User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Shift in approach is encouraged in assessing chronic pain
In many cases, dietary interventions can lead to less inflammation
SAN DIEGO – When clinicians ask patients to quantify their level of chronic pain on a scale of 1-10, and they rate it as a 7, what does that really mean?
Robert A. Bonakdar, MD, said posing such a question as the main determinator of the treatment approach during a pain assessment “depersonalizes medicine to the point where you’re making a patient a number.” Dr. Bonakdar spoke at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
“It considers areas that are often overlooked, such as the role of the gut microbiome, mood, and epigenetics.”
Over the past two decades, the number of American adults suffering from pain has increased from 120 million to 178 million, or to 41% of the adult population, said Dr. Bonakdar, a family physician who is director of pain management at the Scripps Center for Integrative Medicine. Data from the National Institutes of Health estimate that Americans spend more than $600 billion each year on the treatment of pain, which surpasses monies spent on cancer, heart disease, and diabetes. According to a 2016 report from the United States Bone and Joint Initiative, arthritis and rheumatologic conditions resulted in an estimated 6.7 million annual hospitalizations, and the average annual cost per person for treatment of a musculoskeletal condition is $7,800.
“If we continue on our current trajectory, we are choosing to accept more prevalence and incidence of these disorders, spiraling costs, restricted access to needed services, and less success in alleviating pain and suffering – a high cost,” Edward H. Yelin, PhD, cochair of the report’s steering committee, and professor of medicine and health policy at the University of California, San Francisco, said in a prepared statement in 2016. That same year, Brian F. Mandell, MD, PhD, editor of the Cleveland Clinic Journal of Medicine, penned an editorial in which he stated that “The time has come to move past using a one-size-fits-all fifth vital sign . . . and reflexively prescribing an opioid when pain is characterized as severe” (Clev Clin J Med. 2016. Jun;83[6]:400-1). A decade earlier, authors of a cross-sectional review at a single Department of Veterans Affairs medical center set out to assess the impact of the VA’s “Pain as the 5th Vital Sign” initiative on the quality of pain management (J Gen Intern Med. 2006;21[6]:607–12). They found that patients with substantial pain documented by the fifth vital sign often had inadequate pain management. The preponderance of existing evidence suggests that a different approach is needed to prescribing opioids, Dr. Bonakdar said. “It’s coming from every voice in pain care: that what we are doing is not working,” he said. “It’s not only not working; it’s dangerous. That’s the consequence of depersonalized medicine. What’s the consequence of depersonalized nutrition? It’s the same industrialized approach.”
The typical American diet, he continued, is rife with processed foods and lacks an adequate proportion of plant-based products. “It’s basically a setup for inflammation,” Dr. Bonakdar said. “Most people who come into our clinic are eating 63% processed foods, 25% animal foods, and 12% plant foods. When we are eating, we’re oversizing it because that’s the American thing to do. At the end of the day, this process is not only killing us from heart disease and stroke as causes of death, but it’s also killing us as far as pain. The same diet that’s causing heart disease is the same diet that’s increasing pain.”
Dr. Bonakdar said that the ingestion of ultra-processed foods over time jumpstarts the process of dysbiosis, which increases gut permeability. “When gut permeability happens, and you have high levels of polysaccharides and inflammatory markers such as zonulin and lipopolysaccharide (LPS), it not only goes on to affect adipose tissue and insulin resistance, it can affect the muscle and joints,” he explained. “That is a setup for sarcopenia, or muscle loss, which then makes it harder for patients to be fully functional and active. It goes on to cause joint problems as well.”
He likened an increase in gut permeability to “a bomb going off in the gut.” Routine consumption of highly processed foods “creates this wave of inflammation that goes throughout your body affecting joints and muscles, and causes an increased amount of pain. Over time, patients make the connection but it’s much easier to say, ‘take this NSAID’ or ‘take this Cox-2 inhibitor’ to suppress the pain. But if all you’re doing is suppressing, you’re not going to the source of the pain.”
Dr. Bonakdar cited several recent articles that help to make the connection between dysbiosis and pain, including a review that concluded that dysbiosis of gut microbiota can influence the onset and progression of chronic degenerative diseases (Nutrients. 2019;11[8]:1707). Authors of a separate review concluded that human microbiome studies strongly suggest an incriminating role of microbes in the pathophysiology and progression of RA. Lastly, several studies have noted that pain conditions such as fibromyalgia may have microbiome “signatures” related to dysbiosis, which may pave the way for interventions, such as dietary shifting and probiotics that target individuals with microbiome abnormalities (Pain. 2019 Nov;160[11]:2589-602 and EBioMedicine. 2019 Aug 1;46:499-511).
Clinicians can begin to help patients who present with pain complaints “by listening to what their current pattern is: strategies that have worked, and those that haven’t,” he said. “If we’re not understanding the person and we’re just ordering genetic studies or microbiome studies and going off of the assessment, we sometime miss what interventions to start. In many cases, a simple intervention like a dietary shift is all that’s required.”
A survey of more than 1 million individuals found that BMI and daily pain are positively correlated in the United States (Obesity 2012;20[7]:1491-5). “This is increased more significantly for women and the elderly,” said Dr. Bonakdar, who was not affiliated with the study. “If we can change the diet that person is taking, that’s going to begin the process of reversing this to the point where they’re having less pain from inflammation that’s affecting the adipose tissue and adipokines traveling to their joints, which can cause less dysbiosis. It is very much a vicious cycle that patients follow, but if you begin to unwind it, it’s going to help multiple areas.”
In the Intensive Diet and Exercise for Arthritis (IDEA) trial, researchers randomized 450 patients with osteoarthritis to intensive dietary restriction only, exercise only, or a combination of both (BMC Musculoskelet Disord. 2009;10:93). They found that a 5% weight loss over the course of 18 months led to a 30% reduction in pain and a 24% improvement in function.
Inspired by the IDEA trial design, Dr. Bonakdar and his colleagues completed an unpublished 12-week pilot program with 12 patients with a BMI of 27 kg/m2 or greater plus comorbidities. The program consisted of weekly group meetings, including a lecture by team clinicians, dietician, and fitness staff; group support sessions with a behavioral counselor; and a group exercise session. It also included weekly 1:1 personal training sessions and biweekly 1:1 dietitian meetings. The researchers also evaluated several deficiencies linked to pain, including magnesium, vitamin D, vitamins B1, B2, and B12, folate, calcium, amino acids, omega 3s, zinc, coenzyme Q10, carnitine, and vitamin C. The goal was a weight reduction of 5%.
The intervention consisted of a 28-day detox/protein shake consumed 1-3 times per day, which contained 17 g of protein per serving. Nutritional supplementation was added based on results of individual diagnostics.
According to preliminary results from the trial, the intended weight goal was achieved. “More importantly, there were significant improvements in markers of dysbiosis, including zonulin and lipopolysaccharide, as well as the adipokine leptin, which appeared to be associated with improvement in quality of life measures and pain,” Dr. Bonakdar said.
He concluded his presentation by highlighting a pilot study conducted in an Australian tertiary pain clinic. It found that a personalized dietitian-delivered dietary intervention can improve pain scores, quality of life, and dietary intake of people experiencing chronic pain (Nutrients. 2019 Jan 16;11[1] pii: E181). “This is another piece of the puzzle showing that these dietary interventions can be done in multiple settings, including tertiary centers with nutrition staff, and that this important step can improve pain and quality of life,” he said.
Dr. Bonakdar disclosed that he receives royalties from Oxford University Press, Lippincott, and Elsevier. He is also a consultant to Standard Process.
In many cases, dietary interventions can lead to less inflammation
In many cases, dietary interventions can lead to less inflammation
SAN DIEGO – When clinicians ask patients to quantify their level of chronic pain on a scale of 1-10, and they rate it as a 7, what does that really mean?
Robert A. Bonakdar, MD, said posing such a question as the main determinator of the treatment approach during a pain assessment “depersonalizes medicine to the point where you’re making a patient a number.” Dr. Bonakdar spoke at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
“It considers areas that are often overlooked, such as the role of the gut microbiome, mood, and epigenetics.”
Over the past two decades, the number of American adults suffering from pain has increased from 120 million to 178 million, or to 41% of the adult population, said Dr. Bonakdar, a family physician who is director of pain management at the Scripps Center for Integrative Medicine. Data from the National Institutes of Health estimate that Americans spend more than $600 billion each year on the treatment of pain, which surpasses monies spent on cancer, heart disease, and diabetes. According to a 2016 report from the United States Bone and Joint Initiative, arthritis and rheumatologic conditions resulted in an estimated 6.7 million annual hospitalizations, and the average annual cost per person for treatment of a musculoskeletal condition is $7,800.
“If we continue on our current trajectory, we are choosing to accept more prevalence and incidence of these disorders, spiraling costs, restricted access to needed services, and less success in alleviating pain and suffering – a high cost,” Edward H. Yelin, PhD, cochair of the report’s steering committee, and professor of medicine and health policy at the University of California, San Francisco, said in a prepared statement in 2016. That same year, Brian F. Mandell, MD, PhD, editor of the Cleveland Clinic Journal of Medicine, penned an editorial in which he stated that “The time has come to move past using a one-size-fits-all fifth vital sign . . . and reflexively prescribing an opioid when pain is characterized as severe” (Clev Clin J Med. 2016. Jun;83[6]:400-1). A decade earlier, authors of a cross-sectional review at a single Department of Veterans Affairs medical center set out to assess the impact of the VA’s “Pain as the 5th Vital Sign” initiative on the quality of pain management (J Gen Intern Med. 2006;21[6]:607–12). They found that patients with substantial pain documented by the fifth vital sign often had inadequate pain management. The preponderance of existing evidence suggests that a different approach is needed to prescribing opioids, Dr. Bonakdar said. “It’s coming from every voice in pain care: that what we are doing is not working,” he said. “It’s not only not working; it’s dangerous. That’s the consequence of depersonalized medicine. What’s the consequence of depersonalized nutrition? It’s the same industrialized approach.”
The typical American diet, he continued, is rife with processed foods and lacks an adequate proportion of plant-based products. “It’s basically a setup for inflammation,” Dr. Bonakdar said. “Most people who come into our clinic are eating 63% processed foods, 25% animal foods, and 12% plant foods. When we are eating, we’re oversizing it because that’s the American thing to do. At the end of the day, this process is not only killing us from heart disease and stroke as causes of death, but it’s also killing us as far as pain. The same diet that’s causing heart disease is the same diet that’s increasing pain.”
Dr. Bonakdar said that the ingestion of ultra-processed foods over time jumpstarts the process of dysbiosis, which increases gut permeability. “When gut permeability happens, and you have high levels of polysaccharides and inflammatory markers such as zonulin and lipopolysaccharide (LPS), it not only goes on to affect adipose tissue and insulin resistance, it can affect the muscle and joints,” he explained. “That is a setup for sarcopenia, or muscle loss, which then makes it harder for patients to be fully functional and active. It goes on to cause joint problems as well.”
