User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
United Healthcare ditches prior authorization in favor of new policy
Instead, the giant health insurer will adopt an “advance notification” program for nonscreening and nonemergent gastrointestinal procedures.
The company has not made any changes to their policy regarding screening colonoscopies for preventive care, and the advance notification policy does not impact screening colonoscopies.
UHC alerted physicians to changes to the program yesterday, including updated notices on UHCProvider.com with a new Frequently Asked Questions document.
The advance notification program “will not result in the denial of care for clinical reasons or for failure to notify and will help educate physicians who are not following clinical best practices. Provider groups who do not submit advance notification during this period will not be eligible for the United Healthcare Gold Card program,” a spokesperson for the company said.
The previously announced Gold Card program, which is scheduled to start in early 2024, would eliminate prior authorization requirements for providers that meet certain eligibility requirements.
The American Gastroenterological Association remains “extremely concerned” that UHC’s advance notification program is a “temporary patch” likely to have significant repercussions for patient access. The organization says the program only temporarily postpones prior authorization requirements set to impact the insurer’s 27.4 million commercial beneficiaries while increasing the administrative burden on clinicians.
The AGA called the program “nebulous” and “poorly defined.” It would ostensibly require physicians to input “copious” amounts of highly complex and granular patient data prior to performing colonoscopies and endoscopies, the AGA says.
AGA President Barbara H. Jung, MD, AGAF, said UHC’s “slap-dash approach to rolling out a policy that will ultimately control patient access to critical, often life-saving, medical procedures flies in the face of common sense and responsible medical practice.”
“It also indicates that UHC does not currently have data that show any significant overutilization of critical endoscopy and colonoscopy procedures that would ostensibly justify this program or prior authorization. UHC is not acting in good faith, and its actions will compromise patient access to potentially lifesaving procedures,” Dr. Jung added.
Recent data show 62% of high-risk patients in the United States who had polyps removed had evidence of delayed or no use of surveillance colonoscopies after 10 years.
“If other prior authorization requirements imposed on patients for specialty care are any indication, we expect to see negative patient outcomes with an enormous cost to patient well-being and physician resources,” AGA Vice President Lawrence Kim, MD, wrote in a news release.
“Given the high percentage of eventual approvals by insurers mandating prior authorization, we anticipate there will be little to no benefit from this prior authorization requirement. When utilized this way, it becomes a nonsensical and harmful policy,” Dr. Kim added.
AGA says it will continue to work closely with its members to assess the full impact of the new requirements and urges UHC to make endoscopy procedures more accessible to patients.
A recent American Medical Association survey on prior authorization found that one-third (33%) of doctors said the insurance barrier has led to a serious adverse event such as hospitalization, permanent disability, or death for a patient in their care. Nearly half (46%) of physicians reported that prior authorization has led to immediate care and/or emergency department visits.
In a 2023 survey of AGA membership, conducted before UHC announced its proposed prior authorization policy, 95% of respondents said prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes. And 84% said the burdens associated with prior authorization policies have increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
A version of this article first appeared on Medscape.com.
Instead, the giant health insurer will adopt an “advance notification” program for nonscreening and nonemergent gastrointestinal procedures.
The company has not made any changes to their policy regarding screening colonoscopies for preventive care, and the advance notification policy does not impact screening colonoscopies.
UHC alerted physicians to changes to the program yesterday, including updated notices on UHCProvider.com with a new Frequently Asked Questions document.
The advance notification program “will not result in the denial of care for clinical reasons or for failure to notify and will help educate physicians who are not following clinical best practices. Provider groups who do not submit advance notification during this period will not be eligible for the United Healthcare Gold Card program,” a spokesperson for the company said.
The previously announced Gold Card program, which is scheduled to start in early 2024, would eliminate prior authorization requirements for providers that meet certain eligibility requirements.
The American Gastroenterological Association remains “extremely concerned” that UHC’s advance notification program is a “temporary patch” likely to have significant repercussions for patient access. The organization says the program only temporarily postpones prior authorization requirements set to impact the insurer’s 27.4 million commercial beneficiaries while increasing the administrative burden on clinicians.
The AGA called the program “nebulous” and “poorly defined.” It would ostensibly require physicians to input “copious” amounts of highly complex and granular patient data prior to performing colonoscopies and endoscopies, the AGA says.
AGA President Barbara H. Jung, MD, AGAF, said UHC’s “slap-dash approach to rolling out a policy that will ultimately control patient access to critical, often life-saving, medical procedures flies in the face of common sense and responsible medical practice.”
“It also indicates that UHC does not currently have data that show any significant overutilization of critical endoscopy and colonoscopy procedures that would ostensibly justify this program or prior authorization. UHC is not acting in good faith, and its actions will compromise patient access to potentially lifesaving procedures,” Dr. Jung added.
Recent data show 62% of high-risk patients in the United States who had polyps removed had evidence of delayed or no use of surveillance colonoscopies after 10 years.
“If other prior authorization requirements imposed on patients for specialty care are any indication, we expect to see negative patient outcomes with an enormous cost to patient well-being and physician resources,” AGA Vice President Lawrence Kim, MD, wrote in a news release.
“Given the high percentage of eventual approvals by insurers mandating prior authorization, we anticipate there will be little to no benefit from this prior authorization requirement. When utilized this way, it becomes a nonsensical and harmful policy,” Dr. Kim added.
AGA says it will continue to work closely with its members to assess the full impact of the new requirements and urges UHC to make endoscopy procedures more accessible to patients.
A recent American Medical Association survey on prior authorization found that one-third (33%) of doctors said the insurance barrier has led to a serious adverse event such as hospitalization, permanent disability, or death for a patient in their care. Nearly half (46%) of physicians reported that prior authorization has led to immediate care and/or emergency department visits.
In a 2023 survey of AGA membership, conducted before UHC announced its proposed prior authorization policy, 95% of respondents said prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes. And 84% said the burdens associated with prior authorization policies have increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
A version of this article first appeared on Medscape.com.
Instead, the giant health insurer will adopt an “advance notification” program for nonscreening and nonemergent gastrointestinal procedures.
The company has not made any changes to their policy regarding screening colonoscopies for preventive care, and the advance notification policy does not impact screening colonoscopies.
UHC alerted physicians to changes to the program yesterday, including updated notices on UHCProvider.com with a new Frequently Asked Questions document.
The advance notification program “will not result in the denial of care for clinical reasons or for failure to notify and will help educate physicians who are not following clinical best practices. Provider groups who do not submit advance notification during this period will not be eligible for the United Healthcare Gold Card program,” a spokesperson for the company said.
The previously announced Gold Card program, which is scheduled to start in early 2024, would eliminate prior authorization requirements for providers that meet certain eligibility requirements.
The American Gastroenterological Association remains “extremely concerned” that UHC’s advance notification program is a “temporary patch” likely to have significant repercussions for patient access. The organization says the program only temporarily postpones prior authorization requirements set to impact the insurer’s 27.4 million commercial beneficiaries while increasing the administrative burden on clinicians.
The AGA called the program “nebulous” and “poorly defined.” It would ostensibly require physicians to input “copious” amounts of highly complex and granular patient data prior to performing colonoscopies and endoscopies, the AGA says.
AGA President Barbara H. Jung, MD, AGAF, said UHC’s “slap-dash approach to rolling out a policy that will ultimately control patient access to critical, often life-saving, medical procedures flies in the face of common sense and responsible medical practice.”
“It also indicates that UHC does not currently have data that show any significant overutilization of critical endoscopy and colonoscopy procedures that would ostensibly justify this program or prior authorization. UHC is not acting in good faith, and its actions will compromise patient access to potentially lifesaving procedures,” Dr. Jung added.
Recent data show 62% of high-risk patients in the United States who had polyps removed had evidence of delayed or no use of surveillance colonoscopies after 10 years.
“If other prior authorization requirements imposed on patients for specialty care are any indication, we expect to see negative patient outcomes with an enormous cost to patient well-being and physician resources,” AGA Vice President Lawrence Kim, MD, wrote in a news release.
“Given the high percentage of eventual approvals by insurers mandating prior authorization, we anticipate there will be little to no benefit from this prior authorization requirement. When utilized this way, it becomes a nonsensical and harmful policy,” Dr. Kim added.
AGA says it will continue to work closely with its members to assess the full impact of the new requirements and urges UHC to make endoscopy procedures more accessible to patients.
A recent American Medical Association survey on prior authorization found that one-third (33%) of doctors said the insurance barrier has led to a serious adverse event such as hospitalization, permanent disability, or death for a patient in their care. Nearly half (46%) of physicians reported that prior authorization has led to immediate care and/or emergency department visits.
In a 2023 survey of AGA membership, conducted before UHC announced its proposed prior authorization policy, 95% of respondents said prior authorization restrictions have impacted patient access to clinically appropriate treatments and patient clinical outcomes. And 84% said the burdens associated with prior authorization policies have increased “significantly” (60%) or “somewhat” (24%) over the last 5 years.
A version of this article first appeared on Medscape.com.
Preventive antipyretics, antibiotics not needed in stroke
“The results of PRECIOUS do not support preventive use of antiemetic, antipyretic, or antibiotic drugs in older patients with acute stroke,” senior study author Bart van der Worp, MD, professor of acute neurology at University Medical Center, Utrecht, the Netherlands, concluded.
“This trial was all about prevention,” trial co-investigator, Philip Bath, MD, professor of stroke medicine at the University of Nottingham (England), said in an interview.
“It was trying to improve outcomes by preventing infection, fever, and aspiration pneumonia, but the message from these results is that while we should be on the lookout for these complications and treat them early when they occur, we don’t need to give these medications on a prophylactic basis.”
The PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke (PRECIOUS) trial was presented at the annual European Stroke Organisation Conference, held in Munich.
Dr. Van der Worp explained that infections, fever, and aspiration pneumonia frequently occur following stroke, particularly in older patients, and these poststroke complications are associated with an increased risk of death and poor functional outcome.
“We assessed whether a pharmacological strategy to reduce the risk of infections and fever improves outcomes of elderly patients with moderately severe or severe stroke,” he said.
Previous studies looking at this approach have been performed in broad populations of stroke patients who had a relatively low risk of poststroke complications, thereby reducing the potential for benefit from these interventions.
The current PRECIOUS trial was therefore conducted in a more selective elderly population with more severe strokes, a group believed to be at higher risk of infection and fever.
The trial included patients aged 66 years or older with moderately severe to severe ischemic stroke (National Institutes of Health Stroke Scale score ≥ 6) or intracerebral hemorrhage.
They were randomized in a 3 x 2 factorial design to oral, rectal, or intravenous metoclopramide (10 mg three times a day); intravenous ceftriaxone (2,000 mg once daily); oral, rectal, or intravenous paracetamol (1,000 mg four times daily); or usual care.
Medications were started within 24 hours after symptom onset and continued for 4 days or until complete recovery or discharge from hospital, if earlier.
“We assessed these three simple, safe, and inexpensive therapies – paracetamol to prevent fever; the antiemetic, metoclopramide, to prevent aspiration; and ceftriaxone, which is the preferred antibiotic for post-stroke pneumonia in the Netherlands,” Dr. van der Worp said.
The primary outcome was modified Rankin Scale (mRS) score at 90 days.
The trial was aiming to enroll 3,800 patients from 67 European sites but was stopped after 1,493 patients had been included because of lack of funding. After excluding patients who withdrew consent or were lost to follow-up, 1,471 patients were included in the intention-to-treat analysis.
Results showed no effect on the primary outcome of any of the prophylactic treatments.
“None of the medications had any effect on the functional outcome at 90 days. This was a surprise to me,” Dr. Van der Worp commented. “I had expected that at least one of the medications would have been of benefit.”
A secondary outcome was the diagnosis of pneumonia, which again was not reduced by any of the medications.
“Remarkably, neither ceftriaxone nor metoclopramide had any effect on the risk of developing pneumonia. It was all quite disappointing,” van der Worp said.
There was, however, a reduction in the incidence of urinary tract infections in the ceftriaxone group.
Trying to explain why there was a reduction in urinary tract infections but not pneumonia with the antibiotic, Dr. Van der Worp pointed out that poststroke pneumonia is to a large extent caused by a mechanical process (aspiration), and bacteria may only play a minor role in its development.
He said he was therefore surprised that metoclopramide, which should prevent the mechanical process of aspiration, did not reduce the development of pneumonia.
He suggested that some patients may have already experienced aspiration before the metoclopramide was started, noting that many patients with acute stroke already have signs of pneumonia on CT scan in the first few hours after symptom onset.
