User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Biggest challenges practices faced from COVID last year: MGMA
according to a December 2020 report from the Medical Group Management Association.
The report was assembled from the results of weekly Stat polls by MGMA, which consists of 15,000 group practices representing more than 350,000 physicians. During the course of the year, more than 4,800 practice leaders were surveyed, but the individual polls had far fewer respondents.
The 2020 data represents snapshots from different points in the developing public health crisis. Still, much of what practices experienced earlier in the pandemic continues to apply, and it’s likely to persist this year as long as the coronavirus spreads and its toll deepens.
One top-line conclusion of the report: the economic pain felt by practices has resulted in layoffs, furloughs, and/or reduced compensation for providers and staff.
In the May 19 weekly survey, 82% of respondents said some or all of their providers’ compensation had been affected by the crisis. About 62% said every provider had been affected. Provider compensation was cut in several ways, including reduced hours and salaries, reduced or eliminated bonuses, and lower allowances for continuing medical education.
About 61% of health care leaders said in the June 26 poll that their own compensation had decreased.
In the following week’s survey, one in three managers said their organization had reduced staff compensation. Nearly all of the respondents in this category predicted the salary reductions would be temporary.
As of March 17, early in the pandemic, 40% of health care leaders said they were experiencing staff shortages. An April 21 poll found that 53% of health care leaders were taking steps to address their providers’ and staffers’ mental health.
“The mental and emotional toll on everyone continues to be a concern, as public health authorities continue to report alarming numbers of new [COVID-19] cases, hospitalizations, and deaths,” MGMA commented.
Telehealth and remote monitoring
Nearly all of the health care leaders surveyed on March 31 reported that their practices had expanded telehealth access because of COVID-19. The percentage of patient visits handled remotely had dropped substantially by the fall, according to a Harvard University/Commonwealth Fund/Phreesia survey. Still, it remains significantly higher than it was before the pandemic.
“At the end of 2020, telemedicine continues to play a vital role in everyday practice operations and long-term planning,” the MGMA report said. One indication of this, the association said, is that health care leaders are recognizing new best practices in specialty telemedicine, such as pediatrics and ob.gyn.
According to an April 28 poll, the top three coding/billing challenges for telehealth and telephone visits amid COVID-19 were inconsistent payer rules, pay parity and accuracy, and documentation of virtual visits.
While the Centers for Medicare & Medicaid Services has loosened its regulations to allow reimbursement of telehealth in all locations and at the same level as in-person visits, most of those changes will not last beyond the public health crisis without new legislation.
More health care leaders are considering the use of remote patient monitoring, MGMA said, but only 21% of practices offered such services as of Sept. 15. The report drew a connection between these plans and the current challenge of deferred care.
In the July 21 poll, 87% of health care leaders reported that safety concerns were the top reason that patients deferred care amid COVID-19. The MGMA report quoted JaeLynn Williams, CEO of Air Methods, which provides helicopter ambulance services, as saying that many people are staying home even when they face life-threatening conditions such as chest pain, drug symptoms, inflamed appendix, and gallbladder pain.
Operational issues
Overall, MGMA said, practices that have taken a financial risk have done better during the pandemic than fee-for-service practices because their monthly capitation revenue has continued unabated. In contrast, “most groups’ struggles to sustain visits and procedures meant less revenue and lower compensation,” the report said.
In the August 18 survey, one in three health care leaders reported their practices were changing their operational metrics and how often they looked at those measures because of the pandemic. “Practice managers are asking for dashboard data in weeks instead of months to measure the drop in charges and forecast the resulting change in collections,” MGMA noted. “The type of data practice managers are asking for has also changed.”
Among the new metrics that practices are interested in, according to an MGMA article, are measures that track telehealth visits, the productivity of staff working at home, and the number of ancillary services and procedures that new patients might need based on historical data.
Nearly all health care leaders surveyed on Aug. 11 said the cost of obtaining personal protective equipment had increased during 2020. MGMA said it expects this situation to worsen if the pandemic lasts through the summer of 2021.
While everyone is talking about the botched launch of the COVID-19 vaccination campaign, there were also problems with flu vaccination in 2020. In the Sept. 25 poll, 34% of health care leaders reported their practices were experiencing delays in getting the flu vaccine.
Looking ahead
Looking further ahead, the report recommended that practices make plans to boost staff morale by restoring bonuses.
In addition, MGMA suggested that physician groups reassess their space needs. “The equation is simple – fewer nonclinical staff members at your facility means you should repurpose that office space or consider finding a better fit for your new real estate needs in 2021.”
Finally, MGMA noted that the practices expanding rather than contracting their business are those increasing their value-based revenues by taking on more risk. For those groups, “growing the patient panel can help [them] seek better rates in contract negotiations.”
A version of this article first appeared on Medscape.com.
according to a December 2020 report from the Medical Group Management Association.
The report was assembled from the results of weekly Stat polls by MGMA, which consists of 15,000 group practices representing more than 350,000 physicians. During the course of the year, more than 4,800 practice leaders were surveyed, but the individual polls had far fewer respondents.
The 2020 data represents snapshots from different points in the developing public health crisis. Still, much of what practices experienced earlier in the pandemic continues to apply, and it’s likely to persist this year as long as the coronavirus spreads and its toll deepens.
One top-line conclusion of the report: the economic pain felt by practices has resulted in layoffs, furloughs, and/or reduced compensation for providers and staff.
In the May 19 weekly survey, 82% of respondents said some or all of their providers’ compensation had been affected by the crisis. About 62% said every provider had been affected. Provider compensation was cut in several ways, including reduced hours and salaries, reduced or eliminated bonuses, and lower allowances for continuing medical education.
About 61% of health care leaders said in the June 26 poll that their own compensation had decreased.
In the following week’s survey, one in three managers said their organization had reduced staff compensation. Nearly all of the respondents in this category predicted the salary reductions would be temporary.
As of March 17, early in the pandemic, 40% of health care leaders said they were experiencing staff shortages. An April 21 poll found that 53% of health care leaders were taking steps to address their providers’ and staffers’ mental health.
“The mental and emotional toll on everyone continues to be a concern, as public health authorities continue to report alarming numbers of new [COVID-19] cases, hospitalizations, and deaths,” MGMA commented.
Telehealth and remote monitoring
Nearly all of the health care leaders surveyed on March 31 reported that their practices had expanded telehealth access because of COVID-19. The percentage of patient visits handled remotely had dropped substantially by the fall, according to a Harvard University/Commonwealth Fund/Phreesia survey. Still, it remains significantly higher than it was before the pandemic.
“At the end of 2020, telemedicine continues to play a vital role in everyday practice operations and long-term planning,” the MGMA report said. One indication of this, the association said, is that health care leaders are recognizing new best practices in specialty telemedicine, such as pediatrics and ob.gyn.
According to an April 28 poll, the top three coding/billing challenges for telehealth and telephone visits amid COVID-19 were inconsistent payer rules, pay parity and accuracy, and documentation of virtual visits.
While the Centers for Medicare & Medicaid Services has loosened its regulations to allow reimbursement of telehealth in all locations and at the same level as in-person visits, most of those changes will not last beyond the public health crisis without new legislation.
More health care leaders are considering the use of remote patient monitoring, MGMA said, but only 21% of practices offered such services as of Sept. 15. The report drew a connection between these plans and the current challenge of deferred care.
In the July 21 poll, 87% of health care leaders reported that safety concerns were the top reason that patients deferred care amid COVID-19. The MGMA report quoted JaeLynn Williams, CEO of Air Methods, which provides helicopter ambulance services, as saying that many people are staying home even when they face life-threatening conditions such as chest pain, drug symptoms, inflamed appendix, and gallbladder pain.
Operational issues
Overall, MGMA said, practices that have taken a financial risk have done better during the pandemic than fee-for-service practices because their monthly capitation revenue has continued unabated. In contrast, “most groups’ struggles to sustain visits and procedures meant less revenue and lower compensation,” the report said.
In the August 18 survey, one in three health care leaders reported their practices were changing their operational metrics and how often they looked at those measures because of the pandemic. “Practice managers are asking for dashboard data in weeks instead of months to measure the drop in charges and forecast the resulting change in collections,” MGMA noted. “The type of data practice managers are asking for has also changed.”
Among the new metrics that practices are interested in, according to an MGMA article, are measures that track telehealth visits, the productivity of staff working at home, and the number of ancillary services and procedures that new patients might need based on historical data.
Nearly all health care leaders surveyed on Aug. 11 said the cost of obtaining personal protective equipment had increased during 2020. MGMA said it expects this situation to worsen if the pandemic lasts through the summer of 2021.
While everyone is talking about the botched launch of the COVID-19 vaccination campaign, there were also problems with flu vaccination in 2020. In the Sept. 25 poll, 34% of health care leaders reported their practices were experiencing delays in getting the flu vaccine.
Looking ahead
Looking further ahead, the report recommended that practices make plans to boost staff morale by restoring bonuses.
In addition, MGMA suggested that physician groups reassess their space needs. “The equation is simple – fewer nonclinical staff members at your facility means you should repurpose that office space or consider finding a better fit for your new real estate needs in 2021.”
Finally, MGMA noted that the practices expanding rather than contracting their business are those increasing their value-based revenues by taking on more risk. For those groups, “growing the patient panel can help [them] seek better rates in contract negotiations.”
A version of this article first appeared on Medscape.com.
according to a December 2020 report from the Medical Group Management Association.
The report was assembled from the results of weekly Stat polls by MGMA, which consists of 15,000 group practices representing more than 350,000 physicians. During the course of the year, more than 4,800 practice leaders were surveyed, but the individual polls had far fewer respondents.
The 2020 data represents snapshots from different points in the developing public health crisis. Still, much of what practices experienced earlier in the pandemic continues to apply, and it’s likely to persist this year as long as the coronavirus spreads and its toll deepens.
One top-line conclusion of the report: the economic pain felt by practices has resulted in layoffs, furloughs, and/or reduced compensation for providers and staff.
In the May 19 weekly survey, 82% of respondents said some or all of their providers’ compensation had been affected by the crisis. About 62% said every provider had been affected. Provider compensation was cut in several ways, including reduced hours and salaries, reduced or eliminated bonuses, and lower allowances for continuing medical education.
About 61% of health care leaders said in the June 26 poll that their own compensation had decreased.
In the following week’s survey, one in three managers said their organization had reduced staff compensation. Nearly all of the respondents in this category predicted the salary reductions would be temporary.
As of March 17, early in the pandemic, 40% of health care leaders said they were experiencing staff shortages. An April 21 poll found that 53% of health care leaders were taking steps to address their providers’ and staffers’ mental health.
“The mental and emotional toll on everyone continues to be a concern, as public health authorities continue to report alarming numbers of new [COVID-19] cases, hospitalizations, and deaths,” MGMA commented.
Telehealth and remote monitoring
Nearly all of the health care leaders surveyed on March 31 reported that their practices had expanded telehealth access because of COVID-19. The percentage of patient visits handled remotely had dropped substantially by the fall, according to a Harvard University/Commonwealth Fund/Phreesia survey. Still, it remains significantly higher than it was before the pandemic.
“At the end of 2020, telemedicine continues to play a vital role in everyday practice operations and long-term planning,” the MGMA report said. One indication of this, the association said, is that health care leaders are recognizing new best practices in specialty telemedicine, such as pediatrics and ob.gyn.
According to an April 28 poll, the top three coding/billing challenges for telehealth and telephone visits amid COVID-19 were inconsistent payer rules, pay parity and accuracy, and documentation of virtual visits.
While the Centers for Medicare & Medicaid Services has loosened its regulations to allow reimbursement of telehealth in all locations and at the same level as in-person visits, most of those changes will not last beyond the public health crisis without new legislation.
More health care leaders are considering the use of remote patient monitoring, MGMA said, but only 21% of practices offered such services as of Sept. 15. The report drew a connection between these plans and the current challenge of deferred care.
In the July 21 poll, 87% of health care leaders reported that safety concerns were the top reason that patients deferred care amid COVID-19. The MGMA report quoted JaeLynn Williams, CEO of Air Methods, which provides helicopter ambulance services, as saying that many people are staying home even when they face life-threatening conditions such as chest pain, drug symptoms, inflamed appendix, and gallbladder pain.
Operational issues
Overall, MGMA said, practices that have taken a financial risk have done better during the pandemic than fee-for-service practices because their monthly capitation revenue has continued unabated. In contrast, “most groups’ struggles to sustain visits and procedures meant less revenue and lower compensation,” the report said.
In the August 18 survey, one in three health care leaders reported their practices were changing their operational metrics and how often they looked at those measures because of the pandemic. “Practice managers are asking for dashboard data in weeks instead of months to measure the drop in charges and forecast the resulting change in collections,” MGMA noted. “The type of data practice managers are asking for has also changed.”
Among the new metrics that practices are interested in, according to an MGMA article, are measures that track telehealth visits, the productivity of staff working at home, and the number of ancillary services and procedures that new patients might need based on historical data.
