User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Cognitive Behavioral Therapy Plus Placebo Is Inferior to NSAID Therapy for Arthritis Pain
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
Study Overview
Objective. To examine whether discontinuation of nonsteroidal anti-inflammatory drug (NSAID) therapy and initiation of telephone-based cognitive behavioral therapy (CBT) is not worse than continuation of NSAIDs in the management of arthritis pain.
Design. Randomized controlled trial with noninferiority design.
Setting and participants. This study was a multicenter trial conducted across 4 Veterans Affairs health care systems in Boston, Providence, Connecticut, and North Florida/South Georgia that started September 2013 and ended September 2018. Eligibility criteria included being age 20 years or older, radiographic evidence of knee osteoarthritis, and use of an NSAID for knee pain on most days of the month for at least the past 3 months. Exclusion criteria included significant hearing impairments that may impede the conduct of the trial, current opioid prescriptions excluding tramadol, contraindications to NSAID use, recent or scheduled intra-articular injections or surgery, comorbid conditions other than knee pain that limited walking, and bilateral knee replacements or pain only in the replaced knee. Concurrent use of tramadol and other non-NSAID analgesics was permitted.
A total of 490 participants took part in the 2-week run-in period where their NSAID regimen was discontinued and they were started on a standardized dose of the NSAID meloxicam 15 mg daily. During the run-in period, 126 participants were excluded for several reasons, including worsening pain and patient withdrawal, yielding 364 participants who were randomized to continue meloxicam treatment or placebo for 4 weeks with blinding.
Intervention. Subsequent to the 4-week phase 1 placebo controlled trial, participants in the placebo group were given CBT via telephone (unblinded) for 10 weeks, and the meloxicam group continued treatment with meloxicam for phase 2. The CBT group received 10 modules over 10 weeks in 30- to 45-minute telephone contacts with a psychologist using a treatment manual modified for knee osteoarthritis. These modules consisted of 1 introductory module, 8 pain coping skills modules (eg, deep breathing and visual imagery, progressive muscle relaxation, physical activity and bodily mechanics, identifying unhealthy thoughts, balancing unhealthy thoughts, managing stress, time-based pacing, and sleep hygiene), and a final module emphasizing skill consolidation and relapse prevention. Outcomes were assessed at the end of the phase 1 and phase 2 periods.
Main outcome measures. Main study outcome measures included pain as measured with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at 4 weeks. Secondary outcomes included the WOMAC pain score, disability score, and global impression of change after treatment at 14 weeks. The WOMAC pain scale ranges from 0 to 20, and consists of 5 questions regarding severity of pain during walking, stair use, lying in bed at night, sitting, and standing, with 0 indicating no pain; 1, mild pain; 2, moderate pain; 3, severe pain; and 4, very severe pain for each item. The WOMAC disability scale measures self-reported difficulty in performing tasks that reflect lower-extremity physical function, including climbing stairs, rising from a chair, walking, and other activities of daily living. The global impression of change after treatment was measured on a 5-point scale (where 1 indicates much better and 5 indicates much worse). The minimum clinically important difference of the WOMAC pain scale is 2, based on prior literature. With the noninferiority design, the margin was set as a score of 1.
Main results. The placebo group consisted of 180 participants, with an average age of 58.2 years (SD, 11.8 years); 89% of them were male. The meloxicam group consisted of 184 participants, with an average age of 58.6 years (SD, 10 years); 84% of them were male. The average body mass index was 33.9 and 33.4 in each group, respectively. For the primary outcome, the placebo group had a worse pain score than the meloxicam group at 4 weeks (difference of 1.4; 95% confidence interval, 0.8- 2.0). At 14 weeks, the placebo group (with CBT) had a worse pain score than the meloxicam group (difference of 0.8; 95% CI, 0.2-1.4). There was no statistically significant difference in the disability score or global impression of change after treatment score between the 2 groups. The observed difference in pain score did not, however, exceed the minimum clinically important difference.
Conclusion. Placebo treatment and CBT are inferior to NSAIDs in managing pain for patients with knee osteoarthritis. The difference in pain may not be clinically important, and there were no differences in function at 14 weeks.
Commentary
Osteoarthritis is a common chronic condition that causes pain and disability and is often treated with oral analgesics. NSAIDs, despite few high-quality trials demonstrating their efficacy, are among the most commonly used treatment for osteoarthritis pain.1 NSAID therapy, however, does have potential side effects, such as gastric reflux and renal dysfunction.2 This withdrawal trial with placebo control contributes further evidence of the effectiveness of NSAIDs on knee osteoarthritis, demonstrating that indeed NSAIDs improve pain scores to a greater degree than placebo treatment. Augmenting placebo treatment with nonpharmacologic CBT was inferior to NSAIDs in pain management. The authors pointed out that the difference in pain score may not be clinically important, and that lower-extremity function was not different between the groups, concluding that, despite the higher pain score, CBT could be a treatment option, particularly for those who may have difficulty tolerating NSAID treatment.
The study population had a number of chronic conditions in addition to having knee arthritis, and thus likely were taking multiple medications for chronic disease management. Use of multiple medications is associated with an increased risk of rug interactions and adverse effects of medications.3 Thus, this attempt to assess whether a nonpharmacologic alternative treatment is noninferior to a drug treatment is a step toward building the evidence base for deprescribing and enhancing medication safety.4 Previous studies have examined other nonpharmacologic treatments for knee arthritis, such as acupuncture,5 and it is worthwhile to consider combining nonpharmacological approaches as an alternative to oral analgesic medication use.
Applications for Clinical Practice
This study advances our understanding of the effect of NSAID use on knee osteoarthritis when compared to placebo with CBT. Although this is a negative study that failed to show that placebo combined with CBT is noninferior to NSAIDs, it did quantify the expected treatment effect of NSAIDs and showed that this effect likely is not clinically important and/or does not alter lower-extremity function. Further studies are needed to identify other nonpharmacologic approaches and test whether combinations of approaches are effective in the management of chronic pain from osteoarthritis.
–William W. Hung, MD, MPH
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
1. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143-150.
2. Pilotto A, Franceschi M, Leandro G, Di Mario F. NSAID and aspirin use by the elderly in general practice: effect on gastrointestinal symptoms and therapies. Drugs Aging. 2003;20:701-710.
3. Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Intern Med. 2016;176:482-483.
4. Rashid R, Chang C, Niu F, et al. Evaluation of a pharmacist-managed nonsteroidal anti-inflammatory drugs deprescribing program in an integrated health care system. J Manag Care Spec Pharm. 2020;26:918-924.
5. Sun J, Zhao Y, Zhu R, et al. Acupotomy therapy for knee osteoarthritis pain: systematic review and meta-analysis. Evid Based Complement Alternat Med. 2020;2020:2168283.
FDA approves first agent for PSMA-PET imaging in prostate cancer
A radioactive diagnostic agent has been approved by the U.S. Food and Drug Administration for use in patients with prostate cancer, but only for those treated at two institutions in California.
The product, Gallium 68 PSMA-11 (Ga 68 PSMA-11), is the first agent approved specifically for use in positron-emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA)–positive lesions in men with prostate cancer, the FDA noted.
This imaging approach can “detect whether or not the cancer has spread to other parts of the body,” commented Alex Gorovets, MD, acting deputy director of the Office of Specialty Medicine in the FDA’s Center for Drug Evaluation and Research.
Ga 68 PSMA-11 is indicated for use in patients with suspected prostate cancer metastasis whose conditions are potentially curable by surgery or radiotherapy and in patients with suspected prostate cancer recurrence, as determined on the basis of elevated serum prostate-specific antigen (PSA) levels.
Institutional use only
Ga 68 PSMA-11 has been approved for institutional use at the University of California, Los Angeles and the University of California, San Francisco under an academic new drug application (NDA).
The FDA approval was based partly on a clinical trial conducted by the UCSF and UCLA research teams on the effectiveness of PSMA-PET.
“It is rare for academic institutions to obtain FDA approval of a drug, and this unique collaboration has led to what is one of the first coapprovals of a drug at two institutions,” said Thomas Hope, MD, an associate professor at UCSF. “We hope that this first step will lead to a more widespread availability of this imaging test to men with prostate cancer throughout the country.”
Ga 68 PSMA-11 was developed outside the United States at the University of Heidelberg (Germany).
A commercial NDA from Telix Pharmaceuticals for TL591-CDx, a radiopharmaceutical cold kit for the preparation of Ga 68 PSMA-11 injection, is under consideration by the FDA.
The agency noted that two other PET diagnostic agents – fluciclovine F18 and choline C11 – are approved for prostate cancer imaging. However, they are only approved for use in patients with suspected cancer recurrence.
Trial results with PSMA-PET/CT
“PSMA-PET/CT is a novel molecular and functional imaging modality specific for prostate cancer cells that has good sensitivity and outstanding specificity in detecting metastasis,” commented T. Martin Ma, MD, PhD, of UCLA.
Dr. Ma presented a U.S. study on the technique at the recent annual meeting of the American Society for Radiation Oncology. That study showed that PSMA-PET/CT led to nodal upstaging in 19.7% of patients and metastasis upstaging in 9.4%.
He said these results were similar to those from the Australian proPSMA trial, which was published in The Lancet earlier this year. That trial found PSMA-PET/CT to be superior to conventional imaging with CT and bone scanning for primary staging of high-risk prostate cancer.
“These findings carry significant clinical implications and can affect treatment decision-making,” Dr. Ma commented.
“PSMA-PET has been a real game changer in high-risk prostate cancer and has implications in the various stages of prostate cancer management from diagnosis and staging to theranostics,” said Renu Eapen, MBBS, of Peter MacCallum Cancer Center, Melbourne, who was not involved in either study.
“PSMA-PET/CT has challenged conventional imaging in staging before curative-intent surgery or radiotherapy,” Dr. Eapen added.
The accuracy of PSMA-PET/CT was 27% higher than that of conventional imaging in the proPSMA trial, she noted in an interview last month. This superior accuracy can ultimately affect management. The imaging has additional benefits of lower radiation dose as well as reproducibility with high reporter agreement, potentially making it a “one-stop-shop” scan.
Trial results with Ga 68 PSMA-11
The safety and efficacy of Ga 68 PSMA-11 were evaluated in two prospective clinical trials with a total of 960 men with prostate cancer, each of whom received one injection of the product.
The first trial involved 325 patients with biopsy-proven prostate cancer who underwent PET/CT or PET/MRI scans performed with Ga 68 PSMA-11.
“These patients were candidates for surgical removal of the prostate gland and pelvic lymph nodes and were considered at higher risk for metastasis. Among the patients who proceeded to surgery, those with positive readings in the pelvic lymph nodes on Ga 68 PSMA-11 PET had a clinically important rate of metastatic cancer confirmed by surgical pathology,” the FDA noted.
“The availability of this information prior to treatment is expected to have important implications for patient care,” the FDA commented. “For example, it may spare certain patients from undergoing unnecessary surgery.”
The second trial enrolled 635 patients with rising serum PSA levels after initial prostate surgery or radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT scan or PET/MRI scan.
About three-quarters of patients (74%) had at least one positive lesion detected by Ga 68 PSMA-11 PET in at least one region – bone, prostate bed, pelvic lymph node, or extra-pelvic soft tissue.
“In patients with positive Ga 68 PSMA-11 PET readings who had correlative tissue pathology from biopsies, results from baseline or follow-up imaging by conventional methods, and serial PSA levels available for comparison, local recurrence or metastasis of prostate cancer was confirmed in an estimated 91% of cases,” the FDA noted.
“Thus, the second trial demonstrated that Ga 68 PSMA-11 PET can detect sites of disease in patients with biochemical evidence of recurrent prostate cancer, thereby providing important information that may impact the approach to therapy,” the agency added.
The FDA also noted that no serious adverse reactions were attributed to Ga 68 PSMA-11. The most common adverse reactions were nausea, diarrhea, and dizziness.
The FDA said there is a risk for misdiagnosis because Ga 68 PSMA-11 binding may occur in other types of cancer, and certain nonmalignant processes may lead to errors in interpreting images. In addition, there are radiation risks because Ga 68 PSMA-11 contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer.
A version of this article originally appeared on Medscape.com.
A radioactive diagnostic agent has been approved by the U.S. Food and Drug Administration for use in patients with prostate cancer, but only for those treated at two institutions in California.
The product, Gallium 68 PSMA-11 (Ga 68 PSMA-11), is the first agent approved specifically for use in positron-emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA)–positive lesions in men with prostate cancer, the FDA noted.
This imaging approach can “detect whether or not the cancer has spread to other parts of the body,” commented Alex Gorovets, MD, acting deputy director of the Office of Specialty Medicine in the FDA’s Center for Drug Evaluation and Research.
