User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Quick medication, better communication linked to less violence at inpatient psych unit
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
SAN FRANCISCO – Physically violent events at an inpatient psychiatric unit in Pennsylvania dropped by 59.8% in the months after it implemented a plan to administer antipsychotic medications to patients more quickly – both in the emergency department and in the unit – and improve handoffs between providers and nurses, researchers reported.
“We were able to significantly reduce violence,” said Michael Chen, MD, Lehigh Valley Health Network psychiatry resident and lead author of an abstract presented at the annual meeting of the American Psychiatric Association. “Furthermore, the interventions were effective in reducing episodes of violence rather than redirecting it. And the overall feeling of safety on the inpatient psychiatric unit improved.”
Violence is common in psychiatric units, although it’s not clear how often it occurs. “The data has shown that patients with a psychotic disorder such as schizophrenia or a mood disorder with psychotic features such as bipolar disorder tend to account for most of the episodes of violence on the unit,” Dr. Chen said in an interview. “This inevitably results in a higher risk for violence on inpatient psychiatric units as a large portion of patients admitted to inpatient psychiatric units have these diagnoses.”
Enlisting the pharmacy department
For the new study, investigators tracked episodes of violence – including verbal attacks – at an Allentown, Penn.–area inpatient psychiatric unit from December 2021 to September 2022. According to Dr. Chen, unit leaders implemented the new plan in May 2022 in the wake of higher levels of violence during the COVID-19 pandemic and the concurrent staff shortages.
Clinic leaders sought to identify potentially aggressive patients in the emergency department and treat them with antipsychotics prior to admission to the psychiatric unit, ensure that the pharmacy provides access to as-needed or standing medications, and develop “standardized huddles to ensure proper handoffs between providers and nurses.”
Medical staff relied on the Dynamic Appraisal of Situational Aggression scale, risk factors, and clinical judgment to determine which patients had the potential to be violent, Dr. Chen said.
As for treatment, first-line antipsychotics are typically given orally, but they can be injected if patients must be treated over their objections, he said. “We would only consider starting standing medications against objections in patients who are involuntarily committed.”
During the 5 months before the intervention was implemented versus the following 5 months, the average monthly number of physically violent events in the psychiatric unit fell from 12.4 to 4.8 (–61.1%, P = .04), and verbal threats dipped from 7.2 to 4 (–44.4%, P = .15). The total average number of violent events per month, including violence against property, fell from an average of 25.4 to 10.2 (–59.8%, P = .03).
The total patient population didn’t vary significantly over time, Dr. Chen said. “Thus, the decrease in violence was not correlated with a decrease in patient load.”
While “there were concerns that there would just be higher episodes of violence in the ED while psychiatry patients awaited placement,” Dr. Chen said, the numbers actually showed reductions in violence in that setting. The average number of physically violent events per month in the ED fell from 49.6 to 39.4 (–20.6%, P = .03). Verbal threats dropped from 38 to 34.6 (–8.9%, P = .5) and overall violent events dipped from 87.6 to 74 (–15.6%, P = .08).
Why did the interventions seem to work? “Standing doses as well as as-needed medications started for psychiatric patients in the emergency department have been crucial to prevent delay of care,” Dr. Chen said. Enlisting the pharmacy department “helped ensure all patients had appropriate as-needed medications to prevent them from decompensating on the units,” he added, and “involvement of nursing and ancillary staff in high-risk rounds allowed the treatment team to rapidly anticipate and address concerns.”
The study authors also reported that nursing staff felt safer. Scores on a perception-of-safety scale – with 1 most unsafe and 7 most safe – improved from 3.3 to 4.2 (+27%, P < .01).
Dr. Chen said there was a “minimal” increase in cost to implement the intervention, although coordination is necessary. “The emergency department and psychiatry department have to work together to initiate treatment in the ED while awaiting beds,” he said. “The treatment team needs to communicate concerns during rounds. The pharmacist and psychiatrist need to work together to ensure that proper as-needed medications are available.”
‘Good clinical practice’
In an interview, psychiatrist Mark J. Russ, MD, of NewYork-Presbyterian/Westchester Behavioral Health and Weill Cornell Medical College, said violent incidents in inpatient psychiatric units are influenced by many factors, such as history of violence, substance use, history of trauma, psychosis/paranoia, and medical problems.
The units themselves can contribute to the risk of violence through power struggles and lack of attention paid to respect and dignity, he said. “Attention to these issues is important in reducing violence,” he noted. “Generalized training for staff in de-escalation techniques and trauma-informed care is imperative. There may be value in developing specialized psychiatric ICUs where staff are meticulously trained in these and other approaches.”
The new study, Dr. Russ said, suggests that “early identification of patients at risk of engaging in violent behavior on the inpatient unit, pharmacologic treatment, and good communication helps reduce violence.” The findings, he added, suggest that “interventions known to constitute good clinical practice are indeed helpful.”
However, he cautioned that “treating all at-risk patients with antipsychotics, regardless of their psychiatric diagnosis, might well be considered chemical restraint, depending on [the] circumstances.”
There was no study funding. The study authors and Dr. Russ have no disclosures.
AT APA 2023
Lack of paid sick leave is a barrier to cancer screening
“Our results provide evidence for policymakers considering legislative or regulatory solutions to address insufficient screening adherence and highlight an understudied benefit of expanding paid sick leave coverage,” wrote authors who were led by Kevin Callison, PhD, of the Tulane University School of Public Health and Tropical Medicine, New Orleans.
The findings were published earlier this year in the New England Journal of Medicine.
Despite an Affordable Care Act provision eliminating most cost-sharing for cancer screening, the rate for recommended breast and colorectal cancer screening among U.S. adults is lower than 70%. Work commitments, time constraints, and the prospect of lost wages are frequently cited as contributing factors to this underuse of preventive care. Researchers hypothesized that having paid sick leave coverage for the use of preventive services could improve adherence to cancer screening guidelines. With continued failure to pass a bill mandating federal paid sick leave legislation, nearly 30% of the nation’s workforce lacks this coverage. Rates are lower for low-income workers, women, and underserved racial and ethnic groups, the authors write.
Coverage mandates have become politically contentious, as evidenced by the fact that their passage by some states (n = 17), counties (n = 4) and cities (n = 18) has been met by many states (n = 18) passing preemption laws banning municipalities from adopting the laws.
In this study, researchers examined the rate of colorectal and breast cancer screening at 12- and 24-month intervals among people living in one of 61 cities. Before paid sick leave mandates were put in place, cancer screening rates were similar across the board. But once mandates were put in place, cancer screening rates were higher among workers affected by the mandate by 1.31% (95% confidence interval, 0.28-2.34) for 12-month colorectal cancer screening, 1.56% (95% CI, 0.33-2.79) for 24-month colorectal cancer screening, 1.22% (95% CI, −0.20 to 2.64) for 12-month mammography, and 2.07% (95% CI, 0.15-3.99) for 24-month mammography.
“Although these appear to be modest effects, spread across a large population, these indicate a fairly substantial gain in cancer screenings,” Dr. Callison said.
Prior studies showing positive associations between having paid sick leave coverage and whether someone receives cancer screenings are likely confounded by selection bias because they compare workers who have such coverage to those who do not, Dr. Callison and colleagues state in their paper.
“Although the lack of paid sick leave coverage may hinder access to preventive care, current evidence is insufficient to draw meaningful conclusions about its relationship to cancer screening,” the authors write, citing that particularly health conscious workers may take jobs offering sick leave coverage.
Through quasi-experimental design, the present study aimed to overcome such confounding issues. Its analytic sample, using administrative data from the Merative MarketScan Research Databases, encompassed approximately 2.5 million person-specific records per year for the colorectal cancer screening sample. The researchers’ mammography sample included 1.3 million person-specific records per year of the period examined.
The associations cited above translate into relative colorectal cancer screening increases of 8.1% in the 12-month adjusted model and a 5.9% relative increase from the premandate rate in the 24-month adjusted model. The rate was 1.56 percentage points (95% CI, 0.33-2.79) higher in the cities subject to the paid sick leave mandates (a 5.9% relative increase from the premandate rate). For screening mammography in the cities subject to the mandates, the 12-month adjusted 1.22% increase (95% CI, –0.20 to 2.64) represented a 2.5% relative increase from the premandate level. The adjusted 24-month rate increase of 2.07% (95% CI, 0.15-4.00) represented a 3.3% relative increase from premandate rates.
“However, these estimates are averages across all workers in our sample, many of whom likely already had paid sick leave coverage prior to the enactment of a mandate,” Dr. Callison said in the interview. “In fact, in other work related to this project, we estimated that about 28% of private sector workers gain paid sick leave when a mandate is enacted. So then, if we scale our findings by the share of workers actually gaining paid sick leave coverage, our estimates are much larger – a 9%-12% increase in screening mammography and a 21%-29% increase in colorectal cancer screening.”