He likened an increase in gut permeability to “a bomb going off in the gut.” Routine consumption of highly processed foods “creates this wave of inflammation that goes throughout your body affecting joints and muscles, and causes an increased amount of pain. Over time, patients make the connection but it’s much easier to say, ‘take this NSAID’ or ‘take this Cox-2 inhibitor’ to suppress the pain. But if all you’re doing is suppressing, you’re not going to the source of the pain.”
Dr. Bonakdar cited several recent articles that help to make the connection between dysbiosis and pain, including a review that concluded that dysbiosis of gut microbiota can influence the onset and progression of chronic degenerative diseases (Nutrients. 2019;11[8]:1707). Authors of a separate review concluded that human microbiome studies strongly suggest an incriminating role of microbes in the pathophysiology and progression of RA. Lastly, several studies have noted that pain conditions such as fibromyalgia may have microbiome “signatures” related to dysbiosis, which may pave the way for interventions, such as dietary shifting and probiotics that target individuals with microbiome abnormalities (Pain. 2019 Nov;160[11]:2589-602 and EBioMedicine. 2019 Aug 1;46:499-511).
Clinicians can begin to help patients who present with pain complaints “by listening to what their current pattern is: strategies that have worked, and those that haven’t,” he said. “If we’re not understanding the person and we’re just ordering genetic studies or microbiome studies and going off of the assessment, we sometime miss what interventions to start. In many cases, a simple intervention like a dietary shift is all that’s required.”
A survey of more than 1 million individuals found that BMI and daily pain are positively correlated in the United States (Obesity 2012;20[7]:1491-5). “This is increased more significantly for women and the elderly,” said Dr. Bonakdar, who was not affiliated with the study. “If we can change the diet that person is taking, that’s going to begin the process of reversing this to the point where they’re having less pain from inflammation that’s affecting the adipose tissue and adipokines traveling to their joints, which can cause less dysbiosis. It is very much a vicious cycle that patients follow, but if you begin to unwind it, it’s going to help multiple areas.”
In the Intensive Diet and Exercise for Arthritis (IDEA) trial, researchers randomized 450 patients with osteoarthritis to intensive dietary restriction only, exercise only, or a combination of both (BMC Musculoskelet Disord. 2009;10:93). They found that a 5% weight loss over the course of 18 months led to a 30% reduction in pain and a 24% improvement in function.
Inspired by the IDEA trial design, Dr. Bonakdar and his colleagues completed an unpublished 12-week pilot program with 12 patients with a BMI of 27 kg/m2 or greater plus comorbidities. The program consisted of weekly group meetings, including a lecture by team clinicians, dietician, and fitness staff; group support sessions with a behavioral counselor; and a group exercise session. It also included weekly 1:1 personal training sessions and biweekly 1:1 dietitian meetings. The researchers also evaluated several deficiencies linked to pain, including magnesium, vitamin D, vitamins B1, B2, and B12, folate, calcium, amino acids, omega 3s, zinc, coenzyme Q10, carnitine, and vitamin C. The goal was a weight reduction of 5%.
The intervention consisted of a 28-day detox/protein shake consumed 1-3 times per day, which contained 17 g of protein per serving. Nutritional supplementation was added based on results of individual diagnostics.
According to preliminary results from the trial, the intended weight goal was achieved. “More importantly, there were significant improvements in markers of dysbiosis, including zonulin and lipopolysaccharide, as well as the adipokine leptin, which appeared to be associated with improvement in quality of life measures and pain,” Dr. Bonakdar said.
He concluded his presentation by highlighting a pilot study conducted in an Australian tertiary pain clinic. It found that a personalized dietitian-delivered dietary intervention can improve pain scores, quality of life, and dietary intake of people experiencing chronic pain (Nutrients. 2019 Jan 16;11[1] pii: E181). “This is another piece of the puzzle showing that these dietary interventions can be done in multiple settings, including tertiary centers with nutrition staff, and that this important step can improve pain and quality of life,” he said.
Dr. Bonakdar disclosed that he receives royalties from Oxford University Press, Lippincott, and Elsevier. He is also a consultant to Standard Process.
SAN DIEGO – When clinicians ask patients to quantify their level of chronic pain on a scale of 1-10, and they rate it as a 7, what does that really mean?
Robert A. Bonakdar, MD, said posing such a question as the main determinator of the treatment approach during a pain assessment “depersonalizes medicine to the point where you’re making a patient a number.” Dr. Bonakdar spoke at Natural Supplements: An Evidence-Based Update, presented by Scripps Center for Integrative Medicine.
“It considers areas that are often overlooked, such as the role of the gut microbiome, mood, and epigenetics.”
Over the past two decades, the number of American adults suffering from pain has increased from 120 million to 178 million, or to 41% of the adult population, said Dr. Bonakdar, a family physician who is director of pain management at the Scripps Center for Integrative Medicine. Data from the National Institutes of Health estimate that Americans spend more than $600 billion each year on the treatment of pain, which surpasses monies spent on cancer, heart disease, and diabetes. According to a 2016 report from the United States Bone and Joint Initiative, arthritis and rheumatologic conditions resulted in an estimated 6.7 million annual hospitalizations, and the average annual cost per person for treatment of a musculoskeletal condition is $7,800.
“If we continue on our current trajectory, we are choosing to accept more prevalence and incidence of these disorders, spiraling costs, restricted access to needed services, and less success in alleviating pain and suffering – a high cost,” Edward H. Yelin, PhD, cochair of the report’s steering committee, and professor of medicine and health policy at the University of California, San Francisco, said in a prepared statement in 2016. That same year, Brian F. Mandell, MD, PhD, editor of the Cleveland Clinic Journal of Medicine, penned an editorial in which he stated that “The time has come to move past using a one-size-fits-all fifth vital sign . . . and reflexively prescribing an opioid when pain is characterized as severe” (Clev Clin J Med. 2016. Jun;83[6]:400-1). A decade earlier, authors of a cross-sectional review at a single Department of Veterans Affairs medical center set out to assess the impact of the VA’s “Pain as the 5th Vital Sign” initiative on the quality of pain management (J Gen Intern Med. 2006;21[6]:607–12). They found that patients with substantial pain documented by the fifth vital sign often had inadequate pain management. The preponderance of existing evidence suggests that a different approach is needed to prescribing opioids, Dr. Bonakdar said. “It’s coming from every voice in pain care: that what we are doing is not working,” he said. “It’s not only not working; it’s dangerous. That’s the consequence of depersonalized medicine. What’s the consequence of depersonalized nutrition? It’s the same industrialized approach.”
The typical American diet, he continued, is rife with processed foods and lacks an adequate proportion of plant-based products. “It’s basically a setup for inflammation,” Dr. Bonakdar said. “Most people who come into our clinic are eating 63% processed foods, 25% animal foods, and 12% plant foods. When we are eating, we’re oversizing it because that’s the American thing to do. At the end of the day, this process is not only killing us from heart disease and stroke as causes of death, but it’s also killing us as far as pain. The same diet that’s causing heart disease is the same diet that’s increasing pain.”
Dr. Bonakdar said that the ingestion of ultra-processed foods over time jumpstarts the process of dysbiosis, which increases gut permeability. “When gut permeability happens, and you have high levels of polysaccharides and inflammatory markers such as zonulin and lipopolysaccharide (LPS), it not only goes on to affect adipose tissue and insulin resistance, it can affect the muscle and joints,” he explained. “That is a setup for sarcopenia, or muscle loss, which then makes it harder for patients to be fully functional and active. It goes on to cause joint problems as well.”
He likened an increase in gut permeability to “a bomb going off in the gut.” Routine consumption of highly processed foods “creates this wave of inflammation that goes throughout your body affecting joints and muscles, and causes an increased amount of pain. Over time, patients make the connection but it’s much easier to say, ‘take this NSAID’ or ‘take this Cox-2 inhibitor’ to suppress the pain. But if all you’re doing is suppressing, you’re not going to the source of the pain.”
Dr. Bonakdar cited several recent articles that help to make the connection between dysbiosis and pain, including a review that concluded that dysbiosis of gut microbiota can influence the onset and progression of chronic degenerative diseases (Nutrients. 2019;11[8]:1707). Authors of a separate review concluded that human microbiome studies strongly suggest an incriminating role of microbes in the pathophysiology and progression of RA. Lastly, several studies have noted that pain conditions such as fibromyalgia may have microbiome “signatures” related to dysbiosis, which may pave the way for interventions, such as dietary shifting and probiotics that target individuals with microbiome abnormalities (Pain. 2019 Nov;160[11]:2589-602 and EBioMedicine. 2019 Aug 1;46:499-511).
Clinicians can begin to help patients who present with pain complaints “by listening to what their current pattern is: strategies that have worked, and those that haven’t,” he said. “If we’re not understanding the person and we’re just ordering genetic studies or microbiome studies and going off of the assessment, we sometime miss what interventions to start. In many cases, a simple intervention like a dietary shift is all that’s required.”
A survey of more than 1 million individuals found that BMI and daily pain are positively correlated in the United States (Obesity 2012;20[7]:1491-5). “This is increased more significantly for women and the elderly,” said Dr. Bonakdar, who was not affiliated with the study. “If we can change the diet that person is taking, that’s going to begin the process of reversing this to the point where they’re having less pain from inflammation that’s affecting the adipose tissue and adipokines traveling to their joints, which can cause less dysbiosis. It is very much a vicious cycle that patients follow, but if you begin to unwind it, it’s going to help multiple areas.”
In the Intensive Diet and Exercise for Arthritis (IDEA) trial, researchers randomized 450 patients with osteoarthritis to intensive dietary restriction only, exercise only, or a combination of both (BMC Musculoskelet Disord. 2009;10:93). They found that a 5% weight loss over the course of 18 months led to a 30% reduction in pain and a 24% improvement in function.
Inspired by the IDEA trial design, Dr. Bonakdar and his colleagues completed an unpublished 12-week pilot program with 12 patients with a BMI of 27 kg/m2 or greater plus comorbidities. The program consisted of weekly group meetings, including a lecture by team clinicians, dietician, and fitness staff; group support sessions with a behavioral counselor; and a group exercise session. It also included weekly 1:1 personal training sessions and biweekly 1:1 dietitian meetings. The researchers also evaluated several deficiencies linked to pain, including magnesium, vitamin D, vitamins B1, B2, and B12, folate, calcium, amino acids, omega 3s, zinc, coenzyme Q10, carnitine, and vitamin C. The goal was a weight reduction of 5%.
The intervention consisted of a 28-day detox/protein shake consumed 1-3 times per day, which contained 17 g of protein per serving. Nutritional supplementation was added based on results of individual diagnostics.
According to preliminary results from the trial, the intended weight goal was achieved. “More importantly, there were significant improvements in markers of dysbiosis, including zonulin and lipopolysaccharide, as well as the adipokine leptin, which appeared to be associated with improvement in quality of life measures and pain,” Dr. Bonakdar said.
He concluded his presentation by highlighting a pilot study conducted in an Australian tertiary pain clinic. It found that a personalized dietitian-delivered dietary intervention can improve pain scores, quality of life, and dietary intake of people experiencing chronic pain (Nutrients. 2019 Jan 16;11[1] pii: E181). “This is another piece of the puzzle showing that these dietary interventions can be done in multiple settings, including tertiary centers with nutrition staff, and that this important step can improve pain and quality of life,” he said.