Ga 68 PSMA-11 is indicated for use in patients with suspected prostate cancer metastasis whose conditions are potentially curable by surgery or radiotherapy and in patients with suspected prostate cancer recurrence, as determined on the basis of elevated serum prostate-specific antigen (PSA) levels.
Institutional use only
Ga 68 PSMA-11 has been approved for institutional use at the University of California, Los Angeles and the University of California, San Francisco under an academic new drug application (NDA).
The FDA approval was based partly on a clinical trial conducted by the UCSF and UCLA research teams on the effectiveness of PSMA-PET.
“It is rare for academic institutions to obtain FDA approval of a drug, and this unique collaboration has led to what is one of the first coapprovals of a drug at two institutions,” said Thomas Hope, MD, an associate professor at UCSF. “We hope that this first step will lead to a more widespread availability of this imaging test to men with prostate cancer throughout the country.”
Ga 68 PSMA-11 was developed outside the United States at the University of Heidelberg (Germany).
A commercial NDA from Telix Pharmaceuticals for TL591-CDx, a radiopharmaceutical cold kit for the preparation of Ga 68 PSMA-11 injection, is under consideration by the FDA.
The agency noted that two other PET diagnostic agents – fluciclovine F18 and choline C11 – are approved for prostate cancer imaging. However, they are only approved for use in patients with suspected cancer recurrence.
Trial results with PSMA-PET/CT
“PSMA-PET/CT is a novel molecular and functional imaging modality specific for prostate cancer cells that has good sensitivity and outstanding specificity in detecting metastasis,” commented T. Martin Ma, MD, PhD, of UCLA.
Dr. Ma presented a U.S. study on the technique at the recent annual meeting of the American Society for Radiation Oncology. That study showed that PSMA-PET/CT led to nodal upstaging in 19.7% of patients and metastasis upstaging in 9.4%.
He said these results were similar to those from the Australian proPSMA trial, which was published in The Lancet earlier this year. That trial found PSMA-PET/CT to be superior to conventional imaging with CT and bone scanning for primary staging of high-risk prostate cancer.
“These findings carry significant clinical implications and can affect treatment decision-making,” Dr. Ma commented.
“PSMA-PET has been a real game changer in high-risk prostate cancer and has implications in the various stages of prostate cancer management from diagnosis and staging to theranostics,” said Renu Eapen, MBBS, of Peter MacCallum Cancer Center, Melbourne, who was not involved in either study.
“PSMA-PET/CT has challenged conventional imaging in staging before curative-intent surgery or radiotherapy,” Dr. Eapen added.
The accuracy of PSMA-PET/CT was 27% higher than that of conventional imaging in the proPSMA trial, she noted in an interview last month. This superior accuracy can ultimately affect management. The imaging has additional benefits of lower radiation dose as well as reproducibility with high reporter agreement, potentially making it a “one-stop-shop” scan.
Trial results with Ga 68 PSMA-11
The safety and efficacy of Ga 68 PSMA-11 were evaluated in two prospective clinical trials with a total of 960 men with prostate cancer, each of whom received one injection of the product.
The first trial involved 325 patients with biopsy-proven prostate cancer who underwent PET/CT or PET/MRI scans performed with Ga 68 PSMA-11.
“These patients were candidates for surgical removal of the prostate gland and pelvic lymph nodes and were considered at higher risk for metastasis. Among the patients who proceeded to surgery, those with positive readings in the pelvic lymph nodes on Ga 68 PSMA-11 PET had a clinically important rate of metastatic cancer confirmed by surgical pathology,” the FDA noted.
“The availability of this information prior to treatment is expected to have important implications for patient care,” the FDA commented. “For example, it may spare certain patients from undergoing unnecessary surgery.”
The second trial enrolled 635 patients with rising serum PSA levels after initial prostate surgery or radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT scan or PET/MRI scan.
About three-quarters of patients (74%) had at least one positive lesion detected by Ga 68 PSMA-11 PET in at least one region – bone, prostate bed, pelvic lymph node, or extra-pelvic soft tissue.
“In patients with positive Ga 68 PSMA-11 PET readings who had correlative tissue pathology from biopsies, results from baseline or follow-up imaging by conventional methods, and serial PSA levels available for comparison, local recurrence or metastasis of prostate cancer was confirmed in an estimated 91% of cases,” the FDA noted.
“Thus, the second trial demonstrated that Ga 68 PSMA-11 PET can detect sites of disease in patients with biochemical evidence of recurrent prostate cancer, thereby providing important information that may impact the approach to therapy,” the agency added.
The FDA also noted that no serious adverse reactions were attributed to Ga 68 PSMA-11. The most common adverse reactions were nausea, diarrhea, and dizziness.
The FDA said there is a risk for misdiagnosis because Ga 68 PSMA-11 binding may occur in other types of cancer, and certain nonmalignant processes may lead to errors in interpreting images. In addition, there are radiation risks because Ga 68 PSMA-11 contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer.
A version of this article originally appeared on Medscape.com.
A radioactive diagnostic agent has been approved by the U.S. Food and Drug Administration for use in patients with prostate cancer, but only for those treated at two institutions in California.
The product, Gallium 68 PSMA-11 (Ga 68 PSMA-11), is the first agent approved specifically for use in positron-emission tomography (PET) imaging of prostate-specific membrane antigen (PSMA)–positive lesions in men with prostate cancer, the FDA noted.
This imaging approach can “detect whether or not the cancer has spread to other parts of the body,” commented Alex Gorovets, MD, acting deputy director of the Office of Specialty Medicine in the FDA’s Center for Drug Evaluation and Research.
Ga 68 PSMA-11 is indicated for use in patients with suspected prostate cancer metastasis whose conditions are potentially curable by surgery or radiotherapy and in patients with suspected prostate cancer recurrence, as determined on the basis of elevated serum prostate-specific antigen (PSA) levels.
Institutional use only
Ga 68 PSMA-11 has been approved for institutional use at the University of California, Los Angeles and the University of California, San Francisco under an academic new drug application (NDA).
The FDA approval was based partly on a clinical trial conducted by the UCSF and UCLA research teams on the effectiveness of PSMA-PET.
“It is rare for academic institutions to obtain FDA approval of a drug, and this unique collaboration has led to what is one of the first coapprovals of a drug at two institutions,” said Thomas Hope, MD, an associate professor at UCSF. “We hope that this first step will lead to a more widespread availability of this imaging test to men with prostate cancer throughout the country.”
Ga 68 PSMA-11 was developed outside the United States at the University of Heidelberg (Germany).
A commercial NDA from Telix Pharmaceuticals for TL591-CDx, a radiopharmaceutical cold kit for the preparation of Ga 68 PSMA-11 injection, is under consideration by the FDA.
The agency noted that two other PET diagnostic agents – fluciclovine F18 and choline C11 – are approved for prostate cancer imaging. However, they are only approved for use in patients with suspected cancer recurrence.
Trial results with PSMA-PET/CT
“PSMA-PET/CT is a novel molecular and functional imaging modality specific for prostate cancer cells that has good sensitivity and outstanding specificity in detecting metastasis,” commented T. Martin Ma, MD, PhD, of UCLA.
Dr. Ma presented a U.S. study on the technique at the recent annual meeting of the American Society for Radiation Oncology. That study showed that PSMA-PET/CT led to nodal upstaging in 19.7% of patients and metastasis upstaging in 9.4%.
He said these results were similar to those from the Australian proPSMA trial, which was published in The Lancet earlier this year. That trial found PSMA-PET/CT to be superior to conventional imaging with CT and bone scanning for primary staging of high-risk prostate cancer.
“These findings carry significant clinical implications and can affect treatment decision-making,” Dr. Ma commented.
“PSMA-PET has been a real game changer in high-risk prostate cancer and has implications in the various stages of prostate cancer management from diagnosis and staging to theranostics,” said Renu Eapen, MBBS, of Peter MacCallum Cancer Center, Melbourne, who was not involved in either study.
“PSMA-PET/CT has challenged conventional imaging in staging before curative-intent surgery or radiotherapy,” Dr. Eapen added.
The accuracy of PSMA-PET/CT was 27% higher than that of conventional imaging in the proPSMA trial, she noted in an interview last month. This superior accuracy can ultimately affect management. The imaging has additional benefits of lower radiation dose as well as reproducibility with high reporter agreement, potentially making it a “one-stop-shop” scan.
Trial results with Ga 68 PSMA-11
The safety and efficacy of Ga 68 PSMA-11 were evaluated in two prospective clinical trials with a total of 960 men with prostate cancer, each of whom received one injection of the product.
The first trial involved 325 patients with biopsy-proven prostate cancer who underwent PET/CT or PET/MRI scans performed with Ga 68 PSMA-11.
“These patients were candidates for surgical removal of the prostate gland and pelvic lymph nodes and were considered at higher risk for metastasis. Among the patients who proceeded to surgery, those with positive readings in the pelvic lymph nodes on Ga 68 PSMA-11 PET had a clinically important rate of metastatic cancer confirmed by surgical pathology,” the FDA noted.
“The availability of this information prior to treatment is expected to have important implications for patient care,” the FDA commented. “For example, it may spare certain patients from undergoing unnecessary surgery.”
The second trial enrolled 635 patients with rising serum PSA levels after initial prostate surgery or radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT scan or PET/MRI scan.
About three-quarters of patients (74%) had at least one positive lesion detected by Ga 68 PSMA-11 PET in at least one region – bone, prostate bed, pelvic lymph node, or extra-pelvic soft tissue.
“In patients with positive Ga 68 PSMA-11 PET readings who had correlative tissue pathology from biopsies, results from baseline or follow-up imaging by conventional methods, and serial PSA levels available for comparison, local recurrence or metastasis of prostate cancer was confirmed in an estimated 91% of cases,” the FDA noted.
“Thus, the second trial demonstrated that Ga 68 PSMA-11 PET can detect sites of disease in patients with biochemical evidence of recurrent prostate cancer, thereby providing important information that may impact the approach to therapy,” the agency added.
The FDA also noted that no serious adverse reactions were attributed to Ga 68 PSMA-11. The most common adverse reactions were nausea, diarrhea, and dizziness.
The FDA said there is a risk for misdiagnosis because Ga 68 PSMA-11 binding may occur in other types of cancer, and certain nonmalignant processes may lead to errors in interpreting images. In addition, there are radiation risks because Ga 68 PSMA-11 contributes to a patient’s overall long-term cumulative radiation exposure, which is associated with an increased risk for cancer.
A version of this article originally appeared on Medscape.com.
Pandemic increases need for home-based care with remote monitoring of patients
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
First guidelines for keto diets in adults with epilepsy released
Just as in children with epilepsy, ketogenic diet therapies can be safe and effective in adults with epilepsy but should only be undertaken with the support of medical professionals trained in their use, the group said.
“Motivation is the key to successful ketogenic diet therapy adherence,” first author Mackenzie Cervenka, MD, director of the Adult Epilepsy Diet Center and associate professor of neurology at Johns Hopkins University, Baltimore, said in an interview.
“Patients who are autonomous require self-motivation and having a strong support structure is important as well. For those patients who are dependents, their caregivers need to be motivated to manage their diet,” said Dr. Cervenka.
The guidelines were published online Oct. 30 in Neurology Clinical Practice.
Novel in adult neurology
Ketogenic diet therapies are high-fat, low-carbohydrate, and adequate-protein diets that induce fat metabolism and ketone production. Despite its use as an effective antiseizure therapy since the 1920s, ketogenic diet therapies remain novel in adult neurology.
Furthermore, while there are established guidelines for ketogenic diet therapies to reduce seizures in children, there were no formal recommendations for adults, until now.
Drawing on the experience of experts at 20 centers using ketogenic diet therapies in more than 2,100 adults with epilepsy in 10 countries, Dr. Cervenka and an international team developed recommendations on use of ketogenic diet therapies in adults.
The panel noted, “with a relatively mild side effect profile and the potential to reduce seizures in nearly 60% of adults with drug-resistant epilepsy, ketogenic diet therapies should be part of the repertoire of available options.”
Ketogenic diet therapies are appropriate to offer to adults with seizure types and epilepsy syndromes for which these treatments are known to be effective in children, they said. These include tuberous sclerosis complex, Rett syndrome, Lennox-Gastaut syndrome, glucose transporter type 1 deficiency syndrome, genetic generalized epilepsies, and focal epilepsies caused by underlying migrational disorders and resistant to antiseizure medication.
However, adults with drug-resistant focal epilepsy should be offered surgical evaluation first, given the higher anticipated rate of seizure freedom via this route, the panel said.