Nearly all health care leaders surveyed on Aug. 11 said the cost of obtaining personal protective equipment had increased during 2020. MGMA said it expects this situation to worsen if the pandemic lasts through the summer of 2021.
While everyone is talking about the botched launch of the COVID-19 vaccination campaign, there were also problems with flu vaccination in 2020. In the Sept. 25 poll, 34% of health care leaders reported their practices were experiencing delays in getting the flu vaccine.
Looking ahead
Looking further ahead, the report recommended that practices make plans to boost staff morale by restoring bonuses.
In addition, MGMA suggested that physician groups reassess their space needs. “The equation is simple – fewer nonclinical staff members at your facility means you should repurpose that office space or consider finding a better fit for your new real estate needs in 2021.”
Finally, MGMA noted that the practices expanding rather than contracting their business are those increasing their value-based revenues by taking on more risk. For those groups, “growing the patient panel can help [them] seek better rates in contract negotiations.”
A version of this article first appeared on Medscape.com.
ACEIs, ARBs safe to continue in COVID-19: Trial published
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
The BRACE-CORONA trial, the first randomized trial to address the question of whether patients with COVID-19 should continue to take ACE inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs) – has now been published.
The study, which was conducted in patients hospitalized with COVID-19 who were taking ACEIs or ARBs before hospitalization, showed no significant difference in the mean number of days alive and out of the hospital for those assigned to discontinue versus those assigned to continue these medications.
There were, however, hints that continuing to take ACEIs or ARBs may be beneficial for patients with more severe COVID-19.
The study was first presented at last year’s European Society of Cardiology Congress and was reported by this news organization at that time. The study was published online in JAMA on Jan. 19, 2021.
“These findings do not support routinely discontinuing ACEIs or ARBs among patients hospitalized with mild to moderate COVID-19 if there is an indication for treatment,” the authors concluded.
Led by Renato D. Lopes, MD, Duke Clinical Research Institute, Durham, N.C., the researchers explained that there has been conflicting speculation about the effect of renin-angiotensin-aldosterone system (RAAS) inhibitors on the course of COVID-19.
On the one hand, observations from animal models suggest that ACEIs and ARBs up-regulate the expression of ACE2, a receptor involved in SARS-CoV-2 infection of host target cells. This led to suggestions that these medications may enhance viral binding and cell entry. Conversely, RAAS inhibitors could benefit patients with COVID-19 through effects on angiotensin II expression and subsequent increases in angiotensin 1-7 and 1-9, which have vasodilatory and anti-inflammatory effects that might attenuate lung injury.
The BRACE-CORONA trial included 659 patients hospitalized in Brazil with mild to moderate COVID-19 who were taking ACEIs or ARBs prior to hospitalization. The median age of the patients was 55 years. Of these patients, 57.1% were considered to have mild cases at hospital admission, and 42.9% were considered to have moderate cases.
Results showed no significant difference in the number of days alive and out of the hospital for patients in the discontinuation group (mean, 21.9 days) in comparison with patients in the continuation group (mean, 22.9 days). The mean ratio was 0.95 (95% confidence interval, 0.90-1.01).
There also was no statistically significant difference in deaths (2.7% of the discontinuation group vs. 2.8% for the continuation group); cardiovascular death (0.6% vs. 0.3%), or COVID-19 progression (38.3% vs. 32.3%).
The most common adverse events were respiratory failure requiring invasive mechanical ventilation (9.6% in the discontinuation group vs. 7.7% in the continuation group), shock requiring vasopressors (8.4% vs. 7.1%), acute MI (7.5% vs. 4.6%), new or worsening heart failure (4.2% vs. 4.9%), and acute kidney failure requiring hemodialysis (3.3% vs. 2.8%).
The authors note that hypertension is an important comorbidity in patients with COVID-19. Recent data suggest that immune dysfunction may contribute to poor outcomes among patients who have COVID-19 and hypertension.
It has been shown that, when use of long-term medications is discontinued during hospitalization, the use of those medications is often not resumed, owing to clinical inertia. Long-term outcomes worsen as a result, the authors reported. In the current study, all patients had hypertension, and more than 50% were obese; both of these comorbidities increase the risk for poor outcomes with COVID-19.
The investigators pointed out that a sensitivity analysis in which site was regarded as a random effect showed a statistically significant finding in favor of the group that continued ACEIs or ARBs. This finding was similar to that of the on-treatment analysis. There were also statistically significant interactions between treatment effect and some subgroups, such as patients with lower oxygen saturation and greater disease severity at hospital admission. For these patients, continuing ACEIs or ARBs may be beneficial.
“The primary analyses with the null results but wide 95% confidence intervals suggest that the study might have been underpowered to detect a statistically significant benefit of continuing ACEIs or ARBs,” they said.
Dr. Lopes has received grant support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi.
A version of this article first appeared on Medscape.com.
Widespread liver disease missed in patients with T2D
Among these calls is a pending statement from the Endocrine Society, the American Association of Clinical Endocrinologists, the American Gastroenterology Association, and other groups on what the growing appreciation of highly prevalent liver disease in patients with type 2 diabetes (T2D) means for assessing and managing patients. Publication of the statement is expected by spring 2021, said Christos S. Mantzoros, MD, DSc, PhD, chief of endocrinology for the Veterans Affairs Boston Healthcare System and a representative from the Endocrine Society to the statement-writing panel.
This upcoming “Call to Action” from these groups argues for a “need to collaborate across disciplines, and work together on establishing clinical guidelines, and creating new diagnostics and therapeutics,” said Dr. Mantzoros in an interview.
“Over time, it is becoming clearer that management of NAFLD [nonalcoholic fatty liver disease]/NASH [nonalcoholic steatohepatitis] requires a multidisciplinary panel of doctors ranging from primary care practitioners, to endocrinologists, and hepatologists. Given that the nature of the disease crosses scientific discipline boundaries, and that the number of patients is so large (it is estimated that about one in four U.S. adults have NAFLD), not all patients can be treated at the limited number of hepatology centers.
“However, not all stakeholders have fully realized this fact, and no effort had been undertaken so far by any professional society to develop a coordinated approach and clinical care pathway for NAFLD/NASH. The ‘Call to Action’ meeting can be considered as a starting point for such an important effort,” said Dr. Mantzoros, who is also a professor of medicine at Harvard Medical School and director of the human nutrition unit at Beth Israel Deaconess Medical Center, both in Boston.
Dramatic prevalence rates in patients with T2D
Results from two independent epidemiology reports, published in December 2020, documented steatosis (the fatty liver of NAFLD) in 70%-74% of unselected U.S. patients with T2D, advanced liver fibrosis accompanying this disease in 6%-15%, and previously unrecognized cirrhosis in 3%-8%.
One of these reports analyzed 825 patients with T2D included in the National Health and Nutritional Examination Survey of 2017-2018 run by the Centers for Disease Control and Prevention. All these patients, selected to be representative of the overall U.S. adult population with T2D, underwent transient elastography to identify steatosis and fibrosis, the first U.S. National Health Survey to run this type of population-based survey. The results showed an overall steatosis prevalence of 74% with grade 3 steatosis in 58%, advanced liver fibrosis in 15%, and cirrhosis in 8%, reported the team of Italian researchers who analyzed the data .
The second study focused on a single-center series of 561 patients with T2D who also underwent screening by transient elastography during 2018-2020 and had no history of NAFLD or other liver disease, or alcohol abuse. The imaging results showed a NAFLD prevalence of 70%, with 54% of the entire group diagnosed with severe steatosis, severe fibrosis in 6%, and cirrhosis in 3%. Among the 54% of patients with severe steatosis, 30% also had severe liver fibrosis. About 70% of the 561 patients assessed came from either the family medicine or general internal medicine clinics of the University of Florida, Gainesville, with the remaining 30% enrolled from the center’s endocrinology/diabetes outpatient clinic.
Neither report documented a NASH prevalence, which cannot receive definitive diagnosis by imaging alone. “This is the first study of its kind in the U.S. to establish the magnitude of [liver] disease burden in random patients with T2D seeking regular outpatient care,” wrote the University of Florida research team, led by Kenneth Cusi, MD, professor and chief of the university’s division of endocrinology, diabetes, and metabolism. Their finding that patients with T2D and previously unknown to have NAFLD had a 15% prevalence of moderate or advanced liver fibrosis “should trigger a call to action by all clinicians taking care of patients with T2D. Patient and physician awareness of the hepatic and extrahepatic complications of NASH, and reversing current diagnosis and treatment inertia will be the only way to avert the looming epidemic of cirrhosis in patients with diabetes.”
“Endocrinologists don’t ‘see’ NAFLD and NASH” in their patients with T2D “ because they don’t think about it,” Dr. Mantzoros declared.
“Why is NASH underdiagnosed and undertreated? Because many physicians aren’t aware of it,” agreed Dr. Cusi during a talk in December 2020 at the 18th World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease (WCIRDC). “You never find what you don’t look for.”
“Endocrinologists should do the tests for NASH [in patients with T2D], but we’re all guilty of not doing it enough,” Tracey McLaughlin, MD, an endocrinologist and professor of medicine at Stanford (Calif.) University, commented during the WCIRDC.
These prevalence numbers demand that clinicians suspect liver disease “in any patient with diabetes, especially patients with obesity who are older and have components of metabolic syndrome,” said Dr. Mantzoros. “We need to screen, refer the most advanced cases, and treat the early- and mid-stage cases.”
How to find NASH
Both the American Diabetes Association and the European Association for the Study of Diabetes call for routine screening of patients with T2D, starting with a check of liver enzymes, such as ALT, but no clear consensus exists for the specifics of screening beyond that. Dr. Mantzoros, Dr. Cusi, and other experts agree that the scheme for assessing liver disease in patients with T2D starts with regular monitoring of elevations in liver enzymes including ALT. Next is noninvasive ultrasound assessment of the extent of liver fibrosis inferred from the organ’s stiffness using transient elastography. Another frequently cited initial screening tool is the Fibrosis-4 (FIB-4) score, which incorporates a patient’s age, platelet count, and levels of ALT and a second liver enzyme, AST.
“There is more consensus about FIB-4 and then elastography, but some people use tests other than FIB-4. Unfortunately there is no perfect diagnostic test today. A top priority is to define the best diagnostic test,” said Dr. Mantzoros, who is leading an effort to try to refine screening using artificial intelligence.
“FIB-4 is simple, easy, and well validated,” commented Dr. Cusi during the WCIRDC last December. “FIB-4 and elastography should get you pretty close” to identifying patients with T2D and significant liver disease.
But in a recent editorial, Dr. Cusi agreed on the need for “more reliable tests for the diagnosis of NASH and advanced fibrosis in patients with T2D. Significant work is being done in the field to validate novel and more sophisticated fibrosis biomarkers. Future studies will help us enter a new era of precision medicine where biomarkers will identify and target therapy to those with more active disease at risk for cirrhosis,” he wrote.
“The ultimate goal is to diagnose fibrosis at an early stage to prevent people from developing cirrhosis,” Dr. Cusi said in a recent written statement. “We’re trying to identify these problems before they’re unfixable. Once someone has cirrhosis, there isn’t a whole lot you can do.”
Pioglitazone remains the best-documented treatment
Perhaps some of the inertia in diagnosing NAFLD, NASH, and liver fibrosis in patients with T2D is dissatisfaction with current treatment options, although several proven options exist, notably weight loss and diet, and thiazolidinedione (TZD) pioglitazone. But weight loss and diet pose issues for patient compliance and durability of the intervention, and many clinicians consider pioglitazone flawed by its potential adverse effects.
“When we don’t have an established treatment for something, we tend to not measure it or go after it. That’s been true of liver disease” in patients with T2D, said Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif., during the WCIRDC.
Treatment with pioglitazone has resolved NASH in about a third of patients compared with placebo, prevented fibrosis progression, and cut cardiovascular disease events, noted Dr. Cusi during the WCIRDC.
“Pioglitazone is used in only 8% of patients with T2D, or less, but we need to use it more often because of its proven efficacy in patients with T2D and NASH” said Dr. Mantzoros. “The problem is that pioglitazone has side effects, including weight gain and fluid retention, that makes it less attractive unless one thinks about the diagnosis of NASH.”
Others highlight that the adverse effects of pioglitazone have been either misunderstood, or can be effectively minimized with careful dosing.
“The data with the TZDs are much stronger than the data from anything else. TZDs have gotten a bad name because they also work in the kidney and enhance fluid reabsorption. We use modest dosages of pioglitazone, 15 mg or 30 mg a day, to avoid excess fluid retention,” Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio, said during the WCIRDC. “The best drug for NASH is pioglitazone. No other drug beats it” based on current data, Dr. DeFronzo asserted.
Other strategies include the potential to pair pioglitazone with other interventions that can blunt a weight-gain effect. One intriguing combination would combine pioglitazone with a GLP-1 receptor agonist, a drug class that can produce significant weight loss. Results from a phase 2 study showed promise for semaglutide (Rybelsus) in treating patients with NASH.
Getting the name right
Another factor that may be keeping NAFLD and NASH from achieving a higher profile for patients with T2D are those names, which focus on what the diseases are not – nonalcoholic – rather than what they are.