Dr. Bonakdar disclosed that he receives royalties from Oxford University Press, Lippincott, and Elsevier. He is also a consultant to Standard Process.
REPORTING FROM A NATURAL SUPPLEMENTS UPDATE
Colorectal cancer risk elevated in anticoagulated AF patients with lower GI bleeding
A new study has found that patients with atrial fibrillation (AF) who take oral anticoagulants and then suffer from lower GI bleeding have a much higher risk of being diagnosed with colorectal cancer.
“Our data indicate that lower GI bleeding in these patients should not be dismissed as a mere consequence of anticoagulation treatment,” wrote Peter Vibe Rasmussen, MD, of the University of Copenhagen in Denmark and his coauthors, adding that “timely examination could potentially provide early detection of malignant colorectal lesions.” The study was published in the European Heart Journal.
To determine whether being treated with oral anticoagulants (OACs) and subsequently undergoing GI bleeding indicates colorectal cancer, the researchers examined data from 125,418 Danish AF patients gathered from a nationwide registry. Their median age was 73 years old, and 58% (n = 73,271) were males.
Over a 3-year follow-up period, 2,576 cases of lower GI bleeding were identified; 140 of those cases led to a diagnosis of colorectal cancer within a year. (95% confidence interval, 6.1-10.6%) in patients aged 76-80 and 3.7% (95% CI, 2.2-6.2%) in patients 65 years old or younger.
All age groups had a higher risk of colorectal cancer after bleeding, compared with patients without bleeding. Patients 65 or younger had a risk ratio of 24.2 (95% CI, 14.5-40.4) while patients over 85 had a risk ratio of 12.3 (95% CI, 7.9-19.0).
The authors acknowledged their study’s limitations, including a lack of information regarding certain risk factors, such as alcohol consumption, dietary habits, and obesity. In addition, they noted that the absolute risk of colorectal cancer in patients without bleeding is likely underdiagnosed, as “patients without GI bleeding are less likely to undergo diagnostic procedures.”
Two of the authors are employees at Bristol-Myers Squibb and Pfizer, respectively. Six additional authors reported receiving grants, speaker honoraria and consulting fees from various pharmaceutical companies. The remaining authors reported no conflicts of interest.
SOURCE: Rasmussen PV et al. Eur Heart J. 2020 Feb 7. doi: 10.1093/eurheartj/ehz964.
A new study has found that patients with atrial fibrillation (AF) who take oral anticoagulants and then suffer from lower GI bleeding have a much higher risk of being diagnosed with colorectal cancer.
“Our data indicate that lower GI bleeding in these patients should not be dismissed as a mere consequence of anticoagulation treatment,” wrote Peter Vibe Rasmussen, MD, of the University of Copenhagen in Denmark and his coauthors, adding that “timely examination could potentially provide early detection of malignant colorectal lesions.” The study was published in the European Heart Journal.
To determine whether being treated with oral anticoagulants (OACs) and subsequently undergoing GI bleeding indicates colorectal cancer, the researchers examined data from 125,418 Danish AF patients gathered from a nationwide registry. Their median age was 73 years old, and 58% (n = 73,271) were males.
Over a 3-year follow-up period, 2,576 cases of lower GI bleeding were identified; 140 of those cases led to a diagnosis of colorectal cancer within a year. (95% confidence interval, 6.1-10.6%) in patients aged 76-80 and 3.7% (95% CI, 2.2-6.2%) in patients 65 years old or younger.
All age groups had a higher risk of colorectal cancer after bleeding, compared with patients without bleeding. Patients 65 or younger had a risk ratio of 24.2 (95% CI, 14.5-40.4) while patients over 85 had a risk ratio of 12.3 (95% CI, 7.9-19.0).
The authors acknowledged their study’s limitations, including a lack of information regarding certain risk factors, such as alcohol consumption, dietary habits, and obesity. In addition, they noted that the absolute risk of colorectal cancer in patients without bleeding is likely underdiagnosed, as “patients without GI bleeding are less likely to undergo diagnostic procedures.”
Two of the authors are employees at Bristol-Myers Squibb and Pfizer, respectively. Six additional authors reported receiving grants, speaker honoraria and consulting fees from various pharmaceutical companies. The remaining authors reported no conflicts of interest.
SOURCE: Rasmussen PV et al. Eur Heart J. 2020 Feb 7. doi: 10.1093/eurheartj/ehz964.
A new study has found that patients with atrial fibrillation (AF) who take oral anticoagulants and then suffer from lower GI bleeding have a much higher risk of being diagnosed with colorectal cancer.
“Our data indicate that lower GI bleeding in these patients should not be dismissed as a mere consequence of anticoagulation treatment,” wrote Peter Vibe Rasmussen, MD, of the University of Copenhagen in Denmark and his coauthors, adding that “timely examination could potentially provide early detection of malignant colorectal lesions.” The study was published in the European Heart Journal.
To determine whether being treated with oral anticoagulants (OACs) and subsequently undergoing GI bleeding indicates colorectal cancer, the researchers examined data from 125,418 Danish AF patients gathered from a nationwide registry. Their median age was 73 years old, and 58% (n = 73,271) were males.
Over a 3-year follow-up period, 2,576 cases of lower GI bleeding were identified; 140 of those cases led to a diagnosis of colorectal cancer within a year. (95% confidence interval, 6.1-10.6%) in patients aged 76-80 and 3.7% (95% CI, 2.2-6.2%) in patients 65 years old or younger.
All age groups had a higher risk of colorectal cancer after bleeding, compared with patients without bleeding. Patients 65 or younger had a risk ratio of 24.2 (95% CI, 14.5-40.4) while patients over 85 had a risk ratio of 12.3 (95% CI, 7.9-19.0).
The authors acknowledged their study’s limitations, including a lack of information regarding certain risk factors, such as alcohol consumption, dietary habits, and obesity. In addition, they noted that the absolute risk of colorectal cancer in patients without bleeding is likely underdiagnosed, as “patients without GI bleeding are less likely to undergo diagnostic procedures.”
Two of the authors are employees at Bristol-Myers Squibb and Pfizer, respectively. Six additional authors reported receiving grants, speaker honoraria and consulting fees from various pharmaceutical companies. The remaining authors reported no conflicts of interest.
SOURCE: Rasmussen PV et al. Eur Heart J. 2020 Feb 7. doi: 10.1093/eurheartj/ehz964.
FROM the European Heart Journal
Myth busting: Sudden cardiac death in athletes
SNOWMASS, COLO. – Myths and misconceptions abound regarding the merits of universal incorporation of the resting 12-lead ECG into preparticipation cardiovascular screening of young athletes, Aaron L. Baggish, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Baggish, director of the Cardiovascular Performance Program at Massachusetts General Hospital and a cardiologist at Harvard Medical School, Boston, set out to pop the balloons of a handful of these widely floating myths. These are commonly held fictions: In an electronic poll at the outset of his talk, only one in five members of his large audience recognized all of the following boldface statements as false.
“Preparticipation cardiovascular screening (PPCVS) has been shown to reduce the incidence of sudden cardiac death (SCD) among young competitive athletes.”
FALSE. Not for PPCVS by history and physical examination alone, or with the addition of a screening 12-lead ECG. In Italy, where a cluster of high-profile sudden cardiac deaths led to passage of a 1982 national law mandating 12-lead ECG screening as part of the PPCVS, investigators presented studies purporting to demonstrate a subsequent reduction in the risk of SCD. But those studies were subsequently shown to be fraught with problems. And a high-quality study capable of convincingly demonstrating such a benefit would need to be prohibitively large and expensive. “Don’t hold your breath waiting for that to happen anytime soon,” advised Dr. Baggish, who is medical director for the Boston Marathon, as well as team cardiologist for Harvard University Athletics, the New England Patriots, the Boston Bruins, USRowing, and U.S. Soccer.
“Hypertrophic cardiomyopathy is the leading cause of sudden death among young competitive athletes.”
FALSE. A study of the National Collegiate Athletic Association (NCAA) comprehensive database, with 4.2 million athlete-years of follow-up, showed that the most common cause of SCD was autopsy-negative sudden unexplained death (SUD), accounting for 25% of cases. Hypertrophic cardiomyopathy was deemed the cause of 8% of the SCDs (Circulation. 2015 Jul 7;132[1]:10-9).
“The same thing has been shown in studies done in the United Kingdom and in Australia: . Over the next 10 years, I suspect that one of the most important areas that we’ll be looking into will be this SUD area, perhaps using molecular autopsy to make some headway there,” according to the cardiologist.
SCD is rare. In the NCAA study, the incidence was 1 in 53,703 athlete-years. In sobering contrast, accidents, suicide, and homicide accounted for 50% of all deaths in the collegiate athletes.
“When you think about what’s important in terms of educating young people to be safe, the history and physical exam and 12-lead ECG are nowhere near as important as talking with them about minimizing accident risk and staying away from guns,” Dr. Baggish commented.
“Contemporary ECG interpretation criteria designed specifically for use in young athletes have eliminated the problem of false-positive testing.”
FALSE. The story of adding ECG screening to the PPCVS is one of dramatically improved sensitivity over history and physical exam alone, but always at the cost of reduced specificity. In the Harvard Athlete Initiative Study, Dr. Baggish and coworkers reported that adding the 12-lead ECG resulted in a 17% false-positive rate (Ann Intern Med. 2010 Mar 2;152[5]:269-75). Similar findings were reported in independent studies at two other large universities.
“An ECG false-positive rate of 16%-20%? That’s big trouble. Remember, the conditions we’re looking for are uncommon, with a prevalence of maybe 1 in 500 at most. So if you’re flagging one-fifth or one-sixth of your athletes, the ECG is really not an appropriate tool for screening,” he commented.
Recognition of this limitation has led to development of refined, improved ECG criteria: most notably, the 2012 Seattle criteria, with an associated false-positive rate of 4%-8%, followed by the 2017 International Consensus Criteria (J Am Coll Cardiol. 2017 Feb 28;69[8]:1057-75), with a false-positive rate of 1%-2%. That’s a great improvement. Still, when Dr. Baggish, a marathoner himself, thinks about the roughly 32,000 Boston Marathon runners at the starting line each year, that false-positive rate would translate into 320-640 of those individuals being needlessly subjected to the not-insignificant time and expense of further testing, along with considerable anxiety for the runners and their families, and perhaps even inappropriate disqualification.
“Current ACC/AHA guidelines recommend against the use of the 12-lead ECG during the PPCVS.”
FALSE. Dr. Baggish was a coauthor of the current guidelines, which he described as “an open-door invitation to local decisions, with some important caveats” (Circulation. 2015 Dec 1;132[22]:e267-72).