Dr. Callison and his team are in the process of developing a follow-up proposal that would examine the effects of paid sick leave on downstream outcomes of the cancer care continuum, such as timing from diagnosis to treatment initiation. “We also hope to examine who benefits from these additional screens and what they mean for health equity. Data limitations prevented us from exploring that issue in the current study,” he said.
Dr. Callison had no conflicts associated with this study.
“Our results provide evidence for policymakers considering legislative or regulatory solutions to address insufficient screening adherence and highlight an understudied benefit of expanding paid sick leave coverage,” wrote authors who were led by Kevin Callison, PhD, of the Tulane University School of Public Health and Tropical Medicine, New Orleans.
The findings were published earlier this year in the New England Journal of Medicine.
Despite an Affordable Care Act provision eliminating most cost-sharing for cancer screening, the rate for recommended breast and colorectal cancer screening among U.S. adults is lower than 70%. Work commitments, time constraints, and the prospect of lost wages are frequently cited as contributing factors to this underuse of preventive care. Researchers hypothesized that having paid sick leave coverage for the use of preventive services could improve adherence to cancer screening guidelines. With continued failure to pass a bill mandating federal paid sick leave legislation, nearly 30% of the nation’s workforce lacks this coverage. Rates are lower for low-income workers, women, and underserved racial and ethnic groups, the authors write.
Coverage mandates have become politically contentious, as evidenced by the fact that their passage by some states (n = 17), counties (n = 4) and cities (n = 18) has been met by many states (n = 18) passing preemption laws banning municipalities from adopting the laws.
In this study, researchers examined the rate of colorectal and breast cancer screening at 12- and 24-month intervals among people living in one of 61 cities. Before paid sick leave mandates were put in place, cancer screening rates were similar across the board. But once mandates were put in place, cancer screening rates were higher among workers affected by the mandate by 1.31% (95% confidence interval, 0.28-2.34) for 12-month colorectal cancer screening, 1.56% (95% CI, 0.33-2.79) for 24-month colorectal cancer screening, 1.22% (95% CI, −0.20 to 2.64) for 12-month mammography, and 2.07% (95% CI, 0.15-3.99) for 24-month mammography.
“Although these appear to be modest effects, spread across a large population, these indicate a fairly substantial gain in cancer screenings,” Dr. Callison said.
Prior studies showing positive associations between having paid sick leave coverage and whether someone receives cancer screenings are likely confounded by selection bias because they compare workers who have such coverage to those who do not, Dr. Callison and colleagues state in their paper.
“Although the lack of paid sick leave coverage may hinder access to preventive care, current evidence is insufficient to draw meaningful conclusions about its relationship to cancer screening,” the authors write, citing that particularly health conscious workers may take jobs offering sick leave coverage.
Through quasi-experimental design, the present study aimed to overcome such confounding issues. Its analytic sample, using administrative data from the Merative MarketScan Research Databases, encompassed approximately 2.5 million person-specific records per year for the colorectal cancer screening sample. The researchers’ mammography sample included 1.3 million person-specific records per year of the period examined.
The associations cited above translate into relative colorectal cancer screening increases of 8.1% in the 12-month adjusted model and a 5.9% relative increase from the premandate rate in the 24-month adjusted model. The rate was 1.56 percentage points (95% CI, 0.33-2.79) higher in the cities subject to the paid sick leave mandates (a 5.9% relative increase from the premandate rate). For screening mammography in the cities subject to the mandates, the 12-month adjusted 1.22% increase (95% CI, –0.20 to 2.64) represented a 2.5% relative increase from the premandate level. The adjusted 24-month rate increase of 2.07% (95% CI, 0.15-4.00) represented a 3.3% relative increase from premandate rates.
“However, these estimates are averages across all workers in our sample, many of whom likely already had paid sick leave coverage prior to the enactment of a mandate,” Dr. Callison said in the interview. “In fact, in other work related to this project, we estimated that about 28% of private sector workers gain paid sick leave when a mandate is enacted. So then, if we scale our findings by the share of workers actually gaining paid sick leave coverage, our estimates are much larger – a 9%-12% increase in screening mammography and a 21%-29% increase in colorectal cancer screening.”
Dr. Callison and his team are in the process of developing a follow-up proposal that would examine the effects of paid sick leave on downstream outcomes of the cancer care continuum, such as timing from diagnosis to treatment initiation. “We also hope to examine who benefits from these additional screens and what they mean for health equity. Data limitations prevented us from exploring that issue in the current study,” he said.
Dr. Callison had no conflicts associated with this study.
“Our results provide evidence for policymakers considering legislative or regulatory solutions to address insufficient screening adherence and highlight an understudied benefit of expanding paid sick leave coverage,” wrote authors who were led by Kevin Callison, PhD, of the Tulane University School of Public Health and Tropical Medicine, New Orleans.
The findings were published earlier this year in the New England Journal of Medicine.
Despite an Affordable Care Act provision eliminating most cost-sharing for cancer screening, the rate for recommended breast and colorectal cancer screening among U.S. adults is lower than 70%. Work commitments, time constraints, and the prospect of lost wages are frequently cited as contributing factors to this underuse of preventive care. Researchers hypothesized that having paid sick leave coverage for the use of preventive services could improve adherence to cancer screening guidelines. With continued failure to pass a bill mandating federal paid sick leave legislation, nearly 30% of the nation’s workforce lacks this coverage. Rates are lower for low-income workers, women, and underserved racial and ethnic groups, the authors write.
Coverage mandates have become politically contentious, as evidenced by the fact that their passage by some states (n = 17), counties (n = 4) and cities (n = 18) has been met by many states (n = 18) passing preemption laws banning municipalities from adopting the laws.
In this study, researchers examined the rate of colorectal and breast cancer screening at 12- and 24-month intervals among people living in one of 61 cities. Before paid sick leave mandates were put in place, cancer screening rates were similar across the board. But once mandates were put in place, cancer screening rates were higher among workers affected by the mandate by 1.31% (95% confidence interval, 0.28-2.34) for 12-month colorectal cancer screening, 1.56% (95% CI, 0.33-2.79) for 24-month colorectal cancer screening, 1.22% (95% CI, −0.20 to 2.64) for 12-month mammography, and 2.07% (95% CI, 0.15-3.99) for 24-month mammography.
“Although these appear to be modest effects, spread across a large population, these indicate a fairly substantial gain in cancer screenings,” Dr. Callison said.
Prior studies showing positive associations between having paid sick leave coverage and whether someone receives cancer screenings are likely confounded by selection bias because they compare workers who have such coverage to those who do not, Dr. Callison and colleagues state in their paper.
“Although the lack of paid sick leave coverage may hinder access to preventive care, current evidence is insufficient to draw meaningful conclusions about its relationship to cancer screening,” the authors write, citing that particularly health conscious workers may take jobs offering sick leave coverage.
Through quasi-experimental design, the present study aimed to overcome such confounding issues. Its analytic sample, using administrative data from the Merative MarketScan Research Databases, encompassed approximately 2.5 million person-specific records per year for the colorectal cancer screening sample. The researchers’ mammography sample included 1.3 million person-specific records per year of the period examined.
The associations cited above translate into relative colorectal cancer screening increases of 8.1% in the 12-month adjusted model and a 5.9% relative increase from the premandate rate in the 24-month adjusted model. The rate was 1.56 percentage points (95% CI, 0.33-2.79) higher in the cities subject to the paid sick leave mandates (a 5.9% relative increase from the premandate rate). For screening mammography in the cities subject to the mandates, the 12-month adjusted 1.22% increase (95% CI, –0.20 to 2.64) represented a 2.5% relative increase from the premandate level. The adjusted 24-month rate increase of 2.07% (95% CI, 0.15-4.00) represented a 3.3% relative increase from premandate rates.
“However, these estimates are averages across all workers in our sample, many of whom likely already had paid sick leave coverage prior to the enactment of a mandate,” Dr. Callison said in the interview. “In fact, in other work related to this project, we estimated that about 28% of private sector workers gain paid sick leave when a mandate is enacted. So then, if we scale our findings by the share of workers actually gaining paid sick leave coverage, our estimates are much larger – a 9%-12% increase in screening mammography and a 21%-29% increase in colorectal cancer screening.”
Dr. Callison and his team are in the process of developing a follow-up proposal that would examine the effects of paid sick leave on downstream outcomes of the cancer care continuum, such as timing from diagnosis to treatment initiation. “We also hope to examine who benefits from these additional screens and what they mean for health equity. Data limitations prevented us from exploring that issue in the current study,” he said.
Dr. Callison had no conflicts associated with this study.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Unprecedented drop seen in early colorectal cancer cases due to aspirin use
CHICAGO – The authors say that aspirin could prove to be an effective strategy in preventing early-onset colorectal cancer cases.