A focus on compliance
Experts at nearly all of the centers report using two or more ketogenic diet therapies. Ninety percent use the modified Atkins diet, 84% use the classic ketogenic diet, and 63% use the modified ketogenic diet and/or low glycemic index treatment. More than half of the centers (58%) use medium-chain triglyceride oil in combination with another ketogenic diet therapy to boost ketone body production.
The most important factors influencing the choice of ketogenic diet therapy are ease of diet application for the patient (100%) and patient and/or caregiver preference, home setting, and mode of feeding (90% each).
The panel recommended that ketogenic diet therapies be tailored to fit the needs of the individual, taking into account his or her physical and mental characteristics, underlying medical conditions, food preferences, type and amount of support from family and others, level of self-sufficiency, feeding habits, and ease of following the diet.
“Most of the differences between the child and adult recommendations have to do with compliance. Often, it’s more of a challenge for adults than for children,” said Dr. Cervenka.
The panel recommended providing adult patients with recipe ideas, individualized training on the ketogenic diet lifestyle from a dietitian or nutritionist, and guidance for meal planning and preparation before starting the diet. This will provide the greatest likelihood of success, as patients often report difficulties coping with carbohydrate restriction.
“In pediatric practice, positive responders typically remain on a ketogenic diet therapy for 2 years before considering weaning. Ketogenic diet therapy in adults is not time-limited. However, a minimum of 3 months of ketogenic diet therapy is recommended before any judgment of response is made,” the panel advised.
The panel pointed out the absolute metabolic contraindications and cautions related to feeding difficulties, gastrointestinal dysfunction, and digestion remain the same for both children and adults. However, they added that a range of common adult conditions such as hyperlipidemia, heart disease, diabetes, low bone density, and pregnancy “bring additional consideration, caution, and monitoring to ketogenic diet therapy use.”
Beyond epilepsy
The guidelines also call for pre–ketogenic diet therapy biochemical studies to screen adults for preexisting abnormalities and establish a reference for comparing follow-up results after 3, 6, and 12 months, and then annually or as needed.
They also noted that metabolic studies such as urine organic acid and serum amino acid levels are generally not needed in adults unless there is a strong clinical suspicion for an underlying metabolic disorder.
Updated genetic evaluation may also be considered in adults with intellectual disability and epilepsy of unknown etiology. Serial bone mineral density scans may be obtained every 5 years.
The guidelines also call for ketone monitoring (blood beta-hydroxybutyrate or urine amino acids) during the early months of ketogenic diet therapy as an objective indication of compliance and biochemical response.
Dietary adjustments should focus on optimizing the treatment response, minimizing side effects, and maximizing sustainability.
Adults on a ketogenic diet therapy should also be advised to take multivitamin and mineral supplements and drink plenty of fluids.
The panel said emerging evidence also supports the use of ketogenic diet therapies in other adult neurologic disorders such as migraine, Parkinson’s disease, dementia, and multiple sclerosis.
However, the panel said further evidence is needed to guide recommendations on use of ketogenic diet therapies in other neurologic conditions.
The research had no targeted funding. Dr. Cervenka has reported receiving grants from Nutricia, Vitaflo, BrightFocus Foundation, and Army Research Laboratory; honoraria from the American Epilepsy Society, the Neurology Center, Epigenix, LivaNova, and Nutricia; royalties from Demos; and consulting for Nutricia, Glut1 Deficiency Foundation, and Sage Therapeutics. Disclosures for the other authors are listed in the article.
A version of this article originally appeared on Medscape.com.
Just as in children with epilepsy, ketogenic diet therapies can be safe and effective in adults with epilepsy but should only be undertaken with the support of medical professionals trained in their use, the group said.
“Motivation is the key to successful ketogenic diet therapy adherence,” first author Mackenzie Cervenka, MD, director of the Adult Epilepsy Diet Center and associate professor of neurology at Johns Hopkins University, Baltimore, said in an interview.
“Patients who are autonomous require self-motivation and having a strong support structure is important as well. For those patients who are dependents, their caregivers need to be motivated to manage their diet,” said Dr. Cervenka.
The guidelines were published online Oct. 30 in Neurology Clinical Practice.
Novel in adult neurology
Ketogenic diet therapies are high-fat, low-carbohydrate, and adequate-protein diets that induce fat metabolism and ketone production. Despite its use as an effective antiseizure therapy since the 1920s, ketogenic diet therapies remain novel in adult neurology.
Furthermore, while there are established guidelines for ketogenic diet therapies to reduce seizures in children, there were no formal recommendations for adults, until now.
Drawing on the experience of experts at 20 centers using ketogenic diet therapies in more than 2,100 adults with epilepsy in 10 countries, Dr. Cervenka and an international team developed recommendations on use of ketogenic diet therapies in adults.
The panel noted, “with a relatively mild side effect profile and the potential to reduce seizures in nearly 60% of adults with drug-resistant epilepsy, ketogenic diet therapies should be part of the repertoire of available options.”
Ketogenic diet therapies are appropriate to offer to adults with seizure types and epilepsy syndromes for which these treatments are known to be effective in children, they said. These include tuberous sclerosis complex, Rett syndrome, Lennox-Gastaut syndrome, glucose transporter type 1 deficiency syndrome, genetic generalized epilepsies, and focal epilepsies caused by underlying migrational disorders and resistant to antiseizure medication.
However, adults with drug-resistant focal epilepsy should be offered surgical evaluation first, given the higher anticipated rate of seizure freedom via this route, the panel said.
A focus on compliance
Experts at nearly all of the centers report using two or more ketogenic diet therapies. Ninety percent use the modified Atkins diet, 84% use the classic ketogenic diet, and 63% use the modified ketogenic diet and/or low glycemic index treatment. More than half of the centers (58%) use medium-chain triglyceride oil in combination with another ketogenic diet therapy to boost ketone body production.
The most important factors influencing the choice of ketogenic diet therapy are ease of diet application for the patient (100%) and patient and/or caregiver preference, home setting, and mode of feeding (90% each).
The panel recommended that ketogenic diet therapies be tailored to fit the needs of the individual, taking into account his or her physical and mental characteristics, underlying medical conditions, food preferences, type and amount of support from family and others, level of self-sufficiency, feeding habits, and ease of following the diet.
“Most of the differences between the child and adult recommendations have to do with compliance. Often, it’s more of a challenge for adults than for children,” said Dr. Cervenka.
The panel recommended providing adult patients with recipe ideas, individualized training on the ketogenic diet lifestyle from a dietitian or nutritionist, and guidance for meal planning and preparation before starting the diet. This will provide the greatest likelihood of success, as patients often report difficulties coping with carbohydrate restriction.
“In pediatric practice, positive responders typically remain on a ketogenic diet therapy for 2 years before considering weaning. Ketogenic diet therapy in adults is not time-limited. However, a minimum of 3 months of ketogenic diet therapy is recommended before any judgment of response is made,” the panel advised.
The panel pointed out the absolute metabolic contraindications and cautions related to feeding difficulties, gastrointestinal dysfunction, and digestion remain the same for both children and adults. However, they added that a range of common adult conditions such as hyperlipidemia, heart disease, diabetes, low bone density, and pregnancy “bring additional consideration, caution, and monitoring to ketogenic diet therapy use.”
Beyond epilepsy
The guidelines also call for pre–ketogenic diet therapy biochemical studies to screen adults for preexisting abnormalities and establish a reference for comparing follow-up results after 3, 6, and 12 months, and then annually or as needed.
They also noted that metabolic studies such as urine organic acid and serum amino acid levels are generally not needed in adults unless there is a strong clinical suspicion for an underlying metabolic disorder.
Updated genetic evaluation may also be considered in adults with intellectual disability and epilepsy of unknown etiology. Serial bone mineral density scans may be obtained every 5 years.
The guidelines also call for ketone monitoring (blood beta-hydroxybutyrate or urine amino acids) during the early months of ketogenic diet therapy as an objective indication of compliance and biochemical response.
Dietary adjustments should focus on optimizing the treatment response, minimizing side effects, and maximizing sustainability.
Adults on a ketogenic diet therapy should also be advised to take multivitamin and mineral supplements and drink plenty of fluids.
The panel said emerging evidence also supports the use of ketogenic diet therapies in other adult neurologic disorders such as migraine, Parkinson’s disease, dementia, and multiple sclerosis.
However, the panel said further evidence is needed to guide recommendations on use of ketogenic diet therapies in other neurologic conditions.
The research had no targeted funding. Dr. Cervenka has reported receiving grants from Nutricia, Vitaflo, BrightFocus Foundation, and Army Research Laboratory; honoraria from the American Epilepsy Society, the Neurology Center, Epigenix, LivaNova, and Nutricia; royalties from Demos; and consulting for Nutricia, Glut1 Deficiency Foundation, and Sage Therapeutics. Disclosures for the other authors are listed in the article.
A version of this article originally appeared on Medscape.com.
Just as in children with epilepsy, ketogenic diet therapies can be safe and effective in adults with epilepsy but should only be undertaken with the support of medical professionals trained in their use, the group said.
“Motivation is the key to successful ketogenic diet therapy adherence,” first author Mackenzie Cervenka, MD, director of the Adult Epilepsy Diet Center and associate professor of neurology at Johns Hopkins University, Baltimore, said in an interview.
“Patients who are autonomous require self-motivation and having a strong support structure is important as well. For those patients who are dependents, their caregivers need to be motivated to manage their diet,” said Dr. Cervenka.
The guidelines were published online Oct. 30 in Neurology Clinical Practice.
Novel in adult neurology
Ketogenic diet therapies are high-fat, low-carbohydrate, and adequate-protein diets that induce fat metabolism and ketone production. Despite its use as an effective antiseizure therapy since the 1920s, ketogenic diet therapies remain novel in adult neurology.
Furthermore, while there are established guidelines for ketogenic diet therapies to reduce seizures in children, there were no formal recommendations for adults, until now.
Drawing on the experience of experts at 20 centers using ketogenic diet therapies in more than 2,100 adults with epilepsy in 10 countries, Dr. Cervenka and an international team developed recommendations on use of ketogenic diet therapies in adults.
The panel noted, “with a relatively mild side effect profile and the potential to reduce seizures in nearly 60% of adults with drug-resistant epilepsy, ketogenic diet therapies should be part of the repertoire of available options.”
Ketogenic diet therapies are appropriate to offer to adults with seizure types and epilepsy syndromes for which these treatments are known to be effective in children, they said. These include tuberous sclerosis complex, Rett syndrome, Lennox-Gastaut syndrome, glucose transporter type 1 deficiency syndrome, genetic generalized epilepsies, and focal epilepsies caused by underlying migrational disorders and resistant to antiseizure medication.
However, adults with drug-resistant focal epilepsy should be offered surgical evaluation first, given the higher anticipated rate of seizure freedom via this route, the panel said.
A focus on compliance
Experts at nearly all of the centers report using two or more ketogenic diet therapies. Ninety percent use the modified Atkins diet, 84% use the classic ketogenic diet, and 63% use the modified ketogenic diet and/or low glycemic index treatment. More than half of the centers (58%) use medium-chain triglyceride oil in combination with another ketogenic diet therapy to boost ketone body production.
The most important factors influencing the choice of ketogenic diet therapy are ease of diet application for the patient (100%) and patient and/or caregiver preference, home setting, and mode of feeding (90% each).
The panel recommended that ketogenic diet therapies be tailored to fit the needs of the individual, taking into account his or her physical and mental characteristics, underlying medical conditions, food preferences, type and amount of support from family and others, level of self-sufficiency, feeding habits, and ease of following the diet.
“Most of the differences between the child and adult recommendations have to do with compliance. Often, it’s more of a challenge for adults than for children,” said Dr. Cervenka.
The panel recommended providing adult patients with recipe ideas, individualized training on the ketogenic diet lifestyle from a dietitian or nutritionist, and guidance for meal planning and preparation before starting the diet. This will provide the greatest likelihood of success, as patients often report difficulties coping with carbohydrate restriction.
“In pediatric practice, positive responders typically remain on a ketogenic diet therapy for 2 years before considering weaning. Ketogenic diet therapy in adults is not time-limited. However, a minimum of 3 months of ketogenic diet therapy is recommended before any judgment of response is made,” the panel advised.
The panel pointed out the absolute metabolic contraindications and cautions related to feeding difficulties, gastrointestinal dysfunction, and digestion remain the same for both children and adults. However, they added that a range of common adult conditions such as hyperlipidemia, heart disease, diabetes, low bone density, and pregnancy “bring additional consideration, caution, and monitoring to ketogenic diet therapy use.”
Beyond epilepsy
The guidelines also call for pre–ketogenic diet therapy biochemical studies to screen adults for preexisting abnormalities and establish a reference for comparing follow-up results after 3, 6, and 12 months, and then annually or as needed.