The guidelines state that the minimum requirement and legal standard for PPCVS of young competitive athletes is a focused history and physical examination, such as the American College of Cardiology/American Heart Association 14-point screen, which consists of 10 elements addressing personal and family history and 4 focused on the physical examination, or the American Academy of Pediatrics Preparticipation Physical Evaluation. Further, while mandatory universal inclusion of the 12-lead ECG is not recommended – it’s rated Class III, meaning don’t do it – the guidelines state that screening programs are at liberty to choose the 12-lead ECG as an additional tool, “provided that close physician involvement and sufficient quality control can be achieved. If undertaken, such initiatives should recognize the known and anticipated limitations of the 12-lead ECG as a population screening test, including the expected frequency of false-positive and false-negative test results, as well as the cost required to support these initiatives over time.”
Dr. Baggish considers the ACC/AHA guidelines to be one of the two most important developments in the field of SCD during sports in recent years. The other is the NCAA-sponsored multidisciplinary Interassociation Consensus Statement on Cardiovascular Care of College Student-Athletes, which he also coauthored (J Am Coll Cardiol. 2016 Jun 28;67[25]:2981-95).
The report lays out the case for a much broader than traditional view of the PPCVS, with “goals that extend beyond detection of occult high-risk pathology.”
“The NCAA has done something very interesting,” Dr. Baggish explained. “It has said that, if we’re going to be screening, we should be thinking about screening with a much broader rationale. It’s not just about finding the needle-in-a-haystack hypertrophic cardiomyopathy or anomalous coronary arteries, it’s about engaging student-athletes at an early point in their collegiate career and trying to improve their health overall – and not just while they’re in college, but over their lifespan.”
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – Myths and misconceptions abound regarding the merits of universal incorporation of the resting 12-lead ECG into preparticipation cardiovascular screening of young athletes, Aaron L. Baggish, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Baggish, director of the Cardiovascular Performance Program at Massachusetts General Hospital and a cardiologist at Harvard Medical School, Boston, set out to pop the balloons of a handful of these widely floating myths. These are commonly held fictions: In an electronic poll at the outset of his talk, only one in five members of his large audience recognized all of the following boldface statements as false.
“Preparticipation cardiovascular screening (PPCVS) has been shown to reduce the incidence of sudden cardiac death (SCD) among young competitive athletes.”
FALSE. Not for PPCVS by history and physical examination alone, or with the addition of a screening 12-lead ECG. In Italy, where a cluster of high-profile sudden cardiac deaths led to passage of a 1982 national law mandating 12-lead ECG screening as part of the PPCVS, investigators presented studies purporting to demonstrate a subsequent reduction in the risk of SCD. But those studies were subsequently shown to be fraught with problems. And a high-quality study capable of convincingly demonstrating such a benefit would need to be prohibitively large and expensive. “Don’t hold your breath waiting for that to happen anytime soon,” advised Dr. Baggish, who is medical director for the Boston Marathon, as well as team cardiologist for Harvard University Athletics, the New England Patriots, the Boston Bruins, USRowing, and U.S. Soccer.
“Hypertrophic cardiomyopathy is the leading cause of sudden death among young competitive athletes.”
FALSE. A study of the National Collegiate Athletic Association (NCAA) comprehensive database, with 4.2 million athlete-years of follow-up, showed that the most common cause of SCD was autopsy-negative sudden unexplained death (SUD), accounting for 25% of cases. Hypertrophic cardiomyopathy was deemed the cause of 8% of the SCDs (Circulation. 2015 Jul 7;132[1]:10-9).
“The same thing has been shown in studies done in the United Kingdom and in Australia: . Over the next 10 years, I suspect that one of the most important areas that we’ll be looking into will be this SUD area, perhaps using molecular autopsy to make some headway there,” according to the cardiologist.
SCD is rare. In the NCAA study, the incidence was 1 in 53,703 athlete-years. In sobering contrast, accidents, suicide, and homicide accounted for 50% of all deaths in the collegiate athletes.
“When you think about what’s important in terms of educating young people to be safe, the history and physical exam and 12-lead ECG are nowhere near as important as talking with them about minimizing accident risk and staying away from guns,” Dr. Baggish commented.
“Contemporary ECG interpretation criteria designed specifically for use in young athletes have eliminated the problem of false-positive testing.”
FALSE. The story of adding ECG screening to the PPCVS is one of dramatically improved sensitivity over history and physical exam alone, but always at the cost of reduced specificity. In the Harvard Athlete Initiative Study, Dr. Baggish and coworkers reported that adding the 12-lead ECG resulted in a 17% false-positive rate (Ann Intern Med. 2010 Mar 2;152[5]:269-75). Similar findings were reported in independent studies at two other large universities.
“An ECG false-positive rate of 16%-20%? That’s big trouble. Remember, the conditions we’re looking for are uncommon, with a prevalence of maybe 1 in 500 at most. So if you’re flagging one-fifth or one-sixth of your athletes, the ECG is really not an appropriate tool for screening,” he commented.
Recognition of this limitation has led to development of refined, improved ECG criteria: most notably, the 2012 Seattle criteria, with an associated false-positive rate of 4%-8%, followed by the 2017 International Consensus Criteria (J Am Coll Cardiol. 2017 Feb 28;69[8]:1057-75), with a false-positive rate of 1%-2%. That’s a great improvement. Still, when Dr. Baggish, a marathoner himself, thinks about the roughly 32,000 Boston Marathon runners at the starting line each year, that false-positive rate would translate into 320-640 of those individuals being needlessly subjected to the not-insignificant time and expense of further testing, along with considerable anxiety for the runners and their families, and perhaps even inappropriate disqualification.
“Current ACC/AHA guidelines recommend against the use of the 12-lead ECG during the PPCVS.”
FALSE. Dr. Baggish was a coauthor of the current guidelines, which he described as “an open-door invitation to local decisions, with some important caveats” (Circulation. 2015 Dec 1;132[22]:e267-72).
The guidelines state that the minimum requirement and legal standard for PPCVS of young competitive athletes is a focused history and physical examination, such as the American College of Cardiology/American Heart Association 14-point screen, which consists of 10 elements addressing personal and family history and 4 focused on the physical examination, or the American Academy of Pediatrics Preparticipation Physical Evaluation. Further, while mandatory universal inclusion of the 12-lead ECG is not recommended – it’s rated Class III, meaning don’t do it – the guidelines state that screening programs are at liberty to choose the 12-lead ECG as an additional tool, “provided that close physician involvement and sufficient quality control can be achieved. If undertaken, such initiatives should recognize the known and anticipated limitations of the 12-lead ECG as a population screening test, including the expected frequency of false-positive and false-negative test results, as well as the cost required to support these initiatives over time.”
Dr. Baggish considers the ACC/AHA guidelines to be one of the two most important developments in the field of SCD during sports in recent years. The other is the NCAA-sponsored multidisciplinary Interassociation Consensus Statement on Cardiovascular Care of College Student-Athletes, which he also coauthored (J Am Coll Cardiol. 2016 Jun 28;67[25]:2981-95).
The report lays out the case for a much broader than traditional view of the PPCVS, with “goals that extend beyond detection of occult high-risk pathology.”
“The NCAA has done something very interesting,” Dr. Baggish explained. “It has said that, if we’re going to be screening, we should be thinking about screening with a much broader rationale. It’s not just about finding the needle-in-a-haystack hypertrophic cardiomyopathy or anomalous coronary arteries, it’s about engaging student-athletes at an early point in their collegiate career and trying to improve their health overall – and not just while they’re in college, but over their lifespan.”
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – Myths and misconceptions abound regarding the merits of universal incorporation of the resting 12-lead ECG into preparticipation cardiovascular screening of young athletes, Aaron L. Baggish, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Dr. Baggish, director of the Cardiovascular Performance Program at Massachusetts General Hospital and a cardiologist at Harvard Medical School, Boston, set out to pop the balloons of a handful of these widely floating myths. These are commonly held fictions: In an electronic poll at the outset of his talk, only one in five members of his large audience recognized all of the following boldface statements as false.
“Preparticipation cardiovascular screening (PPCVS) has been shown to reduce the incidence of sudden cardiac death (SCD) among young competitive athletes.”
FALSE. Not for PPCVS by history and physical examination alone, or with the addition of a screening 12-lead ECG. In Italy, where a cluster of high-profile sudden cardiac deaths led to passage of a 1982 national law mandating 12-lead ECG screening as part of the PPCVS, investigators presented studies purporting to demonstrate a subsequent reduction in the risk of SCD. But those studies were subsequently shown to be fraught with problems. And a high-quality study capable of convincingly demonstrating such a benefit would need to be prohibitively large and expensive. “Don’t hold your breath waiting for that to happen anytime soon,” advised Dr. Baggish, who is medical director for the Boston Marathon, as well as team cardiologist for Harvard University Athletics, the New England Patriots, the Boston Bruins, USRowing, and U.S. Soccer.
“Hypertrophic cardiomyopathy is the leading cause of sudden death among young competitive athletes.”
FALSE. A study of the National Collegiate Athletic Association (NCAA) comprehensive database, with 4.2 million athlete-years of follow-up, showed that the most common cause of SCD was autopsy-negative sudden unexplained death (SUD), accounting for 25% of cases. Hypertrophic cardiomyopathy was deemed the cause of 8% of the SCDs (Circulation. 2015 Jul 7;132[1]:10-9).
“The same thing has been shown in studies done in the United Kingdom and in Australia: . Over the next 10 years, I suspect that one of the most important areas that we’ll be looking into will be this SUD area, perhaps using molecular autopsy to make some headway there,” according to the cardiologist.
SCD is rare. In the NCAA study, the incidence was 1 in 53,703 athlete-years. In sobering contrast, accidents, suicide, and homicide accounted for 50% of all deaths in the collegiate athletes.
“When you think about what’s important in terms of educating young people to be safe, the history and physical exam and 12-lead ECG are nowhere near as important as talking with them about minimizing accident risk and staying away from guns,” Dr. Baggish commented.
“Contemporary ECG interpretation criteria designed specifically for use in young athletes have eliminated the problem of false-positive testing.”
FALSE. The story of adding ECG screening to the PPCVS is one of dramatically improved sensitivity over history and physical exam alone, but always at the cost of reduced specificity. In the Harvard Athlete Initiative Study, Dr. Baggish and coworkers reported that adding the 12-lead ECG resulted in a 17% false-positive rate (Ann Intern Med. 2010 Mar 2;152[5]:269-75). Similar findings were reported in independent studies at two other large universities.
“An ECG false-positive rate of 16%-20%? That’s big trouble. Remember, the conditions we’re looking for are uncommon, with a prevalence of maybe 1 in 500 at most. So if you’re flagging one-fifth or one-sixth of your athletes, the ECG is really not an appropriate tool for screening,” he commented.
Recognition of this limitation has led to development of refined, improved ECG criteria: most notably, the 2012 Seattle criteria, with an associated false-positive rate of 4%-8%, followed by the 2017 International Consensus Criteria (J Am Coll Cardiol. 2017 Feb 28;69[8]:1057-75), with a false-positive rate of 1%-2%. That’s a great improvement. Still, when Dr. Baggish, a marathoner himself, thinks about the roughly 32,000 Boston Marathon runners at the starting line each year, that false-positive rate would translate into 320-640 of those individuals being needlessly subjected to the not-insignificant time and expense of further testing, along with considerable anxiety for the runners and their families, and perhaps even inappropriate disqualification.