“What we have here is a 15% reduction for all adenomas and 33% for those with advanced histology, which to us is quite substantial. We have not seen that much [33%] in previous studies so I would think it definitely needs more study,” said Cassandra D. Fritz, MD, MPHS, a gastroenterologist with Washington University, St. Louis, in an oral presentation given at the annual Digestive Disease Week®.
“This finding is important given the alarming rise in the incidence and mortality of early-onset colorectal cancer (age < 50 years), and our limited understanding of the underlying drivers to direct prevention efforts,” Dr. Fritz said. Early-onset colorectal cancer cases have doubled since 1995, she said.
The study confirms evidence from 30 years of research that suggests regular aspirin use reduces cancer risk. In patients with Lynch syndrome, the CAPP2 study showed that aspirin has a protective effect against colorectal cancer at 20 years follow-up.
While emerging data have suggested that aspirin use may reduce later-onset colorectal cancer, it was not known if regular aspirin and NSAID use are associated with diminished risk of early-onset conventional adenomas, and especially the high-risk adenomas conferring greater malignant potential known to be the major precursor of early-onset colorectal cancer. An unpublished analysis of molecular markers by the study’s senior author, Yin Cao, ScD, MPH, also of Washington University, found that at least 57% of early-onset colorectal cancers developed from the conventional adenoma-carcinoma pathway.
The objective of the new study was to assess the association between regular aspirin or NSAID use at least twice weekly, with the risk of developing early-onset adenoma. The analysis is based on an evaluation of data from the Nurses’ Health Study II of 32,058 women who had at least one colonoscopy before age 50 (1991-2015). High-risk adenomas included those that were at least 1 cm with tubulovillous/villous histology or high-grade dysplasia, or the presence of at least three adenomas.
There were 1,247 early-onset adenomas, among which 290 were considered high risk. The risk of adenomas among patients who took aspirin or NSAIDs regularly for cardiovascular protection or for inflammatory conditions, was lower than in those who did not take aspirin and/or NSAIDs regularly. While the association was similar for high-risk vs. low-risk adenomas, the benefit was more pronounced for adenomas of tubulovillous/villous histology or with high-grade dysplasia (odds ratio, 0.67; 95% confidence interval, 0.51-0.89), a 33% reduction, compared with tubular adenomas (OR, 0.90; 95% CI, 0.79-1.0; P for heterogeneity = .02).
With later-onset adenomas, risk reduction was confined primarily to large (OR, 0.76; 95% CI, 0.62-0.93) or multiple adenomas (OR, 0.57; 95% CI, 0.40-0.83), but not adenomas of advanced histology (OR, 0.92; 95% CI, 0.73-1.17).
“With colorectal cancer rates increasing, we still don’t have any preventative strategies beyond screening. With this 15% reduction with aspirin/NSAIDS in early-onset adenoma – and particularly for the quite substantial 33% benefit in advanced adenoma with advanced histology, we need to think about a precision-based chemoprevention strategy for early-onset precursors of colorectal cancer,” Dr. Cao said.
The U.S. Preventive Services Task Force issued a new recommendation in 2021 stating that colorectal cancer screening for people with average risk should start 5 years sooner at age 45. “As we know,” Dr. Yin said, “many younger adults are not screened. That’s why we’re looking into potential early-onset colorectal cancer chemopreventative agents.”
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Dr. Fritz had no disclosures and Dr. Cao listed consulting for Geneoscopy.
CHICAGO – The authors say that aspirin could prove to be an effective strategy in preventing early-onset colorectal cancer cases.
“What we have here is a 15% reduction for all adenomas and 33% for those with advanced histology, which to us is quite substantial. We have not seen that much [33%] in previous studies so I would think it definitely needs more study,” said Cassandra D. Fritz, MD, MPHS, a gastroenterologist with Washington University, St. Louis, in an oral presentation given at the annual Digestive Disease Week®.
“This finding is important given the alarming rise in the incidence and mortality of early-onset colorectal cancer (age < 50 years), and our limited understanding of the underlying drivers to direct prevention efforts,” Dr. Fritz said. Early-onset colorectal cancer cases have doubled since 1995, she said.
The study confirms evidence from 30 years of research that suggests regular aspirin use reduces cancer risk. In patients with Lynch syndrome, the CAPP2 study showed that aspirin has a protective effect against colorectal cancer at 20 years follow-up.
While emerging data have suggested that aspirin use may reduce later-onset colorectal cancer, it was not known if regular aspirin and NSAID use are associated with diminished risk of early-onset conventional adenomas, and especially the high-risk adenomas conferring greater malignant potential known to be the major precursor of early-onset colorectal cancer. An unpublished analysis of molecular markers by the study’s senior author, Yin Cao, ScD, MPH, also of Washington University, found that at least 57% of early-onset colorectal cancers developed from the conventional adenoma-carcinoma pathway.
The objective of the new study was to assess the association between regular aspirin or NSAID use at least twice weekly, with the risk of developing early-onset adenoma. The analysis is based on an evaluation of data from the Nurses’ Health Study II of 32,058 women who had at least one colonoscopy before age 50 (1991-2015). High-risk adenomas included those that were at least 1 cm with tubulovillous/villous histology or high-grade dysplasia, or the presence of at least three adenomas.
There were 1,247 early-onset adenomas, among which 290 were considered high risk. The risk of adenomas among patients who took aspirin or NSAIDs regularly for cardiovascular protection or for inflammatory conditions, was lower than in those who did not take aspirin and/or NSAIDs regularly. While the association was similar for high-risk vs. low-risk adenomas, the benefit was more pronounced for adenomas of tubulovillous/villous histology or with high-grade dysplasia (odds ratio, 0.67; 95% confidence interval, 0.51-0.89), a 33% reduction, compared with tubular adenomas (OR, 0.90; 95% CI, 0.79-1.0; P for heterogeneity = .02).
With later-onset adenomas, risk reduction was confined primarily to large (OR, 0.76; 95% CI, 0.62-0.93) or multiple adenomas (OR, 0.57; 95% CI, 0.40-0.83), but not adenomas of advanced histology (OR, 0.92; 95% CI, 0.73-1.17).
“With colorectal cancer rates increasing, we still don’t have any preventative strategies beyond screening. With this 15% reduction with aspirin/NSAIDS in early-onset adenoma – and particularly for the quite substantial 33% benefit in advanced adenoma with advanced histology, we need to think about a precision-based chemoprevention strategy for early-onset precursors of colorectal cancer,” Dr. Cao said.
The U.S. Preventive Services Task Force issued a new recommendation in 2021 stating that colorectal cancer screening for people with average risk should start 5 years sooner at age 45. “As we know,” Dr. Yin said, “many younger adults are not screened. That’s why we’re looking into potential early-onset colorectal cancer chemopreventative agents.”
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Dr. Fritz had no disclosures and Dr. Cao listed consulting for Geneoscopy.
CHICAGO – The authors say that aspirin could prove to be an effective strategy in preventing early-onset colorectal cancer cases.
“What we have here is a 15% reduction for all adenomas and 33% for those with advanced histology, which to us is quite substantial. We have not seen that much [33%] in previous studies so I would think it definitely needs more study,” said Cassandra D. Fritz, MD, MPHS, a gastroenterologist with Washington University, St. Louis, in an oral presentation given at the annual Digestive Disease Week®.
“This finding is important given the alarming rise in the incidence and mortality of early-onset colorectal cancer (age < 50 years), and our limited understanding of the underlying drivers to direct prevention efforts,” Dr. Fritz said. Early-onset colorectal cancer cases have doubled since 1995, she said.
The study confirms evidence from 30 years of research that suggests regular aspirin use reduces cancer risk. In patients with Lynch syndrome, the CAPP2 study showed that aspirin has a protective effect against colorectal cancer at 20 years follow-up.
While emerging data have suggested that aspirin use may reduce later-onset colorectal cancer, it was not known if regular aspirin and NSAID use are associated with diminished risk of early-onset conventional adenomas, and especially the high-risk adenomas conferring greater malignant potential known to be the major precursor of early-onset colorectal cancer. An unpublished analysis of molecular markers by the study’s senior author, Yin Cao, ScD, MPH, also of Washington University, found that at least 57% of early-onset colorectal cancers developed from the conventional adenoma-carcinoma pathway.
The objective of the new study was to assess the association between regular aspirin or NSAID use at least twice weekly, with the risk of developing early-onset adenoma. The analysis is based on an evaluation of data from the Nurses’ Health Study II of 32,058 women who had at least one colonoscopy before age 50 (1991-2015). High-risk adenomas included those that were at least 1 cm with tubulovillous/villous histology or high-grade dysplasia, or the presence of at least three adenomas.