A series of recent publications in both the endocrinology and hepatology literature have called for renaming these disorders either “metabolic (dysfunction)–associated fatty liver disease (MALFD)”, or “dysmetabolism-associated fatty liver disease (DALFD)”.
“The names NAFLD and NASH indicate absence of alcohol as a cause, but the disease is also characterized by the absence of other causes, such as autoimmune disorders or hepatitis. The names were coined when we did not know much about these diseases. We now know that it is dysmetabolism that causes these conditions, and so we need to adopt a new, more accurate name,” explained Dr. Mantzoros, who has published support for a name change.
While many agree, some have raised concerns as to whether a name change now is premature. A group of hepatologists recently published a rebuttal to an immediate name change , saying that, “although we are in agreement that metabolic fatty liver disease may more accurately and positively reflect the relevant risk factors better than the age-old term nonalcoholic fatty liver disease, the term still leaves a great deal of ambiguity. A name change will be appropriate when informed by a new understanding of the molecular basis of the disease entity, insights that fundamentally change risk stratification, or other important aspects of the disease. We may be on the cusp of this, but we are not there yet.”
Dr. Mantzoros agreed, but for somewhat different reasons.
“We need to be careful and deliberate, because there is a significant body of knowledge and a lot of data from clinical trials collected using the old definitions. We need to find an appropriate time frame for a [name] transition. We need to find a nice and robust way to productively bridge the old to the new,” he said. “We also need new diagnostic criteria, and new therapies. A new name and definition will facilitate progress.”
Dr. Mantzoros been a shareholder of and consultant to Coherus and Pangea, he has been a consultant to AstraZeneca, Eisai, Genfit, Intercept, Novo Nordisk, P.E.S., and Regeneron, and has received travel support from the Metabolic Institute of America and the California Walnut Commission. Dr. Cusi has been a consultant to and has received research funding from numerous drug companies. Dr. McLaughlin is a consultant to January AI. Dr. Handelsman has been a consultant to numerous drug companies. Dr. DeFronzo received research grants from AstraZeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk.
Among these calls is a pending statement from the Endocrine Society, the American Association of Clinical Endocrinologists, the American Gastroenterology Association, and other groups on what the growing appreciation of highly prevalent liver disease in patients with type 2 diabetes (T2D) means for assessing and managing patients. Publication of the statement is expected by spring 2021, said Christos S. Mantzoros, MD, DSc, PhD, chief of endocrinology for the Veterans Affairs Boston Healthcare System and a representative from the Endocrine Society to the statement-writing panel.
This upcoming “Call to Action” from these groups argues for a “need to collaborate across disciplines, and work together on establishing clinical guidelines, and creating new diagnostics and therapeutics,” said Dr. Mantzoros in an interview.
“Over time, it is becoming clearer that management of NAFLD [nonalcoholic fatty liver disease]/NASH [nonalcoholic steatohepatitis] requires a multidisciplinary panel of doctors ranging from primary care practitioners, to endocrinologists, and hepatologists. Given that the nature of the disease crosses scientific discipline boundaries, and that the number of patients is so large (it is estimated that about one in four U.S. adults have NAFLD), not all patients can be treated at the limited number of hepatology centers.
“However, not all stakeholders have fully realized this fact, and no effort had been undertaken so far by any professional society to develop a coordinated approach and clinical care pathway for NAFLD/NASH. The ‘Call to Action’ meeting can be considered as a starting point for such an important effort,” said Dr. Mantzoros, who is also a professor of medicine at Harvard Medical School and director of the human nutrition unit at Beth Israel Deaconess Medical Center, both in Boston.
Dramatic prevalence rates in patients with T2D
Results from two independent epidemiology reports, published in December 2020, documented steatosis (the fatty liver of NAFLD) in 70%-74% of unselected U.S. patients with T2D, advanced liver fibrosis accompanying this disease in 6%-15%, and previously unrecognized cirrhosis in 3%-8%.
One of these reports analyzed 825 patients with T2D included in the National Health and Nutritional Examination Survey of 2017-2018 run by the Centers for Disease Control and Prevention. All these patients, selected to be representative of the overall U.S. adult population with T2D, underwent transient elastography to identify steatosis and fibrosis, the first U.S. National Health Survey to run this type of population-based survey. The results showed an overall steatosis prevalence of 74% with grade 3 steatosis in 58%, advanced liver fibrosis in 15%, and cirrhosis in 8%, reported the team of Italian researchers who analyzed the data .
The second study focused on a single-center series of 561 patients with T2D who also underwent screening by transient elastography during 2018-2020 and had no history of NAFLD or other liver disease, or alcohol abuse. The imaging results showed a NAFLD prevalence of 70%, with 54% of the entire group diagnosed with severe steatosis, severe fibrosis in 6%, and cirrhosis in 3%. Among the 54% of patients with severe steatosis, 30% also had severe liver fibrosis. About 70% of the 561 patients assessed came from either the family medicine or general internal medicine clinics of the University of Florida, Gainesville, with the remaining 30% enrolled from the center’s endocrinology/diabetes outpatient clinic.
Neither report documented a NASH prevalence, which cannot receive definitive diagnosis by imaging alone. “This is the first study of its kind in the U.S. to establish the magnitude of [liver] disease burden in random patients with T2D seeking regular outpatient care,” wrote the University of Florida research team, led by Kenneth Cusi, MD, professor and chief of the university’s division of endocrinology, diabetes, and metabolism. Their finding that patients with T2D and previously unknown to have NAFLD had a 15% prevalence of moderate or advanced liver fibrosis “should trigger a call to action by all clinicians taking care of patients with T2D. Patient and physician awareness of the hepatic and extrahepatic complications of NASH, and reversing current diagnosis and treatment inertia will be the only way to avert the looming epidemic of cirrhosis in patients with diabetes.”
“Endocrinologists don’t ‘see’ NAFLD and NASH” in their patients with T2D “ because they don’t think about it,” Dr. Mantzoros declared.
“Why is NASH underdiagnosed and undertreated? Because many physicians aren’t aware of it,” agreed Dr. Cusi during a talk in December 2020 at the 18th World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease (WCIRDC). “You never find what you don’t look for.”
“Endocrinologists should do the tests for NASH [in patients with T2D], but we’re all guilty of not doing it enough,” Tracey McLaughlin, MD, an endocrinologist and professor of medicine at Stanford (Calif.) University, commented during the WCIRDC.
These prevalence numbers demand that clinicians suspect liver disease “in any patient with diabetes, especially patients with obesity who are older and have components of metabolic syndrome,” said Dr. Mantzoros. “We need to screen, refer the most advanced cases, and treat the early- and mid-stage cases.”
How to find NASH
Both the American Diabetes Association and the European Association for the Study of Diabetes call for routine screening of patients with T2D, starting with a check of liver enzymes, such as ALT, but no clear consensus exists for the specifics of screening beyond that. Dr. Mantzoros, Dr. Cusi, and other experts agree that the scheme for assessing liver disease in patients with T2D starts with regular monitoring of elevations in liver enzymes including ALT. Next is noninvasive ultrasound assessment of the extent of liver fibrosis inferred from the organ’s stiffness using transient elastography. Another frequently cited initial screening tool is the Fibrosis-4 (FIB-4) score, which incorporates a patient’s age, platelet count, and levels of ALT and a second liver enzyme, AST.
“There is more consensus about FIB-4 and then elastography, but some people use tests other than FIB-4. Unfortunately there is no perfect diagnostic test today. A top priority is to define the best diagnostic test,” said Dr. Mantzoros, who is leading an effort to try to refine screening using artificial intelligence.
“FIB-4 is simple, easy, and well validated,” commented Dr. Cusi during the WCIRDC last December. “FIB-4 and elastography should get you pretty close” to identifying patients with T2D and significant liver disease.
But in a recent editorial, Dr. Cusi agreed on the need for “more reliable tests for the diagnosis of NASH and advanced fibrosis in patients with T2D. Significant work is being done in the field to validate novel and more sophisticated fibrosis biomarkers. Future studies will help us enter a new era of precision medicine where biomarkers will identify and target therapy to those with more active disease at risk for cirrhosis,” he wrote.
“The ultimate goal is to diagnose fibrosis at an early stage to prevent people from developing cirrhosis,” Dr. Cusi said in a recent written statement. “We’re trying to identify these problems before they’re unfixable. Once someone has cirrhosis, there isn’t a whole lot you can do.”
Pioglitazone remains the best-documented treatment
Perhaps some of the inertia in diagnosing NAFLD, NASH, and liver fibrosis in patients with T2D is dissatisfaction with current treatment options, although several proven options exist, notably weight loss and diet, and thiazolidinedione (TZD) pioglitazone. But weight loss and diet pose issues for patient compliance and durability of the intervention, and many clinicians consider pioglitazone flawed by its potential adverse effects.
“When we don’t have an established treatment for something, we tend to not measure it or go after it. That’s been true of liver disease” in patients with T2D, said Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif., during the WCIRDC.
Treatment with pioglitazone has resolved NASH in about a third of patients compared with placebo, prevented fibrosis progression, and cut cardiovascular disease events, noted Dr. Cusi during the WCIRDC.
“Pioglitazone is used in only 8% of patients with T2D, or less, but we need to use it more often because of its proven efficacy in patients with T2D and NASH” said Dr. Mantzoros. “The problem is that pioglitazone has side effects, including weight gain and fluid retention, that makes it less attractive unless one thinks about the diagnosis of NASH.”
Others highlight that the adverse effects of pioglitazone have been either misunderstood, or can be effectively minimized with careful dosing.
“The data with the TZDs are much stronger than the data from anything else. TZDs have gotten a bad name because they also work in the kidney and enhance fluid reabsorption. We use modest dosages of pioglitazone, 15 mg or 30 mg a day, to avoid excess fluid retention,” Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio, said during the WCIRDC. “The best drug for NASH is pioglitazone. No other drug beats it” based on current data, Dr. DeFronzo asserted.
Other strategies include the potential to pair pioglitazone with other interventions that can blunt a weight-gain effect. One intriguing combination would combine pioglitazone with a GLP-1 receptor agonist, a drug class that can produce significant weight loss. Results from a phase 2 study showed promise for semaglutide (Rybelsus) in treating patients with NASH.
Getting the name right
Another factor that may be keeping NAFLD and NASH from achieving a higher profile for patients with T2D are those names, which focus on what the diseases are not – nonalcoholic – rather than what they are.
A series of recent publications in both the endocrinology and hepatology literature have called for renaming these disorders either “metabolic (dysfunction)–associated fatty liver disease (MALFD)”, or “dysmetabolism-associated fatty liver disease (DALFD)”.
“The names NAFLD and NASH indicate absence of alcohol as a cause, but the disease is also characterized by the absence of other causes, such as autoimmune disorders or hepatitis. The names were coined when we did not know much about these diseases. We now know that it is dysmetabolism that causes these conditions, and so we need to adopt a new, more accurate name,” explained Dr. Mantzoros, who has published support for a name change.
While many agree, some have raised concerns as to whether a name change now is premature. A group of hepatologists recently published a rebuttal to an immediate name change , saying that, “although we are in agreement that metabolic fatty liver disease may more accurately and positively reflect the relevant risk factors better than the age-old term nonalcoholic fatty liver disease, the term still leaves a great deal of ambiguity. A name change will be appropriate when informed by a new understanding of the molecular basis of the disease entity, insights that fundamentally change risk stratification, or other important aspects of the disease. We may be on the cusp of this, but we are not there yet.”
Dr. Mantzoros agreed, but for somewhat different reasons.
“We need to be careful and deliberate, because there is a significant body of knowledge and a lot of data from clinical trials collected using the old definitions. We need to find an appropriate time frame for a [name] transition. We need to find a nice and robust way to productively bridge the old to the new,” he said. “We also need new diagnostic criteria, and new therapies. A new name and definition will facilitate progress.”
Dr. Mantzoros been a shareholder of and consultant to Coherus and Pangea, he has been a consultant to AstraZeneca, Eisai, Genfit, Intercept, Novo Nordisk, P.E.S., and Regeneron, and has received travel support from the Metabolic Institute of America and the California Walnut Commission. Dr. Cusi has been a consultant to and has received research funding from numerous drug companies. Dr. McLaughlin is a consultant to January AI. Dr. Handelsman has been a consultant to numerous drug companies. Dr. DeFronzo received research grants from AstraZeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk.
Among these calls is a pending statement from the Endocrine Society, the American Association of Clinical Endocrinologists, the American Gastroenterology Association, and other groups on what the growing appreciation of highly prevalent liver disease in patients with type 2 diabetes (T2D) means for assessing and managing patients. Publication of the statement is expected by spring 2021, said Christos S. Mantzoros, MD, DSc, PhD, chief of endocrinology for the Veterans Affairs Boston Healthcare System and a representative from the Endocrine Society to the statement-writing panel.
This upcoming “Call to Action” from these groups argues for a “need to collaborate across disciplines, and work together on establishing clinical guidelines, and creating new diagnostics and therapeutics,” said Dr. Mantzoros in an interview.