“Current ACC/AHA guidelines recommend against the use of the 12-lead ECG during the PPCVS.”
FALSE. Dr. Baggish was a coauthor of the current guidelines, which he described as “an open-door invitation to local decisions, with some important caveats” (Circulation. 2015 Dec 1;132[22]:e267-72).
The guidelines state that the minimum requirement and legal standard for PPCVS of young competitive athletes is a focused history and physical examination, such as the American College of Cardiology/American Heart Association 14-point screen, which consists of 10 elements addressing personal and family history and 4 focused on the physical examination, or the American Academy of Pediatrics Preparticipation Physical Evaluation. Further, while mandatory universal inclusion of the 12-lead ECG is not recommended – it’s rated Class III, meaning don’t do it – the guidelines state that screening programs are at liberty to choose the 12-lead ECG as an additional tool, “provided that close physician involvement and sufficient quality control can be achieved. If undertaken, such initiatives should recognize the known and anticipated limitations of the 12-lead ECG as a population screening test, including the expected frequency of false-positive and false-negative test results, as well as the cost required to support these initiatives over time.”
Dr. Baggish considers the ACC/AHA guidelines to be one of the two most important developments in the field of SCD during sports in recent years. The other is the NCAA-sponsored multidisciplinary Interassociation Consensus Statement on Cardiovascular Care of College Student-Athletes, which he also coauthored (J Am Coll Cardiol. 2016 Jun 28;67[25]:2981-95).
The report lays out the case for a much broader than traditional view of the PPCVS, with “goals that extend beyond detection of occult high-risk pathology.”
“The NCAA has done something very interesting,” Dr. Baggish explained. “It has said that, if we’re going to be screening, we should be thinking about screening with a much broader rationale. It’s not just about finding the needle-in-a-haystack hypertrophic cardiomyopathy or anomalous coronary arteries, it’s about engaging student-athletes at an early point in their collegiate career and trying to improve their health overall – and not just while they’re in college, but over their lifespan.”
He reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
CMS proposes second specialty tier for Medicare drugs
The Centers for Medicare & Medicaid Services’ latest maneuver to combat rising drug prices is the proposed addition of a second specialty drug tier for the Medicare Part D prescription drug benefit.
The proposal is part of a broader proposed update to Medicare Parts C and D for contract years 2021 and 2022.
In a fact sheet highlighting various elements of the overall proposal, CMS noted that Part D plan sponsors and pharmacy benefit managers have been requesting the option to add a second “preferred” specialty tier that would “encourage the use of more preferred, less expensive agents, reduce enrollee cost sharing, and reduce costs to CMS.”
Currently, all pharmaceuticals with a cost greater than $670 are placed in a single specialty tier.
During a Feb. 5 press briefing, CMS Administrator Seema Verma described this change as “giving plans more negotiating power so they can lower prices for beneficiaries even further.”
Ms. Verma used a hypothetical example of two rheumatoid arthritis drugs to illustrate how the change will work. Currently, if both are over the $670 threshold, they would both be on the specialty tier with the same cost sharing. “Creating a second preferred specialty tier would allow for a different copay and fosters a more competitive environment that places Part D plans in a better position to negotiate the price of similar drugs and pass those savings onto the patient through lower cost sharing,” she said.
CMS is proposing to allow plans to implement a preferred specialty tier for the 2021 plan year.
The agency is also seeking to drive more generic drug use as a means of lowering costs.
Ms. Verma noted that, typically, even after a generic drug is launched, health plan sponsors prefer to drive patients to the brand name product, if they can secure a greater rebate from the manufacturer.
In a separate Feb. 5 blog post, Ms. Verma noted that when a brand was included on a formulary, the generic was also on the formulary 91.8% of the time. For the times in which the generic was not, it was typically because the wholesale cost of the generic was only 5%-15% lower than the brand wholesale cost.
In an effort to encourage use of generics, CMS is seeking comment on the development of measures of generic and biosimilar use in Medicare Part D that could be incorporated in health plan star ratings.
Some of the measures proposed in the blog post include the generic substitution rate, the generic therapeutic alternative opportunity rate (which measures the number of brand fills divided by the sum of the brand and generic fills when both are available), and the biosimilar utilization rate.
[email protected]
The Centers for Medicare & Medicaid Services’ latest maneuver to combat rising drug prices is the proposed addition of a second specialty drug tier for the Medicare Part D prescription drug benefit.
The proposal is part of a broader proposed update to Medicare Parts C and D for contract years 2021 and 2022.
In a fact sheet highlighting various elements of the overall proposal, CMS noted that Part D plan sponsors and pharmacy benefit managers have been requesting the option to add a second “preferred” specialty tier that would “encourage the use of more preferred, less expensive agents, reduce enrollee cost sharing, and reduce costs to CMS.”
Currently, all pharmaceuticals with a cost greater than $670 are placed in a single specialty tier.
During a Feb. 5 press briefing, CMS Administrator Seema Verma described this change as “giving plans more negotiating power so they can lower prices for beneficiaries even further.”
Ms. Verma used a hypothetical example of two rheumatoid arthritis drugs to illustrate how the change will work. Currently, if both are over the $670 threshold, they would both be on the specialty tier with the same cost sharing. “Creating a second preferred specialty tier would allow for a different copay and fosters a more competitive environment that places Part D plans in a better position to negotiate the price of similar drugs and pass those savings onto the patient through lower cost sharing,” she said.
CMS is proposing to allow plans to implement a preferred specialty tier for the 2021 plan year.
The agency is also seeking to drive more generic drug use as a means of lowering costs.
Ms. Verma noted that, typically, even after a generic drug is launched, health plan sponsors prefer to drive patients to the brand name product, if they can secure a greater rebate from the manufacturer.
In a separate Feb. 5 blog post, Ms. Verma noted that when a brand was included on a formulary, the generic was also on the formulary 91.8% of the time. For the times in which the generic was not, it was typically because the wholesale cost of the generic was only 5%-15% lower than the brand wholesale cost.
In an effort to encourage use of generics, CMS is seeking comment on the development of measures of generic and biosimilar use in Medicare Part D that could be incorporated in health plan star ratings.
Some of the measures proposed in the blog post include the generic substitution rate, the generic therapeutic alternative opportunity rate (which measures the number of brand fills divided by the sum of the brand and generic fills when both are available), and the biosimilar utilization rate.
[email protected]
The Centers for Medicare & Medicaid Services’ latest maneuver to combat rising drug prices is the proposed addition of a second specialty drug tier for the Medicare Part D prescription drug benefit.
The proposal is part of a broader proposed update to Medicare Parts C and D for contract years 2021 and 2022.
In a fact sheet highlighting various elements of the overall proposal, CMS noted that Part D plan sponsors and pharmacy benefit managers have been requesting the option to add a second “preferred” specialty tier that would “encourage the use of more preferred, less expensive agents, reduce enrollee cost sharing, and reduce costs to CMS.”
Currently, all pharmaceuticals with a cost greater than $670 are placed in a single specialty tier.
During a Feb. 5 press briefing, CMS Administrator Seema Verma described this change as “giving plans more negotiating power so they can lower prices for beneficiaries even further.”
Ms. Verma used a hypothetical example of two rheumatoid arthritis drugs to illustrate how the change will work. Currently, if both are over the $670 threshold, they would both be on the specialty tier with the same cost sharing. “Creating a second preferred specialty tier would allow for a different copay and fosters a more competitive environment that places Part D plans in a better position to negotiate the price of similar drugs and pass those savings onto the patient through lower cost sharing,” she said.
CMS is proposing to allow plans to implement a preferred specialty tier for the 2021 plan year.
The agency is also seeking to drive more generic drug use as a means of lowering costs.
Ms. Verma noted that, typically, even after a generic drug is launched, health plan sponsors prefer to drive patients to the brand name product, if they can secure a greater rebate from the manufacturer.
In a separate Feb. 5 blog post, Ms. Verma noted that when a brand was included on a formulary, the generic was also on the formulary 91.8% of the time. For the times in which the generic was not, it was typically because the wholesale cost of the generic was only 5%-15% lower than the brand wholesale cost.
In an effort to encourage use of generics, CMS is seeking comment on the development of measures of generic and biosimilar use in Medicare Part D that could be incorporated in health plan star ratings.
Some of the measures proposed in the blog post include the generic substitution rate, the generic therapeutic alternative opportunity rate (which measures the number of brand fills divided by the sum of the brand and generic fills when both are available), and the biosimilar utilization rate.
[email protected]
IBD quality initiative slashes ED utilization
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.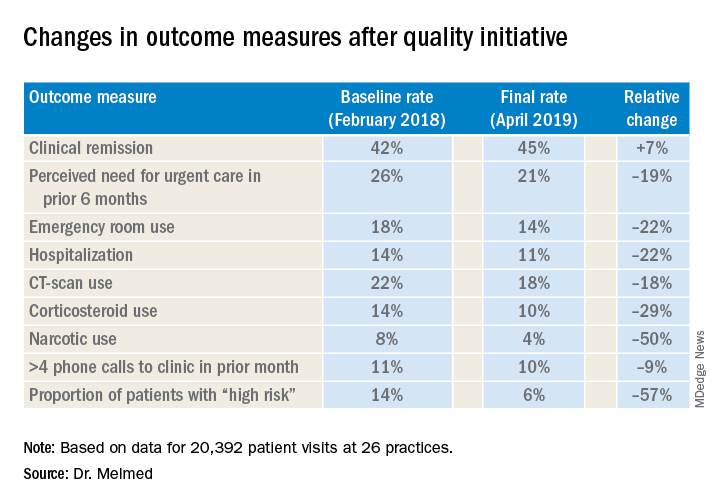
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
AUSTIN, TEX. – A quality improvement initiative aimed at patients with inflammatory bowel disease (IBD) has reduced emergency department visits and hospitalizations by 20% or more and slashed opioid use by half, according to study results presented at the Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
After 15 months, the quality improvement program saw emergency department visit rates decline from 18% to 14%, a 22% relative decrease, Gil Y. Melmed, MD, of Cedars-Sinai Medical Center, Los Angeles, said. Additionally, the study documented a similar decrease in the rate of hospitalization, declining from 14% to 11%, while opioid utilization rates declined from 8% to 4%. “We also found decreases in special-cause variation in other measures of interest, including CT scan utilization as well as corticosteroid use, which was reduced 29% during the course of the program,” he said.
The quality initiative was conducted through the Crohn’s & Colitis Foundation as an outgrowth of its IBD Qorus quality improvement program. The 15-month study involved 20,392 patient visits at 15 academic and 11 private/community practices from January 2018 to April 2019. “This specific project within Qorus is focused specifically around the concept of improving access during times of urgent care need,” Dr. Melmed told this news organization. The goal was to identify practice changes that can drive improvement.
The intervention consisted of 19 different strategies, called a “Change Package,” and participating sites could choose to test and implement one or more of them, Dr. Melmed said. Some examples included designating urgent care slots in the clinic schedule, installing a nurse hotline, a weekly “huddle” to review high-risk patients, and patient education on using urgent care.
