User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
ASCO announces its own COVID-19 and cancer registry
Data will not be commercialized, unlike CancerLinQ
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
Data will not be commercialized, unlike CancerLinQ
Data will not be commercialized, unlike CancerLinQ
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
TWILIGHT-COMPLEX: Tap ticagrelor monotherapy early after complex PCI
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
FROM ACC 2020
Presymptomatic or asymptomatic? ID experts on shifting terminology
They also addressed racial disparities surrounding COVID-19, and announced new IDSA guidelines for diagnosis and treatment of the illness.
Regarding the shifting thinking on symptoms and transmission of the novel coronavirus, when it comes to presymptomatic or asymptomatic, “pre” is really the right terminology, Carlos del Rio, MD, professor of medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, said during the briefing, because it’s not that people are asymptomatic but that they develop symptoms later and start transmitting the virus 24 to 48 hours before they develop symptoms.
“Clearly, this plays a role in transmission,” with some studies suggesting that 6% to 12% of transmissions occur during this presymptomatic stage, he explained.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at University of Alabama at Birmingham, noted that early in the COVID-19 pandemic, the presymptomatic phase “could have been missed because we didn’t realize the wide ranging symptoms this disease has.”
This is turning out to be a “very interesting” virus with “fascinating” symptoms, she told reporters on the call.
The virus seems to have capacity to affect far more than just the respiratory tract. Initially, however, it was viewed “very much like a classic respiratory viral infection. As a result, a lot of people were refused testing because they were not showing the classic signs” of respiratory infection, Marrazzo noted.
It’s now clear that the range of symptoms is quite different, she said.
Notably, loss of smell seems to be “very characteristic and very specific to this infection. I can’t think of another common viral infection that causes loss of smell before you start to see other things,” Marrazzo said.
Data also suggest that gastrointestinal symptoms are common with COVID-19. Early data suggest that diarrhea probably occurs in about one third of patients. Some people have reported abdominal pain as the first sign, she said.
“Now that we know about the more wide range of symptoms associated [with COVID-19], we are being much more open to considering people perhaps having this infection. There is a lower index of suspicion and much lower threshold for diagnostic testing,” Marrazzo said, adding that there are still many barriers to testing and getting test results.
Stark Racial Disparities Need Greater Understanding
The second major topic of discussion at the briefing was the growing realization of racial disparities in COVID-19.
“Racial disparities in our country are not new but racial disparities in this disease are pretty stark,” del Rio said. “We live in a country where disparities have really colored a lot of what our diseases are, from HIV to diabetes to hypertension, and it’s not surprising that we are seeing this now with COVID-19.”
Marrazzo noted that, in Alabama, around 20% of the population is African American, yet almost 40% of COVID-19 deaths are occurring in this population. “The most stark statistics are coming out of Illinois and Michigan, where less than around 15% of the population is African American and yet 70% of the deaths are occurring in that group,” she said.
Both del Rio and Marrazzo agreed that understanding the racial differences in COVID-19 deaths is going to require a lot of analysis in the coming months.
Part of it likely reflects the challenge of social distancing in urban areas, Marrazzo said. “Social distancing is a luxury afforded by having a really big space, and space is money.”
The other long-standing challenge of unequal access to healthcare also likely plays a role, she said. This includes missing out on preventive health appointments and screenings, which can translate into more comorbidities, particularly hypertension.
The evolving evidence about the virus, and the stark conditions that frontline clinicians face, make this an especially challenging public health crisis, del Rio said.
“Taking care of these patients is incredibly taxing and my hat is off to physicians, residents, nurses, everybody working on this in the hospitals because they are really doing a yeoman’s work,” he said.
“These are not easy patients to take care of. Not only are [the frontline clinicians] providing care, they are caring for the patient and providing a comfort and someone to listen to when family can’t be present,” del Rio emphasized.
New Guidelines
The IDSA just released new guidelines for diagnosis and treatment of COVID-19.
“We are learning new things every day about this virus. Things are rapidly changing, and as we learn new things we have to adapt and make changes,” del Rio said.
del Rio noted that the guildelines “will evolve and change as more information comes out.”
This article first appeared on Medscape.com.
They also addressed racial disparities surrounding COVID-19, and announced new IDSA guidelines for diagnosis and treatment of the illness.
Regarding the shifting thinking on symptoms and transmission of the novel coronavirus, when it comes to presymptomatic or asymptomatic, “pre” is really the right terminology, Carlos del Rio, MD, professor of medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, said during the briefing, because it’s not that people are asymptomatic but that they develop symptoms later and start transmitting the virus 24 to 48 hours before they develop symptoms.
“Clearly, this plays a role in transmission,” with some studies suggesting that 6% to 12% of transmissions occur during this presymptomatic stage, he explained.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at University of Alabama at Birmingham, noted that early in the COVID-19 pandemic, the presymptomatic phase “could have been missed because we didn’t realize the wide ranging symptoms this disease has.”
This is turning out to be a “very interesting” virus with “fascinating” symptoms, she told reporters on the call.
The virus seems to have capacity to affect far more than just the respiratory tract. Initially, however, it was viewed “very much like a classic respiratory viral infection. As a result, a lot of people were refused testing because they were not showing the classic signs” of respiratory infection, Marrazzo noted.
It’s now clear that the range of symptoms is quite different, she said.
Notably, loss of smell seems to be “very characteristic and very specific to this infection. I can’t think of another common viral infection that causes loss of smell before you start to see other things,” Marrazzo said.
Data also suggest that gastrointestinal symptoms are common with COVID-19. Early data suggest that diarrhea probably occurs in about one third of patients. Some people have reported abdominal pain as the first sign, she said.
“Now that we know about the more wide range of symptoms associated [with COVID-19], we are being much more open to considering people perhaps having this infection. There is a lower index of suspicion and much lower threshold for diagnostic testing,” Marrazzo said, adding that there are still many barriers to testing and getting test results.
Stark Racial Disparities Need Greater Understanding
The second major topic of discussion at the briefing was the growing realization of racial disparities in COVID-19.