They also noted that metabolic studies such as urine organic acid and serum amino acid levels are generally not needed in adults unless there is a strong clinical suspicion for an underlying metabolic disorder.
Updated genetic evaluation may also be considered in adults with intellectual disability and epilepsy of unknown etiology. Serial bone mineral density scans may be obtained every 5 years.
The guidelines also call for ketone monitoring (blood beta-hydroxybutyrate or urine amino acids) during the early months of ketogenic diet therapy as an objective indication of compliance and biochemical response.
Dietary adjustments should focus on optimizing the treatment response, minimizing side effects, and maximizing sustainability.
Adults on a ketogenic diet therapy should also be advised to take multivitamin and mineral supplements and drink plenty of fluids.
The panel said emerging evidence also supports the use of ketogenic diet therapies in other adult neurologic disorders such as migraine, Parkinson’s disease, dementia, and multiple sclerosis.
However, the panel said further evidence is needed to guide recommendations on use of ketogenic diet therapies in other neurologic conditions.
The research had no targeted funding. Dr. Cervenka has reported receiving grants from Nutricia, Vitaflo, BrightFocus Foundation, and Army Research Laboratory; honoraria from the American Epilepsy Society, the Neurology Center, Epigenix, LivaNova, and Nutricia; royalties from Demos; and consulting for Nutricia, Glut1 Deficiency Foundation, and Sage Therapeutics. Disclosures for the other authors are listed in the article.
A version of this article originally appeared on Medscape.com.
Reducing Inappropriate Laboratory Testing in the Hospital Setting: How Low Can We Go?
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9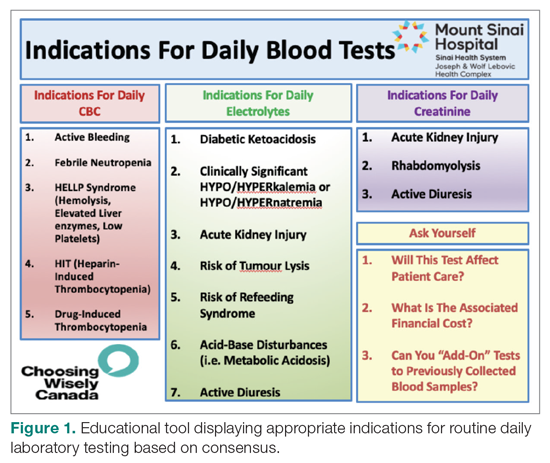
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12
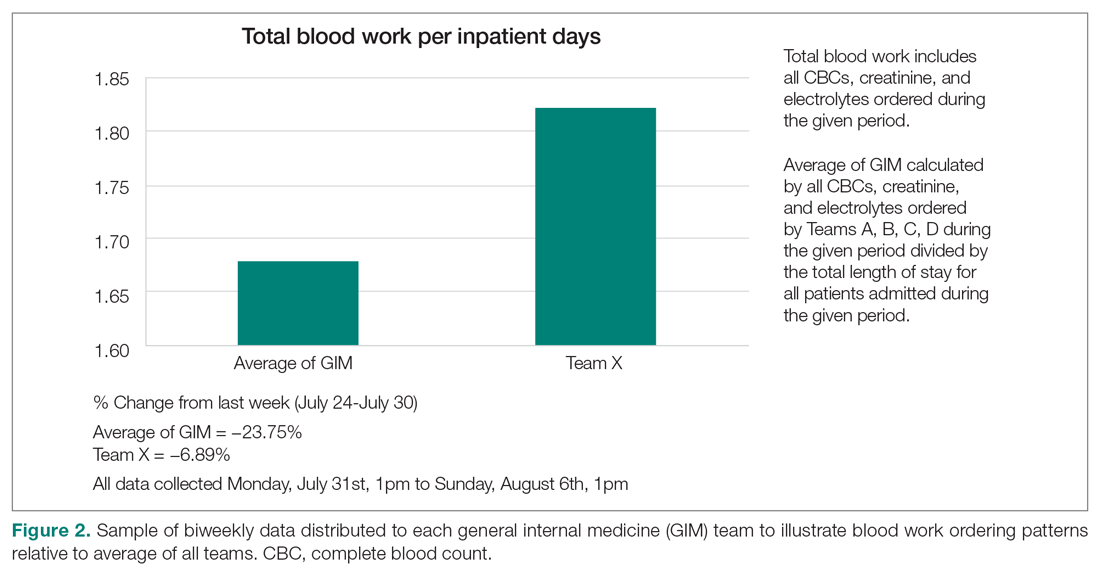
Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 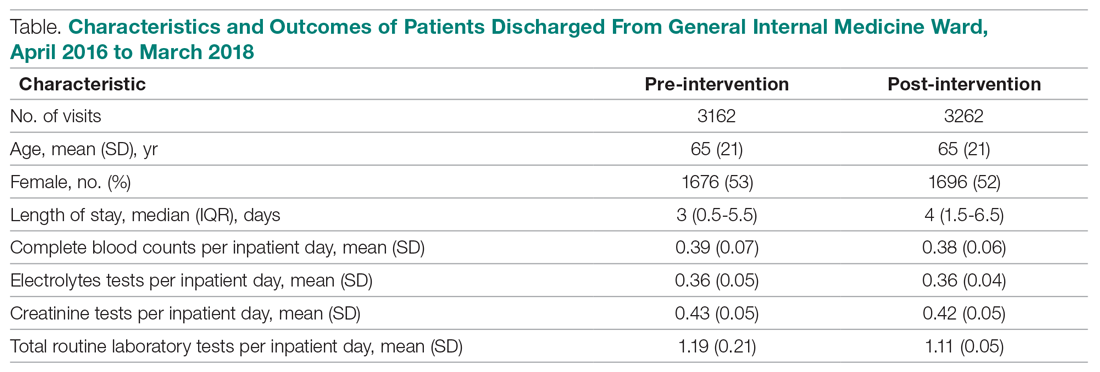
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.
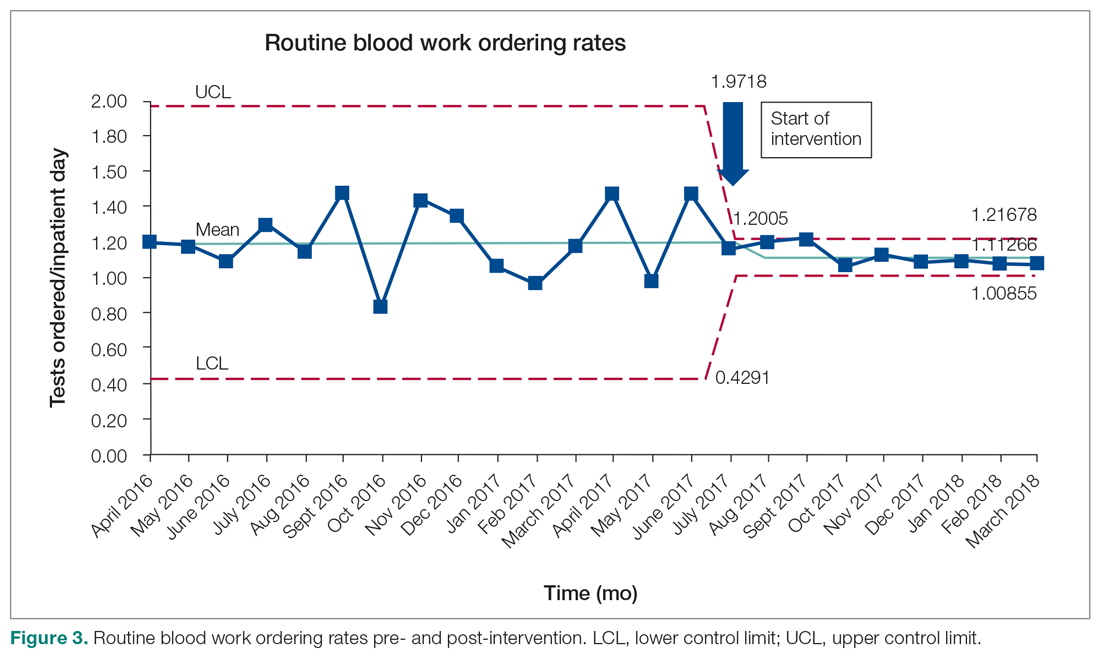
Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12

Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.

Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
From the University of Toronto (Dr. Basuita, Corey L. Kamen, and Dr. Soong) and Sinai Health System (Corey L. Kamen, Cheryl Ethier, and Dr. Soong), Toronto, Ontario, Canada. Co-first authors are Manpreet Basuita, MD, and Corey L. Kamen, BSc.
Abstract
- Objective: Routine laboratory testing is common among medical inpatients; however, when ordered inappropriately testing can represent low-value care. We examined the impact of an evidence-based intervention bundle on utilization.
- Participants/setting: This prospective cohort study took place at a tertiary academic medical center and included 6424 patients admitted to the general internal medicine service between April 2016 and March 2018.
- Intervention: An intervention bundle, whose first components were implemented in July 2016, included computer order entry restrictions on repetitive laboratory testing, education, and audit-feedback.
- Measures: Data were extracted from the hospital electronic health record. The primary outcome was the number of routine blood tests (complete blood count, creatinine, and electrolytes) ordered per inpatient day.
- Analysis: Descriptive statistics were calculated for demographic variables. We used statistical process control charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome.
- Results: The mean number of combined routine laboratory tests ordered per inpatient day decreased from 1.19 (SD, 0.21) tests to 1.11 (SD, 0.05), a relative reduction of 6.7% (P < 0.0001). Mean cost per case related to laboratory tests decreased from $17.24 in the pre-intervention period to $16.17 in the post-intervention period (relative reduction of 6.2%). This resulted in savings of $26,851 in the intervention year.
- Conclusion: A laboratory intervention bundle was associated with small reductions in testing and costs. A routine test performed less than once per inpatient day may not be clinically appropriate or possible.
Keywords: utilization; clinical costs; quality improvement; QI intervention; internal medicine; inpatient.
Routine laboratory blood testing is a commonly used diagnostic tool that physicians rely on to provide patient care. Although routine blood testing represents less than 5% of most hospital budgets, routine use and over-reliance on testing among physicians makes it a target of cost-reduction efforts.1-3 A variety of interventions have been proposed to reduce inappropriate laboratory tests, with varying results.1,4-6 Successful interventions include providing physicians with fee data associated with ordered laboratory tests, unbundling panels of tests, and multicomponent interventions.6 We conducted a multifaceted quality improvement study to promote and develop interventions to adopt appropriate blood test ordering practices.
Methods
Setting
This prospective cohort study took place at Mount Sinai Hospital, a 443-bed academic hospital affiliated with the University of Toronto, where more than 2400 learners rotate through annually. The study was approved by the Mount Sinai Hospital Research Ethics Board.
Participants
We included all inpatient admissions to the general internal medicine service between April 2016 and March 2018. Exclusion criteria included a length of stay (LOS) longer than 365 days and admission to a critical care unit. Patients with more than 1 admission were counted as separate hospital inpatient visits.
Intervention
Based on internal data, we targeted the top 3 most frequently ordered routine blood tests: complete blood count (CBC), creatinine, and electrolytes. Trainee interviews revealed that habit, bundled order sets, and fear of “missing something” contributed to inappropriate routine blood test ordering. Based on these root causes, we used the Model for Improvement to iteratively develop a multimodal intervention that began in July 2016.7,8 This included a change to the computerized provider order entry (CPOE) to nudge clinicians to a restrictive ordering strategy by substituting the “Daily x3” frequency of blood test ordering with a “Daily x1” option on a pick list of order options. Clinicians could still order daily routine blood tests for any specified duration, but would have to do so by manually changing the default setting within the CPOE.
From July 2017 to March 2018, the research team educated residents on appropriate laboratory test ordering and provided audit and feedback data to the clinicians. Diagnostic uncertainty was addressed in teaching sessions. Attending physicians were surveyed on appropriate indications for daily laboratory testing for each of CBC, electrolytes, and creatinine. Appropriate indications (Figure 1) were displayed in visible clinical areas and incorporated into teaching sessions.9
Clinician teams received real-time performance data on their routine blood test ordering patterns compared with an institutional benchmark. Bar graphs of blood work ordering rates (sum of CBCs, creatinine, and electrolytes ordered for all patients on a given team divided by the total LOS for all patients) were distributed to each internal medicine team via email every 2 weeks (Figure 2).1,10-12

Data Collection and Analysis
Data were extracted from the hospital electronic health record (EHR). The primary outcome was the number of routine blood tests (CBC, creatinine, and electrolytes) ordered per inpatient day. Descriptive statistics were calculated for demographic variables. We used statistical process control (SPC) charts to compare the baseline period (April 2016-June 2017) and the intervention period (July 2017-March 2018) for the primary outcome. SPC charts display process changes over time. Data are plotted in chronological order, with the central line representing the outcome mean, an upper line representing the upper control limit, and a lower line representing the lower control limit. The upper and lower limits were set at 3δ, which correspond to 3 standard deviations above and below the mean. Six successive points above or beyond the mean suggests “special cause variation,” indicating that observed results are unlikely due to secular trends. SPC charts are commonly used quality tools for process improvement as well as research.13-16 These charts were created using QI Macros SPC software for Excel V. 2012.07 (KnowWare International, Denver, CO).