“Over time, it is becoming clearer that management of NAFLD [nonalcoholic fatty liver disease]/NASH [nonalcoholic steatohepatitis] requires a multidisciplinary panel of doctors ranging from primary care practitioners, to endocrinologists, and hepatologists. Given that the nature of the disease crosses scientific discipline boundaries, and that the number of patients is so large (it is estimated that about one in four U.S. adults have NAFLD), not all patients can be treated at the limited number of hepatology centers.
“However, not all stakeholders have fully realized this fact, and no effort had been undertaken so far by any professional society to develop a coordinated approach and clinical care pathway for NAFLD/NASH. The ‘Call to Action’ meeting can be considered as a starting point for such an important effort,” said Dr. Mantzoros, who is also a professor of medicine at Harvard Medical School and director of the human nutrition unit at Beth Israel Deaconess Medical Center, both in Boston.
Dramatic prevalence rates in patients with T2D
Results from two independent epidemiology reports, published in December 2020, documented steatosis (the fatty liver of NAFLD) in 70%-74% of unselected U.S. patients with T2D, advanced liver fibrosis accompanying this disease in 6%-15%, and previously unrecognized cirrhosis in 3%-8%.
One of these reports analyzed 825 patients with T2D included in the National Health and Nutritional Examination Survey of 2017-2018 run by the Centers for Disease Control and Prevention. All these patients, selected to be representative of the overall U.S. adult population with T2D, underwent transient elastography to identify steatosis and fibrosis, the first U.S. National Health Survey to run this type of population-based survey. The results showed an overall steatosis prevalence of 74% with grade 3 steatosis in 58%, advanced liver fibrosis in 15%, and cirrhosis in 8%, reported the team of Italian researchers who analyzed the data .
The second study focused on a single-center series of 561 patients with T2D who also underwent screening by transient elastography during 2018-2020 and had no history of NAFLD or other liver disease, or alcohol abuse. The imaging results showed a NAFLD prevalence of 70%, with 54% of the entire group diagnosed with severe steatosis, severe fibrosis in 6%, and cirrhosis in 3%. Among the 54% of patients with severe steatosis, 30% also had severe liver fibrosis. About 70% of the 561 patients assessed came from either the family medicine or general internal medicine clinics of the University of Florida, Gainesville, with the remaining 30% enrolled from the center’s endocrinology/diabetes outpatient clinic.
Neither report documented a NASH prevalence, which cannot receive definitive diagnosis by imaging alone. “This is the first study of its kind in the U.S. to establish the magnitude of [liver] disease burden in random patients with T2D seeking regular outpatient care,” wrote the University of Florida research team, led by Kenneth Cusi, MD, professor and chief of the university’s division of endocrinology, diabetes, and metabolism. Their finding that patients with T2D and previously unknown to have NAFLD had a 15% prevalence of moderate or advanced liver fibrosis “should trigger a call to action by all clinicians taking care of patients with T2D. Patient and physician awareness of the hepatic and extrahepatic complications of NASH, and reversing current diagnosis and treatment inertia will be the only way to avert the looming epidemic of cirrhosis in patients with diabetes.”
“Endocrinologists don’t ‘see’ NAFLD and NASH” in their patients with T2D “ because they don’t think about it,” Dr. Mantzoros declared.
“Why is NASH underdiagnosed and undertreated? Because many physicians aren’t aware of it,” agreed Dr. Cusi during a talk in December 2020 at the 18th World Congress on Insulin Resistance, Diabetes, and Cardiovascular Disease (WCIRDC). “You never find what you don’t look for.”
“Endocrinologists should do the tests for NASH [in patients with T2D], but we’re all guilty of not doing it enough,” Tracey McLaughlin, MD, an endocrinologist and professor of medicine at Stanford (Calif.) University, commented during the WCIRDC.
These prevalence numbers demand that clinicians suspect liver disease “in any patient with diabetes, especially patients with obesity who are older and have components of metabolic syndrome,” said Dr. Mantzoros. “We need to screen, refer the most advanced cases, and treat the early- and mid-stage cases.”
How to find NASH
Both the American Diabetes Association and the European Association for the Study of Diabetes call for routine screening of patients with T2D, starting with a check of liver enzymes, such as ALT, but no clear consensus exists for the specifics of screening beyond that. Dr. Mantzoros, Dr. Cusi, and other experts agree that the scheme for assessing liver disease in patients with T2D starts with regular monitoring of elevations in liver enzymes including ALT. Next is noninvasive ultrasound assessment of the extent of liver fibrosis inferred from the organ’s stiffness using transient elastography. Another frequently cited initial screening tool is the Fibrosis-4 (FIB-4) score, which incorporates a patient’s age, platelet count, and levels of ALT and a second liver enzyme, AST.
“There is more consensus about FIB-4 and then elastography, but some people use tests other than FIB-4. Unfortunately there is no perfect diagnostic test today. A top priority is to define the best diagnostic test,” said Dr. Mantzoros, who is leading an effort to try to refine screening using artificial intelligence.
“FIB-4 is simple, easy, and well validated,” commented Dr. Cusi during the WCIRDC last December. “FIB-4 and elastography should get you pretty close” to identifying patients with T2D and significant liver disease.
But in a recent editorial, Dr. Cusi agreed on the need for “more reliable tests for the diagnosis of NASH and advanced fibrosis in patients with T2D. Significant work is being done in the field to validate novel and more sophisticated fibrosis biomarkers. Future studies will help us enter a new era of precision medicine where biomarkers will identify and target therapy to those with more active disease at risk for cirrhosis,” he wrote.
“The ultimate goal is to diagnose fibrosis at an early stage to prevent people from developing cirrhosis,” Dr. Cusi said in a recent written statement. “We’re trying to identify these problems before they’re unfixable. Once someone has cirrhosis, there isn’t a whole lot you can do.”
Pioglitazone remains the best-documented treatment
Perhaps some of the inertia in diagnosing NAFLD, NASH, and liver fibrosis in patients with T2D is dissatisfaction with current treatment options, although several proven options exist, notably weight loss and diet, and thiazolidinedione (TZD) pioglitazone. But weight loss and diet pose issues for patient compliance and durability of the intervention, and many clinicians consider pioglitazone flawed by its potential adverse effects.
“When we don’t have an established treatment for something, we tend to not measure it or go after it. That’s been true of liver disease” in patients with T2D, said Yehuda Handelsman, MD, an endocrinologist and diabetes specialist who is medical director of the Metabolic Institute of America in Tarzana, Calif., during the WCIRDC.
Treatment with pioglitazone has resolved NASH in about a third of patients compared with placebo, prevented fibrosis progression, and cut cardiovascular disease events, noted Dr. Cusi during the WCIRDC.
“Pioglitazone is used in only 8% of patients with T2D, or less, but we need to use it more often because of its proven efficacy in patients with T2D and NASH” said Dr. Mantzoros. “The problem is that pioglitazone has side effects, including weight gain and fluid retention, that makes it less attractive unless one thinks about the diagnosis of NASH.”
Others highlight that the adverse effects of pioglitazone have been either misunderstood, or can be effectively minimized with careful dosing.
“The data with the TZDs are much stronger than the data from anything else. TZDs have gotten a bad name because they also work in the kidney and enhance fluid reabsorption. We use modest dosages of pioglitazone, 15 mg or 30 mg a day, to avoid excess fluid retention,” Ralph A. DeFronzo, MD, chief of the diabetes division and professor of medicine at the University of Texas Health Science Center, San Antonio, said during the WCIRDC. “The best drug for NASH is pioglitazone. No other drug beats it” based on current data, Dr. DeFronzo asserted.
Other strategies include the potential to pair pioglitazone with other interventions that can blunt a weight-gain effect. One intriguing combination would combine pioglitazone with a GLP-1 receptor agonist, a drug class that can produce significant weight loss. Results from a phase 2 study showed promise for semaglutide (Rybelsus) in treating patients with NASH.
Getting the name right
Another factor that may be keeping NAFLD and NASH from achieving a higher profile for patients with T2D are those names, which focus on what the diseases are not – nonalcoholic – rather than what they are.
A series of recent publications in both the endocrinology and hepatology literature have called for renaming these disorders either “metabolic (dysfunction)–associated fatty liver disease (MALFD)”, or “dysmetabolism-associated fatty liver disease (DALFD)”.
“The names NAFLD and NASH indicate absence of alcohol as a cause, but the disease is also characterized by the absence of other causes, such as autoimmune disorders or hepatitis. The names were coined when we did not know much about these diseases. We now know that it is dysmetabolism that causes these conditions, and so we need to adopt a new, more accurate name,” explained Dr. Mantzoros, who has published support for a name change.
While many agree, some have raised concerns as to whether a name change now is premature. A group of hepatologists recently published a rebuttal to an immediate name change , saying that, “although we are in agreement that metabolic fatty liver disease may more accurately and positively reflect the relevant risk factors better than the age-old term nonalcoholic fatty liver disease, the term still leaves a great deal of ambiguity. A name change will be appropriate when informed by a new understanding of the molecular basis of the disease entity, insights that fundamentally change risk stratification, or other important aspects of the disease. We may be on the cusp of this, but we are not there yet.”
Dr. Mantzoros agreed, but for somewhat different reasons.
“We need to be careful and deliberate, because there is a significant body of knowledge and a lot of data from clinical trials collected using the old definitions. We need to find an appropriate time frame for a [name] transition. We need to find a nice and robust way to productively bridge the old to the new,” he said. “We also need new diagnostic criteria, and new therapies. A new name and definition will facilitate progress.”
Dr. Mantzoros been a shareholder of and consultant to Coherus and Pangea, he has been a consultant to AstraZeneca, Eisai, Genfit, Intercept, Novo Nordisk, P.E.S., and Regeneron, and has received travel support from the Metabolic Institute of America and the California Walnut Commission. Dr. Cusi has been a consultant to and has received research funding from numerous drug companies. Dr. McLaughlin is a consultant to January AI. Dr. Handelsman has been a consultant to numerous drug companies. Dr. DeFronzo received research grants from AstraZeneca, Janssen, and Merck; he has been an adviser to AstraZeneca, Boehringer Ingelheim, Intarcia, Janssen, and Novo Nordisk; and he has been a speaker on behalf of AstraZeneca and Novo Nordisk.
Repeated ketamine infusions linked to rapid relief of PTSD
Repeated intravenous infusions of ketamine provide rapid relief for patients with posttraumatic stress disorder, new research suggests.
In what investigators are calling the first randomized controlled trial of repeated ketamine administration for chronic PTSD, 30 patients received six infusions of ketamine or midazolam (used as a psychoactive placebo) over 2 consecutive weeks.
Between baseline and week 2, those receiving ketamine showed significantly greater improvement than those receiving midazolam. Total scores on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) for the first group were almost 12 points lower than the latter group at week 2, meeting the study’s primary outcome measure.
In addition, 67% vs. 20% of the patients, respectively, were considered to be treatment responders; time to loss of response for those in the ketamine group was 28 days.
Although the overall findings were as expected, “what was surprising was how robust the results were,” lead author Adriana Feder, MD, associate professor of psychiatry, Icahn School of Medicine, Mount Sinai, New York, told this news organization.
It was also a bit surprising that, in a study of just 30 participants, “we were able to show such a clear difference” between the two treatment groups, said Dr. Feder, who is also a coinventor on issued patents for the use of ketamine as therapy for PTSD, and codirector of the Ehrenkranz Lab for the Study of Human Resilience at Mount Sinai.
The findings were published online Jan. 5 in the American Journal of Psychiatry.
Unmet need
Ketamine is a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist that was first approved by the U.S. Food and Drug Administration for anesthetic use in 1970. It has also been shown to be effective for treatment-resistant depression.
PTSD has a lifetime prevalence of about 6% in the United States. “While trauma-focused psychotherapies have the most empirical support, they are limited by significant rates of nonresponse, partial response, and treatment dropout,” the investigators write. Also, there are “few available pharmacotherapies for PTSD, and their efficacy is insufficient,” they add.
“There’s a real need for new treatment interventions that are effective for PTSD and also work rapidly, because it can take weeks to months for currently available treatments to work for PTSD,” Dr. Feder said.
The researchers previously conducted a “proof-of-concept” randomized controlled trial of single infusions of ketamine for chronic PTSD. Results published in 2014 in JAMA Psychiatry showed significant reduction in PTSD symptoms 24 hours after infusion.
For the current study, the investigative team wanted to assess whether ketamine was viable as a longer-term treatment.
“We were encouraged by our initial promising findings” of the earlier trial, Dr. Feder said. “We wanted to do the second study to see if ketamine really works for PTSD, to see if we could replicate the rapid improvement and also examine whether a course of six infusions over 2 weeks could maintain the improvement.”
Thirty patients (aged 18-70; mean age, 39 years) with chronic PTSD from civilian or military trauma were enrolled (mean PTSD duration, 15 years).