There were 1,247 early-onset adenomas, among which 290 were considered high risk. The risk of adenomas among patients who took aspirin or NSAIDs regularly for cardiovascular protection or for inflammatory conditions, was lower than in those who did not take aspirin and/or NSAIDs regularly. While the association was similar for high-risk vs. low-risk adenomas, the benefit was more pronounced for adenomas of tubulovillous/villous histology or with high-grade dysplasia (odds ratio, 0.67; 95% confidence interval, 0.51-0.89), a 33% reduction, compared with tubular adenomas (OR, 0.90; 95% CI, 0.79-1.0; P for heterogeneity = .02).
With later-onset adenomas, risk reduction was confined primarily to large (OR, 0.76; 95% CI, 0.62-0.93) or multiple adenomas (OR, 0.57; 95% CI, 0.40-0.83), but not adenomas of advanced histology (OR, 0.92; 95% CI, 0.73-1.17).
“With colorectal cancer rates increasing, we still don’t have any preventative strategies beyond screening. With this 15% reduction with aspirin/NSAIDS in early-onset adenoma – and particularly for the quite substantial 33% benefit in advanced adenoma with advanced histology, we need to think about a precision-based chemoprevention strategy for early-onset precursors of colorectal cancer,” Dr. Cao said.
The U.S. Preventive Services Task Force issued a new recommendation in 2021 stating that colorectal cancer screening for people with average risk should start 5 years sooner at age 45. “As we know,” Dr. Yin said, “many younger adults are not screened. That’s why we’re looking into potential early-onset colorectal cancer chemopreventative agents.”
DDW is sponsored by the American Association for the Study of Liver Diseases, the American Gastroenterological Association, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Dr. Fritz had no disclosures and Dr. Cao listed consulting for Geneoscopy.
AT DDW 2023
Itchy scaling rash
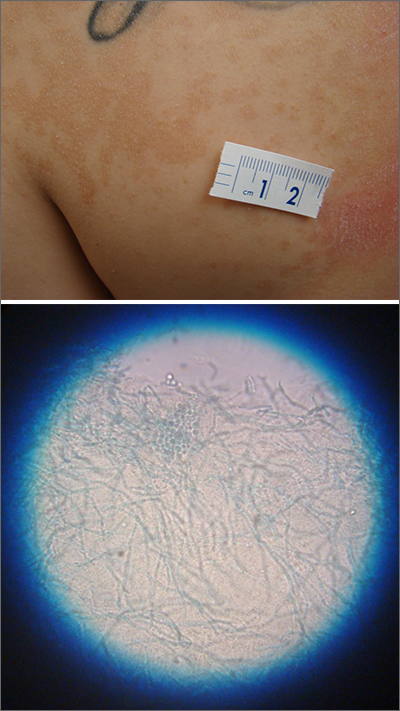
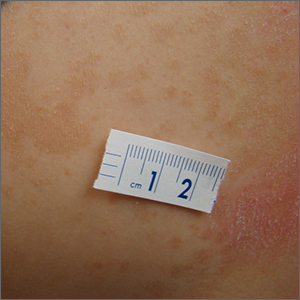
A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A waxing and waning rash with fine scale is classic for tinea versicolor (TV). A potassium hydroxide (KOH) prep with Swartz-Lamkins stain confirmed the presence of the spaghetti-and-meatballs pattern of Malassezia furfur (MF).
TV is a skin infection caused by M furfur. TF is notorious for the variety of colors that are seen clinically, including hyperpigmentation, as seen in a recent installment in this column.1 It can also appear as hypopigmented lesions or tan macules and patches with fine scale, as was seen in this patient. Hypopigmentation is often more pronounced on sun-exposed areas of the body. The MF produces azelaic acid. The azelaic acid blocks tyrosinase, which hinders melanocyte function and leads to hypopigmentation.2 As a result, areas of skin that are affected by TV do not tan as much as the surrounding skin, making the lesions more pronounced.
First line treatment of TV includes topical antifungal preparations, such as the “azoles” (eg, clotrimazole, ketoconazole, miconazole) twice daily for 2 to 4 weeks. However, the large surface areas involved would require a large amount of these antifungal preparations that come in relatively small tubes. Thus, for many years, clinicians have turned to economical over-the-counter dandruff shampoos with either selenium sulfide or zinc pyrithione that provide excellent results. These shampoos are applied to the entire trunk at full strength, allowed to dry, and then washed off later following various timed protocols. If topical therapy is not successful, or if there is a recurrence, systemic antifungal medications are used. Oral options include fluconazole 200 mg to 300 mg orally once a week for 2 weeks and itraconazole 200 mg orally once a day for 7 days.3 Ketoconazole is avoided as a systemic antifungal (except in life-threatening situations) due to its higher rate of liver dysfunction.
This patient was instructed to apply full-strength selenium sulfide shampoo to his entire trunk in the evening, allow it to dry, then wash it off the next morning and repeat in 1 week. An alternate regimen is to leave it on for 1 hour before washing and repeat daily for 1 week. At the patient’s follow-up appointment a month later, the rash and itching had resolved.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
1. Jasser J, Stulberg D. Teen with hyperpigmented skin lesions. J Fam Pract. 2022;71. Published December 2022. Accessed May 26, 2023. www.mdedge.com/familymedicine/article/260076/dermatology/teen-hyperpigmented-skin-lesions. doi: 10.12788/jfp.0529
2. Leung AK, Barankin B, Lam JM, et al. Tinea versicolor: an updated review. Drugs Context. 2022;11:2022-9-2. doi: 10.7573/dic.2022-9-2
3. Gupta AK, Foley KA. Antifungal treatment for pityriasis versicolor. J Fungi (Basel). 2015;1:13-29. doi: 10.3390/jof1010013
Internists in 2022: Increased earnings can’t stop rising dissatisfaction
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.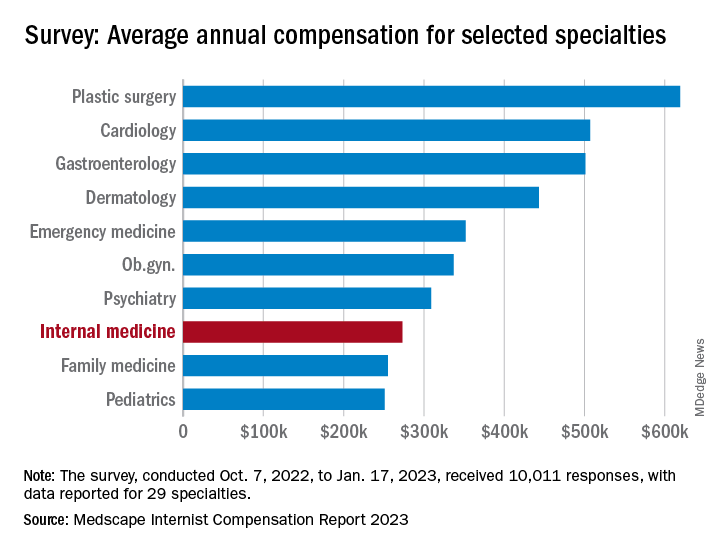
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.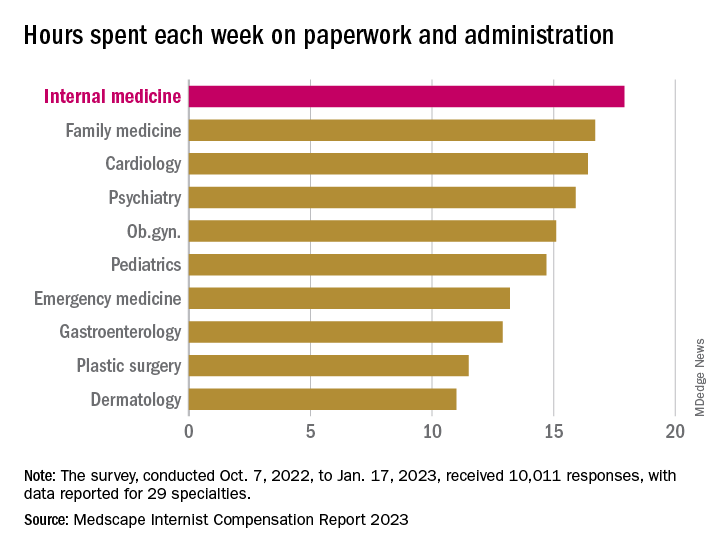
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Internists experienced many of the usual ups and downs regarding nonclinical matters in 2022: Compensation was up, but satisfaction with compensation was down; the percentage of internists who would choose another specialty was up and time spent on paperwork and administration was down only slightly.
A year that began with the COVID-19 Omicron surge ended with many of the same old issues regaining the attention of physicians, according to those who responded to Medscape’s annual compensation survey, which was conducted from Oct. 2, 2022, to Jan. 17, 2023.
“Decreasing Medicare reimbursement and poor payor mix destroy our income,” one physician wrote, and another said that “patients have become rude and come with poor information from social media.” One respondent described the situation this way: “Overwhelming burnout. I had to reduce my hours to keep myself from quitting medicine completely.”