A previous smaller study (MAPS) had shown a lower rate of pneumonia in stroke patients given metoclopramide, but in this study the drug was given for 3 weeks.
Discussing the PRECIOUS trial at the ESOC meeting, Christine Roffe, MD, professor of stroke medicine at Keele (England) University, and senior investigator of the MAPS study, suggested that a longer period of metoclopramide treatment may be needed than the 4 days given in the PRECIOUS study, as the risk of pneumonia persists for longer than just a few days.
She noted that another trial (MAPS-2) is now underway in the United Kingdom to try and confirm the first MAPS result with longer duration metoclopramide.
Dr. Van der Worp responded: “Certainly, I think that the MAPS-2 study should be continued. It is investigating a much longer duration of treatment, which may be beneficial, especially in patients with more severe strokes.”
On the reason for the disappointing results with paracetamol, Dr. Van der Worp elaborated: “We found that only a very few of these older patients developed a fever – only about 5% in the control group. Paracetamol did reduce the risk of fever, but because the proportion of patients who developed fever was so small, this may have been why it didn’t translate into any effect on the functional outcome.”
Dr. Roffe concluded that PRECIOUS was an important study. “There is also a positive message here. We have all been worried about using too many antibiotics. We need to make sure we use these drugs responsibly. I think this trial has told us there is little point in using antibiotics in a preventative way in these patients.”
She added that although the trial was stopped prematurely, it had produced decisive results.
“Yes, I believe that even if the trial was much larger, we still would not have shown an effect,” Dr. Van der Worp agreed.
A version of this article first appeared on Medscape.com.
“The results of PRECIOUS do not support preventive use of antiemetic, antipyretic, or antibiotic drugs in older patients with acute stroke,” senior study author Bart van der Worp, MD, professor of acute neurology at University Medical Center, Utrecht, the Netherlands, concluded.
“This trial was all about prevention,” trial co-investigator, Philip Bath, MD, professor of stroke medicine at the University of Nottingham (England), said in an interview.
“It was trying to improve outcomes by preventing infection, fever, and aspiration pneumonia, but the message from these results is that while we should be on the lookout for these complications and treat them early when they occur, we don’t need to give these medications on a prophylactic basis.”
The PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke (PRECIOUS) trial was presented at the annual European Stroke Organisation Conference, held in Munich.
Dr. Van der Worp explained that infections, fever, and aspiration pneumonia frequently occur following stroke, particularly in older patients, and these poststroke complications are associated with an increased risk of death and poor functional outcome.
“We assessed whether a pharmacological strategy to reduce the risk of infections and fever improves outcomes of elderly patients with moderately severe or severe stroke,” he said.
Previous studies looking at this approach have been performed in broad populations of stroke patients who had a relatively low risk of poststroke complications, thereby reducing the potential for benefit from these interventions.
The current PRECIOUS trial was therefore conducted in a more selective elderly population with more severe strokes, a group believed to be at higher risk of infection and fever.
The trial included patients aged 66 years or older with moderately severe to severe ischemic stroke (National Institutes of Health Stroke Scale score ≥ 6) or intracerebral hemorrhage.
They were randomized in a 3 x 2 factorial design to oral, rectal, or intravenous metoclopramide (10 mg three times a day); intravenous ceftriaxone (2,000 mg once daily); oral, rectal, or intravenous paracetamol (1,000 mg four times daily); or usual care.
Medications were started within 24 hours after symptom onset and continued for 4 days or until complete recovery or discharge from hospital, if earlier.
“We assessed these three simple, safe, and inexpensive therapies – paracetamol to prevent fever; the antiemetic, metoclopramide, to prevent aspiration; and ceftriaxone, which is the preferred antibiotic for post-stroke pneumonia in the Netherlands,” Dr. van der Worp said.
The primary outcome was modified Rankin Scale (mRS) score at 90 days.
The trial was aiming to enroll 3,800 patients from 67 European sites but was stopped after 1,493 patients had been included because of lack of funding. After excluding patients who withdrew consent or were lost to follow-up, 1,471 patients were included in the intention-to-treat analysis.
Results showed no effect on the primary outcome of any of the prophylactic treatments.
“None of the medications had any effect on the functional outcome at 90 days. This was a surprise to me,” Dr. Van der Worp commented. “I had expected that at least one of the medications would have been of benefit.”
A secondary outcome was the diagnosis of pneumonia, which again was not reduced by any of the medications.
“Remarkably, neither ceftriaxone nor metoclopramide had any effect on the risk of developing pneumonia. It was all quite disappointing,” van der Worp said.
There was, however, a reduction in the incidence of urinary tract infections in the ceftriaxone group.
Trying to explain why there was a reduction in urinary tract infections but not pneumonia with the antibiotic, Dr. Van der Worp pointed out that poststroke pneumonia is to a large extent caused by a mechanical process (aspiration), and bacteria may only play a minor role in its development.
He said he was therefore surprised that metoclopramide, which should prevent the mechanical process of aspiration, did not reduce the development of pneumonia.
He suggested that some patients may have already experienced aspiration before the metoclopramide was started, noting that many patients with acute stroke already have signs of pneumonia on CT scan in the first few hours after symptom onset.
A previous smaller study (MAPS) had shown a lower rate of pneumonia in stroke patients given metoclopramide, but in this study the drug was given for 3 weeks.
Discussing the PRECIOUS trial at the ESOC meeting, Christine Roffe, MD, professor of stroke medicine at Keele (England) University, and senior investigator of the MAPS study, suggested that a longer period of metoclopramide treatment may be needed than the 4 days given in the PRECIOUS study, as the risk of pneumonia persists for longer than just a few days.
She noted that another trial (MAPS-2) is now underway in the United Kingdom to try and confirm the first MAPS result with longer duration metoclopramide.
Dr. Van der Worp responded: “Certainly, I think that the MAPS-2 study should be continued. It is investigating a much longer duration of treatment, which may be beneficial, especially in patients with more severe strokes.”
On the reason for the disappointing results with paracetamol, Dr. Van der Worp elaborated: “We found that only a very few of these older patients developed a fever – only about 5% in the control group. Paracetamol did reduce the risk of fever, but because the proportion of patients who developed fever was so small, this may have been why it didn’t translate into any effect on the functional outcome.”
Dr. Roffe concluded that PRECIOUS was an important study. “There is also a positive message here. We have all been worried about using too many antibiotics. We need to make sure we use these drugs responsibly. I think this trial has told us there is little point in using antibiotics in a preventative way in these patients.”
She added that although the trial was stopped prematurely, it had produced decisive results.
“Yes, I believe that even if the trial was much larger, we still would not have shown an effect,” Dr. Van der Worp agreed.
A version of this article first appeared on Medscape.com.
“The results of PRECIOUS do not support preventive use of antiemetic, antipyretic, or antibiotic drugs in older patients with acute stroke,” senior study author Bart van der Worp, MD, professor of acute neurology at University Medical Center, Utrecht, the Netherlands, concluded.
“This trial was all about prevention,” trial co-investigator, Philip Bath, MD, professor of stroke medicine at the University of Nottingham (England), said in an interview.
“It was trying to improve outcomes by preventing infection, fever, and aspiration pneumonia, but the message from these results is that while we should be on the lookout for these complications and treat them early when they occur, we don’t need to give these medications on a prophylactic basis.”
The PREvention of Complications to Improve OUtcome in elderly patients with acute Stroke (PRECIOUS) trial was presented at the annual European Stroke Organisation Conference, held in Munich.
Dr. Van der Worp explained that infections, fever, and aspiration pneumonia frequently occur following stroke, particularly in older patients, and these poststroke complications are associated with an increased risk of death and poor functional outcome.
“We assessed whether a pharmacological strategy to reduce the risk of infections and fever improves outcomes of elderly patients with moderately severe or severe stroke,” he said.
Previous studies looking at this approach have been performed in broad populations of stroke patients who had a relatively low risk of poststroke complications, thereby reducing the potential for benefit from these interventions.
The current PRECIOUS trial was therefore conducted in a more selective elderly population with more severe strokes, a group believed to be at higher risk of infection and fever.
The trial included patients aged 66 years or older with moderately severe to severe ischemic stroke (National Institutes of Health Stroke Scale score ≥ 6) or intracerebral hemorrhage.
They were randomized in a 3 x 2 factorial design to oral, rectal, or intravenous metoclopramide (10 mg three times a day); intravenous ceftriaxone (2,000 mg once daily); oral, rectal, or intravenous paracetamol (1,000 mg four times daily); or usual care.
Medications were started within 24 hours after symptom onset and continued for 4 days or until complete recovery or discharge from hospital, if earlier.
“We assessed these three simple, safe, and inexpensive therapies – paracetamol to prevent fever; the antiemetic, metoclopramide, to prevent aspiration; and ceftriaxone, which is the preferred antibiotic for post-stroke pneumonia in the Netherlands,” Dr. van der Worp said.
The primary outcome was modified Rankin Scale (mRS) score at 90 days.
The trial was aiming to enroll 3,800 patients from 67 European sites but was stopped after 1,493 patients had been included because of lack of funding. After excluding patients who withdrew consent or were lost to follow-up, 1,471 patients were included in the intention-to-treat analysis.
Results showed no effect on the primary outcome of any of the prophylactic treatments.
“None of the medications had any effect on the functional outcome at 90 days. This was a surprise to me,” Dr. Van der Worp commented. “I had expected that at least one of the medications would have been of benefit.”
A secondary outcome was the diagnosis of pneumonia, which again was not reduced by any of the medications.
“Remarkably, neither ceftriaxone nor metoclopramide had any effect on the risk of developing pneumonia. It was all quite disappointing,” van der Worp said.
There was, however, a reduction in the incidence of urinary tract infections in the ceftriaxone group.
Trying to explain why there was a reduction in urinary tract infections but not pneumonia with the antibiotic, Dr. Van der Worp pointed out that poststroke pneumonia is to a large extent caused by a mechanical process (aspiration), and bacteria may only play a minor role in its development.
He said he was therefore surprised that metoclopramide, which should prevent the mechanical process of aspiration, did not reduce the development of pneumonia.
He suggested that some patients may have already experienced aspiration before the metoclopramide was started, noting that many patients with acute stroke already have signs of pneumonia on CT scan in the first few hours after symptom onset.
A previous smaller study (MAPS) had shown a lower rate of pneumonia in stroke patients given metoclopramide, but in this study the drug was given for 3 weeks.
Discussing the PRECIOUS trial at the ESOC meeting, Christine Roffe, MD, professor of stroke medicine at Keele (England) University, and senior investigator of the MAPS study, suggested that a longer period of metoclopramide treatment may be needed than the 4 days given in the PRECIOUS study, as the risk of pneumonia persists for longer than just a few days.
She noted that another trial (MAPS-2) is now underway in the United Kingdom to try and confirm the first MAPS result with longer duration metoclopramide.
Dr. Van der Worp responded: “Certainly, I think that the MAPS-2 study should be continued. It is investigating a much longer duration of treatment, which may be beneficial, especially in patients with more severe strokes.”
On the reason for the disappointing results with paracetamol, Dr. Van der Worp elaborated: “We found that only a very few of these older patients developed a fever – only about 5% in the control group. Paracetamol did reduce the risk of fever, but because the proportion of patients who developed fever was so small, this may have been why it didn’t translate into any effect on the functional outcome.”
Dr. Roffe concluded that PRECIOUS was an important study. “There is also a positive message here. We have all been worried about using too many antibiotics. We need to make sure we use these drugs responsibly. I think this trial has told us there is little point in using antibiotics in a preventative way in these patients.”
She added that although the trial was stopped prematurely, it had produced decisive results.
“Yes, I believe that even if the trial was much larger, we still would not have shown an effect,” Dr. Van der Worp agreed.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Tenecteplase late after stroke misses endpoint: TIMELESS
However, there were some encouraging trends, and there did not appear to be an increase in intracranial hemorrhage (ICH), leading to hope that the option of late thrombolysis in this group of patients may still have potential.
The TIMELESS study tested the approach of giving thrombolysis with tenecteplase (TNK) to patients with a large-vessel occlusion stroke up to 24 hours after symptom onset. Patients were selected by perfusion imaging, and those who had a stroke with a small core and large amount of salvageable brain tissue were included in the placebo-controlled study.
“This is first trial to try giving a thrombolytic so late – up to 24 hours after last known well. While we did not meet the primary outcome, there were some promising findings,” lead author, Gregory Albers, MD, director of the Stanford (Calif.) Stroke Center and professor of neurology at Stanford University, said in an interview.