One of the drivers of the program was to provide immediate care improvement to patients, Dr. Melmed said in the interview. “As opposed to investments into the cure of IBD that we need, but which can take years to develop, this research has immediate, practical applicability for patients today,” he said.
“The fact that we were able to demonstrate reduction in emergency room utilization and hospitalization, steroid use, and narcotic use has really energized the work that we were doing. We can now show that very-low-cost process changes at a site level lead to robust improvement in patient outcomes. These changes are potentially implementable in any practice setting,” Dr. Melmed said in the interview.
After Dr. Melmed’s presentation, Maria T. Abreu, MD, director of the Crohn’s and Colitis Center at the University of Miami, asked about the cost of the interventions. Dr. Melmed said the costs were nominal, such as paying for a new phone line for a patient hotline. “But overall the cost really involved in the program was the time that it took to review the high-risk list on a weekly basis with the team, and that is essentially a 15-minute huddle,” he said.
Later, Dr. Abreu said in an interview that the program was “a terrific example of how measuring outcomes and sharing ideas can make huge impacts in the lives of patients.” She added, “An enormous amount of money is spent on clinical trials of expensive biologics which have revolutionized treatment, yet the humanistic aspects of our care have just as great of an impact. In this study, each center focused on ways they could lower ER visits and hospitalizations. One size did not fit all, yet they could learn from each other. The very platform they used to conduct the study is a model for all of us.”
Corey A. Siegel, MD, of the Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and Dr. Melmed's coprincipal investigator on Qorus, said the quality initiative now includes 49 GI practices across the country with plans to grow to 60 by the end of the year. "We have created this 'collaboratory' for providers from actross the country to work togetherr to learn how to best deliver high-qulaity care for patients with IBD," he said.
Another feature of the quality initiative allowed participating sites to see how they compared with others anonymously, Dr. Melmed said. “Using the data, we called out high-performing sites to teach the rest of us what they were doing that enabled them to improve, so that all of us could learn from their successes,” he said.
The researchers are aiming to evaluate costs and identify the most successful interventions, with the plan to present the latter at Digestive Disease Week® 2020 and use them to develop a toolkit practices can use. “Ultimately,” said Dr. Melmed, “this is scalable.”
Dr. Melmed disclosed financial relationships with AbbVie, Boehringer-Ingelheim, Celgene, Jannsen, GSK, Medtronic, Pfizer, Samsung Bioepis, Takeda, and Techlab; IBD Qorus receives support from Abbvie, AMAG, Helmsley Charitable Trust, Janssen, Nephoroceuticals, Pfizer, Takeda, and UCB.
SOURCE: Melmed GT et al. Crohn’s & Colitis Congress 2020, Session 28.
REPORTING FROM CROHN’S & COLITIS CONGRESS
Funding failures: Tobacco prevention and cessation
When it comes to state funding for tobacco prevention and cessation, the American Lung Association grades on a curve. It did not help.

Each state’s annual funding for tobacco prevention and cessation was calculated and then compared with the Centers for Disease Control and Prevention’s recommended spending level. That percentage became the grade, with any level of funding at 80% or more of the CDC’s recommendation getting an A and anything below 50% getting an F, the ALA explained.
The three A’s went to Alaska – which spent $10.14 million, or 99.4% of the CDC-recommended $10.2 million – California (96.0%), and Maine (83.5%). The lowest levels of spending came from Georgia, which spend just 2.8% of the CDC’s recommendation of $106 million, and Missouri, which spent 3.0%, the ALA reported.
States’ grades were generally better in the four other areas of tobacco-control policy: There were 24 A’s and 9 F’s for smoke-free air laws, 1 A and 35 F’s for tobacco excise taxes, 3 A’s and 17 F’s for access to cessation treatment, and 10 A’s and 30 F’s for laws to raise the tobacco sales age to 21 years, the ALA said in the report.
Despite an overall grade of F, the federal government managed to earn some praise in that last area: “In what could only be described as unimaginable even 2 years ago, in December 2019, Congress passed bipartisan legislation to raise the minimum age of sale for tobacco products to 21,” the ALA said.
The federal government was strongly criticized on the subject of e-cigarettes. “The Trump Administration failed to prioritize public health over the tobacco industry with its Jan. 2, 2020, announcement that will leave thousands of flavored e-cigarettes on the market,” the ALA said, while concluding that the rising use of e-cigarettes in recent years “is a real-world demonstration of the failure of the U.S. Food and Drug Administration to properly oversee all tobacco products. … This failure places the lung health and lives of Americans at risk.”
When it comes to state funding for tobacco prevention and cessation, the American Lung Association grades on a curve. It did not help.

Each state’s annual funding for tobacco prevention and cessation was calculated and then compared with the Centers for Disease Control and Prevention’s recommended spending level. That percentage became the grade, with any level of funding at 80% or more of the CDC’s recommendation getting an A and anything below 50% getting an F, the ALA explained.
The three A’s went to Alaska – which spent $10.14 million, or 99.4% of the CDC-recommended $10.2 million – California (96.0%), and Maine (83.5%). The lowest levels of spending came from Georgia, which spend just 2.8% of the CDC’s recommendation of $106 million, and Missouri, which spent 3.0%, the ALA reported.
States’ grades were generally better in the four other areas of tobacco-control policy: There were 24 A’s and 9 F’s for smoke-free air laws, 1 A and 35 F’s for tobacco excise taxes, 3 A’s and 17 F’s for access to cessation treatment, and 10 A’s and 30 F’s for laws to raise the tobacco sales age to 21 years, the ALA said in the report.
Despite an overall grade of F, the federal government managed to earn some praise in that last area: “In what could only be described as unimaginable even 2 years ago, in December 2019, Congress passed bipartisan legislation to raise the minimum age of sale for tobacco products to 21,” the ALA said.
The federal government was strongly criticized on the subject of e-cigarettes. “The Trump Administration failed to prioritize public health over the tobacco industry with its Jan. 2, 2020, announcement that will leave thousands of flavored e-cigarettes on the market,” the ALA said, while concluding that the rising use of e-cigarettes in recent years “is a real-world demonstration of the failure of the U.S. Food and Drug Administration to properly oversee all tobacco products. … This failure places the lung health and lives of Americans at risk.”
When it comes to state funding for tobacco prevention and cessation, the American Lung Association grades on a curve. It did not help.

Each state’s annual funding for tobacco prevention and cessation was calculated and then compared with the Centers for Disease Control and Prevention’s recommended spending level. That percentage became the grade, with any level of funding at 80% or more of the CDC’s recommendation getting an A and anything below 50% getting an F, the ALA explained.
The three A’s went to Alaska – which spent $10.14 million, or 99.4% of the CDC-recommended $10.2 million – California (96.0%), and Maine (83.5%). The lowest levels of spending came from Georgia, which spend just 2.8% of the CDC’s recommendation of $106 million, and Missouri, which spent 3.0%, the ALA reported.
States’ grades were generally better in the four other areas of tobacco-control policy: There were 24 A’s and 9 F’s for smoke-free air laws, 1 A and 35 F’s for tobacco excise taxes, 3 A’s and 17 F’s for access to cessation treatment, and 10 A’s and 30 F’s for laws to raise the tobacco sales age to 21 years, the ALA said in the report.
Despite an overall grade of F, the federal government managed to earn some praise in that last area: “In what could only be described as unimaginable even 2 years ago, in December 2019, Congress passed bipartisan legislation to raise the minimum age of sale for tobacco products to 21,” the ALA said.
The federal government was strongly criticized on the subject of e-cigarettes. “The Trump Administration failed to prioritize public health over the tobacco industry with its Jan. 2, 2020, announcement that will leave thousands of flavored e-cigarettes on the market,” the ALA said, while concluding that the rising use of e-cigarettes in recent years “is a real-world demonstration of the failure of the U.S. Food and Drug Administration to properly oversee all tobacco products. … This failure places the lung health and lives of Americans at risk.”
FDA not recommending recalls of diabetes drug metformin
The Food and Drug Administration says it has no plans to recall any metformin products, used for the treatment of type 2 diabetes, after tests it conducted did not show any evidence of contamination with N-nitrosodimethylamine (NDMA) at levels that would cause concern.
The FDA began testing samples of metformin for the carcinogen NDMA at the end of 2019. Contamination with this substance has led to recalls of hypertension and heartburn medications within the past 2 years.
That announcement came on the heels of a recall of three versions of metformin in Singapore and the European Medicines Agency’s request that manufacturers test for NDMA.
This week, the FDA posted laboratory results in which NDMA levels in some metformin products ranged from “not detectable to low.”
“To date, no sample of metformin that FDA has tested exceeds the acceptable daily intake for NDMA. FDA has not recommended metformin recalls in the U.S.,” the agency indicates.
More than 30 million people in the United States have diabetes; 90%-95% of cases are of type 2. Metformin is the fourth most prescribed drug in the United States.
“Patients should continue taking metformin to keep their diabetes under control,” the FDA emphasized. “It could be dangerous for patients with this serious condition to stop taking their metformin without first talking to their health care professionals.”
The agency plans to post the methods used in laboratory testing of metformin in the near future. The FDA is collaborating with international regulators to share testing results for metformin, along with testing results for other drugs.
The U.S. agency says it will continue to monitor NDMA in metformin, along with other drug products, and will provide timely updates of new developments, including product recalls.
For more information about NDMA, visit the FDA’s nitrosamines webpage.
The FDA also encourages health care professionals and patients to report adverse reactions or quality problems with any human drugs to the agency’s MedWatch Adverse Event Reporting program.
This article first appeared on Medscape.com.
The Food and Drug Administration says it has no plans to recall any metformin products, used for the treatment of type 2 diabetes, after tests it conducted did not show any evidence of contamination with N-nitrosodimethylamine (NDMA) at levels that would cause concern.
The FDA began testing samples of metformin for the carcinogen NDMA at the end of 2019. Contamination with this substance has led to recalls of hypertension and heartburn medications within the past 2 years.
That announcement came on the heels of a recall of three versions of metformin in Singapore and the European Medicines Agency’s request that manufacturers test for NDMA.
This week, the FDA posted laboratory results in which NDMA levels in some metformin products ranged from “not detectable to low.”
“To date, no sample of metformin that FDA has tested exceeds the acceptable daily intake for NDMA. FDA has not recommended metformin recalls in the U.S.,” the agency indicates.
More than 30 million people in the United States have diabetes; 90%-95% of cases are of type 2. Metformin is the fourth most prescribed drug in the United States.
“Patients should continue taking metformin to keep their diabetes under control,” the FDA emphasized. “It could be dangerous for patients with this serious condition to stop taking their metformin without first talking to their health care professionals.”
The agency plans to post the methods used in laboratory testing of metformin in the near future. The FDA is collaborating with international regulators to share testing results for metformin, along with testing results for other drugs.
The U.S. agency says it will continue to monitor NDMA in metformin, along with other drug products, and will provide timely updates of new developments, including product recalls.
For more information about NDMA, visit the FDA’s nitrosamines webpage.