“Racial disparities in our country are not new but racial disparities in this disease are pretty stark,” del Rio said. “We live in a country where disparities have really colored a lot of what our diseases are, from HIV to diabetes to hypertension, and it’s not surprising that we are seeing this now with COVID-19.”
Marrazzo noted that, in Alabama, around 20% of the population is African American, yet almost 40% of COVID-19 deaths are occurring in this population. “The most stark statistics are coming out of Illinois and Michigan, where less than around 15% of the population is African American and yet 70% of the deaths are occurring in that group,” she said.
Both del Rio and Marrazzo agreed that understanding the racial differences in COVID-19 deaths is going to require a lot of analysis in the coming months.
Part of it likely reflects the challenge of social distancing in urban areas, Marrazzo said. “Social distancing is a luxury afforded by having a really big space, and space is money.”
The other long-standing challenge of unequal access to healthcare also likely plays a role, she said. This includes missing out on preventive health appointments and screenings, which can translate into more comorbidities, particularly hypertension.
The evolving evidence about the virus, and the stark conditions that frontline clinicians face, make this an especially challenging public health crisis, del Rio said.
“Taking care of these patients is incredibly taxing and my hat is off to physicians, residents, nurses, everybody working on this in the hospitals because they are really doing a yeoman’s work,” he said.
“These are not easy patients to take care of. Not only are [the frontline clinicians] providing care, they are caring for the patient and providing a comfort and someone to listen to when family can’t be present,” del Rio emphasized.
New Guidelines
The IDSA just released new guidelines for diagnosis and treatment of COVID-19.
“We are learning new things every day about this virus. Things are rapidly changing, and as we learn new things we have to adapt and make changes,” del Rio said.
del Rio noted that the guildelines “will evolve and change as more information comes out.”
This article first appeared on Medscape.com.
They also addressed racial disparities surrounding COVID-19, and announced new IDSA guidelines for diagnosis and treatment of the illness.
Regarding the shifting thinking on symptoms and transmission of the novel coronavirus, when it comes to presymptomatic or asymptomatic, “pre” is really the right terminology, Carlos del Rio, MD, professor of medicine, Division of Infectious Diseases, Emory University School of Medicine, Atlanta, Georgia, said during the briefing, because it’s not that people are asymptomatic but that they develop symptoms later and start transmitting the virus 24 to 48 hours before they develop symptoms.
“Clearly, this plays a role in transmission,” with some studies suggesting that 6% to 12% of transmissions occur during this presymptomatic stage, he explained.
Jeanne Marrazzo, MD, MPH, director of the Division of Infectious Diseases at University of Alabama at Birmingham, noted that early in the COVID-19 pandemic, the presymptomatic phase “could have been missed because we didn’t realize the wide ranging symptoms this disease has.”
This is turning out to be a “very interesting” virus with “fascinating” symptoms, she told reporters on the call.
The virus seems to have capacity to affect far more than just the respiratory tract. Initially, however, it was viewed “very much like a classic respiratory viral infection. As a result, a lot of people were refused testing because they were not showing the classic signs” of respiratory infection, Marrazzo noted.
It’s now clear that the range of symptoms is quite different, she said.
Notably, loss of smell seems to be “very characteristic and very specific to this infection. I can’t think of another common viral infection that causes loss of smell before you start to see other things,” Marrazzo said.
Data also suggest that gastrointestinal symptoms are common with COVID-19. Early data suggest that diarrhea probably occurs in about one third of patients. Some people have reported abdominal pain as the first sign, she said.
“Now that we know about the more wide range of symptoms associated [with COVID-19], we are being much more open to considering people perhaps having this infection. There is a lower index of suspicion and much lower threshold for diagnostic testing,” Marrazzo said, adding that there are still many barriers to testing and getting test results.
Stark Racial Disparities Need Greater Understanding
The second major topic of discussion at the briefing was the growing realization of racial disparities in COVID-19.
“Racial disparities in our country are not new but racial disparities in this disease are pretty stark,” del Rio said. “We live in a country where disparities have really colored a lot of what our diseases are, from HIV to diabetes to hypertension, and it’s not surprising that we are seeing this now with COVID-19.”
Marrazzo noted that, in Alabama, around 20% of the population is African American, yet almost 40% of COVID-19 deaths are occurring in this population. “The most stark statistics are coming out of Illinois and Michigan, where less than around 15% of the population is African American and yet 70% of the deaths are occurring in that group,” she said.
Both del Rio and Marrazzo agreed that understanding the racial differences in COVID-19 deaths is going to require a lot of analysis in the coming months.
Part of it likely reflects the challenge of social distancing in urban areas, Marrazzo said. “Social distancing is a luxury afforded by having a really big space, and space is money.”
The other long-standing challenge of unequal access to healthcare also likely plays a role, she said. This includes missing out on preventive health appointments and screenings, which can translate into more comorbidities, particularly hypertension.
The evolving evidence about the virus, and the stark conditions that frontline clinicians face, make this an especially challenging public health crisis, del Rio said.
“Taking care of these patients is incredibly taxing and my hat is off to physicians, residents, nurses, everybody working on this in the hospitals because they are really doing a yeoman’s work,” he said.
“These are not easy patients to take care of. Not only are [the frontline clinicians] providing care, they are caring for the patient and providing a comfort and someone to listen to when family can’t be present,” del Rio emphasized.
New Guidelines
The IDSA just released new guidelines for diagnosis and treatment of COVID-19.
“We are learning new things every day about this virus. Things are rapidly changing, and as we learn new things we have to adapt and make changes,” del Rio said.
del Rio noted that the guildelines “will evolve and change as more information comes out.”
This article first appeared on Medscape.com.
FDA approves Koselugo for pediatric neurofibromatosis treatment
The Food and Drug Administration has approved selumetinib (Koselugo) for the treatment of pediatric patients aged 2 years and older with type 1 neurofibromatosis (NF1) with symptomatic, inoperable plexiform neurofibromas.
FDA approval was based on results from the phase 2 SPRINT Stratum 1 trial, in which 50 patients with NF1 received selumetinib as twice-daily oral monotherapy. Of this group, 33 (66%) patients had a partial response of at least a 20% reduction in tumor volume. There were no complete responses, according to a press release.
The most common adverse events were vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain, pyrexia, rash acneiform, stomatitis, headache, paronychia, and pruritus. Dose interruptions, dose reductions, and permanent drug discontinuation occurred in 80%, 24%, and 12% of patients, respectively.
Serious adverse reactions included cardiomyopathy, ocular toxicity, gastrointestinal toxicity, increased creatinine phosphokinase, and increased vitamin E levels and risk of bleeding, according to the press release.
“Previously, there were no medicines approved for this disease. This approval has the potential to change how symptomatic, inoperable NF1 plexiform neurofibromas are treated and provides new hope to these patients,” Roy Baynes, MD, PhD, senior vice president, head of global clinical development, and chief medical officer of Merck Research Laboratories, said in the press release.
The Food and Drug Administration has approved selumetinib (Koselugo) for the treatment of pediatric patients aged 2 years and older with type 1 neurofibromatosis (NF1) with symptomatic, inoperable plexiform neurofibromas.
FDA approval was based on results from the phase 2 SPRINT Stratum 1 trial, in which 50 patients with NF1 received selumetinib as twice-daily oral monotherapy. Of this group, 33 (66%) patients had a partial response of at least a 20% reduction in tumor volume. There were no complete responses, according to a press release.
The most common adverse events were vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain, pyrexia, rash acneiform, stomatitis, headache, paronychia, and pruritus. Dose interruptions, dose reductions, and permanent drug discontinuation occurred in 80%, 24%, and 12% of patients, respectively.
Serious adverse reactions included cardiomyopathy, ocular toxicity, gastrointestinal toxicity, increased creatinine phosphokinase, and increased vitamin E levels and risk of bleeding, according to the press release.
“Previously, there were no medicines approved for this disease. This approval has the potential to change how symptomatic, inoperable NF1 plexiform neurofibromas are treated and provides new hope to these patients,” Roy Baynes, MD, PhD, senior vice president, head of global clinical development, and chief medical officer of Merck Research Laboratories, said in the press release.
The Food and Drug Administration has approved selumetinib (Koselugo) for the treatment of pediatric patients aged 2 years and older with type 1 neurofibromatosis (NF1) with symptomatic, inoperable plexiform neurofibromas.
FDA approval was based on results from the phase 2 SPRINT Stratum 1 trial, in which 50 patients with NF1 received selumetinib as twice-daily oral monotherapy. Of this group, 33 (66%) patients had a partial response of at least a 20% reduction in tumor volume. There were no complete responses, according to a press release.
The most common adverse events were vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain, pyrexia, rash acneiform, stomatitis, headache, paronychia, and pruritus. Dose interruptions, dose reductions, and permanent drug discontinuation occurred in 80%, 24%, and 12% of patients, respectively.
Serious adverse reactions included cardiomyopathy, ocular toxicity, gastrointestinal toxicity, increased creatinine phosphokinase, and increased vitamin E levels and risk of bleeding, according to the press release.
“Previously, there were no medicines approved for this disease. This approval has the potential to change how symptomatic, inoperable NF1 plexiform neurofibromas are treated and provides new hope to these patients,” Roy Baynes, MD, PhD, senior vice president, head of global clinical development, and chief medical officer of Merck Research Laboratories, said in the press release.
COVID-19 hits physician couple: Dramatically different responses
A physician couple who both had COVID-19 had very different responses — one ending up in intensive care, the other asymptomatic.
Their story, one of two people living together but with such different responses to the infection, illustrates how much is still to be learned about COVID-19, says Noopur Raje, MD, professor of medicine at Harvard Medical School and director of the Center for Multiple Myeloma at Massachusetts General Hospital (MGH) in Boston.
“After experiencing #Covid_19 from the patient/caregiver end despite both of us being physicians at a major academic medical center, this has been a challenge like no other I have experienced,” Raje (@NoopurRajeMD) wrote on Twitter.
She outlined their experiences in a Twitter thread and elaborated in an interview with Medscape Medical News.
Raje says that she wants clinicians to know how symptoms can evolve both quickly and suddenly.
She recalls how for 10 days, she cared for her COVID-19–positive husband at home, separated from him by a floor in their Boston townhouse and wearing a surgical mask and gloves to bring him food and fluids, as he was too weak to help himself.
Despite the high fevers, chills, extreme fatigue, and dramatic weight loss, Raje says she felt reasonably confident that her husband was getting better. His temperature had dropped from around 103 to 101, his heart rate was in the 80s, and his blood pressure was “OK,” she recalls.
But then Jag Singh, MD, an otherwise healthy 55-year-old Harvard professor and cardiologist, started to cough — and everything suddenly changed.
The cough sounded chesty, and he was weak and unwell. They decided that he needed medical help.
“I was planning on driving him to the hospital, but I ended up having to call 911, although we literally live across the street,” she said.
“We have stairs here and I wasn’t sure that he would be able to make it coming down with me trying to help him, so the safest thing was for me to call for help.”
Singh was admitted straight to the medical intensive care unit (MICU) while his wife waited at home.
“I was blown away when I saw Jag’s x-ray and CT scan and the bilateral pneumonia he had developed,” she commented. “I would not have believed it, the way he was clinically — and seeing that x-ray.
“Honestly, when I took him in to hospital, I thought he’d be there a couple of days — over the weekend — and I’d get him back Monday. But it didn’t turn out that way. He was there for about 9 days.”
That first night in the hospital, Singh consented to intubation — should he need it. “He called me then,” said Raje. “I said we’ve got to do what we’ve got to do, it’s OK — it is what it is, and we’ll do whatever it takes.”
He remained in the MICU overnight and through the next day, still breathing on his own, but with the looming prospect of mechanical ventilation.
“The good news is he maintained his oxygen saturations throughout,” said Raje. “I was able to see his vitals with EPIC [remote monitoring] ... It was crazy,” she recalls. “Seeing a respiratory rate of 26 was difficult. When you see that, you worry about somebody tiring with the breathing. His inflammatory markers kept climbing, his fevers persisted.”
Thankfully, he never needed the ventilator.
But by this time Raje had another worry: She, too, had tested positive and was now alone at home.
“I was unable to talk to my extended family as they all looked to us as physicians for support,” she tweeted. Both children came to Boston to see her, but she saw them only through a window.
Alone, she waited for the same symptoms that had slammed her husband; but they never came — something she wants caregivers to know.
“The fear and anxiety of taking care of somebody who’s COVID positive ... I am hoping that can be alleviated a little bit at least,” she said. “If you’ve been taking care of someone, chances are you’re probably positive already and if you’re not sick, the chances of you getting sick are really low, so don’t be afraid to take care of that person.”
Singh is recovering well at home now, almost a month into his illness. During the interview, conducted via Zoom, he could be heard coughing in the background.
While in the MICU, Singh was treated with azithromycin and hydroxychloroquine — standard at MGH for critically ill COVID-19 patients — and he was also enrolled into a double-blind, randomized, placebo-controlled trial of the investigational agent remdesivir (Gilead).
Raje is not sure what, if anything, helped him turn the corner.
“I saw his inflammatory markers get worse actually — I don’t think we can know if the drugs made a difference,” she says. “His first dose of hydroxychloroquine was Friday night when he was admitted, and the markers continued to climb until the next Thursday.”
In particular, his C-reactive protein (CRP) kept rising, reaching the 260 to 270 mg/dL range, “which to me was scary,” she said. “I do think he had a cytokine storm going, but I didn’t see those results.”
“Understanding the immune compartment is going to be so, so critically important and what it is that we can do to boost folks’ immune systems,” she said.
“If you have a very high viral load and your immune system is not 100% even though you’re otherwise healthy, you might be the person who ends up with that more serious response to this virus. Trying to study this in a focused way, looking at the immune compartment, looking at the antibody status, looking at the viral load — there’s so much more we need to look at. Until we get the vaccine, which is probably a year-and-a-half away, we need to look at how can we develop that herd immunity so we don’t have folks getting as critically ill as they do.”
Despite feeling perfectly healthy, Raje is still at home. Three weeks after her first test, she is still testing positive for COVID-19, waiting for two consecutive negative results 72 hours apart before she is allowed back to work at the hospital.
When she gets the green light, she plans to go work on the COVID-19 floor, if needed. “It’s people like us [who have had COVID-19] who have to get back in the trenches and do the work now,” she says.
“My biggest concern is that it’s a very isolating experience for the COVID-positive patient. We are doing complete-barrier nursing — they are completely alone. The only person who ever walks into the room is the nurse — and the physician goes in once a day. It’s so important that we don’t lose sight of compassion,” she says.
“That’s why, in terms of alleviating anxiety, it is so important we do antibody testing so that people can actually go in and take care of these folks.”
‘Look for red flag’
Raje wants physicians to warn their self-isolating patients and caregivers to look for red flags. “There are primary care physicians who reached out to me [after my tweets] and said ‘when someone calls me and says it’s been 5-7 days and I am still not feeling well, I am going to look at that more seriously.’
“Part of me wanting to share this experience was basically to dispel the notion that 2 weeks into this you’re going to be fine,” she said, because it is not widely appreciated, she feels that “in week 2, you could become pretty sick.”
This article first appeared on Medscape.com.
A physician couple who both had COVID-19 had very different responses — one ending up in intensive care, the other asymptomatic.
Their story, one of two people living together but with such different responses to the infection, illustrates how much is still to be learned about COVID-19, says Noopur Raje, MD, professor of medicine at Harvard Medical School and director of the Center for Multiple Myeloma at Massachusetts General Hospital (MGH) in Boston.
“After experiencing #Covid_19 from the patient/caregiver end despite both of us being physicians at a major academic medical center, this has been a challenge like no other I have experienced,” Raje (@NoopurRajeMD) wrote on Twitter.
She outlined their experiences in a Twitter thread and elaborated in an interview with Medscape Medical News.
Raje says that she wants clinicians to know how symptoms can evolve both quickly and suddenly.
She recalls how for 10 days, she cared for her COVID-19–positive husband at home, separated from him by a floor in their Boston townhouse and wearing a surgical mask and gloves to bring him food and fluids, as he was too weak to help himself.
Despite the high fevers, chills, extreme fatigue, and dramatic weight loss, Raje says she felt reasonably confident that her husband was getting better. His temperature had dropped from around 103 to 101, his heart rate was in the 80s, and his blood pressure was “OK,” she recalls.
But then Jag Singh, MD, an otherwise healthy 55-year-old Harvard professor and cardiologist, started to cough — and everything suddenly changed.
The cough sounded chesty, and he was weak and unwell. They decided that he needed medical help.
“I was planning on driving him to the hospital, but I ended up having to call 911, although we literally live across the street,” she said.
“We have stairs here and I wasn’t sure that he would be able to make it coming down with me trying to help him, so the safest thing was for me to call for help.”
Singh was admitted straight to the medical intensive care unit (MICU) while his wife waited at home.
“I was blown away when I saw Jag’s x-ray and CT scan and the bilateral pneumonia he had developed,” she commented. “I would not have believed it, the way he was clinically — and seeing that x-ray.
“Honestly, when I took him in to hospital, I thought he’d be there a couple of days — over the weekend — and I’d get him back Monday. But it didn’t turn out that way. He was there for about 9 days.”
That first night in the hospital, Singh consented to intubation — should he need it. “He called me then,” said Raje. “I said we’ve got to do what we’ve got to do, it’s OK — it is what it is, and we’ll do whatever it takes.”
He remained in the MICU overnight and through the next day, still breathing on his own, but with the looming prospect of mechanical ventilation.
“The good news is he maintained his oxygen saturations throughout,” said Raje. “I was able to see his vitals with EPIC [remote monitoring] ... It was crazy,” she recalls. “Seeing a respiratory rate of 26 was difficult. When you see that, you worry about somebody tiring with the breathing. His inflammatory markers kept climbing, his fevers persisted.”
Thankfully, he never needed the ventilator.
But by this time Raje had another worry: She, too, had tested positive and was now alone at home.
“I was unable to talk to my extended family as they all looked to us as physicians for support,” she tweeted. Both children came to Boston to see her, but she saw them only through a window.
Alone, she waited for the same symptoms that had slammed her husband; but they never came — something she wants caregivers to know.
“The fear and anxiety of taking care of somebody who’s COVID positive ... I am hoping that can be alleviated a little bit at least,” she said. “If you’ve been taking care of someone, chances are you’re probably positive already and if you’re not sick, the chances of you getting sick are really low, so don’t be afraid to take care of that person.”
Singh is recovering well at home now, almost a month into his illness. During the interview, conducted via Zoom, he could be heard coughing in the background.
While in the MICU, Singh was treated with azithromycin and hydroxychloroquine — standard at MGH for critically ill COVID-19 patients — and he was also enrolled into a double-blind, randomized, placebo-controlled trial of the investigational agent remdesivir (Gilead).
Raje is not sure what, if anything, helped him turn the corner.
“I saw his inflammatory markers get worse actually — I don’t think we can know if the drugs made a difference,” she says. “His first dose of hydroxychloroquine was Friday night when he was admitted, and the markers continued to climb until the next Thursday.”
In particular, his C-reactive protein (CRP) kept rising, reaching the 260 to 270 mg/dL range, “which to me was scary,” she said. “I do think he had a cytokine storm going, but I didn’t see those results.”
“Understanding the immune compartment is going to be so, so critically important and what it is that we can do to boost folks’ immune systems,” she said.
“If you have a very high viral load and your immune system is not 100% even though you’re otherwise healthy, you might be the person who ends up with that more serious response to this virus. Trying to study this in a focused way, looking at the immune compartment, looking at the antibody status, looking at the viral load — there’s so much more we need to look at. Until we get the vaccine, which is probably a year-and-a-half away, we need to look at how can we develop that herd immunity so we don’t have folks getting as critically ill as they do.”
Despite feeling perfectly healthy, Raje is still at home. Three weeks after her first test, she is still testing positive for COVID-19, waiting for two consecutive negative results 72 hours apart before she is allowed back to work at the hospital.
When she gets the green light, she plans to go work on the COVID-19 floor, if needed. “It’s people like us [who have had COVID-19] who have to get back in the trenches and do the work now,” she says.
“My biggest concern is that it’s a very isolating experience for the COVID-positive patient. We are doing complete-barrier nursing — they are completely alone. The only person who ever walks into the room is the nurse — and the physician goes in once a day. It’s so important that we don’t lose sight of compassion,” she says.
“That’s why, in terms of alleviating anxiety, it is so important we do antibody testing so that people can actually go in and take care of these folks.”
‘Look for red flag’
Raje wants physicians to warn their self-isolating patients and caregivers to look for red flags. “There are primary care physicians who reached out to me [after my tweets] and said ‘when someone calls me and says it’s been 5-7 days and I am still not feeling well, I am going to look at that more seriously.’
“Part of me wanting to share this experience was basically to dispel the notion that 2 weeks into this you’re going to be fine,” she said, because it is not widely appreciated, she feels that “in week 2, you could become pretty sick.”
This article first appeared on Medscape.com.
A physician couple who both had COVID-19 had very different responses — one ending up in intensive care, the other asymptomatic.
Their story, one of two people living together but with such different responses to the infection, illustrates how much is still to be learned about COVID-19, says Noopur Raje, MD, professor of medicine at Harvard Medical School and director of the Center for Multiple Myeloma at Massachusetts General Hospital (MGH) in Boston.
“After experiencing #Covid_19 from the patient/caregiver end despite both of us being physicians at a major academic medical center, this has been a challenge like no other I have experienced,” Raje (@NoopurRajeMD) wrote on Twitter.
She outlined their experiences in a Twitter thread and elaborated in an interview with Medscape Medical News.
Raje says that she wants clinicians to know how symptoms can evolve both quickly and suddenly.
She recalls how for 10 days, she cared for her COVID-19–positive husband at home, separated from him by a floor in their Boston townhouse and wearing a surgical mask and gloves to bring him food and fluids, as he was too weak to help himself.
Despite the high fevers, chills, extreme fatigue, and dramatic weight loss, Raje says she felt reasonably confident that her husband was getting better. His temperature had dropped from around 103 to 101, his heart rate was in the 80s, and his blood pressure was “OK,” she recalls.
But then Jag Singh, MD, an otherwise healthy 55-year-old Harvard professor and cardiologist, started to cough — and everything suddenly changed.
The cough sounded chesty, and he was weak and unwell. They decided that he needed medical help.
“I was planning on driving him to the hospital, but I ended up having to call 911, although we literally live across the street,” she said.
“We have stairs here and I wasn’t sure that he would be able to make it coming down with me trying to help him, so the safest thing was for me to call for help.”
Singh was admitted straight to the medical intensive care unit (MICU) while his wife waited at home.
“I was blown away when I saw Jag’s x-ray and CT scan and the bilateral pneumonia he had developed,” she commented. “I would not have believed it, the way he was clinically — and seeing that x-ray.
“Honestly, when I took him in to hospital, I thought he’d be there a couple of days — over the weekend — and I’d get him back Monday. But it didn’t turn out that way. He was there for about 9 days.”
That first night in the hospital, Singh consented to intubation — should he need it. “He called me then,” said Raje. “I said we’ve got to do what we’ve got to do, it’s OK — it is what it is, and we’ll do whatever it takes.”
He remained in the MICU overnight and through the next day, still breathing on his own, but with the looming prospect of mechanical ventilation.
“The good news is he maintained his oxygen saturations throughout,” said Raje. “I was able to see his vitals with EPIC [remote monitoring] ... It was crazy,” she recalls. “Seeing a respiratory rate of 26 was difficult. When you see that, you worry about somebody tiring with the breathing. His inflammatory markers kept climbing, his fevers persisted.”
Thankfully, he never needed the ventilator.
But by this time Raje had another worry: She, too, had tested positive and was now alone at home.
“I was unable to talk to my extended family as they all looked to us as physicians for support,” she tweeted. Both children came to Boston to see her, but she saw them only through a window.
Alone, she waited for the same symptoms that had slammed her husband; but they never came — something she wants caregivers to know.
“The fear and anxiety of taking care of somebody who’s COVID positive ... I am hoping that can be alleviated a little bit at least,” she said. “If you’ve been taking care of someone, chances are you’re probably positive already and if you’re not sick, the chances of you getting sick are really low, so don’t be afraid to take care of that person.”
Singh is recovering well at home now, almost a month into his illness. During the interview, conducted via Zoom, he could be heard coughing in the background.
While in the MICU, Singh was treated with azithromycin and hydroxychloroquine — standard at MGH for critically ill COVID-19 patients — and he was also enrolled into a double-blind, randomized, placebo-controlled trial of the investigational agent remdesivir (Gilead).
Raje is not sure what, if anything, helped him turn the corner.
“I saw his inflammatory markers get worse actually — I don’t think we can know if the drugs made a difference,” she says. “His first dose of hydroxychloroquine was Friday night when he was admitted, and the markers continued to climb until the next Thursday.”
In particular, his C-reactive protein (CRP) kept rising, reaching the 260 to 270 mg/dL range, “which to me was scary,” she said. “I do think he had a cytokine storm going, but I didn’t see those results.”
“Understanding the immune compartment is going to be so, so critically important and what it is that we can do to boost folks’ immune systems,” she said.
“If you have a very high viral load and your immune system is not 100% even though you’re otherwise healthy, you might be the person who ends up with that more serious response to this virus. Trying to study this in a focused way, looking at the immune compartment, looking at the antibody status, looking at the viral load — there’s so much more we need to look at. Until we get the vaccine, which is probably a year-and-a-half away, we need to look at how can we develop that herd immunity so we don’t have folks getting as critically ill as they do.”
Despite feeling perfectly healthy, Raje is still at home. Three weeks after her first test, she is still testing positive for COVID-19, waiting for two consecutive negative results 72 hours apart before she is allowed back to work at the hospital.
When she gets the green light, she plans to go work on the COVID-19 floor, if needed. “It’s people like us [who have had COVID-19] who have to get back in the trenches and do the work now,” she says.
“My biggest concern is that it’s a very isolating experience for the COVID-positive patient. We are doing complete-barrier nursing — they are completely alone. The only person who ever walks into the room is the nurse — and the physician goes in once a day. It’s so important that we don’t lose sight of compassion,” she says.
“That’s why, in terms of alleviating anxiety, it is so important we do antibody testing so that people can actually go in and take care of these folks.”
‘Look for red flag’
Raje wants physicians to warn their self-isolating patients and caregivers to look for red flags. “There are primary care physicians who reached out to me [after my tweets] and said ‘when someone calls me and says it’s been 5-7 days and I am still not feeling well, I am going to look at that more seriously.’
“Part of me wanting to share this experience was basically to dispel the notion that 2 weeks into this you’re going to be fine,” she said, because it is not widely appreciated, she feels that “in week 2, you could become pretty sick.”
This article first appeared on Medscape.com.
NYC hospitals require health care workers to report in person, even for phone and telehealth work
A social worker in New York City was home, caring for his sick son, when the hospital at which he works ordered him to report back to work. His son had COVID-19, yet his hospital told him he had to show up in person.
The social worker’s situation is just one of many NYC Health + Hospitals employees who could work remotely yet are required to report in person. His circumstances were described in a letter sent by Lichten & Bright, a law firm representing the New York City Health Services Employees Union, Local 768.
“Despite the fact that all or virtually all of the work social workers perform can be done remotely, only a handful…are being permitted to work from home,” said the letter, which was written on behalf of about 1000 social workers and 150 medical records specialists and addressed to NYC H+H CEO Mitchell Katz, MD.
Most social workers stopped seeing patients in person in early March. But many still face crowded conditions at several points during their work day. They take public transportation to work, come face-to-face with other health care workers and patients in elevators, and some attend daily meetings with up to 10 employees in conference rooms too small to stay six feet apart, the letter says.
“The social workers are scared to go to work,” said Daniel Bright, the letter’s author. “They’re baffled by the lack of any management response that would allow them to work from home. They are worried about getting exposed to the coronavirus while riding the subway or the bus to work or at work from a doctor or nurse or patient, and getting sick themselves or taking it home to their families.”
There is no good reason that the social workers should be compelled to be physically at work during the COVID-19 pandemic, Bright said. The handful of social workers at NYC H+H’s World Trade Center Environmental Health Center clinic at Bellevue who have been allowed to work from home on an ad hoc basis, he said, have done so successfully.
In response to Bright’s letter, the hospital system issued a statement that seemed to downplay workers’ assessment of the situation, and included the following: “NYC Health + Hospital social workers…play different roles in our system, from acting as front-line providers to navigating safe discharges and helping patients and families with important health care decisions. Depending on the facility, the department, and the role they play, decisions are made by our hospital leaders on whether their critical work could be done remotely.”
Recently, many medical associations have issued statements supporting the rights of health care workers to speak up without fear of repercussion. But NYC H+H social workers have been complying with the orders because they say they’re scared of retaliation: In daily video conference calls, an administrator at one of NYC H+H’s hospitals has shown exasperation when asked about working from home, multiple employees told Medscape Medical News. And other questions, they said, such as whether staff could receive hazard pay, were scoffed at. Instead, the administration mentioned disciplinary action for those who didn’t show up to work.
During Thursday’s call, a recording of which was obtained by Medscape, the CEO of one NYC H+H hospital chastised his employees for taking their concerns to the press.
“People are just taking things and you know, using things for their benefit to be able to create problems for us who are trying to do our jobs,” he said, adding that he refuses to be bullied or blackmailed and that he’ll continue to do what he needs to do as CEO — but he wanted people to know “some of the garbage I have to deal with.”
He also reminded employees of documentation people need to provide if they don’t come in to work for being sick or taking a personal leave so the hospital can verify that “you have a condition that warrants you being out.”
Christopher Miller, a spokesperson for the hospital system, said that “some employees in certain functions may be approved to telecommute.” But employees contacted by Medscape who see all of their clients remotely said their requests to telecommute have not been approved.
At this point, it’s no longer a theoretical problem. COVID-19 appears to have spread among a cluster of people reporting to work in one of the H+H hospitals, employees said. In some cases, employees’ family members also became ill.
This article first appeared on Medscape.com.
A social worker in New York City was home, caring for his sick son, when the hospital at which he works ordered him to report back to work. His son had COVID-19, yet his hospital told him he had to show up in person.
The social worker’s situation is just one of many NYC Health + Hospitals employees who could work remotely yet are required to report in person. His circumstances were described in a letter sent by Lichten & Bright, a law firm representing the New York City Health Services Employees Union, Local 768.
“Despite the fact that all or virtually all of the work social workers perform can be done remotely, only a handful…are being permitted to work from home,” said the letter, which was written on behalf of about 1000 social workers and 150 medical records specialists and addressed to NYC H+H CEO Mitchell Katz, MD.
Most social workers stopped seeing patients in person in early March. But many still face crowded conditions at several points during their work day. They take public transportation to work, come face-to-face with other health care workers and patients in elevators, and some attend daily meetings with up to 10 employees in conference rooms too small to stay six feet apart, the letter says.
“The social workers are scared to go to work,” said Daniel Bright, the letter’s author. “They’re baffled by the lack of any management response that would allow them to work from home. They are worried about getting exposed to the coronavirus while riding the subway or the bus to work or at work from a doctor or nurse or patient, and getting sick themselves or taking it home to their families.”
There is no good reason that the social workers should be compelled to be physically at work during the COVID-19 pandemic, Bright said. The handful of social workers at NYC H+H’s World Trade Center Environmental Health Center clinic at Bellevue who have been allowed to work from home on an ad hoc basis, he said, have done so successfully.
In response to Bright’s letter, the hospital system issued a statement that seemed to downplay workers’ assessment of the situation, and included the following: “NYC Health + Hospital social workers…play different roles in our system, from acting as front-line providers to navigating safe discharges and helping patients and families with important health care decisions. Depending on the facility, the department, and the role they play, decisions are made by our hospital leaders on whether their critical work could be done remotely.”
Recently, many medical associations have issued statements supporting the rights of health care workers to speak up without fear of repercussion. But NYC H+H social workers have been complying with the orders because they say they’re scared of retaliation: In daily video conference calls, an administrator at one of NYC H+H’s hospitals has shown exasperation when asked about working from home, multiple employees told Medscape Medical News. And other questions, they said, such as whether staff could receive hazard pay, were scoffed at. Instead, the administration mentioned disciplinary action for those who didn’t show up to work.
During Thursday’s call, a recording of which was obtained by Medscape, the CEO of one NYC H+H hospital chastised his employees for taking their concerns to the press.
“People are just taking things and you know, using things for their benefit to be able to create problems for us who are trying to do our jobs,” he said, adding that he refuses to be bullied or blackmailed and that he’ll continue to do what he needs to do as CEO — but he wanted people to know “some of the garbage I have to deal with.”
He also reminded employees of documentation people need to provide if they don’t come in to work for being sick or taking a personal leave so the hospital can verify that “you have a condition that warrants you being out.”
Christopher Miller, a spokesperson for the hospital system, said that “some employees in certain functions may be approved to telecommute.” But employees contacted by Medscape who see all of their clients remotely said their requests to telecommute have not been approved.
At this point, it’s no longer a theoretical problem. COVID-19 appears to have spread among a cluster of people reporting to work in one of the H+H hospitals, employees said. In some cases, employees’ family members also became ill.
This article first appeared on Medscape.com.
A social worker in New York City was home, caring for his sick son, when the hospital at which he works ordered him to report back to work. His son had COVID-19, yet his hospital told him he had to show up in person.
The social worker’s situation is just one of many NYC Health + Hospitals employees who could work remotely yet are required to report in person. His circumstances were described in a letter sent by Lichten & Bright, a law firm representing the New York City Health Services Employees Union, Local 768.
“Despite the fact that all or virtually all of the work social workers perform can be done remotely, only a handful…are being permitted to work from home,” said the letter, which was written on behalf of about 1000 social workers and 150 medical records specialists and addressed to NYC H+H CEO Mitchell Katz, MD.
Most social workers stopped seeing patients in person in early March. But many still face crowded conditions at several points during their work day. They take public transportation to work, come face-to-face with other health care workers and patients in elevators, and some attend daily meetings with up to 10 employees in conference rooms too small to stay six feet apart, the letter says.
“The social workers are scared to go to work,” said Daniel Bright, the letter’s author. “They’re baffled by the lack of any management response that would allow them to work from home. They are worried about getting exposed to the coronavirus while riding the subway or the bus to work or at work from a doctor or nurse or patient, and getting sick themselves or taking it home to their families.”
There is no good reason that the social workers should be compelled to be physically at work during the COVID-19 pandemic, Bright said. The handful of social workers at NYC H+H’s World Trade Center Environmental Health Center clinic at Bellevue who have been allowed to work from home on an ad hoc basis, he said, have done so successfully.
In response to Bright’s letter, the hospital system issued a statement that seemed to downplay workers’ assessment of the situation, and included the following: “NYC Health + Hospital social workers…play different roles in our system, from acting as front-line providers to navigating safe discharges and helping patients and families with important health care decisions. Depending on the facility, the department, and the role they play, decisions are made by our hospital leaders on whether their critical work could be done remotely.”
Recently, many medical associations have issued statements supporting the rights of health care workers to speak up without fear of repercussion. But NYC H+H social workers have been complying with the orders because they say they’re scared of retaliation: In daily video conference calls, an administrator at one of NYC H+H’s hospitals has shown exasperation when asked about working from home, multiple employees told Medscape Medical News. And other questions, they said, such as whether staff could receive hazard pay, were scoffed at. Instead, the administration mentioned disciplinary action for those who didn’t show up to work.
During Thursday’s call, a recording of which was obtained by Medscape, the CEO of one NYC H+H hospital chastised his employees for taking their concerns to the press.
“People are just taking things and you know, using things for their benefit to be able to create problems for us who are trying to do our jobs,” he said, adding that he refuses to be bullied or blackmailed and that he’ll continue to do what he needs to do as CEO — but he wanted people to know “some of the garbage I have to deal with.”
He also reminded employees of documentation people need to provide if they don’t come in to work for being sick or taking a personal leave so the hospital can verify that “you have a condition that warrants you being out.”
Christopher Miller, a spokesperson for the hospital system, said that “some employees in certain functions may be approved to telecommute.” But employees contacted by Medscape who see all of their clients remotely said their requests to telecommute have not been approved.
At this point, it’s no longer a theoretical problem. COVID-19 appears to have spread among a cluster of people reporting to work in one of the H+H hospitals, employees said. In some cases, employees’ family members also became ill.
This article first appeared on Medscape.com.
Use of diabetes technology is lower among black, Hispanic patients
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
FROM ENDO 2020
Doing things right vs. doing the right things
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)
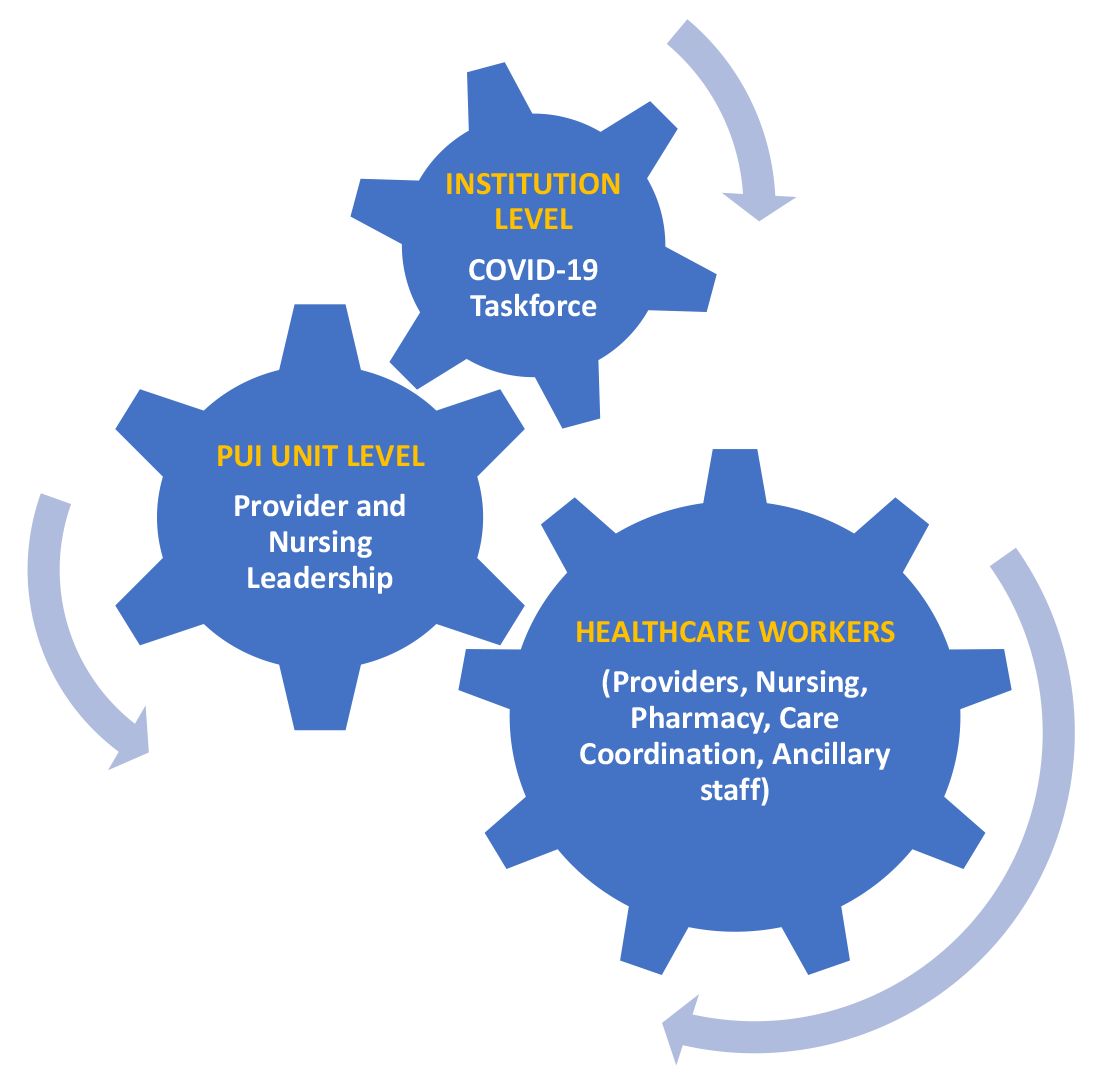
Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
A framework for a COVID-19 Person Under Investigation unit
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
Coronavirus tests are being fast-tracked by the FDA, but it’s unclear how accurate they are
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Hypertension goes unmedicated in 40% of adults
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.
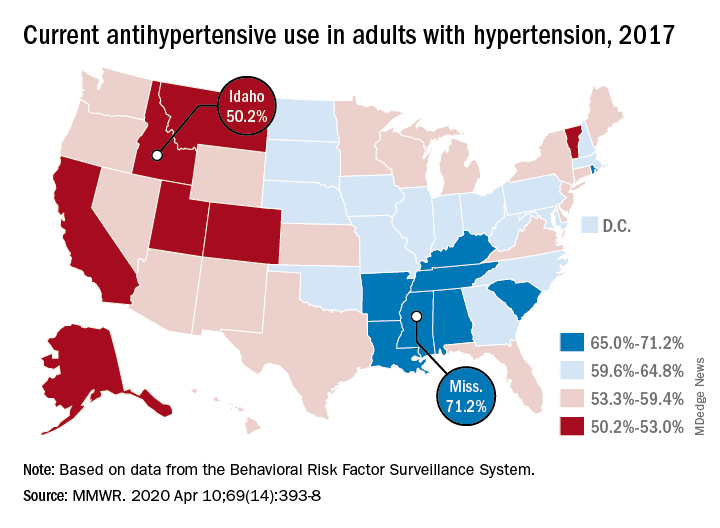
There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
FROM THE MMWR