“The most encouraging observation was that we did not show any safety issues with giving TNK to this population at such a late time. Many people thought this would be too high risk but there was no increase in ICH, which was very low and the same in both groups,” Dr. Albers said.
“And we saw some evidence of drug effect. There appeared to be a benefit in patients with M1 occlusions, the most common type of large-vessel occlusion, who represented half the patients in the study,” he added.
The researchers also gained information on the logistics and timing of TNK administration in this late period which they hope can guide the design of a future trial.
Dr. Albers presented the TIMELESS trial at the annual European Stroke Organisation Conference.
He explained that there is increasing evidence that intravenous thrombolysis can improve outcome in selected patients even beyond the traditional 4.5-hour time window.
The phase 3, double-blind, randomized, placebo-controlled TIMELESS study sought to investigate whether tenecteplase administered to patients with ischemic stroke with large-vessel occlusion presenting between 4.5 and 24 hours after last known well would improve clinical outcome as measured by modified Rankin Scale (mRS) at day 90.
The trial included 458 patients with an internal carotid artery occlusion or middle cerebral artery segment 1 or 2 occlusion and presenting with salvageable tissue on imaging. They were randomly assigned 1:1 to either intravenous tenecteplase (0.25 mg/kg; maximum, 25 mg) or placebo.
The proportion of patients treated with mechanical thrombectomy were similar between the two treatment arms (around 77%). The study completion rate was higher than 96% in both treatment arms.
The primary endpoint analyses showed no significant difference in the odds of a lower mRS score at day 90, but there was a slight trend toward benefit in the TNK group in the shift analysis, with a common odds ratio of 1.13 (95% confidence interval, 0.81-1.56; P = .48).
The percentage of patients achieving a favorable outcome, defined as an mRS of 0-2, was not significantly different between the treatment groups: 46% in the TNK group versus 42% in the placebo group (nominal P = .41).
Promising safety data
There were no significant safety issues, and the risk for bleeding was not significantly increased in the tenecteplase group. Symptomatic ICH occurred in 3.2% of the TNK group versus 2.3% of the placebo group, a nonsignificant difference.
“The low rate of ICH with TNK at this late time point is very reassuring,” Dr. Albers said. “We believe the reason for the low ICH rate is probably because these patients were selected for small core strokes. We also found that there was a trend towards the most benefit from TNK in patients with the smallest cores, supporting the use of imaging to select patients.”
The secondary endpoint of complete recanalization at 24-hours post randomization was higher in the TNK group at 76.7%, compared with 63.9% in the placebo group (P = .006).
Benefit in M1 occlusions?
Subgroup analysis showed that there appeared to be a benefit of TNK in the 227 patients included who had an M1 occlusion. In this group, the common odds ratio for a more favorable outcome in the mRS shift analysis with TNK was 1.59 (95% CI, 1.00-2.52; adjusted nominal P = .051).
The percentage of patients with a favorable outcome (mRS, 0-2) at 90 days in the M1 occlusion subgroup was 45.9% for TNK versus 31.4% for placebo, giving an adjusted odds ratio of 2.03 (95% CI, 1.14-3.66; nominal P = .017).
But Dr. Albers cautioned that this was an exploratory analysis, and no formal conclusions should be drawn from these data.
“We saw very strong results in favor of giving thrombolysis in the patients with M1 occlusions. We had preliminary pilot data suggesting this approach may work in these patients,” he commented.
“But we included the smaller M2 occlusions as well, because we thought that as there should be less clot in an M2 occlusion it might be easier to dissolve with thrombolysis,” he added. “But surprisingly, the M2 occlusion patients seemed to do worse with TNK than placebo, and the M1 patients did better.”
Timing of TNK
Dr. Albers said that there was also information from in the study on the timing of TNK administration.
In patients who also received thrombectomy, who made up of the majority of those in the study, the average time of TNK administration was only 20 minutes before the thrombectomy procedure.
“We had hoped to have a longer time between thrombolysis and thrombectomy so the drug would have more time to work. The idea was that patients would be given TNK at the primary stroke center before being transferred for thrombectomy, but actually only a few patients received TNK at the primary stroke center,” Dr. Albers explained.
“But, again surprisingly, we found that patients given TNK right at the time of the thrombectomy procedure seemed to show a trend toward benefit over placebo,” he reported.
He suggested that this may be caused by the thrombolytic dissolving the small fragments that can sometimes break off and cause further occlusions when the clot is removed by thrombectomy.
“We have learned a lot from this study, and we are planning to go forward with the information gained to plan a second study, in which we will focus on patients with M1 occlusions and try to get the drug on board at primary stroke centers, so it has more time to work before thrombectomy,” he added.
Commenting on the TIMELESS study at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that he thought the trial had shown three important results: “Firstly, TNK appeared to be safe in this late window in these selected patients – that is a very important observation. Secondly, reperfusion rates at 24 hours were increased with TNK and we know that this translates into clinical benefit. And thirdly, there was a neutral effect on primary outcomes, but I think the sample size of 438 patients was not large enough to show efficacy.”
Dr. Tsivgoulis concluded that these points need to be addressed in future trials.
The TIMELESS trial was funded by Genentech.
A version of this article first appeared on Medscape.com.
However, there were some encouraging trends, and there did not appear to be an increase in intracranial hemorrhage (ICH), leading to hope that the option of late thrombolysis in this group of patients may still have potential.
The TIMELESS study tested the approach of giving thrombolysis with tenecteplase (TNK) to patients with a large-vessel occlusion stroke up to 24 hours after symptom onset. Patients were selected by perfusion imaging, and those who had a stroke with a small core and large amount of salvageable brain tissue were included in the placebo-controlled study.
“This is first trial to try giving a thrombolytic so late – up to 24 hours after last known well. While we did not meet the primary outcome, there were some promising findings,” lead author, Gregory Albers, MD, director of the Stanford (Calif.) Stroke Center and professor of neurology at Stanford University, said in an interview.
“The most encouraging observation was that we did not show any safety issues with giving TNK to this population at such a late time. Many people thought this would be too high risk but there was no increase in ICH, which was very low and the same in both groups,” Dr. Albers said.
“And we saw some evidence of drug effect. There appeared to be a benefit in patients with M1 occlusions, the most common type of large-vessel occlusion, who represented half the patients in the study,” he added.
The researchers also gained information on the logistics and timing of TNK administration in this late period which they hope can guide the design of a future trial.
Dr. Albers presented the TIMELESS trial at the annual European Stroke Organisation Conference.
He explained that there is increasing evidence that intravenous thrombolysis can improve outcome in selected patients even beyond the traditional 4.5-hour time window.
The phase 3, double-blind, randomized, placebo-controlled TIMELESS study sought to investigate whether tenecteplase administered to patients with ischemic stroke with large-vessel occlusion presenting between 4.5 and 24 hours after last known well would improve clinical outcome as measured by modified Rankin Scale (mRS) at day 90.
The trial included 458 patients with an internal carotid artery occlusion or middle cerebral artery segment 1 or 2 occlusion and presenting with salvageable tissue on imaging. They were randomly assigned 1:1 to either intravenous tenecteplase (0.25 mg/kg; maximum, 25 mg) or placebo.
The proportion of patients treated with mechanical thrombectomy were similar between the two treatment arms (around 77%). The study completion rate was higher than 96% in both treatment arms.
The primary endpoint analyses showed no significant difference in the odds of a lower mRS score at day 90, but there was a slight trend toward benefit in the TNK group in the shift analysis, with a common odds ratio of 1.13 (95% confidence interval, 0.81-1.56; P = .48).
The percentage of patients achieving a favorable outcome, defined as an mRS of 0-2, was not significantly different between the treatment groups: 46% in the TNK group versus 42% in the placebo group (nominal P = .41).
Promising safety data
There were no significant safety issues, and the risk for bleeding was not significantly increased in the tenecteplase group. Symptomatic ICH occurred in 3.2% of the TNK group versus 2.3% of the placebo group, a nonsignificant difference.
“The low rate of ICH with TNK at this late time point is very reassuring,” Dr. Albers said. “We believe the reason for the low ICH rate is probably because these patients were selected for small core strokes. We also found that there was a trend towards the most benefit from TNK in patients with the smallest cores, supporting the use of imaging to select patients.”
The secondary endpoint of complete recanalization at 24-hours post randomization was higher in the TNK group at 76.7%, compared with 63.9% in the placebo group (P = .006).
Benefit in M1 occlusions?
Subgroup analysis showed that there appeared to be a benefit of TNK in the 227 patients included who had an M1 occlusion. In this group, the common odds ratio for a more favorable outcome in the mRS shift analysis with TNK was 1.59 (95% CI, 1.00-2.52; adjusted nominal P = .051).
The percentage of patients with a favorable outcome (mRS, 0-2) at 90 days in the M1 occlusion subgroup was 45.9% for TNK versus 31.4% for placebo, giving an adjusted odds ratio of 2.03 (95% CI, 1.14-3.66; nominal P = .017).
But Dr. Albers cautioned that this was an exploratory analysis, and no formal conclusions should be drawn from these data.
“We saw very strong results in favor of giving thrombolysis in the patients with M1 occlusions. We had preliminary pilot data suggesting this approach may work in these patients,” he commented.
“But we included the smaller M2 occlusions as well, because we thought that as there should be less clot in an M2 occlusion it might be easier to dissolve with thrombolysis,” he added. “But surprisingly, the M2 occlusion patients seemed to do worse with TNK than placebo, and the M1 patients did better.”
Timing of TNK
Dr. Albers said that there was also information from in the study on the timing of TNK administration.
In patients who also received thrombectomy, who made up of the majority of those in the study, the average time of TNK administration was only 20 minutes before the thrombectomy procedure.
“We had hoped to have a longer time between thrombolysis and thrombectomy so the drug would have more time to work. The idea was that patients would be given TNK at the primary stroke center before being transferred for thrombectomy, but actually only a few patients received TNK at the primary stroke center,” Dr. Albers explained.
“But, again surprisingly, we found that patients given TNK right at the time of the thrombectomy procedure seemed to show a trend toward benefit over placebo,” he reported.
He suggested that this may be caused by the thrombolytic dissolving the small fragments that can sometimes break off and cause further occlusions when the clot is removed by thrombectomy.
“We have learned a lot from this study, and we are planning to go forward with the information gained to plan a second study, in which we will focus on patients with M1 occlusions and try to get the drug on board at primary stroke centers, so it has more time to work before thrombectomy,” he added.
Commenting on the TIMELESS study at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that he thought the trial had shown three important results: “Firstly, TNK appeared to be safe in this late window in these selected patients – that is a very important observation. Secondly, reperfusion rates at 24 hours were increased with TNK and we know that this translates into clinical benefit. And thirdly, there was a neutral effect on primary outcomes, but I think the sample size of 438 patients was not large enough to show efficacy.”
Dr. Tsivgoulis concluded that these points need to be addressed in future trials.
The TIMELESS trial was funded by Genentech.
A version of this article first appeared on Medscape.com.
However, there were some encouraging trends, and there did not appear to be an increase in intracranial hemorrhage (ICH), leading to hope that the option of late thrombolysis in this group of patients may still have potential.
The TIMELESS study tested the approach of giving thrombolysis with tenecteplase (TNK) to patients with a large-vessel occlusion stroke up to 24 hours after symptom onset. Patients were selected by perfusion imaging, and those who had a stroke with a small core and large amount of salvageable brain tissue were included in the placebo-controlled study.
“This is first trial to try giving a thrombolytic so late – up to 24 hours after last known well. While we did not meet the primary outcome, there were some promising findings,” lead author, Gregory Albers, MD, director of the Stanford (Calif.) Stroke Center and professor of neurology at Stanford University, said in an interview.
“The most encouraging observation was that we did not show any safety issues with giving TNK to this population at such a late time. Many people thought this would be too high risk but there was no increase in ICH, which was very low and the same in both groups,” Dr. Albers said.
“And we saw some evidence of drug effect. There appeared to be a benefit in patients with M1 occlusions, the most common type of large-vessel occlusion, who represented half the patients in the study,” he added.
The researchers also gained information on the logistics and timing of TNK administration in this late period which they hope can guide the design of a future trial.
Dr. Albers presented the TIMELESS trial at the annual European Stroke Organisation Conference.
He explained that there is increasing evidence that intravenous thrombolysis can improve outcome in selected patients even beyond the traditional 4.5-hour time window.