The direct cost of each laboratory test was acquired from the hospital laboratory department. The cost of each laboratory test (CBC = $7.54/test, electrolytes = $2.04/test, creatinine = $1.28/test, in Canadian dollars) was subsequently added together and multiplied by the pre- and post-intervention difference of total blood tests saved per inpatient day and then multiplied by 365 to arrive at an estimated cost savings per year.
Results
Over the study period, there were 6424 unique patient admissions on the general internal medicine service, with a median LOS of 3.5 days (Table). 
The majority of inpatient visits had at least 1 test of CBC (80%; mean, 3.6 tests/visit), creatinine (79.3%; mean, 3.5 tests/visit), or electrolytes (81.6%; mean, 3.9 tests/visit) completed. In total, 56,767 laboratory tests were ordered.
Following the intervention, there was a reduction in both rates of routine blood test orders and their associated costs, with a shift below the mean. The mean number of tests ordered (combined CBC, creatinine, and electrolytes) per inpatient day decreased from 1.19 (SD, 0.21) in the pre-intervention period to 1.11 (SD, 0.05) in the post-intervention period (P < 0.0001), representing a 6.7% relative reduction (Figure 3). We observed a 6.2% relative reduction in costs per inpatient day, translating to a total savings of $26,851 over 1 year for the intervention period.

Discussion
Our study suggests that a multimodal intervention, including CPOE restrictions, resident education with posters, and audit and feedback strategies, can reduce lab test ordering on general internal medicine wards. This finding is similar to those of previous studies using a similar intervention, although different laboratory tests were targeted.1,2,5,6,10,17
Our study found lower test result reductions than those reported by a previous study, which reported a relative reduction of 17% to 30%,18 and by another investigation that was conducted recently in a similar setting.17 In the latter study, reductions in laboratory testing were mostly found in nonroutine tests, and no significant improvements were noted in CBC, electrolytes, and creatine, the 3 tests we studied over the same duration.17 This may represent a ceiling effect to reducing laboratory testing, and efforts to reduce CBC, electrolytes, and creatinine testing beyond 0.3 to 0.4 tests per inpatient day (or combined 1.16 tests per inpatient day) may not be clinically appropriate or possible. This information can guide institutions to include other areas of overuse based on rates of utilization in order to maximize the benefits from a resource intensive intervention.
There are a number of limitations that merit discussion. First, observational studies do not demonstrate causation; however, to our knowledge, there were no other co-interventions that were being conducted during the study duration. One important note is that our project’s intervention began in July, at which point there are new internal medicine residents beginning their training. As the concept of resource allocation becomes more important, medical schools are spending more time educating students about Choosing Wisely, and, therefore, newer cohorts of residents may be more cognizant of appropriate blood testing. Second, this is a single-center study, limiting generalizability; however, we note that many other centers have reported similar findings. Another limitation is that we do not know whether there were any adverse clinical events associated with blood work ordering that was too restrictive, although informal tracking of STAT laboratory testing remained stable throughout the study period. It is important to ensure that blood work is ordered in moderation and tailored to patients using one’s clinical judgment.
Future Directions
We observed modest reductions in the quantity and costs associated with a quality improvement intervention aimed at reducing routine blood testing. A baseline rate of laboratory testing of less than 1 test per inpatient day may require including other target tests to drive down absolute utilization.
Corresponding author: Christine Soong, MD, MSc, 433-600 University Avenue, Toronto, Ontario, Canada M5G 1X5; [email protected].
Financial disclosures: None.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
1. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;178:431.
2. May TA, Clancy M, Critchfield J, et al. Reducing unnecessary inpatient laboratory testing in a teaching hospital. Am J Clin Pathol. 2006;126:200-206.
3. Thavendiranathan P, Bagai A, Ebidia A, et al. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20:520-524.
4. Feldman LS, Shihab HM, Thiemann D, et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173:903-908.
5. Attali, M, Barel Y, Somin M, et al. A cost-effective method for reducing the volume of laboratory tests in a university-associated teaching hospital. Mt Sinai J Med. 2006;73:787-794.
6. Faisal A, Andres K, Rind JAK, et al. Reducing the number of unnecessary routine laboratory tests through education of internal medicine residents. Postgrad Med J. 2018;94:716-719.
7. How to Improve. Institute for Healthcare Improvement. 2009. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed June 5, 2019.
8. Langley GL, Moen R, Nolan KM, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nd ed. San Francisco: Jossey-Bass Publishers; 2009.
9. Hicks L. Blood Draws Toolkit. Choosing Wisely Canada. 2017. https://choosingwiselycanada.org/wpcontent/uploads/2017/10/CWC_BloodDraws_Toolkit.pdf. Accessed March 5, 2019.
10. Sadowski BW, Lane AB, Wood SM, et al. High-value, cost-conscious care: iterative systems-based interventions to reduce unnecessary laboratory testing. Am J Med. 2017;130:1112e1-1112e7.
11. Minerowicz C, Abel N, Hunter K, et al. Impact of weekly feedback on test ordering patterns. Am J Manag Care. 2015;21:763-768.
12. Calderon-Margalit R, Mor-Yosef S, et al. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17:243-248.
13. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-64.
14. American Society for Quality. Control chart. ASM website. https://asq.org/quality-resources/control-chart. Accessed November 5, 2020.
15. American Society for Quality. The 7 Basic Quality Tools For Process Improvement. ASM website. https://asq.org/quality-resources/seven-basic-quality-tools. Accessed November 5, 2020.
16. Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12:458-464.
17. Ambasta A, Ma IWY, Woo S, et al. Impact of an education and multilevel social comparison-based intervention bundle on use of routine blood tests in hospitalised patients at an academic tertiary care hospital: a controlled pre-intervention post-intervention study. BMJ Qual Saf. 2020;29:1-2.
18. Lee VS, Kawamoto K, Hess R, et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA. 2016;316:1061-1072.
Practice-changing data at this year’s ASH meeting
Instead of flying out to San Diego in California and soaking up a bit of sunshine in between listening to new research presentations, hematologists from around the world will be glued to their computer screens next weekend, tuning into the 62nd American Society of Hematology annual meeting.
Like many other conferences this year, the ASH meeting will be virtual because of the continuing COVID-19 pandemic, although the dates remain the same: Dec. 5-8.
This is the premier hematology event of the year, and the largest hematology conference in the world, with around 3,500 abstracts presented this year, commented Aaron T. Gerds, MD, chair of ASH’s Committee on Communications.
Ruxolitinib in chronic GvHD
“One of the things that people come to ASH for is to hear about practice-changing clinical trials, and this year is no exception,” said ASH secretary Robert Brodsky, MD.
In a preview webinar, he highlighted four abstracts that offer opportunities to change practice and revamp the current standards of care.
One clinical trial that is “almost certainly a practice changer,” he said, is the REACH 3 study (abstract 77) of the JAK inhibitor ruxolitinib (Jakafi, Incyte) in patients with chronic graft-versus-host disease (GvHD) after a stem cell transplant.
“This has been really hard to treat in patients undergoing allogeneic bone marrow transplants,” said Brodsky. “Steroids are the first-line treatment, but after that, nothing else has shown any improvement, and even steroids don’t work that well.”
There is currently no approved second-line therapy for chronic forms of GvHD, he emphasized. The main endpoint of the trial was overall response rate, which was doubled with ruxolitinib compared to the best available therapy (50% vs 25%).
“This is the first successful phase 3 trial for chronic GvHD,” Brodsky commented.
Transplants for older patients with MDS
Transplant offers the only curative option for myelodysplastic syndromes (MDS), but typically this option is offered to younger patients because benefits for older adults have not been well-defined, Brodsky noted.
New data from a clinical trial conducted in patients with advanced MDS aged 50-75 years (abstract 75) offers the most definitive evidence to date that allogeneic hematopoietic cell transplantation (AHCT) can significantly improve outcomes for older adults.
It’s clear that transplant is the standard of care in younger patients, Brodsky commented, and although there is a trend of offering it to older patients, some are not getting referred and instead are being offered palliative care. “The thinking is that bone marrow transplant would be too toxic in this age group,” he said. “But what is very clear here is that, in an intent-to-treat analysis, there was a significant survival advantage – 48% versus 27% at 3 years for transplantation – and it was seen across all subgroups.”
Subcutaneous daratumumab
New data on a subcutaneous formulation of daratumumab (Darzalex, Janssen), which is usually given by intravenous infusion, will be presented from the APOLLO trial (abstract 412) in patients with relapsed/refractory multiple myeloma.
Patients who received subcutaneous daratumumab combined with pomalidomide and dexamethasone had a 37% reduction in disease progression or death compared to those who received pomalidomide and dexamethasone alone.
“From previous years we’ve learned that daratumumab has had a major impact on outcomes in multiple myeloma,” said Brodsky. “The nice thing about the subcutaneous formulation is that it can be administered quickly and in an outpatient setting, which is especially important in the COVID era.”
Negative data with tranexamic acid
The fourth abstract highlighted by Brodsky is a negative study, but its findings can help guide clinical practice, he said. The a-TREAT study (abstract 2) showed that, despite being routinely used in the clinical setting, tranexamic acid does not prevent bleeding when administered prophylactically to severely thrombocytopenic patients undergoing treatment for hematologic malignancies.
“They found absolutely no difference in bleeding or need for transfusion,” said Brodsky. “What they did find was more catheter-associated blood clots in the tranexamic acid group. This is a practice changer in that it probably should not be given prophylactically to patients with thrombocytopenia.”
‘Very exciting’ news about gene therapy
Brodsky also highlighted several late-breaking abstract that will be presented at the meeting.
In particular, the first data on a gene therapy for hemophilia B (abstract LBA-6) are “very, very exciting,” he said. The HOPE-B trial showed a 96% response rate among patients with hemophilia B who were treated with etranacogene dezaparvovec, an investigational gene therapy composed of an adeno-associated virus serotype 5 (AAV5) vector containing a codon-optimized Padua variant human factor IX.
Brodsky pointed out that this was a large trial with 54 patients, but importantly, it included patients with pre-existing anti-AAV5 neutralizing antibodies. “About 40% of patients have naturally occurring antibodies to AAV5, and they have been excluded from previous trials because it was thought they wouldn’t take the vector,” said Brodsky. “But only one patient didn’t get a response.”
Following a single dose of etranacogene dezaparvovec, Factor IX activity increased into the mild to normal range without the need for prophylactic immunosuppression. Treated patients were able to discontinue prophylaxis and bleeding was controlled in most of the cohort.
“This is a big advance and we are getting very close to the point where gene therapy is going to be standard of care for some forms of hemophilia,” said Brodsky. However, he added that “we will still need to see more patients and have longer follow-up.”
He added that, with time, the technology behind gene therapy will probably become less expensive and more accessible to more patients, which will help become a standard of care.
This is also the hope for the technology behind chimeric antigen receptor T-cell (CAR-T) therapy, he added. At present, this cellular therapy is manufactured individually for each patient and is very expensive, but work on “off-the-shelf” products is underway. This topic will be explored during the presidential symposium, entitled, “Universal Donor Solutions in Hematology.”
New data on one of the currently available CAR-T cell products will be presented at the meeting. The phase 2 ZUMA-5 trial showed that axicabtagene ciloleucel (Axi-Cel) may be a viable option for some patients with high-risk non-Hodgkin lymphoma who have not responded to standard treatments (abstract 700).
At a median follow-up of almost 18 months, 92% of participants achieved an objective response, and 78% achieved a complete response to the treatment. By 12 months, 72% were still in response, and at 17.5 months, 64% were still in response.
“We were very impressed with the magnitude of the responses, and also the durability,” said senior study author Caron Jacobson, MD, of the Dana-Farber Cancer Institute, Boston, in a press release. “I was also struck early on by how favorable the safety profile was compared to what we’ve been seeing in the fast-growing lymphomas, such as large B cell lymphoma.”