For internists at least, some of the survey results were positive. For the 13% of the 10,011 respondents who practice internal medicine, average compensation went from $264,000 in 2021 to $273,000 in 2022, an increase of almost 4% that matched the average for all physicians. Among the other primary care specialists, pediatricians did almost as well with a 3% increase, but ob.gyns. and family physicians only managed to keep their 2022 earnings at 2021 levels.
Overall physician compensation for 2022 was $352,000, an increase of almost 18% since 2018. “Supply and demand is the biggest driver,” Mike Belkin, JD, of physician recruitment firm Merritt Hawkins, said in an interview. “Organizations understand it’s not getting any easier to get good candidates, and so for the most part, physicians are getting good offers.”
The latest increase in earnings among internists also included a decline: The disparity between mens’ and womens’ compensation dropped from 24% in 2021 to 16% in 2022. The gap was slightly larger for all physicians in 2022, with men earning about 19% more than women, and larger again among specialists at 27%, but both of those figures are lower than in recent years, Medscape said.
Satisfaction with their compensation, however, was not high for internists: Only 43% feel that they are fairly paid, coming in above only ophthalmology (42%) and infectious diseases (35%) and well below psychiatry (68%) at the top of the list, the Medscape data show. In the 2022 report, 49% of internists said that they had been fairly paid.
In another source of potential dissatisfaction, internist respondents reported spending an average of 17.9 hours each week on paperwork and administration, just below the survey leaders, physical medicine and rehabilitation (18.5 hours) and nephrology (18.1 hours) and well above anesthesiology, which was the lowest of the 29 specialties at 9.0 hours, and the 2022 average of 15.5 hours for all physicians, Medscape said. A small bright spot comes in the form of a decline from the internists’ time of 18.7 hours per week in 2021.
When asked if they would choose medicine again, 72% of internist respondents and 73% of all physicians said yes, with emergency medicine (65%) and dermatology (86%) representing the two extremes. A question about specialty choice showed internists to be the least likely of the 29 included specialties to follow the same path, with 61% (down from 63% in 2022) approving their initial selection, versus 97% for plastic surgeons, Medscape reported.
Commenters among the survey respondents were not identified by specialty, but dissatisfaction on many fronts was a definite theme:
- “Our costs go up, and our reimbursement does not.”
- “Our practice was acquired by venture capital firms; they slashed costs.”
- “My productivity bonus should have come to $45,000. Instead I was paid only $15,000. Yet cardiologists and administrators who were working from home part of the year received their full bonus.”
- “I will no longer practice cookbook mediocrity.”
Noncardiac mortality is not increased by revascularization in a meta-analysis: New data refute recent study
In response to a randomized trial that associated elective revascularization for ischemia with an increase in noncardiac mortality versus medical therapy alone, a meta-analysis with a far larger dataset challenges this assertion, suggesting the initial conclusion is due to a type 1 error.
, reports William Wijns, MD, PhD, professor of interventional cardiology, National University of Ireland, Galway.
The larger pool of data from the meta-analysis was considered compelling by several experts at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, where it was presented.
“I think these data will close once and forever this controversy,” said Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy).
Evidence for an unexpected increased risk of noncardiac mortality was drawn from the ISCHEMIA-EXTEND study, which was published earlier this year. Numerous prior studies comparing percutaneous intervention (PCI) to medical therapy for relief of ischemia had shown no such safety signal.
The ISCHEMIA-EXTEND study provided long-term follow up of patients enrolled in ISCHEMIA, a study that randomized patients with stable coronary disease and moderate or severe ischemia to PCI or a conservative approach. After 3.2 years of follow up, there was no reduction in risk of cardiovascular events or all-cause death. While this lack of benefit was a disappointing result from the perspective of interventional cardiology, there was also no increase in these risks.
In ISCHEMIA-EXTEND, the more than 5,000 patients originally randomized were followed for an additional 2.5 years (total 5.7 years). During this extended period, the estimated 7-year risk of cardiovascular mortality was 22% lower in the group randomized to PCI (hazard ratio, 0.78; 95% confidence interval, 0.63-0.96) but the noncardiac mortality was increased by 44% (HR, 1.44; 95% CI, 1.08-1.91). Because of the counterbalancing effects on survival, all-cause mortality was similar in the two groups.
The newly completed meta-analysis was undertaken to address this surprising result not least because the increased rates of noncardiac death did not have a plausible explanation, according to Dr. Wijns.
When the patients from the 18 randomized trials were compared, noncardiac death occurred in 4.68% of the 8,665 patients assigned to elective revascularization and in 4.17% of the 8,243 patients assigned to medical therapy alone at an average follow up of 5.7 years.
This difference was not significant overall (HR, 1.09; 95% CI, 0.94-1.26; P = .26) or after sensitivity analyses. For example, there was no difference (P = .52) between an invasive or conservative approach after controlling for length of follow up.
There was also no heterogeneity (I2 = 0%) among the studies when ISCHEMIA-EXTEND was excluded.
Absence of negative effect ‘is confirmed’
On the basis of a Bayesian meta-analysis designed to account for residual uncertainty (relative risk, 1.08, 95% CI, 0.90-1.30) and the consistency of results among all studies with the exception of ISCHEMIA-EXTEND (RR, 1.0; 95% CI, 0.84-1;18; P = .7), “the absence of a negative effect of revascularization on noncardiac death was confirmed,” Dr. Wijns reported.
Based on the preponderance of evidence assembled in this meta-analysis, the “noncardiac mortality excess risk observed following revascularization relative to medical therapy was confined to a single large trial and is likely due to a type 1 error,” Dr. Wijns reported. He noted that this study is “the first large-scale meta-analysis study designed to systematically evaluate potential differences in noncardiac mortality between treatment strategies for chronic coronary syndromes.”
Eliano P. Navarese, MD, PhD, an associate professor of interventional cardiology at Nicolaus Copernicus University, Bydgoszcz, Poland, was the lead author of this study and Dr. Wijns was a coinvestigator. The study was published simultaneously in the Journal of the American College of Cardiology at the time of the EuroPCR meeting.
In the late-breaking session where these data were presented, there was a general consensus among invited panelists that the data are convincing. For example, Michael Joner, MD, PhD, director of early clinical trials, German Heart Centre, Munich, agreed that these data “resolve the issue.”
Bernard de Bruyne, MD, PhD, an interventional cardiologist associated with the Cardiovascular Center Aalst, Kraainem, Belgium, also agreed that these data argue convincingly against the concern raised by publication of ISCHEMIA-EXTEND, but he added that this controversy has raised an important issue.
“We should always be reporting all-cause mortality, not just cardiovascular mortality, in our clinical trials,” he said, emphasizing that extending all-cause survival, not just preventing cardiovascular-related events, should be recognized as the goal of invasive strategies.
In an editorial accompanying the publication, Dr. Harvey D. White, MD, Te Whatu Ora-Health New Zealand, Auckland, writes similarly that the current findings, “alert us to the importance of adjudicating causes of death in clinical trials.
“The current trial-level meta-analysis may seem to dispel concerns about increases in noncardiac and cardiovascular deaths seen in some revascularization trials, but paradoxically, it has raised the need for more and careful analysis of causes of death,” Dr. White notes. He feels the signal of increased noncardiac or noncardiovascular death in ISCHEMIA EXTEND and the REVIVED trials is something “that we should pay attention to and explore the possibility that increased radiation doses with PCI may cause increased rates of cancer.”
Further study, including longer follow-up, other datasets, and quality of life data including cognitive function and “patient-focused outcomes such as day alive out of hospital,” is needed, he concludes.
Dr. Navarese has received research grants from Abbott and Amgen and lecture fees/honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron. Dr. Wijns reports financial relationships with Argonauts, Corrib Core Laboratory, and Rede Optimus Research. Dr. Capodanno reports financial relationships with Amgen, Daiichi Sankyo, and Sanofi. Dr. de Bruyne and Dr. Joner report financial relationships with multiple pharmaceutical and device manufacturers. Prof. White, as the John Neutze scholar, is supported by the Green Lane Research and Educational Fund. Prof. White has received grant support paid to the institution and fees for serving on steering committees of multiple trials sponsored by various companies.
In response to a randomized trial that associated elective revascularization for ischemia with an increase in noncardiac mortality versus medical therapy alone, a meta-analysis with a far larger dataset challenges this assertion, suggesting the initial conclusion is due to a type 1 error.
, reports William Wijns, MD, PhD, professor of interventional cardiology, National University of Ireland, Galway.
The larger pool of data from the meta-analysis was considered compelling by several experts at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, where it was presented.
“I think these data will close once and forever this controversy,” said Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy).
Evidence for an unexpected increased risk of noncardiac mortality was drawn from the ISCHEMIA-EXTEND study, which was published earlier this year. Numerous prior studies comparing percutaneous intervention (PCI) to medical therapy for relief of ischemia had shown no such safety signal.
