apr 18;380[16]:1509-24).
Dr. Bell noted that a meta-analysis of AUGUSTUS and three smaller randomized trials including more than 10,000 AUGUSTUS-type patients with atrial fibrillation concluded that a treatment strategy utilizing a NOAC and a P2Y12 inhibitor resulted in less bleeding than warfarin plus DAPT, and at no cost in terms of excess ischemic events. Moreover, regimens without aspirin resulted in less intracranial and other major bleeding without any difference in major adverse cardiovascular events (JAMA Cardiol. 2019 Jun 19. doi: 10.1001/jamacardio.2019.1880).
A key message of these four trials is that a NOAC is preferable to warfarin, so much so that, in high-risk patients who are already on warfarin, it’s worth considering a switch to a NOAC.
“And we should really be avoiding DAPT,” Dr. Bell added.
How soon after an ACS and/or PCI should patients with atrial fibrillation stop taking aspirin?
“In AUGUSTUS, randomization occurred at a median of 6 days, so we know that half the patients stopped their aspirin by then. In our own practice, we’re just dropping the aspirin for the most part before the patient leaves the hospital. I think if you leave them with instructions to stop the aspirin in a week’s time or a month’s time it just leads to confusion. And we should also remember that half of the major bleeding after PCI or ACS happens in the first 30 days, so it doesn’t make a lot of sense to say that we should continue it for a month and then drop it,” according to the cardiologist.
SMART-CHOICE and STOPDAPT-2
These two large multicenter studies demonstrate that DAPT can safely be stopped early if needed. SMART-CHOICE from South Korea and STOPDAPT-2 from Japan each randomized roughly 3,000 patients undergoing PCI to 12 months of DAPT or to DAPT for only 3 months or 1 month, respectively, at which point the aspirin was dropped and patients in the abbreviated DAPT arm continued on P2Y12 inhibitor monotherapy, mostly clopidogrel, for the remainder of the 12 months. In the Japanese STOPDAPT-2 trial, 1 month of DAPT proved superior to 12 months of DAPT for the primary composite endpoint of cardiovascular death, MI, stroke, definite stent thrombosis, or major or minor bleeding at 12 months (JAMA. 2019 Jun 25;321[24]:2414-27). In the South Korean SMART-CHOICE trial, 3 months of DAPT was noninferior to 12 months for major adverse cardiac and cerebrovascular events, and superior in terms of bleeding risk (JAMA. 2019 Jun 25;321[24]:2428-37). Of note, roughly half of patients in the two trials were lower-risk individuals undergoing PCI for stable angina.
Dr. Bell noted that, while the TWILIGHT trial (Ticagrelor With or Without Aspirin in High-Risk Patients After PCI) didn’t make his top-five list, it certainly fits well with the two East Asian studies. The TWILIGHT investigators randomized more than 7,000 patients to 12 months of DAPT or discontinuation of aspirin after 3 months. The result: a lower incidence of clinically relevant bleeding with ticagrelor monotherapy, and with no increased risk of death, MI, or stroke, compared with 12 months of DAPT (N Engl J Med. 2019 Nov 21;381[21]:2032-42).
“Again, I would just question what the added value of aspirin is here,” Dr. Bell commented. “Many interventional cardiologists are absolutely terrified of their patients having stent thrombosis, but with second-generation drug-eluting stents – the stents we’re putting in day in and day out – the risk of stent thrombosis is less than 1%. And in these two trials it was less than 0.5%. There’s more risk of having major bleeding events than there is of ischemia, so I think the balance is in favor of preventing bleeding. We know that major bleeding predicts short- and long-term mortality.”
COLCOT
This double-blind trial randomized 4,745 patients within 30 days post MI to low-dose colchicine or placebo on top of excellent rates of background guideline-directed medical therapy. The goal was to see if this anti-inflammatory agent could reduce cardiovascular events independent of any lipid-lowering effect, as was earlier seen with canakinumab in the CANTOS trial. It did so to a statistically significant but relatively modest degree, with a 5.5% rate of the composite cardiovascular events endpoint in the colchicine group and 7.1% in placebo-treated controls (N Engl J Med. 2019 Dec 26;381[26]:2497-505). But Dr. Bell was unimpressed.
“All-cause mortality was identical at 1.8% in both groups. So colchicine is not saving lives. In fact, the only real differences were in stroke – but the study wasn’t powered to look at stroke – and in urgent hospitalization for angina leading to revascularization, which is a soft endpoint,” he observed.
Plus, 2.5% of patients were lost to follow-up, which Dr. Bell considers “a little concerning” in a trial conducted in the current era.
