User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
FDA okays new CAR T therapy, first for mantle cell lymphoma
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration granted accelerated approval to brexucabtagene autoleucel (Tecartus, Kite Pharma), the first approved chimeric antigen receptor (CAR) T cell therapy for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
The new agent is the second approved CAR T cell product developed by Kite and follows the 2017 approval of axicabtagene ciloleucel (Yescarta) for diffuse large B-cell lymphoma.
“Despite promising advances, there are still major gaps in treatment for patients with MCL who progress following initial therapy,” investigator Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston, said in a company statement. “Many patients have high-risk disease and are more likely to keep progressing, even after subsequent treatments.”
In the same press statement, Meghan Gutierrez, chief executive officer, Lymphoma Research Foundation, said: “This approval marks the first CAR T cell therapy approved for mantle cell lymphoma patients and represents a new frontier in the treatment of this disease.”
The approval of the single-infusion therapy is based on efficacy and safety data from the ongoing, single-arm ZUMA-2 pivotal trial, which enrolled 74 adult patients. All patients had previously received anthracycline- or bendamustine-containing chemotherapy, an anti-CD20 antibody therapy and a Bruton tyrosine kinase inhibitor (ibrutinib or acalabrutinib).
In the trial, there was an objective response rate, which was the primary outcome measure, of 87% among 60 patients who were evaluable for efficacy analysis; 62% had a complete response.
Among all patients, follow-up was at least 6 months after their first objective disease response. Median duration of response has not yet been reached.
In terms of adverse events, 18% of the 82 patients evaluable for safety experienced > grade 3 cytokine release syndrome and 37% experienced neurologic events, per the company statement. The most common (≥ 10%) grade 3 or higher adverse reactions were anemia, neutropenia, thrombocytopenia, hypotension, hypophosphatemia, encephalopathy, leukopenia, hypoxia, pyrexia, hyponatremia, hypertension, infection-pathogen unspecified, pneumonia, hypocalcemia, and lymphopenia.
Brexucabtagene autoleucel will be manufactured in Kite’s facility in California. In the pivotal trial, there was a 96% manufacturing success rate and a median manufacturing turnaround time of 15 days from leukapheresis to product delivery.
A version of this article originally appeared on Medscape.com.
US News releases latest top hospitals list, adds COVID heroes
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
This year’s rankings include special recognition of the “herculean efforts” by the nation’s healthcare professionals in fighting COVID-19, often at great personal risk.
“The US News Hospital Heroes series is a cornerstone of this year’s rankings package, profiling more than 65 health care heroes from across the country, along with commentary from top executives at hospitals who faced the pandemic head on,” a news release from the magazine explains.
“The pandemic has altered, perhaps permanently, how patients get care and from whom they get it. Amid the disruption, we are steadfastly committed to providing the public with authoritative data for comparing hospital quality,” Ben Harder, managing editor and chief of health analysis at US News, said in the release.
“No hospital’s clinical team came through this unprecedented health crisis unscathed. Our Hospital Heroes series is a tribute to recognizing individuals at urban and rural hospitals in communities across the country who have gone above and beyond during this unparalleled time in history,” said Harder.
Mayo Clinic Still Number One
Following Mayo Clinic, Cleveland Clinic in Ohio takes the number two spot this year (up from number four last year) in the magazine’s annual honor roll, which highlights hospitals that deliver “exceptional treatment across multiple areas of care.”
Johns Hopkins Hospital in Baltimore, Maryland, holds the number three spot, while New York-Presbyterian Hospital–Columbia and Cornell in New York City and UCLA Medical Center, Los Angeles, tie for the number four spot.
Massachusetts General Hospital in Boston, which held the number two spot last year, has fallen to number six. Rounding out the top 10, in order, are Cedars-Sinai Medical Center, Los Angeles; UCSF Medical Center, San Francisco; NYU Langone Hospitals, New York City; Northwestern Memorial Hospital, Chicago, Illinois.
2020–2021 Best Hospitals Honor Roll
1. Mayo Clinic, Rochester, Minnesota
2. Cleveland Clinic, Ohio
3. Johns Hopkins Hospital, Baltimore, Maryland
4. (tie) New York–Presbyterian Hospital–Columbia and Cornell, New York City
4. (tie) UCLA Medical Center, Los Angeles
6. Massachusetts General Hospital, Boston
7. Cedars-Sinai Medical Center, San Francisco
8. UCSF Medical Center, San Francisco
9. NYU Langone Hospitals, New York, New York City
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor
12. Brigham and Women’s Hospital, Boston
13. Stanford Health Care–Stanford Hospital, Palo Alto, California
14. Mount Sinai Hospital, New York City
15. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
16. Mayo Clinic–Phoenix
17. Rush University Medical Center, Chicago
18. (tie) Barnes-Jewish Hospital, Saint Louis
18. (tie) Keck Hospital of USC, Los Angeles
20. Houston Methodist Hospital, Texas
In the 2020–2021 Best Hospitals: Specialty Rankings, University of Texas MD Anderson Cancer Center continues to hold the number one spot in cancer, the Hospital for Special Surgery is number one in orthopedics, and the Cleveland Clinic is number one in cardiology and heart surgery.
For this year’s rankings, US News developed a new cardiac rating that measures the quality of hospitals› transcatheter aortic valve replacement, which is rapidly being adopted as a minimally invasive alternative to aortic valve surgery.
Top Five for Cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York City
3. Mayo Clinic, Rochester, Minnesota
4. Johns Hopkins Hospital, Baltimore, Maryland
5. Cleveland Clinic, Ohio
Top Five for Cardiology and Heart Surgery
1. Cleveland Clinic, Ohio
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, NYC
5. Massachusetts General Hospital, Boston
Top Five for Orthopedics
1. Hospital for Special Surgery, New York City
2. Mayo Clinic, Rochester, Minnesota
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York City
5. Rush University Medical Center, Chicago
For the 2020–2021 rankings and ratings, US News compared more than 4500 medical centers across the country in 16 specialties and 10 procedures and conditions. Of these, 563 were recognized as Best Regional Hospitals on the basis of their strong performance in multiple areas of care. The top 20 hospitals, which deliver exceptional treatment across many areas of care, were also named to the honor roll.
The magazine notes that data for the 2020–2021 Best Hospitals rankings and ratings come from a period predating the COVID-19 pandemic and were not affected by the pandemic’s impact on hospitals. The methodologies are based largely on objective measures, such as risk-adjusted survival and discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report on hospital ranking is available online.
This article first appeared on Medscape.com.
Hypertension medication adjustment less likely with polypill
A secondary analysis of a major study of polypill therapy for hypertension found that patients who don’t reach blood pressure targets are less likely to have their medications adjusted if they’re on fixed-dose combination therapy.
However, hypertension patients on low-dose, triple-pill combination therapy are more likely to achieve blood pressure control than are those on usual care.
The secondary analysis of Triple Pill vs. Usual Care Management for Patients with Mild-to-Moderate Hypertension (TRIUMPH) was published online in JAMA Cardiology (2020 Jul 22. doi: 10.1001/jamacardio.2020.2739). The trial randomized 700 patients with hypertension in Sri Lanka to triple-pill fixed-dose combination (FDC) therapy or usual care during February 2016–May 2017, with follow-up ending in October 2017.
A greater proportion of FDC patients reached target BP by the end of the study compared with usual care, 70% vs. 55%. However, the study found that therapeutic inertia – the failure to intensify therapy in nonresponsive patients – was more common in the FDC group at 6- and 12-week follow-up: 87% vs. 64% and 90% vs. 65%, respectively; both differences were significant different at P < .001).
The once-daily FDC pill contained telmisartan 20 mg, amlodipine 2.5 mg; and chlorthalidone 12.5 mg.
“Using a triple low-dose combination blood-pressure pill reduced the need to uptitrate BP therapy as more patients are at target, but doctors were less likely to uptitrate with triple-pill therapy when it was needed,” lead author Nelson Wang, MD, a research fellow at the George Institute for Global Health in suburban Sydney, said in an interview.
“Overall, there were fewer treatment inertia episodes in the triple-pill group than in the usual care group, but this was driven by the fact that fewer triple-pill patients needed uptitration when coming to their follow-up visits,” Dr. Wang added.
The analysis found that clinicians who prescribed triple-pill FDC used 23 unique drug treatment regimens per 100 treated patients compared with 54 different regiments with usual care (P < .001). “There was a large simplification in care,” Dr. Wang said of the FDC approach.
Dr. Wang and colleagues called for greater efforts to address therapeutic inertia, particularly with FDC therapies, and suggested potential strategies consisting of patient education, incentives for appropriate treatment adjustments, and feedback mechanisms and reminders for physicians.
“There may also be a need for more dosage options with the FDC triple pill to allow physicians to intensify therapy without fear of overtreatment and adverse drug effects,” they wrote.
In an accompanying editorial (JAMA Cardiol. 2020 Jul 22. doi: 10.1001/jamacardio.2020.2693), Ann Marie Navar, MD, PhD, associate professor of cardiology at Duke Clinical Research Institute, Durham, N.C., noted that initiating treatment with FDC therapy doesn’t preclude a more personalized approach for patients who don’t achieve their BP target. “The real choice now is the choice of initial treatment,” she wrote, adding that future treatment guidelines should consider extending an FDC-first approach to patients with less severe levels of hypertension.
“The study showed there’s room for a both a population-based fixed-drug combination approach and a personalized approach to how we think about hypertension management with fixed-dose therapy,” she said in an interview. “It’s not a one-and-done situation.”
Dr. Wang has no financial relationships to disclose. Study coauthors received funding from the Australian National Health and Medical Research Council and the U.K. National Institute for Health Research. Dr. Navar has no relevant financial relationships to report.
SOURCE: Wang N et al. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.2739.
A secondary analysis of a major study of polypill therapy for hypertension found that patients who don’t reach blood pressure targets are less likely to have their medications adjusted if they’re on fixed-dose combination therapy.
However, hypertension patients on low-dose, triple-pill combination therapy are more likely to achieve blood pressure control than are those on usual care.
The secondary analysis of Triple Pill vs. Usual Care Management for Patients with Mild-to-Moderate Hypertension (TRIUMPH) was published online in JAMA Cardiology (2020 Jul 22. doi: 10.1001/jamacardio.2020.2739). The trial randomized 700 patients with hypertension in Sri Lanka to triple-pill fixed-dose combination (FDC) therapy or usual care during February 2016–May 2017, with follow-up ending in October 2017.
A greater proportion of FDC patients reached target BP by the end of the study compared with usual care, 70% vs. 55%. However, the study found that therapeutic inertia – the failure to intensify therapy in nonresponsive patients – was more common in the FDC group at 6- and 12-week follow-up: 87% vs. 64% and 90% vs. 65%, respectively; both differences were significant different at P < .001).