The phase 3, double-blind, randomized, placebo-controlled TIMELESS study sought to investigate whether tenecteplase administered to patients with ischemic stroke with large-vessel occlusion presenting between 4.5 and 24 hours after last known well would improve clinical outcome as measured by modified Rankin Scale (mRS) at day 90.
The trial included 458 patients with an internal carotid artery occlusion or middle cerebral artery segment 1 or 2 occlusion and presenting with salvageable tissue on imaging. They were randomly assigned 1:1 to either intravenous tenecteplase (0.25 mg/kg; maximum, 25 mg) or placebo.
The proportion of patients treated with mechanical thrombectomy were similar between the two treatment arms (around 77%). The study completion rate was higher than 96% in both treatment arms.
The primary endpoint analyses showed no significant difference in the odds of a lower mRS score at day 90, but there was a slight trend toward benefit in the TNK group in the shift analysis, with a common odds ratio of 1.13 (95% confidence interval, 0.81-1.56; P = .48).
The percentage of patients achieving a favorable outcome, defined as an mRS of 0-2, was not significantly different between the treatment groups: 46% in the TNK group versus 42% in the placebo group (nominal P = .41).
Promising safety data
There were no significant safety issues, and the risk for bleeding was not significantly increased in the tenecteplase group. Symptomatic ICH occurred in 3.2% of the TNK group versus 2.3% of the placebo group, a nonsignificant difference.
“The low rate of ICH with TNK at this late time point is very reassuring,” Dr. Albers said. “We believe the reason for the low ICH rate is probably because these patients were selected for small core strokes. We also found that there was a trend towards the most benefit from TNK in patients with the smallest cores, supporting the use of imaging to select patients.”
The secondary endpoint of complete recanalization at 24-hours post randomization was higher in the TNK group at 76.7%, compared with 63.9% in the placebo group (P = .006).
Benefit in M1 occlusions?
Subgroup analysis showed that there appeared to be a benefit of TNK in the 227 patients included who had an M1 occlusion. In this group, the common odds ratio for a more favorable outcome in the mRS shift analysis with TNK was 1.59 (95% CI, 1.00-2.52; adjusted nominal P = .051).
The percentage of patients with a favorable outcome (mRS, 0-2) at 90 days in the M1 occlusion subgroup was 45.9% for TNK versus 31.4% for placebo, giving an adjusted odds ratio of 2.03 (95% CI, 1.14-3.66; nominal P = .017).
But Dr. Albers cautioned that this was an exploratory analysis, and no formal conclusions should be drawn from these data.
“We saw very strong results in favor of giving thrombolysis in the patients with M1 occlusions. We had preliminary pilot data suggesting this approach may work in these patients,” he commented.
“But we included the smaller M2 occlusions as well, because we thought that as there should be less clot in an M2 occlusion it might be easier to dissolve with thrombolysis,” he added. “But surprisingly, the M2 occlusion patients seemed to do worse with TNK than placebo, and the M1 patients did better.”
Timing of TNK
Dr. Albers said that there was also information from in the study on the timing of TNK administration.
In patients who also received thrombectomy, who made up of the majority of those in the study, the average time of TNK administration was only 20 minutes before the thrombectomy procedure.
“We had hoped to have a longer time between thrombolysis and thrombectomy so the drug would have more time to work. The idea was that patients would be given TNK at the primary stroke center before being transferred for thrombectomy, but actually only a few patients received TNK at the primary stroke center,” Dr. Albers explained.
“But, again surprisingly, we found that patients given TNK right at the time of the thrombectomy procedure seemed to show a trend toward benefit over placebo,” he reported.
He suggested that this may be caused by the thrombolytic dissolving the small fragments that can sometimes break off and cause further occlusions when the clot is removed by thrombectomy.
“We have learned a lot from this study, and we are planning to go forward with the information gained to plan a second study, in which we will focus on patients with M1 occlusions and try to get the drug on board at primary stroke centers, so it has more time to work before thrombectomy,” he added.
Commenting on the TIMELESS study at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that he thought the trial had shown three important results: “Firstly, TNK appeared to be safe in this late window in these selected patients – that is a very important observation. Secondly, reperfusion rates at 24 hours were increased with TNK and we know that this translates into clinical benefit. And thirdly, there was a neutral effect on primary outcomes, but I think the sample size of 438 patients was not large enough to show efficacy.”
Dr. Tsivgoulis concluded that these points need to be addressed in future trials.
The TIMELESS trial was funded by Genentech.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
Buprenorphine update: Looser rules and a helpful injectable
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
AT APA 2023
Regular exercise may boost pain tolerance
new research suggests.
In a large observational study of more than 10,000 adults, researchers found those who consistently engage in moderate to vigorous physical activity over the 7- to 8-year study period reported the highest pain tolerance. However, the results also showed that even light exercise was associated with greater pain tolerance.
“There were indications that both total amount of physical activity over time, as well as the direction of change in activity level over time matters to how high your pain tolerance is,” lead investigator Anders Pedersen Årnes, PT, MPH, research fellow and adviser at the University Hospital of North Norway, affiliated with the University of Tromsø, said in an interview. “As an observational study, this points toward the possibility that increased physical activity might increase pain tolerance.”
The findings were published online in PLOS One.
Anything is better than nothing
The researchers drew from the prospective, population-based Tromsø health study, a health survey that draws on surveys conducted periodically since 1974 among residents in northern Norway.
The study included 10,732 participants who completed surveys in 2007-2008 and again in 2015-2016.
Data on physical activity, experimental pain tolerance, sex, sociodemographic covariates, and chronic pain was collected through questionnaires, biological samples and clinical examination.
Pain tolerance was measured using the cold-pressor test (CPT), in which participants submerge their hand in icy water for as long as possible.
CPT tolerance was 7%, 14%, and 16% higher respectively for light, moderate, and vigorous consistent exercise across the two surveys versus the sedentary group.
“Engaging in habitual physical activity in leisure time is associated with higher pain tolerance,” Mr. Årnes said. “Any kind of activity over time is better than being sedentary.”
The researchers also found that people who were sedentary at baseline who reported greater physical activity at follow-up also had higher pain tolerance than those who remained sedentary, although this finding was not statistically significant.
This highest pain tolerance was noted in people who engaged in moderate to vigorous exercise over time, with a 20.4-second longer performance in the CPT than those who were consistently sedentary (P < .001; 95% confidence interval, 13.7-27.1).
There was no significant difference in pain tolerance between men and women and all participants experienced a decline in tolerance over time.
“Results indicate that a positive change in physical activity level over time was associated with higher pain tolerance,” Mr. Årnes said. “Your total activity level might decide how much, as more seems to be better.”
More work needed
The long follow-up and large number of patients are two strengths of the study, Steven Cohen, MD, chief of pain medicine and professor of anesthesiology, neurology, physical medicine & rehabilitation and psychiatry at Johns Hopkins University, Baltimore, said in an interview.
“This study explored the relationship between general physical activity levels and one form of acute pain, but data from other studies show a benefit for other forms of pain,” said Dr. Cohen, who was not part of the research. “Taken together, this suggests that exercise is beneficial for individuals living with pain.”
The findings demonstrate an association between exercise and pain tolerance and other research has shown evidence of a cause-and-effect relationship, Dr. Cohen said. However, “more work is needed to determine what mediates these effects.”
Questions also remain about how exercise might impact tolerance or risk for chronic pain, he added.
Investigators are now working on a follow-up study of how the effect of exercise on pain tolerance might influence chronic pain risk, Mr. Årnes said.
The study received no specific funding. Mr. Årnes and Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a large observational study of more than 10,000 adults, researchers found those who consistently engage in moderate to vigorous physical activity over the 7- to 8-year study period reported the highest pain tolerance. However, the results also showed that even light exercise was associated with greater pain tolerance.
“There were indications that both total amount of physical activity over time, as well as the direction of change in activity level over time matters to how high your pain tolerance is,” lead investigator Anders Pedersen Årnes, PT, MPH, research fellow and adviser at the University Hospital of North Norway, affiliated with the University of Tromsø, said in an interview. “As an observational study, this points toward the possibility that increased physical activity might increase pain tolerance.”
The findings were published online in PLOS One.
Anything is better than nothing
The researchers drew from the prospective, population-based Tromsø health study, a health survey that draws on surveys conducted periodically since 1974 among residents in northern Norway.
The study included 10,732 participants who completed surveys in 2007-2008 and again in 2015-2016.
Data on physical activity, experimental pain tolerance, sex, sociodemographic covariates, and chronic pain was collected through questionnaires, biological samples and clinical examination.
Pain tolerance was measured using the cold-pressor test (CPT), in which participants submerge their hand in icy water for as long as possible.
CPT tolerance was 7%, 14%, and 16% higher respectively for light, moderate, and vigorous consistent exercise across the two surveys versus the sedentary group.
“Engaging in habitual physical activity in leisure time is associated with higher pain tolerance,” Mr. Årnes said. “Any kind of activity over time is better than being sedentary.”
The researchers also found that people who were sedentary at baseline who reported greater physical activity at follow-up also had higher pain tolerance than those who remained sedentary, although this finding was not statistically significant.
This highest pain tolerance was noted in people who engaged in moderate to vigorous exercise over time, with a 20.4-second longer performance in the CPT than those who were consistently sedentary (P < .001; 95% confidence interval, 13.7-27.1).
There was no significant difference in pain tolerance between men and women and all participants experienced a decline in tolerance over time.
“Results indicate that a positive change in physical activity level over time was associated with higher pain tolerance,” Mr. Årnes said. “Your total activity level might decide how much, as more seems to be better.”
More work needed
The long follow-up and large number of patients are two strengths of the study, Steven Cohen, MD, chief of pain medicine and professor of anesthesiology, neurology, physical medicine & rehabilitation and psychiatry at Johns Hopkins University, Baltimore, said in an interview.
“This study explored the relationship between general physical activity levels and one form of acute pain, but data from other studies show a benefit for other forms of pain,” said Dr. Cohen, who was not part of the research. “Taken together, this suggests that exercise is beneficial for individuals living with pain.”
The findings demonstrate an association between exercise and pain tolerance and other research has shown evidence of a cause-and-effect relationship, Dr. Cohen said. However, “more work is needed to determine what mediates these effects.”
Questions also remain about how exercise might impact tolerance or risk for chronic pain, he added.
Investigators are now working on a follow-up study of how the effect of exercise on pain tolerance might influence chronic pain risk, Mr. Årnes said.
The study received no specific funding. Mr. Årnes and Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a large observational study of more than 10,000 adults, researchers found those who consistently engage in moderate to vigorous physical activity over the 7- to 8-year study period reported the highest pain tolerance. However, the results also showed that even light exercise was associated with greater pain tolerance.
“There were indications that both total amount of physical activity over time, as well as the direction of change in activity level over time matters to how high your pain tolerance is,” lead investigator Anders Pedersen Årnes, PT, MPH, research fellow and adviser at the University Hospital of North Norway, affiliated with the University of Tromsø, said in an interview. “As an observational study, this points toward the possibility that increased physical activity might increase pain tolerance.”
The findings were published online in PLOS One.
Anything is better than nothing
The researchers drew from the prospective, population-based Tromsø health study, a health survey that draws on surveys conducted periodically since 1974 among residents in northern Norway.
The study included 10,732 participants who completed surveys in 2007-2008 and again in 2015-2016.
Data on physical activity, experimental pain tolerance, sex, sociodemographic covariates, and chronic pain was collected through questionnaires, biological samples and clinical examination.
Pain tolerance was measured using the cold-pressor test (CPT), in which participants submerge their hand in icy water for as long as possible.
CPT tolerance was 7%, 14%, and 16% higher respectively for light, moderate, and vigorous consistent exercise across the two surveys versus the sedentary group.
“Engaging in habitual physical activity in leisure time is associated with higher pain tolerance,” Mr. Årnes said. “Any kind of activity over time is better than being sedentary.”
The researchers also found that people who were sedentary at baseline who reported greater physical activity at follow-up also had higher pain tolerance than those who remained sedentary, although this finding was not statistically significant.
This highest pain tolerance was noted in people who engaged in moderate to vigorous exercise over time, with a 20.4-second longer performance in the CPT than those who were consistently sedentary (P < .001; 95% confidence interval, 13.7-27.1).
There was no significant difference in pain tolerance between men and women and all participants experienced a decline in tolerance over time.
“Results indicate that a positive change in physical activity level over time was associated with higher pain tolerance,” Mr. Årnes said. “Your total activity level might decide how much, as more seems to be better.”