“In my opinion, the evidence that colchicine is effective is weak, and I don’t think really supports the drug’s routine use post MI. We already send these patients out on numerous medications. We have to think about cost/benefit, and if a patient asks me: ‘Is this going to prevent another heart attack or make me live longer?’ I think the unequivocal answer is no,” he said.
These days colchicine is no longer an inexpensive drug, either, at an average cost of $300-$400 per month, the cardiologist added.
COMPLETE
This study randomized more than 4,000 patients with ST-segment elevation MI (STEMI) and multivessel disease to primary PCI of the culprit lesion only or to staged complete revascularization via PCI of all angiographically significant nonculprit lesions. Complete revascularization proved to be the superior strategy, with a 26% reduction in the risk of the composite of cardiovascular death or MI at a median of 3 years (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
The optimal timing of the staged procedure remains unclear, since the study didn’t specify a protocol.
“I’m still a bit uncomfortable doing multivessel PCI at 2 o’clock in the morning in the setting of STEMI in someone I’ve never met before. I don’t think there’s a rush to do anything then. Often in this middle-of-the-night stuff, we miss things or we overinterpret things. I think it’s better to let the patient cool down, get to know them,” according to Dr. Bell.
EXCEL
Publication of the 5-year outcomes of the largest-ever randomized trial of PCI versus coronary artery bypass grafting (CABG) for left main coronary disease has led to furious controversy, with a few of the surgeons involved in the study opting to publically broadcast allegations of misbehavior on the part of the interventional cardiologist study leadership, charges that have been strongly denied.
The actual results are in line with findings reported from smaller randomized trials. At 5 years in EXCEL, there was no significant difference between the PCI and CABG groups in the primary composite endpoint of death, cerebrovascular accident, or MI (N Engl J Med. 2019 Nov 7;381[19]:1820-30). The all-cause mortality rate was 13% in the PCI arm and 9.9% with CABG, but this finding comes with a caveat.
“I’ll emphasize this trial was never powered to look at mortality. Neither were any of the other randomized trials. On the other hand, I don’t think you can necessarily ignore the finding of an absolute 3.1% difference,” Dr. Bell said.
PCI and CABG are both very good, mature therapies for left main disease, in his view. In the setting of more-complex coronary disease in younger patients, he often views the complete revascularization offered by surgery as the preferred option. On the other hand, in an 80-year-old with severe comorbidities, clearly PCI is attractive.