The once-daily FDC pill contained telmisartan 20 mg, amlodipine 2.5 mg; and chlorthalidone 12.5 mg.
“Using a triple low-dose combination blood-pressure pill reduced the need to uptitrate BP therapy as more patients are at target, but doctors were less likely to uptitrate with triple-pill therapy when it was needed,” lead author Nelson Wang, MD, a research fellow at the George Institute for Global Health in suburban Sydney, said in an interview.
“Overall, there were fewer treatment inertia episodes in the triple-pill group than in the usual care group, but this was driven by the fact that fewer triple-pill patients needed uptitration when coming to their follow-up visits,” Dr. Wang added.
The analysis found that clinicians who prescribed triple-pill FDC used 23 unique drug treatment regimens per 100 treated patients compared with 54 different regiments with usual care (P < .001). “There was a large simplification in care,” Dr. Wang said of the FDC approach.
Dr. Wang and colleagues called for greater efforts to address therapeutic inertia, particularly with FDC therapies, and suggested potential strategies consisting of patient education, incentives for appropriate treatment adjustments, and feedback mechanisms and reminders for physicians.
“There may also be a need for more dosage options with the FDC triple pill to allow physicians to intensify therapy without fear of overtreatment and adverse drug effects,” they wrote.
In an accompanying editorial (JAMA Cardiol. 2020 Jul 22. doi: 10.1001/jamacardio.2020.2693), Ann Marie Navar, MD, PhD, associate professor of cardiology at Duke Clinical Research Institute, Durham, N.C., noted that initiating treatment with FDC therapy doesn’t preclude a more personalized approach for patients who don’t achieve their BP target. “The real choice now is the choice of initial treatment,” she wrote, adding that future treatment guidelines should consider extending an FDC-first approach to patients with less severe levels of hypertension.
“The study showed there’s room for a both a population-based fixed-drug combination approach and a personalized approach to how we think about hypertension management with fixed-dose therapy,” she said in an interview. “It’s not a one-and-done situation.”
Dr. Wang has no financial relationships to disclose. Study coauthors received funding from the Australian National Health and Medical Research Council and the U.K. National Institute for Health Research. Dr. Navar has no relevant financial relationships to report.
SOURCE: Wang N et al. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.2739.
A secondary analysis of a major study of polypill therapy for hypertension found that patients who don’t reach blood pressure targets are less likely to have their medications adjusted if they’re on fixed-dose combination therapy.
However, hypertension patients on low-dose, triple-pill combination therapy are more likely to achieve blood pressure control than are those on usual care.
The secondary analysis of Triple Pill vs. Usual Care Management for Patients with Mild-to-Moderate Hypertension (TRIUMPH) was published online in JAMA Cardiology (2020 Jul 22. doi: 10.1001/jamacardio.2020.2739). The trial randomized 700 patients with hypertension in Sri Lanka to triple-pill fixed-dose combination (FDC) therapy or usual care during February 2016–May 2017, with follow-up ending in October 2017.
A greater proportion of FDC patients reached target BP by the end of the study compared with usual care, 70% vs. 55%. However, the study found that therapeutic inertia – the failure to intensify therapy in nonresponsive patients – was more common in the FDC group at 6- and 12-week follow-up: 87% vs. 64% and 90% vs. 65%, respectively; both differences were significant different at P < .001).
The once-daily FDC pill contained telmisartan 20 mg, amlodipine 2.5 mg; and chlorthalidone 12.5 mg.
“Using a triple low-dose combination blood-pressure pill reduced the need to uptitrate BP therapy as more patients are at target, but doctors were less likely to uptitrate with triple-pill therapy when it was needed,” lead author Nelson Wang, MD, a research fellow at the George Institute for Global Health in suburban Sydney, said in an interview.
“Overall, there were fewer treatment inertia episodes in the triple-pill group than in the usual care group, but this was driven by the fact that fewer triple-pill patients needed uptitration when coming to their follow-up visits,” Dr. Wang added.
The analysis found that clinicians who prescribed triple-pill FDC used 23 unique drug treatment regimens per 100 treated patients compared with 54 different regiments with usual care (P < .001). “There was a large simplification in care,” Dr. Wang said of the FDC approach.
Dr. Wang and colleagues called for greater efforts to address therapeutic inertia, particularly with FDC therapies, and suggested potential strategies consisting of patient education, incentives for appropriate treatment adjustments, and feedback mechanisms and reminders for physicians.
“There may also be a need for more dosage options with the FDC triple pill to allow physicians to intensify therapy without fear of overtreatment and adverse drug effects,” they wrote.
In an accompanying editorial (JAMA Cardiol. 2020 Jul 22. doi: 10.1001/jamacardio.2020.2693), Ann Marie Navar, MD, PhD, associate professor of cardiology at Duke Clinical Research Institute, Durham, N.C., noted that initiating treatment with FDC therapy doesn’t preclude a more personalized approach for patients who don’t achieve their BP target. “The real choice now is the choice of initial treatment,” she wrote, adding that future treatment guidelines should consider extending an FDC-first approach to patients with less severe levels of hypertension.
“The study showed there’s room for a both a population-based fixed-drug combination approach and a personalized approach to how we think about hypertension management with fixed-dose therapy,” she said in an interview. “It’s not a one-and-done situation.”
Dr. Wang has no financial relationships to disclose. Study coauthors received funding from the Australian National Health and Medical Research Council and the U.K. National Institute for Health Research. Dr. Navar has no relevant financial relationships to report.
SOURCE: Wang N et al. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.2739.
FROM JAMA CARDIOLOGY
COVID-19 fears would keep most Hispanics with stroke, MI symptoms home
More than half of Hispanic adults would be afraid to go to a hospital for a possible heart attack or stroke because they might get infected with SARS-CoV-2, according to a new survey from the American Heart Association.
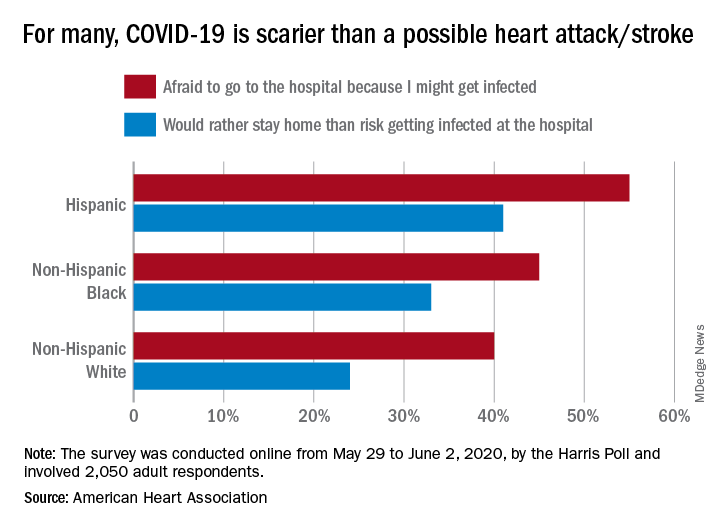
Compared with Hispanic respondents, 55% of whom said they feared COVID-19, significantly fewer Blacks (45%) and Whites (40%) would be scared to go to the hospital if they thought they were having a heart attack or stroke, the AHA said based on the survey of 2,050 adults, which was conducted May 29 to June 2, 2020, by the Harris Poll.
Hispanics also were significantly more likely to stay home if they thought they were experiencing a heart attack or stroke (41%), rather than risk getting infected at the hospital, than were Blacks (33%), who were significantly more likely than Whites (24%) to stay home, the AHA reported.
White respondents, on the other hand, were the most likely to believe (89%) that a hospital would give them the same quality of care provided to everyone else. Hispanics and Blacks had significantly lower rates, at 78% and 74%, respectively, the AHA noted.
These findings are “yet another challenge for Black and Hispanic communities, who are more likely to have underlying health conditions such as cardiovascular disease and diabetes and dying of COVID-19 at disproportionately high rates,” Rafael Ortiz, MD, American Heart Association volunteer medical expert and chief of neuro-endovascular surgery at Lenox Hill Hospital, New York, said in the AHA statement.
The survey was performed in conjunction with the AHA’s “Don’t Die of Doubt” campaign, which “reminds Americans, especially in Hispanic and Black communities, that the hospital remains the safest place to be if experiencing symptoms of a heart attack or a stroke.”
Among all the survey respondents, 57% said they would feel better if hospitals treated COVID-19 patients in a separate area. A number of other possible precautions ranked lower in helping them feel better:
- Screen all visitors, patients, and staff for COVID-19 symptoms when they enter the hospital: 39%.
- Require all patients, visitors, and staff to wear masks: 30%.
- Put increased cleaning protocols in place to disinfect multiple times per day: 23%.
- “Nothing would make me feel comfortable”: 6%.
Despite all the concerns about the risk of coronavirus infection, however, most Americans (77%) still believe that hospitals are the safest place to be in the event of a medical emergency, and 84% said that hospitals are prepared to safely treat emergencies that are not related to the pandemic, the AHA reported.
“Health care professionals know what to do even when things seem chaotic, and emergency departments have made plans behind the scenes to keep patients and healthcare workers safe even during a pandemic,” Dr. Ortiz pointed out.
More than half of Hispanic adults would be afraid to go to a hospital for a possible heart attack or stroke because they might get infected with SARS-CoV-2, according to a new survey from the American Heart Association.

Compared with Hispanic respondents, 55% of whom said they feared COVID-19, significantly fewer Blacks (45%) and Whites (40%) would be scared to go to the hospital if they thought they were having a heart attack or stroke, the AHA said based on the survey of 2,050 adults, which was conducted May 29 to June 2, 2020, by the Harris Poll.
Hispanics also were significantly more likely to stay home if they thought they were experiencing a heart attack or stroke (41%), rather than risk getting infected at the hospital, than were Blacks (33%), who were significantly more likely than Whites (24%) to stay home, the AHA reported.
White respondents, on the other hand, were the most likely to believe (89%) that a hospital would give them the same quality of care provided to everyone else. Hispanics and Blacks had significantly lower rates, at 78% and 74%, respectively, the AHA noted.
These findings are “yet another challenge for Black and Hispanic communities, who are more likely to have underlying health conditions such as cardiovascular disease and diabetes and dying of COVID-19 at disproportionately high rates,” Rafael Ortiz, MD, American Heart Association volunteer medical expert and chief of neuro-endovascular surgery at Lenox Hill Hospital, New York, said in the AHA statement.
The survey was performed in conjunction with the AHA’s “Don’t Die of Doubt” campaign, which “reminds Americans, especially in Hispanic and Black communities, that the hospital remains the safest place to be if experiencing symptoms of a heart attack or a stroke.”