The ISCHEMIA-EXTEND study provided long-term follow up of patients enrolled in ISCHEMIA, a study that randomized patients with stable coronary disease and moderate or severe ischemia to PCI or a conservative approach. After 3.2 years of follow up, there was no reduction in risk of cardiovascular events or all-cause death. While this lack of benefit was a disappointing result from the perspective of interventional cardiology, there was also no increase in these risks.
In ISCHEMIA-EXTEND, the more than 5,000 patients originally randomized were followed for an additional 2.5 years (total 5.7 years). During this extended period, the estimated 7-year risk of cardiovascular mortality was 22% lower in the group randomized to PCI (hazard ratio, 0.78; 95% confidence interval, 0.63-0.96) but the noncardiac mortality was increased by 44% (HR, 1.44; 95% CI, 1.08-1.91). Because of the counterbalancing effects on survival, all-cause mortality was similar in the two groups.
The newly completed meta-analysis was undertaken to address this surprising result not least because the increased rates of noncardiac death did not have a plausible explanation, according to Dr. Wijns.
When the patients from the 18 randomized trials were compared, noncardiac death occurred in 4.68% of the 8,665 patients assigned to elective revascularization and in 4.17% of the 8,243 patients assigned to medical therapy alone at an average follow up of 5.7 years.
This difference was not significant overall (HR, 1.09; 95% CI, 0.94-1.26; P = .26) or after sensitivity analyses. For example, there was no difference (P = .52) between an invasive or conservative approach after controlling for length of follow up.
There was also no heterogeneity (I2 = 0%) among the studies when ISCHEMIA-EXTEND was excluded.
Absence of negative effect ‘is confirmed’
On the basis of a Bayesian meta-analysis designed to account for residual uncertainty (relative risk, 1.08, 95% CI, 0.90-1.30) and the consistency of results among all studies with the exception of ISCHEMIA-EXTEND (RR, 1.0; 95% CI, 0.84-1;18; P = .7), “the absence of a negative effect of revascularization on noncardiac death was confirmed,” Dr. Wijns reported.
Based on the preponderance of evidence assembled in this meta-analysis, the “noncardiac mortality excess risk observed following revascularization relative to medical therapy was confined to a single large trial and is likely due to a type 1 error,” Dr. Wijns reported. He noted that this study is “the first large-scale meta-analysis study designed to systematically evaluate potential differences in noncardiac mortality between treatment strategies for chronic coronary syndromes.”
Eliano P. Navarese, MD, PhD, an associate professor of interventional cardiology at Nicolaus Copernicus University, Bydgoszcz, Poland, was the lead author of this study and Dr. Wijns was a coinvestigator. The study was published simultaneously in the Journal of the American College of Cardiology at the time of the EuroPCR meeting.
In the late-breaking session where these data were presented, there was a general consensus among invited panelists that the data are convincing. For example, Michael Joner, MD, PhD, director of early clinical trials, German Heart Centre, Munich, agreed that these data “resolve the issue.”
Bernard de Bruyne, MD, PhD, an interventional cardiologist associated with the Cardiovascular Center Aalst, Kraainem, Belgium, also agreed that these data argue convincingly against the concern raised by publication of ISCHEMIA-EXTEND, but he added that this controversy has raised an important issue.
“We should always be reporting all-cause mortality, not just cardiovascular mortality, in our clinical trials,” he said, emphasizing that extending all-cause survival, not just preventing cardiovascular-related events, should be recognized as the goal of invasive strategies.
In an editorial accompanying the publication, Dr. Harvey D. White, MD, Te Whatu Ora-Health New Zealand, Auckland, writes similarly that the current findings, “alert us to the importance of adjudicating causes of death in clinical trials.
“The current trial-level meta-analysis may seem to dispel concerns about increases in noncardiac and cardiovascular deaths seen in some revascularization trials, but paradoxically, it has raised the need for more and careful analysis of causes of death,” Dr. White notes. He feels the signal of increased noncardiac or noncardiovascular death in ISCHEMIA EXTEND and the REVIVED trials is something “that we should pay attention to and explore the possibility that increased radiation doses with PCI may cause increased rates of cancer.”
Further study, including longer follow-up, other datasets, and quality of life data including cognitive function and “patient-focused outcomes such as day alive out of hospital,” is needed, he concludes.
Dr. Navarese has received research grants from Abbott and Amgen and lecture fees/honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron. Dr. Wijns reports financial relationships with Argonauts, Corrib Core Laboratory, and Rede Optimus Research. Dr. Capodanno reports financial relationships with Amgen, Daiichi Sankyo, and Sanofi. Dr. de Bruyne and Dr. Joner report financial relationships with multiple pharmaceutical and device manufacturers. Prof. White, as the John Neutze scholar, is supported by the Green Lane Research and Educational Fund. Prof. White has received grant support paid to the institution and fees for serving on steering committees of multiple trials sponsored by various companies.
In response to a randomized trial that associated elective revascularization for ischemia with an increase in noncardiac mortality versus medical therapy alone, a meta-analysis with a far larger dataset challenges this assertion, suggesting the initial conclusion is due to a type 1 error.
, reports William Wijns, MD, PhD, professor of interventional cardiology, National University of Ireland, Galway.
The larger pool of data from the meta-analysis was considered compelling by several experts at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, where it was presented.
“I think these data will close once and forever this controversy,” said Davide Capodanno, MD, PhD, a professor of cardiology and interventional cardiologist at the University of Catania (Italy).
Evidence for an unexpected increased risk of noncardiac mortality was drawn from the ISCHEMIA-EXTEND study, which was published earlier this year. Numerous prior studies comparing percutaneous intervention (PCI) to medical therapy for relief of ischemia had shown no such safety signal.
The ISCHEMIA-EXTEND study provided long-term follow up of patients enrolled in ISCHEMIA, a study that randomized patients with stable coronary disease and moderate or severe ischemia to PCI or a conservative approach. After 3.2 years of follow up, there was no reduction in risk of cardiovascular events or all-cause death. While this lack of benefit was a disappointing result from the perspective of interventional cardiology, there was also no increase in these risks.
In ISCHEMIA-EXTEND, the more than 5,000 patients originally randomized were followed for an additional 2.5 years (total 5.7 years). During this extended period, the estimated 7-year risk of cardiovascular mortality was 22% lower in the group randomized to PCI (hazard ratio, 0.78; 95% confidence interval, 0.63-0.96) but the noncardiac mortality was increased by 44% (HR, 1.44; 95% CI, 1.08-1.91). Because of the counterbalancing effects on survival, all-cause mortality was similar in the two groups.
The newly completed meta-analysis was undertaken to address this surprising result not least because the increased rates of noncardiac death did not have a plausible explanation, according to Dr. Wijns.
When the patients from the 18 randomized trials were compared, noncardiac death occurred in 4.68% of the 8,665 patients assigned to elective revascularization and in 4.17% of the 8,243 patients assigned to medical therapy alone at an average follow up of 5.7 years.
This difference was not significant overall (HR, 1.09; 95% CI, 0.94-1.26; P = .26) or after sensitivity analyses. For example, there was no difference (P = .52) between an invasive or conservative approach after controlling for length of follow up.
There was also no heterogeneity (I2 = 0%) among the studies when ISCHEMIA-EXTEND was excluded.
Absence of negative effect ‘is confirmed’
On the basis of a Bayesian meta-analysis designed to account for residual uncertainty (relative risk, 1.08, 95% CI, 0.90-1.30) and the consistency of results among all studies with the exception of ISCHEMIA-EXTEND (RR, 1.0; 95% CI, 0.84-1;18; P = .7), “the absence of a negative effect of revascularization on noncardiac death was confirmed,” Dr. Wijns reported.
Based on the preponderance of evidence assembled in this meta-analysis, the “noncardiac mortality excess risk observed following revascularization relative to medical therapy was confined to a single large trial and is likely due to a type 1 error,” Dr. Wijns reported. He noted that this study is “the first large-scale meta-analysis study designed to systematically evaluate potential differences in noncardiac mortality between treatment strategies for chronic coronary syndromes.”
Eliano P. Navarese, MD, PhD, an associate professor of interventional cardiology at Nicolaus Copernicus University, Bydgoszcz, Poland, was the lead author of this study and Dr. Wijns was a coinvestigator. The study was published simultaneously in the Journal of the American College of Cardiology at the time of the EuroPCR meeting.
In the late-breaking session where these data were presented, there was a general consensus among invited panelists that the data are convincing. For example, Michael Joner, MD, PhD, director of early clinical trials, German Heart Centre, Munich, agreed that these data “resolve the issue.”
Bernard de Bruyne, MD, PhD, an interventional cardiologist associated with the Cardiovascular Center Aalst, Kraainem, Belgium, also agreed that these data argue convincingly against the concern raised by publication of ISCHEMIA-EXTEND, but he added that this controversy has raised an important issue.