The FDA also encourages health care professionals and patients to report adverse reactions or quality problems with any human drugs to the agency’s MedWatch Adverse Event Reporting program.
This article first appeared on Medscape.com.
The Food and Drug Administration says it has no plans to recall any metformin products, used for the treatment of type 2 diabetes, after tests it conducted did not show any evidence of contamination with N-nitrosodimethylamine (NDMA) at levels that would cause concern.
The FDA began testing samples of metformin for the carcinogen NDMA at the end of 2019. Contamination with this substance has led to recalls of hypertension and heartburn medications within the past 2 years.
That announcement came on the heels of a recall of three versions of metformin in Singapore and the European Medicines Agency’s request that manufacturers test for NDMA.
This week, the FDA posted laboratory results in which NDMA levels in some metformin products ranged from “not detectable to low.”
“To date, no sample of metformin that FDA has tested exceeds the acceptable daily intake for NDMA. FDA has not recommended metformin recalls in the U.S.,” the agency indicates.
More than 30 million people in the United States have diabetes; 90%-95% of cases are of type 2. Metformin is the fourth most prescribed drug in the United States.
“Patients should continue taking metformin to keep their diabetes under control,” the FDA emphasized. “It could be dangerous for patients with this serious condition to stop taking their metformin without first talking to their health care professionals.”
The agency plans to post the methods used in laboratory testing of metformin in the near future. The FDA is collaborating with international regulators to share testing results for metformin, along with testing results for other drugs.
The U.S. agency says it will continue to monitor NDMA in metformin, along with other drug products, and will provide timely updates of new developments, including product recalls.
For more information about NDMA, visit the FDA’s nitrosamines webpage.
The FDA also encourages health care professionals and patients to report adverse reactions or quality problems with any human drugs to the agency’s MedWatch Adverse Event Reporting program.
This article first appeared on Medscape.com.
Fewer complications, better outcomes with outpatient UKA
according to a review from the University of Tennessee Campbell Clinic, Memphis.
“In carefully selected patients, the ASC [ambulatory surgery center] seems to be a safe alternative to the inpatient hospital setting,” concluded investigators led by led by Marcus Ford, MD, a Campbell Clinic orthopedic surgeon.
He and his colleagues have been doing outpatient unicompartmental knee arthroplasty (UKA) since 2009, and “based on the subjective success,” recently increased the number of total knee, hip, and shoulder arthroplasties performed in their ASC.
They wanted to make sure, however, that their impression of good outpatient UKA results was supported by the data, so they compared outcomes in 48 UKA patients treated at their ASC with 48 treated in the hospital. The operations were done by two surgeons using the same technique and same medial UKA implant.
“Naturally, surgeons select those patients who are deemed physically and mentally capable of succeeding with an accelerated discharge plan” for outpatient service, the investigators wrote. To address that potential selection bias, the team matched their subjects by age and comorbidities.
There was only one minor complication in the outpatient group, a superficial stitch abscess. No patient needed a second operation, and all went home the same day.
It was different on the inpatient side. The average length of stay was 2.9 days, and there were four major complications: a deep venous thrombosis, a pulmonary embolus, an acute postoperative infection, and a periprosthetic fracture. All four required hospital readmission, and two patients needed a second operation.
The report didn’t directly address the reasons for the differences, but Dr. Ford and colleagues did note that they “believe that the ASC allows the surgeon greater direct control of perioperative variables that can impact patient outcome.”
Patients were in their late 50s, on average, and there were more women than men in both groups. The mean American Society of Anesthesiologists physical status classification score was 1.94 and mean body mass index was 34.3 kg/m2 in the outpatient group, compared with a mean physical status classification score of 2.08 and mean body mass index of 32.9 kg/m2 in the inpatient group. The differences were not statistically significant.
No funding source was reported. The investigators did not report any disclosures.
SOURCE: Ford M et al. Orthop Clin North Am. 2020 Jan;51[1]:1-5. doi: 10.1016/j.ocl.2019.08.001
according to a review from the University of Tennessee Campbell Clinic, Memphis.
“In carefully selected patients, the ASC [ambulatory surgery center] seems to be a safe alternative to the inpatient hospital setting,” concluded investigators led by led by Marcus Ford, MD, a Campbell Clinic orthopedic surgeon.
He and his colleagues have been doing outpatient unicompartmental knee arthroplasty (UKA) since 2009, and “based on the subjective success,” recently increased the number of total knee, hip, and shoulder arthroplasties performed in their ASC.
They wanted to make sure, however, that their impression of good outpatient UKA results was supported by the data, so they compared outcomes in 48 UKA patients treated at their ASC with 48 treated in the hospital. The operations were done by two surgeons using the same technique and same medial UKA implant.
“Naturally, surgeons select those patients who are deemed physically and mentally capable of succeeding with an accelerated discharge plan” for outpatient service, the investigators wrote. To address that potential selection bias, the team matched their subjects by age and comorbidities.
There was only one minor complication in the outpatient group, a superficial stitch abscess. No patient needed a second operation, and all went home the same day.
It was different on the inpatient side. The average length of stay was 2.9 days, and there were four major complications: a deep venous thrombosis, a pulmonary embolus, an acute postoperative infection, and a periprosthetic fracture. All four required hospital readmission, and two patients needed a second operation.
The report didn’t directly address the reasons for the differences, but Dr. Ford and colleagues did note that they “believe that the ASC allows the surgeon greater direct control of perioperative variables that can impact patient outcome.”
Patients were in their late 50s, on average, and there were more women than men in both groups. The mean American Society of Anesthesiologists physical status classification score was 1.94 and mean body mass index was 34.3 kg/m2 in the outpatient group, compared with a mean physical status classification score of 2.08 and mean body mass index of 32.9 kg/m2 in the inpatient group. The differences were not statistically significant.
No funding source was reported. The investigators did not report any disclosures.
SOURCE: Ford M et al. Orthop Clin North Am. 2020 Jan;51[1]:1-5. doi: 10.1016/j.ocl.2019.08.001
according to a review from the University of Tennessee Campbell Clinic, Memphis.
“In carefully selected patients, the ASC [ambulatory surgery center] seems to be a safe alternative to the inpatient hospital setting,” concluded investigators led by led by Marcus Ford, MD, a Campbell Clinic orthopedic surgeon.
He and his colleagues have been doing outpatient unicompartmental knee arthroplasty (UKA) since 2009, and “based on the subjective success,” recently increased the number of total knee, hip, and shoulder arthroplasties performed in their ASC.
They wanted to make sure, however, that their impression of good outpatient UKA results was supported by the data, so they compared outcomes in 48 UKA patients treated at their ASC with 48 treated in the hospital. The operations were done by two surgeons using the same technique and same medial UKA implant.
“Naturally, surgeons select those patients who are deemed physically and mentally capable of succeeding with an accelerated discharge plan” for outpatient service, the investigators wrote. To address that potential selection bias, the team matched their subjects by age and comorbidities.
There was only one minor complication in the outpatient group, a superficial stitch abscess. No patient needed a second operation, and all went home the same day.
It was different on the inpatient side. The average length of stay was 2.9 days, and there were four major complications: a deep venous thrombosis, a pulmonary embolus, an acute postoperative infection, and a periprosthetic fracture. All four required hospital readmission, and two patients needed a second operation.
The report didn’t directly address the reasons for the differences, but Dr. Ford and colleagues did note that they “believe that the ASC allows the surgeon greater direct control of perioperative variables that can impact patient outcome.”
Patients were in their late 50s, on average, and there were more women than men in both groups. The mean American Society of Anesthesiologists physical status classification score was 1.94 and mean body mass index was 34.3 kg/m2 in the outpatient group, compared with a mean physical status classification score of 2.08 and mean body mass index of 32.9 kg/m2 in the inpatient group. The differences were not statistically significant.
No funding source was reported. The investigators did not report any disclosures.
SOURCE: Ford M et al. Orthop Clin North Am. 2020 Jan;51[1]:1-5. doi: 10.1016/j.ocl.2019.08.001
FROM ORTHOPEDIC CLINICS OF NORTH AMERICA
Serum levels of neurofilament light are increased before clinical onset of MS
(MS), according to research published in the January issue of JAMA Neurology. These results lend weight to the idea that MS has a prodromal phase, and this phase appears to be associated with neurodegeneration, according to the authors.
Patients often have CNS lesions of various stages of development at the time of their first demyelinating event, and this finding was one basis for neurologists’ hypothesis of a prodromal phase of MS. The finding that one-third of patients with radiologically isolated syndrome develop MS within 5 years also lends credence to this idea. Diagnosing MS early would enable early treatment that could prevent demyelination and the progression of neurodegeneration.
Researchers compared presymptomatic and symptomatic samples
With this idea in mind, Kjetil Bjornevik, MD, PhD, a member of the neuroepidemiology research group at Harvard TH Chan School of Public Health in Boston, and colleagues evaluated whether serum levels of NfL, a marker of ongoing neuroaxonal degeneration, were increased in the years before and around the time of clinical onset of MS. For their study population, the investigators chose active-duty U.S. military personnel who have at least one serum sample stored in the U.S. Department of Defense Serum Repository. Samples are collected after routine HIV type 1 antibody testing.
Within this population, Dr. Bjornevik and colleagues identified patients with MS who had at least one presymptomatic serum sample. The date of clinical MS onset was defined as the date of the first neurologic symptoms attributable to MS documented in the medical record. The investigators randomly selected two control individuals from the population and matched them to each case by age, sex, race or ethnicity, and dates of sample collection. Eligible controls were on active duty on the date of onset of the matched case.
Dr. Bjornevik and colleagues identified 245 patients with MS. Among this sample, the researchers selected two groups that each included 30 cases and 30 controls. The first group included patients who had provided at least one serum sample before MS onset and one sample within 2 years after MS onset. The second group included cases with at least two presymptomatic serum samples, one of which was collected more than 5 years before MS diagnosis, and the other of which was collected between 2 and 5 years before diagnosis. The investigators handled pairs of serum samples in the same way and assayed them in the same batch. The order of the samples in each pair was arranged at random.
Levels were higher in cases than in controls
About 77% of the population was male. Sixty percent of participants were white, 28% were black, and 6.7% were Hispanic. The population’s mean age at first sample collection was approximately 27 years. Mean age at MS onset was approximately 31 years.
For patients who provided samples before and after the clinical onset of MS, serum NfL levels were higher than in matched controls at both points. Most patients who passed from the presymptomatic stage to the symptomatic stage had a significant increase in serum NfL level (i.e., from a median of 25.0 pg/mL to a median of 45.1 pg/mL). Serum NfL levels at the two time points in controls did not differ significantly. For any given patient, an increase in serum NfL level from the presymptomatic measurement to the symptomatic measurement was associated with an increased risk of MS.
In patients with two presymptomatic samples, serum NfL levels were significantly higher in both samples than in the corresponding samples from matched controls. In cases, the earlier sample was collected at a median of 6 years before clinical onset of MS, and the later sample was collected at a median of 1 year before clinical onset. The serum NfL levels increased significantly between the two points for cases (i.e., a median increase of 1.3 pg/mL per year), but there was no significant difference in serum NfL level between the two samples in controls. A within-patient increase in presymptomatic serum NfL level was associated with an increased risk of MS.