Among all the survey respondents, 57% said they would feel better if hospitals treated COVID-19 patients in a separate area. A number of other possible precautions ranked lower in helping them feel better:
- Screen all visitors, patients, and staff for COVID-19 symptoms when they enter the hospital: 39%.
- Require all patients, visitors, and staff to wear masks: 30%.
- Put increased cleaning protocols in place to disinfect multiple times per day: 23%.
- “Nothing would make me feel comfortable”: 6%.
Despite all the concerns about the risk of coronavirus infection, however, most Americans (77%) still believe that hospitals are the safest place to be in the event of a medical emergency, and 84% said that hospitals are prepared to safely treat emergencies that are not related to the pandemic, the AHA reported.
“Health care professionals know what to do even when things seem chaotic, and emergency departments have made plans behind the scenes to keep patients and healthcare workers safe even during a pandemic,” Dr. Ortiz pointed out.
More than half of Hispanic adults would be afraid to go to a hospital for a possible heart attack or stroke because they might get infected with SARS-CoV-2, according to a new survey from the American Heart Association.

Compared with Hispanic respondents, 55% of whom said they feared COVID-19, significantly fewer Blacks (45%) and Whites (40%) would be scared to go to the hospital if they thought they were having a heart attack or stroke, the AHA said based on the survey of 2,050 adults, which was conducted May 29 to June 2, 2020, by the Harris Poll.
Hispanics also were significantly more likely to stay home if they thought they were experiencing a heart attack or stroke (41%), rather than risk getting infected at the hospital, than were Blacks (33%), who were significantly more likely than Whites (24%) to stay home, the AHA reported.
White respondents, on the other hand, were the most likely to believe (89%) that a hospital would give them the same quality of care provided to everyone else. Hispanics and Blacks had significantly lower rates, at 78% and 74%, respectively, the AHA noted.
These findings are “yet another challenge for Black and Hispanic communities, who are more likely to have underlying health conditions such as cardiovascular disease and diabetes and dying of COVID-19 at disproportionately high rates,” Rafael Ortiz, MD, American Heart Association volunteer medical expert and chief of neuro-endovascular surgery at Lenox Hill Hospital, New York, said in the AHA statement.
The survey was performed in conjunction with the AHA’s “Don’t Die of Doubt” campaign, which “reminds Americans, especially in Hispanic and Black communities, that the hospital remains the safest place to be if experiencing symptoms of a heart attack or a stroke.”
Among all the survey respondents, 57% said they would feel better if hospitals treated COVID-19 patients in a separate area. A number of other possible precautions ranked lower in helping them feel better:
- Screen all visitors, patients, and staff for COVID-19 symptoms when they enter the hospital: 39%.
- Require all patients, visitors, and staff to wear masks: 30%.
- Put increased cleaning protocols in place to disinfect multiple times per day: 23%.
- “Nothing would make me feel comfortable”: 6%.
Despite all the concerns about the risk of coronavirus infection, however, most Americans (77%) still believe that hospitals are the safest place to be in the event of a medical emergency, and 84% said that hospitals are prepared to safely treat emergencies that are not related to the pandemic, the AHA reported.
“Health care professionals know what to do even when things seem chaotic, and emergency departments have made plans behind the scenes to keep patients and healthcare workers safe even during a pandemic,” Dr. Ortiz pointed out.
Cleaner data confirm severe COVID-19 link to diabetes, hypertension
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
Further refinement of data from patients hospitalized worldwide for COVID-19 disease showed a 12% prevalence rate of patients with diabetes in this population and a 17% prevalence rate for hypertension.
These are lower rates than previously reported for COVID-19 patients with either of these two comorbidities, yet the findings still document important epidemiologic links between diabetes, hypertension, and COVID-19, said the study’s authors.
A meta-analysis of data from 15,794 patients hospitalized because of COVID-19 disease that was drawn from 65 carefully curated reports published from December 1, 2019, to April 6, 2020, also showed that, among the hospitalized COVID-19 patients with diabetes (either type 1 or type 2), the rate of patients who required ICU admission was 96% higher than among those without diabetes and mortality was 2.78-fold higher, both statistically significant differences.
The rate of ICU admissions among those hospitalized with COVID-19 who also had hypertension was 2.95-fold above those without hypertension, and mortality was 2.39-fold higher, also statistically significant differences, reported a team of researchers in the recently published report.
The new meta-analysis was notable for the extra effort investigators employed to eliminate duplicated patients from their database of COVID-19 patients included in various published reports, a potential source of bias that likely introduced errors into prior meta-analyses that used similar data. “We found an overwhelming proportion of studies at high risk of data repetition,” the report said. Virtually all of the included studies were retrospective case studies, nearly two-thirds had data from a single center, and 71% of the studies included only patients in China.
“We developed a method to identify reports that had a high risk for repetitions” of included patients, said Fady Hannah-Shmouni, MD, a senior author of the study. “We also used methods to minimize bias, we excluded certain patients populations, and we applied a uniform definition of COVID-19 disease severity,” specifically patients who died or needed ICU admission, because the definitions used originally by many of the reports were very heterogeneous, said Dr. Hannah-Shmouni, principal investigator for Endocrine, Genetics, and Hypertension at the National Institute of Child Health and Human Development.
Despite the effort to eliminate case duplications, the analysis remains subject to additional confounders, in part because of a lack of comprehensive patient information on factors such as smoking, body mass index, socioeconomic status, and the specific type of diabetes or hypertension a patient had. “Even with these limitations, we were able to show that the prevalence of hypertension and diabetes is elevated in patients with COVID-19, that patients with diabetes have increased risk for both death and ICU admissions, and that there is the potential for reverse causality in the reporting of hypertension as a risk factor for COVID-19,” Dr. Hannah-Shmouni said in an interview. “We believe the explosion of data that associated hypertension and COVID-19 may be partially the result of reverse causality.”
One possible example of this reverse causality is the overlap between hypertension and age as potential risk factors for COVID-19 disease or increased infection severity. People “older than 80 frequently develop severe disease if infected with the novel coronavirus, and 80% of people older than 80 have hypertension, so it’s not surprising that hypertension is highly prevalent among hospitalized COVID-19 patients,” but this “does not imply a causal relationship between hypertension and severe COVID-19; the risk of hypertension probably depends on older age,” noted Ernesto L. Schiffrin, MD, a coauthor of the study, as well as professor of medicine at McGill University and director of the Hypertension and Vascular Research Unit at the Lady Davis Institute for Medical Research, both in Montreal. “My current opinion, on the basis of the totality of data, is that hypertension does not worsen [COVID-19] outcomes, but patients who are elderly, obese, diabetic, or immunocompromised are susceptible to more severe COVID-19 and worse outcomes,” said Dr. Schiffrin in an interview.
The new findings show “there is certainly an interplay between the virus, diabetes, and hypertension and other risk factors,” and while still limited by biases, the new findings “get closer” to correctly estimating the COVID-19 risks associated with these comorbidities,” Dr. Hannah-Shmouni said.
The connections identified between COVID-19, diabetes, and hypertension mean that patients with these chronic diseases should receive education about their COVID-19 risks and should have adequate access to the drugs and supplies they need to control blood pressure and hyperglycemia. Patients with diabetes also need to be current on vaccinations to reduce their risk for pneumonia. And recognition of the heightened COVID-19 risk for people with these comorbidities is important among people who work in relevant government agencies, health care workers, and patient advocacy groups, he added.
The study received no commercial funding. Dr. Hannah-Shmouni and Dr. Schiffrin had no disclosures.
SOURCE: Barrera FJ et al. J Endocn Soc. 2020 July 21. doi: 10.1210/jendso/bvaa102.
FROM JOURNAL OF THE ENDOCRINE SOCIETY
Ultrasound, cardiac CT valuable in COVID-19 assessment
As if the management of patients with severe COVID-19 infections is not complicated enough, an estimated 50%-60% of patients admitted to an ICU with the disease will have some form of cardiovascular involvement, which further increases their already high risk for morbidity and mortality.
Multimodality cardiovascular imaging, chosen wisely, can both help to direct management of cardiovascular complications associated with COVID-19 and lessen risk of exposure of health care workers to SARS-CoV-2, said members of an expert panel from the American College of Cardiology Cardiovascular Imaging Leadership Council.
“When we face a patient with known or suspected COVID-19, it’s not like any other disease because we face potential exposure risk to personnel doing imaging studies and also to other patients,” corresponding author Marcelo F. Di Carli, MD, of Brigham and Women’s Hospital Boston said in an interview.
“Any imaging study that is being considered should be performed only if we think it will help us make a change in the way that we’re going to treat that particular patient. This is true for imaging in any disease – why would you do an imaging study that will make no difference in treatment? – but the stakes are even higher in COVID-19,” he said.
The panel’s recommendations for cardiovascular imaging in patients with COVID-19 are outlined in a guidance document published online in the Journal of the American College of Cardiology.
Testing and biomarkers
The guidance begins by highlighting the importance of diagnostic testing for COVID-19 infection and the use of universal precautions for health care personnel performing imaging studies, as well as disinfection of imaging equipment and rooms after each use.
Circulating biomarkers that measure end-organ stress or injury, inflammation, hypoperfusion, and activation of thrombosis/hemostasis pathways may be prognostically useful, but “almost none of the widely measured biomarkers represent a specific trigger for imaging outside of that supported by clinical judgment,” the guidance states.
In contrast, low to moderate, nonrising concentrations of markers for myocardial stress, such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), or of myocardial injury, such as cardiac troponins (cTn), may be helpful for excluding the need for imaging.
“Importantly, clinicians should be aware that most patients with abnormal BNP/NT-proBNP or cTn do not have acute heart failure or myocardial infarction; and rise in concentration of either class of biomarker presumably reflects complex processes including direct myocardial stress/injury related to systemic illness,” the panel members wrote.
Oldies but goodies
“One thing that we found out in our review of the literature and in our experiences in our own work settings is that cardiac ultrasound plays a huge role in this disease – like in any disease – but this one in particular,” Dr. Di Carli said. “One of the most feared complications in COVID-19 leads to inflammation of the heart muscle, which then leads to heart dysfunction. And of course cardiac ultrasound, because of its portability, can be performed at bedside to help clinicians ascertain an abnormality in the heart.”
Cardiac CT is also extremely helpful for determining whether patients with ECG findings suggestive of infarction have suffered an actual thrombotic event.
“These patients may best be served by a noninvasive study as compared to an invasive coronary angiogram,” he said.
Clinical scenarios
Cardiologists may be called in to consult on the evaluation of possible cardiogenic components of pulmonary abnormalities in patients who present with dyspnea and chest x-rays showing airspace or interstitial infiltrates suggestive of pneumonia, the authors noted.