Race and bloods cancers
ASH president Stephanie Lee, MD, MPH, highlighted several abstracts on disparities that will be presented at the meeting. One of these, which is to be presented during the plenary session, is an analysis of patient survival in acute myeloid leukemia (AML) (abstract 6).
It found that “self-reported race was the best indicator of survival,” noted Lee.
Overall survival at 3 years was 41% in White patients versus 32% in Black patients, a difference that was highly significant, she noted.
Part of the study also evaluated patients who were all on the same chemotherapy protocol, “so there was no effect of different treatment since they were on therapy determined by the trial,” said Lee.
Black patients were less likely to have normal cytogenetics compared with White patients (38% vs 51%; P = .01) and had a lower frequency of prognostically favorable NPM1 mutations (25% vs 38%; P = .04), but higher frequencies of spliceosome gene mutations (24% vs 12%; P = .009). Therefore, the results showed race was an independent prognosticator of poor survival in AML, aside from established molecular markers.
A special scientific session on race will be held on Dec. 5, Lee noted. While other abstracts consider race from the patient side, this session will focus on the scientist’s side, she explained, and address questions such as: “What are the implications of diversity and racism? And how does that impact scientists who are from underrepresented minorities?”
COVID-19 and blood disorders
Lee also highlighted a study (abstract 215) that analyzed emerging data from the ASH Research Collaborative COVID-19 Registry for Hematology, which was developed to look at outcomes of COVID-19 infection in patients with underlying blood disorders.
An analysis of data from 250 patients at 74 sites around the world found that overall mortality was 28%. “This supports the emerging consensus that patients with hematologic malignancies experience significant morbidity and mortality from COVID-19 infection,” say the authors.
“We do need real-world data to see how SARS-CoV-2 is affecting our patients with hematologic diseases or those who don’t have a hematologic disease but who are then infected with the coronavirus and develop a hematologic problem like blood clots,” said Lee.
“More data will be coming in, but this is a good example of trying to harness real-world information to learn things until we have more controlled trials.”
‘Fireside chat’ with Fauci
COVID-19 will be on the agenda for a special session billed as a “fireside chat” with Anthony Fauci, MD, of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
“This will be kicking off our meeting on Saturday morning,” said Lee.
This article first appeared on Medscape.com.
Instead of flying out to San Diego in California and soaking up a bit of sunshine in between listening to new research presentations, hematologists from around the world will be glued to their computer screens next weekend, tuning into the 62nd American Society of Hematology annual meeting.
Like many other conferences this year, the ASH meeting will be virtual because of the continuing COVID-19 pandemic, although the dates remain the same: Dec. 5-8.
This is the premier hematology event of the year, and the largest hematology conference in the world, with around 3,500 abstracts presented this year, commented Aaron T. Gerds, MD, chair of ASH’s Committee on Communications.
Ruxolitinib in chronic GvHD
“One of the things that people come to ASH for is to hear about practice-changing clinical trials, and this year is no exception,” said ASH secretary Robert Brodsky, MD.
In a preview webinar, he highlighted four abstracts that offer opportunities to change practice and revamp the current standards of care.
One clinical trial that is “almost certainly a practice changer,” he said, is the REACH 3 study (abstract 77) of the JAK inhibitor ruxolitinib (Jakafi, Incyte) in patients with chronic graft-versus-host disease (GvHD) after a stem cell transplant.
“This has been really hard to treat in patients undergoing allogeneic bone marrow transplants,” said Brodsky. “Steroids are the first-line treatment, but after that, nothing else has shown any improvement, and even steroids don’t work that well.”
There is currently no approved second-line therapy for chronic forms of GvHD, he emphasized. The main endpoint of the trial was overall response rate, which was doubled with ruxolitinib compared to the best available therapy (50% vs 25%).
“This is the first successful phase 3 trial for chronic GvHD,” Brodsky commented.
Transplants for older patients with MDS
Transplant offers the only curative option for myelodysplastic syndromes (MDS), but typically this option is offered to younger patients because benefits for older adults have not been well-defined, Brodsky noted.
New data from a clinical trial conducted in patients with advanced MDS aged 50-75 years (abstract 75) offers the most definitive evidence to date that allogeneic hematopoietic cell transplantation (AHCT) can significantly improve outcomes for older adults.
It’s clear that transplant is the standard of care in younger patients, Brodsky commented, and although there is a trend of offering it to older patients, some are not getting referred and instead are being offered palliative care. “The thinking is that bone marrow transplant would be too toxic in this age group,” he said. “But what is very clear here is that, in an intent-to-treat analysis, there was a significant survival advantage – 48% versus 27% at 3 years for transplantation – and it was seen across all subgroups.”
Subcutaneous daratumumab
New data on a subcutaneous formulation of daratumumab (Darzalex, Janssen), which is usually given by intravenous infusion, will be presented from the APOLLO trial (abstract 412) in patients with relapsed/refractory multiple myeloma.
Patients who received subcutaneous daratumumab combined with pomalidomide and dexamethasone had a 37% reduction in disease progression or death compared to those who received pomalidomide and dexamethasone alone.
“From previous years we’ve learned that daratumumab has had a major impact on outcomes in multiple myeloma,” said Brodsky. “The nice thing about the subcutaneous formulation is that it can be administered quickly and in an outpatient setting, which is especially important in the COVID era.”
Negative data with tranexamic acid
The fourth abstract highlighted by Brodsky is a negative study, but its findings can help guide clinical practice, he said. The a-TREAT study (abstract 2) showed that, despite being routinely used in the clinical setting, tranexamic acid does not prevent bleeding when administered prophylactically to severely thrombocytopenic patients undergoing treatment for hematologic malignancies.
“They found absolutely no difference in bleeding or need for transfusion,” said Brodsky. “What they did find was more catheter-associated blood clots in the tranexamic acid group. This is a practice changer in that it probably should not be given prophylactically to patients with thrombocytopenia.”
‘Very exciting’ news about gene therapy
Brodsky also highlighted several late-breaking abstract that will be presented at the meeting.
In particular, the first data on a gene therapy for hemophilia B (abstract LBA-6) are “very, very exciting,” he said. The HOPE-B trial showed a 96% response rate among patients with hemophilia B who were treated with etranacogene dezaparvovec, an investigational gene therapy composed of an adeno-associated virus serotype 5 (AAV5) vector containing a codon-optimized Padua variant human factor IX.
Brodsky pointed out that this was a large trial with 54 patients, but importantly, it included patients with pre-existing anti-AAV5 neutralizing antibodies. “About 40% of patients have naturally occurring antibodies to AAV5, and they have been excluded from previous trials because it was thought they wouldn’t take the vector,” said Brodsky. “But only one patient didn’t get a response.”
Following a single dose of etranacogene dezaparvovec, Factor IX activity increased into the mild to normal range without the need for prophylactic immunosuppression. Treated patients were able to discontinue prophylaxis and bleeding was controlled in most of the cohort.
“This is a big advance and we are getting very close to the point where gene therapy is going to be standard of care for some forms of hemophilia,” said Brodsky. However, he added that “we will still need to see more patients and have longer follow-up.”
He added that, with time, the technology behind gene therapy will probably become less expensive and more accessible to more patients, which will help become a standard of care.
This is also the hope for the technology behind chimeric antigen receptor T-cell (CAR-T) therapy, he added. At present, this cellular therapy is manufactured individually for each patient and is very expensive, but work on “off-the-shelf” products is underway. This topic will be explored during the presidential symposium, entitled, “Universal Donor Solutions in Hematology.”
New data on one of the currently available CAR-T cell products will be presented at the meeting. The phase 2 ZUMA-5 trial showed that axicabtagene ciloleucel (Axi-Cel) may be a viable option for some patients with high-risk non-Hodgkin lymphoma who have not responded to standard treatments (abstract 700).
At a median follow-up of almost 18 months, 92% of participants achieved an objective response, and 78% achieved a complete response to the treatment. By 12 months, 72% were still in response, and at 17.5 months, 64% were still in response.
“We were very impressed with the magnitude of the responses, and also the durability,” said senior study author Caron Jacobson, MD, of the Dana-Farber Cancer Institute, Boston, in a press release. “I was also struck early on by how favorable the safety profile was compared to what we’ve been seeing in the fast-growing lymphomas, such as large B cell lymphoma.”
Race and bloods cancers
ASH president Stephanie Lee, MD, MPH, highlighted several abstracts on disparities that will be presented at the meeting. One of these, which is to be presented during the plenary session, is an analysis of patient survival in acute myeloid leukemia (AML) (abstract 6).
It found that “self-reported race was the best indicator of survival,” noted Lee.
Overall survival at 3 years was 41% in White patients versus 32% in Black patients, a difference that was highly significant, she noted.
Part of the study also evaluated patients who were all on the same chemotherapy protocol, “so there was no effect of different treatment since they were on therapy determined by the trial,” said Lee.
Black patients were less likely to have normal cytogenetics compared with White patients (38% vs 51%; P = .01) and had a lower frequency of prognostically favorable NPM1 mutations (25% vs 38%; P = .04), but higher frequencies of spliceosome gene mutations (24% vs 12%; P = .009). Therefore, the results showed race was an independent prognosticator of poor survival in AML, aside from established molecular markers.
A special scientific session on race will be held on Dec. 5, Lee noted. While other abstracts consider race from the patient side, this session will focus on the scientist’s side, she explained, and address questions such as: “What are the implications of diversity and racism? And how does that impact scientists who are from underrepresented minorities?”
COVID-19 and blood disorders
Lee also highlighted a study (abstract 215) that analyzed emerging data from the ASH Research Collaborative COVID-19 Registry for Hematology, which was developed to look at outcomes of COVID-19 infection in patients with underlying blood disorders.
An analysis of data from 250 patients at 74 sites around the world found that overall mortality was 28%. “This supports the emerging consensus that patients with hematologic malignancies experience significant morbidity and mortality from COVID-19 infection,” say the authors.
“We do need real-world data to see how SARS-CoV-2 is affecting our patients with hematologic diseases or those who don’t have a hematologic disease but who are then infected with the coronavirus and develop a hematologic problem like blood clots,” said Lee.
“More data will be coming in, but this is a good example of trying to harness real-world information to learn things until we have more controlled trials.”
‘Fireside chat’ with Fauci
COVID-19 will be on the agenda for a special session billed as a “fireside chat” with Anthony Fauci, MD, of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
“This will be kicking off our meeting on Saturday morning,” said Lee.
This article first appeared on Medscape.com.
Instead of flying out to San Diego in California and soaking up a bit of sunshine in between listening to new research presentations, hematologists from around the world will be glued to their computer screens next weekend, tuning into the 62nd American Society of Hematology annual meeting.
Like many other conferences this year, the ASH meeting will be virtual because of the continuing COVID-19 pandemic, although the dates remain the same: Dec. 5-8.
This is the premier hematology event of the year, and the largest hematology conference in the world, with around 3,500 abstracts presented this year, commented Aaron T. Gerds, MD, chair of ASH’s Committee on Communications.
Ruxolitinib in chronic GvHD
“One of the things that people come to ASH for is to hear about practice-changing clinical trials, and this year is no exception,” said ASH secretary Robert Brodsky, MD.
In a preview webinar, he highlighted four abstracts that offer opportunities to change practice and revamp the current standards of care.
One clinical trial that is “almost certainly a practice changer,” he said, is the REACH 3 study (abstract 77) of the JAK inhibitor ruxolitinib (Jakafi, Incyte) in patients with chronic graft-versus-host disease (GvHD) after a stem cell transplant.
“This has been really hard to treat in patients undergoing allogeneic bone marrow transplants,” said Brodsky. “Steroids are the first-line treatment, but after that, nothing else has shown any improvement, and even steroids don’t work that well.”
There is currently no approved second-line therapy for chronic forms of GvHD, he emphasized. The main endpoint of the trial was overall response rate, which was doubled with ruxolitinib compared to the best available therapy (50% vs 25%).
“This is the first successful phase 3 trial for chronic GvHD,” Brodsky commented.
Transplants for older patients with MDS
Transplant offers the only curative option for myelodysplastic syndromes (MDS), but typically this option is offered to younger patients because benefits for older adults have not been well-defined, Brodsky noted.
New data from a clinical trial conducted in patients with advanced MDS aged 50-75 years (abstract 75) offers the most definitive evidence to date that allogeneic hematopoietic cell transplantation (AHCT) can significantly improve outcomes for older adults.
It’s clear that transplant is the standard of care in younger patients, Brodsky commented, and although there is a trend of offering it to older patients, some are not getting referred and instead are being offered palliative care. “The thinking is that bone marrow transplant would be too toxic in this age group,” he said. “But what is very clear here is that, in an intent-to-treat analysis, there was a significant survival advantage – 48% versus 27% at 3 years for transplantation – and it was seen across all subgroups.”