More work needed
The long follow-up and large number of patients are two strengths of the study, Steven Cohen, MD, chief of pain medicine and professor of anesthesiology, neurology, physical medicine & rehabilitation and psychiatry at Johns Hopkins University, Baltimore, said in an interview.
“This study explored the relationship between general physical activity levels and one form of acute pain, but data from other studies show a benefit for other forms of pain,” said Dr. Cohen, who was not part of the research. “Taken together, this suggests that exercise is beneficial for individuals living with pain.”
The findings demonstrate an association between exercise and pain tolerance and other research has shown evidence of a cause-and-effect relationship, Dr. Cohen said. However, “more work is needed to determine what mediates these effects.”
Questions also remain about how exercise might impact tolerance or risk for chronic pain, he added.
Investigators are now working on a follow-up study of how the effect of exercise on pain tolerance might influence chronic pain risk, Mr. Årnes said.
The study received no specific funding. Mr. Årnes and Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS ONE
Positive top-line results for cannabinoid-based med for nerve pain
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
, new top-line results released by Zelira Therapeutics suggest.
“The implications of these results for patients are incredibly promising,” principal investigator Bryan Doner, DO, medical director of HealthyWays Integrated Wellness Solutions, Gibsonia, Pa., said in a news release.
“Through this rigorously designed study, we have demonstrated that ZLT-L-007 is a safe, effective, and well-tolerated alternative for patients who would typically seek a Lyrica-level of pain relief,” he added.
The observational, nonblinded trial tested the efficacy, safety, and tolerability of ZLT-L-007 against pregabalin in 60 adults with diabetic nerve pain.
The study had three groups with 20 patients each (pregabalin alone, pregabalin plus ZLT-L-007, and ZLT-L-007 alone).
Top-line results show the study met its primary endpoint for change in daily pain severity as measured by the percent change from baseline at 30, 60, and 90 days on the Numerical Rating Scale.
For the pregabalin-only group, there was a reduction in symptom severity at all follow-up points, ranging from 20% to 35% (median percent change from baseline), the company said.
For the ZLT-L-007 only group, there was about a 33% reduction in symptom severity at 30 days, and 71% and 78% reduction, respectively, at 60 and 90 days, suggesting a larger improvement in symptom severity than with pregabalin alone, the company said.
For the pregabalin plus ZLT-L-007 group, there was a moderate 20% reduction in symptom severity at 30 days, but a larger reduction at 60 and 90 days (50% and 72%, respectively), which indicates substantially greater improvement in symptom severity than with pregabalin alone, the company said.
The study also met secondary endpoints, including significant decreases in daily pain severity as measured by the Visual Analog Scale and measurable changes in the short-form McGill Pain Questionnaire and Neuropathic Pain Symptom Inventory.
Dr. Doner noted that the top-line data showed “no serious adverse events, and participants’ blood pressure and other safety vitals remained unaffected throughout. This confirms that ZLT-L-007 is a well-tolerated product that delivers statistically significant pain relief, surpassing the levels achieved by Lyrica.”
The company plans to report additional insights from the full study, as they become available, during fiscal year 2023-2024.
A version of this article first appeared on Medscape.com.
ECG implant tightens AFib management, improves outcomes in MONITOR-AF
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.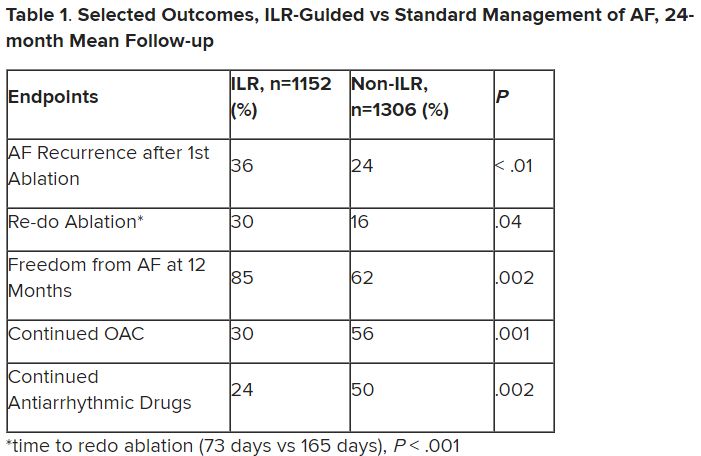
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.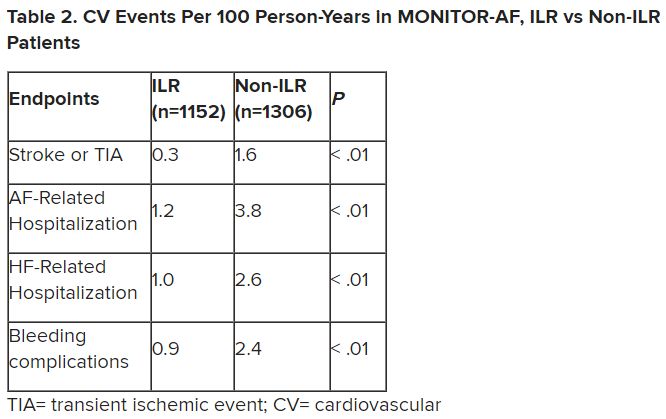
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
Chronic conditions like diabetes or hypertension “often require long-term care through long-term monitoring,” observed a researcher, and “we know that continuous monitoring is superior to intermittent monitoring for long-term outcomes.”
So maybe practice should rely more on continuous ECG monitoring for patients with atrial fibrillation (AFib), also a chronic condition, proposed Dhanunjaya R. Lakkireddy, MD, of the Kansas City Heart Rhythm Institute, Overland Park, Kan., in presenting a new analysis at the annual scientific sessions of the Heart Rhythm Society.
(ILRs), compared with standard care. The latter could include intermittent 12-lead ECG, Holter, or other intermittent monitoring at physicians’ discretion.
Patients with AFib and the ECG implants in the MONITOR-AF study, which was not randomized and therefore only suggestive, were managed “more efficiently” with greater access to electrophysiologists (P < .01) and adherence to oral anticoagulants (P = .020) and other medications.
Followed for a mean of 2 years, patients with ILRs were more likely to undergo catheter ablation, and their time to a catheter ablation “was impressively shorter, 153 days versus 426 days” (P < .001), Dr. Lakkireddy said.
The ILR group also had fewer strokes and bleeding complications and were less likely to be hospitalized for AFib-related reasons, he said, because “a lot of these patients were caught ahead of time through the remote monitoring.”
For example, ILR patients had fewer heart failure (HF) hospitalizations, likely because “you’re not allowing these patients to remain with untreated rapid ventricular rates for a long period of time. You intervene early, thereby mitigating the onset of heart failure.”
Indeed, Dr. Lakkireddy said, their cumulative rate of any cardiovascular complication was “dramatically lower” – 3.4 versus 10.4 events per 100 person-years (P < .001).
Certainly, a routine recommendation to consider AFib patients for continuous monitoring would require randomized-trial evidence, he acknowledged. “This is an observation registry and proof of concept from a very heterogeneous cohort of patients. There were no obvious set criteria for ILR implantation.”
Nonetheless, “continuous and dynamic monitoring enabled quicker decision-making and patient management,” Dr. Lakkireddy said. “Especially in those patients who may have silent atrial fibrillation, an ILR could significantly mitigate the risk of complications from stroke and heart failure exacerbations.”
Several randomized trials have supported “earlier, more aggressive treatment” for AFib, including EAST-AFNET4, EARLY-AF, and CABANA, observed Daniel Morin, MD, MPH, of Ochsner Medical Center, New Orleans, as the invited discussant for Dr. Lakkireddy’s presentation.
So, he continued, if the goal is to “get every single AFib patient to ablation just as soon as possible,” then maybe MONITOR-AF supports the use of ILRs in such cases.
Indeed, it is “certainly possible” that the continuous stream of data from ILRs “allows faster progression of therapy and possibly even better outcomes” as MONITOR-AF suggests, said Dr. Morin, who is director of electrophysiology research at his center.
Moreover, ILR data could potentially “support shared decision-making perhaps by convincing the patient, and maybe their insurers, that we should move forward with ablation.”
But given the study’s observational, registry-based nature, the MONITOR-AF analysis is limited by potential confounders that complicate its interpretation.
For example, Dr. Morin continued, all ILR patients but only 60% of those on standard care˙ had access to an electrophysiologist (P = .001). That means “less access to some antiarrhythmic medications and certainly far less access to ablation therapy.”
Moreover, “during shared decision-making, a patient who sees the results of their ILR monitoring may be more prone to seek out or to accept earlier, more definitive therapy via ablation,” he said. “The presence of an ILR may then be a good way to move the needle toward ablation.”
Of note, an overwhelming majority of ILR patients received ablation, 93.5%, compared with 58.6% of standard-care patients. “It’s unclear how much of that association was caused by the ILR’s presence vs. other factors, such as physician availability, physician aggressiveness, or patient willingness for intervention,” Dr. Morin noted.
MONITOR-AF included 2,458 patients with paroxysmal or persistent AFib who either were implanted with or did not receive an ILR from 2018 to 2021 and were followed for at least 12 months.
The two groups were similar, Dr. Lakkireddy reported, with respect to demographics and baseline history AFib, hypertension, hyperlipidemia, diabetes, coronary disease, neurovascular events, peripheral artery disease, and obstructive sleep apnea.
Dr. Lakkireddy said a subgroup analysis is forthcoming, but that he’d “intuitively” think that the 15%-20% of AFib patients who are asymptomatic would gain the most from the ILR monitoring approach. There is already evidence that such patients tend to have the worst AFib outcomes, often receiving an AFib diagnosis only after presenting with consequences such as stroke or heart failure.
Dr. Lakkireddy disclosed receiving research grants, modest honoraria, or consulting fees from Abbott, Janssen, Boston Scientific, Johnson & Johnson, Biotronik, Bristol-Myers Squibb, Pfizer, Atricure, Northeast Scientific, and Acutus. Dr. Morin disclosed receiving research grants, honoraria, or consulting fees from Abbott and serving on a speakers’ bureau for Boston Scientific, Medtronic, and Zoll Medical.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023
Troponin to ID diabetes patients with silent heart disease?
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Abortion restrictions linked to less evidence-based care for miscarriages
BALTIMORE – , according to a cross-sectional study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists and published in Obstetrics & Gynecology.
The results revealed that “abortion restrictions have far-reaching effects on early pregnancy loss care and on resident education,” the researchers concluded.
“Abortion restrictions don’t just affect people seeking abortions; they affect people also suffering from early pregnancy loss,” Aurora Phillips, MD, an ob.gyn. resident at Albany (N.Y.) Medical Center, said in an interview. “It’s harder to make that diagnosis and to be able to offer interventions, and these institutions that had restrictions also were less likely to have mifepristone or office based human aspiration, which are the most efficient and cost-effective interventions that we have.”
For example, less than half the programs surveyed offered mifepristone to help manage a miscarriage, “with availability varying inversely with abortion restrictions,” they found. After considering all characteristics of residency programs, “institutional abortion restrictions and bans were more important than state policies or religious affiliation in determining whether evidence-based early pregnancy loss treatments were available,” the researchers found, though their findings predated the Supreme Court’s Dobbs ruling that overturned Roe v. Wade. “Training institutions with a commitment to evidence-based family planning care and education are able to ensure access to the most evidence-based, cost-effective, and timely treatments for pregnancy loss even in the face of state abortion restrictions, thereby preserving patient safety, physician competency, and health care system sustainability,” they wrote.
Reduced access leads to higher risk interventions
An estimated 10%-20% of pregnancies result in early miscarriage, totaling more than one million cases in the U.S. each year. But since treatments for miscarriage often overlap with those for abortion, the researchers wondered whether differences existed in how providers managed miscarriages in states or institutions with strict abortion restrictions versus management in hospitals without restrictions.
They also looked at how closely the management strategies adhered to ACOG’s recommendations, which advise that providers consider both ultrasound imaging and other factors, including clinical reasoning and patient preferences, before diagnosing early pregnancy loss and considering possible interventions.
For imaging guidelines, ACOG endorses the criteria established for ultrasound diagnosis of first trimester pregnancy loss from the Society of Radiologists in 2012. But, the authors note, these guidelines are very conservative, exceeding previous measurements that had a 99%-100% predictive value for pregnancy loss, in the interest of “[prioritizing preservation of] fetal potential over facilitating expeditious care.” Hence the reason ACOG advises providers to include clinical judgment and patient preferences in their approach to care.