“Clinicians will rely on history, physical exam, ECG [electrocardiogram] and biomarkers, and recent cardiac imaging tests if available. Underlying cardiac history including [coronary artery disease], cardiomyopathy, heart failure, and arrhythmia should be sought, and frequent contributors to decompensation should be eliminated,” they wrote.
For patients with suspected cardiac injury, either point-of-care ultrasound or limited echocardiography can be used for the initial evaluation, with additional, more advanced technologies called into play for specific clinical scenarios outlined in the guidance.
For example, the guidance recommends that patients with chest pain and abnormal ECG readings with clinical concern for ST-elevation acute coronary syndrome or high clinical risk for in-hospital mortality from conditions such as cardiogenic shock, dynamic ST-segment changes, or left ventricular ejection fraction less than 40% thought to be caused by non–ST-elevation myocardial infarction be referred for emergent coronary angiography and reperfusion.
In contrast, in patients with chest pain and abnormal ECG but equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentations, point-of-care ultrasound or limited echocardiogram could be used to look for regional wall motion abnormalities and left ventricular ejection fraction, whereas in patients with chest pain and ST-elevation without clear evidence of ST-elevation myocardial infarction, coronary CT angiography can help to rule out ACS and point to alternate diagnoses, the authors said.
The guidance also offers recommendations for imaging in patients with hemodynamic instability (shock or hypotension), patients with new left ventricular dysfunction in the absence of shock or hypotension, and patients with subacute and chronic-phase disease.
Development of the guidance document was supported by the ACC. Dr. Di Carli disclosed institutional grant support from Gilead Sciences and Spectrum Dynamics, and consulting income from Janssen and Bayer.
SOURCE: Rudski L et al. J Am Coll Cardiol. 2020 Jul 22. doi: 10.1016/j.jacc.2020.06.080.
As if the management of patients with severe COVID-19 infections is not complicated enough, an estimated 50%-60% of patients admitted to an ICU with the disease will have some form of cardiovascular involvement, which further increases their already high risk for morbidity and mortality.
Multimodality cardiovascular imaging, chosen wisely, can both help to direct management of cardiovascular complications associated with COVID-19 and lessen risk of exposure of health care workers to SARS-CoV-2, said members of an expert panel from the American College of Cardiology Cardiovascular Imaging Leadership Council.
“When we face a patient with known or suspected COVID-19, it’s not like any other disease because we face potential exposure risk to personnel doing imaging studies and also to other patients,” corresponding author Marcelo F. Di Carli, MD, of Brigham and Women’s Hospital Boston said in an interview.
“Any imaging study that is being considered should be performed only if we think it will help us make a change in the way that we’re going to treat that particular patient. This is true for imaging in any disease – why would you do an imaging study that will make no difference in treatment? – but the stakes are even higher in COVID-19,” he said.
The panel’s recommendations for cardiovascular imaging in patients with COVID-19 are outlined in a guidance document published online in the Journal of the American College of Cardiology.
Testing and biomarkers
The guidance begins by highlighting the importance of diagnostic testing for COVID-19 infection and the use of universal precautions for health care personnel performing imaging studies, as well as disinfection of imaging equipment and rooms after each use.
Circulating biomarkers that measure end-organ stress or injury, inflammation, hypoperfusion, and activation of thrombosis/hemostasis pathways may be prognostically useful, but “almost none of the widely measured biomarkers represent a specific trigger for imaging outside of that supported by clinical judgment,” the guidance states.
In contrast, low to moderate, nonrising concentrations of markers for myocardial stress, such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), or of myocardial injury, such as cardiac troponins (cTn), may be helpful for excluding the need for imaging.
“Importantly, clinicians should be aware that most patients with abnormal BNP/NT-proBNP or cTn do not have acute heart failure or myocardial infarction; and rise in concentration of either class of biomarker presumably reflects complex processes including direct myocardial stress/injury related to systemic illness,” the panel members wrote.
Oldies but goodies
“One thing that we found out in our review of the literature and in our experiences in our own work settings is that cardiac ultrasound plays a huge role in this disease – like in any disease – but this one in particular,” Dr. Di Carli said. “One of the most feared complications in COVID-19 leads to inflammation of the heart muscle, which then leads to heart dysfunction. And of course cardiac ultrasound, because of its portability, can be performed at bedside to help clinicians ascertain an abnormality in the heart.”
Cardiac CT is also extremely helpful for determining whether patients with ECG findings suggestive of infarction have suffered an actual thrombotic event.
“These patients may best be served by a noninvasive study as compared to an invasive coronary angiogram,” he said.
Clinical scenarios
Cardiologists may be called in to consult on the evaluation of possible cardiogenic components of pulmonary abnormalities in patients who present with dyspnea and chest x-rays showing airspace or interstitial infiltrates suggestive of pneumonia, the authors noted.
“Clinicians will rely on history, physical exam, ECG [electrocardiogram] and biomarkers, and recent cardiac imaging tests if available. Underlying cardiac history including [coronary artery disease], cardiomyopathy, heart failure, and arrhythmia should be sought, and frequent contributors to decompensation should be eliminated,” they wrote.
For patients with suspected cardiac injury, either point-of-care ultrasound or limited echocardiography can be used for the initial evaluation, with additional, more advanced technologies called into play for specific clinical scenarios outlined in the guidance.
For example, the guidance recommends that patients with chest pain and abnormal ECG readings with clinical concern for ST-elevation acute coronary syndrome or high clinical risk for in-hospital mortality from conditions such as cardiogenic shock, dynamic ST-segment changes, or left ventricular ejection fraction less than 40% thought to be caused by non–ST-elevation myocardial infarction be referred for emergent coronary angiography and reperfusion.
In contrast, in patients with chest pain and abnormal ECG but equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentations, point-of-care ultrasound or limited echocardiogram could be used to look for regional wall motion abnormalities and left ventricular ejection fraction, whereas in patients with chest pain and ST-elevation without clear evidence of ST-elevation myocardial infarction, coronary CT angiography can help to rule out ACS and point to alternate diagnoses, the authors said.
The guidance also offers recommendations for imaging in patients with hemodynamic instability (shock or hypotension), patients with new left ventricular dysfunction in the absence of shock or hypotension, and patients with subacute and chronic-phase disease.
Development of the guidance document was supported by the ACC. Dr. Di Carli disclosed institutional grant support from Gilead Sciences and Spectrum Dynamics, and consulting income from Janssen and Bayer.
SOURCE: Rudski L et al. J Am Coll Cardiol. 2020 Jul 22. doi: 10.1016/j.jacc.2020.06.080.
As if the management of patients with severe COVID-19 infections is not complicated enough, an estimated 50%-60% of patients admitted to an ICU with the disease will have some form of cardiovascular involvement, which further increases their already high risk for morbidity and mortality.
Multimodality cardiovascular imaging, chosen wisely, can both help to direct management of cardiovascular complications associated with COVID-19 and lessen risk of exposure of health care workers to SARS-CoV-2, said members of an expert panel from the American College of Cardiology Cardiovascular Imaging Leadership Council.
“When we face a patient with known or suspected COVID-19, it’s not like any other disease because we face potential exposure risk to personnel doing imaging studies and also to other patients,” corresponding author Marcelo F. Di Carli, MD, of Brigham and Women’s Hospital Boston said in an interview.
“Any imaging study that is being considered should be performed only if we think it will help us make a change in the way that we’re going to treat that particular patient. This is true for imaging in any disease – why would you do an imaging study that will make no difference in treatment? – but the stakes are even higher in COVID-19,” he said.
The panel’s recommendations for cardiovascular imaging in patients with COVID-19 are outlined in a guidance document published online in the Journal of the American College of Cardiology.
Testing and biomarkers
The guidance begins by highlighting the importance of diagnostic testing for COVID-19 infection and the use of universal precautions for health care personnel performing imaging studies, as well as disinfection of imaging equipment and rooms after each use.
Circulating biomarkers that measure end-organ stress or injury, inflammation, hypoperfusion, and activation of thrombosis/hemostasis pathways may be prognostically useful, but “almost none of the widely measured biomarkers represent a specific trigger for imaging outside of that supported by clinical judgment,” the guidance states.
In contrast, low to moderate, nonrising concentrations of markers for myocardial stress, such as B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP), or of myocardial injury, such as cardiac troponins (cTn), may be helpful for excluding the need for imaging.
“Importantly, clinicians should be aware that most patients with abnormal BNP/NT-proBNP or cTn do not have acute heart failure or myocardial infarction; and rise in concentration of either class of biomarker presumably reflects complex processes including direct myocardial stress/injury related to systemic illness,” the panel members wrote.
Oldies but goodies
“One thing that we found out in our review of the literature and in our experiences in our own work settings is that cardiac ultrasound plays a huge role in this disease – like in any disease – but this one in particular,” Dr. Di Carli said. “One of the most feared complications in COVID-19 leads to inflammation of the heart muscle, which then leads to heart dysfunction. And of course cardiac ultrasound, because of its portability, can be performed at bedside to help clinicians ascertain an abnormality in the heart.”
Cardiac CT is also extremely helpful for determining whether patients with ECG findings suggestive of infarction have suffered an actual thrombotic event.
“These patients may best be served by a noninvasive study as compared to an invasive coronary angiogram,” he said.
Clinical scenarios
Cardiologists may be called in to consult on the evaluation of possible cardiogenic components of pulmonary abnormalities in patients who present with dyspnea and chest x-rays showing airspace or interstitial infiltrates suggestive of pneumonia, the authors noted.
“Clinicians will rely on history, physical exam, ECG [electrocardiogram] and biomarkers, and recent cardiac imaging tests if available. Underlying cardiac history including [coronary artery disease], cardiomyopathy, heart failure, and arrhythmia should be sought, and frequent contributors to decompensation should be eliminated,” they wrote.
For patients with suspected cardiac injury, either point-of-care ultrasound or limited echocardiography can be used for the initial evaluation, with additional, more advanced technologies called into play for specific clinical scenarios outlined in the guidance.
For example, the guidance recommends that patients with chest pain and abnormal ECG readings with clinical concern for ST-elevation acute coronary syndrome or high clinical risk for in-hospital mortality from conditions such as cardiogenic shock, dynamic ST-segment changes, or left ventricular ejection fraction less than 40% thought to be caused by non–ST-elevation myocardial infarction be referred for emergent coronary angiography and reperfusion.
In contrast, in patients with chest pain and abnormal ECG but equivocal symptoms, atypical or equivocal ECG abnormalities, or late presentations, point-of-care ultrasound or limited echocardiogram could be used to look for regional wall motion abnormalities and left ventricular ejection fraction, whereas in patients with chest pain and ST-elevation without clear evidence of ST-elevation myocardial infarction, coronary CT angiography can help to rule out ACS and point to alternate diagnoses, the authors said.