The most cited primary trauma was sexual assault or molestation (n = 13), followed by physical assault or abuse (n = 8), witnessing a violent assault or death (n = 4), witnessing the 9/11 attacks (n = 3), and combat exposure (n = 2).
During the 2-week treatment phase, half of the patients were randomly assigned to receive six infusions of ketamine hydrochloride at a dose of 0.5 mg/kg (86.7% women; mean CAPS-5 score, 42), while the other half received six infusions of midazolam at a dose of 0.045 mg/kg (66.7% women; mean CAPS-5 score, 40).
In addition to the primary outcome measure of 2-week changes on the CAPS-5, secondary outcomes included score changes on the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Impact of Event Scale-Revised (IES-R).
Treatment response was defined as a 30% or more improvement in symptoms on the CAPS-5. A number of measures were also used to assess potential treatment-related adverse events (AEs).
Safe, effective
Results showed significantly lower total CAPS-5 scores for the ketamine group vs. the midazolam group at week 1 (score difference, 8.8 points; P = .03) and at week 2 (score difference, 11.88 points; P = .004).
Those receiving ketamine also showed improvements in three of the four PTSD symptom clusters on the CAPS-5: avoidance (P < .0001), negative mood and cognitions (P = .02), and intrusions (P = .03). The fourth symptom cluster – arousal and reactivity – did not show a significant improvement.
In addition, the ketamine group showed significantly greater improvement scores on the MADRS at both week 1 and week 2.
Treatment response at 2 weeks was achieved by 10 members of the ketamine group and by three members of the midazolam group (P = .03).
Secondary analyses showed rapid improvement in the treatment responders within the ketamine group, with a mean change of 26 points on the total IES-R score between baseline and 24 hours after their first infusion, and a mean change of 13.4 points on the MADRS total past-24-hour score, a 53% improvement on average.
“A response at 2 weeks is very rapid but they got better sometimes within the first day,” Dr. Feder noted.
There were no serious AEs reported. Although some dissociative symptoms occurred during ketamine infusions, with the highest levels reported at the end of the infusion, these symptoms had resolved by the next assessment, conducted 2 hours after infusion.
The most frequently reported AE in the ketamine group, compared with midazolam, after the start of infusions was blurred vision (53% vs. 0%), followed by dizziness (33% vs. 13%), fatigue (33% vs. 87%), headache (27% vs. 13%), and nausea or vomiting (20% vs. 7%).
‘Large-magnitude improvement’
The overall findings show that, in this patient population, “repeated intravenous ketamine infusions administered over 2 weeks were associated with a large-magnitude, clinically significant improvement in PTSD symptoms,” the investigators write.
The results “were very satisfying,” added Dr. Feder. “It was heartening also to hear what some of the participants would say. Some told us about how their symptoms and feelings had changed during the course of treatment with ketamine, where they felt stronger and better able to cope with their trauma and memories.”
She noted, however, that this was not a study designed to specifically assess ketamine in treatment-resistant PTSD. “Some patients had had multiple treatments before that hadn’t worked, while others had not received treatment before. Efficacy for treatment-resistant PTSD is an important question for future research,” Dr. Feder said.
Other areas worth future exploration include treatment efficacy in patients with different types of trauma and whether outcomes can last longer in patients receiving ketamine plus psychotherapy treatment, she noted.
“I don’t want to ignore the fact that currently available treatments work for a number of people with chronic PTSD. But because there are many more for whom [the treatments] don’t work, or they’re insufficiently helped by those treatments, this is certainly one potentially very promising approach that can be added” to a clinician’s toolbox, Dr. Feder said.
Speaks to clinical utility
Commenting for this news organization, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Connecticut, called this a “very solid and well-designed” study.
“It definitely builds on what’s been found in the past, but it’s a critical piece of information speaking to the clinical utility of this treatment for PTSD,” said Dr. Sanacora, who is also director of the Yale Depression Research Program and was not involved with the current research.
He agreed with the investigators that PTSD has long been a condition that is difficult to treat.
“It’s an area that has a great unmet need for treatment options. Beyond that, as ketamine is becoming more widely used, there’s increasing demand for off-label uses. This [study] actually provides some evidence that there may be efficacy there,” Dr. Sanacora said.
Although he cautioned that this was a small study, and thus further research with a larger patient population will be needed, it provides a compelling foundation to build upon.
“This study provides clear evidence to support a larger study to really give a definitive statement on the efficacy and safety of its use for PTSD. I don’t think this is the study that provides that definitive evidence, but it is a very strong indication, and it very strongly supports the initiation of a large study to address that,” said Dr. Sanacora.
He noted that, although he’s used the term “cautious optimism” for studies in the past, he has “real optimism” that ketamine will be effective for PTSD based on the results of this current study.
“We still need some more data to really convince us of that before we can say with any clear statement that it is effective and safe, but I’m very optimistic,” Dr. Sanacora concluded.
The study was funded by the Brain and Behavior Research Foundation, Mount Sinai Innovation Partners and the Mount Sinai i3 Accelerator, Gerald and Glenda Greenwald, and the Ehrenkranz Laboratory for Human Resilience. Dr. Feder is a coinventor on issued patents for the use of ketamine as therapy for PTSD. A list of all disclosures for the other study authors are listed in the original article.
A version of this article first appeared on Medscape.com.
Repeated intravenous infusions of ketamine provide rapid relief for patients with posttraumatic stress disorder, new research suggests.
In what investigators are calling the first randomized controlled trial of repeated ketamine administration for chronic PTSD, 30 patients received six infusions of ketamine or midazolam (used as a psychoactive placebo) over 2 consecutive weeks.
Between baseline and week 2, those receiving ketamine showed significantly greater improvement than those receiving midazolam. Total scores on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) for the first group were almost 12 points lower than the latter group at week 2, meeting the study’s primary outcome measure.
In addition, 67% vs. 20% of the patients, respectively, were considered to be treatment responders; time to loss of response for those in the ketamine group was 28 days.
Although the overall findings were as expected, “what was surprising was how robust the results were,” lead author Adriana Feder, MD, associate professor of psychiatry, Icahn School of Medicine, Mount Sinai, New York, told this news organization.
It was also a bit surprising that, in a study of just 30 participants, “we were able to show such a clear difference” between the two treatment groups, said Dr. Feder, who is also a coinventor on issued patents for the use of ketamine as therapy for PTSD, and codirector of the Ehrenkranz Lab for the Study of Human Resilience at Mount Sinai.
The findings were published online Jan. 5 in the American Journal of Psychiatry.
Unmet need
Ketamine is a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist that was first approved by the U.S. Food and Drug Administration for anesthetic use in 1970. It has also been shown to be effective for treatment-resistant depression.
PTSD has a lifetime prevalence of about 6% in the United States. “While trauma-focused psychotherapies have the most empirical support, they are limited by significant rates of nonresponse, partial response, and treatment dropout,” the investigators write. Also, there are “few available pharmacotherapies for PTSD, and their efficacy is insufficient,” they add.
“There’s a real need for new treatment interventions that are effective for PTSD and also work rapidly, because it can take weeks to months for currently available treatments to work for PTSD,” Dr. Feder said.
The researchers previously conducted a “proof-of-concept” randomized controlled trial of single infusions of ketamine for chronic PTSD. Results published in 2014 in JAMA Psychiatry showed significant reduction in PTSD symptoms 24 hours after infusion.
For the current study, the investigative team wanted to assess whether ketamine was viable as a longer-term treatment.
“We were encouraged by our initial promising findings” of the earlier trial, Dr. Feder said. “We wanted to do the second study to see if ketamine really works for PTSD, to see if we could replicate the rapid improvement and also examine whether a course of six infusions over 2 weeks could maintain the improvement.”
Thirty patients (aged 18-70; mean age, 39 years) with chronic PTSD from civilian or military trauma were enrolled (mean PTSD duration, 15 years).
The most cited primary trauma was sexual assault or molestation (n = 13), followed by physical assault or abuse (n = 8), witnessing a violent assault or death (n = 4), witnessing the 9/11 attacks (n = 3), and combat exposure (n = 2).
During the 2-week treatment phase, half of the patients were randomly assigned to receive six infusions of ketamine hydrochloride at a dose of 0.5 mg/kg (86.7% women; mean CAPS-5 score, 42), while the other half received six infusions of midazolam at a dose of 0.045 mg/kg (66.7% women; mean CAPS-5 score, 40).
In addition to the primary outcome measure of 2-week changes on the CAPS-5, secondary outcomes included score changes on the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Impact of Event Scale-Revised (IES-R).
Treatment response was defined as a 30% or more improvement in symptoms on the CAPS-5. A number of measures were also used to assess potential treatment-related adverse events (AEs).
Safe, effective
Results showed significantly lower total CAPS-5 scores for the ketamine group vs. the midazolam group at week 1 (score difference, 8.8 points; P = .03) and at week 2 (score difference, 11.88 points; P = .004).
Those receiving ketamine also showed improvements in three of the four PTSD symptom clusters on the CAPS-5: avoidance (P < .0001), negative mood and cognitions (P = .02), and intrusions (P = .03). The fourth symptom cluster – arousal and reactivity – did not show a significant improvement.
In addition, the ketamine group showed significantly greater improvement scores on the MADRS at both week 1 and week 2.
Treatment response at 2 weeks was achieved by 10 members of the ketamine group and by three members of the midazolam group (P = .03).
Secondary analyses showed rapid improvement in the treatment responders within the ketamine group, with a mean change of 26 points on the total IES-R score between baseline and 24 hours after their first infusion, and a mean change of 13.4 points on the MADRS total past-24-hour score, a 53% improvement on average.
“A response at 2 weeks is very rapid but they got better sometimes within the first day,” Dr. Feder noted.
There were no serious AEs reported. Although some dissociative symptoms occurred during ketamine infusions, with the highest levels reported at the end of the infusion, these symptoms had resolved by the next assessment, conducted 2 hours after infusion.
The most frequently reported AE in the ketamine group, compared with midazolam, after the start of infusions was blurred vision (53% vs. 0%), followed by dizziness (33% vs. 13%), fatigue (33% vs. 87%), headache (27% vs. 13%), and nausea or vomiting (20% vs. 7%).
‘Large-magnitude improvement’
The overall findings show that, in this patient population, “repeated intravenous ketamine infusions administered over 2 weeks were associated with a large-magnitude, clinically significant improvement in PTSD symptoms,” the investigators write.
The results “were very satisfying,” added Dr. Feder. “It was heartening also to hear what some of the participants would say. Some told us about how their symptoms and feelings had changed during the course of treatment with ketamine, where they felt stronger and better able to cope with their trauma and memories.”
She noted, however, that this was not a study designed to specifically assess ketamine in treatment-resistant PTSD. “Some patients had had multiple treatments before that hadn’t worked, while others had not received treatment before. Efficacy for treatment-resistant PTSD is an important question for future research,” Dr. Feder said.
Other areas worth future exploration include treatment efficacy in patients with different types of trauma and whether outcomes can last longer in patients receiving ketamine plus psychotherapy treatment, she noted.
“I don’t want to ignore the fact that currently available treatments work for a number of people with chronic PTSD. But because there are many more for whom [the treatments] don’t work, or they’re insufficiently helped by those treatments, this is certainly one potentially very promising approach that can be added” to a clinician’s toolbox, Dr. Feder said.
Speaks to clinical utility
Commenting for this news organization, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Connecticut, called this a “very solid and well-designed” study.
“It definitely builds on what’s been found in the past, but it’s a critical piece of information speaking to the clinical utility of this treatment for PTSD,” said Dr. Sanacora, who is also director of the Yale Depression Research Program and was not involved with the current research.
He agreed with the investigators that PTSD has long been a condition that is difficult to treat.
“It’s an area that has a great unmet need for treatment options. Beyond that, as ketamine is becoming more widely used, there’s increasing demand for off-label uses. This [study] actually provides some evidence that there may be efficacy there,” Dr. Sanacora said.
Although he cautioned that this was a small study, and thus further research with a larger patient population will be needed, it provides a compelling foundation to build upon.
“This study provides clear evidence to support a larger study to really give a definitive statement on the efficacy and safety of its use for PTSD. I don’t think this is the study that provides that definitive evidence, but it is a very strong indication, and it very strongly supports the initiation of a large study to address that,” said Dr. Sanacora.
He noted that, although he’s used the term “cautious optimism” for studies in the past, he has “real optimism” that ketamine will be effective for PTSD based on the results of this current study.
“We still need some more data to really convince us of that before we can say with any clear statement that it is effective and safe, but I’m very optimistic,” Dr. Sanacora concluded.
The study was funded by the Brain and Behavior Research Foundation, Mount Sinai Innovation Partners and the Mount Sinai i3 Accelerator, Gerald and Glenda Greenwald, and the Ehrenkranz Laboratory for Human Resilience. Dr. Feder is a coinventor on issued patents for the use of ketamine as therapy for PTSD. A list of all disclosures for the other study authors are listed in the original article.
A version of this article first appeared on Medscape.com.
Repeated intravenous infusions of ketamine provide rapid relief for patients with posttraumatic stress disorder, new research suggests.