He considers the highly public nature of this interspecialty spat a regrettable black eye for the entire field of cardiovascular medicine. And he predicted that an ongoing outside neutral-party review of the study data and procedures will conclude, as he has, “there was no malfeasance at all in the trial.”
Dr. Bell reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
Evidence-based tools for premenstrual disorders
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
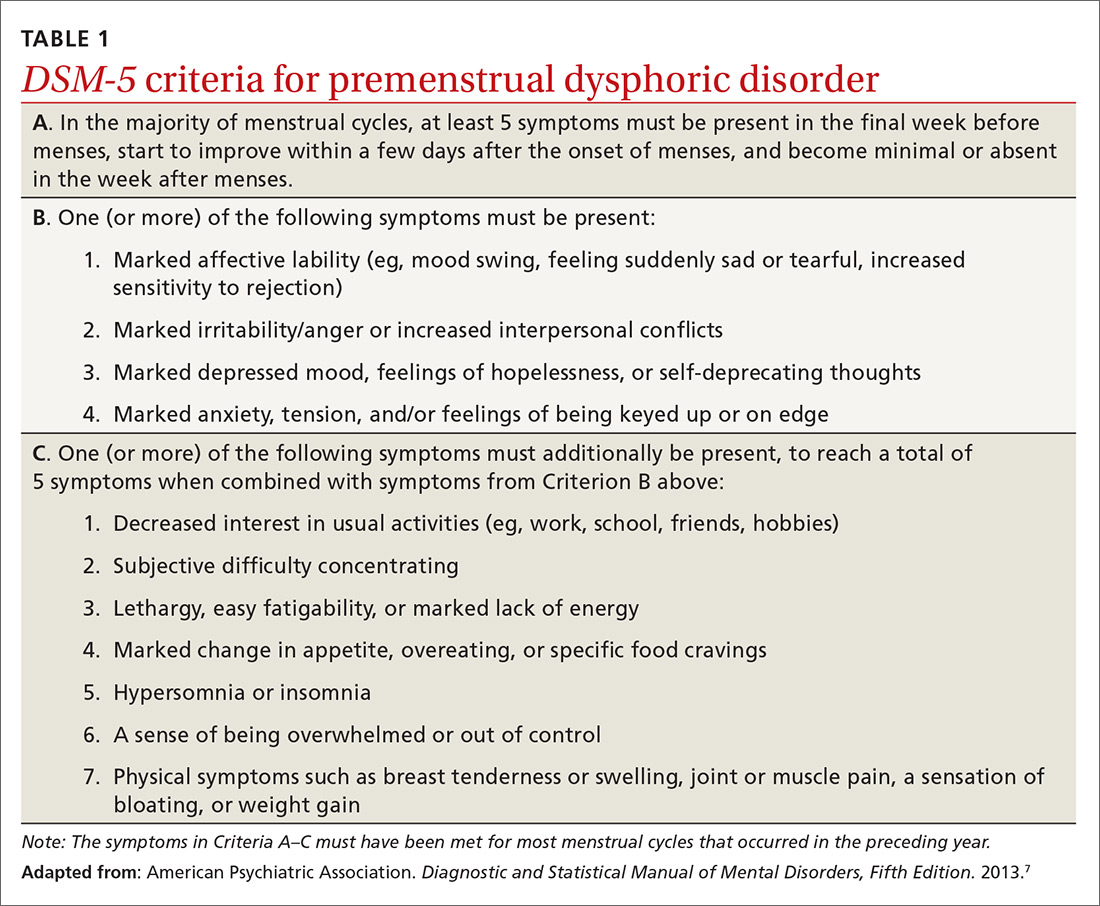
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
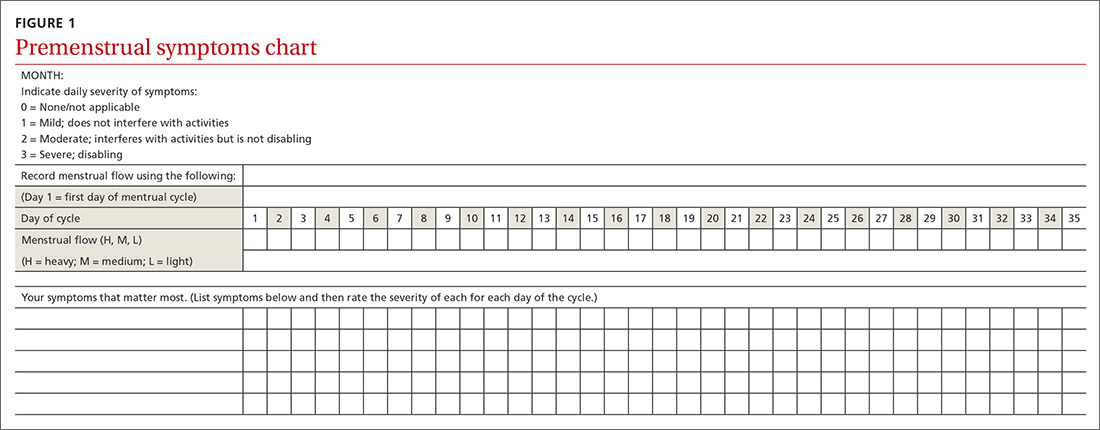
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
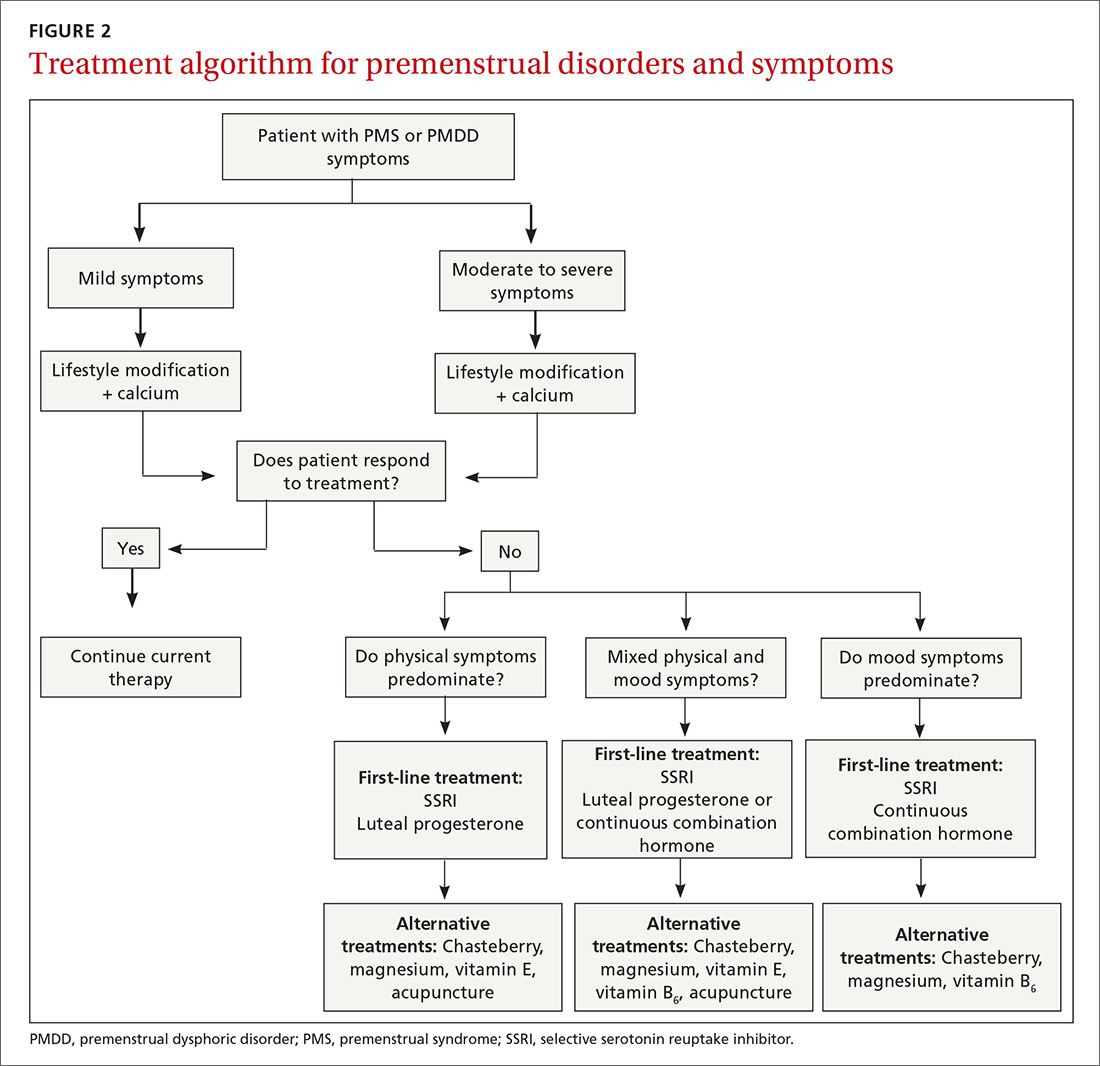
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
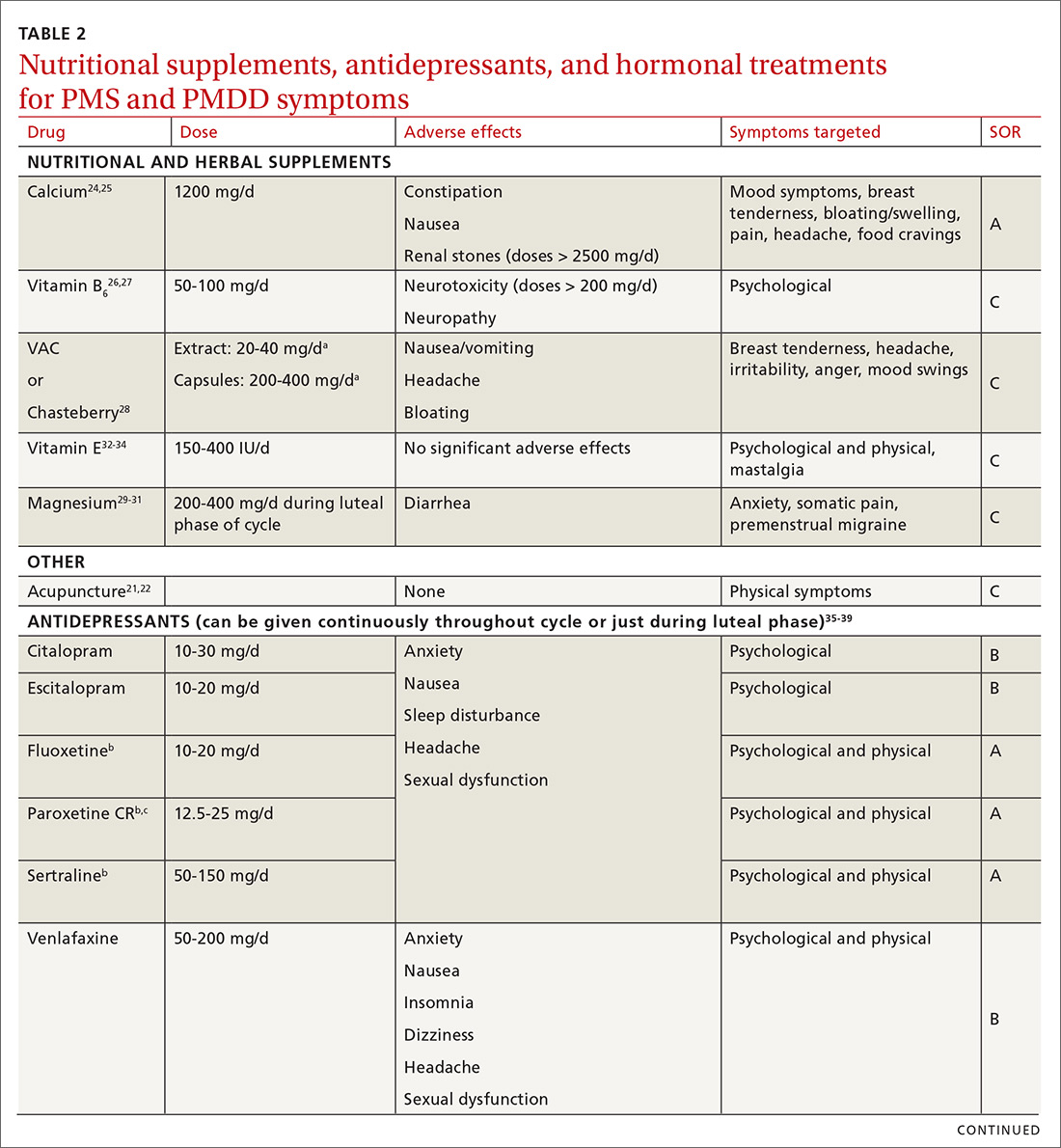
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
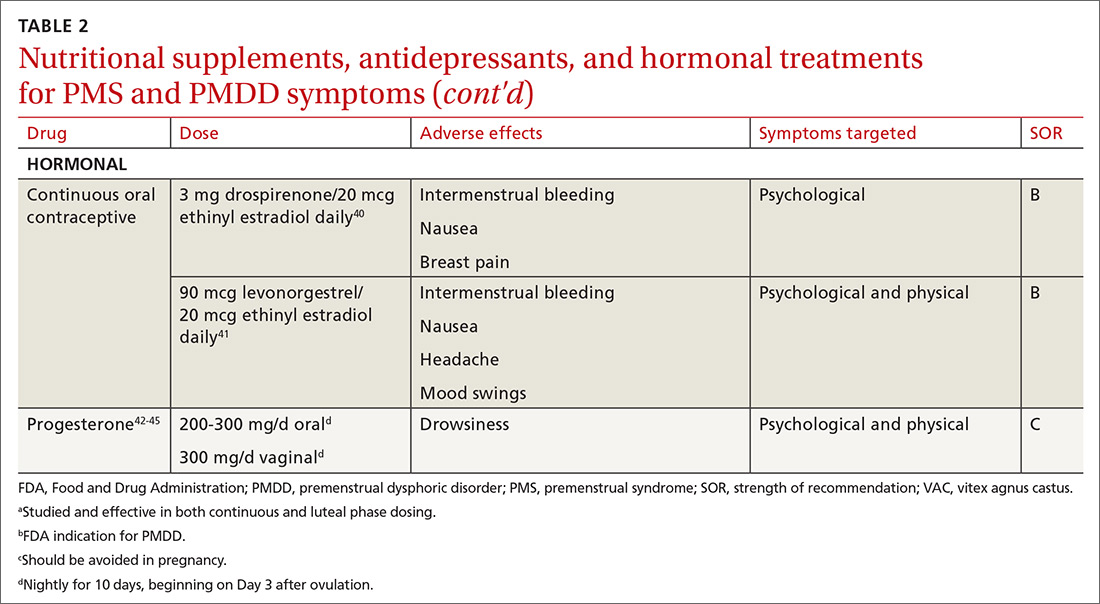
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7

Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.

Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness

Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25

Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27

Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7

Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.

Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness

Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25

Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27

Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; [email protected].
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
PRACTICE RECOMMENDATIONS
› Start calcium supplementation in all patients who report significant premenstrual symptoms. A
› Add a selective serotonin reuptake inhibitor (SSRI) to calcium supplementationfor patients who have more severe premenstrual psychological symptoms. A
› Consider hormonal treatment options for patients who require treatment beyond calcium and an SSRI. B
› Provide nutrition and exercise information to all patients who report significant premenstrual symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
BP levels during endovascular stroke therapy affect neurologic outcomes
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
FROM JAMA NEUROLOGY
Resecting primary tumors fails to boost survival in metastatic CRC
SAN FRANCISCO – The common practice of resecting asymptomatic primary tumors in patients with unresectable metastases of colorectal cancer does not improve survival but does increase morbidity, and it should therefore be discontinued, according to the phase 3 iPACS trial.