Subcutaneous daratumumab
New data on a subcutaneous formulation of daratumumab (Darzalex, Janssen), which is usually given by intravenous infusion, will be presented from the APOLLO trial (abstract 412) in patients with relapsed/refractory multiple myeloma.
Patients who received subcutaneous daratumumab combined with pomalidomide and dexamethasone had a 37% reduction in disease progression or death compared to those who received pomalidomide and dexamethasone alone.
“From previous years we’ve learned that daratumumab has had a major impact on outcomes in multiple myeloma,” said Brodsky. “The nice thing about the subcutaneous formulation is that it can be administered quickly and in an outpatient setting, which is especially important in the COVID era.”
Negative data with tranexamic acid
The fourth abstract highlighted by Brodsky is a negative study, but its findings can help guide clinical practice, he said. The a-TREAT study (abstract 2) showed that, despite being routinely used in the clinical setting, tranexamic acid does not prevent bleeding when administered prophylactically to severely thrombocytopenic patients undergoing treatment for hematologic malignancies.
“They found absolutely no difference in bleeding or need for transfusion,” said Brodsky. “What they did find was more catheter-associated blood clots in the tranexamic acid group. This is a practice changer in that it probably should not be given prophylactically to patients with thrombocytopenia.”
‘Very exciting’ news about gene therapy
Brodsky also highlighted several late-breaking abstract that will be presented at the meeting.
In particular, the first data on a gene therapy for hemophilia B (abstract LBA-6) are “very, very exciting,” he said. The HOPE-B trial showed a 96% response rate among patients with hemophilia B who were treated with etranacogene dezaparvovec, an investigational gene therapy composed of an adeno-associated virus serotype 5 (AAV5) vector containing a codon-optimized Padua variant human factor IX.
Brodsky pointed out that this was a large trial with 54 patients, but importantly, it included patients with pre-existing anti-AAV5 neutralizing antibodies. “About 40% of patients have naturally occurring antibodies to AAV5, and they have been excluded from previous trials because it was thought they wouldn’t take the vector,” said Brodsky. “But only one patient didn’t get a response.”
Following a single dose of etranacogene dezaparvovec, Factor IX activity increased into the mild to normal range without the need for prophylactic immunosuppression. Treated patients were able to discontinue prophylaxis and bleeding was controlled in most of the cohort.
“This is a big advance and we are getting very close to the point where gene therapy is going to be standard of care for some forms of hemophilia,” said Brodsky. However, he added that “we will still need to see more patients and have longer follow-up.”
He added that, with time, the technology behind gene therapy will probably become less expensive and more accessible to more patients, which will help become a standard of care.
This is also the hope for the technology behind chimeric antigen receptor T-cell (CAR-T) therapy, he added. At present, this cellular therapy is manufactured individually for each patient and is very expensive, but work on “off-the-shelf” products is underway. This topic will be explored during the presidential symposium, entitled, “Universal Donor Solutions in Hematology.”
New data on one of the currently available CAR-T cell products will be presented at the meeting. The phase 2 ZUMA-5 trial showed that axicabtagene ciloleucel (Axi-Cel) may be a viable option for some patients with high-risk non-Hodgkin lymphoma who have not responded to standard treatments (abstract 700).
At a median follow-up of almost 18 months, 92% of participants achieved an objective response, and 78% achieved a complete response to the treatment. By 12 months, 72% were still in response, and at 17.5 months, 64% were still in response.
“We were very impressed with the magnitude of the responses, and also the durability,” said senior study author Caron Jacobson, MD, of the Dana-Farber Cancer Institute, Boston, in a press release. “I was also struck early on by how favorable the safety profile was compared to what we’ve been seeing in the fast-growing lymphomas, such as large B cell lymphoma.”
Race and bloods cancers
ASH president Stephanie Lee, MD, MPH, highlighted several abstracts on disparities that will be presented at the meeting. One of these, which is to be presented during the plenary session, is an analysis of patient survival in acute myeloid leukemia (AML) (abstract 6).
It found that “self-reported race was the best indicator of survival,” noted Lee.
Overall survival at 3 years was 41% in White patients versus 32% in Black patients, a difference that was highly significant, she noted.
Part of the study also evaluated patients who were all on the same chemotherapy protocol, “so there was no effect of different treatment since they were on therapy determined by the trial,” said Lee.
Black patients were less likely to have normal cytogenetics compared with White patients (38% vs 51%; P = .01) and had a lower frequency of prognostically favorable NPM1 mutations (25% vs 38%; P = .04), but higher frequencies of spliceosome gene mutations (24% vs 12%; P = .009). Therefore, the results showed race was an independent prognosticator of poor survival in AML, aside from established molecular markers.
A special scientific session on race will be held on Dec. 5, Lee noted. While other abstracts consider race from the patient side, this session will focus on the scientist’s side, she explained, and address questions such as: “What are the implications of diversity and racism? And how does that impact scientists who are from underrepresented minorities?”
COVID-19 and blood disorders
Lee also highlighted a study (abstract 215) that analyzed emerging data from the ASH Research Collaborative COVID-19 Registry for Hematology, which was developed to look at outcomes of COVID-19 infection in patients with underlying blood disorders.
An analysis of data from 250 patients at 74 sites around the world found that overall mortality was 28%. “This supports the emerging consensus that patients with hematologic malignancies experience significant morbidity and mortality from COVID-19 infection,” say the authors.
“We do need real-world data to see how SARS-CoV-2 is affecting our patients with hematologic diseases or those who don’t have a hematologic disease but who are then infected with the coronavirus and develop a hematologic problem like blood clots,” said Lee.
“More data will be coming in, but this is a good example of trying to harness real-world information to learn things until we have more controlled trials.”
‘Fireside chat’ with Fauci
COVID-19 will be on the agenda for a special session billed as a “fireside chat” with Anthony Fauci, MD, of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
“This will be kicking off our meeting on Saturday morning,” said Lee.
This article first appeared on Medscape.com.
International expert group agrees on redefining psoriasis severity
It’s high time to say farewell to the traditional categorization of psoriasis severity into mild, moderate, or severe disease, according to the International Psoriasis Council.
The mild/moderate/severe categorization is vague and defined differently by different organizations and in different countries. It often underestimates disease severity because it ignores psoriasis involvement in particularly tough-to-treat special areas, including the scalp, palms, soles, face, nails, and genitalia, Bruce E. Strober, MD, PhD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year. He chaired an IPC project in which prominent psoriasis experts in 32 countries employed a Delphi consensus approach aimed at achieving agreement on a more practical recategorization of psoriasis severity for use in both daily clinical practice and enrolling appropriate participants in clinical trials. What emerged was a simplified dichotomous categorization system.
“What we came up with is a very sensible approach to defining whether patients should get either topical or systemic therapy. In fact, there are only two groups of patients in psoriasis: those who should get topicals alone, and those who should get systemic therapy. It’s topicals or systemics,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., who also works in private practice in Cromwell, Conn.
Under the IPC classification, psoriasis patients are candidates for systemic therapy if they meet at least one of three criteria: body surface area of involvement greater than 10%, disease involving the previously mentioned special areas, or failure of topical therapy.
“This approach is about practically treating patients who are in need,” Dr. Strober said. “If patients meet just one of these three criteria they can move on to our current toolbox of systemic therapies, be they older systemic treatments, apremilast, phototherapy, or 1 of the 11 biologics currently approved for the treatment of psoriasis. The key point is that for patients with moderate to severe psoriasis – or should I say, systemic therapy–appropriate psoriasis – treatment should be based on individual patient characteristics. We don’t work on a stepwise approach. If a patient walks in with more than 10% body surface area involved, don’t make them fail topicals; you can go right to systemics.”
European dermatologists often use the Psoriasis Area and Severity Index (PASI) score to characterize disease severity and monitor response to therapy. In contrast, American dermatologists generally find PASI too complex and time-consuming for use in clinical practice, relying instead on the amount of body surface area involved with psoriasis. Neither of these measures incorporates disease involvement in special areas, which when present ought to automatically place a patient in the systemic therapy–appropriate category, according to Dr. Strober.
“I find this [IPC recategorization] a very practical approach. I hope you write this down and use this in your own practice,” Dr. Strober said.
The full IPC report was published in the Journal of the American Academy of Dermatology.
The IPC psoriasis severity reclassification project was unfunded. Dr. Strober reported receiving institutional research funding from and serving as a paid consultant to more than two dozen pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
It’s high time to say farewell to the traditional categorization of psoriasis severity into mild, moderate, or severe disease, according to the International Psoriasis Council.
The mild/moderate/severe categorization is vague and defined differently by different organizations and in different countries. It often underestimates disease severity because it ignores psoriasis involvement in particularly tough-to-treat special areas, including the scalp, palms, soles, face, nails, and genitalia, Bruce E. Strober, MD, PhD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year. He chaired an IPC project in which prominent psoriasis experts in 32 countries employed a Delphi consensus approach aimed at achieving agreement on a more practical recategorization of psoriasis severity for use in both daily clinical practice and enrolling appropriate participants in clinical trials. What emerged was a simplified dichotomous categorization system.
“What we came up with is a very sensible approach to defining whether patients should get either topical or systemic therapy. In fact, there are only two groups of patients in psoriasis: those who should get topicals alone, and those who should get systemic therapy. It’s topicals or systemics,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., who also works in private practice in Cromwell, Conn.
Under the IPC classification, psoriasis patients are candidates for systemic therapy if they meet at least one of three criteria: body surface area of involvement greater than 10%, disease involving the previously mentioned special areas, or failure of topical therapy.
“This approach is about practically treating patients who are in need,” Dr. Strober said. “If patients meet just one of these three criteria they can move on to our current toolbox of systemic therapies, be they older systemic treatments, apremilast, phototherapy, or 1 of the 11 biologics currently approved for the treatment of psoriasis. The key point is that for patients with moderate to severe psoriasis – or should I say, systemic therapy–appropriate psoriasis – treatment should be based on individual patient characteristics. We don’t work on a stepwise approach. If a patient walks in with more than 10% body surface area involved, don’t make them fail topicals; you can go right to systemics.”
European dermatologists often use the Psoriasis Area and Severity Index (PASI) score to characterize disease severity and monitor response to therapy. In contrast, American dermatologists generally find PASI too complex and time-consuming for use in clinical practice, relying instead on the amount of body surface area involved with psoriasis. Neither of these measures incorporates disease involvement in special areas, which when present ought to automatically place a patient in the systemic therapy–appropriate category, according to Dr. Strober.
“I find this [IPC recategorization] a very practical approach. I hope you write this down and use this in your own practice,” Dr. Strober said.
The full IPC report was published in the Journal of the American Academy of Dermatology.
The IPC psoriasis severity reclassification project was unfunded. Dr. Strober reported receiving institutional research funding from and serving as a paid consultant to more than two dozen pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
It’s high time to say farewell to the traditional categorization of psoriasis severity into mild, moderate, or severe disease, according to the International Psoriasis Council.
The mild/moderate/severe categorization is vague and defined differently by different organizations and in different countries. It often underestimates disease severity because it ignores psoriasis involvement in particularly tough-to-treat special areas, including the scalp, palms, soles, face, nails, and genitalia, Bruce E. Strober, MD, PhD, asserted at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually this year. He chaired an IPC project in which prominent psoriasis experts in 32 countries employed a Delphi consensus approach aimed at achieving agreement on a more practical recategorization of psoriasis severity for use in both daily clinical practice and enrolling appropriate participants in clinical trials. What emerged was a simplified dichotomous categorization system.
“What we came up with is a very sensible approach to defining whether patients should get either topical or systemic therapy. In fact, there are only two groups of patients in psoriasis: those who should get topicals alone, and those who should get systemic therapy. It’s topicals or systemics,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., who also works in private practice in Cromwell, Conn.
Under the IPC classification, psoriasis patients are candidates for systemic therapy if they meet at least one of three criteria: body surface area of involvement greater than 10%, disease involving the previously mentioned special areas, or failure of topical therapy.
“This approach is about practically treating patients who are in need,” Dr. Strober said. “If patients meet just one of these three criteria they can move on to our current toolbox of systemic therapies, be they older systemic treatments, apremilast, phototherapy, or 1 of the 11 biologics currently approved for the treatment of psoriasis. The key point is that for patients with moderate to severe psoriasis – or should I say, systemic therapy–appropriate psoriasis – treatment should be based on individual patient characteristics. We don’t work on a stepwise approach. If a patient walks in with more than 10% body surface area involved, don’t make them fail topicals; you can go right to systemics.”