In what investigators are calling the first randomized controlled trial of repeated ketamine administration for chronic PTSD, 30 patients received six infusions of ketamine or midazolam (used as a psychoactive placebo) over 2 consecutive weeks.
Between baseline and week 2, those receiving ketamine showed significantly greater improvement than those receiving midazolam. Total scores on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) for the first group were almost 12 points lower than the latter group at week 2, meeting the study’s primary outcome measure.
In addition, 67% vs. 20% of the patients, respectively, were considered to be treatment responders; time to loss of response for those in the ketamine group was 28 days.
Although the overall findings were as expected, “what was surprising was how robust the results were,” lead author Adriana Feder, MD, associate professor of psychiatry, Icahn School of Medicine, Mount Sinai, New York, told this news organization.
It was also a bit surprising that, in a study of just 30 participants, “we were able to show such a clear difference” between the two treatment groups, said Dr. Feder, who is also a coinventor on issued patents for the use of ketamine as therapy for PTSD, and codirector of the Ehrenkranz Lab for the Study of Human Resilience at Mount Sinai.
The findings were published online Jan. 5 in the American Journal of Psychiatry.
Unmet need
Ketamine is a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist that was first approved by the U.S. Food and Drug Administration for anesthetic use in 1970. It has also been shown to be effective for treatment-resistant depression.
PTSD has a lifetime prevalence of about 6% in the United States. “While trauma-focused psychotherapies have the most empirical support, they are limited by significant rates of nonresponse, partial response, and treatment dropout,” the investigators write. Also, there are “few available pharmacotherapies for PTSD, and their efficacy is insufficient,” they add.
“There’s a real need for new treatment interventions that are effective for PTSD and also work rapidly, because it can take weeks to months for currently available treatments to work for PTSD,” Dr. Feder said.
The researchers previously conducted a “proof-of-concept” randomized controlled trial of single infusions of ketamine for chronic PTSD. Results published in 2014 in JAMA Psychiatry showed significant reduction in PTSD symptoms 24 hours after infusion.
For the current study, the investigative team wanted to assess whether ketamine was viable as a longer-term treatment.
“We were encouraged by our initial promising findings” of the earlier trial, Dr. Feder said. “We wanted to do the second study to see if ketamine really works for PTSD, to see if we could replicate the rapid improvement and also examine whether a course of six infusions over 2 weeks could maintain the improvement.”
Thirty patients (aged 18-70; mean age, 39 years) with chronic PTSD from civilian or military trauma were enrolled (mean PTSD duration, 15 years).
The most cited primary trauma was sexual assault or molestation (n = 13), followed by physical assault or abuse (n = 8), witnessing a violent assault or death (n = 4), witnessing the 9/11 attacks (n = 3), and combat exposure (n = 2).
During the 2-week treatment phase, half of the patients were randomly assigned to receive six infusions of ketamine hydrochloride at a dose of 0.5 mg/kg (86.7% women; mean CAPS-5 score, 42), while the other half received six infusions of midazolam at a dose of 0.045 mg/kg (66.7% women; mean CAPS-5 score, 40).
In addition to the primary outcome measure of 2-week changes on the CAPS-5, secondary outcomes included score changes on the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Impact of Event Scale-Revised (IES-R).
Treatment response was defined as a 30% or more improvement in symptoms on the CAPS-5. A number of measures were also used to assess potential treatment-related adverse events (AEs).
Safe, effective
Results showed significantly lower total CAPS-5 scores for the ketamine group vs. the midazolam group at week 1 (score difference, 8.8 points; P = .03) and at week 2 (score difference, 11.88 points; P = .004).
Those receiving ketamine also showed improvements in three of the four PTSD symptom clusters on the CAPS-5: avoidance (P < .0001), negative mood and cognitions (P = .02), and intrusions (P = .03). The fourth symptom cluster – arousal and reactivity – did not show a significant improvement.
In addition, the ketamine group showed significantly greater improvement scores on the MADRS at both week 1 and week 2.
Treatment response at 2 weeks was achieved by 10 members of the ketamine group and by three members of the midazolam group (P = .03).
Secondary analyses showed rapid improvement in the treatment responders within the ketamine group, with a mean change of 26 points on the total IES-R score between baseline and 24 hours after their first infusion, and a mean change of 13.4 points on the MADRS total past-24-hour score, a 53% improvement on average.
“A response at 2 weeks is very rapid but they got better sometimes within the first day,” Dr. Feder noted.
There were no serious AEs reported. Although some dissociative symptoms occurred during ketamine infusions, with the highest levels reported at the end of the infusion, these symptoms had resolved by the next assessment, conducted 2 hours after infusion.
The most frequently reported AE in the ketamine group, compared with midazolam, after the start of infusions was blurred vision (53% vs. 0%), followed by dizziness (33% vs. 13%), fatigue (33% vs. 87%), headache (27% vs. 13%), and nausea or vomiting (20% vs. 7%).
‘Large-magnitude improvement’
The overall findings show that, in this patient population, “repeated intravenous ketamine infusions administered over 2 weeks were associated with a large-magnitude, clinically significant improvement in PTSD symptoms,” the investigators write.
The results “were very satisfying,” added Dr. Feder. “It was heartening also to hear what some of the participants would say. Some told us about how their symptoms and feelings had changed during the course of treatment with ketamine, where they felt stronger and better able to cope with their trauma and memories.”
She noted, however, that this was not a study designed to specifically assess ketamine in treatment-resistant PTSD. “Some patients had had multiple treatments before that hadn’t worked, while others had not received treatment before. Efficacy for treatment-resistant PTSD is an important question for future research,” Dr. Feder said.
Other areas worth future exploration include treatment efficacy in patients with different types of trauma and whether outcomes can last longer in patients receiving ketamine plus psychotherapy treatment, she noted.
“I don’t want to ignore the fact that currently available treatments work for a number of people with chronic PTSD. But because there are many more for whom [the treatments] don’t work, or they’re insufficiently helped by those treatments, this is certainly one potentially very promising approach that can be added” to a clinician’s toolbox, Dr. Feder said.
Speaks to clinical utility
Commenting for this news organization, Gerard Sanacora, MD, PhD, professor of psychiatry at Yale University, New Haven, Connecticut, called this a “very solid and well-designed” study.
“It definitely builds on what’s been found in the past, but it’s a critical piece of information speaking to the clinical utility of this treatment for PTSD,” said Dr. Sanacora, who is also director of the Yale Depression Research Program and was not involved with the current research.
He agreed with the investigators that PTSD has long been a condition that is difficult to treat.
“It’s an area that has a great unmet need for treatment options. Beyond that, as ketamine is becoming more widely used, there’s increasing demand for off-label uses. This [study] actually provides some evidence that there may be efficacy there,” Dr. Sanacora said.
Although he cautioned that this was a small study, and thus further research with a larger patient population will be needed, it provides a compelling foundation to build upon.
“This study provides clear evidence to support a larger study to really give a definitive statement on the efficacy and safety of its use for PTSD. I don’t think this is the study that provides that definitive evidence, but it is a very strong indication, and it very strongly supports the initiation of a large study to address that,” said Dr. Sanacora.
He noted that, although he’s used the term “cautious optimism” for studies in the past, he has “real optimism” that ketamine will be effective for PTSD based on the results of this current study.
“We still need some more data to really convince us of that before we can say with any clear statement that it is effective and safe, but I’m very optimistic,” Dr. Sanacora concluded.
The study was funded by the Brain and Behavior Research Foundation, Mount Sinai Innovation Partners and the Mount Sinai i3 Accelerator, Gerald and Glenda Greenwald, and the Ehrenkranz Laboratory for Human Resilience. Dr. Feder is a coinventor on issued patents for the use of ketamine as therapy for PTSD. A list of all disclosures for the other study authors are listed in the original article.
A version of this article first appeared on Medscape.com.
Low-carb diets boost diabetes remission rates, at least short term
Patients with type 2 diabetes who follow a low-carbohydrate diet (LCD) for at least 6 months appear to have significantly higher remission rates than those following other diets, although the benefits diminish by 12 months, suggests a new analysis of trial data from over 1,300 individuals.
“Based on other evidence, it is likely the degree of weight loss would have been a contributing factor, combined with the lower intake of dietary carbohydrates,” study coauthor Grant D. Brinkworth, PhD, Commonwealth Scientific and Industrial Research Organisation, Sydney, , said in an interview.
He acknowledged, however, that “diets in general can be difficult to sustain over the long term. ... We need to provide patients with easy-to-use support tools and convenient solutions to help them adhere to a low-carb diet long term to gain these greater health improvements.
“In addition, more long-term, well-controlled, randomized trials are needed to determine the effects of low-carb diets on sustained weight loss, diabetes remission, and health outcomes,” Dr. Brinkworth added.
The research was published on Janu. 13 in the BMJ by a consortium of international scientists, led by Joshua Z. Goldenberg, PhD, department of nutrition, Texas A&M University, College Station.
Confusion as to best diet for those with diabetes
Type 2 diabetes is a “significant and worsening” worldwide health problem, wrote Dr. Goldenberg and coauthors, in spite of “many pharmaceutical developments and a global emphasis on glycemic control.”
Although structured diets are “recognized as an essential component of treating diabetes, confusion remains about which diet to choose,” with multiple systemic reviews and meta-analyses of carbohydrate-restricted diets “reporting mixed results,” they noted.
They therefore undertook a systematic review of randomized, controlled trials on the efficacy and safety of LCDs and very-low-carbohydrate diets (VLCDs) using the CENTRAL, Medline, CINAHL, and CAB databases, as well as other literature sources.
Researchers defined LCDs as less than 130 g/day of carbohydrates or less than 26% of calories from carbohydrates as part of a 2,000 kcal/day diet and VLCDs as less than 50 g/day or less than 10% of daily calories. They focused on interventions that lasted at least 12 weeks in adults with type 2 diabetes.
Overall, 23 trials involving 1,357 participants met the inclusion criteria; 52% used VLCDs and the control comparator was a low-fat diet in 78% of the studies. The mean age range of patients was 47-67 years, and treatment duration spanned from 3 months to 2 years.
LCDs were associated with a higher rate of diabetes remission when defined as a hemoglobin A1c level of less than 6.5%, compared with control diets at 6 months, at 57% versus 31% – an increase in remission of 32% associated with LCDs (P < .001 for overall effect).
But when defined more tightly as an A1c level of less than 6.5% in the absence of diabetes medications, remission with LCDs was reduced to a nonsignificant 5% versus control diets at 6 months.
At 12 months, data on remission were sparse, ranging from a small effect to a trivial increased risk of diabetes.
Subgroup analysis demonstrated that patients on an LCD achieved greater weight loss at 6 months than those on a control diet, at a mean reduction of 3.46 kg (approximately 7.6 lb). However, the researchers noted that, at 12 months, any weight-loss benefit was “trivial and nonsignificant.”
A similar pattern was seen for reductions in A1c and fasting glucose levels with LCDs: Notable reductions at 6 months largely disappeared by 12 months.
LCDs were also associated with “greater reductions in diabetes medication and clinically important benefits” in triglycerides and insulin resistance at 6 and 12 months, the team wrote.
VLCDs: Adherence Is key
Finally, the team looked at weight loss achieved with VLCDs.
VLCDs were less effective for weight loss at 6 months than less restrictive LCDs. However, this effect was explained by diet adherence, the researchers noted.
Restricting the analysis to “credible” studies, VLCDs were associated with a larger “clinically important” weight-loss versus control diets when patients were highly adherent to the diet, at a mean reduction of 4.47 kg (9.9 lb) versus a mean increase of 0.55 kg (1.2 lb) among patients who were less adherent.
The team noted that their review has a number of limitations, not least of which is the definition of diabetes remission used, which “is the subject of considerable debate,” as well as the safety concerns raised over LCDs.
Given the latter concerns, “clinicians might consider short-term LCDs for management of type 2 diabetes, while actively monitoring and adjusting diabetes medication as needed,” they concluded.
This study was funded in part by Texas A&M University. One coauthor reported receiving funding from Texas A&M AgriLife Research for a separate research project. Dr. Brinkworth is author of the book “The CSIRO Low Carb Diet,” but does not receive financial royalties or funds either directly or indirectly.
A version of this article first appeared on Medscape.com.
Patients with type 2 diabetes who follow a low-carbohydrate diet (LCD) for at least 6 months appear to have significantly higher remission rates than those following other diets, although the benefits diminish by 12 months, suggests a new analysis of trial data from over 1,300 individuals.
“Based on other evidence, it is likely the degree of weight loss would have been a contributing factor, combined with the lower intake of dietary carbohydrates,” study coauthor Grant D. Brinkworth, PhD, Commonwealth Scientific and Industrial Research Organisation, Sydney, , said in an interview.
He acknowledged, however, that “diets in general can be difficult to sustain over the long term. ... We need to provide patients with easy-to-use support tools and convenient solutions to help them adhere to a low-carb diet long term to gain these greater health improvements.