“We should always be reporting all-cause mortality, not just cardiovascular mortality, in our clinical trials,” he said, emphasizing that extending all-cause survival, not just preventing cardiovascular-related events, should be recognized as the goal of invasive strategies.
In an editorial accompanying the publication, Dr. Harvey D. White, MD, Te Whatu Ora-Health New Zealand, Auckland, writes similarly that the current findings, “alert us to the importance of adjudicating causes of death in clinical trials.
“The current trial-level meta-analysis may seem to dispel concerns about increases in noncardiac and cardiovascular deaths seen in some revascularization trials, but paradoxically, it has raised the need for more and careful analysis of causes of death,” Dr. White notes. He feels the signal of increased noncardiac or noncardiovascular death in ISCHEMIA EXTEND and the REVIVED trials is something “that we should pay attention to and explore the possibility that increased radiation doses with PCI may cause increased rates of cancer.”
Further study, including longer follow-up, other datasets, and quality of life data including cognitive function and “patient-focused outcomes such as day alive out of hospital,” is needed, he concludes.
Dr. Navarese has received research grants from Abbott and Amgen and lecture fees/honoraria from Amgen, AstraZeneca, Bayer, Pfizer, and Sanofi-Regeneron. Dr. Wijns reports financial relationships with Argonauts, Corrib Core Laboratory, and Rede Optimus Research. Dr. Capodanno reports financial relationships with Amgen, Daiichi Sankyo, and Sanofi. Dr. de Bruyne and Dr. Joner report financial relationships with multiple pharmaceutical and device manufacturers. Prof. White, as the John Neutze scholar, is supported by the Green Lane Research and Educational Fund. Prof. White has received grant support paid to the institution and fees for serving on steering committees of multiple trials sponsored by various companies.
FROM EUROPCR 2023
FDA approves new drug, sotagliflozin, for heart failure
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Sotagliflozin, a novel agent that inhibits sodium-glucose cotransporter 1 as well as SGLT2, has received marketing approval from the Food and Drug Administration for reducing the risk for cardiovascular death, hospitalization for heart failure, and urgent heart failure visits in patients with heart failure, and also for preventing these same events in patients with type 2 diabetes, chronic kidney disease (CKD), and other cardiovascular disease risk factors.
This puts sotagliflozin in direct competition with two SGLT2 inhibitors, dapagliflozin (Farxiga) and empagliflozin (Jardiance), that already have indications for preventing heart failure hospitalizations in patients with heart failure as well as approvals for type 2 diabetes and preservation of renal function.
Officials at Lexicon Pharmaceuticals, the company that developed and will market sotagliflozin under the trade name Inpefa, said in a press release that they expect U.S. sales of the agent to begin before the end of June 2023. The release also highlighted that the approval broadly covered use in patients with heart failure across the full range of both reduced and preserved left ventricular ejection fractions.
They base this niche target for sotagliflozin on results from the SOLOIST-WHF trial, which randomized 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure and showed a significant 33% reduction in the rate of deaths from cardiovascular causes and hospitalizations and urgent visits for heart failure, compared with control patients during a median 9 months of follow-up. Nearly half of the enrolled patients received their first dose while still hospitalized, while the other half received their first dose a median of 2 days after hospital discharge. The drug appeared safe.
Cutting heart failure rehospitalizations in half
An exploratory post hoc analysis of SOLOIST-WHF showed that treatment with sotagliflozin cut the rate of rehospitalizations roughly in half after both 30 and 90 days compared with control patients, according to an abstract presented at the 2022 annual scientific sessions of the AHA that has not yet been published in a peer-reviewed journal.
The only SGLT2 inhibitor tested so far when initiated in patients during hospitalization for heart failure is empagliflozin, in the EMPULSE trial, which randomized 530 patients. EMPULSE also showed that starting an SGLT2 inhibitor in this setting was safe and resulted in significant clinical benefit, the study’s primary endpoint, defined as a composite of death from any cause, number of heart failure events, and time to first heart failure event, or a 5-point or greater difference in change from baseline in the Kansas City Cardiomyopathy Questionnaire Total Symptom Score at 90 days.
In the DELIVER trial, which tested dapagliflozin in patients with heart failure with preserved ejection fraction, roughly 10% of patients started study treatment during or within 30 days of heart failure hospitalization, and in this subgroup, dapagliflozin appeared as effective as it was in the other 90% of patients who did not start the drug during an acute or subacute phase.
Despite the SOLOIST-WHF evidence for sotagliflozin’s safety and efficacy in this economically important clinical setting, some experts say the drug faces an uphill path as it contends for market share against two solidly established, albeit dramatically underused, SGLT2 inhibitors. (Recent data document that 20% or fewer of U.S. patients eligible for treatment with an SGLT2 inhibitor receive it, such as a review of 49,000 patients hospitalized during 2021-2022 with heart failure with reduced ejection fraction.)
Others foresee a clear role for sotagliflozin, particularly because of additional facets of the drug’s performance in trials that they perceive give it an edge over dapagliflozin and empagliflozin. This includes evidence that sotagliflozin treatment uniquely (within the SGLT2 inhibitor class) cuts the rate of strokes and myocardial infarctions, as well as evidence of its apparent ability to lower hemoglobin A1c levels in patients with type 2 diabetes and with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a property likely linked to inhibition of SGLT1 in the gut that dampens intestinal glucose absorption.
Sotagliflozin uptake ‘will be a challenge’
“It will be a challenge” for sotagliflozin uptake, given the head start that both dapagliflozin and empagliflozin have had as well-documented agents for patients with heart failure, commented Javed Butler, MD, a heart failure clinician and trialist who is president of the Baylor Scott & White Research Institute in Dallas.
Given the position dapagliflozin and empagliflozin currently have in U.S. heart failure management – with the SGLT2 inhibitor class called out in guidelines as foundational for treating patients with heart failure with reduced ejection fraction and likely soon for heart failure with preserved ejection fraction as well – “I can’t imagine [sotagliflozin] will be considered a preferred option,” Dr. Butler said in an interview.
Another expert was even more dismissive of sotagliflozin’s role.
“There is no persuasive evidence that sotagliflozin has any advantages, compared with the SGLT2 inhibitors, for the treatment of heart failure,” said Milton Packer, MD, a heart failure specialist and trialist at Baylor University Medical Center, Dallas. “I do not see why U.S. physicians might pivot from established SGLT2 inhibitors to sotagliflozin,” unless it was priced “at a very meaningful discount to available SGLT2 inhibitors.”
At the time it announced the FDA’s approval, Lexicon did not provide details on how it would price sotagliflozin. Existing retail prices for dapagliflozin and empagliflozin run about $550-$600/month, a price point that has contributed to slow U.S. uptake of the drug class. But experts anticipate a dramatic shake-up of the U.S. market for SGLT2 inhibitors with expected introduction of a generic SGLT2 inhibitor formulation by 2025, a development that could further dampen sotagliflozin’s prospects.
Other experts are more optimistic about the new agent’s uptake, perhaps none more than Deepak L. Bhatt, MD, MPH, who led both pivotal trials that provide the bulk of sotagliflozin’s evidence package.
In addition to SOLOIST-WHF, Dr. Bhatt also headed the SCORED trial, with 10,584 patients with type 2 diabetes, CKD, and risks for cardiovascular disease randomized to sotagliflozin or placebo and followed for a median of 16 months. The primary result showed that sotagliflozin treatment cut the combined rate of deaths from cardiovascular causes, hospitalizations for heart failure, and urgent visits for heart failure by a significant 26% relative to control patients.
A clear MACE benefit
“The data from SOLOIST-WHF and SCORED look at least as good as the data for the SGLT2 inhibitors for heart failure, and what appears to be different are the rates for MI and stroke in SCORED,” said Dr. Bhatt, director of Mount Sinai Heart, New York.
“I believe the rate of major adverse cardiovascular events [MACE] were reduced [in SCORED], and this is different from the SGLT2 inhibitors,” he said in an interview.
In 2022, Dr. Bhatt reported results from a prespecified secondary analysis of SCORED that showed that treatment with sotagliflozin cut the rate of MACE by a significant 21%-26%, compared with placebo. This finding was, in part, driven by the first data to show a substantial benefit from an SGLT inhibitor on stroke rates.
And while SCORED did not report a significant benefit for slowing progression of CKD, subsequent post hoc analyses have suggested this advantage also in as-yet-unpublished findings, Dr. Bhatt added.
But he said he doubted nephrologists will see it as a first-line agent for slowing CKD progression – an indication already held by dapagliflozin, pending for empagliflozin, and also in place for a third SGLT2 inhibitor, canagliflozin (Invokana) – because sotagliflozin lacks clear significant and prespecified evidence for this effect.