The guidance also offers recommendations for imaging in patients with hemodynamic instability (shock or hypotension), patients with new left ventricular dysfunction in the absence of shock or hypotension, and patients with subacute and chronic-phase disease.
Development of the guidance document was supported by the ACC. Dr. Di Carli disclosed institutional grant support from Gilead Sciences and Spectrum Dynamics, and consulting income from Janssen and Bayer.
SOURCE: Rudski L et al. J Am Coll Cardiol. 2020 Jul 22. doi: 10.1016/j.jacc.2020.06.080.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
CCC19, other registries help define COVID/cancer landscape
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
Initial results from the CCC19 registry were reported as part of the American Society of Clinical Oncology (ASCO) virtual scientific program and published in The Lancet (Lancet. 2020 Jun 20;395[10241]:1907-18).
The latest data were presented at the AACR virtual meeting: COVID-19 and Cancer by Brian I. Rini, MD, of Vanderbilt University, Nashville, Tenn. They were simultaneously published in Cancer Discovery (Cancer Discov. 2020 Jul 22;CD-20-0941).
The CCC19 registry was launched in March by a few institutions as part of “a grassroots idea ... to collect granular data regarding cancer patients and their outcomes with COVID,” Dr. Rini said.
Within a few months of its inception, the registry had partnered with more than 100 institutions worldwide and accrued data from more than 2,000 patients.
The reports in The Lancet and at ASCO included outcomes for the first 928 patients and showed a 13% mortality rate as well as a fivefold increase in the risk of 30-day mortality among patients with COVID-19 and progressing cancer.
The data also showed an increased mortality risk among older patients, men, former smokers, those with poor performance status, those with multiple comorbidities, and those treated with hydroxychloroquine and azithromycin.
The latest data
The CCC19 registry has grown to include 114 sites worldwide, including major comprehensive cancer centers and community sites. As of June 26, there were 2,749 patients enrolled.
Since the last data were reported, the mortality rate increased from 13% to 16% (versus 5% globally). In addition, the increased mortality risk among non-Hispanic black patients and patients with hematologic malignancies reached statistical significance, Dr. Rini said. He noted that the increase in mortality rate was largely attributable to improved follow-up.
Mechanical ventilation was required in 12% of patients, ICU admission was required in 16%, oxygen was required in 45%, and hospitalization was required in 60%. The composite outcome of death, severe illness requiring hospitalization, ICU admission, or mechanical ventilation was reached in 29% of patients, Dr. Rini said.
Mortality rates across cancer types ranged from 3% to 26%, with thyroid and breast cancer patients having the lowest rates (3% and 8%, respectively), and with lymphoma and lung cancer patients having the highest (22% and 26%, respectively), Dr. Rini said.
He noted that the TERAVOLT registry, a COVID-19 registry for patients with thoracic cancers, also showed a very high mortality rate in this subgroup of patients.
Results from TERAVOLT were reported at the AACR virtual meeting I, presented at ASCO, and published in The Lancet (Lancet Oncol. 2020 Jul;21[7]:914-22). The most recent results showed a mortality rate of nearly 36% and reinforce the high mortality rate seen in lung cancer patients in CCC19, Dr. Rini said.
Increased mortality risk
After adjustment for several demographic and disease characteristics, the updated CCC19 data showed a significantly increased risk of mortality among:
- Older patients (adjusted odds ratio [aOR] per decade of age, 1.52).
- Men (aOR, 1.43).
- Current or former smokers vs. never smokers (aOR, 1.28).
- Patients with Eastern Cooperative Oncology Group performance scores of 1 vs. 0 (aOR of 1.80) or 2 vs. 0 (aOR, 4.22).
- Stable cancer vs. remission (aOR, 1.47).
- Progressive cancer vs. remission (aOR, 2.96).
- Non-Hispanic Black vs. White patients (aOR, 1.56).
- Hematologic malignancies vs. solid tumors (aOR, 1.80).
“Importantly, there were some factors that did not reach statistical significance,” Dr. Rini said. These include obesity (aOR, 1.23), recent surgery (aOR, 1.05), receipt of cytotoxic chemotherapy vs. no chemotherapy (aOR, 1.14), and receipt of noncytotoxic chemotherapy vs. no chemotherapy (aOR, 0.75).
“I think this provides some reassurance that cancer care can and should continue for these patients,” Dr. Rini said.
He noted, however, that in TERAVOLT, chemotherapy with or without other treatment was a risk factor for mortality in lung cancer patients when compared with no chemotherapy (OR, 1.71) and when compared with immunotherapy or targeted therapy (OR, 1.64).
NCCAPS and other registries
Dr. Rini discussed a number of registries looking at outcomes in COVID-19 patients with cancer, and he said the findings to date appear to confirm a higher mortality rate among cancer patients, particularly those with lung cancer.
Several factors are emerging that appear to be related to risk, including both cancer-related and non–cancer-related factors, he added.
The ongoing prospective National Cancer Institute COVID-19 in Cancer Patients Study (NCCAPS) “will provide much needed longitudinal data and, importantly, biospecimen collection in a large cohort of patients who have active cancer and are receiving treatment, said Dr. Rini, who is the study’s protocol chair. NCCAPS is a natural history study in that population, he said.
The planned accrual is about 2,000 patients who will be followed for up to 2 years for data collection, imaging scans, and research specimens.
The use of specimens is “a unique and special part of this study,” Dr. Rini said, explaining that the specimens will be used to look for development of antibodies over time, to describe the trajectory of cytokine abnormalities – especially in patients with more acute inpatient courses – to perform DNA-based genome-wide association studies, and to assess coagulation parameters.
NCCAPS is activated at 546 sties, 10 patients were enrolled as of June 21, and rapid accrual is expected over the next several months, he said.
Gypsyamber D’Souza, PhD, session moderator and an infectious disease epidemiologist at Johns Hopkins University in Baltimore, acknowledged the challenge that registry administrators face when trying to balance the need to get data out against the desire to ask the right questions and to have the right comparison groups, stratification, and analyses, especially amid a crisis like the COVID-19 pandemic.
Dr. Rini said it has indeed been a bit of a struggle with CCC19 to determine what information should be published and when, and what constitutes an important update.
“It’s been a learning experience, and frankly, I think we’re still learning,” he said. “This has been such a unique time in terms of a rush to get data out, balanced against making sure that there’s quality data and that you’re actually answering important questions.”
In fact, a number of ongoing registries “should start to produce great data [that will be presented] at upcoming big conferences,” Dr. Rini said. He added that those data “will help piece together different important aspects of this and different hypotheses, and hopefully complement the clinical data that’s starting to come out.”
The CCC19 registry is sponsored by Vanderbilt-Ingram Cancer Center. Dr. Rini disclosed relationships with Pfizer, Merck, Genentech/Roche, Aveo, AstraZeneca, Bristol Myers Squibb, Exelixis, Synthorx, Peloton, Compugen, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, and PTC Therapeutics. Dr. D’Souza did not disclose any conflicts.
SOURCE: Rini BI. AACR: COVID-19 and Cancer. Abstract IA26.
FROM AACR: COVID-19 and CANCER
Combination therapy quells COVID-19 cytokine storm
Treatment with high-dose methylprednisolone plus tocilizumab (Actemra, Genentech) as needed was associated with faster respiratory recovery, a lower likelihood of mechanical ventilation, and fewer in-hospital deaths compared with supportive care alone among people with COVID-19 experiencing a hyperinflammatory state known as a cytokine storm.
Compared with historic controls, participants in the treatment group were 79% more likely to achieve at least a two-stage improvement in respiratory status, for example.
“COVID-19-associated cytokine storm syndrome [CSS] is an important complication of severe acute respiratory syndrome coronavirus-2 infection in up to 25% of the patients,” lead author Sofia Ramiro, MD, PhD, said in an interview.
Furthermore, CSS often leads to death in this population, said Dr. Ramiro, a consultant rheumatologist and senior researcher at Leiden University Medical Center and Zuyderland Medical Center in Heerlen, the Netherlands.
Results of the COVID High-Intensity Immunosuppression in Cytokine Storm Syndrome (CHIC) study were published online July 20 in Annals of the Rheumatic Diseases.
Contrary to guidance?
The World Health Organization (WHO) cautions against administering corticosteroids to some critically ill patients with COVID-19. “WHO recommends against the routine use of systemic corticosteroids for treatment of viral pneumonia,” according to an interim guidance document on the clinical management of COVID-19 published May 27.
Dr. Ramiro and colleagues make a distinction, however, noting “the risk profile of such a short course of glucocorticoid for treatment of CSS needs to be separated from preexisting chronic use of glucocorticoid for conditions like rheumatic and musculoskeletal diseases.”
Participants in the current study tolerated immunosuppressive therapy well without evidence of impaired viral clearance or bacterial superinfection, they added.
Other experts disagree with recent recommendations to use corticosteroids to treat a hyperimmune response or suspected adrenal insufficiency in the setting of refractory shock in patients with COVID-19.
Information about immunosuppressive therapy and CSS linked to COVID-19 remains anecdotal, however, Dr. Ramiro and colleagues noted.
The researchers assessed outcomes of 86 individuals with COVID-19-associated CSS treated with high-dose methylprednisolone plus/minus tocilizumab, an anti-interleukin-6 receptor monoclonal antibody. They compared them with another 86 patients with COVID-19 treated with supportive care before initiation of the combination therapy protocol.
Participants with CSS had an oxygen saturation of 94% or lower at rest or tachypnea exceeding 30 breaths per minute.
They also had at least two of the following: C-reactive protein > 100 mg/L; serum ferritin > 900 mcg/L at one occasion or a twofold increase at admission within 48 hours; or D-dimer levels > 1,500 mcg/L.
The treatment group received methylprednisolone 250 mg intravenously on day 1, followed by 80 mg intravenously on days 2-5. Investigators permitted a 2-day extension if indicated.
Those who failed to clinically improve or experienced respiratory decline could also receive intravenous tocilizumab on day 2 or after. The agent was dosed at 8 mg/kg body weight during a single infusion from day 2-5 up to a maximum of 800 mg.
In all, 37 participants received tocilizumab, including two participants who received a second dose 5 days after initial treatment.
Except for one patient in the treatment group, all participants also received antibiotic treatment and nearly 80% received chloroquine.
Mechanical ventilation and mortality
The primary outcome of at least a two-stage improvement in respiratory status on a WHO scale associated with treatment yielded a hazard ratio (HR) of 1.79. The treatment group achieved this improvement a median 7 days earlier than controls.