Population included few women
“Our study differs from previous studies on the prodromal phase of MS because these have used indirect markers of this phase, which included unspecific symptoms or disturbances occurring before the clinical onset, compared with a marker of neurodegeneration,” wrote Dr. Bjornevik and colleagues. Initiation of treatment with disease-modifying therapy is associated with reductions in serum NfL levels, and this association could explain why some patients in the current study had higher NfL levels before MS onset than afterward. Furthermore, serum NfL levels are highly associated with levels of NfL in cerebrospinal fluid. “Thus, our findings of a presymptomatic increase in serum NfL not only suggest the presence of a prodromal phase in MS, but also that this phase is associated with neurodegeneration,” wrote the investigators.
The study’s well-defined population helped to minimize selection bias, and the blinded, randomized method of analyzing the serum samples eliminated artifactual differences in serum NfL concentrations. But the small sample size precluded analyses that could have influenced clinical practice, wrote Dr. Bjornevik and colleagues. For example, the researchers could not evaluate distinct cutoffs in serum NfL level that could mark the beginning of the prodromal phase of MS. Nor could they determine whether presymptomatic serum NfL levels varied with age at clinical onset, sex, or race. The small number of women in the sample was another limitation of the study.
The Swiss National Research Foundation and the National Institute of Neurologic Disorders and Stroke funded the study. Several of the investigators received fees from various drug companies that were unrelated to the study, and one researcher received grants from the National Institutes of Health during the study.
SOURCE: Bjornevik K et al. JAMA Neurol. 2020;77(1):58-64.
(MS), according to research published in the January issue of JAMA Neurology. These results lend weight to the idea that MS has a prodromal phase, and this phase appears to be associated with neurodegeneration, according to the authors.
Patients often have CNS lesions of various stages of development at the time of their first demyelinating event, and this finding was one basis for neurologists’ hypothesis of a prodromal phase of MS. The finding that one-third of patients with radiologically isolated syndrome develop MS within 5 years also lends credence to this idea. Diagnosing MS early would enable early treatment that could prevent demyelination and the progression of neurodegeneration.
Researchers compared presymptomatic and symptomatic samples
With this idea in mind, Kjetil Bjornevik, MD, PhD, a member of the neuroepidemiology research group at Harvard TH Chan School of Public Health in Boston, and colleagues evaluated whether serum levels of NfL, a marker of ongoing neuroaxonal degeneration, were increased in the years before and around the time of clinical onset of MS. For their study population, the investigators chose active-duty U.S. military personnel who have at least one serum sample stored in the U.S. Department of Defense Serum Repository. Samples are collected after routine HIV type 1 antibody testing.
Within this population, Dr. Bjornevik and colleagues identified patients with MS who had at least one presymptomatic serum sample. The date of clinical MS onset was defined as the date of the first neurologic symptoms attributable to MS documented in the medical record. The investigators randomly selected two control individuals from the population and matched them to each case by age, sex, race or ethnicity, and dates of sample collection. Eligible controls were on active duty on the date of onset of the matched case.
Dr. Bjornevik and colleagues identified 245 patients with MS. Among this sample, the researchers selected two groups that each included 30 cases and 30 controls. The first group included patients who had provided at least one serum sample before MS onset and one sample within 2 years after MS onset. The second group included cases with at least two presymptomatic serum samples, one of which was collected more than 5 years before MS diagnosis, and the other of which was collected between 2 and 5 years before diagnosis. The investigators handled pairs of serum samples in the same way and assayed them in the same batch. The order of the samples in each pair was arranged at random.
Levels were higher in cases than in controls
About 77% of the population was male. Sixty percent of participants were white, 28% were black, and 6.7% were Hispanic. The population’s mean age at first sample collection was approximately 27 years. Mean age at MS onset was approximately 31 years.
For patients who provided samples before and after the clinical onset of MS, serum NfL levels were higher than in matched controls at both points. Most patients who passed from the presymptomatic stage to the symptomatic stage had a significant increase in serum NfL level (i.e., from a median of 25.0 pg/mL to a median of 45.1 pg/mL). Serum NfL levels at the two time points in controls did not differ significantly. For any given patient, an increase in serum NfL level from the presymptomatic measurement to the symptomatic measurement was associated with an increased risk of MS.
In patients with two presymptomatic samples, serum NfL levels were significantly higher in both samples than in the corresponding samples from matched controls. In cases, the earlier sample was collected at a median of 6 years before clinical onset of MS, and the later sample was collected at a median of 1 year before clinical onset. The serum NfL levels increased significantly between the two points for cases (i.e., a median increase of 1.3 pg/mL per year), but there was no significant difference in serum NfL level between the two samples in controls. A within-patient increase in presymptomatic serum NfL level was associated with an increased risk of MS.
Population included few women
“Our study differs from previous studies on the prodromal phase of MS because these have used indirect markers of this phase, which included unspecific symptoms or disturbances occurring before the clinical onset, compared with a marker of neurodegeneration,” wrote Dr. Bjornevik and colleagues. Initiation of treatment with disease-modifying therapy is associated with reductions in serum NfL levels, and this association could explain why some patients in the current study had higher NfL levels before MS onset than afterward. Furthermore, serum NfL levels are highly associated with levels of NfL in cerebrospinal fluid. “Thus, our findings of a presymptomatic increase in serum NfL not only suggest the presence of a prodromal phase in MS, but also that this phase is associated with neurodegeneration,” wrote the investigators.
The study’s well-defined population helped to minimize selection bias, and the blinded, randomized method of analyzing the serum samples eliminated artifactual differences in serum NfL concentrations. But the small sample size precluded analyses that could have influenced clinical practice, wrote Dr. Bjornevik and colleagues. For example, the researchers could not evaluate distinct cutoffs in serum NfL level that could mark the beginning of the prodromal phase of MS. Nor could they determine whether presymptomatic serum NfL levels varied with age at clinical onset, sex, or race. The small number of women in the sample was another limitation of the study.
The Swiss National Research Foundation and the National Institute of Neurologic Disorders and Stroke funded the study. Several of the investigators received fees from various drug companies that were unrelated to the study, and one researcher received grants from the National Institutes of Health during the study.
SOURCE: Bjornevik K et al. JAMA Neurol. 2020;77(1):58-64.
(MS), according to research published in the January issue of JAMA Neurology. These results lend weight to the idea that MS has a prodromal phase, and this phase appears to be associated with neurodegeneration, according to the authors.
Patients often have CNS lesions of various stages of development at the time of their first demyelinating event, and this finding was one basis for neurologists’ hypothesis of a prodromal phase of MS. The finding that one-third of patients with radiologically isolated syndrome develop MS within 5 years also lends credence to this idea. Diagnosing MS early would enable early treatment that could prevent demyelination and the progression of neurodegeneration.
Researchers compared presymptomatic and symptomatic samples
With this idea in mind, Kjetil Bjornevik, MD, PhD, a member of the neuroepidemiology research group at Harvard TH Chan School of Public Health in Boston, and colleagues evaluated whether serum levels of NfL, a marker of ongoing neuroaxonal degeneration, were increased in the years before and around the time of clinical onset of MS. For their study population, the investigators chose active-duty U.S. military personnel who have at least one serum sample stored in the U.S. Department of Defense Serum Repository. Samples are collected after routine HIV type 1 antibody testing.
Within this population, Dr. Bjornevik and colleagues identified patients with MS who had at least one presymptomatic serum sample. The date of clinical MS onset was defined as the date of the first neurologic symptoms attributable to MS documented in the medical record. The investigators randomly selected two control individuals from the population and matched them to each case by age, sex, race or ethnicity, and dates of sample collection. Eligible controls were on active duty on the date of onset of the matched case.
Dr. Bjornevik and colleagues identified 245 patients with MS. Among this sample, the researchers selected two groups that each included 30 cases and 30 controls. The first group included patients who had provided at least one serum sample before MS onset and one sample within 2 years after MS onset. The second group included cases with at least two presymptomatic serum samples, one of which was collected more than 5 years before MS diagnosis, and the other of which was collected between 2 and 5 years before diagnosis. The investigators handled pairs of serum samples in the same way and assayed them in the same batch. The order of the samples in each pair was arranged at random.
Levels were higher in cases than in controls
About 77% of the population was male. Sixty percent of participants were white, 28% were black, and 6.7% were Hispanic. The population’s mean age at first sample collection was approximately 27 years. Mean age at MS onset was approximately 31 years.
For patients who provided samples before and after the clinical onset of MS, serum NfL levels were higher than in matched controls at both points. Most patients who passed from the presymptomatic stage to the symptomatic stage had a significant increase in serum NfL level (i.e., from a median of 25.0 pg/mL to a median of 45.1 pg/mL). Serum NfL levels at the two time points in controls did not differ significantly. For any given patient, an increase in serum NfL level from the presymptomatic measurement to the symptomatic measurement was associated with an increased risk of MS.
In patients with two presymptomatic samples, serum NfL levels were significantly higher in both samples than in the corresponding samples from matched controls. In cases, the earlier sample was collected at a median of 6 years before clinical onset of MS, and the later sample was collected at a median of 1 year before clinical onset. The serum NfL levels increased significantly between the two points for cases (i.e., a median increase of 1.3 pg/mL per year), but there was no significant difference in serum NfL level between the two samples in controls. A within-patient increase in presymptomatic serum NfL level was associated with an increased risk of MS.
Population included few women
“Our study differs from previous studies on the prodromal phase of MS because these have used indirect markers of this phase, which included unspecific symptoms or disturbances occurring before the clinical onset, compared with a marker of neurodegeneration,” wrote Dr. Bjornevik and colleagues. Initiation of treatment with disease-modifying therapy is associated with reductions in serum NfL levels, and this association could explain why some patients in the current study had higher NfL levels before MS onset than afterward. Furthermore, serum NfL levels are highly associated with levels of NfL in cerebrospinal fluid. “Thus, our findings of a presymptomatic increase in serum NfL not only suggest the presence of a prodromal phase in MS, but also that this phase is associated with neurodegeneration,” wrote the investigators.
The study’s well-defined population helped to minimize selection bias, and the blinded, randomized method of analyzing the serum samples eliminated artifactual differences in serum NfL concentrations. But the small sample size precluded analyses that could have influenced clinical practice, wrote Dr. Bjornevik and colleagues. For example, the researchers could not evaluate distinct cutoffs in serum NfL level that could mark the beginning of the prodromal phase of MS. Nor could they determine whether presymptomatic serum NfL levels varied with age at clinical onset, sex, or race. The small number of women in the sample was another limitation of the study.
The Swiss National Research Foundation and the National Institute of Neurologic Disorders and Stroke funded the study. Several of the investigators received fees from various drug companies that were unrelated to the study, and one researcher received grants from the National Institutes of Health during the study.
SOURCE: Bjornevik K et al. JAMA Neurol. 2020;77(1):58-64.
FROM JAMA NEUROLOGY
CRISPR-engineered T cells may be safe for cancer, but do they work?
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
FROM SCIENCE