”In places where abortion is heavily regulated, clinicians managing miscarriages may cautiously rely on the strictest criteria to differentiate early pregnancy loss from potentially viable pregnancy and may not offer certain treatments commonly associated with abortion,” the authors noted. ACOG recommends surgical aspiration and medical treatment with both mifepristone and misoprostol as the safest and most effective options in managing miscarriages.
“Treating early pregnancy loss without the use of mifepristone is more likely to fail, is more likely to require an unscheduled procedure, and people who choose medication management for their miscarriages are usually trying to avoid a procedure, so that is the downside of not using mifepristone,” coauthor Rachel M. Flink-Bochacki, MD, an associate professor at Albany (N.Y.) Medical Center, said in an interview.
“Office-based uterine aspiration has the same safety profile as uterine aspiration in the operating room minus the risks of anesthesia and also helps patients get in faster because they don’t need to wait for OR time,” Dr. Flink-Bochacki explained. “So again, for a patient who wants an aspiration and does not want to pass the pregnancy at home, not having access to office-based aspiration could lead them to miscarry at home, which has higher risks and is not what they wanted.”
Reduced access to miscarriage care options in ‘hostile’ states
Among all 296 U.S. ob.gyn. residency programs that were contacted between November 2021 and January 2022, half (50.3%) responded to the researchers’ survey about their institutional practices around miscarriage, including location of diagnosis, use of ultrasound diagnostic guidelines, treatment options offered by their institution, and institutional restrictions on abortions based on indication.
The survey also collected characteristics of each program, including its state, setting, religious affiliation, and affiliation with the Ryan Training Program in Abortion and Family Planning. The responding sample had similar geographic distribution and state abortion policies as those who did not respond, but the responding programs were slightly more likely to be academic programs and to be affiliated with the Ryan program.
At the time of the study, prior to the Dobbs ruling, more than half the U.S. states had legislation restricting abortion care, and 57% of national teaching hospitals had internal restrictions that limited care based on gestational age and indication, particularly if the indication was elective, the authors reported. The researchers relied on designations from the Guttmacher Institute in December 2020 to categorize states as “hostile” to abortion (very hostile, hostile, and leans hostile) or non-hostile (neutral, leans supportive, supportive, and very supportive).
Most of the programs (80%) had no religious affiliation, but 11% had a Catholic affiliation and 5% had a different Christian affiliation. Institutional policies either had no restrictions on abortion care (38%), had restrictions (39%) based on certain maternal or fetal indications, or completely banned abortion services unless the mother’s life was threatened (23%). Among the Christian-affiliated programs, 60% had bans and 40% had restrictions.
Half (49.7%) of the responding programs relied rigidly on ultrasound criteria before offering any intervention for suspected early pregnancy loss, regardless of patient preferences. The other half (50.3%) incorporated ultrasound criteria and other factors, including clinical judgment and patient preferences, into a holistic determination of what options to present to the patient.
Before accounting for other factors, the researchers found that only a third (33%) of programs in states with severe abortion restrictions considered additional factors besides imaging when offering patients options for miscarriage management. In states without such abortion restrictions, 79% of programs considered both imaging and other factors (P < .001).
In states with “hostile abortion legislation,” only 32% of the programs used mifepristone for miscarriage management, compared with 75% of the programs in states without onerous abortion restrictions (P < .001). The results were similar for use of office-based suction aspiration: Just under half the programs (48%) in states with severe abortion restrictions included this technique as part of standard miscarriage management, compared with 68% of programs in states without such restrictions (P = .014).
Those findings match up with the experience of Cara Heuser, MD, a maternal-fetal medicine specialist from Salt Lake City, who was not involved in this study.
“We had a lot of restrictions even before Roe fell,” including heavy regulation of mifepristone, Dr. Heuser said in an interview. “In non-restricted states, it’s pretty easy to get, but even before Roe in our state, it was very, very difficult to get institutions and individual doctor’s offices to carry mifepristone to treat miscarriages. They were still treating miscarriages in a way that was known to be less effective.” Adding mifepristone to misoprostol reduces the risk of needing an evacuation surgery procedure, she explained, “so adding the mifepristone makes it safer.”
Institutional policies had the strongest impact
Before accounting for the state a hospital was in, 27% of institutions with restrictive abortion policies looked at more than imaging in determining how to proceed, compared with 88% of institutions without abortion restrictions that included clinical judgment and patient preferences in their management.
After controlling for state policies and affiliation with a family planning training program or a religious entity, the odds of an institution relying solely on imaging guidelines were over 12 times greater for institutions with abortion restrictions or bans (odds ratio, 12.3; 95% confidence interval, 3.2-47.9). Specifically, the odds were 9 times greater for institutions with restrictions and 27 times greater for institutions with bans.
Only 12% of the institutions without restrictions relied solely on ultrasound criteria, compared with 67% of the institutions with restrictions and 82% of the institutions that banned all abortions except to save the life of the pregnant individual (P < .001).
Only one in four (25%) of the programs with institutional abortion restrictions used mifepristone, compared with 86% of unrestricted programs (P < .001), and 40% of programs with institutional abortion restrictions used office-based aspiration, compared with 81% of unrestricted programs (P < .001).
Without access to all evidence-based treatments, doctors are often forced to choose expectant management for miscarriages. “So you’re kind of forced to have them to pass the pregnancy at home, which can be traumatic for patients” if that’s not what they wanted, Dr. Phillips said.
Dr. Flink-Bochacki further noted that this patient population is already particularly vulnerable.
“Especially for patients with early pregnancy loss, it’s such a feeling of powerlessness already, so the mental state that many of these patients are in is already quite fraught,” Dr. Flink-Bochacki said. “Then to not even have power to choose the interventions that you want or to be able to access interventions in a timely fashion because you’re being held to some arbitrary guideline further takes away the power and further exacerbates the trauma of the experience.”
The biggest factor likely driving the reduced access to those interventions is the fear that the care could be confused with providing an abortion instead of simply managing a miscarriage, Dr. Flink-Bochacki said. “I think that’s why a lot of these programs don’t have mifepristone and don’t offer outpatient uterine aspiration,” she said. “Because those are so widely used in abortion and the connotation is with abortion, they’re just kind of steering clear of it, but meanwhile, patients with pregnancy loss are suffering because they’re being unnecessarily restrictive.”
The research did not use any external funding, and the authors and Dr. Heuser had no disclosures.
BALTIMORE – , according to a cross-sectional study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists and published in Obstetrics & Gynecology.
The results revealed that “abortion restrictions have far-reaching effects on early pregnancy loss care and on resident education,” the researchers concluded.
“Abortion restrictions don’t just affect people seeking abortions; they affect people also suffering from early pregnancy loss,” Aurora Phillips, MD, an ob.gyn. resident at Albany (N.Y.) Medical Center, said in an interview. “It’s harder to make that diagnosis and to be able to offer interventions, and these institutions that had restrictions also were less likely to have mifepristone or office based human aspiration, which are the most efficient and cost-effective interventions that we have.”
For example, less than half the programs surveyed offered mifepristone to help manage a miscarriage, “with availability varying inversely with abortion restrictions,” they found. After considering all characteristics of residency programs, “institutional abortion restrictions and bans were more important than state policies or religious affiliation in determining whether evidence-based early pregnancy loss treatments were available,” the researchers found, though their findings predated the Supreme Court’s Dobbs ruling that overturned Roe v. Wade. “Training institutions with a commitment to evidence-based family planning care and education are able to ensure access to the most evidence-based, cost-effective, and timely treatments for pregnancy loss even in the face of state abortion restrictions, thereby preserving patient safety, physician competency, and health care system sustainability,” they wrote.
Reduced access leads to higher risk interventions
An estimated 10%-20% of pregnancies result in early miscarriage, totaling more than one million cases in the U.S. each year. But since treatments for miscarriage often overlap with those for abortion, the researchers wondered whether differences existed in how providers managed miscarriages in states or institutions with strict abortion restrictions versus management in hospitals without restrictions.
They also looked at how closely the management strategies adhered to ACOG’s recommendations, which advise that providers consider both ultrasound imaging and other factors, including clinical reasoning and patient preferences, before diagnosing early pregnancy loss and considering possible interventions.
For imaging guidelines, ACOG endorses the criteria established for ultrasound diagnosis of first trimester pregnancy loss from the Society of Radiologists in 2012. But, the authors note, these guidelines are very conservative, exceeding previous measurements that had a 99%-100% predictive value for pregnancy loss, in the interest of “[prioritizing preservation of] fetal potential over facilitating expeditious care.” Hence the reason ACOG advises providers to include clinical judgment and patient preferences in their approach to care.
”In places where abortion is heavily regulated, clinicians managing miscarriages may cautiously rely on the strictest criteria to differentiate early pregnancy loss from potentially viable pregnancy and may not offer certain treatments commonly associated with abortion,” the authors noted. ACOG recommends surgical aspiration and medical treatment with both mifepristone and misoprostol as the safest and most effective options in managing miscarriages.
“Treating early pregnancy loss without the use of mifepristone is more likely to fail, is more likely to require an unscheduled procedure, and people who choose medication management for their miscarriages are usually trying to avoid a procedure, so that is the downside of not using mifepristone,” coauthor Rachel M. Flink-Bochacki, MD, an associate professor at Albany (N.Y.) Medical Center, said in an interview.
“Office-based uterine aspiration has the same safety profile as uterine aspiration in the operating room minus the risks of anesthesia and also helps patients get in faster because they don’t need to wait for OR time,” Dr. Flink-Bochacki explained. “So again, for a patient who wants an aspiration and does not want to pass the pregnancy at home, not having access to office-based aspiration could lead them to miscarry at home, which has higher risks and is not what they wanted.”
Reduced access to miscarriage care options in ‘hostile’ states
Among all 296 U.S. ob.gyn. residency programs that were contacted between November 2021 and January 2022, half (50.3%) responded to the researchers’ survey about their institutional practices around miscarriage, including location of diagnosis, use of ultrasound diagnostic guidelines, treatment options offered by their institution, and institutional restrictions on abortions based on indication.
The survey also collected characteristics of each program, including its state, setting, religious affiliation, and affiliation with the Ryan Training Program in Abortion and Family Planning. The responding sample had similar geographic distribution and state abortion policies as those who did not respond, but the responding programs were slightly more likely to be academic programs and to be affiliated with the Ryan program.
At the time of the study, prior to the Dobbs ruling, more than half the U.S. states had legislation restricting abortion care, and 57% of national teaching hospitals had internal restrictions that limited care based on gestational age and indication, particularly if the indication was elective, the authors reported. The researchers relied on designations from the Guttmacher Institute in December 2020 to categorize states as “hostile” to abortion (very hostile, hostile, and leans hostile) or non-hostile (neutral, leans supportive, supportive, and very supportive).
Most of the programs (80%) had no religious affiliation, but 11% had a Catholic affiliation and 5% had a different Christian affiliation. Institutional policies either had no restrictions on abortion care (38%), had restrictions (39%) based on certain maternal or fetal indications, or completely banned abortion services unless the mother’s life was threatened (23%). Among the Christian-affiliated programs, 60% had bans and 40% had restrictions.
Half (49.7%) of the responding programs relied rigidly on ultrasound criteria before offering any intervention for suspected early pregnancy loss, regardless of patient preferences. The other half (50.3%) incorporated ultrasound criteria and other factors, including clinical judgment and patient preferences, into a holistic determination of what options to present to the patient.
Before accounting for other factors, the researchers found that only a third (33%) of programs in states with severe abortion restrictions considered additional factors besides imaging when offering patients options for miscarriage management. In states without such abortion restrictions, 79% of programs considered both imaging and other factors (P < .001).
In states with “hostile abortion legislation,” only 32% of the programs used mifepristone for miscarriage management, compared with 75% of the programs in states without onerous abortion restrictions (P < .001). The results were similar for use of office-based suction aspiration: Just under half the programs (48%) in states with severe abortion restrictions included this technique as part of standard miscarriage management, compared with 68% of programs in states without such restrictions (P = .014).
Those findings match up with the experience of Cara Heuser, MD, a maternal-fetal medicine specialist from Salt Lake City, who was not involved in this study.
“We had a lot of restrictions even before Roe fell,” including heavy regulation of mifepristone, Dr. Heuser said in an interview. “In non-restricted states, it’s pretty easy to get, but even before Roe in our state, it was very, very difficult to get institutions and individual doctor’s offices to carry mifepristone to treat miscarriages. They were still treating miscarriages in a way that was known to be less effective.” Adding mifepristone to misoprostol reduces the risk of needing an evacuation surgery procedure, she explained, “so adding the mifepristone makes it safer.”
Institutional policies had the strongest impact
Before accounting for the state a hospital was in, 27% of institutions with restrictive abortion policies looked at more than imaging in determining how to proceed, compared with 88% of institutions without abortion restrictions that included clinical judgment and patient preferences in their management.
After controlling for state policies and affiliation with a family planning training program or a religious entity, the odds of an institution relying solely on imaging guidelines were over 12 times greater for institutions with abortion restrictions or bans (odds ratio, 12.3; 95% confidence interval, 3.2-47.9). Specifically, the odds were 9 times greater for institutions with restrictions and 27 times greater for institutions with bans.
Only 12% of the institutions without restrictions relied solely on ultrasound criteria, compared with 67% of the institutions with restrictions and 82% of the institutions that banned all abortions except to save the life of the pregnant individual (P < .001).
Only one in four (25%) of the programs with institutional abortion restrictions used mifepristone, compared with 86% of unrestricted programs (P < .001), and 40% of programs with institutional abortion restrictions used office-based aspiration, compared with 81% of unrestricted programs (P < .001).
Without access to all evidence-based treatments, doctors are often forced to choose expectant management for miscarriages. “So you’re kind of forced to have them to pass the pregnancy at home, which can be traumatic for patients” if that’s not what they wanted, Dr. Phillips said.
Dr. Flink-Bochacki further noted that this patient population is already particularly vulnerable.
“Especially for patients with early pregnancy loss, it’s such a feeling of powerlessness already, so the mental state that many of these patients are in is already quite fraught,” Dr. Flink-Bochacki said. “Then to not even have power to choose the interventions that you want or to be able to access interventions in a timely fashion because you’re being held to some arbitrary guideline further takes away the power and further exacerbates the trauma of the experience.”
The biggest factor likely driving the reduced access to those interventions is the fear that the care could be confused with providing an abortion instead of simply managing a miscarriage, Dr. Flink-Bochacki said. “I think that’s why a lot of these programs don’t have mifepristone and don’t offer outpatient uterine aspiration,” she said. “Because those are so widely used in abortion and the connotation is with abortion, they’re just kind of steering clear of it, but meanwhile, patients with pregnancy loss are suffering because they’re being unnecessarily restrictive.”
The research did not use any external funding, and the authors and Dr. Heuser had no disclosures.
BALTIMORE – , according to a cross-sectional study presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists and published in Obstetrics & Gynecology.
The results revealed that “abortion restrictions have far-reaching effects on early pregnancy loss care and on resident education,” the researchers concluded.
“Abortion restrictions don’t just affect people seeking abortions; they affect people also suffering from early pregnancy loss,” Aurora Phillips, MD, an ob.gyn. resident at Albany (N.Y.) Medical Center, said in an interview. “It’s harder to make that diagnosis and to be able to offer interventions, and these institutions that had restrictions also were less likely to have mifepristone or office based human aspiration, which are the most efficient and cost-effective interventions that we have.”
For example, less than half the programs surveyed offered mifepristone to help manage a miscarriage, “with availability varying inversely with abortion restrictions,” they found. After considering all characteristics of residency programs, “institutional abortion restrictions and bans were more important than state policies or religious affiliation in determining whether evidence-based early pregnancy loss treatments were available,” the researchers found, though their findings predated the Supreme Court’s Dobbs ruling that overturned Roe v. Wade. “Training institutions with a commitment to evidence-based family planning care and education are able to ensure access to the most evidence-based, cost-effective, and timely treatments for pregnancy loss even in the face of state abortion restrictions, thereby preserving patient safety, physician competency, and health care system sustainability,” they wrote.
Reduced access leads to higher risk interventions
An estimated 10%-20% of pregnancies result in early miscarriage, totaling more than one million cases in the U.S. each year. But since treatments for miscarriage often overlap with those for abortion, the researchers wondered whether differences existed in how providers managed miscarriages in states or institutions with strict abortion restrictions versus management in hospitals without restrictions.
They also looked at how closely the management strategies adhered to ACOG’s recommendations, which advise that providers consider both ultrasound imaging and other factors, including clinical reasoning and patient preferences, before diagnosing early pregnancy loss and considering possible interventions.
For imaging guidelines, ACOG endorses the criteria established for ultrasound diagnosis of first trimester pregnancy loss from the Society of Radiologists in 2012. But, the authors note, these guidelines are very conservative, exceeding previous measurements that had a 99%-100% predictive value for pregnancy loss, in the interest of “[prioritizing preservation of] fetal potential over facilitating expeditious care.” Hence the reason ACOG advises providers to include clinical judgment and patient preferences in their approach to care.
”In places where abortion is heavily regulated, clinicians managing miscarriages may cautiously rely on the strictest criteria to differentiate early pregnancy loss from potentially viable pregnancy and may not offer certain treatments commonly associated with abortion,” the authors noted. ACOG recommends surgical aspiration and medical treatment with both mifepristone and misoprostol as the safest and most effective options in managing miscarriages.
“Treating early pregnancy loss without the use of mifepristone is more likely to fail, is more likely to require an unscheduled procedure, and people who choose medication management for their miscarriages are usually trying to avoid a procedure, so that is the downside of not using mifepristone,” coauthor Rachel M. Flink-Bochacki, MD, an associate professor at Albany (N.Y.) Medical Center, said in an interview.
“Office-based uterine aspiration has the same safety profile as uterine aspiration in the operating room minus the risks of anesthesia and also helps patients get in faster because they don’t need to wait for OR time,” Dr. Flink-Bochacki explained. “So again, for a patient who wants an aspiration and does not want to pass the pregnancy at home, not having access to office-based aspiration could lead them to miscarry at home, which has higher risks and is not what they wanted.”
Reduced access to miscarriage care options in ‘hostile’ states
Among all 296 U.S. ob.gyn. residency programs that were contacted between November 2021 and January 2022, half (50.3%) responded to the researchers’ survey about their institutional practices around miscarriage, including location of diagnosis, use of ultrasound diagnostic guidelines, treatment options offered by their institution, and institutional restrictions on abortions based on indication.
The survey also collected characteristics of each program, including its state, setting, religious affiliation, and affiliation with the Ryan Training Program in Abortion and Family Planning. The responding sample had similar geographic distribution and state abortion policies as those who did not respond, but the responding programs were slightly more likely to be academic programs and to be affiliated with the Ryan program.
At the time of the study, prior to the Dobbs ruling, more than half the U.S. states had legislation restricting abortion care, and 57% of national teaching hospitals had internal restrictions that limited care based on gestational age and indication, particularly if the indication was elective, the authors reported. The researchers relied on designations from the Guttmacher Institute in December 2020 to categorize states as “hostile” to abortion (very hostile, hostile, and leans hostile) or non-hostile (neutral, leans supportive, supportive, and very supportive).
Most of the programs (80%) had no religious affiliation, but 11% had a Catholic affiliation and 5% had a different Christian affiliation. Institutional policies either had no restrictions on abortion care (38%), had restrictions (39%) based on certain maternal or fetal indications, or completely banned abortion services unless the mother’s life was threatened (23%). Among the Christian-affiliated programs, 60% had bans and 40% had restrictions.
Half (49.7%) of the responding programs relied rigidly on ultrasound criteria before offering any intervention for suspected early pregnancy loss, regardless of patient preferences. The other half (50.3%) incorporated ultrasound criteria and other factors, including clinical judgment and patient preferences, into a holistic determination of what options to present to the patient.
Before accounting for other factors, the researchers found that only a third (33%) of programs in states with severe abortion restrictions considered additional factors besides imaging when offering patients options for miscarriage management. In states without such abortion restrictions, 79% of programs considered both imaging and other factors (P < .001).
In states with “hostile abortion legislation,” only 32% of the programs used mifepristone for miscarriage management, compared with 75% of the programs in states without onerous abortion restrictions (P < .001). The results were similar for use of office-based suction aspiration: Just under half the programs (48%) in states with severe abortion restrictions included this technique as part of standard miscarriage management, compared with 68% of programs in states without such restrictions (P = .014).
Those findings match up with the experience of Cara Heuser, MD, a maternal-fetal medicine specialist from Salt Lake City, who was not involved in this study.
“We had a lot of restrictions even before Roe fell,” including heavy regulation of mifepristone, Dr. Heuser said in an interview. “In non-restricted states, it’s pretty easy to get, but even before Roe in our state, it was very, very difficult to get institutions and individual doctor’s offices to carry mifepristone to treat miscarriages. They were still treating miscarriages in a way that was known to be less effective.” Adding mifepristone to misoprostol reduces the risk of needing an evacuation surgery procedure, she explained, “so adding the mifepristone makes it safer.”
Institutional policies had the strongest impact
Before accounting for the state a hospital was in, 27% of institutions with restrictive abortion policies looked at more than imaging in determining how to proceed, compared with 88% of institutions without abortion restrictions that included clinical judgment and patient preferences in their management.
After controlling for state policies and affiliation with a family planning training program or a religious entity, the odds of an institution relying solely on imaging guidelines were over 12 times greater for institutions with abortion restrictions or bans (odds ratio, 12.3; 95% confidence interval, 3.2-47.9). Specifically, the odds were 9 times greater for institutions with restrictions and 27 times greater for institutions with bans.
Only 12% of the institutions without restrictions relied solely on ultrasound criteria, compared with 67% of the institutions with restrictions and 82% of the institutions that banned all abortions except to save the life of the pregnant individual (P < .001).
Only one in four (25%) of the programs with institutional abortion restrictions used mifepristone, compared with 86% of unrestricted programs (P < .001), and 40% of programs with institutional abortion restrictions used office-based aspiration, compared with 81% of unrestricted programs (P < .001).
Without access to all evidence-based treatments, doctors are often forced to choose expectant management for miscarriages. “So you’re kind of forced to have them to pass the pregnancy at home, which can be traumatic for patients” if that’s not what they wanted, Dr. Phillips said.
Dr. Flink-Bochacki further noted that this patient population is already particularly vulnerable.
“Especially for patients with early pregnancy loss, it’s such a feeling of powerlessness already, so the mental state that many of these patients are in is already quite fraught,” Dr. Flink-Bochacki said. “Then to not even have power to choose the interventions that you want or to be able to access interventions in a timely fashion because you’re being held to some arbitrary guideline further takes away the power and further exacerbates the trauma of the experience.”
The biggest factor likely driving the reduced access to those interventions is the fear that the care could be confused with providing an abortion instead of simply managing a miscarriage, Dr. Flink-Bochacki said. “I think that’s why a lot of these programs don’t have mifepristone and don’t offer outpatient uterine aspiration,” she said. “Because those are so widely used in abortion and the connotation is with abortion, they’re just kind of steering clear of it, but meanwhile, patients with pregnancy loss are suffering because they’re being unnecessarily restrictive.”
The research did not use any external funding, and the authors and Dr. Heuser had no disclosures.
AT ACOG 2023
COVID boosters effective, but not for long
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.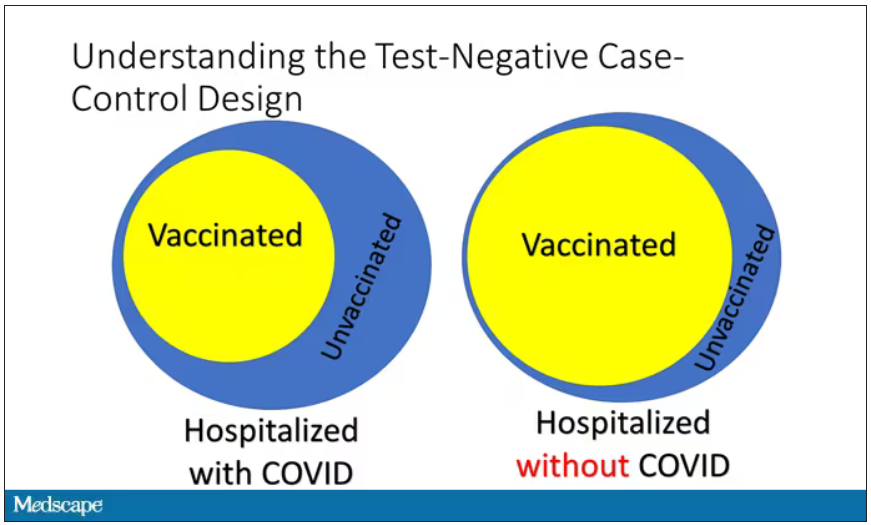
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.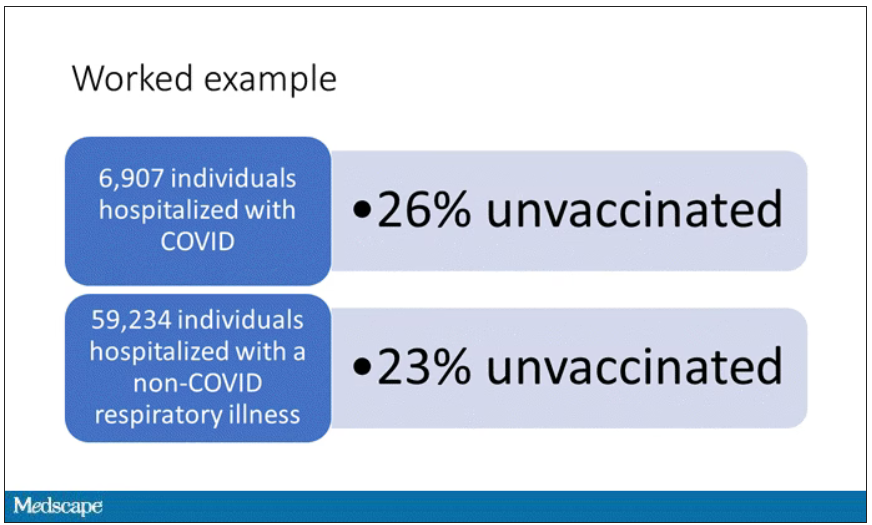
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.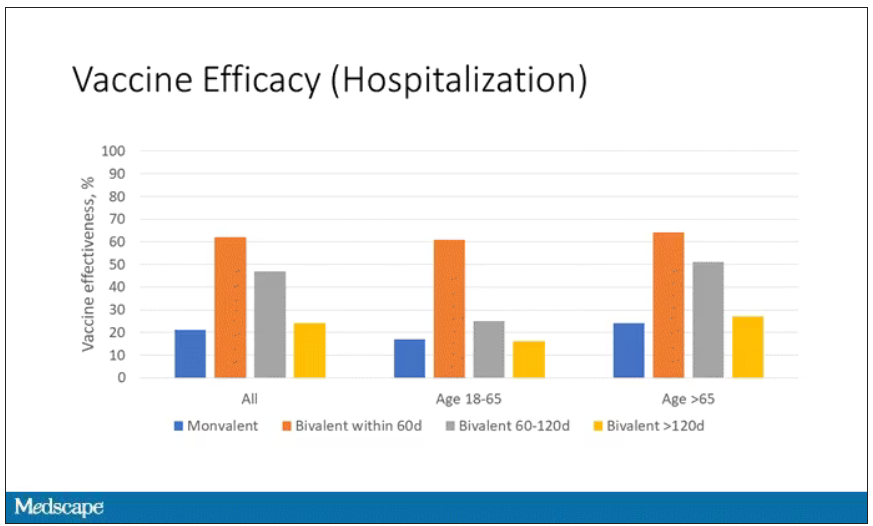
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.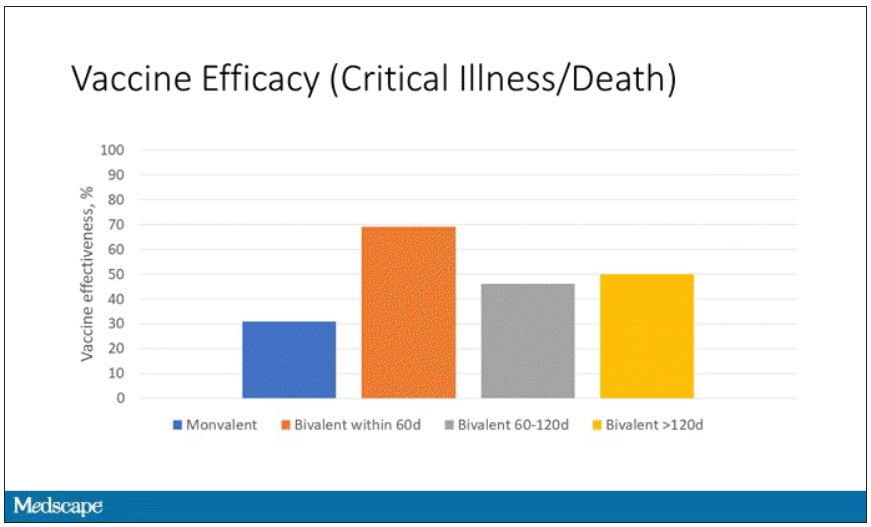
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
I am here today to talk about the effectiveness of COVID vaccine boosters in the midst of 2023. The reason I want to talk about this isn’t necessarily to dig into exactly how effective vaccines are. This is an area that’s been trod upon multiple times. But it does give me an opportunity to talk about a neat study design called the “test-negative case-control” design, which has some unique properties when you’re trying to evaluate the effect of something outside of the context of a randomized trial.
So, just a little bit of background to remind everyone where we are. These are the number of doses of COVID vaccines administered over time throughout the pandemic.
You can see that it’s stratified by age. The orange lines are adults ages 18-49, for example. You can see a big wave of vaccination when the vaccine first came out at the start of 2021. Then subsequently, you can see smaller waves after the first and second booster authorizations, and maybe a bit of a pickup, particularly among older adults, when the bivalent boosters were authorized. But still very little overall pickup of the bivalent booster, compared with the monovalent vaccines, which might suggest vaccine fatigue going on this far into the pandemic. But it’s important to try to understand exactly how effective those new boosters are, at least at this point in time.
I’m talking about Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults – Increasing Community Access to Testing Program, United States, December 2022–January 2023, which came out in the Morbidity and Mortality Weekly Report very recently, which uses this test-negative case-control design to evaluate the ability of bivalent mRNA vaccines to prevent hospitalization.
The question is: Does receipt of a bivalent COVID vaccine booster prevent hospitalizations, ICU stay, or death? That may not be the question that is of interest to everyone. I know people are interested in symptoms, missed work, and transmission, but this paper was looking at hospitalization, ICU stay, and death.
What’s kind of tricky here is that the data they’re using are in people who are hospitalized with various diseases. You might look at that on the surface and say: “Well, you can’t – that’s impossible.” But you can, actually, with this cool test-negative case-control design.
Here’s basically how it works. You take a population of people who are hospitalized and confirmed to have COVID. Some of them will be vaccinated and some of them will be unvaccinated. And the proportion of vaccinated and unvaccinated people doesn’t tell you very much because it depends on how that compares with the rates in the general population, for instance. Let me clarify this. If 100% of the population were vaccinated, then 100% of the people hospitalized with COVID would be vaccinated. That doesn’t mean vaccines are bad. Put another way, if 90% of the population were vaccinated and 60% of people hospitalized with COVID were vaccinated, that would actually show that the vaccines were working to some extent, all else being equal. So it’s not just the raw percentages that tell you anything. Some people are vaccinated, some people aren’t. You need to understand what the baseline rate is.
The test-negative case-control design looks at people who are hospitalized without COVID. Now who those people are (who the controls are, in this case) is something you really need to think about. In the case of this CDC study, they used people who were hospitalized with COVID-like illnesses – flu-like illnesses, respiratory illnesses, pneumonia, influenza, etc. This is a pretty good idea because it standardizes a little bit for people who have access to healthcare. They can get to a hospital and they’re the type of person who would go to a hospital when they’re feeling sick. That’s a better control than the general population overall, which is something I like about this design.
Some of those people who don’t have COVID (they’re in the hospital for flu or whatever) will have been vaccinated for COVID, and some will not have been vaccinated for COVID. And of course, we don’t expect COVID vaccines necessarily to protect against the flu or pneumonia, but that gives us a way to standardize.
If you look at these Venn diagrams, I’ve got vaccinated/unvaccinated being exactly the same proportion, which would suggest that you’re just as likely to be hospitalized with COVID if you’re vaccinated as you are to be hospitalized with some other respiratory illness, which suggests that the vaccine isn’t particularly effective.
However, if you saw something like this, looking at all those patients with flu and other non-COVID illnesses, a lot more of them had been vaccinated for COVID. What that tells you is that we’re seeing fewer vaccinated people hospitalized with COVID than we would expect because we have this standardization from other respiratory infections. We expect this many vaccinated people because that’s how many vaccinated people there are who show up with flu. But in the COVID population, there are fewer, and that would suggest that the vaccines are effective. So that is the test-negative case-control design. You can do the same thing with ICU stays and death.
There are some assumptions here which you might already be thinking about. The most important one is that vaccination status is not associated with the risk for the disease. I always think of older people in this context. During the pandemic, at least in the United States, older people were much more likely to be vaccinated but were also much more likely to contract COVID and be hospitalized with COVID. The test-negative design actually accounts for this in some sense, because older people are also more likely to be hospitalized for things like flu and pneumonia. So there’s some control there.
But to the extent that older people are uniquely susceptible to COVID compared with other respiratory illnesses, that would bias your results to make the vaccines look worse. So the standard approach here is to adjust for these things. I think the CDC adjusted for age, sex, race, ethnicity, and a few other things to settle down and see how effective the vaccines were.
Let’s get to a worked example.
This is the actual data from the CDC paper. They had 6,907 individuals who were hospitalized with COVID, and 26% of them were unvaccinated. What’s the baseline rate that we would expect to be unvaccinated? A total of 59,234 individuals were hospitalized with a non-COVID respiratory illness, and 23% of them were unvaccinated. So you can see that there were more unvaccinated people than you would think in the COVID group. In other words, fewer vaccinated people, which suggests that the vaccine works to some degree because it’s keeping some people out of the hospital.
Now, 26% versus 23% is not a very impressive difference. But it gets more interesting when you break it down by the type of vaccine and how long ago the individual was vaccinated.
Let’s walk through the “all” group on this figure. What you can see is the calculated vaccine effectiveness. If you look at just the monovalent vaccine here, we see a 20% vaccine effectiveness. This means that you’re preventing 20% of hospitalizations basically due to COVID by people getting vaccinated. That’s okay but it’s certainly not anything to write home about. But we see much better vaccine effectiveness with the bivalent vaccine if it had been received within 60 days.
This compares people who received the bivalent vaccine within 60 days in the COVID group and the non-COVID group. The concern that the vaccine was given very recently affects both groups equally so it shouldn’t result in bias there. You see a step-off in vaccine effectiveness from 60 days, 60-120 days, and greater than 120 days. This is 4 months, and you’ve gone from 60% to 20%. When you break that down by age, you can see a similar pattern in the 18-to-65 group and potentially some more protection the greater than 65 age group.
Why is vaccine efficacy going down? The study doesn’t tell us, but we can hypothesize that this might be an immunologic effect – the antibodies or the protective T cells are waning over time. This could also reflect changes in the virus in the environment as the virus seeks to evade certain immune responses. But overall, this suggests that waiting a year between booster doses may leave you exposed for quite some time, although the take-home here is that bivalent vaccines in general are probably a good idea for the proportion of people who haven’t gotten them.
When we look at critical illness and death, the numbers look a little bit better.
You can see that bivalent is better than monovalent – certainly pretty good if you’ve received it within 60 days. It does tend to wane a little bit, but not nearly as much. You’ve still got about 50% vaccine efficacy beyond 120 days when we’re looking at critical illness, which is stays in the ICU and death.
The overriding thing to think about when we think about vaccine policy is that the way you get immunized against COVID is either by vaccine or by getting infected with COVID, or both.
This really interesting graph from the CDC (although it’s updated only through quarter three of 2022) shows the proportion of Americans, based on routine lab tests, who have varying degrees of protection against COVID. What you can see is that, by quarter three of 2022, just 3.6% of people who had blood drawn at a commercial laboratory had no evidence of infection or vaccination. In other words, almost no one was totally naive. Then 26% of people had never been infected – they only have vaccine antibodies – plus 22% of people had only been infected but had never been vaccinated. And then 50% of people had both. So there’s a tremendous amount of existing immunity out there.
The really interesting question about future vaccination and future booster doses is, how does it work on the background of this pattern? The CDC study doesn’t tell us, and I don’t think they have the data to tell us the vaccine efficacy in these different groups. Is it more effective in people who have only had an infection, for example? Is it more effective in people who have only had vaccination versus people who had both, or people who have no protection whatsoever? Those are the really interesting questions that need to be answered going forward as vaccine policy gets developed in the future.
I hope this was a helpful primer on how the test-negative case-control design can answer questions that seem a little bit unanswerable.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.