Mechanical ventilation to treat respiratory deterioration was 71% less likely for the treatment group versus controls (HR, 0.29).
The treatment group were also 65% less likely to die in hospital (HR, 0.35) than were controls.
The researchers also reported a significant difference in the number of deaths at day 14 in the treatment vs. control group, at 10 vs. 33 patients (P < .0001).
Glucocorticoid sufficient for many
In a sensitivity analysis excluding patients who received tocilizumab, the benefits of treatment remained statistically significant, “suggesting that a clinically relevant treatment effect can be reached by high-dose glucocorticoids alone,” the researchers noted.
This finding suggests “that the timely administration of high-dose glucocorticoids alone may provide significant benefit in more than half of the patients, and that tocilizumab is only needed in those cases that had insufficient clinical improvement on methylprednisolone alone,” they added.
“This is an important finding given the limited availability of tocilizumab in many countries and tocilizumab’s high costs.”
Complications were fairly balanced between groups. For example, bacterial infections during hospitalization were diagnosed in eight patients in the treatment group versus seven in the control group.
In addition, cardiac arrhythmias occurred in both groups, but slightly less frequently in the treatment group (P = .265), and there was a trend towards more pulmonary embolisms in the treatment group (P = .059).
Strengths and limitations
“A treatment with high-dose glucocorticoids is a convenient choice since glucocorticoids are safe, widely available, and inexpensive,” the researchers noted. “Longer follow-up, however, is needed to give final resolution about the safety and efficacy of the strategy.”
A strength of the study was “meticulous selection of those patients more likely to benefit from immunosuppressive treatment, namely patients with a CSS,” she added.
The study featured a prospective, observational design for the treatment group and retrospective analysis of the historic controls. “Methodologically, the main limitation of the study is not being a randomized controlled trial,” she noted.
“Ethically it has shown to be very rewarding to consciously decide against a randomized control trial, as we are talking about a disease that if only treated with supportive care can lead to mortality up to almost 50% from COVID-19-associated CSS,” Dr. Ramiro said.
Going forward, Dr. Ramiro plans to continue monitoring patients who experienced CSS to assess their outcome post-COVID-19 infection. “We want to focus on cardiorespiratory, functional, and quality of life outcomes,” she said. “We will also compare the outcomes between patients that have received immunosuppression with those that haven’t.”
‘Quite interesting’ results
“We desperately need better evidence to guide the management of patients hospitalized with COVID-19,” Nihar R. Desai, MD, MPH, who was not affiliated with the study, said in an interview.
“These data from the Netherlands are quite interesting and provide another signal to support the use of corticosteroids, with tocilizumab if needed, among hospitalized patients with COVID-19 to improve outcomes,” added Dr. Desai, associate professor of medicine and investigator at the Center for Outcomes Research and Evaluation, Yale University, New Haven, Conn.
“While these data are not randomized and have a relatively small sample size, we had recently seen the results of the RECOVERY trial, a UK-based randomized trial demonstrating the benefit of steroids in COVID-19,” he said.
“Taken together, these studies seem to suggest that there is a benefit with steroid therapy.” Further validation of these results is warranted, he added.
“While not a randomized clinical trial, and thus susceptible to unmeasured bias, the study adds to mounting evidence that supports targeting the excessive inflammation found in some patients with COVID-19,” Jared Radbel, MD, a pulmonologist, critical care specialist, and assistant professor of medicine at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview.
Dr. Radbel added that he is part of a multicenter group that has submitted a manuscript examining outcomes of critically ill patients with COVID-19 treated with tocilizumab.
Dr. Ramiro, Dr. Desai, and Dr. Radbel have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Treatment with high-dose methylprednisolone plus tocilizumab (Actemra, Genentech) as needed was associated with faster respiratory recovery, a lower likelihood of mechanical ventilation, and fewer in-hospital deaths compared with supportive care alone among people with COVID-19 experiencing a hyperinflammatory state known as a cytokine storm.
Compared with historic controls, participants in the treatment group were 79% more likely to achieve at least a two-stage improvement in respiratory status, for example.
“COVID-19-associated cytokine storm syndrome [CSS] is an important complication of severe acute respiratory syndrome coronavirus-2 infection in up to 25% of the patients,” lead author Sofia Ramiro, MD, PhD, said in an interview.
Furthermore, CSS often leads to death in this population, said Dr. Ramiro, a consultant rheumatologist and senior researcher at Leiden University Medical Center and Zuyderland Medical Center in Heerlen, the Netherlands.
Results of the COVID High-Intensity Immunosuppression in Cytokine Storm Syndrome (CHIC) study were published online July 20 in Annals of the Rheumatic Diseases.
Contrary to guidance?
The World Health Organization (WHO) cautions against administering corticosteroids to some critically ill patients with COVID-19. “WHO recommends against the routine use of systemic corticosteroids for treatment of viral pneumonia,” according to an interim guidance document on the clinical management of COVID-19 published May 27.
Dr. Ramiro and colleagues make a distinction, however, noting “the risk profile of such a short course of glucocorticoid for treatment of CSS needs to be separated from preexisting chronic use of glucocorticoid for conditions like rheumatic and musculoskeletal diseases.”
Participants in the current study tolerated immunosuppressive therapy well without evidence of impaired viral clearance or bacterial superinfection, they added.
Other experts disagree with recent recommendations to use corticosteroids to treat a hyperimmune response or suspected adrenal insufficiency in the setting of refractory shock in patients with COVID-19.
Information about immunosuppressive therapy and CSS linked to COVID-19 remains anecdotal, however, Dr. Ramiro and colleagues noted.
The researchers assessed outcomes of 86 individuals with COVID-19-associated CSS treated with high-dose methylprednisolone plus/minus tocilizumab, an anti-interleukin-6 receptor monoclonal antibody. They compared them with another 86 patients with COVID-19 treated with supportive care before initiation of the combination therapy protocol.
Participants with CSS had an oxygen saturation of 94% or lower at rest or tachypnea exceeding 30 breaths per minute.
They also had at least two of the following: C-reactive protein > 100 mg/L; serum ferritin > 900 mcg/L at one occasion or a twofold increase at admission within 48 hours; or D-dimer levels > 1,500 mcg/L.
The treatment group received methylprednisolone 250 mg intravenously on day 1, followed by 80 mg intravenously on days 2-5. Investigators permitted a 2-day extension if indicated.
Those who failed to clinically improve or experienced respiratory decline could also receive intravenous tocilizumab on day 2 or after. The agent was dosed at 8 mg/kg body weight during a single infusion from day 2-5 up to a maximum of 800 mg.
In all, 37 participants received tocilizumab, including two participants who received a second dose 5 days after initial treatment.
Except for one patient in the treatment group, all participants also received antibiotic treatment and nearly 80% received chloroquine.
Mechanical ventilation and mortality
The primary outcome of at least a two-stage improvement in respiratory status on a WHO scale associated with treatment yielded a hazard ratio (HR) of 1.79. The treatment group achieved this improvement a median 7 days earlier than controls.
Mechanical ventilation to treat respiratory deterioration was 71% less likely for the treatment group versus controls (HR, 0.29).
The treatment group were also 65% less likely to die in hospital (HR, 0.35) than were controls.
The researchers also reported a significant difference in the number of deaths at day 14 in the treatment vs. control group, at 10 vs. 33 patients (P < .0001).
Glucocorticoid sufficient for many
In a sensitivity analysis excluding patients who received tocilizumab, the benefits of treatment remained statistically significant, “suggesting that a clinically relevant treatment effect can be reached by high-dose glucocorticoids alone,” the researchers noted.
This finding suggests “that the timely administration of high-dose glucocorticoids alone may provide significant benefit in more than half of the patients, and that tocilizumab is only needed in those cases that had insufficient clinical improvement on methylprednisolone alone,” they added.
“This is an important finding given the limited availability of tocilizumab in many countries and tocilizumab’s high costs.”
Complications were fairly balanced between groups. For example, bacterial infections during hospitalization were diagnosed in eight patients in the treatment group versus seven in the control group.
In addition, cardiac arrhythmias occurred in both groups, but slightly less frequently in the treatment group (P = .265), and there was a trend towards more pulmonary embolisms in the treatment group (P = .059).
Strengths and limitations
“A treatment with high-dose glucocorticoids is a convenient choice since glucocorticoids are safe, widely available, and inexpensive,” the researchers noted. “Longer follow-up, however, is needed to give final resolution about the safety and efficacy of the strategy.”
A strength of the study was “meticulous selection of those patients more likely to benefit from immunosuppressive treatment, namely patients with a CSS,” she added.
The study featured a prospective, observational design for the treatment group and retrospective analysis of the historic controls. “Methodologically, the main limitation of the study is not being a randomized controlled trial,” she noted.
“Ethically it has shown to be very rewarding to consciously decide against a randomized control trial, as we are talking about a disease that if only treated with supportive care can lead to mortality up to almost 50% from COVID-19-associated CSS,” Dr. Ramiro said.
Going forward, Dr. Ramiro plans to continue monitoring patients who experienced CSS to assess their outcome post-COVID-19 infection. “We want to focus on cardiorespiratory, functional, and quality of life outcomes,” she said. “We will also compare the outcomes between patients that have received immunosuppression with those that haven’t.”
‘Quite interesting’ results
“We desperately need better evidence to guide the management of patients hospitalized with COVID-19,” Nihar R. Desai, MD, MPH, who was not affiliated with the study, said in an interview.
“These data from the Netherlands are quite interesting and provide another signal to support the use of corticosteroids, with tocilizumab if needed, among hospitalized patients with COVID-19 to improve outcomes,” added Dr. Desai, associate professor of medicine and investigator at the Center for Outcomes Research and Evaluation, Yale University, New Haven, Conn.
“While these data are not randomized and have a relatively small sample size, we had recently seen the results of the RECOVERY trial, a UK-based randomized trial demonstrating the benefit of steroids in COVID-19,” he said.
“Taken together, these studies seem to suggest that there is a benefit with steroid therapy.” Further validation of these results is warranted, he added.
“While not a randomized clinical trial, and thus susceptible to unmeasured bias, the study adds to mounting evidence that supports targeting the excessive inflammation found in some patients with COVID-19,” Jared Radbel, MD, a pulmonologist, critical care specialist, and assistant professor of medicine at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview.
Dr. Radbel added that he is part of a multicenter group that has submitted a manuscript examining outcomes of critically ill patients with COVID-19 treated with tocilizumab.
Dr. Ramiro, Dr. Desai, and Dr. Radbel have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Treatment with high-dose methylprednisolone plus tocilizumab (Actemra, Genentech) as needed was associated with faster respiratory recovery, a lower likelihood of mechanical ventilation, and fewer in-hospital deaths compared with supportive care alone among people with COVID-19 experiencing a hyperinflammatory state known as a cytokine storm.
Compared with historic controls, participants in the treatment group were 79% more likely to achieve at least a two-stage improvement in respiratory status, for example.
“COVID-19-associated cytokine storm syndrome [CSS] is an important complication of severe acute respiratory syndrome coronavirus-2 infection in up to 25% of the patients,” lead author Sofia Ramiro, MD, PhD, said in an interview.
Furthermore, CSS often leads to death in this population, said Dr. Ramiro, a consultant rheumatologist and senior researcher at Leiden University Medical Center and Zuyderland Medical Center in Heerlen, the Netherlands.
Results of the COVID High-Intensity Immunosuppression in Cytokine Storm Syndrome (CHIC) study were published online July 20 in Annals of the Rheumatic Diseases.
Contrary to guidance?
The World Health Organization (WHO) cautions against administering corticosteroids to some critically ill patients with COVID-19. “WHO recommends against the routine use of systemic corticosteroids for treatment of viral pneumonia,” according to an interim guidance document on the clinical management of COVID-19 published May 27.
Dr. Ramiro and colleagues make a distinction, however, noting “the risk profile of such a short course of glucocorticoid for treatment of CSS needs to be separated from preexisting chronic use of glucocorticoid for conditions like rheumatic and musculoskeletal diseases.”
Participants in the current study tolerated immunosuppressive therapy well without evidence of impaired viral clearance or bacterial superinfection, they added.
Other experts disagree with recent recommendations to use corticosteroids to treat a hyperimmune response or suspected adrenal insufficiency in the setting of refractory shock in patients with COVID-19.
Information about immunosuppressive therapy and CSS linked to COVID-19 remains anecdotal, however, Dr. Ramiro and colleagues noted.
The researchers assessed outcomes of 86 individuals with COVID-19-associated CSS treated with high-dose methylprednisolone plus/minus tocilizumab, an anti-interleukin-6 receptor monoclonal antibody. They compared them with another 86 patients with COVID-19 treated with supportive care before initiation of the combination therapy protocol.
Participants with CSS had an oxygen saturation of 94% or lower at rest or tachypnea exceeding 30 breaths per minute.
They also had at least two of the following: C-reactive protein > 100 mg/L; serum ferritin > 900 mcg/L at one occasion or a twofold increase at admission within 48 hours; or D-dimer levels > 1,500 mcg/L.
The treatment group received methylprednisolone 250 mg intravenously on day 1, followed by 80 mg intravenously on days 2-5. Investigators permitted a 2-day extension if indicated.
Those who failed to clinically improve or experienced respiratory decline could also receive intravenous tocilizumab on day 2 or after. The agent was dosed at 8 mg/kg body weight during a single infusion from day 2-5 up to a maximum of 800 mg.
In all, 37 participants received tocilizumab, including two participants who received a second dose 5 days after initial treatment.
Except for one patient in the treatment group, all participants also received antibiotic treatment and nearly 80% received chloroquine.
Mechanical ventilation and mortality
The primary outcome of at least a two-stage improvement in respiratory status on a WHO scale associated with treatment yielded a hazard ratio (HR) of 1.79. The treatment group achieved this improvement a median 7 days earlier than controls.
Mechanical ventilation to treat respiratory deterioration was 71% less likely for the treatment group versus controls (HR, 0.29).
The treatment group were also 65% less likely to die in hospital (HR, 0.35) than were controls.
The researchers also reported a significant difference in the number of deaths at day 14 in the treatment vs. control group, at 10 vs. 33 patients (P < .0001).
Glucocorticoid sufficient for many
In a sensitivity analysis excluding patients who received tocilizumab, the benefits of treatment remained statistically significant, “suggesting that a clinically relevant treatment effect can be reached by high-dose glucocorticoids alone,” the researchers noted.
This finding suggests “that the timely administration of high-dose glucocorticoids alone may provide significant benefit in more than half of the patients, and that tocilizumab is only needed in those cases that had insufficient clinical improvement on methylprednisolone alone,” they added.
“This is an important finding given the limited availability of tocilizumab in many countries and tocilizumab’s high costs.”
Complications were fairly balanced between groups. For example, bacterial infections during hospitalization were diagnosed in eight patients in the treatment group versus seven in the control group.
In addition, cardiac arrhythmias occurred in both groups, but slightly less frequently in the treatment group (P = .265), and there was a trend towards more pulmonary embolisms in the treatment group (P = .059).
Strengths and limitations
“A treatment with high-dose glucocorticoids is a convenient choice since glucocorticoids are safe, widely available, and inexpensive,” the researchers noted. “Longer follow-up, however, is needed to give final resolution about the safety and efficacy of the strategy.”
A strength of the study was “meticulous selection of those patients more likely to benefit from immunosuppressive treatment, namely patients with a CSS,” she added.
The study featured a prospective, observational design for the treatment group and retrospective analysis of the historic controls. “Methodologically, the main limitation of the study is not being a randomized controlled trial,” she noted.
“Ethically it has shown to be very rewarding to consciously decide against a randomized control trial, as we are talking about a disease that if only treated with supportive care can lead to mortality up to almost 50% from COVID-19-associated CSS,” Dr. Ramiro said.
Going forward, Dr. Ramiro plans to continue monitoring patients who experienced CSS to assess their outcome post-COVID-19 infection. “We want to focus on cardiorespiratory, functional, and quality of life outcomes,” she said. “We will also compare the outcomes between patients that have received immunosuppression with those that haven’t.”
‘Quite interesting’ results
“We desperately need better evidence to guide the management of patients hospitalized with COVID-19,” Nihar R. Desai, MD, MPH, who was not affiliated with the study, said in an interview.
“These data from the Netherlands are quite interesting and provide another signal to support the use of corticosteroids, with tocilizumab if needed, among hospitalized patients with COVID-19 to improve outcomes,” added Dr. Desai, associate professor of medicine and investigator at the Center for Outcomes Research and Evaluation, Yale University, New Haven, Conn.
“While these data are not randomized and have a relatively small sample size, we had recently seen the results of the RECOVERY trial, a UK-based randomized trial demonstrating the benefit of steroids in COVID-19,” he said.
“Taken together, these studies seem to suggest that there is a benefit with steroid therapy.” Further validation of these results is warranted, he added.
“While not a randomized clinical trial, and thus susceptible to unmeasured bias, the study adds to mounting evidence that supports targeting the excessive inflammation found in some patients with COVID-19,” Jared Radbel, MD, a pulmonologist, critical care specialist, and assistant professor of medicine at Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J., said in an interview.
Dr. Radbel added that he is part of a multicenter group that has submitted a manuscript examining outcomes of critically ill patients with COVID-19 treated with tocilizumab.
Dr. Ramiro, Dr. Desai, and Dr. Radbel have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
One-third of outpatients with COVID-19 are unwell weeks later
, according to survey results in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
Mark W. Tenforde, MD, PhD, for the CDC-COVID-19 Response Team, and colleagues conducted a multistate telephone survey of symptomatic adults who tested positive for SARS-CoV-2. The researchers found that 35% had not returned to their usual state of wellness when they were interviewed 2-3 weeks after testing.
Among the 270 of 274 people interviewed for whom there were data on return to health, 175 (65%) reported that they had returned to baseline health an average of 7 days from the date of testing.
Among the 274 symptomatic outpatients, the median number of symptoms was seven. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms.
Prolonged illness is well described in adults hospitalized with severe COVID-19, especially among the older adult population, but little is known about other groups.
The proportion who had not returned to health differed by age: 26% of interviewees aged 18-34 years, 32% of those aged 35-49 years, and 47% of those at least 50 years old reported not having returned to their usual health (P = .010) within 14-21 days after receiving positive test results.
Among respondents aged 18-34 years who had no chronic medical condition, 19% (9 of 48) reported not having returned to their usual state of health during that time.
Public health messaging targeting younger adults, a group who might not be expected to be sick for weeks with mild disease, is particularly important, the authors wrote.
Kyle Annen, DO, medical director of transfusion services and patient blood management at Children’s Hospital Colorado and assistant professor of pathology at the University of Colorado, Denver, said in an interview that an important message is that delayed recovery (symptoms of fatigue, cough, and shortness of breath) was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization.
“This should impact the perception of this being a mild illness in the young adult population and encourage them to comply with recommendations of social distancing, masking, and hand washing,” she said.
Recovery time of more than 2 weeks will affect work and school performance, especially prolonged fatigue, she noted. This was one of the prominent symptoms that were reported to be slow to dissipate.
“I think the most interesting point in this study is that of underlying conditions; psychiatric conditions were significantly correlated with prolonged recovery. I don’t think that many people think of depression and anxiety as an underlying medical condition in regards to COVID-19 risk. This could potentially have an impact, as depression and anxiety rates will likely increase as COVID-19 continues,” she said.
Buddy Creech, MD, MPH, said in an interview that it is “important to realize that the spectrum of disease with COVID is wide, including mild disease, severe disease, and prolonged disease. This report helps us understand some of the risk factors for those with prolonged symptoms and may allow us to refine even more clearly how we prioritize treatment and vaccine administration, once available.
“It also highlights the challenge of dealing with this virus. Not only do the symptoms vary widely, but so do the incubation period, the duration of symptoms, and the residual symptoms that sometimes occur. Clearly, there is much we still need to understand about this virus,” he said.
The interviews were conducted from April 15 to June 25 with a random sample of adults at least 18 years old who had received a first positive test result for SARS-CoV-2 at an outpatient visit at one of 14 US academic healthcare systems in 13 states.
A version of this article originally appeared on Medscape.com.
, according to survey results in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
Mark W. Tenforde, MD, PhD, for the CDC-COVID-19 Response Team, and colleagues conducted a multistate telephone survey of symptomatic adults who tested positive for SARS-CoV-2. The researchers found that 35% had not returned to their usual state of wellness when they were interviewed 2-3 weeks after testing.
Among the 270 of 274 people interviewed for whom there were data on return to health, 175 (65%) reported that they had returned to baseline health an average of 7 days from the date of testing.
Among the 274 symptomatic outpatients, the median number of symptoms was seven. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms.
Prolonged illness is well described in adults hospitalized with severe COVID-19, especially among the older adult population, but little is known about other groups.
The proportion who had not returned to health differed by age: 26% of interviewees aged 18-34 years, 32% of those aged 35-49 years, and 47% of those at least 50 years old reported not having returned to their usual health (P = .010) within 14-21 days after receiving positive test results.
Among respondents aged 18-34 years who had no chronic medical condition, 19% (9 of 48) reported not having returned to their usual state of health during that time.
Public health messaging targeting younger adults, a group who might not be expected to be sick for weeks with mild disease, is particularly important, the authors wrote.