About 80% of patients with stage IV colorectal cancer at diagnosis have metastases that cannot be resected. “For asymptomatic patients, there is no consensus regarding resection of the primary tumor. There have been no randomized controlled trials comparing upfront chemotherapy with primary tumor resection,” said lead investigator Yukihide Kanemitsu, MD, department of colorectal surgery, National Cancer Center Hospital, Tokyo.
He and coinvestigators with the Japan Clinical Oncology Group enrolled in the randomized controlled trial 160 patients with untreated stage IV colorectal cancer who did not have any symptoms from their primary and had up to three unresectable sites of metastases (liver, lungs, distant lymph nodes, and/or peritoneum).
The trial was stopped early for futility, Dr. Kanemitsu reported at the 2020 GI Cancers Symposium. Median overall survival was slightly more than 2 years regardless of whether patients immediately received chemotherapy or underwent primary tumor resection before receiving chemotherapy.
However, 4% of the patients who underwent resection died in the postoperative period from complications. And the resection group had more frequent and more severe chemotherapy-related morbidity, with a higher rate of grade 3 and 4 adverse events (49% vs. 36%).
“Chemotherapy remains the standard therapy for asymptomatic unresectable stage IV colorectal cancer patients,” Dr. Kanemitsu concluded. “Primary tumor resection is not recommended for these patients.”
RCTs should remain the gold standard
“As someone who has built my career on health services research over the last decade working with real-world data, I have become alarmed in recent years at what appears to be a conversation in our community about leaving the randomized controlled trial behind and just using real-world evidence,” said invited discussant Christopher M. Booth, MD, professor, departments of oncology and medicine, Queen’s University, Kingston, Ont., and Canada Research Chair in Population Cancer Care. “Fundamentally, I think this is a really bad idea. The randomized controlled trial should remain the gold standard for efficacy of new cancer therapies.”
The high level of use of primary tumor resection has largely been driven by a meta-analysis that found a dramatic reduction in risk of death with this surgery (Ann Surg Oncol. 2014;21:3900-8), he noted. However, a subsequent retrospective cohort study showed that the apparent benefit disappeared with application of rigorous statistical methods that compensated for biases in the data (Cancer. 2017;123:1124-33).
“I completely support the conclusions presented today,” Dr. Booth asserted, citing the lack of efficacy and greater morbidity and postoperative mortality of primary tumor resection in iPACS, along with the totality of research evidence. “While we can learn a lot from real-world data, it cannot replace randomized controlled trials. In our patients with an asymptomatic primary tumor in the context of incurable colorectal cancer, I believe there is no longer a role for primary tumor resection.”
Study details
In the iPACS trial, patients in the primary tumor resection group underwent open or laparoscopic colectomy/high anterior resection with a D1-D3 lymph node dissection. Concurrent resection of involved organs was not permitted, except for the mesentery, small intestine, greater omentum, and ovary.
In the resection group, the time between surgery and start of chemotherapy was 8 to 56 days, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
With a median follow-up of 22.0 months, median overall survival was 25.9 months with primary tumor resection followed by chemotherapy and 26.7 months with chemotherapy alone (hazard ratio for death, 1.10; P = .69). Findings were similar in subgroup analyses. Median progression-free survival was 10.4 and 12.1 months, respectively (hazard ratio for progression or death, 1.08).
Patients in the primary tumor resection group had higher rates of chemotherapy-related grade 3 and 4 paresthesia (5% vs. 1%), hypertension (17% vs. 8%), diarrhea (5% vs. 1%), and neuropathy (14% vs. 9%), Dr. Kanemitsu reported.
In terms of secondary surgeries, the R0 (curative) resection rate was 3% in the primary tumor resection group and 5% in the chemotherapy group. Thirteen percent of patients in the chemotherapy group underwent palliative surgery for symptoms caused by their primary tumor.
Dr. Kanemitsu disclosed that he receives honoraria from Chugai Pharma, Covidien, Ethicon, and Intuitive Surgical, and that he has a consulting or advisory role with Covidien. The study was funded by a Health and Labor Sciences Research Grant for Clinical Cancer Research. Dr. Booth disclosed that he has no conflicts of interest.
SOURCE: Kanemitsu Y et al. 2020 GI Cancers Symposium. Abstract 7.