Dr. Bhatt also acknowledged the limitation of sotagliflozin compared with the SGLT2 inhibitors as an agent for glucose control, again because of no evidence for this effect from a prospective analysis and no pending indication for type 2 diabetes treatment. But the SCORED data showed a clear A1c benefit, even in patients with severely reduced renal function.
Mostly for cardiologists? ‘Compelling’ reductions in MIs and strokes
That may mean sotagliflozin “won’t get much use by endocrinologists nor by primary care physicians,” commented Carol L. Wysham, MD, an endocrinologist with MultiCare in Spokane, Wash.
Sotagliflozin “will be a cardiology drug,” and will “have a hard time” competing with the SGLT2 inhibitors, she predicted.
Dr. Bhatt agreed that sotagliflozin “will be perceived as a drug for cardiologists to prescribe. I don’t see endocrinologists, nephrologists, and primary care physicians reaching for this drug if it has a heart failure label.” But, he added, “my hope is that the company files for additional indications. It deserves an indication for glycemic control.”
The evidence for a heart failure benefit from sotagliflozin is “valid and compelling,” and “having this option is great,” commented Mikhail N. Kosiborod, MD, a cardiologist, vice president of research at Saint Luke’s Health System, and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. But, he added, “it will be a reasonably tall task for sotagliflozin to come from behind and be disruptive in a space where there are already two well-established SGLT2 inhibitors” approved for preventing heart failure hospitalizations, “with a lot of data to back them up,”
The feature that sets sotagliflozin apart from the approved SGLT2 inhibitors is the “really compelling decrease” it produced in rates of MIs and strokes “that we simply do not see with SGLT2 inhibitors,” Dr. Kosiborod said in an interview.
He also cited results from SCORED that suggest “a meaningful reduction in A1c” when indirectly compared with SGLT2 inhibitors, especially in patients with more severe CKD. The lack of a dedicated A1c-lowering trial or an approved type 2 diabetes indication “will not be a problem for cardiologists,” he predicted, but also agreed that it is less likely to be used by primary care physicians in low-risk patients.
“I can see myself prescribing sotagliflozin,” said Dr. Kosiborod, a SCORED coinvestigator, especially for patients with coexisting type 2 diabetes, heart failure, CKD, and atherosclerotic cardiovascular disease. These patients may get “more bang for the buck” because of a reduced risk for MI and stroke, making sotagliflozin “a solid consideration in these patients if the economic factors align.”
Like others, Dr. Kosiborod cited the big impact pricing will have, especially if, as expected, a generic SGLT2 inhibitor soon comes onto the U.S. market. “Access and affordability are very important.”
SOLOIST-WHF and SCORED were sponsored initially by Sanofi and later by Lexicon after Sanofi pulled out of sotagliflozin development. Dr. Butler has been a consultant for Lexicon as well as for AstraZeneca (which markets Farxiga), Boehringer Ingelheim and Lilly (which jointly market Jardiance), and Janssen (which markets Invokana), as well as for numerous other companies. Dr. Packer has been a consultant for AstraZeneca, Boehringer Ingelheim, Lilly, and numerous other companies. Dr. Bhatt was lead investigator for SOLOIST-WHF and SCORED and has been an adviser for Boehringer Ingelheim and Janssen and numerous other companies. Dr. Wysham has been an adviser, speaker, and consultant for AstraZeneca, Boehringer Ingelheim, Lilly, Janssen, Novo Nordisk, and Sanofi, an adviser for Abbott, and a speaker for Insulet. Dr. Kosiborod was a member of the SCORED Steering Committee and has been a consultant for Lexicon, AstraZeneca, Boehringer Ingelheim, Janssen, Lilly, Novo Nordisk, and numerous other companies.
A version of this article first appeared on Medscape.com.
Standard measure may underestimate OSA in Black patients
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
FROM ATS 2023
States move to curb insurers’ prior authorization requirements as federal reforms lag
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Common fracture risk predictors often fail for women of any race
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study published in JAMA Internal Medicine.
One of the screenings, the U.S. Fracture Risk Assessment Tool (FRAX), proved relatively ineffective at identifying women who developed osteoporosis. The other screening, the Osteoporosis Self-Assessment Tool (OST), excelled at identifying osteoporosis for women in every racial and ethnic group, but also failed at identifying who was most likely to experience a fracture. Osteoporosis experts say that primary care physicians should test for the condition in anyone with any risk factor for it, even if a screening tool suggests doing so is unnecessary.
The United States Preventive Services Task Force (USPSTF) recommends routine testing of bone mineral density in women age 65 years and older to detect risk of developing osteoporosis, which in turn leads to an increased risk for fractures of the hip, spine, shoulder, or forearm. For women aged 50-64, whether bone mineral density accurately reflects who will develop osteoporosis is less clear. In this age range, the USPSTF recommends using either FRAX or OST rather than routine bone mineral density tests.
“I have the utmost respect for the United States Preventive Services Task Force, which lists both of these as valid screening tools for younger postmenopausal women. What I hope this study does is to inform the next iteration of the screening guidelines,” by maintaining the recommendation to use the OST while not keeping FRAX, said Carolyn J. Crandall, MD, MS, an internal medicine physician and health services researcher at University of California, Los Angeles, who helped conduct the research.
The U.S. version of FRAX requires identifying someone’s race, height, and weight, then answering whether they have different risk factors for a fracture such as a previous fracture, rheumatoid arthritis, or smoking. The result was thought to indicate a cumulative risk for major fracture over the next 10 years. Patients at significant risk should then undergo a bone density test.
The tool can also incorporate information about bone mineral density, if available, but the FRAX analyses in Dr. Crandall’s study did not include those data because the study aimed to test the measure’s predictive ability in the absence of a bone scan.
The OST includes only two variables – weight and age – to calculate risk for osteoporosis, and generally takes seconds to complete. It does not include race. As with FRAX, anyone deemed at significant risk for developing osteoporosis should undergo a bone density test.
“OST is really simple; that makes it very appealing,” Dr. Crandall said. “OST could probably be automatically calculated in the electronic medical record.”
Using data from the Women’s Health Initiative, Dr. Crandall and colleagues tracked more than 67,000 women aged 50-64 years for 10 years following enrollment in the study to see who experienced a fracture or developed osteoporosis over that decade. The investigators found that neither FRAX nor OST was particularly good at predicting who went on to experience a fracture.
The accuracy of FRAX at fracture prediction peaked at 65% for Asian women (area under the receiver operating curve, 0.65; 95% confidence interval, 0.58-0.71), and was lowest for Black women (AUC 0.55; 95% CI, 0.52-0.59). OST also was most accurate for Asian women, but only up to 62% (AUC 0.62; 95% CI, 0.56-0.69), and was again lowest for Black women (AUC 0.53; 95% CI, 0.50 - 0.57)
“It is just very hard to predict fractures in this age group,” Dr. Crandall said, noting that more evidence exists about risk for fracture in people older than 65.
The story diverges with predicting risk of osteoporosis in the neck. The OST did this roughly 80% of the time, for all racial groups. That figure proved better than FRAX, without including race.
Treatment gap
“This evidence supports using OST instead of FRAX” for selecting younger postmenopausal women who should undergo a bone mineral density exam, said E. Michael Lewiecki, MD, director of the New Mexico Clinical Research & Osteoporosis Center in Albuquerque.

Dr. Lewiecki, who was not involved in the new study, noted that the U.S. version of FRAX specifies race because of some clinical evidence that different races have different rates of fracture. But he and Dr. Crandall said the validity of race-based algorithms to guide clinical care is a controversial and evolving topic in medicine. Dr. Lewiecki said the Canadian version of FRAX, which is similarly applied to a diverse population as in the United States, omits race and works as well as the U.S. version. Future iterations of the instrument in the United States may not include race, Dr. Lewiecki said.
“The study is perfectly valid as far as it goes. But the big gorilla in the room is that most patients who need a bone density test are not getting it,” Dr. Lewiecki added. Sometimes a patient might break a bone in their wrist, for example, and tell their primary care provider that anyone would have broken that bone because the fall was so hard. Even if that’s true, Dr. Lewiecki said, any woman older than 45 who has broken a bone should undergo a bone density test to determine if they have osteoporosis, even if it seems like there are other possible reasons for why the break occurred.
“Most of the clinical practice guidelines that are used by physicians recommend getting a bone density test in postmenopausal women under the age of 65 who have a risk factor for fracture,” Dr. Lewiecki said, with a primary risk factor being a prior fracture. Dr. Lewiecki said he would rather that anyone who could benefit from a bone density test receive it, rather than someone foregoing a scan based on a screening tool that may be flawed.
“Most patients – men and women – who have osteoporosis are currently not being identified. Even when they are being identified, they are commonly not being treated. And when they are started on treatment, many patients discontinue treatment before they’ve taken it long enough to benefit,” Dr. Lewiecki said.
Dr. Crandall and Dr. Lewiecki report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE