User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
AHA updates management when CAD and T2DM coincide
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
FROM CIRCULATION
‘We’re in great distress here,’ infusion center CMO says
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Count Vikram Sengupta, MD, among the slew of health care workers feeling overwhelmed by the impact that COVID-19 is having on the delivery of health care in Manhattan and its surrounding boroughs.
“Nobody in the country is suffering like New York City,” said Dr. Sengupta, chief medical officer of Thrivewell Infusion, which operates three stand-alone infusion centers in the region: a four-chair center in Crown Heights, a 10-chair center in Borough Park, Brooklyn, and an eight-chair center in Manhasset. “We have 30%-50% of all cases in the country. I’ve been reading the news, and some people think this thing is going away. We’re in great distress here. There need to be new strategies moving forward. The whole world has changed. Our whole approach to ambulatory care has changed.”
In early March 2020, when it became clear that New York hospitals would face a tidal wave of citizens infected with COVID-19, Thrivewell began to receive an influx of referrals originating from concerned patients, providers, payers, and even large integrated health care systems, all in an effort to help prevent infectious exposure through infusion in hospital-based settings. “We are trying to accommodate them as swiftly as possible,” said Dr. Sengupta, who was interviewed for this story on April 9. “There’s been a huge uptick from that standpoint. We’ve made sure that we’ve kept our facilities clean by employing standards that have been released by the CDC, as well as by the major academic centers who are dealing with this firsthand, and also with guidance from the National Infusion Center Association.”
He and his colleagues launched a pop-up infusion center in the Bronx to help offload Montefiore Medical Center, “because they’re so overwhelmed with COVID-19 patients that they need help taking care of the autoimmune patients,” Dr. Sengupta said. “That’s the role we’re playing. We’ve made our resources available to these centers in a very flexible way in order to ensure that we do the best thing we can for everybody.”
Thrivewell is also deploying a mobile infusion unit to recovered COVID-19 patients who require an infusion for their autoimmune disease, in order to minimize the risk of contamination and transmission in their stand-alone centers. The RV-sized unit, about the size of a Bloodmobile, is equipped with infusion chairs and staffed by a physician and nurse practitioner. “The objective is continuant care and reduction of cross-contamination, and also, on a broader health care systems level, to ensure that we as ambulatory infusion center providers can offload an overburdened system,” he said.
Dr. Sengupta, who has assisted on COVID-19 inpatient wards at New York University as a volunteer, is also leading a trial of a stem cell-derived therapy developed by Israel-based Pluristem Therapeutics, to treat New York–area patients severely ill from COVID-19 infection. “There are reports from Wuhan, China, in which clinicians are delivering IV mesenchymal stem cells to patients who are on mechanical ventilators, and the patients are getting better,” he said. “I have initiated a study in which we have three cohorts: One is the outpatient setting in which we are trying to treat COVID-19 patients who have hypoxia but have been turned away from overwhelmed EDs and need some therapy. We will be converting one of our infusion centers to conduct this trial. We are also going to be administering this [stem cell-derived therapy] to COVID-19 patients in ICUs, in EDs, and on med-surg floors throughout the city.”
Infusion center directors shuffle treatment services in the era of COVID-19
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
When to treat, delay, or omit breast cancer therapy in the face of COVID-19
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
CDC issues new return-to-work guidelines
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
The Centers for Disease Control and Prevention is releasing new guidance on return-to-work rules for critical workers exposed to a COVID-19 case, or a suspected case, replacing previous guidance to stay home for 14 days.
“One of the most important things we can do is keep our critical workforce working,” CDC Director Robert Redfield said at a White House briefing on April 8. “In certain circumstances they can go back to work,” he said.
Neither Redfield nor the other governmental officials specified what counts as an essential worker, although it has generally referred to food-service and health care workers.
They must take their temperature before work, wear a facial mask at all times and practice social distancing when at work, the new guidance says. They cannot share headsets or other objects used near the face.
Employers must take the worker’s temperature and assess each one for symptoms before work starts, sending a worker home if he or she is sick. Employers must increase the cleaning of frequently used surfaces, increase air exchange in the building and test the use of face masks to be sure they do not interfere with workflow.
Pressed on whether he would reopen the country at the end of the 30-day Stop the Spread effort on April 30 — since one model has revised the U.S. death toll down from 100,000-240,000 to 61,000 — President Donald Trump said meetings will take place soon to discuss the decision and that he will ‘’rely very heavily” on health experts.
“We know now for sure that the mitigation we have been doing is having a positive effect,” said Anthony Fauci, MD, a coronavirus task force member and director of the National Institute of Allergy and Infectious Diseases.
This article first appeared on WebMD.
Low-risk TAVR loses ground at 2 years in PARTNER 3
Transcatheter aortic valve replacement (TAVR) continued to show superiority over surgical replacement in terms of the primary composite endpoint in low-surgical-risk patients at 2 years of follow-up in the landmark randomized PARTNER 3 trial, but the between-group differences favoring the transcatheter procedure in some key outcomes have narrowed considerably, Michael J. Mack, MD, reported in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“On the basis of 1-year data, many physicians were counseling patients that TAVR outcomes were better than surgery. Now we see that the outcomes are roughly the same at 2 years,” said Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
PARTNER 3 randomized 1,000 patients with severe symptomatic aortic stenosis with a tricuspid valve and a very low mean Society of Thoracic Surgeons risk score of 1.9% to TAVR with the Sapien 3 valve or surgical aortic valve replacement (SAVR). The 1-year results presented at ACC 2019 caused a huge stir, with the primary composite outcome of death, stroke, or cardiovascular rehospitalization occurring in 8.5% of TAVR patients and 15.6% of the SAVR group, representing a 48% relative risk reduction and a resounding win for TAVR (N Engl J Med. 2019 May 2;380:1695-705). At 2 years, the difference in the composite outcome remained statistically significant, but the gap had closed: 11.5% with TAVR and 17.4% with SAVR for a 37% relative risk reduction.
Moreover, the between-group difference in stroke, which at 1 year was significantly in favor of TAVR at 1.2% versus 3.3%, was no longer significant at 2 years, with rates of 2.4% versus 3.6%. Nor was the difference in mortality significant: 2.4% with TAVR, 3.2% with SAVR.
What was a statistically significant between-group difference at 2 years – and an eye-catching one at that – involved the cumulative incidence of valve thrombosis confirmed by CT or echocardiography: 2.6% in the TAVR arm, compared with 0.7% with SAVR, with most of these unwanted events coming in year 2.
The good news was there was no echocardiographic evidence of deterioration in valve structure or function in either study arm at 2 years. The mean gradients and aortic valve areas remained unchanged in both arms between 1 and 2 years, as did the frequency of mild or moderate paravalvular leak. Prospective follow-up will continue annually out to 10 years.
“I think it’s way too early to expect to see a signal, but I think it’s somewhat comforting at this point that there’s no signal of early structural valve deterioration,” Dr. Mack said.
Discussant Howard C. Hermann, MD, commented: “I guess the biggest concern in looking at the data is the increase in stroke and valve thrombosis, both numerically and relative to SAVR, between years 1 and 2.”
Dr. Mack offered a note of reassurance regarding the valve thrombosis findings: The rates he presented were based upon the now-outdated second Valve Academic Research Consortium (VARC-2) definition, per study protocol. When he and his coinvestigators recalculated the valve thrombosis rates using the contemporary VARC-3 definition of valve deterioration and bioprosthetic valve failure, the incidence was very low and not significantly different in the two study arms, at roughly 1%.
Dr. Hermann, professor of medicine and director of the cardiac catheterization laboratories at the University of Pennsylvania, Philadelphia, had a question: As a clinician taking care of TAVR patients, what clinical or hemodynamic findings should prompt an imaging study looking for valve thrombus or deterioration that might prompt initiating oral anticoagulation?
“If there’s a change in hemodynamics, an increasing valve gradient, if there’s increasing paravalvular leak, or if there’s a change in symptoms, that should prompt an imaging study. Only with confirmation of valve thrombosis on an imaging study should anticoagulation be considered. Oral anticoagulation is not benign: Of the six clinical events associated with valve thrombosis in the study, two were related to anticoagulation,” Dr. Mack replied.
“Regarding whether patients should receive warfarin or a novel anticoagulant, I don’t think we have evidence that there’s benefit to anything other than warfarin at the current time,” he added.
Dr. Mack reported receiving research support from Edwards Lifesciences, the sponsor of PARTNER 3, as well as from Abbott, Gore, and Medtronic.
Transcatheter aortic valve replacement (TAVR) continued to show superiority over surgical replacement in terms of the primary composite endpoint in low-surgical-risk patients at 2 years of follow-up in the landmark randomized PARTNER 3 trial, but the between-group differences favoring the transcatheter procedure in some key outcomes have narrowed considerably, Michael J. Mack, MD, reported in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“On the basis of 1-year data, many physicians were counseling patients that TAVR outcomes were better than surgery. Now we see that the outcomes are roughly the same at 2 years,” said Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
PARTNER 3 randomized 1,000 patients with severe symptomatic aortic stenosis with a tricuspid valve and a very low mean Society of Thoracic Surgeons risk score of 1.9% to TAVR with the Sapien 3 valve or surgical aortic valve replacement (SAVR). The 1-year results presented at ACC 2019 caused a huge stir, with the primary composite outcome of death, stroke, or cardiovascular rehospitalization occurring in 8.5% of TAVR patients and 15.6% of the SAVR group, representing a 48% relative risk reduction and a resounding win for TAVR (N Engl J Med. 2019 May 2;380:1695-705). At 2 years, the difference in the composite outcome remained statistically significant, but the gap had closed: 11.5% with TAVR and 17.4% with SAVR for a 37% relative risk reduction.
Moreover, the between-group difference in stroke, which at 1 year was significantly in favor of TAVR at 1.2% versus 3.3%, was no longer significant at 2 years, with rates of 2.4% versus 3.6%. Nor was the difference in mortality significant: 2.4% with TAVR, 3.2% with SAVR.
What was a statistically significant between-group difference at 2 years – and an eye-catching one at that – involved the cumulative incidence of valve thrombosis confirmed by CT or echocardiography: 2.6% in the TAVR arm, compared with 0.7% with SAVR, with most of these unwanted events coming in year 2.
The good news was there was no echocardiographic evidence of deterioration in valve structure or function in either study arm at 2 years. The mean gradients and aortic valve areas remained unchanged in both arms between 1 and 2 years, as did the frequency of mild or moderate paravalvular leak. Prospective follow-up will continue annually out to 10 years.
“I think it’s way too early to expect to see a signal, but I think it’s somewhat comforting at this point that there’s no signal of early structural valve deterioration,” Dr. Mack said.
Discussant Howard C. Hermann, MD, commented: “I guess the biggest concern in looking at the data is the increase in stroke and valve thrombosis, both numerically and relative to SAVR, between years 1 and 2.”
Dr. Mack offered a note of reassurance regarding the valve thrombosis findings: The rates he presented were based upon the now-outdated second Valve Academic Research Consortium (VARC-2) definition, per study protocol. When he and his coinvestigators recalculated the valve thrombosis rates using the contemporary VARC-3 definition of valve deterioration and bioprosthetic valve failure, the incidence was very low and not significantly different in the two study arms, at roughly 1%.
Dr. Hermann, professor of medicine and director of the cardiac catheterization laboratories at the University of Pennsylvania, Philadelphia, had a question: As a clinician taking care of TAVR patients, what clinical or hemodynamic findings should prompt an imaging study looking for valve thrombus or deterioration that might prompt initiating oral anticoagulation?
“If there’s a change in hemodynamics, an increasing valve gradient, if there’s increasing paravalvular leak, or if there’s a change in symptoms, that should prompt an imaging study. Only with confirmation of valve thrombosis on an imaging study should anticoagulation be considered. Oral anticoagulation is not benign: Of the six clinical events associated with valve thrombosis in the study, two were related to anticoagulation,” Dr. Mack replied.
“Regarding whether patients should receive warfarin or a novel anticoagulant, I don’t think we have evidence that there’s benefit to anything other than warfarin at the current time,” he added.
Dr. Mack reported receiving research support from Edwards Lifesciences, the sponsor of PARTNER 3, as well as from Abbott, Gore, and Medtronic.
Transcatheter aortic valve replacement (TAVR) continued to show superiority over surgical replacement in terms of the primary composite endpoint in low-surgical-risk patients at 2 years of follow-up in the landmark randomized PARTNER 3 trial, but the between-group differences favoring the transcatheter procedure in some key outcomes have narrowed considerably, Michael J. Mack, MD, reported in a video presentation of his research during the joint scientific sessions of the American College of Cardiology and the World Heart Federation, which was presented online this year. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“On the basis of 1-year data, many physicians were counseling patients that TAVR outcomes were better than surgery. Now we see that the outcomes are roughly the same at 2 years,” said Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
PARTNER 3 randomized 1,000 patients with severe symptomatic aortic stenosis with a tricuspid valve and a very low mean Society of Thoracic Surgeons risk score of 1.9% to TAVR with the Sapien 3 valve or surgical aortic valve replacement (SAVR). The 1-year results presented at ACC 2019 caused a huge stir, with the primary composite outcome of death, stroke, or cardiovascular rehospitalization occurring in 8.5% of TAVR patients and 15.6% of the SAVR group, representing a 48% relative risk reduction and a resounding win for TAVR (N Engl J Med. 2019 May 2;380:1695-705). At 2 years, the difference in the composite outcome remained statistically significant, but the gap had closed: 11.5% with TAVR and 17.4% with SAVR for a 37% relative risk reduction.
Moreover, the between-group difference in stroke, which at 1 year was significantly in favor of TAVR at 1.2% versus 3.3%, was no longer significant at 2 years, with rates of 2.4% versus 3.6%. Nor was the difference in mortality significant: 2.4% with TAVR, 3.2% with SAVR.
What was a statistically significant between-group difference at 2 years – and an eye-catching one at that – involved the cumulative incidence of valve thrombosis confirmed by CT or echocardiography: 2.6% in the TAVR arm, compared with 0.7% with SAVR, with most of these unwanted events coming in year 2.
The good news was there was no echocardiographic evidence of deterioration in valve structure or function in either study arm at 2 years. The mean gradients and aortic valve areas remained unchanged in both arms between 1 and 2 years, as did the frequency of mild or moderate paravalvular leak. Prospective follow-up will continue annually out to 10 years.
“I think it’s way too early to expect to see a signal, but I think it’s somewhat comforting at this point that there’s no signal of early structural valve deterioration,” Dr. Mack said.
Discussant Howard C. Hermann, MD, commented: “I guess the biggest concern in looking at the data is the increase in stroke and valve thrombosis, both numerically and relative to SAVR, between years 1 and 2.”
Dr. Mack offered a note of reassurance regarding the valve thrombosis findings: The rates he presented were based upon the now-outdated second Valve Academic Research Consortium (VARC-2) definition, per study protocol. When he and his coinvestigators recalculated the valve thrombosis rates using the contemporary VARC-3 definition of valve deterioration and bioprosthetic valve failure, the incidence was very low and not significantly different in the two study arms, at roughly 1%.
Dr. Hermann, professor of medicine and director of the cardiac catheterization laboratories at the University of Pennsylvania, Philadelphia, had a question: As a clinician taking care of TAVR patients, what clinical or hemodynamic findings should prompt an imaging study looking for valve thrombus or deterioration that might prompt initiating oral anticoagulation?
“If there’s a change in hemodynamics, an increasing valve gradient, if there’s increasing paravalvular leak, or if there’s a change in symptoms, that should prompt an imaging study. Only with confirmation of valve thrombosis on an imaging study should anticoagulation be considered. Oral anticoagulation is not benign: Of the six clinical events associated with valve thrombosis in the study, two were related to anticoagulation,” Dr. Mack replied.
“Regarding whether patients should receive warfarin or a novel anticoagulant, I don’t think we have evidence that there’s benefit to anything other than warfarin at the current time,” he added.
Dr. Mack reported receiving research support from Edwards Lifesciences, the sponsor of PARTNER 3, as well as from Abbott, Gore, and Medtronic.
FROM ACC 2020
Home-based chemo skyrockets at one U.S. center
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Major organization opposes concept
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
The 7 strategies of highly effective people facing the COVID-19 pandemic
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
A few weeks ago, I saw more than 60 responses to a post on Nextdoor.com entitled, “Toilet paper strategies?”
Asking for help is a great coping mechanism when one is struggling to find a strategy, even if it’s for toilet paper. What other kinds of coping strategies can help us through this historic and unprecedented time?
The late Stephen R. Covey, PhD, wrote about the coping strategies of highly effective people in his book, “The 7 Habits of Highly Effective People.”1 For, no matter how smart, perfect, or careful you may be, life will never be trouble free. When trouble comes, it’s important to have coping strategies that help you navigate through choppy waters. Whether you are a practitioner trying to help your patients or someone who wants to maximize their personal resilience during a worldwide pandemic, here are my conceptualizations of the seven top strategies highly effective people use when facing challenges.
Strategy #1: Begin with the end in mind
In 2007, this strategy helped me not only survive but thrive when I battled for my right to practice as a holistic psychiatrist against the Maryland Board of Physicians.2 From the first moment when I read the letter from the board, to the last when I read the administrative law judge’s dismissal, I turned to this strategy to help me cope with unrelenting stress.
I imagined myself remembering being the kind of person I wanted to be, wrote that script for myself, and created those memories for my future self. I wanted to remember myself as being brave, calm, strong, and grounded, so I behaved each day as if I were all of those things.
As Dr. Covey wrote, “ ‘Begin with the end in mind’ is based on the principle that all things are created twice. There’s a mental or first creation, and a physical or second creation to all things.” Imagine who you would like to remember yourself being a year or two down the road. Do you want to remember yourself showing good judgment and being positive and compassionate during this pandemic? Then, follow the script you’ve created in your mind and be that person now, knowing that you are forming memories for your future self. Your future self will look back at who you are right now with appreciation and satisfaction. Of course, this is a habit that you can apply to your entire life.
Strategy #2: Be proactive
Between the event and the outcome is you. You are the interpreter and transformer of the event, with the freedom to apply your will and intention on the event. Whether it is living through a pandemic or dealing with misplaced keys, every day you are revealing your nature through how you deal with life. To be proactive is different from being reactive. Within each of us there is a will, the drive, to rise above our difficult environments.
Dr. Covey wrote, “the ability to subordinate an impulse to a value is the essence of the proactive person.” A woman shared with me that she created an Excel spreadsheet with some of the things she plans to do with her free time while she stays in her NYC apartment. She doesn’t want to slip into a passive state and waste her time. That’s being proactive.
Strategy #3: Set proper priorities
Or, as Dr. Covey would say, “Put first things first.” During a pandemic, when the world seems to be precariously tilting at an angle, it’s easy to cling to outdated standards, expectations, and behavioral patterns. Doing so heightens our sense of regret, fear, and scarcity. Valuing gratitude will empower you to deal with financial loss differently because you can still remain grateful despite uncontrollable losses. We can choose “to have or to be” as psychoanalyst, Erich Fromm, PhD, would say.3 If your happiness is measured by how much money you have, then it would make sense that, when the amount shrinks, so does your happiness. However, if your happiness is a side effect of who you are, you will remain a mountain before the winds and tides of circumstance.
Strategy #4: Create a win/win mentality
This state of mind is built on character. Dr. Covey separates character into three categories: integrity, maturity, and abundance mentality. A lack of character resulted in the hoarding of toilet paper in many communities and the cry for help from Nextdoor.com. I noticed that, in the 60+ responses that included advice about using bidets, old towels, and even leaves, no one offered to share a bag of toilet paper. That’s because people experienced the fear of scarcity, in turn, causing the scarcity they feared.
During a pandemic, a highly effective person or company thinks beyond themselves to create a win/win scenario. At a grocery store in my neighborhood, a man stands at its entrance with a bottle of disinfectant spray in one hand for the shoppers and a sign on the sidewalk with guidelines for purchasing products to avoid hoarding. He tells you where the wipes are for the carts as you enter the store. People line up 6 feet apart, waiting to enter, to limit the number of shoppers inside the store, facilitating proper physical distancing. Instead of maximizing profits at the expense of everyone’s health and safety, the process is a win/win for everyone, from shoppers to employees.
Strategy #5: Develop empathy and understanding
Seeking to first understand and then be understood is one of the most powerful tools of effective people. In my holistic practice, every patient comes in with their own unique needs that evolve and transform over time. I must remain open, or I fail to deliver appropriately.
Learning to listen and then to clearly communicate ideas is essential to effective health care. During this time, it is critical that health care providers and political leaders first listen/understand and then communicate clearly to serve everyone in the best way possible.
In our brains, the frontal lobes (the adult in the room) manages our amygdala (the child in the room) when we get enough sleep, meditate, spend time in nature, exercise, and eat healthy food.4 Stress can interfere with the frontal lobe’s ability to maintain empathy, inhibit unhealthy impulses, and delay gratification. During the pandemic, we can help to shift from the stress response, or “fight-or-flight” response, driven by the sympathetic nervous system to a “rest-and-digest” response driven by the parasympathetic system through coherent breathing, taking slow, deep, relaxed breaths (6 seconds on inhalation and 6 seconds on exhalation). The vagus nerve connected to our diaphragm will help the heart return to a healthy rhythm.5
Strategy #6: Synergize and integrate
All of life is interdependent, each part no more or less important than any other. Is oxygen more important than hydrogen? Is H2O different from the oxygen and hydrogen atoms that make it?
During a pandemic, it’s important for us to appreciate each other’s contributions and work synergistically for the good of the whole. Our survival depends on valuing each other and our planet. This perspective informs the practice of physical distancing and staying home to minimize the spread of the virus and its impact on the health care system, regardless of whether an individual belongs in the high-risk group or not.
Many high-achieving people train in extremely competitive settings in which survival depends on individual performance rather than mutual cooperation. This training process encourages a disregard for others. Good leaders, however, understand that cooperation and mutual respect are essential to personal well-being.
Strategy #7: Practice self-care
There are five aspects of our lives that depend on our self-care: spiritual, mental, emotional, physical, and social. Unfortunately, many kind-hearted people are kinder to others than to themselves. There is really only one person who can truly take care of you properly, and that is yourself. In Seattle, where many suffered early in the pandemic, holistic psychiatrist David Kopacz, MD, is reminding people to nurture themselves in his post, “Nurture Yourself During the Pandemic: Try New Recipes!”6 Indeed, that is what many must do since eating out is not an option now. If you find yourself stuck at home with more time on your hands, take the opportunity to care for yourself. Ask yourself what you really need during this time, and make the effort to provide it to yourself.
After the pandemic is over, will you have grown from the experiences and become a better person from it? Despite our current circumstances, we can continue to grow as individuals and as a community, armed with strategies that can benefit all of us.
References
1. Covey SR. The 7 Habits of Highly Effective People. New York: Simon & Schuster; 1989.
2. Lee AW. Townsend Letter. 2009 Jun;311:22-3.
3. Fromm E. To Have or To Be? New York: Continuum International Publishing; 2005.
4. Rushlau K. Integrative Healthcare Symposium. 2020 Feb 21.
5. Gerbarg PL. Mind Body Practices for Post-Traumatic Stress Disorder. Presentation at Integrative Medicine for Mental Health Conference. 2016 Sep.
6. Kopacz D. Nurture Yourself During the Pandemic: Try New Recipes! Being Fully Human. 2020 Mar 22.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
CDC: Screen nearly all adults, including pregnant women, for HCV
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
In the latest issue of the Morbidity and Mortality Weekly Report, the Centers for Disease Control and Prevention recommended hepatitis C virus screening for all adults and all pregnant women – during each of their pregnancies – in areas where prevalence of the infection is 0.1% or greater.
That’s essentially the entire United States; there’s no state with a statewide adult prevalence below 0.1%, and “few settings are known to exist” otherwise, the CDC noted (MMWR Recomm Rep. 2020 Apr 10;69(2):1-17).
The agency encouraged providers to consult state or local health departments or the CDC directly to determine local HCV prevalence. “As a general guide ... approximately 59% of anti-HCV positive persons are HCV RNA positive,” indicating active infection, the agency noted.
The advice was an expansion from the CDC’s last universal screening recommendation in 2012, which was limited to people born from 1945 to 1965; the incidence of acute infections has climbed since then and is highest now among younger people, so the guideline needed to be revisited, explained authors led by Sarah Schillie, MD, of the CDC’s Division of Viral Hepatitis, Atlanta.
The U.S. Preventive Services Task Force also recently recommended universal adult screening after previously limiting it to baby boomers.
As for pregnancy, the CDC’s past advice was to screen pregnant women with known risk factors, but that needed to be revisited as well. For one thing, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America have since recommended testing all pregnant women.
But also, the CDC said, it’s an opportune time for screening because “many women only have access to health care during pregnancy and the immediate postpartum period,” when treatment, if needed, can be started. Plus, HCV status is important for management decisions, such as using amniocentesis in positive women instead of chorionic villus sampling.
The rest of CDC’s 2012 recommendations stand, including screening all people with risk factors and repeating screening while they persist. Also, “any person who requests hepatitis C testing should receive it, regardless of disclosure of risk,” because people might be reluctant to report things like IV drug use, the authors said.
Screening in the guidelines means an HCV antibody test, followed by a nucleic acid test to check for active infection. The CDC encouraged automatic reflex testing, meaning immediately checking antibody positive samples for HCV RNA. RNA in the blood indicates active, replicating virus.
The new recommendations penciled out in modeling, with an incremental cost-effectiveness ratio (ICER) for universal adult screening of approximately $36,000 per quality-adjusted life year (QALY) gained, and an ICER of approximately $15,000 per QALY gained for pregnancy screening, where HCV prevalence is 0.1%; the 0.1% cost/benefit cutpoint was one of the reasons it was chosen as the prevalence threshold. An ICER under $50,000 is the conservative benchmark for cost-effectiveness, the authors noted.
There was no external funding, and the authors had no disclosures.
SOURCE: Schillie S et al. MMWR Recomm Rep. 2020 Apr 10;69[2]:1-17).
Almost 90% of COVID-19 admissions involve comorbidities
The hospitalization rate for COVID-19 is 4.6 per 100,000 population, and almost 90% of hospitalized patients have some type of underlying condition, according to the Centers for Disease Control and Prevention.
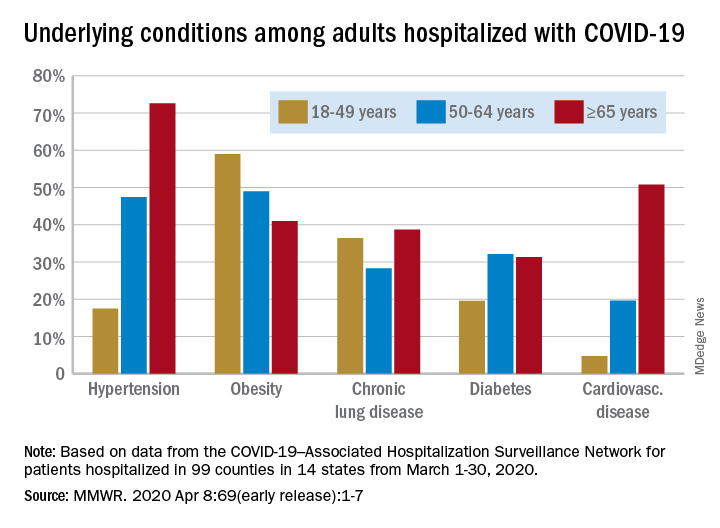
Data collected by the newly created COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) put the exact prevalence of underlying conditions at 89.3% for patients hospitalized during March 1-30, 2020, Shikha Garg, MD, of the CDC’s COVID-NET team and associates wrote in the MMWR.
The hospitalization rate, based on COVID-NET data for March 1-28, increased with patient age. Those aged 65 years and older were admitted at a rate of 13.8 per 100,000, with 50- to 64-year-olds next at 7.4 per 100,000 and 18- to 49-year-olds at 2.5, they wrote.
The patients aged 65 years and older also were the most likely to have one or more underlying conditions, at 94.4%, compared with 86.4% of those aged 50-64 years and 85.4% of individuals who were aged 18-44 years, the investigators reported.
Hypertension was the most common comorbidity among the oldest patients, with a prevalence of 72.6%, followed by cardiovascular disease at 50.8% and obesity at 41%. In the two younger groups, obesity was the condition most often seen in COVID-19 patients, with prevalences of 49% in 50- to 64-year-olds and 59% in those aged 18-49, Dr. Garg and associates wrote.
“These findings underscore the importance of preventive measures (e.g., social distancing, respiratory hygiene, and wearing face coverings in public settings where social distancing measures are difficult to maintain) to protect older adults and persons with underlying medical conditions,” the investigators wrote.
COVID-NET surveillance includes laboratory-confirmed hospitalizations in 99 counties in 14 states: California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah. Those counties represent about 10% of the U.S. population.
SOURCE: Garg S et al. MMWR. 2020 Apr 8;69(early release):1-7.
The hospitalization rate for COVID-19 is 4.6 per 100,000 population, and almost 90% of hospitalized patients have some type of underlying condition, according to the Centers for Disease Control and Prevention.

Data collected by the newly created COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) put the exact prevalence of underlying conditions at 89.3% for patients hospitalized during March 1-30, 2020, Shikha Garg, MD, of the CDC’s COVID-NET team and associates wrote in the MMWR.
The hospitalization rate, based on COVID-NET data for March 1-28, increased with patient age. Those aged 65 years and older were admitted at a rate of 13.8 per 100,000, with 50- to 64-year-olds next at 7.4 per 100,000 and 18- to 49-year-olds at 2.5, they wrote.
The patients aged 65 years and older also were the most likely to have one or more underlying conditions, at 94.4%, compared with 86.4% of those aged 50-64 years and 85.4% of individuals who were aged 18-44 years, the investigators reported.
Hypertension was the most common comorbidity among the oldest patients, with a prevalence of 72.6%, followed by cardiovascular disease at 50.8% and obesity at 41%. In the two younger groups, obesity was the condition most often seen in COVID-19 patients, with prevalences of 49% in 50- to 64-year-olds and 59% in those aged 18-49, Dr. Garg and associates wrote.
“These findings underscore the importance of preventive measures (e.g., social distancing, respiratory hygiene, and wearing face coverings in public settings where social distancing measures are difficult to maintain) to protect older adults and persons with underlying medical conditions,” the investigators wrote.
COVID-NET surveillance includes laboratory-confirmed hospitalizations in 99 counties in 14 states: California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah. Those counties represent about 10% of the U.S. population.
SOURCE: Garg S et al. MMWR. 2020 Apr 8;69(early release):1-7.
The hospitalization rate for COVID-19 is 4.6 per 100,000 population, and almost 90% of hospitalized patients have some type of underlying condition, according to the Centers for Disease Control and Prevention.

Data collected by the newly created COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) put the exact prevalence of underlying conditions at 89.3% for patients hospitalized during March 1-30, 2020, Shikha Garg, MD, of the CDC’s COVID-NET team and associates wrote in the MMWR.
The hospitalization rate, based on COVID-NET data for March 1-28, increased with patient age. Those aged 65 years and older were admitted at a rate of 13.8 per 100,000, with 50- to 64-year-olds next at 7.4 per 100,000 and 18- to 49-year-olds at 2.5, they wrote.
The patients aged 65 years and older also were the most likely to have one or more underlying conditions, at 94.4%, compared with 86.4% of those aged 50-64 years and 85.4% of individuals who were aged 18-44 years, the investigators reported.
Hypertension was the most common comorbidity among the oldest patients, with a prevalence of 72.6%, followed by cardiovascular disease at 50.8% and obesity at 41%. In the two younger groups, obesity was the condition most often seen in COVID-19 patients, with prevalences of 49% in 50- to 64-year-olds and 59% in those aged 18-49, Dr. Garg and associates wrote.
“These findings underscore the importance of preventive measures (e.g., social distancing, respiratory hygiene, and wearing face coverings in public settings where social distancing measures are difficult to maintain) to protect older adults and persons with underlying medical conditions,” the investigators wrote.
COVID-NET surveillance includes laboratory-confirmed hospitalizations in 99 counties in 14 states: California, Colorado, Connecticut, Georgia, Iowa, Maryland, Michigan, Minnesota, New Mexico, New York, Ohio, Oregon, Tennessee, and Utah. Those counties represent about 10% of the U.S. population.
SOURCE: Garg S et al. MMWR. 2020 Apr 8;69(early release):1-7.
FROM THE MMWR