“In addition, more long-term, well-controlled, randomized trials are needed to determine the effects of low-carb diets on sustained weight loss, diabetes remission, and health outcomes,” Dr. Brinkworth added.
The research was published on Janu. 13 in the BMJ by a consortium of international scientists, led by Joshua Z. Goldenberg, PhD, department of nutrition, Texas A&M University, College Station.
Confusion as to best diet for those with diabetes
Type 2 diabetes is a “significant and worsening” worldwide health problem, wrote Dr. Goldenberg and coauthors, in spite of “many pharmaceutical developments and a global emphasis on glycemic control.”
Although structured diets are “recognized as an essential component of treating diabetes, confusion remains about which diet to choose,” with multiple systemic reviews and meta-analyses of carbohydrate-restricted diets “reporting mixed results,” they noted.
They therefore undertook a systematic review of randomized, controlled trials on the efficacy and safety of LCDs and very-low-carbohydrate diets (VLCDs) using the CENTRAL, Medline, CINAHL, and CAB databases, as well as other literature sources.
Researchers defined LCDs as less than 130 g/day of carbohydrates or less than 26% of calories from carbohydrates as part of a 2,000 kcal/day diet and VLCDs as less than 50 g/day or less than 10% of daily calories. They focused on interventions that lasted at least 12 weeks in adults with type 2 diabetes.
Overall, 23 trials involving 1,357 participants met the inclusion criteria; 52% used VLCDs and the control comparator was a low-fat diet in 78% of the studies. The mean age range of patients was 47-67 years, and treatment duration spanned from 3 months to 2 years.
LCDs were associated with a higher rate of diabetes remission when defined as a hemoglobin A1c level of less than 6.5%, compared with control diets at 6 months, at 57% versus 31% – an increase in remission of 32% associated with LCDs (P < .001 for overall effect).
But when defined more tightly as an A1c level of less than 6.5% in the absence of diabetes medications, remission with LCDs was reduced to a nonsignificant 5% versus control diets at 6 months.
At 12 months, data on remission were sparse, ranging from a small effect to a trivial increased risk of diabetes.
Subgroup analysis demonstrated that patients on an LCD achieved greater weight loss at 6 months than those on a control diet, at a mean reduction of 3.46 kg (approximately 7.6 lb). However, the researchers noted that, at 12 months, any weight-loss benefit was “trivial and nonsignificant.”
A similar pattern was seen for reductions in A1c and fasting glucose levels with LCDs: Notable reductions at 6 months largely disappeared by 12 months.
LCDs were also associated with “greater reductions in diabetes medication and clinically important benefits” in triglycerides and insulin resistance at 6 and 12 months, the team wrote.
VLCDs: Adherence Is key
Finally, the team looked at weight loss achieved with VLCDs.
VLCDs were less effective for weight loss at 6 months than less restrictive LCDs. However, this effect was explained by diet adherence, the researchers noted.
Restricting the analysis to “credible” studies, VLCDs were associated with a larger “clinically important” weight-loss versus control diets when patients were highly adherent to the diet, at a mean reduction of 4.47 kg (9.9 lb) versus a mean increase of 0.55 kg (1.2 lb) among patients who were less adherent.
The team noted that their review has a number of limitations, not least of which is the definition of diabetes remission used, which “is the subject of considerable debate,” as well as the safety concerns raised over LCDs.
Given the latter concerns, “clinicians might consider short-term LCDs for management of type 2 diabetes, while actively monitoring and adjusting diabetes medication as needed,” they concluded.
This study was funded in part by Texas A&M University. One coauthor reported receiving funding from Texas A&M AgriLife Research for a separate research project. Dr. Brinkworth is author of the book “The CSIRO Low Carb Diet,” but does not receive financial royalties or funds either directly or indirectly.
A version of this article first appeared on Medscape.com.
Patients with type 2 diabetes who follow a low-carbohydrate diet (LCD) for at least 6 months appear to have significantly higher remission rates than those following other diets, although the benefits diminish by 12 months, suggests a new analysis of trial data from over 1,300 individuals.
“Based on other evidence, it is likely the degree of weight loss would have been a contributing factor, combined with the lower intake of dietary carbohydrates,” study coauthor Grant D. Brinkworth, PhD, Commonwealth Scientific and Industrial Research Organisation, Sydney, , said in an interview.
He acknowledged, however, that “diets in general can be difficult to sustain over the long term. ... We need to provide patients with easy-to-use support tools and convenient solutions to help them adhere to a low-carb diet long term to gain these greater health improvements.
“In addition, more long-term, well-controlled, randomized trials are needed to determine the effects of low-carb diets on sustained weight loss, diabetes remission, and health outcomes,” Dr. Brinkworth added.
The research was published on Janu. 13 in the BMJ by a consortium of international scientists, led by Joshua Z. Goldenberg, PhD, department of nutrition, Texas A&M University, College Station.
Confusion as to best diet for those with diabetes
Type 2 diabetes is a “significant and worsening” worldwide health problem, wrote Dr. Goldenberg and coauthors, in spite of “many pharmaceutical developments and a global emphasis on glycemic control.”
Although structured diets are “recognized as an essential component of treating diabetes, confusion remains about which diet to choose,” with multiple systemic reviews and meta-analyses of carbohydrate-restricted diets “reporting mixed results,” they noted.
They therefore undertook a systematic review of randomized, controlled trials on the efficacy and safety of LCDs and very-low-carbohydrate diets (VLCDs) using the CENTRAL, Medline, CINAHL, and CAB databases, as well as other literature sources.
Researchers defined LCDs as less than 130 g/day of carbohydrates or less than 26% of calories from carbohydrates as part of a 2,000 kcal/day diet and VLCDs as less than 50 g/day or less than 10% of daily calories. They focused on interventions that lasted at least 12 weeks in adults with type 2 diabetes.
Overall, 23 trials involving 1,357 participants met the inclusion criteria; 52% used VLCDs and the control comparator was a low-fat diet in 78% of the studies. The mean age range of patients was 47-67 years, and treatment duration spanned from 3 months to 2 years.
LCDs were associated with a higher rate of diabetes remission when defined as a hemoglobin A1c level of less than 6.5%, compared with control diets at 6 months, at 57% versus 31% – an increase in remission of 32% associated with LCDs (P < .001 for overall effect).
But when defined more tightly as an A1c level of less than 6.5% in the absence of diabetes medications, remission with LCDs was reduced to a nonsignificant 5% versus control diets at 6 months.
At 12 months, data on remission were sparse, ranging from a small effect to a trivial increased risk of diabetes.
Subgroup analysis demonstrated that patients on an LCD achieved greater weight loss at 6 months than those on a control diet, at a mean reduction of 3.46 kg (approximately 7.6 lb). However, the researchers noted that, at 12 months, any weight-loss benefit was “trivial and nonsignificant.”
A similar pattern was seen for reductions in A1c and fasting glucose levels with LCDs: Notable reductions at 6 months largely disappeared by 12 months.
LCDs were also associated with “greater reductions in diabetes medication and clinically important benefits” in triglycerides and insulin resistance at 6 and 12 months, the team wrote.
VLCDs: Adherence Is key
Finally, the team looked at weight loss achieved with VLCDs.
VLCDs were less effective for weight loss at 6 months than less restrictive LCDs. However, this effect was explained by diet adherence, the researchers noted.
Restricting the analysis to “credible” studies, VLCDs were associated with a larger “clinically important” weight-loss versus control diets when patients were highly adherent to the diet, at a mean reduction of 4.47 kg (9.9 lb) versus a mean increase of 0.55 kg (1.2 lb) among patients who were less adherent.
The team noted that their review has a number of limitations, not least of which is the definition of diabetes remission used, which “is the subject of considerable debate,” as well as the safety concerns raised over LCDs.
Given the latter concerns, “clinicians might consider short-term LCDs for management of type 2 diabetes, while actively monitoring and adjusting diabetes medication as needed,” they concluded.
This study was funded in part by Texas A&M University. One coauthor reported receiving funding from Texas A&M AgriLife Research for a separate research project. Dr. Brinkworth is author of the book “The CSIRO Low Carb Diet,” but does not receive financial royalties or funds either directly or indirectly.
A version of this article first appeared on Medscape.com.
Women physicians and the pandemic: A snapshot
“Women physicians do not have trouble balancing competing demands any more than men physicians do. It is simply a more common expectation that women physicians will adjust their professional lives,” she observed.
The daily grind of caring for patients during a global pandemic is taking an emotional and mental toll on doctors as well as a physical one. “The recently publicized suicide of emergency physician Lorna Breen, MD, following her intense work during the pandemic in New York should cause every physician to reflect on their culture in medicine,” Dr. Brubaker wrote in the article. In an interview, she expounded on the current climate for women psychiatrists and physicians in general, offering some coping techniques.
Question: The pandemic has amplified disparities among men and women physicians. What may be the repercussions from this, not just for patient care, but for work-life balance among women physicians?
Answer: Focusing on women in academic roles, both research and clinical productivity have changed in the professional arena. Many women continue to bear a disproportionate share of family responsibilities and have reduced paid work to accommodate these needs. These changes can impact academic promotion and, therefore, subsequent academic opportunities for leadership. These gaps will add to the well-recognized gender wage gap. Women physicians are more likely to experience reduced wages associated with reduced professional activities. This reduces their annual earnings, which reduces their contributions to Social Security and other retirement programs. This can adversely impact their financial security later in life, at a time when women are already disadvantaged, compared with men.
Q: Are women psychiatrists facing additional burdens, given that many patients are suffering from anxiety and depression right now, and seeking out prescriptions?
A: We know that mental health concerns are on the rise. Although I cannot point to specific evidence, as a result. Similar to those on the more well-recognized “front lines” in the ED and critical care units, I consider my psychiatric colleagues to be on the front lines as well, as they are addressing this marked increase in care needs, for patients and for other members of the health care team.
Q: You mentioned the suicide of Dr. Breen. What might women psychiatrists take away from this incident?
A: Physicians are drawn to our vocation with a commitment to be of service to others. During such demanding times as these, the “safety” rails between service to others and self-care shift – clearly this can endanger individual doctors.
Q: What advice might you have for women in this profession? Any resources that could provide support?
A: My advice is to ensure your own well-being, knowing that this differs for each woman. Be realistic with your time and commitments, allowing time for restoration and rest. Sometimes I tell my peers to meditate or do some other form of contemplative practice. Exercise (preferably outdoors) and sleep, including preparing for good sleep, such as not reading emails or patient charts right up until sleep time, are all important. Most importantly, identify your support team and check in regularly with them. Never hesitate to reach out for help. People truly do care and want to help you.
“Women physicians do not have trouble balancing competing demands any more than men physicians do. It is simply a more common expectation that women physicians will adjust their professional lives,” she observed.
The daily grind of caring for patients during a global pandemic is taking an emotional and mental toll on doctors as well as a physical one. “The recently publicized suicide of emergency physician Lorna Breen, MD, following her intense work during the pandemic in New York should cause every physician to reflect on their culture in medicine,” Dr. Brubaker wrote in the article. In an interview, she expounded on the current climate for women psychiatrists and physicians in general, offering some coping techniques.
Question: The pandemic has amplified disparities among men and women physicians. What may be the repercussions from this, not just for patient care, but for work-life balance among women physicians?
Answer: Focusing on women in academic roles, both research and clinical productivity have changed in the professional arena. Many women continue to bear a disproportionate share of family responsibilities and have reduced paid work to accommodate these needs. These changes can impact academic promotion and, therefore, subsequent academic opportunities for leadership. These gaps will add to the well-recognized gender wage gap. Women physicians are more likely to experience reduced wages associated with reduced professional activities. This reduces their annual earnings, which reduces their contributions to Social Security and other retirement programs. This can adversely impact their financial security later in life, at a time when women are already disadvantaged, compared with men.
Q: Are women psychiatrists facing additional burdens, given that many patients are suffering from anxiety and depression right now, and seeking out prescriptions?
A: We know that mental health concerns are on the rise. Although I cannot point to specific evidence, as a result. Similar to those on the more well-recognized “front lines” in the ED and critical care units, I consider my psychiatric colleagues to be on the front lines as well, as they are addressing this marked increase in care needs, for patients and for other members of the health care team.
Q: You mentioned the suicide of Dr. Breen. What might women psychiatrists take away from this incident?
A: Physicians are drawn to our vocation with a commitment to be of service to others. During such demanding times as these, the “safety” rails between service to others and self-care shift – clearly this can endanger individual doctors.
Q: What advice might you have for women in this profession? Any resources that could provide support?
A: My advice is to ensure your own well-being, knowing that this differs for each woman. Be realistic with your time and commitments, allowing time for restoration and rest. Sometimes I tell my peers to meditate or do some other form of contemplative practice. Exercise (preferably outdoors) and sleep, including preparing for good sleep, such as not reading emails or patient charts right up until sleep time, are all important. Most importantly, identify your support team and check in regularly with them. Never hesitate to reach out for help. People truly do care and want to help you.
“Women physicians do not have trouble balancing competing demands any more than men physicians do. It is simply a more common expectation that women physicians will adjust their professional lives,” she observed.