Kyle Annen, DO, medical director of transfusion services and patient blood management at Children’s Hospital Colorado and assistant professor of pathology at the University of Colorado, Denver, said in an interview that an important message is that delayed recovery (symptoms of fatigue, cough, and shortness of breath) was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization.
“This should impact the perception of this being a mild illness in the young adult population and encourage them to comply with recommendations of social distancing, masking, and hand washing,” she said.
Recovery time of more than 2 weeks will affect work and school performance, especially prolonged fatigue, she noted. This was one of the prominent symptoms that were reported to be slow to dissipate.
“I think the most interesting point in this study is that of underlying conditions; psychiatric conditions were significantly correlated with prolonged recovery. I don’t think that many people think of depression and anxiety as an underlying medical condition in regards to COVID-19 risk. This could potentially have an impact, as depression and anxiety rates will likely increase as COVID-19 continues,” she said.
Buddy Creech, MD, MPH, said in an interview that it is “important to realize that the spectrum of disease with COVID is wide, including mild disease, severe disease, and prolonged disease. This report helps us understand some of the risk factors for those with prolonged symptoms and may allow us to refine even more clearly how we prioritize treatment and vaccine administration, once available.
“It also highlights the challenge of dealing with this virus. Not only do the symptoms vary widely, but so do the incubation period, the duration of symptoms, and the residual symptoms that sometimes occur. Clearly, there is much we still need to understand about this virus,” he said.
The interviews were conducted from April 15 to June 25 with a random sample of adults at least 18 years old who had received a first positive test result for SARS-CoV-2 at an outpatient visit at one of 14 US academic healthcare systems in 13 states.
A version of this article originally appeared on Medscape.com.
, according to survey results in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
Mark W. Tenforde, MD, PhD, for the CDC-COVID-19 Response Team, and colleagues conducted a multistate telephone survey of symptomatic adults who tested positive for SARS-CoV-2. The researchers found that 35% had not returned to their usual state of wellness when they were interviewed 2-3 weeks after testing.
Among the 270 of 274 people interviewed for whom there were data on return to health, 175 (65%) reported that they had returned to baseline health an average of 7 days from the date of testing.
Among the 274 symptomatic outpatients, the median number of symptoms was seven. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms.
Prolonged illness is well described in adults hospitalized with severe COVID-19, especially among the older adult population, but little is known about other groups.
The proportion who had not returned to health differed by age: 26% of interviewees aged 18-34 years, 32% of those aged 35-49 years, and 47% of those at least 50 years old reported not having returned to their usual health (P = .010) within 14-21 days after receiving positive test results.
Among respondents aged 18-34 years who had no chronic medical condition, 19% (9 of 48) reported not having returned to their usual state of health during that time.
Public health messaging targeting younger adults, a group who might not be expected to be sick for weeks with mild disease, is particularly important, the authors wrote.
Kyle Annen, DO, medical director of transfusion services and patient blood management at Children’s Hospital Colorado and assistant professor of pathology at the University of Colorado, Denver, said in an interview that an important message is that delayed recovery (symptoms of fatigue, cough, and shortness of breath) was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization.
“This should impact the perception of this being a mild illness in the young adult population and encourage them to comply with recommendations of social distancing, masking, and hand washing,” she said.
Recovery time of more than 2 weeks will affect work and school performance, especially prolonged fatigue, she noted. This was one of the prominent symptoms that were reported to be slow to dissipate.
“I think the most interesting point in this study is that of underlying conditions; psychiatric conditions were significantly correlated with prolonged recovery. I don’t think that many people think of depression and anxiety as an underlying medical condition in regards to COVID-19 risk. This could potentially have an impact, as depression and anxiety rates will likely increase as COVID-19 continues,” she said.
Buddy Creech, MD, MPH, said in an interview that it is “important to realize that the spectrum of disease with COVID is wide, including mild disease, severe disease, and prolonged disease. This report helps us understand some of the risk factors for those with prolonged symptoms and may allow us to refine even more clearly how we prioritize treatment and vaccine administration, once available.
“It also highlights the challenge of dealing with this virus. Not only do the symptoms vary widely, but so do the incubation period, the duration of symptoms, and the residual symptoms that sometimes occur. Clearly, there is much we still need to understand about this virus,” he said.
The interviews were conducted from April 15 to June 25 with a random sample of adults at least 18 years old who had received a first positive test result for SARS-CoV-2 at an outpatient visit at one of 14 US academic healthcare systems in 13 states.
A version of this article originally appeared on Medscape.com.
Small NY study: Mother-baby transmission of COVID-19 not seen
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
according to a study out of New York-Presbyterian Hospital.
“It is suggested in the cumulative data that the virus does not confer additional risk to the fetus during labor or during the early postnatal period in both preterm and term infants,” concluded Jeffrey Perlman, MB ChB, and colleagues in Pediatrics.
But other experts suggest substantial gaps remain in our understanding of maternal transmission of SARS-CoV-2.
“Much more needs to be known,” Munish Gupta, MD, and colleagues from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, said in an accompanying editorial.
The prospective study is the first to describe a cohort of U.S. COVID-19–related deliveries, with the prior neonatal impact of COVID-19 “almost exclusively” reported from China, noted the authors. They included a cohort of 326 women who were tested for SARS-CoV-2 on admission to labor and delivery at New York-Presbyterian Hospital between March 22 and April 15th, 2020. Of the 31 (10%) mothers who tested positive, 15 (48%) were asymptomatic and 16 (52%) were symptomatic.
Two babies were born prematurely (one by Cesarean) and were isolated in negative pressure rooms with continuous positive airway pressure. Both were moved out of isolation after two negative test results and “have exhibited an unremarkable clinical course,” the authors reported.
The other 29 term babies were cared for in their mothers’ rooms, with breastfeeding allowed, if desired. These babies and their mothers were discharged from the hospital between 24 and 48 hours after delivery.
“Visitor restriction for mothers who were positive for COVID-19 included 14 days of no visitation from the start of symptoms,” noted the team.
They added “since the prepublication release there have been a total of 47 mothers positive for COVID-19, resulting in 47 infants; 4 have been admitted to neonatal intensive care. In addition, 32 other infants have been tested for a variety of indications within the unit. All infants test results have been negative.”
The brief report outlined the institution’s checklist for delivery preparedness in either the operating room or labor delivery room, including personal protective equipment, resuscitation, transportation to the neonatal intensive care unit, and early postresuscitation care. “Suspected or confirmed COVID-19 alone in an otherwise uncomplicated pregnancy is not an indication for the resuscitation team or the neonatal fellow,” they noted, adding delivery room preparation and management should include contact precautions. “With scrupulous attention to infectious precautions, horizontal viral transmission should be minimized,” they advised.
Dr. Perlman and associates emphasized that rapid turnaround SARSCoV-2 testing is “crucial to minimize the likelihood of a provider becoming infected and/or infecting the infant.”
Although the findings are “clearly reassuring,” Dr. Gupta and colleagues have reservations. “To what extent does this report address concerns for infection risk with a rooming-in approach to care?” they asked in their accompanying editorial. “The answer is likely some, but not much.”
Many questions remain, they said, including: “What precautions were used to minimize infection risk during the postbirth hospital course? What was the approach to skin-to-skin care and direct mother-newborn contact? Were restrictions placed on family members? Were changes made to routine interventions such as hearing screens or circumcisions? What practices were in place around environmental cleaning? Most important, how did the newborns do after discharge?”
The current uncertainty around neonatal COVID-19 infection risk has led to “disparate” variations in care recommendations, they pointed out. Whereas China’s consensus guidelines recommend a 14-day separation of COVID-19–positive mothers from their healthy infants, a practice supported by the American Academy of Pediatrics “when possible,” the Italian Society of Neonatology, the Royal College of Paediatrics and Child Health, and the Canadian Paediatric Society advise “rooming-in and breastfeeding with appropriate infection prevention measures.”
Dr. Gupta and colleagues pointed to the following as at least three “critical and time-sensitive needs for research around neonatal care and outcomes related to COVID-19”:
- Studies need to have much larger sample sizes and include diverse populations. This will allow for reliable measurement of outcomes.
- Descriptions of care practices must be in detail, especially about infection prevention; these should be presented in a way to compare the efficacy of different approaches.
- There needs to be follow-up information on outcomes of both the mother and the neonate after the birth hospitalization.
Asked to comment, Lillian Beard, MD, of George Washington University in Washington welcomed the data as “good news.”
“Although small, the study was done during a 3-week peak period at the hottest spot of the pandemic in the United States during that period. It illustrates how delivery room preparedness, adequate personal protective equipment, and carefully planned infection control precautions can positively impact outcomes even during a seemingly impossible period,” she said.
“Although there are many uncertainties about maternal COVID-19 transmission and neonatal infection risks ... in my opinion, during the after birth hospitalization, the inherent benefits of rooming in for breast feeding and the opportunities for the demonstration and teaching of infection prevention practices for the family home, far outweigh the risks of disease transmission,” said Dr. Beard, who was not involved with the study.
The study and the commentary emphasize the likely low risk of vertical transmission of the virus, with horizontal transmission being the greater risk. However, cases of transplacental transmission have been reported, and the lead investigator of one recent placental study cautions against complacency.
“Neonates can get infected in both ways. The majority of cases seem to be horizontal, but those who have been infected or highly suspected to be vertically infected are not a small percentage either,” said Daniele de Luca, MD, PhD, president-elect of the European Society for Pediatric and Neonatal Intensive Care (ESPNIC) and a neonatologist at Antoine Béclère Hospital in Clamart, France.
“Perlman’s data are interesting and consistent with other reports around the world. However, two things must be remembered,” he said in an interview. “First, newborn infants are at relatively low risk from SARS-CoV-2 infections, but this is very far from zero risk. Neonatal SARS-CoV-2 infections do exist and have been described around the world. While they have a mild course in the majority of cases, neonatologists should not forget them and should be prepared to offer the best care to these babies.”
“Second, how this can be balanced with the need to promote breastfeeding and avoid overtreatment or separation from the mother is a question far from being answered. Gupta et al. in their commentary are right in saying that we have more questions than answers. While waiting for the results of large initiatives (such as the ESPNIC EPICENTRE Registry that they cite) to answer these open points, the best we can do is to provide a personalised case by case approach, transparent information to parents, and an open counselling informing clinical decisions.”
The study received no external funding. Dr. Perlman and associates had no financial disclosures. Dr. Gupta and colleagues had no relevant financial disclosures. Neither Dr. Beard nor Dr. de Luca had any relevant financial disclosures.
SOURCE: Perlman J et al. Pediatrics. 2020;146(2):e20201567.
FROM PEDIATRICS