European dermatologists often use the Psoriasis Area and Severity Index (PASI) score to characterize disease severity and monitor response to therapy. In contrast, American dermatologists generally find PASI too complex and time-consuming for use in clinical practice, relying instead on the amount of body surface area involved with psoriasis. Neither of these measures incorporates disease involvement in special areas, which when present ought to automatically place a patient in the systemic therapy–appropriate category, according to Dr. Strober.
“I find this [IPC recategorization] a very practical approach. I hope you write this down and use this in your own practice,” Dr. Strober said.
The full IPC report was published in the Journal of the American Academy of Dermatology.
The IPC psoriasis severity reclassification project was unfunded. Dr. Strober reported receiving institutional research funding from and serving as a paid consultant to more than two dozen pharmaceutical companies.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
CMS launches hospital-at-home program to free up hospital capacity
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
As an increasing number of health systems implement “hospital-at-home” (HaH) programs to increase their traditional hospital capacity, the Centers for Medicare & Medicaid Services has given the movement a boost by changing its regulations to allow acute care to be provided in a patient’s home under certain conditions.
The CMS announced Nov. 25 that it was launching its Acute Hospital Care at Home program “to increase the capacity of the American health care system” during the COVID-19 pandemic.
At the same time, the agency announced it was giving more flexibility to ambulatory surgery centers (ASCs) to provide hospital-level care.
The CMS said its new HaH program is an expansion of the Hospitals Without Walls initiative that was unveiled last March. Hospitals Without Walls is a set of “temporary new rules” that provide flexibility for hospitals to provide acute care outside of inpatient settings. Under those rules, hospitals are able to transfer patients to outside facilities, such as ASCs, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving Medicare hospital payments.
Under CMS’ new Acute Hospital Care at Home, which is not described as temporary, patients can be transferred from emergency departments or inpatient wards to hospital-level care at home. The CMS said the HaH program is designed for people with conditions such as the acute phases of asthma, heart failure, pneumonia, and chronic obstructive pulmonary disease. Altogether, the agency said, more than 60 acute conditions can be treated safely at home.
However, the agency didn’t say that facilities can’t admit COVID-19 patients to the hospital at home. Rami Karjian, MBA, cofounder and CEO of Medically Home, a firm that supplies health systems with technical services and software for HaH programs, said in an interview that several Medically Home clients plan to treat both COVID-19 and non-COVID-19 patients at home when they begin to participate in the CMS program in the near future.
The CMS said it consulted extensively with academic and private industry leaders in building its HaH program. Before rolling out the initiative, the agency noted, it conducted successful pilot programs in leading hospitals and health systems. The results of some of these pilots have been reported in academic journals.
Participating hospitals will be required to have specified screening protocols in place before beginning acute care at home, the CMS announced. An in-person physician evaluation will be required before starting care at home. A nurse will evaluate each patient once daily in person or remotely, and either nurses or paramedics will visit the patient in person twice a day.
In contrast, Medicare regulations require nursing staff to be available around the clock in traditional hospitals. So the CMS has to grant waivers to hospitals for HaH programs.
While not going into detail on the telemonitoring capabilities that will be required in the acute hospital care at home, the release said, “Today’s announcement builds upon the critical work by CMS to expand telehealth coverage to keep beneficiaries safe and prevent the spread of COVID-19.”
More flexibility for ASCs
The agency is also giving ASCs the flexibility to provide 24-hour nursing services only when one or more patients are receiving care on site. This flexibility will be available to any of the 5,700 ASCs that wish to participate, and will be immediately effective for the 85 ASCs currently participating in the Hospital Without Walls initiative, the CMS said.
The new ASC regulations, the CMS said, are aimed at allowing communities “to maintain surgical capacity and other life-saving non-COVID-19 [care], like cancer surgeries.” Patients who need such procedures will be able to receive them in ASCs without being exposed to known COVID-19 cases.
Similarly, the CMS said patients and families not diagnosed with COVID-19 may prefer to receive acute care at home if local hospitals are full of COVID-19 patients. In addition, the CMS said it anticipates patients may value the ability to be treated at home without the visitation restrictions of hospitals.
Early HaH participants
Six health systems with extensive experience in providing acute hospital care at home have been approved for the new HaH waivers from Medicare rules. They include Brigham and Women’s Hospital (Massachusetts); Huntsman Cancer Institute (Utah); Massachusetts General Hospital (Massachusetts); Mount Sinai Health System (New York City); Presbyterian Healthcare Services (New Mexico); and UnityPoint Health (Iowa).
The CMS said that it’s in discussions with other health care systems and expects new applications to be submitted soon.
To support these efforts, the CMS has launched an online portal to streamline the waiver request process. The agency said it will closely monitor the program to safeguard beneficiaries and will require participating hospitals to report quality and safety data on a regular basis.
Support from hospitals
The first health systems participating in the CMS HaH appear to be supportive of the program, with some hospital leaders submitting comments to the CMS about their view of the initiative.
“The CMS has taken an extraordinary step today, facilitating the rapid expansion of Hospitalization at Home, an innovative care model with proven results,” said Kenneth L. Davis, MD, president and CEO of the Mount Sinai Health System in New York City. “This important and timely move will enable hospitals across the country to use effective tools to safely care for patients during this pandemic.”
David Levine, MD, assistant professor of medicine and medical director of strategy and innovation for Brigham Health Home Hospital in Boston, was similarly laudatory: “Our research at Brigham Health Home has shown that we can deliver hospital-level care in our patients’ homes with lower readmission rates, more physical mobility, and a positive patient experience,” he said. “During these challenging times, a focus on the home is critical. We are so encouraged that CMS is taking this important step, which will allow hospitals across the country to increase their capacity while delivering the care all patients deserve.”
Scaling up quickly
If other hospitals and health systems recognize the value of HaH, how long might it take them to develop and implement these programs in the midst of a pandemic?
Atrium Health, a large health system in the Southeast, ramped up a hospital-at-home initiative last spring for its 10 hospitals in the Charlotte, N.C., area, in just 2 weeks. However, it had been working on the project for some time before the pandemic struck. Focusing mostly on COVID-19 patients, the initiative reduced the COVID-19 patient load by 20%-25% in Atrium’s hospitals.
Medically Home, the HaH infrastructure company, said in a news release that it “enables health systems to establish new hospital-at-home services in as little as 30 days.” Medically Home has partnered in this venture with Huron Consulting Group, which has about 200 HaH-trained consultants, and Cardinal Health, a large global medical supplies distributor.
Mr. Karjian said in an interview that he expects private insurers to follow CMS’ example, as they often do. “We think this decision will cause not only CMS but private insurers to cover hospital at home after the pandemic, if it becomes the standard of care, because patients have better outcomes when treated at home,” he said.
Asked for his view on why the CMS specified that patients could be admitted to an HaH only from emergency departments or inpatient settings, Mr. Karjian said that the CMS wants to make sure that patients have access to brick-and-mortar hospital care if that’s what they need. Also, he noted, this model is new to most hospitals, so the CMS wants to make sure it starts “with all the safety guardrails” in place.
Overall, Mr. Karjian said, “This is an exciting development for patients across the country. What CMS has done is terrific in terms of letting patients get the care they want, where they want it, and get the benefit of better outcomes while the nation is going through this capacity crunch for hospital beds.”
A version of this article originally appeared on Medscape.com.
Patient health suffers amid pandemic health care shortages
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
More than half (56%) of responding clinicians reported seeing a decline in patient health because of delayed or inaccessible care amid the pandemic, according to the results of the latest survey by the Larry A. Green Center and the Primary Care Collaborative. The survey was conducted in mid-October and the results were published online Nov. 17.
In addition, 37% of respondents said their patients with chronic conditions showed “noticeably worse health resulting from the pandemic.” And a resounding 85% said patient mental health had worsened.
“I think it’s worse than we thought,” said Rebecca Etz, PhD, codirector of the Larry Green Center. “It’s the outcome of not sufficiently sending resources to primary care either before or during the pandemic.” According to Dr. Etz, survey respondents noted substantial increases in patient weight gain as well as weight loss, anxiety and depression, sleep issues, domestic abuse, and poor oral and eye health, among others.
One clinician from Pennsylvania wrote: “Patients are becoming sicker during the pandemic. I’m seeing more uncontrolled [diabetes]and new [patients with diabetes]. They prefer telehealth yet [have] no access to glucose monitoring or a blood pressure cuff. I am concerned about patients’ isolation and mental health. People are delaying care.”
Now, with COVID numbers peaking across much of the country, many clinicians are trying to close the gap in care with telehealth – something they’re more prepared to do now than they were in March. Over two-thirds of practices are using telehealth for visits to keep up with patients who have stable chronic conditions, according to the survey.
Over 60% of physicians report using telehealth for mental health visits. But a much smaller number – only 16% of respondents – said their practice had added staff to help manage the rising number of behavioral and mental health cases. About one-third (35%) of practices say they’re not financially able to take on new staff.
“We’ve been looking for more ways for patients to do self-support. A big part of chronic disease is health behaviors,” Alex Krist, MD, MPH, a family doctor in Fairfax, Va., and chairperson of the U.S. Preventive Services Task Force, said in an interview. And unfortunately, on top of limited access to basic care, healthy habits that are essential to managing many chronic conditions have become more difficult and less consistent during the pandemic.
The survey – the 22nd iteration in a series of surveys the Green Center and the Primary Care Collaborative have conducted – received 580 respondents from 47 states and Guam. Over two-thirds of respondents were primary care physicians (MDs and DOs). Over half were owners, partners, or employees of a private practice, 66% of which were family medicine practices. And one fifth of respondents provided care in a rural area.
Funding and support for primary care has been wildly insufficient, Dr. Etz said in an interview. If that doesn’t change, patient health, clinic staffing, and public health strategies amid the pandemic will continue to suffer.
“When you think of the COVID vaccine, who do you think is going to be sending that out?” Dr. Etz asked. “If we don’t bolster primary care now how are they going to handle that.”
A version of this article originally appeared on Medscape.com.
Moderna filing for FDA emergency COVID-19 vaccine approval, reports 94.1% efficacy
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.
The Moderna COVID-19 vaccine in development was 94.1% effective in the final analysis of its 30,000-participant phase 3 study. Bolstered by the new findings, the company plans to file for an emergency use authorization (EUA) from the Food and Drug Administration (FDA) today, according to a company release.
A total of 11 people in the mRNA-1273 vaccinated group later tested positive for COVID-19, compared with 185 participants given two placebo injections, resulting in a point estimate of 94.1% efficacy. This finding aligns with the 94.5% efficacy in interim trial results announced on November 16, as reported by Medscape Medical News.
Furthermore, Moderna announced that the vaccine prevented serious cases of infection. All 30 severe infections occurred among those people randomly assigned to placebo.
The FDA plans to review the Moderna vaccine safety and efficacy data at the next Vaccines and Related Biological Products Advisory Committee (VRBPAC) meeting scheduled for December 17. If and when approved, healthcare providers can use the new 91301 CPT code specific to mRNA-1273 vaccination.
“This positive primary analysis confirms the ability of our vaccine to prevent COVID-19 disease with 94.1% efficacy and, importantly, the ability to prevent severe COVID-19 disease,” said Stéphane Bancel, MBA, MEng, chief executive officer of Moderna, in the news release. “We believe that our vaccine will provide a new and powerful tool that may change the course of this pandemic and help prevent severe disease, hospitalizations, and death.”
Vaccine efficacy remained consistent across different groups analyzed by age, race/ethnicity, and gender. The 196 COVID-19 cases in the trial included 33 adults older than 65 years and 42 people from diverse communities, including 29 Hispanic or Latinx, six Black or African Americans, four Asian Americans, and three multiracial participants, the company reported.
No serious vaccine-related safety issues
The mRNA-1273 vaccine was generally well tolerated and no serious safety concerns with the vaccine have been identified to date, the company reported.
Injection site pain, fatigue, myalgia, arthralgia, headache, and erythema/redness at the injection site were the most common solicited adverse events in a prior analysis. The company noted that these solicited adverse reactions increased in frequency and severity after the second vaccine dose. A continuous review of safety data is ongoing.
One COVID-19-related death in the study occurred in the placebo group.
Ready to start shipping
Moderna expects to have approximately 20 million doses of mRNA-1273 available in the United States by the end of this year. The company reports that it’s on track to manufacture 500 million to 1 billion doses globally in 2021.
The company also is seeking approval from nations and organizations worldwide, including a conditional approval from the European Medicines Agency (EMA). The study is being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services.
Moderna will be the second company to file an EUA with the FDA for a COVID vaccine, after Pfizer requested one for its mRNA vaccine earlier this month.
This article first appeared on Medscape.com.