The daily grind of caring for patients during a global pandemic is taking an emotional and mental toll on doctors as well as a physical one. “The recently publicized suicide of emergency physician Lorna Breen, MD, following her intense work during the pandemic in New York should cause every physician to reflect on their culture in medicine,” Dr. Brubaker wrote in the article. In an interview, she expounded on the current climate for women psychiatrists and physicians in general, offering some coping techniques.
Question: The pandemic has amplified disparities among men and women physicians. What may be the repercussions from this, not just for patient care, but for work-life balance among women physicians?
Answer: Focusing on women in academic roles, both research and clinical productivity have changed in the professional arena. Many women continue to bear a disproportionate share of family responsibilities and have reduced paid work to accommodate these needs. These changes can impact academic promotion and, therefore, subsequent academic opportunities for leadership. These gaps will add to the well-recognized gender wage gap. Women physicians are more likely to experience reduced wages associated with reduced professional activities. This reduces their annual earnings, which reduces their contributions to Social Security and other retirement programs. This can adversely impact their financial security later in life, at a time when women are already disadvantaged, compared with men.
Q: Are women psychiatrists facing additional burdens, given that many patients are suffering from anxiety and depression right now, and seeking out prescriptions?
A: We know that mental health concerns are on the rise. Although I cannot point to specific evidence, as a result. Similar to those on the more well-recognized “front lines” in the ED and critical care units, I consider my psychiatric colleagues to be on the front lines as well, as they are addressing this marked increase in care needs, for patients and for other members of the health care team.
Q: You mentioned the suicide of Dr. Breen. What might women psychiatrists take away from this incident?
A: Physicians are drawn to our vocation with a commitment to be of service to others. During such demanding times as these, the “safety” rails between service to others and self-care shift – clearly this can endanger individual doctors.
Q: What advice might you have for women in this profession? Any resources that could provide support?
A: My advice is to ensure your own well-being, knowing that this differs for each woman. Be realistic with your time and commitments, allowing time for restoration and rest. Sometimes I tell my peers to meditate or do some other form of contemplative practice. Exercise (preferably outdoors) and sleep, including preparing for good sleep, such as not reading emails or patient charts right up until sleep time, are all important. Most importantly, identify your support team and check in regularly with them. Never hesitate to reach out for help. People truly do care and want to help you.
Further warning on SGLT2 inhibitor use and DKA risk in COVID-19
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
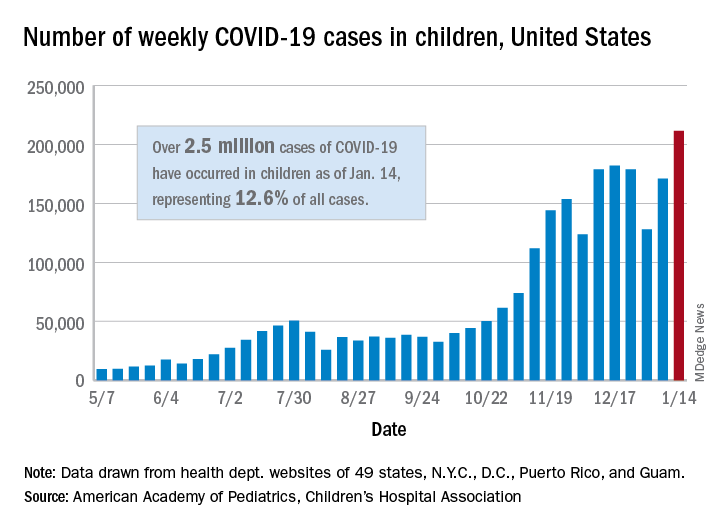
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Biomarker HF risk score envisioned as SGLT2 inhibitor lodestar in diabetes
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
A scoring system that predicts risk for new heart failure over 5 years that is based solely on a few familiar, readily available biomarkers could potentially help steer patients with diabetes or even prediabetes toward HF-preventive therapies, researchers proposed based on a new study.
They foresee the risk-stratification tool, based on data pooled from three major community-based cohort studies but not independently validated, as a way to select patients with diabetes and prediabetes for treatment with SGLT2 inhibitors.
Several members of that drug class, conceived as antidiabetic agents, have been shown to help in prevention or treatment of HF in patients with diabetes and those without diabetes but at increased cardiovascular (CV) risk. Yet their uptake in practice has been lagging, the group noted.
Most HF benefits in the SGLT2 inhibitor trials “were seen in patients who have established cardiovascular disease – basically a history of heart attack or stroke,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, said in an interview.
“So we wanted to see how we can identify high-risk patients without a history of cardiovascular disease using these biomarkers, as an approach to targeting SGLT2 inhibitors, which are fairly expensive therapies,” he said. Without such risk stratification, “you end up treating so many more patients to get very modest returns.”
The group developed a scoring system based on four biomarkers that are “easily measured with inexpensive tests,” Dr. Pandey said: high-sensitivity-assay cardiac troponin T (hs-cTnT) and C-reactive protein (hs-CRP) levels, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) levels, and electrocardiography for evidence of left-ventricular hypertrophy (ECG-LVH).
The derivation cohort consisted of participants in the Atherosclerosis Risk in Communities RIC, Dallas Heart Study, and Multi-Ethnic Study of Atherosclerosis Multi-Ethnic Study of Atherosclerosis epidemiologic studies who were free of coronary heart disease, stroke, or HF for whom there were sufficient data on CV risk factors and the four biomarkers. None were taking SGLT2 inhibitors at enrollment in their respective studies, the researchers noted.
Members of the pooled cohorts who had diabetes or prediabetes were assigned 1 point for each abnormal biomarker. The 5-year risk for incident HF went up continuously along with the score in people with diabetes and in those with prediabetes, the latter defined as a fasting plasma glucose level from 100 mg/dL to less than 126 mg/dL.
For those with a score of 1, compared with 0, for example, the risk for HF went up 82% with diabetes and 40% with prediabetes. But for those with a score of 3 or 4, the risk went up more than four and a half times with diabetes and more than three and a half times for those with prediabetes. Risk increases were independent of other likely HF risk factors and consistently significant.
The analysis was published Jan. 6 in JACC: Heart Failure.
The biomarker score should be especially useful in patients considered at low to intermediate risk, based on clinical characteristics, as a means to identify residual HF risk and, potentially, select candidates for SGLT2-inhibitor therapy, Dr. Pandey said.
“The other purpose of the study was to broaden the scope of heart failure prevention in dysglycemia by looking also at prediabetes, not just diabetes,” he said. There isn’t much high-quality evidence supporting SGLT2-inhibitor therapy in prediabetes, but it follows that the drugs may be helpful in prediabetes because they are protective in patients with and without diabetes.
“Our work suggests that prediabetes patients who have elevated biomarkers are at a higher risk of heart failure,” Dr. Pandey said, suggesting that the HF risk score could potentially help select their drug therapy as well.
The current study seems “to provide a proof of concept that one can use circulating biomarkers to more precisely identify patients in whom therapies might be expected to exert greatest benefit,” which is especially important for potentially expensive agents like the SGLT2 inhibitors, James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Importantly in the analysis, a greater number of biomarker abnormalities not only corresponded to rising levels of risk, the risk increases were “dramatic,” and therefore so was the supposed potential benefit of SGLT2-inhibitor therapy, said Dr. Januzzi, who isn’t a coauthor but was an editor for its publication in JACC: Heart Failure.
The uptake of SGLT2 inhibitors for heart failure in practice has been less rapid than hoped, he observed, so if “this hypothetical construct holds up” for the drug class, “it might actually help kick-start focusing on who might optimally receive the drugs.”
Elevated levels of hs-cTnT, hs-CRP, and NT-proBNP, as well as presence of ECG-LVH, were each independently associated with a significantly increased 5-year risk for HF in unadjusted and adjusted analyses of the 6,799 people in the pooled cohort, 33.2% of whom had diabetes and 66.8% of whom had prediabetes, the group writes.
The scoring system would require validation in other cohorts before it could be used, Dr. Pandey observed; once there is “robust validation,” it might be applied first to patients with dysglycemia at intermediate CV risk by standard clinical measures.
Certainly the HF risk-stratification scoring system requires validation in other studies, Dr. Januzzi agreed. But it is intuitively appealing, and the study’s results are consistent with “data that we’re submitting for publication imminently” based on the CANVAS CV-outcomes trial of the SGLT2 inhibitor canagliflozin (Invokana) in patients with diabetes.
Dr. Pandey disclosed receiving support from the Gilead Sciences Research Scholar Program and serving on an advisory board of Roche Diagnostics. Dr. Januzzi disclosed receiving grant support from Novartis, Applied Therapeutics, and Innolife; consulting for Abbott Diagnostics, Janssen, Novartis, Quidel, and Roche Diagnostics; and serving on end-point committees or data safety monitoring boards for trials supported by Abbott, AbbVie, Amgen, CVRx, Janssen, MyoKardia, and Takeda.
A version of this article first appeared on Medscape.com.
HHS will drop buprenorphine waiver rule for most physicians
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.
Federal officials on Thursday announced a plan to largely drop the so-called X-waiver requirement for buprenorphine prescriptions for physicians in a bid to remove an administrative procedure widely seen as a barrier to opioid use disorder (OUD) treatment.
The Department of Health & Human Services unveiled new practice guidelines that include an exemption from current certification requirements. The exemption applies to physicians already registered with the Drug Enforcement Administration.
A restriction included in the new HHS policy is a limit of treating no more than 30 patients with buprenorphine for OUD at any one time. There is an exception to this limit for hospital-based physicians, such as those working in emergency departments, HHS said.
, such as buprenorphine, and does not apply to methadone. The new guidelines say the date on which they will take effect will be added after publication in the Federal Register. HHS did not immediately answer a request from this news organization for a more specific timeline.
Welcomed change
The change in prescribing rule was widely welcomed, with the American Medical Association issuing a statement endorsing the revision. The AMA and many prescribers and researchers had seen the X-waiver as a hurdle to address the nation’s opioid epidemic.
There were more than 83,000 deaths attributed to drug overdoses in the United States in the 12 months ending in June 2020. This is the highest number of overdose deaths ever recorded in a 12-month period, HHS said in a press release, which cited data from the Centers for Disease Control and Prevention.
In a tweet about the new policy, Peter Grinspoon, MD, a Boston internist and author of the memoir “Free Refills: A Doctor Confronts His Addiction,” contrasted the relative ease with which clinicians can give medicines that carry a risk for abuse with the challenge that has existed in trying to provide patients with buprenorphine.
“Absolutely insane that we need a special waiver for buprenorphine to TREAT opioid addiction, but not to prescribe oxycodone, Vicodin, etc., which can get people in trouble in the first place!!” Dr. Grinspoon tweeted.
Patrice Harris, MD, chair of the AMA’s Opioid Task Force and the organization’s immediate past president, said removing the X-waiver requirement can help lessen the stigma associated with this OUD treatment. The AMA had urged HHS to change the regulation.
“With this change, office-based physicians and physician-led teams working with patients to manage their other medical conditions can also treat them for their opioid use disorder without being subjected to a separate and burdensome regulatory regime,” Dr. Harris said in the AMA statement.
Researchers have in recent years sought to highlight what they described as missed opportunities for OUD treatment because of the need for the X-waiver.
Buprenorphine is a cost-effective treatment for opioid use disorder, which reduces the risk of injection-related infections and mortality risk, notes a study published online last month in JAMA Network Open.
However, results showed that fewer than 2% of obstetrician-gynecologists who examined women enrolled in Medicaid were trained to prescribe buprenorphine. The study, which was based on data from 31, 211 ob.gyns. who accepted Medicaid insurance, was created to quantify how many were on the list of Drug Addiction Treatment Act buprenorphine-waived clinicians.
The Drug Addiction Treatment Act has required 8 hours of training for physicians and 24 hours for nurse practitioners and physician assistants for the X-waiver needed to prescribe buprenorphine, the investigators report.
‘X the X-waiver’
Only 10% of recent family residency graduates reported being adequately trained to prescribe buprenorphine and only 7% reported actually prescribing the drug, write Kevin Fiscella, MD, University of Rochester (N.Y.) Medical Center and colleagues in a 2018 Viewpoint article published in JAMA Psychiatry.
In the article, which was subtitled “X the X Waiver,” they called for deregulation of buprenorphine as a way of mainstreaming treatment for OUD.
“The DATA 2000 has failed – too few physicians have obtained X-waivers,” the authors write. “Regulations reinforce the stigma surrounding buprenorphine prescribers and patients who receive it while constraining access and discouraging patient engagement and retention in treatment.”
The change, announced Jan. 14, leaves in place restrictions on prescribing for clinicians other than physicians. On a call with reporters, Adm. Brett P. Giroir, MD, assistant secretary for health, suggested that federal officials should take further steps to remove hurdles to buprenorphine prescriptions.
“Many people will say this has gone too far,” Dr. Giroir said of the drive to end the X-waiver for clinicians. “But I believe more people will say this has not gone far enough.”
A version of this article first appeared on Medscape.com.