SAN FRANCISCO – The common practice of resecting asymptomatic primary tumors in patients with unresectable metastases of colorectal cancer does not improve survival but does increase morbidity, and it should therefore be discontinued, according to the phase 3 iPACS trial.
About 80% of patients with stage IV colorectal cancer at diagnosis have metastases that cannot be resected. “For asymptomatic patients, there is no consensus regarding resection of the primary tumor. There have been no randomized controlled trials comparing upfront chemotherapy with primary tumor resection,” said lead investigator Yukihide Kanemitsu, MD, department of colorectal surgery, National Cancer Center Hospital, Tokyo.
He and coinvestigators with the Japan Clinical Oncology Group enrolled in the randomized controlled trial 160 patients with untreated stage IV colorectal cancer who did not have any symptoms from their primary and had up to three unresectable sites of metastases (liver, lungs, distant lymph nodes, and/or peritoneum).
The trial was stopped early for futility, Dr. Kanemitsu reported at the 2020 GI Cancers Symposium. Median overall survival was slightly more than 2 years regardless of whether patients immediately received chemotherapy or underwent primary tumor resection before receiving chemotherapy.
However, 4% of the patients who underwent resection died in the postoperative period from complications. And the resection group had more frequent and more severe chemotherapy-related morbidity, with a higher rate of grade 3 and 4 adverse events (49% vs. 36%).
“Chemotherapy remains the standard therapy for asymptomatic unresectable stage IV colorectal cancer patients,” Dr. Kanemitsu concluded. “Primary tumor resection is not recommended for these patients.”
RCTs should remain the gold standard
“As someone who has built my career on health services research over the last decade working with real-world data, I have become alarmed in recent years at what appears to be a conversation in our community about leaving the randomized controlled trial behind and just using real-world evidence,” said invited discussant Christopher M. Booth, MD, professor, departments of oncology and medicine, Queen’s University, Kingston, Ont., and Canada Research Chair in Population Cancer Care. “Fundamentally, I think this is a really bad idea. The randomized controlled trial should remain the gold standard for efficacy of new cancer therapies.”
The high level of use of primary tumor resection has largely been driven by a meta-analysis that found a dramatic reduction in risk of death with this surgery (Ann Surg Oncol. 2014;21:3900-8), he noted. However, a subsequent retrospective cohort study showed that the apparent benefit disappeared with application of rigorous statistical methods that compensated for biases in the data (Cancer. 2017;123:1124-33).
“I completely support the conclusions presented today,” Dr. Booth asserted, citing the lack of efficacy and greater morbidity and postoperative mortality of primary tumor resection in iPACS, along with the totality of research evidence. “While we can learn a lot from real-world data, it cannot replace randomized controlled trials. In our patients with an asymptomatic primary tumor in the context of incurable colorectal cancer, I believe there is no longer a role for primary tumor resection.”
Study details
In the iPACS trial, patients in the primary tumor resection group underwent open or laparoscopic colectomy/high anterior resection with a D1-D3 lymph node dissection. Concurrent resection of involved organs was not permitted, except for the mesentery, small intestine, greater omentum, and ovary.
In the resection group, the time between surgery and start of chemotherapy was 8 to 56 days, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
With a median follow-up of 22.0 months, median overall survival was 25.9 months with primary tumor resection followed by chemotherapy and 26.7 months with chemotherapy alone (hazard ratio for death, 1.10; P = .69). Findings were similar in subgroup analyses. Median progression-free survival was 10.4 and 12.1 months, respectively (hazard ratio for progression or death, 1.08).
Patients in the primary tumor resection group had higher rates of chemotherapy-related grade 3 and 4 paresthesia (5% vs. 1%), hypertension (17% vs. 8%), diarrhea (5% vs. 1%), and neuropathy (14% vs. 9%), Dr. Kanemitsu reported.
In terms of secondary surgeries, the R0 (curative) resection rate was 3% in the primary tumor resection group and 5% in the chemotherapy group. Thirteen percent of patients in the chemotherapy group underwent palliative surgery for symptoms caused by their primary tumor.
Dr. Kanemitsu disclosed that he receives honoraria from Chugai Pharma, Covidien, Ethicon, and Intuitive Surgical, and that he has a consulting or advisory role with Covidien. The study was funded by a Health and Labor Sciences Research Grant for Clinical Cancer Research. Dr. Booth disclosed that he has no conflicts of interest.
SOURCE: Kanemitsu Y et al. 2020 GI Cancers Symposium. Abstract 7.
SAN FRANCISCO – The common practice of resecting asymptomatic primary tumors in patients with unresectable metastases of colorectal cancer does not improve survival but does increase morbidity, and it should therefore be discontinued, according to the phase 3 iPACS trial.
About 80% of patients with stage IV colorectal cancer at diagnosis have metastases that cannot be resected. “For asymptomatic patients, there is no consensus regarding resection of the primary tumor. There have been no randomized controlled trials comparing upfront chemotherapy with primary tumor resection,” said lead investigator Yukihide Kanemitsu, MD, department of colorectal surgery, National Cancer Center Hospital, Tokyo.
He and coinvestigators with the Japan Clinical Oncology Group enrolled in the randomized controlled trial 160 patients with untreated stage IV colorectal cancer who did not have any symptoms from their primary and had up to three unresectable sites of metastases (liver, lungs, distant lymph nodes, and/or peritoneum).
The trial was stopped early for futility, Dr. Kanemitsu reported at the 2020 GI Cancers Symposium. Median overall survival was slightly more than 2 years regardless of whether patients immediately received chemotherapy or underwent primary tumor resection before receiving chemotherapy.
However, 4% of the patients who underwent resection died in the postoperative period from complications. And the resection group had more frequent and more severe chemotherapy-related morbidity, with a higher rate of grade 3 and 4 adverse events (49% vs. 36%).
“Chemotherapy remains the standard therapy for asymptomatic unresectable stage IV colorectal cancer patients,” Dr. Kanemitsu concluded. “Primary tumor resection is not recommended for these patients.”
RCTs should remain the gold standard
“As someone who has built my career on health services research over the last decade working with real-world data, I have become alarmed in recent years at what appears to be a conversation in our community about leaving the randomized controlled trial behind and just using real-world evidence,” said invited discussant Christopher M. Booth, MD, professor, departments of oncology and medicine, Queen’s University, Kingston, Ont., and Canada Research Chair in Population Cancer Care. “Fundamentally, I think this is a really bad idea. The randomized controlled trial should remain the gold standard for efficacy of new cancer therapies.”
The high level of use of primary tumor resection has largely been driven by a meta-analysis that found a dramatic reduction in risk of death with this surgery (Ann Surg Oncol. 2014;21:3900-8), he noted. However, a subsequent retrospective cohort study showed that the apparent benefit disappeared with application of rigorous statistical methods that compensated for biases in the data (Cancer. 2017;123:1124-33).
“I completely support the conclusions presented today,” Dr. Booth asserted, citing the lack of efficacy and greater morbidity and postoperative mortality of primary tumor resection in iPACS, along with the totality of research evidence. “While we can learn a lot from real-world data, it cannot replace randomized controlled trials. In our patients with an asymptomatic primary tumor in the context of incurable colorectal cancer, I believe there is no longer a role for primary tumor resection.”
Study details
In the iPACS trial, patients in the primary tumor resection group underwent open or laparoscopic colectomy/high anterior resection with a D1-D3 lymph node dissection. Concurrent resection of involved organs was not permitted, except for the mesentery, small intestine, greater omentum, and ovary.
In the resection group, the time between surgery and start of chemotherapy was 8 to 56 days, according to data reported at the symposium, sponsored by the American Gastroenterological Association, the American Society of Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
With a median follow-up of 22.0 months, median overall survival was 25.9 months with primary tumor resection followed by chemotherapy and 26.7 months with chemotherapy alone (hazard ratio for death, 1.10; P = .69). Findings were similar in subgroup analyses. Median progression-free survival was 10.4 and 12.1 months, respectively (hazard ratio for progression or death, 1.08).
Patients in the primary tumor resection group had higher rates of chemotherapy-related grade 3 and 4 paresthesia (5% vs. 1%), hypertension (17% vs. 8%), diarrhea (5% vs. 1%), and neuropathy (14% vs. 9%), Dr. Kanemitsu reported.
In terms of secondary surgeries, the R0 (curative) resection rate was 3% in the primary tumor resection group and 5% in the chemotherapy group. Thirteen percent of patients in the chemotherapy group underwent palliative surgery for symptoms caused by their primary tumor.
Dr. Kanemitsu disclosed that he receives honoraria from Chugai Pharma, Covidien, Ethicon, and Intuitive Surgical, and that he has a consulting or advisory role with Covidien. The study was funded by a Health and Labor Sciences Research Grant for Clinical Cancer Research. Dr. Booth disclosed that he has no conflicts of interest.
SOURCE: Kanemitsu Y et al. 2020 GI Cancers Symposium. Abstract 7.
REPORTING FROM THE 2020 GI CANCERS SYMPOSIUM